Filed Pursuant to Rule 424(b)(3) and Rule 424(c)
Registration No. 333-198847
Prospectus Supplement No. 4
(To Prospectus filed on November 10, 2014, as supplemented
by Prospectus Supplement No. 1 dated November 12, 2014,
Prospectus Supplement No. 2 dated November 14, 2014, and Prospectus Supplement No. 3 dated December 18, 2014)
ENUMERAL BIOMEDICAL HOLDINGS, INC.
This Prospectus Supplement No. 4 supplements the information contained in the Prospectus, dated as of November 10, 2014, as amended by Prospectus Supplement No. 1 dated November 12, 2014, Prospectus Supplement No. 2 dated November 14, 2014, and Prospectus Supplement No. 3 dated December 18, 2014, relating to the resale of up to 53,035,356 shares of our common stock by selling stockholders.
This Prospectus Supplement No. 4 is being filed to include the information set forth in our Current Report on Form 8-K, which was filed with the Securities and Exchange Commission on January 9, 2015.
You should read this Prospectus Supplement No. 4 in conjunction with the Prospectus. This Prospectus Supplement No. 4 is qualified by reference to the Prospectus, except to the extent that the information contained in this Prospectus Supplement No. 4 supersedes the information contained in the Prospectus. This Prospectus Supplement No. 4 is not complete without, and may not be utilized except in connection with, the Prospectus.
You should consider carefully the risks that we have described in “Risk Factors” beginning on page 9 of the Prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this Prospectus Supplement is January 9, 2015
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):January 9, 2015
Enumeral Biomedical Holdings, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 333-185891 | 99-0376434 |
| (State or Other Jurisdiction | (Commission File | (I.R.S. Employer |
| of Incorporation) | Number) | Identification Number) |
One Kendall Square, Building 400, 4th Floor
Cambridge, Massachusetts | 02139 |
| (Address of Principal Executive Offices) | (Zip Code) |
(617) 674-1865
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
¨ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
¨ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
¨ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
¨ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Item 7.01 Regulation FD Disclosure.
Enumeral Biomedical Holdings, Inc. (the “Company”) may use a slide presentation, in whole or in part, from time to time in presentations to potential partners, investors, analysts and others. A copy of the slide presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K and incorporated by reference herein. A copy of the slide presentation is also available on the Company’s website atwww.enumeral.com.
The information in this Item 7.01 of this Current Report on Form 8-K and in the accompanying Exhibit 99.1 shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in this Item 7.01 of this Current Report on Form 8-K and in the accompanying Exhibit 99.1 shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
Exhibit
Number | | Description |
| | | |
| 99.1 | | Enumeral Biomedical Holdings, Inc. January 2015 Overview Presentation |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | ENUMERAL BIOMEDICAL HOLDINGS, INC. |
| | |
| Dated: January 9, 2015 | By: | /s/ Kevin G. Sarney |
| | | Name: Kevin G. Sarney |
| | | Title: Vice President of Finance and Chief Accounting Officer |
Exhibit 99.1

THE POWER of HUMAN™ Enumeral Overview January 2015 Arthur H. Tinkelenberg , Ph.D. President and CEO

Forward Looking Statements OTC QB: ENUM THIS PRESENTATION CONTAINS FORWARD - LOOKING STATEMENTS THAT ARE BASED ON THE COMPANY’S CURRENT EXPECTATIONS, ASSUMPTIONS, ESTIMATES AND PROJECTIONS ABOUT THE COMPANY AND THE PHARMACEUTICAL INDUSTRY. THE COMPANY MAKES NO REPRESENTATIONS ABOUT THE ACCURACY OF SUCH STATEMENTS ESTIMATES OR PROJECTIONS. FORWARD - LOOKING STATEMENTS ARE INDICATED BY WORDS SUCH AS: MAY, WILL, SHOULD, PREDICT, CONTINUE, PLAN, EXPECT, ANTICIPATE, ESTIMATE, INTEND, BELIEVE, COULD, GOAL OBJECTIVES AND SIMILAR EXPRESSIONS. FORWARD - LOOKING STATEMENTS MAY INCLUDE, BUT ARE NOT LIMITED TO, STATEMENTS CONCERNING THE COMPANY’S ANTICIPATED PERFORMANCE, INCLUDING REVENUE AND PROFIT EXPECTATIONS; DEVELOPMENT AND IMPLEMENTATION OF OUR COLLABORATIONS; DURATION; SIZE; SCOPE AND REVENUE ASSOCIATED WITH COLLABORATION PARTNERSHIPS; BENEFITS PROVIDED TO COLLABORATION PARTNERS BY OUR TECHNOLOGY; BUSINESS MIX; REVENUES AND GROWTH IN OUR PARTNER BASE; MARKET OPPORTUNITIES; COMPETING TECHNOLOGIES, INDUSTRY CONDITIONS AND TRENDS; AND REGULATORY DEVELOPMENTS. ACTUAL RESULTS MAY DIFFER MATERIALLY FROM THE ANTICIPATED RESULTS DUE TO SUBSTANTIAL RISKS AND UNCERTAINTIES RELATED TO THE COMPANY AND THE BIOPHARMACEUTICAL INDUSTRY IN WHICH THE COMPANY OPERATES. 2

Enumeral’s Mission Discover and Develop Best - In - Class Antibody Immunotherapies Using Our Proprietary Platform that Uniquely Leverages Human Cell Biology 3
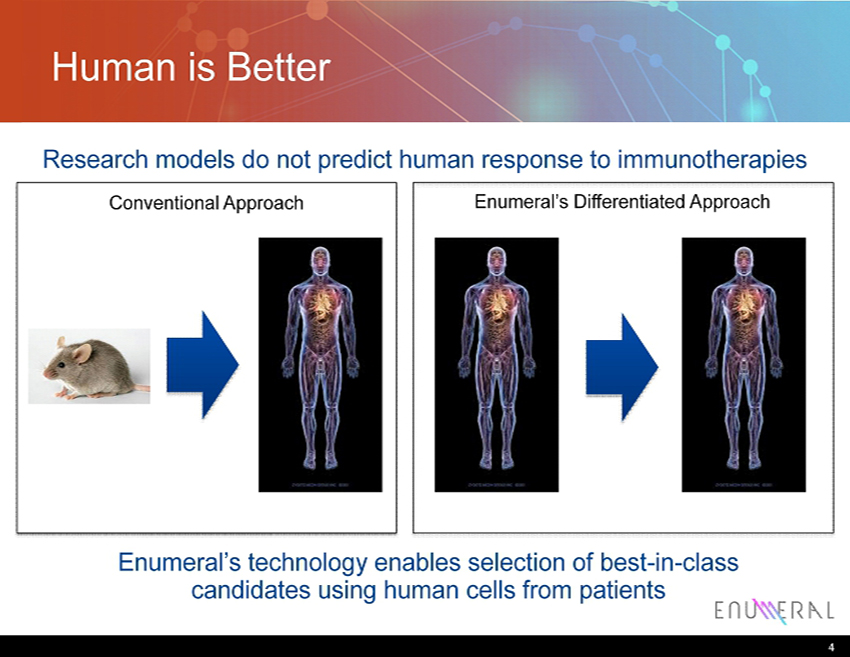
Human is Better Research models do not predict human response to immunotherapies 4 Conventional Approach Enumeral’s Differentiated Approach Enumeral’s technology enables selection of best - in - class candidates using human cells from patients

Immunotherapy market opportunity • Immunotherapy predicted to generate sales of up to $35 billion per year over next 10 years and treat up to 60% of all cancers* • Yervoy proves clinical and commercial success is tenable - launched in 2011 , >$ 1B in revenue year ended June 30th, 2014** • Strong interest among pharmaceutical companies, especially for antibodies • Merck, Bristol Myers Squibb, AstraZeneca, Roche lead; others actively investing 5 *Citigroup **Bristol Myers Squibb
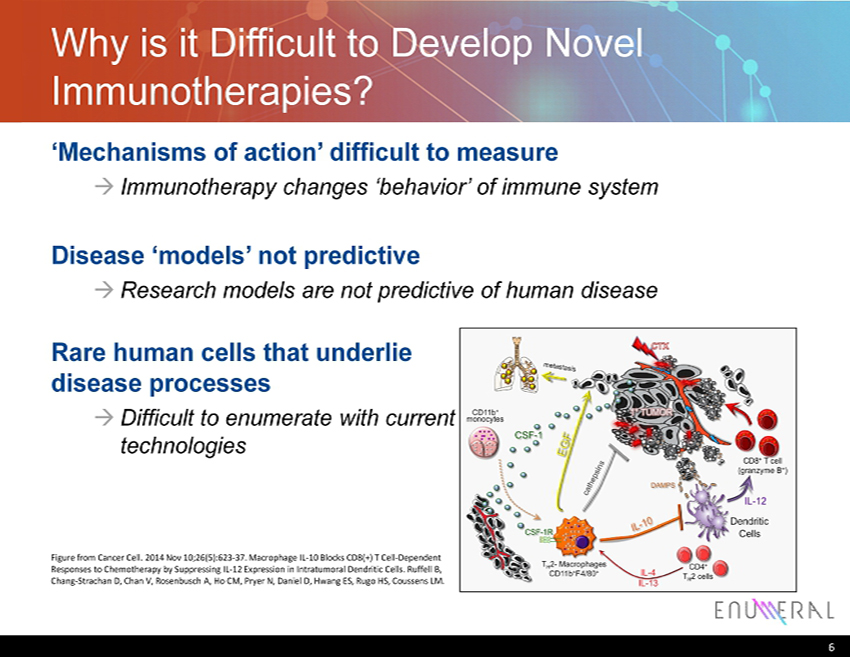
Why is it Difficult to Develop Novel Immunotherapies? ‘Mechanisms of action’ difficult to measure → Immunotherapy changes ‘behavior’ of immune system Disease ‘models’ not predictive → Research models are not predictive of human disease 6 Rare human cells that underlie disease processes → Difficult to enumerate with current technologies Figure from Cancer Cell. 2014 Nov 10;26(5):623 - 37. Macrophage IL - 10 Blocks CD8(+) T Cell - Dependent Responses to Chemotherapy by Suppressing IL - 12 Expression in Intratumoral Dendritic Cells. Ruffell B, Chang - Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS, Coussens LM.
Enumeral opportunity • Antibody immunotherapies : proven immunotherapy class; product category understood; high demand • Ongoing programs targeting checkpoint proteins: PD - 1, OX40, Lag - 3, others; candidates and indications selected using Enumeral’s ‘Human Approach’ for cancer and non - cancer indications • Differentiated approach: platform confirms function and enables selection of best - in - class candidates using human cells from patients 7
Enumeral’s Differentiation • Many technologies focused on drug candidate discovery • Few platforms that can also address will they work , and if so, in whom … • Proprietary platform developed at Harvard and MIT – Enables rapid discovery of antibody candidates , faster, cheaper, and with substantially greater candidate diversity than any established technology of which we are aware – Informs selection of potential best - in - class candidates , based on direct measure of rare immune cell function using human cells from patients 8
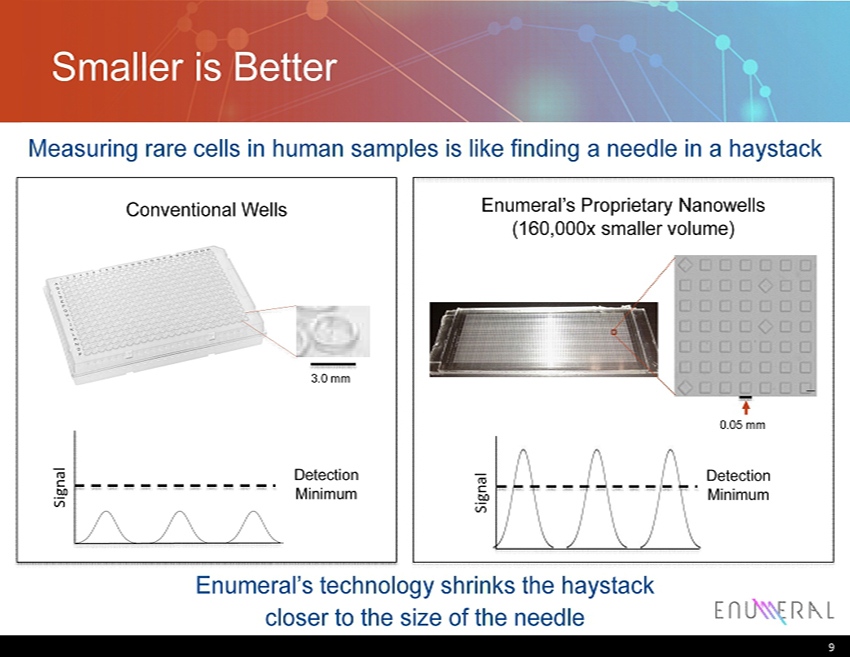
Smaller is Better Measuring rare cells in human samples is like finding a needle in a haystack Enumeral’s technology shrinks the haystack closer to the size of the needle 9 3.0 mm Conventional Wells Enumeral’s Proprietary Nanowells (160,000x smaller volume) 0.05 mm Detection Minimum Signal Signal Detection Minimum
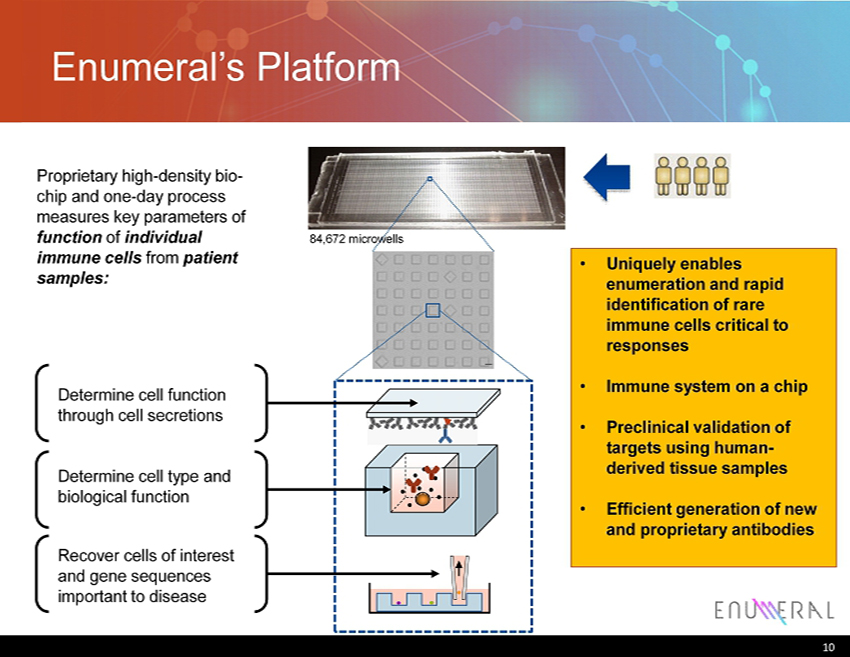
Enumeral’s Platform 10 84,672 microwells Proprietary high - density bio - chip and one - day process measures key parameters of function of individual immune cells from patient samples: Determine cell function through cell secretions Determine cell type and biological function R ecover cells of interest and gene sequences important to disease • Uniquely enables enumeration and rapid identification of rare immune cells critical to responses • Immune system on a chip • Preclinical validation of targets using human - derived tissue samples • Efficient generation of new and proprietary antibodies 10
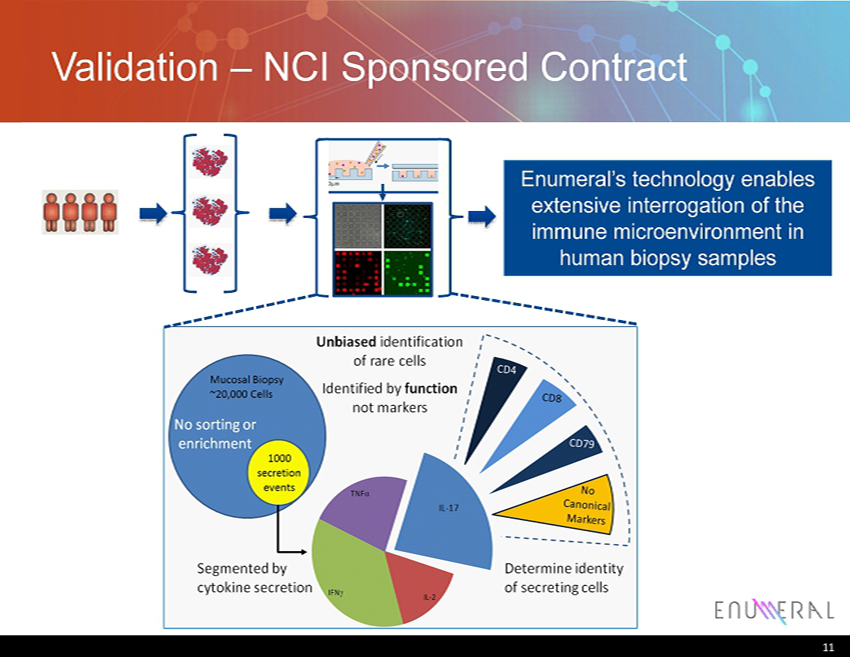
Validation – NCI Sponsored Contract 11 Enumeral’s technology enables extensive interrogation of the immune microenvironment in human biopsy samples

Enumeral’s Platform Enables Study of Disease in Humans at Disease Site 12 Enumeral’s platform enables human - based development paradigm Proprietary functional immune signatures determined from both patient groups Human Population – Biopsy Tissues Enumeral Platform Patients who benefit from treatment Patients who do not benefit from treatment

Enumeral’s human - based target validation and lead selection 13 [Indication 1] [Indication 2] [Indication 3] … [Indication X] Patients Biopsy Focus on validated targets of high commercial value TILs Enumerate “Tissue - infiltrating Lymphocytes” Targets Leads Expression of targets by cell type: PD - 1 OX40 Lag - 3 Target 4 Target 5 Target 6 Target 7 Antibodies from ENUM proprietary libraries Selected based on predicted effects from ex vivo human studies

Faster is Better 14 Enumeral believes its technology platform is faster where time to market can mean the difference of billions of dollars Target to Lead Candidate Enumeral - Estimated Development Timeline Target to Lead Candidate Traditional Pharma/Biotech 1 1 Paul et al, "How to improve R&D productivity: the pharmaceutical industry's grand challenge". Nature Reviews Drug Discovery 9, 203 - 21 4 (March 2010) | doi:10.1038/nrd3078 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4
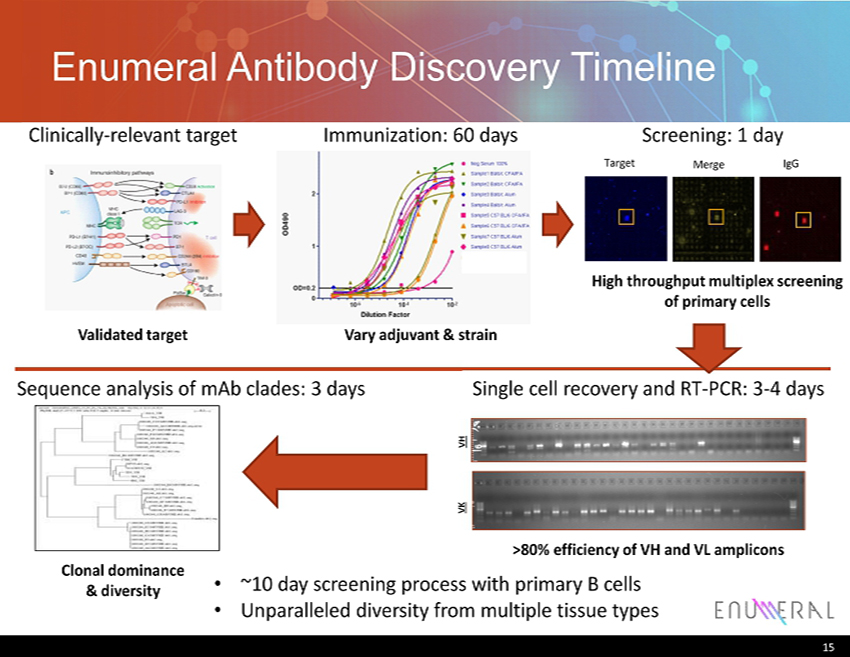
Enumeral Antibody Discovery Timeline Freeman & Sharpe 2012 VH VK Sequence analysis of mAb clades: 3 days • ~10 day screening process with primary B cells • Unparalleled diversity from multiple tissue types Single cell recovery and RT - PCR: 3 - 4 days Clinically - relevant target Immunization: 60 days Target Merge IgG Screening: 1 day Vary adjuvant & strain Validated target High throughput multiplex screening of primary cells >80% efficiency of VH and VL amplicons Clonal dominance & diversity 15
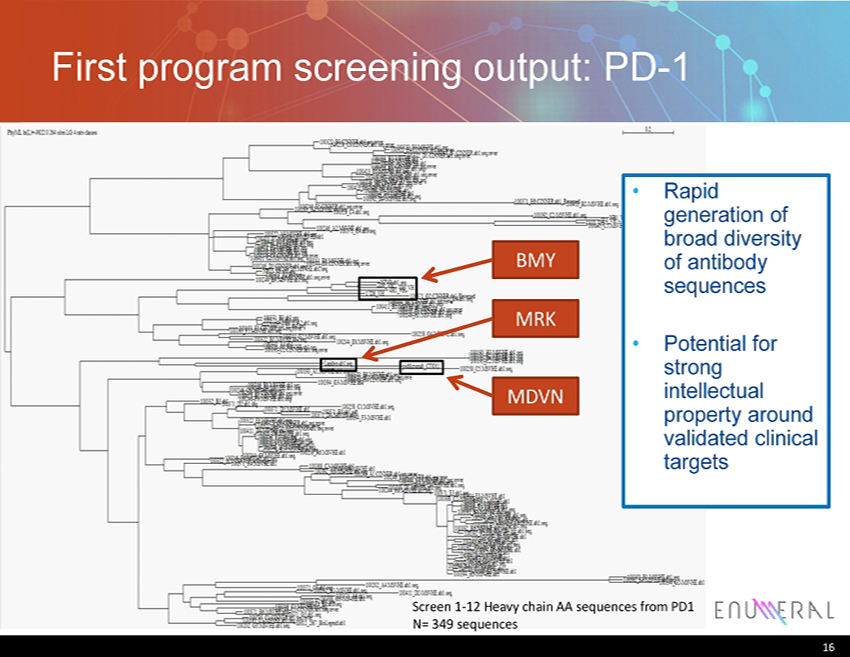
First program screening output: PD - 1 16 Screen 1 - 12 Heavy chain AA sequences from PD1 N= 349 sequences BMY MRK MDVN • Rapid generation of broad diversity of antibody sequences • P otential for strong intellectual property around validated clinical targets
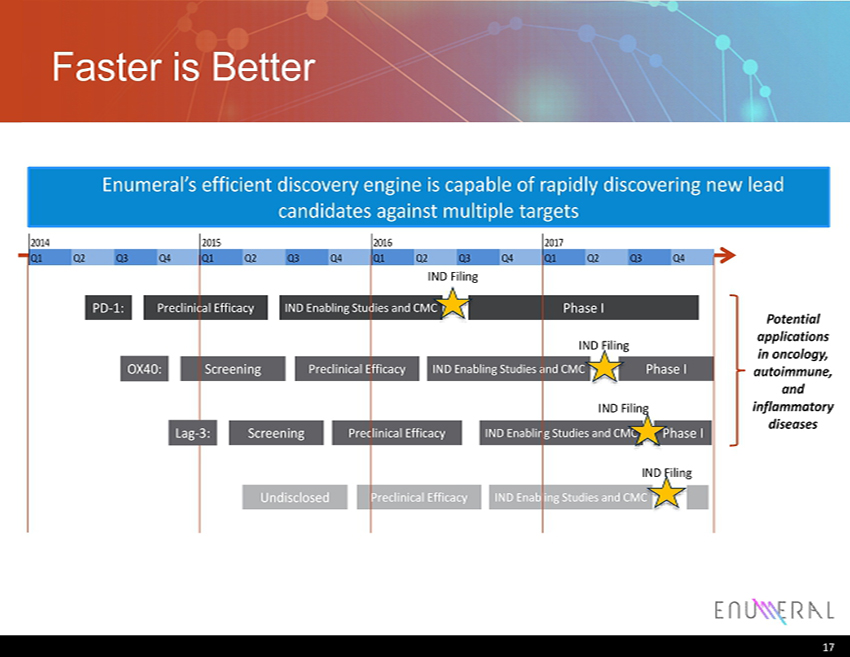
17 Faster is Better 2014 2015 2016 2017 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 IND Filing IND Enabling Studies and CMC Preclinical Efficacy Phase I PD - 1: Phase I Screening IND Enabling Studies and CMC Preclinical Efficacy IND Filing Phase I Screening IND Enabling Studies and CMC Preclinical Efficacy IND Filing Undisclosed IND Enabling Studies and CMC Preclinical Efficacy IND Filing Enumeral’s efficient discovery engine is capable of rapidly discovering new lead candidates against multiple targets OX40: Lag - 3: Potential applications in oncology, autoimmune, and inflammatory diseases
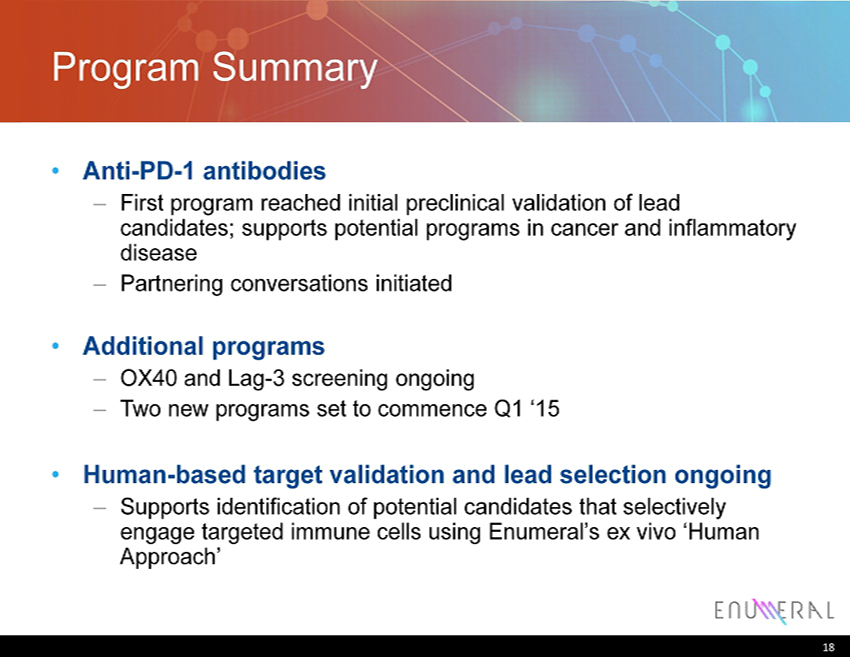
Program Summary • Anti - PD - 1 antibodies – First program reached initial preclinical validation of lead candidates; supports potential programs in cancer and inflammatory disease – Partnering conversations initiated • Additional programs – OX40 and Lag - 3 screening ongoing – Two new programs set to commence Q1 ‘15 • Human - based target validation and lead selection ongoing – Supports identification of potential candidates that selectively engage targeted immune cells using Enumeral’s ex vivo ‘Human Approach’ 18

Anti - PD - 1 key questions: “What is the drug mechanism?” 19 http://www.hematology.org/Thehematologist/Diffusion/2560.aspx • Ipilimumab , N ivolumab – Do these drug ‘deplete’ cells? Induce anergy ? Induce apoptosis? Activate T cells? What else happens? – Why are these agents active in melanoma but not in colorectal cancer? – What accounts for patient variability in efficacy vs. adverse events? • Enumeral’s platform: potential to solve these questions based on ex vivo human profiling
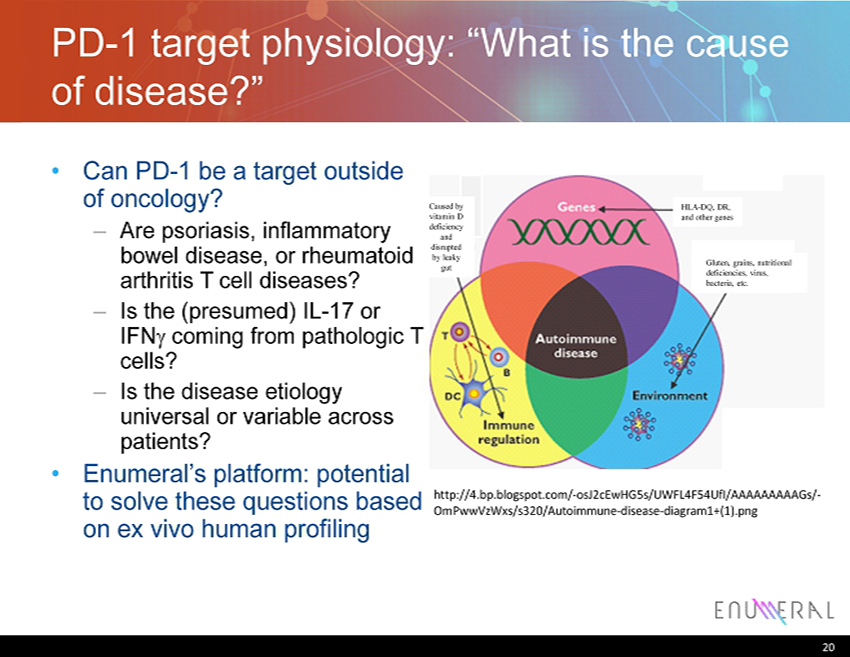
PD - 1 target physiology: “What is the cause of disease?” • Can PD - 1 be a target outside of oncology? – Are psoriasis, inflammatory bowel disease, or rheumatoid arthritis T cell diseases? – Is the (presumed) IL - 17 or IFN g coming from pathologic T cells? – Is the disease etiology universal or variable across patients? • Enumeral’s platform: potential to solve these questions based on ex vivo human profiling 20 http://4.bp.blogspot.com/ - osJ2cEwHG5s/UWFL4F54UfI/AAAAAAAAAGs/ - OmPwwVzWxs/s320/Autoimmune - disease - diagram1+(1).png HLA - DQ, DR, and other genes Gluten, grains, nutritional deficiencies, virus, bacteria, etc. Caused by vitamin D deficiency and disrupted by leaky gut
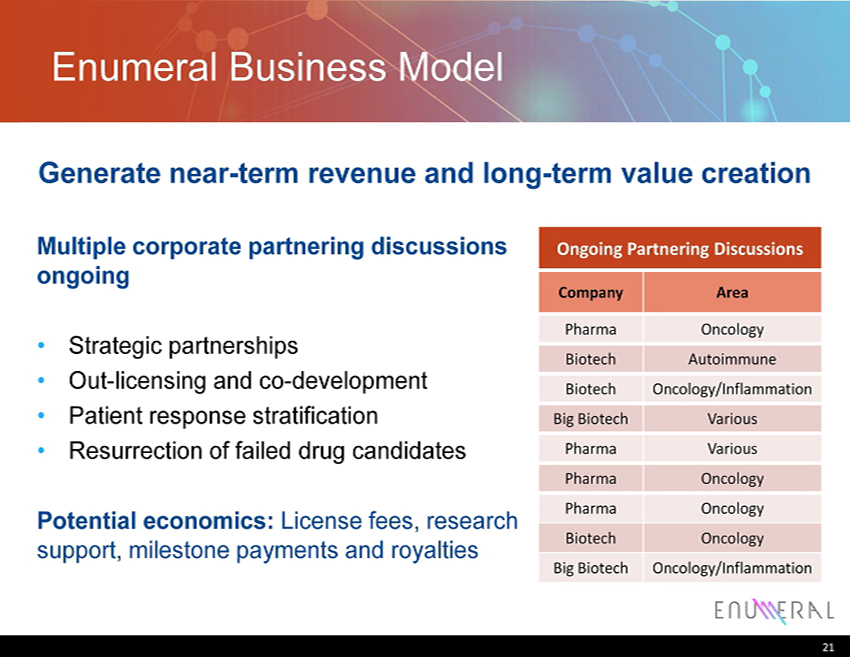
Enumeral Business Model Multiple corporate partnering discussions ongoing • Strategic partnerships • Out - licensing and co - development • Patient response stratification • Resurrection of failed drug candidates Potential economics: License fees, research support, milestone payments and royalties 21 Ongoing Partnering Discussions Company Area Pharma Oncology Biotech Autoimmune Biotech Oncology/Inflammation Big Biotech Various Pharma Various Pharma Oncology Pharma Oncology Biotech Oncology Big Biotech Oncology/Inflammation Generate near - term revenue and long - term value creation
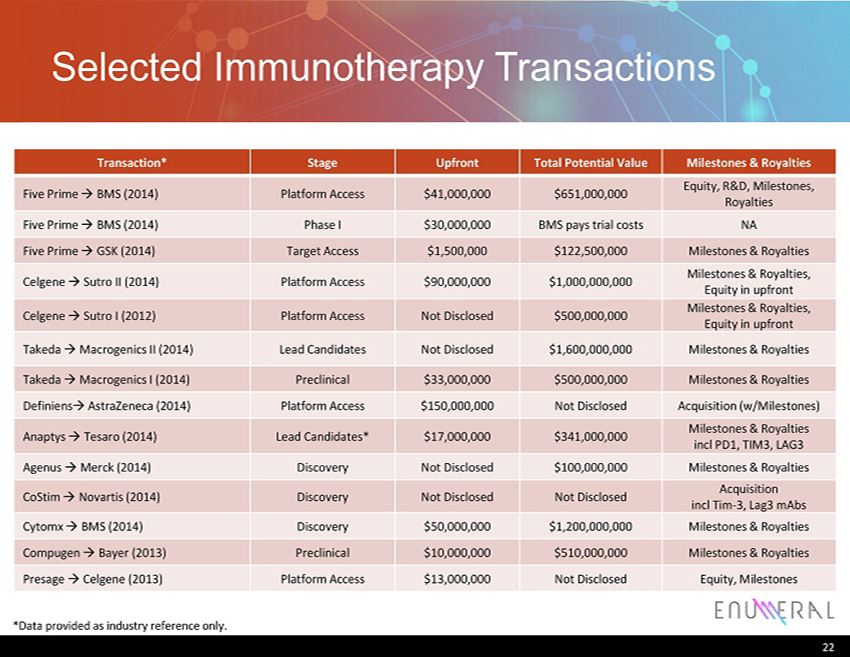
Selected Immunotherapy Transactions Transaction* Stage Upfront Total Potential Value Milestones & Royalties Five Prime → BMS (2014) Platform Access $41,000,000 $651,000,000 Equity, R&D, Milestones, Royalties Five Prime → BMS (2014) Phase I $30,000,000 BMS pays trial costs NA Five Prime → GSK (2014) Target Access $1,500,000 $122,500,000 Milestones & Royalties Celgene → Sutro II (2014) Platform Access $90,000,000 $1,000,000,000 Milestones & Royalties, Equity in upfront Celgene → Sutro I (2012) Platform Access Not Disclosed $500,000,000 Milestones & Royalties, Equity in upfront Takeda → Macrogenics II (2014) Lead Candidates Not Disclosed $1,600,000,000 Milestones & Royalties Takeda → Macrogenics I (2014) Preclinical $33,000,000 $500,000,000 Milestones & Royalties Definiens → AstraZeneca (2014) Platform Access $150,000,000 Not Disclosed Acquisition ( w/Milestones) Anaptys → Tesaro (2014) Lead Candidates* $17,000,000 $341,000,000 Milestones & Royalties incl PD1, TIM3, LAG3 Agenus → Merck (2014) Discovery Not Disclosed $100,000,000 Milestones & Royalties CoStim → Novartis (2014) Discovery Not Disclosed Not Disclosed Acquisition incl Tim - 3, Lag3 mAbs Cytomx → BMS (2014 ) Discovery $50,000,000 $1,200,000,000 Milestones & Royalties Compugen → Bayer (2013) Preclinical $10,000,000 $510,000,000 Milestones & Royalties Presage → Celgene (2013) Platform Access $13,000,000 Not Disclosed Equity, Milestones 22 *Data provided as industry reference only.

Enumeral and Merck Form Collaboration for Predicting Clinical Drug Response with Human - driven Immune Profiling Platform • December 18, 2014 • O ncology - focused collaboration using Enumeral's human approach to interrogate the tumor microenvironment in colorectal cancer tissues obtained directly from patients in order to identify functional cellular responses to immuno - oncology therapies being developed by Merck . • Enumeral will receive R&D funding and is eligible to receive undisclosed future milestone payments if certain goals are achieved. Merck has exclusive rights to data related to its proprietary compounds that are generated from the studies. 23

Recent Achievements • Collaboration deal signed with top immuno - oncology pharmaceutical company (Merck, December 18, 2014) • Received notice that seven patents in the Company’s intellectual property estate recently issued: three patents in the U.S. and four patents in international jurisdictions • Awarded $1M Phase II SBIR contract from the National Cancer Institute for technology development for human immuno - oncology profiling (September 2014) • Appointments of veteran biotech executives to Board of Directors – Paul J. Sekhri , Senior Vice President, Integrated Care at Sanofi Robert L. Van Nostrand , former CFO of OSI Pharmaceuticals • Raised $21.5M through private placement offering and began public trading on the OTC Markets (OTCQB:ENUM; July 2014) 24

Near - term Corporate Objectives • 2015 (1H): – Sign additional corporate partnership – Achieve key human ex vivo preclinical milestone in lead program (PD - 1) – Commence screening programs for next two targets • 2015 (2H): – Commence IND - enabling studies in first program (with a partner) – Sign additional corporate partnership – Achieve key human ex vivo preclinical milestones on next two programs (OX40 and Lag - 3) • 2016 – IND filing in first program; initiate Phase 1 ( with a partner) – Next two programs proceed to IND - enabling work – Sign additional corporate partnership 25

Management Team • John J. Rydzewski, Executive Chairman, Co - Founder, Director Co Chair, RAND Healthcare Advisory Board, Christofferson - Robb, Kidder - Peabody, Price Waterhouse & Co., Wharton • Arthur H. Tinkelenberg , Ph.D., President & CEO, Co - Founder, Director Ascent Biomedical Ventures, Robertson - Stephens, Columbia, Rockefeller • Kevin G. Sarney, Vice President, Finance, and Chief Accounting Officer Avaxia , Archemix , NitroMed and PricewaterhouseCoopers • Isabel Chiu , Ph.D., Vice President, Vice President of Translational and Clinical Sciences AVEO, GPC - Biotech, Mitotix , Johns Hopkins • Cokey Nguyen, Ph.D., Vice President of Research and Development FORMA , Genzyme, Novartis, MIT, Washington University, Harvard • Derek Brand, Vice President, Business Development New York Academy of Sciences, HyperMed , Argose , GE Sensing • Matthew A. Ebert, General Counsel Stream Global Services, NitroMed , Hale and Dorr LLP (now WilmerHale LLP) 26

Board of Directors • John J. Rydzewski, Executive Chairman, Co - Founder • Arthur H. Tinkelenberg , Ph.D., President & CEO, Co - Founder • Barry Buckland , Ph.D., Co - Founder, Co - Chairman of the Scientific Advisory Board Merck’s bioprocess R&D Group; launched immunotherapy products, including GARDASIL, ROTATEQ, and ZOSTAVAX • Allan Rothstein Managing Director, Hedge Capital Partners • Paul J. Sekhri Independent Director, Senior Vice President, Integrated Care at Sanofi • Robert L. Van Nostrand Independent Director, former Chief Financial Officer , OSI Pharmaceuticals • Daniel Wolfe, Ph.D. President , Chief Operating Officer, & Managing Director of Harris & Harris Group, Inc. (NASDAQ:TINY) 27
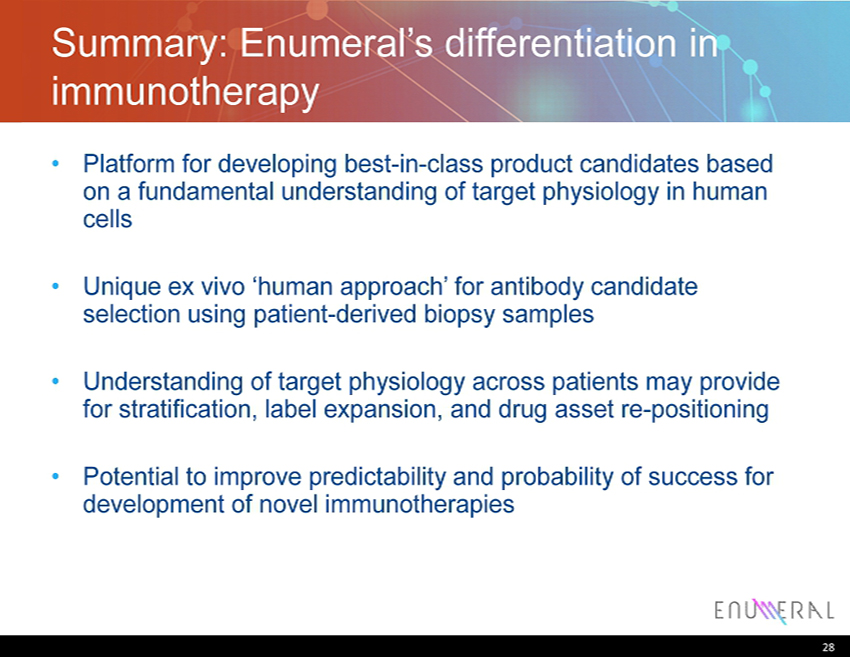
Summary: Enumeral’s differentiation in immunotherapy • Platform for developing best - in - class product candidates based on a fundamental understanding of target physiology in human cells • Unique ex vivo ‘human approach’ for antibody candidate selection using patient - derived biopsy samples • Understanding of target physiology across patients may provide for stratification, label expansion, and drug asset re - positioning • Potential to improve predictability and probability of success for development of novel immunotherapies 28
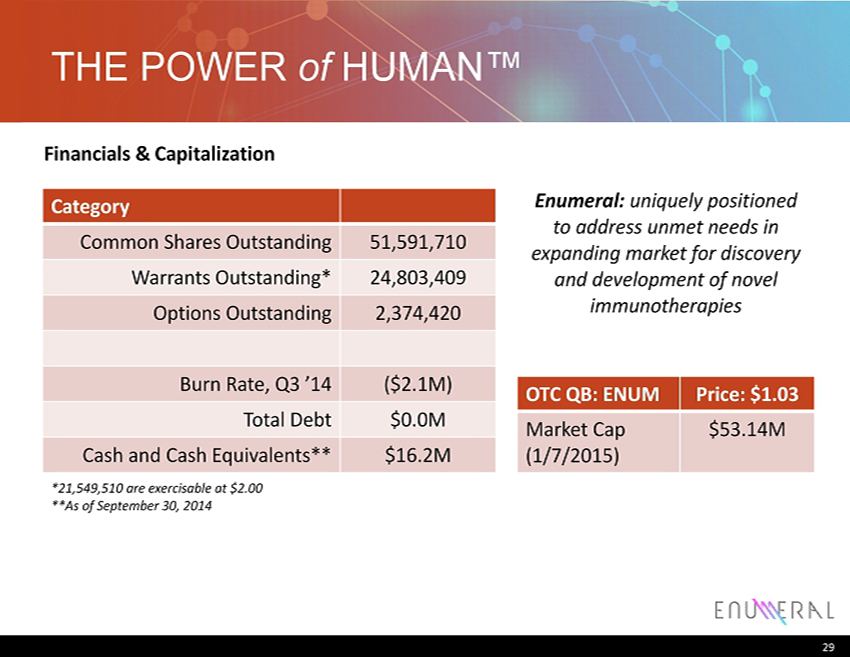
THE POWER of HUMAN ™ Category Common Shares Outstanding 51,591,710 Warrants Outstanding* 24,803,409 Options Outstanding 2,374,420 Burn Rate , Q3 ’14 ($2.1M) Total Debt $0.0M Cash and Cash Equivalents** $16.2M 29 OTC QB: ENUM Price: $1.03 Market Cap (1/7/2015) $53.14M Enumeral : uniquely positioned to address unmet needs in expanding market for discovery and development of novel immunotherapies Financials & Capitalization *21,549,510 are exercisable at $2.00 **As of September 30, 2014

THE POWER of HUMAN™ Arthur H. Tinkelenberg , Ph.D. President and CEO arthur@enumeral.com 617 - 500 - 2647





























