
A Phase 2 Trial of Belzupacap Sarotalocan (AU-011) A First-in-Class Targeted Therapy for Choroidal Melanoma via Suprachoroidal Administration AAO 2022 October 2, 2022 Ivana K. Kim, MD, MBA On Behalf of the AU-011 Investigator Group AU-011-202, NCT04417530 Co-Director Ocular Melanoma Center Massachusetts Eye and Ear Associate Professor of Ophthalmology Harvard Medical School Exhibit 99.3
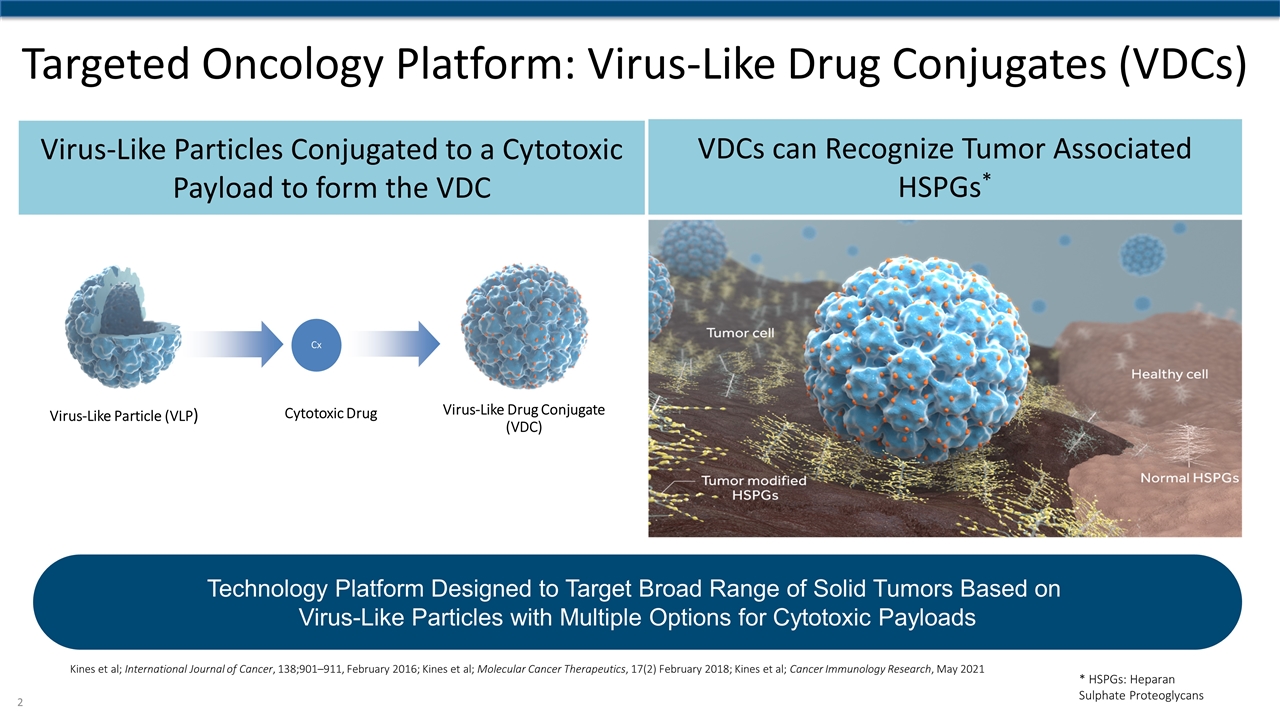
Targeted Oncology Platform: Virus-Like Drug Conjugates (VDCs) Virus-Like Particles Conjugated to a Cytotoxic Payload to form the VDC VDCs can Recognize Tumor Associated HSPGs* Virus-Like Particle (VLP) Virus-Like Drug Conjugate (VDC) Cx Cytotoxic Drug Kines et al; International Journal of Cancer, 138;901–911, February 2016; Kines et al; Molecular Cancer Therapeutics, 17(2) February 2018; Kines et al; Cancer Immunology Research, May 2021 Technology Platform Designed to Target Broad Range of Solid Tumors Based on Virus-Like Particles with Multiple Options for Cytotoxic Payloads * HSPGs: Heparan Sulphate Proteoglycans
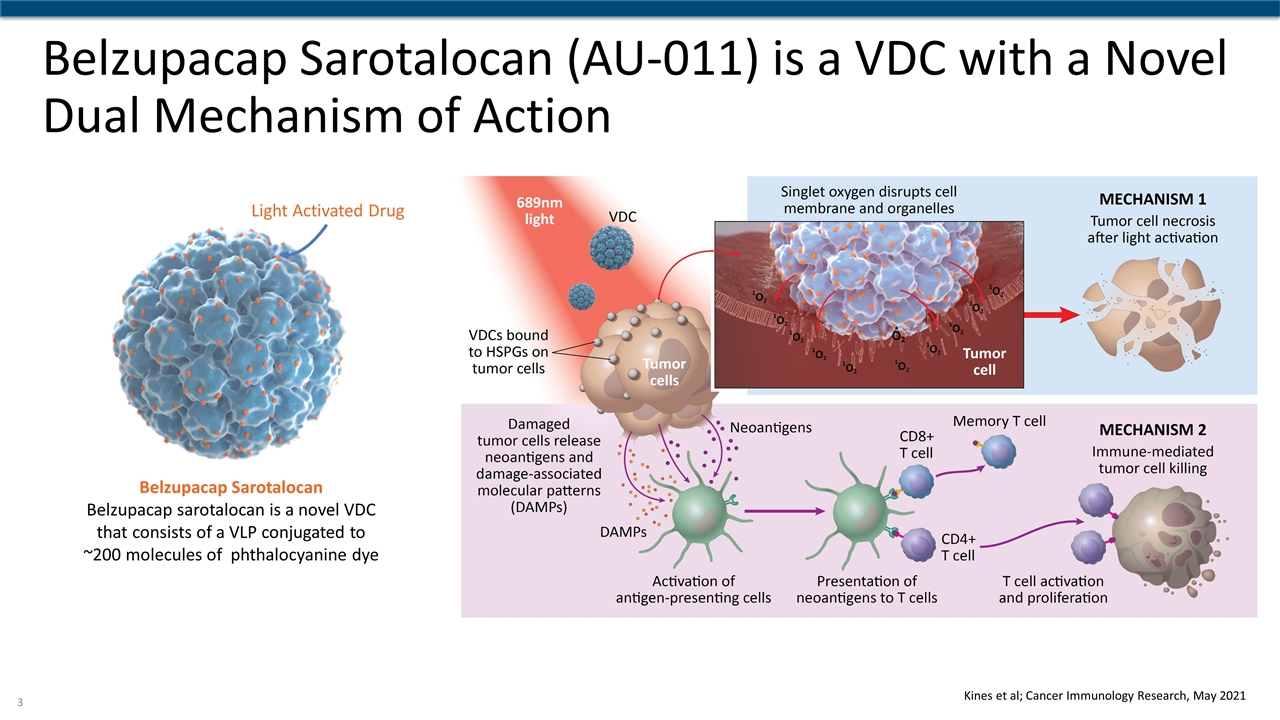
Belzupacap Sarotalocan (AU-011) is a VDC with a Novel Dual Mechanism of Action Light Activated Drug Belzupacap Sarotalocan Belzupacap sarotalocan is a novel VDC that consists of a VLP conjugated to ~200 molecules of phthalocyanine dye Kines et al; Cancer Immunology Research, May 2021
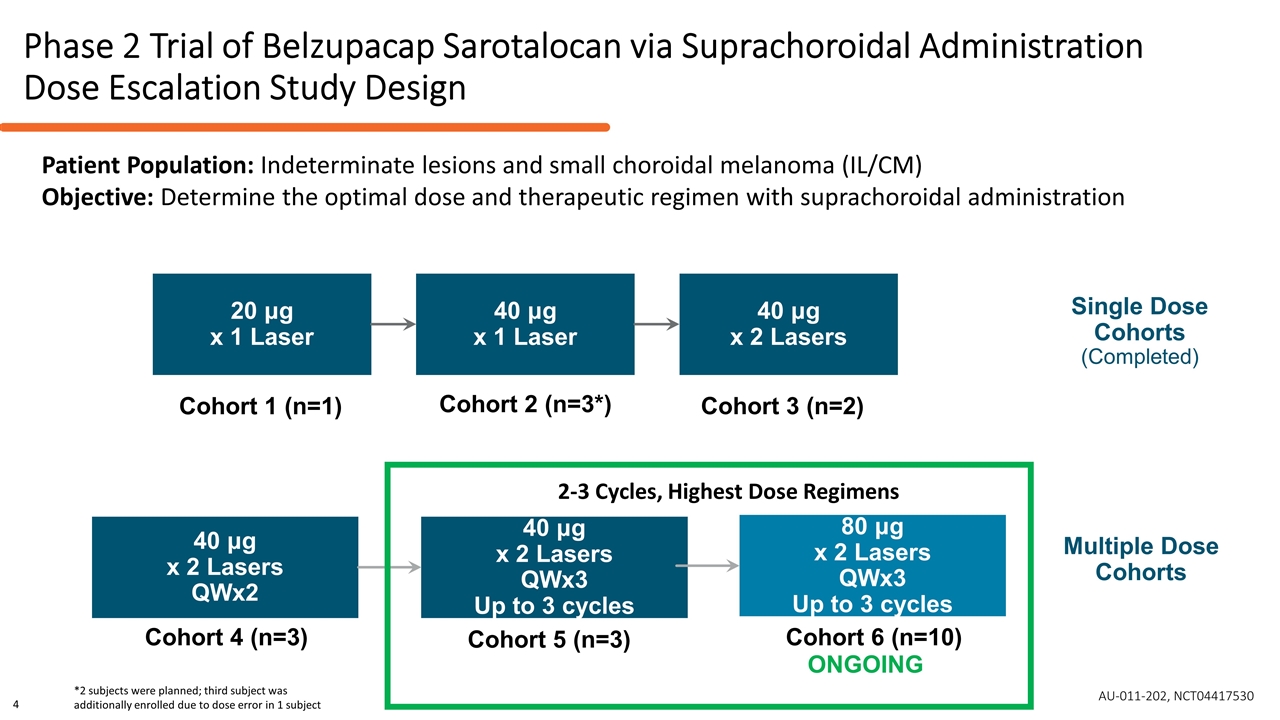
Phase 2 Trial of Belzupacap Sarotalocan via Suprachoroidal Administration Dose Escalation Study Design Single Dose Cohorts (Completed) Multiple Dose Cohorts 20 μg x 1 Laser 40 μg x 1 Laser 40 μg x 2 Lasers 40 μg x 2 Lasers QWx2 40 μg x 2 Lasers QWx3 Up to 3 cycles Cohort 1 (n=1) Cohort 2 (n=3*) Cohort 3 (n=2) Cohort 4 (n=3) Cohort 5 (n=3) 80 μg x 2 Lasers QWx3 Up to 3 cycles Cohort 6 (n=10) ONGOING *2 subjects were planned; third subject was additionally enrolled due to dose error in 1 subject 2-3 Cycles, Highest Dose Regimens Patient Population: Indeterminate lesions and small choroidal melanoma (IL/CM) Objective: Determine the optimal dose and therapeutic regimen with suprachoroidal administration AU-011-202, NCT04417530 4
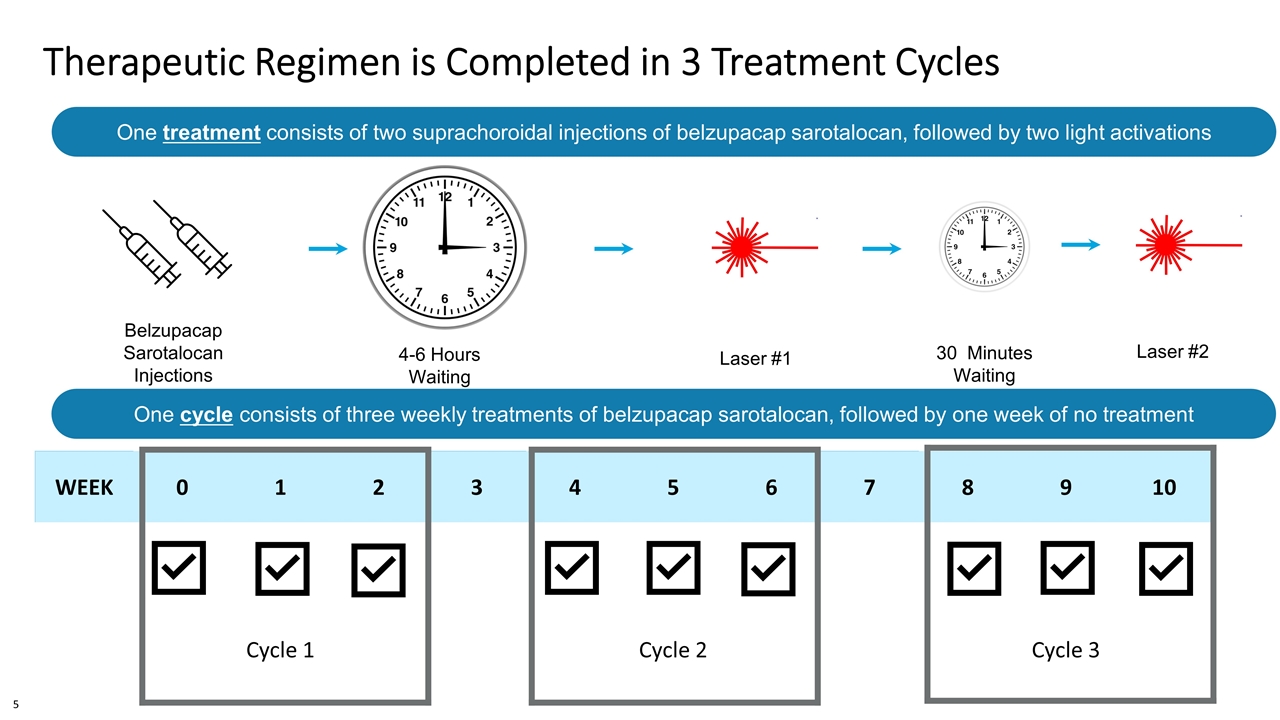
Therapeutic Regimen is Completed in 3 Treatment Cycles WEEK 0 1 2 3 4 5 6 7 8 9 10 Cycle 1 Cycle 2 Cycle 3 4-6 Hours Waiting Period Belzupacap Sarotalocan Injections Laser #1 30 Minutes Waiting Period Laser #2 One treatment consists of two suprachoroidal injections of belzupacap sarotalocan, followed by two light activations 4-4.5mm from the limbus, quadrant of tumor or adjacent quadrant One cycle consists of three weekly treatments of belzupacap sarotalocan, followed by one week of no treatment 5
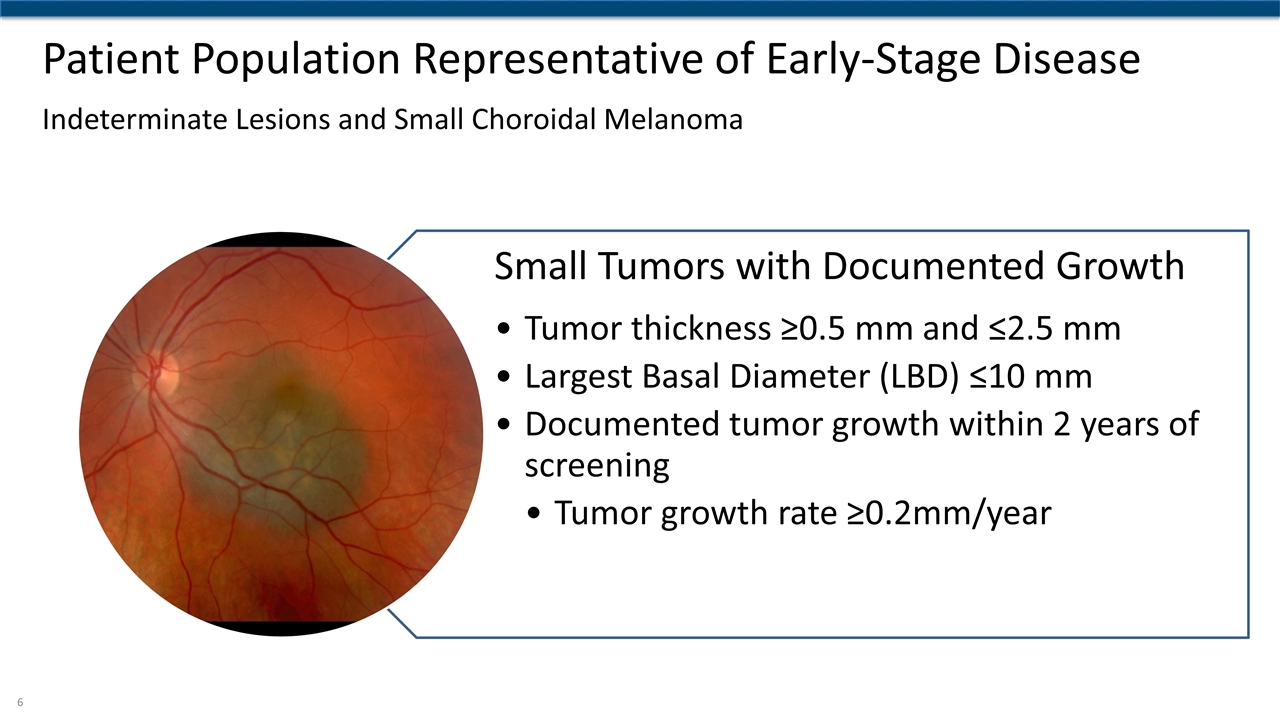
Patient Population Representative of Early-Stage Disease Indeterminate Lesions and Small Choroidal Melanoma Small Tumors with Documented Growth Tumor thickness ≥0.5 mm and ≤2.5 mm Largest Basal Diameter (LBD) ≤10 mm Documented tumor growth within 2 years of screening Tumor growth rate ≥0.2mm/year
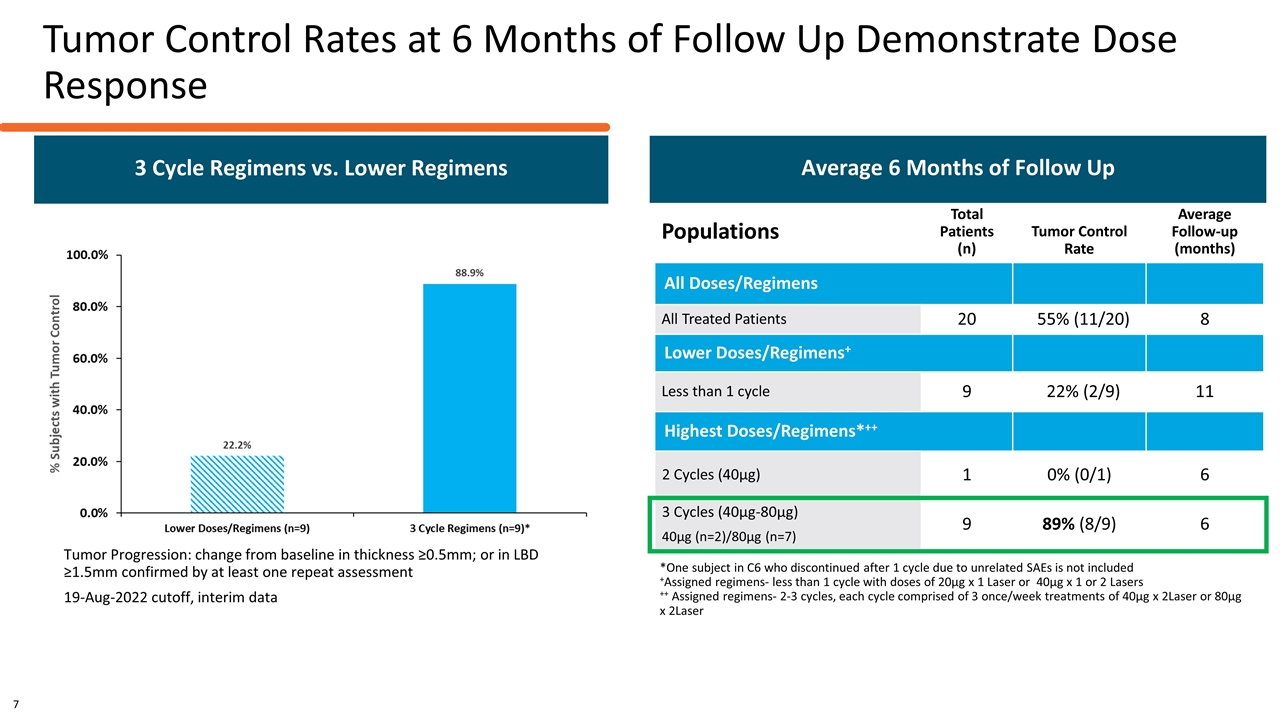
Tumor Control Rates at 6 Months of Follow Up Demonstrate Dose Response Tumor Progression: change from baseline in thickness ≥0.5mm; or in LBD ≥1.5mm confirmed by at least one repeat assessment 19-Aug-2022 cutoff, interim data Populations Total Patients (n) Tumor Control Rate Average Follow-up (months) All Doses/Regimens All Treated Patients 20 55% (11/20) 8 Lower Doses/Regimens+ Less than 1 cycle 9 22% (2/9) 11 Highest Doses/Regimens*++ 2 Cycles (40µg) 1 0% (0/1) 6 3 Cycles (40µg-80µg) 40µg (n=2)/80µg (n=7) 9 89% (8/9) 6 Average 6 Months of Follow Up *One subject in C6 who discontinued after 1 cycle due to unrelated SAEs is not included +Assigned regimens- less than 1 cycle with doses of 20µg x 1 Laser or 40µg x 1 or 2 Lasers ++ Assigned regimens- 2-3 cycles, each cycle comprised of 3 once/week treatments of 40µg x 2Laser or 80µg x 2Laser 3 Cycle Regimens vs. Lower Regimens 7
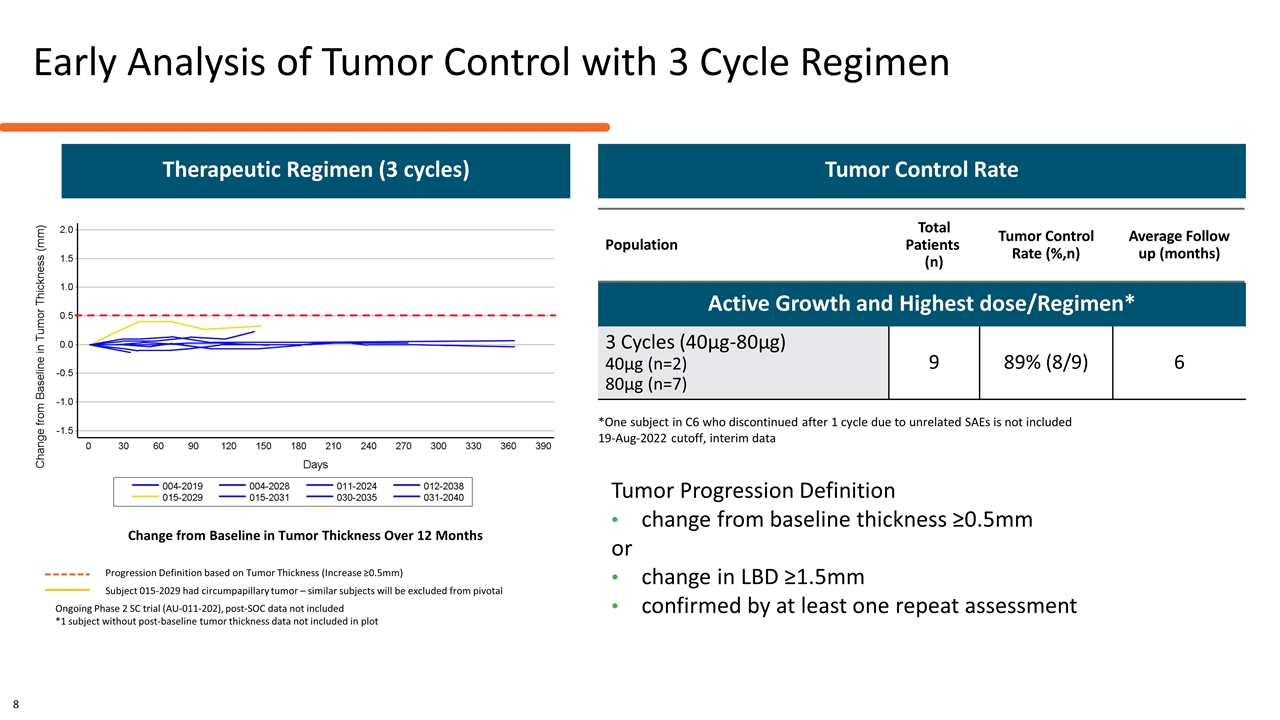
Early Analysis of Tumor Control with 3 Cycle Regimen Change from Baseline in Tumor Thickness Over 12 Months Ongoing Phase 2 SC trial (AU-011-202), post-SOC data not included *1 subject without post-baseline tumor thickness data not included in plot Population Total Patients (n) Tumor Control Rate (%,n) Average Follow up (months) Active Growth and Highest dose/Regimen* 3 Cycles (40µg-80µg) 40µg (n=2) 80µg (n=7) 9 89% (8/9) 6 *One subject in C6 who discontinued after 1 cycle due to unrelated SAEs is not included 19-Aug-2022 cutoff, interim data Tumor Control Rate Progression Definition based on Tumor Thickness (Increase ≥0.5mm) Subject 015-2029 had circumpapillary tumor – similar subjects will be excluded from pivotal Therapeutic Regimen (3 cycles) 8 Tumor Progression Definition change from baseline thickness ≥0.5mm or change in LBD ≥1.5mm confirmed by at least one repeat assessment
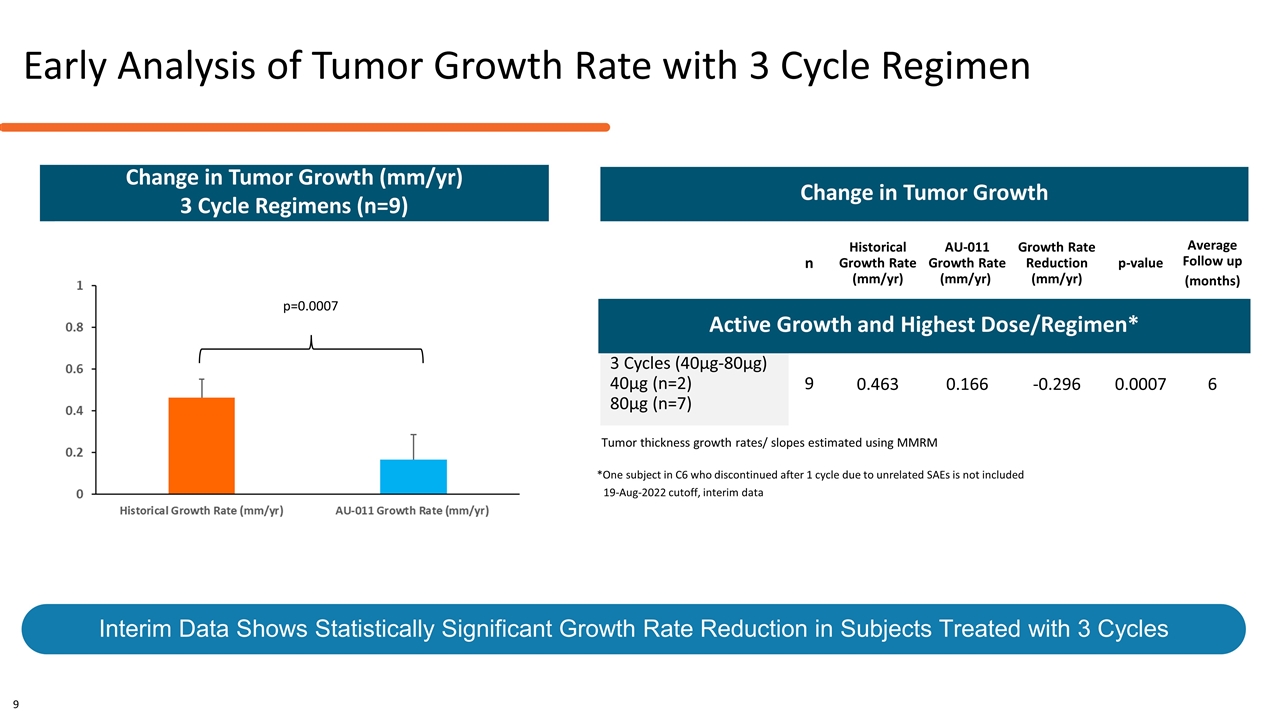
n Historical Growth Rate (mm/yr) AU-011 Growth Rate (mm/yr) Growth Rate Reduction (mm/yr) p-value Average Follow up (months) Active Growth and Highest Dose/Regimens* 3 Cycles (40µg-80µg) 40µg (n=2) 80µg (n=7) 9 0.463 0.166 -0.296 0.0007 6 Tumor thickness growth rates/ slopes estimated using MMRM Reduction in Tumor Growth Rate is Statistically Significant and Supports Planned Pivotal Key Endpoint Change in Tumor Growth Change in Tumor Growth (mm/yr) 3 Cycle Regimens (n=9) *One subject in C6 who discontinued after 1 cycle due to unrelated SAEs is not included 19-Aug-2022 cutoff, interim data Early Analysis of Tumor Growth Rate with 3 Cycle Regimen Interim Data Shows Statistically Significant Growth Rate Reduction in Subjects Treated with 3 Cycles 9 p=0.0007 Active Growth and Highest Dose/Regimen*
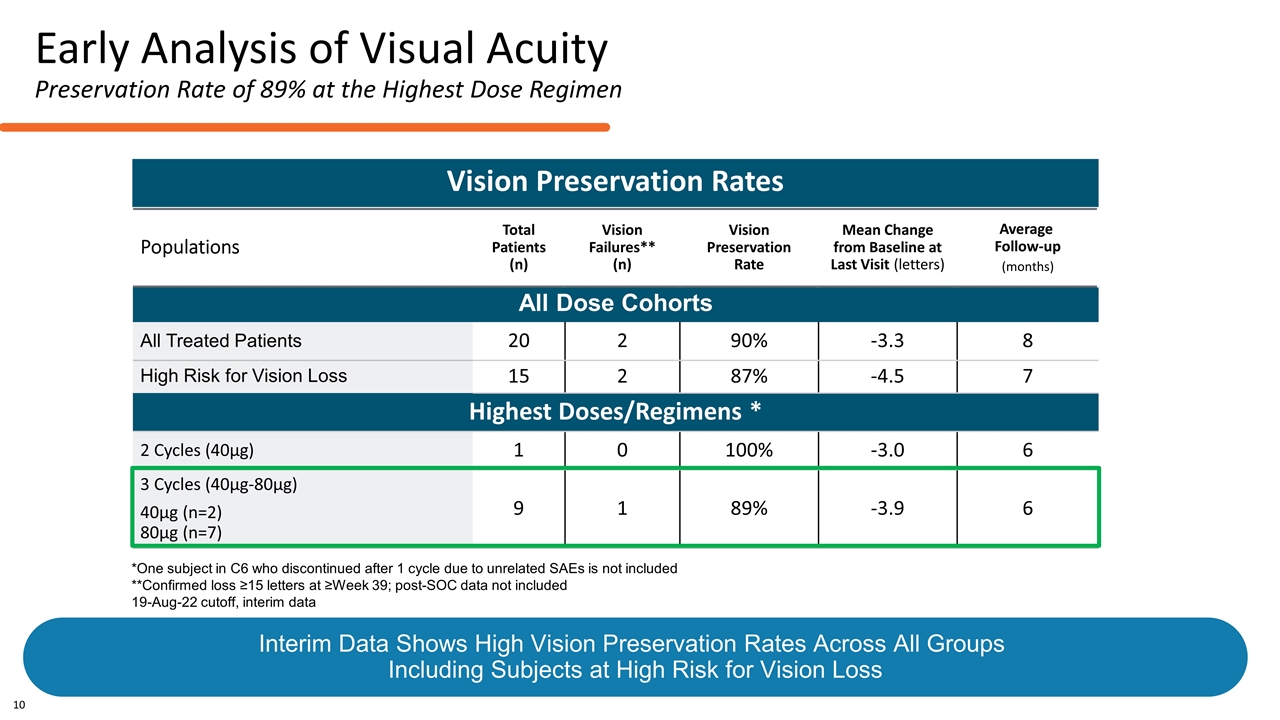
Populations Total Patients (n) Vision Failures** (n) Vision Preservation Rate Mean Change from Baseline at Last Visit (letters) Average Follow-up (months) All Dose Cohorts All Treated Patients 20 2 90% -3.3 8 High Risk for Vision Loss 15 2 87% -4.5 7 Highest Doses/Regimens * 2 Cycles (40µg) 1 0 100% -3.0 6 3 Cycles (40µg-80µg) 40µg (n=2) 80µg (n=7) 9 1 89% -3.9 6 Vision Preservation Rates *One subject in C6 who discontinued after 1 cycle due to unrelated SAEs is not included **Confirmed loss ≥15 letters at ≥Week 39; post-SOC data not included 19-Aug-22 cutoff, interim data Early Analysis of Visual Acuity Preservation Rate of 89% at the Highest Dose Regimen 10 Interim Data Shows High Vision Preservation Rates Across All Groups Including Subjects at High Risk for Vision Loss
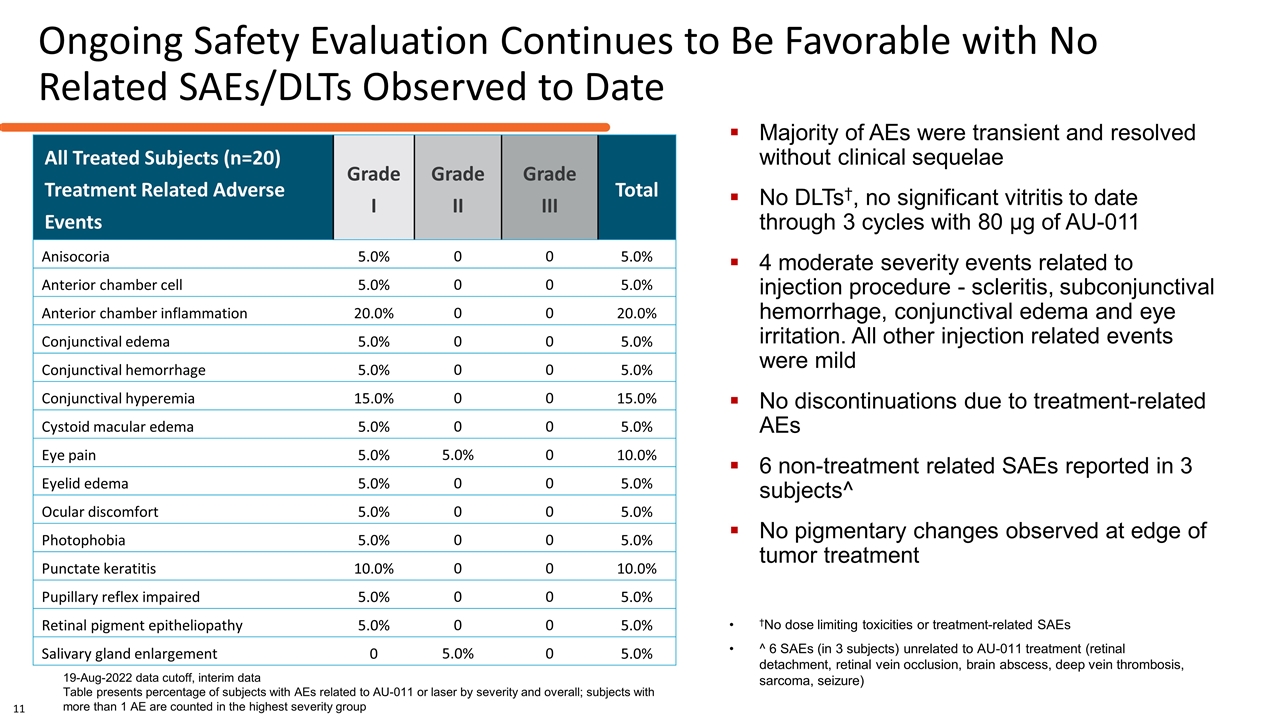
Ongoing Safety Evaluation Continues to Be Favorable with No Related SAEs/DLTs Observed to Date 19-Aug-2022 data cutoff, interim data Table presents percentage of subjects with AEs related to AU-011 or laser by severity and overall; subjects with more than 1 AE are counted in the highest severity group †No dose limiting toxicities or treatment-related SAEs ^ 6 SAEs (in 3 subjects) unrelated to AU-011 treatment (retinal detachment, retinal vein occlusion, brain abscess, deep vein thrombosis, sarcoma, seizure) Majority of AEs were transient and resolved without clinical sequelae No DLTs†, no significant vitritis to date through 3 cycles with 80 µg of AU-011 4 moderate severity events related to injection procedure - scleritis, subconjunctival hemorrhage, conjunctival edema and eye irritation. All other injection related events were mild No discontinuations due to treatment-related AEs 6 non-treatment related SAEs reported in 3 subjects^ No pigmentary changes observed at edge of tumor treatment All Treated Subjects (n=20) Treatment Related Adverse Events Grade I Grade II Grade III Total Anisocoria 5.0% 0 0 5.0% Anterior chamber cell 5.0% 0 0 5.0% Anterior chamber inflammation 20.0% 0 0 20.0% Conjunctival edema 5.0% 0 0 5.0% Conjunctival hemorrhage 5.0% 0 0 5.0% Conjunctival hyperemia 15.0% 0 0 15.0% Cystoid macular edema 5.0% 0 0 5.0% Eye pain 5.0% 5.0% 0 10.0% Eyelid edema 5.0% 0 0 5.0% Ocular discomfort 5.0% 0 0 5.0% Photophobia 5.0% 0 0 5.0% Punctate keratitis 10.0% 0 0 10.0% Pupillary reflex impaired 5.0% 0 0 5.0% Retinal pigment epitheliopathy 5.0% 0 0 5.0% Salivary gland enlargement 0 5.0% 0 5.0% 11
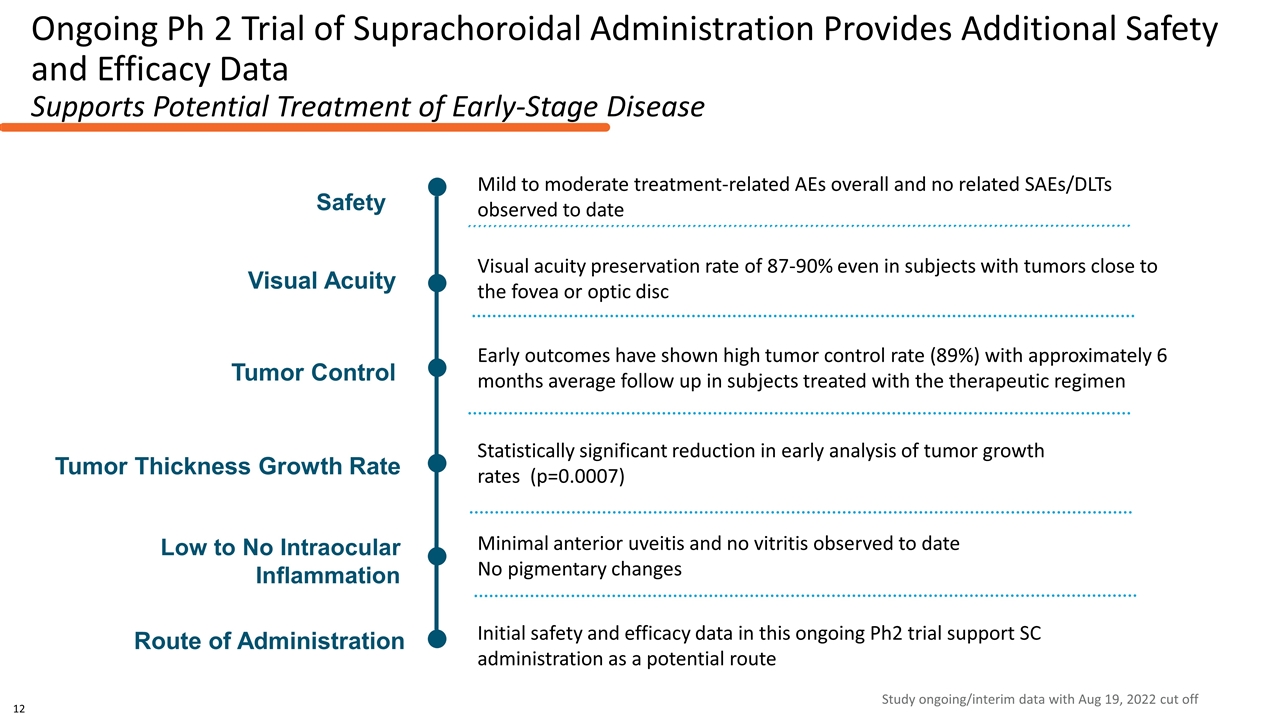
Ongoing Ph 2 Trial of Suprachoroidal Administration Provides Additional Safety and Efficacy Data Supports Potential Treatment of Early-Stage Disease Statistically significant reduction in early analysis of tumor growth rates (p=0.0007) Early outcomes have shown high tumor control rate (89%) with approximately 6 months average follow up in subjects treated with the therapeutic regimen Initial safety and efficacy data in this ongoing Ph2 trial support SC administration as a potential route Visual acuity preservation rate of 87-90% even in subjects with tumors close to the fovea or optic disc Route of Administration Tumor Thickness Growth Rate Visual Acuity Tumor Control Safety Mild to moderate treatment-related AEs overall and no related SAEs/DLTs observed to date Low to No Intraocular Inflammation Minimal anterior uveitis and no vitritis observed to date No pigmentary changes Study ongoing/interim data with Aug 19, 2022 cut off 12

Clinical Investigators & Participating Sites Belzupacap Sarotalocan Ocular Oncology Investigator Group Dr. Carol Shields Philadelphia, PA Dr. Ivana Kim Boston, MA Dr. Tara McCannel Los Angeles, CA Dr. Abdhish Bhavsar Minneapolis, MN Dr. Antonio Capone Jr. Royal Oak, MI Dr. Amy Schefler Houston, TX Dr. Brian Marr New York, NY Dr. Hakan Demirci Ann Arbor, MI Dr. Prithvi Mruthyunjaya Palo Alto, CA Dr. Cameron Javid Tucson, AZ Dr. James Howard Salt Lake City, UT Dr. Chris Bergstrom Greenville, SC Dr. Michael Seider San Francisco, CA Dr. Tony Tsai Sacramento, CA Dr. Timothy Fuller Dallas, TX Dr. David Reichstein Nashville, TN Peter Hovland Denver, CO












