Filed Pursuant to Rule 424(b)(3) and Rule 424(c)
Registration No. 333-198847
Prospectus Supplement No. 6
(To Prospectus filed on July 6, 2015, as supplemented
by Prospectus Supplement No. 1 dated July 14, 2015, Prospectus Supplement No. 2 dated July 28, 2015, Prospectus Supplement No. 3 dated August 4, 2015, Prospectus Supplement No. 4 dated August 12, 2015, and Prospectus Supplement No. 5 dated September 17, 2015)
ENUMERAL BIOMEDICAL HOLDINGS, INC.
This Prospectus Supplement No. 6 supplements the information contained in the Prospectus, dated as of July 6, 2015, as amended by Prospectus Supplement No. 1 dated July 14, 2015, Prospectus Supplement No. 2 dated July 28, 2015, Prospectus Supplement No. 3 dated August 4, 2015, Prospectus Supplement No. 4 dated August 12, 2015, and Prospectus Supplement No. 5 dated September 17, 2015, relating to the resale of up to 52,154,760 shares of our common stock by selling stockholders.
This Prospectus Supplement No. 6 is being filed to include the information set forth in our Current Report on Form 8-K, which was filed with the Securities and Exchange Commission on September 18, 2015.
You should read this Prospectus Supplement No. 6 in conjunction with the Prospectus. This Prospectus Supplement No. 6 is qualified by reference to the Prospectus, except to the extent that the information contained in this Prospectus Supplement No. 6 supersedes the information contained in the Prospectus. This Prospectus Supplement No. 6 is not complete without, and may not be utilized except in connection with, the Prospectus.
You should consider carefully the risks that we have described in “Risk Factors” beginning on page 7 of the Prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this Prospectus Supplement is September 18, 2015
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):September 18, 2015
Enumeral Biomedical Holdings, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | Delaware | 99-0376434 |
| (State or Other Jurisdiction of Incorporation) | (Commission File Number) | (I.R.S. Employer Identification Number) |
200 CambridgePark Drive, Suite 2000 Cambridge, Massachusetts (Address of Principal Executive Offices) | | 02140 (Zip Code) |
(617) 945-9146
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Item 7.01 Regulation FD Disclosure.
Enumeral Biomedical Holdings, Inc. (the “Company”) may use a presentation, in whole or in part, from time to time in presentations to potential partners, investors, analysts and others. A copy of the presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K and incorporated by reference herein. A copy of the slide presentation is also available on the Company’s website at www.enumeral.com.
The information in this Item 7.01 of this Current Report on Form 8-K and in the accompanying Exhibit 99.1 shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in this Item 7.01 of this Current Report on Form 8-K and in the accompanying Exhibit 99.1 shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| Exhibit Number | | Description |
| 99.1 | | Enumeral Biomedical Holdings, Inc. September 2015 Presentation Entitled “Human Tumor-Based Identification of Immune Checkpoint Targets” |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | ENUMERAL BIOMEDICAL HOLDINGS, INC. |
| | | |
| Dated: September 18, 2015 | By: | /s/ Kevin G. Sarney |
| | | Name: Kevin G. Sarney |
| | | Title: Vice President of Finance, Chief Accounting Officer and Treasurer |
EXHIBIT INDEX
Exhibit
No. | | Description |
| | | |
| 99.1 | | Enumeral Biomedical Holdings, Inc. September 2015 Presentation Entitled “Human Tumor-Based Identification of Immune Checkpoint Targets” |
Exhibit 99.1
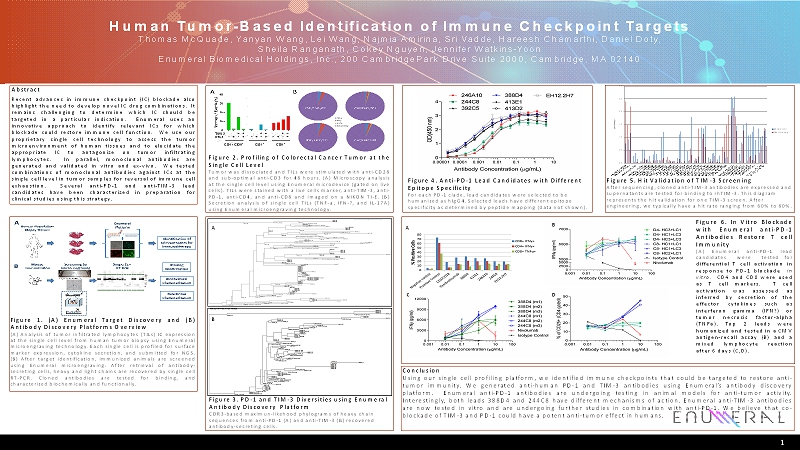
C D 1 Human Tumor-Based Identification of Immune Checkpoint Targets Thomas McQuade, Yanyan Wang, Lei Wang, Najmia Amirina, Sri Vadde, Hareesh Chamarthi, Daniel Doty, Sheila Ranganath, Cokey Nguyen, Jennifer Watkins-Yoon EnumeralBiomedical Holdings, Inc., 200 CambridgeParkDrive Suite 2000, Cambridge, MA 02140 Abstract Recentadvancesinimmunecheckpoint(IC)blockadealso highlighttheneedtodevelopnovelICdrugcombinations.It remains challenging to determine which IC should be targeted in a particular indication. Enumeral uses an innovative approach to identify relevant ICs for which blockadecouldrestoreimmunecellfunction. Weuseour proprietary single cell technology to assess the tumor microenvironmentofhumantissuesandtoelucidatethe appropriate IC to antagonize on tumor infiltrating lymphocytes. In parallel, monoclonal antibodies are generatedandvalidatedinvitroandex-vivo. Wetested combinationsofmonoclonalantibodiesagainstICsatthe singlecelllevelintumorsamplesforreversalofimmunecell exhaustion. Several anti-PD-1 and anti-TIM-3 lead candidates have been characterized in preparation for clinicalstudiesusingthisstrategy. Figure 1. (A) Enumeral TargetDiscovery and (B) AntibodyDiscoveryPlatformsOverview (A)Analysisoftumorinfiltratedlymphocytes(TILs)ICexpression atthesinglecelllevelfromhumantumorbiopsyusingEnumeral microengravingtechnology.Eachsinglecellisprofiledforsurface markerexpression,cytokinesecretion,andsubmittedforNGS. (B)Aftertargetidentification,immunizedanimalsarescreened using Enumeral microengraving. After retrieval of antibody- secretingcells,heavyandlightchainsarerecoveredbysinglecell RT-PCR. Cloned antibodies are tested for binding, and characterizedbiochemicallyandfunctionally. Figure2.ProfilingofColorectalCancerTumoratthe SingleCellLevel TumorwasdissociatedandTILswerestimulatedwithanti-CD28 andsub-optimalanti-CD3for48hours.(A)Microscopyanalysis atthesinglecelllevelusingEnumeralmicrodevice(gatedonlive cells).TILswerestainedwithalivecellsmarker,anti-TIM-3,anti- PD-1,anti-CD4,andanti-CD8andimagedonaNIKONTI-E.(B) SecretionanalysisofsinglecellTILs(TNF-a,IFN-?,andIL-17A) usingEnumeralmicroengravingtechnology. Figure 3. PD-1 and TIM-3 Diversities using Enumeral Antibody Discovery Platform CDR3-based maximun-likehoodphylogramsof heavy chain sequences from anti-PD-1 (A) and anti-TIM-3 (B) recovered antibody-secreting cells. Conclusion Usingoursinglecellprofilingplatform,weidentifiedimmunecheckpointsthatcouldbetargetedtorestoreanti- tumorimmunity.Wegeneratedanti-humanPD-1andTIM-3antibodiesusingEnumeral’santibodydiscovery platform. Enumeral anti-PD-1 antibodies are undergoing testing in animal models for anti-tumor activity. Interestingly,bothleads388D4and244C8havedifferentmechanismsofaction.Enumeralanti-TIM-3antibodies arenowtestedinvitroandareundergoingfurtherstudiesincombinationwithanti-PD-1.Webelievethatco- blockadeofTIM-3andPD-1couldhaveapotentanti-tumoreffectinhumans. 0.0 0.5 1.0 1.5 2.0 2.5 3.0 O D ( 4 5 0 n m ) IgExpression TIM-3 Binding Figure 5. Hit Validation of TIM-3 Screening After sequencing, cloned anti-TIM-3 antibodies are expressed and supernatants are tested for binding to rhTIM-3. This diagram represents the hit validation for one TIM-3 screen. After engineering, we typically have a hit rate ranging from 60% to 80%. B Figure6.InVitroBlockade with Enumeral anti-PD-1 Antibodies Restore T cell Immunity (A) Enumeral anti-PD-1 lead candidates were tested for differentialTcellactivationin responsetoPD-1blockade in vitro. CD4andCD8wereused as T cell markers. T cell activation was assessed as inferred by secretion of the effector cytokines such as interferon gamma (IFN?) or tumor necrosis factor-alpha (TNFa). Top 2 leads were humanizedandtestedinaCMV antigen-recallassay(B) and a mixed lymphocyte reaction after6days(C,D). B A A Figure 4. Anti-PD-1 Lead Candidates with Different Epitope Specificity For each PD-1 clade, lead candidates were selected to be humanized as hIgG4. Selected leads have different epitope specificity as determined by peptide mapping (data not shown).
