Exhibit 99.1

Citius Pharmaceuticals, Inc. Corporate Presentation Winter 2020 - 21 NASDAQ: CTXR

2 NASDAQ: CTXR This presentation has been prepared by Citius Pharmaceuticals, Inc. (the “Company”) for informational purposes only and not for any other purpose. Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the Company or any director, employee, agent, or adviser of the Company. This presentation does not purport to be all - inclusive or to contain all of the information you may desire. The information contained in this presentation and the comments and remarks of the representatives of the Company made during any presentation to which this presentation relates are integrally related and, as such, are intended to be delivered and understood together. Information provided in this presentation speaks only as of the date hereof. The Company assumes no obligation to update any statement after the date of this presentation as a result of new information, subsequent events or any other circumstances. This presentation also includes express and implied forward - looking statements regarding the current expectations, estimates, opinions and beliefs of the Company that are not historical facts. Such forward - looking statements may be identified by words such as “believes”, “expects”, “endeavors”, “anticipates”, “intends”, “plans”, “estimates”, “projects”, “should”, “objective ” a nd variations of such words and similar words. The accuracy of such statements is dependent upon future events, and involves known and unknown risks, uncertainties and other factors beyond the Company’s control that may cause actual results to diffe r materially from what is presented herein. Investors are strongly encouraged to carefully review the Company’s SEC filings f or a listing of the risks that could cause actual results to differ from these forward looking statements. These forward - looking statements speak only as of the date of this presentation and should not be construed as statements of facts. Disclaimer

3 NASDAQ: CTXR Olympic Motto: “ Citius , Altius, Fortius ” ( Faster , Higher, Stronger) Company Overview Four Active Programs with a Late Stage Lead Asset Mino - Lok in late Phase 3; favorable review received for Futility Analysis by Data Monitoring Committee (12/2019); favorable review for safety and efficacy analysis in September (2020). Large Market Needs CRBSI market estimated to be >$1.8 B worldwide; ARDS market large with no approved therapies; TE infection prevention estimated at >$0.4 B worldwide; Rx hemorrhoid market estimated at >$2.0 B in US. Expert Team to Execute Management has history of >$1B in pharma M&A; Scientific Advisors are key KOL’s in infectious disease, breast surgery, pulmonology (ARDS) Management Commitment Management / Founders have invested $26.5 million into the company Have persevered with clinical research in the Covid - 19 pandemic 3

4 NASDAQ: CTXR Management Leonard Mazur, Director and Executive Chairman • Founder/co - founder of: Genesis, Triax , Akrimax , and others Myron Holubiak, Director, CEO and President • Former President of Roche Laboratories, Inc. Jaime Bartushak, EVP, Chief Financial Officer • Over 20 years experience in corporate finance, M&A, and strategic planning Myron Czuczman, M.D., EVP, Chief Medical Officer • Recent Therapeutic Area Head and Vice President of the Clinical Research and Development Global Lymphoma/CLL Program at Celgene • Former Chief, Lymphoma/Myeloma service at Roswell Park Comprehensive Cancer Center in Buffalo • Has published more than 180 peer - reviewed journal articles Gary Talarico, EVP, Operations • Has led commercial activities for many corporate expansions and start - ups, including Reliant Pharma and Ventiv Health Alan Lader, Ph.D , VP, Clinical Operations • Over 25 years of experience in medical research, former Instructor in Medicine at Harvard Medical School and Brigham and Women’s Hospital Andrew Scott, VP, Business Development • 20 years of transactional experience in strategic planning, product identification, asset acquisition, and capital markets communication

5 NASDAQ: CTXR Key Scientific/Medical Advisors (ID and CA) Isaam Raad , M.D • Chair of MD Anderson Cancer Center’s Dept. of Infectious Diseases • Author of the underlying patents for Mino - Lok® • Dr. Raad’s innovations have been endorsed at the highest level (Category 1A) by the Center for Disease Control (CDC) Mark Rupp, M.D. • Professor and Chief of the Division of Infectious Diseases in the Dept. of Internal Medicine at the U. of Nebraska Medical Ce nte r • Past - President of SHEA and Past - President of ASM Division L • Has served as consultant to FDA, CDC, NIH, and VA Leonard A. Mermel, D.O. • Technical Expert Panel Member of Medicare Patient Safety Monitoring System, US Dept. of Health & Human Services • Has co - authored US guidelines dealing with prevention and management of intravascular catheter infections Jesse Selber , M.D. • Director of Clinical Research and an Associate Professor in the Department of Plastic Surgery at the University of Texas MD Anderson Cancer Center • Dr. Selber is an microvascular reconstructive surgeon, whose research involves head and neck reconstruction, breast reconstruction, microsurgery, abdominal wall reconstruction, microsurgical training, robotic - assisted reconstructive surgery, an d other areas George Viola, M.D. • Associate Professor of Department of Infectious Diseases, Infection Control and Employee Health, and Division of Internal Medicine at The University of Texas MD Anderson Cancer Center • Associate Professor, Department of Infectious Diseases, Division of Internal Medicine, Baylor College of Medicine

6 NASDAQ: CTXR Key Cellular Therapy Advisors Michael A. Matthay, MD • Senior Associate at the Cardiovascular Research Institute, and Associate Director of the Critical Care Medicine & Professor o f M edicine and Anesthesia at the University of California at San Francisco (UCSF) • Research focused on the pathogenesis and resolution of the acute respiratory distress syndrome (ARDS) focused on the biology and potential clinical use of allogeneic bone marrow derived mesenchymal stromal cells (MSCs) for ARDS • Lead investigator “Mesenchymal Stromal Cells for Acute Respiratory Distress Syndrome (START),” a United States Department of Def ense supported study of MSCs for ARDS Mitchell M. Levy, MD • Chief of the Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, and Professor of Medicine at B row n University • Medical Director of the Medical ICU at Rhode Island Hospital. He has been an investigator on numerous pharmacologic and biolo gic trials intended to treat sepsis, cardiovascular and pulmonary pathology Lorraine B. Ware, MD • Professor of Medicine, Pathology, Microbiology, and Immunology at Vanderbilt University & Director of Vanderbilt’s Medical Sc hol ars Program • Research centers on the pathogenesis and treatment of sepsis and acute lung injury with a current focus on mechanisms of lung ep ithelial and endothelial oxidative injury by cell - free hemoglobin. • Dr. Ware is also a lead investigator for the “Mesenchymal Stromal Cells for Acute Respiratory Distress Syndrome (START)” stud y Perenlei Enkhbaatar MD., PhD., FAHA • Charles Robert Allen Professor of Anesthesiology & Director of Translational Intensive Care Unit, Department of Anesthesiolog y a t University of Texas Medical Branch (UTMB), Galveston, TX • Research focus on investigate pathophysiologic mechanisms of multi - organ failures with especial emphasis tissue regeneration, st em cell biology, and modified mRNA • Principal or co - investigator on three NIH RO1 grants and one industry sponsored grant (Citius MSCs). Two of the RO1 grants are in ARDS

7 NASDAQ: CTXR Program Estimated Market (Worldwide) Preclinical Phase I Phase II Phase III Mino - Lok® Treat CVC Infections > $1.5B CITI - 002 (Halo - Lido) Rx Therapy for Hemorrhoids > $2B CITI - 101 (Mino - Wrap) Prevent Infections Associated with Breast Implants > $400M CITI - 401 ( i - MSC) Treat ARDS Multi - billion Unique Pipeline in Progressive Stages (current as of 1/2021*) Next milestone: IND (exp Q2 ‘21); Ph 2B Initiated (exp Q2 ’21) IND est. Q4 ’21 Interim Safety/Efficacy Analysis (Sep); Last Pat. Visit est. Q2 ’21 IND est. Q2 ’22 * Best estimate, subject to impact of Covid pandemic on clinical trial conduct. 7

LEAD PRODUCT Minocycline/EDTA/Ethanol Antibiotic Lock Therapy for Salvaging Catheters That Cause Bloodstream Infections Mino - Lok®

9 Central Venous Catheters Central Venous Catheters (CVCs), Peripherally Inserted Central Catheter (PICCs), and Hemodialysis Central Venous Catheter PICC Hemodialysis CTXR
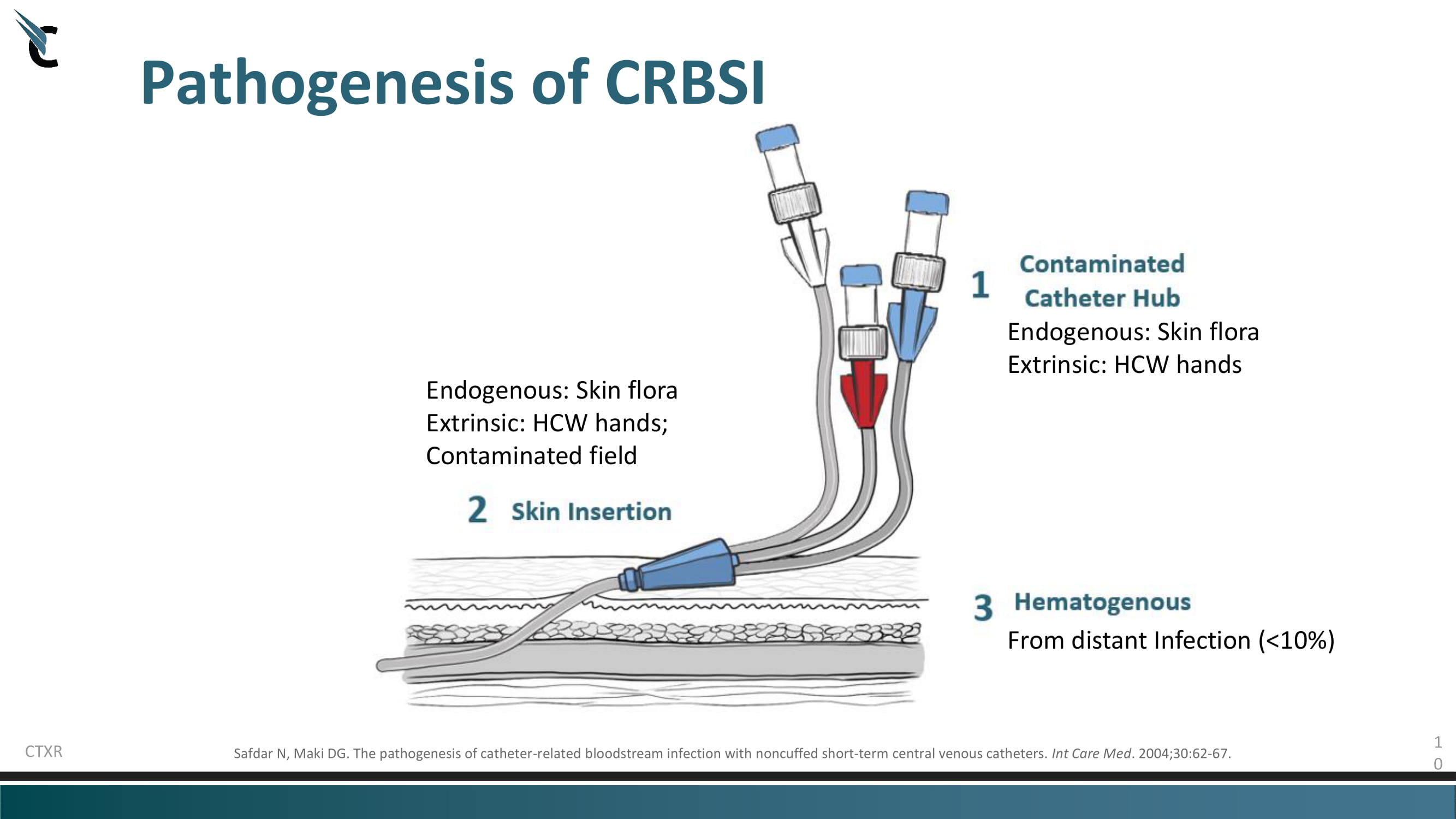
1 0 Pathogenesis of CRBSI Safdar N, Maki DG. The pathogenesis of catheter - related bloodstream infection with noncuffed short - term central venous catheters. Int Care Med . 2004;30:62 - 67. CTXR Endogenous: Skin flora Extrinsic: HCW hands; Contaminated field Endogenous: Skin flora Extrinsic: HCW hands From distant Infection (<10%)
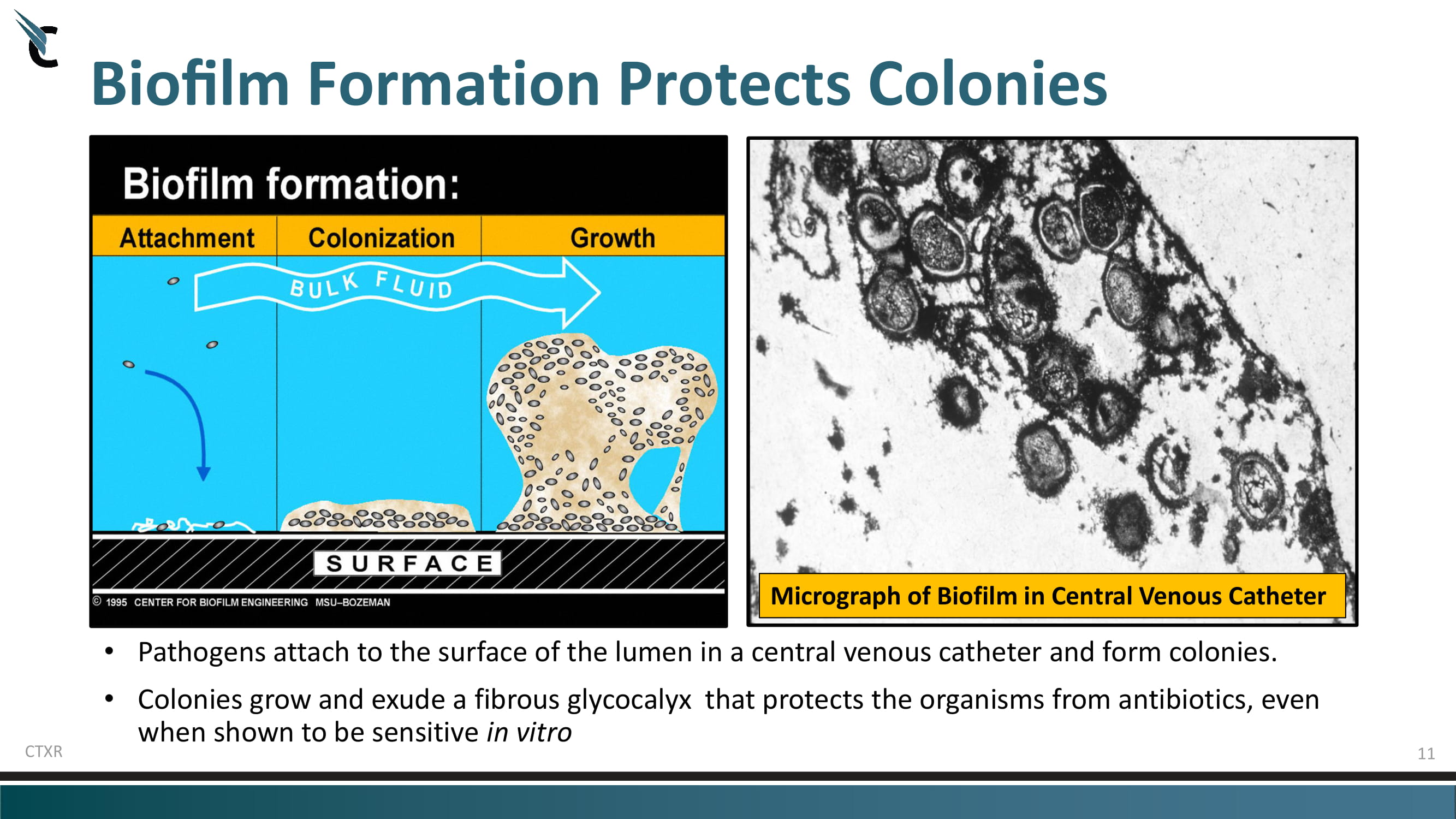
11 Biofilm Formation Protects Colonies • Pathogens attach to the surface of the lumen in a central venous catheter and form colonies. • Colonies grow and exude a fibrous glycocalyx that protects the organisms from antibiotics, even when shown to be sensitive in vitro CTXR Micrograph of Biofilm in Central Venous Catheter

12 NASDAQ: CTXR BUT Infection Rates + Poor SOC Leads to Death & Morbidity Infections are Common & Dangerous Of the 7,000,000 CVCs used annually in US, up to 472,000 become infected leading to serious, life threatening infections called CRBSI/CLABSI. 1 These infections are associated with 12 - 25% mortality and morbidity. 2 Hospitals are penalized for reporting high infection rates, not to mention, incur an attributable cost of $46,000 to $65,000 per episode SOC is a Poor Option for Patients & Hospitals Current SOC is to remove and replace (R&R) the CVC, while treating with systemic antibiotics Catheter R&R causes physical and psychological symptoms in 57% to 67% of patients. 3 R&R is difficult for many patients, due to unavailability of other accessible vascular sites and the need to maintain infusion therapy Cost for just the R&R procedure is ~$10,000 Mino - Lok is the first – and only – therapy under investigation that can be used to sterilize and salvage the infected CVC avoiding the complications, discomfort and costs of removal and replacement. Sources: 1. Shah H., Bosch W., Hellinger W. C., Thompson K. M. (2013). Intravascular catheter - related bloodstream infection. Neurohospitalist 3, 144 – 151. doi : 10.1177/1941874413476043. 2. Antoňáková Němčíková A, Bednárovská E. Catheter - related bloodstream infections: do we know all of it? Klin Onkol . 2017;30(6):405 – 411. doi : 10.14735/amko2017405. 3. Chaftari , AM et al,. Unnecessary Removal of CVCs in Cancer Patients with CRBSI: Impact on Symptom Burden. Poster presentation at ID W eek 2017, Infectious Diseases Society of America (IDSA)Oct 04 - 08, 2017 THE PROBLEM: CVCs are a Lifeline for Cancer Patients

13 NASDAQ: CTXR R&R procedures are invasive and discomforting to patient R&R Procedures are costly and usually require additional hospital stay. Complications include infection, thrombosis, occlusion, and mechanical complications. • Infectious complications are reported to occur in 5% to 26% of patients; • Mechanical complications in 5% to 19%; and, • Thrombotic complications in 2% to 26%. 1,2 Mechanical complications associated with the insertion of CVCs include arterial puncture, hematoma, hemothorax, pneumothorax, arterial - venous fistula, venous air embolism, nerve injury, thoracic duct injury (left side only), intraluminal dissection, and puncture of the aorta. 3 Catheter removal and reinsertion causes physical and psychological symptoms in 57% to 67% of patients, respectively. 4 Sources (NCBI: Annals of Translational Medicine): 1. McGee DC, Gould MK.. Preventing complications of central venous catheterization. N Engl J Med 2003;348:1123 - 33. 2. Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled tr ial. JAMA 2001;286:700 - 7. 3. Polderman KH, Girbes AJ.. Central venous catheter use. Part 1: mechanical complications. Intensive Care Med 2002;28:1 - 17. 4. Chaftari , AM et al,. Unnecessary Removal of CVCs in Cancer Patients with CRBSI: Impact on Symptom Burden. Poster presentation at ID W eek 2017, Infectious Diseases Society of America (IDSA)Oct 04 - 08, 2017 CVC Remove and Replace (R&R) Complications

14 NASDAQ: CTXR Locking a Catheter is a Standard Operating Procedure 1. Using Mino - Lok does not require any novel methodologies. 2. Any RN or LPN or Technician can perform the procedure. 3. There is no change in normal workflow and does not require exceptional training. 4. The patient does not experience any sensations similar to the threading of a central line through a vein or artery. 5. The procedure does not require any change to the tunneling or change in placement of the central line. 6. No anesthesia (general or local) is needed. 7. Standard sterile techniques still apply. *Mino - Lok Ρ is not flushed into the venous system. Locking a Central Venous Line with Mino - Lok®
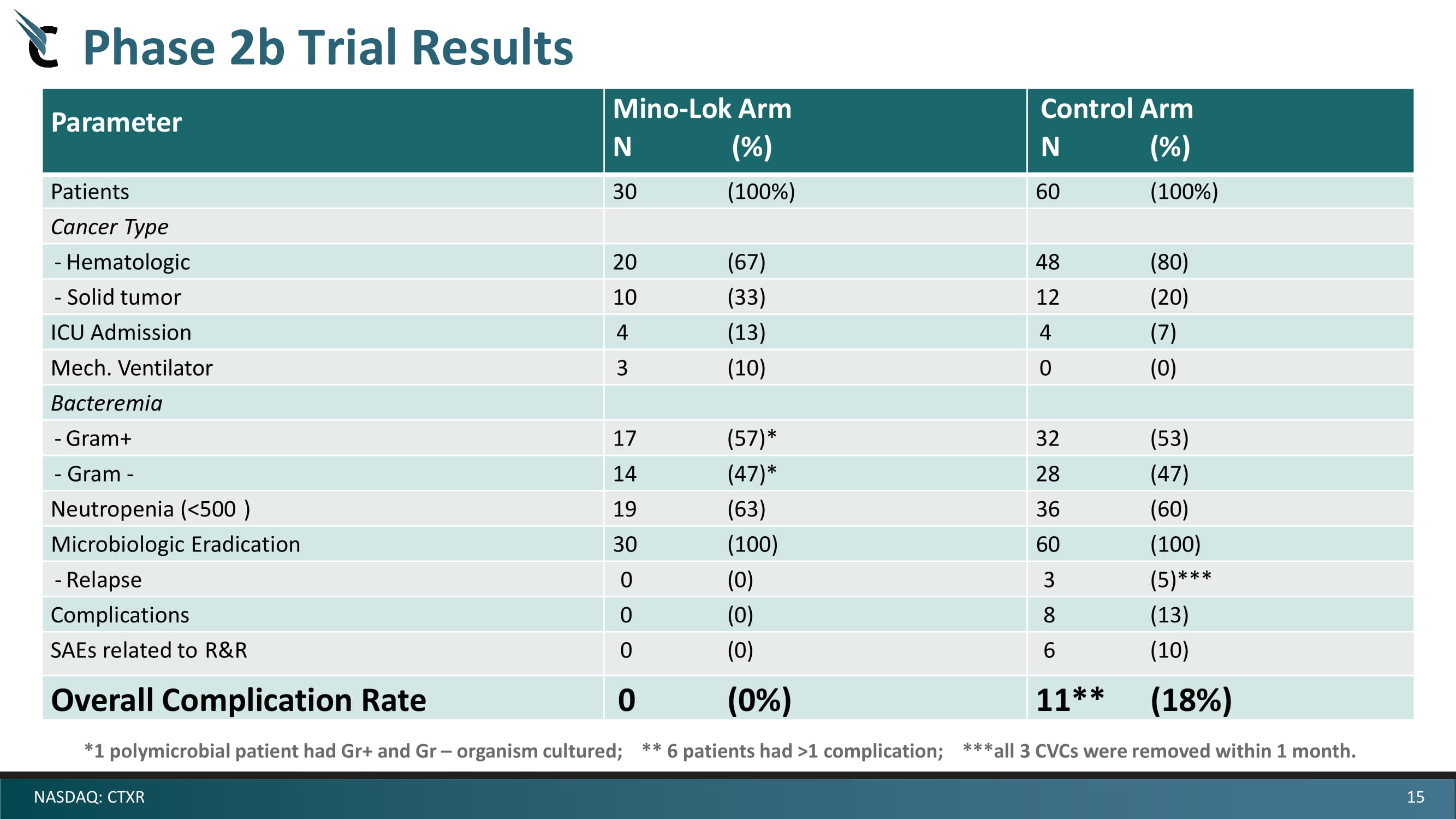
15 NASDAQ: CTXR Phase 2b Trial Results Parameter Mino - Lok Arm N ( % ) Control Arm N ( % ) Patients 30 (100%) 60 (100%) Cancer Type - Hematologic 20 (67) 48 (80) - Solid tumor 10 (33) 12 (20) ICU Admission 4 (13) 4 (7) Mech. Ventilator 3 (10) 0 (0) Bacteremia - Gram+ 17 (57)* 32 (53) - Gram - 14 (47)* 28 (47) Neutropenia (<500 ) 19 (63) 36 (60) Microbiologic Eradication 30 (100) 60 (100) - Relapse 0 (0) 3 (5) *** Complications 0 (0) 8 (13) SAEs related to R&R 0 (0) 6 (10) Overall Complication Rate 0 (0%) 11** (18%) *1 polymicrobial patient had Gr+ and Gr – organism cultured; ** 6 patients had >1 complication; ***all 3 CVCs were removed within 1 month.

16 NASDAQ: CTXR Active Arm (n=72) Mino - Lok® Solution Control Arm (n=72) Antibiotic Lock Patients with CRBSI/CLABSI (n~ 144) R Multi - center, randomized, open label, blinded assessor, active control superiority study (80% powered) Futility Performed at 35 - 40 Events and 60 - 70 Events for Superior Efficacy Adjunct in CLABSI/CRBSI Comparison of “Time to Catheter Failure”, TOC = 6 weeks Primary End Point Interim Analysis Anticipated median time of 21 days vs. 38 days to achieve significance Mino - Lok® Phase 3 Pivotal Trial Design

Mino - Lok® Time - To - Catheter Failure Trial Design 0 10 20 30 40 50 60 70 80 90 100 0 10 20 30 40 50 60 Catheter Survival (%) Time (days) Kaplan - Meier Model ALT MLT 21 vs. 38 Day Difference in Median Number of Catheter Failures 17

18 NASDAQ: CTXR 2015 2016 2017 2018 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Pivotal Phase 3 ALT Study 2019 Q1 Q2 Q3 Q4 2020 Q1 Q2 Q3 Q4 2021 Q1 Q2 Q3 Q4 First Patient In Interim Data Safety/Efficacy FDA Interim Mtg. FDA Review Mtg. Commercial Preparation Phase 2 FDA EOP2 Meeting Registration Manufacturing and Stability FDA CMC Meeting 2 nd Trial Pediatric (if needed) Chemistry and Manufacturing Control (CMC) Development Mino - Lok® Development Plan (estimated as of 2/2021*) Interim Data Futility Analysis NDA Submission DMC Efficacy Review * Best estimate, subject to impact of Covid pandemic on clinical trial conduct.

19 NASDAQ: CTXR Worldwide Ex - US United States Regulatory Updates Reimbursement Pricing With modest penetration at conservative pricing, we believe that >$500M peak year U.S. sales is achievable. DISARM Act is pending in the Senate, which would create a DRG carveout for QIDP products. This would allow for full reimbursement for CMS programs and not be part of the payment bundle. Conservative pricing to allow for rapid market uptake would be ~$1.4k treatment Pricing should have elasticity upwards, given the alternative, R&R (~$10k) Company expects to apply for NTAP, which was just increased to 75% of list price, which would apply to all QIDP products Company expects to apply for transitional pass - through Pursue Directly Partnerable * DelveInsight “Catheter - Related Blood Stream Infections (CRBSI) - Market Insights, Epidemiology & Market Forecast – 2028” >4 million CLABSI’s per year* Market Opportunity

20 NASDAQ: CTXR Mino - Lok TM is supported by a robust intellectual property portfolio Composition of Matter patent that provides protection until June 7, 2024 . Formulation Patent has been issued and will add protection through 2036. Creators Description of Patent All U.S. and Foreign Patent Applications / Patent Numbers Issam Raad , M.D. et al Antimicrobials in Combination with Chelators and Ethanol for the Rapid Eradication of Micro - organisms Embedded in Biofilm (Composition of Matter) • U.S. Patent No.: 7,601,731; • EP Ser. No.: 04754538.9; • CA Ser. No.: 2,528,522; Issam Raad , M.D. Joel Rosenblatt, Ph.D. et al Antimicrobial Catheter Lock / Flush Solutions with Enhanced Stability (Formulation) • Pub.No .: US 2017/051373 A1 • Global IP: UTFC.P1283WO U.S. Patent No. 7,601,731 (Composition of Matter) was filed on June 7, 2004 priority date of Provisional Application No. 60/476,555 of June 6, 2003 and issued on October 13, 2009. The expiration date is June 7, 2024 . U.S. Patent No. 9,078,441 (Method of Use) was issued on July 14, 2015. The expiration date is June 7, 2024 . There are corresponding patents granted in Europe and Canada (European Patent No. EP 1644024, and Canadian Patent No. 2528522 ). U.S. Patent No. 10,086,114 (Formulation/Enhanced Stability) was filed on November 4, 2016 and issued on Oct. 2, 2018. The expiration date is November 4, 2036. Patent applications for Global IP filed on June 12, 2018 incl. Canada, China, Japan, Korea, European Patent Office. Intellectual Property

21 NASDAQ: CTXR Regulatory Protection Qualified Infectious Disease Product (US) • Eligibility for Fast Track Status, enables frequent communication and collaboration with FDA; • Priority Review, reduces the NDA review time from 12 to 6 months; and, • Market Exclusivity, grants an additional 5 years of market exclusivity at NDA, combined with Hatch - Waxman. Fast Track Designation (US) • Fast Track expedites review of drugs which treat a serious or life - threatening condition and fills an unmet medical need. • More frequent meetings with FDA to discuss the development plan and ensure collection of appropriate data needed to support approval; • More frequent correspondence with FDA about the design of the clinical trials; • Rolling review allows for completed sections of the New Drug Application (NDA) to be submitted and reviewed by FDA rather than waiting until the entire application is compiled and submitted for review. Supplementary Protection Certificate (EU) • Extends patent protection up to 5 years for medicinal products which must undergo lengthy testing and clinical trials. 21
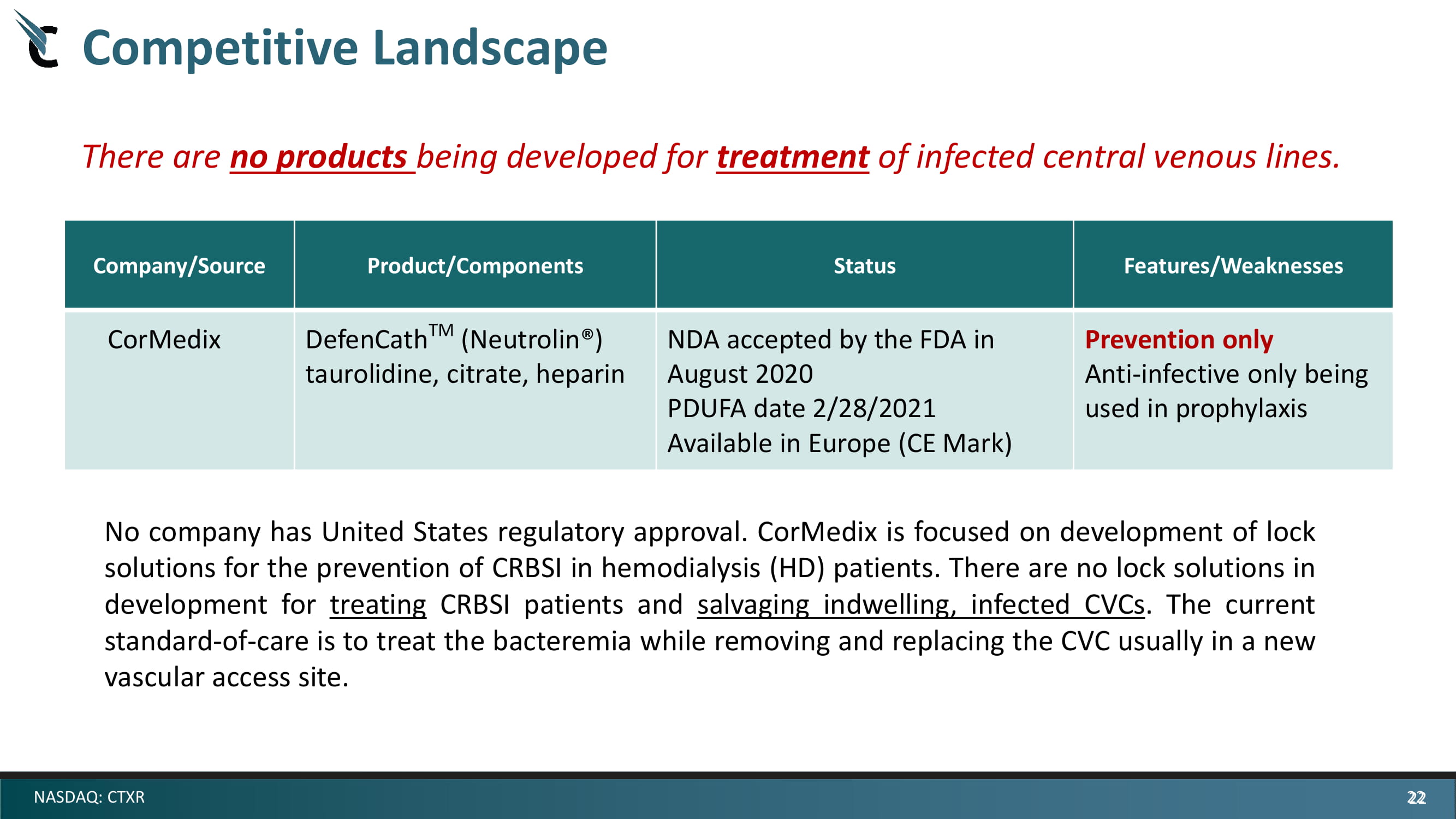
22 NASDAQ: CTXR Company/Source Product/Components Status Features/Weaknesses CorMedix DefenCath TM ( Neutrolin ®) taurolidine, citrate, heparin NDA accepted by the FDA in August 2020 PDUFA date 2/28/2021 Available in Europe (CE Mark) Prevention only Anti - infective only being used in prophylaxis No company has United States regulatory approval . CorMedix is focused on development of lock solutions for the prevention of CRBSI in hemodialysis (HD) patients . There are no lock solutions in development for treating CRBSI patients and salvaging indwelling, infected CVCs . The current standard - of - care is to treat the bacteremia while removing and replacing the CVC usually in a new vascular access site . There are no products being developed for treatment of infected central venous lines. Competitive Landscape 22

23 NASDAQ: CTXR Mino - Lok® (minocycline/disodium EDTA/ethyl alcohol) Treats catheter - related blood stream infections (CRBSIs). Penetrates biofilm, eradicates bacteria and salvages infected, indwelling vascular catheters while providing anti - clotting properties. Salvages central venous access in patients highly dependent on central lines and avoids the serious and expensive complications and morbidities associated with catheter removal and reinsertion. Expected to be indicated as adjunctive therapy for the treatment of Catheter - Related Blood Stream Infections (CRBSI) in combination with appropriate systemic antibiotic(s). Would have worldwide rights with appx. 16 years of exclusivity at time of launch. A major step forward in addressing a serious unmet medical need.

NoveCite induced Mesenchymal Stem Cells (“ i - MSCs”)

25 Citius Pharmaceuticals, Inc. • late - stage specialty pharmaceutical company • critical care products (anti - infectives and cancer care) • Mino - Lok, Mino - Wrap, and Halo - Lido. NASDAQ (CTXR) Novellus Therapeutics • pre - clinical stage biotechnology company • patented non - immunogenic mRNA technology • gene editing, mutation - free & footprint - free cell reprogramming. • Novellus is privately held. NoveCite Biotherapeutics • Worldwide license for i - MSCs for acute respiratory conditions • main focus is on ARDS associated with COVID - 19 • developing the next generation of mesenchymal stem cells • NoveCite cells are called i - MSCs • derived from an i - PSC master cell bank • NoveCite is a subsidiary of Citius

NoveCite i - MSC Compelling Unique Advantages 26 • Novellus mRNA cell reprogramming efficiently creates i - PSC cells ( i - PSCs are an ideal building - block for cell - based therapies) • Clonal i - PSC master cell bank is created – no batch - to - batch variation and footprint - free (no viral vectors) • Rapid production of i - MSCs (4 week differentiation from i - PSC master cell bank) • Significantly greater expansion ability than donor - derived cells • Higher level secretion of many immunomodulatory proteins (vs. donor - derived MSCs) results in higher potency and anti - inflammatory effects • NoveCite i - MSCs will be allogeneic (‘off - the - shelf’) and supplied in frozen, ready - to - use IV bags.

Novellus mRNA Cell Reprogramming Proprietary induced Pluripotent Stem Cell Platform – Single donor, NO repeat harvesting adult - derived cells; NO repeat donor testing – Clonal i - PSCs differentiated into clonal i - MSCs for genetically identical manufacturing batches – i - MSCs exhibit enormous expansion potential (trillions of cells) – Footprint - free (“viral - vector free”) i - MSCs – Allogeneic cells (‘off - the - shelf”) – mRNA process restores telomeres (rejuvenated embryonic - like cells) – i - MSCs secrete higher levels of immunomodulatory proteins Mesenchymal Stem Cells (MSCs) Adipocytes Oil Red O stain Osteoblasts Alizarin Red S stain Chondrocytes Alcian Blue stain Typical Results Neurons β - tubulin Retinal Pigment Epithelial Cells Cardiomyocytes – Thorsten M Schlaeger, PhD, Boston Children’s Hospital Schlaeger, et al. A comparison of non - integrating reprogramming methods. Nature Biotechnology. 2015. “The main advantages of the RNA method are speed of colony emergence, high efficiency, a complete absence of integration, a very low aneuploidy rate and a low donor cell requirement” • iPSC colonies from dermal fibroblasts • 6 daily transfections, 5 mRNA constructs expressing reprogramming factors • animal - component free

Proprietary mRNA Technology – Non - Immunogenic Ribolog Œ evades the innate immune system and is recognized by the ribosome as mRNA, resulting in no immunogenicity, high protein expression and ability for repeat dose 28 Novellus’s RiboLog Ρ Native mRNA is recognized by the innate immune system so is highly immunogenic, resulting in lower protein expression and no ability for repeat dose
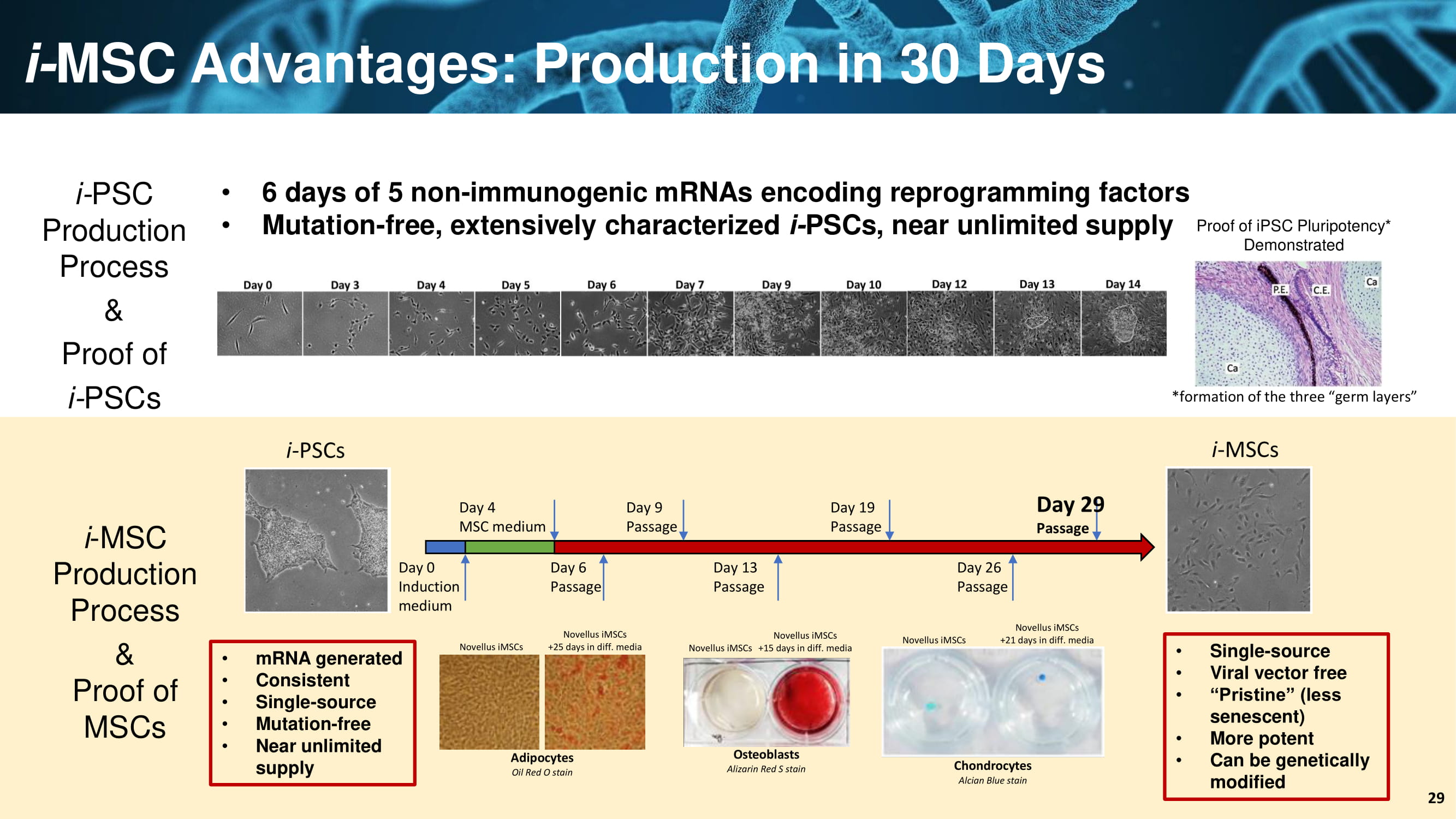
i - PSCs Day 0 Induction medium Day 4 MSC medium Day 6 Passage Day 9 Passage Day 13 Passage Day 19 Passage Day 26 Passage Day 29 Passage i - MSCs i - MSC Advantages: Production in 30 Days • 6 days of 5 non - immunogenic mRNAs encoding reprogramming factors • Mutation - free, extensively characterized i - PSCs, near unlimited supply i - MSC Production Process & Proof of MSCs Adipocytes Oil Red O stain Osteoblasts Alizarin Red S stain Chondrocytes AlcianBlue stain Novellus iMSCs Novellus iMSCs Novellus iMSCs Novellus iMSCs +25 days in diff. media Novellus iMSCs +15 days in diff. media Novellus iMSCs +21 days in diff. media iMSCDifferentiation i - PSC Production Process & Proof of i - PSCs • Single - source • Viral vector free • “Pristine” (less senescent) • More potent • Can be genetically modified • mRNA generated • Consistent • Single - source • Mutation - free • Near unlimited supply 29 Proof of iPSC Pluripotency* Demonstrated *formation of the three “germ layers”

i - MSCs Advantages: Greater Expansion Potential 30 Growth Curve Telomere Length i - MSCs exhibit greater expansion potential – mRNA process restores telomeres to yield >70 population doublings “BM - MSCs” = bone marrow - derived MSCs *Data on file (Novellus) BM - MSCs

NoveCite i - MSC Summary 31 • Novellus mRNA cell reprogramming efficiently creates i - PSC cells ( i - PSCs are an ideal building - block for cell - based therapies) • Clonal i - PSC master cell bank is created – no batch - to - batch variation and footprint - free (no viral vectors) • Rapid production of i - MSCs (4 week differentiation from i - PSC master cell bank) • Significantly greater expansion ability than donor - derived cells • Higher level secretion of many immunomodulatory proteins (vs. donor - derived MSCs) results in higher potency and anti - inflammatory effects • NoveCite i - MSCs will be allogeneic (‘off - the - shelf’) and supplied in frozen, ready - to - use IV bags.
NoveCite i - MSCs: ARDS in COVID - 19 SARS - CoV - 2 Severe acute respiratory syndrome coronavirus 2

ARDS Lung Normal ARDS a type of respiratory failure characterized by rapid onset of widespread inflammation and fluid accumulation in the lungs. ARDS impairs the lungs’ ability to exchange oxygen for carbon dioxide Symptoms includes shortness of breath, rapid breathing, and bluish skin coloration; for those who survive, a decreased quality of life is common Drug Treatments No FDA approved drug therapy for ARDS Clinical Management supportive care, through the use of ventilator, as well as fluid management, and in some instances, extracorporeal membrane oxygenation and/or glucocorticoids Acute Respiratory Distress Syndrome (ARDS)

ARDS (and sepsis) is a dysfunction of the immune system with the concomitant presence of pro - inflammatory and anti - inflammatory states • Immunosuppressive state is evidenced by : • increase in lymphocyte apoptosis , • alteration of immune cell functions , • less antigen presentation by dendritic cells , • promotion of reactive oxygen species (ROS), neutrophil production, or phagocytic activities • anti - inflammatory phenotypes • Therapeutic management must adapt to the inflammatory context and at the same time, improve organ failures and survival • Mesenchymal stromal cells (MSCs) possess the unique ability to modulate inflammation while limiting concomitant immunosuppression and has great potential in providing a safe and well - tolerated therapeutic for patients with ARDS ( and sepsis) Reference: Laroye C, Gibot S, Huselstein C,Bensoussan D. Mesenchymal stromal cells for sepsis and septic shock: Lessons for treatment of COVID - 19. STEMCELLS Transl Med. 2020;9:1488 – 1494.https://doi.org/10.1002/sctm.20 - 02391494 LAROYE ET AL. Immune Response to MSCs in SARS - CoV - 2 Infected Lung

i - MSC Advantages: Levels of Immunomodulator Secretion 35 i - MSCs secrete higher levels of many immunomodulatory proteins NoveCite Example: MMP - 1 = 6,500 pg/ml MMP - 1 = 330 pg/ml Example: VEGF - A = 10,800 pg/ml VEGF - A = 3,000 pg/ml Example: MMP - 1 = 6,500 pg/ml MMP - 1 = 330 pg/ml *MMP - 1: member of the MMP family which contribute to homeostasis and a variety of physiological processes (e.g. immunity, wound healing, angiogenesis) **VEGF - A: mediates the growth of new blood vessels from pre - existing vessels (angiogenesis) *Data on file (Novellus) Novellus iMSCs Commercial BM - MSCs

NoveCite i - MSC Mouse Study
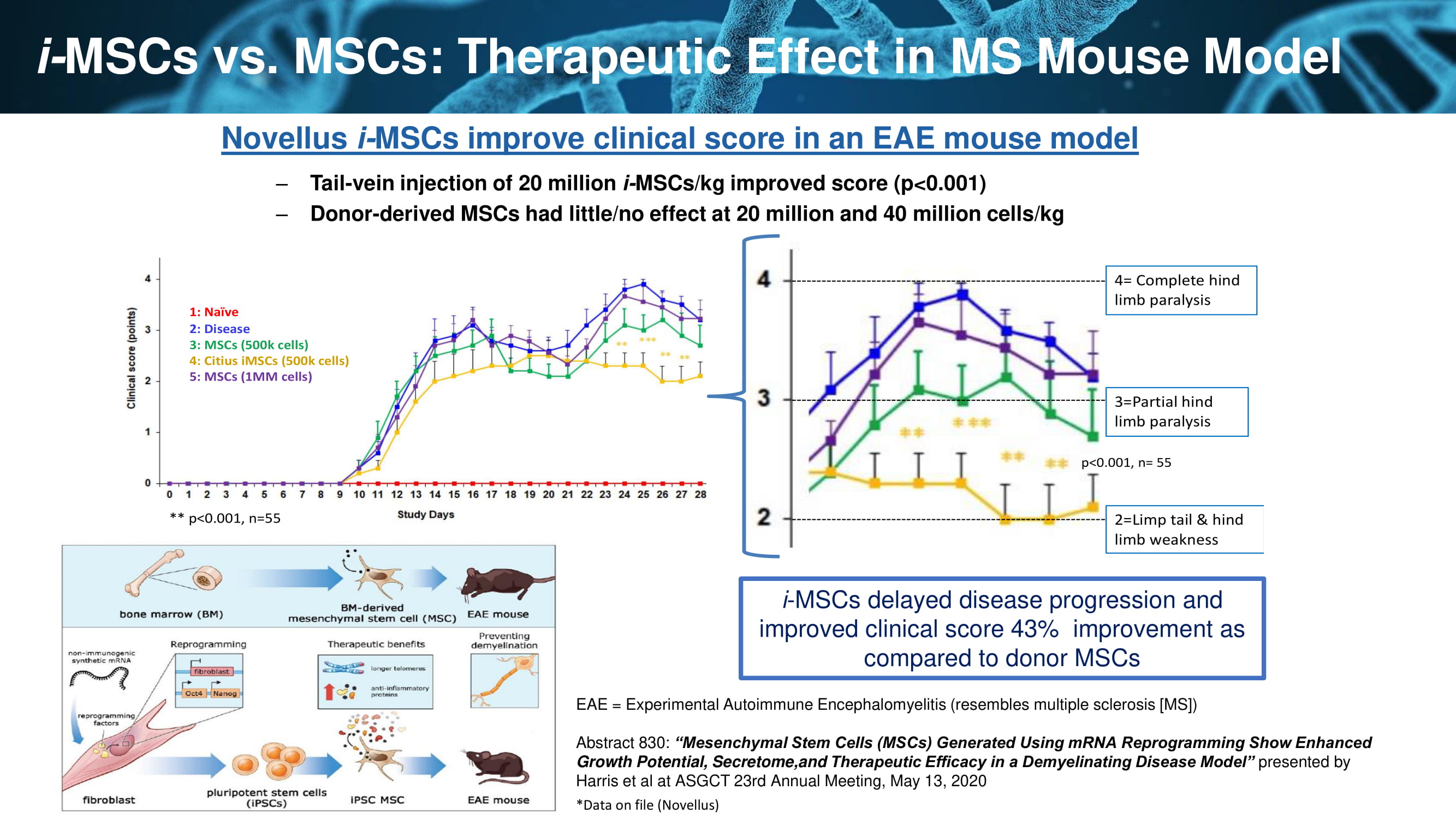
i - MSCs vs. MSCs: Therapeutic Effect in MS Mouse Model 37 Novellus i - MSCs improve clinical score in an EAE mouse model – Tail - vein injection of 20 million i - MSCs/kg improved score (p<0.001) – Donor - derived MSCs had little/no effect at 20 million and 40 million cells/kg 25 3=Partial hind limb paralysis 2=Limp tail & hind limb weakness 4= Complete hind limb paralysis p<0.001, n= 55 1: Naïve 2: Disease 3: MSCs (500k cells) 4: CitiusiMSCs(500k cells) 5: MSCs (1MM cells) ** p<0.001, n=55 i - MSCs delayed disease progression and improved clinical score 43% improvement as compared to donor MSCs EAE = Experimental Autoimmune Encephalomyelitis (resembles multiple sclerosis [MS]) Abstract 830: “Mesenchymal Stem Cells (MSCs) Generated Using mRNA Reprogramming Show Enhanced Growth Potential, Secretome,and Therapeutic Efficacy in a Demyelinating Disease Model” presented by Harris et al at ASGCT 23rd Annual Meeting, May 13, 2020 *Data on file (Novellus)

ARDS Sheep Study
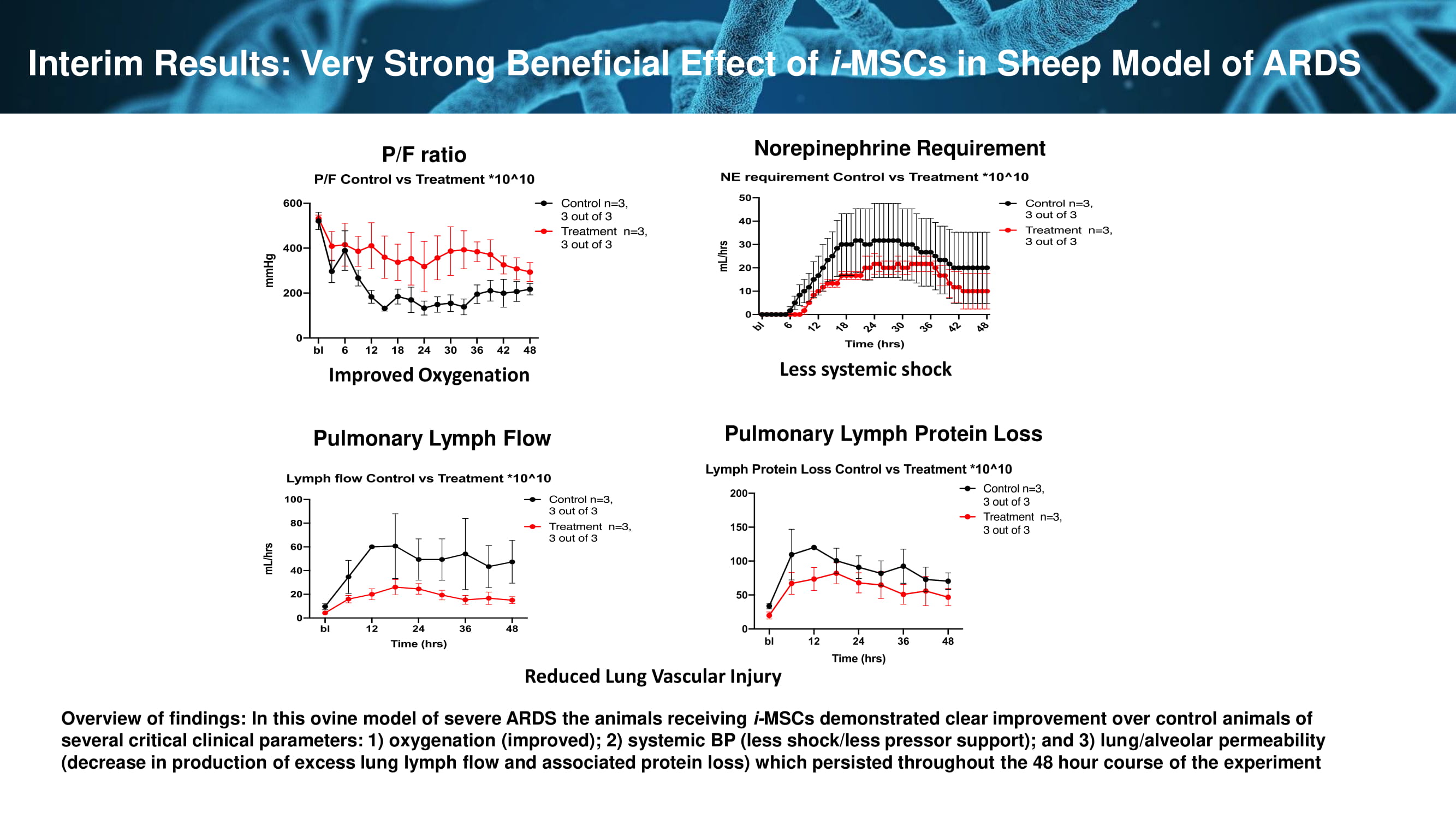
P/F ratio Pulmonary Lymph Flow Pulmonary Lymph Protein Loss Norepinephrine Requirement Interim Results: Very Strong Beneficial Effect of i - MSCs in Sheep Model of ARDS Improved Oxygenation Reduced Lung Vascular Injury Less systemic shock Overview of findings: In this ovine model of severe ARDS the animals receiving i - MSCs demonstrated clear improvement over control animals of several critical clinical parameters: 1) oxygenation (improved); 2) systemic BP (less shock/less pressor support); and 3) lun g/a lveolar permeability (decrease in production of excess lung lymph flow and associated protein loss) which persisted throughout the 48 hour course of the experiment

Our Solution – NoveCite i - MSCs Description: • Allogeneic (“off - the - shelf”) i - PSC - derived induced Mesenchymal Stem Cells • High potency i - MSCs – in vitro data showing exponentially higher secretion of immunomodulatory proteins vs. BM - derived MSCs • Footprint - free i - PSCs created using patented non - immunogenic mRNA process • Clonal cells, telomere restored, near unlimited supply, lower COGs • “Superior” MSCs How Supplied/Administered: • Cells cryo - frozen 250 ml IV bag (saline), thawed and reconstituted with Plasmalyte prior to use • IV infusion: will evaluate dosing range between 1 - 10 million cells/kg to determine OBD (optimal biological dose); a second dose may be given, at 48 - 96 hours, based on patient’s clinical status Target Indication: • Treatment of ARDS in Covid - 19 patients Future Indications: • Treatment of acute inflammatory respiratory conditions 40

Clinical Development Plan (as of 2/2021) Phase Type n Description 1/2 Multi - center Phase 1 dose - finding study followed by a randomized placebo - controlled Phase 2 expansion phase to assess the safety, tolerability, and efficacy of iMSCs in patients with moderate to severe ARDS due to COVID - 19 40 in phase 1 and 200 in phase 2 This is an adaptive design trial . The first part of the trial will serve as the phase 1 portion of the trial and will serve to establish safety in human subjects . The phase 1 portion will also serve as a dose - finding study . The phase 2 portion of the trial will take the selected dose forward into a larger patient population . The phase 2 portion will serve to establish safety of the selected dose in a larger population as well as an evaluation of efficacy endpoints . The results of this study will serve to establish the primary endpoint and help determine the sample size for the phase 3 study . 3 Multi - centered randomized placebo - controlled safety and efficacy evaluation of iMSCs for treatment of acute respiratory distress syndrome in patients with COVID - 19 TBD The primary objectives of the Phase 3 study are to confirm and expand on safety and effectiveness results from the Phase 1/2 studies of iMSCs as a treatment for subjects with moderate to severe ARDS due to COVID - 19. The secondary objectives of this study are to evaluate tolerability, pulmonary function, mortality, and quality of life among survivors associated with iMSCs therapy as a treatment for subjects with moderate to severe ARDS due to COVID - 19. This trial to serve as a pivotal trial for the NDA.
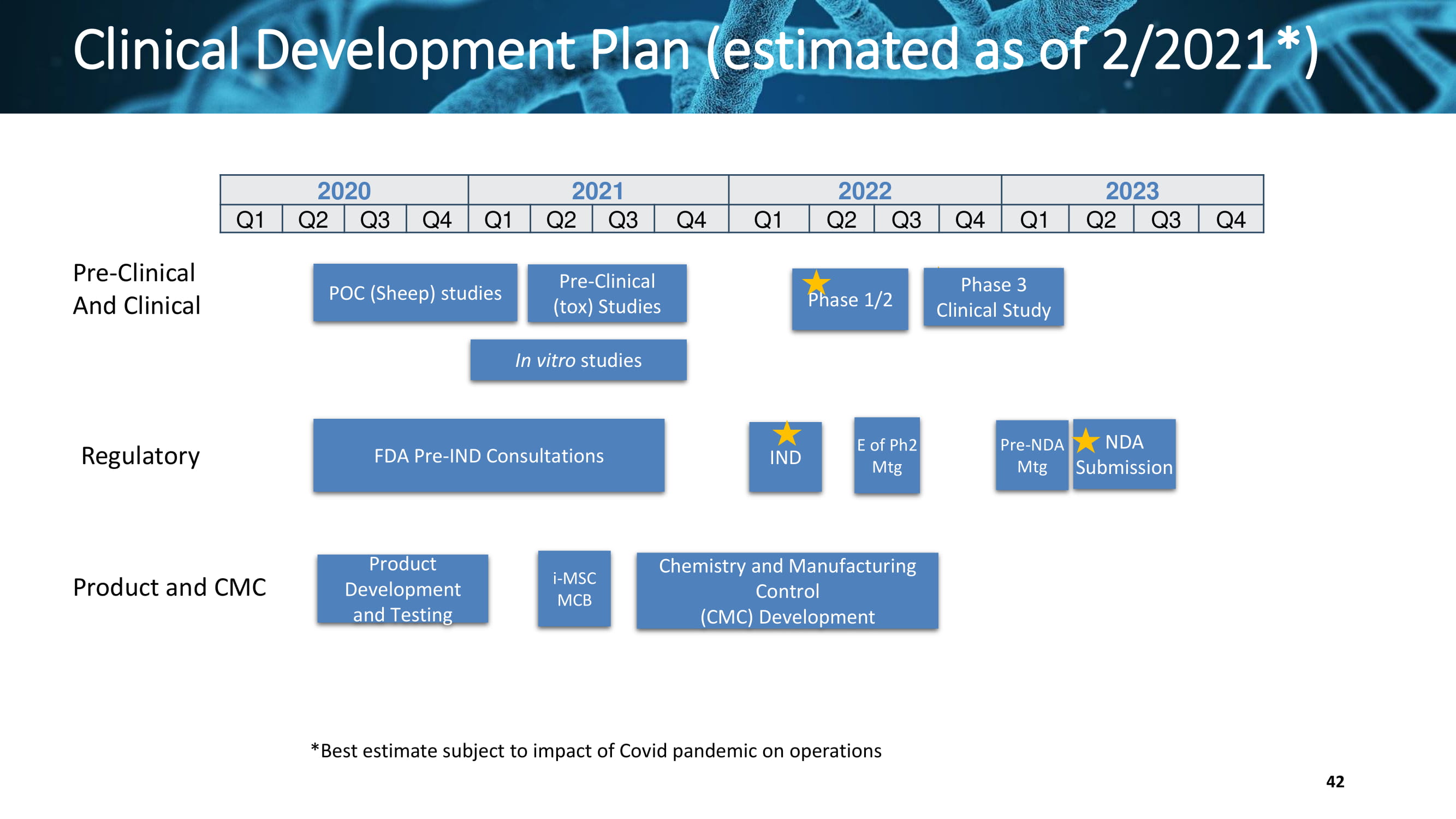
Clinical Development Plan (estimated as of 2/2021*) Pre - Clinical And Clinical Regulatory Product and CMC 2020 2021 2022 2023 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Pre - Clinical (tox) Studies Chemistry and Manufacturing Control (CMC) Development Phase 3 Clinical Study POC (Sheep) studies Product Development and Testing FDA Pre - IND Consultations IND Phase 1/2 E of Ph2 Mtg NDA Submission In vitro studies i - MSC MCB Pre - NDA Mtg *Best estimate subject to impact of Covid pandemic on operations 42

Mino - Wrap CITI - 101 Minocycline/Rifampin (M/R) Gelatin Film Bioabsorbable Extended Release Antimicrobial Wrap for the Prevention of Breast Tissue Expander Infections
44 NASDAQ: CTXR Background: Rate of Infection Post - Mastectomy • The rate of infection following mastectomy with tissue expander (TE) is 2.4 to 24%. Estimated mean is 12 - 14%*. • Once the implant becomes infected, the patient is usually hospitalized requiring approximate 2 weeks of IV and/or oral antimicrobials; and the TE is removed leading to a delay of lifesaving chemoradiation therapy, and a more complex reconstruction in the future. • The preventive measures used to decrease the rate of TE infections are (a) systemic perioperative antimicrobial agents, (b) perioperative immersion of the implant or irrigation of the surgical pocket with an antimicrobial solution prior to insertion of the device, and (c) immediate postoperative oral antimicrobials. Except for (a), all of the other preventive modalities are of debatable use. Armstrong RW. Ann Plast Surg 1989;23:284 - 8 Francis SH. Plast Reconstr Surg 2009;124:1790 - 6 Rosenblatt et al. 2015. Novel in situ liquefying antimicrobial wrap for preventing tissue expander infections following breas t r econstructive surgeries. J Biomed Mater Res Part B 2015:00B. * Please note that the 12 - 14% estimate for mean infection rates is an estimate from clinicians and is not a published data point.

45 NASDAQ: CTXR Mino - Wrap: Thesis • The highest risk for TE - related infections occurs at the time of surgery and as long as drains remain in place (about two weeks post - operatively) and there are portals for microbial colonization. • Mino - Wrap is a malleable, bioabsorbable, antimicrobial wrap that is placed over the TE in the surgical pocket as a solid film. It swells and liquefies in situ for a specified period of time providing extended protection against infection from the most likely pathogens. • Mino - Wrap is designed to allow the temporary tissue expander to be inflated without any restrictions, and to prevent infection and biofilm formation on the implant over longer durations than current practice. • The current standard of care (SOC) appears to be inadequate as the mean infection rate is very high compared to common surgical infection rates.
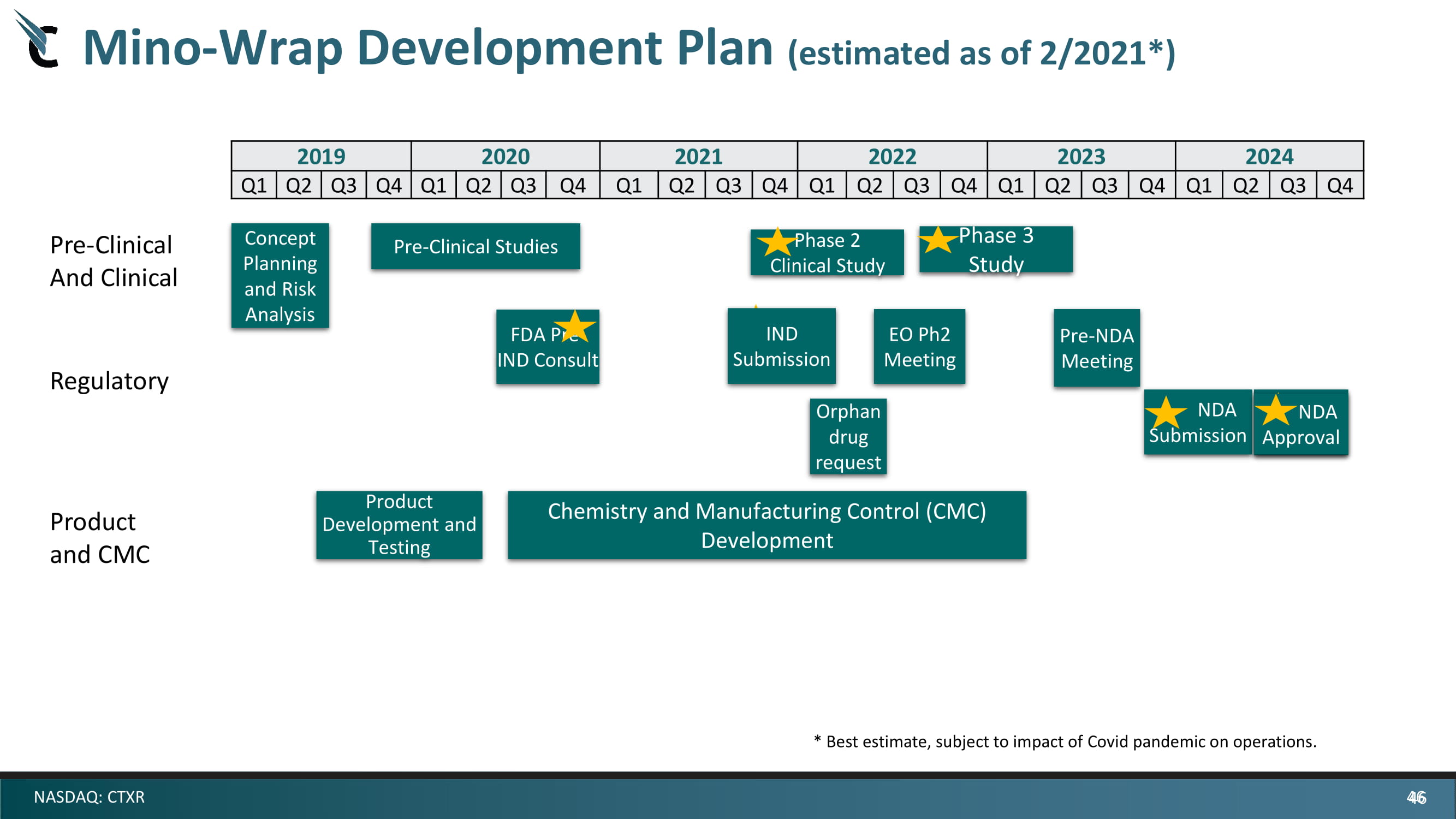
46 NASDAQ: CTXR 2019 2020 2021 2022 2023 2024 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Orphan drug request Pre - Clinical And Clinical Regulatory Product and CMC NDA Approval Mino - Wrap Development Plan (estimated as of 2/2021*) Pre - Clinical Studies Chemistry and Manufacturing Control (CMC) Development Concept Planning and Risk Analysis Product Development and Testing IND Submission EO Ph2 Meeting Phase 2 Clinical Study Phase 3 Study Pre - NDA Meeting FDA Pre - IND Consult NDA Submission NDA Approval * Best estimate, subject to impact of Covid pandemic on operations. 46

Halobetasol /Lidocaine Prescription Strength Topical for Symptomatic Hemorrhoid Treatment Halo - Lido CITI - 002

48 NASDAQ: CTXR CITI - 002 ( halobetasol + lidocaine) Citius’ product candidate would be the first FDA - approved prescription product to treat hemorrhoids in the US OTC Products are the Mainstay for Treatment of Grade I and II • Up to 5% of the U.S. population suffers from hemorrhoids, but there are no FDA - approved prescription products on the market • Over 10 million patients admit to symptoms of hemorrhoidal disease and one - third of them seek physician treatment • OTC hemorrhoid product sales are approximately 20 million units annually Existing Rx Treatments: “Grandfathered Products” • Several DESI topical cream formulations containing hydrocortisone and lidocaine are commonly prescribed to treat grade I and II hemorrhoids, but none are FDA - approved • In 2011, more than 4 million prescriptions were written in the U.S. for hemorrhoidal medications • Other topical DESI products for hemorrhoids contain hydrocortisone and pramoxine and have annual sales in excess of $80 million Commonly Used OTC Treatments Prescription, Non - approved Treatments
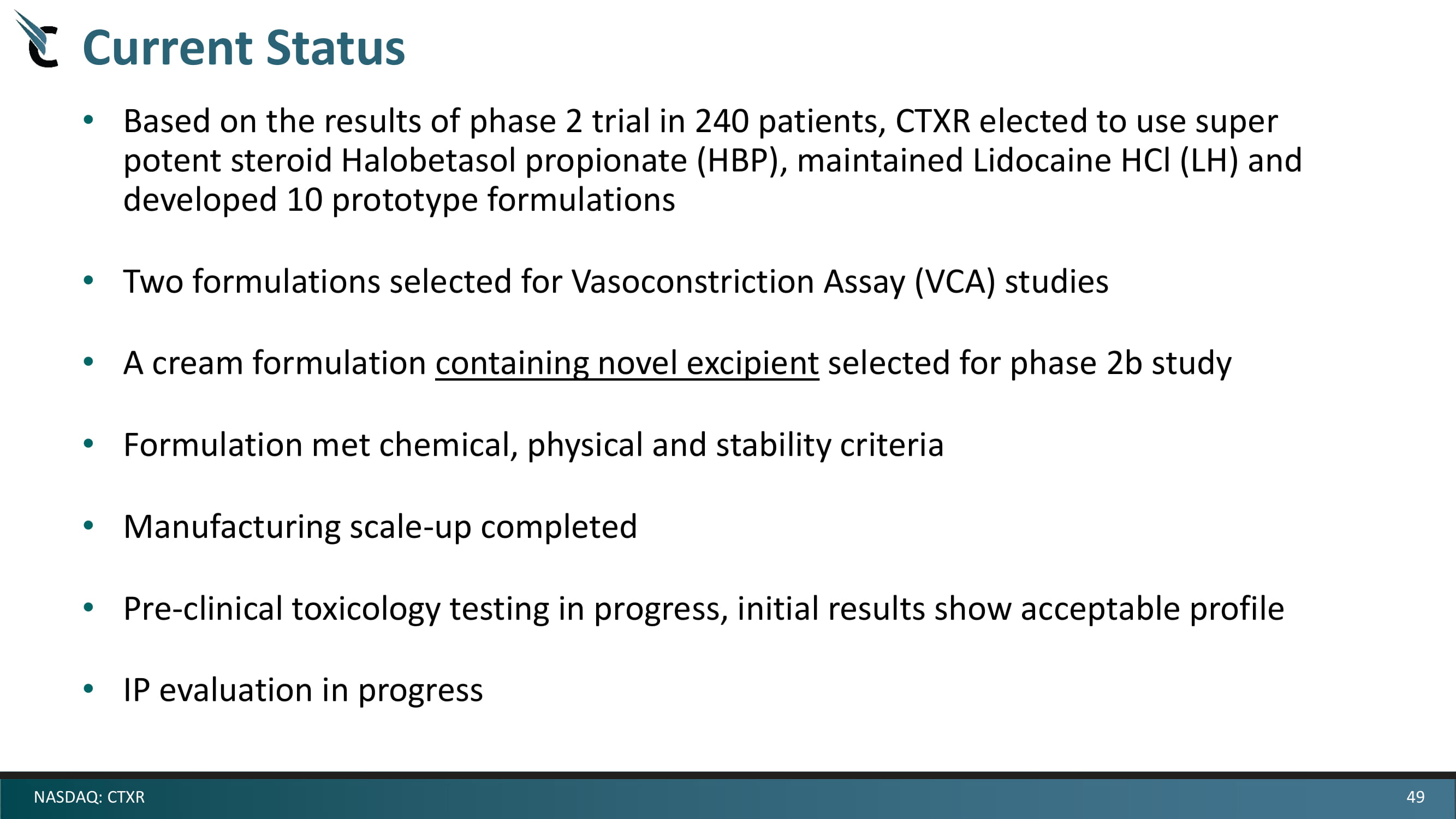
49 NASDAQ: CTXR Current Status • Based on the results of phase 2 trial in 240 patients, CTXR elected to use super potent steroid Halobetasol propionate (HBP), maintained Lidocaine HCl (LH) and developed 10 prototype formulations • Two formulations selected for Vasoconstriction Assay (VCA) studies • A cream formulation containing novel excipient selected for phase 2b study • Formulation met chemical, physical and stability criteria • Manufacturing scale - up completed • Pre - clinical toxicology testing in progress, initial results show acceptable profile • IP evaluation in progress
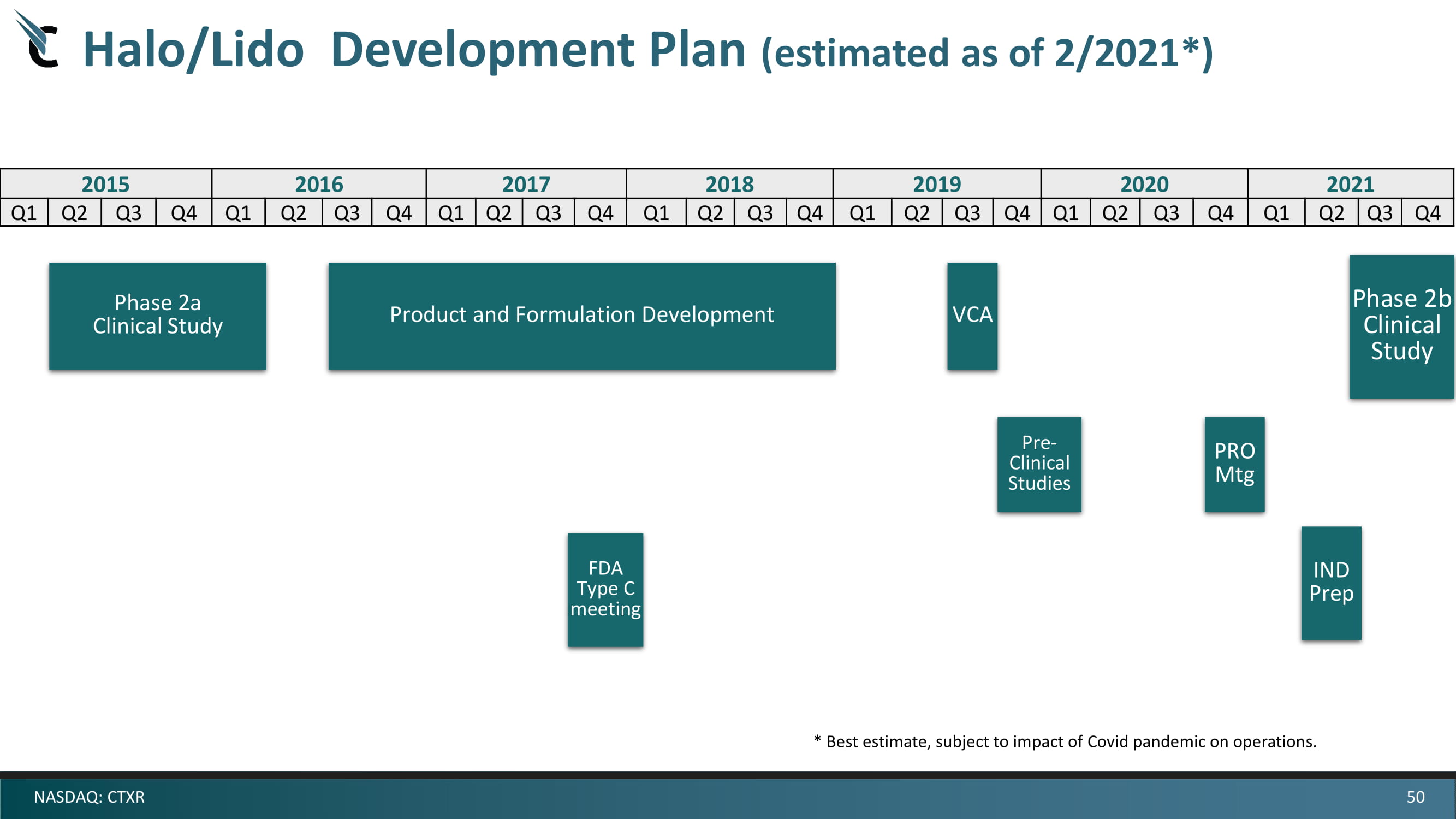
50 NASDAQ: CTXR Halo/Lido Development Plan (estimated as of 2/2021*) 2015 2016 2017 2018 2019 2020 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Phase 2a Clinical Study Product and Formulation Development Phase 2b Clinical Study Pre - Clinical Studies FDA Type C meeting IND Prep VCA 2021 Q1 Q2 Q3 Q4 PRO Mtg * Best estimate, subject to impact of Covid pandemic on operations.

51 NASDAQ: CTXR CITIUS Corporate Summary • Addressing attractive diversified multi - billion dollar opportunities – Adjunctive Cancer Care/Infectious Disease and Gastrointestinal Disease • Partnership with Novellus in developing highly promising i - MSC therapies • Partnership with MD Anderson Cancer Center in developing novel anti - infectives in cancer • Portfolio addressing recognized unmet medical needs with cost - saving or cost - effective solutions with low risk development pathways • Multiple staged near - term milestones anticipated • Highly experienced and successful Management Team, Board of Directors, and Scientific Advisory Board
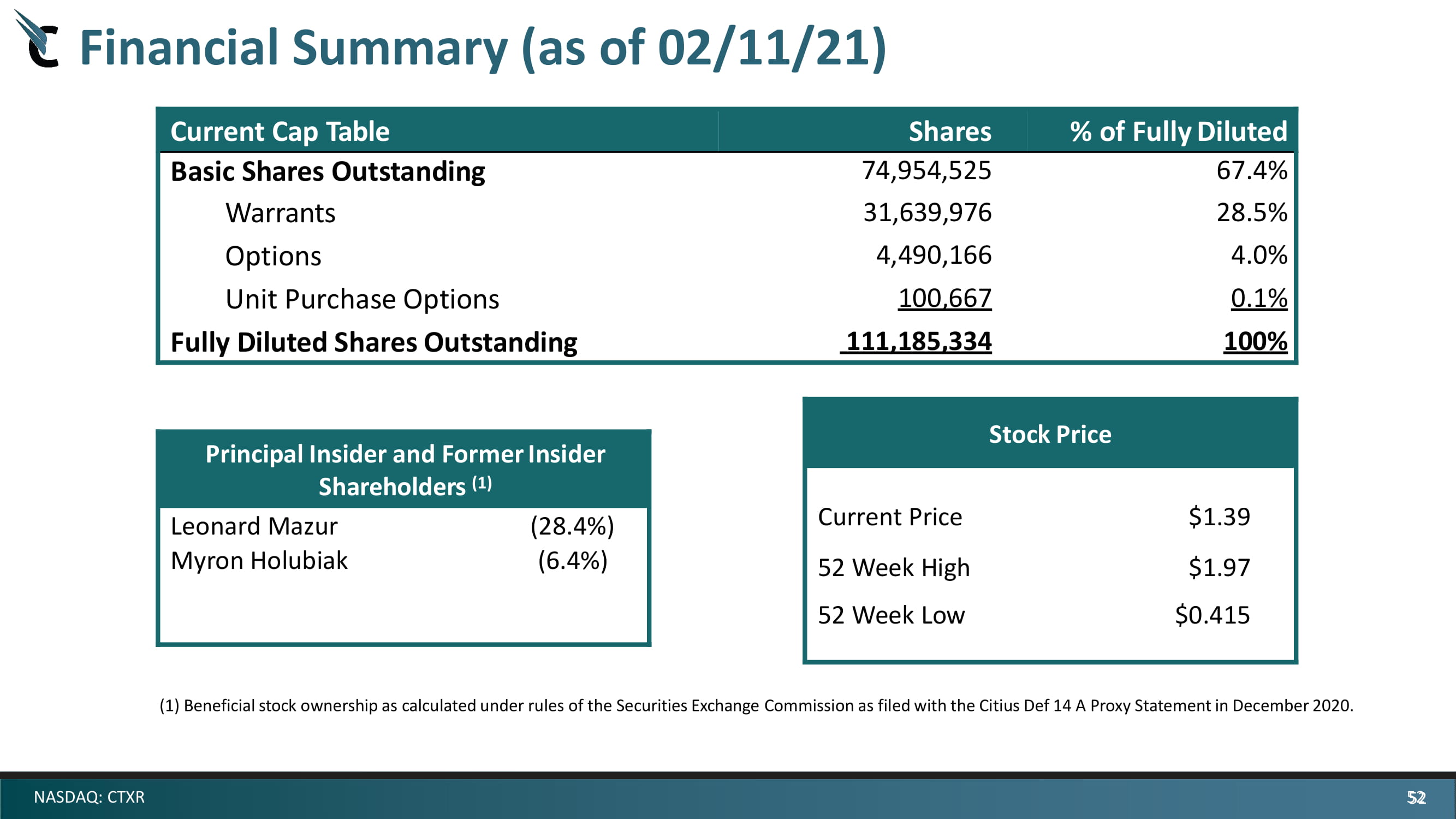
52 NASDAQ: CTXR Financial Summary (as of 02/11/21) Current Cap Table Sha r es % of Fully Diluted Basic Shares Outstanding 74,954,525 67.4 % Warrants 31,639,976 28.5 % Options 4,490,166 4.0 % Unit Purchase Options 100 , 667 0. 1 % Fully Diluted Shares Outstanding 111,185,334 100% ( 1 ) Beneficial stock ownership as calculated under rules of the Securities Exchange Commission as filed with the Citius Def 14 A Proxy Statement in December 2020. Stock Price Current Price $ 1.39 52 Week High $ 1.97 52 Week Low $ 0 . 415 Principal Insider and Former Insider Shareholders (1) Leonard Mazur ( 28.4 %) Myron Holubiak ( 6.4 %) 52

53 NASDAQ: CTXR Citius Pharmaceuticals, Inc. 11 Commerce Drive First Floor Cranford, NJ 07016 www.citiuspharma.com Investor Relations Contact : Andrew Scott – V.P., Corporate Development (908) 967 - 6677 x105 ascott@citiuspharma.com




















































