- SERA Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
Sera Prognostics (SERA) 8-KResults of Operations and Financial Condition
Filed: 31 Jan 25, 1:31pm

Exhibit 99.1 { WE AT SERA AIM TO CHANGE PREGNANCY CARE } In order to transform the experience into one with fewer uncertainties ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. The company has no obligation to provide any updates to these forward-looking statements, even if its expectations change, whether as a result of new information, future events or otherwise, except as required by law. All forward-looking statements are expressly qualified in their entirety by this cautionary statement. Further information on potential factors, risks and uncertainties that could affect operating and financial results is included in the company’s Registration Statement on Form S-3, most recent Annual Report on Form 10-K, and/or subsequent Forms 10-Q, including in each case under the heading RISK FACTORS, and in the company’s other filings with the SEC. The information in this presentation should be considered in conjunction with a review of the company’s filings with the SEC including the information in the company’s Registration Statement on Safe Harbor Form S-3, most recent Annual Report on Form 10-K, and/or subsequent Forms 10-Q, under the heading MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIALCONDITION AND RESULTS OF OPERATIONS. Statement
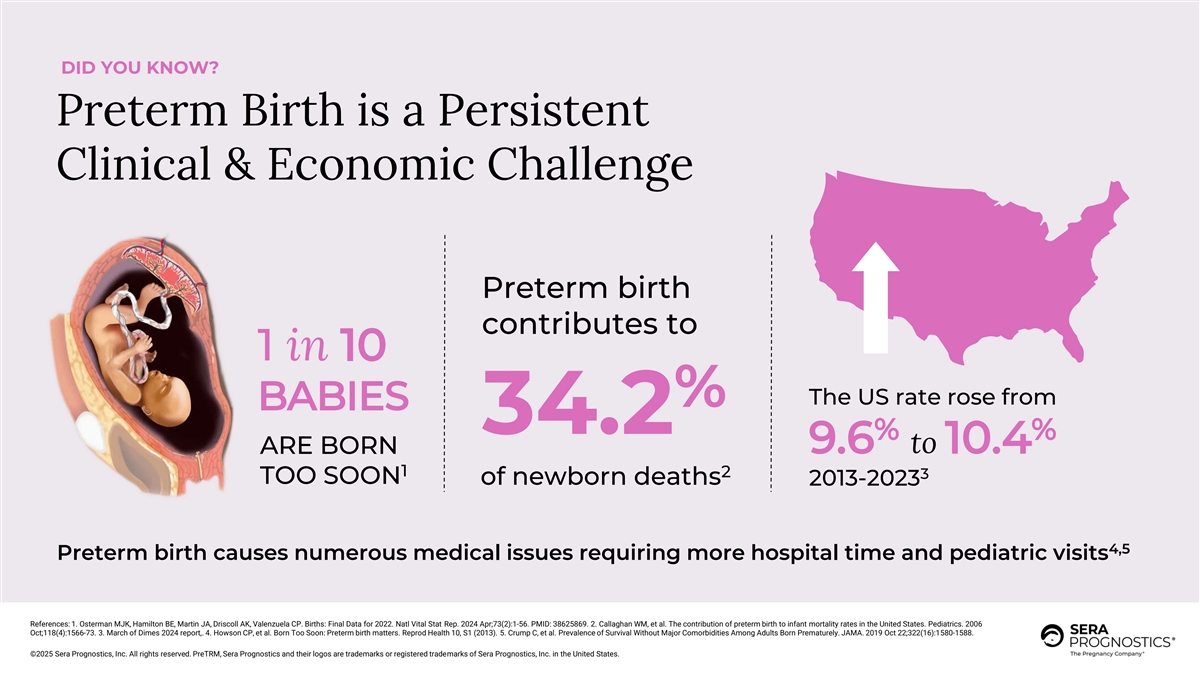
DID YOU KNOW? Preterm Birth is a Persistent Clinical & Economic Challenge Preterm birth contributes to 1 in 10 The US rate rose from % BABIES 34.2 % % ARE BORN 9.6 to 10.4 1 2 3 TOO SOON of newborn deaths 2013-2023 4,5 Preterm birth causes numerous medical issues requiring more hospital time and pediatric visits References: 1. Osterman MJK, Hamilton BE, Martin JA, Driscoll AK, Valenzuela CP. Births: Final Data for 2022. Natl Vital Stat Rep. 2024 Apr;73(2):1-56. PMID: 38625869. 2. Callaghan WM, et al. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006 Oct;118(4):1566-73. 3. March of Dimes 2024 report,. 4. Howson CP, et al. Born Too Soon: Preterm birth matters. Reprod Health 10, S1 (2013). 5. Crump C, et al. Prevalence of Survival Without Major Comorbidities Among Adults Born Prematurely. JAMA. 2019 Oct 22;322(16):1580-1588. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
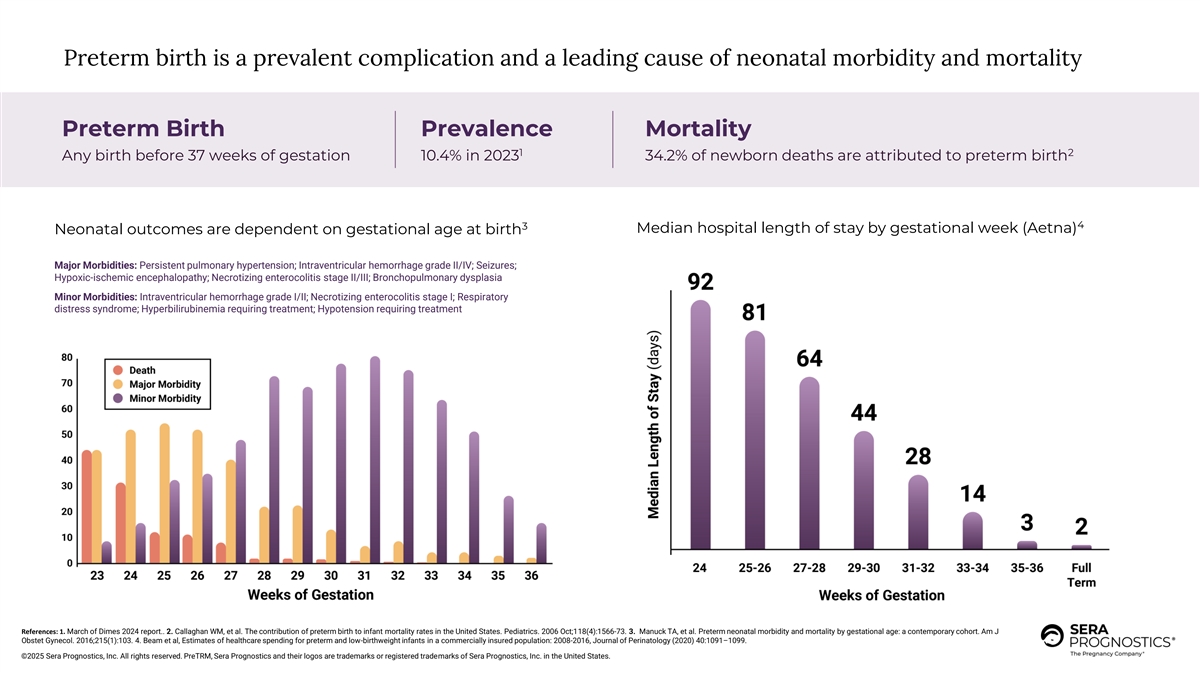
Preterm birth is a prevalent complication and a leading cause of neonatal morbidity and mortality Preterm Birth Prevalence Mortality 1 2 Any birth before 37 weeks of gestation 10.4% in 2023 34.2% of newborn deaths are attributed to preterm birth 4 3 Median hospital length of stay by gestational week (Aetna) Neonatal outcomes are dependent on gestational age at birth Major Morbidities: Persistent pulmonary hypertension; Intraventricular hemorrhage grade II/IV; Seizures; Hypoxic-ischemic encephalopathy; Necrotizing enterocolitis stage II/III; Bronchopulmonary dysplasia Minor Morbidities: Intraventricular hemorrhage grade I/II; Necrotizing enterocolitis stage I; Respiratory distress syndrome; Hyperbilirubinemia requiring treatment; Hypotension requiring treatment References: 1. March of Dimes 2024 report.. 2. Callaghan WM, et al. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006 Oct;118(4):1566-73. 3. Manuck TA, et al. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol. 2016;215(1):103. 4. Beam et al, Estimates of healthcare spending for preterm and low-birthweight infants in a commercially insured population: 2008-2016, Journal of Perinatology (2020) 40:1091–1099. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
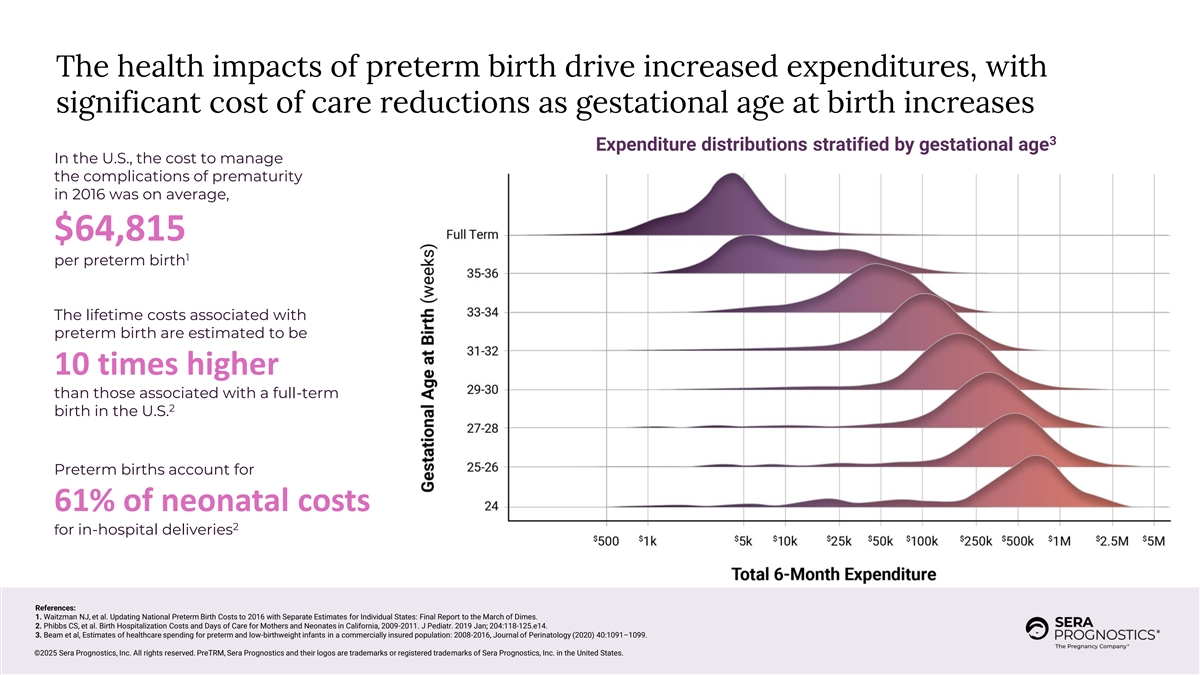
The health impacts of preterm birth drive increased expenditures, with significant cost of care reductions as gestational age at birth increases 3 Expenditure distributions stratified by gestational age In the U.S., the cost to manage the complications of prematurity in 2016 was on average, $64,815 1 per preterm birth The lifetime costs associated with preterm birth are estimated to be 10 times higher than those associated with a full-term 2 birth in the U.S. Preterm births account for 61% of neonatal costs 2 for in-hospital deliveries References: 1. Waitzman NJ, et al. Updating National Preterm Birth Costs to 2016 with Separate Estimates for Individual States: Final Report to the March of Dimes. 2. Phibbs CS, et al. Birth Hospitalization Costs and Days of Care for Mothers and Neonates in California, 2009-2011. J Pediatr. 2019 Jan; 204:118-125.e14. 3. Beam et al, Estimates of healthcare spending for preterm and low-birthweight infants in a commercially insured population: 2008-2016, Journal of Perinatology (2020) 40:1091–1099. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

Importantly, an 50% effective treatment to reduce PTB OF PREGNANT “ ACOG defined characteristics of should be available, WOMEN who deliver an effective screening program and the screening prematurely have no 1 program should be known risk factors feasible, cost- effective, and accessible to all IDENTIFYING PATIENTS 2 patients. AT HIGHER RISK OF PRETERM BIRTH IS A CLINICAL CHALLENGE References: 1. Institute of Medicine Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): National Academies Press; 2007. 2. American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Obstetrics. Prediction and Prevention of Spontaneous Preterm Birth: ACOG Practice Bulletin, Number 234. Obstetrics Gynecol. 2021 Aug 1;138(2):e65-e90. doi: 10.1097/AOG.0000000000004479. PMID: 34293771. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

Given the limitations of current screening practices for pregnancy complications, scientists at Sera discovered a biomarker risk predictor that fills a critical diagnostic gap Answers provided by PROTEOMICS Proteomics provide insights into the biology of each pregnancy and go beyond genetic inheritance Proteins reflect the functions of specific cells allowing for early detection and intervention before symptoms arise ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
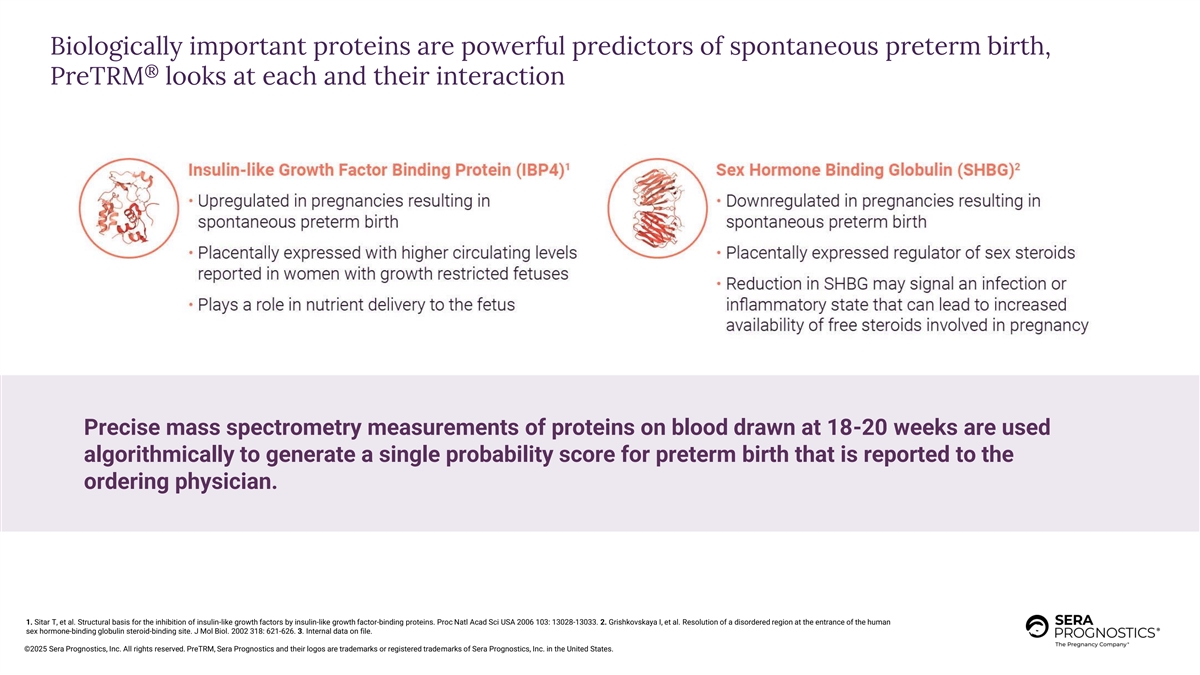
Biologically important proteins are powerful predictors of spontaneous preterm birth, ® PreTRM looks at each and their interaction Precise mass spectrometry measurements of proteins on blood drawn at 18-20 weeks are used algorithmically to generate a single probability score for preterm birth that is reported to the ordering physician. 1. Sitar T, et al. Structural basis for the inhibition of insulin-like growth factors by insulin-like growth factor-binding proteins. Proc Natl Acad Sci USA 2006 103: 13028-13033. 2. Grishkovskaya I, et al. Resolution of a disordered region at the entrance of the human sex hormone-binding globulin steroid-binding site. J Mol Biol. 2002 318: 621-626. 3. Internal data on file. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
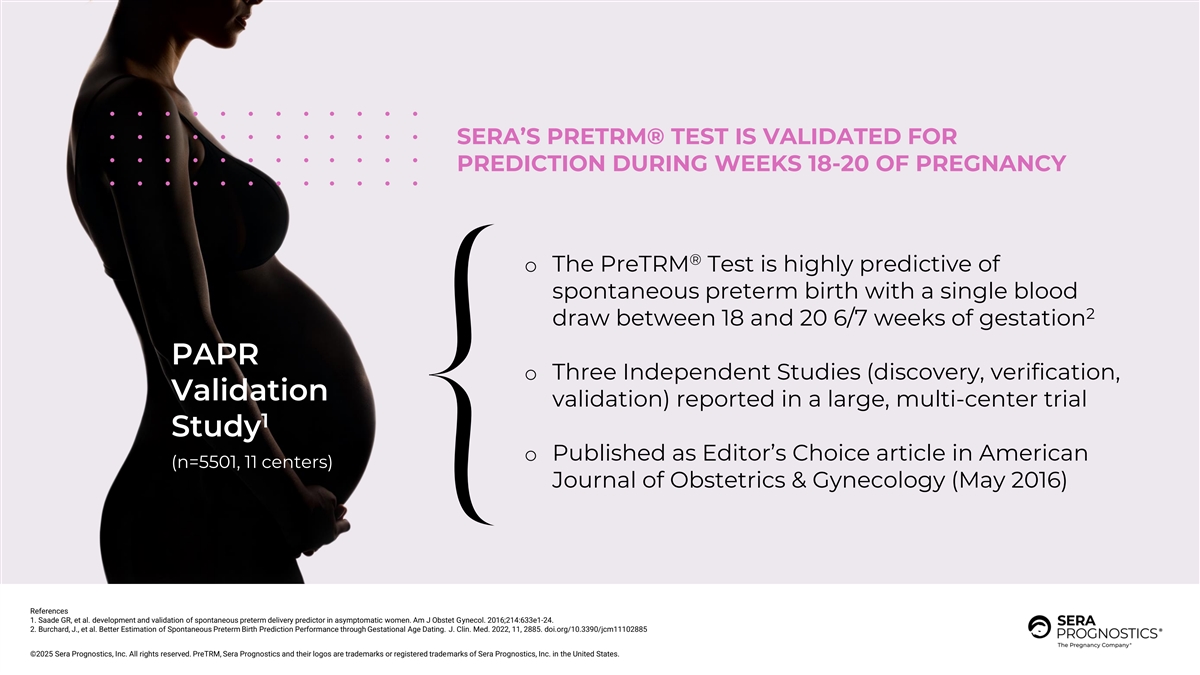
SERA’S PRETRM® TEST IS VALIDATED FOR PREDICTION DURING WEEKS 18-20 OF PREGNANCY ® o The PreTRM Test is highly predictive of spontaneous preterm birth with a single blood 2 draw between 18 and 20 6/7 weeks of gestation PAPR o Three Independent Studies (discovery, verification, Validation validation) reported in a large, multi-center trial 1 Study o Published as Editor’s Choice article in American (n=5501, 11 centers) Journal of Obstetrics & Gynecology (May 2016) References 1. Saade GR, et al. development and validation of spontaneous preterm delivery predictor in asymptomatic women. Am J Obstet Gynecol. 2016;214:633e1-24. 2. Burchard, J., et al. Better Estimation of Spontaneous Preterm Birth Prediction Performance through Gestational Age Dating. J. Clin. Med. 2022, 11, 2885. doi.org/10.3390/jcm11102885 ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

® PreTRM was designed to screen women without obvious risk factors for preterm birth After a physician places the order { OUR TESTING PROCESS SEAMLESSLY INTEGRATES INTO WOMEN’S HEALTH CLINICS } Blood sample kits are Sample collection can be Results delivered in an provided for testing performed by staff and average of five days 0/7- 6/7 during 18 20 weeks aligns with preexisting from a CLIA-certified, with prepaid shipment appointments CAP-accredited lab to Sera’s lab ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

® THE PRETRM TEST The first of its kind to detect premature birth ® The PreTRM Test predicts a patient’s individual risk of spontaneous preterm delivery in asymptomatic singleton pregnancies through a single blood collection performed during weeks 18 through 20 of gestational age. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

TEST REPORT Communicates a patient’s risk of spontaneous preterm birth & helps Final physicians engage with patients Pregnancies identified as higher risk by the PreTRM Test are at increased risk for*: Longer neonatal Spontaneous hospital length preterm birth of stay Severe adverse neonatal outcomes *Reference: Burchard J, et al. Clinical Validation of a Proteomic Biomarker Threshold for Increased Risk of Spontaneous Preterm Birth and Associated Clinical Outcomes: A Replication Study. J. Clin. Med. 2021, 10, 5088. doi: 10.3390/jcm10215088. Available at https://www.mdpi.com/2077-0383/10/21/5088 ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
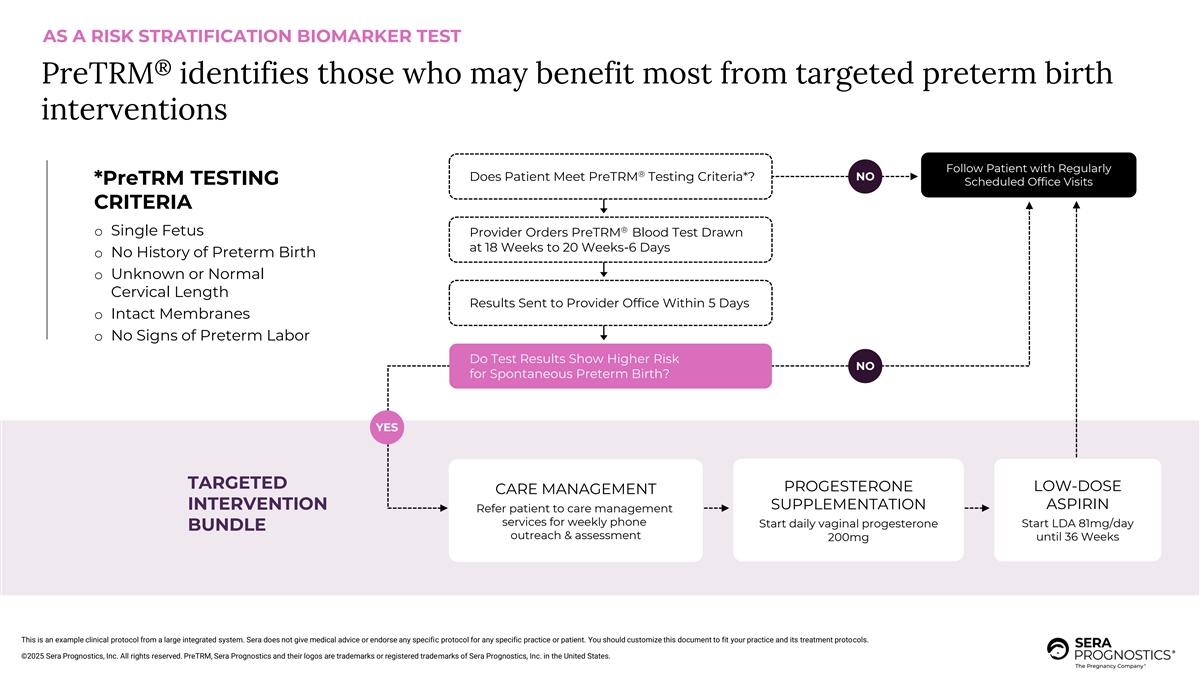
AS A RISK STRATIFICATION BIOMARKER TEST ® PreTRM identifies those who may benefit most from targeted preterm birth interventions Follow Patient with Regularly ® Does Patient Meet PreTRM Testing Criteria*? NO *PreTRM TESTING Scheduled Office Visits CRITERIA ® o Single Fetus Provider Orders PreTRM Blood Test Drawn at 18 Weeks to 20 Weeks-6 Days o No History of Preterm Birth o Unknown or Normal Cervical Length Results Sent to Provider Office Within 5 Days o Intact Membranes o No Signs of Preterm Labor Do Test Results Show Higher Risk NO for Spontaneous Preterm Birth? YES TARGETED PROGESTERONE LOW-DOSE CARE MANAGEMENT ASPIRIN INTERVENTION SUPPLEMENTATION Refer patient to care management services for weekly phone Start daily vaginal progesterone Start LDA 81mg/day BUNDLE outreach & assessment 200mg until 36 Weeks This is an example clinical protocol from a large integrated system. Sera does not give medical advice or endorse any specific protocol for any specific practice or patient. You should customize this document to fit your practice and its treatment protocols. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
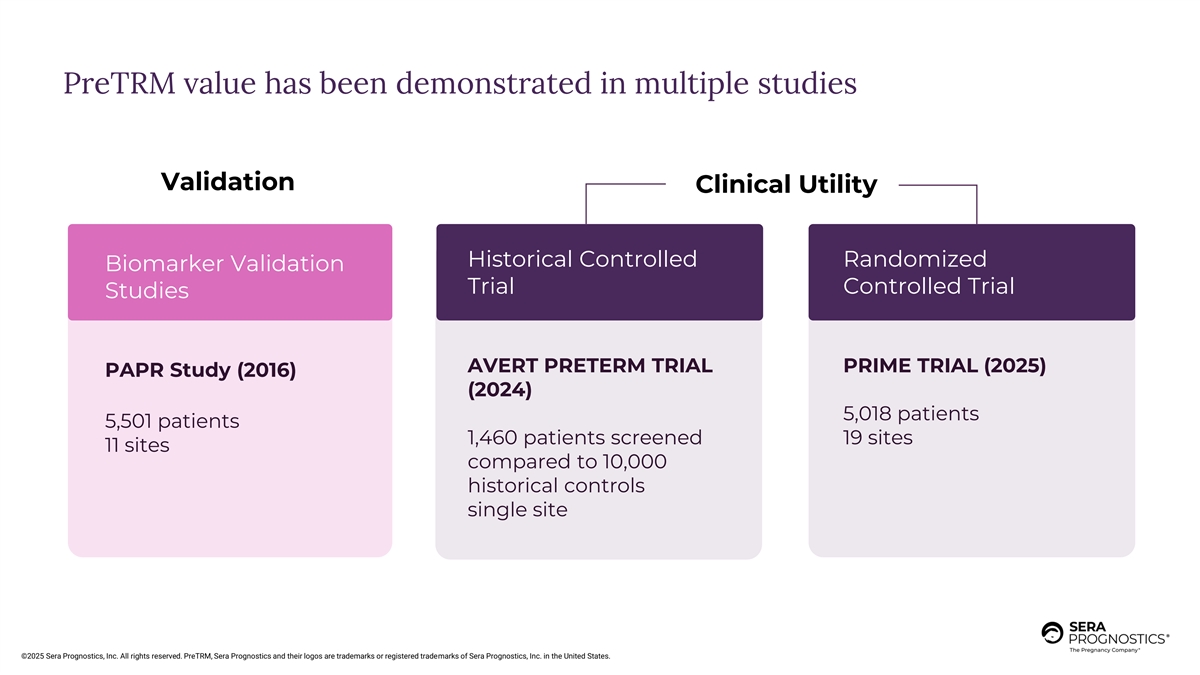
PreTRM value has been demonstrated in multiple studies Validation Clinical Utility Historical Controlled Randomized Biomarker Validation Trial Controlled Trial Studies AVERT PRETERM TRIAL PRIME TRIAL (2025) PAPR Study (2016) (2024) 5,018 patients 5,501 patients 1,460 patients screened 19 sites 11 sites compared to 10,000 historical controls single site ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
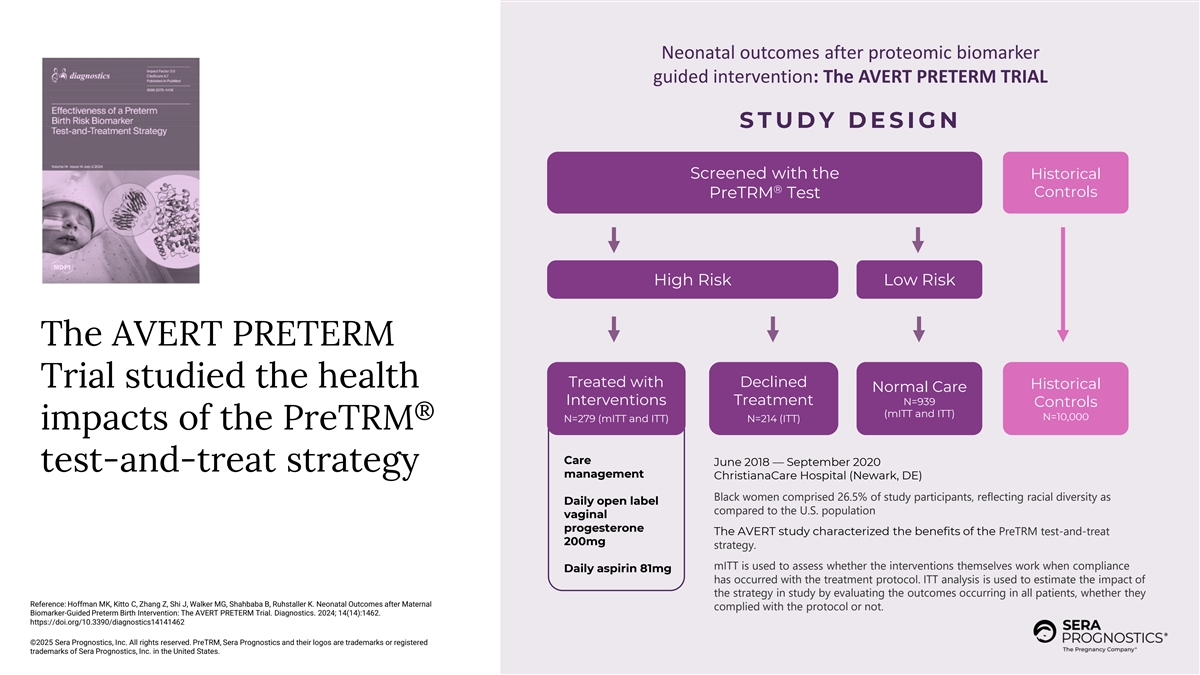
Neonatal outcomes after proteomic biomarker guided intervention: The AVERT PRETERM TRIAL S T U D Y D E S I G N Screened with the Historical ® Controls PreTRM Test High Risk Low Risk The AVERT PRETERM Trial studied the health Treated with Declined Historical Normal Care Interventions Treatment N=939 Controls (mITT and ITT) ® N=10,000 N=279 (mITT and ITT) N=214 (ITT) impacts of the PreTRM Care June 2018 — September 2020 test-and-treat strategy management ChristianaCare Hospital (Newark, DE) Black women comprised 26.5% of study participants, reflecting racial diversity as Daily open label compared to the U.S. population vaginal progesterone The AVERT study characterized the benefits of the PreTRM test-and-treat 200mg strategy. mITT is used to assess whether the interventions themselves work when compliance Daily aspirin 81mg has occurred with the treatment protocol. ITT analysis is used to estimate the impact of the strategy in study by evaluating the outcomes occurring in all patients, whether they Reference: Hoffman MK, Kitto C, Zhang Z, Shi J, Walker MG, Shahbaba B, Ruhstaller K. Neonatal Outcomes after Maternal complied with the protocol or not. Biomarker-Guided Preterm Birth Intervention: The AVERT PRETERM Trial. Diagnostics. 2024; 14(14):1462. https://doi.org/10.3390/diagnostics14141462 ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
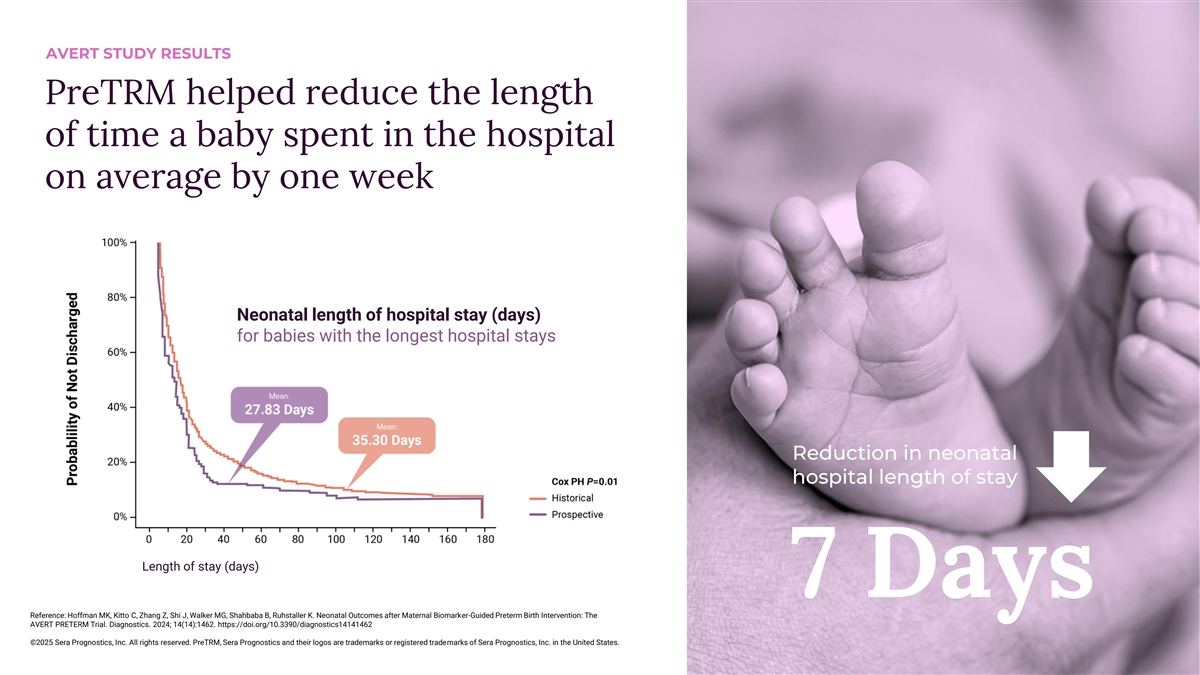
AVERT STUDY RESULTS PreTRM helped reduce the length of time a baby spent in the hospital on average by one week Neonatal length of hospital stay (days) for babies with the longest hospital stays Reduction in neonatal hospital length of stay Length of stay (days) 7 Days Reference: Hoffman MK, Kitto C, Zhang Z, Shi J, Walker MG, Shahbaba B, Ruhstaller K. Neonatal Outcomes after Maternal Biomarker-Guided Preterm Birth Intervention: The AVERT PRETERM Trial. Diagnostics. 2024; 14(14):1462. https://doi.org/10.3390/diagnostics14141462 ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
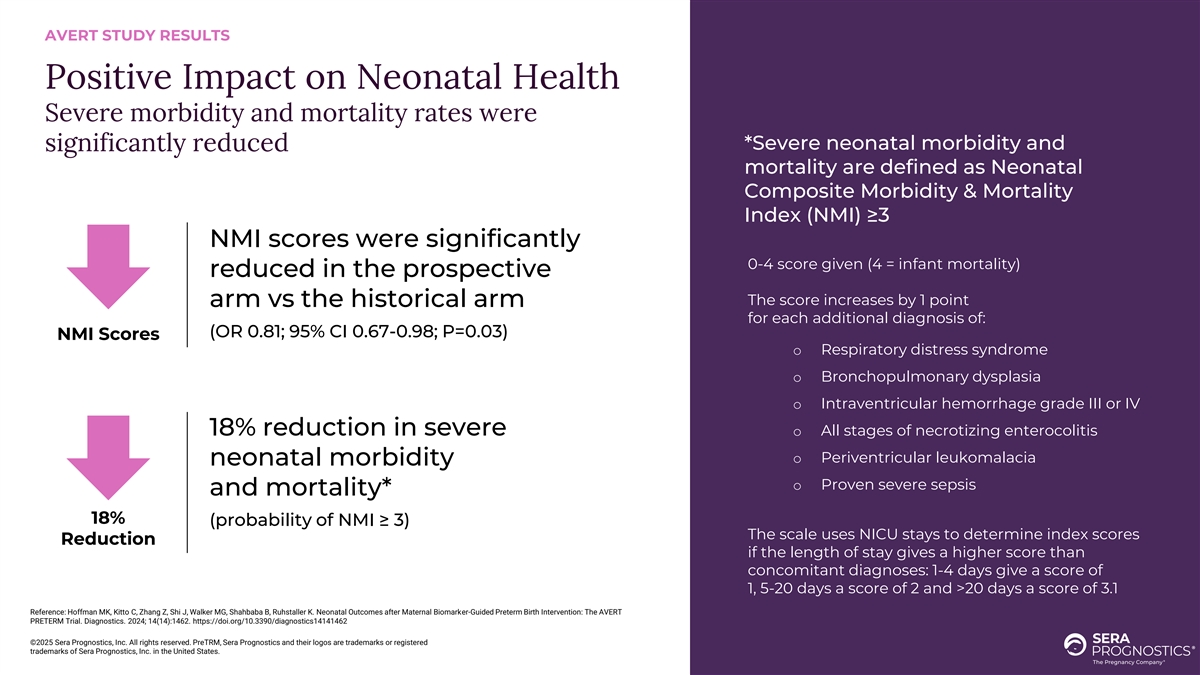
AVERT STUDY RESULTS Positive Impact on Neonatal Health Severe morbidity and mortality rates were *Severe neonatal morbidity and significantly reduced mortality are defined as Neonatal Composite Morbidity & Mortality Index (NMI) ≥3 NMI scores were significantly 0-4 score given (4 = infant mortality) reduced in the prospective The score increases by 1 point arm vs the historical arm for each additional diagnosis of: (OR 0.81; 95% CI 0.67-0.98; P=0.03) NMI Scores o Respiratory distress syndrome o Bronchopulmonary dysplasia o Intraventricular hemorrhage grade III or IV 18% reduction in severe o All stages of necrotizing enterocolitis o Periventricular leukomalacia neonatal morbidity o Proven severe sepsis and mortality* 18% (probability of NMI ≥ 3) The scale uses NICU stays to determine index scores Reduction if the length of stay gives a higher score than concomitant diagnoses: 1-4 days give a score of 1, 5-20 days a score of 2 and >20 days a score of 3.1 Reference: Hoffman MK, Kitto C, Zhang Z, Shi J, Walker MG, Shahbaba B, Ruhstaller K. Neonatal Outcomes after Maternal Biomarker-Guided Preterm Birth Intervention: The AVERT PRETERM Trial. Diagnostics. 2024; 14(14):1462. https://doi.org/10.3390/diagnostics14141462 ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
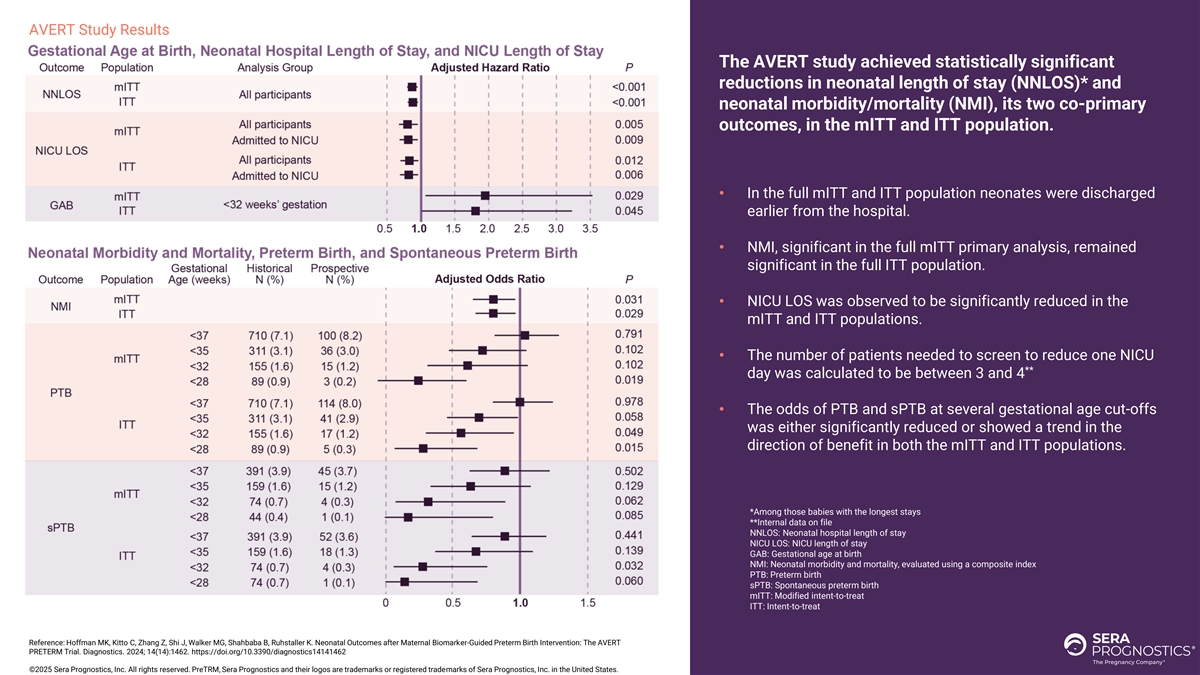
AVERT Study Results The AVERT study achieved statistically significant reductions in neonatal length of stay (NNLOS)* and neonatal morbidity/mortality (NMI), its two co-primary outcomes, in the mITT and ITT population. • In the full mITT and ITT population neonates were discharged earlier from the hospital. • NMI, significant in the full mITT primary analysis, remained significant in the full ITT population. • NICU LOS was observed to be significantly reduced in the mITT and ITT populations. • The number of patients needed to screen to reduce one NICU ** day was calculated to be between 3 and 4 • The odds of PTB and sPTB at several gestational age cut-offs was either significantly reduced or showed a trend in the direction of benefit in both the mITT and ITT populations. *Among those babies with the longest stays **Internal data on file NNLOS: Neonatal hospital length of stay NICU LOS: NICU length of stay GAB: Gestational age at birth NMI: Neonatal morbidity and mortality, evaluated using a composite index PTB: Preterm birth sPTB: Spontaneous preterm birth mITT: Modified intent-to-treat ITT: Intent-to-treat Reference: Hoffman MK, Kitto C, Zhang Z, Shi J, Walker MG, Shahbaba B, Ruhstaller K. Neonatal Outcomes after Maternal Biomarker-Guided Preterm Birth Intervention: The AVERT PRETERM Trial. Diagnostics. 2024; 14(14):1462. https://doi.org/10.3390/diagnostics14141462 ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
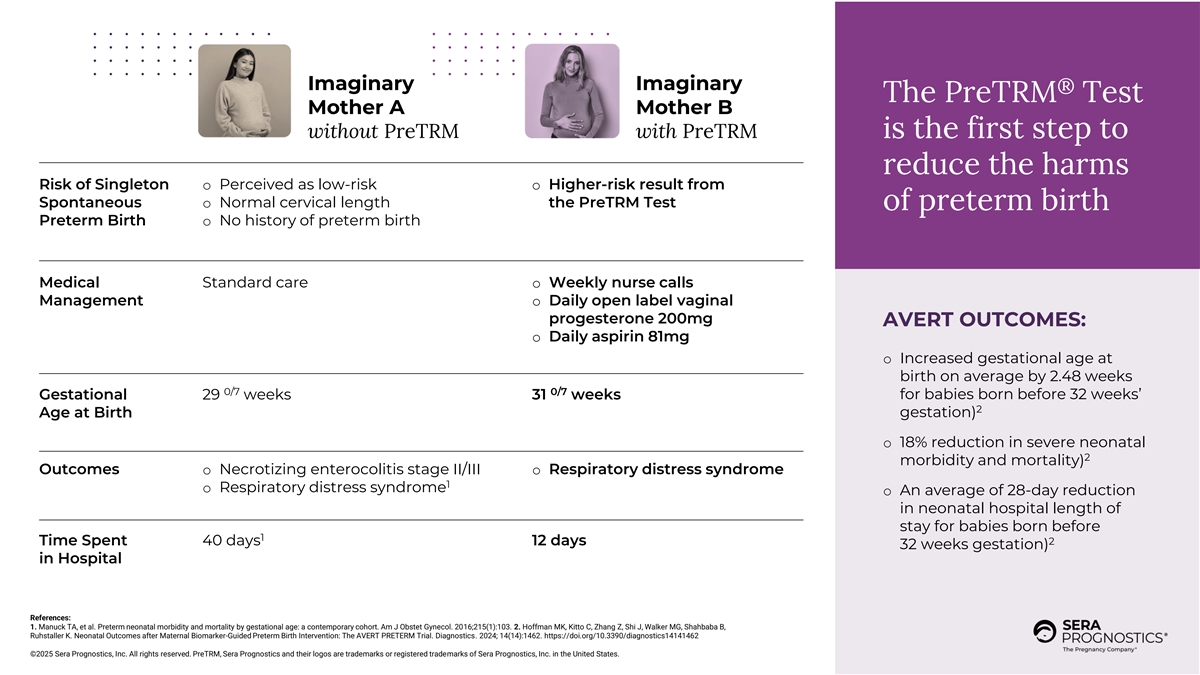
Imaginary Imaginary ® The PreTRM Test Mother A Mother B without PreTRM with PreTRM is the first step to reduce the harms Risk of Singleton o Perceived as low-risk o Higher-risk result from Spontaneous o Normal cervical length the PreTRM Test of preterm birth Preterm Birth o No history of preterm birth Medical Standard care o Weekly nurse calls Management o Daily open label vaginal progesterone 200mg AVERT OUTCOMES: o Daily aspirin 81mg o Increased gestational age at birth on average by 2.48 weeks 0/7 0/7 Gestational 29 weeks 31 weeks for babies born before 32 weeks’ 2 Age at Birth gestation) o 18% reduction in severe neonatal 2 morbidity and mortality) Outcomes o Necrotizing enterocolitis stage II/III o Respiratory distress syndrome 1 o Respiratory distress syndrome o An average of 28-day reduction in neonatal hospital length of stay for babies born before 1 Time Spent 40 days 12 days 2 32 weeks gestation) in Hospital References: 1. Manuck TA, et al. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol. 2016;215(1):103. 2. Hoffman MK, Kitto C, Zhang Z, Shi J, Walker MG, Shahbaba B, Ruhstaller K. Neonatal Outcomes after Maternal Biomarker-Guided Preterm Birth Intervention: The AVERT PRETERM Trial. Diagnostics. 2024; 14(14):1462. https://doi.org/10.3390/diagnostics14141462 ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

PRIME STUDY In 2024, Sera completed a pivotal multicenter national trial: PRIME Prematurity Risk Assessment Combined With Clinical Interventions for Improving Neonatal outcoMEs (PRIME) A pioneering clinical trial assessing the efficacy of the Achievement of one or more PreTRM Test and preventive interventions in lowering primary endpoints resulted in the the occurrence of adverse pregnancy outcomes early termination of the study ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
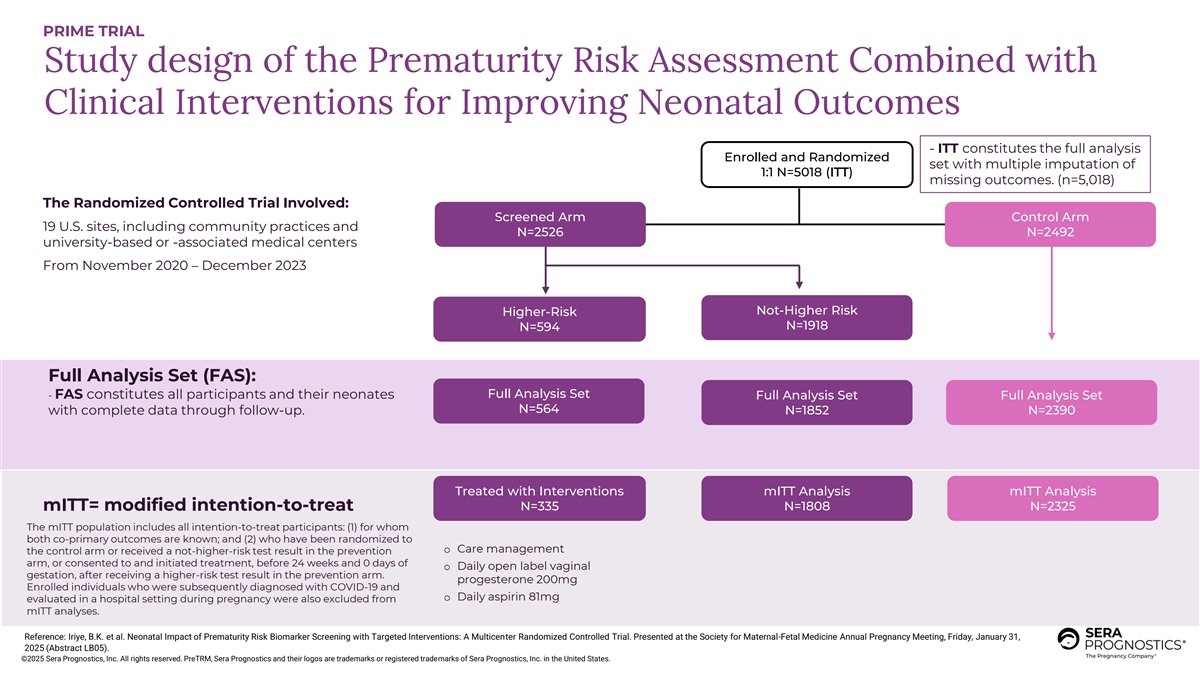
PRIME TRIAL Study design of the Prematurity Risk Assessment Combined with Clinical Interventions for Improving Neonatal Outcomes - ITT constitutes the full analysis Enrolled and Randomized set with multiple imputation of 1:1 N=5018 (ITT) missing outcomes. (n=5,018) The Randomized Controlled Trial Involved: Screened Arm Control Arm 19 U.S. sites, including community practices and N=2526 N=2492 university-based or -associated medical centers From November 2020 – December 2023 Not-Higher Risk Higher-Risk N=1918 N=594 Full Analysis Set (FAS): Full Analysis Set - FAS constitutes all participants and their neonates Full Analysis Set Full Analysis Set N=564 with complete data through follow-up. N=1852 N=2390 Treated with Interventions mITT Analysis mITT Analysis N=335 N=1808 N=2325 mITT= modified intention-to-treat The mITT population includes all intention-to-treat participants: (1) for whom both co-primary outcomes are known; and (2) who have been randomized to o Care management the control arm or received a not-higher-risk test result in the prevention arm, or consented to and initiated treatment, before 24 weeks and 0 days of o Daily open label vaginal gestation, after receiving a higher-risk test result in the prevention arm. progesterone 200mg Enrolled individuals who were subsequently diagnosed with COVID-19 and o Daily aspirin 81mg evaluated in a hospital setting during pregnancy were also excluded from mITT analyses. Reference: Iriye, B.K. et al. Neonatal Impact of Prematurity Risk Biomarker Screening with Targeted Interventions: A Multicenter Randomized Controlled Trial. Presented at the Society for Maternal-Fetal Medicine Annual Pregnancy Meeting, Friday, January 31, CONFIDENTIAL 2025 (Abstract LB05). ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
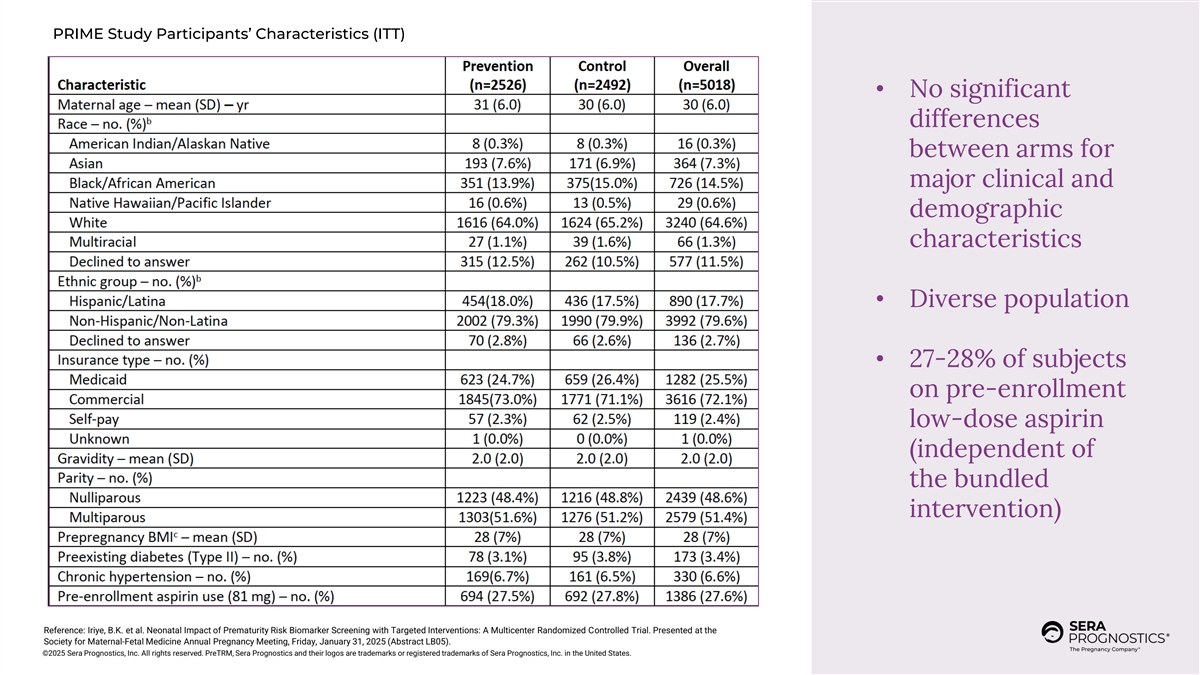
PRIME Study Participants’ Characteristics (ITT) • No significant differences between arms for major clinical and demographic characteristics • Diverse population • 27-28% of subjects on pre-enrollment low-dose aspirin (independent of the bundled intervention) Reference: Iriye, B.K. et al. Neonatal Impact of Prematurity Risk Biomarker Screening with Targeted Interventions: A Multicenter Randomized Controlled Trial. Presented at the Society for Maternal-Fetal Medicine Annual Pregnancy Meeting, Friday, January 31, 2025 (Abstract LB05). ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
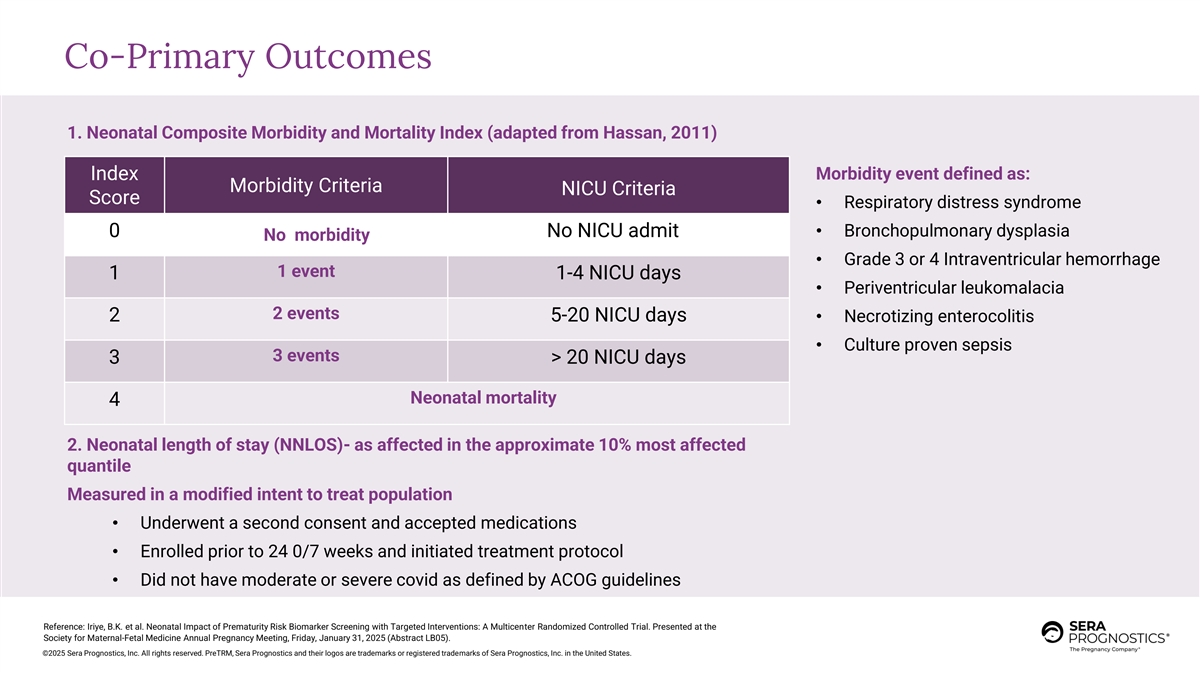
Co-Primary Outcomes 1. Neonatal Composite Morbidity and Mortality Index (adapted from Hassan, 2011) Index Morbidity event defined as: Morbidity Criteria NICU Criteria Score • Respiratory distress syndrome • Bronchopulmonary dysplasia 0 No NICU admit No morbidity • Grade 3 or 4 Intraventricular hemorrhage 1 event 1 1-4 NICU days • Periventricular leukomalacia 2 events 2 5-20 NICU days • Necrotizing enterocolitis • Culture proven sepsis 3 events 3 > 20 NICU days Neonatal mortality 4 2. Neonatal length of stay (NNLOS)- as affected in the approximate 10% most affected quantile Measured in a modified intent to treat population • Underwent a second consent and accepted medications • Enrolled prior to 24 0/7 weeks and initiated treatment protocol • Did not have moderate or severe covid as defined by ACOG guidelines Reference: Iriye, B.K. et al. Neonatal Impact of Prematurity Risk Biomarker Screening with Targeted Interventions: A Multicenter Randomized Controlled Trial. Presented at the Society for Maternal-Fetal Medicine Annual Pregnancy Meeting, Friday, January 31, 2025 (Abstract LB05). ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
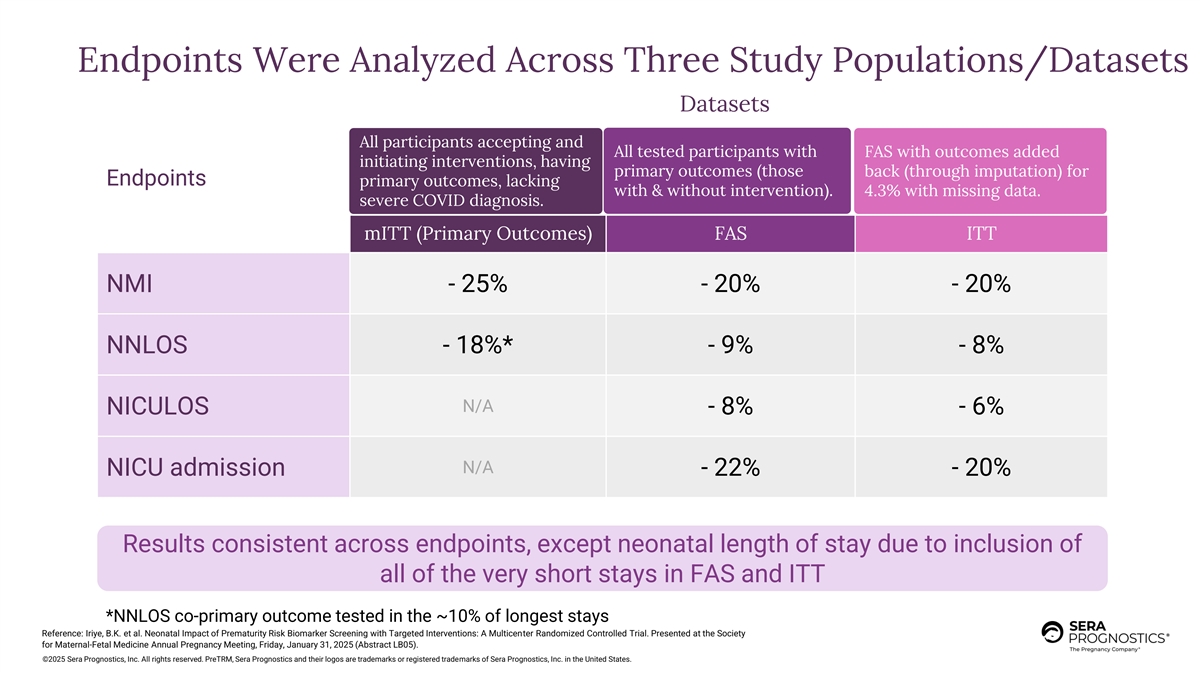
Endpoints Were Analyzed Across Three Study Populations/Datasets Datasets All participants accepting and All tested participants with FAS with outcomes added initiating interventions, having Congest 10% of those All tested (with or without FAST added back 4.3% primary outcomes (those back (through imputation) for Endpoints primary outcomes, lacking accepting intervention (65%) w inte ithrvent & with ion) out intervention). m 4.3 issing % with missing data. severe COVID diagnosis. mITT (Primary Outcomes) FAS ITT NMI - 25% - 20% - 20% NNLOS - 18%* - 9% - 8% N/A NICULOS - 8% - 6% N/A NICU admission - 22% - 20% Results consistent across endpoints, except neonatal length of stay due to inclusion of all of the very short stays in FAS and ITT *NNLOS co-primary outcome tested in the ~10% of longest stays Reference: Iriye, B.K. et al. Neonatal Impact of Prematurity Risk Biomarker Screening with Targeted Interventions: A Multicenter Randomized Controlled Trial. Presented at the Society for Maternal-Fetal Medicine Annual Pregnancy Meeting, Friday, January 31, 2025 (Abstract LB05). ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
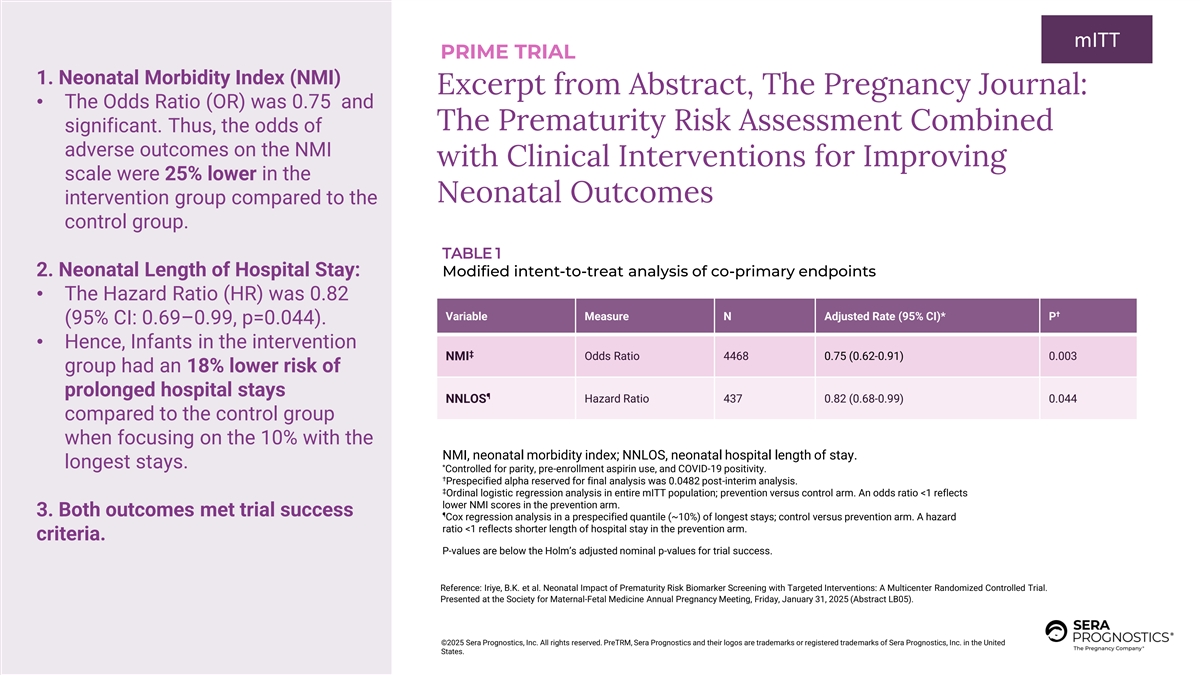
mITT PRIME TRIAL 1. Neonatal Morbidity Index (NMI) Excerpt from Abstract, The Pregnancy Journal: • The Odds Ratio (OR) was 0.75 and The Prematurity Risk Assessment Combined significant. Thus, the odds of adverse outcomes on the NMI with Clinical Interventions for Improving scale were 25% lower in the Neonatal Outcomes intervention group compared to the control group. TABLE 1 2. Neonatal Length of Hospital Stay: Modified intent-to-treat analysis of co-primary endpoints • The Hazard Ratio (HR) was 0.82 † Variable Measure N Adjusted Rate (95% CI)* P (95% CI: 0.69–0.99, p=0.044). • Hence, Infants in the intervention ‡ NMI Odds Ratio 4468 0.75 (0.62-0.91) 0.003 group had an 18% lower risk of prolonged hospital stays ¶ NNLOS Hazard Ratio 437 0.82 (0.68-0.99) 0.044 compared to the control group when focusing on the 10% with the NMI, neonatal morbidity index; NNLOS, neonatal hospital length of stay. longest stays. * Controlled for parity, pre-enrollment aspirin use, and COVID-19 positivity. † Prespecified alpha reserved for final analysis was 0.0482 post-interim analysis. ‡ Ordinal logistic regression analysis in entire mITT population; prevention versus control arm. An odds ratio <1 reflects lower NMI scores in the prevention arm. 3. Both outcomes met trial success ¶ Cox regression analysis in a prespecified quantile (~10%) of longest stays; control versus prevention arm. A hazard ratio <1 reflects shorter length of hospital stay in the prevention arm. criteria. P-values are below the Holm’s adjusted nominal p-values for trial success. Reference: Iriye, B.K. et al. Neonatal Impact of Prematurity Risk Biomarker Screening with Targeted Interventions: A Multicenter Randomized Controlled Trial. Presented at the Society for Maternal-Fetal Medicine Annual Pregnancy Meeting, Friday, January 31, 2025 (Abstract LB05). ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
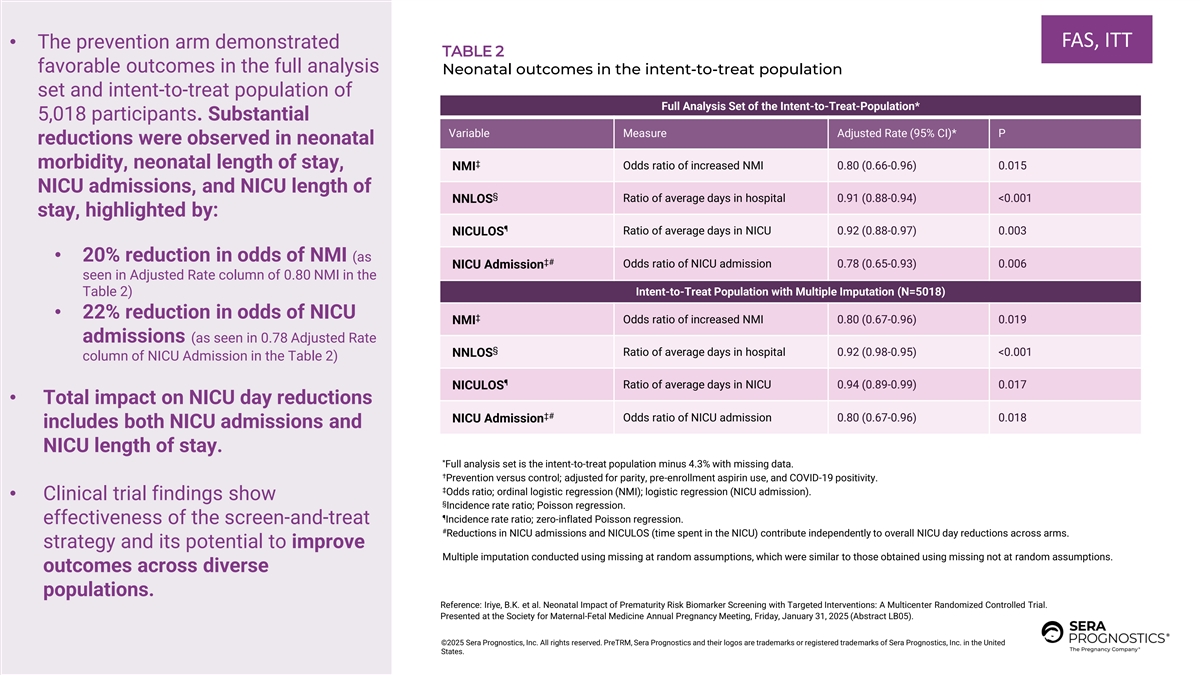
FAS, ITT • The prevention arm demonstrated TABLE 2 favorable outcomes in the full analysis Neonatal outcomes in the intent-to-treat population set and intent-to-treat population of Full Analysis Set of the Intent-to-Treat-Population* 5,018 participants. Substantial Variable Measure Adjusted Rate (95% CI)* P reductions were observed in neonatal ‡ morbidity, neonatal length of stay, NMI Odds ratio of increased NMI 0.80 (0.66-0.96) 0.015 NICU admissions, and NICU length of § NNLOS Ratio of average days in hospital 0.91 (0.88-0.94) <0.001 stay, highlighted by: ¶ Ratio of average days in NICU 0.92 (0.88-0.97) 0.003 NICULOS • 20% reduction in odds of NMI (as ‡# Odds ratio of NICU admission 0.78 (0.65-0.93) 0.006 NICU Admission seen in Adjusted Rate column of 0.80 NMI in the Table 2) Intent-to-Treat Population with Multiple Imputation (N=5018) • 22% reduction in odds of NICU ‡ Odds ratio of increased NMI 0.80 (0.67-0.96) 0.019 NMI admissions (as seen in 0.78 Adjusted Rate § Ratio of average days in hospital 0.92 (0.98-0.95) <0.001 NNLOS column of NICU Admission in the Table 2) ¶ Ratio of average days in NICU 0.94 (0.89-0.99) 0.017 NICULOS • Total impact on NICU day reductions ‡# Odds ratio of NICU admission 0.80 (0.67-0.96) 0.018 NICU Admission includes both NICU admissions and NICU length of stay. * Full analysis set is the intent-to-treat population minus 4.3% with missing data. † Prevention versus control; adjusted for parity, pre-enrollment aspirin use, and COVID-19 positivity. ‡ Odds ratio; ordinal logistic regression (NMI); logistic regression (NICU admission). • Clinical trial findings show § Incidence rate ratio; Poisson regression. ¶ Incidence rate ratio; zero-inflated Poisson regression. effectiveness of the screen-and-treat # Reductions in NICU admissions and NICULOS (time spent in the NICU) contribute independently to overall NICU day reductions across arms. strategy and its potential to improve Multiple imputation conducted using missing at random assumptions, which were similar to those obtained using missing not at random assumptions. outcomes across diverse populations. Reference: Iriye, B.K. et al. Neonatal Impact of Prematurity Risk Biomarker Screening with Targeted Interventions: A Multicenter Randomized Controlled Trial. Presented at the Society for Maternal-Fetal Medicine Annual Pregnancy Meeting, Friday, January 31, 2025 (Abstract LB05). ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
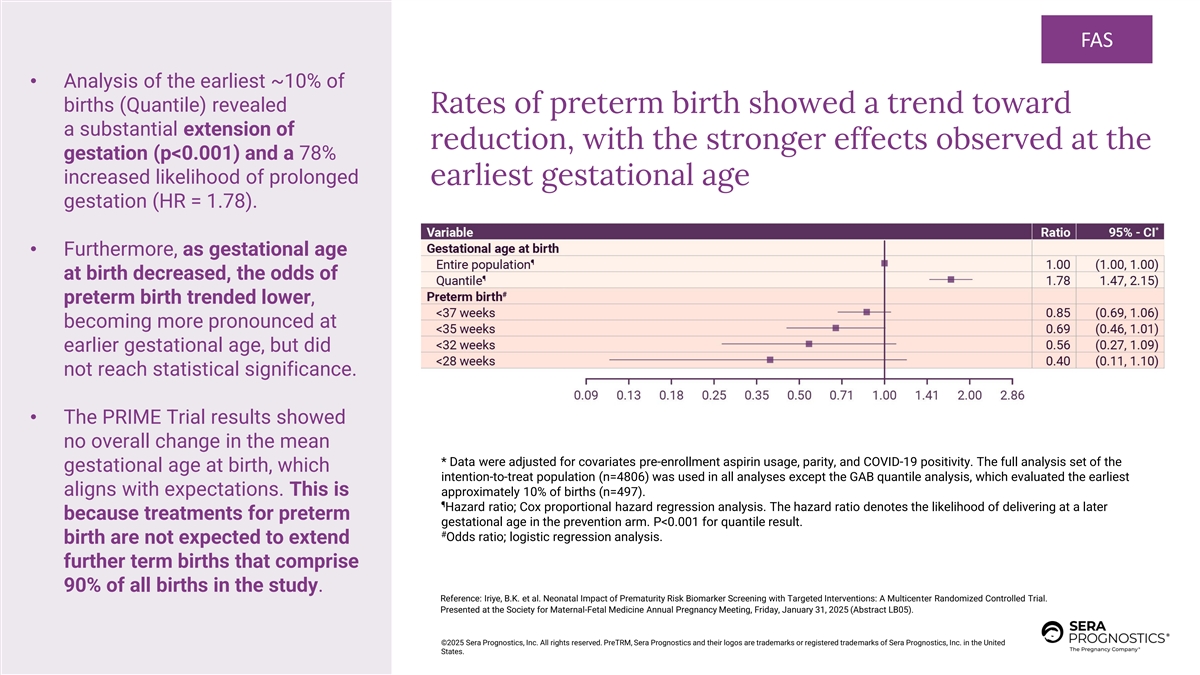
FAS • Analysis of the earliest ~10% of births (Quantile) revealed Rates of preterm birth showed a trend toward a substantial extension of reduction, with the stronger effects observed at the gestation (p<0.001) and a 78% increased likelihood of prolonged earliest gestational age gestation (HR = 1.78). • Furthermore, as gestational age at birth decreased, the odds of preterm birth trended lower, becoming more pronounced at earlier gestational age, but did not reach statistical significance. • The PRIME Trial results showed no overall change in the mean * Data were adjusted for covariates pre-enrollment aspirin usage, parity, and COVID-19 positivity. The full analysis set of the gestational age at birth, which intention-to-treat population (n=4806) was used in all analyses except the GAB quantile analysis, which evaluated the earliest aligns with expectations. This is approximately 10% of births (n=497). ¶ Hazard ratio; Cox proportional hazard regression analysis. The hazard ratio denotes the likelihood of delivering at a later because treatments for preterm gestational age in the prevention arm. P<0.001 for quantile result. # Odds ratio; logistic regression analysis. birth are not expected to extend further term births that comprise 90% of all births in the study. Reference: Iriye, B.K. et al. Neonatal Impact of Prematurity Risk Biomarker Screening with Targeted Interventions: A Multicenter Randomized Controlled Trial. Presented at the Society for Maternal-Fetal Medicine Annual Pregnancy Meeting, Friday, January 31, 2025 (Abstract LB05). ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
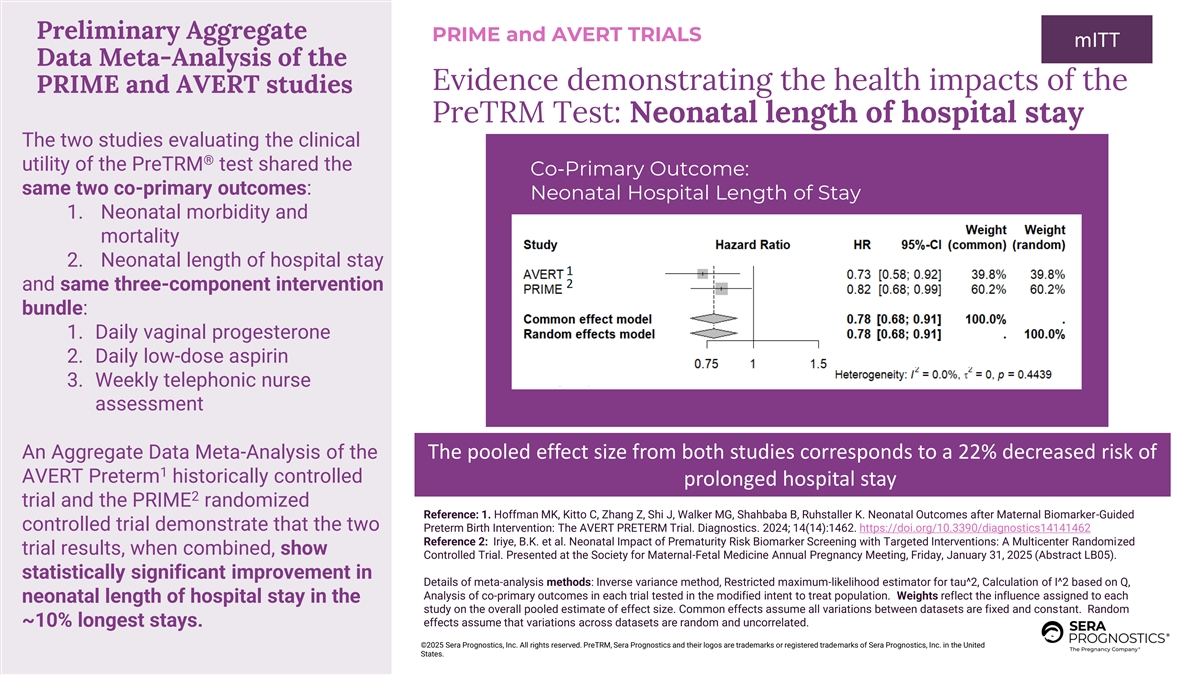
Preliminary Aggregate PRIME and AVERT TRIALS mITT Data Meta-Analysis of the Evidence demonstrating the health impacts of the PRIME and AVERT studies PreTRM Test: Neonatal length of hospital stay The two studies evaluating the clinical ® utility of the PreTRM test shared the Co-Primary Outcome: same two co-primary outcomes: Neonatal Hospital Length of Stay 1. Neonatal morbidity and mortality 2. Neonatal length of hospital stay 1 2 and same three-component intervention bundle: 1. Daily vaginal progesterone 2. Daily low-dose aspirin 3. Weekly telephonic nurse assessment An Aggregate Data Meta-Analysis of the The pooled effect size from both studies corresponds to a 22% decreased risk of 1 AVERT Preterm historically controlled prolonged hospital stay 2 trial and the PRIME randomized Reference: 1. Hoffman MK, Kitto C, Zhang Z, Shi J, Walker MG, Shahbaba B, Ruhstaller K. Neonatal Outcomes after Maternal Biomarker-Guided controlled trial demonstrate that the two Preterm Birth Intervention: The AVERT PRETERM Trial. Diagnostics. 2024; 14(14):1462. https://doi.org/10.3390/diagnostics14141462 Reference 2: Iriye, B.K. et al. Neonatal Impact of Prematurity Risk Biomarker Screening with Targeted Interventions: A Multicenter Randomized trial results, when combined, show Controlled Trial. Presented at the Society for Maternal-Fetal Medicine Annual Pregnancy Meeting, Friday, January 31, 2025 (Abstract LB05). statistically significant improvement in Details of meta-analysis methods: Inverse variance method, Restricted maximum-likelihood estimator for tau^2, Calculation of I^2 based on Q, Analysis of co-primary outcomes in each trial tested in the modified intent to treat population. Weights reflect the influence assigned to each neonatal length of hospital stay in the study on the overall pooled estimate of effect size. Common effects assume all variations between datasets are fixed and constant. Random effects assume that variations across datasets are random and uncorrelated. ~10% longest stays. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

Preliminary Aggregate PRIME and AVERT TRIALS mITT Data Meta-Analysis of the Evidence demonstrating the health impacts of the PRIME and AVERT studies PreTRM Test: Neonatal morbidity and mortality The two studies evaluating the clinical ® Co-Primary Outcome: utility of the PreTRM test shared the Neonatal Morbidity and Mortality same two co-primary outcomes: 1. Neonatal morbidity and mortality 1 2. Neonatal length of hospital 2 stay and same three-component intervention bundle: 1. Daily vaginal progesterone 2. Daily low-dose aspirin 3. Weekly telephonic nurse assessment The pooled effect size from both studies corresponds to a 22% decreased odds of NMI An Aggregate Data Meta-Analysis of 1 the AVERT Preterm historically Reference: 1. Hoffman MK, Kitto C, Zhang Z, Shi J, Walker MG, Shahbaba B, Ruhstaller K. Neonatal Outcomes after Maternal Biomarker-Guided 2 controlled trial and the PRIME Preterm Birth Intervention: The AVERT PRETERM Trial. Diagnostics. 2024; 14(14):1462. https://doi.org/10.3390/diagnostics14141462 Reference 2: Iriye, B.K. et al. Neonatal Impact of Prematurity Risk Biomarker Screening with Targeted Interventions: A Multicenter Randomized randomized controlled trial Controlled Trial. Presented at the Society for Maternal-Fetal Medicine Annual Pregnancy Meeting, Friday, January 31, 2025 (Abstract LB05). demonstrate that the two trial results, Details of meta-analysis methods: Inverse variance method, Restricted maximum-likelihood estimator for tau^2, Calculation of I^2 based on Q, Analysis of co-primary outcomes in each trial tested in the modified intent to treat population. Weights reflect the influence assigned to each when combined, show statistically study on the overall pooled estimate of effect size. Pooled effect sizes. Common effects assume all variations between datasets are fixed and constant. Random effects assume that variations across datasets are random and uncorrelated. significant improvement in neonatal ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United morbidity and mortality. States.
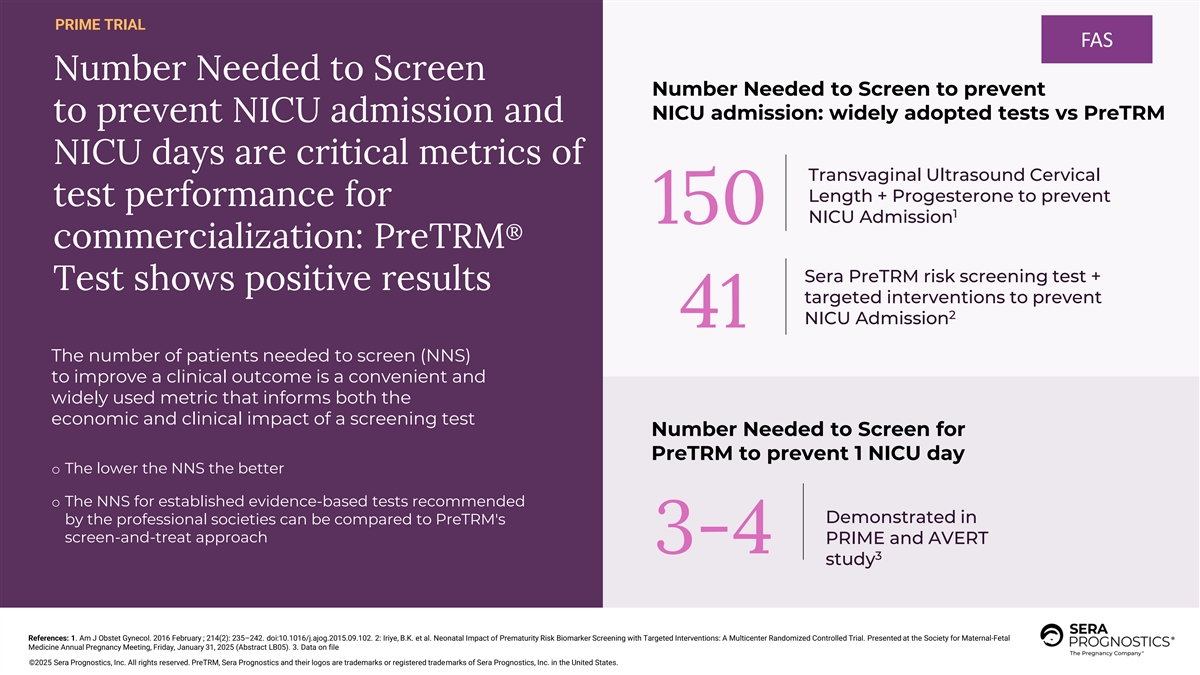
PRIME TRIAL FAS Number Needed to Screen Number Needed to Screen to prevent NICU admission: widely adopted tests vs PreTRM to prevent NICU admission and NICU days are critical metrics of Transvaginal Ultrasound Cervical Length + Progesterone to prevent test performance for 1 NICU Admission 150 ® commercialization: PreTRM Sera PreTRM risk screening test + Test shows positive results targeted interventions to prevent 2 NICU Admission 41 The number of patients needed to screen (NNS) to improve a clinical outcome is a convenient and widely used metric that informs both the economic and clinical impact of a screening test Number Needed to Screen for PreTRM to prevent 1 NICU day o The lower the NNS the better o The NNS for established evidence-based tests recommended Demonstrated in by the professional societies can be compared to PreTRM's screen-and-treat approach PRIME and AVERT 3-4 3 study References: 1. Am J Obstet Gynecol. 2016 February ; 214(2): 235–242. doi:10.1016/j.ajog.2015.09.102. 2: Iriye, B.K. et al. Neonatal Impact of Prematurity Risk Biomarker Screening with Targeted Interventions: A Multicenter Randomized Controlled Trial. Presented at the Society for Maternal-Fetal Medicine Annual Pregnancy Meeting, Friday, January 31, 2025 (Abstract LB05). 3. Data on file ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
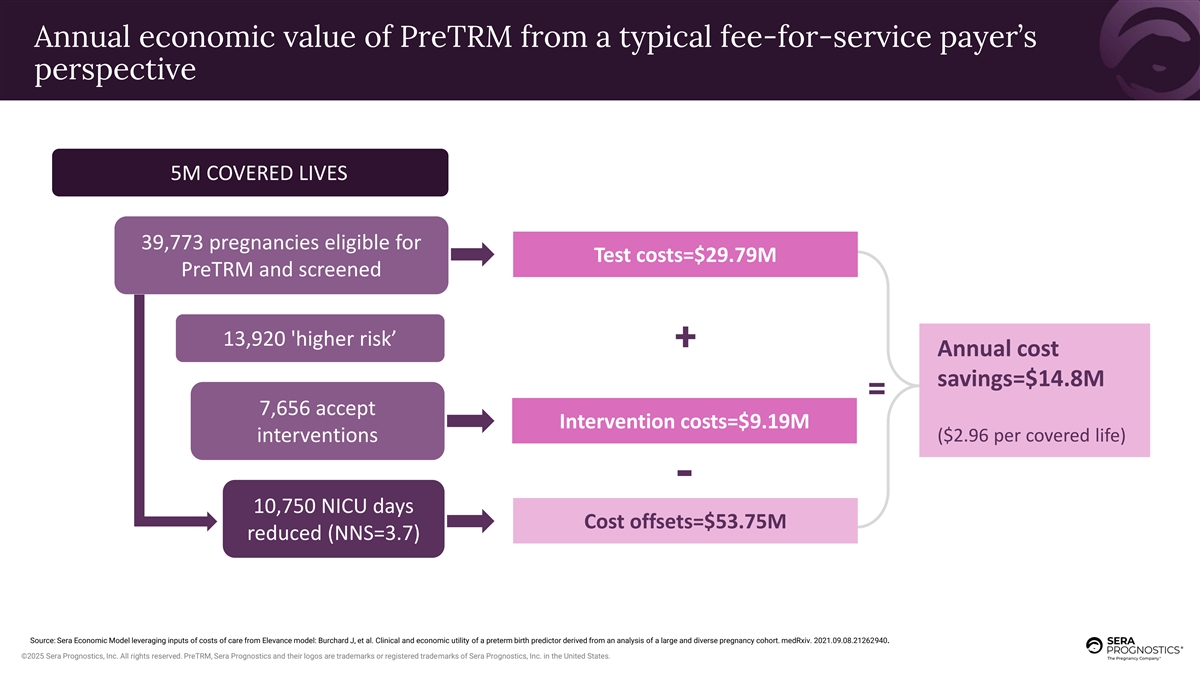
Annual economic value of PreTRM from a typical fee-for-service payer’s perspective 5M COVERED LIVES 39,773 pregnancies eligible for Test costs=$29.79M PreTRM and screened 13,920 'higher risk’ + Annual cost savings=$14.8M = 7,656 accept Intervention costs=$9.19M ($2.96 per covered life) interventions - 10,750 NICU days Cost offsets=$53.75M reduced (NNS=3.7) Source: Sera Economic Model leveraging inputs of costs of care from Elevance model: Burchard J, et al. Clinical and economic utility of a preterm birth predictor derived from an analysis of a large and diverse pregnancy cohort. medRxiv. 2021.09.08.21262940. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

At a 6-week post-partum appointment, I walked into the exam room and my patient greeted me with a big smile and said… “I’m so grateful for the PreTRM Test!” Because after she took it, the results showed an extremely high risk for preterm labor. We worked together during the next 4 months and the outcome was a vaginal delivery, at term, with a healthy baby. My patient then told me… “ “I came home from the hospital with a healthy baby I can hold. My friend can only touch her baby in an incubator.” My patient then explained she’d just returned from a visit with a friend who was also pregnant—with a due Dr. Barbi Phelps-Sandall date close to her own. The friend had a preterm delivery. She did not have the PreTRM test available to shares a patient her and now both mom and baby were in the NICU. pregnancy story “I’m so glad we did the PreTRM Test.” ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

o Publications o Commercialization: Driving Next Inflection Point o Reimbursement Anticipated o Guidelines o Regulatory Milestones o Additional Evidence ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
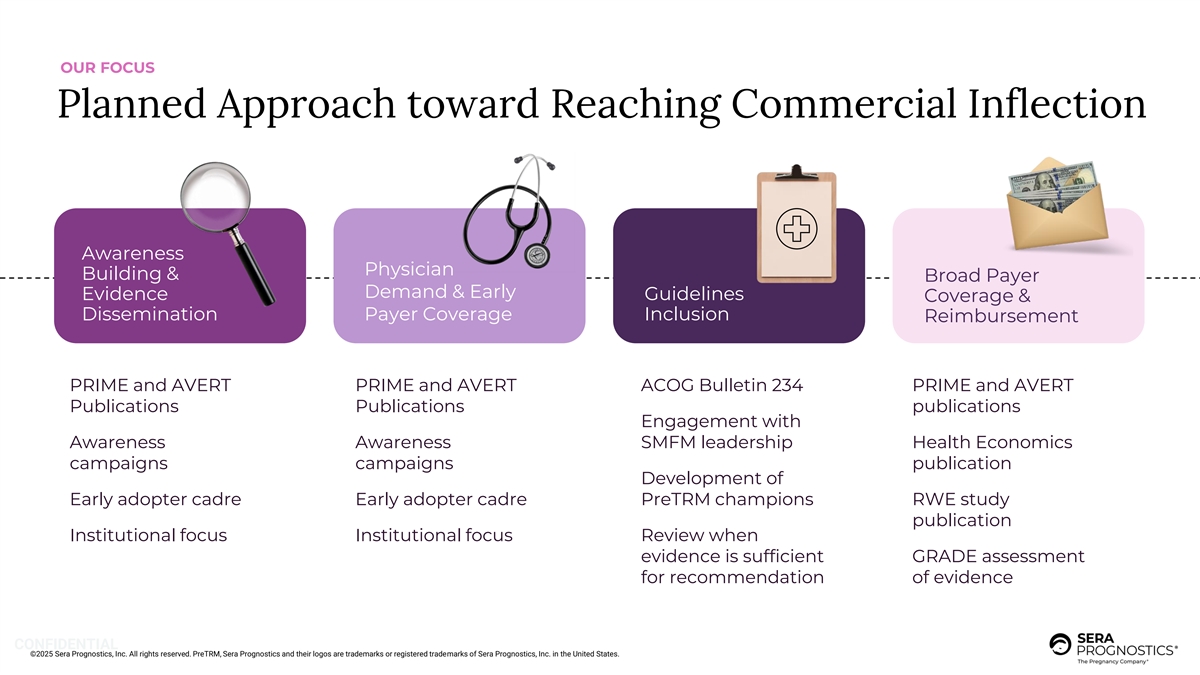
OUR FOCUS Planned Approach toward Reaching Commercial Inflection Awareness Physician Building & Broad Payer Demand & Early Evidence Guidelines Coverage & Dissemination Payer Coverage Inclusion Reimbursement PRIME and AVERT PRIME and AVERT ACOG Bulletin 234 PRIME and AVERT Publications Publications publications Engagement with Awareness Awareness SMFM leadership Health Economics campaigns campaigns publication Development of Early adopter cadre Early adopter cadre PreTRM champions RWE study publication Institutional focus Institutional focus Review when evidence is sufficient GRADE assessment for recommendation of evidence CONFIDENTIAL ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

There are examples of a pivotal RCT leading to a speedy ACOG practice advisory announcement...and then others that required more time & evidence to change guidelines. HERE ARE (3) CASE STUDIES 1 2 3 Chronic Hypertension ACOG recommended NIPT as an option to ACOG recommended and Pregnancy (CHAP) be “discussed and offered to all patients antenatal steroids in Study led to an update early in pregnancy, regardless of maternal October 2017 with one in ACOG guidance age or baseline risk” in Oct ‘20 pivotal study: via practice bulletin with 87 references: SMFM presentation January Study published in April 2022 in o 1 Level I (Harmony/Roche RCT) 2016 by Cynthia Gyamfi- NEJM: demonstrated that o 23 Level II-2 Bannerman, published in utilizing a treatment threshold of 1 o 27 Level II-3 NEJM later that year 140/90 (vs. 160/105) for pregnant o 27 Level III & 9 systemic revie/meta-analysis people with chronic hypertension provides improved outcomes ACOG is conservative with their approach to recommending novel diagnostics Time to commercial inflection point influenced by guideline inclusion, can be supported by continued physician awareness building & community 1 Source: ACOG website, expert interviews. Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311-1320. doi:10.1056/NEJMoa1516783 ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
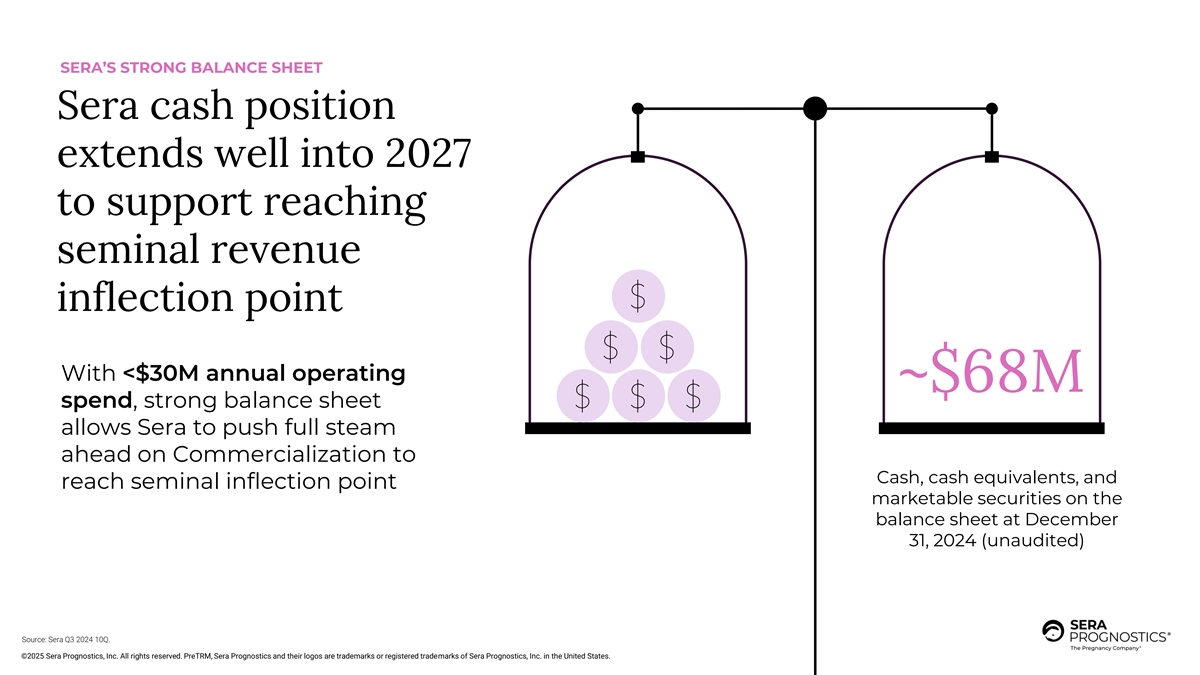
SERA’S STRONG BALANCE SHEET Sera cash position extends well into 2027 to support reaching seminal revenue inflection point With <$30M annual operating ~$68M spend, strong balance sheet allows Sera to push full steam ahead on Commercialization to Cash, cash equivalents, and reach seminal inflection point marketable securities on the balance sheet at December 31, 2024 (unaudited) Source: Sera Q3 2024 10Q. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

Zhenya Lindgardt President & CEO • Former VP of Platform and Customer Engagement, Uber Technologies • Former Senior Partner and Managing Director, The Boston Consulting Group • Former CEO, The Commons Project Foundation • MBA, Harvard University Sera Austin Aerts Chief Financial Officer • Former VP, Finance and Corporate Controller, Sera Leadership • Former finance team member, Myriad • Former auditor, Ernst & Young LLP • Master of Accounting University of Utah; CPA Team Jay Boniface, Ph.D. Michael Foley, M.D. Paul Kearney, Ph.D, Doug Roach Benjamin Jackson Robert G. Harrison Chief Scientific Officer Medical Advisor Chief Data Officer VP of Commercial General Counsel Chief Information Officer ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

As a result of the weekly “ check-ins & overall care management, I was well-informed and empowered to advocate for myself and my baby. BONNIE ® Actual PreTRM patient ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

TOGETHER, WE CAN DO BETTER Educate Providers. Empower MOMS. HELP Babies. Advance Science. Elevate Care. Reach Maternity Deserts. Inform Payers. Evoke Change. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.