UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
Form 10-K
(Mark One)
☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2020
or
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 001-36075
Evoke Pharma, Inc.
(Exact Name of Registrant as Specified in its Charter)
Delaware |
| 20-8447886 |
(State or Other Jurisdiction of Incorporation or Organization) |
| (I.R.S. Employer Identification No.) |
|
|
|
420 Stevens Avenue, Suite 370 Solana Beach, California |
| 92075 |
(Address of Principal Executive Offices) |
| (Zip Code) |
858-345-1494
(Registrant’s Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class | Trading Symbol(s) | Name of Each Exchange on Which Registered |
Common Stock, par value $0.0001 per share | EVOK | The Nasdaq Capital Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). ☒ Yes ☐ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
| ☐ |
| Accelerated filer |
| ☐ |
|
|
|
| |||
Non-accelerated filer |
| ☒ |
| Smaller reporting company |
| ☒ |
|
|
|
|
|
|
|
Emerging growth company |
| ☐ |
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes Oxley Act (15 U.S.C. 7262 (b)) by the registered public accounting firm that prepared or issued its audit report. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the registrant’s common stock held by non-affiliates of the registrant as of the last business day of the registrant’s most recently completed second fiscal quarter was approximately $88.6 million, based on the closing price of the registrant’s common stock on the Nasdaq Capital Market of $3.54 per share.
The number of outstanding shares of the registrant’s common stock, par value $0.0001 per share, as of February 28, 2021 was 32,371,954.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement to be filed with the Securities and Exchange Commission pursuant to Regulation 14A in connection with the registrant’s 2021 Annual Meeting of Stockholders, which will be filed subsequent to the date hereof, are incorporated by reference into Part III of this Form 10-K. Such proxy statement will be filed with the Securities and Exchange Commission not later than 120 days following the end of the registrant’s fiscal year ended December 31, 2020.
EVOKE PHARMA, INC.
FORM 10-K — ANNUAL REPORT
For the Fiscal Year Ended December 31, 2020
Table of Contents
i
Forward-Looking Statements and Market Data
This Annual Report on Form 10-K contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. All statements other than statements of historical facts contained in this Annual Report on Form 10-K, including statements regarding our future results of operations and financial position, business strategy, commercial activities to be conducted by Eversana Life Science Services, LLC, or Eversana, the pricing and reimbursement for Gimoti, future regulatory developments, research and development costs, timing and likelihood of regulatory filings and approvals, commercialization plans, pricing and reimbursement, the potential to develop future product candidates, timing and likelihood of success, plans and objectives of management for future operations, future results of current and anticipated products and the impact of the COVID-19 pandemic, on us or on third parties on whom we rely, are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statement. The forward-looking statements are contained principally in the sections entitled “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business.” In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. Although we believe the expectations reflected in these forward-looking statements are reasonable, such statements are inherently subject to risk and we can give no assurances that our expectations will prove to be correct. Given these risks, uncertainties and other factors, you should not place undue reliance on these forward-looking statements, which speak only as of the date of this Annual Report on Form 10-K. You should read this Annual Report on Form 10-K completely. As a result of many factors, including without limitation those set forth under “Risk Factors” under Item 1A of this Part I below, and elsewhere in this Annual Report on Form 10-K, our actual results may differ materially from those anticipated in these forward-looking statements. Except as required by applicable law, we undertake no obligation to update these forward-looking statements to reflect events or circumstances after the date of this report or to reflect actual outcomes. For all forward-looking statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
This Annual Report on Form 10-K also contains estimates, projections and other information concerning our industry, our business, and the potential markets for Gimoti™ (metoclopramide) nasal spray, including data regarding the estimated size of those markets, their projected growth rates, the incidence of certain medical conditions, statements that certain drugs or classes of drugs are the most widely prescribed in the United States or other markets, the perceptions and preferences of patients and physicians regarding certain therapies and other prescription, prescriber and patient data, as well as data regarding market research, estimates and forecasts prepared by our management. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained this industry, business, market and other data from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources.
We use our registered trademark, EVOKE PHARMA, and other trademarks, including GIMOTI and EvokeAssist, in this Annual Report on Form 10-K. This Annual Report on Form 10-K also includes trademarks, tradenames and service marks that are the property of other organizations. Solely for convenience, trademarks and tradenames referred to in this Annual Report on Form 10-K appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or that the applicable owner will not assert its rights, to these trademarks and tradenames.
Unless the context requires otherwise, references in this Annual Report on Form 10-K to “Evoke,” “we,” “us” and “our” refer to Evoke Pharma, Inc.
Summary of Risks Related to our Business
Our business is subject to numerous risks and uncertainties, including those described in Part I, Item 1A, “Risk Factors.” The principal risks and uncertainties affecting our business include the following:
| • | Our business is entirely dependent on the success of Gimoti, which may never generate sufficient sales to become profitable. |
| • | We may require substantial additional funding and may be unable to raise capital when needed. |
1
|
| • | Any termination or suspension of, or delays in the completion of, the post-marketing pharmacokinetics, or PK, trial of Gimoti or any other future clinical trials could result in increased costs to us, delay or limit our ability to generate revenue and adversely affect our commercial prospects. |
| • | We have no internal sales, marketing or distribution capabilities currently and rely on Eversana, and will rely on other third parties, for the commercialization of Gimoti, and we and they may not be able to effectively market, sell and distribute Gimoti. |
| • | Use of Gimoti or any future product candidates we may develop could be associated with side effects, adverse events or other properties or safety risks, which could delay or preclude approval, cause us to suspend or discontinue clinical trials, abandon a product candidate, limit the commercial profile of the approved labeling, or result in other significant negative consequences. |
| • | Our business may continue to be impacted by epidemic diseases such as the COVID-19 pandemic. |
| • | Even though FDA has approved Gimoti for the relief of symptoms in adults with acute and recurrent diabetic gastroparesis, we will remain subject to significant post-marketing regulatory requirements and oversight. |
| • | It will be difficult for us to profitably sell Gimoti if insurance coverage and reimbursement are limited. |
| • | We rely and will continue to rely on outsourcing arrangements for many of our activities, including commercialization activities and supply of Gimoti. |
| • | We face substantial competition, which may result in others selling their products more effectively than we do, and in others discovering, developing or commercializing product candidates before, or more successfully, than we do. |
| • | Enacted and future legislation may increase the difficulty and cost for us to commercialize Gimoti and affect the prices we may obtain. |
| • | If we fail to develop and commercialize other product candidates, we may be unable to grow our business. |
| • | It is difficult and costly to protect our intellectual property rights, and we cannot ensure the protection of these rights. |
| • | Claims by third parties that we infringe their proprietary rights may result in liability for damages or prevent or delay our developmental and commercialization efforts. |
| • | Changes in patent laws or patent jurisprudence could diminish the value of patents in general, thereby impairing our ability to protect our products. |
| • | Our recurring losses from operations have raised substantial doubt regarding our ability to continue as a going concern. |
| • | We have incurred significant operating losses since inception, and we expect to incur losses for the foreseeable future. We may never become profitable or, if achieved, be able to sustain profitability. |
Overview
We are a specialty pharmaceutical company focused primarily on the development and commercialization of drugs to treat gastrointestinal, or GI, disorders and diseases. Since our inception, we have devoted our efforts to developing our sole product, Gimoti (metoclopramide) nasal spray, the first and only nasally-administered product indicated for the relief of symptoms in adults with acute and recurrent diabetic gastroparesis. On June 19, 2020, we received approval from the U.S. Food and Drug Administration, or FDA, for our 505(b)(2) New Drug Application, or NDA, for Gimoti. We launched commercial sales of Gimoti in the United States in October 2020 through our commercial partner Eversana.
Diabetic gastroparesis is a GI disorder affecting millions of patients worldwide, in which food in an individual’s stomach takes too long to empty resulting in a variety of serious GI symptoms and systemic metabolic complications. The gastric delay caused by gastroparesis can compromise absorption of orally administered medications.
2
Gastroparesis frequently occurs in individuals with diabetes, but is also observed in patients with prior gastric surgery, a preceding infectious illness, pseudo-obstruction, collagen vascular disorders and anorexia nervosa. In some patients with gastroparesis, no cause can be identified, which is referred to as idiopathic gastroparesis. According to the American Motility Society Task Force on Gastroparesis, the prevalence of gastroparesis is estimated to be up to 4% of the United States population. Signs and symptoms of gastroparesis may include nausea, early satiety, bloating, prolonged fullness, upper abdominal pain, vomiting and retching. Patients may experience any combination of signs and symptoms with varying degrees of severity.
Patients with diabetic gastroparesis may experience impaired glucose control due to unpredictable gastric emptying and altered absorption of orally administered hypoglycemic drugs, which may affect the severity of their signs and symptoms. Severe signs and symptoms may cause complications such as malnutrition, esophagitis, and Mallory‑Weiss tears. Gastroparesis adversely affects the lives of patients with the disease, resulting in decreased social interaction, poor work functionality, and the development of anxiety and/or depression.
We believe nasal spray administration has the potential to provide our target population of diabetic gastroparesis patients with a preferred treatment option over the tablet formulation for several important reasons: (1) unlike metoclopramide tablets which may be absorbed erratically due to gastroparesis itself, Gimoti is designed to bypass the digestive system to allow for more predictable absorption without needing to determine if a patient’s stomach is functioning; (2) during episodes of vomiting, Gimoti may provide predictable drug absorption through the nasal mucosa; and (3) for gastroparesis patients experiencing nausea and are not wanting to swallow a pill or water, a nasal spray may be better tolerated than an oral medication.
On January 21, 2020, we entered into an agreement with Eversana, or the Eversana Agreement. Pursuant to the Eversana Agreement, Eversana commercializes and distributes Gimoti in the United States. Eversana also manages the marketing of Gimoti to targeted health care providers, as well as the sales and distribution of Gimoti within the United States. Eversana also provided a $5 million revolving credit facility, or Eversana Credit Facility, which became available upon FDA approval of the Gimoti NDA. In June 2020 we borrowed $2 million and in December 2020 we borrowed the remaining $3 million under the Eversana Credit Facility. For additional details regarding the Eversana Agreement and the revolving credit facility, see “Business—Commercialization—Commercial Services and Loan Agreements with Eversana” below.
We have primarily funded our operations through the sale of our convertible preferred stock prior to our initial public offering, or IPO, in September 2013, borrowings under bank loans and the sale of shares of our common stock on the Nasdaq Capital Market. We launched commercial sales of Gimoti in late October 2020 with Eversana, and, to date, have generated modest sales given the launch occurred during the COVID-19 pandemic and we were entering the holiday season.
We have incurred losses in each year since our inception. These operating losses resulted from expenses incurred in connection with advancing Gimoti through development activities and general and administrative costs associated with our operations. We expect to continue to incur operating losses until revenues from the sales of Gimoti exceed our expenses, if ever. We may never become profitable, or if we do, we may not be able to sustain profitability on a recurring basis.
Business Strategy
Our objective is to develop and bring to market products to treat acute and chronic GI disorders that are not satisfactorily treated with current therapies and represent significant market opportunities. Our business strategy is to:
| ● | Successfully commercialize Gimoti in the United States. Through our commercialization agreement with Eversana, we have built a commercial infrastructure to allow us to directly market Gimoti in the United States. We have engaged Eversana to utilize its internal sales organization, along with other commercial functions, for market access, marketing, distribution, and other related patient support services. |
| ● | Expand on the Gimoti technology to develop a next generation product to expand our market potential. We have initiated planning for a PK trial of Gimoti, based on an FDA post-marketing commitment requirement. This trial will be designed to characterize dose proportionality of a lower dosage strength of Gimoti to accommodate patients that may require further dosage adjustments. We expect to initiate this trial in the second half of 2021. |
| ● | Seek partnerships to accelerate and maximize the potential for Gimoti. We continue to evaluate partnering opportunities with pharmaceutical companies that have established development and sales and marketing capabilities to potentially enhance and accelerate the development and commercialization of Gimoti, including the potential to explore regulatory approval outside the United States. |
| ● | In-license or acquire additional clinical or commercial stage product candidates for the treatment of GI diseases in a capital efficient manner. We may opportunistically in-license or acquire additional programs targeting GI diseases, leveraging our prior development experience. |
3
The Gastrointestinal Market
The health of the GI system has a major effect on an individual’s daily activities and quality of life. A retrospective review published by the National Institute of Diabetes and Digestive and Kidney Diseases estimated that in 2004 there were more than 72 million ambulatory care visits with a diagnosis of a GI disorder in the United States alone. The annual cost of these GI disorders in 2004, not including digestive cancers and viral diseases, was estimated to be greater than $114 billion in direct and indirect expenditures, including hospital, physician and nursing services as well as over-the-counter and prescription drugs.
In 2004, the total cost of GI prescription drugs in the United States was $12.3 billion, and over half of this cost ($7.7 billion) was associated with drugs prescribed for gastroesophageal reflux disease, or GERD. Peptic ulcer disease, hepatitis C, irritable bowel syndrome, or IBS, and inflammatory bowel disease, or IBD, were major contributors to the remaining drug cost. Historically GI product development efforts have focused on indications with the largest patient populations such as GERD, constipation, peptic ulcers and IBS. As a result, limited innovation has occurred in other segments of the GI market, such as upper GI motility disorders, even though these disorders affect several million patients worldwide. Consequently, due to the limited treatment options available for upper GI motility disorders, we believe there is a substantial market opportunity for us to address significant unmet medical needs, initially for diabetic gastroparesis.
GI Motility Disorders
Motility disorders are some of the most common GI disorders. Motility disorders affect the orderly contractions or relaxation of the GI tract which move contents forward and prevent backward egress. This is important in the normal movement of food through the GI tract. Motility disorders are sometimes referred to as functional GI disorders to highlight that many abnormalities in stomach function can occur even when anatomic structures appear normal. Functional GI disorders affect the upper and lower GI tract and include gastroparesis, GERD, functional dyspepsia, constipation and IBS. It has been estimated by the International Foundation for Functional Gastrointestinal Disorders that one in four people in the United States suffer from functional GI disorders, having signs and symptoms such as abdominal pain, nausea, constipation, diarrhea, bloating, decreased appetite, early satiety, swallowing difficulties, heartburn, vomiting and/or incontinence.
Gastroparesis
Gastroparesis is a debilitating, chronic condition that has a significant impact on patients’ lives. It is characterized by slow or delayed gastric emptying and evidence of gastric retention in the absence of mechanical obstruction. Muscular contractions in the stomach, which move food into the intestine, may be too slow, out of rhythm or erratic. The following graph depicts the timing associated with the emptying of solids in patients with diabetic gastroparesis compared to normal individuals:
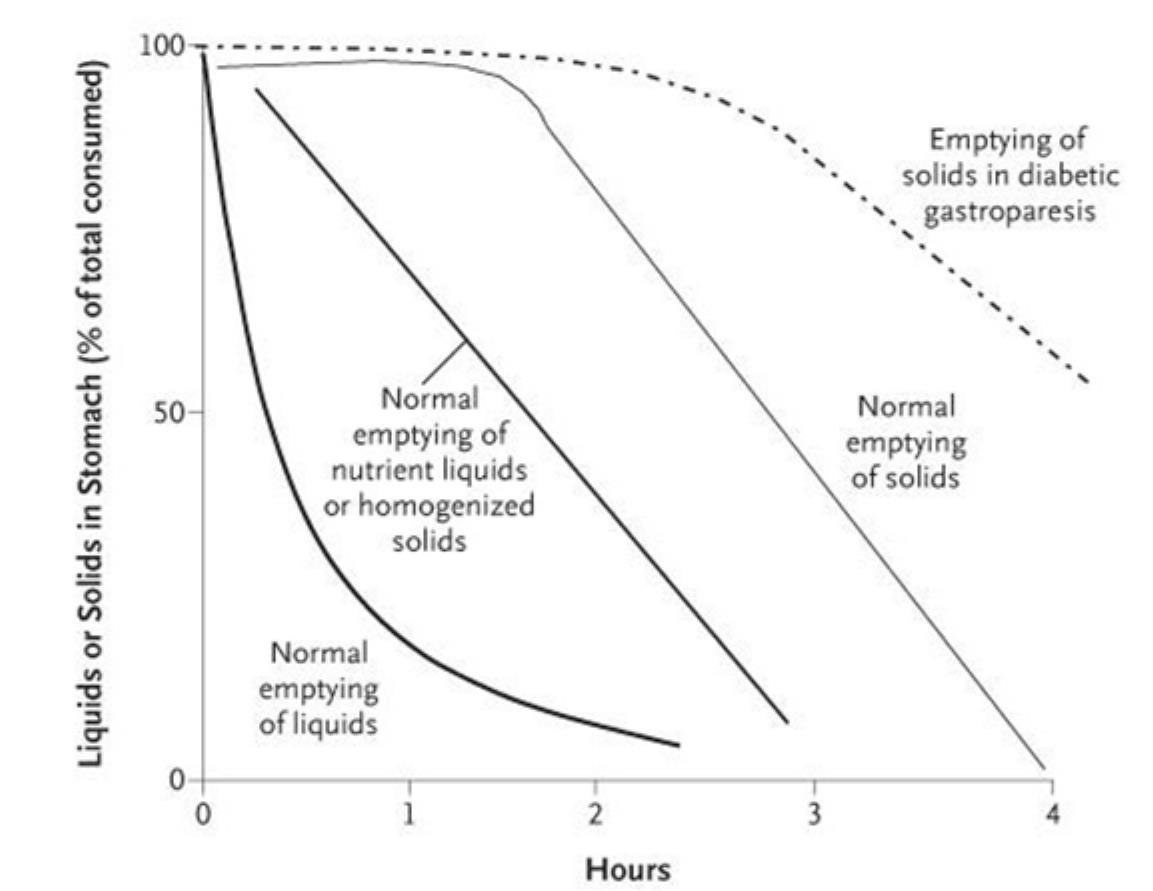
Camilleri M. New England Journal of Medicine 2007
The stomach is a muscular sac between the esophagus and the small intestine where the digestion of food begins. The stomach makes acids and enzymes referred to as gastric juices which are mixed with food by the churning action of the stomach muscles. Peristalsis is the contraction and relaxation of the stomach muscles to physically breakdown food and propel it forward. The crushed and mixed food is liquefied to form chyme and is pushed through the pyloric canal into the small intestine in a controlled and regulated manner.
4
In gastroparesis, the stomach does not perform these functions normally, causing characteristic flares of signs and symptoms that include nausea, early satiety, prolonged fullness, bloating, upper abdominal pain, vomiting and retching. As a result of these signs and symptoms, patients may limit their food and liquid intake leading to poor nutrition, dehydration and electrolyte disturbances, and have poor blood glucose control, ultimately requiring hospitalization. If left untreated or not adequately treated, gastroparesis causes significant acute and chronic medical problems, including additional diabetic complications resulting from poor glucose control.
Gastroparesis in the Hospital Setting
When patients experience a flare of their gastroparesis symptoms that cannot be adequately managed by oral medications, they may be hospitalized for hydration, parenteral nutrition, and correction of abnormal blood glucose or electrolyte levels. In this setting, intravenous metoclopramide is the first line of treatment. Typically, these diabetic patients with gastroparesis symptoms remain in the hospital until they are stabilized and able to be effectively treated with oral metoclopramide. These hospitalizations are costly and expose patients to increased risks, including hospital-acquired infections. The number of patients with gastroparesis that require hospitalization due to their disease is growing, according to a study published in the American Journal of Gastroenterology in 2008. Additionally, the study reported, from 1995 to 2004, total hospitalizations with a primary diagnosis of gastroparesis increased 158%. Hospital admissions for patients with gastroparesis as the secondary diagnosis increased 136%. The average length of stay for a patient is approximately six days at an estimated cost of approximately $22,000. Compared to the other four most common upper GI admission diagnoses (GERD, gastric ulcer, gastritis and nonspecific nausea/vomiting), gastroparesis had the longest length of stay and one of the highest total charges per stay. Additionally, the study estimates that costs associated with gastroparesis as the primary or secondary diagnosis for admission exceeded $3.5 billion in 2004.
A study of patients in clinics at the University of Pittsburgh Medical Center between January 2004 and December 2008, published in the Journal of Gastroenterology and Hepatology, showed that patients with diabetic or post-surgical gastroparesis had significantly more emergency room visits than other gastroparesis groups. The study reinforced the view that gastroparesis constitutes a significant burden for patients and the healthcare system, with more than one-third of patients requiring hospitalization. The number of emergency room visits and annual days of inpatient treatment were comparable to patients with Crohn’s disease. The study indicated that patients received an average of 6.7 prescriptions on admission. Eighty percent of the patients identified in the University of Pittsburgh study were women. According to a study conducted by Baylor College of Medicine and published in Gastroenterology & Endoscopy in December 2017, hospitalizations for gastroparesis have risen significantly since the early 1990s. This study noted that the number of hospitalizations increased from roughly 900 in 1994 to 16,400 in 2014, with median costs climbing from $6,000 to approximately $24,500 during the period. The number of people who visited the emergency department because of gastroparesis rose from 15,549 in 2006 to 39,470 in 2014, with an average annual increase of nearly 13% over that time.
Etiology
Gastroparesis can be a manifestation of many systemic illnesses, arise as a complication of select surgical procedures, or develop due to unknown causes. Any disease inducing neuromuscular dysfunction of the GI tract can result in gastroparesis, with diabetes being one of the leading known causes. In a 2007 study published in Current Gastroenterology Reports, 29% of gastroparesis cases were found in association with diabetes, 13% developed as a complication of surgery and 36% were due to unknown causes. According to the American Motility Society Task Force on Gastroparesis, up to 4% of the U.S. population experiences symptomatic manifestations of gastroparesis. As the incidence of diabetes rises worldwide, the prevalence of gastroparesis is expected to rise correspondingly.
The most common identified cause of gastroparesis is diabetes mellitus. The underlying mechanism of diabetic gastroparesis is unknown, though it is thought to be related in part to neuropathic changes in the vagus nerve and/or the myenteric plexus. Prolonged elevated serum glucose levels are also associated with vagus nerve damage. The vagus nerve controls the movement of food through the digestive tract and when it is damaged, movement of food through the GI tract may be abnormal. The prevalence of diabetes in the United States is rapidly rising, with the Centers for Disease Control estimating that one in ten adults currently suffer from the disease. Sedentary lifestyles, poor dietary habits and a consequent rising prevalence of obesity are expected to cause this number to grow substantially. According to a study published in the Journal of Gastrointestinal and Liver Diseases in July 2010, between 25% and 55% of type 1 and 15% and 30% of type 2 diabetics suffer from symptoms associated with the condition and diabetics are 29% of the total gastroparesis population.
A 2007 study published in Current Gastroenterology Reports states that approximately 36% of gastroparesis patients suffer from idiopathic gastroparesis. The development of idiopathic gastroparesis is thought to be related to loss of myenteric ganglion cells in the distal large bowel (myenteric hypoganglionosis) and reduction in the interstitial cells of Cajal, which help control contraction of the smooth muscle in the GI tract.
Post-surgical gastroparesis is a smaller subset of the total patient pool and accounts for approximately 13% of all cases of the disease, according to a 2007 study published in Current Gastroenterology Reports. Post-surgical gastroparesis is often
5
associated with peptic ulcer surgery, bariatric procedures or esophageal procedures and is thought to result from damage/desensitization of the vagus nerve.
Prevalence
In 2012, the American Diabetes Association estimated that diabetes affects approximately 29.1 million people of all ages in the United States, equating to about 9.3% of the population. Based on prevalence data, the potential gastroparesis patient pool in the United States is approximately 12 to 16 million adults with women making up 82% of this population, according to a 2007 study published in Current Gastroenterology Reports.
There are approximately 2.3 million diabetic patients with moderate or severe gastroparesis symptoms who are seeking treatment in the United States by a health care professional, according to a study presented at the Digestive Disease Week 2013 conference in Orlando, Florida. When patients do receive treatment for gastroparesis, multiple medications are frequently used to address the individual signs and symptoms of gastroparesis. For example, patients may receive anti-emetics for nausea and vomiting and opioids for abdominal pain, which can exacerbate delayed gastric emptying in patients with gastroparesis.
Unmet Needs in Gastroparesis Treatment
Market research and physician interviews demonstrate that existing treatment options for diabetic gastroparesis are inadequate and there is a high level of interest in effective outpatient options for managing patients with gastroparesis symptoms. The market is currently served by oral metoclopramide, intravenous metoclopramide, and the oral disintegrating tablet, or ODT, formulation of metoclopramide (Metozolv® ODT), with approximately 4.0 million prescriptions in the United States per year, according to IMS Health (2015).
Due to the limited availability of FDA-approved treatments for gastroparesis, physicians may resort to using medications “off-label” in an attempt to address individual symptoms experienced by patients. Off-label therapies are pharmaceuticals prescribed by physicians for an unapproved indication or in an unapproved age group, unapproved dose or unapproved form of administration. Examples of drugs used without FDA approval in gastroparesis include erythromycin and Botox® injected via endoscopic procedure directly into the lower gastric sphincter. Previously-approved drugs, such as cisapride and tegaserod, are no longer commercially available in the United States because of safety concerns. Domperidone has never been approved by FDA but is obtained through certain compounding pharmacies for individual patients under special FDA usage rules.
Gimoti is a non-oral, promotility and anti-emetic treatment that we believe has the potential to significantly improve the standard of care for gastroparesis patients. With Gimoti being approved for the treatment of diabetic gastroparesis, patients and physicians now have access to an outpatient therapy that could be administered and absorbed even when patients are experiencing delayed gastric emptying or nausea and vomiting.
Our Solution: Gimoti (Metoclopramide) Nasal Spray
We developed Gimoti, a dopamine antagonist / mixed 5-HT3 antagonist / 5-HT4 agonist with promotility and anti-emetic effects, for the relief of symptoms associated with acute and recurrent diabetic gastroparesis. For over 40 years, the only FDA approved products for the treatment of diabetic gastroparesis had been an oral tablet and injection formulations of metoclopramide. Gimoti is a novel formulation of metoclopramide offering systemic delivery by nasal spray administration.
We developed the nasal formulation of metoclopramide to provide our targeted patient population with acute or recurrent symptoms of diabetic gastroparesis with a product that can be systemically delivered as an alternative to the oral or intravenous routes of administration. Nasal delivery is possible because the mucosa of the nasal cavity is a single epithelial cell layer which is well‑vascularized and allows metoclopramide molecules to be transferred directly to the systemic circulation. There is no first pass liver metabolism required prior to onset of action. Since gastroparesis is a disease that halts or slows the movement of the contents of the stomach to the small intestine, oral drug administration is often compromised. The nasal formulation may also provide a predictable and consistent means of delivering metoclopramide in patients with delayed gastric emptying and/or frequent vomiting. Also, unlike the oral tablet formulation of metoclopramide, we believe that Gimoti may be tolerated even when patients are experiencing nausea.
A nasal spray formulation of metoclopramide could offer an alternative route of administration for patients with severe symptoms of diabetic gastroparesis receiving the parenteral formulation of metoclopramide. Following hospitalization for intravenous metoclopramide, a nasal spray formulation would also provide a non-oral option for the transition to an outpatient treatment.
6
Future Clinical Trials
We have initiated planning for an FDA post-marketing commitment PK trial of Gimoti. This trial will be designed to characterize dose proportionality of a lower dosage strength of Gimoti to accommodate patients that may require dose adjustments. We expect to initiate this trial in the second half of 2021.
Commercialization
We are commercializing Gimoti in the United States through our partnership with Eversana. Our strategy is to establish Gimoti as the prescription product of choice for diabetic gastroparesis. Gimoti is initially being marketed to gastroenterologists, internal medicine specialists, primary care physicians and select health care providers. We have engaged Eversana to utilize its internal sales organization, along with additional commercial functions, for market access, marketing, distribution, and other related patient support services.
Commercial Services and Loan Agreements with Eversana
On January 21, 2020, we entered into the Eversana Agreement for the commercialization of Gimoti. Pursuant to the Eversana Agreement, Eversana commercializes and distributes Gimoti in the United States. Eversana also manages the marketing of Gimoti to targeted health care providers, as well as the sales and distribution of Gimoti in the United States.
Under the terms of the Eversana Agreement, we maintain ownership of the Gimoti NDA, as well as legal, regulatory, and manufacturing responsibilities for Gimoti. Eversana will utilize its internal sales organization, along with other commercial functions, for market access, marketing, distribution and other related patient support services. We will record sales for Gimoti and retain more than 80% of net product profits once the parties’ costs are reimbursed. As of December 31, 2020, unreimbursed commercialization costs to Eversana were approximately $9.0 million. Such costs will generally be payable only as net product profits are recognized. Eversana will receive reimbursement of its commercialization costs pursuant to an agreed upon budget and a percentage of product profits in the mid-to-high teens. Net product profits are the net sales (as defined in the Eversana Agreement) of Gimoti, less (i) reimbursed commercialization costs, (ii) manufacturing and administrative costs set at a fixed percentage of net sales, and (iii) third party royalties. During the term of the Eversana Agreement, Eversana agreed to not market, promote, or sell a competing product in the United States.
The Eversana Agreement terminates on June 19, 2025, unless terminated earlier pursuant to its terms. Upon expiration or termination of the agreement, we will retain all profits from product sales and assume all corresponding commercialization responsibilities. Within 30 days after each of the first three annual anniversaries of commercial launch, either party may terminate the agreement if net sales of Gimoti do not meet certain annual thresholds. Either party may terminate the agreement: for the material breach of the other party, subject to a 60-day cure period; in the event an insolvency, petition of the other party is pending for more than 60 days; upon 30 days written notice to the other party if Gimoti is subject to a safety recall; the other party is in breach of certain regulatory compliance representations under the agreement; if we discontinue the development or production of Gimoti; if the net profit is negative for any two consecutive calendar quarters beginning with the first full calendar quarter 24 months following commercial launch; if the cumulative product profits fail to reach certain thresholds in the first three years following launch; or if there is a change in applicable laws that makes operation of the services as contemplated under the agreement illegal or commercially impractical. Either party may also terminate the Eversana Agreement upon a change of control of our ownership, subject, in the event that we initiate such termination, to a one-time payment equal to between two times and one times annualized service fees paid by us under the Eversana Agreement, with such amount based on which year after commercial launch the change of control occurs. Such payment amount would be reduced by the amount of previously reimbursed commercialization costs and profit split paid for the related prior twelve-month period and any revenue which occurred prior to the termination yet to be collected. If Eversana terminates the agreement due to an uncured material breach by us, or if we terminate the Eversana Agreement in certain circumstances, we have agreed to reimburse Eversana for its unreimbursed commercialization costs for the prior year and certain other costs. In addition, Eversana may terminate the Eversana Agreement if we withdraw Gimoti from the market for more than 90 days.
In connection with the Eversana Agreement, we and Eversana have entered into the Eversana Credit Facility, pursuant to which Eversana agreed to provide a revolving credit facility of up to $5 million to us upon FDA approval of the Gimoti NDA. The Eversana Credit Facility terminates on June 25, 2025, unless terminated earlier pursuant to its terms. The Eversana Credit Facility is secured by all of our personal property other than our intellectual property. Under the terms of the Eversana Credit Facility, we cannot grant an interest in our intellectual property to any other person. Each loan under the Eversana Credit Facility will bear interest at an annual rate equal to 10.0%, with such interest due at the end of the loan term. In June 2020 we borrowed $2 million and in December 2020 we borrowed the remaining $3 million under the Eversana Credit Facility.
We may prepay any amounts borrowed under the Eversana Credit Facility at any time without penalty or premium. The maturity date of all amounts, including interest, borrowed under the Eversana Credit Facility will be 90 days after the
7
expiration or earlier termination of the Eversana Agreement. The Eversana Credit Facility also includes events of default, the occurrence and continuation of which provide Eversana with the right to exercise remedies against us and the collateral securing the loans under the Eversana Credit Facility, including our cash. These events of default include, among other things, our failure to pay any amounts due under the Eversana Credit Facility, an uncured material breach of the representations, warranties and other obligations under the Eversana Credit Facility, the occurrence of insolvency events and the occurrence of a change in control.
Gimoti Product Launch
The U.S. launch of Gimoti occurred in October 2020 through our commercial partner Eversana and its specialty pharmacy. Eversana currently has 27 Gimoti dedicated sales representatives located throughout the U.S. In addition to the field sales team, Eversana telemarketing representatives field inbound calls and contact targeted physicians outside of the currently covered geographies. Sales representatives are communicating the benefits of Gimoti and the process to secure a prescription through EvokeAssist, a patient support and reimbursement team managed by Eversana. The reimbursement team receives and processes all patient prescriptions from healthcare providers and then manages insurance coverage, fulfillment and shipment of orders through their specialty pharmacy.
The commercial strategy has focused on educating targeted healthcare professionals, or HCPs, that are predominately gastroenterologists, about the clinical benefits of Gimoti. To date, the majority of prescriptions that have been enrolled in our patient reimbursement and distribution system have come from gastroenterologists. Future promotional initiatives for Gimoti will include social media and digital promotion through patient support groups and other online resources. Additionally, through Eversana, we are partnering with OptimizeRx, an electronic medical records and prescribing platform, to include Gimoti on many HCP electronic prescribing platforms. This will allow HCPs to more rapidly and easily prescribe Gimoti when deemed medically necessary and clinically appropriate for their patients by such HCPs. Additionally, when requested by a patient seeking treatment, an HCP can be directed to the EvokeAssist support process and the specialty pharmacy fulfillment center. The launch of these platforms was a result of feedback received following the initial contact with our key gastroenterology targets.
HCP feedback regarding Gimoti has generally been positive. We believe this is due to the fact that patients diagnosed with gastroparesis have delayed gastric emptying resulting in unpredictable absorption of oral medications. The only products currently approved to treat diabetic gastroparesis in an outpatient setting are Gimoti and oral metoclopramide. This limited choice of treatments has led to notable interest in Gimoti. Because Gimoti is absorbed through the nasal passage and bypasses the potential issues associated with oral absorption, physicians have noted that Gimoti is appropriate for many of their patients. The primary messaging to physicians about the benefits of a non-oral treatment for diabetic gastroparesis remains the focus of our marketing strategy.
We have also begun government program access initiatives. In December 2020, certain Medicare Part D plans began including Gimoti on their formularies, and in February 2021, certain state Medicaid programs also began covering Gimoti. These access points allow physicians to prescribe Gimoti to patients covered under these government programs and for Eversana’s specialty pharmacy to seek reimbursement under those programs. Because no uniform policy of coverage and reimbursement for drugs exists among third-party payors in the U.S., coverage and reimbursement can differ significantly from payor to payor, including government healthcare programs and commercial payors.
Manufacturer Support/Co-pay Program
The EvokeAssist program offers benefits verification support, submits orders to the pharmacy, and provides co-pay assistance to eligible patients. Co-pay assistance is available to commercially insured and cash paying patients, and varies in amount based on the patient’s insurance plan. For patients with commercial insurance coverage, there is no out-of-pocket cost. For those patients where coverage is denied, patients may elect to pay $50 for Gimoti. Patients without any insurance may also pay $50. Government insured patients are not eligible for co-pay assistance due to legal restrictions. To assure that each patient receives the maximum benefit of EvokeAssist, all patients must enroll in EvokeAssist to fill their Gimoti prescription.
Market Research
During December 2020, Eversana conducted an ATU (Awareness, Trial, and Usage) Study, a quantitative survey to measure physician awareness, trial, and product usage, for Gimoti. Approximately 104 total physician responses were captured. Survey respondents were split into three groups drawn from the healthcare practitioner, or HCP, community; “target” gastroenterologists currently being called on by the field sales force (n = 61), other “non-target” gastroenterologists (n = 19), and primary care physicians, or PCPs, who are not currently targeted for messaging (n = 24). Areas of interest that were queried included initial and future potential prescribing trends, and how HCPs viewed the suitability of Gimoti in certain gastroparesis patient populations.
8
Key Findings:
| • | Indicated an intent to prescribe Gimoti: |
| - | 79% of target gastroenterologists. |
| - | 89% of non-target gastroenterologists. |
| - | 50% of PCPs. |
| • | Out of those target gastroenterologists indicating an intent to prescribe Gimoti, 94% indicated Gimoti would be “appropriate” to use in moderate to severe patients. |
| • | A majority of each of the target and non-target gastroenterologists noted they intend to prescribe Gimoti for both new and existing gastroparesis patients. |
| • | Nineteen of all participating HCPs indicated that they have already written a prescription for Gimoti. |
| - | HCPs indicated that the primary driver for prescribing Gimoti was patients being switched to Gimoti due to lack of efficacy of current treatments. |
Manufacturing
We do not own or operate manufacturing facilities for the production of Gimoti, nor do we have plans to develop our own manufacturing operations in the foreseeable future. We currently depend on third-party contract manufacturers for all of our required raw materials, drug substance and finished product for our product development and clinical trials. We currently use a third-party consultant, which we engage on an as-needed, hourly basis, to manage product development and manufacturing contractors.
In November 2017, we entered into a Manufacturing Services Agreement with Patheon UK Limited, or Patheon, a wholly-owned subsidiary of Thermo Fisher, Inc., pursuant to which Patheon has agreed to manufacture commercial quantities of Gimoti. Under the terms of the agreement, we are required to purchase a certain percentage of our requirements for our Gimoti product intended for commercial sale, provided certain terms and conditions are met. The initial term of the agreement commenced in November 2017 and will continue in effect until December 31, 2025. This initial term shall be automatically renewed for additional one-year terms, unless either party provides written notice of its intention to terminate the agreement upon notice within a specified time prior to the end of the then current term. Either party may terminate the agreement effective immediately upon written notice to the other in the event that (i) the other party dissolves, is declared insolvent or bankrupt by a court of competent jurisdiction, (ii) a voluntary petition of bankruptcy is filed in any court of competent jurisdiction, or (iii) the agreement is assigned for the benefit of creditors. We may terminate the agreement upon specified prior written notice if any governmental or regulatory authority, including, but not limited to, FDA, takes any action, or raises any objection, that prevents us from importing, exporting, purchasing, or selling Gimoti. Patheon or we may terminate the agreement upon specified prior written notice to the other party if Patheon or we, as applicable, assigns any of our rights under the agreement to an assignee that is (i) not a credit worthy substitute for the assigning party; or (ii) a competitor of assigning party. Moreover, either party may terminate the agreement upon written notice to the other party where the other party has failed to remedy a material breach of any of its representations, warranties, or other obligations under the agreement within a specified period of time following receipt of a written notice of the breach, subject to specified terms and conditions.
In May 2016, we entered into a Master Supply Agreement with Cosma S.p.A., or Cosma, pursuant to which Cosma will be the exclusive commercial supplier of metoclopramide for the manufacture of Gimoti. Under the supply agreement, Cosma will supply metoclopramide pursuant to purchase orders which we may deliver to Cosma from time to time, and there is no minimum supply requirement. In the event Cosma discontinues supply of metoclopramide for any reason, including by reason of a force majeure event, or materially changes the metoclopramide specifications, then we may require Cosma to supply up to a two years’ supply of the metoclopramide based on our purchase orders over the preceding two years. The term of the supply agreement is three years, which term shall be automatically extended (1) for an additional period equivalent to the time elapsing from May 2016 to the date of the first commercial launch of Gimoti and (2) for successive one-year periods thereafter, unless terminated earlier. Either party may terminate the supply agreement on 180 days’ written notice to the other party or on a 30 days’ written notice to the other party for such party’s material uncured breach.
Competition
The pharmaceutical industry is characterized by intense competition and rapid innovation. Our potential competitors include large pharmaceutical and biotechnology companies, specialty pharmaceutical and generic drug companies, academic institutions, government agencies and research institutions. We believe the key competitive factors that will affect the development and commercial success of our product candidates are efficacy, safety and tolerability profile, reliability, convenience of dosing, coverage pricing and reimbursement.
Many of our potential competitors have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of product candidates, obtaining FDA and other regulatory approvals of products and the commercialization of those products. Accordingly, our competitors may be more successful
9
than we may be in obtaining FDA approval for drugs and achieving widespread market acceptance. Our competitors’ drugs may be more effective, or more effectively marketed and sold, than any drug we may commercialize and may render our product candidates obsolete or non-competitive before we can recover the expenses of developing and commercializing any of our product candidates. We anticipate that we will face intense and increasing competition as new drugs enter the market and advanced technologies become available. Finally, the development of new treatment methods for the diseases we are targeting could render our drugs non-competitive or obsolete.
Gimoti competes directly with metoclopramide oral, erythromycin and domperidone as a treatment for gastroparesis. Metoclopramide is the only product currently approved in the United States to treat gastroparesis. Metoclopramide is available from a number of generic pharmaceutical manufacturers as well as in branded form in the United States under the tradename Reglan® Tablets from Ani Pharmaceuticals.
Salix Pharmaceuticals, Inc. launched an orally dissolving tablet formulation of metoclopramide in 2009. Other programs in the gastroparesis pipeline include new chemical entities in earlier-stage clinical trials. In addition to Gimoti, we are aware of the following development candidates, all of which are in clinical development.
Gastroparesis Treatment Development Pipeline
Product |
| Class |
| Route |
| Company |
| Status |
Tradipitant |
| NK-1 antagonist |
| oral |
| Vanda |
| Phase 3 |
Velusetrag |
| 5-HT4 receptor agonist |
| oral |
| Theravance/Alfasigma |
| Phase 2 |
Metopimazine |
| D2/D3 antagonist |
| oral |
| Neurogastrx |
| Phase 2 |
CIN-102 |
| Deuterated Domperidone |
| oral |
| CinRx |
| Phase 2 |
TAK-906 |
| D2/D3 antagonist |
| oral |
| Takeda/Altos |
| Phase 2 |
Tradipitant is a NK-1 antagonist that has been tested in various other indications by Vanda Pharmaceuticals Inc. In December 2018, a Phase 2 study reached statistical significance for the primary endpoint for treatment of nausea. Vanda proposed a 12-month open-label extension trial for patients who completed the Phase 2 clinical trial of tradipitant. This trial is currently subject to an FDA partial clinical hold. A Phase 3 clinical trial is targeting complete enrollment in the first half of 2021.
Velusetrag, also called TAK-954, is a 5-HT4 receptor agonist compound under development for the treatment of gastroparesis by Theravance Biopharma, Inc., in collaboration with Alfasigma S.p.A. In August 2018, Theravance announced that its Phase 2 study failed to reach statistical significance in the two higher doses tested, but did show statistical significance in the lower dose tested.
Neurogastrx is currently developing Metopimazine, a selective and peripherally restricted dopamine D2/D3 receptor antagonist to treat gastroparesis. It is approved in countries outside the U.S. in other indications.
CinRx is developing CIN-102 to treat gastroparesis. CIN-102 is a dopamine D2/D3 receptor antagonist that is a deuterated version of Domperidone. Domperidone is a product approved outside the U.S. that was in clinical trials in the U.S., but has not received FDA approval to date. A 60-person Phase 2 trial has been initiated with an estimated completion of the trial in March 2021.
Takeda is developing TAK-906 to treat gastroparesis. TAK-906 is a dopamine D2/D3 receptor antagonist. A 205-person Phase 2 trial has been initiated with an estimated completion of the trial in July 2021.
One additional medication, Motilium (domperidone), a dopamine receptor modulator, is not FDA-approved, but is available in the United States through various compounding pharmacies under a specific FDA restricted-access program. The safety and efficacy of Motilium as a promotility agent is not fully established.
Intellectual Property and Proprietary Rights
Overview
We are building an intellectual property portfolio for Gimoti in the United States and abroad. We seek patent protection in the United States and internationally for our product candidate, its methods of use and processes for its manufacture, and for other technologies, where appropriate. Our policy is to actively seek to protect our proprietary position by, among other things, filing patent applications in the United States and abroad relating to proprietary technologies that are important to the development of our business. We also rely on trade secrets, know-how, continuing technological innovation and in-licensing opportunities to develop and maintain our proprietary position. We cannot be sure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future, nor can we be sure that any of our existing patents or any patents that may be granted to us in the future will be commercially useful in protecting our technology.
10
Our business success will depend significantly on our ability to:
| ● | secure, maintain and enforce patent and other proprietary protection for our core technologies, inventions and know-how; |
| ● | obtain and maintain licenses to key third-party intellectual property owned by such third parties; |
| ● | preserve the confidentiality of our trade secrets; and |
| ● | operate without infringing upon valid, enforceable third-party patents and other rights. |
Patent Portfolio
Our patent portfolio consists of patents and patent applications, including the following U.S. patents and patent applications as of February 28, 2021:
| ● | U.S. Patent 6,770,262—Nasal Administration of Agents for the Treatment of Gastroparesis. This patent is expected to expire no earlier than March 2021. |
|
| ● | U.S. Patent 8,334,281—Nasal Formulations of Metoclopramide. This patent is expected to expire no earlier than 2030 and has a pending Continuation application (U.S. Non-Provisional Patent Application No. 16/181,841). |
|
| ● | U.S. Non-Provisional Patent Application No. 16/016,246 —Treatment of Symptoms Associated with Female Gastroparesis. If granted, this patent is not expected to expire earlier than 2032. |
|
| ● | U.S. Non-Provisional Patent Application No. 16/469,092 – Treatment of Moderate and Severe Gastroparesis. If granted, this patent is not expected to expire earlier than 2037. |
|
| ● | U.S. Non-Provisional Patent Application No. 16/646,527 – Methods of Intranasal Metoclopramide Dosing. If granted, this patent is not expected to expire earlier than 2038. |
|
We have also been granted European and Canadian patents for the method of use of metoclopramide via nasal delivery for gastroparesis. These patents are expected to expire no earlier than 2021. We have also been granted European and Canadian patents for pharmaceutical compositions comprising metoclopramide. These patents are expected to expire no earlier than 2029. We have also been granted European, Japanese, Russian and Mexican patents for the use of intranasal metoclopramide for treating diabetic gastroparesis in human females. These patents are expected to expire no earlier than 2032. Additional patent applications have been filed in the United States and abroad related to more recent clinical trial findings.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidate are obtained, once the patent life has expired, we may be open to competition from competitive products, including generics. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
Other Intellectual Property Rights
We currently have a registered trademark for EVOKE PHARMA and other trademarks, including GIMOTI and EvokeAssist in the United States.
Confidential Information and Inventions Assignment Agreements
We require our employees and consultants to execute confidentiality agreements upon the commencement of employment, consulting or collaborative relationships with us. These agreements provide that all confidential information developed or made known during the course of the relationship with us be kept confidential and not disclosed to third parties except in specific circumstances.
In the case of employees, the agreements provide that all inventions resulting from work performed for us, utilizing our property or relating to our business and conceived or completed by the individual during employment shall be our exclusive property to the extent permitted by applicable law. Our consulting agreements also provide for assignment to us of any intellectual property resulting from services performed for us.
Technology Acquisition Agreement
In June 2007, we acquired all worldwide rights, data, patents and other related assets associated with Gimoti from Questcor Pharmaceuticals, Inc., or Questcor, pursuant to an asset purchase agreement. We paid Questcor $650,000 in the form of an upfront payment and $500,000 in May 2014 as a milestone payment based upon the initiation of the first patient dosing in our
11
Phase 3 clinical trial for Gimoti. In August 2014, Mallinckrodt, plc, or Mallinckrodt, acquired Questcor. As a result of that acquisition, Questcor transferred its rights included in the asset purchase agreement with us to Mallinckrodt. In addition to the payments previously made to Questcor, we may also be required to make additional milestone payments totaling up to $52 million. In March 2018, we amended the asset purchase agreement with Mallinckrodt to defer development and approval milestone payments, such that rather than paying two milestone payments based on FDA acceptance for review of the NDA and final product marketing approval, we would be required to make a single $5 million payment on the one-year anniversary after we receive FDA approval to market Gimoti. At the time of the Gimoti NDA approval by FDA, we recorded the $5 million payable owed to Mallinckrodt with a due date of June 19, 2021, along with a $5 million research and development expense.
The remaining $47 million in milestone payments depend on Gimoti’s commercial success. We will be required to pay to Mallinckrodt a low single digit royalty on net sales of Gimoti. Our obligation to pay such royalties will terminate upon the expiration of the last patent right covering Gimoti, which is expected to occur in 2030, subject to possible extension should any additional, later expiring, licensed patents be granted.
Government Regulation
FDA Regulations
In the United States, pharmaceutical products are subject to extensive regulation by FDA. The Federal Food, Drug, and Cosmetic Act, or FFDCA, and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products. Failure to comply with applicable FDA or other requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA’s refusal to approve pending applications, a clinical hold, warning letters, recall or seizure of products, partial or total suspension of production, withdrawal of the product from the market, injunctions, fines, civil penalties or criminal prosecution.
FDA approval is required before any new unapproved drug or dosage form, including a new use of a previously approved drug, can be marketed in the United States. The process required by FDA before a drug may be marketed in the United States generally involves:
| ● | completion of pre-clinical laboratory and animal testing and formulation studies in compliance with FDA’s good laboratory practice regulations; |
| ● | submission to FDA of an Investigational New Drug Application, or IND, for human clinical testing which must become effective before human clinical trials may begin in the United States; |
| ● | approval by an independent institutional review board, or IRB, at each clinical trial site before each trial may be initiated; |
| ● | performance of adequate and well-controlled human clinical trials in accordance with good clinical practice, or GCP, regulations to establish the safety and efficacy of the proposed drug product for each intended use; |
| ● | satisfactory completion of an FDA pre-approval inspection of the facility or facilities at which the product is manufactured to assess compliance with FDA current good manufacturing practices, or cGMP, regulations, including, for devices and device components, the Quality System Regulation, or QSR, and to assure that the facilities, methods and controls are adequate to preserve the product’s identity, strength, quality and purity; |
| ● | submission to FDA of an NDA; |
| ● | satisfactory completion of an FDA advisory committee review, if applicable; and |
| ● | FDA review and approval of the NDA. |
Pre-clinical tests include laboratory evaluation of product chemistry, formulation, stability and toxicity, as well as animal studies to assess the characteristics and potential safety and efficacy of the product. The results of pre-clinical tests, together with manufacturing information, analytical data and a proposed clinical trial protocol and other information, are submitted as part of an IND to FDA. Some pre-clinical testing may continue even after the IND is submitted. The IND automatically becomes effective 30 days after receipt by FDA, unless FDA, within the 30-day time period, raises concerns or questions relating to one or more proposed clinical trials and places the clinical trial on a clinical hold, including concerns that human research subjects will be exposed to unreasonable health risks. In such a case, the IND sponsor and FDA must resolve any outstanding concerns before the clinical trial can begin. As a result, our submission of an IND may not result in FDA authorization to commence a clinical trial. A separate submission to an existing IND must also be made for each successive clinical trial conducted during product development.
12
Further, an IRB covering each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial and informed consent information for subjects before the trial commences at that site, and it must monitor the study until completed. FDA, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk or for failure to comply with the IRB’s or regulatory requirements, or for other reasons, or FDA or IRB may impose other conditions.
Clinical trials involve the administration of the investigational new drug to human subjects under the supervision of qualified investigators in accordance with GCP requirements, which include the requirement that all research subjects provide their informed consent in writing for their participation in any clinical trial. Sponsors of clinical trials generally must register and report, at the National Institutes of Health-maintained website ClinicalTrials.gov, key parameters of certain clinical trials. For purposes of an NDA submission and approval, human clinical trials are typically conducted in the following sequential phases, which may overlap or be combined:
| ● | Phase 1: The drug is initially introduced into healthy human subjects or patients and tested for safety, dose tolerance, absorption, metabolism, distribution and excretion and, if possible, to gain an early indication of its effectiveness. |
| ● | Phase 2: The drug is administered to a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted indications and to determine dose tolerance and optimal dosage. Multiple Phase 2 clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more extensive Phase 3 clinical trials. |
| ● | Phase 3: The drug is administered to a large patient populations to further evaluate dosage, to obtain additional evidence of clinical efficacy and safety in an expanded patient population at multiple, geographically-dispersed clinical trial sites, to establish the overall risk-benefit relationship of the drug and to provide adequate information for the labeling of the drug. |
| ● | Phase 4: In some cases, FDA may condition approval of an NDA for a product candidate on the sponsor’s agreement to conduct additional clinical trials to further assess the drug’s safety and effectiveness after NDA approval. Such post-approval trials are typically referred to as Phase 4 studies. |
The results of product development, pre-clinical studies and clinical trials are submitted to FDA as part of an NDA. NDAs must also contain extensive information relating to the product’s pharmacology, chemistry, manufacturing and controls, or CMC, and proposed labeling, among other things.
Under federal law, the submission of most NDAs is subject to a substantial application user fee, and the manufacturer and/or sponsor under an approved NDA are also subject to annual program fees. FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. FDA may request additional information rather than accept an NDA for filing. In this event, the NDA must be resubmitted with the additional information and is subject to payment of additional user fees. The resubmitted application is also subject to review before FDA accepts it for filing.
Once the submission has been accepted for filing, FDA begins an in-depth substantive review. Under PDUFA, FDA agrees to specific performance goals for NDA review time through a two-tiered classification system, Standard Review and Priority Review. Standard Review NDAs have a goal of being completed within ten months of the date of receipt by FDA (for drugs that do not contain new molecular entities) and ten months of the 60-day filing date (for drugs that contain new molecular entities). A Priority Review designation is given to drugs that treat a serious condition and, if approved, would provide a significant improvement in safety or effectiveness. The goal for completing a Priority Review is six months from the date of receipt by FDA (for drugs that do not contain new molecular entities) and six months of the 60-day filing date (for drugs that contain new molecular entities). However, FDA does not always complete its review within these timelines and the review can take substantially longer.
FDA may refer applications for novel drug products or drug products which present difficult questions of safety or efficacy to an advisory committee for review, evaluation and recommendation as to whether the application should be approved and under what conditions. FDA is not bound by the recommendation of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving an NDA, FDA may inspect the facility or facilities where the product is manufactured. FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements, and are adequate to assure consistent production of the product within required specifications. Additionally, FDA will typically inspect one or more clinical sites to assure compliance with GCP requirements before approving an NDA.
After FDA evaluates an NDA and conducts inspections of manufacturing facilities where the investigational product and/or its drug substance will be produced, FDA may issue an approval letter or a Complete Response Letter, or CRL. An approval
13
letter authorizes commercial marketing of the product with specific prescribing information for specific indications. A CRL will describe all of the deficiencies that FDA has identified in the NDA, except that where FDA determines that the data supporting the application are inadequate to support approval, FDA may issue the CRL without first conducting required inspections and/or reviewing proposed labeling. In issuing the CRL, FDA may recommend actions that the applicant might take to place the NDA in condition for approval, including requests for additional information or clarification. FDA may delay or refuse approval of an NDA if applicable regulatory criteria are not satisfied, require additional testing or information and/or require post-marketing testing and surveillance to monitor safety or efficacy of a product.
If regulatory approval of a product is granted, such approval will be granted for particular indications and may entail limitations on the indicated uses for which such product may be marketed. For example, FDA may approve the NDA with a Risk Evaluation and Mitigation Strategy, or REMS to ensure the benefits of the product outweigh its risks. A REMS is a safety strategy to manage a known or potential serious risk associated with a medicine and to enable patients to have continued access to such medicines by managing their safe use, and could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries, and other risk minimization tools. FDA also may condition approval on, among other things, changes to proposed labeling or the development of adequate controls and specifications. FDA may also require one or more Phase 4 post- market studies and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization, and may limit further marketing of the product based on the results of these post-marketing studies.
Post-Approval Requirements
Once an NDA is approved, the product will be subject to pervasive and continuing regulation by FDA, including, among other things, requirements relating to drug/device listing, recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims, are subject to prior FDA review and approval. There also are continuing, annual program fees for any marketed products. FDA may also require post-approval studies and clinical trials if FDA finds that scientific data, including information regarding related drugs, deem such studies appropriate. The purpose of such studies would be to assess a known serious risk or signals of serious risk related to the drug or to identify an unexpected serious risk when available data indicate the potential for a serious risk. FDA may also require a labeling change if it becomes aware of new safety information that it believes should be included in the labeling of a drug.
In addition, drug manufacturers and other entities involved in the manufacture and distribution of approved products are required to register their establishments with FDA and state agencies, and are subject to periodic unannounced inspections by FDA and these state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated and generally require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon us and any third-party manufacturers that we may decide to use. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance.
Once an approval is granted, FDA may suspend, restrict or withdraw the approval, require a product recall, or impose additional restrictions or limitations if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| ● | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| ● | fines, warning letters or holds on post-approval clinical trials; |
| ● | refusal of FDA to approve pending applications or supplements to approved applications, or suspension or revocation of product license approvals; |
| ● | product seizure or detention, or refusal to permit the import or export of products; or |
| ● | injunctions or the imposition of civil or criminal penalties. |
In addition, FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market, and FDA imposes a number of complex regulations on entities that advertise and promote pharmaceuticals, which include, among others, standards for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities, and promotional activities involving the internet. While physicians may prescribe for off-label uses, manufacturers may only promote for the approved indications and in accordance with the provisions of the approved label. FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability. Indeed, FDA has very broad
14
enforcement authority under the FFDCA, and failure to abide by these regulations can result in penalties, including the issuance of a warning letter directing entities to correct deviations from FDA standards, a requirement that future advertising and promotional materials are pre-cleared by FDA, and state and federal civil and criminal investigations and prosecutions.
The distribution of prescription pharmaceutical products is also subject to the Prescription Drug Marketing Act, or PDMA, which regulates the distribution of drugs and drug samples at the federal level and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution, including a drug pedigree which tracks the distribution of prescription drugs.
Section 505(b)(2) New Drug Applications
As an alternate path to FDA approval for modifications to formulations or uses of products previously approved by FDA, an applicant may submit an NDA under Section 505(b)(2) of the FFDCA. Section 505(b)(2) was enacted as part of the Drug Price Competition and Patent Term Restoration Act of 1984, also known as the Hatch-Waxman Amendments, and permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. The applicant may rely upon published literature and FDA’s findings of safety and effectiveness based on certain pre-clinical or clinical studies conducted for an approved product. FDA may also require companies to perform additional studies or measurements to support the change from the approved product. FDA may then approve the new product candidate for all or some of the label indications for which the referenced product has been approved, as well as for any new indication sought by the Section 505(b)(2) applicant.
To the extent that a Section 505(b)(2) NDA relies on studies conducted for a previously approved drug product, the applicant is required to certify to FDA concerning any patents listed for the approved product in FDA Orange Book. FDA Orange Book is where patents associated with an FDA-approved product are listed. Specifically, the applicant must certify for each listed patent that (1) the required patent information has not been filed; (2) the listed patent has expired; (3) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (4) the listed patent is invalid, unenforceable or will not be infringed by the new product. A certification that the new product will not infringe the already approved product’s listed patent or that such patent is invalid is known as a Paragraph IV certification. If the applicant does not challenge the listed patents through a Paragraph IV certification, the Section 505(b)(2) NDA application will not be approved until all the listed patents claiming the referenced product have expired. The Section 505(b)(2) NDA application also will not be accepted or approved until any non-patent exclusivity, such as exclusivity for obtaining approval of a New Chemical Entity, listed in the Orange Book for the referenced product has expired.
If the 505(b)(2) NDA applicant has provided a Paragraph IV certification to FDA, the applicant must also send notice of the Paragraph IV certification to the referenced NDA and patent holders once the 505(b)(2) NDA has been accepted for filing by FDA. The NDA and patent holders may then initiate a legal challenge to the Paragraph IV certification. Under the FFDCA, the filing of a patent infringement lawsuit within 45 days of the NDA and patent holders’ receipt of a Paragraph IV certification in most cases automatically prevents FDA from approving the Section 505(b)(2) NDA for 30 months, or until a court decision or settlement finding that the patent is invalid, unenforceable or not infringed, whichever is earlier. The court also has the ability to shorten or lengthen the 30-month stay if either party is found not to be reasonably cooperating in expediting the litigation. Thus, the Section 505(b)(2) applicant may invest a significant amount of time and expense in the development of its product only to be subject to significant delay and patent litigation before its product may be commercialized.
The 505(b)(2) NDA applicant also may be eligible for its own regulatory exclusivity period, such as three-year exclusivity. Specifically, a product may be granted three-year Hatch-Waxman exclusivity if one or more clinical studies, other than bioavailability or bioequivalence studies, was essential to the approval of the application and was conducted/sponsored by the applicant. Should this occur, FDA would be precluded from making effective any other application for the same condition of use or for a change to the drug product that was granted exclusivity until after that three-year exclusivity period has expired. Additional non-patent exclusivities may also apply.
Additionally, the 505(b)(2) NDA applicant may have relevant patents in the Orange Book, and if so, it can initiate patent infringement litigation against those applicants that challenge such patents, which could result in a 30-month stay delaying those applicants.
Manufacturing Requirements
We and our third-party manufacturers must comply with applicable FDA regulations relating to cGMP, including applicable QSR requirements for the device component of Gimoti. The cGMP regulations include requirements relating to, among other things, organization of personnel, buildings and facilities, equipment, control of components and drug product containers and closures, production and process controls, packaging and labeling controls, holding and distribution, laboratory controls, records and reports, and returned or salvaged products. The manufacturing facilities for our products must meet cGMP
15
requirements to the satisfaction of FDA pursuant to a pre-approval inspection before we can use them to manufacture our products. We and our third-party manufacturers are also subject to periodic unannounced inspections of facilities by FDA and other authorities, including procedures and operations used in the testing and manufacture of our products to assess our compliance with applicable regulations. Failure to comply with statutory and regulatory requirements subjects a manufacturer to possible legal or regulatory action, including, among other things, warning letters, the seizure or recall of products, injunctions, consent decrees placing significant restrictions on or suspending manufacturing operations and civil and criminal penalties.
Insurance Coverage and Reimbursement
Sales of our products depend, in part, on the extent to which our products are covered by third-party payors, such as commercial insurance, managed healthcare organizations and government health care programs. These third-party payors are increasingly limiting coverage and reducing reimbursements for medical products and services. In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost-containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. Decreases in third-party reimbursement for Gimoti or any of our drug candidates or a decision by a third-party payor to not cover Gimoti or any of our drug candidates could reduce physician utilization of our products and have a material adverse effect on our sales, results of operations and financial condition.
Other Healthcare Laws
We are subject to healthcare regulation and enforcement by the federal government and the states and foreign governments in which we conduct our business. These laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, and physician and other health care provider payment transparency laws and regulations.
The federal Anti-Kickback Statute prohibits, among other things, any person from knowingly and willfully offering, soliciting, receiving or providing remuneration, directly or indirectly, to induce either the referral of an individual, for an item or service or the purchasing or ordering of a good or service, for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs. The Anti-Kickback Statute is subject to evolving interpretations. In the past, the government has enforced the Anti-Kickback Statute to reach large settlements with healthcare companies based on sham consulting and other financial arrangements with physicians. Further, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. The majority of states also have anti-kickback laws which establish similar prohibitions and, in some cases, may apply to items or services reimbursed by any third-party payor, including commercial insurers.
Additionally, the False Claims Act prohibits knowingly presenting or causing the presentation of a false, fictitious or fraudulent claim for payment to the U.S. government. Actions under the False Claims Act may be brought by the Attorney General or as a qui tam action by a private individual in the name of the government. Violations of the False Claims Act can result in very significant monetary penalties and treble damages. The federal government is using the False Claims Act, and the accompanying threat of significant liability, in its investigation and prosecution of pharmaceutical and biotechnology companies throughout the country, for example, in connection with the promotion of products for unapproved uses and other sales and marketing practices. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act. The government has obtained multi-million and multi-billion dollar settlements under the False Claims Act in addition to individual criminal convictions under applicable criminal statutes. Given the significant size of actual and potential settlements, it is expected that the government will continue to devote substantial resources to investigating healthcare providers’ and manufacturers’ compliance with applicable fraud and abuse laws.
The federal criminal false claims laws prohibit, among other things, knowingly and willfully making, or causing to be made, a false statement or representation of a material fact for use in determining the right to any benefit or payment under a federal health care program. A violation of these laws may constitute a felony or misdemeanor and may result in fines or imprisonment.
The federal Civil Monetary Penalties Law prohibits, among other things, the offering or transferring of remuneration to a Medicare or Medicaid beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular supplier of Medicare or Medicaid payable items or services. Noncompliance with such beneficiary inducement provision of the federal Civil Monetary Penalties Law can result in civil money penalties for each wrongful act, assessment of three times the amount claimed for each item or service and exclusion from the federal healthcare programs.
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, also created new federal criminal statutes that prohibit among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud
16
any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians and other healthcare providers. The Physician Payment Transparency Act imposes reporting requirements on certain drug manufacturers for payments made by them to physicians (as defined by statute), certain other health care professionals beginning in 2022, and teaching hospitals, as well as ownership and investment interests held by such physicians and their immediate family members. Failure to submit required information may result in significant civil monetary penalties for any payments, transfers of value or ownership or investment interests that are not timely, accurately and completely reported in an annual submission. Drug manufacturers are required to submit reports to the government by the 90th day of each calendar year. Certain states also mandate implementation of commercial compliance programs, impose restrictions on drug manufacturer marketing practices and/or require the tracking and reporting of marketing expenditures and pricing information, as well as gifts, compensation and other remuneration to physicians.
The shifting commercial compliance environment and the need to build and maintain robust and expandable systems to comply with different compliance and/or reporting requirements in multiple jurisdictions increase the possibility that a healthcare company may violate one or more of the requirements. If our operations are found to be in violation of any of such laws or any other governmental regulations that apply to us, we may be subject to penalties, including, without limitation, civil and criminal penalties, damages, fines, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs and imprisonment, any of which could adversely affect our ability to operate our business and our financial results.
Healthcare Reform
In March 2010, the Affordable Care Act, which substantially changed the way healthcare is financed by both governmental and private insurers in the United States, was signed into law and significantly affected the pharmaceutical industry. The Affordable Care Act contains a number of provisions, including those governing enrollment in federal healthcare programs, reimbursement adjustments and fraud and abuse changes. Additionally, the Affordable Care Act increases the minimum level of Medicaid rebates payable by manufacturers of brand name drugs from 15.1% to 23.1%; imposes a non-deductible annual fee on pharmaceutical manufacturers or importers who sell “branded prescription drugs” to specified federal government programs; and addresses a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected.
Since its enactment, there have been judicial and Congressional challenges to certain aspects of the Affordable Care Act, and we expect there will be additional challenges and amendments to the Affordable Care Act in the future. For example, on December 14, 2018, a U.S. District Court Judge in the Northern District of Texas, ruled that the individual mandate is a critical and inseverable feature of the Affordable Care Act, and therefore, because it was repealed as part of the Tax Cuts and Jobs Act, the remaining provisions of the Affordable Care Act are invalid as well. On December 18, 2019, the U.S. Court of Appeals for the 5th Circuit upheld the district court’s decision that the individual mandate was unconstitutional, but remanded the case back to the District Court to determine whether the remaining provisions of the Affordable Care Act are invalid as well. The U.S. Supreme Court is currently reviewing the case, although it is unclear how the Supreme Court will rule. It is also unclear how other efforts, if any, to challenge, repeal or replace the Affordable Care Act will impact the law.
Other legislative changes have been proposed and adopted since the Affordable Care Act was enacted, including aggregate reductions of Medicare payments to providers of 2% per fiscal year and reduced payments to several types of Medicare providers, which was temporarily suspended from May 1, 2020 through March 31, 2021. Moreover, there has recently been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed bills designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. Individual states in the United States have also become increasingly active in implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
Data Privacy and Security
We may also be subject to U.S. federal and state and foreign health information privacy, security and data breach notification laws, which may govern the collection, use, disclosure and protection of health-related and other personal information. In the U.S., HIPAA imposes privacy, security and breach reporting obligations with respect to individually identifiable health
17
information upon “covered entities” (health plans, health care clearinghouses and certain health care providers), and their respective business associates, individuals or entities that create, receive, maintain or transmit protected health information in connection with providing a service for or on behalf of a covered entity. HIPAA mandates the reporting of certain breaches of health information to the United States Department of Health and Human Services, or HHS, affected individuals and if the breach is large enough, the media. Entities that are found to be in violation of HIPAA as the result of a breach of unsecured protected health information, a complaint about privacy practices or an audit by HHS, may be subject to significant civil, criminal and administrative fines and penalties and/or additional reporting and oversight obligations if required to enter into a resolution agreement and corrective action plan with HHS to settle allegations of HIPAA non-compliance. Even when HIPAA does not apply, according to the Federal Trade Commission, or FTC, failing to take appropriate steps to keep consumers’ personal information secure constitutes unfair acts or practices in or affecting commerce in violation of Section 5(a) of the Federal Trade Commission Act. The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities. Individually identifiable health information is considered sensitive data that merits stronger safeguards.
In addition, certain states govern the privacy and security of health information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. For example, California recently enacted legislation, the California Consumer Privacy Act, or CCPA, which went into effect January 1, 2020. The CCPA, among other things, creates new data privacy obligations for covered companies and provides new privacy rights to California residents, including the right to opt out of certain disclosures of their information. The CCPA also creates a private right of action with statutory damages for certain data breaches, thereby potentially increasing risks associated with a data breach. Although the law includes limited exceptions, including for “protected health information” maintained by a covered entity or business associate, it may regulate or impact our processing of personal information depending on the context. Further, the California Privacy Rights Act, or CPRA, recently passed in California. The CPRA will impose additional data protection obligations on covered businesses, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data, and opt outs for certain uses of sensitive data. It will also create a new California data protection agency authorized to issue substantive regulations and could result in increased privacy and information security enforcement. The majority of the provisions will go into effect on January 1, 2023, and additional compliance investment and potential business process changes may be required.
In Europe, the General Data Protection Regulation, or GDPR, went into effect in May 2018 and imposes stringent data protection requirements for controllers and processors of personal data of persons within the European Economic Area, or EEA. The GDPR applies to any company established in the EEA as well as to those outside the EEA if they collect and use personal data in connection with the offering of goods or services to individuals in the EEA or the monitoring of their behavior. Companies that must comply with the GDPR face increased compliance obligations and risk, including more robust regulatory enforcement of data protection requirements and potential fines for noncompliance of up to €20 million or 4% of the annual global revenues of the noncompliant company, whichever is greater. Among other requirements, the GDPR regulates transfers of personal data subject to the GDPR to third countries that have not been found to provide adequate protection to such personal data, including the United States, and the efficacy and longevity of current transfer mechanisms between the European Union, or EU, and the United States remains uncertain. For example, in 2016, the EU and United States agreed to a transfer framework for data transferred from the EU to the United States, called the Privacy Shield, but the Privacy Shield was invalidated in July 2020 by the Court of Justice of the European Union. Further, from January 1, 2021, companies have to comply with the GDPR and also the United Kingdom GDPR, or UK GDPR, which, together with the amended UK Data Protection Act 2018, retains the GDPR in UK national law. The UK GDPR mirrors the fines under the GDPR, e.g. fines up to the greater of €20 million (£17.5 million) or 4% of global turnover. The relationship between the United Kingdom and the European Union in relation to certain aspects of data protection law remains unclear, and it is unclear how United Kingdom data protection laws and regulations will develop in the medium to longer term, and how data transfers to and from the United Kingdom will be regulated in the long term. Currently there is a four to six-month grace period agreed in the EU and United Kingdom Trade and Cooperation Agreement, ending June 30, 2021 at the latest, whilst the parties discuss an adequacy decision. However, it is not clear whether (and when) an adequacy decision may be granted by the European Commission enabling data transfers from EU member states to the United Kingdom long term without additional measures. These changes may lead to additional costs and increase our overall risk exposure.
18
Human Capital
Our human capital resources objectives include, as applicable, identifying, attracting, retaining and motivating our highly qualified management and our other employees, non-employee directors and consultants. The principal purposes of our long-term, equity-based incentive awards are to align the interests of our named executive officers and other employees, non-employee directors and consultants with the interests of our stockholders.
As of February 28, 2021, we had five full-time employees and several consultants in the regulatory, clinical, manufacturing and finance areas. None of our employees are represented by a collective bargaining arrangement, and we believe our relationship with our employees is good.
About Evoke
We were incorporated under the laws of the state of Delaware in January 2007. Our principal executive offices are located at 420 Stevens Avenue, Suite 370, Solana Beach, California 92075, and our telephone number is (858) 345-1494.
Financial Information about Segments
We have one operating segment, which is the development and commercialization of pharmaceutical products. See Note 2 to our financial statements included in this Annual Report on Form 10-K. For financial information regarding our business, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and those financial statements and related notes.
Available Information
We file electronically with the Securities and Exchange Commission, or SEC, our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K pursuant to Sections 13(a) and 15(d) of the Securities Exchange Act of 1934, as amended. We make available copies of these reports, free of charge, on our website at www.evokepharma.com, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. The SEC maintains a website that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that website is www.sec.gov. The information in or accessible through the SEC and our website are not incorporated into, and are not considered part of, this report. Further, our references to the URLs for these websites are intended to be inactive textual references only.
19
We operate in a dynamic and rapidly changing environment that involves numerous risks and uncertainties. Certain factors may have a material adverse effect on our business prospects, financial condition and results of operations, and you should carefully consider them. Accordingly, in evaluating our business, we encourage you to consider the following discussion of risk factors, in its entirety, in addition to other information contained in this Annual Report on Form 10-K and our other public filings with the SEC. Other events that we do not currently anticipate or that we currently deem immaterial may also affect our business, prospects, financial condition and results of operations.
Risks Related to our Business, including the Regulatory Compliance and Commercialization of our Product, Gimoti
Our business is entirely dependent on the success of Gimoti, which may never generate sufficient sales to become profitable.
To date, we have devoted all of our research, development and clinical efforts and financial resources toward the development of our only product, Gimoti. Because our business is entirely dependent on the success of Gimoti, if we are unable to successfully commercialize this product, we will be required to curtail all of our activities and may be required to liquidate, dissolve or otherwise wind down our operations. Any of these events could result in the complete loss of an investment in our securities.
The future commercial success of Gimoti is subject to a number of risks, including the following:
| • | Gimoti competes with well-established products, including oral and intravenous forms of metoclopramide, the same active ingredient in the nasal spray for Gimoti; |
| • | we may not be able to develop market demand for, and later increase sales of, Gimoti through our sales and marketing efforts; |
| • | our ability to obtain adequate levels of coverage and reimbursement for Gimoti from commercial health plans and government health programs; |
| • | we may not be able to maintain commercial manufacturing arrangements with third-party manufacturers or establish and maintain commercial-scale manufacturing capabilities; |
| • | patients taking Gimoti may suffer adverse effects for reasons that may or may not be related to Gimoti, which may adversely affect Gimoti’s commercial profile; and |
| • | we may not be able to obtain, maintain and enforce our patents and other intellectual property rights. |
We may require substantial additional funding and may be unable to raise capital when needed, which would force us to liquidate, dissolve or otherwise wind down our operations.
Our operations have consumed substantial amounts of cash since inception. We believe, based on our current operating plan, that our cash and cash equivalents as of December 31,2020 of approximately $8.1 million, along with the approximately $13.1 million of net proceeds raised from our public offering in January 2021, will be sufficient to fund our operations into the first quarter of 2022, excluding future Gimoti revenue. This period could be shortened if there are any significant increases in planned spending other than anticipated. We anticipate that we will be required to raise additional funds through debt, equity or other forms of financing, such as potential collaboration arrangements, to fund future operations and continue as a going concern. There can be no assurance that we will be able to raise additional funds on acceptable terms, or at all. Because our business is entirely dependent on the success of Gimoti, if we are unable to secure additional financing, successfully commercialize Gimoti or identify and execute on other commercialization or strategic alternatives for Gimoti or our company, we will be required to curtail all of our activities and may be required to liquidate, dissolve or otherwise wind down our operations. Any of these events could result in a complete loss of your investment in our securities.
20
Our estimates of the amount of cash necessary to fund our activities may prove to be wrong and we could spend our available financial resources much faster than we currently expect. Our future funding requirements will depend on many factors, including, but not limited to:
| • | the costs of commercialization activities, including costs associated with commercial manufacturing; |
| • | our ability to manufacture sufficient quantities of Gimoti to meet demand, including whether our contract manufacturers, suppliers, and/or consultants are able to meet appropriate timelines; |
| • | our ability to obtain, maintain and enforce our patents and other intellectual property rights and the costs incurred in doing so; |
| • | the terms and timing of any collaborative, licensing, co-promotion or other arrangements that we may establish; and |
Additional funding may not be available to us on acceptable terms or at all. In addition, the terms of any financing may adversely affect the holdings or the rights of our stockholders. Furthermore, the issuance of additional shares or other securities by us, or the possibility of such issuance, may cause the market price of our shares to decline and dilute the holdings of our existing stockholders. If we raise additional funds by incurring debt, the terms of the debt may involve significant cash payment obligations, as well as covenants and specific financial ratios that may restrict our ability to operate our business. We cannot provide any assurance that our existing capital resources will be sufficient to enable us to identify or execute a viable plan for continued clinical development of Gimoti or to otherwise survive as a going concern.
Any termination or suspension of, or delays in the completion of, the post-marketing PK trial of Gimoti or any other future clinical trials could result in increased costs to us, delay or limit our ability to generate revenue and adversely affect our commercial prospects.
In connection with FDA’s approval of Gimoti, we committed to conduct a PK trial to characterize dose proportionality of a lower dose strength compared to the current 15 mg dose strength. We expect to initiate this trial in the second half of 2021. We do not know whether any trials will produce data on schedule, if at all. The commencement and completion of clinical trials can be delayed for a number of reasons, including delays related to:
21
| ● | an IRB refusing to approve, suspending or terminating the trial at an investigational site, precluding enrollment of additional subjects, or withdrawing its approval of the trial. |
In addition, disruptions caused by the COVID-19 pandemic may increase the likelihood that we encounter such difficulties or delays in initiating, enrolling, conducting or completing our planned and ongoing clinical trials. Product development costs will increase if we need to perform more or larger clinical trials than planned. Additionally, changes in regulatory requirements and policies may occur and we may need to amend clinical trial protocols to reflect these changes. Amendments may require us to resubmit our clinical trial protocols to IRBs for reexamination, which may impact the costs, timing or successful completion of a clinical trial. If we experience delays in completion of or if we, FDA or other regulatory authorities, the IRB, or other reviewing entities, or any of our clinical trial sites suspend or terminate any of our clinical trials, the commercial prospects for our product candidate may be harmed and our ability to generate product revenues will be delayed. In addition, many of the factors that cause, or lead to, termination or suspension of, or a delay in the commencement or completion of, clinical trials may also ultimately lead to the denial of regulatory approval of a product candidate.
Delays in the completion of any clinical trials and studies we may conduct for Gimoti could be harmful to our business and cause us to require additional funding.
We have no internal sales, marketing or distribution capabilities currently and rely on Eversana, and will rely on other third parties, for the commercialization of Gimoti, and we and they may not be able to effectively market, sell and distribute Gimoti.
Currently, we have no internal sales, marketing or distribution capabilities, and we may not be able to effectively market and distribute the product. Eversana manages substantially all activities related to marketing, market access, distribution, sales team, patient reimbursement, and provides related support services. To the extent we and Eversana are not successful in retaining qualified sales and marketing personnel, we may not be able to effectively market Gimoti. Further, there can be no assurance that the capabilities of the Eversana will be effective in marketing and selling Gimoti, or that their personnel will be more effective than an internally developed sales organization. In addition, Eversana may terminate our agreement under certain circumstances, including failure to make payments when due, if we are in material breach of the agreement and fail to remedy the breach following notice, if we enter into bankruptcy, or if we are excluded from participation in certain federal governmental programs or have similar actions taken against us. If we and Eversana fail to hire, train, retain and manage qualified sales personnel, market our product successfully or on a cost-effective basis or otherwise terminate our relationship, our ability to generate revenue will be limited and we will need to identify and retain an alternative organization, or develop our own sales and marketing capability. In such an event, we would have to invest significant amounts of financial and management resources to develop internal sales, distribution and marketing capabilities. This could involve significant delays and costs, including the diversion of our management’s attention from other activities. We may also need to retain additional consultants or external service providers to assist us in sales, marketing and distribution functions, and may be unsuccessful in retaining such services on acceptable financial terms or at all.
If we do perform sales, marketing and distribution functions ourselves, we could face a number of additional related risks, including:
| • | inability to attract and build an effective marketing department or sales force; |
| • | the cost of establishing a marketing department or sales force may exceed our available financial resources and the revenues generated by Gimoti or any other product candidates that we may develop, in-license or acquire; and |
| • |
If we are unsuccessful in building and managing a sales and marketing infrastructure internally or through a third-party partner for Gimoti or any future approved product, we will have difficulty commercializing the product, which would adversely affect our business and financial condition.
We and Eversana will need to retain qualified sales and marketing personnel and collaborate in order to successfully commercialize Gimoti.
In January 2020, we entered into the Eversana Agreement, pursuant to which Eversana provides sales representatives to promote Gimoti. These representatives are employees of Eversana and are hired and managed by Eversana. To the extent Eversana is not successful in retaining qualified sales and marketing personnel, we may not be able to effectively market Gimoti.
We and Eversana each have the right to terminate the Eversana Agreement subject to certain conditions, as described above under “Business—Commercialization—Commercial Services and Loan Agreements with Eversana.” While our agreement with Eversana requires sales representatives to undergo onboarding and training, we cannot be sure that Eversana’s efforts will be successful or generate sufficient awareness or demand for Gimoti.
Revenues we receive from sales of Gimoti will largely depend upon the efforts of Eversana, which in many instances are not within our control. If we are unable to maintain the Eversana Agreement or to effectively establish alternative arrangements
22
to market Gimoti or any other products, our business could be adversely affected. In addition, despite our arrangement with Eversana, we still may not be able to cover all of the prescribing physicians for gastroparesis at the same level of reach and frequency as our competitors, and we ultimately may need to further expand our selling efforts in order to effectively compete.
Use of Gimoti or any future product candidates we may develop could be associated with side effects, adverse events or other properties or safety risks, which could delay or preclude approval, cause us to suspend or discontinue clinical trials, abandon a product candidate, limit the commercial profile of the approved labeling, or result in other significant negative consequences that could severely harm our business, prospects, operating results and financial condition.
If we or others identify undesirable side effects, or other previously unknown problems, with Gimoti, a number of potentially significant negative consequences could result, including:
| • | regulatory authorities may require the addition of warnings in the product label or narrowing of the indication in the product label; |
| • | FDA could suspend or withdraw approval of the product, or refuse to approve pending NDA supplements; |
| • | FDA may require us to conduct additional clinical trials or costly post-marketing testing and surveillance to monitor the safety or efficacy of the product; |
Moreover, if any future product candidates we may develop are associated with undesirable side effects in clinical trials or have characteristics that are unexpected, we may elect to abandon their development or limit their development to more narrow uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective, which may limit the commercial prospects for the product candidate, if approved. Undesirable side effects could cause us or regulatory authorities to interrupt, delay or halt clinical trials, result in a more restrictive label than proposed, or delay or cause the denial of regulatory approvals by FDA or comparable foreign regulatory authorities. The drug-related side effects could also affect patient recruitment for our clinical trials, or the ability of enrolled patients to complete the trials, or result in potential product liability claims. We may also be required to modify our plans for future studies based on findings in our ongoing clinical trials. Many compounds that initially showed promise in early-stage testing have later been found to cause side effects that prevented further development of the compound. In addition, regulatory authorities may draw different conclusions or require additional testing to confirm these determinations. Any of these occurrences may harm our business, financial condition and prospects significantly.
Undesirable side effects or other previously unknown problems could prevent us from achieving or maintaining market acceptance of Gimoti, or our future product candidates, if approved, and could substantially increase the costs of commercializing and developing such products or product candidates.
Our business may continue to be impacted by epidemic diseases such as the COVID-19 pandemic.
In March 2020, the World Health Organization declared the outbreak of COVID-19, which has spread across the United States and worldwide, to be a pandemic. A pandemic, including COVID-19 or other public health epidemics, poses the risk that we or our employees, or our third-party suppliers and manufacturers may be prevented from conducting business activities for an indefinite period of time, including due to spread of the disease within these groups or due to shutdowns that may be requested or mandated by governmental authorities. To date, we have experienced various disruptions to our sales activities. For example, Eversana’s commercialization efforts have been adversely affected by operational restrictions imposed on its sales force from quarantines, travel restrictions and bans, and other governmental restrictions related to COVID-19. As a result of these restrictions, their sales force has been restricted from conducting in-person interactions with certain physicians and customers and has been restricted to conducting educational and promotional activities for Gimoti virtually in certain circumstances, which has impacted Eversana’s ability to more actively market Gimoti. The continued spread of COVID-19 and the measures taken by the governments of countries affected could disrupt the raw material supply chain and the manufacture or shipment of Gimoti for commercial sale or cause us and Eversana to delay or materially modify our commercial plans, which could increase costs or decrease potential Gimoti revenues and have a material adverse effect on our business, financial condition and results of operations. Although we have not experienced any material disruptions or delays in manufacturing or supplying Gimoti to date, there can be no assurance that the COVID-19 pandemic will not disrupt the operations of our third-party suppliers and manufacturers and delay our manufacturing timelines of Gimoti in the future, which may negatively impact our ability to successfully commercialize Gimoti and generate product sales. COVID-19 may also impact the costs and timing of our planned post-marketing commitment PK trial of Gimoti. Moreover, to the extent any
23
of these risks and uncertainties adversely impact us in the ways described above or otherwise, they may also have the effect of heightening many of the other risks set forth in this Annual Report on Form 10-K. The COVID-19 pandemic and mitigation measures have also had an adverse impact on global economic conditions which could have an adverse effect on our business and financial condition, including impairing our ability to raise capital when needed. The extent to which the COVID-19 pandemic impacts our manufacturing capabilities and commercial plans and other results will depend on future developments that are highly uncertain and cannot be predicted, including new information that may emerge concerning the actions to contain its impact and treat the disease.
Disruptions at FDA and other government agencies caused by funding shortages or global health concerns could hinder their ability to hire, retain or deploy key leadership and other personnel, or otherwise prevent new or modified products from being developed, approved or commercialized in a timely manner or at all, which could negatively impact our business.
The ability of FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, statutory, regulatory, and policy changes, FDA’s ability to hire and retain key personnel and accept the payment of user fees, and other events that may otherwise affect FDA’s ability to perform routine functions. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of other government agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable. Disruptions at FDA and other agencies may also slow the time necessary for new drugs or modifications to approved drugs to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, including for 35 days beginning on December 22, 2018, the U.S. government has shut down several times and certain regulatory agencies, such as FDA, have had to furlough critical FDA employees and stop critical activities.
Separately, in response to the COVID-19 pandemic, on March 10, 2020 FDA announced its intention to postpone most foreign inspections of manufacturing facilities and products through April 2020, and subsequently, on March 18, 2020, FDA announced its intention to temporarily postpone routine surveillance inspections of domestic manufacturing facilities. Regulatory authorities outside the United States may adopt similar restrictions or other policy measures in response to the COVID-19 pandemic. If a prolonged government shutdown occurs, or if global health concerns continue to prevent FDA or other regulatory authorities from conducting their regular inspections, reviews, or other regulatory activities, it could significantly impact the ability of FDA or other regulatory authorities to timely review and process our regulatory submissions, which could have a material adverse effect on our business.
Even though FDA has approved Gimoti for the relief of symptoms in adults with acute and recurrent diabetic gastroparesis, we will remain subject to significant post-marketing regulatory requirements and oversight.
Any regulatory approvals that we may receive for Gimoti or any future product candidates will require the submission of reports to regulatory authorities and surveillance to monitor the safety and efficacy of the product, may contain significant limitations related to use restrictions for specified age groups, warnings, precautions or contraindications, and may include burdensome post-approval study or risk management requirements. For example, the approved labeling for Gimoti includes a black box warning regarding the risks of tardive dyskinesia associated with metoclopramide, the active ingredient in Gimoti. FDA may also require a REMS in order to approve a product candidate, which could entail requirements for a medication guide, physician training and communication plans or additional elements to ensure safe use, such as restricted distribution methods, patient registries and other risk minimization tools.
In addition, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion, import, export and recordkeeping for Gimoti are subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as on-going compliance with current good manufacturing practices, or cGMPs, and GCPs for any clinical trials that we conduct post-approval. In addition, manufacturers of drug products and their facilities are subject to continual review and periodic, unannounced inspections by FDA and other regulatory authorities for compliance with cGMP regulations and standards. If we or a regulatory authority discover previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facilities where the product is manufactured, a regulatory authority may impose restrictions on that product, the manufacturing facility or us, including requiring recall or withdrawal of the product from the market or suspension of manufacturing. In addition, failure to comply with FDA and other comparable foreign regulatory requirements may subject our company to administrative or judicially imposed sanctions, including:
| • | delays in or the rejection of product approvals; |
| • | restrictions on our ability to conduct clinical trials, including full or partial clinical holds on ongoing or planned trials; |
| • | restrictions on the products, manufacturers or manufacturing process; |
24
|
| • | warning or untitled letters; |
| • | civil and criminal penalties; |
| • | injunctions; |
| • | suspension or withdrawal of regulatory approvals; |
| • | product seizures, detentions or import bans; |
| • | voluntary or mandatory product recalls and publicity requirements; |
| • | total or partial suspension of production; and |
| • | imposition of restrictions on operations, including costly new manufacturing requirements. |
The occurrence of any event or penalty described above may inhibit our ability to commercialize Gimoti and generate revenue and could require us to expend significant time and resources in response and could generate negative publicity. In addition, FDA’s and other regulatory authorities’ policies may change, and additional government regulations may be enacted that could impair our business. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may be subject to enforcement action, and we may not achieve or sustain profitability.
We also cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action, either in the United States or abroad. For example, the results of the 2020 U.S. Presidential Election may impact our business and industry. Namely, the Trump administration took several executive actions, including the issuance of a number of executive orders, that could impose significant burdens on, or otherwise materially delay, FDA’s ability to engage in routine regulatory and oversight activities such as implementing statutes through rulemaking, issuance of guidance, and review and approval of marketing applications. It is difficult to predict whether or how these executive actions will be implemented, or whether they will be rescinded and replaced under the new Biden administration. The policies and priorities of a new administration are unknown and could materially impact the regulations governing our products.
FDA and other regulatory agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses.
FDA strictly regulates marketing, labeling, advertising and promotion of prescription drugs. These regulations include standards and restrictions for direct-to-consumer advertising, industry-sponsored scientific and educational activities, promotional activities involving the internet and off-label promotion. Any regulatory approval that FDA grants is limited to those specific diseases and indications for which a product is deemed to be safe and effective by FDA. For example, the FDA-approved label for Gimoti is limited to the relief of symptoms in adults with acute and recurrent diabetic gastroparesis. While physicians in the United States may choose, and are generally permitted, to prescribe drugs for uses that are not described in the product’s labeling and for uses that differ from those tested in clinical trials and approved by the regulatory authorities, our ability to promote the products is narrowly limited to those indications that are specifically approved by FDA. These “off-label” uses are common across medical specialties and may constitute an appropriate treatment for some patients in varied circumstances. For example, other formulations of metoclopramide, the active ingredient in Gimoti, have been approved for uses beyond those authorized in Gimoti’s approved labeling, such as for the treatment of gastroesophageal reflux symptoms. We do not market or promote Gimoti for these uses.
Regulatory authorities in the United States generally do not regulate the behavior of physicians in their choice of treatments. Regulatory authorities do, however, restrict communications by pharmaceutical companies on the subject of off-label use. Although recent court decisions suggest that certain off-label promotional activities may be protected under the First Amendment, the scope of any such protection is unclear. If our promotional activities fail to comply with FDA’s regulations or guidelines, we may be subject to warnings from, or enforcement action by, these authorities. In addition, our failure to follow FDA rules and guidelines relating to promotion and advertising may cause FDA to issue warning letters or untitled letters, bring an enforcement action against us, suspend or withdraw an approved product from the market, require a recall or institute fines or civil fines, or could result in disgorgement of money, operating restrictions, injunctions or criminal prosecution, any of which could harm our reputation and our business.
It will be difficult for us to profitably sell Gimoti if coverage and reimbursement are limited.
Market acceptance and sales of our product candidate will depend on coverage and reimbursement policies and may be affected by healthcare reform measures. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities, pharmacy benefit managers and other third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for
25
particular medications. Increasingly, third-party payors have been challenging the prices charged for products. They may also refuse to provide any coverage of uses of approved products for medical indications other than those for which FDA has granted marketing approval. This trend may impact the reimbursement for treatments for GI disorders especially, including Gimoti, as physicians typically focus on symptoms rather than underlying conditions when treating patients with these disorders and drugs are often prescribed for uses outside of their approved indications. In instances where alternative products are available, it may be required that those alternative treatment options are tried before coverage and reimbursement are available for Gimoti. Although Gimoti is a novel nasal spray formulation of metoclopramide, this is the same active ingredient that is already available in other formulations approved for the treatment of gastroparesis that are already widely available at generic prices. We cannot be sure that coverage will be available for Gimoti and, if coverage is available, the level of reimbursement. Reimbursement may impact the demand for, or the price of, this product candidate. In addition, in certain foreign countries, particularly the countries of the EU, the pricing of prescription pharmaceuticals is subject to governmental control. If reimbursement is not available or is available only to limited levels, we may not be able to successfully commercialize our product candidate.
We rely and will continue to rely on outsourcing arrangements for many of our activities, including commercialization activities and supply of Gimoti.
As of February 28, 2021, we had five full-time employees and, as a result, we rely on outsourcing arrangements with third-party vendors for a significant portion of our activities, including commercial sales and marketing, data analysis, assistance with regulatory discussions, manufacturing, and the functions required of being a public company. Any failure of our third-party vendors to continue their support could adversely affect our ability to commercialize Gimoti.
The manufacture of pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. We do not own or operate manufacturing facilities for the production of any component of Gimoti, including metoclopramide, the nasal spray device or associated bottle, nor do we have plans to develop our own manufacturing operations in the foreseeable future. We currently depend on third-party contract manufacturers for all of our required raw materials, drug substance and drug product for our clinical trials and commercialization activities. We are currently using, and relying on, single suppliers and single manufacturers for starting materials, the final drug substance and nasal spray delivery device for Gimoti, including Cosma as the sole-source supplier of metoclopramide and Thermo Fisher Scientific Inc., as the sole manufacturer of Gimoti. Although potential alternative suppliers and manufacturers for some components have been identified, we have not qualified these vendors to date. If we were required to change vendors, it could result in a failure to meet regulatory requirements or projected timelines and necessary quality standards for successful manufacturing of the various required lots of material for our development and commercialization efforts.
If we change to other manufacturers in the future, FDA and comparable foreign regulators must approve these manufacturers’ facilities and processes prior to use, which could require new clinical studies, testing and compliance inspections, and the new manufactures would have to be educated in, or demonstrate successful technology transfer of, the processes necessary for the production of Gimoti.
In addition, our reliance on third-party vendors and contract manufacturing organizations, or CMOs, entails further risks including:
| ● | non-compliance by third parties with regulatory and quality control standards; |
| ● | breach by third parties of our agreements with them; |
| ● | termination or non-renewal of an agreement with third parties; and |
| ● | sanctions imposed by regulatory authorities if compounds supplied or manufactured by a third-party supplier or manufacturer fail to comply with applicable regulatory standards. |
Any performance failure on the part of our third-party manufacturers could delay commercialization and we may be required to replace such manufacturers, and we may be unable to replace them on a timely basis or at all. Further, our third-party manufacturers may experience manufacturing difficulties due to resource constraints or as a result of natural disasters, labor disputes, unstable political environments, or public health emergencies such as the COVID-19 pandemic. If our third-party manufacturers were to encounter any manufacturing difficulties or delays due to these factors, our ability to provide Gimoti for treatment of patients would be jeopardized.
26
We face substantial competition, which may result in others selling their products more effectively than we do, and in others discovering, developing or commercializing product candidates before, or more successfully, than we do.
Our future success depends on our ability to demonstrate and maintain a competitive advantage with respect to the design, development and commercialization of Gimoti, which competes directly with metoclopramide, erythromycin and domperidone, each of which is available under various trade names sold by several major pharmaceutical companies, including generic manufacturers. Metoclopramide is the only molecule currently approved in the United States to treat gastroparesis. Metoclopramide is generically-available and indicated for the relief of symptoms associated with acute and recurrent diabetic gastroparesis.
Many of our potential competitors have substantially greater financial, technical and personnel resources than we have. In addition, many of these competitors have significantly greater commercial infrastructures than we have. We will not be able to compete successfully unless we successfully:
| ● | assure health care providers, patients and health care payors that Gimoti is beneficial compared to other products in the market; |
| ● | obtain patent and/or other proprietary protection for Gimoti; |
| ● | obtain and maintain required regulatory approvals for Gimoti; and |
| ● | collaborate with others to effectively market, sell and distribute Gimoti. |
Established competitors may invest heavily to quickly discover and develop novel compounds that could make Gimoti obsolete. We are aware of other product candidates in the gastroparesis pipeline in clinical development. Any of these product candidates could advance quickly through clinical development and, if approved, could attain faster and greater market acceptance than Gimoti. If we are not able to compete effectively against our current and future competitors, our business will not grow and our financial condition and operations will suffer.
If we fail to attract and retain senior management and key commercial personnel, we may be unable to successfully commercialize Gimoti.
Our success depends in part on our continued ability to attract, retain and motivate highly qualified management, clinical and commercial personnel. We are highly dependent upon our senior management team composed of three individuals: David A. Gonyer, R.Ph., our President and Chief Executive Officer, Matthew J. D’Onofrio, our Executive Vice President and Chief Business Officer, and Marilyn Carlson, D.M.D., M.D., our Chief Medical Officer. The loss of services of any of these individuals could delay or prevent the successful commercialization of Gimoti.
In addition to the team at Eversana, we may need to hire and retain qualified personnel to pursue the commercialization of Gimoti. We could experience problems in the future attracting and retaining qualified employees. For example, competition for qualified personnel in the biotechnology and pharmaceuticals field is intense, particularly in the San Diego, California area where we are headquartered. We may not be able to attract and retain quality personnel on acceptable terms who have the expertise we need to sustain and grow our business.
We may encounter difficulties in managing our growth and expanding our operations successfully.
We may need to grow our organization to pursue the commercialization of Gimoti and to potentially conduct additional unplanned development activities. As we commercialize Gimoti, we will need to expand our regulatory, finance, manufacturing, marketing and sales capabilities or contract with third parties to provide these capabilities for us. As our operations expand, we expect that we will need to manage additional relationships with various strategic partners, suppliers and other third parties. Future growth will impose significant added responsibilities on members of management and require us to retain additional internal capabilities. Our future financial performance and our ability to commercialize Gimoti and to compete effectively will depend, in part, on our ability to manage any future growth effectively. To that end, we must be able to manage our development efforts and clinical trials effectively and hire, train and integrate additional management, clinical and regulatory, financial, administrative and sales and marketing personnel. We may not be able to accomplish these tasks, and our failure to accomplish any of them could prevent us from successfully growing our company.
Enacted and future legislation may increase the difficulty and cost for us to commercialize Gimoti and affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could restrict or regulate post-approval activities and affect our ability to profitably sell Gimoti.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We are not sure whether additional legislative changes will be enacted, or whether
27
FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the commercialization of Gimoti, if any, may be. In addition, increased scrutiny by the U.S. Congress of FDA’s approval process may subject us to more stringent product labeling and post-marketing testing and other requirements.
In 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, the Affordable Care Act, was signed into law. The Affordable Care Act was intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. The Affordable Care Act, among other things, increased the Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program for both branded and generic drugs and revised the definition of “average manufacturer price” for reporting purposes, which could further increase the amount of Medicaid drug rebates to states. Further, the law imposes a significant annual fee on companies that manufacture or import branded prescription drug products, increased the number of entities eligible for discounts under the 340B program and included a discount on brand name drugs for Medicare Part D beneficiaries in the coverage gap, or “donut hole.” Substantial provisions affecting compliance have also been enacted, which may require us to modify our business practices with healthcare practitioners.
Since its enactment, there have been judicial and Congressional challenges to certain aspects of the Affordable Care Act. For example, the Tax Cuts and Jobs Act, or Tax Act, was enacted, which, among other things, removes penalties for not complying with Affordable Care Act’s individual mandate to carry health insurance. On December 14, 2018, a U.S. District Court Judge in the Northern District of Texas, ruled that the individual mandate is a critical and inseverable feature of the Affordable Care Act, and therefore, because it was repealed as part of the Tax Act, the remaining provisions of the Affordable Care Act are invalid as well. On December 18, 2019, the U.S. Court of Appeals for the 5th Circuit upheld the District Court’s decision that the individual mandate was unconstitutional, but remanded the case back to the District Court to determine whether the remaining provisions of the Affordable Care Act are invalid as well. The U.S. Supreme Court is currently reviewing the case, although it is unclear how the Supreme Court will rule. It is also unclear how other efforts, if any, to challenge, repeal or replace the Affordable Care Act will impact the law. We cannot predict the ultimate content, timing or effect of any healthcare reform legislation or the impact of potential legislation on us.
There have been a number of recent regulatory and legislative initiatives designed to encourage generic competition for pharmaceutical products, including expedited review procedures for generic manufacturers and incentives designed to spur generic competition of branded drugs. In particular, FDA and FTC have been focused on brand companies’ denial of drug supply to potential generic competitors for testing. In December 2019, the Creating and Restoring Equal Access to Equivalent Samples Act, or the CREATES Act, was enacted, which provides a legislatively defined private right of action under which eligible product developers can bring suit against companies who refuse to sell sufficient quantities of their branded products on commercially reasonable, market-based terms to support such eligible product developers’ marketing applications. We cannot currently predict the specific outcome or impact on our business of such regulatory and legislative initiatives. However, it is our policy, which is in compliance with the CREATES Act, to evaluate requests for samples of our branded products, and to provide samples in response to bona fide requests from qualified third parties, including generic manufacturers, subject to specified conditions. We have received a request for samples of Gimoti and are in the process of responding to that request in compliance with the requirements of the CREATES Act.
In addition, other legislative changes have been proposed and adopted in the United States since the Affordable Care Act was enacted. These changes include aggregate reductions to Medicare payments to providers of two percent per fiscal year, which went into effect on April 1, 2013, and due to subsequent legislative amendments, will remain in effect through 2030, with the exception of a temporary suspension from May 1, 2020 through March 31, 2021, unless additional Congressional action is taken and the American Taxpayer Relief Act of 2012 which, among other things, further reduced Medicare payments to several types of providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. Recently there has also been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted legislation designed to, among other things, reform government program reimbursement methodologies. Individual states in the United States have also become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. These laws and the regulations and policies implementing them, as well as other healthcare reform measures that may be adopted in the future, may have a material adverse effect on our industry generally and on our ability to successfully develop and commercialize our products.
28
If we or our commercialization partners market products in a manner that violates healthcare laws, we may be subject to civil or criminal penalties.
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal healthcare fraud and abuse laws have been applied in recent years to restrict business activities in the pharmaceutical industry, including certain marketing practices. These laws include false claims, anti-kickback, and physician and other health care provider payment transparency laws and regulations. Because of the breadth of these laws and the narrowness of the safe harbors, it is possible that some of our business activities could be subject to challenge under one or more of these laws.
The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce, or in return for, purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Although there are several statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution, the exceptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exception or safe harbor. Our practices may not in all cases meet all of the criteria for safe harbor protection from anti-kickback liability. Further a person or entity does not need to have actual knowledge of these statutes or specific intent to violate them in order to have committed a violation.
Federal civil and criminal false claims laws, including the False Claims Act, prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government or knowingly making, or causing to be made, a false statement to get a false claim paid. Violations of the False Claims Act can result in very significant monetary penalties and treble damages. Over the past few years, several pharmaceutical and other healthcare companies have been prosecuted under these laws for a variety of alleged promotional and marketing activities, such as: allegedly providing free trips, free goods, sham consulting fees and grants and other monetary benefits to prescribers; reporting to pricing services inflated average wholesale prices that were then used by federal programs to set reimbursement rates; engaging in off-label promotion that caused claims to be submitted to Medicaid for non-covered, off-label uses; and submitting inflated best price information to the Medicaid Rebate Program to reduce liability for Medicaid rebates. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act. Most states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
Federal civil monetary penalties laws impose civil fines for, among other things, the offering or transfer of remuneration to a Medicare or state healthcare program beneficiary if the person knows or should know it is likely to influence the beneficiary’s selection of a particular provider, practitioner, or supplier of services reimbursable by Medicare or a state healthcare program, unless an exception applies.
HIPAA created additional federal criminal statutes that prohibit among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Like the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Federal price reporting laws require manufactures to calculate and report complex pricing metrics to government programs, where such reported prices may be used in the calculation of reimbursement and/or discounts on approved products.
Federal and state consumer protection and unfair competition laws broadly regulate marketplace activities and activities that potentially harm consumers.
With the approval of Gimoti by FDA in June 2020, and our commencement of sales in the United States in October 2020, we are required to comply with the federal Physician Payment Sunshine Act, which requires manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the government information related to payments or other “transfers of value” made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain other health care professionals beginning in 2022, and teaching hospitals, and applicable manufacturers and group purchasing organizations to report annually to the government ownership and investment interests held by physicians (as defined above) and their immediate family members. Manufacturers are required to report such data to the government by the 90th calendar day of each year. There are also several states with similar laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures and pricing
29
information, and/or require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers.
The risk of our being found in violation of these laws and regulations is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from governmental health care programs, a corporate integrity agreement or other agreement to resolve allegations of non-compliance, individual imprisonment, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our financial results.
We may be subject to foreign, federal, and state data privacy and security laws, and failure to protect our information systems against security breaches, service interruptions, or misappropriation of data could disrupt operations, compromise sensitive data, and expose us to liability, possibly causing our business and reputation to suffer.
We and our collaborators and third-party providers may be subject to federal, state and foreign data privacy and security laws and regulations. In the United States, numerous federal and state laws and regulations, including federal health information privacy laws, state data breach notification laws, state health information privacy laws and federal and state consumer protection laws that govern the collection, use, disclosure and protection of health-related and other personal information could apply to our operations or the operations of our collaborators and third-party providers. In addition, we may obtain health information from third parties (including research institutions from which we obtain clinical trial data) that are subject to privacy and security requirements under HIPAA. Depending on the facts and circumstances, we could be subject to significant penalties if we violate HIPAA.
Even when HIPAA does not apply, according to the FTC, failing to take appropriate steps to keep consumers’ personal information secure constitutes unfair acts or practices in or affecting commerce in violation of Section 5(a) of the Federal Trade Commission Act. The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities. Individually identifiable health information is considered sensitive data that merits stronger safeguards.
Certain state laws also govern the privacy and security of health-related and other personal information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. For example, the CCPA went into effect on January 1, 2020. The CCPA, among other things, creates new data privacy obligations for covered companies and provides new privacy rights to California residents, including the right to opt out of certain disclosures of their information. The CCPA also creates a private right of action with statutory damages for certain data breaches, thereby potentially increasing risks associated with a data breach. Although the law includes limited exceptions, including for “protected health information” maintained by a covered entity or business associate, it may regulate or impact our processing of personal information depending on the context. Further, the CPRA was recently voted into law by California residents. The CPRA significantly amends the CCPA, and imposes additional data protection obligations on covered companies doing business in California, including additional consumer rights processes and opt outs for certain uses of sensitive data. It also creates a new California data protection agency specifically tasked to enforce the law, which would likely result in increased regulatory scrutiny of California businesses in the areas of data protection and security. The substantive requirements for businesses subject to the CPRA will go into effect on January 1, 2023, and become enforceable on July 1, 2023.
Similar healthcare laws and regulations exist in Europe and other jurisdictions, including reporting requirements detailing interactions with and payments to healthcare providers and requirements regarding the collection, distribution, use, security, and storage of personally identifiable information and other data relating to individuals, including the GDPR, which went into effect in May 2018. The GDPR applies to any company established in the EEA, as well as to those outside the EEA, if they collect and use personal data in connection with the offering of goods or services to individuals in the EEA or the monitoring of their behavior. Companies that must comply with the GDPR face increased compliance obligations and risk, including more robust regulatory enforcement of data protection requirements and potential fines for noncompliance of up to €20 million or 4% of the annual global revenues of the noncompliant company, whichever is greater. The GDPR provides that EU and EEA member states may introduce further conditions, including limitations, to the processing of genetic, biometric or health data, which could limit our ability to collect, use and share personal data, or could cause our compliance costs to increase, ultimately having an adverse impact on our business. Among other requirements, the GDPR regulates transfers of personal data subject to the GDPR to third countries that have not been found to provide adequate protection to such personal data, including the United States, and the efficacy and longevity of current transfer mechanisms between the European Union, or EU, and the United States remains uncertain. For example, in 2016, the EU and United States agreed to a
30
transfer framework for data transferred from the EU to the United States, called the Privacy Shield, but the Privacy Shield was invalidated in July 2020 by the Court of Justice of the European Union.
Further, from January 1, 2021, companies have to comply with the GDPR and also the UK GDPR, which, together with the amended UK Data Protection Act 2018, retains the GDPR in UK national law. The UK GDPR mirrors the fines under the GDPR, e.g. fines up to the greater of €20 million (£17.5 million) or 4% of global turnover. The relationship between the United Kingdom and the European Union in relation to certain aspects of data protection law remains unclear, and it is unclear how United Kingdom data protection laws and regulations will develop in the medium to longer term, and how data transfers to and from the United Kingdom will be regulated in the long term. Currently there is a four to six-month grace period agreed in the EU and United Kingdom Trade and Cooperation Agreement, ending June 30, 2021 at the latest, whilst the parties discuss an adequacy decision. However, it is not clear whether (and when) an adequacy decision may be granted by the European Commission enabling data transfers from EU member states to the United Kingdom long term without additional measures. These changes may lead to additional costs and increase our overall risk exposure.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of Gimoti.
We face an inherent risk of product liability as a result of the clinical testing of Gimoti and will face an even greater risk as we commercialize Gimoti. For example, we may be sued if Gimoti allegedly causes injury or is found to be otherwise unsuitable during product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability and a breach of warranties. Claims could also be asserted under state consumer protection acts.
In particular, products containing metoclopramide have been reported to cause side effects, including TD. It is possible that a patient taking Gimoti will be found to experience a variety of side effects. In 2009, FDA required a boxed warning on all metoclopramide product labels concerning the chance of TD for patients taking these products. The label for Gimoti contains a similar warning regarding TD. Several manufactures of metoclopramide products have been sued by patients regarding TD.
If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our product candidate. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
| ● | decreased demand for Gimoti; |
| ● | injury to our reputation; |
| ● | withdrawal of clinical trial participants; |
| ● | costs to defend the related litigation; |
| ● | a diversion of management’s time and our resources; |
| ● | substantial monetary awards to trial participants or patients; |
| ● | product recalls, withdrawals or labeling, marketing or promotional restrictions; |
| ● | loss of revenue; |
| ● | the inability to commercialize Gimoti; and |
| ● | a decline in our stock price. |
We may form strategic alliances in the future, and we may not realize the benefits of such alliances.
We may form strategic alliances, create joint ventures or collaborations or enter into licensing arrangements with third parties that we believe will complement or augment our existing business, including for the continued development or commercialization of Gimoti. These relationships or those like them may require us to incur non-recurring and other charges, increase our near- and long-term expenditures, issue securities that dilute our existing stockholders or disrupt our management and business. In addition, we face significant competition in seeking appropriate strategic partners and the negotiation process is time-consuming and complex. Moreover, we may not be successful in our efforts to establish a strategic partnership or other alternative arrangements for Gimoti because third parties may view the development or commercialization risk of Gimoti as too significant or the commercial opportunity for our product candidate as too limited. We cannot be certain that, following a strategic transaction or license, we will achieve the revenues or specific net income that justifies such transaction.
31
Our business and operations would suffer in the event of system failures, including cyberattacks.
Despite the implementation of security measures, our internal computer systems and those of our current and any future CROs and other contractors and consultants and collaborators are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Attacks upon information technology systems are increasing in their frequency, levels of persistence, sophistication and intensity, and are being conducted by sophisticated and organized groups and individuals with a wide range of motives and expertise. As a result of the COVID-19 pandemic, we may also face increased cybersecurity risks due to our reliance on internet technology and the number of our employees who are working remotely, which may create additional opportunities for cybercriminals to exploit vulnerabilities. Furthermore, because the techniques used to obtain unauthorized access to, or to sabotage, systems change frequently and often are not recognized until launched against a target, we may be unable to anticipate these techniques or implement adequate preventative measures. We may also experience security breaches that may remain undetected for an extended period. While we do not believe that we have experienced any such material system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development program for Gimoti and our business operations. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we rely on third parties to manufacture Gimoti and conduct clinical trials, and similar events relating to their computer systems could also have a material adverse effect on our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our product candidate could be delayed, or otherwise adversely affected.
Business disruptions could seriously harm our future revenues and financial condition and increase our costs and expenses.
Our operations could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or manmade disasters or business interruptions, for which we are predominantly self-insured. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. We rely on third-party manufacturers to produce our Gimoti. Our ability to obtain clinical supplies of Gimoti could be disrupted, if the operations of these suppliers are affected by a man-made or natural disaster or other business interruption, including the COVID-19 pandemic.
Our operations are located in Solana Beach, California near major earthquake faults and fire zones. The ultimate impact on us, our significant suppliers and our general infrastructure of being located near major earthquake faults and fire zones and being consolidated in certain geographical areas is unknown, but our operations and financial condition could suffer in the event of a major earthquake, fire or other natural disaster, or public health emergency.
If we fail to develop and commercialize other product candidates, we may be unable to grow our business.
As part of our growth strategy, we plan to evaluate the development and/or commercialization of other therapies for GI motility disorders. Similar to our initial focus on gastroparesis, we will evaluate opportunities to in-license or acquire other product candidates as well as commercial products to treat patients suffering from predominantly GI disorders, seeking to identify areas of high unmet medical needs with limited treatment options. These other product candidates will require additional, time-consuming development efforts prior to commercial sale, including preclinical studies, extensive clinical trials and approval by FDA and applicable foreign regulatory authorities. All product candidates are prone to the risks of failure that are inherent in pharmaceutical product development, including the possibility that the drug candidate will not be shown to be sufficiently safe and/or effective for approval by regulatory authorities. In addition, we cannot assure you that any such products that are approved will be manufactured or produced economically, successfully commercialized or widely accepted in the marketplace or be more effective than other commercially available alternatives.
If we engage in an acquisition, reorganization or business combination, we will incur a variety of risks that could adversely affect our business operations or our stockholders.
From time to time we have considered, and we will continue to consider in the future, strategic business initiatives intended to further the development of our business. These initiatives may include acquiring businesses, technologies or products or entering into a business combination with another company. If we do pursue such a strategy, we could, among other things:
| ● | issue equity securities that would dilute our current stockholders’ percentage ownership; |
| ● | incur substantial debt that may place strains on our operations; |
| ● | spend substantial operational, financial and management resources in integrating new businesses, technologies and products; and |
32
| ● | assume substantial actual or contingent liabilities. |
We may be unable to maintain sufficient product liability insurance.
Our inability to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of products we develop. We currently carry product liability insurance covering Gimoti’s commercial sales. Although we maintain such insurance, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. If we determine that it is prudent to increase our product liability coverage due to the commercial launch of any product, we may be unable to obtain such increased coverage on acceptable terms or at all. Our insurance policies also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. We will have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts.
Risks Relating to Our Intellectual Property
It is difficult and costly to protect our intellectual property rights, and we cannot ensure the protection of these rights. Any impairment of our intellectual property rights may materially affect our business.
We place considerable importance on obtaining patent protection for new technologies, products and processes because our commercial success will depend, in large part, on obtaining patent protection for new technologies, products and processes, successfully defending these patents against third-party challenges and successfully enforcing our patents against third-party competitors. To that end, we have acquired and will file applications for patents covering formulations containing or uses of Gimoti or our proprietary processes as well as other intellectual property important to our business. One of our patent families related to Gimoti was acquired from Questcor, which was acquired by Mallinckrodt in August 2014. The method of use patents in this patent family were not written by us or our attorneys, and we did not have control over the drafting and prosecution of these patents. Further, Questcor and other predecessors might not have given the same attention to the drafting and prosecution of these patents as we would have if we had been the owners of the patents and application and had control over the drafting and prosecution.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain and involves complex legal and factual questions for which legal principles remain unresolved. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued which protect our technology or products or which effectively prevent others from commercializing competitive technologies and products. In recent years patent rights have been the subject of significant litigation, in particular due to inter partes review, introduced by the America Invents Act of 2012, which allows for quicker patent challenges decided by the U.S. Patent and Trademark Office’s, or USPTO, Patent Trial and Appeal Board rather than a lay jury. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection. The laws of foreign countries may not protect our rights to the same extent as the laws of the United States. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we or our predecessors were the first to make the inventions claimed in our owned and licensed patents or pending patent applications, or that we or our predecessors were the first to file for patent protection of such inventions One or more of these factors could possibly result in findings of invalidity or unenforceability of one or more of the patents we own.
With respect to challenges to the validity of our patents, for example, there might be invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on a product candidate. Even if a defendant does not prevail on a legal assertion of invalidity and/or unenforceability, our patent claims may be construed in a manner that would limit our ability to enforce such claims against the defendant and others. The cost of defending such a challenge, particularly in a foreign jurisdiction, and any resulting loss of patent protection could have a material adverse impact on one or more of our product candidates and our business.
Enforcing our intellectual property rights against third parties may also cause such third parties to file other counterclaims against us, which could be costly to defend, particularly in a foreign jurisdiction, and could require us to pay substantial damages, cease the sale of certain products or enter into a license agreement and pay royalties (which may not be possible on commercially reasonable terms or at all). Any efforts to enforce our intellectual property rights are also likely to be costly and may divert the efforts of our scientific and management personnel.
The patent rights we own covering Gimoti are directed to specific methods of use and formulations of metoclopramide. As a result, our ability to prevent others from marketing products related to Gimoti may be limited by the lack of patent protection
33
for the active ingredient itself and other metoclopramide formulations may be developed by competitors. The active ingredient in Gimoti is metoclopramide. No patent protection is available for metoclopramide itself. As a result, competitors who develop and receive required regulatory approval for competing products using the same active ingredient as Gimoti may market their competing products so long as they do not infringe any of the method or formulation patents owned by us.
Third parties may seek approval to market their own products similar to or otherwise competitive with our product candidates. In these circumstances, we may need to defend or assert our patents, including by filing lawsuits alleging patent infringement. The outcome following legal assertions of invalidity and unenforceability is unpredictable. In any of these types of proceedings, a court or agency with jurisdiction may find our patents invalid or unenforceable. Even if we have valid and enforceable patents, these patents still may not provide protection against competing products or processes sufficient to achieve our business objectives. Even after they have issued, our patents and any patents that we license may be challenged, narrowed, invalidated or circumvented. If our patents are invalidated or otherwise limited or will expire prior to the commercialization of our product candidates, other companies may be better able to develop products that compete with ours, which could adversely affect our competitive business position, business prospects and financial condition. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates. The following are examples of litigation and other adversarial proceedings or disputes that we could become a party to involving our patents or patents licensed to us:
| ● | we may initiate litigation or other proceedings against third parties to enforce our patent and trade secret rights; |
| ● | third parties may initiate litigation or other proceedings seeking to invalidate patents owned by or licensed to us or to obtain a declaratory judgment that their product or technology does not infringe our patents or patents licensed to us; |
| ● | third parties may initiate opposition or reexamination proceedings challenging the validity or scope of our patent rights, requiring us to participate in such proceedings to defend the validity and scope of our patents; |
| ● | there may be a challenge or dispute regarding inventorship or ownership of patents or trade secrets currently identified as being owned by or licensed to us; |
| ● | the USPTO may initiate an interference between patents or patent applications owned by or licensed to us and those of our competitors, requiring us to participate in an interference proceeding to determine the priority of invention, which could jeopardize our patent rights; or |
| ● | third parties may seek approval to market similar versions of our future approved products prior to expiration of relevant patents owned by or licensed to us, requiring us to defend our patents, including by filing lawsuits alleging patent infringement. |
These lawsuits and proceedings would be costly and could affect our results of operations and divert the attention of our managerial and scientific personnel. Adversaries in these proceedings may have the ability to dedicate substantially greater resources to prosecuting these legal actions than we or our licensors can. There is a risk that a court or administrative body would decide that our patents are invalid or not infringed or trade secrets not misappropriated by a third party’s activities, or that the scope of certain issued claims must be further limited. An adverse outcome in a litigation or proceeding involving our own patents or trade secrets could limit our ability to assert our patents or trade secrets against these or other competitors, affect our ability to receive royalties or other licensing consideration from any licensees, and may curtail or preclude our ability to exclude third parties from making, using and selling similar or competitive products. Any of these occurrences could adversely affect our competitive business position, business prospects and financial condition. We may not be able to prevent, alone or with our licensors, infringement or misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States. Any litigation or other proceedings to enforce our intellectual property rights may fail, and even if successful, may result in substantial costs and distract our management and other employees. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have an adverse effect on the price of our common shares. The degree of future protection for our proprietary rights is uncertain because legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage. For example:
| ● | third parties may seek approval to market similar versions of our future approved products prior to expiration of relevant patents owned by or licensed to us, requiring us to defend our patents, including by filing lawsuits alleging patent infringement. |
| ● | others may be able to develop a platform that is similar to, or better than, ours in a way that is not covered by the claims of our patents; |
34
| ● | others may be able to make products that are similar to our product candidates but that are not covered by the claims of our patents; |
| ● | we might not have been the first to make the inventions covered by patents or pending patent applications or we might not have been the first to file patent applications for these inventions; |
| ● | any patents that we obtain may not provide us with any competitive advantages or may ultimately be found invalid or unenforceable; or |
| ● | we may not develop additional proprietary technologies that are patentable or that afford meaningful trade secret protection. |
Others have filed, and in the future are likely to file, patent applications covering products and technologies that are similar, identical or competitive to ours, or important to our business. We cannot be certain that any patent application owned by a third party will not have priority over patent applications filed or in-licensed by us, or that we will not be involved in interference, opposition or invalidity proceedings before U.S. or foreign patent offices.
We have focused our intellectual property efforts on the United States. To the extent that our patent portfolio differs from country to country outside the United States, this may make protecting Gimoti as a product outside the United States even more difficult and unpredictable. Various countries maintain their own standards and interpretation of intellectual property law, potentially creating additional patent risk beyond even that experienced within the United States.
We also rely on trade secrets to protect technology in cases when we believe patent protection is not appropriate or obtainable. However, trade secrets are difficult to protect. While we require employees, consultants and other contractors to enter into confidentiality agreements, we may not be able to adequately protect our trade secrets or other proprietary information. Our research collaborators and scientific advisors may have rights to publish data and information in which we have rights. If we cannot maintain the confidentiality of our technology and other confidential information in connection with our collaborators and advisors, our ability to receive patent protection or protect our proprietary information may be imperiled.
Claims by third parties that we infringe their proprietary rights may result in liability for damages or prevent or delay our developmental and commercialization efforts.
The biotechnology industry has been characterized by frequent litigation regarding patent and other intellectual property rights. Because patent applications are maintained in secrecy until the application is published, we may be unaware of third-party patent applications which may issue as patents that may be infringed by commercialization of Gimoti. In addition, identification of third-party patent rights that may be relevant to our technology is difficult because patent searching is imperfect due to differences in terminology among patents, incomplete databases and the difficulty in assessing the meaning of patent claims. Any claims of patent infringement asserted by third parties would be time consuming and would likely:
| ● | result in costly litigation; |
| ● | divert the time and attention of our technical personnel and management; |
| ● | cause development delays; |
| ● | prevent us from commercializing Gimoti until the asserted patent expires or is held finally invalid or not infringed in a court of law; |
| ● | require us to develop non-infringing technology; and/or |
| ● | require us to enter into royalty or licensing agreements. |
Although no third party has asserted a claim of infringement against us, others may hold proprietary rights that could prevent Gimoti from being marketed. Any patent-related legal action against us claiming damages or seeking to enjoin commercial activities relating to our product candidate or processes could subject us to potential liability for damages and could require us to obtain a license to continue to manufacture or market Gimoti, or, if no such license were available on commercially viable terms, could require us to cease manufacturing and marketing of Gimoti. We cannot predict whether we would prevail in any such actions or that any license required under any of these patents would be made available on commercially acceptable terms, if at all. In addition, we cannot be sure that we could redesign our product candidate or processes to avoid infringement, if necessary. Accordingly, an adverse determination in a judicial or administrative proceeding, or the failure to obtain necessary licenses, could prevent us from developing and commercializing Gimoti, which could harm our business, financial condition and operating results. Whatever the outcome, any patent litigation would be costly and time consuming, could be distracting to our management, and could have a material adverse effect on our business.
35
We may be subject to claims that we have wrongfully hired an employee from a competitor or that we or our employees have wrongfully used or disclosed alleged confidential information or trade secrets of their former employers.
As is commonplace in our industry, we employ and consult with individuals who were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject in the future to claims that our employees or consultants are subject to a continuing obligation to their former employers or clients (such as non-competition or non-solicitation obligations) or claims that our employees, our consultants or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers or clients. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Changes in patent laws or patent jurisprudence could diminish the value of patents in general, thereby impairing our ability to protect our products.
The patent positions of pharmaceutical and biotechnology companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. Changes in either the patent laws or in the interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property. We cannot predict the breadth of claims that may be allowed or found to be enforceable in our patents, in our strategic partners’ patents or in third-party patents. The United States has enacted and is currently implementing wide-ranging patent reform legislation. Further, recent U.S. Supreme Court rulings have either narrowed the scope of patent protection available in certain circumstances or weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the validity, scope and value of patents, once obtained.
For our U.S. patent applications containing a priority claim after March 16, 2013, there is a greater level of uncertainty in the patent law. In September 2011, the Leahy-Smith America Invents Act, also known as the America Invents Act, or AIA, was signed into law. The AIA includes a number of significant changes to U.S. patent law, including provisions that affect the way patent applications will be prosecuted and may also affect patent litigation.
The AIA and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have an adverse effect on our business. An important change introduced by the AIA is that, as of March 16, 2013, the United States transitioned to a “first-to-file” system for deciding which party should be granted a patent when two or more patent applications are filed by different parties disclosing or claiming the same invention. A third party that has filed, or does file a patent application in the USPTO after March 16, 2013 but before us, could be awarded a patent covering a given invention, even if we had made the invention before it was made by the third party. This requires us to be cognizant going forward of the time from invention to filing of a patent application.
Among some of the other changes introduced by the AIA are changes that limit where a patentee may file a patent infringement suit and providing opportunities for third parties to challenge any issued patent in the USPTO. This applies to all of our U.S. patents, even those issued before March 16, 2013. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in United States federal court necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action.
Depending on decisions by the U.S. Congress, the U.S. federal courts, the USPTO or similar authorities in foreign jurisdictions, the laws and regulations governing patents could change in unpredictable ways that may weaken our and our licensors’ ability to obtain new patents or to enforce existing patents we and our licensors or partners may obtain in the future.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our current or future products, if any, and our patents or other intellectual property rights may not be effective or
36
sufficient to prevent them from competing. Recent United States Supreme Court cases have narrowed the scope of what is considered patentable subject matter, for example, in the areas of software and diagnostic methods involving the association between treatment outcome and biomarkers. This could impact our ability to patent certain aspects of our technology in the United States.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license. Additionally, the requirements for patentability may differ in certain countries, particularly developing countries. For example, unlike other countries, China has a heightened requirement for patentability, and specifically requires a detailed description of medical uses of a claimed drug. In India, unlike the United States, there is no link between regulatory approval of a drug and its patent status. In addition to India, certain countries in Europe and developing countries, including China, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In those countries, we and our licensors may have limited remedies if patents are infringed or if we or our licensors are compelled to grant a license to a third party, which could materially diminish the value of those patents. This could limit our potential revenue opportunities. Accordingly, our efforts to enforce intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we own or license.
Risks Related to Our Financial Position and Need for Capital
Our recurring losses from operations have raised substantial doubt regarding our ability to continue as a going concern.
Our recurring losses from operations raise substantial doubt about our ability to continue as a going concern, and as a result, management concluded that there is substantial doubt about our ability to continue as a going concern. Our independent registered public accounting firm also included an explanatory paragraph in its report on our financial statements as of and for the year ended December 31, 2020 with respect to this uncertainty. This doubt about our ability to continue as a going concern could materially limit our ability to raise additional funds through the issuance of new debt or equity securities or otherwise. In addition, the perception that we may not be able to continue as a going concern may cause others to choose not to deal with us due to concerns about our ability to meet our contractual obligations. Future reports on our financial statements may also include an explanatory paragraph with respect to our ability to continue as a going concern. We have incurred significant losses since our inception and have never been profitable, and it is possible we will never achieve profitability. We have devoted our resources to developing Gimoti, which we launched in October 2020.
Our operations have consumed substantial amounts of cash since inception. We believe, based on our current operating plan, that our existing cash and cash equivalents as of December 31, 2020 of approximately $8.1 million, along with the approximately $13.1 million of net proceeds raised from a public offering in January 2021, will be sufficient to fund our operations into the first quarter of 2022, excluding any future Gimoti product revenue. This period could be shortened if there are any significant increases in planned spending other than anticipated. We anticipate that we will be required to raise additional funds in order to continue as a going concern. There is no assurance that other financing will be available on acceptable terms, or at all, when needed to allow us to continue as a going concern. There can be no assurance that we will be able to further develop Gimoti, if required. Because our business is entirely dependent on the success of Gimoti, if we are unable to secure additional financing, successfully commercialize Gimoti or identify and execute on strategic alternatives for Gimoti or our company, we will be required to curtail all of our activities and may be required to liquidate, dissolve or otherwise wind down our operations. Any of these events could result in a complete loss of your investment in our securities.
We have incurred significant operating losses since inception, and we expect to incur losses for the foreseeable future. We may never become profitable or, if achieved, be able to sustain profitability.
We have incurred significant operating losses since we were founded in 2007 and expect to incur significant losses for the next several years primarily related to funding commercialization activities for Gimoti, manufacturing commercial batches of Gimoti, and conducting the post-marketing commitment PK trial of Gimoti. Our net loss for the year ended December 31, 2020, was approximately $13.2 million. As of December 31, 2020, we had an accumulated deficit of approximately $98.9 million. Losses have resulted principally from costs incurred in our clinical trials, research and development programs and from our general and administrative expenses, especially since we became a public company in September 2013. In the future, we intend to continue the commercial activities for Gimoti, including manufacturing commercial batches, conduct the
37
post-marketing commitment PK trial and any additional development activities should we seek additional indications, maintain, expand and protect our intellectual property portfolio and continue to fund general and administrative expenses and costs of being a public company. These costs will likely result in our incurring further significant losses until net sales from Gimoti exceed such costs, if ever.
Our ability to generate revenue and become profitable depends on our ability to successfully commercialize Gimoti, which we launched in October 2020 through our commercial partner Eversana. If we or Eversana fail to successfully launch Gimoti and grow and maintain sales, we may never generate significant revenues and our results of operations and financial position will be adversely affected, which could impair our ability to sustain operations or obtain any required additional funding. If we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods.
If we fail to obtain the capital necessary to fund our operations, we will be unable to successfully commercialize Gimoti.
We may require additional capital in the future. The amount and timing of any expenditure needed to implement our development and commercialization programs will depend on numerous factors, including:
| • | the timing and costs related to commercialization activities for Gimoti by us and our commercial partner Eversana; |
| • | the timing and costs to manufacture commercial batches of Gimoti; |
| • | the market acceptance of Gimoti; |
| • | the costs to conduct the post-marketing commitment PK trial of Gimoti, including the timing and costs to manufacture product for such trial, and any additional development activities should we seek additional indications; |
| • | the outcome, costs and timing of seeking and obtaining regulatory approvals from FDA, and any similar regulatory agencies for any new indications; |
| • | our need and ability to hire additional management, development and scientific personnel, if necessary; |
| • | the cost to maintain, expand and defend the scope of our intellectual property portfolio, including the amount and timing of any payments we may be required to make, or that we may receive, in connection with licensing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights; |
| • | the extent to which we are required to pay milestone or other payments under our Mallinckrodt asset purchase agreement and the timing of such payments; |
| • | the costs of acquiring, licensing or investing in additional businesses, products, product candidates and technologies; |
| • | our need to implement additional internal systems and infrastructure, including financial and reporting systems: and |
| • | the costs necessary to fund general and administrative activities to support operations. |
Some of these factors are outside of our control. We cannot provide any assurance that our existing capital will be sufficient to enable us to fund the items noted and, in any event, we may need to raise additional capital to complete such activities.
We may seek additional funding through collaboration agreements, public or private equity financings, debt financings or receivables financings. For example, we currently may sell from time to time, at our option, up to an aggregate of $30.0 million of shares of our common stock through B. Riley FBR, Inc., or FBR, and H.C. Wainwright & Co., LLC, or HCW and together with FBR, the Sales Agents, pursuant to a sales agreement, or ATM Sales Agreement. Sales pursuant to the ATM Sales Agreement are registered pursuant to a shelf registration statement on Form S-3 which was declared effective by the SEC on January 6, 2021. There can be no assurance that the Sales Agents will be successful in consummating future sales based on prevailing market conditions or in the quantities or at the prices that we deem appropriate.
In addition, the Sales Agents are permitted to terminate the ATM Sales Agreement in their sole discretion upon ten days’ notice, or at any time in certain circumstances, including the occurrence of an event that would be reasonably likely to have a material adverse effect on our assets, business, operations, earnings, properties, condition (financial or otherwise), prospects, stockholders’ equity or results of operations.
Additional funding may not be available to us on acceptable terms or at all. In addition, the terms of any financing may adversely affect the holdings or the rights of our stockholders. The issuance of additional shares by us, or the possibility of such issuance, may cause the market price of our shares to decline and dilute the holdings of our existing stockholders. If we raise additional funds by incurring debt, the terms of the debt may involve significant cash payment obligations as well as covenants and specific financial ratios that may restrict our ability to operate our business.
If we are unable to obtain funding on a timely basis, if required, we will be unable to complete additional clinical development of Gimoti and may be required to significantly curtail all of our activities. We also could be required to seek
38
funds through arrangements with collaborative partners or otherwise that may require us to relinquish rights to our product candidate or some of our technologies or otherwise agree to terms unfavorable to us.
Our ability to use net operating loss and tax credit carryforwards and certain built-in losses to reduce future tax payments is limited by provisions of the Internal Revenue Code, and may be subject to further limitation as a result of the transactions completed in connection with our initial public offering.
Under Section 382 of the Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change” (generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period), the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes to offset its post-change income may be limited. As a result of our most recent private placement and other transactions that have occurred over the past three years, we may have experienced an “ownership change.” We may also experience ownership changes in the future as a result of subsequent shifts in our stock ownership. As of December 31, 2020, we had federal and state net operating loss carryforwards of approximately $81.3 million and $47.7 million, respectively, and federal and state research and development credits of approximately $2.4 million and $1.5 million, respectively, which could be limited if we experience an “ownership change.” Furthermore, under U.S. tax legislation enacted in December 2017, although the treatment of tax losses generated before December 31, 2017 has generally not changed, tax losses generated in calendar year 2018 and beyond do not expire, but may only offset 80% of our taxable income. This change may require us to pay federal income taxes in future years despite generating a loss for federal income tax purposes in prior years.
U.S. tax legislation may materially adversely affect our financial condition, results of operations and cash flows.
U.S. tax legislation enacted in December 2017 has significantly changed the U.S. federal income taxation of U.S. corporations, including by reducing the U.S. corporate income tax rate, limiting interest deductions, adopting elements of a territorial tax system, imposing a one-time transition tax on all undistributed earnings and profits of certain U.S.-owned foreign corporations, revising the rules governing net operating losses and the rules governing foreign tax credits, and introducing new anti-base erosion provisions. Many of these changes are effective immediately, without any transition periods or grandfathering for existing transactions. The legislation is unclear in many respects and could be subject to potential amendments and technical corrections, as well as interpretations and implementing regulations by the Treasury and Internal Revenue Service, any of which could lessen or increase certain adverse impacts of the legislation. In addition, it is unclear how these U.S. federal income tax changes will affect state and local taxation, which often uses federal taxable income as a starting point for computing state and local tax liabilities.
While some of the changes made by the tax legislation may adversely affect us in one or more reporting periods and prospectively, other changes may be beneficial on a going forward basis. We urge our investors to consult with their legal and tax advisors with respect to such legislation.
Risks Related to Ownership of Our Common Stock
An active trading market for our common stock may not be sustained.
An active trading market may not be sustained. If an active trading market is not sustained, it may be difficult to sell shares of our common stock at a price that is desirable or at all. In addition, an inactive market may impair our ability to raise capital by selling shares and may impair our ability to acquire other companies or technologies by using our shares as consideration, which, in turn, could materially adversely affect our business. Since the commencement of trading in connection with our initial public offering in September 2013 through February 28, 2021, the sale price per share of our common stock on the Nasdaq Capital Market has ranged from a low of $0.50 to a high of $14.25.
The price of the shares of our common stock could be highly volatile, and purchasers of our common stock could incur substantial losses.
Our stock price is likely to be volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. The stock market in general and the market for biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, investors may not be able to sell their common stock at or above the price at which they purchased the shares. The market price for our common stock may be influenced by many factors, including:
| ● | regulatory developments in the United States and foreign countries; |
| ● | the timing, progress and results of any additional trials we may conduct, and the results of trials of our competitors or those of other companies in our market sector; |
| ● | variations in our financial results or those of companies that are perceived to be similar to us; |
| ● | changes in the structure of healthcare payment systems, especially in light of current reforms to the U.S. healthcare system; |
39
| ● | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
| ● | business interruptions resulting from geopolitical actions, including war and terrorism, or natural disasters, such as earthquakes, typhoons, floods and fires, or public health emergencies or pandemics, such as the COVID-19 pandemic; |
| ● | market conditions in the pharmaceutical and biotechnology sectors and issuance of securities analysts’ reports or recommendations; |
| ● | sales of our stock by insiders and 5% stockholders; |
| ● | trading volume of our common stock; |
| ● | general economic, industry and market conditions other events or factors, many of which are beyond our control; |
| ● | additions or departures of key personnel; and |
| ● | intellectual property, product liability or other litigation against us. |
In addition, in the past, stockholders have initiated class action lawsuits against biotechnology and pharmaceutical companies following periods of volatility in the market prices of these companies’ stock. Such litigation, if instituted against us, could cause us to incur substantial costs and divert management’s attention and resources, which could have a material adverse effect on our business, financial condition and results of operations.
Our quarterly operating results may fluctuate significantly.
We expect our operating results to be subject to quarterly fluctuations. Our net loss and other operating results will be affected by numerous factors, including:
| ● | variations in the level of Gimoti sales; |
| ● | additional clinical trials and related manufacturing and regulatory costs; |
| ● | any intellectual property infringement lawsuit in which we may become involved; |
| ● | regulatory developments affecting Gimoti; and |
| ● | our execution of any collaborative, licensing or similar arrangements, and the timing of payments we may make or receive under these arrangements. |
If our quarterly operating results fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Furthermore, any quarterly fluctuations in our operating results may, in turn, cause the price of our stock to fluctuate substantially.
Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our amended and restated certificate of incorporation and amended and restated bylaws may delay or prevent an acquisition of us or a change in our management. These provisions include:
| ● | authorizing the issuance of “blank check” preferred stock, the terms of which may be established and shares of which may be issued without stockholder approval; |
| ● | limiting the removal of directors by the stockholders; |
| ● | creating a staggered board of directors; |
| ● | prohibiting stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of our stockholders; |
| ● | eliminating the ability of stockholders to call a special meeting of stockholders; |
| ● | permitting our board of directors to accelerate the vesting of outstanding option grants upon certain transactions that result in a change of control; and |
| ● | establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings. |
40
In addition, because we are incorporated under the laws of the state of Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which limits the ability of stockholders owning in excess of 15% of our outstanding voting stock to merge or combine with us. Although we believe these provisions collectively provide for an opportunity to obtain greater value for stockholders by requiring potential acquirors to negotiate with our board of directors, they would apply even if an offer rejected by our board were considered beneficial by some stockholders. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management.
We do not intend to pay dividends on our common stock and, consequently, the ability of our stockholders to achieve a return on their investment will depend on appreciation in the price of our common stock.
We have never declared or paid any cash dividend on our common stock and do not currently intend to do so for the foreseeable future. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business. In addition, any future debt financing arrangement may contain terms prohibiting or limiting the amount of dividends that may be declared or paid on our common stock. Any return to stockholders will therefore be limited to the appreciation of their stock. Therefore, the success of an investment in shares of our common stock will depend upon any future appreciation in their value. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which our stockholders have purchased their shares.
Sales of a substantial number of shares of our common stock by our existing stockholders in the public market could cause our stock price to fall.
Persons who were our stockholders prior to the sale of shares in our initial public offering in September 2013 continue to hold a substantial number of shares of our common stock that they are able to sell in the public market, subject in some cases to certain legal restrictions. Significant portions of these shares are held by a small number of stockholders. Sales of a substantial number of shares of our common stock in the public market, or the perception that these sales might occur, could significantly reduce the market price of our common stock and impair our ability to raise adequate capital through the sale of additional equity securities.
As of February 28, 2021, we had 32,371,954 shares of common stock outstanding. All of these shares are freely tradable without restriction in the public market, except for 988,510 shares that are held by directors and executive officers that are subject to volume limitations under Rule 144 under the Securities Act. In addition, shares of common stock that are either subject to outstanding options or reserved for future issuance under our employee benefit plans will become eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules and Rule 144 and Rule 701 under the Securities Act. If these additional shares of common stock are sold, or if it is perceived that they will be sold, in the public market, the trading price of our common stock could decline.
We will continue to incur significant costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
As a public company, we have incurred and will continue to incur significant legal, accounting and other expenses under the Sarbanes-Oxley Act and the Dodd-Frank Wall Street Reform and Consumer Protection Act, as well as rules adopted by the SEC and the Nasdaq Stock Market. These rules impose significant requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls, changes in corporate governance practices, proxy access and “say on pay” votes. Stockholder activism, the current political environment and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business in ways we cannot currently anticipate.
The rules and regulations applicable to public companies have substantially increased our legal and financial compliance costs and made some activities more time-consuming and costly. If these requirements divert the attention of our management and personnel from other business concerns, they could have a material adverse effect on our business, financial condition and results of operations. The increased costs will decrease our net income or increase our net loss, and may require us to reduce costs in other areas of our business or increase the prices of our products or services. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to incur substantial costs to maintain the same or similar coverage. We cannot predict or estimate the amount or timing of additional costs we may incur to respond to these requirements. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers.
41
If securities or industry analysts publish unfavorable research or reports about our business, our stock price and trading volume could decline.
The trading market for our common stock depends in part on the research and reports that securities or industry analysts publish about us, our business, our market or our competitors. We currently have limited research coverage by securities and industry analysts. If one or more of the analysts who covers us downgrades our stock, our stock price would likely decline. If one or more of these analysts ceases to cover us or fails to regularly publish reports on us, interest in our stock could decrease, which could cause our stock price or trading volume to decline.
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because pharmaceutical companies have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
If we fail to meet all applicable Nasdaq Capital Market requirements and Nasdaq determines to delist our common stock, the delisting could adversely affect the market liquidity of our common stock and the market price of our common stock could decrease.
Our common stock is listed on the Nasdaq Capital Market. In order to maintain our listing, we must meet minimum financial and other requirements, including requirements for a minimum amount of capital, a minimum closing bid price per share of $1.00 and continued business operations so that we are not characterized as a “public shell company.”
In the event that our common stock is delisted from the Nasdaq Capital Market and is not eligible for quotation or listing on another market or exchange, trading of our common stock could be conducted only in the over-the-counter market or on an electronic bulletin board established for unlisted securities such as the Pink Sheets or the OTC Bulletin Board. In such event, it could become more difficult to dispose of, or obtain accurate price quotations for, our common stock, and there would likely also be a reduction in our coverage by securities analysts and the news media, which could cause the price of our common stock to decline further. Also, it may be difficult for us to raise additional capital if we are not listed on a major exchange.
Item 1B. Unresolved Staff Comments
Not applicable.
We occupy approximately 3,000 square feet of office space in Solana Beach, California under a lease that we entered into in December 2016. The lease was amended in September 2018, December 2019 and December 2020 to extend the expiration date through January 2022. We believe that our facility is adequate to meet our needs and that, if necessary, additional space can be leased to accommodate any future growth on commercially reasonable terms.
We are not currently a party to any material legal proceedings.
Item 4. Mine Safety Disclosures
Not applicable.
42
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our common stock is traded on the Nasdaq Capital Market under the symbol “EVOK.”
Holders of Common Stock
As of February 28, 2021, there were 9 holders of record of our common stock.
Dividend Policy
We have never declared or paid any cash dividends on our capital stock and do not anticipate paying any cash dividends in the foreseeable future. We expect to retain available cash to finance ongoing operations and the potential growth of our business. Any future determination to pay dividends on our common stock will be at the discretion of our board of directors and will depend upon, among other factors, our results of operations, financial condition, capital requirements, contractual restrictions, business prospects and other factors our board of directors may deem relevant.
Unregistered Sales of Equity Securities
None.
Issuer Repurchases of Equity Securities
None.
Securities Authorized for Issuance Under Equity Compensation Plans
Information about our equity compensation plans is incorporated herein by reference to Item 12 of Part III of this Annual Report on Form 10-K.
Item 6. Selected Financial Data
Not required.
43
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our financial statements and the accompanying notes and other financial information included elsewhere in this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis, or set forth elsewhere in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of certain factors, including, but not limited to, those set forth under “Risk Factors” under Item 1A of Part I of this Annual Report on Form 10-K and elsewhere in this Annual Report on Form 10-K.
Overview
We are a specialty pharmaceutical company focused primarily on the development and commercialization of drugs to treat GI disorders and diseases. Since our inception, we have devoted our efforts to developing our sole product, Gimoti (metoclopramide) nasal spray, the first and only nasally-administered product indicated for the relief of symptoms in adults with acute and recurrent diabetic gastroparesis. On June 19, 2020, we received approval from FDA for our NDA for Gimoti. We launched commercial sales of Gimoti in the United States in October 2020 through our commercial partner Eversana.
Diabetic gastroparesis is a GI disorder affecting millions of patients worldwide, in which food in an individual’s stomach takes too long to empty resulting in a variety of serious GI symptoms and system metabolic complications. The gastric delay caused by gastroparesis can compromise absorption of orally administered medications.
On January 21, 2020, we entered into the Eversana Agreement for the commercialization of Gimoti. Pursuant to the Eversana Agreement, Eversana commercializes and distributes Gimoti in the United States. Eversana also manages the marketing of Gimoti to targeted health care providers, as well as the sales and distribution of Gimoti in the United States. Eversana also provided a $5 million revolving credit facility that became available upon FDA approval of the Gimoti NDA. In June 2020 we borrowed $2 million and in December 2020 we borrowed the remaining $3 million under the Eversana Credit Facility.
We have primarily funded our operations through the sale of our convertible preferred stock prior to our initial public offering in September 2013, borrowings under our bank loans and the sale of shares of our common stock on the Nasdaq Capital Market. We launched commercial sales of Gimoti in late October 2020 with Eversana and, to date, have generated modest sales given the launch occurred during the COVID-19 pandemic and we were entering the holiday season.
We have incurred losses in each year since our inception. These operating losses resulted from expenses incurred in connection with advancing Gimoti through development activities and general and administrative costs associated with our operations. We expect to continue to incur operating losses until revenues from sales of Gimoti exceed our expenses, if ever. We may never become profitable, or if we do, we may not be able to sustain profitability on a recurring basis.
As of December 31, 2020, we had cash and cash equivalents of approximately $8.1 million, which excludes our receipt of approximately $13.1 million in net proceeds raised from our public offering of our common stock in January 2021. Current cash on hand is intended to fund commercialization activities for Gimoti, manufacture commercial batches of Gimoti, conduct the post-marketing commitment PK trial of Gimoti and any additional development activities should we seek additional indications, protect our intellectual property portfolio and for general and administrative costs to support operations. Our operations have consumed substantial amounts of cash since inception. We believe, based on our current operating plan, that our existing cash and cash equivalents as of December 31, 2020, along with the net proceeds raised from our public offering in January 2021, will be sufficient to fund our operations into the first quarter of 2022, excluding future Gimoti revenue. This period could be shortened if there are any significant increases in planned spending other than anticipated. We anticipate that we will be required to raise additional funds in order to continue as a going concern. Because our business is entirely dependent on the success of Gimoti, if we are unable to secure additional financing or identify and execute on other development or strategic alternatives for Gimoti or our company, we will be required to curtail all of our activities and may be required to liquidate, dissolve or otherwise wind down our operations. Any of these events could result in a complete loss of your investment in our securities.
Impact of COVID-19
Despite the COVID-19 pandemic, we began our commercial sales of Gimoti with Eversana in October 2020. We have experienced various disruptions to our sales activities, but have continued our efforts to reach physicians and customers. For example, Eversana’s commercialization efforts have been adversely affected by operational restrictions imposed on its sales force from quarantines, travel restrictions and bans, and other governmental restrictions related to COVID-19. As a result of these restrictions, their sales force has been restricted from conducting in-person interactions with certain physicians and customers and has been restricted to conducting educational and promotional activities for Gimoti virtually in certain circumstances, which has impacted Eversana’s ability to more actively market Gimoti. Research conducted by IOVIA stated that as a result of COVID-19, fewer patients are visiting physician offices resulting in lower patient volumes than normal,
44
and the Centers for Disease Control and Prevention reported during 2020 that over 40% of patients were avoiding care due to COVID-19. We anticipate that we and Eversana will continue to be impacted by the COVID-19 pandemic.
The COVID-19 pandemic has not significantly disrupted the operations of our third-party suppliers and manufacturers or delayed our manufacturing timelines of Gimoti, but may negatively impact our ability to successfully commercialize Gimoti and generate product sales in the future. Further, the COVID-19 pandemic and mitigation measures have also had an adverse impact on global economic conditions which could have an adverse effect on our future business and financial condition, including impairing our ability to raise capital when needed.
In March 2020, the Coronavirus Aid, Relief, and Economic Security, or CARES, Act was enacted in response to the COVID-19 pandemic. In April 2020, we applied for and were approved for a Small Business Administration, or SBA, loan under the Paycheck Protection Program, or PPP, established by the CARES Act. On May 1, 2020, we received the loan proceeds of approximately $104,000. In January 2021, we received notice that our loan and accrued interest were forgiven by the SBA.
Technology Acquisition Agreement
In June 2007, we acquired all worldwide rights, data, patents and other related assets associated with Gimoti from Questcor Pharmaceuticals, Inc., or Questcor, pursuant to an asset purchase agreement. We paid Questcor $650,000 in the form of an upfront payment and $500,000 in May 2014 as a milestone payment based upon the initiation of the first patient dosing in our Phase 3 clinical trial for Gimoti. In August 2014, Mallinckrodt, plc, or Mallinckrodt, acquired Questcor. As a result of that acquisition, Questcor transferred its rights included in the asset purchase agreement with us to Mallinckrodt. In addition to the payments previously made to Questcor, we may be required to make additional milestone payments totaling up to $52 million. In March 2018, we amended the asset purchase agreement with Mallinckrodt to defer development and approval milestone payments, such that rather than paying two milestone payments based on FDA acceptance for review of the NDA and final product marketing approval, we would be required to make a single $5 million payment on the one-year anniversary after we receive FDA approval to market Gimoti. At the time of the Gimoti NDA approval by FDA, we recorded the $5 million payable owed to Mallinckrodt with a due date of June 19, 2021, along with a $5 million research and development expense.
The remaining $47 million in milestone payments depend on Gimoti’s commercial success. We will be required to pay to Mallinckrodt a low single digit royalty on net sales of Gimoti. Our obligation to pay such royalties will terminate upon the expiration of the last patent right covering Gimoti, which is expected to occur in 2030, subject to possible extension should any additional, later expiring, licensed patents be granted.
Financial Operations Overview
Revenue Recognition
Our ability to generate revenue and become profitable depends on our ability to successfully commercialize Gimoti, which we launched in the United States through prescription in October 2020 through our commercial partner Eversana. If we or Eversana fail to successfully launch Gimoti and grow and maintain sales, we may never generate significant revenues and our results of operations and financial position will be adversely affected.
In accordance with Accounting Standards Codification, or ASC 606, Revenue from Contracts with Customers, we recognize revenue when a customer obtains control of promised goods in an amount that reflects the consideration we expect to receive in exchange for the goods provided. Customer control is determined upon the customer’s physical receipt of the product. To determine revenue recognition for arrangements within the scope of ASC 606, we perform the following five steps: identify the contracts with the customer; identify the performance obligations in the contract; determine the transaction price; allocate the transaction price to the performance obligations in the contract; and recognize revenue when (or as) it satisfies a performance obligation. At contract inception, we assess the goods promised within each contract and determine those that are performance obligations and assess whether each promised good is distinct. We then recognize as revenue the amount of the transaction price that is allocated to the respective performance obligation when the customer obtains control of the product.
Product sales are recorded at the transaction price, which includes variable considerations for co-payment assistance to commercially insured patients meeting certain eligibility requirements, as well as to uninsured patients. Co-payment assistance is recorded as an offset to gross revenue at the time revenue from the product sale is recognized based on expected and actual program participation.
Co-pay liabilities are estimated using prescribing data available from customers. Actual amounts of consideration ultimately received may differ from our estimates. If actual results in the future vary from estimates, we will adjust these estimates, which would affect net product revenue and earnings in the period such variances become known.
Liabilities for co-pay assistance are classified as accounts payable and accrued expenses in the balance sheets.
45
Research and Development Expenses
We expense all research and development expenses as they are incurred. Research and development expenses primarily include:
| ● | clinical and regulatory-related costs; |
| ● | expenses incurred under agreements with contract research organizations, or CROs; |
| ● | manufacturing and stability testing costs and related supplies and materials; and |
| ● | employee-related expenses, including salaries, benefits, travel and stock-based compensation expense. |
All of our research and development expenses to date have been incurred in connection with the development of Gimoti. With FDA approval of Gimoti, we expect research and development costs to decrease and shift to commercialization and selling costs. However, we have initiated planning for an FDA post-marketing commitment PK trial of Gimoti. This trial will be designed to characterize dose proportionality of a lower dosage strength of Gimoti to accommodate patients that may require further dosage adjustments. We are unable to estimate with any certainty the costs we will incur related to this trial, or the regulatory review of such lower dosage of Gimoti, though such costs may be significant. Clinical development timelines, the probability of success and development costs can differ materially from expectations.
The costs of clinical trials may vary significantly over the life of a project owing to, but not limited to, the following:
| ● | per subject trial costs; |
| ● | the number of sites included in the trials; |
| ● | the length of time required to enroll eligible subjects; |
| ● | the number of subjects that participate in the trials; |
| ● | the number of doses that subjects receive; |
| ● | the cost of comparative agents used in trials; |
| ● | the drop-out or discontinuation rates of subjects; |
| ● | potential additional safety monitoring or other studies requested by regulatory agencies; and |
| ● | the duration of patient follow-up. |
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related benefits, including stock-based compensation. Other general and administrative expenses include professional fees for accounting, tax, patent costs, legal services, insurance, facility costs and costs associated with being a publicly-traded company, including fees associated with investor relations and directors and officers liability insurance premiums. We expect that general and administrative expenses will increase in the future as we continue to progress with the commercialization of Gimoti and we reimburse Eversana from the net profits attained from the sales of Gimoti.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which we have prepared in accordance with generally accepted accounting principles in the United States, or GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported expenses during the reporting periods. We evaluate these estimates and judgments on an ongoing basis. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Our actual results may differ materially from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Note 2 to our financial statements appearing elsewhere in this Annual Report on Form 10-K, we believe that the following accounting policies are the most critical for fully understanding and evaluating our financial condition and results of operations.
Stock-Based Compensation
Stock-based compensation expense for stock option grants and employee stock purchases under our Employee Stock Purchase Plan, or ESPP, is recorded at the estimated fair value of the award as of the grant date and is recognized as expense
46
on a straight-line basis over the employee’s requisite service period, except awards with a performance condition. Awards with a performance condition commence vesting when the satisfaction of the performance condition is probable. The estimation of stock option and ESPP fair value requires management to make estimates and judgments about, among other things, employee exercise behavior, forfeiture rates and volatility of our common stock. The judgments directly affect the amount of compensation expense that will be recognized.
We grant stock options to purchase common stock to employees and members of the board of directors with exercise prices equal to our closing market price on the date the stock options are granted. The risk-free interest rate assumption was based on the yield of an applicable rate for U.S. Treasury instruments with maturities similar to those of the expected term of the award being valued. The weighted-average expected term of options and employee stock purchases was calculated using the simplified method as prescribed by accounting guidance for stock-based compensation. This decision was based on the lack of relevant historical data due to our limited historical experience. In addition, due to our limited historical data, the estimated volatility was calculated based upon our historical volatility and, if necessary, supplemented with historical volatility of comparable companies in the biotechnology industry whose share prices are publicly available for a sufficient period of time. The assumed dividend yield was based on our history of never paying cash dividends and having no expectation of paying cash dividends in the foreseeable future. We account for forfeitures as the forfeitures occur.
We granted options to purchase 1,172,000 and 829,500 shares of common stock in 2020 and 2019, respectively. In addition, in June 2019, we effected a one-time option exchange, wherein employees were offered the opportunity to exchange certain outstanding stock options for the grant of a lesser number of replacement stock options. The participants received three new stock options for every four stock options tendered for exchange. As a result, 2,456,999 stock options were exchanged for 1,842,746 replacement options.
Other Information
Net Operating Loss Carryforwards
As of December 31, 2020, we had federal and California tax net operating loss carryforwards of approximately $81.3 million and $47.7 million, respectively. The federal and California net operating loss carryforwards will begin to expire in 2027 and 2028, respectively, unless previously utilized. The portion of federal net operating losses created after 2017 of approximately $19.5 million do not expire and will carry forward indefinitely. As of December 31, 2020, we also had federal and California research and development tax credit carryforwards of $2.4 million and $1.5 million, respectively. The federal research and development tax credit carryforwards will begin to expire in 2027 unless previously utilized. The California research and development tax credit will carry forward indefinitely. Furthermore, under the U.S. tax legislation enacted in December 2017, although the treatment of tax losses generated before December 31, 2017 has generally not changed, tax losses generated in calendar year 2018 and beyond do not expire, but may only offset 80% of our taxable income. This change may require us to pay federal income taxes in future years despite generating a loss for federal income tax purposes in prior years.
Under Section 382 of the Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change” (generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period), the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes to offset its post-change income may be limited. We have not completed our analysis to determine what, if any, impact any prior ownership change has had on our ability to utilize our net operating loss carryforwards.
Results of Operations
Comparison of Years Ended December 31, 2020 and 2019
The following table summarizes the results of our operations for the fiscal years ended December 31, 2020 and 2019:
|
| Year Ended December 31, |
|
| Increase/ |
| ||||||
|
| 2020 |
|
| 2019 |
|
| (Decrease) |
| |||
Research and development expense |
| $ | 6,554,825 |
|
| $ | 3,416,466 |
|
| $ | 3,138,359 |
|
Selling general and administrative expense |
| $ | 6,428,832 |
|
| $ | 3,737,987 |
|
| $ | 2,690,845 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and Development Expenses. Research and development expenses for the year ended December 31, 2020 compared to the year ended December 31, 2019 increased by approximately $3.1 million. The increase during the year ended December 31, 2020 is primarily due to recording a $5 million expense in June 2020 upon achieving a technology acquisition milestone related to FDA’s approval of Gimoti. Although the expense was recorded when incurred, the payment is not due to Mallinckrodt until June 19, 2021. During the year ended December 31, 2020, we also incurred expenses responding to requests for additional information from FDA related to the NDA and preparing for future manufacturing and the commercial launch of Gimoti. Excluding the Mallinckrodt milestone expense, research and development expenses decreased during 2020 as we have shifted our focus to commercialization and selling activities. Costs incurred in 2020 included approximately $832,000 for wages, taxes and employee insurance, including approximately $317,000 of stock-based compensation expense,
47
and approximately $625,000 related to acquiring raw material prior to obtaining FDA approval of Gimoti and to continued testing of Gimoti batches that were manufactured prior to FDA approval.
In 2019, we incurred expenses primarily related to responding to requests for additional information from FDA and manufacturing registration batches of Gimoti as required by FDA. Costs incurred in 2019 included approximately $2.1 million for wages, taxes and employee insurance, including approximately $710,000 of stock-based compensation expense, approximately $945,000 related to manufacturing, and approximately $286,000 related to responding to FDA information requests regarding the NDA and preparing for the NDA resubmission.
Selling, General and Administrative Expenses. Selling, general and administrative expenses for the year ended December 31, 2020 compared to the year ended December 31, 2019 increased by approximately $2.7 million. Costs incurred in 2020 primarily included approximately $3.5 million for wages, taxes and employee insurance, including approximately $1.3 million of stock-based compensation expense, approximately $2.4 million for legal, accounting, directors and officers liability insurance and other costs associated with being a public company, approximately $296,000 related to pre‑commercialization and commercialization activities and $173,000 for facility-related expenses. Of the total selling, general and administrative expenses incurred during the year ended December 31, 2020, approximately $2.0 million related to wages, taxes, employee insurance, stock-based compensation, pre-commercialization and commercialization activities were selling-based expenses. Costs incurred in 2019 primarily included approximately $1.7 million for wages, taxes and employee insurance, including approximately $664,000 of stock-based compensation expense, approximately $1.6 million for legal, accounting, directors and officers liability insurance and other costs associated with being a public company, approximately $165,000 for facility-related expenses, approximately $132,000 for outside consultants, and approximately $68,000 for pre‑commercialization costs.
Liquidity and Capital Resources
Since our inception in 2007, we have funded our operations primarily from the sale of equity securities and borrowings under loan and security agreements. Prior to our IPO, we received $17.7 million in net proceeds from the sale of our Series A convertible preferred stock and advances of $5.5 million under the loan and security agreements. During 2013, we completed our IPO and raised approximately $25.1 million, net of offering costs and commissions.
In November 2017, we filed a shelf registration statement with the SEC on Form S-3. The shelf registration statement included a prospectus for an at-the-market offering to sell up to an aggregate of $16.0 million of shares of our common stock through FBR as a sales agent, pursuant to a sales agreement with FBR, or the FBR Sales Agreement. During the year ended December 31, 2020, we sold 1,490,813 shares of common stock at a weighted-average price per share of $2.46 pursuant to the FBR Sales Agreement and received proceeds of approximately $3.6 million, net of commissions and fees. During the year ended December 31, 2019, we sold 7,004,381 shares of common stock at a weighted-average price per share of $0.89 pursuant to the FBR Sales Agreement and received proceeds of approximately $6.1 million, net of commission and fees. Effective January 6, 2021, we terminated the FBR Sales Agreement.
Under current SEC regulations, if at the time we file our Annual Report on Form 10-K our public float is less than $75 million, and for so long as our public float remains less than $75 million, the amount we can raise through primary public offerings of securities in any twelve-month period using shelf registration statements is limited to an aggregate of one-third of our public float, which is referred to as the baby shelf rules. As of the date we filed this Annual Report on Form 10-K, our public float exceeded $75 million, thereby allowing us to conduct primary offerings without being constrained by the baby shelf rules. We will remain unconstrained by the baby shelf rules under our Form S-3 shelf registration statement until the date we file a new registration statement or our Form 10-K for the fiscal year ending December 31, 2021, at which time if our public float is less than $75 million, the number of securities we may sell under a Form S-3 registration statement will again be limited by the baby shelf rules.
In December 2020, we filed a new shelf registration statement with the SEC on Form S-3, or the replacement shelf registration statement. The replacement shelf registration statement replaced the registration statement on Form S-3 we originally filed with the SEC in November 2017, which registration statement expired in December 2020. The replacement shelf registration was declared effective by the SEC on January 6, 2021. In December 2020, we also entered into the ATM Sales Agreement with FBR and H.C. Wainwright & Co., LLC pursuant to which we may sell from time to time, at our option, up to an aggregate of $30 million worth of shares of our common stock through the Sales Agents. The ATM Sales Agreement provides, among other things, that sales under the ATM Sales Agreement will be made pursuant to the registration statement, including the base prospectus filed as part of such registration statement.
Future sales under the ATM Sales Agreement will depend on a variety of factors including, but not limited to, market conditions, the trading price of our common stock and our capital needs. There can be no assurance that the Sales Agents will be successful in consummating future sales based on prevailing market conditions or in the quantities or at the prices that we deem appropriate.
48
In addition, we will not be able to make future sales of common stock pursuant to the ATM Sales Agreement unless certain conditions are met, which include the accuracy of representations and warranties made to the Sales Agents under the ATM Sales Agreement. Furthermore, each of the Sales Agents is permitted to terminate the ATM Sales Agreement with respect to itself in its sole discretion upon ten days’ notice, or at any time in certain circumstances, including the occurrence of an event that would be reasonably likely to have a material adverse effect on our assets, business, operations, earnings, properties, condition (financial or otherwise), prospects, stockholders’ equity or results of operations. We have no obligation to sell the shares available for sale pursuant to the ATM Sales Agreement.
In connection with the Eversana Agreement, we entered into the Eversana Credit Facility, pursuant to which Eversana agreed to provide a revolving credit facility of up to $5 million to us upon FDA approval of the Gimoti NDA, as well as certain other customary conditions. The Eversana Credit Facility terminates on June 19, 2025, unless terminated earlier pursuant to its terms. The Eversana Credit Facility is secured by all of the Company’s personal property other than its intellectual property. Under the terms of the Eversana Credit Facility, we cannot grant an interest in our intellectual property to any other person. Each loan under the Eversana Credit Facility will bear interest at an annual rate equal to 10.0%, with such interest due at the end of the loan term. In June 2020 we borrowed $2 million and in December 2020 we borrowed $3 million from the Eversana Credit Facility.
In January 2021, we completed the sale of 5,750,000 shares of our common stock in an underwritten public offering led by Laidlaw & Company (UK) Ltd. The price to the public in this offering was $2.50 per share resulting in gross proceeds to us of approximately $14.4 million. After deducting underwriting discounts and commissions, and offering expenses paid by us, the net proceeds to us raised from this offering were approximately $13.1 million.
Management concluded that there is substantial doubt about our ability to continue as a going concern. Our independent registered public accounting firm also included an explanatory paragraph in their report on our financial statements as of and for the year ended December 31, 2020 with respect to our ability to continue as a going concern. This doubt about our ability to continue as a going concern for at least twelve months from the date of the financial statements could materially limit our ability to raise additional funds through the issuance of new debt or equity securities or otherwise. Future reports on our financial statements may also include an explanatory paragraph with respect to our ability to continue as a going concern. We have incurred significant losses since our inception and have never been profitable, and it is possible we will never achieve profitability. We believe, based on our current operating plan, that our existing cash and cash equivalents will be sufficient to fund our operations into the first quarter of 2022, excluding any future Gimoti revenue. This period could be shortened if there are any significant increases in planned spending other than anticipated. Even with the Eversana Credit Facility, we will be required to raise additional funds in order to continue as a going concern. Because our business is entirely dependent on the success of Gimoti, if we are unable to secure additional financing or identify and execute on other development or strategic alternatives for Gimoti or our company, we will be required to curtail all of our activities and may be required to liquidate, dissolve or otherwise wind down our operations. Any of these events could result in a complete loss of your investment in our securities.
These estimates of cash runway could be shortened if there are any significant increases in planned spending on commercialization activities, including for marketing and manufacturing of Gimoti, and our general and administrative costs to support operations. There is no assurance that other financing will be available when needed to allow us to continue as a going concern. The perception that we may not be able to continue as a going concern may cause others to choose not to deal with us due to concerns about our ability to meet our contractual obligations.
We expect to continue to incur expenses as we:
| ● | continue the commercial activities for Gimoti; |
| ● | manufacture commercial batches of Gimoti; |
| ● | conduct the post-marketing commitment PK trial of Gimoti and any additional development activities should we seek additional indications; |
| ● | maintain, expand and protect our intellectual property portfolio; and |
| ● | continue to fund the accounting, legal, insurance and other costs associated with being a public company. |
49
|
The following table summarizes our cash flows for the years ended December 31, 2020 and 2019:
|
| Year Ended December 31, |
| |||||
|
| 2020 |
|
| 2019 |
| ||
Net cash used in operating activities |
| $ | (6,630,007 | ) |
| $ | (5,762,093 | ) |
Net cash provided by financing activities |
| $ | 9,035,113 |
|
| $ | 6,106,922 |
|
Net increase in cash and cash equivalents |
| $ | 2,405,106 |
|
| $ | 344,829 |
|
|
|
|
|
|
|
|
|
|
Operating Activities. The primary use of our cash has been to fund our clinical research, prepare our NDA, manufacture Gimoti, and other general operations. The cash used in operating activities during the year ended December 31, 2020 was primarily related to ongoing communication with FDA related to the resubmitted NDA, pre-approval and commercialization activities and other ongoing costs of operating the business. The cash used in operating activities during the year ended December 31, 2019 was primarily related to ongoing communication with FDA related to the NDA and to manufacturing registration batches of Gimoti. We expect that cash used in operating activities will increase due to commercialization activities, including manufacturing of Gimoti.
Financing Activities. During the year ended December 31, 2020, we received $5 million from borrowings under the Eversana Credit Facility, net proceeds of approximately $3.6 million from the sale of 1,490,813 shares of common stock pursuant to the FBR Sales Agreement, approximately $216,000 from the exercise of stock options to purchase 199,111 shares of common stock, approximately $119,000 from the sale of 118,491 shares of common stock pursuant to our ESPP and approximately $104,000 from the PPP loan.
During the year ended December 31, 2019, we received net proceeds of approximately $6.1 million from the sale of 7,004,381 shares of common stock pursuant to the FBR Sales Agreement. We did not sell any shares through our ESPP during 2019.
The amount and timing of our future funding requirements will depend on many factors, including but not limited to:
| ● | the costs of commercialization activities, including costs associated with commercial manufacturing; |
| ● | the commercial success of Gimoti, including competition with well-established products approved earlier by FDA, including oral and intravenous forms of metoclopramide, the same active ingredient in the nasal spray for Gimoti; |
| ● | the impact of the COVID-19 pandemic on us or on third parties on whom we rely; |
| ● | our ability to manufacture sufficient quantities of Gimoti to meet demand, including whether our contract manufacturers, suppliers, and/or consultants are able to meet appropriate timelines; |
| ● | our ability to obtain, maintain and enforce our patents and other intellectual property rights, and the costs incurred to do so; |
| ● | the terms and timing of any collaborative, licensing, co-promotion or other arrangements that we may establish; and |
Off-Balance Sheet Arrangements
Through December 31, 2020, we have not entered into and did not have any relationships with unconsolidated entities or financial collaborations, such as entities often referred to as structured finance or special purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purpose.
Contractual Obligations and Commitments
In December 2016, we entered into an operating lease for office space in Solana Beach, California. The lease commenced on January 1, 2017, was extended in September 2018, December 2019 and December 2020, and has an amended expiration date of January 31, 2022. We also pay pass through costs and utility costs, which are expensed as incurred.
As of December 31, 2020, future minimum lease payments for our facility lease are approximately $149,000.
In accordance with the technology acquisition with Mallinckrodt, we may be required to make milestone payments totaling up to $52 million. The first $5 million is required on June 19, 2021, the one-year anniversary of our receipt of approval from
50
FDA to market Gimoti. The remaining $47 million in milestone payments depend on Gimoti’s commercial success. We will be required to pay a low single digit royalty on net sales of Gimoti. Our obligation to pay such royalties will terminate upon the expiration of the last patent right covering Gimoti, which is expected to occur in 2030, subject to possible extension should any additional, later expiring, licensed patents be granted.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
As a smaller reporting company, we are not required to provide the information required by this Item.
Item 8. Financial Statements and Supplementary Data
Our financial statements and the report of our independent registered public accounting firm are included in this report on the pages indicated in Item 15 of Part IV of this Annual Report on Form 10-K.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Conclusions Regarding the Effectiveness of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported within the timelines specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Business Officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can only provide reasonable assurance of achieving the desired control objectives, and in reaching a reasonable level of assurance, management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures. In addition, the design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, control may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
As required by SEC Rule 13a-15(b), as of December 31, 2020 we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Business Officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as of the end of the period covered by this report. Based on the foregoing, our Chief Executive Officer and Chief Business Officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level as of December 31, 2020.
Management’s Report on Internal Control Over Financial Reporting
Internal control over financial reporting refers to the process designed by, or under the supervision of, our Chief Executive Officer and Chief Business Officer, and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles, and includes those policies and procedures that: (1) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
Internal control over financial reporting cannot provide absolute assurance of achieving financial reporting objectives because of its inherent limitations. Internal control over financial reporting is a process that involves human diligence and compliance and is subject to lapses in judgment and breakdowns resulting from human failures. Internal control over financial reporting also can be circumvented by collusion or improper management override. Because of such limitations, there is a risk that material misstatements may not be prevented or detected on a timely basis by internal control over financial reporting. However, these inherent limitations are known features of the financial reporting process. Therefore, it is possible to design into the process safeguards to reduce, though not eliminate, this risk.
Management is responsible for establishing and maintaining adequate internal control over our financial reporting, as such term is defined in Rule 13a-15(f) under the Exchange Act. Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Business Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting. Management has used the framework set forth in the report
51
entitled “Internal Control — Integrated Framework (2013 Framework)” published by the Committee of Sponsoring Organizations of the Treadway Commission to evaluate the effectiveness of our internal control over financial reporting. Based on its evaluation, management has concluded that our internal control over financial reporting was effective as of December 31, 2020, the end of our most recent fiscal year.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting identified in management’s evaluation pursuant to Rules 13a-15(d) or 15d-15(d) of the Exchange Act during the quarter ended December 31, 2020 that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting, other than controls implemented over revenue recognition as a result of the commercial launch of Gimoti.
None.
52
Item 10. Directors, Executive Officers and Corporate Governance
Information required by this item will be contained in our definitive proxy statement to be filed with the Securities and Exchange Commission in connection with our 2021 Annual Meeting of Stockholders, or the Definitive Proxy Statement, and which is expected to be filed not later than 120 days after the end of our fiscal year ended December 31, 2020, under the headings “Election of Directors,” “Corporate Governance and Other Matters,” and “Executive Officers,” and is incorporated herein by reference.
We have adopted a Code of Business Conduct and Ethics that applies to our officers, directors and employees which is available on our internet website at www.evokepharma.com. The Code of Business Conduct and Ethics contains general guidelines for conducting the business of our company consistent with the highest standards of business ethics, and is intended to qualify as a “code of ethics” within the meaning of Section 406 of the Sarbanes-Oxley Act of 2002 and Item 406 of Regulation S-K. In addition, we intend to promptly disclose (1) the nature of any amendment to our Code of Business Conduct and Ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or controller or persons performing similar functions and (2) the nature of any waiver, including an implicit waiver, from a provision of our code of ethics that is granted to one of these specified officers, the name of such person who is granted the waiver and the date of the waiver on our website in the future.
Item 11. Executive Compensation
Information required by this item will be contained in our Definitive Proxy Statement under the heading “Executive Compensation and Other Information” and is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Information required by this item will be contained in our Definitive Proxy Statement under the headings “Security Ownership of Certain Beneficial Owners and Management” and is incorporated herein by reference.
Item 13. Certain Relationships, Related Transactions and Director Independence
Information required by this item will be contained in our Definitive Proxy Statement under the headings “Certain Relationships and Related Party Transactions” and “Independence of the Board of Directors” and is incorporated herein by reference.
Item 14. Principal Accounting Fees and Services
Information required by this item will be contained in our Definitive Proxy Statement under the heading “Independent Registered Public Accounting Firm’s Fees” and is incorporated herein by reference.
53
Item 15. Exhibits, Financial Statement Schedules
(a) Documents filed as part of this report.
1. Financial Statements. The following financial statements of Evoke Pharma, Inc., together with the report thereon of BDO USA, LLP, an independent registered public accounting firm, are included in this Annual Report on Form 10-K:
|
| Page |
|
| 57 |
| |
| 58 |
| |
| 59 |
| |
| 60 |
| |
| 61 |
|
2. Financial Statement Schedules.
None.
3. Exhibits.
A list of exhibits to this Annual Report on Form 10-K is set forth on the Exhibit Index immediately preceding the signature page and is incorporated herein by reference.
(b) See Exhibit Index.
(c) See Item 15(a)(2) above.
None.
54
Report of Independent Registered Public Accounting Firm
Shareholders and Board of Directors
Evoke Pharma, Inc.
Solana Beach, California
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Evoke Pharma, Inc. (the “Company”) as of December 31, 2020 and 2019, the related statements of operations, stockholders’ (deficit) equity, and cash flows for each of the two years in the period ended December 31, 2020, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Going Concern Uncertainty – See also Critical Audit Matter section below
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has suffered recurring losses from operations and has not generated significant revenues or positive cash flows from operations. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which it relates.
Going Concern Uncertainty – See also Going Concern Uncertainty explanatory paragraph above
As described in Note 1 to the Company’s financial statements, the Company has had recurring losses and negative cash flows from operations since inception and expects to continue to incur net losses for the foreseeable future. The determination as to whether the Company can continue as a going concern includes consideration of managements operating plan and anticipated timing of future cash flows. This matter is also described in the “Going Concern Uncertainty” section of our report.
55
We identified management’s evaluation of going concern uncertainty and related financial statement disclosures as a critical audit matter. Management is required to make subjective judgments and assumptions in concluding on going concern uncertainty, preparing a forecast of future cash flows and providing complete and accurate disclosures related to the Company’s current and future operations. Auditing these judgments and assumptions involved especially challenging auditor judgment due to the nature and extent of audit evidence and effort required to address these matters.
The primary procedures we performed to address this critical audit matter included:
| • | Testing the existence of the total liquid assets at December 31, 2020. |
| • | Evaluating the fluctuations in forecasted research and development, general and administrative expenses, and related payments thereof, as compared to historic amounts and underlying management assumptions. |
| • | Assessing management’s plans in the context of other audit evidence obtained during the audit to determine whether such information supported or contradicted the assumptions used by management. |
| • | Evaluating the adequacy of the Company’s disclosure of the going concern uncertainty in the notes to the financial statements. |
/s/ BDO USA, LLP
We have served as the Company's auditor since 2014.
San Diego, California
March 11, 2021
56
Evoke Pharma, Inc.
|
| December 31, |
| |||||
|
| 2020 |
|
| 2019 |
| ||
Assets |
|
|
|
|
|
|
|
|
Current Assets: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
| $ | 8,068,939 |
|
| $ | 5,663,833 |
|
Accounts receivable, net |
|
| 23,311 |
|
| — |
| |
Prepaid expenses |
|
| 921,762 |
|
|
| 581,706 |
|
Inventory |
|
| 236,480 |
|
| — |
| |
Other current assets |
|
| 30,300 |
|
| — |
| |
Total current assets |
|
| 9,280,792 |
|
|
| 6,245,539 |
|
Operating lease right-of-use asset |
|
| 141,705 |
|
|
| 138,538 |
|
Other assets |
|
| 11,551 |
|
|
| 11,551 |
|
Total assets |
| $ | 9,434,048 |
|
| $ | 6,395,628 |
|
|
|
|
|
|
|
|
|
|
Liabilities and stockholders' (deficit) equity |
|
|
|
|
|
|
|
|
Current Liabilities: |
|
|
|
|
|
|
|
|
Accounts payable and accrued expenses |
| $ | 1,273,572 |
|
| $ | 1,033,383 |
|
Accrued compensation |
|
| 1,016,232 |
|
|
| 843,162 |
|
Operating lease liability |
|
| 141,705 |
|
|
| 138,538 |
|
Paycheck protection program loan |
|
| 104,168 |
|
| — |
| |
Milestone payable |
|
| 5,000,000 |
|
| — |
| |
Total current liabilities |
|
| 7,535,677 |
|
|
| 2,015,083 |
|
Long-term Liabilities: |
|
|
|
|
|
|
|
|
Note payable |
|
| 5,000,000 |
|
| — |
| |
Accrued interest payable |
|
| 112,994 |
|
| — |
| |
Total long-term liabilities |
|
| 5,112,994 |
|
| — |
| |
Total liabilities |
|
| 12,648,671 |
|
|
| 2,015,083 |
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies (Note 3) |
|
|
|
|
|
|
|
|
Stockholders' (deficit) equity: |
|
|
|
|
|
|
|
|
Preferred stock, $0.0001 par value; authorized shares — 5,000,000 at December 31, 2020 and 2019; issued and outstanding shares — 0 at December 31, 2020 and 2019 |
|
| — |
|
|
| — |
|
Common stock, $0.0001 par value; authorized shares — 50,000,000 at December 31, 2020 and 2019; issued and outstanding shares — 26,621,954 and 24,431,914 at December 31, 2020 and 2019, respectively |
|
| 2,662 |
|
|
| 2,443 |
|
Additional paid-in capital |
|
| 95,667,776 |
|
|
| 90,108,492 |
|
Accumulated deficit |
|
| (98,885,061 | ) |
|
| (85,730,390 | ) |
Total stockholders' (deficit) equity |
|
| (3,214,623 | ) |
|
| 4,380,545 |
|
Total liabilities and stockholders' (deficit) equity |
| $ | 9,434,048 |
|
| $ | 6,395,628 |
|
|
|
|
|
|
|
|
|
|
See accompanying notes.
57
Evoke Pharma, Inc.
|
| Year Ended December 31, |
| |||||
|
| 2020 |
|
| 2019 |
| ||
|
|
|
|
|
|
|
|
|
Net product sales |
| $ | 23,020 |
|
| $ | — |
|
Operating expenses: |
|
|
|
|
|
|
|
|
Cost of goods sold |
|
| 86,712 |
|
|
| — |
|
Research and development |
|
| 6,554,825 |
|
|
| 3,416,466 |
|
Selling, general and administrative |
|
| 6,428,832 |
|
|
| 3,737,987 |
|
Total operating expenses |
|
| 13,070,369 |
|
|
| 7,154,453 |
|
Loss from operations |
|
| (13,047,349 | ) |
|
| (7,154,453 | ) |
Other income (expense): |
|
|
|
|
|
|
|
|
Interest income |
|
| 5,672 |
|
|
| 28,798 |
|
Interest expense |
|
| (112,994 | ) |
|
| — |
|
Total other income (expense) |
|
| (107,322 | ) |
|
| 28,798 |
|
Net loss |
| $ | (13,154,671 | ) |
| $ | (7,125,655 | ) |
Net loss per share of common stock, basic and diluted |
| $ | (0.52 | ) |
| $ | (0.32 | ) |
Weighted-average shares used to compute basic and diluted net loss per share |
|
| 25,492,169 |
|
|
| 22,296,089 |
|
|
|
|
|
|
|
|
|
|
See accompanying notes.
58
Evoke Pharma, Inc.
Statements of Stockholders’ (Deficit) Equity
|
|
|
|
|
|
|
|
|
| Additional |
|
|
|
|
|
| Total |
| ||
|
| Common Stock |
|
| Paid-In |
|
| Accumulated |
|
| Stockholders' |
| ||||||||
|
| Shares |
|
| Amount |
|
| Capital |
|
| Deficit |
|
| (Deficit) Equity |
| |||||
Balance at December 31, 2018 |
|
| 17,427,533 |
|
| $ | 1,743 |
|
| $ | 82,628,312 |
|
| $ | (78,604,735 | ) |
| $ | 4,025,320 |
|
Stock-based compensation expense |
| — |
|
| — |
|
|
| 1,373,958 |
|
| — |
|
|
| 1,373,958 |
| |||
Issuance of common stock from ATM, |
|
| 7,004,381 |
|
|
| 700 |
|
|
| 6,106,222 |
|
| — |
|
|
| 6,106,922 |
| |
Net loss |
| — |
|
| — |
|
| — |
|
|
| (7,125,655 | ) |
|
| (7,125,655 | ) | |||
Balance at December 31, 2019 |
|
| 24,431,914 |
|
|
| 2,443 |
|
|
| 90,108,492 |
|
|
| (85,730,390 | ) |
|
| 4,380,545 |
|
Stock-based compensation expense |
| — |
|
| — |
|
|
| 1,628,558 |
|
| — |
|
|
| 1,628,558 |
| |||
Issuance of common stock from employee |
|
| 118,491 |
|
| 12 |
|
|
| 119,404 |
|
| — |
|
|
| 119,416 |
| ||
Issuance of common stock from warrant |
|
| 381,625 |
|
| 38 |
|
|
| (38 | ) |
| — |
|
| — |
| |||
Issuance of common stock from stock |
|
| 199,111 |
|
| 20 |
|
|
| 215,796 |
|
| — |
|
|
| 215,816 |
| ||
Issuance of common stock from ATM, |
|
| 1,490,813 |
|
| 149 |
|
|
| 3,595,564 |
|
| — |
|
|
| 3,595,713 |
| ||
Net loss |
| — |
|
| — |
|
| — |
|
|
| (13,154,671 | ) |
|
| (13,154,671 | ) | |||
Balance at December 31, 2020 |
|
| 26,621,954 |
|
| $ | 2,662 |
|
| $ | 95,667,776 |
|
| $ | (98,885,061 | ) |
| $ | (3,214,623 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes.
59
Evoke Pharma, Inc.
|
| Year Ended December 31, |
| |||||
|
| 2020 |
|
| 2019 |
| ||
Operating activities |
|
|
|
|
|
|
|
|
Net loss |
| $ | (13,154,671 | ) |
| $ | (7,125,655 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
Stock-based compensation expense |
|
| 1,628,558 |
|
|
| 1,373,958 |
|
Change in operating assets and liabilities: |
|
|
|
|
|
|
|
|
Prepaid expenses and other assets |
|
| (396,834 | ) |
|
| (116,676 | ) |
Inventory |
|
| (236,480 | ) |
| — |
| |
Accounts payable and accrued liabilities |
|
| 416,426 |
|
|
| 106,280 |
|
Accrued interest expense |
|
| 112,994 |
|
| — |
| |
Milestone payable |
|
| 5,000,000 |
|
| — |
| |
Net cash used in operating activities |
|
| (6,630,007 | ) |
|
| (5,762,093 | ) |
|
|
|
|
|
|
|
|
|
Financing activities |
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock from ATM, net |
|
| 3,595,713 |
|
|
| 6,106,922 |
|
Proceeds from issuance of common stock from employee stock purchase plan |
|
| 119,416 |
|
| — |
| |
Proceeds from issuance of common stock from stock options exercises |
|
| 215,816 |
|
| — |
| |
Proceeds from paycheck protection program |
|
| 104,168 |
|
| — |
| |
Proceeds from Eversana line of credit |
|
| 5,000,000 |
|
| — |
| |
Net cash provided by financing activities |
|
| 9,035,113 |
|
|
| 6,106,922 |
|
Net increase in cash and cash equivalents |
|
| 2,405,106 |
|
|
| 344,829 |
|
Cash and cash equivalents at beginning of period |
|
| 5,663,833 |
|
|
| 5,319,004 |
|
Cash and cash equivalents at end of period |
| $ | 8,068,939 |
|
| $ | 5,663,833 |
|
|
|
|
|
|
|
|
|
|
See accompanying notes.
60
Evoke Pharma, Inc.
1. Organization and Basis of Presentation
Evoke Pharma, Inc. (the “Company”) was incorporated under the laws of the state of Delaware in January 2007. The Company is a specialty pharmaceutical company focused primarily on the development of drugs to treat gastroenterological disorders and disease.
Since its inception, the Company has devoted its efforts to developing its sole product, Gimoti™ (metoclopramide) nasal spray, the first and only nasally-administered product indicated for the relief of symptoms in adults with acute and recurrent diabetic gastroparesis. On June 19, 2020, the Company received approval from the U.S. Food and Drug Administration (“FDA”) for its 505(b)(2) New Drug Application (“NDA”) for Gimoti. The Company launched U.S. commercial sales of Gimoti in October 2020 through its commercial partner Eversana Life Science Services, LLC (“Eversana”).
The Company’s activities are subject to the significant risks and uncertainties associated with any specialty pharmaceutical company that has launched its first commercial product, including market acceptance of the product and the potential need to obtain additional funding for its operations.
Going Concern
The financial statements have been prepared assuming the Company will continue as a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company has incurred recurring losses and negative cash flows from operations since inception and expects to continue to incur net losses for the foreseeable future until such time, if ever, that it can generate significant revenues from the sale of Gimoti. The Company ended 2020 with approximately $8.1 million in cash and cash equivalents, and raised net proceeds of approximately $13.1 million from the sale of 5,750,000 shares of common stock in its public offering in January 2021. The Company anticipates that it will continue to incur losses from operations due to commercialization activities, including manufacturing commercial batches of Gimoti, and general and administrative costs to support operations. As a result, the Company believes that there is substantial doubt about its ability to continue as a going concern for one year after the date these financial statements are issued. The financial statements do not include any adjustments that may result from the outcome of this uncertainty.
The Company’s net losses may fluctuate significantly from quarter to quarter and year to year. The Company believes, based on its current operating plan, that its cash and cash equivalents as of December 31, 2020, along with the net proceeds raised from its public offering in January 2021, will be sufficient to fund its operations into the first quarter of 2022, less than one year after the date these financial statements are issued, excluding any future Gimoti revenue. This period could be shortened if there are any significant increases in planned spending other than anticipated. The Company anticipates that it will be required to raise additional funds through debt, equity or other forms of financing, such as potential collaboration arrangements, to fund future operations and continue as a going concern.
There can be no assurance that additional financing will be available when needed or on acceptable terms. If the Company is not able to secure adequate additional funding, the Company may be forced to make reductions in spending, extend payment terms with suppliers, and/or suspend or curtail commercialization activities. Any of these actions could materially harm the Company’s business, results of operations, financial condition and future prospects. There can be no assurance that the Company will be able to successfully commercialize Gimoti. Because the Company’s business is entirely dependent on the success of Gimoti, if the Company is unable to secure additional financing, successfully commercialize Gimoti or identify and execute on strategic alternatives for Gimoti or the Company, the Company will be required to curtail all of its activities and may be required to liquidate, dissolve or otherwise wind down its operations.
Impact of COVID-19
Despite the COVID-19 pandemic, the Company began its commercial sales of Gimoti with Eversana in October 2020. The Company has experienced various disruptions to its sales activities, but have continued its efforts to reach physicians and customers. For example, Eversana’s commercialization efforts have been affected by operational restrictions imposed on its sales force from quarantines, travel restrictions and bans and other governmental restrictions related to COVID-19. As a result of these restrictions, Eversana’s sales force has been restricted from conducting in-person interactions with certain physicians and customers and has been restricted to conducting educational and promotional activities for Gimoti virtually in certain circumstances, which has impacted Eversana’s ability to more actively market Gimoti. Third-party research stated that as a result of COVID-19, fewer patients are visiting physician offices resulting in lower patient volumes than normal. The Company anticipates that it and Eversana will continue to be impacted by the COVID-19 pandemic.
The COVID-19 pandemic has not significantly disrupted the operations of the Company’s third-party suppliers and manufacturers or delayed the Company’s manufacturing timelines of Gimoti, but may negatively impact the Company’s ability to successfully commercialize Gimoti and generate product sales in the future. Further, the COVID-19 pandemic and
61
mitigation measures have also had an adverse impact on global economic conditions which could have an adverse effect on the Company’s future business and financial condition, including impairing its ability to raise capital when needed.
In March 2020, the Coronavirus Aid, Relief, and Economic Security (“CARES”) Act was enacted in response to the COVID-19 pandemic. In April 2020, the Company applied for and was approved for a Small Business Administration (“SBA”) loan under the Paycheck Protection Program, established by the CARES Act. On May 1, 2020, the Company received the loan proceeds of approximately $104,000. In January 2021, the Company received notice that its loan and accrued interest were forgiven by the SBA.
2. Summary of Significant Accounting Policies
Use of Estimates
The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States (“GAAP”). The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Actual results could differ materially from those estimates.
Segment Reporting
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker in making decisions regarding resource allocation and assessing performance. The Company views its operations and manages its business in one operating segment operating in the United States.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less from the date of purchase to be cash equivalents. Cash and cash equivalents include cash in readily available checking and savings accounts.
Fair Value of Financial Instruments
The carrying amounts of all financial instruments, including accounts receivable and accounts payable and accrued expenses, are considered to be representative of their respective fair values because of the short-term nature of those instruments. The carrying value of other short-term and long-term borrowings approximates fair value because of the relative short maturity of these instruments and the interest rates the Company could currently obtain.
Concentrations of Risk
Financial instruments that potentially subject the Company to significant credit risk consist primarily of cash and cash equivalents. The Company maintains deposits in a federally insured financial institution in excess of federally insured limits. The Company has established guidelines designed to maintain safety and liquidity, has not experienced any losses in such accounts and believes the exposure to significant risk to the cash balance is minimal.
The Company relies on contract research organizations (“CROs”) and consultants to assist with ongoing regulatory activities. If the CROs and consultants are unable to continue their support, this could adversely affect the Company’s operations.
In addition, the Company relies on third-party manufacturers for the production of Gimoti. If the third-party manufacturers are unable to continue manufacturing Gimoti, or if the Company loses one of its sole source suppliers used in its manufacturing processes, the Company may not be able to meet any development needs or commercial supply demand for Gimoti, and the development and/or commercialization of Gimoti could be materially and adversely affected.
The Company also relies on a dedicated third-party sales team to sell Gimoti. If such third-party organization is unable to continue serving as a dedicated sales team, the commercialization of Gimoti could be materially and adversely affected.
Accounts Receivable
Accounts receivable are recorded net of allowance for doubtful accounts. Estimates for allowances for doubtful accounts are determined based on existing contractual obligations and historical payment patterns. The allowance for doubtful accounts was zero at December 31, 2020 and no bad debt expense was recorded for the year ended December 31, 2020.
Inventory
The Company does not own or operate manufacturing facilities for the production of Gimoti, nor does it plan to develop its own manufacturing operations in the foreseeable future. The Company depends on third-party contract manufacturers for all of its required raw materials, drug substance and finished product for its commercial manufacturing. The Company has agreements with Cosma S.p.A. to supply metoclopramide for the manufacture of Gimoti, and with Thermo Fisher Scientific,
62
Inc., through its subsidiary Patheon UK Limited, for the manufacturing of Gimoti. The Company currently utilizes third-party consultants, which it engages on an as-needed, hourly basis, to manage the manufacturing contractors.
Prior to FDA approval of Gimoti in June 2020, the cost of materials and expenses associated with the manufacturing of Gimoti were recorded as research and development expense. Subsequent to FDA approval, the Company began manufacturing Gimoti for commercialization and began capitalizing inventory at that time. The Company’s inventory, consisting of approximately $150,000 of raw materials and approximately $86,000 of finished goods at December 31, 2020. Inventories are stated at the lower of cost (first-in first-out basis) or net realizable value. Inventory when written down to net realizable value establishes a new cost basis and its value is not subsequently increased based upon changes in underlying facts and circumstances. The Company’s raw materials inventory is held at its third-party suppliers and its finished goods inventory is held at its manufacturer and at Eversana. The Company records such inventory as consigned inventory.
The Company’s ability to generate revenue and become profitable depends on its ability to successfully commercialize Gimoti, which was launched in the United States through prescription in October 2020 through the Company’s commercial partner Eversana. If the Company or Eversana fail to successfully launch Gimoti and grow and maintain sales, the Company may never generate significant revenues and its results of operations and financial position will be adversely affected.
In accordance with Accounting Standards Codification (“ASC”) 606, Revenue from Contracts with Customers, the Company recognizes revenue when a customer obtains control of promised goods in an amount that reflects the consideration the Company expects to receive in exchange for the goods provided. Customer control is determined upon the customer’s physical receipt of the product. To determine revenue recognition for arrangements within the scope of ASC 606, the Company performs the following five steps: identify the contracts with the customer; identify the performance obligations in the contract; determine the transaction price; allocate the transaction price to the performance obligations in the contract; and recognize revenue when (or as) it satisfies a performance obligation. At contract inception, the Company assesses the goods promised within each contract and determines those that are performance obligations and assesses whether each promised good is distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective performance obligation when the customer obtains control of the product.
Product sales are recorded at the transaction price, which includes variable considerations for co-payment assistance to commercially insured patients meeting certain eligibility requirements, as well as to uninsured patients. Co-payment assistance is recorded as an offset to gross revenue at the time revenue from the product sale is recognized based on expected and actual program participation. Co-pay liabilities are estimated using prescribing data available from customers. Actual amounts of consideration ultimately received may materially differ from the Company’s estimates. If actual results in the future vary from estimates, the Company will adjust these estimates, which would affect net product revenue and earnings in the period such variances become known.
Liabilities for co-pay assistance are classified as accounts payable and accrued expenses in the balance sheets.
Stock-Based Compensation
Stock-based compensation expense for stock option grants and employee stock purchases under the Company’s Employee Stock Purchase Plan (the “ESPP”) is recorded at the estimated fair value of the award as of the grant date and is recognized as expense on a straight-line basis over the employee’s requisite service period, except awards with a performance condition. Awards with a performance condition commence vesting when the satisfaction of the performance condition is probable. The estimation of stock option and ESPP fair value requires management to make estimates and judgments about, among other things, employee exercise behavior, forfeiture rates and volatility of the Company’s common stock. The judgments directly affect the amount of compensation expense that will be recognized.
The Company grants stock options to purchase common stock to employees and members of the board of directors with exercise prices equal to the Company’s closing market price on the date the stock options are granted. The risk-free interest rate assumption was based on the yield of an applicable rate for U.S. Treasury instruments with maturities similar to those of the expected term of the award being valued. The weighted average expected term of options and employee stock purchases was calculated using the simplified method as prescribed by accounting guidance for stock-based compensation. This decision was based on the lack of relevant historical data due to the Company’s limited historical experience. In addition, due to the Company’s limited historical data, the estimated volatility was calculated based upon the Company’s historical volatility, supplemented, as necessary, with historical volatility of comparable companies in the biotechnology industry whose share prices are publicly available for a sufficient period of time. The assumed dividend yield was based on the Company never paying cash dividends and having no expectation of paying cash dividends in the foreseeable future. The Company accounts for forfeitures as the forfeitures occur.
63
Research and Development Expenses
Research and development costs are expensed as incurred and primarily include compensation and related benefits, stock-based compensation expense, costs paid to third-party contractors for product development activities and drug product materials, and technology acquisition milestones. The Company has expensed costs relating to the purchase and production of pre-approval inventories as research and development expense in the period incurred prior to FDA approval received on June 19, 2020. The Company will expense the clinical, regulatory and manufacturing costs related to the post-marketing commitment to conduct a PK trial of Gimoti to characterize dose proportionality of a lower dose strength of Gimoti, as well as other costs that may occur for any additional clinical trials the Company may pursue to expand the indication of Gimoti.
Income Taxes
The Company accounts for income taxes in accordance with ASC 740, Income Taxes. Under ASC 740, deferred tax assets and liabilities reflect the future tax consequences of the differences between the financial reporting and tax basis of assets and liabilities using current enacted tax rates. The Company provides a valuation allowance against net deferred tax assets unless, based upon the available evidence, it is more likely than not that the deferred tax assets will be realized.
The Company’s policy related to accounting for uncertainty in income taxes prescribes a recognition threshold and measurement attributed criteria for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities.
Net Loss Per Share
Basic net loss per share is calculated by dividing the net loss by the weighted-average number of common stock outstanding for the period, without consideration for common stock equivalents. Diluted net loss per share is calculated by dividing the net loss by the weighted-average number of common share equivalents outstanding for the period determined using the treasury-stock method. Dilutive common stock equivalents are comprised of warrants to purchase common stock, options to purchase common stock under the Company’s equity incentive plans and potential shares to be purchased under the ESPP. For the periods presented, the following table sets forth the outstanding potentially dilutive securities that have been excluded from the calculation of diluted net loss per share because to do so would be anti-dilutive:
|
| Year Ended December 31, |
| |||||
|
| 2020 |
|
| 2019 |
| ||
Warrants to purchase common stock |
|
| 1,841,879 |
|
|
| 2,713,561 |
|
Common stock options |
|
| 4,077,259 |
|
|
| 3,114,371 |
|
Employee stock purchase plan |
|
| 12,242 |
|
|
| 25,000 |
|
Total excluded securities |
|
| 5,931,380 |
|
|
| 5,852,932 |
|
|
|
|
|
|
|
|
|
|
64
Recent Accounting Pronouncements
In June 2016, the Financial Accounting Standards Board, (“FASB”) issued ASU 2016-13, Financial Instruments – Credit Losses: Measurement of Credit Losses on Financial Instruments, which amends the impairment model by requiring entities to use a forward-looking approach based on expected losses to estimate credit losses on certain types of financial instruments, including trade receivables and available-for-sale debt securities. This update is effective for annual periods beginning after December 15, 2022, and interim periods within those periods, and early adoption is permitted. We expect to adopt the standard on its effective date in the first quarter of 2023. We believe the adoption will modify the way we analyze financial instruments, but currently do not expect the adoption to have a material financial impact on our financial statements.
3. Commitments
In December 2016, the Company entered into an operating lease for office space in Solana Beach, California. The lease commenced on January 1, 2017, was extended in September 2018, December 2019 and December 2020, and has an expiration date of January 31, 2022. According to ASU No. 2016-02, the Company recognized an operating lease ROU asset and liability based on the present value of the future minimum lease payments over the lease term at the commencement date, using the Company’s assumed incremental borrowing rate, and then amortizes the ROU assets over the lease term. The Company applies a discount rate to the minimum lease payments within the lease agreement to determine the value of right-of-use assets and lease liabilities. Unless the rate implicit in the lease is determinable, ASU No. 2016-02 requires the use of the rate of interest that a lessee would have to pay to borrow on a collateralized basis over a similar term for a similar amount to the lease payments in a similar economic environment. The Company noted that the implicit rate in the lease was not determinable and calculated its incremental borrowing rate primarily based on the Company’s assumed borrowing rate. On January 1, 2019, the Company recorded an operating lease ROU asset and liability of approximately $136,000 based on the present value of the remaining minimum lease payments. As of December 31, 2019, the Company had an operating lease ROU asset and liability of approximately $139,000 based on the present value of the remaining minimum lease payments. During the years ended December 31, 2020 and 2019, changes in operating lease ROU asset and payments of the lease liability were included in prepaid expenses and other assets and accounts payable and accrued expense, respectively, on the statement of cash flows.
Upon amendment and renewal of the term of the lease in December 2020, the Company updated the operating lease ROU asset and liability based on the present value of the future minimum lease payments over the lease term at the commencement date of the lease amendment, with an assumed borrowing rate of 10%, and recorded an operating lease ROU asset and liability of approximately $142,000.
Rent expense for the years ended December 31, 2020 and 2019 was approximately $151,000 and $145,000, respectively. The Company also pays pass through costs and utility costs, which are expensed as incurred.
As of December 31, 2020, the Company has future minimum lease payments under its facility lease of approximately $137,000 in 2021 and approximately $12,000 in 2022.
4. Technology Acquisition Agreement
In June 2007, the Company acquired all worldwide rights, data, patents and other related assets associated with Gimoti from Questcor Pharmaceuticals, Inc. (“Questcor”) pursuant to an Asset Purchase Agreement. The Company paid Questcor $650,000 in the form of an upfront payment and $500,000 in May 2014 as a milestone payment based upon the initiation of the first patient dosing in the Company’s Phase 3 clinical trial for Gimoti. In August 2014, Mallinckrodt, plc (“Mallinckrodt”) acquired Questcor. As a result of that acquisition, Questcor transferred its rights included in the Asset Purchase Agreement with the Company to Mallinckrodt. In addition to the payments previously made to Questcor, the Company may also be required to make additional milestone payments totaling up to $52 million. In March 2018, the Company and Mallinckrodt amended the Asset Purchase Agreement to defer development and approval milestone payments, such that, rather than paying two milestone payments based on FDA acceptance for review of the NDA and final product marketing approval, the Company would be required to make a single $5 million payment on the one-year anniversary after the Company receives FDA approval to market Gimoti. At the time of the Gimoti NDA approval by FDA, the Company recorded the $5 million payable owed to Mallinckrodt with a due date of June 19, 2021, along with a $5 million research and development expense.
The remaining $47 million in milestone payments depend on Gimoti’s commercial success. The Company will be required to pay Mallinckrodt a low single digit royalty on net sales of Gimoti. The Company’s obligation to pay such royalties will terminate upon the expiration of the last patent right covering Gimoti, which is expected to occur in 2030, subject to possible extension should any additional, later expiring, licensed patents be granted.
65
5. Preferred Stock, Common Stock and Stockholders’ Equity
Preferred Stock
Under the Company’s amended and restated certificate of incorporation, the Company is authorized to issue 5,000,000 shares of preferred stock with a $0.0001 par value. No shares of preferred stock were outstanding as of December 31, 2020 or 2019.
Common Stock
As of December 31, 2020, there were 26,621,954 shares of common stock outstanding. Each share of common stock is entitled to one vote. The holders of the common stock are also entitled to receive dividends whenever funds are legally available and when declared by the board of directors of the Company. To date, no dividends have been declared.
Sale of Common Stock in Public Offering
In January 2021, the Company completed the sale of 5,750,000 shares of its common stock in an underwritten public offering led by Laidlaw & Company (UK) Ltd. The price to the public in this offering was $2.50 per share resulting in gross proceeds to the Company of approximately $14.4 million. After deducting underwriting discounts and commissions and offering expenses paid by the Company, the net proceeds to the Company raised from this offering were approximately $13.1 million.
At the Market Equity Offering Program
In November 2017, the Company filed a shelf registration with the SEC on Form S-3. The shelf registration statement included a prospectus for the at-the-market offering to sell up to an aggregate of $16.0 million of shares of the Company’s common stock through B. Riley FBR, Inc. (“FBR”) as a sales agent (the “FBR Sales Agreement”). During the year ended December 31, 2020, the Company sold 1,490,813 shares of common stock at a weighted-average price per share of $2.46 pursuant to the FBR Sales Agreement and received proceeds of approximately $3.6 million, net of commissions and fees. During the year ended December 31, 2019, the Company sold 7,004,381 shares of common stock at a weighted-average price per share of $0.89 pursuant to the FBR Sales Agreement and received proceeds of approximately $6.1 million, net of commissions and fees. Effective January 6, 2021, the Company terminated the FBR Sales Agreement.
In December 2020, the Company filed a new shelf registration statement with the SEC on Form S-3, or the replacement shelf registration statement. The replacement shelf registration statement replaced the registration statement on Form S-3 the Company originally filed with the SEC in November 2017, which registration statement expired in December 2020. The replacement shelf registration was declared effective by the SEC on January 6, 2021. In December 2020, the Company also entered into a new At Market Issuance Sales Agreement (the “ATM Sales Agreement”), with FBR and H.C. Wainwright & Co. (together with FBR, the “Sales Agents”), pursuant to which the Company may sell from time to time, at its option, up to an aggregate of $30 million worth of shares of the Company’s common stock through the Sales Agents. The ATM Sales Agreement provides, among other things, that sales under the ATM Sales Agreement will be made pursuant to the registration statement, including the base prospectus filed as part of such registration statement.
Future sales under the ATM Sales Agreement will depend on a variety of factors including, but not limited to, market conditions, the trading price of the Company’s common stock and the Company’s capital needs. There can be no assurance that the Sales Agents will be successful in consummating future sales based on prevailing market conditions or in the quantities or at the prices that the Company deems appropriate.
In addition, the Company will not be able to make future sales of common stock pursuant to the ATM Sales Agreement unless certain conditions are met, which include the accuracy of representations and warranties made to the Sales Agents under the ATM Sales Agreement. Furthermore, each of the Sales Agents is permitted to terminate the ATM Sales Agreement with respect to itself in its sole discretion upon ten days’ notice, or at any time in certain circumstances, including the occurrence of an event that would be reasonably likely to have a material adverse effect on the Company’s assets, business, operations, earnings, properties, condition (financial or otherwise), prospects, stockholders’ equity or results of operations. The Company has no obligation to sell the shares available for sale pursuant to the ATM Sales Agreement.
Warrants
The Company has issued warrants to purchase common stock to banks that have previously loaned funds to the Company, as well as to representatives of the underwriters of the Company’s public offerings and certain of their affiliates.
For the year ended December 31, 2020, certain holders of warrants exercised their warrants to purchase 871,682 shares of the Company’s common stock by a “cashless” exercise and received 381,625 shares of the Company’s common stock. The warrants had a weighted average exercise price of $2.77 per share. The shares were issued, and the warrants were originally sold, in reliance upon the registration exemption set forth in Section 4(a)(2) of the Securities Act of 1933. There were no warrants exercised in 2019.
66
At December 31, 2020 and 2019, there were warrants outstanding to purchase 1,841,879 and 2,713,561 shares of the Company’s common stock with a weighted average exercise price of $2.98 and $2.91, respectively. At December 31, 2020, the weighted average remaining contractual term of the outstanding warrants is 1.04 years.
Equity Incentive Award Plans
In August 2013, the Company adopted the 2013 Equity Incentive Award Plan (the “2013 Plan”). Under the 2013 Plan, the Company may grant stock options, stock appreciation rights, restricted stock, restricted stock units and other awards to individuals who are then employees, officers, non-employee directors or consultants of the Company. Since its adoption, the Company’s stockholders have amended and restated the 2013 Plan. As of April 2018, the Company’s stockholders increased the number of shares of common stock authorized for issuance under the 2013 Plan to an aggregate of 6,286,425 shares, and extended the term of the 2013 Plan to February 2028. In addition, the number of shares available for issuance is annually increased on the first day of each fiscal year by that number of shares equal to the least of (a) four percent of the outstanding shares of common stock on the last day of the immediately preceding calendar year, and (b) such other amount determined by the Company’s board of directors. Notwithstanding the foregoing, the number of shares of common stock that may be issued or transferred pursuant to incentive stock options under the Restated Plan may not exceed an aggregate of 8,000,000 shares.
In June 2019, the Company effected a one-time option exchange, wherein employees were offered the opportunity to exchange certain outstanding stock options for the grant of a lesser number of replacement stock options. The participants received three new stock options for every four stock options tendered for exchange. As a result, 2,456,999 stock options were exchanged for 1,842,746 replacement stock options. The replacement stock options have a four-year vesting schedule and an exercise price of $0.62 per share, which was the closing price of the Company’s common stock on the date of the option exchange. All other terms of the replacement stock options remain the same as the original stock options that were exchanged. As a result of this transaction, the Company recognized approximately $84,000 of additional stock-based compensation expense over the four-year vesting term of the exchanged options.
As a result of the annual increases since the 2013 Plan originated, and the increase of stock options reserved under the restatements of the 2013 Plan approved by the Company’s stockholders through April 2018, the Company has increased the number shares reserved for issuance under the 2013 Plan by 4,073,526 shares. As of December 31, 2020, 1,402,433 options remain available for future grant under the 2013 Plan. On January 1, 2021, the Company further increased the number of shares reserved for issuance under the 2013 Plan by 1,064,878 shares, making 2,467,311 options available for future grant under the 2013 Plan.
Options granted under the 2013 Plan have ten-year terms from the date of grant and generally vest over a one to four year period. The Company granted options to purchase 1,172,000 and 829,500 shares of common stock in 2020 and 2019, respectively. The exercise price of all options granted during the years ended December 31, 2020 and 2019 was equal to the market value per share of the Company’s common stock on the date of grant.
A summary of the Company’s stock option activity under the 2007 Equity Incentive Plan and the 2013 Plan is as follows:
|
| Shares |
|
| Weighted Average Exercise Price |
|
| Weighted Average Remaining Contractual Term (Years) |
|
| Aggregate Intrinsic Value |
| ||||
Outstanding at December 31, 2019 |
|
| 3,114,371 |
|
| $ | 1.71 |
|
|
| 8.32 |
|
| $ | 2,143,756 |
|
Granted |
|
| 1,172,000 |
|
| $ | 1.58 |
|
|
| 9.22 |
|
| — |
| |
Exercised |
|
| (199,111 | ) |
| $ | 1.08 |
|
|
| 2.78 |
|
| $ | 484,188 |
|
Expired/Forfeited/Exchanged |
|
| (10,001 | ) |
| $ | 2.42 |
|
|
| 6.93 |
|
| $ | 30,620 |
|
Outstanding at December 31, 2020 |
|
| 4,077,259 |
|
| $ | 1.70 |
|
|
| 8.09 |
|
| $ | 5,134,768 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vested and expected to vest at |
|
| 4,077,259 |
|
| $ | 1.70 |
|
|
| 8.09 |
|
| $ | 5,134,768 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable at December 31, 2020 |
|
| 1,943,106 |
|
| $ | 2.32 |
|
|
| 7.32 |
|
| $ | 1,967,591 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The intrinsic values above represent the aggregate value of the total pre-tax intrinsic value based upon a common stock price of $2.58 and $1.62 at December 31, 2020 and 2019, respectively, and the contractual exercise price.
67
The weighted average grant date fair value per share of employee stock options granted during the years ended December 31, 2020 and 2019, was $1.23 and $0.91, respectively.
Employee Stock Purchase Plan
In June 2013, the Company’s board of directors adopted the ESPP, and the Company’s stockholders approved the ESPP on August 29, 2013. The ESPP became effective on the day prior to the effectiveness of the IPO. The ESPP permits participants to purchase the Company’s common stock at 85% of the fair market value through payroll deductions of up to 20% of their eligible compensation. A total of 30,000 shares of common stock were initially reserved for issuance under the ESPP. In addition, the number of shares of common stock available for issuance under the ESPP has been annually increased on the first day of each fiscal year during the term of the ESPP by an amount equal to the lesser of: (i) 30,000 shares; (ii) one percent of the outstanding shares of common stock as of the last day of the immediately preceding fiscal year; or (iii) such other amount as the Company’s board of directors may determine.
In May 2017, the Company’s stockholders approved an amendment and restatement of the Company’s ESPP to increase the number of shares of common stock reserved under the ESPP by 100,000 shares (to an aggregate of 1,250,000 shares), to increase the annual evergreen provision from 30,000 shares to 100,000 shares, and to extend the term of the ESPP into 2027. The Company increased the number shares reserved for issuance under the ESPP by 520,000 shares since the inception of the ESPP. As of December 31, 2020, 244,574 shares remain available for future issuance under the ESPP. On January 1, 2021, the Company further increased the number of shares reserved for future issuance under the ESPP by 100,000 shares, making 344,574 shares available for future issuance under the ESPP after that increase.
Payroll withholdings from the Company’s employees of approximately $119,000 resulted in the issuance of 118,491 shares of common stock through its ESPP during the year ended December 31, 2020. No shares of common stock were issued through the ESPP during 2019.
Stock-Based Compensation
Stock-based compensation expense includes charges related to employee stock purchases under the ESPP and stock option grants. The Company measures stock-based compensation expense based on the grant date fair value of any awards granted to its employees. Such expense is recognized over the period of time that employees provide service and earn rights to the awards.
The estimated fair value of each stock option award granted was determined on the date of grant using the Black Scholes option-pricing valuation model with the following weighted-average assumptions for option grants during the years ended December 31, 2020 and 2019:
|
| Year Ended December 31, |
| |||||
|
| 2020 |
|
| 2019 |
| ||
Risk free interest rate |
| 0.39% - 0.96% |
|
| 1.80% - 2.55% |
| ||
Expected option term |
| 5.5 - 6.0 years |
|
| 4.27 - 6.0 years |
| ||
Expected volatility of common stock |
| 99.73% - 103.99% |
|
| 90.34% - 112.58% |
| ||
Expected dividend yield |
| 0.0% |
|
| 0.0% |
| ||
|
|
|
|
|
|
|
|
|
The estimated fair value of the shares to be acquired under the ESPP was determined on the initiation date of each six-month purchase period using the Black-Scholes option-pricing valuation model with the following weighted-average assumptions for ESPP shares to be purchased during the years ended December 31, 2020 and 2019 as follows:
|
| Year Ended December 31, |
| |||||
|
| 2020 |
|
| 2019 |
| ||
Risk free interest rate |
| 0.13% - 1.11% |
|
| 1.89% - 2.52% |
| ||
Expected term |
| 0.5 years |
|
| 0.5 years |
| ||
Expected volatility of common stock |
| 69.72% - 111.98% |
|
| 130.36% - 170.68% |
| ||
Expected dividend yield |
| 0.0% |
|
| 0.0% |
| ||
|
|
|
|
|
|
|
|
|
68
The Company recognized stock-based compensation expense to employees and directors in its research and development and its general and administrative functions during the years ended December 31, 2020 and 2019 as follows:
|
| Year Ended December 31, |
| |||||
|
| 2020 |
|
| 2019 |
| ||
Research and development |
| $ | 316,892 |
|
| $ | 710,155 |
|
Selling, general and administrative |
|
| 1,311,666 |
|
|
| 663,803 |
|
Total stock-based compensation expense |
| $ | 1,628,558 |
|
| $ | 1,373,958 |
|
|
|
|
|
|
|
|
|
|
As of December 31, 2020, there was approximately $2.0 million of unrecognized compensation costs related to outstanding employee and board of director options, which are expected to be recognized over a weighted-average period of 1.09 years.
Common Stock Reserved for Future Issuance
Common stock reserved for future issuance consists of the following at December 31, 2020 and 2019:
|
| December 31, |
| |||||
|
| 2020 |
|
| 2019 |
| ||
Stock options issued and outstanding |
|
| 4,077,259 |
|
|
| 3,114,371 |
|
Authorized for future option grants |
|
| 1,402,433 |
|
|
| 1,587,155 |
|
Warrants to purchase common stock |
|
| 1,841,879 |
|
|
| 2,713,561 |
|
Authorized for employee stock purchase plan |
|
| 244,574 |
|
|
| 263,065 |
|
Total common stock reserved for future issuance |
|
| 7,566,145 |
|
|
| 7,678,152 |
|
|
|
|
|
|
|
|
|
|
6. Employee Benefit Plan
The Company has established a defined contribution 401(k) plan (the “Plan”) for all employees who are at least 21 years of age. Employees are eligible to participate in the Plan beginning on the date of employment. Under the terms of the Plan, employees may make voluntary contributions as a percentage of compensation. The Company’s contributions to the Plan are discretionary, and no contributions have been made by the Company to date. For the years ended December 31, 2020 and 2019, the Company adopted Safe Harbor 401(k) provisions. No contributions were required to be made to the accounts of employees for the years ended December 31, 2020 and 2019 in order to maintain the Plan’s compliance with Internal Revenue Service regulations.
7. Commercial Services and Loan Agreements with Eversana
On January 21, 2020, the Company entered into a commercial services agreement (the “Eversana Agreement”) with Eversana for the commercialization of Gimoti. Pursuant to the Eversana Agreement, Eversana commercializes and distributes Gimoti in the United States. Eversana also manages the marketing of Gimoti to targeted health care providers, as well as the sales and distribution of Gimoti in the United States.
Under the terms of the Eversana Agreement, the Company maintains ownership of the Gimoti NDA, as well as legal, regulatory, and manufacturing responsibilities for Gimoti. Eversana will utilize its internal sales organization, along with other commercial functions, for market access, marketing, distribution and other related patient support services. The Company will record sales for Gimoti and retain more than 80% of net product profits once the parties’ costs are reimbursed. As of December 31, 2020, unreimbursed commercialization costs to Eversana were approximately $9.0 million. Such costs will generally be payable only as net product profits are recognized. Eversana will receive reimbursement of its commercialization costs pursuant to an agreed upon budget and a percentage of product profits in the mid-to-high teens. Net product profits are the net sales (as defined in the Eversana Agreement) of Gimoti, less (i) reimbursed commercialization costs, (ii) manufacturing and administrative costs set at a fixed percentage of net sales, and (iii) third party royalties. During the term of the Eversana Agreement, Eversana agreed to not market, promote, or sell a competing product in the United States.
The Eversana Agreement terminates on June 19, 2025, unless terminated earlier pursuant to its terms. Upon expiration or termination of the agreement, the Company will retain all profits from product sales and assume all corresponding commercialization responsibilities. Within 30 days after each of the first three annual anniversaries of commercial launch, either party may terminate the agreement if net sales of Gimoti do not meet certain annual thresholds. Either party may terminate the agreement: for the material breach of the other party, subject to a 60-day cure period; in the event an insolvency, petition of the other party is pending for more than 60 days; upon 30 days written notice to the other party if Gimoti is subject to a safety recall; the other party is in breach of certain regulatory compliance representations under the agreement; if the
69
Company discontinues the development or production of Gimoti; if the net profit is negative for any two consecutive calendar quarters beginning with the first full calendar quarter 24 months following commercial launch; if the cumulative net product profits fail to reach certain thresholds in the first three years following launch; or if there is a change in applicable laws that makes operation of the services as contemplated under the agreement illegal or commercially impractical. Either party may also terminate the Eversana Agreement upon a change of control of the Company’s ownership, subject, in the event that the Company initiates such termination, to a one-time payment equal to between two times and one times annualized service fees paid by the Company under the Eversana Agreement, with such amount based on which year after commercial launch the change of control occurs. Such payment amount would be reduced by the amount of previously reimbursed commercialization costs and profit split paid for the related prior twelve-month period and any revenue which occurred prior to the termination yet to be collected. If Eversana terminates the agreement due to an uncured material breach by the Company, or if the Company terminates the Eversana Agreement in certain circumstances, the Company has agreed to reimburse Eversana for its unreimbursed commercialization costs for the prior twelve-month period and certain other costs. In addition, Eversana may terminate the Eversana Agreement if the Company withdraws Gimoti from the market for more than 90 days.
In connection with the Eversana Agreement, the Company and Eversana have entered into the Eversana Credit Facility, pursuant to which Eversana has agreed to provide a revolving Credit Facility of up to $5 million to the Company upon FDA approval of the Gimoti NDA under certain customary conditions. The Eversana Credit Facility terminates on June 25, 2025, unless terminated earlier pursuant to its terms. The Eversana Credit Facility is secured by all of the Company’s personal property other than the Company’s intellectual property. Under the terms of the Eversana Credit Facility, the Company cannot grant an interest in the Company’s intellectual property to any other person. Each loan under the Eversana Credit Facility will bear interest at an annual rate equal to 10.0%, with such interest due at the end of the loan term. In June 2020 the Company borrowed $2 million and in December 2020 it borrowed the remaining $3 million under the Eversana Credit Facility.
The Company may prepay any amounts borrowed under the Eversana Credit Facility at any time without penalty or premium. The maturity date of all amounts, including interest, borrowed under the Eversana Credit Facility will be 90 days after the expiration or earlier termination of the Eversana Agreement. The Eversana Credit Facility also includes events of default, the occurrence and continuation of which provide Eversana with the right to exercise remedies against the Company and the collateral securing the loans under the Eversana Credit Facility, including the Company’s cash. These events of default include, among other things, the Company’s failure to pay any amounts due under the Eversana Credit Facility, an uncured material breach of the representations, warranties and other obligations under the Eversana Credit Facility, the occurrence of insolvency events and the occurrence of a change in control.
8. Income Taxes
The Company accounts for uncertain tax positions in accordance with ASC Topic 740, Income Taxes. The application of income tax law and regulations is inherently complex. Interpretations and guidance surrounding income tax laws and regulations change over time. As such, changes in the Company’s subjective assumptions and judgments can materially affect amounts recognized in its financial statements.
The Company’s policy is to recognize interest and/or penalties related to income tax matters in income tax expense. The Company had no accrual for interest and penalties on the balance sheet at December 31, 2020. The Company has an uncertain tax position (“UTP”) of approximately $2.0 million related to California net operating losses at December 31, 2020. The Company is subject to taxation in the United States and state jurisdictions, and the Company’s tax years beginning 2007 to date are subject to examination by taxing authorities.
Deferred income taxes result from temporary differences between the tax basis of assets and liabilities and their reported amounts in the financial statements that will result in taxable or deductible amounts in future years. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in years in which those temporary differences are expected to be recovered or settled. As changes in tax laws or rates are enacted, deferred tax assets and liabilities are adjusted through income tax expense.
70
A reconciliation of the federal statutory income tax rate and the effective income tax rate is as follows for the years ended December 31, 2020 and 2019:
|
| December 31, |
| |||||
|
| 2020 |
|
| 2019 |
| ||
|
| (%) |
|
| (%) |
| ||
Federal statutory rate |
|
| 21 |
|
|
| 21 |
|
Change in valuation allowance |
|
| (6 | ) |
|
| (4 | ) |
State income taxes, net of federal effect |
| — |
|
|
| 7 |
| |
Research and development credits |
|
| (1 | ) |
|
| 3 |
|
Removal of net operating losses and other credits |
|
| (11 | ) |
|
| (27 | ) |
Impact of state tax rate change |
|
| (2 | ) |
|
| 4 |
|
Stock compensation and other permanent items |
|
| (1 | ) |
|
| (4 | ) |
Effective income tax rate |
| — |
|
| — |
| ||
|
|
|
|
|
|
|
|
|
Pursuant to Internal Revenue Code of 1986 (“IRC”) Sections 382 and 383, annual use of the Company’s net operating loss and research and development credit carryforwards may be limited in the event a cumulative change in ownership of more than 50% occurs within a three-year period. The Company has not completed an IRC Section 382/383 analysis regarding the limitation of net operating loss and research and development credit carryforwards. Until this analysis has been completed, the Company has removed the deferred tax assets for net operating losses of approximately $20.4 million and a research and development credit of approximately $3.5 million generated through December 31, 2020 from its deferred tax asset schedule, and has recorded a corresponding decrease to its valuation allowance. When this analysis is finalized, the Company plans to update its unrecognized tax benefits accordingly. The Company does not expect this analysis to be completed within the next twelve months and, as a result, the Company does not expect that the unrecognized tax benefits will change within twelve months of this reporting date. Due to the existence of the valuation allowance, future changes in the Company’s unrecognized tax benefits will not impact the Company’s effective tax rate.
Significant components of the Company’s deferred tax assets at December 31, 2020 and 2019 are as follows:
|
| December 31, |
| |||||
|
| 2020 |
|
| 2019 |
| ||
Deferred tax assets: |
|
|
|
|
|
|
|
|
Acquired technology |
| $ | 1,089,000 |
|
| $ | 80,000 |
|
Stock compensation expense |
|
| 695,000 |
|
|
| 846,000 |
|
Lease liability |
|
| 59,000 |
|
|
| 39,000 |
|
Accruals and other |
|
| 214,000 |
|
|
| 237,000 |
|
Total deferred tax assets |
|
| 2,057,000 |
|
|
| 1,202,000 |
|
Deferred tax liabilities: |
|
|
|
|
|
|
|
|
Right of use asset |
|
| (59,000 | ) |
|
| (39,000 | ) |
Total deferred tax liabilities |
|
| (59,000 | ) |
|
| (39,000 | ) |
Less valuation allowance |
|
| (1,998,000 | ) |
|
| (1,163,000 | ) |
Net deferred tax assets (liabilities) |
| $ | — |
|
| $ | — |
|
At December 31, 2020, the Company has federal and California net operating loss carryforwards of approximately $81.3 million and $47.7 million, respectively. The federal and California net operating loss carryforwards begin to expire in 2027 and 2028, respectively, unless previously utilized. The portion of federal net operating losses created after 2017 of approximately $19.5 million do not expire and will carry forward indefinitely. At December 31, 2020, the Company also has federal and California research tax credit carryforwards of approximately $2.4 million and $1.5 million, respectively. The federal research credit carryforwards will begin expiring in 2027 unless previously utilized. The California research credit will carry forward indefinitely. Furthermore, under U.S. tax legislation enacted in December 2017, although the treatment of tax losses generated before December 31, 2017 has generally not changed, tax losses generated in calendar year 2018 and beyond do not expire, but may only offset 80% of the Company’s taxable income. This change may require us to pay federal income taxes in future years despite generating a loss for federal income tax purposes in prior years.
The CARES Act, among other things, permits net operating loss carryforwards generated in taxable years beginning after December 31, 2017, to offset 100% of taxable income for taxable years beginning before January 1, 2021, and 80% of
71
taxable income in taxable years beginning after December 31, 2020. The CARES Act did not have a material impact on the Company’s financial results for the year ended December 31, 2020.
California Assembly Bill 85 (“AB 85”), which intends to close a gap in the budget created by the COVID-19 pandemic, was signed into law on June 29, 2020. AB 85 disallows California net operating losses for any taxable year beginning on or after January 1, 2020 and before January 1, 2023 for any corporation with a net business or modified adjusted gross income of more than $1 million for the taxable year. This bill also limits any business credit to offset a maximum of $5 million of California tax, including the California research credit. The Company has a taxable loss for the year ended December 31, 2020, and therefore is not affected by the limitation.
There were no changes to unrecognized tax benefits in 2020 and 2019. As such, the balance of unrecognized tax benefits (excluding interest and penalties) was approximately $2.0 million at December 31, 2020 and 2019. The Company will recognize interest and penalties related to unrecognized tax benefits as income tax expense when incurred. To date, since no benefit has been taken related to the UTP, there has been no interest and penalties recognized.
Due to the full valuation allowance that the Company has on the deferred tax assets, there are no unrecognized tax benefits that would impact the effective tax rate, if recognized.
9. Subsequent Events
For the purposes of the financial statements as of December 31, 2020 and the year then ended, the Company has evaluated subsequent events through the date the audited annual financial statements were issued. The Company has concluded that no subsequent event has occurred that required disclosure in the financial statements other than what has been disclosed.
72
Exhibit Index
Exhibit Number |
| Description of Exhibit |
|
|
|
3.1(2) |
| Amended and Restated Certificate of Incorporation of the Company |
|
|
|
3.2(2) |
| |
|
|
|
4.1(3) |
| |
|
|
|
4.2(4) |
| Warrant dated June 1, 2012 issued by the Company to Silicon Valley Bank |
|
|
|
4.3(10) |
| |
|
|
|
4.4(11) |
| |
|
|
|
4.5(13) |
| |
|
|
|
4.6(18) |
| |
|
|
|
4.7(19) |
| |
|
|
|
4.8(23) |
| |
|
|
|
10.1(4)# |
| |
|
|
|
10.2(5)# |
| |
|
|
|
10.3(1)# |
| 2013 Equity Incentive Award Plan and form of option agreement thereunder |
|
|
|
10.4(6)# |
| 2013 Employee Stock Purchase Plan restated effective May 3, 2017 |
|
|
|
10.5(5)# |
| Amended and Restated Retention Letter, dated May 22, 2013, between the Company and David A. Gonyer |
|
|
|
10.6(5)# |
| Amended and Restated Retention Letter, dated May 22, 2013, between the Company and Matthew D’Onofrio |
|
|
|
10.7(7)† |
| |
|
|
|
10.8(8)# |
| Employment Agreement, effective as of December 1, 2013, between the Company and Marilyn R. Carlson |
|
|
|
10.9(9)# |
| Non-Employee Director Compensation Policy, as Amended and Restated Effective January 28, 2016 |
|
|
|
10.10(10) |
| |
|
|
|
10.11(10) |
| |
|
|
|
10.12(11) |
| |
|
|
|
10.13(11) |
| |
|
|
|
10.14(14) |
| |
|
|
|
73
Exhibit Number |
| Description of Exhibit |
|
|
|
10.15(14)# |
| |
|
|
|
10.16(14)# |
| |
|
|
|
10.17(16)† |
| Manufacturing Services Agreement dated November 7, 2017, between the Company and Patheon UK Limited |
|
|
|
10.18(22) |
| |
|
|
|
10.19(15) |
| |
|
|
|
10.20(12)† |
| Master Supply Agreement dated as of May 11, 2016 by and between the Company and Cosma S.p.A. |
|
|
|
10.21(20) |
| |
|
|
|
10.22(17) |
| 2013 Equity Incentive Award Plan, as amended and restated, effective February 7, 2018 |
|
|
|
10.23(21) |
| |
|
|
|
10.24(21)# |
| Non-Employee Director Compensation Policy, as Amended and Restated Effective February 6, 2019 |
|
|
|
10.25(23) |
| |
|
|
|
10.26(24)† |
| |
|
|
|
10.27(24)† |
| |
|
|
|
10.28(24)# |
| Non-Employee Director Compensation Policy, as Amended and Restated, Effective February 28, 2020 |
|
|
|
10.29(25)† |
| 3PL Agreement between the Company and Eversana Life Science Services, LLC dated August 27, 2020 |
|
|
|
10.30 |
| |
|
|
|
10.31(26) |
| |
|
|
|
10.32# |
| Non-Employee Director Compensation Policy, as Amended and Restated Effective January 27, 2021 |
|
|
|
23.1 |
| Consent of BDO USA, LLP, Independent Registered Public Accounting Firm |
|
|
|
31.1 |
| |
|
|
|
31.2 |
| |
|
|
|
32.1* |
| |
|
|
|
32.2* |
| |
|
|
|
74
Exhibit Number |
| Description of Exhibit |
|
|
|
101.INS |
| XBRL Instance Document |
|
|
|
101.SCH |
| XBRL Taxonomy Extension Schema Document |
|
|
|
101.CAL |
| XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
101.DEF |
| XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
101.LAB |
| XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
101.PRE |
| XBRL Taxonomy Extension Presentation Linkbase Document |
(1) | Incorporated by reference to the Company’s Amendment No. 4 to Registration Statement on Form S-1 filed with the SEC on August 30, 2013. |
(2) | Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on September 30, 2013. |
(3) | Incorporated by reference to the Company’s Amendment No. 3 to Registration Statement on Form S-1 filed with the SEC on August 16, 2013. |
(4) | Incorporated by reference to the Company’s Registration Statement on Form S-1 filed with the SEC on May 24, 2013. |
(5) | Incorporated by reference to the Company’s Amendment No. 1 to Registration Statement on Form S-1 filed with the SEC on June 14, 2013. |
(6) | Incorporated by reference to Appendix A to the Company’s Definitive Proxy Statement on Schedule 14A filed with the SEC on March 22, 2017. |
(7) | Incorporated by reference to the Company’s Amendment No. 2 to Registration Statement on Form S-1 filed with the SEC on July 3, 2013. |
(8) | Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on December 2, 2013. |
(9) | Incorporated by reference to the Company’s Annual Report on Form 10-K filed with the SEC on March 10, 2016. |
(10) | Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on July 20, 2016. |
(11) | Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on August 1, 2016. |
(12) | Incorporated by reference to the Company’s Quarterly Report on Form 10-Q filed with the SEC on August 15, 2016. |
(13) | Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on December 16, 2016. |
(14) | Incorporated by reference to the Company’s Annual Report on Form 10-K filed with the SEC on March 15, 2017. |
(15) | Incorporated by reference to the Company’s Registration Statement on Form S-3 filed with the SEC on November 14, 2017. |
(16) | Incorporated by reference to the Company’s Annual Report on Form 10-K filed with the SEC on March 7, 2018. |
(17) | Incorporated by reference to Appendix A to the Company’s Definitive Proxy Statement on Schedule 14A filed with the SEC on March 16, 2018. |
(18) | Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on March 23, 2018. |
(19) | Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on April 4, 2018. |
(20) | Incorporated by reference to the Company’s Quarterly Report on Form 10-Q filed with the SEC on May 14, 2018. |
(21) | Incorporated by reference to the Company’s Annual Report on Form 10-K filed with the SEC on March 6, 2019. |
(22) | Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on April 15, 2016. |
(23) | Incorporated by reference to the Company’s Annual Report on Form 10-K filed with the SEC on March 12, 2020. |
(24) | Incorporated by reference to the Company’s Quarterly Report on Form 10-Q filed with the SEC on May 12, 2020. |
(25) | Incorporated by reference to the Company’s Quarterly Report on Form 10-Q filed with the SEC on November 10, 2020. |
(26) | Incorporated by reference to the Company’s Registration Statement on Form S-3 filed with the SEC on December 22, 2020. |
† | Portions of this exhibit have been omitted pursuant to Item 601(b)(10)(iv) of Regulation S-K. |
# | Management contract or compensatory plan or arrangement. |
* | These certifications are being furnished solely to accompany this annual report pursuant to 18 U.S.C. Section 1350, and are not being filed for purposes of Section 18 of the Securities Exchange Act of 1934 and are not to be incorporated by reference into any filing of Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing. |
75
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
| EVOKE PHARMA, INC. | ||
|
|
|
| |||
Date: March 11, 2021 |
|
|
| By: |
| /s/ David A. Gonyer |
|
|
|
|
|
| David A. Gonyer |
|
|
|
|
|
| President and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature |
| Title |
| Date |
|
|
| ||
/s/ David A. Gonyer |
| President, Chief Executive Officer and Director (principal executive officer) |
| March 11, 2021 |
David A. Gonyer, R.Ph. |
|
|
| |
|
|
| ||
/s/ Matthew J. D’Onofrio |
| Executive Vice President, Chief Business Officer, Treasurer |
| March 11, 2021 |
Matthew J. D’Onofrio |
| and Secretary (principal financial and accounting officer) |
|
|
|
|
| ||
/s/ Cam L. Garner |
| Chairman of the Board of Directors |
| March 11, 2021 |
Cam L. Garner |
|
|
|
|
|
|
| ||
/s/ Todd C. Brady, M.D., Ph.D. |
| Director |
| March 11, 2021 |
Todd C. Brady, M.D., Ph.D. |
|
|
|
|
|
|
| ||
/s/ Malcolm R. Hill, Pharm. D. |
| Director |
| March 11, 2021 |
Malcolm R. Hill, Pharm. D. |
|
|
|
|
|
|
| ||
/s/ Ann D. Rhoads |
| Director |
| March 11, 2021 |
Ann D. Rhoads |
|
|
|
|
|
|
| ||
/s/ Kenneth J. Widder, M.D. |
| Director |
| March 11, 2021 |
Kenneth J. Widder, M.D. |
|
|
|
|
76