Filed Pursuant to Rule 424(b)(3) and Rule 424(c)
Registration No. 333-198847
Prospectus Supplement No. 9
(To Prospectus filed on July 6, 2015, as supplemented
by Prospectus Supplement No. 1 dated July 14, 2015, Prospectus Supplement No. 2 dated July 28, 2015, Prospectus Supplement No. 3 dated August 4, 2015, Prospectus Supplement No. 4 dated August 12, 2015, Prospectus Supplement No. 5 dated September 17, 2015, Prospectus Supplement No. 6 dated September 18, 2015, Prospectus Supplement No. 7 dated September 24, 2015, and Prospectus Supplement No. 8 dated September 25, 2015)
ENUMERAL BIOMEDICAL HOLDINGS, INC.
This Prospectus Supplement No. 9 supplements the information contained in the Prospectus, dated as of July 6, 2015, as amended by Prospectus Supplement No. 1 dated July 14, 2015, Prospectus Supplement No. 2 dated July 28, 2015, Prospectus Supplement No. 3 dated August 4, 2015, Prospectus Supplement No. 4 dated August 12, 2015, Prospectus Supplement No. 5 dated September 17, 2015, Prospectus Supplement No. 6 dated September 18, 2015, Prospectus Supplement No. 7 dated September 24, 2015, and Prospectus Supplement No. 8 dated September 25, 2015, relating to the resale of up to 52,154,760 shares of our common stock by selling stockholders.
This Prospectus Supplement No. 9 is being filed to include the information set forth in our Current Report on Form 8-K, which was filed with the Securities and Exchange Commission on September 30, 2015.
You should read this Prospectus Supplement No. 9 in conjunction with the Prospectus. This Prospectus Supplement No. 9 is qualified by reference to the Prospectus, except to the extent that the information contained in this Prospectus Supplement No. 9 supersedes the information contained in the Prospectus. This Prospectus Supplement No. 9 is not complete without, and may not be utilized except in connection with, the Prospectus.
You should consider carefully the risks that we have described in “Risk Factors” beginning on page 7 of the Prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this Prospectus Supplement is September 30, 2015
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):September 30, 2015
Enumeral Biomedical Holdings, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 000-55415 | 99-0376434 |
| (State or Other Jurisdiction | (Commission File | (I.R.S. Employer |
| of Incorporation) | Number) | Identification Number) |
200 CambridgePark Drive, Suite 2000 Cambridge, Massachusetts (Address of Principal Executive Offices) | | 02140 (Zip Code) |
(617) 945-9146
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Item 7.01 Regulation FD Disclosure.
Enumeral Biomedical Holdings, Inc. (the “Company”) may use a slide presentation, in whole or in part, from time to time in presentations to potential partners, investors, analysts and others. A copy of the slide presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K and incorporated by reference herein. A copy of the slide presentation is also available on the Company’s website atwww.enumeral.com.
The information in this Item 7.01 of this Current Report on Form 8-K and in the accompanying Exhibit 99.1 shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in this Item 7.01 of this Current Report on Form 8-K and in the accompanying Exhibit 99.1 shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
Exhibit
Number | | Description |
| 99.1 | | Enumeral Biomedical Holdings, Inc. Fall 2015 Presentation |
| | | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | |
| | ENUMERAL BIOMEDICAL HOLDINGS, INC. |
| | | | |
| Dated: September 30, 2015 | By: | /s/ Kevin G. Sarney | |
| | | Name: Kevin G. Sarney | |
| | | Title: Vice President of Finance, Chief Accounting Officer and Treasurer | |
EXHIBIT INDEX
Exhibit
No. | | Description |
| | | |
| 99.1 | | Enumeral Biomedical Holdings, Inc. Fall 2015 Presentation |
Exhibit 99.1

THE POWER of HUMAN™ Fall 2015

Forward Looking S tatements OTC QB: ENUM THIS PRESENTATION CONTAINS FORWARD - LOOKING STATEMENTS THAT ARE BASED ON THE COMPANY’S CURRENT EXPECTATIONS, ASSUMPTIONS, ESTIMATES AND PROJECTIONS ABOUT THE COMPANY AND THE PHARMACEUTICAL INDUSTRY . THE COMPANY MAKES NO REPRESENTATIONS ABOUT THE ACCURACY OF SUCH STATEMENTS ESTIMATES OR PROJECTIONS . FORWARD - LOOKING STATEMENTS ARE INDICATED BY WORDS SUCH AS : MAY, WILL, SHOULD, PREDICT, CONTINUE, PLAN, EXPECT, ANTICIPATE, ESTIMATE, INTEND, BELIEVE, COULD, GOAL OBJECTIVES AND SIMILAR EXPRESSIONS . FORWARD - LOOKING STATEMENTS MAY INCLUDE, BUT ARE NOT LIMITED TO, STATEMENTS CONCERNING THE COMPANY’S ANTICIPATED PERFORMANCE, INCLUDING REVENUE AND PROFIT EXPECTATIONS ; DEVELOPMENT AND IMPLEMENTATION OF OUR COLLABORATIONS ; DURATION ; SIZE ; SCOPE AND REVENUE ASSOCIATED WITH COLLABORATION PARTNERSHIPS ; BENEFITS PROVIDED TO COLLABORATION PARTNERS BY OUR TECHNOLOGY ; BUSINESS MIX ; REVENUES AND GROWTH IN OUR PARTNER BASE ; MARKET OPPORTUNITIES ; COMPETING TECHNOLOGIES, INDUSTRY CONDITIONS AND TRENDS ; AND REGULATORY DEVELOPMENTS .. ACTUAL RESULTS MAY DIFFER MATERIALLY FROM THE ANTICIPATED RESULTS DUE TO SUBSTANTIAL RISKS AND UNCERTAINTIES RELATED TO THE COMPANY AND THE BIOPHARMACEUTICAL INDUSTRY IN WHICH THE COMPANY OPERATES . 2

Vision Leverage broad antibody diversity and human cell biology to develop cures for all cancer patients 3

Enumeral Opportunity • Differentiated lead anti - PD - 1 antibody program as backbone for combination immunotherapy • Broad antibody pipeline against important immunotherapy targets • Translational science approach provides insights into target biology to drive combination therapy development • Strategy leverages breadth of proprietary antibody diversity and depth of patient - centric biology 4

Enumeral Opportunity Immune system target complexity: Breadth of antibody diversity and patient - centric insights provide for development of optimal combinations “Novel combinations with an anti - PD1 pathway backbone may produce better response rates in more tumor types with fewer side effects .” 5 Figure and quote from Mahoney et. al., Nature Reviews Drug Discovery, August 2015

Opportunity Improve Cure Rate 6 Sharma and Allison Cell 2015 ENUM translational science approach: differentiated insights drive development of combinations
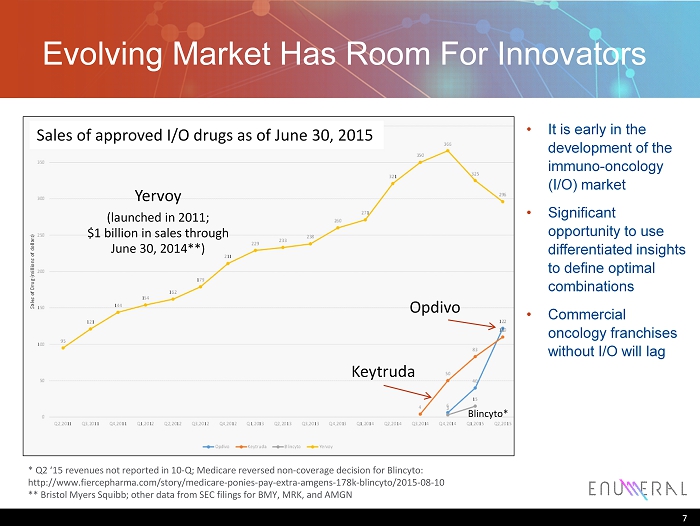
Evolving Market H as R oom F or I nnovators * Q2 ‘15 revenues not reported in 10 - Q; Medicare reversed non - coverage decision for Blincyto : http:// www.fiercepharma.com/story/medicare - ponies - pay - extra - amgens - 178k - blincyto/2015 - 08 - 10 ** Bristol Myers Squibb; other data from SEC filings for BMY, MRK, and AMGN Yervoy (launched in 2011; $1 billion in sales through June 30, 2014**) Keytruda Opdivo Blincyto * • It is early in the development of the immuno - oncology (I/O) market • Significant opportunity to use differentiated insights to define optimal combinations • Commercial oncology franchises without I/O will lag Sales of approved I/O drugs as of June 30, 2015 7

Pipeline of Antibody Immunotherapies to Drive Combination Opportunities 8 Represents potential value inflection points *Assumes program partnered after preclinical efficacy studies; additional costs covered by partner; and additional capital raised or obtained through partnership. Leads Preclinical Studies IND - enabling / IND Filed Phase 1 Screening / Lead Candidates Preclinical Studies IND - enabling / IND Filed Phase 1 Screening/Lead Candidates PD - 1 TIM - 3 LAG - 3 VISTA OX40 CD39 TIGIT CD66A Q1 Q2 Q3 Q4 2018 Q1 Q2 Q3 Q4 2017 Q1 Q2 Q3 Q4 2016 Q2 Q3 Q4 2015 Screening/Lead Candidates Screening/Lead Candidates Immunization/Screening/Lead Candidates Immunization/Screening/Lead Candidates Immunization/Screening/Lead Candidates Anticipate two drugs in Phase 1 clinical trial in 2017* Patient - centric paradigm to guide optimal development of combinatorial immunotherapy leveraging differentiated approach and proprietary platform

Enumeral Advantage: The Power of Human TM 9 function phenotype genomics Resolution of immunomodulatory targets on immune cells based on function Patient biopsy Differentiating insights into target biology Patient selection and optimal target combinations for development path After Tsioris et al 2015 Differentiated translational science platform and approach: Patient - centric integrated single cell functional immune profiling

Validation of Technology Platform* 10 *Publications from laboratory of scientific founder, J. Christopher Love, Ph.D.

Recognition of Differentiated Approach • MERCK : c ollaboration with a leading immuno - oncology pharmaceutical company – Focused on using Enumeral's platform to interrogate the tumor microenvironment in colorectal cancer tissues to identify functional cellular responses to therapies being developed by Merck – R&D funding and undisclosed milestone payments – Merck has exclusive rights to data related to its proprietary compounds – ENUM recently achieved first milestone in the collaboration • NCI: awarded Phase 2 contract for ~$1 million over two years – Automation of human tissue immuno - oncology profiling – Opens door to broader pipeline and potentially accelerated development – Collaboration with leading scientists: 11 – Jedd Wolchok’s group at MSKCC − genetic basis for response to checkpoint inhibitors and novel immunotherapeutics – Doug Kwon’s group at MGH/ Ragon Institute − pioneering techniques for single cell immune cell analysis in biopsy This image cannot currently be displayed. This image cannot currently be displayed.
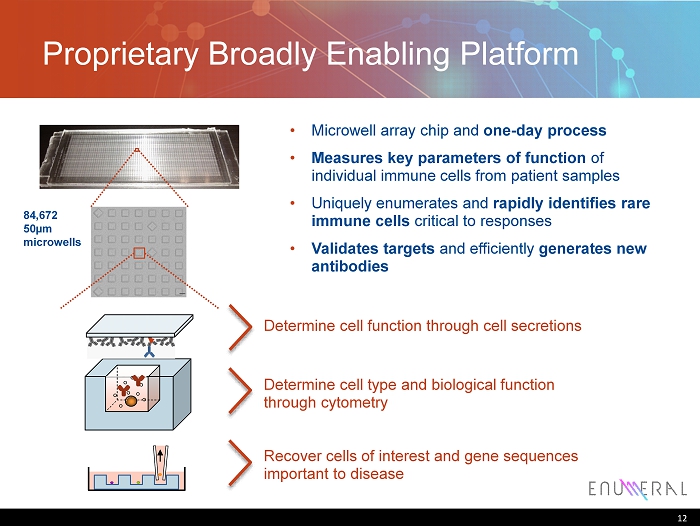
Proprietary Broadly E nabling P latform 12 84,672 50µm microwells • Microwell array chip and one - day process • Measures key parameters of function of individual immune cells from patient samples • Uniquely enumerates and rapidly identifies rare immune cells critical to responses • Validates targets and efficiently generates new antibodies Determine cell function through cell secretions Determine cell type and biological function through cytometry R ecover cells of interest and gene sequences important to disease

Approach to Developing Differentiated Antibodies Starts with Diversity • Enumeral antibody discovery results in exceptional diversity * • Proprietary compositions of matter of Anti - PD - 1 antibodies • Potential for strong IP position • Breadth of diversity: keys to unlocking the target physiology • Multiple potential program opportunities OPDIVO® KEYTRUDA® Pidilizumab 392C5 246A10 413D2 413E1 244C8 388D4 *Based on ENUM evaluation of published literature Cladogram representing heavy chain AA sequences N= 159 sequences shown 28 families of antibodies Potential allosteric modulator: novel epitope 13
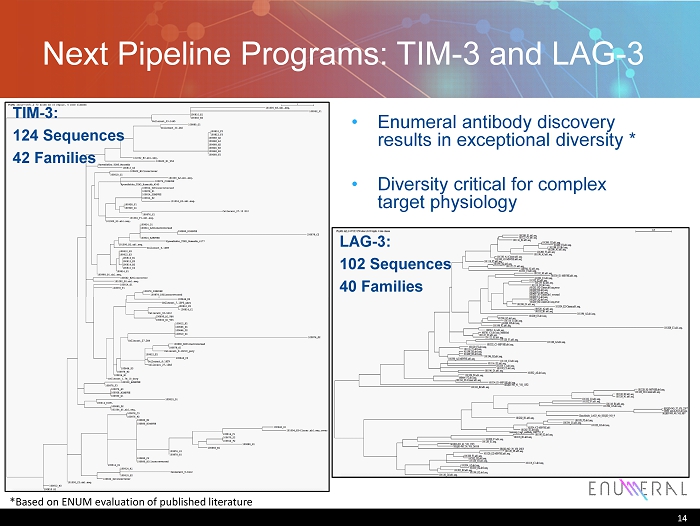
Next Pipeline Programs: TIM - 3 and LAG - 3 • Enumeral antibody discovery results in exceptional diversity * • Diversity critical for complex target physiology 14 LAG - 3: 102 Sequences 40 Families TIM - 3: 124 Sequences 42 Families *Based on ENUM evaluation of published literature

PD - 1 Overview • Proven target commercially and clinically • Backbone of future immuno - oncology combination therapies • Mechanism of action of PD - 1 - targeted drugs poorly understood – Reported overall response rates (ORR): 32% for Opdivo and 24% for Keytruda in advanced metastatic melanoma – Dose - dependency for therapeutic effect unclear – Reported affinities for PD - 1 markedly different 15
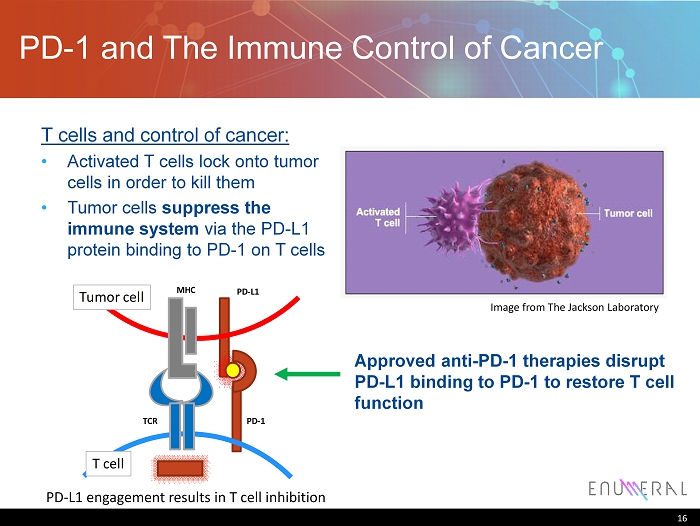
PD - 1 and The Immune Control of Cancer T cells and control of cancer: • Activated T cells lock onto tumor cells in order to kill them • T umor cells suppress the immune system via the PD - L1 protein binding to PD - 1 on T cells 16 Approved anti - PD - 1 therapies disrupt PD - L1 binding to PD - 1 to restore T cell function Image from The Jackson Laboratory PD - L1 engagement results in T cell inhibition Tumor cell T cell MHC TCR PD - 1 PD - L1

Lead Program: Potential Allosteric PD - 1 Modulator 17 Current anti - PD1 agents allow for T cell activation; disrupt PD - L1 binding to PD - 1 PD - L1 engagement results in T cell inhibition; tumor cell protected ENUM anti - PD1: novel epitope and PD - L1 independent binding; IL - 2R autocrine activation enhanced T cell activation PD - 1 T cell Tumor cell PD - L1 Tumor cell PD - L1 PD - 1 T cell Tumor cell PD - L1 T cell PD - 1 CD25 IL - 2 Potential allosteric mechanism of action via novel PD - 1 epitope
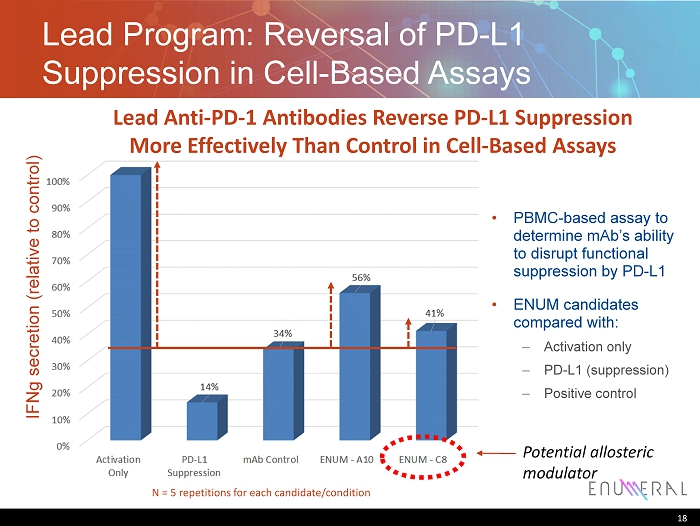
Lead Program: Reversal of PD - L1 Suppression in Cell - Based Assays 18 IFNg secretion (relative to control) N = 5 repetitions for each candidate/condition • PBMC - based assay to determine mAb’s ability to disrupt functional suppression by PD - L1 • ENUM candidates compared with : – Activation only – PD - L1 (suppression) – Positive control Potential allosteric modulator Lead Anti - PD - 1 Antibodies Reverse PD - L1 Suppression More Effectively Than Control in Cell - Based Assays

D 4 - H C 3 + L C 1 D 4 - H C 1 + L C 3 D 4 - H C 3 + L C 3 C 8 - H C 1 + L C 1 C 8 - H C 1 + L C 3 C 8 - H C 2 + L C 1 N i v o - I g G 4 h I g G 4 - H C A 2 4 7 0 5000 10000 MLR Donor 337+23 (IFNy) I F N y ( p g / m l ) 10 ug/ml 1 ug/ml 0.1 ug/ml 0.01 ug/ml Antibody concentration Antibody concentration D 4 - H C 3 + L C 1 D 4 - H C 1 + L C 3 D 4 - H C 3 + L C 3 C 8 - H C 1 + L C 1 C 8 - H C 1 + L C 3 C 8 - H C 2 + L C 1 N i v o - I g G 4 h I g G 4 - H C A 2 4 7 0 10 20 30 40 50 % o f C D 2 5 + ( C D 4 g a t e d ) 1 ug/ml 10 ug/ml 0.1 ug/ml 0.01 ug/ml MLR Donor 337+23 (T cell activation analysis by FACS) Stimulation of IFN g production Antibody concentration D 4 - H C 3 + L C 1 D 4 - H C 1 + L C 3 D 4 - H C 3 + L C 3 C 8 - H C 1 + L C 1 C 8 - H C 1 + L C 3 C 8 - H C 2 + L C 1 N i v o - I g G 4 h I g G 4 - H C A 2 4 7 0 10 20 30 40 50 % o f C D 2 5 + ( C D 4 g a t e d ) 1 ug/ml 10 ug/ml 0.1 ug/ml 0.01 ug/ml MLR Donor 337+23 (T cell activation analysis by FACS) Stimulation of CD25 expression (high affinity IL - 2R) Nivo IgG4 D4 - 1 D4 - 2 D4 - 3 C8 - 1 C8 - 2 C8 - 3 Nivo IgG4 D4 - 1 D4 - 2 D4 - 3 C8 - 1 C8 - 2 C8 - 3 Lead Anti - PD - 1 Antibodies Elicit Enhanced T Cell Function 19 ENUM antibodies show enhanced activation of T cells in mixed lymphocyte reaction (dose - dependent stimulation of IFN g production) and induce the high affinity IL - 2R on activated T cells Data shown for humanized clones compared to Nivolumab and IgG4 control

Differentiated PD - 1 Antagonist with Potential Allosteric Mechanism 20 PD - L1 engagement results in T cell inhibition Hypothesis: approved PD - 1 agents allows for T cell activation; T cell – Tumor cell interaction altered when PD - L1 interaction disrupted Hypothesis: PD - 1 blockade with ENUM allosteric modulator maintains T cell – Tumor cell interaction, allows for enhanced T cell activation via novel IL - 2 autocrine activation ENUM antibody: PD - L1 independent binding and enhanced activation via autocrine IL - 2 signaling

Enhanced T Cell Immunomodulation: Potential Clinical Implications • Monotherapy : Differentiated mechanism of action; potential higher initial response rate and durable responses • Potential to treat PD - 1 refractory or relapse patients (non - responders) • Potential synergistic Rx: with TIM - 3 blockade, other checkpoints in ENUM pipeline 21

Enumeral Advantage: Translational Science Platform Drives Antibody Development • Proprietary bioinformatics capability to define target correlation with disease • Define cell types on which the target is expressed • Define subsets of functional immune cells that express the target in biopsy from intent - to - treat patients • Supports indication selection and clinical development path 22

Proprietary ‘ Enumeral Pathway Annotation’ (EPA) I nforms I ndication Selection 23 EPA Lymphoid index (inc. PD-1) EPA Myeloid index (inc. TIM3) EPA TIM3-index (3 gene index) Solid Tumor #1 Solid Tumor #1 Solid Tumor #2 Solid Tumor #5 Solid Tumor #2 Solid Tumor #1 Solid Tumor #2 Solid Tumor #3 Solid Tumor #3 Solid Tumor #4 Solid Tumor #4 Solid Tumor #5 Solid Tumor #3 Solid Tumor #5 Solid Tumor #9 Solid Tumor #11 Solid Tumor #6 Solid Tumor #4 Solid Tumor #8 Solid Tumor #7 Solid Tumor #7 Solid Tumor #7 Solid Tumor #8 Solid Tumor #6 Solid Tumor #12 Solid Tumor #9 Solid Tumor #10 Solid Tumor #13 Solid Tumor #10 Solid Tumor #8 Solid Tumor #10 Solid Tumor #11 Solid Tumor #11 Solid Tumor #9 Solid Tumor #12 Solid Tumor #12 Solid Tumor #6 Solid Tumor #13 Solid Tumor #13 Ranked Indications using EPA Indexes: EPA Myeloid index (cluster 28: inc. TIM - 3 , VISTA, CSF1R) from tumor gene expression datasets Lymphoid Neoplasm Diffuse Large B - cell Lymphoma Acute myeloid leukemia [Solid Tumor #1] [Solid Tumor #2] [Solid Tumor #3] Glioblastoma multiforme [Solid Tumor #4] Testicular Germ Cell Tumors Thymoma [Solid Tumor #5] [Solid Tumor #6] [Solid Tumor #7] [Solid Tumor #8] [Solid Tumor #9] [Solid Tumor #10] [Solid Tumor #11] Cholangiocarcinoma Cervical squamous cell carcinoma and endocervical adenocarcinoma [Solid Tumor #12] Brain Lower Grade Glioma Thyroid carcinoma Liver hepatocellular carcinoma Uterine Corpus Endometrioid Carcinoma [Solid Tumor #13] Bladder Urothelial Carcinoma Uterine Carcinosarcoma Prostate adenocarcinoma Pheochromocytoma and Paraganglioma Kidney Chromophobe Adrenocortical carcinoma Uveal Melanoma No. of samples Myeloid index expression level Differentiated insights into target biology Enables indication selection and development path
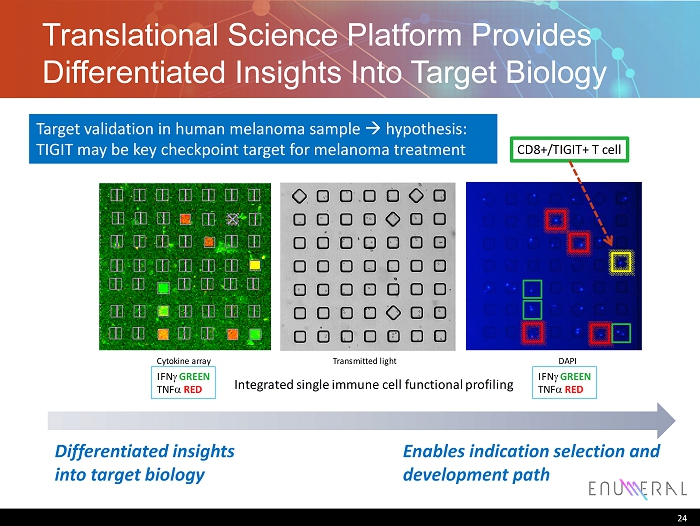
Translational Science Platform Provides Differentiated Insights Into Target Biology 24 Target validation in human melanoma sample h ypothesis: TIGIT may be key checkpoint target for melanoma treatment Differentiated insights into target biology Enables indication selection and development path Cytokine array Transmitted light DAPI IFN g GREEN TNF a RED IFN g GREEN TNF a RED CD8+/TIGIT+ T cell Integrated single immune cell functional profiling

Translational Science Platform Provides Differentiated Insights Into Target Biology 25 Platform uniquely identifies rare events at high resolution: identification of TIM - 3 expression on non - T cells suggests combination approach Differentiated insights into target biology Enables development of potential novel treatment strategies Target validation in human colorectal cancer sample h ypothesis: TIM - 3 expression not restricted to T cells – novel treatment strategy
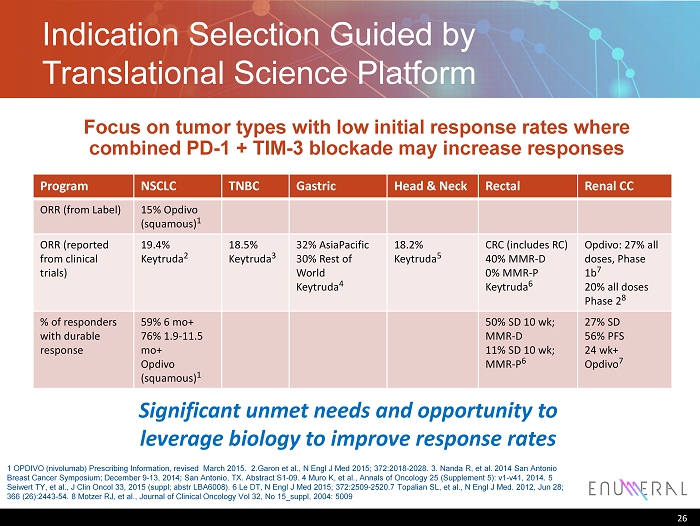
Indication Selection Guided by Translational Science Platform Program NSCLC TNBC Gastric Head & Neck Rectal Renal CC ORR (from Label) 15% Opdivo (squamous) 1 ORR (reported from clinical trials ) 19.4% Keytruda 2 18.5% Keytruda 3 32% AsiaPacific 30% Rest of World Keytruda 4 18.2% Keytruda 5 CRC (includes RC) 40% MMR - D 0% MMR - P Keytruda 6 Opdivo : 27% a ll doses, Phase 1b 7 20% all doses Phase 2 8 % of responders with durable response 59% 6 mo+ 76% 1.9 - 11.5 mo+ Opdivo (squamous) 1 50% SD 10 wk ; MMR - D 11% SD 10 wk ; MMR - P 6 27% SD 56% PFS 24 wk + Opdivo 7 26 Significant unmet needs and opportunity to leverage biology to improve response rates Focus on tumor types with low initial response rates where combined PD - 1 + TIM - 3 blockade may increase responses 1 OPDIVO (nivolumab) Prescribing Information, revised March 2015. 2.Garon et al., N Engl J Med 2015; 372:2018 - 2028. 3 . Nanda R, et al. 2014 San Antonio Breast Cancer Symposium; December 9 - 13, 2014; San Antonio, TX. Abstract S1 - 09. 4 Muro K, et al., Annals of Oncology 25 (Supplement 5): v1 - v41, 2014. 5 Seiwert TY, et al., J Clin Oncol 33, 2015 ( suppl ; abstr LBA6008). 6 Le DT, N Engl J Med 2015; 372:2509 - 2520.7 Topalian SL, et al., N Engl J Med. 2012, Jun 28; 366 (26): 2443 - 54. 8 Motzer RJ, et al., Journal of Clinical Oncology Vol 32, No 15_suppl, 2004: 5009

Clinical T rial P lans S upport Testing of Multiple Combinations 27 IND TIM 3 Phase 1 PD 1 Phase 1 IND PD1 Allosteric TIM3 PD1 Vaccine or Targeted Rx Checkpoint blockade improves efficacy of other modalities PD1 TIM3 PD 1 Allosteric Phase 2 : Monotherapy IND PD 1 Allosteric Phase 1 Treat relapse and refractory patients Checkpoint combination inhibition may lead to higher response rates Multiple potential co - development opportunities with existing modalities and checkpoint inhibitors

Strong Intellectual Property Position • Exclusive worldwide license with MIT/Harvard for platform technology – 6 issued patents in US and 29 issued in international jurisdictions – 26 pending patent applications (US & International) • Patents covering compositions of matter and methods – 4 a pplications pending or in preparation covering compositions of matter, methods of making, and methods of treating disease, using anti - PD - 1, TIM - 3, LAG - 3 antibodies – Application pending for methods for cellular response profiling • Freedom to operate – Ability to navigate crowded patent landscape – Extensive searches and detailed analyses conducted at outset of each program – Formal opinions of outside counsel obtained, where appropriate 28

Strategy: Development Partnerships • Out - license or co - develop antibodies for combination with oncology drugs – Chemotherapy, targeted therapy, vaccines, radiation • Pipeline and translational approach drive combination development – TIM - 3, LAG - 3, OX40, VISTA, TIGIT, CD39, CD66a • Retain long - term ownership/value through royalties and/or other commercial rights – Research partnerships drive revenues and platform development 29 Leverage Proprietary Antibodies and Biology Insights

Opportunities for New Entrants and Co - Development Partnerships 30 Only companies with disclosed clinical I/O programs against the targets listed are shown**; bold indicate ENUM targets. *Source: Defined Health, presentation at Sachs I/O Investment Forum, Chicago May 2015. **Based on publicly available data, companies without disclosed clinical stage antibody programs against PD - 1 include: Takeda, Astellas , Baxalta , Shire, Gilead , Kyowa Hakko Kirin Pharma, Boehringer Ingelheim , Bayer, Eisai, Otsuka, others I/O Clinical Landscape*: Target BMY MRK AZN Roche PFE NVS SNY GSK CELG AMGN JNJ LLY CTLA4 M III w/MRK (+TVEC) w/AZN In MM 41BB II I Ox40 I/II I KIR II LAG3 I Ph I in 2015 GITR I TIM3 Ph I in 2015 PD1 M M I/II I I w/BMY w/MRK & BMY PDL1 IIIIII III

Projected Growth Driven by Checkpoint Inhibitors and Costimulators 31 C heckpoint inhibitors and costimulators Strong interest in new immunotherapies among big pharma companies, especially for antibody approaches – Merck, Bristol Myers Squibb, AstraZeneca and Roche are leading development

Experienced Leadership T eam 32 John J. Rydzewski Executive Chairman, Co - Founder, Director Arthur H. Tinkelenberg, Ph.D. President & CEO, Co - Founder, Director Cokey Nguyen, Ph.D. Vice President, Research & Development Isabel Chiu, Ph.D. Vice President, Translational & Clinical Sciences Kevin G. Sarney Vice President, Finance, & Chief Accounting Officer Derek Brand Vice President, Business Development Matthew A. Ebert General Counsel Gary L. Creason , Ph.D. Vice President, Intellectual Property

Accomplished Board of Directors 33 John J. Rydzewski Executive Chairman, Co - Founder, Director Arthur H. Tinkelenberg, Ph.D. President & CEO, Co - Founder, Director Barry Buckland, Ph.D. Co - Founder, Chairman, Scientific Advisory Board Robert J. Easton Director Allan Rothstein Director Paul J. Sekhri Director Robert L. Van Nostrand Director
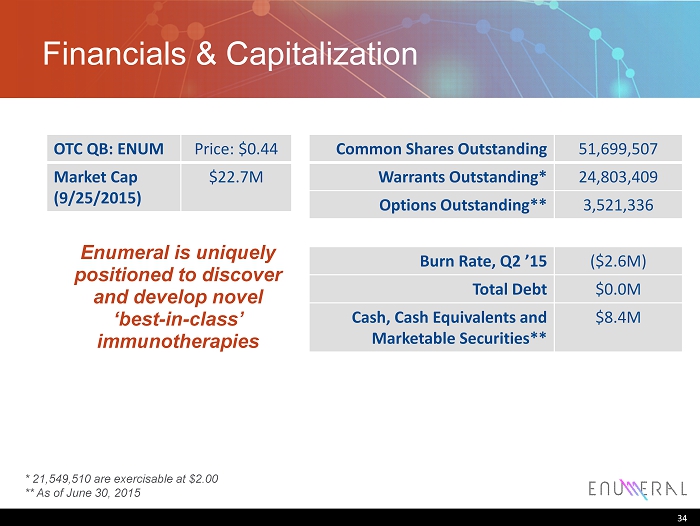
Financials & Capitalization Common Shares Outstanding 51,699,507 Warrants Outstanding* 24,803,409 Options Outstanding** 3,521,336 Burn Rate , Q2 ’15 ($2.6M) Total Debt $0.0M Cash, Cash Equivalents and Marketable Securities** $8.4M 34 OTC QB: ENUM Price: $0.44 Market Cap (9/25/2015) $22.7M Enumeral is uniquely positioned to discover and develop novel ‘best - in - class’ immunotherapies * 21,549,510 are exercisable at $2.00 ** As of June 30, 2015

THE POWER of HUMAN™ Arthur H. Tinkelenberg, Ph.D. President and CEO arthur@enumeral.com 617 - 500 - 2647


































