Exhibit 99.2

1 Avutometinib and Defactinib in Recurrent Low - Grade Serous Ovarian Cancer (LGSOC) October 17, 2024 Corporate Update Call Amanda, real patient living with recurrent LGSOC Diagnosed at 26 with LGSOC

2 Forward - Looking Statements This presentation includes forward - looking statements about, among other things, Verastem Oncology’s (the “Company”) programs an d product candidates, strategy, future plans and prospects, including statements related to the scope and expecting timing for the completion of the NDA submission for the avutometinib and defactinib combination in LGSOC, the o ngo ing discussions with the FDA and the ability to obtain Accelerated Approval and Priority Review of the mature RAMP 201 data, the potential of the combination of avutometinib and defactinib to change the way patients with recurre nt LGSOC are treated, the status of enrollments for and potential of the results of the RAMP 301 Phase 3 trial to expand the indication regardless of KRAS mutation status, the structure of our planned and pending clinical trials, the potential clinical value of various of the Company's clinical trials, including the RAMP 201, RAMP 205 and RAMP 301 trials, the timing of commencing and completing trials, including topline data reports, interactions with regulators, the ti meline and indications for clinical development, regulatory submissions, the potential for and timing of commercialization of product candidates and the potential market opportunities of, and estimated addressable markets for, our dr ug candidates. The words "anticipate," "believe," "estimate," "expect," "intend," "may," "plan," "predict," "project," "target," "potential," "will," "would," "could," "should," "continue," “can,” “promising” and similar expressions are intended to identify forward - looking statements, although not all forward - looking statements contain these identifying words. Each forward - looking statement is subject to risks and uncertainties that could cause actual results to diffe r materially from those expressed or implied in such statement. Applicable risks and uncertainties include the risks and uncertainties, among other things, regarding: the success in the dev elo pment and potential commercialization of our product candidates, including avutometinib in combination with other compounds, including defactinib, LUMAKRAS and others; the uncertainties inherent in research and development, such as n ega tive or unexpected results of clinical trials, the occurrence or timing of applications for our product candidates that may be filed with regulatory authorities in any jurisdictions; whether and when regulatory authorities in any ju risdictions may approve any such applications that may be filed for our product candidates, and, if approved, whether our product candidates will be commercially successful in such jurisdictions; our ability to obtain, maintain and enf orc e patent and other intellectual property protection for our product candidates; the scope, timing, and outcome of any legal proceedings; decisions by regulatory authorities regarding trial design, labeling and other matters that could a ffe ct the timing, availability or commercial potential of our product candidates; whether preclinical testing of our product candidates and preliminary or interim data from clinical trials will be predictive of the results or success of ongoi ng or later clinical trials; that the timing, scope and rate of reimbursement for our product candidates is uncertain; that the market opportunities of our drug candidates are based on internal and third - party estimates which may prove to be incor rect; that third - party payors (including government agencies) may not reimburse; that there may be competitive developments affecting our product candidates; that data may not be available when expected; that enrollment of c lin ical trials may take longer than expected, which may delay our development programs, including delays in submission or review by the FDA of our NDA submission in recurrent KRAS mutant LGSOC if enrollment in our confirmatory trial is not well underway at the time of submission or that the FDA may require the company to enroll additional patients in the Company's ongoing RAMP 301 confirmatory Phase 3 clinical trial prior to Verastem submitting or the FD A taking action on our NDA seeking accelerated approval; risks associated with preliminary and interim data, which may not be representative of more mature data, including with respect to interim duration of therapy data; that o ur product candidates will cause adverse safety events and/or unexpected concerns may arise from additional data or analysis, or result in unmanageable safety profiles as compared to their levels of efficacy; that we may be unable to su ccessfully validate, develop and obtain regulatory approval for companion diagnostic tests for our product candidates that require or would commercially benefit from such tests, or experience significant delays in doing so; that the ma ture RAMP 201 data and associated discussions with the FDA may not support the scope of our rolling NDA submission for the avutometinib and defactinib combination in LGSOC, including with respect to KRAS wild type LGSOC; that our pr oduct candidates may experience manufacturing or supply interruptions or failures; that any of our third - party contract research organizations, contract manufacturing organizations, clinical sites, or contractors, among others, who we rely on fail to fully perform; that we face substantial competition, which may result in others developing or commercializing products before or more successfully than we do which could result in reduced market share or m ark et potential for our product candidates; that we will be unable to successfully initiate or complete the clinical development and eventual commercialization of our product candidates; that the development and commercialization of our product candidates will take longer or cost more than planned, including as a result of conducting additional studies or our decisions regarding execution of such commercialization; that we may not have sufficient cash to fund our contemplated op erations, including certain of our product development programs; that we may not attract and retain high quality personnel; that we or Chugai Pharmaceutical Co., Ltd. will fail to fully perform under the av uto metinib license agreement; that our total addressable and target markets for our product candidates might be smaller than we are presently estimating; that we or Secura Bio, Inc. will fail to fully perform under the asset purchase agreement with Secura Bio, Inc., including in relation to milestone payments; that we will not see a return on investment on the payments we have and may continue to make pursuant to the collaboration and option agreement with GenFleet The rapeutics (Shanghai), Inc. (GenFleet), or that GenFleet will fail to fully perform under the agreement; that we may not be able to establish new or expand on existing collaborations or partnerships, including with respect to in - lice nsing of our product candidates, on favorable terms, or at all; that we may be unable to obtain adequate financing in the future through product licensing, co - promotional arrangements, public or private equity, debt financing or othe rwise; that we will not pursue or submit regulatory filings for our product candidates; and that our product candidates will not receive regulatory approval, become commercially successful products, or result in new treatment options bei ng offered to patients. Other risks and uncertainties include those identified under the heading “Risk Factors” in the Company’s Annual Report on For m 1 0 - K for the year ended December 31, 2023, as filed with the Securities and Exchange Commission (SEC) on March 14, 2024, and in any subsequent filings with the SEC, which are available at www.sec.gov and www.verastem.com . The forward - looking statements in this presentation speak only as of the original date of this presentation, and we undertake no obligation to update or revise any of these stateme nts whether as a result of new informatio n, future events or otherwise, except as required by law. Third - Party Sources Certain information contained in this presentation, including industry and market data and other statistical information, rel ate s to or is based on studies, publications, surveys and other data obtained from third - party sources and the Company’s own internal estimates and research. While the Company believes these third - party sources to be reliable as of the dat e of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third - party sources. In addition, all of the market data in cluded in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions
3 Today’s Agenda Closing Remarks Dan Paterson, President & Chief Executive Officer Mature RAMP 201 Data Results John Hayslip, M.D., Chief Medical Officer Q&A Dan Paterson, President & Chief Executive Officer John Hayslip, M.D., Chief Medical Officer Mike Crowther, Chief Commercial & Strategy Officer Dan Calkins, Chief Financial Officer Changing the Treatment Paradigm Mike Crowther, Chief Commercial & Strategy Officer Opening Remarks Dan Paterson, President & Chief Executive Officer

4 Opening Remarks Dan Paterson, President and CEO
5 Avutometinib + Defactinib Demonstrated Durable Results Across Various Efficacy Measures in Heavily Pretreated Patients in RAMP 201 • 31% Overall ORR, 44% in KRAS mt, 17% in KRAS wt • 82% of all patients had tumor shrinkage • 14.5 months estimated mean DoT, 18.3 months in KRAS mt and 10.7 months in KRAS wt • 12.9 months median PFS, 22 months in KRAS mt, 12.8 months in KRAS wt • 10% discontinuation rates due to adverse events Clear Regulatory Path for KRAS Mutant • On track to complete the NDA submission in October for recurrent KRAS mutant LGSOC; Pursuing Accelerated Approval with Priority Review • RAMP 301 enrollment remains on track and will continue enrolling all comers • Committed to make the combination available to patients with KRAS wild - type in several ways, including a path for regulatory approval Verastem Aims to Deliver First FDA - Approved Treatment Specifically for Recurrent KRAS mutant LGSOC in 2025 Significant Market Opportunity in Area of High Unmet Need • SoC (Chemo/Hormonal) is associated with low response rates (6 - 13%) with PFS below 12 months and high discontinuation rates due to toxicity • Plan to be launch ready in 2025 to maximize market opportunity in recurrent KRAS mutant LGSOC • Plan to submit RAMP 201 for NCCN guideline review • NCCN guideline inclusion may enable patients with KRAS wild - type LGSOC to access therapy, if approved Source for all data: RAMP 201 data cut off as of June 30, 2024; LGSOC: Low - grade serous ovarian cancer; ORR: Objective Response Rate; KRAS, kirsten rat sarcoma virus; KRAS mt: mutant; KRAS wt : wild - type; PFS: Progression - free Survival; NDA: New Drug Application; SOC: Standard of Care; NCCN: National Comprehensive Cancer Network; The combination of Avutometinib and Defactinib is an investigational drug. It has not been proven to be safe or effective and h as not been approved by FDA or any other comparable regulatory authority.
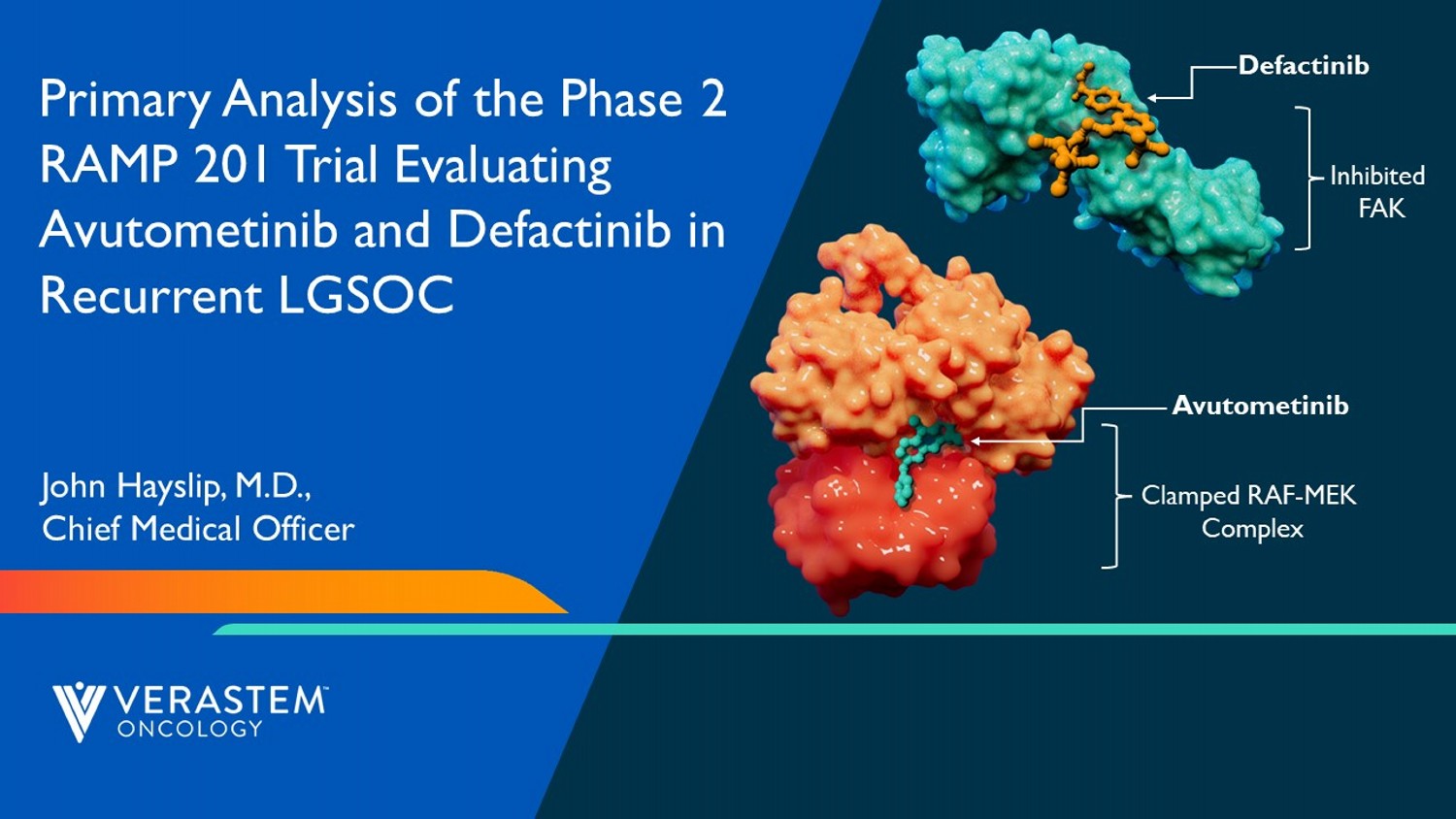
6 Primary Analysis of the Phase 2 RAMP 201 Trial Evaluating Avutometinib and Defactinib in Recurrent LGSOC John Hayslip, M.D., Chief Medical Officer Clamped RAF - MEK Complex Avutometinib Inhibited FAK Defactinib

7 High Unmet Need for an Effective & Tolerable Therapy in Recurrent LGSOC “When you get told that you have a recurrence, the mental load is a lot. You’re thinking, okay, what did I have to do for treatment the first time? Now I have to repeat that. And will there even be something available for me to take for a second, or a third recurrence?” - Amanda, real patient living with recurrent LGSOC Diagnosed at 26 with LGSOC • U.S. Incidence / Prevalence: 1k - 2k 1 / 6k - 8k 2 / W orldwide : 80,000 • Affects younger population (20 - 30s) and disproportionately impacts health, fertility, and long - term quality of life 3, 4 • 80%+ of patients will experience a recurrence 5 • Disease currently managed by NCCN guidelines, with no FDA approved treatments • Current S o C offer poor to moderate response rates (6 - 13%) and patients cycle through therapy 6,7,8 • Median OS of ~10 years from time of diagnosis 9 • KRAS mt – ~12 years 10 and KRAS wt – ~7 years 10 1. Verastem DOF; 13. US Cancer Statistics. Accessed 2024. 3. Slomovitz Gynecol Oncol 2020; 4. Manning - Geist B et al. Clin Cancer Res 2022;28(20):4456 - 4465; 5. Babaier 2022/p1/para1/ln6,7; 6. Gershenson Gynecol Oncol 2022; 7. Slomovitz Gynecol Oncol 2020; 10. Manning - Geist B et al. Clin Cancer Res 2022;28(20):4456 - 4465; 8. Monk 2020/p3758/table2/footnote - b; 9. Banerjee SN). J Clin Oncol. 41. No 16_suppl (June 1, 2023) 5515 - 5515; 10. Calculated using figures in Gershenson Gynecol Oncol 2022.
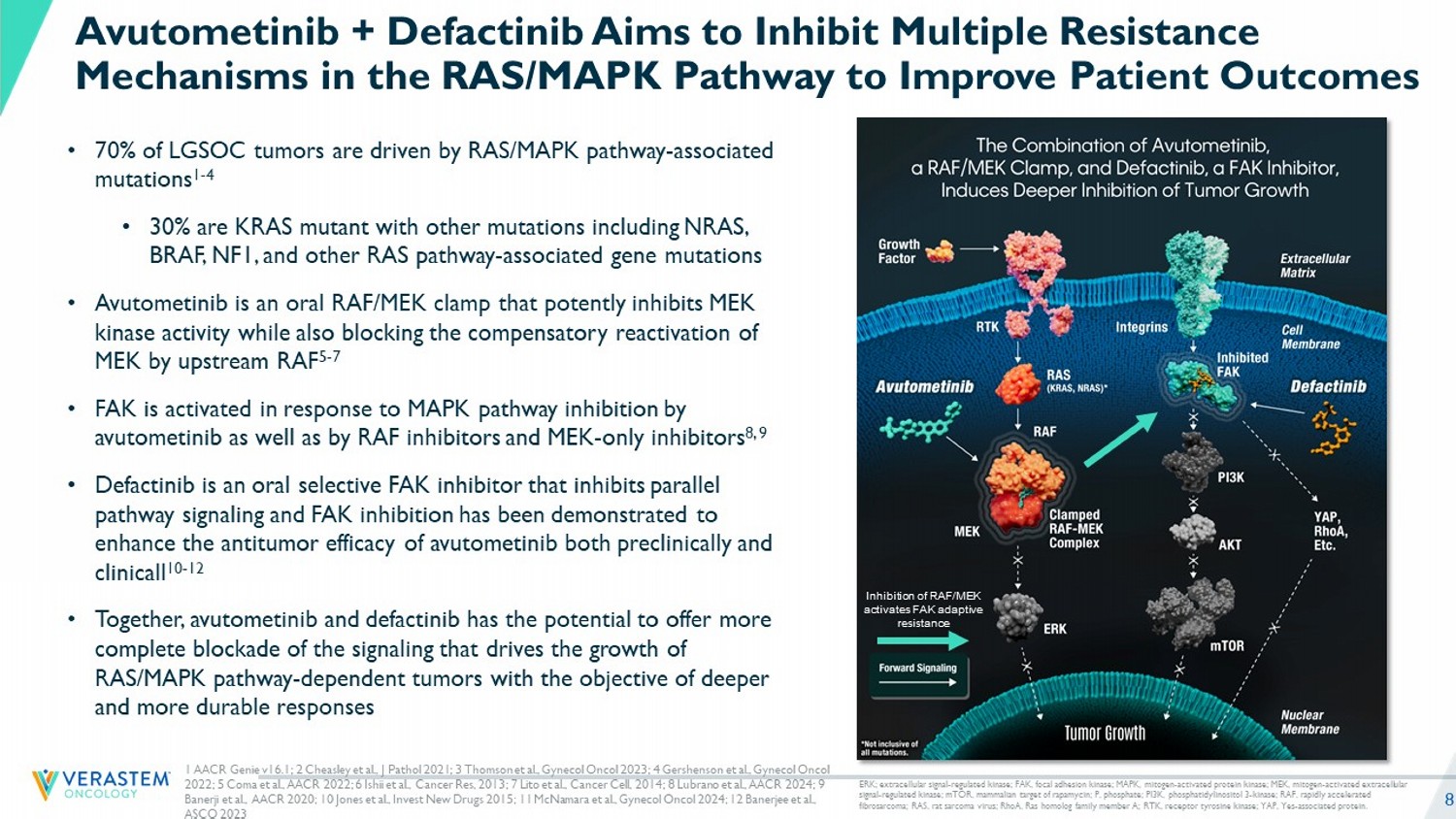
8 • 70% of LGSOC tumors are driven by RAS/MAPK pathway - associated mutations 1 - 4 • 30% are KRAS mutant with other mutations including NRAS, BRAF, NF1, and other RAS pathway - associated gene mutations • Avutometinib is an oral RAF/MEK clamp that potently inhibits MEK kinase activity while also blocking the compensatory reactivation of MEK by upstream RAF 5 - 7 • FAK is activated in response to MAPK pathway inhibition by avutometinib as well as by RAF inhibitors and MEK - only inhibitors 8, 9 • Defactinib is an oral selective FAK inhibitor that inhibits parallel pathway signaling and FAK inhibition has been demonstrated to enhance the antitumor efficacy of avutometinib both preclinically and clinicall 10 - 12 • Together, avutometinib and defactinib has the potential to offer more complete blockade of the signaling that drives the growth of RAS/MAPK pathway - dependent tumors with the objective of deeper and more durable responses Avutometinib + Defactinib Aims to Inhibit Multiple Resistance Mechanisms in the RAS/MAPK Pathway to Improve Patient Outcomes Inhibition of RAF/MEK activates FAK adaptive resistance ERK; extracellular signal - regulated kinase; FAK, focal adhesion kinase; MAPK, mitogen - activated protein kinase ; MEK, mitogen - activated extracellular signal - regulated kinase; mTOR, mammalian target of rapamycin; P, phosphate; PI3K, phosphatidylinositol 3 - kinase; RAF, rapidly ac celerated fibrosarcoma; RAS, rat sarcoma virus; RhoA , Ras homolog family member A; RTK, receptor tyrosine kinase; YAP, Yes - associated protein. 1 AACR Genie v16.1; 2 Cheasley et al., J Pathol 2021; 3 Thomson et al., Gynecol Oncol 2023; 4 Gershenson et al., Gynecol Oncol 2022; 5 Coma et al., AACR 2022 ; 6 Ishii et al., Cancer Res, 2013 ; 7 Lito et al., Cancer Cell, 2014 ; 8 Lubrano et al., AACR 2024 ; 9 Banerji et al., AACR 2020 ; 10 Jones et al., Invest New Drugs 2015; 11 McNamara et al., Gynecol Oncol 2024 ; 12 Banerjee et al., ASCO 2023

9 Key Inclusion Criteria • Recurrent LGSOC • Prior chemotherapy • Measurable disease (RECIST 1.1) • Prior MEKi allowed Avutometinib + Defactinib 3.2 mg BIW 200 mg BID KRAS mt (n=20) KRAS wt (n=22) Avutometinib + Defactinib 3.2 mg BIW 200 mg BID KRAS mt (n=22) KRAS wt (n=20) Avutometinib + Defactinib 1.6 mg BIW 200 mg BID KRAS mt (n=11) KRAS wt (n=16) Avutometinib + Defactinib 3.2 mg BIW 200 mg BID KRAS mt (n=16) KRAS wt (n=15) Go Forward Regimen Selection Criteria (Selection Phase): • Observed ORR is comparatively greater than the other regimen • Observed ORR of the leading regimen is ≥15% Primary Endpoint: ORR (BICR) Evaluation of ORR in Combination Arm: • In KRAS mt patients • All patients (KRAS mt & wt ) Actual Enrollment at RP2D: 115 Treated Patients Avutometinib Mono 4.0 mg BIW KRAS mt (n=15) KRAS wt (n=22) Avutometinib Mono 4.0 mg BIW KRAS mt (n=16) KRAS wt (n=16) Part A Selection Phase Part B Expansion Phase Part C Expansion Phase Combo Part D Low - dose Avuto Combo x x x x Go Forward Regimen Numbers represent patients treated on study. RECIST: Response Evaluation Criteria in Solid Tumors; MEKi : Mitogen - activated extracellular signal - regulated kinase inhibitor; BICR, blinded independent central review; BID, twice daily; BIW, twice weekly; RP2D: Recommended Phase 2 Dose RAMP 201: Registration - Directed Phase 2 Trial of Avutometinib ± Defactinib in Patients with Recurrent LGSOC RAMP 201 (ENGOT - ov60/GOG - 3052)
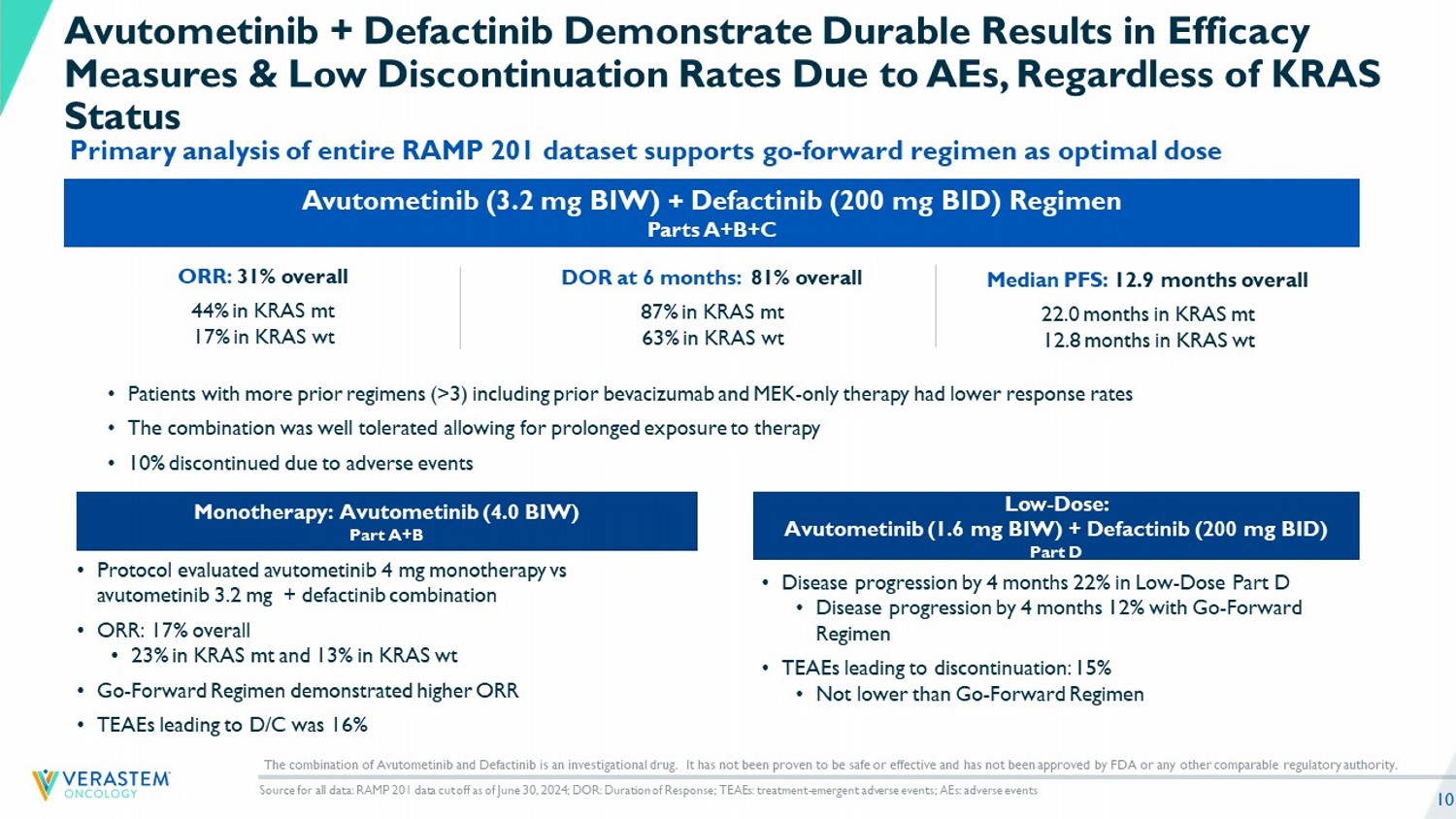
10 Avutometinib (3.2 mg BIW) + Defactinib (200 mg BID) Regimen Parts A+B+C ORR: 31% overall 44% in KRAS mt 17% in KRAS wt Low - Dose: Avutometinib (1.6 mg BIW) + Defactinib (200 mg BID) Part D • Disease progression by 4 months 22% in Low - Dose Part D • Disease progression by 4 months 12% with Go - Forward Regimen • TEAEs leading to discontinuation: 15% • Not lower than Go - Forward Regimen Monotherapy: Avutometinib (4.0 BIW) Part A+B • Protocol evaluated avutometinib 4 mg monotherapy vs avutometinib 3.2 mg + defactinib combination • ORR: 17% overall • 23% in KRAS mt and 13% in KRAS wt • Go - Forward Regimen demonstrated higher ORR • TEAEs leading to D/C was 16% Avutometinib + Defactinib Demonstrate Durable Results in Efficacy Measures & Low Discontinuation Rates Due to AEs, Regardless of KRAS Status Primary analysis of entire RAMP 201 dataset supports go - forward regimen as optimal dose Source for all data: RAMP 201 data cut off as of June 30, 2024; DOR: Duration of Response; TEAEs: treatment - emergent adverse eve nts; AEs: adverse events • Patients with more prior regimens (>3) including prior bevacizumab and MEK - only therapy had lower response rates • The combination was well tolerated allowing for prolonged exposure to therapy • 10% discontinued due to adverse events Median PFS: 12.9 months overall 22.0 months in KRAS mt 12.8 months in KRAS wt DOR a t 6 months: 81% overall 87% in KRAS mt 63% in KRAS wt The combination of Avutometinib and Defactinib is an investigational drug. It has not been proven to be safe or effective and h as not been approved by FDA or any other comparable regulatory authority.
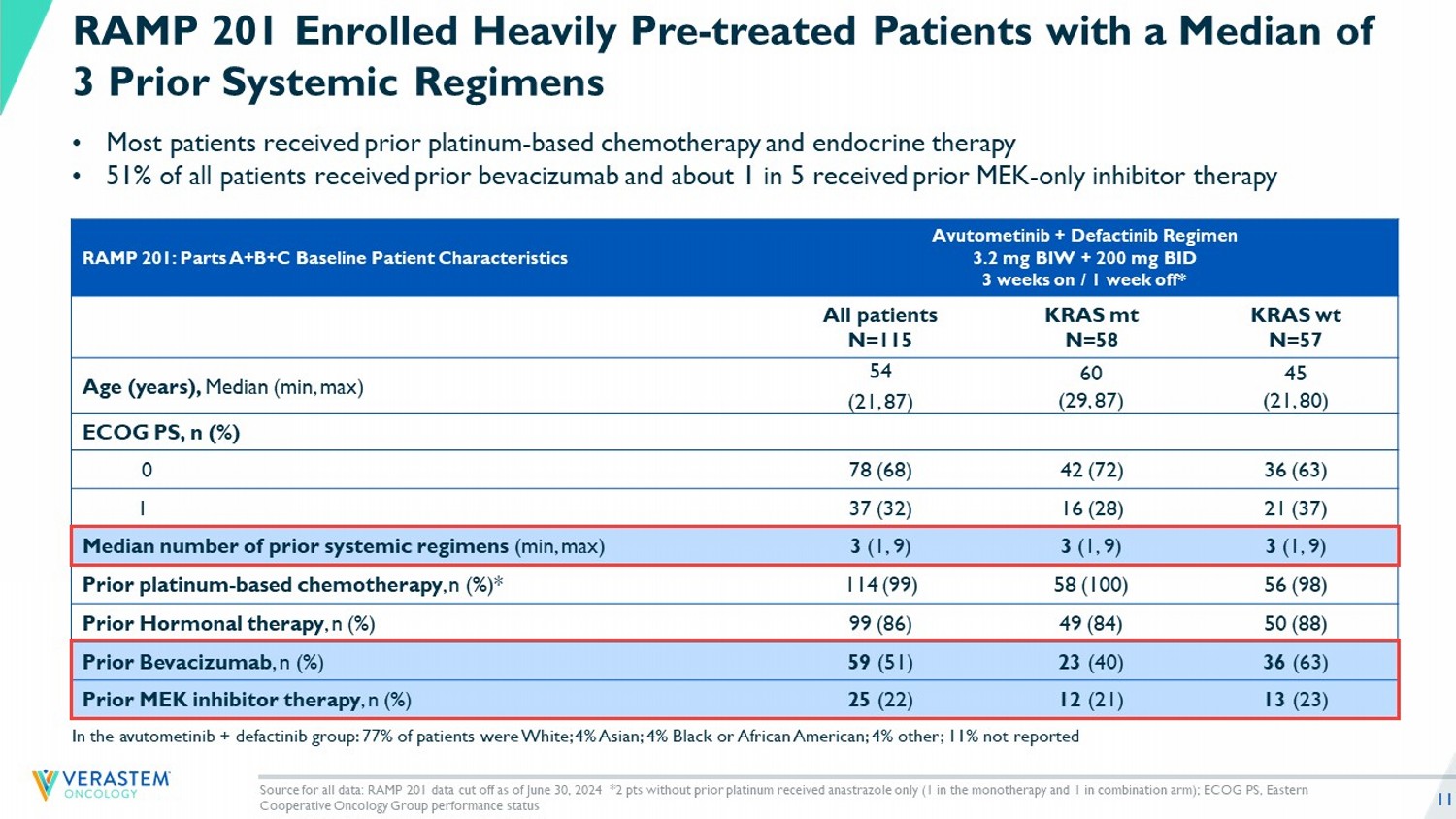
11 Avutometinib + Defactinib Regimen 3.2 mg BIW + 200 mg BID 3 weeks on / 1 week off* RAMP 201: Parts A+B+C Baseline Patient Characteristics KRAS wt N=57 KRAS mt N=58 All patients N=115 45 (21, 80) 60 (29, 87) 54 (21, 87) Age (years), Median (min, max) ECOG PS, n (%) 36 (63) 42 (72) 78 (68) 0 21 (37) 16 (28) 37 (32) 1 3 (1, 9) 3 (1, 9) 3 (1, 9) Median number of prior systemic regimens (min, max) 56 (98) 58 (100) 114 (99) Prior platinum - based chemotherapy , n (%)* 50 (88) 49 (84) 99 (86) Prior Hormonal therapy , n (%) 36 (63) 23 (40) 59 (51) Prior Bevacizumab , n (%) 13 (23) 12 (21) 25 (22) Prior MEK inhibitor therapy , n (%) RAMP 201 Enrolled Heavily Pre - treated Patients with a Median of 3 Prior Systemic Regimens • Most patients received prior platinum - based chemotherapy and endocrine therapy • 51% of all patients received prior bevacizumab and about 1 in 5 received prior MEK - only inhibitor therapy In th e avutometinib + defactinib group: 77% of patients were White; 4% Asian ; 4% Black or African American; 4% other; 11% not reported Source for all data: RAMP 201 data cut off as of June 30, 2024 *2 pts without prior platinum received anastrazole only (1 in the monotherapy and 1 in combination arm); ECOG PS, Eastern Cooperative Oncology Group performance status
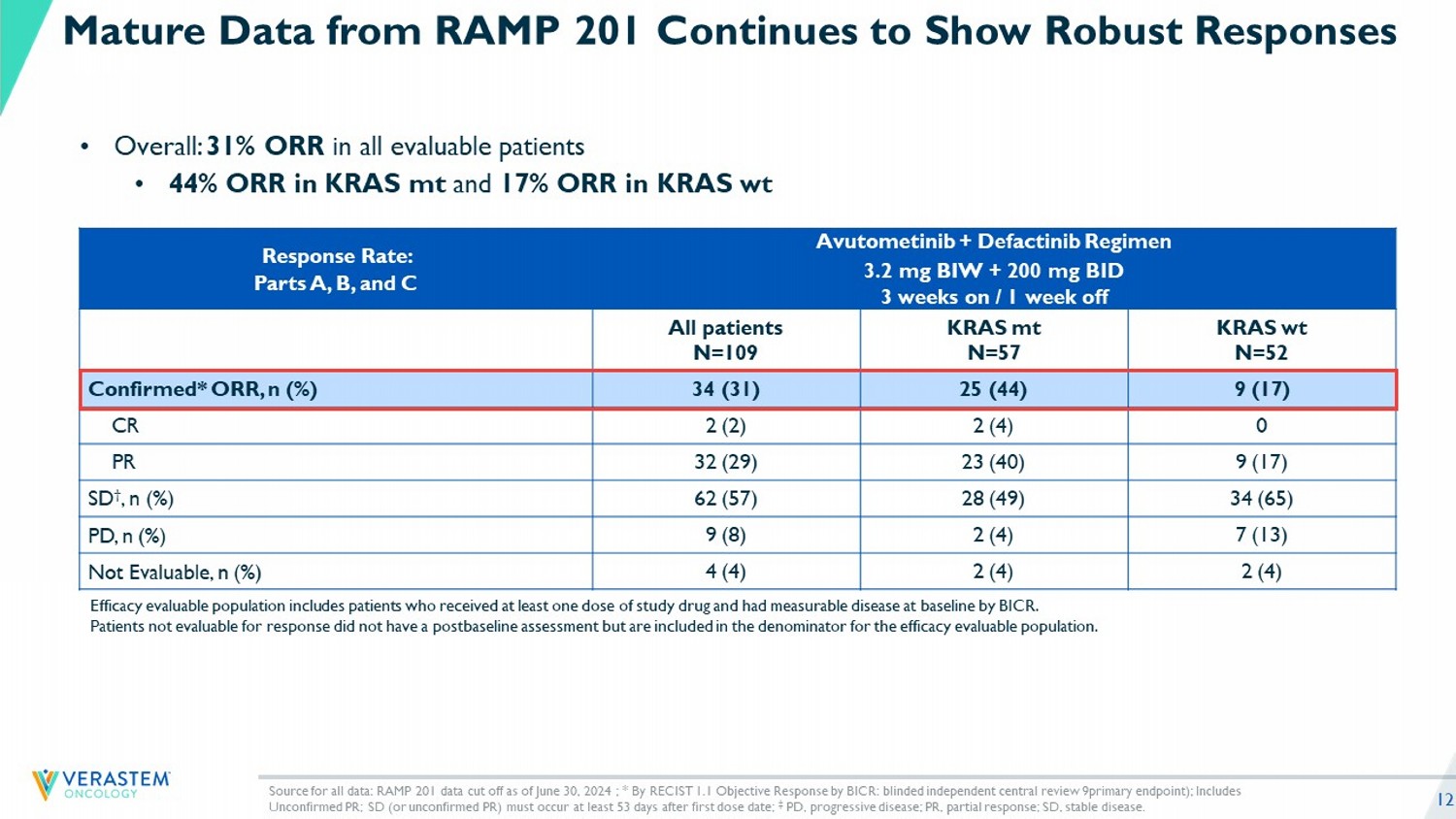
12 Avutometinib + Defactinib Regimen 3.2 mg BIW + 200 mg BID 3 weeks on / 1 week off Response Rate: Parts A, B, and C KRAS wt N=52 KRAS mt N=57 All patients N=109 9 (17) 25 (44) 34 (31) Confirmed* ORR, n (%) 0 2 (4) 2 (2) CR 9 (17) 23 (40) 32 (29) PR 34 (65) 28 (49) 62 (57) SD † , n (%) 7 (13) 2 (4) 9 (8) PD, n (%) 2 (4) 2 (4) 4 (4) Not Evaluable, n (%) Source for all data: RAMP 201 data cut off as of June 30, 2024 ; * By RECIST 1.1 Objective Response by BICR: blinded independ ent central review 9primary endpoint); Includes Unconfirmed PR; SD (or unconfirmed PR) must occur at least 53 days after first dose date; ‡ PD, progressive disease; PR, partial response; SD, stable disease. • Overall: 31% ORR in all evaluable patients • 44% ORR in KRAS mt and 17% ORR in KRAS wt Mature Data from RAMP 201 Continues to Show Robust Responses Efficacy evaluable population includes patients who received at least one dose of study drug and had measurable disease at ba sel ine by BICR. Patients not evaluable for response did not have a postbaseline assessment but are included in the denominator for the effica cy evaluable population.
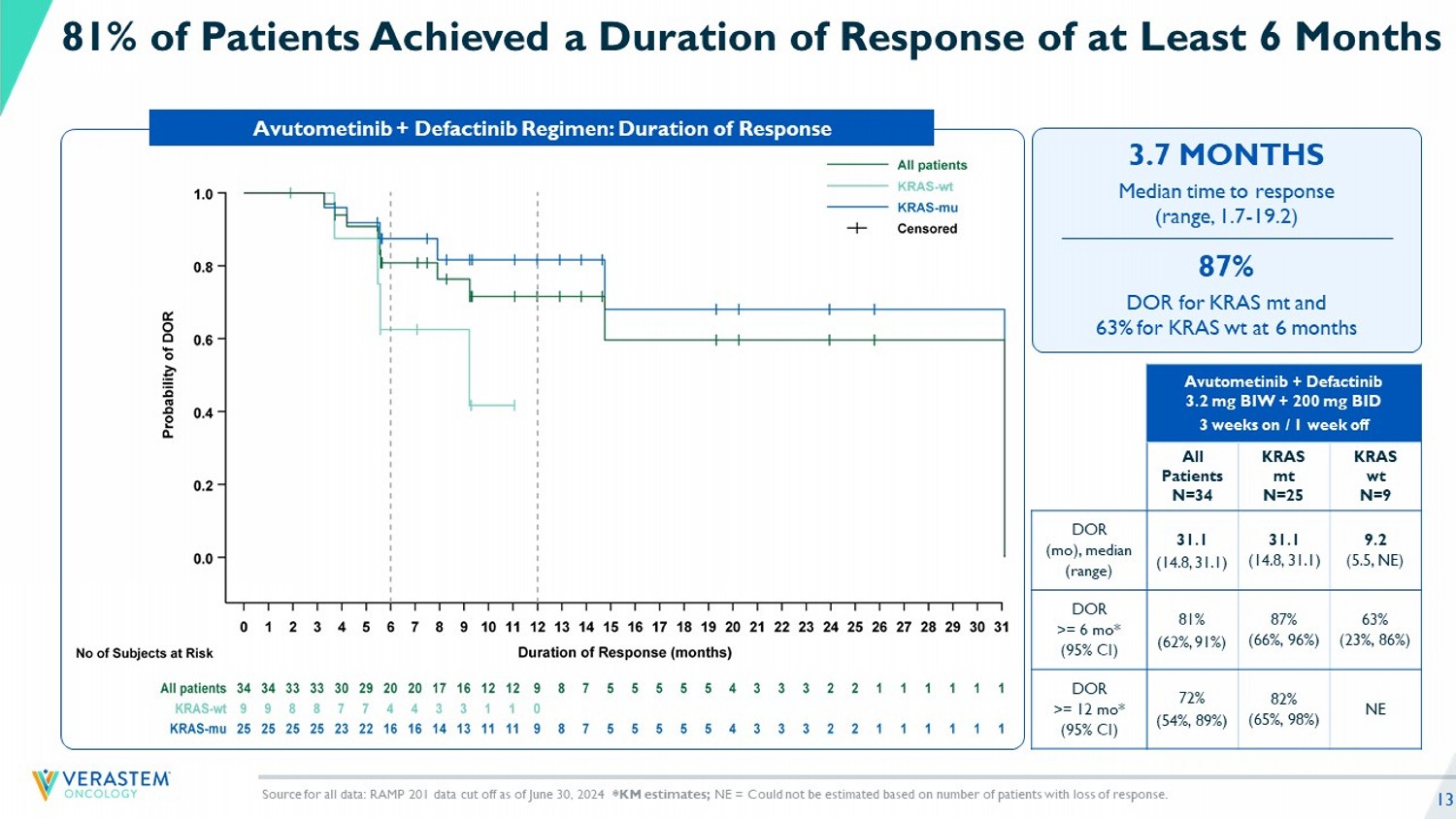
13 Median time to response (range, 1.7 - 19.2) 3.7 MONTHS DOR for KRAS mt and 63% for KRAS wt at 6 months 87% Avutometinib + Defactinib 3.2 mg BIW + 200 mg BID 3 weeks on / 1 week off KRAS wt N=9 KRAS mt N=25 All Patients N=34 9.2 (5.5, NE) 31.1 (14.8, 31.1) 31.1 (14.8, 31.1) DOR ( mo ), median (range) 63% (23%, 86%) 87% (66%, 96%) 81% (62%, 91%) DOR >= 6 mo * (95% CI) NE 82% (65%, 98%) 72% (54%, 89%) DOR >= 12 mo * (95% CI) 81% of Patients Achieved a Duration of Response of at Least 6 Months Avutometinib + Defactinib Regimen: Duration of Response Source for all data: RAMP 201 data cut off as of June 30, 2024 *KM estimates; NE = Could not be estimated based on number of patients with loss of response.
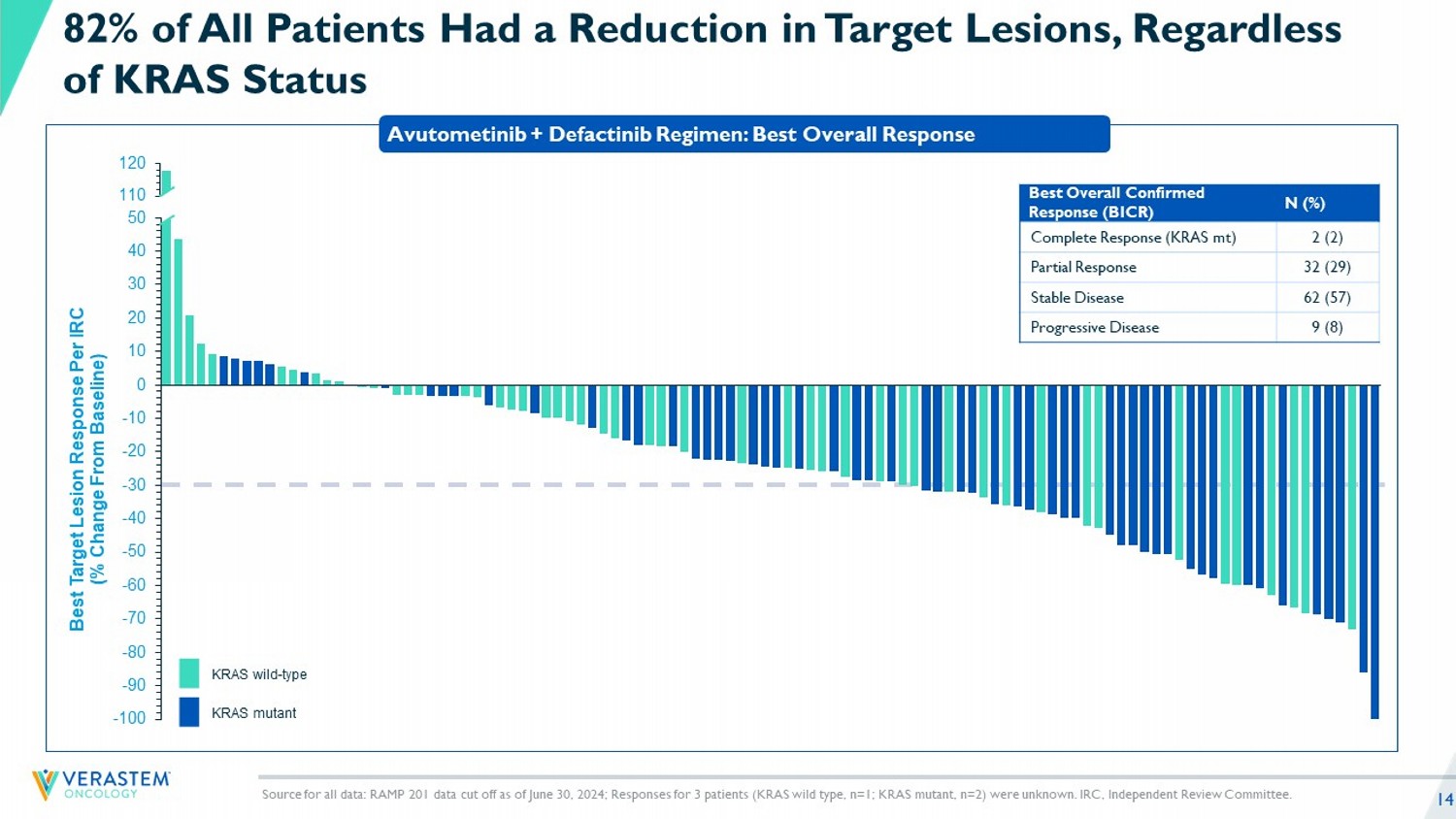
14 -100 -90 -80 -70 -60 -50 -40 -30 -20 -10 0 10 20 30 40 50 Best Target Lesion Response Per IRC (% Change From Baseline) 110 120 Best Target Lesion Response Per IRC (% Change From Baseline) Avutometinib + Defactinib Regimen: Best Overall Response 82% of All Patients Had a Reduction in Target Lesions, Regardless of KRAS Status Source for all data: RAMP 201 data cut off as of June 30, 2024; Responses for 3 patients (KRAS wild type, n=1; KRAS mutant, n =2) were unknown. IRC, Independent Review Committee. N (%) Best Overall Confirmed Response (BICR) 2 (2) Complete Response (KRAS mt) 32 (29) Partial Response 62 (57) Stable Disease 9 (8) Progressive Disease KRAS wild - type KRAS mutant
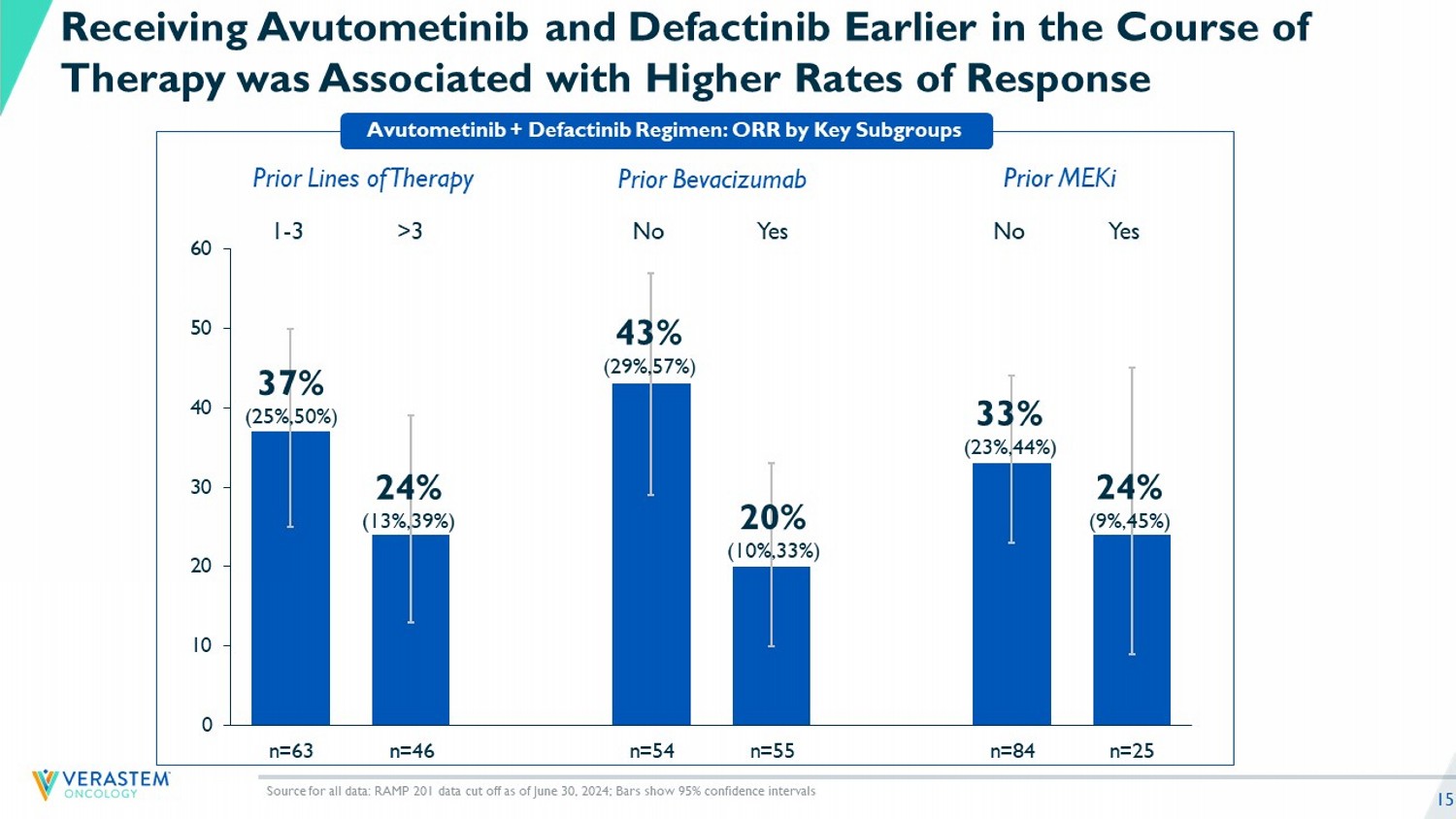
15 Avutometinib + Defactinib Regimen: ORR by Key Subgroups Source for all data: RAMP 201 data cut off as of June 30, 2024; Bars show 95% confidence intervals Receiving Avutometinib and Defactinib Earlier in the Course of Therapy was Associated with Higher Rates of Response 0 10 20 30 40 50 60 n=63 n=46 n=54 n=55 n=84 n=25 24% (9%,45%) 33% (23%,44%) 20% (10%,33%) 43% (29%,57%) 24% (13%,39%) 37% (25%,50%) Prior MEKi Prior Bevacizumab Prior Lines of Therapy Yes No Yes No >3 1 - 3
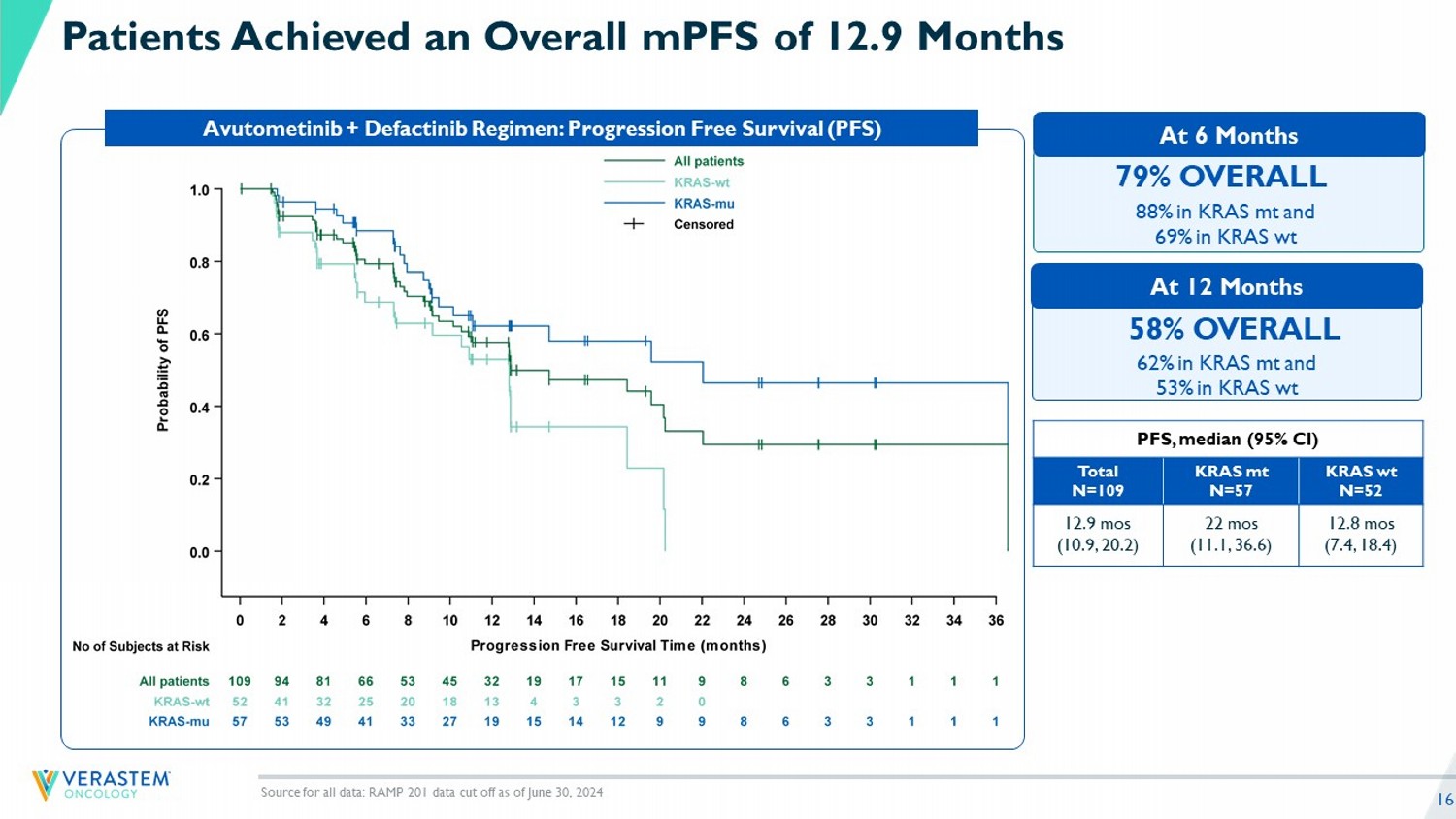
16 88% in KRAS mt and 69% in KRAS wt 79% OVERALL 62% in KRAS mt and 53% in KRAS wt 58% OVERALL Patients Achieved an Overall mPFS of 12.9 Months Avutometinib + Defactinib Regimen: Progression Free Survival (PFS) PFS, median (95% CI) KRAS wt N=52 KRAS mt N=57 Total N=109 12.8 mos (7.4, 18.4) 22 mos (11.1, 36.6) 12.9 mos (10.9, 20.2) Source for all data: RAMP 201 data cut off as of June 30, 2024 At 12 Months At 6 Months
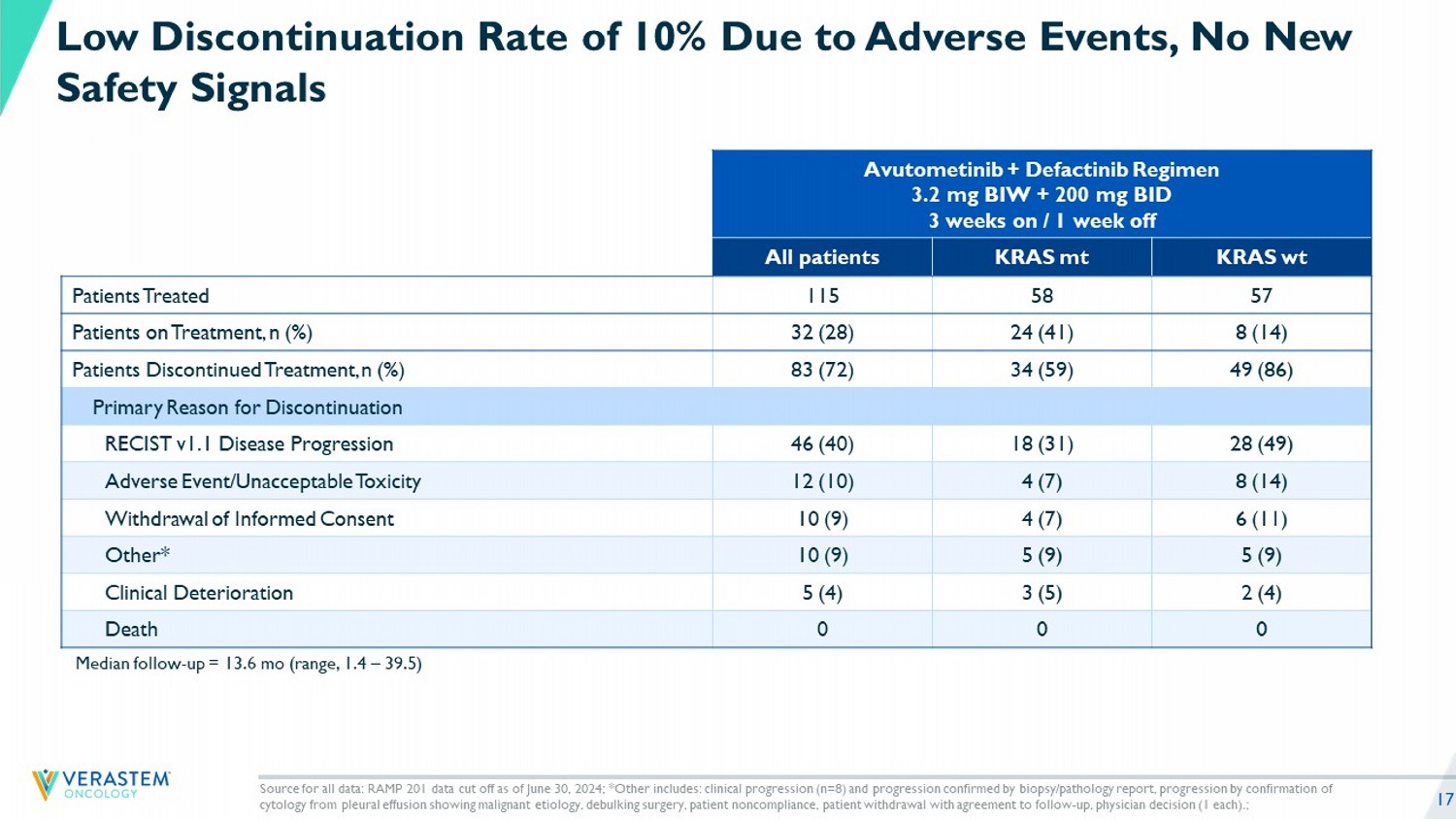
17 Median follow - up = 13.6 mo (range, 1.4 – 39.5) Source for all data: RAMP 201 data cut off as of June 30, 2024; *Other includes: clinical progression (n=8) and progression c onf irmed by biopsy/pathology report, progression by confirmation of cytology from pleural effusion showing malignant etiology, debulking surgery, patient noncompliance, patient withdrawal with agr eement to follow - up, physician decision (1 each).; Low Discontinuation Rate of 10% Due to Adverse Events, No New Safety Signals Avutometinib + Defactinib Regimen 3.2 mg BIW + 200 mg BID 3 weeks on / 1 week off KRAS wt KRAS mt All patients 57 58 115 Patients Treated 8 (14) 24 (41) 32 (28) Patients on Treatment, n (%) 49 (86) 34 (59) 83 (72) Patients Discontinued Treatment, n (%) Primary Reason for Discontinuation 28 (49) 18 (31) 46 (40) RECIST v1.1 Disease Progression 8 (14) 4 (7) 12 (10) Adverse Event/Unacceptable Toxicity 6 (11) 4 (7) 10 (9) Withdrawal of Informed Consent 5 (9) 5 (9) 10 (9) Other* 2 (4) 3 (5) 5 (4) Clinical Deterioration 0 0 0 Death

18 Avutometinib Plus Defactinib C ontinue to Demonstrate a W ell - Tolerated Safety Pr ofile Severe adverse events are generally uncommon and typically managed by a treatment pause 10% (12/115) discontinued for AEs (any cause); most common increased CPK (n=4) 80% (92/115) had AEs leading to dose interruption • 38% (44/115) for elevations in CPK 36.5% (42/115) had AEs leading to dose reduction • Mean relative dose intensity of 0.84 for avutometinib and 0.77 for defactinib 7% (8/115) of patients had serious AEs considered by the investigator to be related to study treatment: the only event occurring in more than 1 patient was abdominal pain 4 deaths (within 30 days of discontinuation) but were not considered related to the study treatment: • GI hemorrhage, large intestine perforation, clinical progression, clinical deterioration Avutometinib + Defactinib Regimen 3.2 mg BIW + 200 mg BID 3 weeks on/1 week off N= 115 Treatment - Related Adverse Events (>20% of patients)* n (%) Grade ≥3 All Grades Preferred term Non - laboratory AEs 3 (2.6) 77 (67.0) Nausea 9 (7.8) 67 (58.3) Diarrhea 1 (0.9) 61 (53.0) Oedema peripheral 3 (2.6) 50 (43.5) Fatigue 3 (2.6) 49 (42.6) Vomiting 0 47 (40.9) Vision blurred 2 (1.7) 41 (35.7) Rash 5 (4.3) 39 (33.9) Dermatitis acneiform 0 30 (26.1) Dry skin 6 (5.2) 26 (22.6) Anemia Laboratory - related AEs 28 (24.3) 69 (60.0) Increased blood CPK 5 (4.3) 38 (33.0) Increased blood bilirubin increased/ hyperbilirubinemia 2 (1.7) 36 (31.3) AST increased Source for all data: RAMP 201 data cut off as of June 30, 2024; *Most common adverse events (preferred term) considered by th e i nvestigator to be related to study drug (either avutometinib or defactinib); AE, adverse event; AST; aspartate aminotransferase; CPK, creatine phosphokinase; GI, gastrointestinal.
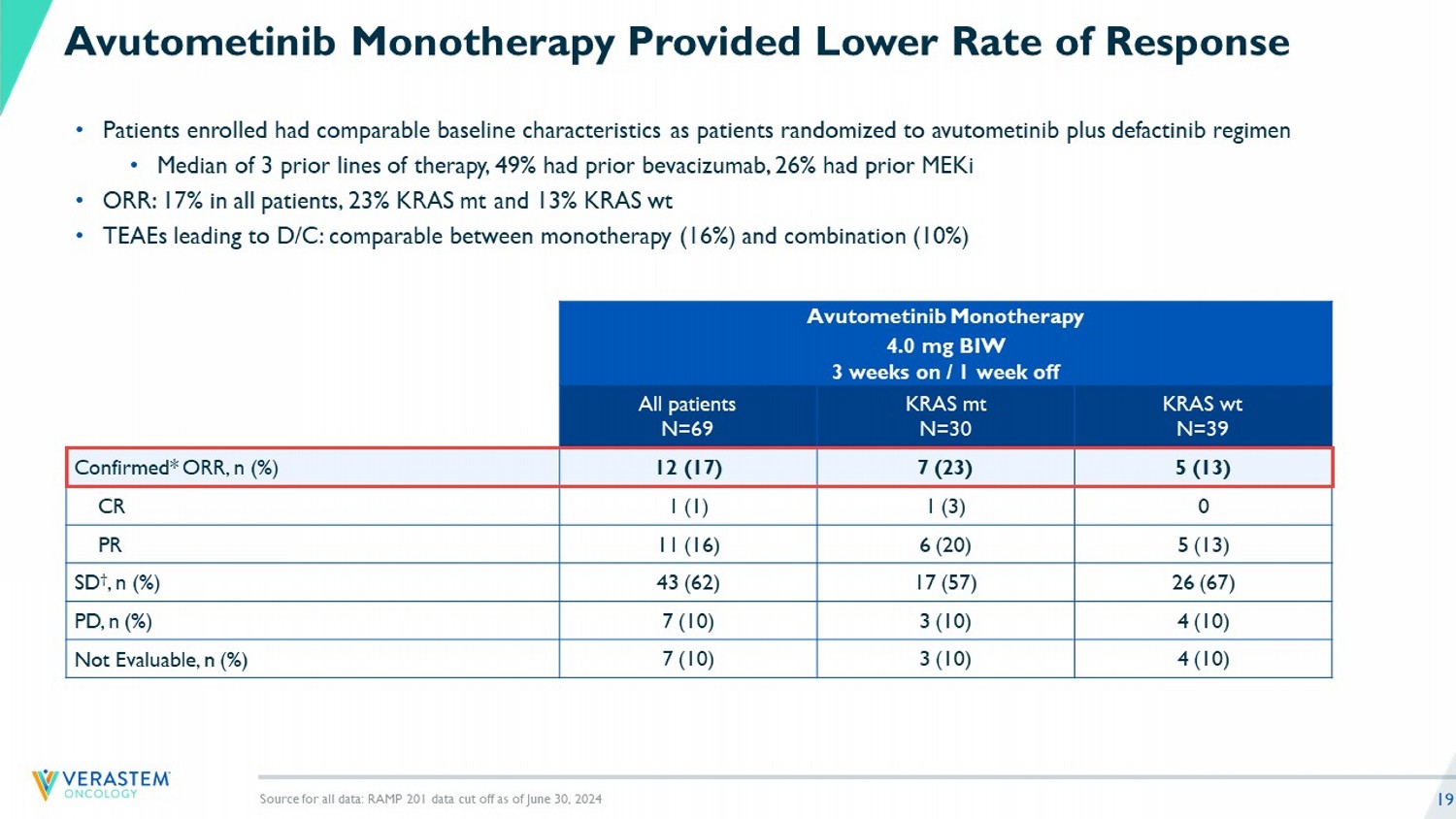
19 Avutometinib Monotherapy Provided Lower Rate of Response • Patients enrolled had comparable baseline characteristics as patients randomized to avutometinib plus defactinib regimen • Median of 3 prior lines of therapy, 49% had prior bevacizumab, 26% had prior MEKi • ORR: 17% in all patients, 23% KRAS mt and 13% KRAS wt • TEAEs leading to D/C: comparable between monotherapy (16%) and combination (10%) Source for all data: RAMP 201 data cut off as of June 30, 2024 Avutometinib Monotherapy 4.0 mg BIW 3 weeks on / 1 week off KRAS wt N=39 KRAS mt N=30 All patients N=69 5 (13) 7 (23) 12 (17) Confirmed* ORR, n (%) 0 1 (3) 1 (1) CR 5 (13) 6 (20) 11 (16) PR 26 (67) 17 (57) 43 (62) SD † , n (%) 4 (10) 3 (10) 7 (10) PD, n (%) 4 (10) 3 (10) 7 (10) Not Evaluable, n (%)
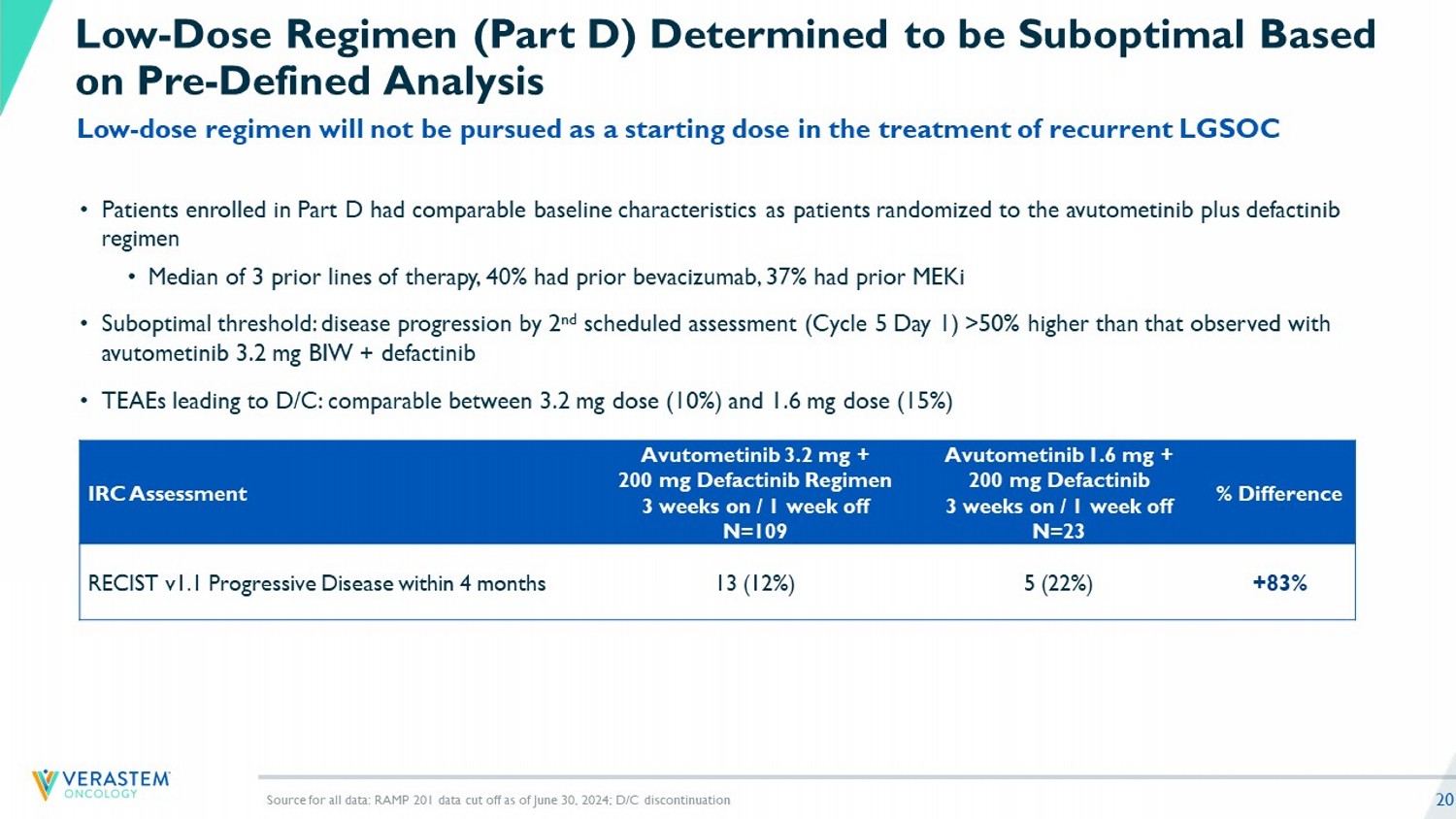
20 Low - Dose Regimen (Part D) Determined to be Suboptimal Based on Pre - Defined Analysis Source for all data: RAMP 201 data cut off as of June 30, 2024; D/C discontinuation % Difference Avutometinib 1.6 mg + 200 mg Defactinib 3 weeks on / 1 week off N=23 Avutometinib 3.2 mg + 200 mg Defactinib Regimen 3 weeks on / 1 week off N=109 IRC Assessment +83% 5 (22%) 13 (12%) RECIST v1.1 Progressive Disease within 4 months Low - dose regimen will not be pursued as a starting dose in the treatment of recurrent LGSOC • Patients enrolled in Part D had comparable baseline characteristics as patients randomized to the avutometinib plus defactini b regimen • Median of 3 prior lines of therapy, 40% had prior bevacizumab, 37% had prior MEKi • Suboptimal threshold: disease progression by 2 nd scheduled assessment (Cycle 5 Day 1) >50% higher than that observed with avutometinib 3.2 mg BIW + defactinib • TEAEs leading to D/C: comparable between 3.2 mg dose (10%) and 1.6 mg dose (15%)

21 RAMP 301: First Randomized Prospective Study to Fully Characterize KRAS Status of all Enrolled LGSOC Patients • Patients enrolling is similar to patient population in RAMP 201, with recurrent KRAS mt and KRAS wt LGSOC; prior MEKi and bevacizumab use allowed and post one line of platinum chemotherapy • Primary Endpoint: PFS • Stratification Factors: KRAS mutation status ( wt vs. mt) • Investigator choice of treatment • May crossover to avutometinib + defactinib arm upon BICR - confirmed PD • Study sites include the U.S., Australia, UK, Canada, and Europe RAMP 301: Phase 3 International Confirmatory Trial Enrollment is on track, targeting full enrollment by end of 2025

22 Changing the Treatment Paradigm in Recurrent LGSOC Mike Crowther Chief Commercial and Strategy Officer
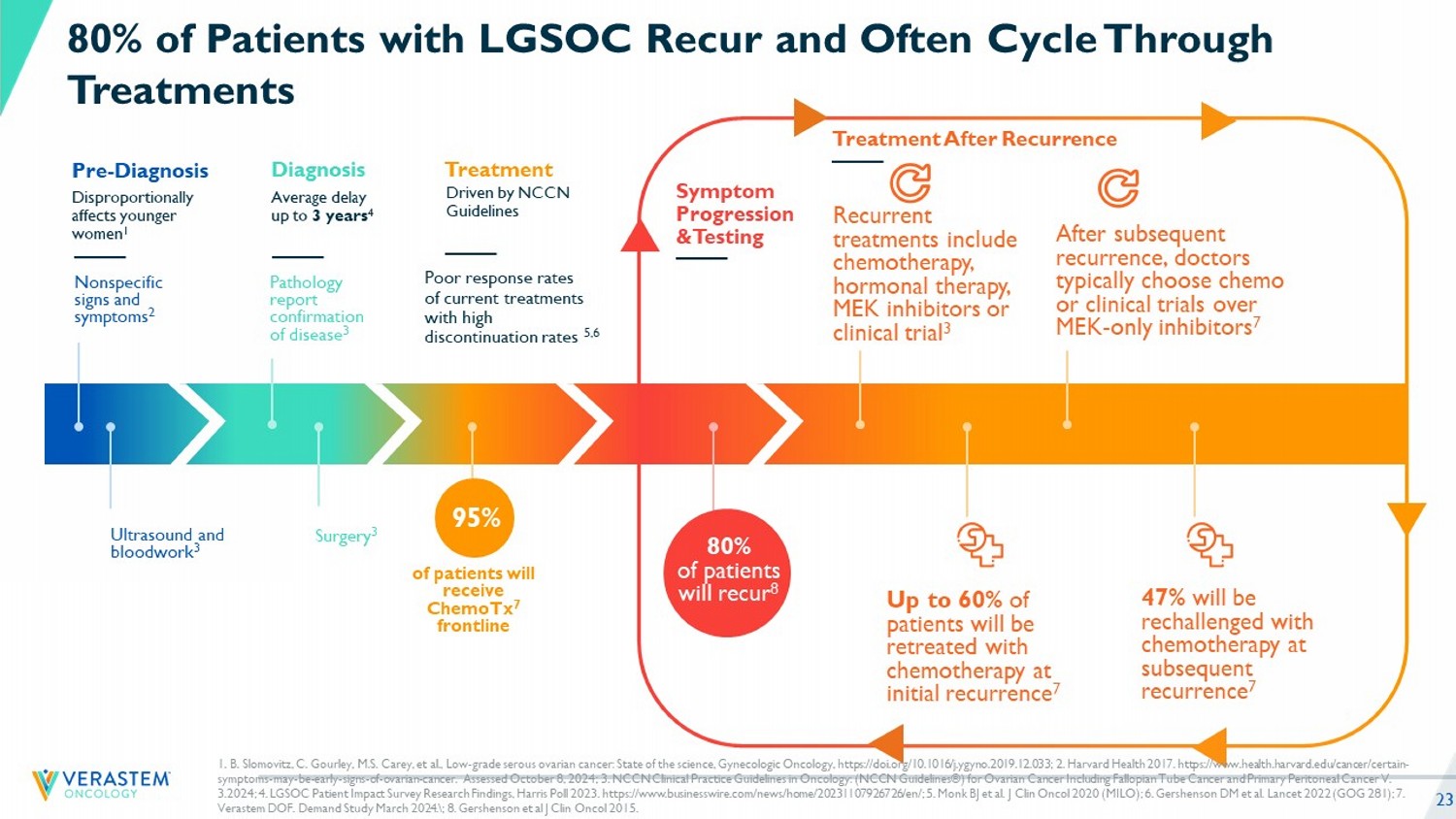
23 Disproportionally affects younger women 1 Pre - Diagnosis Nonspecific signs and symptoms 2 Ultrasound and bloodwork 3 Average delay up to 3 years 4 Diagnosis Poor r esponse rates of current treatments w ith high discontinuation rates 5,6 Treatment Symptom Progression & Testing Treatment A fter Recurrence Pathology report confirmation of disease 3 Surgery 3 of patients will receive ChemoTx 7 frontline 95% Recurrent treatments include chemotherapy, hormonal therapy, MEK inhibitors or clinical trial 3 Up to 60% of patients will be retreated with chemotherapy at initial recurrence 7 After subsequent recurrence, doctors typically choose chemo or clinical trials over MEK - only inhibitors 7 47% will be rechallenged with chemotherapy at subsequent recurrence 7 80% of Patients with LGSOC Recur and Often Cycle Through Treatments &UHDWHG�E\�5DQDK�3L[HO�6WXGLR IURP�WKH�1RXQ�3URMHFW 80% of patients will recur 8 Driven by NCCN Guidelines 1. B. Slomovitz , C. Gourley, M.S. Carey, et al., Low - grade serous ovarian cancer: State of the science, Gynecologic Oncology, https://doi.org/1 0.1016/j.ygyno.2019.12.033; 2. Harvard Health 2017. https://www.health.harvard.edu/cancer/certain - symptoms - may - be - early - signs - of - ovarian - cancer. Assessed October 8, 2024; 3. NCCN Clinical Practice Guidelines in Oncology: (NCCN Guidelines®) for Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer V. 3.2024; 4. LGSOC Patient Impact Survey Research Findings, Harris Poll 2023. https://www.businesswire.com/news/home/2023110792 672 6/en/ ; 5. Monk BJ et al. J Clin Oncol 2020 (MILO); 6. Gershenson DM et al. Lancet 2022 (GOG 281); 7. Verastem DOF. Demand Study March 2024. \ ; 8. Gershenson et al J Clin Oncol 2015.

24 Discontinuati on Rate due to AEs mPFS KRAS wt mPFS KRAS mt Median PFS Months (95% CI) ORR KRAS wt ORR KRAS mt Response Rate ORR Image assessment Therapy Trial 30% 6.3 95% CI: (3.7, 9.9) 11.4 95% CI: (3.7, 13.3) 7.2 (5.6 - 9.9) 7.1%, 95% CI: (2.1%, 17.9%) 9.1%, 95% CI: (1.9%, 26.1%) 6% 95% CI: (3%, 12%) INV SoC (n=130) (n=22 KRAS/NRAS/ BRAF mt; n=42 KRAS/NRAS/ BRAF wt ) GOG 281 1 36% 7.3 95% CI: (5.6, 12.7) 13.2 95% CI: (9.4, 20.8) 13.0 (9.9 - 15.0) 8.3%, 95% CI: (2.9%, 18.6%) 50%, 95% CI: (30.2%, 69.8%) 26% 95% CI: (19%, 35%) INV Trametinib (n=130) (n=22 KRAS/NRAS/ BRAF mt; n=42 KRAS/NRAS/ BRAF wt ) 17% 11.5 (5.7, 26.6) 14.6 (9.4, NA) 10.6 (9.2 - 14.5) 19% (8.6%, 34%) 33%, 95% CI: (16%, 55%) 13% 95% CI: (7%, 21%) BICR SoC (n=101) (n=24 KRAS mt; n=42 KRAS wt ) MILO 2 31% 10.8 (5.5, 16.7) 17.7 (12, NR) 9.1 (7.3 - 11.3) 19%, 95% CI: (11%, 29%) 44%, 95% CI: (30%, 60%) 16% 95% CI: (11%, 22%) BICR Binimetinib 2 (n=198) (n=45 KRAS mt; n=90 KRAS wt ) 1 Study GOG 281 trial Gershenson et al., Lancet 2022; 2 MILO Study. Grisham et al Clinical Cancer Research 2023, 3 MILO Study Monk et al., J Clin Oncol 2020.; SoC = Standard of Care (endocrine / chemotherapy); INV = Investigator; BICR = Blinded Independent Central Review; PFS = Progression Free Survival; CI = Confidence Interval; NR = Not Re ached Current Available Therapies Offer Relatively Poor Response Rates, High Discontinuation Rates • These studies started in 2013 and 2014 • Both MILO and GOG studies had low historical use of bevacizumab during trial conduct; % not reported • Mutation category is KRAS/BRAF/NRAS rather than just KRAS for GOG 281 • In both studies, n ot all patients had mutation status available • In the MILO study no more than 3 lines of prior chemotherapy • No prior MEK was allowed in either GOG 281 or MILO • The number of prior systemic therapies median (range) were 2 (1 - 10) in GOG 281 and 2 (1 - 8) in MILO
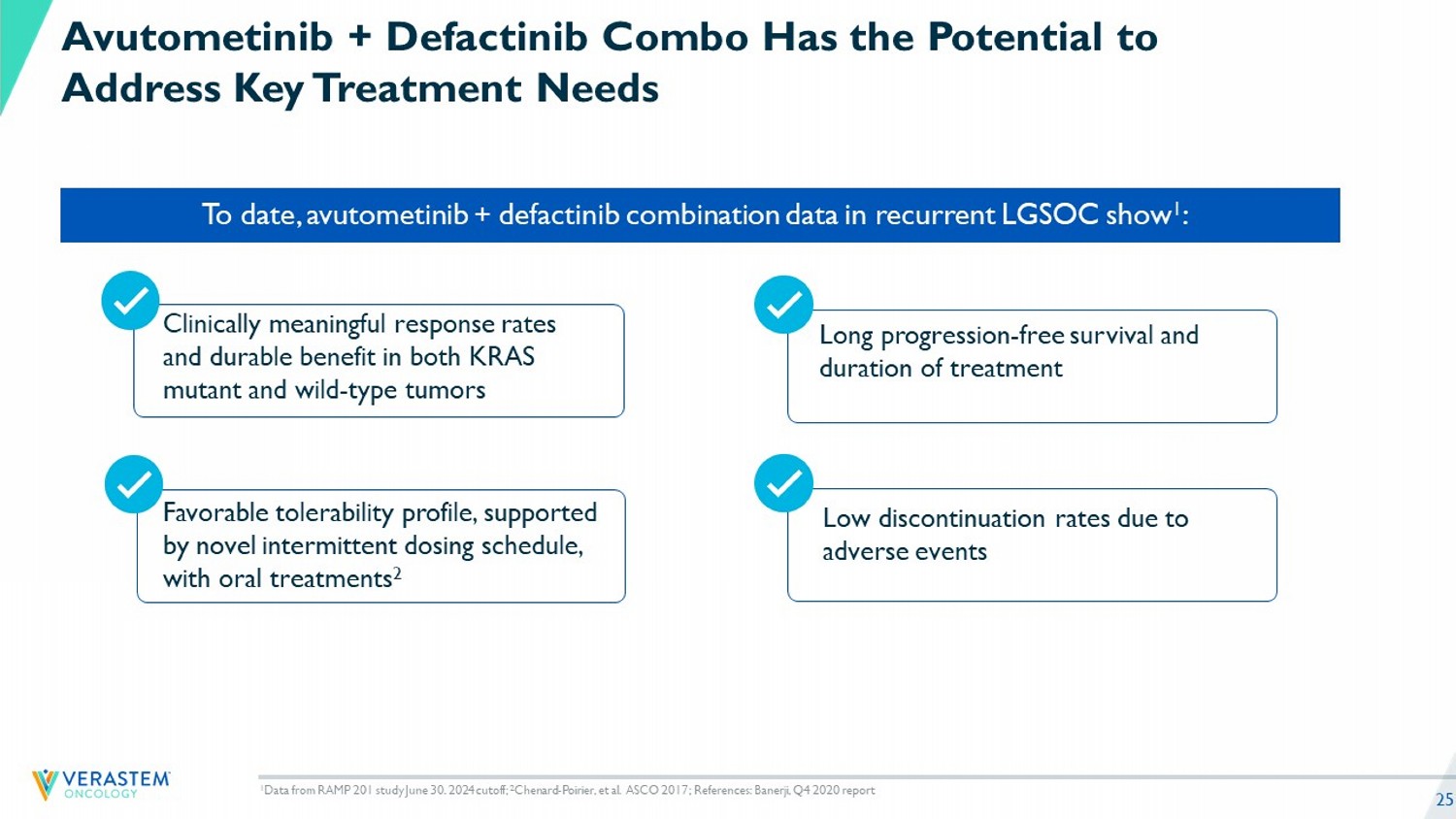
25 To date, avutometinib + defactinib combination data in recurrent LGSOC show 1 : Clinically meaningful response rates and durable benefit in both KRAS mutant and wild - type tumors Long progression - free survival and duration of treatment 1 Data from RAMP 201 study June 30. 2024 cutoff; 2 Chenard - Poirier, et al. ASCO 2017; References: Banerji, Q4 2020 report Avutometinib + Defactinib Combo Has the Potential to Address Key Treatment Needs Favorable tolerability profile, supported by novel intermittent dosing schedule, with oral treatments 2 Low discontinuation rates due to adverse events
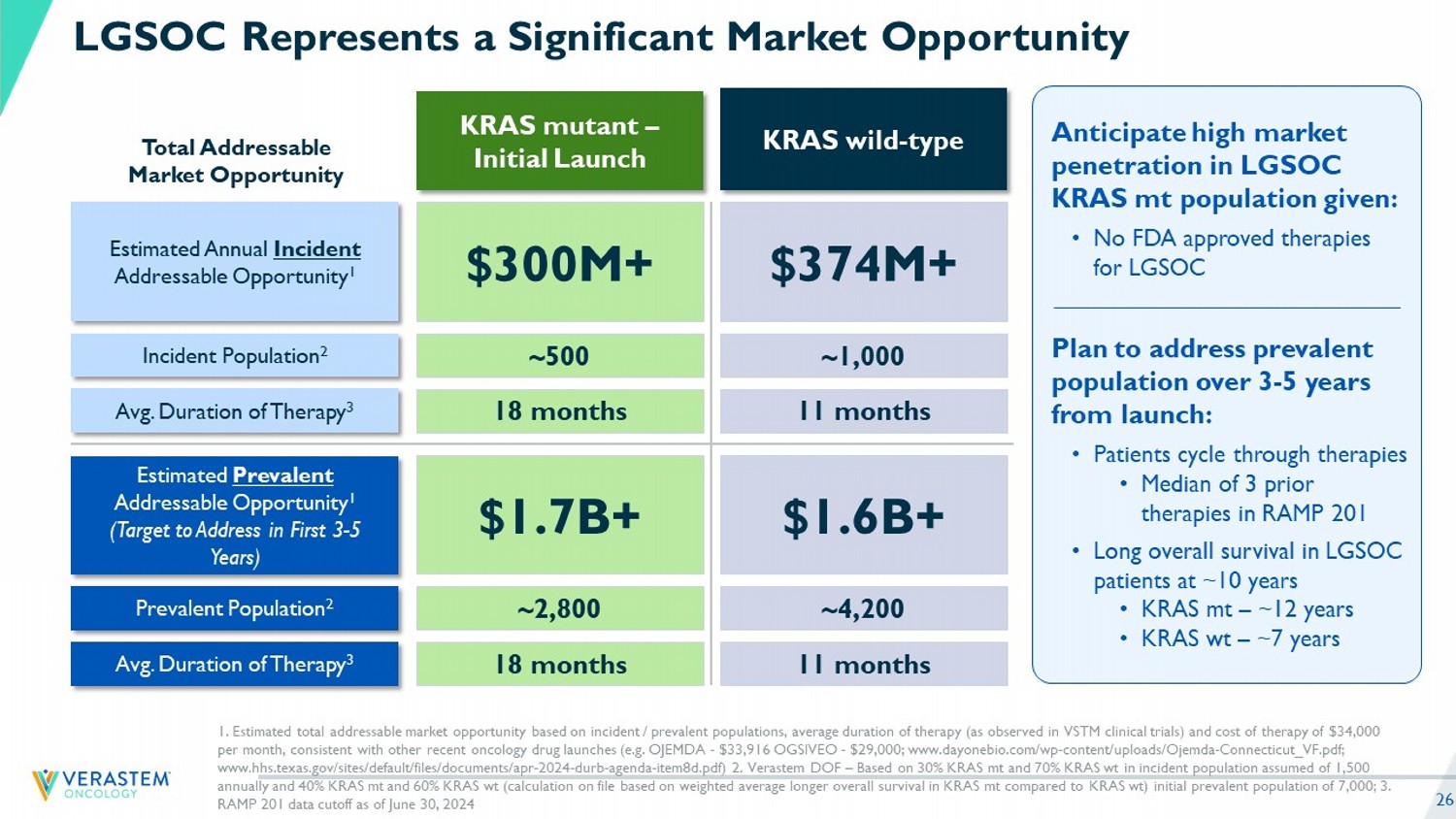
26 LGSOC Represents a Significant Market Opportunity 1. Estimated total addressable market opportunity based on incident / prevalent populations, average duration of therapy (as obs erved in VSTM clinical trials) and cost of therapy of $34,000 per month, consistent with other recent oncology drug launches (e.g. OJEMDA - $33,916 OGSIVEO - $29,000; www.dayonebio.com/wp - co ntent/uploads/Ojemda - Connecticut_VF.pdf; www.hhs.texas.gov/sites/default/files/documents/apr - 2024 - durb - agenda - item8d.pdf) 2. Verastem DOF – Based on 30% KRAS mt and 70% KRAS wt in incident population assumed of 1,500 annually and 40% KRAS mt and 60% KRAS wt (calculation on file based on weighted average longer overall survival in KRAS mt compared to KRAS wt ) initial prevalent population of 7,000; 3. RAMP 201 data cutoff as of June 30, 2 024 KRAS mutant – Initial Launch KRAS wild - type Estimated Prevalent Addressable Opportunity 1 (Target to Address in First 3 - 5 Years) Prevalent Population 2 Avg. Duration of Therapy 3 Estimated Annual Incident Addressable Opportunity 1 Incident Population 2 Avg. Duration of Therapy 3 $1.7B+ $1.6B+ ~2,800 ~4,200 18 months 11 months $300M+ $374M+ ~500 ~1,000 18 months 11 months Total Addressable Market Opportunity Anticipate high market penetration in LGSOC KRAS mt population given: • No FDA approved therapies for LGSOC Plan to address prevalent population over 3 - 5 years from launch: • Patients cycle through therapies • Median of 3 prior therapies in RAMP 201 • Long overall survival in LGSOC patients at ~10 years • KRAS mt – ~12 years • KRAS wt – ~7 years
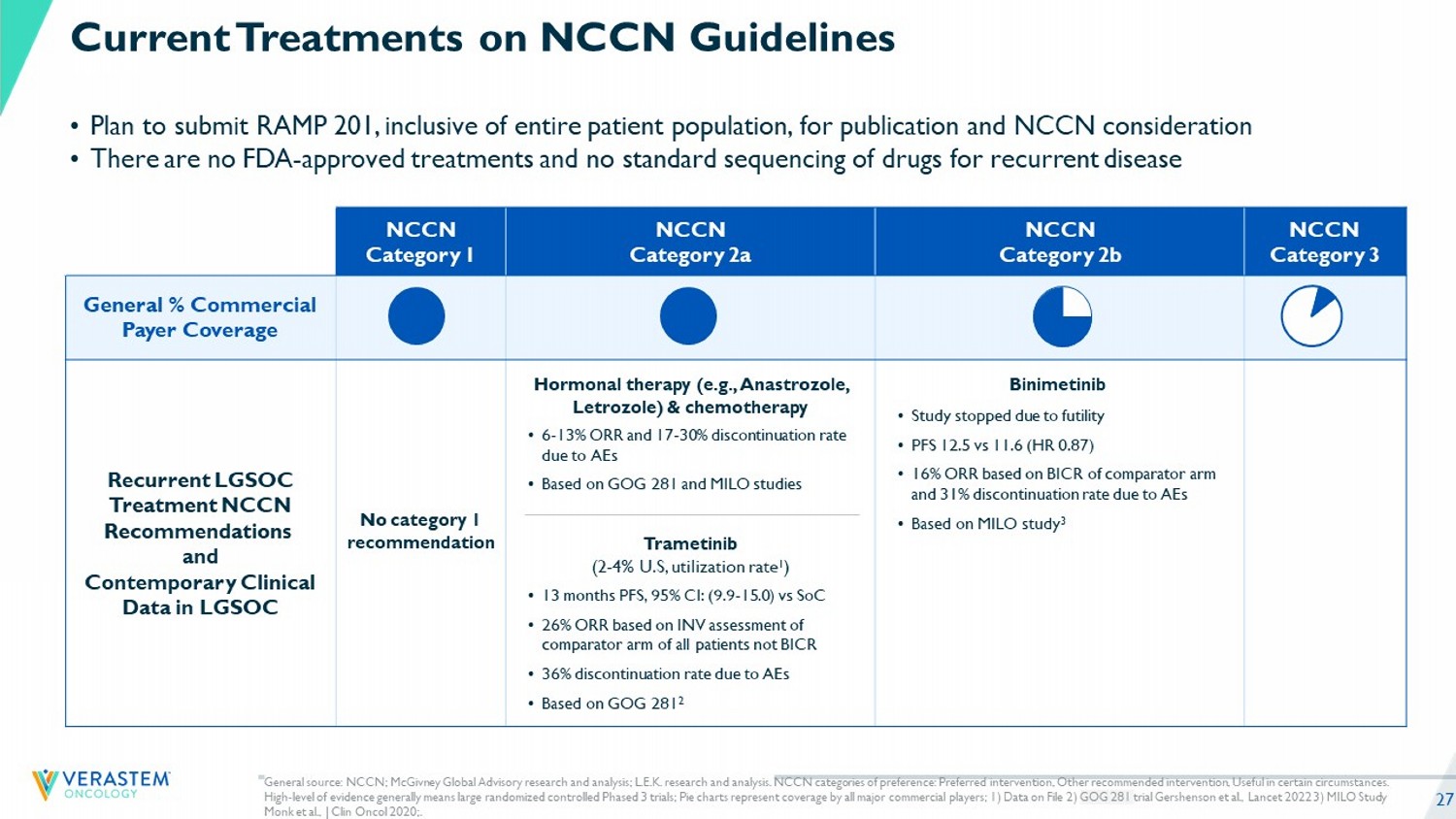
27 NCCN Category 3 NCCN Category 2b NCCN Category 2a NCCN Category 1 General % Commercial Payer Coverage No category 1 recommendation Recurrent LGSOC Treatment NCCN Recommendations and Contemporary Clinical Data in LGSOC • 6 - 13% ORR and 17 - 30% discontinuation rate due to AEs • Based on GOG 281 and MILO studies Trametinib (2 - 4% U.S, utilization rate 1 ) Hormonal therapy (e.g., Anastrozole, Letrozole) & chemotherapy • 13 months PFS, 95% CI: (9.9 - 15.0) vs SoC • 26% ORR based on INV assessment of comparator arm of all patients not BICR • 36% discontinuation rate due to AEs • Based on GOG 281 2 • Study stopped due to futility • PFS 12.5 vs 11.6 (HR 0.87) • 16% ORR based on BICR of comparator arm and 31% discontinuation rate due to AEs • Based on MILO study 3 Binimetinib • Plan to submit RAMP 201, inclusive of entire patient population, for publication and NCCN consideration • There are no FDA - approved treatments and no standard sequencing of drugs for recurrent disease Current Treatments on NCCN Guidelines General s ource: NCCN; McGivney Global Advisory research and analysis; L.E.K. research and analysis. NCCN categories of preference: Preferred intervention, Other recommended intervention, Useful in certain circumstances. High - level of evidence generally means large randomized controlled Phased 3 trials; Pie charts represent coverage by all major c ommercial players; 1 ) Data on File 2) GOG 281 trial Gershenson et al., Lancet 2022 3) MILO Study Monk et al., J Clin Oncol 2020;.
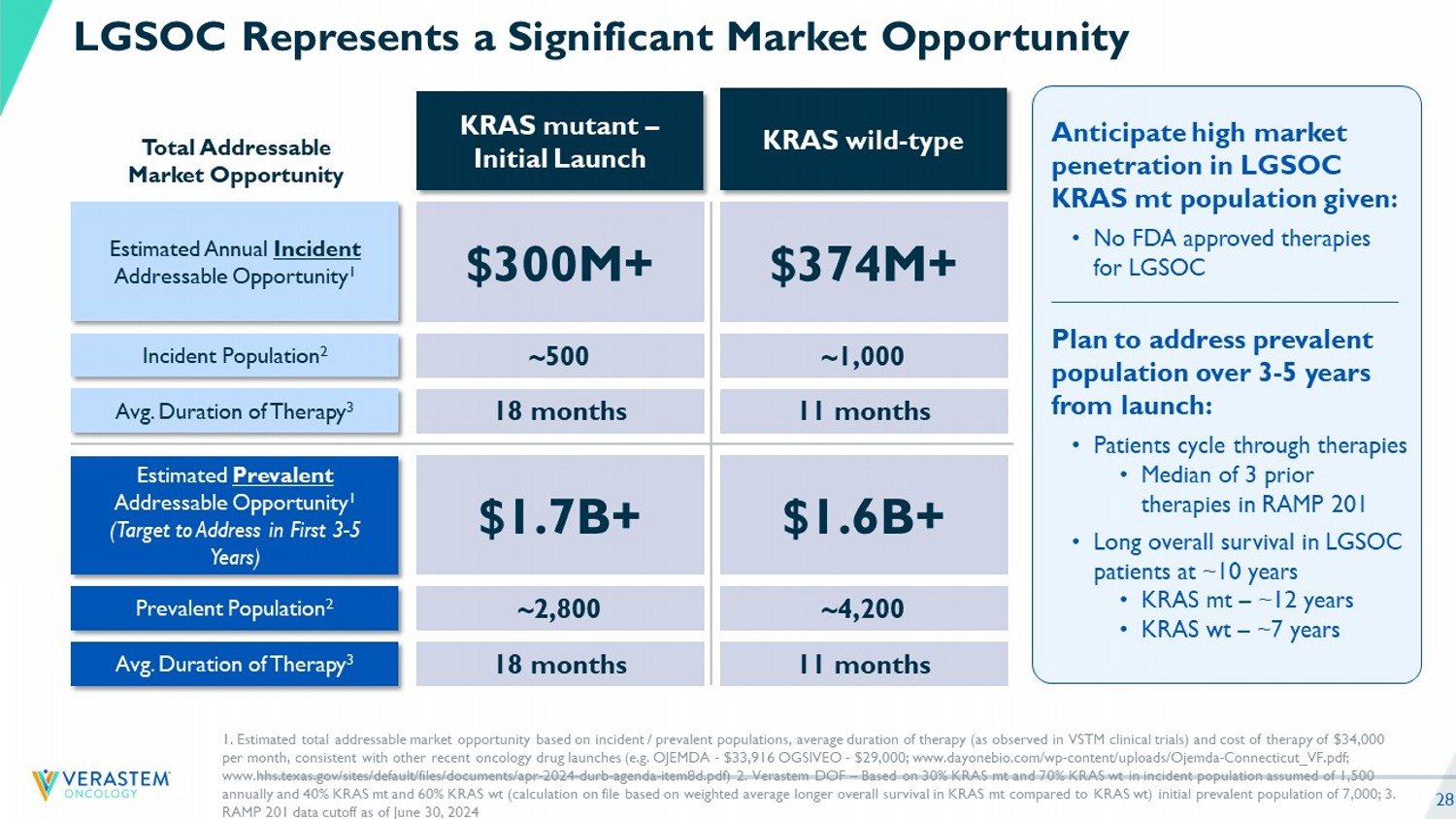
28 LGSOC Represents a Significant Market Opportunity 1. Estimated total addressable market opportunity based on incident / prevalent populations, average duration of therapy (as obs erved in VSTM clinical trials) and cost of therapy of $34,000 per month, consistent with other recent oncology drug launches (e.g. OJEMDA - $33,916 OGSIVEO - $29,000; www.dayonebio.com/wp - co ntent/uploads/Ojemda - Connecticut_VF.pdf; www.hhs.texas.gov/sites/default/files/documents/apr - 2024 - durb - agenda - item8d.pdf) 2. Verastem DOF – Based on 30% KRAS mt and 70% KRAS wt in incident population assumed of 1,500 annually and 40% KRAS mt and 60% KRAS wt (calculation on file based on weighted average longer overall survival in KRAS mt compared to KRAS wt ) initial prevalent population of 7,000; 3. RAMP 201 data cutoff as of June 30, 2 024 KRAS mutant – Initial Launch KRAS wild - type Estimated Prevalent Addressable Opportunity 1 (Target to Address in First 3 - 5 Years) Prevalent Population 2 Avg. Duration of Therapy 3 Estimated Annual Incident Addressable Opportunity 1 Incident Population 2 Avg. Duration of Therapy 3 $1.7B+ $1.6B+ ~2,800 ~4,200 18 months 11 months $300M+ $374M+ ~500 ~1,000 18 months 11 months Total Addressable Market Opportunity Anticipate high market penetration in LGSOC KRAS mt population given: • No FDA approved therapies for LGSOC Plan to address prevalent population over 3 - 5 years from launch: • Patients cycle through therapies • Median of 3 prior therapies in RAMP 201 • Long overall survival in LGSOC patients at ~10 years • KRAS mt – ~12 years • KRAS wt – ~7 years
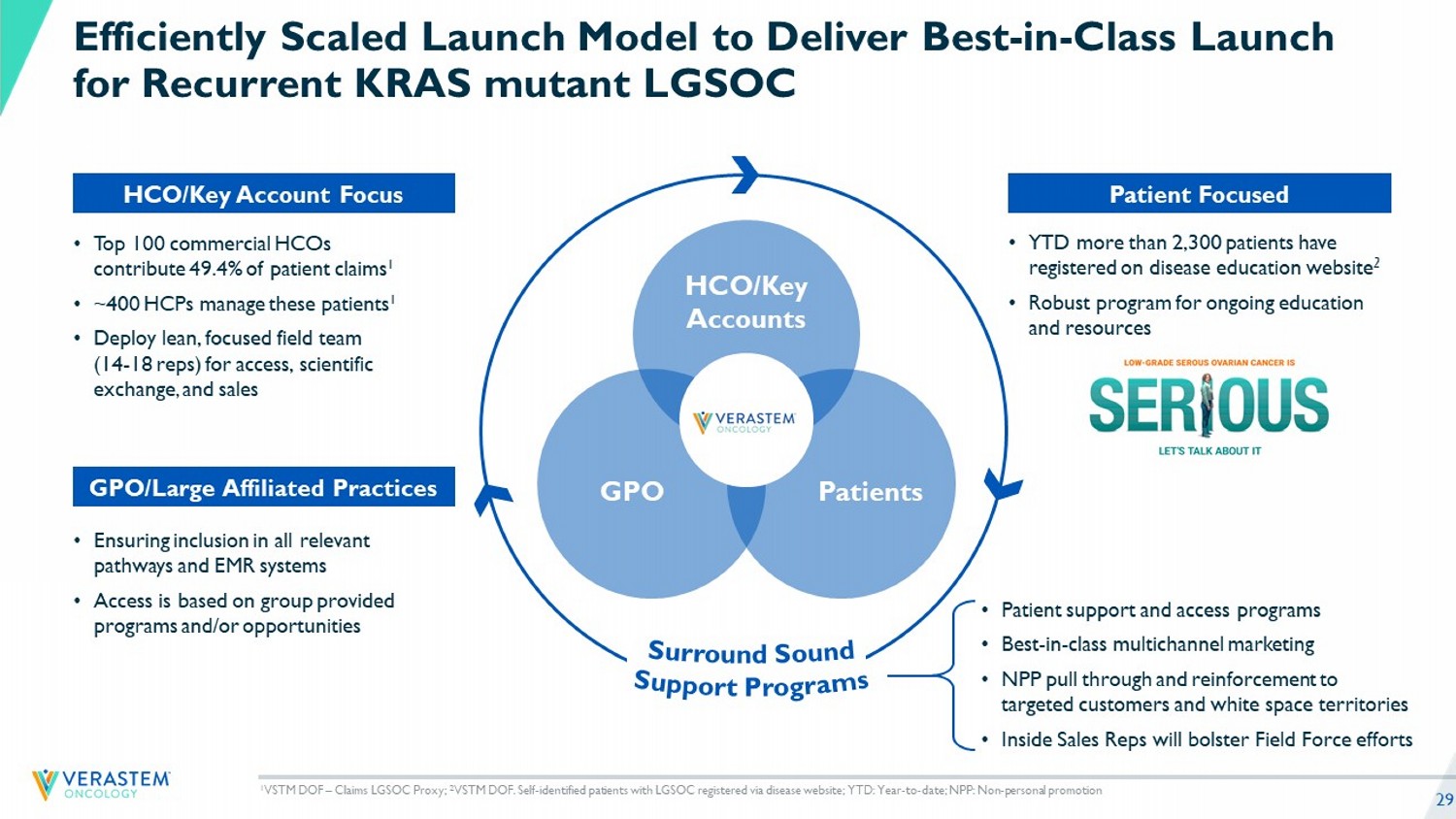
29 • Patient support and access programs • Best - in - class multichannel marketing • NPP pull through and reinforcement to targeted customers and white space territories • Inside Sales Reps will bolster Field Force efforts • YTD more than 2,300 patients have registered on disease education website 2 • Robust program for ongoing education and resources HCO/Key Accounts Patients GPO Efficiently Scaled Launch Model to Deliver Best - in - Class Launch for Recurrent KRAS mutant LGSOC Patient Focused • Top 100 commercial HCOs contribute 49.4% of patient claims 1 • ~400 HCPs manage these patients 1 • Deploy lean, focuse d field team (14 - 18 reps) for access, scientific exchange, and sales HCO/Key Account Focus • Ensuring inclusion in all relevant pathways and EMR systems • Access is based on group provided programs and/or opportunities GPO/Large Affiliated Practices 1 VSTM DOF – Claims LGSOC Proxy; 2 VSTM DOF. Self - identified patients with LGSOC registered via disease website; YTD: Year - to - date; NPP: Non - personal promotion
30 Current available therapies offer limited efficacy, relatively high discontinuation rates due to AEs; no FDA - approved therapies and no active promotion NCCN guidelines help to drive treatment decision ; will submit entire RAMP 201 dataset for NCCN consideration Avutometinib in combination with defactinib is differentiated on multiple efficacy measures , relatively low rates of discontinuation due to AEs and favorable tolerability 1k - 2k incidence with a prevalence of 6k - 8k; potential for high market penetration in KRAS mutant at launch enriching overtime with the prevalent patient population Efficiently scaled launch model to deliver best - in - class launch for recurrent KRAS mutant LGSOC Potential to Change Treat Paradigm and Improve Patient Outcomes The combination of Avutometinib and Defactinib is an investigational drug. It has not been proven to be safe or effective and h as not been approved by FDA or any other comparable regulatory authority.

31 Closing Remarks Dan Paterson, President and CEO

32 Avutometinib + Defactinib Demonstrated Durable Results Across Various Efficacy Measures in Heavily Pretreated Patients in RAMP 201 • 31% Overall ORR, 44% in KRAS mt, 17% in KRAS wt • 82% of all patients had tumor shrinkage • 14.5 months estimated mean DoT, 18.3 months in KRAS mt and 10.7 months in KRAS wt • 12.9 months median PFS, 22 months in KRAS mt, 12.8 months in KRAS wt • 10% discontinuation rates due to adverse events Clear Regulatory Path for KRAS Mutant • On track to complete the NDA submission in October for recurrent KRAS mutant LGSOC; Pursuing Accelerated Approval with Priority Review • RAMP 301 enrollment remains on track and will continue enrolling all comers • Committed to make the combination available to patients with KRAS wild - type in several ways, including a path for regulatory approval Verastem Aims to Deliver First FDA - Approved Treatment Specifically for Recurrent KRAS mutant LGSOC in 2025 Significant Market Opportunity in Area of High Unmet Need • SoC (Chemo/Hormonal) is associated with low response rates (6 - 13%) with PFS below 12 months and high discontinuation rates due to toxicity • Plan to be launch ready in 2025 to maximize market opportunity in recurrent KRAS mutant LGSOC • Plan to submit RAMP 201 for NCCN guideline review • NCCN guideline inclusion may enable patients with KRAS wild - type LGSOC to access therapy, if approved Source for all data: RAMP 201 data cut off as of June 30, 2024; LGSOC: Low - grade serous ovarian cancer; ORR: Objective Response Rate; KRAS, kirsten rat sarcoma virus; KRAS mt: mutant; KRAS wt : wild - type; PFS: Progression - free Survival; NDA: New Drug Application; SOC: Standard of Care; NCCN: National Comprehensive Cancer Network; The combination of Avutometinib and Defactinib is an investigational drug. It has not been proven to be safe or effective and h as not been approved by FDA or any other comparable regulatory authority.
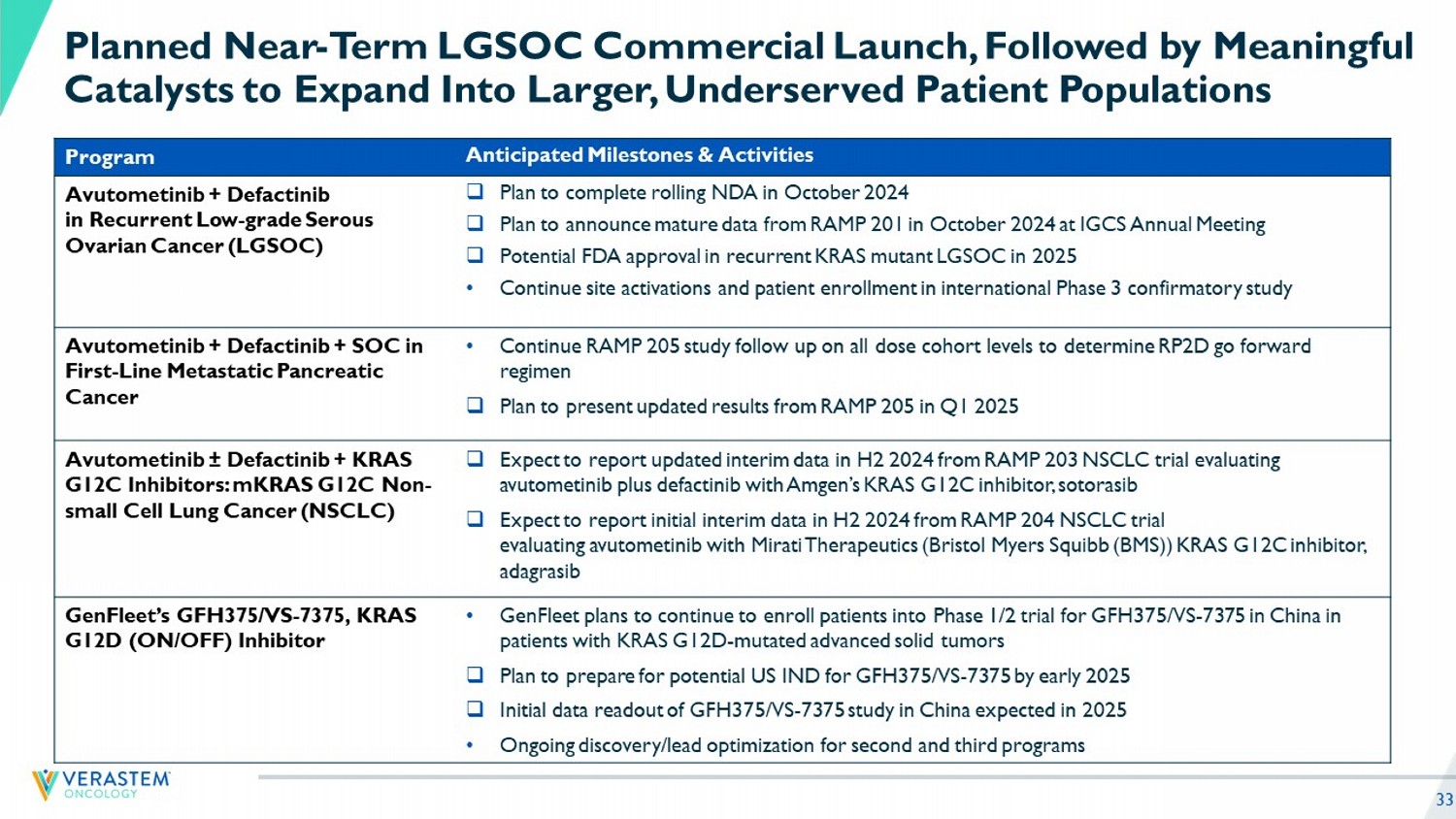
33 Planned Near - Term LGSOC Commercial Launch, Followed by Meaningful Catalysts to Expand Into Larger, Underserved Patient Populations Anticipated Milestones & Activities Program □ Plan to complete rolling NDA in October 2024 □ Plan to announce mature data from RAMP 201 in October 2024 at IGCS Annual Meeting □ Potential FDA approval in recurrent KRAS mutant LGSOC in 2025 • Continue site activations and patient enrollment in international Phase 3 confirmatory study Avutometinib + Defactinib in Recurrent Low - grade Serous Ovarian Cancer (LGSOC) • Continue RAMP 205 study follow up on all dose cohort levels to determine RP2D go forward regimen □ Plan to present updated results from RAMP 205 in Q1 2025 Avutometinib + Defactinib + SOC in First - Line Metastatic Pancreatic Cancer □ Expect to report updated interim data in H2 2024 from RAMP 203 NSCLC trial evaluating avutometinib plus defactinib with Amgen’s KRAS G12C inhibitor, sotorasib □ Expect to report initial interim data in H2 2024 from RAMP 204 NSCLC trial evaluating avutometinib with Mirati Therapeutics (Bristol Myers Squibb (BMS)) KRAS G12C inhibitor, adagrasib Avutometinib ± Defactinib + KRAS G12C Inhibitors: mKRAS G12C Non - small Cell Lung Cancer (NSCLC) • GenFleet plans to continue to enroll patients into Phase 1/2 trial for GFH375/VS - 7375 in China in patients with KRAS G12D - mutated advanced solid tumors □ Plan to prepare for potential US IND for GFH375/VS - 7375 by early 2025 □ Initial data readout of GFH375/VS - 7375 study in China expected in 2025 • Ongoing discovery/lead optimization for second and third programs GenFleet’s GFH375/VS - 7375, KRAS G12D (ON/OFF) Inhibitor

34 Thank you to all the patients, their families, and the site investigators and staff that worked on RAMP 201.

35 Q&A


































