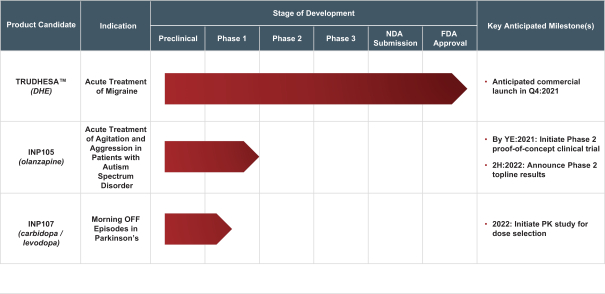
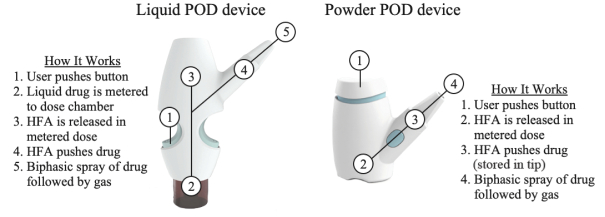
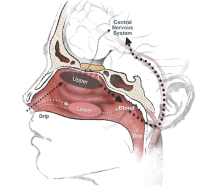
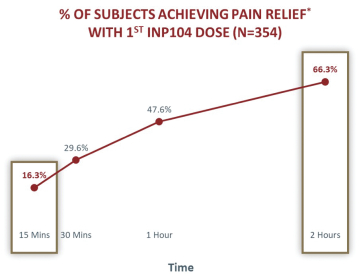
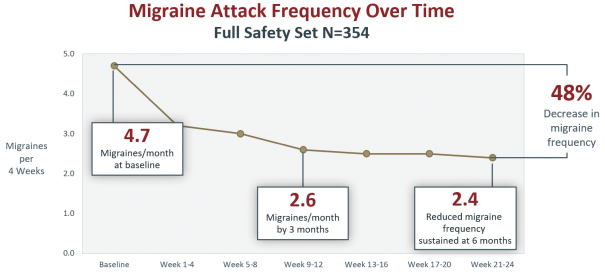
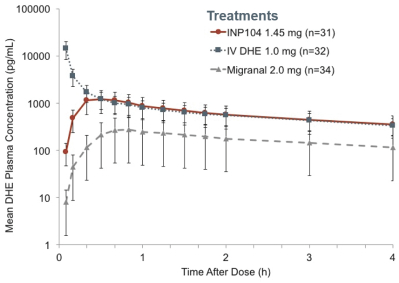
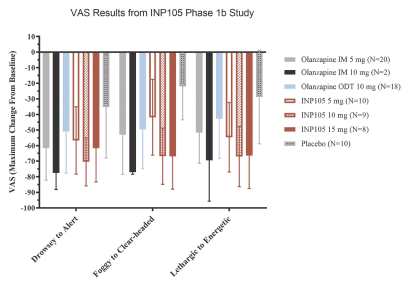
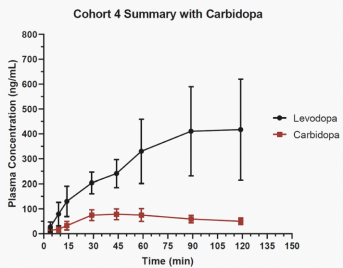
competitors may also obtain regulatory approvals before us, resulting in our competitors building a strong market position in advance of the entry of our product candidates. We believe the factors determining the success of our programs will be the efficacy, safety and convenience of our product candidates.
Intellectual Property
We strive to protect and enhance the proprietary technology, inventions, and improvements that are commercially important to our business, including seeking, maintaining, and defending patent rights, whether developed internally or licensed from third parties. Our policy is to seek to protect our proprietary position by, among other methods, pursuing and obtaining patent protection in the United States and in jurisdictions outside of the United States related to our proprietary technology, inventions, improvements, platforms and product candidates that are important to the development and implementation of our business. Our patent portfolio is intended to cover, but is not limited to, our POD technology, our product candidates and components thereof, their methods of use and processes for their manufacture, and any other inventions that are commercially important to our business. We expect to rely on data exclusivity, market exclusivity, patent term adjustment and patent term extensions when available. Our commercial success may depend in part on our ability to obtain and maintain patent and other proprietary protection for our technology, inventions, and improvements; to maintain our licenses to use intellectual property owned or controlled by third parties; to defend and enforce our proprietary rights, including our patents; to defend against and challenge the assertion by third parties of their purported intellectual property rights; and to operate without the unauthorized infringement on the valid and enforceable patents and other proprietary rights of third parties.
We believe that we have a strong global intellectual property position relating to our POD device, TRUDHESA and our other product candidates. Our patent portfolio as of June 30, 2021 contained six U.S. issued patents and 29 patents issued in ex-U.S. jurisdictions including Australia, Canada, China, Switzerland, Germany, France, Great Britain, Japan, and Russia and 13 U.S. pending applications as well as 67 patent applications pending in ex-U.S. jurisdictions including Australia, Brazil, Canada, China, Europe, Hong Kong, Israel, India, Japan, Korea, Mexico, New Zealand, Russia, South Africa and two pending international patent applications owned solely by us.
TRUDHESA is covered by five patent families: three of the families include claims relating to the POD device used for delivery of DHE to the upper nasal cavity; the other two families include claims relating to methods of delivering DHE to the upper nasal cavity. As of June 30, 2021, from the three device patent families, five U.S. patents have issued, 24 patents have issued in ex-U.S. jurisdictions including Australia, Canada, China, Switzerland, Germany, France, Great Britain, Japan, and Russia, and 25 applications were pending in the U.S., Australia, Brazil, Canada, China, Europe, Hong Kong, Israel, India, Japan, Korea, Mexico, New Zealand, and South Africa. Upon FDA approval, we intend to list the issued U.S. patents on the FDA Orange Book. As of June 30, 2021, from the two method of use patent families, three applications were pending in the U.S., and 10 applications were pending in ex-U.S. jurisdictions including Australia, Brazil, Canada, China, Europe, India, Japan, Hong Kong, Korea, and Russia and there is one pending international application. Our issued patents, and any patents that may issue from our pending patent applications, are expected to expire between 2032 and 2039, absent any patent term adjustments or extensions.
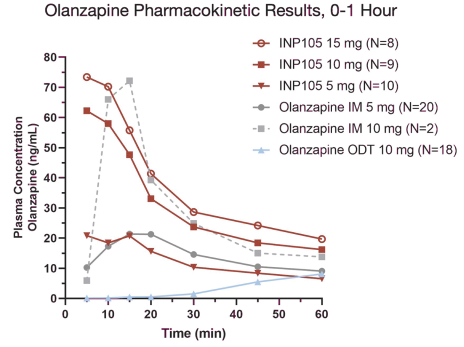
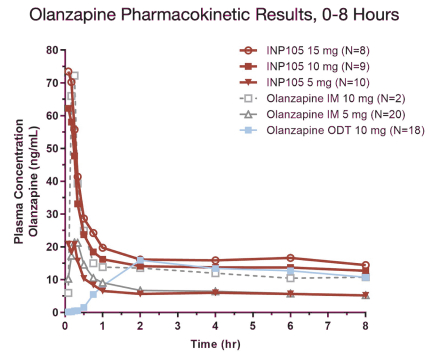
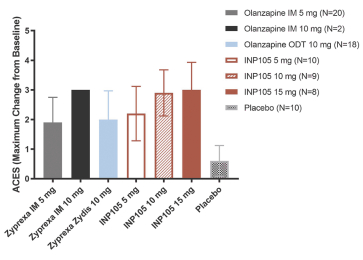
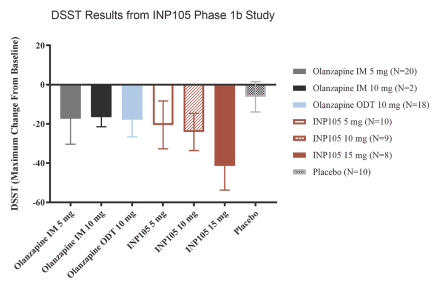
Our INP105 product candidate is covered by three patent families: two of the families include claims relating to the POD device used for delivery of olanzapine to the upper nasal cavity; one patent family includes composition of matter claims covering our dry powder formulation and claims directed to methods of delivering olanzapine to the upper nasal cavity. As of June 30, 2021, from the two device patent families, two U.S. patents have issued, ten patents have issued in ex-U.S. jurisdictions including Australia, Canada, China, Switzerland, Germany, France, Great Britain, Japan, and Russia, and 11 applications were pending in the U.S., Australia, Brazil, Canada, Europe, India, Israel, New Zealand,
114