
YTX-7739 Phase 1B Trial in Patients with Parkinson’s Disease (PD) Summary of Key Findings November 2021 Exhibit 99.1

Forward-Looking Statements This presentation contains forward-looking statements, including statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements may be identified by words and phrases such as “aims,” “anticipates,” “believes,” “could,” “designed to,” “estimates,” “expects,” “forecasts,” “goal,” “intends,” “may,” “plans,” “possible,” “potential,” “seeks,” “will,” and variations of these words and phrases or similar expressions that are intended to identify forward-looking statements. These forward-looking statements include, without limitation, statements regarding our business strategy for and the potential therapeutic benefits of YTX-7739 and the design, commencement, enrollment, and timing of planned clinical trials, clinical trial results, product approvals and regulatory pathways, the anticipated benefits of our drug discovery platform, and statements regarding our financial and cash position and expected cash runway. Any such statements in this press release that are not statements of historical fact may be deemed to be forward-looking statements. Results in preclinical and early-stage clinical trials may not be indicative of results from later stage or larger scale clinical trials and do not ensure regulatory approval. You should not place undue reliance on these statements, or the scientific data presented. The forward-looking statements in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.
Executive Summary – Positive Phase 1b Results for YTX-7739, Well-Positioned for Phase 2 POC Trial Generally well tolerated: No serious adverse events Favorable PK/PD profile: Clear dose-dependent PK/PD relationships, consistent with previous studies Results inform dose selection for Phase 2 program Target successfully engaged: Recap: Target enzyme = SCD; Biomarker = Fatty Acid Desaturation Index (FA-DI) Results in patients: FA-DI reduction within the 20-40% range hypothesized to be therapeutically relevant Exploratory markers: No statistically significant difference in clinical or biochemical markers (as expected for 28 days) In a subset of patients, quantitative EEG suggests early signs of potential improvement in synaptic function PK/PD = Pharmacokinetics/Pharmacodynamics The Company plans to advance YTX-7739 to Phase 2 proof-of-concept (POC) trial in Parkinson’s disease in 2022
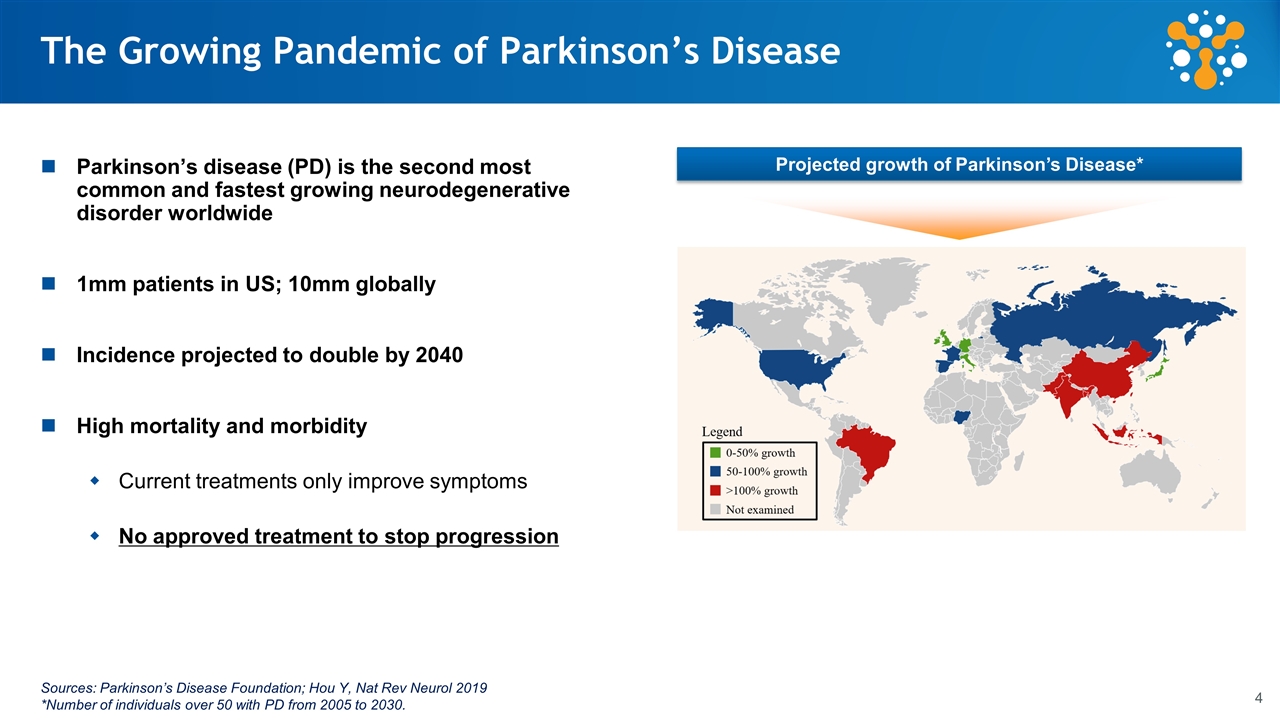
The Growing Pandemic of Parkinson’s Disease Parkinson’s disease (PD) is the second most common and fastest growing neurodegenerative disorder worldwide 1mm patients in US; 10mm globally Incidence projected to double by 2040 High mortality and morbidity Current treatments only improve symptoms No approved treatment to stop progression Projected growth of Parkinson’s Disease* Sources: Parkinson’s Disease Foundation; Hou Y, Nat Rev Neurol 2019 *Number of individuals over 50 with PD from 2005 to 2030.
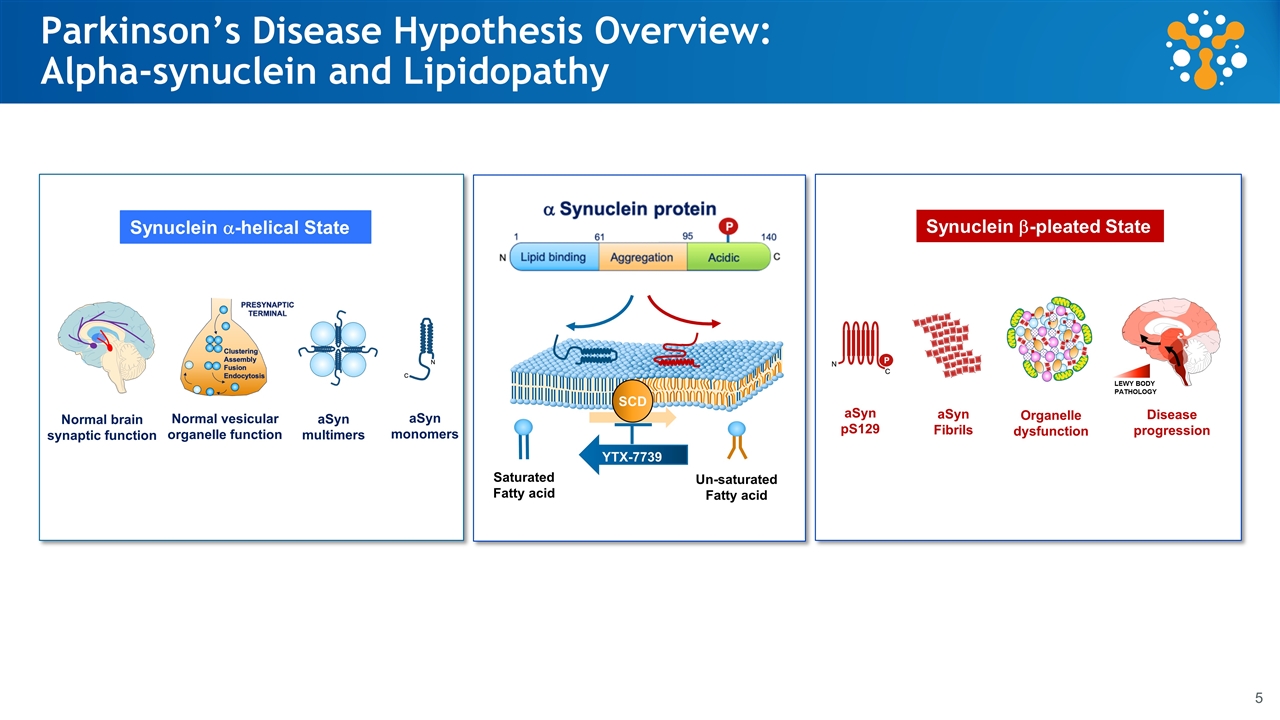
Synuclein a-helical State aSyn monomers aSyn multimers Normal vesicular organelle function Normal brain synaptic function Parkinson’s Disease Hypothesis Overview: Alpha-synuclein and Lipidopathy Synuclein b-pleated State aSyn pS129 aSyn Fibrils Organelle dysfunction Disease progression LEWY BODY PATHOLOGY SCD Saturated Fatty acid Un-saturated Fatty acid YTX-7739
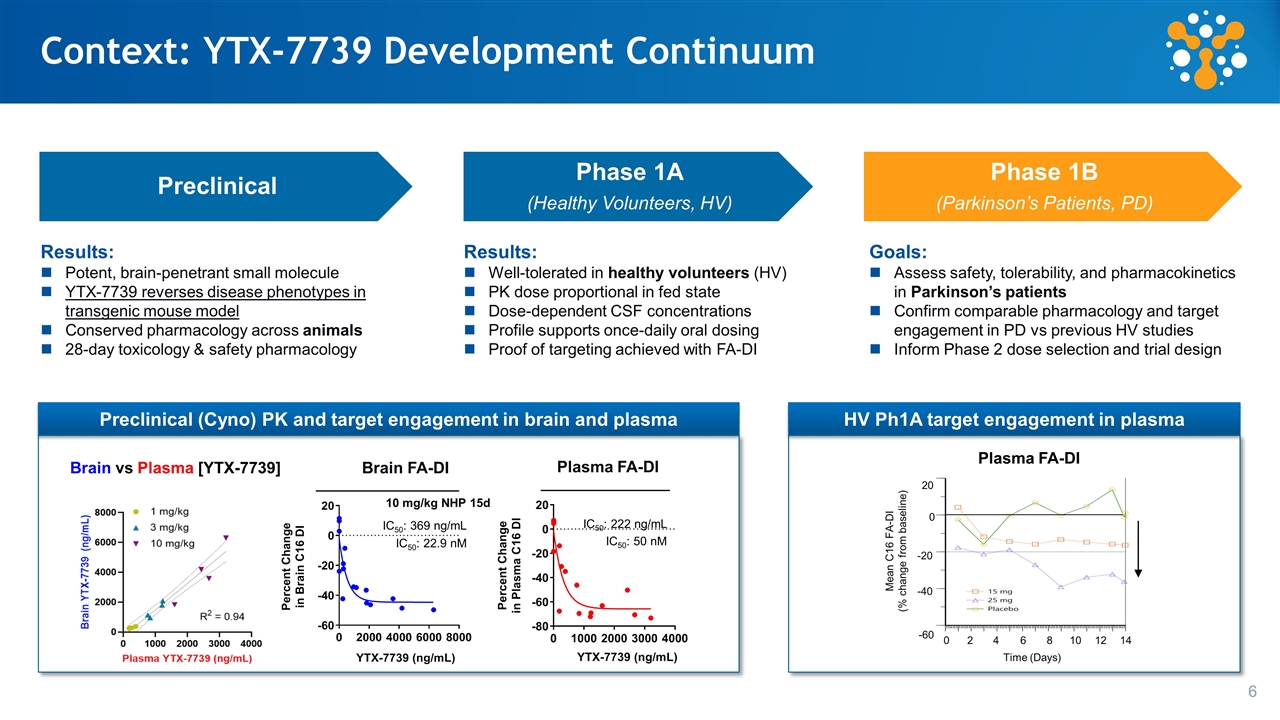
IC50: 369 ng/mL IC50: 22.9 nM Plasma FA-DI IC50: 222 ng/mL IC50: 50 nM 10 mg/kg NHP 15d Brain vs Plasma [YTX-7739] Brain FA-DI HV Ph1A target engagement in plasma Context: YTX-7739 Development Continuum Results: Potent, brain-penetrant small molecule YTX-7739 reverses disease phenotypes in transgenic mouse model Conserved pharmacology across animals 28-day toxicology & safety pharmacology Results: Well-tolerated in healthy volunteers (HV) PK dose proportional in fed state Dose-dependent CSF concentrations Profile supports once-daily oral dosing Proof of targeting achieved with FA-DI Goals: Assess safety, tolerability, and pharmacokinetics in Parkinson’s patients Confirm comparable pharmacology and target engagement in PD vs previous HV studies Inform Phase 2 dose selection and trial design Mean C16 FA-DI (% change from baseline) 20 0 -20 -40 -60 Time (Days) 2 4 6 8 10 12 14 0 Preclinical Phase 1A (Healthy Volunteers, HV) Phase 1B (Parkinson’s Patients, PD) Plasma FA-DI Preclinical (Cyno) PK and target engagement in brain and plasma
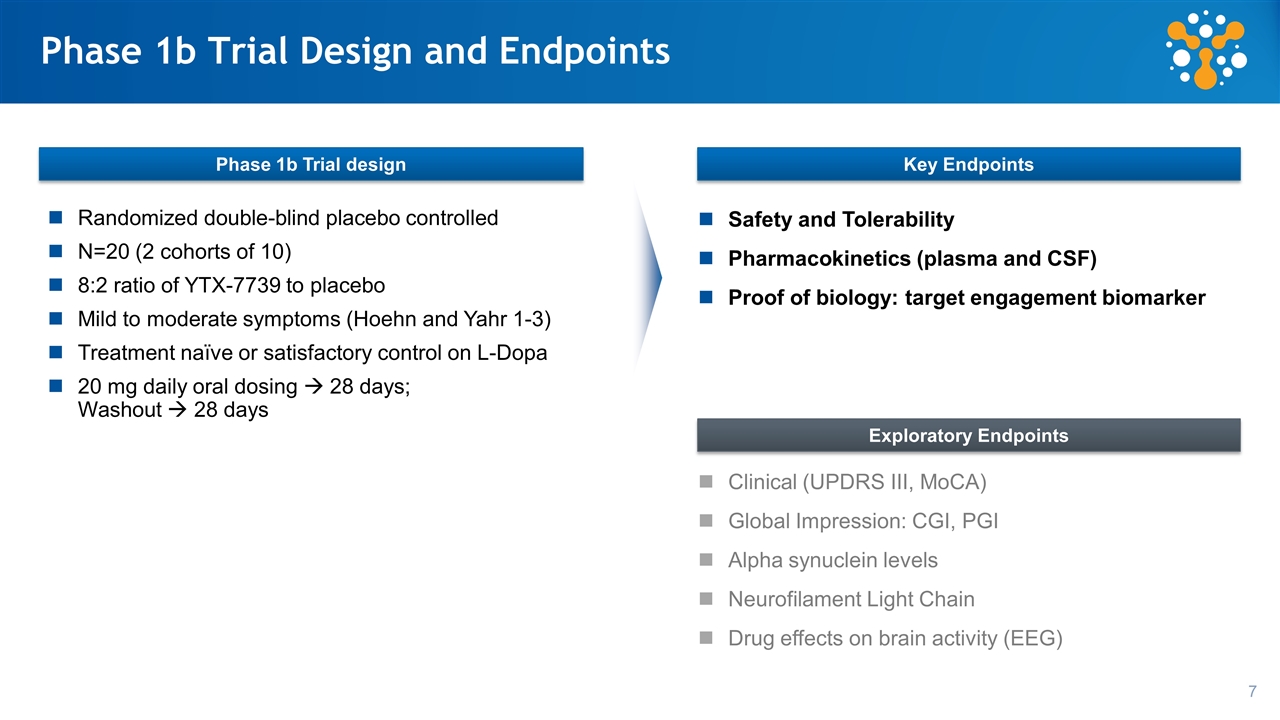
Phase 1b Trial Design and Endpoints Randomized double-blind placebo controlled N=20 (2 cohorts of 10) 8:2 ratio of YTX-7739 to placebo Mild to moderate symptoms (Hoehn and Yahr 1-3) Treatment naïve or satisfactory control on L-Dopa 20 mg daily oral dosing à 28 days; Washout à 28 days Safety and Tolerability Pharmacokinetics (plasma and CSF) Proof of biology: target engagement biomarker Phase 1b Trial design Key Endpoints Exploratory Endpoints Clinical (UPDRS III, MoCA) Global Impression: CGI, PGI Alpha synuclein levels Neurofilament Light Chain Drug effects on brain activity (EEG)
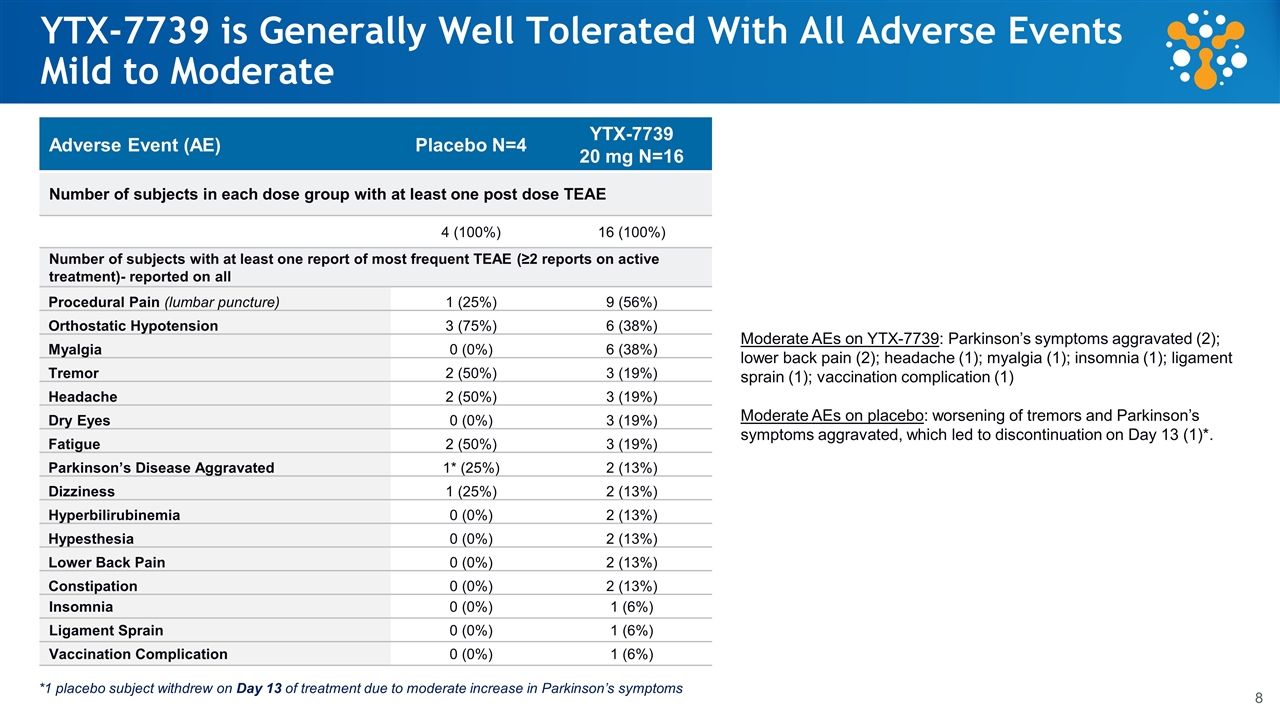
YTX-7739 is Generally Well Tolerated With All Adverse Events Mild to Moderate Adverse Event (AE) Placebo N=4 YTX-7739 20 mg N=16 Number of subjects in each dose group with at least one post dose TEAE 4 (100%) 16 (100%) Number of subjects with at least one report of most frequent TEAE (≥2 reports on active treatment)- reported on all Procedural Pain (lumbar puncture) 1 (25%) 9 (56%) Orthostatic Hypotension 3 (75%) 6 (38%) Myalgia 0 (0%) 6 (38%) Tremor 2 (50%) 3 (19%) Headache 2 (50%) 3 (19%) Dry Eyes 0 (0%) 3 (19%) Fatigue 2 (50%) 3 (19%) Parkinson’s Disease Aggravated 1* (25%) 2 (13%) Dizziness 1 (25%) 2 (13%) Hyperbilirubinemia 0 (0%) 2 (13%) Hypesthesia 0 (0%) 2 (13%) Lower Back Pain 0 (0%) 2 (13%) Constipation 0 (0%) 2 (13%) Insomnia 0 (0%) 1 (6%) Ligament Sprain 0 (0%) 1 (6%) Vaccination Complication 0 (0%) 1 (6%) *1 placebo subject withdrew on Day 13 of treatment due to moderate increase in Parkinson’s symptoms Moderate AEs on YTX-7739: Parkinson’s symptoms aggravated (2); lower back pain (2); headache (1); myalgia (1); insomnia (1); ligament sprain (1); vaccination complication (1) Moderate AEs on placebo: worsening of tremors and Parkinson’s symptoms aggravated, which led to discontinuation on Day 13 (1)*.
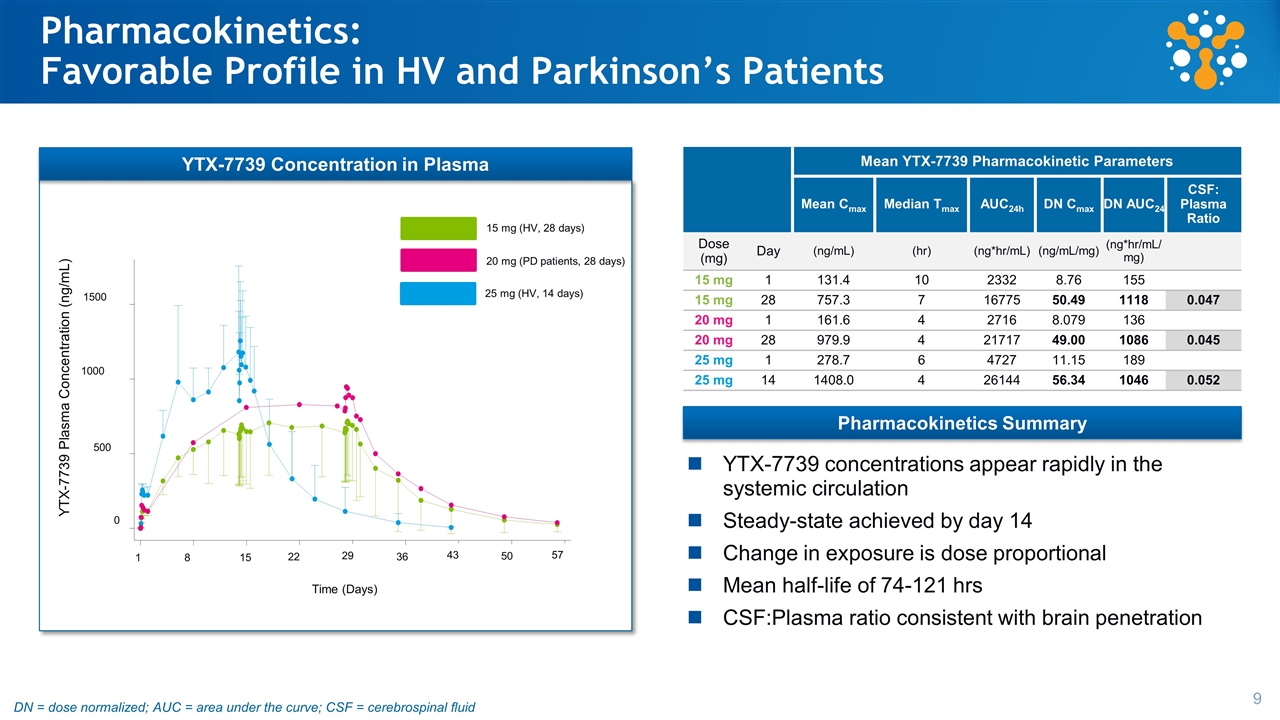
Pharmacokinetics: Favorable Profile in HV and Parkinson’s Patients 1500 1000 500 0 YTX-7739 Plasma Concentration (ng/mL) Time (Days) 1 8 15 22 36 43 50 57 29 25 mg (HV, 14 days) 15 mg (HV, 28 days) 20 mg (PD patients, 28 days) Mean YTX-7739 Pharmacokinetic Parameters Mean Cmax Median Tmax AUC24h DN Cmax DN AUC24 CSF: Plasma Ratio Dose (mg) Day (ng/mL) (hr) (ng*hr/mL) (ng/mL/mg) (ng*hr/mL/mg) 15 mg 1 131.4 10 2332 8.76 155 15 mg 28 757.3 7 16775 50.49 1118 0.047 20 mg 1 161.6 4 2716 8.079 136 20 mg 28 979.9 4 21717 49.00 1086 0.045 25 mg 1 278.7 6 4727 11.15 189 25 mg 14 1408.0 4 26144 56.34 1046 0.052 YTX-7739 concentrations appear rapidly in the systemic circulation Steady-state achieved by day 14 Change in exposure is dose proportional Mean half-life of 74-121 hrs CSF:Plasma ratio consistent with brain penetration YTX-7739 Concentration in Plasma Pharmacokinetics Summary DN = dose normalized; AUC = area under the curve; CSF = cerebrospinal fluid
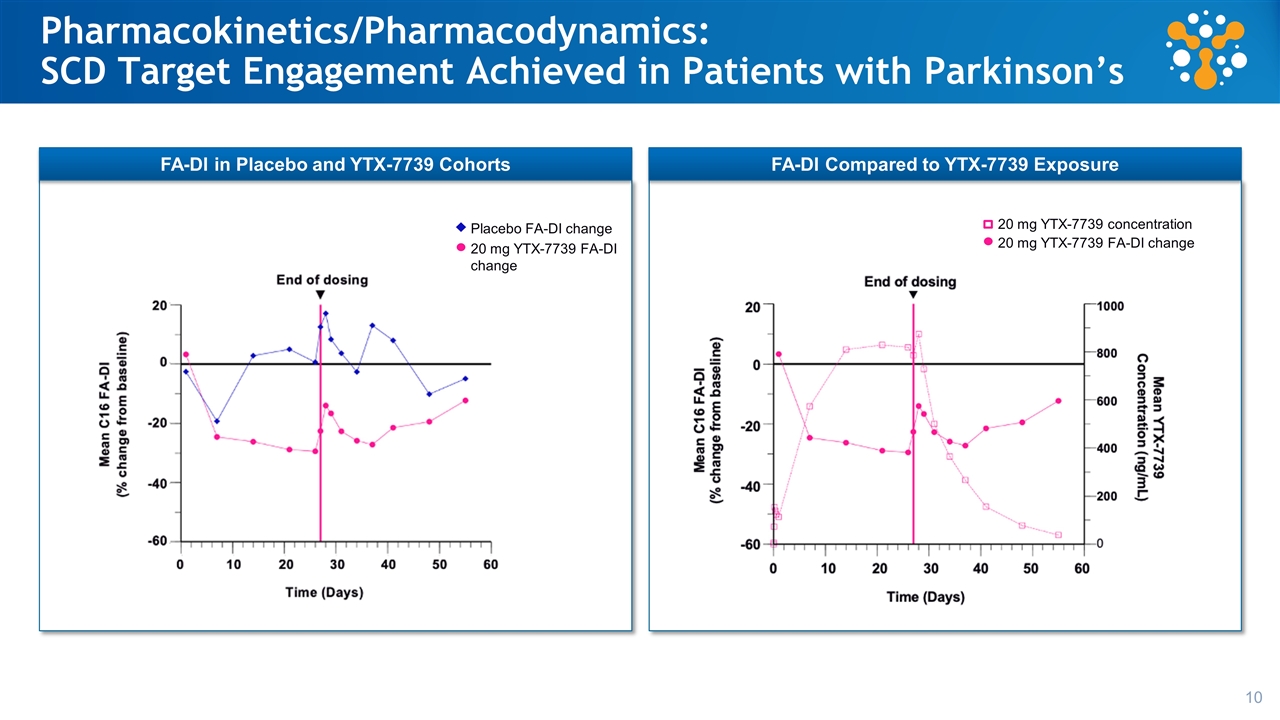
Pharmacokinetics/Pharmacodynamics: SCD Target Engagement Achieved in Patients with Parkinson’s 20 mg YTX-7739 FA-DI change 20 mg YTX-7739 concentration 20 mg YTX-7739 FA-DI change Placebo FA-DI change FA-DI in Placebo and YTX-7739 Cohorts FA-DI Compared to YTX-7739 Exposure
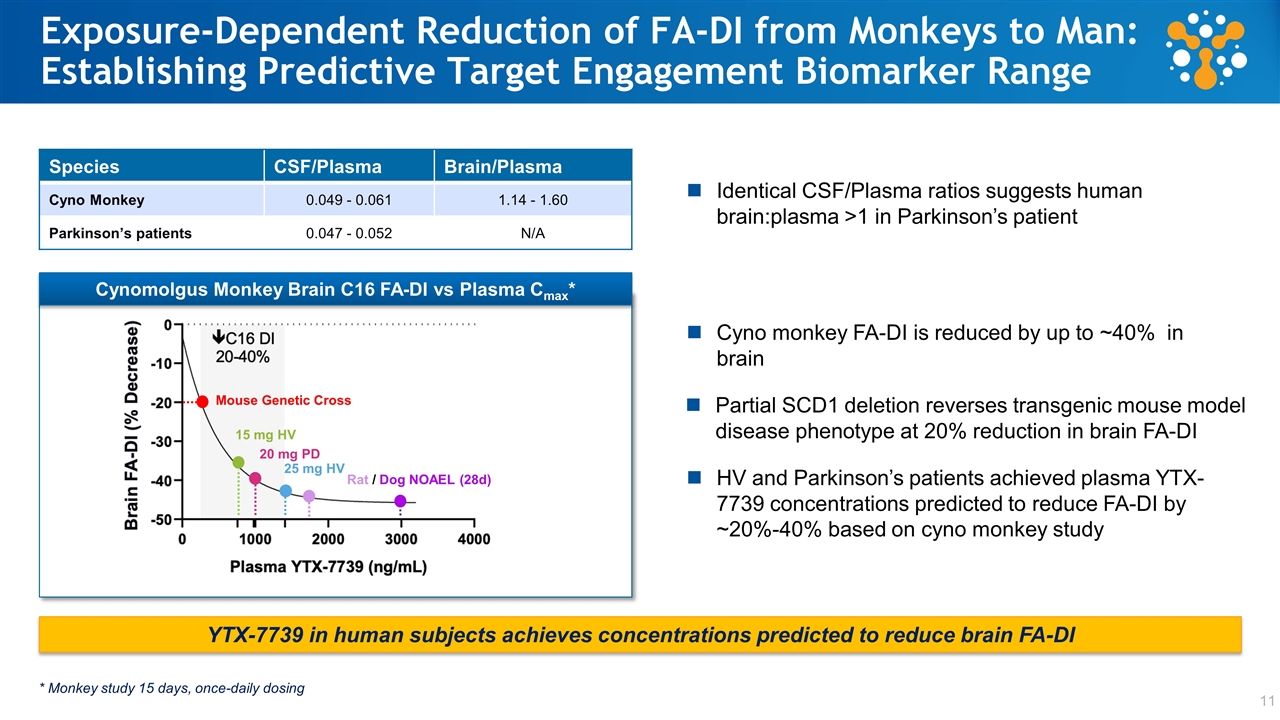
Exposure-Dependent Reduction of FA-DI from Monkeys to Man: Establishing Predictive Target Engagement Biomarker Range 1 Species CSF/Plasma Brain/Plasma Cyno Monkey 0.049 - 0.061 1.14 - 1.60 Parkinson’s patients 0.047 - 0.052 N/A YTX-7739 in human subjects achieves concentrations predicted to reduce brain FA-DI Identical CSF/Plasma ratios suggests human brain:plasma >1 in Parkinson’s patient Cyno monkey FA-DI is reduced by up to ~40% in brain * Monkey study 15 days, once-daily dosing Cynomolgus Monkey Brain C16 FA-DI vs Plasma Cmax* Cyno 15 mg HV 20 mg PD 25 mg HV HV and Parkinson’s patients achieved plasma YTX-7739 concentrations predicted to reduce FA-DI by ~20%-40% based on cyno monkey study Mouse Genetic Cross Rat / Dog NOAEL (28d) Partial SCD1 deletion reverses transgenic mouse model disease phenotype at 20% reduction in brain FA-DI
Phase 1b Trial Design and Endpoints Randomized double-blind placebo controlled N=20 (2 cohorts of 10) 8:2 ratio of YTX-7739 to placebo Mild to moderate symptoms (Hoehn and Yahr 1-3) Treatment naïve or satisfactory control on L-Dopa 20 mg daily oral dosing à 28 days; Washout à 28 days Safety and Tolerability Pharmacokinetics (plasma and CSF) Proof of biology: target engagement biomarker Phase 1b Trial design Exploratory Endpoints Clinical (UPDRS III, MoCA) Global Impression: CGI, PGI Alpha synuclein levels Neurofilament light chain Drug effects on brain activity (EEG) Key Endpoints
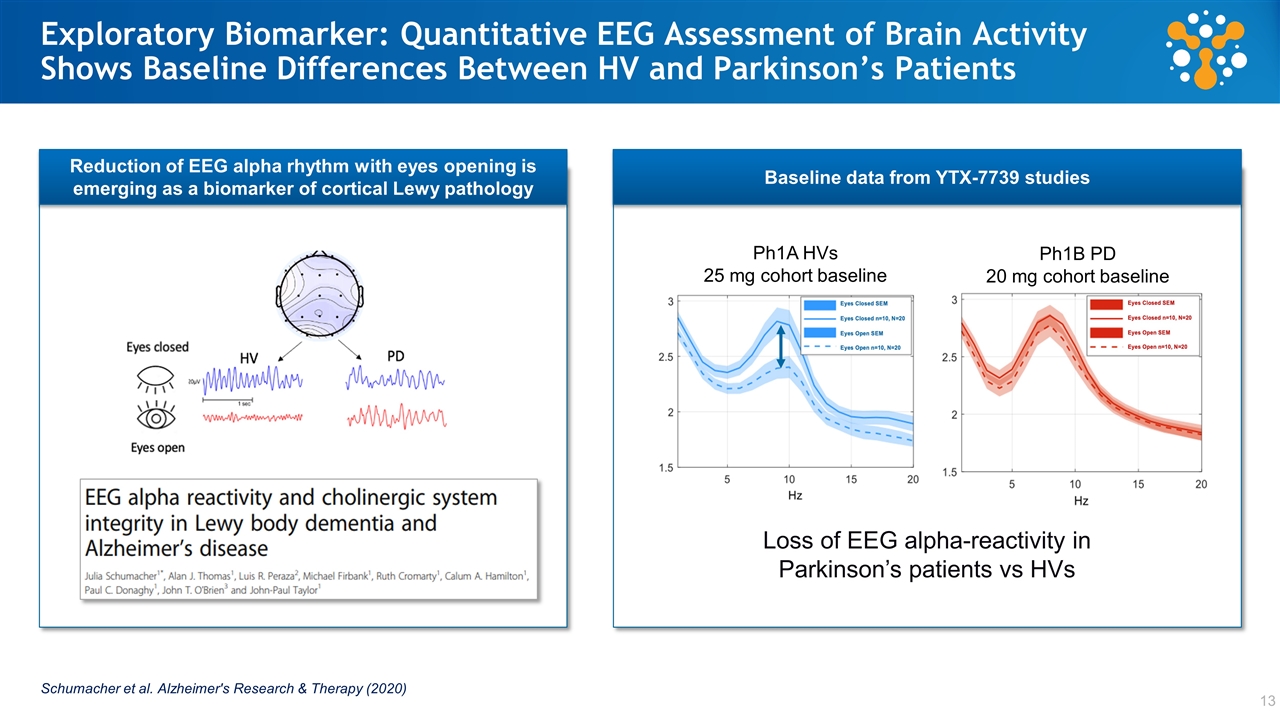
Schumacher et al. Alzheimer's Research & Therapy (2020) Loss of EEG alpha-reactivity in Parkinson’s patients vs HVs Ph1A HVs 25 mg cohort baseline Ph1B PD 20 mg cohort baseline Exploratory Biomarker: Quantitative EEG Assessment of Brain Activity Shows Baseline Differences Between HV and Parkinson’s Patients Reduction of EEG alpha rhythm with eyes opening is emerging as a biomarker of cortical Lewy pathology Baseline data from YTX-7739 studies
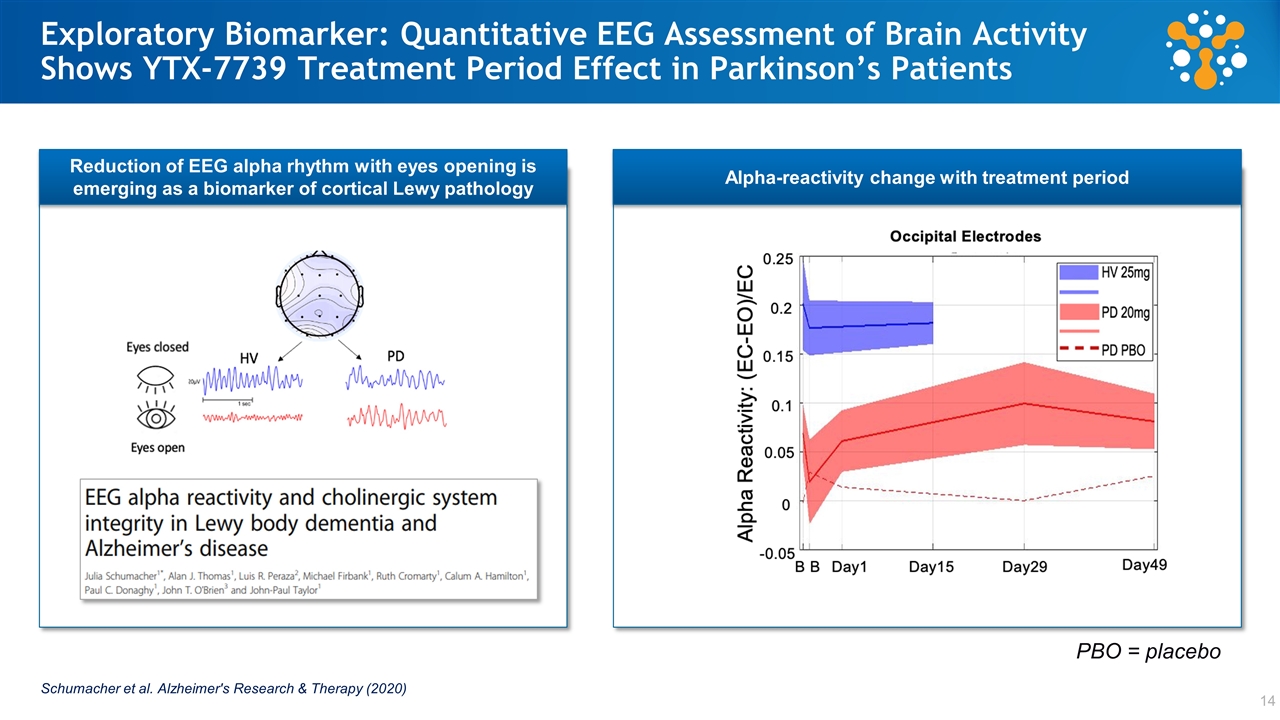
Exploratory Biomarker: Quantitative EEG Assessment of Brain Activity Shows YTX-7739 Treatment Period Effect in Parkinson’s Patients Alpha-reactivity change with treatment period Reduction of EEG alpha rhythm with eyes opening is emerging as a biomarker of cortical Lewy pathology Schumacher et al. Alzheimer's Research & Therapy (2020) PBO = placebo
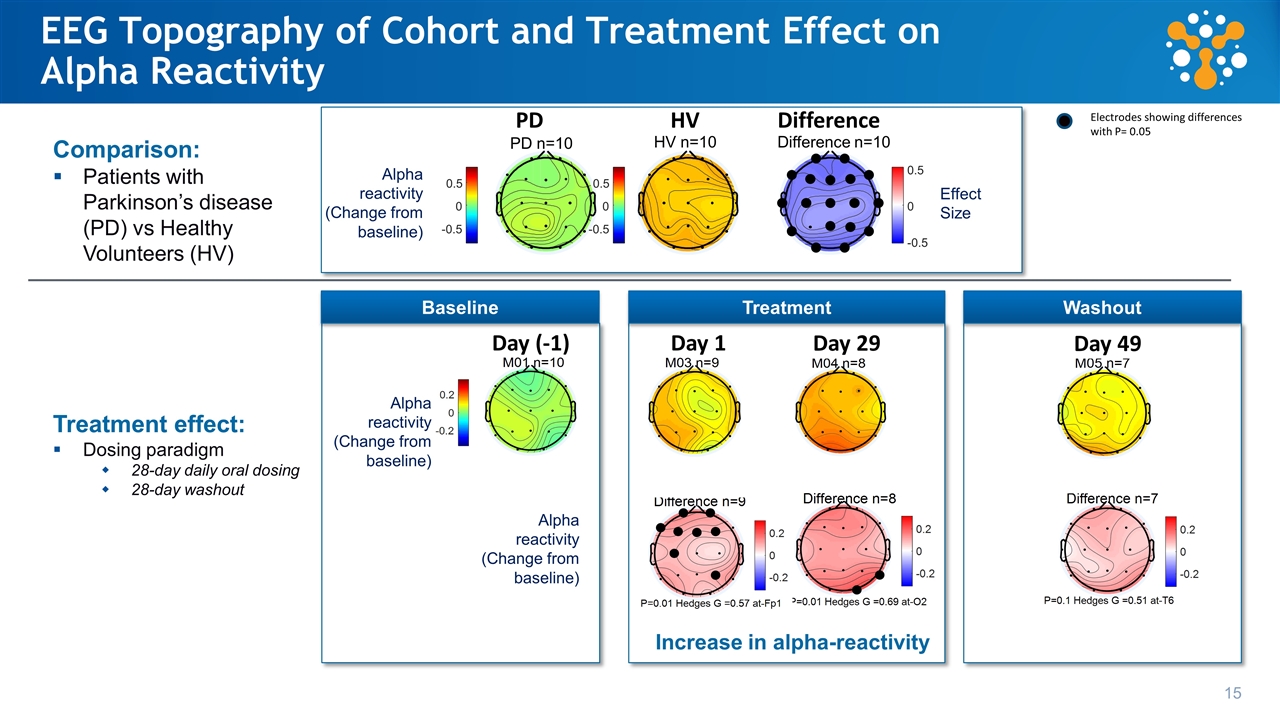
EEG Topography of Cohort and Treatment Effect on Alpha Reactivity Day (-1) Day 1 Day 29 Day 49 PD HV Difference Electrodes showing differences with P= 0.05 15 Comparison: Patients with Parkinson’s disease (PD) vs Healthy Volunteers (HV) Treatment effect: Dosing paradigm 28-day daily oral dosing 28-day washout Increase in alpha-reactivity Alpha reactivity (Change from baseline) Effect Size Alpha reactivity (Change from baseline) Alpha reactivity (Change from baseline) -0.5 0 0.5 -0.5 0 0.5 -0.5 0 0.5 PD n=10 HV n=10 Difference n=10 Washout Treatment Baseline

Phase 2 Plans for YTX-7739 The Company plans to initiate a Phase 2 trial for YTX-7739 in 2022 The trial will be a proof-of-concept (POC) study exploring safety and efficacy in Parkinson’s disease Early trial design considerations Early stages of disease (e.g., Hoehn & Yahr stages 1-3) Subjects stable on dopaminergic treatment Randomized, double-blind, placebo-controlled – will explore 2 doses vs placebo Endpoints: delay in time to disability progression on motor, non-motor and QOL endpoints (e.g., MDS-UPDRS – parts II and III) Interim analysis on disease-related biomarkers
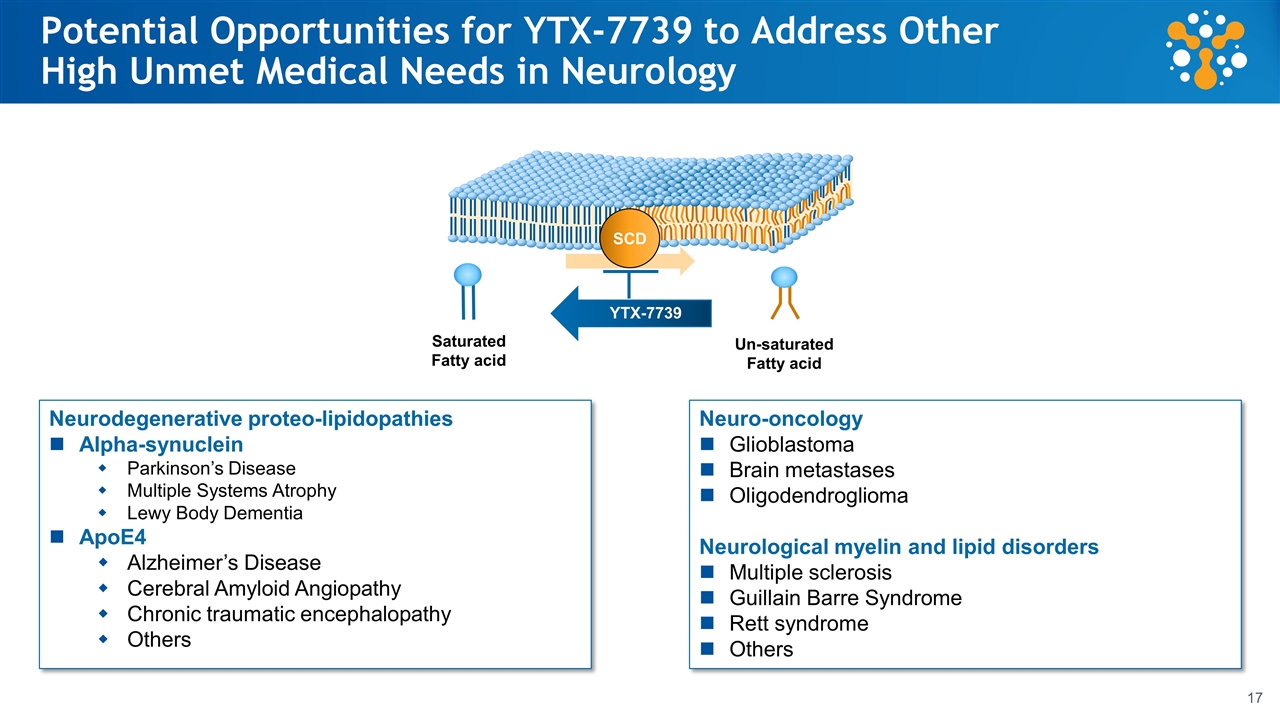
Potential Opportunities for YTX-7739 to Address Other High Unmet Medical Needs in Neurology SCD Saturated Fatty acid Un-saturated Fatty acid YTX-7739 Neurodegenerative proteo-lipidopathies Alpha-synuclein Parkinson’s Disease Multiple Systems Atrophy Lewy Body Dementia ApoE4 Alzheimer’s Disease Cerebral Amyloid Angiopathy Chronic traumatic encephalopathy Others Neuro-oncology Glioblastoma Brain metastases Oligodendroglioma Neurological myelin and lipid disorders Multiple sclerosis Guillain Barre Syndrome Rett syndrome Others
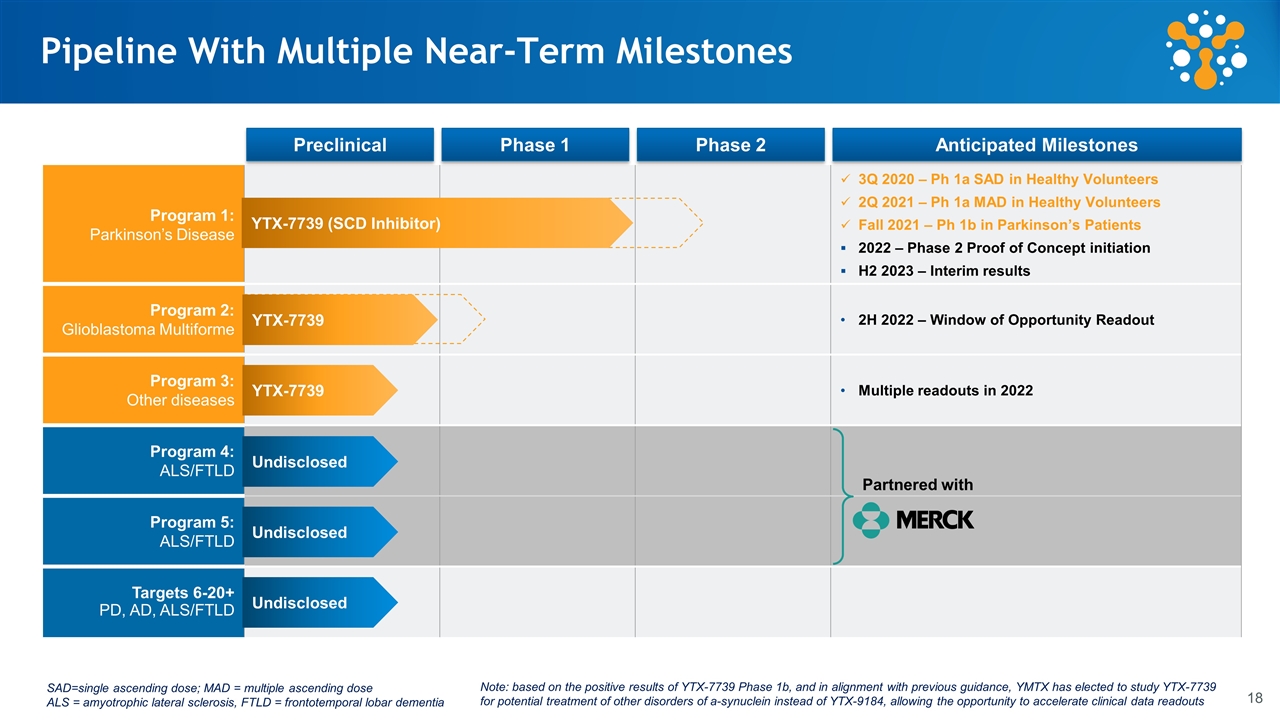
Pre-clinical Phase 1 Phase 2 Anticipated Catalysts Program 1: Parkinson’s Disease 3Q 2020 – Ph 1a SAD in Healthy Volunteers 2Q 2021 – Ph 1a MAD in Healthy Volunteers Fall 2021 – Ph 1b in Parkinson’s Patients 2022 – Phase 2 Proof of Concept initiation H2 2023 – Interim results Program 2: Glioblastoma Multiforme 2H 2022 – Window of Opportunity Readout Program 3: Other diseases Multiple readouts in 2022 Program 4: ALS/FTLD Program 5: ALS/FTLD Targets 6-20+ PD, AD, ALS/FTLD Undisclosed Undisclosed Undisclosed Pipeline With Multiple Near-Term Milestones Partnered with SAD=single ascending dose; MAD = multiple ascending dose ALS = amyotrophic lateral sclerosis, FTLD = frontotemporal lobar dementia YTX-7739 YTX-7739 Preclinical Phase 1 Phase 2 Anticipated Milestones Note: based on the positive results of YTX-7739 Phase 1b, and in alignment with previous guidance, YMTX has elected to study YTX-7739 for potential treatment of other disorders of a-synuclein instead of YTX-9184, allowing the opportunity to accelerate clinical data readouts YTX-7739 (SCD Inhibitor)

BACKUP

YTX-7739 Safety Summary – All Adverse Events Mild-Moderate YTX-7739 was generally well tolerated, all treatment emergent adverse events (AEs) were mild to moderate in severity No serious adverse events Moderate AEs on YTX-7739: Parkinson’s symptoms aggravated (2); lower back pain (2); headache (1); myalgia (1); insomnia (1); ligament sprain (1); vaccination complication (1) Moderate AEs on placebo: worsening of tremors and Parkinson’s symptoms aggravated, which led to discontinuation on Day 13 (1) AEs occurring in more than 2 subjects: At a higher percentage in YTX-7739 vs placebo: procedural pain, myalgia, dry eye, hyperbilirubinemia, hypesthesia, lower back pain, and constipation At a higher percentage in placebo vs YTX-7739: orthostatic hypotension, headache, tremor, fatigue and dizziness No deaths occurred in the study 20
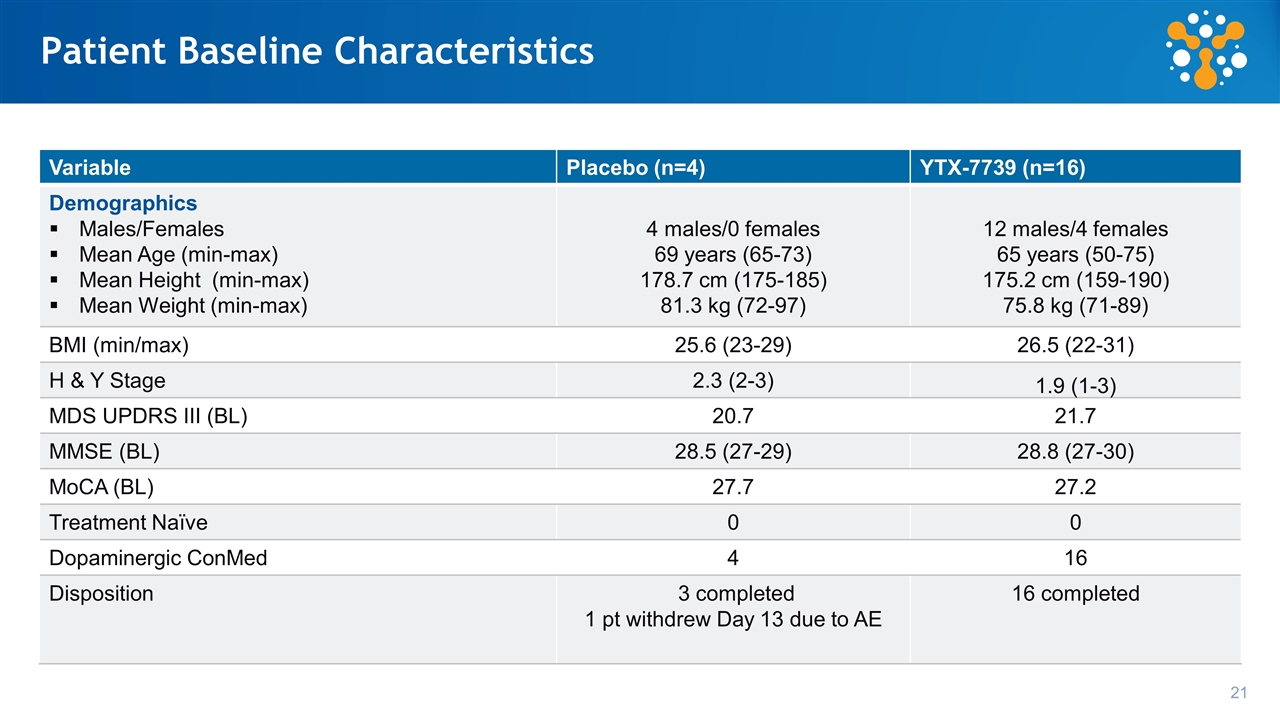
Patient Baseline Characteristics Variable Placebo (n=4) YTX-7739 (n=16) Demographics Males/Females Mean Age (min-max) Mean Height (min-max) Mean Weight (min-max) 4 males/0 females 69 years (65-73) 178.7 cm (175-185) 81.3 kg (72-97) 12 males/4 females 65 years (50-75) 175.2 cm (159-190) 75.8 kg (71-89) BMI (min/max) 25.6 (23-29) 26.5 (22-31) H & Y Stage 2.3 (2-3) 1.9 (1-3) MDS UPDRS III (BL) 20.7 21.7 MMSE (BL) 28.5 (27-29) 28.8 (27-30) MoCA (BL) 27.7 27.2 Treatment Naïve 0 0 Dopaminergic ConMed 4 16 Disposition 3 completed 1 pt withdrew Day 13 due to AE 16 completed 21
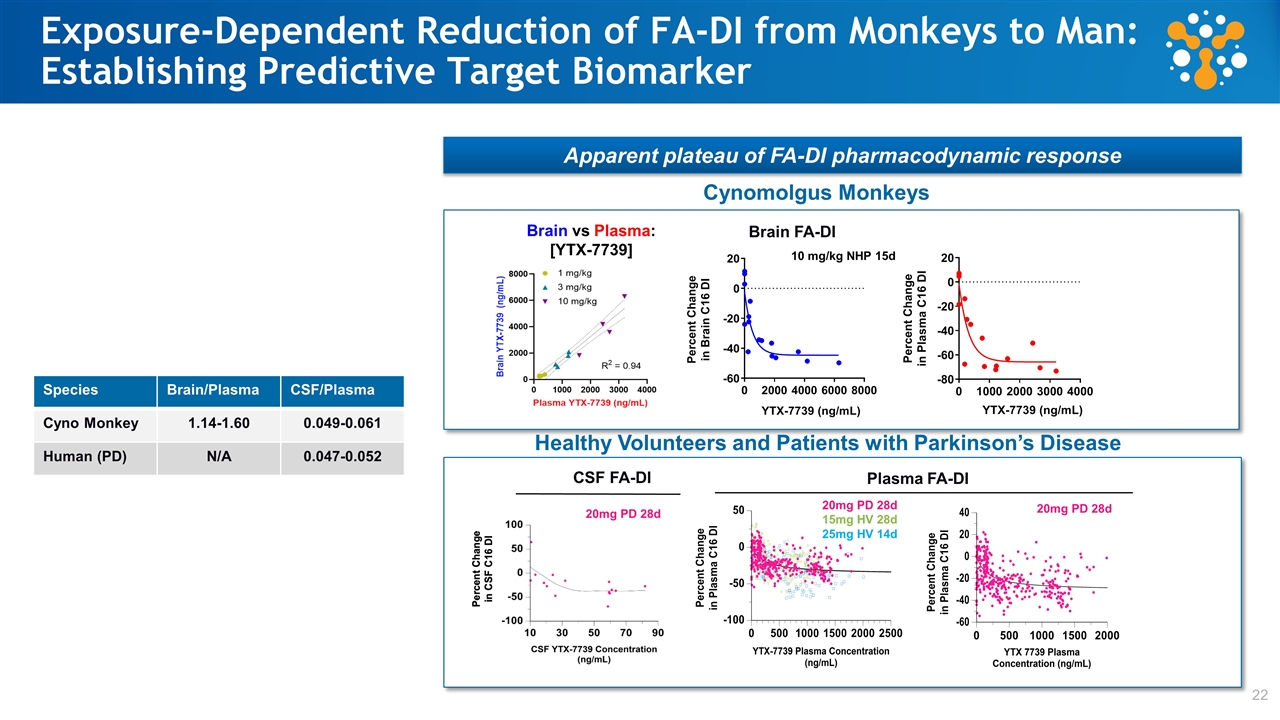
Exposure-Dependent Reduction of FA-DI from Monkeys to Man: Establishing Predictive Target Biomarker 22 Apparent plateau of FA-DI pharmacodynamic response IC50: 369 ng/mL IC50: 22.9 nM Plasma FA-DI IC50: 222 ng/mL IC50: 50 nM Cynomolgus Monkeys 20mg PD 28d 15mg HV 28d 25mg HV 14d 20mg PD 28d Healthy Volunteers and Patients with Parkinson’s Disease 10 mg/kg NHP 15d Brain vs Plasma: [YTX-7739] 20mg PD 28d Brain FA-DI Species Brain/Plasma CSF/Plasma Cyno Monkey 1.14-1.60 0.049-0.061 Human (PD) N/A 0.047-0.052 CSF FA-DI Plasma FA-DI
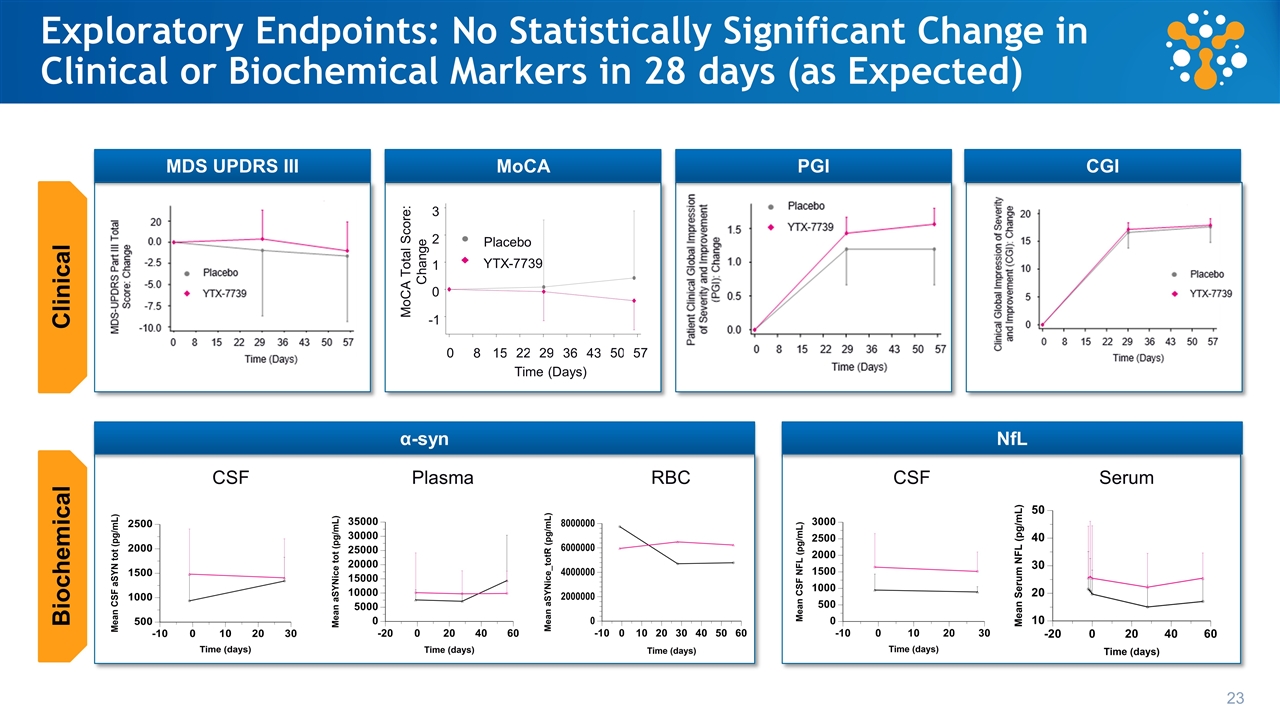
Exploratory Endpoints: No Statistically Significant Change in Clinical or Biochemical Markers in 28 days (as Expected) 3 2 1 0 -1 MoCA Total Score: Change Placebo YTX-7739 0 8 15 22 29 36 43 Time (Days) 50 57 23 Plasma RBC CSF Serum CSF MDS UPDRS III MoCA PGI CGI α-syn NfL Clinical Biochemical
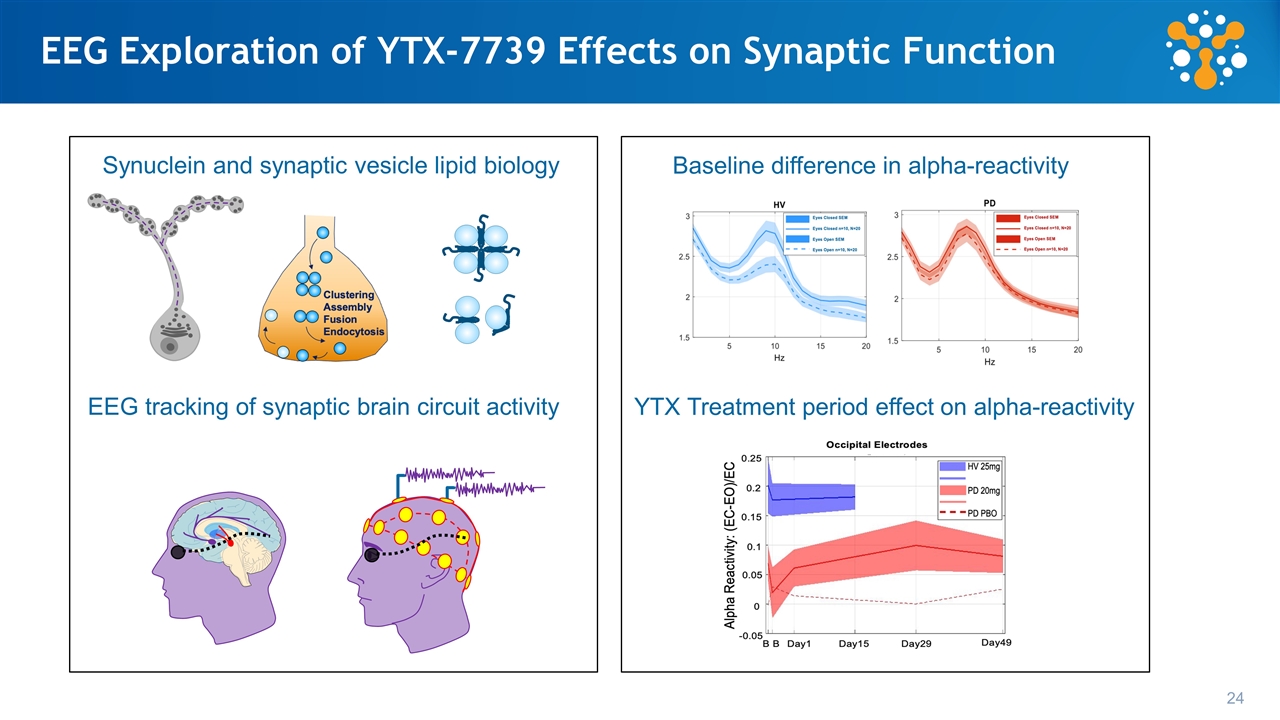
EEG Exploration of YTX-7739 Effects on Synaptic Function Synuclein and synaptic vesicle lipid biology EEG tracking of synaptic brain circuit activity Baseline difference in alpha-reactivity YTX Treatment period effect on alpha-reactivity 24























