UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington D.C. 20549
FORM 10-Q
| x | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended: June 30, 2016
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ______to______.
| | XG SCIENCES, INC. | |
| | (Exact name of registrant as
specified in its
charter) | |
| Michigan | | 333-209131 | | 20-4998896 |
(State or other jurisdiction of
incorporation or organization) | | (Commission File No.) | | (I.R.S. Employer Identification
No.) |
3101 Grand Oak Drive
Lansing, MI 48911
(Address of principal executive offices) (zip code)
(517) 703-1110
(Issuer Telephone number)
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yesx No¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes¨ Nox
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company filer. See definition of “accelerated filer” and “large accelerated filer” in Rule 12b-2 of the Exchange Act (Check one):
| Large Accelerated Filer¨ | Accelerated Filer¨ | Non-Accelerated Filer¨ | Smaller Reporting Companyx |
Indicate by check mark whether the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Yes¨ Nox
As of August 9, 2016, there were 1,155,548 shares outstanding of the registrant’s common stock.
XG SCIENCES, INC.
FORM 10-Q
JUNE 30, 2016
INDEX
FORWARD-LOOKING STATEMENTS
The information in this Quarterly Report on Form 10-Q contains “forward-looking statements” and information within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) relating to XG Sciences, Inc., a Michigan corporation and its subsidiary, XG Sciences IP, LLC, a Michigan corporation (collectively referred to as “we”, “us”, “our”, “XG Sciences”, “XGS”, or the “Company”), which are subject to the “safe harbor” created by those sections. These forward-looking statements include, but are not limited to, statements concerning our strategy, future operations, future financial position, future revenues, projected costs, prospects and plans and objectives of management. The words “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “projects,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements and you should not place undue reliance on our forward-looking statements. These forward-looking statements involve known and unknown risks and uncertainties that could cause our actual results, performance or achievements to differ materially from those expressed or implied by the forward-looking statements, including, without limitation, the risks set forth under the section entitled “Risk Factors” in Amendment No. 4 to our Registration Statement on Form S-1 (File No. 333-209131) as filed with the Securities and Exchange Commission (the “SEC”) on April 12, 2016, and declared effective on April 13, 2016.
XG SCIENCES, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
| | | June 30,
2016 | | | December 31, 2015 | |
| | | (unaudited) | | | | |
| ASSETS | | | | | | | | |
| | | | | | | | | |
| CURRENT ASSETS | | | | | | | | |
| Cash | | $ | 959,787 | | | $ | 1,060,224 | |
| Accounts receivable, less allowance for doubtful accounts of $10,000 at June 30, 2016 and December 31, 2015 | | | 48,483 | | | | 54,413 | |
| Inventories | | | 216,409 | | | | 229,034 | |
| Other current assets | | | 273,412 | | | | 194,096 | |
| | | | 1,498,091 | | | | 1,537,767 | |
| Total current assets | | | | | | | | |
| | | | | | | | | |
| PROPERTY, PLANT AND EQUIPMENT, NET | | | 3,336,428 | | | | 3,753,248 | |
| | | | | | | | | |
| RESTRICTED CASH FOR LETTER OF CREDIT | | | 195,351 | | | | 195,206 | |
| | | | | | | | | |
| INTANGIBLE ASSETS, NET | | | 447,532 | | | | 411,789 | |
| | | | | | | | | |
| TOTAL ASSETS | | $ | 5,477,402 | | | $ | 5,898,010 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | | | | | | | | |
| | | | | | | | | |
| CURRENT LIABILITIES | | | | | | | | |
| Accounts payable and other liabilities | | $ | 1,075,784 | | | $ | 704,177 | |
| Short-term promissory notes | | | 363,968 | | | | 497,324 | |
| Current portion of capital lease obligations | | | 209,647 | | | | 178,487 | |
| Total current liabilities | | | 1,649,399 | | | | 1,379,988 | |
| | | | | | | | | |
| LONG TERM LIABILITIES | | | | | | | | |
| Long term portion of capital lease obligations | | | 241,094 | | | | 354,483 | |
| Derivative liability - warrants | | | 7,951,132 | | | | 8,235,163 | |
| Total long term liabilities | | | 8,192,226 | | | | 8,589,646 | |
| | | | | | | | | |
| TOTAL LIABILITIES | | | 9,841,625 | | | | 9,969,634 | |
| | | | | | | | | |
| STOCKHOLDERS’ DEFICIT | | | | | | | | |
| Series A convertible preferred stock, 3,000,000 shares authorized, 1,814,976 and 1,800,696 shares issued and outstanding, liquidation value of $21,779,712 and $21,608,376 at June 30, 2016 and December 31, 2015, respectively | | | 21,463,254 | | | | 21,291,912 | |
| Series B Preferred Stock, 1,500,000 shares authorized, 269,987 shares issued and outstanding, liquidation value of $4,319,792 | | | 3,651,533 | | | | 3,651,533 | |
| Common stock, no par value, 25,000,000 shares authorized, 1,122,173 and 836,544 shares issued and outstanding at June 30, 2016 and December 31, 2015, respectively | | | 10,850,257 | | | | 8,565,225 | |
| Additional paid in capital | | | 5,758,078 | | | | 5,791,074 | |
| Accumulated deficit | | | (46,087,345 | ) | | | (43,371,368 | ) |
| Total stockholders’ deficit | | | (4,364,223 | ) | | | (4,071,624 | ) |
| | | | | | | | | |
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | | $ | 5,477,402 | | | $ | 5,898,010 | |
See notes to unaudited condensed consolidated financial statements.
XG SCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE THREE AND SIX MONTHS ENDED JUNE 30, 2016 AND 2015 (unaudited)
| | | For��the Three Months Ended
June 30, | | | For the Six Months ended
June 30, | |
| | | 2016 | | | 2015 | | | 2016 | | | 2015 | |
| | | | | | | | | | | | | |
| REVENUES | | | | | | | | | | | | | | | | |
| Product sales | | $ | 82,035 | | | $ | 81,008 | | | $ | 141,777 | | | $ | 118,313 | |
| Grants | | | 137,055 | | | | 121,407 | | | | 158,365 | | | | 218,911 | |
| Licensing revenue | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | |
| Total revenue | | | 244,090 | | | | 227,415 | | | | 350,142 | | | | 387,224 | |
| | | | | | | | | | | | | | | | | |
| COST OF GOODS SOLD | | | | | | | | | | | | | | | | |
| Direct costs | | | 25,020 | | | | 30,667 | | | | 57,052 | | | | 52,668 | |
| Unallocated manufacturing expenses | | | 404,232 | | | | 418,453 | | | | 747,947 | | | | 816,560 | |
| Total cost of goods sold | | | 429,252 | | | | 449,120 | | | | 804,999 | | | | 869,228 | |
| | | | | | | | | | | | | | | | | |
| GROSS LOSS | | | (185,162 | ) | | | (221,705 | ) | | | (454,857 | ) | | | (482,004 | ) |
| | | | | | | | | | | | | | | | | |
| OPERATING EXPENSES | | | | | | | | | | | | | | | | |
| Research and development | | | 419,007 | | | | 393,338 | | | | 635,357 | | | | 775,987 | |
| Sales, general and administrative | | | 625,381 | | | | 1,089,107 | | | | 1,721,603 | | | | 2,135,971 | |
| Total operating expenses | | | 1,044,388 | | | | 1,482,445 | | | | 2,356,960 | | | | 2,911,958 | |
| | | | | | | | | | | | | | | | | |
| OPERATING LOSS | | | (1,229,550 | ) | | | (1,704,150 | ) | | | (2,811,817 | ) | | | (3,393,962 | ) |
| | | | | | | | | | | | | | | | | |
| OTHER INCOME (EXPENSE) | | | | | | | | | | | | | | | | |
| Incentive refund and interest income | | | 24,073 | | | | 27,173 | | | | 48,223 | | | | 51,628 | |
| Interest expense | | | (101,196 | ) | | | (545,858 | ) | | | (184,997 | ) | | | (1,056,685 | ) |
| Gain from change in fair value of derivative liability – warrants | | | 142,848 | | | | 174,715 | | | | 232,614 | | | | 271,014 | |
| Total other income (expense) | | | 65,725 | | | | (343,970 | ) | | | 95,840 | | | | (734,043 | ) |
| | | | | | | | | | | | | | | | | |
| NET LOSS | | $ | (1,163,825 | ) | | $ | (2,048,120 | ) | | $ | (2,715,977 | ) | | $ | (4,128,005 | ) |
| | | | | | | | | | | | | | | | | |
WEIGHTED AVERAGE NUMBER OF SHARES OUTSTANDING – Basic and diluted | | | 887,595 | | | | 835,544 | | | | 862,069 | | | | 835,906 | |
| | | | | | | | | | | | | | | | | |
| NET LOSS PER SHARE – Basic and diluted | | $ | (1.31 | ) | | $ | (2.45 | ) | | $ | (3.15 | ) | | $ | (4.94 | ) |
See notes to unaudited condensed consolidated financial statements.
XG SCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS’ (DEFICIT) (unaudited)
FOR THE SIX MONTHS ENDED JUNE 30, 2016
| | | Preferred stock (A) | | | Preferred stock (B) | | | Common stock | | | Additional
paid-in | | | Accumulated | | | | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | capital | | | deficit | | | Total | |
| Balances, December 31, 2015 | | | 1,800,696 | | | $ | 21,291,912 | | | | 269,987 | | | $ | 3,651,533 | | | | 836,544 | | | $ | 8,565,225 | | | $ | 5,791,074 | | | $ | (43,371,368 | ) | | $ | (4,071,624 | ) |
| Stock issued for cash | | | - | | | | - | | | | - | | | | - | | | | 285,629 | | | | 2,285,032 | | | | - | | | | - | | | | 2,285,032 | |
| Stock issuance fees and expenses | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (336,600) | | | | - | | | | (336,600) | |
| Reclassification of Derivative Liability Warrants to Equity | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 51,418 | | | | - | | | | 51,418 | |
| Warrants issued with Bridge Financings | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 24,060 | | | | - | | | | 24,060 | |
| Preferred stock issued to pay capital lease obligations | | | 14,280 | | | | 171,342 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 171,342 | |
| Stock based compensation expense | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 228,126 | | | | - | | | | 228,126 | |
| Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (2,715,977) | | | | (2,715,977 | ) |
| Balances, June 30, 2016 | | | 1,814,976 | | | $ | 21,463,254 | | | | 269,987 | | | $ | 3,651,533 | | | | 1,122,173 | | | $ | 10,850,257 | | | $ | 5,758,078 | | | $ | (46,087,345) | | | $ | (4,364,223 | ) |
See notes to unaudited condensed consolidated financial statements.
XG SCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE SIX MONTHS ENDED JUNE 30, 2016 AND 2015 (unaudited)
| | | 2016 | | | 2015 | |
| CASH FLOWS FROM OPERATING ACTIVITIES | | | | | | | | |
| Net loss | | $ | (2,715,977 | ) | | $ | (4,128,005 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Depreciation and amortization | | | 456,133 | | | | 427,536 | |
| Amortization of intangible assets | | | 17,904 | | | | 14,070 | |
| Provision for bad debts | | | - | | | | 5,955 | |
| Stock based compensation expense | | | 228,126 | | | | 270,642 | |
| Non-cash interest expense | | | 157,903 | | | | 991,103 | |
| Gain from change in fair value of derivative liability - warrants | | | (232,614 | ) | | | (271,014 | ) |
| (Increase) Decrease in: | | | | | | | | |
| Accounts receivable | | | 5,930 | | | | (64,516 | ) |
| Inventory | | | 12,624 | | | | (77,559 | ) |
| Other current and non-current assets | | | (79,461 | ) | | | 56,310 | |
| Increase (Decrease) in: | | | | | | | | |
| Accounts payable and other liabilities | | | 371,606 | | | | (505,253 | ) |
| NET CASH USED IN OPERATING ACTIVITIES | | | (1,777,826 | ) | | | (3,280,731 | ) |
| | | | | | | | | |
| CASH FLOWS FROM INVESTING ACTIVITIES | | | | | | | | |
| Purchases of property and equipment | | | (39,314 | ) | | | (108,478 | ) |
| Purchases of intangible assets | | | (53,647 | ) | | | (23,429 | ) |
| NET CASH USED IN INVESTING ACTIVITIES | | | (92,961 | ) | | | (131,907 | ) |
| | | | | | | | | |
| CASH FLOWS FROM FINANCING ACTIVITIES | | | | | | | | |
| Repayments of capital lease obligations | | | (2,832 | ) | | | (26,588 | ) |
| Repayments of short-term notes | | | (750,000 | ) | | | - | |
| Advances on short-term notes | | | 574,750 | | | | - | |
| Proceeds from issuance of preferred stock and warrants | | | - | | | | 4,319,792 | |
| Proceeds from issuance of common stock | | | 2,285,032 | | | | 14,000 | |
| Common stock issuance fees and expenses | | | (336,600 | ) | | | - | |
| NET CASH PROVIDED BY FINANCING ACTIVITIES | | | 1,770,350 | | | | 4,307,204 | |
| | | | | | | | | |
| NET INCREASE (DECREASE) IN CASH | | | (100,437 | ) | | | 894,566 | |
| CASH AT BEGINNING OF PERIOD | | | 1,060,224 | | | | 2,088,866 | |
| | | | | | | | | |
| CASH AT END OF PERIOD | | $ | 959,787 | | | $ | 2,983,432 | |
| | | | | | | | | |
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | | | | | | | | |
| Cash paid for interest | | $ | 77,646 | | | $ | 65,582 | |
| | | | | | | | | |
| SUPPLEMENTAL DISCLOSURE OF NON-CASH INVESTING AND FINANCING: | | | | | | | | |
| Value of preferred stock issued for AAOF capital lease obligations | | $ | 171,342 | | | $ | 171,343 | |
See notes to unaudited condensed consolidated financial statements.
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2016
NOTE 1 - NATURE OF BUSINESS AND BASIS OF PRESENTATION
XG Sciences, Inc., a Michigan company located in Lansing, Michigan and its subsidiary, XGS IP, LLC (collectively referred to as “we”, “us”, “our”, or the “Company”) manufactures graphene nanoplatelets made from graphite, using a proprietary manufacturing process to split natural flakes of crystalline graphite into very small and thin particles, which we sell as xGnP® graphene nanoplatelets. These particles are then used in products like battery electrodes, thin sheets, films, inks and coatings that we sell to other companies. We also sell our nanoparticles in the form of bulk powders or dispersions to other companies for use as additives to make composite and other materials with specially engineered characteristics. Additionally, we license our technology to other companies in exchange for royalties and other fees.
Basis of Presentation
The accompanying interim condensed consolidated financial statements are unaudited and have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) for interim financial information and the instructions to Form 10-Q and do not include all of the information and footnotes required by GAAP for complete financial statements. All intercompany transactions have been eliminated in consolidation.
Certain information and footnote disclosures normally included in the Company’s annual audited consolidated financial statements and accompanying notes have been condensed or omitted in these interim condensed consolidated financial statements. Accordingly, the unaudited condensed consolidated financial statements included herein should be read in conjunction with the audited consolidated financial statements for the year ended December 31, 2015, as filed with the Securities and Exchange Commission (“SEC”) on Form S-1 (Registration No. 333-209131) with an effective date of April 13, 2016.
The results of operations presented in this quarterly report are not necessarily indicative of the results of operations that may be expected for any future periods. In the opinion of management, these unaudited condensed consolidated financial statements include all adjustments and accruals, consisting only of normal recurring adjustments that are necessary for a fair statement of the results of all interim periods reported herein.
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Going Concern
We have historically incurred recurring losses from operations and we may continue to generate negative cash flows as we implement our business plan. Our unaudited condensed consolidated financial statements are prepared using GAAP as applicable to a going concern, which contemplates the realization of assets and liquidation of liabilities in the normal course of business.
We currently do not have sufficient cash or commitments for financing to sustain our operations for the next twelve months. Our plan is to develop customer relationships and increase our revenues derived from our products and IP licensing. Although we have historically incurred operating losses, we have been able to fund such losses primarily by selling common and preferred stock and convertible notes. We expect that our cash on hand at June 30, 2016, of $959,787 and proceeds from our initial public offering of common stock (“IPO”) will sustain our operations for the next twelve months. However, we cannot make any assurances that additional financing will be available to us and, if available, completed on a timely basis, on acceptable terms, or at all.
There has been no public market for our securities and a public market may never develop, or, if any market does develop, it may not be sustained. Our common stock is not currently quoted on or traded on any exchange or to our knowledge, on any over-the-counter market. In the event we are unable to fund our operations from existing cash on hand, operating cash flows, additional borrowings or raising equity capital, we may be forced to reduce our expenses, slow down our growth rate, or discontinue operations. Our condensed consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should we be unable to continue as a going concern.
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2016
Use of Estimates
The preparation of our condensed consolidated financial statements in conformity with GAAP requires us to make estimates, judgments and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, together with amounts disclosed in the related notes to the financial statements. Actual results and outcomes may differ from our estimates, judgments and assumptions. Significant estimates, judgments and assumptions used in these condensed consolidated financial statements include, but are not limited to, those related to revenues, accounts receivable and related allowances, contingencies, useful lives and recovery of long-term assets, income taxes, the fair value of stock-based compensation and derivative financial instrument liabilities. These estimates, judgments, and assumptions are reviewed periodically and the effects of material revisions in estimates are reflected in the financial statements prospectively from the date of the change in estimate.
Inventory
Inventory consists of raw materials, work-in-process and finished goods, all of which are valued at standard cost, which approximates average cost.
Derivative Financial Instruments
We do not use derivative instruments to hedge exposures to cash flow, market or foreign currency risk. The terms of convertible preferred stock and convertible notes that we issue are reviewed to determine whether or not they contain embedded derivative instruments that are required by ASC 815: “Derivatives and Hedging” to be accounted for separately from the host contract, and recorded at fair value. In addition, freestanding warrants are also reviewed to determine if they achieve equity classification. Certain warrants that we have issued did not meet the conditions for equity classification and are classified as derivative instrument liabilities measured at fair value. The fair values of these derivative liabilities are revalued at each reporting date, with the change in fair value recognized in earnings.
Fair Value Measurements
The following is a reconciliation of the beginning and ending balances for liabilities measured at fair value on a recurring basis using significant unobservable inputs (Level 3) during the six months ended June 30, 2016 and 2015:
| | | 2016 | | | 2015 | |
| | | | | | | |
| Balance at January 1 | | $ | 8,235,163 | | | $ | 5,000,752 | |
| Warrants issued with private placement of Series B Preferred Stock | | | — | | | | 660,378 | |
| Warrants issued with preferred stock sold under preemptive rights | | | — | | | | 7,881 | |
| Warrants reclassified to equity | | | (51,418 | ) | | | — | |
| Gain recognized in earnings | | | (232,613 | ) | | | (271,013 | ) |
| Balance at June 30 | | $ | 7,951,132 | | | $ | 5,397,998 | |
NOTE 3 – PRIVATE PLACEMENT AND PREEMPTIVE RIGHTS
Private Placement
In April 2015, we commenced a private placement offering of up to $18,000,000 in Series B Units consisting of up to 1,125,000 shares of Series B convertible preferred stock (“Series B Preferred Stock”) and warrants to purchase common stock (the “Warrants”) at an offering price of $16.00 per Unit. The offering terminated on August 31, 2015 and as of such date, we had sold 266,987 shares of Series B Preferred Stock and Warrants to purchase 222,262 shares of common stock, for aggregate gross proceeds of $4,270,192.
The Series B Preferred Stock has a stated value of $16.00 per share and is convertible, at the option of the holder into common shares, at a conversion price of $16.00 per share, subject to adjustments for stock dividends, splits, combinations and similar events. The Warrants have an exercise price of $16.00 per share and expire 7 years from issuance. During the period from closing of the offering and ending on the earlier of i) December 31, 2017 and ii) the date the Company consummates the sale of new securities resulting in gross proceeds of at least $18,000,000, the holder has the right to exchange their Series B Units (Series B Preferred Stock and Warrants) into any new security sold to third parties at the same relative price per share and other terms at which such new security is sold to such third parties.
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2016
The cash proceeds from the private placement were allocated first to the derivative liabilities resulting from warrants, at their fair values, with the residual being allocated to the Series B Preferred Stock.
Preemptive Rights
On January 15, 2014, as part of our financing agreements with Samsung Ventures (“Samsung”), Aspen Advanced Opportunity Fund LP (“AAOF”) and XGS II, LLC (“XGS II”), we agreed to allow all shareholders to purchase one share of Series A convertible preferred stock (“Series A Preferred Stock”) at a price of $12.00 per share for every two (2) shares of Series A Preferred Stock or common stock owned by the shareholder. In addition, for every two preemptive shares purchased, the Company issued the shareholder a warrant to purchase one additional share of Series A Preferred Stock with the same terms as the warrants issued to AAOF and XGS II. The Company also agreed to issue warrants with the same terms to those shareholders who exercised preemptive rights in October 2013.
Under the January 15, 2014 preemptive rights offering, 101,000 shares of Series A Preferred Stock were sold to existing stockholders at a price of $12.00 per share. In addition, warrants indexed to 56,054 shares of Series A Preferred Stock were issued in conjunction with these stock purchases, including 5,554 warrants related to the preemptive rights exercised in October 2013.
As part of our Series B Unit private placement in April 2015, shareholders and holders of our convertible notes were provided the right to purchase their pro rata share of any class of stock that the Company sells or issues. The sale of Series B Preferred Stock in the April 2015 offering triggered the preemptive rights. As of June 30, 2016, 3,100 shares of Series B Convertible Stock have been sold to existing shareholders at a price of $16.00 per share. In addition, the Warrants indexed to 2,635 shares of common stock were issued as part of the Series B Units.
As of June 30, 2016, the total number of warrants issued due to the preemptive rights offerings was 58,689.
NOTE 4 – BRIDGE FINANCINGS
From December 31, 2015 through April 7, 2016, the Company entered into private placement bridge financings with 14 investors, seven of whom are board members or affiliates of board members, totaling $1,124,750 (the “Bridge Financings”). The investors in the Bridge Financings received common stock warrant coverage of 30% for investments made prior to December 31, 2015 and 20% coverage thereafter.
During June 2016 the Company repaid i) outstanding principal of $550,000 plus accrued interest of $22,000 to the December 2015 Bridge Financing investors and ii) outstanding principal of $200,000 plus accrued interest of $5,032 to two of the March 2016 Bridge Financing investors. These investors, who are also members of the board of directors of the Company, used the proceeds from repayment of their notes, plus additional funds, to purchase 199,879 additional shares of the Company’s common stock for approximately $1.6 million.
The following tables provide additional details regarding the Bridge Financings:
| | | December 2015 | | | March 2016 | | | April 2016 | | | Total | |
| | | Bridge Financing | | | Bridge Financing | | | Bridge Financing | | | Bridge Financing | |
| Face value of notes at issuance | | $ | 550,000 | | | $ | 530,000 | | | $ | 44,750 | | | $ | 1,124,750 | |
| Outstanding principal on June 30, 2016 | | $ | -- | | | $ | 330,000 | | | $ | 44,750 | | | $ | 374,750 | |
| Interest rate | | | 8.0 | % | | | 8.0 | % | | | 8.0 | % | | | 8.0 | % |
| Maturity Date | | | June 30, 2016 | | | | December 31, 2016 | | | | December 31, 2016 | | | | | |
| Common Stock Warrant Shares | | | 20,625 | | | | 10,600 | | | | 895 | | | | 32,120 | |
| Warrant Exercise Price | | $ | 8.00 | | | $ | 10.00 | | | $ | 10.00 | | | | | |
| Warrant Term | | | 5 years | | | | 5 years | | | | 5 years | | | | 5 years | |
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2016
The Bridge Financing Warrants issued in December 2015 inadvertently provided the holder with the right to exchange their warrants on a price per share basis into a new security on the same relative price per share terms as any new securities sold to third parties resulting in gross proceeds of at least $18,000,000. As a result of these exchange rights, the December 2015 Bridge Financing warrants did not achieve equity classification at inception and were recorded as derivative liabilities, at fair value. During the second quarter of 2016, the warrant holders agreed to waive their exchange rights at which time the warrants were reclassified to equity and $52,676 of derivative liabilities related to such December 2015 Bridge Financing warrants was reclassified to equity.
The following table reconciles the Bridge Financings balance recorded on the balance sheet at June 30, 2016:
| | | 2016 | |
| | | | |
| Balance at January 1 | | $ | 550,000 | |
| Proceeds from Bridge Financings received January through April 7 | | | 574,750 | |
| Subtotal | | | 1,124,750 | |
| Proceeds allocated to warrants – liability | | | (52,676 | ) |
| Proceeds allocated to warrants – equity | | | (24,059 | ) |
| Accrued interest January through June 30 | | | 92,985 | |
| Payoff of principal ($750,000) and accrued interest | | | (777,032 | ) |
| Balance at June 30, 2016 | | $ | 363,968 | |
NOTE 5 – DERIVATIVE LIABILITY WARRANTS
As of June 30, 2016, all 1,197,617 derivative liability classified warrants issued to AAOF, XGS II, and holders of Series A and Series B Preferred Stock have vested.
Shares indexed to derivative liabilities as of June 30, 2016 and December 31, 2015 were as follows:
| | | Type of
shares
indexed | | Exercise
Price | | | June
30, 2016 | | | December
31, 2015 | |
| | | | | | | | | | | | |
| Warrants issued with Secured Convertible Notes | | Series A PS | | $ | 6.40 | | | | 833,333 | | | | 833,333 | |
| Warrants issued with equipment financing leases | | Series A PS | | $ | 6.40 | | | | 83,333 | | | | 83,333 | |
| Warrants issued with Series A preemptive rights | | Series A PS | | $ | 6.40 | | | | 56,054 | | | | 56,054 | |
| Warrants issued with Series B preemptive rights | | Common | | $ | 16.00 | | | | 2,635 | | | | 2,635 | |
| Warrants issued with Series B Units | | Common | | $ | 16.00 | | | | 222,262 | | | | 222,262 | |
| Warrants issued with Bridge Financings | | Common | | $ | 8.00 | | | | — | | | | 20,625 | |
| Total shares indexed to derivative liabilities | | | | | | | | | 1,197,617 | | | | 1,218,242 | |
The following table summarizes the fair value of the derivative liabilities as of June 30, 2016 and December 31, 2015:
| | | June 30, 2016 | | | December 31,2015 | |
| | | | | | | |
| Warrants issued with Secured Convertible Notes | | $ | 6,565,326 | | | $ | 6,743,997 | |
| Warrants issued with equipment financing leases | | | 656,535 | | | | 674,397 | |
| Warrants issued with preemptive rights | | | 444,984 | | | | 457,265 | |
| Warrants issued with 2015 Series B Unit private placement | | | 284,287 | | | | 306,828 | |
| Warrants issued with Bridge Financings | | | — | | | | 52,676 | |
| | | | | | | | | |
| Total derivative liabilities | | $ | 7,951,132 | | | $ | 8,235,163 | |
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2016
The Company estimated the fair value of their warrant derivative liabilities as of June 30, 2016 and December 31, 2015, using a lattice model and the following assumptions:
| | | June 30, 2016 | | | December 31, 2015 | |
| Fair value of underlying stock | | | $7.63 - $12.64 | | | | $7.63 - $12.64 | |
| Equivalent risk free interest rate | | | 0.68%- 0.79% | | | | 1.06%- 1.39% | |
| Expected term (in years) | | | 5.84- 7.55 | | | | 5.01- 8.05 | |
| Equivalent stock price volatility | | | 39.03%- 39.21% | | | | 38.00%- 38.61% | |
| Expected dividend yield | | | — | | | | — | |
The fair value of the warrants is estimated using a binomial lattice model. Equivalent amounts reflect the net results of multiple modeling simulations that the lattice model applies to underlying assumptions. Because the Company’s common stock is not publicly traded on a national exchange or to our knowledge, an over-the-counter market, the expected volatility of the Company’s stock was developed using historical volatility for a peer group for a period equal to the expected term of the warrants. The fair value of the warrants will be significantly influenced by the fair value of our common stock, stock price volatility and the risk free interest components of the lattice technique. Changes in the fair value of Derivative Liabilities, carried at fair value, are reported as “Change in fair value of derivative liability - warrants” in the Statement of Operations, and were as follows:
| | | Three months ended June 30, | |
| | | 2016 | | | 2015 | |
| Warrants issued with Secured Convertible Notes | | $ | 103,084 | | | $ | 120,833 | |
| Warrants issued with equipment financing leases | | | 10,308 | | | | 12,083 | |
| Warrants issued with preemptive rights | | | 7,197 | | | | 8,580 | |
| Warrants issued with 2015 private placement | | | 22,259 | | | | 33,219 | |
| Total Derivative Gain | | $ | 142,848 | | | $ | 174,715 | |
| | | Six months ended June 30, | |
| | | 2016 | | | 2015 | |
| Warrants issued with Secured Convertible Notes | | $ | 178,671 | | | $ | 203,333 | |
| Warrants issued with equipment financing leases | | | 17,862 | | | | 20,333 | |
| Warrants issued with preemptive rights | | | 12,281 | | | | 14,129 | |
| Warrants issued with 2015 private placement | | | 22,541 | | | | 33,219 | |
| Warrants issued with Bridge Financings | | | 1,259 | | | | — | |
| | | | | | | | | |
| Total Derivative Gain | | $ | 232,614 | | | $ | 271,014 | |
NOTE 6 – OTHER COMMON STOCK WARRANTS
In addition to the warrants described in Note 5, we had 42,694 warrants to purchase common stock that were issued in 2012 and prior years which are accounted for as equity instruments. As of June 30, 2016, the remaining warrants, all of which are exercisable, have exercise prices ranging from $8.00 to $12.00 and expire at various dates through 2027, as follows:
| Date Issued | | Expiration Date | | Exercise Price | | | Number of
Warrants | |
| | | | | | | | | |
| 7/1/2009 | | 7/1/2019 | | $ | 8.00 | | | | 6,000 | |
| 10/8/2012 | | 10/8/2027 | | $ | 12.00 | | | | 5,000 | |
| | | | | | | | | | 11,000 | |
NOTE 7 - INCENTIVE STOCK OPTION PLAN
We have established an incentive stock option plan (the “Plan”) under which the Company may grant key employees and directors options to purchase common stock of the Company at not less than fair market value as of the grant date. Options for up to 600,000 shares may be awarded under the Plan. Each option is exercisable into one share of common stock of the Company. The Plan expires in December 2017. The fair value of the options granted was estimated on the dates of grant using the Black Scholes option-pricing model. As of June 30, 2016, 419,750 option shares have been granted and are outstanding, of which 221,824 are exercisable at an exercise price of $12.00. Vesting of the options ranges from immediately to 20% per year, with most options vesting on a straight-line basis over a three or four year period from the date issued. Rights to exercise the options vest immediately upon a change in control of the Company or termination of the employee’s continuous service due to death or disability. The options expire at various dates through October 2023.
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2016
NOTE 8 – CAPITAL LEASES
As of June 30, 2016 and December 31, 2015, we have capital lease obligations as follows:
| | | June 30, 2016 | | | December 31, 2015 | |
| | | | | | | |
| Capital lease obligations | | $ | 555,433 | | | $ | 682,564 | |
| Unamortized warrant discount | | | (104,692 | ) | | | (149,594 | ) |
| Net obligations | | | 450,741 | | | | 532,970 | |
| Short-term portion of obligations | | | (209,647 | ) | | | (178,487 | ) |
| | | | | | | | | |
| Long-term portion of obligations | | $ | 241,094 | | | $ | 354,483 | |
The 83,333 common stock warrants issued as consideration for the equipment financing leases are recorded as derivative liabilities at fair value. The initial value of these warrants was recorded as a reduction of the capital lease obligation and is being amortized as part of the effective interest cost on the capital lease obligations.
NOTE 9 - RELATED PARTY TRANSACTIONS
We have a licensing agreement for exclusive use of patents and pending patents with Michigan State University (“MSU”), a shareholder of the Company via the MSU Foundation. During the three and six months ended June 30, 2016 and 2015 we incurred expenses of $12,500 and $25,000, respectively. We have also entered into product licensing agreements with certain other shareholders. No royalty revenue or expenses have been recognized related to these agreements during the six months ended June 30, 2016 and 2015.
Beginning in 2014, POSCO Corporation (“POSCO”), one of our shareholders, has a contractual obligation to pay us a minimum of $100,000 per year to license certain technologies we license from MSU. This obligation is due annually on February 28 of the following year. We record this license revenue at a rate of $25,000 per quarter. POSCO is disputing that they are obligated to pay the royalties. A petition for arbitration has been filed for this matter by the Company on March 9, 2016. On July 7 we received a letter from the International Court of Arbitration and they have assigned an arbitrator to the case. No assessment or decision has made by the arbitrator as of the filing date of these financial statements. An allowance in the amount of $125,000 and $100,000 has been recorded at June 30, 2016 and December 31, 2015, respectively, to reflect an estimate of the portion of the 2016, 2015 and 2014 royalties that we believe may not be collectible. The accrued royalty and allowance are netted together and reflected in other current assets on the condensed consolidated balance sheet.
The financing arrangements as previously disclosed were provided by AAOF and XGS II, two private funds that were formed for the sole purpose of investing in the Company by two investors affiliated with ASC-XGS, LLC, a shareholder of the Company. Pursuant to the Company’s Shareholders’ Agreement dated March 18, 2013 (as amended on February 26, 2016), a principal of each private fund serves as a director of the Company.
The Bridge Financings discussed in Note 4 above include loans from entities controlled by existing shareholders. Three of these shareholders are also directors of the Company. In conjunction with these short-term borrowings, the Company issued Warrants (see also discussed in Note 5).
NOTE 10 – SUBSEQUENT EVENTS
During the period from July1 through August 9, 2016, we received common stock proceeds of $267,000 for the sale of 33,375 shares.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Forward-Looking Statements
In this Quarterly Report on Form 10-Q, unless otherwise indicated, the words “we”, “us”, “our”, “XG”, “XGS”, “XG Sciences” or the “Company” refer to XG Sciences, Inc. and its wholly owned subsidiary, XG Sciences IP, LLC, a Michigan limited liability company.
Introduction
The following discussion and analysis should be read in conjunction with the unaudited condensed consolidated financial statements, and the notes thereto included herein. The information contained below includes statements of the Company’s or management’s beliefs, expectations, hopes, goals and plans that, if not historical, are forward-looking statements subject to certain risks and uncertainties that could cause actual results to differ materially from those anticipated in the forward-looking statements. For a discussion on forward-looking statements, see the information set forth in the introductory note to this quarterly report on Form 10-Q under the caption “Forward-Looking Statements”, which information is incorporated herein by reference.
Overview of our Business
XG Sciences was formed in May 2006 for the purpose of commercializing certain technology to produce graphene nanoplatelets. First isolated and characterized in 2004, graphene is a single layer of carbon atoms configured in an atomic-scale honeycomb lattice. Among many noted properties, graphene is harder than diamonds, lighter than steel but significantly stronger, and conducts electricity better than copper. Graphene nanoplatelets are particles consisting of multiple layers of graphene. Graphene nanoplatelets have unique capabilities for energy storage, thermal conductivity, electrical conductivity, barrier properties, lubricity and the ability to impart strength when incorporated into plastics or other matrices.
We believe the unique properties of graphene and graphene nanoplatelets will enable numerous new product applications and the market for such products will quickly grow to be a significant market opportunity. Our business model is to design, manufacture and sell advanced materials we call xGnP® graphene nanoplatelets and value-added products based on these nanoplatelets. We currently have hundreds of customers trialing our products for numerous applications, including, but not limited to lithium ion batteries, supercapacitors, thermal shielding and heat transfer, inks and coatings, printed electronics, construction materials, composites, and military uses.
We target our xGnP®nanoplatelets for use in a range of large and growing end-use markets. Our proprietary manufacturing processes allow us to produce nanoplatelets with varying performance characteristics that can be tuned to specific end-use applications based on customer requirements. We currently offer three commercial “grades” of bulk materials, each of which is available in various particle sizes, which allows for surface areas ranging from 50 to 800 square meters of surface area per gram of material depending on the product. Other grades may be made available, depending on the needs for specific applications. In addition to selling bulk graphene nanoplatelets, we also offer the following value added products that contain our graphene nanoplatelets in various forms.
| | 1. | Energy storage materials.These consist of specialty advanced materials that have been formulated for specific applications in the energy storage segment. Chief among these is our proprietary, specially formulated silicon-graphene composite material (also referred to as “SiG” or “XG SiG®”) for use in lithium-ion battery anodes. XG SiG® targets the never-ending need for higher battery capacity and longer life. In several customer trials, our SiG material has demonstrated the potential to increase battery energy storage capacity by 3-5x what is currently available with conventional lithium ion batteries today. Additionally, we offer various bulk materials for use as conductive additives for cathodes and anodes in li-ion batteries, as an additive to anode slurries for lead-carbon batteries and are investigating the use of our materials as part of other battery components. |
| | 2. | XG Leaf®. XG Leaf is a family of sheet products for a variety of thermal management and other applications. XG Leaf® is ideally suited for use in thermal management in portable electronics, which may include cell phones, tablets and notebook PC’s. As these devices continue to adopt faster electronics, higher data management capabilities, brighter displays with ever increasing definition, they generate more and more heat. Managing that heat is a key requirement for the portable electronics market and our XG Leaf®product line is well suited to address the need. These sheets are made using special formulations of xGnP® graphene nanoplatelets as precursors, along with other materials for specific applications. There are several different types of XG Leaf® available in various thicknesses, depending on the end-use requirements for thermal conductivity, electrical conductivity, or resistive heating. |
| | 3. | Inks and Coatings. These consist of specially-formulated dispersions of xGnP® together with solvents, binders, and other additives to make electrically or thermally conductive products designed for printing or coating and which are showing promise in diverse customer applications such as advanced packaging, electrostatic dissipation and thermal management. |
We sell products to customers around the world and have sold materials to over 1,000 customers (entities that have purchased our materials) in 47 countries since 2008. Some of these customers are research organizations and some are commercial organizations. Because graphene is a new material, our customers are developing new uses for our products and initially purchase them in quantities consistent with development purposes. A few of our customers have indicated to us that they have introduced commercial products that use our materials, but our customers are under no obligation to report to us on the usage of our materials. Our customers have included well-known automotive and OEM suppliers around the world, world-scale lithium ion battery manufacturers in the US, South Korea and China, diverse specialty material companies, as well as many others. We have also licensed some of our base manufacturing technology to other companies and we consider technology licensing a component of our business model. Our licensees include POSCO and Cabot Corporation, who further extend our technology through their customer networks. Ultimately, we expect to benefit in terms of royalties on sales of xGnP® produced and sold by our licensees.
The process of “designing-in” new materials is a relatively complex process that involves the use of relatively small amounts of the new material in laboratory and engineering development for an extended period of time. Following successful development, we expect customers that incorporate our materials into their products will then order much larger quantities of material to support commercial production. Thus, while many of our customers are currently purchasing our materials in kilogram (one or two pound) quantities, we expect many of our customers will require tons or even hundreds of tons of material when they commercialize products that incorporate our materials. The majority of our customers are still in the development stage and our product sales thus consist mainly of orders for relatively small quantities of materials being used in a variety of research or development activities and in early phase limited commercial applications.
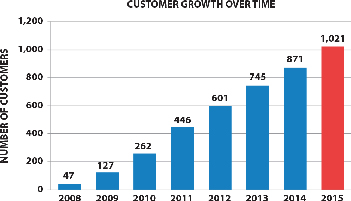 | 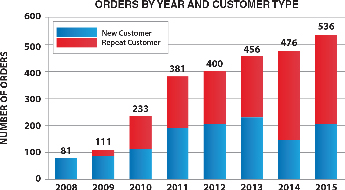 |
The above graphs show total orders and customers based on actual purchases of our materials and do not include free samples or materials used in joint development programs. The average order size for the six months ending June 30, 2016 was $865, as compared to $600 for the full year 2015, which indicates that most of these orders were for materials that were not yet incorporated into large-volume commercial products.
We currently have five customers who are using our materials in their products and actively selling them to their customers or actively promoting them for future sales. We anticipate seeing the average order size for these customers increase in the second half of 2016 and into 2017 as their demand grows. In addition, we have another five customers who have indicated that they expect to begin shipping product incorporating our materials in the second half of 2016, and have another seven customers who have indicated an intent to commercialize in the second half of 2016 and into the first quarter of 2017. We also have tens of customers with whom we are working that have not yet indicated an exact date for commercialization, but for whom we believe have the potential to contribute to revenue in 2017. As a result, we expect to begin shipping significantly greater quantities of our products in the second half of 2016 and into 2017. Based on the status of current discussions with such customers, we believe that we will continue to ramp revenue as 2016 progresses and that we will be able to recognize approximately $2-4 million of revenue in 2016 and approximately $10-25 million of revenue in 2017.
The following are examples of commercial and development uses of our products:
| | • | Construction company demonstrating less than one weight percent of our product in construction material composites improves flexural strength by more than 30%, and |
| | • | Large oil and lubricant supplier showing gear and friction improvements when incorporated into industrial and automotive greases, and |
| | • | Engineering design firm for automotive manufacturers found 20% reduction in operating temperature and in thermal uniformity when XG Leaf®replaces standard cooling fins in lithium ion battery packs’, and |
| | • | Auto manufacturer showing increased tensile and flexural strength and reduced weight in automotive composites, and |
| | • | Battery manufacturers demonstrating improved cycle life and energy storage when used as additives in lead acid batteries. |
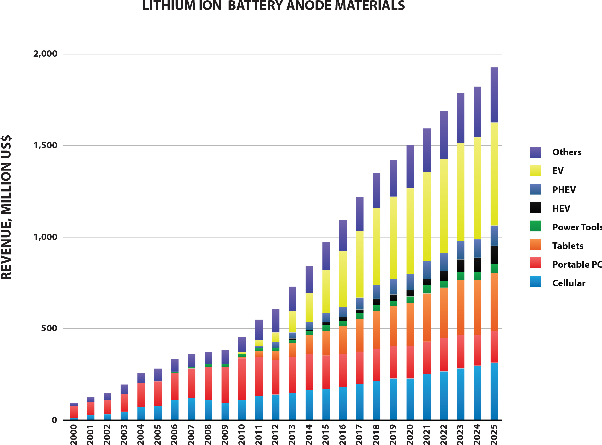
The markets that we serve are large and rapidly growing. For example, as shown in the figure below (Avicenne Energy, “The Rechargeable Battery Market, 2014–2025”, July 2015), the market for materials used in lithium ion battery anodes is currently approximately $1 billion, but is expected to approximately double over the next ten years. We believe our ability to address next generation anode materials represents a significant opportunity for us.
According to Prismark Partners, LLC, a leading electronics industry consulting firm specializing in advanced materials, the 2014 market for finished graphitic heat spreaders as sold to the OEM and EMS companies with adhesive, PET, and/or copper backing for selected portable applications was $600 million, and is expected to reach $900 million in 2018. The market is currently in a significant expansion period driven by the demand for portable devices. In a press release dated March 3, 2015, Gartner, Inc., a leading research organization, estimated the 2014 global cell phone market at 1.88 billion units. Every cell phone has some form of thermal management system, and we believe many of the new smart phones being developed can benefit from the thermal management properties of our XG Leaf® product line. In August 2015, International Data Corporation (IDC) in their Worldwide Quarterly Tablet Tracker, estimated the global shipment of tablets in 2015 at 212 million units. Thus, we believe our XG Leaf®product line is well positioned to address a very large and rapidly growing market.
Operating Segment
We have one reportable operating segment that manufactures xGnP® graphene nanoplatelets and value-added products produced therefrom, conducts research on graphene nanoplatelets and related products, and licenses our technology as appropriate. As of June 30, 2016 we shipped products on a worldwide basis, but all of our assets were located within the United States.
Results of Operations for the Three and Six Months Ended June 30, 2016 Compared with the Three and Six Months Ended June 30, 2015
The following table summarizes the results of our operations for the three and six months ended June 30, 2016 and 2015.
| Summary Income Statement | | For the Three Months Ended June 30, | | | | | | For the Six Months Ended June 30, | | | | |
| | | 2016 | | | 2015 | | | Change | | | 2016 | | | 2015 | | | Change | |
| Revenues | | | | | | | | | | | | | | | | | | | | | | | | |
| Product Sales | | $ | 82,035 | | | $ | 81,008 | | | $ | 1,027 | | | $ | 141,777 | | | $ | 118,313 | | | | 23,464 | |
| Grants | | | 137,055 | | | | 121,407 | | | | 15,648 | | | | 158,365 | | | | 218,911 | | | | (60,546 | ) |
| Licensing Revenue | | | 25,000 | | | | 25,000 | | | | - | | | | 50,000 | | | | 50,000 | | | | - | |
| Total Revenues | | | 244,090 | | | | 227,415 | | | | 16,675 | | | | 350,142 | | | | 387,224 | | | | (37,082 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Cost of Goods Sold | | | 429,252 | | | | 449,120 | | | | (19,868 | ) | | | 804,999 | | | | 869,228 | | | | (64,229 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Gross Loss | | | (185,162 | ) | | | (221,705 | ) | | | 36,543 | | | | (454,857 | ) | | | (482,004 | ) | | | 27,147 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Research & Development Expense | | | 419,007 | | | | 393,338 | | | | 25,669 | | | | 635,357 | | | | 775,987 | | | | (140,630 | ) |
| Sales, General & Administative Expense | | | 625,381 | | | | 1,089,107 | | | | (463,726 | ) | | | 2,058,203 | | | | 2,135,971 | | | | (414,368 | ) |
| Total Operating Expense | | | 1,044,388 | | | | 1,482,445 | | | | (438,057 | ) | | | 2,693,560 | | | | 2,911,958 | | | | (554,998 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Operating Loss | | | (1,229,550 | ) | | | (1,704,150 | ) | | | 474,600 | | | | (3,148,417 | ) | | | (3,393,962 | ) | | | 582,145 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Other Income (Expense) | | | 65,725 | | | | (343,970 | ) | | | 409,695 | | | | 95,840 | | | | (734,043 | ) | | | 829,883 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net Loss | | $ | (1,163,825 | ) | | $ | (2,048,120 | ) | | $ | 884,295 | | | $ | (3,052,577 | ) | | $ | (4,128,005 | ) | | $ | 1,412,028 | |
Product sales consist of two broad categories: (1) material sold to customers for research or development purposes; and (2) production orders for customers. Typically, the order sizes for the first category are relatively small, however we expect orders in the second category to be much larger in the future. In the six months ended June 30, 2016, product sales increased by $23,464, or 19.8%. The main reason for the increase was customers moving through development programs towards commercialization, requiring larger quantities of our materials for advanced testing and pilot production activities.
We ship our products from our Lansing manufacturing facilities to customers around the world. During the six months ended June 30, 2016, we shipped materials to customers in 21 different countries, versus 23 countries during the six months ended June 30, 2015. Shipments to South Korea accounted for approximately 38% and 13% of total product revenues during the six months ended June 30, 2016 and 2015. No other countries accounted for more than 10% of product revenue in each of these respective periods.
The table below shows a comparison of orders received, both domestic and international. The table also includes the average order size for product sales reflected in our Statement of Operations. These numbers indicate that our customer base remains active with development or research projects that use our materials and indicate the breadth of our geographic coverage. The average order size for the product revenue reflected in our statement of operations increased 65% for the three months ended June 30, 2016 as compared to the same period in 2015. For the six months ended June 30, 2016, the increase was 54% as compared to the same period in 2015. Although the average size of these orders is still relatively small, we are encouraged that the trend is increasing. The current average order size indicates that most of our orders are for R&D and development activity. We expect that our average order size will begin to increase significantly once our customers begin to commercialize products that incorporate our materials within them.
| Order Summary | | For the Three Months Ended June 30, | | | | | | For the Six Months Ended June 30, | | | | |
| | | 2016 | | | 2015 | | | Change | | | 2016 | | | 2015 | | | Change | |
| | | | | | | | | | | | | | | | | | | |
| Number of orders - domestic | | | 74 | | | | 70 | | | | 4 | | | | 155 | | | | 142 | | | | 13 | |
| Number of orders - international | | | 73 | | | | 54 | | | | 19 | | | | 142 | | | | 112 | | | | 30 | |
| Number of orders - total | | | 147 | | | | 124 | | | | 23 | | | | 297 | | | | 254 | | | | 43 | |
| Average order size for product sales recorded in our Statement of Operations | | $ | 973 | | | $ | 588 | | | $ | 385 | | | $ | 865 | | | $ | 561 | | | $ | 304 | |
| | | | | | | | | | | | 65 | % | | | | | | | | | | | 54 | % |
Grant revenues of $158,365 and $218,911 during the six months ended June 30, 2016 and 2015, respectively, consisted entirely of revenue from the Phase II SBIR grant from the US Department of Energy. This Phase II SBIR was a grant awarded from the DOE as a follow-on for continued development of the materials investigated under the Phase I program entitled “Low-cost, High-Energy Si/Graphene Anodes for Li-Ion Batteries.” This award was for a total of $999,899 to fund a research project over a planned two-year period commencing in January 2014 and originally expiring December 2015. A no cost contract extension had been approved with a new expiration date of June 22, 2016. The grant has been billed in full and is now considered completed. All grant revenues are recorded as time and expenses are incurred according to the grant contracts.
Cost of Goods Sold
We use a standard cost system to estimate the direct costs of products sold. Direct costs include estimates of raw material costs, packaging, freight charges net of those billed to customers, and an allocation for direct labor and manufacturing overhead. Because of the nature of our production processes, there is a substantial fixed manufacturing expense requirement that represents the ongoing cost of maintaining production facilities that are not directly related to products sold, so we use a “full capacity” allocation of overhead based on an estimate of what product costs would be if the manufacturing facilities were operating on a full-time basis and producing products at the designed capacity. This estimate involves estimating both the level of expenses as well as production amounts as if the manufacturing facility were operating on a continuous, three-shift, production basis.
The following table shows the relationship of direct costs to product sales for the three and six months ended June 30, 2016 and 2015:
| Gross Profit Summary | | For the Three Months Ended June 30, | | | | | | For the Six Months Ended June 30, | | | | |
| | | 2016 | | | 2015 | | | Change | | | 2016 | | | 2015 | | | Change | |
| | | | | | | | | | | | | | | | | | | |
| Product Sales | | $ | 82,035 | | | $ | 81,008 | | | $ | 1,027 | | | $ | 141,777 | | | $ | 118,313 | | | $ | 23,464 | |
| Direct Costs | | | 25,020 | | | | 30,667 | | | | (5,647 | ) | | | 57,052 | | | | 52,668 | | | | 4,384 | |
| Direct Cost Margin | | $ | 57,015 | | | $ | 50,341 | | | $ | (4,620 | ) | | $ | 84,725 | | | $ | 65,645 | | | $ | 27,850 | |
| % of Sales | | | 69.5 | % | | | 62.1 | % | | | | | | | 59.8 | % | | | 55.5 | % | | | | |
| Unallocated Manufacturing Expense | | | 404,232 | | | | 418,453 | | | | (14,221 | ) | | | 747,947 | | | | 816,560 | | | | (68,613 | ) |
| Gross Loss on Product Sales | | $ | (347,217 | ) | | $ | (368,112 | ) | | $ | 20,895 | | | $ | (663,222 | ) | | $ | (750,915 | ) | | $ | 87,693 | |
We believe that the fluctuations in direct cost from period to period are not indicative of overall performance or of future margins because of the relatively small size of our sales in comparison to our future expectations. Direct costs vary depending on the size of an order, the specific products being ordered, and other factors like shipping destination.
Costs associated with grant revenues tend to be a mixture of facilities use, management time, labor from scientists, technicians and manufacturing personnel, and some supplies. Because of the difficulty of developing and maintaining an administrative system to gather direct costs for grants, together with the relatively small size of grant revenues, we do not track direct costs for grant revenues as a separate cost category. Therefore, we do not calculate direct cost margins associated with grant revenues but, rather, we view these revenues as being supported by indirect corporate expenses.
Costs associated with licensing revenue tend to be a mixture of IP costs as well as management and administrative expenses that are indirect in nature. As such, we do not assign direct costs to licensing revenues. Where revenues from a license agreement can be assigned to specific product revenues, we classify these revenues as product sales and, using our standard cost system, assign direct costs to those sales.
The remaining “non-direct” costs of operating our manufacturing facilities are recorded as unallocated manufacturing expenses. These expenses include personnel costs, rent, utilities, indirect supplies, depreciation, and related indirect expenses. Unallocated manufacturing expenses are expensed as incurred. We allocate these costs as direct product costs on the basis of the proportion of these expenses that would be representative product costs if we were operating our factory at full capacity.
For the six months ended June 30, 2016, unallocated manufacturing expenses decreased by $68,613 or 8.4% from the comparable period in 2015. The largest part of this decrease was due to decreased payroll, tax and benefit costs as compared with the prior year.
Research and Development Expenses
Research and development expenses totaled $635,357 for the six months ended June 30, 2016. This is a decrease of $140,630 or 18% from the previous year. The largest part of this decrease was due to reduced payroll, tax and benefit costs as compared with the prior year.
Selling, General and Administrative Expenses
During the first six months of 2016 we incurred selling, general and administrative expenses (SGA) of $1.7 million. This is a decrease of $414,368 or 19% from the same period in the prior year. This decrease was driven by a reduction in payroll and related expenses as well as a reduction in professional fees. Common stock issuance costs incurred in 2016 for legal, accounting, and other fees were $336,600 and these expenses are recorded as an offset to proceeds as part of additional paid in capital on the balance sheet. As we continue to grow and gain traction in the marketplace our SGA expenses will fluctuate but should stabilize and become more fixed in nature as we achieve economies of scale.
Other Income (Expense)
The following table shows a comparison of other income and expense by major component for the three and six months ended June 30, 2016 and 2015:
| Other Income (Expense) | | For the Three Months Ended June 30, | | | | | | For the Six Months Ended June 30, | | | | |
| | | 2016 | | | 2015 | | | Change | | | 2016 | | | 2015 | | | Change | |
| | | | | | | | | | | | | | | | | | | |
| Incentive Refund & Interest Income | | $ | 24,073 | | | $ | 27,173 | | | $ | (3,100 | ) | | $ | 48,224 | | | $ | 51,628 | | | $ | (3,404 | ) |
| Interest Expense | | | (101,196 | ) | | | (545,858 | ) | | | 444,662 | | | | (184,997 | ) | | | (1,056,685 | ) | | | 871,688 | |
| Gain from change in fair value of derivative liability - warrants | | | 142,848 | | | | 174,715 | | | | (31,867 | ) | | | 232,613 | | | | 271,014 | | | | (38,401 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total | | $ | 65,725 | | | $ | (343,970 | ) | | $ | 409,695 | | | $ | 95,840 | | | $ | (734,043 | ) | | $ | 829,883 | |
Interest expense for the three and six months ended June 30, 2016 was substantially lower than the same period(s) prior year because the convertible notes outstanding in 2015 were converted to stock on December 31, 2015.
Cash Flow Summary
The following condensed cash flow statement compares cash flow from operating, investing and financing activities for the six months ended June 30, 2016 and 2015. Net cash used by operating activities decreased by 46% during the six months ended June 30, 2016 as compared to the same period in 2015, because of reduced operating expenses, as discussed above.
| | | Six Months Ended June 30 | | | Change 2015 - 2016 | |
| | | 2016 | | | 2015 | | | $ | | | % | |
| Cash, beginning of period | | $ | 1,060,224 | | | $ | 2,088,866 | | | $ | (1,028,642 | ) | | | (49.2 | ) |
| Net Cash provided (used) by: | | | | | | | | | | | | | | | | |
| Operating activities | | | (1,777,826 | ) | | | (3,280,731 | ) | | | 1,502,905 | | | | (45.8 | ) |
| Investing Activities | | | (92,961 | ) | | | (131,907 | ) | | | (38,946 | ) | | | (29.5 | ) |
| Financing Activities | | | 1,770,350 | | | | 4,307,204 | | | | (2,536,854 | ) | | | (58.9 | ) |
| Net increase (decrease) in cash | | | (100,437 | ) | | | 894,566 | | | | (995,003 | ) | | | (111.2 | ) |
| Cash, end of period | | $ | 959,787 | | | $ | 2,983,432 | | | $ | (2,023,645 | ) | | | (67.8 | ) |
Investment activities for the six months ended June 30, 2016 included net capital expenditures for the purchase of property and equipment of $39,314 and $53,647 for intellectual property as compared with $108,478 for property and equipment and $23,429 for intellectual property during the corresponding period in 2015. These levels of capital expenditures are significantly lower than expected in the future as we begin to ramp up our production capacity to meet customer orders. Therefore, these expenditures should not be interpreted as indicative of future expenditures in this area.
Liquidity and Capital Expenditures
Ongoing cash flow from operations has been negative throughout our history. In addition, we have invested significant amounts in research and manufacturing capabilities. We anticipate that we will need to invest further to support ongoing operations for a minimum of another one to two years. We believe that cash on hand of $959,787 as of June 30, 2016 is sufficient to finance our business through the end of September 2016. Our registration statement on Form S-1 for our IPO was declared effective on April 13, 2016, and we are now in the process of conducting our IPO. We expect that our cash on hand at June 30, 2016, of $959,787 and proceeds from our initial public offering of common stock (“IPO”) will sustain our operations for the next twelve months. Our plan is also to further develop customer relationships and increase our revenues derived from our products and IP licensing.
The Company’s financial projections show that the Company may need to raise an additional $15,000,000 or more before it is capable of achieving sustainable cash flow from operations. We intend that the primary means for raising such funds will be through our currently ongoing IPO. However, we cannot make any assurance that we will be able to raise these funds or that the terms and conditions of future financing will be workable or acceptable for the Company and its shareholders.
We also currently forecast a need for additional capital expenditures to execute on our business plan. The amount and timing of such expenditures will be dependent on the timing and magnitude of sales orders received from customers, but we currently anticipate a need to invest approximately $1,000,000 to $6,000,000 during the next twelve months. We plan to fund these investments through a mixture of capital lease financing, debt financing, and from the proceeds of our IPO. If we are unable to obtain such financing, we may be forced to curtail our investment activities and this may limit our ability to grow our revenues as fast as we would like.
Additionally, we anticipate that as our sales increase we will need to invest funds in working capital to support additional inventory and accounts receivable. Taken together, these multiple cash requirements will likely exceed our cash flow from operations for at least one to two more years.
Although there can be no guarantee of successful funding in the future, we intend to pursue the sale of additional equity securities, as well as additional debt or lease financing in the future, until such time as ongoing revenues allow us to generate a positive monthly cash flow from the business sufficient to cover our cash requirements.
In the event we are unable to fund our operations from existing cash on hand, operating cash flows, or additional debt or equity capital, we may be forced to reduce our expenses by curtailing operations, slow down our growth rate, or discontinue operations. Our condensed consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should we be unable to continue as a going concern.
Critical Accounting Estimates
In preparing the consolidated financial statements in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”), we have adopted various accounting policies. Our most significant accounting policies are disclosed in Note 2 to the consolidated financial statements included in our registration statement on Form S-1 for the year ended December 31 2015, which became effective April 13, 2016.
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires us to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Our estimates and assumptions, including those related to inventories, intangible assets, property, plant and equipment, legal proceedings, research and development, warranty obligations, product liability, fair valued liabilities, sales returns and discounts, and income taxes are updated as appropriate, which in most cases is at least quarterly. We base our estimates on historical experience, or various judgements about the reported values of assets, liabilities, revenues and expenses. Actual results may materially differ from these estimates.
Item 3. Quantitative and Qualitative Disclosures about Market Risk
Smaller reporting companies are not required to provide this information.
Item 4. Controls and Procedures
(a)Evaluation of disclosure controls and procedures. At the conclusion of the period ended June 30, 2016, we carried out an evaluation, under the supervision and with the participation of our management, including our Principal Executive Officer/Principal Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934 (the “Exchange Act”)). Based upon that evaluation, our Principal Executive Officer/Principal Financial Officer concluded that as of June 30, 2016, our disclosure controls and procedures were effective and adequately designed to ensure that the information required to be disclosed by us in the reports we submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the applicable rules and forms and that such information was accumulated and communicated to our Principal Executive Officer/Principal Financial Officer, in a manner that allowed for timely decisions regarding required disclosure.
We do not expect that our disclosure controls and procedures will prevent all errors and all instances of fraud. Disclosure controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the disclosure controls and procedures are met. Further, the design of disclosure controls and procedures must reflect the fact that there are resource constraints, and the benefits must be considered relative to their costs. Because of the inherent limitations in all disclosure controls and procedures, no evaluation of disclosure controls and procedures can provide absolute assurance that we have detected all our control deficiencies and instances of fraud, if any. The design of disclosure controls and procedures also is based partly on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions.
(b)Changes in internal controls. There were no changes in our internal control over financial reporting that occurred during our last fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II - OTHER INFORMATION
Item 1. Legal Proceedings.
Beginning in 2014, POSCO, one of our licensees and a shareholder, has had a contractual obligation to pay us a minimum fee of $100,000 per year to license certain technologies. This obligation is due annually on February 28 of the following year. We record this license revenue at a rate of $25,000 per quarter. POSCO is disputing that they are obligated to pay the royalties. A petition for arbitration has been filed for this matter by the Company on March 9, 2016. On July 7 we received a letter from the International Court of Arbitration and they have assigned an arbitrator to the case. No assessment or decision has made by the arbitrator as of the issuance date of this report.
Item 1A. Risk Factors.
Smaller reporting companies are not required to provide this information.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
During the three months ended June 30, 2016, we issued 8% promissory notes in an aggregate amount of $44,750 which mature on December 31, 2016 and issued 5 year warrants to purchase 895 shares of common stock having a strike price of $10.00 per share.
No underwriters were utilized and no commissions or fees were paid with respect to any of the above transactions. These persons were the only offerees in connection with these transactions. We relied on Section 4(a)(2) and/or Rule 506 of Regulation D of the Securities Act for the transactions set forth above since none of the transactions involved any public offering.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
None.
Item 5. Other Information.
None.
Item 6. Exhibits.
EXHIBIT
NUMBER | | DESCRIPTION | | LOCATION |
| | | | | |
31.1 | | Certifications of the Chief Executive Officer and Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | | Filed herewith |
| | | | | |
| 32.1 | | Certification Pursuant To 18 U.S.C. Section 1350, As Adopted Pursuant To Section 906 of the Sarbanes-Oxley Act Of 2002* | | Furnished herewith |
| | | | | |
| 101. INS | | XBRL Instance Document | | Filed herewith |
| | | | | |
| 101. CAL | | XBRL Taxonomy Extension Calculation Link base Document | | Filed herewith |
| | | | | |
| 101. DEF | | XBRL Taxonomy Extension Definition Link base Document | | Filed herewith |
| | | | | |
| 101. LAB | | XBRL Taxonomy Label Link base Document | | Filed herewith |
| | | | | |
| 101. PRE | | XBRL Extension Presentation Link base Document | | Filed herewith |
| | | | | |
| 101. SCH | | XBRL Taxonomy Extension Scheme Document | | Filed herewith |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this Quarterly Report on Form 10-Q to be signed on its behalf by the undersigned, thereunto duly authorized.
XG SCIENCES, INC.
| Dated: August 9, 2016 | By: | | /s/ Philip L. Rose |
| | Name: | | Philip L. Rose |
| | Title: | | Chief Executive Officer, President,
Treasurer, Principal Executive Officer and
Principal Financial Officer |
| | | | |
| Dated: August 9, 2016 | By: | | /s/ Corinne Lyon |
| | Name: | | Corinne Lyon |
| | Title: | | Controller and Principal Accounting Officer |


