Exhibit 99.2
 | (908) 967-6677 x105 ascott@citiuspharma.com |
LETTER TO SHAREHOLDERS
February 2021
Dear Fellow Shareholders,
Citius Pharmaceuticals, Inc. (“Company”) has successfully traversed 2020 following what was a challenging year for so many. Thankfully, all of our employees and their loved ones have been able to avoid COVID-19 infections as we implemented various measures to keep our employees and visitors safe and virus-free. As for our business, we have continued to make solid progress since our last shareholder update in October 2020. We began 2021 with great enthusiasm and are building already what we expect to be a seminal year for advancing three of our product platforms in the clinic.
Mino-Lok® Phase 3 Superiority Review Imminent
As reported in my last letter, data from the Mino-Lok® (M-L) Phase 3 program was reviewed by our independent Data Monitoring Committee (DMC) for safety and efficacy and found to be progressing as planned with no recommended changes to trial design. Our Mino-Lok product is an antibiotic lock solution used to treat patients with catheter-related bloodstream infections (CRBSIs). CRBSIs are very serious, especially in cancer patients receiving therapy through central venous catheters (CVCs). Infected catheters can cause life-threatening bacteremia in these compromised patients. Currently, there are no approved therapies to salvage infected CVCs, and the standard treatment is removing and replacing (R&R) infected catheters. However, through our novel therapy, catheters can be disinfected and salvaged to avoid the significant costs and potential serious complications associated with R&R.
Because of the subsequent reduction in the number of participating clinical trial sites and the slowdown in patient recruitment due to the impact of the COVID-19 pandemic, we were short of the originally planned number of events that could have generated a “superiority” review by the DMC. Based on the comments and recommendations made by the DMC, which is further supported by the June 2020 FDA guidance document titled, “Statistical Considerations for Clinical Trials During the COVID-19 Public Health Emergency,” we have amended the DMC charter to enable a “superiority” review at 65% of the expected events rather than 75% as originally planned. The protocol, the Statistical Analysis Plan (SAP), and the DMC charter have been amended to reflect that change. To counter the impact of COVID-19, we have aggressively pursued outreach programs with webinars and other remote communications, and have now been able to add randomized patients bringing us closer to the number of events for the “superiority” analysis and to schedule a meeting with the DMC, both expected in the second quarter of 2021.
Halo-Lido PRO Nearing Completion
Halo-Lido, another one of our advanced clinical programs, is being developed to provide symptomatic relief of hemorrhoidal symptoms. Halo-Lido combines the high-potency steroid halobetasol with lidocaine and, if and when approved, would be the first FDA-approved prescription product to treat hemorrhoids in the U.S. Over the past six months, we have had extensive communication with the FDA regarding the Phase 2b clinical trial protocol. In our earlier interaction with the FDA, we were guided to implement a novel patient-reported outcome (PRO) instrument to assess clinical outcomes and efficacy as our primary endpoint. We have completed most of the work related to the development of the PRO and are now in what we believe to be the final round of comments with the FDA. Once we have the PRO completed, we will revise the protocol accordingly and expect to submit the investigational new drug application (IND) in the second quarter of 2021. We are also planning to implement electronic data collection for this study and are working with an experienced group that has developed proprietary tools to collect daily diary data, ensure compliance and facilitate statistical analysis. We believe we are the first to implement electronic data capture in a hemorrhoid treatment trial.
| 11 Commerce Drive | www.citiuspharma.com |
| First Floor | |
| Cranford, NJ 07016 | |
 | (908) 967-6677 x105 ascott@citiuspharma.com |
Mino-Wrap Pre-Clinical Development
Our early-stage development project is Mino-Wrap, a novel approach to significantly reduce the rate of infection in post-mastectomy breast cancer patients that elect to undergo reconstructive breast surgery. We believe that this serious condition impacts about 100,000 women in the U.S. and many more in the rest of the world. Mino-Wrap is a bio-absorbable, antimicrobial semi-solid film that is wrapped around a tissue expander and placed in the surgical pocket following a mastectomy to prevent post-surgical infections. Once implanted, Mino-Wrap slowly dissolves in situ for a specified period of time, providing extended protection against infection. In 2020, we submitted a pre-investigational new drug (PIND) consultation request with the FDA. We received written comments from the FDA, in which they provided direction about the pre-clinical development program. Following the FDA’s feedback, we are conducting the requested in vitro experiments and product characterization studies. Once this work is completed, we will begin non-clinical pharmacology and toxicology studies for inclusion in the IND submission. In parallel, we are working on a PIND consultation request that we plan to submit to the FDA by the end of the second quarter of 2021 for chemistry, manufacturing, and controls (CMC) development.
Unique Pipeline in Progressive Stages (current as of 2/2021*)
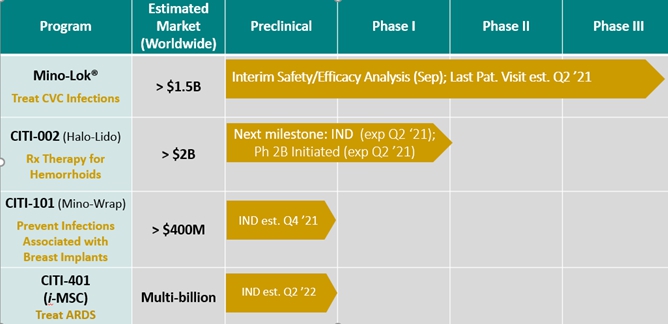
* Best estimate, subject to impact of COVID-19 pandemic on clinical trial conduct and operations.
Status report on our induced mesenchymal stem cells (i-MSCs) for ARDS
In the third quarter of 2020, we announced that we signed an exclusive worldwide licensing agreement with Novellus Therapeutics Limited to in-license second-generation cellular therapy for acute inflammatory respiratory conditions including acute respiratory distress syndrome (ARDS).
This technology is based on a unique and patented approach of engineering mesenchymal stem cells (MSCs) from an induced pluripotent stem cell (iPSC) bank. We call these cells induced mesenchymal stem cells (i-MSCs). The starting point in this semi-synthetic process is a patented, non-immunogenic, mRNA gene programming and editing procedure to create mutation-free, extensively characterized iPSCs that provide a near unlimited source of pristine i-MSCs. Currently, donor-derived MSCs are sourced from human bone marrow, adipose tissue, placenta, umbilical tissue, etc., and have significant challenges such as variable donor and tissue sources, limited supply, low potency, and inefficient and expensive manufacturing processes.
| 11 Commerce Drive | www.citiuspharma.com |
| First Floor | |
| Cranford, NJ 07016 | |
 | (908) 967-6677 x105 ascott@citiuspharma.com |
| We have recently initiated the manufacturing process development for our i-MSCs. Stem cell manufacturing is a complex process. Worldwide, there are a limited number of contract and development manufacturing companies (CDMOs) that provide this service for producing clinical-grade trial materials or long-term commercial product. Our program begins with our partner, Novellus, performing the proprietary processes of mRNA reprogramming and generating the initial cells. These cells will then be transferred to Waisman Biotechnology Institute of University of Wisconsin (WB) for further expansion, testing, and extensive characterization. At the end of this WB process, the finished product is considered the master cell bank (MCB). We will then transfer the MCB to Catalent, a commercial CDMO. | | 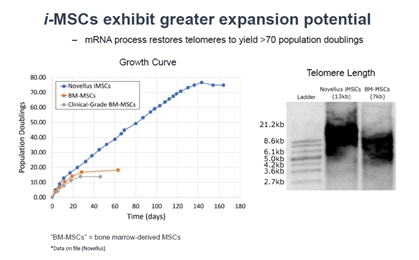 |
The multimodal mechanisms-of-action of our novel i-MSCs enables simultaneous targeting and treatment of a large number of dysregulated inflammatory cytokines and pathways, along with important tissue repair and pathogen clearing capabilities. We believe that the powerful multipotent single therapeutic advantage of i-MSCs is expected to prove superior to any currently studied single therapeutic or even limited combination approach of agents targeting ARDS-associated dysregulated cytokines/cell pathways that would likely be given in combination with anti-viral therapy.
We submitted a PIND to the FDA in April 2020 with respect to our development plans. Based on feedback and guidance in the written response from the FDA, both in-vitro and in-vivo (preclinical mouse toxicology) studies are underway. Preclinical work includes the development and validation of sensitive flow cytometric and reverse transcription polymerase chain reaction (RT-PCR) assays to confirm that our final i-MSC product is iPSC-free. A pilot study in an immunodeficient mouse model is planned to be performed in the second quarter of 2021 to guide the design of a larger definitive GLP mouse toxicology safety study, in which we would evaluate the toxicity, cell distribution, and tumorigenicity profile of our i-MSCs.
We are evaluating the potential clinical activity of our i-MSCs in an ongoing proof-of-concept large animal (sheep) severe ARDS model. Our interim results have been positively reviewed and found to be promising by our expert panel of ARDS medical advisors. Interim findings indicate that animals receiving i-MSCs demonstrated noticeable improvement over control animals of several critical clinical parameters: (i) oxygenation; (ii) systemic blood pressure (BP) (less shock/less pressor support); and (iii) lung/alveolar permeability (decrease in the production of excess lung lymph flow and associated protein loss). We plan to complete an evaluation of different doses and schedules of i-MSCs in our sheep ARDS model by the end of the second quarter of 2021. Results from our sheep ARDS experiments will be utilized to help inform the design and dosing of our planned human clinical trials. We have submitted an abstract of our interim data (first quarter 2021) to be considered for presentation at a large national/international conference in the second quarter of 2021. We plan to submit a detailed manuscript of our completed work to a major peer-reviewed medical journal during the third quarter of 2021.
| 11 Commerce Drive | www.citiuspharma.com |
| First Floor | |
| Cranford, NJ 07016 | |
 | (908) 967-6677 x105 ascott@citiuspharma.com |
Currently, there is no FDA-approved drug therapy for ARDS. We plan to submit an IND to the FDA and initiate our Phase 1 study by the end of the second quarter of 2022. Our first-in-human clinical trial is entitled: “i-MSCs in Subjects with Acute Respiratory Distress Syndrome (ARDS) Due to SARS-CoV-2 Disease (COVID-19): i-MARCO.” Following the completion of a multi-center Phase 1 pilot study, we would expect to proceed onto a double-blinded, randomized Phase 2/3 trial to demonstrate the safety, efficacy, and multimodal healing capabilities of our i-MSCs in patients with moderate to severe ARDS due to COVID-19.
While initially targeted to treat ARDS in COVID-19 patients, we plan to further expand the application of our i-MSC drug candidate in the future by testing it in ARDS associated with any cause or condition (e.g., bacteria, other viral pathogens, trauma, noxious exposure).
Recent Financing
On January 27, 2021, we closed a private placement round of financing for approximately $20.0 million in gross proceeds from certain institutional and accredited investors. The capital raise will continue to help advance our pre-clinical and clinical development of our product candidates.
Also, in early February 2021, 3.9 million investor warrants from an August 2018 securities offering were exercised at $1.15 per share for net proceeds of approximately $4.5 million.
Summary
We believe that 2021 will be a highly significant time for Citius (all planned events, however, could still be impacted by the COVID-19 pandemic):
| ● | For Mino-Lok, we will continue to push forward with the Phase 3 pivotal trial until it is fully enrolled, which we expect in 2021, and look forward with great anticipation to our DMC guidance on interim analysis for superior efficacy. The antibiotic lock solution market is still estimated to be a $750 million opportunity in the U.S. and more than $1.5 billion worldwide. |
| ● | For Halo-Lido, we expect to file an IND for the combination in the second quarter of 2021and to initiate our Phase 2b trial by the end of the year. |
| ● | For Mino-Wrap, we plan to start pre-clinical pharmacology and toxicology studies within the next few months along with CMC development, and seek to file an IND by the end of 2021. |
| ● | For our stem cell program, our goal is to become a leader in next-generation stem cells for the treatment of COVID-related ARDS and other acute respiratory indications. |
| ● | The Company recently closed a private round of financing and investors exercised a number of warrants to provide a significant financial runway for the Company. |
On behalf of the Citius Pharmaceuticals team, thank you for your interest in our Company. We look forward to sharing future important corporate developments and our clinical progress with you. In this current healthcare crisis, we hope you and your family stay healthy and safe.
| Sincerely, | |
| | |
| /s/ Myron Holubiak | |
| Myron Holubiak | |
| Chief Executive Officer, President, and Director | |
| 11 Commerce Drive | www.citiuspharma.com |
| First Floor | |
| Cranford, NJ 07016 | |
 | (908) 967-6677 x105 ascott@citiuspharma.com |
Safe Harbor
This communication may contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Such statements are made based on our expectations and beliefs concerning future events impacting Citius. You can identify these statements by the fact that they use words such as “will,” “anticipate,” “estimate,” “plan,” “expect,” “should,” and “may,” and other words and terms of similar meaning or use of future dates. Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition, and stock price. Factors that could cause actual results to differ materially from those currently anticipated are: risks associated with conducting our Phase 3 trial for Mino-Lok®, including completing patient enrollment, opening study sites, and achieving the required number of catheter failure events; risks associated with conducting our planned Phase 2b trial for Halo-Lido, including development of the PRO element of the trial; risks associated with developing Mino-Wrap™ and our planned ARDS therapy, including that preclinical results may not be predictive of clinical results and our ability to file an IND for each of those product candidates; the estimated markets for our product candidates and the acceptance thereof by any market; our need for substantial additional funds; risks related to our growth strategy; our ability to identify, acquire, close, and integrate product candidates and companies successfully and on a timely basis; risks relating to the results of research and development activities; uncertainties relating to preclinical and clinical testing; the early stage of products under development; our ability to obtain, perform under, and maintain financing and strategic agreements and relationships; our ability to attract, integrate, and retain key personnel; government regulation; patent and intellectual property matters; competition; as well as other risks described in our SEC filings. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations or any changes in events, conditions, or circumstances on which any such statement is based, except as required by law.
| 11 Commerce Drive | www.citiuspharma.com |
| First Floor | |
| Cranford, NJ 07016 | |
5


