
The FANTOM Scaffold Expanding the use of Fantom BRS in worldwide markets: REVA’s global clinical trial programme and beyond Gregg W. Stone MD Columbia University Medical Center The Cardiovascular Research Foundation Exhibit 99.5

Potential conflicts of interest Speaker's name: Dr. Gregg W. Stone I have the following potential conflicts of interest to report: Consultant to REVA Medical, Inc.
Fantom Clinical Programs & Beyond Clinical Trials that have Reached Their Primary Endpoint New Clinical Programs: Now Enrolling Planned Future Clinical Programs New Product Advancements Potential Product Applications
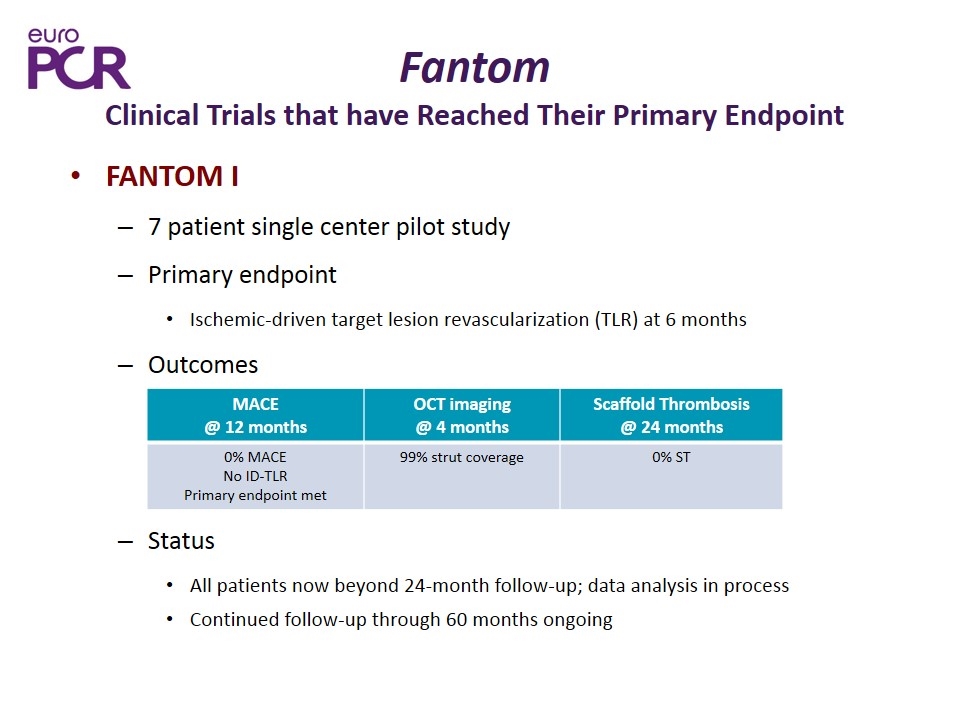
Fantom Clinical Trials that have Reached Their Primary Endpoint FANTOM I 7 patient single center pilot study Primary endpoint Ischemic-driven target lesion revascularization (TLR) at 6 months Outcomes Status All patients now beyond 24-month follow-up; data analysis in process Continued follow-up through 60 months ongoing MACE @ 12 months OCT imaging @ 4 months Scaffold Thrombosis @ 24 months 0% MACE No ID-TLR Primary endpoint met 99% strut coverage 0% ST
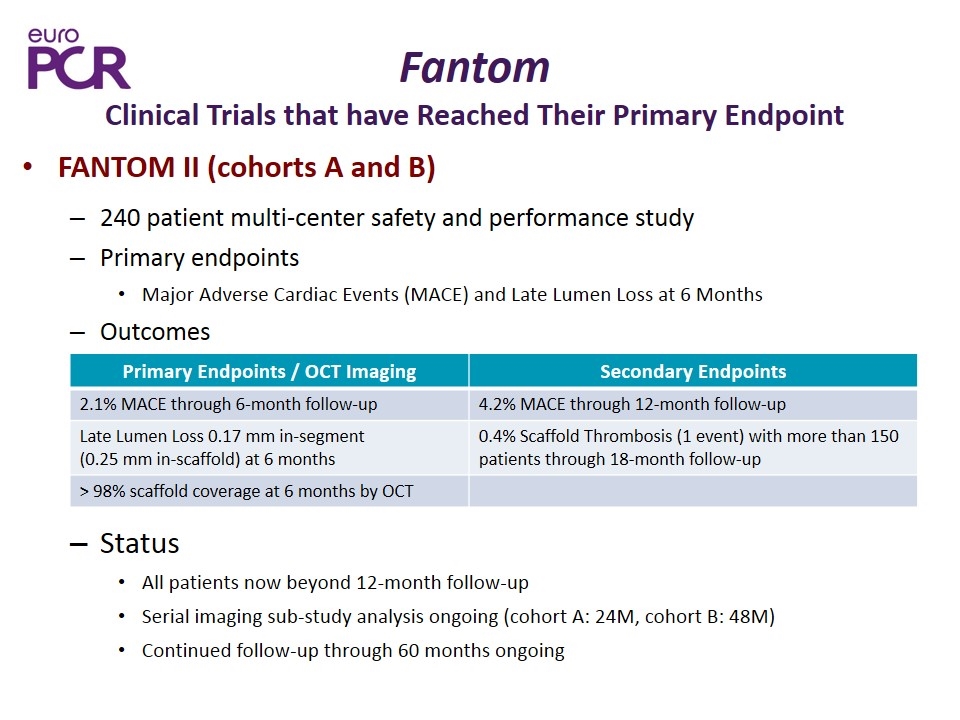
FANTOM II (cohorts A and B) 240 patient multi-center safety and performance study Primary endpoints Major Adverse Cardiac Events (MACE) and Late Lumen Loss at 6 Months Outcomes Status All patients now beyond 12-month follow-up Serial imaging sub-study analysis ongoing (cohort A: 24M, cohort B: 48M) Continued follow-up through 60 months ongoing Primary Endpoints / OCT Imaging Secondary Endpoints 2.1% MACE through 6-month follow-up 4.2% MACE through 12-month follow-up Late Lumen Loss 0.17 mm in-segment (0.25 mm in-scaffold) at 6 months 0.4% Scaffold Thrombosis (1 event) with more than 150 patients through 18-month follow-up > 98% scaffold coverage at 6 months by OCT Fantom Clinical Trials that have Reached Their Primary Endpoint

Fantom New Clinical Programs: Enrolling More Complex Patients FANTOM II (Cohort C) – Long Lesions / Multiple Vessels 50 patient multicenter study Population Patients with lesions >20 mm in length requiring two or more scaffolds in the same vessel, and patients requiring treatment of multiple lesions in more than one vessel Primary Endpoints Major Adverse Cardiac Events (MACE) at 6 Months Late Lumen Loss at 6 Months Status Initiated April 2017, enrollment ongoing
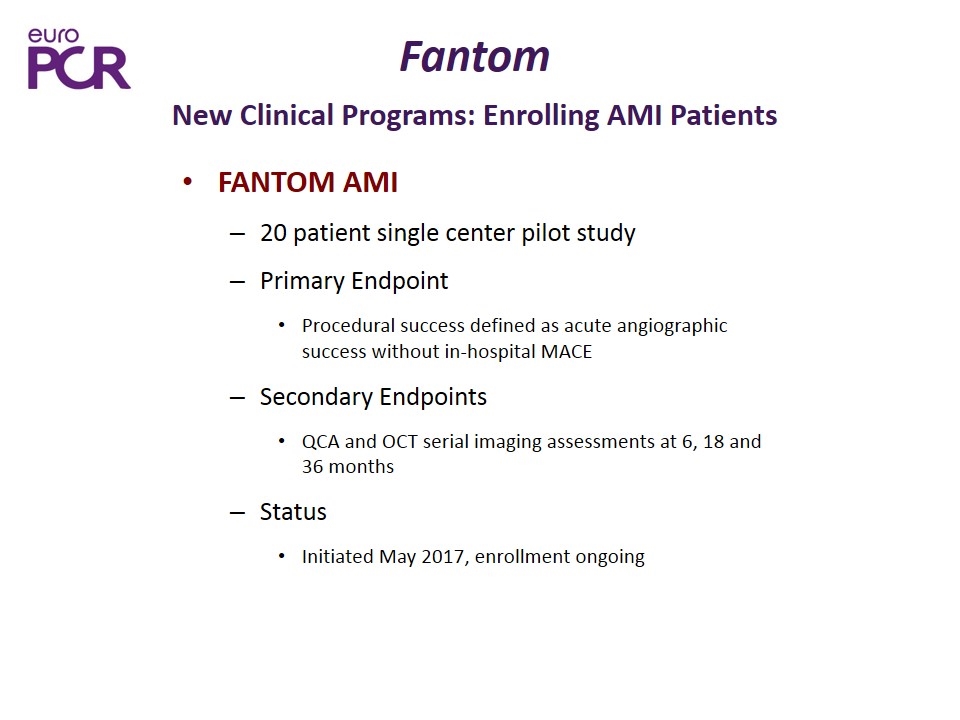
Fantom New Clinical Programs: Enrolling AMI Patients FANTOM AMI 20 patient single center pilot study Primary Endpoint Procedural success defined as acute angiographic success without in-hospital MACE Secondary Endpoints QCA and OCT serial imaging assessments at 6, 18 and 36 months Status Initiated May 2017, enrollment ongoing
Fantom Planned Future Clinical Programs FANTOM III (US Pivotal Trial) Randomized controlled multicenter study Sample size: 1800 – 2200 patients Control: Metallic drug-eluting stent Primary Endpoint: Target Lesion Failure (TLF) at 12 months (non-inferiority) Secondary Endpoints (tentative): QCA & OCT derived parameters at 3 years Status Currently in active discussions with FDA on study design Planned Initiation: Q1/Q2 2018

Fantom Planned Future Clinical Programs FANTOM Japan Trial design currently in early planning phase Typical pivotal trial design Randomized controlled multi-center study Sample size: 350 - 400 patients Control: Metallic drug-eluting stent Primary Endpoint: Target Lesion Failure at 12 Months Status Actively evaluating trial design options
Fantom New Product Advancements Fantom Gen 2 Advanced Fantom scaffold with sub-100 micron strut thickness Designed for use in smaller vessels (≤2.5 mm) First product size 2.5 mm diameter in multiple lengths Status Actively qualifying design enhancements Planned introduction Q1/Q2 2018; pending regulatory approval
Fantom Potential New Product Applications FANTOM Peripheral Fantom scaffold with design features needed for peripheral applications Design Considerations Evaluating potential use in SFA application Evaluating potential use in BTK application Status Actively qualifying design enhancements

Fantom Global Clinical Trial Programme and Device Development Conclusions Fantom has demonstrated initial device safety through 12 months 4.2% MACE 0.4% scaffold thrombosis Good late lumen loss, with excellent strut coverage at 6 months 0.17 mm in-segment, 0.25 mm in-scaffold >98% scaffold coverage at 6 months by OCT Fantom clinical program & product development expanding Expanding geographical approvals Evaluating coronary and non-coronary indication expansion Evaluating design enhancements (e.g. thinner struts)











