UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2012
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ______________to ______________
Commission File Number:
Emmaus Life Sciences, Inc.
(Exact name of small business issuer as specified in its charter)
| Delaware | 2834 | 41-2254389 | ||
| (State or Other Jurisdiction of | (Primary Standard Industrial | (I.R.S. Employer Identification No.) | ||
Incorporation or Organization) | Classification Code Number) |
20725 S. Western Avenue, Suite 136
Torrance, CA 90501
(Address of principal executive offices)
310-214-0065
(Registrant’s telephone number, including area code)
SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT:
None.
SECURITIES REGISTERED PURSUANT TO SECTION 12(g) OF THE ACT:
Common Stock, $0.001 par value
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ¨ No x
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act:
Large accelerated filer ¨ | Accelerated filer ¨ |
Non-accelerated filer ¨ | Smaller reporting company x |
| (Do not check if a smaller reporting company) |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act).
Yes ¨ No x
There was no aggregate market value of shares of common stock held by non-affiliates of the registrant as of June 30, 2012, the last business day of the registrant’s most recently completed second fiscal quarter, because the registrant’s common stock was not trading on any exchange on that date.
There were 24,878,436 shares outstanding of the registrant’s common stock, par value $0.001 per share, as of March 20, 2013. The registrant’s common stock is not traded or listed on any exchange.
TABLE OF CONTENTS
EMMAUS LIFE SCIENCES, INC.
TABLE OF CONTENTS TO ANNUAL REPORT ON FORM 10-K
For the Fiscal Year Ended December 31, 2012
| ITEM | PAGE | ||||
| 3 | |||||
| 16 | |||||
| 31 | |||||
| 31 | |||||
| 31 | |||||
| 32 | |||||
| 33 | |||||
| 35 | |||||
| 35 | |||||
| 45 | |||||
| 45 | |||||
| 45 | |||||
| 45 | |||||
| 46 | |||||
| 47 | |||||
| 51 | |||||
| 58 | |||||
| 60 | |||||
| 64 | |||||
| 65 | |||||
| 70 | |||||
1
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
The information contained in this Annual Report on Form 10-K includes some statements that are not purely historical and that are “forward-looking statements.” Such forward-looking statements include, but are not limited to, statements regarding our company and our management’s expectations, hopes, beliefs, intentions or strategies regarding the future, including our company’s financial condition and results of operations. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “anticipates,” “believes,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “might,” “plans,” “possible,” “potential,” “predicts,” “projects,” “seeks,” “should,” “will,” “would” and similar expressions, or the negatives of such terms, may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking.
The forward-looking statements contained in this Annual Report on Form 10-K are based on current expectations and beliefs concerning future developments. There can be no assurance that future developments actually affecting our company will be those anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond our control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements, including the following:
| · | our ability to raise additional capital to fund our operations; |
| · | our ability to obtain FDA and other regulatory approvals to market our drug products under development, including our L-glutamine treatment for SCD; |
| · | successful completion of our clinical trials; |
| · | our ability to commercialize our L-glutamine treatment for SCD; |
| · | our reliance on third party manufacturers for our drug products; |
| · | the ability of our third party manufacturers to meet regulatory requirements consistently; |
| · | the approval and market entry of competitor drug products; |
| · | market acceptance of our products; |
| · | our dependence on licenses for certain of our products; |
| · | our reliance on the expected growth in demand for our products; |
| · | exposure to product liability and defect claims; |
| · | the costs and uncertain outcome of ongoing litigation; |
| · | exposure to intellectual property claims from third parties; |
| · | the lack of a current public trading market for our securities; |
| · | the cost of complying with current and future governmental regulations and the impact of any changes in the regulations upon our operations; and |
| · | such other factors referenced in this Report, including, without limitation, under the sections entitled “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and “Business.” |
These risks and uncertainties, along with others, are also described below under the heading “Risk Factors.” Should one or more of these risks or uncertainties materialize, or should any of the parties’ assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
2
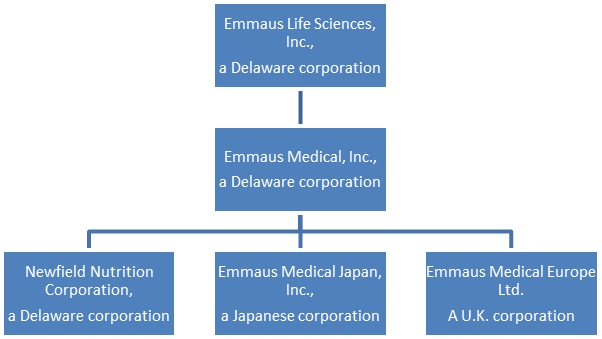
With respect to this discussion, the terms, “we,” “us,” “our” or the “Company” refer to Emmaus Life Sciences, Inc., and its wholly-owned subsidiary Emmaus Medical, Inc., a Delaware corporation (“Emmaus Medical”) and Emmaus Medical’s wholly-owned subsidiaries, Newfield Nutrition Corporation, a Delaware corporation (“Newfield Nutrition”); Emmaus Medical Japan, Inc., a Japanese corporation (“EM Japan”) and Emmaus Medical Europe Ltd., a U.K. corporation (“EM Europe”).
Overview
We are engaged in the discovery, development, and commercialization of innovative and cost-effective treatments and therapies for rare diseases, areas that we believe have traditionally been underserved by large pharmaceutical companies. We believe that there are attractive niche markets and financial opportunities for companies such as ours that specialize in treatments for rare diseases. Over time, we plan to expand our business to include developing and marketing products to treat more common diseases. The primary focus of our business is the late-stage development of the amino acid L-glutamine as a prescription drug for the treatment of sickle cell disease (“SCD”). To a lesser extent, we are also engaged in the marketing and sale of NutreStore® L-glutamine powder for oral solution, a treatment for short bowel syndrome (“SBS”) and the sale of L-glutamine as a nutritional supplement under the brand name AminoPure®. Since inception, we have generated minimal revenue from the sale and/or promotion of NutreStore® and AminoPure®.
Corporate Information
Emmaus Medical, LLC was organized on December 20, 2000. In October 2003, Emmaus Medical, LLC conducted a reorganization and merged with and into Emmaus Medical, which was originally incorporated on September 12, 2003. Through this merger of Emmaus Medical, LLC into Emmaus Medical, Emmaus Medical acquired the exclusive patent rights for a treatment for SCD.
AFH Acquisition IV, Inc. was incorporated in the state of Delaware on September 24, 2007 and was originally organized as a “blank check” shell company. On May 3, 2011, pursuant to an Agreement and Plan of Merger dated April 21, 2011, Emmaus Medical merged with and into AFH Merger Sub, Inc., a wholly-owned subsidiary of AFH Acquisition IV, with Emmaus Medical continuing as the surviving entity (the “Merger”). Upon the closing of the Merger, we (i) became the 100% parent of Emmaus Medical, (ii) assumed the operations of Emmaus Medical and its subsidiaries, and (iii) changed our name from “AFH Acquisition IV, Inc.” to “Emmaus Holdings, Inc.” On September 14, 2011, we changed our name from “Emmaus Holdings, Inc.” to “Emmaus Life Sciences, Inc.”
Our corporate structure is illustrated as follows:
Our principal executive offices and corporate offices are located at 20725 S. Western Avenue, Ste. 136, Torrance, CA 90501-1884. Our telephone number is 310-214-0065. There have been no bankruptcy, receivership or similar proceedings during the past three years.
Industry and Market Opportunity
We focus on developing treatments and therapies for rare diseases. A “rare disease,” pursuant to the Rare Diseases Act of 2002, is defined as any disease or condition that affects fewer than 200,000 persons residing in the United States. In Japan, a rare disease is one that affects fewer than 50,000 persons residing in Japan. The European Commission on Public Health defines a rare disease as a life-threatening or chronically debilitating disease which is of such low prevalence, namely, that it affects fewer than 1 in 2,000 people, and special combined efforts are required to treat it.
3
Sickle Cell Disease
We are currently engaged in a Phase III clinical trial of L-glutamine as a treatment for SCD. Upon completion of the clinical trial, if the data results demonstrate the safety and effectiveness of L-glutamine as a treatment for SCD, we intend to submit a marketing application to the U.S. Food and Drug Administration (“FDA”) by the end of 2013 and would expect to obtain a decision on our marketing application from the FDA in 2014. It is estimated that SCD affects approximately 90,000 to 100,000 persons in the United States, according to information posted by the US Department of Health and Human Services (DHHS), National Institutes of Health (NIH), National Heart, Lung, and Blood Institute, at http://www.nhlbi.nih.gov/health/dci/Diseases/Sca/SCA_WhoIsAtRisk.html, last updated September 2012, and millions of people worldwide. The disease is particularly common among those whose ancestors come from sub-Saharan Africa; Spanish-speaking regions in the Western Hemisphere such as South America, the Caribbean, and Central America; Saudi Arabia; India; and Mediterranean countries such as Turkey, Greece and Italy.
SCD is an inherited blood disorder that affects red blood cells. Red blood cells contain hemoglobin which allows red blood cells to carry oxygen from the air in the lungs to all parts of the body. Normal red blood cells contain hemoglobin A. In contrast, the bone marrow of people with SCD produces red blood cells with a different form of hemoglobin called hemoglobin S (S stands for sickle). When a person has SCD, rather than remaining round, smooth and flexible, the red blood cells become sickle (crescent) shaped, inflexible, and sticky as they release oxygen to other tissues in the body. These abnormally shaped cells become rigid and become lodged in the capillaries when oxygen is released from the cells’ hemoglobin, causing blockages and preventing the normal flow of oxygen to the surrounding tissue. SCD diseased red blood cells also tend to clump together, further impeding circulation.
According to information posted on the NIH National Heart, Lung, and Blood Institute’s website regarding SCD (the “NHLBI SCD Website”), normal red blood cells live for about 120 days before they are replaced with new ones. In sharp contrast, sickle-shaped red blood cells are destroyed faster, in about 10 to 20 days, and cannot always be replaced quickly. As a result, people with SCD are often anemic. Sickle cell disease includes sickle cell anemia (which results from two hemoglobin S genes), sickle ß-thalassemia (one hemoglobin S and one ß-thalassemia gene), hemoglobin SC disease (one hemoglobin S and one hemoglobin C), and the somewhat rare disease hemoglobin C Harlem. According to the NHLBI SCD Website, these hereditary diseases often affect individuals of African American heritage, and increasingly, Hispanic populations.
The complications of sickle cell disease occur when sickle-shaped red blood cells block veins which then can cause pain in the arms, legs, back and stomach, bones, skin and other parts of the body, as well as long-term organ damage, diminished exercise tolerance, increased stroke and infection rate, and decreased lifespan. According to Hematology in Clinical Practice, by Hillman et al. published by McGraw Hill in 2005 (the “Hillman Treatise”), “sickle cell crisis” is a broad term that describes several different conditions, particularly aplastic crisis, which is temporary bone marrow aplasia; hemolytic crisis, which is acute red cell destruction, leading to jaundice; and vaso-occlusive crisis, which is severe pain due to infarctions located in the bones, joints, lungs, liver, spleen, kidney, eye, or central nervous system. The acute pain of a sickle cell crisis generally persists for several days, and is usually followed by a dull, aching pain, which generally ends after several weeks, although it may persist between crises.
According to the article In the Clinic-Sickle Cell Disease by M.H. Steinberg published in Annals of Intern. Med. in September 2011 (the “Steinberg Article”), acute chest syndrome (ACD), which can often lead to death, is a particularly serious complication of SCD and is commonly accompanied by infections in the lungs, characterized by fever, chest pain, cough, and lung infiltrates, this sometimes-lethal complication affects more than half of all patients with sickle cell disease and is the second most common reason for hospitalization.
Pain is the hallmark of sickle cell disease (see the Steinberg Article). This pain is called a sickle cell crisis and often affects the bones, lungs, abdomen and joints. The pain can be acute or chronic, but acute pain is more common according to the NHLBI SCD Website. Typical painful episodes last 5 to 10 days in adults but some seem to persist for weeks. The frequency of acute pain episodes varies within and among individuals from rare to several times a month. Approximately one third of patients have a few pain episodes, 50% have occasional episodes, and 20% have weekly or monthly episodes. A few patients account for most patients with acute pain episodes. The frequency of pain episodes increases late in the second decade of life and decreases after the fourth decade. More than 3 episodes per year is associated with reduced life expectancy (see the Steinberg Article). According to the Hillman Treatise, sickle cell pain is treated with nonsteroidal anti-inflammatory drugs (NSAIDs), narcotics (e.g. morphine, codeine and oxycodone), other pain relief medicines, and blood transfusions. SCD patients also require frequent visits to emergency rooms and urgent care facilities. Aside from pain and ACD, there are other complications of SCD that includes proliferative retinopathy, gallstone, aseptic necrosis of the femoral and humeral heads, leg ulcers and renal insufficiency (see the Hillman Treatise), infection, priapism, digestive system disease (biliary tract and liver disease) (see the Steinberg Article), stroke and hand-foot disease (see the NHLBI SCD Website).
We have acquired the rights to develop a treatment approach for SCD covered under U.S. Patent No. 5,693,671, entitled “L-glutamine Therapy for Sickle Cell Disease and Thalassemia” issued on December 2, 1997 to Niihara et al. Emmaus Medical is the exclusive worldwide licensee of this patent. The license agreement is effective until the expiration of the patent in 2016. For further information on the key terms of the license agreement, see “Intellectual Property” below. L-glutamine is a conditionally essential amino acid that has long been used as a non-pharmaceutical nutritional supplement. A conditionally essential amino acid is an amino acid that the body can naturally synthesize, but under certain circumstances, the body may be unable to synthesize such amino acid and it must be supplied, instead, by diet or supplement. Emmaus’ treatment under development involves SCD patients orally consuming 30 gm/day of USP-grade L-glutamine for adults, and 0.6 gm/kg of body weight for infants and children, up to 30 gm/day.
4
In red blood cells, pyridine nucleotides, nicotinamide adenine dinucleotide (NAD) and its reduced form NADH, are the major molecules that regulate and prevent oxidative damage. According to various articles including L-Glutamine Therapy Reduces Endothelial Adhesion of Sickle Red Blood Cells to Human Umbilical Vein Endothelial Cells by Yutaka Niihara et al., published in BMC Blood Disorders in 2005, Oral L-Glutamine Therapy for Sickle Cell Anemia: I. Subjective Clinical Improvement and Favorable Change in Red Cell NAD Redox Potential by Yutaka Niihara et al., published in the American Journal of Hematology in 1998 and Increased Red Cell Glutamine Availability In Sickle Cell Anemia: Demonstration of Increased Active Transport, Affinity, and Increased Glutamate Level in Intact Red Cells by Yutaka Niihara et al., published in 1997 in the Journal of Laboratory and Clinical Medicine, sickle red blood cells have a significantly increased rate of transport of one of the major precursors of NAD, glutamine. It was proposed that the SCD red blood cell is attempting to improve NAD redox potential by increasing transport of glutamine. Analysis of the chemistry of NAD synthesis in red blood cells has suggested that with glutamine supplementation to sickle red blood cells, the NAD synthesis will further increase, which would prevent the sickle red blood cells from being oxidative damaged, and make the sickle red blood cells less adhesive to small blood vessels. This would lead to less obstruction or blockage of small blood vessels, which is a major cause of the problems that sickle cell patients face.
Before we can submit an application to the FDA to obtain marketing approval, we are required to complete the Phase III clinical trial of L-glutamine as a treatment for SCD, analyze all of the data collected during the trial, and demonstrate with such data and other information that the drug product is safe and effective for its intended use. We believe that the Phase III clinical trial will be completed before the end of 2013. Upon confirmation that data demonstrate the safety and effectiveness of our drug, we intend to submit a New Drug Application (NDA) to the FDA. Should the trial be completed by the end of 2013 as anticipated, we believe we will be permitted and able to submit portions of the NDA to the FDA as they are completed on a rolling basis possibly beginning before the end of 2013 or early 2014. A rolling submission is intended to facilitate early review of completed portions of the application; however, the FDA is not obligated to begin review until the FDA receives and accepts or “files” the complete NDA. The FDA has 60 days after its submission to decide whether to file the NDA. If the NDA is filed, the review clock will officially begin from the date the FDA received the original submission. The FDA can refuse to file an application for review if it is incomplete for reasons such as the omission of required clinical study data. Because our product has obtained Orphan Drug Designation and fast-track designation, the FDA is expected to prioritize the review with the intent of completing the review and rendering a decision within six months of the date the NDA is submitted to the FDA.
After reviewing the NDA, the FDA will make a determination as to whether the drug is safe and effective for its intended use(s); whether the benefits of the drug outweigh the risks; whether the drug’s proposed labeling (package insert) is adequate and complete; and whether the methods used in, and the facilities or controls used for, the manufacture, processing, packing, and holding of the drug conform to and are operated or administered in conformity with current good manufacturing practice to ensure that the drug meets the requirements for safety and has the identity and strength, and meets the quality and purity characteristics it is represented to possess. Upon completion of the NDA review, the FDA will either approve or not approve the application, or issue a Complete Response Letter (a letter from the FDA indicating that it cannot approve the application in its present form and informing the applicant of changes that should be made before the application could be approved). If the FDA approves the NDA, we will be able to commercialize the drug.
Should the FDA issue a Complete Response Letter, the FDA is required to provide the basis for its decision and we would have an opportunity to meet with FDA officials to discuss any deficiencies noted in the letter. At that point, we can choose to request a hearing and appeal the agency’s decision, or correct any deficiencies and, if necessary, submit additional data or information, or withdraw the application. Common problems which may delay or prevent the FDA from approving an NDA include, but are not limited to, unexpected safety issues, inadequate data analysis, or the failure, in the FDA’s judgment, to demonstrate a drug’s effectiveness. We may need to conduct additional studies, perhaps studies of more people or, different types of people, or conduct studies for a longer period of time.
Manufacturing issues also may be a reason for a delay or denial of approval. Drugs must be manufactured in accordance with regulations establishing “current good manufacturing practice” (“cGMP”). The FDA inspects manufacturing facilities for compliance with cGMP regulations before deciding whether to approve a drug for marketing. If a facility is not ready for inspection, or cGMP deficiencies are found, FDA approval could be delayed unless and until the deficiencies are corrected.
If the FDA does not approve the NDA, we will not be able to commercialize the drug, which will have a material adverse impact on us. If we must conduct new studies to address any deficiencies identified in a Complete Response Letter, we may not have sufficient funding to conduct such additional studies. While we do not expect that the FDA will reject our NDA or that we would be unable to correct any deficiencies listed in a Complete Response Letter, if either occurs, we may consider whether to market the L-glutamine product as a medical food, which does not require premarket approval. Medical foods are required to be manufactured in compliance with other requirements, such as cGMP, and the food manufacturing facilities must be registered with the FDA. Medical foods do not have to include nutrition information on their labels, and any claims in their labeling must be truthful and non-misleading. We will also continue to develop other products in our pipeline, including the cell sheet cornea technology we are developing with CellSeed, Inc. described below.
5
NutreStore® Business
We currently sell one prescription pharmaceutical product in the United States, the NutreStore® L-glutamine powder for oral solution. NutreStore® is distributed through local treating medical centers and physicians and through contracts with certain federal government agencies. NutreStore® has received FDA approval as a treatment for SBS.
SBS is a condition affecting people who have had half or more of their small intestine surgically removed or who have a congenital defect or a disease affecting the small intestine, such as Crohn’s disease and inflammatory bowel disease. The small bowel plays a significant role in nutrient absorption and those with SBS experience malnourishment due to an inadequate absorption of nutrients and fluids. As described in the K. Migliaccio-Walle 2006 article Economic Implications of Growth Hormone Use in Patients with Short Bowel Syndrome published in Current Medical Research and Opinion in 2006, SBS is a life threatening condition that results in nutritional mal-absorption causing diarrhea, dehydration, weight loss, increased susceptibility to infections, osteoporosis and shortened life expectancy.
According to an article titled Long-term Survival and Parenteral Nutrition Dependence in Adult Patients With the Short Bowel Syndrome which was published in 1999 in Gastroenterology and an article titled Management of Complications in Patients Receiving Home Parenteral Nutrition, which was published in 2003 in Gastroenterology, the standard treatment for SBS for many years has been changes in diet, intravenous (IV) feeding (also called “parenteral nutrition”), vitamin and mineral supplements, and medication to relieve symptoms. Long term IV feeding is expensive, inconvenient for the patient and can be harmful to the body.
In 2004, a new and patented treatment regime was approved by the FDA, which was covered by US Patent No. 5,288,703 (the “SBS Patent”). The treatment is comprised of NutreStore® used in combination with a recombinant human growth hormone approved for SBS and a specialized diet. The recombinant human growth hormone, namely Zorbtive® somatropin (rDNA origin), is administered by injection for four weeks, and NutreStore® is taken orally for 16 weeks. Treatment with NutreStore® alone or with a specialized diet, recombinant human growth hormone alone or with a specialized diet, and recombinant human growth hormone and NutreStore® used in combination with a specialized diet, are all intended to help the small intestine take in more water, electrolytes and nutrients and reduce the volume and frequency of IV feedings and the associated problems. Study results cited in an article entitled Growth Hormone, Glutamine, and an Optimal Diet Reduces Parenteral Nutrition in Patients With Short Bowel Syndrome, A Prospective, Randomized, Placebo-Controlled, Double-Blind Clinical Trial published in Annals of Surgery in 2005 (the “Ann Surg Article”) and cited in the NutreStore® Full Package Information available on our website, show that the treatment has the potential to reduce the mean weekly frequency of intravenous parenteral nutrition from 5.4 days to 1.2 days per week after 4 weeks of treatment and to reduce the required weekly volume and caloric content.
In October 2007, we became the exclusive sublicensee of the SBS Patent for the U.S. market, including the rights to distribute the L-glutamine treatment for SBS under the trademark NutreStore® in the United States. We commercially launched NutreStore® in June 2008 and the patent expired on October 7, 2011.
In December 2010, we were awarded a five-year contract by the U.S. Department of Veterans Affairs for our NutreStore® prescription drug product. The contract is effective from December 15, 2010 to December 14, 2015. The estimated value of the award is $125,000. Pursuant to the agreement, we agreed to sell NutreStore® to the Department of Veterans Affairs from time to time during the term of the agreement at a rate of $201.20 per carton. The discount pricing that we have offered to the Department of Veterans Affairs under this contract is also extended to the Department of Defense, Public Health Service (Indian Health Service), and U.S. Coast Guard customers.
AminoPure® Business
We sell L-glutamine as a nutritional supplement (also called dietary supplement) under the brand name AminoPure® through our indirect wholly owned subsidiary, Newfield Nutrition Corporation. AminoPure® is made up of USP-grade L-glutamine that the body requires when a person is under physical exertion, stress, or is sick. Data from scientific research has shown that L-glutamine helps with gastrointestinal health and to support the body’s natural immune response. In the United States, AminoPure® may be marketed and sold as a nutrient or dietary ingredient that is intended to affect the structure or function of the body, but may not be marketed or sold for the diagnosis, treatment, cure or prevention of any disease.
AminoPure® is currently sold through retail stores in several states and via importers and distributors in Japan. We have started to export AminoPure® to Taiwan, Ghana and South Korea. We added a new distributor in December 2012, Nishimoto Trading Co., Ltd., a leading distributor of Japanese foods in Asian-American communities in the United States, to take advantage of its retail outlet network in the United States.
6
AminoPure® is considered a dietary supplement and subject to regulation under the Dietary Supplement Health and Education Act of 1994 (DSHEA). Under the FDA regulations all domestic and foreign companies that manufacture, test, package, label, or hold, dietary supplements for distribution in the United States, must comply with the cGMP regulations specific to dietary supplements. In addition, the manufacturer, packer, or distributor whose name appears on the label of a dietary supplement is required to submit to the FDA all serious adverse event reports associated with use of the dietary supplement in the United States. Our name appears on the label of AminoPure® and, therefore, we are responsible for making such reports to the FDA if they should occur and we become aware of them. The FDA also regulates dietary supplements with respect to the dissemination of product information, such as labeling, claims, package inserts, and accompanying literature. The FDA shares oversight responsibility for dietary supplements with the Federal Trade Commission (FTC). The FTC regulates dietary supplement advertising, which must be truthful, not misleading, and substantiated.
Sales of AminoPure® have steadily increased in recent years. Sales increased 53.1% in the year ended December 31, 2012 as compared to the year ended December 31, 2011. However, despite the increase in sales of AminoPure®, we have generated minimal revenues from the sale of AminoPure® and NutreStore® since our inception, and the sale of such products are not part of our principal operations.
CellSeed Investment
In January 2009, Emmaus made a strategic investment in CellSeed, Inc. (“CellSeed”), a Japanese company engaged in the research and development, manufacture and sale of temperature-responsive cell culture equipment, which is a cell sheet tissue-engineering platform tool, and application products, as well as cell sheet tissue engineered medical products and application products. Emmaus owns a 1.2% stake in CellSeed, a public company traded on the JASDAQ NEO market in Tokyo, Japan. In April 2011, we entered into a Joint Research and Development Agreement and an Individual Agreement with CellSeed, described below under the heading “Intellectual Property” to pursue collaborative opportunities with CellSeed.
Governmental Regulation of Pharmaceutical and Biotechnology Industries
Regulation by the United States and foreign governmental authorities is a significant factor in the development, manufacture, and expected marketing of our drug product candidates and in our ongoing research and development activities. The nature and extent to which such regulation will apply to us will vary depending on the nature of any drug product candidates developed.
In particular, human therapeutic drug products are subject to rigorous preclinical and clinical testing and other pre-approval requirements of the FDA and similar regulatory authorities in other countries. Various federal and state statutes and regulations govern and influence pre- and post-approval requirements related to research, testing, manufacturing, labeling, packaging, storage, distribution, and record-keeping of such products to ensure the safety and effectiveness for their intended uses. The process of obtaining marketing approval and ensuring post-approval compliance with the applicable federal and state statutes and regulations requires substantial time and financial resources. Any failure by us or our collaborators to obtain, or any delay in obtaining, marketing approval could adversely affect the marketing of any of our drug product candidates, our ability to receive product revenues, and our liquidity and capital resources.
Before obtaining regulatory approvals for the commercial sale of L-glutamine as a treatment for any of our products under development, we must demonstrate through preclinical studies and clinical trials that the product is safe and effective for its intended use in each target indication. The results from preclinical studies and early clinical trials might not be predictive of results that will be obtained in large-scale, pivotal clinical trials. Our clinical trials might not successfully demonstrate the safety and effectiveness of any product candidates or result in marketable products.
In order to clinically test, manufacture, and market products for therapeutic use, we and our third-party collaborators will have to meet the regulatory requirements of each country and jurisdiction in which we intend to market our products. In the United States, the Public Health Service Act and the Federal Food, Drug, and Cosmetic Act, as amended (“FD&C Act”), and the regulations promulgated thereunder, and other federal and state statutes and regulations govern, among other things, the research, testing, manufacture, labeling, packaging, storage, distribution, record keeping, advertising, and promotion of our product candidates. Product development and approval within this regulatory framework takes a number of years and involves the expenditure of substantial resources.
The steps required by the FDA before new drug products may be marketed in the United States include:
| · | completion of preclinical studies; |
| · | the submission to the FDA of our proposal for the design of our clinical trial program. This submission is referred to as an investigational new drug application (“IND”). The IND becomes effective within thirty days after the FDA receives the IND, unless the FDA notifies us that the investigations described in the IND are deficient and cannot begin. The FDA reviews the IND to ensure it adequately protects the safety and rights of study participants, and the design of the studies are adequate to permit an evaluation of the drug's effectiveness and safety; |
7
| · | the conduct of adequate and well-controlled clinical trials, usually completed in three phases, to establish and confirm the safety and effectiveness of a drug candidate for its intended use; |
| · | the submission to the FDA of a marketing application, an NDA if it is a drug product or a Biologics License Application (“BLA”) if it is a biological drug product, that provides data and other information to demonstrate the product is safe and effective for its intended use; and |
| · | the review and approval of the NDA or BLA by the FDA before the product may be shipped or sold commercially. |
In addition to obtaining FDA approval for each product, each product manufacturing establishment must be registered with the FDA and undergo an inspection prior to the approval of a marketing application. Each manufacturing facility and its quality control and manufacturing procedures must also conform and adhere at all times to the FDA’s current good manufacturing practice (“cGMP”) regulations. In addition to preapproval inspections, the FDA regularly inspects manufacturing facilities for post-approval ongoing compliance with cGMP requirements. If, as a result of these inspections, the FDA determines that any equipment, facilities, laboratories, procedures, or processes do not comply with applicable FDA regulations and the conditions of the product approval, the FDA may seek civil, criminal, or administrative sanctions and/or remedies against us, including the suspension of the manufacturing operations and market withdrawal of marketed product. Manufacturers must expend substantial time, money and effort in the area of production, quality assurance, and quality control to ensure compliance with these standards.
Preclinical testing includes laboratory evaluation of the safety of a drug and characterization of the drug product formulation. Preclinical testing results are submitted to the FDA as a part of an IND which must become effective prior to commencement of clinical trials. Clinical trials are typically conducted in three sequential phases following submission of an IND. In Phase I, the drug is initially administered to a small group of humans, either patients or healthy volunteers, primarily to test for safety (e.g., to identify any adverse effects), dosage tolerance, absorption, distribution, metabolism, excretion and clinical pharmacology, and, if possible, to gain early evidence of effectiveness. In Phase II, a slightly larger sample of patients who have the condition or disease being studied receive the study drug to assess the effectiveness of the drug, to determine dose tolerance and the optimal dose range, and to gather additional information relating to safety and potential adverse effects. If the data show the investigational drug may be effective and has an acceptable safety profile in the targeted patient population, Phase III studies, also referred to as pivotal studies, are initiated to further establish clinical safety and provide substantial evidence of the effectiveness of the therapy in a broader sample of the general patient population, to determine the overall risk-benefit ratio of the drug, and provide an adequate basis for physician and patient labeling. During all clinical studies, we must adhere to Good Clinical Practice (“GCP”) standards and applicable human subject protection requirements. The results of the research and product development, manufacturing, preclinical studies, clinical studies, and related information are submitted in an NDA to the FDA.
The process of completing clinical testing and obtaining FDA approval for a new drug is likely to take a number of years and require the expenditure of substantial resources. If an application is submitted, there can be no assurance that the FDA will file, review, and approve the NDA. Even after initial FDA approval has been obtained, post-market studies could be required to provide additional data on safety or effectiveness. Additional pivotal studies would be required to support adding other indications to the labeling. Also, the FDA will require post-market reporting and could require specific surveillance or risk mitigation programs to monitor for known and unknown side effects of the drug. Results of post-marketing programs could limit or expand our ability to market the products. Further, if there are any modifications to the drug product, including changes in indication, manufacturing process, labeling, or the location of the manufacturing facility, an NDA or BLA supplement would be required to be submitted to the FDA prior to or corresponding with that change.
The rate of completion of any clinical trial will be dependent upon, among other factors, the rate of patient enrollment and retention. Patient enrollment is a function of many factors, including the size of the patient population, the nature of the trial, the number of clinical sites, the availability of alternative therapies and drugs, the proximity of patients to clinical sites, and the eligibility and exclusion criteria for the study. Delays in planned patient enrollment might result in increased costs and delays, which could have a material adverse effect on us. Patient retention could be affected by patient non-compliance, adverse events, or any change in circumstances making the patient no longer eligible to remain in the study.
In addition to unforeseen safety issues, failure to comply with applicable FDA requirements could materially and adversely affect us. Failure to adhere to regulatory requirements and GCP standards in conducting clinical trials could cause the FDA to place a “clinical hold” on one or more studies, which would stop the clinical study and delay or preclude further research and data collection necessary for product approval. Noncompliance with GCP standards would also have a negative impact on the FDA’s evaluation of an NDA. Noncompliance could result in the FDA refusing to accept an NDA and, possible enforcement actions if the noncompliance affects the safety of study participants. Enforcement actions could include civil and administrative actions, civil money penalties, criminal prosecution, and criminal fines. Whether or not FDA approval has been obtained, approval of a product by regulatory authorities in foreign countries must be obtained prior to the commencement of marketing of the product in those countries.
8
The requirements governing the conduct of clinical trials and product approvals vary widely from country to country, and the time required for approval might be longer or shorter than that required for FDA approval. Although there are some procedures for unified filings for some European countries, in general, each country at this time has its own procedures and requirements. There can be no assurance that any foreign approvals would be obtained.
In most cases, if the FDA has not approved a drug product candidate for sale in the United States, the unapproved drug product may be exported to any country in the world for clinical study or sale if it meets U.S. export requirements and has marketing authorization in any one of the following countries: Canada, Australia, New Zealand, Japan, Israel, Switzerland, South Africa, or any member nation in the European Union or the European Economic Area, without submitting an export request to FDA or receiving FDA approval to export the drug, as long as the drug meets the regulatory requirements of the country to which the product is being exported. If an unapproved drug product is not approved in one of the listed countries, the unapproved drug may be exported directly to an unlisted country if the drug has valid marketing authorization from the regulatory authority of that country, and FDA determines that the foreign country has statutory or regulatory requirements similar or equivalent to the United States.
In addition to the regulatory framework for product approvals, we and our collaborative partners must comply with federal, state, and local laws and regulations regarding occupational safety, laboratory practices, the use, handling and disposition of radioactive materials, environmental protection and hazardous substance control, and other local, state, federal and foreign regulation. All facilities and manufacturing processes used by third parties to produce our drug candidates for clinical use in the United States must be in compliance with cGMP requirements and are subject to periodic regulatory inspections. The failure of third party manufacturers to comply with applicable regulations could extend, delay, or cause the termination of clinical trials conducted for our drug candidates. The impact of government regulation upon us cannot be predicted and could be material and adverse. We cannot accurately predict the extent of government regulation that might result from future legislation or administrative action.
Outside of the United States, we sell AminoPure® in Japan, Taiwan, South Korea and Ghana. There are no regulatory requirements to sell AminoPure® in Japan because it is classified as a nutritional supplement product. To sell AminoPure® in Taiwan, we are required to obtain a Certificate of Free Sale from the FDA, which we provide to our distributor. FDA issues a Certificate of Free Sale upon request for drug products that either meet the applicable requirements of the FD&C Act and may be legally marketed in the United States or may be legally exported under the FD&C Act although they may not be legally marketed in the United States. Once the Certificate of Free Sale is furnished to our distributor in Taiwan, it is the distributor’s responsibility to comply with local regulations, including but not limited to, obtaining the proper import license. The Certificate of Free Sale for AminoPure® expired on August 16, 2012 and is in the process of being renewed. We are unable to determine whether this lapse may impact our business. In South Korea, AminoPure® is imported and sold as a food supplement which does not require any regulatory approval. In Ghana, AminoPure® is a registered product with the Food and Drug Board of the Ministry of Health.
Clinical Trials
We have conducted a number of clinical trials with the goal of obtaining FDA approval to market and sell L-glutamine as a treatment for SCD. In July 1994, Dr. Niihara conducted a pilot clinical trial using L-glutamine as an oral supplement to SCD patients. The study data supported the hypothesis that oral consumption of L-glutamine by SCD patients may increase the concentration of the reduced form of NAD, NADH, and its redox status. Clinically, all patients who participated in the study reported various levels of increased energy and decreases in the severity of chronic pain with treatment.
In a small pilot Phase II, 12-week open label study conducted in 1995 by Dr. Niihara, the data again showed a decrease in the incidence and severity of sickle cell crisis in selected patients who experienced unusually frequent episodes of sickle cell crisis. A small Phase II blind clinical trial to assess the reduction of sickle cell crisis and chronic pain, which started in 1997 and was funded by the National Institutes of Health, the results of which were released in January 2003, also showed a reduction in sickle cell crises. Another Phase II small open label clinical trial directed to exercise tolerance, which started in 2000 and was funded by the FDA, the results of which were reported to the FDA in April 2009, also indicated that patients on L-glutamine had improved physical stamina with no significant side effects.
In April 2009, Emmaus completed an 80 patient Phase II controlled, double blind clinical trial funded in large part by the FDA to measure the reduction in the incidence of sickle cell crisis as its primary endpoint. The trial took place at the following institutions: the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center (“LABioMed”); Emory University, Atlanta, Georgia; Kaiser Permanente, Bellflower, California; the University of Medicine and Dentistry of New Jersey (Robert Wood Johnson Medical School), New Brunswick, NJ; and the Jacobi Medical Center-North Bronx Healthcare Network, Bronx, New York. This study showed clinical significance for reducing the incidence of sickle cell crisis (more than a 50% reduction in incidence of sickle cell crisis) but due to a higher than expected drop out rate, the study endpoints did not achieve statistical significance, except for the reduction in hospital admittance at 24 weeks. However, the safety of L-glutamine in this trial, as in previous trials, continued to be demonstrated. This Phase II clinical trial was managed by the contract research organization, ClinDatrix, Inc.
9
In April 2009, after consultation with the FDA, we initiated a large Phase III clinical trial to study L-glutamine as an experimental agent to reduce sickle cell crisis. Patient enrollment began in mid-2010 and by December 2012, we had enrolled over 230 patients, thus completing enrollment. An interim analysis of a subset of data from the Phase III clinical trial was completed by an independent third party. The results of the interim analysis were then reported to the FDA by way of the independent third party in August 2012. A meeting was held with the FDA on November 5, 2012. The Company received unanimous support to continue the trial without any modification to protocol. We aim to complete the trial in 2013. This Phase III clinical trial is managed by ClinDatrix, Inc.
Status of FDA Approvals and Orphan Drug Designation
The FDA has already approved L-glutamine as a treatment for short bowel syndrome under Section 505(b)(1) of FD&C Act, which requires a “full” NDA that contains all original data produced by or owned by the applicant. Emmaus intends to proceed under Section 505(b)(2) of the FD&C Act, which allows an applicant to rely on data in published literature, or owned, or submitted to FDA by another person. Consequently, we believe we will be able to rely on certain data in the approved application for L-glutamine as a treatment for short bowel syndrome and will need to submit only our Phase III clinical trial data demonstrating a reduction of sickle cell crises.
The Food and Drug Administration Modernization Act of 1997 codified the FDA’s policy of granting “fast track” review of certain therapies targeting “orphan” indications and other therapies intended to treat severe or life threatening diseases and having potential to address unmet medical needs. The Orphan Drug Act (“ODA”) provides several financial benefits to persons developing an orphan drug, which is a drug intended to treat a rare disease or condition, including seven years market exclusivity and waiver of filing fees. A drug may be designated as an “orphan drug” if it is intended for the safe and effective treatment, diagnosis, or prevention of rare diseases/disorders that affect fewer than 200,000 people in the United States, or drugs for the treatment of diseases/disorders that affect more than 200,000 persons but for which the drug developer is not expected to recover the costs of developing and marketing the drug from the commercialization and sale of such drug.
We applied for Orphan Drug Designation on June 11, 2001, and the FDA granted it on August 1, 2001. There is no expiration date on the status of the Orphan Drug Designation. This designation waives the new drug application fee (presently over $1 million) and annual establishment and product fees, which were $520,100 and $98,970, respectively, for the fiscal year ended December 31, 2012, provided a 50% tax credit for clinical work and, if the product is approved, will provide us exclusive marketing rights for the SCD indication for seven years. The seven years of marketing exclusivity is measured from the date of the approval of the application or the issuance of the license and runs concurrently with any remaining patent term. Because we were granted Orphan Drug Designation, the 50% tax credit is absolute and is provided pursuant to the ODA, which provides tax credits for testing expenses for drugs for rare disease or conditions, and the Internal Revenue Code, which specifically provides a tax credit equal to 50% of the qualified clinical testing expenses for the taxable year.
The waiver of the annual establishment fee under section 526 of the FD&C Act, as amended by the Food and Drug Administration Amendments Act of 2007 (the “FDAAA”), applies if the orphan drug meets all of the following conditions: the drug had received Orphan Drug Designation from the FDA; and the drug is owned or licensed and is marketed by a company that had less than $50,000,000 in gross worldwide revenue during the previous year. We are eligible for waivers of such fees because the experimental treatment for SCD meets all of the above conditions: (1) L-glutamine treatment for SCD received orphan designation under on August 1, 2001 and (2) the L-glutamine treatment for SCD is licensed by Emmaus Medical, Inc. whose gross worldwide revenue during the previous 12 months was less than $50,000,000. We expect to continue to meet such conditions in the future and to remain eligible to receive waivers of the establishment and product fees. However, a marketing application for an orphan drug product is not eligible for fee waivers if the application includes another indication that is not for a rare disease or condition.
We have obtained Fast Track Designation for the L-glutamine therapy for SCD. Fast Track Designation will provide us with many advantages over the normal FDA approval process, including the right to submit completed sections of the NDA before the entire NDA has been completed (referred to as a “rolling submission” or “rolling review”), and the opportunity to have more FDA interaction.
Fast track is a process designed to facilitate the development, and expedite the review of, drugs to treat serious diseases and fill an unmet medical need. The purpose is to get important new drugs to the patient sooner. As long as we are able to maintain Fast Track Designation, we will be eligible for some or all the following:
| · | More frequent meetings with the FDA to discuss the drug’s development plan and ensure collection of appropriate data needed to support drug approval; |
| · | More frequent written correspondence from the FDA about such things as the design of the proposed clinical trials; |
10
| · | Accelerated Approval, i.e., approval on an effect on a surrogate, or substitute endpoint reasonably likely to predict clinical benefit; |
| · | Rolling review, which means that we can submit completed sections of the NDA for review by FDA before the entire application is completed. NDA review usually does not begin until the entire application has been submitted to the FDA; and |
| · | Priority Review, which is an expedited review that the FDA generally completes within six months after the date the completed NDA is originally submitted to FDA. The normal review process typically takes 10-12 months after submission of the NDA. |
We anticipate completing our Phase III clinical trial in late 2013 and that we will begin submitting completed portions of the NDA for rolling review before the end of 2013. If we receive Priority Review of our NDA and no application deficiencies are identified by FDA, we expect to receive an approval decision within six months after the completed NDA is filed, which we estimate would be in 2014. We intend to communicate with the FDA on a regular basis to ensure that questions and issues are resolved quickly, which may help lead to earlier drug approval and access by patients.
The expected cost to obtain FDA approval is difficult to estimate because it is a dynamic process. Based on our assumption of receiving a Priority Review, we estimate that our regulatory and administrative costs to obtain FDA approval will be approximately $400,000. Actual costs can vary greatly from this figure based on how the FDA review process proceeds. The bulk of these costs would be associated with the consultants retained by us to assist with the FDA application and response process. Should the FDA find deficiencies with our study data, additional studies may be required. Additionally, if the FDA finds issues with the manufacturing of the product, such issues would need to be resolved prior to the approval of the NDA. The occurrence of either of these events would increase the costs dramatically.
Product Sourcing and Packaging
We plan to obtain our USP-grade L-glutamine from Ajinomoto Aminoscience LLC, a subsidiary of Ajinomoto U.S.A. (collectively, “Ajinomoto”), a Japanese food, amino acid and pharmaceutical company, and from Kyowa Hakko, a Japanese pharmaceutical company. Ajinomoto and Kyowa Hakko together produce the vast majority of USP-grade L-glutamine approved for sale in the United States. The manufacturing of large quantities of USP-grade L-glutamine is a complex and expensive undertaking, and is therefore not an easy market for third parties to enter. As a result, there are limited alternative suppliers from whom we could obtain the USP-grade L-glutamine required to manufacture our current products and SCD treatment product under development.
Ajinomoto, through its parent company, the Ajinomoto Company, has previously provided L-glutamine to us free of charge for our clinical work, including our completed Phase II clinical trials. Ajinomoto is also providing L-glutamine for our Phase III clinical trials without charge. Pursuant to a letter of intent between Emmaus Medical and Ajinomoto, we agreed to purchase or cause relevant third party purchasers to purchase from Ajinomoto all of the L-glutamine that we will need for our commercial products. Pursuant to the letter of intent, we will be permitted to source L-glutamine from third party suppliers of up to 10% of our requirement for L-glutamine on a back-up basis. However, if a third party competitor of Ajinomoto offers us a more favorable pricing on L-glutamine of a similar grade with similar terms and conditions, we may ask Ajinomoto to adjust its pricing. Although the letter of intent contemplates that we will enter into a supply agreement with Ajinomoto, we have not yet entered into the supply agreement.
We currently source L-glutamine from Kyowa Hakko U.S.A. for our NutreStore® product. We do not have an agreement with Kyowa Hakko for the supply of L-glutamine. We purchase L-glutamine from Kyowa Hakko on an as-needed basis pursuant to individual purchase orders.
Eventually we plan to enter into exclusive long term supply contracts with these manufacturers for L-glutamine for SCD treatment that will require these companies to agree not to sell L-glutamine as a nutritional supplement or pharmaceutical for sickle cell disease applications to third parties. However, there is no assurance that we will be able to obtain such terms or economically attractive terms for obtaining pharmaceutical grade L-glutamine from these proposed suppliers, or that the suppliers will not experience an interruption in supply that could materially and adversely affect our business.
Our products will be packaged by a third party facility in compliance with FDA requirements. Anderson Packaging, Inc., of Rockville, Illinois, has handled the packaging for our Phase II and III clinical trials of L-glutamine for SCD and we plan to use the same company for commercial packaging of the product. Anderson Packaging, Inc. packaged L-glutamine for the clinical trials that resulted in the FDA’s marketing approval for L-glutamine for short bowel syndrome using the same dose and packaging protocol as the Company expects to use for treatment of SCD. Prior FDA approval of packaging types and protocols does not guarantee future approval of packaging types and protocols.
Sales and Marketing
We have one full time pharmaceutical sales representative. Our sales representative is in constant contact with management and other employees at our headquarters to share current product information and sales strategies to assist with any immediate patient and physician needs.
11
As we expand our sales of L-glutamine for SBS and commercialize our L-glutamine treatment for SCD, we intend to increase the size of our sales staff. We intend to employ experienced sales representatives in the United States to promote our prescription pharmaceuticals. Sales representatives will receive appropriate training, education and development to ensure they have current knowledge of our products, approved labeling, disease states, policies and procedures as well as the current Compliance Program Guidance for Pharmaceutical Manufacturers published by the U.S. Department of Health and Human Services, Office of Inspector General, the provisions of the Code on Interactions with Healthcare Professionals created by the Pharmaceutical Research and Manufacturers of America (“PhRMA Code”), and the FDA’s regulatory limitations on promotional activities. We also intend to seek partnerships if deemed necessary.
If we obtain FDA approval for the SCD indication, we intend to focus our sales and marketing efforts across several different groups, including patients, physicians, health care providers, hospitals, treatment centers, insurance carriers, non-profit associations, and collaborating pharmaceutical companies. Our in-house product specialists and sales representatives will focus on the following tasks as part of our marketing strategy:
· | promote our L-glutamine therapy to SCD specialist physicians; |
· | promote awareness of our L-glutamine therapy at all U.S. community-based treatment centers; |
· | develop L-glutamine therapy collateral materials and informational packets to educate patients and physicians and garner industry support; |
· | establish collaborative relationships with non-profit organizations that focus on SCD; and |
· | identify international opportunities for our L-glutamine therapy. |
Our target customers for NutreStore® are SBS patients, as well as their local treating medical centers and physicians. Patient and physician awareness of the NutreStore® brand will be key to our success. We intend to exhibit at trade shows and other events and maintain websites with current information on NutreStore® to strengthen this brand. In addition, we will work with patient support organizations, such as the Oley Foundation, to promote our SBS treatment.
Research and Development
For the years ended December 31, 2012 and 2011, we expended $3.0 million and $1.6 million, respectively, in research and development costs related primarily to our L-glutamine treatment for SCD.
The estimated cost to complete the Phase III clinical trial we are currently conducting for our L-glutamine treatment for SCD is $4.0 million. This estimate is based on the total number of active clinical trial sites and study participants on protocol across the United States and assumes that the trial is conducted in accordance with our projected timeline to complete the trial by the end of 2013. Should the trial take longer than expected to complete or we use more trial sites than currently anticipated, our costs related to the Phase III trial will increase.
We believe that the research and development cost for corneal cell sheet technology in the United States is approximately $3.0 million. Such estimate includes the cost of obtaining FDA approval for the corneal cell sheets. We have assumed that we will need obtain approval through the BLA process for the corneal cell sheets, which is considered a biological drug product, rather than pharmaceutical approval, and that we will only have to run a small trial here in the United States to test the safety and efficacy of the corneal cell sheets because we believe that we will be able to submit, and the FDA will accept, the data regarding corneal cell sheets submitted to the European Medicines Agency (“EMA”).
The estimated cost to initiate the cardiac cell sheet work is $2.8 million. This estimate is based on our current plan to work with regulatory consultants and medical advisors. We expect that these costs will increase in the future when the clinical study program has been developed.
Intellectual Property
We rely on a combination of patent licenses, trademark and trade secret protection and other unpatented proprietary information to protect our intellectual property rights and to maintain and enhance our competitiveness in the pharmaceutical industry. While we do not currently have any issued patents, we have 2 patent licenses with third parties. We have submitted patent applications which have yet to be published.
12
We also rely on unpatented technologies to protect the proprietary nature of our products. We require that our management team and key employees enter into confidentiality agreements that require the employees to assign the rights to any inventions developed by them during the course of their employment with us. All of the confidentiality agreements include non-solicitation provisions that remain effective during the course of employment and for periods following termination of employment.
Licenses and Promotional Rights Agreements
On March 1, 2001, Emmaus Medical became the exclusive worldwide licensee of U.S. Patent No. 5,693,671, entitled “L-glutamine Therapy for Sickle Cell Disease and Thalassemia” (the “SCD Patent”) to develop a treatment for SCD and thalassemia using L-glutamine pursuant to a license agreement. The license agreement is effective until the expiration of the SCD Patent, which will be in 2016 unless a patent term extension is granted by the United States Patent & Trademark Office due to regulatory (e.g., FDA) delay related to product commercialization. The maximum possible term extension one can receive is five years. Pursuant to the license agreement, we acquired the exclusive right to test, gain governmental approval of, make, have made, use, distribute and sell products designed for use in carrying out methods covered under the SCD Patent (“Licensed Methods”) and/or incorporating technical information provided by the licensor or by any of certain doctors affiliated with the licensor (“Licensed Products”). Pursuant to an addendum to the license agreement, we agreed to pay royalties to the licensor during the term of the agreement equal to 4.5% of net sales of Licensed Products in the United States until lifetime royalty payments made to the licensor total $100,000, at which time the royalty rate will decrease to 2.5% of net sales of the Licensed Products. No royalties will be paid to the licensor for Licensed Products sold or distributed, or Licensed Methods practiced, on a non-profit basis. Royalty payments are due within 45 days of the end of each fiscal quarter, with the last payment due 45 days after the termination of the agreement. Any payments not made when due accrue interest on and after the due date at a rate equal to the prime interest rate quoted by the Bank of America on the date the payment is due, with interest being compounded on the last day of each calendar quarter.
We are also responsible for paying all fees and costs relating to the maintenance of the SCD Patent. Before any Licensed Products are commercially sold or Licensed Methods are practiced on humans, we are required to obtain comprehensive general liability insurance policies, including product liability insurance coverage in the minimum amount of $0.5 million. If we fail to obtain the required insurance policies, the licensor may terminate the agreement or obtain such insurance at our sole cost and expense. We currently have product liability insurance for NutreStore® and also have clinical trial insurance for the SCD study.
The license agreement will terminate upon the expiration of the patent in 2016 unless an extension is granted, or earlier upon a court’s determination that the patent is invalid or unenforceable. If we fail to pay royalties when due and payable, the licensor may terminate the agreement upon 90 days’ written notice, unless we pay all outstanding royalties and interest due, during such 90-day period. The licensor may also terminate the agreement upon our material breach of the agreement upon providing us with 90 days’ written notice. The agreement will automatically terminate at the end of such 90-day period unless we cure the breach or default during such period.
Currently, we estimate we will need approximately $4.0 million to complete our Phase III clinical trial and $400,000 to obtain regulatory approval of L-glutamine as a therapy for SCD.
In October 2007, Emmaus Medical became the exclusive sublicensee of US Patent No. 5,288,703 (the “SBS Patent”) for the U.S. market, including the rights to distribute the L-glutamine treatment for SBS under the trademark NutreStore® in the United States (the “SBS License”). We commercially launched NutreStore® in June 2008. The SBS License was amended in February 2011 and ownership of the NutreStore® NDA and Drug Master Files ("DMF") containing the proprietary information relating to the manufacturing and packaging specifications of NutreStore® product were transferred to us. Pursuant to the SBS License, as amended by an assignment and transfer agreement, we are required to pay a royalty of 10% of adjusted gross sales of NutreStore® to Cato Holding Company (“Cato”) through 2016. We are also required to pay to Cato a royalty of 1% of gross sales of L-glutamine as a treatment for SCD and thalassemia for a period of five years from the date of the first commercial sale of such product as outlined in the Sublicense Agreement with Cato. The SBS License is subject to a sublicense that Cato holds from Ares Trading, S.A. (the “Ares License”).
The SBS Patent expired on October 7, 2011 and the SBS License terminated on the date the SBS Patent expired. We agreed to pay the royalties to Cato noted above after the expiration of the SBS License from 2012 to 2016 in consideration of the transfer of the NDA for NutreStore® from Cato to us, the transfer of the NutreStore® related trademarks from Cato to us, the transfer of the NutreStore Drug Master File (“DMF”) Nos. 16633 and 16639 from Cato to us and for the know-how represented by the NutreStore® NDA and DMFs.
We do not anticipate that the expiration of the SBS License will have a significant impact on us and we intend to sell NutreStore® in accordance with the terms of the assignment and transfer agreement. We currently do not believe there should be significant competition in the marketplace for a generic version of NutreStore® given the small population of SBS patients (<10,000 adults).
On April 8, 2011, Emmaus Medical entered into a Joint Research and Development Agreement (the “Research Agreement”) and an Individual Agreement for CAOMECS (the “Individual Agreement”) with CellSeed. Pursuant to the Research Agreement, Emmaus Medical and CellSeed formed a relationship regarding the future research and development of cell sheet engineering regenerative medicine products (the “Products”) and the future commercialization of such Products. In particular, Emmaus Medical and CellSeed are interested in the joint research and development of Cultured Autologous Oral Mucosal Epithelial Cell-Sheet (“CAOMECS”) for generated medicine of cornea cells, and potentially Cell-Sheets for Cardiac Muscle Regeneration and Regenerated Cartilage Sheets.
13
The parties will enter into separate individual agreements for each project or task conducted pursuant to the Research Agreement defining the details of such project. CellSeed will transfer to Emmaus laboratories in the United States the engineering and know-how technology necessary for Emmaus Medical to create cell sheets under the Individial Agreement and each future individual agreement.
All intellectual property rights created in the course of the Research Agreement, the Individual Agreement and any future individual agreement, including rights made jointly by the employees of Emmaus Medical and CellSeed or made solely by the employee(s) of the other party based on confidential information or intellectual property rights exchanged between the parties, will be owned jointly by Emmaus Medical and CellSeed. Intellectual property rights related to the Products that are developed solely by one party’s employees independently from confidential information and intellectual property rights of the other party, shall be owned by the party whose employees made such invention, provided however, that such party will grant a worldwide, perpetual, irrevocable, non-exclusive, royalty free, fully paid up, sub-licensable, transferable license of such rights to the other party.
Pursuant to the Research Agreement, Emmaus Medical agreed to pay CellSeed $8,500,000 (the “Research Agreement Payment”) within 30 days of the completion of all of the following: (i) the execution of the Research Agreement; (ii) the execution of the Individual Agreement; and (iii) CellSeed’s delivery of the accumulated information package, as defined in the Research Agreement, to Emmaus. Also, pursuant to the addendum to the Research Agreement, CellSeed and Emmaus Medical further acknowledged that all obligations of CellSeed corresponding to the Research Agreement Payment shall be fully discharged by CellSeed’s delivery of the applicable accumulated information package, which delivery shall be documented by written confirmation of acceptance by Emmaus Medical. The Company is obligated to make the Research Agreement Payment only after Emmaus Medical provides its written confirmation of acceptance of the complete accumulated information package to CellSeed.
Pursuant to the Individual Agreement for CAOMECS, CellSeed granted Emmaus Medical an exclusive right to manufacture, sell, market and distribute CAOMECS for the cornea in the United States. CellSeed agreed to disclose its accumulated information package for the joint development of CAOMECS to Emmaus Medical. Also pursuant to the Individual Agreement, Emmaus Medical agreed to pay $1,500,000 to CellSeed and a royalty to be agreed upon by the parties. The Company made the Individual Agreement payment of $1,500,000 to CellSeed in February 2012.
The parties will determine the rate at which profits from the net sales of CAOMECS in the United States will be split between the parties. The Individual Agreement will remain in effect until CellSeed’s patents used for the CAOMECS expire in the United States in February 2023 and February 2024, unless terminated earlier by the parties.
Cell sheet technology is a method that allows us to grow and harvest tissue in a sheet form rather than individual cells. Clinical trials to repair damaged cornea and heart muscle using cell sheets have been initiated by CellSeed in Japan and Europe. A relatively large single center clinical trial entitled “Multicenter Study of Cultured Autologous Oral Mucosal Epithelial Cell-sheet (CAOMECS) Transplantation to Patients with Total Limbal Stem Cell Deficiency,” was conducted from 2007 to 2011 at the Les Hospices Civils De Lyon in France involving 25 patients with limbal stem cell deficiencies (“LCSD”) who have suffered loss of vision. The Company believes that this treatment may provide a cure for a group of patients diagnosed with LSCD, which is currently considered incurable. An interim report of the results of the study was presented at the World Cornea Congress in April 2010. CellSeed issued a press release in April 2010 regarding such interim report, which can be viewed on CellSeed’s website at www.cellseed.com.
Although CellSeed had submitted an application for the marketing authorization to the EMA in June 2011, it has formally notified the EMA of its decision to withdraw its application. Additional information on the withdrawal is available at the EMA web site.
We intend to apply for FDA approval of the corneal cell sheets, for which we have the exclusive rights to manufacture, sell, market and distribute in the United States pursuant to the Individual Agreement.
Currently, cell sheets for cornea cells and cardiac cells are not being sold. The potential market for the cornea cell sheet products includes patients with damaged corneas, which we believe includes a small percentage of the approximate 40,000 corneal transplants in the United States each year, based on information published in the National Eye Institute section of the National Institutes of Health website. The potential market for cardiac cell sheets includes the approximate 3,000 persons who are awaiting heart transplants in the United States, according to the section of the National Heart, Lung and Blood Institute’s website regarding heart transplants.
While many large pharmaceutical and biotechnology companies throughout the world, especially in Europe, have contracts with CellSeed to promote this technology, Emmaus is the first company to obtain the marketing and development contracts with CellSeed for this technology in the United States The principal steps to develop the corneal and cardiac cell sheets include engaging a manufacturer compliant with applicable cGMP regulations, obtaining FDA approval of the products, training physicians who will use the products and perform procedures with the products and promoting the availability of the products.
14
The FDA has different divisions that review products and the designation of the review division would depend on the classification of the product. Biological products include a wide range of things such as vaccines, blood and blood components, allergenics, toxins, antitoxins, human cells, tissues and cellular and tissue-based products including gene therapy, and recombinant therapeutic proteins. Biologics can be composed of sugars, proteins, or nucleic acids or complex combinations of these substances, or may be live cells and tissues. The review and approval process for biologics is handled by the FDA’s Center for Biologics Evaluation and Research (“CBER”). Pharmaceutical drugs are typically chemically synthesized and have a known structure, whereas most biologics are complex mixtures that are not as easily identified or characterized. The review and approval process for pharmaceuticals (“pharmaceutical approval”) is generally handled by the FDA’s Center for Drug Evaluation and Research (“CDER”). Although the review process for biologics and pharmaceuticals is generally handled by different Centers, the regulatory pathway is similar. We believe the corneal cell sheets will be considered biological products for which a BLA submission to CBER for review and approval will be required.
If FDA approval is obtained, we estimate that we will need an additional $2.0 million to commercialize the corneal cell sheet technology. Based on data available for cornea treatment using this technology, we anticipate it will be two to three years before we may be able to submit a BLA and obtain a marketing decision from the FDA to commercialize this product in the United States.
We plan to participate in a multi-center international study involving cardiac cell sheets within the next two years and, if the studies are successful, we anticipate that we would be able to submit an application for marketing approval to the FDA to commercialize these products in about five years. We estimate that we will need $2.8 million to initiate the cardiac cell sheet work. The total estimated costs for development and commercialization of the cardiac cell sheets has not yet been determined. In addition, we estimate that we will need $2.5 million for research related to other cell sheet applications and cGMP laboratory costs to establish the infrastructure and production capabilities related to regenerative medicine products.
Trademarks
We currently own three U.S. trademarks, including “Emmaus Medical,” “NutreStore” and “AminoPure,” a Japanese trademark for “AminoPure,” and a Philippines trademark for “AminoPure.” We are also pursuing trademark registration for “AminoPure” in South Korea, Taiwan, Ghana and Kenya.
Our success will depend in part on our ability to obtain patents and preserve other intellectual property rights covering the design and operation of our products. We intend to seek patents on our products when we deem it commercially appropriate. The process of seeking patent protection can be lengthy and expensive, and there can be no assurance that patents will be issued for currently pending or future applications or that our existing patents or any new patents issued will be of sufficient scope or strength or provide meaningful protection or any commercial advantage to us. We may be subject to, or may initiate, litigation or patent office interference proceedings, which may require significant financial and management resources. The failure to obtain necessary licenses or other rights or the advent of litigation arising out of any such intellectual property claims could have a material adverse effect on our operations.
Competition
The development and commercialization of pharmaceutical products is very competitive and characterized by extensive research efforts and rapid technological progress. Competition in our industry occurs on a number of fronts, including developing and bringing new products to market before our competitors, developing new products to provide the same benefits as existing products at lower cost and developing new products to provide benefits superior to those of existing products. We face competition from other pharmaceutical companies, particularly those that provide alternative drugs to treat SCD and SBS, as well as other entities that develop alternative therapies and dietary supplements that could limit the market for our L-glutamine product.
We currently face two competing treatments for SCD treatment, one of which being Bristol-Myers Squibb’s Hydroxyurea and the other being bone marrow transplants. Additionally, gene therapy techniques hold promise as a potential treatment for a variety of genetic diseases, including SCD; however, there are currently many questions about the effectiveness of gene therapy to treat diseases such as SCD and when such therapies could become available.
Presently, the most common therapy for patients with SBS is parenteral nutrition. However, as outlined above, Emmaus’ product NutreStore®, when used in conjunction with a recombinant human growth hormone approved for this indication and a specialized diet, can be used to reduce the volume and frequency of parenteral nutrition therapy for most patients. In December 2012, NPS Pharmaceuticals Inc. announced that its product, Gattex, won U.S. approval as a treatment for patients with short-bowel syndrome to help absorb nutrients. The impact of this new product is not yet determined.
Because L-glutamine is currently sold as a nutritional supplement, there is risk that both of the Company’s pharmaceutical products for treatment of SCD and SBS may experience competition with providers of L-glutamine in nutritional supplement form. In fact, when dealing with a method patent directed to new uses for old compounds, there is always a risk that the medication can be obtained from unauthorized sources, and sold at cut-rate prices, known as the “generic leakage” problem. More generally, generic leakage results when a barrier to competition is suddenly eliminated, e.g., a patent expires or is invalidated, thus exposing the formerly price protected (and relatively expensive) product to competition from relatively inexpensive products. As a result, consumers will tend to purchase more of the cheaper product and less of the expensive product. However, the Company believes generic leakage will not be a major factor for a number of reasons, including but not limited to insurance/reimbursement factors, pricing strategies, regulatory barriers to market entry, state laws governing the prescribing and dispensing of pharmaceuticals and the substitution of therapeutic equivalents, distribution mechanisms, FDA-market exclusivity protections, intellectual property protections, and other factors inherent in the FDA regulatory differences between pharmaceuticals and nutritional supplements including but not limited to advertising laws and manufacturing practices. The FDA market exclusivity protection that we would be eligible to receive is based on Orphan Drug Designation granted by the FDA.
15
Our competitors may have products that have been approved or are in advanced development and may succeed in developing drugs that are clinically superior in that they are more effective, safer, more easily administered than ours and more affordable, or that achieve commercialization sooner than our products.
Employees
As of December 31, 2012, we had 15 employees, 14 of whom are full time, and three consultants. We have not experienced any work stoppages and we consider our relations with our employees to be good.
The following risk factors should be considered in conjunction with the other information included in this Annual Report on Form 10-K. This report may include forward-looking statements that involve risks and uncertainties. In addition to those risk factors discussed elsewhere in this report, we identify the following risk factors, which could affect our actual results and cause actual results to differ materially from those in the forward-looking statements.
RISKS RELATED TO OUR OPERATIONS
We have incurred losses since inception, have limited cash resources and anticipate that we will continue to incur substantial losses for the foreseeable future.
We are in the development stage. As of December 31, 2012, we had an accumulated deficit of $36.0 million since our inception in 2000. Our net losses were $14.4 million and $8.8 million for the years ended December 31, 2012 and 2011, respectively. These losses resulted principally from costs incurred in our research and development programs and from our general and administrative expenses. We have had limited revenue and have sustained significant operating losses since inception, and are likely to sustain operating losses in the foreseeable future. Since inception, we have funded our operations through the private placement of equity securities, convertible notes and loans from stockholders and expect that we will continue to fund our operations through public or private equity or debt financings or other sources, such as strategic partnerships. Such financings may not be available in amounts or on terms acceptable to us, if at all. Our failure to raise capital as and when needed would inhibit our ability to continue operations and implement our business strategies.
We expect to continue to incur significant and increasing negative cash flow and operating losses as we continue our research activities, conduct clinical trials, and seek regulatory approvals for our L-glutamine treatment for SCD. These losses, among other things, have had and will continue to have an adverse effect on our stockholders’ equity, total assets and working capital. Because of the numerous risks and uncertainties associated with drug development, we are unable to predict the extent of any future losses, whether or when we will be able to commercialize our L-glutamine treatment for SCD, or when we will become profitable, if at all. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis.
Our recurring operating losses have raised substantial doubt regarding our ability to continue as a going concern.
Our recurring operating losses raise substantial doubt about our ability to continue as a going concern. As a result, our independent registered public accounting firm included an explanatory paragraph in its report on our financial statements as of and for the year ended December 31, 2012 with respect to this uncertainty. The continuation and expansion of our business is dependent upon obtaining further financing, successful and sufficient market acceptance of our products, and, finally, achieving a profitable level of operations. The perception of our ability to continue as a going concern may make it more difficult for us to obtain financing for the continuation of our operations and could result in the loss of confidence by investors, suppliers and employees.
We will require substantial additional funding and may be unable to raise capital when needed, which could force us to delay, reduce or eliminate planned activities or result in our inability to continue as a going concern.
We will require additional capital to pursue planned clinical trials and regulatory approvals, as well as further research and development and marketing efforts for our products and potential products. Our future capital requirements will depend on, and could increase significantly as a result of, many factors, including:
16
· | the duration and results of the clinical trials for our various products going forward; |
| · | unexpected delays or developments in seeking regulatory approvals; |
| · | the time and cost in preparing, filing, prosecuting, maintaining and enforcing patent claims; |
| · | other unexpected developments encountered in implementing our business development and commercialization strategies; and |
| · | the outcome of litigation and further arrangements, if any, with collaborators. |
We may attempt to raise additional funds through public or private financings, collaborations with other pharmaceutical companies or financing from other sources. Additional funding may not be available on terms which are acceptable to us. If adequate funding is not available to us on reasonable terms, we may need to delay, reduce or eliminate one or more of our product development programs or obtain funds on terms less favorable than we would otherwise accept.
We have had limited revenue, have sustained significant operating losses, and are likely to sustain operating losses in the foreseeable future. As of December 31, 2012, we had cash and cash equivalents of approximately $0.4 million. We anticipate that we will continue to fund our operations through public or private equity or debt financings or other sources, such as strategic partnership agreements. Such financings may not be available in amounts or on terms acceptable to us, if at all. There is substantial doubt about our ability to continue as a going concern as the continuation and expansion of our business is dependent upon obtaining further financing, successful and sufficient market acceptance of our products, and, finally, achieving a profitable level of operations.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights.
We may seek additional capital through a combination of private and public equity offerings, debt financings and collaborations and strategic and licensing arrangements. To the extent that additional capital is raised through the sale of equity securities or securities convertible into or exchangeable for equity securities, the issuance of those securities could result in dilution to our stockholders. The terms of such securities may include liquidation or other preferences that could adversely affect the rights of our existing shareholders. Moreover, the incurrence of debt financing could result in a substantial portion of our future operating cash flow, if any, being dedicated to the payment of principal and interest on such indebtedness and could impose restrictions on our operations. This could render us more vulnerable to competitive pressures and economic downturns.
Debt financing, if available, would result in increased fixed payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring debt, making capital expenditures or declaring dividends. If we raise additional funds through collaboration, strategic alliance and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or product candidates, or grant licenses on terms that are not favorable to us.
In addition, if we do not meet our payment obligations to third parties as they come due, we may be subject to litigation claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and would be a distraction to management, and may result in unfavorable results that could further adversely impact our financial condition.
Our business is subject to extensive government regulation, which could cause delays in the development and commercialization of our drug products, impose significant costs on us or provide advantages to our larger competitors.
The FDA and similar regulatory authorities in foreign countries impose substantial requirements upon the development, manufacture, and marketing of drugs. The FDA and most other regulatory authorities impose requirements for laboratory and clinical testing, manufacturing, labeling, registration, marketing, distribution, recordkeeping, reporting, and advertising and promotion, and other costly and time-consuming processes and procedures, applicable to drug products. Satisfaction of approval commitments, if any are imposed by the FDA as a condition of approval, could take several years or more and varies substantially from country to country as well as upon the type, complexity and novelty of the therapeutic product. Post-approval requirements regarding safety surveillance, cGMP compliance, advertising and promotion, adverse event reporting, and recordkeeping must be maintained at all times.
The effect of government regulation may be to delay approval of marketing of products for a considerable or indefinite period of time, to impose costly processes and procedures upon our activities, and to furnish a competitive advantage to larger companies that compete with us. There can be no assurance that marketing authorization for any of our drug products (including biological drug products) would be granted by the FDA or other regulatory authority on a timely basis, if at all, or, once granted, that the marketing authorization would not be withdrawn or other regulatory actions taken which might limit our ability to market our proposed products. Any such delay in obtaining or failing to obtain such authorizations or imposition of regulatory actions would adversely affect us, the manufacturing and marketing of the products we intend to develop, and our ability to generate product revenue.
17
We cannot assure you that we will be able to complete our clinical trial programs successfully within any specific time period or at all and if such clinical trials take longer to complete than we project, our ability to execute our current business strategy will be adversely affected.
We do not know if our current clinical trials for our L-glutamine treatment for SCD or future clinical trials for other of our contemplated products will be completed on schedule or at all. Even if completed, we do not know if these trials will produce statistically significant or clinically meaningful results sufficient to support an application for marketing approval.
Generally speaking, whether or not and how quickly we complete clinical trials is dependent in part upon: (i) the date the IND becomes effective enabling us to commence the clinical studies (which occurs no more than thirty days after the FDA receives the IND, unless the FDA notifies us that the IND cannot begin); (ii) the engagement of clinical trial sites and medical investigators;(iii) reaching an agreement on acceptable clinical trial agreement terms, clinical trial protocols or informed consent forms with medical investigators; (iv) obtaining approval from institutional review boards; and, thereafter, (v) the rate of patient enrollment and the rate to collect, clean, lock and analyze the clinical trial database.
Patient enrollment in trials is a function of many factors, including the design of the protocol, the size of the patient population, the proximity of patients to and availability of clinical sites, the eligibility criteria for the study, the perceived risks and benefits of the drug under study and of the control drug, if any, the medical investigator’s efforts to facilitate timely enrollment in clinical trials, the patient referral practices of local physicians, the existence of competitive clinical trials, and whether other investigational, existing or new therapies are available for the indication. If we experience delays in identifying and contracting with appropriate medical investigators and sites, in enrolling patients, and/or in completing our clinical trial programs, we may incur additional costs and delays in our development programs, and may not be able to complete our clinical trials on a cost-effective or timely basis, if at all. If we or any third party have difficulty obtaining sufficient clinical drug materials or enrolling a sufficient number of patients in a timely or cost-effective manner to conduct clinical trials as planned, or if enrolled patients do not complete the trial as planned, we or a third party may need to delay or terminate ongoing clinical trials, which could negatively affect our business.
Clinical trials often require the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit for our clinical trials. Our ability to enroll sufficient numbers of patients in our clinical trials depends on many factors, including the size of the relevant patient population, the nature and design of the protocol, the proximity of patients to clinical sites, the eligibility and exclusion criteria applicable for the trial, existence of competing clinical trials and the availability of already approved effective drugs for the indications being studied. In addition, patients may withdraw from a clinical trial or be unwilling to follow our clinical trial protocols for a variety of reasons, such as adverse events or noncompliance with study requirements. If we fail to enroll and maintain the number of patients for which the clinical trial was designed, the statistical power of that clinical trial may be reduced which would make it harder to demonstrate that the product candidate being tested in such clinical trial is safe and effective for its intended use.
The drug development process to obtain FDA approval is very costly and time consuming and if we cannot complete our clinical trials in a cost-effective manner, our operations may be adversely affected.
Even with the granting of Orphan Drug Designation and Fast Track Designation, the cost associated with the successful development of the L-glutamine treatment for SCD is uncertain. Costs of clinical trials may vary significantly over the life of a project owing, but not limited to, the following:
| · | the duration of the clinical trials; |
| · | the number of sites included in the trials; |
| · | the countries in which the trials are conducted; |
| · | the length of time required to enroll eligible patients; |
| · | the number of patients that participate in the trials; |
| · | the number of doses that patients receive; |
| · | adverse events experienced by study participants; |
| · | the drop-out or discontinuation rates of patients; |
| · | per patient trial costs; |
| · | potential additional safety monitoring or other studies requested by regulatory agencies; |
18
| · | the duration of patient follow-up; |
| · | the efficacy and safety profile of the product candidate; |
| · | the costs and timing of obtaining regulatory approvals; and |
| · | the costs involved in enforcing or defending patent claims or other intellectual property rights. |
If we are unable to control the costs of our clinical trials and conduct our trials in a cost-effective manner, our results of operations may be adversely affected.
We may be required to suspend, repeat or terminate our clinical trials if they do not meet regulatory requirements, the results are negative or inconclusive, human subject protections are inadequate, the trials are not well designed, or clinical investigators fail to comply with all requirements for the conduct of trials under an IND, all of which may result in significant negative repercussions on our business and financial condition.
We must be evaluated in light of the uncertainties and complexities affecting a development stage company. Our L-glutamine treatment for SCD, which is currently our only product in development, has not yet received marketing approval. We cannot market a pharmaceutical product in any jurisdiction until we have completed rigorous preclinical testing and clinical trials, demonstrated the product’s safety and effectiveness for its intended use, and met the requirements of such jurisdiction’s extensive regulatory approval process. Pre-clinical testing and clinical development are long, expensive and uncertain processes. Data obtained from pre-clinical and clinical tests can be interpreted in different ways and could ultimately be deemed insufficient by regulatory authorities, which could delay, limit or prevent regulatory approval. It may take us many years to complete the required testing of our products to support an application for marketing approval and failure can occur at any stage during this process.
We cannot provide assurance that our preclinical and clinical testing will be completed successfully within any specified time period by us, or without significant additional resources or expertise provided by third parties to conduct such testing. We cannot provide assurance that any such testing will demonstrate our drug products to be safe and effective or that any such product will be approved for a specific indication. Results from early clinical trials may not be indicative of the results that will be obtained in later-stage clinical trials. In addition, negative or inconclusive results from the clinical trials we conduct or adverse medical events could cause us to have to suspend, repeat or terminate the clinical trials. Clinical trials are subject to continuing oversight by governmental regulatory authorities and institutional review boards and must meet the requirements of these authorities, including but not limited to requirements for informed consent, human subject protection, and good clinical practices; and we cannot guarantee that we will be able to comply or that a regulatory authority will agree that we have complied with such requirements. We will rely on third parties, such as contract research organizations and/or contract laboratories, regulatory consultants, and data management companies to assist us in overseeing and monitoring clinical trials as well as to process the clinical data and manage test requests, which may result in delays or failure to complete trials, if the third parties fail to perform or meet applicable regulatory requirements and standards. A failure by us or any such third parties to comply with the terms of a product development program and regulatory requirements for any particular product candidate or to complete the clinical trials for a product candidate in the projected time frame could have a significant negative effect on our business and financial condition.
There are significant requirements imposed on us and on clinical investigators who conduct clinical trials under an IND. Although we are responsible for selecting qualified investigators, providing them with the information they need to conduct an investigation properly, ensuring proper monitoring of the investigation(s), and ensuring that the investigation(s) is conducted in accordance with the general investigational plan and protocols contained in the IND, we cannot ensure the investigators will maintain compliance with all regulatory requirements at all times. Clinical investigators have been found at times to incorrectly record data, omit data, or even falsify data. We cannot ensure that the clinical investigators in our trials will not make mistakes or otherwise compromise the integrity or validity of data, all of which would have a significant negative effect on our ability to obtain marketing approval, our business, and our financial condition.
There are known adverse side effects to our NutreStore® product.
We market and sell NutreStore® L-glutamine powder for oral solution, a prescription pharmaceutical product that has received FDA approval for the treatment of SBS when used in combination with a recombinant human growth hormone approved for this indication and a specialized nutritional support. Patients receiving intravenous parenteral nutrition (IPN) and NutreStore® should be routinely monitored for kidney and liver function, particularly patients with kidney or liver impairment. Common reported side effects of NutreStore® include, but are not limited to, nausea or vomiting, feeling the need or urge to empty bowels, gas, abdominal pain, hemorrhoids, constipation, aggravation of Crohn’s disease, gastric ulcer or gastric fistula. The approved indication of the product in combination with recombinant human growth hormone and specialized diets, and any of these known side effects or any associated warning statements or labeling requirements may limit the commercial profile of this product and prevent us from achieving or maintaining market acceptance of such product.
Changes in regulatory requirements and guidance or unanticipated events during our clinical trials may occur, which may result in necessary changes to clinical trial protocols, informed consents and study budgets, which could result in increased costs to us, delay our development timeline or reduce the likelihood of successful completion of the clinical trial.
19
Changes in regulatory requirements or the FDA’s interpretation of those requirements, which may be provided through guidance documents, or the occurrence of unanticipated events during our clinical trials, could require us to amend clinical trial protocols, informed consent forms, and study budgets. If we experience delays in initiation, conduct or completion of, or if we terminate, any of our clinical trials, due to changes in regulatory requirements or guidance documents, unexpected and serious adverse events, or other unanticipated events, we may incur additional costs, have difficulty enrolling subjects or achieving medical investigator or institutional review board acceptance of the changes, and successfully completing the trial, the commercial prospects for our L-glutamine treatment for SCD may be harmed and our ability to generate product revenue could be delayed, possibly materially.
Even if we are able to develop our L-glutamine treatment for SCD, we may not be able to receive regulatory approval, or if approved, we may not be able to generate significant revenues or successfully commercialize our L-glutamine treatment for SCD, which would adversely affect our financial results and financial condition.
Although our L-glutamine treatment for SCD is in Phase III clinical trials, regulatory approval to market the product is still required. We cannot ensure a successful outcome of our Phase III clinical trial of this product and cannot assure you that we will obtain the necessary regulatory approvals to permit commercialization. There are many reasons that we may fail in our efforts to develop and commercialize our L-glutamine treatment for SCD and other drug product candidates, including:
| · | the chance that our clinical trials could show that our L-glutamine treatment for SCD or other drug product candidates are ineffective and/or cause harmful side effects; |
· | the failure of our drug product candidates to receive necessary regulatory approvals from the FDA or foreign regulatory authorities in a timely manner, or at all; |
· | the failure of our drug product candidates, once approved, to be produced in commercial quantities or at reasonable costs; |
· | physicians’ reluctance to switch from existing treatment methods, including traditional therapy agents, to our products; |
· | the failure of our drug product candidates, once approved, to achieve commercial acceptance; |
· | the introduction of products by our competitors that are more effective or have a different safety profile than our products; |
· | the application of restrictions to our drug product candidates by regulatory or governmental authorities; |
· | the proprietary rights of other parties preventing us or our potential collaborative partners from marketing our drug product candidates; |
· | the possibility that we may not be able to maintain the Orphan Drug Designation or obtain or maintain orphan drug exclusivity for our product; |
· | the possibility, if we obtain orphan drug market exclusivity, that a L-glutamine product that is the same as ours would be approved for another indication and made available; |
· | the possibility, if we obtain orphan drug market exclusivity, that another company with the same drug for the same condition, would demonstrate clinically superiority over our drug product, thus making it eligible for approval despite our market exclusivity; |
· | the possibility, if we obtain orphan drug market exclusivity, that we are unable to assure adequate supply of the orphan drug thus allowing FDA to approve another drug product our orphan drug exclusivity; and |
· | the possibility that our Fast Track Designation may be rescinded or not actually lead to a faster regulatory review and approval. |
Even if the FDA and other regulatory authorities approve our L-glutamine treatment for SCD or any of our products, the manufacture, packaging, labeling, distribution, marketing and sale of such products will be subject to strict and ongoing post-approval regulations. Compliance with such regulations will be expensive and consume substantial financial and management resources. The FDA has the authority to regulate the claims we make in marketing our prescription drug products to ensure that such claims are true, not misleading, supported by scientific evidence, and consistent with the approved labeling of the drug. The FDA also has the authority to regulate the claims we make in marketing our dietary supplement AminoPure®. Failure to comply with FDA requirements in this regard could result in, among other things, warning letters, suspensions of approvals, seizures, recalls , injunctions prohibiting a product's manufacture and distribution, prohibiting and requiring corrective actions regarding sales and marketing activities, other operating restrictions, civil money penalties, prosecution under the False Claims Act, and criminal prosecutions. Any of these government enforcement actions could negatively impact our product sales and profitability. Additionally, an approval for a product may be conditioned on our agreement to conduct costly post-marketing follow-up studies to monitor the safety or effectiveness of the products or to implement risk mitigation strategies. In addition, as clinical experience with a drug expands after approval because the drug is used by a greater number and more diverse group of patients than during clinical trials, unknown side effects or other problems may be observed that were not observed or anticipated during pre-approval clinical trials. In such a case, a regulatory authority could restrict the indications for which the product may be sold, or restrict the distribution channels, or revoke the product’s regulatory approval, which could hinder our ability to generate revenues from our products. If we fail to develop and commercialize our drug product candidates as planned, our financial results and financial condition will be adversely affected, we will have to delay or terminate some or all of our research product development programs and may be forced to cease operations.
20
We are subject to various regulations pertaining to healthcare fraud and abuse, violations of which could have a material adverse effect on our business.
We are subject to various federal and state laws pertaining to healthcare fraud and abuse, including inducing, facilitating or encouraging submission of false claims to government programs and prohibitions on the offer or payment or acceptance of kickbacks or other remuneration for the purchase of our products. The False Claims Act makes it illegal to defraud federal government programs, such as Medicare. Drug manufacturers have been held responsible for claims filed by physicians for reimbursement of the cost of medical services related to uses of a drug product that are not on a the approved labeling, known as “off-label use,” if the manufacturer promoted the drug for such off-label use. Violations of the False Claims Act may result in treble damages based on the amount of overpayment and additional civil fines of $5,000 to $10,000 for each false claim. Drug manufactures could also be held responsible for reimbursement claims submitted by any physician for drug products that were knowingly not manufactured in compliance with cGMP regulations.
The Anti-Kickback Statute makes it illegal for a prescription drug manufacturer to solicit, offer, or pay any remuneration in exchange for purchasing, leasing, or ordering any service or items including the purchase or prescribing of a particular drug for which payment may be made under a federal healthcare program. Because of the sweeping language of the federal Anti-Kickback Statute, many potentially beneficial business arrangements would be prohibited if the statute were strictly applied. To avoid this outcome, the Department of Health and Human Services has published regulations, known as “safe harbors,” that identify exceptions or exemptions to the statute’s prohibitions. Arrangements that do not fit within the safe harbors are not automatically deemed to be illegal, but must be evaluated on a case by case basis for compliance with the statute. We seek to comply with the Anti-Kickback Statute and, if necessary, to fit within one of the defined "safe harbors.” We are unaware of any violations of these laws. However, due to the breadth of the statutory provisions and the absence of uniform guidance in the form of regulations or court decisions, there can be no assurance that our practices will not be challenged under anti-kickback or similar laws. Violations of such restrictions may be punishable by civil or criminal sanctions, including fines and civil monetary penalties, as well as the possibility of exclusion from U.S. federal healthcare programs (including Medicaid and Medicare). Any such violations could have a material adverse effect on our business, financial condition, results or operations and cash flows.
If the manufacturers upon whom we rely fail to timely produce in the volumes and quality that we require, or fail to comply with stringent regulations applicable to pharmaceutical manufacturers, we may face delays in the development and commercialization of, or be unable to meet demand for, our products, if any, and may lose any marketing exclusivity and potential revenues.
We do not currently have or intend to develop our own manufacturing capabilities. We intend to enter into various arrangements with contract manufacturers and others to manufacture our products and, thus, will significantly depend upon the subsequent success of these outside parties in performing their manufacturing responsibilities. The manufacture of pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of pharmaceutical products are required to comply with cGMP regulations but may encounter difficulties in production, including problems with quality control, quality assurance testing, shortages of qualified personnel, and compliance with strictly enforced federal, state, and foreign requirements. Our third-party manufacturers and key suppliers may experience manufacturing difficulties due to resource constraints or as a result of labor disputes, unstable political environments at foreign facilities or financial difficulties. If these manufacturers or key suppliers were to encounter any of these difficulties, or otherwise fail to comply with their regulatory and contractual obligations, our ability to timely launch any potential product candidate, if approved, would be jeopardized. If we are unable to ensure adequate supply of an orphan drug for which we have obtained marketing exclusivity, FDA may approve another drug for marketing, which could have a material adverse effect on our business and financial condition.
We currently obtain our pharmaceutical grade L-glutamine from two Japanese companies, which together produce the vast majority of pharmaceutical grade L-glutamine approved for sale in the United States, and obtain all of our L-glutamine for our NutreStore® product from one of these companies. If these suppliers were to experience any manufacturing or production difficulties producing pharmaceutical grade L-glutamine, our ability to complete our clinical trials, to commercialize L-glutamine for the treatment of SCD, to maintain marketing exclusivity if granted, and to continue to sell NutreStore® would be harmed.
21
We intend to enter into long term supply agreements with one or more manufacturers for our products. There can be no assurance that we will be successful in entering into such long term supply contracts, or that such contracts will be at prices that are acceptable to us. Our manufacturing partners may not be able to expand capacity or to produce additional product requirements for us in the event that demand for our products increases. There can be no assurance that we or our manufacturers will be able to continue purchasing raw materials or single source ingredients for our products from current suppliers or any other supplier on terms similar to current terms or at all. Any interruption in the availability of certain raw materials or ingredients, or significant increases in the prices paid by us for them, could have a material adverse effect on our business, financial condition, liquidity and operating results.
In addition, all manufacturers and suppliers of pharmaceutical products must comply with applicable cGMP regulations for the manufacture of drug products, which are enforced by the FDA through its facilities inspection program. The FDA is likely to conduct inspections of our third party manufacturer and key supplier facilities as part of the Agency’s pre-approval review of any of our NDAs and post-approval, ongoing compliance programs. If our third party manufacturers and key suppliers are not in compliance with cGMP requirements, it may result in a delay of approval for products undergoing regulatory review or the inability to meet market demands for any approved products, particularly if these sites are supplying single source ingredients required for the manufacture of any potential product. These cGMP requirements include quality control, quality assurance and the maintenance of records and documentation, among other items. Furthermore, each manufacturing facility used to manufacture drug or biological products is subject to FDA inspection and must meet cGMP requirements. As a result, if one of the manufacturers that we rely on shifts production from one facility to another, the new facility must go through a complete regulatory qualification and be approved by regulatory authorities prior to being used for commercial supply. Our manufacturers may be unable or fail to comply with these cGMP requirements and with other FDA, state, and foreign regulatory requirements. A failure to comply with any applicable manufacturing requirements may result in warning or untitled letters, fines, product recalls, seizures, corrective actions involving public notifications, injunctions, total or partial suspension of production, civil money penalties, suspension or withdrawals of previously granted regulatory approvals, refusal to approve new applications or supplements to applications for marketing of new products, import or export bans or restrictions, and criminal prosecution and criminal penalties. Any of these penalties could delay or prevent the promotion, marketing or sale of our products. If the safety of any quantities supplied is compromised due to a third party manufacturer’s or key supplier’s failure to comply with or adhere to applicable laws or for other reasons, we may not be able to obtain regulatory approval for or successfully commercialize our products.
The failure of our products to gain market acceptance will hinder our ability to generate revenues from the sale of our products.
Even if our products are approved for commercialization, they may not be successful in the marketplace. Market acceptance of any of our products will depend on a number of factors including, but not limited to:
· | demonstration of significant clinical efficacy and safety; |
· | the prevalence and severity of any adverse side effects; |
· | limitations or warnings contained in the product’s approved labeling; |
· | the availability of alternative treatments for the indications; |
· | the advantages and disadvantages of our products relative to current or alternative treatments; |
· | the availability of acceptable pricing and adequate third-party reimbursement; and |
· | the effectiveness of marketing and distribution methods for the products. |
If our products do not gain market acceptance among physicians, patients, treatment centers, healthcare payors, and others in the health care community, which may not accept or utilize our products, our ability to generate significant revenues from our products would be limited and our financial conditions will be materially adversely affected. In addition, if we fail to successfully penetrate our core markets and successfully expand our business into new markets, the growth in sales of our products, along with its operating results, could be negatively impacted.
Our ability to successfully penetrate our core markets in which we compete or to expand our business successfully into additional countries in Africa, Europe, Asia or elsewhere is subject to numerous factors, many of which are beyond our control. Our products, if successfully developed, may compete with a number of drugs and therapies currently manufactured and marketed by major pharmaceutical companies. Our products may also compete with new products currently under development by others or with products which may be less expensive than our products. There is no assurance that our efforts to increase market penetration in our core markets and existing geographic markets will be successful. Our failure to do so could have an adverse effect on our operating results.
22
We lack experience in commercializing products, which may have an adverse effect on our business.
If we obtain FDA approval of our products under development, we will need to transition from a company with a development focus to a company capable of supporting commercial activities. We may not be successful in such a transition. We have not yet demonstrated an ability to obtain marketing approval for product candidates and have limited experience in commercializing products. As a result, we may not be as successful as companies that have previously obtained marketing approval for drug candidates and have more experience commercially launching drugs.
We are required to make a significant payment to CellSeed pursuant to the Research Agreement, and if we cannot make such payment when due, CellSeed may terminate its agreements with us, which will negatively impact our financial condition and our ability to implement our business strategies.
In April 2011, Emmaus Medical entered into the Research Agreement and the Individual Agreement with CellSeed and, in August 2011, an addendum to the agreements. Pursuant to the Individual Agreement, CellSeed granted us the exclusive right to manufacture, sell, market and distribute CAOMECS for the cornea in the United States and agreed to disclose its accumulated information package for the joint development of CAOMECS to us. Under the Individual Agreement, we agreed to pay CellSeed $1.5 million, which we paid in February 2012. Pursuant to the Research Agreement, we formed a relationship with CellSeed regarding the future research and development of cell sheet engineering regenerative medicine products, and the future commercialization of such products. Under the Research Agreement, as supplemented by the addendum, we agreed to pay CellSeed $8.5 million within 30 days of the completion of all of the following: (i) the execution of the Research Agreement; (ii) the execution of the Individual Agreement; and (iii) CellSeed’s delivery of the accumulated information package, as defined in the Research Agreement, to Emmaus and Emmaus providing written confirmation of its acceptance of the complete accumulated information package. Our failure to raise capital as and when needed could impact our ability to pay the amounts required under the Research Agreement to CellSeed.
If our payment obligation under the Research Agreement becomes due and payable prior to our generation of revenue from the commercialization of our L-glutamine SCD treatment under development, we will need to seek other funding sources, including the sale of additional equity or debt securities, in order to make the payments to CellSeed required under the Research Agreement. CellSeed may terminate these agreements with us if we are unable to make timely payments, which are required under the agreements. If these agreements were terminated, the Company would become more reliant on its SCD product and funding may be more difficult to obtain.
There are various uncertainties related to the research, development and commercialization of cell sheet engineering regenerative medicine products pursuant to the Research Agreement and Individual Agreement which could negatively affect our ability to commercialize such products.
We have historically focused on the research and development of our L-glutamine treatment for SCD and have no experience in the research, development or commercialization of cell sheet regenerative medicine products or any other biological product. Such products must be manufactured in conformance with cGMP requirements as well as Good Tissue Practice (“GTP”) requirements and demonstrate that they are safe and effective for their intended uses to obtain FDA approval. The GTP requirements, which are specifically applicable to all cellular-based products, are intended to prevent communicable disease transmission. It is uncertain what type of scientific data would be required to sufficiently demonstrate the safety and effectiveness of cell sheet regenerative medicine products for their intended uses. Such uncertainties could delay our ability to obtain FDA approval for and to commercialize such products. In addition, the research and commercialization of cell sheet regenerative medicine products could be hindered if third party manufacturers of such products are not compliant with cGMP, GTP, or other applicable regulations. Any delay in the development of, obtaining FDA approval for, or the occurrence of any problems with third party manufacturers of cell sheet regenerative medicine products would negatively affect our ability to commercialize such products.
We are currently engaged in significant litigation with Amir Heshmatpour and AFH Advisory.
Since July 2012, we have been engaged in significant litigation with Amir Heshmatpour and AFH Holding & Advisory, LLC (“AFH Advisory”). Mr. Heshmatpour, a former director of the Company, owns AFH Advisory, which was the sole stockholder of AFH Acquisition IV until its combination with Emmaus Medical pursuant to the Merger in May 2011. In September 2012, AFH Advisory and related parties filed a complaint against us in the Superior Court of Delaware. In October 2012 we filed counterclaims against the plaintiffs and third-party claims against Mr. Heshmatpour. The case is scheduled for trial in May 2013. For a detailed description of the case, please see "ITEM 3. LEGAL PROCEEDINGS" below in this Annual Report on Form 10-K.
Conduct of the litigation has entailed significant expense to the Company and will continue to do so until it is resolved. It has also required, and will continue to require, significant amounts of time and attention from our management. In connection with the litigation, we placed our planned initial public offering on hold, and the existence of the litigation has been one of several factors that has prevented us from resuming our efforts to conduct a public offering of common stock and has required Company management to devote large amounts of time and effort to securing private sources of capital. Although management believes its claims against Mr. Heshmatpour and AFH Advisory are strong and that the claims asserted against the Company are without merit, we might not be entirely or even partially successful in the lawsuit. If we are not successful in defending the claims brought by Mr. Heshmatpour and AFH Advisory or asserting our own claims, it could result in our incurring liabilities and expenses that would have a material adverse effect on our financial condition and cash flows.
23
Failure to obtain acceptable prices or adequate reimbursement for our products may cause an adverse impact on our results of operations.
Our ability to successfully commercialize our products will depend significantly on our ability to obtain acceptable prices and the availability of reimbursement to the patient from third-party payors, such as governmental and private insurance plans. These third-party payors frequently require companies to provide predetermined discounts from list prices, and they are increasingly challenging the prices charged for pharmaceuticals and other medical products. Our products may not be considered medically necessary or cost-effective, may not compare favorably on price with other L-glutamine products, and reimbursement to the patient may not be available or sufficient to allow us or our partners to sell our products on a competitive basis. It may not be possible to negotiate favorable reimbursement rates for our products.
Significant uncertainty exists as to the reimbursement status of newly approved healthcare products and third-party payors are increasingly challenging the prices charged for pharmaceuticals and other medical products. In addition, the continuing efforts of third-party payors to contain or reduce the costs of healthcare through various means may limit our commercial opportunity and reduce any associated revenue and profits. For example, in some foreign markets, the pricing or profitability of healthcare products is subject to government control. In other foreign markets, including Africa where the largest population of SCD patients exists, there is a limited number of third party payors from which reimbursement can be sought. In the United States, there have been, and we expect there will continue to be, a number of federal and state proposals to implement similar government control, as evidenced by the passing of the Patient Protection and Affordable Care Act and its amendment, the Health Care and Education Reconciliation Act. Such government-adopted reform measures may adversely impact the pricing of healthcare products and services in the United States or internationally and the amount of reimbursement available from governmental agencies or other third party payors. In addition, increasing emphasis on managed care will continue to put pressure on the pricing of pharmaceutical products. Cost control initiatives could decrease the price that we or any current or potential collaborators could receive for any of our products and could adversely affect our profitability. If we fail to obtain acceptable prices or an adequate level of reimbursement for our products, the sales of our products would be adversely affected or there may be no commercially viable market for our products.
If we do not achieve our projected development goals in the time frames we expect and announce, the credibility of our management and our drug products may be adversely affected.
For business planning purposes, we estimate the timing of the accomplishment of various scientific, clinical, regulatory and other product development goals, which we sometimes refer to as milestones. These milestones may include the commencement or completion of preclinical studies, clinical trials and the submission of regulatory filings for our products.
From time to time, we may publicly announce the expected timing of some of these milestones. All of these milestones will be based on a variety of assumptions. The actual timing of these milestones can vary dramatically compared to our estimates, in many cases for reasons beyond our control. If we do not meet these milestones as publicly announced, our stockholders may lose confidence in our ability to meet these milestones and, as a result, we may be unable to obtain additional financing on terms acceptable to us, or at all.
If we do not obtain the support of new, and maintain the support of existing, key scientific collaborators, it may be difficult to research medical indications for L-glutamine other than SCD and to expand our product offerings, which may limit our revenue growth and profitability and could have a material adverse effect on our business, financial condition and operating results.
We will need to establish relationships with additional leading scientists and research institutions in order to develop new products and expand our product offerings and to explore other medical indications for L-glutamine for SCD. Although we have established research collaborations, we cannot assure you that our relationships with our research collaborators will continue or that we will be able to attract additional research partners. If we are not able to maintain existing or establish new scientific relationships to assist in our research and development, we may not be able to successfully develop our drug product candidates or expand our products offerings.
If our competitors succeed in developing products and technologies that are more effective than our own, or if scientific developments change our understanding of the potential scope and utility of our drug product candidates, then our technologies and future drug product candidates may be rendered less competitive.
We face significant competition from industry participants that are pursuing similar technologies that we are pursuing and are developing pharmaceutical products that are competitive with our drug product candidates. Nearly all of our industry competitors have greater capital resources, larger overall research and development staffs and facilities, and a longer history in drug discovery and development, obtaining regulatory approval and pharmaceutical product manufacturing and marketing than we do. With these additional resources and experience, our competitors may be able to respond to the rapid and significant technological changes in the biotechnology and pharmaceutical industries faster than we can. Our future success will depend in large part on our ability to maintain a competitive position with respect to these technologies. Rapid technological development, as well as new scientific developments, may result in our compounds, drug product candidates or processes becoming obsolete or less effective or otherwise clinically inferior for some or all patients before we can recover any of the expenses incurred to develop them. For example, changes in our understanding of the appropriate population of patients who should be treated with a targeted therapy like we are developing may limit the drug’s market potential if it is subsequently demonstrated that only certain subsets of patients should be treated with the targeted therapy. If a competitor develops the same drug for the same indication that is clinically superior to our drug product, or if the same drug product is intended for a different indication, or if we are unable to ensure adequate supplies of the orphan drug product, the FDA may approve the marketing application of that other drug product regardless of any marketing exclusivity we may obtain. Any of these events could have a material adverse effect on our ability to successfully commercialize our product and thus on our business and financial condition.
24
The use of any of our drug product candidates in clinical trials and in the market may expose us to liability claims.
The nature of our business exposes us to potential liability risks inherent in the testing, manufacturing and marketing of our drug product candidates. While we are in clinical stage testing, our drug product candidates could potentially harm people or allegedly harm people and we may be subject to costly and damaging product liability claims. Some of the patients who participate in clinical trials are already critically ill when they enter a trial. Informed consent and contractual limitations on payments for subject injury or waivers we obtain may not be enforceable and may not protect us from liability or the costs of product liability litigation. Although we currently carry a $5 million clinical product liability insurance policy, it may not be sufficient to cover future claims. We could be materially and adversely affected if we were required to pay damages or incur defense costs in connection with a claim outside the scope of indemnity or insurance coverage, if the indemnity is not performed or enforced in accordance with its terms, or if our liability exceeds the amount of applicable insurance. In addition, there can be no assurance that insurance will continue to be available on terms acceptable to us, if at all, or that if obtained, the insurance coverage will be sufficient to cover any potential claims or liabilities. Similar risks would exist upon the commercialization or marketing of any products by us or our partners. We currently do not have any clinical or product liability claims or threats of claims filed against us.
The pharmaceutical and biotechnology industries are subject to rapid technological change, and if we fail to keep up with such change, our results of operations and financial condition could be adversely impacted.
Biotechnology and related pharmaceutical technology have undergone and are subject to rapid and significant change. We expect that the technologies associated with biotechnology research and development will continue to develop rapidly. Our failure to keep pace with such rapid change could result in our products becoming obsolete and we may be unable to recoup any expenses incurred with developing such products, which may adversely affect our future revenues and financial condition.
We rely heavily on the founder of Emmaus Medical, Yutaka Niihara, M.D., MPH, our current President and Chief Executive Officer. The loss of his services would have a material adverse effect upon us and our business and prospects.
Our success depends, to a significant extent, upon the continued services of Yutaka Niihara, M.D. MPH, who is the founder of Emmaus Medical and our current President and Chief Executive Officer. Since inception, we have been dependent upon Dr. Niihara, who was one of the initial patentees for the technology for treatment of SCD. While Dr. Niihara and the rest of our executive officers are parties to confidentiality agreements that prevent them from soliciting our existing customers or disclosing information deemed confidential to us, we do not have any agreement with Dr. Niihara or any key members of management that would prohibit them from joining our competitors or forming competing companies. In addition, we do not maintain key man life insurance policies on any of our executive officers. If Dr. Niihara or any key management personnel resign to join a competitor or form a competing company, the loss of such personnel, together with the loss of any customers or potential customers due to such executive’s departure, could materially and adversely affect our business and results of operations.
We are dependent on a technically trained workforce and an inability to retain or effectively recruit such employees could have a material adverse effect on our business, financial condition and results of operations.
Our ability to compete effectively depends largely on our ability to attract and retain certain key personnel, including our clinical, regulatory and scientific staff members. Industry demand for such skilled employees, however, exceeds the number of personnel available, and the competition for attracting and retaining these employees is intense. Because of this intense competition for skilled employees, we may be unable to retain our existing personnel or attract additional qualified employees to keep up with future business needs. If this should happen, our business, operating results and financial condition could be adversely affected.
In addition, we intend to hire in-house marketing personnel to promote and market and sell our SCD and SBS treatment products to patients, physicians and treatment centers, and obtain the approval of insurance companies and healthcare payors for reimbursement of the cost of these treatments. We cannot assure you that we will be able to recruit and retain qualified personnel to perform these marketing functions. Our inability to hire and then retain such personnel and scientists could have a materially adverse effect on our business and our projects.
25
We are dependent on licenses and sublicenses for certain patents for our products. If the licenses of any superior sublicenses terminates or if the patents of our licensors are challenged and we are limited in our ability to utilize the licensed patents, we may be unable to develop, out-license, market and sell our products, which would cause a material adverse effect on our business, prospects, financial condition, and operating results.
Our ability to develop products depends on licenses we have obtained to patents for treatment of SCD and SBS. We hold a license as a sublicense of certain patent rights needed to operate our business. If any of the licenses of any of the superior sublicensees terminate, our license may terminate also.
There can be no assurance that, in the event any claims in such sublicensed patents are challenged, that any court or patent authority would determine that such patent claims are valid and enforceable or sufficiently broad in scope to protect our proprietary rights. Our commercial success will depend, in part, on not infringing patents or proprietary rights of others, and there can be no assurance that the technologies and products used or developed by us will not infringe such rights. Infringement actions could result in litigation and we could incur significant costs and diversion of resources in defending such claims. The party making such claims could secure a judgment awarding substantial damages, as well as injunctive or other equitable relief. Such relief could effectively block our ability to make, use, sell, distribute or market our products and services in such jurisdiction. If such infringement occurs and we are unable to obtain a license from the relevant third party, we will not be able to continue the development, manufacture, use or sale of any such infringing technology or product. There can be no assurance that necessary license to third party technology will be available at all, or on commercially reasonable terms. Our failure to obtain a license to technology that it may require to utilize its technologies or commercialize its products could have a material adverse effect on us. In some cases, litigation or other proceedings may be necessary to defend against or assert claims of infringement, to enforce patents issued to us, to protect trade secrets, know-how or other intellectual property rights owned by us or to determine the scope and validity of the proprietary rights of third parties. Any potential litigation could result in substantial costs to, and diversion of, resources by us and could have a material and adverse impact on us. There can be no assurance that any of our issued or licensed patents would ultimately be held valid or that efforts to defend any of our patents, trade secrets, know-how or other intellectual property rights would be successful. An adverse outcome in any such litigation or proceeding could subject us to significant liabilities, require us to cease using the subject technology or require us to license the subject technology from the third party, all of which could have a material adverse effect on our business.
If we are unable to protect our proprietary technology, we may not be able to compete effectively and our business and financial prospects may be harmed.
Where appropriate, we seek patent protection for certain aspects of our technology. Patent protection may not be available for some of the drug product candidates we are developing. If we must spend significant time and money protecting our patents, designing around patents held by others or licensing, potentially for large fees, patents or other proprietary rights held by others, our business and financial prospects may be harmed.
We will incur significant ongoing expenses in maintaining our patent portfolio. Should we lack the funds to maintain our patent portfolio or to enforce our rights against infringers, we could be adversely impacted. Even if claims of infringement are without merit, any such action could divert the time and attention of management and impair our ability to access additional capital and/or cost us significant funds to defend.
Companies and universities that have licensed product candidates to us for research, clinical development and marketing are sophisticated competitors that could develop similar products to compete with our products which could reduce our future revenues.
Licensing our product candidates from other companies, universities or individuals does not always prevent them from developing non-identical but competitive products for their own commercial purposes, nor from pursuing patent protection in areas that are competitive with us. While we seek patent protection for all of our owned and licensed product candidates, our licensors or assignors who created these product candidates are experienced scientists and business people who may continue to do research and development and seek patent protection in the same areas that led to the discovery of the product candidates that they licensed or assigned to us. By virtue of the previous research that led to the discovery of the drugs or product candidates that they licensed or assigned to us, these companies, universities, or individuals may be able to develop and market competitive products in less time than might be required to develop a product with which they have no prior experience and may reduce our future revenues from such product candidates.
Our success depends on our ability to manage our growth.
If we are able to raise significant additional financing, we expect to continue to grow, which could strain our managerial, operational, financial and other resources. With the addition of our clinical-stage programs and with our plan to in-license and acquire additional clinical-stage product candidates, we will be required to retain experienced personnel in the regulatory, clinical and medical areas over the next several years. Also, as our preclinical pipeline diversifies through the acquisition or in-licensing of new molecules, we will need to hire additional scientists to supplement our existing scientific expertise over the next several years.
26
Our staff, financial resources, systems, procedures or controls may be inadequate to support our operations and our management may be unable to take advantage of future market opportunities or manage successfully our relationships with third parties if we are unable to adequately manage our anticipated growth and the integration of new personnel.
Our business and results of operations may be negatively impacted by general economic and financial market conditions and such conditions may exacerbate the other risks that affect our business.
The world’s financial markets have recently experiencing significant turmoil, resulting in reductions in available credit, constraints in access to capital, extreme volatility in security prices, rating downgrades of investments and reduced valuations of securities generally. These economic conditions have had, and we expect will continue to have, an adverse impact on the pharmaceutical industries. Our business depends on our ability to raise substantial additional capital and to maintain and enter into new collaborative research, development and commercialization agreements with leading pharmaceutical companies. Turmoil or deterioration in market conditions could impair our ability to raise additional capital when needed for our clinical trials. Recent economic conditions may result in prospective collaborators electing to defer entering into collaborative agreements with us or reduce the amount of discretionary investment that prospective collaborators may have available to invest in our business.
We may pursue future growth through strategic acquisitions and alliances which may not yield anticipated benefits and may adversely affect our operating results, financial condition and existing business.
We may seek to grow in the future through strategic acquisitions and alliances in order to complement and expand our business. The success of our acquisition strategy will depend on, among other things:
· | the availability of suitable candidates; |
· | competition from other companies for the purchase of available candidates; |
· | our ability to value those candidates accurately and negotiate favorable terms for those acquisitions; |
· | the availability of funds to finance acquisitions; |
· | the ability to establish new informational, operational and financial systems to meet the needs of our business; |
· | the ability to achieve anticipated synergies, including with respect to complementary products; and |
· | the availability of management resources to oversee the integration and operation of the acquired businesses. |
If we are not successful in integrating acquired businesses and completing acquisitions in the future, we may be required to reevaluate our acquisition strategy. We also may incur substantial expenses and devote significant management time and resources in seeking to complete acquisitions. Acquired businesses may fail to meet our performance expectations. If we do not achieve the anticipated benefits of an acquisition as rapidly as expected, or at all, investors or analysts may not perceive the same benefits of the acquisition as we do. If these risks materialize, our stock price could be materially adversely affected.
We have adopted an equity incentive plan under which we may grant securities to compensate employees and other services providers, which could result in increased share-based compensation expenses and, therefore, reduce net income.
Under current accounting rules, we are required to recognize share-based compensation as compensation expense in our statement of operations, based on the fair value of equity awards on the date of the grant, and recognize the compensation expense over the period in which the recipient is required to provide service in exchange for the equity award. We made grants of equity awards in 2012, and accordingly our results of operations for the year ended December 31, 2012 contain share-based compensation charges. Additional expenses associated with share-based compensation may reduce the attractiveness of issuing stock options under an equity incentive plan that we may adopt in the future. If we grant equity compensation to attract and retain key personnel, the expenses associated with share-based compensation may adversely affect our net income. However, if we do not grant equity compensation, we may not be able to attract and retain key personnel or be forced to expend cash or other compensation instead. Furthermore, the issuance of equity awards would dilute existing stockholders’ ownership interests in our company. In February 2013 our board of directors amended our equity incentive plan to increase the number of shares of our common stock that may be subject to awards granted under the plan from 3 million shares to 6 million shares.
27
We have issued and may continue to issue debt instruments that are convertible and/or may include warrant features, which could result in the calculation of discounts on the debt issued and increased amortization of discount expenses and, therefore, reduce net income.
Under current accounting rules, we are required to recognize discounts on debt issued with stock conversion features or with warrants attached which can result in amortization of the discount as an expense in our statement of operations, based on the fair value of conversion feature or warrant on the date of issue. If we grant stock conversion features or warrants with debt instruments to attract capital, the expenses associated with amortization of the calculated discount may adversely affect our net income. However, if we do not grant stock conversion features or warrants with debt instruments, we may not be able to attract debt capital.
Our certificate of incorporation and bylaws and Delaware law may have anti-takeover effects that could discourage, delay or prevent a change in control, which may cause our stock price to decline.
Our certificate of incorporation and bylaws and Delaware law could make it more difficult for a third party to acquire us, even if closing such a transaction would be beneficial to our stockholders. We are authorized to issue up to 20,000,000 shares of preferred stock. This preferred stock may be issued in one or more series, the terms of which may be determined at the time of issuance by our board of directors without further action by stockholders. The terms of any series of preferred stock may include voting rights (including the right to vote as a series on particular matters), preferences as to dividend, liquidation, conversion and redemption rights and sinking fund provisions. No preferred stock is currently outstanding. The issuance of any preferred stock could materially adversely affect the rights of the holders of our common stock, and therefore, reduce the value of our common stock. In particular, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell our assets to, a third party and thereby preserve control by the present management.
Provisions of our certificate of incorporation and bylaws and Delaware law also could have the effect of discouraging potential acquisition proposals or making a tender offer or delaying or preventing a change in control, including changes a stockholder might consider favorable. Such provisions may also prevent or frustrate attempts by our stockholders to replace or remove our management. In particular, the certificate of incorporation and bylaws and Delaware law, as applicable, among other things:
· | provide the board of directors with the ability to alter the bylaws without stockholder approval; |
· | place limitations on the removal of directors; and |
· | provide that vacancies on the board of directors may be filled by a majority of directors in office, although less than a quorum. |
These provisions are expected to discourage certain types of coercive takeover practices and inadequate takeover bids and to encourage persons seeking to acquire control of our company to first negotiate with its board. These provisions may delay or prevent someone from acquiring or merging with us, which may cause the market price of our common stock to decline.
RISKS RELATED TO OWNERSHIP OF OUR COMMON STOCK
There is no current trading market for our common stock, and we cannot predict when or if an established public trading market will develop.
Our common stock is not currently listed or quoted for trading on any national securities exchange or national quotation system. If and when we are able to conduct an initial public offering, we intend to resume our efforts related to our application for the listing of our common stock on the NASDAQ Global Market. There can be no assurance that the NASDAQ Global Market, or any other securities exchange or quotation system, will permit our shares to be listed and traded or that a market for shares of our common stock will in fact develop at any time. Because of the unlisted status of, and lack of a current trading market for, our common stock, it may be difficult for our stockholders to sell these shares without considerable delay.
The market price and trading volume of shares of our common stock may be volatile.
When and if a market develops for our securities, the market price of our common stock could fluctuate significantly for many reasons, including reasons unrelated to our specific performance, such as reports by industry analysts, investor perceptions, or announcements by our competitors regarding their own performance, as well as general economic and industry conditions. For example, to the extent that other large companies within our industry experience declines in their share price, our share price may decline as well. Fluctuations in operating results or the failure of operating results to meet the expectations of public market analysts and investors may negatively impact the price of our securities. Quarterly operating results may fluctuate in the future due to a variety of factors that could negatively affect revenues or expenses in any particular quarter, including vulnerability of our business to a general economic downturn; changes in the laws that affect our products or operations; competition; compensation related expenses; application of accounting standards; and our ability to obtain and maintain all necessary government certifications and/or licenses to conduct our business. In addition, when the market price of a company’s shares drops significantly, stockholders could institute securities class action lawsuits against the company. A lawsuit against us could cause us to incur substantial costs and could divert the time and attention of our management and other resources.
28
Shares eligible for future sale may adversely affect the market price of our common stock, as the future sale of a substantial amount of outstanding stock in the public marketplace could reduce the price of our common stock.
Following the one year anniversary of the filing of our current report on Form 8-K reporting the Merger, or May 3, 2012, our pre-Merger stockholders and the former stockholders of Emmaus Medical may be eligible to sell all or some of our shares of common stock by means of ordinary brokerage transactions in the open market pursuant to Rule 144, promulgated under the Securities Act (“Rule 144”), subject to certain limitations and the terms of any lock-up agreements entered into by such stockholders. Additionally, we granted “piggyback” registration rights to certain pre-Merger stockholders of the Company. All of the shares included in an effective registration statement, if any, may be freely sold and transferred, subject to a lock-up agreement. Under Rule 144, an affiliate stockholder who has satisfied the required holding period may, under certain circumstances, sell within any three-month period a number of securities which does not exceed the greater of 1% of the then outstanding shares of common stock or the average weekly trading volume of the class during the four calendar weeks prior to such sale. As of the date of this Form 10-K, 1% of our issued and outstanding shares of common stock is approximately 248,784 shares. Non-affiliate stockholders are not subject to volume limitations. Any substantial sale of common stock pursuant to any resale prospectus or Rule 144 may have an adverse effect on the market price of our common stock.
Members of our management team have significant influence over us.
As of March 20, 2013, our officers and directors beneficially owned approximately 48.7% of the outstanding shares of common stock on an undiluted basis. These stockholders, therefore, have a controlling influence in determining the outcome of any corporate transaction or other matters submitted to our stockholders for approval, including mergers, consolidations and the sale of all or substantially all of our assets, election of directors, and other significant corporate actions. These stockholders may also have the power to prevent or cause a change in control. In addition, without the consent of these stockholders, we could be prevented from entering into transactions that could be beneficial to us. The interests of these stockholders may differ from the interests of our other stockholders.
If we fail to maintain effective internal control over financial reporting, the price of shares of our common stock may be adversely affected.
We are required to establish and maintain appropriate internal controls over financial reporting. Failure to establish those controls, or any failure of those controls once established, could adversely impact our public disclosures regarding our business, financial condition or results of operations. Any failure of these controls could also prevent us from maintaining accurate accounting records and discovering accounting errors and financial frauds. Rules adopted by the SEC pursuant to Section 404 of the Sarbanes-Oxley Act of 2002 require us to assess the effectiveness of our internal control over financial reporting annually and to report our conclusion in our Annual Report on Form 10-K. The standards that must be met for management to assess the internal control over financial reporting as effective are complex, and require significant documentation, testing and possible remediation. We may encounter problems or delays in completing activities necessary to make an assessment of our internal control over financial reporting. If we cannot assess our internal control over financial reporting as effective, investor confidence and share value may be negatively impacted.
In addition, management’s assessment of internal controls over financial reporting may identify weaknesses and conditions that need to be addressed in our internal controls over financial reporting or other matters that may raise concerns for investors. In March 2012, our auditor issued a management letter to us which noted a material weakness in our internal control over financial reporting related to our lack of personnel with adequate knowledge of U.S. generally accepted accounting principles. We have remediated the material weakness; however, we could be subject to the same or other material weaknesses in the future. Any actual or perceived weaknesses and conditions that need to be addressed in our internal control over financial reporting or disclosure of management’s assessment of our internal controls over financial reporting may have an adverse impact on the price of our common stock.
Our efforts to achieve the anticipated benefits of the Merger will continue to require significant time and attention from our management and might not be successful.
On May 3, 2011, we closed the Merger. As a result of the Merger, Emmaus Medical became our wholly-owned subsidiary and our operations became that of Emmaus Medical.
We may not realize the benefits that we anticipated as a result of the Merger, including:
· | the increased market liquidity expected to result from exchanging stock in a private company for securities of a public company that may eventually be traded; |
29
· | the ability to use registered securities to make acquisition of assets or businesses; |
· | increased visibility in the financial community; |
· | enhanced access to the capital markets; |
· | improved transparency of operations; and |
· | perceived credibility and enhanced corporate image of being a publicly traded company. |
Even though we have not yet been able to complete an initial public offering, we are nonetheless a public reporting company, subject to reporting requirements and other securities regulations. Compliance with these obligations and our ongoing efforts to achieve the anticipated benefits of the Merger have required and will continue to require significant time and attention from our management.
We incur significant costs as a result of operating as a public company, and our management is required to devote substantial time to compliance efforts.
As a public company, we incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002 and related SEC regulations have created uncertainty for public companies and significantly increased the costs and risks associated with accessing the public markets and public reporting. For example, on January 30, 2009, the SEC adopted rules requiring companies to provide their financial statements in interactive data format using the eXtensible Business Reporting Language, or XBRL. We are required to comply with these rules. Our management and other personnel need to devote a substantial amount of time and financial resources to comply with these requirements, as well any new requirements implemented by the SEC. Moreover, these rules and regulations increase our legal and financial compliance costs and will make some activities more time-consuming and costly and could lead to a diversion of management time and attention from revenue generating activities to compliance activities. We are currently unable to estimate these costs with any degree of certainty. These rules and regulations could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors and board committees or as executive officers and more expensive for us to obtain director and officer liability insurance.
If the NASDAQ Global Market or any other national securities exchange or national quotation system does not list our shares of common stock, our shares of common stock may be considered a “penny stock,” and thereby be subject to additional sale and trading regulations that may make the shares more difficult to sell.
Our common stock, which is not currently listed or quoted for trading, may be considered to be a “penny stock” if it does not qualify for one of the exemptions from the definition of “penny stock” under Section 3a51-1 of the Exchange Act, once, and if, it starts trading. Our common stock may be a “penny stock” if it meets one or more of the following conditions (i) the stock trades at a price less than $5.00 per share; (ii) it is NOT traded on a “recognized” national exchange; (iii) it is NOT quoted on an automated quotation system sponsored by a registered national securities association, or even if so, has a price less than $5.00 per share; or (iv) is issued by a company that has been in business less than three years with net tangible assets less than $5 million.
The principal result or effect of being designated a “penny stock” is that securities broker-dealers participating in sales of our common stock will be subject to the “penny stock” regulations set forth in Rules 15-2 through 15g-9 promulgated under the Exchange Act. For example, Rule 15g-2 requires broker-dealers dealing in penny stocks to provide potential investors with a document disclosing the risks of penny stocks and to obtain a manually signed and dated written receipt of the document at least two business days before effecting any transaction in a penny stock for the investor’s account. Moreover, Rule 15g-9 requires broker-dealers in penny stocks to approve the account of any investor for transactions in such stocks before selling any penny stock to that investor. This procedure requires the broker-dealer to (i) obtain from the investor information concerning his or her financial situation, investment experience and investment objectives; (ii) reasonably determine, based on that information, that transactions in penny stocks are suitable for the investor and that the investor has sufficient knowledge and experience as to be reasonably capable of evaluating the risks of penny stock transactions; (iii) provide the investor with a written statement setting forth the basis on which the broker-dealer made the determination in (ii) above; and (iv) receive a signed and dated copy of such statement from the investor, confirming that it accurately reflects the investor’s financial situation, investment experience and investment objectives. Compliance with these requirements may make it more difficult and time consuming for holders of our common stock to resell their shares to third parties or to otherwise dispose of them in the market or otherwise.
There can be no assurance that the NASDAQ Global Market, or any other securities exchange or quotation system, will permit our shares of common stock to be listed and traded, or that our shares of common stock will not be considered “penny stock” and thereby subject to the regulations described above.
30
If securities or industry analysts do not publish research or reports or publish unfavorable research about our business, the price and trading volume of our common stock could decline.
In the event a public trading market develops for our common stock, the price and trading volume of our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. We do not currently have and may never obtain research coverage by securities and industry analysts. If no securities or industry analysts commence coverage of us, the trading price for our common stock and other securities would be negatively affected. In the event we obtain securities or industry analyst coverage, if one or more of the analysts who covers us downgrades our securities, the price of our securities would likely decline. If one or more of these analysts ceases to cover us or fails to publish regular reports on us, interest in the purchase of our securities could decrease, which could cause the price of our common stock and other securities and their trading volume to decline.
We do not foresee paying cash dividends in the foreseeable future and, as a result, our investors’ sole source of gain, if any, will depend on capital appreciation, if any.
We do not plan to declare or pay any cash dividends on our shares of common stock in the foreseeable future and currently intend to retain any future earnings for funding growth. As a result, investors should not rely on an investment in our securities if they require the investment to produce dividend income. Capital appreciation, if any, of our shares may be investors’ sole source of gain for the foreseeable future. Moreover, investors may not be able to resell their shares of our common stock at or above the price they paid for them.
Not applicable.
We lease approximately 4,540 square feet of office space at our headquarters at 20725 S. Western Avenue, Ste. 136, Torrance, CA 90501-1884, at a base rent of $5,552 per month. This lease was extended by the parties for an additional term beginning on December 1, 2012 and expiring on November 30, 2013. During the extension period, the monthly rent will be $4,994. In addition, we lease two warehouse suites at 3870 Del Amo Boulevard, Torrance, CA under two separate leases: Suite 506 (approximately 1,400 square feet) at a base rent of $1,636 per month; and Suite 507 (approximately 1,300 square feet) at a base rent of $1,690 per month. The lease for Suite 506 will expire on August 11, 2013; the lease for Suite 507 will expire on February 28, 2015. We are working with the landlord to extend the lease for Suite 506 for an additional 2 year term. The entire Suite 507 is currently subleased to an unaffiliated entity on a month to month basis. We do not expect to experience any difficulties in renewing our leases, or finding additional or replacement office and warehouse space, at their current or more favorable rates.
The 4,540 square feet office space at 20725 S. Western Ave. is adequate and suitable for our corporate headquarters and we currently use all of such office space. Additionally, the two warehouse facilities at 3870 Del Amo Blvd., a total of 2,700 square feet, are adequate and suitable for the storage and distribution of the sickle cell study medication, study placebo and AminoPure®. Suite 506 is used to store and distribute our SCD study medication and study placebo and we are able to store all study medication and study placebo required at this location. A separate section of Suite 506 is used to store AminoPure® and can store up to a six month supply of AminoPure®, which is adequate for operations.
EM Japan leases two office suites of approximately 512 square feet and 532 square feet, respectively, in Tokyo, Japan at a base rent of 130,000 Japanese Yen (or $1,625, assuming an exchange rate of 0.0125 dollars per yen) per month each. These leases will expire on October 14, 2014 and September 15, 2013, respectively. We anticipate that the lease expiring on September 15, 2013 will be extended for another two years with the same terms.
The 1,044 total square feet of office space in Tokyo, Japan is adequate and suitable for our operations, which includes general office work and the storage and distribution of AminoPure® in Japan. We currently use all of such office space.
On July 23, 2012, the Company filed a complaint in Los Angeles Superior Court against AFH Advisory and Amir F. Heshmatpour. Mr. Heshmatpour is a former officer of AFH Acquisition IV, Inc. (prior to the Merger) and former director of the Company and is the Managing Partner of AFH Advisory. Pursuant to the complaint, the Company sought return of approximately $1.2 million in proceeds (the “Placement Proceeds”) raised in a private placement conducted by AFH Acquisition IV, Inc. in April 2011 prior to the Merger, which Placement Proceeds were not received by the Company at the time of the Merger. On December 27, 2012, the court granted a motion to stay the case on the grounds that the forum-selection clause in the Amended and Restated Letter of Intent dated September 30, 2011 by and between the Company and AFH Advisory (the “LOI”) required the Company’s claims to be brought in the State of Delaware.
31
On July 19, 2012, the Company elected to terminate the offering contemplated by the LOI. Based on the Company’s termination of the offering pursuant to the terms of the LOI and separate from the Company’s lawsuit (described above) to recover the Placement Proceeds, the Company is seeking to cancel certain shares of its common stock that were previously issued to AFH Advisory, Mr. Heshmatpour and their affiliates.
On September 7, 2012, AFH Advisory and related parties filed a complaint against the Company in the Superior Court of Delaware. The complaint alleges that the Company does not have the right to cancel the plaintiffs’ shares and asks the court to issue a declaratory judgment to that effect. In addition, the complaint alleges that the Company has breached the LOI by attempting to cancel plaintiffs’ shares and failing to reimburse AFH for expenses incurred in connection with the Merger and asks the Court to award plaintiffs damages of approximately $9.4 million. The Company believes AFH’s claims are completely without merit and is defending them vigorously.
On October 12, 2012, the Company filed counterclaims against the plaintiffs and Mr. Heshmatpour in the Superior Court of Delaware. The Company is asking the Court for an order declaring that the plaintiffs’ and Mr. Heshmatpour’s shares are canceled and that, because of fraudulent inducement on their part, the LOI is void and of no further effect. In addition, the Company is asking the Court to award compensatory and punitive damages, in amounts to be determined, for breach of contract, unjust enrichment, and fraud. The parties are currently engaged in discovery, with trial scheduled for May 2013.
Except as described above, the Company is not aware of any legal proceedings in which any director, nominee, officer or affiliate of the Company, any owner of record or beneficially of more than five percent of any class of voting securities of the Company, or any associate of any such director, nominee, officer, affiliate of the Company, or security holder is a party adverse to the Company or any of its subsidiaries or has a material interest adverse to the Company or any of its subsidiaries.
Not applicable.
32
Market Information
There is no established public trading market for our common stock and our shares of common stock are not currently listed or quoted for trading on any national securities exchange or national quotation system.
Holders
As of March 20, 2013, we had 326 stockholders of record of 24,881,436 shares of our common stock.
Dividends
We do not expect to declare or pay any cash dividends on our common stock in the foreseeable future, and we currently intend to retain future earnings, if any, to finance the expansion of our business. The decision whether to pay cash dividends on our common stock will be made by our board of directors in its discretion and will depend on our financial condition, operating results, capital requirements and other factors that the board of directors considers significant. We did not pay cash dividends in the years ended December 31, 2012 and 2011.
Recent Sales of Unregistered Securities
On October 23, 2012, we issued 400,000 shares of common stock to an investor at a price of $3.30 per share for cash for an aggregate offering price of $1,320,000.
On December 10, 2012, we issued 17,146 share of common stock to a nonaffiliated individual investor at a price of $3.60 per share for cash for an aggregate offering price of $61,726.
On December 11, 2012, we issued 17,042 shares of common stock to a nonaffiliated individual investor at a price of $3.60 per share for cash for an aggregate offering price of $61,351.
On December 27, 2012, we issued 27,000 shares of common stock to a nonaffiliated individual investor at a price of $3.60 per share for cash for an aggregate offering price of $97,200.
During the quarter ended December 31, 2012, the Company issued the convertible notes and promissory notes described below.
| Lender | Annual Interest Rate | Date of loan | Term of Loan | Loan Principal Outstanding | Conversion Price | Shares Underlying Principal as of December 31, 2012 | ||||||||||||||
| Paul Shitabata(1) | 10.00 | % | 10/3/2012 | 1 year | $ | 1,620,540 | $ | 3.60 | 450,151 | |||||||||||
| Willis Lee(2) | 10.00 | % | 10/5/2012 | 1 year | $ | 138,242 | $ | 3.60 | 38,401 | |||||||||||
| Alison Brown(3) | 10.00 | % | 12/21/2012 | 2 years | $ | 100,800 | $ | 3.60 | 28,001 | |||||||||||
| Yoshiko Takemoto(3) | 10.00 | % | 12/27/2012 | 2 years | $ | 100,800 | $ | 3.60 | 28,001 | |||||||||||
| Yutaka Niihara(4) | 10.00 | % | 12/5/2012 | Six months | $ | 1,213,700 | NA | NA | ||||||||||||
| For Days Co., Ltd.(5) | 2.00 | % | 12/26/2012 | 2 years | $ | 700,000 | NA | NA | ||||||||||||
| (1) | In October 2012, the Company refinanced two convertible notes payable to Paul Shitabata, a stockholder of the Company, in the amounts of $486,002 and $1,050,000, with a new convertible note in the amount of $1,620,540 which bears interest at 10% per annum and is a one-year note that is due upon demand. The principal amount and any unpaid interest due under the note are convertible into shares of the Company’s common stock at $3.60 per share. |
| (2) | In October 2012, the Company refinanced a convertible note payable to Willis Lee, an officer of the Company, in the amount of $128,002 with a new convertible note in the amount of $138,242 which bears interest at 10% per annum and matures on the one-year anniversary date of the note. The principal amount and any unpaid interest due under the note are convertible into shares of the Company’s common stock at $3.60 per share. |
33
| (3) | In December 2012, the Company issued convertible notes to third parties totaling $201,600 in aggregate principal amount, which bear interest at 10% per annum and mature on the respective two-year anniversary dates of the notes. The principal amount plus the unpaid accrued interest due under each of the convertible notes is convertible into shares of the Company’s common stock at $3.60 per share. |
| (4) | In December 2012, the Company issued a promissory note to Dr. Niihara, the CEO of the Company, in the principal amount of $1,213,700 which bears interest at 10% per annum and matures on the six-month anniversary date of the note. This note refinanced a note for JPY 100,000,000 ($1,213,700 at an exchange rate of 0.012137 as of the date of issuance) that was payable to related parties. |
| (5) | In December 2012, the Company issued a promissory note payable to For Days Co., Ltd. in the principal amount of $700,000 which bears interest at 2% per annum and matures on the two-year anniversary date of the note. |
All such securities were issued in reliance upon the exemption from registration provided by Section 4(2) of the Securities Act and Rule 506 promulgated thereunder. These securities qualified for exemption under Rule 506 promulgated under Section 4(2) of the Securities Act because the issuance of securities by the Company did not involve a “public offering.” The issuance was not a public offering based upon the following factors: (i) a limited number of securities were issued to a limited number of offerees; (ii) there was no public solicitation; (iii) each offeree was an “accredited investor” as such term is defined by Rule 501 under the Securities Act; and (iv) the investment intent of the offerees. No underwriters were used in connection with such sales of unregistered securities.
On July 11, 2011, we filed a Registration Statement on Form S-1, as subsequently amended. To date, the Registration Statement has not been declared effective. As further described under “Item 3. Legal Proceedings”, we are engaged in litigation relating to the Merger. Partly as a result of the litigation, we are not currently proceeding with our efforts to conduct a public offering of shares of our common stock. Although we will reassess this status after the filing of this Annual Report on Form 10-K, there can be no assurance that we will resume these efforts or, if resumed, that such efforts will result in a completed public offering or that our common stock will be accepted for listing on any national securities exchange.
Issuer Purchases of Equity Securities
We did not repurchase any shares of common stock in 2012.
Additional Information
Copies of our annual reports, quarterly reports, current reports, and any amendments to those reports, are available free of charge on the Internet at www.sec.gov. All statements made in any of our filings, including all forward-looking statements, are made as of the date of the document in which the statement is included, and we do not assume or undertake any obligation to update any of those statements or documents unless we are required to do so by law.
34
Not required for a smaller reporting company.
Forward-Looking Statements
The following discussion of our financial condition and results of operations should be read in conjunction with our financial statements and the related notes, and the other financial information included in this Annual Report on Form 10-K.
This Form 10-K contains forward-looking statements. The words “anticipated,” “believe,” “expect,” “plan,” “intend,” “seek,” “estimate,” “project,” “could,” “may,” and similar expressions are intended to identify forward-looking statements. These statements include, among others, information regarding future operations, future capital expenditures, and future net cash flow. Such statements reflect our management’s current views with respect to future events and financial performance and involve risks and uncertainties, including, without limitation, our ability to raise additional capital to fund our operations, obtaining FDA and other regulatory authorization to market our drug and biologic products, successful completion of our clinical trials, our ability to achieve regulatory authorization to market our L-glutamine treatment for SCD, our ability to commercialize our L-glutamine treatment for SCD; our reliance on third party manufacturers for our drug products, market acceptance of our products, our dependence on licenses for certain of our products, our reliance on the expected growth in demand for our products, exposure to product liability and defect claims, development of a public trading market for our securities, and various other matters, many of which are beyond our control. Should one or more of these risks or uncertainties occur, or should underlying assumptions prove to be incorrect, actual results may vary materially and adversely from those anticipated, believed, estimated or otherwise indicated. Consequently, all of the forward-looking statements made in this Form 10-K are qualified by these cautionary statements and accordingly there can be no assurances made with respect to the actual results or developments.
Company Overview
We develop and commercialize treatments and therapies for rare diseases and are primarily focused on the late-stage development of our lead product candidate currently in Phase III clinical trials studying the use of the amino acid L-glutamine as a prescription drug for the treatment of SCD. To a lesser extent, we are also engaged in the marketing and sale of NutreStore® L-glutamine powder for oral solution, which has received FDA approval, as a treatment for Short Bowel Syndrome (“SBS”) in patients receiving specialized nutritional support when used in conjunction with a recombinant human growth hormone that is approved for this indication. Our indirect wholly owned subsidiary, Newfield Nutrition Corporation, sells L-glutamine as a nutritional supplement under the brand name AminoPure® through retail stores in multiple states and via importers and distributors in Japan, Taiwan and South Korea. Since inception, we have generated minimal revenues from the sale and promotion of NutreStore® and AminoPure®.
We also own a minority interest in CellSeed, Inc., a Japanese company listed on the JASDAQ Growth market in Tokyo, which is engaged in research and development of regenerative medicine products and the manufacture and sale of temperature-responsive cell culture equipment.
The Company also has certain rights to regenerative medicine products owned by CellSeed and is involved in research focused on providing innovative solutions for tissue-engineering through the development of novel cell harvest methods and 3-dimensional living tissue replacement products for “cell-sheet therapy” and regenerative medicine and the commercialization of such products. In April 2011, we entered into a Joint Research and Development Agreement (the “Research Agreement”) with CellSeed regarding the future research and development of cell sheet engineering regenerative medicine products and the future commercialization of such products. Pursuant to the Individual Agreement between us and CellSeed executed in April 2011, CellSeed granted us the exclusive right to manufacture, sell, market and distribute Cultured Autologous Oral Mucosal Epithelial Cell-Sheet (“CAOMECS”) to be used on the cornea of appropriate patients residing in the United States. We intend to work on commercializing the CAOMECS for the cornea and to expand our relationship with CellSeed to develop cardiac cell sheets and cell sheets for other types of cells in the future.
Emmaus Medical, LLC was organized on December 20, 2000. In October 2003, Emmaus Medical, LLC undertook a reorganization and merged with Emmaus Medical, Inc., which was originally incorporated in September 2003.
Pursuant to an Agreement and Plan of Merger dated April 21, 2011 (the “Merger Agreement”), by and among the Company, AFH Merger Sub, Inc., a wholly-owned subsidiary of the Company (“AFH Merger Sub”), AFH Advisory, and Emmaus Medical, Emmaus Medical merged with and into AFH Merger Sub on May 3, 2011 with Emmaus Medical continuing as the surviving entity (the “Merger”). Upon the closing of the Merger, the Company changed its name from “AFH Acquisition IV, Inc.” to “Emmaus Holdings, Inc.” Subsequently, on September 14, 2011, we changed our name from “Emmaus Holdings, Inc.” to “Emmaus Life Sciences, Inc.”
35
Upon consummation of the Merger, (i) each outstanding share of Emmaus Medical common stock was exchanged for 29.48548924976 shares of our common stock, (ii) each outstanding Emmaus Medical option and warrant, which was exercisable for one share of Emmaus Medical common stock, was exchanged for an option or warrant, as applicable, exercisable for 29.48548924976 shares of our common stock; and (iii) each outstanding convertible note of Emmaus Medical, which was convertible for one share of Emmaus Medical common stock, was exchanged for a convertible note exercisable for 29.48548924976 shares of our common stock. As a result of the Merger, holders of Emmaus Medical common stock, options, warrants and convertible notes received 20,628,305 shares of our common stock (excluding 47,178 shares held by stockholders who exercised dissenters’ rights in connection with the Merger), options and warrants to purchase an aggregate of 326,508 shares of our common stock, and convertible notes to purchase an aggregate of 271,305 shares of our common stock. Securityholders of Emmaus Medical held 85% of our issued and outstanding common stock on a fully diluted basis upon the closing of the Merger. Immediately after the closing of the Merger, we had issued and outstanding 24,378,305 (excluding 47,178 shares held by stockholders who exercised dissenters’ rights) shares of common stock, no shares of preferred stock, options to purchase 23,590 shares of common stock, warrants to purchase 302,918 shares of common stock and convertible notes exercisable for 271,305 shares of common stock.
Our future capital requirements are substantial and may increase beyond our current expectations depending on many factors including: the duration and results of the clinical trials for our various products going forward; unexpected delays or developments when seeking regulatory approvals; the time and cost in preparing, filing, prosecuting, maintaining and enforcing patent claims; current and future unexpected developments encountered in implementing our business development and commercialization strategies; the outcome of litigation; and further arrangements, if any, with collaborators. Until we can generate a sufficient amount of product revenue, future cash requirements are expected to be financed through public or private equity offerings, debt financings or corporate collaboration and licensing arrangements. As of December 31, 2012, our accumulated deficit since inception is $36.0 million and we had cash and cash equivalents of $0.4 million. Since inception we have had minimal revenues and have been required to rely on funding from sales of equity securities and borrowings from officers and stockholders. Currently, we estimate we will need approximately $4.0 million to complete our Phase III clinical trial and $400,000 to obtain regulatory approval of L-glutamine as a therapy for SCD.
In addition, we agreed to pay CellSeed an aggregate of $8.5 million pursuant to the Research Agreement, which is still payable, and we believe that we will need approximately $3.0 million for research and development to develop corneal cell sheet technology in the United States and $2.8 million to initiate the cardiac cell sheet work. In addition, we estimate that we will need $2.5 million for research related to other cell sheet applications and cGMP laboratory costs for regenerative medicine.
Recent Highlights
In April 2009, the FDA authorized us to begin a large Phase III clinical trial directed to study L-glutamine as an experimental agent to reduce sickle cell crisis. Patient enrollment began in mid-2010 and as of December 2012, we have signed contracts with 32 study sites across the United States and have enrolled 230 patients. An interim analysis of a subset of data from the Phase III clinical trial was completed by an independent third party. The results of the interim analysis were then reported to the FDA by way of the independent third party in August 2012. A meeting was held with the FDA on November 5, 2012. The Company received unanimous support to continue the trial without any modification to protocol. We completed enrollment for the Phase III clinical trial in November of 2012 and aim to complete the trial in 2013.
In October 2010, we formed EM Japan, a wholly-owned subsidiary of Emmaus Medical that markets and sells AminoPure® in Japan and other neighboring regions. EM Japan also manages our distributors in Japan and may also import other medical products and drugs in the future. The results of EM Japan have been included in our consolidated financial statements since the date of our formation. EM Japan recorded approximately $266,000 in net sales in the year ended December 31, 2012 and approximately $160,000 in the year ended December 31, 2011.
In December 2010, we were awarded a five-year contract by the U.S. Department of Veterans Affairs for our NutreStore® product. In October 2007, we became the exclusive sublicensee of the SBS Patent for the U.S. market, including the rights to distribute the L-glutamine treatment for SBS under the trademark NutreStore®, in the United States, and commercially launched NutreStore® in June 2008. Previously we promoted Zorbtive® in the United States pursuant to a promotional rights agreement with EMD Serono, Inc. We terminated the agreement effective July 31, 2011 and no longer promote Zorbtive®. We do not anticipate that the termination of the promotional rights agreement with EMD Serono will have a significant impact on the Company as we had generated no revenues from the promotion of Zorbtive® pursuant to the promotional rights agreement and we continue to sell NutreStore® and AminoPure®.
We sell L-glutamine as a nutritional supplement under the brand name AminoPure® through our wholly-owned subsidiary Newfield Nutrition Corporation. The product is currently sold online and through retail stores in multiple states and via importers and distributors in Japan, Taiwan, Ghana and South Korea. As part of the growth strategy, Newfield Nutrition is focused on adding additional distributors both domestically and internationally. In November 2011, we added a new distributor in South Korea for the distribution of AminoPure® and received our first order from this South Korean distributor in January 2012.
36
In November 2011, we formed EM Europe, a wholly owned subsidiary of Emmaus Medical. EM Europe’s primary focus is expanding the business of Emmaus Medical in Europe. EM Europe submitted an application for orphan medicinal product designation with the EMA in January 2012.
On February 28, 2012, our board of directors and stockholders holding a majority of the voting power of our outstanding shares of common stock approved an amendment to our Certificate of Incorporation to effect a reverse stock split of all outstanding shares of our common stock at an exchange ratio of up to one-for-three (1:3) (the “Reverse Stock Split”), with our board of directors maintaining the discretion of whether or not to implement the Reverse Stock Split and which exchange ratio to implement prior to the closing of a public offering of our common stock, if any. The board of directors will effect the Reverse Stock Split, if at all, by filing the amendment with the Delaware Secretary of State. The par value and number of authorized shares of our common stock will remain unchanged.
On July 17, 2012, Emmaus Medical, Inc., a subsidiary of Emmaus Life Sciences, Inc., announced that the European Commission (EC) has granted Orphan Medicinal Product designation for the Company’s investigational drug Levoglutamide (L-glutamine) for the treatment of sickle cell disease. The EC designation follows the recommendation of the EMA’s Committee for Orphan Medicinal Products announced in May. The Company already has Orphan Drug status designation from the FDA.
On December 3, 2012, Emmaus Medical, Inc., a subsidiary of Emmaus Life Sciences, Inc., announced that it reached full enrollment for its Phase 3 clinical trial studying L-glutamine for the treatment of sickle cell disease.
Financial Overview
Revenue
As noted above, we are in the development stage. Since our inception in 2000, we have had limited revenue from the sale of NutreStore®, an FDA approved prescription drug to treat SBS, and AminoPure®, a nutritional supplement. We have funded operations principally through the private placement of equity securities and debt financings. Emmaus Medical’s operations to date have been primarily limited to staffing, licensing and promoting products for SBS, outsourcing distribution and sales activities, developing clinical trials for sickle cell treatment, establishing manufacturing for products and maintaining and improving its patent portfolio. In November 2011, we added a new distributor in South Korea for the distribution of AminoPure® and received our first order from this South Korean distributor in January 2012.
Currently, we generate revenue through the sale of NutreStore® L-glutamine powder for oral solution as a treatment for SBS, as well as AminoPure®, a nutritional supplement. Pursuant to the sublicense agreement for the SBS Patent, we are required to pay an annual royalty equal to 10% of adjusted gross sales of NutreStore® to Cato, the sublicensor. We made a royalty payment to Cato in the amount of $469 in October 2011 and $855 in May 2012 which represents the 10% royalty of the NutreStore® adjusted gross sales for 2010 and 2011, respectively. Management expects that any revenues generated from the sale of NutreStore® and AminoPure® will fluctuate from quarter to quarter as a result of the timing of orders and the amount of product sold.
Research and Development Expenses
Research and development costs consist of expenditures for new products and technologies, which primarily involve fees paid to the contract research organization (CRO), payroll-related expenses, study site payments, consultant fees, activities related to regulatory filings, manufacturing development costs and other related supplies. Product candidates in late stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of late stage clinical trials. We plan to increase our research and development expenses for the foreseeable future as we seek to complete the development of our most advanced product candidate, the amino acid L-glutamine as a prescription drug for the treatment of SCD.
Expenses related to the Phase III clinical trial are based on estimates of the services received and efforts expended pursuant to contracts with study sites and the CRO that conducts and manages the clinical trial on our behalf. We expect to incur increased research and development expenses as we continue to enroll patients in our Phase III clinical trial for sickle cell disease. The most significant clinical trial expenditures are related to the CRO costs and the payments to study sites. The contract with the CRO is based on time and material expended whereas the study site agreements are based on per patient costs as well as other pass-through costs including but not limited to start-up costs and institutional review board (IRB) fees. The financial terms of these agreements are subject to negotiation and vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts depend on factors such as the successful enrollment of patients and the completion of clinical trial milestones. Management estimates the expenses based on the time period over which the services will be performed and the level of effort to be expended by the CRO in each period. Although we do not expect the estimate to be materially different from amounts actually incurred, our estimate of the status and timing of services performed relative to the actual status and timing of services performed may vary and consequently, result in us reporting amounts that are higher or lower in any given period.
37
While we currently are focused on advancing the sickle cell clinical trials, future research and development expenses will depend on any new products or technologies that may be introduced in the pipeline. In addition, we cannot forecast with any degree of certainty which product candidate(s) may be subject to future collaborations, when such arrangements will be secured, if at all, and to what degree, if any, such arrangements would affect our development plans and capital requirements.
At this time, due to the inherently unpredictable nature of the drug development process and the interpretation of the regulatory requirements, we are unable to estimate with any degree of certainty the amount of costs which will be incurred in the continued development of the sickle cell treatment and other clinical programs. Clinical development timelines, the probability of success and development costs can differ materially from expectations and can vary widely. Our current estimated cost to complete the Phase III clinical trial is $4.0 million, which is based on the assumptions that the total number of trial sites does not increase beyond our current estimates and that we remain on the projected timeline. Should the total number of trial sites need to be increased or should the timeline require extension, there will be a commensurate increase in costs associated with the additional time and effort required by the CRO and Company staff.
The drug development process to obtain FDA approval is very costly and time consuming. Even with the granting of orphan drug status and Fast Track Designation, the successful development of the L-glutamine treatment for SCD is uncertain and subject to a number of variables, in addition to other risks described above under the caption “Risk Factors.”
The L-glutamine treatment for SCD is investigational in nature and has not yet received FDA approval. In order to grant marketing approval, the FDA must conclude that the clinical data establishes the safety and efficacy of the L-glutamine treatment for SCD and that the manufacturing processes and controls are adequate. Despite our efforts, the L-glutamine treatment for SCD may not be proven safe and effective in clinical trials, or meet applicable regulatory standards. We are focused on completing the Phase III clinical trial and submitting the new drug application (NDA) to the FDA for consideration. As a result of the uncertainties discussed above, the uncertainty associated with clinical trial enrollment and the risks inherent in the development process, we are unable to determine with any degree of certainty the duration and completion of costs or when, and to what extent, we will generate revenues from the commercialization and sale of the L-glutamine treatment for SCD.
In connection with our agreements with CellSeed related to the development of corneal cell sheet technology in the United States, we believe the cost to develop the corneal cell sheet technology in the United States will be approximately $3.0 million. Such estimate includes the anticipated cost of obtaining FDA approval for the corneal cell sheets and assumes that we will need biologic approval of the FDA for the corneal cell sheets, rather than pharmaceutical approval. We estimate that we will need another $2.0 million to commercialize the corneal cell sheet technology. Based on data currently available for cornea treatment using this technology, we anticipate it will be two to three years before we would be able to submit a BLA and obtain a marketing decision from the FDA to commercialize this product in the United States.
We also plan to participate in a multi-center international study involving cardiac cell sheets within the next two years and, if the studies are successful, we anticipate that we would be able to submit an application for marketing approval to the FDA to commercialize these products in about five years. We estimate that we will need $2.8 million to initiate the cardiac cell sheet work. The total estimated costs for development and commercialization of the cardiac cell sheets has not yet been determined. In addition, we estimate that we will need $2.5 million for research related to other cell sheet applications and cGMP laboratory costs to establish the infrastructure and production capabilities related to regenerative medicine products.
At this time, no research and development costs are associated with the SBS treatment.
General and Administrative Expenses
General and administrative expenses consist principally of salaries and related costs for personnel in executive, finance, business development, information technology, marketing, and legal functions. Other general and administrative expenses include facility costs, patent filing costs, and professional fees for legal, consulting, auditing and tax services. Inflation has not had a material impact on our general and administrative expenses over the past two years.
Environmental Expenses
The cost of compliance with environmental laws has not been material over the past two years and any such costs are included in general and administrative costs.
Inventories
Inventories consist of finished goods and work-in-process and are valued based on a first-in, first-out basis and at the lower of cost or market value. All of the purchases during the year ended December 31, 2012 were from one vendor and during the year ended December 31, 2011 were from two vendors.
38
Results of Operations
| Year Ended December 31, | From December 20, 2000 (date of inception) | |||||||||||
| 2012 | 2011 | to December 31, 2012 | ||||||||||
| REVENUES | ||||||||||||
| Sales | $ | 571,606 | $ | 339,156 | $ | 1,285,261 | ||||||
| Sales return & allowance | (28,548 | ) | (2,182 | ) | (61,087 | ) | ||||||
| Total revenues | $ | 543,058 | $ | 336,974 | $ | 1,224,174 | ||||||
| COST OF GOODS SOLD | ||||||||||||
| Cost of goods sold | 183,673 | 132,104 | 541,811 | |||||||||
| Scrapped inventory | — | — | 235,537 | |||||||||
| Total cost of goods sold | 183,673 | 132,104 | 777,348 | |||||||||
| GROSS PROFIT (LOSS) | 359,385 | 204,870 | 446,826 | |||||||||
| OPERATING EXPENSES | ||||||||||||
| Research and development | 2,966,583 | 1,552,205 | 9,418,440 | |||||||||
| Selling | 402,601 | 696,758 | 2,901,567 | |||||||||
| General and administrative | 8,307,376 | 5,047,886 | 18,968,002 | |||||||||
| Transaction costs | — | 788,893 | 788,893 | |||||||||
| 11,676,560 | 8,085,742 | 32,076,902 | ||||||||||
| LOSS FROM OPERATIONS | (11,317,175 | ) | (7,880,872 | ) | (31,630,076 | ) | ||||||
| OTHER INCOME (EXPENSE) | ||||||||||||
| Realized gain on securities available-for-sale | 275,822 | — | 275,822 | |||||||||
| Interest income | 37,649 | 30,493 | 153,376 | |||||||||
| Interest expense | (3,422,220 | ) | (979,170 | ) | (4,791,383 | ) | ||||||
| (3,108,749 | ) | (948,677 | ) | (4,362,185 | ) | |||||||
| LOSS BEFORE INCOME TAXES | (14,425,924 | ) | (8,829,549 | ) | (35,992,261 | ) | ||||||
| INCOME TAXES | 7,246 | 3,209 | 25,303 | |||||||||
| NET LOSS | (14,433,170 | ) | (8,832,758 | ) | (36,017,564 | ) | ||||||
| NET LOSS PER COMMON SHARE | $ | (0.59 | ) | $ | (0.38 | ) | ||||||
| WEIGHTED AVERAGE COMMON SHARES OUTSTANDING | 24,482,759 | 23,068,206 | ||||||||||
Years ended December 31, 2012 and 2011
Net Losses. Net losses increased by $5.6 million, or 63%, to $14.4 million from $8.8 million for the years ended December 31, 2012 and 2011, respectively. The increase in losses is primarily a result of increased operating expenses as discussed below. As of December 31, 2012, we had an accumulated deficit of approximately $36.0 million. Losses, partially offset by revenue from commercialized products, will continue as we advance our sickle cell treatment toward regulatory approval and potential commercialization. As a result, we anticipate that we will continue to incur net losses and be unprofitable for the foreseeable future.
Revenues. Sales increased $232,450, or 69%, to $571,606 from $339,156 for the years ended December 31, 2012 and 2011, respectively. Sales increased primarily due to increases in the number of units sold of our AminoPure® product. There were no sales returns of NutreStore® product in the year ended December 31, 2012 as compared to $1,540 in the year ended December 31, 2011. The Company estimates its sales returns based upon its prior sales and return history. Historically, sales returns have been very nominal. The Company continues to monitor its returns and will adjust its estimates based on its actual sales return experience. However, the Company recorded 5% of Sales Return Allowance for both NutreStore® and AminoPure® in the total amount of $28,548 for the year ended December 31, 2012.
39
Cost of Goods Sold. Cost of goods sold increased $51,569, or 39%, to $183,673 from $132,104 for the years ended December 31, 2012 and 2011, respectively. Cost of goods sold includes costs for raw material, packaging, testing, shipping and costs related to scrapped inventory. No scrapped inventory expense was realized for the years ended December 31, 2012 and 2011. All of the purchases during the year ended December 31, 2012 were from one vendor and during the year ended December 31, 2011 were from two vendors. Purchases from the two vendors amounted to 90% and 10% during the year ended December 31, 2011.
Research and Development Expenses. Research and development expenses increased $1.4 million, or 91%, to $3.0 million from $1.6 million for the years ended December 31, 2012 and 2011, respectively. This increase was primarily due to increases in our CRO costs and study site costs due to the increase in the patient enrollment for the Phase III clinical trial of our L-glutamine treatment for SCD. For the year ended December 31, 2012, the CRO spent a tremendous amount of time with Phase III study related activities and a total of 31 clinical study sites were actively recruiting patients during 2012 versus 25 during 2011. Research and development costs increased as follows: $1.1 million for CRO costs and $0.4 million for study site expenses.
Selling Expenses. Selling expenses decreased $0.3 million, or 42%, to $0.4 million from $0.7 million for the year ended December 31, 2012 and 2011, respectively. The decrease largely resulted from a scaling down of the NutreStore promotion efforts. Selling expenses included the costs for distribution, promotion, travel, tradeshows and exhibits related to NutreStore®, Zorbtive® (in 2011), and AminoPure®.
General and Administrative Expenses. General and administrative expenses increased $3.3 million, or 64%, to $8.3 million from $5.0 million for the years ended December 31, 2012 and December 31, 2011, respectively. The increase was primarily due to a $3.4 million increase in stock based compensation which had been less than $0.1 in 2011. The Company utilized stock based compensation to retain and provide an incentive for the management, staff and board of directors. Increases in payroll, insurance and other expenses were offset by decreases in professional fees.
We anticipate that our operating expenses will continue to increase for, among others, the following reasons:
| · | as a result of increased payroll, expanded infrastructure and higher consulting, legal, accounting and investor relations costs, and director and officer insurance premiums associated with being a public company; |
| · | to support research and development activities, which the Company expects to expand as development of our product candidate(s) continue; and |
| · | to build a sales and marketing team before we receive regulatory approval of a product candidate in anticipation of commercial launch. |
Liquidity and Capital Resources
Based on our losses to date, anticipated future revenue and operating expenses and our cash and cash equivalents balance of $0.4 million as of December 31, 2012, we do not have sufficient operating capital for our business without raising additional capital. We incurred losses of $14.4 million for the year ended December 31, 2012 and $8.8 million for the year ended December 31, 2011. We had an accumulated deficit since inception to December 31, 2012 of $36.0 million. We anticipate that we will continue to incur net losses for the foreseeable future as we incur expenses for the development and commercialization of L-glutamine as a prescription drug for the treatment of sickle cell disease, the corneal cell sheets technology and the expansion of corporate infrastructure, including costs associated with being a public company. We have previously relied on private equity offerings, debt financings, and loans, including loans from related parties. As part of this effort, we have received many loans from stockholders as discussed below. As of December 31, 2012, we had total outstanding notes payable of $11.3 million, consisting of $5.5 million of non-convertible promissory notes and $5.8 million of convertible notes. Of the $11.3 million aggregate outstanding principal amount of notes outstanding as of December 21, 2012, approximately $9.5 million will become due and payable within the twelve months ending December 31, 2013. Our failure to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategies, including the development of cell sheet technology in the United States.
Due to the uncertainty of our ability to meet our current operating and capital expenses, in their report on our audited annual financial statements as of and for the years ended December 31, 2012 and 2011, our independent auditors included an explanatory paragraph regarding concerns about our ability to continue as a going concern. Our financial statements contain additional note disclosures describing the circumstances that led to this disclosure by our independent auditors. There is substantial doubt about our ability to continue as a going concern as the continuation and expansion of our business is dependent upon obtaining further financing, successful and sufficient market acceptance of our products, and, finally, achieving a profitable level of operations.
On April 8, 2011, pursuant to a Research Agreement with CellSeed, we agreed to pay CellSeed $8.5 million within 30 days of the completion of all of the following: (i) the execution of the Research Agreement; (ii) the execution of the Individual Agreement; and (iii) CellSeed’s delivery of the accumulated information package, as defined in the Research Agreement, to us. Pursuant to the Individual Agreement with CellSeed, the Company agreed to pay $1.5 million to CellSeed and a royalty to be agreed upon by the parties. We paid the $1.5 million due to CellSeed pursuant to the Individual Agreement in February 2012. We currently anticipate that the additional $8.5 million payment obligation under the Research Agreement will become due and payable after we begin to generate revenues from the commercialization of our L-glutamine treatment for SCD. If the payment obligation becomes due and payable prior to our generation of revenue from the commercialization of our L-glutamine SCD treatment, we will need to seek other funding sources, including the sale of additional equity or debt securities, in order to make the payment. CellSeed may terminate these agreements if we are unable to make timely payments as required under the agreements.
40
In addition to the $8.5 million we have agreed to pay CellSeed pursuant to the Research Agreement, we currently estimate that we will need an additional $4.0 million to complete our Phase III clinical trial and $0.4 million to obtain FDA approval for our L-glutamine treatment for SCD. Our current cash burn rate is approximately $0.6 million per month.
Our future capital requirements will be substantial and may increase beyond our current expectations depending on many factors including, but not limited to: the number, duration and results of the clinical trials for our various products going forward; unexpected delays or developments in seeking regulatory approvals; the time and cost in preparing, filing, prosecuting, maintaining and enforcing patent claims; other unexpected developments encountered in implementing our business development and commercialization strategies; the outcome of litigation; and further arrangements, if any, with collaborators. We will rely, in part, on sales of AminoPure® for revenues, which we expect will increase. Revenues from NutreStore® are currently not significant and we are unsure whether sales of NutreStore® will increase. Until we can generate a sufficient amount of product revenue, future cash needs are expected to be financed through public or private equity offerings, debt financings, loans, including loans from related parties, or other sources, such as strategic partnership agreements and corporate collaboration and licensing arrangements. If we do not receive adequate funding to complete our clinical trials or to obtain FDA approval for our L-glutamine treatment for SCD, we may be required to delay our trial. If we are required to delay our trial, we will delay the approval of our L-glutamine treatment for SCD. If no funds are available to continue the trial, we risk losing the data gathered to date and may need to start over with additional subjects.
Our cash flow from operations is not adequate and our future capital requirements are substantial and may increase beyond our current expectations depending on many factors including, but not limited to: the duration and results of the clinical trials for our various products going forward; unexpected delays or developments in seeking regulatory approvals; the time and cost in preparing, filing, prosecuting, maintaining and enforcing patent claims; other unexpected developments encountered in implementing our business development and commercialization strategies; the outcome of litigation, if any; and further arrangements, if any, with collaborators. We intend to fund our cash flow needs through public or private equity offerings, debt financings, loans, and other sources such as strategic partnership agreements and corporate collaboration and licensing arrangements. Until we can generate a sufficient amount of product revenue, there can be no assurance of the availability of such capital on terms acceptable to the Company (or at all).
For the years ended December 31, 2012 and 2011, we borrowed varying amounts pursuant to promissory notes, the majority of which have been issued to our stockholders. As of December 31, 2012 and December 31, 2011, the aggregate amounts outstanding under promissory notes totaled $11.3 million and $5.7 million, respectively. The promissory notes carry interest from 0% to 11%. All notes are unsecured except for one promissory note in the principal amounts of $0.5 million. Interest on 0% loans was imputed at the incremental borrowing rate of 6.25% per annum. The net proceeds of the notes were used for working capital.
The table below lists our outstanding notes payable as of December 31, 2012, and the material terms of our outstanding borrowings:
| Lender | Annual Interest Rate | Date of loan | Term of Loan | Loan Principal Outstanding | Conversion Price | Shares Underlying Principal as of December 31, 2012 | |||||||||||||||
| Shigeru Matsuda (1) | 6.50 | % | 1/12/2009 | 5 years | $ | 294,355 | $ | 3.05 | 96,436 | ||||||||||||
| Kazuo Murakami (1) | 0.00 | % | 8/16/2010 | 5 years | $ | 18,000 | $ | 3.05 | 5,898 | ||||||||||||
| Makoto Murakami (1) | 0.00 | % | 8/16/2010 | 5 years | $ | 18,000 | $ | 3.05 | 5,898 | ||||||||||||
| Nami Murakami (1) | 0.00 | % | 8/16/2010 | 5 years | $ | 18,000 | $ | 3.05 | 5,898 | ||||||||||||
| M’s Support Co. Ltd. (1) | 0.00 | % | 8/17/2010 | 5 years | $ | 18,000 | $ | 3.05 | 5,898 | ||||||||||||
| Yumiko Takemoto (1) | 6.00 | % | 11/23/2010 | 5 years | $ | 2,000 | $ | 3.05 | 656 | ||||||||||||
| Mitsubishi UFJ Capital III, Limited Partnership | 10.00 | % | 3/14/2011 | 5 years | $ | 500,000 | $ | 3.05 | 163,809 | ||||||||||||
| Tracey & Mark Doi (3) | 8.00 | % | 2/10/2012 | 1 year | $ | 108,000 | $ | 3.60 | 30,001 | ||||||||||||
| Yukio Hasegawa (1) | 8.00 | % | 2/15/2012 | 1 year (2) | $ | 133,333 | $ | 3.60 | 37,037 | ||||||||||||
| Robert Jo (1) | 8.00 | % | 2/18/2012 | 1 year (2) | $ | 100,000 | $ | 3.60 | 27,778 | ||||||||||||
| Hiroshi Iguchi | 8.00 | % | 2/20/2012 | 1 year (2) | $ | 133,333 | $ | 3.60 | 37,037 | ||||||||||||
| J. R. Downey | 8.00 | % | 3/2/2012 | 1 year (2) | $ | 150,005 | $ | 3.60 | 41,669 | ||||||||||||
| Paul Terasaki (1) (7) | 10.00 | % | 5/1/2012 | 1 year | $ | 500,000 | $ | 3.30 | 151,516 | ||||||||||||
41
| Yasushi Nagasaki (5) | 10.00 | % | 6/29/2012 | On Demand | $ | 388,800 | $ | 3.30 | 117,819 | ||||||||||||
| Yumiko Duchane | 10.00 | % | 7/5/2012 | 1 year | $ | 30,000 | $ | 3.30 | 9,091 | ||||||||||||
| Andrew K. Wood (4) | 10.00 | % | 7/8/2012 | 1 year | $ | 3,240 | $ | 3.30 | 982 | ||||||||||||
| Hiromi Saito | 10.00 | % | 7/10/2012 | 1 year | $ | 25,000 | $ | 3.30 | 7,576 | ||||||||||||
| Suh Yung Min | 10.00 | % | 7/11/2012 | 1 year | $ | 1,180,716 | $ | 3.30 | 357,793 | ||||||||||||
| Kiyohiro Sugashita | 10.00 | % | 7/30/2012 | 1 year | $ | 6,600 | $ | 3.30 | 2,001 | ||||||||||||
| Yumiko Nakamura | 10.00 | % | 8/9/2012 | 2 years | $ | 49,500 | $ | 3.30 | 15,001 | ||||||||||||
| Masayuki Makino | 10.00 | % | 8/17/2012 | 1 year | $ | 6,600 | $ | 3.30 | 2,001 | ||||||||||||
| The Saito Family Trust (1) | 8.00 | % | 9/5/2012 | 1 year (2) | $ | 50,000 | $ | 3.60 | 13,889 | ||||||||||||
| Hideki & Eiko Uehara (1) | 10.00 | % | 9/7/2012 | 1 year | $ | 32,400 | $ | 3.60 | 9,001 | ||||||||||||
| Dennis Y. Teranishi | 10.00 | % | 9/9/2012 | 1 year | $ | 116,640 | $ | 3.60 | 32,401 | ||||||||||||
| Paul Shitabata (1) | 10.00 | % | 10/3/2012 | 1 year (2) | $ | 1,620,540 | $ | 3.60 | 450,151 | ||||||||||||
| Willis Lee (5) | 10.00 | % | 10/5/2012 | 1 year | $ | 138,242 | $ | 3.60 | 38,401 | ||||||||||||
| Alison Brown | 10.00 | % | 12/21/2012 | 2 years | $ | 100,800 | $ | 3.60 | 28,001 | ||||||||||||
| Yoshiko Takemoto | 10.00 | % | 12/27/2012 | 2 years | $ | 100,800 | $ | 3.60 | 28,001 | ||||||||||||
| Convertible Notes | $ | 5,842,904 | 1,721,640 | ||||||||||||||||||
| Yutaka Niihara (5) | 6.50 | % | 1/12/2009 | On Demand | $ | 272,800 | NA | ||||||||||||||
| Hope Int’l Hospice (6) | 8.00 | % | 1/12/2011 | 2 years | $ | 200,000 | NA | ||||||||||||||
| Hope Int’l Hospice (6) | 8.00 | % | 1/17/2012 | On Demand | $ | 200,000 | NA | ||||||||||||||
| Shigeru Matsuda (1) | 11.00 | % | 2/15/2012 | 2 years (2) | $ | 833,335 | NA | ||||||||||||||
| Hideki & Eiko Uehara (1) | 11.00 | % | 2/15/2012 | 2 years (2) | $ | 133,333 | NA | ||||||||||||||
| Hope Int’l Hospice (6) | 8.00 | % | 6/14/2012 | On Demand | $ | 200,000 | NA | ||||||||||||||
| Hope Int’l Hospice (6) | 8.00 | % | 6/21/2012 | On Demand | $ | 100,000 | NA | ||||||||||||||
| Cuc T. Tran | 11.00 | % | 6/27/2012 | 1 year | $ | 10,000 | NA | ||||||||||||||
| Yukio Hattori (1) | 11.00 | % | 7/20/2012 | On Demand | $ | 248,725 | NA | ||||||||||||||
| Yutaka Niihara (5) | 1.00 | % | 8/29/2012 | 1 year | $ | 1,270,100 | NA | ||||||||||||||
| Yutaka Niihara (5) | 10.00 | % | 12/5/2012 | Six months. | $ | 1,213,700 | NA | ||||||||||||||
| Lan T. Tran (5) | 11.00 | % | 2/10/2012 | 2 years (2) | $ | 80,000 | NA | ||||||||||||||
| For Days Co., Ltd. | 2.00 | % | 12/26/2012 | 2 years | $ | 700,000 | NA | ||||||||||||||
| Non-Convertible Notes | $ | 5,461,993 | |||||||||||||||||||
| Total, undiscounted (9) | $ | 11,304,897 | |||||||||||||||||||
| (1) | Related party – Shareholder |
| (2) | Due on Demand |
| (3) | Related party – Director |
| (4) | Employee |
| (5) | Related party – Officer |
| (6) | Dr. Niihara is also the CEO of Hope International Hospice, Inc. |
| (7) | Convertible into shares of the Company’s common stock at $3.30 per share or, if then publicly traded, at the average closing sale price per share for the three (3) trading days immediately preceding the exercise thereof, whichever is lower |
| (8) | Secured by the Company’s minority interest in CellSeed common stock |
| (9) | Total amount due on notes without discounts for fair market value of warrants issued and conversion features 9,485,442 |
42
Subsequent to the fiscal year ended December 31, 2012, the Company entered into additional financings arrangements, as set forth below. The total amount of these loans provided to the Company consists of $0.6 million of refinancings of notes payable outstanding as of December 31, 2012 and $0.7 million of additional borrowings, the proceeds of which were used for working capital.
| Lender | Annual Interest Rate | Date of loan | Term of Loan | Loan Principal Amount | Conversion Price | Shares Underlying Original Principal Amount | |||||||||||||||
| Hope Int’l Hospice(1) | 8.00 | % | 01/12/2013 | Due on Demand | $ | 200,000 | NA | NA | |||||||||||||
| For Days Co., Ltd. (2) | 2.00 | % | 01/29/2013 | 2 years | $ | 500,000 | NA | NA | |||||||||||||
| Tracey and Mark Doi(3) | 10.00 | % | 02/10/2013 | Due on Demand | $ | 116,640 | $ | 3.60 | 32,400 | ||||||||||||
| Hope Int’l Hospice(4) | 8.00 | % | 02/11/2013 | Due on Demand | $ | 50,000 | NA | NA | |||||||||||||
| Yukio Hasegawa(5) | 10.00 | % | 02/15/2013 | Due on Demand | $ | 144,000 | $ | 3.60 | 40,000 | ||||||||||||
| Sun Moo & Hyon Sil Lee(6) | 10.00 | % | 02/21/2013 | 2 years | $ | 100,800 | $ | 3.60 | 28,000 | ||||||||||||
| J. R. Downey(7) | 10.00 | % | 03/02/2013 | One year | $ | 162,005 | $ | 3.60 | 45,001 | ||||||||||||
Shigenori Yoshida(8) | 10.00 | % | 03/12/2013 | 2 years | $ | 100,800 | $ | 3.60 | 28,000 | ||||||||||||
| (1) | In January 2013, the Company refinanced a promissory note payable to Hope International Hospice in the principal amount of $200,000 bearing interest at 8% per annum. The term of this note is due on demand. Dr. Niihara is also the CEO of Hope International Hospice, Inc. |
| (2) | In January 2013, the Company issued a promissory note payable to For Days Co., Ltd. in the principal amount of $500,000 which bears interest at 2% per annum and matures on the two-year anniversary date of the note. |
| (3) | In February 2013, the Company refinanced a convertible note payable to Tracey and Mark Doi totaling $108,000 with new convertible notes totaling $116,640 which bear interest at 10% per annum. The term of this note is due on demand up to one year. Principal amounts and any unpaid interests due under the notes are convertible into shares of the Company’s common stock at $3.60 per share. Tracey Doi is a Director of the Company. |
| (4) | In February 2013, the Company issued a promissory note payable to Hope International Hospice in the principal amount of $50,000 bearing interest at 8% per annum. The term of this note is due on demand. Dr. Niihara is also the CEO of Hope International Hospice, Inc. |
| (5) | In February 2013, the Company refinanced convertible notes payable to Yukio Hasegawa, a shareholder, totaling $133,333 with new convertible notes totaling $144,000 which bear interest at 10% per annum. The term of this note is due on demand up to one year from the date of issuance. Principal amounts and any unpaid interests due under the notes are convertible into shares of the Company’s common stock at $3.60 per share. |
| (6) | In February 2013, the Company issued a convertible note payable to a third party in the principal amount of $100,800, which bears interest at 10% per annum. The note has two-year term, but the lender can demand repayment of the note at any time after two months from the date of issuance. The principal amount plus the unpaid accrued interest due under the convertible note is convertible into shares of the Company’s common stock at $3.60 per share. |
| (7) | In March 2013, the Company refinanced a convertible note payable to J. R. Downey in the amount of $150,005 with a new convertible note in the amount of $162,005 which bears interest at 10% per annum. The loan has one-year term but the lender can demand the loan after three months from the loan date. The principal amount and any unpaid interest due under the note are convertible into shares of the Company’s common stock at $3.60 per share. |
| (8) | In March 2013, the Company issued a convertible note payable to Shigenori Yoshida, a third party, in the principal amount of $100,800, which bears interest at 10% per annum and matures on the two-year anniversary date of the note. The principal amount plus the unpaid accrued interest due under the convertible note is convertible into shares of the Company’s common stock at $3.60 per share. |
Cash Flows
Net cash used in operating activities
Net cash flows used in operating activities increased by $0.5 million, or 8%, to $6.5 million from $6.0 million for the years ended December 31, 2012 and 2011, respectively. The increase in operating expenses of $3.6 million was a direct result of research and development expenses increasing by $1.4 million to support our SCD trial and selling, general, administrative and transaction expenses increasing by $2.1 million. The cash flow impact of this increase was further affected by a decrease in the fair market value of warrants issued for services of $1.1 million which were offset by an increase in share based compensation of $3.4 million, an increase in interest expense from amortizing loan discounts of $1.8 million and an increase in the accounts payable outstanding balance of $1.2 million. In addition, in 2011 we issued warrants to a consultant in exchange for services rendered, which resulted in a $1.1 million decrease in cash flows used in operating activities. These warrants have not been exercised and were recorded in operating activities as non-cash item. Another non-cash item in 2011 was interest expense accrued from discount of convertible notes of $0.8 million.
43
Net cash used in investing activities
Net cash flows used in investing activities increased to $1.5 million from $0.0 million for the year ended December 31, 2012 and 2011, respectively. The increase was mainly due to an increase in the payments toward license fees. No other investing activities occurred in the years ended December 31, 2012 and 2011.
Net cash from financing activities
Net cash flows from financing activities increased by $2.0 million, or 33%, to $8.1 million from $6.1 million for the years ended December 31, 2012 and 2011, respectively, primarily as a result of a $1.5 million increase in the proceeds from the issuance of notes payable and convertible notes payable, a decrease of $0.1 million in payment of principal on notes payable and convertible notes payable, and an increase of $0.4 million in proceeds from the issuance of shares of our common stock. Since mid-2011 the Company has mainly relied on the sale and issuance of convertible notes for its financial and working capital needs.
Off-Balance-Sheet Arrangements
Since our inception, Emmaus has not engaged in any off-balance sheet arrangements, including the use of structured finance, special purpose entities or variable interest entities.
Critical Accounting Policies
Management’s discussion and analysis of financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States, or GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses. On an ongoing basis, we evaluate these estimates and judgments, including those described below. We base our estimates on our historical experience and on various other assumptions that we believe to be reasonable under the present circumstances. These estimates and assumptions form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ materially from these estimates.
While our significant accounting policies are more fully described in Note 2 to our financial statements which is provided at the end of this Form 10-K, we believe that the following accounting policies are the most critical to assist you in fully understanding and evaluating our reported financial results and affect the more significant judgments and estimates that we use in the preparation of our financial statements.
Revenue recognition
We recognize revenue in accordance with ASC 605, “Revenue Recognition.”
Revenue is recognized when there is persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed and determinable and collection is reasonably assured.
With prior written approval of the Company, product is returnable only by our direct customers for a returned goods credit, provided the product meets any of the following criteria:
| A. | Product expiring within six (6) months of the expiration date printed on the package/container that is in the manufacturer’s original package/container and bears the original label. |
| B. | Expired product that is in the manufacturer’s original package/container and bears the manufacturer’s original label, provided, however, that expired product must be returned within 12 months of the expiration date printed on the package/container. |
| C. | Product shipped directly by the Company that is damaged in transit, subject to Free on Board (“FOB”) Destination, or material shipped in error by the Company. |
| D. | Product that is discontinued, withdrawn, or recalled. |
Credits will only be issued for full cartons without any missing packets of product. No credit is issued, nor does the Company accept charges or deductions for administrative, handling, or freight charges associated with the return of product to the Company. No credit is issued for product destroyed by anyone other than the Company. Customers must return the product within 60 days of receiving our written approval for the return or the return will not be issued a credit. The amount of the credit provided for returned product is based on the current wholesale acquisition cost of the returned product less 5%. When product is returned, a credit memo is applied to the customer’s current account balance or applied to future purchases. Credit memos expire one hundred eighty (180) days from date issued.
44
We estimate our sales return based upon our prior sales and return history. Historically, sales returns have been very nominal. The extraordinary sales returns in the year ended December 31, 2010 were all related to the same batch of NutreStore® product produced in 2008 that expired. The expiry date of that batch of NutreStore® product was two years after the date of manufacture. Currently-manufactured batches of NutreStore® product have an expiration date that is four years after the date of manufacture. All products sold in 2009 were part of the batch that had a two year expiry period and expired in 2010. All NutreStore® products produced in 2010 and sold from 2010 through 2012 have an expiry date of 2014 and we anticipate that the product will be consumed before expiration and not returned. We continue to monitor our returns and will adjust our estimates based on its actual sales return experience. As of December 31, 2012, we recorded a 5% sales return allowance for the NutreStore® sales in the accompanying financial statements.
We are required to pay royalties, which are recognized as an expense upon sale of the products. We are required to pay a royalty to Cato equivalent to 10% of our adjusted gross sales of NutreStore® calculated on an annual basis. The 10% royalty is calculated at the end of the year and accrued on an annual basis.
Share-based compensation
We recognize compensation cost for share-based compensation awards during the service term of the recipients of the share-based awards. The fair value of share-based is calculated using the Black-Scholes-Merton pricing model. The Black-Scholes-Merton model requires subjective assumptions regarding future stock price volatility and expected time to exercise, which greatly affect the calculated values. The expected term of awards granted is derived from historical data on awards exercised and post-vesting employment termination behavior. The risk-free rate selected to value any particular grant is based on the U.S. Treasury rate that corresponds to the vesting period of the grant effective as of the date of the grant. The expected volatility is based on the historical volatility of the common stock of comparable publicly traded companies. These factors could change, affecting the determination of stock-based awards expense in future periods.
Marketable securities
Securities available-for-sale are recorded at cost and any increases or decreases in fair market value are recorded as unrealized gains or losses, net of taxes in accumulated other comprehensive income. We monitor these investments for impairment and make appropriate reductions in carrying values when necessary.
Not required for a smaller reporting company.
The information required by this Item 8 is incorporated by reference to information begins on Page F-1 of this Form 10-K.
None.
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission's rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports that we file under the Exchange Act is accumulated and communicated to our management, including our principal executive and financial officers, as appropriate to allow timely decisions regarding required disclosure.
As of the end of the period covered by this Annual Report on Form 10-K, we conducted an evaluation, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, of the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) of the Exchange Act). Based upon this evaluation as of December 31, 2012 (described below), our Chief Executive Officer and Chief Financial Officer concluded that the Company’s disclosure controls and procedures are effective.
45
Internal Control over Financial Reporting
(a) Management's Annual Report on Internal Control over Financial Reporting
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) under the Exchange Act. Under the supervision and with the participation of the Company’s management, including its Chief Executive Officer and Chief Financial Officer, the Company conducted an evaluation of the effectiveness of its internal control over financial reporting based on criteria established in the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, the Company’s management concluded that its internal control over financial reporting was effective as of December 31, 2012.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risks that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
(b) Attestation Report
This annual report does not include an attestation report of our independent registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to the attestation by our independent registered public accounting firm because smaller reporting companies are exempt from this requirement.
Changes in Internal Control Over Financial Reporting
Based on the evaluation for the year ended December 31, 2011, the Company’s management concluded that its internal control over financial reporting was not effective. We had identified a material weakness consisting of a lack of personnel with adequate knowledge of U.S. Generally Accepted Accounting Principles (GAAP). On April 2, 2012 we hired additional personnel with in-depth knowledge of GAAP to evaluate and improve our existing internal control documentation and procedures and to develop clear identification of key financial and reporting controls in order to remediate the material weakness described herein. Our evaluation for the year ended December 31, 2012 has concluded that the prior material weakness has been remediated.
Based on the evaluation of our management as required by paragraph (d) of Rule 13a-15 of the Exchange Act, we believe that there were no changes in our internal control over financial reporting that occurred during the quarter ended December 31, 2012 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
None.
46
Executive Officers, Directors and Key Employees
The following individuals constitute our board of directors and executive management:
| Name | Age | Position | ||
| Yutaka Niihara, M.D., MPH | 53 | President, Chief Executive Officer and Director | ||
| Willis C. Lee | 52 | Chief Operating Officer and Director | ||
| Peter B. Ludlum | 57 | Executive Vice President and Chief Financial Officer | ||
| Lan T. Tran | 37 | Chief Administrative Officer and Corporate Secretary | ||
| Yasushi Nagasaki | 45 | Senior Vice President Finance | ||
| Henry A. McKinnell, Jr., Ph.D. | 70 | Chairman of the Board | ||
| Tracey C. Doi | 52 | Director | ||
| Maurice J. DeWald | 73 | Director |
All officers of the Company devote at least forty (40) hours per week, the equivalent of a full-time employee, to their positions with Company.
Background of Officers and Directors
The following is a brief summary of the background of each director and executive officer of the Company:
Yutaka Niihara, M.D., MPH has served as the President, Chief Executive Officer and Director of the Company since the closing of the Merger. Previously, Dr. Niihara served as the President, Chief Executive Officer and Chairman of the Board of Emmaus Medical from 2003 until the closing of the Merger. In addition, Dr. Niihara has served as the President, Chief Executive Officer and Medical Director of Hope International Hospice since May 2005 . From June 1992 to October 2009, Dr. Niihara served as a physician specialist for Los Angeles County. Dr. Niihara is the principal inventor of the patented L-glutamine therapy for treatment of SCD. Dr. Niihara has been involved in patient care and research for sickle cell disease during most of his career and is a widely published author in the area of sickle cell disease. Dr. Niihara is board-certified by the American Board of Internal Medicine/Medical Oncology and the American Board of Internal Medicine/Hematology. He is licensed to practice medicine in both the United States and Japan. Dr. Niihara is a Professor of Medicine at the David Geffen School of Medicine at UCLA. Dr. Niihara received his B.A. in Religion from Loma Linda University in 1982 and obtained his MD degree from the Loma Linda University School of Medicine in 1986. Dr. Niihara is qualified to serve on our board of directors due to his knowledge and experience.
Henry A. McKinnell, Jr., Ph.D. has served as Chairman of the Board of the Company since the closing of the Merger and as a director of Emmaus Medical since April 2010. Dr. McKinnell served as the Chairman of the Board of Pfizer Inc. (NYSE: PFE), a pharmaceutical company, from May 2001 until December 2006. He retired from Pfizer and its Board in February 2007. He also served as Chief Executive Officer of Pfizer from January 2001 to July 2006. He served as President of Pfizer from May 1999 to May 2001, and as President of Pfizer Pharmaceuticals Group from January 1997 to April 2001. Dr. McKinnell served as Chief Operating Officer of Pfizer from May 1999 to December 2000 and as Executive Vice President from 1992 to 1999. Since October 1997, Dr. McKinnell has served as a member of the board of directors of Moody’s Corporation (NYSE: MCO), where he serves as Chairman and a member of the audit committee, the governance and compensation committee and MIS committee. Dr. McKinnell has served as a director of Optimer Pharmaceuticals, Inc. (NasdaqGM: OPTR) since January 2011 and serves as Chairman of the Board and Chief Executive Officer. Dr. McKinnell also serves as Chairman of the Board of the Accordia Global Health Foundation. He is Chairman Emeritus of the Connecticut Science Center, and is a member of the Academic Alliance for AIDS Care and Prevention in Africa. He served as director of ExxonMobil Corporation from 2002 to 2007 and John Wiley & Sons until 2005. Dr. McKinnell holds a Bachelor’s Degree in business from the University of British Columbia, and M.B.A. and Ph.D. degrees from the Stanford University Graduate School of Business. We believe that Dr. McKinnell is qualified to serve on our board of directors due to his extensive experience and leadership in the pharmaceutical industry, in addition to his substantial involvement with business and civic organizations and years of experience as an officer and director of publicly traded companies.
47
Tracey C. Doi has served as a director of the Company since August 2011. Ms. Doi serves as the Group Vice President and Chief Financial Officer of Toyota Motor Sales, U.S.A., Inc., the marketing, sales, distribution and customer service arm of Toyota, Lexus and Scion in the United States. Ms. Doi is responsible for corporate finance, tax, customs, treasury, accounting, procurement and corporate services at Toyota Motor Sales. Ms. Doi serves on Toyota Motor Sales’ audit committee, risk committee and benefit committee, as well as an officer or director of several subsidiaries. Ms. Doi joined Toyota in 2000 as vice president, corporate controller and was promoted to CFO in 2003. Prior to that, she held financial executive positions with AT&T Wireless, L.A. Cellular Telephone Company, and L.A. Gear, Inc. Ms. Doi received a bachelor’s degree in business economics from UCLA in 1983 and is a certified public accountant (inactive). Ms. Doi serves on the Federal Reserve Bank of San Francisco’s Economic Advisory Council, the board of directors for the Food Allergy & Anaphylaxis Network (FAAN), the board of governors for the Japanese American National Museum, and the board of directors for the Japan America Society. The Company believes that Ms. Doi is qualified to serve as a director of the Company due to her business and financial management experience, including her position as a Chief Financial Officer, and her knowledge of audit committee functions.
Maurice J. DeWald has served as a director of the Company since August 2011. Mr. DeWald has served as the Chairman and Chief Executive Officer of Verity Financial Group, Inc., a financial advisory firm he founded, since 1992. From 1962 to 1991, Mr. DeWald served in various positions with KPMG LLP, including managing partner of the Orange County office from 1976 to 1984, managing partner of the Chicago office from 1985 to 1986 and managing partner of the Los Angeles office from 1986 to 1991. Mr. DeWald has served as a director of Targeted Medical Pharma, Inc. (OTCBB: TRGM) since February 2011 and as non-executive Chairman since October 2011, as a director of Healthcare Trust of America, Inc. since September 2006 and as a director of Integrated Healthcare Holdings, Inc. (OTCBB: IHCH.OB) since August 2005, including as Chairman of the Board since October 2008. Mr. DeWald served as a director of Mizuho Corporate Bank of California from 1992 to 2011. From 1991 to July 2003 he served on the board of directors of Tenet Healthcare Corporation (NYSE: THC) and from 1995 to 2003 he served on the board of directors of ARV Assisted Living, Inc. Mr. DeWald received a B.B.A. in finance and accounting from the University of Notre Dame in 1962 and is a certified public accountant (inactive). Mr. DeWald also sits on the School of Business Advisory Council of the University of Notre Dame and has previously served as the Chairman and a director of the United Way of Greater Los Angeles. The Company believes that Mr. DeWald is qualified to sit on the Company’s board of directors due to his extensive management, finance, public accounting and public company directorship experience, as well as his experience in the healthcare industry.
Willis C. Lee, MS has served as the Chief Operating Officer and a director of the Company since the closing of the Merger. He has served as the co-Chief Operating Officer and Chief Financial Officer and as a director of Emmaus Medical from April 2010 until the closing of the Merger. Prior to that, he was the Controller at Emmaus from February 2009 to February 2010. From 2004 to 2010, Mr. Lee led worldwide sales and business development of Yield Dynamics product group at MKS Instruments, Inc., a provider of instruments, subsystems, and process control solutions for the semiconductor, flat panel display, solar cell, data storage media, medical equipment, pharmaceutical manufacturing, and energy generation and environmental monitoring industries. Mr. Lee also served as President and Managing Director of Kenos Inc. from January 2004 to December 2008. Mr. Lee held various managerial and senior positions at semiconductor companies such as MicroUnity Systems Engineering, Inc. from August 1995 to July 1996, HPL, Inc. from June 2000 to October 2002, Syntricity, Inc. from November 2002 to April 2004 and also at a healthcare actuarial consulting firm, Reden & Anders from September 1996 to June 2000, which was acquired by United Health Care. Mr. Lee received his B.S. and M.S. in Physics from University of Hawaii (1984) and University of South Carolina (1986) respectively. Mr. Lee’s knowledge of our business and operations and his business, leadership and management experience qualify him to serve as a member of the Company’s board of directors.
Peter Ludlum was appointed Executive Vice President and Chief Financial Officer of the Company effective April 2, 2012. Mr. Ludlum previously served as the Chief Financial Officer of Energy and Power Solutions, Inc. from April 2008 to March 2012. Mr. Ludlum also served as the Financial Compliance Officer from April 2006 to March 2008 and the Chief Financial Officer from April 2005 to April 2006 of Applied Medical Resources Corporation. Mr. Ludlum also served as Group Controller for IsoTis S.A. from October 2003 to April 2005. IsoTis S.A. acquired GenSci Regeneration Sciences Inc. in October 2003 where Mr. Ludlum served as Vice President and Chief Financial Officer from December 1999 to October 2003. Prior to his position at GenSci, Mr. Ludlum had served as the Vice President Finance and Chief Financial Officer from November 1996 to December 1999 of Pacific Biometrics, Inc. Earlier in his career Mr. Ludlum had worked for Derlan Industries, Ltd. as Corporate Controller and Treasurer and in various positions at Mobil Oil Corporation, and for subsidiaries of Bechtel Corporation and PacifiCorp. Mr. Ludlum is a Certified Management Accountant (CMA). He received a B.S. in Business and Economics with a major in accounting from Lehigh University in 1977 and a Masters in Business Administration with a concentration in Finance from California State University, Fullerton in 1991.
Lan T. Tran, MPH has served as the Chief Administrative Officer and Corporate Secretary of the Company since the closing of the Merger. She has served as the Co-Chief Operating Officer and Corporate Secretary of Emmaus Medical since April 2010 and as the Chief Compliance Officer of Emmaus Medical since May 2008. Prior to joining Emmaus Medical, Ms. Tran was with LABioMed from September 1999 to April 2008 and held positions of increasing responsibility including Grants and Contracts Trainee from September 1999 to March 2000, Grants and Contracts Officer from April 2000 to August 2004, Associate Director, Pre-Clinical/Clinical Trials Unit from September 2004 to June 2005, Director, Pre-Clinical/Clinical Trials Unit from July 2005 to June 2007, and Assistant Vice President, Research Administration from June 2007 to April 2008. In her position as Director, Pre-Clinical/Clinical Trials Unit and Assistant Vice President, Research Administration, Ms. Tran was part of the executive management team of LABioMed and responsible for all administrative aspects of research in her assigned area at LABioMed, which had a research budget of $61,000,000 in 2008. Ms. Tran holds a B.S. in Psychobiology from UCLA, which was awarded in 1999, and a Masters of Public Health from UCLA which was awarded in 2002.
48
Yasushi Nagasaki was appointed as the Senior Vice President, Finance of the Company effective April 2, 2012. From May 2011 to April 1, 2012, Mr. Nagasaki served as the Company’s Chief Financial Officer. From September 2005 until joining our Company, Mr. Nagasaki was the Chief Financial Officer of Hexadyne Corporation, an aerospace and defense supplier. From May 2003 to August 2005, Mr. Nagasaki was the Controller at Upsilon Intertech Corporation. Mr. Nagasaki received a B.A. in Commerce in 1992 from Waseda University and an M.A. in International Policy Studies in 1994 from the Monterey Institute of International Studies.
Our success depends, to a significant extent, upon the continued services of Yutaka Niihara, M.D. MPH, who is the founder of Emmaus Medical and our current President and Chief Executive Officer. Since inception, we have been dependent upon Dr. Niihara, who was one of the initial patentees for the technology for treatment of SCD.
Family Relationships
There are no family relationships among any of the officers and directors.
Involvement in Certain Legal Proceedings
There have been no events under any bankruptcy act, criminal proceedings, judgments, injunctions, orders or decrees material to the evaluation of the ability and integrity of any director, executive officer, promoter or control person of the Company during the past ten years.
On July 23, 2012, the Company filed a complaint in Los Angeles Superior Court against AFH Advisory and Amir F. Heshmatpour. Mr. Heshmatpour is a former officer of AFH Acquisition IV, Inc. (prior to the Merger) and former director of the Company and is the Managing Partner of AFH Advisory. Pursuant to the complaint, the Company sought return of approximately $1.2 million in proceeds (the “Placement Proceeds”) raised in a private placement conducted by AFH Acquisition IV, Inc. in April 2011 prior to the Merger, which Placement Proceeds were not received by the Company at the time of the Merger. On December 27, 2012, the court granted a motion to stay the case on the grounds that the forum-selection clause in the Amended and Restated Letter of Intent dated September 30, 2011 by and between the Company and AFH Advisory (the “LOI”) required the Company’s claims to be brought in the State of Delaware.
On July 19, 2012, the Company elected to terminate the offering contemplated by the LOI. Based on the Company’s termination of the offering pursuant to the terms of the LOI and separate from the Company’s lawsuit (described above) to recover the Placement Proceeds, the Company is seeking to cancel certain shares of its common stock that were previously issued to AFH Advisory, Mr. Heshmatpour and their affiliates.
On September 7, 2012, AFH Advisory and related parties filed a complaint against the Company in the Superior Court of Delaware. The complaint alleges that the Company does not have the right to cancel the plaintiffs’ shares and asks the court to issue a declaratory judgment to that effect. In addition, the complaint alleges that the Company has breached the LOI by attempting to cancel plaintiffs’ shares and failing to reimburse AFH for expenses incurred in connection with the Merger and asks the Court to award plaintiffs damages of approximately $9.4 million. The Company believes AFH’s claims are completely without merit and is defending them vigorously.
On October 12, 2012, the Company filed counterclaims against the plaintiffs and third-party claims against Mr. Heshmatpour in the Superior Court of Delaware. The Company is asking the Court for an order declaring that the plaintiffs’ and Mr. Heshmatpour’s shares are canceled and that, because of fraudulent inducement on their part, the LOI is void and of no further effect. In addition, the Company is asking the Court to award compensatory and punitive damages, in amounts to be determined, for breach of contract, unjust enrichment, and fraud. The parties are currently engaged in discovery, with trial scheduled for May 2013.
Except as described above, the Company is not aware of any legal proceedings in which any director, nominee, officer or affiliate of the Company, any owner of record or beneficially of more than five percent of any class of voting securities of the Company, or any associate of any such director, nominee, officer, affiliate of the Company, or security holder is a party adverse to the Company or any of its subsidiaries or has a material interest adverse to the Company or any of its subsidiaries.
Board of Directors and Committees and Director Independence
Currently, our board of directors has determined that each of Henry A. McKinnell, Jr., Tracey C. Doi and Maurice J. DeWald is an “independent” director as defined by the NASDAQ Marketplace Rules, currently in effect and all applicable rules and regulations of the SEC. All members of the Audit, Compensation and Nominating Committees satisfy the “independence” standards applicable to members of each such committee. The board of directors made this affirmative determination regarding these directors’ independence based on discussions with the directors and on its review of the directors’ responses to a standard questionnaire regarding employment and compensation history; affiliations, family and other relationships; and transactions with the Company. The board of directors considered relationships and transactions between each director or any member of his immediate family and the Company and its subsidiaries and affiliates.
49
Audit Committee
We established our Audit Committee on May 3, 2011. The Audit Committee consists of Dr. McKinnell, Ms. Doi and Mr. DeWald each of whom is an independent director as defined by the NASDAQ Marketplace Rules. Mr. DeWald serves as Chairman of the Audit Committee. Each of Ms. Doi and Mr. DeWald qualifies as an “audit committee financial expert” as defined under Item 407(d) of Regulation S-K. The purpose of the Audit Committee is to represent and assist our board of directors in its general oversight of our accounting and financial reporting processes, audits of the financial statements and internal control and audit functions. The Audit Committee’s responsibilities include:
| · | The appointment, replacement, compensation, and oversight of work of the independent auditor, including resolution of disagreements between management and the independent auditor regarding financial reporting, for the purpose of preparing or issuing an audit report or performing other audit, review or attest services. |
| · | Reviewing and discussing with management and the independent auditor various topics and events that may have significant financial impact on our company or that are the subject of discussions between management and the independent auditors. |
The board of directors has adopted a written charter for the Audit Committee. A copy of the Audit Committee Charter is available on our website at www.Emmausmedical.com.
Compensation Committee
We established our Compensation Committee on May 3, 2011. The Compensation Committee consists of Dr. McKinnell, Ms. Doi and Mr. DeWald, each of whom is an independent director as defined by the NASDAQ Marketplace Rules. Ms. Doi is the Chairman of the Compensation Committee. The Compensation Committee is responsible for the design, review, recommendation and approval of compensation arrangements for our directors, executive officers and key employees, and for the administration of our equity incentive plans, including the approval of grants under such plans to our employees, consultants and directors. The Compensation Committee also reviews and determines compensation of our executive officers, including our Chief Executive Officer. The board of directors has adopted a written charter for the Compensation Committee. A copy of the Compensation Committee Charter is available on our website at www.Emmausmedical.com.
Nominating and Corporate Governance Committee
The Nominating and Corporate Governance Committee consists of Dr. McKinnell, Ms. Doi and Mr. DeWald, each of whom is an independent director as defined by the NASDAQ Marketplace Rules. Ms. Doi is the Chairman of the Nominating and Corporate Governance Committee. The Nominating and Corporate Governance Committee assists in the selection of director nominees, approves director nominations to be presented for stockholder approval at our annual general meeting, fills any vacancies on our board of directors, considers any nominations of director candidates validly made by stockholders, and reviews and considers developments in corporate governance practices. The board of directors has adopted a written charter for the Nominating and Corporate Governance Committee. A copy of the Nominating and Corporate Governance Committee Charter is available on our website at www.Emmausmedical.com.
Section 16(a) Beneficial Ownership Reporting Compliance
The Company’s securities are currently registered under Section 12 of the Securities Exchange Act of 1934, as amended. As a result, and pursuant to Rule 16a-2, the Company’s directors and officers and holders of 10% or more of its common stock are currently required to file statements of beneficial ownership with regards to their ownership of equity securities under Sections 13 or 16 of the Exchange Act. Based on a review of written representations from our executive officers and directors and a review of Forms 3, 4 and 5 furnished to us, we believe that during the fiscal year ended December 31, 2012 the following directors, officers and owners of more than 10% of our common stock failed to file, on a timely basis, reports required by Section 16(a) of the Exchange Act:
| · | Yasushi Nagasaki failed to timely file a Form 4 in June 2012, upon his receipt of convertible notes from the Company, due to an administrative oversight. |
| · | Yutaka Niihara failed to timely file a Form 4 in August 2012, upon his receipt of warrants related to a loan to the Company, due to an administrative oversight. |
| · | Willis Lee failed to timely file a Form 4 in October 2012, upon his receipt of convertible notes from the Company, due to an administrative oversight. |
50
Code of Business Conduct and Ethics
On May 3, 2011, our board of directors approved a Code of Conduct and Ethics (the “Code of Ethics”) that applies to all of the directors, officers and employees of the Company. The Code of Ethics addresses, among other things, honesty and ethical conduct, conflicts of interest, compliance with laws, regulations and policies, including disclosure requirements under the federal securities laws, confidentiality, trading on inside information, and reporting of violations of the code. A copy of the Code of Ethics is available on our website at www.Emmausmedical.com. Requests for copies of the Code of Ethics should be sent in writing to Emmaus Medical, Inc., Attention: Secretary, 20725 S. Western Avenue, Ste. 136, Torrance, CA 90501-1884.
Summary Compensation Table
The following table sets forth information concerning the compensation earned by our chief executive officer, chief financial officer and our next three most highly compensated executive officers for the two fiscal years ended December 31, 2012 and 2011.
| Name and Position | Year | Salary ($) | Bonus ($) | Option awards ($) (1) | Total ($) | |||||||||||||
| Yutaka Niihara, M.D., MPH | 2012 | 250,000 | — | 840,686 | 1,090,686 | |||||||||||||
| President, Chief Executive Officer and Director | 2011 | 218,750 | — | — | 218,750 | |||||||||||||
| Willis C. Lee | 2012 | 180,000 | — | 840,686 | 1,020,686 | |||||||||||||
| Chief Operating Officer and Director | 2011 | 170,000 | — | — | 170,000 | |||||||||||||
| Peter B. Ludlum | 2012 | 135,000 | — | — | 135,000 | |||||||||||||
| Chief Financial Officer | 2011 | — | — | — | — | |||||||||||||
| Lan T. Tran | 2012 | 180,000 | — | 840,686 | 1,020,686 | |||||||||||||
| Chief Administrative Officer and Corporate Secretary | 2011 | 161,000 | 50,000 | — | 211,000 | |||||||||||||
| Yasushi Nagasaki | 2012 | 180,000 | — | 840,686 | 1,020,686 | |||||||||||||
| Senior Vice President, Finance | 2011 | 120,000 | — | — | 120,000 | |||||||||||||
__________________
| (1) | The Company has calculated the grant date fair value of the option grants utilizing the Black-Scholes-Merton Option pricing model. Accordingly, the fair value of the underlying shares was determined based on recent transactions by the Company to sell shares to third parties and other factors determined by management to be relevant to the valuation of such shares. The fair value of the options was determined through the Black Scholes Option pricing model with the following inputs: |
| Stock Price | $3.60 |
| Exercise Price | $3.60 |
| Term | 10 years |
| Risk-Free Rate | 2.22% |
| Dividend Yield | 0% |
| Volatility | 141.70% |
Total compensation for Dr. Niihara, Mr. Lee and Ms. Tran for the year ended December 31, 2012 and 2011 listed in the table above does not include the amount of the annual performance bonus to be paid pursuant to their respective employment agreements based on performance in 2012 and 2011. Pursuant to the employment agreements, the amounts of the target annual performance bonuses for 2011 for Dr. Niihara, Mr. Lee and Ms. Tran were to be $50,000, $25,000 and $20,000, respectively. However, the Company did not grant performance-based cash bonuses in 2011 and 2012, in large part because of management’s emphasis on preserving available capital to fund operating expenses. Cash incentive bonus targets for 2013, if any, have not been determined.
51
On April 2, 2012, the Board of Directors of the Company authorized and approved option grants to the named executive officers in the amounts set forth below, with a term of 10 years and an exercise price of $3.60 per share, the fair market value of a share of Common Stock as of the grant date, as determined by the Board of Directors.
| Name | Title | Number of Options | ||
| Dr. Yutaka Niihara | CEO, President and Director | 250,000 | ||
| Willis Lee | COO and Director | 250,000 | ||
| Yasushi Nagasaki | Senior Vice President, Finance | 250,000 | ||
| Lan Tran | Chief Administrative Officer and Corporate Secretary | 250,000 |
The options granted to each named executive officer will vest as follows: each option will vest annually in equal amounts (or as close to an equal amount as possible) over a period of three years with the first one-third (1/3) of such amount to vest one year from the Grant Date.
Outstanding Equity Awards at 2012 Fiscal Year End
The following table sets forth information regarding outstanding equity awards held by our named executive officers at the end of fiscal 2012.
| Outstanding Equity Awards at Fiscal Year-End for Fiscal Year 2012 | |||||||||||||||||||||||||||||||||||
| Option Awards | Stock Awards | ||||||||||||||||||||||||||||||||||
Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable (1) | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($) | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested ($) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) | ||||||||||||||||||||||||||
| Yutaka Niihara, M.D., MPH | — | 250,000 | — | $ | 3.60 | 4/1/2022 | — | — | — | — | |||||||||||||||||||||||||
| Willis C. Lee | — | 250,000 | — | $ | 3.60 | 4/1/2022 | — | — | — | — | |||||||||||||||||||||||||
| Lan T. Tran | — | 250,000 | — | $ | 3.60 | 4/1/2022 | — | — | — | — | |||||||||||||||||||||||||
| Yasushi Nagasaki | — | 250,000 | — | $ | 3.60 | 4/1/2022 | — | — | — | — | |||||||||||||||||||||||||
| (1) | Each option will vest annually in equal amounts (or as close to an equal amount as possible) over a period of three years with the first one-third (1/3) amount to vest one year from the Grant Date. |
Employment Agreements
On April 5, 2011, we entered into employment agreements with Yutaka Niihara, M.D., MPH, our Chief Executive Officer, Willis C. Lee, our Chief Operating Officer, and Lan T. Tran, our Chief Administrative Officer. On April 8, 2011, we entered into an employment agreement with Yasushi Nagasaki, our former Chief Financial Officer and current Senior Vice President, Finance on April 2, 2012, we entered into an employment agreement with Peter Ludlum, our Executive Vice President and Chief Financial Officer (the employment agreements set forth herein, collectively, the “Employment Agreements”). Each of the Employment Agreements has an initial 2-year term, unless terminated earlier. The Employment Agreements for Dr. Niihara, Mr. Lee and Ms. Tran automatically renew for additional one year periods unless the Company or the officer provides notice of non-renewal at least sixty (60) days prior to the expiration of the then current term.
Base Salary, Bonus and Other Compensation. Dr. Niihara’s, Mr. Lee’s, Ms. Tran’s, Mr. Nagasaki’s and Mr. Ludlum’s base salary is $250,000, $180,000, $180,000, $180,000 and $180,000 per year, respectively, which will be reviewed at least annually. In addition to base salary, the officers may be entitled to receive an annual performance bonus based on the officer’s performance for the previous year. The target bonuses for 2011 for Dr. Niihara, Mr. Lee and Ms. Tran were $50,000, $25,000 and $20,000, respectively. There was no target bonus for 2011 for Mr. Nagasaki or performance target bonus for 2012 for Mr. Ludlum. The officers are also eligible to receive paid vacation, and participate in health and other benefit plans and will be reimbursed for reasonable and necessary business expenses. 2011 and 2012 cash performance bonuses were not awarded to our executive officers in order to preserve capital to fund operating expenses.
52
Equity Compensation. The employment contracts state that effective December 31, 2011 and at the end of each calendar year on December 31, or as soon as reasonably practicable after each such December 31 (each a “Grant Date”), the Company will grant non-qualified ten-year stock options with a Black Scholes value of $100,000 to Dr. Niihara, $50,000 to Mr. Lee, and $40,000 to Ms. Tran, in each case with an exercise price per share equal to the “Fair Market Value” (as such term is defined in the Company’s 2011 Stock Incentive Plan) on the applicable Grant Date. Mr. Nagasaki will receive a grant of non-qualified ten-year options with a Black Scholes value of $40,000 on December 31, 2012; however, there is no predetermined grant of options to Mr. Nagasaki for 2011. One-third of the options granted to each officer will vest on the first anniversary of the applicable Grant Date, one-third will vest on the second anniversary of the applicable Grant Date and the final one-third will vest on the third anniversary of the applicable Grant Date. Any unvested options will vest immediately upon a change in control (as defined below), termination of the officer’s employment other than a voluntary termination by the officer or a termination of the officer for cause. In the event that the officer is terminated for any reason other than cause, death or disability or retirement, each option, to the extent that it is exercisable at the time of such termination, shall remain exercisable for the 90 day period following such termination, but in no event following the expiration of its term. In the event that the officer’s employment terminates on account of death, disability or, with respect to any non-qualified stock option, retirement, each option granted that is outstanding and vested as of the date of such termination shall remain exercisable by such officer (or the officer’s legal representatives, heirs or legatees) for the one year period following such termination, but in no event following the expiration of its term.
The employment contract for Mr. Ludlum states that effective December 31, 2012 and on each successive Grant Date, the Compensation Committee may grant ten-year options to purchase shares of common stock to Mr. Ludlum based on his performance for the previous year. There is no predetermined option grant for Mr. Ludlum for 2012. Any unvested options will vest upon a change of control (as defined below) or any termination of Mr. Ludlum’s employment other than a termination by Mr. Ludlum or a termination of Mr. Ludlum by the Company for cause or during the Initial Employment Period. If Mr. Ludlum is terminated for any reason other than (i) cause, (ii) death or (iii) disability or retirement, each option granted to Mr. Ludlum, to the extent that it is exercisable at the time of such termination, shall remain exercisable for the 90 day period following such termination, but in no event following the expiration of its term. In the event of the termination of Mr. Ludlum’s employment for cause, each outstanding option granted to Mr. Ludlum shall terminate at the commencement of business on the date of such termination. In the event that Mr. Ludlum’s employment with the Company terminates on account of death, disability or, with respect to any non-qualified stock option, retirement of Mr. Ludlum, each option granted that is outstanding and vested as of the date of such termination shall remain exercisable by Mr. Ludlum (or Mr. Ludlum’s legal representatives, heirs or legatees) for the one year period following such termination, but in no event following the expiration of its term.
Recent Grants
On February 28, 2013, the Board of Directors of the Company authorized and approved option grants to the named executive officers in the amounts set forth below with a term of 10 years and an exercise price of $3.60 per share, the fair market value of a share of Common Stock as of the grant date, as determined by the Board of Directors.
| Name | Title | Number of Options | ||
| Dr. Yutaka Niihara | CEO, President and Director | 500,000 | ||
| Willis Lee | COO and Director | 500,000 | ||
| Peter Ludlum | Executive Vice President and CFO | 500,000 | ||
| Yasushi Nagasaki | Senior Vice President, Finance | 500,000 | ||
| Lan Tran | Chief Administrative Officer and Corporate Secretary | 500,000 |
The options granted to each named executive officer will vest as follows: one-third (1/3) will vest on the first anniversary of the grant date, and the remaining two-thirds (2/3) will vest in approximately equal monthly installments over a period of two years thereafter.
Severance Compensation. If Dr. Niihara’s, Mr. Lee’s, Ms. Tran’s, Mr. Nagasaki’s or Mr. Ludlum’s employment is terminated for any reason, other than without cause or good reason, each will be entitled to receive his or her base salary prorated through the termination date, any expense reimbursement due and owing for reasonable and necessary business expenses, and unpaid vacation benefits (the “Voluntary Termination Benefits”). If Dr. Niihara’s, Mr. Lee’s, Ms. Tran’s or Mr. Nagasaki’s termination is due to death or disability of such officer, then such officer will also receive an amount equal to his or her target annual performance bonus, and for a termination due to disability only, 6 additional months of his or her base salary to be paid out over a 6-month period and payment of COBRA benefits of up to 6 months following the termination. If Mr. Ludlum’s termination is due to death or disability, he will receive the Voluntary Termination Benefits and an amount equal to 6 months of his target annual performance bonus, and for a termination due to disability only (provided Mr. Ludlum signs a binding release of all claims relating to his employment (a “Release”)), 6 additional months of his or her base salary to be paid out over a 6-month period and payment of COBRA benefits of up to 6 months following the termination. If Dr. Niihara is terminated without cause or resigns with good reason (not within 2 years following a change in control), he will receive the Voluntary Termination Benefits and, provided he signs a Release, a severance package equal to one year of his base salary to be paid out over a 12-month period, a pro rata amount of the annual performance bonus for the calendar year in which the termination date occurs based on the achievement of any applicable performance terms or goals for the year, and payment of COBRA benefits of up to 12 months following the termination. If any of Mr. Lee, Ms. Tran or Mr. Nagasaki is terminated without cause or resigns with good reason (not within 2 years following a change in control), he or she will receive the Voluntary Termination Benefits and, provided he or she signs a Release, a severance package equal to 6 months of his or her base salary to be paid out over a 6-month period, an amount equal to half of the targeted annual performance bonus, and payment of COBRA benefits of up to 6 months following the termination. If Mr. Ludlum is terminated without cause after the Initial Employment Period or resigns with good reason (not within 1 year following a change in control), he will receive the Voluntary Termination Benefits and, provided he signs a Release, a severance package equal to 6 months of his base salary to be paid out over a 6-month period, a pro-rata amount of the targeted annual performance bonus based on his achievement of applicable terms or performance goals at the time of termination, and payment of COBRA benefits of up to 6 months following the termination.
53
Termination with cause includes a proven act of dishonesty, fraud, embezzlement or misappropriation of Company proprietary information; a conviction of, or plea of nolo contendere to, a felony or a crime involving moral turpitude; willful misconduct which cannot be cured on reasonable notice to the officer; or the officer’s habitual failure or refusal to perform his duties if such failure or refusal is not cured within 20 days after receiving written notice thereof from the board of directors. Good reason includes a reduction of more than 10% (or 25% in the case of Mr. Nagasaki and Mr. Ludlum) to the officer’s base salary or other compensation (except as part of a general reduction for all executive employees); a material diminution of the officer’s authority, responsibilities, reporting or job duties (except for any reduction for cause) (and except in Mr. Nagasaki’s case if his position is reduced to Treasurer, Comptroller or Controller during the first 14 months of employment); the Company’s material breach of the Employment Agreement; or, in the case of Dr. Niihara, Mr. Lee and Ms. Tran, a relocation of the business requiring the officer to move or drive to work more than 40 miles from its current location. The officer may terminate the Employment Agreement for good reason if he or she provides written notice to the Company within ninety (90) days of the event constituting good reason and the Company fails to cure the good reason within thirty (30) days after receiving such notice.
Change of Control. The Employment Agreements will not be terminated upon a change of control. A change of control means any merger or reorganization where the holders of the Company’s capital stock prior the transaction own fewer than 50% of the shares of capital stock after the transaction, an acquisition of 50% of the voting power of the Company’s outstanding securities by another entity, or a transfer of at least 50% of the fair market value of the Company’s assets. Upon Dr. Niihara’s termination without cause or good reason that occurs within 2 years after a change of control, he will receive the Voluntary Termination Benefits and, provided he signs a release of all claims relating to his employment, a severance package equal to 2 years of his base salary to be paid out over a 12-month period, an amount equal to double the targeted annual performance bonus, payment of COBRA benefits of up to 18 months following the termination; and a one-time cash payment of $3.0 million. Upon Mr. Lee’s or Ms. Tran’s termination without cause or good reason that occurs within 2 years after a change of control or upon Mr. Nagasaki’s or Mr. Ludlum’s termination without cause or good reason that occurs within 1 year after a change of control (provided Mr. Ludlum’s termination is after the Initial Termination Period), he or she will receive the Voluntary Termination Benefits and, provided he or she signs a Release, a severance package equal to 1 year of his or her base salary to be paid out over a 12-month period, an amount equal to the full year targeted annual performance bonus, payment of COBRA benefits of up to 12 months following the termination. Mr. Lee and Ms. Tran will also receive a one-time cash payment of $200,000. In addition each officer’s unvested equity awards shall vest upon such termination and the officer will have 36 months, except for Mr. Nagasaki and Mr. Ludlum who will have 4 months, in which to sell or exercise such awards (subject to expiration of the term of such options). The officer will also be free from all lock-up or other contractual restrictions upon the free sale of shares that are subject to waiver by the Company upon such termination.
Director Compensation
Our non-employee directors receive compensation of $700 for each in-person board meeting that they attend, $400 for each telephonic board meeting that they attend and $400 for each committee meeting. Our employee directors do not receive additional compensation for their services as a director. We expect the board of directors to hold four in-person meetings and two telephonic meetings each calendar year. Pursuant to the 2011 Stock Incentive Plan, non-officer directors receive an annual grant of 10,000 options. Such grants will vest annually. Additionally, the Chairman of the Board receives a one-time retainer grant of 10,000 options and each committee chair receives a one-time retainer grant of 2,000 options.
On April 2, 2012, the Board of Directors of the Company authorized and approved option grants to non-employee directors in the amounts set forth below with a term of 10 years and an exercise price of $3.60 per share, the fair market value of a share of Common Stock as of the grant date, as determined by the Board of Directors.
Name | Shares Underlying Outstanding Options | |||
| Tracey C. Doi | 75,000 | |||
| Maury J. DeWald | 75,000 | |||
The options granted to each named non-employee director will vest as follows: each option will vest annually in equal amounts (or as close to an equal amount as possible) over a period of three years with the first one-third (1/3) amount to vest one year from the Grant Date.
54
On February 29, 2012, we issued warrants to purchase 1,000,000 and 500,000 to Henry McKinnell and Alfred Osborne, respectively, with an exercise price of $1.00 per share. Each warrantholder may purchase one-half of the shares underlying his respective warrant in 2013 only and the other half of the shares underlying his warrant in 2014 only. The warrants were issued to each of the directors as compensation for their contributions to various projects on the Company’s behalf. In September 2012, the Company canceled Alfred Osborne‘s warrants to purchase 500,000 shares of common stock when he advised the Company that he would not be standing for re-election to the board of directors.
The following table shows information regarding the compensation earned during the fiscal year ended December 31, 2012 by the non-employee members of our board of directors. Compensation earned by our employee directors, Yutaka Niihara, M.D., MPH and Willis C. Lee, who do not receive compensation for their services as a director, is reported above under the heading “Summary Compensation Table.”
| Name | Fees Earned or Paid in Cash ($) | Stock Awards ($) | Option Awards ($)(1) (2) | Non-Equity Incentive Plan Compensation ($) | Nonqualified Deferred Compensation Earnings | All Other Compensation ($) | Total ($) | ||||||||||||
| Henry A. McKinnell, Jr., Ph.D. | $ | 4,700 | — | — | — | — | $2,878,825 | $ | 2,883,525 | ||||||||||
| Alfred E. Osborne, Jr., Ph.D. (3) | $ | 3,400 | — | — | — | — | $1,439,412 | $ | 3,400 | ||||||||||
| Tracey C. Doi | $ | 5,500 | — | $ | 252,206 | — | — | — | $ | 257,706 | |||||||||
| Maury J. DeWald | $ | 4,900 | — | $ | 252,206 | — | — | — | $ | 257,106 | |||||||||
| Amir Heshmatpour (4) | — | — | — | — | — | — | — | ||||||||||||
_________
(1) The amounts in this column represent the aggregate grant date fair value of option awards granted to the directors in 2012, calculated in accordance with the provisions of FASB ASC Topic 718, “Stock Compensation”. For a description of the assumptions used to calculate these amounts, see Note 7 to our audited consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2012. The actual value of any option award to a director, if any, will depend on the excess of our stock price over the exercise price on the date the option award is exercised. The actual value realized by the director upon exercise of the option awards may be higher or lower than the value shown in this column.
(2) As of December 31, 2012, our non-employee directors had option awards outstanding pursuant to the 2011 Stock Incentive Plan to purchase the following number of shares of our common stock:
Name | Shares Underlying Outstanding Options | |||
| Henry A. McKinnell, Jr., Ph.D.(1) | 38,795 | |||
| Tracey C. Doi(2) | 85,000 | |||
| Maury J. DeWald(3) | 87,000 | |||
| (1) | Includes options to purchase 11,795 shares that vested as of December 31, 2012 and are exercisable at $3.05 per share through 2015, which award was issued prior to the commencement of the 2011 Stock Incentive Plan, and options to purchase 27,000 shares that vested December 19, 2012 and are exercisable at $3.60 per share through December 19, 2021. |
| (2) | Includes options to purchase 10,000 shares that vested December 19, 2012 and are exercisable at $3.60 per share through December 19, 2021 and options to purchase 75,000 shares which vest annually in equal amounts (or as close to an equal amount as possible) over a period of three years with the first one-third (1/3) amount to vest one year from the Grant Date. |
| (3) | Includes options to purchase 12,000 shares that vested December 19, 2012 and are exercisable at $3.60 per share through December 19, 2021 and options to purchase 75,000 shares which vest annually in equal amounts (or as close to an equal amount as possible) over a period of three years with the first one-third (1/3) amount to vest one year from the Grant Date. |
(3) Dr. Osborne informed the Board on September 24, 2012 that he would not stand for re-election to the Board. The warrants issued in February 2012 to Dr. Osborne, reported under the column entitled “All Other Compensation,” were canceled in connection with his departure from the board.
(4) Mr. Heshmatpour resigned from the board of directors on April 24, 2012.
Recent Grants
On February 28, 2013, the Board of Directors of the Company authorized and approved the option grants to non-employee directors in the amounts set forth below with a term of 10 years and an exercise price of $3.60 per share, the fair market value of a share of Common Stock as of the grant date, as determined by the Board of Directors.
Name | Shares Underlying Outstanding Options | |||
| Henry A. McKinnell, Jr., Ph.D. | 250,000 | |||
| Tracey C. Doi | 250,000 | |||
| Maury J. DeWald | 250,000 | |||
The options granted to each named non-employee director will vest as follows: each option will vest annually in equal amounts (or as close to an equal amount as possible) over a period of three years with the first one-third (1/3) amount to vest one year from the Grant Date.
55
2011 Stock Incentive Plan
Background
On May 3, 2011, the board of directors and stockholders adopted the Emmaus Life Sciences, Inc. 2011 Stock Incentive Plan (the “Incentive Plan”). Stockholder approval of the Incentive Plan enables the Company to satisfy stock exchange listing requirements, and to make awards that qualify as performance-based compensation that is exempt from the deduction limitation set forth under Section 162(m) of the Internal Revenue Code of 1986, as amended (the “Code”). Subject to certain exceptions, Section 162(m) generally limits the corporate income tax deductions to $1,000,000 annually for compensation paid to each of the Chief Executive Officer and the other three highest paid executive officers of the Company required to be reported under the proxy disclosure rules. The Company intends to cause the shares of common stock that will become available for issuance to be registered on a Form S-8 registration statement to be filed with the SEC at the Company’s expense.
The Incentive Plan permits grants of stock options, stock appreciation rights, or SARs, restricted stock or units, unrestricted stock, deferred stock and performance awards. The Company’s board of directors believes that the Incentive Plan will be an important factor in attracting, retaining and motivating employees, consultants, agents, and directors of the Company and its affiliates, collectively referred to herein as Eligible Persons. Our board of directors believes that the Company needs the flexibility both to have an ongoing reserve of common stock available for future equity-based awards, and to make future awards in a variety of forms.
On February 28, 2013, the Board of Directors of the Company authorized and adopted an amendment to Section 1.5(a) of the Incentive Plan. The amendment increases the total number of shares of common stock of the Company with respect to which awards may be granted pursuant to the Incentive Plan (the “Cap”) from 3,000,000 to 6,000,000 (subject to adjustment as set forth in the Incentive Plan). Except for this increase in the Cap, the terms of the Incentive Plan, including the adjustment mechanisms set forth therein, are unchanged. The 3,000,000 additional shares authorized for issuance pursuant to this amendment have not been approved by the Company’s shareholders and awards granted with respect to these additional shares will not qualify as performance-based compensation for purposes of Section 162(m) of the Code.
Capitalized terms used in this summary and not otherwise defined herein will have the meanings ascribed to such terms in the Incentive Plan.
Purpose
The purpose of the Incentive Plan is to attract, retain and motivate select employees, consultants, agents, and directors of the Company and its affiliates (“Eligible Persons”), and to provide incentives and rewards for superior performance.
Shares Subject to the Incentive Plan
The Incentive Plan provides that no more than 6,000,000 shares of common stock may be issued pursuant to Awards under the Incentive Plan. The number of shares available for Awards, as well as the terms of outstanding Awards, is subject to adjustment as provided in the Incentive Plan for stock splits, stock dividends, recapitalizations and other similar events. The maximum awards that can be granted under the Incentive Plan to a single participant in any calendar year shall be 500,000 shares of common stock in the form of options or SARs, and 500,000 shares of common stock in the form of restricted shares, restricted share units, stock bonus and other stock-based awards.
Administration
The Company’s Compensation Committee of the board of directors or another committee appointed by the Company’s board of directors administers the Incentive Plan. The Compensation Committee of the Company’s board of directors and any other committee exercising discretion under the Incentive Plan from time to time are referred to herein as the “Committee.”
Subject to the terms of the Incentive Plan, the Committee has express authority to determine the Eligible Persons who will receive Awards, the number of shares of common stock, units or dollars to be covered by each Award, and the terms and conditions of Awards. The Committee has broad discretion to prescribe, amend, and rescind rules relating to the Incentive Plan and its administration, to interpret and construe the terms of the Incentive Plan and the terms of all Award agreements, and to take all actions necessary or advisable to administer the Incentive Plan.
Stock awards granted under the Incentive Plan (other than annual director stock grants described below) will have a minimum forfeiture period of at least three years (but such forfeiture periods may lapse in installments). However, performance-based stock awards may have a minimum vesting or forfeiture period of one year with certain exceptions.
56
The Incentive Plan provides that the Company will indemnify members of the Committee and their delegates against any claims, liabilities or costs arising from the good faith performance of their duties under the Incentive Plan. The Incentive Plan releases these individuals from liability for good faith actions associated with the Incentive Plan’s administration.
Eligibility
The Committee may grant options that are intended to qualify as incentive stock options, or ISOs, only to employees of the Company or its affiliates, and may grant all other Awards to Eligible Persons. The Incentive Plan and the discussion below use the term “Participant” to refer to an Eligible Person who has received an Award.
Types of Awards
Options. Options granted under the Incentive Plan provide Participants with the right to purchase shares of common stock at a predetermined exercise price. The Committee may grant options that are intended to qualify as ISOs or options that are not intended to so qualify, referred to herein as Non-ISOs. The Incentive Plan also provides that ISO treatment may not be available for options that become first exercisable in any calendar year to the extent the value of the underlying shares that are the subject of the option exceeds $100,000 (based upon the fair market value of the shares of common stock on the option grant date).
Share Appreciation Rights (SARs). A share appreciation right generally permits a Participant who receives it to receive, upon exercise, cash and/or shares of common stock equal in value to an amount determined by multiplying (a) the excess of the fair market value, on the date of exercise, of the shares of common stock with respect to which the SAR is being exercised, over the exercise price of the SAR for such shares by (b) the number of shares with respect to which the SARs are being exercised. The Committee may grant SARs in tandem with options or independently of them. SARs that are independent of options may limit the value payable on its exercise to a percentage, not exceeding 100%, of the excess value.
Exercise Price for Options and SARs. The exercise price of ISOs, Non-ISOs, and SARs may not be less than 100% of the fair market value on the grant date of the shares of common stock subject to the Award (110% of fair market value for ISOs granted to employees who, on the grant date, own stock representing more than 10% of the combined voting power of all classes of stock of the Company).
Exercise of Options and SARs. To the extent exercisable in accordance with the agreement granting them, an option or SAR may be exercised in whole or in part, and from time to time during its term, subject to earlier termination relating to a holder’s termination of employment or service. The term over which Participants may exercise options and SARs may not exceed ten years from the date of grant (five years in the case of ISOs granted to employees who, on the grant date, own more than 10% of the combined voting power of all classes of stock of the Company).
Unless otherwise provided under the terms of the agreement evidencing a grant, options and SARs that have vested may be exercised during the six-month period after the optionee retires, during the one-year period after the optionee’s termination of service due to death or permanent disability, and during the 90-day period after the optionee’s termination of employment other than for cause (but in no case later than the termination date of the option or SAR). Each option or SAR that remains unexercisable at the time of termination shall be terminated at the time of termination.
Restricted Shares, Stock Units, Stock Bonus, and Other Stock-Based Awards. Under the Incentive Plan, the Committee may grant restricted shares that are forfeitable until certain vesting requirements are met, may grant restricted stock units which represent the right to receive shares of common stock after certain vesting requirements are met, and may grant unrestricted shares as to which the Participant’s interest is immediately vested (subject to the exceptions to the minimum vesting requirements described above). The Incentive Plan provides the Committee with discretion to determine the terms and conditions under which a Participant’s interests in such Awards becomes vested, which may include the achievement of financial or other objective performance goals or other objectives.
Annual Non-Employee Director Grants. The Incentive Plan provides for annual grants of 10,000 options to non-employee directors (the “Annual Director Award”). Each Annual Director Award will vest in four substantially equal quarterly installments.
Clawback of Awards
Unless otherwise provided in an agreement granting an Award, the Company may terminate any outstanding, unexercised, unexpired or unpaid Award, rescind any exercise, payment or delivery pursuant to the Award, or recapture any common stock (whether restricted or unrestricted) or proceeds from the Participant’s sale of shares issued pursuant to the Award in the event of the discovery of the Participant’s fraud or misconduct, or otherwise in connection with a financial restatement.
57
Income Tax Withholding
As a condition for the issuance of shares pursuant to Awards, the Incentive Plan requires satisfaction of any applicable federal, state, local, or foreign withholding tax obligations that may arise in connection with the award or the issuance of shares.
Transferability
Awards may not be sold, pledged, assigned, hypothecated, transferred or disposed of other than by will or the laws of descent and distribution, except to the extent the Committee permits.
Certain Corporate Transactions
The Committee shall equitably adjust the number of shares covered by each outstanding Award, the number of shares that have been authorized for issuance under the Incentive Plan but have not yet been granted or that have been returned to the Incentive Plan upon cancellation, forfeiture or expiration of an Award, and the maximum number of shares that may be granted in any calendar year to individual participants, as well as the price per share covered by each outstanding Award, to reflect any increase or decrease in the number of issued shares resulting from a stock split, reverse stock split, stock dividend, combination, recapitalization or reclassification of the shares, or any other increase or decrease in the number of issued shares effected without receipt of consideration by the Company.
In addition, in the event of a Change in Control (as defined in the Incentive Plan), each outstanding Award shall be assumed or substituted by a substantially equivalent award granted by the surviving corporation or its affiliate upon the consummation of the transaction. Alternatively, the Committee has the authority to cause the Company to accelerate the vesting of Awards for any period and cause the Company’s repurchase rights to lapse, offer Participants cash payments in exchange for the satisfaction and cancellation of awards or provide for the automatic vesting and cancellation of awards in connection with the consummation of a Change in Control. To the extent that an Award is not exercised prior to consummation of a transaction in which the Award is not being assumed or substituted, such Award shall terminate upon such consummation.
Term of the Incentive Plan; Amendments or Termination
The term of the Incentive Plan is ten years from the date of adoption by the board of directors. The Company’s board of directors may from time to time, amend, alter, suspend, discontinue or terminate the Incentive Plan; provided that no amendment, suspension or termination of the Incentive Plan shall materially and adversely affect Awards already granted. Furthermore, the Incentive Plan specifically prohibits the repricing of stock options or SARs without stockholder approval. For this purpose, a “repricing” means any of the following (or any other action that has the same effect as any of the following): (i) changing the terms of a stock option or SAR to lower its exercise price; (ii) any other action that is treated as a “repricing” under generally accepted accounting principles; and (iii) repurchasing for cash or canceling a stock option or SAR at a time when its exercise price is greater than the fair market value of the underlying stock in exchange for another award, unless the cancellation and exchange occurs in connection with a change in capitalization or similar change. Such cancellation and exchange would be considered a “repricing” regardless of whether it is treated as a “repricing” under generally accepted accounting principles and regardless of whether it is voluntary on the part of the participant.
Beneficial ownership is determined in accordance with the rules of the SEC. In computing the number of shares beneficially owned by a person and the percentage of ownership of that person, shares of common stock subject to options, warrants and convertible notes held by that person that are currently exercisable or become exercisable within 60 days of March 20, 2013 are deemed outstanding even if they have not actually been exercised. Those shares, however, are not deemed outstanding for the purpose of computing the percentage ownership of any other person.
The following table sets forth certain information with respect to beneficial ownership of our common stock based on issued and outstanding shares of common stock before and after the offering, by:
| · | Each person known to be the beneficial owner of 5% or more of our outstanding common stock; |
| · | Each executive officer; |
| · | Each director; and |
| · | All of the executive officers and directors as a group. |
58
The number of shares of our common stock outstanding as of the date of March 20, 2013 excludes up to 4,789,000 shares of common stock underlying outstanding options, 3,408,795 shares of common stock underlying outstanding warrants and 1,721,640 shares of common stock underlying outstanding principal amount on the convertible notes (as of March 20, 2013). Unless otherwise indicated, the persons and entities named in the table have sole voting and sole investment power with respect to the shares set forth opposite the stockholder’s name, subject to community property laws, where applicable. Unless otherwise indicated in the table or its footnotes, the address of each stockholder listed in the table is c/o Emmaus Life Sciences, Inc., 20725 S. Western Avenue, Ste. 136, Torrance, CA 90501-1884.
Name and Address of Beneficial Owner | Title | Amount and Nature of Beneficial Ownership of Shares of Common Stock | Percent of Class(1) | |||
| Directors and Executive Officers | ||||||
| Yutaka Niihara, M.D., MPH | President, Chief Executive Officer and Director | 10,712,829(2) | 43.1% | |||
| Peter B. Ludlum | Chief Financial Officer | 3,000 | * | |||
| Yasushi Nagasaki | Senior Vice President, Finance | 234,530(3) | * | |||
| Willis C. Lee | Chief Operating Officer and Director | 335,973(4) | 1.4% | |||
| Lan T. Tran | Chief Administrative Officer and Corporate Secretary | 163,572(5) | * | |||
| Henry A. McKinnell, Jr., Ph.D. | Chairman of the Board | 538,795 (6) | 2.2% | |||
| Tracey C. Doi | Director | 97,693(7) | * | |||
| Maurice J. DeWald | Director | 37,000(8) | * | |||
| Officers and Directors as a Group (total of 8 persons) | 12,123,393 (9) | 48.7% | ||||
| 5% or More Owners | ||||||
AFH Holding & Advisory, LLC 9595 Wilshire Blvd, Suite 700 Beverly Hills, CA 90212 | 2,170,000 (10) | 8.8% | ||||
Amir Heshmatpour 9595 Wilshire Blvd, Suite 700 Beverly Hills, CA 90212 | 2,504,249 (10) | 10.3% | ||||
Daniel R. and Yuka I. Kimbell 350 W. Colorado Blvd., Ste. 350 Pasadena, CA 91105 | 2,434,028 (11) | 9.8% |
* Less than 0.1%.
_______
(1) | Based on 24,878,436 shares of common stock issued and outstanding as of March 20, 2013. |
| (2) | Includes 9,529,711 shares that are held jointly by Yutaka and Soomi Niihara, his wife. Also includes 44,229 shares of common stock for which Dr. Niihara is custodian. Dr. Niihara may be deemed the indirect beneficial owner of these securities since he has sole voting and investment control over the securities. Also includes 1,000,000 shares underlying warrants to purchase shares of common stock, 83,333 shares underlying stock options and 55,556 shares underlying warrants to purchase shares of common stock owned by Hope International Hospice, Inc. (“Hope Hospice”). Dr. Niihara is the chief executive officer of Hope Hospice and has voting and investment power over such shares. |
| (3) | Includes 126,197 shares of common stock underlying outstanding convertible notes (as of March 20, 2013), 25,000 shares underlying warrants to purchase shares of common stock and 83,333 shares underlying stock options. |
59
| (4) | Includes 40,171 shares of common stock underlying outstanding convertible notes (as of March 20, 2013), 83,333 shares underlying stock options and 35,556 shares underlying warrants to purchase shares of common stock owned by MLPF&S Cust. FBO Willis C. Lee. |
| (5) | Includes 56,945 shares underlying warrants to purchase shares of common stock and 83,333 shares underlying stock options. |
| (6) | Includes 500,000 shares underlying warrants to purchase shares of common stock and options to purchase 38,795 shares of common stock. |
| (7) | Includes 32,693 shares of common stock underlying outstanding convertible notes (as of March 20, 2013), 35,000 shares underlying stock options and 30,000 shares underlying warrants to purchase shares of common stock owned by Tracey and Mark Doi. |
| (8) | Represents options to purchase 37,000 shares of common stock. |
| (9) | Includes options to purchase 444,128 shares of common stock, warrants to purchase 1,703,057 shares of common stock and 199,061 (as of March 20, 2013) shares of common stock underlying outstanding convertible notes. |
| (10) | Mr. Heshmatpour is the managing partner of AFH Advisory and may be deemed to have voting and dispositive controls with respect to these shares. Mr. Heshmatpour disclaims beneficial ownership of any shares in which he does not have a pecuniary interest. Pursuant to the lawsuit described elsewhere in this annual report under “ITEM 3. LEGAL PROCEEDINGS,” the Company is seeking a court order that these shares are canceled. |
| (11) | Includes 44,229 shares of common stock held by the holder as custodian. Daniel and Yuka Kimbell may be deemed the indirect beneficial owner of these securities since they have sole voting and investment control over the securities. |
Securities Authorized for Issuance under Equity Compensation Plans
The following table provides information as of December 31, 2012 regarding compensation plans, including any individual compensation arrangements, under which equity securities of Emmaus Life Sciences, Inc. are authorized for issuance. Please see Part III. Item 11 “2011 Stock Incentive Plan” for a description of the terms of our 2011 Stock Incentive Plan.
| Plan Category | Number of Securities to be issued upon exercise of outstanding options, warrants and rights | Weighted- average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans | |||||||||
| Equity compensation plans approved by security holders (1) | 1,539,000 | $ | 3.60 | 1,461,000 | ||||||||
| Equity compensation plans not approved by security holders (2) | 1,000,000 | 1.00 | — | |||||||||
| Total | 2,539,000 | 2.58 | 1,461,000 | |||||||||
| (1) | In May 2011, the Company adopted the Incentive Plan. |
| (2) | On February 28, 2013, the Board of Directors approved an increase of 3,000,000 additional options for the Incentive Plan and such additional shares have not been approved by the Company shareholders. The securities provided reflect warrants issued to non-employee directors in 2012. |
Emmaus Medical, Inc.
Emmaus Life Sciences, Inc., Emmaus Medical Inc., Newfield Nutrition Corporation, Emmaus Medical Japan, Inc. (“EM Japan”) and Emmaus Medical Europe Ltd. (“EM Europe”) which are either directly or indirectly wholly-owned subsidiaries of the Company, each have interlocking executive and director positions with us and with each other. Dr. Niihara and Mr. Lee are directors of Newfield Nutrition Corporation. Dr. Niihara is a director of EM Japan. Dr. Niihara and Ms. Tran are directors of EM Europe. The officers of Emmaus Life Sciences are also the officers of Emmaus Medical and Newfield Nutrition and hold the same officer positions in Emmaus Medical and Newfield as they do in Emmaus Life Sciences.
60
Merger and AFH Advisory
Pursuant to the Merger Agreement AFH Merger Sub merged with and into Emmaus Medical with Emmaus Medical continuing as the surviving entity. As a result of the Merger, Emmaus Medical became a wholly-owned subsidiary of the Company. In connection with the Merger, the Company changed its corporate name from “AFH Acquisition IV, Inc.” to “Emmaus Holdings, Inc.” Subsequently, on September 14, 2011, we changed our name from “Emmaus Holdings, Inc.” to “Emmaus Life Sciences, Inc.”
Upon consummation of the Merger, (i) each outstanding share of Emmaus Medical common stock was exchanged for 29.48548924976 shares of our common stock, (ii) each outstanding Emmaus Medical option and warrant, which was exercisable for one share of Emmaus Medical common stock, was exchanged for an option or warrant, as applicable, exercisable for 29.48548924976 shares of our common stock and (iii) each outstanding convertible note of Emmaus Medical, which was convertible for one share of Emmaus Medical common stock, was exchanged for a convertible note exercisable for 29.48548924976 shares of our common stock. As a result of the Merger, Emmaus Medical securityholders received 20,673,714 of our shares of common stock, options and warrants to purchase an aggregate of 326,507 shares of common stock, and convertible notes to purchase an aggregate of 271,305 shares of our common stock, or 85% of our issued and outstanding common stock on a fully diluted basis. Immediately after the closing of the Merger, we had 24,423,714 shares of common stock, no shares of preferred stock, options to purchase 23,590 shares of common stock, warrants to purchase 302,918 shares of common stock and convertible notes convertible into 271,305 shares of our common stock issued and outstanding.
The Merger resulted in a change in control of our company from AFH Advisory, which is owned by Mr. Amir F. Heshmatpour, to the former securityholders of Emmaus Medical. In connection with the change in control, we appointed new persons to our board of directors and elected new officers of the Company. Mr. Heshmatpour, an officer and director of the Company prior to the consummation of the Merger, resigned from all of his officer positions with the Company at the time the transaction was consummated. Mr. Heshmatpour resigned from the board of directors on April 24, 2012.
Since July 2012 we have been engaged in litigation with Amir Heshmatpour and AFH Advisory. Mr. Heshmatpour owns AFH Advisory, which was the sole stockholder of AFH Acquisition IV until its combination with Emmaus Medical pursuant to the Merger in May 2011. In September 2012 AFH Advisory and related parties filed a complaint against the Company in the Superior Court of Delaware. In October 2012 the Company filed counterclaims against the plaintiffs and third-party claims against Mr. Heshmatpour. The case is scheduled for trial in May 2013. For a detailed description of the case please see "ITEM 3. LEGAL PROCEEDINGS" above in this Annual Report on Form 10-K.
Loans by Related Persons
The following table sets forth information relating to our loans from related persons outstanding as of December 31, 2012.
| Lender | Annual Interest Rate | Date of loan | Term of Loan | Principal Amount Outstanding as of December 31, 2012 | Highest Principal Outstanding | Amount of Principal Repaid | Amount of Interest Paid | Conversion Price | Shares Underlying Principal as of December 31, 2012 | ||||||||||||||||||||||
| Yutaka Niihara (3) | 6.50 | % | 1/12/2009 | On Demand | $ | 272,800 | $ | 350,000 | — | $ | 18,763 | NA | NA | ||||||||||||||||||
| Hope Int’l Hospice (4) | 8.00 | % | 1/12/2011 | 2 years | $ | 200,000 | $ | 200,000 | — | $ | 12,000 | NA | NA | ||||||||||||||||||
| Hope Int’l Hospice (4) | 8.00 | % | 1/17/2012 | On Demand | $ | 200,000 | $ | 200,000 | — | $ | 8,000 | NA | NA | ||||||||||||||||||
| Lan T. Tran (3) | 11.00 | % | 2/10/2012 | 2 years (1) | $ | 80,000 | $ | 205,000 | $ | 125,000 | — | NA | NA | ||||||||||||||||||
| Tracey & Mark Doi (2) | 8.00 | % | 2/10/2012 | 1 year | $ | 108,000 | $ | 108,000 | — | — | $ | 3.60 | 30,000 | ||||||||||||||||||
| Hope Int’l Hospice (4) | 8.00 | % | 6/14/2012 | On Demand | $ | 200,000 | $ | 200,000 | — | — | NA | NA | |||||||||||||||||||
| Hope Int’l Hospice (4) | 8.00 | % | 6/21/2012 | On Demand | $ | 100,000 | $ | 100,000 | — | $ | 8,000 | NA | NA | ||||||||||||||||||
| Yasushi Nagasaki (3) | 10.00 | % | 6/29/2012 | On Demand | $ | 388,800 | $ | 388,800 | — | — | $ | 3.30 | 117,818 | ||||||||||||||||||
| Yutaka Niihara (3) | 1.00 | % | 8/29/2012 | 1 year | $ | 1,270,000 | $ | 1,270,100 | — | — | NA | NA | |||||||||||||||||||
| Willis Lee (3) | 10.00 | % | 10/5/2012 | 1 year | $ | 183,242 | $ | 138,242 | — | — | $ | 3.60 | 38,400 | ||||||||||||||||||
| Yutaka Niihara (3) | 10.00 | % | 12/5/2012 | Six months | $ | 1,213,700 | $ | 1,213,700 | — | — | NA | NA | |||||||||||||||||||
| (1) | Due on Demand |
| (2) | Director |
| (3) | Officer |
| (4) | Dr. Niihara is also the CEO of Hope International Hospice, Inc. |
61
The above loans were made to provide us with needed working capital.
In January 2009, the Company issued a promissory note to Dr. Niihara, the CEO of the Company, in the principal amount of $350,000 which bears interest at 6.5% per annum and is due on demand.
In January 2011, the Company issued a promissory note to Hope International Hospice, in the principal amount of $200,000. The note bears interest at 8% per annum and all unpaid principal and interest are due on the two year anniversary date of the note. Dr. Niihara is also the CEO of Hope International Hospice, Inc.
In January 2012, the Company issued a promissory note to Hope International Hospice, in the principal amount of $200,000. The note bears interest at 8% per annum and all unpaid principal and interest are due upon demand at the lender’s option. In connection with the issuance of the note, the Company issued three-year warrants to purchase 55,556 shares of the Company’s common stock at a per share exercise price of $1.00. Dr. Niihara is also the CEO of Hope International Hospice, Inc.
In February 2012, the Company issued a promissory note to Lan Tran, an office of the Company, in the amount of $205,000, which bears interest at 11% per annum and matures on the two year anniversary date of the note. The lender may, at any time after the six month anniversary date of the note, declare the entire unpaid principal and unpaid accrued interest immediately due and payable. In connection with the issuance of the note, the Company issued three-year warrants to purchase 56,945 shares of the Company’s common stock at a per share exercise price of $1.00.
In February 2012, the Company issued a one-year convertible note to Tracey and Mark Doi in the amount of $108,000, which bears interest at 8% per annum and matures on the anniversary date of the note. The principal amount plus the unpaid accrued interest due under the convertible note is convertible into shares of the Company’s common stock at $3.60 per share. In connection with the issuance of the note, the Company issued three-year warrants to purchase 30,000 shares of the Company’s common stock at a per share exercise price of $1.00. Tracey Doi is a Director of the Company.
In June 2012, the Company issued two promissory notes to Hope International Hospice totaling $300,000 bearing interest at 8% per annum. The entire principal amount of each note and any outstanding accrued interest thereon is due on demand. Dr. Niihara is also the CEO of Hope International Hospice, Inc.
In June 2012, the Company refinanced a convertible note payable to Yasushi Nagasaki, an officer of the Company, in the amount of $360,000 with a new convertible note in the amount of $388,800 which bears interest at 10% per annum and is due upon demand. The principal amount and any unpaid interest due under the promissory note are convertible into shares of the Company’s common stock at $3.30 per share.
In August 2012, the Company issued a promissory note to Dr. Niihara, the CEO of the Company, in the principal amount of $1,270,100 which bears interest at 1% per annum and matures on the one-year anniversary date of the note. In connection with the issuance of the note, the Company issued three-year warrants to purchase a total of 1,000,000 shares of the Company’s common stock at a per share exercise price of $2.50.
In October 2012, the Company refinanced a convertible note payable to Willis Lee, an officer of the Company, in the amount of $128,002 with a new convertible note in the amount of $138,242 which bears interest at 10% per annum and matures on the one-year anniversary date of the note. The principal amount and any unpaid interest due under the note are convertible into shares of the Company’s common stock at $3.60 per share.
62
In December 2012, the Company issued a promissory note to Dr. Niihara, the CEO of the Company, in the principal amount of $1,213,700 bearing interest at 10% per annum and matures on six-month anniversary date of the note. This note refinanced a note for JPY 100,000,000 ($1,213,700 @0.012137) that was payable to related parties who were certain family members of Dr. Niihara.
The following table sets forth information relating to loans provided to the Company by related persons during the first quarter of 2013.
| Lender | Annual Interest Rate | Date of loan | Term of Loan | Loan Principal Outstanding | Conversion Price | Shares Underlying Original Principal Amount | |||||||||||||
| Tracey & Mark Doi | 10.00 | % | 2/10/2013 | Due on Demand | $ | 116,640 | $ | 3.60 | 32,400 | ||||||||||
| Hope Int’l Hospice | 8.00 | % | 01/12/2013 | Due on Demand | $ | 200,000 | NA | ||||||||||||
| Hope Int’l Hospice | 8.00 | % | 02/11/2013 | Due on Demand | $ | 50,000 | NA | ||||||||||||
In January 2013, the Company renewed a promissory note payable to Hope International Hospice in the principal amount of $200,000 bearing interest at 8% per annum. The term of this note is due on demand. Dr. Niihara is also the CEO of Hope International Hospice, Inc.
In February 2013, the Company issued a promissory note in the principal amount of $50,000 to Hope International Hospice bearing interest at 8% per annum. The term of this note is due on demand. Dr. Niihara is also the CEO of Hope International Hospice, Inc.
In February 2013, the Company refinanced a convertible note payable to Tracey and Mark Doi totaling $108,000 with new convertible notes totaling $116,640 which bear interest at 10% per annum. The term of this note is due on demand up to one year. Principal amounts and any unpaid interests due under the notes are convertible into shares of the Company’s common stock at $3.60 per share. Tracey Doi is a Director of the Company.
Guarantees by Officers
On January 12, 2009, Emmaus Medical entered into a promissory note with Mr. Shigeru Matsuda for 20 million Japanese Yen, equivalent to $221,895. As of December 31, 2012, the aggregate outstanding principal amount of the note was $294,355 which includes the aggregate amount of accrued and unpaid interest was $57,045 that has been added to the original principal. Dr. Niihara has provided a guarantee on the promissory note entered into with Mr. Matsuda.
On October 3, 2012, Emmaus Medical refinanced a promissory note with the Shitabata Family Trust for $1,620,540. Dr. Niihara and Mr. Lee have provided a guarantee on the promissory note entered into with the Shitabata Family Trust.
Policy for Approval of Related Party Transactions
We do not currently have a formal related party approval policy for review and approval of transactions required to be disclosed pursuant to Item 404(a) of Regulation S-K. All such transactions through February 28, 2013 have been reviewed and approved by the independent directors of our board of directors. We expect to adopt such a policy that will identify the types of transactions covered by such policy and the standards to be applied pursuant to such policy. We expect that the Nominating and Corporate Governance Committee will be responsible for applying such policy.
Director Independence
Currently, our board of directors has determined that each of Henry A. McKinnell, Jr., Tracey C. Doi and Maurice J. DeWald is an “independent” director as defined by the NASDAQ Marketplace Rules, currently in effect and all applicable rules and regulations of the SEC. All members of the Audit, Compensation and Nominating Committees satisfy the “independence” standards applicable to members of each such committee. The board of directors made this affirmative determination regarding these directors’ independence based on discussions with the directors and on its review of the directors’ responses to a standard questionnaire regarding employment and compensation history; affiliations, family and other relationships; and transactions with the Company. The board of directors considered relationships and transactions between each director or any member of his immediate family and the Company and its subsidiaries and affiliates. Dr. McKinnell, Ms. Doi and Mr. DeWald, each of whom is an independent director as defined by the NASDAQ Marketplace Rules, are the sole members of the Audit Committee, the Compensation Committee and the Nominating and Corporate Governance Committee.
63
The following table presents fees, including reimbursements for expenses, for professional audit services rendered by EFP Rotenberg LLP for the audits of the Company’s annual financial statements and interim reviews of the Company’s quarterly financial statements for the years ended December 31, 2012 and December 31, 2011 and fees billed or to be billed for other services rendered by EFP Rotenberg LLP during those periods.
| Year ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| Audit Fees(1) | $ | 62,998 | $ | 47,500 | ||||
| Audit-Related Fees | $ | 36,355 | $ | 45,930 | ||||
| Tax Fees | $ | — | $ | — | ||||
| All Other Fees | $ | — | $ | — | ||||
| Total | $ | 99,353 | $ | 88,430 | ||||
(1) These are fees for professional services performed by EFP Rotenberg LLP for the audit of our annual financial statements, review of our quarterly reports and review of our Registration Statement on Form S-1 in 2011.
Pre-Approval Policy
The Audit Committee on an annual basis reviews audit and non-audit services performed by the independent registered public accounting firm for such services. The audit committee pre-approves (i) auditing services (including those performed for purposes of providing comfort letters and statutory audits) and (ii) non-auditing services that exceed a de minimis standard established by the committee, which are rendered to the Company by its outside auditors (including fees).
64
1. Financial Statements: See “Index to Consolidated Financial Statements” on page F-1 of this Annual Report on Form 10-K.
2. Financial Statement Schedule: See Notes to the Consolidated Financial Statements in Part II, Item 8 of this Annual Report on Form 10-K.
3. Exhibits: The exhibits listed in the accompanying “Exhibit Index” are filed or incorporated by reference as part of this Annual Report on Form 10-K.
Exhibit Index
| Exhibit No. | Exhibit Description | |
2.1 | Merger Agreement dated as of April 21, 2011 by and among the registrant, AFH Merger Sub, Inc., AFH Holding and Advisory, LLC, and Emmaus Medical, Inc. (incorporated by reference from Exhibit 2.1 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on April 25, 2011). | |
| 3.1 | Certificate of Incorporation (incorporated by reference from Exhibit 3.1 to the registrant’s Form 10-SB filed with the Securities and Exchange Commission on February 1, 2008). | |
| 3.2 | Bylaws (incorporated by reference from Exhibit 3.2 to the registrant’s Form 10-SB filed with the Securities and Exchange Commission on February 1, 2008). | |
| 3.2(a) | Amendment No. 1 to Bylaws (incorporated by reference from Exhibit 3.1 to the registrant’s Form 8-K filed with the Securities and Exchange Commission on March 6, 2013). | |
| 3.3 | Certificate of Ownership and Merger filed with the Office of Secretary of State of Delaware on May 3, 2011 (incorporated by reference from Exhibit 3.3 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 3.4 | Certificate of Ownership and Merger effecting the name change to Emmaus Life Sciences, Inc. filed with the Delaware Secretary of State on September 14, 2011 (incorporated by reference from Exhibit 3.1 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on September 15, 2011). | |
| 4.1 | Form of Warrant (incorporated by reference from Exhibit 4.1 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 4.2 | Convertible Promissory Note (Cash Interest) dated March 14, 2011 (incorporated by reference from Exhibit 4.2 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 4.3 | Form of Convertible Note (No Interest) entered into with the persons indicated in Schedule A attached to the Form of Convertible Note (incorporated by reference from Exhibit 4.3 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 4.4 | Convertible Promissory Note (2-5 Years) dated January 12, 2009 (incorporated by reference from Exhibit 4.4 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 4.5 | Convertible Promissory Note (Cash Interest) dated November 23, 2010 (incorporated by reference from Exhibit 4.5 to the registrant’s Current Report on Form 8-K/A filed with the Securities and Exchange Commission on July 5, 2011). | |
| 4.6 | Convertible Promissory Note dated June 29, 2011 issued by the registrant to Yasushi Nagasaki (incorporated by reference from Exhibit 4.8 to the registrant’s Registration Statement on Form S-1 filed with the Securities and Exchange Commission on July 11, 2011). | |
| 4.7 | Loan Agreement dated June 9, 2011 by and between Emmaus Medical, Inc. and Equities First Holdings, LLC (incorporated by reference to Exhibit 4.2 to the registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 15, 2011). | |
| 4.8 | Pledge Agreement dated June 9, 2011 by and between Emmaus Medical, Inc. and Equities First Holdings, LLC (incorporated by reference to Exhibit 4.3 to the registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 15, 2011). |
65
| 4.9 | Form of Convertible Promissory Note (1 Year Cash Interest) issued by the registrant to the persons indicated in Schedule A attached to the Form of Convertible Promissory Note (incorporated by reference from Exhibit 4.11 to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on October 6, 2011). | |
| 4.10 | Convertible Promissory Note dated July 11, 2011 issued by the registrant to Suh Yung Min (incorporated by reference from Exhibit 4.12 to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on October 6, 2011). | |
| 4.11 | Form of Convertible Promissory Note (On Demand Up to 1 Year) issued by the registrant to the persons indicated in Schedule A attached to the Form of Convertible Promissory Note (incorporated by reference from Exhibit 4.13 to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on October 6, 2011). | |
| 4.12 | Form of Warrant issued to the individuals listed in Schedule A thereto (incorporated by reference to Exhibit 4.4 to the registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 14, 2011). | |
| 4.13 | Warrant dated August 31, 2011 issued by the registrant to Gregoire De Rothschild (incorporated by reference from Exhibit 4.15 to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on October 6, 2011). | |
| 4.13(a) | Amendment No. 1 dated January 9, 2011 to Warrant dated August 31, 2011 issued by the registrant to Gregoire De Rothschild (incorporated by reference from Exhibit 4.15(a) to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on February 14, 2012). | |
| 4.14 | Convertible Promissory Notes issued by the registrant to The Shitabata Family Trust on September 29, 2011 and October 3, 2011 (incorporated by reference to Exhibit 4.6 to the registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 14, 2011). | |
| 4.15 | Convertible Promissory Note dated October 5, 2011 issued by the registrant to MLPF&S Cust. FBO Willis C. Lee (incorporated by reference from Exhibit 4.7 to the registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 14, 2011). | |
| 4.16 | Warrant dated October 5, 2011 issued to MLPF&S Cust. FBO Willis C. Lee (incorporated by reference to Exhibit 4.8 to the registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 14, 2011). | |
| 4.17 | Warrant dated January 17, 2012 issued by the registrant to Hope International Hospice, Inc. (incorporated by reference from Exhibit 4.19 to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on February 14, 2012). | |
| 4.18 | Convertible Promissory Note, dated February 10, 2012, issued by the registrant to Tracey and Mark Doi (incorporated by reference from Exhibit 4.21 to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on April 10, 2012). | |
| 4.19 | Form of Convertible Promissory Note (On Demand Up to 1 Year) issued by the registrant to the persons indicated in Schedule A attached to the Form of Convertible Promissory Note (incorporated by reference from Exhibit 4.22 to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on April 10, 2012). | |
| 4.20 | Form of Warrant issued by the registrant to the persons indicated in Schedule A attached to the Form of Warrant (incorporated by reference from Exhibit 4.23 to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on April 10, 2012). | |
| 4.21 | Warrant issued by the registrant to the persons indicated in Schedule A attached to the Warrant (incorporated by reference from Exhibit 4.24 to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on April 10, 2012). | |
| 4.22 | Form of Convertible Promissory Note issued by the registrant to the persons indicated in Schedule A. | |
| 4.23 | Promissory Note dated December 5, 2012 issued by the registrant to Dr. Yutaka Niihara. | |
| 4.24 | Promissory Note dated December 26, 2012 issued by the registrant to For Days Co. Ltd. | |
| 10.1 | Share Cancellation Agreement dated as of April 21, 2011 by and between the registrant and AFH Holding and Advisory, LLC (incorporated by reference from Exhibit 10.1 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.2 | Registration Rights Agreement dated as of May 3, 2011 by and among the registrant and the individuals listed on Schedule A thereto (incorporated by reference from Exhibit 10.2 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.3* | Amended and Restated Emmaus Life Sciences, Inc. 2011 Stock Incentive Plan. | |
| 10.3(a)* | Form of Incentive Stock Option Agreement (Time-based and Performance-based Vesting) under the Emmaus Life Sciences, Inc. 2011 Stock Incentive Plan (incorporated by reference from Exhibit 10.3(a) to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.3(b)* | Form of Incentive Stock Option Agreement (Time-based Vesting) under the Emmaus Life Sciences, Inc. 2011 Stock Incentive Plan (incorporated by reference from Exhibit 10.3(b) to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.3(c)* | Form of Non-Qualified Stock Option Agreement (Time-based and Performance-based Vesting) under the Emmaus Life Sciences, Inc. 2011 Stock Incentive Plan (incorporated by reference from Exhibit 10.3(c) to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.3(d)* | Form of Non-Qualified Stock Option Agreement (Time-based Vesting) under the Emmaus Life Sciences, Inc. 2011 Stock Incentive Plan (incorporated by reference from Exhibit 10.3(d) to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). |
66
| 10.3(e)* | Form of Restricted Stock Agreement (Time-based and Performance-based Vesting) under the Emmaus Life Sciences, Inc. 2011 Stock Incentive Plan (incorporated by reference from Exhibit 10.3(e) to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.3(f)* | Form of Restricted Stock Agreement (Time-based Vesting) under the Emmaus Life Sciences, Inc. 2011 Stock Incentive Plan (incorporated by reference from Exhibit 10.3(f) to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.4 | License Agreement dated as of March 7, 2001 by and between Harbor-UCLA Research and Education Institute and Orphan Drug International, L.L.C (i.e. Emmaus Medical, Inc.) and corresponding Addendum to the License Agreement entered into December 19, 2003 between Harbor-UCLA Research and Education Institute and Emmaus Medical, Inc. (incorporated by reference from Exhibit 10.4 to the registrant’s Current Report on Form 8-K/A filed with the Securities and Exchange Commission on July 5, 2011). | |
| 10.5 | Federal Supply Schedule Contract Award dated December 8, 2010 by and between the United States Department of Veterans Affairs and Emmaus Medical, Inc. (incorporated by reference from Exhibit 10.5 to the registrant’s Current Report on Form 8-K/A filed with the Securities and Exchange Commission on July 5, 2011). | |
| 10.6 | Office Lease, dated March 12, 2008, by and between Emmaus Medical, Inc. and 20655 S. Western Avenue, LLC (incorporated by reference from Exhibit 10.6 to the registrant’s Current Report on Form 8-K/A filed with the Securities and Exchange Commission on July 5, 2011). | |
| 10.7 | Sublicense Agreement dated as of October 18, 2007 by and between Cato Holding Company and Emmaus Medical, Inc. (incorporated by reference from Exhibit 10.7 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.8 | Assignment and Transfer Agreement dated as of February 1, 2011 by and among Cato Holding Company, Nutritional Restart Pharmaceutical Limited Partnership and Emmaus Medical, Inc. (incorporated by reference from Exhibit 10.8 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.9 | Promotional Rights Agreement effective as of March 12, 2008 by and between Ares Trading S.A. and Emmaus Medical, Inc. (incorporated by reference from Exhibit 10.9 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.10 | Joint Research and Development Agreement dated as of April 8, 2011 by and between Emmaus Medical, Inc. and CellSeed, Inc. (incorporated by reference from Exhibit 10.10 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.10(a) | Addendum to the Joint Research and Development Agreement effective August 8, 2011 (incorporated by reference from Exhibit 10.10(a) to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on October 6, 2011). | |
| 10.11 | Individual Agreement dated as of April 8, 2011 by and between Emmaus Medical, Inc. and CellSeed, Inc. (incorporated by reference from Exhibit 10.11 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.12* | Employment Agreement dated as of April 5, 2011 by and between Emmaus Medical, Inc. and Yutaka Niihara, M.D., MPH (incorporated by reference from Exhibit 10.12 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.13* | Employment Agreement dated as of April 5, 2011 by and between Emmaus Medical, Inc. and Willis C. Lee (incorporated by reference from Exhibit 10.13 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.14* | Employment Agreement dated as of April 5, 2011 by and between Emmaus Medical, Inc. and Lan T. Tran (incorporated by reference from Exhibit 10.14 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.15* | Employment Agreement dated as of April 8, 2011 by and between Emmaus Medical, Inc. and Yasushi Nagasaki (incorporated by reference from Exhibit 10.15 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.16 | Promissory Note dated as of January 12, 2009 by and between Emmaus Medical, Inc. and Yutaka Niihara, M.D., MPH (incorporated by reference from Exhibit 10.16 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.17 | Promissory Note dated as of April 23, 2009 by and between Emmaus Medical, Inc. and Yutaka Niihara, M.D., MPH (incorporated by reference from Exhibit 10.17 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.18 | Promissory Note dated as of January 12, 2011 by and between Emmaus Medical, Inc. and Willis C. Lee (incorporated by reference from Exhibit 10.18 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
67
| 10.19 | Promissory Note dated as of January 12, 2011 by and between Emmaus Medical, Inc. and Hope International Hospice, Inc. (incorporated by reference from Exhibit 10.19 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.20 | Form of Indemnification Agreement and List of Officers and Directors (incorporated by reference from Exhibit 10.20 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
| 10.21 | Promissory Note dated as of April 29, 2009 by and between Emmaus Medical, Inc. and Daniel Kimbell (incorporated by reference from Exhibit 10.21 to the registrant’s Current Report on Form 8-K/A filed with the Securities and Exchange Commission on July 5, 2011). | |
| 10.22 | Promissory Note dated as of May 11, 2009 by and between Emmaus Medical, Inc. and Daniel Kimbell (incorporated by reference from Exhibit 10.22 to the registrant’s Current Report on Form 8-K/A filed with the Securities and Exchange Commission on July 5, 2011). | |
| 10.23 | Trademark Assignment dated as of February 14, 2011 by and between Cato Research Ltd. and Emmaus Medical, Inc. (incorporated by reference from Exhibit 10.23 to the registrant’s Current Report on Form 8-K/A filed with the Securities and Exchange Commission on July 5, 2011). | |
| 10.24 | Letter of Intent by and between Ajinomoto Company and Emmaus Medical, Inc. (incorporated by reference from Exhibit 10.24 to the registrant’s Current Report on Form 8-K/A filed with the Securities and Exchange Commission on July 5, 2011). | |
| 10.25 | Services Agreement dated as of March 16, 2004 by and between Emmaus Medical, Inc. and ClinDatrix, Inc. (incorporated by reference from Exhibit 10.25 to the registrant’s Current Report on Form 8-K/A filed with the Securities and Exchange Commission on August 18, 2011). | |
| 10.26 | Amended and Restated Letter of Intent by and between the registrant and AFH Holding & Advisory LLC dated September 30, 2011 (incorporated by reference from Exhibit 10.26 to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on October 6, 2011). | |
| 10.27 | Promissory Note dated June 21, 2011 issued by the registrant to Yutaka Niihara, M.D., MPH (incorporated by reference from Exhibit 10.28 to the registrant’s Registration Statement on Form S-1 filed with the Securities and Exchange Commission on July 11, 2011). | |
| 10.28 | Form of Promissory Note issued to Yoko Hagiike issued on the dates and in the amounts indicated in Schedule A attached to the Form of Promissory Note (incorporated by reference from Exhibit 10.29 to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on October 6, 2011). | |
| 10.29 | Promissory Note dated January 17, 2012 issued by the registrant to Hope International Hospice, Inc. (incorporated by reference from Exhibit 10.30 to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on February 14, 2012). | |
| 10.30 | Form of Warrant issued by the registrant to the persons indicated in Schedule A attached to the Form of Warrant (incorporated by reference from Exhibit 10.20 to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on April 10, 2012). | |
| 10.31 | Form of Promissory Note, dated February 10, 2012, issued by the registrant to Lan T. Tran (incorporated by reference from Exhibit 10.32 to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on April 10, 2012). | |
| 10.32 | Form of Promissory Note issued by the registrant to the persons indicated in Schedule A attached to the Form of Promissory Note (incorporated by reference from Exhibit 10.33 to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on April 10, 2012). | |
| 10.33 | Form of Promissory Note issued by the registrant to the persons indicated in Schedule A attached to the Form of Promissory Note (incorporated by reference from Exhibit 10.34 to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on April 10, 2012). | |
| 10.34* | Employment Agreement dated as of April 2, 2012 by and between the registrant and Peter B. Ludlum (incorporated by reference from Exhibit 10.1 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on April 6, 2012). |
68
| 21.1 | List of Subsidiaries (incorporated by reference from Exhibit 21.1 to the registrant’s Registration Statement on Form S-1/A filed with the Securities and Exchange Commission on February 14, 2012). | |
| 31.1 | ||
| 31.2 | ||
| 32.1 | ||
| 101.INS | XBRL Instance Document | |
| 101.SCH | XBRL Taxonomy Extension Schema Document | |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document |
* Designates management contract or compensatory plan or arrangement.
69
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Torrance, California, on March 29, 2013.
| Emmaus Life Sciences, Inc. | |||
| By: | /s/ Yutaka Niihara | ||
| Name: | Yutaka Niihara, MD, MPH | ||
| Title: | President and Chief Executive Officer | ||
| (principal executive officer and duly authorized officer) | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Company in the capacities and on the dates indicated.
| SIGNATURE | TITLE | DATE | ||
| President and Chief Executive Officer (Principal | ||||
/s/ Yutaka Niihara | Executive Officer) and Director | March 29, 2013 | ||
| Yutaka Niihara, M.D., MPH | ||||
| Chief Financial Officer | ||||
/s/ Peter Ludlum | (Principal Financial and Accounting Officer) | March 29, 2013 | ||
| Peter Ludlum | ||||
| /s/ Henry A. McKinnell, Jr. | Chairman of the Board | March 29, 2013 | ||
| Dr. Henry A. McKinnell, Jr. | ||||
/s/ Willis C. Lee | Director | March 29, 2013 | ||
| Willis C. Lee | ||||
| /s/ Tracey C. Doi | Director | March 29, 2013 | ||
| Tracey C. Doi | ||||
| /s/ Maurice J. DeWald | Director | March 29, 2013 | ||
| Maurice J. DeWald |
70
INDEX TO FINANCIAL STATEMENTS
EMMAUS LIFE SCIENCES, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| PAGE | ||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-10 | ||||
| F-11 | ||||
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Emmaus Life Sciences, Inc.
We have audited the accompanying consolidated balance sheets of Emmaus Life Sciences, Inc. as of December 31, 2012 and 2011, and the related consolidated statements of comprehensive loss, changes in stockholders’ equity (deficit), and cash flows for each of the years in the two-year period ended December 31, 2012 and for the period since inception (December 20, 2000) through December 31, 2012. Emmaus Life Sciences, Inc.’s management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Emmaus Life Sciences, Inc. as of December 31, 2012 and 2011, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2012 and for the period since inception (December 20, 2000) through December 31, 2012 in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, these conditions raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments relating to the recoverability and classification of asset carrying amounts or the amount and classification of liabilities that might result should the Company be unable to continue as a going concern.
/s/ EFP Rotenberg, LLP
EFP Rotenberg, LLP
Rochester, New York
March 29, 2013
F-2
Emmaus Life Sciences, Inc.
(A Development Stage Company)
| As of | ||||||||
| December 31, 2012 | December 31, 2011 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash and cash equivalents | $ | 402,823 | $ | 313,684 | ||||
| Accounts receivable | 80,279 | 17,996 | ||||||
| Inventories, net | 203,389 | 195,114 | ||||||
| Marketable securities | — | — | ||||||
| Prepaid expenses and other current assets | 62,833 | 62,774 | ||||||
| Total current assets | 749,324 | 589,568 | ||||||
| PROPERTY AND EQUIPMENT, net | 38,769 | 60,018 | ||||||
| OTHER ASSETS | ||||||||
| Marketable securities, long-term | 561,521 | 1,387,153 | ||||||
| Intangibles, net | 1,321,429 | — | ||||||
| Notes receivable | 18,000 | 18,000 | ||||||
| Deposits | 195,197 | 427,572 | ||||||
| Total other assets | 2,096,147 | 1,832,725 | ||||||
| Total Assets | $ | 2,884,240 | $ | 2,482,311 | ||||
LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable and accrued expenses | $ | 2,560,278 | $ | 756,714 | ||||
| Due to related party | 394,446 | 394,446 | ||||||
| Dissenting stockholders payable | 200,000 | 200,000 | ||||||
| Notes payable | 3,939,041 | 380,000 | ||||||
| Convertible notes payable, net | 4,627,695 | 1,846,010 | ||||||
| Total current liabilities | 11,721,460 | 3,577,170 | ||||||
| LONG-TERM LIABILITIES | ||||||||
| Notes payable | 700,000 | 1,041,728 | ||||||
| Convertible notes payable | 1,106,035 | 838,492 | ||||||
| Total long-term liabilities | 1,806,035 | 1,880,220 | ||||||
| Total Liabilities | 13,527,495 | 5,457,390 | ||||||
| STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||
| Preferred stock – par value $0.001 per share, 20,000,000 shares authorized, none issued and outstanding | — | — | ||||||
Common stock – par value $0.001 per share, 100,000,000 shares authorized, 24,878,436 and 24,393,461 shares issued and outstanding at December 31, 2012 and December 31, 2011, respectively (both excluding 47,178 shares held by stockholders who exercised dissenters’ rights). | 24,878 | 24,394 | ||||||
| Additional paid-in capital | 25,361,863 | 18,332,508 | ||||||
| Accumulated other comprehensive income | (12,432 | ) | 252,413 | |||||
| Deficit accumulated during the development stage | (36,017,564 | ) | (21,584,394 | ) | ||||
| Total Stockholders’ Equity (Deficit) | (10,643,255 | ) | (2,975,079 | ) | ||||
| Total Liabilities & Stockholders’ Equity (Deficit) | $ | 2,884,240 | $ | 2,482,311 | ||||
The accompanying notes are an integral part of these financial statements.
F-3
Emmaus Life Sciences, Inc.
(A Development Stage Company)
| Year Ended December 31, | From December 20, 2000 (date of inception) | |||||||||||
| 2012 | 2011 | to December 31, 2012 | ||||||||||
| SALES | $ | 571,606 | $ | 339,156 | $ | 1,285,261 | ||||||
| SALES RETURN & ALLOWANCE | (28,548 | ) | (2,182 | ) | (61,087 | ) | ||||||
| REVENUES | 543,058 | 336,974 | 1,224,174 | |||||||||
| COST OF GOODS SOLD | ||||||||||||
| Cost of goods sold | 183,673 | 132,104 | 541,811 | |||||||||
| Scrapped inventory | — | — | 235,537 | |||||||||
| Total cost of goods sold | 183,673 | 132,104 | 777,348 | |||||||||
| GROSS PROFIT | 359,385 | 204,870 | 446,826 | |||||||||
| OPERATING EXPENSES | ||||||||||||
| Research and development | 2,966,583 | 1,552,205 | 9,418,440 | |||||||||
| Selling | 402,601 | 696,758 | 2,901,567 | |||||||||
| General and administrative | 8,307,376 | 5,047,886 | 18,968,002 | |||||||||
| Transaction costs | — | 788,893 | 788,893 | |||||||||
| 11,676,560 | 8,085,742 | 32,076,902 | ||||||||||
| LOSS FROM OPERATIONS | (11,317,175 | ) | (7,880,872 | ) | (31,630,076 | ) | ||||||
| OTHER INCOME (EXPENSE) | ||||||||||||
| Realized gain on securities available-for sale | 275,822 | — | 275,822 | |||||||||
| Interest income | 37,649 | 30,493 | 153,376 | |||||||||
| Interest expense | (3,422,220 | ) | (979,170 | ) | (4,791,383 | ) | ||||||
| (3,108,749 | ) | (948,677 | ) | (4,362,185 | ) | |||||||
| LOSS BEFORE INCOME TAXES | (14,425,924 | ) | (8,829,549 | ) | (35,992,261 | ) | ||||||
| INCOME TAXES | 7,246 | 3,209 | 25,303 | |||||||||
| NET LOSS | (14,433,170 | ) | (8,832,758 | ) | (36,017,564 | ) | ||||||
| OTHER INCOME (LOSS) | ||||||||||||
| Unrealized holding gain (loss) on securities available-for-sale, net of tax | (259,725 | ) | (287,233 | ) | (4,385 | ) | ||||||
| Unrealized foreign translation | (5,119 | ) | (2,927 | ) | (8,046 | ) | ||||||
| COMPREHENSIVE LOSS | $ | (14,698,014 | ) | $ | (9,122,918 | ) | $ | (36,029,995 | ) | |||
| NET LOSS PER COMMON SHARE | $ | (0.59 | ) | $ | (0.38 | ) | ||||||
| WEIGHTED AVERAGE COMMON SHARES OUTSTANDING | 24,482,759 | 23,068,206 | ||||||||||
The accompanying notes are an integral part of these financial statements
F-4
Emmaus Life Sciences, inc.
(A Development Stage Company)
Consolidated Statement of Changes in Stockholders’ Equity (Deficit)
| Common stock – par value $0.001 per share, 100,000,000 shares authorized | Gross $/Share | Additional Paid-in Capital | Accumulated Other Comprehensive income | Deficit Accumulated during Development Stage | ||||||||||||||||||||||||
| Shares | Common stock | Total | ||||||||||||||||||||||||||
| Balance, December 31, 2000 (1) (2) | 12,531,125 | $ | 12,531 | — | $ | (2,931 | ) | — | $ | — | $ | 9,600 | ||||||||||||||||
| Net loss | — | — | — | — | — | (21,942 | ) | (21,942 | ) | |||||||||||||||||||
| Balance, December 31, 2001 | 12,531,125 | 12,531 | — | (2,931 | ) | — | (21,942 | ) | (12,342 | ) | ||||||||||||||||||
| Net loss | — | — | — | — | — | (12,464 | ) | (12,464 | ) | |||||||||||||||||||
| Balance, December 31, 2002 | 12,531,125 | 12,531 | — | (2,931 | ) | — | (34,406 | ) | (24,806 | ) | ||||||||||||||||||
| Constructive distribution of retained loss to Additional Paid-in Capital | — | — | — | (34,406 | ) | — | 34,406 | — | ||||||||||||||||||||
| Common stock issued | 737,125 | 737 | 0.34 | 249,263 | — | — | 250,000 | |||||||||||||||||||||
| — | ||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | (97,481 | ) | (97,481 | ) | |||||||||||||||||||
| Balance, December 31, 2003 | 13,268,250 | 13,268 | — | 211,926 | — | (97,481 | ) | 127,713 | ||||||||||||||||||||
(1) Reflects recapitalization of members’ equity into (425,000 pre-merger) 12,531,125 shares of common stock of Emmaus Medical, Inc.
(2) The stockholders’ equity has been recapitalized to give effect to the share exchanged by existing stockholders pursuant to the merger agreement dated April 21, 2011, more fully discussed in the Recapitalization and change in legal status of entity footnotes to these financial statements.
F-5
Emmaus Life Sciences, Inc.
(A Development Stage Company)
Consolidated Statement of Changes in Stockholders’ Equity (Deficit)
for the period from December 20, 2000 (Inception) to December 31, 2012 (Continued)
| Common stock – par value $0.001 per share, 100,000,000 shares authorized | Gross $/Share | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | ||||||||||||||||||||||||
| Shares | Common Stock | Total | ||||||||||||||||||||||||||
| Balance, December 31, 2003 | 13,268,250 | $ | 13,268 | — | $ | 211,926 | $ | — | $ | (97,481 | ) | $ | 127,713 | |||||||||||||||
| Common stock issued | 1,459,270 | 1,459 | 0.35 | 503,616 | — | — | 505,075 | |||||||||||||||||||||
| Common stock issued | 88,455 | 89 | 0.06 | 4,911 | — | — | 5,000 | |||||||||||||||||||||
| Common stock issued | 67,817 | 68 | 2.03 | 137,932 | — | — | 138,000 | |||||||||||||||||||||
| Net loss | — | — | — | — | (624,936 | ) | (624,936 | ) | ||||||||||||||||||||
| Balance, December 31, 2004 | 14,883,792 | 14,884 | — | 858,385 | — | (722,417 | ) | 150,852 | ||||||||||||||||||||
| Common stock issued | 398,549 | 399 | 2.03 | 327,886 | — | — | 328,285 | |||||||||||||||||||||
| Net loss | — | — | — | — | (668,091 | ) | (668,091 | ) | ||||||||||||||||||||
| Balance, December 31, 2005 | 15,282,341 | 15,283 | — | 1,186,271 | — | (1,390,508 | ) | (188,954 | ) | |||||||||||||||||||
| Common stock issued | 523,388 | 523 | 2.03 | 824,517 | — | — | 825,040 | |||||||||||||||||||||
| Net loss | — | — | — | — | (759,962 | ) | (759,962 | ) | ||||||||||||||||||||
| Balance, December 31, 2006 | 15,805,729 | 15,806 | — | 2,010,788 | — | (2,150,470 | ) | (123,876 | ) | |||||||||||||||||||
| Common stock issued | 1,344,162 | 1,344 | 2.03 | 2,732,516 | — | — | 2,733,860 | |||||||||||||||||||||
| Net loss | — | — | — | — | — | (1,282,212 | ) | (1,282,212 | ) | |||||||||||||||||||
| Balance, December 31, 2007 | 17,149,891 | 17,150 | — | 4,743,304 | — | (3,432,682 | ) | 1,327,772 | ||||||||||||||||||||
F-6
Emmaus Life Sciences, Inc.
(A Development Stage Company)
Consolidated Statement of Changes in Stockholders’ Equity (Deficit)
for the period from December 20, 2000 (Inception) to December 31, 2012 (Continued)
| Common stock – par value $0.001 per share, 100,000,000 shares authorized | Gross $/Share | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | ||||||||||||||||||||||||
| Shares | Common Stock | Total | ||||||||||||||||||||||||||
| Balance, December 31, 2007 | 17,149,891 | $ | 17,150 | — | $ | 4,743,304 | $ | — | $ | (3,432,682 | ) | $ | 1,327,772 | |||||||||||||||
| Common stock issued | 1,226,959 | 1,227 | 2.03 | 3,389,464 | — | — | 3,390,691 | |||||||||||||||||||||
| Net loss | — | — | — | — | — | (2,993,777 | ) | (2,993,777 | ) | |||||||||||||||||||
| Balance, December 31, 2008 | 18,376,850 | 18,377 | — | 8,132,768 | — | (6,426,459 | ) | 1,724,686 | ||||||||||||||||||||
| Warrants issued | — | — | — | 160,000 | — | — | 160,000 | |||||||||||||||||||||
| Common stock issued, net of issuance cost of $160,000 | 854,446 | 854 | 2,078,071 | — | — | 2,078,925 | ||||||||||||||||||||||
| Net loss | — | — | — | — | — | (2,567,807 | ) | (2,567,807 | ) | |||||||||||||||||||
| Balance, December 31, 2009 | 19,231,296 | 19,231 | — | 10,370,839 | — | (8,994,266 | ) | 1,395,804 | ||||||||||||||||||||
| Warrants issued | — | — | — | 480,000 | — | — | 480,000 | |||||||||||||||||||||
| Common stock issued, net of issuance cost of $480,000 | 705,900 | 706 | 3.05 | 1,643,588 | — | — | 1,644,294 | |||||||||||||||||||||
| Conversion of notes payable to common stock | 427,857 | 428 | 3.05 | 1,305,572 | — | — | 1,306,000 | |||||||||||||||||||||
| Unrealized gain on securities available for sale | — | — | — | — | 542,573 | — | 542,573 | |||||||||||||||||||||
| Net loss | — | — | — | — | — | (3,757,370 | ) | (3,757,370 | ) | |||||||||||||||||||
| Balance, December 31, 2010 | 20,365,053 | 20,365 | — | 13,799,999 | 542,573 | (12,751,636 | ) | 1,611,301 | ||||||||||||||||||||
F-7
Emmaus Life Sciences, Inc.
(A Development Stage Company)
Consolidated Statement of Changes in Stockholders’ Equity (Deficit)
for the period from December 20, 2000 (Inception) to December 31, 2012 (Continued)
| Common stock – par value $0.001 per share, 100,000,000 shares authorized | Gross $/Share | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | ||||||||||||||||||||||||
| Shares | Common Stock | Total | ||||||||||||||||||||||||||
| Balance, December 31, 2010 | 20,365,053 | $ | 20,365 | — | $ | 13,799,999 | $ | 542,573 | $ | (12,751,636 | ) | $ | 1,611,301 | |||||||||||||||
| Common stock issued, net of issuance cost | 272,147 | 272 | 4.24 | 1,153,402 | — | — | 1,153,674 | |||||||||||||||||||||
| Conversion of notes payable to common stock | 36,514 | 37 | 3.05 | 109,993 | — | — | 110,030 | |||||||||||||||||||||
| Shares issued to existing shell stockholders in the reorganization | 3,750,000 | 3,750 | — | (3,750 | ) | — | — | — | ||||||||||||||||||||
| Shares issued for stock option exercised | 11,794 | 12 | 3.05 | 35,960 | — | — | 35,972 | |||||||||||||||||||||
| Proceeds from exercise of warrants | 4,718 | 5 | 3.05 | 14,395 | — | — | 14,400 | |||||||||||||||||||||
| Stock options vested | — | — | — | 35,196 | — | — | 35,196 | |||||||||||||||||||||
| Cashless exercise of warrants | 413 | — | — | — | — | — | — | |||||||||||||||||||||
| Common stock repurchased and cancelled from dissenting stockholders | (47,178 | ) | (47 | ) | 4.24 | (199,953 | ) | — | — | (200,000 | ) | |||||||||||||||||
| Warrants issued as a payment of consulting fee | — | — | — | 1,053,150 | — | — | 1,053,150 | |||||||||||||||||||||
| Warrants issued in conjunction with convertible note | — | — | — | 864,773 | — | — | 864,773 | |||||||||||||||||||||
| Impact of beneficial conversion feature | — | — | — | 1,469,343 | — | — | 1,469,343 | |||||||||||||||||||||
| Unrealized loss on securities | — | — | — | — | (287,233 | ) | — | (287,233 | ) | |||||||||||||||||||
| Foreign currency translation effect | — | — | — | — | (2,927 | ) | — | (2,927 | ) | |||||||||||||||||||
| Net loss | — | — | — | — | — | (8,832,758 | ) | (8,832,758 | ) | |||||||||||||||||||
| Balance, December 31, 2011 | 24,393,461 | 24,394 | - | 18,332,508 | 252,413 | (21,584,394 | ) | (2,975,079 | ) | |||||||||||||||||||
F-8
Emmaus Life Sciences, Inc.
(A Development Stage Company)
Consolidated Statement of Changes in Stockholders’ Equity (Deficit)
for the period from December 20, 2000 (Inception) to December 31, 2012
Common stock – par value $0.001 per share, 100,000,000 shares authorized | Gross $/Share | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | ||||||||||||||||||||||||
| Shares | Common Stock | Total | ||||||||||||||||||||||||||
| Balance, December 31, 2011 | 24,393,461 | $ | 24,394 | — | $ | 18,332,508 | $ | 252,413 | $ | (21,584,394 | ) | $ | (2,975,079 | ) | ||||||||||||||
| Warrants issued in conjunction with convertible note | — | — | — | 175,961 | — | — | 175,961 | |||||||||||||||||||||
| Warrants issued in conjunction with promissory note | — | — | — | 1,485,835 | — | — | 1,485,835 | |||||||||||||||||||||
| Impact of beneficial conversion feature | — | — | — | 256,761 | — | — | 256,761 | |||||||||||||||||||||
| Discount on noninterest bearing convertible note | — | — | — | 25,562 | — | — | 25,562 | |||||||||||||||||||||
| Stock based compensation | — | — | — | 3,466,410 | — | — | 3,466,410 | |||||||||||||||||||||
| Stock issued as a payment of professional fee | 12,787 | 12 | 3.60 | 46,021 | — | — | 46,033 | |||||||||||||||||||||
| Stock issued for cash | 472,188 | 472 | 3.33 | 1,572,805 | — | 1,573,277 | ||||||||||||||||||||||
| Realized gain on securities | — | — | — | — | 24,490 | — | 24,490 | |||||||||||||||||||||
| Unrealized gain on securities | — | — | — | — | (284,215 | ) | — | (284,215 | ) | |||||||||||||||||||
| Foreign currency translation effect | — | — | — | — | (5,120 | ) | — | (5,120 | ) | |||||||||||||||||||
| Net loss | — | — | — | — | — | (14,433,170 | ) | (14,433,170 | ) | |||||||||||||||||||
| Balance, December 31, 2012 | 24,878,436 | $ | 24,878 | — | $ | 25,361,863 | $ | (12,432 | ) | $ | (36,017,564 | ) | $ | (10,643,255 | ) | |||||||||||||
The accompanying notes are an integral part of these financial statements.
F-9
EMMAUS LIFE SCIENCES, INC.
(A Development Stage Company)
| Year ended December 31, | From December 20, 2000 (date of inception) to December 31, | |||||||||||
| 2012 | 2011 | 2012 | ||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||||||
| Net loss | $ | (14,433,170 | ) | $ | (8,832,758 | ) | $ | (36,017,564 | ) | |||
| Adjustments to reconcile net loss to net cash flows from operating activities | ||||||||||||
| Depreciation and amortization | 207,598 | 170,271 | 1,084,617 | |||||||||
| Cost of scrapped inventory written off | — | — | 235,537 | |||||||||
| Fair value of warrants issued for services | — | 1,053,150 | 1,053,150 | |||||||||
| Interest expense accrued from discount of convertible note | 2,570,025 | 776,084 | 3,346,109 | |||||||||
| Gain on termination of secured debt | (275,822 | ) | — | (275,822 | ) | |||||||
| Share-based compensation | 3,466,410 | 35,196 | 3,501,606 | |||||||||
| Net changes in operating assets and liabilities, net of acquisition | ||||||||||||
| Accounts receivable | (62,893 | ) | 3,750 | (86,995 | ) | |||||||
| Inventory | (11,902 | ) | (64,541 | ) | (438,543 | ) | ||||||
| Prepaid expenses and other current assets | (1,011 | ) | (51,295 | ) | (81,785 | ) | ||||||
| Deposits | 231,776 | (79,164 | ) | (145,160 | ) | |||||||
| Accounts payable and accrued expenses | 1,859,601 | 566,607 | 2,556,177 | |||||||||
| Due to related party | — | 394,446 | 394,446 | |||||||||
| Net cash flows used in operating activities | (6,449,388 | ) | (6,028,254 | ) | (24,874,227 | ) | ||||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||||||
| Payment towards license | (1,500,000 | ) | — | (2,250,000 | ) | |||||||
| Purchases of marketable securities | — | — | (1,131,813 | ) | ||||||||
| Purchases of property and equipment | (7,385 | ) | (1,171 | ) | (194,363 | ) | ||||||
| Net cash flows used in investing activities | (1,507,385 | ) | (1,171 | ) | (3,576,176 | ) | ||||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||||||
| Borrowings from line of credit | — | — | 299,500 | |||||||||
| Repayment of line of credit | — | — | (299,500 | ) | ||||||||
| Proceeds from notes payable issued | 5,273,660 | 1,841,728 | 7,857,851 | |||||||||
| Proceeds from convertible notes payable issued | 1,950,470 | 3,921,645 | 7,362,145 | |||||||||
| Payments of notes payable | (391,667 | ) | (880,000 | ) | (1,307,243 | ) | ||||||
| Payments of convertible notes payable issued | (350,100 | ) | — | (350,100 | ) | |||||||
| Proceeds from issuance of common stock | 1,573,277 | 1,204,046 | 15,291,687 | |||||||||
| Net cash flows from financing activities | 8,055,640 | 6,087,419 | 28,854,340 | |||||||||
| Effect of exchange rate changes on cash | (9,728 | ) | (2,986 | ) | (12,714 | ) | ||||||
| Net increase/decrease in cash and cash equivalents | 89,139 | 55,008 | 391,223 | |||||||||
| Cash and cash equivalents, beginning of period | 313,684 | 258,676 | — | |||||||||
| Cash acquired | — | — | 11,600 | |||||||||
| Cash and cash equivalents, end of period | $ | 402,823 | $ | 313,684 | $ | 402,823 |
| SUPPLEMENTAL DISCLOSURES OF CASH FLOW ACTIVITIES | ||||||||||||
| Interest paid | $ | 248,880 | $ | 63,284 | $ | 702,157 | ||||||
| Income taxes paid | $ | 7,246 | $ | 3,209 | $ | 25,303 | ||||||
Non-cash financing activities:
| Stock issued as a payment of professional fee | $ | 46,033 | $ | 0 | $ | 46,033 | ||||||
| Conversion of notes payable to common stock | $ | — | $ | 110,030 | $ | 1,416,030 | ||||||
| Disposal of pledged marketable securities | (565,907 | ) | $ | — | $ | (565,907 | ) | |||||
| Cancellation of secured debt | $ | 841,728 | $ | — | $ | 841,728 |
The accompanying notes are an integral part of these financial statements
F-10
Emmaus Life Sciences, Inc.
(A Development Stage Company)
NOTE 1 – DESCRIPTION OF BUSINESS
Organization – Emmaus Life Sciences, Inc. (the “Company” or “Emmaus”), which is engaged in the discovery, development, and commercialization of treatments and therapies for rare diseases, was incorporated in the state of Delaware on September 24, 2007. Pursuant to an Agreement and Plan of Merger, dated April 21, 2011 (the “Merger Agreement”), by and among the Company, AFH Merger Sub, Inc., a wholly-owned subsidiary of the Company (“AFH Merger Sub”), AFH Holding and Advisory, LLC, and Emmaus Medical, Inc. (“Emmaus Medical”), Emmaus Medical merged with and into AFH Merger Sub with Emmaus Medical continuing as the surviving entity (the “Merger”). Upon the closing of the Merger, the Company changed its name from “AFH Acquisition IV, Inc.” to “Emmaus Holdings, Inc.” and became the parent company of Emmaus Medical. The Company changed its name from “Emmaus Holdings, Inc.” to “Emmaus Life Sciences, Inc.” on September 14, 2011.
Emmaus Medical is a Delaware corporation originally incorporated on September 12, 2003. Emmaus Medical, LLC was organized on December 20, 2000. In October 2003, Emmaus Medical, LLC conducted a reorganization and merged with Emmaus Medical. As a result of the merger, Emmaus Medical acquired the exclusive patent rights for a treatment for sickle cell disease.
In October 2010, the Company established Emmaus Medical Japan, Inc., a Japanese corporation (“EM Japan”) by paying 97% of the initial capital. EM Japan is engaged in the business of trading in nutritional supplements and other medical products and drugs. The results of EM Japan have been included in the consolidated financial statements of the Company since the date of formation. The aggregate formation cost was $52,500. Emmaus Medical acquired the additional 3% of the outstanding shares of EM Japan during the three months ended March 31, 2011 and is now the 100% owner of the outstanding share capital.
In November 2011, the Company formed Emmaus Medical Europe, Ltd. (“EM Europe”), a wholly owned subsidiary of Emmaus Medical. EM Europe’s primary focus is expanding the business of Emmaus Medical in Europe.
Emmaus, its wholly-owned subsidiary, Emmaus Medical, and Emmaus Medical’s wholly-owned subsidiaries, Newfield Nutrition Corporation, EM Japan and EM Europe, are collectively referred to herein as the “Company.”
Nature of Business – The Company has undertaken the business of developing and commercializing cost-effective treatments and therapies for rare diseases. The Company’s primary business purpose is to continue its late-stage development of the amino acid L-glutamine as a prescription drug for the treatment of sickle cell disease (“SCD”). The Company’s current focus is to complete the Phase III clinical trial on SCD that involves over 30 research sites and at least 220 patients.
To a lesser extent, we are also engaged in the marketing and sale of NutreStore® [L-glutamine powder for oral solution], which has received FDA approval, as a treatment for Short Bowel Syndrome (“SBS”) in patients receiving specialized nutritional support when used in conjunction with a recombinant human growth hormone that is approved for this indication. Our indirect wholly owned subsidiary, Newfield Nutrition Corporation, sells L-glutamine as a nutritional supplement under the brand name AminoPure® through retail stores in multiple states and via importers and distributors in Japan, Taiwan and South Korea. The Company also owns a minority interest in CellSeed, Inc., a Japanese company listed on the JASDAQ NEO Growth market in Tokyo, which is engaged in research and development of regenerative medicine products and the manufacture and sale of temperature-responsive cell culture equipment.
The Company also has certain rights to regenerative medicine products owned by CellSeed and is involved in research focused on providing innovative solutions for tissue-engineering through the development of novel cell harvest methods and 3-dimensional living tissue replacement products for "cell-sheet therapy" and regenerative medicine and the commercialization of such products.
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of presentation – The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America.
Going concern – The accompanying consolidated financial statements have been prepared on the basis that the Company will continue as a going concern. The Company has losses for the year ended December 31, 2012 totaling $14,433,170 as well as an accumulated deficit since inception amounting to $36,017,564. Further, the Company appears to have inadequate cash and cash equivalents of $402,823 as of December 31, 2012 considering that revenues from operations since inception totaled only $1,224,174. As a result, the Company is dependent upon funds from private investors and the support of certain stockholders.
F-11
These factors raise substantial doubt about the ability of the Company to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of these uncertainties. In this regard, management is planning to raise any necessary additional funds through loans and additional sales of its common stock. There is no assurance that the Company will be successful in raising additional capital.
Recapitalization and change in legal status of entity – In October 2003, Emmaus Medical acquired substantially all of the assets of Emmaus Medical, LLC. The stockholders of Emmaus Medical were substantially the same as the members of Emmaus Medical, LLC. As such, the transaction was accounted for as a transfer of assets between entities under common control pursuant to accounting standards codification 805, Business Combinations.
For a transferred set of activities and assets to be a business, it must contain all of the inputs and processes necessary for it to continue to conduct normal operations after the transferred set of assets is separated from the transferor, which include the ability to sustain a revenue stream by providing its outputs to customers. Emmaus Medical obtained the inputs and processes necessary for normal operations. The transaction has been accounted for as a recapitalization of Emmaus Medical, LLC. Accordingly, the assets were carried over to Emmaus Medical, Inc. at the historical carrying values and the historical operations of those assets owned by Emmaus Medical are presented in the accompanying financial statements as the historical operations of Emmaus Medical, Inc. for all periods presented.
The effect of the recapitalization was to retroactively present the stockholders’ equity of Emmaus Medical, Inc. (the surviving entity) to the earliest period presented in the financial statements. This recapitalization had no effect on results of operations for any period presented. Also, concurrent with the recapitalization, Emmaus Medical changed its legal status from a Limited Liability Company to a “C” Corporation. In connection with this change, deficits accumulated in the Limited Liability Company were transferred to additional paid in capital.
Pursuant to the Merger Agreement, Emmaus Medical merged with and into AFH Merger Sub with Emmaus Medical continuing as the surviving entity (the “Merger”).
Upon the closing of the Merger on May 3, 2011, the Company changed its name from “AFH Acquisition IV, Inc.” to “Emmaus Holdings, Inc.” The Company subsequently changed its name from “Emmaus Holdings, Inc.” to “Emmaus Life Sciences, Inc.” on September 14, 2011.
Upon consummation of the Merger, (i) each outstanding share of Emmaus Medical common stock was exchanged for 29.48548924976 shares of Company common stock, (ii) each outstanding Emmaus Medical option and warrant, which was exercisable for one share of Emmaus Medical common stock, was exchanged for an option or warrant, as applicable, exercisable for 29.48548924976 shares of Company common stock; and (iii) each outstanding convertible note of Emmaus Medical, which was converted for one share of Emmaus Medical common stock, was exchanged for a convertible note exercisable for 29.48548924976 shares of Company common stock.
As a result of the Merger, security holders of Emmaus Medical received 20,628,305 shares of Company common stock, options and warrants to purchase an aggregate of 326,507 shares of Company common stock, and convertible notes to purchase an aggregate of 271,305 shares of Company common stock.
Four stockholders exercised their dissenters’ rights in connection with the Merger and returned an aggregate of 47,178 shares for an aggregate of $200,000. The shares were cancelled as of May 3, 2011, the closing date of the Merger.
For accounting purposes, the Merger transaction is being accounted for as a reverse merger. The transaction has been treated as a recapitalization of Emmaus Medical and its subsidiaries, with Emmaus Life Sciences, Inc. (the legal acquirer of Emmaus Medical and its subsidiaries) considered the accounting acquiree and Emmaus Medical, whose management took control of Emmaus Life Sciences, Inc. (the legal acquiree of Emmaus Medical), considered the accounting acquirer.
Principles of consolidation – The financial statements include the accounts of the Company (and its wholly-owned subsidiary, Emmaus Medical, Inc., and Emmaus Medical’s wholly-owned subsidiaries, Newfield Nutrition Corporation, EM Japan and EM Europe. All significant intercompany transactions have been eliminated.
Development stage company – The Company is a development stage company as defined in accounting principles generally accepted in the United States of America. The Company is considered a development stage company because it devotes substantially all of its time to research and development for potential pharmaceutical products and to establish its business and operations. The minimal sales for the period from inception to December 31, 2012 are from NutreStore and the products of Newfield Nutrition Corporation, which is not considered to be a part of its principal operations.
F-12
Estimates – Financial statements prepared in accordance with accounting principles generally accepted in the United States require management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Among other things, management has estimated the useful lives of equipment and other assets, along with the variables used to calculate the valuation of stock options and warrants using the Black-Scholes-Merton option valuation model. Actual results could differ from those estimates.
Cash and cash equivalents – Cash and cash equivalents include all short-term securities with original maturities of less than ninety days. The Company maintains its cash and cash equivalents at insured financial institutions, the balances of which may, at times, exceed federally insured limits. Management believes that the risk of loss due to the concentrations is minimal.
Inventories – Inventories as of December 31, 2012 consisted of 100% finished goods and are valued based on first-in, first-out and at the lesser of cost or market value. Work-in-process inventories consist of raw material L-Glutamine for the Company’s AminoPure and NutreStore products that has not yet been packaged and labeled for sale.
All of the purchases during the year ended December 31, 2012 were from one vendor and in 2011 were from two vendors.
Deposits – Carrying value of amounts transferred to third parties for security purposes that are expected to be returned or applied towards payment after one year or beyond the operating cycle, if longer. Deposit amounts consist primarily of the 20% patient site enrollment deposit paid to the Company’s contract research organization for the FDA Phase III clinical trial activities.
Revenue recognition – the Company recognizes revenue in accordance with ASC 605, “Revenue Recognition”.
Revenue is recognized when there is persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed and determinable and collection is reasonably assured.
With prior written approval of the Company, product is returnable only by our direct customers for a returned goods credit, if the product meets any of the following criteria:
A. | Product expiring within six (6) months of the expiration date printed on the package/container that is in the manufacturer’s original package/container and bears the original label. |
| B. | Expired product that is in the manufacturer’s original package/container and bears the manufacturer’s original label, provided, however, that expired product must be returned within 12 months of the expiration date printed on the package/container. |
| C. | Product shipped directly by the Company that is damaged in transit, subject to Free on Board (“FOB”) Destination, or material shipped in error by the Company. |
D. | Product that is discontinued, withdrawn, or recalled. |
Credits will only be issued for full cartons without any missing packets of product. No credit is issued, nor does the Company accept charges or deductions for administrative, handling, or freight charges associated with the return of product to the Company. No credit is issued for product destroyed by anyone other than the Company. Customers must return the product within 60 days of receiving the Company’s written approval for the return or the return will not be issued a credit. The amount of the credit provided for returned product is based on the current wholesale acquisition cost of the returned product less 5%. When product is returned, a credit memo is applied to the customer’s current account balance or applied to future purchases. Credit memos expire one hundred eighty (180) days from date issued.
The Company estimates its sales returns based upon its prior sales and return history. Historically, sales returns have been very nominal. The Company continues to monitor its returns and will adjust its estimates based on its actual sales return experience. The Company records a 5% Sales Return Allowance for its sales in the accompanying financial statements.
The Company is currently required to pay royalties to CATO Holding Company on an annual basis, which is recognized as an expense upon sale of the products, primarily NutreStore.
Allowance for doubtful accounts – The Company provides an allowance for uncollectible accounts based upon prior experience and management’s assessment of the collectability of existing specific accounts.
Advertising cost – Advertising costs are expensed as incurred. Advertising costs for the year ended December 31, 2012 and 2011 were $172,359 and $104,168, respectively. Advertising costs from inception to December 31, 2012 were approximately $416,527.
Property and equipment – Leaseholds, furniture, and fixtures are recorded at historical cost and depreciated on a straight-line basis over their estimated useful lives of 5 to 7 years. Maintenance and repairs are expensed as incurred, while major additions and improvements are capitalized. Gains and losses on disposition are included in current operations.
F-13
Intangibles – The Company’s intangible assets include license issue fees and patent costs relating to a license agreement (Note 4). These intangible assets are amortized over a period of 3 to 7 years, the estimated legal life of the patents and economic life of the license agreements. The intangible assets are assessed by management, annually, for potential impairment. No impairment existed as of December 31, 2012 and December 31, 2011.
Impairment of long-lived assets – In accordance with FASB ASC 360-10-5, Accounting for the Impairment or Disposal of Long-Lived Assets, the Company evaluates the carrying value of its long-lived assets for impairment whenever events or changes in circumstances indicate that such carrying values may not be recoverable. The Company uses its best judgment based on the current facts and circumstances relating to its business when determining whether any significant impairment factors exist. The Company considers the following factors or conditions, among others, that could indicate the need for an impairment review:
| • | significantly lower performance relative to expected historical or projected future operating results; | |
| • | market projections; | |
| • | its ability to obtain patents, including continuation patents, on technology; | |
| • | significant changes in its strategic business objectives and utilization of the assets; | |
| • | significant negative industry or economic trends, including legal factors; | |
| • | potential for strategic partnerships for the development of its patented technology; and | |
| • | changing or implementation of rules regarding manufacture. |
If the Company determines that the carrying values of long-lived assets may not be recoverable based upon the existence of one or more of the above indicators of impairment, the Company’s management performs an undiscounted cash flow analysis to determine if impairment exists. If impairment exists, the Company measures the impairment based on the difference between the asset’s carrying amount and its fair value, and the impairment is charged to operations in the period in which the long-lived asset impairment is determined by management.
Based on existing indicators of impairment and on the related analysis, the Company believes that no impairment of the carrying value of its long-lived assets existed at December 31, 2012.
There can be no assurance, however, that market conditions will not change or demand for the Company’s products will continue or allow the Company to realize the value of its long-lived assets and prevent future impairment.
Research and development – Research and development consist of expenditures for the research and development of new products and technologies, which primarily involve contract research, payroll-related expenses, and other related supplies. Research and development costs are expensed as incurred.
Share-based compensation – The Company recognizes compensation cost for share-based compensation awards during the service term of the recipients of the share-based awards. The fair value of share-based compensation is calculated using the Black-Scholes-Merton pricing model. The Black-Scholes-Merton model requires subjective assumptions regarding future stock price volatility and expected time to exercise, which greatly affect the calculated values. The expected term of awards granted is derived from historical data on awards exercised and post-vesting employment termination behavior. The risk-free rate selected to value any particular grant is based on the U.S. Treasury rate on the grant date that corresponds to the expected term of the award. The expected volatility is based on the historical volatility of the common stock of comparable publicly traded companies. These factors could change, affecting the determination of stock-based awards expense in future periods.
Income taxes – The Company accounts for income taxes under the asset and liability method, wherein deferred tax assets and liabilities are recognized for the future tax consequences attributable to the differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period the enactment occurs. A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize tax assets through future operations.
The Company's short-term and long-term deferred tax liability is based on the calculation of the deferred taxes on the Company's unrealized gain on available-for-sale securities using a 40% effective tax rate based on a 31% federal income tax rate (net of state tax deduction) combined with an 8.84% California state income tax rate. The Company recognizes a deferred tax asset (through changes in the valuation allowance) for the exact amount of the deferred tax liability. The classification of these deferred taxes is concurrent with the classification of investments for which the unrealized gain is derived. For balance sheet presentation, current deferred tax assets and liabilities have been offset and presented as a single amount and non-current deferred tax assets and liabilities within each tax jurisdiction have been offset and presented as a single amount.
F-14
When tax returns are filed, it is highly probable that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. The benefit of a tax position is recognized in the financial statements in the period during which, based on all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50 percent likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken that exceeds the amount measured as described above is reflected as a liability for unrecognized tax benefits along with any associated interest and penalties that would be payable to the taxing authorities upon examination. As of December 31, 2012, the Company had no unrecognized tax benefits, and the Company had no positions which, in the opinion of management, would be reversed if challenged by a taxing authority. The Company’s evaluation of tax positions was performed for those tax years which remain open to audit. The Company may from time to time be assessed interest or penalties by the taxing authorities, although any such assessments historically have been minimal and immaterial to the Company’s financial results. In the event the Company is assessed interest and/or penalties, such amounts will be classified as income tax expense in the financial statements.
As of December 31, 2012, all federal tax returns since 2009 and state tax returns since 2008 are still subject to adjustment upon audit. No tax returns are currently being examined by taxing authorities.
Comprehensive income (loss) – Comprehensive income (loss) includes net income (loss) and other comprehensive income (loss). The items of other comprehensive income (loss) for the Company are unrealized gains and losses on securities classified as available-for-sale and foreign translation adjustments from its subsidiaries. When the Company realizes a gain or loss on available-for-sale securities for which an unrealized gain or loss was previously recognized, a corresponding reclassification adjustment is made to remove the unrealized gain or loss from other comprehensive income and reflect the realized gain or loss in current operations.
Marketable securities – Investment securities as of December 31, 2012 and December 31, 2011 are classified as available-for-sale. Securities available-for-sale are recorded at cost and any increases or decreases in fair market value are recorded as unrealized gain or loss, net of taxes in accumulated other comprehensive income. The Company monitors these investments for impairment and makes appropriate reductions in carrying values when necessary. CellSeed, Inc. securities are the only marketable security the Company currently carries on its books. The Company’s marketable securities consist of 73,550 shares of CellSeed stock which are part of 147,100 shares acquired in January 2009 for 100,028,000 Japanese Yen (equivalent to $1,109,819), at 680 Yen per share. CellSeed’s IPO (JASDAQ Growth symbol 7776) was completed on March 16, 2010. As of December 31, 2012 and December 31, 2011, the closing price per share was 661 Yen and 730 Yen, respectively. Historical JASDEQ closing prices can be found from http://www.bloomberg.com/apps/quote?ticker=7776:JP. The Company’s security position in CellSeed is many times the average daily trading volume of the stock on the JASDAQ exchange. Any attempt to sell the Company’s position in a short period of time may have an adverse impact on the price of the stock.
As of December 31, 2012 and December 31, 2011, 100% of the investment in CellSeed is classified as a long term asset in the accompanying balance sheet as the investment is assigned as collateral on certain borrowings.
Fair value measurements – The Company defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The Company measures fair value under a framework that provides a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (level 1 measurements) and the lowest priority to unobservable inputs (level 3 measurements). The three levels of the fair value hierarchy are described as follows:
Level 1: Inputs to the valuation methodology are unadjusted quoted prices for identical assets or liabilities in active markets that the Company has the ability to access.
Level 2: Inputs to the valuation methodology include:
| • | Quoted prices for similar assets or liabilities in active markets; | |
| • | Quoted prices for identical or similar assets or liabilities in inactive markets; | |
| • | Inputs other than quoted prices that are observable for the asset or liability; and | |
| • | Inputs that are derived principally from or corroborated by observable market data by correlation or other means. |
If the asset or liability has a specified (contractual) term, the level 2 input must be observable for substantially the full term of the asset or liability.
F-15
Level 3: Inputs to the valuation methodology are unobservable and significant to the fair value measurement.
The asset’s or liability’s fair value measurement level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. Valuation techniques used need to maximize the use of observable inputs and minimize the use of unobservable inputs. The fair value assigned to marketable securities is determined by obtaining quoted prices on nationally recognized securities exchanges, and are classified as Level 1 investments at December 31, 2012. The fair value of the Company’s debt instruments is not materially different from their carrying values as presented. The fair value of the Company’s convertible debt instruments was determined based on Level 2 inputs. The carrying value of the debt was discounted based on allocating proceeds to other financial instruments within the arrangement as discussed in Note 6.
Net loss per share – In accordance with FASB ASC Topic 260, “Earnings per Share,” the basic loss per common share is computed by dividing net loss available to common stockholders by the weighted average number of common shares outstanding. Dilutive loss per share is computed similar to basic loss per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive. As of December 31, 2012 and 2011, there were 6,743,922 and 2,261,213 shares of potentially dilutive securities outstanding, respectively. As the Company reported a net loss, none of the potentially dilutive securities were included in the calculation of diluted earnings per share since their effect would be anti-dilutive for that reporting period.
Recent accounting pronouncements
In December 2011, the FASB issued an accounting standards update that will require us to disclose information about offsetting and related arrangements associated with certain financial and derivative instruments to enable users of our financial statements to better understand the effect of those arrangements on our financial position. The new guidance will be applicable to us for fiscal years, and interim periods within those years, beginning after January 1, 2013. We do not expect the adoption of this guidance to have a material impact on our consolidated financial statements.
In July 2012, the FASB issued an accounting standards update with new guidance on annual impairment testing of indefinite-lived intangible assets. The standards update allows an entity to first assess qualitative factors to determine if it is more likely than not that the fair value of an indefinite-lived intangible asset is less than its carrying amount. If based on its qualitative assessment an entity concludes it is more likely than not that the fair value of an indefinite-lived intangible asset is less than its carrying amount, quantitative impairment testing is required. However, if an entity concludes otherwise, quantitative impairment testing is not required. The standards update is effective for annual and interim impairment tests performed for fiscal years beginning after September 15, 2012, with early adoption permitted. We do not expect the adoption of this guidance to have a material impact on our consolidated financial statements.
In January 2013, the FASB issued ASU 2013-01, Balance Sheet (Topic 210): Clarifying the Scope of Disclosures about Offsetting Assets and Liabilities which provides further clarification relating to the scope of ASU 2011-11, Balance Sheet (Topic 210): Disclosure about Offsetting Assets and Liabilities. Effective for fiscal years beginning on or after January 1, 2013, ASU 2011-11 requires an entity to include additional disclosures about financial instruments and transactions eligible for offset in the statement of financial position, as well as financial instruments subject to a master netting agreement or similar arrangement. ASU 2013-01 added further scope clarification that ASU 2011-11 applies to derivatives, including bifurcated embedded derivatives, repurchase agreements and reverse repurchase agreements, and securities borrowing and securities lending transactions that are either offset or subject to an enforceable master netting arrangement or similar agreement. We do not expect the adoption of this guidance to have a material impact on our consolidated financial statements. This ASU will be effective for fiscal years beginning on or after January 1, 2013, including interim periods within those fiscal years.
In February 2013, the FASB issued amendments to the accounting guidance for presentation of comprehensive income to improve the reporting of reclassifications out of accumulated other comprehensive income. The amendments do not change the current requirements for reporting net income or other comprehensive income, but do require an entity to provide information about the amounts reclassified out of accumulated other comprehensive income by component. In addition, an entity is required to present, either on the face of the statement where the net income is presented or in the notes, significant amounts reclassified out of accumulated other comprehensive income by the respective line items of net income but only if the amount reclassified is required under GAAP to be reclassified to net income in its entirety in the same reporting period. For other amounts that are not required under GAAP to be reclassified in their entirety to net income, an entity is required to cross-reference to other disclosures required under GAAP that provide additional detail about these amounts. For public companies, these amendments are effective prospectively for reporting periods beginning after December 15, 2012. Other than a change in presentation, we do not believe the adoption of this guidance will have a material impact on our consolidated financial statements.
F-16
NOTE 3 – PROPERTY AND EQUIPMENT
Property and equipment consisted of the following at:
| December 31, 2012 | December 31, 2011 | |||||||
| Equipment | $ | 120,603 | $ | 111,655 | ||||
| Leasehold improvements | 23,054 | 23,054 | ||||||
| Furniture and fixtures | 52,269 | 52,269 | ||||||
| 195,926 | 186,978 | |||||||
| Less: accumulated depreciation | (157,157 | ) | (126,960 | ) | ||||
| $ | 38,769 | $ | 60,018 | |||||
During the years ended December 31, 2012 and 2011, the depreciation expense was $30,197 and $35,332, respectively. Depreciation expense from inception to December 31, 2012 was $157,157.
NOTE 4 – INTANGIBLE ASSETS
The Company is licensed to market and sell NutreStore® [L-glutamine powder for oral solution] as a treatment for short bowel syndrome (“SBS”). The Company previously promoted Zorbtive® [somatropin (rDNA origin) for injection] prior to July 31, 2011. Subsequent to July 31, 2011, the Company has not promoted Zorbtive.
In April 2011, Emmaus Medical entered into the Research Agreement and the Individual Agreement with CellSeed and, in August 2011, an addendum to the agreements. Pursuant to the Research Agreement, the Company and CellSeed formed a relationship regarding the future research and development of cell sheet engineering regenerative medicine products, and the future commercialization of such products. Pursuant to the Individual Agreement, CellSeed granted the Company the exclusive right to manufacture, sell, market and distribute Cultured Autologous Oral Mucosal Epithelial Cell-Sheet (“CAOMECS”) for the cornea in the United States and agreed to disclose its accumulated information package for the joint development of CAOMECS to the Company. Under the Research Agreement, as supplemented by the addendum, the Company agreed to pay CellSeed $8.5 million within 30 days of the completion of all of the following: (i) the execution of the Research Agreement; (ii) the execution of the Individual Agreement; and (iii) CellSeed’s delivery of the accumulated information package ,as defined in the Research Agreement, to Emmaus and Emmaus providing written confirmation of its acceptance of the complete Package, which has not yet been completed. Under the Individual Agreement, the Company agreed to pay CellSeed $1.5 million, which it paid in February 2012.
Intangible assets consisted of the following at:
| December 31, 2012 | December 31, 2011 | |||||||
| License fees and patent filing costs | $ | 2,250,000 | $ | 750,000 | ||||
| Less: accumulated amortization | (928,571 | ) | (750,000 | ) | ||||
| $ | 1,321,429 | $ | — | |||||
During the years ended December 31, 2012 and 2011, the amortization expense was $178,571 and $134,880, respectively. Amortization expense from inception to December 31, 2012 was $928,571.
As of December 31, 2012 estimated aggregate amortization expense for the next five years is as follows:
| Year ending December 31 | Amount | |||
| 2013 | $ | 214,286 | ||
| 2014 | 214,286 | |||
| 2015 | 214,286 | |||
| 2016 | 214,286 | |||
| 2017 | 214,286 | |||
| Thereafter | 249,999 | |||
| $ | 1,321,429 | |||
NOTE 5 – ACCOUNTS PAYABLE AND ACCRUED EXPENSES
Accounts payable and accrued expenses consisted of the following at:
| Accounts payable | December 31, 2012 | December 31, 2011 | ||||||
| Clinical trial management expenses | $ | 527,868 | $ | 237,202 | �� | |||
| Legal Expenses | 609,823 | 33,310 | ||||||
| Miscellaneous vendors | 619,886 | 228,817 | ||||||
| Subtotal | 1,757,577 | 499,329 | ||||||
| Accrued expenses | 401,034 | 96,719 | ||||||
| Accrued expenses (Deferred Salary) | 401,667 | 160,666 | ||||||
| Total accounts payable and accrued expenses | $ | 2,560,278 | $ | 756,714 | ||||
F-17
There are no amounts due to related parties included in accounts payable. However, accrued expenses include accrued interest for the Convertible Notes issued to related parties. Amounts due to related parties have been separately presented on the balance sheet.
NOTE 6 – NOTES PAYABLE
Notes payable consisted of the following at December 31, 2012:
| Lender | Annual Interest Rate | Date of loan | Term of Loan | Loan Principal Outstanding | Conversion Price | Shares Underlying Principal as of December 31, 2012 | |||||||||||||
| Shigeru Matsuda (1) | 6.50 | % | 1/12/2009 | 5 years | $ | 294,355 | $ | 3.05 | 96,436 | ||||||||||
| Kazuo Murakami (1) | 0.00 | % | 8/16/2010 | 5 years | $ | 18,000 | $ | 3.05 | 5,898 | ||||||||||
| Makoto Murakami (1) | 0.00 | % | 8/16/2010 | 5 years | $ | 18,000 | $ | 3.05 | 5,898 | ||||||||||
| Nami Murakami (1) | 0.00 | % | 8/16/2010 | 5 years | $ | 18,000 | $ | 3.05 | 5,898 | ||||||||||
| M’s Support Co. Ltd. (1) | 0.00 | % | 8/17/2010 | 5 years | $ | 18,000 | $ | 3.05 | 5,898 | ||||||||||
| Yumiko Takemoto (1) | 6.00 | % | 11/23/2010 | 5 years | $ | 2,000 | $ | 3.05 | 656 | ||||||||||
| Mitsubishi UFJ Capital III, Limited Partnership | 10.00 | % | 3/14/2011 | 5 years | $ | 500,000 | $ | 3.05 | 163,809 | ||||||||||
| Tracey & Mark Doi (3) | 8.00 | % | 2/10/2012 | 1 year | $ | 108,000 | $ | 3.60 | 30,001 | ||||||||||
| Yukio Hasegawa (1) | 8.00 | % | 2/15/2012 | 1 year (2) | $ | 133,333 | $ | 3.60 | 37,037 | ||||||||||
| Robert Jo (1) | 8.00 | % | 2/18/2012 | 1 year (2) | $ | 100,000 | $ | 3.60 | 27,778 | ||||||||||
| Hiroshi Iguchi | 8.00 | % | 2/20/2012 | 1 year (2) | $ | 133,333 | $ | 3.60 | 37,037 | ||||||||||
| J. R. Downey | 8.00 | % | 3/2/2012 | 1 year (2) | $ | 150,005 | $ | 3.60 | 41,669 | ||||||||||
| Paul Terasaki (1) (7) | 10.00 | % | 5/1/2012 | 1 year | $ | 500,000 | $ | 3.30 | 151,516 | ||||||||||
| Yasushi Nagasaki (5) | 10.00 | % | 6/29/2012 | On Demand | $ | 388,800 | $ | 3.30 | 117,819 | ||||||||||
| Yumiko Duchane | 10.00 | % | 7/5/2012 | 1 year | $ | 30,000 | $ | 3.30 | 9,091 | ||||||||||
| Andrew K. Wood (4) | 10.00 | % | 7/8/2012 | 1 year | $ | 3,240 | $ | 3.30 | 982 | ||||||||||
| Hiromi Saito | 10.00 | % | 7/10/2012 | 1 year | $ | 25,000 | $ | 3.30 | 7,576 | ||||||||||
| Suh Yung Min | 10.00 | % | 7/11/2012 | 1 year | $ | 1,180,716 | $ | 3.30 | 357,793 | ||||||||||
| Kiyohiro Sugashita | 10.00 | % | 7/30/2012 | 1 year | $ | 6,600 | $ | 3.30 | 2,001 | ||||||||||
| Yumiko Nakamura | 10.00 | % | 8/9/2012 | 2 years | $ | 49,500 | $ | 3.30 | 15,001 | ||||||||||
| Masayuki Makino | 10.00 | % | 8/17/2012 | 1 year | $ | 6,600 | $ | 3.30 | 2,001 | ||||||||||
| The Saito Family Trust (1) | 8.00 | % | 9/5/2012 | 1 year (2) | $ | 50,000 | $ | 3.60 | 13,889 | ||||||||||
| Hideki & Eiko Uehara (1) | 10.00 | % | 9/7/2012 | 1 year | $ | 32,400 | $ | 3.60 | 9,001 | ||||||||||
| Dennis Y. Teranishi | 10.00 | % | 9/9/2012 | 1 year | $ | 116,640 | $ | 3.60 | 32,401 | ||||||||||
| Paul Shitabata (1) | 10.00 | % | 10/3/2012 | 1 year (2) | $ | 1,620,540 | $ | 3.60 | 450,151 | ||||||||||
| Willis Lee (5) | 10.00 | % | 10/5/2012 | 1 year | $ | 138,242 | $ | 3.60 | 38,401 | ||||||||||
| Alison Brown | 10.00 | % | 12/21/2012 | 2 years | $ | 100,800 | $ | 3.60 | 28,001 | ||||||||||
| Yoshiko Takemoto | 10.00 | % | 12/27/2012 | 2 years | $ | 100,800 | $ | 3.60 | 28,001 | ||||||||||
| Convertible Notes | $ | 5,842,904 | 1,721,640 | ||||||||||||||||
F-18
| Yutaka Niihara (5) | 6.50 | % | 1/12/2009 | On Demand | $ | 272,800 | NA | ||||||||||||
| Hope Int’l Hospice (6) | 8.00 | % | 1/12/2011 | 2 years | $ | 200,000 | NA | ||||||||||||
| Hope Int’l Hospice (6) | 8.00 | % | 1/17/2012 | On Demand | $ | 200,000 | NA | ||||||||||||
| Shigeru Matsuda (1) | 11.00 | % | 2/15/2012 | 2 years (2) | $ | 833,335 | NA | ||||||||||||
| Hideki & Eiko Uehara (1) | 11.00 | % | 2/15/2012 | 2 years (2) | $ | 133,333 | NA | ||||||||||||
| Hope Int’l Hospice (6) | 8.00 | % | 6/14/2012 | On Demand | $ | 200,000 | NA | ||||||||||||
| Hope Int’l Hospice (6) | 8.00 | % | 6/21/2012 | On Demand | $ | 100,000 | NA | ||||||||||||
| Cuc T. Tran | 11.00 | % | 6/27/2012 | 1 year | $ | 10,000 | NA | ||||||||||||
| Yukio Hattori (1) | 11.00 | % | 7/20/2012 | On Demand | $ | 248,725 | NA | ||||||||||||
| Yutaka Niihara (5) | 1.00 | % | 8/29/2012 | 1 year | $ | 1,270,100 | NA | ||||||||||||
| Yutaka Niihara (5) | 10.00 | % | 12/5/2012 | Six months. | $ | 1,213,700 | NA | ||||||||||||
| Lan T. Tran (5) | 11.00 | % | 2/10/2012 | 2 years (2) | $ | 80,000 | NA | ||||||||||||
| For Days Co., Ltd. | 2.00 | % | 12/26/2012 | 2 years | $ | 700,000 | NA | ||||||||||||
| Non-Convertible Notes | $ | 5,461,993 | |||||||||||||||||
| Total, undiscounted (9) | $ | 11,304,897 | |||||||||||||||||
| (1) | Related party – Shareholder |
| (2) | Due on Demand |
| (3) | Director |
| (4) | Employee |
| (5) | Officer |
| (6) | Dr. Niihara is also the CEO of Hope International Hospice, Inc. |
| (7) | Convertible into shares of the Company’s common stock at $3.30 per share or, if then publicly traded, at the average closing sale price per share for the three (3) trading days immediately preceding the exercise thereof, whichever is lower |
| (8) | Secured by the Company’s minority interest in CellSeed common stock |
| (9) | Total amount due on notes without discounts for fair market value of warrants issued and conversion features |
On June 9, 2011, the Company entered into a Loan Agreement and Pledge Agreement with Equities First Holdings, LLC, whereby the Company borrowed an aggregate of $841,728 in a non-recourse loan and pledged shares of third-party common stock as collateral. On May 31, 2012, the Company received notice from Equities First Holdings, LLC that there was a technical event of default based on a decrease of the fair market value of the pledged shares of third-party common stock below the loan to value ratio required pursuant to the terms of the Loan Agreement. On June 1, 2012, according to the terms of the Loan Agreement, the Company provided notice to EFH to terminate the Loan Agreement and Pledge Agreement. As a result of the termination, the Company forfeited the shares of third-party common stock assigned to EFH as collateral pursuant to the Pledge Agreement in full satisfaction of the Company’s obligations and recognized a realized gain of $275,822 since the cancelation of non-recourse loan exceeded the value of the securities available-for-sale.
Contractual principal payments due on loans and notes payable are as follows:
| Year Ending | at December 31, 2012 | |||
| 2013 | $ | 9,485,442 | ||
| 2014 | 1,245,455 | |||
| 2015 | 574,000 | |||
| total | $ | 11,304,897 | ||
The Company estimated the total fair value of the convertible note and warrant in allocating the debt proceeds. The proceeds were allocated to the warrant and convertible note based on the pro-rata fair value. The proceeds allocated to the beneficial conversion were determined by taking the estimated fair value of shares issuable under the convertible note less the fair value of the convertible note determined above. The fair value of the warrant was determined through the Black Scholes Option pricing model with the following inputs:
| Stock Price | $ | 3.60 | ||
| Exercise Price | $ | 1.00 ~ 3.60 | ||
| Term | 2 ~ 10 years | |||
| Risk-Free Rate | 0.30 ~ 2.22% | |||
| Dividend Yield | 0 | % | ||
| Volatility | 99.89 ~ 141.70% | |||
F-19
Original Principal Loan Amount at December 31, 2012 | Discount Amount at December 31, 2012 | Carrying Amount at December 31, 2012 | ||||||||
| $ | 11,304,897 | $ | (932,126 | ) | $ | 10,372,771 | ||||
NOTE 7 – STOCKHOLDERS’ EQUITY (DEFICIT)
Common stock – As of the year ended December 31, 2012, the Company had issued a total of 24,878,436 shares of its common stock, which includes 20,628,305 shares issued to the former stockholders of Emmaus Medical pursuant to the Merger Agreement (excluding the 47,178 shares held by stockholders who exercised dissenter’s right in connection with the Merger), and 15,156 shares issued upon the exercise of warrants and options. As discussed in Note 2, in connection with the closing of the Merger, stockholders of the Company prior to the Merger cancelled an aggregate of 1,827,750 shares of common stock owned by them such that they held an aggregate of 3,750,000 shares of common stock upon the closing of the Merger. Pursuant to the Merger Agreement on May 3, 2011, four stockholders of Emmaus Medical exercised their dissenter’s rights and returned 47,178 shares for $200,000, which was outstanding as of December 31, 2012. The shares were cancelled as of May 3, 2011, the closing date of the Merger.
Stock warrants – During the year ended December 31, 2012, the Company issued warrants in connection with the issuance of convertible notes to purchase an aggregate of 56,573 shares of common stock at a per share exercise price equal to 75% of the per share fair market value of the Company’s common stock on the date prior to exercise. During this period, the Company also issued warrants to purchase a total of 1,911,020 shares of common stock at an exercise price of $1.00 per share and 1,000,000 shares of common stock at an exercise price of $2.50 per share. In September 2012, the Company canceled warrants to purchase 500,000 shares of common stock at an exercise price of $1.00 per share that had previously been issued to a director who advised the Company that he would not be standing for e-election to the board of directors.
A summary of outstanding warrants as of December 31, 2012 is presented below.
| Year ended December 31, 2012 | ||||
| Warrants outstanding, beginning of period | 941,202 | |||
| Granted | 2,967,593 | |||
| Exercised | — | |||
| Cancelled, forfeited and expired | (500,000 | ) | ||
| Warrants outstanding, end of period | 3,408,795 | |||
| Outstanding | Exercisable | |||||||||||||||||||||
| Exercise Price | Number of Warrants | Weighted Average Remaining Contractual Life (Years) | Weighted Average Exercise Price | Total | Weighted Average Exercise Price | |||||||||||||||||
| During 2012 | ||||||||||||||||||||||
| $ | 1.00 | 1,411,020 | 2.03 | $ | 1.00 | 411,020 | $ | 1.00 | ||||||||||||||
| 75% of FMV | 56,573 | 2.24 | 75% of FMV | 56,573 | 75% of FMV | |||||||||||||||||
| $ | 2.50 | 1,000,000 | 2.66 | $ | 2.50 | 1,000,000 | $ | 2.50 | ||||||||||||||
| During 2011 | ||||||||||||||||||||||
| $ | 1.00 | 311,038 | 1.68 | $ | 1.00 | 311,038 | $ | 1.00 | ||||||||||||||
| 75% of FMV | 331,670 | 1.68 | 75% of FMV | 331,670 | 75% of FMV | |||||||||||||||||
| $ | 3.05 | 5,898 | 2.22 | $ | 3.05 | 5,898 | $ | 3.05 | ||||||||||||||
| During 2010 | ||||||||||||||||||||||
| $ | 3.05 | 197,146 | 2.63 | $ | 3.05 | 197,146 | $ | 3.05 | ||||||||||||||
| During 2009 | ||||||||||||||||||||||
| $ | 3.05 | 95,450 | 1.77 | $ | 3.05 | 95,450 | $ | 3.05 | ||||||||||||||
Stock options – Management has valued the options at their date of grant utilizing the Black-Scholes-Merton Option pricing model. Accordingly, the fair value of the underlying shares was determined based on recent transactions by the Company to sell shares to third parties and other factors determined by management to be relevant to the valuation of such shares. The expected volatility was calculated using the historical volatility of a similar public entity in the industry.
F-20
In making this determination and finding another similar company, the Company considered the industry, stage of life cycle, size and financial leverage of such other entities. Based on the development stage of the Company, similar companies with enough historical data are not available. The Company was able to find one entity that met the industry criterion and as a result has based its expected volatility off of this Company’s historical stock prices for a period similar to the expected term of the option.
The risk–free interest rate is based on the implied yield available on U.S. Treasury issues with an equivalent term approximating the expected life of the options depending on the date of the grant and expected life of the options. The expected life of options used was based on the contractual life of the option granted.
The Company had 72,795 options outstanding held by directors of the Company as of December 31, 2011. Of these options 11,795 were vested as of December 31, 2012 and are exercisable at $3.05 per share through 2015 and were issued prior to the commencement of the 2011 Stock Incentive Plan. During 2011, 61,000 options were granted from the 2011 Stock Incentive Plan, are exercisable at $3.60 per share and vested in 2012. During the year ended December 31, 2012, the Company issued 1,490,000 options to its directors, officers, employees and consultants. The fair value of these options issued is approximately $5 million. These options will be vested equally over 3 years, starting April 2, 2013 and are exercisable at $3.60 per share through 2022. During the year ended December 31, 2012, 12,000 options were canceled or forfeited. The total outstanding options as of December 31, 2012 are 1,550,795.
A summary of outstanding options as of December 31, 2012 for the 2011 Stock Incentive Plan is presented below.
| Year ended December 31, 2012 | ||||
| Options outstanding, beginning of period | 61,000 | |||
| Granted | 1,490,000 | |||
| Exercised | — | |||
| Cancelled, forfeited and expired | 12,000 | |||
| Options outstanding, end of period | 1,539,000 | |||
Registration Rights – In connection with the consummation of the Merger, the Company entered into the Registration Rights Agreement for the benefit of certain pre-Merger Company stockholders. Pursuant to the Registration Rights Agreement, the such stockholders have certain “piggyback” registration rights on registration statements filed after the Merger is consummated other than registration statements (i) filed in connection with any employee stock option or other benefit plan, (ii) for an exchange offer or offering of securities solely to the Company’s existing stockholders, (iii) for an offering of debt that is convertible into equity securities of the Company; (iv) for a dividend reinvestment plan or (v) for an offering of equity securities of the Company. The Company will bear the expenses incurred in connection with the filing of any such registration statements.
NOTE 8 - INCOME TAXES
The provision (benefit) for income taxes consists of the following for the year ended December 31:
| 2012 | 2011 | ||||||||
| Current | U.S. | $ | 3,405 | $ | 1,997 | ||||
| International | 3,841 | 1,212 | |||||||
| Deferred | U.S. | — | — | ||||||
| International | — | — | |||||||
| $ | 7,246 | $ | 3,209 | ||||||
A valuation allowance for the net deferred tax assets has been recorded as it is more likely than not that these benefits will not be realized through future operations.
F-21
Deferred tax assets consist of the following as of December 31, 2012 and 2011:
| 2012 | 2011 | |||||||
| Net operating loss carryforward | $ | 8,775,557 | $ | 5,800,000 | ||||
| General business tax credit | 3,519,185 | 2,035,893 | ||||||
| Stock options | 1,317,101 | — | ||||||
| Charitable contribution | 70,077 | 128,601 | ||||||
| Accrued expenses | 34,060 | 42,256 | ||||||
| Unrealized loss on available-for-sale securities | 1,491 | — | ||||||
| Other | 51,609 | 6,804 | ||||||
| 13,769,080 | 8,013,554 | |||||||
| Valuation allowance | (13,769,080 | ) | (7,911,418 | ) | ||||
| $ | - | $ | 102,136 | |||||
Deferred tax liabilities consist of the following as of December 31, 2012 and 2011:
| 2012 | 2011 | |||||||
| Unrealized appreciation on available-for-sale securities | $ | — | $ | 102,136 | ||||
During 2012 and 2011, the valuation allowance increased by $5,857,662 and $2,988,447, respectively.
As of December 31, 2012 and 2011, the Company had net operating loss carryforwards (“NOL”) for federal and state reporting purposes of approximately $22,500,000 and $14,500,000, and $19,000,000 and $13,900,000 respectively, which expire in various years through 2032. The Federal and state tax codes provide for restrictive limitations on the annual utilization of NOLs to offset taxable income when the stock ownership of a company significantly changes, as defined. As of December 31, 2012 and 2011, the Company has general business tax credits of $3,519,000 and $2,036,000, respectively, for federal tax purposes. The tax credits are available to offset future tax liabilities, if any, through 2022.
The income tax provision differs from that computed using the statutory federal tax rate of 34%, due to the following:
| 2012 | 2011 | |||||||
| Tax benefit at statutory federal rate | $ | (4,915,586 | ) | $ | (2,947,420 | ) | ||
| State taxes, net of federal tax benefit | (656,452 | ) | (235,764 | ) | ||||
| Increase (decrease) in valuation allowance | 5,857,662 | 2,908,447 | ||||||
| Other | (509,585 | ) | 28,985 | |||||
| Permanent Items | 1,714,499 | 1,292,979 | ||||||
| General business tax credit | (1,483,292 | ) | (1,044,018 | ) | ||||
| $ | 7,246 | $ | 3,209 | |||||
NOTE 9 – COMMITMENTS AND CONTINGENCIES
Distribution contract – Cardinal Health Specialty Pharmacy Services has been contracted to distribute NutreStore to other wholesale distributors and some independent pharmacies since April 2008. For its service, Emmaus Medical pays a monthly commercialization management fee of $5,000 with discount.
Operating leases – The Company leases its office space under operating leases from unrelated entities. The rent expense during the year ended December 31, 2012 and 2011 amounted to $139,302 and $142,960, respectively.
The Company leases its approximately 4,540 square foot headquarters offices in Torrance, CA, at a base rental of $4,994 per month plus $320 per month as its share of common area expenses. The lease expires on November 30, 2013. In addition, the Company leases two office suites in Torrance, California at a base rent of $1,636 per month plus share of common area expenses of $90 per month, and at a base rent of $1,750 per month plus share of common area expenses of $90 per month. These leases will expire on August 19, 2013 and February 28, 2015, respectively. Approximately 490 square feet from one office and 1,079 square feet from the other office are currently subleased to an unaffiliated entity on a month to month basis. The Company does not expect to experience any difficulties in renewing its leases, or finding additional or replacement office and warehouse space, at its current or more favorable rates.
The Company also leases two office suites of approximately 512 square feet and 532 square feet, respectively, in Tokyo, Japan at a base rent of JPY130,000 ($1,625 @0.0125) per month each. These leases will expire on October 14, 2014 and September 15, 2013, respectively.
Future minimum lease payments under the agreements are as follows:
| 2013 | $ | 121,735 | ||
| 2014 | 35,718 | |||
| 2015 | 3,380 | |||
| $ | 160,833 |
F-22
Licensing agreement - On April 8, 2011, pursuant to a Research Agreement, the Company agreed to pay CellSeed $8.5 million within 30 days of the completion of all of the following: (i) the execution of the Research Agreement; (ii) the execution of the Individual Agreement; and (iii) CellSeed’s delivery of the accumulated information package, as defined in the Research Agreement, to the Company. Pursuant to the Individual Agreement, the Company agreed to pay $1.5 million to CellSeed within 30 days of CellSeed’s delivery of the accumulated information package for the joint development of CAOMECS to the Company and a royalty to be agreed upon by the parties. The Company made a payment of $1.5 million to CellSeed in February 2012, pursuant to the Individual Agreement. CellSeed may terminate these agreements with the Company if the Company is unable to make timely payments required under the agreements.
NOTE 10 – RELATED PARTY TRANSACTIONS
As of December 31, 2012 an aggregate of $8,234,903 in principal amount of promissory notes from certain stockholders and officers was outstanding as per the detail in Note 6. The debt is unsecured and carries interest rates from 0% to 11%.
The Company had agreed to pay AFH Holding and Advisory, LLC (“AFH Advisory”) certain costs to allow Emmaus Medical stockholders to acquire shares of common stock of the Company and become the majority owners in the aggregate of the Company and to achieve the desired post-merger capitalization of the Company.
Since July 2012 we have been engaged in litigation with AFH Advisory, which was the sole stockholder of AFH Acquisition IV immediately prior to its combination with Emmaus Medical pursuant to the Merger in May 2011. In September 2012 AFH Advisory and related parties filed a complaint against the Company in the Superior Court of Delaware. In October 2012 the Company filed counterclaims against the plaintiffs and third-party claims against Amir Heshmatpour. The case is scheduled for trial in May 2013.
During the year ended December 31, 2011, the Company paid $394,446 to AFH Advisory for certain costs leaving an aggregate of $394,446 remaining unpaid.
NOTE 11 – GEOGRAPHIC INFORMATION
For the years ended December 31, 2012 and 2011, the Company earned revenue from countries outside of the United States as outlined in the table below. The Company did not have any significant currency translation or foreign transaction adjustments during the years ended December 31, 2012 and 2011.
Country | Sales year ended December 31, 2012 | % of Total Revenue year ended December 31, 2012 | Sales year ended December 31, 2011 | % of Total Revenue year ended December 31, 2011 | ||||||||||||
| Ghana | — | 0 | % | $ | 15,400 | 5 | % | |||||||||
| Japan | $ | 265,913 | 49 | % | $ | 161,602 | 48 | % | ||||||||
| Taiwan | — | 0 | % | $ | 50,000 | 15 | % | |||||||||
| South Korea | $ | 92,000 | 17 | % | ||||||||||||
NOTE 12 – SUBSEQUENT EVENTS
| Lender | Annual Interest Rate | Date of loan | Term of Loan | Loan Principal Outstanding | Conversion Price | Shares Underlying Principal as of December 31, 2012 | |||||||||||||
| Sun Moo & Hyon Sil Lee | 10.00 | % | 2/21/2013 | 2 years | $ | 100,800 | $ | 3.60 | 28,000 | ||||||||||
| Tracey and Mark Doi | 10.00 | % | 2/10/2013 | Due on Demand | $ | 116,640 | $ | 3.60 | 32,400 | ||||||||||
| Yukio Hasegawa | 10.00 | % | 2/15/2013 | Due on Demand | $ | 144,000 | $ | 3.60 | 40,000 | ||||||||||
| J. R. Downey | 10.00 | % | 3/2/2013 | One year | $ | 162,005 | $ | 3.60 | 45,001 | ||||||||||
Shigenori Yoshida | 10.00 | % | 3/12/2013 | 2 years | $ | 100,800 | $ | 3.60 | 28,000 | ||||||||||
| For Days Co., Ltd. | 2.00 | % | 01/29/2013 | 2 years | $ | 500,000 | NA | ||||||||||||
| Hope Int’l Hospice | 8.00 | % | 01/12/2013 | Due on Demand | $ | 200,000 | NA | ||||||||||||
| Hope Int’l Hospice | 8.00 | % | 02/11/2013 | Due on Demand | $ | 50,000 | NA | ||||||||||||
F-23
In January 2013, the Company issued a promissory note payable to For Days Co., Ltd. in the principal amount of $500,000 bearing interest at 2% per annum and maturing on the two-year anniversary date of the note.
In January 2013, the Company renewed a promissory note payable to Hope International Hospice in the principal amount of $200,000 bearing interest at 8% per annum. The term of this note is due on demand. Dr. Niihara is also the CEO of Hope International Hospice, Inc.
In February 2013, the Company issued a promissory note in the principal amount of $50,000 bearing interest at 8% per annum. The term of this note is due on demand. Dr. Niihara is also the CEO of Hope International Hospice, Inc.
In February 2013, the Company issued a convertible note payable to Sun Moo & Hyon Sil Lee in the principal amount of $100,800, which bears interest at 10% per annum. The loan has two-year term but the lender can demand the loan after two months from the loan date. The principal amount plus the unpaid accrued interest due under the convertible note is convertible into shares of the Company’s common stock at $3.60 per share. In February 2013, the Company refinanced a convertible note payable to Tracey and Mark Doi totaling $108,000 with new convertible notes totaling $116,640 which bear interest at 10% per annum. The term of this note is due on demand up to one year. Principal amounts and any unpaid interests due under the notes are convertible into shares of the Company’s common stock at $3.60 per share. Tracey Doi is a Director of the Company.
In February 2013, the Company refinanced convertible notes payable to Yukio Hasegawa, a shareholder, totaling $133,333 with new convertible notes totaling $144,000 which bear interest at 10% per annum. The term of this note is due on demand up to one year. Principal amounts and any unpaid interests due under the notes are convertible into shares of the Company’s common stock at $3.60 per share.
In March 2013, the Company refinanced a convertible note payable to J. R. Downey in the amount of $150,005 with a new convertible note in the amount of $162,005 which bears interest at 10% per annum. The loan has one-year term but the lender can demand the loan after three months from the loan date. The principal amount and any unpaid interest due under the note are convertible into shares of the Company’s common stock at $3.60 per share.
In March 2013, the Company issued a convertible note payable to Shigenori Yoshida, a third party, in the principal amount of $100,800, which bears interest at 10% per annum and matures on two-year anniversary date of the note. The principal amount plus the unpaid accrued interest due under the convertible note is convertible into shares of the Company’s common stock at $3.60 per share.
F-24