Filed Pursuant to Rule 424(b)(3) and Rule 424(c)
Registration No. 333-198847
Prospectus Supplement No. 7
(To Prospectus filed on November 10, 2014, as supplemented
by Prospectus Supplement No. 1 dated November 12, 2014, Prospectus Supplement No. 2 dated
November 14, 2014, Prospectus Supplement No. 3 dated December 18, 2014, Prospectus Supplement No. 4 dated January 9, 2015, Prospectus Supplement No. 5 dated February 23, 2015, and Prospectus Supplement No. 6 dated March 19, 2015)
ENUMERAL BIOMEDICAL HOLDINGS, INC.
This Prospectus Supplement No. 7 supplements the information contained in the Prospectus, dated as of November 10, 2014, as amended by Prospectus Supplement No. 1 dated November 12, 2014, Prospectus Supplement No. 2 dated November 14, 2014, Prospectus Supplement No. 3 dated December 18, 2014, Prospectus Supplement No. 4 dated January 9, 2015, Prospectus Supplement No. 5 dated February 23, 2015, and Prospectus Supplement No. 6 dated March 19, 2015 relating to the resale of up to 53,035,356 shares of our common stock by selling stockholders.
This Prospectus Supplement No. 7 is being filed to include the information set forth in our Current Report on Form 8-K, which was filed with the Securities and Exchange Commission on April 7, 2015.
You should read this Prospectus Supplement No. 7 in conjunction with the Prospectus. This Prospectus Supplement No. 7 is qualified by reference to the Prospectus, except to the extent that the information contained in this Prospectus Supplement No. 7 supersedes the information contained in the Prospectus. This Prospectus Supplement No. 7 is not complete without, and may not be utilized except in connection with, the Prospectus.
You should consider carefully the risks that we have described in “Risk Factors” beginning on page 9 of the Prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this Prospectus Supplement is April 7, 2015
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):April 7, 2015
Enumeral Biomedical Holdings, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 333-185891 | 99-0376434 |
| (State or Other Jurisdiction | (Commission File | (I.R.S. Employer |
| of Incorporation) | Number) | Identification Number) |
200 CambridgePark Drive, Suite 2000 Cambridge, Massachusetts (Address of Principal Executive Offices) | | 02140 (Zip Code) |
(617) 945-9146
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Item 7.01 Regulation FD Disclosure.
Enumeral Biomedical Holdings, Inc. (the “Company”) may use a slide presentation, in whole or in part, from time to time in presentations to potential partners, investors, analysts and others. A copy of the slide presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K and incorporated by reference herein. A copy of the slide presentation is also available on the Company’s website at www.enumeral.com.
The information in this Item 7.01 of this Current Report on Form 8-K and in the accompanying Exhibit 99.1 shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in this Item 7.01 of this Current Report on Form 8-K and in the accompanying Exhibit 99.1 shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
Exhibit Number | | Description |
| 99.1 | | Enumeral Biomedical Holdings, Inc. April 2015 Antibody Immunotherapy Programs Presentation |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | ENUMERAL BIOMEDICAL HOLDINGS, INC. |
| | | |
| | | |
| Dated: April 7, 2015 | By: | /s/ Kevin G. Sarney |
| | | Name: Kevin G. Sarney |
| | | Title: Vice President of Finance and Chief Accounting Officer |
| | | |
EXHIBIT INDEX
Exhibit
No. | | Description |
| | | |
| 99.1 | | Enumeral Biomedical Holdings, Inc. April 2015 Antibody Immunotherapy Programs Presentation |
Exhibit 99.1

Enumeral Biomedical Antibody Immunotherapy Programs April 2015

Enumeral Overview Enumeral’s mission is to discover and develop best - in - class antibody immunotherapies using our proprietary platform that uniquely leverages human cell biology The field agrees that combination therapies will be necessary to drive higher response rates… … And that there is currently little to no data to sort patients or to inform combination choices Insights into functional biology - driven by “breaking the code” of immuno - oncology at the cellular level – is the driver behind Enumeral’s pipeline of next - generation immunotherapies 2
Executive Summary Enumeral Program Overview – Sequence diversity, binding data, and initial functional data provided for PD1 program. – Enumeral anti - PD1 candidates show comparable binding characteristics and superior EC 50 data vs Keytruda and superior ability to disrupt functional inhibition from PDL1 vs Keytruda – Update on status of programs targeting Tim3, Ox40, Lag3, and VISTA – Introductory data on target validation and combination strategy Enumeral Advantage: Human - driven platform allows development based on a specific functional profile within the intent - to - treat population rational development for new indications Enumeral is seeking a partner for co - development of one or multiple programs in certain indications 3

Enumeral Immunotherapy Programs Anti - PD1 mAbs & Pipeline Update
Enumeral Discovery Advantages Discovery from primary cells, from any tissue and species leverages the mammalian immune system to its full potential Samples the full breadth of clonal diversity – Combinatorial in vitro methods like phage display lose all advantages of somatic hypermutation and affinity maturation – Hybridoma loses 99.99% of diversity through transformation and serial dilution – Thus, typical lead selection is based on “what established technology can generate” rather than being driven by mechanism of action Functional heterogeneity at lead selection maximizes opportunity for pharmacologic and mechanism of action differentiation of assets Enumeral’s functionally - driven approach is ideal for program development in a crowded oncology field and for expansion of indications 5
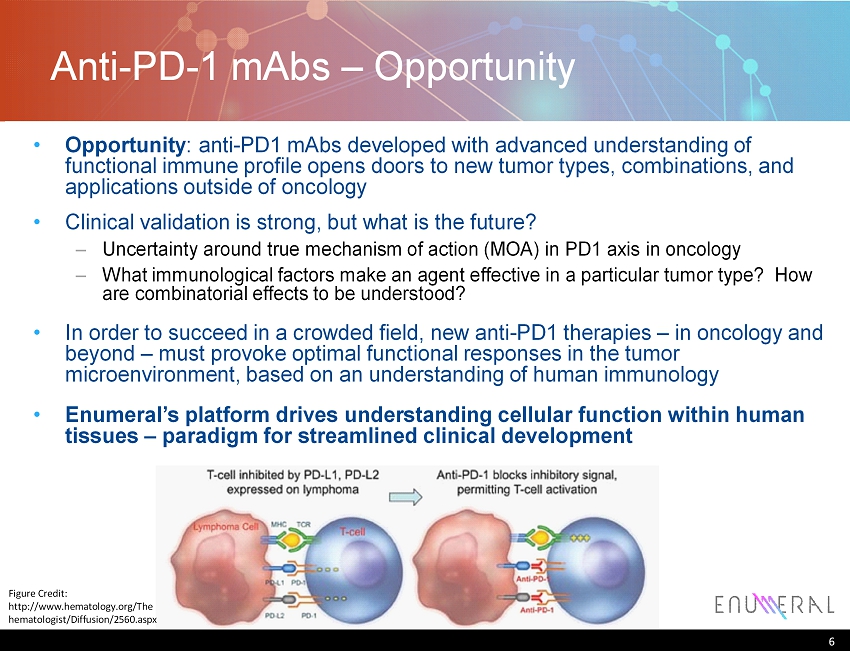
Anti - PD - 1 mAbs – Opportunity 6 Figure Credit: http ://www.hematology.org/The hematologist/Diffusion/2560.aspx Opportunity : anti - PD1 mAbs developed with advanced understanding of functional immune profile opens doors to new tumor types, combinations , and applications outside of oncology Clinical validation is strong, but what is the future? – Uncertainty around true mechanism of action (MOA) in PD1 axis in oncology – What immunological factors make an agent effective in a particular tumor type? How are combinatorial effects to be understood? In order to succeed in a crowded field, new anti - PD1 therapies – in oncology and beyond – must provoke optimal functional responses in the tumor microenvironment, based on an understanding of human immunology Enumeral’s platform drives understanding cellular function within human tissues – paradigm for streamlined clinical development
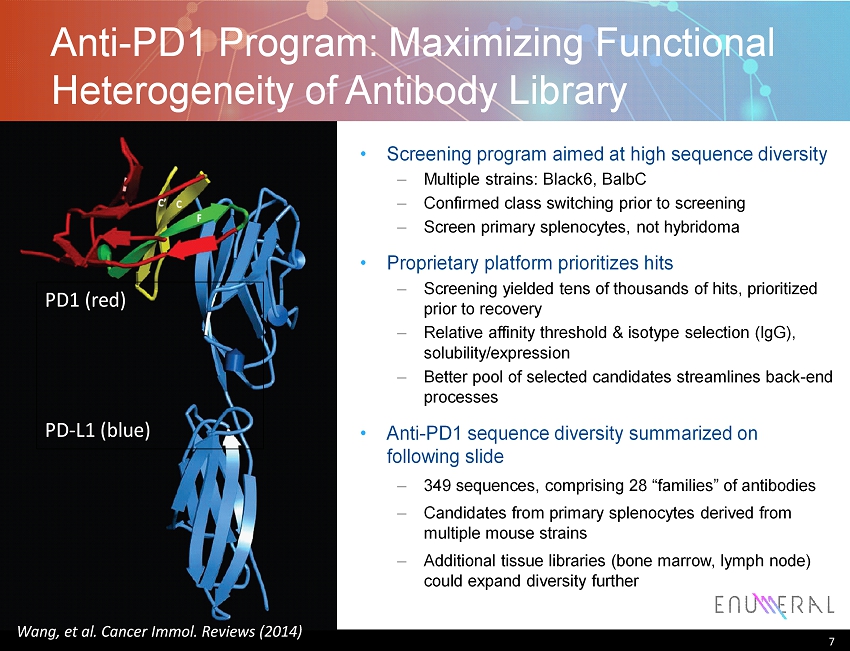
Anti - PD1 Program: Maximizing Functional Heterogeneity of Antibody Library Screening program aimed at high sequence diversity – Multiple strains: Black6 , BalbC – Confirmed class switching prior to screening – Screen primary splenocytes , not hybridoma Proprietary platform prioritizes hits – Screening yielded tens of thousands of hits, prioritized prior to recovery – Relative affinity threshold & isotype selection (IgG), solubility/expression – B etter pool of selected candidates streamlines back - end processes Anti - PD1 sequence diversity summarized on following slide – 349 sequences, comprising 28 “families” of antibodies – Candidates from primary splenocytes derived from multiple mouse strains – Additional tissue libraries (bone marrow, lymph node) could expand diversity further 7 PD1 (red) PD - L1 (blue) Wang, et al. Cancer Immol . Reviews (2014)
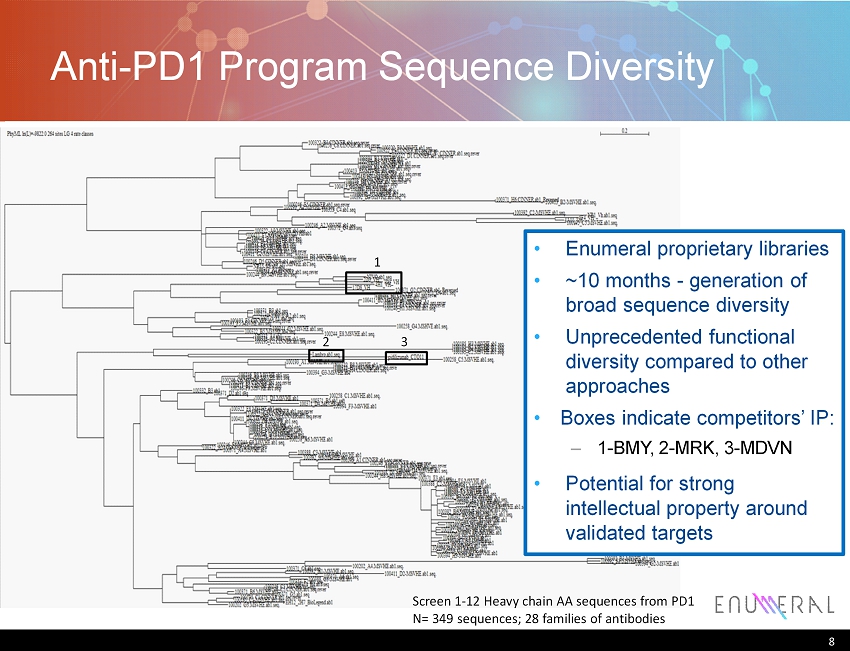
Anti - PD1 Program Sequence Diversity 8 1 2 3 Enumeral proprietary libraries ~10 months - generation of broad sequence diversity Unprecedented functional diversity compared to other approaches Boxes indicate competitors’ IP: – 1 - BMY, 2 - MRK, 3 - MDVN Potential for strong intellectual property around validated targets Screen 1 - 12 Heavy chain AA sequences from PD1 N= 349 sequences; 28 families of antibodies
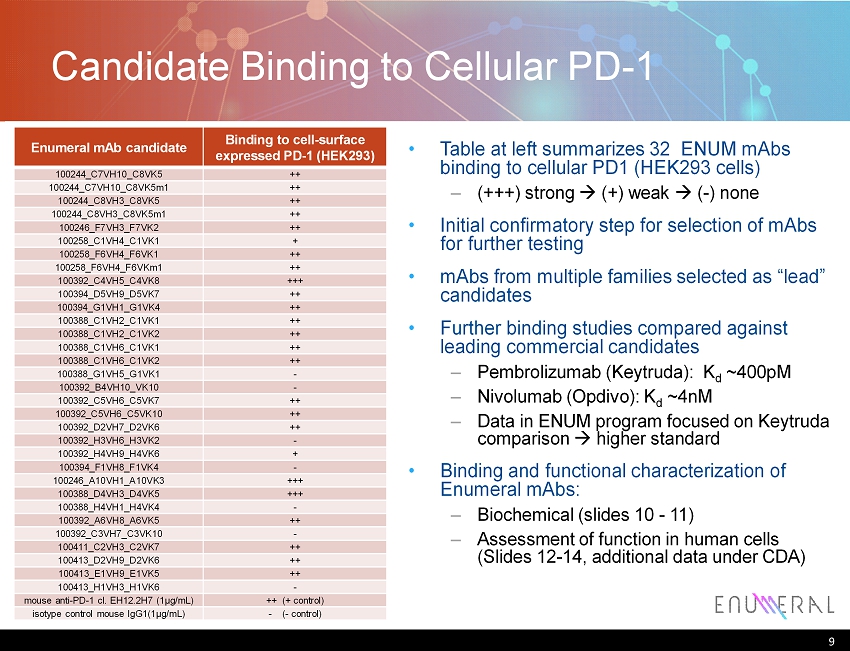
Enumeral mAb candidate Binding to cell - surface expressed PD - 1 (HEK293) 100244_C7VH10_C8VK5 ++ 100244_C7VH10_C8VK5m1 ++ 100244_C8VH3_C8VK5 ++ 100244_C8VH3_C8VK5m1 ++ 100246_F7VH3_F7VK2 ++ 100258_C1VH4_C1VK1 + 100258_F6VH4_F6VK1 ++ 100258_F6VH4_F6VKm1 ++ 100392_C4VH5_C4VK8 +++ 100394_D5VH9_D5VK7 ++ 100394_G1VH1_G1VK4 ++ 100388_C1VH2_C1VK1 ++ 100388_C1VH2_C1VK2 ++ 100388_C1VH6_C1VK1 ++ 100388_C1VH6_C1VK2 ++ 100388_G1VH5_G1VK1 - 100392_B4VH10_VK10 - 100392_C5VH6_C5VK7 ++ 100392_C5VH6_C5VK10 ++ 100392_D2VH7_D2VK6 ++ 100392_H3VH6_H3VK2 - 100392_H4VH9_H4VK6 + 100394_F1VH8_F1VK4 - 100246_A10VH1_A10VK3 +++ 100388_D4VH3_D4VK5 +++ 100388_H4VH1_H4VK4 - 100392_A6VH8_A6VK5 ++ 100392_C3VH7_C3VK10 - 100411_C2VH3_C2VK7 ++ 100413_D2VH9_D2VK6 ++ 100413_E1VH9_E1VK5 ++ 100413_H1VH3_H1VK6 - mouse anti - PD - 1 cl. EH12.2H7 (1μg/mL) ++ (+ control) isotype control mouse IgG1(1μg/mL) - ( - control) Candidate Binding to Cellular PD - 1 9 Table at left summarizes 32 ENUM mAbs binding to cellular PD1 (HEK293 cells) – (+++) strong (+) weak ( - ) none Initial confirmatory step for selection of mAbs for further testing mAbs from multiple families selected as “lead” candidates Further binding studies compared against leading commercial candidates – Pembrolizumab ( Keytruda ): K d ~400pM – Nivolumab ( Opdivo ): K d ~ 4nM – Data in ENUM program focused on Keytruda comparison higher standard Binding and functional characterization of Enumeral mAbs : – Biochemical (slides 10 - 11) – Assessment of function in human cells (Slides 12 - 14, additional data under CDA)
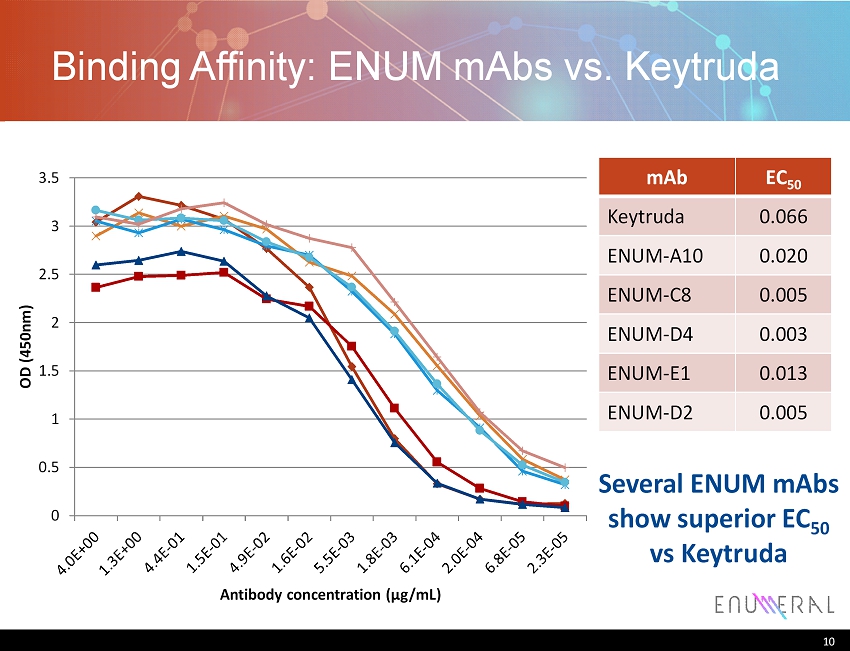
Binding Affinity: ENUM mAbs vs. Keytruda mAb EC 50 Keytruda 0.066 ENUM - A10 0.020 ENUM - C8 0.005 ENUM - D4 0.003 ENUM - E1 0.013 ENUM - D2 0.005 10 0 0.5 1 1.5 2 2.5 3 3.5 OD (450nm) Antibody concentration ( μ g/mL) Several ENUM mAbs show superior EC 50 vs Keytruda
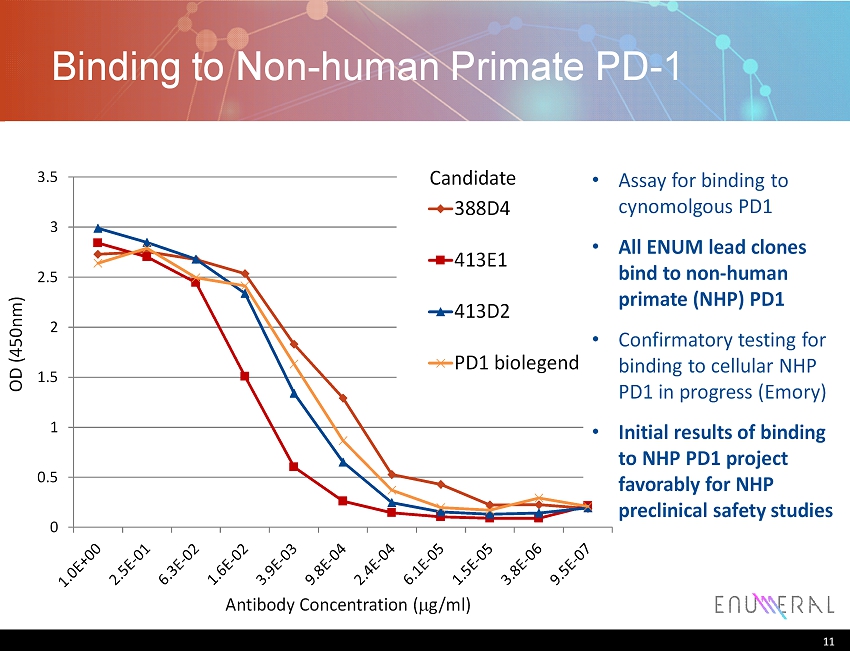
Binding to Non - human Primate PD - 1 11 Antibody Concentration ( m g/ml) OD (450nm) 0 0.5 1 1.5 2 2.5 3 3.5 388D4 413E1 413D2 PD1 biolegend Assay for binding to cynomolgous PD1 All ENUM lead clones bind to non - human primate (NHP) PD1 Confirmatory testing for binding to cellular NHP PD1 in progress (Emory) Initial results of binding to NHP PD1 project favorably for NHP preclinical safety studies Candidate
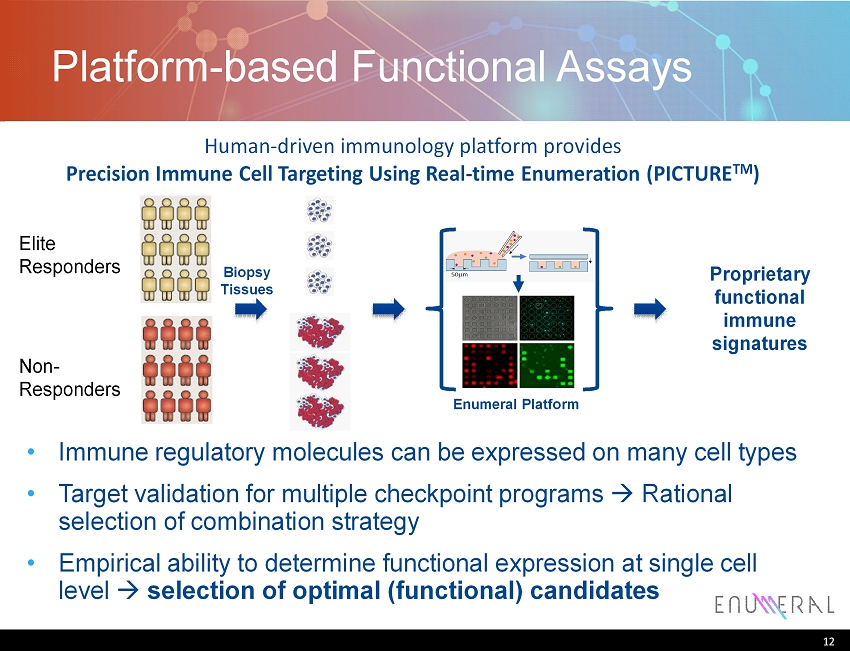
Platform - based Functional Assays 12 Proprietary functional immune signatures Biopsy Tissues Enumeral Platform Elite Responders Non - Responders Immune regulatory molecules can be expressed on many cell types Target validation for multiple checkpoint programs Rational selection of combination strategy Empirical ability to determine functional expression at single cell level selection of optimal (functional) candidates Human - driven immunology platform provides Precision Immune Cell Targeting Using Real - time Enumeration (PICTURE TM )
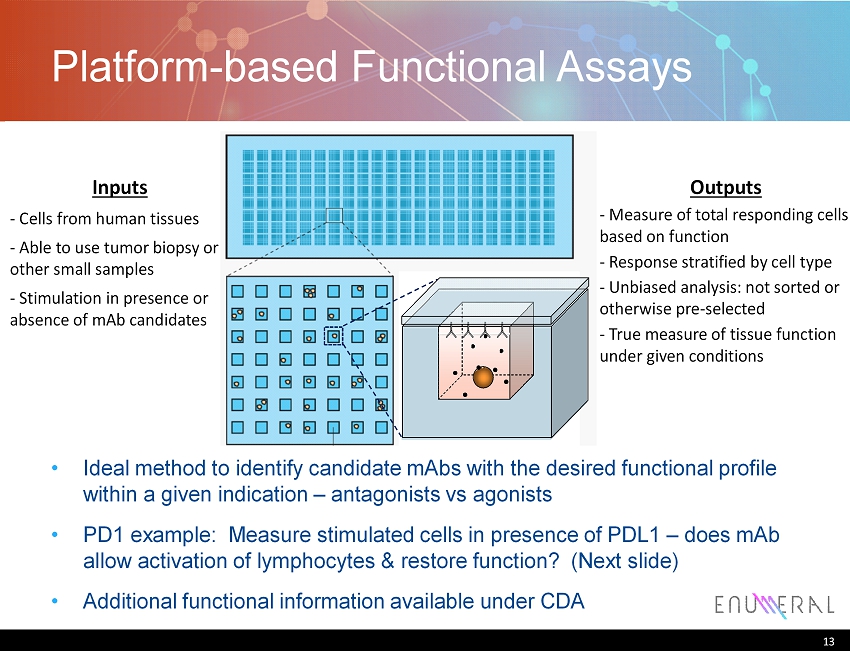
Platform - based Functional Assays Ideal method to identify candidate mAbs with the desired functional profile within a given indication – antagonists vs agonists PD1 example: Measure stimulated cells in presence of PDL1 – does mAb allow activation of lymphocytes & restore function? (Next slide) Additional functional information available under CDA 13 Inputs - Cells from human tissues - Able to use tumor biopsy or other small samples - Stimulation in presence or absence of mAb candidates Outputs - Measure of total responding cells based on function - Response stratified by cell type - Unbiased analysis: not sorted or otherwise pre - selected - True measure of tissue function under given conditions

PDL1 Suppression Assay 14 IFNg secretion (relative to control) N = 5 repetitions for each candidate/condition PBMC - based assay to determine mAb’s ability to disrupt functional suppression from PDL1 ENUM candidates compared with : – Control ( stim /no PDL1) – PDL1 (suppress resp.) – Keytruda A10 and C8 shown, all ENUM candidates tested via this and other functional assays ENUM A10 and C8 reverse PDL1 suppression more effectively than Keytruda in these experiments

Pipeline Update Program Screening Initiated Hit Selection Status PD1 Q2 2014 349 Vh amino acid sequences , 28 families Analysis of ex - vivo function via PICTURE; Affinity, epitope mapping, humanization TIM3 Q1 2015 200 + hits in - process Lead - selection from n=11 antibody clones LAG3 Q1 2015 30+ hits in - process RT - PCR sequence/clade analyses OX40 Q1 2015 76+ hits in - process Second immunization underway with cell - based antigen presentation VISTA Q2 2015 n/a Screening 15 Development status of disclosed targets in Enumeral pipeline Screening performed on multiple tissue types and multiple mouse strains to generate greatest functional diversity Second wave of programs progressing rapidly, will support testing and further validation of combination therapies Development and functional characterization continually supplemented by profiling and target validation in human tissues
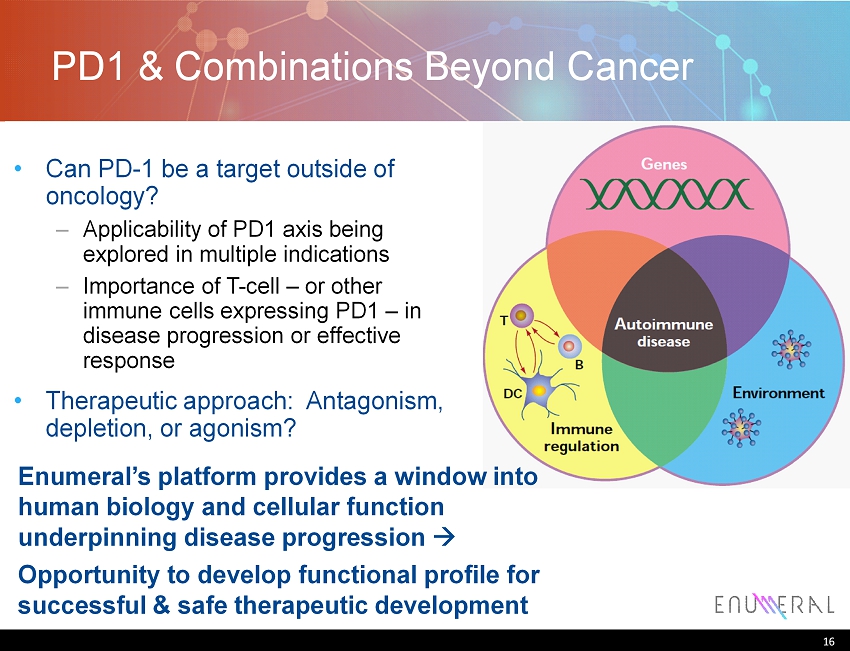
PD1 & Combinations Beyond Cancer Can PD - 1 be a target outside of oncology? – Applicability of PD1 axis being explored in multiple indications – Importance of T - cell – or other immune cells expressing PD1 – in disease progression or effective response Therapeutic approach: Antagonism, depletion, or agonism ? 16 Enumeral’s platform provides a window into human biology and cellular function underpinning disease progression Opportunity to develop functional profile for successful & safe therapeutic development

Target Validation in Human Tissues and Rational Selection of Combination Therapies
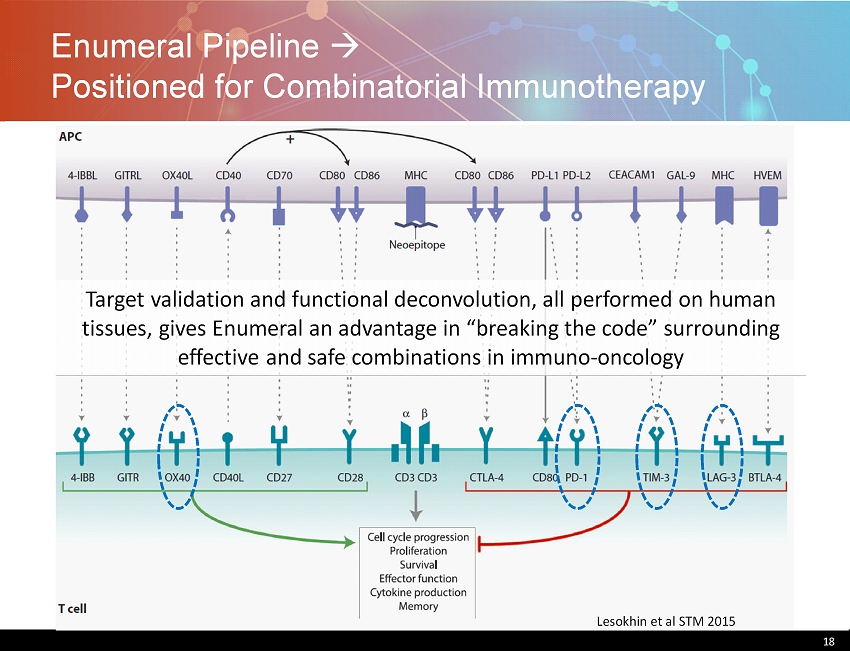
Enumeral Pipeline Positioned for Combinatorial Immunotherapy 18 Lesokhin et al STM 2015 Target validation and functional deconvolution, all performed on human tissues, gives Enumeral an advantage in “breaking the code” surrounding effective and safe combinations in immuno - oncology
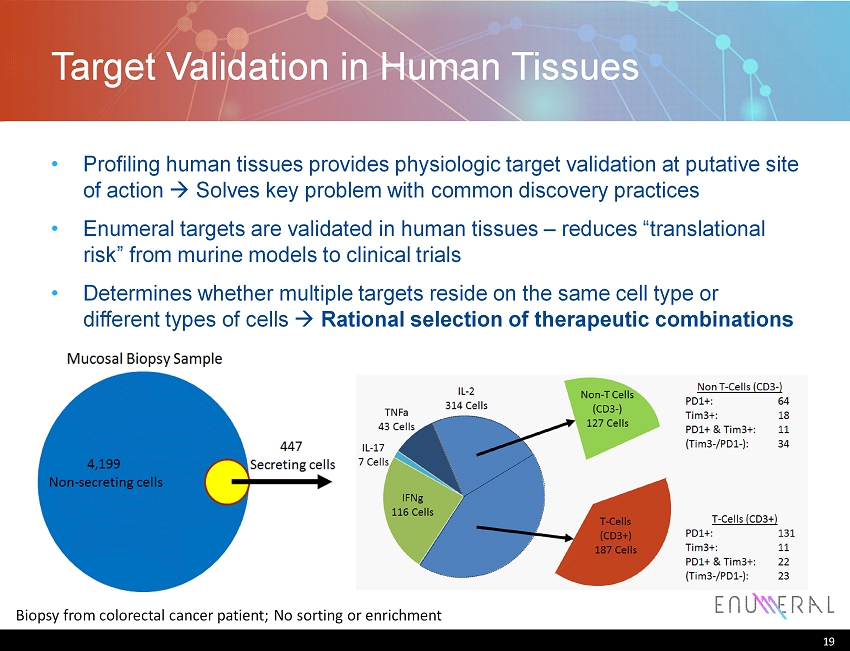
Target Validation in Human Tissues Profiling human tissues provides physiologic target validation at putative site of action Solves key problem with common discovery practices Enumeral targets are validated in human tissues – reduces “translational risk” from murine models to clinical trials Determines whether multiple targets reside on the same cell type or different types of cells Rational selection of therapeutic combinations 19 Biopsy from colorectal cancer patient; No sorting or enrichment
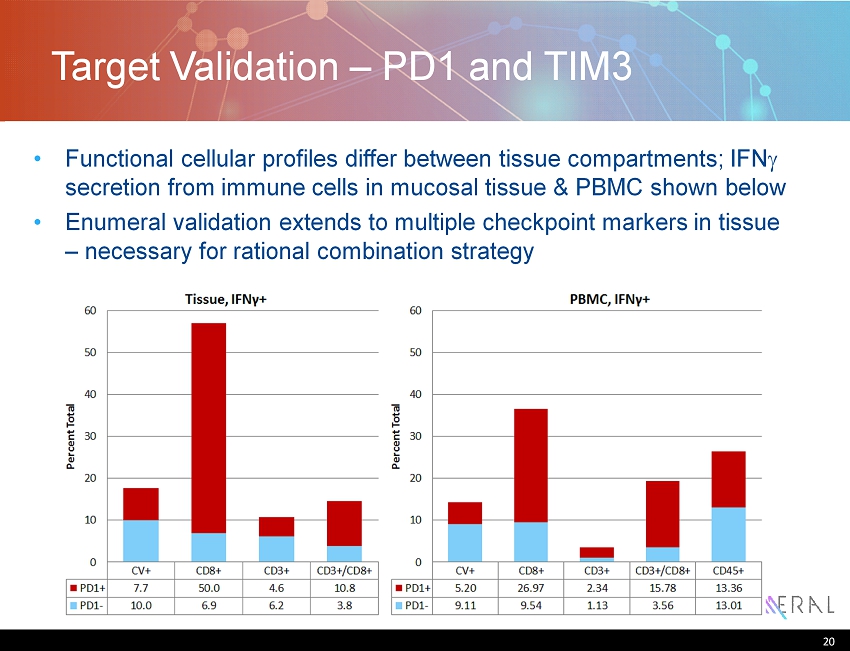
Target Validation – PD1 and TIM3 Functional cellular profiles differ between tissue compartments; IFN g secretion from immune cells in mucosal tissue & PBMC shown below Enumeral validation extends to multiple checkpoint markers in tissue – necessary for rational combination strategy 20

Next Wave of Targets: TIM family 21 Baghdadi and Jinushi 2014 TIM - 3 biology originally centered around “exhausted” T cells – Upregulated as negative feedback regulator of activation – Dampen immune response Emerging data suggests TIM - 3 is expressed on multiple cell types in the tumor microenvironment Enumeral data on PD1/TIM3 expression and validation in human tissues is encouraging for combination therapy

Enumeral Clinical Impact Does the selective activation of certain immune cells result in better efficacy? – Does this correlate with response rate or durability? How do we know elevated IFN g or granzyme B is only from effector cells? (And what cells are they…. T cells? NK cells?) Can we functionally define suppressor cells from the TME? Enumeral aims to leverage its platform to inform selection of best - in - class therapeutics and rational combinations: – Potential for immunological insight into function in human tissues to guide development and treatment – When leveraged with multiple candidates, this serves as the basis for rational selection of combination strategies 22

THE POWER of HUMAN™ Contact: Derek Brand Vice President of Business Development 917 - 225 - 8669 Derek@Enumeral.com






















