
LEADERSHIP THROUGH INNOVATION J.P. Morgan 37th Annual Healthcare Conference January 2019 Exhibit 99.2

SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS In addition to historical information, this presentation contains forward-looking statements with respect to our business, capital resources, strategic initiatives and growth reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, including regarding continuing adoption of, and interest in, Senza in the U.S. and international markets; our beliefs regarding market size and share for Senza; our beliefs regarding the advantages of Senza and HF10 therapy, including additional opportunities around our clinical efforts and potential indication expansion; and our expectations regarding our commercialization efforts. These forward-looking statements are based upon information that is currently available to us or our current expectations, speak only as of the date hereof, and are subject to numerous risks and uncertainties, including our ability to continue to successfully commercialize our products; our ability to manufacture our products to meet demand; the level and availability of third-party payor reimbursement for our products; our ability to effectively manage our anticipated growth; our ability to protect our intellectual property rights and proprietary technologies; our ability to operate our business without infringing the intellectual property rights and proprietary technology of third parties; competition in our industry; additional capital and credit availability; our ability to attract and retain qualified personnel; and product liability claims. These factors, together with those that are described in greater detail in our Annual Report on Form 10-K filed on February 22, 2018 and our Quarterly Report on Form 10-Q filed on November 5, 2018, as well as any reports that we may file with the Securities and Exchange Commission in the future, may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements. We expressly disclaim any obligation, except as required by law, or undertaking to update or revise any such forward-looking statements. Forward-Looking Statements
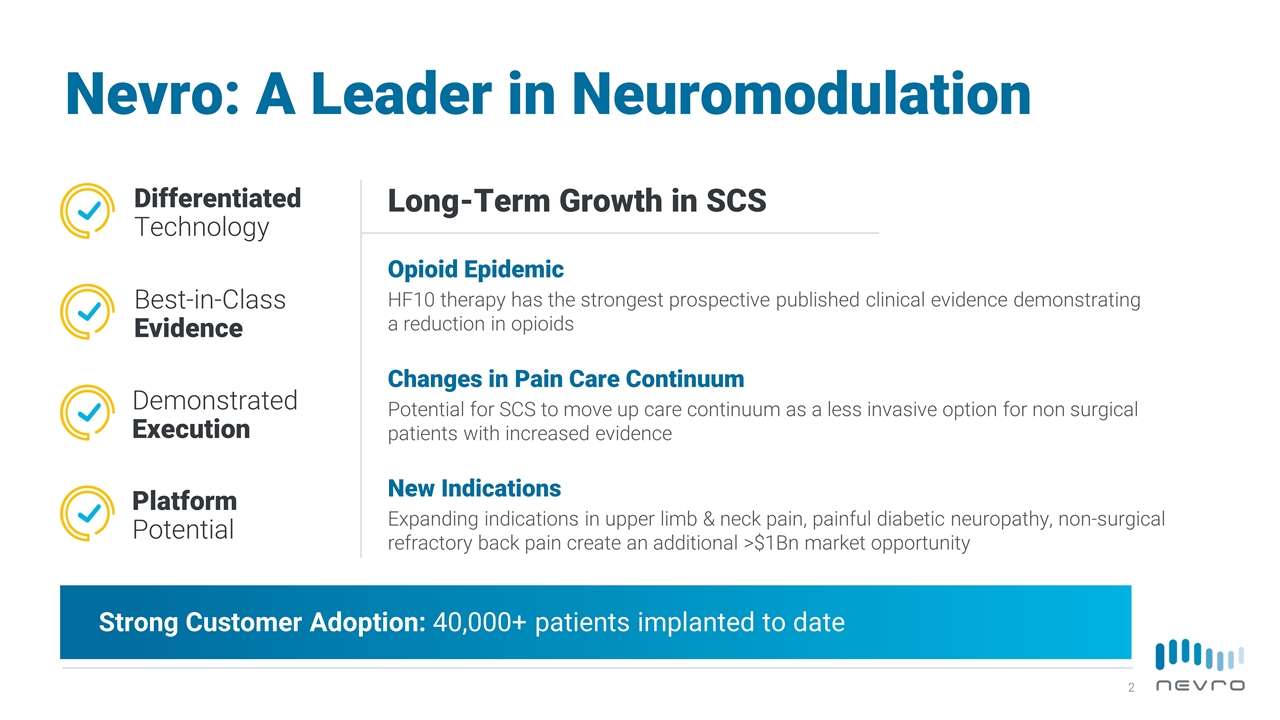
Nevro: A Leader in Neuromodulation Long-Term Growth in SCS Opioid Epidemic HF10 therapy has the strongest prospective published clinical evidence demonstrating a reduction in opioids Changes in Pain Care Continuum Potential for SCS to move up care continuum as a less invasive option for non surgical patients with increased evidence Differentiated Technology Best-in-Class Evidence Demonstrated Execution Platform Potential New Indications Expanding indications in upper limb & neck pain, painful diabetic neuropathy, non-surgical refractory back pain create an additional >$1Bn market opportunity Strong Customer Adoption: 40,000+ patients implanted to date
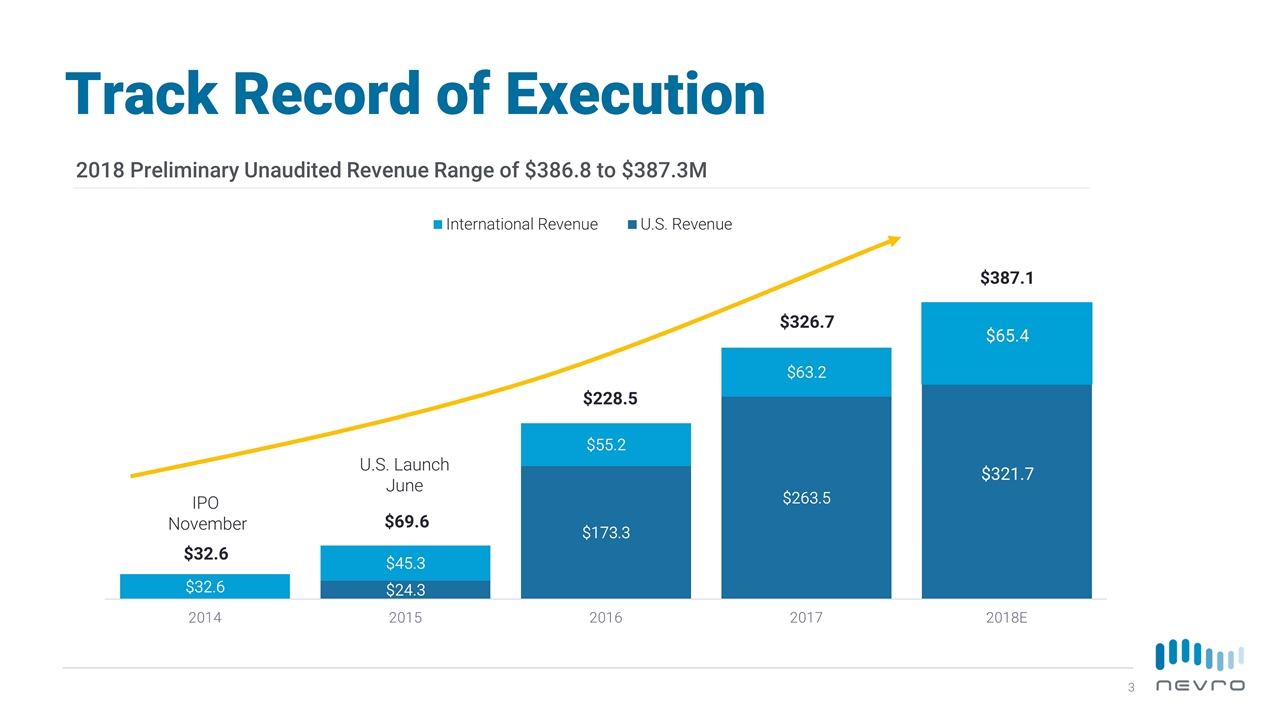
[13] Consecutive Quarters of Double-Digit Growth 2018 Preliminary Unaudited Revenue Range of $386.8 to $387.3M $321.7 $32.6 $387.1 IPO November U.S. Launch June $69.6 $228.5 $326.7 $65.4 Track Record of Execution
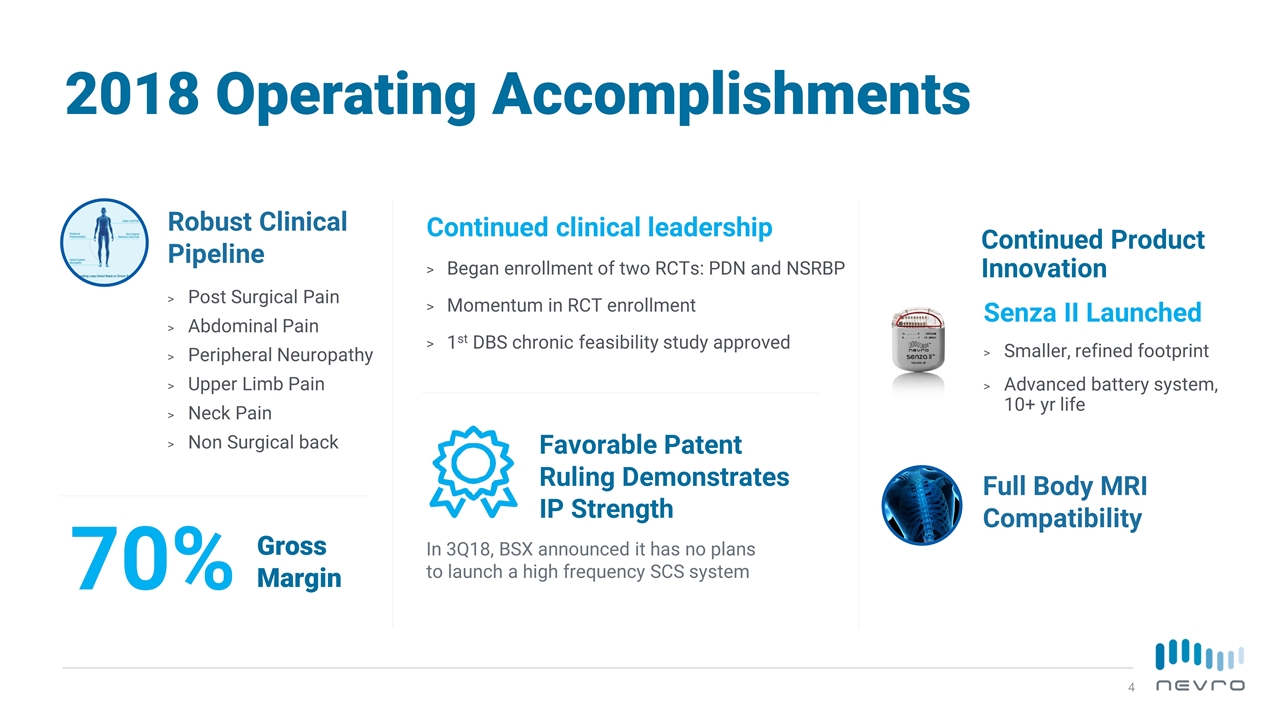
Continued Product Innovation Senza II Launched Smaller, refined footprint Advanced battery system, 10+ yr life Gross Margin Favorable Patent Ruling Demonstrates IP Strength In 3Q18, BSX announced it has no plans to launch a high frequency SCS system Continued clinical leadership Began enrollment of two RCTs: PDN and NSRBP Momentum in RCT enrollment 1st DBS chronic feasibility study approved 2018 Operating Accomplishments Robust Clinical Pipeline Post Surgical Pain Abdominal Pain Peripheral Neuropathy Upper Limb Pain Neck Pain Non Surgical back Full Body MRI Compatibility 70%

Innovation Leadership Clinical Leadership Commercial Scale Positioned for Market Leadership 3 2 1

Innovation Leadership Best-in-Class Therapy Operates with Different Mechanism of Action Value Across Stakeholders Cadence of Innovation
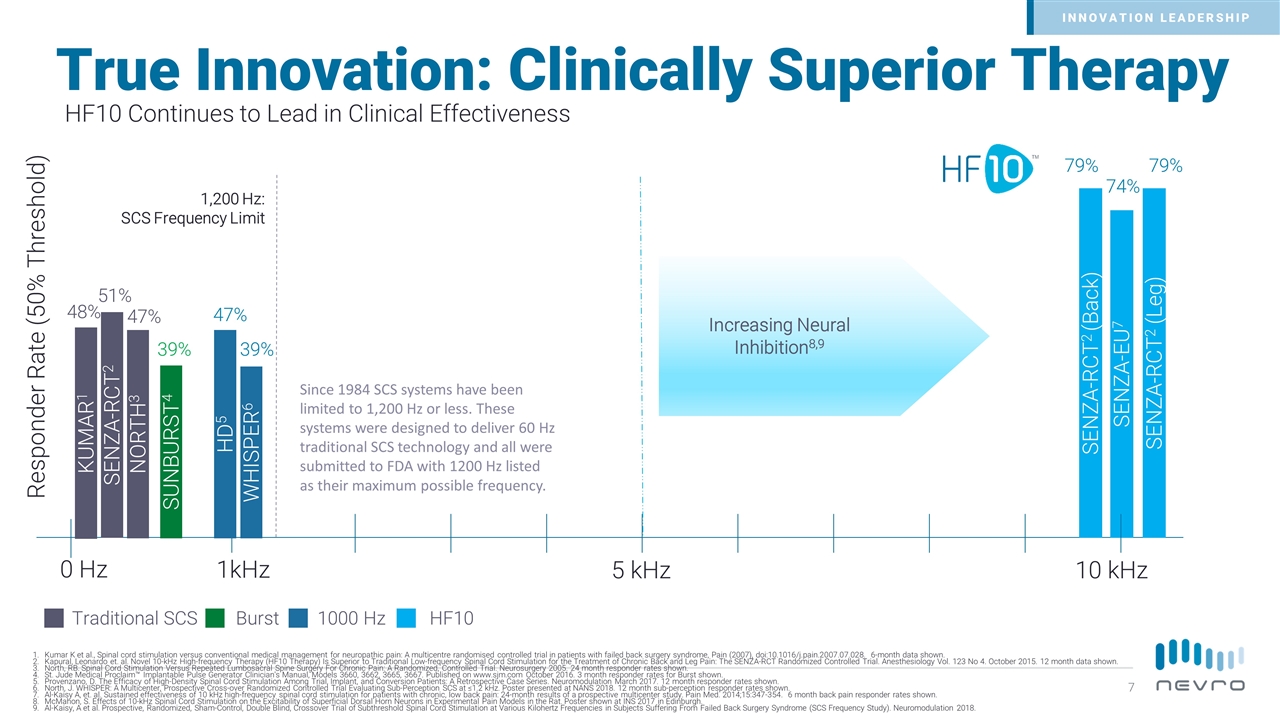
True Innovation: Clinically Superior Therapy SENZA-RCT2 (Leg) SENZA-EU7 SENZA-RCT2 (Back) Traditional SCS WHISPER6 SUNBURST4 39% 39% 79% 74% 79% KUMAR1 SENZA-RCT2 48% 51% 10 kHz 1kHz HD5 47% 1,200 Hz: SCS Frequency Limit Responder Rate (50% Threshold) 5 kHz Burst 1000 Hz 0 Hz Kumar K et al., Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome, Pain (2007), doi:10.1016/j.pain.2007.07.028. 6-month data shown. Kapural, Leonardo et. al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) Is Superior to Traditional Low-frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: The SENZA-RCT Randomized Controlled Trial. Anesthesiology Vol. 123 No 4. October 2015. 12 month data shown. North, RB. Spinal Cord Stimulation Versus Repeated Lumbosacral Spine Surgery For Chronic Pain: A Randomized, Controlled Trial. Neurosurgery 2005. 24 month responder rates shown. St. Jude Medical Proclaim™ Implantable Pulse Generator Clinician's Manual, Models 3660, 3662, 3665, 3667. Published on www.sjm.com October 2016. 3 month responder rates for Burst shown. Provenzano, D. The Efficacy of High-Density Spinal Cord Stimulation Among Trial, Implant, and Conversion Patients: A Retrospective Case Series. Neuromodulation March 2017. 12 month responder rates shown. North, J. WHISPER: A Multicenter, Prospective Cross-over Randomized Controlled Trial Evaluating Sub-Perception SCS at ≤1.2 kHz. Poster presented at NANS 2018. 12 month sub-perception responder rates shown. Al-Kaisy A, et. al. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med. 2014;15:347-354. 6 month back pain responder rates shown. McMahon, S. Effects of 10-kHz Spinal Cord Stimulation on the Excitability of Superficial Dorsal Horn Neurons in Experimental Pain Models in the Rat. Poster shown at INS 2017 in Edinburgh. Al-Kaisy, A et al. Prospective, Randomized, Sham-Control, Double Blind, Crossover Trial of Subthreshold Spinal Cord Stimulation at Various Kilohertz Frequencies in Subjects Suffering From Failed Back Surgery Syndrome (SCS Frequency Study). Neuromodulation 2018. Increasing Neural Inhibition8,9 HF10 Since 1984 SCS systems have been limited to 1,200 Hz or less. These systems were designed to deliver 60 Hz traditional SCS technology and all were submitted to FDA with 1200 Hz listed as their maximum possible frequency. HF10 Continues to Lead in Clinical Effectiveness NORTH3 47% INNOVATION LEADERSHIP
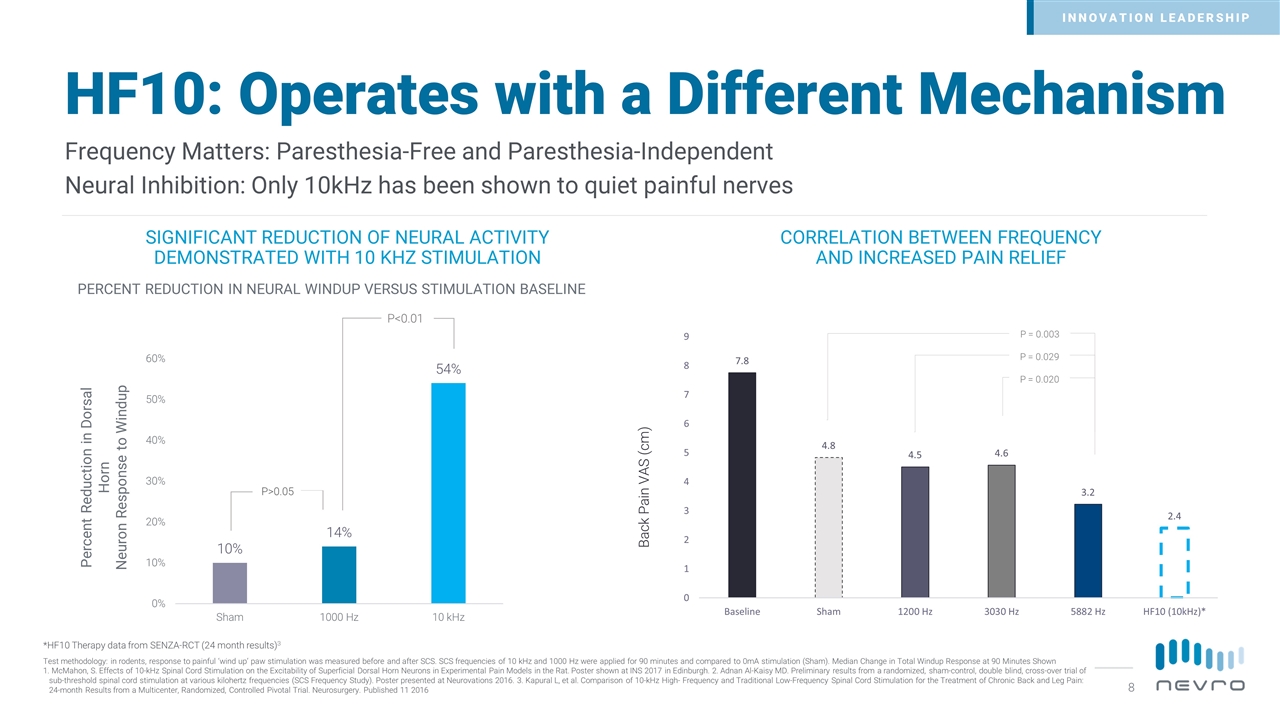
HF10: Operates with a Different Mechanism SIGNIFICANT REDUCTION OF NEURAL ACTIVITY DEMONSTRATED WITH 10 KHZ STIMULATION CORRELATION BETWEEN FREQUENCY AND INCREASED PAIN RELIEF Frequency Matters: Paresthesia-Free and Paresthesia-Independent Neural Inhibition: Only 10kHz has been shown to quiet painful nerves Percent Reduction in Dorsal Horn Neuron Response to Windup P>0.05 P<0.01 PERCENT REDUCTION IN NEURAL WINDUP VERSUS STIMULATION BASELINE *HF10 Therapy data from SENZA-RCT (24 month results)3 Back Pain VAS (cm) P = 0.020 P = 0.003 P = 0.029 Test methodology: in rodents, response to painful ‘wind up’ paw stimulation was measured before and after SCS. SCS frequencies of 10 kHz and 1000 Hz were applied for 90 minutes and compared to 0mA stimulation (Sham). Median Change in Total Windup Response at 90 Minutes Shown 1. McMahon, S. Effects of 10-kHz Spinal Cord Stimulation on the Excitability of Superficial Dorsal Horn Neurons in Experimental Pain Models in the Rat. Poster shown at INS 2017 in Edinburgh. 2. Adnan Al-Kaisy MD. Preliminary results from a randomized, sham-control, double blind, cross-over trial of sub-threshold spinal cord stimulation at various kilohertz frequencies (SCS Frequency Study). Poster presented at Neurovations 2016. 3. Kapural L, et al. Comparison of 10-kHz High- Frequency and Traditional Low-Frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: 24-month Results from a Multicenter, Randomized, Controlled Pivotal Trial. Neurosurgery. Published 11 2016 INNOVATION LEADERSHIP
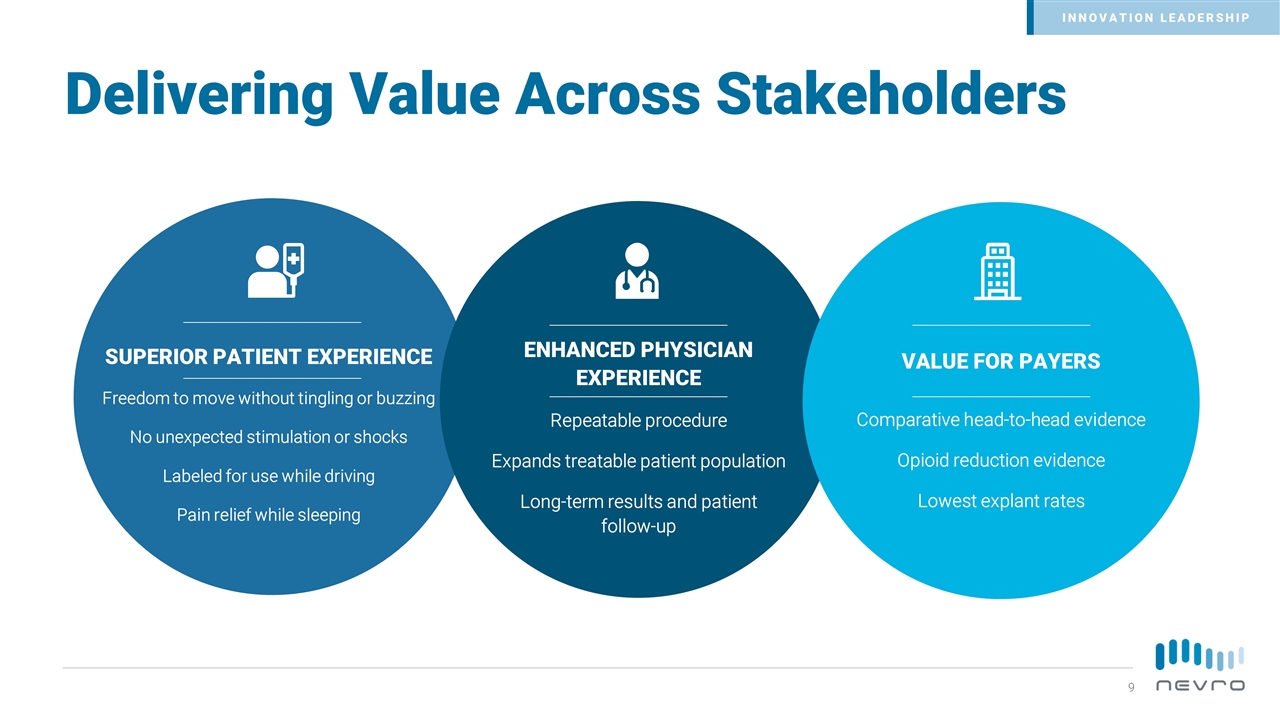
Delivering Value Across Stakeholders SUPERIOR PATIENT EXPERIENCE Freedom to move without tingling or buzzing No unexpected stimulation or shocks Labeled for use while driving Pain relief while sleeping ENHANCED PHYSICIAN EXPERIENCE Repeatable procedure Expands treatable patient population Long-term results and patient follow-up VALUE FOR PAYERS Comparative head-to-head evidence Opioid reduction evidence Lowest explant rates INNOVATION LEADERSHIP
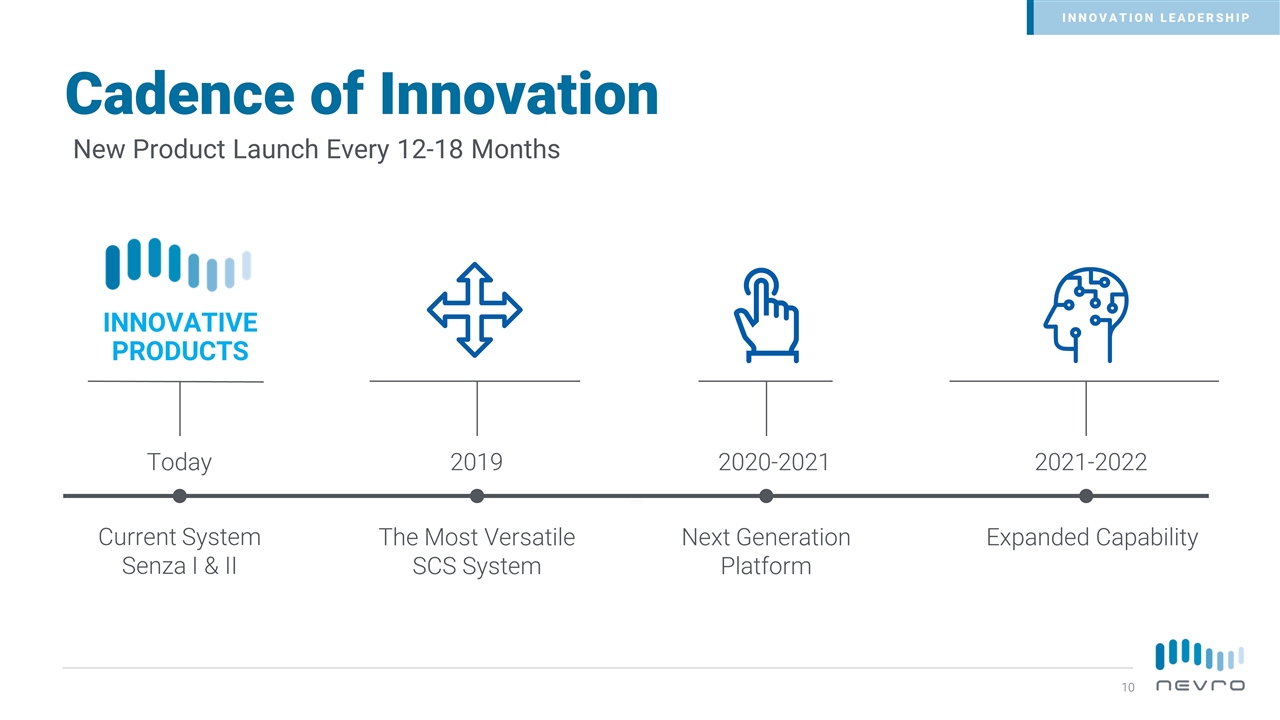
Cadence of Innovation Today 2019 2020-2021 2021-2022 Current System Senza I & II The Most Versatile SCS System Next Generation Platform Expanded Capability INNOVATIVE PRODUCTS INNOVATION LEADERSHIP New Product Launch Every 12-18 Months

Clinical Leadership Clinical Evidence Base Expanding the Scope of Neuromodulation Progress in Pipeline Indications NANS 2019 Preview Focused on Attractive Markets
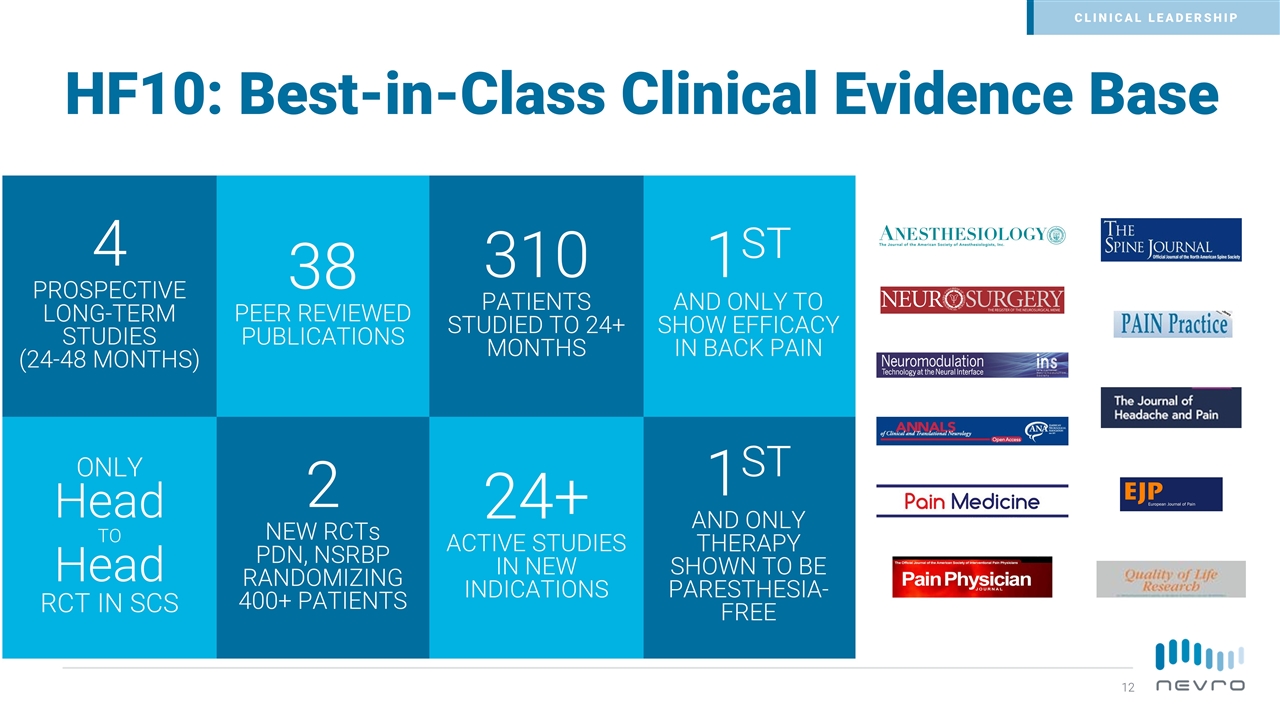
HF10: Best-in-Class Clinical Evidence Base 38 PEER REVIEWED PUBLICATIONS 1ST AND ONLY TO SHOW EFFICACY IN BACK PAIN 4 PROSPECTIVE LONG-TERM STUDIES (24-48 MONTHS) 310 PATIENTS STUDIED TO 24+ MONTHS 2 NEW RCTs PDN, NSRBP RANDOMIZING 400+ PATIENTS ONLY Head TO Head RCT IN SCS 1ST AND ONLY THERAPY SHOWN TO BE PARESTHESIA-FREE 24+ ACTIVE STUDIES IN NEW INDICATIONS CLINICAL LEADERSHIP
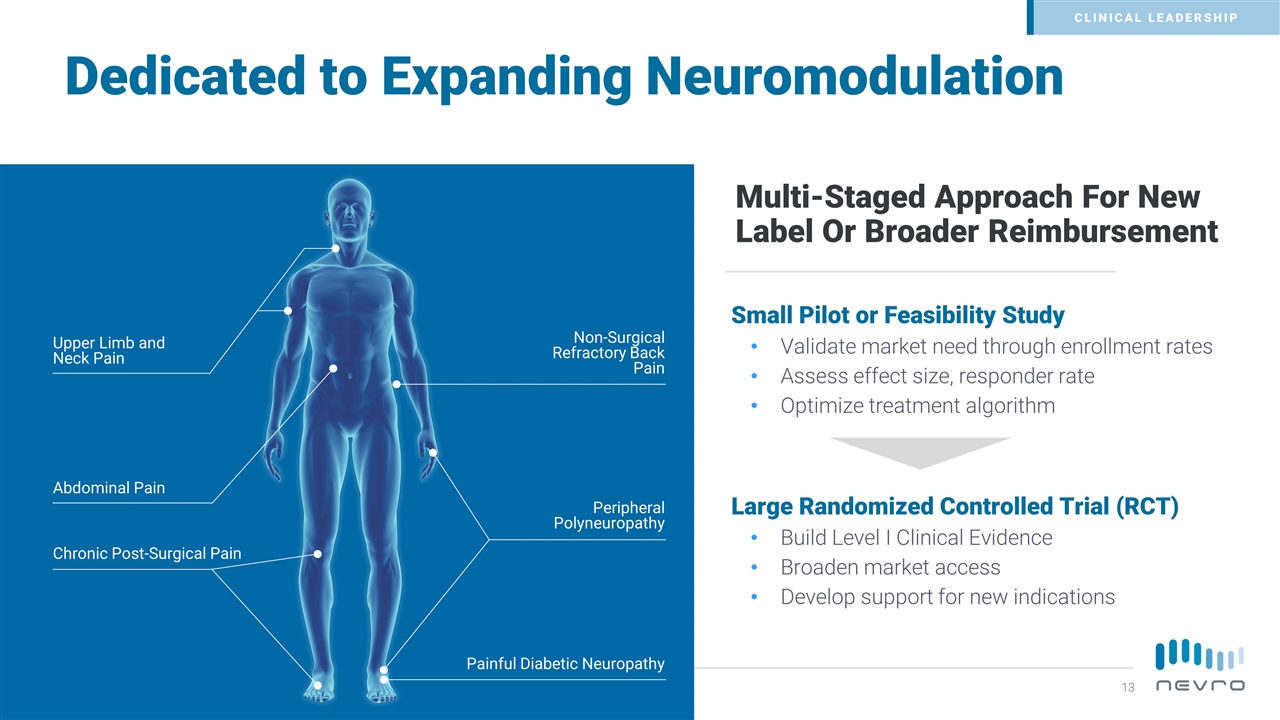
Small Pilot or Feasibility Study Validate market need through enrollment rates Assess effect size, responder rate Optimize treatment algorithm Large Randomized Controlled Trial (RCT) Build Level I Clinical Evidence Broaden market access Develop support for new indications Multi-Staged Approach For New Label Or Broader Reimbursement Upper Limb and Neck Pain Abdominal Pain Chronic Post-Surgical Pain Non-Surgical Refractory Back Pain Peripheral Polyneuropathy Painful Diabetic Neuropathy Dedicated to Expanding Neuromodulation CLINICAL LEADERSHIP
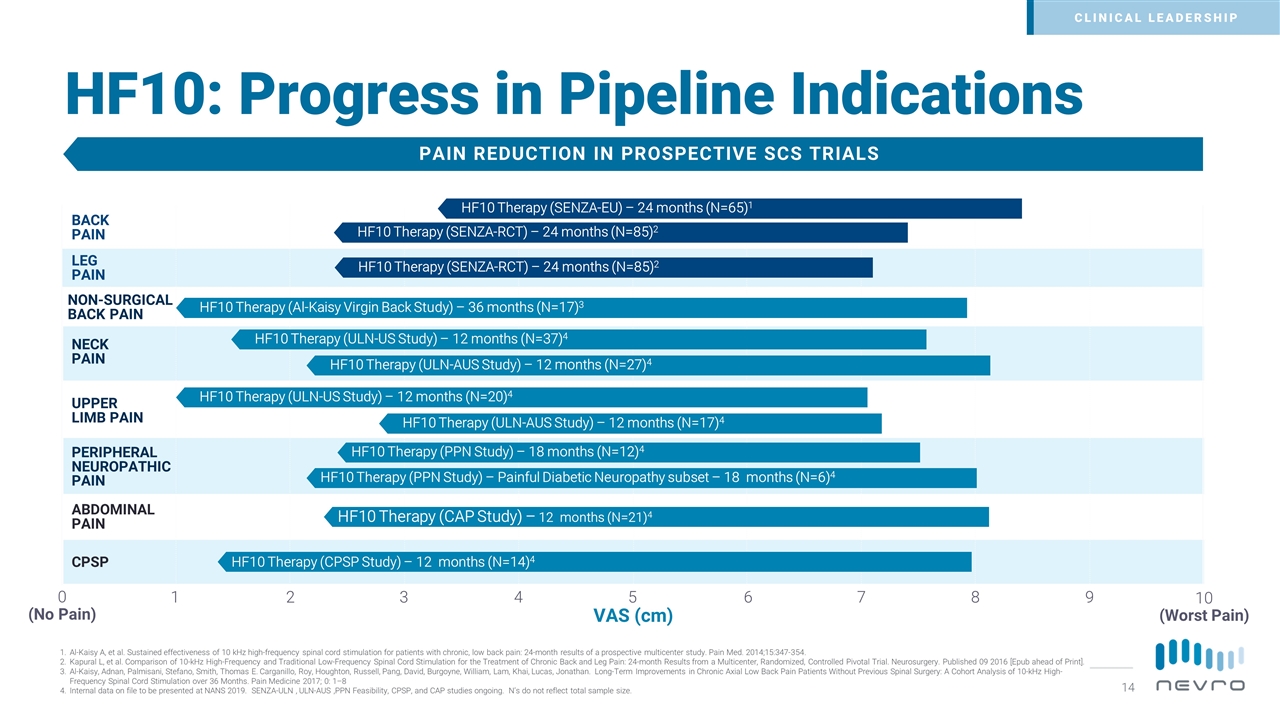
HF10: Progress in Pipeline Indications PAIN REDUCTION IN PROSPECTIVE SCS TRIALS 0 (No Pain) 10 (Worst Pain) 5 1 3 2 6 8 7 9 4 BACK PAIN LEG PAIN PERIPHERAL NEUROPATHIC PAIN VAS (cm) NON-SURGICAL BACK PAIN NECK PAIN UPPER LIMB PAIN ABDOMINAL PAIN CPSP HF10 Therapy (SENZA-EU) – 24 months (N=65)1 HF10 Therapy (SENZA-RCT) – 24 months (N=85)2 HF10 Therapy (SENZA-RCT) – 24 months (N=85)2 HF10 Therapy (PPN Study) – 18 months (N=12)4 HF10 Therapy (Al-Kaisy Virgin Back Study) – 36 months (N=17)3 HF10 Therapy (ULN-US Study) – 12 months (N=37)4 HF10 Therapy (ULN-US Study) – 12 months (N=20)4 HF10 Therapy (PPN Study) – Painful Diabetic Neuropathy subset – 18 months (N=6)4 HF10 Therapy (ULN-AUS Study) – 12 months (N=27)4 HF10 Therapy (ULN-AUS Study) – 12 months (N=17)4 HF10 Therapy (CAP Study) – 12 months (N=21)4 HF10 Therapy (CPSP Study) – 12 months (N=14)4 CLINICAL LEADERSHIP Al-Kaisy A, et al. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med. 2014;15:347-354. Kapural L, et al. Comparison of 10-kHz High-Frequency and Traditional Low-Frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: 24-month Results from a Multicenter, Randomized, Controlled Pivotal Trial. Neurosurgery. Published 09 2016 [Epub ahead of Print]. Al-Kaisy, Adnan, Palmisani, Stefano, Smith, Thomas E. Carganillo, Roy, Houghton, Russell, Pang, David, Burgoyne, William, Lam, Khai, Lucas, Jonathan. Long-Term Improvements in Chronic Axial Low Back Pain Patients Without Previous Spinal Surgery: A Cohort Analysis of 10-kHz High-Frequency Spinal Cord Stimulation over 36 Months. Pain Medicine 2017; 0: 1–8 Internal data on file to be presented at NANS 2019. SENZA-ULN , ULN-AUS ,PPN Feasibility, CPSP, and CAP studies ongoing. N’s do not reflect total sample size.
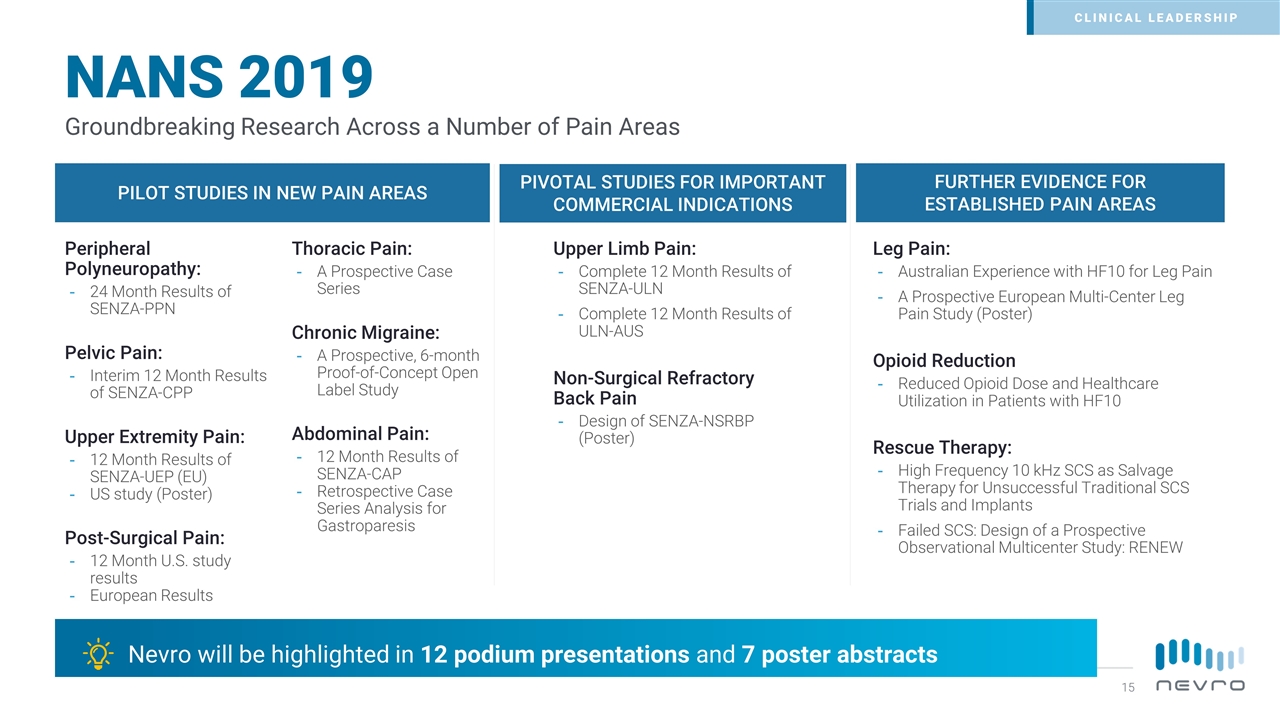
Nevro will be highlighted in 12 podium presentations and 7 poster abstracts PILOT STUDIES IN NEW PAIN AREAS PIVOTAL STUDIES FOR IMPORTANT COMMERCIAL INDICATIONS FURTHER EVIDENCE FOR ESTABLISHED PAIN AREAS Upper Limb Pain: Complete 12 Month Results of SENZA-ULN Complete 12 Month Results of ULN-AUS Non-Surgical Refractory Back Pain Design of SENZA-NSRBP (Poster) Leg Pain: Australian Experience with HF10 for Leg Pain A Prospective European Multi-Center Leg Pain Study (Poster) Opioid Reduction Reduced Opioid Dose and Healthcare Utilization in Patients with HF10 Rescue Therapy: High Frequency 10 kHz SCS as Salvage Therapy for Unsuccessful Traditional SCS Trials and Implants Failed SCS: Design of a Prospective Observational Multicenter Study: RENEW Peripheral Polyneuropathy: 24 Month Results of SENZA-PPN Pelvic Pain: Interim 12 Month Results of SENZA-CPP Upper Extremity Pain: 12 Month Results of SENZA-UEP (EU) US study (Poster) Post-Surgical Pain: 12 Month U.S. study results European Results Thoracic Pain: A Prospective Case Series Chronic Migraine: A Prospective, 6-month Proof-of-Concept Open Label Study Abdominal Pain: 12 Month Results of SENZA-CAP Retrospective Case Series Analysis for Gastroparesis Groundbreaking Research Across a Number of Pain Areas NANS 2019 CLINICAL LEADERSHIP
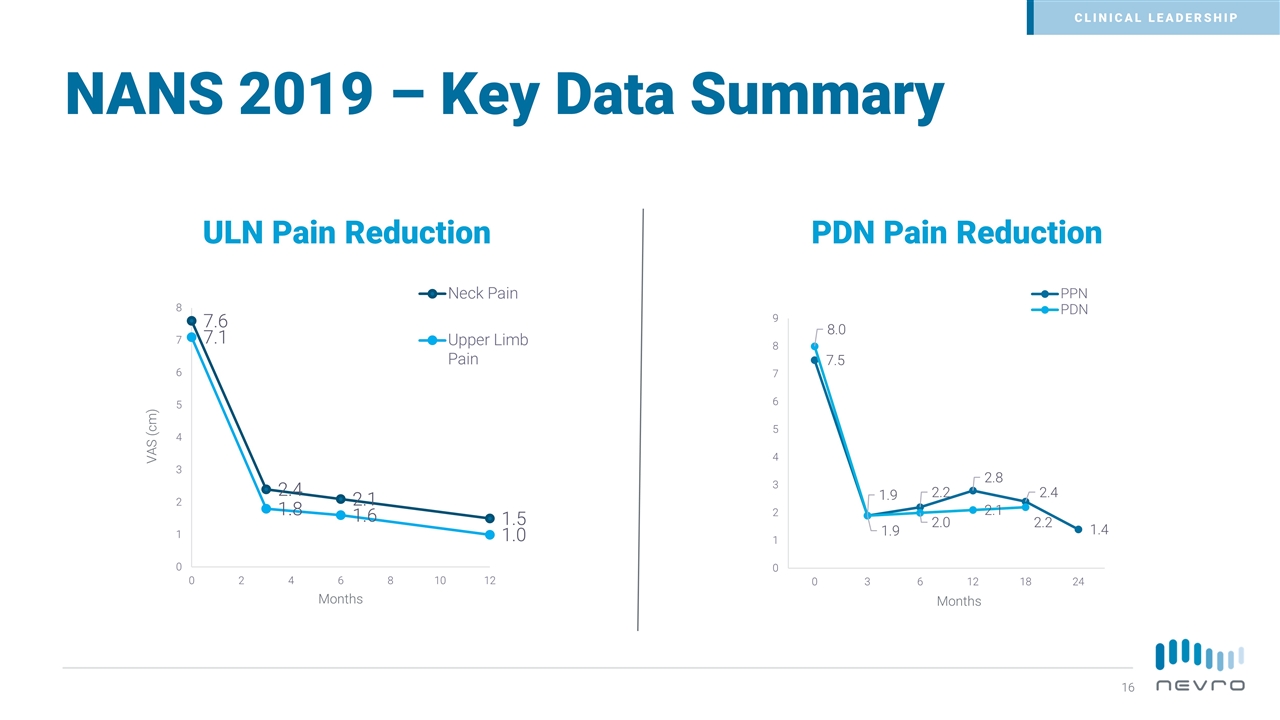
NANS 2019 – Key Data Summary ULN Pain Reduction PDN Pain Reduction CLINICAL LEADERSHIP Months

Focused on Attractive Markets Nevro can address $4B+ market opportunity CONTINUED MARKET EXPANSION IN BACK & LEG Back & Leg Pain EXPANDING PAIN -RELATED INDICATIONS Expanding reimbursement, market development or labeling Addressing significant unmet needs Opioid epidemic Upper Limb & Neck Pain, Painful Diabetic Neuropathy, Non-Surgical Refractory Back Pain, CRPS New sales force and/or new reimbursement codes Demonstration of clinical effectiveness INVESTIGATE BROADER INDICATIONS Abdominal Pain Deep Brain Stimulation CLINICAL LEADERSHIP Today 2019-2021 2022 and Beyond Clinical efficacy for back pain Superior long-term outcomes Translating clinical outcomes commercially Intraoperative efficiencies

Commercial Scale Scaling Organization & Sales Management Improving Account Access Patient Awareness Program New Product Launch

Expanding Sales Capacity and Management to Drive Topline Growth COMMERCIAL SCALE Scaling Commercial Organization Reaccelerated hiring in 2H18 Building sales bench Increasing sales density Improving sales retention Significant expansion of area and regional sales leaders in 2H18
Improving Account Access Penetrate historically difficult to access accounts Streamline selling process for sales team Validate differentiation Upcoming Contracts with Two GPOs Representing A Significant Portion of U.S. SCS Market COMMERCIAL SCALE
Patient Awareness Program Nevro is identifying new well qualified patients and developing HF10 Brand Preference through a comprehensive Direct to Patient Marketing Program Multi-Channel Digital and Social Strategy Introduction of Patients to Provider Network Video Ads Facebook and Google Display Ads Search Engine Marketing Pain Assessment to Qualify Potential Patients Physician Finder Patient Education Website Engagement on Social Media COMMERCIAL SCALE
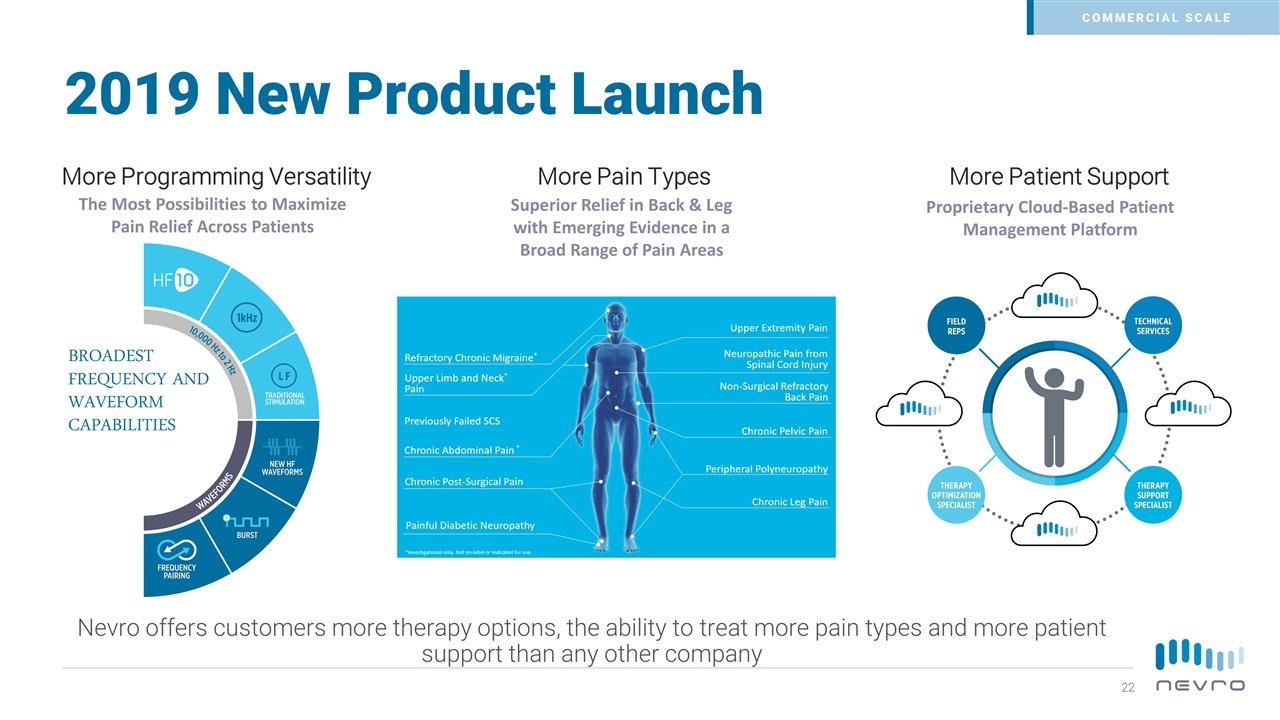
2019 New Product Launch Nevro offers customers more therapy options, the ability to treat more pain types and more patient support than any other company More Programming Versatility More Pain Types More Patient Support Superior Relief in Back & Leg with Emerging Evidence in a Broad Range of Pain Areas Proprietary Cloud-Based Patient Management Platform The Most Possibilities to Maximize Pain Relief Across Patients COMMERCIAL SCALE BROADEST FREQUENCY AND WAVEFORM CAPABILITIES

HF10 Matters Clinical Effectiveness Addressing Opioid Epidemic Lowest Explant Rate Strong Validation from Real-world Results
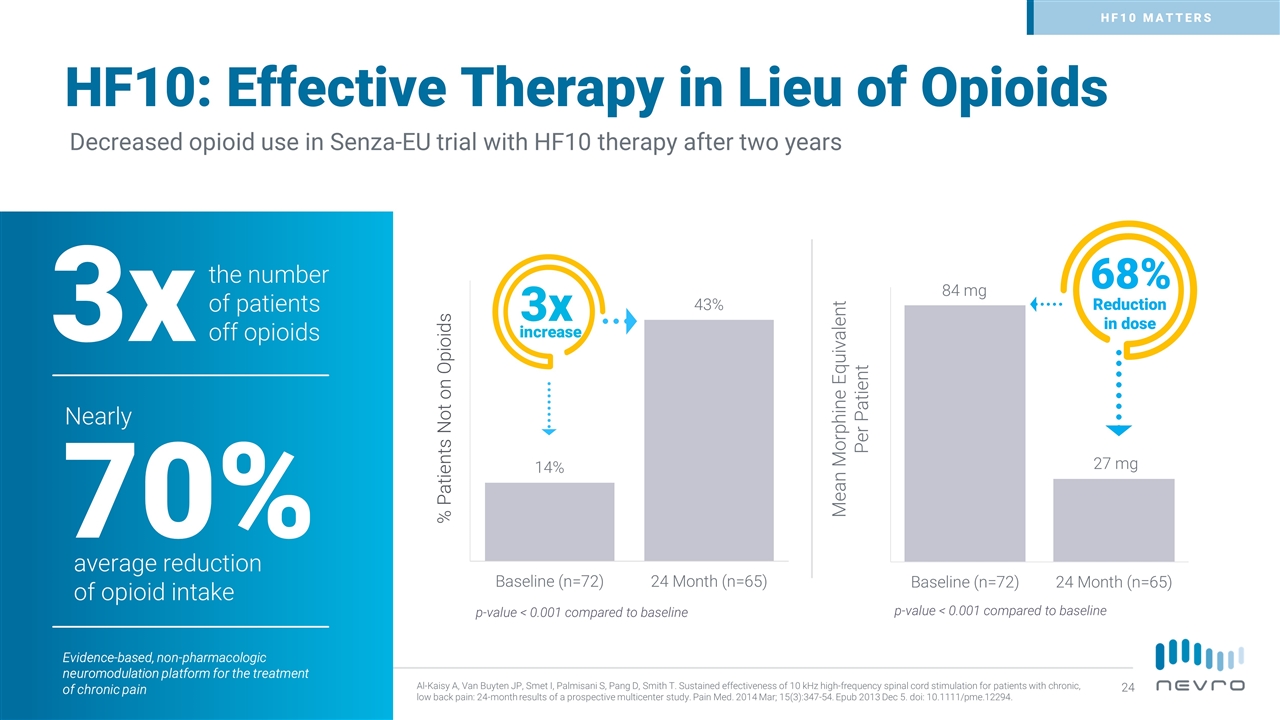
HF10: Effective Therapy in Lieu of Opioids Decreased opioid use in Senza-EU trial with HF10 therapy after two years % Patients Not on Opioids 3x increase 68% Reduction in dose Mean Morphine Equivalent Per Patient the number of patients off opioids 3x average reduction of opioid intake 70% Nearly p-value < 0.001 compared to baseline p-value < 0.001 compared to baseline Al-Kaisy A, Van Buyten JP, Smet I, Palmisani S, Pang D, Smith T. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med. 2014 Mar; 15(3):347-54. Epub 2013 Dec 5. doi: 10.1111/pme.12294. Evidence-based, non-pharmacologic neuromodulation platform for the treatment of chronic pain HF10 MATTERS
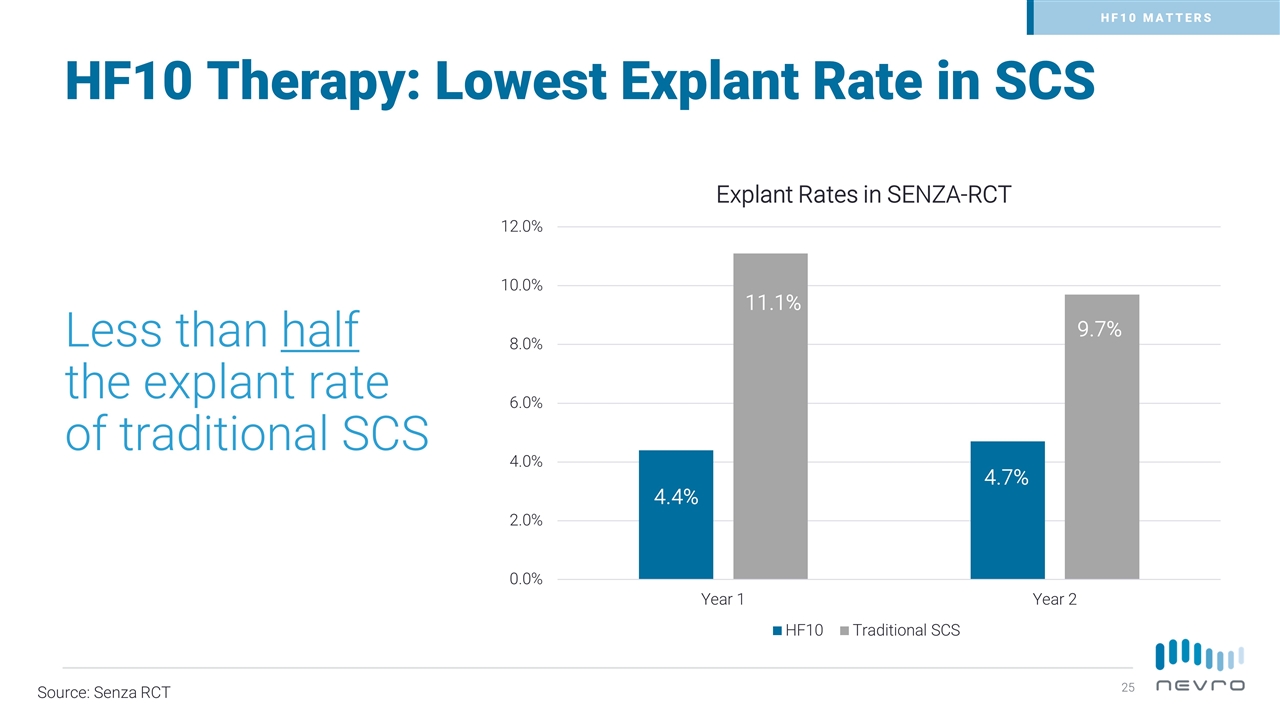
HF10 MATTERS Less than half the explant rate of traditional SCS Source: Senza RCT HF10 Therapy: Lowest Explant Rate in SCS
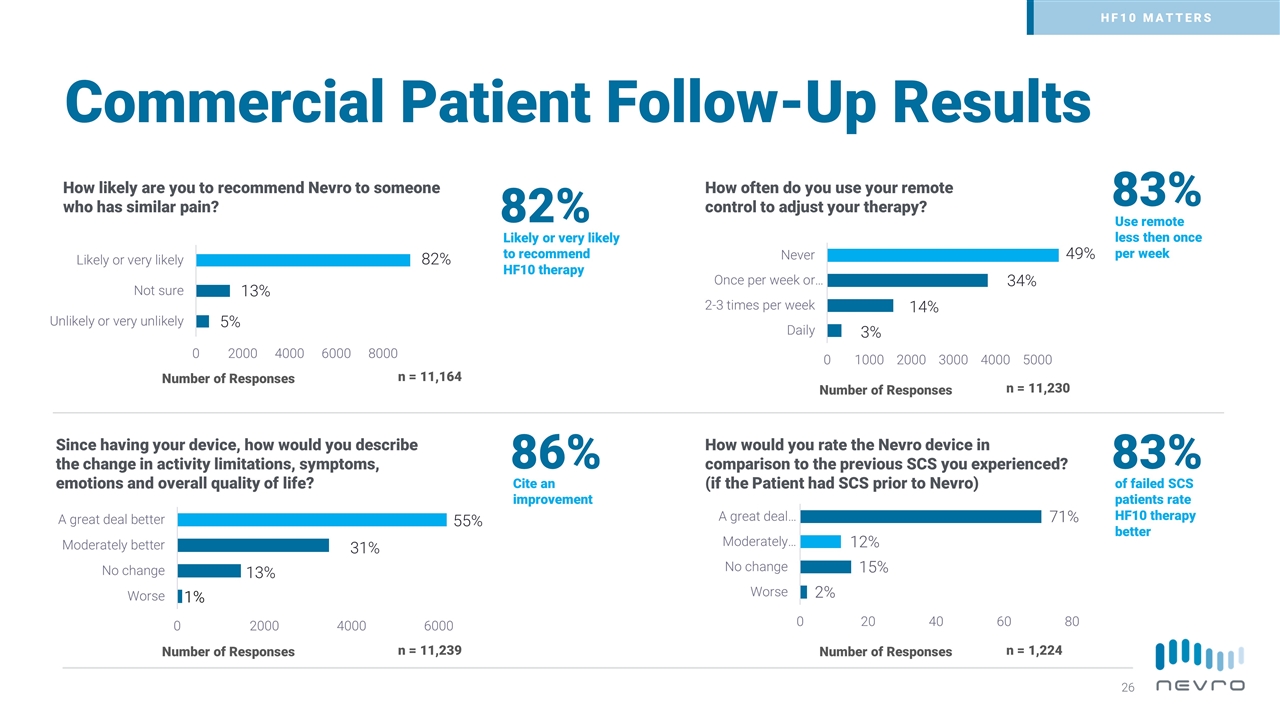
Commercial Patient Follow-Up Results Number of Responses How likely are you to recommend Nevro to someone who has similar pain? n = 11,164 82% Number of Responses Since having your device, how would you describe the change in activity limitations, symptoms, emotions and overall quality of life? How often do you use your remote control to adjust your therapy? n = 11,239 n = 11,230 Number of Responses Likely or very likely to recommend HF10 therapy 86% Cite an improvement 83% Use remote less then once per week How would you rate the Nevro device in comparison to the previous SCS you experienced? (if the Patient had SCS prior to Nevro) 83% of failed SCS patients rate HF10 therapy better n = 1,224 Number of Responses HF10 MATTERS

Transforming Patient Lives: Raymond Raymond, a disabled veteran with significant service-related lower back issues, is finally free from chronic pain and able to be an active father to his young family. About six years after having spinal fusion surgery to relieve his chronic back pain, Raymond began experiencing debilitating nerve pain, numbness in his lower legs and constant lower back pain. “I tried all sorts of different treatments to manage the pain,” Raymond recalled. “At best I was able to make things manageable with a large amount of medication, but that was not sustainable.” Traditional spinal cord stimulation didn’t provide relief. Then Dr. Mehul Shah introduced Raymond to HF10 and scheduled a trial. “After the second day of my trial, I was almost entirely pain-free!” Raymond said. “Now, seven months after my June 2017 implant, I have more than 85% relief from my chronic pain and can finally be the active dad I want to be,” Raymond said. “I may never feel like I am 20-years-old again, but I am very happy with my huge transformation.” Learn More -> https://www.hf10.com/ #HF10Matters #Nevro #ChronicPain Results may vary. Important safety and risk information: https://www.hf10.com/safety HF10 MATTERS

First Acute DBS Case DBS OFF HF10 DBS ON HF10 MATTERS

Building to Drive Long-Term Growth Investing in Commercial Organization Expanding sales capacity and management for broad U.S. coverage, improving account access, increasing patient awareness, and launching new products Innovative Leader Positioned for Growth Proprietary best-in-class SCS (HF10) platform delivering superior clinical effectiveness and strong long-term financial growth and performance Long-Term Commitment to Grow Market and Market Share Product innovation and clinical pipeline to drive long-term market growth and share on the path to market leadership HF10 MATTERS





























