
The Science Behind Annapurna R. Crystal Department of Genetic Medicine Weill Cornell Medical College 2-17-16 Exhibit 99.1

Cautionary Statements Regarding Forward-Looking Statements Statements contained in this document regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Examples of such statements include, but are not limited to, statements regarding plans, potential opportunities, expectations, projections, goals, objectives, milestones, strategies, product pipeline, the occurrence or effect of the proposed transaction between Avalanche Biotechnologies, Inc. (“Avalanche”) and Annapurna Therapeutics SAS (“Annapurna”), the sufficiency of the combined company’s resources to fund the advancement of any development program or the completion of any clinical trials, and the safety, efficacy and projected development timeline and commercial potential of products under development by Avalanche and Annapurna, all of which are based on certain assumptions made by us based on current conditions, expected future developments and other factors we believe are appropriate in the circumstances. Actual results, the timing of events, product development programs, performance or achievements could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks and uncertainties inherent in the product development and the regulatory approval process, delays in clinical trials and other matters that could affect the availability or commercial potential of product candidates, the ability to consummate the proposed transaction with Annapurna, the ability to project future cash utilization, the availability of sufficient resources to conduct or continue planned development programs, and the ability to successfully develop any of Avalanche’s or Annapurna’s product candidates. Risks and uncertainties facing Avalanche are described more fully in Avalanche’s periodic reports filed with the SEC. All forward-looking statements contained in this document speak only as of the date on which they were made. Avalanche undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. This document contains estimates, projections and other information concerning Avalanche’s and Annapurna’s industry, business and the markets for certain drugs, including data regarding the estimated size of those markets, their projected growth rates and the incidence of certain medical conditions. Information that is based on estimates, forecasts, projections or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained this industry, business, market and other data from reports, research surveys, studies and similar data prepared by third parties, industry, medical and general publications, government data and similar sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as representations made by, Avalanche or Annapurna.

Additional Information and Where To Find It This document not constitute a solicitation of any vote or approval. In connection with the proposed transaction, Avalanche intends to file with the SEC a proxy statement of Avalanche, as well as other relevant documents concerning the proposed transaction. INVESTORS AND SECURITYHOLDERS OF AVALANCHE ARE URGED TO READ THE PROXY STATEMENT REGARDING THE PROPOSED TRANSACTION WHEN IT BECOMES AVAILABLE AND ANY OTHER RELEVANT DOCUMENTS FILED WITH THE SEC, AS WELL AS ANY AMENDMENTS OR SUPPLEMENTS TO THOSE DOCUMENTS, BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION. A free copy of the proxy statement and other filings containing information about Avalanche may be obtained at the SEC website at www.sec.gov. You will also be able to obtain these documents, free of charge, from Avalanche by directing a written request to: Avalanche Biotechnologies, Inc., 1035 O’Brien Drive, Suite A, Menlo Park, CA 94025, Attention: Investor Relations. Investors and security holders are urged to read the proxy statement and the other relevant materials when they become available before making any voting decision with respect to the issuance of shares of the Avalanche common stock in connection with the proposed transaction and any other matters relating to the proposed transaction. Certain Information Regarding Participants Avalanche and its directors and executive officers may be deemed to be participants in the solicitation of proxies from the stockholders of Avalanche in connection with the proposed transaction and the issuance of additional Avalanche common stock. Information regarding the special interests of these directors and executive officers in the proposed transaction will be included in the proxy statement referred to above. Additional information regarding the directors and executive officers of Avalanche is also included in Avalanche’s Annual Report on Form 10-K for the year ended December 31, 2014 and the proxy statement for the Company’s 2015 Annual Meeting of Stockholders. Additional information about the interests of potential participants will be contained in the proxy statement (when filed) and other relevant materials to be filed with the SEC in connection with the proposed transaction. These documents may be obtained free of charge at the SEC web site at www.sec.gov and from Investor Relations at Avalanche in the manner set forth above.
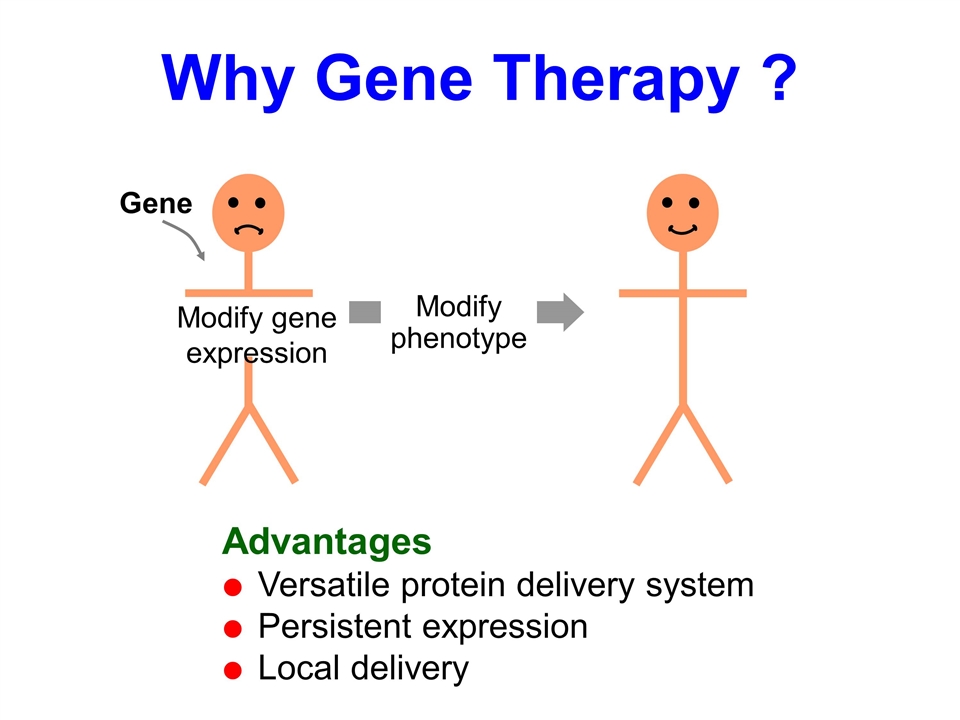
Why Gene Therapy ? Gene Modify gene expression Modify phenotype Advantages Versatile protein delivery system Persistent expression Local delivery
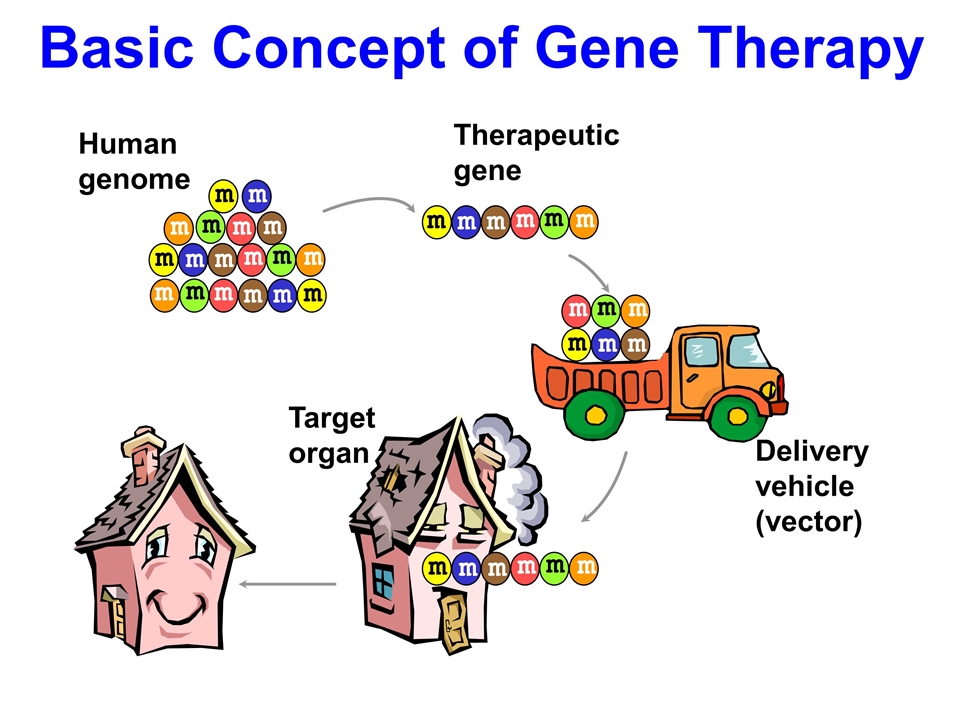
Basic Concept of Gene Therapy Therapeutic gene Human genome m m m m m m m m m m m m m m m m m m Delivery vehicle (vector) Target organ m m m m m m m m m m m m m m m m m m

Challenges of Gene Therapy Challenges Target choice – need, feasibility Targeting to site of disease Level of expression required Robust phenotype, clear demonstration of efficacy using FDA-acceptable parameters Safety – risk/benefit, dose-limiting immune related toxicity, lack of control of expression and inability to reverse Manufacturing Registration

Annapurna Programs Alpha 1-antitrypsin deficiency Hereditary angioedema Friedreich’s ataxia cardiac disease Severe allergy
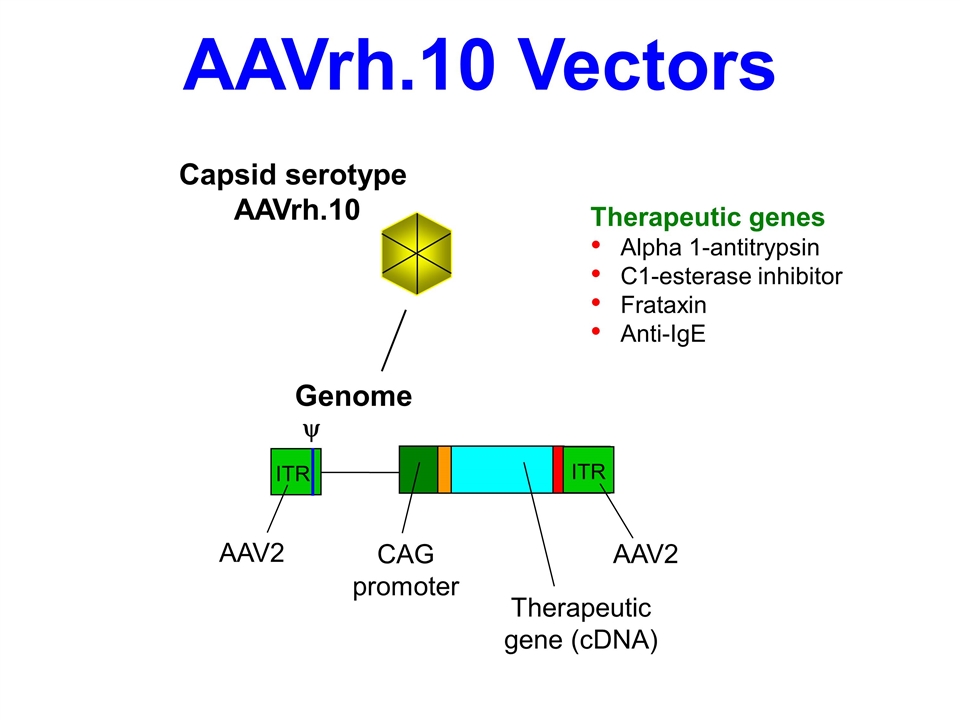
AAVrh.10 Vectors ITR y ITR ITR CAG promoter Therapeutic gene (cDNA) Genome Capsid serotype AAVrh.10 AAV2 AAV2 Therapeutic genes Alpha 1-antitrypsin C1-esterase inhibitor Frataxin Anti-IgE

Why Gene Therapy? Alpha 1-antitrypsin deficiency, hereditary angioedema, severe allergy Reduced treatment burden Ideal pharmacokinetics - constant levels For hereditary angioedema and severe allergy, eliminate risk for attacks without need for immediate medical care Friedreich’s ataxia cardiac disease Intracellular delivery of the deficient protein in the target organ Unmet medical need for a fatal disease
Alpha 1-Antitrypsin Deficiency Common autosomal recessive disorder characterized by a marked reduction in serum a1AT levels Estimated 90,000 affected individuals in US Emphysema develops at ages 35-45 in cigarette smokers, 55-65 in non-smokers Small % develop liver cirrhosis a1AT normally protects the lung from the destructive potential of neutrophil elastase; if a1AT levels are low, neutrophil elastase slowly destroys the lung parenchyma Current therapy to protect the lung - weekly intravenous infusions of 4 gm of human a1AT purified from pooled plasma
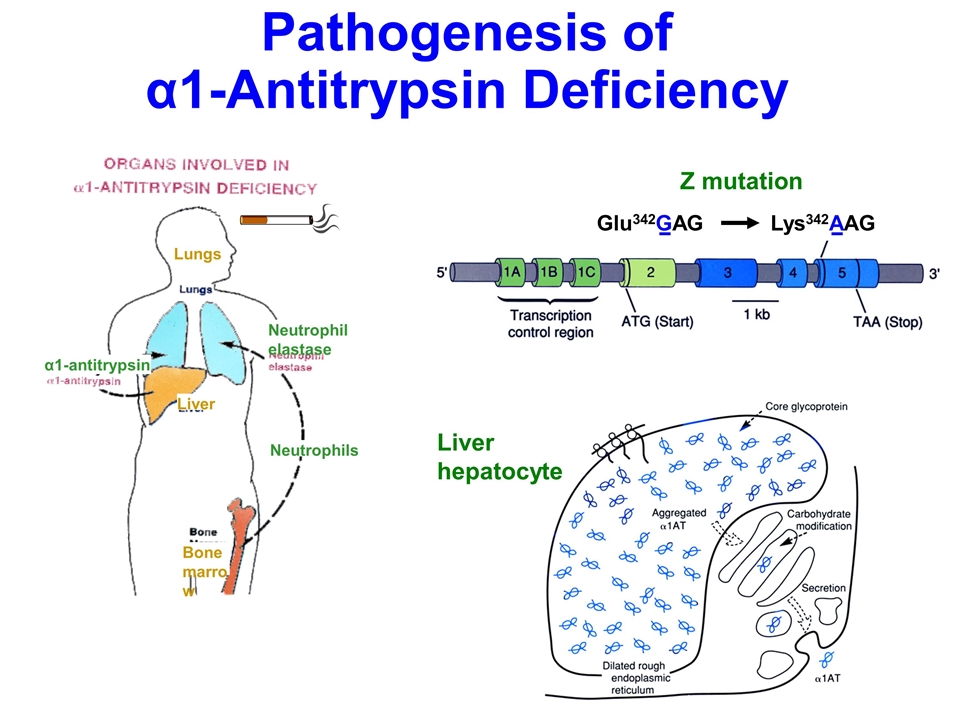
Pathogenesis of α1-Antitrypsin Deficiency α1-antitrypsin Neutrophil elastase Lungs Liver Neutrophils Bone marrow Glu342GAG Lys342AAG Z mutation Liver hepatocyte
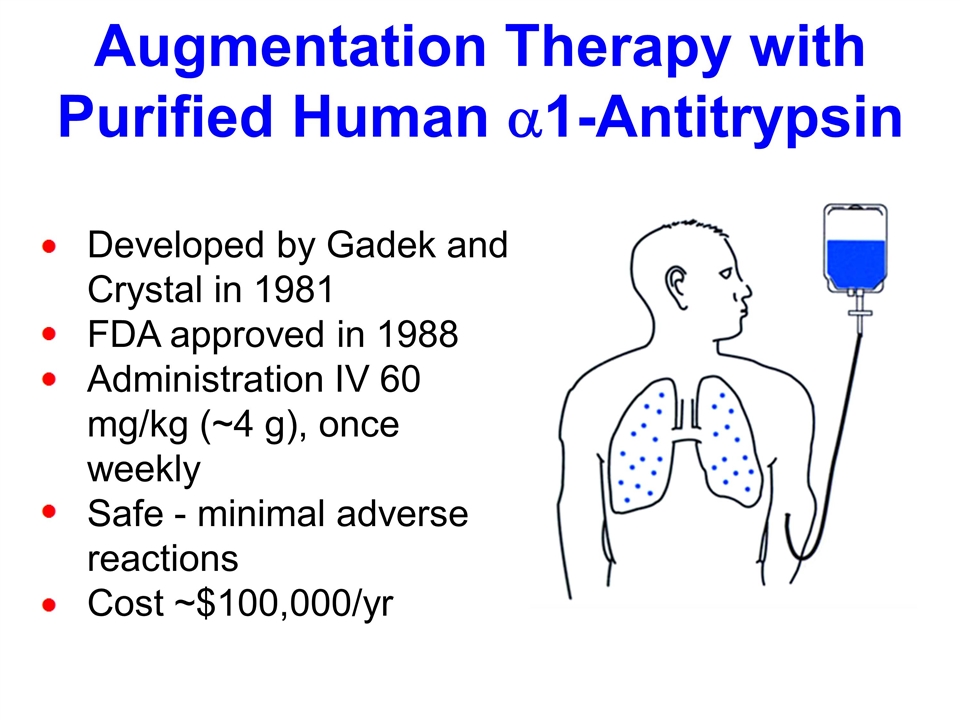
Augmentation Therapy with Purified Human a1-Antitrypsin Developed by Gadek and Crystal in 1981 FDA approved in 1988 Administration IV 60 mg/kg (~4 g), once weekly Safe - minimal adverse reactions Cost ~$100,000/yr
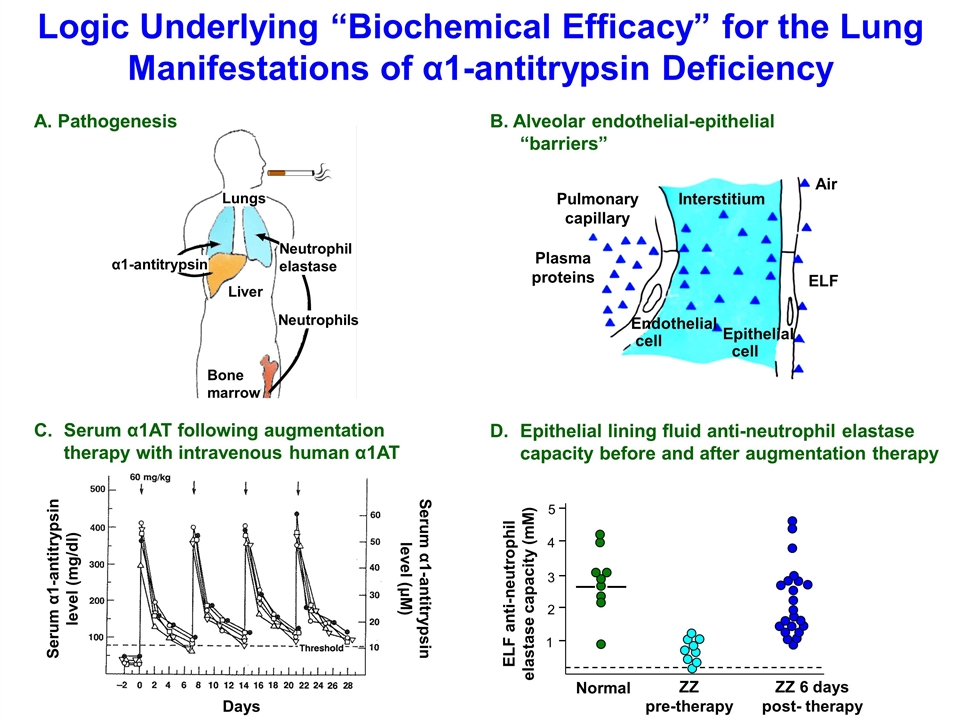
Lungs Liver Bone marrow Neutrophils Neutrophil elastase α1-antitrypsin A. Pathogenesis D.Epithelial lining fluid anti-neutrophil elastase capacity before and after augmentation therapy Normal ZZ pre-therapy ZZ 6 days post- therapy 1 2 3 4 5 ELF anti-neutrophil elastase capacity (mM) C.Serum α1AT following augmentation therapy with intravenous human α1AT pathogenesis Pulmonary capillary Interstitium Air Plasma proteins Endothelial cell Epithelial cell B. Alveolar endothelial-epithelial “barriers” ELF Logic Underlying “Biochemical Efficacy” for the Lung Manifestations of α1-antitrypsin Deficiency Serum α1-antitrypsin level (mg/dl) Serum α1-antitrypsin level (μM) Days
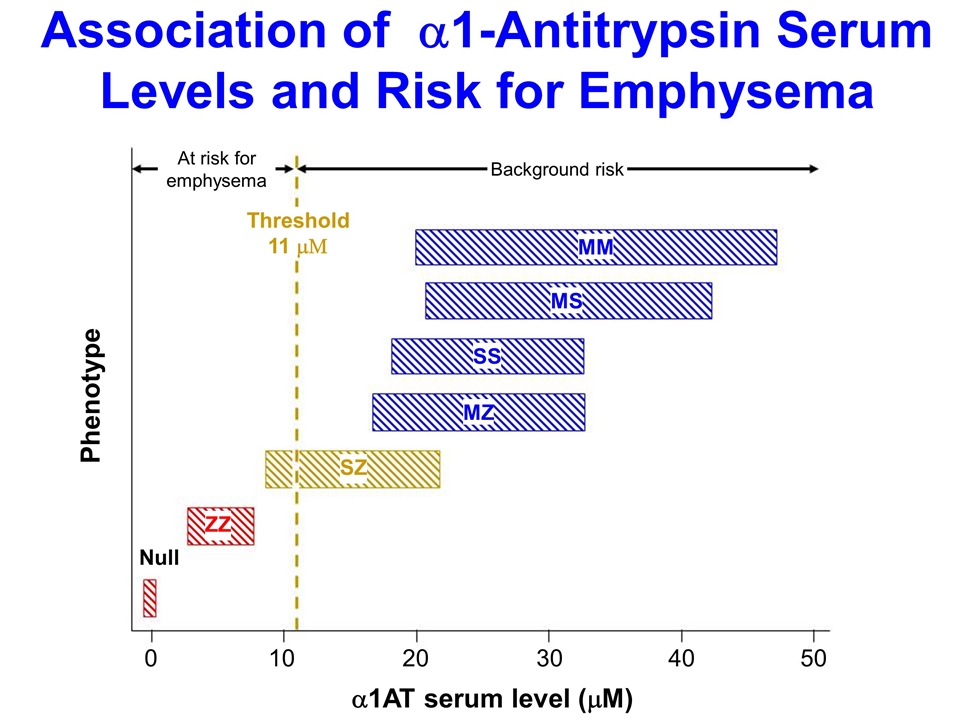
Association of a1-Antitrypsin Serum Levels and Risk for Emphysema MM MS SS MZ SZ ZZ Threshold 11 mM Null 0 10 20 30 40 50 a1AT serum level (mM) Phenotype At risk for emphysema Background risk
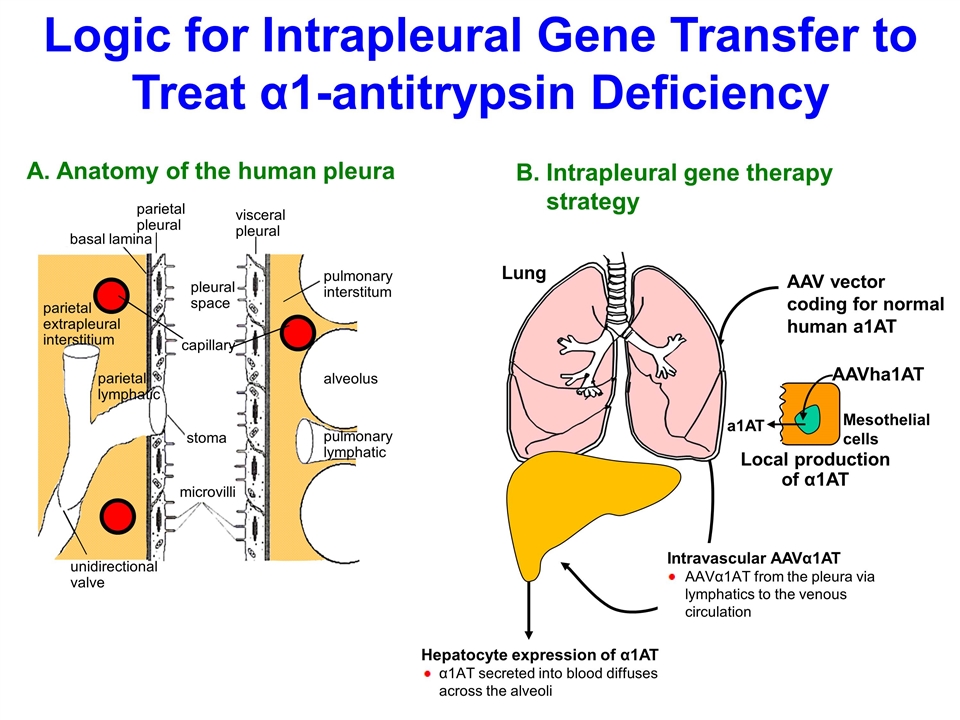
unidirectional valve parietal extrapleural interstitium parietal lymphatic pleural space stoma microvilli basal lamina parietal pleural visceral pleural capillary pulmonary interstitum alveolus pulmonary lymphatic A. Anatomy of the human pleura AAV vector coding for normal human a1AT B.Intrapleural gene therapy strategy Local production of α1AT AAVha1AT a1AT Mesothelial cells Lung Intravascular AAVα1AT AAVα1AT from the pleura via lymphatics to the venous circulation Hepatocyte expression of α1AT α1AT secreted into blood diffuses across the alveoli Logic for Intrapleural Gene Transfer to Treat α1-antitrypsin Deficiency
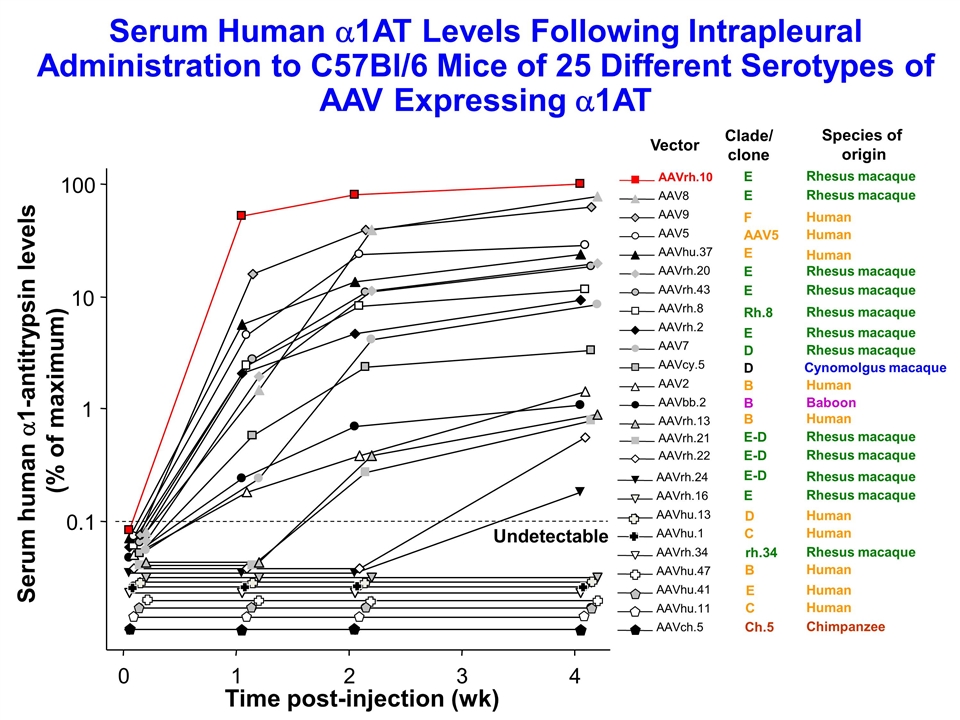
Time post-injection (wk) Serum human a1-antitrypsin levels (% of maximum) 0.1 1 10 100 0 1 2 3 4 Undetectable AAVch.5 AAVhu.11 AAVhu.41 AAVhu.47 AAVrh.34 AAVhu.1 AAVhu.13 AAVrh.16 AAVrh.24 AAVrh.22 AAVrh.21 AAVrh.13 AAVbb.2 AAV2 AAVcy.5 AAV7 AAVrh.2 AAVrh.8 AAVrh.43 AAVrh.20 AAVhu.37 AAV5 AAV9 AAV8 AAVrh.10 Vector Clade/ clone Species of origin E Rhesus macaque E Rhesus macaque E-D Rhesus macaque E-D Rhesus macaque E-D Rhesus macaque Serum Human a1AT Levels Following Intrapleural Administration to C57Bl/6 Mice of 25 Different Serotypes of AAV Expressing a1AT E Rhesus macaque F Human AAV5 Human E Human E Rhesus macaque E Rhesus macaque Rh.8 Rhesus macaque E Rhesus macaque D Rhesus macaque D Cynomolgus macaque B Human B Baboon B Human D Human C Human rh.34 Rhesus macaque B Human E Human C Human Ch.5 Chimpanzee
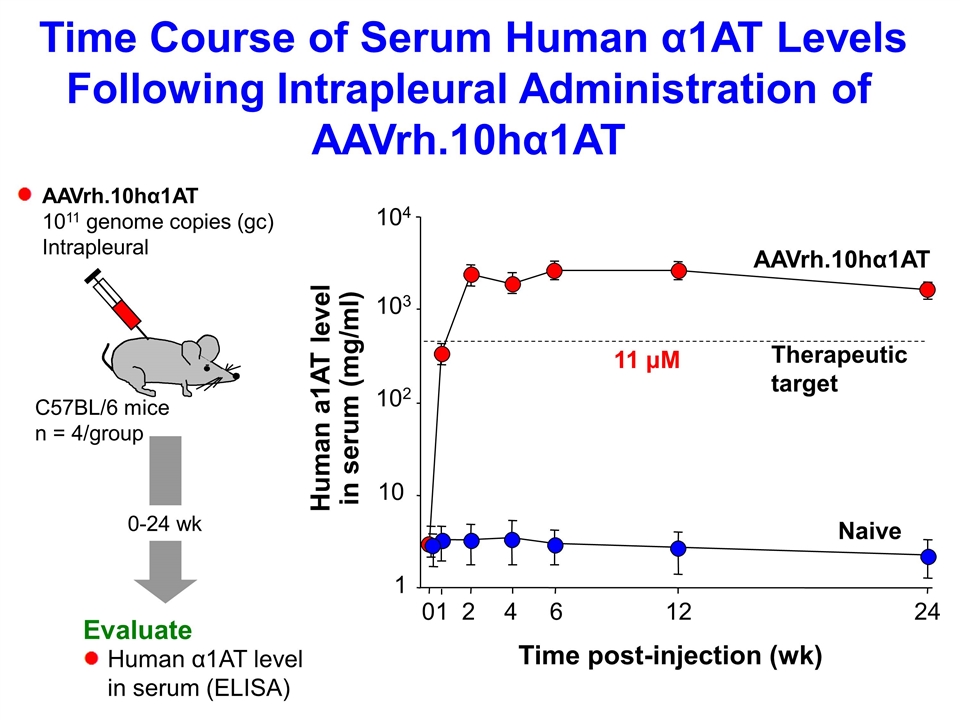
0 1 2 4 6 12 1 10 102 103 104 24 Time post-injection (wk) Human a1AT level in serum (mg/ml) Naive AAVrh.10hα1AT 0-24 wk Evaluate AAVrh.10hα1AT 1011 genome copies (gc) Intrapleural Human α1AT level in serum (ELISA) C57BL/6 mice n = 4/group Time Course of Serum Human α1AT Levels Following Intrapleural Administration of AAVrh.10hα1AT Therapeutic target 11 μM
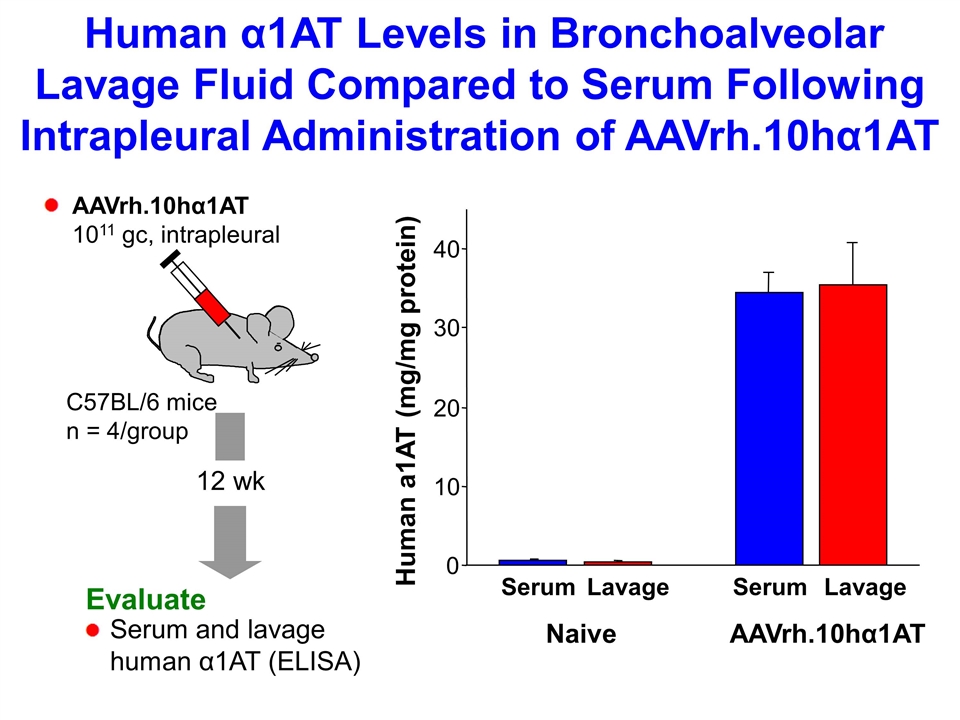
12 wk Evaluate Serum and lavage human α1AT (ELISA) C57BL/6 mice n = 4/group Human α1AT Levels in Bronchoalveolar Lavage Fluid Compared to Serum Following Intrapleural Administration of AAVrh.10hα1AT Human a1AT (mg/mg protein) Naive AAVrh.10hα1AT Serum Serum Lavage Lavage 0 10 20 30 40 AAVrh.10hα1AT 1011 gc, intrapleural
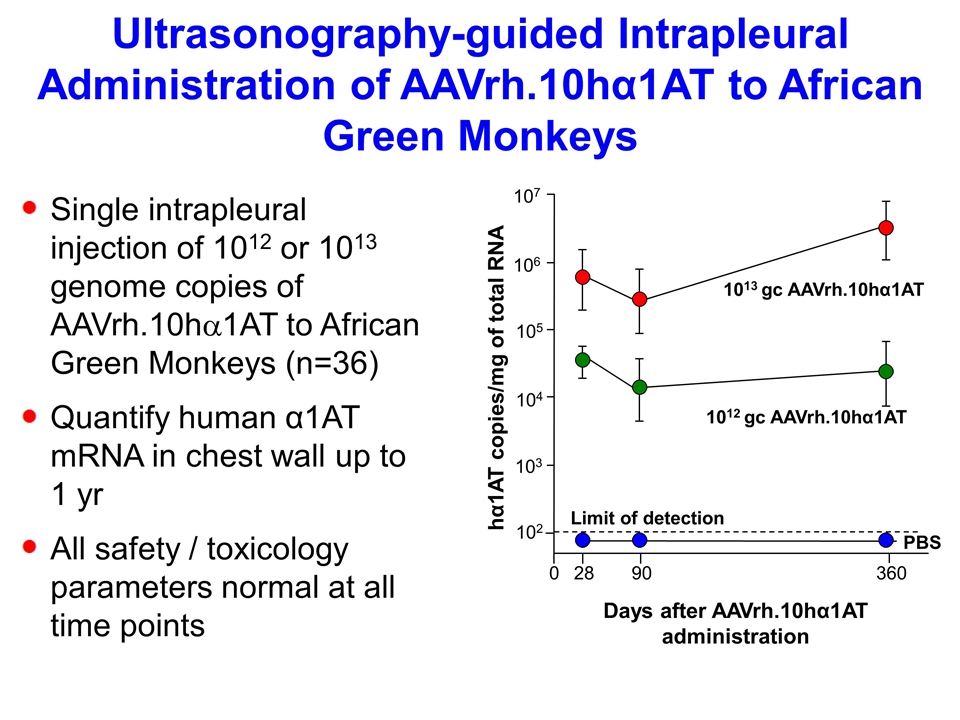
Ultrasonography-guided Intrapleural Administration of AAVrh.10hα1AT to African Green Monkeys Single intrapleural injection of 1012 or 1013 genome copies of AAVrh.10ha1AT to African Green Monkeys (n=36) Quantify human α1AT mRNA in chest wall up to 1 yr All safety / toxicology parameters normal at all time points Days after AAVrh.10hα1AT administration 107 105 104 103 102 28 90 360 0 Limit of detection 1013 gc AAVrh.10hα1AT 1012 gc AAVrh.10hα1AT PBS 106 hα1AT copies/mg of total RNA
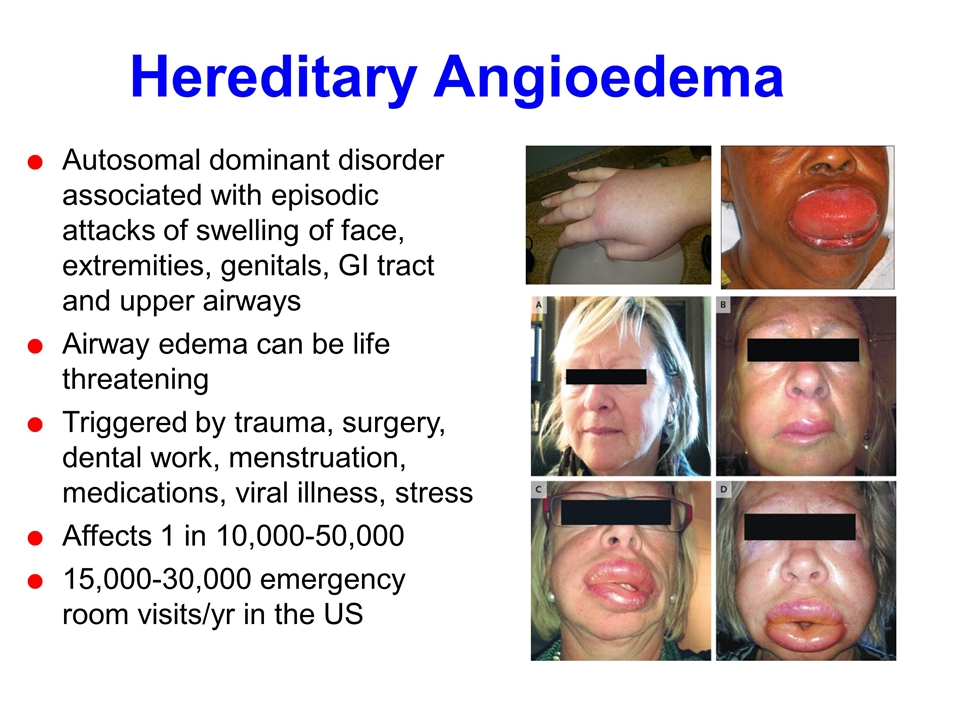
Hereditary Angioedema Autosomal dominant disorder associated with episodic attacks of swelling of face, extremities, genitals, GI tract and upper airways Airway edema can be life threatening Triggered by trauma, surgery, dental work, menstruation, medications, viral illness, stress Affects 1 in 10,000-50,000 15,000-30,000 emergency room visits/yr in the US
Hereditary Angioedema (2) Caused by mutations of the SERPING1 gene, coding for C1 esterase inhibitor (C1EI) that regulates the complement system C1EI - single chain, 510 residue, 105 kDa, glycosylated serine anti-protease expressed 10 by hepatocytes SERPING1 mutations result in reduced serum levels in C1EI (80-85% cases) or functional C1EI deficiency (15-20%) C1EI deficiency results in up-regulation of bradykinin, causing edema via leaky vessels 6-8,000 patients in the US Approved/reimbursed prophylactic recombinant C1EI therapy reduces number and severity of attacks, but requires 2x/week infusion and costs $500,000/yr

Gene Therapy for Hereditary Angioedema Proof-of-concept studies cannot be discussed at this time due to patent filings in progress

Friedreich Ataxia Approximately 5,000 patients in the US, 5,000-10,000 in Europe Autosomal recessive, results from variants in the frataxin (FXN) gene, a nuclear gene coding for a mitochondrial protein associated with iron sulfur clusters Impairs dorsal root ganglia, spinal cord and cerebellum resulting in gait ataxia, gradually worsening and spreading to the arms and the trunk, slurred and slowed speech Hypertrophic cardiomyopathy, >60% of patients die from cardiac events No effective therapy
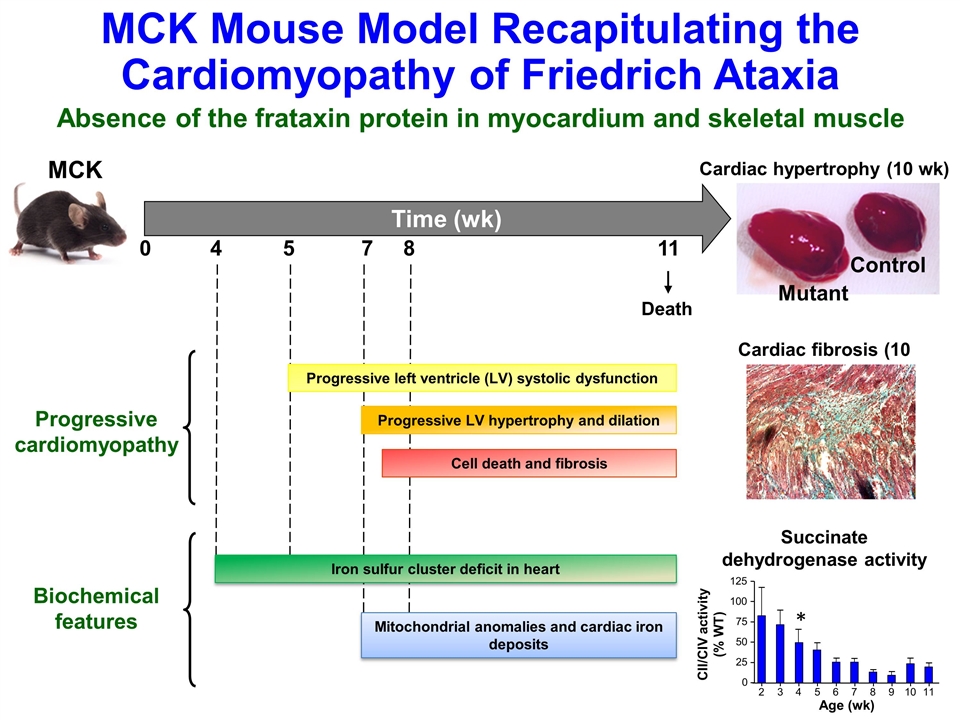
MCK Mouse Model Recapitulating the Cardiomyopathy of Friedrich Ataxia Absence of the frataxin protein in myocardium and skeletal muscle MCK 4 5 7 11 0 Death 8 Progressive left ventricle (LV) systolic dysfunction Cell death and fibrosis Progressive LV hypertrophy and dilation Progressive cardiomyopathy Iron sulfur cluster deficit in heart Mitochondrial anomalies and cardiac iron deposits Biochemical features Cardiac hypertrophy (10 wk) Cardiac fibrosis (10 wk) Time (wk) Mutant Control Age (wk) CII/CIV activity (% WT) Succinate dehydrogenase activity 2 3 4 5 6 7 8 9 10 11 25 125 100 75 50 0 *
3 MCK Evaluation of survival and multiple cardiac phenotypes 5 7 Intravenous AAVrh10.hFXN 5.4x1013 vg/kg) 22 35 60 Time (wk) MCK Mice in Heart Failure Treated with AAVrh.10.hFXN Advanced heart failure
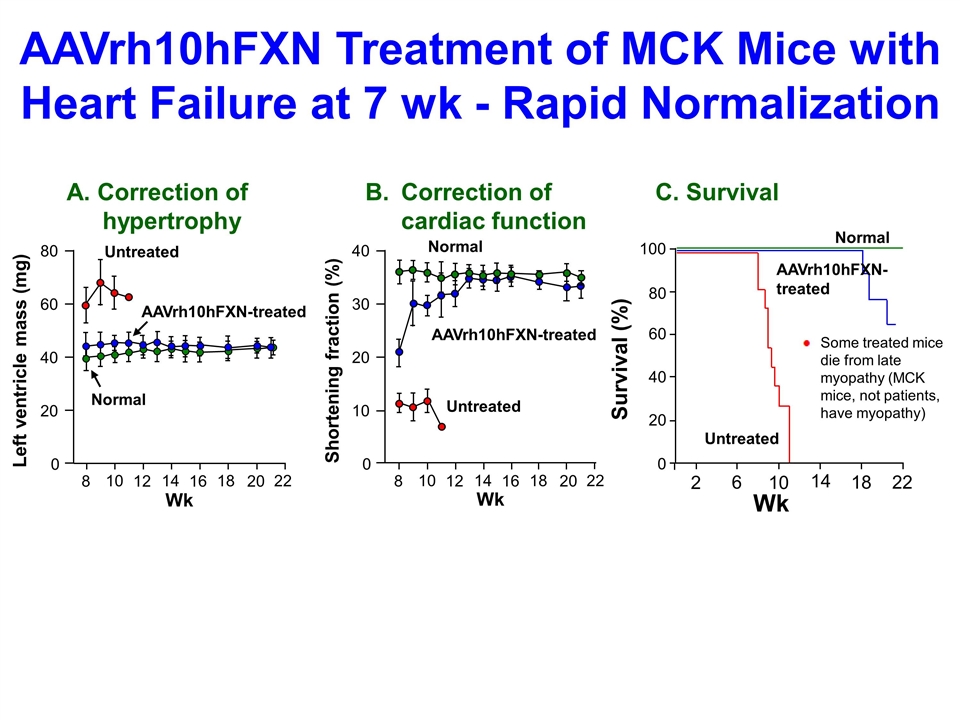
Untreated AAVrh10hFXN-treated Normal A. Correction of hypertrophy B. Correction of cardiac function C. Survival Some treated mice die from late myopathy (MCK mice, not patients, have myopathy) AAVrh10hFXN Treatment of MCK Mice with Heart Failure at 7 wk - Rapid Normalization 22 18 14 10 20 16 12 8 0 80 60 40 20 Wk Left ventricle mass (mg) 40 30 20 10 0 22 18 14 10 20 16 12 8 Wk Shortening fraction (%) 22 18 14 6 10 2 Wk 100 80 40 20 0 60 Survival (%) Untreated AAVrh10hFXN-treated Normal Untreated AAVrh10hFXN-treated Normal

Identifying a Robust Cardiac Phenotype for Cardiac Freidreich’s Ataxia Gene Therapy Studies to identify cardiac parameters Department of Genetic Medicine, Weill Cornell Hôpital Universitaire Pitié-Salpêtrière, Paris Parameters Genetic Neurologic Cardiac Serology Cardiac echo Cardiac mri Exercise CDG PET CT

Severe Allergy – Example Peanut Allergy Common food allergy, manifests with itchiness, urticaria, swelling, eczema, asthma, abdominal pain, hypotension and anaphylaxis Can be fatal 0.4–0.6% US population, 4,000 diagnosed/yr (11/day); most common cause of anaphylaxis in children presenting to the emergency ward Significant adverse effect on the quality of life No definitive therapy Strict avoidance Epinephrine Desensitization Omalizumab (Xolair) anti-IgE
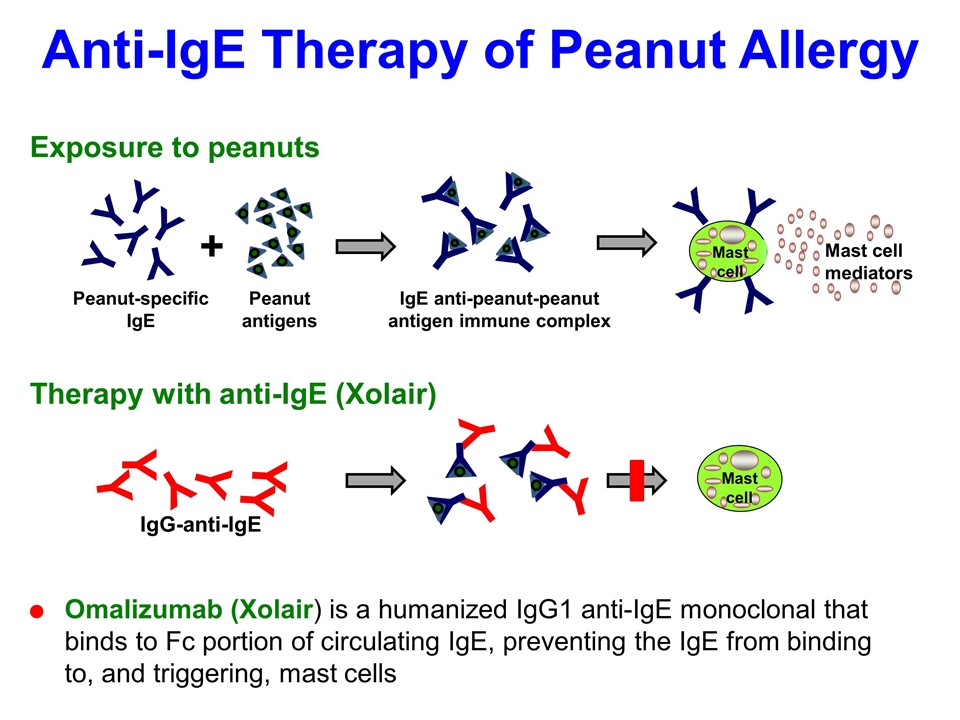
Anti-IgE Therapy of Peanut Allergy Omalizumab (Xolair) is a humanized IgG1 anti-IgE monoclonal that binds to Fc portion of circulating IgE, preventing the IgE from binding to, and triggering, mast cells Mast cell mediators Y Y Y Y Peanut-specific IgE Y Y Mast cell Exposure to peanuts + Peanut antigens IgE anti-peanut-peanut antigen immune complex Y Y Y Y Therapy with anti-IgE (Xolair) IgG-anti-IgE Y Y Y Y Y Y Mast cell Y Y Y Y Y Y Y Y Y Y Y Y Y Y
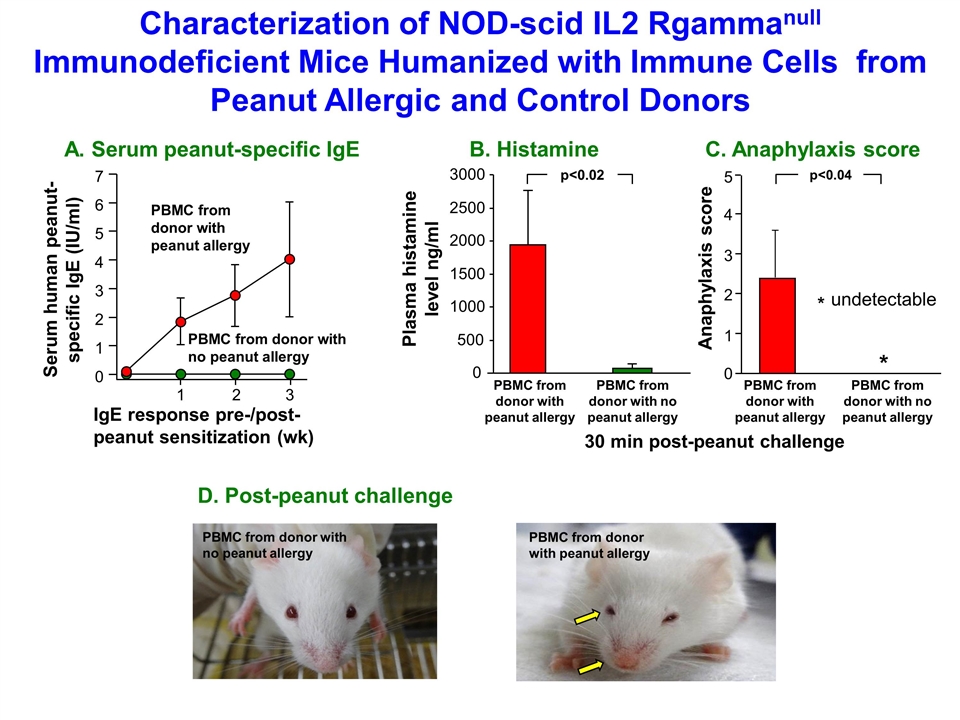
Characterization of NOD-scid IL2 Rgammanull Immunodeficient Mice Humanized with Immune Cells from Peanut Allergic and Control Donors PBMC from donor with no peanut allergy D. Post-peanut challenge PBMC from donor with peanut allergy PBMC from donor with no peanut allergy IgE response pre-/post-peanut sensitization (wk) A. Serum peanut-specific IgE Serum human peanut-specific IgE (IU/ml) 0 1 2 3 4 5 6 7 1 2 3 PBMC from donor with peanut allergy PBMC from donor with no peanut allergy B. Histamine C. Anaphylaxis score PBMC from donor with peanut allergy PBMC from donor with no peanut allergy PBMC from donor with peanut allergy 30 min post-peanut challenge Plasma histamine level ng/ml 0 500 1000 1500 2000 2500 3000 Anaphylaxis score p<0.02 p<0.04 * 0 2 3 4 1 * undetectable 5
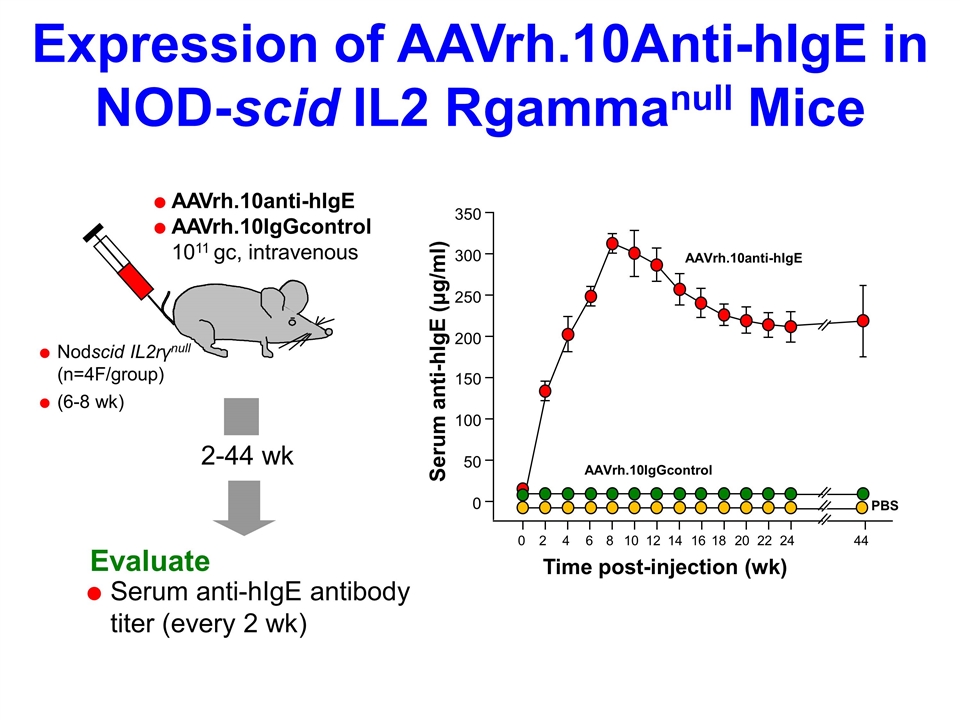
Expression of AAVrh.10Anti-hIgE in NOD-scid IL2 Rgammanull Mice 2-44 wk Evaluate AAVrh.10anti-hIgE AAVrh.10IgGcontrol 1011 gc, intravenous Serum anti-hIgE antibody titer (every 2 wk) Nodscid IL2rγnull (n=4F/group) (6-8 wk) Time post-injection (wk) Serum anti-hIgE (μg/ml) AAVrh.10anti-hIgE AAVrh.10IgGcontrol PBS 0 50 100 150 200 250 300 350 0 2 4 6 8 10 12 14 16 18 20 22 24 44
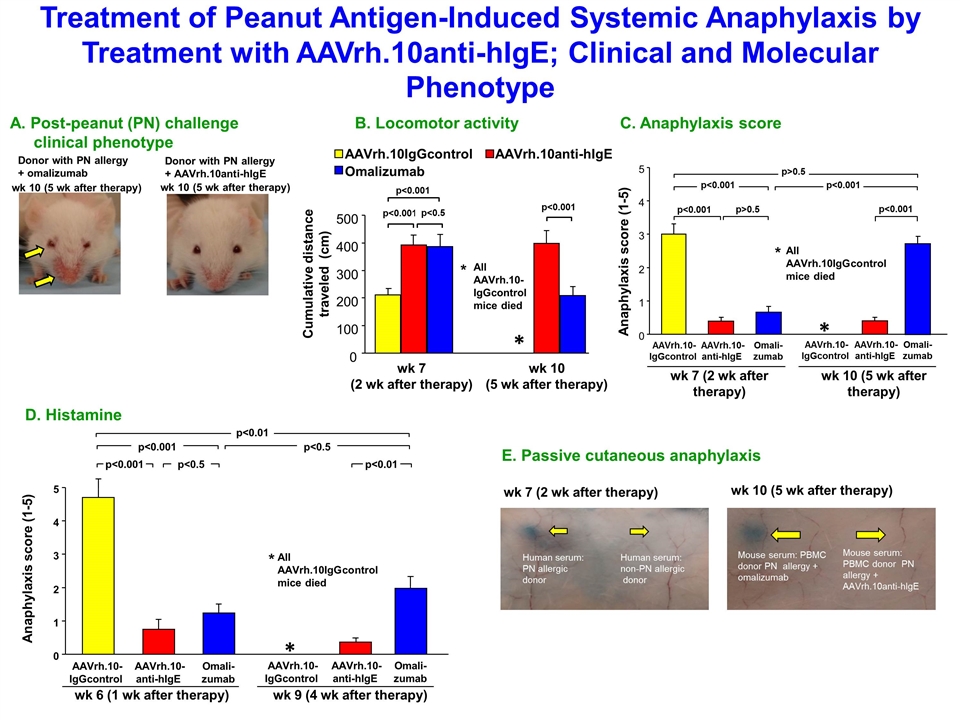
Treatment of Peanut Antigen-Induced Systemic Anaphylaxis by Treatment with AAVrh.10anti-hIgE; Clinical and Molecular Phenotype A. Post-peanut (PN) challenge clinical phenotype Donor with PN allergy + omalizumab Donor with PN allergy + AAVrh.10anti-hIgE wk 10 (5 wk after therapy) wk 10 (5 wk after therapy) wk 10 (5 wk after therapy) B. Locomotor activity Cumulative distance traveled (cm) AAVrh.10IgGcontrol AAVrh.10anti-hIgE Omalizumab 0 100 200 300 400 500 wk 7 (2 wk after therapy) All AAVrh.10- IgGcontrol mice died * * p<0.001 p<0.001 p<0.5 p<0.001 C. Anaphylaxis score Anaphylaxis score (1-5) All AAVrh.10IgGcontrol mice died 0 1 2 3 4 5 AAVrh.10- IgGcontrol AAVrh.10-anti-hIgE Omali-zumab AAVrh.10- IgGcontrol AAVrh.10-anti-hIgE Omali-zumab * wk 7 (2 wk after therapy) wk 10 (5 wk after therapy) * p<0.001 p<0.001 p>0.5 p<0.001 p<0.001 p>0.5 0 1 2 AAVrh.10- IgGcontrol AAVrh.10-anti-hIgE Omali-zumab AAVrh.10- IgGcontrol AAVrh.10-anti-hIgE Omali-zumab * wk 6 (1 wk after therapy) wk 9 (4 wk after therapy) D. Histamine Anaphylaxis score (1-5) All AAVrh.10IgGcontrol mice died 3 4 5 * p<0.001 p<0.001 p<0.5 p<0.01 p<0.5 p<0.01 E. Passive cutaneous anaphylaxis Mouse serum: PBMC donor PN allergy + omalizumab Mouse serum: PBMC donor PN allergy + AAVrh.10anti-hIgE Human serum: PN allergic donor Human serum: non-PN allergic donor wk 7 (2 wk after therapy) wk 10 (5 wk after therapy)
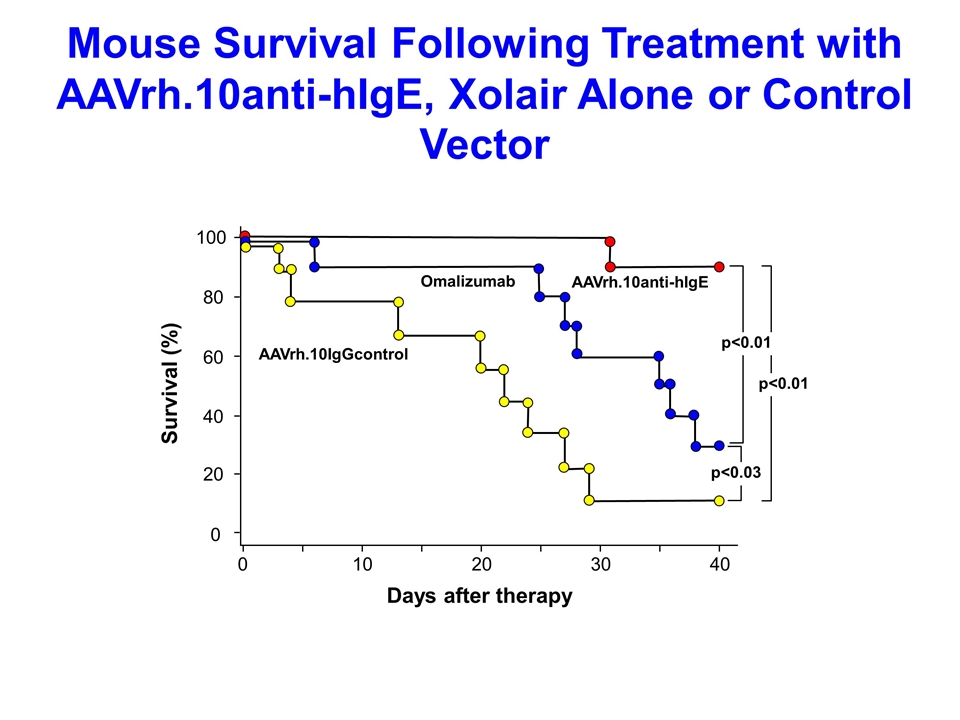
Mouse Survival Following Treatment with AAVrh.10anti-hIgE, Xolair Alone or Control Vector p<0.03 p<0.01 p<0.01 AAVrh.10IgGcontrol Omalizumab AAVrh.10anti-hIgE 100 60 40 20 0 80 0 30 20 10 40 Survival (%) Days after therapy
Gene Therapy Gene Modify gene expression Modify phenotype

































