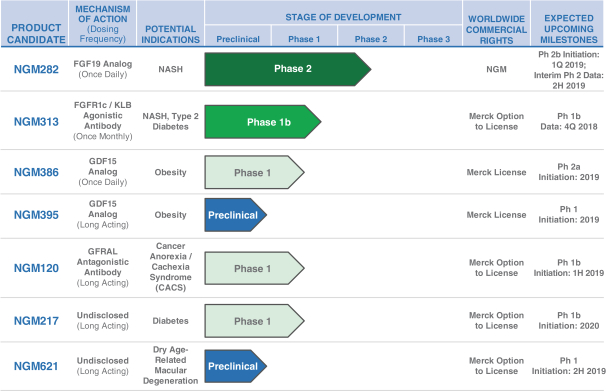
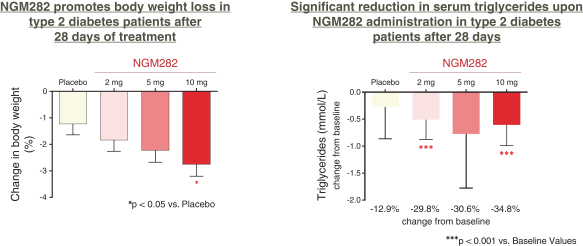
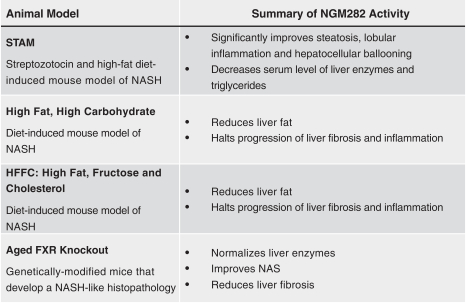
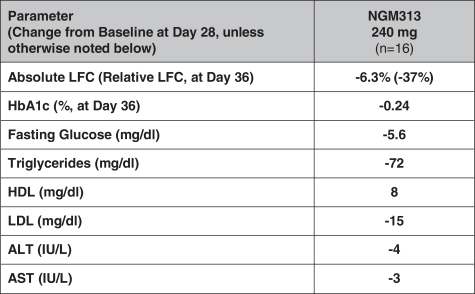
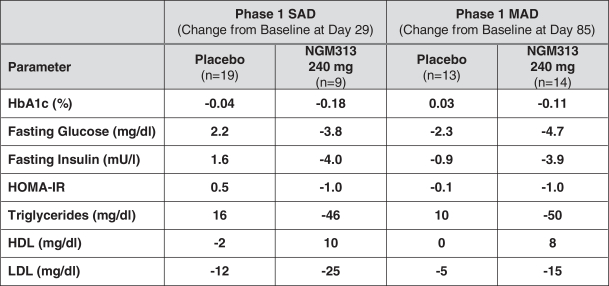
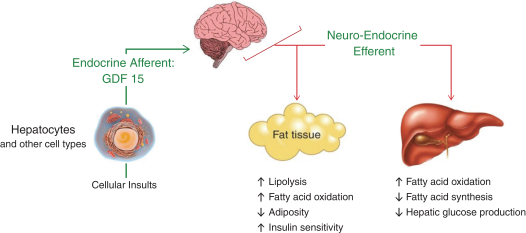
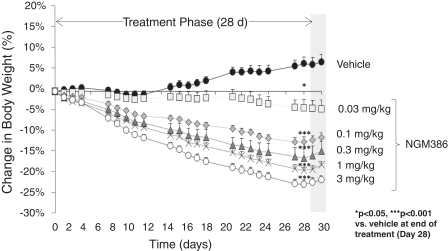
the African Regional Intellectual Property Organization, Algeria, Argentina, Australia, Barbados, Belize, Brunei Darussalam, Brazil, Canada, Chile, China, Colombia, Costa Rica, Dominican Republic, El Salvador, Ecuador, Egypt, the Eurasian Patent Office, the EPO, Georgia, Guatemala, Gulf Cooperation Council, Honduras, Hong Kong, India, Indonesia, Iran, Israel, Jamaica, Japan, Jordan, Republic of Korea, Malaysia, Mexico, Republic of Moldova, Mongolia, New Zealand, Nicaragua, Nigeria, Pakistan, Panama, Peru, Philippines, Singapore, Sri Lanka, South Africa, Taiwan R.O.C., Thailand, Trinidad and Tobago, Tunisia, Ukraine, Venezuela and Vietnam. The earliest expected expiration date for these patents and patent applications is July 2035, exclusive of possible patent term extensions or adjustments. Any changes we make to the NGM386 molecule to cause it to have what we view as more advantageous properties may not be covered by our existing patents and patent applications, and we may be required to file new applications and/or seek other forms of protection for any such altered molecule. The patent landscape surrounding GDF15, the naturally-occurring hormone upon which NGM386 is based, is crowded, and there can be no assurance that we would be able to secure patent protection that would adequately cover an alternative to our NGM386 molecule.
NGM395 Patent Portfolio
Our NGM395 product candidate, and relatedcompositions-of-matter and methods of use, are covered by one issued U.S. patent and one issued Lebanese patent; and are disclosed and claimed in other applications that are pending in the United States and the following foreign countries and regions: the African Regional Intellectual Property Organization, Algeria, Argentina, Australia, Barbados, Belize, Brazil, Brunei Darussalam, Canada, Chile, China, Colombia, Costa Rica, Dominican Republic, El Salvador, Ecuador, Egypt, the Eurasian Patent Office, the EPO, Georgia, Guatemala, Gulf Cooperation Council, Honduras, Hong Kong, India, Indonesia, Iran, Israel, Jamaica, Japan, Jordan, Republic of Korea, Malaysia, Republic of Moldova, Mongolia, Mexico, New Zealand, Nicaragua, Nigeria, Pakistan, Panama, Peru, Philippines, Singapore, Sri Lanka, South Africa, Taiwan R.O.C., Thailand, Trinidad and Tobago, Tunisia, Ukraine, Venezuela and Vietnam. The earliest expected expiration date for these patents and patent applications is October 2035, exclusive of possible patent term extensions or adjustments. Any changes we make to the NGM395 molecule to cause it to have what we view as more advantageous properties may not be covered by our existing patents and patent applications, and we may be required to file new applications and/or seek other forms of protection for any such altered molecule. The patent landscape surrounding GDF15, the naturally occurring hormone upon which NGM395 is based, is crowded, and there can be no assurance that we would be able to secure patent protection that would adequately cover an alternative to our NGM395 molecule.
NGM120 Patent Portfolio
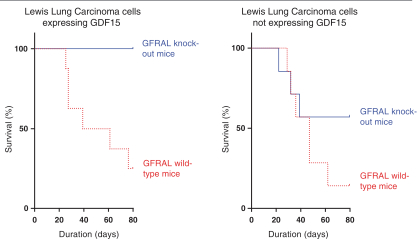
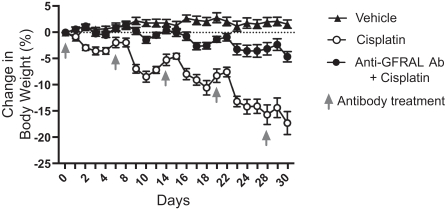
We do not currently own or have a license to any issued patent that covers our NGM120 product candidate. However, our NGM120 product candidate, and relatedcompositions-of-matter and methods of use, are disclosed and claimed in pending United States, Taiwan and PCT applications. The earliest expected expiration date for any patents issuing from these patent applications is October 2037, exclusive of possible patent term extensions or adjustments. Any changes we make to the NGM120 molecule to cause it to have what we view as more advantageous properties may not be covered by our existing patent applications, and we may be required to file new applications and/or seek other forms of protection for any such altered molecule. The patent landscape surrounding antibodies to GFRAL is crowded, and there can be no assurance that we would be able to secure patent protection that would adequately cover an alternative to our NGM120 molecule, nor can there be any assurance that we will obtain sufficiently broad claims to be able to prevent others from selling competing products.
158