Exhibit 99.3
Updated Results from the Phase 1/2 FRAME Study in Low - Grade Serous Ovarian Cancer September 16, 2020 NASDAQ: VSTM

2 On Today’s Call John Doyle, Vice President, Investor Relations and Finance Dan Paterson, Chief Operating Officer Rachel Grisham, MD, Medical Oncologist, Memorial Sloan Kettering Cancer Cente r ; Brian Stuglik, Chief Executive Officer Rob Gagnon, Chief Financial Officer Prepared Remarks Jonathan Pachter, PhD, Chief Scientific Officer Joining for Q&A Session

3 This presentation includes forward - looking statements about, among other things, Verastem Oncology’s products and product candidates, including anticipated regulatory submissions, approvals, performance and potential benefits of Verastem Oncology products and product candidates, that are subject to substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements. Applicable risks and uncertainties include the risks and uncertainties, among other things, regarding: the satisfaction of closing conditions with respect to the sale of the COPIKTRA assets to Secura Bio; the ability of Secura Bio to achieve the clinical and sales milestones necessary to result in additional consideration payable to Verastem. Additional information regarding these factors can be found in Verastem Oncology’s Annual Report on Form 10 - K for the fiscal year ended December 31, 2019 and in any subsequent filings with the SEC, including in the sections thereof captioned “Risk Factors” and “Forward - Looking Information and Factors that May Affect Future Results,” as well as in our subsequent reports on Form 8 - K, all of which are filed with the U.S. Securities and Exchange Commission (SEC) and available at www.sec.gov and www.verastem.com. The forward - looking statements in this presentation speak only as of the original date of this presentation, and we undertake no obligation to update or revise any of these statements. Safe Harbor Statement

Updated Phase 1/2 FRAME Study Data in Low - Grade Serous Ovarian Cancer Dan Paterson President and Chief Operating Officer
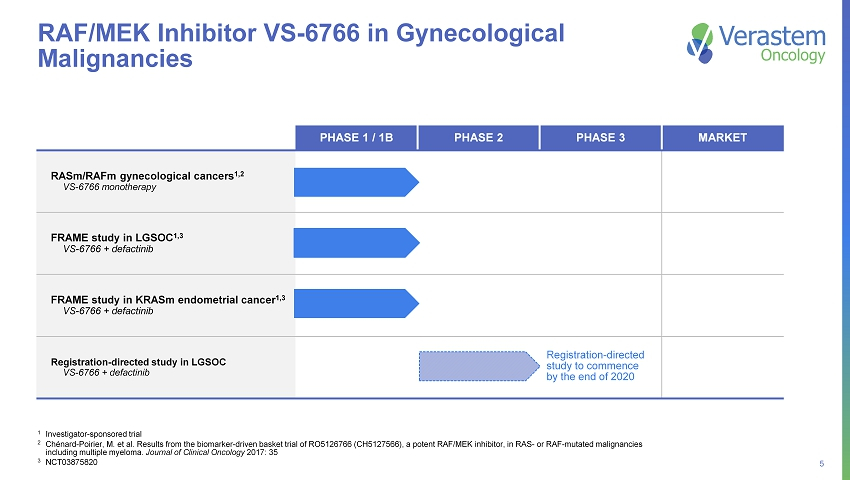
5 PHASE 1 / 1B PHASE 2 PHASE 3 MARKET RASm/RAFm gynecological cancers 1,2 VS - 6766 monotherapy FRAME study in LGSOC 1,3 VS - 6766 + defactinib FRAME study in KRASm endometrial cancer 1,3 VS - 6766 + defactinib Registration - directed study in LGSOC VS - 6766 + defactinib RAF/MEK Inhibitor VS - 6766 in Gynecological Malignancies 1 Investigator - sponsored trial 2 Chénard - Poirier, M. et al. Results from the biomarker - driven basket trial of RO5126766 (CH5127566), a potent RAF/MEK inhibitor, in RAS - or RAF - mutated malignancies including multiple myeloma. Journal of Clinical Oncology 2017: 35 3 NCT03875820 Registration - directed study to commence by the end of 2020

6 Favorable Tolerability Profile for Novel Intermittent Dosing Regimen of VS - 6766 plus Defactinib Daily at MTD N=6 28 - day cycle 4mg twice weekly N=26 28 - day cycle RP2D (VS - 6766 3.2mg twice weekly + defactinib 200mg twice daily) N=26 21 days of 28 - day cycle Adverse Event Grade ≥3 Grade ≥3 Grade ≥3 Rash related 3 (50%) 5 (19%) 1 (4%) CK elevation 1 (17%) 2 (8%) 1 (4%) Blurred vision - - - Peripheral edema - - - Diarrhea - 1 (4%) - Mucositis - 1 (4%) - Fatigue - 1 (4%) - Nausea - - - 1 Chenard - Poirier, et al . ASCO 2017.
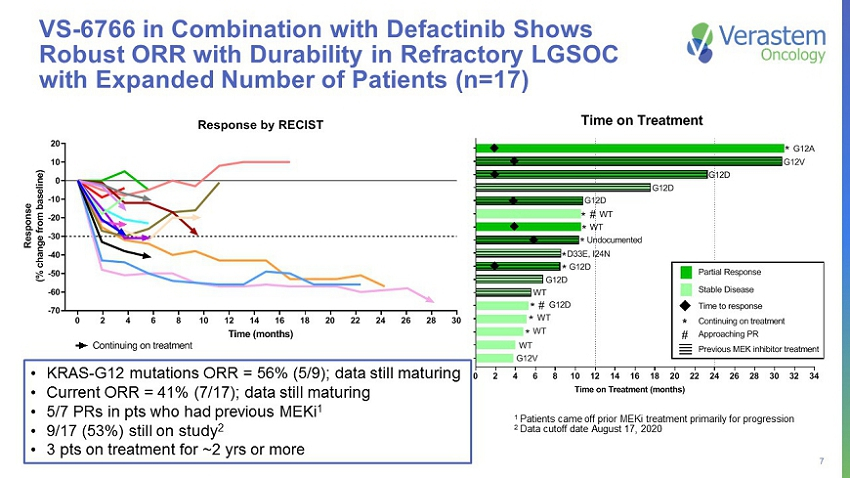
7 VS - 6766 in Combination with Defactinib Shows Robust ORR with Durability in Refractory LGSOC with Expanded Number of Patients (n=17) 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 -70 -60 -50 -40 -30 -20 -10 0 10 20 Response by RECIST Time (months) R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) FRA101001 - KRAS G12V FRA101002 - KRAS G12A FRA101009 - KRAS G12D FRA101012 - KRAS WT FRA101007 - KRAS WT FRA101014 - KRAS G12D FRA101015 - KRAS - G12V FRA101019 - KRAS G12D FRA101024 - KRAS WT FRA101025 - KRAS WT FRA102010 - KRAS G12D FRA101028 - Undocumented FRA101032 - KRAS D33E, I24N FRA101033 - KRAS G12D FRA101035 - KRAS G12D FRA101037 - KRAS WT FRA101038 - KRAS WT Continuing on treatment 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 FRA101015 FRA101012 FRA101038 FRA101037 FRA101035 FRA101007 FRA102010 FRA101033 FRA101032 FRA101028 FRA101024 FRA101025 FRA101019 FRA101014 FRA101009 FRA101001 FRA101002 Time on Treatment (months) * * * * * # * * * # * G12A G12V G12D G12D G12D WT WT Undocumented D33E, I24N G12D G12D WT G12D WT WT WT G12V Time on Treatment Previous MEK inhibitor treatment Continuing on treatment * Approaching PR # Time to response Partial Response Stable Disease • KRAS - G12 mutations ORR = 56% (5/9); data still maturing • Current ORR = 41% (7/17); data still maturing • 5/7 PRs in pts who had previous MEKi 1 • 9/17 (53%) still on study 2 • 3 pts on treatment for ~2 yrs or more 1 Patients came off prior MEKi treatment primarily for progression 2 Data cutoff date August 17, 2020
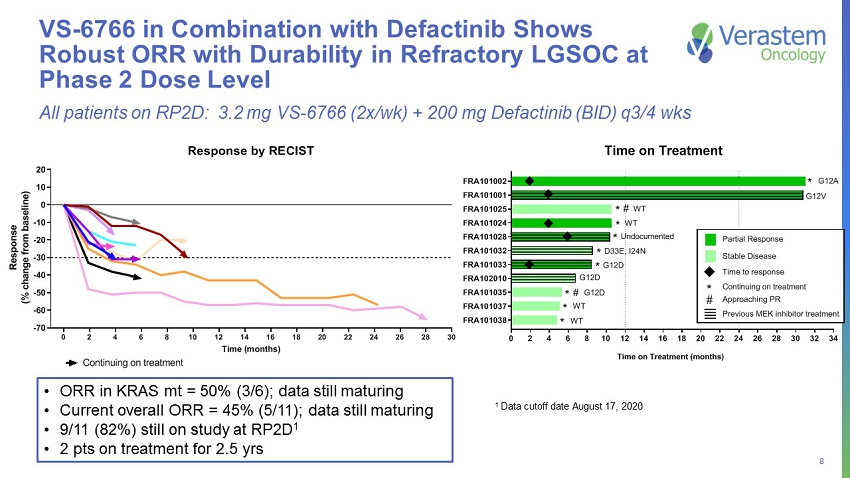
8 VS - 6766 in Combination with Defactinib Shows Robust ORR with Durability in Refractory LGSOC at Phase 2 Dose Level • ORR in KRAS mt = 50% (3/6); data still maturing • Current overall ORR = 45% (5/11); data still maturing • 9/11 (82%) still on study at RP2D 1 • 2 pts on treatment for 2.5 yrs 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 FRA101038 FRA101037 FRA101035 FRA102010 FRA101033 FRA101032 FRA101028 FRA101024 FRA101025 FRA101001 FRA101002 Time on Treatment (months) * * * * * # * * * # * G12A G12V WT WT Undocumented D33E, I24N G12D G12D G12D WT WT Time on Treatment Previous MEK inhibitor treatment Continuing on treatment * Approaching PR # Time to response Partial Response Stable Disease 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 -70 -60 -50 -40 -30 -20 -10 0 10 20 Response by RECIST Time (months) R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) FRA101001 - KRAS G12V FRA101002 - KRAS G12A FRA101024 - KRAS WT FRA101025 - KRAS WT FRA102010 - KRAS G12D FRA101028 - Undocumented FRA101032 - KRAS D33E, I24N FRA101033- KRAS G12D FRA101035 - KRAS G12D FRA101037 - KRAS WT FRA101038 - KRAS WT Continuing on treatment All patients on RP2D: 3.2 mg VS - 6766 (2x/wk) + 200 mg Defactinib (BID) q3/4 wks 1 Data cutoff date August 17, 2020

9 KRAS Mutated (mt) and Wild Type (wt), Phase 2, Recurrent LGSOC Adaptive Design for Potential Accelerated Approval * Selection Phase – KRAS mt only ** Expansion Phase – final sample size to be adjusted based on adaptive design FDA Was Supportive of Development Strategy and Adaptive Design This Registration - directed Phase 2 Study is Expected to Commence by Year End 2020 Recurrent LGSOC Measurable disease (RECIST 1.1) Prior MEKi allowed Approximately 32 subjects Primary Endpoint ORR Hierarchical evaluation of: 1) In KRAS mt subjects 2) All subjects (KRASmt & wt) Defactinib+VS - 6766 Defactinib 200 mg PO BID 21/28 days + VS - 6766 3.2 mg PO 2x/wk 21/28 days VS - 6766 Mono VS - 6766 4.0 mg PO 2x/wk 21/28 days Selected Regimen based on ORR Additional 20 - 30 subjects with KRAS mt Additional 36 - 56 subjects with KRAS wt Total Range of Subjects: 88 - 118 Selection Phase* Expansion Phase**

Low - Grade Serous Ovarian Cancer Treatment Landscape and Clinical Perspective Rachel Grisham, MD Memorial Sloan Kettering Cancer Center
11 What is Low - Grade Serous Ovarian Cancer (LGSOC)? LGSOC is a type of ovarian cancer that disproportionately affects younger women Patients often experience significant pain and suffering from their disease over time A slow growing cancer, that has a median survival of almost 10 years, so patients remain in treatment for a long time Source: Monk, Randall, Grisham, The Evolving Landscape of Chemotherapy in Newly Diagnosed Advanced Epithelial Ovarian Cancer, Am Soc Cli n Oncol Educ Book; 2019; Slomovitz, Gourley, Carey, Malpica, Shih, Huntsman, Fader., Grisham et al, Low - Grade serous ovarian cancer: State of the Science; Gynecol Oncol; 202 0. Grisham, Iyer, Low - Grade Serous Ovarian Cancer: Current Treatment Paradigms and Future Directions; Curr Treat Options Oncology; 2018. Most prior research has focused on high grade serous ovarian cancer (HGSOC). However, LGSOC is clinically, histologically and molecularly unique from HGSOC with limited treatments available 1,000 to 2,000 patients in the U.S. and 15,000 to 30,000 worldwide diagnosed with LGSOC each year

12 Treatment Landscape and Clinical Perspective Rachel Grisham, MD Memorial Sloan Kettering Cancer Center Bio: Dr. Grisham is a medical oncologist with clinical expertise in the diagnosis and treatment of women with gynecologic malignan ci es including ovarian, uterine, and cervical cancers as well as other less common tumors. Her clinical research focuses on develo pin g novel treatments and improving treatment strategies for women with gynecologic malignancies. She has a particular interest in th e use of targeted therapies for the treatment of recurrent ovarian cancer. She has developed, and serves as the principal investiga tor for, ongoing clinical trials in this area. Dr. Grisham earned her M.D. degree from Duke University School of Medicine. She comple ted her residency at Massachusetts General Hospital and subsequently held fellowships at Weill Cornell Medical College and Memorial S loa n Kettering Cancer Center.

Low - Grade Serous Ovarian Cancer Market Opportunity Brian Stuglik Chief Executive Officer
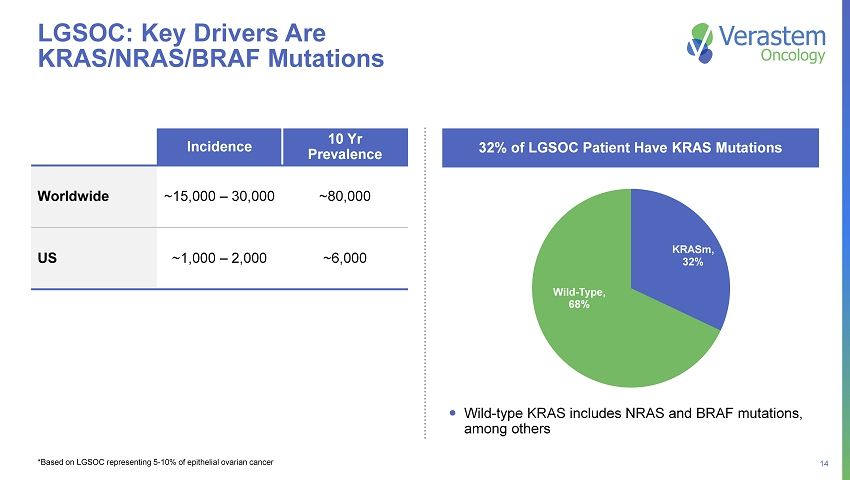
14 KRASm , 32% Wild - Type , 68% Incidence 10 Yr Prevalence Worldwide ~15,000 – 30,000 ~80,000 US ~1,000 – 2,000 ~6,000 32% of LGSOC Patient Have KRAS Mutations LGSOC: Key Drivers Are KRAS/NRAS/BRAF Mutations *Based on LGSOC representing 5 - 10% of epithelial ovarian cancer Wild - type KRAS includes NRAS and BRAF mutations, among others
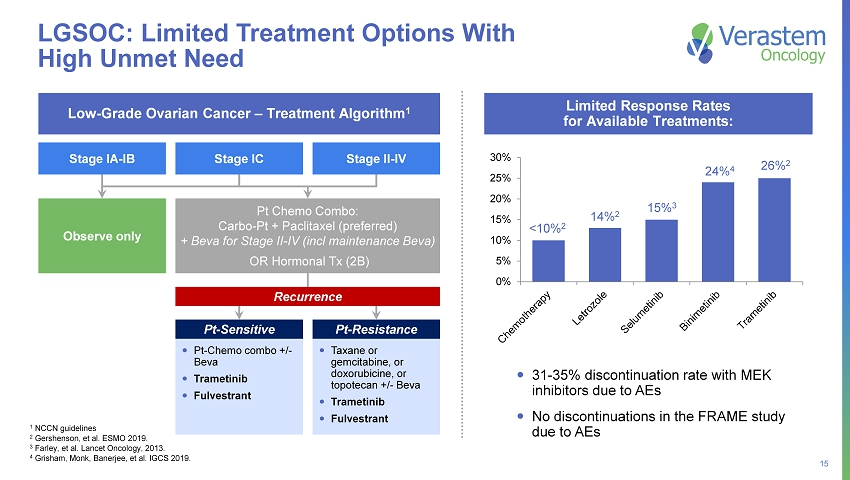
15 31 - 35% discontinuation rate with MEK inhibitors due to AEs No discontinuations in the FRAME study due to AEs Low - Grade Ovarian Cancer – Treatment Algorithm 1 Observe only Pt Chemo Combo: Carbo - Pt + Paclitaxel (preferred) + Beva for Stage II - IV (incl maintenance Beva) OR Hormonal Tx (2B) Pt - Chemo combo +/ - Beva Trametinib Fulvestrant Recurrence Taxane or gemcitabine, or doxorubicine, or topotecan +/ - Beva Trametinib Fulvestrant LGSOC: Limited Treatment Options With High Unmet Need 1 NCCN guidelines 2 Gershenson, et al. ESMO 2019. 3 Farley, et al. Lancet Oncology, 2013. 4 Grisham, Monk, Banerjee, et al. IGCS 2019. Pt - Sensitive Pt - Resistance Limited Response Rates for Available Treatments: 0% 5% 10% 15% 20% 25% 30% Stage IC Stage II - IV Stage IA - IB <10% 2 14% 2 15% 3 26% 2 24% 4
16 Commence Phase 2 registration - directed trial by the end of 2020 Report updated data from FRAME LGSOC cohort in mid - 2021 Validating Clinical Data in LGSOC VS - 6766 ± Defactinib Represents Best in Class Market Opportunity in LGSOC 1 AACR Project Genie, cBioportal KRAS mutations account for 32% 1 of LGSOC cases No FDA - approved therapy; limited treatment options Unmet medical need creates large market opportunity ~6,000 patients living with the disease; ultra - orphan opportunity FRAME study: 56% ORR in KRAS - G12m LGSOC and 41% ORR in overall LGSOC represents best - in - class opportunity FDA supportive of development strategy and registration trial design Key Takeaways Next Steps

Other Program Updates Brian Stuglik Chief Executive Officer
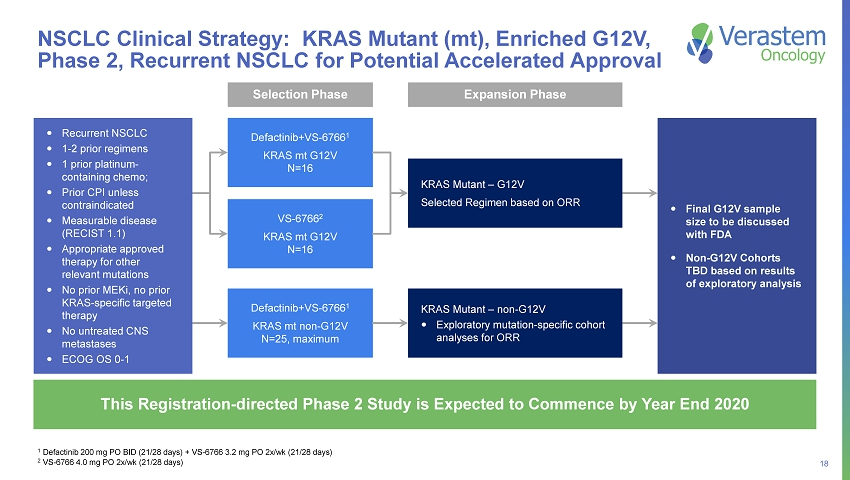
18 NSCLC Clinical Strategy: KRAS Mutant (mt), Enriched G12V, Phase 2, Recurrent NSCLC for Potential Accelerated Approval 1 Defactinib 200 mg PO BID (21/28 days) + VS - 6766 3.2 mg PO 2x/wk (21/28 days) 2 VS - 6766 4.0 mg PO 2x/wk (21/28 days) This Registration - directed Phase 2 Study is Expected to Commence by Year End 2020 Recurrent NSCLC 1 - 2 prior regimens 1 prior platinum - containing chemo; Prior CPI unless contraindicated Measurable disease (RECIST 1.1) Appropriate approved therapy for other relevant mutations No prior MEKi, no prior KRAS - specific targeted therapy No untreated CNS metastases ECOG OS 0 - 1 Defactinib+VS - 6766 1 KRAS mt G12V N=16 VS - 6766 2 KRAS mt G12V N=16 KRAS Mutant – G12V Selected Regimen based on ORR Selection Phase Expansion Phase Defactinib+VS - 6766 1 KRAS mt non - G12V N=25, maximum KRAS Mutant – non - G12V Exploratory mutation - specific cohort analyses for ORR Final G12V sample size to be discussed with FDA Non - G12V Cohorts TBD based on results of exploratory analysis
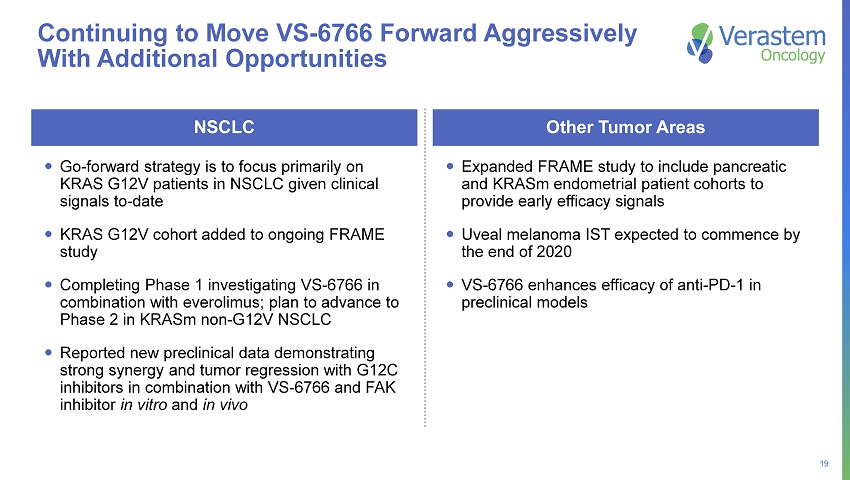
19 Continuing to Move VS - 6766 Forward Aggressively With Additional Opportunities Go - forward strategy is to focus primarily on KRAS G12V patients in NSCLC given clinical signals to - date KRAS G12V cohort added to ongoing FRAME study Completing Phase 1 investigating VS - 6766 in combination with everolimus; plan to advance to Phase 2 in KRASm non - G12V NSCLC Reported new preclinical data demonstrating strong synergy and tumor regression with G12C inhibitors in combination with VS - 6766 and FAK inhibitor in vitro and in vivo NSCLC Expanded FRAME study to include pancreatic and KRASm endometrial patient cohorts to provide early efficacy signals Uveal melanoma IST expected to commence by the end of 2020 VS - 6766 enhances efficacy of anti - PD - 1 in preclinical models Other Tumor Areas
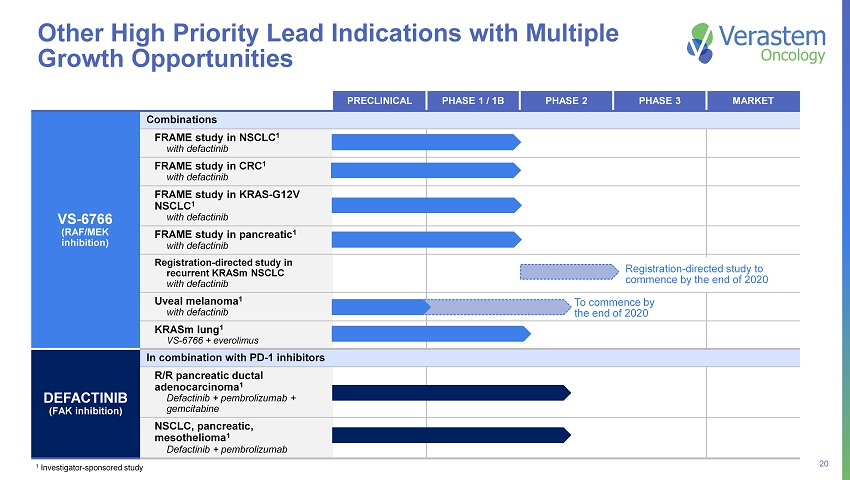
20 PRECLINICAL PHASE 1 / 1B PHASE 2 PHASE 3 MARKET Combinations FRAME study in NSCLC 1 with defactinib FRAME study in CRC 1 with defactinib FRAME study in KRAS - G12V NSCLC 1 with defactinib FRAME study in pancreatic 1 with defactinib Registration - directed study in recurrent KRASm NSCLC with defactinib Uveal melanoma 1 with defactinib KRASm lung 1 VS - 6766 + everolimus DEFACTINIB (FAK inhibition) In combination with PD - 1 inhibitors R/R pancreatic ductal adenocarcinoma 1 Defactinib + pembrolizumab + gemcitabine NSCLC, pancreatic, mesothelioma 1 Defactinib + pembrolizumab Other High Priority Lead Indications with Multiple Growth Opportunities 1 Investigator - sponsored study VS - 6766 (RAF/MEK inhibition ) Registration - directed study to commence by the end of 2020 To commence by the end of 2020

Corporate Rob Gagnon Chief Financial Officer

22 Selling COPIKTRA ® (duvelisib) Rights to Secura Bio Secura Bio to Assume All Operational and Financial Responsibilities, Including Existing Royalty Obligations Beginning in 2021 Annual OPEX Expected to be ~$50 Million New Verastem Headcount of ~50 Provides Cash Runway Through at Least 2024 Total Deal Value Up to $311 Million, Plus Royalties
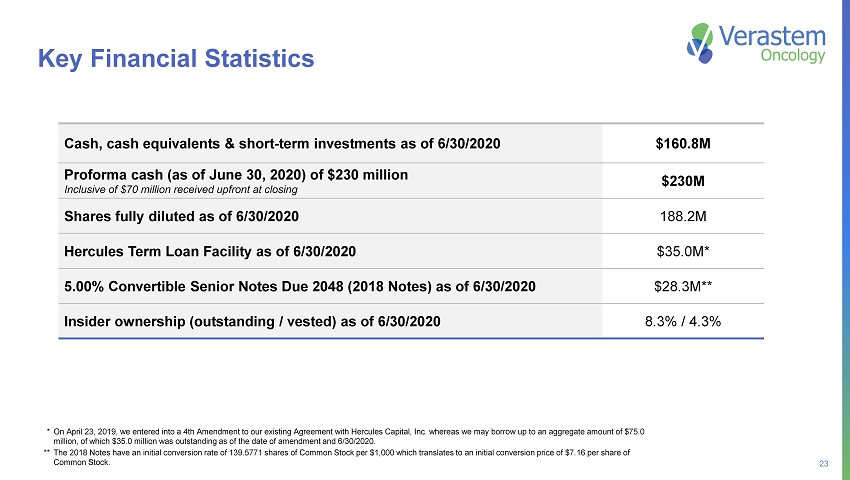
23 Key Financial Statistics * On April 23, 2019, we entered into a 4th Amendment to our existing Agreement with Hercules Capital, Inc. whereas we may borro w up to an aggregate amount of $75.0 million, of which $35.0 million was outstanding as of the date of amendment and 6/30/2020. ** The 2018 Notes have an initial conversion rate of 139.5771 shares of Common Stock per $1,000 which translates to an initial conversion price of $7.16 per share of Common Stock. Cash, cash equivalents & short - term investments as of 6/30/2020 $160.8M Proforma cash (as of June 30, 2020) of $230 million Inclusive of $70 million received upfront at closing $230M Shares fully diluted as of 6/30/2020 188.2M Hercules Term Loan Facility as of 6/30/2020 $35.0M* 5.00% Convertible Senior Notes Due 2048 (2018 Notes) as of 6/30/2020 $28.3M** Insider ownership (outstanding / vested) as of 6/30/2020 8.3% / 4.3%
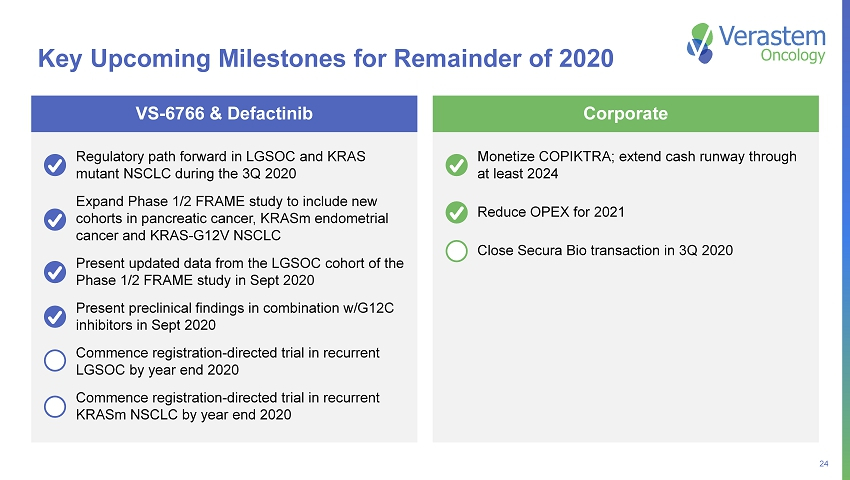
24 Monetize COPIKTRA; extend cash runway through at least 2024 Reduce OPEX for 2021 Close Secura Bio transaction in 3Q 2020 Regulatory path forward in LGSOC and KRAS mutant NSCLC during the 3Q 2020 Expand Phase 1/2 FRAME study to include new cohorts in pancreatic cancer, KRASm endometrial cancer and KRAS - G12V NSCLC Present updated data from the LGSOC cohort of the Phase 1/2 FRAME study in Sept 2020 Present preclinical findings in combination w/G12C inhibitors in Sept 2020 Commence registration - directed trial in recurrent LGSOC by year end 2020 Commence registration - directed trial in recurrent KRASm NSCLC by year end 2020 Key Upcoming Milestones for Remainder of 2020 Corporate VS - 6766 & Defactinib

Thank you www.verastem.com Questions and Answers
























