
Studies beyond CE-mark: Fantom in long lesion & Multi-Vessel Disease Matthias Lutz University Medical Center Schleswig-Holstein, Campus Kiel, Germany Exhibit 99.4

Speaker's name: Matthias Lutz I have the following potential conflicts of interest to report: Receipt of grants / research supports: REVA Medical, St. Jude Medical Receipt of honoraria or consultation fees: Abbott, St. Jude Medical Potential conflicts of interest
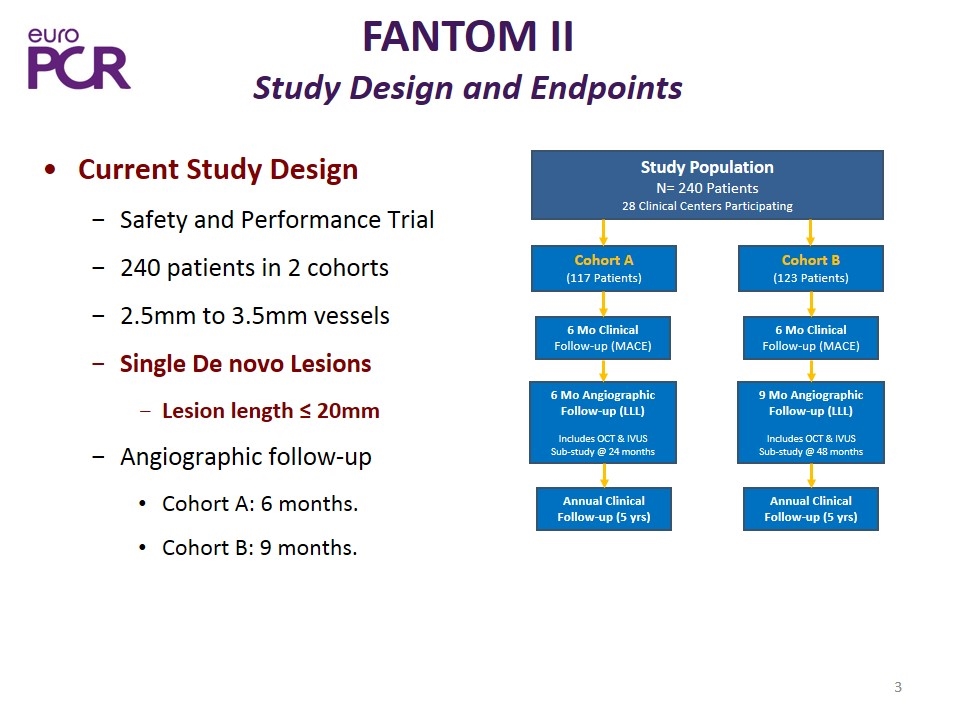
FANTOM II Study Design and Endpoints Current Study Design Safety and Performance Trial 240 patients in 2 cohorts 2.5mm to 3.5mm vessels Single De novo Lesions Lesion length ≤ 20mm Angiographic follow-up Cohort A: 6 months. Cohort B: 9 months. Study Population N= 240 Patients 28 Clinical Centers Participating Cohort A (117 Patients) 6 Mo Clinical Follow-up (MACE) Cohort B (123 Patients) 6 Mo Clinical Follow-up (MACE) 6 Mo Angiographic Follow-up (LLL) Includes OCT & IVUS Sub-study @ 24 months 9 Mo Angiographic Follow-up (LLL) Includes OCT & IVUS Sub-study @ 48 months Annual Clinical Follow-up (5 yrs) Annual Clinical Follow-up (5 yrs)
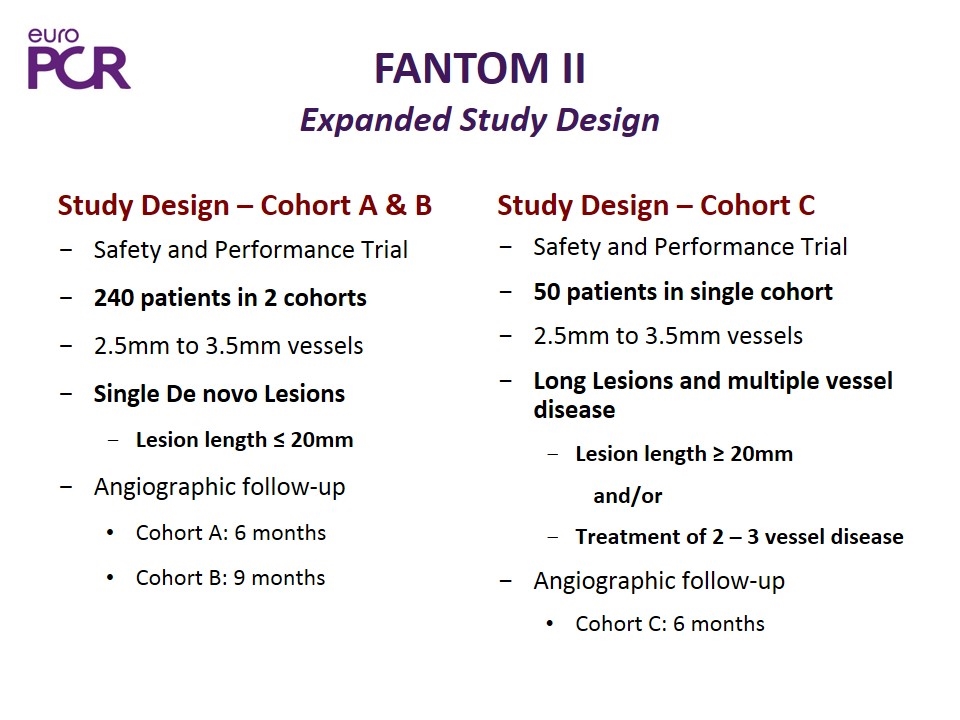
FANTOM II Expanded Study Design Study Design – Cohort A & B Safety and Performance Trial 240 patients in 2 cohorts 2.5mm to 3.5mm vessels Single De novo Lesions Lesion length ≤ 20mm Angiographic follow-up Cohort A: 6 months Cohort B: 9 months Study Design – Cohort C Safety and Performance Trial 50 patients in single cohort 2.5mm to 3.5mm vessels Long Lesions and multiple vessel disease Lesion length ≥ 20mm and/or Treatment of 2 – 3 vessel disease Angiographic follow-up Cohort C: 6 months
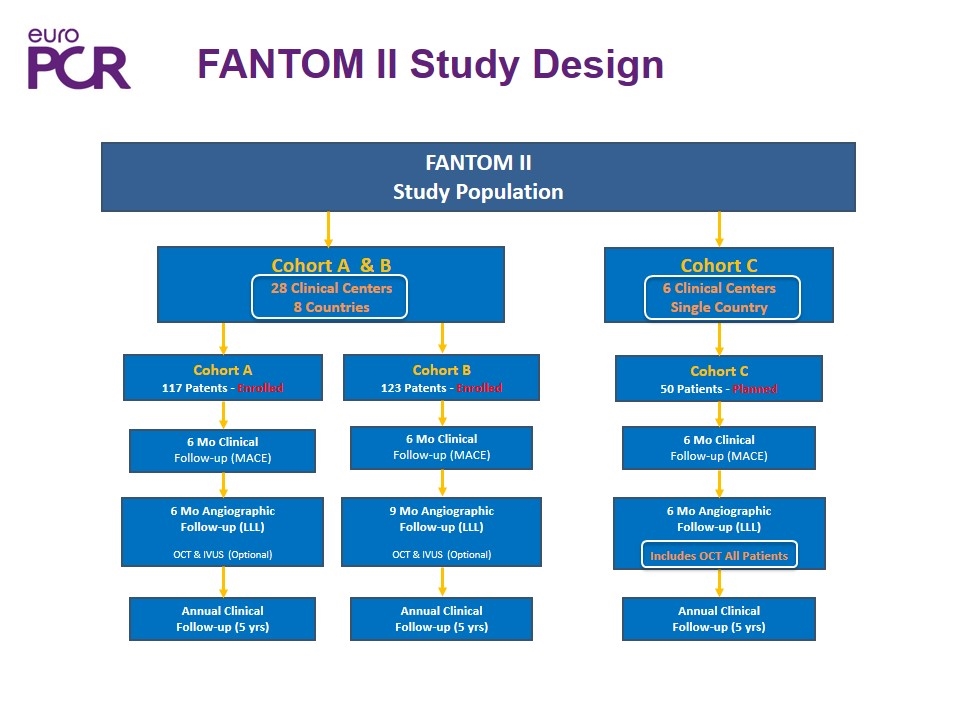
FANTOM II Study Design FANTOM II Study Population Cohort A 117 Patents - Enrolled 6 Mo Clinical Follow-up (MACE) Cohort B 123 Patents - Enrolled 6 Mo Clinical Follow-up (MACE) 6 Mo Angiographic Follow-up (LLL) OCT & IVUS (Optional) 9 Mo Angiographic Follow-up (LLL) OCT & IVUS (Optional) Annual Clinical Follow-up (5 yrs) Annual Clinical Follow-up (5 yrs) Cohort C 50 Patients - Planned 6 Mo Clinical Follow-up (MACE) 6 Mo Angiographic Follow-up (LLL) Includes OCT All Patients Annual Clinical Follow-up (5 yrs) Cohort A & B 28 Clinical Centers 8 Countries Cohort C 6 Clinical Centers Single Country
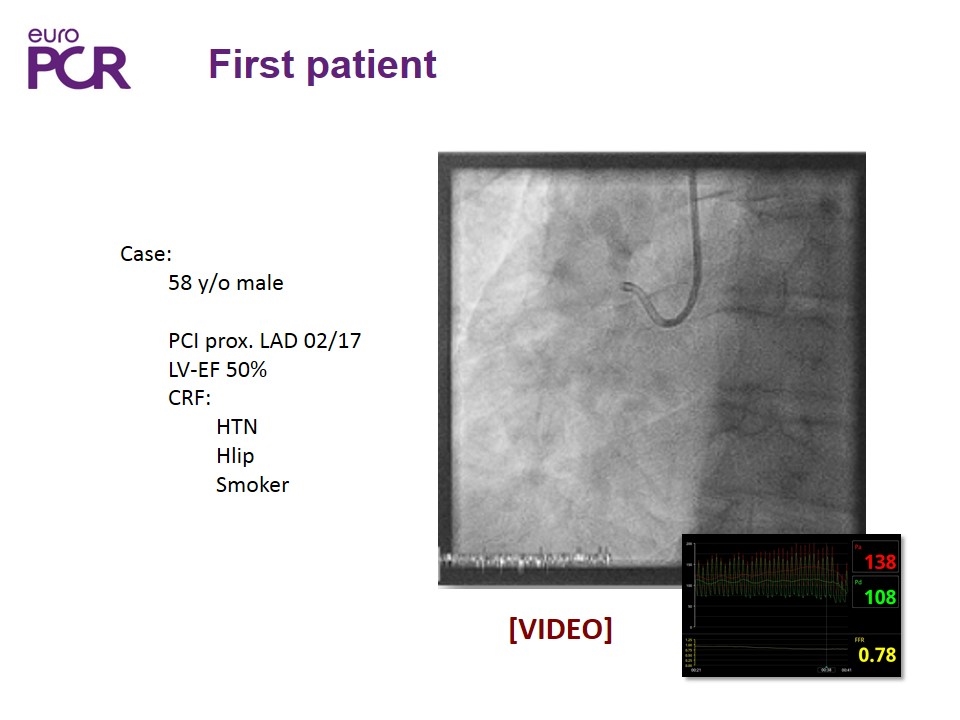
First patient Case: 58 y/o male PCI prox. LAD 02/17 LV-EF 50% CRF: HTN Hlip Smoker [VIDEO]
NC 3.0 x 15 mm @ 14 bar First patient - RCA Delivery 3.0 x 24 mm Fantom [VIDEO] [VIDEO]
First patient - RCA NC 3.0 x 15 mm @ 14 bar 3.0 x 24 mm Fantom @ 12 bar [VIDEO]
2. Fantom crossing First patient - RCA [VIDEO]
3.0 x 24 mm Fantom @ 16 bar [VIDEO] First patient - RCA
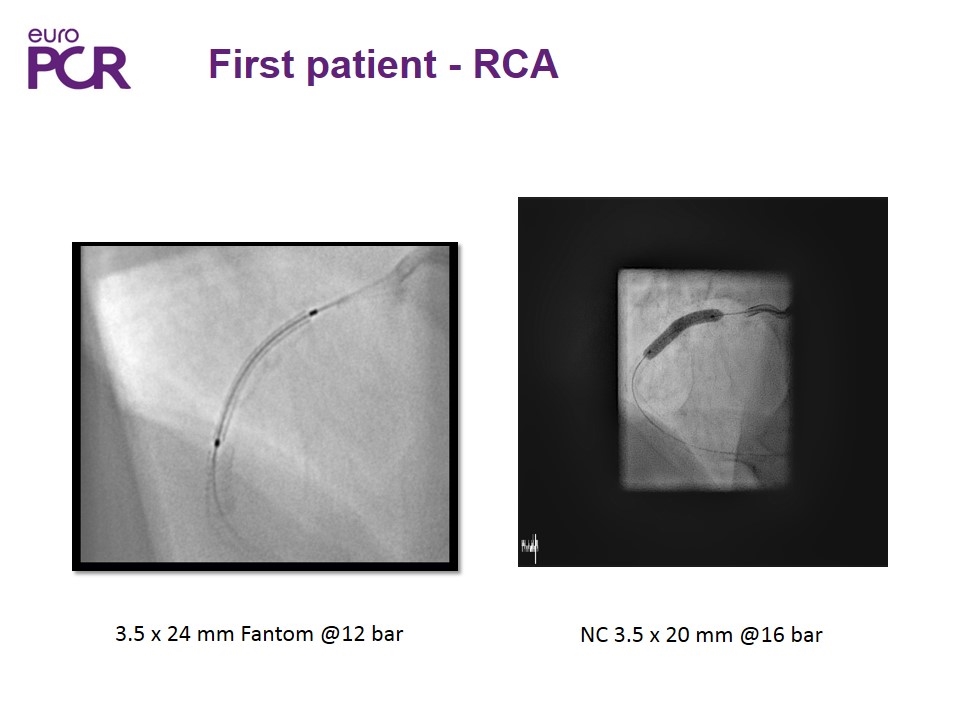
3.5 x 24 mm Fantom @12 bar First patient - RCA NC 3.5 x 20 mm @16 bar
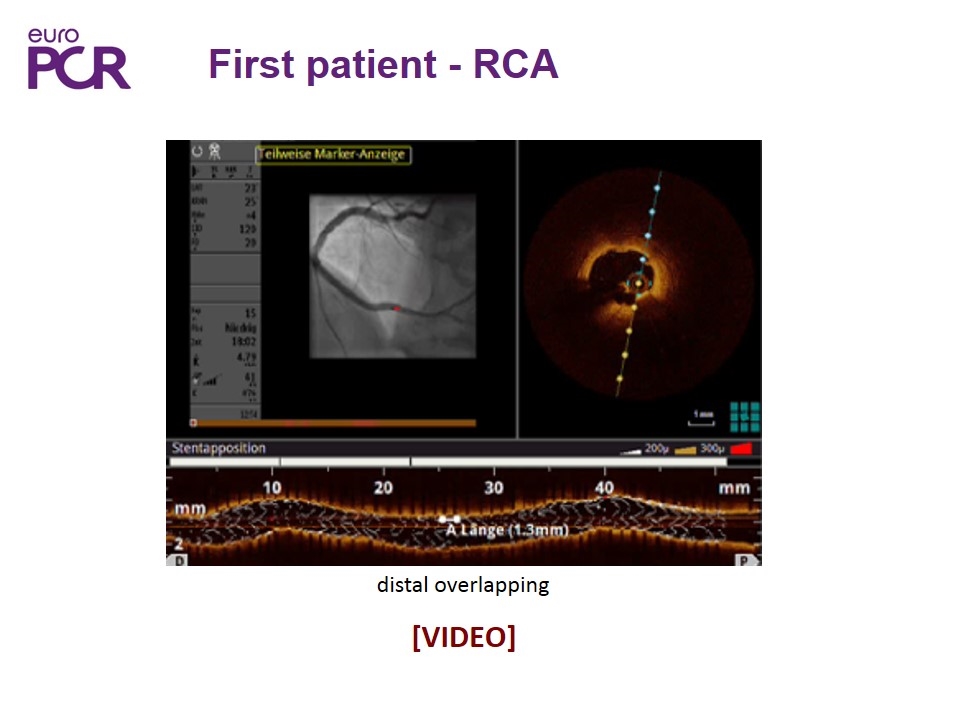
First patient - RCA distal overlapping [VIDEO]
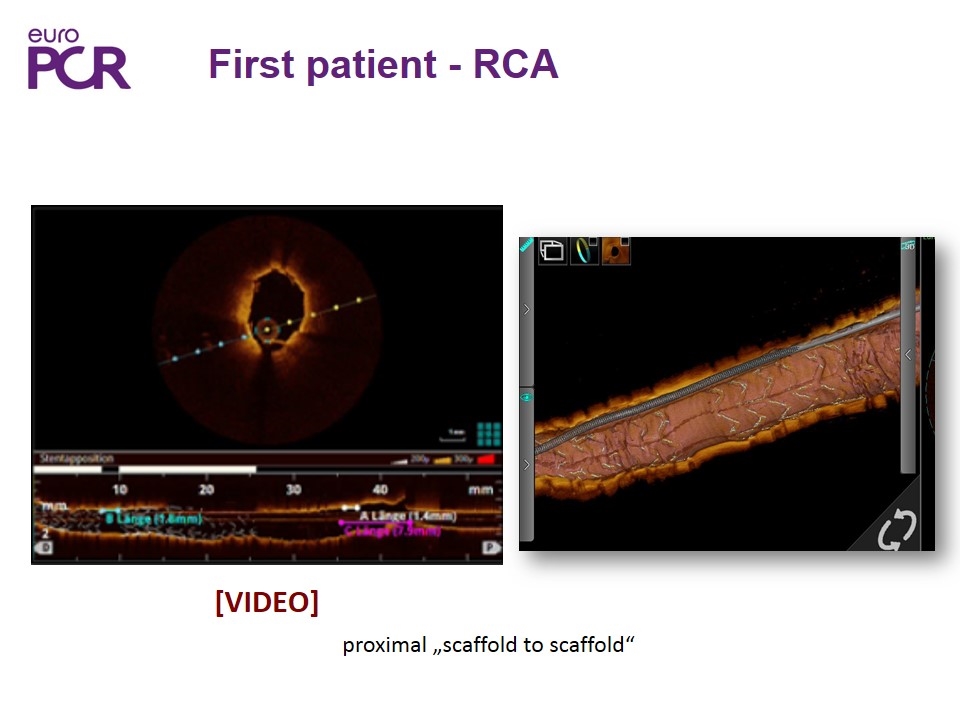
First patient - RCA proximal „scaffold to scaffold“ [VIDEO]
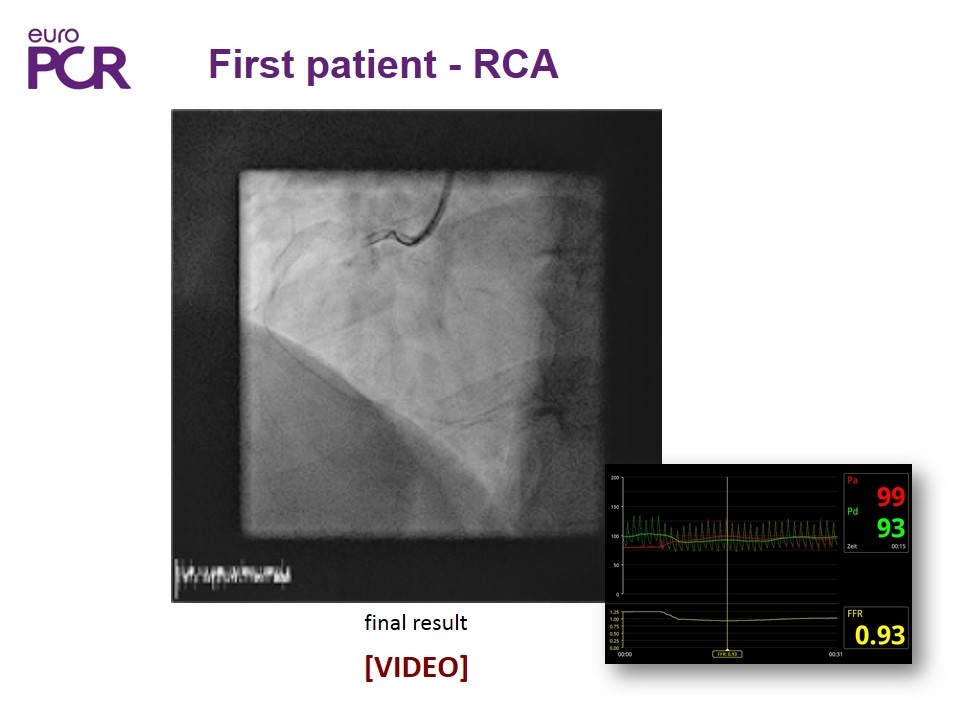
First patient - RCA final result [VIDEO]
Case observations: Able to deliver multiple scaffolds in a single vessel Radiopacity makes visualisation of edge to edge or slight overlap possible Acute performance in first case positive - clinical study ongoing Fantom II – Cohort C














