c
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 20‑F
(Mark one)
☐ | Registration statement pursuant to Section 12(b) or (g) of the Securities Exchange Act of 1934 |
or
☒ | Annual report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the fiscal year ended June 30, 2019
or
☐ | Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
or
☐ | Shell company report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
Commission file number: 001-37518
Benitec Biopharma Limited
(Exact name of Registrant as specified in its charter)
Australia
(Jurisdiction of incorporation or organization)
14/114 William Street,
Melbourne, VIC, 3000, Australia
(Address of principal executive offices)
Dr. Jerel Banks
Chief Executive Officer
14/114 William Street,
Melbourne, VIC, 3000, Australia
Tel: +61 3 8692 7222
Fax: +613 9966 9923
(Name, telephone, e‑mail and/or facsimile number and address of company contact person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
Title of each class | | Name of each exchange on which registered |
American Depositary Shares, each representing twenty Ordinary Shares* | | The NASDAQ Capital Market |
Warrants, each exercisable for one American Depositary Share | | The NASDAQ Capital Market |
Securities registered or to be registered pursuant to Section 12(g) of the Act:
None
* | Not for trading, but only in connection with the registration of American Depositary Shares. |
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
None
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the date of the close of the period covered by the annual report.
257,029,426 Ordinary Shares at June 30, 2019
Indicate by check mark if the registrant is a well‑known seasoned issuer, as defined in Rule 405 of the Securities Act. ☐ Yes ☒ No
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. ☐ Yes ☒ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒ Yes ☐ No
Indicate by check mark whether the registrant has submitted electronically, every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S‑T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files) ☒Yes ☐No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non‑accelerated filer or an emerging growth company. See definition of “large accelerated filer”, “accelerated filer”, and “emerging growth company” in Rule 12b‑2 of the Exchange Act:
| Large accelerated filer ☐ | Non‑accelerated filer ☒ |
| | |
| Accelerated filer ☐ | Emerging growth company ☒ |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
U.S. GAAP ☐ | International Financial Reporting Standards as issued | Other ☐ |
| by the International Accounting Standards Board ☒ | |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow. ☐ Item 17 ☐ Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b‑2 of the Exchange Act). ☐ Yes ☒ No
Table of contents
EXPLANATORY NOTES
Unless otherwise indicated or the context implies otherwise:
| • | “we,” “us,” “our” or “Benitec” refers to Benitec Biopharma Limited, an Australian corporation, and its subsidiaries; |
| • | “shares” or “ordinary shares” refers to our ordinary shares; |
| • | “ADSs” refers to American Depositary Shares, each of which represents 20 ordinary shares; |
| • | “ADRs” refers to American Depositary Receipts, which evidence the ADSs; and |
Our reporting and functional currency is the Australian dollar. Solely for the convenience of the reader, this Annual Report on Form 20-F contains translations of some Australian dollar amounts into U.S. dollars at specified rates. Except as otherwise stated in this Annual Report on Form 20-F, all translations from Australian dollars to U.S. dollars are based on the rate published by the Reserve Bank of Australia on the date indicated. See Item 3.A “Key Information—Selected Financial Data.” No representation is made that the Australian dollar amounts referred to in this Annual Report on Form 20-F could have been or could be converted into U.S. dollars at such rate.
Unless otherwise noted, all industry and market data in this Annual Report on Form 20-F, including information provided by independent industry analysts, are presented in U.S. dollars. Unless otherwise noted, all other financial and other data related to Benitec Biopharma Limited in this Annual Report on Form 20-F are presented in Australian dollars. All references to “$” in this Annual Report on Form 20-F refer to Australian dollars or U.S. dollars, as the context requires based on the foregoing. All references to “A$” in this Annual Report on Form 20-F mean Australian dollars. All references to “US$” in this Annual Report on Form 20-F mean U.S. dollars.
Our fiscal year end is June 30. References to a particular “fiscal year” are to our fiscal year ended June 30 of that calendar year.
Unless otherwise indicated, the consolidated financial statements and related notes included in this Annual Report on Form 20-F have been prepared in accordance with International Accounting Standards and also comply with International Financial Reporting Standards, or IFRS, and interpretations issued by the International Accounting Standards Board, or IASB, which differ in certain significant respects from Generally Accepted Accounting Principles in the United States, or GAAP. See Item 3.A “Key Information—Selected Financial Data.”
TRADEMARKS AND TRADENAMES
We have proprietary and licensed rights to trademarks used in this Annual Report on Form 20-F which are important to our business, many of which are registered under applicable intellectual property laws. These trademarks are as follows:
| • | SILENCING GENES FOR LIFE® |
Solely for convenience, trademarks and trade names referred to in this Annual Report on Form 20-F appear without the “®” or “™” symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent possible under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend use or display of other companies' trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other companies. Each trademark, trade name or service mark of any other company appearing in this Annual Report on Form 20-F is the property of its respective holder.
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 20-F contains forward-looking statements that are subject to a number of risks and uncertainties, many of which are beyond our control. All statements, other than statements of historical fact included in this Annual Report on Form 20-F, regarding our strategy, future operations, financial position, projected costs, prospects, plans and objectives of management are forward-looking statements. When used in this Annual Report on Form 20-F, the words “could,” “believe,” “anticipate,” “intend,” “estimate,” “expect,” “may,” “continue,” “predict,” “potential,” “project,” or the negative of these terms, and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain such identifying words. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. Although we believe that we have a reasonable basis for each forward-looking statement contained in this Annual Report on Form 20-F, we caution you that these statements are based on a combination of facts and important factors currently known by us and our expectations of the future, about which we cannot be certain.
Forward-looking statements may include statements about:
| • | our plans to develop and potentially commercialize our product candidates; |
| • | the timing of the initiation and completion of preclinical studies and clinical trials; |
| • | the timing of patient enrollment and dosing in any future clinical trials; |
| • | the timing of the availability of data from clinical trials; |
| • | the timing of expected regulatory filings; |
| • | the development of novel AAV vectors; |
| • | expectations about the plans of licensees of our technology; |
| • | the clinical utility and potential attributes and benefits of ddRNAi and our product candidates, including the potential duration of treatment effects and the potential for a “one shot” cure; |
| • | potential future out-licenses and collaborations; |
| • | our expectations regarding expenses, ongoing losses, future revenue, capital needs and needs for additional financing; |
| • | the length of time over which we expect our cash and cash equivalents, including the proceeds from our U.S. initial public offering, to be sufficient; and |
| • | our intellectual property position and the duration of our patent portfolio. |
All forward-looking statements speak only as of the date of this Annual Report on Form 20-F. You should not place undue reliance on these forward-looking statements. Although we believe that our plans, objectives, expectations and intentions reflected in or suggested by the forward-looking statements we make in this Annual Report on Form 20-F are reasonable, we can give no assurance that these plans, objectives, expectations or intentions will be achieved. We disclose important factors that could cause our actual results to differ materially from our expectations under Item 3.D “Key Information—Risk Factors” and elsewhere in this Annual Report on Form 20-F.
The forward-looking statements made in this Annual Report on Form 20-F relate only to events or information as of the date on which the statements are made in this Annual Report on Form 20-F. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events.
2
PART I
Item 1. Identity of Directors, Senior Management and Advisors
Not applicable.
Item 2. Offer Statistics and Expected Timetable
Not applicable.
Item 3. Key Information
A. | Selected Financial Data. |
The following tables set forth summary historical financial data for the periods indicated.
The following selected consolidated financial data as of June 30, 2019 and 2018 and for the fiscal years ended June 30, 2019, 2018 and 2017 have been derived from our audited consolidated financial statements and notes thereto included elsewhere in this Annual Report on Form 20-F. The selected consolidated financial data as of June 30, 2017, 2016 and 2015 and for the fiscal years ended June 30, 2016 and 2015 have been derived from our audited consolidated financial statements and notes thereto which are not included in this Annual Report on Form 20-F. Certain prior year amounts have been reclassified for consistency with the current year presentation. These reclassifications had no effect on the reported results of operations.
Our financial statements have been prepared in Australian dollars and in accordance with International Financial Reporting Standards (IFRS). Our financial statements comply with IFRS, as issued by the IASB.
3
You should read the selected consolidated financial data in conjunction with our consolidated financial statements and related notes included elsewhere in this Annual Report on Form 20-F, including Item 5. “Operating and Financial Review and Prospects” and “Item 18. Financial Statements.” Our historical results do not necessarily indicate our expected results for any future periods.
(in thousands, except per share data) | | For the year ended June 30 | |
| | 2019 | | | 2018 | | | 2017 | | | 2016 | | | 2015 | |
Statement of Profit or Loss and Other Comprehensive Income Data: | | | | | | | | | | | | | | | | | | | | | | | | | |
Revenue and other income | | | | | | | | | | | | | | | | | | | | | | | | | |
Revenue | | A$ | | 16,159 | | | A$ | | 378 | | | A$ | | 333 | | | A$ | | 247 | | | A$ | | 307 | |
Other income | | | | 1,350 | | | | | 4,087 | | | | | 10,507 | | | | | 3,590 | | | | | 2,891 | |
Total revenue and other income | | | | 17,509 | | | | | 4,465 | | | | | 10,840 | | | | | 3,837 | | | | | 3,198 | |
Costs and expenses: | | | | | | | | | | | | | | | | | | | | | | | | | |
Royalties and license fees | | | | (609 | ) | | | | (451 | ) | | | | (272 | ) | | | | (139 | ) | | | | (40 | ) |
Research and development | | | | (3,104 | ) | | | | (6,890 | ) | | | | (6,925 | ) | | | | (13,287 | ) | | | | (6,228 | ) |
Employment related | | | | (5,025 | ) | | | | (5,094 | ) | | | | (5,015 | ) | | | | (6,283 | ) | | | | (3,425 | ) |
Share based expenses | | | | (939 | ) | | | | (434 | ) | | | | (386 | ) | | | | (1,746 | ) | | | | (1,503 | ) |
Travel related expenses | | | | (350 | ) | | | | (468 | ) | | | | (629 | ) | | | | (1,023 | ) | | | | (1,039 | ) |
Consultants costs | | | | (662 | ) | | | | (783 | ) | | | | (976 | ) | | | | (1,020 | ) | | | | (882 | ) |
Occupancy costs | | | | (648 | ) | | | | (587 | ) | | | | (550 | ) | | | | (500 | ) | | | | (275 | ) |
Depreciation | | | | (221 | ) | | | | (194 | ) | | | | (217 | ) | | | | (290 | ) | | | | (61 | ) |
Corporate expenses | | | | (1,884 | ) | | | | (1,360 | ) | | | | (1,540 | ) | | | | (1,139 | ) | | | | (957 | ) |
Net loss foreign exchange | | | | (106 | ) | | | | (44 | ) | | | | (266 | ) | | | | (414 | ) | | | | — | |
IPO costs | | | | — | | | | | — | | | | | — | | | | | (1,191 | ) | | | | (1,071 | ) |
Change in market value of listed investment | | | | (28 | ) | | | | (41 | ) | | | | — | | | | | — | | | | | — | |
Loss on disposal of fixed assets | | | | (9 | ) | | | | (1 | ) | | | | (7 | ) | | | | — | | | | | — | |
Write-off of clinical trial prepayment | | | | — | | | | | — | | | | | — | | | | | (1,800 | ) | | | | — | |
Finance Income | | | | 170 | | | | | 242 | | | | | 253 | | | | | 217 | | | | | 774 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Profit/(Loss) before income tax | | | | 4,094 | | | | | (11,640 | ) | | | | (5,690 | ) | | | | (24,778 | ) | | | | (11,509 | ) |
Income tax benefit | | | | — | | | | | — | | | | | — | | | | | — | | | | | — | |
Profit/(Loss) for the period | | | | 4,094 | | | | | (11,640 | ) | | | | (5,690 | ) | | | | (24,778 | ) | | | | (11,509 | ) |
Profit/(Loss)per share, basic and diluted | | | | 0.0159 | | | | | (0.0553 | ) | | | | (0.0324 | ) | | | | (0.1741 | ) | | | | (0.0996 | ) |
Weighted-average shares outstanding, basic and diluted | | | | 257,029,426 | | | | 210,454,829 | | | | 175,433,909 | | | | 142,312,486 | | | | 115,507 | |
4
(in thousands) | | | | | | | | | | | | | | | | | | | | | | | | | |
| | As of June 30 | |
| | 2019 | | | 2018 | | | 2017 | | | 2016 | | | 2015 | |
Statement of Financial Position Data: | | | | | | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | A$ | | 22,411 | | | A$ | | 16,085 | | | A$ | | 17,375 | | | A$ | | 18,230 | | | A$ | | 21,787 | |
Total current assets | | | | 26,743 | | | | | 20,895 | | | | | 22,162 | | | | | 19,384 | | | | | 25,064 | |
Total assets | | | | 27,426 | | | | | 21,339 | | | | | 22,666 | | | | | 19,890 | | | | | 25,520 | |
Net assets | | | | 23,660 | | | | | 18,744 | | | | | 21,506 | | | | | 18,837 | | | | | 23,878 | |
Total current liabilities | | | | 3,766 | | | | | 2,547 | | | | | 1,125 | | | | | 1,035 | | | | | 1,642 | |
Total liabilities | | | | 3,766 | | | | | 2,595 | | | | | 1,160 | | | | | 1,053 | | | | | 1,642 | |
Total equity | | | | 23,660 | | | | | 18,744 | | | | | 21,506 | | | | | 18,837 | | | | | 23,878 | |
Exchange Rate Information
The Australian dollar is convertible into U.S. dollars at freely floating rates. There are no legal restrictions on the flow of Australian dollars between Australia and the United States. Any remittances of dividends or other payments by us to persons in the United States are not and will not be subject to any exchange controls.
Our financial statements are prepared and presented in Australian dollars.
The table below sets forth for the periods identified the number of U.S. dollars per Australian dollar as published by the Reserve Bank of Australia. We make no representation that any Australian dollar or U.S. dollar amounts could have been, or could be, converted into U.S. dollars or Australian dollars, as the case may be, at any particular rate, the rates stated below, or at all.
| | At Period | | | Average | | | | | | | | | |
| | End (1) | | | Rate (2) | | | High (3) | | | Low (4) | |
Fiscal year ended June 30, | | | | | | | | | | | | | | | | |
2019 | | | 0.7013 | | | | 0.7156 | | | | 0.7467 | | | | 0.6840 | |
2018 | | | 0.7391 | | | | 0.7753 | | | | 0.8121 | | | | 0.7353 | |
2017 | | | 0.7692 | | | | 0.7542 | | | | 0.7724 | | | | 0.7202 | |
2016 | | | 0.7426 | | | | 0.7272 | | | | 0.7812 | | | | 0.6867 | |
2015 | | | 0.7680 | | | | 0.8288 | | | | 0.9458 | | | | 0.7590 | |
2014 | | | 0.9420 | | | | 0.9148 | | | | 0.9672 | | | | 0.8716 | |
Month ended: | | | | | | | | | | | | | | | | |
September 30, 2019 | | | | | | | | | | | | | | | | |
August 31, 2019 | | | 0.6718 | | | | 0.6772 | | | | 0.6851 | | | | 0.6716 | |
July 31, 2019 | | | 0.6895 | | | | 0.6987 | | | | 0.7065 | | | | 0.6894 | |
June 30, 2019 | | | 0.7013 | | | | 0.6942 | | | | 0.7013 | | | | 0.6840 | |
May 31, 2019 | | | 0.6916 | | | | 0.6949 | | | | 0.7054 | | | | 0.6875 | |
April 30, 2019 | | | 0.7039 | | | | 0.7115 | | | | 0.7200 | | | | 0.7025 | |
(1) | Determined by the published rate on the last trading day of the month or fiscal year indicated, as applicable. |
(2) | Determined by averaging the published rate on the last trading day of each full month during the fiscal year indicated or by averaging the published rates for all trading days during the month indicated, as applicable. |
(3) | Determined by the highest published rate during the month or fiscal year indicated, as applicable. |
(4) | Determined by the lowest published rate during the month or fiscal year indicated, as applicable. |
5
B. | Capitalization and Indebtedness. |
Not applicable.
C. | Reasons for the Offer and Use of Proceeds. |
Not applicable.
An investment in ADSs involves significant risks. You should carefully consider the risks described below and the other information in this Annual Report on Form 20-F, including our consolidated financial statements and related notes included elsewhere in this Annual Report on Form 20-F. If any of the following risks actually occurs, our business, prospects, financial condition and results of operations could be materially and adversely affected, the trading price of the ADSs could decline and you could lose all or part of your investment.
Risks Related to Our Financial Condition, Capital Requirements and Largest Shareholder
We anticipate that we will incur significant net losses for the foreseeable future, and we may never achieve or maintain profitability.
As of June 30, 2019, we had accumulated losses of A$141.3 million. We have devoted most of our financial resources to research and development, including our clinical and preclinical development activities. To date, we have financed our operations primarily through the issuance of equity securities (including a private placement in May 2018 as well as an entitlement offer in June 2018 and September 2019), research and development grants from the Australian government and payments from our collaboration partners. We do not expect to generate, any significant revenue for the foreseeable future, and we expect to incur significant operating losses for the foreseeable future due to the cost of research and development, preclinical studies and clinical trials and the regulatory approval process for product candidates. The amount of our future net losses is uncertain and will depend, in part, on the rate of our future expenditures. Our ability to continue operations will depend on, among other things, our ability to obtain funding through equity or debt financings, strategic collaborations or additional grants.
We expect to continue to incur significant expenses and we may incur operating losses for the foreseeable future. We anticipate that our expenses will increase substantially if and as we:
| • | continue our research and preclinical development of our product candidates; |
| • | expand the scope of our current preclinical studies for our product candidates or initiate clinical, additional preclinical or other studies for product candidates; |
| • | seek regulatory and marketing approvals for any of our product candidates that successfully complete clinical trials; |
| • | further develop the manufacturing process for our product candidates; |
| • | change or add additional manufacturers or suppliers; |
| • | seek to identify and validate additional product candidates; |
| • | acquire or in-license other product candidates and technologies, which may or may not include those related to our ddRNAi technology and delivery vectors for our therapeutic candidates; |
| • | maintain, protect and expand our intellectual property portfolio; |
| • | create additional infrastructure to support our operations as a public company in the United States and our product development and future commercialization efforts; and |
| • | experience any delays or encounter issues with any of the above. |
6
The net losses we incur may fluctuate significantly from quarter to quarter and year to year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance. In any particular quarter or quarters, our operating results could be below the expectations of securities analysts or investors, which could cause the price of the ADSs to decline.
We have never generated any revenue from product sales and may never be profitable.
Our ability to generate significant revenue and achieve profitability depends on our ability to, alone or with strategic collaboration partners, successfully complete the development of and obtain the regulatory approvals for our product candidates, to manufacture sufficient supply of our product candidates, to establish a sales and marketing organization or suitable third-party alternative for the marketing of any approved products and to successfully commercialize any approved products on commercially reasonable terms. All of these activities will require us to raise sufficient funds to finance business activities.
In the past we received milestone payments from our collaborative partners. However, we do not anticipate generating revenue from commercializing product candidates for the foreseeable future, if ever.
Our ability to generate future revenues from commercializing product candidates depends heavily on our success in:
| • | establishing proof of concept in preclinical studies and clinical trials for our product candidates; |
| • | successfully initiating and completing clinical trials of our product candidates; |
| • | obtaining regulatory and marketing approvals for product candidates for which we complete clinical trials; |
| • | maintaining, protecting and expanding our intellectual property portfolio, and avoiding infringing on intellectual property of third parties; |
| • | establishing and maintaining successful licenses, collaborations and alliances with third parties; |
| • | developing a sustainable, scalable, reproducible and transferable manufacturing process for our product candidates; |
| • | establishing and maintaining supply and manufacturing relationships with third parties that can provide products and services adequate, in amount and quality, to support clinical development and commercialization of our product candidates, if approved; |
| • | launching and commercializing any product candidates for which we obtain regulatory and marketing approval, either by collaborating with a partner or, if launched independently, by establishing a sales, marketing and distribution infrastructure; |
| • | obtaining market acceptance of any product candidates that receive regulatory approval as viable treatment options; |
| • | obtaining favorable coverage and reimbursement rates for our products from third-party payers; |
| • | addressing any competing technological and market developments; |
| • | identifying and validating new product candidates; and |
| • | negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter. |
The process of developing product candidates for ddRNAi-based and antisense RNA-based therapeutics contains a number of inherent risks and uncertainties. For example, with regard to ddRNAi, it may not be possible to identify a target region of a disease-associated gene that has not been previously identified and/or patented by others, resulting in restrictions on freedom to operate for that target sequence. Silencing the target gene may not ultimately result in curing the disease as there may be more factors contributing to the development of the disease than the target gene. Silencing the target gene using ddRNAi may lead to short-term or long-term adverse effects that were not predicted or observed in preclinical studies. The delivery of the DNA construct to the target cells may not be possible, or complete or adequate to provide sufficient therapeutic benefit.
7
Even if one or more of our product candidates is approved for commercial sale, we may incur significant costs associated with commercializing any approved product candidate. As one example, our expenses could increase beyond expectations if we are required by the Food and Drug Administration, or FDA, or other regulatory agencies, domestic or foreign, to perform clinical and other studies in addition to those that we currently anticipate. Even if we are able to generate revenues from the sale of any approved products, we may not become profitable and may need to obtain additional funding to continue operations, which could have an adverse effect on our business, financial condition, results of operations and prospects.
We will need to continue our efforts to raise additional funding, which may not be available on acceptable terms, or at all. Failure to obtain capital when needed may negatively impact our ability to continue as a going concern.
Developing ddRNAi products is expensive, and we expect our research and development expenses to increase substantially in connection with our ongoing activities, particularly as we advance our product candidates in preclinical studies and in future clinical trials and as we undertake preclinical studies of new product candidates.
As of June 30, 2019, our cash and cash equivalents were A$22.4 million. We estimate that our cash and cash equivalents will be sufficient to fund our operations until approximately the end of calendar 2021. However, our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings, government grants or other third-party funding, strategic alliances and licensing arrangements or a combination of these approaches. In addition, because the length of time and activities associated with successful development of our product candidates is highly uncertain, we are unable to estimate the actual funds we will require for development and any approved marketing and commercialization activities. In any event, we will require additional capital to obtain regulatory approval for our product candidates and to commercialize any product candidates that receive regulatory approval.
Any additional fundraising efforts may divert our management from their day-to-day activities, which may compromise our ability to develop and commercialize our product candidates. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may adversely affect the holdings or the rights of our shareholders, and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our ordinary shares, ADSs and Warrants to decline. If we incur indebtedness we may be required to agree to restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could compromise our ability to conduct our business. We could also seek financing through arrangements with collaborative partners at an earlier stage than would otherwise be desirable and we may be required to relinquish rights to some or all of our technologies or product candidates or otherwise agree to terms unfavorable to us.
If we are unable to obtain funding on a timely basis or on acceptable terms, we may be required to significantly curtail, delay or discontinue one or more of our research or development programs or the commercialization of any approved product candidates.
Nant Capital, LLC is our largest shareholder and may have interests that are different from Benitec's and our other shareholders.
Following the October 2016 and March 2017 private placements of an aggregate 58,611,638 shares to Nant Capital, LLC and an entitlement offer subscribed by Nant Capital for additional 29,305,819 ordinary shares, Nant Capital currently holds as at September 30, 2019 28.08% of our outstanding ordinary shares. Nant Capital is entitled to appoint, and has appointed, a director to Benitec’s board of directors. Nant Capital's representative on the Board, Dr. Jerel Banks, also became Chief Executive Officer of Benitec in June 2018.
We have also entered into an exclusive sublicense agreement with an affiliate of Nant Capital and a research collaboration agreement with Nant Capital pursuant to which we have agreed to develop and commercialize an Epidermal Growth Factor Receptor, or EGFR, antisense product candidate for HNSCC, which we call BB-401. We are required to make periodic, milestone and royalty payments to Nant Capital pursuant to the exclusive sublicense agreement in connection with the development and sale of BB-401.
8
As a result of its ownership of our ordinary shares and its nomination of a director to our board of directors, Nant Capital may exert influence over Benitec, including election of our board of directors, corporate transactions and strategic decisions. The interests of Nant Capital may differ from or conflict with the interests of our other shareholders. In the future, if Nant Capital does not support a merger, tender offer, sale of assets or other business combination because it judges that transaction to be inconsistent with Nant Capital’s investment strategy, Benitec may be unable to enter into or consummate a transaction that would enable other shareholders to realize a premium over the then-prevailing market prices for our ordinary shares. Furthermore, if Nant Capital sells substantial amounts of the Benitec’s ordinary shares to enhance its liquidity position, fund alternative investments or for other reasons, the trading price of Benitec’s ADSs and the Warrants could decline significantly, and other shareholders may be unable to sell their ADSs or Warrants at favorable prices. We cannot predict or control how Nant Capital may use the influence it has as a result of its ordinary share holdings.
We may be unable to conduct and conclude clinical trials for our product candidate for OPMD if we are unable to raise additional financing.
We have sufficient funds at this time to complete the pre-clinical and clinical studies for BB-301 however we expect that we will need to raise additional funds to continue our pre-clinical and clinical studies. If we are unable to raise additional funds, and thus unable to continue our studies, our business operations may be adversely affected.
We receive Australian government research and development grants. If we lose funding from these research and development grants, we may encounter difficulties in the funding of future research and development projects, which could harm our operating results.
We have historically received, and expect to continue to receive, grants through the Australian federal government's Research and Development Tax Incentive program, under which the government provides a cash refund for the 43.5% (2018:43.5%) of eligible research and development expenditures by small Australian entities, which are defined as Australian entities with less than A$20 million in revenue, having a tax loss. The Research and Development Tax Incentive grant is made by the Australian federal government for eligible research and development purposes based on the filing of an annual application. We expect to receive a Research and Development Tax Incentive grant of A$0.9 million for the period end June 30, 2019. We have received Research and Development Tax Incentive grants in the fiscal years ended June 30, 2018, 2017 and 2016 of A$4 million, A$10.5 million and A$3.6 million, respectively. This grant is available for our research and development activities in Australia, as well as activities in the United States to the extent such U.S.-based expenses relate to our activities in Australia, do not exceed half the expenses for the relevant activities and are approved by the Australian government. To the extent our research and development expenditures are deemed to be "ineligible," then our grants would decrease. In addition, the Australian government may in the future decide to modify the requirements of, reduce the amounts of the grants available under, or discontinue the Research and Development Tax Incentive program. Changes to the Tax Incentive program could have a material adverse effect on our future cash flows and financial position.
Risks Related to the Product Development and Regulatory Approval of Our Product Candidates
Our product candidates are based on ddRNAi technology. Currently, no product candidates utilizing ddRNAi technology have been approved for commercial sale and our approach to the development of ddRNAi technology may not result in safe, effective or marketable products.
We have concentrated our product research and development efforts on our ddRNAi technology, and our future success depends on successful clinical development of this technology. We plan to progress our product candidates using our ddRNAi technology and deliver therapeutics for the life-threatening conditions of OPMD and hepatitis B.
The scientific research that forms the basis of our efforts to develop product candidates is based on the therapeutic use of ddRNAi, and the identification, optimization and delivery of ddRNAi-based product candidates is relatively new. The scientific evidence to support the feasibility of successfully developing therapeutic treatments based on ddRNAi is preliminary and limited. There can be no assurance that any development and technical problems we experience in the future will not cause significant delays or unanticipated costs, or that such
9
development problems can be solved. We may be unable to reach agreement on favorable terms, or at all, with providers of vectors needed to optimize delivery of our product candidates to target disease cells and we may also experience unanticipated problems or delays in expanding our manufacturing capacity or transferring our manufacturing process to commercial partners, any of which may prevent us from completing our preclinical trials, initiating clinical trials or commercializing our products on a timely or profitable basis, if at all.
Only a few product candidates based on ddRNAi have been tested in either animals or humans, and a number of clinical trials conducted by other companies using other forms of RNAi technologies have not been successful. We may discover that application of ddRNAi does not possess properties required for a therapeutic benefit, such as the ability to continually express shRNAs for the period of time required to be maximally effective or the ability of viral vectors or other technologies to effectively deliver ddRNAi constructs to target cells in therapeutically relevant concentrations. In addition, application of ddRNAi-based products in humans may result in safety issues. We currently have only limited data, and no conclusive evidence, to suggest that we can effectively produce effective therapeutic treatments using our ddRNAi technology.
We are early in our product development efforts and our current product candidates are still in preclinical or early clinical development. We may not be able to obtain regulatory approvals for the commercialization of our product candidates.
The research, testing, manufacturing, labeling, approval, selling, marketing and distribution of biologics is subject to extensive regulation by the FDA and other regulatory authorities, and these regulations differ from country to country. We do not have any products on the market and are early in our development efforts. All of our ddRNAi product candidates are in preclinical development. Our current product candidates are subject to the risks of failure typical for development of biologics. The development and approval process is expensive and can take many years to complete, and its outcome is inherently uncertain. In addition, the outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results.
We have not submitted a marketing application, or received marketing approval, for any of our product candidates and will not submit any applications for marketing approval for several years. We have limited experience in conducting and managing clinical trials necessary to obtain regulatory approvals, including marketing approval by the FDA. To receive marketing approval, we must, among other things, demonstrate with evidence from clinical trials that the product candidate is both safe and effective for each indication for which approval is sought, and failure can occur in any stage of development. Satisfaction of the marketing approval requirements typically takes several years and the time needed to satisfy them may vary substantially, based on the type, complexity and novelty of the biopharmaceutical product. We cannot predict if or when we might receive regulatory approvals for any of our product candidates currently under development.
The FDA and foreign regulatory authorities also have substantial discretion in the biopharmaceutical product approval process. The numbers, types and sizes of preclinical studies and clinical trials that will be required for regulatory approval varies depending on the product candidate, the disease or condition that the product candidate is designed to address and the regulations applicable to any particular product candidate. Approval policies, regulations or the type and amount of clinical data necessary to gain marketing approval may change during the course of a product candidate's clinical development and may vary among jurisdictions, and there may be varying interpretations of data obtained from preclinical studies or clinical trials, any of which may cause delays or limitations in the marketing approval or the decision not to approve an application. Regulatory agencies can delay, limit or deny marketing approval of a product candidate for many reasons, including:
| • | the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of any future clinical trials; |
| • | we may be unable to demonstrate to the satisfaction of the FDA or comparable foreign regulatory authorities that a product candidate is safe and effective for its proposed indication; |
| • | the results of any future clinical trials may not meet the level of statistical or clinical significance required by the FDA or comparable foreign regulatory authorities for approval; |
10
| • | the patients recruited for a particular clinical program may not be sufficiently broad or representative to assure safety and effectiveness in the full population for which we seek approval; |
| • | the results of any future clinical trials may not confirm the positive results from earlier preclinical studies or clinical trials; |
| • | we may be unable to demonstrate that a product candidate's clinical and other benefits outweigh its safety risks; |
| • | the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials; |
| • | the data collected from any future clinical trials of our product candidates may not be sufficient to the satisfaction of FDA or comparable foreign regulatory authorities to support the submission of a biologics license application, or BLA, or other comparable submission in foreign jurisdictions or to obtain regulatory approval in the United States or elsewhere; |
| • | the FDA or comparable foreign regulatory authorities may only agree to approve a product candidate under conditions that are so restrictive that the product is not commercially viable; |
| • | regulatory agencies might not approve or might require changes to our manufacturing processes or facilities; or |
| • | regulatory agencies may change their approval policies or adopt new regulations in a manner rendering our clinical data insufficient for approval. |
Any delay in obtaining or failure to obtain required approvals could materially and adversely affect our ability to generate revenue from the particular product candidate, which likely would result in significant harm to our financial position and adversely impact the price of the ADSs and Warrants. Furthermore, any regulatory approval to market a product may be subject to limitations on the indicated uses for which we may market the product. These limitations may limit the size of the market for the product.
We are not permitted to market our product candidates in the United States or in other countries until we receive approval of a BLA from the FDA or marketing approval from applicable regulatory authorities outside the United States. Obtaining approval of a BLA can be a lengthy, expensive and uncertain process. If we fail to obtain FDA approval to market our product candidates, we will be unable to sell our product candidates in the United States, which will significantly impair our ability to generate revenues. In addition, failure to comply with FDA and non-U.S. regulatory requirements may, either before or after product approval, if any, subject us to administrative or judicially imposed sanctions, including:
| • | restrictions on our ability to conduct clinical trials, including full or partial clinical holds on ongoing or planned trials; |
| • | restrictions on the products, manufacturers or manufacturing process; |
| • | civil and criminal penalties; |
| • | suspension or withdrawal of regulatory approvals; |
| • | product seizures, detentions or import bans; |
| • | voluntary or mandatory product recalls and publicity requirements; |
| • | total or partial suspension of production; |
| • | imposition of restrictions on operations, including costly new manufacturing requirements; and |
| • | refusal to approve pending BLAs or supplements to approved BLAs. |
11
Even if we do receive regulatory approval to market a product candidate, any such approval may be subject to limitations on the indicated uses for which we may market the product. It is possible that none of our existing product candidates or any product candidates we may seek to develop in the future will ever obtain the appropriate regulatory approvals necessary for us or our collaborators to commence product sales. Any delay in obtaining, or an inability to obtain, applicable regulatory approvals would prevent us from commercializing our product candidates, generating revenues and achieving and sustaining profitability.
Because our ddRNAi product candidates are considered gene therapies, it is difficult to predict the time and cost of product candidate development as well as subsequently obtaining regulatory approval.
The clinical trial requirements of the FDA and comparable foreign regulatory authorities and the criteria these regulators use to determine the safety and efficacy of a product candidate vary substantially according to the type, complexity, novelty and intended use and market of such product candidates. The regulatory approval process for novel product candidates such as ours can be more expensive and take longer than for other pharmaceutical product candidates. The FDA and comparable foreign regulatory authorities have relatively limited experience with ddRNAi-based therapeutics, which makes it difficult to determine how long it will take or how much it will cost to obtain regulatory approvals for our product candidates in the United States or other countries. We and our current collaborators, or any future collaborators, may never receive approval to market and commercialize any product candidate. Even if we or a collaborator obtain regulatory approval, the approval may be for disease indications or patient populations that are not as broad as we intended or desired and may require labeling that includes significant use or distribution restrictions or safety warnings.
Regulatory requirements governing gene and cell therapy products have changed frequently and may continue to change in the future. Also, before a clinical trial can begin at any institution, that institution's institutional review board, or IRB, and its institutional biosafety committee, or IBC, if it has one, have to review the proposed clinical trial to assess the safety of the trial. In addition, adverse developments in clinical trials of gene therapy products conducted by others may cause the FDA or comparable foreign regulatory bodies to change the requirements for approval of any of our product candidates.
These committees and advisory groups and the new guidelines they promulgate and new requirements they may impose may lengthen the clinical development and regulatory review process, require us to perform additional studies, increase our development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization of these product candidates or lead to significant post-approval limitations or restrictions. As we advance our product candidates, we will be required to consult with these regulatory committees and advisory groups, and comply with applicable guidelines and requirements as they may change from time to time. If we fail to do so, we may be required to delay or discontinue development of our product candidates. These additional processes may result in a development, review and approval process that is longer than we otherwise would have expected for our product candidates. Delay or failure to obtain, or unexpected costs in obtaining, the regulatory approval necessary to bring a potential product to market would delay or prevent us from commercializing our product candidates, generating revenues and achieving and sustaining profitability.
Positive results from preclinical studies of our product candidates are not necessarily predictive of the results of our planned clinical trials of our product candidates.
Positive results in preclinical proof of concept and animal studies of our product candidates may not result in positive results in clinical trials in humans. Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in clinical trials after achieving positive results in preclinical development or early stage clinical trials, and we cannot be certain that we will not face similar setbacks. These setbacks have been caused by, among other things, preclinical findings made while clinical trials were underway or safety or efficacy observations made in clinical trials, including adverse events. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that believed their product candidates performed satisfactorily in preclinical studies and clinical trials nonetheless failed to obtain FDA or other regulatory authority approval. If we fail to produce positive results in our clinical trials of our product candidates, the development timeline and regulatory approval and commercialization prospects for our product candidates, and, correspondingly, our business and financial prospects, would be negatively impacted.
12
Issues that may impact delivery of our therapeutics to the cell could adversely affect or limit our ability to develop and commercialize product candidates.
Successful clinical development of ddRNAi-based therapeutics is largely dependent on using the appropriate delivery methodologies, including viral vectors, to obtain therapeutically relevant concentrations of the DNA constructs that express the shRNAs in the appropriate target cells. To develop effective product candidates, we will need to license delivery technologies from third parties or develop delivery technologies with research collaborators. Although delivery technologies, including AAV vectors, have been identified and are well defined for specific tissue types, we continue to seek vectors with ideal delivery properties for indications we are pursuing, including OPMD. The tissue tropism and other physical properties of AAV vectors are limited and may not be effective for other product candidates or delivery into a wide array of tissues types. AAV vectors can also trigger immune responses in some patients, and those patients will not derive clinical benefit from administration of a product candidate unless steps are taken to clinically address the issue. If we or our collaborators are not successful in identifying effective delivery methodologies to achieve a therapeutically relevant concentration for our product candidates in the target tissues, we may not succeed in developing marketable products. In addition, if we are unable to reach agreement on favorable terms, or at all, with providers of any effective vectors that we do identify, we may not succeed in completing our clinical trials or commercializing our products on a timely or profitable basis, if at all.
We use AAV vectors as part of our ddRNAi approach for several indications. As such, we require licenses and the ability to manufacture large quantities of AAV particles under the FDA's current good manufacturing practices, or cGMP, requirements and those of comparable foreign regulatory authorities in order to commercialize a product candidate using an AAV vector.
We may find it difficult to enroll patients in our current and any future clinical trials, and patients could discontinue their participation in our current and any future clinical trials, which could delay or prevent our current and any future clinical trials of our product candidates and make those trials more expensive to undertake.
Identifying and qualifying patients to participate in current and any future clinical trials of our product candidates is critical to our success. The timing of our clinical trials depends on the speed at which we can recruit patients to participate in testing our product candidates. Patients may be unwilling to participate in any future clinical trials because of negative publicity from adverse events in the biotechnology, RNAi or gene therapy industries. Patients may be unavailable for other reasons, including competitive clinical trials for similar patient populations, and the timeline for recruiting patients, conducting trials and obtaining regulatory approval of potential products may be delayed. If we have difficulty enrolling a sufficient number of patients to conduct any future clinical trials as planned, we may need to delay, limit or discontinue those clinical trials. Clinical trial delays could result in increased costs, slower product development, setbacks in testing the safety and effectiveness of our technology or discontinuation of the clinical trials altogether.
We may not be able to identify, recruit and enroll a sufficient number of patients, or those with required or desired characteristics to achieve diversity in a trial, to complete any future clinical trials in a timely manner. Patient enrollment is affected by factors including:
| • | finding and diagnosing patients; |
| • | severity of the disease under investigation; |
| • | design of the clinical trial protocol; |
| • | size and nature of the patient population; |
| • | eligibility criteria for the trial in question; |
| • | perceived risks and benefits of the product candidate under study; |
| • | proximity and availability of clinical trial sites for prospective patients; |
| • | availability of competing therapies and clinical trials; |
13
| • | clinicians' and patients' perceptions of the potential advantages of the product being studied in relation to other available therapies, including any new products that may be approved for the indications we are investigating; |
| • | patient referral practices of physicians; and |
| • | ability to monitor patients adequately during and after treatment. |
For example, we experienced some difficulties in enrolling patients in our clinical trial of TT-034, which was discontinued for commercial reasons. These difficulties were due to several factors, including sudden changes in patients' viral load, liver enzymes and other clinical parameters immediately prior to their dosing, as well as late withdrawal due to personal reasons. We believe the increased availability of new and effective therapies such as Sovaldi and Harvoni, which were approved for treatment of the hepatitis C virus in 2013 and 2014, respectively, and the fact that the early lower-dose cohort patients receive a sub-therapeutic dose of TT-034, may also have been contributing factors. We may face similar difficulties enrolling patients in future clinical trials for other product candidates for these and other reasons.
We or any potential collaborators plan to seek initial marketing approval for our product candidates in the United States, Australia and Europe. We may not be able to initiate or continue any future clinical trials in a timely manner if we cannot enroll a sufficient number of eligible patients to participate in the clinical trials required by the FDA or comparable foreign regulatory agencies. Our ability to successfully initiate, enroll and complete a clinical trial in any foreign country is subject to numerous risks unique to conducting business in foreign countries, including:
| • | difficulty in establishing or managing relationships with contract research organizations, or CROs, clinical sites and physicians; |
| • | different standards for the conduct of clinical trials; |
| • | our inability to locate and engage qualified local consultants, physicians and partners; and |
| • | the potential burden of complying with a variety of foreign laws, medical standards and regulatory requirements, including the regulation of biopharmaceutical and biotechnology products and treatments. |
In addition, patients enrolled in current and any future clinical trials may discontinue their participation at any time during the trial as a result of a number of factors, including experiencing adverse events, which may or may not be judged related to our product candidates under evaluation. The discontinuation of patients in any one of our trials may cause us to delay or discontinue our clinical trial, or cause the results from that trial not to be positive or sufficient to support either partnering with a pharmaceutical or biotechnology company to further develop and commercialize the product candidate or filing for regulatory approval of the product candidate.
We may encounter substantial delays in current and any future clinical trials or we may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities.
Before obtaining marketing approval from regulatory authorities for the sale of any of our product candidates, we must conduct extensive clinical trials to demonstrate the safety and efficacy of the product candidates in humans. Clinical trials are expensive and time-consuming, and their outcome is uncertain. We cannot guarantee that any clinical trials will be initiated or conducted as planned or completed on schedule, if at all. A failure of one or more clinical trials can occur at any stage of testing. Events that may prevent successful or timely completion of clinical development include:
| • | inability to generate sufficient preclinical, toxicology or other data to support the initiation of human clinical trials; |
| • | delays in reaching consensus with regulatory agencies on trial design; |
| • | identifying, recruiting and training suitable clinical investigators; |
| • | delays in reaching agreement on acceptable terms with prospective CROs and clinical trial sites; |
14
| • | delays in obtaining required IRB or IBC approval at each clinical trial site; |
| • | delays in recruiting suitable patients to participate in our clinical trials; |
| • | imposition of a clinical hold by regulatory agencies, including after an inspection of our clinical trial operations or trial sites; |
| • | failure by our CROs, other third parties or us to adhere to clinical trial requirements; |
| • | failure to perform in accordance with the FDA's good clinical practices, or GCP, or applicable regulatory requirements in other countries; |
| • | inability to manufacture, test, release, import or export for use sufficient quantities of our product candidates for use in clinical trials; |
| • | failure to manufacture our product candidate in accordance with cGMP requirements or applicable regulatory guidelines in other countries; |
| • | delays in the testing, validation and manufacturing of our product candidates; |
| • | delays in the delivery of our product candidates to the clinical trial sites; |
| • | delays in having patients complete participation in a trial or return for post-treatment follow-up; |
| • | clinical trial sites dropping out of a trial; |
| • | occurrence of serious adverse events associated with the product candidate that are viewed to outweigh its potential benefits; |
| • | changes in regulatory requirements and guidance that require amending or submitting new clinical trial protocols; or |
| • | clinical trials of our product candidates may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical trials or discontinue product development programs. |
Further, a clinical trial may be suspended or discontinued by us, our collaborators, the IRBs or the IBCs at the sites in which such trials are being conducted, the data safety monitoring board, or DSMB, for such trial, or by the FDA or comparable foreign regulatory authorities due to a number of factors, including the imposition of a clinical hold or termination of a trial due to failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, unforeseen safety issues or adverse side effects of our product candidate, or a product candidate from another company that shares similar properties, failure to demonstrate adequate benefit from using a product candidate, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. If we experience discontinuation of, or delays in the completion of, any clinical trial of our product candidates, the commercial prospects of our product candidates may be harmed, and our ability to generate product revenues from any of these product candidates may be eliminated or delayed. Furthermore, many of the factors that lead to a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
In addition, if we or our third-party collaborators make significant manufacturing or formulation changes to our product candidates, we or they may need to conduct additional studies to bridge the modified product candidates to earlier versions to ensure comparability, safety and efficacy of the two different product candidates.
Any inability to successfully complete preclinical and clinical development could result in additional costs to us or impair our ability to commercialize our programs and product candidates. Clinical trial delays could also shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates.
15
If the results of our current or any future clinical trials are inconclusive or if there are safety concerns or adverse events associated with our product candidates, we may:
| • | fail to obtain, or be delayed in obtaining, marketing approval for our product candidates; |
| • | obtain approval for indications or patient populations that are not as broad as intended or desired; |
| • | obtain approval with labeling that includes significant use or distribution restrictions or safety warnings; |
| • | need to change the way the product is administered; |
| • | be required to perform additional clinical trials to support approval or be subject to additional post-marketing testing requirements; |
| • | have regulatory authorities withdraw their marketing approval of the product after granting it; |
| • | have regulatory authorities impose restrictions on distribution of the product in the form of a risk evaluation and mitigation strategy, or REMS, or modified REMS, that limit our ability to commercialize the product; |
| • | be subject to the addition of labeling statements, such as warnings or contraindications; |
| • | be sued and held liable for harm caused to patients; or |
| • | experience damage to our reputation. |
Any of these events could prevent us from achieving or maintaining market acceptance of our product candidates and impair our ability to commercialize our product candidates.
In addition, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and may receive cash or equity compensation in connection with such services. If these relationships and any related compensation result in perceived or actual conflicts of interest, or the FDA concludes that the financial relationship may have affected the interpretation of any particular study, the integrity of the data generated at the applicable clinical trial site may be questioned and the utility of the clinical trial itself may be jeopardized, which could result in the delay or rejection of any BLA we submit to the FDA or any comparable foreign regulating authorities. Any such delay or rejection could prevent us from commercializing our product candidates.
Our product candidates and the process for administering our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following any potential marketing approval.
Treatment with our product candidates may produce undesirable side effects or adverse reactions or events. For example, the AAV vector and related capsid protein, which we are currently using to deliver many of our ddRNAi product candidates, could cause adverse immunological side effects due to preexisting and/or recall responses to the naturally occurring virus from which the vector is engineered, or to the DNA construct product itself. These responses may also interfere with therapeutic efficacy if not identified and managed optimally. Preexisting immune responses to AAV manifesting as neutralizing antibodies are common within the general population and may be a limitation to the enrollment of patients in gene therapy clinical trials using AAV vectors, the successful use of AAV vectors in gene therapy clinical trials and the market acceptance of product candidates, if approved, that are delivered using AAV vectors. Patients with neutralizing antibodies to AAV will not derive clinical benefit from administration of such a product candidate unless steps are taken to clinically address the issue and those treatments themselves may cause adverse effects. In previous clinical trials undertaken by other companies involving systemic administration of AAV viral vectors for gene therapy, some subjects experienced adverse events, including the development of a negative T cell response against the AAV capsid protein. If our vectors cause similar adverse events, we may be required to delay or discontinue further clinical development of our product candidates. It is also possible that we may discover new adverse events related to AAV or other vectors, which could potentially enhance the risk to patients who use our product candidates delivered with that vector.
16
If any such adverse events occur, our current and any future clinical trials could be suspended or discontinued and the FDA or comparable foreign regulatory authorities could order us to cease further development or deny approval of our product candidates for any or all targeted indications. The product-related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial. If we elect or are required to delay, suspend or discontinue any clinical trial of any of our product candidates, the commercial prospects of such product candidates will be harmed and our ability to generate product revenues from any of these product candidates will be delayed or eliminated. Any of these occurrences may harm our business, financial condition and prospects significantly.
Additionally, if any of our biopharmaceutical product candidates receive marketing approval, the FDA could require us to adopt a REMS to ensure that the benefits of the product outweigh its risks, which may include, among other things, a medication guide outlining the risks of gene therapies for distribution to patients and a communication plan to patients and healthcare practitioners. Other elements to assure safe use in a mandated REMS could include, but are not limited to, restrictions upon distribution and prescribing, additional prescriber training, establishment of patient registries and other measures that could limit commercialization of the product. Comparable foreign regulating authorities might require adoption of measures similar to a REMS. Furthermore, if we or others later identify undesirable side effects caused by our product candidate, a number of potentially significant negative consequences could result, including:
| • | regulatory authorities may withdraw approvals of such product candidate; |
| • | regulatory authorities may require additional warnings on the label; |
| • | we may be required to create a medication guide outlining the risks of such side effects for distribution to patients; |
| • | we may be required to change the way a product candidate is administered or conduct additional clinical trials; |
| • | we could be sued and held liable for harm caused to patients; and |
| • | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of our product candidates and could significantly harm our business, prospects, financial condition and results of operations.
If we are unable to successfully develop related diagnostics for our therapeutic product candidates, or experience significant delays in doing so, we may not achieve marketing approval or realize the full commercial potential of our therapeutic product candidates.
We may develop related diagnostics for some of our therapeutic product candidates. Such related diagnostics are subject to regulation by the FDA and typically to comparable foreign regulatory authorities as medical devices and typically require separate regulatory approval or clearance prior to commercialization. Marketing approval or clearance of the diagnostic will require sufficient data to support its safety and efficacy. In addition, at least in some cases, the FDA and comparable foreign regulatory authorities may require the development and regulatory approval or clearance of a related diagnostic as a condition to approving our therapeutic product candidates. While we have some, limited experience in developing diagnostics, we plan to rely in large part on third parties to perform these functions. We may seek to enter into arrangements with one or more third parties to create a related diagnostic for use with our current or future product candidates.
If we or any third parties that we engage to assist us, are unable to successfully develop or obtain marketing approval or clearance for related diagnostics for our therapeutic product candidates, or experience delays in doing so:
| • | the development of relevant product candidates may be delayed or impaired altogether if we are unable to appropriately select patients for enrollment in our clinical trials; |
| • | our relevant therapeutic product candidate may not receive marketing approval if its effective use depends on a related diagnostic in the regulatory authority’s judgment; and |
17
| • | we may not realize the full commercial potential of any therapeutic product candidates that receive marketing approval if, among other reasons, we are unable to appropriately identify patients with the specific genetic alterations targeted by our therapeutic product candidates. |
If any of these events were to occur, our business would be harmed.
Even if we complete the necessary preclinical studies and clinical trials, we cannot predict when or if we will obtain regulatory approval to commercialize a product candidate or the approval may be for a more narrow indication than we expect.
We cannot commercialize a product until the appropriate regulatory authorities have reviewed and approved the product candidate. Even if our product candidates demonstrate safety and efficacy in clinical trials, the regulatory agencies may not complete their review processes in a timely manner, or we may not be able to obtain regulatory approval. Additional delays may result if an FDA Advisory Committee or comparable foreign regulatory authority recommends non-approval or restrictions on approval. In addition, we may experience delays or rejections based upon additional government regulation from future legislation or administrative action, or changes in regulatory agency policy during the period of product development, clinical trials and the review process. Regulatory agencies may also approve a treatment candidate for fewer or more limited indications than requested, may not approve the price we intend to charge for our product candidate, may limit our ability to promote the product, may impose significant limitations upon the approval of the product, including, but not limited to, narrow indications, significant warnings, precautions or contraindications with respect to conditions of use, or may grant approval subject to the performance of post-marketing studies. In addition, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of our product candidates. The FDA or comparable foreign regulatory authorities may impose a REMS or other conditions upon our approval that limit our ability to commercialize the product candidate.
Even if we obtain regulatory approval for a product candidate, our products will remain subject to regulatory scrutiny.
Even if we obtain regulatory approval in a jurisdiction, the regulatory authority may still impose significant restrictions on the indicated uses or marketing of our product candidates, or impose ongoing requirements for potentially costly post-approval studies or post-market surveillance. For example, the holder of an approved BLA is obligated to monitor and report to the FDA adverse events and any failure of a product to meet the specifications in the BLA. FDA guidance advises that patients treated with some types of gene therapy undergo follow-up observations for potential adverse events for as long as 15 years. The holder of an approved BLA must also submit new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling or manufacturing process. Advertising and promotional materials must comply with FDA rules and are subject to FDA review, in addition to other potentially applicable foreign, federal and state laws.
In addition, product manufacturers and their establishments, products and applications are subject to payment of user fees/or and continual review and periodic inspections by the FDA and comparable foreign regulatory authorities for compliance with cGMP and comparable foreign requirements, and adherence to commitments made in the BLA. If we or a regulatory agency discover previously unknown problems with a product such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions relative to that product or the manufacturing facility, including requiring recall or withdrawal of the product from the market or suspension of manufacturing.
If we fail to comply with applicable regulatory requirements following approval of any of our product candidates, a regulatory agency may:
| • | issue a warning letter asserting that we are in violation of the law; |
| • | seek an injunction or impose civil or criminal penalties or monetary fines; |
| • | suspend or withdraw regulatory approval; |
| • | suspend any ongoing clinical trials; |
18
| • | refuse to permit government reimbursement of our product by government-sponsored third-party payers; |
| • | refuse to approve a pending BLA or supplements to a BLA submitted by us for other indications or new product candidates; |
| • | refuse to allow us to enter into or continue supply contracts, including government contracts. |
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. The occurrence of any event or penalty described above may inhibit our ability to commercialize our product candidates and generate revenues.
Even if we obtain and maintain approval for our product candidates from the FDA, we may never obtain approval for our product candidates outside of the United States, which would limit our market opportunities and adversely affect our business.
Approval of a product candidate in the United States by the FDA does not ensure approval of such product candidate by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. Sales of our product candidates outside of the United States will be subject to foreign regulatory requirements governing clinical trials and marketing approval. Even if the FDA grants marketing approval for a product candidate, comparable regulatory authorities of foreign countries must also approve the manufacturing and marketing of the product candidates in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the United States, including additional preclinical studies or clinical trials. In many countries outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that country. In some cases, the price that we intend to charge for our products, if approved, is also subject to approval. Even if a product candidate is approved, the FDA or comparable regulatory authorities in other countries, as the case may be, may limit the indications for which the product may be marketed, require extensive warnings on the product labeling or require expensive and time-consuming clinical trials or reporting as conditions of approval. Regulatory authorities in countries outside of the United States also have requirements for approval of product candidates with which we must comply prior to marketing in those countries. Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our product candidates in certain countries.
Further, clinical trials conducted in one country may not be accepted by regulatory authorities in other countries. Regulatory approval of a product candidate in one country does not ensure approval in any other country, but a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory approval process in other countries. Also, regulatory approval for any of our product candidates may be withdrawn based on adverse events reported or regulatory decisions made in other countries. If we fail to comply with the regulatory requirements in international markets and/or fail to receive applicable marketing approvals, our target market will be reduced, our ability to realize the full market potential of our product candidates will be compromised and our business may be adversely affected.
Our future prospects may also depend on our or our collaborators' ability to successfully develop a pipeline of additional product candidates, and we and our collaborators may not be successful in efforts to use our platform technologies to identify or discover additional product candidates.
The success of our business depends primarily upon our ability to identify, develop and commercialize products based on our platform technology. We do not have any products on the market and are early in our development efforts. All of our product candidates are in preclinical development or early clinical development. Our product candidates derived from our platform technology may not successfully complete investigational new drug, or IND, enabling studies, and our research programs may fail to identify other potential product candidates for clinical development for a number of reasons. Our and our collaborators' research methodology may be unsuccessful in identifying potential product candidates, our potential product candidates may not demonstrate the necessary
19
preclinical outcomes to progress to clinical studies, or our product candidates may be shown to have harmful side effects or may have other characteristics that may make the products unmarketable or unlikely to receive marketing approval.
If any of these events occur, we may be forced to discontinue our development efforts for a program or programs. Research programs to identify new product candidates require substantial technical, financial and human resources. We may focus our efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful.
We may not be able to obtain orphan drug exclusivity, where relevant, in all markets for our product candidates.
Of our current pipeline product candidates, only our ddRNAi therapeutic for the treatment of OPMD has been designated with orphan drug status. In January 2018, the FDA granted such designation after OPMD had been designated an orphan drug in January 2017 by the European Commission. Regulatory authorities in some jurisdictions, including the United States, may designate drugs or biological products for relatively small patient populations as orphan drugs. Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a product intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals annually in the United States. The FDA may also designate a product as an orphan drug if it is intended to treat a disease or condition of more than 200,000 individuals in the United States and there is no reasonable expectation that the cost of developing and making a drug or biological product available in the United States for this type of disease or condition will be recovered from sales of the product candidate. Generally, if a product with an orphan drug designation subsequently receives the first marketing approval for the indication for which it has such designation, the product is entitled to a period of marketing exclusivity, which precludes the FDA from approving another marketing application for the same drug for such indication for that time period. The applicable period is seven years in the United States. Orphan drug exclusivity may be lost if the FDA or EMA determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition. While there is no guarantee, FDA orphan drug designation may provide a range of benefits, including a potential fast track process for clinical regulatory approval, potential tax credits for qualified clinical trials and an exemption from FDA application user fees.
Even if we obtain orphan drug exclusivity for a product in the United States or for additional products in the European Union, that exclusivity may not effectively protect the product from competition because different drugs can be approved for the same condition, and the same drug could be approved for a different condition. Even after an orphan drug is approved, the FDA can subsequently approve the same drug, made by a competitor, for the same condition if the FDA concludes that the competitive product is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care. In the European Union, the European Medicines Agency, or the EMA, can approve a competitive product if the orphan drug no longer meets the criteria for orphan designation (including sufficient profitability), if the competitive product is safer, more effective or otherwise clinically superior, or if the orphan drug cannot be supplied in sufficient quantities.
Risks Related to Our Reliance on Third Parties
Our prospects for successful development and commercialization of our products are dependent to varying degrees upon the research, development, commercialization, and marketing efforts of any potential collaborators.
We rely on third parties for certain aspects of the research, development, commercialization and marketing of our current and any future product candidates. Other than as provided for in our collaboration agreements, we have no control over the resources, time and effort that our collaborators may devote to the development of product candidates. We are dependent on our collaborators to conduct some aspects of the research and development of each of our product candidates, and expect to need access to them to facilitate and/or to complete the regulatory process. We will likely rely on a pharmaceutical company for the successful marketing and commercialization of any such product candidates for which they/we receive approval, if any. There can be no guarantee at this stage that we will conclude a partnership with such a company on favorable terms, or at all, nor even if we do so, that success will be achieved.
20
Our ability to recognize revenues from successful potential collaborations may be impaired by multiple factors including:
| • | a collaborator may shift its priorities and resources away from our programs due to a change in business strategies, or a merger, acquisition, sale or downsizing of its company or business unit; |
| • | a collaborator may cease development in an area that is the subject of a collaboration agreement; |
| • | a collaborator may change the success criteria for a particular program or product candidate in development, thereby delaying or ceasing development of such program or product candidate in development; |
| • | a collaborator with commercialization obligations may not commit sufficient financial or human resources to the marketing, distribution or sale of a product; |
| • | a collaborator with manufacturing responsibilities may encounter regulatory, resource or quality issues and be unable to meet demand requirements; |
| • | a collaborator may exercise its rights under the agreement to discontinue our collaboration; |
| • | a dispute may arise between us and a collaborator concerning the development and commercialization of a product candidate in development, resulting in a delay in milestones, royalty payments, or discontinuation of a program and possibly resulting in costly litigation or arbitration that may divert management attention and resources; |
| • | a collaborator may not adequately protect the intellectual property rights associated with a product candidate; and |
| • | a collaborator may use our proprietary information or intellectual property in such a way as to expose us to litigation from a third party. |
If our potential collaborators do not perform in the manner we expect or fulfill their responsibilities in a timely manner, or at all, the development, regulatory and commercialization process could be delayed or discontinued or otherwise be unsuccessful. Conflicts between us and our collaborators may arise. In the event of discontinuation of one or more of our collaboration agreements, it may become necessary for us to assume the responsibility for any such product candidates at our own expense or seek new collaborators. In that event, we likely would be required to limit the size and scope of one or more of our independent programs or increase our expenditures and seek additional funding, which may not be available on acceptable terms or at all, and our business may be harmed.
We rely on third parties to conduct our preclinical studies and clinical trials.
We do not have the ability to conduct all aspects of our preclinical testing or our current or any future clinical trials ourselves. We are dependent on third parties to conduct the preclinical studies for our product candidates and will depend on third parties to conduct our current and any future clinical trials for our product candidates, and therefore the timing of the initiation and completion of these trials and studies is reliant on third parties and may occur at times substantially different from our estimates or expectations.
In the case of clinical trials, we expect to rely on CROs and third-party collaborators to conduct any future clinical trials in accordance with our clinical protocols and regulatory requirements. We expect our CROs, investigators and third-party collaborators will play a significant role in the conduct of these trials and subsequent collection and analysis of data. There is no guarantee that any CROs, investigators or the other third-party collaborators on which we rely for administration and conduct of our current and any future clinical trials will devote adequate time and resources to such trials or perform as contractually required. If any of these third parties fails to meet expected deadlines, fails to adhere to our clinical protocols, fails to meet regulatory requirements or otherwise performs in a substandard manner, any future clinical trials may be extended, delayed or terminated. If our current or any of our future clinical trial sites terminates for any reason, we may lose all of the information on subjects enrolled in any such clinical trials.
21
If we cannot contract with acceptable third parties on commercially reasonable terms, or if these third parties do not carry out their contractual duties, satisfy legal and regulatory requirements for the conduct of preclinical studies or clinical trials or meet expected deadlines, our clinical development programs could be delayed or discontinued.
In all events, we are responsible for ensuring that each of our preclinical studies, our current clinical trials and any future clinical trials are conducted in accordance with the general investigational plan and protocols for the study or trial. The FDA requires clinical trials to be conducted in accordance with current GCP, including for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights and confidentiality of clinical trial participants are protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements, and any failure to satisfy these responsibilities and requirements, whether caused by us or by third parties upon whom we rely, could have a material adverse effect on our business, financial condition, results of operations and prospects.
Because we rely on third-party manufacturing and supply partners, our supply of research and development, preclinical and clinical development materials may become limited or interrupted or may not be of satisfactory quantity or quality.
We rely on third-party supply and manufacturing partners to manufacture and supply the materials for our research and development and preclinical and clinical study supplies. We do not own manufacturing facilities or supply sources for such materials.
There can be no assurance that our supply of research and development, preclinical and clinical development biologics and other materials will not be limited, interrupted or restricted in certain geographic regions, be of satisfactory quality or continue to be available at acceptable prices. Replacement of a third-party manufacturer could require significant effort, cost and expertise because there may be a limited number of qualified replacements.
The manufacturing process for a product candidate is subject to FDA and foreign regulatory authority review. Suppliers and manufacturers must meet applicable manufacturing requirements and undergo rigorous facility and process validation tests required by regulatory authorities in order to comply with regulatory standards, such as cGMP. In the event that any of our suppliers or manufacturers fails to comply with such requirements or to perform its obligations to us in relation to quality, timing or otherwise, or if our supply of components or other materials becomes limited or interrupted for other reasons, we may be forced to manufacture the materials ourselves or enter into an agreement with another third party, which would be costly and delay any future clinical trials.
In some cases, the technical skills or technology required to manufacture our product candidates may be unique or proprietary to the original manufacturer and we may have difficulty, or there may be contractual restrictions prohibiting us from, transferring such skills or technology to another third party. These factors increase our reliance on our manufacturers and may require us to obtain a license from a manufacturer in order to have another third-party manufacture our product candidates. If we are required to change manufacturers for any reason, we will be required to verify that the new manufacturer maintains facilities and procedures that comply with quality standards and with all applicable regulations and guidelines of the FDA and comparable foreign regulatory authorities. The delays and costs associated with the verification of a new manufacturer could increase our costs and delay the development of our product candidates.
We expect to continue to rely on third-party manufacturers for preclinical and clinical grade product candidates and if we receive regulatory approval for any product candidate. To the extent that we have existing, or enter into future, manufacturing arrangements with third parties, we will depend on these third parties to perform their obligations in a timely manner consistent with contractual and regulatory requirements, including those related to quality control and assurance. If we are unable to obtain or maintain third-party manufacturing for product candidates, or to do so on commercially reasonable terms, we may not be able to develop and commercialize our product candidates successfully. Our or a third party's failure to execute on our manufacturing requirements could adversely affect our business in a number of ways, including:
| • | an inability to conduct necessary preclinical studies to progress our product candidates to clinical trials; |
| • | an inability to initiate or continue any future clinical trials of product candidates under development; |
| • | delay in submitting regulatory applications, or receiving regulatory approvals, for product candidates; |
22
| • | loss of the cooperation of a collaborator; |
| • | subjecting our product candidates to additional inspections by regulatory authorities; |
| • | requirements to cease distribution or to recall batches of our product candidates; and |
| • | in the event of approval to market and commercialize a product candidate, an inability to meet commercial demands for our products. |
We and our potential collaborators may disagree over our right to receive payments under any potential collaboration agreements, potentially resulting in costly litigation and loss of reputation.
Our ability to receive payments under any potential collaboration agreements depends on our ability to clearly delineate our rights under those agreements. We have out-licensed portions of our intellectual property to our collaborators with the intent that our collaborators will develop product candidates based on our ddRNAi technology to address specific conditions. However, a collaborator may use our intellectual property without our permission, dispute our ownership of intellectual property rights, or argue that our intellectual property does not cover, or add value to, any product candidates they develop. If a dispute arises, it may result in costly patent office procedures and litigation, and our collaborator may refuse to pay us while the dispute is ongoing. Furthermore, regardless of any resort to legal action, a dispute with a collaborator over intellectual property rights may damage our relationship with that collaborator and may also harm our reputation in the industry. Even if we are entitled to payments from our collaborators, we may not actually receive these payments, or we may experience difficulties in collecting the payments to which we believe we are entitled. After our collaborators launch commercial products containing our licensed traits, we will need to rely on the good faith of our collaborators to report to us the sales they earn from these products and to accurately calculate the payments we are entitled to, a process that will involve complicated and difficult calculations. Although we seek to address these concerns in our collaboration agreements by reserving our right to audit financial records, such provisions may not be effective.
We have only limited experience in regulatory affairs and intend to rely on consultants and other third parties for regulatory matters, which may affect our ability or the time we require to obtain necessary regulatory approvals.
We have limited experience in filing and prosecuting the applications necessary to gain regulatory approvals for gene therapy or ddRNAi product candidates. Moreover, the product candidates that are likely to result from our development programs are based on novel technologies that have not been extensively tested in humans. The regulatory requirements governing these types of product candidates may be less well defined or more rigorous than for conventional products. As a result, we may experience a longer regulatory process in connection with obtaining regulatory approvals of any products that we develop. We intend to rely on independent consultants for purposes of our regulatory compliance and product development and approvals in the United States and elsewhere. Any failure by our consultants to properly advise us regarding, or properly perform tasks related to, regulatory compliance requirements could compromise our ability to develop and seek regulatory approval of our product candidates.
Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
Because we rely on third parties to manufacture our product candidates, and because we collaborate with various organizations and academic institutions on the advancement of our technology and product candidates, we may, at times, share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, collaborative research agreements, consulting agreements or other similar agreements with our collaborators, advisors, employees and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose our confidential information, such as trade secrets. Despite these contractual provisions, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known by potential competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, discovery by a third party of our trade secrets or other unauthorized use or disclosure would impair our intellectual property rights and protections in our product candidates.
23
In addition, these agreements typically restrict the ability of our collaborators, advisors, employees and consultants to publish data potentially relating to our trade secrets. Our academic collaborators typically have rights to publish data, provided that we are notified in advance and may delay publication for a specified time in order to secure our intellectual property rights arising from the collaboration. In other cases, publication rights are controlled exclusively by us. In other cases we may share these rights with other parties. Despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of these agreements, independent development or publication of information including our trade secrets in cases where we do not have proprietary or otherwise protected rights at the time of publication.
Risks Related to Commercialization of Our Product Candidates
We have not entered into agreements with any third-party manufacturers to support commercialization of our product candidates.
We have not yet secured manufacturing capabilities for commercial quantities of our product candidates or established facilities in the desired locations to support commercialization of our product candidates. We intend to rely on third-party manufacturers for commercialization, but have not entered into any agreements with such manufacturers to support our product candidates currently in development. We may be unable to negotiate agreements with third-party manufacturers to support our commercialization activities on commercially reasonable terms.
We may encounter technical or scientific issues related to manufacturing or development that we may be unable to resolve in a timely manner or with available funds. Currently, we do not have the capacity to manufacture our product candidates on a commercial scale. In addition, our product candidates are novel, and no manufacturer currently has experience producing our product candidates on a large scale. If we are unable to engage manufacturing partners to produce our product candidates on a larger scale on reasonable terms, our commercialization efforts will be harmed.
Even if we timely develop a manufacturing process and successfully transfer it to the third-party manufacturers of our product candidates, if such third-party manufacturers are unable to produce the necessary quantities of our product candidates, or do so in compliance with cGMP or with pertinent foreign regulatory requirements, and within our planned time frame and cost parameters, the development and sales of our product candidates, if approved, may be impaired. In some jurisdictions, approval of the manufacturer may be required. There is no guarantee such approval can be obtained.
If we are unable to enter into agreements with third parties to commercialize our product candidates or establish sales and marketing capabilities to market and sell our product candidates, we may be unable to generate any revenues.
We currently have no sales and marketing organization and have no experience selling and marketing pharmaceutical products. To successfully commercialize any product candidates that may be approved, we will need to develop these capabilities, either through our relationships with collaborators or our own. We may seek to enter into collaborations with other entities to utilize their marketing and distribution capabilities, but we may be unable to enter into marketing agreements on favorable terms, if at all. If our future collaborators do not commit sufficient resources to commercialize our future products, if any, and we are unable to develop the necessary marketing capabilities on our own, we will be unable to generate sufficient product revenue to sustain our business. The establishment and development of our own sales force or the establishment of a contract sales force to market any products we may develop will be expensive and time-consuming and could delay any product launch. Moreover, we cannot be certain that we will be able to successfully develop this capability. We will be competing with many companies that currently have extensive and well-funded marketing and sales operations to recruit, hire, train and retain marketing and sales personnel. We also face competition in our search for third parties to assist us with the sales and marketing efforts of our product candidates. Without an internal team or the support of a third party to perform marketing and sales functions, we may be unable to compete successfully against these more established companies.
24
Physicians, patients, third-party payers or others in the medical community may not be receptive to our product candidates, and we may not generate any future revenue from the sale or licensing of our product candidates.
Even if we obtain approval for a product candidate, we may not generate or sustain revenue from sales of the product if the product cannot be sold at a competitive cost or if it fails to achieve market acceptance by physicians, patients, third-party payers or others in the medical community. These market participants may be hesitant to adopt a novel treatment based on ddRNAi or antisense RNA technology, and we may not be able to convince the medical community and third-party payers to accept and use, or to provide favorable reimbursement for, any product candidates developed by us or our existing or future collaborators. Market acceptance of our product candidates will depend on, among other factors:
| • | the safety and efficacy of our product candidates; |
| • | our ability to offer our products for sale at competitive prices; |
| • | the relative convenience and ease of administration of our product candidates; |
| • | the prevalence and severity of any adverse side effects associated with our product candidates; |
| • | the terms of any approvals and the countries in which approvals are obtained; |
| • | limitations or warnings contained in any labeling approved by the FDA or comparable foreign regulatory authorities; |
| • | conditions upon the approval imposed by FDA or comparable foreign regulatory authorities, including, but not limited to, a REMS; |
| • | the willingness of patients to try new treatments and of physicians to prescribe these treatments; |
| • | the availability of government and other third-party payer coverage and adequate reimbursement; and |
| • | availability of alternative effective treatments for the disease indications our product candidates are intended to treat and the relative risks, benefits and costs of those treatments. |
Since we are focused on the emerging therapeutic modality of ddRNAi, these risks may increase if new competitors are able to market ddRNAi-based therapeutics or if these treatments become less favored in the commercial marketplace. In addition, we believe that one of the benefits of our ddRNAi technology is the expected length of time of its effect. If our treatments do not have a long-term effect after administration, such a development would likely significantly and adversely affect market acceptance of our product candidates, if approved.
Additional risks apply in relation to any disease indications we pursue which are classified as rare diseases and allow for orphan drug designation by regulatory agencies in major commercial markets, such as the United States or European Union. If pricing is not approved or accepted in the market at an appropriate level for any approved product for which we pursue and receive an orphan drug designation, such product may not generate enough revenue to offset costs of development, manufacturing, marketing and commercialization despite any benefits received from the orphan drug designation, such as market exclusivity, for a period of time. Orphan exclusivity could temporarily delay or block approval of one of our products if a competitor obtains orphan drug designation for its product first. However, even if we obtain orphan exclusivity for one of our products upon approval, our exclusivity may not block the subsequent approval of a competitive product that is shown to be clinically superior to our product.
Market size is also a variable in disease indications not classified as rare. Our estimates regarding potential market size for any indication may be materially different from what we discover to exist at the time we commence commercialization, if any, for a product, which could result in significant changes in our business plan and have a material adverse effect on our business, financial condition, results of operations and prospects.
25
We face competition from entities that have developed or may develop product candidates for our target disease indications, including companies developing novel treatments and technology platforms based on modalities and technology similar to ours.
The development and commercialization of pharmaceutical products is highly competitive. We compete with a variety of multinational pharmaceutical companies and specialized biotechnology companies, as well as technology being developed at universities and other research institutions. Our competitors have developed, are developing or could develop product candidates and processes competitive with our product candidates. Competitive therapeutic treatments include those that have already been approved and accepted by the medical community, patients and third-party payers, and any new treatments that enter the market.
There may be a significant number of products that are currently under development, and may become commercially available in the future, for the treatment of conditions for which we are developing, and may in the future try to develop, product candidates.
This increasingly competitive landscape may compromise the development of our product candidates. For example, improvements in the efficacy, delivery and success rates of competitors’ product candidates, in conjunction with a reduction in the price and duration of their treatments, diminished partnering interest from pharmaceutical companies in our product candidate TT-034 for the treatment of the hepatitis C virus, or HCV. This caused us to announce in February 2016 the discontinuation of our program to develop TT-034 before the conclusion of its clinical trial.
We are aware of multiple companies that are working in the field of RNAi therapeutics, including Alnylam, Arbutus, Arrowhead, Silence Therapeutics plc, RXi Pharmaceuticals Corporation, Quark Pharmaceuticals, Inc., Marina Biotech, Inc. and Dicerna. Arrowhead, Arbutus and Alnylam are all developing siRNA-based therapeutics for HBV. All of our current product candidates, if approved, would compete with approved and currently marketed treatments.
In addition, our ddRNAi-based product candidates would compete with antisense and other RNA-based pharmaceutical products currently under development. Like RNAi therapeutics, antisense products target mRNA with the objective of suppressing the activity of specific genes. The development of antisense products is more advanced than that of RNAi therapeutics, and antisense technology may become the preferred technology for products that target mRNAs. Our HNSCC product candidate BB-401 is based on antisense RNA technology.
Many of our competitors have significantly greater financial, technical, manufacturing, marketing, sales and supply resources and experience than we have. If we successfully obtain approval for any product candidate, we will face competition based on many different factors, including the safety and effectiveness of our products, the ease with which our products can be administered and the extent to which patients accept relatively new routes of administration, the timing and scope of regulatory approvals for these products, the availability and cost of manufacturing, marketing and sales capabilities, price, reimbursement coverage and patent position.
Competing products could present superior treatment alternatives, including by being more effective, safer, less expensive or marketed and sold more effectively than any products we may develop. Competitive products may make any products we develop obsolete or non-competitive before we recover the expense of developing and commercializing our product candidates. Such competitors could also recruit our employees, which could negatively impact our level of expertise and our ability to execute our business plan.
A variety of risks outside of our control associated with international operations could adversely affect our business.
If any of our product candidates are approved for commercialization, it is our current intention to market them on a worldwide basis, either alone or in collaboration with others. In addition, we conduct development activities in various jurisdictions throughout the world. We expect that we will be subject to additional risks related to engaging in international operations, including:
| • | different regulatory requirements for approval of biopharmaceutical products in foreign countries; |
| • | reduced protection for intellectual property rights; |
26
| • | unexpected changes in tariffs, trade barriers and regulatory requirements; |
| • | economic weakness, including inflation, or political instability in particular foreign economies and markets; |
| • | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
| • | foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country; |
| • | workforce uncertainty in countries where labor unrest is more common than in Australia or the United States; |
| • | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
| • | business interruptions resulting from geopolitical actions, including war and terrorism, or natural disasters including earthquakes, typhoons, floods and fires. |
The insurance coverage and reimbursement status of newly approved products is uncertain.
The availability of coverage and adequate reimbursement by governmental and private payers is essential for most patients to be able to afford expensive treatments. Sales of our product candidates will depend substantially, both in the United States and abroad, on the extent to which the costs of our product candidates will be paid by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or reimbursed by third-party payers. If reimbursement is not available, or is available only to limited levels, we may not be able to successfully commercialize our product candidates. Even if coverage is provided, the reimbursement amounts approved by third-party payers may not be high enough to allow us to establish or maintain pricing sufficient to realize a return on our investment.
There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products. In the United States, the principal decisions about reimbursement for new medicines are typically made by the Centers for Medicare & Medicaid Services, or CMS, an agency within the U.S. Department of Health and Human Services, as CMS decides whether and to what extent a new medicine will be covered and reimbursed under Medicare. Private payers tend to follow CMS to a substantial degree. It is difficult to predict what CMS will decide with respect to reimbursement for fundamentally novel products such as ours, as there is no body of established practices and precedents for these new products.
The intended use of a biopharmaceutical product by a physician can also affect pricing. For example, CMS could initiate a National Coverage Determination administrative procedure, by which the agency determines which uses of a therapeutic product would and would not be reimbursable under Medicare. This determination process can be lengthy, thereby creating a long period during which the future reimbursement for a particular product may be uncertain.
Outside the United States, international operations are generally subject to extensive governmental price controls and other market regulations, and we believe the increasing emphasis on cost-containment initiatives in Europe, Canada and other countries is likely to put pressure on the pricing and usage of any of our product candidates that may be approved for marketing in the future. In many countries, the prices of medical products are subject to varying price control mechanisms as part of national health systems. In general, the prices of medicines under such systems can be substantially lower than in the United States. Other countries allow companies to fix their own prices for medicines, but monitor and control company profits. In some countries, we or our collaborators may be required to conduct a clinical trial or other studies that compare the cost-effectiveness of our product candidates to other available therapies in order to obtain or maintain reimbursement or pricing approval. Additional foreign price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our product candidates. Accordingly, in markets outside the United States, the reimbursement for our products may be reduced compared with the United States and may be insufficient to generate commercially reasonable revenue and profits.
27
Moreover, increasing efforts by governmental and other third-party payers, in the United States and abroad, to cap or reduce healthcare costs, have resulted in legislation and reforms such as, in the United States, the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care Education Reconciliation Act, or the ACA. The ACA may cause such organizations to limit both coverage and level of reimbursement for new products approved and, as a result, they may not cover or provide adequate payment for our product candidates. In addition, other legislative changes have been adopted since the ACA was enacted. These changes included aggregate reductions to Medicare payments to providers of up to 2% per fiscal year effective April 2013 that, due to subsequent legislative amendments to the statute, will stay in effect through 2024 unless additional Congressional action is taken. Additionally, in January 2013, former President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding, which could negatively affect coverage or reduce the reimbursement for any of our product candidates that receive regulatory approval.
We expect to experience pricing pressures in connection with the sale of any of our product candidates due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription pharmaceutical products and surgical procedures and other treatments, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. We cannot predict what healthcare reform initiatives may be adopted in the future. Further federal, state and foreign legislative and regulatory developments are likely, and we expect ongoing initiatives to increase pressure on biopharmaceutical product pricing. Such reforms could depress pricing for any product candidates that we may successfully develop and for which we may obtain regulatory approval and may negatively affect our overall financial condition and ability to develop additional product candidates.
Our relationships with third-party payers, healthcare professionals and customers in the United States and elsewhere may be subject to anti-kickback, fraud and abuse, false claims, transparency, health information privacy and security and other healthcare laws and regulations, which could expose us to significant penalties.
Our relationships with third-party payers, healthcare professionals and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations, including, without limitation, the federal Anti-Kickback Statute and the federal False Claims Act, that may constrain the business or financial arrangements and relationships through which we sell, market and distribute any biopharmaceutical products for which we obtain marketing approval. In addition, we may be subject to transparency laws and patient privacy regulation by the federal government and by the U.S. states and foreign jurisdictions in which we conduct our business. The applicable federal, state and foreign healthcare laws and regulations that may affect our ability to operate include, but are not limited to, the following:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid; |
| • | federal civil and criminal false claims laws and civil monetary penalty laws, including the federal False Claims Act, which impose criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| • | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for, among other things, executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
28
| • | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, which impose obligations on covered healthcare providers, health plans and healthcare clearinghouses, as well as their business associates that create, receive, maintain or transmit individually identifiable health information for or on behalf of a covered entity, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| • | the federal Open Payments program, created under the ACA, and its implementing regulations, which requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children's Health Insurance Program, with specific exceptions, to report annually to the CMS information related to payments or other transfers of value made to physicians, and applicable group purchasing organizations to report annually to CMS ownership and investment interests held by the physicians and their immediate family members by the 90th day of each subsequent calendar year, and disclosure of such information will be made by CMS on a publicly available website; and |
| • | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payers, including private insurers; state and foreign laws that require biopharmaceutical or biotechnology companies to comply with the industry voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; state and foreign laws that require biopharmaceutical or biotechnology manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations may involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, including, without limitation, damages, fines, disgorgement, imprisonment, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations, which could have a material adverse effect on our business. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found not to be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from participation in government healthcare programs, which could also materially affect our business.
Negative public opinion and increased regulatory scrutiny of gene therapy and genetic research may damage public perception of our product candidates or compromise our ability to conduct our business or obtain regulatory approvals for our product candidates.
Gene therapy remains a novel technology and no gene therapy product utilizing ddRNAi has been approved to date in the United States. Public perception may be influenced by claims that gene therapy is unsafe, and gene therapy may not gain the acceptance of the public or the medical community. In particular, our success will depend upon physicians specializing in the treatment of those diseases that our product candidates target prescribing treatments that involve the use of our product candidates in lieu of, or in addition to, existing treatments they are already familiar with and for which greater clinical data may be available. More restrictive government regulations or negative public opinion would have a negative effect on our business or financial condition and may delay or impair the development and commercialization of our product candidates or demand for any products we may develop. Our product candidates, including our viral delivery systems, could produce adverse events. Adverse events in our clinical trials or following approval of any of our product candidates, even if not ultimately attributable to our product candidates, could result in increased governmental regulation, unfavorable public perception, potential regulatory delays in the testing or approval of our product candidates, stricter labeling requirements for those product candidates that are approved and a decrease in demand for any such product candidates.
29
Risks Related to Our Business Operations
We may not successfully engage in strategic transactions or enter into new collaborations, which could adversely affect our ability to develop and commercialize product candidates, impact our cash position, increase our expenses and present significant distractions to our management.
From time to time, we may consider additional strategic transactions, such as collaborations, acquisitions, asset purchases or sales and out- or in-licensing of product candidates or technologies. In particular, we will evaluate and, if strategically attractive, seek to enter into additional collaborations, including with major biotechnology or pharmaceutical companies. The competition for collaborators is significant, and the negotiation process is time-consuming and complex. Any new collaboration may be on terms that are not optimal for us, and we may not be able to maintain any new or existing collaboration if, for example, development or approval of a product candidate is delayed, sales of an approved product candidate do not meet expectations or the collaborator discontinues the collaboration. Any such collaboration, or other strategic transaction, may require us to incur non-recurring or other charges, increase our expenditures, pose significant integration or implementation challenges or disrupt our management or business.
These transactions would entail numerous operational and financial risks, including exposure to unknown liabilities, incurrence of substantial debt or dilutive issuances of equity securities to pay transaction consideration or costs, higher than expected collaboration, acquisition or integration costs, write-downs of assets or goodwill or impairment charges, increased amortization expenses, difficulty and cost in facilitating the collaboration or combining the operations and personnel of any acquired business, impairment of relationships with key suppliers, manufacturers or customers of any acquired business due to changes in management and ownership and the inability to retain key employees of any acquired business.
Accordingly, although there can be no assurance that we will undertake or successfully complete any additional transactions of the nature described above, any transactions that we do complete may be subject to the foregoing or other risks and have a material adverse effect on our business, results of operations, financial condition and prospects. Conversely, any failure to enter any collaboration or other strategic transaction that would be beneficial to us could delay and make more expensive the development and potential commercialization of our product candidates and have a negative impact on the competitiveness of any product candidate that reaches market.
Any inability to attract and retain qualified key management and technical personnel would impair our ability to implement our business plan.
Our success largely depends on the continued service of key management and other specialized personnel, including Dr. Jerel Banks (Chief Executive Officer) and Ms. Megan Boston (Executive Director and Head of Operations Australia). The loss of one or more members of our management team or other key employees or advisors, if not adequately replaced, could delay or increase the cost of our research and development programs and materially harm our business, financial condition, results of operations and prospects. The relationships that our key managers have cultivated within our industry make us particularly dependent upon their continued employment with us. We are dependent on the continued service of our technical personnel because of the highly technical nature of our product candidates and the specialized nature of the regulatory approval process for our product candidates. Because our management team and key employees are not obligated to provide us with continued service, they could terminate their employment with us at any time without penalty. We do not maintain key person life insurance policies on any of our management team members or key employees. Our future success will depend in large part on our continued ability to attract and retain other highly qualified scientific, technical and management personnel, as well as personnel with expertise in clinical testing, manufacturing, governmental regulation and commercialization. We face competition for personnel from other companies, universities, public and private research institutions, government entities and other organizations.
Our collaborations with outside scientists and consultants may be subject to restriction and change.
We work with medical experts, chemists, biologists and other scientists at academic and other institutions, and consultants who assist us in our research, development and regulatory efforts, including the members of our scientific advisory board. In addition, these scientists and consultants have provided, and we expect that they will continue to provide, valuable advice regarding our programs and regulatory approval processes. These scientists and consultants are not our employees and may have other commitments that would limit their future availability to us.
30
If a conflict of interest arises between their work for us and their work for another entity, we may lose their services. In addition, we are limited in our ability to prevent them from establishing competing businesses or developing competing products. For example, if a key scientist acting as a principal investigator in any of our future clinical trials identifies a potential product or compound that is more scientifically interesting to his or her professional interests, his or her availability to remain involved in any future clinical trials could be restricted or eliminated.
We may experience difficulties in managing our growth and expanding our operations.
We have limited experience in development and commercialization of pharmaceutical products. As our product candidates continue to advance through preclinical studies and any future clinical trials and potentially toward regulatory approval and commercial sale, we will need to expand our development, regulatory, manufacturing and sales capabilities or contract with other organizations to provide these capabilities for us. In the future, we expect to have to manage additional relationships with collaborators or partners, suppliers and other organizations. Our ability to manage our operations and future growth will require us to continue to improve our operational, financial and management controls, reporting systems and procedures. We may not be able to implement improvements to our management information and control systems in an efficient or timely manner and may discover deficiencies in existing systems and controls.
Our employees, independent contractors, principal investigators, CROs, consultants and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and insider trading.
We are exposed to the risk of fraud or other misconduct by our employees, independent contractors, principal investigators, CROs, consultants, commercial partners and vendors. Misconduct by these parties could include intentional failures to comply with the regulations of the FDA and comparable foreign regulators, provide accurate information to the FDA and comparable foreign regulators, comply with healthcare fraud and abuse laws and regulations in the United States and abroad, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Such misconduct could also involve the improper use of information obtained in the course of any future clinical trials, which could result in regulatory sanctions and cause serious harm to our reputation. We have adopted a code of conduct applicable to all of our employees, but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
We could face potential product liability and, if successful claims are brought against us, we may incur substantial liability and costs.
The use of our product candidates in clinical trials and the sale of any products for which we may in the future obtain marketing approval exposes us to the risk of product liability claims. Product liability claims might be brought against us by consumers, healthcare providers, pharmaceutical companies or others selling or otherwise coming into contact with our product candidates. There is a risk that our product candidates may induce adverse events. If we cannot successfully defend against product liability claims, we could incur substantial liability and costs. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| • | impairment of our business reputation; |
| • | withdrawal of clinical trial participants; |
| • | costs due to related litigation; |
| • | distraction of management's attention from our primary business; |
31
| • | substantial monetary awards to patients or other claimants; |
| • | the inability to commercialize our product candidates; |
| • | decreased demand for our product candidates, if approved for commercial sale; and |
| • | increased cost, or impairment of our ability, to obtain or maintain product liability insurance coverage. |
We carry combined public and products liability (including human clinical trials extension) insurance of A$20 million per occurrence with a A$20 million aggregate limit. We believe our product liability insurance coverage is sufficient in light of our current clinical programs. However, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. If we obtain marketing approval for any product candidates, we intend to expand our insurance coverage to include the sale of commercial products, but we may not be able to obtain or maintain product liability insurance on commercially reasonable terms or in adequate amounts. On occasion, large judgments have been awarded against other pharmaceutical companies in class action lawsuits based on pharmaceutical products, or medical treatments that had unanticipated adverse effects. A successful product liability claim or series of claims brought against us could cause the price of the ADSs and Warrants to decline and, if judgments exceed our insurance coverage, could materially and adversely affect our financial position.
During the course of treatment, patients may suffer adverse events, including death, for reasons that may be related to our product candidates. Such events could subject us to costly litigation, require us to pay substantial amounts of money to injured patients, delay, negatively impact or end our opportunity to receive or maintain regulatory approval to market our products, or require us to suspend or discontinue our commercialization efforts. Even in a circumstance in which we do not believe that an adverse event is related to our product candidate, the investigation into the circumstance may be time-consuming or inconclusive. These investigations may harm our reputation, delay our regulatory approval process, limit the type of regulatory approvals our product candidates receive or maintain, and compromise the market acceptance of any of our product candidates that may in the future receive regulatory approval. As a result of these factors, a product liability claim, even if successfully defended, could hurt our business and impair our ability to generate revenue.
We and our development partners, third-party manufacturers and suppliers use biological materials and may use hazardous materials, and any claims relating to improper handling, storage or disposal of these materials could be time consuming or costly.
We and our development partners, third-party manufacturers and suppliers may use hazardous materials, including chemicals and biological agents and compounds that could be dangerous to human health and safety or the environment. Our operations and the operations of our third-party manufacturers and suppliers also produce hazardous waste products. National, state and local laws and regulations in the United States, Australia and other countries govern the use, generation, manufacture, storage, handling and disposal of these materials and wastes. Compliance with applicable environmental laws and regulations may be expensive, and current or future environmental laws and regulations may impair our product development and commercialization efforts. In addition, we cannot entirely eliminate the risk of accidental injury or contamination from these materials or wastes. We do not carry specific biological or hazardous waste insurance coverage, and our property, casualty and general liability insurance policies specifically exclude coverage for damages and fines arising from biological or hazardous waste exposure or contamination. Accordingly, in the event of contamination or injury, we could be held liable for damages or be penalized with fines in an amount exceeding our resources, and any future clinical trials, regulatory approvals or product commercialization progress could be suspended.
We may use our limited financial and human resources to pursue a particular research program or product candidate and fail to capitalize on programs or product candidates that may be more profitable or for which there is a greater likelihood of success.
Because we have limited resources, we may forego or delay pursuit of opportunities with certain programs or product candidates or for indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs for product candidates may not yield any
32
commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through strategic collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate, or we may allocate internal resources to a product candidate in a therapeutic area in which it would have been more advantageous to enter into a collaboration arrangement.
Our internal computer and information technology systems, or those of our collaborators and other development partners, third-party CROs or other contractors or consultants, may fail or suffer security breaches, which could result in a disruption of our product development programs.
Despite the implementation of security measures, our internal computer and information technology systems and those of our current and any future CROs and other contractors, consultants and collaborators are vulnerable to damage from computer viruses, cyber-attacks, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Such events could cause interruptions of our operations. While we have not experienced any material system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a disruption of our development programs and our business operations, whether due to a loss of our trade secrets or other similar disruptions. For example, the loss of clinical trial data from ongoing or future clinical trials or data from preclinical studies could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we rely on third parties to manufacture our product candidates and will rely on third parties to conduct future clinical trials, and similar events relating to their computer systems could also have similar consequences to our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our product candidates could be delayed and become more expensive.
Our current laboratory operations are concentrated in one location and any events affecting this location may seriously compromise our ability to operate our business and continue the development of our product candidates.
Our current laboratory operations are located in our facility situated in Hayward, California. Any unplanned event, such as flood, fire, explosion, earthquake, extreme weather condition, medical epidemics, power shortage, telecommunication failure or other natural or manmade accidents or incidents that result in us being unable to fully utilize the facility, may compromise our ability to operate our business, particularly on a daily basis, cause us financial losses and inhibit or delay our continued development of our product candidates. Loss of access to this facility may result in increased costs, delays in the development of our product candidates or interruption of our business operations. As part of our risk management policy, we maintain insurance coverage at levels that we believe are appropriate for our business. However, in the event of an accident or incident at this facility, we cannot assure you that the amounts of insurance will be sufficient to satisfy any damages and losses. If our facility is unable to operate because of an accident or incident or for any other reason, even for a short period of time, any or all of our research and development programs may be harmed. Any business interruption may have a material adverse effect on our business, financial position, results of operations and prospects.
The investment of our cash and cash equivalents is subject to risks which may cause losses and affect the liquidity of these investments.
As of June 30, 2019, we had A$22.4 million in cash and cash equivalents. We historically have invested substantially all of our available cash and cash equivalents, including the net proceeds of our U.S. initial public offering, in cash deposits meeting the criteria of our investment policy, which is focused on the preservation of our capital. These investments are subject to general credit, liquidity, market and interest rate risks. We may realize losses in the fair value of these investments, which would have a negative effect on our financial results. In addition, should our investments cease paying or reduce the amount of interest paid to us, our interest income would suffer. The market risks associated with our investment portfolio may have an adverse effect on our results of operations, liquidity and financial condition.
33
Our ability to utilize our net operating losses and certain other tax attributes may be limited.
We have substantial carried forward tax losses which may not be available to offset any future assessable income. In order for an Australian corporate taxpayer to carry forward and utilize tax losses, the taxpayer must pass either the Continuity of Ownership test, or COT, or the Similar Business Test, or SBT, in respect of relevant tax losses. We have carried out an analysis as to whether we have met the SBT over the most recent period and confirm that the criteria have been met.
Risks Related to Our Intellectual Property
If we are unable to obtain or protect intellectual property rights related to our product candidates, we may not be able to obtain exclusivity for our product candidates or prevent others from developing similar competitive products.
We rely upon a combination of patents, know-how, trade secret protection and confidentiality agreements to protect the intellectual property related to our product candidates.
The patent application process, also known as patent prosecution, is expensive and time-consuming, and we and our current or future licensors and licensees may not be able to prepare, file, and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we or our current licensors or licensees, or any future licensors or licensees, will fail to identify patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection on them. Therefore, our patents and patent applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. It is possible that defects of form in the preparation or filing of our patents or patent applications may exist, or may arise in the future, for example with respect to proper priority claims, inventorship, claim scope, patent term adjustments, etc., although we are unaware of any such defects. If we or our current licensors or licensees, or any future licensors or licensees, fail to establish, maintain or protect such patents and other intellectual property rights, such rights may be reduced or eliminated. If our current licensors or licensees, or any future licensors or licensees, are not fully cooperative or disagree with us as to the prosecution, maintenance or enforcement of any patent rights, such patent rights could be compromised. If there are material defects in the form or preparation of our patents or patent applications, such patents or applications may be invalid and unenforceable. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business.
The strength of patents in the biotechnology and pharmaceutical field involves complex legal and scientific questions and can be uncertain. The patent applications that we own or in-license may fail to result in issued patents with claims that cover our product candidates in the United States or other jurisdictions. In addition, we cannot guarantee that any patents will issue from any pending or future patent applications owned by or licensed to us. There is no assurance that all of the potentially relevant prior art relating to our patents and patent applications has been found. If such prior art exists, it can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue and even if such patents cover our product candidates, third parties may initiate opposition, interference, re-examination, post-grant review, inter partes review, nullification or derivation action in court or before patent offices or similar proceedings challenging the validity, enforceability or scope of such patents, which may result in the patent claims being narrowed or invalidated. For example, three of our patents licensed from CSIRO that expire in 2019 have been subject to oppositions before the European Patent Office (EPO), and these oppositions have resulted in appealable decisions to maintain one of the European patents and to revoke two others. Each of those EPO decisions has been appealed. One of the appeals filed by CSIRO has been dismissed and the relevant European patent was revoked. The remaining two appeals are still underway and we cannot know whether these appeals, if carried through, will be decided favorably for us. Furthermore, even if our patents and patent applications are unchallenged, they may not adequately protect our intellectual property, provide exclusivity for our product candidates or prevent others from designing around our claims. Any of these outcomes could impair our ability to prevent competition from third parties.
34
If the patent applications we hold or have in-licensed with respect to our programs or product candidates fail to issue, or are revoked, if the breadth or strength of our patent protection is threatened, or if our patent portfolio fails to provide meaningful exclusivity for our product candidates, it could dissuade companies from collaborating with us to develop product candidates and threaten our ability to commercialize future products. Any successful opposition to any patents owned by or licensed to us could deprive us of rights necessary for the successful commercialization of any product candidates that we may develop. Further, if we encounter delays in regulatory approvals, the period of time during which we could market a product candidate under patent protection could be reduced. Since patent applications in the United States and most other countries are confidential for a period of time after filing, and some remain so until issued, we cannot be certain that we or our licensors were the first to file any patent application related to a product candidate. Furthermore, if third parties have filed such patent applications before March 16, 2013, an interference proceeding in the United States can be initiated by such third parties to determine who was the first to invent any of the subject matter covered by the patent claims of our applications. If third parties have filed such applications on or after March 16, 2013, a derivation proceeding in the United States can be initiated by such third parties to determine whether our invention was derived from theirs. Even where we have a valid and enforceable patent, we may not be able to exclude others from practicing our invention where the other party can show that they used the invention in commerce before our filing date or the other party benefits from a compulsory license. In addition, patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years after it is filed. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates are obtained, once the patent life has expired for a product, we may be open to competition from competitive medications, including biosimilar or generic medications. This risk is material in light of the length of the development process of our products and lifespan of our current patent portfolio.
In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes for which patents are difficult to enforce and any other elements of our product candidate discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. However, trade secrets can be difficult to protect. What constitutes a trade secret and what protections are available for trade secrets varies from state to state in the United States and country by country worldwide. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. Security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. Although we expect all of our employees and consultants to assign their inventions to us, and all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology to enter into confidentiality agreements, we cannot provide any assurances that all such agreements have been duly executed or that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Misappropriation or unauthorized disclosure of our trade secrets can be difficult to detect, could impair our competitive position and may have a material adverse effect on our business. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating the trade secret. In addition, others may independently discover our trade secrets and proprietary information. For example, the FDA, as part of its Transparency Initiative, is currently considering whether to make additional information publicly available on a routine basis, including information that we may consider to be trade secrets or other proprietary information, and it is not clear at the present time how the FDA's disclosure policies may change in the future, if at all.
Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. Further, the laws of some foreign countries such as India and China do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, we may encounter significant problems in protecting and defending our intellectual property both in the United States and abroad. If we are unable to prevent material disclosure of the non-patented intellectual property related to our technologies to third parties, and there is no guarantee that we will have any such enforceable trade secret protection, we may not be able to establish or maintain a competitive advantage in our markets.
35
We rely on license relationships with a number of third parties for portions of our intellectual property, including platform technology patents relating to our ddRNAi technology.
We have in-licensed certain ddRNAi-related and antisense RNA intellectual property from third-party licensors. We rely on some of these third parties to file and prosecute patent applications and maintain patents and otherwise protect the intellectual property we license. We have not had and do not have primary control over these activities for certain of our patents or patent applications and other intellectual property rights we license, and therefore cannot guarantee that these patents and applications will be prosecuted or enforced in a manner consistent with the best interests of our business. We cannot be certain that such activities by third parties have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents and other intellectual property rights. Additionally, we may not be able to control the publication or other disclosures of research carried out by our licensors relating to technology that could otherwise prove patentable. Pursuant to the terms of some of our license agreements with third parties, some of our third-party licensors have the right, but not the obligation, to control enforcement of our licensed patents or defense of any claims asserting the invalidity of these patents. Even if we are permitted to pursue such enforcement or defense, we will require the cooperation of our licensors, and cannot guarantee that we would receive it and on what terms. We cannot be certain that our licensors will allocate sufficient resources or prioritize their or our enforcement of such patents or defense of such claims to protect our interests in the licensed patents. If we cannot obtain patent protection, or enforce existing or future patents against third parties, our competitive position and our financial condition could suffer.
Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. There is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions and inter partes review proceedings before the USPTO, and corresponding foreign patent offices. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are pursuing development candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our product candidates may be subject to claims of infringement of the patent rights of third parties.
Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our product candidates. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that our product candidates may be accused of infringing. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. If any third-party patents were held by a court of competent jurisdiction to cover the manufacturing process of any of our product candidates, any DNA constructs formed during the manufacturing process or any final product itself, the holders of any such patents may be able to block our ability to commercialize such product candidate unless we obtained a license under the applicable patents, or until such patents expire. Similarly, if any third-party patents were held by a court of competent jurisdiction to cover aspects of our formulations, processes for manufacture or methods of use, the holders of any such patents may be able to block our ability to develop and commercialize the applicable product candidate unless we obtained a license or until such patent expires. In either case, such a license may not be available on commercially reasonable terms or at all.
Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of management and employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys' fees for willful infringement, pay royalties, redesign our infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure. Even if a license can be obtained on acceptable terms, the rights may be non-exclusive, which could give our competitors access to the same technology or intellectual property rights licensed to us. If we fail to obtain a required license, we may be unable to effectively market product candidates based on our technology, which could limit our ability to generate revenue or achieve profitability and possibly prevent us from generating revenue sufficient to sustain our operations.
36
We may not be successful in obtaining or maintaining necessary rights to gene therapy product components and processes for our development pipeline through acquisitions and in-licenses.
Presently we have rights to intellectual property to develop our current gene therapy product candidates. However, our product candidates may require specific formulations to work effectively and efficiently and rights to such formulations may be held by others. In addition, we may need additional intellectual property rights as we develop future therapy product candidates. We may be unable to acquire or in-license any compositions, methods of use, processes or other third-party intellectual property rights from third parties that we identify on terms that we find acceptable, or at all. The licensing and acquisition of third-party intellectual property rights is a competitive area, and a number of more established companies are also pursuing strategies to license or acquire third-party intellectual property rights that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities.
For example, we sometimes collaborate with U.S. and foreign academic institutions to accelerate our preclinical research or development under written agreements with these institutions. Typically, these institutions provide us with an option to negotiate a license to any of the institution's rights in technology resulting from the collaboration. Regardless of such right of first negotiation for intellectual property, we may be unable to negotiate a license within the specified time frame or under terms that are acceptable to us. If we are unable to do so, the institution may offer the intellectual property rights to other parties, potentially blocking our ability to pursue our program.
In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment. If we are unable to successfully obtain rights to required third-party intellectual property rights, our business, financial condition and prospects for growth could suffer.
We may enter into license agreements with third parties, and if we fail to comply with our obligations in such agreements under which we license intellectual property rights from third parties or otherwise experience disruptions to our business relationships with our licensors, we could lose license rights that are important to our business.
We may need to obtain licenses from third parties to advance our research or allow commercialization of our product candidates, and we have done so from time to time. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we may be required to expend significant time and resources to develop or license replacement technology. If we are unable to do so, we may be unable to develop or commercialize the affected product candidates.
In many cases, patent prosecution of our in-licensed technology is controlled solely by the licensor. If our licensors fail to obtain and maintain patent or other protection for the proprietary intellectual property we license from them, we could lose our rights to the intellectual property or our exclusivity with respect to those rights, and our competitors could market competing products using the intellectual property. In some cases, we control the prosecution of patents resulting from licensed technology. In the event we breach any of our obligations related to such prosecution, we may incur significant liability to our licensing partners. Licensing of intellectual property is of critical importance to our business and involves complex legal, business and scientific issues and is complicated by the rapid pace of scientific discovery in our industry. Disputes may arise regarding intellectual property subject to a licensing agreement, including:
| • | the scope of rights granted under the license agreement and other interpretation-related issues; |
| • | the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; |
| • | the sublicensing of patent and other rights under any collaboration relationships we might enter into in the future; |
| • | our diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
37
| • | the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and |
| • | the priority of invention of patented technology. |
If disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful, and issued patents covering our product candidates could be found invalid or unenforceable if challenged in court.
Competitors may infringe our patents or the patents of our licensors. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours or our licensors is not valid, is unenforceable or is not infringed, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
For example, in patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, for example, lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post grant review, and equivalent proceedings in foreign jurisdictions, such as opposition proceedings. Such proceedings could result in revocation, amendments to our patent claims or statements being made on the record such that our claims may no longer be construed to cover our product candidates. For example, three of our patents licensed from CSIRO have been subject to oppositions before the EPO, and these oppositions have resulted in appealable decisions to maintain one European patent and to revoke two others. Each of those EPO decisions has been appealed. One of the appeals filed by CSIRO has been dismissed and the relevant European patent was revoked. The remaining two appeals are still underway. Outcomes or statements on the record in one country could have a disadvantageous effect on prosecution or enforcement of a patent or patent application in another country. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that no invalidating prior art exists or that the patent examiner was aware of all material prior art during prosecution. Moreover, in some circumstances, we do not have the right to control the preparation, filing and prosecution of patent applications, or to maintain, enforce or defend the patents, covering technology that we license from third parties. Therefore, these patents and applications may not be prosecuted, enforced and defended in a manner consistent with the best interests of our business. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we could lose at least part, and perhaps all, of the patent protection on one or more of our products or certain aspects of our platform technology. Such a loss of patent protection could have a material adverse impact on our business. Patents and other intellectual property rights also will not protect our technology if competitors design around our protected technology without legally infringing our patents or other intellectual property rights. Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses and could distract our technical and management personnel from their normal responsibilities. In addition, enforcement of a favorable decision by a court can depend on cooperation of a governmental authority which may or may not be available in every jurisdiction. There could be public announcements of the results of hearings, motions or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could depress the market price of our ADSs and Warrants. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities.
38
For our patents and patent applications filed in the United States before March 16, 2013, interference proceedings provoked by third parties or brought by us may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Litigation or interference proceedings may result in a decision adverse to our interests and, even if successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could cause the trading price of our ADSs and Warrants to fall.
Our results of operations will be affected by the level of royalty payments that we are required to pay to third parties.
We are a party to license agreements that require us to remit royalty payments and other payments related to in-licensed intellectual property. Under our in-license agreements, we may pay up-front fees and milestone payments and be subject to future royalties. We cannot precisely predict the amount, if any, of royalties we will owe in the future, and if our calculations of royalty payments are incorrect, we may owe additional royalties, which could negatively affect our results of operations. As our product sales increase, we may, from time to time, disagree with our third-party collaborators as to the appropriate royalties owed, and the resolution of such disputes may be costly, may consume management's time, and may damage our relationship with our collaborators. Furthermore, we may enter into additional license agreements in the future, which may also include royalty, milestone and other payments.
The licenses we grant to our collaborators to use our ddRNAi technology are exclusive to the development of product candidates for certain conditions.
Some of the out-licenses we grant to our collaborators to use our ddRNAi technology are exclusive to the development of product candidates for certain conditions, so long as our collaborators comply with certain requirements. That means that once our ddRNAi technology is licensed to a collaborator for a specified condition, we are generally prohibited from developing product candidates for that condition and from licensing the ddRNAi to any third party for that condition. The limitations imposed by these exclusive licenses could prevent us from expanding our business and increasing our development of product candidates with new collaborators, both of which could adversely affect our business and results of operations.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Certain of our key employees and personnel are or were previously employed at universities, medical institutions or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants and independent contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any of our employee's former employer or other third parties. Litigation may be necessary to defend against these claims. Furthermore, universities or medical institutions who employ some of our key employees and personnel in parallel to their engagement by us may claim that intellectual property developed by such person is owned by the respective academic or medical institution under the respective institution intellectual property policy or applicable law. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs, be a distraction to management and other employees, and damage our relationships with the academic and medical institutions.
39
We may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property.
We may be subject to claims that former employees, collaborators or other third parties have an ownership interest in our patents or other intellectual property. We may in the future have ownership disputes arising, for example, from conflicting obligations of consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and applications are required to be paid to the USPTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and applications. The USPTO and various corresponding governmental patent agencies outside of the United States require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process and after a patent has issued. There are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other biotechnology companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biotechnology industry involve both technological and legal complexity, and it therefore is costly, time-consuming and inherently uncertain. In addition, on September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law, including provisions that affect the way patent applications will be prosecuted and may also affect patent litigation.
An important change introduced by the Leahy-Smith Act is that, as of March 16, 2013, the United States transitioned to a "first-to-file" system for deciding which party should be granted a patent when two or more patent applications are filed by different parties claiming the same invention. A third party that files a patent application in the USPTO after that date but before us could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by the third party. This will require us to be cognizant going forward of the time from invention to filing of a patent application. It is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
Among some of the other changes introduced by the Leahy-Smith Act are changes that limit where a patentee may file a patent infringement suit and providing opportunities for third parties to challenge any issued patent in the USPTO. This applies to all of our U.S. patents, even those issued before March 16, 2013. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in U.S. federal court necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action.
40
Recent U.S. Supreme Court rulings such as Association for Molecular Pathology v. Myriad Genetics, Inc. (Myriad I); BRCA1- &BRCA2-Based Hereditary Cancer Test Patent Litig. (Myriad II); and Promega Corp. v. Life Technologies Corp. have also narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in some situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
Our success depends, in part, on our ability to protect our intellectual property and our technologies outside the United States.
Our commercial success depends, in part, on our ability to obtain and maintain patent and trade secret protection for our technologies, our traits, and their uses, as well as our ability to operate without infringing upon the proprietary rights of others outside the United States. If we do not adequately protect our intellectual property, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability.
Filing, prosecuting and defending patents on product candidates in all countries around the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. In addition, we may at times in-license third-party technologies for which limited international patent protection exists and for which the time period for filing international patent applications has passed. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Potential competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our product candidates, if approved, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Risks Related to the ADSs and Warrants
On July 22, 2019 we received from The NASDAQ Capital Markets, or NASDAQ a deficiency notice following the decrease of our ADSs market price below $1.00. If we fail to address the deficiency notice within 180 calendar days from the receipt of the notice, the ADSs may be delisted.
NASDAQ listing rules require listed securities to maintain a minimum bid price of $1.00 per security. On July 22, 2019, as a result of our ADSs’ closing bid price being below $1.00 dollar for 30 consecutive business days, NASDAQ sent us a deficiency notice. Thus, we have 180 calendar days to regain compliance with the NASDAQ listing rules. This can be achieved either through a reverse stock split or by having our ADSs’ closing bid price above $1.00 for 10 consecutive business days. If we fail to regain compliance with the NASDAQ listing rules, our ADSs may be delisted from NASDAQ
41
The market price and trading volume of the ADSs and Warrants may be volatile and may be affected by economic conditions beyond our control.
The market price of the ADSs and Warrants may be highly volatile and subject to wide fluctuations. In addition, the trading volume of the ADSs and Warrants may fluctuate and cause significant price variations to occur. If the market price of the ADSs and Warrants declines significantly, you may be unable to resell your ADSs and Warrants at or above your purchase price, if at all. We cannot assure you that the market price of the ADSs or Warrants will not fluctuate or significantly decline in the future.
Some specific factors that could negatively affect the price of the ADSs and Warrants or result in fluctuations in their price and trading volume include:
| • | actual or expected fluctuations in our operating results; |
| • | changes in market valuations of similar companies; |
| • | changes in our key personnel; |
| • | changes in financial estimates or recommendations by securities analysts; |
| • | trading prices of our ordinary shares on the Australian Securities Exchange (ASX); |
| • | changes in trading volume of ADSs or Warrants on the NASDAQ, and of our ordinary shares on the ASX; |
| • | sales of the ADSs or Warrants or ordinary shares by us, our executive officers or our shareholders in the future; and |
| • | conditions in the financial markets or changes in general economic conditions. |
An active trading market for the ADSs or Warrants may not continue to develop or may not be liquid enough for you to sell your ADSs or Warrants quickly or at market price.
Prior to our U.S. initial public offering, our securities were not listed on any U.S. stock exchange, we were not a reporting company under the Exchange Act and there had been only a limited public market in the United States for the ADSs and no public market in the United States for the Warrants. Although the ADSs and Warrants are listed on The NASDAQ Capital Market, if an active public market in the United States for the ADSs and Warrants does not continue to develop, the market price and liquidity of the ADSs and Warrants may be adversely affected. The initial public offering price for the ADSs and Warrants was determined by negotiation between us and the underwriter. The price at which the ADSs are traded has declined below the initial public offering price and the price at which the Warrants are traded has not exceeded the Warrant exercise price. The ADS price and/or the Warrant price may continue to decline, which means you may experience a decrease in the value of your ADSs and Warrants regardless of our operating performance or prospects. In the past, following periods of volatility in the market price of a company's securities, shareholders often instituted securities class action litigation against that company. If we were involved in a class action suit, it could divert the attention of senior management and, if adversely determined, could cause us significant financial harm.
As a foreign private issuer, we are permitted and we expect to follow certain home country corporate governance practices in lieu of certain NASDAQ requirements applicable to domestic issuers.
As a foreign private issuer whose ADSs and Warrants are listed on NASDAQ, we will be permitted to follow certain home country corporate governance practices in lieu of certain NASDAQ requirements. For example, we may follow home country practice with regard to the composition of the board of directors and quorum requirements applicable to shareholder meetings. A foreign private issuer must disclose in its annual reports filed with the SEC the requirements with which it does not comply followed by a description of its applicable home country practice. The Australian home country practices described above may afford less protection to holders of the ADSs and Warrants than that provided under NASDAQ rules. See Item 16G “Corporate Governance.”
42
As a foreign private issuer, we are permitted to disclose less information with the SEC than a company incorporated in the United States.
As a foreign private issuer, we are exempt from certain rules under the Exchange Act, that impose disclosure requirements as well as procedural requirements for proxy solicitations under Section 14 of the Exchange Act. In addition, our officers, directors and principal shareholders are exempt from the reporting and "short-swing" profit recovery provisions of Section 16 of the Exchange Act. Moreover, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as listed domestic issuers, nor are we required to comply with the SEC's Regulation FD, which restricts the selective disclosure of material non-public information. Accordingly, there may be less information publicly available concerning us than there is for a domestic issuer.
If we lose our status as a "foreign private issuer", then we will be subject to additional reporting obligations and costs like a U.S. domestic issuer under U.S. securities laws.
As discussed above, as long as we are a foreign private issuer, we are exempt from certain rules under the Exchange Act. We would cease to be a foreign private issuer at such time as more than 50% of our outstanding voting securities are held by U.S. residents and any of the following three circumstances applies: (i) the majority of our executive officers or directors are U.S. citizens or residents; (ii) more than 50% of our assets are located in the United States or (iii) our business is administered principally in the United States. We are required to determine our status as a foreign private issuer on an annual basis at the end of our second fiscal quarter.
If we were to lose that status, then the exemptions under the Exchange Act that we currently enjoy would no longer be available and, as a result, our costs for complying with the requirements of the Exchange Act would increase.
If we fail to establish and maintain proper internal financial reporting controls, our ability to produce accurate financial statements or comply with applicable regulations could be impaired.
Section 404(a) of the Sarbanes-Oxley Act requires that our management assess and report annually on the effectiveness of our internal controls over financial reporting and identify any material weaknesses in our internal controls over financial reporting. Although Section 404(b) of the Sarbanes-Oxley Act requires our independent registered public accounting firm to issue an annual report that addresses the effectiveness of our internal controls over financial reporting, we rely on the exemption provided in the JOBS Act, and consequently will not be required to comply with SEC rules that implement Section 404(b) of the Sarbanes-Oxley Act until such time as we are no longer an emerging growth company and are an accelerated or large accelerated filer.
The presence of any material weaknesses in our internal control over financial reporting could result in financial statement errors which, in turn, could lead to errors in our financial reports and/or delays in our financial reporting, which could require us to restate our operating results. We might not identify one or more material weaknesses in our internal controls in connection with evaluating our compliance with Section 404(a) of the Sarbanes-Oxley Act. In order to maintain and improve the effectiveness of our disclosure controls and procedures and internal controls over financial reporting, we will need to expend significant resources and provide significant management oversight. Implementing any appropriate changes to our internal controls may require specific compliance training of our directors and employees, entail substantial costs in order to modify our existing accounting systems, take a significant period of time to complete and divert management's attention from other business concerns. These changes may not, however, be effective in maintaining the adequacy of our internal control.
If we are unable to conclude that we have effective internal controls over financial reporting, investors may lose confidence in our operating results, the price of the ADSs or Warrants could decline and we may be subject to litigation or regulatory enforcement actions. In addition, if we are unable to meet the requirements of Section 404 of the Sarbanes-Oxley Act, the ADSs and Warrants may not be able to remain listed on NASDAQ.
43
ADS holders and Warrants holders may be subject to additional risks related to holding ADSs and Warrants rather than ordinary shares.
ADS holders and Warrants holders do not hold ordinary shares directly and, as such, are subject to, among others, the following additional risks:
| • | As an ADS holder, we will not treat you as one of our shareholders and you will not be able to exercise shareholder rights, except through the ADR depositary as permitted by the deposit agreement. As a Warrant holder, you will have no rights as an ADS holder until you exercise your Warrant. |
| • | Distributions, if any, on the ordinary shares represented by your ADSs will be paid to the ADR depositary, and before the ADR depositary makes a distribution to you on behalf of your ADSs, any withholding taxes that must be paid will be deducted. Additionally, if the exchange rate fluctuates during a time when the ADR depositary cannot convert the foreign currency, you may lose some or all of the value of the distribution. |
| • | We and the ADR depositary may amend or terminate the deposit agreement without the ADS holders' consent in a manner that could prejudice ADS holders. We may also amend the ADS Warrant Agent Agreement without the consent of any Warrant holder for the purpose of curing any ambiguity, or curing, correcting or supplementing any defective provision contained in the ADS Warrant Agent Agreement or adding or changing any other provisions with respect to matters or questions arising under that agreement as we and the Warrant Agent deem shall not adversely affect the interest of the Warrant holders. We may also modify or amend the ADS Warrant Agent Agreement in other respects with the vote or written consent of the holders of at least 65% of the then outstanding Warrants. |
The Warrants are a risky investment. You may be unable to exercise your Warrants for a profit.
The value of the Warrants will depend on the value of our ADSs, which will depend on factors related and unrelated to the success of our commercialization and product development activities, and cannot be predicted at this time, as well as the factors described herein that may affect the value of our ordinary shares. The Warrants have an exercise period of 5 years.
If the price of our ADSs does not increase to an amount sufficiently above the exercise price of the Warrants during the exercise period of the Warrants, you may be unable to recover any of your investment in the Warrants. In addition, because we are an Australian corporation whose ordinary shares are listed on the ASX, the anti-dilution adjustments included in the Warrants are limited to those permitted by the rules of the ASX. As a result, the Warrants do not include any value-weighted average price or similar adjustment provision for issuances of ADSs at a price below the exercise price of the Warrants or the market price of our ADSs or ordinary shares. There can be no assurance that any of the factors that could impact the trading price of our ADSs will result in the trading price increasing to an amount that will exceed the exercise price or the price required for you to achieve a positive return on your investment in the Warrants.
You must act through the ADR depositary to exercise your voting rights and, as a result, you may be unable to exercise your voting rights on a timely basis. Holders of Warrants will have no rights as ADS holders until they acquire ADSs.
As a holder of ADSs (and not the ordinary shares underlying your ADSs), we will not treat you as one of our shareholders, and you will not be able to exercise shareholder rights. The ADR depositary will be the holder of the ordinary shares underlying your ADSs, and ADS holders will be able to exercise voting rights with respect to the ordinary shares represented by the ADSs only in accordance with the deposit agreement relating to the ADSs. There are practical limitations on the ability of ADS holders to exercise their voting rights due to the additional procedural steps involved in communicating with these holders. For example, holders of our ordinary shares will receive notice of shareholders' meetings by mail and will be able to exercise their voting rights by either attending the shareholders meeting in person or voting by proxy. ADS holders, by comparison, will not receive notice directly from us. Instead, in accordance with the deposit agreement, we will provide notice to the ADR depositary of any such shareholders meeting and details concerning the matters to be voted upon at least 30 days in advance of the meeting date. If we so instruct, the ADR depositary will mail to holders of ADSs the notice of the meeting and a statement as to the manner
44
in which voting instructions may be given by holders as soon as practicable after receiving notice from us of any such meeting. To exercise their voting rights, ADS holders must then instruct the ADR depositary as to voting the ordinary shares represented by their ADSs. Due to these procedural steps involving the ADR depositary, the process for exercising voting rights may take longer for ADS holders than for holders of ordinary shares. The ordinary shares represented by ADSs for which the ADR depositary fails to receive timely voting instructions will not be voted.
Until you acquire ADSs upon exercise of the Warrants, you will have no rights with respect to our ADSs or the ordinary shares underlying the ADSs, including the right they receive dividend payments, vote or respond to tender offers. Upon exercise of your Warrants, you will be entitled to exercise the rights of ADS holders as to matters for which the record date occurs after the exercise date.
Although we are required to use our reasonable best efforts to have an effective registration statement covering the issuance of the ADSs underlying the Warrants at the time that holders of our Warrants exercise their Warrants, we cannot guarantee that a registration statement will be effective, in which case holders of our Warrants may not be able to receive freely tradable ADSs upon exercise of the Warrants.
Holders of our Warrants will be able to exercise the Warrants and receive freely tradable shares only if (i) a current registration statement under the Securities Act relating to the ADSs underlying the Warrants is then effective, or an exemption from such registration is available, and (ii) such ADSs are qualified for sale or exempt from qualification under the applicable securities laws of the states in which the various holders of Warrants reside, as further described in the ADS Warrant Agent Agreement. Although we have undertaken in the ADS Warrant Agent Agreement, and therefore have a contractual obligation, to use our reasonable best efforts to maintain a current registration statement covering the ADSs underlying the Warrants to the extent required by federal securities laws, we may not be able to do so. If we are not able to do so, holders will not be able to exercise their Warrants and receive freely tradable ADSs but rather will have the exercise price for the Warrants returned to them. The value of the Warrants may be greatly reduced if a registration statement covering the ADSs issuable upon exercise of the Warrants is not kept current.
The Warrants may not have any value.
Benitec issued Warrants at the time of the US initial public offering which will expire on August 21, 2020, the 5-year anniversary of the closing of our U.S. initial public offering. In the event the price of the ADSs does not exceed the exercise price of the Warrants during the period in which the Warrants are exercisable, the Warrants may not have any value.
We believe that we were likely a "passive foreign investment company," or PFIC, for U.S. federal income tax purposes for our 2019 fiscal year.
Generally, if, for any taxable year, at least 75% of our gross income is passive income or at least 50% of the average quarterly value of our gross assets is attributable to assets that produce passive income or are held for the production of passive income, including cash, we would be characterized as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. For purposes of these tests, passive income includes dividends, interest, gains from the sale or exchange of investment property and rents and royalties other than rents and royalties which are received from unrelated parties in connection with the active conduct of a trade or business. If we directly or indirectly own at least 25% by value of the shares of another corporation, we are treated as if we hold our proportionate share of the assets of such corporation and receive directly our proportionate share of the income of such corporation.
We believe we were likely a PFIC in fiscal 2019. This arose due to the decline in the value of our shares and the fact that the applicable PFIC rules treat our working capital as a passive asset for purposes of the PFIC asset test. Accordingly, a U.S. holder of our ADSs may suffer adverse U.S. federal income tax consequences, including (i) the treatment of all or a portion of any gain on disposition as ordinary income, (ii) the application of a deferred interest charge on such gain and the receipt of certain dividends and (iii) compliance with certain reporting requirements.
45
For further discussion of the U.S. federal income tax considerations with respect to our classification as a PFIC, see “Item 10. Additional Information – E. Taxation – U.S. Taxation – Passive Foreign Investment Company.”
If a U.S. person is treated as owning at least 10% of our equity, such holder may be subject to adverse U.S. federal income tax consequences.
If a U.S. person is treated as owning (directly, indirectly or constructively) at least 10% of the value or voting power of our equity, such person may be treated as a “United States shareholder” with respect to each “controlled foreign corporation” in our group (if any). If our group includes one or more U.S. subsidiaries, certain of our non-U.S. subsidiaries could be treated as controlled foreign corporations (regardless of whether we are or are not treated as a controlled foreign corporation) with respect to such United States shareholders, such that these United States shareholders will be subject to the controlled foreign corporation rules. A United States shareholder of a controlled foreign corporation may be required to annually report and include in such shareholder's U.S. taxable income such shareholder's pro rata share of “Subpart F income,” “global intangible low-taxed income” and investments in U.S. property by controlled foreign corporations, whether or not we make any distributions. An individual that is a United States shareholder with respect to a controlled foreign corporation generally would not be allowed certain tax deductions or foreign tax credits that would be allowed to a United States shareholder that is a U.S. corporation. A failure to comply with the foregoing obligations may subject you to significant monetary penalties and may prevent the statute of limitations from starting with respect to your U.S. federal tax return for the year for which reporting was due. We cannot provide any assurances that we will assist investors in determining whether any of our non-U.S. subsidiaries are treated as a controlled foreign corporation or whether any investor is treated as a United States shareholder with respect to any of such controlled foreign corporations, or furnish to any United States shareholders information that may be necessary to comply with the aforementioned reporting and tax paying obligations. United States investors should consult their own tax advisors regarding the potential application of the foregoing rules to its investment in ordinary shares, ADSs and Warrants.
Currency fluctuations may adversely affect the price of our ordinary shares ADSs and Warrants.
Our ordinary shares are quoted in Australian dollars on the ASX and the ADSs and Warrants are quoted in U.S. dollars on NASDAQ. Movements in the Australian dollar/U.S. dollar exchange rate may adversely affect the U.S. dollar price of the ADSs and Warrants. In the past year the Australian dollar has generally weakened against the U.S. dollar. However, this trend may not continue and may be reversed. If the Australian dollar weakens against the U.S. dollar, the U.S. dollar price of the ADSs and Warrants could decline, even if the price of our ordinary shares in Australian dollars increases or remains unchanged.
We have never declared or paid dividends on our ordinary shares and we do not anticipate paying dividends in the foreseeable future.
We have never declared or paid cash dividends on our ordinary shares. For the foreseeable future, we currently intend to retain all available funds and any future earnings to support our operations and to finance the growth and development of our business. Any future determination to declare cash dividends will be made at the discretion of our board of directors, subject to compliance with applicable laws and covenants under current or future credit facilities, which may restrict or limit our ability to pay dividends, and will depend on our financial condition, operating results, capital requirements, general business conditions and other factors that our board of directors may deem relevant. We do not anticipate paying any cash dividends on our ordinary shares in the foreseeable future. As a result, a return on your investment in the ADS and Warrants will only occur if our ADS price and the Warrants price appreciate.
You may not receive distributions on our ordinary shares represented by the ADSs or any value for such distribution if it is illegal or impractical to make them available to holders of ADSs.
While we do not anticipate paying any dividends on our ordinary shares in the foreseeable future, if such a dividend is declared, the depositary for the ADSs has agreed to pay to you the cash dividends or other distributions it or the custodian receives on our ordinary shares or other deposited securities after deducting its fees and expenses. You will receive these distributions in proportion to the number of our ordinary shares your ADSs represent.
46
However, in accordance with the limitations set forth in the deposit agreement, it may be unlawful or impractical to make a distribution available to holders of ADSs. We have no obligation to take any other action to permit the distribution of the ADSs, ordinary shares, rights or anything else to holders of the ADSs. This means that you may not receive the distributions we make on our ordinary shares or any value from them if it is unlawful or impractical to make them available to you. These restrictions may have a material adverse effect on the value of your ADSs.
Australian takeover laws may discourage takeover offers being made for us or may discourage the acquisition of a significant position in our ordinary shares, ADSs and Warrants.
We are incorporated in Australia and are subject to the takeover laws of Australia. Among other things, we are subject to the Australian Corporations Act 2001, or the Corporations Act. Subject to a range of exceptions, the Corporations Act prohibits the acquisition of a direct or indirect interest in our issued voting shares if the acquisition of that interest will lead to a person's voting power in us increasing to more than 20% or increasing from a starting point that is above 20% and below 90%. Australian takeover laws may discourage takeover offers being made for us or may discourage the acquisition of a significant position in our ordinary shares. This may have the ancillary effect of entrenching our board of directors and may deprive or limit our shareholders', ADS holders' and Warrants holders’ opportunity to sell their ordinary shares, ADSs or Warrants and may further restrict the ability of our shareholders, ADS holders and Warrant holders to obtain a premium from such transactions. See Item 10.B “Additional Information – Memorandum and Articles of Association.”
Our Constitution and Australian laws and regulations applicable to us may adversely affect our ability to take actions that could be beneficial to our shareholders.
As an Australian company, we are subject to different corporate requirements than a corporation organized under the laws of the states of the United States. Our Constitution, as well as the Australian Corporations Act, set forth various rights and obligations that are unique to us as an Australian company. These requirements may operate differently than those of many U.S. companies. You should carefully review the summary of these matters set forth under Item 10.B “Additional Information – Memorandum and Articles of Association.”
Item 4. Information on Benitec
A. | History and Development of the Company. |
Benitec Biopharma Limited was incorporated under the laws of Australia in 1995 and has been listed on the Australian Securities Exchange, or ASX, since 1997. Since we were incorporated in Australia in 1995, we have devoted the majority of our resources to development efforts relating to ddRNAi. While we have established some licensing arrangements, we do not have any products approved for sale and have not generated any revenue from product sales. We have funded our operations primarily from private placements of ordinary shares, including A$31.5 million of gross proceeds raised from private placements in February 2014 and from our U.S. initial public offering in August 2015, the gross proceeds of which equaled US$13.8 million (A$18.84million). as well as A$8.1 million of aggregate gross proceeds raised from private placements to Nant Capital in October 2016 and March 2017, A$8.8 million from private placement in May 2018 as well as an entitlement offer in June 2018, and in September 2019 we have raised US$2.3 million (A$3.3 million) from securities purchase agreement with certain investors. We have also been awarded research and development grants from the Australian federal government, totaling A$3.6 million in fiscal 2016, A$10.5 million in fiscal 2017, A$4.0 million in fiscal 2019 and we expect to receive A$0.9 million in fiscal 2020. We have earned licensing revenue from licensing our ddRNAi technology to five biopharmaceutical companies, totaling A$0.4 million in fiscal 2018 and A$16.2 million in fiscal 2019.
In October 2012, we acquired Tacere Therapeutics, Inc., or Tacere, an RNA interference therapeutics company based in California with a development program focused on hepatitis C. As consideration for the acquisition, we issued a total of 4,092,854 ordinary shares (taking into account a 25:1 share consolidation that became effective in July 2013), representing 9.8% of our issued capital immediately after the transaction, having an aggregate value of A$1.5 million.
In November 2014, we entered into a collaboration research and license agreement with 4DMT to develop a delivery vector for a ddRNAi-based therapy that we are developing for the treatment of wet AMD, which is designated BB-201. The company has elected to terminate this program.
47
In August 2015, we completed our U.S. initial public offering in which we issued 30,000,000 ordinary shares (represented by 1,500,000 ADSs) and 575,000 Warrants. The ADSs and Warrants are listed on The NASDAQ Capital Market.
In October 2016, we entered into a strategic engagement with Nant Capital. The strategic engagement included a scientific collaboration in clinical programs and an immediate private placement to Nant Capital, LLC of 29,305,819 ordinary shares in Benitec, representing 19.9% of our outstanding capital (for a post-issuance holding of 16.7%). The shares were priced at A$0.0895 per share, representing the 7-day volume weighted average price of the ordinary shares on the ASX prior to the execution of a share purchase subscription agreement. We received A$2.62 million in gross proceeds from Nant Capital in connection with the October 2016 issuance.
In March 2017, an additional 29,305,819 fully paid ordinary shares were issued to Nant Capital at A$0.1859 per share. We received A$5.45 million in gross proceeds from Nant Capital in connection with the March 2017 issuance.
In May 2018, we issued 15,444,020 fully paid ordinary shares representing 772,201 American Depositary Shares (ADS) at a market price of A$0.17 per share to Highbridge Capital Management LLC ("Highbridge"). We received A$2,625,483 in gross proceeds. On June 4, 2018, we issued 36,442,672 in a 1-for-2 entitlement offer, with Nant Capital subscribing 29,305,819 ordinary shares. We received A$6,195,254 in gross proceeds. As a result of the institutional placement and the entitlement offer, Nant Capital increased its holding to 34.21% of Benitec's issued capital. Currently Nant Capital holds 28.08% of our ordinary shares.
In fiscal 2018, we underwent a restructuring of our management team. In January 2018, Dr. Cliff Hollway resigned as Chief Business and Operations Officer. In June 2018, Mr. Greg West resigned as Chief Executive Officer and Company Secretary. Mr. West was replaced by Dr. Jerel Banks as Chief Executive Officer and by Mr. Oliver Kidd, as Company Secretary. In June 2018, Ms. Megan Boston was appointed to the role of Executive Director as Head of Operations Australia. In June 2018, Dr. David Suhy resigned as Chief Scientific Officer. This restructuring is part of our strategy to redefine the core proprietary programs on which we will focus our efforts.
In fiscal 2019, on July 9, 2018 Benitec announced that it had licensed to Axovant Sciences the exclusive global rights for BB-301 intended for the treatment of OPMD and had also entered into a fully funded research collaboration for the development of five additional gene therapy products in neurological disorders.
On June 6, 2019 the License and Collaboration Agreement with Axovant Sciences was terminated, effective as at September 3, 2019. The Benitec team endeavour to conduct several additional exploratory analyses of BB-301 prior to the initiation of the clinical study in order to potentially improve the biological efficacy of the compound via further optimization of the proprietary delivery method employed to dose the target tissues.
On July 22, 2019, as a result of our ADSs’ closing bid price being below $1.00 dollar for 30 consecutive business days, NASDAQ sent us a deficiency notice. Thus, we have 180 calendar days to regain compliance with The NASDAQ listing rules.
On September 16, 2019, we announced our plans to complete three non-clinical studies regarding BB-301 that will facilitate the filing of an Investigational New Drug application ("IND") and the formal initiation of a Phase I clinical trial in patients suffering from Oculopharyngeal Muscular Dystrophy (OPMD).
On September 30, 2019, we announced that we had entered into a securities purchase agreement with certain institutional, sophisticated and professional investors to issue 2,800,000 ADSs, each ADS representing 20 ordinary shares, at a purchase price per ADS of US$0.70, and warrants to purchase 412,863 ADSs in a registered direct offering, for total gross proceeds of approximately US$2.25 million.
Our headquarters are located at Level 14, 114 William Street, Melbourne, Victoria, 3000 Australia.
Our telephone number is +61 3 8692 7222. Our website address is www.benitec.com. Our agent for service of process in the United States is Tacere Therapeutics, Inc., 3940 Trust Way, Hayward, CA 94545.
48
Overview
Benitec Biopharma is a clinical-stage biotechnology company focused on the development of novel genetic medicines. The proprietary platform, called DNA-directed RNA interference, or ddRNAi, combines RNA interference, or RNAi, with gene therapy to create medicines that facilitate sustained silencing of disease-causing genes following a single administration. Benitec endeavours to develop and commercialise BB-301 for the treatment of Oculopharyngeal Muscular Dystrophy, or OPMD.
The ddRNAi-based genetic medicine currently under development by Benitec (BB-301) represents a proprietary product candidate that can, potentially, be used to meaningfully improve upon the existing standard of care for a rare, chronic, life-threatening form of muscular dystrophy. In the past, the research and development efforts of the Company have been directed towards disorders that include head and neck squamous cell carcinoma, or HNSCC, OPMD, wet age-related macular degeneration, or AMD, and chronic hepatitis B or HBV. Through the combination of the targeted gene silencing effect of RNAi together with the durable gene expression associated with the use of modified viral vectors, ddRNAi has the potential to produce durable silencing of disease-causing genes following a single administration of the proprietary genetic medicine. This novel attribute of the investigational agent that is being advanced through nonclinical development could facilitate the achievement of robust clinical activity while greatly reducing the dosing frequencies traditionally expected for medicines employed for the management of chronic diseases. Additionally, the establishment of chronic gene silencing via ddRNAi-based genetic medicines could significantly reduce the risk of patient non-compliance during the course of medical management of potentially fatal clinical disorders.
We may require additional financing to progress our product candidates through to key inflection points.
Our objective is to become the leader in discovering, developing, clinically validating and commercializing ddRNAi-based therapeutics for a range of human diseases with high unmet clinical need or large patient populations and, as a result, provide a better life for patients with these diseases.
Many human diseases are known to be caused by the inappropriate expression of a gene or multiple genes. It has been observed that RNAi is a potential mechanism to specifically turn off, or silence, genes whose sequences are known. Thus, RNAi can potentially be used to treat or cure diseases with a genetic basis by targeting a specific region of the molecular sequence of the disease-causing gene. RNAi is potentially applicable to over 20,000 human genes and a large number of disease-causing microorganism-specific genes. The mechanism of action of RNAi involves the introduction of pre-synthesized complexes of duplex short interfering RNA, or siRNA, into a cell. The siRNA's sequence is constructed to match a short region of the target gene. The siRNA is processed by the cell's own enzymes to destroy the target gene's messenger RNA, or mRNA, thus preventing the disease-causing gene from being expressed. This occurs as long as the siRNA remains prevalent in the cell.
In this standard RNAi approach, double-stranded siRNA is produced synthetically and subsequently introduced into the target cell either by chemical modification of the RNA or by a range of other delivery methods. While clinical efficacy has been demonstrated for a number of indications utilizing this approach, it has a number of limitations, including:
| • | treatment requires repeat administration for multiple cycles in order to maintain its efficacy; |
| • | patient adherence challenges due to dosing frequency and treatment duration; |
| • | therapeutic concentrations of siRNA are not stably maintained because the levels of synthetic siRNA in the cells decrease over time; |
| • | novel chemical modifications or novel delivery materials are typically required to introduce the siRNA into the target cells, making it complicated to develop therapeutics; |
49
| • | can have an adverse immune response, or interferon response, potentially resulting in serious adverse effects; |
| • | requirement for specialized delivery formulations for those diseases caused by multiple disease-causing genes; and |
| • | siRNA only acts to silence genes but cannot be used to replace defective genes with normally functioning genes. |
This siRNA approach is being developed by several other companies, including Alnylam Pharmaceuticals, Inc. or Alnylam, Arbutus Biopharma Corporation, or Arbutus (formerly known as Tekmira Pharmaceuticals Corporation before its name change and integration with OnCore BioPharma), and Dicerna Pharmaceuticals, Inc., or Dicerna.
Our ddRNAi technology differs from the standard siRNA approach and is designed to utilize the specificity and gene silencing effect of RNA interference while overcoming many of the limitations associated with the ongoing administration of siRNA. Our ddRNAi approach combines the specificity of gene silencing using RNAi with the delivery capability of gene therapy vectors. Unlike siRNA, our ddRNAi technology starts with a DNA construct. Gene therapy vectors, which are carrier molecules, often viruses, that deliver genetic material into the cell, are used to deliver the DNA construct to the nucleus of the targeted cells. The DNA construct then generates double-stranded short hairpin RNAs, or shRNAs, which are processed by the cell resulting in the conversion of the shRNA into siRNAs, which in turn silence the disease-associated genes. Advantages of our ddRNAi approach include:
| • | when paired with gene therapy delivery vectors, ddRNAi is designed to produce sustained, long-lasting silencing of the disease-causing gene, following a single administration, leading to the potential for "one shot" cures for a wide range of diseases, which could eliminate the requirement for patient compliance to take regular doses of medicine for long-term management of their disease; |
| • | ddRNAi technology can potentially use a range of clinically validated gene therapy vectors, enabling it to target a wide range of tissues, including, but not limited to, the liver; |
| • | because ddRNAi uses the cell's own transcriptional mechanisms to produce shRNA, a constant level of shRNA can potentially be produced so that intracellular levels of siRNA do not fall below threshold levels required for disease suppression; |
| • | modulation of transcriptional activity in the cells can potentially be fine-tuned to achieve optimal concentrations of shRNA; |
| • | off-tissue effects may be minimized by the use of tissue specific promoters; |
| • | the DNA constructs are shielded in gene therapy vectors that could potentially be designed to avoid activating the interferon response; |
| • | ddRNAi provides the option to both silence the defective gene and replace the defective gene with a normal version, using the same gene therapy vector, thus the silencing and replacement of the mutant gene occurs in the same cell, which we believe is ideally suited to developing therapeutics for a number of genetic disorders; |
| • | ddRNAi constructs can be designed to express multiple siRNAs in the same cell, targeting either a single gene at several different sites to maximize gene silencing or multiple genes in distinct cellular pathways, potentially enabling treatment of complex genetic diseases such as cancer, diabetes and heart disease; and |
| • | ddRNAi constructs can potentially be designed to simultaneously express siRNAs as well as other genetic sequences, such as those that encode for proteins. |
Our strategy is to discover, develop and commercialize treatments that leverage the capabilities of ddRNAi. We intend to do so by progressing our pipeline of ddRNAi-based therapeutics designed to treat and cure a number of human diseases, thereby demonstrating the broad clinical application of ddRNAi.
50
For selected product candidates, at the appropriate stage, we may collaborate with large pharmaceutical companies to further co-develop and, if approved, commercialize our ddRNAi products to achieve broad product distribution. For certain disease indications we deem to be outside of our immediate focus, we will continue to out-license, where appropriate, applications of our ddRNAi technology for the development of a range of therapeutics, which we believe could provide further validation of our technology's potential to address numerous diseases.
Our cash and cash equivalents will advance our product candidates for OPMD and we will seek a partnership to support the progression of hepatitis B into the clinic. We expect that our cash expenditures to particular product candidates would be reduced to the extent we enter into collaborative partnerships.
Head and Neck Squamous Cell Carcinoma – HNSCC
BB-401 is a DNA plasmid that expresses an antisense RNA molecule targeting the EGFR mRNA, thus, preventing its translation into its cognate protein via post-transcriptional gene silencing. Benitec acquired the rights to BB-401 from Nant Capital in 2016, and BB-401 has undergone clinical evaluation in a Phase 2 study in patients with advanced HNSCC. EGFR is the cell-surface receptor for members of the epidermal growth factor family, or EGF family, of extracellular protein ligands.
Key updates include:
| • | In December 2018, the Company completed the investigation of the single agent activity of BB-401 in a Phase 2 clinical trial which was designed as an open label study to explore the safety, tolerability and efficacy of BB-401 following intratumoral injections. The Phase 2 study patients were refractory to all standard therapies such as surgery, chemotherapy and immunotherapy. The study was conducted at clinical trial sites in Australia and Russia. |
| • | On December 21, 2018 Benitec announced the interim clinical trial results for the Phase 2 study involving the assessment of the single agent activity of BB-401. |
| • | An interim analysis was conducted to evaluate the objective response rate observed for the initial 12-patient cohort treated in Stage 1 of the Phase 2 study. |
| • | Benitec’s scientific and clinical teams will continue to follow patients that were treated in the first cohort of this Phase 2 study. |
| • | However, based on the initial analysis, the objective response rate required to support continued patient enrollment into the Phase 2 study was not achieved. |
| • | There are several critical points to note regarding the underlying nature of BB-401 as it relates to the other distinct investigational agent in the Benitec pipeline: |
| • | At the molecular level, the investigational agent that is currently under development by Benitec (BB-301) is fundamentally different from BB-401. BB-301 employs ddRNAi which facilitates gene silencing via the production of short hairpin RNA-based molecules whereas BB-401 represents a modified antisense oligonucleotide. |
| • | BB-301 functions by a mechanism of action that is completely distinct from that of BB-401, as BB-401 achieves gene-silencing via a mechanism described as post-transcriptional interference. BB-301 ultimately achieves gene-silencing via RNA interference driven by activation of the RNA-Induced Silencing Complex. |
| • | BB-301 employs a tissue-specific delivery vector (AAV9) whereas BB-401 has no delivery vector and was delivered intratumorally as a “naked” plasmid. |
The Company has terminated the clinical development of BB-401 along with the discovery stage programs directed at the engineering of follow-on anti-EGFR strategies (BB-501).
51
Oculopharyngeal Muscular Dystrophy – OPMD
OPMD is an insidious, autosomal-dominant, late-onset degenerative muscle disorder that typically presents in patients at 40-to-50 years of age. The disease is characterized by progressive swallowing difficulties (dysphagia) and eyelid drooping (ptosis). OPMD is caused by a specific mutation in the poly(A)-binding protein nuclear 1, or PABPN1, gene. OPMD is a rare disease and has been reported in at least 33 countries. Patients suffering with OPMD are well identified and are geographically clustered, which we believe should simplify clinical development and global commercialisation efforts.
BB-301 is a monotherapy delivered using an innovative AAV single vector system with the capability to both ‘silence and replace’ disease causing genes. In addition to using RNA interference to ‘silence’ the mutant PABPN1 gene expression that causes the OPMD, BB-301 simultaneously introduces a normal copy of the same gene, thus, providing the potential to restore normal function to the treated tissues and, in the process, improve treatment outcomes. This single gene therapy product, versus an equivalent system with two or more vectors, vastly simplifies the manufacturing and regulatory processes and reduces the complexity of the clinical strategy for BB-301.
Key updates include:
| • | On July 9, 2018 Benitec announced that it had licensed to Axovant Sciences the exclusive global rights for BB-301 intended for the treatment of OPMD, and had also entered into a fully funded research collaboration for the development of five additional gene therapy products in neurological disorders. |
| • | Under the terms of the agreement, Benitec received an upfront cash payment of US$10m (AUD$14.2m) and was slated to receive additional cash payments totalling US$17.5m (AUD$23.6m) upon completion of four specific near-term manufacturing, regulatory and clinical milestones. |
Axovant was granted worldwide rights to BB-301 and was slated to assume all future development costs. The total potential value of all of the development, regulatory and commercial milestones achievable by Benitec, of which there were eight milestones including the four near-term milestones, was US$187.5m (AUD$253.3m). As previously reported, there could be no assurance as to the total amount of payments that the Company would actually receive or when they would be received.
| • | Upon commercialisation, Benitec was slated to retain 30% of the net profits on worldwide sales of BB-301. |
| • | On June 6, 2019 the notice of termination of the License and Collaboration Agreement with Axovant Sciences was announced. The 90 day termination period will end on September 3, 2019. The Benitec team will endeavour to conduct several additional exploratory analyses of BB-301 prior to the initiation of the clinical study in order to potentially improve the biological efficacy of the compound via further optimization of the proprietary delivery method employed to dose the target tissues. |
| • | Preclinical data derived from recently concluded in vivo evaluations of BB-301 in two distinct large animal species suggests that the opportunity exists to further improve the biological efficacy of the compound via additional optimization of the proprietary delivery method employed to dose key target tissues that underlie the morbidity and mortality associated with the progression of OPMD. The initial biological efficacy profile observed for BB-301 following in vivo testing in the murine model of OPMD, including full correction of the disease phenotype, remains unchanged. However, the Benitec team plans to conduct several additional exploratory analyses prior to the initiation of BB-301 clinical testing. |
| • | Completion of the experimental work noted above will delay the initiation of the BB-301 clinical study beyond the timelines that were initially outlined by Axovant Sciences following the execution of the License and Collaboration Agreement between Benitec Biopharma and Axovant Sciences. As such, the License and Collaboration Agreement between Benitec Biopharma and Axovant Sciences was terminated, and all rights and licenses granted to Axovant Sciences will cease, including the rights to BB-301, which was in preclinical development for the treatment of OPMD, and all other early stage research collaboration programs. |
52
| • | The termination of the License and Collaboration Agreement was received on June 6 2019 and is effective 90 days post this date, September 3, 2019. |
| • | Benitec will retain all financial payments that were received during the term of the Axovant License and Collaboration Agreement prior to the termination of the Agreement. |
| • | On July 31, 2019 Benitec announced the completion of a workforce reduction of approximately 50%. Through this streamlining of operations, the Company retained staff members who are key to the achievement of the core research and development goals. The current team will continue to work diligently on Benitec’s primary asset, BB-301. The rationalization of resources will result in an extended financial runway for the Company. Benitec will continue development of BB-301. |
Wet Age-related Macular Degeneration – AMD
The Company was exploring the development of a ddRNAi-based therapy for the treatment of wet AMD, which is designated BB-201. The delivery vector for BB-201 was comprised of a novel AAV capsid that was developed in collaboration with 4DMT and was designed to deliver ddRNAi constructs to the retina using a direct intravitreal injection.
The key updates achieved include:
| • | The Company completed the molecular analyses of the retinal tissues from an in vivo proof of concept study in a non-human primate. These data indicated that additional optimization work on the BB-201 AMD program was required to progress the program forward. |
| • | The Company has elected to terminate this program. |
Hepatitis B
The Company is developing BB-103 for the treatment of HBV. Results of in vivo and in vitro studies, from December 2016, March 2016 and December 2015, demonstrated the potential utility of an approach that combines RNAi with gene therapy to treat HBV. In April 2017, the Company completed a pre-IND submission with the FDA in which the feedback provided by the agency included details regarding steps required to initiate a clinical trial for BB-103. The Company is seeking partnerships to support the progression of BB-103 into the clinic. Benitec will continue to seek a partner for HBV.
Collaboration Programs with Axovant
In July 2018, we entered into a collaboration agreement with Axovant to collaborate on five research plans regarding investigational gene therapy products for neurological disorders, however this was terminated on September 3, 2019 after receiving the notice of termination on June 6, 2019.
The termination of the License and Collaboration Agreement with Axovant Sciences was announced as the Benitec team endeavored to conduct several additional exploratory analyses of BB-301 prior to the initiation of a clinical study in order to potentially improve the biological efficacy of the compound via further optimization of the proprietary delivery method employed to dose the target tissues.
Preclinical data derived from recently concluded in vivo evaluations of BB-301 in two distinct large animal species suggest that the opportunity exists to further improve the biological efficacy of the compound via additional optimization of the proprietary delivery method employed to dose key target tissues that underlie the morbidity and mortality associated with the progression of OPMD. The initial biological efficacy profile observed for BB-301 following in vivo testing in the A17 mouse model of OPMD, including full correction of the disease phenotype, remains unchanged. However, the Benitec team plans to conduct several additional exploratory analyses prior to the initiation of clinical testing.
53
Completion of the experimental work noted above will delay the initiation of the BB-301 clinical study beyond the timelines that were initially outlined by Axovant Sciences following the execution of the License and Collaboration Agreement between Benitec Biopharma and Axovant Sciences. As such, the License and Collaboration Agreement between Benitec Biopharma and Axovant Sciences was terminated, and all rights and licenses granted to Axovant Sciences will cease, including the rights to BB-301, which was in preclinical development for the treatment of OPMD, and all other early stage research collaboration programs.
The termination of the License and Collaboration Agreement is effective as of September 3, 2019.
Our Strengths
We believe that the combination of our proprietary ddRNAi technology and our deep expertise and know-how in designing and clinical development of gene therapy, and specifically ddRNAi-based therapeutics, will enable us to achieve and maintain a leading position in gene silencing for treatment of human disease. Our key strengths include:
| • | a first mover advantage for ddRNAi-based therapeutics; |
| • | exclusive rights to a novel, proprietary ddRNAi technology platform that is potentially the basis of single-administration therapies with sustained, long-term silencing of disease-causing genes; |
| • | a pipeline of programs focused on life threatening or chronic diseases with either large patient populations, including hepatitis, or rare disease status potentially supporting an orphan drug classification, including OPMD; |
| • | our development team has significant experience in designing and developing ddRNAi therapeutics; |
| • | rights to intellectual property that includes a patent portfolio protecting our ddRNAi technology platform in numerous jurisdictions through 2019, and a growing portfolio of patents protecting improvements to our ddRNAi technology and product candidates through at least 2034, with additional patent life anticipated through at least 2036. |
Our Strategy
Benitec endeavours to become the leader in discovering, developing, and commercializing ddRNAi-based therapeutics for a range of human diseases with high unmet clinical need. The following strategy has been put in place to drive the company towards these goals:
| • | Selectively develop proprietary and partnered programs |
| • | Continue to explore and secure research and development partnerships with global biopharmaceutical companies supported by the differentiated nature of our scientific platform and intellectual property portfolio |
Our senior leadership team will continue to explore partnership opportunities with global pharmaceutical companies, as we expect the unique attributes of the proprietary ddRNAi approach and the breadth of potential clinical applications to support the formation of collaborations over a broad range of disorders with significant unmet medical need. Our development team has significant experience in designing and developing ddRNAi therapeutics.
We actively protect our intellectual property and proprietary technology that are important to our business, which includes seeking and maintaining patents claiming our ddRNAi technology, and other inventions relating to our specific products in development, or otherwise commercially and/or strategically important to the development of our business. We have rights to intellectual property that includes a patent portfolio protecting our ddRNAi technology platform in numerous jurisdictions through 2019, and a growing portfolio of patents protecting improvements to our ddRNAi technology and product candidates through at least 2034, with additional patent life anticipated through at least 2036.
54
Our Technology—ddRNAi
Our proprietary technology platform is called DNA-directed RNA interference, or ddRNAi, which is designed to produce long-term silencing of disease-causing genes, by combining RNA interference, or RNAi, with delivery agents typically associated with gene therapy.
Standard gene therapy is normally used to compensate for abnormal or malfunctioning genes or to make a beneficial protein to address a defect. If a mutated gene causes a necessary protein to be faulty or missing, gene therapy is used to introduce a wild type, or normal, copy of the gene to restore the function of the protein. This is the approach to gene therapy taken by a number of other companies to date.
With our ddRNAi approach, gene therapy vectors are used to deliver a DNA construct that produces shRNAs, which are processed by the cell into siRNAs, which then silence the disease-associated genes.
Overview of RNAi and siRNA approach
Many diseases are known to be caused by the inappropriate expression of a gene or multiple genes. These disease-associated genes can be turned off, or silenced, by the use of RNAi, resulting in a treatment or cure of the disease. Thus, RNAi provides the ability to develop therapeutics against diseases caused by inappropriate gene expression, by targeting a specific region of the molecular sequence of the disease-causing gene. RNAi is potentially applicable to over 20,000 human genes and a large number of disease-causing microorganism-specific genes.
The mechanism of action of RNAi involves the introduction of siRNA into a cell. The siRNA's sequence is constructed to match a short region of the target gene. The siRNA is processed by the cell's own enzymes to destroy the target gene's mRNA, thus preventing the disease-causing gene from being expressed. This occurs as long as the siRNA remains prevalent in the cell. In the standard RNAi approach, siRNA is produced synthetically in the laboratory and introduced into the target cell either by chemical modification of the RNA or by a range of delivery materials. A number of other companies, including Alnylam, Arbutus, and Dicerna, utilize this approach in their RNAi product candidates.
Figure 1. The siRNA approach
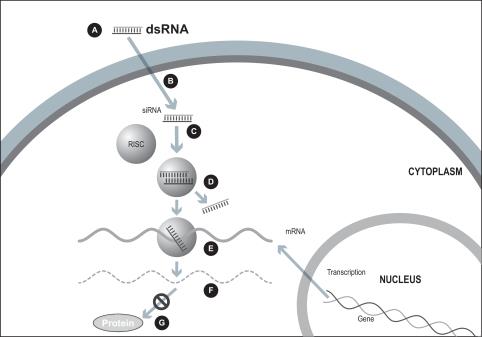
55
A small double stranded RNA, or dsRNA, molecule (A), comprising one strand known as the sense strand and another strand known as the antisense strand, which are complementary to each other, is synthesized in the laboratory. These small dsRNAs are called small interfering RNAs, or siRNAs. The sequence of the sense strand corresponds to a short region of the target gene mRNA. The siRNA is delivered to the target cell (B), where a group of enzymes, referred to as the RNA-Induced Silencing Complex, or RISC, process the siRNA (C), where one of the strands (usually the sense strand) is released (D). RISC uses the antisense strand to find the mRNA that has a complementary sequence (E) leading to the cleavage of the target mRNA (F). As a consequence, the output of the mRNA (protein production) does not occur (G).
Our Approach to Gene Silencing—ddRNAi
Our ddRNAi technology is designed to utilize the specificity and gene silencing effect of RNAi while overcoming many of the limitations of siRNA. Our ddRNAi approach combines RNA interference with gene therapy vectors to deliver a DNA compound to the target diseased tissue in order to silence the disease-associated genes.
Gene therapy
Gene therapy is designed to introduce genetic material into cells, usually to compensate for abnormal or malfunctioning genes or to make a beneficial protein to address a defect. If a mutated gene causes a necessary protein to be faulty or missing, gene therapy is used typically to introduce a normal copy of the gene to restore the function of the protein. Genetic material that is inserted directly into a cell usually does not function. Instead, a carrier called a vector is genetically engineered to deliver the gene. Certain viruses are often used as vectors because they can deliver the new gene by infecting the cell. The vector viruses are designed not to cause disease when used in people. Some types of vector viruses, such as lentivirus, integrate their genetic material, including the new gene, into a chromosome in the human cell. Other vector viruses, such as adenoviruses and adeno-associated viruses, or AAV, introduce their DNA into the nucleus of the cell, but the DNA of the vector virus is not integrated into a chromosome; only the therapeutic gene is integrated. Most of our ddRNAi programs utilize AAV as the delivery vector. A number of viral vectors can produce gene expression for months or years following a single administration, depending on the target tissue.
The vector can be given intravenously or injected directly into a specific tissue in the body, where it enters individual cells. Alternatively, a sample of the patient's cells can be removed and exposed to the vector in a laboratory setting. The cells containing the vector are then returned to the patient where they produce the expressed RNA or protein.
ddRNAi
Our ddRNAi technology utilizes DNA as the therapeutic molecule designed to generate shRNAs continuously in the target cell. A range of viral and non-viral gene therapy vectors can be used to deliver the DNA construct into the cell's nucleus. Once delivered, the DNA sequence codes for specific shRNAs, which are then processed by the cell's endogenous machinery into siRNA. The siRNA created by the cell then completes the RNAi cycle described above by cleaving the mRNA of the target gene thus preventing the disease-causing gene from being expressed.
56
Figure 2. The ddRNAi approach
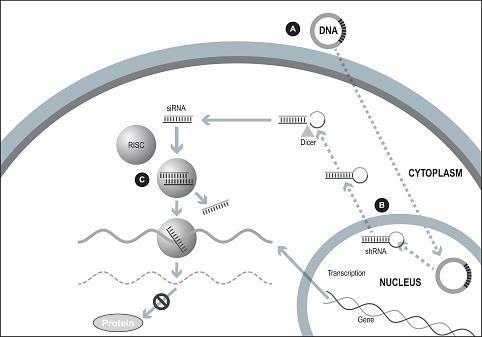
A DNA construct is delivered to the target cell's nucleus by a gene therapy vector (A) such as an AAV. Once in the nucleus, the DNA construct continuously produces shRNAs (B) which are processed by an enzyme called Dicer into siRNAs (C). The processed siRNA is incorporated into RISC and silences the target gene using the same mechanism shown in Figure 1.
Our Pipeline
We are developing a portfolio of product candidates based on our proprietary ddRNAi gene silencing technology focused on chronic and life-threatening conditions with disease-associated genes.
In-House Programs
Company Pipeline
The following table sets out our product candidates and their development status.
Figure 3. Pipeline: Oculopharyngeal Muscular Dystrophy (OPMD)
and Chronic Hepatitis B Virus Infection

57
BB-301 for Treatment of Oculopharyngeal Muscular Dystrophy
We are developing BB-301 for the treatment of OPMD, a heritable, autosomal-dominant, slowly-progressing, late-onset degenerative muscle disorder. OPMD patients typically present with clinically significant symptoms between age 40 and age 60. OPMD patients commonly present with progressive swallowing difficulties, or dysphagia, and eyelid drooping, or ptosis, due to pathophysiological mechanisms impacting specific craniofacial and pharyngeal muscles. The disease is caused by the presence of a trinucleotide repeat expansion in the poly(A)-binding protein nuclear 1, or PABPN1, gene. Dense intranuclear inclusions comprise one histopathological hallmark of OPMD.
BB-301 accomplishes the “silence and replace” approach to disease treatment via the employment of a bifunctional ddRNAi construct, delivered via a modified AAV vector, to silence the expression of the mutant PABPN1 gene and to deliver a normal PABPN1 replacement gene.
OPMD is a rare disease and has been reported in at least 33 countries. Patients suffering with OPMD are well identified and are aggregated in particular regions, which we believe should simplify clinical development and commercialization of BB-301, if it is approved. The largest OPMD cluster is in the French-Canadian population, with estimated prevalence of one in every one thousand people, and its highest prevalence is among Bukhara Jews living in Israel, where it affects one in six hundred people. In Europe, the estimated prevalence is one in one hundred thousand people. The relatively low abundance of patients afflicted by this disease allows this indication to be characterized as a rare disease. In January 2017, the European Commission granted orphan drug designation for BB-301 as an orphan medicinal product for the treatment of OPMD. In January 2018, BB-301 received orphan drug designation in the United States. In July 2018, we licensed BB-301 to Axovant for continued development. However, this contract was terminated on September 3, 2019 after receiving the notice of termination on June 6, 2019.
Current OPMD Treatments and Products in Development
No curative or disease-modifying therapies currently exist for OPMD patients. Surgical interventions can be undertaken for palliative purposes, including the use of cricopharyngeal myotomy.
Investigational therapies under development include intravenous administration of trehalose and the use of autologous myoblast transplant.
Our ddRNAi-Based OPMD Therapeutic — BB-301
BB-301 is a bifunctional ddRNAi-based gene therapy designed to correct the genetic defect underlying OPMD following a single administration. BB-301 is administered as a monotherapy via the use of a modified AAV vector and is designed to silence the expression of the mutant PABPN1 gene while simultaneously introducing a silencing-resistant, normal form of the PABPN1 gene to restore the biological function of the diseased tissue. We believe OPMD is well suited for this "silence and replace" approach since the genetic mutation is well characterized and the targeted tissue domains are relatively small. Once validated, we believe a similar approach could be applied to other genetic disorders.
58
BB-301 —Design and Mechanism of Action
BB-301 is designed to target three distinct regions of the PABPN1 mRNA via the concomitant expression of three distinct shRNAs from a single DNA construct and the simultaneous expression of a codon-optimized, silencing-resistant version of the wildtype PABPN1 gene (Figure 4).
Figure 4. BB-301 AAV "silence and replace" combination vector
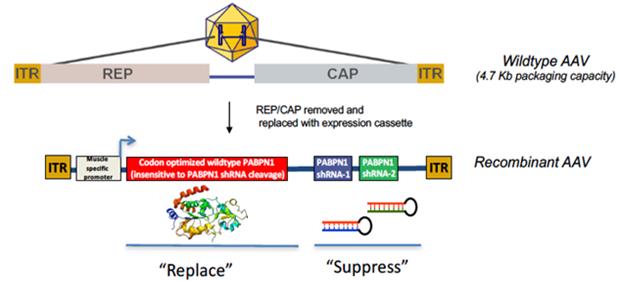
BB-301 is comprised of a ddRNAi DNA construct expressing three separate shRNAs targeting three separate regions of the PABPN1 gene, designed to silence the defective PABPN1 gene in OPMD patients, combined with a gene expression construct that produces a silencing-resistant version of the normal PABPN1 gene, delivered using an AAV vector.
59
In collaboration with researchers at the Royal Holloway University of London and the Institut de Myologie in Paris, we have observed effective silencing of the PABPN1 gene in vitro by the ddRNAi construct. Furthermore, we have generated a gene expression construct that produces a silencing-resistant version of the normal PABPN1 gene. The mechanism of action of BB-301 is shown in Figure 5.
Figure 5. The mechanism of action of BB-301
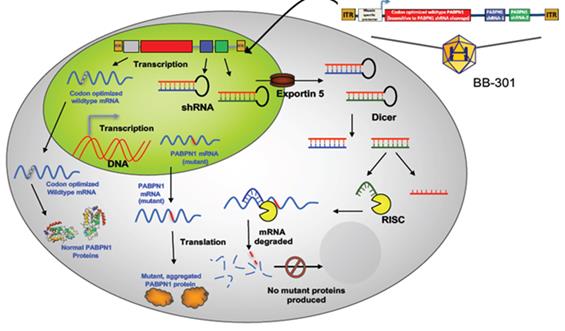
The DNA construct is delivered to the affected muscle cells via an AAV vector. Upon reaching the nucleus, the construct expresses multiple independent shRNAs that are ultimately processed by the endogenous machinery of the target cell to produce siRNAs that cleave the mutant PABPN1 mRNA and silence the expression of the mutant gene. In addition, the bifunctional gene construct drives the expression of a silencing-resistant version of the normal PABPN1 gene, which has been shown to promote the restoration of muscle function in several non-clinical studies.
60
In in vivo studies using a transgenic mouse model of OPMD at the Royal Holloway University of London and the Institut de Myologie, we observed a normalization of muscle strength following administration of the ddRNAi and gene expression constructs (Figure 6). The full results of the studies were published in Nature Communications in April 2017.
Figure 6. Restoration of muscle function in vivo following gene silencing and replacement
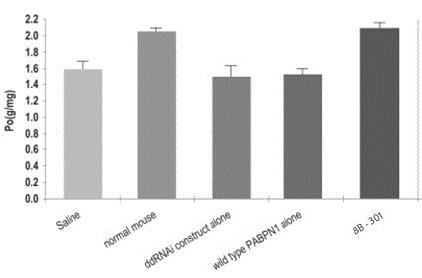
Figure 6 demonstrates the capacity of BB-301 to drive restoration of muscle function in vivo following suppression of the mutant PABPN1 and replacement with the normal PABPN1 gene, with muscle function measured by specific tetanic force (Po). Neither the expression of a triple hairpin construct to down-regulate the mutant form of the PABPN1 protein, nor the expression of the normal protein, was sufficient by itself to restore specific force levels. The combination of the silencing of the mutant gene with the triple hairpin construct and replacement with the normal gene was observed to restore specific force capacity to healthy levels.
Ongoing Development Plans for BB-301
In August 2017, following the results of the in vitro studies conducted by researchers at the Royal Holloway University of London and the Institut de Myologie in Paris, we announced our innovative vector design to ‘silence and replace’ the disease-causing gene in a single construct. The single vector system has shown activity consistent with the dual vector system in which the ‘silence’ and ‘replace’ are delivered in separate vectors. Benitec will complete three non-clinical studies for BB-301 that will facilitate the filing of an Investigational New Drug (IND) application and the formal initiation of a Phase I clinical trial in patients suffering from OPMD. The three non-clinical BB-301 studies will be conducted in canine subjects and will support the optimization of the methods of administration, confirm the efficiency of vector transduction in the key tissue compartments underlying the disease phenotype, confirm the optimal drug doses in advance of initiation of human clinical studies, and finalize experiments designed to characterize any toxicological data-points.
BB-103 for the Treatment of Hepatitis B
We are developing BB-103 for the treatment of HBV. Results of in vivo and in vitro studies, from December 2016, March 2016 and December 2015, have, we believe, demonstrated the potential utility of an approach that combines RNAi with gene therapy to treat HBV. We are currently seeking strategic partners to work with the Benitec team to advance the HBV program towards the clinic.
61
We have completed a Phase I/IIa clinical trial for our now discontinued product candidate, TT-034, which we were developing to treat patients chronically infected with the most common genotype of HCV. The trial achieved its primary end point of safety of TT-034 in HCV patients, based on safety within the liver and other organs of the nine patients that were dosed in the study. There was one serious adverse event observed, a pulmonary embolism resulting from a fall, and it was classified as unrelated to the therapy. That event was resolved within four weeks. Three other adverse events (diarrhoea, light-headedness and bradycardia) were also observed and considered possibly treatment related. All of those events were mild in nature and resolved completely. No adverse event met the pre-defined criteria for a dose-limiting toxicity. In addition, no T-cell capsid response was seen in any of the subjects, as has been previously reported at similar high-dose levels in other systemic trials with AAV. While transduction of hepatic tissues was seen, there was no reduction in viral load in treated patients, which was a secondary endpoint of the study. We believe that our experience with the TT-034 clinical trial will be of value in designing and implementing our other programs, in particular HBV, as both HBV and HCV replicate in the liver. We have improved specific elements of BB-103 as a result of the nonclinical and clinical study results obtained during the evaluation of TT-034.
The human hepatitis B virus is a small DNA virus that, according to the WHO, infects up to 240 million people worldwide, resulting in up to 780,000 deaths per year. Infection with HBV occurs in phases ranging from a silent, acute phase that can be resolved by the immune system to a persistent chronic infection requiring life-long therapy. In the case of a chronic HBV infection, the presence of viral proteins, particularly the s-antigen, causes hepatic inflammation leading to liver dysfunction, acute hepatic failure, cirrhosis or hepatocellular carcinoma.
Current Hepatitis B Treatments
HBV predominantly exists as eight genotypes, designated A through H, with distinct geographic distribution. We believe the keys to developing a successful HBV therapeutic are to stop viral replication and to prevent production of or clear specific antigens generated by the virus, in particular the HBV surface antigen, or HBsAg.
According to GlobalData, a market research firm, the global hepatitis B therapeutics market was worth $2.4 billion in 2014 and is expected to reach a total of $3.0 billion by 2024 at a Compound Annual Growth Rate of 2.4%. The current therapies used as standard of care for HBV consist of antivirals composed of nucleotide and nucleoside analogues, or NUC, and, less commonly, interferon therapy.
The most common anti-viral medications are taken as tablets each day for a year or longer and primarily act to inhibit viral replication:
| • | Lamivudine (Zeffix). There are almost no side effects to Lamivudine, however a significant concern is the possible development of hepatitis B virus mutations and antiviral drug resistance after long-term use. |
| • | Adefovir (Hepsera). Adefovir is often used for people who have developed a hepatitis B virus mutation after taking Lamivudine. There are almost no side effects except for the possibility of developing hepatitis B virus mutations and antiviral drug resistance. |
| • | Entecavir (Baraclude). Entecavir has potent activity against chronic hepatitis B. There are almost no side effects except for the possibility of developing hepatitis B virus mutations and antiviral drug resistance. |
| • | Tenofovir (Viread). Tenofovir has potent activity against chronic hepatitis B. It is particularly useful in patients who have developed drug resistance to other medications. |
| • | Pegylated Interferon (Pegasys). Interferon is given by injection once a week, usually for six months to a year. The drug has many potential side effects, such as flu symptoms and depression, but is reported to control the hepatitis B virus in a third of patients without need for long-term medication. |
62
Most of these therapies can provide long-term viral load suppression but have low cure rates and have the additional risk of drug-resistant mutations. The long-term use of interferon, particularly in high doses, may also be associated with significant side effects, including nausea, vomiting, shortness of breath, dizziness and fatigue, adding to issues with patient compliance for the course of treatment. We believe that there is significant unmet medical need for HBV treatment due to the following factors:
| • | inability of existing therapies to address the risk of recurrence of the infection, once an antiviral therapeutic is removed, due to the persistence of HBV covalently closed circular DNA, or cccDNA. cccDNA is a supercoiled DNA molecule that is present in the nucleus of HBV-infected cells and acts as a reservoir for further HBV infectivity. cccDNA is responsible for persistence of infection in the natural course of chronic HBV infection and during prolonged antiviral therapy; |
| • | mutations in the HBV genome conferring resistance to existing therapies; and |
| • | long treatment regimens and, in some cases, significant debilitating side effects associated with current therapies, which lead to a risk of patient non-compliance. |
A problem inherent to all of the current HBV antiviral treatment approaches is their inability to achieve a curative outcome. According to a study published in the Journal of Viral Hepatitis in 2014, HBsAg clearance occurs in only 3% of patients treated with a NUC and 7.8% of patients treated with interferon. Sustained suppression of HBV cccDNA by treatment with a NUC is only possible with continued treatment for many years, and clear-cut guidelines on when to stop treatment are not yet available. The study's authors concluded that there is a need for alternative therapeutic approaches such as drugs that can preferentially target stages in the life cycle of HBV, referred to as direct acting anti-virals, or DAAs, as well as new immunotherapeutic approaches. BB-103 is designed to be single administration ddRNAi-based therapy that is delivered using a gene therapy vector that targets the liver and inhibits viral replication and s-antigen production on a long-term basis.
There are several late-stage pipeline agents in development for the treatment of hepatitis B, including TAF, GS- 9620 and GS-4774. These products aim to fulfill key unmet needs by targeting improvements in safety and tolerability, and by more effectively lowering serum HBsAg which may indicate better long-term disease outcomes.
Our ddRNAi-based Hepatitis B Therapeutic—BB-103
We are developing BB-103 to address many of the limitations of therapeutics for HBV currently on the market and those in development. BB-103 is designed to be a single administration ddRNAi-based therapy that is delivered using a gene therapy vector that targets the liver and inhibits viral replication and s-antigen production on a long-term basis. Although initially developed as a monotherapy, we believe that combining BB-103 with a NUC, a class of drugs currently used to treat the HBV in infected individuals, will help spur the patient’s own immune system to produce anti-s-antigen antibodies and eliminate their daily anti-viral treatments to control disease.
BB-103 has been designed to target the liver and produce steady state levels of anti-HBV shRNAs that inhibit viral replication and viral protein production. This includes the hepatitis B surface-antigen, or s-antigen, which is thought to be the principal component of the long-term immunosuppression and chronicity associated with disease progression. The BB-103 therapeutic vector is being developed to be used in combination with other anti-viral compounds.
BB-103 has been designed to mimic certain design elements of TT-034, our now discontinued product candidate for the treatment of HCV, and we believe useful information was obtained from the TT-034 clinical trial which could be beneficial for the clinical development of BB-103, the IND-enabling studies, the design and regulatory approval pathway for initial clinical trials, and potentially the starting clinical dose, in light of the similarities between TT-034 and BB-103. Although HBV and HCV are a DNA and RNA virus respectively, both replicate predominantly in the hepatocytes of infected subjects, the target tissue for BB-103 and TT-034:
| • | both use identical AAV8 capsids for delivery; |
| • | many of the components of the recombinant expression construct remain unchanged including the use of three simultaneously expressed ddRNAi components directed to cleave viral RNA; |
63
| • | the data from the TT-034 clinical study has provided a solid understanding of the safety of a ddRNAi product in humans as well as establishing a relationship between the dose administered and the level of DNA transduction and transgene expression; and |
| • | the extensive pre-clinical toxicology package from the development of TT-034 provides relevant information for BB-103. |
BB-103—Design and Mechanism of Action
The design of the BB-103 DNA construct takes advantage of the structure of the HBV genome. The hepatitis B virus is a small DNA virus with four overlapping open reading frames, meaning several genes are produced from the same DNA sequence by shifting the starting point of the translation process (Figure 10). These four genes are known as the core, surface, X and polymerase genes. The core gene encodes the core nucleocapsid protein, which is important in viral packaging and thought to help stabilize cccDNA, and hepatitis B e-antigen. The surface gene encodes proteins, including s-antigen. The X gene encodes the X protein, which has properties that may be important in liver carcinogenesis. The polymerase gene encodes a large protein with functions critical for viral packaging and replication. Although HBV is a DNA virus, it replicates through an RNA intermediate. BB-103 targets the viral mRNA at three overlapping regions of the genome (Figure 10), simultaneously silencing the surface, X, core and polymerase genes. As a result, we believe that the long-term suppression of HBV viral replication, through silencing of the polymerase gene and targeting the HBV RNA used for replication, the inhibition of HBV viral proteins production, including the s-antigen production, through silencing of the surface gene, and the inhibition of the cccDNA, through silencing of the core protein gene, could lead to eradication of HBV infection in patients by a single administration of BB-103 when paired with a NUC.
In vitro Development Highlights
Our bioinformatics analysis of the major HBV genotypes, A through H, has identified several well-conserved regions of the genome for targeting with ddRNAi therapeutics. We have designed numerous shRNAs to target these regions, and we have identified lead candidates based on several factors, including activity, hyperfunctionality, meaning the highest activity at lowest levels, and the ability of the shRNA strand to be incorporated into the cell's natural RNA processing mechanism. The final clinical construct we have chosen is illustrated in Figure 7.
Figure 7. The design for BB-103
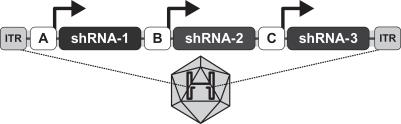
64
A
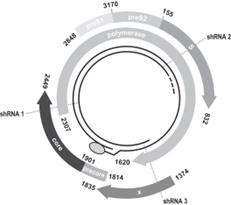
B
The design for BB-103 (Figure 7) is based on the design of TT-034, utilizing an AAV capsid and a triple construct targeting three separate conserved regions on the HBV mRNA (A); The shRNAs target regions on the overlapping reading frames of the HBV genome (B), allowing simultaneous targeting of the mRNAs that express viral proteins including DNA polymerase, s-antigen and core proteins.
Figure 8. The mechanism of action of BB-103

The DNA construct is delivered to the nucleus of hepatocytes via an AAV vector. Upon reaching the nucleus the construct expresses three different types of shRNAs (B) that are processed by the cell's endogenous machinery to produce siRNAs that cleave the HBV mRNA and thus prevent the virus from replicating and producing viral proteins.
65
In vivo Development Highlights and Ongoing Development Plan for BB-103
Benitec is developing BB-103 for the treatment of HBV. Results of in vivo and in vitro studies, from December 2016, March 2016 and December 2015, demonstrated the potential utility of an approach that combines RNAi with gene therapy to treat HBV. In April 2017, we completed a pre-IND submission with the FDA in which the feedback provided by the agency included details regarding steps required to initiate a clinical trial for BB-103. We are seeking strategic partnerships to facilitate the advancement of BB-103 into clinical testing.
TT-034 for the Treatment of Hepatitis C
Hepatitis C is a complex public health problem, characterized by a high prevalence of chronic infection by an RNA virus, an increasing burden of HCV-associated disease, low rates of testing and treatment, and the prospect of increasing incidence associated with injectable drug abuse. According to the WHO, over 170 million individuals worldwide have chronic hepatitis C. Chronic infection can result in cirrhosis and death in 20% of patients due to end-stage liver disease or hepatocellular carcinoma. We have been developing a ddRNAi-based therapeutic, TT-034, for the treatment of the most common genotype of the human hepatitis C virus. We began a Phase I/IIa first-in-human clinical trial of TT-034 in January 2014. Following the commencement of the clinical trial of TT-034, a number of new and effective therapeutics were approved for the treatment of HCV. Competitors' products showed improvements in the efficacy, delivery and success rate of treatments for HCV while, at the same time, reducing the price and duration of their treatments.
As a result of the increasingly competitive landscape, in February 2016 we concluded that the TT-034 program no longer offered the commercial value necessary to attract a worthwhile commercial partnership deal and, as a result, did not warrant additional expenditure or focus of our resources beyond completion of the existing patients.
This phase I/IIa clinical study enrolled nine patients who received a single intravenous infusion of TT-034 at escalating doses. Patients were monitored for safety and efficacy assessments over 24 weeks following the administration of TT-034. A liver biopsy, collected 21 days post dosing, was used to assess hepatic TT-034 DNA levels and shRNA expression.
These results show the administered doses of TT-034 to be well tolerated with no treatment related serious adverse events observed. In the nine patients dosed, one serious adverse event, a pulmonary embolism resulting from a fall, was observed and classified as unrelated to therapy. That event resolved within four weeks. Three other adverse events (diarrhoea, light-headedness and bradycardia) were also observed and considered possibly treatment related. All of those events were mild in nature and resolved completely. No adverse event met the pre-defined criteria for a dose-limiting toxicity. In addition, no T-cell capsid response was seen in any of the subjects, as has been previously reported at similar high dose levels in other companies' systemic trials with AAV. The patients dosed with TT-034 have been followed in a pre-planned safety follow-up study. In 2018 we received agreement from the FDA to terminate this safety study.
While transduction of hepatic tissues was seen, the assessment of TT-034 treatment on viral load, a secondary endpoint of the study, showed no reduction in viral load in the treated subjects.
We believe the data collected from this study will have a positive impact not only on Benitec’s other therapeutic programs, but also for the field of gene therapy as a whole. This is the first time DNA transduction and transgene expression could be measured directly in hepatic tissues following systemic administration. We have been able to use the data from this study to implement design changes in our clinical constructs and to ensure that our other therapeutics programs benefit from this study.
Our Out-Licensed Programs
In addition to the proprietary development program, the Company has licensed its ddRNAi technology to companies who are developing therapeutic programs in other disease areas.
66
HIV/AIDS: In March 2012, Benitec granted a non-exclusive, royalty-bearing, worldwide license to a U.S. based biotechnology company, Calimmune, Inc. Under the agreement, Calimmune could develop, use and commercialise ddRNAi to silence up to three targets for the treatment or prevention of HIV/AIDS. Calimmune's approach was developed with core technology from the laboratory of Dr. David Baltimore, a Nobel Laureate in the area of HIV/AIDS, and involves silencing the gene that codes for a receptor protein known as CCR5. Calimmune's HIV/AIDS treatment is known as CAL-1. In August 2017, the CSL Behring subsidiary of CSL Ltd. announced that it would acquire Calimmune Inc. gaining two ex vivo autologous gene therapy candidates and two stem cell therapy technologies.
As part of this deal, CSL Behring also acquired CAL-1, the autologous T cell and blood stem cell therapy in Phase I/II testing to treat HIV infection. The announcement indicated that CSL Behring was evaluating options for developing this candidate, including licensing or partnering as the company is "unlikely" to develop the candidate on its own.
On December 19, 2018 the license was terminated by Calimmune Inc.
Cancer Immunotherapy: In August 2013, an exclusive, royalty-bearing, worldwide license was granted to a U.S.-based biotechnology company, Regen Biopharma Inc. to use ddRNAi for silencing expression of indoleamine 2,3—dioxygenase, or IDO, in dendritic cells. Regen is developing a cancer immunotherapy using the licensed technology. IDO is associated with immune-suppression and is overexpressed in some cancers. Regen has reported preclinical evidence that modification of these cells using ddRNAi targeting the silencing of IDO may significantly enhance their efficacy in cancer immunotherapy. Regen's first treatment, which is for breast cancer, is called dCellVax.
Regen advised Benitec in July 2019 that they intend to terminate the agreement.
Intractable Neuropathic Pain: In November 2014, an exclusive, royalty-bearing, worldwide license was granted to a U.S.-based biotechnology company, Circuit Therapeutics, Inc. to use ddRNAi for the development of treatments for and the prevention of pain. This license has been terminated in February 2019.
Intellectual Property
Benitec Biopharma Limited and its controlled entities (collectively Benitec) actively seeks to protect the intellectual property and proprietary technology that it believes is important to its business, which includes seeking and maintaining patents directed to its proprietary ddRNAi technology, as well as inventions arising from in-house product development, or otherwise commercially and/or strategically important to the development of the business. Benitec also relies on know-how and trade secrets and actively seek to protect the confidentiality of such know-how and trade secrets.
As of September 30, 2019, Benitec owns a total of nineteen patent families directed to its product candidate programs, of which four are in the provisional phase, two are in the Patent Cooperation Treaty (PCT) phase and the others having progressed to national phase and are under prosecution or are granted. Within this portfolio, Benitec has 72 pending national/regional or convention applications and 17 granted patents, in a total of 23 jurisdictions (counting as one jurisdiction the member states of the European Patent Convention).
Benitec also has a portfolio of in-licensed patents relating to its ddRNAi platform technology, which includes irrevocable, exclusive rights in the field of human therapeutics to CSIRO's patents claiming ddRNAi, non-exclusive rights to Carnegie’s patents relating to genetic inhibition methods using double-stranded RNA, and non-exclusive rights to Hairpin Technologies’ (as exclusive licensing agent for Cold Spring Harbor Laboratories) patents relating to short hairpin RNA (shRNA) technology.
67
ddRNAi Platform Technology (in-licensed)
In December 2009, Benitec entered into a commercial license arrangement with CSIRO for two patent families relating to ddRNAi technology. This worldwide license in the field of human therapeutics is exclusive and irrevocable.
The two patent families exclusively licensed from CSIRO include different aspects of the ddRNAi technology. The first of these patent families relates to DNA constructs and methods of using same to deliver RNA molecules, particularly shRNA, directed to the target gene within a cell and thereby reduce expression of a target gene by RNA interference (RNAi). This family is entitled “Control of gene expression”. The remaining US patent in this family is due to expire in April 2020.
The second patent family is an extension of the first patent family, and relates to chimeric DNA and methods for using DNA to deliver RNA molecules, particularly shRNA. This family is entitled “Methods and means for obtaining modified phenotypes”. The remaining US patents in this family are due to expire in August 2021.
Technology Improvements
Benitec owns two patent families relating to improvements to ddRNAi technology. The first patent family, which is entitled “Genetic silencing”, relates to compositions of matter and methods for delivering shRNA molecules to animal cells for a variety of target genes. This patent family includes a granted patent in the United Kingdom which is due to expire in March 2021.
The second patent family, which is entitled “Double-stranded nucleic acids”, relates to nucleic acid constructs and methods for using DNA to produce hairpin RNA molecules that can target multiple genes within one molecule. This patent family includes a granted patent Australia which is due to expire in June 2024.
ddRNAi-based treatment of Hepatitis B
The Benitec patent portfolio includes three patent families relevant to Benitec’s ddRNAi-based candidate for treatment of hepatitis B virus (HBV) infection (BB-103). BB-103 is a triple construct encoding three shmiRs targeting the HBV pol gene, with each shmiR-coding sequence operably linked to its own U6 promoter. Two variants of BB-103 have been developed at Benitec, each with a different combination of shmiRs. The first variant of BB-103 comprises the shmiRs internally designated All-4.1_minus3, shRNA8v2_plus1, and All-9.1_plus1. The second variant of BB-103 comprises the shmiRs internally designated All-4.1_minus3, All-5, and All-9.1_plus1.
The first patent family, entitled “HBV Treatment (HBV family #1)”, relates to single-stranded RNA and shRNA sequences to a range of target regions of the hepatitis B viral genome. This patent family was jointly filed with Biomics Biotech Co., Ltd., with ownership interests subsequently being assigned to Benitec. This patent family includes a number of granted patents with claims covering a ddRNAi agent comprising at least two effector sequences, wherein one of the effector sequences corresponds to the shRNA (or shmiR) in BB-103 designated “shRNA8v2_plus1”. Although drafted to explicitly cover shRNAs (as this was the RNAi format under development at the time), the claims encompass shRNAs comprised within a microRNA backbone (i.e., short hairpin microRNA (shmiR)) which is the format of RNAi currently in use within Benitec’s HBV program.
A further patent family relating to shRNA sequences to a range of additional target regions of the hepatitis B viral genome was filed solely in the name of Benitec. This patent family, entitled “Reagents for treatment of hepatitis B virus (HBV) infection and use thereof (HBV family #2)”, includes claims relating to the shRNA designated All-4.1_minus3, All-5, and All-9.1_plus1 in BB-103, as well as shRNA targeting other regions of the HBV genome. These new shRNAs are described individually, as well as in the context of the triple construct BB-103. Although drafted to cover shRNAs (as this was the RNAi format under development at the time), the claims encompass shRNA and shmiR.
The third patent family relevant to HBV relates to the development of constructs. This patent family, entitled “Reagents for treatment of hepatitis B virus (HBV) infection and use thereof (HBV family #3)”, includes claims relating to shmiRs designated All-4.1_minus3, All-5, and All-9.1_plus1 in BB-103. The sequences covered
68
in this family correspond to those described in HBV family #2 with the same name. This patent family was filed in order to cover single and triple shmiR constructs currently under development at Benitec. This patent family names Benitec as the sole applicant.
ddRNAi-based treatment of AMD
The Benitec patent portfolio also includes one patent family relevant to Benitec’s ddRNAi-based candidate for treatment of age-related macular degeneration (BB-201), which has been discontinued. BB-201 is a triple construct encoding three shmiRs targeting VEGFb, VEGFa and PGF respectively, with each shmiR-coding sequence operably linked to its own U6 promoter. Two variants of BB-201 have been developed at Benitec, each with a different combination of shmiRs. The first variant of BB-201 comprises the shmiRs internally designated VEGFb shmiR-1, VEGFa shmiR-8 and PGF shmiR-3. The second variant of BB-201comprises the shmiRs internally designated VEGFb shmiR-1, VEGFa shmiR-8 and PGF shmiR-7.
The AMD patent family entitled “Age-Related Macular Degeneration Treatment (AMD family #1)”, relates to a first set of target gene sequences considered for the ddRNAi- based therapeutic candidate being developed for AMD. Of the first set of genes described in this patent family, target regions within VEGFa have been the focus of prosecution and a patent has been granted in the USA. This patent family was filed solely in the name of Benitec.
ddRNAi-based treatment for OPMD
Benitec’s patent portfolio for OPMD includes five patent families relating to shRNA and shmiRs targeting PABPN1 (the causative gene for OPMD), as well as ‘silence and replace’ therapeutics and treatment strategies for OPMD. These five families cover the OPMD therapeutic candidate, BB-301, under development at Benitec, treatment strategies for OPMD which silence PABPN1 which is causative of OPMD and replace with functional PABPN1, and Benitec’s AAV patent family which covers the delivery system for BB-301. BB-301 is a ‘silence and replace’ construct encoding two shmiRs targeting the endogenous PABPN1 (including variants causative of OPMD) internally designated shmiR-13 and shmiR-17, as well as a codon-optimized PABPN1 replacement construct, the transcript of which is not targeted by shmiR-13 and shmiR-17. Both shmiRs and the codon-optimized PABPN1 replacement construct are under the control of a muscle-specific promoter.
The first patent family, entitled “Reagents for treatment of oculopharyngeal muscular dystrophy (OPMD) and use thereof (OPMD family #1)”, arose out of a collaboration with Royal Holloway University of London (RHUL) and relates to three shRNA target regions within PABPN1. RHUL assigned its ownership interests in this patent family to Benitec, and the PCT application and the related U.S. priority document were filed solely in the name of Benitec. This patent family is directed to RNAi agents targeting specific regions within mutant PABPN1 variants causative of OPMD, as well as use of those RNAi agents in combination with PABPN1 replacement constructs to treat OPMD. More specifically, this family includes claims covering shmiR17 of BB-301 This patent family entered the national/regional phase in October/November 2018.
The second patent family, entitled “Reagents for treatment of oculopharyngeal muscular dystrophy (OPMD) and use thereof (OPMD family #2)” relates to a second set of target gene sequences within PABPN1 as well as ‘silence and replace’ construct BB-301 under development at Benitec. The PCT application and the related U.S. priority document were filed solely in the name of Benitec, and this family entered the national/regional phase in June/July 2019. This patent family is directed to RNAi agents targeting specific regions within mutant PABPN1 variants causative of OPMD, as well as ‘silence and replace’ constructs and use of same for treatment of OPMD. More specifically, this family includes claims covering shmiR13 and shmiR17 of BB-301 separately, as well as the full BB-301 ‘knockdown and replacement’ construct.
A third patent family, entitled “Methods for Treating Oculopharyngeal Muscular Dystrophy (OPMD) (OPMD family #3)”, has been filed and exists as an unpublished US provisional application. This US provisional application was filed to pursue claims directed to the ‘silence and replace’ treatment concept for OPMD relying on RNAi agents to knockdown PABPN1 causative OPMD and replacing with functional PABPN1 which is not targeted by the RNAi agents. The claims in this application are not limited to BB-301. This US provisional application was filed solely in the name of Benitec.
69
A fourth patent family, entitled “Methods for Treating Oculopharyngeal Muscular Dystrophy (OPMD) (OPMD family #4)” has been filed and exists as an unpublished US provisional application. This US provisional application was filed to specifically claim the OPMD therapeutic candidate described in OPMD family#2 which is packaged in a modified adeno-associated virus (AAV) delivery system developed by Benitec (described below). The modified AAV was not described in OPMD family#2. This US provisional application was filed solely in the name of Benitec.
AAV with modified phospholipase domain
The Benitec patent portfolio includes a single patent family, entitled “Adeno-associated virus (AAV) with modified phospholipase domain”, which relates to an AAV having a modified phospholipase (PLA2) domain in the capsid. This patent family exists as a PCT application, with convention applications filed in Uruguay and Taiwan. The priority document, PCT and convention application were filed solely in the name of Benitec. The modified AAV will be used as the delivery system for the OPMD therapeutic candidate.
RNAi-based treatment for HNSCC
Pursuant to an exclusive sublicense agreement with NantWorks, Benitec has licensed two patents relating to the inhibition of human squamous cell carcinoma in vivo by EGFR antisense RNA. These in-licensed patents support the development of Benitec’s Head and Neck Squamous Cell Carcinoma (HNSCC) candidate BB-401, which is an antisense RNA therapeutic targeting EGFR for delivery by intratumoral injection.
In addition to the licensed-in patents, the Benitec patent portfolio includes two patent families based on technology developed in house for its ddRNAi-based HNSCC therapeutic candidate, BB-501. BB-501 is a dual expression construct designed to express one or more shmiRs targeting EGFR, as well as an immunomodulatory cytokine (e.g., IL2).
The first patent family, entitled “Reagents for treatment of head and neck cancer and use thereof”, exists as an unpublished US provisional application and relates to target regions within EGFR for inhibition using shmiRs expressed from ddRNAi constructs. This patent family describes ddRNAi constructs coding for shmiRs which target EGFR, and dual constructs for expressing shmiRs and an immunomodulatory cytokine (e.g., IL2).
The second patent family, entitled “Expression Cassettes and use thereof in cancer therapy”, also exists as an unpublished US provisional application and relates to expression cassettes comprising specific combinations of promoters and polyadenylation terminator sequences which are particularly good at expressing a gene of interest e.g., immunomodulatory cytokine, in cancer cells e.g., head and neck cancer cells. This patent family also describes dual expression constructs for expressing immunomodulatory cytokine (e.g., IL2) and a shmiR. This US provisional application was filed solely in the name of Benitec.
The first and second patent families relate to different aspects of BB-501 i.e., the ddRNAi construct and the expression cassette. As they include overlapping datasets, they were filed on the same day. Both of these US provisional applications were filed solely in the name of Benitec. Benitec does not intend to file a PCT application and/or any convention applications claiming priority to the two provisional applications.
TT-034—ddRNAi-based treatment for hepatitis C
The Benitec patent portfolio also includes two patent families relating to the now discontinued Hepatitis C program. These patent families relate primarily to the shRNA sequences of TT-034 and the expression cassette design of the therapeutic candidate.
The first patent family in the HCV program, entitled “Multiple Promoter Expression Cassettes for Simultaneous Delivery of RNAi Agents”, includes claims to methods and genetic constructs that use the shRNA sequences of TT-034 in the treatment of hepatitis C. The patent family relates to a range of different shRNA sequences and includes the three candidate shRNA sequences incorporated in TT-034. This family also exemplifies
70
and claims the use of ddRNAi technology to deliver the shRNA. The design of the expression cassette in this patent family is for three shRNA sequences with independent promoters driving the expression of each shRNA. A number of the patent and applications in this family have been abandoned in view of the decision to discontinue the HCV program.
The second patent family in the HCV program, entitled “RNAi Expression Constructs”, also includes claims for methods and genetic constructs using the shRNA sequences of TT-034 in the treatment of Hepatitis C. The design of the expression in this patent family is for a single promoter to drive the expression of multiple shRNA sequences. Most of the patent and applications in this family have been abandoned in view of the decision to discontinue the HCV program.
Targeting the T-Cell Receptor
The Benitec patent portfolio also includes two PCT applications relating to CAR-T cell therapeutics incorporating ddRNAi technology.
The first patent family in the CAR-T program, entitled “Reagents for Producing T-Cells with Non-Functional TCR Compositions Comprising Same and Use Thereof”, relates to the use of Benitec’s ddRNAi technology to target specific T-cell subunits and inhibit endogenous T-cell receptors. This PCT application was filed solely in the name of Benitec. Benitec may continue to pursue this family in limited jurisdictions.
The second patent family in the CAR-T program, entitled “CD-70 deficient CAR-T cells, and reagents and method for producing same”, relates to the use of Benitec’s ddRNAi platform to inhibit expression of CD-70 in CAR-T cells. As the excessive presence of CD70 on activated T cells can lead to “fratricide”, Benitec has used its ddRNAi technology to develop reagents for producing CD70 deficient CAR-T cells which may improve survival and persistence of CAR-T cells during cancer therapy. Benitec does not intend to proceed to the national/regional phase.
Know-How
In addition to patent protection of ddRNAi technology and our product candidates, we also rely on proprietary know-how that is not patentable or that we elect not to patent, as valuable intellectual property for our business. This know-how is related to the areas of, among others, identifying nucleic acid targets for ddRNAi technology and designing ddRNAi constructs for targeting preferred genes. We have implemented a number of security measures to safeguard our know-how including limiting access to our research facilities, databases and networks. We also protect know-how by way of confidentiality agreements when engaging with external providers for progressing our pipeline of therapeutic candidates.
Laws and Regulations Regarding Patent Terms
The term of individual patents depends upon the legal terms of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing a non-provisional patent application. In the United States, a patent term may be shortened if a patent is terminally disclaimed over another patent or as a result of delays in patent prosecution by the patentee. A patent's term may be lengthened by a patent term adjustment, which compensates a patentee for administrative delays by the USPTO in granting a patent. The patent term of a European patent is 20 years from its filing date, which, unlike in the United States, is not subject to patent term adjustments.
The term of a patent that covers an FDA-approved biologic may also be eligible for patent term extension, which permits patent term restoration as compensation for the patent term lost during the FDA regulatory review process. The Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Act, permits a patent term extension of up to five years beyond the expiration of the patent. The length of the patent term extension is related to the length of time the biologic is under clinical testing regulatory review. Patent extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved biologic may be extended. Similar provisions are available in Europe and other
71
jurisdictions to extend the term of a patent that covers an approved biologic although the eligibility requirements for any duration of such extension vary. In the future, if and when our products receive FDA approval, we expect to apply for patent term extensions on patents covering those products.
Trademarks
Our trademarks include registrations for company branding and product names for our pipeline in development.
Trade Mark (program) | | Trade Mark Number | | Filing date | | Jurisdiction | | Status |
BENITEC® | | 1103049 | | 10 Mar 2006 | | Australia | | Registered |
BENITEC™ | | 86795296 | | 21 Oct 2015 | | United States | | Registered |
BENITEC™ | | 1728797 | | 16 Sep 2015 | | Australia | | Registered |
BENITEC™ | | 14680003 | | 16 Oct 2015 | | Europe | | Registered |
BENITEC BIOPHARMA® | | 1448046 | | 13 Sep 2011 | | Australia | | Registered |
BENITEC BIOPHARMA® | | 4636053 | | 11 Feb 2014 | | United States | | Registered |
GIVING DISEASE THE SILENT TREATMENT® | | 1851660 | | 11 Jan 2018 | | Australia | | Registered |
GIVING DISEASE THE SILENT TREATMENT® | | 1389399 | | 15 Feb 2018 | | Europe | | Registered |
SILENCING GENES FOR LIFE® | | 1448041 | | 13 Sep 2011 | | Australia | | Registered |
SILENCING GENES FOR LIFE® | | 4807242 | | 22 Dec 2014 | | United States | | Registered |
Manufacturing
The manufacture of the complex biological products required for gene therapy is complex and difficult. We do not currently own or operate manufacturing facilities for the production of preclinical, clinical or commercial quantities of any of our product candidates. We are exploring long-term manufacturing alliances with a number of potential partners to investigate manufacturing processes in order to produce materials at reasonable scale and cost of goods to support future commercialization efforts. We do not have a long-term agreement with any third-party manufacturer, but we plan to establish such a relationship with an appropriate manufacturer to serve our long-term needs.
Manufacturing is subject to extensive regulations that impose various procedural and documentation requirements, which govern record keeping, manufacturing processes and controls, personnel, quality control and quality assurance, among others. Our contract manufacturing organizations manufacture our product candidates under cGMP conditions. cGMP is a regulatory standard for the production of pharmaceuticals that will be used in humans.
Sales and Marketing
We have not yet established sales, marketing or product distribution operations because our product candidates are in preclinical or clinical development. If we receive marketing and commercialization approval for any of our product candidates, we intend to market the product through strategic alliances and distribution agreements with third parties. In certain cases, we may market an approved product directly worldwide or in selected geographical segments. The ultimate implementation of our strategy for realizing the financial value of our product candidates is dependent on the results of clinical trials for our product candidates, the availability of funds and the ability to negotiate acceptable commercial terms with third parties.
72
Competition
The biopharmaceutical industry is characterized by intense and dynamic competition to develop new technologies and proprietary therapies.
Any product candidates that we successfully develop and commercialize will have to compete with existing therapies and new therapies that may become available in the future. While we believe that our proprietary technology estate and scientific expertise in gene silencing using ddRNAi provide us with competitive advantages, we face potential competition from many different sources, including larger and better-funded pharmaceutical, specialty pharmaceutical and biotechnology companies, as well as from academic institutions and governmental agencies and public and private research institutions that may develop potentially competitive products or technologies. We are aware of several companies focused on developing gene therapy or gene silencing product candidates, including:
| • | Alnylam, Arbutus and Arrowhead—developing siRNA-based therapeutics for hepatitis B; |
| • | Adverum Biotechnologies, Inc., Applied Genetic Technologies Corporation and Oxford Biomedica—developing gene therapies for wet AMD. |
We are not aware of any companies developing a gene therapy or gene silencing approach for OPMD. There are other therapies either being marketed or currently in development for all of these diseases, some of which already have significant market share. Our product candidates, if approved, would also compete with treatments that have already been approved and accepted by the medical community, patients and third-party payers.
Many of our competitors and potential competitors, alone or with their strategic partners, have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of product candidates, obtaining FDA and other regulatory approvals of treatments and the commercialization of those treatments. Mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated among a smaller number of our competitors. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical study sites and subject registration for clinical studies, as well as in acquiring technologies complementary to, or necessary for, our programs. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
We anticipate that we will face intense and increasing competition as new products enter the market and advanced technologies become available. We expect any treatments that we develop and commercialize to compete on the basis of, among other things, efficacy, safety, convenience of administration and delivery, price, the level of competition and the availability of reimbursement from government and other third-party payers.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In addition, we expect that our therapeutic products, if approved, will be priced at a significant premium over competitive products and our ability to compete may be affected in many cases by insurers or other third-party payers seeking to encourage the use of competitive products including biosimilar or generic products.
This increasingly competitive landscape may compromise the development of our product candidates. For example, improvements in the efficacy, delivery and success rates of competitors’ product candidates, in conjunction with a reduction in the price and duration of their treatments, diminished partnering interest from pharmaceutical companies in our product candidate TT-034 for the treatment of HCV. This caused us to announce in February 2016 the discontinuation of our program to develop TT-034 before the conclusion of its clinical trial, despite the promising clinical results regarding the safety of that product candidate achieved to date.
73
Government Regulation
As a pharmaceutical and biological product company that wishes to conduct clinical trials and ultimately obtain marketing approval in the United States, we are subject to extensive regulation by the FDA, and other federal, state, and local regulatory agencies. The Federal Food, Drug, and Cosmetic Act, or the FDC Act, the Public Health Service Act, or PHS Act, and their implementing regulations set forth, among other things, requirements for the research, testing, development, manufacture, quality control, safety, effectiveness, approval, labeling, storage, record keeping, reporting, distribution, import, export, advertising and promotion of our products. A failure to comply explicitly with any requirements during the product development, approval, or post-approval periods, may lead to administrative or judicial sanctions. These sanctions could include the imposition by the FDA or an IRB, of a suspension on clinical trials, refusal to approve pending marketing applications or supplements, withdrawal of approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution.
Although the discussion below focuses on regulation in the United States, we anticipate seeking approval for the testing and marketing of our products in other countries. Generally, our activities in other countries will be subject to regulation that is similar in nature and scope as that imposed in the United States, although there can be important differences. Additionally, some significant aspects of regulation in the European Union are addressed in a centralized way through the EMA, but country-specific regulation remains essential in many respects.
Government regulation may delay or prevent testing or marketing of our products and impose costly procedures upon our activities. The testing and marketing approval process, and the subsequent compliance with appropriate statutes and regulations, requires substantial time, effort, and financial resources, and we cannot be certain that the FDA or any other regulatory agency will grant marketing approvals for our products or any future products on a timely basis, if at all. The FDA's or any other regulatory agency's policies may change and additional governmental regulations may be enacted that could prevent or delay regulatory approval of our products or any future products or approval of new indications or label changes. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative, judicial, or administrative action, either in the United States or abroad.
Recent Developments in Regulation of Gene Therapy
The FDA has provided guidance for the development of gene therapy products. For example, the FDA has established the Office of Tissues and Advanced Therapies (formerly Office of Cellular, Tissue and Gene Therapies) within CBER, to consolidate the review of gene therapy and related products, and the Cellular, Tissue and Gene Therapies Advisory Committee to advise CBER on its reviews. In addition, the FDA has issued a growing body of clinical guidelines, chemical, manufacturing and control, or CMC, guidelines, regenerative medicine guidelines a and other guidelines, all of which are intended to facilitate industry's development of gene therapy products.
In 2016, Section 3033 of the 21st Century Cures Act created a new product category called “regenerative medicine advanced therapy”, or the RMAT designation. The RMAT designation gives the sponsor of a new investigational biologic access to increased meeting opportunities with the FDA, in a manner comparable to those offered to sponsors of therapies designated as “breakthrough therapies” by the FDA. Because the designated products meet the criteria for unmet medical need in the treatment of a serious condition, they may be eligible for priority review, in which the initial assessment of the BLA is reduced from 12 months to eight months, and accelerated approval, which bases approval on an effect on a predictive surrogate endpoint or an intermediate clinical endpoint. RMATs qualifying for such accelerated approval may be able to satisfy licensing requirements through commitment to post-approval clinical studies as well as real-world data such as patient registries and health record analysis. The eligibility of the RMAT-designated product for these expedited programs can be discussed with the FDA at specific development meetings, but we do not know whether any of our current or future product candidates will be eligible for RMAT designation. We believe the increased access to the FDA during early development is a benefit for sponsors, because the typical Type B development meetings are normally restricted to one each at the stages of pre-IND, end of Phase II/pre-Phase III and pre-BLA submission. In addition, the option to qualify for a fast-track program, also based on the potential to serve an unmet medical need in the treatment of a serious condition, allows for a so-called “rolling review” of parts of the BLA, which can be submitted for assessment following agreement of a review timetable with CBER.
74
The FDA plans to include certain gene therapy products that permanently alter tissue and produce a sustained therapeutic benefit as part of the products that will meet the definition of being eligible to come under the pathway enabled by RMAT designation. RMAT designation enables gene therapy products to access the FDA’s existing expedited programs to help foster the development and approval of gene therapy products. Our product candidates may not be eligible for RMAT designation now or in the future.
In May 2016, the EMA approved a second gene therapy product called Strimvelis, the first approved ex vivo stem cell gene therapy, to treat patients with a very rare disease called ADA-SCID (Severe Combined Immunodeficiency due to Adenosine Deaminase deficiency).
In August 2017, the FDA approved the first gene therapy product in the United States. The FDA approved Kymriah (tisagenlecleucel) for the treatment of certain pediatric and young adult patients with a form of acute lymphoblastic leukemia (ALL). Kymriah is a genetically-modified autologous T-cell immunotherapy. Because of the risk of cytokine release syndrome and neurological events, Kymriah is being approved with a REMS. In December 2017, the FDA approved Luxturna (voretigene neparvovec-rzyl), a gene therapy to treat children and adult patients with an inherited form of vision loss that may result in blindness. Luxturna is the first directly administered gene therapy approved in the United States that targets a disease caused by mutations in a specific gene.
Marketing Approval
In the United States, for premarket approval purposes, the FDA regulates gene therapy products as biologics under the FDC Act, the PHS Act and related regulations.
The steps required before a new biologic may be marketed in the United States generally include:
| • | nonclinical pharmacology and toxicology laboratory and animal tests according to good laboratory practices, or GLPs, and applicable requirements for the humane use of laboratory animals or other applicable regulations; |
| • | submission of an IND application which must become effective before human clinical trials may begin; |
| • | adequate and well-controlled human clinical trials according to GCPs and any additional requirements for the protection of human research subjects and their health information to establish the safety and efficacy of the investigational product for each targeted indication; |
| • | submission of a biologics license application, or BLA, to the FDA; |
| • | FDA's pre-approval inspection of manufacturing facilities to assess compliance with cGMPs and, if applicable, the FDA's good tissue practices, or GTPs, for the use of human cellular and tissue products to prevent the introduction, transmission, or spread of communicable diseases; |
| • | FDA's audit of clinical trial sites that generated data in support of the BLA; and |
| • | FDA approval of a BLA, which must occur before a product can be marketed or sold. |
Product Development Process
Before testing any biologic in humans, the product enters the nonclinical, or preclinical, testing stage. Nonclinical tests include laboratory evaluations of product chemistry, toxicity, and formulation, as well as animal studies to assess the potential safety and activity of the product. The conduct of nonclinical tests must comply with federal regulations and requirements including GLPs.
Where a gene therapy trial is conducted at, or sponsored by, institutions receiving NIH funding for recombinant DNA research, prior to the submission of an IND to the FDA, a protocol and related documentation is submitted to and the trial is registered with the NIH Office of Science Policy, or OSP.
75
The product sponsor then submits the results of the nonclinical testing, together with manufacturing information, analytical data, any available clinical data or literature, and a proposed clinical protocol, to the FDA in an IND, which is a request for authorization from the FDA to administer an investigational product to humans. Some nonclinical testing may continue even after the IND application is submitted. IND authorization is required before interstate shipping and administration of any new product to humans that is not the subject of an approved BLA. The IND automatically becomes effective 30 days after receipt by the FDA unless the FDA, within the 30-day time period, raises concerns or questions about the conduct of the clinical trial and places the clinical trial on a clinical hold. In such case, the IND sponsor must resolve any outstanding concerns with the FDA before the clinical trial may begin. Further, an IRB for each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that site. If the site has an IBC, it may also have to review and approve the proposed clinical trial. Clinical trials involve the administration of the investigational product to patients under the supervision of qualified investigators following GCPs, requirements meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, investigators, and monitors. Clinical trials are conducted under protocols that detail, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria, the parameters to be used in monitoring safety, including stopping rules that assure a clinical trial will be stopped if certain adverse events should occur, and the efficacy criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND. The informed written consent of each participating subject is required and the form and content of the informed consent must be approved by each IRB.
The clinical investigation of an investigational product is generally divided into three phases. Although the phases are usually conducted sequentially, they may overlap or be combined in some cases. The three phases of an investigation are as follows:
| • | Phase I includes the initial introduction of an investigational product into humans. Phase I clinical trials may be conducted in patients with the target disease or condition or on healthy volunteers. These studies are designed to evaluate the safety, metabolism, pharmacokinetics and pharmacologic actions of the investigational product in humans, the side effects associated with increasing doses, and if possible, to gain early evidence on effectiveness. During Phase I clinical trials, sufficient information about the investigational product's pharmacokinetics and pharmacological effects may be obtained to permit the design of Phase II clinical trials. The total number of participants included in Phase I clinical trials varies, but is generally in the range of 20 to 80. |
| • | Phase II includes the controlled clinical trials conducted to evaluate the effectiveness of the investigational product for a particular indication(s) in patients with the disease or condition under study, to determine dosage tolerance and optimal dosage, and to identify possible adverse side effects and safety risks associated with the product. Phase II clinical trials are typically well-controlled, closely monitored, and conducted in a limited patient population, usually involving no more than several hundred participants. Phase IIa trials provide information on the impact of dose ranging on safety, biomarkers and proof of concept, while Phase IIb trials are patient dose-ranging efficacy trials. |
| • | Phase III clinical trials are controlled clinical trials conducted in an expanded patient population at geographically dispersed clinical trial sites. They are performed after preliminary evidence suggesting effectiveness of the investigational product has been obtained, and are intended to further evaluate dosage, clinical effectiveness and safety, to establish the overall benefit-risk relationship of the product, and to provide an adequate basis for product approval. Phase III clinical trials usually involve several hundred to several thousand participants. In most cases, the FDA requires two adequate and well controlled Phase III clinical trials to demonstrate the efficacy of the product. FDA may accept a single Phase III trial with other confirmatory evidence in rare instances where the trial is a large multicenter trial demonstrating internal consistency and a statistically very persuasive finding of a clinically meaningful effect on mortality, irreversible morbidity or prevention of a disease with a potentially serious outcome and confirmation of the result in a second trial would be practically or ethically impossible. |
76
Annual progress reports detailing the results of the clinical trials must be submitted to the FDA. Written IND safety reports must be promptly submitted to the FDA and the investigators for serious and unexpected adverse events; any findings from other studies, tests in laboratory animals or in vitro testing that suggest a significant risk for human subjects; or any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. The sponsor must submit an IND safety report within 15 calendar days after the sponsor determines that the information qualifies for reporting. The sponsor also must notify the FDA of any unexpected fatal or life-threatening suspected adverse reaction within seven calendar days after the sponsor's initial receipt of the information. The FDA typically recommends that sponsors observe subjects for potential gene therapy-related delayed adverse events for a 15-year period, including a minimum of five years of annual examinations followed by 10 years of annual queries, either in person or by questionnaire, of trial subjects.
The decision to terminate a clinical trial of an investigational biologic may be made by the FDA or other regulatory authority, an IRB, an IBC, or institutional ethics committee, or by a company for various reasons. The FDA may place a clinical hold and order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial either is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. If the FDA imposes a clinical hold, trials may not recommence without FDA and IRB authorization and then only under terms authorized by the FDA and IRB. In some cases, clinical trials are overseen by an independent group of qualified experts organized by the trial sponsor, or the clinical monitoring board or DSMB. This group provides authorization for whether or not a trial may move forward at designated check points. These decisions are based on the limited access to data from the ongoing trial. The suspension or termination of a clinical trial can occur during any phase of clinical trials if it is determined that the participants or patients are being exposed to an unacceptable health risk. In addition, there are requirements for the registration of ongoing clinical trials of drugs and biologics on public registries and the disclosure of certain information pertaining to the trials as well as clinical trial results after completion.
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, detailed investigational product information is submitted to the FDA in the form of a BLA for a biologic to request marketing approval for the product in specified indications.
Human gene therapy products are a new category of therapeutics. Because this is a relatively new and expanding area of novel therapeutic interventions, there can be no assurance as to the length of the trial period, the number of patients the FDA will require to be enrolled in the trials in order to establish the safety, efficacy, purity and potency of human gene therapy products, or that the data generated in these trials will be acceptable to the FDA to support marketing approval. The NIH and the FDA have a publicly accessible database, the Genetic Modification Clinical Research Information System, which includes information on gene transfer trials and serves as an electronic tool to facilitate the reporting and analysis of adverse events on these trials. Over the last several years the FDA has issued helpful guidance on development of gene therapy products and shown a willingness to work closely with developers, especially with those working in orphan disease areas.
Biologics License Application Approval Process
In order to obtain approval to market a biologic in the United States, a BLA must be submitted to the FDA that provides data from nonclinical studies and clinical trials and manufacturing information establishing to the FDA's satisfaction the safety, purity, and potency or efficacy of the investigational product for the proposed indication. The BLA must be accompanied by a substantial user fee payment unless a waiver or exemption applies.
The FDA will initially review the BLA for completeness before it accepts it for filing. Under the FDA's procedures, the agency has 60 days from its receipt of a BLA to determine whether the application will be accepted for filing based on the agency's threshold determination that the application is sufficiently complete to permit substantive review. After the BLA submission is accepted for filing, the FDA reviews the BLA to determine, among other things, whether the proposed product is safe, pure and potent, which includes determining whether it is effective for its intended use, and whether the product is being manufactured in accordance with cGMP, to assure and preserve the product's identity, safety, strength, quality, potency and purity, and in accordance with biological product standards. The FDA will inspect the facilities at which the product is manufactured to ensure the manufacturing processes and facilities are in compliance with cGMP requirements and are adequate to assure consistent production of the product within required specifications. For a human cellular or tissue product, the FDA
77
also will not approve the product if the manufacturer is not in compliance with the GTPs. These are FDA regulations that govern the methods used in, and the facilities and controls used for, the manufacture of human cells, tissues, and cellular and tissue based products, which are human cells or tissue intended for implantation, transplant, infusion, or transfer into a human recipient. The primary intent of the GTP requirements is to ensure that cell and tissue based products are manufactured in a manner designed to prevent the introduction, transmission, and spread of communicable disease. Additionally, before approving a BLA, the FDA may inspect one or more clinical sites to assure compliance with GCP.
If the FDA determines the application, manufacturing process or manufacturing facilities are not acceptable, it typically will outline the deficiencies and often will request additional testing or information, or corrective action for a manufacturing facility. This may significantly delay further review of the application. If the FDA finds that a clinical site did not conduct the clinical trial in accordance with GCP, the FDA may determine the data generated by the clinical site should be excluded from the primary efficacy analyses provided in the BLA. Additionally, notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
The FDA may refer applications for novel products or products that present difficult questions of safety or efficacy to an advisory committee. The FDA also may determine a REMS is necessary to assure the safe use of the biologic, in which case the BLA sponsor must submit a proposed REMS. The REMS may include, but is not limited to, a Medication Guide, a communications plan, and other elements to assure safe use, such as restrictions on distribution, prescribing, and dispensing.
After the FDA completes its initial review of a BLA, it will either license, or approve, the product, or issue a complete response letter to communicate that it will not approve the BLA in its current form and to inform the sponsor of changes that the sponsor must make or additional clinical, nonclinical or manufacturing data that must be received before the FDA can approve the application, with no implication regarding the ultimate approvability of the application. If a complete response letter is issued, the sponsor may either resubmit the BLA, addressing all of the deficiencies identified in the letter, or withdraw the application.
The testing and approval process for both a drug and biologic requires substantial time, effort and financial resources and this process may take several years to complete. Data obtained from clinical trials is not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals, which could delay or preclude us from marketing our products.
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biological product candidate intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making a drug or biological product available in the United States for this type of disease or condition will be recovered from sales of the product candidate. Orphan product designation must be requested before submitting a BLA. After the FDA grants orphan product designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan product designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product candidate that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug or biological product for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity. Competitors, however, may receive approval of different products for the indication for which the orphan product has exclusivity or obtain approval for the same product but for a different indication than the one for which the orphan product has exclusivity. Orphan product exclusivity also could block the approval of one of our products for seven years if a competitor obtains approval of the same biological product as defined by the FDA or if our product candidate is determined to be contained within the competitor's product for the same indication or disease. If a drug or biological product designated as an orphan product receives marketing approval for an indication broader than what is designated, it may not be entitled to orphan product exclusivity. Orphan drug status in the European Union has similar, but not identical, benefits.
78
Expedited Development and Review Programs
The FDA has a fast track program that is intended to expedite or facilitate the process for reviewing new drugs and biological products that meet certain criteria. Specifically, new drugs and biological products are eligible for fast track designation if they are intended to treat a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for the condition. Fast track designation applies to the combination of the product and the specific indication for which it is being studied. The sponsor of a new biologic or drug may request the FDA to designate the biologic or drug as a fast track product at any time during the clinical development of the product. Unique to a fast track product, the FDA may consider for review sections of the marketing application on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the application, the FDA agrees to accept sections of the application and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the application.
Any product submitted to the FDA for marketing, including under a fast track program, may be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. Any product is eligible for priority review if it would, if approved, be a significant improvement in the safety, effectiveness, treatment, diagnosis or prevention of a disease compared to marketed products. The FDA will attempt to direct additional resources to the evaluation of an application for a new biological or drug product designated for priority review in an effort to reduce the review period from 12 to eight months. Additionally, a product may be eligible for accelerated approval. Biological or drug products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval, which means that they may be approved on the basis of adequate and well-controlled clinical trials establishing that the product has an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit, or on the basis of an effect on an intermediate clinical endpoint. As a condition of approval, the FDA may require that a sponsor of a biological or drug product receiving accelerated approval perform adequate and well-controlled post-marketing clinical trials. In addition, the FDA currently requires as a condition for accelerated approval pre-approval of promotional materials, which could adversely impact the timing of the commercial launch of the product. Lastly, under the provisions of the new Food and Drug Administration Safety and Innovation Act, enacted in 2012, a sponsor can request designation of a product candidate as a "breakthrough therapy." A breakthrough therapy is defined as a drug or biological that is intended, alone or in combination with one or more other drugs or biological, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug or biological may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Drugs and biologicals designated as breakthrough therapies receive the same benefits as drugs and biologicals with Fast Track designation. In addition, the FDA must take certain additional actions, such as intensive guidance on an efficient drug development program (beginning as early as Phase 1), and organizational commitment involving senior managers, intended to expedite the development and review of an application for approval of a breakthrough therapy.
Fast Track designation and breakthrough therapy designation may expedite the product development and approval process, and priority review may expedite the approval process. However, these three paths do not change the standards for approval. Accelerated approval designation changes the standards for product approval and thus may expedite the development and/or approval process.
FDA Additional Requirements
The FDA may require, or companies may pursue, additional clinical trials after a product is approved. These so-called Phase 4 clinical trials may be made a condition to be satisfied for continuing drug and biologic approval. The results of Phase 4 clinical trials can confirm the efficacy of a product candidate and can provide important safety information. In addition, the FDA has expressed statutory authority to require sponsors to conduct post-market studies to specifically address safety issues identified by the agency.
Even if a product candidate receives regulatory approval, the approval may be limited to specific disease states, patient populations and dosages, or might contain significant limitations on use in the form of warnings, precautions or contraindications, or in the form of an onerous REMS, restrictions on distribution, or post-marketing study requirements. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. In addition, we cannot predict what adverse governmental regulations may arise from future U.S. or foreign governmental action.
79
Medical device requirements
Our contemplated diagnostics, for use with certain of our therapeutic products, are regulated by FDA as in vitro diagnostic, or IVD, medical devices. Such IVD devices must comply with applicable FDA IVD-specific regulations as well as FDA regulations applicable more broadly to medical devices. These FDA regulations include requirements for registering establishments with FDA; listing IVD devices with FDA; reporting certain adverse events related to IVD devices to FDA; complying with the Quality System Regulation (current good manufacturing practices for devices); labeling IVD devices; and obtaining premarket approval or clearance prior to marketing IVD devices (unless exempt). There are also regulations covering the requirements for investigational devices and the conduct of clinical investigations of devices. Like drugs and biologics, failure to comply with applicable device/IVD requirements can result in legal or administrative enforcement actions against an IVD device firm, its officers or employees, and/or its products.
FDA Post-Approval Requirements
Any products manufactured or distributed by us or on our behalf pursuant to FDA approvals are subject to continuing regulation by the FDA, including requirements for record-keeping, reporting of adverse experiences with the biologic or drug, and submitting biological product deviation reports to notify the FDA of unanticipated changes in distributed products. Manufacturers are required to register their facilities with the FDA and certain state agencies, and are subject to periodic announced or unannounced inspections by the FDA and certain state agencies for compliance with cGMP requirements, which impose certain quality processes, manufacturing controls and documentation requirements upon us and our third-party manufacturers in order to ensure that the product is safe, has the identity and strength, and meets the quality, purity and potency characteristics that it purports to have. In November 2013, the Drug Quality and Security Act, or DQSA, became law and establishes requirements to facilitate the tracing of prescription drug and biological products through the supply distribution chain. This law includes a number of new requirements that are being implemented over time and require us to devote additional resources to satisfy these requirements, including serializing the product and using new technology and data storage to electronically trace the product from manufacturer to dispenser. If our products are not covered by the serialization and tracing requirements of the DQSA, they may be subject to state pedigree and traceability requirements. We cannot be certain that we or our present or future suppliers will be able to comply with the cGMP and other FDA regulatory requirements. If our present or future suppliers are not able to comply with these requirements, the FDA may halt our clinical trials, refuse to approve any BLA, force us to recall a product from distribution, shut down manufacturing operations or withdraw approval of the applicable BLA. Noncompliance with cGMP or other requirements can result in issuance of warning or untitled letters, civil and criminal penalties, seizures, and injunctive action.
The FDA and other federal and state agencies closely regulate the labeling, marketing and promotion of drugs and biologics. Government regulators, including the Department of Justice and the Office of the Inspector General of the Department of Health and Human Services, as well as state authorities, recently have increased their scrutiny of the promotion and marketing of drugs and biologics. While doctors are free to prescribe any product approved by the FDA for any use, a company can only make claims relating to safety and efficacy of a product that are consistent with FDA approval, and the company is allowed to market a product only for the particular use and treatment approved by the FDA. In addition, any claims we make for our products in advertising or promotion must, among other things, be appropriately balanced with important safety information and otherwise be adequately substantiated. Failure to comply with these requirements can result in adverse publicity, warning or untitled letters, corrective advertising, injunctions, potential civil and criminal penalties, criminal prosecution, and agreements with governmental agencies that materially restrict the manner in which a company promotes or distributes products.
Pediatric Research Equity Act
Under the Pediatric Research Equity Act, or PREA, as amended, a BLA or supplement must contain data to assess the safety and efficacy of the product for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. Manufacturers must submit a pediatric study plan to the IND not later than 60 days after the end-of-phase 2 meeting with the FDA; if there is no such meeting, before the initiation of any phase 3 studies or a combined phase 2 and phase 3 study; or if no such study will be conducted, no later than 210 days before a marketing application or supplement is submitted. The
80
intent of PREA is to compel sponsors whose products have pediatric applicability to study those products in pediatric populations, rather than ignoring pediatric indications for adult indications that could be more economically desirable. The FDA may grant deferrals for submission of data or full or partial waivers. By its terms, PREA does not apply to any product for an indication for which orphan designation has been granted, unless the FDA issues regulations stating otherwise. Because the FDA has not issued any such regulations, submission of a pediatric assessment is not required for an application to market a product for an orphan-designated indication. In a July 2018 guidance, the FDA announced that it does not expect to grant any additional orphan drug designations to drugs for pediatric subpopulations of common diseases (i.e., diseases or conditions with an overall prevalence of 200,000 or greater). Pediatric subpopulation orphan designations that have already been granted will not be affected by this change.
Patent Term Restoration and Marketing Exclusivity
Depending on the timing, duration and specifics of FDA marketing approval of our product candidates, some of our U.S. patents may be eligible for limited patent term extension under the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product's approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of a BLA plus the time between the submission date of a BLA and the approval of that application. Only one patent applicable to an approved biological is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent and within sixty days of approval of the biological product. The USPTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration.
The Biologics Price Competition and Innovation Act of 2009, which was included within the Patient Protection and Affordable Care Act, created an abbreviated approval pathway for biological products shown to be similar to, or interchangeable with, an FDA-licensed reference biological product, and grants a reference biologic twelve years of exclusivity from the time of first licensure. Biosimilarity, which requires that there be no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency, can be shown through analytical studies, animal studies, and a clinical study or studies. Interchangeability requires that a product is biosimilar to the reference product and the product must demonstrate that it can be expected to produce the same clinical results as the reference product and, for products administered multiple times, the biologic and the reference biologic may be switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic. However, complexities associated with the larger, and often more complex, structure of biological products, as well as the process by which such products are manufactured, pose significant hurdles to implementation that are still being worked out by the FDA.
Pediatric exclusivity is another type of exclusivity in the United States. Pediatric exclusivity, if granted, provides an additional six months of exclusivity to be attached to any existing marketing exclusivity, e.g., twelve year exclusivity, or patent protection for a drug. This six month exclusivity, which runs from the end of other exclusivity protection or patent delay, may be granted based on the voluntary completion of a pediatric trial in accordance with an FDA-issued "Written Request" for such a trial.
Government regulation outside the United States
In addition to regulations in the United States, we will be subject to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our products.
Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical trials or marketing of the product in those countries. Certain countries outside of the United States have a similar process that requires the submission of a clinical trial application much like the IND prior to the commencement of human clinical trials. In the European Union, for example, a request for a clinical trial authorization, or CTA, must be submitted to each country's national health authority and an independent ethics committee, much like the FDA and the IRB, respectively. Once the CTA is approved in accordance with a country's requirements, clinical trial development may proceed.
81
The requirements and process governing the conduct of clinical trials, product approval or licensing, pricing and reimbursement vary from country to country. In all cases, the clinical trials are conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
To obtain regulatory approval of a biological product under European Union regulatory systems, we must submit a marketing authorization application. The application required in the European Union is similar to a BLA in the United States, with the exception of, among other things, country-specific document requirements. The European Union also provides opportunities for market exclusivity. For example, in the European Union, upon receiving marketing authorization, a new biological generally receives eight years of data exclusivity and an additional two years of market exclusivity. If granted, data exclusivity prevents regulatory authorities in the European Union from referencing the innovator's data to assess a biosimilar application. During the additional two-year period of market exclusivity, a biosimilar marketing authorization can be submitted, and the innovator's data may be referenced, but no biosimilar product can be marketed until the expiration of the market exclusivity. The innovator may obtain an additional one year of market exclusivity if the innovator obtains an additional authorization during the initial eight year period for one or more new indications that demonstrate significant clinical benefit over existing therapies. This data and market exclusivity regime in the European Union of a total of 10 or 11 years protects against generic competition, but does not protect against the launch of a competing product if the competitor, rather than referencing the clinical data of the originator, has conducted its own clinical trials to support its marketing authorization application.
Orphan drugs in the European Union are eligible for 10-year market exclusivity. This 10-year market exclusivity may be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan designation, for example, if the product is sufficiently profitable not to justify maintenance of market exclusivity. Additionally, marketing authorization may be granted to a similar product for the same indication at any time if:
| • | the second applicant can establish that its product, although similar, is safer, more effective or otherwise clinically superior; |
| • | the applicant consents to a second orphan medicinal product application; or |
| • | the applicant cannot supply enough orphan medicinal product. |
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Pharmaceutical Coverage, Pricing and Reimbursement
Sales of our products, when and if approved for marketing, will depend, in part, on the extent to which our products will be covered by third-party payers, such as federal, state, and foreign government healthcare programs, commercial insurance and managed healthcare organizations. These third-party payers are increasingly reducing reimbursements for medical products, biologicals, drugs and services. In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of interchangeable products. Adoption of price controls and cost containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. Decreases in third-party reimbursement for our product candidates or a decision by a third-party payer not to cover our product candidates could reduce physician usage of our products once approved and have a material adverse effect on our sales, results of operations and financial condition.
The containment of healthcare costs has become a priority of federal, state and foreign governments. Third-party payers are increasingly challenging the prices charged for drug products and medical services, examining the medical necessity and reviewing the cost effectiveness of drug products and medical services, in addition to questioning safety and efficacy. If these third-party payers do not consider our products to be cost-effective compared to other available therapies, they may not cover our products after FDA approval or, if they do, the level of payment may not be sufficient to allow us to sell our products at a profit.
82
In the United States and some foreign jurisdictions, there have been, and likely will continue to be, a number of legislative and regulatory changes and proposed changes regarding the healthcare system directed at broadening the availability of healthcare and containing or lowering the cost of healthcare. In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care Education Reconciliation Act, or the ACA, was enacted. The ACA includes measures that have significantly changed, and are expected to continue to significantly change, the way healthcare is financed by both governmental and private insurers. Other legislative changes have been proposed and adopted in the United States since the ACA was enacted. In August 2011, the Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation's automatic reduction to several government programs. This includes aggregate reductions of Medicare payments to providers up to 2% per fiscal year, which went into effect in April 2013 and will remain in effect through 2024 unless additional Congressional action is taken. In January 2013, former President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
Other Healthcare Laws
Although we currently do not have any products on the market, we may be subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states and other countries in which we conduct our business. Such laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, privacy and security and physician sunshine and open payment laws and regulations, many of which may become more applicable to us if our product candidates are approved and we begin commercialization. If our operations are found to be in violation of any of such laws or any other governmental regulations that apply to us, we may be subject to penalties, including, without limitation, administrative, civil and criminal penalties, damages, fines, disgorgement, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs and imprisonment, any of which could adversely affect our ability to operate our business and our financial results.
C. | Organizational Structure. |
We have four significant subsidiaries:
Name | | Country of Incorporation | | Percentage Owned | |
Benitec Limited | | U.K. | | | 100 | % |
Benitec, Inc. | | Delaware, U.S. | | | 100 | % |
Tacere Therapeutics, Inc. | | Delaware, U.S. | | | 100 | % |
Benitec Australia Limited | | Australia | | | 100 | % |
D. | Property, Plant and Equipment. |
Facilities
Our corporate headquarters are located in Melbourne. Our research and development facility is located in Hayward, California, and consists of approximately 7,295 square feet of leased office space under a lease that expires in June 2022.
We believe that our existing facilities are adequate for our current needs.
Item 4A. Unresolved Staff Comments
Not applicable.
83
Item 5. Operating and Financial Review and Prospects
As at June 30, 2019, the Company had cash on hand of A$22.4 million. This was an increase of A$6.3million from June 30, 2018. This represents payments to suppliers of A$16.1 million offset by receipts from Axovant of A$2.6 million and grant income of A$4.1million, revenue and other income of A$15.2 million, purchase of plant and equipment of A$0.6 million, and foreign exchange gain of A$1.0 million.
Review of Operations
Benitec Biopharma is a clinical-stage biotechnology company focused on the development of novel genetic medicines. The proprietary platform, called DNA-directed RNA interference, or ddRNAi, combines RNA interference, or RNAi, with gene therapy to create medicines that facilitate sustained silencing of disease-causing genes following a single administration. Benitec endeavours to develop and commercialise BB-301 for the treatment of Oculopharyngeal Muscular Dystrophy, or OPMD.
The ddRNAi-based genetic medicine currently under development by Benitec (BB-301) represents a proprietary product candidate that can, potentially, be used to meaningfully improve upon the existing standard of care for a rare, chronic, life-threatening form of muscular dystrophy. In the past, the research and development efforts of the Company have been directed towards disorders that include head and neck squamous cell carcinoma, or HNSCC, OPMD, wet age-related macular degeneration, or AMD, and chronic hepatitis B or HBV. Through the combination of the targeted gene silencing effect of RNAi together with the durable gene expression associated with the use of modified viral vectors, ddRNAi has the potential to produce durable silencing of disease-causing genes following a single administration of the proprietary genetic medicine. This novel attribute of the investigational agent that is being advanced through nonclinical development could facilitate the achievement of robust clinical activity while greatly reducing the dosing frequencies traditionally expected for medicines employed for the management of chronic diseases. Additionally, the establishment of chronic gene silencing via ddRNAi-based genetic medicines could significantly reduce the risk of patient non-compliance during the course of medical management of potentially fatal clinical disorders.
Axovant Termination
On June 6, 2019 the termination of the License and Collaboration Agreement with Axovant Sciences was announced. The Benitec team endeavor to conduct several additional exploratory analyses of BB-301 prior to the initiation of a clinical study in order to potentially improve the biological efficacy of the compound via further optimization of the proprietary delivery method employed to dose the target tissues.
Preclinical data derived from recently concluded in vivo evaluations of BB-301 in two distinct large animal species suggest that the opportunity exists to further improve the biological efficacy of the compound via additional optimization of the proprietary delivery method employed to dose key target tissues that underlie the morbidity and mortality associated with the progression of OPMD. The initial biological efficacy profile observed for BB-301 following in vivo testing in the A17 mouse model of OPMD, including full correction of the disease phenotype, remains unchanged. However, the Benitec team plans to conduct several additional exploratory analyses prior to the initiation of clinical testing.
Completion of the experimental work noted above will delay the initiation of the BB-301 clinical study beyond the timelines that were initially outlined by Axovant Sciences following the execution of the License and Collaboration Agreement between Benitec Biopharma and Axovant Sciences. As such, the License and Collaboration Agreement between Benitec Biopharma and Axovant Sciences was terminated, and all rights and licenses granted to Axovant Sciences will cease, including the rights to BB-301, which was in preclinical development for the treatment of OPMD, and all other early stage research collaboration programs.
The termination of the License and Collaboration Agreement was provided to Benitec on June 6, 2019 and is effective as at September 3, 2019.
84
Preclinical Programs
Preclinical research efforts supporting the development of proprietary ddRNAi-based therapeutics targeted towards the treatment of HBV and AMD have concluded.
Workforce Reduction
On July 31, 2019 Benitec announced the completion of a workforce reduction of approximately 50%. Through this streamlining of operations, the Company retained staff members who are key to the achievement of the core research and development goals. The rationalization of resources will result in an extended financial runway for
the Company while allowing Benitec to continue to advance the BB-301 program through development.
Financial operations overview
Revenue & other income
To date, we have derived revenues and other income from licensing fees and interest income. We have not generated any revenues from the sales of products. Revenues from licensing fees and interest income are included in the revenue line item on our statements of profit or loss. The Research and Development Tax Incentive is recognized as other income.
Our licensing fees have been generated through the licensing of our ddRNAi technology to biopharmaceutical companies.
Our grant income is generated through the Australian federal government's Research and Development Tax Incentive program, under which the government provides a cash refund for the 43.5% (2018:43.5%) of eligible research and development expenditures, including salaries, by small Australian entities having a tax loss. For this purpose, small Australian entities are defined as those with less than A$20 million in revenue. This grant is available for our research and development activities in Australia, as well as activities in the United States to the extent such U.S.-based expenses relate to our activities in Australia, do not exceed half the expenses for the relevant activities and are approved by the Australian government. Prior to June 30, 2017 the grant income was only taken up on the lodgement of the previous year’s tax return, which was the time at which it was considered a reliable estimate could be made. In determining the estimate management reviews historical claims, Government overseas findings enabling the claim of overseas expenditure and the allocation of staff and overheads costs within approved projects. To the extent our research and development expenditures are deemed to be "ineligible," then our grants would decrease. In addition, the Australian government may in the future decide to modify the requirements of, reduce the amounts of the grants available under, or discontinue the Research and Development Tax Incentive program. Previously, we have been awarded research and development grants from the Australian federal government, totaling A$3.6 million in fiscal 2016, A$10.5 million in fiscal 2017, A$4.0 million in fiscal 2019 and A$0.9 million we expect to receive in in fiscal 2020. Any such change in the Research and Development Tax Incentive program could have a material adverse effect on our future cash flows and financial position.
We also record interest and other financial income earned from bank accounts, term deposits and short-term investments as other revenues in our statements of profit or loss.
Employment related costs
Employment related costs include salaries for all our employees and related benefits, including the grant of share options, which are valued and included in the statements of profit or loss and other comprehensive income as share based expenses.
85
Impairment
We assess at the end of each fiscal year and half year whether there is an indication that an asset may be impaired. If any such indication exists, or when annual impairment testing is required for an asset, such as goodwill, intangible assets with indefinite useful lives and intangible assets not yet available for use, we make an estimate of the asset's recoverable amount. An asset's recoverable amount is the higher of its fair value less costs to sell and its value in use and is determined for an individual asset, unless the asset does not generate cash inflows that are largely independent of those from other assets or groups of assets and the asset's value in use cannot be estimated to be close to its fair value. In such cases, the asset is tested for impairment as part of the cash generating unit to which it belongs. When the carrying amount of an asset or cash-generating unit exceeds its recoverable amount, the asset or cash-generating unit is considered impaired and is written down to its recoverable amount. No impairment was recorded in fiscal years 2019, 2018 or 2017. In fiscal 2016, we recorded an impairment of A$1.8 million in relation to the write-off of a clinical trial prepayment that had originally been made for a non-small cell lung cancer program. The non-small cell lung cancer program was cancelled in 2016.
In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset. Impairment losses relating to continuing operations are recognized in those expense categories consistent with the function of the impaired asset.
Royalties and license fees
We pay royalties and license fees in connection with our licensing of intellectual property from third parties. In December 2016, we entered into an exclusive sublicence agreement with NantWorks, pursuant to which we have agreed to develop and commercialize an antisense RNA product candidate for HNSCC, which we call BB-401. We are required to make periodic, milestone and royalty payments to Nant Capital pursuant to the exclusive sublicense agreement in connection with the development and sale of BB-401.
In August 2009, we entered into a collaborative agreement with Biomics Biotech Co., Ltd. pursuant to which we agreed to share any revenue generated from commercializing our jointly filed patents which relate to single-stranded RNA and shRNA sequences for treatment of hepatitis B. In July 2015, we entered into an earn-out agreement with Biomics pursuant to which we acquired all rights, title and interest in these patents in exchange for upfront and milestone payments. At the time of signing the agreement, we paid Biomics A$2.5 million consisting of A$2.0 million in cash and 647,333 ordinary shares (having a value of A$500,000 at the time the agreement was entered into). These shares could not be traded until October 1, 2015 and thereafter Biomics may only sell up to A$100,000 in value of those shares in any calendar month.
Foreign exchange translation
The foreign currency translation reserve represents the currency translation movements of subsidiary company balances denominated in foreign currencies at year end. Foreign currency monetary items are translated at the year-end exchange rate. Non-monetary items measured at historical cost continue to be carried at the exchange rate at the date of the transaction. Non-monetary items measured at fair value are reported at the exchange rate at the date when fair values were determined. Movements in the foreign currency translation reserve are shown in our Statement of Profit or Loss and Other Comprehensive Income.
Foreign currency transactions are translated into functional currency using the exchange rates prevailing at the date of the transactions. Exchange rate differences are recognized in the Statement of Profit or Loss and Other Comprehensive Income.
Critical Accounting Policies and Estimates
The preparation of our financial statements requires us to make estimates and judgments that can affect the reported amounts of assets, liabilities, revenues and expenses, as well as the disclosure of contingent assets and liabilities at the date of our financial statements. We analyze our estimates and judgments and we base our estimates and judgments on historical experience and various other assumptions that we believe to be reasonable under the
86
circumstances. Actual results may vary from our estimates. Our significant accounting policies are detailed in Note 1 to our consolidated financial statements for the fiscal year ended June 30, 2019, appearing elsewhere in this report. We have summarized below the accounting policies of particular importance to the portrayal of our financial position and results of operations and that require the application of significant judgment or estimates by our management.
Research and development expenses
Management does not consider the development programs to be sufficiently advanced to reliably determine the economic benefits and technical feasibility to justify capitalization of development costs. These costs have been recognized as an expense when incurred. Research and development expenses relate primarily to the cost of conducting clinical and pre-clinical trials. Clinical development costs are a significant component of research and development expenses. Estimates have been used in determining the expense liability under certain clinical trial contracts where services have been performed but not yet invoiced. Generally, the costs, and therefore estimates, associated with clinical trial contracts are based on the number of patients, drug administration cycles, the type of treatment and the outcome being the length of time before actual amounts can be determined will vary depending on length of the patient cycles and the timing of the invoices by the clinical trial partners.
Research and development refundable tax offsets
The Company accounts for the federal government research and development grant tax incentive when a reliable estimate of the amounts receivable can be made. In the year ended June 30, 2017 reporting period detailed reporting systems were implemented to allow for the first time a reliable estimate to be made of the grant income that is expected to be received for the current period. In determining the estimate management reviews historical claims, Government overseas findings enabling the claim of overseas expenditure and the allocation of staff and overheads costs within approved projects. Judgement is also applied in determining the eligibility of the activities undertaken in Australia and overseas. Other income for the year ended June 30, 2019 includes an estimate of Research and Development grant receivable for June 30, 2019 of A$0.9 million.
Share-based payment transactions
The Company measures the cost of equity-settled transactions with employees by reference to the fair value of the equity instruments at the date at which they are granted. The fair value is determined by using the Black-Scholes model taking into account the terms and conditions upon which the instruments were granted. The accounting estimates and assumptions relating to equity-settled share-based payments would have no impact on the carrying amounts of assets and liabilities within the next annual reporting period but may impact profit or loss and equity.
Recovery of deferred tax assets
Deferred tax assets are recognized for deductible temporary differences only if the Company considers it is probable that future taxable amounts will be available to utilise those temporary differences and losses. Given the Company’s and each individual entities’ history of recent losses, the Company has not recognized a deferred tax asset with regard to unused tax losses and other temporary differences, as it has not been determined whether the Company or its subsidiaries will generate sufficient taxable income against which the unused tax losses and other temporary differences can be utilized. The Company applies judgments in determining whether the tests for utilisation of carry forward tax losses are satisfied in a year where taxable income is generated. This involves management considering the Continuity of Ownership and/or Similar Business tests.
Results of Operations
The following discussion relates to our consolidated results of operations, financial condition and capital resources. You should read this discussion in conjunction with our consolidated financial statements and the notes thereto contained elsewhere in this report.
87
Comparison of the fiscal years ended June 30, 2019 and 2018
| | For the fiscal year ended June 30, | | | Increase | |
(in thousands) | | 2019 | | | 2018 | | | (Decrease) | |
Revenue: | | | | | | | | | | | | | | | |
Licensing revenue and royalties | | A$ | | 14,627 | | | A$ | | 378 | | | A$ | | 14,249 | |
Service revenue | | | | 1,532 | | | | | — | | | | | 1,532 | |
| | | | | | | | | | | | | | | |
Other income: | | | | | | | | | | | | | | | |
Australian Government Research and Development grants | | | | 907 | | | | | 3,999 | | | | | (3,092 | ) |
Foreign exchange unrealized gain | | | | 443 | | | | | 87 | | | | | 356 | |
Other | | | | — | | | | | 1 | | | | | (1 | ) |
| | | | | | | | | | | | | | | |
Total revenue and other income | | A$ | | 17,509 | | | A$ | | 4,465 | | | | | 13,044 | |
Licensing revenue and royalties increased by A$14.2 million, from A$0.4 million in fiscal 2018 to A$14.6 million in fiscal 2019, primarily due to the license agreement with Axovant, which resulted in upfront license payment of A$14.2 million.
Service revenue consist of payments for services provided to Axovant during the license agreement signed in July 2018. The License agreement was terminated in September 2019.
Other income, consisting of our Research and Development refundable tax offset from the Australian Government, decreased by A$3.1 million, from A$4 million in fiscal 2018 to A$0.9 million in fiscal 2019. This decrease is primarily due to excluding the OPMD program from the R&D claim. It is noted that grant income recognized in the current period, will be received subsequent to the claim being made, on lodgement, of the June 2019 income tax return. See Note 2 and Note 4 of our June 30, 2019 financial statements included in this Annual Report on Form 20-F for more detailed information on research and development incentives.
Expenses
Research and development expense. Research and development expense decreased by A$3.8 million from A$6.9 million in fiscal 2018 to A$3.1 million in fiscal 2019 due to the reimbursement of costs from Axovant relating to the OPMD program and the termination of the AMD program.
Employment related expenses. Employment-related expenses decreased by A$0.1 million in fiscal 2019 compared to fiscal 2018 primarily due to payments related to our management restructuring.
Share based expenses. Share based expenses increased by A$0.5 million from A$0.4 million in fiscal 2018 to A$0.9 million in fiscal 2019, due to new options issued to employees. Share based expenses are calculated using a Black-Scholes model. The share based expense model uses a data set that includes share price and exercise price, exercise probability, volatility, exercise time and interest rates. We recognize share based expenses over the service period in which the employee earns the award, which is the vesting period of the award.
Travel related costs. Travel related costs decreased by A$0.1 million, from A$0.5 million in fiscal 2018 to A$0.4 million in fiscal 2019, primarily due to a decrease in overseas business trips.
Consultants costs. Consultants costs decreased by A$0.1 million from A$0.8million in fiscal 2018 to A$0.7 million in fiscal 2019 as a result of more administrative tasks being performed in house, such as the Research and Development returns. We retained specialist advisers in relation to our key product candidate programs and for media and shareholder relations capabilities.
Occupancy costs. Occupancy costs remained relatively unchanged at approximately A$0.6 million in fiscal 2019, as a result of largely unchanged lease liabilities in California and Australia.
88
Corporate expenses. Corporate expenses increased by A$0.5 million from A$1.4 million in fiscal 2018 to A$1.9 million in fiscal 2019 primarily due to increase in legal fees.
Result for the period
As a result of the foregoing, our profit for the period after income tax benefit was A$4.1 million compared to a loss of A$11.6 million in fiscal 2018. The change in profit is predominantly due to an increase in revenue of A$15.8 million, which includes the upfront license payment of A$14.2 million as well as payment for services provided to Axovant totaling A$1.5 million.
Given our and our subsidiaries’ history of losses, we have not recognized a deferred tax asset with regard to unused tax losses and other temporary differences, as it has not been determined whether we or our subsidiaries will generate sufficient taxable income against which the unused tax losses and other temporary differences can be utilized.
Comparison of the fiscal years ended June 30, 2018 and 2017
Revenue
| | For the fiscal year ended June 30, | | | Increase | |
(in thousands) | | 2018 | | | 2017 | | | (Decrease) | |
Revenue: | | | | | | | | | | | | | | |
Licensing revenue and royalties | | A$ | | 378 | | | A$ | | 333 | | | | 45 | |
| | | | | | | | | | | | | | |
Other income: | | | | | | | | | | | | | | |
Australian Government Research and Development refundable tax offset | | | | 3,999 | | | | | 10,507 | | | | (6,508 | ) |
Foreign exchange unrealized gain | | | | 87 | | | | | — | | | | 87 | |
Other | | | | 1 | | | | | — | | | | 1 | |
| | | | | | | | | | | | | | |
Total revenue and other income | | A$ | | 4,465 | | | A$ | | 10,840 | | | | (6,375 | ) |
Licensing revenue and royalties increased by A$0.05 million, from A$0.33 million in fiscal 2017 to A$0.38 million in fiscal 2018, primarily due to timing differences in the recognition of such revenue.
Other income, consisting of our Research and Development refundable tax offset from the Australian Government, decreased by A$6.5 million, from A$10.5 million in fiscal 2017 to A$4 million in fiscal 2018. This decrease is primarily due to the inclusion of an estimation of the grant income for the twelve months ended June 30, 2018, of A$4 million, whilst in the previous corresponding period we included grant income of A$6 million for the fiscal year 2016 and an estimation for twelve months to June 30, 2017, of A$4 million. In 2017 a new reporting system was implemented to allow a reliable estimate to be made of the grant income. As a result, an estimation of grant income for each quarter is now taken to account on a quarterly basis. Previously the grant income was only taken up on the lodgement of the previous year’s tax return, which was the time at which it was considered a reliable estimate could be made. It is noted that grant income recognized in the current period, will be received subsequent to the claim being made, on lodgement, of the June 2018 income tax return. See Note 2 and Note 4 of our June 30, 2018 financial statements included in this Annual Report on Form 20-F for more detailed information on research and development incentives.
Expenses
Research and development expense. Research and development expense remained at A$6.9 million in fiscal 2018, as the increase in costs of the OPMD and the HNSCC programs was offset by a decrease in costs of the programs related to HVB, HCV and AMD.
89
Employment related expenses. Employment-related expenses increased by A$0.1 million in fiscal 2018 compared to fiscal 2017 primarily due to payments related to our management restructuring.
Share based expenses. Share based expenses slightly increased by A$48 thousand from A$386 thousand to A$434 thousand in fiscal 2017 to A$0.4 million in fiscal 2018, largely due to new options issued to employees. Share based expenses are calculated using a Black-Scholes model. The share based expense model uses a data set that includes share price and exercise price, exercise probability, volatility, exercise time and interest rates. We recognize share based expenses over the service period in which the employee earns the award, which is the vesting period of the award.
Travel related costs. Travel related costs decreased by A$0.2 million, from A$0.6 million in fiscal 2017 to A$0.5 million in fiscal 2018, primarily due to a decrease in overseas business trips.
Consultants costs. Consultants costs decreased by A$0.2 million from A$1.0 million in fiscal 2017 to A$0.8 million in fiscal 2018 as a result of more administrative tasks being performed in house, such as the Research and Development returns. We retained specialist advisers in relation to our key product candidate programs and for media and shareholder relations capabilities.
Occupancy costs. Occupancy costs remained relatively unchanged at approximately A$0.6 million in fiscal 2018, as a result of largely unchanged lease liabilities in California and Australia.
Corporate expenses. Corporate expenses decreased by A$0.2 million from A$1.5 million in fiscal 2017 to A$1.4 million in fiscal 2018 primarily due to reduction in legal fees.
Loss for the period
As a result of the foregoing, our loss for the period after income tax benefit increased by A$6.0 million, from A$5.7 million in fiscal 2017 to A$11.6 million in fiscal 2018.
Given our and our subsidiaries’ history of recent losses, we have not recognized a deferred tax asset with regard to unused tax losses and other temporary differences, as it has not been determined whether we or our subsidiaries will generate sufficient taxable income against which the unused tax losses and other temporary differences can be utilized.
B. | Liquidity and Capital Resources. |
We have incurred cumulative losses and negative cash flows from operations since our inception in 1995, except for the current period where we had a profit of A$4.1 million and, as of June 30, 2019, we had accumulated losses of A$141.3 million. We expect that our research and development and general and administrative expenses will proceed at lower rate compare to previous year due to staff rationalisation and the single focus on OPMD program due to the loss of the Axovant contract.
We had no borrowings in fiscal 2019, fiscal 2018 or fiscal 2017 and do not currently have a credit facility.
As of June 30, 2019, we had cash and cash equivalents of A$22.4 million. Cash in excess of immediate requirements is invested in accordance with our investment policy, primarily with a view to liquidity and capital preservation. Currently, our cash and cash equivalents are held in bank accounts. Our short-term investments consist of term deposits with maturity within 180 days.
90
Cash flows
The following table sets forth the sources and uses of cash for each of the periods set forth below:
| | For the fiscal year ended June 30, | |
(in thousands) | | 2019 | | | 2018 | | | 2017 | |
Net cash provided by (used in) operating activities | | A$ | | 5,857 | | | A$ | | (9,794 | ) | | A$ | | (8,304 | ) |
Net cash provided by (used in) investing activities | | | | (570 | ) | | | | (147 | ) | | | | (302 | ) |
Net cash provided by financing activities | | | | — | | | | | 8,507 | | | | | 7,939 | |
Operating activities. Net cash provided by operating activities for fiscal 2019 was A$5.9 million. Net cash used in operating activities for fiscal 2018 and 2017 was A$9.8 million and A$8.3 million, respectively. The use of net cash in all periods is primarily related to payments to suppliers and employees.
Investing activities. Net cash used in investing activities in fiscal 2019, 2018 and 2017 was A$0.6 million, A$0.1 million and A$0.3 million, respectively, and primarily related to purchases of equipment and, for fiscal 2018, a clinical trial deposit.
Financing activities. Net cash provided by financing activities was nil in fiscal 2019, A$8.5 million and A$7.9 million for fiscal 2018 and 2017 respectively. All such cash from financing activities related to the issuance of ordinary shares, including A$8.8 million in gross proceeds from a private placement and entitlement offer in fiscal 2018, A$8.0 million in gross proceeds from private placements in fiscal 2017, partially offset by share issue transaction costs.
Operating capital requirements
To date, our sources of liquidity have been licensing revenue and royalties, Australian government research and development grants, interest on invested cash in excess of immediate requirements and proceeds of the issuance of equity securities.
In the future, we expect our revenue stream will be generated mostly from licensing, strategic alliances and collaboration arrangements with pharmaceutical companies. While we continue to progress discussions and advance opportunities to engage with pharmaceutical companies and continue to seek licensing partners for ddRNAi in disease areas that are not our focus, there can be no assurance as to whether we will enter into such arrangements or what the terms of any such arrangement could be.
While we have established some licensing arrangements, we do not have any products approved for sale and have not generated any revenue from product sales. We do not know when, or if, we will generate any revenue from product sales. We do not expect to generate significant revenue from product sales unless and until we obtain regulatory approval of and commercialize one of our current or future product candidates.
Unless and until we establish significant revenues from licensing programs, strategic alliances or collaboration arrangements with pharmaceutical companies, or from product sales, we anticipate that we will continue to generate losses for the foreseeable future, and we expect the losses to increase as we continue the development of product candidates and begin to prepare to commercialize any product that receives regulatory approval. We are subject to the risks inherent in the development of new gene therapy products, and we may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business.
We expect that our cash and cash equivalents will be sufficient to support preclinical and Phase I clinical studies for BB-301 and the OPMD program until approximately the fourth quarter of calendar 2021.
91
We have based our projections of operating capital requirements on assumptions that may prove to be incorrect and we may use all of our available capital resources sooner than we expect. Because of the numerous risks and uncertainties associated with research, development and commercialization of pharmaceutical products, we are unable to estimate the exact amount of our operating capital requirements. Our future funding requirements will depend on many factors, including, but not limited to:
| • | the timing and costs of our planned clinical trials for our ddRNAi product candidates; |
| • | the timing and costs of our planned preclinical studies for our ddRNAi product candidates; |
| • | the number and characteristics of product candidates that we pursue; |
| • | the outcome, timing and costs of seeking regulatory approvals; |
| • | revenue received from commercial sales of any of our product candidates that may receive regulatory approval; |
| • | the terms and timing of any future collaborations, licensing, consulting or other arrangements that we may establish; |
| • | the amount and timing of any payments we may be required to make, or that we may receive, in connection with the licensing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights; |
| • | the costs of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights and defending against intellectual property related claims; and |
| • | the extent to which we need to in-license or acquire other products and technologies. |
C. | Research and Development, Patents and Licenses, etc. |
Research and development expenses consist primarily of costs incurred for the development of our product candidates, which include:
| • | expenses incurred under agreements with academic research centers, clinical research organizations and investigative sites that conduct our clinical trials; and |
| • | the cost of acquiring, developing, and manufacturing clinical trial materials. |
Research and development expenses do not include employment related expenses, which are included in our Statement of Profit or Loss and Other Comprehensive Income as a separate line item.
Research and development costs are expensed as incurred. Costs for certain development activities are recognized based on an evaluation of the progress to completion of specific tasks using information and data provided to us by our vendors and our clinical sites.
We cannot determine with certainty the duration and completion costs of the current or future product development, preclinical studies or clinical trials of our product candidates. The duration, costs, and timing of clinical trials and development of our product candidates will depend on a variety of factors, including:
| • | the scope, rate of progress, and expense of our ongoing as well as any additional clinical trials and other research and development activities; |
| • | the countries in which trials are conducted; |
| • | future clinical trial results; |
| • | our relationship with collaborators; |
| • | uncertainties in clinical trial enrollment rates or drop-out or discontinuation rates of patients; |
| • | potential additional safety monitoring or other studies requested by regulatory agencies; |
92
| • | significant and changing government regulation; and |
| • | the timing and receipt of any regulatory approvals. |
A change in the outcome of any of these variables with respect to the development of a product candidate could mean a significant change in the costs and timing associated with the development of that product candidate. For example, if the FDA, or another regulatory authority were to require us to conduct clinical trials beyond those that we anticipate will be required to complete clinical development of a product candidate or if we experience significant delays in enrollment in any of our clinical trials, we could be required to expend significant additional financial resources and time on the completion of clinical development.
Our research and development expenses, categorized by product candidate or program, in fiscal 2019, fiscal 2018 and fiscal 2017 were as follows:
| | For the year ended June 30, | |
Product candidate or program | | 2019 | | | 2018 | | | 2017 | |
TT-034 for the treatment of HCV | | A$ | | 5,918 | | | A$ | | 85,128 | | | A$ | | 1,066,875 | |
BB-103 for the treatment of HBV | | | | — | | | | | 221,055 | | | | | 1,353,351 | |
BB-201 for the treatment of AMD | | | | 88,225 | | | | | 709,495 | | | | | 2,049,897 | |
BB-301 for treatment of OPMD | | | | 396,393 | | | | | 2,814,783 | | | | | 595,060 | |
BB-401 and BB-501 for the treatment of HNSCC* | | | | 1,005,696 | | | | | 1,832,261 | | | | | 518,438 | |
New technologies | | | | 1,104 | | | | | 20,304 | | | | | 74,915 | |
Other project related research and development costs (includes insurance, legal, IP and lab equipment costs) | | | | 1,606,861 | | | | | 1,207,283 | | | | | 1,266,834 | |
Total | | A$ | | 3,104,197 | | | A$ | | 6,890,309 | | | A$ | | 6,925,370 | |
* | The Company has terminated the clinical development of BB-401 along with the discovery stage programs directed at the engineering of follow-on anti EGFR strategies (BB-501) in second half of fiscal 2019. |
Our objective is to become the leader in discovering, developing, clinically validating and commercializing ddRNAi-based therapeutics for a range of human diseases with high unmet clinical need or large patient populations, and to thereby provide a better life for patients with these diseases. Our strategy to accomplish this goal is to progress our BB-301 OPMD program based on our proprietary ddRNAi-based therapeutics, continue our leadership position in ddRNAi-based therapeutics, develop drugs in our core disease area, partner selectively to commercialize and expand our pipeline and pursue indications with high unmet medical need or a large patient population.
The scientific research that forms the basis of our efforts to develop product candidates is based on the therapeutic use of ddRNAi, and the identification, optimization and delivery of ddRNAi-based product candidates is relatively new. The scientific evidence to support the feasibility of successfully developing therapeutic treatments based on ddRNAi is preliminary and limited. There can be no assurance that any development and technical problems we experience in the future will not cause significant delays or unanticipated costs, or that such development problems can be solved.
We are currently working to advance our product candidates through preclinical proof of concept studies and IND-enabling studies. Based on cash requirements and financing, we plan to continue to advance our product candidates through to submission of an IND application and potentially completion of clinical proof of concept.
E. | Off-Balance Sheet Arrangements. |
We did not have over the past three fiscal years, and we currently do not have, any off-balance sheet arrangements as defined in the rules and regulations of the Securities and Exchange Commission.
93
F. | Tabular Disclosure of Contractual Obligations. |
The following table summarizes our contractual obligations as of June 30, 2019:
| | Payments due by period (A$ thousand) | |
| | Total | | | Less than 1 year | | | 1-3 years | | | 3-5 years | | | More than 5 years | |
Operating lease obligations | | A$ | | 920 | | | A$ | | 289 | | | A$ | | 631 | | | A$ | | — | | | A$ | | — | |
Total | | A$ | | 920 | | | A$ | | 289 | | | A$ | | 631 | | | A$ | | — | | | A$ | | — | |
Item 6. Directors, Senior Management and Employees
A. | Directors and Senior Management. |
The following table sets forth information covering our current directors and executive officers.
Name | Position |
Jerel Banks | Chief Executive Officer and Executive Chairman of the Board of Directors |
Megan Boston | Executive Director and Head of Operations Australia |
J. Kevin Buchi | Non-Executive Director |
Peter Francis | Non-Executive Director |
Dr. Jerel Banks has been a Director since October 2016, Chairman of our Board since October 2017 and Chief Executive Officer since June 2018. Dr. Banks was formerly the Chief Investment Officer of Nant Capital, LLC. Prior to joining Nant Capital, LLC, Dr. Banks served as vice president, portfolio manager and research analyst for the Franklin Biotechnology Discovery Fund at Franklin Templeton Investments from 2012 to 2015. Prior to his tenure at Franklin Templeton Investments, he worked as a biotechnology senior equity research analyst at Sectoral Asset Management from 2011 to 2012. From 2008 to 2011, Dr. Banks worked as a biotechnology equity research analyst at Apothecary Capital, the healthcare investment management team for the family investment office of the Bass Family of Fort Worth, Texas. Dr. Banks began his career in investment management as a healthcare equity research associate at Capital Research Company where he was a member of the equity research team from 2006 to 2008. Dr. Banks earned an M.D. from the Brown University School of Medicine and a Ph.D. in Organic Chemistry from Brown University, and he holds an A.B. in Chemistry from Princeton University.
Megan Boston has previously been Chief Executive Officer and Managing Director of several ASX listed entities. With over 13 years of experience, Ms. Boston has been a director across a range of industries where she chaired company boards as well as board sub-committees particularly in the area of finance and risk management. Specifically, Ms. Boston has been a Director of Benitec since August 2016 and Executive Director and Head of Operations Australia since June 2018. Previously, Ms. Boston held senior executive roles at various banking institutions in the area of risk and compliance, as well as working for PricewaterhouseCoopers. Ms. Boston holds a Bachelor of Commerce and is an Australian Chartered Accountant. Ms. Boston has also completed the company directors course diploma administered by the Australian Institute of Company Directors.
J. Kevin Buchi has been a Director since April 2013. Mr. Buchi previously served as Chief Executive Officer of TetraLogic Pharmaceuticals Corporation. Mr. Buchi served as Chief Executive Officer of Cephalon, Inc., or Cephalon, from December 2010 through its acquisition by Teva Pharmaceutical Industries Ltd in October 2011. After the acquisition Mr. Buchi served as Corporate Vice President, Global Branded Products of Teva Pharmaceuticals Industries Ltd. Mr. Buchi joined Cephalon in 1991 and held various positions, including Chief Operating Officer, from January 2010 to December 2010, Chief Financial Officer and Head of Business Development prior to being appointed Chief Executive Officer. Mr. Buchi is also on the board of directors of Amneal Pharmaceuticals and Dicerna Pharmaceuticals. Mr. Buchi has a B.A. in chemistry from Cornell University and a Masters in Management from Kellogg Graduate School of Management at Northwestern University. He is also a Certified Public Accountant.
94
Peter Francis has been a director since February 2006 and was previously Chairman of the Board until October 2017. Since 1993, Mr. Francis has been a partner at Francis Abourizk Lightowlers, a firm of commercial and technology lawyers with offices in Melbourne, Australia. He is a legal specialist in the areas of intellectual property and licensing and provides legal advice to corporations and research bodies. Mr. Francis completed his studies in law and jurisprudence at Monash University.
There are no family relationships among any of our directors or executive officers and no arrangements or understandings with major shareholders, customers, suppliers or others pursuant to which any of our directors or members of senior management was selected as such, except Dr. Banks was appointed as a Director in October 2016 following the acquisition by Nant Capital, LLC of 19.9% of Benitec's then-outstanding ordinary shares.
The business addresses for each of our directors and executive officers is Benitec Biopharma Limited, Level 14, 114 William Street, Melbourne, Victoria, 3000 Australia.
Remuneration
The remuneration policy of Benitec is to align director and executive objectives with shareholder and business objectives by providing a fixed remuneration component and typically offering long-term incentives based on key performance areas. Our board of directors believes the remuneration policy to be appropriate and effective in its ability to attract and retain the best executives and directors to run and manage the consolidated entity, as well as create goal congruence between directors, executives, and shareholders.
Our board of directors is responsible for determining the appropriate remuneration package for our Chief Executive Officer, and our Chief Executive Officer is in turn responsible for determining the appropriate remuneration packages for senior management.
Executives typically receive a base salary based on factors such as experience and comparable industry information, options and performance incentives. Our board of directors reviews our Chief Executive Officer's remuneration package, and our Chief Executive Officer reviews the other senior executives' remuneration packages, annually by reference to the consolidated entity's performance, executive performance, and comparable information within the industry.
The performance of executives is measured against criteria agreed annually with each executive and is based predominantly on the overall success of Benitec in achieving its broader corporate goals. Bonuses and incentives are linked to predetermined performance criteria. Our board of directors may, however, exercise its discretion in relation to approving incentives, bonuses, and options, and can recommend changes to our Chief Executive Officer's recommendations. The policy is designed to attract the highest caliber of executives and reward them for performance that results in long-term growth in shareholder wealth.
Executives may be invited to participate in the employee share option plan at the Board's discretion. Options vest according to Board's determination and cannot be transferred or encumbered.
Australian executives or directors receive a superannuation guarantee contribution required by the government and do not receive any other retirement benefits.
All remuneration paid to directors and executives is valued at the cost to us and expensed. Options are valued using the Black-Scholes methodology. The board of directors' policy is to remunerate non-executive directors at market rates for comparable companies for time, commitment, and responsibilities. Our board of directors as a whole determines payments to the non-executive directors and reviews their remuneration annually, based on market practice, duties and accountability. The maximum aggregate amount of fees that can be paid to non-executive directors is subject to approval by shareholders at our annual general meeting. Fees for non-executive directors are not linked to the performance of the consolidated entity. However, to align directors' interests with shareholder interests, the directors are encouraged to hold our shares.
95
Our directors are paid remuneration for their services as directors (but excluding any remuneration payable to a director under any executive services contract with us or one of our related bodies corporate) which is determined in a general meeting of shareholders. The aggregate, fixed sum for directors’ remuneration is to be divided among the directors in such proportion as the directors themselves agree and in accordance with our Constitution. The fixed sum remuneration for directors may not be increased except at a general meeting of shareholders and the particulars of the proposed increase are required to have been provided to shareholders in the notice convening the meeting. In addition, executive directors may be paid remuneration as employees of Benitec.
Fees payable to our non-executive directors must be by way of a fixed sum and not by way of a commission on or a percentage of profits or operating revenue. Remuneration paid to our executive directors must also not include a commission or percentage of operating revenue.
Pursuant to our Constitution, any director who performs services that in the opinion of our board of directors, are outside the scope of the ordinary duties of a director may be paid extra remuneration, which is determined by our board of directors.
In addition to other remuneration provided in our Constitution, all of our directors are entitled to be paid by us for reasonable travel, accommodation and other expenses incurred by the directors in attending general meetings, board meetings, committee meetings or otherwise in connection with our business.
In addition, in accordance with our Constitution, a director may be paid a retirement benefit as determined by our board of directors subject to the limits set out in the Corporations Act and the ASX Listing Rules which broadly restrict our ability to pay our officers a termination benefit in the event of a change of control of the Company or our subsidiaries as well as impose requirements for shareholder approval to be obtained to pay certain retirement benefits to our officers.
Performance Based Remuneration
Each executive's remuneration package typically has a performance-based component. The intention of this approach is to facilitate goal congruence between executives with the business and shareholders. Generally, the executive's performance based remuneration is tied to Benitec's successful achievement of certain key milestones relating to its operating activities, as well as Benitec's overall financial position.
The remuneration policy has been tailored to align the goals of shareholders, directors, and executives. Two methods are applied in achieving this aim, the first being a performance-based bonus linked to achievement of key corporate milestones, and the second being the issuance of options to the majority of directors and executives to encourage the alignment of personal and shareholder interests.
96
Details of Remuneration for fiscal 2019
The following tables set forth all of the compensation awarded to, earned by or paid to each individual who served as director and executive officer in fiscal 2019.
| | Short Term Benefits | | | Post- Employment | | | Long-Term Benefits | |
| | Cash Salary and Fees A$ | | | Cash Bonus A$ | | | Non- Monetary A$ | | | Benefits Super- annuation A$ | | | Employee Leave A$ | | | Share-Based Payments Options A$ | | | Total A$ | |
2019 Directors | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Jerel Banks | | | 578,985 | | | | — | | | | — | | | | — | | | | — | | | | 714,263 | | | | 1,293,248 | |
Peter Francis | | | 70,000 | | | | — | | | | — | | | | 6,650 | | | | — | | | | — | | | | 76,650 | |
Megan Boston | | | 266,509 | | | | — | | | | 15,287 | | | | 19,312 | | | | — | | | | 92,914 | | | | 394,022 | |
Kevin Buchi | | | 76,650 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 76,650 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Other Key management Personnel | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Georgina Kilfoil(1) | | | 168,533 | | | | — | | | | (15,689 | ) | | | 10,728 | | | | — | | | | — | | | | 163,572 | |
| | | 1,160,677 | | | | — | | | | (402 | ) | | | 36,690 | | | | — | | | | 807,177 | | | | 2,004,142 | |
(1) | Georgina Kilfoil appointed as Chief Development Officer on February 9, 2018, resigned on January 9, 2019. |
The proportion of remuneration at risk and the fixed proportion are as follows:
| | Fixed remuneration | | | At risk – STI (bonus) | | | At risk – LTI (options) | |
| | 2019 | | | 2018 | | | 2019 | | | 2018 | | | 2019 | | | 2018 | |
Directors | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Jerel Banks | | | 45 | % | | | 95 | % | | | — | % | | | — | % | | | 55 | % | | | 5 | % |
Peter Francis | | | 100 | % | | | 83 | % | | | — | % | | | — | % | | | — | % | | | 17 | % |
Megan Boston | | | 76 | % | | | 100 | % | | | — | % | | | — | % | | | 24 | % | | | — | % |
Kevin Buchi | | | 100 | % | | | 87 | % | | | — | % | | | — | % | | | — | % | | | 13 | % |
John Chiplin(1) | | | — | % | | | 100 | % | | | — | % | | | — | % | | | — | % | | | — | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
Other Key Management Personnel | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Georgina Kilfoil(2) | | | 100 | % | | | 87 | % | | | — | % | | | — | % | | | — | % | | | 13 | % |
Greg West(3) | | | — | % | | | 87 | % | | | — | % | | | — | % | | | — | % | | | 13 | % |
David Suhy(4) | | | — | % | | | 84 | % | | | — | % | | | — | % | | | — | % | | | 16 | % |
Cliff Holloway(5) | | | — | % | | | 100 | % | | | — | % | | | — | % | | | — | % | | | — | % |
(2) | John Chiplin resigned as a director on October 23, 2017. |
(3) | Georgina Kilfoil appointed as Chief Development Officer on February 9, 2018, resigned on January 9, 2019. |
(4) | Greg West resigned as CEO and Company Secretary June 15, 2018. |
(5) | David Suhy resigned as CSO on June 22, 2018. |
(6) | Cliff Holloway resigned as Chief Business and Operations Officer on January 7, 2018. |
97
The proportion of the cash bonus paid/payable or forfeited is as follows:
| | Cash bonus paid/payable | | | Cash bonus forfeited | |
Name | | 2019 | | | 2018 | | | 2019 | | | 2018 | |
Executive Officers: | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
David Suhy | | | — | | | | 100 | % | | | — | | | | — | |
There were no shares issued to directors and executive officers as part of compensation during the year ended June 30, 2019.
The terms and conditions of each grant of options over ordinary shares affecting remuneration of directors and executive officers during fiscal 2019 is as follows:
Grant date | | No. granted | | | Expiry date | | Exercise price | | | Fair value per option at grant date | |
March 12, 2019 | | | 5,000,000 | | | March 12, 2024 | | A$ | | 0.2001 | | | A$ | | 0.1009 | |
Details of options over ordinary shares granted, vested and lapsed for directors and executive officers as part of compensation during the year ended June 30, 2019 are set out below:
Name | | Number of options granted | | | Grant date | | Value per options at grant date | | | Value of options at grant date | | | Number vested/ (forfeited) | | | Exercise Price | | | Vested and first exercised date | | Last exercise date |
Jerel Banks | | | 10,000,000 | | | 06/26/2018 | | A$ | | 0.1003 | | | A$ | | 1,003,000 | | | | 3,333,333 | | | | 0.2278 | | | 06/26/2019 | | 06/26/2023 |
Megan Boston | | | 5,000,000 | | | 03/12/2019 | | A$ | | 0.1009 | | | A$ | | 504,500 | | | | — | | | | 0.2001 | | | 03/12/2020 | | 03/12/2024 |
Georgina Kilfoil | | | 800,000 | | | 07/17/2017 | | A$ | | 0.9090 | | | A$ | | 72,720 | | | | (800,000 | ) | | | 0.1960 | | | 07/17/2018 | | 04/09/2019 |
(already expired)
Options granted carry no dividend or voting rights. Options for J.Banks and M.Boston vest over three years with vesting based on remaining in service. There are no other performance criteria.
Employment Agreements with Executive Officers
The key provisions of the employment agreements (other than remuneration) are set out below for each of our executive officers. None of these employment agreements have termination dates.
Name of executive officer | | Title of executive officer | | Date employment agreement commenced | | Notice period required to terminate without cause by either party |
Jerel Banks | | Chief Executive Officer | | June 15, 2018 | | Six months |
Megan Boston | | Executive Director and Head of Operations Australia | | June 15, 2018 | | Six months |
Mr. Banks and Ms. Boston are entitled to no non-statutory benefits in the case of termination of employment.
98
Consequences of Performance on Shareholder Wealth
The earnings of Benitec and its subsidiaries for the five years ended June 30, 2019, are summarized below:
| | 2015 A$‘000 | | | 2016 A$‘000 | | | 2017 A$‘000 | | | 2018 A$‘000 | | | 2019 A$‘000 | |
Profit/(Loss) after income tax | | | (11,509 | ) | | | (24,778 | ) | | | (5,690 | ) | | | (11,640 | ) | | | 4,094 | |
The factors that are considered to affect total shareholders return are summarized below:
| | 2015 | | | 2016 | | | 2017 | | | 2018 | | | 2019 | |
Share price at financial year end (A$) | | | 0.690 | | | | 0.097 | | | | 0.125 | | | | 0.135 | | | | 0.060 | |
Basic earnings/(loss) per share (cents per share) | | | (9.96 | ) | | | (17.41 | ) | | | (3.24 | ) | | | (5.53 | ) | | | 1.59 | |
Additional Disclosures Relating to Key Management Personnel
The number of shares in Benitec held during fiscal 2019 by each director and executive officer, including their personally related parties, is set out below:
| | Balance at the start of the year | | | Received as part of remuneration | | | Purchased | | | Disposals / other | | | Balance at the end of the year | |
Ordinary shares | | | | | | | | | | | | | | | | | | | | |
Jerel Banks | | | — | | | | — | | | | — | | | | — | | | | — | |
Peter Francis | | | 636,261 | | | | — | | | | — | | | | — | | | | 636,261 | |
Megan Boston | | | 100,000 | | | | — | | | | — | | | | — | | | | 100,000 | |
Kevin Buchi | | | 1,448,210 | | | | — | | | | — | | | | — | | | | 1,448,210 | |
Georgina Kilfoil | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | 2,184,471 | | | | — | | | | — | | | | — | | | | 2,184,471 | |
The number of options over ordinary shares in Benitec held during the financial year ended June 30, 2019, by each director and other executive officers, including their personally related parties, is set out below:
| | Balance at the start of the year | | | Granted | | | Exercised | | | Expired /forfeited /other | | | Balance at the end of the year | | | Vested and exercisable | | | Vested and unexercisable | |
Options over ordinary shares | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Jerel Banks | | | 10,000,000 | | | | — | | | | — | | | | — | | | | 10,000,000 | | | | 3,333,333 | | | | — | |
Peter Francis | | | 1,400,000 | | | | — | | | | — | | | | — | | | | 1,400,000 | | | | 1,400,000 | | | | — | |
Megan Boston | | | — | | | | 5,000,000 | | | | — | | | | — | | | | 5,000,000 | | | | — | | | | — | |
Kevin Buchi | | | 840,000 | | | | — | | | | — | | | | — | | | | 840,000 | | | | 840,000 | | | | — | |
Georgina Kilfoil(1) | | | 1,400,000 | | | | — | | | | — | | | | (1,400,000 | ) | | | — | | | | — | | | | — | |
Greg West(2) | | | 3,013,332 | | | | — | | | | — | | | | (3,013,332 | ) | | | — | | | | — | | | | — | |
David Suhy(3) | | | 2,300,000 | | | | — | | | | — | | | | (800,000 | ) | | | 1,500,000 | | | | 500,000 | | | | — | |
| | | 18,953,332 | | | | 5,000,000 | | | | — | | | | (5,213,332 | ) | | | 18,740,000 | | | | 6,073,333 | | | | — | |
(1) | Georgina Kilfoil resigned on January 9, 2019. |
(2) | Greg West resigned as CEO and Company Secretary on June 15, 2018. Mr. West’s options expired on September 15, 2018. |
(3) | David Suhy resigned as Chief Scientific Officer on June 22, 2018. His options terms were varied, the options will expire on November 19, 2019. |
99
Employee Share Option Plan
We had an employee share option plan that was approved by our shareholders in November 2009 and was in effect until November 2014. Our shareholders approved a new Officers’ and Employees’ Option Plan at our 2015 Annual General Meeting in November 2015.
The following employee options to purchase ordinary shares of Benitec Biopharma Limited were outstanding as at June 30, 2019:
| | | | Share options outstanding at June 30, 2019 | |
| | | | Exercise | | | Number | |
Grant date | | Expiry date | | price | | | under option | |
December 17, 2014 ** | | December 17, 2019 | A$ | 1.25 | | | | 700,000 | |
May 6, 2015 ** | | May 6, 2020 | A$ | | 1.25 | | | | 350,000 | |
November 12, 2015* | | November 12, 2020 | A$ | | 0.77 | | | | 2,240,000 | |
July 17, 2017** | | July 17, 2022 | A$ | | 0.1960 | | | | 3,800,000 | |
April 11, 2018** | | April 11, 2023 | A$ | | 0.2980 | | | | 650,000 | |
June 26, 2018** | | June 26, 2023 | A$ | | 0.2278 | | | | 10,000,000 | |
March 12, 2019** | | March 12, 2024 | A$ | | 0.2000 | | | | 5,000,000 | |
March 21, 2019** | | March 21, 2024 | A$ | | 0.206 | | | | 1,575,000 | |
April 11, 2019** | | April 11, 2024 | A$ | | 0.208 | | | | 1,150,000 | |
May 2, 2019** | | May 2, 2024 | A$ | | 0.198 | | | | 275,000 | |
May 16, 2019** | | May 16, 2024 | A$ | | 0.206 | | | | 200,000 | |
| | | | | | | | | 25,940,000 | |
* | Non-Executive Directors options. |
** | Employee Share Option Plan options. |
No employee options were exercised during fiscal 2019. No person entitled to exercise the options had or has any right by virtue of the option to participate in any share issue of the Company or of any other body corporate.
Board of Directors
Our board of directors currently consists of four members. Our directors are elected at each annual general meeting of our shareholders and serve until their successors are elected or appointed, unless their office is earlier vacated. We believe that each of our directors has relevant industry experience. The membership of our board of directors is based on the following principles:
| • | our Constitution specifies that there must be a minimum of three directors and a maximum of 10, and our board of directors may determine the number of directors within those limits; |
| • | the membership of the board of directors has the experience needed to conduct our business operations; and |
| • | our board of directors should, collectively, have the appropriate level of personal qualities, skills, experience, and time commitment to properly fulfill its responsibilities or have ready access to such skills where they are not available. |
Our board of directors has delegated responsibility for the conduct of our businesses to the Chief Executive Officer, but remains responsible for overseeing the performance of management. Our board of directors has established delegated limits of authority, which define the matters that are delegated to management and those that require board of directors approval. Under the Corporations Act, at least two of our directors must be resident Australians. None of our directors have any service contracts with Benitec that provide for benefits upon termination of employment.
100
Committees
To assist our board of directors with the effective discharge of its duties, it has established a Remuneration and Nomination Committee and an Audit and Risk Committee, which committees operate under a specific charter approved by our board of directors.
Remuneration and Nomination Committee. The members of our Remuneration and Nomination Committee are Peter Francis and Kevin Buchi. Kevin Buchi acts as chairman of the committee. The committee's role involves:
| • | identifying, evaluating and recommending qualified nominees to serve on our board of directors; |
| • | developing and overseeing our internal corporate governance processes; |
| • | maintaining a management succession plan; |
| • | evaluating, adopting and administering our compensation plans and similar programs advisable for us, as well as modifying or terminating existing plans and programs; |
| • | establishing policies with respect to equity compensation arrangements; and |
| • | overseeing, reviewing and reporting on various remuneration matters to our board of directors. |
Audit and Risk Committee. The members of our Audit and Risk Committee are Peter Francis and Kevin Buchi. Our board of directors has determined that each of them meets the criteria for independence of audit committee members set forth in Rule 10A-3(b)(1) under the Securities Exchange Act of 1934 and the applicable rules of The NASDAQ Capital Market and Rule 10A-3(b)(1)(iv)(2) under the Exchange Act. In March 2017 Benitec elected to follow home country practice in compliance with Australian law and ASX rules with respect to the NASDAQ rule that requires a company to maintain an audit committee of at least three members. As a result, Benitec is required to maintain an audit committee of at least two members, each of whom is independent under relevant rules of NASDAQ and the SEC. See Item 16D. "Exemptions from the Listing Standards for Audit Committees."
Each member of our audit committee meets the financial literacy requirements of the listing standards of The NASDAQ Capital Market. Peter Francis acts as the chairman of the audit committee and our board of directors has determined that Peter Francis and Kevin Buchi as audit committee members satisfy the "financial expert" requirement, as defined by Item 407(d) of Regulation S-K under the Securities Act.
The principal duties and responsibilities of our audit committee include:
| • | overseeing and reporting on various auditing and accounting matters to our board of directors, including the selection of our independent accountants, the scope of our annual audits, fees to be paid to the independent accountants, the performance of our independent accountants and our accounting practices; |
| • | overseeing and reporting on various risk management matters to our board of directors. |
| • | considering and approving or disapproving all related-party transactions; |
| • | reviewing our annual and semi-annual financial statements and reports and discussing the statements and reports with our independent registered public accounting firm and management; |
| • | reviewing and pre-approving the engagement of our independent registered public accounting firm to perform audit services and any permissible non-audit services; |
| • | evaluating the performance of our independent registered public accounting firm and deciding whether to retain their services; and |
| • | establishing procedures for the receipt, retention and treatment of complaints received by us regarding financial controls, accounting or auditing matters. |
101
Code of Conduct
We have established a Code of Conduct, which sets out the standards of behavior that apply to every aspect of our dealings and relationships, both within and outside Benitec. The following standards of behavior apply to all directors, executive officers and employees of Benitec:
| • | comply with all laws that govern us and our operations; |
| • | act honestly and with integrity and fairness in all dealings with others and each other; |
| • | avoid or manage conflicts of interest; |
| • | use our assets responsibly and in the best interests of Benitec; and |
| • | be responsible and accountable for our actions. |
The Code of Conduct is available on our website at www.benitec.com.
As of June 30, 2019, we had 21 full-time employees, 11 of whom have a PhD or other post-graduate degrees. Of these full-time employees, 16 are engaged in research and development activities and 5 are engaged in finance, legal, human resources, facilities and general management. Our employees are located in Sydney, Australia and at our scientific laboratories located in Hayward, California. None of our employees is represented by any labor union. As of June 30, 2019, 18 of our employees were located in Hayward, California, and 3 of our employees were located in Sydney, Australia.
The following table and accompany footnotes set forth information regarding the beneficial ownership of our ordinary shares based on 313,029,426 ordinary shares outstanding as of September 30, 2019 by each of our directors and executive officers individually and as a group.
Beneficial ownership is determined according to the rules of the SEC and generally means that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power of that security, including options that are exercisable within 60 days of June 30, 2019. Information with respect to beneficial ownership has been furnished to us by each director, executive officer, or 5% or more shareholder, as the case may be. Ordinary shares subject to options currently exercisable or exercisable within 60 days of June 30, 2019 are deemed to be outstanding for computing the percentage ownership of the person holding these options and the percentage ownership of any group of which the holder is a member but are not deemed outstanding for computing the percentage of any other person.
Unless otherwise indicated, to our knowledge each shareholder possesses sole voting and investment power over the ordinary shares listed subject to community property laws, where applicable. None of our shareholders has different voting rights from other shareholders. Unless otherwise indicated, the address for each of the persons listed in the table below is Benitec Biopharma Limited, Level 14, 114 William Street, Melbourne, Victoria, 3000 Australia.
| | Ordinary Shares Beneficially Owned(6) | | | Options(6) | |
Shareholder | | Number | | | Percent | | | Number | |
Officers and Directors | | | | | | | | | | | | |
Jerel Banks | | | — | | | | — | | | | 10,000,000 | |
Peter Francis | | | 636,261 | | | | (1 | ) | | | 1,400,000 | |
Megan Boston | | | 100,000 | | | | (1 | ) | | | 5,000,000 | |
Kevin Buchi | | | 1,448,210 | | | | (1 | ) | | | 840,000 | |
Georgina Kilfoil(2) | | | — | | | | — | | | | — | |
Officers and directors as a group (5 persons) | | | 2,184,471 | | | | (1 | ) | | | 17,240,000 | |
(1) | Represents beneficial ownership of less than 1% of the outstanding ordinary shares of Benitec. |
102
(2) | Georgina Kilfoil resigned on January 9, 2019. |
(3) | Table includes shares and options which vested or vest within 60 days of June 30, 2019. |
Item 7. Major Shareholders and Related Party Transactions
The following table presents certain information regarding the beneficial ownership of our ordinary shares based on 313,029,426 ordinary shares outstanding as of September 30, 2019 by each person known by us to be the beneficial owner of more than 5% of our ordinary shares.
| | Ordinary Shares Beneficially Owned | |
Shareholder | | Number | | | Percent | |
5% Shareholders | | | | | | | | |
Nant Capital, LLC | | | 87,917,457 | | | | 28.08 | % |
Sabby Management | | | 28,000,000 | | | | 8.94 | % |
Empery Asset Management | | | 28,000,000 | | | | 8.94 | % |
As at September 30, 2019, 90,454,760 of our outstanding ordinary shares were held in the form of ADSs (ADS 4,522,738) representing 28.90% of our outstanding ordinary shares.
A large number of our ordinary shares are held by nominee companies so we cannot be certain of the identity of those beneficial owners. All shareholders have the same voting rights.
In October 2016, Nant Capital, LLC acquired 29,305,819 ordinary shares, or 16.7% interest of our total post-issuance voting power, in connection with a private placement. Nant Capital, LLC increased its shareholding to 28.57% of our total post-issuance voting power in March 2017 through a private placement. On June 4, 2018, we issued 36,442,672 in a 1-for-2 entitlement offer, with Nant Capital subscribing 29,305,819 ordinary shares. As a result, Nant Capital increased its holding to 34.21% of Benitec's issued capital. Following the capital raising which occurred on September 30, 2019, Nant Capital's ownership decreased to 28.08%.
In May 2018, Highbridge acquired 15,444,020 ordinary shares as represented by American Depositary Shares, or 7.00% interest of our outstanding shares. With respect to these shares, 1992 MSF International Ltd (“MSF”) also had beneficial ownership as a result of Highbridge being its trading manager (the Highbridge’s and MSF’s beneficial ownerships, collectively, the “Highbridge ownership”). In June 2018, the Highbridge ownership decreased to 6.00% as a result of the sale of 23,220 ordinary shares. On July 3, 2018, the Highbridge ownership decreased its interest to 2.64%.
On September 30, 2019, following the acquisition of 1,400,000 ADSs, Sabby Volatility Warrant Master Fund, Ltd., Sabby Management LLC, acquired ownership of 8.94% of our ordinary shares.
On September 30, 2019, following the acquisition of 1,400,000 ADSs, Empery Tax Efficient LP, Empery Tax Efficient II LP, Empery Asset Master LTD, as controlled entities of Empery AM GP, LLC, Empery Asset Management, LP, acquired ownership of 8.94% of our ordinary shares.
B. | Related Party Transactions. |
Other than as disclosed below, since July 1, 2015, we did not enter into any transactions or loans with any: (i) enterprises that directly or indirectly, through one or more intermediaries, control, are controlled by or are under common control with us; (ii) associates; (iii) individuals owning, directly or indirectly, an interest in our voting power that gives them significant influence over us, and close members of any such individual's family; (iv) executive officers and close members of such individuals' families; or (v) enterprises in which a substantial interest in our voting power is owned, directly or indirectly, by any person described in (iii) or (iv) or over which such person is able to exercise significant influence.
103
Nant Capital, LLC
| • | In October 2016, we entered into a strategic relationship with Nant Capital. As part of that strategic relationship, we initially issued Nant Capital a total of 58,611,638 of our ordinary shares for a combined total consideration of A$8.1 million. Nant Capital is entitled to appoint, and has appointed, a director to Benitec’s board of directors. |
In December 2016, we entered into an exclusive sublicense agreement with NantWorks, LLC, an affiliate of Nant Capital, pursuant to which we acquired rights to intellectual property, including preclinical and clinical data, relating to a product candidate, now named BB-401, for the treatment of HNSCC. We are required to make periodic, milestone and royalty payments to Nant Capital pursuant to the exclusive sublicense agreement in connection with the development and sale of BB-401. In fiscal 2017, we made royalty payments to Nant Capital of A$79,449, and reimbursed patent expense of A$44,486. Moreover, in fiscal 2018, we paid (i) in August 2017, A$5,214 as reimbursement in patent application extension, (ii) in October 2017, A$38,260 in annual maintenance fee and (iii) A$39,861 in annual maintenance fee.
In January 2017, we entered into a research collaboration agreement with Nant Capital pursuant to which we will manage the clinical development of BB-401, a recombinant DNA construct that produces an antisense RNA with specificity against EGFR for the treatment of HNSCC. The research collaboration agreement with Nant Capital allocates the A$5.4 million received from Nant Capital in the March 2017 private placement to the development of BB-401 and BB-501. Nant Capital will have a controlling vote on the joint steering committee that directs and oversees such development of BB-401 and BB-501 until those funds have been expended. BB-401 and BB-501 programs have been terminated.
Other Related Parties
| • | Legal services at normal commercial rates totaling A$726 for fiscal 2019, A$8,212 for fiscal 2018 and A$191,050 for fiscal 2017 were provided by Francis Abourizk Lightowlers, a law firm in which Mr. Peter Francis is a partner and has a beneficial interest. In addition, Benitec temporarily rented office space in Melbourne from Francis Abourizk Lightowlers and the associated rental cost was A$11,102 during fiscal 2016. |
| • | Annabel West, the wife of Greg West, our former Chief Executive Officer, was employed by us as a part-time clerical and administrative assistant. Annabel West was paid wages of A$42,278 and A$36,248, respectively, for fiscal 2018 and fiscal 2017. |
Transactions between related parties are on normal commercial terms and the conditions no more favorable than those available to other non-related parties.
C. | Interests of Experts and Counsel. |
Not applicable.
Item 8. Financial Information
A. | Consolidated Statements and Other Financial Information. |
Our financial statements are included in Item 18 “Financial Statements.”
Legal Proceedings
We are not currently a party to any material legal proceedings. From time to time, we may be subject to various legal proceedings and claims that arise in the ordinary course of our business activities. Although the results of litigation and claims cannot be predicted with certainty, any such future litigation could have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
104
Dividends
We have never declared or paid cash dividends on our ordinary shares. For the foreseeable future, we currently intend to retain all available funds and any future earnings to support our operations and to finance the growth and development of our business. Any future determination to declare cash dividends will be made at the discretion of our board of directors, subject to compliance with applicable laws and covenants under current or future credit facilities, which may restrict or limit our ability to pay dividends, and will depend on our financial condition, operating results, capital requirements, general business conditions and other factors that our board of directors may deem relevant. We do not anticipate paying any cash dividends on our ordinary shares in the foreseeable future. Should we determine to pay dividends in the future, all dividends unclaimed for one year after having been declared may be invested or otherwise made use of by our board of directors for our benefit until claimed or otherwise disposed of in accordance with our Constitution.
No significant change occurred since the end of the latest fiscal year.
Item 9. The Offer and Listing.
See Item 9C for more information.
Not applicable.
Our ordinary shares have been trading on the ASX since 1997 under the symbol BLT.
On August 18, 2015 our ADSs and Warrants were listed on The NASDAQ Capital Market under the ticker symbols “BNTC” and “BNTCW,” respectively. Prior to our initial public offering in the United States, we did not list our ADSs on any other stock exchange.
Not applicable.
Not applicable.
Item 10. Additional Information.
Not applicable.
B. | Memorandum and Articles of Association. |
Our Constitution is similar in nature to the bylaws of a U.S. corporation. It does not provide for or prescribe any specific objectives or purposes of Benitec. Our Constitution is subject to the terms of the ASX Listing Rules and the Corporations Act. It may be amended or repealed and replaced by special resolution of shareholders, which is a resolution passed by at least 75% of the votes cast by shareholders entitled to vote on the resolution.
105
Under Australian law, a company has the legal capacity and powers of an individual both within and outside Australia. The material provisions of our Constitution are summarized below. This summary is not intended to be complete nor to constitute a definitive statement of the rights and liabilities of our shareholders, and is qualified in its entirety by reference to the complete text of our Constitution, a copy of which is on file with the Securities and Exchange Commission.
Interested Directors
Subject to the Corporations Act and the ASX Listing Rules, neither a director nor that director's alternate may vote in respect of any contract or arrangement in which the director has, directly or indirectly, any material interest according to our Constitution. Such director must not be counted in a quorum, must not vote on the matter and must not be present at the meeting while the matter is being considered. However, that director may execute or otherwise act in respect of that contract or arrangement notwithstanding any material personal interest.
Unless a relevant exception applies, the Corporations Act requires our directors to provide disclosure of any material personal interest, and prohibits directors from voting on matters in which they have a material personal interest and from being present at the meeting while the matter is being considered. In addition, the Corporations Act and the ASX Listing Rules require shareholder approval of any provision of related party benefits to our directors.
Directors’ compensation
Our directors are paid remuneration for their services as directors (but excluding any remuneration payable to a director under any executive services contract with us or one of our related bodies corporate) which is determined in a general meeting of shareholders. The aggregate, fixed sum for directors’ remuneration is to be divided among the directors in such proportion as the directors themselves agree and in accordance with our Constitution. The fixed sum remuneration for directors may not be increased except at a general meeting of shareholders and the particulars of the proposed increase are required to have been provided to shareholders in the notice convening the meeting. In addition, subject to the ASX Listing Rules and the terms of any agreement entered into with any executive director, our Board may fix the remuneration of each executive director, which may comprise salary or commission on or participation in profits of Benitec (or comprising a combination of each) as our directors determine.
Fees payable to our non-executive directors must be by way of a fixed sum and not by way of a commission on or a percentage of profits or operating revenue. Remuneration paid to our executive directors must also not include a commission or percentage of operating revenue.
Pursuant to our Constitution, any director who performs extra services or makes any special exertions, whether in going or residing abroad or otherwise for any of the purposes of the Company, that director may be paid an additional sum for those services and exertions.
In addition to other remuneration provided in our Constitution, all of our directors are entitled to be paid by us for all travelling and other expenses properly incurred by the directors in attending general meetings, board meetings, committee meetings or otherwise in connection with our business.
In addition, in accordance with our Constitution, a director may be paid a retirement benefit as determined by our board of directors subject to the limits set out in the Corporations Act and the ASX Listing Rules which broadly restrict our ability to pay our officers a termination benefit in the event of a change of control of the Company or our subsidiaries as well as impose requirements for shareholder approval to be obtained to pay certain retirement benefits to our officers.
Borrowing powers exercisable by Directors
Pursuant to our Constitution, the management and control of our business affairs are vested in our board of directors. Our board of directors has the power to raise or borrow money or obtain other financial accommodation for the purposes of the Company, and may grant security for the repayment of that sum or sums or the payment, performance or fulfilment of any debts, liabilities, contracts or obligations incurred or undertaken by the Company in any manner and upon any terms and conditions as our Board thinks fit.
106
Retirement of Directors
Pursuant to our Constitution, one-third of our directors, other than the managing director, must retire from office at every annual general meeting. If the number of directors is not a multiple of three, then the number nearest, to but not exceeding, one-third must retire from office. The directors who retire in this manner are required to be the directors or director longest in office since last being elected. A director, other than the managing director, must retire from office at the conclusion of the third annual general meeting after which the director was elected. Retired directors are eligible for a re-election to the board of directors unless disqualified from acting as a director under the Corporations Act or our Constitution.
Rights and restrictions on classes of shares
The rights attaching to our ordinary shares are detailed in our Constitution. Our Constitution provides that our directors may issue shares with preferential, deferred or special rights, privileges or conditions or with any restrictions, whether in relation to dividends, voting, return of share capital, or otherwise as our board of directors may determine. Subject to any approval which is required from our shareholders under the Corporations Act and the ASX Listing Rules (see “—Exemptions from Certain NASDAQ Corporate Governance Rules” and “—Change of Control”), any rights and restrictions attached to a class of shares, we may issue further shares on such terms and conditions as our board of directors resolve. Currently, our outstanding share capital consists of only one class of ordinary shares
Dividend rights
Our board of directors may from time to time determine to pay and declare dividends to shareholders. Except as otherwise provided by the Corporations Act, all dividends unclaimed for one year after having been declared may be invested or otherwise made use of by our board of directors for our benefit until claimed or until dealt with under any law relating to unclaimed moneys.
Voting rights
Under our Constitution, and subject to any voting exclusions imposed under the ASX Listing Rules (which typically exclude parties from voting on resolutions in which they have an interest), the rights and restrictions attaching to a class of shares, each shareholder has one vote on a show of hands at a meeting of the shareholders unless a poll is demanded under the Constitution or the Corporations Act. On a poll vote, each shareholder shall have one vote for each fully paid share and a fractional vote for each share held by that shareholder that is not fully paid, such fraction being equivalent to the proportion of the amount that has been paid to such date on that share. Shareholders may vote in person or by proxy, attorney or representative. Under Australian law, shareholders of a public company are generally not permitted to approve corporate matters by written consent. Our Constitution does not provide for cumulative voting.
Note that ADS holders may not directly vote at a meeting of the shareholders but may instruct the depositary to vote the number of deposited ordinary shares their ADSs represent.
Right to share in our profits
Pursuant to our Constitution, our shareholders are entitled to participate in our profits only by payment of dividends. Our board of directors may from time to time determine to pay dividends to the shareholders; however, no dividend is payable except in accordance with the thresholds set out in the Corporations Act.
Rights to share in the surplus in the event of winding up
Our Constitution provides for the right of shareholders to participate in a surplus in the event of our winding up, subject to the rights attaching to a class of shares, the Constitution, the Corporations Act and the ASX Listing Rules.
107
No redemption provision for ordinary shares
There are no redemption provisions in our Constitution in relation to ordinary shares. Under our Constitution, any preference shares may be issued on the terms that they are, or may at our option be, liable to be redeemed.
Variation or cancellation of share rights
Subject to the Corporations Act, the ASX Listing Rules and the terms of issue of shares of that class, the rights and privileges attached to shares in a class of shares may only be varied or cancelled by a special resolution of Benitec, together with either:
| • | a special resolution passed at a meeting of members holding shares in the class; or |
| • | the written consent of members with at least 75% of the shares in the class. |
Directors may make calls
Our Constitution provides that subject to compliance with the Corporations Act, the ASX Listing Rules and the terms on which the shares have been issued, directors may make calls they think fit on a shareholder for amounts unpaid on shares held by that shareholder, other than monies payable at fixed times under the conditions of allotment.
General Meetings of Shareholders
General meetings of shareholders may be called by our board of directors. Except as permitted under the Corporations Act, shareholders may not convene a meeting. The Corporations Act requires the directors to call and arrange to hold a general meeting on the request of shareholders with at least 5% of the votes that may be cast at a general meeting or at least 100 shareholders who are entitled to vote at the general meeting. Notice of the proposed meeting of our shareholders is required at least 28 days prior to such meeting under the Corporations Act.
Foreign Ownership Regulation
Our Constitution does not impose specific limitations on the rights of non-residents to own securities imposed by our Constitution. However, acquisitions and proposed acquisitions of securities in Australian companies may be subject to review and approval by the Australian Federal Treasurer under the Foreign Acquisitions and Takeovers Act 1975 (Cth), or the FATA, which generally applies to acquisitions or proposed acquisitions:
| • | by a foreign person (as defined in the FATA) or associated foreign persons that would result in such persons having an interest in 20% or more of the issued shares of, or control of 20% or more of the voting power in, an Australian company; and |
| • | by non-associated foreign persons that would result in such foreign persons having an aggregate interest in 40% or more of the issued shares of, or control of 40% or more of the voting power in, an Australian company, where the Australian company is valued above the monetary threshold prescribed by FATA. |
However, in general terms, in respect of proposals for investment in non-sensitive sectors, no such review or approval under the FATA is required if the foreign acquirer is a U.S. entity or an entity from certain other countries and the value of the Australian target is less than A$1,154 million.
The Australian Federal Treasurer may prevent a proposed acquisition in the above categories or impose conditions on such acquisition if the Treasurer is satisfied that the acquisition would be contrary to the national interest. If a foreign person acquires shares or an interest in shares in an Australian company in contravention of the FATA, the Australian Federal Treasurer may make a range of orders including an order the divestiture of such person’s shares or interest in shares in that Australian company.
108
Ownership Threshold
There are no specific provisions in our Constitution that require a shareholder to disclose ownership above a certain threshold. The Corporations Act, however, requires a shareholder to notify us and the ASX once it, together with its associates, acquires a 5% interest in our ordinary shares, at which point the shareholder will be considered to be a “substantial” shareholder. Further, once a shareholder owns (alone or together with associates) a 5% interest in us, such shareholder must notify us and the ASX of any increase or decrease of 1% or more in its holding of our ordinary shares, and must also notify us and the ASX on its ceasing to be a “substantial” shareholder.
Issues of Shares and Change in Capital
Subject to our Constitution, the Corporations Act, the ASX Listing Rules and any other applicable law, we may at any time issue shares and give any person a call or option over any shares on any terms, with preferential, deferred or other special rights, privileges or conditions or with restrictions and for the consideration and other terms that the directors determine.
Subject to the requirements of our Constitution, the Corporations Act, the ASX Listing Rules and any other applicable law, including relevant shareholder approvals, we may consolidate or divide our share capital into a larger or smaller number by resolution, reduce our share capital in any manner (provided that the reduction is fair and reasonable to our shareholders as a whole, does not materially prejudice our ability to pay creditors and obtains the necessary shareholder approval) or buy back our ordinary shares whether under an equal access buy-back or on a selective basis.
Change of Control
Takeovers of listed Australian public companies, such as Benitec, are regulated by the Corporations Act, which prohibits the acquisition of a “relevant interest” in issued voting shares in a listed company if the acquisition will lead to that person’s or someone else’s voting power in Benitec increasing from 20% or below to more than 20% or increasing from a starting point that is above 20% and below 90% (“Takeovers Prohibition”), subject to a range of exceptions.
Generally, a person will have a relevant interest in securities if the person:
| • | is the holder of the securities; |
| • | has power to exercise, or control the exercise of, a right to vote attached to the securities; or |
| • | has the power to dispose of, or control the exercise of a power to dispose of, the securities, including any indirect or direct power or control. |
If, at a particular time,
| • | a person has a relevant interest in issued securities; and |
| o | entered or enters into an agreement with another person with respect to the securities; |
| o | given or gives another person an enforceable right, or has been or is given an enforceable right by another person, in relation to the securities (whether the right is enforceable presently or in the future and whether or not on the fulfillment of a condition); |
| o | granted or grants an option to, or has been or is granted an option by, another person with respect to the securities; and |
| • | the other person would have a relevant interest in the securities if the agreement were performed, the right enforced or the option exercised, |
109
then the other person is taken to already have a relevant interest in the securities.
There are a number of exceptions to the above prohibition on acquiring a relevant interest in issued voting shares above 20%. In general terms, some of the more significant exceptions include:
| • | when the acquisition results from the acceptance of an offer under a formal takeover bid; |
| • | when the acquisition is conducted on market by or on behalf of the bidder during the bid period for a full takeover bid that is unconditional or only conditional on certain 'prescribed' matters set out in the Corporations Act; |
| • | when the acquisition has been previously approved by shareholders of Benitec by resolution passed at general meeting; |
| • | an acquisition by a person if, throughout the six months before the acquisition, that person or any other person has had voting power in Benitec of at least 19% and, as a result of the acquisition, none of the relevant persons would have voting power in Benitec more than three percentage points higher than they had six months before the acquisition; |
| • | when the acquisition results from the issue of securities under a rights issue; |
| • | when the acquisition results from the issue of securities under a dividend reinvestment scheme or bonus share plan; |
| • | when the acquisition results from the issue of securities under certain underwriting arrangements; |
| • | when the acquisition results from the issue of securities through a will or through operation of law; |
| • | an acquisition that arises through the acquisition of a relevant interest in another listed company which is listed on a prescribed financial market or a financial market approved by ASIC; |
| • | an acquisition arising from an auction of forfeited shares conducted on-market; or |
| • | an acquisition arising through a compromise, arrangement, liquidation or buy-back. |
Breaches of the takeovers provisions of the Corporations Act are criminal offenses. ASIC and the Australian Takeover Panel have a wide range of powers relating to breaches of takeover provisions, including the ability to make orders canceling contracts, freezing transfers of, and rights attached to, securities, and forcing a party to dispose of securities. There are certain defenses to breaches of the takeover provisions provided in the Corporations Act.
Access to and Inspection of Documents
Inspection of our records is governed by the Corporations Act. Any member of the public has the right to inspect or obtain copies of our registers on the payment of a prescribed fee. Shareholders are not required to pay a fee for inspection of our registers or minute books of the meetings of shareholders. Other corporate records, including minutes of directors’ meetings, financial records and other documents, are not open for inspection by shareholders. Where a shareholder is acting in good faith and an inspection is deemed to be made for a proper purpose, a shareholder may apply to the court to make an order for inspection of our books.
Nant Capital, LLC Strategic Engagement and Scientific Collaboration
In October 2016, we entered into a strategic relationship with Nant Capital. As part of that strategic relationship, pursuant to a share subscription agreement we initially issued Nant Capital a total of 58,611,638 of our ordinary shares for a combined total consideration of A$8.1 million. Under the terms of the share subscription agreement, Nant Capital is entitled to appoint, and has appointed, a director to Benitec’s board of directors. Upon completion of the initial placement of ordinary shares in October 2016, Dr. Jerel Banks, the Chief Investment Officer of Nant Capital, was appointed to the Board of Directors of Benitec on October 25, 2016.
110
In December 2016, we entered into an exclusive sublicense agreement with NantWorks, LLC, or NantWorks, an affiliate of Nant Capital, pursuant to which we acquired rights to intellectual property, including preclinical and clinical data, relating to a product candidate, now named BB-401, for the treatment of HNSCC. The license from NantWorks includes an exclusive worldwide sublicense to make, use and sell technology using the listed intellectual property for a term of ten years from the date of the first sale of a therapeutic using the intellectual property or the expiration of the last claim of the licensed patent rights, whichever is sooner. We paid NantWorks an initial licensing fee as well as closing costs in connection with the execution of the exclusive sublicense agreement in the high tens of thousands of dollars in aggregate and will be required to pay NantWorks maintenance fees in the low to mid tens of thousands of dollars annually, as well as aggregate milestone payments of over A$5 million relating to the development of a therapeutic product candidate for the treatment of HNSCC. We are also required to pay royalties on net sales of any products and services using the licensed intellectual property equal to a low double-digit percentage of such net sales. The initial term of the exclusive sublicense is the earlier of 10 years after the date of the first commercial sale of licensed technology or the expiration of the relevant patent rights.
In January 2017, we entered into a research collaboration agreement with Nant Capital pursuant to which we will manage the clinical development of BB-401. Our research collaboration agreement with Nant Capital requires us to allocate the A$5.4 million received from Nant Capital in connection with the March 2017 private placement to the development of BB-401 and future product candidates for HNSCC. Under the terms of the research collaboration agreement, Nant Capital will have a controlling vote on the joint steering committee that directs and oversees such development of BB-401 until those funds have been expended. We also agreed to sublicense certain intellectual property to NantOmics, LLC, or NantOmics, an affiliate of Nant Capital, to allow NantOmics to develop and commercialize a companion diagnostics to therapeutics developed pursuant to the research and collaboration agreement and to enter into a related services agreement with NantOmics. Under these agreements, Benitec would grant NantOmics an exclusive world-wide license to and the rights to use the results for such companion diagnostics. This program has subsequently been terminated and there are no remaining funds quarantined for allocation to the project.
Australia has largely abolished exchange controls on investment transactions. The Australian dollar is freely convertible into U.S. dollars at floating rates. In addition, there are currently no specific rules or limitations regarding the export from Australia of profits, dividends, capital or similar funds belonging to foreign investors, except that certain payments to non-residents must be reported to the Australian Cash Transaction Reports Agency, which monitors such transaction
111
The following is a summary of material U.S. federal and Australian income tax considerations to U.S. holders, as defined below, of the acquisition, ownership and disposition of ordinary shares, ADSs and Warrants. This discussion is based on the laws in force as of the date of this Annual Report, and is subject to changes in the relevant income tax law, including changes that could have retroactive effect. The following summary does not take into account or discuss the tax laws of any country or other taxing jurisdiction other than the United States and Australia. Holders are advised to consult their tax advisors concerning the overall tax consequences of the acquisition, ownership and disposition of ordinary shares, ADSs or Warrants in their particular circumstances. This discussion is not intended, and should not be construed, as legal or professional tax advice.
This summary does not address the 3.8% U.S. federal “Medicare tax” on net investment income, the effects of U.S. federal estate and gift tax laws, the alternative minimum tax, or any state and local tax considerations within the United States, and is not a comprehensive description of all U.S. federal or Australian income tax considerations that may be relevant to a decision to acquire or dispose of ordinary shares, ADSs or Warrants. Furthermore, this summary does not address U.S. federal or Australian income tax considerations relevant to holders subject to taxing jurisdictions other than, or in addition to, the United States and Australia, and does not address all possible categories of holders, some of which may be subject to special tax rules.
U.S. Taxation
This section describes certain material U.S. federal income tax considerations to a U.S. holder of owning ordinary shares, ADSs or Warrants, based on the U.S. Internal Revenue Code of 1986, as amended (the “Code”), U.S. Treasury regulations promulgated thereunder, published administrative interpretations of the U.S. Internal Revenue Service (“IRS”) and the U.S. Treasury, judicial decisions and the income tax treaty between the United States and Australia (the “Treaty”), all of which are subject to differing interpretations and to change, possibly with retroactive effect. It applies only to ordinary shares, ADSs or Warrants that are held as capital assets for tax purposes. This section does not apply to a holder of ordinary shares, ADSs or Warrants that is a member of a special class of holders subject to special rules, including, but not limited to, banks, insurance companies and other financial institutions, regulated investment companies, real estate investment trusts, individual retirement and other tax-deferred accounts, certain former U.S. citizens or long-term residents, brokers or dealers in securities, a trader in securities who elects to use a mark-to-market method of accounting for its securities holdings, a tax-exempt organization, a person that may have been liable for alternative minimum tax, a person who directly, indirectly or constructively owns 10 percent or more of the value or voting stock of the Company, a person that holds ordinary shares, ADSs or Warrants as part of a straddle or a hedging or conversion transaction, a person that purchases or sells ordinary shares, ADSs or Warrants as part of a wash sale for tax purposes, a person whose functional currency is not the U.S. dollar, a person subject to special tax accounting rules as a result of any item of gross income with respect to ordinary shares or ADSs being taken into account in an applicable financial statement, a person who acquires ordinary shares or ADSs pursuant to the exercise of any employee share option or otherwise as compensation, or a person that is not a U.S. holder.
In this section, a “U.S. holder” means a beneficial owner of ordinary shares or ADSs, other than a partnership or other entity treated as a partnership for U.S. federal income tax purposes, that is, for U.S. federal income tax purposes:
| • | an individual who is a citizen or resident of the United States (for U.S. federal income tax purposes); |
| • | a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States or any state thereof or the District of Columbia; |
| • | an estate the income of which is includable in gross income for U.S. federal income tax purposes regardless of its source; or |
| • | a trust (i) the administration of which is subject to the primary supervision of a court in the United States and for which one or more U.S. persons have the authority to control all substantial decisions or (ii) that has an election in effect under applicable U.S. income tax regulations to be treated as a U.S. person. |
112
If a partnership holds the ordinary shares, ADSs or Warrants, the U.S. federal income tax treatment of a partner generally will depend on the status of the partner and the tax treatment of the partnership. A partner in a partnership holding the ordinary shares, ADSs or Warrants should consult its tax adviser with regard to the U.S. federal income tax treatment of an investment in the ordinary shares, ADSs or Warrants. This section is in part based on the representations of the Depositary and the assumption that each obligation in the deposit agreement and any related agreement will be performed in accordance with its terms.
In general, for U.S. federal income tax purposes, a holder of ADSs will be treated as the owner of the ordinary shares represented by those ADSs. Exchanges of ordinary shares for ADSs, and ADSs for ordinary shares generally will not be subject to U.S. federal income tax.
Prospective purchasers should consult their own tax advisors concerning the U.S. federal, state, local, foreign and other tax consequences of acquiring, owning and disposing of ordinary shares or ADSs, in light of their particular circumstances.
Distributions
Subject to the passive foreign investment company (“PFIC”) rules discussed below, U.S. holders generally will include as dividend income the U.S. dollar value of the gross amount of any distributions of cash or property (without deduction for any withholding tax), other than certain pro rata distributions of ordinary shares, with respect to ordinary shares to the extent the distributions are made from our current or accumulated earnings and profits, as determined for U.S. federal income tax purposes. A U.S. holder will include the dividend income on the day actually or constructively received by the holder, in the case of ordinary shares, or by the depositary, in the case of ADSs. Distribution in excess of our current or accumulated earnings and profits, generally will first be treated as a tax-free return of capital to the extent of a U.S. holder’s adjusted U.S. federal income tax basis in such U.S holder’s ordinary shares or ADSs, and then as capital gain.
Dividends paid to a non-corporate U.S. holder on ordinary shares or ADSs will generally be taxable at the preferential rates applicable to long-term capital gains provided that (a) certain holding period requirements are satisfied, (b) the Treaty is a qualified treaty and we are eligible for benefits under the Treaty or our ordinary shares or ADSs are readily tradable on a U.S. securities market, and (c) we were not, in the taxable year prior to the year in which the dividend was paid, and are not, in the taxable year in which the dividend is paid, a PFIC. The Treaty has been approved for the purposes of the qualified dividend rules and the ADSs and Warrants are listed on NASDAQ. If, as is likely, we are currently a PFIC, any dividends paid to a non-corporate U.S. holder will not qualify for the preferential tax rates ordinarily applicable to “qualified dividends.” In the case of a corporate U.S. holder, dividends on ordinary shares and ADSs are taxed as ordinary income and will not be eligible for the dividends received deduction generally allowed to U.S. corporations in respect of dividends received from other U.S. corporations.
The amount of any cash distribution paid in any foreign currency will be equal to the U.S. dollar value of such currency, calculated by reference to the spot rate in effect on the date such distribution is received by the U.S. holder or, in the case of ADSs, by the Depositary, regardless of whether and when the foreign currency is in fact converted into U.S. dollars. If the foreign currency is converted into U.S. dollars on the date received, the U.S. holder generally should not recognize foreign currency gain or loss on such conversion. If the foreign currency is not converted into U.S. dollars on the date received, the U.S. holder will have a basis in the foreign currency equal to its U.S. dollar value on the date received, and generally will recognize foreign currency gain or loss on a subsequent conversion or other disposal of such currency. Such foreign currency gain or loss generally will be treated as U.S. source ordinary income or loss for foreign tax credit limitation purposes.
Dividends will be income from sources outside the United States, and generally will be “passive category” income or, for certain taxpayers, “general category” income, which are treated separately from each other for the purpose of computing the foreign tax credit allowable to a U.S. holder. In general, a taxpayer’s ability to use foreign tax credits may be limited and is dependent on the particular circumstances. U.S. holders should consult their own tax advisors with respect to the forgoing.
113
Sale, Exchange or other Disposition of Ordinary shares, ADSs or Warrants
Subject to the PFIC rules discussed below, a U.S. holder who sells or otherwise disposes of ordinary shares, ADSs or Warrants will recognize a capital gain or loss for U.S. federal income tax purposes equal to the difference between the U.S. dollar value of the amount realized and the holder’s adjusted tax basis, determined in U.S. dollars, in those ordinary shares, ADSs or Warrants. The gain or loss will generally be income or loss from sources within the United States for foreign tax credit limitation purposes. The capital gain of a non-corporate U.S. holder is generally taxed at preferential rates where the holder has a holding period greater than 12 months in the ordinary shares, ADSs or Warrants sold. The deductibility of capital losses is subject to limitations.
The U.S. dollar value of any foreign currency received upon a sale or other disposition of ordinary shares, ADSs or Warrants will be calculated by reference to the spot rate in effect on the date of sale or other disposal (or, in the case of a cash basis or electing accrual basis taxpayer, at the spot rate of exchange on the settlement date). A U.S. holder will have a tax basis in the foreign currency received equal to that U.S. dollar amount, and generally will recognize foreign currency gain or loss on a subsequent conversion or other disposal of the foreign currency. This foreign currency gain or loss generally will be treated as U.S.-source ordinary income or loss for foreign tax credit limitation purposes. However, if such foreign currency is converted into U.S. dollars on the date received by the U.S. holder, a cash basis or electing accrual basis U.S. holder should not recognize any gain or loss on such conversion.
Passive Foreign Investment Company
A non-U.S. corporation will be a PFIC for U.S. federal income tax purposes for any taxable year if either:
| (a) | 75 percent or more of its gross income for such year is “passive income” which for this purpose generally includes dividends, interest, royalties, rents and gains from commodities and securities transactions and gains from assets that produce passive income (the “Income Test”); or |
| (b) | 50 percent or more of the value of its gross assets (based on an average of the quarterly values of the gross assets) during such year is attributable to assets that produce passive income or are held for the production of passive income (the “Asset Test”). |
We believe it is likely that we qualified as a PFIC for fiscal year 2019. This arose because of the decline in the Company's stock price coupled with the fact that the applicable PFIC rules treat working capital as passive assets for purposes of the Asset Test. Assuming we are a PFIC, any gain realized on the sale or other disposition of ordinary shares, ADSs or Warrants would in general not be treated as a capital gain. Instead, a U.S. holder would be treated as if it had realized such gain and certain “excess distributions” ratably over its holding period for the ordinary shares, ADSs or Warrants and would be taxed at the highest tax rate in effect for each such year to which the gain was allocated, together with an interest charge in respect of the tax attributable to each such year. In addition, dividends received with respect to ordinary shares or ADSs would not be eligible for the special tax rates applicable to qualified dividend income either in the taxable year of the distribution or the preceding taxable year, but instead would be taxable under the tax rules described above. Assuming the ordinary shares or ADSs are “marketable stock”, however, a U.S. holder may mitigate the adverse tax consequences described above by timely electing to be taxed annually on a mark-to-market basis with respect to such shares or ADSs. Under certain attribution rules, if we are a PFIC, U.S. holders may be deemed to own a proportionate share of any of our subsidiaries that is also a PFIC, in which case U.S. holders will generally be subject to the PFIC rules described above with respect to any of our subsidiaries that is also a PFIC.
A U.S. holder that makes a mark-to-market election must include in ordinary income for each year that we are a PFIC an amount equal to the excess, if any, of the fair market value of such U.S. holder’s ordinary shares or ADSs at the close of the taxable year, over such U.S. holder’s adjusted tax basis therein. An electing U.S. holder may also claim an ordinary loss deduction for the excess, if any, of such U.S. holder’s adjusted tax basis in ordinary shares or ADSs over their fair market value at the close of the taxable year, but this deduction is allowable only to the extent of any net mark-to-market gains for prior years. Any income or deductions taken into account under these mark-to-market rules will also increase or decrease a U.S. holder’s adjusted tax basis in such U.S. holder’s ordinary shares or ADSs. Gains from an actual sale or other taxable disposition of ordinary shares or ADSs will generally be treated as ordinary income, and any losses incurred on a sale or other taxable disposition of ordinary shares or ADSs will generally be treated as an ordinary loss to the extent of any net mark-to-market gains for prior years. Once made, the election cannot be revoked without the consent of the IRS unless the ordinary shares or ADSs cease to be marketable.
114
In addition to the mark-to-market election, a U.S. holder may, in the first taxable year that we are treated as a PFIC with respect to such U.S. holder, make a “qualified electing fund election” (a “QEF election”) with respect to us and each of our subsidiaries that is a PFIC, in order to mitigate the adverse U.S. federal income tax consequences as a result of our being a PFIC. A U.S. holder must make the QEF election for each PFIC by attaching a separate properly completed IRS Form 8621 (“Information Return by a Shareholder of a PFIC or Qualified Electing Fund”) for each PFIC to its timely filed U.S. federal income tax return. A QEF election will not be available with respect to any Warrants. Upon request of a U.S. holder, we intend to provide the information necessary for such U.S. holder to make a QEF election with respect to us, and we will use commercially reasonable efforts to cause each direct and indirect subsidiary that we control that is a PFIC to provide such information. However, there is no assurance that we will have timely knowledge of our or our subsidiaries’ status as a PFIC in the future, and no assurance can be given that QEF election information will be available for any subsidiary that is a PFIC.
A U.S. holder that makes a QEF election with respect to a PFIC is required to annually include in income such U.S. holder's pro rata share of the PFIC’s ordinary earnings and net capital gain, which will be taxed as ordinary income and long-term capital gain, respectively, regardless of whether such amounts are actually distributed to U.S. holders. If a U.S. holder makes a QEF election with respect to us, any distributions paid by us out of our earnings and profits that were previously included in the income of such U.S. holder under the QEF election should not be taxable to such U.S. holder. The tax basis of a U.S. holder in ordinary shares or ADSs should be increased by an amount equal to any income included under the QEF election, and decreased by any amount distributed on the ordinary shares or ADSs that is not included in income. A U.S. holder should recognize capital gain or loss on the disposition of ordinary shares or ADSs in an amount equal to the difference between (i) the amount realized and (ii) the U.S. holder’s adjusted tax basis in the ordinary shares or ADSs. U.S. holders should note that if they make QEF elections with respect to us and any of our direct or indirect subsidiaries that are PFICs, such U.S. holders may have U.S. federal income tax liabilities with respect to their ordinary shares or ADSs for any taxable year that are in excess of any cash distributions received on their ordinary shares or ADSs for such taxable year. U.S. holders are strongly encouraged to consult their independent tax advisers regarding the PFIC rules and the making of QEF elections, in light of their particular circumstances.
Holders of PFIC stock are subject to additional U.S. information reporting rules. If a U.S. holder owns ordinary shares, ADSs or Warrants during any year in which we are a PFIC, the U.S. holder generally will be required to file IRS Form 8621 with such U.S. holder's federal income tax return for that year.
Backup Withholding Tax and Information Reporting Requirements
U.S. backup withholding tax and information reporting requirements generally apply to payments to non-corporate holders of ordinary shares or ADSs. Information reporting will apply to payments of dividends on ordinary shares or ADSs, and to proceeds from the disposition of ordinary shares, ADSs or Warrants, by a paying agent within the United States to a U.S. holder, other than U.S. holders that are exempt from information reporting and properly certify their exemption. A paying agent within the United States will be required to withhold at the applicable statutory rate, currently 24%, payments of dividends on ordinary shares or ADSs, and to proceeds from the disposition of ordinary shares, ADSs or Warrants, within the United States to a U.S. holder (other than U.S. holders that are exempt from backup withholding and properly certify their exemption) if the holder fails to furnish its correct taxpayer identification number or otherwise fails to comply with applicable backup withholding requirements. U.S. holders who are required to establish their exempt status generally must provide a properly completed IRS Form W-9.
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against a U.S. holder’s U.S. federal income tax liability. A U.S. holder generally may obtain a refund of any amounts withheld under the backup withholding rules in excess of such holder’s U.S. federal income tax liability by filing the appropriate claim for refund with the IRS in a timely manner and furnishing any required information.
Foreign Financial Asset Reporting
Certain U.S. holders that own “specified foreign financial assets” with an aggregate value in excess of USD$50,000 are generally required to file an information statement along with their U.S. federal tax returns, currently on IRS Form 8938, with respect to such assets. “Specified foreign financial assets” include any financial accounts held at a non-U.S. financial institution, as well as securities issued by a non-U.S. issuer that are not held in
115
accounts maintained by financial institutions. If a U.S. holder does not include in such holder’s gross income an amount relating to one or more specified foreign financial assets, and the amount such U.S. holder omits is more than USD$5,000, any tax such U.S. holder owes for the tax year can be assessed at any time within 6 years after the filing of such U.S. holder’s federal tax return, U.S. holders who fail to report the required information could be subject to substantial penalties. Prospective investors are encouraged to consult with their own tax advisors regarding the possible application of the foregoing to an investment in ordinary shares, ADSs or Warrants in light of their particular circumstances.
U.S. holders are strongly encouraged to consult their tax advisors with respect to our status as a PFIC, the availability and desirability of a mark-to-market election, and all information reporting obligations, in each case with respect to ordinary shares, ADSs and Warrants and in light of such U.S. holder's particular circumstances.
Australian Tax Considerations
In this section, we discuss the material Australian income tax, stamp duty and goods and services tax considerations related to the acquisition, ownership and disposal by the absolute beneficial owners of the ordinary shares, ADSs or Warrants.
It is based upon existing Australian tax law as of the date of this registration statement, which is subject to change, possibly retrospectively. This discussion does not address all aspects of Australian tax law which may be important to particular investors in light of their individual investment circumstances, such as shares held by investors subject to special tax rules (for example, financial institutions, insurance companies or tax exempt organizations). In addition, this summary does not discuss any foreign or state tax considerations, other than stamp duty and goods and services tax.
Prospective investors are urged to consult their tax advisors regarding the Australian and foreign income and other tax considerations of the acquisition, ownership and disposition of the shares or warrants. This summary is based upon the premise that the holder is not an Australian tax resident and is not carrying on business in Australia through a permanent establishment (referred to as a “Non-Australian Shareholder” in this summary).
Australian Income Tax
Nature of ADSs for Australian Taxation Purposes
Ordinary shares represented by ADSs held by a U.S. holder will be treated for Australian taxation purposes as held under a “bare trust” for such holder. Consequently, the underlying ordinary shares will be regarded as owned by the ADS holder for Australian income tax and capital gains tax purposes. Dividends paid on the underlying ordinary shares will also be treated as dividends paid to the ADS holder, as the person beneficially entitled to those dividends. Therefore, in the following analysis we discuss the tax consequences to Non-Australian Shareholders which, for Australian taxation purposes, will be the same as to U.S. holders of ADSs.
Taxation of Dividends
Australia operates a dividend imputation system under which dividends may be declared to be “franked” to the extent of tax paid on company profits. Fully franked dividends are not subject to dividend withholding tax. Dividends payable to Non-Australian Shareholders will be subject to dividend withholding tax, to the extent the dividends are not foreign (i.e., non-Australian) sourced and declared to be conduit foreign income, or CFI, and are unfranked. Dividend withholding tax will be imposed at 30%, unless a shareholder is a resident of a country with which Australia has a double taxation agreement and qualifies for the benefits of the treaty. Under the provisions of the current Double Taxation Convention between Australia and the United States, the Australian tax withheld on unfranked dividends that are not CFI paid by us to which a resident of the United States is beneficially entitled is limited to 15%.
If a company that is a Non-Australian Shareholder directly owns a 10% or more interest, the Australian tax withheld on unfranked dividends (that are not CFI) paid by us to which a resident of the United States is beneficially entitled is limited to 5%. In limited circumstances, the rate of withholding can be reduced to zero.
116
Exercise of Warrants
Any capital gain or loss on exercise of a warrant is disregarded. The amount paid to acquire the warrant, and the amount paid to exercise the warrant, are both included in the cost base and reduced cost base of the ordinary shares underlying the Warrants.
Tax on Sales or other Dispositions of Shares or Warrants—Capital Gains Tax
Non-Australian Shareholders will not be subject to Australian capital gains tax on the gain made on a sale or other disposal of ordinary shares or warrants (or recognize a capital loss on the lapse of a warrant), unless they, together with associates, hold 10% or more of our issued capital, at the time of disposal or for 12 months of the last two years prior to disposal.
Non-Australian Shareholders who own a 10% or more interest would be subject to Australian capital gains tax if more than 50% of our assets held directly or indirectly, determined by reference to market value, consists of Australian real property (which includes land and leasehold interests) or Australian mining, quarrying or prospecting rights. The Double Taxation Convention between the United States and Australia is unlikely to limit the amount of this taxable gain. Australian capital gains tax applies to net capital gains of Foreign Shareholders at the Australian tax rates for non-Australian resident individuals, which start at a marginal rate of 32.5%. Net capital gains are calculated after reduction for capital losses, which may only be offset against capital gains.
The 50% capital gains tax discount is not available to Non-Australian Shareholders on gains accrued after May 8, 2012. Companies are not entitled to a capital gains tax discount.
Broadly, where there is a disposal of certain taxable Australian property, the purchaser will be required to withhold and remit to the Australian Taxation Office (“ATO”) 12.5% of the proceeds from the sale. A transaction is excluded from the withholding requirements in certain circumstances, including where the value of the taxable Australian property is less than A$750,000, the transaction is an on-market transaction conducted on an approved stock exchange, a securities lending, or the transaction is conducted using a broker operated crossing system. There is also an exception to the requirement to withhold where the ATO Commissioner issues a clearance certificate which broadly certifies that the vendor is not a foreign person. The Non-Australian Shareholder may be entitled to receive a tax credit for the tax withheld by the purchaser which they may claim in their Australian income tax return.
Tax on Sales or other Dispositions of Shares or Warrants—Shareholders Holding Shares and Warrants on Revenue Account
Some Non-Australian Shareholders may hold ordinary shares on revenue rather than on capital account for example, share traders. These shareholders may have the gains made on the sale or other disposal of the ordinary shares and/or warrants included in their assessable income under the ordinary income provisions of the income tax law, if the gains are sourced in Australia.
Non-Australian Shareholders assessable under these ordinary income provisions in respect of gains made on ordinary shares and/or warrants held on revenue account would be assessed for such gains at the Australian tax rates for non-Australian resident individuals , which start at a marginal rate of 32.5%. Some relief from Australian income tax may be available to Non-Australian Shareholders under the Double Taxation Convention between the United States and Australia.
To the extent an amount would be included in a Non-Australian Shareholder’s assessable income under both the capital gains tax provisions and the ordinary income provisions, the capital gain amount would generally be reduced, so that the shareholder would not be subject to double tax on any part of the income gain or capital gain.
The comments above in “Tax on Sales or Other Dispositions of Shares or Warrants—Capital Gains Tax” regarding a purchaser being required to withhold 12.5% tax on the acquisition of certain taxable Australian property equally applies where the disposal of the Australian real property asset by a foreign resident is likely to generate gains on revenue account, rather than a capital gain.
117
Dual Residency
If a shareholder is a resident of both Australia and the United States under those countries’ domestic taxation laws, that shareholder may be subject to tax as an Australian resident. If, however, the shareholder is determined to be a U.S. resident for the purposes of the Double Taxation Convention between the United States and Australia, the Australian tax would be subject to limitation by the Double Taxation Convention. Shareholders should obtain specialist taxation advice in these circumstances.
Stamp Duty
No Australian stamp duty is payable by Australian residents or non-Australian residents on the issue, transfer and/or surrender of the ADSs, warrants or the ordinary shares in Benitec, provided that the shares issued, transferred and/or surrendered do not represent 90% or more of the issued shares in Benitec.
Australian Death Duty
Australia does not have estate or death duties. As a general rule, no capital gains tax liability is realized upon the inheritance of a deceased person’s shares. The disposal of inherited shares by beneficiaries may, however, give rise to a capital gains tax liability if the gain falls within the scope of Australia’s jurisdiction to tax.
Goods and Services Tax
The supply of ADSs, warrants and/or ordinary shares in Benitec will not be subject to Australian goods and services tax.
F. | Dividends and Paying Agents. |
Not applicable.
Not applicable.
Inspection of our records is governed by the Australian Corporations Act. Any member of the public has the right to inspect or obtain copies of our registers on the payment of a prescribed fee. Shareholders are not required to pay a fee for inspection of our registers or minute books of the meetings of shareholders. Other corporate records, including minutes of directors’ meetings, financial records and other documents, are not open for inspection by shareholders. Where a shareholder is acting in good faith and an inspection is deemed to be made for a proper purpose, a shareholder may apply to the court to make an order for inspection of our books.
We file periodic reports and information with the Securities and Exchange Commission. You may inspect a copy of these reports without charge at the Public Reference Room of the Securities and Exchange Commission at 100 F Street N.E., Washington, D.C. 20549 or at the Securities and Exchange Commission's regional offices 233 Broadway, New York, New York 10279 and 500 West Madison Street, Suite 1400, Chicago, Illinois 60661. The public may obtain information on the operation of the Public Reference Room by calling the Securities and Exchange Commission at 1-800-SEC-0330. The Securities and Exchange Commission also maintains an Internet site that contains reports, proxy and information statements and other information regarding registrants that file electronically with the Securities and Exchange Commission. The Securities and Exchange Commission's World Wide Web address is http://www.sec.gov.
Our ADSs are listed on The NASDAQ Capital Market. As a result, we are subject to the periodic reporting requirements of the Exchange Act and we file reports and other information with the Securities and Exchange Commission. These reports and other information and the registration statement and exhibits and schedules thereto may be inspected without charge at, and copies thereof may be obtained at prescribed rates from, the public reference facilities of the Securities and Exchange Commission and the electronic sources listed in the preceding paragraph.
118
We prepare annual and other reports. Our annual reports contain financial statements examined and reported upon, with opinions expressed by our independent auditors. Our consolidated financial statements included in these annual reports are prepared in conformity with IFRS. Our annual and other reports to our shareholders are posted on the “Investor Centre” page of our website at http://www.benitec.com. In furnishing our web site address in this report, however, we do not intend to incorporate any information on our web site into this report, and any information on our web site should not be considered to be part of this report.
We will also furnish the depositary with all notices of shareholder meetings and other reports and communications that are made generally available to our shareholders. The depositary, to the extent permitted by law, shall arrange for the transmittal to the registered holders of American Depositary Receipts of all notices, reports and communications, together with the governing instruments affecting our shares and any amendments thereto. Such documents are also available for inspection by registered holders of American Depositary Receipts at the principal office of the depositary.
I. | Subsidiary Information. |
Not applicable.
Item 11. Quantitative and Qualitative Disclosures About Market Risk.
We are exposed to market risk related to changes in interest rates and exchange rates.
As of June 30, 2019, 2018 and 2017, we had cash and cash equivalents of A$22.4 million, A$16.1 million and A$17.4 million, respectively, primarily held in bank accounts and term deposits. Our primary exposure to market risk is interest rate sensitivity, which is affected primarily by changes in the general level of Australian interest rates. Our available for sale securities are subject to interest rate risk and will fall in value if market interest rates increase. Due to the short-term duration of our investment portfolio and the low risk profile of our investments, an immediate 10% increase in interest rates would not have a material effect on the fair market value of our portfolio.
We are exposed to fluctuations in foreign currencies that arise from foreign currencies held in bank accounts and the translation of results from our operations outside Australia. Our foreign exchange exposure is primarily the U.S. dollar. Foreign currency risks arising from commitments in foreign currencies are managed by holding cash in that currency. Foreign currency translation risk is not hedged.
See Note 19 of our June 30, 2019, financial statements included in this Annual Report on Form 20-F for more detailed information on our financial risk management.
Item 12. Description of Securities Other than Equity Securities.
Not applicable.
Not applicable.
Not applicable.
119
D. | American Depositary Shares. |
Fees and Expenses
Persons depositing or withdrawing ordinary shares or ADS holders must pay the depositary: | | For: |
$5.00 (or less) per 100 ADSs (or portion of 100 ADSs) | | • Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property • Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates |
| | |
$.05 (or less) per ADS | | • Any cash distribution to you |
| | |
A fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited for issuance of ADSs | | • Distribution of securities distributed to holders of deposited securities which are distributed by the depositary to you |
| | |
$.05 (or less) per ADS per calendar year | | • Depositary services |
| | |
Registration or transfer fees | | • Transfer and registration of shares on our share register to or from the name of the depositary or its agent when you deposit or withdraw shares |
| | |
Expenses of the depositary | | • Cable, telex and facsimile transmissions (when expressly provided in the deposit agreement) • Converting foreign currency to U.S. dollars |
| | |
Taxes and other governmental charges the depositary or the custodian have to pay on any ADS or share underlying an ADS, for example, stock transfer taxes, stamp duty or withholding taxes | | • As necessary |
| | |
Any charges incurred by the depositary or its agents for servicing the deposited securities | | • As necessary |
The depositary collects its fees for delivery and surrender of ADSs directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deduction from cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The depositary may generally refuse to provide fee-attracting services until its fees for those services are paid. The depositary may collect any of its fees by deduction from any cash distribution payable to you that are obligated to pay those fees.
From time to time, the depositary may make payments to us to reimburse or share revenue from the fees collected from you, or waive fees and expenses for services provided, generally relating to costs and expenses arising out of establishment and maintenance of the ADS program. In performing its duties under the deposit agreement, the depositary may use brokers, dealers or other service providers that are affiliates of the depositary and that may earn or share fees or commissions.
120
PART II
Item 13. Defaults, Dividend Arrearages and Delinquencies
Not applicable.
Item 14. Material Modifications to the Rights of Security Holders and the Use of Proceeds
Not applicable.
Item 15. Controls and Procedures
Disclosure Controls and Procedures
As of June 30, 2019, under the supervision and with the participation of our management, including our Chief Executive Officer and Executive Director and Head of Australian Operations, we performed an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act).
Based on such evaluation, our Chief Executive Officer and Executive Director and Head of Australian Operations concluded that our disclosure controls and procedures are effective to provide reasonable assurance that the information we are required to disclose in the reports we file or submit under the Exchange Act is (1) recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and (2) accumulated and communicated to our management to allow timely decisions regarding required disclosures.
There are inherent limitations to the effectiveness of any disclosure controls and procedures system, including the possibility of human error and circumventing or overriding them. Even if effective, disclosure controls and procedures can provide only reasonable assurance of achieving their control objectives.
Management’s Annual Report on Internal Control over Financial Reporting
The management of Benitec is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) under the Exchange Act. Under the supervision and with the participation of our management, including our Chief Executive Officer and Executive Director and Head of Australian Operations, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of June 30, 2019 based on the criteria set forth in "Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission" ("COSO 2013"). Based on our evaluation under the criteria set forth in COSO 2013, our management concluded that our internal control over financial reporting was effective as of June 30, 2019.
Attestation Report of the Registered Public Accounting Firm
Not applicable. As an emerging growth company, we are not required to provide an attestation report of the company’s registered public accounting firm on management’s assessment regarding internal control over financial reporting.
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting that occurred during the period covered by this annual report that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
121
Item 16A. Audit Committee Financial Expert
Each member of our audit committee meets the financial literacy requirements of the listing standards of The NASDAQ Capital Market. Peter Francis acts as the chairman of the audit committee, and our board of directors has determined that Kevin Buchi is an audit committee “financial expert,” as defined by Item 407(d) of Regulation S-K under the Securities Act. Peter Francis meets the independence requirements of the NASDAQ Capital Market and SEC’s rules and regulations.
Item 16B. Code of Ethics
We have established a Code of Conduct, which sets out the standards of behavior that apply to every aspect of our dealings and relationships, both within and outside Benitec. The Code of Conduct applies to all directors, executive officers and employees of Benitec. The Code of Conduct is available on our website at www.benitec.com. For additional information regarding our Code of Conduct, see Item 6.C., “Directors, Senior Management and Employees - Board Practices - Code of Conduct.”
Item 16C. Principal Accountant Fees and Services
During the financial year the following fees were paid or payable for services provided by Grant Thornton Audit Pty Ltd and affiliated entities, the auditor of the Company:
| | 2019 | | | 2018 | |
| | A$ | | | A$ | |
Audit Fees | | | 168,398 | | | | 240,806 | |
Audit-Related Fees | | | — | | | | 24,650 | |
Tax Fees | | | 15,650 | | | | 42,617 | |
Total | | | 184,048 | | | | 308,073 | |
Audit fees
The audit fees include the aggregate fees incurred in fiscal years 2019 and 2018 for professional services rendered in connection with the audit of the Company’s annual financial statements and for related services that are reasonably related to the performance of the audit or services that are normally provided by the auditor in connection with regulatory filings of engagements for those financial years (including review of the Company’s Annual Report on Form 20-F, consents and other services related to SEC matters).
Audit-Related fees
Fees paid in respect of filing of SEC Form F-1 and F-3 consent services, which relates to procedures required by the auditor to issue their consent in the document.
Tax fees
Grant Thornton provides tax compliance services to the Company.
Pre-approval policies and procedures
Our audit committee reviews and pre-approves all audit services and permitted non-audit services (including the fees and other terms) to be provided by our independent auditors.
In fiscal year 2019, the amount paid for services other than strictly Audit fees, as approved by the audit committee, corresponded to 8.5% of the total paid to Grant Thornton.
122
Item 16D. Exemptions from the Listing Standards for Audit Committees.
Not applicable.
Item 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers
Not applicable.
Item 16F. Changes in Registrant’s Certifying Accountant
Not applicable.
Item 16G. Corporate Governance
Implications of Being an Emerging Growth Company
Pursuant to The Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), we are classified as an “Emerging Growth Company.” Under the JOBS Act, Emerging Growth Companies are exempt from certain reporting requirements, including the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act. Under this exemption, our auditor will not be required to attest to and report on management’s assessment of our internal controls over financial reporting during a five-year transition period. We may avail ourselves of these disclosure exemptions until we are no longer an emerging growth company.
Pursuant to the JOBS Act, we will remain an Emerging Growth Company until the earliest of:
| • | the end of the fiscal year in which the fifth anniversary of completion of our initial public offering in the United States; |
| • | the end of the first fiscal year in which the market value of our ordinary shares held by non-affiliates exceeds US$700 million as of the end of the second quarter of such fiscal year; |
| • | the end of the first fiscal year in which we have total annual gross revenues of at least US$1 billion; and |
| • | the date on which we have issued more than US$1 billion in non-convertible debt securities in any rolling three-year period. |
Implications of being a Foreign Private Issuer
We are also considered a “foreign private issuer.” In our capacity as a foreign private issuer, we are exempt from certain rules under the U.S. Securities Exchange Act of 1934, as amended (the “Exchange Act”), that impose certain disclosure obligations and procedural requirements for proxy solicitations under Section 14 of the Exchange Act. In addition, our officers, directors and principal shareholders are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and the rules under the Exchange Act with respect to their purchases and sales of our ordinary shares. Moreover, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. In addition, we are not required to comply with Regulation FD, which restricts the selective disclosure of material information.
123
Exemptions from certain NASDAQ Corporate Governance Rules
The NASDAQ listing rules allow for a foreign private issuer, such as Benitec, to follow its home country practices in lieu of certain of the NASDAQ's corporate governance standards. In connection with our NASDAQ Listing Application, we have relied on and expect to continue to rely on exemptions from certain corporate governance standards that are contrary to the laws, rules, regulations or generally accepted business practices in Australia. These exemptions are described below:
| • | Although the majority of our directors currently qualify as independent under the NASDAQ Listing Rules, we have relied on and expect in the future to continue to rely on an exemption from these independence requirements for a majority of our board of directors as prescribed by NASDAQ Listing Rules. The ASX Listing Rules do not require us to have a majority of independent directors although ASX Corporate Governance Principles and Recommendations do recommend a majority of independent directors. During fiscal 2017, we did, however, have a majority of directors who were "independent" as defined in the ASX Corporate Governance Principles and Recommendations, however the definition differs from NASDAQ's definition. Accordingly, because Australian law and generally accepted business practices in Australia regarding director independence differ from the independence requirements under NASDAQ Listing Rules, we may seek to claim this exemption in the future. |
| • | We have relied on and expect to continue to rely on an exemption from the requirement that our independent directors meet regularly in executive sessions under NASDAQ Listing Rules. The ASX Listing Rules and the Corporations Act do not require the independent directors of an Australian company to have such executive sessions and, accordingly, we seek to claim this exemption. |
| • | We have relied on and expect to continue to rely on an exemption from the quorum requirements applicable to meetings of shareholders under NASDAQ Listing Rules. In compliance with Australian law, our Constitution provides that three shareholders present, in person or by proxy, attorney or a representative, shall constitute a quorum for a general meeting. NASDAQ Listing Rules require that an issuer provide for a quorum as specified in its by-laws for any meeting of the holders of ordinary shares, which quorum may not be less than 331/3% of the outstanding shares of an issuer's voting ordinary shares. Accordingly, because applicable Australian law and rules governing quorums at shareholder meetings differ from NASDAQ's quorum requirements, we seek to claim this exemption. |
| • | We will rely an exemption from the requirement that at least two members of a compensation committee be "independent" as defined in NASDAQ Rule 5605(a)(2). The ASX Listing Rules and Australian law do not require an Australian company to establish a compensation committee, known in Australia as a remuneration committee, which is comprised solely of non-executive directors if the company is not included in the S&P/ASX300 Index at the beginning of its fiscal year. Benitec was not included on the S&P/ASX300 Index at the beginning of its last fiscal year and, hence, is not required under ASX Listing Rules to have a remuneration (compensation) committee. The ASX Corporate Governance Principles and Recommendations contain a non-binding recommendation that all ASX-listed companies should have a remuneration committee comprised of at least three members, a majority of whom (including the chair) are "independent". While these recommendations contain guidelines for assessing independence, ASX-listed entities are able to adopt their own definitions of an independent director for this purpose and is different from the definition in NASDAQ Rule 5605(a)(2). That being said, Benitec has, and expects to continue to have, a Remuneration and Nominations Committee consisting of two non-executive directors. |
124
| • | We have relied on and expect to continue to rely on an exemption from the requirement prescribed by NASDAQ Listing Rules that issuers obtain shareholder approval prior to the issuance of securities in connection with certain acquisitions, private placements of securities, or the establishment or amendment of certain stock option, purchase or other compensation plans. Applicable Australian law and the ASX Listing Rules differ from NASDAQ requirements, with the ASX Listing Rules providing generally for prior shareholder approval in numerous circumstances, including (i) issuance of equity securities exceeding 15% (or 25% under certain circumstances) of our issued share capital in any 12-month period (but, in determining the 15% limit, securities issued under an exception to the rule or with shareholder approval are not counted), (ii) issuance of equity securities to related parties (as defined in the ASX Listing Rules) and (iii) issuances of securities to directors or their associates under an employee incentive plan. Due to differences between Australian law and rules and the NASDAQ shareholder approval requirements, we seek to claim this exemption. |
| • | We have relied on and expect to continue to rely on an exemption from NASDAQ audit committee requirements set forth in NASDAQ Listing Rule 5605(c)(2), whereby the audit committee must have three independent members. The ASX Listing Rules and Australian law do not require an Australian company to have three independent audit committee members unless it is included in the S&P/ASX300 Index at the beginning of its fiscal year. Benitec was not included on the S&P/ASX300 Index at the beginning of its last fiscal year and, hence, is not required under ASX Listing Rules to have three independent audit committee members. NASDAQ has agreed that Benitec may have two directors on its audit committee so long as both directors satisfy NASDAQ's definition of "independence," as is currently the case. |
Item 16H. Mine Safety Disclosure
Not applicable.
125
PART III
Item 17. Financial Statements
Refer to “Item 18 – Financial Statements” below
Item 18. Financial Statements
Our Consolidated Financial Statements commencing on page F-1, as set forth in the following index, are hereby incorporated herein by reference. These Consolidated Financial Statements are filed as part of this Annual Report.
126
Index to Financial Statements
F-1
BENITEC BIOPHARMA LIMITED
Statement of Profit or Loss and Other Comprehensive Income
for the year ended June 30, 2019
| | | | Consolidated | |
| | Note | | 2019 | | | 2018 | | | 2017 | |
| | | | A$’000 | | | A$’000 | | | A$’000 | |
Revenue | | 4a | | | 16,159 | | | | 378 | | | | 333 | |
Other income | | 4b | | | 1,350 | | | | 4,087 | | | | 10,507 | |
Total Income | | | | | 17,509 | | | | 4,465 | | | | 10,840 | |
| | | | | | | | | | | | | | |
Expenses | | | | | | | | | | | | | | |
Royalties and licence fees | | | | | (609 | ) | | | (451 | ) | | | (272 | ) |
Research and development | | 5 | | | (3,104 | ) | | | (6,890 | ) | | | (6,925 | ) |
Employee benefits expense | | 5 | | | (5,025 | ) | | | (5,094 | ) | | | (5,015 | ) |
Share-based expense | | | | | (939 | ) | | | (434 | ) | | | (386 | ) |
Travel related costs | | | | | (350 | ) | | | (468 | ) | | | (629 | ) |
Consultants costs | | | | | (662 | ) | | | (783 | ) | | | (976 | ) |
Occupancy costs | | | | | (648 | ) | | | (587 | ) | | | (550 | ) |
Depreciation | | 5 | | | (221 | ) | | | (194 | ) | | | (217 | ) |
Corporate expenses | | | | | (1,884 | ) | | | (1,360 | ) | | | (1,540 | ) |
Foreign exchange realized loss | | | | | (106 | ) | | | (39 | ) | | | (98 | ) |
Foreign exchange unrealized loss | | | | | — | | | | (5 | ) | | | (168 | ) |
Change in market value of listed investment | | | | | (28 | ) | | | (41 | ) | | | — | |
Net loss on disposal of fixed assets | | | | | (9 | ) | | | (1 | ) | | | (7 | ) |
Total Expenses | | | | | (13,585 | ) | | | (16,347 | ) | | | (16,783 | ) |
| | | | | | | | | | | | | | |
Finance Income | | | | 170 | | | | 242 | | | | 253 | |
| | | | | | | | | | | | | | |
Profit/(Loss) before income tax | | | | | 4,094 | | | | (11,640 | ) | | | (5,690 | ) |
Income tax | | 6 | | | — | | | | — | | | | — | |
Profit/(Loss) after income tax for the year attributable to the owners of Benitec Biopharma Limited | | 17 | | | 4,094 | | | | (11,640 | ) | | | (5,690 | ) |
| | | | | | | | | | | | | | |
Other comprehensive income/(loss) | | | | | | | | | | | | | | |
Items that may be reclassified subsequently to profit and loss | | | | | | | | | | | | | | |
Foreign currency translation gain/( loss) | | | | | (117 | ) | | | (63 | ) | | | 34 | |
Income tax on items that may be reclassified to profit and loss | | | | | — | | | | — | | | | | |
Total comprehensive Profit/(Loss) for the year attributable to the owners of Benitec Biopharma Limited | | | | | 3,977 | | | | (11,703 | ) | | | (5,656 | ) |
| | | | | | | | | | | | | | |
Basic earnings income/(loss) cents per share | | 28 | | 1.59 | | | | (5.53 | ) | | | (3.24 | ) |
Diluted earnings income/(loss) cents per share | | 28 | | 1.59 | | | | (5.53 | ) | | | (3.24 | ) |
The above statement of profit or loss and other comprehensive income should be read in conjunction with the accompanying notes.
F-2
BENITEC BIOPHARMA LIMITED
Consolidated Statement of Financial Position
as at June 30, 2019
| | Note | | 2019 | | | 2018 | |
| | | | A$’000 | | | A$’000 | |
ASSETS | | | | | | | | | | |
Current Assets | | | | | | | | | | |
Cash and cash equivalents | | 7 | | | 22,411 | | | | 16,085 | |
Other financial assets | | 8 | | | 181 | | | | 130 | |
Trade and other receivables | | 9 | | | 3,616 | | | | 4,255 | |
Other assets | | 10 | | | 535 | | | | 425 | |
Total Current Assets | | | | | 26,743 | | | | 20,895 | |
| | | | | | | | | | |
Non-Current Assets | | | | | | | | | | |
Deposits | | 11 | | | 13 | | | | 125 | |
Plant and equipment | | 12 | | | 670 | | | | 319 | |
Total Non-Current Assets | | | | | 683 | | | | 444 | |
| | | | | | | | | | |
TOTAL ASSETS | | | | | 27,426 | | | | 21,339 | |
| | | | | | | | | | |
LIABILITIES | | | | | | | | | | |
Current liabilities | | | | | | | | | | |
Trade and other payables | | 13 | | | 3,556 | | | | 2,376 | |
Provisions | | 14 | | | 210 | | | | 171 | |
Total Current Liabilities | | | | | 3,766 | | | | 2,547 | |
| | | | | | | | | | |
Non-Current Liabilities | | | | | | | | | | |
Provisions | | | | | — | | | | 48 | |
Total Non-Current Liabilities | | | | | — | | | | 48 | |
| | | | | | | | | | |
TOTAL LIABILITIES | | | | | 3,766 | | | | 2,595 | |
| | | | | | | | | | |
NET ASSETS | | | | | 23,660 | | | | 18,744 | |
| | | | | | | | | | |
EQUITY | | | | | | | | | | |
Issued capital | | 15 | | | 164,087 | | | | 164,087 | |
Reserves | | 16 | | | 831 | | | | 1,492 | |
Accumulated losses | | 17 | | | (141,258 | ) | | | (146,835 | ) |
TOTAL EQUITY | | | | | 23,660 | | | | 18,744 | |
The above statement of financial position should be read in conjunction with the accompanying notes
F-3
BENITEC BIOPHARMA LIMITED
Consolidated Statement of Changes in Equity
for the year ended June 30, 2019
| | Issued capital A$'000 | | | Reserves A$'000 | | | Accumulated losses A$'000 | | | Total equity A$'000 | |
Balance at June 30, 2016 | | | 147,641 | | | | 2,565 | | | | (131,369 | ) | | | 18,837 | |
| | | | | | | | | | | | | | | | |
Loss after income tax | | | — | | | | — | | | | (5,690 | ) | | | (5,690 | ) |
Other comprehensive income- Foreign exchange translation reserve | | | — | | | | 34 | | | | — | | | | 34 | |
Total comprehensive income | | | — | | | | 34 | | | | (5,690 | ) | | | (5,656 | ) |
Contributions of equity, net of transaction costs | | | 7,939 | | | | — | | | | — | | | | 7,939 | |
Share based payments | | | — | | | | 386 | | | | — | | | | 386 | |
Transfer of expired share based payments | | | — | | | | (1,311 | ) | | | 1,311 | | | | — | |
At June 30, 2017 | | | 155,580 | | | | 1,674 | | | | (135,748 | ) | | | 21,506 | |
| | | | | | | | | | | | | | | | |
Balance at July 1, 2017 | | | 155,580 | | | | 1,674 | | | | (135,748 | ) | | | 21,506 | |
| | | | | | | | | | | | | | | | |
Loss after income tax | | | — | | | | — | | | | (11,640 | ) | | | (11,640 | ) |
Other comprehensive income- Foreign exchange translation reserve | | | — | | | | (63 | ) | | | — | | | | (63 | ) |
Total comprehensive income | | | — | | | | (63 | ) | | | (11,640 | ) | | | (11,703 | ) |
| | | | | | | | | | | | | | | | |
Contributions of equity, net of transaction costs | | | 8,507 | | | | — | | | | — | | | | 8,507 | |
Share-based payments | | | — | | | | 434 | | | | — | | | | 434 | |
Transfer of expired share-based payments | | | — | | | | (553 | ) | | | 553 | | | | — | |
Balance at June 30, 2018 | | | 164,087 | | | | 1,492 | | | | (146,835 | ) | | | 18,744 | |
Balance at July 1, 2018 | | | 164,087 | | | | 1,492 | | | | (146,835 | ) | | | 18,744 | |
| | | | | | | | | | | | | | | | |
Profit after income tax | | | — | | | | — | | | | 4,094 | | | | 4,094 | |
Other comprehensive income- Foreign exchange translation reserve | | | — | | | | (117 | ) | | | — | | | | (117 | ) |
Total comprehensive income | | | — | | | | (117 | ) | | | 4,094 | | | | 3,977 | |
| | | | | | | | | | | | | | | | |
Contributions of equity, net of transaction costs | | | — | | | | — | | | | — | | | | — | |
Share-based payments | | | — | | | | 939 | | | | — | | | | 939 | |
Transfer of expired share-based payments | | | — | | | | (1,483 | ) | | | 1,483 | | | | — | |
Balance at June 30, 2019 | | | 164,087 | | | | 831 | | | | (141,258 | ) | | | 23,660 | |
The above statement of changes in equity should be read in conjunction with the accompanying notes
F-4
BENITEC BIOPHARMA LIMITED
Consolidated Statement of Cash Flows
for the year ended June 30, 2019
| | Note | | 2019 | | | 2018 | | | 2017 | |
| | | | A$’000 | | | A$’000 | | | A$’000 | |
Cash flows from operating activities | | | | | | | | | | | | | | |
Receipts from customers | | | | | 17,664 | | | | 237 | | | | 333 | |
Interest received | | | | | 164 | | | | 246 | | | | 242 | |
Government grants | | | | | 4,121 | | | | 4,112 | | | | 6,274 | |
Receipts of CRO prepayment | | | | | — | | | | 109 | | | | 791 | |
Payments to suppliers and employees | | | | | (16,092 | ) | | | (14,498 | ) | | | (15,944 | ) |
Net cash provided by/(used in) operating activities | | 27 | | | 5,857 | | | | (9,794 | ) | | | (8,304 | ) |
| | | | | | | | | | | | | | |
Cash flows from investing activities | | | | | | | | | | | | | | |
Payments for plant and equipment | | 12 | | | (576 | ) | | | (83 | ) | | | (171 | ) |
Proceeds from disposal of plant and equipment | | | | | 6 | | | | 2 | | | | — | |
Security deposit | | | | | — | | | | — | | | | (131 | ) |
Clinical trial deposit | | | | | — | | | | (66 | ) | | | — | |
Net cash used in investing activities | | | | | (570 | ) | | | (147 | ) | | | (302 | ) |
| | | | | | | | | | | | | | |
Cash flows from financing activities | | | | | | | | | | | | | | |
Proceeds from issue of shares | | | | | — | | | | 8,820 | | | | 8,072 | |
IPO and share issue transaction costs | | | | | — | | | | (313 | ) | | | (133 | ) |
Net cash from financing activities | | | | | — | | | | 8,507 | | | | 7,939 | |
| | | | | | | | | | | | | | |
Net increase/(decrease) in cash and cash equivalents | | | | | 5,287 | | | | (1,434 | ) | | | (667 | ) |
Cash and cash equivalents at the beginning of the financial year | | | | | 16,085 | | | | 17,375 | | | | 18,230 | |
Effects of exchange rate changes on cash and cash equivalents | | | | | 1,039 | | | | 144 | | | | (188 | ) |
Cash and cash equivalents at the end of the financial year | | 7 | | | 22,411 | | | | 16,085 | | | | 17,375 | |
The above statement of cash flows should be read in conjunction with the accompanying notes
F-5
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019
Note 1. Significant accounting policies
The principal accounting policies adopted in the preparation of the financial statements are set out below. These policies have been consistently applied to all the years presented, unless otherwise stated.
Basis of preparation
These general purpose financial statements have been prepared in accordance with Australian Accounting Standards and Interpretations issued by the Australian Accounting Standards Board ('AASB') and the Corporations Act 2001, as appropriate for for-profit oriented entities. These financial statements also comply with International Financial Reporting Standards (‘IFRS’) as issued by the International Accounting Standards Board ('IASB').
Historical cost convention
The financial statements have been prepared under the historical cost convention.
Critical accounting estimates
The preparation of the financial statements requires the use of certain critical accounting estimates. It also requires management to exercise its judgement in the process of applying the Group's accounting policies. The areas involving a higher degree of judgement or complexity, or areas where assumptions and estimates are significant to the financial statements, are disclosed in note 2.
New, revised or amending Accounting Standards and Interpretations adopted
The annual financial statements have been prepared in accordance with the accounting policies adopted in the Group’s last annual financial statements for the year ended June 30, 2018, with the exception of new accounting standards IFRS 15 Revenue from Contracts with Customers and IFRS 9 Financial Instruments (2014) which became mandatorily effective for financial years beginning on or after January 1, 2018.
The nature and effect of the changes arising from these standards are summarised below.
New Standards adopted as at July 1, 2018
IFRS 15 Revenue from Contracts with Customers
IFRS 15 replaces IAS 18 and covers contracts for goods and services. IFRS 15 is based on the principle that revenue is recognised when control of a good or service transfers to a customer; so the notion of control replaces the existing notion of risks and rewards.
The Group has adopted IFRS 15 from July 1, 2018, using a modified retrospective approach. Under this approach, transitional adjustments are recognised in retained earnings as at July 1, 2018 (the date of initial application), without restating the comparative period.
Many of the Group’s contracts comprise a variety of performance obligations including, but not limited to, licensing fees, ongoing support, reimbursement of know how. Under IFRS 15, the Group must evaluate the separability of the promised goods or services based on whether they are ‘distinct’. A promised good or service is ‘distinct’ if both:
• | the customer benefits from the item either on its own or together with other readily available resources: and |
• | it is ‘separately identifiable’ (i.e. the Group does not provide significant service integrating, modifying or customising it). |
While this represents significant new guidance, the implementation of this new guidance did not have a significant impact on the timing or amount of revenue recognised during the year. No adjustments were required to account for the impact of IFRS 15 on initial adoption.
F-6
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 1. Significant accounting policies continued
IFRS 9 Financial Instruments
IFRS 9 Financial Instruments replaces IAS 39 Financial Instruments: Recognition and Measurement requirements. It makes changes to the previous guidance to the classification and measurement of financial assets and includes an ‘expected credit loss’ model for impairment of financial assets. Our financial assets include those outlined in note 9 and trade and other receivables. There was no change to the classification of listed equity investments. They remain fair value through the profit and loss. The security deposit also remains unchanged and therefore no adjustment was required to be made to retained earnings.
The classification of trade and other receivables changed from loans and receivables to amortised cost. No adjustment was required as a result of this change.
Changes in significant accounting policies
The Group’s accounting policies, which have changed as a result of the changes to accounting standards noted above, are summarised below:
Revenue
Revenue arises mainly from licensing revenues and royalties, as well through the provision of research and development services.
To determine whether to recognise revenue, the Group follows a 5-step process:
1. Identifying the contract with a customer
2. Identifying the performance obligations
3. Determining the transaction price
4. Allocating the transaction price to the performance obligations
5. Recognising revenue when/as performance obligation(s) are satisfied
Further information about each source of revenue from contracts with customers and the criteria for recognition follows.
Licensing revenues
Revenue from licensees of Benitec’s intellectual property reflects the transfer of a right to use the intellectual property as it exists at the point in time in which the licence is transferred to the customer. Consideration can be variable and is estimated using the most likely amount method. Subsequently, the estimate is constrained until it is highly probable that a significant revenue reversal will not occur when the uncertainty is resolved. Revenue is recognised as or when the performance obligations are satisfied.
The Group recognises contract liabilities for consideration received in respect of unsatisfied performance obligations and reports these amounts as other liabilities in the statement of financial position. Similarly, if the Group satisfies a performance obligation before it receives the consideration, the Group recognises either a contract asset or a receivable in its statement of financial position, depending on whether something other than the passage of time is required before the consideration is due.
Royalties
Revenue from licensees of Benitec’s intellectual property reflect a right to use the intellectual property as it exists at the point in time in which the licence is granted. Where consideration is based on sales of product by the licensee, revenue is recognised when the customer’s subsequent sales of product occurs.
F-7
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 1. Significant accounting policies continued
Services revenue
Revenue is earned (constrained by variable considerations) from the provision of research and development services to customers. Services revenue is recognised when performance obligations are either satisfied over time or at a point in time. Generally, the provision of research and development services under a contract with a customer will represent satisfaction of a performance obligation over time where Benitec retains the right to payment for services performed but not yet completed.
Financial Instruments
Recognition and derecognition
Financial assets and financial liabilities are recognised when the Group becomes a party to the contractual provisions of the financial instrument and are measured initially at fair value adjusted by transactions costs, except for those carried at fair value through profit or loss, which are measured initially at fair value. Subsequent measurement of financial assets and financial liabilities are described below.
Financial assets are derecognised when the contractual rights to the cash flows from the financial asset expire, or when the financial asset and substantially all the risks and rewards are transferred. A financial liability is derecognised when it is extinguished, discharged, cancelled or expires.
Classification and initial measurement of financial assets
Except for those trade receivables that do not contain a significant financing component and are measured at the transaction price in accordance with IFRS 15, all financial assets are initially measured at fair value adjusted for transaction costs (where applicable).
Subsequent measurement of financial assets
For the purpose of subsequent measurement, financial assets, other than those designated and effective as hedging instruments, are classified into the following categories upon initial recognition:
• | financial assets at amortised cost |
• | financial assets at fair value through profit or loss (FVPL) |
Classifications are determined by both:
• | The entity’s business model for managing the financial asset |
• | The contractual cash flow characteristics of the financial assets |
All income and expenses relating to financial assets that are recognised in profit or loss are presented within finance costs, finance income or other financial items, except for impairment of trade receivables which is presented within other expenses.
Financial assets at amortised cost
Financial assets are measured at amortised cost if the assets meet the following conditions (and are not designated as FVPL):
• | they are held within a business model whose objective is to hold the financial assets and collect its contractual cash flows |
• | the contractual terms of the financial assets give rise to cash flows that are solely payments of principal and interest on the principal amount outstanding |
F-8
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 1. Significant accounting policies continued
After initial recognition, these are measured at amortised cost using the effective interest method. Discounting is omitted where the effect of discounting is immaterial. The Group’s cash and cash equivalents, trade and most other receivables fall into this category of financial instruments.
Financial assets at fair value through profit or loss (FVPL)
Financial assets that are held within a business model other than ‘hold to collect’ or ‘hold to collect and sell’ are categorised at fair value through profit and loss. Further, irrespective of business model, financial assets whose contractual cash flows are not solely payments of principal and interest are accounted for at FVPL. All derivative financial instruments fall into this category, except for those designated and effective as hedging instruments, for which the hedge accounting requirements apply. The Group’s investments in equity instruments fall under this category.
Impairment of financial assets
IFRS 9’s new impairment model uses more forward-looking information to recognize expected credit losses - the ‘expected credit losses (ECL) model’. The application of the new impairment model depends on whether there has been a significant increase in credit risk.
The Group considers a broader range of information when assessing credit risk and measuring expected credit losses, including past events, current conditions, reasonable and supportable forecasts that affect the expected collectability of the future cash flows of the instrument.
In applying this forward-looking approach, a distinction is made between:
• | financial instruments that have not deteriorated significantly in credit quality since initial recognition or that have low credit risk (‘Stage 1’) and |
• | financial instruments that have deteriorated significantly in credit quality since initial recognition and whose credit risk is not low (‘Stage 2’). |
• | ‘Stage 3’ would cover financial assets that have objective evidence of impairment at the reporting date. |
12-month expected credit losses are recognised for the first category while ‘lifetime expected credit losses’ are recognised for the second category.
Measurement of the expected credit losses is determined by a probability-weighted estimate of credit losses over the expected life of the financial instrument.
Trade and other receivables
The Group makes use of a simplified approach in accounting for trade and other receivables as well as contract assets and records the loss allowance at the amount equal to the expected lifetime credit losses. In using this practical expedient, the Group uses its historical experience, external indicators and forward-looking information to calculate the expected credit losses using a provision matrix.
The Group assess impairment of trade receivables on a collective basis as they possess credit risk characteristics based on the days past due. The Group allows 1% for amounts that are 30 to 60 days past due, 1.5% for amounts that are between 60 and 90 days past due and writes off fully any amounts that are more than 90 days past due.
All financial assets, except for those at fair value through profit or loss (FVPL), are subject to review for impairment at least at each reporting date to identify whether there is any objective evidence that a financial asset or a group of financial assets is impaired.
F-9
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 1. Significant accounting policies continued
Classification and measurement of financial liabilities
As the accounting for financial liabilities remains largely unchanged from IAS 39, the Group’s financial liabilities were not impacted by the adoption of IFRS 9. However, for completeness, the accounting policy is disclosed below.
The Group’s financial liabilities include trade and other payables. Financial liabilities are initially measured at fair value, and, where applicable, adjusted for transaction costs unless the Group designated a financial liability at fair value through profit or loss. Subsequently, financial liabilities are measured at amortised cost using the effective interest method except for financial liabilities designated at FVPL, which are carried subsequently at fair value with gains or losses recognised in profit or loss.
All interest-related charges and, if applicable, changes in an instrument’s fair value that are reported in profit or loss are included within finance costs or finance income.
New Accounting Standards and Interpretations not yet mandatory or early adopted
IFRS 16 Leases - The IASB has issued a new standard for the recognition of leases. This will replace IAS 17: Leases. The new standard introduces a single lessee accounting model that no longer requires leases to be classified as operating or financing.
Other major changes include, the recognition of a right-to-use asset and liability, depreciation of right-to-use assets in line with IAS 16: Property Plant and Equipment, variable lease payments that depend on an index or rate are included in the initial measurement of lease liability, option for lessee to not separate non-lease components and account for all components as a lease, and additional disclosure requirements.
Impact - The entity has undertaken a detailed review and has concluded that it will have a material impact on its financial position on the transactions and balances recognized in the financial statements when it is first adopted for the year ending June 30, 2020 due to the material size of lease entered into by the Company. The Company’s only lease is the lease on its research and development facilities. The Group’s existing lease commitments are set out in note 22.
The following is a reconciliation of total operating lease commitments as at June 30, 2019 to the lease liability recognised at July 1, 2019.
Total operating lease commitments disclosed at June 30, 2019 | A$ | | 920,883 | |
| | | | |
Recognition exceptions: | | | | |
Lease with remaining lease term of less than 12 months | A$ | | (7,621 | ) |
| | | | |
Operating leases liabilities before discounting | A$ | | 913,262 | |
Discounted using incremental borrowing rate | A$ | | (70,169 | ) |
Operating lease liabilities | A$ | | 843,093 | |
The Mandatory application date/date of adoption by group - Must be applied for financial years commencing on or after January 1, 2019. Expected date of adoption by the group: July 1, 2019.
There are no other standards that are not yet effective and that would be expected to have a material impact on the entity in future reporting periods and on foreseeable future transactions.
Going concern
The directors have prepared the financial statements on a going concern basis after taking into consideration the net income for the year of A$4.094m (2018 loss: A$11.640m) and the cash and cash equivalents balance of A$22.411m (2018:A$16.085m). The directors have recognised the capital raisings in the last 3 years, performed a review of the cash flow forecasts, considered the cash flow needs of the Group, and believe that there will be sufficient cash to maintain the going concern status of the Group.
F-10
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 1. Significant accounting policies continued
We expect that our research and development and general and administrative expenses will proceed at a lower rate compared to the previous year due to staff rationalisation and the single focus on OPMD program due to the loss of the Axovant contract.
The financial report does not contain any adjustments to the amounts or classifications of recorded assets or liabilities that might be necessary if the Group does not continue as a going concern.
The financial statements take no account of the consequences, if any, of the effects of unsuccessful product development or commercialisation, nor of the inability of the Group to obtain adequate funding in the future.
Parent entity information
In accordance with the Corporations Act 2001, these financial statements present the results of the Group only. Supplementary information about the parent entity is disclosed in note 24.
Principles of consolidation
The consolidated financial statements incorporate the assets and liabilities of all subsidiaries of Benitec Biopharma Limited ('Company' or 'parent entity') as at June 30, 2019 and the results of all subsidiaries for the year then ended. Benitec Biopharma Limited and its subsidiaries together are referred to in these financial statements as the 'Group'.
Subsidiaries are all those entities over which the Group has control. The Group controls an entity when it is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power to direct the activities of the entity. Subsidiaries are fully consolidated from the date on which control is transferred to the Group. They are de-consolidated from the date that control ceases.
The Company’s 100% owned subsidiary, Tacere Therapeutics, Inc. has a 31 December year end. The Company is reviewing the appropriate time to align the subsidiary year end to the parent’s year end. For consolidation purposes Tacere prepares financial statements for the 12 month period ended 30 June that are used to consolidate into the group accounts.
Intercompany transactions, balances and unrealised gains on transactions between entities in the Group are eliminated. Unrealised losses are also eliminated unless the transaction provides evidence of the impairment of the asset transferred. Accounting policies of subsidiaries have been changed where necessary to ensure consistency with the policies adopted by the Group.
The acquisition of subsidiaries is accounted for using the acquisition method of accounting. A change in ownership interest, without the loss of control, is accounted for as an equity transaction, where the difference between the consideration transferred and the book value of the share of the non-controlling interest acquired is recognised directly in equity attributable to the parent.
Where the Group loses control over a subsidiary, it derecognises the assets including goodwill, liabilities and non-controlling interest in the subsidiary together with any cumulative translation differences recognised in equity. The Group recognises the fair value of the consideration received and the fair value of any investment retained together with any gain or loss in profit or loss.
Operating segments
Operating segments are presented using the 'management approach', where the information presented is on the same basis as the internal reports provided to the Chief Operating Decision Makers ('CODM'). The CODM is responsible for the allocation of resources to operating segments and assessing their performance.
F-11
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 1. Significant accounting policies continued
Foreign currency translation
The financial statements are presented in Australian dollars, which is Benitec Biopharma Limited's functional and presentation currency.
Foreign currency transactions
Foreign currency transactions are translated into Australian dollars using the exchange rates prevailing at the dates of the transactions. Foreign exchange gains and losses resulting from the settlement of such transactions and from the translation at financial year-end exchange rates of monetary assets and liabilities denominated in foreign currencies are recognised in profit or loss.
Foreign operations
The assets and liabilities of foreign operations are translated into Australian dollars using the exchange rates at the reporting date. The revenues and expenses of foreign operations are translated into Australian dollars using the average exchange rates, which approximate the rates at the dates of the transactions, for the period. All resulting foreign exchange differences are recognised in other comprehensive income through the foreign currency reserve in equity. The foreign currency reserve is recognised in profit or loss when the foreign operation or net investment is disposed of.
Government research and development grants
Government grants are recognised at fair value where there is reasonable assurance that the grant will be received and all grant conditions will be met. Grants relating to expense items are recognised as income over the periods necessary to match the grant costs they are compensating.
Grant income is generated through the Australian federal government’s Research and Development Tax Incentive program, under which the government provides a cash refund for the 43.5% (2018: 43.5%) of eligible research and development expenditures. Grants are recorded when a reliable estimate can be made. In the twelve months ended `June 30, 2019 the Company estimated the grant income that will be receivable following the lodgement of the 2019 tax return. Prior to June 30, 2017 the grant income was only taken up on the lodgement of the previous year’s tax return, which was the time at which it was considered a reliable estimate could be made.
Income tax
The income tax expense or benefit for the period is the tax payable on that period's taxable income based on the applicable income tax rate for each jurisdiction, adjusted by the changes in deferred tax assets and liabilities attributable to temporary differences, unused tax losses and the adjustment recognised for prior periods, where applicable.
Deferred tax assets and liabilities are recognised for temporary differences at the tax rates expected to be applied when the assets are recovered or liabilities are settled, based on those tax rates that are enacted or substantively enacted, except for:
• | When the deferred income tax asset or liability arises from the initial recognition of goodwill or an asset or liability in a transaction that is not a business combination and that, at the time of the transaction, affects neither the accounting nor taxable profits; or |
• | When the taxable temporary difference is associated with interests in subsidiaries, associates or joint ventures, and the timing of the reversal can be controlled, and it is probable that the temporary difference will not reverse in the foreseeable future. |
F-12
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 1. Significant accounting policies continued
Deferred tax assets are recognised for deductible temporary differences and unused tax losses only if it is probable that future taxable amounts will be available to utilise those temporary differences and losses.
The carrying amount of recognised and unrecognised deferred tax assets are reviewed at each reporting date. Deferred tax assets recognised are reduced to the extent that it is no longer probable that future taxable profits will be available for the carrying amount to be recovered. Previously unrecognised deferred tax assets are recognised to the extent that it is probable that there are future taxable profits available to recover the asset.
Deferred tax assets and liabilities are offset only where there is a legally enforceable right to offset current tax assets against current tax liabilities and deferred tax assets against deferred tax liabilities; and they relate to the same taxable authority on either the same taxable entity or different taxable entities which intend to settle simultaneously.
Benitec Biopharma Limited (the 'head entity') and its wholly-owned Australian subsidiaries have formed an income tax consolidated group under the tax consolidation regime. The head entity and each subsidiary in the tax consolidated group continue to account for their own current and deferred tax amounts. The tax consolidated group has applied the 'separate taxpayer within group' approach in determining the appropriate amount of taxes to allocate to members of the tax consolidated group. No tax sharing agreement has been entered between entities in the tax consolidated group.
In addition to its own current and deferred tax amounts, the head entity also recognises the current tax liabilities (or assets) and the deferred tax assets arising from unused tax losses and unused tax credits assumed from each subsidiary in the tax consolidated group.
Current and non-current classification
Assets and liabilities are presented in the statement of financial position based on current and non-current classification.
An asset is classified as current when: it is either expected to be realised or intended to be sold or consumed in normal operating cycle; it is held primarily for the purpose of trading; it is expected to be realised within 12 months after the reporting period; or the asset is cash or cash equivalent unless restricted from being exchanged or used to settle a liability for at least 12 months after the reporting period. All other assets are classified as non-current.
A liability is classified as current when: it is either expected to be settled in normal operating cycle; it is held primarily for the purpose of trading; it is due to be settled within 12 months after the reporting period; or there is no unconditional right to defer the settlement of the liability for at least 12 months after the reporting period. All other liabilities are classified as non-current.
Deferred tax assets and liabilities are always classified as non-current.
Cash and cash equivalents
Cash and cash equivalents includes cash on hand, deposits held at call with financial institutions, other short-term, highly liquid investments with original maturities of three months or less that are readily convertible to known amounts of cash and which are subject to an insignificant risk of changes in value.
Plant and equipment
Plant and equipment is stated at historical cost less accumulated depreciation and impairment. Historical cost includes expenditure that is directly attributable to the acquisition of the items.
F-13
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 1. Significant accounting policies continued
Depreciation is calculated on a straight-line basis to write off the net cost of each item of property, plant and equipment (excluding land) over their expected useful lives as follows:
Leasehold improvements | | period of the lease term |
Plant and equipment | | 3-7 years |
The residual values, useful lives and depreciation methods are reviewed, and adjusted if appropriate, at each reporting date.
An item of plant and equipment is derecognised upon disposal or when there is no future economic benefit to the Group. Gains and losses between the carrying amount and the disposal proceeds are taken to profit or loss.
Leases
The determination of whether an arrangement is or contains a lease is based on the substance of the arrangement and requires an assessment of whether the fulfilment of the arrangement is dependent on the use of a specific asset or assets and the arrangement conveys a right to use the asset.
Impairment of non-financial assets
Other intangible assets that have an indefinite useful life are not subject to amortisation and are tested annually for impairment, or more frequently if events or changes in circumstances indicate that they might be impaired. Other non-financial assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. An impairment loss is recognised for the amount by which the asset's carrying amount exceeds its recoverable amount.
Recoverable amount is the higher of an asset's fair value less costs of disposal and value-in-use. The value-in-use is the present value of the estimated future cash flows relating to the asset using a pre-tax discount rate specific to the asset or cash-generating unit to which the asset belongs. Assets that do not have independent cash flows are grouped together to form a cash-generating unit.
Trade and other payables
These amounts represent liabilities for goods and services provided to the Group prior to the end of the financial year and which are unpaid. Due to their short-term nature, they are measured at amortised cost and are not discounted. The amounts are unsecured and are usually paid within 30 days of recognition.
Employee benefits
Short-term employee benefits
Liabilities for wages and salaries and other employee benefits expected to be settled within 12 months of the reporting date are measured at the amounts expected to be paid when the liabilities are settled.
Other long-term employee benefits
Employee benefits not expected to be settled within 12 months of the reporting date are measured as the present value of expected future payments to be made in respect of services provided by employees up to the reporting date using the projected unit credit method. Consideration is given to expected future wage and salary levels, experience of employee departures and periods of service. Expected future payments are discounted using market yields at the reporting date on high quality corporate bonds with terms to maturity and currency that match, as closely as possible, the estimated future cash outflows.
F-14
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 1. Significant accounting policies continued
Defined contribution superannuation expense
Contributions to defined contribution superannuation plans are expensed in the period in which they are incurred.
Share-based payments
Equity-settled share-based compensation benefits are provided to directors and senior executives. The plan currently in place to provide these benefits is the Employee Share Option Plan ('ESOP').
Equity-settled transactions are awards of shares, or options over shares that are provided to employees in exchange for the rendering of services.
The cost of equity-settled transactions are measured at fair value on grant date. Fair value is independently determined using Black-Scholes option pricing model that takes into account the exercise price, the term of the option, the impact of dilution, the share price at grant date and expected price volatility of the underlying share, the expected dividend yield and the risk free interest rate for the term of the option, together with non-vesting conditions that do not determine whether the Group receives the services that entitle the employees to receive payment. No account is taken of any other vesting conditions.
The cost of equity-settled transactions are recognised as an expense with a corresponding increase in equity over the vesting period. The cumulative charge to profit or loss is calculated based on the grant date fair value of the award, the best estimate of the number of awards that are likely to vest and the expired portion of the vesting period. The amount recognised in profit or loss for the period is the cumulative amount calculated at each reporting date less amounts already recognised in previous periods.
Market conditions are taken into consideration in determining fair value. Therefore, any awards subject to market conditions are considered to vest irrespective of whether or not that market condition has been met, provided all other conditions are satisfied.
If equity-settled awards are modified, as a minimum an expense is recognised as if the modification has not been made. An additional expense is recognised, over the remaining vesting period, for any modification that increases the total fair value of the share-based compensation benefit as at the date of modification.
If the non-vesting condition is within the control of the Group or employee, the failure to satisfy the condition is treated as a cancellation. If the condition is not within the control of the Group or employee and is not satisfied during the vesting period, any remaining expense for the award is recognised over the remaining vesting period, unless the award is forfeited. If equity-settled awards are cancelled, it is treated as if it has vested on the date of cancellation, and any remaining expense is recognised immediately. If a new replacement award is substituted for the cancelled award, the cancelled and new award is treated as if they were a modification. The dilutive effect, if any, of outstanding options is reflected as additional share dilution in the computation of earnings per share.
Fair value measurement
When an asset or liability, financial or non-financial, is measured at fair value for recognition or disclosure purposes, the fair value is based on the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date; and assumes that the transaction will take place either: in the principal market; or in the absence of a principal market, in the most advantageous market.
Fair value is measured using the assumptions that market participants would use when pricing the asset or liability, assuming they act in their economic best interests. For non-financial assets, the fair value measurement is based on its highest and best use. Valuation techniques that are appropriate in the circumstances and for which sufficient data are available to measure fair value, are used, maximising the use of relevant observable inputs and minimising the use of unobservable inputs.
F-15
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 1. Significant accounting policies continued
Issued capital
Ordinary shares are classified as equity.
Incremental costs directly attributable to the issue of new shares or options are shown in equity as a deduction, net of tax, from the proceeds.
Costs related to an initial offering are expensed in the statement of profit or loss and other comprehensive income.
Earnings per share
Basic earnings per share
Basic earnings per share is calculated by dividing the profit attributable to the owners of Benitec Biopharma Limited, excluding any costs of servicing equity other than ordinary shares, by the weighted average number of ordinary shares outstanding during the financial year, adjusted for bonus elements in ordinary shares issued during the financial year.
Diluted earnings per share
Diluted earnings per share adjusts the figures used in the determination of basic earnings per share to take into account the after income tax effect of interest and other financing costs associated with dilutive potential ordinary shares and the weighted average number of shares assumed to have been issued for no consideration in relation to dilutive potential ordinary shares.
Goods and Services Tax ('GST') and other similar taxes
Revenues, expenses and assets are recognised net of the amount of associated GST, unless the GST incurred is not recoverable from the tax authority. In this case it is recognised as part of the cost of the acquisition of the asset or as part of the expense.
Receivables and payables are stated inclusive of the amount of GST receivable or payable. The net amount of GST recoverable from, or payable to, the tax authority is included in other receivables or other payables in the statement of financial position.
Cash flows are presented on a gross basis. The GST components of cash flows arising from investing or financing activities which are recoverable from, or payable to the tax authority, are presented as operating cash flows.
Commitments and contingencies are disclosed net of the amount of GST recoverable from, or payable to, the tax authority.
Rounding of amounts
The Parent entity has applied the relief available to it under ASIC Corporations (Rounding in Financial/Directors’ Reports). Instrument 2016/191 and accordingly amounts in the financial statements and Directors Report have been rounded off to the nearest $1,000, or in certain cases, to the nearest dollars.
F-16
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 2. Critical accounting judgements, estimates and assumptions
The preparation of the financial statements requires management to make judgements, estimates and assumptions that affect the reported amounts in the financial statements. Management continually evaluates its judgements and estimates in relation to assets, liabilities, contingent liabilities, revenue and expenses. Management bases its judgements, estimates and assumptions on historical experience and on other various factors, including expectations of future events, management believes to be reasonable under the circumstances. The resulting accounting judgements and estimates will seldom equal the related actual results. The judgements, estimates and assumptions that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities (refer to the respective notes) within the next financial year are discussed below.
Research and development expenses
Management does not consider the development programs to be sufficiently advanced to reliably determine the economic benefits and technical feasibility to justify capitalisation of development costs. These costs have been recognised as an expense when incurred. Research and development expenses relate primarily to the cost of conducting clinical and pre-clinical trials. Clinical development costs are a significant component of research and development expenses. Estimates have been used in determining the expense liability under certain clinical trial contracts where services have been performed but not yet invoiced. Generally, the costs, and therefore estimates, associated with clinical trial contracts are based on the number of patients, drug administration cycles, the type of treatment and the outcome being the length of time before actual amounts can be determined will vary depending on length of the patient cycles and the timing of the invoices by the clinical trial partners.
Research and development refundable tax offsets
The Group accounts for the federal government research and development grant tax incentive when a reliable estimate of the amounts receivable can be made. In determining the estimate management reviews historical claims, Government overseas findings enabling the claim of overseas expenditure and the allocation of staff and overheads costs within approved projects. Judgement is also applied in determining the eligibility of the activities undertaken in Australia and overseas. Grant Income for the year ended June 30, 2019 includes an estimate of Research and Development grant receivable for June 30, 2019 of A$907k (refer Note 4b).
Share-based payment transactions
The Group measures the cost of equity-settled transactions with employees by reference to the fair value of the equity instruments at the date at which they are granted. The fair value is determined by using the Black-Scholes model taking into account the terms and conditions upon which the instruments were granted. The accounting estimates and assumptions relating to equity-settled share-based payments would have no impact on the carrying amounts of assets and liabilities within the next annual reporting period but may impact profit or loss and equity.
Recovery of deferred tax assets
Deferred tax assets are recognised for deductible temporary differences only if the Group considers it is probable that future taxable amounts will be available to utilise those temporary differences and losses. Given the Company’s and each individual entities’ history of recent losses, the Group has not recognised a deferred tax asset with regard to unused tax losses and other temporary differences, as it has not been determined whether the Company or its subsidiaries will generate sufficient taxable income against which the unused tax losses and other temporary differences can be utilised. The Group applies judgments in determining whether the tests for utilisation of carry forward tax losses are satisfied in a year where taxable income is generated. This involves management considering the Continuity of Ownership and/or Similar Business tests.
F-17
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 2. Critical accounting judgements, estimates and assumptions continued
Revenue recognition
The Group applies judgement in determining whether contracts entered into fall within the scope of IFRS 15 ‘Revenue from Contracts with Customers’. In doing so, management considers the commercial substance of the transaction and how risks and benefits of the contract accrue to the various parties to the contract. In determining the accounting treatment of the contract with Axovant management assessed that the contract was within the scope of IFRS 15 ‘Revenue from Contracts with Customers’.
Management has also made the judgement that the grant of the licence and transfer of associated know-how and materials are accounted for as one performance obligation as they are not considered to be distinct; they are highly interrelated and could not provide benefits to the customer independently from each other. Judgements were made in relation to the transfer of the licence and know-how and whether this should be recognised over time or a point in time. The point in time has been determined with regard to the point at which the transfer of know-how has substantially been completed and the customer has control of the asset and the ability to direct the use of and receive substantially all of the remaining benefits.
On June 6, 2019 the termination of the License and Collaboration Agreement with Axovant Sciences was announced. The termination of the License and Collaboration Agreement is effective as of September 3, 2019. The termination discharges all future performance obligations at termination date under the contract. As such, there are no contract liabilities recognised at June 30, 2019.
Costs of capital raising
Costs directly attributable to an equity transaction are held in the statement of financial position until the completion of the transaction. On completion, the costs will be applied against issued capital. Costs associated with abandoned or sub-optimal equity transactions are expensed to profit or loss in the year the transaction is determined to no longer be viable under existing conditions.
Note 3. Operating segments
The Group had only one business segment during the period, being the global commercialisation by licensing and partnering of and licences in biotechnology, with applications in biomedical research and human therapeutics. Business operations are conducted in Australia. However, there are controlled entities based in the USA and United Kingdom. The United Kingdom entity has no segment revenues, results or assets.
Geographical locations | | Segment Revenues from External Customers | | | Segment Results | | | Carrying Amount of Segment Assets | |
| | June 2019 | | | June 2018 | | | June 2017 | | | June 2019 | | | June 2018 | | | June 2017 | | | June 2019 | | | June 2018 | | | June 2017 | |
| | A$’000 | | | A$’000 | | | A$’000 | | | A$’000 | | | A$’000 | | | A$’000 | | | A$’000 | | | A$’000 | | | A$’000 | |
Australia | | | 14,627 | | | | 378 | | | | 333 | | | | 3,755 | | | | (11,733 | ) | | | (5,835 | ) | | | 25,112 | | | | 19,639 | | | | 21,580 | |
USA | | | — | | | | — | | | | — | | | | 339 | | | | 93 | | | | 145 | | | | 2,314 | | | | 1,700 | | | | 1,086 | |
| | | 14,627 | | | | 378 | | | | 333 | | | | 4,094 | | | | (11,640 | ) | | | (5,690 | ) | | | 27,426 | | | | 21,339 | | | | 22,666 | |
F-18
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 3. Operating segments continued
Accounting Policies
Segment revenues and expenses are directly attributable to the identified segments. Segment assets include all assets used by a segment and consist mainly of cash, receivables, inventories, intangibles and property, plant and equipment, net of any allowances, accumulated depreciation and amortisation. Segment liabilities include mainly accounts payable, employee entitlements, accrued expenses, provisions and borrowings. Deferred income tax provisions are not included in segment assets and liabilities
Note 4. Revenue and other income
| | 2019 | | | 2018 | | | 2017 | |
| | A$’000 | | | A$’000 | | | A$’000 | |
Licensing revenue and royalties | | | 14,627 | | | | 378 | | | | 333 | |
Service revenue* | | | 1,532 | | | | — | | | | — | |
Total | | | 16,159 | | | | 378 | | | | 333 | |
Australian Government R&D grants | | | 907 | | | | 3,999 | | | | 10,507 | |
Foreign exchange unrealized gain | | | 443 | | | | 87 | | | | — | |
Other | | | — | | | | 1 | | | | — | |
Total | | | 1,350 | | | | 4,087 | | | | 10,507 | |
*On June 6, 2019 termination of Licence and Collaboration Agreement with Axovant Science was announced. The termination of the License and Collaboration Agreement is effective as of September 3, 2019.
| | | | | | | | | | Twelve months to June 30, 2019 | |
| | Licensing A$’000 | | | Royalties A$’000 | | | Development activities A$’000 | | | Total A$’000 | |
Services transferred at a point of time | | | 14,179 | | | | — | | | | — | | | | 14,179 | |
Services transferred over time | | | 192 | | | | 256 | | | | 1,532 | | | | 1,980 | |
| | | 14,371 | | | | 256 | | | | 1,532 | | | | 16,159 | |
| | | | | | | | | | Twelve months to June 30, 2018 | |
| | Licensing A$’000 | | | Royalties A$’000 | | | Development activities A$’000 | | | Total A$’000 | |
Services transferred at a point of time | | | — | | | | — | | | | — | | | | — | |
Services transferred over time | | | 235 | | | | 143 | | | | — | | | | 378 | |
| | | 235 | | | | 143 | | | | — | | | | 378 | |
F-19
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 5. Expenses
| | 2019 | | | 2018 | | | 2017 | |
| | A$’000 | | | A$’000 | | | A$’000 | |
Profit/(Loss) before income tax includes the following specific expenses: | | | | | | | | | | | | |
| | | | | | | | | | | | |
Depreciation | | | | | | | | | | | | |
Leasehold improvements | | | 31 | | | | 25 | | | | 53 | |
Plant and equipment | | | 190 | | | | 169 | | | | 164 | |
Total depreciation | | | 221 | | | | 194 | | | | 217 | |
| | | | | | | | | | | | |
Research and development | | | | | | | | | | | | |
Project expenses | | | 2,152 | | | | 6,219 | | | | 6,456 | |
Other IP related expenses | | | 952 | | | | 671 | | | | 469 | |
Total research and development | | | 3,104 | | | | 6,890 | | | | 6,925 | |
| | | | | | | | | | | | |
Employee benefits expense | | | | | | | | | | | | |
Defined contribution superannuation expense | | | 197 | | | | 241 | | | | 240 | |
Employee benefits expense excluding superannuation | | | 4,828 | | | | 4,853 | | | | 4,775 | |
| | | 5,025 | | | | 5,094 | | | | 5,015 | |
| | | | | | | | | | | | |
Rental expense relating to operating leases | | | | | | | | | | | | |
Minimum lease payments | | | 491 | | | | 384 | | | | 376 | |
F-20
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 6. Income tax benefit
| | 2019 | | | 2018 | | | 2017 | |
| | A$’000 | | | A$’000 | | | A$’000 | |
Income tax benefit | | | | | | | | | | | | |
Current tax | | | — | | | | — | | | | — | |
Aggregate income tax benefit | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
Numerical reconciliation of income tax benefit and tax at the statutory rate | | | | | | | | | | | | |
Income/(Loss) before income tax benefit | | | 4,094 | | | | (11,640 | ) | | | (5,690 | ) |
| | | | | | | | | | | | |
Tax at the statutory tax rate of 27.5% (27.5%) | | | 1,126 | | | | (3,201 | ) | | | (1,565 | ) |
| | | | | | | | | | | | |
Tax effect amounts which are not deductible/(taxable) in calculating taxable income: | | | | | | | | | | | | |
R&D expenses | | | 573 | | | | 2,605 | | | | 2,676 | |
R&D incentive income | | | (249 | ) | | | (1,124 | ) | | | (2,889 | ) |
Legal expenses | | | 225 | | | | 70 | | | | 154 | |
Share-based payments | | | 258 | | | | 119 | | | | 106 | |
Timing differences utilised not previously recognised | | | (264 | ) | | | (196 | ) | | | (506 | ) |
Impact of foreign exchange rate differences | | | — | | | | — | | | | 2 | |
| | | 1,669 | | | | (1,727 | ) | | | (2,022 | ) |
(Utilisation of carried forward losses)/Tax losses not brought to account | | | (1,669 | ) | | | 1,727 | | | | 2,022 | |
Income tax benefit | | | — | | | | — | | | | — | |
Tax losses are recognised only if the consolidated entity considers it is probable that future taxable amounts will be available to utilise those temporary differences and losses.
Tax losses for which no deferred tax asset has been recognised - Australia | | | | | | | | | | | | |
- Tax losses not recognised | | 54,083 | | | | 61,471 | | | | 60,382 | |
- Capital losses not recognised | | | 1,272 | | | | 1,272 | | | | 1,272 | |
- Other deferred tax assets not recognised | | | 980 | | | | 627 | | | | 2,776 | |
| | | 56,335 | | | | 63,370 | | | | 64,430 | |
| | | | | | | | | | | | |
Potential tax benefit of tax assets not recognised at 27.5% (27.5%) | | | 15,492 | | | | 17,427 | | | | 17,718 | |
| | | | | | | | | | | | |
Tax losses for which no deferred tax asset has been recognised - US (Tacere) | | | | | | | | | | | | |
- Tax losses not recognised | | | 771 | | | | 846 | | | | 955 | |
| | | | | | | | | | | | |
Potential tax benefit of tax assets not recognised at 21% - US | | | 162 | | | | 178 | | | | 324 | |
The above potential tax benefit, which excludes tax losses, for deductible temporary differences has not been recognised in the statement of financial position as the recovery of this benefit is uncertain.
F-21
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 7. Cash and cash equivalents
| | 2019 | | | 2018 | | | 2017 | |
| | A$’000 | | | A$’000 | | | A$’000 | |
Cash at bank | | | 15,369 | | | | 9,575 | | | | 4,349 | |
Cash on deposit | | | 7,042 | | | | 6,510 | | | | 13,026 | |
| | | 22,411 | | | | 16,085 | | | | 17,375 | |
Note 8. Other financial assets
| | 2019 | | | 2018 | | | 2017 | |
| | A$’000 | | | A$’000 | | | A$’000 | |
Market value of listed shares | | | 1 | | | | 30 | | | | — | |
Security deposit | | | 147 | | | | 100 | | | | 100 | |
Deposit other | | | 33 | | | | — | | | | — | |
| | | 181 | | | | 130 | | | | 100 | |
Note 9. Trade and other receivables
| | 2019 | | | 2018 | | | 2017 | |
| | A$’000 | | | A$’000 | | | A$’000 | |
R&D grant receivable | | | 907 | | | | 4,121 | | | | 4,233 | |
Settlement Receivable | | | — | | | | — | | | | 109 | |
Receivables | | | 2,709 | | | | 134 | | | | 64 | |
| | | 3,616 | | | | 4,255 | | | | 4,406 | |
There are no receivable balances that are past due that are not impaired.
Note 10. Current assets – other
| | 2019 | | 2018 | | | 2017 | |
| | A$’000 | | A$’000 | | | A$’000 | |
Prepayments | | 535 | | | 425 | | | | 281 | |
| | 535 | | | 425 | | | | 281 | |
Note 11. Deposits non – current
| | 2019 | | 2018 | | | 2017 | |
| | A$’000 | | A$’000 | | | A$’000 | |
Other | | 13 | | | 125 | | | | 59 | |
| | 13 | | | 125 | | | | 59 | |
F-22
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 12. Property, plant and equipment
| | 2019 | | | 2018 | |
| | A$’000 | | | A$’000 | |
Leasehold improvements - at cost | | | 109 | | | | 79 | |
Less: Accumulated depreciation | | | (73 | ) | | | (44 | ) |
| | | 36 | | | | 35 | |
| | | | | | | | |
Plant and equipment - at cost | | | 1,516 | | | | 975 | |
Less: Accumulated depreciation | | | (882 | ) | | | (691 | ) |
| | | 634 | | | | 284 | |
| | | | | | | | |
| | | 670 | | | | 319 | |
Reconciliations
Reconciliations of the written down values at the beginning and end of the current and previous financial year are set out below:
| | Leasehold | | | Plant and | | | | | |
| | improvement | | | equipment | | | Total | |
| | A$’000 | | | A$’000 | | | A$’000 | |
Balance at June 30, 2017 | | | 60 | | | | 385 | | | | 445 | |
Additions | | | — | | | | 86 | | | | 86 | |
Disposals | | | — | | | | (27 | ) | | | (27 | ) |
Depreciation expense | | | (25 | ) | | | (169 | ) | | | (194 | ) |
FX loss | | | — | | | | 9 | | | | 9 | |
Balance at June 30, 2018 | | | 35 | | | | 284 | | | | 319 | |
| | Leasehold | | | Plant and | | | | | |
| | improvement | | | equipment | | | Total | |
| | A$’000 | | | A$’000 | | | A$’000 | |
Balance at June 30, 2018 | | | 35 | | | | 284 | | | | 319 | |
Additions | | | 30 | | | | 541 | | | | 571 | |
Disposals | | | — | | | | (54 | ) | | | (54 | ) |
Depreciation expense | | | (31 | ) | | | (190 | ) | | | (221 | ) |
FX loss | | | 2 | | | | 53 | | | | 55 | |
Balance at June 30, 2019 | | | 36 | | | | 634 | | | | 670 | |
Note 13. Trade and other payables
| | 2019 | | | 2018 | |
| | A$’000 | | | A$’000 | |
Trade creditors | | | 2,101 | | | | 580 | |
Sundry creditors and accrued expenses | | | 1,455 | | | | 1,796 | |
Total | | | 3,556 | | | | 2,376 | |
F-23
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 14. Provisions
| | 2019 | | | 2018 | |
| | A$’000 | | | A$’000 | |
Employee benefits | | | 200 | | | | 146 | |
Provision for make good | | | 10 | | | | 25 | |
Total | | | 210 | | | | 171 | |
Note 15. Issued Capital
| | 2019 | | | 2018 | | | 2017 | | | 2019 | | | 2018 | | | 2017 | |
| | Shares | | | Shares | | | Shares | | | A$’000 | | | A$’000 | | | A$’000 | |
Ordinary shares - fully paid | | | 257,029,426 | | | | 257,029,426 | | | | 205,142,734 | | | | 164,087 | | | | 164,087 | | | | 155,580 | |
Movements in ordinary share capital
Details | | Date | | Shares | | | Issue price | | $'000 | |
Balance | | June 30, 2018 | | | 257,029,426 | | | | | | 164,087 | |
Balance | | June 30, 2019 | | | 257,029,426 | | | | | | 164,087 | |
The weighted average number of shares on issue during the twelve months to June 30, 2019 was | | | | | 257,029,426 | | | | | | | |
Ordinary shares
Ordinary shares entitle the holder to participate in dividends and the proceeds on the winding up of the Company in proportion to the number of and amounts paid on the shares held. The fully paid ordinary shares have no par value and the Company does not have a limited amount of authorised capital.
On a show of hands every member present at a meeting in person or by proxy shall have one vote and upon a poll each share shall have one vote.
Share buy-back
There is no current on-market share buy-back.
Capital risk management
The Group's objectives when managing capital is to safeguard its ability to continue as a going concern, so that it can provide returns for shareholders and benefits for other stakeholders and to maintain an optimum capital structure to reduce the cost of capital.
The capital structure of the Group consists of cash and cash equivalents and equity attributable to equity holders. Operating globally, the Group develops specialty pharmaceutical products. The overall strategy of the Group is to continue its drug development programs, which depends on selling assets and raising additional equity to fund the activities.
The capital risk management policy remains unchanged from the prior year.
F-24
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 16. Reserves
| | 2019 | | | 2018 | |
| | A$’000 | | | A$’000 | |
Foreign currency translation reserve | | | (1,465 | ) | | | (1,348 | ) |
Share-based payments reserve | | | 2,296 | | | | 2,840 | |
| | | 831 | | | | 1,492 | |
Foreign currency reserve
The reserve is used to recognise exchange differences arising from the translation of the financial statements of foreign operations to Australian dollars.
Share-based payments reserve
The reserve is used to recognise the value of equity benefits provided to employees and directors as part of their remuneration, and other parties as part of their compensation for services.
Movements in reserves
Movements in each class of reserve during the current and previous financial year are set out below:
| | Foreign | | | Share-based | | | | | |
| | currency | | | payments | | | Total | |
| | A$’000 | | | A$’000 | | | A$’000 | |
Balance at June 30, 2017 | | | (1,285 | ) | | | 2,959 | | | | 1,674 | |
Foreign currency translation | | | (63 | ) | | | — | | | | (63 | ) |
Share-based payments | | | — | | | | (119 | ) | | | (119 | ) |
Balance at June 30, 2018 | | | (1,348 | ) | | | 2,840 | | | | 1,492 | |
Foreign currency translation | | | (117 | ) | | | — | | | | (117 | ) |
Share-based payments | | | — | | | | (544 | ) | | | (544 | ) |
Balance at June 30, 2019 | | | (1,465 | ) | | | 2,296 | | | | 831 | |
Note 17. Accumulated losses
| | 2019 | | | 2018 | |
| | A$’000 | | | A$’000 | |
Accumulated losses at the beginning of the financial year | | | (146,835 | ) | | | (135,748 | ) |
Income/(Loss) after income tax benefit for the year | | | 4,094 | | | | (11,640 | ) |
Transfer from share-based payment reserve for expired options | | | 1,483 | | | | 553 | |
Accumulated losses at the end of the financial year | | | (141,258 | ) | | | (146,835 | ) |
Note 18. Dividends
There were no dividends paid, recommended or declared during the current or previous financial year.
F-25
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 19. Financial instruments
Financial risk management objectives
The Group's activities expose it to a variety of financial risks: market risk (including foreign currency risk and interest rate risk) and liquidity risk. The Group’s principal financial instruments comprise receivables, payables, cash and short-term deposits. The Group manages its exposure to key financial risks, including interest rate and currency risk in accordance with the Company financial risk management policy. The objective of the policy is to protect the assets and provide a solid return.
| | 2019 | | | 2018 | |
| | A$’000 | | | A$’000 | |
Financial Assets | | | | | | | | |
Cash and cash equivalents | | | 22,411 | | | | 16,085 | |
Trade and other receivables | | | 3,616 | | | | 4,255 | |
Total Financial Assets | | | 26,027 | | | | 20,340 | |
| | | | | | | | |
Financial Liabilities | | | | | | | | |
Trade and other payables | | | 3,556 | | | | 2,376 | |
Total Financial Liabilities | | | 3,556 | | | | 2,376 | |
Market risk
Foreign currency risk
The Group undertakes certain transactions denominated in foreign currency and is exposed to foreign currency risk through foreign exchange rate fluctuations.
Foreign exchange risk arises from future commercial transactions and recognised financial assets and financial liabilities denominated in a currency that is not the entity's functional currency. The risk is measured using sensitivity analysis and cash flow forecasting.
At June 30, 2019 the Company held USD cash or cash equivalents of AUD$12m and trade payables and accruals of AUD$2.43m. Net USD exposure in AUD of $9.59m. Each 1 cent movement in the AUD/USD exchange rate has a +/- effect of AUD $139k on profit and net assets of the Company. Exposure to foreign exchange rates vary during the year depending on the volume of overseas transactions. Nonetheless the analysis above is considered to be appropriate of the Group’s exposure to currency risk.
Interest rate risk
The Group generates income from interest on surplus funds. At reporting date, the Group had the following assets exposed to Australian variable interest rate risk that are not designated in cash flow hedges.
As at the reporting date, the Group had the following variable rate cash and cash equivalents outstanding:
| | Weighted average interest rate | | | Balance 2019 | | | Weighted average interest rate | | | Balance 2018 | |
| | % | | | A$’000 | | | % | | | A$’000 | |
Cash and cash equivalents | | | 2 | % | | | 22,411 | | | | 2 | % | | | 16,085 | |
Net exposure to cash flow interest rate risk | | | | | | | 22,411 | | | | | | | | 16,085 | |
F-26
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 19. Financial instruments continued
Credit risk
Credit risk refers to the risk that a counterparty will default on its contractual obligations resulting in financial loss to the Group. The maximum exposure to credit risk at the reporting date to recognised financial assets is the carrying amount, net of any provisions for impairment of those assets, as disclosed in the statement of financial position and notes to the financial statements. The Group does not hold any collateral.
Liquidity risk
Vigilant liquidity risk management requires the Group to maintain sufficient liquid assets (mainly cash and cash equivalents) to be able to pay debts as and when they become due and payable.
The Group manages liquidity risk by maintaining adequate cash reserves and available borrowing facilities by continuously monitoring actual and forecast cash flows and matching the maturity profiles of financial assets and liabilities.
Remaining contractual maturities
The following tables detail the Group's remaining contractual maturity for its financial instrument liabilities. The tables have been drawn up based on the undiscounted cash flows of financial liabilities based on the earliest date on which the financial liabilities are required to be paid.
| | Weighted average interest rate | | | 1 year or less | | | Between 1 and 2 years | | | Between 2 and 5 years | | | Over 5 years | | | Remaining contractual maturities | |
2019 | | % | | | A$’000 | | | A$’000 | | | A$’000 | | | A$’000 | | | A$’000 | |
Non-derivatives | | | | | | | | | | | | | | | | | | | | | | | | |
Non-interest bearing | | | | | | | | | | | | | | | | | | | | | | | | |
Trade payables | | | — | % | | | 2,101 | | | | — | | | | — | | | | — | | | | 2,101 | |
Other payables | | | — | % | | | 1,455 | | | | — | | | | — | | | | — | | | | 1,455 | |
Total non-derivatives | | | | | | | 3,556 | | | | — | | | | — | | | | — | | | | 3,556 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
2018 | | % | | | A$’000 | | | A$’000 | | | A$’000 | | | A$’000 | | | A$’000 | |
Non-derivatives | | | | | | | | | | | | | | | | | | | | | | | | |
Non-interest bearing | | | | | | | | | | | | | | | | | | | | | | | | |
Trade payables | | | — | % | | | 580 | | | | — | | | | — | | | | — | | | | 580 | |
Other payables | | | — | % | | | 1,796 | | | | — | | | | — | | | | — | | | | 1,796 | |
Total non-derivatives | | | | | | | 2,376 | | | | — | | | | — | | | | — | | | | 2,376 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
2017 | | % | | | A$’000 | | | A$’000 | | | A$’000 | | | A$’000 | | | A$’000 | |
Non-derivatives | | | | | | | | | | | | | | | | | | | | | | | | |
Non-interest bearing | | | | | | | | | | | | | | | | | | | | | | | | |
Trade payables | | | — | % | | | 174 | | | | — | | | | — | | | | — | | | | 174 | |
Other payables | | | — | % | | | 745 | | | | — | | | | — | | | | — | | | | 745 | |
Total non-derivatives | | | | | | | 919 | | | | — | | | | — | | | | — | | | | 919 | |
The cash flows in the maturity analysis above are not expected to occur significantly earlier than contractually disclosed above.
Fair value of financial instruments
Unless otherwise stated, the carrying amounts of financial instruments reflect their fair value.
F-27
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 20. Remuneration of auditors
During the financial year the following fees were paid or payable for services provided by Grant Thornton Audit Pty Ltd and affiliated entities, the auditor of the Company:
| | 2019 | | | 2018 | | | 2017 | |
Audit services | | A$ | | | A$ | | | A$ | |
Audit or review of the financial statements | | | 168,398 | | | | 240,806 | | | | 241,933 | |
Other audit services | | | | | | | | | | | | |
- F1 review | | | — | | | | 17,990 | | | | 20,800 | |
- F3 review | | | — | | | | 6,660 | | | | 9,561 | |
| | | | | | | | | | | | |
Other services | | | | | | | | | | | | |
Tax compliance services | | | 15,650 | | | | 42,617 | | | | 23,150 | |
| | | 184,048 | | | | 308,073 | | | | 295,444 | |
Note 21. Contingent liabilities and commitments
There are no contingent liabilities as at June 30, 2019.
Note 22. Commitments
| | 2019 | | | 2018 | | | 2017 | |
| | A$’000 | | | A$’000 | | | A$’000 | |
Lease commitments - operating | | | | | | | | | | | | |
Committed at the reporting date but not recognised as liabilities, payable: | | | | | | | | | | | | |
Within one year | | | 289 | | | | 219 | | | | 169 | |
One to five years | | | 632 | | | | 293 | | | | 89 | |
| | | 921 | | | | 512 | | | | 258 | |
Operating lease commitments includes contracted amounts for offices under non-cancellable operating leases expiring within 3 years with, in some cases, options to extend. The leases have various escalation clauses. On renewal, the terms of the leases are renegotiated.
F-28
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 23. Related party transactions
Parent entity
Benitec Biopharma Limited is the parent entity.
Subsidiaries
Interests in subsidiaries are set out in note 25.
Key management personnel
Disclosures relating to key management personnel are set out in the table below and in Item 6. Directors, Senior Management and Employees on pages 95 to 104.
Compensation
The aggregate compensation made to directors and other members of key management personnel of the Group is set out below:
| | 2019 | | | 2018 | | | 2017 | |
| | A$ | | | A$ | | | A$ | |
Short-term employee benefits | | | 1,160,275 | | | | 1,843,334 | | | | 1,539,777 | |
Post-employment benefits | | | 36,690 | | | | 89,780 | | | | 76,623 | |
Long-term employee benefits | | | — | | | | — | | | | 32,537 | |
Share-based payments | | | 807,177 | | | | 257,001 | | | | 418,986 | |
Total key management compensation | | | 2,004,142 | | | | 2,190,115 | | | | 2,067,923 | |
| | | | | | | | | | | | |
The following transactions occurred with related parties: | | | | | | | | | | | | |
Payment for other expenses: | | | | | | | | | | | | |
Legal services paid / payable to Francis Abourizk Lightowlers, a law firm in which Mr Peter Francis is a partner and has a beneficial interest. | | | 726 | | | | 8,212 | | | | 191,050 | |
Annabel West, the wife of Greg West, our former Chief Executive Officer, was employed as a part-time clerical and administrative assistant. | | | — | | | | 42,278 | | | | 36,248 | |
Receivable from and payable to related parties
There were no trade receivables from or trade payables to related parties at the current and previous reporting date.
Loans to/from related parties
There were no loans to or from related parties at the current and previous reporting date.
Terms and conditions
All transactions were made on normal commercial terms and conditions and at market rates.
F-29
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 24. Parent entity information
Set out below is the supplementary information about the parent entity.
| | 2019 | | | 2018 | |
| | A$’000 | | | A$’000 | |
Statement of profit or loss and other comprehensive income | | | | | | | | |
Profit/(loss) after income tax | | | 3,542 | | | | (13,566 | ) |
Total comprehensive income | | | 3,542 | | | | (13,566 | ) |
| | | | | | | | |
Statement of financial position | | | | | | | | |
Total current assets | | | 25,095 | | | | 19,461 | |
Total assets | | | 25,112 | | | | 19,639 | |
| | | | | | | | |
Total current liabilities | | | 3,390 | | | | 2,351 | |
Total liabilities | | | 3,390 | | | | 2,399 | |
| | | | | | | | |
Equity | | | | | | | | |
Issued capital | | | 164,087 | | | | 164,087 | |
Share-based payments reserve | | | 2,296 | | | | 2,840 | |
Accumulated losses | | | (144,661 | ) | | | (149,687 | ) |
Total equity | | | 21,722 | | | | 17,240 | |
Guarantees entered into by the parent entity in relation to the debts of its subsidiaries
The parent entity had no guarantees in relation to the debts of its subsidiaries as at June 30, 2019 and June 30, 2018.
Contingent liabilities
The parent entity had no contingent liabilities as at June 30, 2019 (2018: nil).
Capital commitments - Property, plant and equipment
The parent entity had no capital commitments for property, plant and equipment as at June 30, 2019 and June 30, 2018.
Significant accounting policies
The accounting policies of the parent entity are consistent with those of the Group, as disclosed in note 1, except for the following:
• | Investments in subsidiaries are accounted for at cost, less any impairment, in the parent entity. |
• | Dividends received from subsidiaries are recognised as other income by the parent entity and its receipt may be an indicator of an impairment of the investment. |
F-30
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 25. Interests in subsidiaries
The consolidated financial statements incorporate the assets, liabilities and results of the following subsidiaries in accordance with the accounting policy described in note 1:
| | Principal place of business / | | 2019 | | | 2018 | | | 2017 | |
Name | | Country of incorporation | | % | | | % | | | % | |
Benitec Australia Limited | | Australia | | 100% | | | 100% | | | 100% | |
Benitec Biopharma Limited | | United Kingdom | | 100% | | | 100% | | | 100% | |
Benitec, Inc. | | USA | | 100% | | | 100% | | | 100% | |
Benitec LLC | | USA | | 100% | | | 100% | | | 100% | |
RNAi Therapeutics, Inc. | | USA | | 100% | | | 100% | | | 100% | |
Tacere Therapeutics, Inc.* | | USA | | 100% | | | 100% | | | 100% | |
All companies in the Group adopt the same accounting policies.
* Note Tacere year end is 31 December which was the year end date when the Company was acquired.
Note 26. Events after the reporting period
On September 30, 2019 Benitec entered into a securities purchase agreement ("SPA") with certain sophisticated and professional investors in the United States to issue 2,800,000 American Depositary Shares ("ADSs"), with each ADS representing 20 fully paid ordinary shares, at a purchase price of US$0.70 per ADS, in a registered direct offering. The Investors were also issued warrants to purchase up to 412,863 ADSs in aggregate, at a purchase price per warrant equal to US$0.6999 per ADS to be issued on exercise of the warrant ("Pre-Funded Warrants"). The Pre-Funded Warrants may be exercised at any time from issue, in whole or in part, at an exercise price of US$0.0001 per ADS issued on exercise (subject to certain adjustments), provided that the beneficial ownership of the relevant Investor in the total number of ADSs on issue may not exceed 9.99%. The Pre-Funded Warrants do not expire.
The issue of the ADSs and Pre-Funded Warrants raised gross proceeds of approximately US$2.25 million (approximately A$3.33 million) exclusive of costs. The ADSs (and the underlying ordinary shares) and Pre-Funded Warrants were issued without shareholder approval under the Company's existing placement capacity under ASX Listing Rules 7.1 and 7.1A.
In a concurrent private placement, the Company has also agreed to issue additional warrants to the Investors to purchase up to a further 3,212,864 ADSs in aggregate ("Purchase Warrants"). The issue of the Purchase Warrants is subject to shareholder approval. If approved by shareholders, the Purchase Warrants will be issued for nil consideration, will have an exercise price of US$0.70 per ADS and will expire in five years from the date of issuance.
F-31
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 27. Reconciliation of profit/(loss) after income tax to net cash used in operating activities
| | 2019 A$'000 | | | 2018 A$'000 | | | 2017 A$'000 | |
Profit/(Loss) after income tax benefit for the year | | | 4,094 | | | | (11,640 | ) | | | (5,690 | ) |
| | | | | | | | | | | | |
Adjustments for: | | | | | | | | | | | | |
Loss on disposal of fixed assets | | | 9 | | | | 1 | | | | 6 | |
Depreciation and amortisation | | | 221 | | | | 194 | | | | 217 | |
Share-based payments | | | 939 | | | | 434 | | | | 386 | |
Loss on assets held for sale | | | 29 | | | | — | | | | | |
Net unrealised foreign exchange | | | (1,053 | ) | | | (82 | ) | | | 242 | |
Change in operating assets and liabilities: | | | | | | | | | | | | |
(Decrease)/Increase in trade and other receivables | | | (2,542 | ) | | | 72 | | | | 814 | |
(Decrease) in other current assets | | | (104 | ) | | | (121 | ) | | | (182 | ) |
Decrease in trade and other payables | | | 1,066 | | | | 1,259 | | | | 114 | |
Decrease in R&D grant receivable | | | 3,214 | | | | 112 | | | | (4,233 | ) |
(Decrease) in employee benefits | | | (1 | ) | | | (23 | ) | | | (3 | ) |
(Decrease) in provision | | | (15 | ) | | | — | | | | 25 | |
Net cash used in operating activities | | | 5,857 | | | | (9,794 | ) | | | (8,304 | ) |
F-32
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 28. Earnings per share
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | 2019 A$'000 | | | 2018 A$'000 | | | 2017 A$'000 | |
Profit/(Loss) after income tax attributable to the owners of Benitec Biopharma Limited | | | 4,094 | | | | (11,640 | ) | | | (5,690 | ) |
| | Number | | | Number | | | Number | |
Weighted average number of ordinary shares used in calculating basic earnings per share | | | 257,029,426 | | | | 210,454,829 | | | | 175,433,909 | |
Weighted average number of ordinary shares used in calculating diluted earnings per share | | | 257,029,426 | | | | 210,454,829 | | | | 175,433,909 | |
| | Cents | | | Cents | | | Cents | |
Basic earnings per share | | | 1.59 | | | | (5.53 | ) | | | (3.24 | ) |
Diluted earnings per share | | | 1.59 | | | | (5.53 | ) | | | (3.24 | ) |
Outstanding options to acquire ordinary shares are not considered dilutive for the years ended June 30, 2019 and June 30, 2018, because they are anti-dilutive, as the strike price was lower than share price.
Note 29. Share-based payments
Benitec Biopharma Limited Employees Share Option Plan (ESOP):
Description of plan
The Group may from time to time issue employee’s options to acquire shares in the parent at a fixed price. Each option when exercised entitles the option holder to one share in the Parent Company. Options are exercisable on or before an expiry date, do not carry any voting or dividend rights and are not transferable except on death of the option holder.
The following table shows the number and weighted average exercise price (WAEP) of share options issued under the ESOP:
| | 2019 | | | 2019 | | | 2018 | | | 2018 | | | 2017 | | | 2017 | |
| | Number | | | WAEP | | | Number | | | WAEP | | | Number | | | WAEP | |
Outstanding at the beginning of the year | | | 24,477,332 | | | | 0.416 | | | | 9,724,000 | | | | 0.832 | | | | 12,220,000 | | | | 1.079 | |
Granted during the year | | | 8,200,000 | | | | 0.202 | | | | 19,950,000 | | | | 0.218 | | | | 2,200,000 | | | | 0.166 | |
Exercised during the year | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Lapsed or forfeited during the year | | | (6,737,332 | ) | | | 0.585 | | | | (5,196,668 | ) | | | 0.426 | | | | (4,696,000 | ) | | | 1.164 | |
Outstanding at the end of the year | | | 25,940,000 | | | | 0.305 | | | | 24,477,332 | | | | 0.416 | | | | 9,724,000 | | | | 0.832 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Options exercisable at the end of the year | | | 8,106,667 | | | | | | | | 6,527,333 | | | | | | | | 6,497,333 | | | | | |
F-33
BENITEC BIOPHARMA LTD
Notes to the consolidated financial statements 30 June 2019 continued
Note 29. Share-based payments continued
Details of ESOP share options outstanding as at end of year:
| | | | | | 2019 | | | 2018 | | | 2017 | |
| | | | Exercise | | Number | | | Number | | | Number | |
Grant date | | Expiry date | | price | | under option | | | Under option | | | Under option | |
November 17, 2012** | | November 17, 2017 | | A$ 1.25 | | | — | | | | — | | | | 400,000 | |
November 10, 2013* | | May 18, 2018 | | A$ 0.62 | | | — | | | | — | | | | 400,000 | |
August 22, 2013 ** | | August 22, 2018 | | A$ 1.25 | | | — | | | | 480,000 | | | | 480,000 | |
May 15, 2014 ** | | May 15, 2019 | | A$ 1.50 | | | — | | | | 90,000 | | | | 180,000 | |
December 17, 2014 ** | | December 17, 2019 | | A$ 1.25 | | | 700,000 | | | | 2,334,000 | | | | 2,334,000 | |
May 6, 2015 ** | | May 6, 2020 | | A$ 1.25 | | | 350,000 | | | | 650,000 | | | | 650,000 | |
November 12, 2015 * | | November 12, 2020 | | A$ 0.77 | | | 2,240,000 | | | | 2,240,000 | | | | 3,080,000 | |
August 9, 2016 ** | | August 9, 2021 | | A$ 0.1665 | | | — | | | | 1,466,666 | | | | 2,200,000 | |
July 17, 2017 ** | | July 17, 2022 | | A$ 0.196 | | | 3,800,000 | | | | 6,566,666 | | | | — | |
April 11, 2018** | | April 11, 2023 | | A$ 0.298 | | | 650,000 | | | | 650,000 | | | | — | |
June 26, 2018** | | June 26, 2023 | | A$ 0.228 | | | 10,000,000 | | | | 10,000,000 | | | | — | |
March 12, 2019** | | March 12, 2024 | | A$ 0.200 | | | 5,000,000 | | | | — | | | | — | |
March 21, 2019** | | March 21, 2024 | | A$ 0.206 | | | 1,575,000 | | | | — | | | | — | |
April 11, 2019** | | April 11, 2024 | | A$ 0.208 | | | 1,150,000 | | | | — | | | | — | |
May 2, 2019** | | May 2, 2024 | | A$ 0.198 | | | 275,000 | | | | — | | | | — | |
May 16, 2019** | | May 16, 2024 | | A$ 0.206 | | | 200,000 | | | | — | | | | — | |
| | | | | | | 25,940,000 | | | | 24,477,332 | | | | 9,724,000 | |
* | Non-Executive Directors options |
The weighted average remaining life of the options issued under the ESOP at June 30, 2019 was 3 years and 7 months (2018: 3 years and 10 months).
For the options granted during the year, the valuation model inputs used to determine the fair value at the grant date are as follows:
| | | | Share price at grant | | Exercise | | Expected * | | | Dividend | | | Risk-free | | | Fair value |
Grant date | | Expiry date | | date | | price | | volatility | | | yield | | | interest rate | | | at grant date |
12/03/2019 | | 12/03/2024 | | A$ 0.135 | | A$ 0.200 | | | 104.10 | % | | | — | % | | | 1.670 | % | | A$ 0.1009 |
21/03/2019 | | 21/03/2024 | | A$ 0.130 | | A$ 0.206 | | | 103.73 | % | | | — | % | | | 1.530 | % | | A$ 0.0916 |
11/04/2019 | | 11/04/2024 | | A$ 0.140 | | A$ 0.208 | | | 103.84 | % | | | — | % | | | 1.520 | % | | A$ 0.0999 |
02/05/2019 | | 02/05/2024 | | A$ 0.140 | | A$ 0.198 | | | 103.24 | % | | | — | % | | | 1.400 | % | | A$ 0.1003 |
16/05/2019 | | 16/05/2024 | | A$ 0.130 | | A$ 0.206 | | | 102.92 | % | | | — | % | | | 1.280 | % | | A$ 0.0908 |
Total expenses arising from share-based payment transactions recognised during the period as part of employee benefit expense were A$0.939m (2018: A$0.434m).
* | expected volatility was determined with reference to the Benitec share price based on historical volatility |
F-34

Level 17, 383 Kent Street
Sydney NSW 2000
Correspondence to:
Locked Bag Q800
QVB Post Office
Sydney NSW 1230
T +61 2 8297 2400
F +61 2 9299 4445
E info.nsw@au.gt.com
W www.grantthornton.com.au
Report of independent registered public accounting firm
Board of Directors and Shareholders
Benitec Biopharma Limited
Opinion on the financial statements
We have audited the accompanying consolidated statements of financial position of Benitec Biopharma Limited and subsidiaries (the “Company”) as of June 30, 2019 and 2018, the related consolidated statements of profit or loss and other comprehensive income, changes in shareholders’ equity, and cash flows for each of the three years in the period ended June 30, 2019, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of June 30, 2019 and 2018, and the results of its operations and its cash flows for each of the three years in the period ended June 30, 2019, in conformity with International Financial Reporting Standards, as issued by the International Accounting Standards Board.
Basis for opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ GRANT THORNTON AUDIT PTY LTD
We have served as the Company’s auditor since 2010.
Sydney, Australia
October 30, 2019
F-35
Item 19. Exhibits
Exhibits | | Description |
| | |
1.1 | | Constitution of Benitec Biopharma Limited (incorporated by reference to Exhibit 3.1 to the Company’s Registration Statement on Form F-1 filed with the SEC on June 22, 2015) |
| | |
2.1 | | Deposit Agreement, dated May 30, 2014, between Benitec Biopharma Limited and The Bank of New York Mellon, as depositary, and Owners and Holders of the American Depositary Shares (incorporated by reference to the Company's Registration Statement on Form F-6 (No. 333-196105) filed with the SEC on May 20, 2014) |
| | |
2.2 | | Amendment to Deposit Agreement between Benitec Biopharma Limited and The Bank of New York Mellon, as depositary, and Owners and Holders of the American Depositary Shares (incorporated by reference to Post-Effective Amendment No. 1 to the Company's Registration Statement on Form F-6 (No. 333-196105) filed with the SEC on July 21, 2015) |
| | |
2.3 | | Form of American Depositary Receipt evidencing American Depositary Shares (included in Exhibit 2.2) |
| | |
2.4 | | Form of Global Warrant to Purchase ADSs (included in Exhibit 2.5) |
| | |
2.5 | | Form of ADS Warrant Agent Agreement, between Benitec Biopharma Limited and The Bank of New York Mellon, as warrant agent (incorporated by reference to Exhibit 4.5 to Amendment No. 2 to the Company’s Registration Statement on Form F-1 filed with the SEC on August 10, 2015) |
| | |
2.6 | | Form of American Depositary Shares Pre-Funded Warrant (incorporated by reference to Exhibit 99.3 to the Company's 6-K filed with the SEC on September 30, 2019) |
| | |
2.7 | | Form of American Depositary Shares Purchase Warrant (incorporated by reference to Exhibit 99.4 to the Company's 6-K filed with the SEC on September 30, 2019 |
| | |
4.1 | | Share Subscription Agreement, dated October 24, 2016, between Nant Capital, LLC and Benitec Biopharma Limited (incorporated by reference to Exhibit 10.1 to the Company's Registration Statement on Form F-3 filed with the SEC on June 1, 2017) |
| | |
4.2 | | Commercial Lease Agreement between Hayward Point Eden I Limited Partnership and Benitec Biopharma Limited (incorporated by reference to Exhibit 10.5 to the Company's Registration Statement on Form F-1 filed with the SEC on June 22, 2015) |
| | |
4.3 | | Form of Executive Employment Agreement for executive officers (incorporated by reference to Exhibit 10.8 to the Company’s Registration Statement on Form F-1 filed with the SEC on June 22, 2015) |
| | |
4.4 | | Form of Deed of Access, Insurance and Indemnity for Directors and Officers (incorporated by reference to Exhibit 10.9 to the Company’s Registration Statement on Form F-1 filed with the SEC on June 22, 2015) |
| | |
4.5 | | Benitec Officers' and Employees' Share Option Plan (incorporated by reference to Exhibit 4.16 to the Company's Annual Report on Form 20-F filed with the SEC on November 17, 2015) |
| | |
4.6 | | Exclusive Sublicense Agreement, dated December 23, 2016, between Benitec Biopharma Limited and NantWorks, LLC (incorporated by reference to Exhibit 10.2 to the Registration Statement on Form F-3 filed with the SEC on June 1, 2017) † |
| | |
4.7 | | Research Collaboration Agreement, dated January 27, 2017, between Benitec Biopharma Limited and Nant Capital, LLC (incorporated by reference to Exhibit 10.3 to the Registration Statement on Form F-3 filed with the SEC on June 1, 2017) |
| | |
4.8 | | Form of Securities Purchase Agreement, dated September 30, 2019, between Benitec Biopharma Limited and the Purchasers (incorporated by reference to Exhibit 99.2 to the Company's 6-K filed with the SEC on September 30, 2019) |
| | |
† | Confidential treatment requested. Confidential materials omitted and filed separately with the Securities and Exchange Commission |
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
Benitec Biopharma Limited |
| | |
By: | | /S/ Jerel Banks |
| | Name: | Dr. Jerel Banks |
| | Title: | Chief Executive Officer |
Date: October 30, 2019









