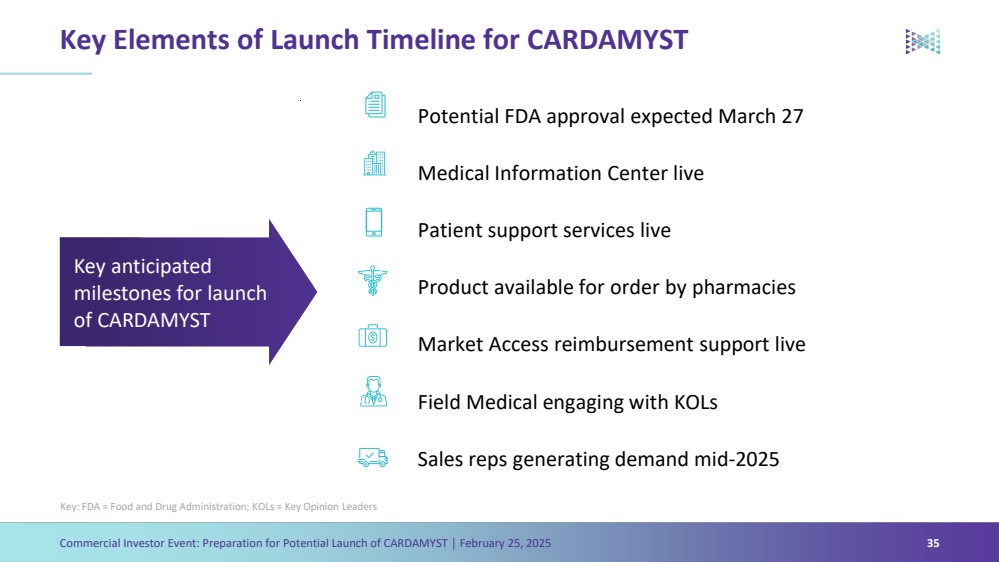
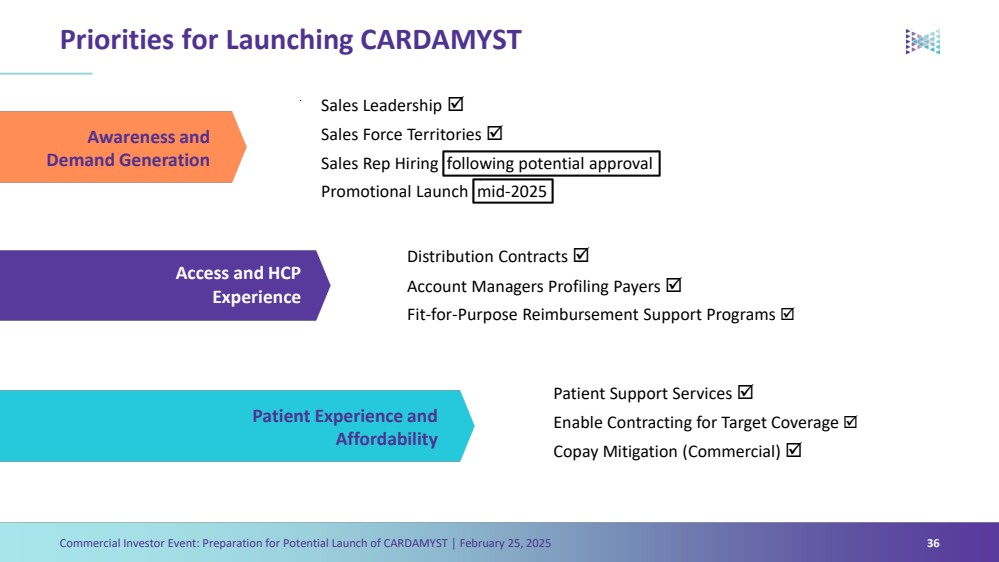
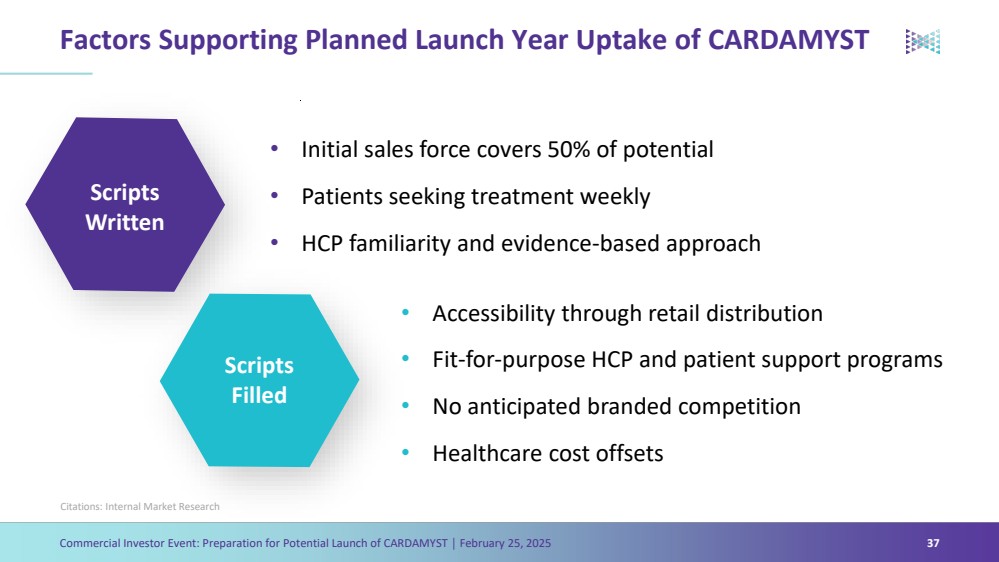
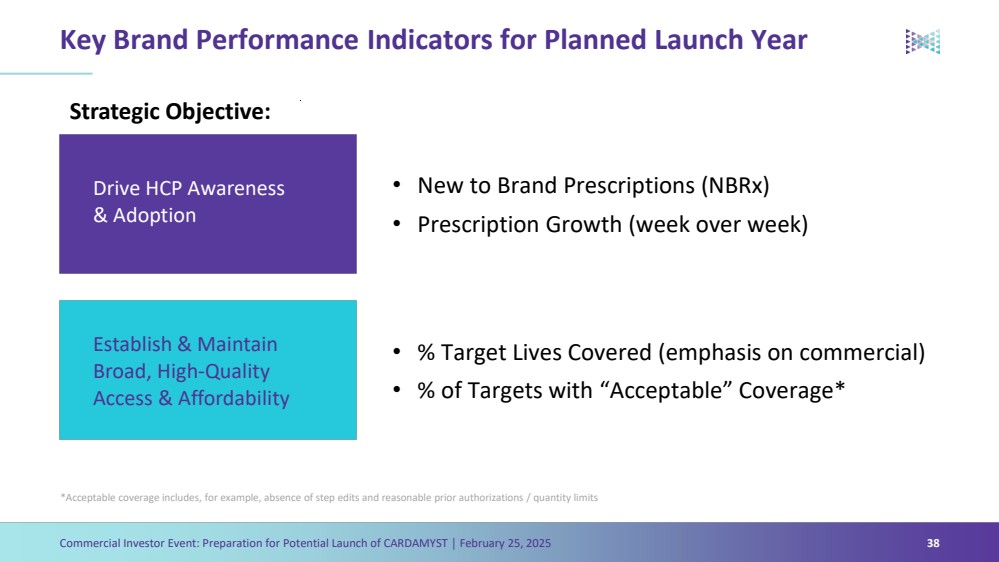
| Forward Looking Statements Commercial Investor Event: Preparation for Potential Launch of CARDAMYST │ February 25, 2025 3 The Presentation contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, as amended. Words such as ‘‘aim,’’ ‘‘anticipate,’’ ‘‘assume,’’ ‘‘believe,’’ ‘‘contemplate,’’ ‘‘continue,’’ ‘‘could,’’ ‘‘design,’’ ‘‘due,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘goal,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘objective,’’ ‘‘plan,’’ ‘‘predict,’’ ‘‘positioned,’’ ‘‘potential,’’ ‘‘project,’’ ‘‘seek,’’ ‘‘should,’’ ‘‘target,’’ ‘‘will,’’ ‘‘would’’ (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. These forward- looking statements are based on Milestone's expectations and assumptions as of the date of this Presentation. Each of these forward-looking statements involves risks and uncertainties. Actual results may differ materially from these forward-looking statements. Forward-looking statements contained in this Presentation include statements regarding: (i) the approval by the U.S. Food and Drug Administration of CARDAMYST on the anticipated timeline, or at all; (ii) the design, progress, timing, scope and results of the etripamil clinical trial in AFib-RVR; (iii) the potential efficacy, safety and tolerability of etripamil for AFib-RVR; (iv) the potential of CARDAMYST to (a) deliver a new PSVT therapeutic option to market, (b) decrease costs for the healthcare system and reduce emergency department visits and hospital admissions, (c) empower patients to treat symptomatic attacks; (v) plans relating to commercializing CARDAMYST, if approved, including timing, the geographic areas of focus and sales strategy; (vi) the potential market size and the rate and degree of market acceptance of CARDAMYST (etripamil) and any future product candidates; (vii) the projected use of CARDAMYST in the future; (viii) anticipated commercial and Medicare coverage of CARDAMYST; (ix) the implementation of Milestone’s business model and strategic plans for its business, etripamil and any future product candidates; (x) Milestone’s expected cash runway; and (xi) potential royalty payments. Important factors that could cause actual results to differ materially from those in the forward-looking statements include, but are not limited to, the risks inherent in biopharmaceutical product development and clinical trials; whether our future interactions with the FDA will have satisfactory outcomes; whether and when, if at all, our NDA for etripamil will be approved by the FDA; uncertainties related to the timing of initiation, enrollment, completion, evaluation and results of our clinical trials; risks and uncertainty related to the complexity inherent in cleaning, verifying and analyzing trial data; and whether the clinical trials will validate the safety and efficacy of etripamil for PSVT or other indications, among others, general economic, political, and market conditions, including deteriorating market conditions due to investor concerns regarding inflation, international tariffs; Russian hostilities in Ukraine and ongoing disputes in Israel and Gaza and overall fluctuations in the financial markets in the United States and abroad; risks related to pandemics and public health emergencies; and risks related the sufficiency of Milestone's capital resources and its ability to raise additional capital in the current economic climate. These and other risks are set forth in Milestone's filings with the U.S. Securities and Exchange Commission (“SEC”), including in its annual report on Form 10-K for the year ended December 31, 2023, under the caption "Risk Factors,” as such discussion may be updated in future filings we make with the SEC. Except as required by law, Milestone assumes no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available. This Presentation contains trademarks, trade names and service marks of other companies, which are the property of their respective owners. Certain information contained in this Presentation and statements made orally during this Presentation relate to or is based on studies, publications, surveys and other data obtained from third-party sources and Milestone's own internal estimates and research. While Milestone believes these third-party studies, publications, surveys and other data to be reliable as of the date of the Presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent sources has evaluated the reasonableness or accuracy of Milestone's internal estimates or research and no reliance should be made on any information or statements made in this Presentation relating to or based on such internal estimates and research. |