
Exhibit 99.2 October 2022 Corporate Presentation

Legal Disclosure This presentation contains forward-looking statements, all of which are qualified in their entirety by this cautionary statement. Many of the forward-looking statements contained herein can be identified by the use of forward-looking words such as may , anticipate , believe , could', expect , should , plan , intend , estimate , will , potential and ongoing , among others, although not all forward-looking statements contain these identifying words. These forward-looking statements include statements about the initiation, timing, progress, results, and cost of our research and development programs and our current and future preclinical studies and clinical trials, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available, and our research and development programs; our ability to successfully manufacture our drug substances and product candidates for preclinical use, for clinical trials and on a larger scale for commercial use, if approved; the ability and willingness of our third-party strategic collaborators to continue research and development activities relating to our development candidates and product candidates; our ability to obtain funding for our operations necessary to complete further development and commercialization of our product candidates; our ability to obtain and maintain regulatory approval of our product candidates; the size and growth potential of the markets for our product candidates, and our ability to serve those markets; our financial performance; the effect of the COVID-19 pandemic, including mitigation efforts and economic effects, on any of the foregoing or other aspects of our business operations, including but not limited to our preclinical studies and clinical trials and any future studies or trials; our ability to commercialize our products, if approved; and the implementation of our business model, and strategic plans for our business and product candidates. Except as otherwise noted, these forward-looking statements speak only as of the date of this presentation, and we undertake no obligation to update or revise any of such statements to reflect events or circumstances occurring after this presentation. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. We caution you not to place undue reliance on the forward-looking statements contained in this presentation. This presentation discusses product candidates that are under preclinical or clinical evaluation and that have not yet been approved for marketing by the U.S. Food and Drug Administration or any other regulatory authority. Until finalized in a clinical study report, clinical trial data presented herein remain subject to adjustment as a result of clinical site audits and other review processes. No representation is made as to the safety or effectiveness of these product candidates for the use for which such product candidates are being studied. 2
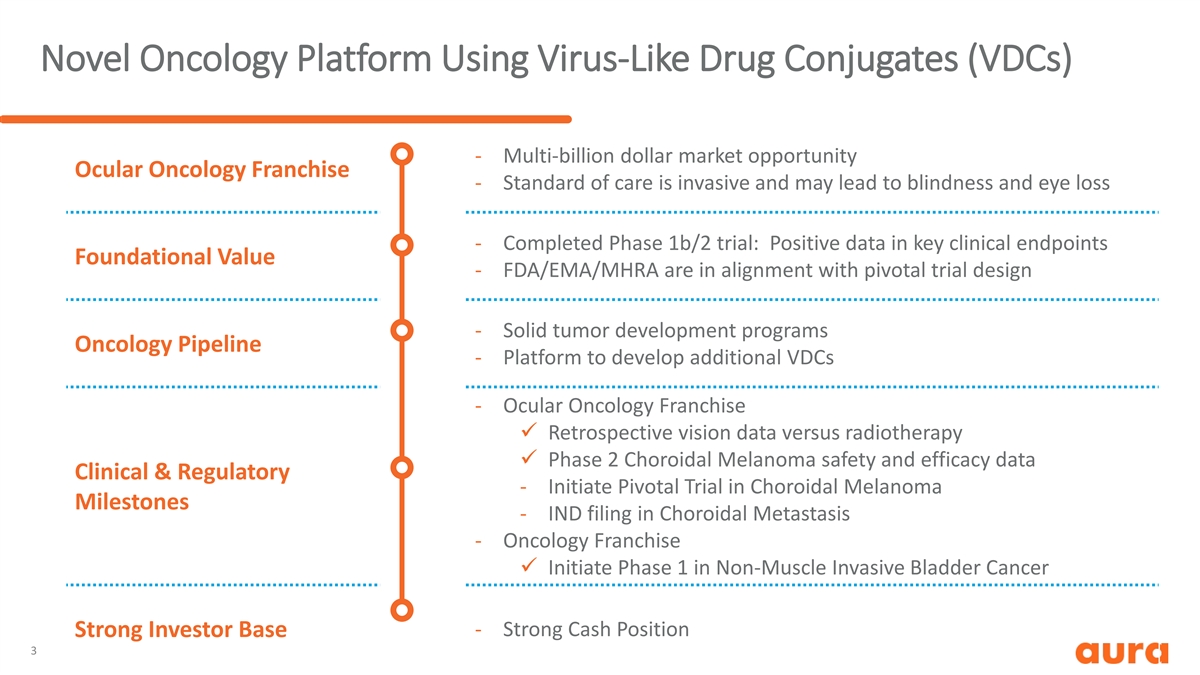
Novel Oncology Platform Using Virus-Like Drug Conjugates (VDCs) - Multi-billion dollar market opportunity Ocular Oncology Franchise - Standard of care is invasive and may lead to blindness and eye loss - Completed Phase 1b/2 trial: Positive data in key clinical endpoints Foundational Value - FDA/EMA/MHRA are in alignment with pivotal trial design - Solid tumor development programs Oncology Pipeline - Platform to develop additional VDCs - Ocular Oncology Franchise ü Retrospective vision data versus radiotherapy ü Phase 2 Choroidal Melanoma safety and efficacy data Clinical & Regulatory - Initiate Pivotal Trial in Choroidal Melanoma Milestones - IND filing in Choroidal Metastasis - Oncology Franchise ü Initiate Phase 1 in Non-Muscle Invasive Bladder Cancer - Strong Cash Position Strong Investor Base 3
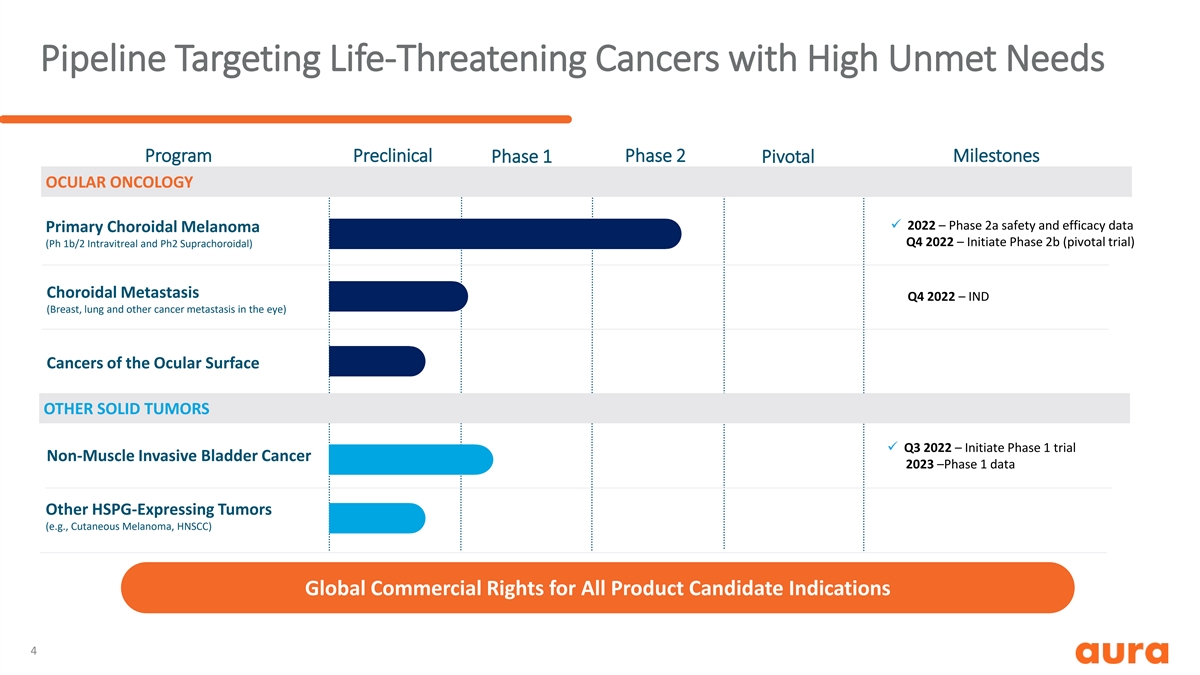
Pipeline Targeting Life-Threatening Cancers with High Unmet Needs Program Preclinical Phase 1 Phase 2 Pivotal Milestones OCULAR ONCOLOGY ü 2022 – Phase 2a safety and efficacy data Primary Choroidal Melanoma Q4 2022 – Initiate Phase 2b (pivotal trial) (Ph 1b/2 Intravitreal and Ph2 Suprachoroidal) Choroidal Metastasis Q4 2022 – IND (Breast, lung and other cancer metastasis in the eye) Cancers of the Ocular Surface OTHER SOLID TUMORS ü Q3 2022 – Initiate Phase 1 trial Non-Muscle Invasive Bladder Cancer 2023 –Phase 1 data Other HSPG-Expressing Tumors (e.g., Cutaneous Melanoma, HNSCC) Global Commercial Rights for All Product Candidate Indications 4

Experienced David Johnson Elisabet de los Pinos, PhD Cadmus Rich, MD Julie Feder Mark De Rosch, PhD Executive Board Chair Founder & Chief Medical Officer, Chief Operating Officer Chief Financial Officer Chief Executive Officer Head of R&D Team and CEO (acq Merck) Board CEO (acq AstraZeneca) 20+ 20+ average years of Regulatory drug experience and device approvals 5
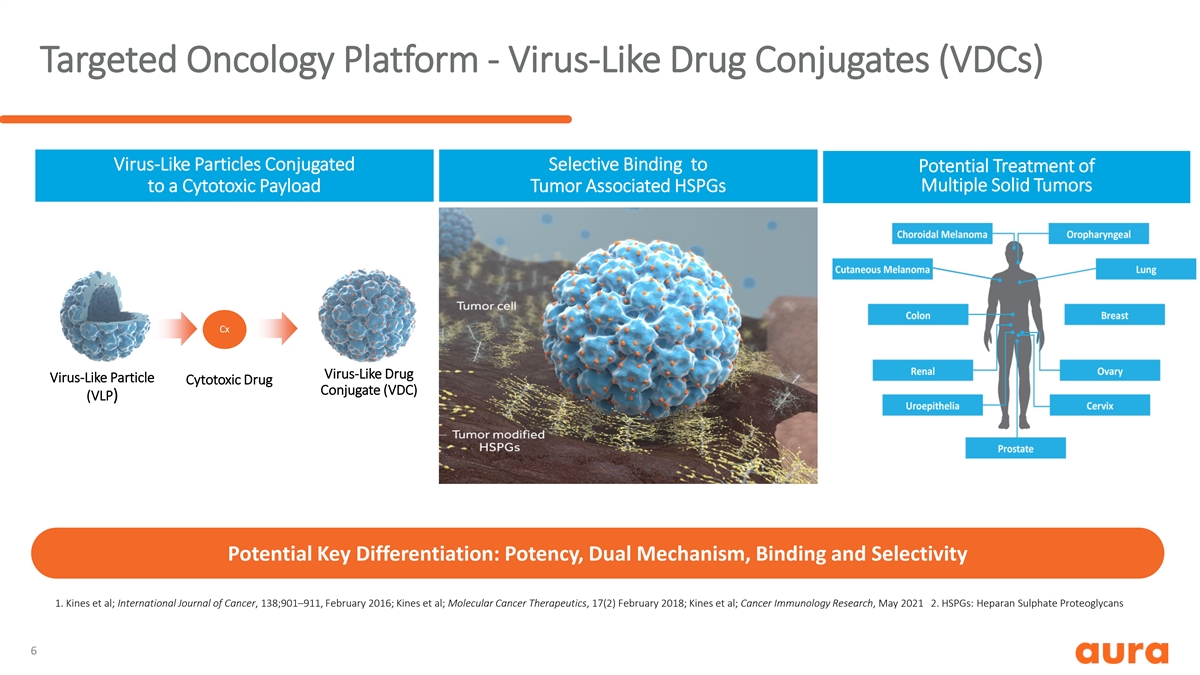
Targeted Oncology Platform - Virus-Like Drug Conjugates (VDCs) Virus-Like Particles Conjugated Selective Binding to Potential Treatment of Multiple Solid Tumors to a Cytotoxic Payload Tumor Associated HSPGs Cx Virus-Like Drug Virus-Like Particle Cytotoxic Drug Conjugate (VDC) (VLP) Potential Key Differentiation: Potency, Dual Mechanism, Binding and Selectivity 1. Kines et al; International Journal of Cancer, 138;901–911, February 2016; Kines et al; Molecular Cancer Therapeutics, 17(2) February 2018; Kines et al; Cancer Immunology Research, May 2021 2. HSPGs: Heparan Sulphate Proteoglycans 6

Bel-Sar (AU-011) Is a VDC with a Novel Dual Mechanism of Action Light Activated Drug Bel-Sar Bel-Sar is a novel VDC that consists of VLP conjugated to ~200 molecules of light activated drug Potential Key Differentiation: Physical ablation is agnostic to genetic mutations and may reduce risk of developing resistance Kines et al; Cancer Immunology Research, May 2021 Bel-Sar – Belzupacap Sarotalocan 7

Ocular Oncology AU-011 Franchise INN: belzupacap sarotalocan Target Indications: • Early-Stage Choroidal Melanoma • Choroidal Metastasis • Other Ocular Cancers 8

Choroidal Melanoma – High Unmet Medical Need with No Drugs Approved The choroid is the part of the uvea that is behind the retina Standard of Care is Radiotherapy or Enucleation Melanocytic Most common primary Lesion intraocular cancer in adults Impacts 11,000 patients in US/Europe per year Melanoma cells ~80% patients diagnosed Benign nevus cells with early-stage disease Blindness, Eye Loss, and Disfiguration Choroidal Melanoma is a Rare and Life-Threatening Ocular Cancer Kaliki et al; Eye (Lond) 2017 Feb; 31(2): 241–257; Clearview & Putnam & Assoc. Market Research; Source: Peddada. J Contemp Brachytherapy. August 2019 9

Bel-Sar has the Potential to be the First Approved Therapy in Primary Choroidal Melanoma Bel-Sar is Delivered by Simple Intravitreal Light Activation with Standard or Suprachoroidal Injection Goals of Treatment Ophthalmic Laser Local tumor control intravitreal Preservation of vision No radioactive co-morbidities Opportunity to treat early and reduce risk of metastases suprachoroidal Improvement in safety and quality of life Bel-Sar – Belzupacap Sarotalocan 10
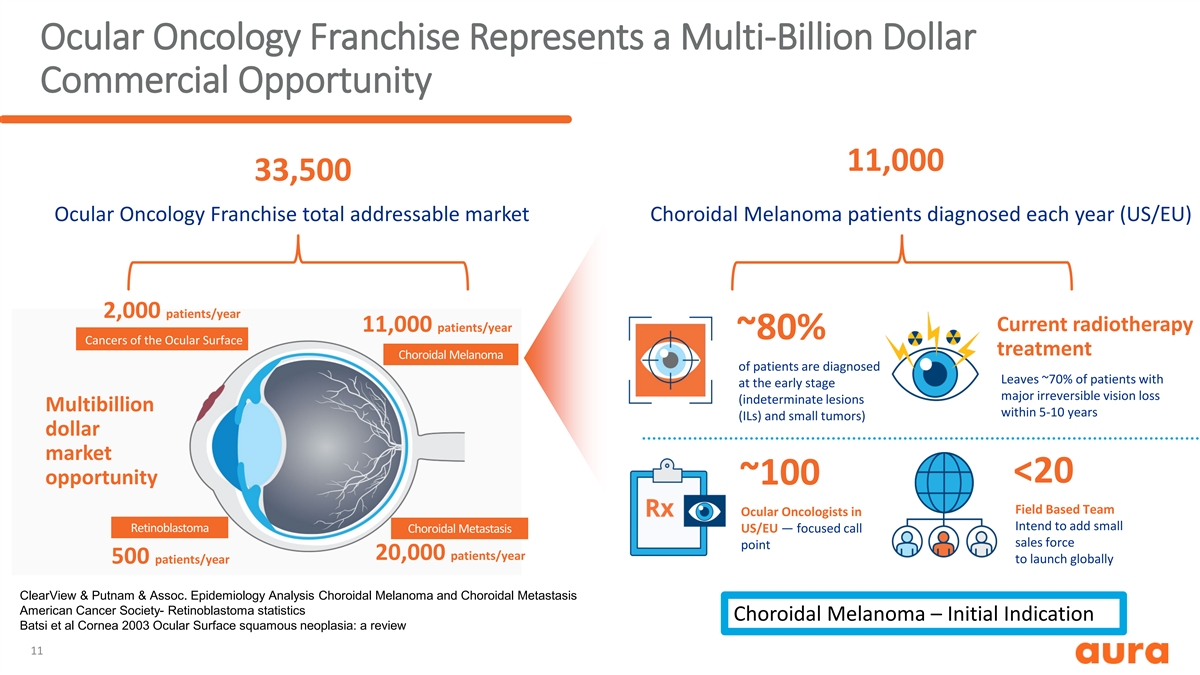
Ocular Oncology Franchise Represents a Multi-Billion Dollar Commercial Opportunity 11,000 33,500 Ocular Oncology Franchise total addressable market Choroidal Melanoma patients diagnosed each year (US/EU) 2,000 patients/year Current radiotherapy 11,000 patients/year ~80% Cancers of the Ocular Surface treatment Choroidal Melanoma of patients are diagnosed Leaves ~70% of patients with at the early stage major irreversible vision loss (indeterminate lesions Multibillion within 5-10 years (ILs) and small tumors) dollar market <20 opportunity ~100 Field Based Team Ocular Oncologists in Intend to add small Retinoblastoma Choroidal Metastasis US/EU — focused call sales force point 20,000 patients/year 500 patients/year to launch globally ClearView & Putnam & Assoc. Epidemiology Analysis Choroidal Melanoma and Choroidal Metastasis American Cancer Society- Retinoblastoma statistics Choroidal Melanoma – Initial Indication Batsi et al Cornea 2003 Ocular Surface squamous neoplasia: a review 11
Clinical program Ocular Oncology AU-011 Franchise INN: belzupacap sarotalocan Initial Target Indication: Early-Stage Choroidal Melanoma 12

Current Standard of Care is Invasive with Significant Co-Morbidities Standard of Care is Radiotherapy or Enucleation Standard of Care Often Results in Irreversible Vision Loss — Does Not Reduce Rate of Developing Metastasis 13
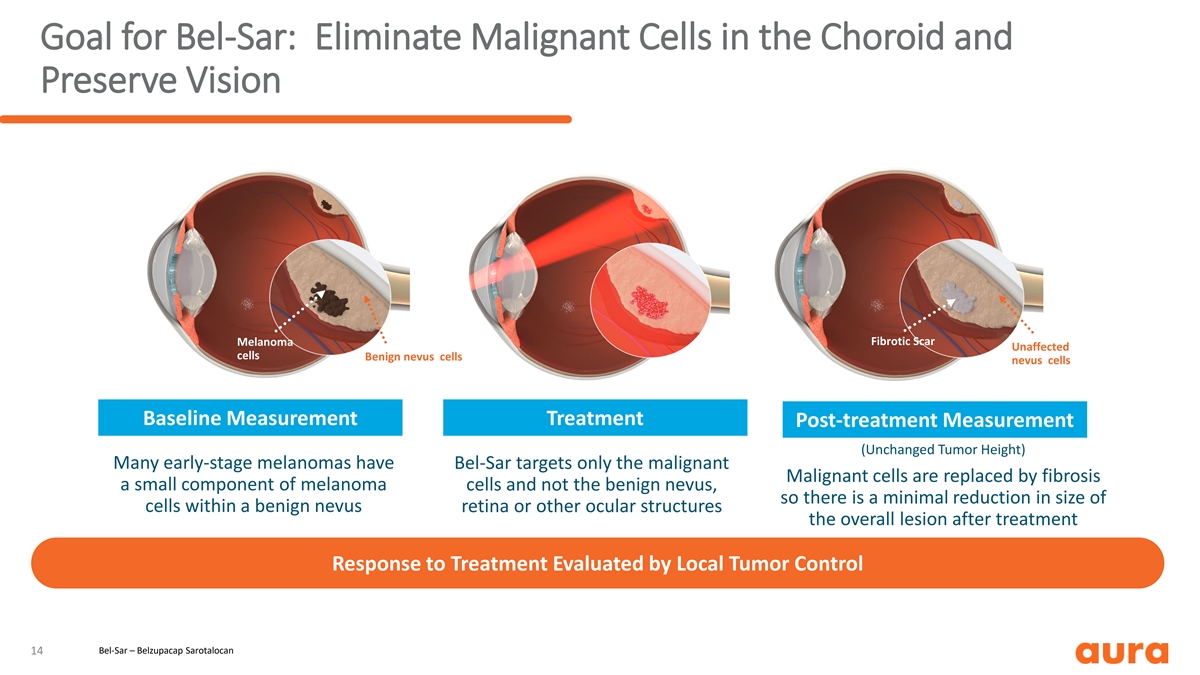
Goal for Bel-Sar: Eliminate Malignant Cells in the Choroid and Preserve Vision 3mm 3mm 3mm Fibrotic Scar Melanoma Unaffected cells Benign nevus cells nevus cells Baseline Measurement Treatment Post-treatment Measurement (Unchanged Tumor Height) Many early-stage melanomas have Bel-Sar targets only the malignant Malignant cells are replaced by fibrosis a small component of melanoma cells and not the benign nevus, so there is a minimal reduction in size of cells within a benign nevus retina or other ocular structures the overall lesion after treatment Response to Treatment Evaluated by Local Tumor Control Bel-Sar – Belzupacap Sarotalocan 14
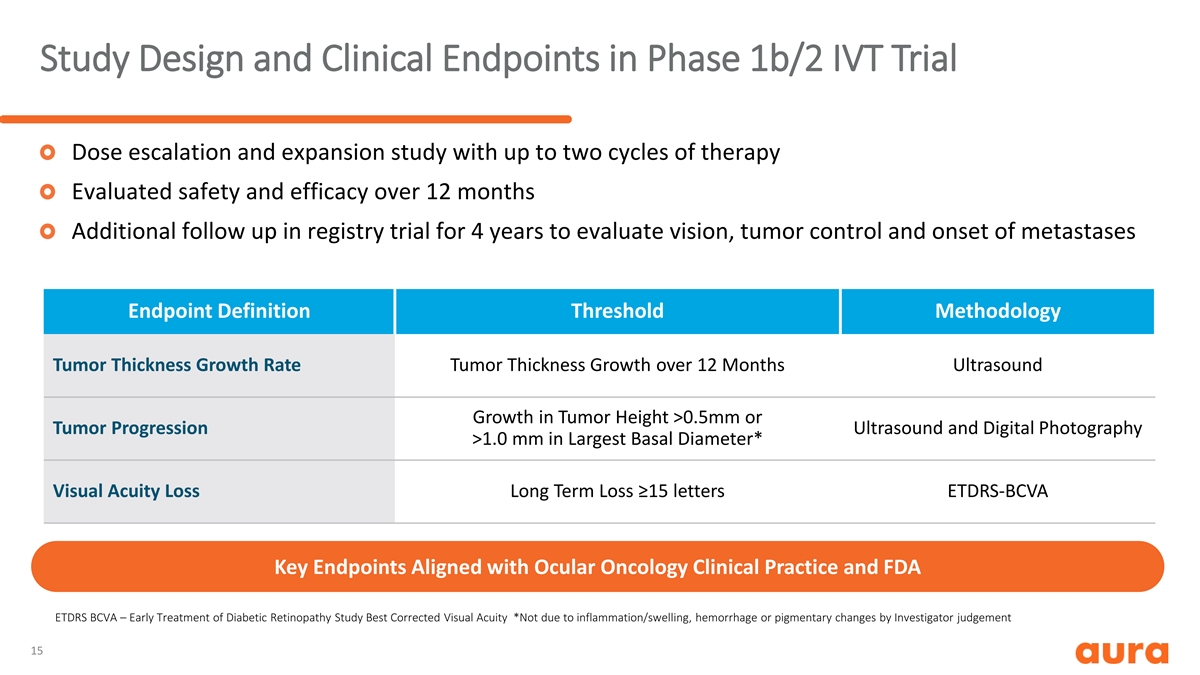
Study Design and Clinical Endpoints in Phase 1b/2 IVT Trial Dose escalation and expansion study with up to two cycles of therapy Evaluated safety and efficacy over 12 months Additional follow up in registry trial for 4 years to evaluate vision, tumor control and onset of metastases Endpoint Definition Threshold Methodology Tumor Thickness Growth Rate Tumor Thickness Growth over 12 Months Ultrasound Growth in Tumor Height >0.5mm or Tumor Progression Ultrasound and Digital Photography >1.0 mm in Largest Basal Diameter* Visual Acuity Loss Long Term Loss ≥15 letters ETDRS-BCVA Key Endpoints Aligned with Ocular Oncology Clinical Practice and FDA ETDRS BCVA – Early Treatment of Diabetic Retinopathy Study Best Corrected Visual Acuity *Not due to inflammation/swelling, hemorrhage or pigmentary changes by Investigator judgement 15
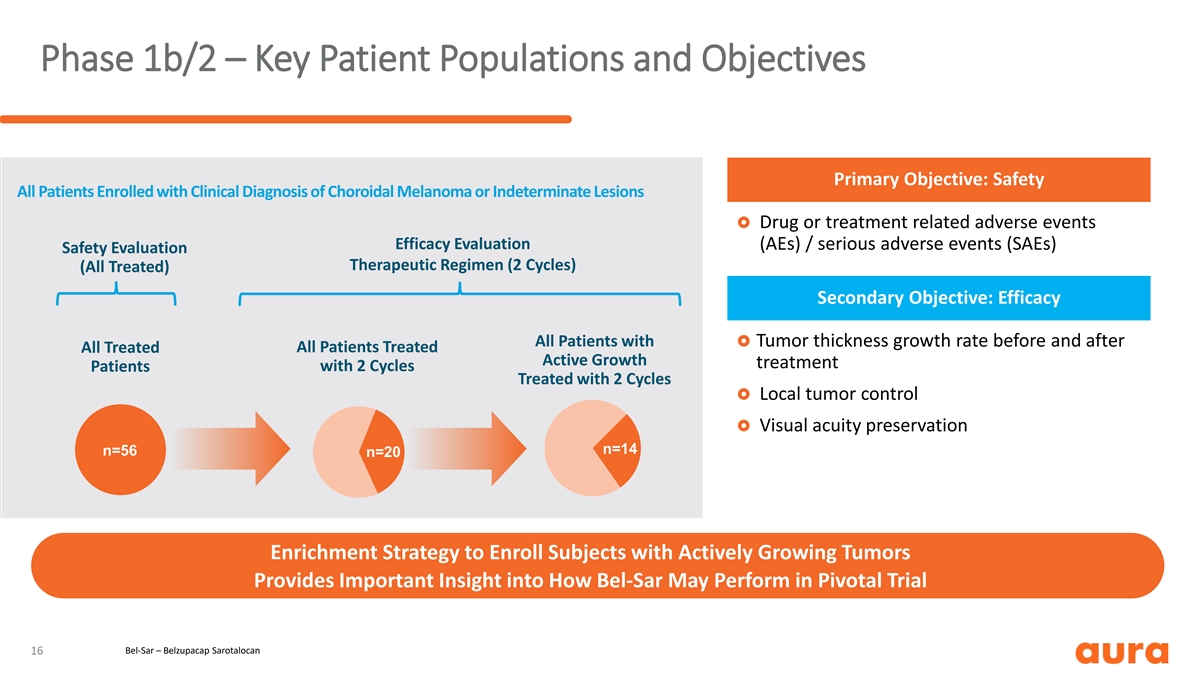
Phase 1b/2 – Key Patient Populations and Objectives Primary Objective: Safety All Patients Enrolled with Clinical Diagnosis of Choroidal Melanoma or Indeterminate Lesions £ Drug or treatment related adverse events Efficacy Evaluation (AEs) / serious adverse events (SAEs) Safety Evaluation Therapeutic Regimen (2 Cycles) (All Treated) Secondary Objective: Efficacy All Patients with £ Tumor thickness growth rate before and after All Treated All Patients Treated Active Growth treatment with 2 Cycles Patients Treated with 2 Cycles £ Local tumor control £ Visual acuity preservation n=14 n=56 n=20 Enrichment Strategy to Enroll Subjects with Actively Growing Tumors Provides Important Insight into How Bel-Sar May Perform in Pivotal Trial Bel-Sar – Belzupacap Sarotalocan 16
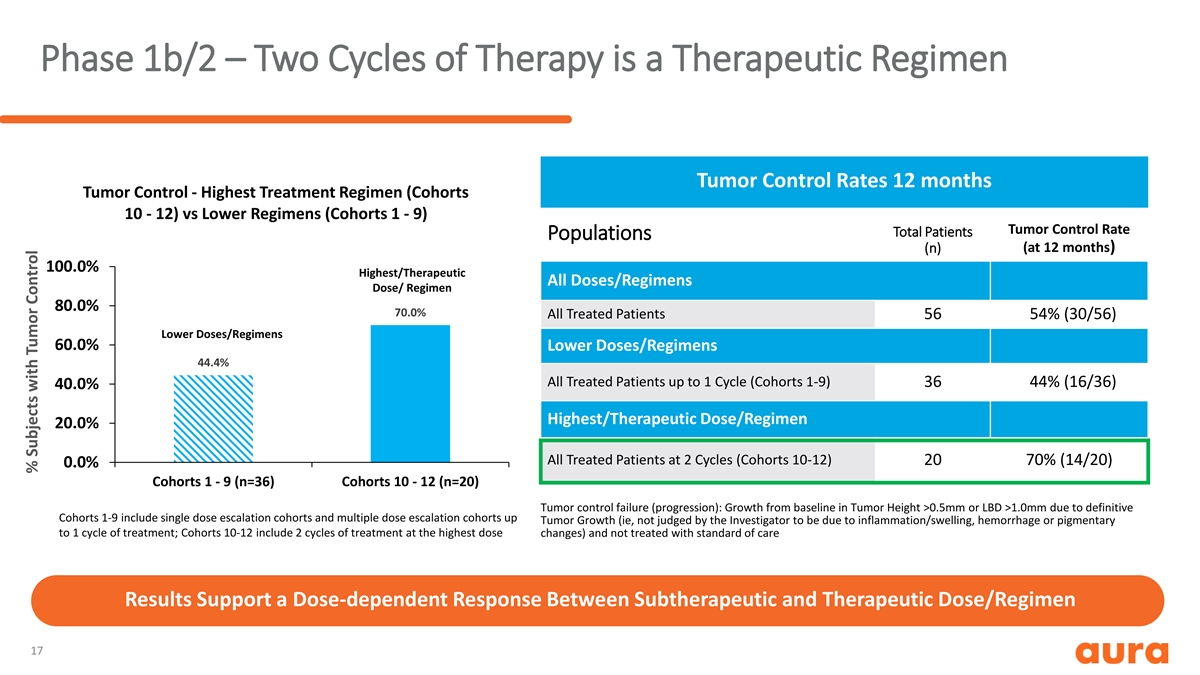
Phase 1b/2 – Two Cycles of Therapy is a Therapeutic Regimen Tumor Control Rates 12 months Tumor Control - Highest Treatment Regimen (Cohorts 10 - 12) vs Lower Regimens (Cohorts 1 - 9) Tumor Control Rate Total Patients Populations (n) (at 12 months) 100.0% Highest/Therapeutic All Doses/Regimens Dose/ Regimen 80.0% 70.0% All Treated Patients 56 54% (30/56) Lower Doses/Regimens 60.0% Lower Doses/Regimens 44.4% All Treated Patients up to 1 Cycle (Cohorts 1-9) 36 44% (16/36) 40.0% Highest/Therapeutic Dose/Regimen 20.0% All Treated Patients at 2 Cycles (Cohorts 10-12) 20 70% (14/20) 0.0% Cohorts 1 - 9 (n=36) Cohorts 10 - 12 (n=20) Tumor control failure (progression): Growth from baseline in Tumor Height >0.5mm or LBD >1.0mm due to definitive Cohorts 1-9 include single dose escalation cohorts and multiple dose escalation cohorts up Tumor Growth (ie, not judged by the Investigator to be due to inflammation/swelling, hemorrhage or pigmentary to 1 cycle of treatment; Cohorts 10-12 include 2 cycles of treatment at the highest dose changes) and not treated with standard of care Results Support a Dose-dependent Response Between Subtherapeutic and Therapeutic Dose/Regimen 17 % Subjects with Tumor Control

Phase 1b/2 – Tumor Control Achieved with Therapeutic Regimen All Patients with Active Growth Tumor Control Rate at 12 months Treated with 2 Cycles (n=14) Tumor Control Rate Total Patients Populations (n) (at 12 months) Therapeutic Dose/Regimen (2 Cycles) All Patients Treated with 2 Cycles 20 70% (14/20) All Patients with Active Growth Treated with 2 Cycles 14 64% (9/14) Tumor control failure (progression): Growth from baseline in Tumor Height >0.5mm or LBD >1.0mm due to definitive Tumor Growth (ie, not judged by the Investigator to be due to inflammation/swelling, hemorrhage or pigmentary changes) and not treated with standard of care Change from Baseline in Tumor Thickness Over 12 Months Progression Definition Tumor Height Increase >0.5mm Completed Ph1b/2 IVT trial (AU-011-101) We Believe Results Support Bel-Sar as First Line Treatment to Help Many Patients Avoid the Need for Radiotherapy Bel-Sar – Belzupacap Sarotalocan 18
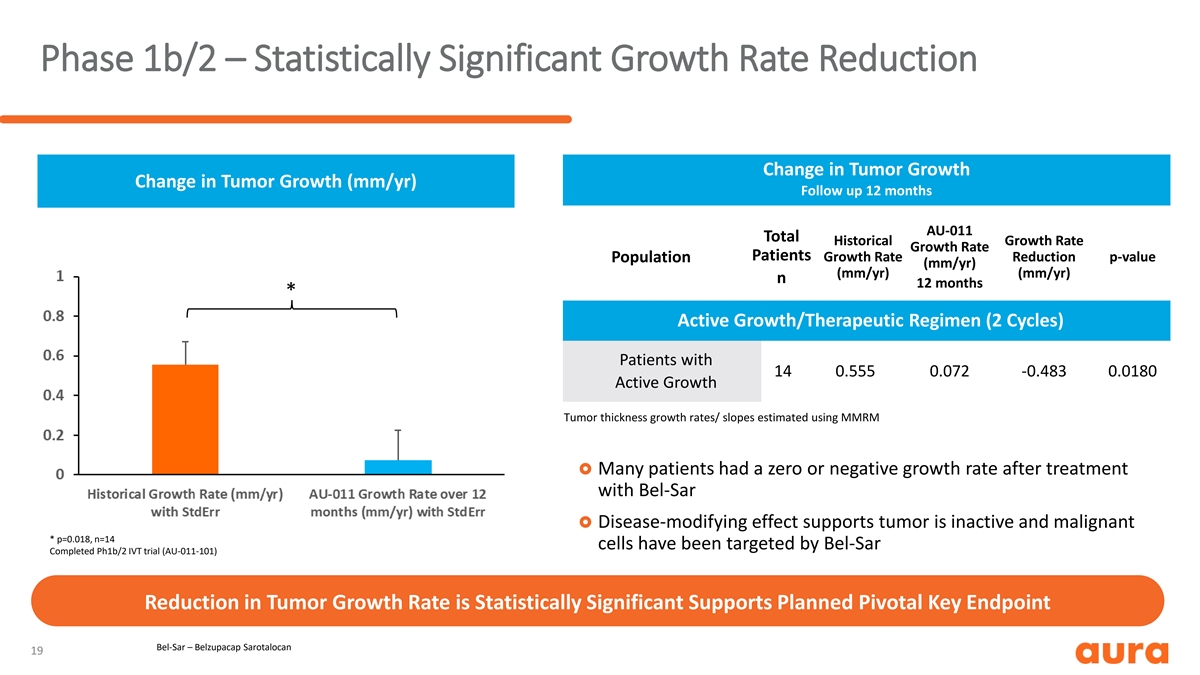
Phase 1b/2 – Statistically Significant Growth Rate Reduction Change in Tumor Growth Change in Tumor Growth (mm/yr) Follow up 12 months AU-011 Total Historical Growth Rate Growth Rate Patients Growth Rate Reduction p-value Population (mm/yr) (mm/yr) (mm/yr) n 12 months * Active Growth/Therapeutic Regimen (2 Cycles) Patients with 14 0.555 0.072 -0.483 0.0180 Active Growth Tumor thickness growth rates/ slopes estimated using MMRM £ Many patients had a zero or negative growth rate after treatment with Bel-Sar £ Disease-modifying effect supports tumor is inactive and malignant * p=0.018, n=14 cells have been targeted by Bel-Sar Completed Ph1b/2 IVT trial (AU-011-101) Reduction in Tumor Growth Rate is Statistically Significant Supports Planned Pivotal Key Endpoint Bel-Sar – Belzupacap Sarotalocan 19

Phase 1b/2 – Visual Acuity was Preserved in Majority of Patients Vision Preservation Rates Follow up 12 months Vision Preservation £ Vision loss was transient but recovered in Rate Total Patients Failure: Long term most patients after inflammation or Populations (n) loss ≥15 letters transient AEs resolved All Dose Cohorts All Treated Patients 56 86% (48/56) £ Vision was preserved in patients with Patients with Active Growth - High Risk for Vision Loss 17 76% (13/17) tumors near the fovea or optic nerve that had a high risk for vision loss Therapeutic Regimen (2 cycles) All Treated Patients 20 75% (15/20) Patients with Active Growth 14 71% (10/14) 1 patient had loss ≥15 letters at Week 52 visit which recovered within 15 letters at the next visit which was ~3 weeks after standard of care (SOC); all other post-SOC data excluded for all subjects Completed Ph1b/2 IVT trial (AU-011-101) Vision was Preserved in Majority of Patients Whereas Radiotherapy Often Leads to Irreversible and Long-Term Severe Vision Loss 20

Phase 1b/2 – Demonstrated Favorable Safety Profile Majority of AEs Were Transient and Resolved Without Clinical Sequelae AU-011 Treatment Related AEs Adverse Event Radiotherapy* Bel-Sar Grade I/II Grade III ≥15% Subjects (n=56) Final Surgeries secondary to AEs (e.g., Cataracts) 40%+ ~13% Vitreous Inflammation 83.9% 7.1% Radiation Retinopathy 40%+ 0% Anterior Chamber Inflammation 67.9% 3.6% Neovascular Glaucoma 10% 0% Increase in Intraocular Pressure 46.4% 0 Dry Eye Syndrome 20% ~2% Pigmentary Changes/Peritumoral 37.5% 0 Strabismus 2%+ 0% Keratic Precipitates 23.2% 0 Retinal Detachment 1-2% ~2% Floaters/Vitreous Opacity 19.7% 1.8% Vision Loss (≥15 letters) ~70% ~21% Decreased visual acuity 19.6% 1.8% Serious Adverse Event Radiotherapy* Bel-Sar Treatment Related SAEs (n=56) Scleral Necrosis 0-5% 0% Vision Loss (juxtafoveal tumor, n=2) 3.6% Enucleation/Eye Loss 10-15% 0% SAE of vision loss in two subjects with tumors close to fovea due to pigmentary changes at the edge of the tumor + SAEs are listed separately in the SAE table Completed Ph 1b/2 IVT trial (AU-011-101) Vision Loss in High-Risk Subjects** (≥30 letters) ~90% 4.6% + Cross-trial comparison of AU-011-101 and Radiotherapy 77% (43/56) of patients in Ph1b/2 IVT trial were at high risk for vision loss ; 2/43= 4.6% Safety Profile Supports Indication as a First Line Treatment in Early-Stage Disease *J. Contemp Brachytherapy. J. 2019 Aug; 11(4): 392–397.; Arch Ophthalmol. 2000;118(9):1219-1228; Curr. Opin. Ophthalmol. 2019 May;30(3):206-214; Eye 2017 Feb;31(2):241-257 **High-Risk Subjects are those with tumors <3mm to fovea or optic nerve Bel-Sar – Belzupacap Sarotalocan 21
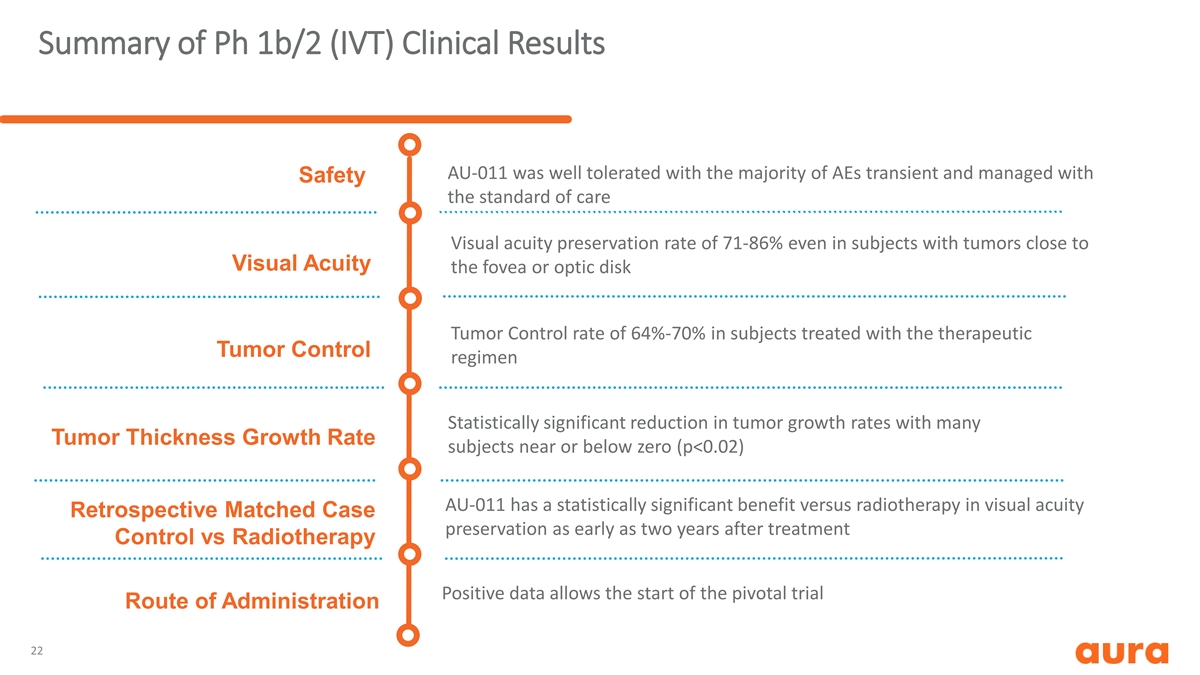
Summary of Ph 1b/2 (IVT) Clinical Results AU-011 was well tolerated with the majority of AEs transient and managed with Safety the standard of care Visual acuity preservation rate of 71-86% even in subjects with tumors close to Visual Acuity the fovea or optic disk Tumor Control rate of 64%-70% in subjects treated with the therapeutic Tumor Control regimen Statistically significant reduction in tumor growth rates with many Tumor Thickness Growth Rate subjects near or below zero (p<0.02) AU-011 has a statistically significant benefit versus radiotherapy in visual acuity Retrospective Matched Case preservation as early as two years after treatment Control vs Radiotherapy Positive data allows the start of the pivotal trial Route of Administration 22

Retrospective Matched Case-Control Study 23

Retrospective MCC Study to Evaluate Visual Acuity Outcomes of Bel-Sar vs. Radiotherapy • Matching criteria: baseline tumor thickness, LBD, distance to fovea/ optic disk, visual acuity (all 4 must match) • Matching performed by Independent Statistician (n=43 AU-011 and n=150 plaque matched case control subjects) Subjects with lesion ≤3mm from the fovea or optic disc and received AU- 011 treatment (IVT) in Ph1b/2 trial (n=43*) AU-011 subjects followed in primary trial Up to 5:1 and vision results extrapolated Data from patients treated with AU-011 Patients matched based on in the Ph1b/2 study compared to patients tumor size, location, and treated with plaque at 1, 2, 3, 4 and 5 years Subjects treated with plaque radiotherapy baseline vision with long term follow up from large center dataset (Dr. Shields) to support visual acuity benefit Patients treated with plaque radiotherapy matched based on criteria (n=150) AU-011 with IVT Administration has a Long-Term Vision Benefit Compared to Radiotherapy *43 AU-011 subjects included in matching; 2 AU-011 subjects did not have any matches; results presented for 41 AU-011 subjects with at least 1 match Bel-Sar – Belzupacap Sarotalocan 24 MCC – Matched Case Control
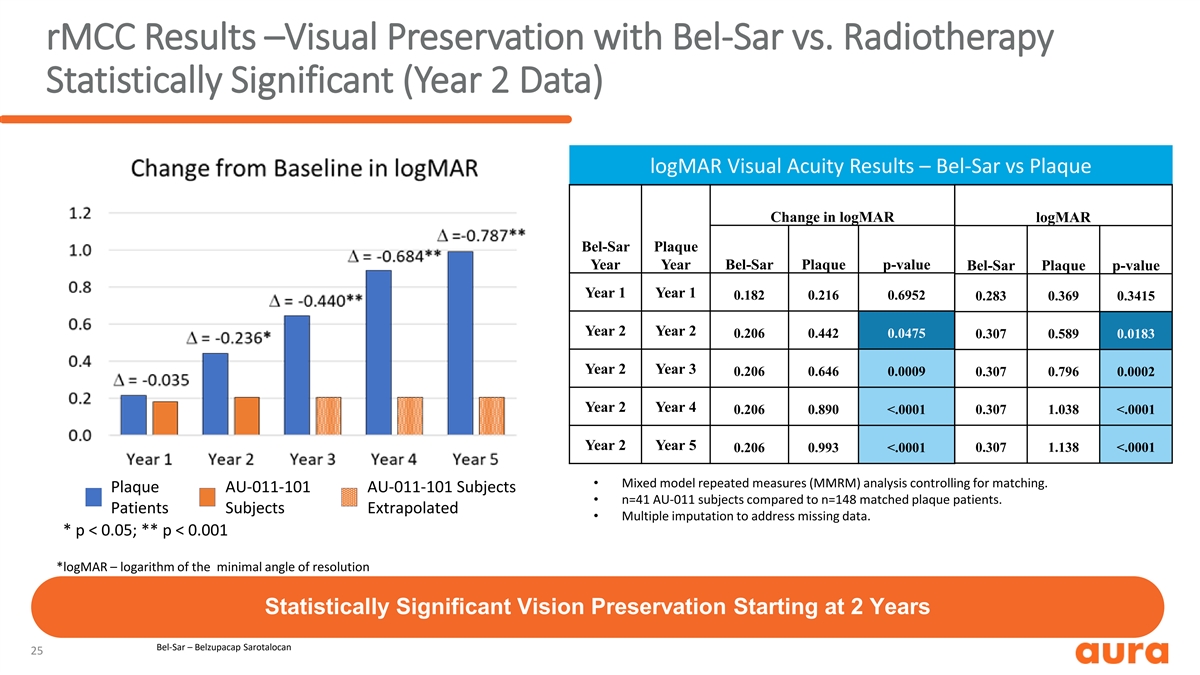
rMCC Results –Visual Preservation with Bel-Sar vs. Radiotherapy Statistically Significant (Year 2 Data) logMAR Visual Acuity Results – Bel-Sar vs Plaque Change in logMAR logMAR Bel-Sar Plaque Year Year Bel-Sar Plaque p-value Bel-Sar Plaque p-value Year 1 Year 1 0.182 0.216 0.6952 0.283 0.369 0.3415 Year 2 Year 2 0.206 0.442 0.0475 0.307 0.589 0.0183 Year 2 Year 3 0.206 0.646 0.0009 0.307 0.796 0.0002 Year 2 Year 4 0.206 0.890 <.0001 0.307 1.038 <.0001 Year 2 Year 5 0.206 0.993 <.0001 0.307 1.138 <.0001 • Mixed model repeated measures (MMRM) analysis controlling for matching. Plaque AU-011-101 AU-011-101 Subjects • n=41 AU-011 subjects compared to n=148 matched plaque patients. Patients Subjects Extrapolated • Multiple imputation to address missing data. * p < 0.05; ** p < 0.001 *logMAR – logarithm of the minimal angle of resolution Statistically Significant Vision Preservation Starting at 2 Years Bel-Sar – Belzupacap Sarotalocan 25
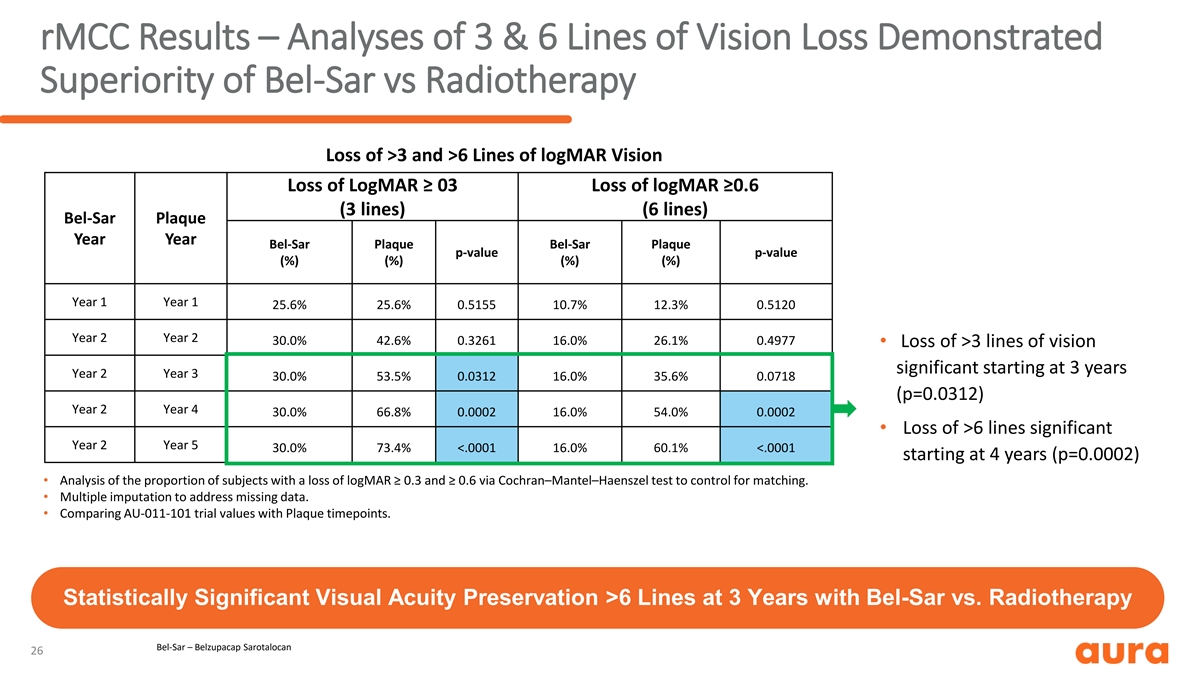
rMCC Results – Analyses of 3 & 6 Lines of Vision Loss Demonstrated Superiority of Bel-Sar vs Radiotherapy Loss of >3 and >6 Lines of logMAR Vision Loss of LogMAR ≥ 03 Loss of logMAR ≥0.6 (3 lines) (6 lines) Bel-Sar Plaque Year Year Bel-Sar Plaque Bel-Sar Plaque p-value p-value (%) (%) (%) (%) Year 1 Year 1 25.6% 25.6% 0.5155 10.7% 12.3% 0.5120 Year 2 Year 2 30.0% 42.6% 0.3261 16.0% 26.1% 0.4977 • Loss of >3 lines of vision significant starting at 3 years Year 2 Year 3 30.0% 53.5% 0.0312 16.0% 35.6% 0.0718 (p=0.0312) Year 2 Year 4 30.0% 66.8% 0.0002 16.0% 54.0% 0.0002 • Loss of >6 lines significant Year 2 Year 5 30.0% 73.4% <.0001 16.0% 60.1% <.0001 starting at 4 years (p=0.0002) • Analysis of the proportion of subjects with a loss of logMAR ≥ 0.3 and ≥ 0.6 via Cochran–Mantel–Haenszel test to control for matching. • Multiple imputation to address missing data. • Comparing AU-011-101 trial values with Plaque timepoints. Statistically Significant Visual Acuity Preservation >6 Lines at 3 Years with Bel-Sar vs. Radiotherapy Bel-Sar – Belzupacap Sarotalocan 26

Results Demonstrated Bel-Sar Preserves Visual Acuity at 1 & 2 Years • Objective to compare 2 Year Bel-Sar vision value to yearly plaque values for 5 years • Statistical significance starting in Year 2 @ p<0.05 level • Primary endpoint – Change from baseline in logMAR vision at 5 years • Statistically significant at 5 years (p<0.0001) • Secondary endpoints o logMAR vision comparison significant starting at 2 years (p=0.0134) o Loss of logMAR vision ≥ 0.3 (3 lines) significant starting at 3 years (p=0.0312) o Loss of logMAR vision ≥ 0.6 (6 lines) significant starting at 4 years (p=0.0002) Statistically Significant Vision Preservation Compared to Radiotherapy Bel-Sar – Belzupacap Sarotalocan 27

Phase 2 Suprachoroidal Study 28

Evaluating Suprachoroidal Administration to Determine Optimal Administration Route for Pivotal Trial Ph2 SC Trial Design: Dose Escalation Phase Patient Population Representative of Early- Single Dose Cohorts – Completed Stage Disease (IL/CM) 20 μg 40 μg 40 μg x 1 Laser x 1 Laser x 2 Lasers suprachoroidal Cohort 1 (n=1) Cohort 3 (n=2) Cohort 2 (n=3*) Small Tumors with Active Growth Multiple Dose Cohorts ONGOING 40 μg 80 μg • Tumor thickness ≥0.5 mm and ≤2.5 mm 40 μg x 2 Lasers x 2 Lasers x 2 Lasers QWx3 QWx3 QWx2 • LBD ≤10 mm Up to 3 cycles Up to 3 cycles Cohort 4 (n=3) Cohort 6 Cohort 5 (n=3) • Active tumor growth within 2 years of (n=10) screening *2 subjects were planned; third subject was additionally enrolled due to dose error in 1 subject ClinicalTrials.gov Identifier: NCT04417530 Goal to Determine Optimal Dose and Treatment Regimen with Suprachoroidal Administration SC – Suprachoroidal; IL – Indeterminate Lesion; CM- Choroidal Melanoma; LBD – Largest Basal Diameter 29
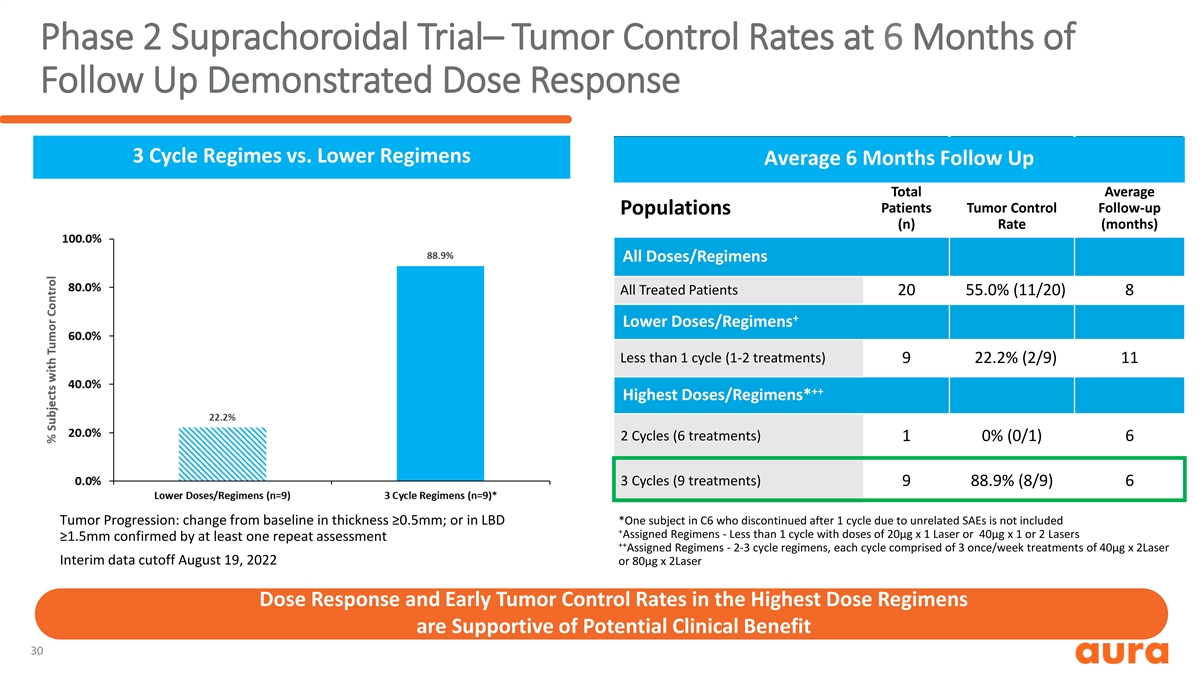
Phase 2 Suprachoroidal Trial– Tumor Control Rates at 6 Months of Follow Up Demonstrated Dose Response 3 Cycle Regimes vs. Lower Regimens Average 6 Months of Follow Up Average 6 Months Follow Up Total Average Patients Tumor Control Follow-up Populations (n) Rate (months) All Doses/Regimens All Treated Patients 20 55.0% (11/20) 8 + Lower Doses/Regimens Less than 1 cycle (1-2 treatments) 9 22.2% (2/9) 11 ++ Highest Doses/Regimens* 2 Cycles (6 treatments) 1 0% (0/1) 6 3 Cycles (9 treatments) 9 88.9% (8/9) 6 Tumor Progression: change from baseline in thickness ≥0.5mm; or in LBD *One subject in C6 who discontinued after 1 cycle due to unrelated SAEs is not included + Assigned Regimens - Less than 1 cycle with doses of 20µg x 1 Laser or 40µg x 1 or 2 Lasers ≥1.5mm confirmed by at least one repeat assessment ++ Assigned Regimens - 2-3 cycle regimens, each cycle comprised of 3 once/week treatments of 40µg x 2Laser Interim data cutoff August 19, 2022 or 80µg x 2Laser Dose Response and Early Tumor Control Rates in the Highest Dose Regimens are Supportive of Potential Clinical Benefit 30
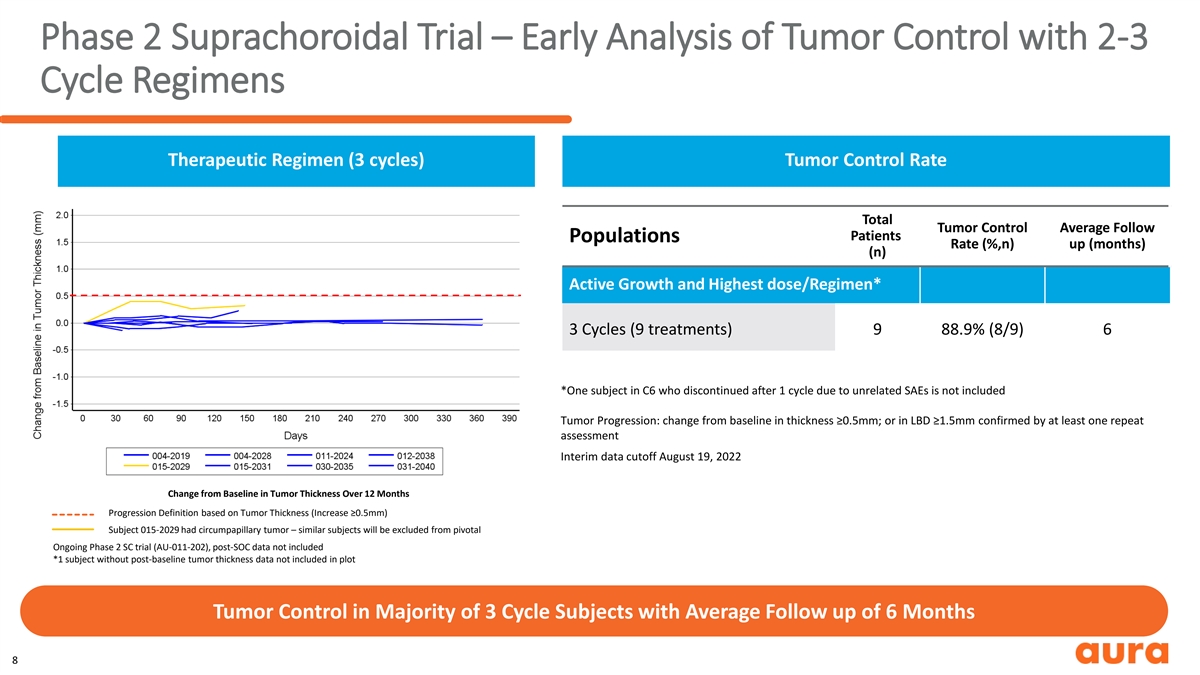
Phase 2 Suprachoroidal Trial – Early Analysis of Tumor Control with 2-3 Cycle Regimens Therapeutic Regimen (3 cycles) Tumor Control Rate Total Tumor Control Average Follow Patients Populations Rate (%,n) up (months) (n) Active Growth and Highest dose/Regimen* 3 Cycles (9 treatments) 9 88.9% (8/9) 6 *One subject in C6 who discontinued after 1 cycle due to unrelated SAEs is not included Tumor Progression: change from baseline in thickness ≥0.5mm; or in LBD ≥1.5mm confirmed by at least one repeat assessment Interim data cutoff August 19, 2022 Change from Baseline in Tumor Thickness Over 12 Months Progression Definition based on Tumor Thickness (Increase ≥0.5mm) Subject 015-2029 had circumpapillary tumor – similar subjects will be excluded from pivotal Ongoing Phase 2 SC trial (AU-011-202), post-SOC data not included *1 subject without post-baseline tumor thickness data not included in plot Tumor Control in Majority of 3 Cycle Subjects with Average Follow up of 6 Months 8

Phase 2 Suprachoroidal Trial – Early Analysis of Tumor Growth Rate with 3 Cycles of Therapy Change in Tumor Growth (mm/yr) Change in Tumor Growth 3 Cycle Regimens (n=9) Total Average Historical AU-011 Growth Rate Patients Follow up Population Growth Rate Growth Rate Reduction p-value (mm/yr) (mm/yr) (mm/yr) (months) n p=0.0007 A Acctt ii v v e e G G rr o ow w tt h h a a n n d d H H ii gh gh e e ss tt D D o oss e e // R R e e gi gi m m e e n n ts ** 3 Cycles 9 0.463 0.166 -0.296 0.0007 6 Tumor thickness growth rates/ slopes estimated using MMRM *One subject in C6 who discontinued after 1 cycle due to unrelated SAEs is not included Interim data cutoff August 19, 2022 Interim Data Showed Statistically Significant Growth Rate Reduction in 3 Cycle Regimen Subjects Reduction in Tumor Growth Rate is Statistically Significant and Supports Planned Pivotal Key Endpoint 32
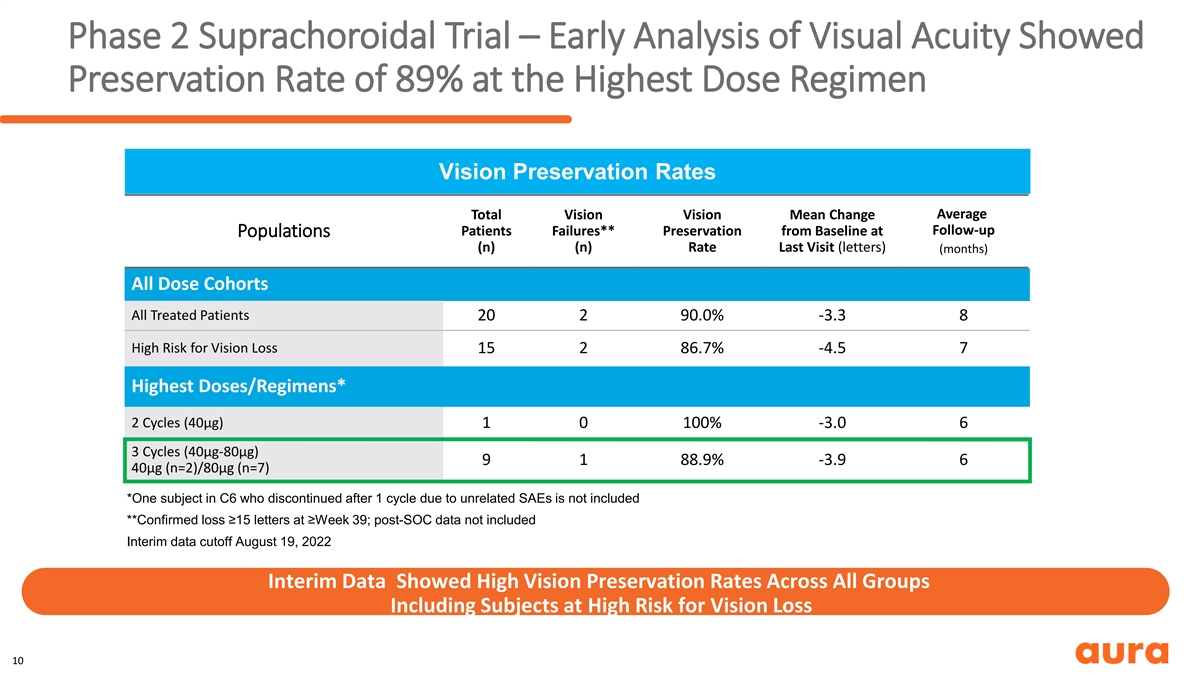
Phase 2 Suprachoroidal Trial – Early Analysis of Visual Acuity Showed Preservation Rate of 89% at the Highest Dose Regimen Vision Preservation Rates Average Total Vision Vision Mean Change Follow-up Patients Failures** Preservation from Baseline at Populations (n) (n) Rate Last Visit (letters) (months) All Dose Cohorts All Treated Patients 20 2 90.0% -3.3 8 High Risk for Vision Loss 15 2 86.7% -4.5 7 Highest Doses/Regimens* 2 Cycles (40µg) 1 0 100% -3.0 6 3 Cycles (40µg-80µg) 9 1 88.9% -3.9 6 40µg (n=2)/80µg (n=7) *One subject in C6 who discontinued after 1 cycle due to unrelated SAEs is not included **Confirmed loss ≥15 letters at ≥Week 39; post-SOC data not included Interim data cutoff August 19, 2022 Interim Data Showed High Vision Preservation Rates Across All Groups Including Subjects at High Risk for Vision Loss 10
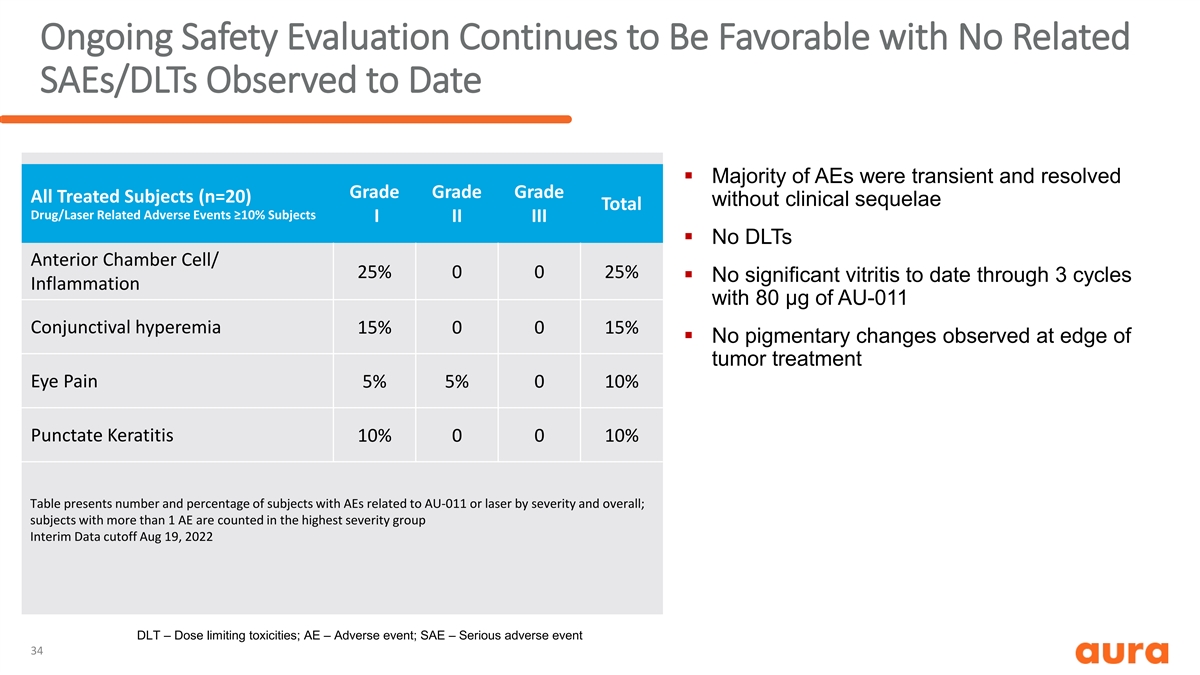
Ongoing Safety Evaluation Continues to Be Favorable with No Related SAEs/DLTs Observed to Date § Majority of AEs were transient and resolved Grade Grade Grade All Treated Subjects (n=20) without clinical sequelae Total Drug/Laser Related Adverse Events ≥10% Subjects I II III § No DLTs Anterior Chamber Cell/ 25% 0 0 25% § No significant vitritis to date through 3 cycles Inflammation with 80 µg of AU-011 Conjunctival hyperemia 15% 0 0 15% § No pigmentary changes observed at edge of tumor treatment Eye Pain 5% 5% 0 10% Punctate Keratitis 10% 0 0 10% Table presents number and percentage of subjects with AEs related to AU-011 or laser by severity and overall; subjects with more than 1 AE are counted in the highest severity group Interim Data cutoff Aug 19, 2022 DLT – Dose limiting toxicities; AE – Adverse event; SAE – Serious adverse event 34

Suprachoroidal Delivery Provides Additional Safety and Efficacy to Support Potential Treatment of Early-Stage Disease (IL/CM) Mild to moderate AEs overall and no related SAEs/DLTs observed to date Safety Visual acuity preservation rate of 87-90% even in subjects with tumors close to Visual Acuity the fovea or optic disk Early outcomes show high tumor control rate (~90%) with approximately Tumor Control 6 months of follow up in subjects treated with the therapeutic regimen Statistically significant reduction in tumor growth rates with many Tumor Thickness Growth Rate subjects near or below zero (p=0.0007) Minimal anterior uveitis and no vitritis observed to date Low to No Intraocular No pigmentary changes Inflammation Initial safety and efficacy in this ongoing Ph2 dose escalation study support Route of Administration SC administration Study ongoing/interim data cutoff August 19, 2022 35 IL – indeterminate lesion; CM – choroidal melanoma; SC - suprachoroidal

Choroidal Metastasis 36
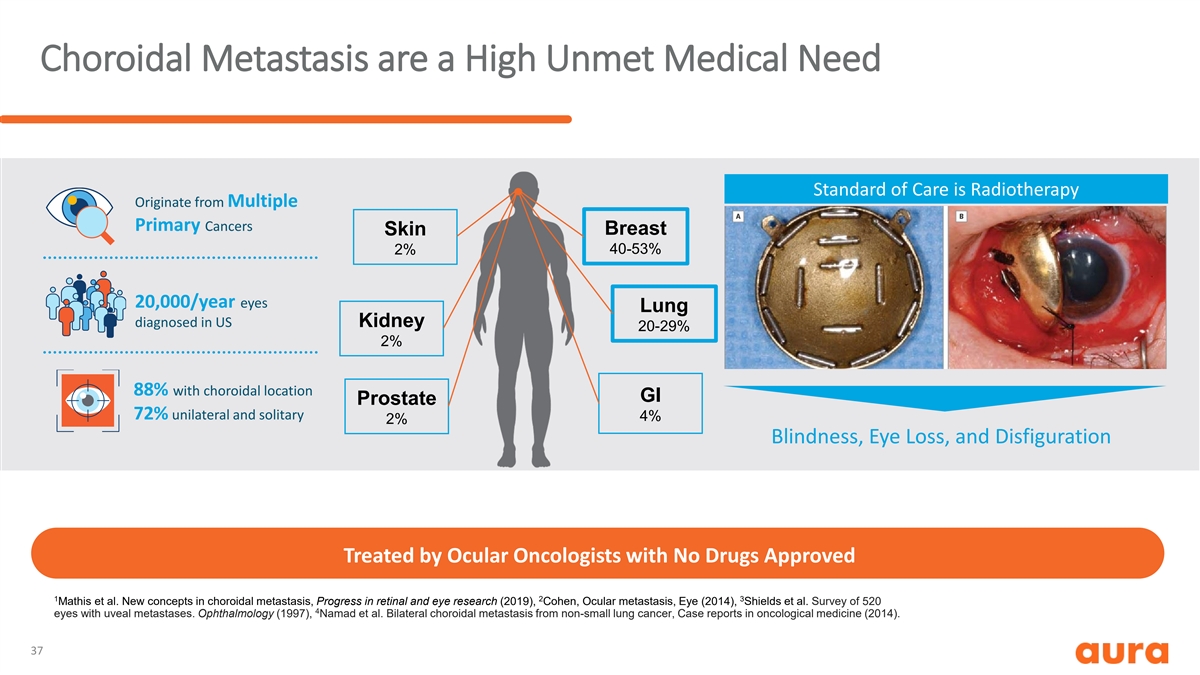
Choroidal Metastasis are a High Unmet Medical Need Standard of Care is Radiotherapy Originate from Multiple Primary Cancers Breast Skin 40-53% 2% 20,000/year eyes Lung diagnosed in US Kidney 20-29% 2% 88% with choroidal location GI Prostate 72% unilateral and solitary 4% 2% Blindness, Eye Loss, and Disfiguration Treated by Ocular Oncologists with No Drugs Approved 1 2 3 Mathis et al. New concepts in choroidal metastasis, Progress in retinal and eye research (2019), Cohen, Ocular metastasis, Eye (2014), Shields et al. Survey of 520 4 eyes with uveal metastases. Ophthalmology (1997), Namad et al. Bilateral choroidal metastasis from non-small lung cancer, Case reports in oncological medicine (2014). 37
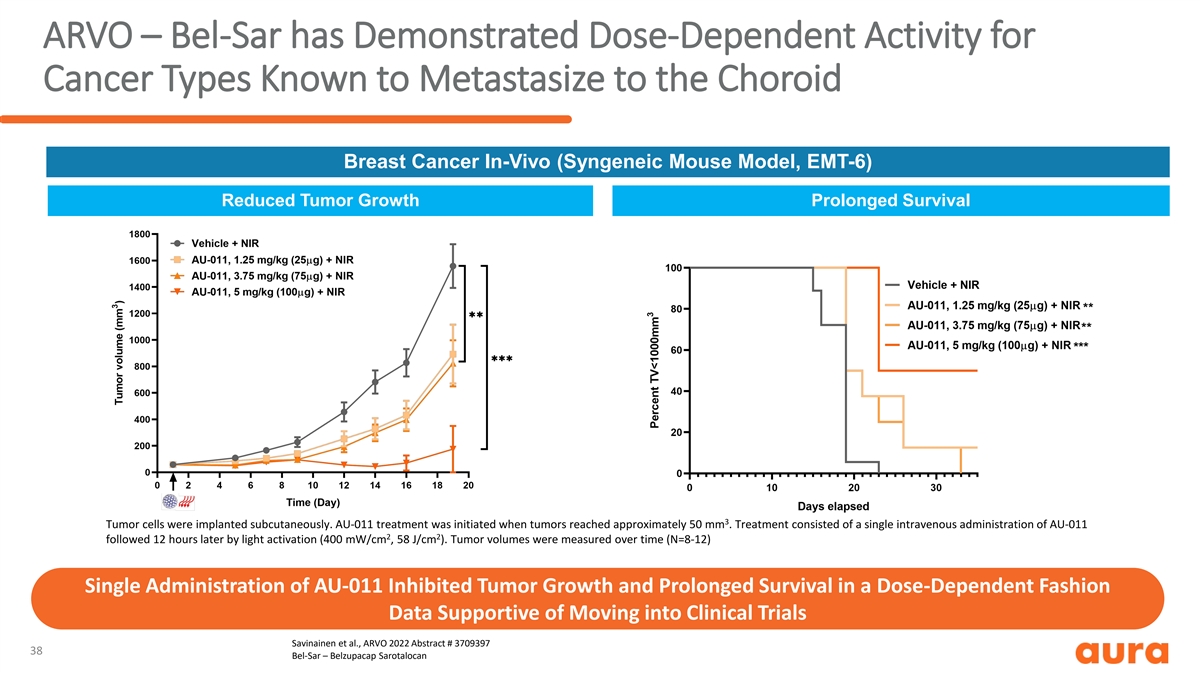
ARVO – Bel-Sar has Demonstrated Dose-Dependent Activity for Cancer Types Known to Metastasize to the Choroid Breast Cancer In-Vivo (Syngeneic Mouse Model, EMT-6) Reduced Tumor Growth Prolonged Survival 3 Tumor cells were implanted subcutaneously. AU-011 treatment was initiated when tumors reached approximately 50 mm . Treatment consisted of a single intravenous administration of AU-011 2 2 followed 12 hours later by light activation (400 mW/cm , 58 J/cm ). Tumor volumes were measured over time (N=8-12) Single Administration of AU-011 Inhibited Tumor Growth and Prolonged Survival in a Dose-Dependent Fashion Data Supportive of Moving into Clinical Trials Savinainen et al., ARVO 2022 Abstract # 3709397 38 Bel-Sar – Belzupacap Sarotalocan
Oncology Franchise AU-011 INN: belzupacap sarotalocan Target Indications: Non-Muscle Invasive Bladder Cancer 39
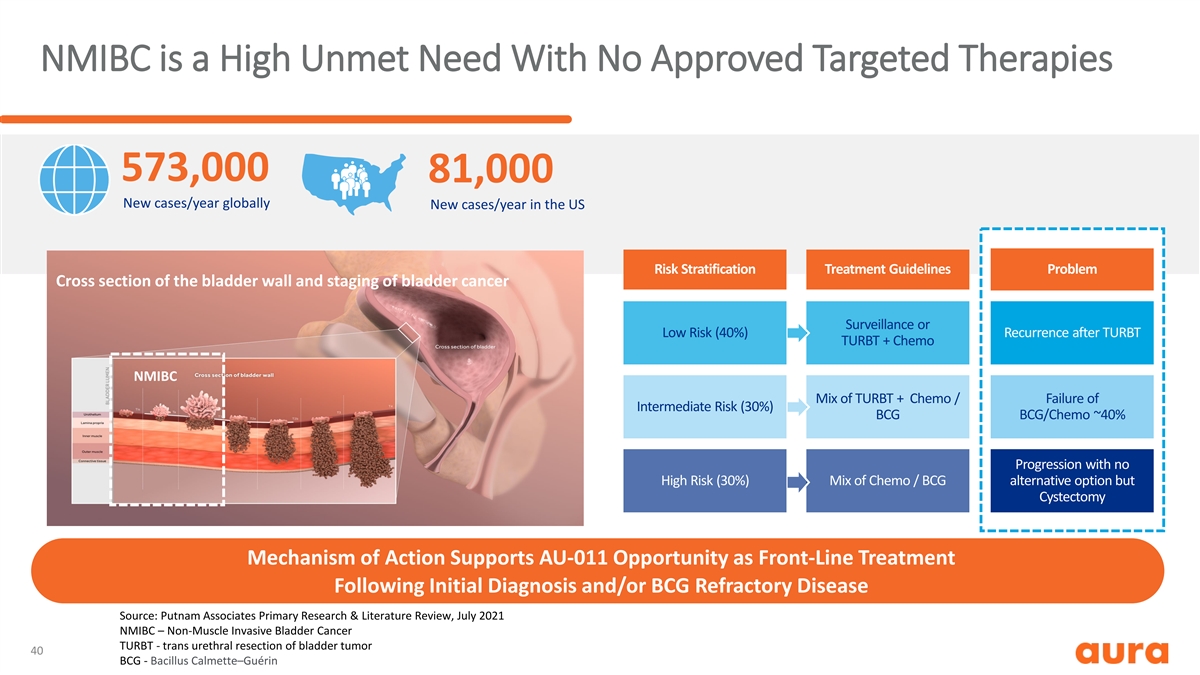
NMIBC is a High Unmet Need With No Approved Targeted Therapies 573,000 81,000 New cases/year globally New cases/year in the US Risk Stratification Treatment Guidelines Problem Cross section of the bladder wall and staging of bladder cancer Surveillance or Low Risk (40%) Recurrence after TURBT TURBT + Chemo NMIBC Mix of TURBT + Chemo / Failure of Intermediate Risk (30%) BCG BCG/Chemo ~40% Progression with no High Risk (30%) Mix of Chemo / BCG alternative option but Cystectomy Mechanism of Action Supports AU-011 Opportunity as Front-Line Treatment Following Initial Diagnosis and/or BCG Refractory Disease Source: Putnam Associates Primary Research & Literature Review, July 2021 NMIBC – Non-Muscle Invasive Bladder Cancer TURBT - trans urethral resection of bladder tumor 40 BCG - Bacillus Calmette–Guérin

Pre-clinical Activity Supports Initiation of Clinical Trials in NMIBC Treatment of Tumor Caused Anti-Tumor Immune Response and Prevented Tumor Growth After Re-Challenge Tumor Growth Survival Survival After Re-challenge 100 100 1600 Control 1400 anti-PD-1 80 80 AU-011 1200 anti-PD-1 + AU-011 1000 60 60 Control (Naive) Control AU-011 anti-PD-1 800 anti-PD-1 + AU-011 AU-011 40 40 600 anti-PD-1 + AU-011 ✱✱ 400 20 20 200 0 0 0 10 20 30 40 0 25 50 75 100 0 25 50 75 100 Day post-treatment Day post-rechallenge Day Post Implantation AU-011 Single Dose Syngeneic Mouse Tumor Bladder Model (MB49 Model in C57BL/6 Mice) (N=8 -10/group) Data Demonstrates Robust Efficacy Supporting Development of Bel-Sar as Single Agent and in Combination with Checkpoint Inhibitors Kines et al; Cancer Immunology Research, May 2021 Bel-Sar – Belzupacap Sarotalocan 41 3 Tumor volume (mm ) (Mean±SEM) Percent survival Percent survival
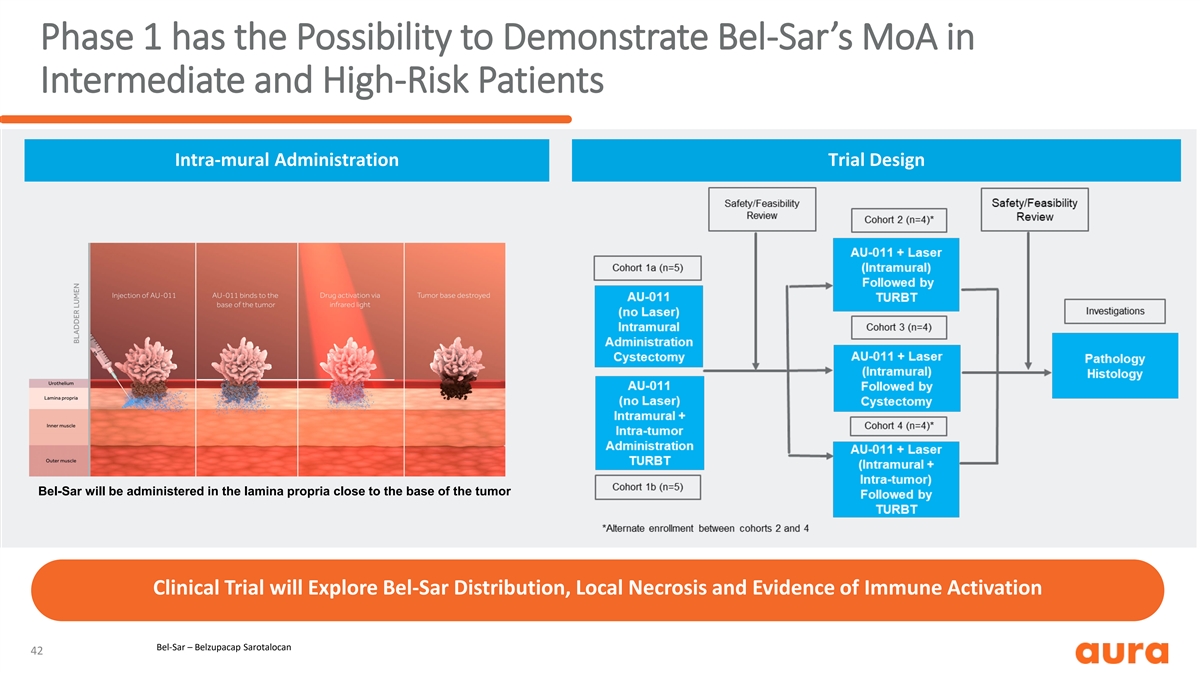
Phase 1 has the Possibility to Demonstrate Bel-Sar’s MoA in Intermediate and High-Risk Patients Intra-mural Administration Trial Design Bel-Sar will be administered in the lamina propria close to the base of the tumor Clinical Trial will Explore Bel-Sar Distribution, Local Necrosis and Evidence of Immune Activation Bel-Sar – Belzupacap Sarotalocan 42

Treatment of Primary and Distant Tumors is Enhanced by Bel-Sar with Immune Checkpoint Inhibitors Primary Tumor Distant Tumors Bel-Sar is Synergistic with Immune Checkpoint Inhibitors and has Improved Primary and Distant Lesion Efficacy when Combined With PD-L1 and LAG-3 Antibodies Huis in‘t Veld, et al., ASCO Abstract #e14544 43 LAG-3 – Lymphocyte Activating Gene-3 Bel-Sar – Belzupacap Sarotalocan
Drug portfolio Strategy & Key Milestones 44

Novel Oncology Platform Using Virus-Like Drug Conjugates (VDCs) - Multi-billion dollar market opportunity Ocular Oncology Franchise - Standard of care is invasive and may lead to blindness and eye loss - Completed Phase 1b/2 trial: Positive data in key clinical endpoints Foundational Value - FDA/EMA/MHRA are in alignment with pivotal trial design - Solid tumor development programs Oncology Pipeline - Platform to develop additional VDCs - Ocular Oncology Franchise ü Retrospective vision data versus radiotherapy ü Phase 2 Choroidal Melanoma safety and efficacy data Clinical & Regulatory - Initiate Pivotal Trial in Choroidal Melanoma Milestones - IND filing in Choroidal Metastasis - Oncology Franchise ü Initiate Phase 1 in Non-Muscle Invasive Bladder Cancer - Strong Cash Position Strong Investor Base 45














































