Exhibit 99.1

Corporate Presentation Nasdaq: PLXP Confidential

Confidential 2 Forward - Looking Statements This presentation includes or incorporates by reference statements that constitute forward - looking statements within the meaning of Section 27 A of the Securities Act of 1933 , as amended, and Section 21 E of the Securities Exchange Act of 1934 , as amended . These statements relate to future events or to our future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance, or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward - looking statements . These statements include, but are not limited to information or assumptions about expenses, capital and other expenditures, financing plans, capital structure, cash flow, liquidity, management’s plans, goals and objectives for future operations and growth . In some cases, you can identify forward - looking statements by the use of words such as “may,” “could,” “expect,” “intend,” “plan,” “seek,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue,” or the negative of these terms or other comparable terminology . You should not place undue reliance on forward - looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases beyond our control and which could cause actual performance or results to differ materially from those expressed in or suggested by forward - looking statements . Important factors that could cause such differences include, but are not limited to ( i ) our ability to bring both VAZALORE™ 81 mg and VAZALORE 325 mg to market - readiness ; (ii) our ability to maintain regulatory approval of VAZALORE 325 mg or obtain and maintain regulatory approval of VAZALORE 81 mg and any future product candidates ; (iii) the benefits of the use of VAZALORE 325 mg and VAZALORE 81 mg ; (iv) our ability to successfully commercialize our VAZALORE products, or any future product candidates ; (v) the rate and degree of market acceptance of our VAZALORE products or any future product candidates ; (vi) our ability to scale up manufacturing of our VAZALORE products to commercial scale ; (vii) our ability to successfully build a specialty sales force and commercial infrastructure or collaborate with a firm that has these capabilities ; (viii) our ability to compete with companies currently producing GI - safer technologies for NSAIDs and other analgesics ; (ix) our reliance on third parties to conduct our clinical studies ; (x) our reliance on third - party contract manufacturers to manufacture and supply our product candidates for us ; (xi) our ability to retain and recruit key personnel, including development of a sales and marketing function ; and (xii) our ability to obtain and maintain intellectual property protection for our VAZALORE products or any future product candidates . Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, actual results may vary in material respects from those projected in these forward - looking statements . We do not undertake any obligation to update or revise any forward - looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws .

Confidential 3 Our Mission PLx Pharma is focused on improving the performance of established therapeutic agents with the proprietary PLxGuard ™ targeted drug delivery platform . We are driven to transform the standard of care for millions of patients.
Confidential 4 PLx Pharma Investment Highlights: Innovative Drug Delivery Platform Overcomes limitations of enteric - coated aspirin with better absorption and safety Large OTC opportunity launching late 2020 , with $10 billion market opportunity A better, liquid aspirin for over 40 million patients at risk for vascular events World - renowned scientific advisory board chaired by Drs. Deepak Bhatt & Gabriel Steg High physician interest with >80% of specialists intending to prescribe VAZALORE ● Better efficacy and improved GI safety for superior benefit - risk profile ● Novel mechanism of action enables strong patent life for multiple APIs Lead Product: VAZALORE ™

Confidential 5 Advancing the Standard of Care Coming Soon

Confidential 6 Current Aspirin Therapy Landscape Primary Prevention – People without vascular disease ● Daily aspirin for these low risk individuals not routinely recommended ● Doctors can recommend on a case by case basis (i.e. patients with diabetes or low risk of bleeding) Secondary Prevention – Patients with Vascular Disease ● Daily aspirin therapy is foundational therapy for 25 million Americans with vascular disease ● Highest recommendation from American Heart Association and American College of Cardiology Current SOC: EC aspirin Unmet need because: • Erratic absorption • Unreliable platelet inhibition • Does not improve GI safety Formulation Matters Advancing the SOC: VAZALORE Improved performance because: x Fast, predictable absorption x Reliable platelet inhibition x Lower risk for stomach ulcers CV : cardiovascular; EC : enteric coated; SOC : standard of care Our Target Not new news
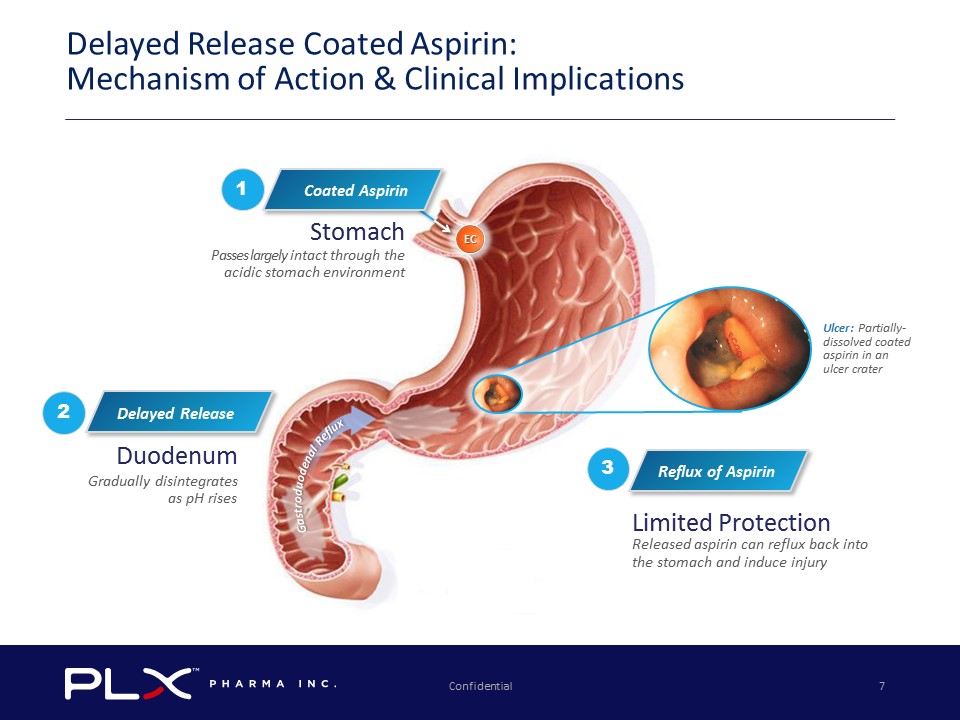
Confidential 7 Delayed Release Coated Aspirin: Mechanism of Action & Clinical Implications Reflux of Aspirin 3 Ulcer: Partially - dissolved coated aspirin in an ulcer crater Stomach P asses largely i ntact through the acidic s tomach environment Duodenum Gradually disintegrates as pH rises Limited Protection Released aspirin can reflux back into the stomach and induce injury 2 EC Coated Aspirin 1 Delayed Release
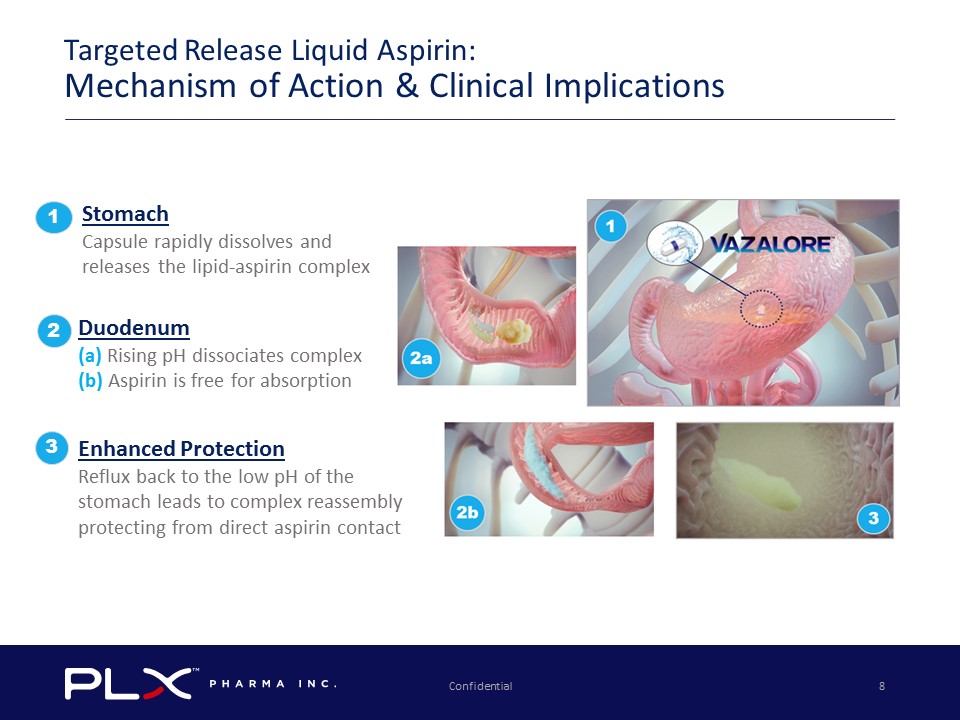
Targeted Release Liquid Aspirin: Mechanism of Action & Clinical Implications Stomach Capsule rapidly dissolves and releases the lipid - aspirin complex 1 Duodenum (a) Rising pH dissociates complex (b) Aspirin is free f or absorption 2 Enhanced Protection Reflux back to the low pH of the stomach leads to complex reassembly protecting from direct aspirin contact 3 Confidential 8
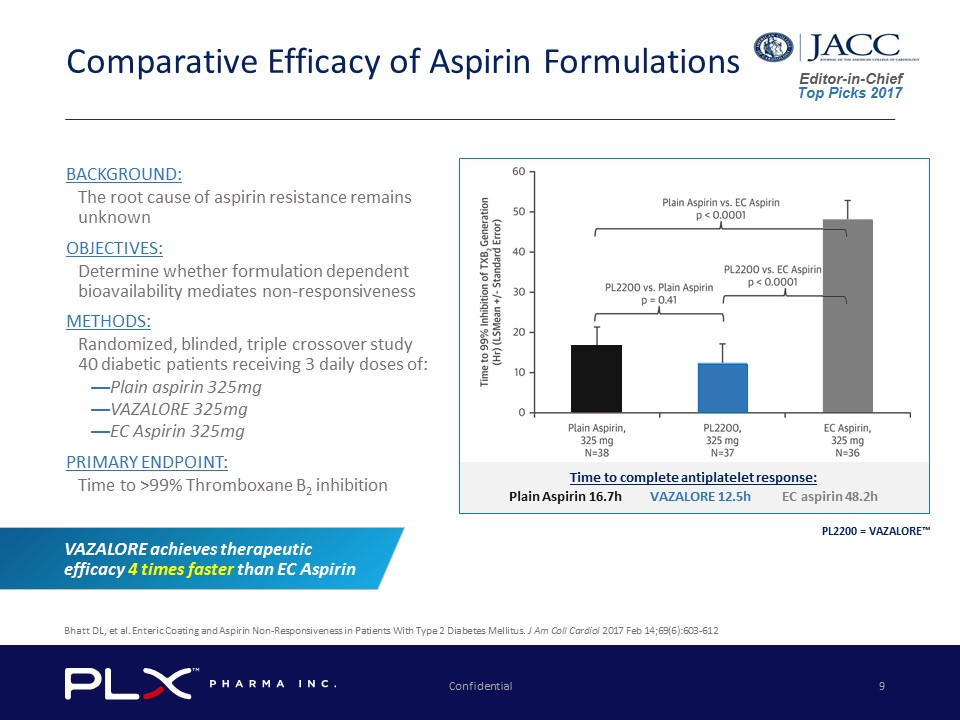
Editor - in - Chief Top Picks 2017 Confidential 9 Comparative Efficacy of Aspirin Formulations Time to complete antiplatelet response: Plain Aspirin 16.7h VAZALORE 12.5h EC aspirin 48.2h BACKGROUND: The root cause of aspirin resistance remains unknown OBJECTIVES: Determine whether formulation dependent bioavailability mediates non - responsiveness METHODS: Randomized, blinded, triple crossover study 40 diabetic patients receiving 3 daily doses of: — Plain aspirin 325mg — VAZALORE 325mg — EC Aspirin 325mg PRIMARY ENDPOINT: Time to >99% Thromboxane B 2 inhibition VAZALORE achieves therapeutic efficacy 4 times faster than EC Aspirin Bhatt DL, et al. Enteric Coating and Aspirin Non - Responsiveness in Patients With Type 2 Diabetes Mellitus. J Am Coll Cardiol 2017 Feb 14;69(6):603 - 612 PL2200 = VAZALORE™
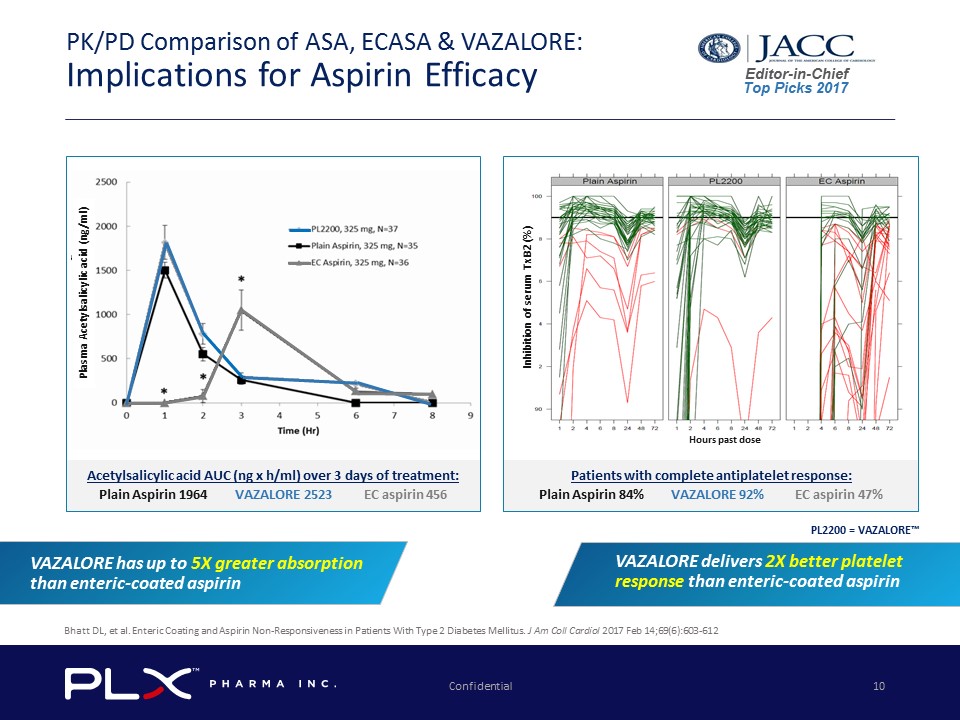
Confidential 10 PK/PD Comparison of ASA, ECASA & VAZALORE: Implications for Aspirin Efficacy Inhibition of serum TxB2 (%) Hours past dose Plasma Acetylsalicylic acid (ng/ml) PL2200 = VAZALORE™ Patients with complete antiplatelet response: Plain Aspirin 84% VAZALORE 92% EC aspirin 47% Acetylsalicylic acid AUC (ng x h/ml) over 3 days of treatment: Plain Aspirin 1964 VAZALORE 2523 EC aspirin 456 Editor - in - Chief Top Picks 2017 Bhatt DL, et al. Enteric Coating and Aspirin Non - Responsiveness in Patients With Type 2 Diabetes Mellitus. J Am Coll Cardiol 2017 Feb 14;69(6):603 - 612 VAZALORE has up to 5X greater absorption than enteric - coated aspirin VAZALORE delivers 2X better platelet response than enteric - coated aspirin
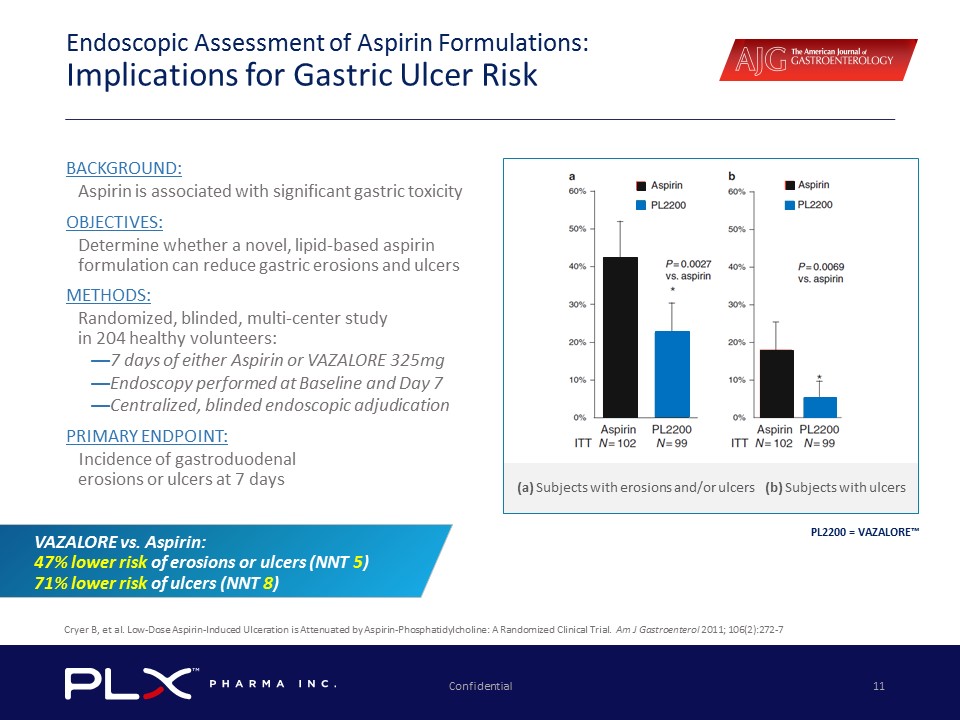
Confidential 11 Endoscopic Assessment of Aspirin Formulations: Implications for Gastric Ulcer Risk Cryer B, et al. Low - Dose Aspirin - Induced Ulceration is Attenuated by Aspirin - Phosphatidylcholine: A Randomized Clinical Trial. Am J Gastroenterol 2011; 106(2):272 - 7 PL2200 = VAZALORE™ (a) Subjects with erosions and/or ulcers (b) Subjects with ulcers VAZALORE vs. Aspirin: 47% lower risk of erosions or ulcers (NNT 5 ) 71% lower risk of ulcers (NNT 8 ) BACKGROUND: Aspirin is associated with significant gastric toxicity OBJECTIVES: Determine whether a novel, lipid - based aspirin formulation can reduce gastric erosions and ulcers METHODS: Randomized, blinded, multi - center study in 204 healthy volunteers: — 7 days of either Aspirin or VAZALORE 325mg — Endoscopy performed at Baseline and Day 7 — Centralized, blinded endoscopic adjudication PRIMARY ENDPOINT: Incidence of gastroduodenal erosions or ulcers at 7 days
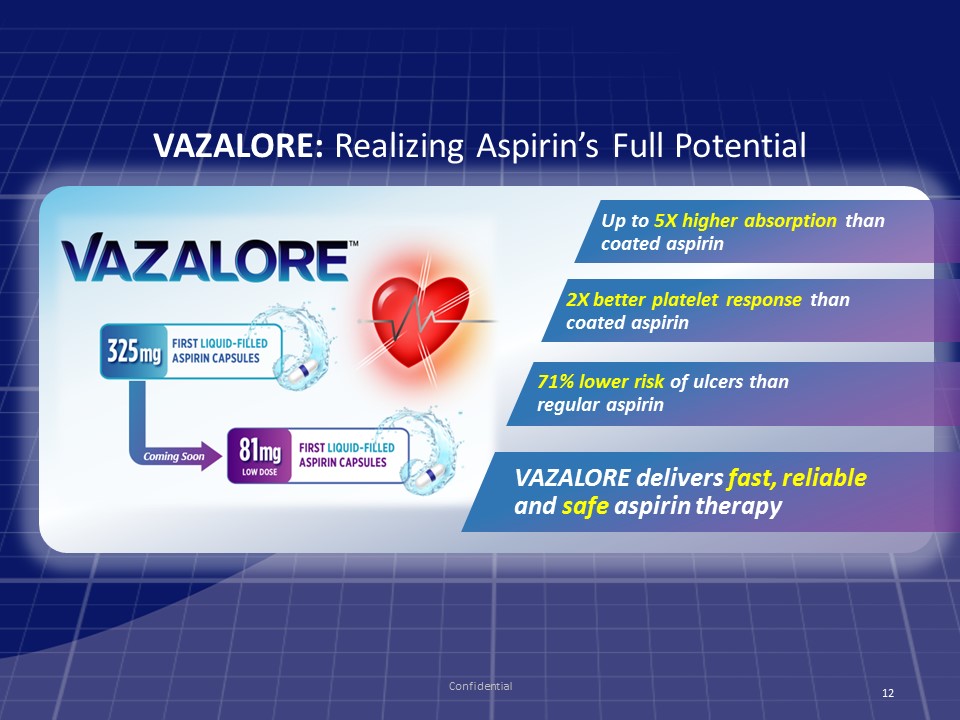
VAZALORE: Realizing Aspirin’s Full Potential 71% lower risk of ulcers than regular aspirin Confidential Up to 5X higher absorption than coated aspirin 2X better platelet response than coated aspirin 12 VAZALORE delivers fast, reliable and safe aspirin therapy
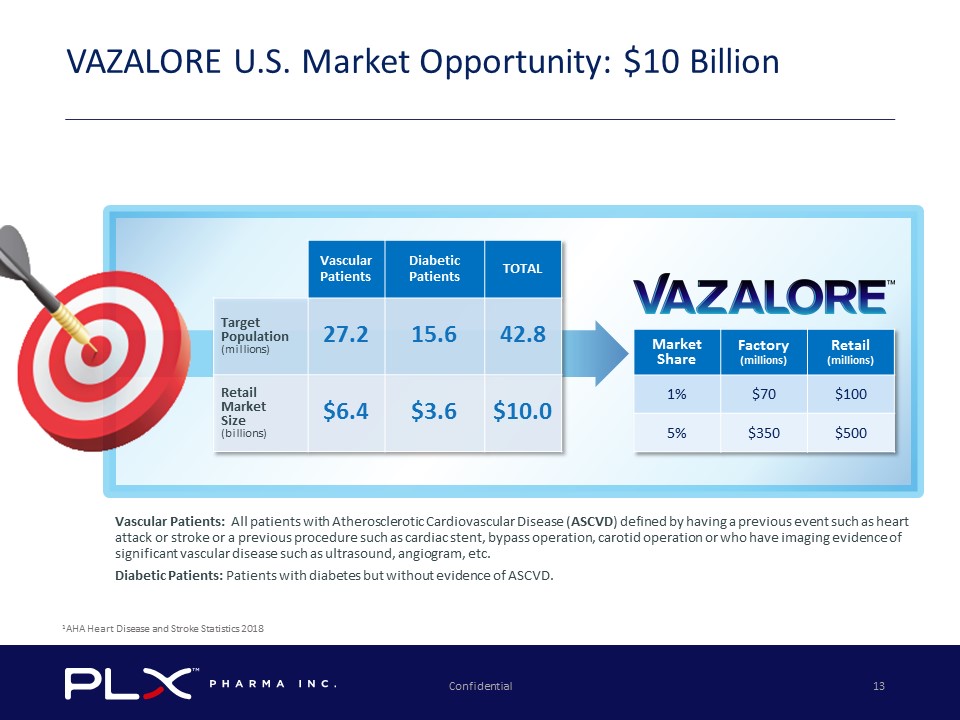
Market Share Factory (millions) Retail (millions) 1% $70 $100 5% $350 $500 Vascular Patients: All patients with Atherosclerotic Cardiovascular Disease ( ASCVD ) defined by having a previous event such as heart attack or stroke or a previous procedure such as cardiac stent, bypass operation, carotid operation or who have imaging evide nce of significant vascular disease such as ultrasound, angiogram, etc. Diabetic Patients: Patients with diabetes but without evidence of ASCVD. Confidential 13 VAZALORE U.S. Market Opportunity: $10 Billion Vascular Patients Diabetic Patients TOTAL Target Population (millions) 27.2 15.6 42.8 Retail Market Size (billions) $6.4 $3.6 $10.0 1 AHA Heart Disease and Stroke Statistics 2018

Confidential 14 High Intent to Prescribe VAZALORE Consumers (2,000 surveyed) were more likely to purchase specific OTC products when prescribed by a physician 1 Weinman Schnee Morais Inc. Cardiologists Neurologists Endocrinologists Diabetologists GPs Number of Physicians: 201 100 100 104 WOULD PRESCRIBE: 81% 86% 80% 77% Over 80% of specialists intending to prescribe VAZALORE based on quantitative and qualitative research 1

Confidential 15 VAZALORE Commercial Strategy ● Thought Leaders ● New Standard of Care PROFESSIONAL ● New life to category ● Premium $ TRADE ● Awareness/Education ● Compliance CONSUMER

Confidential 16 Key VAZALORE Messages to HCPs Better safety with lower risk for ulcers VAZALORE will transform the standard of care Innovative liquid aspirin formula Faster, predictable and reliable antiplatelet efficacy

Confidential 17 Milestones - 2019 325mg Labeling Approved Stability & Dissolution Testing Registration Batches FDA Meeting Development Scale Batches Q1 2019 Q2 2019 Q3 2019 4Q 2019 End of 2019
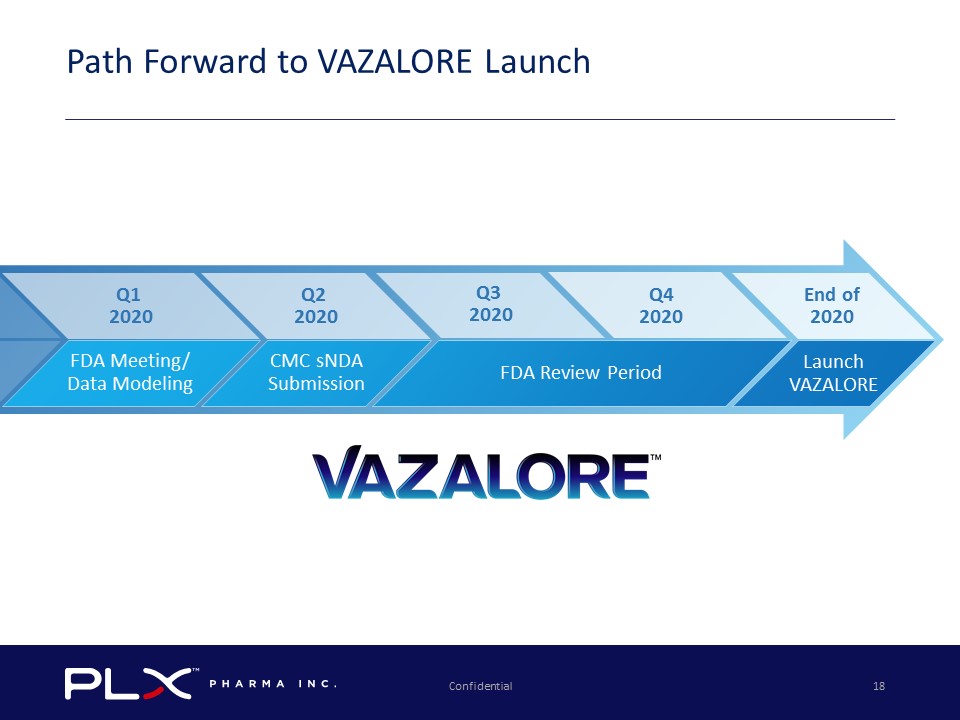
Confidential 18 Path Forward to VAZALORE Launch Launch VAZALORE FDA Review Period CMC sNDA Submission FDA Meeting/ Data Modeling Q1 2020 Q2 2020 End of 2020 Q4 2020 Q3 2020
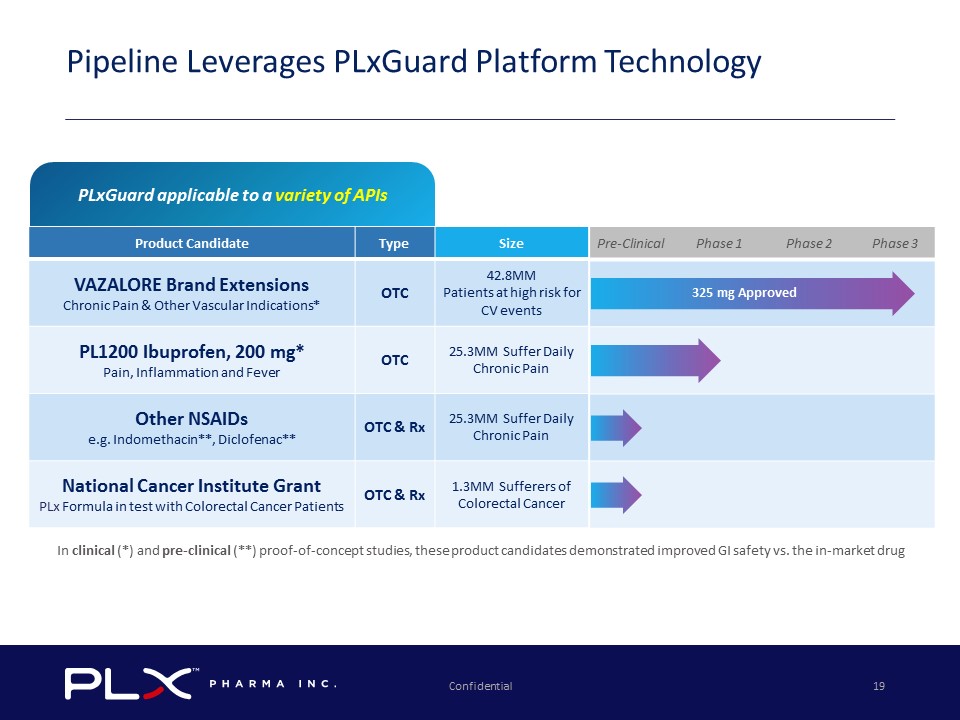
Confidential 19 Pipeline Leverages PLxGuard Platform Technology In clinical ( * ) and pre - clinical ( ** ) proof - of - concept studies, these product candidates demonstrated improved GI safety vs. the in - market drug Product Candidate Type Size VAZALORE Brand Extensions Chronic Pain & Other Vascular Indications * OTC 42.8MM Patients at high risk for CV events PL1200 Ibuprofen, 200 mg * Pain, Inflammation and Fever OTC 25.3MM Suffer Daily Chronic Pain Other NSAIDs e.g. Indomethacin ** , Diclofenac ** OTC & Rx 25.3MM Suffer Daily Chronic Pain National Cancer Institute Grant PLx Formula in test with Colorectal Cancer Patients OTC & Rx 1.3MM Sufferers of Colorectal Cancer Pre - Clinical Phase 1 Phase 2 Phase 3 325 mg Approved PLxGuard applicable to a variety of APIs

Michael (Mike) J. Valentino Executive Chairman of the Board 35+ years CEO and senior management with successful OTC and Rx brands (OTC brand, Mucinex®: $2.3 billion exit in 4.5 years) Natasha Giordano President and CEO 25+ years CEO and senior management commercialization experience Rita M. O’Connor, CPA Chief Financial Officer 25+ years finance leadership in public & private Rx & OTC companies Efthymios N. Deliargyris, MD FACC, FESC, FSCAI Chief Medical Officer Internationally - recognized expert in cardiovascular disease and thrombosis Steven Valentino VP, Trade Sales 25+ years in OTC and consumer healthcare including Rx - to - OTC switches, brand management, trade sales Joanne Cotignola VP, Marketing 20+ years in OTC healthcare brand management at public and private companies Confidential 20 PLx Management Team Name Experience

Director Experience Gary S. Balkema • Former global head of Bayer Healthcare LLC and Worldwide Consumer Care Division • Prior VP and General Manager for American Cyanamid Co.’s Lederle Consumer Health Division Tony Bartsh • Portfolio manager and partner at Park West Asset Management • Former investment analyst at Emrose Capital and Crosslink Capital Kirk Calhoun • Former audit committee chair, Adams Respiratory • Former Partner, Ernst & Young LLP Robert (Bob) Casale • Former Adams Respiratory COO (Mucinex® launch, Adams’ IPO and $2.3 billion sale) • Former senior manager at Pfizer, Warner Lambert and CEO of Scerene Healthcare John W. Hadden II • Former CEO of IRX Therapeutics (private) • Former healthcare investment banker at JP Morgan & Co Confidential 21 Independent Board of Directors & Advisors Efthymios N. Deliargyris , MD, FACC, FESC, FSCAI Chief Medical Officer PLX Pharma Inc. Sparta, NJ, USA Deepak L. Bhatt, MD, MPH, FACC, FAHA, FSCAI, FESC Executive Director of Interventional CV Programs Brigham and Women’s Hospital Heart & Vascular Center Professor of Medicine, Harvard Medical School Boston, MA, USA P. Gabriel Steg, MD, FESC, FACC Director of the Coronary Care Unit, Hôpital Bichat - Claude Professor of Cardiology, Univ. Paris VII - Denis Diderot Professor at the National Heart and Lung Institute, Imperial College, London, UK Paris, FRANCE Scientific Advisory Board Dominick J. Angiolillo , MD, PhD, FACC, FESC, FSCAI Program Director, Interventional Cardiology Fellowship Professor of Medicine, Director, Cardiovascular Research University of Florida College of Medicine - Jacksonville Jacksonville, FL, USA Roxana Mehran, MD, FACC FACP, FCCP, FESC, FAHA, FSCAI Professor of Medicine and Director of Interventional Cardiovascular Research and Clinical Trials at the Zena and Michael A. Wiener Cardiovascular Institute at Mount Sinai School of Medicine Board of Directors Carey Kimmelstiel , MD, FACC, FACP, FSCAI Director, Catheterization Laboratory and Interventional Cardiology, Tufts Medical Center Professor of Medicine, Tufts University School of Medicine Boston, MA, USA Byron Cryer, MD Associate Dean for Faculty Diversity and Development Professor of Medicine, UT Southwestern Medical School Dallas, TX, USA Todd K. Rosengart , MD Professor and Chairman, DeBakey - Bard Chair of Surgery Michael E. DeBakey Department of Surgery Baylor College of Medicine Houston, TX, USA Jayne Prats, PhD Elysis Medical Scientific Solutions Boston, MA, USA Mark J. Alberts, MD Physician - in - Chief Ayer Neuroscience Institute Hartford HealthCare Chief of Neurology Hartford Hospital, Professor of Neurology UConn School of Medicine

Confidential 22 PLx Pharma Investment Highlights: Innovative Drug Delivery Platform Overcomes limitations of enteric - coated aspirin with better absorption and safety Large OTC opportunity launching late 2020 , with $10 billion market opportunity A better, liquid aspirin for over 40 million patients at risk for vascular events World - renowned scientific advisory board chaired by Drs. Deepak Bhatt & Gabriel Steg High physician interest with >80% of specialists intending to prescribe VAZALORE ● Better efficacy and improved GI safety for superior benefit - risk profile ● Novel mechanism of action enables strong patent life for multiple APIs Lead Product: VAZALORE ™

Thank You! Confidential 23






















