Exhibit 99.2

1 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 Synergistic Combinations with the Dual RAF/MEK Inhibitor VS - 6766 to Overcome Resistance Mechanisms Jonathan Pachter, PhD, Chief Scientific Officer – Verastem Oncology RAS - Targeted Drug Development, Sept 16, 2020

2 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 2 Disclosures ▪ I am an employee of Verastem Oncology ▪ I will be discussing investigational/off - label uses of VS - 6766 (RAF/MEK inhibitor) and defactinib (focal adhesion kinase inhibitor)
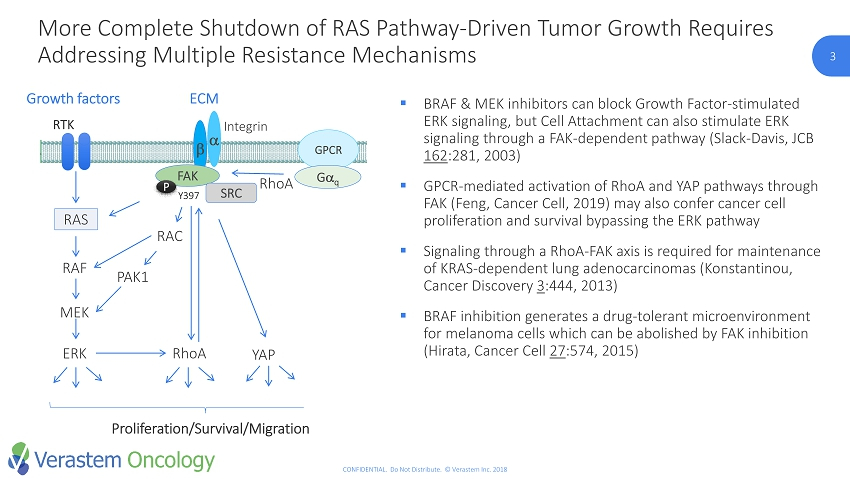
3 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 3 ▪ BRAF & MEK inhibitors can block Growth Factor - stimulated ERK signaling, but Cell Attachment can also stimulate ERK signaling through a FAK - dependent pathway (Slack - Davis, JCB 162 :281, 2003) ▪ GPCR - mediated activation of RhoA and YAP pathways through FAK (Feng, Cancer Cell, 2019) may also confer cancer cell proliferation and survival bypassing the ERK pathway ▪ Signaling through a RhoA - FAK axis is required for maintenance of KRAS - dependent lung adenocarcinomas (Konstantinou, Cancer Discovery 3 :444, 2013) ▪ BRAF inhibition generates a drug - tolerant microenvironment for melanoma cells which can be abolished by FAK inhibition (Hirata, Cancer Cell 27 :574, 2015) RTK RAS RAF MEK ERK RhoA Growth factors PAK1 b a Y397 Integrin FAK ECM SRC RAC GPCR G q YAP RhoA Proliferation/Survival/Migration PLC Ca 2+ /DAG PKC ERK P More Complete Shutdown of RAS Pathway - Driven Tumor Growth Requires Addressing Multiple Resistance Mechanisms
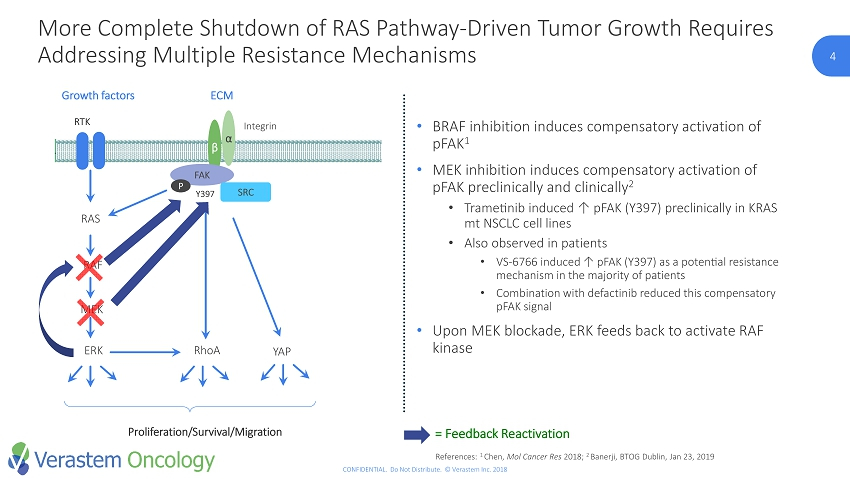
4 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 RTK RAS RAF MEK ERK RhoA Growth factors β α Y397 Integrin FAK ECM SRC YAP Proliferation/Survival/Migration P • BRAF inhibition induces compensatory activation of pFAK 1 • MEK inhibition induces compensatory activation of pFAK preclinically and clinically 2 • Trametinib induced ↑ pFAK (Y397) preclinically in KRAS mt NSCLC cell lines • Also observed in patients • VS - 6766 induced ↑ pFAK (Y397) as a potential resistance mechanism in the majority of patients • Combination with defactinib reduced this compensatory pFAK signal • Upon MEK blockade, ERK feeds back to activate RAF kinase = Feedback Reactivation References: 1 Chen, Mol Cancer Res 2018; 2 Banerji, BTOG Dublin, Jan 23, 2019 More Complete Shutdown of RAS Pathway - Driven Tumor Growth Requires Addressing Multiple Resistance Mechanisms
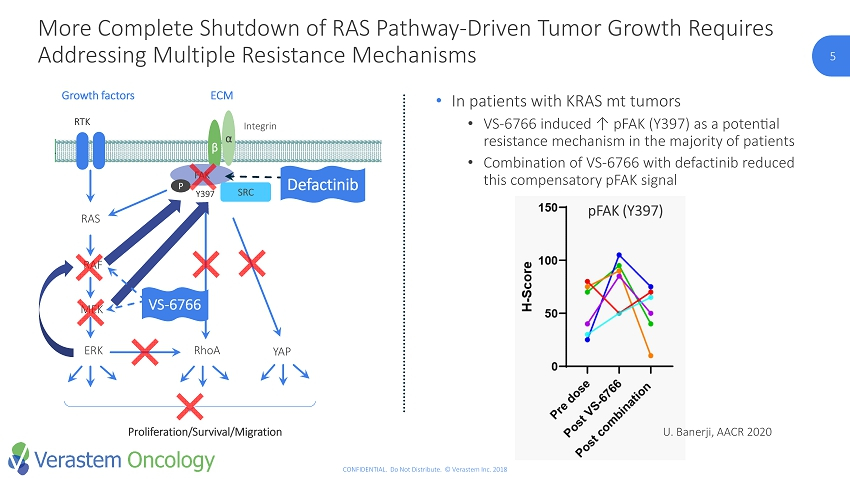
5 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 RTK RAS RAF MEK ERK RhoA Growth factors β α Y397 Integrin FAK ECM SRC YAP Proliferation/Survival/Migration P • In patients with KRAS mt tumors • VS - 6766 induced ↑ pFAK (Y397) as a potential resistance mechanism in the majority of patients • Combination of VS - 6766 with defactinib reduced this compensatory pFAK signal VS - 6766 Defactinib More Complete Shutdown of RAS Pathway - Driven Tumor Growth Requires Addressing Multiple Resistance Mechanisms pFAK (Y397) U. Banerji, AACR 2020

6 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 6 ▪ VS - 6766 inhibits both MEK & RAF kinase activities ▪ Most MEK inhibitors paradoxically induce MEK phosphorylation (pMEK) by relieving ERK - dependent feedback inhibition of RAF ▪ By inhibiting RAF phosphorylation of MEK, VS - 6766 has advantage of not inducing pMEK ▪ VS - 6766 inhibits ERK signaling more completely; may confer enhanced therapeutic activity VS - 6766 is a Unique Small Molecule RAF/MEK Dual Inhibitor Reference: Ishii et al., Cancer Res , 2013; Lito et al., Cancer Cell, 2014; Blasco, R. B. et al. Cancer Cell (2011); Sanclemente, M. et al. Cancer Cell (2018) RAS RAF MEK ERK Proliferation & Survival
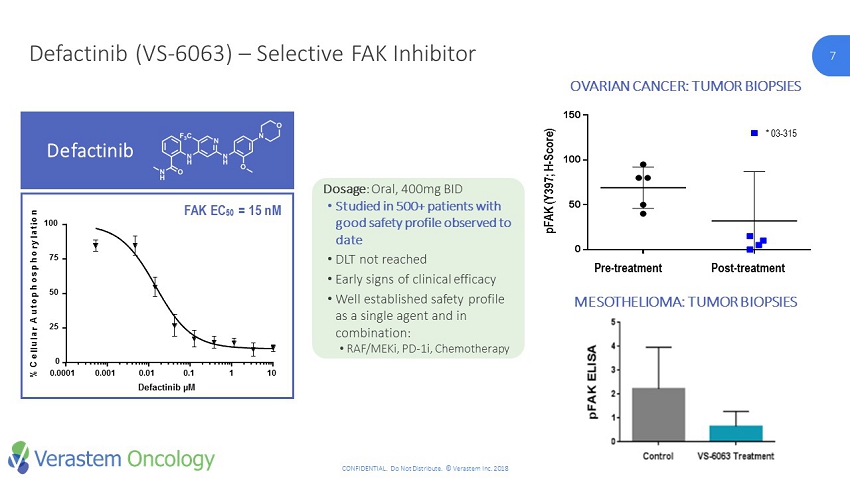
7 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 7 Defactinib (VS - 6063) – Selective FAK Inhibitor p F A K ( Y 3 9 7 ; H S c o r e ) D a y 1 D a y 1 0 0 50 100 150 Pre - treatment Post - treatment pFAK (Y397; H - Score) * 03 - 315 OVARIAN CANCER: TUMOR BIOPSIES MESOTHELIOMA: TUMOR BIOPSIES FAK EC 50 = 15 nM Defactinib Defactinib µM % C e l l u l a r A u t o p h o s p h o r y l a t i o n 0.0001 0.001 0.01 0.1 1 10 0 25 50 75 100 Dosage : Oral, 400mg BID • Studied in 500+ patients with good safety profile observed to date • DLT not reached • Early signs of clinical efficacy • Well established safety profile as a single agent and in combination: • RAF/ MEKi , PD - 1i, Chemotherapy
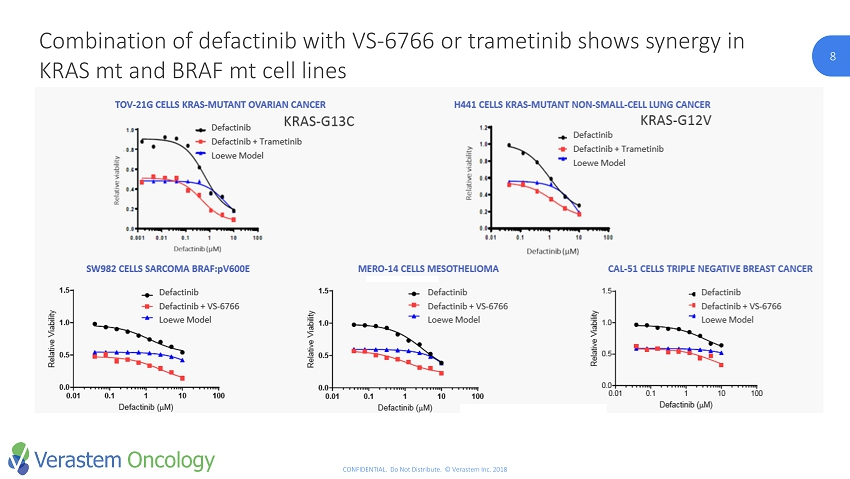
8 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 8 Combination of defactinib with VS - 6766 or trametinib shows synergy in KRAS mt and BRAF mt cell lines
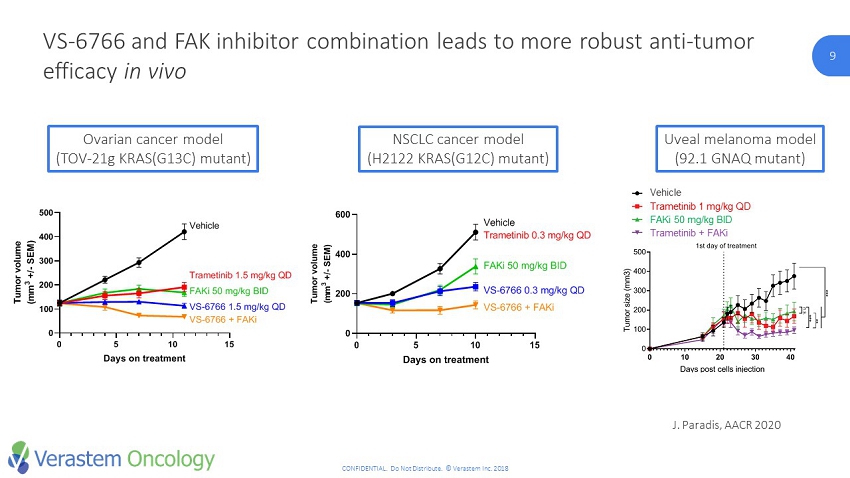
9 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 9 VS - 6766 and FAK inhibitor combination leads to more robust anti - tumor efficacy in vivo Ovarian cancer model (TOV - 21g KRAS(G13C) mutant) NSCLC cancer model (H2122 KRAS(G12C) mutant) J. Paradis, AACR 2020 0 5 10 15 0 200 400 600 Tumor growth Days on treatment T u m o r v o l u m e ( m m 3 + / - S E M ) Vehicle FAKi 50 mg/kg BID VS-6766 0.3 mg/kg QD VS-6766 + FAKi Trametinib 0.3 mg/kg QD 0 5 10 15 0 100 200 300 400 500 Tumor growth VS-4718 + CH5126766 Days on treatment T u m o r v o l u m e ( m m 3 + / - S E M ) Vehicle Trametinib 1.5 mg/kg QD FAKi 50 mg/kg BID VS-6766 1.5 mg/kg QD VS-6766 + FAKi FAKi + MEKicombination iscytotoxic in vivo CTL MEKi FAKi Combo *** *** *** Trametinib + VS-4718 is more ecient than one alone. Each inhbitor iscytostatic , thecombination iscytotoxic In vitro Trametinib + VS-4718 induced more apoptosis in vitro than each one alone Vehicle Trametinib 1 mg/kg QD FAKi 50 mg/kg BID Trametinib + FAKi Uveal melanoma model (92.1 GNAQ mutant)
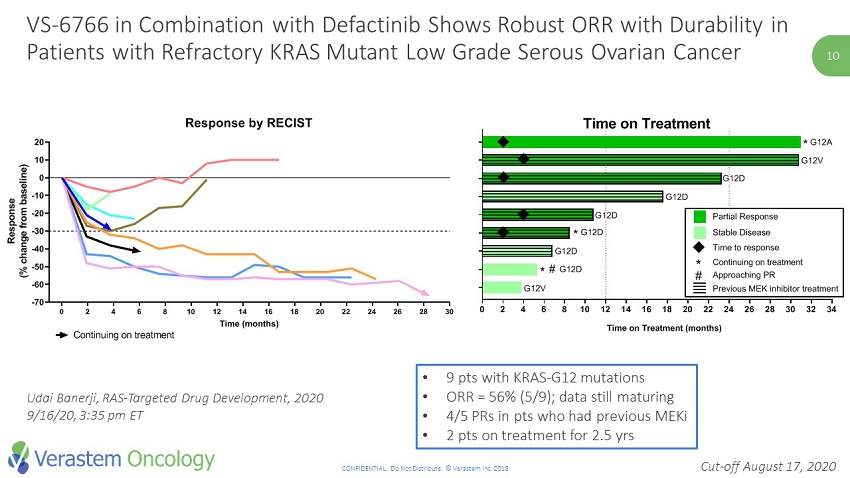
10 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 10 • 9 pts with KRAS - G12 mutations • ORR = 56% (5/9); data still maturing • 4/5 PRs in pts who had previous MEKi • 2 pts on treatment for 2.5 yrs Cut - off August 17, 2020 VS - 6766 in Combination with Defactinib Shows Robust ORR with Durability in Patients with Refractory KRAS Mutant Low Grade Serous Ovarian Cancer 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 FRA101015 FRA101035 FRA102010 FRA101033 FRA101019 FRA101014 FRA101009 FRA101001 FRA101002 Time on Treatment (months) * * G12A G12V G12D G12D G12D G12D G12D G12V Time on Treatment * G12D Previous MEK inhibitor treatment Continuing on treatment * Approaching PR # Time to response Partial Response Stable Disease # 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 -70 -60 -50 -40 -30 -20 -10 0 10 20 Response by RECIST Time (months) R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) FRA101001 - KRAS G12V FRA101002 - KRAS G12A FRA101009 - KRAS G12D FRA101014 - KRAS G12D FRA101015 - KRAS G12V FRA101019 - KRAS G12D FRA102010 - KRAS G12D FRA101033 - KRAS G12D FRA101035 - KRAS G12D Continuing on treatment Udai Banerji, RAS - Targeted Drug Development, 2020 9/16/20, 3:35 pm ET
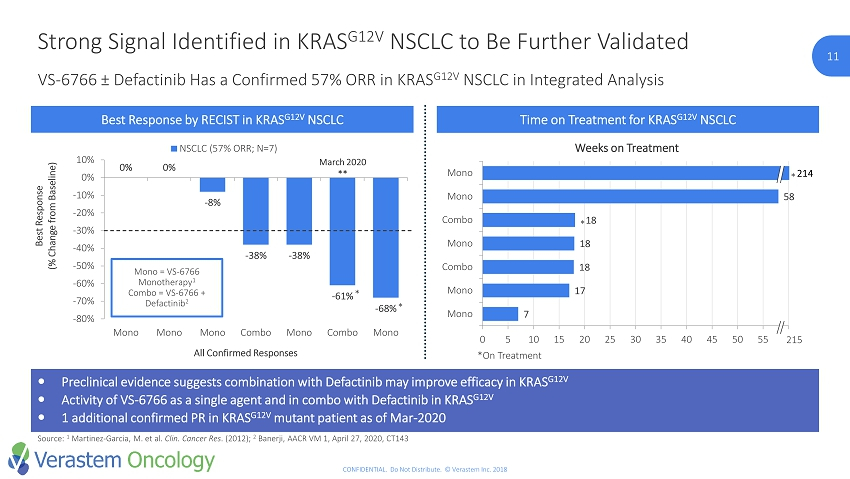
11 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 Preclinical evidence suggests combination with Defactinib may improve efficacy in KRAS G12V Activity of VS - 6766 as a single agent and in combo with Defactinib in KRAS G12V 1 additional confirmed PR in KRAS G12V mutant patient as of Mar - 2020 VS - 6766 ± Defactinib Has a Confirmed 57% ORR in KRAS G12V NSCLC in Integrated Analysis Strong Signal Identified in KRAS G12V NSCLC to Be Further Validated Source: 1 Martinez - Garcia, M. et al. Clin. Cancer Res . (2012); 2 Banerji, AACR VM 1, April 27, 2020, CT143 *On Treatment Best Response by RECIST in KRAS G12V NSCLC Time on Treatment for KRAS G12V NSCLC 0% 0% - 8% - 38% - 38% - 61% - 68% -80% -70% -60% -50% -40% -30% -20% -10% 0% 10% Mono Mono Mono Combo Mono Combo Mono Best Response (% Change from Baseline) All Confirmed Responses NSCLC (57% ORR; N=7) Mono = VS - 6766 Monotherapy 1 Combo = VS - 6766 + Defactinib 2 * * March 2020 ** 7 17 18 18 18 58 0 5 10 15 20 25 30 35 40 45 50 55 60 Mono Mono Combo Mono Combo Mono Mono Weeks on Treatment 215 214 * *
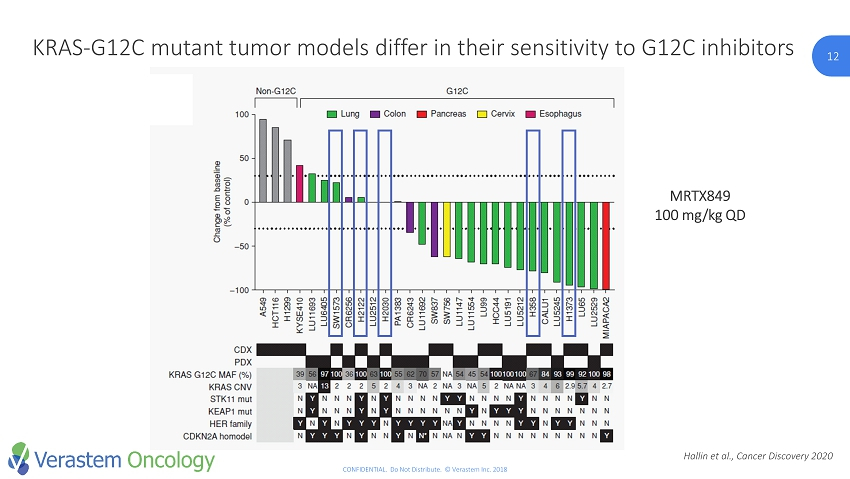
12 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 12 KRAS - G12C mutant tumor models differ in their sensitivity to G12C inhibitors MRTX849 100 mg/kg QD Hallin et al., Cancer Discovery 2020
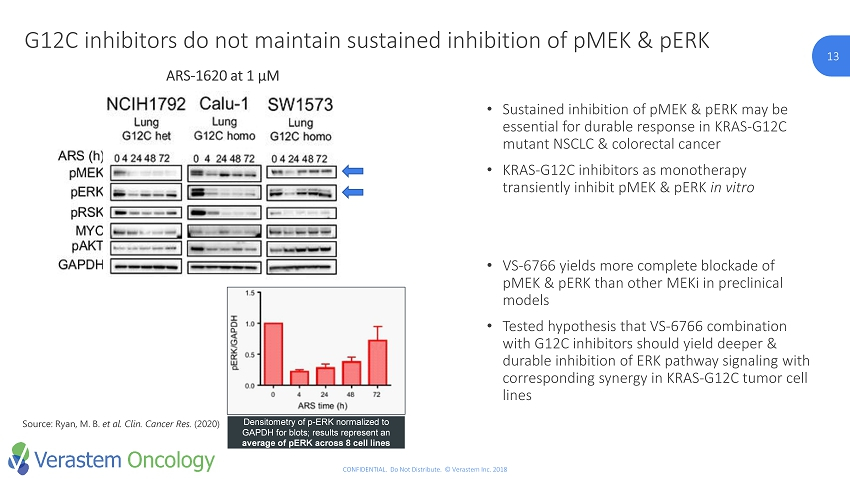
13 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 13 G12C inhibitors do not maintain sustained inhibition of pMEK & pERK Densitometry of p - ERK normalized to GAPDH for blots; results represent an average of pERK across 8 cell lines Source: Ryan, M. B. et al. Clin. Cancer Res . (2020) • Sustained inhibition of pMEK & pERK may be essential for durable response in KRAS - G12C mutant NSCLC & colorectal cancer • KRAS - G12C inhibitors as monotherapy transiently inhibit pMEK & pERK in vitro • VS - 6766 yields more complete blockade of pMEK & pERK than other MEKi in preclinical models • Tested hypothesis that VS - 6766 combination with G12C inhibitors should yield deeper & durable inhibition of ERK pathway signaling with corresponding synergy in KRAS - G12C tumor cell lines ARS - 1620 at 1 µM
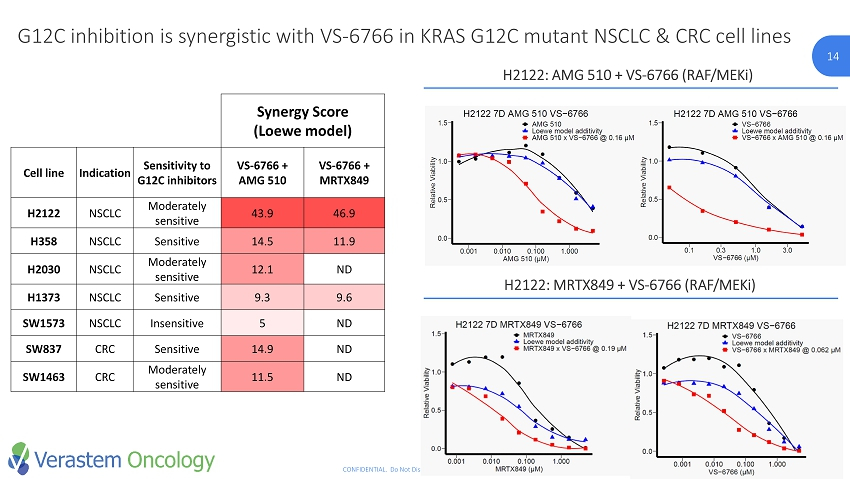
14 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 14 G12C inhibition is synergistic with VS - 6766 in KRAS G12C mutant NSCLC & CRC cell lines H2122: AMG 510 + VS - 6766 (RAF/ MEKi ) H2122: MRTX849 + VS - 6766 (RAF/ MEKi ) Synergy Score (Loewe model) Cell line Indication Sensitivity to G12C inhibitors VS - 6766 + AMG 510 VS - 6766 + MRTX849 H2122 NSCLC Moderately sensitive 43.9 46.9 H358 NSCLC Sensitive 14.5 11.9 H2030 NSCLC Moderately sensitive 12.1 ND H1373 NSCLC Sensitive 9.3 9.6 SW1573 NSCLC Insensitive 5 ND SW837 CRC Sensitive 14.9 ND SW1463 CRC Moderately sensitive 11.5 ND
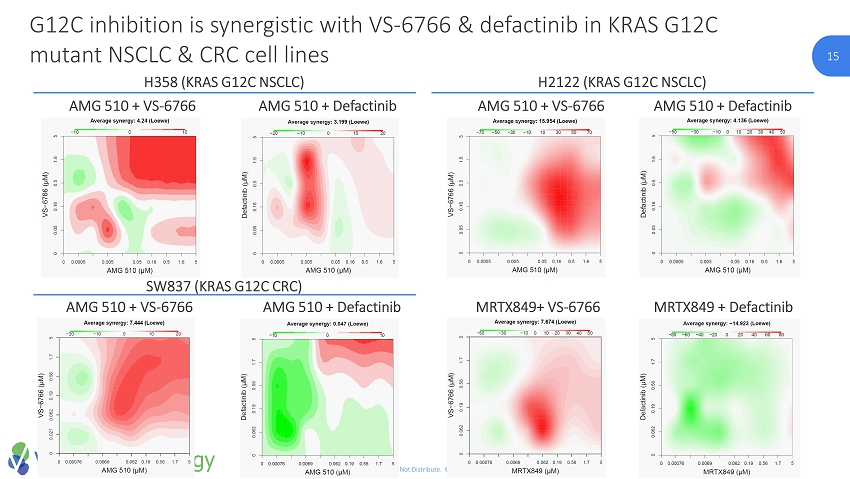
15 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 15 G12C inhibition is synergistic with VS - 6766 & defactinib in KRAS G12C mutant NSCLC & CRC cell lines AMG 510 + VS - 6766 AMG 510 + Defactinib SW837 (KRAS G12C CRC) AMG 510 + VS - 6766 AMG 510 + Defactinib Loewe Synergy Score H2122 (KRAS G12C NSCLC) AMG 510 + VS - 6766 AMG 510 + Defactinib MRTX849+ VS - 6766 MRTX849 + Defactinib H358 (KRAS G12C NSCLC)
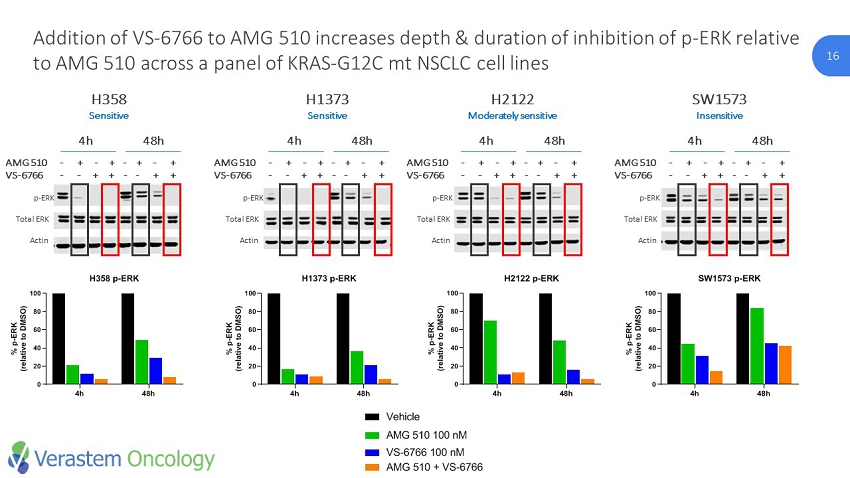
16 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 16 Addition of VS - 6766 to AMG 510 increases depth & duration of inhibition of p - ERK relative to AMG 510 across a panel of KRAS - G12C mt NSCLC cell lines p - ERK Actin AMG 510 - + - + - + - + VS - 6766 - - + + - - + + H2122 Moderately sensitive SW1573 Insensitive p - ERK Actin AMG 510 - + - + - + - + VS - 6766 - - + + - - + + H1373 Sensitive H358 Sensitive p - ERK Actin AMG 510 - + - + - + - + VS - 6766 - - + + - - + + p - ERK Actin AMG 510 - + - + - + - + VS - 6766 - - + + - - + + Total ERK Total ERK Total ERK Total ERK 4h 48h 0 20 40 60 80 100 H358 p-ERK % p - E R K ( r e l a t i v e t o D M S O ) AMG 510 VS-6766 AMG 510 + VS-6766 Vehicle 4h 48h 0 20 40 60 80 100 H1373 p-ERK % p - E R K ( r e l a t i v e t o D M S O ) AMG 510 VS-6766 AMG 510 + VS-6766 Vehicle 4h 48h 0 20 40 60 80 100 H2122 p-ERK % p - E R K ( r e l a t i v e t o D M S O ) AMG 510 VS-6766 AMG 510 + VS-6766 Vehicle 4h 48h 0 20 40 60 80 100 SW1573 p-ERK % p - E R K ( r e l a t i v e t o D M S O ) AMG 510 VS-6766 AMG 510 + VS-6766 Vehicle 4h 48h 4h 48h 4h 48h 4h 48h 4h 48h 0 20 40 60 80 100 H2122 p-p90RSK % p - p 9 0 R S K ( r e l a t i v e t o D M S O ) AMG 510 100 nM VS-6766 100 nM AMG 510 + VS-6766 Vehicle
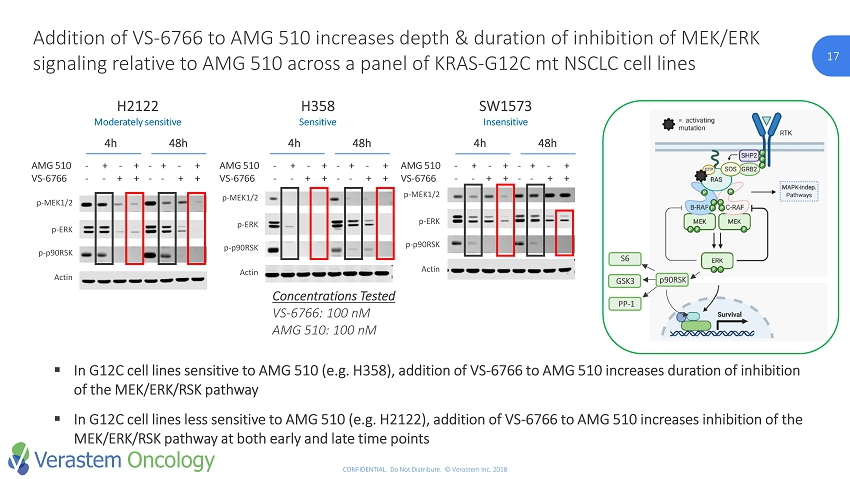
17 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 17 Addition of VS - 6766 to AMG 510 increases depth & duration of inhibition of MEK/ERK signaling relative to AMG 510 across a panel of KRAS - G12C mt NSCLC cell lines Concentrations Tested VS - 6766: 100 nM AMG 510: 100 nM H2122 Moderately sensitive p - MEK1/2 p - ERK p - p90RSK Actin AMG 510 - + - + - + - + VS - 6766 - - + + - - + + 4h 48h H358 Sensitive p - MEK1/2 p - ERK p - p90RSK Actin AMG 510 - + - + - + - + VS - 6766 - - + + - - + + 4h 48h SW1573 Insensitive p - MEK1/2 p - ERK p - p90RSK Actin AMG 510 - + - + - + - + VS - 6766 - - + + - - + + 4h 48h ▪ In G12C cell lines sensitive to AMG 510 (e.g. H358), addition of VS - 6766 to AMG 510 increases duration of inhibition of the MEK/ERK/RSK pathway ▪ In G12C cell lines less sensitive to AMG 510 (e.g. H2122), addition of VS - 6766 to AMG 510 increases inhibition of the MEK/ERK/RSK pathway at both early and late time points p90RSK S6 GSK3 PP - 1
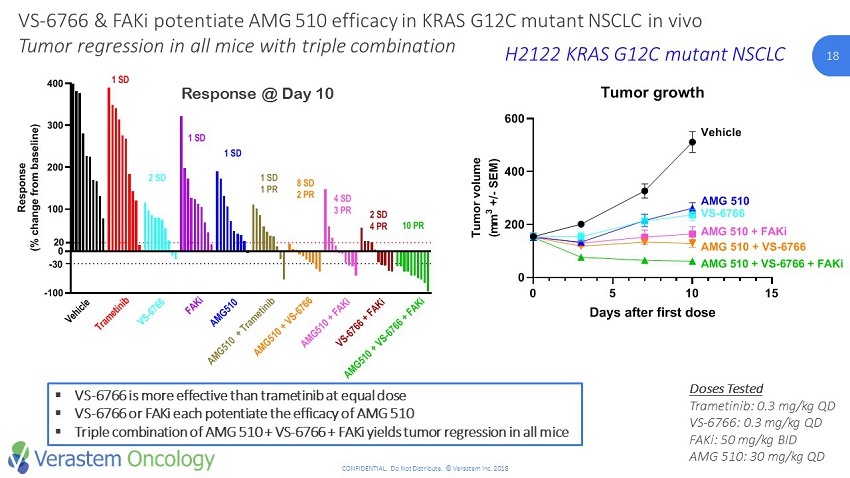
18 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 18 ▪ VS - 6766 is more effective than trametinib at equal dose ▪ VS - 6766 or FAKi each potentiate the efficacy of AMG 510 ▪ Triple combination of AMG 510 + VS - 6766 + FAKi yields tumor regression in all mice Doses Tested Trametinib: 0.3 mg/kg QD VS - 6766: 0.3 mg/kg QD FAKi : 50 mg/kg BID AMG 510: 30 mg/kg QD VS - 6766 & FAKi potentiate AMG 510 efficacy in KRAS G12C mutant NSCLC in vivo Tumor regression in all mice with triple combination H2122 KRAS G12C mutant NSCLC -100 0 100 200 300 400 Response at Day 10 R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) Vehicle VS-6766 0.3mg/kg QD AMG 510 30mg/kg QD VS-6766 + AMG 510 VS-4718 50mg/kg BID VS-6766 + VS-4718 AMG 510 + VS-4718 VS-6766 + AMG 510 + VS-4718 Trametinib 0.3mg/kg QD Trametinib + AMG 510 V e h i c l e T r a m e t i n i b 10 PR V S - 6 7 6 6 F A K i A M G 5 1 0 A M G 5 1 0 + T r a m e t i n i b A M G 5 1 0 + V S - 6 7 6 6 A M G 5 1 0 + F A K i V S - 6 7 6 6 + F A K i A M G 5 1 0 + V S - 6 7 6 6 + F A K i -30 20 1 SD 2 SD 1 SD 1 SD 1 SD 1 PR 8 SD 2 PR 4 SD 3 PR 2 SD 4 PR Response @ Day 10 0 5 10 15 0 200 400 600 Tumor growth Days after first dose T u m o r v o l u m e ( m m 3 + / - S E M ) Vehicle AMG 510 AMG 510 + FAKi AMG 510 + VS-6766 AMG 510 + VS-6766 + FAKi VS-6766
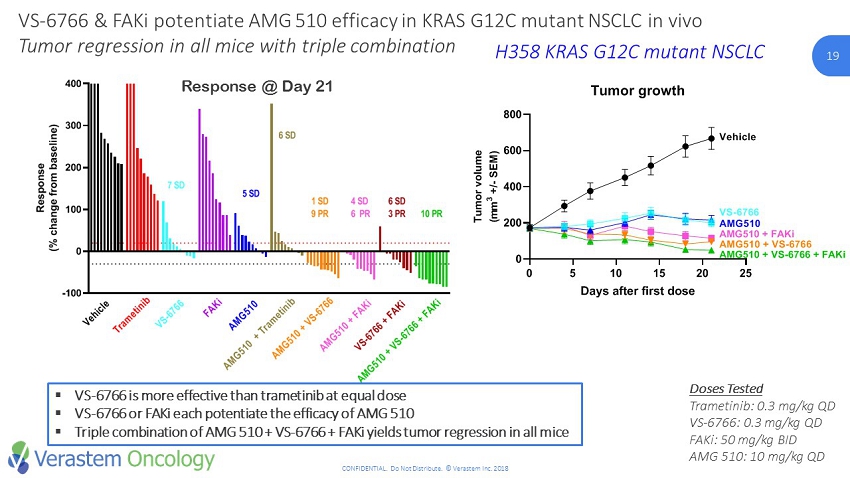
19 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 19 -100 0 100 200 300 400 Waterfall at Day 21 R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) Vehicle VS-6766 0.3mg/kg QD AMG 510 10mg/kg QD VS-6766 + AMG 510 VS-4718 50mg/kg BID VS-6766 + VS-4718 AMG 510 + VS-4718 VS-6766 + AMG 510 + VS-4718 Trametinib 0.3mg/kg QD Trametinib + AMG 510 V e h i c l e T r a m e t i n i b 10 PR V S - 6 7 6 6 F A K i A M G 5 1 0 A M G 5 1 0 + T r a m e t i n i b A M G 5 1 0 + V S - 6 7 6 6 A M G 5 1 0 + F A K i V S - 6 7 6 6 + F A K i A M G 5 1 0 + V S - 6 7 6 6 + F A K i 5 SD 6 SD 1 SD 9 PR 4 SD 6 PR 6 SD 3 PR 7 SD SD: Stable disease PR: Partial response VS - 6766 & FAKi potentiate AMG 510 efficacy in KRAS G12C mutant NSCLC in vivo Tumor regression in all mice with triple combination H358 KRAS G12C mutant NSCLC Response @ Day 21 ▪ VS - 6766 is more effective than trametinib at equal dose ▪ VS - 6766 or FAKi each potentiate the efficacy of AMG 510 ▪ Triple combination of AMG 510 + VS - 6766 + FAKi yields tumor regression in all mice Doses Tested Trametinib: 0.3 mg/kg QD VS - 6766: 0.3 mg/kg QD FAKi : 50 mg/kg BID AMG 510: 10 mg/kg QD 0 5 10 15 20 25 0 200 400 600 800 Tumor growth Days after first dose T u m o r v o l u m e ( m m 3 + / - S E M ) Vehicle AMG510 AMG510 + FAKi AMG510 + VS-6766 AMG510 + VS-6766 + FAKi VS-6766
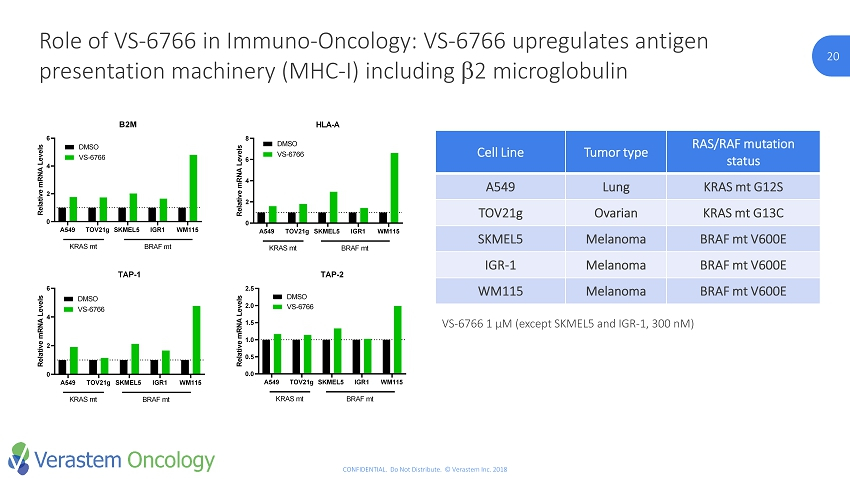
20 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 20 Role of VS - 6766 in Immuno - Oncology: VS - 6766 upregulates antigen presentation machinery (MHC - I) including b 2 microglobulin Cell Line Tumor type RAS/RAF mutation status A549 Lung KRAS mt G12S TOV21g Ovarian KRAS mt G13C SKMEL5 Melanoma BRAF mt V600E IGR - 1 Melanoma BRAF mt V600E WM115 Melanoma BRAF mt V600E A549 TOV21g SKMEL5 IGR1 WM115 0 2 4 6 B2M R e l a t i v e m R N A L e v e l s DMSO VS-6766 KRAS mt BRAF mt A549 TOV21g SKMEL5 IGR1 WM115 0 2 4 6 8 HLA-A R e l a t i v e m R N A L e v e l s DMSO VS-6766 KRAS mt BRAF mt A549 TOV21g SKMEL5 IGR1 WM115 0 2 4 6 TAP-1 R e l a t i v e m R N A L e v e l s DMSO VS-6766 KRAS mt BRAF mt A549 TOV21g SKMEL5 IGR1 WM115 0.0 0.5 1.0 1.5 2.0 2.5 TAP-2 R e l a t i v e m R N A L e v e l s DMSO VS-6766 KRAS mt BRAF mt VS - 6766 1 µM (except SKMEL5 and IGR - 1, 300 nM )
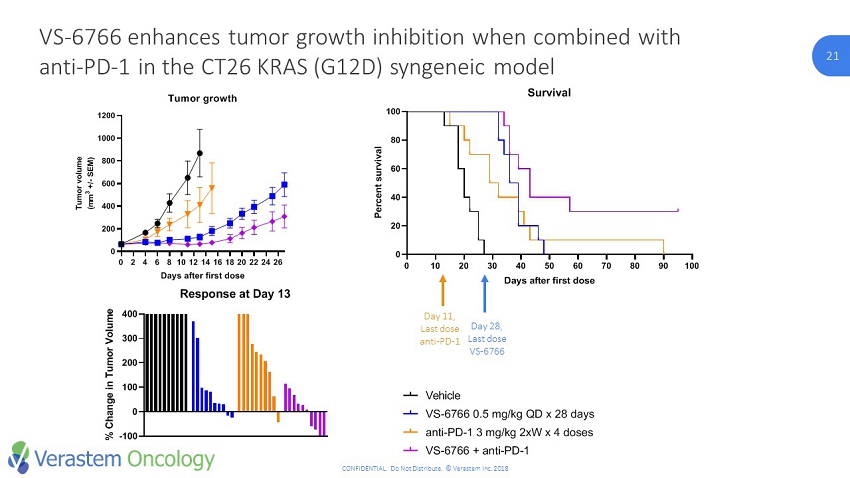
21 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 21 VS - 6766 enhances tumor growth inhibition when combined with anti - PD - 1 in the CT26 KRAS (G12D) syngeneic model -100 0 100 200 300 400 Response at Day 13 % C h a n g e i n T u m o r V o l u m e Vehicle VS-6766 anti-PD-1 VS-6766 + anti-PD-1 20 40 60 80 100 0 20 40 60 80 100 Survival Time P e r c e n t s u r v i v a l VS-6766 + anti-PD-1 anti-PD-1 3 mg/kg 2xW x 4 doses VS-6766 0.5 mg/kg QD x 28 days Vehicle Day 11, Last dose anti - PD - 1 Day 28, Last dose VS - 6766 0 10 20 30 40 50 60 70 80 90 100 0 20 40 60 80 100 Survival Days after first dose P e r c e n t s u r v i v a l VS-6766 + anti-PD-1 anti-PD-1 3 mg/kg 2xW x 4 doses VS-6766 0.5 mg/kg QD x 28 days Vehicle 0 2 4 6 8 101214161820222426 0 200 400 600 800 1000 1200 Tumor growth Days after first dose T u m o r v o l u m e ( m m 3 + / - S E M ) Vehicle VS-6766 0.5 mg/kg QD anti-PD-1 3 mg/kg 2xW VS-6766 + anti-PD-1
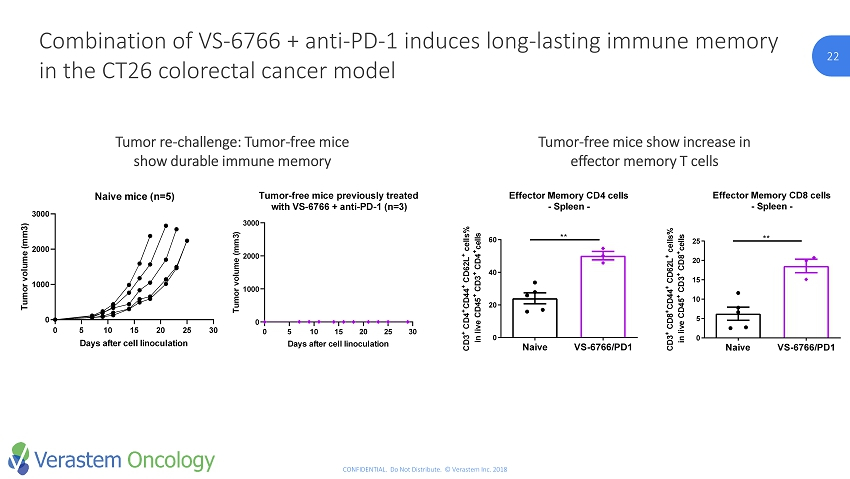
22 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 22 Combination of VS - 6766 + anti - PD - 1 induces long - lasting immune memory in the CT26 colorectal cancer model Tumor re - challenge: Tumor - free mice show durable immune memory Tumor - free mice show increase in effector memory T cells Effector Memory CD4 cells - Spleen - Naive VS-6766/PD1 0 20 40 60 C D 3 + C D 4 + C D 4 4 + C D 6 2 L + c e l l s % i n l i v e C D 4 5 + C D 3 + C D 4 + c e l l s ** Effector Memory CD8 cells - Spleen - Naive VS-6766/PD1 0 5 10 15 20 25 C D 3 + C D 8 + C D 4 4 + C D 6 2 L + c e l l s % i n l i v e C D 4 5 + C D 3 + C D 8 + c e l l s ** 0 5 10 15 20 25 30 0 1000 2000 3000 Naive mice (n=5) Days after cell linoculation T u m o r v o l u m e ( m m 3 ) 0 5 10 15 20 25 30 0 1000 2000 3000 Tumor-free mice previously treated with VS-6766 + anti-PD-1 (n=3) Days after cell linoculation T u m o r v o l u m e ( m m 3 )
23 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 23 VS - 6766 Combinations to Overcome Resistance Mechanisms: Conclusions ▪ Combination of dual RAF/MEK inhibitor VS - 6766 + FAKi may yield more complete RAS pathway shutdown o Synergy in cellular models with tumor regression in vivo o Clinical activity in KRAS mt ovarian cancer & KRAS G12V mt NSCLC patients ▪ Combination of RAF/MEK inhibitor VS - 6766 is synergistic with KRAS - G12C inhibitors across G12C mt NSCLC & CRC cell lines o Strong & durable inhibition of pERK pathway signaling o Tumor regressions in KRAS G12C mt NSCLC models in vivo o In KRAS G12C NSCLC models, triple combination of G12Ci + VS - 6766 + FAKi yields PRs in all mice ▪ Combination of RAF/MEK inhibitor with anti - PD - 1 yields enhanced efficacy & immune memory in vivo o VS - 6766 increases MHC - I expression across KRAS & BRAF mt cell lines
24 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 24 VS - 6766 Combinations: Next Steps ▪ Registration - directed phase II study of VS - 6766 + defactinib vs. VS - 6766 monotherapy in Low Grade Serous Ovarian Cancer ▪ Registration - directed phase II study of VS - 6766 + defactinib vs. VS - 6766 monotherapy in KRAS - G12V mt NSCLC ▪ Potential for a combination study of VS - 6766 ц defactinib with a G12C inhibitor in patients with KRAS - G12C mt NSCLC and/or CRC

25 CONFIDENTIAL. Do Not Distribute. © Verastem Inc. 2018 Acknowledgments Verastem Oncology ▪ Silvia Coma ▪ David Weaver Emory University ▪ C. Ronald Funk ▪ Edmund Waller Thanks for your attention! Acknowledgments Verastem Oncology ▪ Silvia Coma ▪ Sanjib Chowdhury ▪ Winnie Tam Royal Marsden Hospital ▪ Udai Banerji Thanks for your attention! UCSD ▪ J. Silvio Gutkind
























