Filed Pursuant to Rule 424(b)(3) and Rule 424(c)
Registration No. 333-198847
Prospectus Supplement No. 5
(To Prospectus filed on July 6, 2015, as supplemented
by Prospectus Supplement No. 1 dated July 14, 2015, Prospectus Supplement No. 2 dated July 28, 2015, Prospectus Supplement No. 3 dated August 4, 2015, and Prospectus Supplement No. 4 dated August 12, 2015)
ENUMERAL BIOMEDICAL HOLDINGS, INC.
This Prospectus Supplement No. 5 supplements the information contained in the Prospectus, dated as of July 6, 2015, as amended by Prospectus Supplement No. 1 dated July 14, 2015, Prospectus Supplement No. 2 dated July 28, 2015, Prospectus Supplement No. 3 dated August 4, 2015, and Prospectus Supplement No. 4 dated August 12, 2015, relating to the resale of up to 52,154,760 shares of our common stock by selling stockholders.
This Prospectus Supplement No. 5 is being filed to include the information set forth in our Current Report on Form 8-K, which was filed with the Securities and Exchange Commission on September 17, 2015.
You should read this Prospectus Supplement No. 5 in conjunction with the Prospectus. This Prospectus Supplement No. 5 is qualified by reference to the Prospectus, except to the extent that the information contained in this Prospectus Supplement No. 5 supersedes the information contained in the Prospectus. This Prospectus Supplement No. 5 is not complete without, and may not be utilized except in connection with, the Prospectus.
You should consider carefully the risks that we have described in “Risk Factors” beginning on page 7 of the Prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this Prospectus Supplement is September 17, 2015
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):September 17, 2015
Enumeral Biomedical Holdings, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 000-55415 | 99-0376434 |
(State or Other Jurisdiction
of Incorporation) | (Commission File Number) | (I.R.S. Employer
Identification Number) |
200 CambridgePark Drive, Suite 2000 Cambridge, Massachusetts (Address of Principal Executive Offices) | | 02140 (Zip Code) |
(617) 945-9146
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Item 7.01 Regulation FD Disclosure.
Enumeral Biomedical Holdings, Inc. (the “Company”) may use a slide presentation, in whole or in part, from time to time in presentations to potential partners, investors, analysts and others. A copy of the slide presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K and incorporated by reference herein. A copy of the slide presentation is also available on the Company’s website at www.enumeral.com.
The information in this Item 7.01 of this Current Report on Form 8-K and in the accompanying Exhibit 99.1 shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in this Item 7.01 of this Current Report on Form 8-K and in the accompanying Exhibit 99.1 shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| Exhibit Number | | Description |
| 99.1 | | Enumeral Biomedical Holdings, Inc. September 17, 2015 Biopharm America Presentation |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | ENUMERAL BIOMEDICAL HOLDINGS, INC. |
| | | |
| Dated: September 17, 2015 | By: | /s/ Kevin G. Sarney |
| | | Name: Kevin G. Sarney |
| | | Title: Vice President of Finance, Chief Accounting Officer and Treasurer |
EXHIBIT INDEX
Exhibit
No. | | Description |
| | | |
| 99.1 | | Enumeral Biomedical Holdings, Inc. September 17, 2015 Biopharm America Presentation |
Exhibit 99.1

BiopharmAmerica 2015 September 17, 2015

Forward Looking Statements OTC QB: ENUM THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS THAT ARE BASED ON THE COMPANY’S CURRENT EXPECTATIONS, ASSUMPTIONS, ESTIMATES AND PROJECTIONS ABOUT THE COMPANY AND THE PHARMACEUTICAL INDUSTRY. THE COMPANY MAKES NO REPRESENTATIONS ABOUT THE ACCURACY OF SUCH STATEMENTS ESTIMATES OR PROJECTIONS. FORWARD-LOOKING STATEMENTS ARE INDICATED BY WORDS SUCH AS: MAY, WILL, SHOULD, PREDICT, CONTINUE, PLAN, EXPECT, ANTICIPATE, ESTIMATE, INTEND, BELIEVE, COULD, GOAL OBJECTIVES AND SIMILAR EXPRESSIONS. FORWARD-LOOKING STATEMENTS MAY INCLUDE, BUT ARE NOT LIMITED TO, STATEMENTS CONCERNING THE COMPANY’S ANTICIPATED PERFORMANCE, INCLUDING REVENUE AND PROFIT EXPECTATIONS; DEVELOPMENT AND IMPLEMENTATION OF OUR COLLABORATIONS; DURATION; SIZE; SCOPE AND REVENUE ASSOCIATED WITH COLLABORATION PARTNERSHIPS; BENEFITS PROVIDED TO COLLABORATION PARTNERS BY OUR TECHNOLOGY; BUSINESS MIX; REVENUES AND GROWTH IN OUR PARTNER BASE; MARKET OPPORTUNITIES; COMPETING TECHNOLOGIES, INDUSTRY CONDITIONS AND TRENDS; AND REGULATORY DEVELOPMENTS. ACTUAL RESULTS MAY DIFFER MATERIALLY FROM THE ANTICIPATED RESULTS DUE TO SUBSTANTIAL RISKS AND UNCERTAINTIES RELATED TO THE COMPANY AND THE BIOPHARMACEUTICAL INDUSTRY IN WHICH THE COMPANY OPERATES. 2

EnumeralMission 3 A biopharma company developing potential ‘best- in-class’ antibodies against both proven and new targets using a proprietary immuno-oncology profiling technology.

Enumeral’sDifferentiated Approach 4 Create broad antibody diversity Measure antibody function using our ‘Human immune system on a chip’ platform Select best-in-class candidates that modulate desired immune effector cells

Discovery and Profiling Processes 5
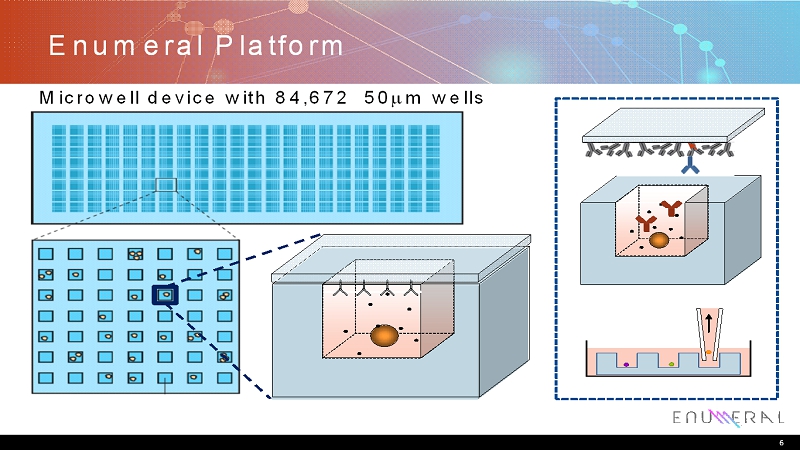
EnumeralPlatform 6 Microwell device with 84,672 50m wells

Platform Enables All IO Entry Points 7
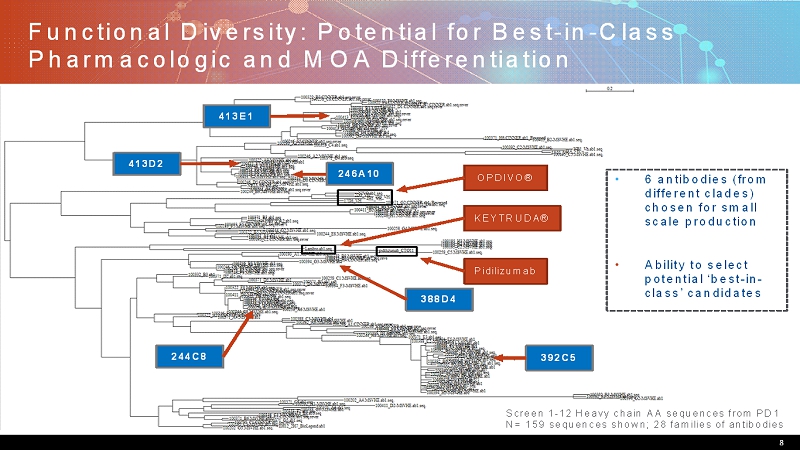
Functional Diversity: Potential for Best-in-Class Pharmacologic and MOA Differentiation 8 Screen 1-12 Heavy chain AA sequences from PD1 N= 159 sequences shown; 28 families of antibodies OPDIVO® KEYTRUDA® Pidilizumab • 6 antibodies (from different clades) chosen for small scale production • Ability to select potential ‘best-in- class’ candidates 246A10 392C5 413D2 413E1 244C8 388D4
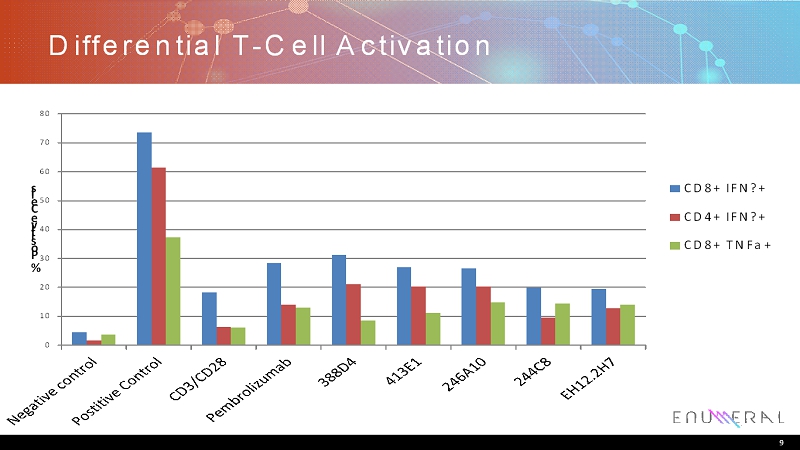
Differential T-Cell Activation 9 0 10 20 30 40 50 60 70 80 % P o s i t i v e C e l l s CD8+ IFN?+ CD4+ IFN?+ CD8+ TNFa+
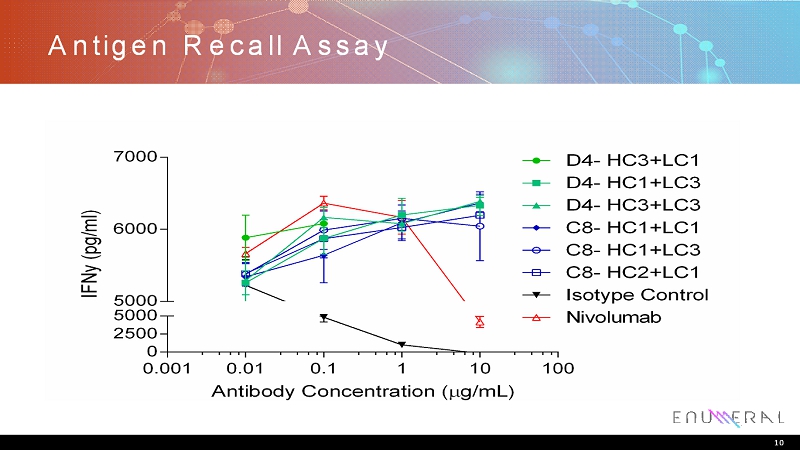
Antigen Recall Assay 10

Mixed Lymphocyte Reaction (6 days) 11

Our approach to discovery has resulted in consistent, repeatable levels of diversity for additional checkpoint targets 12

13 TIM-3 Program Diversity • CDR3 cladistics • 114 sequences • Greenboxes indicate a confirmed binder (ELISA) *indicates confirmed cell- based binder * * Screen1: 100600-100605 Screen4: 100646-100671 Screen6: 100730-100734 Screen12: 100810-100816 Screen13: 100874-100881 Screen14: 100928-100931 Screen15: 100952-100957 Screen16: 100998-101005

14 LAG-3 Program Diversity • CDR3 cladistics shown • 64 sequences CONFIDENTIAL

Why Diversity Matters: • TIM-3 biology much more complex than any other I/O target: – Ligands: – Galectin-9 – Phosphatidylserine – CEACAM1 • Correct epitope is unknown for therapeutic TIM-3 blockade • TIM-3 also a marker for cancer progression 15 Anderson et al 2007 Immunity

Tissue Profiling: Human Translational Link Between Discovery and Development 16 Use of human tissues serves as a bridge between mAbfunction in vitro and cellular function in tumors in relevant indications

Tumor Profiling at the Single Cell Level 17

O v a r i a n R e n a l C a n c e r A v a s t i n R a f / M E K i PD1 Blockade Indication Mapping - T I M 3

Conclusions • Our PD1 program gives us an independent benchmark therapeuticfor partnering in immuno-oncology • Our pipeline places us in an ideal position to further combination IO strategies in diverse indications with urgent unmet need • Our single-cell analysis platform will allow us to better understand humanimmune responses • We focus on understanding patient immunology and functional responses of the immune system, which will drive development of clinically effectivenovel therapeutics and combinations 19


















