
New 24-month data from the FANTOM II clinical trial Dr. Alexandre Abizaid Instituto Dante Pazzanese de Cardiologia Sao Paulo, Brazil Exhibit 99.1

Speaker's name: Alexandre Abizaid, MD x I have the following potential conflicts of interest to report: None Potential conflicts of interest
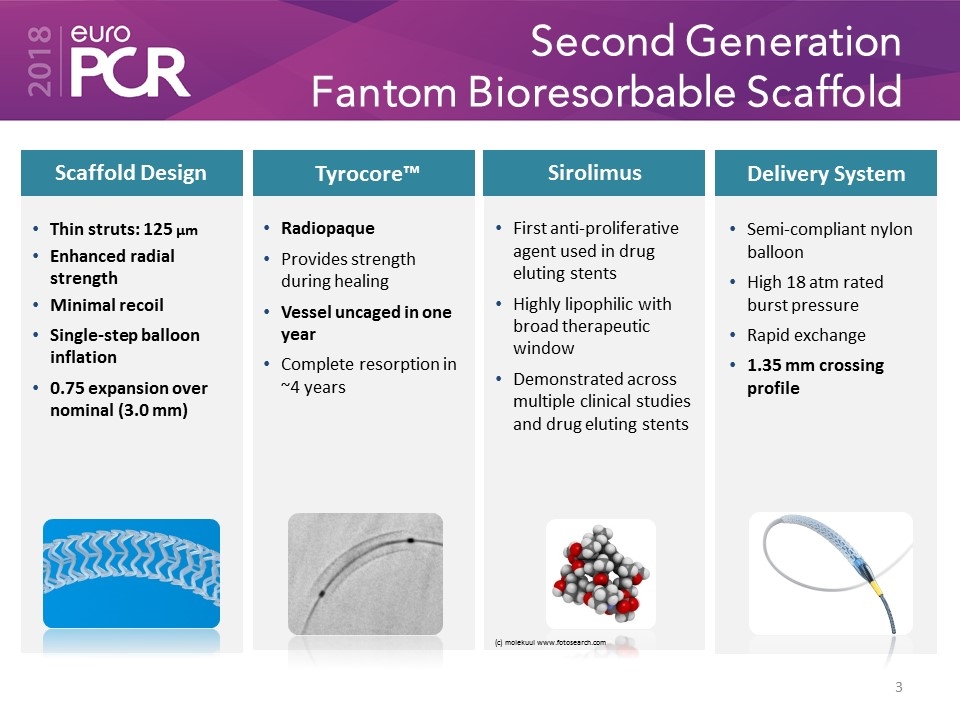
Second Generation Fantom Bioresorbable Scaffold Sirolimus Scaffold Design Delivery System Thin struts: 125 µm Enhanced radial strength Minimal recoil Single-step balloon inflation 0.75 expansion over nominal (3.0 mm) Semi-compliant nylon balloon High 18 atm rated burst pressure Rapid exchange 1.35 mm crossing profile First anti-proliferative agent used in drug eluting stents Highly lipophilic with broad therapeutic window Demonstrated across multiple clinical studies and drug eluting stents (c) molekuul www.fotosearch.com Tyrocore™ Radiopaque Provides strength during healing Vessel uncaged in one year Complete resorption in ~4 years

FANTOM II Trial Safety & Performance Study for the Fantom Bioresorbable Scaffold

FANTOM II Study Investigators Australia Dr. Muller, Dr. Jepson, Dr. Walters Belgium Dr. De Bruyne Brazil Dr. Abizaid, Dr. Costa, Dr. Chamie, Dr. Perin Denmark Dr. Christiansen, Dr. Lassen, Dr. Okkels-Jensen France Dr. Carrié, Dr. Chevalier, Dr. Fajadet, Dr. Collet Germany Dr. Weber-Albers, Dr. Naber, Dr. Achenbach, Dr. Frey, Dr. Lutz, Dr. Kische, Dr. Ince, Dr. Brachmann Netherlands Dr. Amoroso, Dr. Wykrzykowska, Dr. Daemen Poland Dr. Dudek, Dr. Kochman, Dr. Koltowski, Dr. Lesiak, Dr. Wojdyla
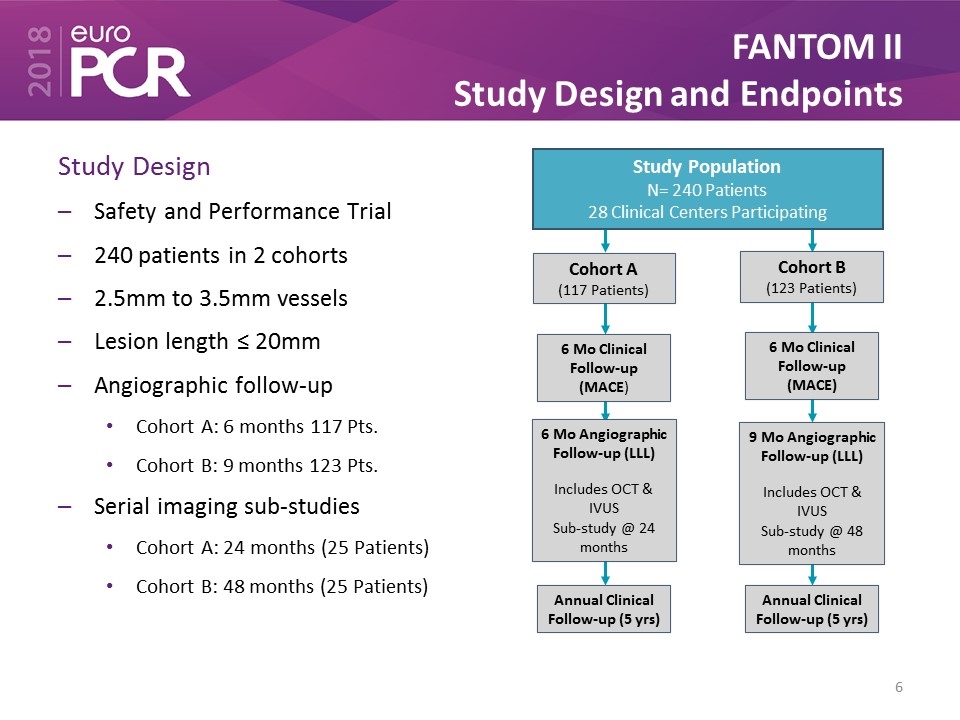
FANTOM II Study Design and Endpoints Study Design Safety and Performance Trial 240 patients in 2 cohorts 2.5mm to 3.5mm vessels Lesion length ≤ 20mm Angiographic follow-up Cohort A: 6 months 117 Pts. Cohort B: 9 months 123 Pts. Serial imaging sub-studies Cohort A: 24 months (25 Patients) Cohort B: 48 months (25 Patients) Study Population N= 240 Patients 28 Clinical Centers Participating Cohort A (117 Patients) 6 Mo Clinical Follow-up (MACE) Cohort B (123 Patients) 6 Mo Clinical Follow-up (MACE) 6 Mo Angiographic Follow-up (LLL) Includes OCT & IVUS Sub-study @ 24 months 9 Mo Angiographic Follow-up (LLL) Includes OCT & IVUS Sub-study @ 48 months Annual Clinical Follow-up (5 yrs) Annual Clinical Follow-up (5 yrs)

FANTOM II – Cohorts A & B Patient Flow and Baseline Characteristics Patient Characteristics (N=240) Patient Age (average years) 62.7 ± 10.1 Male 70.4% Diabetes 23.8% Current/Former Smoker 59.6% Hypertension 73.8% Hyperlipidemia 70.8% Prior PCI 43.8% Prior CABG 2.9% Prior MI 26.3% Recent LVEF <40% 0.0% (N=231) 6 & 9 Month Follow-up Clinical & Imaging Cohort A: 6 months Cohort B: 9 months 12 Month Follow-up Clinical Angiographic (cohort A N=100) (cohort B N=105) OCT (cohort A N=73) (cohort B N= 80) IVUS (cohort A N=45) (cohort B N=27) 24 Month Follow-up Clinical Imaging Sub-set Annual Follow-up Through 5 years Study Population N= 240 Patients 28 Clinical Centers
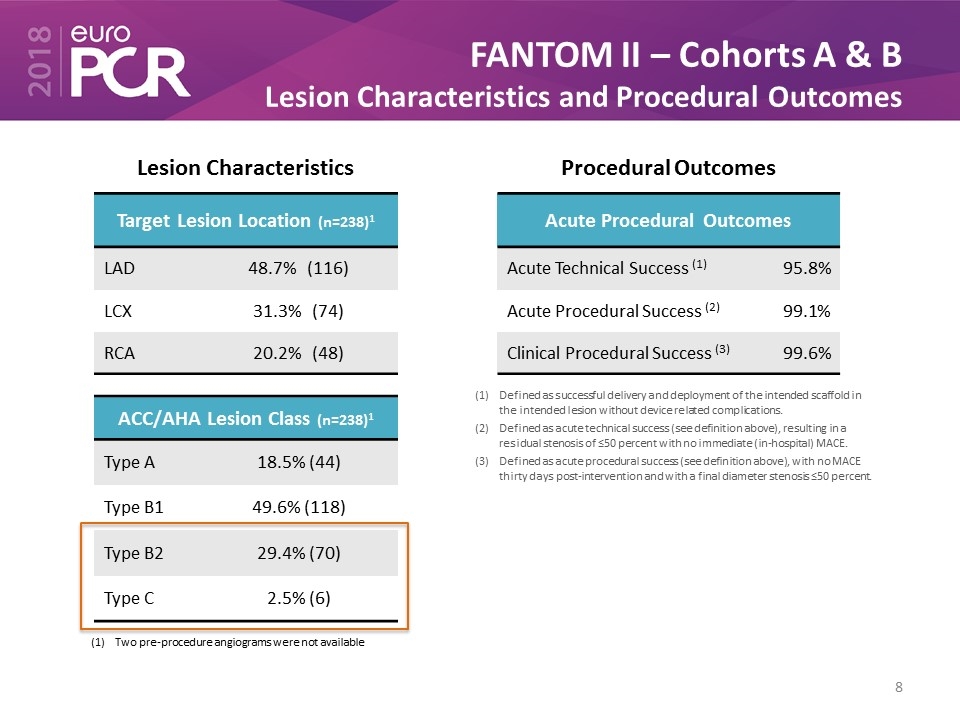
FANTOM II – Cohorts A & B Lesion Characteristics and Procedural Outcomes Target Lesion Location (n=238)1 LAD 48.7% (116) LCX 31.3% (74) RCA 20.2% (48) ACC/AHA Lesion Class (n=238)1 Type A 18.5% (44) Type B1 49.6% (118) Type B2 29.4% (70) Type C 2.5% (6) Two pre-procedure angiograms were not available Lesion Characteristics Procedural Outcomes Defined as successful delivery and deployment of the intended scaffold in the intended lesion without device related complications. Defined as acute technical success (see definition above), resulting in a residual stenosis of ≤50 percent with no immediate (in-hospital) MACE. Defined as acute procedural success (see definition above), with no MACE thirty days post-intervention and with a final diameter stenosis ≤50 percent. Acute Procedural Outcomes Acute Technical Success (1) 95.8% Acute Procedural Success (2) 99.1% Clinical Procedural Success (3) 99.6%
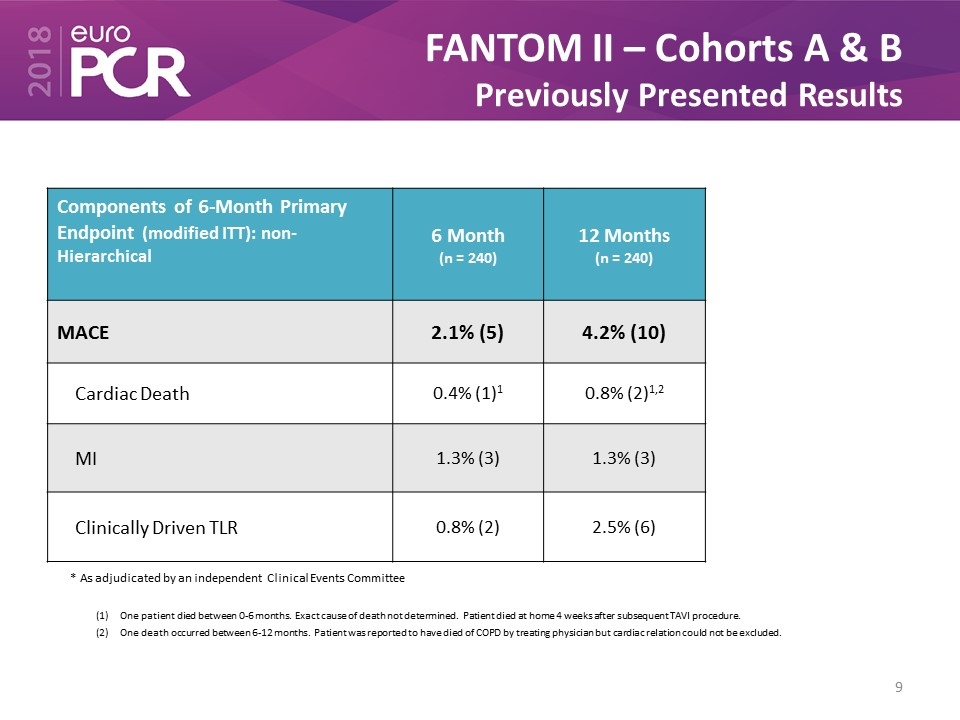
FANTOM II – Cohorts A & B Previously Presented Results * As adjudicated by an independent Clinical Events Committee One patient died between 0-6 months. Exact cause of death not determined. Patient died at home 4 weeks after subsequent TAVI procedure. One death occurred between 6-12 months. Patient was reported to have died of COPD by treating physician but cardiac relation could not be excluded. Components of 6-Month Primary Endpoint (modified ITT): non-Hierarchical 6 Month (n = 240) 12 Months (n = 240) MACE 2.1% (5) 4.2% (10) Cardiac Death 0.4% (1)1 0.8% (2)1,2 MI 1.3% (3) 1.3% (3) Clinically Driven TLR 0.8% (2) 2.5% (6)
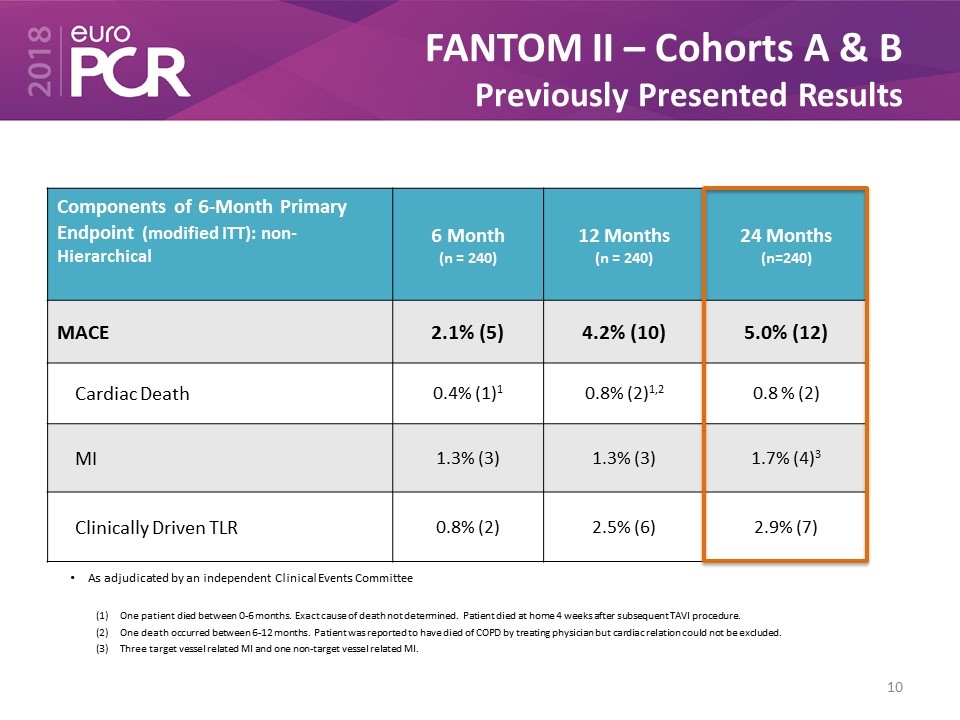
FANTOM II – Cohorts A & B Previously Presented Results Components of 6-Month Primary Endpoint (modified ITT): non-Hierarchical 6 Month (n = 240) 12 Months (n = 240) 24 Months (n=240) MACE 2.1% (5) 4.2% (10) 5.0% (12) Cardiac Death 0.4% (1)1 0.8% (2)1,2 0.8 % (2) MI 1.3% (3) 1.3% (3) 1.7% (4)3 Clinically Driven TLR 0.8% (2) 2.5% (6) 2.9% (7) As adjudicated by an independent Clinical Events Committee One patient died between 0-6 months. Exact cause of death not determined. Patient died at home 4 weeks after subsequent TAVI procedure. One death occurred between 6-12 months. Patient was reported to have died of COPD by treating physician but cardiac relation could not be excluded. Three target vessel related MI and one non-target vessel related MI.

FANTOM II – Cohorts A & B 24-Month Scaffold Thrombosis As adjudicated by an independent Clinical Events Committee ** Maximum day=761 days Definite or Probable Scaffold Thrombosis (N = 240 Patients) Acute (0 – 1 day) 0.0% (0) Sub-acute (2 – 30 days) 0.4% (1)1 Late (31 – 365 days) 0.0% (0) Very Late (>365 days)** 0.4% (1)2 Target lesion was not fully covered with scaffold. Significant untreated stenosis was present at index procedure. Patient returned 5 days post procedure with a scaffold thrombosis Distal segment of scaffold was in a 2.0mm vessel and the scaffold had significant malaposition that was not corrected
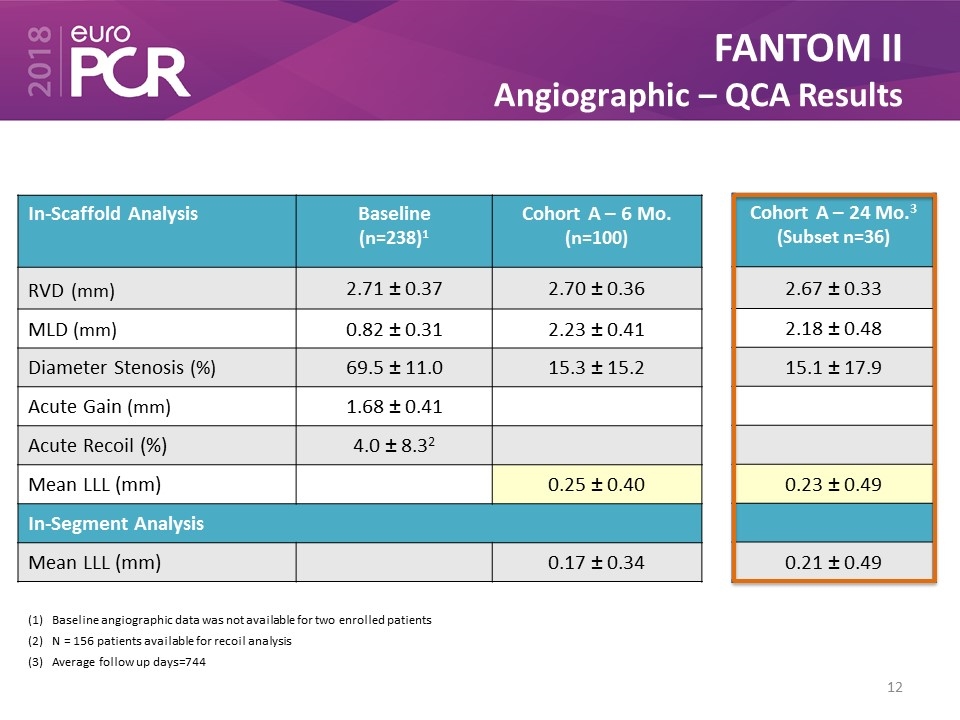
FANTOM II Angiographic – QCA Results In-Scaffold Analysis Baseline (n=238)1 Cohort A – 6 Mo. (n=100) RVD (mm) 2.71 ± 0.37 2.70 ± 0.36 MLD (mm) 0.82 ± 0.31 2.23 ± 0.41 Diameter Stenosis (%) 69.5 ± 11.0 15.3 ± 15.2 Acute Gain (mm) 1.68 ± 0.41 Acute Recoil (%) 4.0 ± 8.32 Mean LLL (mm) 0.25 ± 0.40 In-Segment Analysis Mean LLL (mm) 0.17 ± 0.34 Baseline angiographic data was not available for two enrolled patients N = 156 patients available for recoil analysis Average follow up days=744 Cohort A – 24 Mo.3 (Subset n=36) 2.67 ± 0.33 2.18 ± 0.48 15.1 ± 17.9 0.23 ± 0.49 0.21 ± 0.49
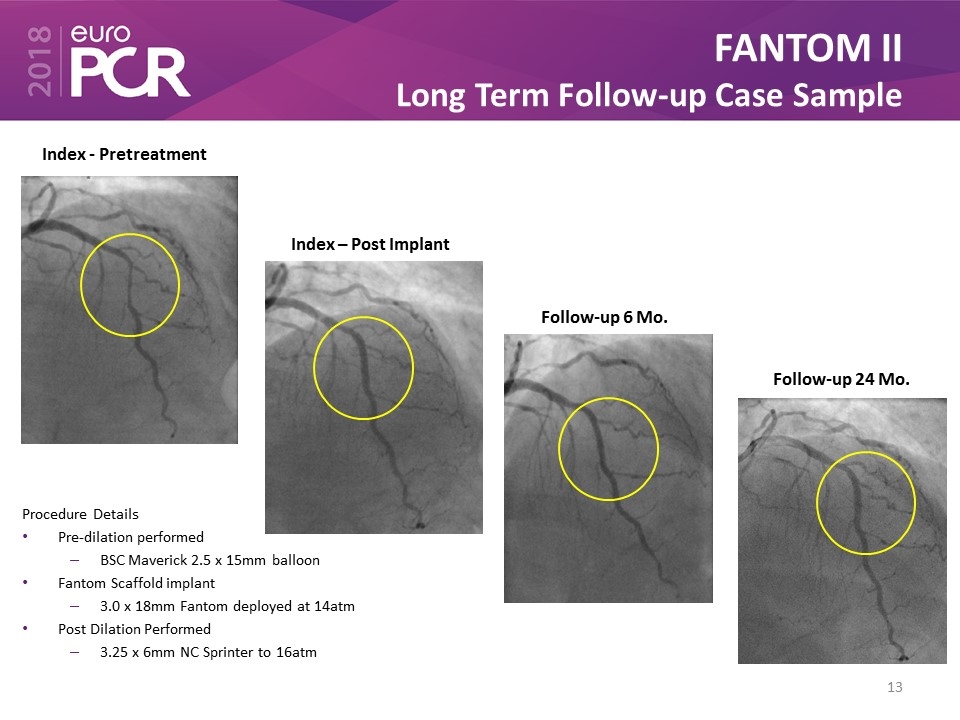
FANTOM II Long Term Follow-up Case Sample Index - Pretreatment Index – Post Implant Follow-up 6 Mo. Follow-up 24 Mo. Procedure Details Pre-dilation performed BSC Maverick 2.5 x 15mm balloon Fantom Scaffold implant 3.0 x 18mm Fantom deployed at 14atm Post Dilation Performed 3.25 x 6mm NC Sprinter to 16atm
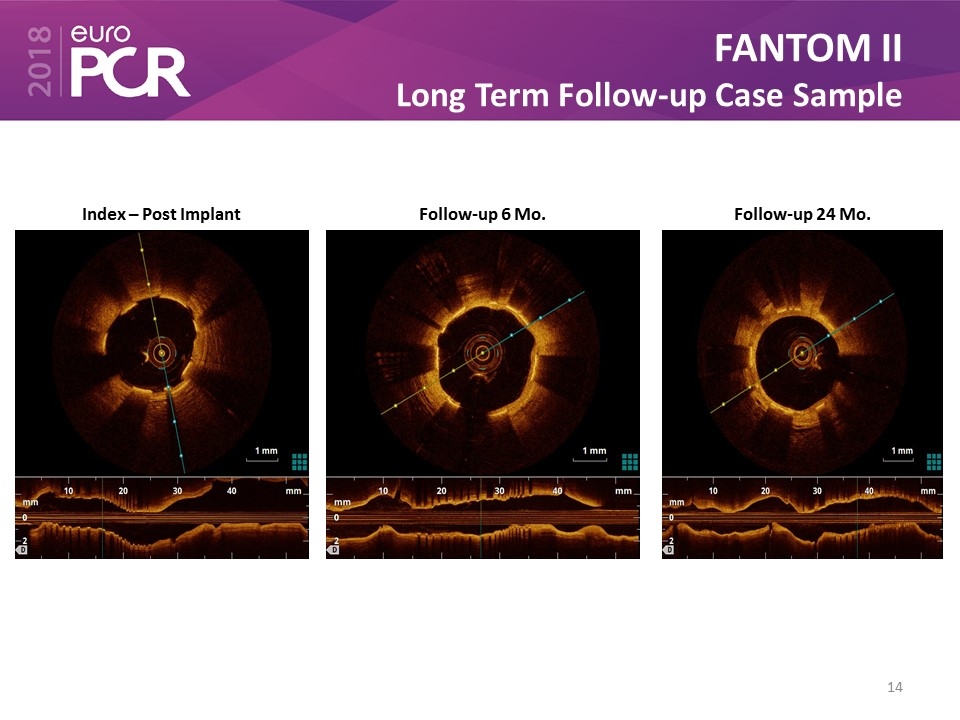
FANTOM II Long Term Follow-up Case Sample Index – Post Implant Follow-up 6 Mo. Follow-up 24 Mo.
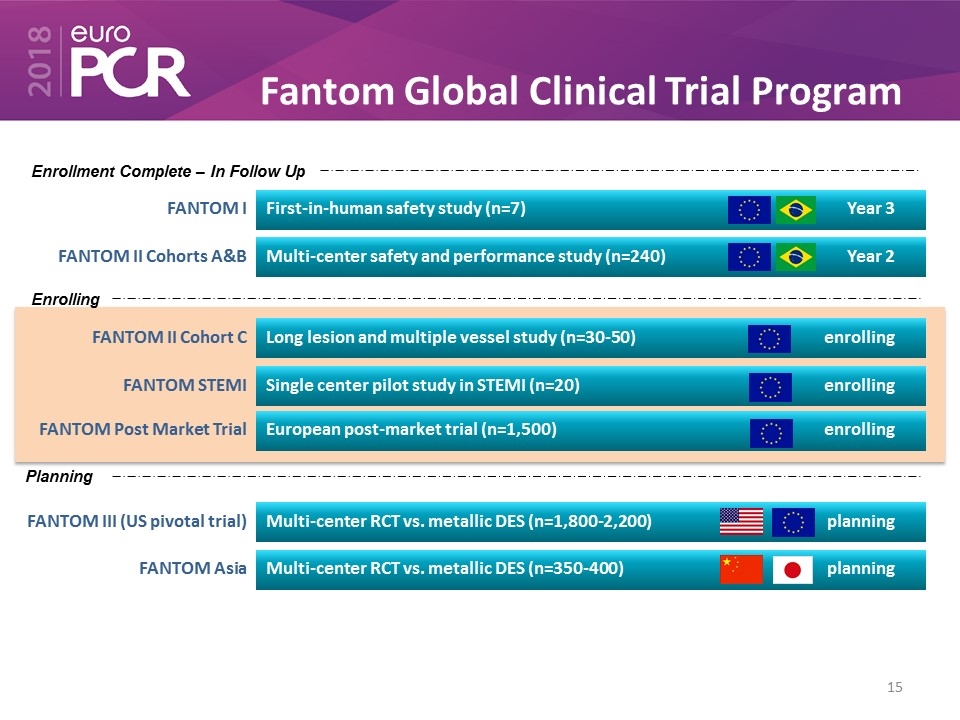
Fantom Global Clinical Trial Program FANTOM I First-in-human safety study (n=7) Year 3 FANTOM II Cohorts A&B Multi-center safety and performance study (n=240) Year 2 FANTOM II Cohort C Long lesion and multiple vessel study (n=30-50) enrolling FANTOM STEMI Single center pilot study in STEMI (n=20) enrolling FANTOM Post Market Trial European post-market trial (n=1,500) enrolling FANTOM III (US pivotal trial) Multi-center RCT vs. metallic DES (n=1,800-2,200) planning FANTOM Asia Multi-center RCT vs. metallic DES (n=350-400) planning Enrollment Complete – In Follow Up Enrolling Planning
FANTOM Program Clinical Summary Only BRS with unique Tyrocore polymer Thin struts, radiopaque, enhanced radial force, biocompatible Sustained safety and efficacy through 24-months: Low MACE Rate (5.0%) Low rate of VLST (0.4%) Vessel lumen is maintained through 24 months: No change in average late lumen loss No evidence of chronic scaffold recoil

Thank you!
















