Exhibit 99.1

In Collaboration With Efficacy and Safety of Avutometinib ± Defactinib in Recurrent Low - Grade Serous Ovarian Cancer: Primary Analysis of ENGOT - OV60/GOG - 3052/RAMP 201 Susana N. Banerjee , Carol Aghajanian, Els Van Nieuwenhuysen, Alessandro D. Santin, Kari L. Ring, Nicoletta Colombo, Premal H. Thaker, Emily N. Prendergast, Kathleen N. Moore, Hye Sook Chon, Andrew R. Clamp, David M. O’Malley, Bradley J. Monk, Alfonso Cortés Salgado, Michel Fabbro, Elsa Kalbacher, Toon Van Gorp, Stephanie Lustgarten, Hagop Youssoufian, Rachel N. Grisham

IGCS | 2024 Annual Global Meeting Disclosure No, nothing to disclose Yes, please specify: x Other (please specify) Employee Ownership/ Equity Position Stock Options Royalties/ Patent Funded Research Consulting/ Advisory Board Honoraria/ Expenses Company Name x AbbVie, AstraZeneca, BioNTech, Eisai, Gilead, GlaxoSmithKline, Immunogen, Incyte, ITM Oncologics , Merck Sharpe Dohme, Mersana, Myriad, Oncxerna , Pharma&, Seagen , Verastem, Zymeworks x AbbVie, AstraZeneca, GlaxoSmithKline, Immunogen, Merck Sharpe Dohme, Mersana, Takeda, Verastem x Institution AstraZeneca, GlaxoSmithKline, Verastem (PI) 2
IGCS | 2024 Annual Global Meeting • LGSOC is a rare, histopathologically, molecularly, and clinically distinct cancer accounting for <10% of new epithelial ovarian cancers 1,2 • LGSOC is commonly driven by alterations in the RAS/MAPK pathway, including KRAS mutations, which occur in approximately 30% of patients 3,4 • Molecular alterations may influence patient outcomes • KRAS mutations/MAPK alterations are associated with improved prognosis 1,5,6 • Chemotherapy options have shown limited efficacy in LGSOC (ORR 0% – 13%) 5,7 • Response rates of 26% and 16% were observed with trametinib and binimetinib , respectively, but with discontinuation rates of 36% and 31% due to toxicity 5,7 3 New Treatment Options Are Needed for Patients With LGSOC KRAS, kirsten rat sarcoma virus; LGSOC, low - grade serous ovarian cancer; MAPK, mitogen - activated protein kinase; ORR, objective response rate. 1 . Grisham RN, et al. Int J Gynecol Cancer. 2023;33(9):1331 - 1344; 2 . Matsuo K, et al. J Gynecol Oncol . 2018;29(1 a ):e15 ; 3 . Manning - Geist B, et al. Clin Cancer Res . 2022;28(20)4456 - 4465 ; 4 . ElNaggar A, et al. Gynecol Oncol. 2022;167(2):306 - 313; 5 . Gershenson DM, et al. Lancet. 2022;399(10324):541 - 553; 6 . Manning - Geist BL, et al. Clin Adv Hematol Oncol . 2024:22(5):205 - 226; 7 . Monk BJ, et al. J Clin Oncol. 2020;38(32):3753 - 3762.
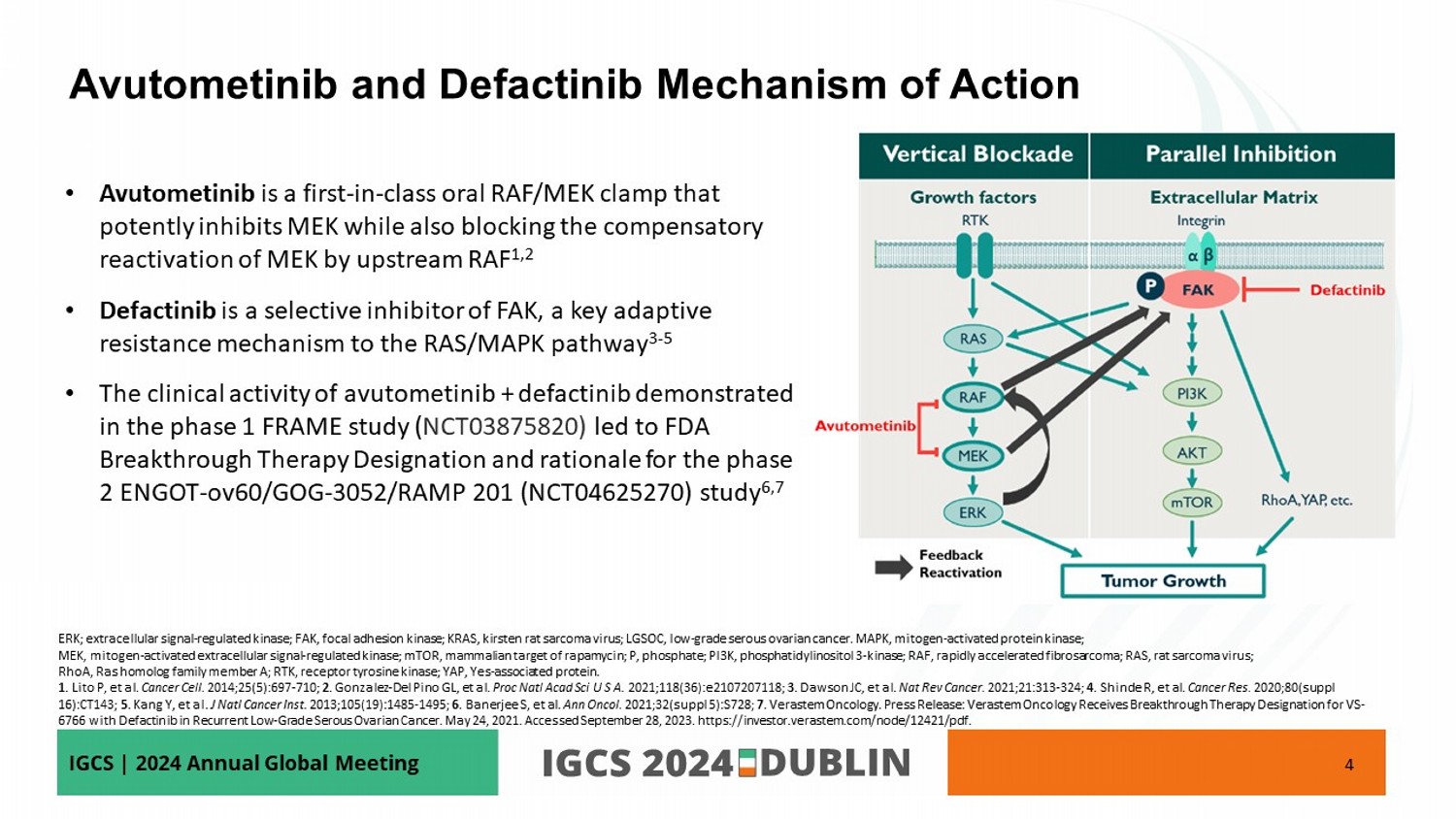
IGCS | 2024 Annual Global Meeting • Avutometinib is a first - in - class oral RAF/MEK clamp that potently inhibits MEK while also blocking the compensatory reactivation of MEK by upstream RAF 1,2 • Defactinib is a selective inhibitor of FAK, a key adaptive resistance mechanism to the RAS/MAPK pathway 3 - 5 • The clinical activity of avutometinib + defactinib demonstrated in the phase 1 FRAME study ( NCT03875820) led to FDA Breakthrough Therapy Designation and rationale for the phase 2 ENGOT - ov60/GOG - 3052/RAMP 201 (NCT04625270) study 6,7 4 Avutometinib and Defactinib Mechanism of Action ERK; extracellular signal - regulated kinase; FAK, focal adhesion kinase; KRAS, kirsten rat sarcoma virus; LGSOC, low - grade serous ovarian cancer . MAPK, mitogen - activated protein kinase ; MEK, mitogen - activated extracellular signal - regulated kinase; mTOR, mammalian target of rapamycin; P, phosphate; PI3K, phosphati dylinositol 3 - kinase; RAF, rapidly accelerated fibrosarcoma; RAS, rat sarcoma virus; RhoA , Ras homolog family member A; RTK, receptor tyrosine kinase; YAP, Yes - associated protein. 1 . Lito P, et al. Cancer Cell. 2014;25(5):697 - 710; 2 . Gonzalez - Del Pino GL, et al. Proc Natl Acad Sci U S A . 2021;118(36):e2107207118; 3 . Dawson JC, et al. Nat Rev Cancer. 2021;21:313 - 324; 4 . Shinde R, et al. Cancer Res. 2020;80(suppl 16):CT143; 5 . Kang Y, et al. J Natl Cancer Inst. 2013;105(19):1485 - 1495; 6 . Banerjee S, et al. Ann Oncol. 2021;32( suppl 5):S728 ; 7 . Verastem Oncology. Press Release: Verastem Oncology Receives Breakthrough Therapy Designation for VS - 6766 with Defactinib in Recurrent Low - Grade Serous Ovarian Cancer. May 24, 2021. Accessed September 28, 2023. https://investor.v erastem.com/node/12421/pdf.
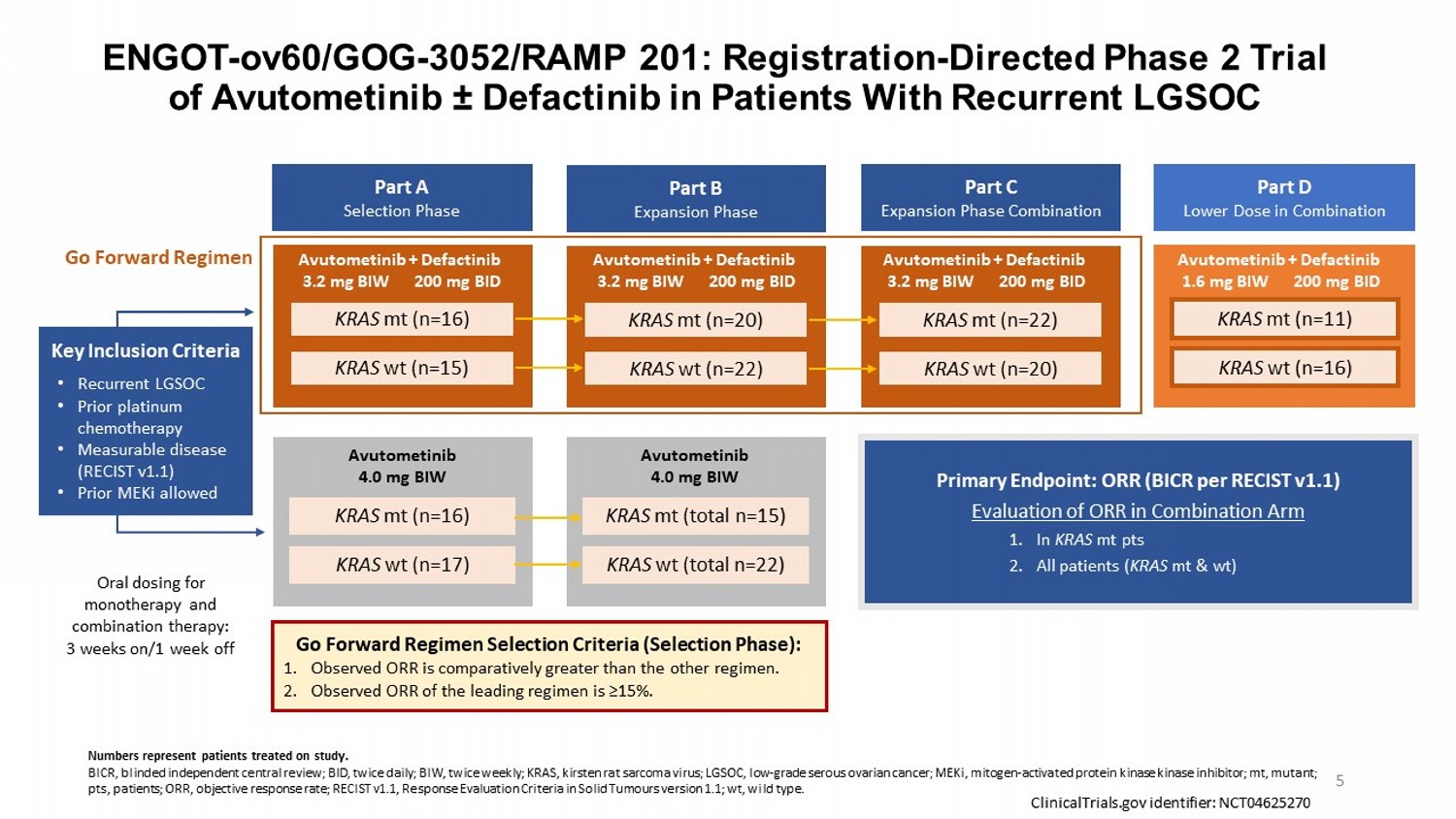
ENGOT - ov60/GOG - 3052/RAMP 201: Registration - Directed Phase 2 Trial of Avutometinib “ Defactinib in Patients With Recurrent LGSOC Part A Selection Phase Part B Expansion Phase Part C Expansion Phase Combination Part D Lower Dose in Combination Key Inclusion Criteria • Recurrent LGSOC • Prior platinum chemotherapy • Measurable disease (RECIST v1.1) • Prior MEKi allowed KRAS mt (n=16) KRAS wt (n=15) KRAS wt (n=17) KRAS mt (n=16) KRAS mt (n=20) KRAS wt (n=22) KRAS mt (total n=15) KRAS wt (total n=22) KRAS mt (n=22) KRAS wt (n=20) KRAS mt (n=11) KRAS wt (n=16) Primary Endpoint: ORR (BICR per RECIST v1.1) Evaluation of ORR in Combination Arm 1. In KRAS mt pts 2. All patients ( KRAS mt & wt) Go Forward Regimen Selection Criteria (Selection Phase): 1. Observed ORR is comparatively greater than the other regimen. 2. Observed ORR of the leading regimen is ≥15%. Go Forward Regimen ClinicalTrials.gov identifier: NCT04625270 Oral dosing for monotherapy and combination therapy: 3 weeks on/1 week off Avutometinib + Defactinib 3.2 mg BIW 200 mg BID Avutometinib + Defactinib 3.2 mg BIW 200 mg BID Avutometinib + Defactinib 3.2 mg BIW 200 mg BID Avutometinib + Defactinib 1.6 mg BIW 200 mg BID 5 Numbers represent patients treated on study. BICR, blinded independent central review; BID, twice daily; BIW, twice weekly; KRAS, kirsten rat sarcoma virus; LGSOC, low - grade serous ovarian cancer; MEKi , mitogen - activated protein kinase kinase inhibitor ; mt, mutant; pts, patients; ORR, objective response rate; RECIST v1.1, Response Evaluation Criteria in Solid Tumours version 1.1; wt , wild type. Avutometinib 4.0 mg BIW Avutometinib 4.0 mg BIW
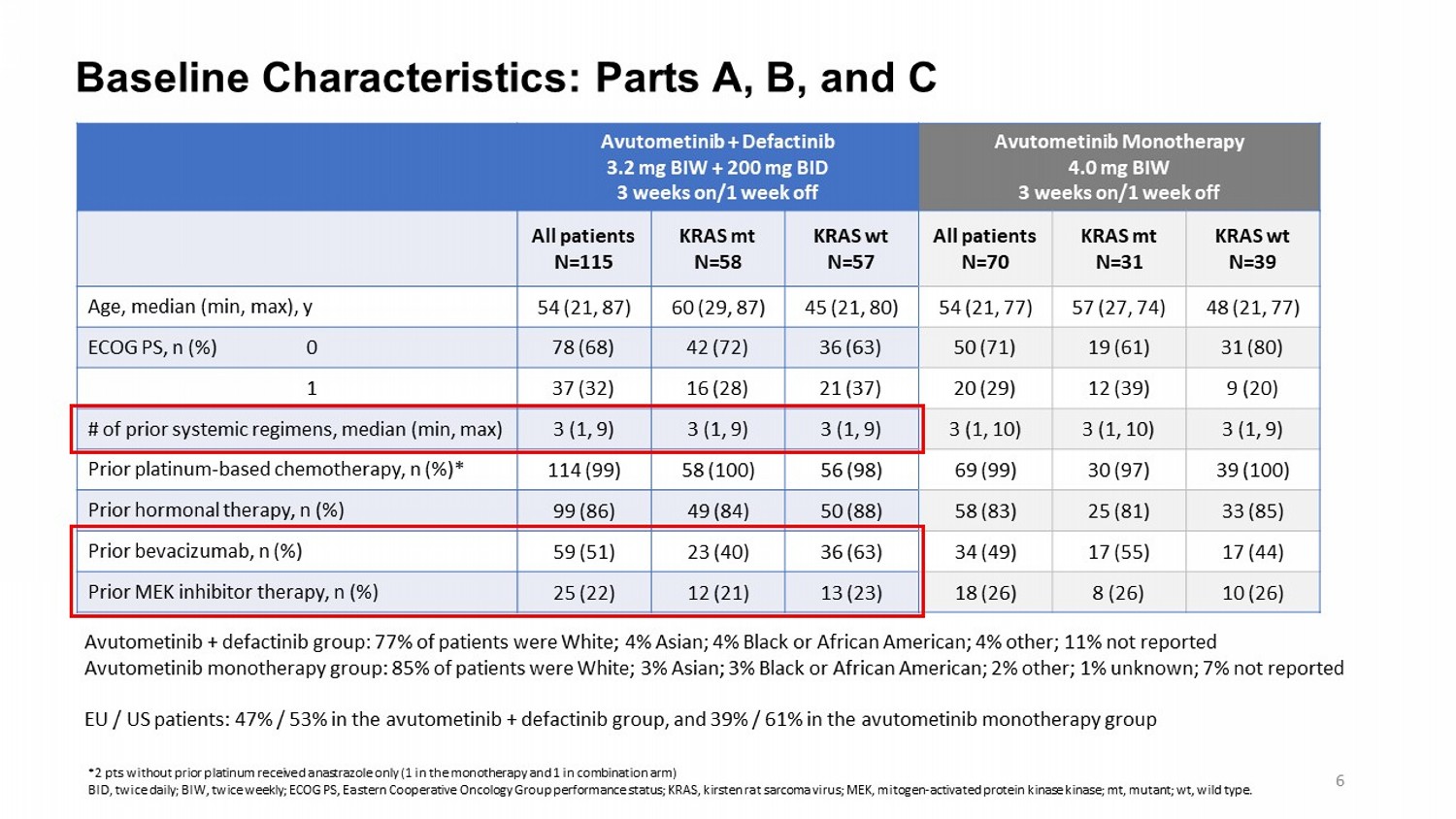
Baseline Characteristics: Parts A, B, and C Avutometinib Monotherapy 4.0 mg BIW 3 weeks on/1 week off Avutometinib + Defactinib 3.2 mg BIW + 200 mg BID 3 weeks on/1 week off KRAS wt N=39 KRAS mt N=31 All patients N=70 KRAS wt N=57 KRAS mt N=58 All patients N=115 48 (21, 77) 57 (27, 74) 54 (21, 77) 45 (21, 80) 60 (29, 87) 54 (21, 87) Age, median (min, max), y 31 (80) 19 (61) 50 (71) 36 (63) 42 (72) 78 (68) ECOG PS, n (%) 0 9 (20) 12 (39) 20 (29) 21 (37) 16 (28) 37 (32) 1 3 (1, 9) 3 (1, 10) 3 (1, 10) 3 (1, 9) 3 (1, 9) 3 (1, 9) # of prior systemic regimens, median (min, max) 39 (100) 30 (97) 69 (99) 56 (98) 58 (100) 114 (99) Prior platinum - based chemotherapy, n (%)* 33 (85) 25 (81) 58 (83) 50 (88) 49 (84) 99 (86) Prior hormonal therapy, n (%) 17 (44) 17 (55) 34 (49) 36 (63) 23 (40) 59 (51) Prior bevacizumab, n (%) 10 (26) 8 (26) 18 (26) 13 (23) 12 (21) 25 (22) Prior MEK inhibitor therapy, n (%) Avutometinib + defactinib group: 77% of patients were White; 4% Asian ; 4% Black or African American; 4% other; 11% not reported Avutometinib monotherapy group: 85 % of patients were White; 3% Asian ; 3% Black or African American; 2% other; 1% unknown; 7% not reported EU / US patients: 47% / 53% in the avutometinib + defactinib group, and 39% / 61% in the avutometinib monotherapy group *2 pts without prior platinum received anastrazole only (1 in the monotherapy and 1 in combination arm) BID, twice daily; BIW, twice weekly; ECOG PS, Eastern Cooperative Oncology Group performance status; KRAS, kirsten rat sarcoma virus; MEK, mitogen - activated protein kinase kinase ; mt, mutant; wt , wild type. 6
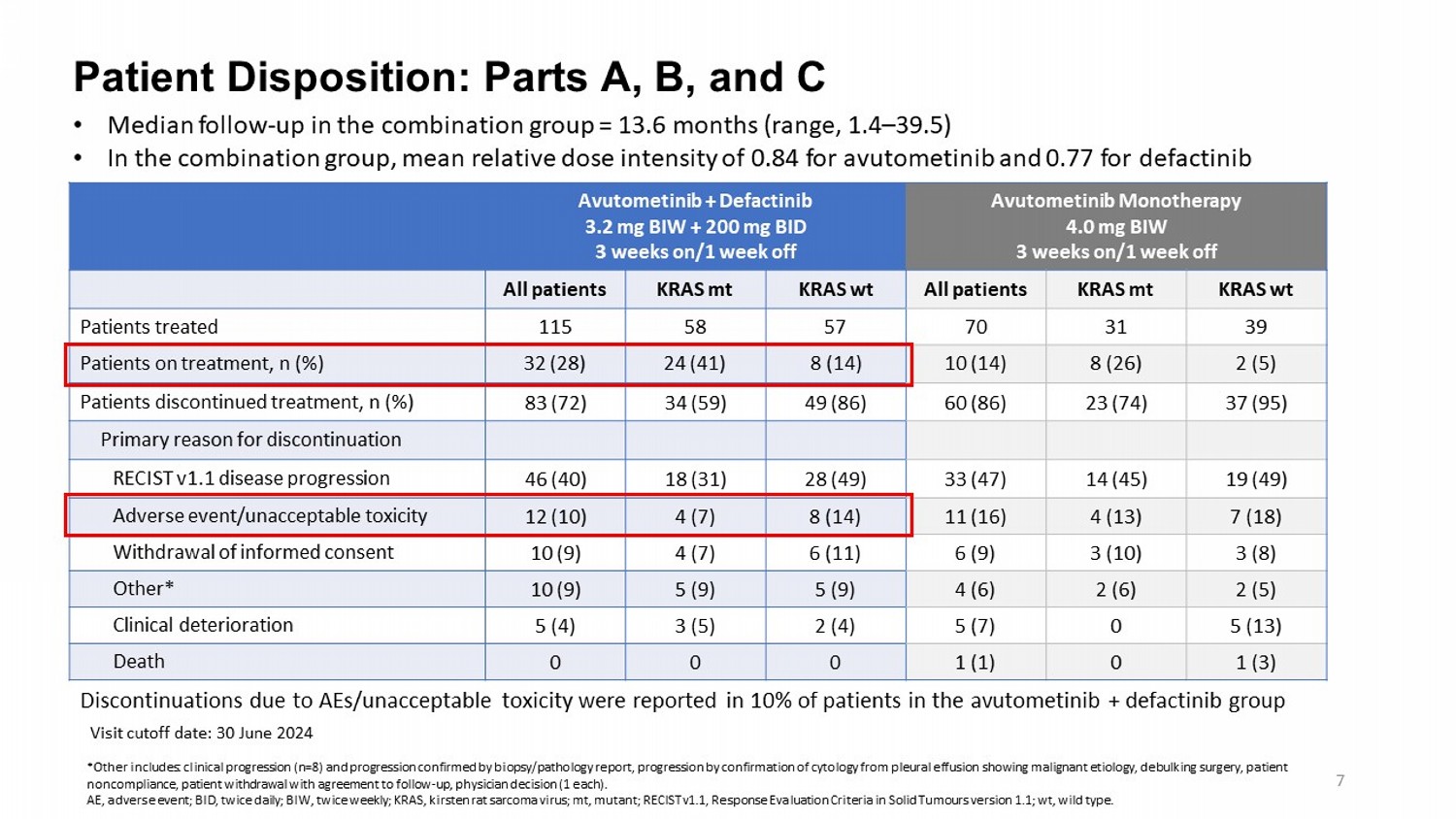
Patient Disposition: Parts A, B, and C Visit cutoff date: 30 June 2024 Avutometinib Monotherapy 4.0 mg BIW 3 weeks on/1 week off Avutometinib + Defactinib 3.2 mg BIW + 200 mg BID 3 weeks on/1 week off KRAS wt KRAS mt All patients KRAS wt KRAS mt All patients 39 31 70 57 58 115 Patients treated 2 (5) 8 (26) 10 (14) 8 (14) 24 (41) 32 (28) Patients on treatment, n (%) 37 (95) 23 (74) 60 (86) 49 (86) 34 (59) 83 (72) Patients discontinued treatment, n (%) Primary reason for discontinuation 19 (49) 14 (45) 33 (47) 28 (49) 18 (31) 46 (40) RECIST v1.1 disease progression 7 (18) 4 (13) 11 (16) 8 (14) 4 (7) 12 (10) Adverse event/unacceptable toxicity 3 (8) 3 (10) 6 (9) 6 (11) 4 (7) 10 (9) Withdrawal of informed consent 2 (5) 2 (6) 4 (6) 5 (9) 5 (9) 10 (9) Other* 5 (13) 0 5 (7) 2 (4) 3 (5) 5 (4) Clinical deterioration 1 (3) 0 1 (1) 0 0 0 Death Discontinuations due to AEs/unacceptable toxicity were reported in 10% of patients in the avutometinib + defactinib group • Median follow - up in the combination group = 13.6 months (range, 1.4 – 39.5) • In the combination group, mean relative dose intensity of 0.84 for avutometinib and 0.77 for defactinib 7 *Other includes: clinical progression (n=8) and progression confirmed by biopsy/pathology report, progression by confirmation of cytology from pleural effusion showing malignant etiology, debulking surgery, patient noncompliance, patient withdrawal with agreement to follow - up, physician decision (1 each). AE, adverse event; BID, twice daily; BIW, twice weekly ; KRAS, kirsten rat sarcoma virus; mt, mutant; RECIST v1.1, Response Evaluation Criteria in Solid Tumours version 1.1; wt , wild type .
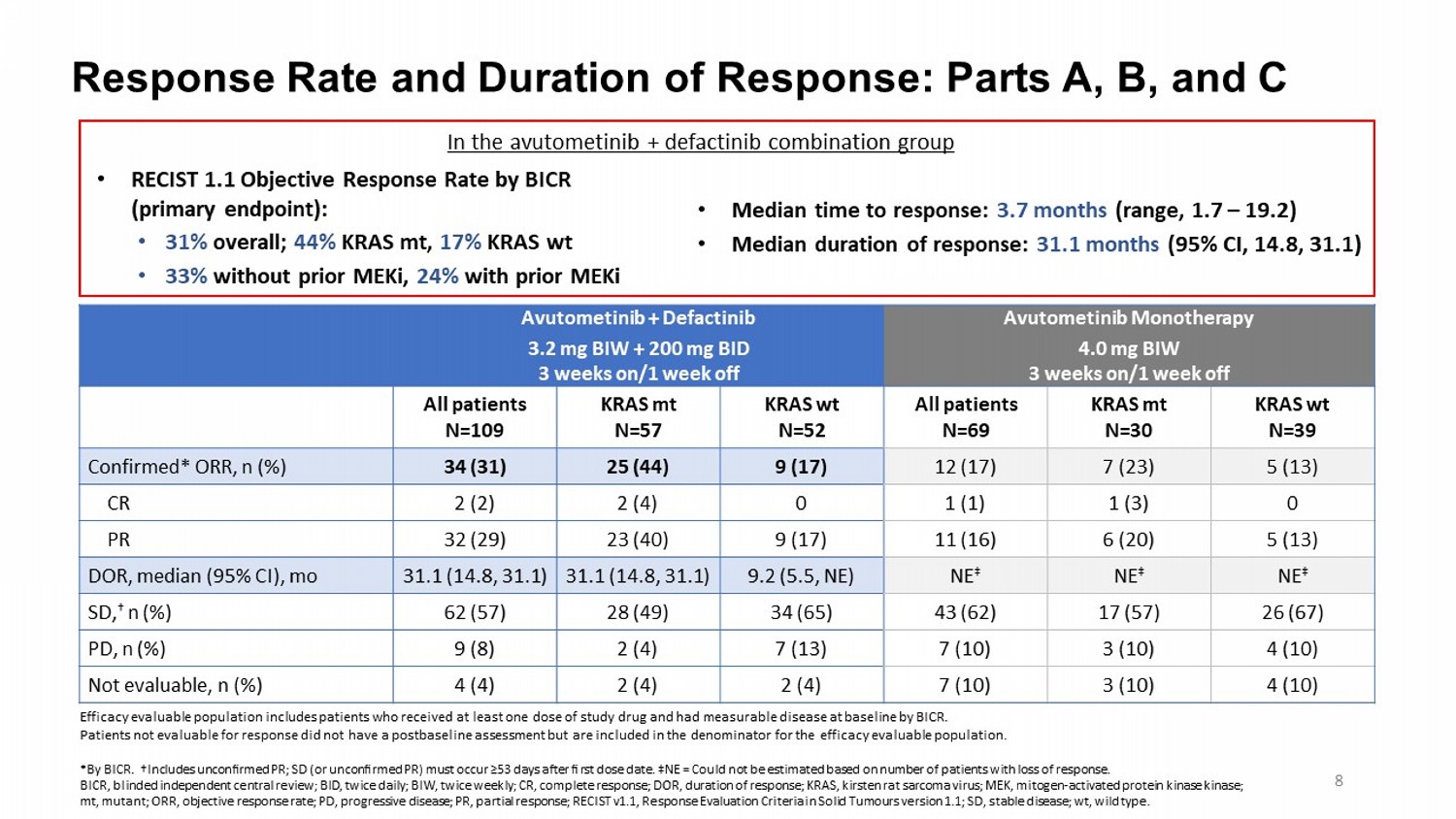
Response Rate and Duration of Response: Parts A, B, and C Avutometinib Monotherapy 4.0 mg BIW 3 weeks on/1 week off Avutometinib + Defactinib 3.2 mg BIW + 200 mg BID 3 weeks on/1 week off KRAS wt N=39 KRAS mt N=30 All patients N=69 KRAS wt N=52 KRAS mt N=57 All patients N=109 5 (13) 7 (23) 12 (17) 9 (17) 25 (44) 34 (31) Confirmed* ORR, n (%) 0 1 (3) 1 (1) 0 2 (4) 2 (2) CR 5 (13) 6 (20) 11 (16) 9 (17) 23 (40) 32 (29) PR NE ‡ NE ‡ NE ‡ 9.2 (5.5, NE) 31.1 (14.8, 31.1) 31.1 (14.8, 31.1) DOR, median (95% CI), mo 26 (67) 17 (57) 43 (62) 34 (65) 28 (49) 62 (57) SD, † n (%) 4 (10) 3 (10) 7 (10) 7 (13) 2 (4) 9 (8) PD, n (%) 4 (10) 3 (10) 7 (10) 2 (4) 2 (4) 4 (4) Not evaluable, n (%) Efficacy evaluable population includes patients who received at least one dose of study drug and had measurable disease at ba sel ine by BICR. Patients not evaluable for response did not have a postbaseline assessment but are included in the denominator for the effica cy evaluable population. *By BICR. †Includes unconfirmed PR; SD (or unconfirmed PR) must occur ≥53 days after first dose date. ‡ NE = Could not be estimated based on number of patients with loss of response. BICR, blinded independent central review; BID, twice daily; BIW, twice weekly; CR, complete response; DOR, duration of respon se; KRAS, kirsten rat sarcoma virus; MEK, mitogen - activated protein kinase kinase ; mt, mutant; ORR, objective response rate; PD, progressive disease; PR, partial response; RECIST v1.1, Response Evaluation Cri ter ia in Solid Tumours version 1.1; SD, stable disease; wt , wild type. 8 • RECIST 1.1 Objective Response Rate by BICR (primary endpoint): • 31% overall; 44% KRAS mt, 17% KRAS wt • 33% without prior MEKi , 24% with prior MEKi • Median time to response: 3.7 months (range, 1.7 – 19.2) • Median duration of response: 31.1 months (95% CI, 14.8, 31.1) In the avutometinib + defactinib combination group
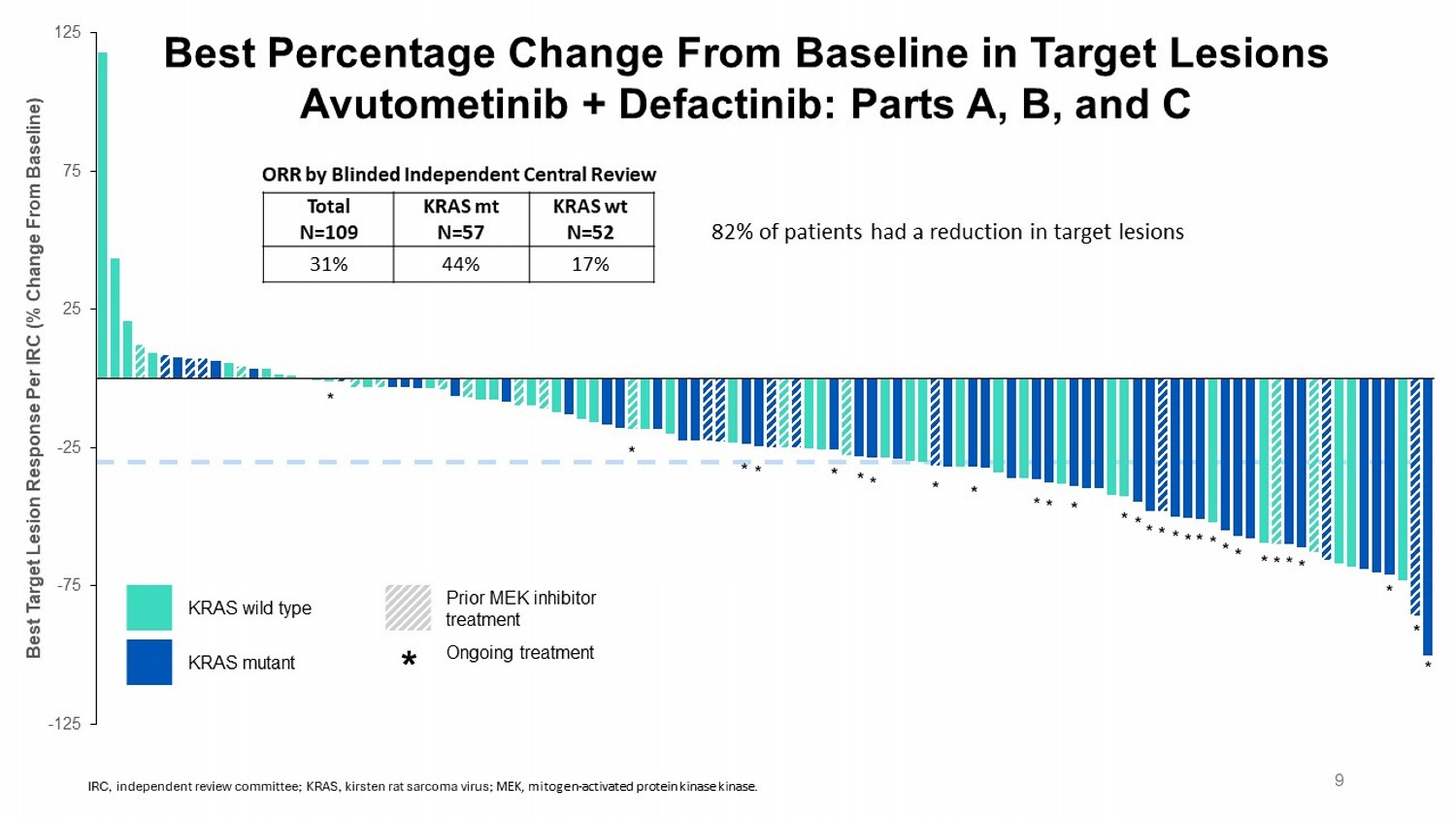
-125 -75 -25 25 75 125 Best Target Lesion Response Per IRC (% Change From Baseline) * * * * * * * * * * * * * * * * * * * * * * * * * * * * * Best Percentage Change From Baseline in Target Lesions Avutometinib + Defactinib : Parts A, B, and C Prior MEK inhibitor treatment KRAS wild type Ongoing treatment KRAS mutant * 9 82% of patients had a reduction in target lesions KRAS wt N=52 KRAS mt N=57 Total N=109 17% 44% 31% ORR by Blinded Independent Central Review IRC, independent review committee; KRAS, kirsten rat sarcoma virus; MEK, mitogen - activated protein kinase kinase .
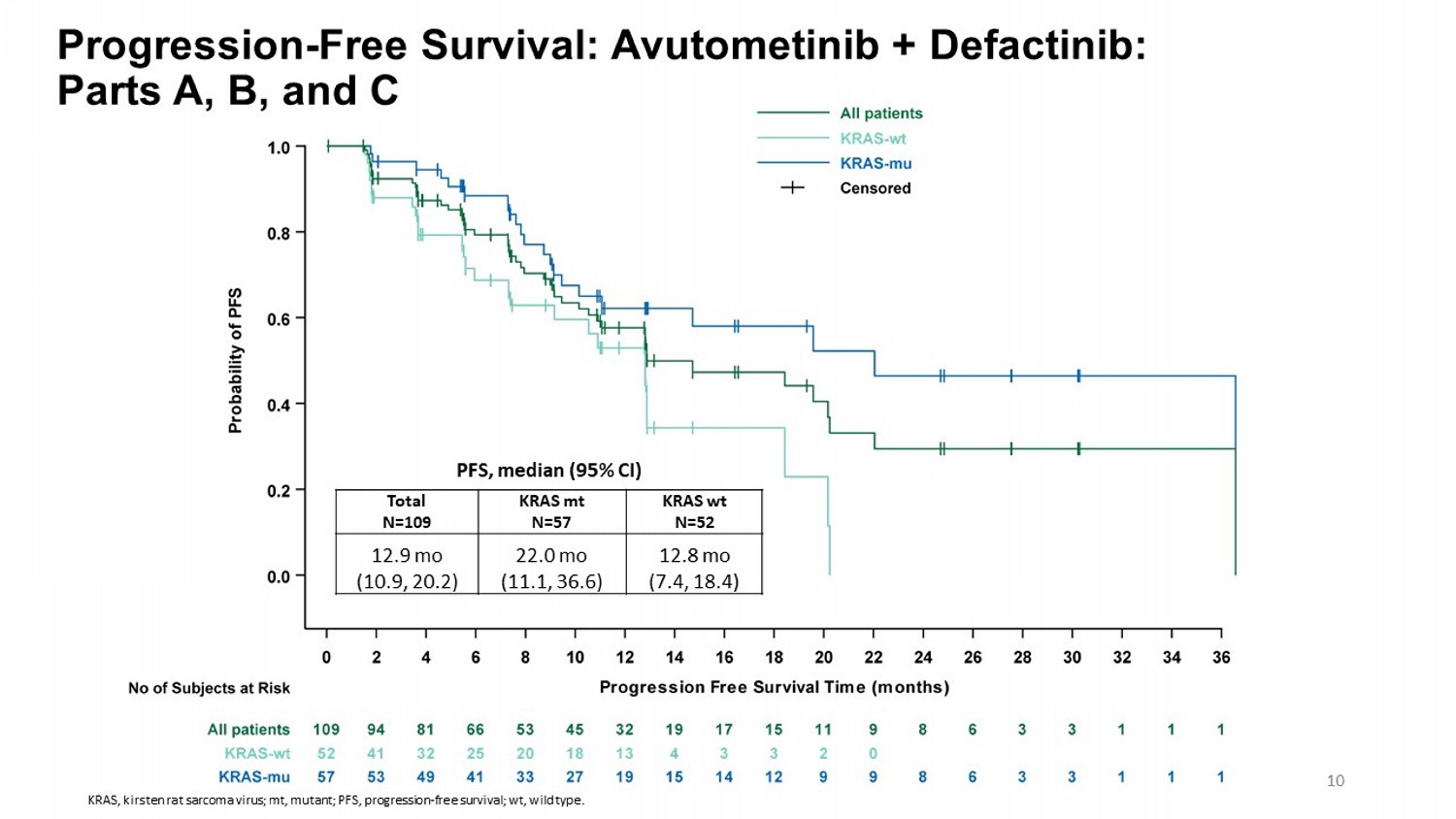
Progression - Free Survival: Avutometinib + Defactinib : Parts A, B, and C KRAS wt N=52 KRAS mt N=57 Total N=109 12.8 mo (7.4, 18.4) 22.0 mo (11.1, 36.6) 12.9 mo (10.9, 20.2) PFS, median (95% CI) 10 KRAS, kirsten rat sarcoma virus; mt, mutant; PFS, progression - free survival; wt , wild type.
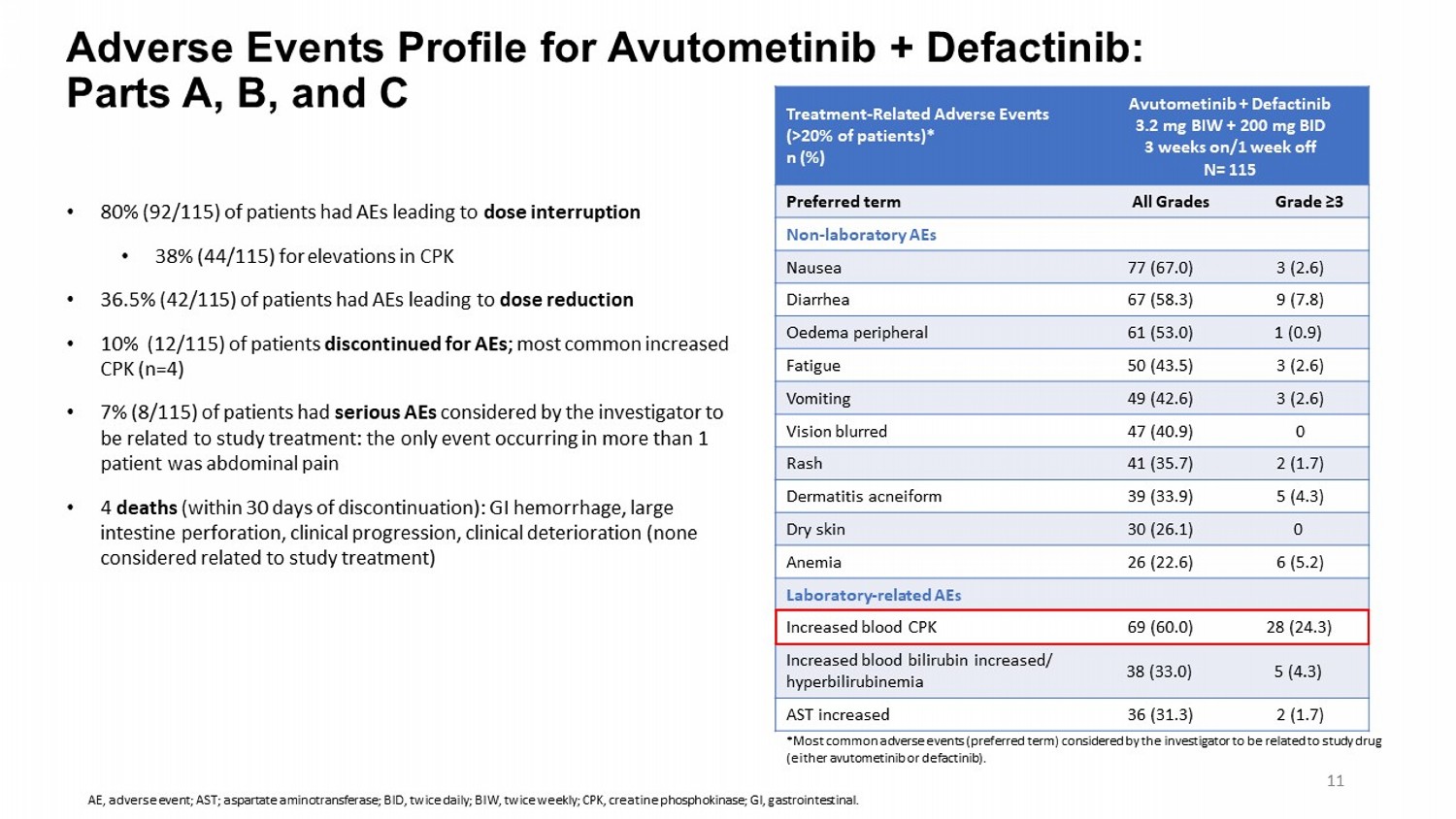
Adverse Events Profile for Avutometinib + Defactinib : Parts A, B, and C Avutometinib + Defactinib 3.2 mg BIW + 200 mg BID 3 weeks on/1 week off N= 115 Treatment - Related Adverse Events (>20% of patients)* n (%) Grade ≥3 All Grades Preferred term Non - laboratory AEs 3 (2.6) 77 (67.0) Nausea 9 (7.8) 67 (58.3) Diarrhea 1 (0.9) 61 (53.0) Oedema peripheral 3 (2.6) 50 (43.5) Fatigue 3 (2.6) 49 (42.6) Vomiting 0 47 (40.9) Vision blurred 2 (1.7) 41 (35.7) Rash 5 (4.3) 39 (33.9) Dermatitis acneiform 0 30 (26.1) Dry skin 6 (5.2) 26 (22.6) Anemia Laboratory - related AEs 28 (24.3) 69 (60.0) Increased blood CPK 5 (4.3) 38 (33.0) Increased blood bilirubin increased/ hyperbilirubinemia 2 (1.7) 36 (31.3) AST increased • 80% (92/115) of patients had AEs leading to dose interruption • 38% (44/115) for elevations in CPK • 36.5% (42/115) of patients had AEs leading to dose reduction • 10% (12/115) of patients discontinued for AEs ; most common increased CPK (n=4) • 7% (8/115) of patients had serious AEs considered by the investigator to be related to study treatment: the only event occurring in more than 1 patient was abdominal pain • 4 deaths (within 30 days of discontinuation): GI hemorrhage, large intestine perforation, clinical progression, clinical deterioration (none considered related to study treatment) *Most common adverse events (preferred term) considered by the investigator to be related to study drug (either avutometinib or defactinib ). 11 AE, adverse event; AST; aspartate aminotransferase; BID, twice daily; BIW, twice weekly; CPK, creatine phosphokinase; GI, gas tro intestinal.
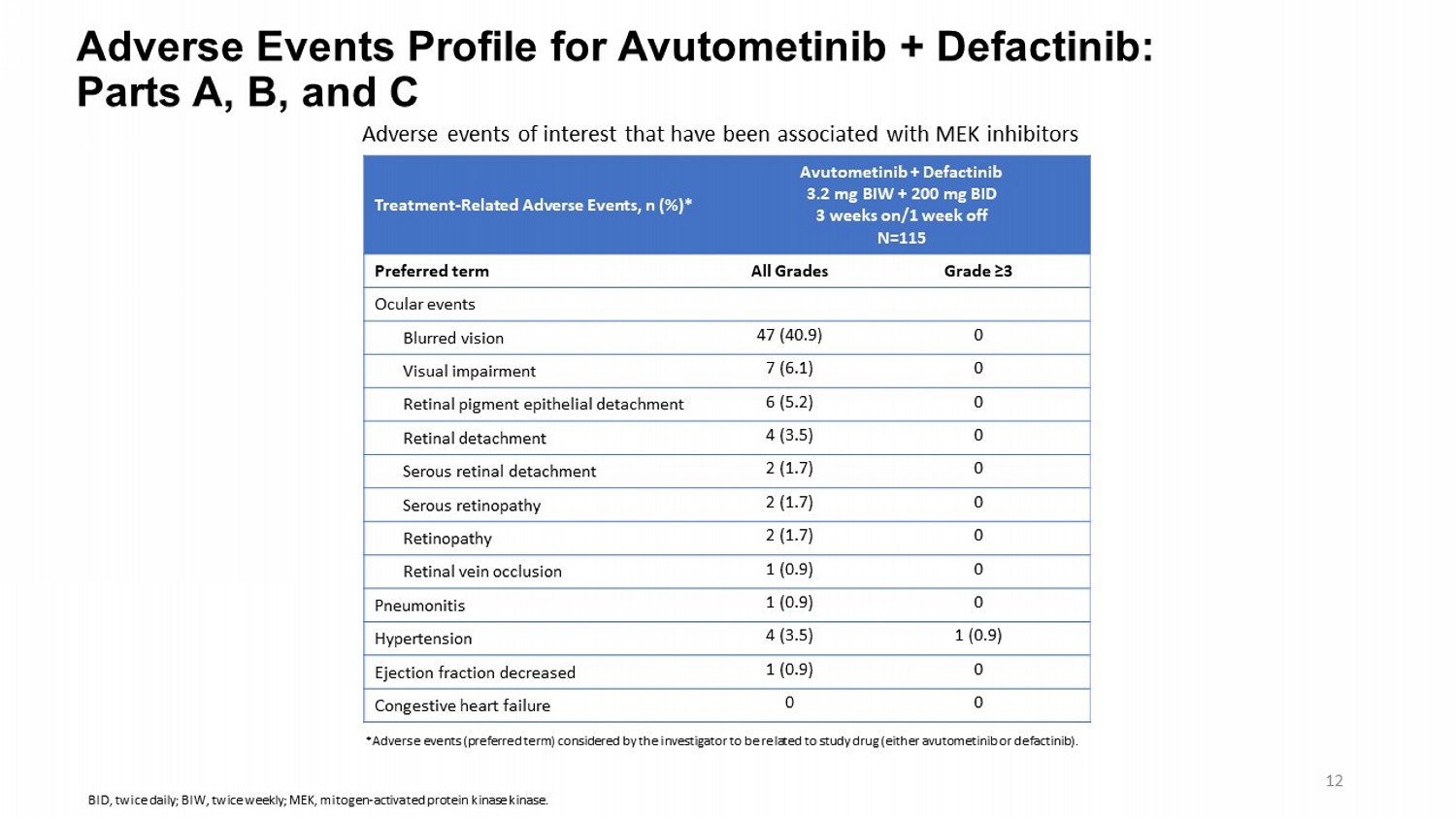
Adverse Events Profile for Avutometinib + Defactinib : Parts A, B, and C Avutometinib + Defactinib 3.2 mg BIW + 200 mg BID 3 weeks on/1 week off N=115 Treatment - Related Adverse Events, n (%)* Grade ≥3 All Grades Preferred term Ocular events 0 47 (40.9) Blurred vision 0 7 (6.1) Visual impairment 0 6 (5.2) Retinal pigment epithelial detachment 0 4 (3.5) Retinal detachment 0 2 (1.7) Serous retinal detachment 0 2 (1.7) Serous retinopathy 0 2 (1.7) Retinopathy 0 1 (0.9) Retinal vein occlusion 0 1 (0.9) Pneumonitis 1 (0.9) 4 (3.5) Hypertension 0 1 (0.9) Ejection fraction decreased 0 0 Congestive heart failure Adverse events of interest that have been associated with MEK inhibitors *Adverse events (preferred term) considered by the investigator to be related to study drug (either avutometinib or defactinib ). 12 BID, twice daily; BIW, twice weekly; MEK, mitogen - activated protein kinase kinase .
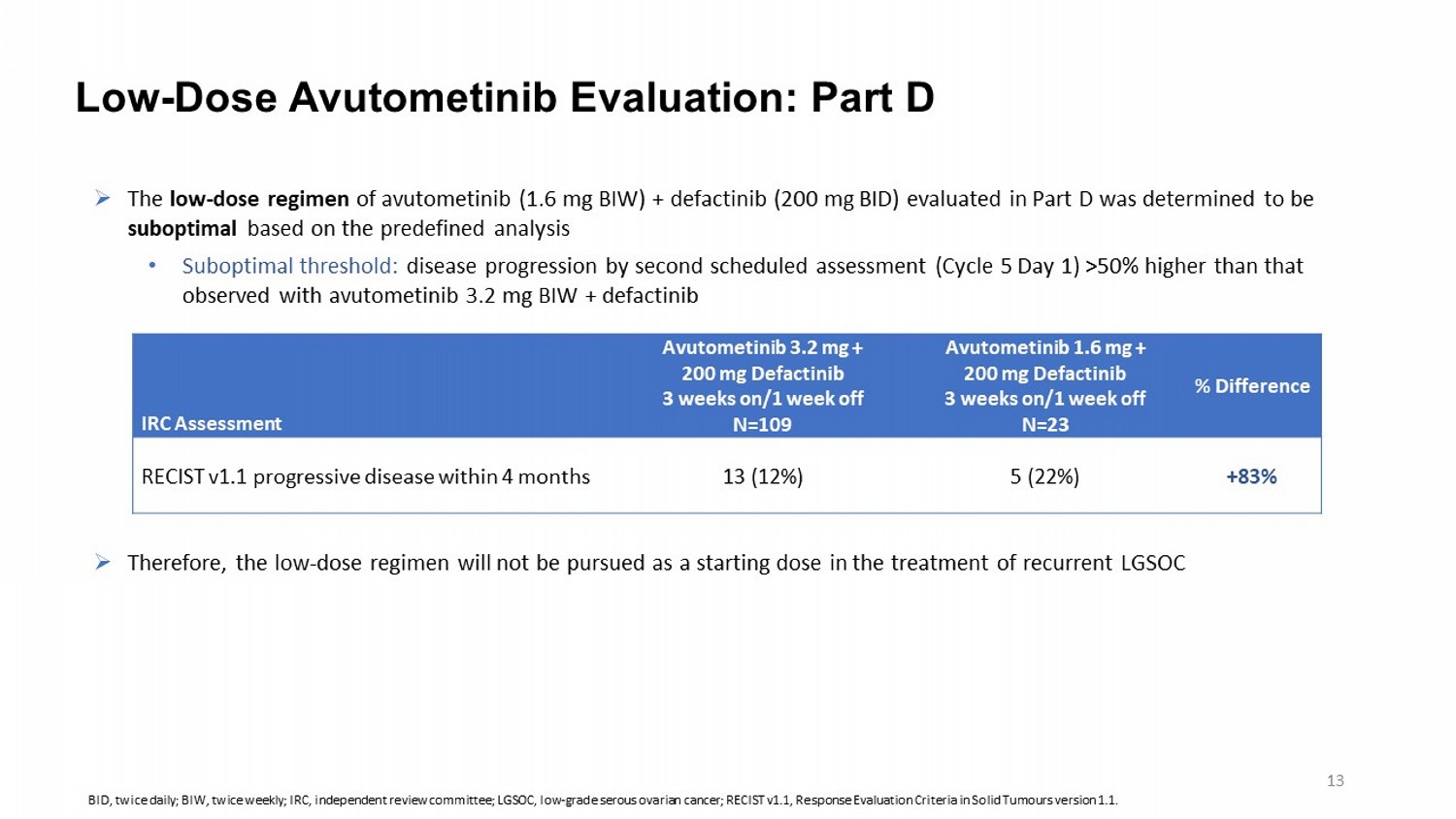
» The low - dose regimen of avutometinib (1.6 mg BIW) + defactinib (200 mg BID) evaluated in Part D was determined to be suboptimal based on the predefined analysis • Suboptimal threshold: disease progression by second scheduled assessment (Cycle 5 Day 1) >50% higher than that observed with avutometinib 3.2 mg BIW + defactinib » Therefore, the low - dose regimen will not be pursued as a starting dose in the treatment of recurrent LGSOC Low - Dose Avutometinib Evaluation: Part D % Difference Avutometinib 1.6 mg + 200 mg Defactinib 3 weeks on/1 week off N=23 Avutometinib 3.2 mg + 200 mg Defactinib 3 weeks on/1 week off N=109 IRC Assessment +83% 5 (22%) 13 (12%) RECIST v1.1 progressive disease within 4 months BID, twice daily; BIW, twice weekly ; IRC, independent review committee; LGSOC, low - grade serous ovarian cancer; RECIST v1.1, Response Evaluation Criteria in Solid Tumours version 1.1. 13

IGCS | 2024 Annual Global Meeting Summary and Conclusions • In women with recurrent LGSOC with few available treatment options, the combination of avutometinib 3.2 mg BIW + defactinib 200 mg BID resulted in clinically meaningful responses, duration of response, and progression - free survival • ORR: 31% overall; 44% in KRAS mt and 17% in KRAS wt • Median DOR: 31 months overall • Median PFS : 12.9 months overall; 22.0 months in KRAS mt and 12.8 months in KRAS wt • The safety profile of the combination was consistent with previous reports • The majority of adverse events were grade 1 and 2 • The majority of adverse events were managed with dose interruptions and reductions • Discontinuation rate of 10% for adverse events • These data support the potential for avutometinib + defactinib as a new standard of care for recurrent LGSOC, regardless of KRAS status 14 A phase 3 trial ( GOG - 3097/ENGOT - OV81/NCRI/RAMP 301) comparing avutometinib + defactinib to investigator’s choice of therapy in recurrent LGSOC is enrolling BID, twice daily; BIW, twice weekly ; DOR, duration of response; KRAS, kirsten rat sarcoma virus; LGSOC, low - grade serous ovarian cancer; mt, mutant; ORR, objective response rate; PFS, progression - free survival; wt , wild type.

IGCS | 2024 Annual Global Meeting We thank the patients and their families , the trial teams at the participating centers, ENGOT, and GOG for supporting this study United Kingdom ( GTG - UK ) Royal Marsden NHS Foundation Trust (Susana Banerjee) Beatson West of Scotland Cancer Centre (Rosalind Glasspool) The Christie NHS Foundation Trust (Andrew Clamp) UCLH Cancer Clinical Trials Unit (Rowan Miller) Western General Hospital (Charlie Gourley ) Belgium (BGOG) CHU de Liège (Christine Gennigens ) UZ Gent Medische Oncologie ( Hannelorre Denys) UZ Leuven ( Els Van Nieuwenhuysen and Toon Van Gorp) France (GINECO) Centre Leon Berard (Isabelle Ray - Coquard) Hospital Jean Minjoz (Elsa Kalbacher ) ICM Vall d’Aurelle (Michel Fabbro ) Institut Curie (Manuel Rodrigues) Italy ( MaNGO ) Instituto Europeo di Oncologia IRCCS (Nicoletta Colombo) UOC Oncologia 2, Istituto Oncologico Veneto IRCCS (Valentina Guarneri) Spain (GEICO) Hospital Clínico Universitario de Valencia ( Jose Alejandro Perez Fidalgo) Hospital Universitario Ramon y Cajal (Alfonso Cortés - Salgado) Hospital Universitario Reina Sofia (Maria Jesus Rubio) Hospital Universitario Vall D'Hebron (Ana Oaknin) Canada (ENGOT) Centre de recherche di Centre Hospitalier de i'Universite de Montreal (Diane Provencher) Princess Margaret Cancer Centre (Amit Oza ) United States (GOG) Memorial Sloan Kettering Cancer Center (Rachel Grisham) Advent Health (Robert Holloway) Florida Cancer Specialists and Research Institute (Bradley J. Monk) Cleveland Clinic Women’s Health Institute (Peter Rose) Comprehensive Cancer Centers of Nevada (Anu Thummala ) H. Lee Moffitt Cancer Center and Research Institute (Hye Sook Chon) Maryland Oncology and Hematology (Carol Tweed) Minnesota Oncology Hematology (Lauren Bollinger) Northwest Cancer Specialists (Erin Salinas) United States (GOG, continued) Sansum Clinic (Gregg Newman) Sarah Cannon Research Institute (Erika Hamilton) The Ohio State University Wexner Medical Center & James Cancer Hospital (David O’Malley) Texas Oncology Austin (Lynne Knowles) Texas Oncology Dallas (Kristi McIntyre) Texas Oncology Longview (Anna M. Priebe) Texas Oncology McAllen (Suresh Ratnam) Texas Oncology San Antonio (Antonio Santillian - Gomez) Texas Oncology The Woodlands (Christine Lee) University of Chicago (John Maroney) University of New Mexico Comprehensive Cancer Center (Carolyn Muller) University of Oklahoma Medical Center (Kathleen Moore) University of Virginia (Kari Ring) UT Southwestern Medical Center (David S. Miller) Washington University School of Medicine (Premal Thaker ) Willamette Valley Cancer Institute and Research Center (Charles Anderson) Yale School of Medicine (Alessandro Santin) Virginia Cancer Specialists (Mitul Gandhi) ENGOT - ov60/GOG - 3052/RAMP 201 was sponsored by Verastem Oncology 15














