Exhibit 99.2

Investor Presentation January 2025 NASDAQ: NMTC

2 Caution: Federal law restricts this device to sale by or on the order of a physician. Forward - Looking Statements This presentation contains forward - looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Except for statements of historical fact, any information contained in this presentation may be a forward – looking statement that reflects NeuroOne's current views about future events. In some cases, you can identify forward – looking statements by the words "may," "might," "will," "could," "would," "should," "expect," "intend," "plan," "upcoming," "target," "objective," "anticipate," "believe," "estimate," "predict," "project," "potential," "target," "seek," "contemplate," "continue" and "ongoing," or the negative of these terms, or other comparable terminology. Forward – looking statements may include statements regarding the fiscal 2025 guidance, the potential receipt of any revenue related to facial pain ablation, additional potential strategic partnerships, fiscal 2025 guidance, the potential receipt of any revenue related to facial pain ablation, additional potential strategic partnerships, the completion of drug delivery system in 2025, development of the Company's ablation electrode technology program, applications for, or receipt of, regulatory clearance, the timing and extent of product launch and commercialization of our technology, expected milestone payments, clinical and pre - clinical testing, what the future may hold for electrical stimulation and NeuroOne's potential role, business strategy, market size, potential growth opportunities, future operations, future efficiencies, and other financial and operating information. Our actual future results may be materially different from what we expect due to known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward - looking statements, including risks that the partnership with Zimmer Biomet may not facilitate the commercialization or market acceptance of our technology; risks that our sEEG electrodes may not be ready for commercialization in a timely manner or at all, whether due to supply chain disruptions and the impact of COVID - 19, or otherwise; risks that our technology will not perform as expected based on results of our pre - clinical and clinical trials; risks related to uncertainties associated with the Company's capital requirements to achieve its business objectives and ability to raise additional funds; the risk that the COVID - 19 pandemic will continue to adversely impact our business; the risk that we may not be able to secure or retain coverage or adequate reimbursement for our technology; uncertainties inherent in the development process of our technology; risks related to changes in regulatory requirements or decisions of regulatory authorities; that we may not have accurately estimated the size and growth potential of the markets for our technology; risks related to clinical trial patient enrollment and the results of clinical trials; that we may be unable to protect our intellectual property rights; and other risks, uncertainties and assumptions, including those described under the heading "Risk Factors" in our filings with the Securities and Exchange Commission. These forward – looking statements speak only as of the date of this presentation and NeuroOne undertakes no obligation to revise or update any forward – looking statements for any reason, even if new information becomes available in the future. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market share and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. NASDAQ: NMTC

NeuroOne is a medical technology company that is transforming the diagnosis and treatment of neurological disorders 3 FDA 510(k) cleared products for use in the brain, with the only FDA cleared product that uses the same sEEG electrode for both diagnostic and therapeutic applications. Additional submissions planned for 2025. Exclusive partnership with Zimmer Biomet, the global leader in robotic surgical technology, and the Mayo Clinic. Patented and disruptive technology unlocking multi - billion markets in neurology. NASDAQ: NMTC 3

Thin Film & Flexibility 4 NeuroOne’s next - generation electrode platform is highly disruptive and differentiated Multi - Purpose Device Since the 1950s, clinicians and researchers have used first generation electrodes for the recording and stimulation of brain tissue. NeuroOne’s multi - purpose electrodes are trying to reduce the number of surgeries, and hospitalizations while potentially improving efficacy. NASDAQ: NMTC

Mayo Clinic partnership • Mayo Clinic began testing technology in pre - clinical models and clinical research in 2015 • Mayo Clinic leading neurologist, Dr. Worrell, chairs the NeuroOne Scientific Advisory Board • First commercial human use of Evo® Cortical Electrodes performed at Mayo Clinic in November 2020 • Currently using our drug delivery system in pre - clinical studies Mayo Clinic board representation Current Investor 5 NASDAQ: NMTC

Zimmer Biomet partnership • Zimmer is a worldwide leader in robotic technology used in minimally invasive neurosurgeries • Evo® electrodes are complementary to Zimmer’s ROSA ONE ® robotic neurosurgery platform • Partnership initiated in 2020: $5.5 million total paid to NeuroOne under initial contract 6 NASDAQ: NMTC
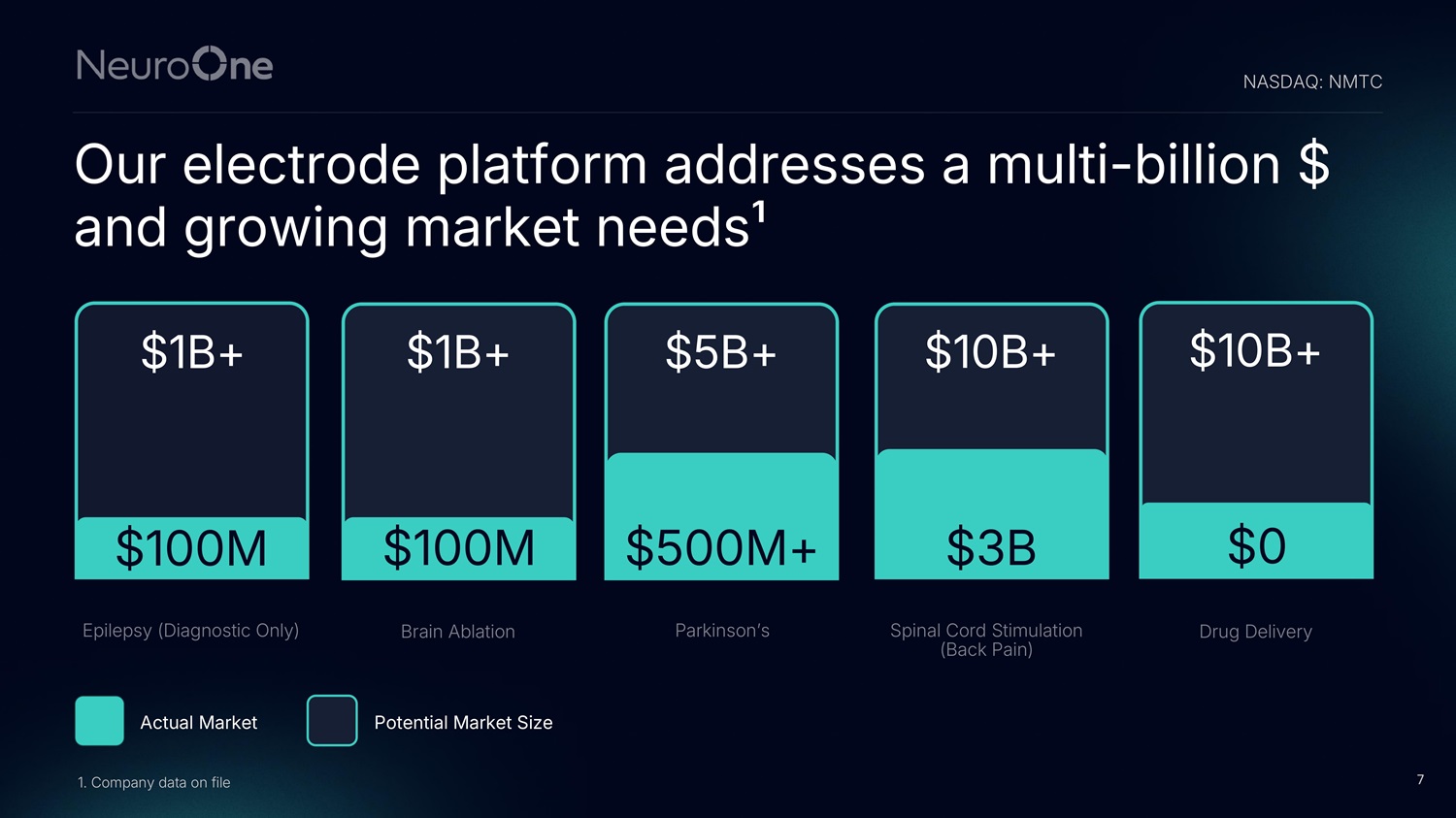
7 Our electrode platform addresses a multi - billion $ and growing market needs¹ $100M Brain Ablation $500M+ Parkinson’s $3B Spinal Cord Stimulation (Back Pain) $100M Epilepsy (Diagnostic Only) $1B+ $1B+ $5B+ $10B+ $0 Drug Delivery $10B+ Actual Market Potential Market Size 1. Company data on file NASDAQ: NMTC

Technology platform 8 NASDAQ: NMTC
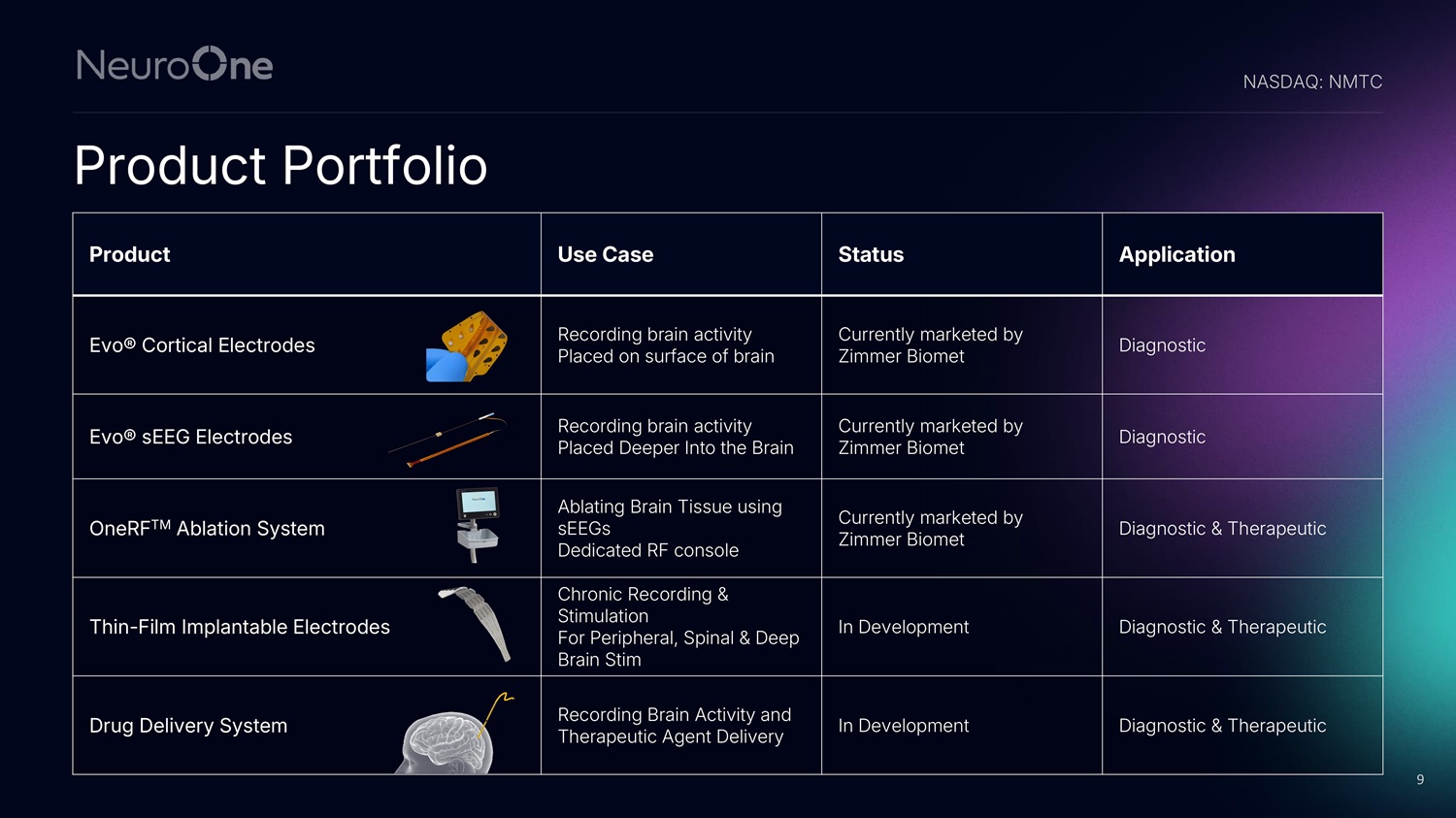
Product Portfolio Application Status Use Case Product Diagnostic Currently marketed by Zimmer Biomet Recording brain activity Placed on surface of brain Evo® Cortical Electrodes Diagnostic Currently marketed by Zimmer Biomet Recording brain activity Placed Deeper Into the Brain Evo® sEEG Electrodes Diagnostic & Therapeutic Currently marketed by Zimmer Biomet Ablating Brain Tissue using sEEGs Dedicated RF console OneRF TM Ablation System Diagnostic & Therapeutic In Development Chronic Recording & Stimulation For Peripheral, Spinal & Deep Brain Stim Thin - Film Implantable Electrodes Diagnostic & Therapeutic In Development Recording Brain Activity and Therapeutic Agent Delivery Drug Delivery System NASDAQ: NMTC 9

Thin - Film Evo® sEEG & Cortical Electrodes NASDAQ: NMTC 10

OneRF TM Ablation System Recently expanded Zimmer Biomet Partnership to distribute system NASDAQ: NMTC 11
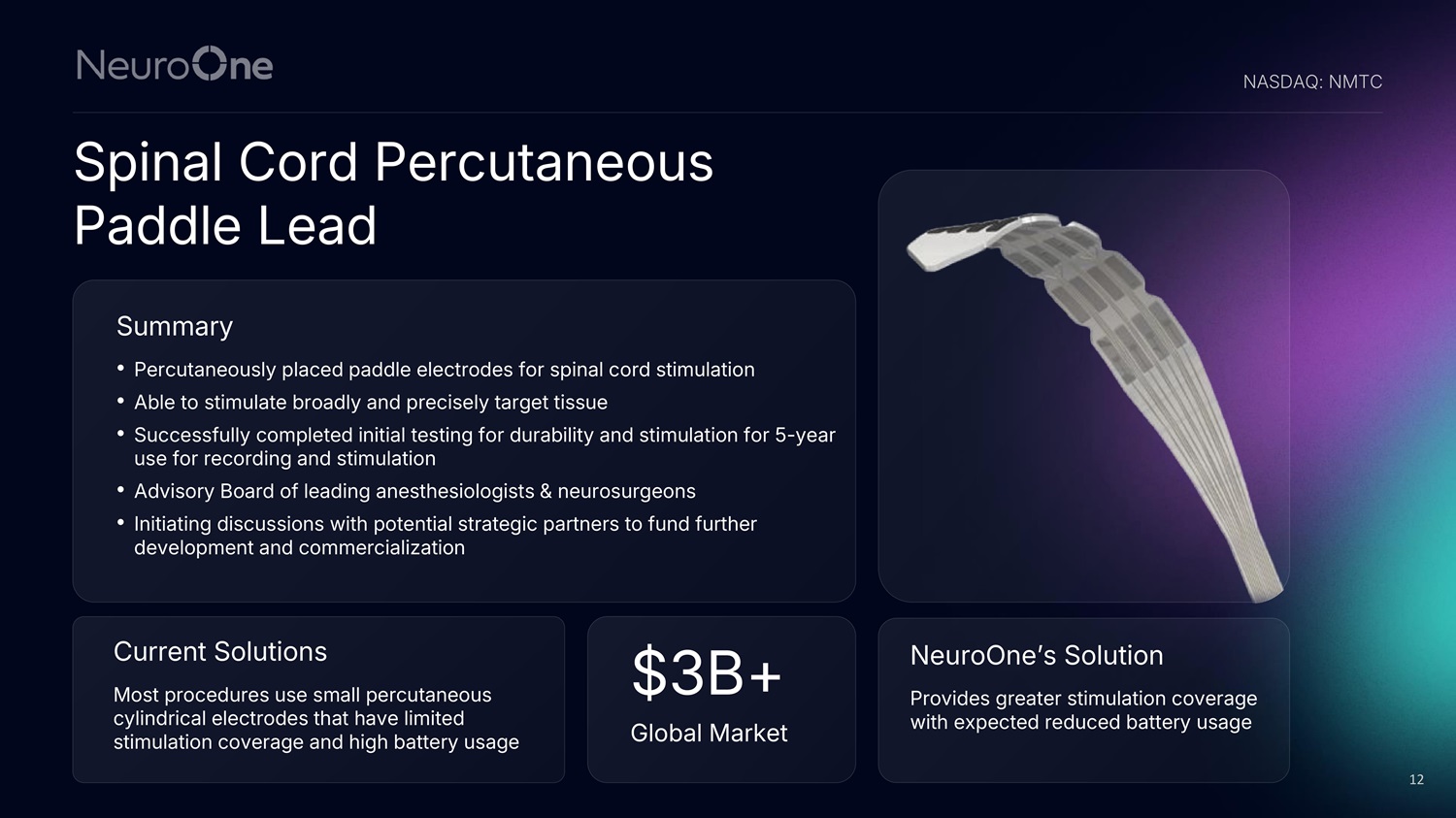
Spinal Cord Percutaneous Paddle Lead NASDAQ: NMTC 12
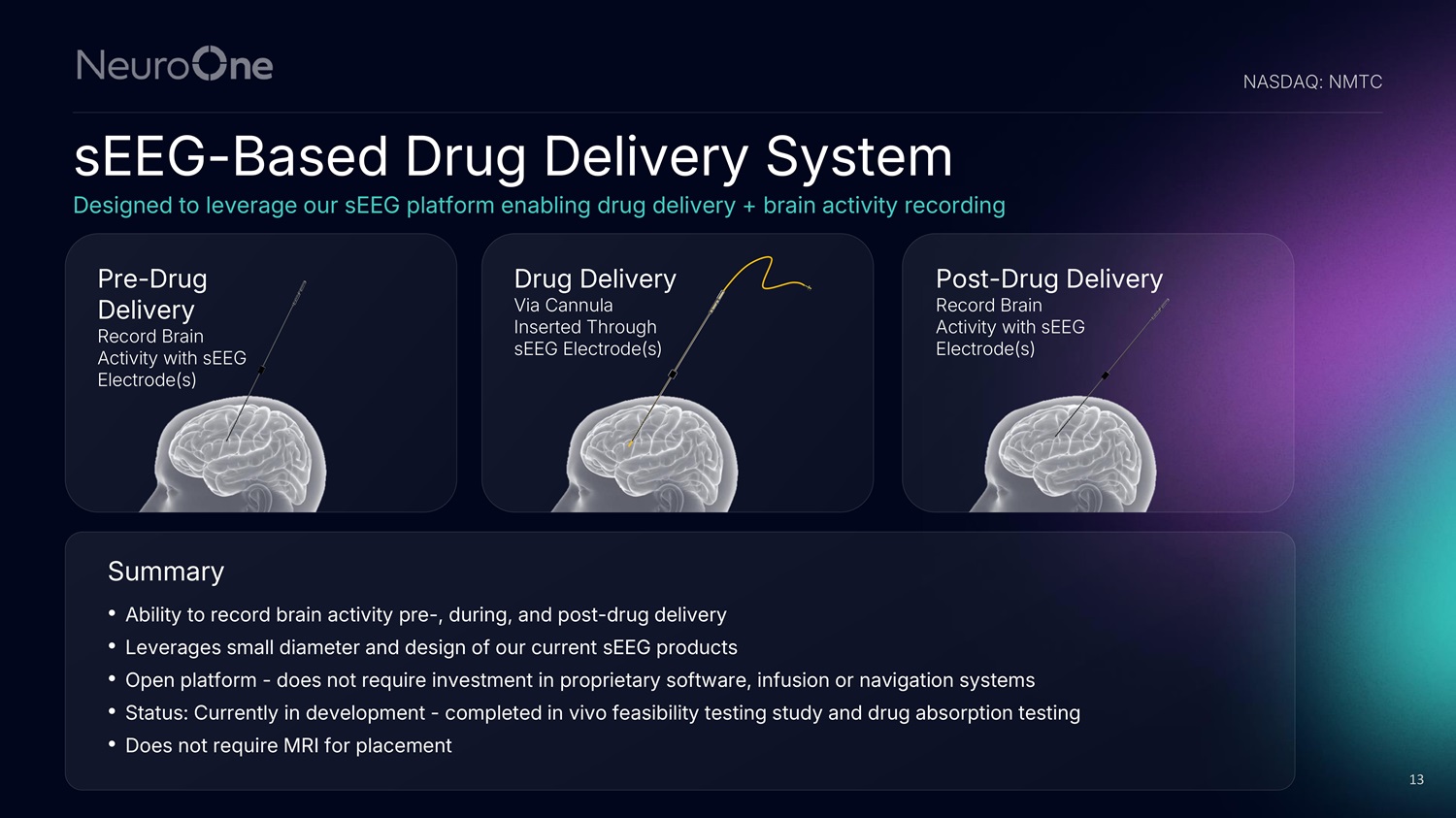
sEEG - Based Drug Delivery System Designed to leverage our sEEG platform enabling drug delivery + brain activity recording NASDAQ: NMTC 13
Our management team Dave Rosa President & Chief Executive Officer Ron McClurg Chief Financial Officer Ron McClurg Chief Technology Officer Mark Christianson Co - Founder, Business Development Director, Medical Sales Liaison Hijaz Haris Vice - President of Marketing Camilo Diaz Botia Director of Electrode Development Chad Wilhelmy Vice President of Quality Control and Regulatory Affairs Scientific advisory board Greg Worrell MD PhD, Chairman of the Scientific Advisory Board Bob Gross MD, PhD Jamie Van Gompel MD Justin Williams PhD Greg Esper MD, MBA Kip Ludwig PhD Chris Volker Chief Operating Officer 14 NASDAQ: NMTC

• Decreasing burn rate in FY2024 • Expected FY2025 product revenue of $8 - $10M, an increase of 132% - 190% vs FY2024 • Expected FY2025 gross margin of 47% - 51% • More potential partnerships focused on pain management and drug delivery • Debt free balance sheet 15 Financial overview Highlights & Financial Catalysts Capital Position ($ in millions) $1.60 Cash (as of 9/30) $0 Debt $2.65 August 2024 pvt. placement $3.00 Upfront licensing fee payment $3.50 TTM revenue NASDAQ: NMTC
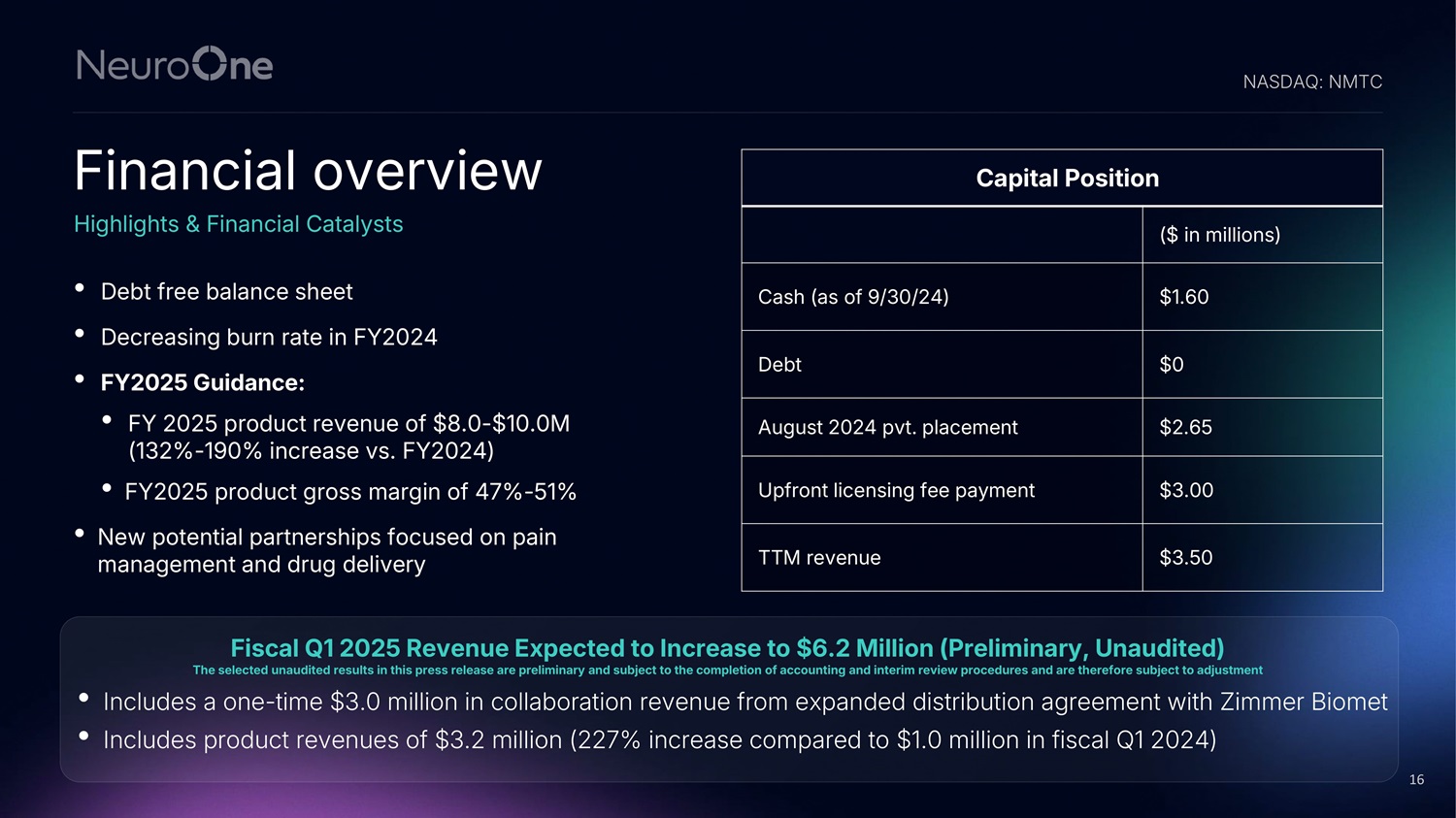
• Debt free balance sheet • Decreasing burn rate in FY2024 • FY2025 Guidance: • FY 2025 product revenue of $8.0 - $10.0M (132% - 190% increase vs. FY2024) • FY2025 product gross margin of 47% - 51% • New potential partnerships focused on pain management and drug delivery Financial overview Highlights & Financial Catalysts Capital Position ($ in millions) $1.60 Cash (as of 9/30/24) $0 Debt $2.65 August 2024 pvt. placement $3.00 Upfront licensing fee payment $3.50 TTM revenue NASDAQ: NMTC Fiscal Q1 2025 Revenue Expected to Increase to $6.2 Million (Preliminary, Unaudited) The selected unaudited results in this press release are preliminary and subject to the completion of accounting and interim review procedures and are therefore subject to adjustment 16

Upcoming potential catalysts 17 • Zimmer Biomet OneRF® Ablation System full launch • Potential revenue in calendar 2025 for facial pain ablation system • Electrode revenue expected to more than double in FY2025 • Non - dilutive licensing agreement for ablation system with Zimmer Biomet with future potential milestone payment • Potential strategic partnerships to leverage NeuroOne’s core technology for ablation and stimulation • Completion of drug delivery system in calendar 2025 followed by FDA 510(k) submission • Potential for strategic partnerships with pharma companies NASDAQ: NMTC

Key takeaways NASDAQ: NMTC 18

Thank you NASDAQ: NMTC Dave Rosa Chief Executive Officer ir@nmtc1.com


















