Filed Pursuant to Rule 424(b)(3)
Registration No. 333-198847
ENUMERAL BIOMEDICAL HOLDINGS, INC.
Prospectus
47,674,386 Shares
Common Stock
This prospectus relates to the resale of up to 47,674,386 shares of common stock, par value $0.001 per share (“Common Stock”), of Enumeral Biomedical Holdings, Inc. (“we” or the “Company”) held by certain selling stockholders, consisting of the following:
| · | 39,016,432 shares of Common Stock issued to investors in our July 2014 private placement, consisting of 17,466,922 shares of Common Stock currently outstanding and 21,549,510 shares of Common Stock issuable upon exercise of common stock purchase warrants; |
| · | 2,000,000 shares of Common Stock issuable upon exercise of common stock purchase warrants issued to the placement agents in our July 2014 private placement (and subsequently transferred to employees of the placement agents); |
| · | 4,964,207 shares of Common Stock issued to the former holders of common and preferred stock of Enumeral Biomedical Corp (“Enumeral”) pursuant to the terms of the merger agreement (the “Merger Agreement”) in connection with the merger (the “Merger”) of a wholly owned subsidiary of the Company with and into Enumeral, consisting of 4,780,525 shares of Common Stock currently outstanding and 183,682 shares of Common Stock issuable upon exercise of common stock purchase warrants; and |
| · | 1,693,747 shares of Common Stock issued to the pre-Merger stockholders of the Company pursuant to anti-dilution provisions of the Merger Agreement. |
The distribution of the shares by the selling stockholders is not subject to any underwriting agreement. The selling stockholders may offer all or part of the shares for resale from time to time through public or private transactions, at either prevailing market prices or at privately negotiated prices, or a combination of these methods
We will not receive any proceeds from the resale of any of the shares of Common Stock being registered hereby. However, we received $21,549,510 in gross proceeds in the July 2014 private placement, and in the future may receive up to an aggregate of $45,186,979 in additional gross proceeds upon the exercise of the warrants for which shares issuable upon exercise thereof are being registered (to the extent the registration statement of which this prospectus is a part is then effective and, if applicable, the “cashless exercise” provision is not utilized by the holder).
The Company is paying all of the registration expenses incurred in connection with the registration of the shares. We will not pay any of the selling commissions, brokerage fees and related expenses.
Our common stock is quoted on the OTC Markets under the symbol “ENUM.” On July 21, 2016, the last reported sale price of our common stock on the OTC Markets was $0.18 per share.
Our business and an investment in our securities involve a high degree of risk. Before making any investment in our securities, you should read and carefully consider risks described in the “Risk Factors” section beginning on page 8 of this prospectus.
You should rely only on the information contained in this prospectus. We have not authorized any dealer, salesperson or other person to provide you with information concerning us, except for the information contained in this prospectus. The information contained in this prospectus is complete and accurate only as of the date on the front cover page of this prospectus, regardless when the time of delivery of this prospectus or the sale of any common stock. This prospectus is not an offer to sell, nor is it a solicitation of an offer to buy, our Common Stock in any jurisdiction in which the offer or sale is not permitted.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
This prospectus is dated July 26, 2016.
TABLE OF CONTENTS
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING INFORMATION
This Prospectus contains forward-looking statements, including, without limitation, within the sections captioned “Business,” “Risk Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and elsewhere. Any and all statements contained in this Prospectus that are not statements of historical fact may be deemed forward-looking statements. Terms such as “may,” “might,” “would,” “should,” “could,” “project,” “estimate,” “pro-forma,” “predict,” “potential,” “strategy,” “anticipate,” “attempt,” “develop,” “plan,” “help,” “believe,” “continue,” “intend,” “expect,” “future,” and terms of similar import (including the negative of any of the foregoing) may be intended to identify forward-looking statements; however, not all forward-looking statements may contain one or more of these identifying terms. Forward-looking statements in this Prospectus may include, without limitation, statements regarding (i) the plans and objectives of management for future operations, including plans or objectives relating to the development of commercially viable pharmaceuticals, (ii) a projection of income (including income/loss), earnings (including earnings/loss) per share, capital expenditures, dividends, capital structure or other financial items, (iii) our future financial performance, including any such statement contained in a discussion and analysis of financial condition by management or in the results of operations included pursuant to the rules and regulations of the SEC, and (iv) the assumptions underlying or relating to any statement described in points (i), (ii) or (iii) above.
The forward-looking statements are not meant to predict or guarantee actual results, performance, events or circumstances and may not be realized because they are based upon our current projections, plans, objectives, beliefs, expectations, estimates and assumptions and are subject to a number of risks and uncertainties and other influences, many of which we have no control over. Actual results and the timing of certain events and circumstances may differ materially from those described by the forward-looking statements as a result of these risks and uncertainties. Factors that may influence or contribute to the inaccuracy of the forward-looking statements or cause actual results to differ materially from expected or desired results may include, without limitation, our inability to obtain adequate financing, the significant length of time associated with drug development and related insufficient cash flows and resulting illiquidity, our inability to expand our business, significant government regulation of pharmaceuticals and the healthcare industry, lack of product diversification, volatility in the price of our raw materials, existing or increased competition, results of arbitration and litigation, stock volatility and illiquidity of our securities, and our failure to implement effectively our business plans or strategies. A description of some of the risks and uncertainties that could cause our actual results to differ materially from those described by the forward-looking statements in this Prospectus appears in the section captioned “Risk Factors” and elsewhere in this Prospectus.
Readers are cautioned not to place undue reliance on forward-looking statements because of the risks and uncertainties related to them and to the risk factors. We disclaim any obligation to update the forward-looking statements contained in this Prospectus to reflect any new information or future events or circumstances or otherwise, except as required by law.
Readers should read this Prospectus in conjunction with the discussion under the caption “Risk Factors,” our financial statements and the related notes thereto in this Prospectus, and other documents which we may file from time to time with the Securities and Exchange Commission (the “SEC”).
OTHER PERTINENT INFORMATION
Unless the context indicates otherwise, all references in this Prospectus to “Enumeral Biomedical,” the “Company,” “we,” “us” and “our” refer to Enumeral Biomedical Holdings, Inc., and its wholly-owned subsidiaries, Enumeral Biomedical Corp. and Enumeral Securities Corporation; and references to “Enumeral” refer to Enumeral Biomedical Corp.
PROSPECTUS SUMMARY
The following summary highlights information contained elsewhere in this prospectus. This summary is not complete and does not contain all of the information that should be considered before investing in our common stock. Potential investors should read the entire prospectus carefully, including the more detailed information regarding our business provided below in the “Business” section, the risks of purchasing our common stock discussed under the “Risk Factors” section, and our consolidated financial statements and the accompanying notes to the consolidated financial statements.
Overview
We are a biopharmaceutical company focused on discovering and developing novel antibody immunotherapies that help the immune system fight cancer and other diseases. We utilize a proprietary platform technology that facilitates the rapid high resolution measurement of immune cell function within small tissue biopsy samples. Our initial focus is on the development of a pipeline of next generation monoclonal antibody drugs targeting established and novel immuno-modulatory receptors.
The concept of stimulating the immune system to fight cancer was first advanced more than a century ago, but it is only recently that the field of immuno-oncology has seen clinical success, with marketing approvals being granted for antibodies that block CTLA-4 (Yervoy® (ipilimumab)) and PD-1 (Keytruda® (pembrolizumab) and Opdivo® (nivolumab)). Use of these drugs has established that durable anti-tumor responses can be elicited in some patients by blocking the checkpoints that normally suppress the human immune response against cancer cells. The success of these drugs suggests that immuno-oncology may fundamentally alter the course of cancer treatment.
In our lead antibody program, we have characterized certain anti-PD-1 antibodies, or simply “PD-1 antibodies,” using patient biopsy samples, in an effort to identify next generation PD-1 antagonists with enhanced selectivity for the immune effector cells that carry out anti-tumor functions. We have identified two antagonist PD-1 antibodies that inhibit PD-1 activity in different ways. The distinction is that one of the antibodies (ENUM 388D4) blocks binding of the ligand PD-L1 to PD-1, while the other antibody (ENUM 244C8) does not inhibit PD-L1 binding. However, both display activity in various biological assays. In addition to our PD-1 antibody program, we are developing antibody drug candidates for a number of immunomodulatory protein targets, including TIM-3, LAG-3, OX40, TIGIT, and VISTA. We are also pursuing several antibody programs for which we have not yet publicly disclosed the targets.
Our proprietary platform technology, exclusively licensed from the Massachusetts Institute of Technology, or MIT, is a microwell array technology that detects secreted molecules (such as antibodies and cytokines) and cell surface markers, at the level of single, live cells – and enables recovery of single, live cells of interest. The platform technology can be used to achieve at least three separate, but complementary, objectives. First, we use the platform to rapidly produce antibody libraries with high diversity. Second, the platform has the potential to guide rational selection of lead candidates derived from these libraries, through characterization of immune function at the level of single cells from human biopsy samples. Third, it has the potential to identify patients more likely than others to benefit from treatment with a given therapeutic antibody. Thus, our platform is a multipurpose tool that is valuable for activities ranging from antibody discovery to target discovery to patient stratification in clinical development. The platform yields multidimensional, functional read-outs from single live cells, such as tumor infiltrating lymphocytes, or TILs, from human tumor biopsy samples, and it enables us to examine the responses of different classes of human immune cells to treatment with immuno-modulators in the context of human disease, as opposed to animal models of disease. Consequently, we call our approach The Human Approach®.
To date, our proof-of-concept corporate collaborations have provided modest revenues. However,our business has not generated (nor do we anticipate that in the foreseeable future it will generate) the cash necessary to finance our operations. We expect to continue to incur losses and use cash during fiscal year 2016, and we will require additional capital to continue our operations beyond July 2016.
Our near-term capital needs depend on many factors, including:
| · | our ability to carefully manage our costs; |
| · | the amount and timing of revenue received from grants or our collaboration and license arrangements; and |
| · | our ability to raise additional capital through public or private equity offerings, debt financings, or strategic collaborations and licensing arrangements, and/or our success in promptly establishing a strategic alternative that is in our stockholders’ best interests. |
At March 31, 2016, we had cash and cash equivalents totaling approximately $1.8 million, excluding restricted cash.
In June 2016, we entered into a Definitive License and Transfer Agreement with Pieris Pharmaceuticals, Inc. and Pieris Pharmaceuticals GmbH (collectively, “Pieris”), as contemplated in the License and Transfer Agreement that we had entered into with Pieris in April 2016 (the “License Agreement”). Pieris paid us an upfront license fee in the amount of $250,000 in connection with execution of the License Agreement, and paid us a $750,000 license maintenance fee, which was due by May 31, 2016, to continue the licensing arrangements under the License Agreement.
Capital Needs
As of the date of this filing, we believe that, with the receipt of the $750,000 maintenance fee from Pieris described above, we now have sufficient liquidity to fund operations through July 2016. However, we will still require additional capital to continue its operations through the third quarter of 2016.We are currently exploring a range of potential transactions, which may includepublic or private equity offerings, debt financings, collaborations and licensing arrangements, and/or other strategic alternatives, including a merger, sale of assets or other similar transactions. If we are unable to preserve or raise additional capital through one or more of the means listed above prior to the end of July 2016, we could face substantial liquidity problems and might be required to implement cost reduction strategies in addition to the cash conservation steps that we have already taken. These reductions could significantly affect our research and development activities, and could result in significant harm to our business, financial condition and results of operations. In addition, we may be required to downsize or wind down our operations through liquidation, bankruptcy, or a sale of our assets.
No assurance can be given that additional financing or strategic alliances and licensing arrangements will be available when needed or that, if available, such financing could be obtained on terms favorable to us or our stockholders. See “Risk Factors.”
About This Offering
This prospectus relates to the public offering, which is not being underwritten, by the selling stockholders listed in this prospectus, of up to 47,674,386 shares of our common stock. Of the shares being offered, 23,941,194 are presently issued and outstanding, and 23,733,192 are issuable upon exercise of common stock purchase warrants.
The selling stockholders may offer all or part of the shares for resale from time to time through public or private transactions, at either prevailing market prices or at privately negotiated prices, or a combination of these methods
We will not receive any proceeds from the resale of any of the shares of Common Stock being registered hereby. However, we received $21,549,510 in gross proceeds in the July 2014 private placement, and in the future may receive up to an aggregate of $45,186,979 in additional gross proceeds upon the exercise of the warrants for which shares issuable upon exercise thereof are being registered (to the extent the registration statement of which this prospectus is a part is then effective and, if applicable, the “cashless exercise” provision is not utilized by the holder).
The Company is paying all of the registration expenses incurred in connection with the registration of the shares. We will not pay any of the selling commissions, brokerage fees and related expenses.
Corporate Information
Our principal executive offices are located at 200 CambridgePark Drive, Suite 2000, Cambridge, MA 02140. Our telephone number is (617) 945-9146. Our website address iswww.enumeral.com.
THE OFFERING
Common stock currently outstanding | | 52,073,481 shares (1) |
| | | |
| Common stock offered by the Company | | None |
| | | |
| Common stock offered by the selling stockholders | | 47,674,386 shares (2) |
| | | |
| Use of proceeds | | We will not receive any of the proceeds from the sales of our common stock by the selling stockholders. However, we received $21,549,510 in gross proceeds in the July 2014 private placement, and in the future may receive up to an aggregate of $45,186,979 in additional gross proceeds upon the exercise of the warrants for which shares issuable upon exercise thereof are being registered. |
| | | |
| OTC Markets symbol | | ENUM |
| | | |
| Risk Factors | | You should carefully consider the information set forth in this prospectus and, in particular, the specific factors set forth in the “Risk Factors” section beginning on page 8 of this prospectus before deciding whether or not to invest in shares of our common stock. |
| (2) | Consists of 23,941,194 outstanding shares of common stock and 23,733,192 shares of common stock issuable upon exercise of common stock purchase warrants. |
RISK FACTORS
An investment in our securities is speculative and involves various risks that may affect our operations or financial results. Many of those risks are driven by factors and events that we cannot control or predict. Before investing in our securities you should carefully consider the following risks, together with the financial and other information contained in this Prospectus.
This report contains certain statements relating to future events or the future financial performance of our company. Prospective investors are cautioned that such statements are only predictions and involve risks and uncertainties, and actual events or results may differ materially. In evaluating such statements, prospective investors should specifically consider the various factors identified in this Prospectus, including the matters set forth below, which could cause actual results to differ materially from those indicated by such forward-looking statements.
If any of the following or other risks materialize, our business, financial condition, and results of operations could be materially adversely affected which could adversely affect the value of our common stock. In such a case, investors in our common stock could lose all or part of their investment.
Prospective investors should consider carefully whether an investment in our company is suitable for them in light of the information contained in this Prospectus and the financial resources available to them. The risks described below do not purport to be all the risks to which we could be exposed. This section is a summary of certain risks and is not set out in any particular order of priority. They are the risks that we presently believe are material to our operations. Additional risks of which we are not presently aware or which we presently deem immaterial may also impair our business, financial condition or results of operations.
Risks Related to our Business and the Industry in Which We Operate
We face technological uncertainties.
To date, we have not developed or commercialized any products utilizing our proprietary platform technology. There can be no assurance that our approach will enable us to successfully discover and develop novel therapeutics that help the immune system attack diseased cells. The discovery and development of such novel therapeutics for use in the diagnosis and treatment of cancer, infectious, autoimmune and inflammatory diseases also will be subject to the risks of failure inherent in the development of products based on new technologies. These risks include the possibilities that products based on these technologies will be ineffective or toxic, or otherwise fail to receive necessary regulatory approvals; that the products, if safe and effective, will be difficult or uneconomical to manufacture on a large scale; that third party patent rights will preclude us or our partners from marketing products; or that third parties will market equivalent or superior competing products. As a result, there can be no assurance that our research and development activities will lead to any commercially viable products in a relevant timeframe.
Biotechnology and pharmaceutical technologies have undergone and are expected to continue to undergo rapid and significant change. Our future success will depend in large part on our ability to maintain a competitive position with respect to these technologies. Rapid technological developments by our company or others may result in products or processes becoming obsolete before we recover any expenses that we incur in connection with the development of such products.
We may not achieve profitability.
We have historically incurred operating losses and expect to continue to have negative cash flow from operations. As of March 31, 2016, we have accumulated losses of approximately $16.0 million. Our future profitability will depend on our ability to increase our revenues, which is subject to a number of factors, including our ability to successfully enter into collaborations with third parties, the success of our core platform technology and research and development efforts, our ability to compete effectively in a crowded field, availability of public funding through grants and other federal funds, the time required to reach commercial revenue and profitability, if at all, and global economic and political conditions.
Our future profitability also depends on our expense levels, which are influenced by a number of factors, including the resources we devote to developing and supporting our projects and potential products, the continued progress of our research and development of potential products, our ability to improve research and development efficiencies, license fees or royalties we may be required to pay, and the potential need to acquire licenses to new technology, the availability of intellectual property for licensing or acquisition, or to use our technology in new markets, which could require us to pay unanticipated license fees and royalties in connection with these licenses.If we fail to grow our revenue and manage our expenses, we may never achieve profitability, which would adversely and materially affect our ability to provide a return to our investors.
Our fiscal year 2015 consolidated financial statements state that our recurring losses from operations and limited cash resources raise substantial doubt about our ability to continue as a going concern.
Our continuation as a going concern is dependent upon our attaining and maintaining profitable operations, generating continued cash payments from partners under new or existing contracts and/or raising additional capital, such as through public or private equity offerings, debt financings, or strategic collaborations and licensing arrangements. In addition, our consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Our liquidity is highly dependent on our ability to obtain additional capital in the future. Our failure to raise new capital would impair our ability to both continue our current collaborations and develop new collaborative partnerships and could result in our failure to continue to operate as a going concern.
Because of a working capital deficit, we may not be able to continue as a going concern.
There is doubt as to our ability to continue as a going concern. As of March 31, 2016, the Company had current assets of $2,408,659 and current liabilities of $2,878,082, resulting in a working capital deficit of approximately $469,423. As of the date of this filing, we believe that we only have sufficient liquidity to fund operations through July 2016. We are currently exploring a range of potential transactions, which may include public or private equity offerings, debt financings, collaborations and licensing arrangements, and/or other strategic alternatives, including a merger, sale of assets or other similar transactions. If we are unable to raise additional capital on terms acceptable to us and on a timely basis, we may be required to downsize or wind down our operations through liquidation, bankruptcy, or a sale of our assets. Our financial statements do not include any adjustments related to the recovery and classification of asset carrying amounts or the amount and classification of liabilities that might result should we be unable to continue as a going concern. Should the going concern assumption not be appropriate and we are not able to realize our assets and settle our liabilities, commitments and contingencies in the normal course of operations, adjustments would be required to our unaudited condensed consolidated financial statements to the amounts and classifications of assets and liabilities, and these adjustments could be significant. Additionally, we expect to incur significant expenses and operating losses for the foreseeable future, and our net losses may fluctuate significantly from quarter to quarter and from year to year. These factors raise substantial doubt about our ability to continue as a going concern.
We need additional funding to continue our operations and meet our research and development, capital and general and administrative expenses. Such funding may not be available on favorable terms, if at all, and may be dilutive to our existing stockholders. Without modifications to our existing payment obligations or receipt of additional funding, our existing cash and other sources of liquidity may not be sufficient to fund our operations beyond July 2016. If additional capital is not available, we may have to further curtail our operations, or take other actions that could adversely affect our stockholders.
Our business has not generated (nor do we anticipate that in the foreseeable future it will generate) the cash necessary to finance our operations. We expect to continue to incur losses and use cash during fiscal year 2016, and we will require additional capital to continue our operations beyond July 2016. At March 31, 2016, we had cash and cash equivalents totaling approximately $1.8 million, excluding restricted cash.
Our near-term capital needs depend on many factors, including:
| · | our ability to carefully manage our costs; |
| · | the amount and timing of revenue received from grants or our collaboration and license arrangements; and |
| · | our ability to raise additional capital through public or private equity offerings, debt financings, or strategic collaborations and licensing arrangements, and/or our success in promptly establishing a strategic alternative that is in our stockholders’ best interests. |
If we are unable to preserve or raise additional capital through one or more of the means listed above prior to the end of July 2016, we could face substantial liquidity problems and might be required to implement cost reduction strategies in addition to the cash conservation steps that we have already taken. These reductions could significantly affect our research and development activities, and could result in significant harm to our business, financial condition and results of operations. In addition, these reductions could cause us to further curtail our operations, or take other actions that would adversely affect our stockholders. If we are unable to raise additional capital on terms acceptable to us and on a timely basis, we may be required to downsize or wind down our operations through liquidation, bankruptcy, or a sale of our assets.
We will require additional capital to support operations and business growth, and such capital might not be available when needed or on terms satisfactory to us.
Continued investment will be needed to support our operations and business growth. Our future capital requirements will depend on a number of factors, including, but not limited to:
| · | the size and complexity of, and continued scientific progress in, our research and development programs; |
| · | our entry into new partnerships, and the terms of such partnering agreements; |
| · | competing technological and market developments; |
| · | the time and expense of building and maintaining our patent portfolio and enforcing patent claims; and |
| · | the cost of conducting clinical development and commercialization activities and potentially in-licensing products, if it proves necessary to do so. |
We intend to continue to make investments to support operations and business growth and will require additional funds to respond to business challenges, which may include the need to develop new products, conduct clinical trials (on our own or with our partners), enhance our operating infrastructure, and acquire complementary businesses and technologies.To continue to make such investments, we will likely need to obtain equity or debt financing or consummate acollaboration or licensing arrangement, or some other strategic transaction. However,such financing or potential transaction might not be available when needed or, if available, might not be available on terms satisfactory to us. From time to time, capital markets may experience periods of disruption and instability, which can contribute to worsening economic conditions that materially adversely affect broader financial and credit markets and reducethe availability of debt and equity capital for the market as a whole and for small capitalization businesses such as ours in particular.
In addition, to the extent additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in dilution to our shareholders. These securities may also be sold at a discount from the market price of our common stock.For example, the shares included in the Units and the warrants issued in connection with the PPO contain anti-dilution protection in the event that within certain specified time periods we issue common stock or securities convertible into or exercisable for shares of our common stock at a price lower than the Unit purchase price or the warrant exercise price, as applicable. The anti-dilution protection provisions apply to issuances within two years after the closing of the PPO in the case of the Units and, with respect to the warrants, prior to the warrant expiration date, in both cases subject to exceptions for certain issuances.
Additionally, if we fail to meet the criteria set forth in SEC regulations, various requirements would be imposed by law on broker-dealers who sell our securities to persons other than established customers and accredited investors. Consequently, such regulations may deter broker-dealers from recommending or selling our common stock, which may make it more difficult for us to raise capital.If we are unable to obtain adequate financing or financing on terms satisfactory to us, our ability to continue to support our operations and business growth and to respond to business challenges could be significantly impaired and there would be a material adverse effect on our business and financial condition.
If we are unable to raise capital on terms acceptable to us and on a timely basis, we may be required to downsize or wind down our operations through liquidation, bankruptcy, or a sale of our assets.
We rely on relationships with third parties for product development and commercialization, and those third parties could fail to perform as expected.
We believe that our success depends on developing and maintaining scientific and business relationships with other entities, including companies, academic institutions, and other organizations. Relying on such collaborative relationships entails risk to our future success because, among other things:
| · | our partners may not devote sufficient resources to the success of our collaboration; |
| · | our partners may not obtain regulatory approvals necessary to continue the collaborations in a timely manner, if at all; |
| · | our partners may be acquired by another company and decide to terminate our collaborative partnership or cease doing business or become insolvent; |
| · | our partners may develop or license technologies or components competitive with our products; |
| · | disagreements with partners could result in litigation or termination of the relationship; |
| · | collaborators may not have sufficient capital resources; |
| · | our existing collaborations may preclude us from entering into additional future arrangements; and |
| · | we may not be able to negotiate future partnerships, or renew existing collaborative agreements, on acceptable terms, if at all. |
Because these and other factors may be beyond our control, the development or commercialization of our products may be delayed or otherwise adversely affected.
If we or any of our partners terminate a collaborative arrangement, we may be required to devote additional resources to product development and commercialization, or we may need to cancel some development programs, which could adversely affect our product pipeline and business.
Successful development of products is uncertain.
Our development of future product candidates is subject to the risks inherent in the development of new biotechnology products, which include:
| · | delays in research and development, clinical testing or manufacturing; |
| · | unplanned expenditures in product development, clinical testing or manufacturing; |
| · | failure in clinical trials or failure to receive and maintain regulatory approvals; |
| · | emergence of equivalent or superior products; |
| · | inability to manufacture product candidates on a commercial scale; |
| · | inability to market products due to third-party patent rights; |
| · | decisions by our partners not to pursue product development; and |
| · | failure to achieve market acceptance after regulatory approval. |
Because of these and other risks, our research and development efforts or those of our partners may not result in any commercially viable products. If a significant portion of these development efforts is not successfully completed, required regulatory approvals are not obtained, or any approved products are not commercially successful, our business, financial condition and results of operations may be materially adversely affected.
The regulatory approval process is lengthy, expensive and uncertain.
Prior to marketing, any new therapeutic product must undergo an extensive regulatory approval process in the United States (and in other countries, as applicable) to establish that the product meets minimum requirements for safety and efficacy. This regulatory process, which includes preclinical studies and clinical trials (and may also include post-marketing surveillance), can take many years to complete and require the expenditure of substantial resources. The commencement or completion of clinical trials may be delayed or halted for various reasons, including difficulty in patient accrual, inadequate drug supply, adverse medical events, lack of efficacy, and issues with evaluator institutional review boards. Data obtained from preclinical studies and clinical trials are susceptible to varying interpretations that could delay, limit or prevent regulatory approval. In addition, previously unknown problems associated with a product that come to light after marketing approval might lead to a requirement for additional clinical studies or withdrawal of the product from the market.
We have not submitted an investigational new drug, or IND, application for any product candidate, and no product candidate has been approved for commercialization in the United States or elsewhere. No assurance can be given that we or any of our partners will be able to identify a product candidate to submit for approval, conduct clinical testing, or obtain the necessary approvals from the U.S. Food and Drug Administration or other regulatory authorities for any products.
Our use of our platform technology depends on a third party license that could be terminated.
We utilize our platform technology under an exclusive patent license agreement with the Massachusetts Institute of Technology. The term of the license agreement remains in effect until the expiration or abandonment of all issued patents and filed patent applications within the patent rights set forth in the agreement. The license agreement contains certain diligence obligations relating to research and development milestones, clinical milestones, and commercialization milestones. If we fail to achieve those milestones, we could lose certain rights under the license, or even lose the license entirely, which would have a material adverse effect on our business.
We operate in a highly competitive industry, and if our competitors develop superior products and technologies, our competitive position could be compromised.
We face various types of actual and potential competition. Competition could come from both established and emerging pharmaceutical and biotechnology companies (including companies specializing in immunotherapy), academic institutions, governmental agencies, and public and private research institutions. Many of the companies against which we are likely to compete have significantly greater financial resources and expertise in research and development, preclinical testing, clinical trials, manufacturing, regulatory affairs, and marketing than we do. We also compete with small and early-stage companies, particularly with respect to collaborative arrangements with large and established companies and with respect to acquiring technologies that may be complementary to our programs. In addition, we face competition in recruiting and retaining qualified scientific and management personnel. If we are unable to compete effectively against these companies, our business, financial condition and results of operations could be materially adversely affected. Additional information concerning competition is set forth in the section entitled “Description of Business – Competition.”
Claims that our platform technology or our products infringe third party patents might result in costly litigation
We cannot be certain that our platform technology, our products, or their respective use, do not infringe third party patents. Third parties might allege that we are infringing their patent rights and resort to litigation against us. Although we have conducted freedom-to-operate studies, it is possible that we have failed to identify relevant patents or applications. New patent applications in the United States and elsewhere are published approximately 18 months after their initial filing. Therefore, it is possible that relevant third party patent applications have been filed, but are not yet publicly available. During the examination process, which is sometimes called patent prosecution, amendment of existing claims or introduction of new claims is permissible in the U.S. Patent and Trademark Office and in most foreign patent offices. Therefore, potentially problematic claims may be introduced into pending third party applications that do not currently contain such claims.
We are aware of third party patents that contain broad claims potentially relevant to certain therapeutic uses of our anti-PD-1 antibodies. If we were to challenge the validity of any claim in an issued U.S. patent in court, we would need to overcome a statutory presumption of validity that attaches to every U.S. patent. This means that in order to prevail, we would have to present clear and convincing evidence as to the invalidity of the claim(s) in question. There is no assurance that a court would find in our favor on questions of infringement or validity.
In order to avoid or to settle actual or potential patent infringement claims, we may seek a license from a third party and pay license fees, or royalties, or both. Necessary licenses might not be available on commercially reasonable terms, if at all. Ultimately, we could be prevented from commercializing a product, or forced to cease using our technology platform, as a result of patent infringement claims. Defending against claims of patent infringement or misappropriation of trade secrets could be costly and time consuming, even if we prevail in the litigation, which could materially adversely affect our business, financial condition and results of operations.
We might be unsuccessful in obtaining adequate patent protection for one or more of our potential products.
The patent position of biotechnology and pharmaceutical companies is often uncertain because it involves complex legal and factual considerations. The standards applied by the U.S. Patent and Trademark Office and foreign patent offices in granting patents are not always applied uniformly or predictably, and such standards are subject to change based on court decisions, such as recent U.S. Supreme Court cases involving patents. Consequently, patents might not issue from some or all of our patent applications. In the event that we are unsuccessful in obtaining adequate patent protection for one or more of our products in the future, our business could be adversely affected.
Our competitors might successfully evade our patent protection.
Notwithstanding valid patents that we may own or control through an exclusive license, our competitors may independently develop and market alternative products similar to ours, without infringing any of our patent rights. In addition, they may design around our patented platform technology. This could diminish our competitive advantage, particularly if the competitor is a company with resources greater than ours.
One or more of our patents might be found invalid or unenforceable if challenged in court.
If we were to initiate legal proceedings against a third party to enforce a patent covering our platform technology or one of our products, the defendant might argue successfully that the claims we are asserting are invalid or unenforceable. Even though all of our issued patents will have undergone examination by the U.S. Patent and Trademark Office or a foreign patent office, we cannot be certain that there is no invalidating prior art. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we could lose part or all of the patent protection on one or our products, or on our platform technology, which could have a materially adverse effect on our business.
In the course of litigation, we might be subject to unfavorable publicity.
During the course of any patent litigation, there could be public announcements regarding hearings, rulings on motions, or other interim developments. If securities analysts or investors perceive such announcements as negative, our business could be adversely affected and the market price of our stock could decline.
If we fail to retain key members of our staff, or fail to attract and train skilled new employees, our ability to conduct and expand our business would be impaired.
The loss of any of our key employees could seriously harm our product development and commercialization efforts. Furthermore, as our company continues to grow, we will require additional highly trained technical and business personnel. The market for such individuals is highly competitive in the biotechnology industry, particularly in the greater Cambridge, Massachusetts area where our main office and laboratory is located. If we fail to hire, train and retain a sufficient number of qualified employees to match our growth, our ability to conduct and expand our business could be impaired. We have entered into employment agreements with certain of our executive officers. However, the existence of an employment agreement does not guarantee retention of members of our management team and we may not be able to retain those individuals for the duration of or beyond the end of their respective terms.
If we become subject to claims relating to improper handling, storage or disposal of hazardous materials, we could expend significant resources to bring our company into compliance.
Our research and development processes involve the controlled storage, use and disposal of hazardous materials. We are subject to federal, state and local regulations governing the use, manufacture, storage, handling and disposal of hazardous materials and waste products. We may incur significant costs complying with both existing and future environmental laws and regulations, and we are unable to predict whether any agency will adopt regulations in the future that would have a material adverse effect on our operations. The risk of accidental contamination or injury from hazardous materials cannot be eliminated completely. In the event of an accident, we could be held liable for damages that result, and the associated liability could exceed the limits or fall outside our existing insurance coverage.
If a catastrophe strikes our research facilities, we might be unable to complete our projects and our business operations would suffer, including but not limited to lost revenue.
Our research and development facilities are located in Cambridge, Massachusetts. Although we have commercial insurance, our facilities and some pieces of equipment and biological samples are difficult to replace and could require substantial replacement lead-time. Various types of disasters, including earthquakes, fires, floods, and acts of terrorism, may affect our research and development facilities. In the event our existing research and development facilities or equipment is affected by man-made or natural disasters, we may be unable to continue research and development activities, and may fail to meet our internal objectives or our partner demands. If our research and development operations were curtailed or ceased, it would seriously harm our business. In the event of a prolonged or even short-term power failure, we may lose biological samples stored in our freezers or growing in our incubators that could affect our ability to complete projects for ourselves or partners.
We might face product liability claims related to the use or misuse of products employing our antibody technology.
The administration of drugs to humans, in clinical trials or after commercialization, may expose us to product liability claims. Consumers, healthcare producers or persons selling products derived from our research and development programs may be able to bring claims against us based on the use of those products in clinical trials or the sale of those products. Product liability claims may be expensive to defend and may result in significant judgments against us, which could exceed our insurance coverage.
Pharmaceutical pricing, reimbursement and related matters are uncertain.
Our business, financial condition and results of operations may be materially and adversely affected by the continuing efforts of the government and third party payors to contain or reduce the costs of healthcare. Federal and state proposals to implement government controls, as well as ongoing emphasis on managed care in the U.S. healthcare industry, may continue to put pressure on the pricing of pharmaceutical products and diagnostic tests. Cost control initiatives could decrease the price that we or our partners receive for products commercialized in the future, and also may have a material adverse effect on our business, financial condition and results of operations. To the extent that cost control initiatives have a material adverse effect on our partners, our ability to commercialize products and to realize royalties may be adversely affected.
Investment Risks
You could lose all of your investment.
An investment in our securities is speculative and involves a high degree of risk. Potential investors should be aware that the value of an investment in our securities may go down as well as up. In addition, there can be no certainty that the market value of an investment in our securities will fully reflect its underlying value. You could lose your entire investment.
Failure to develop or maintain a trading market could negatively affect the value of our common stock and make it difficult or impossible for you to sell your shares.
Our common stock is currently quoted on the OTCQB. The OTCQB is a thinly traded market and lacks the liquidity of certain other public markets with which some investors may have more experience. We may not be able to satisfy the requirements of a national securities exchange for our common stock to be listed on such an exchange, which is often a more widely-traded and liquid market. Among the factors which may delay or prevent the listing of our common stock on a more widely-traded and liquid market are the following: our stockholders’ equity may be insufficient; the market value of our outstanding securities may be too low; our net income from operations may be too low; our common stock may not be sufficiently widely held; we may not be able to secure market makers for our common stock; and we may fail to meet the rules and requirements mandated by the various national exchanges and markets to have our common stock listed. Should we fail to satisfy the listing standards of the national exchanges or if our common stock is otherwise rejected for listing, or if our common stock remains listed on the OTCQB or is suspended from the OTCQB, the trading price of our common stock could suffer. In addition, the trading market for our common stock may continue to be less liquid and our common stock price may be subject to increased volatility, making it difficult or impossible for stockholders to sell shares of our common stock. Further, an unestablished trading market for our common stock may also impair our ability to raise capital by selling additional equity in the future, and may impair our ability to enter into strategic partnerships or acquire companies or products by using shares of our common stock as consideration.
Our stock may be traded infrequently and in low volumes, so you may be unable to sell your shares at or near the quoted bid prices if you need to sell your shares.
Unless we are able to meet the listing requirements of a national securities exchange, such as the New York Stock Exchange or the Nasdaq Stock Market, we expect our common stock to remain eligible for quotation on the OTCQB, or on another over-the-counter quotation system, or in the “pink sheets.” In those venues, however, the shares of our common stock may trade infrequently and in low volumes, meaning that the number of persons interested in purchasing our common shares at or near bid prices at any given time may be relatively small or non-existent. An investor may find it difficult to obtain accurate quotations as to the market value of our common stock or to sell his, her or its shares at or near bid prices, if at all. In addition, if we fail to meet the criteria set forth in SEC regulations, various requirements would be imposed by law on broker-dealers who sell our securities to persons other than established customers and accredited investors. Consequently, such regulations may deter broker-dealers from recommending or selling our common stock, which may further affect the liquidity of our common stock. This would also make it more difficult for us to raise capital.
You may experience dilution of your ownership interests because of the future issuance of additional shares of our common or preferred stock or other securities that are convertible into or exercisable for our common or preferred stock.
In the future, we may issue our authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our present stockholders. We are authorized to issue an aggregate of 300,000,000 shares of our common stock and 10,000,000 shares of “blank check” preferred stock. We will need to raise additional capital in the near future to meet our working capital needs, and there can be no assurance that we will not be required to issue additional shares, warrants or other convertible securities in the future in conjunction with these capital raising efforts, including at a price (or exercise price) below the price you paid for your stock. We may issue additional shares of our common stock or other securities that are convertible into or exercisable for our common stock in connection with hiring, promoting or retaining employees, future acquisitions, future sales of our securities for capital raising purposes, or for other business purposes. The future issuance of any such additional shares of our common stock may create downward pressure on our stock’s trading price.
Our common stock is subject to the “penny stock” rules of the SEC and the trading market in the securities is limited, which makes transactions in the stock cumbersome and may reduce the value of an investment in the stock.
The Securities Exchange Act of 1934, as amended (the “Exchange Act”), establishes the definition of a “penny stock,” for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require that a broker or dealer take a number of additional steps to approve a person’s account for transactions in penny stocks, including obtaining financial information and investment experience objectives of the person, making a reasonable determination that the transactions in penny stocks are suitable for that person, and making a reasonable determination that the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks. In addition, the rules require that the broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased. Due to the additional burdens imposed by these rules, brokers may be less willing to execute transactions in securities subject to the penny stock rules, and this may make it more difficult for investors to dispose of our common stock and consequently cause a decline in the market value of our common stock.
We do not anticipate paying dividends on our common stock, and investors may lose the entire amount of their investment.
Cash dividends have never been declared or paid on our common stock, and we do not anticipate such a declaration or payment for the foreseeable future. We expect to use future earnings, if any, to fund business growth. Therefore, stockholders will not receive any funds absent a sale of their shares of common stock. If we do not pay dividends, our common stock may be less valuable because a return on your investment will only occur if our stock price appreciates. We cannot assure stockholders of a positive return on their investment when they sell their shares, nor can we assure stockholders that they will not lose the entire amount of their investment.
Being a public company is expensive and administratively burdensome.
As a public reporting company, we are subject to the information and reporting requirements of the Securities Act, the Exchange Act, and other federal securities laws, rules and regulations related thereto, including compliance with the Sarbanes-Oxley Act. Complying with these laws, regulations and standards requires and will continue to require the time and attention of our board of directors and management, and increases our expenses. Among other things, we are required to:
| · | prepare and distribute periodic reports in compliance with our obligations under federal securities laws; |
| · | institute a comprehensive compliance function, including with respect to corporate governance; |
| · | maintain and evaluate a system of internal controls over financial reporting in compliance with the requirements of Section 404 of the Sarbanes-Oxley Act and the related rules and regulations of the SEC and the Public Company Accounting Oversight Board; |
| · | maintain policies relating to our disclosure controls and procedures; and |
| · | involve, to a greater degree, our outside legal counsel and accountants in the above activities. |
The costs of preparing and filing annual and quarterly reports, proxy statements and other information with the SEC and furnishing audited reports to stockholders are significant. Continued compliance with the rules and regulations applicable to publicly-traded companies may require us to hire additional financial reporting, internal controls and other finance personnel, and involve significant regulatory, legal and accounting expenses, investor relations costs, as well as the attention of management. There can be no assurance that we will be able to continue to comply with the applicable regulations in a timely manner, if at all. In addition, being a public company makes it more expensive for us to obtain director and officer liability insurance, and, in the future, we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified executives and members of our board of directors, particularly directors willing to serve on our audit committee.
As an emerging growth company, we may follow certain permitted corporate governance practices, and may delay adoption of new or revised accounting standards, which may make our stock less attractive and result in less protection than is accorded to investors in a non-emerging growth company.
As an emerging growth company, we are permitted to follow certain corporate governance practices and disclosure requirements that are less robust than those otherwise required by the SEC. This may provide our stockholders with less information and less protection than what is accorded to investors under a national stock exchange’s listing requirements applicable to non-emerging growth company issuers.
In addition, as an emerging growth company, we have elected to take advantage of an extended transition period for any new or revised accounting standards that may be issued by the Financial Accounting Standards Board or the SEC. This means that when a standard is issued or revised and it has different application dates for public or private companies, we can delay adoption of the standard until it applies to private companies. This may make a comparison of our financial statements with any other public company that is not an emerging growth company, or is an emerging growth company that has opted out of using the extended transition period, difficult, as different or revised standards may be used. As a result, investors may find our common stock less attractive and there may be a less active trading market for our common stock. Consequently, our stock price may be more volatile and could decline.
Any failure to maintain effective internal control over our financial reporting could materially adversely affect us.
Section 404 of the Sarbanes-Oxley Act of 2002 requires us to include in our annual reports on Form 10-K an assessment by management of the effectiveness of our internal control over financial reporting. In addition, at such time, if any, as we are no longer a “smaller reporting company,” our independent registered public accounting firm will have to attest to and report on management’s assessment of the effectiveness of such internal control over financial reporting. Our compliance with Section 404 may require that we incur substantial accounting expense and expend significant management efforts. We currently do not have an internal audit group, and we may need to retain the services of additional accounting and financial staff or consultants with appropriate public company experience and technical accounting knowledge to satisfy the ongoing requirements of Section 404.
While we intend to diligently and thoroughly document, review, test and improve our internal control over financial reporting in order to ensure compliance with Section 404, in the future management may not be able to conclude that our internal control over financial reporting is effective. Furthermore, even if management were to reach such a conclusion, if our independent registered public accounting firm is not satisfied with the adequacy of our internal control over financial reporting, or if the independent auditors interpret the requirements, rules or regulations differently than we do, then they may decline to attest to management’s assessment or may issue a report that is qualified. Any of these events could result in a loss of investor confidence in the reliability of our financial statements, which in turn could negatively affect the price of our common stock.
Provisions in our charter documents and under Delaware law could discourage a takeover that stockholders may consider favorable.
Certain provisions in our certificate of incorporation and by-laws may have the effect of delaying or preventing a change of control or changes in our management. These provisions include the following:
| · | authorize “blank check” preferred stock that could be issued by our board of directors to defend against a takeover attempt; |
| · | establish a classified board of directors, as a result of which the successors to the directors whose terms have expired will be elected to serve from the time of election and qualification until the third annual meeting following their election; |
| · | require that directors only be removed from office for cause and only upon a supermajority stockholder vote; |
| · | provide that vacancies on the board of directors, including newly created directorships, may be filled only by a majority vote of directors then in office rather than by stockholders; |
| · | prevent stockholders from calling special meetings; and |
| · | prohibit stockholder action by written consent, requiring all actions to be taken at a meeting of the stockholders. |
In addition, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation from engaging in a broad range of business combinations with any “interested” stockholder for a period of three years following the date on which the stockholder becomes an “interested” stockholder.
Also, in connection with the Merger, certain of our stockholders (who hold a majority of our common stock), including all of the investors in the PPO, all of our pre-Merger stockholders and certain of the Enumeral stockholders (including all of Enumeral’s officers directors and principal stockholders) entered into a Voting Agreement in which they have agreed to vote their shares of our stock to elect certain directors specified in the Voting Agreement. The Voting Agreement will terminate two years from the date of closing of the Merger.
Our ability to issue additional stock may prevent or make more difficult certain transactions, including a sale or merger of our company.
Our board of directors may authorize our issuance of up to 10,000,000 shares of preferred stock with powers, rights and preferences designated by it. Shares of voting or convertible preferred stock could be issued, or rights to purchase such shares could be issued, to create voting impediments or to frustrate persons seeking to effect a takeover or otherwise gain control of our company. The ability of our board of directors to authorize the issuance of such additional shares of preferred stock, with rights and preferences it deems advisable, could discourage an attempt by a party to acquire control of our company by tender offer or other means. Such issuances could therefore deprive stockholders of benefits that could result from such an attempt, such as the realization of a premium over the market price for their shares or the temporary increase in market price that such an attempt could cause. Moreover, the issuance of such additional shares of preferred stock to persons friendly to our board of directors could make it more difficult to remove incumbent officers and directors from office even if such change were to be favorable to stockholders generally.
The sale of a significant number of registered shares of our common stock may cause our stock price to decline.
This prospectus relates to the public offering, which is not being underwritten, by the selling stockholders listed in this prospectus, of up to 47,674,386 shares of our common stock. Of the shares being offered, 23,941,194 are presently issued and outstanding, and 23,733,192 are issuable upon exercise of common stock purchase warrants. The sale of these shares of our common stock will lead to an increase in the public float of our common stock, and such increase may cause the market price of our common stock to decline or fluctuate significantly.
***
The risks above do not necessarily comprise all of those associated with an investment in our company. This Prospectus contains forward looking statements that involve unknown risks, uncertainties and other factors that may cause the actual results, financial condition, performance or achievements of our company to be materially different from any future results, performance or achievements expressed or implied by such forward looking statements. Factors that might cause such a difference include, but are not limited to, those set out above.
SELLING STOCKHOLDERS
This Prospectus covers the resale from time to time by the selling stockholders identified in the table below of:
| · | up to 39,016,432 shares of Common Stock (the “PPO Shares”) issued to investors in our July 2014 private placement, consisting 17,466,922 shares of Common Stock currently outstanding and 21,549,510 shares of common stock issuable upon exercise of common stock purchase warrants; |
| · | up to 2,000,000 shares of Common Stock (the “Agent Warrant Shares”) issuable upon exercise of common stock purchase warrants issued to the placement agents in our July 2014 private placement (and subsequently transferred to employees of the placement agents); |
| · | 4,964,207 shares of Common Stock (the “Enumeral Shares”) issued to the former holders of common and preferred stock of Enumeral pursuant to the terms of the Merger Agreement, consisting 4,780,525 shares of Common Stock currently outstanding and 183,682 shares of common stock issuable upon exercise of common stock purchase warrants; and |
| · | 1,693,747 shares of Common Stock (the “True-Up Shares”) issued to the pre-Merger stockholders of the Company pursuant to anti-dilution provisions of the Merger Agreement. |
The selling stockholders identified in the table below may from time to time offer and sell under this Prospectus any or all of the shares of common stock described under the columns “Shares of common stock owned prior to this Offering and Registered hereby” and “Shares Issuable Upon Exercise of Warrants owned Prior to this Offering and Registered hereby” in the table below.
Certain selling stockholders may be deemed to be “underwriters” as defined in the Securities Act. Any profits realized by such selling stockholder may be deemed to be underwriting commissions.
The table below has been prepared based upon the information furnished to us by the selling stockholders as of the date of this prospectus. The selling stockholders identified below may have sold, transferred or otherwise disposed of some or all of their shares since the date on which the information in the following table is presented in transactions exempt from or not subject to the registration requirements of the Securities Act. Information concerning the selling stockholders may change from time to time and, if necessary, we will amend or supplement this prospectus accordingly. We cannot give an estimate as to the number of shares of common stock that will actually be held by the selling stockholders upon termination of this offering because the selling stockholders may offer some or all of their common stock under the offering contemplated by this prospectus or acquire additional shares of common stock. The total number of shares that may be sold hereunder will not exceed the number of shares offered hereby. Please read the section entitled “Plan of Distribution” in this prospectus.
The following table sets forth the name of each selling stockholder, the number of shares of our common stock beneficially owned by such stockholder before this offering, the number of shares to be offered for such stockholder’s account and the number and (if one percent or more) the percentage of the class to be beneficially owned by such stockholder after completion of the offering. The number of shares owned are those beneficially owned, as determined under the rules of the SEC, and such information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares of our common stock as to which a person has sole or shared voting power or investment power and any shares of common stock which the person has the right to acquire within 60 days after July 22, 2016 (the “Determination Date”), through the exercise of any option, warrant or right, through conversion of any security or pursuant to the automatic termination of a power of attorney or revocation of a trust, discretionary account or similar arrangement, and such shares are deemed to be beneficially owned and outstanding for computing the share ownership and percentage of the person holding such options, warrants or other rights, but are not deemed outstanding for computing the percentage of any other person.
Unless otherwise set forth below, based upon the information furnished to us, (a) the persons and entities named in the table have sole voting and sole investment power with respect to the shares set forth opposite the selling stockholder’s name, subject to community property laws, where applicable, (b) no selling stockholder had any position, office or other material relationship within the past three years, with us or with any of our predecessors or affiliates, and (c) no selling stockholder is a broker-dealer or an affiliate of a broker-dealer. Selling stockholders who are broker-dealers or affiliates of broker-dealers are indicated by footnote. We have been advised that these broker-dealers and affiliates of broker-dealers purchased our common stock in the ordinary course of business, not for resale, and at the time of purchase, did not have any agreements or understandings, directly or indirectly, with any person to distribute such common stock. The number of shares of common stock shown as beneficially owned before the offering is based on information furnished to us or otherwise based on information available to us at the timing of the filing of the registration statement of which this prospectus forms a part.
| Selling Stockholder | | Shares of
common stock
beneficially
owned prior to
the Offering | | | Shares of
common
stock owned
prior to this
Offering and
registered
hereby | | | Shares
issuable upon
exercise of
warrants
owned prior to
this Offering
and registered
hereby (1) | | | Shares of
common stock
beneficially
owned upon
completion of
this Offering (2) | | | Percentage
of
Common
Stock
beneficially
owned upon
completion
of this
Offering (3) | |
| | | | | | | | | | | | | | | | |
| A.G. Family L.P. (4)(6) | | | 600,000 | | | | 300,000 | | | | 300,000 | | | | - | | | | - | |
| Aaron Segal (5) | | | 756,628 | | | | 55,798 | | | | 363,624 | | | | 337,206 | | | | * | |
| Alan F. Bilzi (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Alden Thomas Leith (6) | | | 416,000 | | | | 208,000 | | | | 208,000 | | | | - | | | | - | |
| Alec H. Jaret (6) | | | 70,000 | | | | 35,000 | | | | 35,000 | | | | - | | | | - | |
| Alfred J. Difiore, Jr. (6) | | | 30,000 | | | | 15,000 | | | | 15,000 | | | | - | | | | - | |
| Allan Rothstein (7) | | | 2,338,136 | | | | 50,000 | | | | 50,000 | | | | 2,238,136 | | | | 4.4 | % |
| Allen Chase Foundation - Special Investment Account (6)(8) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Allan Lipkowitz Revocable Living Trust 8/26/2005 (6)(9) | | | 150,000 | | | | 75,000 | | | | 75,000 | | | | - | | | | - | |
| Alyson D. Schlosser (6) | | | 80,000 | | | | 40,000 | | | | 40,000 | | | | - | | | | - | |
| AME Capital Group, LLC (6)(10) | | | 500,000 | | | | 250,000 | | | | 250,000 | | | | - | | | | - | |
| American Portfolios Financial Services Inc. (11) | | | 43,917 | | | | - | | | | 43,917 | | | | - | | | | - | |
| Amir Zaghloul and Mona Zaghloul (6) (12) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Anders J. Maxwell and Carlene S. Maxwell (13) | | | 137,766 | | | | 68,883 | | | | - | | | | 68,883 | | | | * | |
| Andrea Bedan (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Adrienne M. Baker (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Andrew Fisher and Melissa Fisher (6)(14) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Andrew S. Brenner (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Anthony Iannotta (6) | | | 40,000 | | | | 20,000 | | | | 20,000 | | | | - | | | | - | |
| Anthony Liberti and Barbara Liberti (6)(15) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Anthony Naer and Mary Naer (6)(16) | | | 40,000 | | | | 20,000 | | | | 20,000 | | | | - | | | | - | |
| Anthony Ziniti (6) | | | 45,000 | | | | 20,000 | | | | 25,000 | | | | - | | | | - | |
| Anton Bogner and Barbara D. Bogner (6)(17) | | | 600,000 | | | | 300,000 | | | | 300,000 | | | | - | | | | - | |
| Arielle E. Crothers(6) | | | 20,000 | | | | 10,000 | | | | 10,000 | | | | - | | | | - | |
| Arun Penmetsa (18) | | | 137,766 | | | | 68,883 | | | | - | | | | 68,883 | | | | * | |
| Ashish Jhingan and Dolly Jhingan (6)(19) | | | 45,000 | | | | 20,000 | | | | 25,000 | | | | - | | | | - | |
| Bantam Group, LLC (6)(20) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Barclay Armitage (6) | | | 40,000 | | | | 20,000 | | | | 20,000 | | | | - | | | | - | |
| Barry Cohn (21) | | | 21,959 | | | | - | | | | 21,959 | | | | - | | | | - | |
| Barry J. Bentley (18) | | | 344,414 | | | | 172,207 | | | | - | | | | 172,207 | | | | * | |
| Barry J. Shemaria (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Biological E. Limited (18)(22) | | | 344,414 | | | | 172,207 | | | | - | | | | 172,207 | | | | * | |
| BlackBook (18)(23) | | | 112,004 | | | | - | | | | 56,002 | | | | 56,002 | | | | * | |
| Borca Dynasty Trust (6)(24) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Boris Shames (6) | | | 40,000 | | | | 20,000 | | | | 20,000 | | | | - | | | | - | |
| Brian E. Peierls (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Brian Fischhoff and Andrea Fischhoff (6)(25) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Brian M. Miller (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Brio Capital Master Fund LTD (6)(26) | | | 575,700 | | | | 275,700 | | | | 300,000 | | | | - | | | | - | |
| Selling Stockholder | | Shares of
common stock
beneficially
owned prior to
the Offering | | | Shares of
common
stock owned
prior to this
Offering and
registered
hereby | | | Shares
issuable upon
exercise of
warrants
owned prior to
this Offering
and registered
hereby (1) | | | Shares of
common stock
beneficially
owned upon
completion of
this Offering (2) | | | Percentage
of
Common
Stock
beneficially
owned upon
completion
of this
Offering (3) | |
| | | | | | | | | | | | | | | | | | | | | |
| Bruce A. Haverberg and Donna R. Haverberg (6)(27) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Bruce Rosen (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Sergey Brudanin and Inna Mamuta Haverberg (6)(28) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Byron Hughey (6) | | | 30,000 | | | | 15,000 | | | | 15,000 | | | | - | | | | - | |
| Carnahan Trust (6)(29) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Carol Cody (6) | | | 300,000 | | | | 150,000 | | | | 150,000 | | | | - | | | | - | |
| Carol Giorello and Anthony Giorello (6)(30) | | | 20,000 | | | | 10,000 | | | | 10,000 | | | | - | | | | - | |
| Cathy D. Guerriera (6) | | | 20,000 | | | | 10,000 | | | | 10,000 | | | | - | | | | - | |
| Cede & Co. (31) | | | 12,329 | | | | 3,089 | | | | - | | | | 9,240 | | | | * | |
| Chad Forti (6) | | | 40,000 | | | | 20,000 | | | | 20,000 | | | | - | | | | - | |
| Charles Freeland (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Charles Freeland and Beverly Freeland (6)(32) | | | 300,000 | | | | 150,000 | | | | 150,000 | | | | - | | | | - | |
| Charles Loegering (6) | | | 40,000 | | | | 20,000 | | | | 20,000 | | | | - | | | | - | |
| Charles Medici and Diane Medici (6)(33) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Chinn Family Limited Partnership (6)(34) | | | 500,000 | | | | 250,000 | | | | 250,000 | | | | - | | | | - | |
| Choi Family Trust Dated March 15, 2001 (6)(35) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Christopher Cozzolino (6) | | | 11,700 | | | | - | | | | 11,700 | | | | - | | | | - | |
| Christopher Innace (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Christopher Warren Lamar (6) | | | 25,000 | | | | - | | | | 25,000 | | | | - | | | | - | |
| Christopher William Washburn (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Clay Godfrey Lebhar | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Clayton A. Struve (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Clyde S. McGregor Revocable Trust dtd 6/6/97 (6)(36) | | | 500,000 | | | | 250,000 | | | | 250,000 | | | | - | | | | - | |
| Coover Multi-Generational Trust (6)(37) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Craig R. Francis (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Craig H. Millet (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Craig I. Leipold (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Craig Whited (6) | | | 150,000 | | | | 75,000 | | | | 75,000 | | | | - | | | | - | |
| Currie Family Trust (6)(38) | | | 260,000 | | | | 130,000 | | | | 130,000 | | | | - | | | | - | |
| Cynthia Warenik Serini (6) | | | 40,000 | | | | 20,000 | | | | 20,000 | | | | - | | | | - | |
| Daniel Regan (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Daniel Chestler (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Daniel Michael (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Daniel Salvas (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Daniel Woods (18) | | | 68,884 | | | | 34,442 | | | | - | | | | 34,442 | | | | * | |
| Daryl Squicciarini(6) | | | 500,000 | | | | 250,000 | | | | 250,000 | | | | - | | | | - | |
| Selling Stockholder | | Shares of
common stock
beneficially
owned prior to
the Offering | | | Shares of
common
stock owned
prior to this
Offering and
registered
hereby | | | Shares
issuable upon
exercise of
warrants
owned prior to
this Offering
and registered
hereby (1) | | | Shares of
common stock
beneficially
owned upon
completion of
this Offering (2) | | | Percentage
of
Common
Stock
beneficially
owned upon
completion
of this
Offering (3) | |
| | | | | | | | | | | | | | | | | | | | | |
| Dave Rickey & Daughters Foundation Charitable Trust (6)(39) | | | 25,000 | | | | 10,000 | | | | 15,000 | | | | - | | | | - | |
| David Blau (6) | | | 250,000 | | | | 125,000 | | | | 125,000 | | | | - | | | | - | |
| David E. Weiss, Jr. (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| David Frydrych (18) | | | 289,306 | | | | 144,653 | | | | - | | | | 144,653 | | | | * | |
| David Gerald Tompkins (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| David Landskowsky (40) | | | 465,774 | | | | 89,523 | | | | 150,722 | | | | 225,529 | | | | * | |
| David M. Rickey Trust dtd 5/8/02 (6)(41) | | | 45,000 | | | | 10,000 | | | | 35,000 | | | | - | | | | - | |
| David Routenberg (18)(143) | | | 35,132 | | | | 690 | | | | - | | | | 34,442 | | | | * | |
| David Schechter (18) | | | 34,442 | | | | 17,221 | | | | - | | | | 17,221 | | | | * | |
| David Stollwerk and Susan S.Stollwerk (42) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Dean Greenberg (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Delux Investment Group LLC (6)(43) | | | 30,000 | | | | 15,000 | | | | 15,000 | | | | - | | | | - | |
| Dennis McKee (6) | | | 20,000 | | | | 10,000 | | | | 10,000 | | | | - | | | | - | |
| Dennis T. Brooks (6) | | | 60,000 | | | | 30,000 | | | | 30,000 | | | | - | | | | - | |
| Diana Pocapalia Trust dated July 8, 2013 (18)(44) | | | 96,438 | | | | 48,219 | | | | - | | | | 48,219 | | | | * | |
| Domenic Strazzulla (6) | | | 80,000 | | | | 40,000 | | | | 40,000 | | | | - | | | | - | |
| Donald Cooper (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Donald P. Sesterhenn (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Double Add Investments I LLC(6)(45) | | | 30,000 | | | | 15,000 | | | | 15,000 | | | | - | | | | - | |
| Douglas Kohrs (6) | | | 400,000 | | | | 200,000 | | | | 200,000 | | | | - | | | | - | |
| Douglas Lee Krohn (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Due Mondi Investments, LTD (6)(46) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| E. Jeffrey Peierls (6) | | | 240,000 | | | | 120,000 | | | | 120,000 | | | | - | | | | - | |
| Edward King (6) | | | 300,000 | | | | 150,000 | | | | 150,000 | | | | - | | | | - | |
| Edward J. O'Connell (6) | | | 60,000 | | | | 30,000 | | | | 30,000 | | | | - | | | | - | |
| EFD Capital, Inc. (47) | | | 20,000 | | | | - | | | | 20,000 | | | | - | | | | - | |
| Elizabeth Crothers (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Emanuele Ostuni (18)(48) | | | 281,279 | | | | 18,039 | | | | 5,102 | | | | 258,138 | | | | * | |
| Eric Gordon (18) | | | 68,884 | | | | 34,442 | | | | - | | | | 34,442 | | | | * | |
| Eric Rubenstein (49) | | | 495,368 | | | | 89,524 | | | | 180,315 | | | | 225,529 | | | | * | |
| Four Jr. Investment Ltd. (6)(50) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Francesco Ostuni (18)(51) | | | 97,346 | | | | 48,673 | | | | - | | | | 48,673 | | | | * | |
| Frank Delillo (6) | | | 30,000 | | | | 15,000 | | | | 15,000 | | | | - | | | | - | |
| Fred Saltzman and Marilyn Saltzman Stollwerk (6)(52) | | | 240,000 | | | | 120,000 | | | | 120,000 | | | | - | | | | - | |
| Ganmukhi Irrevocable Trust (6)(53) | | | 400,000 | | | | 200,000 | | | | 200,000 | | | | - | | | | - | |
| Gary D. Sweeney (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| General Hospital Corporation (18) | | | 645 | | | | - | | | | - | | | | 645 | | | | * | |
| George Adamoyurka (18) | | | 34,442 | | | | 17,221 | | | | - | | | | 17,221 | | | | * | |
| Selling Stockholder | | Shares of
common stock
beneficially
owned prior to
the Offering | | | Shares of
common
stock owned
prior to this
Offering and
registered
hereby | | | Shares
issuable upon
exercise of
warrants
owned prior to
this Offering
and registered
hereby (1) | | | Shares of
common stock
beneficially
owned upon
completion of
this Offering (2) | | | Percentage
of
Common
Stock
beneficially
owned upon
completion
of this
Offering (3) | |
| | | | | | | | | | | | | | | | | | | | | |
| George Elefther and Karen A. Elefther (6)(54) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| George Kingsley and Nadine Kingsley (6)(55) | | | 150,000 | | | | 75,000 | | | | 75,000 | | | | - | | | | - | |
| Gerald P. Mondell (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Gerard B. Dotterweich (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Gibralt Capital Corporation (6)(56) | | | 800,000 | | | | 400,000 | | | | 400,000 | | | | - | | | | - | |
| Gilbert S. Omenn (6) | | | 120,000 | | | | 60,000 | | | | 60,000 | | | | - | | | | - | |
| Glen Ackermann (6) | | | 30,000 | | | | 15,000 | | | | 15,000 | | | | - | | | | - | |
| Glenn Turturro (6) | | | 150,000 | | | | 75,000 | | | | 75,000 | | | | - | | | | - | |
| Greenover Group, LP (6)(57) | | | 400,000 | | | | 200,000 | | | | 200,000 | | | | - | | | | - | |
| Gregory D. Vogel (6) | | | 40,000 | | | | 20,000 | | | | 20,000 | | | | - | | | | - | |
| Gregory J. Borca and Heidi H. Borca (6)(58) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Gregory S. Bentley (18) | | | 344,414 | | | | 172,207 | | | | - | | | | 172,207 | | | | * | |
| Hans C. Bodmer (6) | | | 400,000 | | | | 200,000 | | | | 200,000 | | | | - | | | | - | |
| Harold Doran (18) | | | 518,368 | | | | 259,184 | | | | - | | | | 259,184 | | | | * | |
| Harris & Harris Group, Inc. (59) | | | 9,801,488 | | | | 1,500,000 | | | | 1,500,000 | | | | 6,801,488 | | | | 12.7 | % |
| Helmsbridge Holdings Limited (6)(60) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Helmut H. Albrecht Trust (6)(61) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Howard A. Kalka and Susan Kalka (6)(62) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Howard Stringer (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Ian Jacobs(63) | | | 106,839 | | | | 10 | | | | - | | | | 106,829 | | | | * | |
| India Young (64) | | | 400 | | | | - | | | | 400 | | | | - | | | | | |
| Irving Jablon (6) | | | 10,000 | | | | - | | | | 10,000 | | | | - | | | | - | |
| J. Christopher Love (18)(65) | | | 409,830 | | | | 6,867 | | | | - | | | | 402,963 | | | | * | |
| Jacob L. Bojman (6) | | | 15,000 | | | | 7,500 | | | | 7,500 | | | | - | | | | - | |
| James L. Dritz (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| James L. Kennedy(18) | | | 275,528 | | | | 137,764 | | | | - | | | | 137,764 | | | | * | |
| James Smith (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| James T. Lenehan (18) | | | 835,286 | | | | 417,643 | | | | - | | | | 417,643 | | | | * | |
| James Taylor Hanan III (6) | | | 60,000 | | | | 30,000 | | | | 30,000 | | | | - | | | | - | |
| James Zhu (18) | | | 140,664 | | | | 70,332 | | | | - | | | | 70,332 | | | | * | |
| Jason Camp (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Jay Bradbury(6) | | | 150,000 | | | | 75,000 | | | | 75,000 | | | | - | | | | - | |
| Jay M. Haft (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Jeff Rennell and Christine Kellye Rennell (6)(66) | | | 1,000,000 | | | | 500,000 | | | | 500,000 | | | | - | | | | - | |
| Jeffrey Reidenouer (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Joel L. Hochman Revocable Trust UAD 12/8/1994 (6)(67) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Joel R. Jacks (6) | | | 300,000 | | | | 150,000 | | | | 150,000 | | | | - | | | | - | |
| Joel Yanowitz (6) | | | 40,000 | | | | 20,000 | | | | 20,000 | | | | - | | | | - | |
| Selling Stockholder | | Shares of
common stock
beneficially
owned prior to
the Offering | | | Shares of
common
stock owned
prior to this
Offering and
registered
hereby | | | Shares
issuable upon
exercise of
warrants
owned prior to
this Offering
and registered
hereby (1) | | | Shares of
common stock
beneficially
owned upon
completion of
this Offering (2) | | | Percentage
of
Common
Stock
beneficially
owned upon
completion
of this
Offering (3) | |
| | | | | | | | | | | | | | | | | | | | | |
| John A. Cotter and Wendy M. Cotter (6)(68) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| John and Mary Cooper Multigenerational Trust (6)(69) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| John Burrus (18) | | | 253,289 | | | | 4,967 | | | | - | | | | 248,322 | | | | * | |
| John C. Blazier and Fleur A. Christensen Cotter (6)(70) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| John E. Larsen (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| John Herbst (6) | | | 30,000 | | | | 15,000 | | | | 15,000 | | | | - | | | | - | |
| John O. Gallant (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| John P. Funkey Revocable Trust dtd 2/26/90 (6)(71) | | | 300,000 | | | | 150,000 | | | | 150,000 | | | | - | | | | - | |
| John Patrick White (72) | | | 269,915 | | | | 169,915 | | | | 100,000 | | | | - | | | | - | |
| John V. Wagner Jr. (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Jonas A. Edstrom (6) | | | 29,540 | | | | 4,540 | | | | 25,000 | | | | - | | | | - | |
| Jonathan Blatt and Gina Blatt (6)(73) | | | 70,000 | | | | 35,000 | | | | 35,000 | | | | - | | | | - | |
| Jonathan Kyle Leipold (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Jonathan Wayne Byrd (18) | | | 688,826 | | | | 344,413 | | | | - | | | | 344,413 | | | | * | |
| Jordan Rothstein (18)(74) | | | 110,212 | | | | 55,106 | | | | - | | | | 55,106 | | | | * | |
| Joseph & Francis Simek Family Investments Limited Partnership (6)(75) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Joseph Codi (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Joseph Grassi (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Joseph O. Manzi (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Joseph P. Sullivan (18) | | | 172,208 | | | | 86,104 | | | | - | | | | 86,104 | | | | * | |
| Joseph S. Konowiecki & Miriam B. Konowiecki Family Trust (18)(76) | | | 314,320 | | | | 157,160 | | | | - | | | | 157,160 | | | | * | |
| Joseph Torsiello and Cynthia Torsiello (6)(77) | | | 80,000 | | | | 40,000 | | | | 40,000 | | | | - | | | | - | |
| Judith E. Bailey Revocable Trust dtd 5/8/04, amended 6/2/08 (6)(78) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Juli-Ann Cialone (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Karl David Tritsch (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Kent Tucker Andersen (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Kamran Givpoor (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Karen Crothers (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Karen Katen (18) | | | 482,180 | | | | 241,090 | | | | - | | | | 241,090 | | | | * | |
| Karl H. Wiedenman (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Kathleen L. Rush (79) | | | 438 | | | | - | | | | 438 | | | | - | | | | - | |
| Keith A. Hanson and Irene Hanson (6)(80) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Kenneth E. Normann (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Kenneth Rosenblatt and David Rosenblatt (6) | | | 60,000 | | | | 30,000 | | | | 30,000 | | | | - | | | | - | |
| Kerry Routenberg Love (18)(81) | | | 179,426 | | | | 1,985 | | | | - | | | | 177,441 | | | | * | |
| Selling Stockholder | | Shares of
common stock
beneficially
owned prior to
the Offering | | | Shares of
common
stock owned
prior to this
Offering and
registered
hereby | | | Shares
issuable upon
exercise of
warrants
owned prior to
this Offering
and registered
hereby (1) | | | Shares of
common stock
beneficially
owned upon
completion of
this Offering (2) | | | Percentage
of
Common
Stock
beneficially
owned upon
completion
of this
Offering (3) | |
| | | | | | | | | | | | | | | | | | | | | |
| Kevin Bracken (18) | | | 55,108 | | | | 27,554 | | | | - | | | | 27,554 | | | | * | |
| Kevin J. Koch and Susan M. Koch (6)(82) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Kevin Roe (6) | | | 170,000 | | | | 85,000 | | | | 85,000 | | | | - | | | | - | |
| Kimberly Hanley (83) | | | 6,344 | | | | - | | | | 6,344 | | | | - | | | | - | |
| KT4 Partners, LLC (18)(84) | | | 34,442 | | | | 17,221 | | | | - | | | | 17,221 | | | | * | |
| Larry Caillouet (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Lauren Lung (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Lauren Simek (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Lee Harrison Corbin (6) | | | 180,000 | | | | 80,000 | | | | 100,000 | | | | - | | | | - | |
| Lee J. Seidler Revocable Trust dtd 4/12/1990 (6)(85) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Leon S. Frenkel (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Lewis Pell (18) | | | 482,178 | | | | 241,089 | | | | - | | | | 241,089 | | | | * | |
| Lga Design Corp. (86) | | | 604,106 | | | | 151,346 | | | | - | | | | 452,760 | | | | * | |
| Lincoln Park Capital Fund, LLC (6)(87) | | | 366,600 | | | | 166,600 | | | | 200,000 | | | | - | | | | - | |
| Linda Brooks (18) | | | 68,884 | | �� | | 34,442 | | | | - | | | | 34,442 | | | | * | |
| Lindsey McGrandy (88) | | | 3,340 | | | | - | | | | 3,340 | | | | - | | | | - | |
| Lisa Torsiello (6) | | | 250,000 | | | | 125,000 | | | | 125,000 | | | | - | | | | - | |
| Bell Family Trust dtd 2/2/1195, as amended, Lon E. Bell Trustee (6)(144) | | | 500,000 | | | | 250,000 | | | | 250,000 | | | | - | | | | - | |
| Long Island Auto Realty LLC (6)(89) | | | 40,000 | | | | 20,000 | | | | 20,000 | | | | - | | | | - | |
| Louis Marghella (6) | | | 10,000 | | | | 5,000 | | | | 5,000 | | | | - | | | | - | |
| Mackie Klingbeil (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Mahesh N. Ganmukhi (6) | | | 400,000 | | | | 200,000 | | | | 200,000 | | | | - | | | | - | |
| Mark K. Borca (6) | | | 70,000 | | | | 35,000 | | | | 35,000 | | | | - | | | | - | |
| Mark E. Brooks (18) | | | 68,884 | | | | 34,442 | | | | - | | | | 34,442 | | | | * | |
| Mark L. Eurek (6) | | | 40,000 | | | | 20,000 | | | | 20,000 | | | | - | | | | - | |
| Mark Leopold (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Mark N. Tompkins (90) | | | 1,359,219 | | | | - | | | | 1,200,000 | | | | 159,219 | | | | * | |
| Mark P. Pacchini Revocable Living Trust (6)(91) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Mark S. Whiting Profit Sharing Plan (6)(92) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Martillo Finance Limited (6)(94) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Martin J. Junge (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Martin W. Prinz (95) | | | 188,732 | | | | 24,498 | | | | 85,663 | | | | 78,571 | | | | * | |
| Massachusetts Institute of Technology (18) | | | 209,720 | | | | 104,860 | | | | - | | | | 104,860 | | | | * | |
| Matthew J. Longo (6) | | | 240,000 | | | | 120,000 | | | | 120,000 | | | | - | | | | - | |
| Maxim Jacobs (18) | | | 27,554 | | | | 13,777 | | | | - | | | | 13,777 | | | | * | |
| Meryle Evans Family Trust (6)(96) | | | 300,000 | | | | 150,000 | | | | 150,000 | | | | - | | | | - | |
| Michael B. Weiss (18)(97) | | | 540,990 | | | | 176,836 | | | | 25,511 | | | | 338,643 | | | | * | |
| Michael Crawford (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Michael J. Pierce (6) | | | 400,000 | | | | 200,000 | | | | 200,000 | | | | - | | | | - | |
| Selling Stockholder | | Shares of
common stock
beneficially
owned prior to
the Offering | | | Shares of
common
stock owned
prior to this
Offering and
registered
hereby | | | Shares
issuable upon
exercise of
warrants
owned prior to
this Offering
and registered
hereby (1) | | | Shares of
common stock
beneficially
owned upon
completion of
this Offering (2) | | | Percentage
of
Common
Stock
beneficially
owned upon
completion
of this
Offering (3) | |
| | | | | | | | | | | | | | | | | | | | | |
| Michael P. Ziniti and Alexandra Ziniti (6)(98) | | | 35,000 | | | | - | | | | 35,000 | | | | - | | | | - | |
| Michael Prendergast (6) | | | 20,000 | | | | 10,000 | | | | 10,000 | | | | - | | | | - | |
| Michael Silverman (99) | | | 761,978 | | | | - | | | | 217,800 | | | | 544,178 | | | | 1.0 | % |
| Michael Zimmerman (6) | | | 40,000 | | | | 20,000 | | | | 20,000 | | | | - | | | | - | |
| Mukund Halthore (6) | | | 20,000 | | | | 10,000 | | | | 10,000 | | | | - | | | | - | |
| N. Williams Family Investments Limited Partnership (6)(100) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Nale Developments (Florida) Inc. (6)(101) | | | 100,020 | | | | 50,010 | | | | 50,010 | | | | - | | | | - | |
| Nancy E. Williams (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Nancy E. Williams Multi-Generational Trust (6)(102) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Narendra Kadaba (6) | | | 30,000 | | | | 5,000 | | | | 25,000 | | | | - | | | | - | |
| NCTW Group Limited (18)(103) | | | 68,884 | | | | 34,442 | | | | - | | | | 34,442 | | | | * | |
| Neal Bloom (6) | | | 15,000 | | | | - | | | | 15,000 | | | | - | | | | - | |
| Nicole Rothstein (18)(104) | | | 110,212 | | | | 55,106 | | | | - | | | | 55,106 | | | | * | |
| Nitin Bhatnagar (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Norman Rothstein (18)(105) | | | 1,001,268 | | | | 418,027 | | | | 51,024 | | | | 532,217 | | | | 1.0 | % |
| Northlea Partners LLP (6)(106) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| O. Stuart Chase (6) | | | 20,000 | | | | 10,000 | | | | 10,000 | | | | - | | | | - | |
| Olive Tree Holdings, LLC (6)(107) | | | 500,000 | | | | 250,000 | | | | 250,000 | | | | - | | | | - | |
| Omega Cambridge SPV Fund, L.P. (18)(108) | | | 30,838 | | | | 15,419 | | | | - | | | | 15,419 | | | | * | |
| Opaleye L.P. (6)(109) | | | 1,000,000 | | | | - | | | | 1,000,000 | | | | - | | | | - | |
| Opus Point Healthcare Innovations Fund L.P. (110) | | | 500,000 | | | | - | | | | 500,000 | | | | - | | | | - | |
| Ostuni Family Irrevocable Trust u/d/t 12/23/2011 (18)(111) | | | 115,318 | | | | 32,659 | | | | - | | | | 82,659 | | | | * | |
| Paul Ehrenstein (112) | | | 28,399 | | | | - | | | | 28,399 | | | | - | | | | - | |
| Paul Russo (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Peter C. Gould (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Peter Candito (6) | | | 20,000 | | | | 10,000 | | | | 10,000 | | | | - | | | | - | |
| Peter H. Colettis (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Peter J. Green (18) | | | 185,850 | | | | 80,169 | | | | 12,756 | | | | 92,925 | | | | * | |
| Peter Karp (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Peter Scott Kastner (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Philip Kempisty (113) | | | 28,399 | | | | - | | | | 28,399 | | | | - | | | | - | |
| Philip W. Faucette II (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Polaris Investors, LLC (114) | | | 84,442 | | | | 59,442 | | | | 25,000 | | | | - | | | | - | |
| Pradeep Kaul (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Prathit Holdings (115) | | | 300,300 | | | | - | | | | - | | | | 300,300 | | | | * | |
| President and Fellows of Harvard College (18) | | | 54,786 | | | | 27,393 | | | | - | | | | 27,393 | | | | * | |
| Selling Stockholder | | Shares of
common stock
beneficially
owned prior to
the Offering | | | Shares of
common
stock owned
prior to this
Offering and
registered
hereby | | | Shares
issuable upon
exercise of
warrants
owned prior to
this Offering
and registered
hereby (1) | | | Shares of
common stock
beneficially
owned upon
completion of
this Offering (2) | | | Percentage
of
Common
Stock
beneficially
owned upon
completion
of this
Offering (3) | |
| | | | | | | | | | | | | | | | | | | | | |
| Randall Sebring and Alice Sebring (6)(116) | | | 250,000 | | | | 125,000 | | | | 125,000 | | | | - | | | | - | |
| Raymond Warenik (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Red Top Forever LLC (18)(117) | | | 199,760 | | | | 99,880 | | | | - | | | | 99,880 | | | | * | |
| Revocable Trust Agreement between Roland F. Hartman and Roland T. Hartman dtd 06/05/1998 (6)(118) | | | 60,000 | | | | 30,000 | | | | 30,000 | | | | - | | | | - | |
| Richard A. Salzer and Anca M. Cretan-Salzer (6)(119) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Richard D. Cohen (6) | | | 150,000 | | | | 75,000 | | | | 75,000 | | | | - | | | | - | |
| Richard Gostanian (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Richard Pisacano (6) | | | 5,000 | | | | - | | | | 5,000 | | | | - | | | | - | |
| Richard Earl Stites(6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Rick A. Condrey 18) | | | 146,376 | | | | 73,188 | | | | - | | | | 73,188 | | | | * | |
| Robert Burkhardt (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Robert Crothers (120) | | | 353,299 | | | | - | | | | 353,299 | | | | - | | | | - | |
| Robert G. Curtin (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Robert Holm (6) | | | 20,000 | | | | 10,000 | | | | 10,000 | | | | - | | | | - | |
| Robert McGrath (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Robert Rhea Exempt Family Trust (6)(121) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Robert W. Harrigan (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Robert W. Streett (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Robinson Crothers (122) | | | 15,000 | | | | - | | | | 15,000 | | | | - | | | | - | |
| Roger Barbaro and Laura Barbaro (6)(123) | | | 10,000 | | | | 5,000 | | | | 5,000 | | | | - | | | | - | |
| Roger D. Bozarth (6) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Roman Livson (124) | | | 26,798 | | | | - | | | | 26,798 | | | | - | | | | - | |
| Ronald Bartlett and Valerie Bartlett (6)(125) | | | 160,000 | | | | 80,000 | | | | 80,000 | | | | - | | | | - | |
| Russell S. Dritz (6) | | | 80,000 | | | | 40,000 | | | | 40,000 | | | | - | | | | - | |
| Ryan Konik (126) | | | 4,600 | | | | - | | | | 4,600 | | | | - | | | | - | |
| Sack Family Investment Fund, LLC (6)(127) | | | 200,000 | | | | 100,000 | | | | 100,000 | | | | - | | | | - | |
| Salvatore Iannotta (6) | | | 20,000 | | | | 10,000 | | | | 10,000 | | | | - | | | | - | |
| Scott Cardone (128) | | | 159,153 | | | | 19,582 | | | | 89,996 | | | | 49,575 | | | | - | |
| Scott P. Murray (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Scott Rapfogel(6) | | | 60,000 | | | | 30,000 | | | | 30,000 | | | | - | | | | - | |
| Silver Dice Inc. (129) | | | 428,569 | | | | - | | | | - | | | | 428,569 | | | | * | |
| Square 1 Bank (18) | | | 66,574 | | | | - | | | | 33,287 | | | | 33,287 | | | | * | |
| Stephen A. Dichiara (6) | | | 80,000 | | | | 40,000 | | | | 40,000 | | | | - | | | | - | |
| Stephen P. Ziniti, TTE Stephen P. Ziniti 401k (6)(130) | | | 67,442 | | | | 17,442 | | | | 50,000 | | | | - | | | | - | |
| Stephen Pisacano (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Stephen Renaud (131) | | | 736,978 | | | | 115,609 | | | | 192,800 | | | | 428,569 | | | | * | |
| Steven Rothstein (18)(93) | | | 50,940 | | | | 1,000 | | | | - | | | | 49,940 | | | | * | |
| Selling Stockholder | | Shares of
common stock
beneficially
owned prior to
the Offering | | | Shares of
common
stock owned
prior to this
Offering and
registered
hereby | | | Shares
issuable upon
exercise of
warrants
owned prior to
this Offering
and registered
hereby (1) | | | Shares of
common stock
beneficially
owned upon
completion of
this Offering (2) | | | Percentage
of
Common
Stock
beneficially
owned upon
completion
of this
Offering (3) | |
| | | | | | | | | | | | | | | | | | | | | |
| The M.W. Benedetto Revocable Trust dated 09/14/2012 (18)(132) | | | 55,108 | | | | 27,554 | | | | - | | | | 27,554 | | | | * | |
| The Peierls Bypass Trust (6)(133) | | | 32,000 | | | | 16,000 | | | | 16,000 | | | | - | | | | - | |
| The Peierls Foundation, Inc. (6)(134) | | | 1,270,000 | | | | 635,000 | | | | 635,000 | | | | - | | | | - | |
| Thomas Degirolamo and Dawn Degirolamo (6)(135) | | | 30,000 | | | | 15,000 | | | | 15,000 | | | | - | | | | - | |
| Thomas Ghirardi (6) | | | 40,000 | | | | 20,000 | | | | 20,000 | | | | - | | | | - | |
| Thomas L. Eisenberg (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Thomas Norgiel (6) | | | 175,000 | | | | 75,000 | | | | 100,000 | | | | - | | | | - | |
| Thomas Zahavi (6) | | | 100,000 | | | | 50,000 | | | | 50,000 | | | | - | | | | - | |
| Thore Karlsen (136) | | | 269,915 | | | | 169,915 | | | | 100,000 | | | | - | | | | * | |
| Timothy E. Needham (18) | | | 103,326 | | | | 51,663 | | | | - | | | | 51,663 | | | | * | |
| Timothy Herrmann (137) | | | 91,753 | | | | 11,249 | | | | 52,024 | | | | 28,480 | | | | - | |
| Todd Harrigan (138) | | | 225,758 | | | | 16,046 | | | | 122,463 | | | | 87,249 | | | | * | |
| Trust Under Article III of The Thomas E. Brophy 2004 Grantor Retained Annuity Trust dtd 3/2/04 (6)(139) | | | 299,500 | | | | 149,500 | | | | 150,000 | | | | - | | | | - | |
| UD E.F. Peierls for Brian E. Peierls (6)(140) | | | 58,000 | | | | 29,000 | | | | 29,000 | | | | - | | | | - | |
| UD E.F. Peierls for E. Jeffrey Peierls (6)(140) | | | 58,000 | | | | 29,000 | | | | 29,000 | | | | - | | | | - | |
| UD E.S. Peierls for E.F. Peierls et al (6)(140) | | | 36,000 | | | | 18,000 | | | | 18,000 | | | | - | | | | - | |
| UD Ethel F. Peierls Charitable Lead Trust (6)(140) | | | 126,000 | | | | 63,000 | | | | 63,000 | | | | - | | | | - | |
| UD J.N. Peierls for Brian E. Peierls (6)(140) | | | 80,000 | | | | 40,000 | | | | 40,000 | | | | - | | | | - | |
| UD J.N. Peierls for E. Jeffrey Peierls (6)(140) | | | 80,000 | | | | 40,000 | | | | 40,000 | | | | - | | | | - | |
| UW E.S. Peierls for Brian E. Peierls – Accumulation (6)(140) | | | 52,000 | | | | 26,000 | | | | 26,000 | | | | - | | | | - | |
| UW E.S. Peierls for E. Jeffrey Peierls – Accumulation (6)(140) | | | 32,000 | | | | 16,000 | | | | 16,000 | | | | - | | | | - | |
| UW J.N. Peierls for Brian E. Peierls (6)(140) | | | 68,000 | | | | 34,000 | | | | 34,000 | | | | - | | | | - | |
| UW J.N. Peierls for E. Jeffrey Peierls (6)(140) | | | 68,000 | | | | 34,000 | | | | 34,000 | | | | - | | | | - | |
| Victor G. Kelly (6) | | | 32,000 | | | | 16,000 | | | | 16,000 | | | | - | | | | - | |
| Walter G. Gans (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| Whitehead Institute for Biomedical Research (18)(145) | | | 16,776 | | | | 8,388 | | | | - | | | | 8,388 | | | | * | |
| William A. Crothers, Jr. (6) | | | 80,000 | | | | 40,000 | | | | 40,000 | | | | - | | | | - | |
| William Barthelme (6) | | | 240,000 | | | | 120,000 | | | | 120,000 | | | | - | | | | - | |
| William C. Purdon (6) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
| William C. Sykes (6) | | | 256,000 | | | | 128,000 | | | | 128,000 | | | | - | | | | - | |
| William J. Benedetto (18) | | | 13,778 | | | | 6,889 | | | | - | | | | 6,889 | | | | * | |
| Selling Stockholder | | Shares of
common stock
beneficially
owned prior to
the Offering | | | Shares of
common
stock owned
prior to this
Offering and
registered
hereby | | | Shares
issuable upon
exercise of
warrants
owned prior to
this Offering
and registered
hereby (1) | | | Shares of
common stock
beneficially
owned upon
completion of
this Offering (2) | | | Percentage
of
Common
Stock
beneficially
owned upon
completion
of this
Offering (3) | |
| | | | | | | | | | | | | | | | | | | | | |
| William Jeffrey Kadi (6) | | | 400,000 | | | | 200,000 | | | | 200,000 | | | | - | | | | - | |
| Windham Life Sciences Partners LP (18)(141) | | | 500,628 | | | | 250,314 | | | | - | | | | 250,314 | | | | * | |
| Yuan Chung Yang (18) | | | 68,884 | | | | 34,442 | | | | - | | | | 34,442 | | | | * | |
| Zhaohui Ren (6) | | | 150,000 | | | | 75,000 | | | | 75,000 | | | | - | | | | - | |
| Yury Minkovsky and Eleanora Minkovsky Degirolamo (6)(142) | | | 50,000 | | | | 25,000 | | | | 25,000 | | | | - | | | | - | |
______________________________
*Less than 1%
| (1) | An aggregate of 23,733,192 of the shares of common stock being offered by the selling security holders are issuable upon exercise of common stock purchase warrants. |
| (2) | Assumes all of the shares of common stock to be registered on the registration statement of which this prospectus is a part, including all shares of common stock underlying common stock purchase warrants held by the selling stockholders, are sold in the offering and that shares of common stock beneficially owned by such selling stockholder but not being registered by this prospectus (if any) are not sold. |
| (3) | Percentages are based on the 52,073,481 shares of common stock issued and outstanding as of the Determination Date. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Shares of common stock underlying shares of preferred stock, options or warrants currently exercisable or convertible, or exercisable or convertible within 60 days after the Determination Date are deemed outstanding for computing the percentage of the person holding such shares of preferred stock, options or warrants but are not deemed outstanding for computing the percentage of any other person. |
| (4) | Thomas A. Satterfield, Jr. is the managing investment partner of A.G. Family L.P. and has sole voting and investment power over the shares owned thereby. |
| (5) | Shares beneficially owned consist of shares of Common Stock owned by Mr. Segal prior to the Merger, Agent Warrant Shares and True-Up Shares. Mr. Segal is an affiliate of a broker-dealer which acted as a sub-agent in the PPO. Mr. Segal received the warrants in the ordinary course of business for his own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (6) | Shares beneficially owned consist of PPO Shares. |
| (7) | See “Security Ownership Of Certain Beneficial Owners and Management” for information as to Mr. Rothstein’s beneficial ownership of the Company’s common stock. Mr. Rothstein is a director of the Company and was a director of Enumeral prior to the Merger. Shares being registered consist of PPO Shares. |
| (8) | O. Stuart Chase is the Headmaster Emeritus of Allen Chase Foundation - Special Investment Account and has sole voting and investment power over the shares owned thereby. |
| (9) | Allan Lipkowitz is the trustee of Allan Lipkowitz Revocable Living Trust 8/26/2005 and has sole voting and investment power over the shares owned thereby. |
| (10) | Avi Schron is the managing member of AME Capital Group, LLC and has sole voting and investment power over the shares owned thereby. |
| (11) | Shares beneficially owned consist of Agent Warrant Shares. American Portfolios Financial Services Inc. is a registered broker-dealer which acted as a sub-agent in the PPO. American Portfolios Financial Services Inc. received the warrants as compensation for investment banking services. |
| (12) | Amir Zaghloul and Mona Zaghloul are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (13) | Anders J. Maxwell and Carlene S. Maxwell are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (14) | Andrew Fisher and Melissa Fisher are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (15) | Anthony Liberti and Barbara Liberti are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (16) | Anthony Naer and Mary Naer are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (17) | Anton Bogner and Barbara D. Bogner are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (18) | Shares being registered consist of Enumeral Shares. See “Business - The Merger and Related Transactions—Registration Rights.” |
| (19) | Ashish Jhingan and Dolly Jhingan are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (20) | Joseph Caruso is the President of Bantam Group, LLC and has sole voting and investment power over the shares owned thereby. |
| (21) | Shares beneficially owned consist of Agent Warrant Shares. Mr. Cohn is an affiliate of a broker-dealer which acted as a sub-agent in the PPO. Mr. Cohn received the warrants in the ordinary course of business for his own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (22) | Narender Dev Mantena is the Senior Vice President of Biological E. Limited and has sole voting and investment power over the shares owned thereby. |
| (23) | Consists of warrants to purchase common stock. |
| (24) | Troy Borca is the trustee of Borca Dynasty Trust and has sole voting and investment power over the shares owned thereby. |
| (25) | Brian Fischhoff and Andrea Fischhoff are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (26) | Shaye Hirsch is the director of Brio Capital Master Fund LTD and has sole voting and investment power over the shares owned thereby. |
| (27) | Bruce A. Haverberg and Donna R. Haverberg are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (28) | Sergey Brudanin and Inna Mamuta Haverberg are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (29) | Kevin Carnahan is the trustee of Carnahan Trust and has sole voting and investment power over the shares owned thereby. |
| (30) | Carol Giorello and Anthony Giorello are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (31) | Shares beneficially owned consist of shares of Common Stock held in street name by Cede & Co. prior to the Merger, True-Up Shares held in street name by Cede & Co. |
| (32) | Charles Freeland and Beverly Freeland are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (33) | Charles Medici and Diane Medici are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (34) | Tom Chinn is the general partner of Chinn Family Limited Partnership and has sole voting and investment power over the shares owned thereby. |
| (35) | Jong Tae Choi is the trustee of Choi Family Trust Dated March 15, 2001 and has sole voting and investment power over the shares owned thereby. |
| (36) | Clyde S. McGregor is the trustee of Clyde S. McGregor Revocable Trust dtd 6/6/97 and has sole voting and investment power over the shares owned thereby. |
| (37) | Erik Coover is the trustee of The Coover Multi-Generational Trust and has sole voting and investment power over the shares owned thereby. |
| (38) | Malcolm Currie is the trustee of Currie Family Trust and has sole voting and investment power over the shares owned thereby. |
| (39) | David M. Rickey is the trustee of Dave Rickey & Daughters Foundation Charitable Trust and has sole voting and investment power over the shares owned thereby. |
| (40) | Shares beneficially owned consist of shares of Common Stock owned by Mr. Landskowsky prior to the Merger, Agent Warrant Shares and True-Up Shares. Mr. Landskowsky is an affiliate of a broker-dealer which acted as a sub-agent in the PPO. Mr. Landskowsky received the warrants in the ordinary course of business for his own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (41) | David M. Rickey is the trustee of David M. Rickey Trust dtd 5/8/02 and has sole voting and investment power over the shares owned thereby. |
| (42) | David Stollwerk and Susan S.Stollwerk are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (43) | Adam Passaglia is the manager of Delux Investment Group LLC and has sole voting and investment power over the shares owned thereby. |
| (44) | Diana Pocapalia is the trustee of Diana Pocapalia Trust dated July 8, 2013 and has sole voting and investment power over the shares owned thereby. |
| (45) | Adam Passaglia is the manager of Double Add Investments I LLC and has sole voting and investment power over the shares owned thereby. |
| (46) | Robert Beodle is the President of Due Mondi Investments, LTD and has sole voting and investment power over the shares owned thereby. |
| (47) | Shares beneficially owned consist of Agent Warrant Shares. EFD Capital, Inc. is a registered broker-dealer which acted as a sub-agent in the PPO. EFD Capital, Inc. received the warrants as compensation for investment banking services. |
| (48) | Emanuele Ostuni is a former director of Enumeral. |
| (49) | Shares beneficially owned consist of shares of Common Stock owned by Mr. Rubenstein, Agent Warrant Shares and True-Up Shares. Mr. Rubenstein is an affiliate of a broker-dealer which acted as a sub-agent in the PPO. Mr. Rubenstein received the warrants in the ordinary course of business for his own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (50) | Robert Burke is the General Partner of Four Jr. Investment Ltd. and has sole voting and investment power over the shares owned thereby. |
| (51) | Francesco Ostuni is the brother of Emanuele Ostuni. |
| (52) | Fred Saltzman and Marilyn Saltzman Stollwerk are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (53) | Vivek J. Kulkarni is the trustee of Ganmukhi Irrevocable Trust and has sole voting and investment power over the shares owned thereby. |
| (54) | George Elefther and Karen A. Elefther are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (55) | George Kingsley and Nadine Kingsley are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (56) | Ryan Chan is the Chief Financial Officer of Gibralt Capital Corporation and has sole voting and investment power over the shares owned thereby. |
| (57) | J. Kelly Williams, Jr. is the general partner of Greenover Group LP and has sole voting and investment power over the shares owned thereby. |
| (58) | Gregory J. Borca and Heidi H. Borca are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (59) | See “Security Ownership Of Certain Beneficial Owners and Management” for information as to Harris & Harris Group. Inc.’s beneficial ownership of the Company’s common stock. Shares being registered consist of PPO Shares. |
| (60) | Sruli Weinreb is the Vice President of Helmsbridge Holdings Limited and has sole voting and investment power over the shares held thereby. |
| (61) | Helmut H. Albrecht is the trustee of The Helmut H. Albrecht Trust and has sole voting and investment power over the shares owned thereby. |
| (62) | Howard A. Kalka and Susan Kalka are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (63) | Shares beneficially owned consist of shares of Common Stock owned by Mr. Jacobs prior to the Merger and True-Up Shares. |
| (64) | Shares beneficially owned consist of Agent Warrant Shares. Ms. Young is an affiliate of a broker-dealer which acted as a placement agent in the PPO. Ms. Young received the warrants in the ordinary course of business for her own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (65) | Mr. Love is a Scientific Founder of the Company. See “Directors, Executive Officers, Promoters and Control Persons.” |
| (66) | Jeff Rennell and Christine Kellye Rennell are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (67) | Joel Hochman is the trustee of Joel L. Hochman Revocable Trust UAD 12/8/1994 and has sole voting and investment power over the shares owned thereby. |
| (68) | John A. Cotter and Wendy M. Cotter are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (69) | David Weiss is the trustee of John and Mary Cooper Multigenerational Trust and has sole voting and investment power over the shares owned thereby. |
| (70) | John C. Blazier and Fleur A. Christensen Cotter are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (71) | John P. Funkey is the trustee of John P. Funkey Revocable Trust dtd 2/26/90 and has sole voting and investment power over the shares owned thereby. |
| (72) | Shares being registered consist of PPO Share and Enumeral Shares. See “Business - The Merger and Related Transactions—Registration Rights.” |
| (73) | Jonathan Blatt and Gina Blatt are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (74) | Jordan Rothstein is the son of Allan Rothstein, a director of the Company. Jordan Rothstein is also a registered representative Series 7 and Series 63. |
| (75) | David Weiss is the general partner of Joseph & Francis Simek Family Investments Limited Partnership and has sole voting and investment power over the shares owned thereby. |
| (76) | Joseph S. Konowiecki is the trustee of Joseph S. Konowiecki & Miriam B. Konowiecki Family Trust and has sole voting and investment power over the shares owned thereby. |
| (77) | Joseph Torsiello and Cynthia Torsiello are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (78) | Robert Bailey is the trustee of Judith E. Bailey Revocable Trust dtd 5/8/04, as amended and has sole voting and investment power over the shares owned thereby. |
| (79) | Shares being registered consist of Agent Warrants. Kathleen Rush is an employee of Crone Kline Rinde LLP, which served as legal counsel to the Company prior to the Merger. |
| (80) | Keith A. Hanson and Irene Hanson are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (81) | Kerry Love was a former employee of Enumeral and is the wife of J. Christopher Love, a Scientific Founder of the Company. Shares beneficially owned consist of Enumeral Shares. See “Directors, Executive Officers, Promoters and Control Persons.” |
| (82) | Kevin J. Koch and Susan M. Koch are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (83) | Shares beneficially owned consist of Agent Warrant Shares. Ms. Hanley is an affiliate of a broker-dealer which acted as a sub- agent in the PPO. Ms. Hanley received the warrants in the ordinary course of business for her own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (84) | Marc Abramowitz is the managing member of KT4 Partners, LLC and has sole voting and investment power over the shares owned thereby. |
| (85) | Lee J. Seidler is the trustee of Lee J. Seidler Revocable Trust dtd 4/12/1990, as amended and has sole voting and investment power over the shares owned thereby. |
| (86) | Shares beneficially owned consist of shares of Common Stock owned by Lga Design Corp. prior to the Merger and True-Up Shares. Lori Guarini is the President of Lga Design Corp. and has sole voting and investment power over the shares owned thereby. |
| (87) | Joshua Scheinfeld is the Founder and Managing Member of Lincoln Park Capital Fund, LLC and has sole voting and investment power over the shares owned thereby. |
| (88) | Shares beneficially owned consist of Agent Warrant Shares. Ms. McGrandy is an affiliate of a broker-dealer which acted as a sub-agent in the PPO. Ms. McGrandy received the warrants in the ordinary course of business for her own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (89) | Robert Gomes is the managing member of Long Island Auto Realty LLC and has sole voting and investment power over the shares owned thereby. |
| (90) | Shares beneficially owned includes shares of Common Stock owned by Mr. Tompkins prior to the Merger. .. Based on information provided by Mr. Tompkins in a Schedule 13G filed with the SEC on February 16, 2016. See “Security Ownership Of Certain Beneficial Owners and Management” for information as to Mr. Tompkins’s beneficial ownership of the Company’s common stock. |
| (91) | Mark P. Pacchini is the trustee of Mark P. Pacchini Revocable Living Trust and has sole voting and investment power over the shares owned thereby. |
| (92) | Mark S. Whiting is the trustee of Mark S. Whiting Profit Sharing Plan and has sole voting and investment power over the shares owned thereby. |
| (93) | Steven Rothstein is the brother of Allan Rothstein, a director of the Company. |
| (94) | Paul Conway and N.S. Cairns are the directors of Martillo Finance Limited and have equal voting and investment power over the shares owned thereby. |
| (95) | Shares beneficially owned consist of shares of Common Stock owned by Mr. Prinz prior to the Merger, Agent Warrant Shares and True-Up Shares. Mr. Prinz is an affiliate of a broker-dealer which acted as a sub-agent in the PPO. Mr. Prinz received the warrants in the ordinary course of business for his own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (96) | Steven Evans is the trustee of Meryle Evans Family Trust and has sole voting and investment power over the shares owned thereby. |
| (97) | Shares beneficially owned consist of Enumeral Shares and common stock which may be acquired upon exercise of stock options or warrants which are currently exercisable or which become exercisable within 60 days. Mr. Weiss is a former director of Enumeral. |
| (98) | Michael P. Ziniti and Alexandra Ziniti are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (99) | Shares beneficially owned consist of PPO Shares, Agent Warrant Shares and shares owned by Silver Dice, Inc. (see note 129 below). Mr. Silverman is an affiliate of a broker-dealer which acted as a placement agent in the PPO. Mr. Silverman received the warrants in the ordinary course of business for his own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (100) | James A. Westerfied is the senior vice president of N. Williams Family Investments Limited Partnership and has sole voting and investment power over the shares owned thereby. |
| (101) | Anc Levy is the president of Nale Developments (Florida) Inc. and has sole voting and investment power over the shares owned thereby. |
| (102) | David Weiss is the trustee of Nancy E. Williams Multi-Generational Trust and has sole voting and investment power over the shares owned thereby. |
| (103) | Chih Tang Wang is a director of NCTW Group Limited and has sole voting and investment power over the shares owned thereby. |
| (104) | Nicole Rothstein is the daughter of Allan Rothstein, a director of the Company. |
| (105) | Norman Rothstein was a former consultant to Enumeral and is the father of Allan Rothstein, a director of the Company. |
| (106) | John Abeles is the managing member of Northlea Partners LLP and has sole voting and investment power over the shares owned thereby. |
| (107) | Avi Schron is the managing member of Olive Tree Holdings, LLC and has sole voting and investment power over the shares owned thereby. |
| (108) | Richard Lim is a partner of Omega Cambridge SPV Fund, L.P. and has sole voting and investment power over the shares owned thereby. |
| (109) | James Silverman is the managing director of Opaleye L.P. and has sole voting and investment power over the shares owned thereby. |
| (110) | Michael Weiss is the manager of Opus Point Healthcare Innovations Fund L.P. and has sole voting and investment power over the shares owned thereby. |
| (111) | Megan Ostuni is the trustee of Ostuni Family Irrevocable Trust u/d/t 12/23/2011 and has sole voting and investment power over the shares owned thereby. Emanuele Ostuni, husband of Megan Ostuni and former director of Enumeral, gifted the shares to this trust. |
| (112) | Shares beneficially owned consist of Agent Warrant Shares. Mr. Ehrenstein is an affiliate of a broker-dealer which acted as a placement agent in the PPO. Mr. Ehrenstein received the warrants in the ordinary course of business for his own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (113) | Shares beneficially owned consist of Agent Warrant Shares. Mr. Kempisty is an affiliate of a broker-dealer which acted as a placement agent in the PPO. Mr. Kempisty received the warrants in the ordinary course of business for his own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (114) | Shares being registered consist of PPO Share and Enumeral Shares. See “Business - The Merger and Related Transactions—Registration Rights.”Minor Hinson is the Chief Executive Officer of Polaris Investors, LLCand has sole voting and investment power over the shares owned thereby. |
| (115) | Shares beneficially owned consist of shares of Common Stock owned by Prathit Holdings prior to the Merger and True-Up Shares. Carl Stadelhofer is the President of Prathit Holdings and has sole voting and investment power over the shares owned thereby. |
| (116) | Randall Sebring and Alice Sebring are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (117) | Louis R. Piscatelli is the manager of Red Top Forever LLC and has sole voting and investment power over the shares owned thereby. |
| (118) | Robert F. Hartman is the trustee of Revocable Trust Agreement between Roland F. Hartman and Roland T. Hartman dtd 06/05/1998 and has sole voting and investment power over the shares owned thereby. |
| (119) | Richard A. Salzer and Anca M. Cretan-Salzer are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (120) | Shares beneficially owned consist of Agent Warrant Shares. Mr. Crothers is an affiliate of a broker-dealer which acted as a sub-agent in the PPO. Mr. Crothers received the warrants in the ordinary course of business for his own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (121) | Ida Rhea is the trustee of Robert Rhea Exempt Family Trust and has sole voting and investment power over the shares owned thereby. |
| (122) | Shares beneficially owned consist of Agent Warrant Shares. Mr. Crothers is an affiliate of a broker-dealer which acted as a sub-agent in the PPO. Mr. Crothers received the warrants in the ordinary course of business for his own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (123) | Roger Barbaro and Laura Barbaro are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (124) | Shares beneficially owned consist of Agent Warrant Shares. Mr. Livson is an affiliate of a broker-dealer which acted as a placement agent in the PPO. Mr. Livson received the warrants in the ordinary course of business for his own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (125) | Ronald Bartlett and Valerie Bartlett are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (126) | Shares beneficially owned consist of Agent Warrant Shares. Mr. Konik is an affiliate of a broker-dealer which acted as a sub-agent in the PPO. Mr. Konik received the warrants in the ordinary course of business for his own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (127) | Burton Sack is the managing member of Sack Family Investment Fund, LLC and has sole voting and investment power over the shares owned thereby. |
| (128) | Shares beneficially owned consist of Agent Warrant Shares. Mr. Cardone is an affiliate of a broker-dealer which acted as a placement agent in the PPO. Mr. Cardone received the warrants in the ordinary course of business for his own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (129) | Shares beneficially owned consist of shares of Common Stock owned by Silver Dice Inc. prior to the Merger and True-Up Shares. Mr. Michael Silverman is the sole owner of Silver Dice Inc. and has sole voting and investment power over the shares owned thereby. |
| (130) | Stephen P. Ziniti is the trustee of Stephen P. Ziniti 401k and has sole voting and investment power over the shares owned thereby. |
| (131) | Shares beneficially owned consist of shares of Common Stock owned by Mr. Renaud prior to the Merger, Agent Warrant Shares and True-Up Shares. Mr. Renaud is an affiliate of a broker-dealer which acted as a placement agent in the PPO. Mr. Renaud received the warrants in the ordinary course of business for his own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (132) | M. William Benedetto is the trustee of The M.W. Benedetto Revocable Trust dated 09/14/2012 and has sole voting and investment power over the shares owned thereby. |
| (133) | Brian E. Peierls is the trustee of The Peierls Bypass Trust and has sole voting and investment power over the shares owned thereby. |
| (134) | E. Jeffrey Peierls is the authorized officer of The Peierls Foundation, Inc. and has sole voting and investment power over the shares owned thereby. |
| (135) | Thomas Degirolamo and Dawn Degirolamo are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (136) | Shares being registered consist of PPO Share and Enumeral Shares. See “Business - The Merger and Related Transactions—Registration Rights.” |
| (137) | Shares beneficially owned consist of Agent Warrant Shares. Mr. Herrmann is an affiliate of a broker-dealer which acted as a sub-agent in the PPO. Mr. Herrmann received the warrants in the ordinary course of business for his own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (138) | Shares beneficially include Agent Warrant Shares. Mr. Harrigan is an affiliate of a broker-dealer which acted as a sub-agent in the PPO. Mr. Harrigan received the warrants in the ordinary course of business for his own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. |
| (139) | Karen Brophy is the trustee of Trust Under Article III of The Thomas E. Brophy 2004 Grantor Retained Annuity Trust dtd 3/2/04 and has sole voting and investment power over the shares owned thereby. |
| (140) | E. Jeffrey Peierls is the trustee of UD E.F. Peierls for Brian E. Peierls, UD E.F. Peierls for E. Jeffrey Peierls, UD E.S. Peierls for E.F. Peierls et al., UD Ethel F. Peierls Charitable Lead Trust, UD J.N. Peierls for Brian E. Peierls, UD J.N. Peierls for E. Jeffrey Peierls, UW E.S. Peierls for Brian E. Peierls – Accumulation, UW E.S. Peierls for E. Jeffrey Peierls – Accumulation, UW J.N. Peierls for Brian E. Peierls and UW J.N. Peierls for E. Jeffrey Peierls and has sole voting and investment power over the shares owned thereby. |
| (141) | Adam E. Fine is the Chief Executive Officer of Windham Life Sciences Partners LP and has sole voting and investment power over the shares owned thereby. |
| (142) | Yury Minkovsky and Eleanora Minkovsky Degirolamo are joint tenants and have equal voting and investment power over the shares owned thereby. |
| (143) | David Routenberg is the father of Kerry Routenberg Love, a former employee of Enumeral, and is the father-in-law of John Christopher Love, a Scientific Founder of the Company. |
| (144) | Lon E. Bell is the trustee of Bell Family Trust dtd 2/2/1195, as amended, Lon E. Bell Trustee and has sole voting and investment power over the shares owned thereby. |
| (145) | Laura Sander, the Chief Financial Officer and Treasurer, and Martin Mullins, Vice President, of the Whitehead Institute for Biomedical Research, have shared voting and investment power over the shares owned thereby. |
USE OF PROCEEDS
We will not receive any proceeds from the resale of any of the shares of Common Stock being registered hereby. However, we received $21,549,510 in gross proceeds in the July 2014 private placement, and in the future may receive up to an aggregate of $45,186,979 in additional gross proceeds upon the exercise of the warrants for which shares issuable upon exercise thereof are being registered (to the extent the registration statement of which this prospectus is a part is then effective and, if applicable, the “cashless exercise” provision is not utilized by the holder).
DETERMINATION OF OFFERING PRICE
There currently is a limited public market for our common stock. The selling stockholders will determine at what price they may sell the offered shares, and such sales may be made at prevailing market prices or at privately negotiated prices. See “Plan of Distribution” below for more information.
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Our common stock is currently eligible for quotation and trades on the OTCQB under the symbol “ENUM.” Prior to the Merger, there was very limited trading in the Common Stock, and since the Merger our common stock continues to be thinly traded. The following table sets forth (since the Merger on July 31, 2014) the high and low closing sale prices for our common stock for the fiscal quarter indicated as reported on OTCQB. The quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions.
| Period | | High | | | Low | |
| Fiscal 2016 | | | | | | | | |
| Quarter ending September 30, 2016 (through July 21, 2016) | | $ | 0.21 | | | $ | 0.18 | |
| Quarter ended June 30, 2016 | | $ | 0.24 | | | $ | 0.13 | |
| Quarter ended March 31, 2016 | | $ | 0.29 | | | $ | 0.16 | |
| | | | | | | | | |
| Fiscal 2015 | | | | | | | | |
| Quarter ended December 31, 2015 | | $ | 0.39 | | | $ | 0.22 | |
| Quarter ended September 30, 2015 | | $ | 0.65 | | | $ | 0.36 | |
| Quarter ended June 30, 2015 | | $ | 0.85 | | | $ | 0.57 | |
| Quarter ended March 31, 2015 | | $ | 1.05 | | | $ | 0.70 | |
| | | | | | | | | |
| Fiscal 2014 | | | | | | | | |
| Quarter ended December 31, 2014 | | $ | 1.50 | | | $ | 0.92 | |
| Quarter ended September 30, 2014 (beginning July 31, 2014) | | $ | 2.10 | | | $ | 1.14 | |
Holders
As of July 21, 2016, we had 52,073,481 shares of our common stock issued and outstanding held by approximately 318 stockholders of record.
Securities Authorized for Issuance Under Equity Compensation Plans
The Company’s 2014 Equity Incentive Plan
On July 31, 2014, the Company’s Board of Directors adopted, and the Company’s stockholders approved, the 2014 Equity Incentive Plan (the “2014 Plan”), which reserves a total of 8,100,000 shares of the Company’s common stock for incentive awards.The following table provides information with respect to the 2014 Plan, as well as grants outside of the 2014 Plan, as of December 31, 2015.
The following table provides information with respect to the 2014 Plan, as well as grants outside of the 2014 Plan, as of December 31, 2015:
| Equity Compensation Plan Information |
| Plan Category | | Number of
securities
to be
issued
upon
exercise of
outstanding
options,
warrants
and rights | | | Weighted-
average
exercise
price of
outstanding
options,
warrants
and rights | | | Number of
securities
remaining
available for
future
issuance
under equity
compensation
plans
(excluding
securities
reflected in
column (a)) | |
| Equity compensation plans approved by security holders | | | 5,926,654 | (1) | | $ | 0.62 | | | | 1,399,097 | |
| Equity compensation plans not approved by security holders | | | 309,966 | (2) | | $ | 0.73 | | | | — | |
| Total | | | 6,236,620 | | | $ | 0.63 | | | | 1,399,097 | |
| | (1) | A total of 8,100,000 shares of our common stock were authorized for issuance pursuant to awards under the 2014 Plan as of December 31, 2015, of which 5,926,654 stock options have been awarded to participants under the 2014 Plan and are outstanding as of December 31, 2015. The amount listed above does not include 282,119 shares of restricted stock granted under the 2014 Plan as of December 31, 2015 for which restrictions have not lapsed and 367,224 shares of restricted stock granted under the 2014 Plan for which restrictions have lapsed as of December 31, 2015. |
| | (2) | On April 15, 2014, Mr. Rydzewski and Dr. Tinkelenberg received warrants to purchase 47,058 and 58,823 shares of Enumeral Series B Preferred Stock, respectively, in relation to the executives agreeing to take a temporary salary reduction, as detailed in the Summary Compensation Table above. In connection with the Merger, on July 31, 2014 Mr. Rydzewski’s and Dr. Tinkelenberg’s warrants to purchase Enumeral Series B Preferred Stock were converted into warrants to purchase 137,762 and 172,204 shares of our common stock, respectively. These warrants were granted outside of our 2014 Plan. |
Adjustment for Awards and Payouts:
Unless determined otherwise by the compensation committee or the Board of Directors in the absence of such a committee, unless a participant has received a benefit of ownership such as dividend or voting rights with respect to the incentive award, the following transactions will restore, on a one-for-one basis, the number of shares available for issuance under the Plan:
| 1. | A payout of a stock appreciation right (“SAR”) or a tandem SAR in cash; |
| 2. | A cancellation, termination, expiration, forfeiture or lapse for any reason (with the exception of the termination of a tandem SAR upon exercise of the related options, or the termination of a related option upon exercise of the corresponding tandem SAR) of any award payable in shares; |
| 3. | Shares tendered in payment of the exercise price of an option; |
| 4. | Shares withheld for payment of federal, state or local taxes; |
| 5. | Shares repurchased by the Company with proceeds collected in connection with the exercise of outstanding options; and |
| 6. | The net shares issued in connection with the exercise of SARs (as opposed to the full number of shares underlying the exercised portion of the SAR). |
In addition, the number of shares of our Common Stock subject to the 2014 Plan, any number of shares subject to any numerical limit in the 2014 Plan, and the number of shares and terms of any incentive award are expected to be adjusted in the event of any change in our outstanding our Common Stock by reason of any stock dividend, spin-off, split-up, stock split, reverse stock split, recapitalization, reclassification, merger, consolidation, liquidation, business combination or exchange of shares or similar transaction.
Administration:
The compensation committee of the Board of Directors, or the Board of Directors in the absence of such a committee, will administer the 2014 Plan. Subject to the terms of the 2014 Plan, the compensation committee of the Board of Directors, or the Board of Directors in the absence of such a committee, has complete authority and discretion to determine the terms of awards under the 2014 Plan.
Grants:
The 2014 Plan authorizes the grant by the compensation committee to participants of nonqualified stock options, incentive stock options, restricted stock awards, restricted stock units, performance units and performance shares (which may be designed to comply with Section 162(m) of the Internal Revenue Code (as amended, the “Code”)) and SARs, as described below:
Options granted entitle the grantee, upon exercise, to purchase a specified number of shares from us at a specified exercise price per share. The exercise price for shares of our Common Stock covered by an option cannot be less than the fair market value of our Common Stock on the date of grant. In addition, in the case of an incentive stock option granted to an employee who, at the time the incentive stock option is granted, owns stock representing more than 10% of the voting power of all classes of stock of the Company or any parent or subsidiary, the per share exercise price will be no less than 110% of the fair market value of our Common Stock on the date of grant.
Restricted stock awards and restricted stock units may be awarded on terms and conditions established by the compensation committee, or the Board of Directors in the absence of such a committee, which may include time-based and performance based-conditions for restricted stock awards and the lapse of restrictions on the achievement of one or more performance goals for restricted stock units.
A performance share award and/or a performance unit award may be granted to participants. Each performance unit will have an initial value that is established by the compensation committee, or the Board of Directors in the absence of such a committee, at the time of grant. Each performance share will have an initial value equal to the fair market value of one share of Common Stock on the date of grant. Such awards may be earned based upon satisfaction of certain specified performance criteria, subject to such other terms and conditions as the compensation committee, or the Board of Directors in the absence of such a committee, deems appropriate.
SARs entitle the participant to receive a distribution in an amount not to exceed the number of shares of our Common Stock subject to the portion of the SAR exercised multiplied by the difference between the market price of a share of our Common Stock on the date of exercise of the SAR and the market price of a share of our Common Stock on the date of grant of the SAR. An Option and a SAR may be granted “in tandem” with each other. An option and a SAR are considered to be in tandem with each other because the exercise of the option aspect of the tandem unit automatically cancels the right to exercise the SAR aspect of the tandem unit, and vice versa. The option may be an incentive stock option or a nonqualified stock option.
Change in Control:
Generally upon the occurrence of a Change in Control:
| · | any and all options and SARs granted shall become fully-vested and immediately exercisable; |
| · | any periods of restriction and any restrictions imposed on restricted stock or RSUs which are not intended to qualify for the performance-based exception shall lapse; and |
| · | any award intended to qualify for the performance-based exception under Section 162(m) of the Code shall be earned in accordance with the applicable award agreement. |
For purposes of the 2014 Plan, a “Change in Control” shall be deemed to have occurred as of the first day that any one or more of the following conditions shall have been satisfied:
| · | the “Beneficial Ownership” of securities as defined in Rule 13d-3 under the Exchange Act representing more than thirty-three percent (33%) of the combined voting power of the Company is acquired by any “person” as defined in Section 3(a)(9) of the Exchange Act (other than the Company, any trustee or other fiduciary holding securities under an employee benefit plan of the Company, or any corporation owned, directly or indirectly, by the stockholders of the Company in substantially the same proportions as their ownership of stock of the Company); or |
| · | the consummation of a definitive agreement to merge or consolidate the Company with or into another corporation or to sell or otherwise dispose of all or substantially all of its assets, or adopt a plan of liquidation; or |
| · | during any period of three consecutive years, individuals who at the beginning of such period were members of the Board of Directors cease for any reason to constitute at least a majority thereof (unless the election, or the nomination for election by the Company’s stockholders, of each new director was approved by a vote of at least a majority of the directors then still in office who were directors at the beginning of such period or whose election or nomination was previously so approved). |
Notwithstanding the foregoing, with respect to any incentive award subject to Code Section 409A, a “change in control” of the Company is defined in a manner to insure compliance with Code Section 409A.
Duration, Amendment, and Termination:
The Board of Directors upon recommendation of the compensation committee, has the power to amend, suspend or terminate the 2014 Plan without stockholder approval or ratification at any time or from time to time. No change may be made that increases the total number of shares of our Common Stock reserved for issuance pursuant to incentive awards, materially increase, the benefits accruing to participants or materially modify the requirements for participation in the 2014 Plan, unless such change is authorized by our stockholders. Unless sooner terminated, the 2014 Plan will terminate ten years after it is adopted.
As of July 22, 2016, options to purchase an aggregate of 6,814,841 shares of our Common Stockhave been issued and are outstanding under the 2014 Plan. In addition, as of July 22, 2016, and restricted stock awards totaling 790,253 shares of our Common Stock have been granted under the 2014 Plan. With respect to the restricted stock awards granted under the 2014 Plan, the restrictions lapse on such shares in accordance with the terms of the respective restricted stock award agreements. See “Description of Securities—Options” below for more information.
Dividend Policy
We have never paid any cash dividends on our capital stock and do not anticipate paying any cash dividends on our Common Stock in the foreseeable future. We intend to retain future earnings to fund ongoing operations and future capital requirements. Any future determination to pay cash dividends will be at the discretion of our Board of Directors and will be dependent upon financial condition, results of operations, capital requirements and such other factors as the Board of Directors deems relevant.
DESCRIPTION OF BUSINESS
Overview
We are a biopharmaceutical company focused on discovering and developing novel antibody immunotherapies that help the immune system fight cancer and other diseases. We utilize a proprietary platform technology that facilitates the rapid high resolution measurement of immune cell function within small tissue biopsy samples. Our initial focus is on the development of a pipeline of next generation monoclonal antibody drugs targeting established and novel immuno-modulatory receptors.
The concept of stimulating the immune system to fight cancer was first advanced more than a century ago, but it is only recently that the field of immuno-oncology has seen clinical success, with marketing approvals being granted for antibodies that block CTLA-4 (Yervoy® (ipilimumab)) and PD-1 (Keytruda® (pembrolizumab) and Opdivo® (nivolumab)). Use of these drugs has established that durable anti-tumor responses can be elicited in some patients by blocking the checkpoints that normally suppress the human immune response against cancer cells. The success of these drugs suggests that immuno-oncology may fundamentally alter the course of cancer treatment.
In our lead antibody program, we have characterized certain anti-PD-1 antibodies, or simply “PD-1 antibodies,” using patient biopsy samples, in an effort to identify next generation PD-1 antagonists with enhanced selectivity for the immune effector cells that carry out anti-tumor functions. We have identified two antagonist PD-1 antibodies that inhibit PD-1 activity in different ways. The distinction is that one of the antibodies (ENUM 388D4) blocks binding of the ligand PD-L1 to PD-1, while the other antibody (ENUM 244C8) does not inhibit PD-L1 binding. However, both display activity in various biological assays. In addition to our PD-1 antibody program, we are developing antibody drug candidates for a number of immunomodulatory protein targets, including TIM-3, LAG-3, OX40, TIGIT, and VISTA. We are also pursuing several antibody programs for which we have not yet publicly disclosed the targets.
Our proprietary platform technology, exclusively licensed from the Massachusetts Institute of Technology, or MIT, is a microwell array technology that detects secreted molecules (such as antibodies and cytokines) and cell surface markers, at the level of single, live cells – and enables recovery of single, live cells of interest. The platform technology can be used to achieve at least three separate, but complementary, objectives. First, we use the platform to rapidly produce antibody libraries with high diversity. Second, the platform has the potential to guide rational selection of lead candidates derived from these libraries, through characterization of immune function at the level of single cells from human biopsy samples. Third, it has the potential to identify patients more likely than others to benefit from treatment with a given therapeutic antibody. Thus, our platform is a multipurpose tool that is valuable for activities ranging from antibody discovery to target discovery to patient stratification in clinical development. The platform yields multidimensional, functional read-outs from single live cells, such as tumor infiltrating lymphocytes, or TILs, from human tumor biopsy samples, and it enables us to examine the responses of different classes of human immune cells to treatment with immuno-modulators in the context of human disease, as opposed to animal models of disease. Consequently, we call our approach The Human Approach®.
To date, our proof-of-concept corporate collaborations have provided modest revenues. However,our business has not generated (nor do we anticipate that in the foreseeable future it will generate) the cash necessary to finance our operations. We expect to continue to incur losses and use cash during fiscal year 2016, and we will require additional capital to continue our operations beyond July 2016.
Our near-term capital needs depend on many factors, including:
| · | our ability to carefully manage our costs; |
| · | the amount and timing of revenue received from grants or our collaboration and license arrangements; and |
| · | our ability to raise additional capital through public or private equity offerings, debt financings, or strategic collaborations and licensing arrangements, and/or our success in promptly establishing a strategic alternative that is in our stockholders’ best interests. |
At March 31, 2015, we had cash and cash equivalents totaling approximately $1.8 million, excluding restricted cash.
In June 2016, we entered into a Definitive License and Transfer Agreement with Pieris Pharmaceuticals, Inc. and Pieris Pharmaceuticals GmbH (collectively, “Pieris”), as contemplated in the License and Transfer Agreement that we had entered into with Pieris in April 2016 (the “License Agreement”). Pieris paid us an upfront license fee in the amount of $250,000 in connection with execution of the License Agreement, and paid us a $750,000 license maintenance fee due by May 31, 2016 to continue the licensing arrangements under the License Agreement.
As of the date of this filing, we believe that, with the receipt of the $750,000 maintenance fee from Pieris described above, we now have sufficient liquidity to fund operations through July 2016. However, we will still require additional capital to continue its operations through the third quarter of 2016.We are currently exploring a range of potential transactions, which may includepublic or private equity offerings, debt financings, collaborations and licensing arrangements, and/or other strategic alternatives, including a merger, sale of assets or other similar transactions. If we are unable to preserve or raise additional capital through one or more of the means listed above prior to the end of July 2016, we could face substantial liquidity problems and might be required to implement cost reduction strategies in addition to the cash conservation steps that we have already taken. These reductions could significantly affect our research and development activities, and could result in significant harm to our business, financial condition and results of operations. In addition, we may be required to downsize or wind down our operations through liquidation, bankruptcy, or a sale of our assets.
No assurance can be given that additional financing or strategic alliances and licensing arrangements will be available when needed or that, if available, such financing could be obtained on terms favorable to us or our stockholders. See “Risk Factors.”
Our Strategy
In general, our strategy is to exploit our proprietary platform technology to accelerate the discovery and development of monoclonal antibodies, for the treatment of cancer and other diseases. Key elements of our strategy include:
| | · | Integrating primary cell screening of our high diversity antibody libraries with cellular response profiling – both made possible by our proprietary platform technology. Our platform enables us to screen high-diversity mammalian cell libraries at the single cell level, in antibody discovery; and to examine rare immune cells in human biopsies to understand how different cell types respond to treatment with targeted immuno-modulators. Eventually, our platform may enable us to identify which patients are more likely to benefit from such treatments. We are currently pursuing this approach against a number of immunomodulatory protein targets, including PD-1, TIM-3, LAG-3, OX40, TIGIT, and VISTA. |
| | · | Advancing our PD-1 program (referred to internally as ENUM003). We are conducting pre-clinical characterization of certain PD-1 antibodies, using ex vivo human tissues, with the aim of generating next generation PD-1 antagonists for use in treating cancer. We have identified two anti-PD-1 antibodies that bind to different locations on the PD-1 target. We refer to these antibodies as ENUM 388D4 and ENUM 244C8, or simply 388D4 and 244C8. Our antibody 388D4 is a PD-1 antagonist that blocks binding of PD-L1 to PD-1. Our antibody 244C8 is a PD-1 antagonist that inhibits PD-1 function without blocking binding of PD-L1 to PD-1. We are exploring ways in which one or both of these antibodies might be used to develop desirable new PD-1 antibody-based therapies. We have preliminary evidence that 244C8 activates T cell function in a way distinct from currently marketed anti-PD-1 therapies. Through ex vivo assays performed on tumor biopsy samples, we are exploring the possibility that 244C8 causes stimulation of both adaptive (T cell) and innate (dendritic cell) function. |
| | · | Future Collaborations. We seek to collaborate with companies advancing therapies that might provide superior patient benefit when combined with an immunotherapy. We believe such collaborations will enable us to improve and accelerate product development, regulatory approval, and commercialization of novel immuno-modulators from our pipeline. Such collaborations might involve antibodies, peptide therapeutics, cell therapies, or other therapeutic approaches. In addition, we seek collaborations with key opinion leaders in academic and clinical institutions to conduct preclinical, translational and investigator-sponsored studies involving our proprietary molecules. |
Our Programs
Anti-PD-1 Immunotherapy
Our lead program involves antibodies against the “Programmed Cell Death” receptor, commonly known as “PD-1.” Our PD-1 program has yielded twenty-five families of PD-1 antibodies. We are conducting functional characterization of our lead antibodies, using patient biopsy samples, in an effort to identify next generation PD-1 antagonists with enhanced selectivity for the immune effector cells that have anti-tumor functions.
We have employedex vivo methods to examine how TILs derived from human lung biopsies respond to various PD-1 antibodies. Measurements include production of interferon gamma (IFNγ), which is a cytokine indicative of T cell activation. We have found that approximately half of assayed biopsies harbored TILs responsive to different PD-1 antibodies. We have also found that TILs isolated from lung tumor biopsies that display elevated TIM-3 expression levels have lower responsiveness to the effects of PD-1 antibodies inex vivo assays.
Our two lead PD-1 antibodies, 388D4 and 244C8, both enhance T cell function through reversal of PD-1-dependent immunosuppression, but they appear to act on PD-1 through different mechanisms. Antibody 388D4 blocks binding of PD-L1 to PD-1 (as do the currently-marketed PD-1 antibodies), while 244C8 does not block binding of PD-L1 to PD-1. Using TILs derived from human lung biopsy samples, we have performed experiments comparing the activities of 244C8 and a currently marketed PD-1 antibody, in reversing T cell exhaustion. In some experiments, 244C8 has appeared to restore T cell function to a higher level than did a currently-marketed PD-1 antibody, which we used as a comparator. We have also presented research results in which 244C8 has appeared to elicit cytokine secretion from cell types associated with innate immunity. Results from other experiments are consistent with the observed pattern of cytokine secretion being dependent on the activity of CD40L, a receptor involved in T cell engagement with dendritic cells, which are involved in anti-tumor immunity.
Despite the clinical breakthroughs achieved with the currently marketed PD-1 antibody drugs, OPDIVO® (nivolumab) and KEYTRUDA® (pembrolizumab), those drugs are not effective in all patients, or in all cancers. We hope to develop anti-PD-1 treatments for certain solid tumor indications, where effective therapies do not exist.
The remarkable diversity of PD-1 antibodies obtained by using our platform to screen individual primary B cells from mouse strains that possess fully intact immune systems is illustrated by the dendrogram in Figure 1. Bioinformatics analysis clusters these sequences into “families” of antibodies, based on sequence relatedness. The twenty-five families in the dendrogram are the pool from which our initial candidates have been selected for further testing and optimization. We included published sequences of various commercial and development-stage antibodies in our bioinformatics analysis. This analysis shows that our screening has identified not only antibodies similar to those described previously, but also a large number of less closely related families.
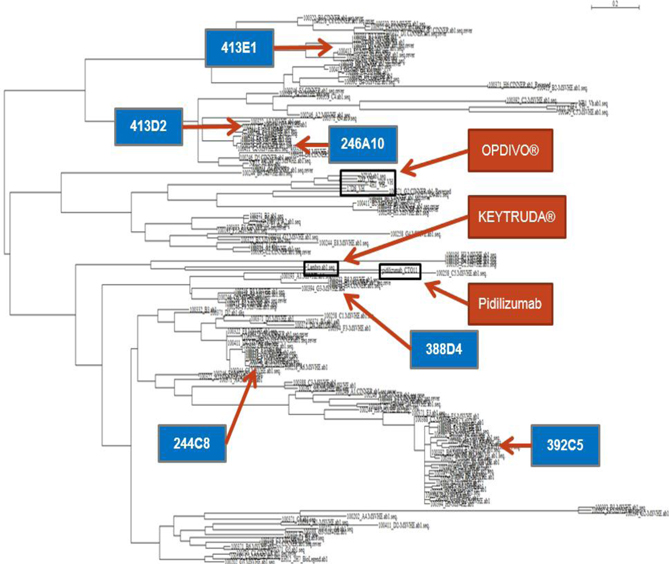
Figure 1: Dendrogram representing discovered sequences and associated “families” of antibodies in our PD-1 program. Data presented are relatedness of different antibodies based on amino acid sequences predicted by recovered variable region heavy chain genes. Sequence identifiers are our internal designations (highlighted by the blue boxes), and additional sequences representing antibodies commercialized or in development with other companies (highlighted by the red boxes).
After DNA cloning and sequence analysis, antibodies selected from different families underwent further analysis. Following recloning into expression constructs and purification, confirmatory testing was performed to determine whether the antibodies bind to PD-1 expressed on the surface of cells. Following selection of antibodies that met rigorous binding criteria, and passed initial characterization, a smaller group of antibodies underwent further testing and selection. Our antibody characterization included standard measurements of biochemical properties, and evaluation in appropriate preclinical studies. Figure 2 shows data for three therapeutic antibody candidates (and one control) binding to cynomolgus monkey PD-1.
ENUM 003 Antibody Binding to NHP PD-1
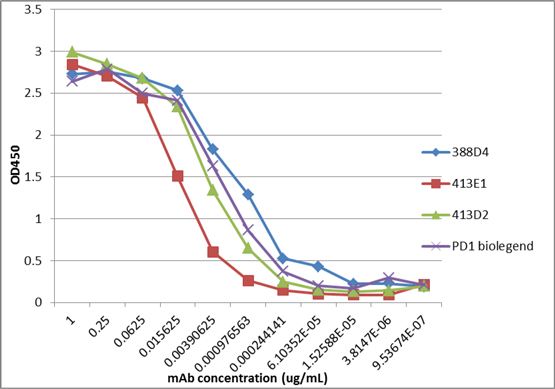
Figure 2: ELISA results showing binding confirmation to cynomolgus monkey PD-1. These measurements are the first step prior to primate toxicity studies.
Figure 3 shows the results of testing of two of our PD-1 antibodies for activity in restoring immune function of human peripheral blood mononuclear cells, or PBMCs, in the presence of PD-L1, a PD-1 ligand. In this test, human PBMCs were activated and then exposed to PD-L1, which binds to PD-1, thereby suppressing immune function of the cells. IFNγ secretion was used as an indicator of immune function. In this particular experiment, PD-L1 treatment caused IFNγ levels to decrease to 14% of the level in the untreated controls. After PD-L1 was added, a therapeutic antibody candidate, or a commercially available anti-PD-1 reagent antibody was added. The number of secreting cells was measured as an index of immune function restoration. In this test, the commercial antibody restored IFNγ secretion to 34%, antibody ENUM A10 restored secretion to 56%, and antibody ENUM C8 restored secretion to 41% of the control level.
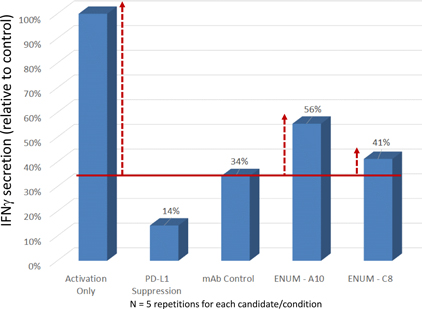
Figure 3: Reversal of PD-L1-based suppression of T cell function.
In order to elucidate mechanisms of action, and to develop a next generation PD-1 antibody, it is important to know not only that the antibody binds to PD-1, but also to understand which cell types the binding modulates in human patients. We believe the ability of our platform to discriminate among cell types can be used to identify candidates that elicit an immune response associated with anti-tumor activity. This is an important advantage of The Human Approach. In addition, we believe that integratingex vivo analysis of human tissues, at the level of single cells, will not only improve our chances of developing best-in-class therapeutics as single agents, but that it also will improve our chances of identifying rational immunotherapy combinations for testing in human clinical trials.
Figure 4 illustrates how data from our platform can be used to classify human cell types in terms of cellular function. Figure 4 is based on measurements of secretory activity of mucosal cells from biopsy tissue, along with the identified lineage of the functional cells. In particular, these data relate to secretion of the effector cell cytokine IL-2, specifically looking at cells expressing PD-1 and TIM-3. These markers are expressed on multiple cell types, and in some cases are co-expressed on the same cell types. This type of information can help improve our understanding of cancer disease mechanisms, and mechanisms of action of anti-cancer drugs. In turn, this understanding can be used to identify which cellular responses are desirable, in the search for improved drugs.
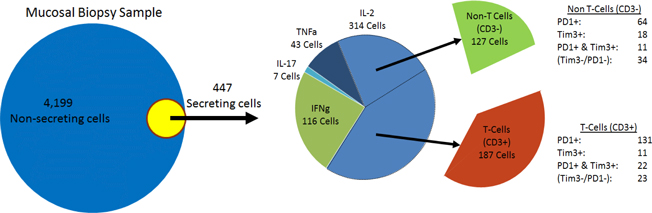
Figure 4: Distribution of cytokine secretion and cell lineage in a human mucosal biopsy sample from a CRC (colorectal cancer) tumor.
In order to test the effect of our two lead PD-1 antibodies on tumor growth, we conducted a study using a patient-derived tumor (PDX) model on humanized NSG (NOD scid gamma) mice, which are engineered to allow forin vivo study of human T cell response. This enabledin vivo testing of our PD-1 antibodies. Seventy-five humanized NSG mice were implanted with a lung tumor PDX derived from a patient with metastatic non-small cell lung carcinoma (LG1306). The mice were randomized into five cohorts (n=12 per group). Dosing and analyses were done according to scheme. As shown in Figure 5 below, 388D4 and 244C8 displayed significantin vivo anti-tumor activity when tested in a non-small cell lung cancer (or NSCLC) PDX model.
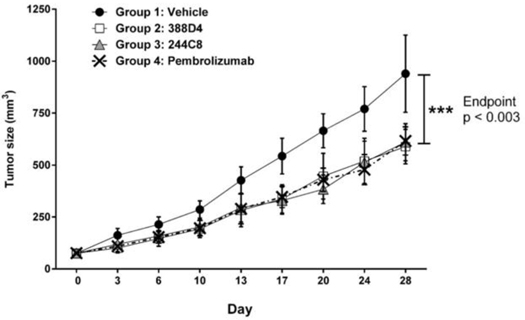
Figure 5: Tumor growth inhibition by ENUM 388D4, ENUM 244C8, pembrolizumab, and vehicle control. Lung tumor PDX (LG1306) growth was measured by tumor volume (error bars = 95% Confidence Interval).
We think The Human Approach can help inform our clinical development plan for an anti-PD1 monotherapy, based on results from our ongoing preclinical evaluations. One possible clinical goal would be to develop a PD-1 inhibitor that will yield response rates higher than those obtained with existing anti-PD-1 therapies, in approved indications. This could be tested in phase Ib/II clinical trials by enrolling Keytruda® and Opdivo® treatment failures among the patients dosed. Alternatively, or concurrently, a PD-1 antibody drug candidate might be developed in new indications with unmet clinical need, where currently marketed PD-1 inhibitors are not being developed.
Figure 6 summarizes results from recent experiments to test theex vivo effect of antibody 244C8 on lung biopsy tissue. Collectively, the data in Figure 6 constitute a cytokine signature that we think is consistent with antibody 244C8 eliciting activation of innate immunity.
Figure 6: Ex vivo cytokine production in NSCLC tumor biopsy consistent with focal activation of both innate and adaptive anti-tumor immunity.
Figure 7 below shows results from a CMV (cytomegalovirus) recall assay involving blockade of PD-1 with antibody 244C8 and blockade of the CD40 ligand with a CD40 ligand antibody. These results are consistent with 244C8-based PD-1 blockade potentiating both T cell activation and antigen presenting cell (APC) activation.
Figure 7: CMV recall assay with 244C8-based PD-1 blockade with or without blockade of CD40 ligand.
We have also conducted experiments in which our anti-PD-1 antibodies appeared to be effective in reversing T cell suppression inex vivo assays. This activity was evident from increased secretion of interferon gamma and increased expression of the IL-2 receptor alpha chain (CD25), both of which are hallmarks of T cell activation. In other studies, individual cells isolated from a human melanoma tumor biopsy were identified and characterized using our cell profiling technology. These cells were selected on the basis of functional properties, and their T cell receptor (TCR) genes were cloned and sequenced. Thus, TCR specific sequences were associated with specific cellular functions at the single cell level.
Anti-TIM-3 Immunotherapy
In early 2015, we initiated screening for antibodies against the “T Cell Immunoglobulin and Mucin Protein 3,” commonly known as TIM-3. This program (designated internally as ENUM005) has yielded 42 families of TIM-3 antibodies, which represent high diversity with respect to sequence relatedness. Our lead selection work in this program is ongoing. Figure 8 below is a dendrogram that illustrates the sequence diversity of our TIM-3 antibodies, in relation to each other, and in relation to TIM-3 sequences published by third parties. We have chosen clones from ten different families for scale-up. In addition to biophysical characterization, we are conducting functional characterization of potential lead antibodies, in cell based assays.
Figure 8: Dendrogram representing discovered sequences and associated “families” of antibodies in our TIM-3 program. Data are represented in terms of relatedness of different antibodies, based on amino acid sequences predicted by recovered variable region heavy chain genes. Sequence identifiers are our internal designations.
Figure 9 below shows the results of an experiment to measure human T cell stimulation by five of our TIM-3 antibodies, following suboptimal CD3/CD28-based stimulation of human donor PBMCs. In this experiment, the tested antibodies displayed greater potency than a commercial reagent TIM-3 antibody (F38-2e2), as well a negative (isotype) control mIgG1.
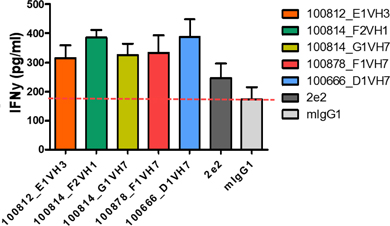
Figure 9: Stimulation of T cell activation by Enumeral TIM-3 antibodies.
Figure 10 below shows the results of anex vivo experiment performed with biopsy material from a human NSCLC tumor containing TILs that were refractory to (not activated by) PD-1 blockade. The Enumeral TIM-3 antibody tested here displayed activity in restoring T cell effector function to the PD-1 blockade-refractory TILs.
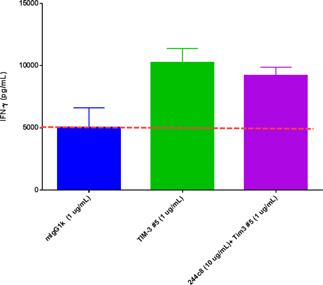
Figure 10: Enumeral TIM-3 antibody activates PD-1 blockade-refractory NSCLC TILsex vivo.
Our future plans for our PD-1 and TIM-3 programs, as well as our other antibody programs described below, will depend on our ability to raise additional capital through public or private equity offerings, debt financings, or strategic collaborations and licensing arrangements, and/or our success in promptly establishing a strategic alternative that is in our stockholders’ best interests.
Other Antibody Programs
In addition to our PD-1 program (ENUM003) and TIM-3 program (ENUM005), we have several other antibody programs, including OX40 (ENUM004), LAG-3 (ENUM006), VISTA (ENUM007), and TIGIT (ENUM009). We are also pursuing several antibody programs for which we have not yet publicly disclosed the targets.
| | · | LAG-3 (ENUM006). LAG-3 (also known as CD223) is believed to be a negative immunomodulatory receptor expressed on T cells and NK cells that mediate tumor lysis. Our objective for the ENUM006 program is to generate therapeutic antibody candidates that block the interaction of LAG-3 on effector cells with cognate ligand in tumors. We initiated screening for ENUM006 in early 2015, and we have isolated the immunoglobulin (Ig) genes from antigen positive B cells. |
| | · | OX40 (ENUM004). OX40 is believed to be a key activating immunomodulatory receptor expressed on CD8+ T cells, NK cells, NK-T cells and neutrophils. Our goal for the ENUM004 program is to identify therapeutic antibody candidates that can enhance activation of immune effector cells. We initiated screening for ENUM004 in the first quarter of 2015, and isolated Ig genes from recovered B cells. We are currently conducting a second immunization with a cell-based antigen presentation. |
| | · | VISTA (ENUM007). VISTA (also known as PD-L3) is believed to be a negative immunomodulatory receptor expressed on lymphoid and non-lymphoid cells. Our objective for the ENUM007 program is to generate therapeutic antibody candidates that block the interaction of VISTA with cognate ligand on effector T cells. We initiated screening for ENUM007 in 2015, and screening efforts are ongoing. |
| | · | TIGIT (ENUM009). TIGIT is believed to be a negative immunomodulatory receptor expressed on lymphoid cells. Our objective for the ENUM009 program is to generate therapeutic antibody candidates that can block the interaction of TIGIT (on NK or T cells) with cognate ligand on accessory cells. We have completed an immunization with a cell-based antigen presentation. |
Our Proprietary Platform Technology
In our drug discovery programs, our platform enables us to use primary cells from fully intact immune systems in various strains of mice (or other species) for antibody screening. As discussed in the Antibody Discovery section below, using primary cells from a fully intact immune system provides a significant advantage over established technologies such as phage display and mouse strains genetically engineered to produce human antibodies, because those technologies place certain limits on recovery of functional antibody diversity.
Our platform is built around a proprietary microwell array chip, which contains 84,672 spatially addressable sub-nanoliter microwells. Our microwell array chips are manufactured exclusively for us by a third-party provider, using custom molds and industrialized processes. In conjunction with the microwell array chips, we employ commercial laboratory equipment and proprietary software to create an integrated system.
Using this system, we can analyze cells from various sources, including human peripheral blood mononuclear cells (PBMCs), bone marrow, tumor biopsy, mucosal biopsy, and cerebrospinal fluid (CSF). Figures 11A and 11B below illustrate the three major elements of our platform:
| | 1) | Cell culture or cell handling, which includes preparation (or dissociation) of cells from primary tissue samples. Our system generally avoids complex cell handling processes. This provides for rapid measurements involving fragile, individual live cells (element 2, below), which can be difficult with technologies other than ours. |
| | 2) | Measurement of cellular functioning at the level of single cells, including independent identification of cell surface markers and secreted proteins, such as antibodies and cytokines; and |
| | 3) | Retrieval of individual cells, after which they can be subjected to molecular analyses such as reverse transcription polymerase chain reaction, or RT-PCR, RNA sequencing, and other molecular biological methods. These data link cell type (surface markers), and cell function (identified by secretion), to underlying genomic information. We believe that without our platform, this linkage of information at the single cell level would be difficult or impossible to achieve with cell-limited biopsy material from human patients. |
The microwells containing individual cells allow for rapid and sensitive detection of secreted factors, and eliminate the need for common procedures such as cell proliferation (for example, Enzyme-Linked ImmunoSpot Assay, or ELISPOT), cell fusion (hybridoma techniques), and cell sorting and enrichment (such as fluorescence activated cell sorting, or FACS, and B cell cloning), all of which can bias results.
Cell Loading
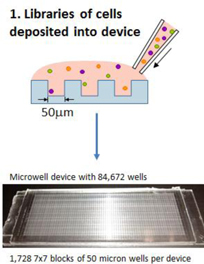 | 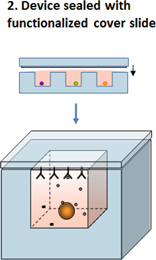 |  |
| Simple pipetting step achieves desired cell density, e.g. single cell occupancy | Secreted proteins bind to capture antibodies on cover slide | Spatially-registered custom protein microarray allows identification of cells with desired functionality for recovery |
Figure 11A: Platform Overview
Cells are loaded directly on the microwell array without manipulations that could lead to loss of cellular function or viability. This process effectively preserves the entire original population of cells for analysis. Loading is rapid, which is critical for analysis of live primary cells derived from limited biopsy materials. A glass cover slide treated with commercially available capture reagents specific to secreted proteins of interest is sealed across the top of the chip. Multiple capture reagents can be used at the same time. We routinely analyze four secreted factors in parallel, across the entire cell population. The sealed chip is incubated (usually between 2 and 6 hours). Proteins secreted in each microwell during the incubation period are capturedon the slide. This yields a spatially registered protein array corresponding to each position (microwell) on the chip.
Analysis & Recovery
Figure 11B: Platform Overview
The cover slide is treated with conventional fluorescently-labeled detection reagents, and array scanners are used to identify proteins secreted from individual cells. In parallel, the cells are labeled with reagents specific for cell surface protein markers and examined through high-speed multiplexed imaging cytometry to determine cell lineage. Data are integrated to produce independent, unbiased measurements of lineage and function for the cells within each microwell. Based on this information, cells are retrieved by automated micromanipulation. Ongoing research and development efforts aimed at automation of all steps in our platform process are currently being supported by our Phase II research contract from the National Cancer Institute.
Antibody Discovery
Our antibody discovery programs exploit our proprietary, high diversity antibody libraries by screening primary cells from murine tissues such as splenocytes or bone marrow cells, following target-specific immunizations that are performed exclusively for us by a third party. In contrast to the limited diversity of human antibodies from transgenic mouse platforms, we employ multiple mouse strains with intact, fully diverse murine immune systems, and we use multiple adjuvants during our immunization campaigns. This avoids loss in the natural diversity of the antibodies produced in the mouse for antibody screening and selection. Our platform enables direct screening of antibody-secreting cells without any requirement for intervening cell fusion, enrichment or sorting. This direct screening enables the exceptional degree of antibody diversity provided by our system.
In our screening process, after secretion and capture of the antibodies, we measure the isotype, amount secreted, target specificity, and relative target affinity. This information is used to derive a priority list for retrieval of individual cells that secreted the antibodies. Cells are retrieved using an automated commercial system customized to our platform and the genes encoding the secreted antibodies are cloned through single cell RT-PCR protocols which we have optimized. Our standard procedures can result in approximately 80% recovery of paired heavy-chain and light-chain genes. Figure 12 illustrates the binding of a secreted antibody to a functionalized cover slide in the context of antibody discovery.
Figure 12: Detection of antibody secretion events from single cells.
Figure 13 below illustrates the detection of secreted antibodies specific for a target of interest captured on the cover slide, showing parallel detection of both antibody (Ig+) and target (Ag+) positive signals, indicating cells producing antibodies which bind to the target of interest.
Figure 13: Antibodies secreted from single cells screened for binding to an antigen target.
Each colored square in the photo micrographs at right is 50 microns on each side.
The design of our proprietary microwell array provides for spatial registration of the secreted antibodies with the microwell containing the secreting cells. This enables retrieval of specific cells of interest. After cloning and sequencing of the antibody genes, bioinformatics analysis enables classification of the antibodies into “families.”
Cellular Response Profiling
Cellular response profiling is what we call our analysis of human tissue samples to measure specific functions of individual cells, and to elucidate how cells in the human immune system respond to a given set of circumstances. As illustrated in Figure 14, we can measure the frequency and functional status of tumor-infiltrating lymphocytes to generate a functional immune “signature.” Such signatures might be generated, for example, using biopsies from patients that have responded to a particular treatment, and compared to signatures generated using biopsies from non-responders. This primary biopsy-based approach also can be used to compare among a series of antibody drug candidates, to profile the immune responses they elicitex vivo, and to facilitate selection of those with the best immune modulation properties. Single-cell functional profiling enables simultaneous and unbiased analysis of cellular parameters from individual cells within the same population of cells, including antibodies, cytokines, other proteins, and cell lineage.
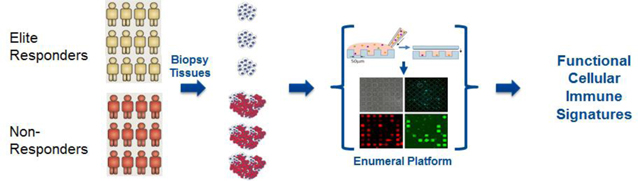
Figure 14: Profiling of human tissue samples for immunological signatures.
Certain of our methods for profiling of immune cells in the tumor microenvironment were developed under a Phase I Small Business Innovation Research contract with the National Cancer Institute in 2012. These Phase I efforts focused on characterizing the performance of our platform technology and generation of multiplexed immunological data from mucosal biopsy samples. We generated data from the primary human tissue (gut mucosa) of patients with colorectal cancer (CRC). In these studies, we examined diseased tissue (tumor), as well as normal (adjacent) tissue from each patient studied. The data demonstrated that matched normal and tumor tissues showed similar secretion levels of immunoglobulin (Ig), and notable differences in the secretion of certain cytokines.
We believe that there are measurable differences in how cells in the human immune system respond to different antibodies, even when those antibodies bind the same target (e.g., PD-1). A composite of those differences, as measured at the level of single cells, is what we call a cellular response profile. The differences in cellular response profiles may translate into differences in how certain patients respond to certain therapies.
Cellular response profiling is a versatile method that can be employed in various ways. Cellular response profiling complements our antibody programs. Once a desired cellular response profile has been defined, testing of human tissue samples on our platform can support either (or both) of two different courses of action: (1) screening for patients with tumors that display a cellular response profile indicating suitability for treatment with a particular drug; or (2) screening for antibodies that elicit a desired cellular response profile in samples from different patient subpopulations,i.e., screening for an antibody that produces higher initial response rates. Whether screening antibodies or screening patients, the concept and principles are the same. Accumulated data from cellular response profiling may lead to valuable insight into details of the mechanisms of action of different antibodies in different tissues or disease indications. In turn, this may guide selection of particular drug combinations for testing in clinical trials. These points are illustrated by our studies usingex vivo TIL analysis in our PD-1 and TIM-3 programs, as described above.
Collaborations
Merck
In December 2014, we entered into a collaboration with Merck Sharp & Dohme Corp., or Merck. Pursuant to our study agreement with Merck, we are conducting a specified research program using our platform technology to identify functional response of single cell types in colorectal cancer in the presence or absence of Merck’s proprietary immunomodulatory receptor (IMR) modulators. In February 2016, we and Merck subsequently amended the work plan under the study agreement to include non-small cell lung cancer tissue samples.
In this collaboration, Merck is reimbursing us for the cost of performing the work specified in the agreement, for up to a specified number of full time employees, at an agreed annual rate. In addition, Merck will make certain milestone payments to us upon the completion of specified objectives set forth in the Merck agreement and related work plan. In September 2015, we announced the achievement of the first milestone. Merck retains ownership of the results generated from the studies on the IMR modulators identified by Merck, and we retain a royalty-free, non-exclusive, non-sublicensable license to use the study results for our own internal research purposes.
National Cancer Institute (NCI)
We previously completed a Phase I research contract with the NCI to analyze B- and T-cells (at the single cell level) in parallel with cell surface markers in both diseased and healthy intestinal tissue samples from patients with colorectal cancer. This work demonstrated our capabilities in measuring samples with limited numbers of cells, such as pinch biopsies. Our final report was accepted in November 2013.
The Phase I contract provided for funding the development of assays and capabilities that our scientific team continues to use in our internal programs. An example of this is profiling human mucosal tissues, which we believe will be highly relevant in cancer immunotherapy development and, potentially, in autoimmune and inflammatory diseases as well. These capabilities may provide the basis for other collaborations.
In September 2014, we were awarded a Phase II Small Business Innovation Research contract from the NCI for up to $999,967 over two years. As part of this Phase II contract, we are developing and deploying an automated prototype system for human tissue immuno-oncology profiling at the Ragon Institute of Massachusetts General Hospital, MIT and Harvard University and also at Memorial Sloan Kettering Cancer Center. At the Ragon Institute, we are collaborating with the laboratory of Douglas S. Kwon, M.D., Ph.D., whose research focuses on the immunology of mucosal surfaces and tissues, which are home to between sixty and ninety percent of the human body’s lymphocytes. At Sloan-Kettering, we are collaborating with the laboratory of Jedd D. Wolchok, M.D., Ph.D., who played an important role in the clinical development of Yervoy® (ipilimumab) and Opdivo® (nivolumab).
M.D. Anderson
In January 2016, we entered into a collaborative research and development agreement with The University of Texas M.D. Anderson Cancer Center, or MDACC. Under this agreement, we and MDACC plan to collaborate on the discovery and development of novel monoclonal antibodies against selected targets in immuno-oncology, utilizing our antibody discovery and immune profiling platform and MDACC’s preclinical and development expertise and infrastructure.
We and MDACC will share the costs of research and development activities necessary to take development candidates through successful completion of a Phase I clinical trial. The agreement provides for a structure whereby we and MDACC are each granted the right to receive a percentage of the net income from product sales or any payments associated with licensing or otherwise partnering a program with a third party.
The agreement contemplates that, in conjunction with the research and development activities, either we or MDACC will seek to enter into licensing transactions with third parties to engage in development or commercialization activities involving the identified antibodies, subject to approval by the other party. We and MDACC also have the option of continuing collaboration efforts into advanced development and commercialization, with one party taking the lead, and both parties sharing costs equally.
We and MDACC each has the right to opt out and cease further funding of future collaboration activities with respect to a collaboration antibody at specified stages, provided that a party which elects to opt out may continue to be responsible for certain expenses incurred prior to such election. A party that exercises its opt out right will also have its percentage of net income from product sales or payments associated with such collaboration antibody program adjusted in accordance with the terms of the agreement.
Pieris
In April 2016, we entered into a License and Transfer Agreement (the “License Agreement”) with Pieris Pharmaceuticals, Inc. and Pieris Pharmaceuticals GmbH (collectively, “Pieris”). Pursuant to the terms and conditions of the License Agreement, Pieris is licensing from us specified intellectual property related to our anti-PD-1 antibody program ENUM 388D4 for the potential development and commercialization by Pieris of novel multispecific therapeutic proteins comprising fusion proteins based on Pieris’ Anticalins® class of therapeutic proteins and our antibodies in the field of oncology. In June 2016, we entered into a Definitive License and Transfer Agreement (the “Definitive Agreement”) with Pieris, as contemplated in the License Agreement. Pieris paid us an upfront license fee in the amount of $250,000 in connection with execution of the License Agreement, and paid us a $750,000 license maintenance fee due by May 31, 2016 to continue the licensing arrangements under the License Agreement. In accordance with its terms, the Definitive Agreement supersedes the License Agreement.
Under the Definitive Agreement, we have granted Pieris an option until May 31, 2017 to license specified patent rights and know-how of ours covering two additional undisclosed antibody programs on the same terms and conditions as for our 388D4 anti-PD-1 antibody (each, a “Subsequent Option”). Pieris may exercise the Subsequent Antibody Options separately and on different dates during the Option Period. Pieris will pay us additional license fees in the event that Pieris exercises one or both Subsequent Options.
Intellectual Property
From MIT, we have in-licensed a patent portfolio (which includes patents owned by Harvard or co-owned by MIT and The Whitehead Institute, or MIT and Massachusetts General Hospital) that we refer to as the Platform Portfolio. It broadly covers our platform technology in the United States, and also covers certain aspects of it in certain foreign countries. The license, referred to as the Platform License, gives us exclusive worldwide rights under patents and patent applications covering the platform technology. The Platform License provides us with worldwide rights under the Platform Portfolio, in all fields of use. This enables us to use the platform in drug discovery and development (including partnered programs), and the right to commercialize its application for purposes such as diagnostics. The Platform License obligates us to achieve certain diligence milestones within certain time frames.
The Platform Portfolio protects our platform technology through eight issued U.S. patents that cover various aspects of the platform technology, including the microarray apparatus, microarrays with micro-channels, a method of capturing products secreted from single cells, a method of making a microarray of secreted antibodies, microarray methods for screening antibodies, single cell cytotoxicity assays, methods of immuno-profiling, and methods of performing RT-PCR from single cells. There are also corresponding issued patents or pending applications in certain foreign jurisdictions. The basic patent coverage of our platform technology in the United States will expire in 2027. The U.S. patent on microarrays with micro-channels will expire in 2029.
We have begun to build our own patent portfolio to protect our novel antibodies and other inventions. In December 2014, we filed two provisional patent applications of which we are the sole owners. One application covers a method of using our platform for rapid identification of compounds that elicit specific, desired cellular response profiles. The second application covers novel PD-1 antibodies (and their therapeutic use), which were identified using our platform, and tested in cell-based assays. The application covers 26 individual heavy chain variable sequences and 27 individual light chain variable sequences, as well as matched pairs of heavy and light chains. Both of these provisional applications were updated and re-filed as non-provisional applications in December 2015. In addition, in March 2016 we filed a new provisional patent application that covers our TIM-3 antibodies. As we discover additional antibodies against selected therapeutics targets, we plan to file patent applications covering those antibodies and their therapeutic uses. We also plan to file patent applications that cover novel research tools and methods that we discover, and any improvements that we make in the platform technology. For example, in March 2016, we filed a new patent application covering certain small accessories that we designed to facilitate processing and imaging of microwell arrays. In addition to filing and prosecuting patent applications in the United States, we plan to file counterpart applications in Australia, Canada, Europe, Japan, and additional jurisdictions, in cases where we think such filings are likely to be cost-effective and important to our business objectives.
For some aspects of our proprietary technology, trade secret protection is more suitable than patent protection. For example, certain proprietary bioinformatics software, methods and databases, which enable us to store, analyze and interpret the large volume of data generated from our platform technology, are protected as trade secrets.
Many pharmaceutical companies, biotechnology companies and academic institutions are competing with us in the field of immunotherapy and oncology, and they have obtained, or may obtain in the future, patents potentially relevant to our business. In order to identify and mitigate the risk of third party intellectual property conflicts, we conduct freedom-to-operate studies, and where appropriate, we obtain opinion of counsel, as an ongoing part of our business operations. We are aware of certain third party patents that contain broad claims potentially relevant to certain therapeutic uses of our PD-1 antibodies. We also are aware of certain third party patents that contain claims potentially relevant to other antibodies (against other targets) in our pipeline, and certain uses of those antibodies. Based on our analyses, if any claims in these patents were asserted against us, we do not believe our activities would be found to infringe any valid claim.
From time to time, we may find it necessary or prudent to obtain licenses from third party patent owners. Where licenses are available at reasonable cost, such licenses are considered a normal cost of doing business. In other instances, we may use the results of our freedom-to-operate studies to guide our early stage research away from areas where we are likely to encounter obstacles in the form of third party intellectual property. We strive to identify potential third party intellectual property issues in the early stages of research in our programs in order to minimize the cost and disruption of resolving such issues. In some cases, otherwise potentially relevant third party patents will expire before marketing approval by the U.S. Food and Drug Administration is likely to have been granted. In such instances, under U.S. law, our pre-clinical and clinical activities are exempt from claims of patent infringement. This exemption is sometimes called the “safe harbor” provision of U.S. law.
The Merger and Related Transactions
Overview
We were incorporated in Nevada as Cerulean Group, Inc. on February 27, 2012, and converted to a Delaware corporation on July 10, 2014. Our original business was to develop and operate a website for self-travelers and backpackers that would allow a person with access to the Internet to build an itinerary and plan a trip. Prior to the Merger (as defined below), our Board of Directors determined to discontinue operations in this area to seek a new business opportunity. As a result of the Merger, we acquired the business of Enumeral and changed our name to Enumeral Biomedical Holdings, Inc.
Our authorized capital stock currently consists of 300,000,000 shares of common stock, par value $0.001 (the “Common Stock”), and 10,000,000 shares of “blank check” preferred stock, par value $0.001. Our Common Stock is quoted on the OTC Markets (OTCQB) under the symbol “ENUM,” which changed from “CEUL” on July 21, 2014.
Enumeral was incorporated on December 11, 2009 under the laws of the State of Delaware.
On July 25, 2014, we completed a 4.62-for-1 forward split of our Common Stock in the form of a dividend, with the result that the 6,190,000 shares of Common Stock outstanding immediately prior to the stock split became 28,597,804 shares of Common Stock outstanding immediately thereafter. All share and per share numbers in this Prospectus relating to our Common Stock have been adjusted to give effect to this stock split, unless otherwise stated.
On July 31, 2014 (the “Closing Date”), our wholly owned subsidiary, Enumeral Acquisition Corp. (“Acquisition Sub”) merged with and into Enumeral (the “Merger”). Enumeral was the surviving corporation in the Merger and became our wholly owned subsidiary. All of the outstanding Enumeral stock was converted into shares of our Common Stock, as described in more detail below.
Upon the closing of the Merger and under the terms of a split-off agreement and a general release agreement (the “Split-Off Agreement”), we transferred all of our pre-Merger operating assets and liabilities to our wholly-owned special-purpose subsidiary, Cerulean Operating Corp. (“Split-Off Subsidiary”). Thereafter, pursuant to the Split-Off Agreement, we transferred all of the outstanding shares of capital stock of Split-Off Subsidiary to our pre-Merger majority stockholder, and our former sole officer and director (the “Split-Off”), in consideration of and in exchange for (i) the surrender and cancellation of an aggregate of 23,100,000 shares of our Common Stock held by such stockholder (which were cancelled and resumed the status of authorized but unissued shares of our Common Stock) and (ii) certain representations, covenants and indemnities.
As a result of the Merger and transactions effected pursuant to the Split-Off Agreement, we discontinued our pre-Merger business and acquired the business of Enumeral, and will continue the existing business operations of Enumeral as a publicly-traded company under the name Enumeral Biomedical Holdings, Inc. On the Closing Date, we changed our fiscal year from a fiscal year ending on October 31 of each year to one ending on December 31 of each year, which is the fiscal year end of Enumeral.
Also on the Closing Date, we closed a private placement offering (the “PPO”) of 21,549,510 Units of our securities, at a purchase price of $1.00 per Unit, each Unit consisting of one share of our Common Stock and a warrant to purchase one share of Common Stock at an exercise price of $2.00 per share with a term of five years (the “PPO Warrants”). Additional information concerning the PPO and PPO Warrants is presented below under “—The Merger and Related Transactions—the PPO” in this Section.
Merger Agreement
On the Closing Date, Enumeral Biomedical, Acquisition Sub and Enumeral entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”), which closed on the same date. Pursuant to the terms of the Merger Agreement, Acquisition Sub merged with and into Enumeral, which was the surviving corporation and thus became our wholly-owned subsidiary.
Pursuant to the Merger, we acquired the business of Enumeral to discover and develop novel therapeutics known as immunomodulators or immunotherapies that help the human immune system attack diseased cells. (See “Description of Business” above.)
At the closing of the Merger (a) each share of Enumeral’s common stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.102121 shares of our Common Stock, (b) each share of Enumeral’s Series A Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.598075 shares of our Common Stock, (c) each share of Enumeral’s Series A-1 Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.790947 shares of our Common Stock, (d) each share of Enumeral’s Series A-2 Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.997594 shares of our Common Stock, (e) each share of Enumeral’s Series B Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 2.927509 shares of our Common Stock, and (f) a convertible note was converted into 3,230,869 shares of our Common Stock. As a result, an aggregate of 22,700,645 shares of our Common Stock were issued to the holders of Enumeral’s stock.
In addition, pursuant to the Merger Agreement:
| · | warrants to purchase 694,443 shares of Enumeral’s common stock issued and outstanding immediately prior to the closing of the Merger were converted into warrants to purchase shares of our Common Stock at a conversion ratio of 1.102121 for one; |
| · | warrants to purchase 41,659 shares of Enumeral’s Series A Preferred Stock issued and outstanding immediately prior to the closing of the Merger were converted into warrants to purchase shares of our Common Stock at a conversion ratio of 1.598075 for one; |
| · | warrants to purchase 144,140 shares of Enumeral’s Series B Preferred Stock issued and outstanding immediately prior to the closing of the Merger were converted into warrants to purchase shares of our Common Stock at a conversion ratio of 2.927509 for one; and |
| · | options to purchase 948,567 shares of Enumeral’s common stock issued and outstanding immediately prior to the closing of the Merger were converted into options to purchase shares of our Common Stock at a conversion ratio of 1.102121 for one. |
As a result, warrants to purchase an aggregate of 1,253,899 shares of our Common Stock and options to purchase an aggregate of 1,045,419 shares of our Common Stock were issued in connection with the Merger.
The Merger Agreement provided certain anti-dilution protection to our Common Stock holders immediately prior to the Merger (after giving effect to the Split-Off), in the event that the aggregate number of Units sold in the PPO after the final closing thereof were to exceed 15,000,000. Accordingly, based on the final amount of gross proceeds raised in the PPO, we issued 1,690,658 additional shares of Common Stock to our Common Stock holders immediately prior to the Merger.
The Merger Agreement contained customary representations and warranties and pre- and post-closing covenants of each party and customary closing conditions. Breaches of the representations and warranties were subject to indemnification provisions. Each of the stockholders of Enumeral as of the date of the Merger initially received in the Merger 98% of the shares to which each such stockholder is entitled, with the remaining 2% of such shares held in escrow for 18 months to satisfy post-closing claims for indemnification by us (“Indemnity Shares”). The indemnification period expired on January 31, 2016 without any claims being made and all of the Indemnity Shares were distributed to the pre-Merger stockholders of Enumeral on a pro rata basis. The Merger Agreement also contained a provision providing for a post-Merger share adjustment as a means for which claims for indemnity could have been made by the pre-Merger stockholders of Enumeral. Pursuant to this provision, up to 500,000 additional shares (“R&W Shares”) of Common Stock could have been issued to the pre-Merger stockholders of Enumeral, pro rata, during the 18-month period following the Merger for breaches of representations and warranties by us. The indemnification period expired on January 31, 2016 with no claims being made. The value of the Indemnity Shares and the R&W Shares issued pursuant to the foregoing adjustment mechanisms was fixed at $1.00 per share. The foregoing mechanisms were our exclusive remedies on one hand and the pre-Merger stockholders of Enumeral on the other hand for satisfying indemnification claims under the Merger Agreement.
The Merger was treated as a recapitalization for financial accounting purposes. Enumeral was considered the acquirer for accounting purposes, and our historical financial statements prior to the Merger have been replaced with the historical financial statements of Enumeral prior to the Merger in all filings with the SEC subsequent to the Merger.
The Merger is intended to be treated as a tax-free reorganization under Section 368 of the Internal Revenue Code of 1986, as amended.
We also agreed not to register under the Securities Act of 1933, as amended (the “Securities Act”), the resale of the shares of our Common Stock received in the Merger by our officers, directors and key employees and holders of 10% or more of our Common Stock for a period of one year following the closing of the Merger.
The Merger Agreement is filed as Exhibit 2.1 to the Registration Statement of which this Prospectus forms a part.All descriptions of the Merger Agreement herein are qualified in their entirety by reference to the text thereof filed as an exhibit hereto, which is incorporated herein by reference.
Split-Off
Upon the closing of the Merger and under the terms of the Split-Off Agreement and a general release agreement, we transferred all of our pre-Merger operating assets and liabilities to the Split-Off Subsidiary. Thereafter, pursuant to the Split-Off Agreement, we transferred all of the outstanding shares of capital stock of Split-Off Subsidiary to Olesya Didenko, our pre-Merger majority stockholder, and our former sole officer and director, in consideration of and in exchange for (i) the surrender and cancellation of an aggregate of 23,100,000 shares of our Common Stock held by Olesya Didenko (which were cancelled and will resume the status of authorized but unissued shares of our Common Stock) and (ii) certain representations, covenants and indemnities. All descriptions of the Split-Off agreement and the general release agreement herein are qualified in their entirety by reference to the text thereof filed as Exhibits 10.1 and 10.2 to the Registration Statement of which this Prospectus forms a part.
The PPO
Concurrently with the closing of the Merger and in contemplation of the Merger, we closed our PPO in which we sold 21,549,510 Units of our securities, at a purchase price of $1.00 per Unit, each Unit consisting of one share of the our Common Stock and PPO Warrant to purchase one share of Common Stock at an exercise price of $2.00 per share and with a term of five years. The aggregate gross proceeds of the PPO were $21,549,510 (before deducting placement agent fees and expenses of approximately $3,294,000).
The investors in the PPO (for so long as they hold shares of our common stock) have anti-dilution protection on the shares of Common Stock included in Units purchased in the PPO and not subsequently transferred or sold (other than transfers to trusts or affiliates of such investors for the purpose of estate planning) in the event that within two years after the closing of the PPO we issue Common Stock or securities convertible into or exercisable for shares of Common Stock at a price lower than the Unit purchase price. The anti-dilution protection provisions are subject to exceptions for certain issuances, including but not limited to (a) shares of Common Stock issued in an underwritten public offering, (b) issuances of awards under our 2014 Equity Incentive Plan and (c) other exempt issuances, as set forth in the PPO Subscription Agreement.
In addition, PPO Warrants not subsequently transferred or sold (other than transfers to trusts or affiliates of such investors for the purpose of estate planning) have anti-dilution protection in the event that prior to the warrant expiration date we issue Common Stock or securities convertible into or exercisable for shares of Common Stock at a price lower than the warrant exercise price, subject to exceptions for certain issuances, including but not limited to (a) shares of Common Stock issued in an underwritten public offering, (b) issuances of awards under our 2014 Equity Incentive Plan and (c) other exempt issuances, as set forth in the PPO Warrants.
In connection with the PPO, we agreed to pay our placement agents, EDI Financial, Inc. and Katalyst Securities LLC (the “Placement Agents”), a commission equal to 10% of the gross proceeds raised from investors in the PPO. In addition, the Placement Agents collectively received warrants to purchase 10% of the number of shares of Common Stock included in the Units sold in the PPO, provided, however, that the Placement Agents were not entitled to any warrants on the sale of Units in excess of 20,000,000, with a term of five (5) years and an exercise price of $1.00 per share (the “Agent Warrants”). Any sub-agent of the Placement Agents or certain individuals identified by us that introduced investors to the PPO were entitled to share in the cash fees and warrants attributable to those investors as described above. We also agreed to pay to the Placement Agents a cash fee on the amount that any person or entity contacted by the Placement Agents, in connection with the Offering, invested in us at any time prior to the date that was eighteen (18) months after the PPO. No such fee was earned or paid.
As a result of the foregoing, the Placement Agents and their respective sub-agents were collectively paid an aggregate commission of $2,154,951 and were issued Agent Warrants to purchase an aggregate of 2,000,000 shares of our Common Stock. We were also required to reimburse the Placement Agents up to $30,000 of legal expenses incurred in connection with the PPO, in the aggregate.
We agreed to indemnify the Placement Agents and their respective sub-agents to the fullest extent permitted by law, against certain liabilities that may be incurred in connection with the PPO, including certain civil liabilities under the Securities Act, and, where such indemnification is not available, to contribute to the payments the Placement Agents and their respective sub-agents may be required to make in respect of such liabilities.
All descriptions of the PPO Warrants and the Agent Warrants herein are qualified in their entirety by reference to the text of the forms of such documents filed as Exhibits 10.5 and 10.8 to the Registration Statement of which this Prospectus forms a part.
Registration Rights
In connection with the PPO, we entered into a Registration Rights Agreement, pursuant to which we have agreed to promptly, but no later than 90 calendar days from the final closing of the PPO, file a registration statement with the SEC (the “Registration Statement”) covering (a) the shares of Common Stock issued in the PPO, (b) the shares of Common Stock issuable upon exercise of the PPO Warrants, (c) the shares of Common Stock underlying the Agent Warrants, (d) up to 50% of shares of Common Stock issued in the Merger in exchange for the preferred and common stock held by the former stockholders of Enumeral prior to the Merger (the “Enumeral Stockholders”) who are not parties to a lock-up agreement (provided that any registered Enumeral Stockholder that purchased Units in the PPO having a purchase price equal to at least 50% of the total amount invested by such holder in Enumeral stock prior to the PPO, the Registration Statement will include 100% of shares of Common Stock issued in the Merger to an Enumeral Stockholder in exchange for Enumeral’s preferred and common stock held by such person) and (e) the True-Up Shares, if any(clauses (a) through (e), collectively, the “Registrable Shares”). We agreed to use commercially reasonable efforts to ensure that such Registration Statement is declared effective within 180 calendar days of filing with the SEC. The Registration Statement was filed on September 19, 2014 and declared effective on November 10, 2014. If we had been late in filing the Registration Statement or if the Registration Statement had not been declared effective within 180 days of filing with the SEC, we would have been required to pay the holders of Registrable Shares that have not been so registered, liquidated damages at a rate equal to 1.00% of the Offering Price per share for each full month that (i) we were late in filing the Registration Statement, (ii) the Registration Statement was late in being declared effective by the SEC or (iii) after the Registration Statement is declared effective, the Registration Statement ceases for any reason to remain continuously effective or the holders of the Registrable Shares are otherwise not permitted to utilize the prospectus therein to resell the Registrable Securities for a period of more than 30 consecutive trading days; provided, however, that in no event shall the aggregate of any such liquidated damages exceed 8% of the PPO offering price per share. No liquidated damages will accrue and accumulate with respect to (a) any Registrable Shares removed from the Registration Statement in response to a comment from the staff of the SEC limiting the number of shares of Common Stock which may be included in the Registration Statement (a “Cutback Comment”), or (b) after the shares may be resold under Rule 144 under the Securities Act or another exemption from registration under the Securities Act.
We are required to use commercially reasonable efforts to keep the Registration Statement “evergreen” for two years from the date it is declared effective by the SEC (i.e., November 10, 2016) or until Rule 144 is available to the holders of Registrable Shares who are not and have not been our affiliates with respect to all of their registrable shares, whichever is earlier.
Prior to the Merger, we were a “shell company” as defined in Rule 12b-2 under the Exchange Act. Pursuant to Rule 144(i), securities issued by a current or former shell company that otherwise meet the holding period and other requirements of Rule 144 nevertheless cannot be sold in reliance on Rule 144 until twelve (12) months after the company (a) is no longer a shell company; and (b) has filed current “Form 10 information” (as defined in Rule 144(i)) with the SEC reflecting that it is no longer a shell company, and provided that at the time of a proposed sale pursuant to Rule 144, the company is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act and has filed all reports and other materials required to be filed by Section 13 or 15(d) of the Exchange Act, as applicable, during the preceding twelve (12) months (or for such shorter period that the issuer was required to file such reports and materials), other than Form 8-K reports. As a result, the restrictive legends on certificates for our Common Stock and Warrants cannot be removed (a) except in connection with an actual sale meeting the foregoing requirements or (b) pursuant to an effective registration statement.
The holders of Registrable Shares (including any shares of Common Stock removed from the Registration Statement as a result of a Cutback Comment) and the holders of our common stock prior to the Merger (but not holders of the shares issued to the stockholders of Enumeral in consideration for the Merger) will have two “piggyback” registration rights for such shares with respect to any registration statement filed by us following the effectiveness of the Registration Statement that would permit the inclusion of such shares, subject to customary cut-backs on a pro rata basis if the underwriter or we determine that marketing factors require a limitation on the number of shares of stock or other securities to be underwritten.
We will pay all expenses in connection with any registration obligation provided in the Registration Rights Agreement, including, without limitation, all registration, filing, stock exchange fees, printing expenses, all fees and expenses of complying with applicable securities laws, and the fees and disbursements of our counsel and of our independent accountants. Each investor will be responsible for its own sales commissions, if any, transfer taxes and the expenses of any attorney or other advisor that such investor decides to employ.
All descriptions of theRegistration Rights Agreement herein are qualified in their entirety by reference to the text of the form of such document filed as Exhibit 10.9 to the Registration Statement of which this Prospectus forms a part.
Composition of the Board; Voting Agreement
In connection with the Merger, the parties agreed that our Board of Directors will consist of seven members, as follows:
| (i) | six directors nominated by Enumeral, who shall be composed of |
| | (a) | John J. Rydzewski, Arthur H. Tinkelenberg, Allan P. Rothstein and Barry Buckland, |
| | (b) | one of whom shall be a director nominated by Harris & Harris Group, Inc. (the “H&H” Director), who is reasonably acceptable to us and who initially was Daniel Wolfe, Ph.D., until Dr. Wolfe’s resignation from the board in July 2015 (the appointment of a new director to replace Dr. Wolfe is detailed below); provided, that the right of H&H shall cease at such time as the number of shares of Common Stock owned directly by H&H is less than five percent of the total number of shares of Common Stock outstanding, and |
| | (c) | one additional person to be designated by the directors specified in clauses (a) and (b) above, who is initially Robert L. Van Nostrand (who was appointed on December 1, 2014), and |
| (ii) | one independent director who shall be nominated by Montrose Capital Limited and the Placement Agents, who is reasonably acceptable to us and who is originally Paul Sekhri (who was appointed on December 16, 2014). |
In connection with Dr. Wolfe’s resignation from the board on July 30, 2015, the board appointed Robert J. Easton to serve as a director.
In connection with the Merger, certain of our stockholders (holding a majority of our common stock), including all of the investors in the PPO, all of our pre-Merger stockholders and certain of the Enumeral Stockholders (including all officers and directors and certain principal stockholders), entered into a Voting Agreement in which they have agreed to vote their Enumeral Biomedical stock to maintain the composition of our Board of Directors as described above.
The Voting Agreement will terminate two years from the date of closing of the Merger.All descriptions of theVoting Agreement herein are qualified in their entirety by reference to the text thereof filed as Exhibit 10.10 to the Registration Statement of which this Prospectus forms a part.
Accounting Treatment; Change of Control
The Merger was accounted for as a “reverse merger,” and Enumeral is deemed to be the acquirer in the reverse merger. Consequently, the assets and liabilities and the historical operations that are reflected in the consolidated financial statements prior to the Merger are those of Enumeral and are recorded at the historical cost basis of Enumeral, and the consolidated financial statements after completion of the Merger will include the assets and liabilities of Enumeral, historical operations of Enumeral and operations our company and our subsidiaries from the closing date of the Merger. As a result of the issuance of the shares of our Common Stock pursuant to the Merger, a change in control of Enumeral Biomedical occurred as of the date of consummation of the Merger. Except as described in this Prospectus, no arrangements or understandings exist among present or former controlling stockholders with respect to the election of members of our Board of Directors and, to our knowledge, no other arrangements exist that might result in a change of control of Enumeral Biomedical.
We continue to be a “smaller reporting company,” as defined under the Exchange Act, and an “emerging growth company” under the Jumpstart Our Business Startups (JOBS) Act of 2012. We believe that as a result of the Merger we have ceased to be a “shell company” (as such term is defined in Rule 12b-2 under the Exchange Act).
Competition
The biotechnology industry, especially early-stage companies, is characterized by rapidly advancing technologies, intense competition, and a strong emphasis on intellectual property. While we believe our technology, experience and scientific resources provide us with certain competitive advantages, we face actual and potential competition from various sources, including large, established pharmaceutical companies, emerging pharmaceutical and biotechnology companies (including companies specializing in immuno-oncology), academic institutions, governmental agencies, and public and private research institutions.
Many of the companies against which we are competing, or against which we are likely to compete in the future, have significantly greater financial resources and greater expertise in research and development, preclinical testing, clinical trials, manufacturing, regulatory affairs, and marketing than we do. We also compete with small and early-stage companies, particularly in our attempts to secure collaborative arrangements with large and established companies, and in our attempts to acquire technologies complementary to our programs. These companies also compete with us in recruiting and retaining qualified scientific and management personnel. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated in some of our competitors.
Although we are an early-stage company focused on the discovery and development of novel therapeutics, and have not yet begun any clinical trials, we anticipate that any pharmaceutical products that we develop and commercialize in the future will face competition from existing therapies and from new therapies that become available. The key competitive factors affecting the success of those products, if developed and approved, are likely to be their efficacy, safety, convenience, price, the level of generic competition and the availability of reimbursement from government and other third-party payors. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize drugs that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any drugs that we may develop. Our competitors also may obtain FDA or other regulatory approval for their drugs more rapidly than we obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market.
Government Regulation
Regulatory authorities in the United States and in other countries regulate numerous aspects of the pharmaceutical industry, including pre-clinical testing, human clinical trials, manufacturing, marketing approval, labeling, promotion, advertising, distribution, post-approval monitoring, and export and import of pharmaceutical products.
Marketing Approval of Drugs in the United States
In the United States, the Food and Drug Administration, or FDA, regulates drugs under the Federal Food, Drug, and Cosmetic Act and its implementing regulations. The steps ordinarily required before a new pharmaceutical product may be marketed in the United States include:
| | · | preclinical laboratory studies involving animals; |
| | · | submission of an Investigational New Drug, or IND, application for human clinical testing; |
| | · | rigorous human clinical trials to establish the safety and efficacy of the drug, on an indication-by-indication basis; |
| | · | FDA inspections of the manufacturing facility at which the product is manufactured; |
| | · | FDA inspections of clinical trial sites; |
| | · | submission of a New Drug Application, or NDA; |
| | · | payment of user fees; and |
| | · | FDA review and approval of the NDA. |
Preclinical Studies:
Preclinical studies include laboratory testing and animal studies. Preclinical studies must comply with federal regulations, which specify certain requirements such as compliance with good laboratory practices. Results of the preclinical studies, manufacturing information, analytical data and a proposed clinical trial protocol are submitted to the FDA as part of an IND, which is an exemption that allows an unapproved drug to be shipped in interstate commerce and administered to humans. The IND must become effective before clinical trials may be commenced.
Clinical Trials:
Clinical trials must be conducted under the supervision of a qualified medical professional acting as the principal investigator, in compliance with detailed protocols that have been reviewed and approved by the FDA and by an independent Institutional Review Board (known as an IRB) at, or affiliated with, the medical facility where the new drug is being administered.
Human clinical trials are typically conducted in three sequential (and sometimes overlapping) phases:
| | · | In Phase I, the drug is administered to a small population of healthy human subjects or patients having the indication for which the drug is being tested. The primary purpose of Phase I is initial assessment of safety, although preliminary indications of effectiveness are sometimes sought. |
| | · | In Phase II, the drug is administered to a somewhat larger, but limited, patient population. The primary purposes of Phase II are to obtain additional safety data, to assess effectiveness in patients with the relevant indication, and to obtain data concerning dosage and dosage regimen. |
| | · | In Phase III, the drug is administered to an expanded patient population, generally at geographically dispersed clinical trial sites. The primary purposes of Phase III are to obtain additional data on safety and efficacy in a population large enough to permit rigorous statistical analysis of results, and to provide information such as a list of side effects, for use in drug labeling. |
NDA:
The results of preclinical studies and clinical trials, together with detailed information on the drug’s chemistry, pharmacology, manufacture, formulation, safety and effectiveness, are submitted to the FDA in an NDA requesting approval to market the drug. The cost of an NDA is substantial, both in terms of studies required to generate and compile the requisite data, as well as the mandatory user fees submitted with the application.
As part of its review, the FDA may refer the application to an outside expert advisory committee for review, evaluation and a non-binding recommendation as to whether the drug should be approved. If the FDA is satisfied that all requirements have been met, it issues an approval letter, which authorizes commercial marketing of the drug for specific indications. As a condition of approval, the FDA may require post-marketing testing and surveillance to monitor the drug’s safety or efficacy, or impose other conditions. Once granted, marketing approvals may be withdrawn if compliance with regulatory standards is not maintained or if other problems occur.
Hazardous Materials
Our research and development processes involve the use of certain hazardous materials. We are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposing of hazardous materials and waste products, including certain regulations promulgated by the U.S. Environmental Protection Agency.
Employees
As of July 22, 2016, we employed five business executives and ten scientists and other employees. None of our employees is represented by a collective bargaining agreement, nor have we experienced work stoppages. We believe that our relations with our employees are good.
Description of Property
In March 2015, we relocated our offices and research laboratories to 200 CambridgePark Drive in Cambridge, Massachusetts. We are leasing 16,825 square feet at this facility pursuant to a lease that we entered into in November 2014. The term of this lease is for five years, and the initial base rent is $42.50 per square foot, or approximately $715,062 on an annual basis. The base rent will increase incrementally over the term of the lease, reaching approximately $804,739 on an annual basis in the fifth year of the term. In addition, we are obligated to pay a proportionate share of the operating expenses and applicable taxes associated with the premises, as calculated pursuant to the terms of the lease. We have delivered a security deposit to the landlord in the amount of $529,699, in the form of an irrevocable letter of credit, which may be reduced to $411,988 following the second anniversary of our commencement date under the lease, provided that we meet certain financial conditions set forth in the lease.
We previously occupied offices and research laboratories in approximately 4,782 square feet of space at One Kendall Square in Cambridge, Massachusetts, at an annual rent of $248,664, which we refer to as the Kendall Lease. For the year ended December 31, 2015, we recorded an expense of $55,352, representing all exit costs associated with our move to new offices and research laboratories in March 2015. In June 2015, we entered into a lease termination agreement with the landlord for our former facility at One Kendall Square, pursuant to which the Kendall Lease was terminated as of June 17, 2015. In accordance with the terms of the lease termination agreement, we are not obligated to pay rent for the One Kendall Square facility after May 31, 2015. We had maintained a security deposit relating to the facility, recorded as restricted cash on the accompanying consolidated balance sheet, which was returned to us pursuant to the lease termination agreement.
In addition, we maintain a small corporate office at 1370 Broadway in New York, New York, at an annual rent of $23,100. Our lease for our New York office expires December 31, 2016.
Legal Proceedings
From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business.
We are currently not aware of any pending legal proceedings to which we are a party or of which any of our property is the subject, nor are we aware of any such proceedings that are contemplated by any governmental authority.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
As a result of the Merger and the change in business and operations of Enumeral Biomedical, a discussion of our financial results prior to July 31, 2014 is not pertinent, and under applicable accounting principles the historical financial results of Enumeral, the accounting acquirer, prior to the Merger are considered our historical financial results.
The following discussion highlights our results of operations and the principal factors that have affected our financial condition as well as our liquidity and capital resources for the periods described, and provides information that management believes is relevant for an assessment and understanding of the statements of financial condition and results of operations presented herein. The following discussion and analysis are based on our audited and unaudited consolidated financial statements contained in this Prospectus, which we have prepared in accordance with generally accepted accounting principles in the United States, or GAAP. You should read the discussion and analysis together with such financial statements and the related notes thereto.
Principles of Consolidation and Presentation
The consolidated financial statements include the accounts of Enumeral Biomedical and its subsidiaries. In these consolidated financial statements, “subsidiaries” are companies that are wholly owned by us, the accounts of which are consolidated with ours. Significant intercompany transactions and balances are eliminated in consolidation.
Overview
We were incorporated in Nevada as Cerulean Group, Inc. on February 27, 2012, and converted to a Delaware corporation on July 10, 2014. Our original business was to develop and operate a website for self-travelers and backpackers that would allow a person with access to the Internet to build an itinerary and plan a trip. Prior to the Merger, which is described in further detail below, our Board of Directors determined to discontinue operations in this area to seek a new business opportunity. As a result of the Merger, we acquired the business of Enumeral and changed our name to Enumeral Biomedical Holdings, Inc.
On July 25, 2014, we completed a 4.62-for-1 forward split of our common stock in the form of a dividend, with the result that the 6,190,000 shares of our common stock outstanding immediately prior to the stock split became 28,597,804 shares of common stock outstanding immediately thereafter. All share and per share numbers in this Prospectus relating to our common stock have been adjusted to give effect to this stock split, unless otherwise stated.
On July 31, 2014, our wholly owned subsidiary, Enumeral Acquisition Corp. merged with and into Enumeral (the “Merger”). Enumeral was the surviving corporation in the Merger and became our wholly owned subsidiary. All of the outstanding Enumeral stock was converted into shares of our common stock, as described in more detail below.
Upon the closing of the Merger and under the terms of a split-off agreement and a general release agreement (the “Split-Off Agreement”), we transferred all of our pre-Merger operating assets and liabilities to a wholly-owned special-purpose subsidiary, Cerulean Operating Corp. (the “Split-Off Subsidiary”). Thereafter, pursuant to the Split-Off Agreement, we transferred all of the outstanding shares of capital stock of Split-Off Subsidiary to our pre-Merger majority stockholder, and our former sole officer and director (the “Split-Off”), in consideration of and in exchange for (i) the surrender and cancellation of an aggregate of 23,100,000 shares of our common stock held by such stockholder (which were cancelled and will resume the status of authorized but unissued shares of our common stock) and (ii) certain representations, covenants and indemnities.
As a result of the Merger and transactions effected pursuant to the Split-Off Agreement, we discontinued our pre-Merger business and acquired the business of Enumeral, and are continuing the existing business operations of Enumeral as a publicly-traded company under the name Enumeral Biomedical Holdings, Inc.
Also on July 31, 2014, we closed a private placement offering (the “PPO”) of 21,549,510 Units of our securities, at a purchase price of $1.00 per Unit, each Unit consisting of one share of our common stock and a warrant to purchase one share of common stock at an exercise price of $2.00 per share with a term of five years (the “PPO Warrants”).
Also on July 31, 2014, we changed our fiscal year from a fiscal year ending on October 31 of each year to one ending on December 31 of each year, which is the fiscal year end of Enumeral.
Enumeral was incorporated on December 11, 2009 and has devoted substantially all of its resources to the discovery of monoclonal antibodies and other novel biologics for use in the diagnosis and treatment of cancer, infectious and inflammatory diseases. To date, all of our revenue has resulted from payments from strategic partners and we have not received any revenue from the sale of products or services. As of March 31, 2016, we had total stockholders’ equity of $1,251,816, including an accumulated deficit of $15,964,028.
We are in the business of discovering and developing novel antibody immunotherapies that help the immune system fight cancer and other diseases. We utilize a proprietary platform technology that facilitates the rapid high resolution measurement of immune cell function within small tissue biopsy samples. Our initial focus is on the development of a pipeline of next generation monoclonal antibody drugs targeting established and novel immuno-modulatory receptors.
In our lead antibody program, we have characterized certain anti-PD-1 antibodies, or simply “PD-1 antibodies,” using patient biopsy samples, in an effort to identify next generation PD-1 antagonists with enhanced selectivity for the immune effector cells that carry out anti-tumor functions. We have identified two antagonist PD-1 antibodies that inhibit PD-1 activity in distinctly different ways. One of the antibodies blocks binding of the ligand PD-L1 to PD-1, while the other antibody does not. However, both display activity in various biological assays. In addition to our PD-1 antibody program, we are developing antibody drug candidates for a number of immunomodulatory protein targets, including TIM-3, LAG-3, OX40, TIGIT and VISTA. We are also pursuing several antibody programs for which we have not yet publicly disclosed the targets.
Our proprietary platform technology, exclusively licensed from the Massachusetts Institute of Technology, or MIT, is a microwell array technology that detects secreted molecules (such as antibodies and cytokines) and cell surface markers, at the level of single, live cells – and enables recovery of single, live cells of interest. The platform technology can be used to achieve at least three separate, but complementary, objectives. First, we use the platform to rapidly produce antibody libraries with high diversity. Second, the platform has the potential to guide rational selection of lead candidates derived from these libraries, through characterization of immune function at the level of single cells from human biopsy samples. Third, it has the potential to identify patients more likely than others to benefit from treatment with a given therapeutic antibody. Thus, our platform is a multipurpose tool that is valuable for activities ranging from antibody discovery to target discovery to patient stratification in clinical development. The platform yields multidimensional, functional read-outs from single live cells, such as tumor infiltrating lymphocytes, or TILs, from human tumor biopsy samples, and it enables us to examine the responses of different classes of human immune cells to treatment with immuno-modulators in the context of human disease, as opposed to animal models of disease. Consequently, we call our approach The Human Approach®.
Our proof-of-concept corporate collaborations have provided modest revenues. However,our business has not generated (nor do we anticipate that in the foreseeable future it will generate) the cash necessary to finance our operations. We expect to continue to incur losses and use cash during fiscal year 2016, and we will require additional capital to continue our operations beyond July 2016.
Our near-term capital needs depend on many factors, including:
| · | our ability to carefully manage our costs; |
| · | the amount and timing of revenue received from grants or our collaboration and license arrangements; and |
| · | our ability to raise additional capital through public or private equity offerings, debt financings, or strategic collaborations and licensing arrangements, and/or our success in promptly establishing a strategic alternative that is in our stockholders’ best interests. |
If we are unable to preserve or raise additional capital through one or more of the means listed above prior to the end of July 2016, we could face substantial liquidity problems and might be required to implement cost reduction strategies in addition to the cash conservation steps that we have already taken. These reductions could significantly affect our research and development activities, and could result in significant harm to our business, financial condition and results of operations. In addition, these reductions could cause us to further curtail our operations, or take other actions that would adversely affect our stockholders. If we are unable to raise additional capital on terms acceptable to us and on a timely basis, we may be required to downsize or wind down our operations through liquidation, bankruptcy, or a sale of our assets.
No assurance can be given that additional financing or strategic alliances and licensing arrangements will be available when needed or that, if available, such financing could be obtained on terms favorable to us or our stockholders. See “Risk Factors.”
Results of Operations
Year ended December 31, 2015 as compared to year ended December 31, 2014
| | | Years Ended
December 31, | | | Increase | |
| | | 2015 | | | 2014 | | | (Decrease) | |
| | | | | | | | | | |
| Revenue: | | | | | | | | | | | | |
| Collaboration and license revenues | | $ | 1,100,000 | | | $ | 115,714 | | | $ | 984,286 | |
| Grant revenue | | | 389,385 | | | | 48,312 | | | | 341,073 | |
| | | | | | | | | | | | | |
| Total revenue | | | 1,489,385 | | | | 164,026 | | | | 1,325,359 | |
| | | | | | | | | | | | | |
| Cost of revenue and expenses: | | | | | | | | | | | | |
| Research and development | | | 6,493,859 | | | | 3,575,695 | | | | 2,918,164 | |
| General and administrative | | | 5,695,932 | | | | 3,019,101 | | | | 2,676,831 | |
| | | | | | | | | | | | | |
| Total cost of revenue and expenses | | | 12,189,791 | | | | 6,594,796 | | | | 5,594,995 | |
| | | | | | | | | | | | | |
| Loss from operations | | | (10,700,406 | ) | | | (6,430,770 | ) | | | (4,269,636 | ) |
| | | | | | | | | | | | | |
| Other income (expense): | | | | | | | | | | | | |
| Interest income (expense) | | | 9,699 | | | | (242,430 | ) | | | 252,129 | |
| Other expense | | | - | | | | (1,690,658 | ) | | | 1,690,658 | |
| Change in fair value of derivative liabilities | | | 13,980,711 | | | | 184,448 | | | | 13,796,263 | |
| | | | | | | | | | | | | |
| Total other income (expense), net | | | 13,990,410 | | | | (1,748,640 | ) | | | 15,739,050 | |
| Net income (loss) | | $ | 3,290,004 | | | $ | (8,179,410 | ) | | $ | 11,469,414 | |
Collaboration and license revenues. Collaboration and license revenues increased by $984,286, or 851%, to $1,100,000 for the year ended December 31, 2015, as compared to $115,714 for the year ended December 31, 2014. The increase is attributable to our collaboration agreement with Merck.
Grant revenue. Grant revenue increased by $341,073, or 706%, to $389,385 for the year ended December 31, 2015, as compared to $48,312 for the year ended December 31, 2014. The increase is attributable to our Phase II Small Business Innovation Research agreement with the National Cancer Institute, or NCI.
Research and development expenses. Research and development expenses increased by $2,918,164, or 82%, to $6,493,859 for the year ended December 31, 2015, as compared to $3,575,695 for the year ended December 31, 2014. The increase was primarily attributable to an increase in laboratory expenses of $1,058,570 associated with increased laboratory activities in connection with additional research and development personnel, an increase in payroll and personnel expenses of $725,324, as we hired additional research and development personnel, an increase in facility expenses of $729,588 in connection with our office relocation, an increase in depreciation expense of $312,068, and an increase in stock compensation expense of $135,966.
General and administrative expenses. General and administrative expenses increased by $2,676,831, or 89%, to $5,695,932 for the year ended December 31, 2015, as compared to $3,019,101 for the year ended December 31, 2014. This increase was primarily attributable to an increase in payroll and personnel expenses of $1,774,607, as we hired additional general and administrative personnel, an increase in professional service fees of $557,519, which included additional fees related to being a public company, and an increase in facility expenses of $133,706 in connection with our office relocation.
Interest income (expense). Interest income (expense) changed by $252,129, or 104%, to $9,699 for the year ended December 31, 2015, as compared to ($242,430) the year ended December 31, 2014. This change is primarily attributable to the interest expense recorded for the year ended December 31, 2014 associated with the extinguishment of previously issued Enumeral debt and convertible notes in connection with the PPO. No such instruments were outstanding during the year ended December 31, 2015.
Other expense. Other expense decreased $1,690,658, or 100%, to $0 for the year ended December 31, 2015, as compared to $1,690,658 for the year ended December 31, 2014. Based on the final amount of gross proceeds raised in the PPO, we issued 1,690,658 additional shares of common stock to our holders of common stock immediately prior to the Merger. These additional shares were issued at $1.00 per share. Consequently, we recorded $1,690,658 in expenses related to these additional shares for the year ended December 31, 2014. We had no such similar expense for the year ended December 31, 2015.
Change in fair value of derivative liabilities. Change in fair value of derivative liability increased $13,796,263, or 7,480%, to $13,980,711 for the year ended December 31, 2015, as compared to $184,448 for the year ended December 31, 2014. In connection with the PPO, we issued warrants to purchase an aggregate of 23,549,510 shares of our common stock. We also previously issued warrants to purchase an aggregate of 66,574 shares of our common stock related to a prior financing transaction with Square 1 Bank. As of December 31, 2015, these warrants remained unexercised. The fair value of the warrants is recorded in the liability section of the consolidated balance sheet and at December 31, 2015 was estimated at $2,138,091. The fair value of the warrant liability is determined at the end of each reporting period, with the resulting gains and losses recorded as the change in fair value of warrant liability on the consolidated statement of operations and comprehensive income (loss). During the year ended December 31, 2015, we realized a gain of $13,980,711, due to the change in the fair value of the warrant liability. This gain is principally a result of the decrease of the fair value of our stock price between December 31, 2014 and December 31, 2015. We expect that future changes in the fair value of the warrant liability will be due primarily to fluctuations in the value of our common stock and potential exercises of outstanding warrants.
Net income (loss). Net income increased $11,469,414, or 140%, to $3,290,004 for the year ended December 31, 2015, as compared to a loss of ($8,179,410) for the year ended December 31, 2014. This change in net income was primarily due to an increase in the change in fair value of derivative liabilities of $13,796,263 and an increase in revenues of $1,325,359, offset by an increase of $5,594,995 in research and development and general and administrative expenses.
As of December 31, 2015, we have accumulated losses of $14,400,643 since inception and, therefore, have not paid any federal income taxes. Realization of deferred tax assets is dependent on future earnings, if any, the timing and amount of which are uncertain. Accordingly, valuation allowances in amounts equal to the deferred tax assets have been established to reflect these uncertainties. Utilization of the deferred tax asset, consisting of net operating loss and research and development credit carryforwards, may be subject to a substantial annual limitation under Section 382 of the Internal Revenue Code of 1986 due to ownership change limitations that have occurred previously or that could occur in the future. These ownership changes may limit the amount of net operating loss and research and development credit carryforwards that can be utilized annually to offset future taxable income and tax, respectively.
Three months ended March 31, 2016 as compared to three months ended March 31, 2015
| | | Three Months Ended March 31, | | | Increase | |
| | | 2016 | | | 2015 | | | (Decrease) | |
| | | | | | | | | | |
| Collaboration and license revenue | | $ | 316,018 | | | $ | 209,635 | | | $ | 106,383 | |
| Grant revenue | | | 118,439 | | | | 65,087 | | | | 53,352 | |
| Total revenue | | | 434,457 | | | | 274,722 | | | | 159,735 | |
| Cost of revenue and expenses: | | | | | | | | | | | | |
| Research and development | | | 1,470,043 | | | | 1,230,504 | | | | 239,539 | |
| General and administrative | | | 1,194,334 | | | | 1,424,943 | | | | (230,609 | ) |
| Total cost of revenue and expenses | | | 2,664,377 | | | | 2,655,447 | | | | 8,930 | |
| Loss from operations | | | (2,229,920 | ) | | | (2,380,725 | ) | | | 150,805 | |
| Other income (expense): | | | | | | | | | | | | |
| Interest income (expense) | | | (3,567 | ) | | | 2,635 | | | | (6,202 | ) |
| Change in fair value of derivative liabilities | | | 678,435 | | | | 4,669,884 | | | | (3,991,449 | ) |
| Total other income (expense), net | | | 674,868 | | | | 4,672,519 | | | | (3,997,651 | ) |
| Net income (loss) | | $ | (1,555,052 | ) | | $ | 2,291,794 | | | $ | (3,846,846 | ) |
Collaboration and license revenue. Collaboration and license revenue increased by $106,383, or 51%, to $316,018 for the three months ended March 31, 2016, as compared to $209,635 for the three months ended March 31, 2015. This increase in revenue is attributable to our collaboration agreement with Merck.
Grant revenue. Grant revenue increased by $53,352, or 82%, to $118,439 for the three months ended March 31, 2016, as compared to $65,087 for the three months ended March 31, 2015. This increase is attributable to our Phase II Small Business Innovation Research agreement with the National Cancer Institute, or NCI.
Research and development expenses. Research and development expenses increased by $239,539, or 19%, to $1,470,043 for the three months ended March 31, 2016, as compared to $1,230,504 for the three months ended March 31, 2015. This increase is primarily attributable to increased facilities costs of $186,081 in connection with our office relocation, and an increase in stock-based compensation expense of $62,455 resulting from options granted to purchase our common stock during the three months ended March 31, 2016.
General and administrative expenses. General and administrative expenses decreased by $230,609, or 16%, to $1,194,334 for the three months ended March 31, 2016, as compared to $1,424,943 for the three months ended March 31, 2015. This decrease was primarily attributable to a decrease in professional services fees of $158,530 associated with lower legal costs and a decrease in exit costs of $55,352 associated with the termination of the Kendall Lease.
Interest income (expense). Interest income (expense) changed by $6,202, or 235%, to ($3,567) for the three months ended March 31, 2016, as compared to $2,635 for the three months ended March 31, 2015. This change is largely attributable to interest expense associated with the equipment lease financing.
Change in fair value of derivative liabilities. Change in fair value of derivative liabilities decreased by $3,991,449, or 85%, to $678,435 for the three months ended March 31, 2016, as compared to $4,669,884 for the three months ended March 31, 2015. In connection with the PPO, we issued warrants to purchase an aggregate of 23,549,510 shares of our common stock. We also previously issued warrants to purchase an aggregate of 66,574 shares of our common stock related to a prior financing transaction with Square 1 Bank. As of March 31, 2016, these warrants remained unexercised. The fair value of the warrants is recorded in the liability section of the unaudited condensed consolidated balance sheets and as of March 31, 2016 was estimated at $1,459,656. The fair value of the warrant liabilities is determined at the end of each reporting period, with the resulting gains and losses recorded as the change in fair value of derivative liabilities on the unaudited condensed consolidated statements of operations and comprehensive income (loss). During the three months ended March 31, 2016, we realized a gain of $678,435, due to the change in the fair value of the derivative liabilities. This gain is principally a result of the decrease of the fair value of our stock price between December 31, 2015 and March 31, 2016. We expect that future changes in the fair value of the warrant liabilities will be due primarily to fluctuations in the value of our common stock and potential exercises of outstanding warrants.
Net income (loss). Net income (loss) decreased $3,846,846, or 168%, to ($1,555,052) for the three months ended March 31, 2016, as compared to $2,291,794 for the three months ended March 31, 2015. This decrease was primarily due to a decrease of $3,991,449 in the change in the fair value of derivative liabilities offset by an increase of $159,735 in revenue.
As of March 31, 2016, we had accumulated losses of $15,964,028 since inception and, therefore, have not paid any federal income taxes. Realization of deferred tax assets is dependent on future earnings, if any, the timing and amount of which are uncertain. Accordingly, valuation allowances in amounts equal to the deferred tax assets have been established to reflect these uncertainties. Utilization of the deferred tax asset, consisting of net operating loss and research and development credit carryforwards, may be subject to a substantial annual limitation under Section 382 of the Internal Revenue Code of 1986 due to ownership change limitations that have occurred previously or that could occur in the future. These ownership changes may limit the amount of net operating loss and research and development credit carryforwards that can be utilized annually to offset future taxable income and tax, respectively.
Liquidity and Capital Resources
We have financed our operations since inception primarily through equity financings of both preferred and common stock, venture debt, and revenues from corporate collaborations and our contracts with NCI. We have raised gross proceeds of approximately $32.8 million in six financing rounds, consisting of: (i) a $3.2 million Series A Preferred Stock financing completed in early 2011; (ii) a $2.7 million Series A-1 Preferred Stock financing completed in mid-2012; (iii) a $2.7 million Series A-2 Preferred Stock financing completed in mid-2013; (iv) a $2.0 million Series B Preferred Stock financing in April 2014; (v) a $750,000 bridge note financing; and (vi) $21.5 million in the PPO in July 2014. The securities issued in the above financing rounds prior to the PPO were converted into Enumeral Biomedical common stock in the Merger.
The unaudited condensed consolidated financial statements have been prepared in conformity with generally accepted accounting principles in the United States which contemplate our continuation as a going concern. At March 31, 2016, we had a working capital deficit of $469,423 including $1,459,656 of derivative liabilities, and an accumulated deficit of $15,964,028. As of the date of this filing, we believe that we only have sufficient liquidity to fund operations through July 2016. If we are unable to raise additional capital on terms acceptable to us and on a timely basis, we may be required to downsize or wind down our operations through liquidation, bankruptcy, or a sale of our assets. The unaudited condensed consolidated financial statements do not include any adjustments related to the recovery and classification of asset carrying amounts or the amount and classification of liabilities that might result should we be unable to continue as a going concern.
To date, revenue from contracts with our collaboration partners and NCI grant revenue totaled $2,603,022, of which $434,457 and $274,722 was recognized for the three months ended March 31, 2016 and 2015, respectively.
Cash Flows
Year ended December 31, 2015 as compared to year ended December 31, 2014
The following table summarizes our sources and uses of cash for the years ended December 31, 2015 and 2014:
| | | Years Ended | |
| | | 2015 | | | 2014 | |
| | | | | | | |
| Net cash used in operating activities | | $ | (9,285,052 | ) | | $ | (5,238,414 | ) |
| Net cash provided by (used in) investing activities | | | 1,977,923 | | | | (4,113,335 | ) |
| Net cash provided by financing activities | | | 443,274 | | | | 19,547,956 | |
| Net Increase (decrease) in cash and cash equivalents | | | (6,863,855 | ) | | | 10,196,207 | |
The decrease in net cash for the year ended December 31, 2015 as compared to the year ended December 31, 2014 was $17,060,062, representing the aggregate of (i) a decrease in net cash provided by financing activities of $19,104,682, (ii) an increase in net cash provided by investing activities of $6,091,258, and (iii) an increase in net cash used in operating activities of $4,046,638.
Operating Activities
Net cash used in operating activities was $9,285,052 for the year ended December 31, 2015, which primarily resulted from change in fair value of derivative liabilities of $13,980,711, offset by income of $3,290,004.
Net cash used in operating activities was $5,238,414 for the year ended December 31, 2014, which consisted primarily of a net loss of $8,179,410, adjusted for non-cash items including costs associated with the reverse merger of $1,690,658, stock based compensation of $659,435, depreciation and amortization of $304,456 and a net increase of $293,183 in operating assets and liabilities.
Investing Activities
Net cash provided by investing activities was $1,977,923 for the year ended December 31, 2015. Net cash provided by investing activities for the year ended December 31, 2015 primarily resulted from net proceeds of marketable securities of $3,000,799, offset by purchases of property and equipment of $1,050,506.
Net cash used in investing activities was $4,113,335 for the year ended December 31, 2014. Net cash used in investing activities for the year ended December 31, 2014 resulted primarily from net purchases of marketable securities of $3,019,896, purchases of property and equipment of $563,695 and security deposits of $529,744.
Financing Activities
Net cash provided by financing activities was $443,274 for the year ended December 31, 2015. Net cash provided by financing activities for the year ended December 31, 2015 consisted of net proceeds from equipment lease financing of $413,599 and proceeds from the excise of stock options of $29,675.
Net cash provided by financing activities was $19,547,956 for the year ended December 31, 2014. Net cash provided by financing activities for the year ended December 31, 2014 consisted of net proceeds from issuance of common stock of $18,255,444, net proceeds from the issuance of preferred stock of $1,597,860, proceeds from the issuance of promissory notes of $750,000, offset by payments on long-term debt of $1,055,348.
Three months ended March 31, 2016 as compared to three months ended March 31, 2015
The following table summarizes our sources and uses of cash for the three months ended March 31, 2016 and 2015:
| | | Three Months Ended | |
| | | March 31, | |
| | | 2016 | | | 2015 | |
| | | | | | | |
| Net cash used in operating activities | | $ | (1,699,858 | ) | | $ | (2,064,248 | ) |
| Net cash provided by investing activities | | | - | | | | 608,875 | |
| Net cash used in financing activities | | | (59,100 | ) | | | - | |
| Net decrease in cash and cash equivalents | | | (1,758,958 | ) | | | (1,455,373 | ) |
The decrease in net cash for the three months ended March 31, 2016 as compared to the three months ended March 31, 2015 was $303,585, representing the aggregate of (i) a decrease in net cash used in operating activities of $364,390, (ii) a decrease in net cash provided by investing activities of $608,875 and (iii) an increase in net cash used in financing activities of $59,100.
Operating Activities
Net cash used in operating activities was $1,699,858 for the three months ended March 31, 2016, which consisted primarily of a net loss of $1,555,052, adjusted for non-cash items including the change in the fair value of the derivative liabilities of $678,435, stock-based compensation of $333,811, and depreciation and amortization of $185,855.
Net cash used in operating activities was $2,064,248 for the three months ended March 31, 2015, which consisted primarily of net income of $2,291,794, adjusted for non-cash items including the change in the fair value of the derivative liabilities of $4,669,884, stock-based compensation of $166,391, depreciation and amortization of $136,298 and exit costs associated with a write-down of leasehold improvements of $22,962.
Investing Activities
Net cash provided by investing activities was $0 for the three months ended March 31, 2016.
Net cash provided by investing activities was $608,875 for the three months ended March 31, 2015. Net cash provided by investing activities for the three months ended March 31, 2015 primarily resulted from proceeds from sales of marketable securities of $1,000,805, offset by purchases of property and equipment of $391,930.
Financing Activities
Net cash used in financing activities was $59,100 for the three months ended March 31, 2016. Net cash used in financing activities for the three months ended March 31, 2016 consisted of payments on equipment lease financing of $59,100.
Net cash used in financing activities was $0 for the three months ended March 31, 2015.
Our business does not generate (nor do we anticipate that in the foreseeable future it will generate) the cash necessary to finance our operations. We expect to continue to incur losses and use cash during fiscal year 2016, and we will require additional capital to continue our operations beyond July 2016. As of March 31, 2016, we had cash and cash equivalents totaling $1,837,304, excluding restricted cash.
Our near-term capital needs depend on many factors, including:
| · | our ability to carefully manage our costs; |
| · | the amount and timing of revenue received from grants or our collaboration and license arrangements; and |
| · | our ability to raise additional capital through public or private equity offerings, debt financings, or strategic collaborations and licensing arrangements, and/or our success in promptly establishing a strategic alternative that is in our stockholders’ best interests. |
If we are unable to preserve or raise additional capital through one or more of the means listed above prior to the end of July 2016, we could face substantial liquidity problems and might be required to implement cost reduction strategies in addition to the cash conservation steps that we have already taken. These reductions could significantly affect our research and development activities, and could result in significant harm to our business, financial condition and results of operations. In addition, these reductions could cause us to further curtail our operations, or take other actions that would adversely affect our stockholders. If we are unable to raise additional capital on terms acceptable to us and on a timely basis, we may be required to downsize or wind down our operations through liquidation, bankruptcy, or a sale of our assets.
In addition, to the extent additional capital is raised through the sale of equity or convertible debt securities, such securities may be sold at a discount from the market price of our common stock. The issuance of these securities could also result in significant dilution to some or all of our stockholders, depending on the terms of the transaction. For example, the shares included in the Units and the warrants issued in connection with the PPO contain anti-dilution protection in the event that within certain specified time periods we issue common stock or securities convertible into or exercisable for shares of our common stock at a price lower than the Unit purchase price or the warrant exercise price, as applicable. The anti-dilution protection provisions apply to issuances within two years after the closing of the PPO in the case of the Units and, with respect to the warrants, prior to the warrant expiration date. In addition, the anti-dilution protection provisions are subject to exceptions for certain issuances, including but not limited to (a) shares of common stock issued in an underwritten public offering, (b) issuances of awards under our 2014 Equity Incentive Plan, and (c) other exempt issuances.
No assurance can be given that additional financing or strategic alliance and licensing arrangements will be available when needed or that, if available, such financing could be obtained on terms favorable to us or our stockholders. See “Risk Factors.”
Critical Accounting Policies, Estimates, and Judgments
Basis of Presentation
The unaudited condensed consolidated financial statements for the three month period ended March 31, 2016 and 2015 have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”). Any reference to applicable guidance is meant to refer to the authoritative United States generally accepted accounting principles as found in the Accounting Standards Codifications and Accounting Standards Updates (“ASU”) of the Financial Accounting Standards Board (“FASB”). The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the unaudited condensed consolidated financial statements and accompanying notes. On an ongoing basis, our management evaluates its estimates, which include, but are not limited to, estimates related to accruals, stock-based compensation expense, warrants to purchase securities, and reported amounts of revenues and expenses during the reported period. We base our estimates on historical experience and other market-specific or other relevant assumptions that we believe to be reasonable under the circumstances. Actual results may differ from those estimates or assumptions.
Principles of Consolidation
The unaudited condensed consolidated financial statements for the three month period ended March 31, 2016 and 2015 include our accounts and the accounts of our subsidiaries, Enumeral Biomedical Corp. and Enumeral Securities Corporation. In these unaudited condensed consolidated financial statements, “subsidiaries” are companies that are wholly owned, the accounts of which are consolidated with those of ours. Intercompany transactions and balances are eliminated in consolidation.
Cash and Cash Equivalents
We consider all highly liquid investments with maturities of 90 days or less from the purchase date to be cash equivalents. Cash and cash equivalents are held in depository and money market accounts and are reported at fair value.
Marketable Securities
Marketable securities consist of U.S. treasury securities with maturities of more than 90 days. We have determined the appropriate balance sheet classification of the securities as current since they are available for use in current operating activities, regardless of actual maturity dates, and are recorded on the balance sheet at fair value, with the unrealized gains and losses reported in accumulated other comprehensive income (loss), which is a separate component of stockholders’ equity.
Fair Value of Financial Instruments
Fair values of financial instruments included in current assets and current liabilities are estimated to approximate their book values, due to the short maturity of such instruments. All debt is based on current rates at which we could borrow funds with similar remaining maturities and approximates fair value. Our assets and liabilities that are measured at fair value on a recurring basis are measured in accordance with FASB’s ASC Topic 820,Fair Value Measurements and Disclosures, which establishes a three-level valuation hierarchy for measuring fair value and expands financial statement disclosures about fair value measurements.
Revenue Recognition
Collaboration and License Revenue
Non-refundable license fees are recognized as revenue when we have a contractual right to receive such payment, the contract price is fixed or determinable, the collection of the resulting receivable is reasonably assured and we have no further performance obligations under the license agreement. Multiple element arrangements, such as license and development arrangements are analyzed to determine whether the deliverables, which often include license and performance obligations such as research and steering committee services, can be separated or whether they must be accounted for as a single unit of accounting in accordance with GAAP.
We recognize up-front license payments as revenue upon delivery of the license only if the license has stand-alone value and the fair value of the undelivered performance obligations, typically including research and/or steering committee services, can be determined. If the fair value of the undelivered performance obligations can be determined, such obligations would then be accounted for separately as performed. If the license is considered to either (i) not have stand-alone or (ii) have stand-alone value but the fair value of any of the undelivered performance obligations cannot be determined, the arrangement would then be accounted for as a single unit of accounting and the license payments and payments for performance obligations are recognized as revenue over the estimated period of when the performance obligations are performed.
Whenever we determine that an arrangement should be accounted for as a single unit of accounting, we must determine the period over which the performance obligations will be performed and revenue will be recognized. Revenue will be recognized using either a relative performance or straight-line method. We recognize revenue using the relative performance method provided that we can reasonably estimate the level of effort required to complete our performance obligations under an arrangement and such performance obligations are provided on a best-efforts basis. Direct labor hours or full-time equivalents are typically used as a measure of performance. Revenue recognized under the relative performance method would be determined by multiplying the total payments under the contract, excluding royalties and payments contingent upon achievement of substantive milestones, by the ratio of level of effort incurred to date to estimated total level of effort required to complete our performance obligations under the arrangement. Revenue is limited to the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned, as determined using the relative performance method, as of each reporting period.
If we cannot reasonably estimate the level of effort required to complete our performance obligations under an arrangement, the performance obligations are provided on best-efforts basis and we can reasonably estimate when the performance obligation ceases or the remaining obligations become inconsequential and perfunctory. At that time, the total payments under the arrangement, excluding royalties and payments contingent upon achievement of substantive milestones, would be recognized as revenue on a straight-line basis over a period we expect to complete our performance obligations. Revenue is limited to the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned, as determined using the straight-line basis, as of the period ending date.
Grant Revenue
We recognize nonrefundable grant revenue that is earned in connection with our Small Business Innovation Research (“SBIR”) contracts with the NCI. In September 2012, we entered into a Phase I SBIR contract with the NCI, and in September 2014, we were awarded a Phase II SBIR contract from the NCI. Grant revenue consists of a portion of the funds received to date from the NCI, which allows us to conduct research on colon cancer tissues. Revenue is recognized as the related research services are performed in accordance with the terms of the contract.
Research and Development Expenses
Research and development expenditures are charged to the unaudited condensed consolidated statements of operations and comprehensive income (loss) as incurred. Research and development expenses are comprised of costs incurred in performing research and development activities, including salaries and benefits, facilities costs, clinical supply costs, contract services, depreciation and amortization expense and other related costs. Costs associated with acquired technology, in the form of upfront fees or milestone payments, are charged to research and development expense as incurred. Legal fees incurred in connection with patent applications along with fees associated with the license to our core technology are expensed as research and development expense.
Derivative Liabilities
Our derivative liabilities relate to (a) warrants to purchase an aggregate of 23,549,510 shares of our common stock that were issued in connection with the PPO and (b) warrants to purchase 41,659 shares of Enumeral Series A Preferred Stock that were issued in December 2011 and June 2012 pursuant to Enumeral’s debt financing arrangement with Square 1 Bank that were subsequently converted into warrants to purchase 66,574 shares of our common stock in connection with the July 2014 Merger.
Due to the price protection provision included in the warrant agreements, the warrants were deemed to be derivative liabilities and, therefore, the fair value of the warrants is recorded in the current liabilities section of the unaudited condensed consolidated balance sheet. As such, the outstanding warrants are revalued each reporting period with the resulting gains and losses recorded as the change in fair value of derivative liabilities on the unaudited condensed consolidated statements of operations and comprehensive income (loss).
We used the Black-Scholes option-pricing model to estimate the fair value of the issued and outstanding warrants upon issuance and as of March 31, 2016. As of January 1, 2016, we began using a blended average of our historical volatility and the historical volatility of a group of similarly situated companies (as described in detail below) to calculate the expected volatility when valuing our derivative liabilities.
Comprehensive Income (Loss)
Other comprehensive income (loss) is comprised of unrealized holding gains and losses arising during the period on available-for-sale securities that are not other-than-temporarily impaired. The unrealized gains and losses are reported in accumulated other comprehensive income (loss), until sold or mature, at which time they are reclassified to earnings.
Stock-Based Compensation
We account for our stock-based compensation awards to employees and directors in accordance with FASB ASC Topic 718, Compensation—Stock Compensation (“ASC 718”). ASC 718 requires all stock-based payments to employees, including grants of employee stock options and restricted stock, to be recognized in the unaudited condensed consolidated statements of operations and comprehensive income (loss) based on their grant date fair values. Compensation expense related to awards to employees is recognized on a straight-line basis based on the grant date fair value over the associated service period of the award, which is generally the vesting term. Share-based payments issued to non-employees are recorded at their fair values, and are periodically revalued as the equity instruments vest and are recognized as expense over the related service period in accordance with the provisions of ASC 718 and ASC Topic 505, Equity, and are expensed using an accelerated attribution model.
We estimate the fair value of our stock options using the Black-Scholes option pricing model, which requires the input of subjective assumptions, including (a) the expected stock price volatility, (b) the expected term of the award, (c) the risk-free interest rate, (d) expected dividends, and (e) the estimated fair value of our common stock on the measurement date. As of January 1, 2016, we began using a blended average of our historical volatility and the historical volatility of a group of similarly situated companies to calculate the expected volatility when valuing our stock options. For purposes of calculating this blended volatility, we selected companies with comparable characteristics to us, including enterprise value, risk profiles, position within the industry, and with historical share price information sufficient to meet the expected term of the stock-based awards. We compute historical volatility data using the daily closing prices for our company and the selected companies’ shares during the equivalent period of the calculated expected term of the stock-based awards. Prior to January 1, 2016, due to the lack of a public market for the trading of our common stock and a lack of company specific historical and implied volatility data, we have based our estimate of expected volatility only on the historical volatility of a group of similarly situated companies that were publicly traded. Due to the lack of our specific historical option activity, we have estimated the expected term of our employee stock options using the “simplified” method, whereby, the expected term equals the arithmetic average of the vesting term and the original contractual term of the option. The expected term for non-employee awards is the remaining contractual term of the option. The risk-free interest rates are based on the U.S. Treasury securities with a maturity date commensurate with the expected term of the associated award. We have never paid dividends, and do not expect to pay dividends in the foreseeable future.
Prior to the Merger, Enumeral engaged a third party to develop an estimate of the fair value of a share of Enumeral’s common stock on a fully-diluted, minority, non-marketable basis. Based upon unaudited historical, pro-forma and/or forecast financial and operational information, which Enumeral management represented as accurately reflecting Enumeral’s operating results and financial position, the third party utilized both a market approach (using various financial statement metrics of similar enterprises’ equity securities to estimate the fair value of Enumeral’s equity securities) and an income approach (which bases value on expectations of future income and cash flows) in their analyses. The fair value of a single share of common stock was determined using the option pricing method, which treats common and preferred stock as call options on the aggregate enterprise value and using traditional models, including Black-Scholes or binomial models, to calculate share values.
Following the Merger, our common stock became publicly traded, and fair market value is determined based on the closing sales price of our common stock on the OTC Markets.
During the year ended December 31, 2014, we engaged a third party to develop a binomial lattice model to estimate the fair value of options to purchase a total of 450,000 shares with vesting based on the future performance of a share of our common stock.
Effective January 1, 2016, we have elected to account for forfeitures as they occur, as permitted by ASU 2016-09,Compensation—Stock Compensation (Topic 718), Improvements to Employee Share-Based Payment Accounting. See the Accounting Standards Adopted in the Period section below for further details.
Prior to the adoption of ASU 2016-09, we estimated the number stock-based awards that were expected to vest, and only recognized compensation expense for such awards. The estimation of stock awards that will ultimately vest required judgment, and to the extent actual results or updated estimates differed from current estimates, such amounts were recorded as a cumulative adjustment in the period estimates were revised. We considered many factors when estimating expected forfeitures, including type of awards granted, employee class, and historical experience.
Earnings (Loss) Per Share
Basic earnings (loss) per common share amounts are based on the weighted average number of common shares outstanding. Diluted earnings (loss) per common share amounts are based on the weighted average number of common shares outstanding, plus the incremental shares that would have been outstanding upon the assumed exercise of all potentially dilutive stock options, warrants and convertible debt, subject to anti-dilution limitations. As of March 31, 2016 and 2015, the number of shares underlying options and warrants that were anti-dilutive were approximately 31.7 million and 25.5 million, respectively.
Recent Authoritative Guidance
In May 2014, the FASB issued ASU No. 2014-09,Revenue from Contracts with Customers (Topic 606). The ASU provides for a single comprehensive model for use in accounting for revenue arising from contracts with customers and supersedes most current revenue recognition guidance. The accounting standard is effective for interim and annual periods beginning after December 15, 2016 with no early adoption permitted. We are required to adopt the amendments in the ASU using one of two acceptable methods. Our management is currently in the process of determining which adoption method we will apply and evaluating the impact of the guidance on our unaudited condensed consolidated financial statements. In April 2016, the FASB issued ASU No. 2016-10,Revenue from Contracts with Customers (Topic 606): Identifying Performance Obligations and Licensing.The ASU clarifies the following two aspects of Topic 606: (a) identifying performance obligations; and (b) the licensing implementation guidance. The amendments do not change the core principle of the guidance in Topic 606. The effective date and transition requirements for the ASU are the same as the effective date and transition requirements in Topic 606. Public entities should apply the ASU for annual reporting periods beginning after December 15, 2017, including interim reporting periods therein (i.e., January 1, 2018, for a calendar year entity). Early application for public entities is permitted only as of annual reporting periods beginning after December 15, 2016, including interim reporting periods within that reporting period. We are currently evaluating the impact of this new standard on our unaudited condensed consolidated financial statements.
In August 2014, the FASB issued ASU No. 2014-15,Presentation of Financial Statements—Going Concern (Subtopic 205-40). The ASU requires all entities to evaluate for the existence of conditions or events that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the issuance date of the financial statements. The accounting standard is effective for interim and annual periods ending after December 15, 2016, and will not have a material impact on our unaudited condensed consolidated financial statements, but may impact our footnote disclosures.
In February 2016, the FASB issued ASU No. 2016-02,Leases (Topic 842). The ASU is effective for annual periods beginning after December 15, 2018, and requires a lessee to recognize assets and liabilities for leases with a maximum possible term of more than 12 months. A lessee would recognize a liability to make lease payments (the lease liability) and a right-of-use asset representing its right to use the leased asset (the underlying asset) for the lease term. Early application is permitted. We are currently evaluating the impact the adoption of the accounting standard will have on our unaudited condensed consolidated financial statements.
Accounting Standards Adopted in the Period
In March 2016 the FASB issued ASU 2016-09, which simplified several aspects of employee share-based payment accounting. In particular, the ASU permits entities to make an accounting policy election to either estimate forfeitures on share-based payment awards, as previously required, or to recognize forfeitures as they occur. Effective January 1, 2016, the Company elected to recognize forfeitures as they occur. The impact of that change in accounting policy has been recorded as an $8,333 cumulative effect adjustment to accumulated deficit, as of December 31, 2015. We expect that we will recognize slightly higher share-based payment expense for the remainder of 2016, relative to prior periods, as the effects of forfeitures will not be recognized until they occur, rather than being estimated at the time of grant and subsequently adjusted as and when necessary. The effects of adopting the remaining provisions in ASU 2016-09 affecting the income tax consequences of share-based payments, classification of awards as either equity or liabilities when an entity partially settles the award in cash in excess of the employer’s minimum statutory withholding requirements and classification in the unaudited condensed consolidated statements of cash flows did not have any impact on our financial position, results of operations or cash flows.
Other accounting standards that have been issued by the FASB or other standards-setting bodies that do not require adoption until a future date are not expected to have a material impact on our financial statements upon adoption.
Off-Balance Sheet Arrangements
We did not engage in any “off-balance sheet arrangements” (as that term is defined in Item 303(a)(4)(ii) of Regulation S-K) as of March 31, 2016 or December 31, 2015.
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
Directors and Executive Officers
Below are the names of and certain information regarding our current executive officers and directors as of July 22, 2016:
| Name | | Age | | Position | | Date Named to
Board of
Directors/as
Executive Officer | | Director
Class (if
applicable) |
| John J. Rydzewski | | 63 | | Executive Chairman of the Board | | July 31, 2014 | | I |
| Arthur H. Tinkelenberg, Ph.D. | | 49 | | President, Chief Executive Officer and Director | | July 31, 2014 | | I |
| Anhco Nguyen, Ph.D. | | 43 | | Vice President of Research and Development | | July 31, 2014 | | - |
| Kevin G. Sarney | | 51 | | Vice President of Finance, Chief Accounting Officer and Treasurer | | July 31, 2014 | | - |
| Barry Buckland, Ph.D. | | 68 | | Director | | July 31, 2014 | | II |
| Robert J. Easton | | 71 | | Director | | July 30, 2015 | | III |
| Allan P. Rothstein | | 58 | | Director | | July 31, 2014 | | II |
| Paul J. Sekhri | | 58 | | Director | | December 16, 2014 | | III |
| Robert L. Van Nostrand | | 59 | | Director | | December 1, 2014 | | III |
Our board of directors is divided into three classes, designated Class I, Class II and Class III. Each class consists, as nearly as may be possible, of one-third of the total number of directors constituting the entire board of directors. Class I directors were elected initially for a one-year term which expired at the 2015 Annual Meeting of Stockholders, Class II directors were elected initially for a two-year term which will expire in 2016 and Class III directors were elected initially for a three-year term which will expire in 2017. At each succeeding annual meeting of stockholders beginning with this annual meeting of stockholders, successors to the class of directors whose term expires at that annual meeting will be elected for a three-year term. If the number of directors is changed, any increase or decrease will be apportioned among the classes so as to maintain the number of directors in each class as nearly equal as possible, and any additional director of any class will hold office for a term that will coincide with the remaining term of that class, but in no case will a decrease in the number of directors shorten the term of any incumbent director.
A majority of the authorized number of directors constitutes a quorum of the board of directors for the transaction of business. The directors must be present at the meeting to constitute a quorum. However, any action required or permitted to be taken by the board of directors may be taken without a meeting if all members of the board of directors individually or collectively consent in writing to the action.
Executive officers are appointed by the board of directors and serve at its pleasure.
The principal occupation and business experience during the past five years for our executive officers and directors is as follows:
John J. Rydzewski – Executive Chairman of the Board of Directors and Co-Founder
Mr. Rydzewski has served as the Executive Chairman of our board of directors since the Merger (as defined below), and prior thereto served as a director of Enumeral Biomedical Corp. (“Enumeral”) since September 2010 and as Executive Chairman of Enumeral’s board since October 2011. From July 2006 to September 2011, Mr. Rydzewski was managing director of Christofferson, Robb & Co., an investment firm where he monetized life-sciences intellectual property covering large and small molecule therapeutics. His banking career included partnership responsibilities at Benedetto, Gartland & Co., an investment bank serving corporate and institutional clients, and officer positions in the merchant banking and healthcare specialty finance units of Kidder, Peabody & Co. Incorporated and Dean Witter Reynolds Inc. Prior to banking, he was a manager in the Bankruptcy Services Group of Price Waterhouse & Co., and he also previously held positions in industry. He has previously served as a director of publicly traded and privately held healthcare companies, and he currently serves as a director of Fidelis Care, Inc., a provider of health insurance coverage. Mr. Rydzewski is also Co-Chairman of the Board of Advisors of the RAND Corporation’s health policy research unit. He received an M.B.A. and a B.S. in Economics with Honors from The Wharton School of the University of Pennsylvania. The board of directors has concluded that Mr. Rydzewski should serve on our board because of his significant life sciences industry experience as an institutional investor, investment banker and corporate director.
Arthur H. Tinkelenberg, Ph.D. – President, Chief Executive Officer, Director and Co-Founder
Dr. Tinkelenberg has served as our President and Chief Executive Officer and a member of our board of directors since the Merger, and prior thereto was President, Chief Executive Officer and a director of Enumeral since September 2010. From 2003 through 2009, Dr. Tinkelenberg was a partner at Ascent Biomedical Ventures, a life sciences venture capital firm focused on early stage biomedical technologies, where he was responsible for originating and managing numerous biotechnology and medical device company investments. From January 2001 through July 2002, he served in the healthcare corporate finance and mergers and acquisitions group of Robertson Stephens. Prior to industry, Dr. Tinkelenberg conducted research in lipid metabolism, genomics, biochemistry, and cell biology as a Research Scientist at Columbia University from 1996 through 2000. He is a published author and inventor on a number of patents from projects initiated by him during his tenure in academic research. He received his Ph.D. in molecular genetics from The Rockefeller University and his B.A. in Biology from Grinnell College. The board of directors has concluded that Dr. Tinkelenberg should serve on our board because of his significant experience in the life sciences industry as an investment banker and venture capitalist, as well as his previous life sciences academic research.
Anhco Nguyen, Ph.D. - Vice President of Research and Development
Dr. Nguyen has served as our Vice President of Research and Development since the Merger, and prior thereto held positions of increasing responsibility since joining Enumeral in January 2012. Dr. Nguyen leads our research and development team and is responsible for both our proprietary discovery and corporate partnership programs. He participates with our business development team in structuring new research collaboration initiatives. He brings to this role significant industry experience in the development of therapeutic antibodies for treatment of cancer and multiple sclerosis, including as staff scientist at Forma Therapeutics from June 2009 to January 2012, and as staff scientist at Genzyme Corp. from June 2006 to May 2009. He completed postdoctoral training at MIT’s Cancer Center and was the recipient of a Presidential Postdoctoral Fellowship, which he completed at the Novartis Institute for Biomedical Research. He received his Ph.D. in Immunology from Washington University and his A.B. in Biology from Harvard College. He has co-authored numerous articles in the fields of cancer and immunology.
Kevin G. Sarney – Vice President of Finance, Chief Accounting Officer and Treasurer
Kevin G. Sarney has served as our Vice President of Finance, Chief Accounting Officer and Treasurer since the Merger. From September 2013 to July 2014, Mr. Sarney served as Vice President, Finance and Administration for Avaxia Biologics, Inc., a privately-held clinical-stage biopharmaceutical company developing gut-targeted therapeutics. Prior to joining Avaxia, Mr. Sarney provided financial consulting services to various life sciences companies from May 2009 to August 2013, including at Helicos BioSciences Corporation, a publicly traded medical device sequencing company, from December 2009 to May 2010 and at Archemix Corp., a privately held aptamer therapeutics company, from June 2010 to February 2012. In addition, from February 2012 to August 2013, Mr. Sarney provided consulting services for various life sciences companies, including Tetraphase Pharmaceuticals, Inc., Tesaro Inc., Karyopharm Therapeutics Inc., and Genocea Biosciences, Inc. From March 2005 to April 2009, Mr. Sarney held positions of increasing responsibility at NitroMed, Inc., a publicly traded cardiovascular-focused pharmaceutical company, culminating as Corporate Controller and Principal Accounting Officer. Mr. Sarney earned a B.S. in business management from the University of Hartford, an M.B.A. from Boston University, and an M.A. in accounting from Suffolk University. Mr. Sarney is a certified public accountant in the Commonwealth of Massachusetts.
Barry Buckland, Ph.D. – Director, Co-Founder and Chairman of the Scientific Advisory Board
Dr. Buckland has been a member of our board of directors since the Merger and prior thereto served as member of the board of directors of Enumeral since December 2009. Dr. Buckland’s career in the pharmaceutical industry includes his leadership of Merck’s bioprocess Research and Development Group, which saw the market launch of major biological products, including Gardasil, Rotateq, and Zostavax. Since May 2009, Dr. Buckland has been the Chief Executive Officer of BiologicB LLC, a consulting firm working with biotechnology and vaccine products. Dr. Buckland is a member of the supervisory board of Mucosis BV, a privately held Netherlands company focused on developing a proprietary mucosal vaccine technology platform. Dr. Buckland also serves on the board of directors for Engineering Conferences International, a not-for-profit global engineering conferences program. Dr. Buckland is the recipient of numerous awards, among them the Donald Medal, UK Institute of Chemical Engineering in 2002, Prix Galien award as a member of the team that received the Vaccine Award for Gardasil in 2007, the Marvin Johnson award by ACS for lifetime contribution to Biotechnology in 2008, and the PhRMA Discoverer of the Year for development of the Merck HPV vaccine (along with Eliav Barr and Kathrin Jansen) in 2009. He has chaired multiple International Conferences related to Bioprocess Research and Development and Vaccine Technology and is an author on over 70 papers. He has been a Visiting Professor at University College London since 1995. Dr. Buckland earned his Ph.D. in Biochemical Engineering at University College London in 1974. The board of directors has concluded that Dr. Buckland should serve on our board because of his significant experience in the pharmaceutical and biotechnology industries.
Robert J. Easton – Director
Mr. Easton has been a member of our board of directors since 2015. He is a recognized leader in the life sciences and healthcare industries, having led strategy development and supervised opportunity assessments for hundreds of clients across the globe, including large and specialty pharmaceutical companies, early-stage and publicly traded biopharmaceutical companies, and diagnostics businesses. Mr. Easton currently serves as Co-chairman of Bionest Partners, Inc., a consulting firm he co-founded that specializes in strategic planning for pharmaceutical, medical device and diagnostic companies. Prior to co-founding Bionest Partners, Mr. Easton built and led two consulting firms, Easton Associates and The Wilkerson Group, which provided advisory services in the healthcare, pharmaceuticals and medical products industries. Previously, Mr. Easton spent 12 years in various management roles at Union Carbide and Union Carbide Europe, including in marketing and engineering in the clinical diagnostics business unit. Mr. Easton has served since 2002 on the Board of Directors of Cepheid, a molecular diagnostics company, where he also serves on the Compensation Committee and the Nominating and Governance Committee. He currently serves as chairman of the New York Biotechnology Association, and also serves on the Board of Advisors of the Global Cardiovascular Innovation Center at the Cleveland Clinic. Mr. Easton holds B.S. and B.A. degrees in chemical engineering from Rice University, and an M.B.A. from Harvard University. Mr. Easton serves as Chairman of our Audit Committee. The board of directors has concluded that Mr. Easton should serve on our board because of his significant experience in the life sciences and healthcare industries.
Allan P. Rothstein – Director
Mr. Rothstein has been a member of our board of directors since the Merger, and prior thereto served as member of the board of directors of Enumeral since September 2013. Since April 2004, Mr. Rothstein has owned and managed Hedge Capital Partners LLC, an investment vehicle for venture capital, portfolio management and asset management. Mr. Rothstein has also owned Shizoom LLC, a business funding company, since it was formed in March 2013. Mr. Rothstein has served as founder and president of Empire Spirits Project, Inc., a New York-based distillery, since 2013. Since April 2015, Mr. Rothstein has also served as a partner and Chairman of The Agency Worx LLC, which specializes in staffing, recruitment and relationship management. From September 2010 to August 2011, Mr. Rothstein was head of trading at Centurion Partners. Mr. Rothstein served as a principal of Healthcare Recovery Services LLC, which services financial aspects and enhances revenue cycles of hospitals and other healthcare service enterprises, from December 2011 through November 2014. From 2002 through 2007, Mr. Rothstein was co-founder and Chairman of NanoDynamics Inc. He has served as a member of the National Nanotechnology Business Alliance and the New York Nanotechnology Business Alliance advisory boards and the Dean’s Council of the Graduate School of SUNY Stonybrook. Mr. Rothstein has over 30 years’ experience in the financial industry, having started his career at Shearson Loeb Rhoades where he handled arbitrage lines for domestic and international customers. In 1982, he became a member of the New York Futures Exchange. In 1990 he joined Fahnestock & Company (presently, Oppenheimer & Company) as a Nasdaq market maker. In his role as Senior Vice President and co-head of Nasdaq Trading, Mr. Rothstein was responsible for trading in over 500 markets, oversight of all Nasdaq market making and trading systems construction and integration. Mr. Rothstein graduated as a Benjamin Franklin Scholar from The University of Pennsylvania,magna cum laude, in 1980. The board of directors has concluded that Mr. Rothstein should serve on our board because of his significant experience as an investor in, and advisor to, emerging technology companies.
Paul J. Sekhri – Director
Mr. Sekhri has been a member of our board of directors since December 2014. Since February 2015, Mr. Sekhri has served as President and Chief Executive Officer and a member of the board of directors of Lycera Corp., a biopharmaceutical company that is developing medicines to treat cancer and autoimmune disease. From April 2014 to February 2015, Mr. Sekhri was Senior Vice President, Integrated Care at Sanofi S.A., a multinational pharmaceutical company, where he led the company’s efforts to create innovative solutions and business models to meet patient needs. Prior to Sanofi, Mr. Sekhri served from June 2013 to March 2014 as Group Executive Vice President, Global Business Development and Chief Strategy Officer for Teva Pharmaceutical Industries, Ltd., a multinational pharmaceutical company. From January 2009 to May 2014, Mr. Sekhri served as Operating Partner and Head of the Biotechnology Operating Group at TPG Biotech, the life sciences venture capital arm of TPG Capital. From 2004 to 2009, Mr. Sekhri was Founder, President, and Chief Executive Officer of Cerimon Pharmaceuticals, Inc. Prior to founding Cerimon, Mr. Sekhri was President and Chief Business Officer of ARIAD Pharmaceuticals, Inc. from 2003 to 2004. From 2002 to 2003, Mr. Sekhri was a partner at New Leaf Ventures, where he focused on healthcare investments. From 1999 to 2003, Mr. Sekhri held various positions at Novartis Pharma AG, including Senior Vice President and Head of Global Search and Evaluation, Business Development and Licensing. Mr. Sekhri serves on the board of directors of Veeva Systems Inc., a publicly-held cloud-based software company focused on the life sciences industry. Mr. Sekhri is also a member of the board of directors of Ascendancy Healthcare Inc., a privately held company focused on the development and registration of pharmaceutical products in China and other high-growth Asian markets. Mr. Sekhri serves on the board of directors of the non-profit Cancer Research Institute, the BioExec Institute, Inc., the Industry Advisory Board of the Michael J. Fox Foundation, the TB Alliance, the Tectonic Theatre Project, and Young Concert Artists, Inc. Mr. Sekhri is a member of the Patrons Council of Carnegie Hall, where he served as a member of the Board of Trustees from 2010 to 2012. Mr. Sekhri holds a B.S. in Zoology from the University of Maryland. The board of directors has concluded that Mr. Sekhri should serve on our board because of his significant experience as a senior executive and director in the life sciences industry.
Robert L. Van Nostrand– Director
Mr. Van Nostrand has been a member of our board of directors since December 2014. Since 2010, Mr. Van Nostrand has served as an advisor and board member to several biotechnology companies. From January 2010 to July 2010, Mr. Van Nostrand was Executive Vice President and Chief Financial Officer of Aureon Laboratories, Inc., a pathology life sciences company. Prior to joining Aureon Laboratories, Mr. Van Nostrand served as Executive Vice President and Chief Financial Officer of AGI Dermatics, Inc., a private biotechnology company, from July 2007 to September 2008, at which time the company was acquired. From May 2005 to July 2007, Mr. Van Nostrand served as the Senior Vice President and Chief Compliance Officer of OSI Pharmaceuticals, Inc. a biotechnology company, where he previously served as Vice President and Chief Financial Officer from December 1996 through May 2005, and as Vice President, Finance and Administration prior to that. He also served as OSI’s Treasurer from March 1992 to May 2005, and as Secretary from March 1995 to January 2004. Mr. Van Nostrand joined OSI as Controller and Chief Accounting Officer in September 1986. Prior to joining OSI, Mr. Van Nostrand served in a managerial position with the accounting firm of Touche Ross & Co. (now Deloitte). Mr. Van Nostrand serves on the board of directors of Achillion Pharmaceuticals, Inc., a publicly-held biotechnology company, where he is chairman of the audit committee and a member of the compensation committee. Mr. Van Nostrand also serves on the board of directors and is chairman of the audit committee of Intra-Cellular Therapies, Inc. and Metabolix, Inc., both publicly-held biotechnology companies. In addition, Mr. Van Nostrand is chairman of the board of directors at Metabolix. Mr. Van Nostrand is on the board of the Biomedical Research Alliance of New York, a private company providing clinical trial services, as well as the Board of the New York Biotechnology Association, where he previously served as chairman. In addition, he is on the Foundation Board of Farmingdale University. Mr. Van Nostrand holds a B.S. in Accounting from Long Island University, New York, and he is a Certified Public Accountant.The board of directors has concluded that Mr. Van Nostrand should serve on our board because of his significant experience as a senior executive and director in the life sciences industry.
Daniel B. Wolfe, Ph.D., resigned from our board on July 30, 2015.
Director Relationships and Arrangements
Except for Messrs. Easton, Rydzewski, Sekhri and Van Nostrand, none of our current directors or executive officers have served as a director of another public company within the past five years.
On July 31, 2014, our wholly owned subsidiary, Enumeral Acquisition Corp. (“Acquisition Sub”) merged (the “Merger”) with and into Enumeral. Enumeral was the surviving corporation in the Merger and became our wholly owned subsidiary. All of the outstanding shares of Enumeral stock were converted into shares of our common stock. Also on July 31, 2014, we closed a private placement offering (the “PPO”) of 21,549,510 Units of our securities, at a purchase price of $1.00 per unit, each unit consisting of one share of our common stock and a warrant to purchase one share of common stock at an exercise price of $2.00 per share. In connection with the transactions pursuant to the Merger, certain stockholders of our company (holding a majority of our common stock), including all of the investors in the PPO, all of our pre-Merger stockholders and certain of the Enumeral stockholders (including all of its officers directors and principal stockholders) entered into a voting agreement in which they have agreed to vote their shares of our stock to maintain the composition of our board of directors (the “Voting Agreement”). The Voting Agreement will terminate on July 31, 2016.
To our knowledge, there are no arrangements or understandings between any director, director nominee or executive officer and any other person pursuant to which any person was selected as a director, director nominee or executive officer, other than the Voting Agreement. There are no family relationships between any of our directors, director nominees or executive officers. To our knowledge, there have been no material legal proceedings as described in Item 401(f) of Regulation S-K during the last ten years that are material to an evaluation of the ability or integrity of any of our directors, director nominees or executive officers. In addition, to our knowledge, there are no pending legal proceedings to which any of the directors or executive officers is a party adverse to or has a material interest that is adverse to our company or Enumeral.
Board of Directors and Corporate Governance
While we are currently quoted on the over-the-counter market, we have adopted the corporate governance standards of a listed company on the Nasdaq Stock Market, or Nasdaq. These standards require that a majority of the members of our board of directors be “independent,” as Nasdaq defines that term, and that our board make an affirmative determination as to the independence of each director. Consistent with these rules, our board of directors undertook its annual review of director independence in April 2016. During the review, our board considered relationships and transactions during 2015 and during the past three fiscal years between each director or any member of his or her immediate family, on the one hand, and our company and its subsidiaries and affiliates, on the other hand. The purpose of this review was to determine whether any such relationships or transactions were inconsistent with a determination that the director is independent. Based on this review, our board of directors determined that Dr. Buckland and Messrs. Easton, Sekhri, and Van Nostrand are independent under the criteria established by Nasdaq and by our board of directors.
Our board of directors held 7 meetings during 2015, and each incumbent director standing for election attended at least 75% of the meetings of the board and the meetings of those committees on which each incumbent director served, in each case during the period that such person was a director. The permanentcommittees established by our board of directors are the audit committee, the compensation committee, and the nominating and governance committee, descriptions of which are set forth in more detail below.
Each member of our board of directors is expected to participate, either in person or via teleconference, in meetings of the board and meetings of committees of which each director is a member, and to spend the time necessary to properly discharge such director’s respective duties and responsibilities. Although we do not have a written policy regarding directors’ attendance at annual meetings of stockholders, all directors are encouraged to attend. Four directors attended our annual meeting of stockholders in 2015.
Board Leadership Structure
Our board of directors is currently chaired by Mr. Rydzewski. The Board Chairman has authority, among other things, to call and preside over board of directors meetings, to set meeting agendas and to determine materials to bedistributed to the board. We believe that having a Board Chairman separate from the chief executive officer can enhance the effectiveness of the board of directors as a whole. In addition, our board believes it should be able to freely select the Board Chairman based on criteria that it deems to be in our best interests and the interests of our stockholders, and therefore one person may, in the future, serve as both our Chief Executive Officer and Board Chairman.
The board hasdesignated Mr. Van Nostrand as the lead independent director. We believe that the position of lead independent director reinforces the independence of the board in its oversight of our business and affairs. The lead independent director, among his other duties and responsibilities, serves as chairman at sessions of the independent and non-employee directors, and is the principal liaison between the independent and non-management directors and the Board Chairman, the President and Chief Executive Officer, and our senior management. The lead independent director consults with the Board Chairman regarding the schedule of board meetings, as well as the agenda and information to be presented at such meetings. When the Board Chairman is not present, the lead independent director acts as chairman of board meetings. Pursuant to the lead independent director charter, the individual holding that position must meet the independence requirements set forth in the applicable rules and listing standards of Nasdaq, or such other national securities exchange on which our securities are then listed.
Role of the Board in Risk Oversight
Our board of directors has an active role in overseeing management of our risks, which the board administers directly as well as through various standing committees of the board that address risks inherent in their respective areas of oversight. In particular, our board of directors is responsible for monitoring and assessing strategic risk exposure, including information regarding our credit, liquidity and operations and the risks associated with each. Our primary risks are currently associated with the development of our platform technology, including our ability to raise additional capital to complete the development and potential commercialization of product candidates using our platform technology. The audit committee of the board of directors has the responsibility to consider and discuss our major financial risk exposures and the steps that our management has taken to monitor and control these exposures. The audit committee also monitors compliance with legal and regulatory requirements, in addition to oversight of the performance of our internal controls over financial reporting. The nominating and governance committee of the board of directors monitors the effectiveness of our corporate governance guidelines, and manages risks associated with the independence of the board of directors and potential conflicts of interest. The compensation committee of the board of directors assesses and monitors whether any of our compensation policies and programs has the potential to encourage excessive risk-taking. While each committee is responsible for evaluating certain risks and overseeing management of such risks, the entire board of directors is regularly informed through committee reports about such risks.
Communicating with the Board of Directors
Stockholders can mail communications to our board of directors to our Corporate Secretary, Enumeral Biomedical Holdings, Inc., 200 CambridgePark Drive, Suite 2000, Cambridge, Massachusetts 02140, who will forward the correspondence to each addressee.
Board Committees
Audit Committee
The audit committee was established on August 4, 2014 and currently consists of Mr. Easton, as chairman, and Messrs. Sekhri and Van Nostrand. Mr. Easton was appointed to replace Dr. Wolfe in July 2015 in connection with Dr. Wolfe’s resignation from our board. Messrs. Sekhri and Van Nostrand were appointed to replace Dr. Buckland and Mr. Rothstein in February 2015. Dr. Buckland and Mr. Rothstein were appointed to the audit committee in August 2014 when the committee was formed following the Merger. The audit committee held 5 committee meetings during 2015.
The duties and responsibilities of the audit committee are set forth in the charter of the audit committee. A copy of the charter of the audit committee is available under the Investors—Corporate Governancesection of our corporate website atwww.enumeral.com. Among other things, the duties and responsibilities of the audit committee include reviewing and monitoring our financial statements and internal accounting procedures, the selection of our independent registered public accounting firm and consulting with and reviewing the services provided by our independent registered public accounting firm. The audit committee has sole discretion over the retention, compensation, evaluation and oversight of our independent registered public accounting firm.
The Unites States Securities and Exchange Commission, or SEC, and Nasdaq have established rules and regulations regarding the composition of audit committees and the qualifications of audit committee members. Our board of directors has examined the composition of the audit committee and the qualifications of the audit committee members in light of the current rules and regulations governing audit committees. Based upon this examination, our board of directors has determined that each member of the audit committee is independent and is otherwise qualified to be a member of the audit committee in accordance with the rules of the SEC and Nasdaq.
Additionally, the SEC requires that at least one member of the audit committee have a “heightened” level of financial and accounting sophistication. Such a person is known as the “audit committee financial expert” under the SEC’s rules. Our board of directors has determined that Mr. Van Nostrand satisfies the definition of “audit committee financial expert” in the rules and regulations of the SEC, and is an independent member of the board of directors and the audit committee. Our board had previously determined that Dr. Wolfe, during his service on the audit committee, also satisfied the definition of audit committee financial expert, and was an independent member of the board of directors and the audit committee.
Compensation Committee
The compensation committee was established on August 4, 2014 and currently consists of Mr. Sekhri, as chairman, and Dr. Buckland and Mr. Van Nostrand. Messrs. Sekhri and Van Nostrand were appointed to replace Mr. Rothstein and Dr. Wolfe in February 2015. Mr. Rothstein and Dr. Wolfe were appointed to the compensation committee in August 2014 when the committee was formed following the Merger. The compensation committee held 4 committee meetings during 2015.
The duties and responsibilities of the compensation committee are set forth in the charter of the compensation committee. A copy of the charter of the compensation committee is available under the Investors—Corporate Governancesection of our corporate website atwww.enumeral.com. As discussed in its charter, among other things, the duties and responsibilities of the compensation committee include evaluating the performance of our executive officers, determining the overall compensation of our executive officers and administering all executive compensation programs, including, but not limited to, our incentive and equity-based plans. The compensation committee evaluates the performance of our executive officers on an annual basis and reviews and approves on an annual basis all compensation programs and awards relating to such officers. The compensation committee applies discretion in the determination of individual executive compensation packages to ensure compliance with our compensation philosophy. Our chief executive officer makes recommendations to the compensation committee with respect to the compensation packages for officers other than himself.
Nasdaq has established rules and regulations regarding the composition of compensation committees and the qualifications of compensation committee members. Our board of directors has examined the composition of the compensation committee and the qualifications of the compensation committee members in light of the current rules and regulations governing compensation committees. Based upon this examination, our board of directors has determined that each member of the compensation committee is independent and is otherwise qualified to be a member of the compensation committee in accordance with such rules.
None of our executive officers has served as a member of the compensation committee (or other committee serving an equivalent function) of any other entity.
Nominating and Governance Committee
The nominating and governance committee was established in February 2015 and currently consists of Dr. Buckland, as chairman, and Messrs. Sekhri and Van Nostrand. The nominating and governance committee conducted all of their business by written consent in 2015 and did not hold any committee meetings during 2015.
The duties and responsibilities of the nominating and governance committee are set forth in the nominating and governance committee charter. A copy of the nominating and governance committee charter is available under the Investors—Corporate Governancesection of our corporate website atwww.enumeral.com.Among other things, the duties and responsibilities of the nominating and governance committee include identifying individuals qualified to become board members, recommending director nominees to the board for the next annual meeting of stockholders, evaluating the overall effectiveness of the board, developing, mentoring and evaluating our corporate governance practices, and performing such other duties as enumerated in and consistent with its charter.
The nominating and governance committee will also consider candidates recommended by stockholders for nomination to the board of directors. A stockholder who wishes to recommend a candidate for nomination to the board of directors must submit such recommendation to Enumeral Biomedical Holdings, Inc., 200 CambridgePark Drive, Suite 2000, Cambridge, Massachusetts 02140; Attention: Corporate Secretary. All stockholder recommendations of candidates for nomination for election to the board of directors must be in writing and must set forth the following: (i) the candidate’s name, age, business address, and other contact information, (ii) the number of shares of our common stock beneficially owned by the candidate, (iii) a complete description of the candidate’s qualifications, experience, background and affiliations, as would be required to be disclosed in the proxy statement pursuant to Schedule 14A under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), (iv) a sworn or certified statement by the candidate in which he or she consents to being named in the proxy statement as a nominee and to serve as director if elected, and (v) the name and address of the stockholder(s) of record making such a recommendation and the number of shares owned by the recommending stockholder(s).
We believe that our board of directors as a whole should encompass a range of talent, skill and expertise enabling it to provide sound guidance with respect to our operations and interests. The nominating and governance committee evaluates all candidates for the board of directors by reviewing their biographical information and qualifications. The nominating and governance committee then determines, based on the background information and the information obtained in the interviews, whether to recommend to the board of directors that the candidate be nominated for approval by the stockholders to fill a directorship. With respect to an incumbent director whom the nominating and governance committee is considering as a potential nominee for re-election, the nominating and governance committee reviews and considers the incumbent director’s service during his or her term, including the number of meetings attended, level of participation, and overall contribution to the board of directors. The manner in which the nominating and governance committee evaluates a potential nominee will not differ based on whether the candidate is recommended by the directors or stockholders.
Nasdaq has established rules and regulations regarding the composition of nominations committees and the qualifications of nominations committee members. The board of directors has examined the composition of the nominating and governance committee and the qualifications of the members of the nominating and governance committee in light of the current rules and regulations governing nominations committees. Based upon this examination, the board of directors has determined that each member of the nominating and governance committee is independent and is otherwise qualified to be a member of the nominating and governance committee in accordance with such rules.
We do not have a formal policy in place with regard to the consideration of diversity of candidates for the board of directors, but the board of directors strives to nominate candidates with a variety of complementary skills so that, as a group, the board of directors will possess the appropriate talent, skills and expertise to oversee our business.
Code of Ethics and Business Conduct
We have adopted a written code of ethics and business conduct that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. A current copy of the code of ethics and business conduct is available under the Investors—Corporate Governance section of our corporate website atwww.enumeral.com. A copy of the code of ethics and business conduct will also be provided to any person, without charge, upon written request sent to Enumeral Biomedical Holdings, Inc., 200 CambridgePark Drive, Suite 2000, Cambridge, Massachusetts 02140; Attention: Corporate Secretary. Any amendments to or waivers of the code of ethics and business conduct will be promptly posted on our website atwww.enumeral.com or in a Current Report on Form 8-K, as required by applicable laws.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors and officers and holders of more than 10% of our common stock to file with the SEC initial reports of ownership of our common stock and other equity securities on a Form 3 and reports of changes in such ownership on a Form 4 or Form 5. Directors and officers and holders of 10% of our common stock are required by SEC regulations to furnish us with copies of all Section 16(a) forms they file. We registered our common stock under Section 12(g) of the Exchange Act on April 3, 2015. To our knowledge, based solely on a review of our records and representations made by our directors and officers regarding their filing obligations, all Section 16(a) filing requirements were satisfied with respect to the applicable period in 2015.
Related Person Transaction Approval Policy
While we have no written policy regarding approval of transactions between us and a related person, our board of directors, as matter of appropriate corporate governance, reviews and approves all such transactions to the extent required by applicable rules and regulations. Generally, management would present any related person transactions proposed to be entered into by us to the board of directors for approval. The board may approve the transaction if it is deemed to be in the best interests of our stockholders and our company.
EXECUTIVE COMPENSATION
Executive Compensation Overview
Our compensation committee sets base salaries and bonuses and grants equity incentive awards to our executive officers. In setting base salaries and bonuses and granting equity incentive awards, our compensation committee considers compensation for comparable positions in the market, the historical compensation levels of our executives, individual and corporate performance as compared to our expectations and objectives, our desire to motivate our employees to achieve short- and long-term results that are in the best interests of our stockholders, and a long-term commitment to our company. The compensation committee also consults with the board of directors from time to time regarding compensation for our executive officers.
In 2015, our compensation committee engaged J. Thelander Consulting as its independent compensation consultant to review our executive compensation and director compensation and assess such compensation relative to comparable companies. J. Thelander Consulting worked with our compensation committee to determine a peer group of comparable companies, and review comparable compensatory arrangements for our executive officers and our board of directors and the committees thereof. Following consultation with J. Thelander Consulting, our compensation committee made certain adjustments to the compensatory arrangements of executive officers and the board of directors, as noted herein.
The material terms of the elements of our executive compensation program for 2015 are described below.
Base Salary
Base salaries are used to recognize the experience, skills, knowledge and responsibilities required of all our employees, including our named executive officers. None of our named executive officers is currently party to an employment agreement or other agreement or arrangement that provides for automatic or scheduled increases in base salary.
In May 2015, our compensation committee, following consultation with J. Thelander Consulting, increased Dr. Nguyen’s annual base salary to $275,000.
Performance Bonus
Each named executive officer is eligible for an annual performance cash bonus based upon the achievement of certain corporate and individual goals and objectives approved by our board or our compensation committee. Cash bonuses are based on the executive officer’s base salary.
All final bonus payments to our named executive officers are determined by our compensation committee, which retains full discretion to adjust individual target bonus awards. The actual bonuses, if any, awarded in a given year may vary from target, depending on individual performance and the achievement of corporate objectives and may also vary based on other factors at the discretion of our compensation committee.
Pursuant to their July 2014 Amended and Restated Employment Agreements, each of Dr. Tinkelenberg and Mr. Rydzewski is entitled to participate in bonus plans approved by our board or compensation committee, with a target incentive bonus percentage equal to 40% of their then-current annual base salary. In September 2015, our compensation committee awarded cash bonus payments to each of Dr. Tinkelenberg and Mr. Rydzewski in the amount of $105,000, representing their target bonus percentage of 40% of annual base salary, in recognition of corporate achievements in 2014. In May 2015, our compensation committee awarded a cash bonus payment to Dr. Nguyen in the amount of $25,000, representing over-achievement of his then-target bonus of 10% of annual base salary. Also in May 2015, our compensation committee, following consultation with J. Thelander Consulting, increased Dr. Nguyen’s target bonus percentage to 30% of his base salary.
Our compensation committee did not establish formal corporate goals and objectives in calendar year 2015.
Equity Incentives
Our equity incentive program is the primary vehicle for offering long-term incentives to our executives, although the compensation committee has the authority to award other forms of equity-based compensation under our stock incentive plan. We believe that grants of equity incentive awards provide our executives with a strong link to our long-term performance and create an ownership culture that helps align the interests of our executives and stockholders. In addition, the vesting feature of our equity incentive grants furthers our goal of executive retention because this feature provides an incentive to our executives to remain in our employ during the vesting period.
In September 2015, following consultation with J. Thelander Consulting, our compensation committee awarded Dr. Tinkelenberg and Mr. Rydzewski options to purchase 880,000 and 720,000 shares of our common stock, respectively, at an exercise price of $0.36 per share. Fifty percent (50%) of the shares underlying these options vest and become exercisable in 48 equal monthly installments over 4 years from the date of grant, twenty-five percent (25%) of the shares shall vest and become exercisable, if at all, upon us entering into a collaboration agreement with either an academic institution or a corporate partner on terms acceptable to our board, and twenty-five percent (25%) of the shares shall vest and become exercisable, if at all, upon the closing of an equity financing transaction on terms acceptable to our board with gross proceeds to our company in excess of a specified amount.
Also in September 2015, again following consultation with J. Thelander Consulting, our compensation committee awarded Dr. Nguyen an option to purchase 400,000 shares of our common stock at an exercise price of $0.47 per share. This option vests and becomes exercisable in 48 equal monthly installments over 4 years from the date of grant.
Summary Compensation Table
The following table sets forth information concerning the total compensation paid or accrued by the Company and Enumeral during the last two fiscal years indicated to (i) all individuals that served as our or Enumeral’s principal executive officer or acted in a similar capacity for the Company or Enumeral at any time during the most recent fiscal year indicated; (ii) the two most highly compensated executive officers who were serving as executive officers of the Company or Enumeral at the end of the most recent fiscal year indicated; and (iii) up to two additional individuals for whom disclosure would have been provided pursuant to clause (ii) above but for the fact that the individual was not serving as an executive officer of the Company or Enumeral at the end of the most recent fiscal year indicated.
Name & Principal
Position | | Fiscal
Year
ended
December 31, | | | Salary ($) | | | Bonus ($) | | | Option
Awards
($)(1) | | | Total ($) | |
| John J. Rydzewski | | | 2015 | | | | 262,500 | | | | 105,000 | (2) | | | 216,235 | (3) | | | 583,735 | |
| Executive Chairman | | | 2014 | | | | 167,359 | (4) | | | — | | | | 233,461 | (5) | | | 400,820 | |
Arthur H.
Tinkelenberg, Ph.D. | | | 2015 | | | | 262,500 | | | | 105,000 | (2) | | | 264,287 | (6) | | | 631,787 | |
| President and Chief Executive Officer | | | 2014 | | | | 174,609 | (4) | | | — | | | | 248,769 | (7) | | | 423,378 | |
Anhco Nguyen, Ph.D.
Vice President of
Research and | | | 2015 | | | | 241,597 | (8) | | | 25,000 | (9) | | | 156,860 | (10) | | | 423,457 | |
| Development | | | 2014 | | | | 162,083 | (11) | | | 43,885 | (12) | | | 94,804 | (13) | | | 300,772 | |
| | (1) | These amounts represent the aggregate grant date fair value of (a) stock option awards granted in fiscal years 2015 and 2014 under our 2014 Equity Incentive Plan and (b) with respect to Mr. Rydzewski and Dr. Tinkelenberg, warrants to purchase shares of Enumeral Series B Preferred Stock granted in fiscal 2014, as further detailed in notes (4), (5) and (7) below. In all cases, these amounts are computed in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification Topic 718 (“Topic 718”). These amounts do not represent the actual amounts paid to or realized by the Named Executive Officers during fiscal years 2015 and 2014, and there can be no assurance that the Topic 718 amount will ever be realized. A description of the assumptions we used for purposes of determining grant date fair value is set forth in the notes to our consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2015, which was filed with the SEC on March 30, 2016. The named executive officers will only realize compensation to the extent the trading price of our common stock is greater than the exercise price of such stock options or warrants, as applicable. |
| (2) | These amounts reflect bonuses paid in 2015 for service in calendar year 2014, representing the target bonus of 40% of annual salary. |
| (3) | This amount reflects a September 28, 2015 grant of 720,000 incentive stock options to purchase shares of our common stock. |
| | (4) | During the first half of 2014, Mr. Rydzewski and Dr. Tinkelenberg each agreed to a reduction in salary until such time as Enumeral obtained at least $2 million in proceeds in connection with either a financing transaction or a corporate collaboration or similar arrangement. Mr. Rydzewski’s and Dr. Tinkelenberg’s full salaries were restored in connection with the Merger. In relation to this salary reduction, Mr. Rydzewski and Dr. Tinkelenberg received warrants to purchase 47,058 and 58,823 shares of Enumeral Series B Preferred Stock, respectively, as further detailed in notes (5) and (7) below. In connection with the Merger, Mr. Rydzewski’s and Dr. Tinkelenberg’s Enumeral Series B Preferred Stock warrants were converted into warrants to purchase 137,762 and 172,204 shares of the Company’s common stock, respectively. |
| | (5) | This amount reflects (a) $172,230 related to a July 31, 2014 grant of 300,000 incentive stock options to purchase shares of our common stock and (b) $61,231 related to the grant of warrants to purchase 47,058 shares of Enumeral Series B Preferred Stock, as detailed in Note (4) above. |
| | (6) | This amount reflects a September 28, 2015 grant of 880,000 incentive stock options to purchase shares of our common stock. |
| | (7) | This amount reflects (a) $172,230 related to a July 31, 2014 grant of 300,000 incentive stock options to purchase shares of our common stock and (b) $76,539 related to the grant of warrants to purchase 58,823 shares of Enumeral Series B Preferred Stock, as detailed in Note (4) above. |
| (8) | On May 15, 2015, the compensation committee increased Dr. Nguyen’s annual salary from $185,000 to $275,000. |
| | (9) | This amount reflects a bonus paid in 2015 for service in calendar year 2014, representing over-achievement of Dr. Nguyen’s then-target bonus of 10% of annual base salary. |
| | | |
| | (10) | This amount reflects a September 22, 2015 grant of 400,000 incentive stock options to purchase shares of our common stock. |
| (11) | As of July 29, 2014, Dr. Nguyen’s annual salary was increased from $145,000 to $185,000. |
| (12) | This amount reflects a bonus paid in mid-2014 for service in calendar year 2013, as well as additional achievements in the first half of calendar year 2014, representing over-achievement of Dr. Nguyen’s then-target bonus of 10% of annual base salary. |
| (13) | This amount reflects (a) a July 18, 2014 grant of 28,655 incentive stock options to purchase shares of our common stock and (b) a July 31, 2014 grant of 90,000 incentive stock options to purchase shares of our common stock. |
We have no plans in place and have never maintained any plans that provide for the payment of retirement benefits or benefits that will be paid primarily following retirement including, but not limited to, tax qualified deferred benefit plans, supplemental executive retirement plans, tax-qualified deferred contribution plans and nonqualified deferred contribution plans.
Except as indicated below, we have no contracts, agreements, plans or arrangements, whether written or unwritten, that provide for payments to the named executive officers listed above.
Outstanding Equity Awards at Fiscal Year-End
As of December 31, 2015, we have one compensation plan, the Enumeral Biomedical Holdings, Inc. 2014 Equity Incentive Plan, or the 2014 Plan. The 2014 Plan was approved by our stockholders on July 31, 2014 and provides for awards of stock options, stock appreciation rights, restricted stock, restricted stock units, performance shares, and performance units. The table below provides information with respect to the outstanding equity awards of Mr. Rydzewski, Dr. Tinkelenberg and Dr. Nguyen as of December 31, 2015.
| | | Option Awards | | Stock Awards | |
| Name | | Number
of
Securities
Underlying
Unexercised
Options
Exercisable
(#) | | | Number
of
Securities
Underlying
Unexercised
Options
Unexercisable
(#) | | |
Number
of
Securities
Underlying
Unexercised
Unearned
Options
(#) | | | Option
Exercise
Price
($) | | | Option
Expiration
Date | | Number of
Shares
or Units
of Stock
That Have
Not Vested
(#) | | | Market
Value of
Shares or
Units of
Stock That
Have Not
Vested
($) | |
| John J. | | | 22,500 | (1) | | | 337,500 | (1) | | | 360,000 | (1) | | | 0.36 | | | 09/27/2025 | | | 74,393 | (2) | | | 17,029 | (3) |
| Rydzewski | | | 37,510 | (4) | | | 62,490 | (4) | | | 200,000 | (4) | | | 1.00 | | | 07/30/2024 | | | | | | | | |
| Arthur H. | | | 27,501 | (5) | | | 412,499 | (5) | | | 440,000 | (5) | | | 0.36 | | | 09/27/2025 | | | 74,393 | (2) | | | 17,029 | (3) |
| Tinkelenberg, Ph.D. | | | 37,510 | (4) | | | 62,490 | (4) | | | 200,000 | (4) | | | 1.00 | | | 07/30/2024 | | | — | | | | — | |
| Anhco Nguyen, Ph.D. | | | 25,002 | (6) | | | 374,998 | (6) | | | — | | | | 0.47 | | | 09/21/2025 | | | — | | | | — | |
| | | | 30,634 | (7) | | | 9,366 | (7) | | | 50,000 | (7) | | | 1.00 | | | 07/30/2024 | | | — | | | | — | |
| | | | 17,910 | (8) | | | 10,745 | (8) | | | — | | | | 1.00 | | | 07/17/2024 | | | — | | | | — | |
| | | | 33,063 | (9) | | | — | | | | 16,532 | (9) | | | 0.245 | | | 07/25/2023 | | | — | | | | — | |
| | | | 35,360 | (10) | | | 459 | (10) | | | — | | | | 0.245 | | | 10/26/2022 | | | — | | | | — | |
| | | | 30,849 | (11) | | | 4,970 | (11) | | | — | | | | 0.245 | | | 10/26/2022 | | | — | | | | — | |
| | (1) | Consists of incentive stock options granted on September 28, 2015 for (a) 360,000 shares of our common stock which vest monthly over 48 equal monthly installments, beginning on October 28, 2015, and (b) 360,000 shares of our common stock which vest upon satisfactory achievement of performance-based objectives. These objectives have not yet been met. |
| | (2) | Represents the unvested portion of restricted stock awards made to each of Mr. Rydzewski and Dr. Tinkelenberg in the amount of 324,000 shares of Enumeral common stock granted under the Enumeral 2009 Equity Incentive Plan (the “2009 Plan”) as of January 2, 2013, of which 13,500 shares were immediately vested upon grant and the balance vests in 46 equal monthly installments beginning on January 26, 2013. In connection with the Merger, Mr. Rydzewski’s and Dr. Tinkelenberg’s Enumeral restricted stock awards were each converted into restricted stock awards for 357,086 shares of our common stock under the 2014 Plan. |
| | (3) | The market value of the unvested shares of restricted stock is calculated based on a market value of $0.229 per share (the closing price of our common stock on the OTCQB on December 31, 2015) multiplied by the number of shares. |
| | (4) | Consists of incentive stock options granted on July 31, 2014 for (a) 100,000 shares of our common stock, which vest monthly over four years in 48 equal monthly installments, beginning on July 31, 2014, and (b) 200,000 shares of our common stock which vest upon satisfactory achievement of performance-based objectives. These objectives have not yet been met. |
| | (5) | Consists of incentive stock options granted on September 28, 2015 for (a) 440,000 shares of our common stock which vest monthly over 48 equal monthly installments, beginning on October 28, 2015, and (b) 440,000 shares of our common stock which vest upon satisfactory achievement of performance-based objectives. These objectives have not yet been met. |
| | (6) | Consists of incentive stock options granted on September 22, 2015 for 400,000 shares of our common stock which vest monthly over 48 equal monthly installments, beginning on October 22, 2015. |
| | (7) | Consists of incentive stock options granted on July 31, 2014 for 90,000 shares of our common stock, of which (a) 15,000 options vest monthly over four years in 48 equal monthly installments, beginning on July 31, 2014, and (b) 75,000 options vest upon satisfactory achievement of performance-based objectives. Some of these objectives have been met. |
| | (8) | Consists of incentive stock options originally granted on July 18, 2014 to purchase 26,000 shares of Enumeral common stock under the 2009 Plan at an exercise price of $1.102 per share, which vest monthly over four years in 48 equal monthly installments, beginning on July 25, 2013. In connection with the Merger, these options were converted into incentive stock options to purchase 28,655 shares of our common stock under the 2014 Plan at an exercise price of $1.00 per share, on the same vesting terms. |
| | (9) | Consists of incentive stock options originally granted on July 25, 2013 to purchase 45,000 shares of Enumeral common stock under the 2009 Plan at an exercise price of $0.27 per share, which vest upon satisfactory achievement of performance-based objectives. In connection with the Merger, these options were converted into incentive stock options to purchase 49,595 shares of our common stock under the 2014 Plan at an exercise price of $0.245 per share, on the same vesting terms. Some of these objectives have been met. |
| | (10) | Consists of incentive stock options originally granted on October 26, 2012 to purchase 32,500 shares of Enumeral common stock under the 2009 Plan at an exercise price of $0.27 per share. In connection with the Merger, these options were converted into incentive stock options to purchase 35,819 shares of our common stock under the 2014 Plan at an exercise price of $0.245 per share, of which 23,878 options vest monthly over four years in 48 equal monthly installments, beginning on November 26, 2012, and 11,941 options vest upon satisfactory achievement of performance-based objectives. All of these objectives have been met. |
| | (11) | Consists of incentive stock options originally granted on October 26, 2012 to purchase 32,500 shares of Enumeral common stock under the 2009 Plan at an exercise price of $0.27 per share. In connection with the Merger, these options were converted into incentive stock options to purchase 35,819 shares of our common stock under the 2014 Plan at an exercise price of $0.245 per share, of which 3,857 options vested on October 26, 2012, 5,511 options vested on January 4, 2013, 16,532 options vested over three years in 36 equal monthly installments, beginning on February 4, 2013, and 9,919 options vest upon satisfactory achievement of a performance-based objective. The performance-based objective has been met. |
The following table sets forth certain information with respect to grants of stock options to the Named Executive Officers during fiscal year 2015:
| GRANTS OF PLAN BASED AWARDS TABLE |
| Name | | Grant Date | | All Other
Option
Awards:
Number of
Securities
Underlying
Options (#) | | | Exercise Price
of Option
Awards
($/Share) | | | Grant Date
Fair Value
($)(1) | |
| | | | | | | | | | | | |
| John J. Rydzewski | | 09/28/2015 | | | 720,000 | | | | 0.36 | | | | 216,235 | |
| | | | | | | | | | | | | | | |
| Arthur H. Tinkelenberg, Ph. D. | | 09/28/2015 | | | 880,000 | | | | 0.36 | | | | 264,287 | |
| | | | | | | | | | | | | | | |
| Anhco Nguyen, Ph.D. | | 09/22/2015 | | | 400,000 | | | | 0.47 | | | | 156,860 | |
| | (1) | These amounts represent the aggregate grant date fair value of stock option awards granted in fiscal year 2015 under our 2014 Equity Incentive Plan. These amounts are computed in accordance with FASB ASC Topic 718. A description of the assumptions used for purposes of determining grant date fair value is set forth in the notes to our consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2015, which was filed with the SEC on March 30, 2016. The named executive officers will only realize compensation to the extent the trading price of our common stock is greater than the exercise price of such stock options or warrants, as applicable. |
Employment Agreements
John J. Rydzewski
On July 21, 2014, Enumeral and Mr. Rydzewski amended and restated his existing employment agreement, dated as of October 1, 2011. His amended and restated employment agreement provides, following the closing of the Merger and PPO, for (i) a term of employment of two years from July 21, 2014 that automatically renews for additional two-year terms unless terminated by Enumeral or Mr. Rydzewski, (ii) a base salary of $262,500, (iii) entitlement to participate in bonus plans which are approved by the board for Enumeral employees generally, with a target incentive bonus percentage equal to 40% of his then-current annual salary, (iv) an incentive stock option award to purchase 100,000 shares of Enumeral’s or, if applicable, the Company’s common stock with an exercise price equal to the fair market value of Enumeral’s common stock as determined by Enumeral’s board of directors and vesting in 48 equal monthly installments over 4 years beginning on July 31, 2014, (v) an incentive stock option award to purchase 200,000 shares of Enumeral’s or, if applicable, the Company’s common stock with an exercise price equal to the fair market value of Enumeral’s common stock as determined by Enumeral’s board of directors and vesting subject to performance-based criteria set forth in the employment agreement, and (vi) a warrant to purchase 47,058 shares of Enumeral’s Series B Preferred Stock subject to vesting conditions set forth in the employment agreement and exercisable at $2.125 per share.
Pursuant to the October 1, 2011 employment agreement, Mr. Rydzewski received (a) a restricted stock grant of 100,000 shares and (b) a stock option award to purchase 50,000 shares of Enumeral’s common stock, in each case subject to the vesting conditions set forth in the employment agreement and with an exercise price equal to the fair market value of Enumeral’s common stock as determined by Enumeral’s board of directors. Mr. Rydzewski exercised his option to purchase 50,000 options on July 25, 2012. Additionally, on January 2, 2013, Mr. Rydzewski received a restricted stock grant of 324,000 shares of Enumeral common stock, of which 13,500 shares were immediately vested upon grant and the balance vests in 46 equal monthly installments beginning on January 26, 2013.
Contemporaneous with the closing of the Merger and PPO, Mr. Rydzewski’s warrants and options to purchase shares of Enumeral common stock, as well as restricted grants of Enumeral common stock, were converted into warrants and options to purchase shares of our common stock, as well as restricted grants of our common stock, respectively.
On July 31, 2014, we agreed to assume the obligations of Enumeral under the amended and restated employment agreement.
Arthur H. Tinkelenberg, Ph.D.
On July 21, 2014, Enumeral and Dr. Tinkelenberg amended and restated his existing employment agreement, dated as of July 1, 2010. His amended and restated employment agreement provides, following the closing of the Merger and PPO, for (i) a term of employment of two years from July 21, 2014 that automatically renews for additional two-year terms unless terminated by Enumeral or Dr. Tinkelenberg, (ii) a base salary of $262,500, (iii) entitlement to participate in bonus plans which are approved by the Board for Enumeral employees generally, (iv) an incentive stock option award to purchase 100,000 shares of Enumeral’s or, if applicable, the Company’s common stock with an exercise price equal to the fair market value of Enumeral’s common stock as determined by Enumeral’s board of directors and vesting in 48 equal monthly installments over 4 years beginning on July 31, 2014, (v) an incentive stock option award to purchase 200,000 shares of Enumeral’s or, if applicable, the Company’s common stock with an exercise price equal to the fair market value of Enumeral’s common stock as determined by Enumeral’s board of directors and vesting subject to performance-based criteria set forth in the employment agreement, and (vi) a warrant to purchase 58,823 shares of Enumeral’s Series B Preferred Stock subject to vesting conditions set forth in the employment agreement and exercisable at $2.125 per share.
Pursuant to the July 1, 2010 employment agreement, Dr. Tinkelenberg received (a) a restricted stock grant of 393,750 shares and (b) a stock option award to purchase 51,576 shares of Enumeral’s common stock, in each case subject to the vesting conditions set forth in the employment agreement (and which were fully vested prior to the closing of the Merger) and with an exercise price equal to the fair market value of Enumeral’s common stock as determined by Enumeral’s board of directors. Dr. Tinkelenberg exercised his 51,576 options on July 27, 2012. Additionally, on January 2, 2013, Dr. Tinkelenberg received a restricted stock grant of 324,000 shares of Enumeral common stock,of which 13,500 shares were immediately vested upon grant and the balance vests in 46 equal monthly installments beginning on January 26, 2013.
Contemporaneous with the closing of the Merger and PPO, Dr. Tinkelenberg’s warrants and options to purchase shares of Enumeral common stock, as well as restricted grants of Enumeral common stock, were converted into warrants and options to purchase shares of our common stock, as well as restricted grants of our common stock, respectively.
On July 31, 2014, we agreed to assume the obligations of Enumeral under the amended and restated employment agreement.
Anhco Nguyen, Ph.D.
Dr. Nguyen was initially hired as our Research Director pursuant to an offer letter dated December 17, 2011. Dr. Nguyen has subsequently been promoted to increasing roles of responsibility within our company, and now serves as our Vice President of Research and Development. On March 24, 2016, we entered into a new employment letter with Dr. Nguyen (the “Nguyen Agreement”), which states our desire to continue to employ Dr. Nguyen in his position and confirms the terms of his employment including, among other things, certain benefits in the event his employment with our company is terminated. The Nguyen Agreement provides that Dr. Nguyen will continue to serve as our Vice President of Research and Development, reporting to our President and Chief Executive Officer. Dr. Nguyen will continue to receive a base salary at the rate of $275,000 per annum, and he will continue to be eligible to earn a target bonus up to 30% of his base salary, based on the achievement of corporate objectives as determined by our board or our compensation committee.
Pursuant to Dr. Nguyen’s original December 2011 offer letter, Dr. Nguyen received an incentive stock option to purchase 32,500 shares of Enumeral common stock, which were converted into 35,819 shares of our common stock in connection with the Merger. With respect to this option grant, 9,000 of the original Enumeral shares granted were to vest upon the satisfactory achievement of performance-based objectives, with the remainder of shares vesting on a time-based schedule.
As Dr. Nguyen progressed in our company, he has been awarded additional equity grants. See “Outstanding Equity Awards at Fiscal Year-End” above for a description of these awards. Contemporaneous with the closing of the Merger and PPO, Dr. Nguyen’s options to purchase shares of Enumeral common stock were converted into options to purchase shares of our common stock.
Potential Payments upon Termination or Change in Control
Employment Agreements of Mr. Rydzewski and Dr. Tinkelenberg
If Mr. Rydzewski’s or Dr. Tinkelenberg’s employment were terminated for cause, death, disability or if they resign for any reason (other than resignation for Good Reason, as such term is defined below), their employment agreements provide that such executive would have received the following:
| | · | any accrued but unpaid salary through the date of termination plus any accrued vacation; |
| | · | any earned and declared but unpaid bonus for the most recently completed year; |
| | · | reimbursement of any unreimbursed expenses; and |
| | · | benefits in accordance with the terms of the applicable plans and programs of the Company. |
In addition, all of the executive’s unvested restricted stock or options shall immediately be forfeited and be cancelled.
If Mr. Rydzewski’s or Dr. Tinkelenberg’s employment is terminated by us other than for Cause or by Mr. Rydzewski or Dr. Tinkelenberg for Good Reason where a Change in Control has not occurred (as such terms are defined below), and provided that the executive executes our form of termination and release agreement, the executive will be entitled to the following:
| | · | base salary for the greater of twelve months or the remaining time until two years from the date of the closing of the Merger and PPO (the “Severance Period”); |
| | · | all earned and declared but unpaid bonuses which are due and payable, including a pro rata portion of the current year’s potential bonus, calculated based on the then-current target bonus percentage (but no less than 40%), payable in lump sum, based upon achievement of then-current target milestones through the termination date of employment as determined in good faith by our compensation committee or board of directors, as applicable; |
| | · | continued coverage for the Severance Period of all our group insurance (including life, health, accident and disability insurance) and, to the extent permitted by applicable law, all other employee benefit plans, programs or arrangements in which the executive was participating at any time during the six month period preceding the termination of employment, provided that we will no longer be obligated to fund the cost of premiums for such plans and benefits in the event that the executive becomes eligible for similar insurance or other employee benefits from a new employer during the Severance Period; |
| | · | continued vesting of any non-vested stock options and restricted stock granted pursuant to the employment agreement for the Severance Period pursuant to the vesting schedule set forth therein, as well as extension of the period to exercise vested stock options until five years following the expiration of the Severance Period, provided that if the executive breaches the terms of his employment agreement, all of the executive’s unvested options and shares of restricted stock will be immediately forfeited; and |
| | · | payment for all accrued but unused vacation on the termination date of employment. |
In addition, if a Change in Control occurs during the Severance Period, all non-vested stock options and shares of restricted stock shall immediately accelerate and become exercisable or non-forfeitable, as the case may be.
If Mr. Rydzewski’s or Dr. Tinkelenberg’s employment is terminated, or Mr. Rydzewski or Dr. Tinkelenberg resigned for Good Reason where a Change in Control has occurred, and provided that the executive executes our form of termination and release agreement, the executive will receive:
| | · | an amount equal to one year of base salary, payable immediately in one lump sum; |
| | · | all bonuses which are due and payable, including the full amount of the current year’s target incentive bonus as if the then-current target milestones were achieved for the applicable year; |
| | · | continued coverage for the Severance Period of all our group insurance (including life, health, accident and disability insurance) and, to the extent permitted by applicable law, all other employee benefit plans, programs or arrangements in which the executive was participating at any time during the six month period preceding the termination of employment; |
| | · | all non-vested stock options or restricted stock shall vest immediately and become exercisable or non-forfeitable and shall be exchanged for cash or freely tradable stock of the acquiring company, provided that in the event it is not possible for such stock options or restricted stock to be exchanged for cash or freely tradable stock of the acquiring company, the period to exercise vested stock options shall be extended until five years following the date such shares vested; and |
| | · | payment for all accrued but unused vacation on the termination date of employment. |
“Cause” is defined in Mr. Rydzewski’s and Dr. Tinkelenberg’s employment agreements as:
| | · | the executive’s conviction by a court of competent jurisdiction of any felony involving dishonesty, breach of trust or misappropriation or entry of a plea of nolo contendere thereto; |
| | · | the commission of an act of fraud upon, breaching the duty of loyalty to, us or any of our subsidiaries; |
| | · | a conviction for willful violation of any law, rule or regulation governing operation of our company or any of its subsidiaries that is punishable by six months or more imprisonment; |
| | · | the substantial and continuing failure or refusal of the executive, after seven days’ written notice thereof, to perform his or her job duties and responsibilities which failure or refusal is committed in bad faith (other than failure or refusal resulting from incapacity due to physical disability or mental illness); |
| | · | the executive’s breach of his employment agreement that continues for more than seven days after written notice has been given to the executive; or |
| | · | the deliberate and willful disregard for our rules or policies that results in a material and substantial loss, damage or injury to our company. |
“Good Reason” is defined in Mr. Rydzewski’s and Dr. Tinkelenberg’s employment agreements as:
| | · | a reduction in the executive’s then-current base salary or target bonus percentage; |
| | · | any failure to offer to the executive at least the same level of benefits offered to similarly situated employees; |
| | · | a significant diminution in the executive’s managerial authority, duties and responsibilities following a Change in Control of our company; |
| | · | the relocation of the executive’s primary business location to a location outside of the New York metropolitan area; |
| | · | failure to pay to the executive any portion of his current base salary, bonus or benefits within 20 days of when such compensation is due, based upon the payment terms currently in effect, unless such payment is prohibited by law, regulation or rule; or |
| | · | our failure to obtain a reasonably satisfactory agreement from any successor to our company to assume and agree to perform the executive’s employment agreement. |
“Change in Control” is defined in Mr. Rydzewski’s and Dr. Tinkelenberg’s employment agreements as:
| | · | the merger or consolidation of our company with another entity, where, immediately after the transaction: |
| | o | our stockholders immediately prior to the merger or consolidation beneficially own, directly or indirectly, less than 50% of the voting shares of the surviving entity in the consolidation or merger (or of its ultimate parent corporation, if any); or |
| | o | persons who constitute our board of directors prior to the transaction cease to constitute at least a majority of the board of directors of the surviving entity in the consolidation or merger (or of its ultimate parent corporation, if any); |
| | · | sale, lease or other transfer of all or substantially all of our assets or the exclusive license of all or substantially all of its patents, provisional patent applications and patent applications for substantially all uses to a third party; |
| | · | any person (other than our company, any of its subsidiaries, or any trustee, fiduciary or other person or entity holding securities under any employee benefit plan), together with all affiliates and associates of such person, becomes the beneficial owner of our securities representing 50% or more of the combined voting power of our then outstanding securities having the right to vote in an election of our board of directors. |
Employment Letter of Dr. Nguyen
Pursuant to the terms of the Nguyen Agreement, in the event that Dr. Nguyen’s employment with our company terminates without Cause (as defined below) or should Dr. Nguyen resign for Good Reason (as defined below), Dr. Nguyen will be entitled to the following severance benefits, provided that he executes a separation and release agreement in a form proposed by and acceptable to our company:
| | · | Dr. Nguyen will continue to receive his base salary at the then-current rate for a period of six (6) months following the date of his termination of employment with our company, provided that he continues to comply with and not breach the terms of the separation and release agreement and, as applicable, any other agreement that he has previously entered into with us; and |
| | · | Dr. Nguyen will receive all earned and declared but unpaid bonuses which are due and payable, including without limitation a pro rata portion of the current year’s potential bonus calculated based on the executive’s then-current target bonus percentage, payable in a lump sum, based on the number of days in any given year that he was employed through the date of termination, and based upon achievement of then-current corporate objectives as determined in good faith by our President and Chief Executive Officer. |
The Nguyen Agreement also provides that upon a Change of Control (as defined in our 2014 Equity Incentive Plan, as amended), Dr. Nguyen will be entitled, in addition to the severance benefits set forth above, to immediate and full vesting of the shares underlying his equity awards which remain unvested as of the date of such Change in Control.
For purposes of the Nguyen Agreement, “Cause” means any of the following, as determined by our President and Chief Executive Officer, acting in good faith: (a) any failure of the executive to take or refrain from taking any corporate action consistent with the executive’s duties as specified in written directions of our President and Chief Executive Officer or our board of directors; (b) the executive’s willful engagement in illegal conduct or gross misconduct that is injurious to our company; (c) the conviction of the executive of, or the entry of a pleading of guilty or nolo contendere by the executive to, any crime involving moral turpitude or any felony; (d) fraud upon our company including, without limitation, falsification of company records or financial information; or (e) the executive’s breach of any of the non-compete, non-solicitation, and proprietary information provisions of the Nguyen Agreement (including the terms of any obligations agreement entered into between the executive and us).
In addition, for purposes of the Nguyen Agreement, “Good Reason” means the occurrence, without the executive’s prior written consent, of any of these events or circumstances:
| | · | a material diminution in the executive’s rate of base salary; |
| | · | a material diminution in the executive’s authority, duties, or responsibilities; or |
| | · | a material breach by us of the Executive Agreement; |
provided that any of these events described above shall constitute Good Reason only if we fail to cure such event within thirty (30) days after receipt from the executive of written notice of the event which constitutes Good Reason; and provided further that Good Reason shall cease to exist for an event on the sixtieth (60th) day following its occurrence, unless the executive has given us written notice thereof prior to such date.
For purposes of the Nguyen Agreement, “Change in Control” has the meaning ascribed to such term in the 2014 Plan. The 2014 Plan provides that a Change in Control shall be deemed to have occurred as of the first day that any one or more of the following conditions shall have been satisfied:
| | · | the beneficial ownership of securities (as defined in Rule 13d-3 under the Exchange Act) representing more than thirty-three percent (33%) of the combined voting power of our company is acquired by any person (other than our company, any trustee or other fiduciary holding securities under an employee benefit plan of our company, or any corporation owned, directly or indirectly, by the stockholders of our company in substantially the same proportions as their ownership of stock of our company); or |
| | · | the consummation of a definitive agreement to merge or consolidate our company with or into another corporation or to sell or otherwise dispose of all or substantially all of its assets, or adopt a plan of liquidation; or |
| | · | during any period of three consecutive years, individuals who at the beginning of such period were members of our board cease for any reason to constitute at least a majority thereof (unless the election, or the nomination for election by our stockholders, of each new director was approved by a vote of at least a majority of the directors then still in office who were directors at the beginning of such period or whose election or nomination was previously so approved). |
The 2014 Plan also details additional triggers for a Change in Control applicable to equity awards subject to Section 409A of the Internal Revenue Code of 1986, as amended, including threshold changes of ownership, changes in effective control, and changes in ownership of substantial assets. The full definition of “Change in Control” set forth in the 2014 Plan is incorporated herein by reference.
Director Compensation
Non-employee directors’ compensation generally is determined and awarded by our board of directors, or the compensation committee thereof. Our board is responsible for, among other things, reviewing, evaluating and designing a director compensation package of a reasonable total value, typically based on comparisons with similar firms, and aligned with long-term interests of our stockholders, and reviewing director compensation levels and practices and considering, from time to time, changes in such compensation levels and practices. These matters also include making equity awards to non-employee directors from time to time under our equity-based plans. As part of these responsibilities, our board may request that our management provide the board with recommendations on non-employee director compensation and/or common director compensation practices, although the board retains its ultimate authority to take compensatory actions.
Prior to the Merger, we did not pay our non-employee directors an annual retainer. In August 2014, our board of directors approved a director compensation program for non-employee directors, which we refer to as the 2014 Program. Under the 2014 Program, newly elected non-employee board members received an option to purchase 60,000 shares of our common stock that vested on a monthly basis over three years from date of grant, and a one-time option grant of 20,000 shares that was fully vested upon their election to the board. The 2014 Program also provided that non-employee directors received an annual retainer at the rate of $20,000 per annum, which individual directors could elect to take in the form of cash, restricted stock or options to purchase shares of our common stock.
Under the 2014 Program, directors who served as members of the audit committee were entitled to additional compensation at the rate of $3,000 per annum, with the chairman of the audit committee being compensated at the rate of $6,000 per annum. Directors who served as members of the compensation committee were entitled to additional compensation at the rate of $2,000 per annum, with the chairman of the compensation committee being compensated at the rate of $4,000 per annum. The 2014 Program also included a $6,000 per annum retainer to a director who serves in the position of lead independent director. Pursuant to the terms of the 2014 Program, all board and committee fees were paid quarterly in advance.
In September 2015, our compensation committee approved a revised director compensation program for non-employee directors, which we refer to as the 2015 Program. Under the 2015 Program, newly elected non-employee board members will continue to receive an option to purchase 20,000 shares of our common stock that will be fully vested upon their election to the board. Non-employee directors are also entitled to receive an annual grant of options to purchase common stock, as determined by the compensation committee. In September 2015, the compensation committee awarded each non-employee director an option to purchase 76,000 shares of our common stock. In addition, the 2015 Program provides that non-employee directors are entitled to an annual retainer in the amount of $35,000, which individual directors may elect to take in the form of cash, restricted stock or options to purchase common stock. Compensation taken in the form of restricted stock or options to purchase common stock shall vest over one year in four equal quarterly amounts on the first day of each fiscal quarter, provided the individual continues to serve as a director.
Pursuant to the 2015 Program, directors who serve as members of the audit committee are entitled to additional compensation at the rate of $10,000 per annum, with the chairman of the audit committee being compensated at the rate of $20,000 per annum. Directors who serve as members of the compensation committee are entitled to additional compensation at the rate of $7,500 per annum, with the chairman of the compensation committee being compensated at the rate of $15,000 per annum. Directors who serve as members of the nominating and governance committee are entitled to additional compensation at the rate of $5,000 per annum, with the chairman of the nominating and governance committee being compensated at the rate of $10,000 per annum. In addition, the 2015 Program provides that a director who serves in the position of lead independent director shall be entitled to compensation at the rate of $30,000 per annum. Pursuant to the terms of the 2015 Program, all such board and committee fees shall be paid quarterly in advance.
The following table sets forth compensation paid to our non-employee directors during fiscal year 2015:
| Name | | Fees earned or
paid in cash
($)(1) | | | Stock
awards
($)(2) | | | Option
awards
($)(2) | | | Total
($) | |
| Barry Buckland, Ph.D. | | | 76,000 | (3) | | | — | | | | 29,803 | | | | 105,803 | |
| Allan P. Rothstein | | | 18,250 | | | | 20,000 | | | | 44,509 | | | | 82,759 | |
| Paul J. Sekhri | | | 44,250 | | | | — | | | | 29,803 | | | | 74,053 | |
| Robert L. Van Nostrand | | | 56,500 | | | | — | | | | 29,803 | | | | 86,303 | |
| Daniel B. Wolfe, Ph.D. (4) | | | 15,667 | | | | — | | | | — | | | | 15,667 | |
| Robert J. Easton (5) | | | 22,917 | | | | — | | | | 39,827 | | | | 62,744 | |
| | (1) | Except as otherwise specifically noted, the amounts set forth in this column represent fees paid for service on our board of directors and committees thereof. |
| | | |
| | (2) | These amounts represent the aggregate grant date fair value of stock option awards and restricted stock awards granted in fiscal year 2015 under the 2014 Plan, computed in accordance with FASB ASC Topic 718. These amounts do not represent the actual amounts paid to or realized by the directors during fiscal year 2015, and there can be no assurance that the Topic 718 amount will ever be realized. A description of the assumptions we used for purposes of determining grant date fair value is set forth in the notes to our consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2015, which was filed with the SEC on March 30, 2016. |
| | | |
| | (3) | Represents the aggregate of (i) $38,000 paid to Dr. Buckland pursuant to his service on our Scientific Advisory Board (“SAB”) and (ii) $38,000 paid to Dr. Buckland for service on our board of directors and committees thereof. For additional information on Dr. Buckland’s SAB agreement, please refer to “Certain Relationships and Related Transactions, and Director Independence.” |
| | | |
| | (4) | Dr. Wolfe resigned from our board on July 30, 2015. |
| | | |
| | (5) | Mr. Easton joined our board on July 30, 2015. |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
SEC rules require us to disclose any transaction or currently proposed transaction in which we are a participant and in which any related person has or will have a direct or indirect material interest involving the lesser of $120,000 or one percent (1%) of the average of our total assets as of the end of last two completed fiscal years. A related person is any executive officer, director, nominee for director, or holder of 5% or more of our common stock, or an immediate family member of any of those persons.
We completed the following offerings in which a person who was a related party at the time of the transaction participated (share and per share numbers below are without giving effect to the conversion of all Enumeral shares into shares of our common stock in the Merger):
| (a) | During April and September, 2013, Enumeral completed a private placement with 17 accredited investors pursuant to which we sold 1,833,798 shares of Series A-2 Preferred Stock, at price of $1.45 per share, or approximately $2,659,007 in the aggregate. In connection with the Merger, each share of Series A-2 Preferred Stock was converted into 1.997594 shares of our common stock, representing 3,663,177 shares in the aggregate. Mr. Rydzewski, Dr. Tinkelenberg, Mr. Rothstein (who became a director of Enumeral in September 2013), Jay and Tammy Levine (Mr. Rothstein’s brother-in-law and sister), Steven Rothstein (Mr. Rothstein’s brother), and Harris & Harris Group, Inc. purchased 34,483, 34,483, 100,000, 100,000, 50,000, and 724,138, respectively, shares of the securities in this offering. |
| (b) | During February and March 2014 and to fund operations prior to the Series B offering, Enumeral completed a private placement with 7 accredited investors pursuant to which we sold $750,000 principal amount of 12% Convertible Promissory Notes (the “Bridge Notes”). The Bridge Notes had a maturity of July 1, 2015 or if earlier, a Liquidity Event (as defined in Enumeral’s charter) and bore interest at the rate of 12% payable annually and automatically converts into either Series B Preferred Stock or common stock, at the option of holder of the Bridge Notes, upon achieving certain fundraising goals. Each noteholder also received a warrant to purchase 925.924 shares of common stock, for each $1,000 principal amount of Bridge Notes, or 694,443 in the aggregate. The warrants have an exercise price of $0.27 per share. In connection with the Merger, the principal and interest on the Bridge Notes were converted into 3,230,869 shares of our common stock. Mr. Rydzewski, Mr. Ostuni, Mr. Rothstein, Norman Rothstein (Mr. Rothstein’s father), Michael Weiss (a director of Enumeral at the time), and Harris & Harris Group, Inc. purchased $50,000 principal amount of the Bridge Notes and warrants to purchase 46,296 shares of Enumeral common stock, $10,000 principal amount of the Bridge Notes and warrants to purchase 9,259 shares of Enumeral common stock, $265,000 principal amount of the Bridge Notes and warrants to purchase 245,370 shares of Enumeral common stock, $100,000 principal amount of the Bridge Notes and warrants to purchase 92,593 shares of Enumeral common stock, $50,000 principal amount of the Bridge Notes and warrants to purchase 46,296 shares of Enumeral common stock, and $250,000 principal amount of the Bridge Notes and warrants to purchase 231,481 shares of Enumeral common stock, respectively, in this offering. |
| (c) | During April 2014, Enumeral completed a private placement of 948,823 shares of Series B Preferred Stock. Harris & Harris Group, Inc. purchased 470,588 of the securities in this offering. |
In September 2014, we and Dr. Buckland entered into a Scientific Advisory Board Agreement (the “SAB Agreement”) pursuant to which Dr. Buckland serves as chairman of our Scientific Advisory Board. The SAB Agreement supersedes Dr. Buckland’s previous consulting agreement entered into with Enumeral in April 2011, as subsequently amended, pursuant to which Dr. Buckland received 159,045 shares of Enumeral restricted common stock, as well as cash compensation for consulting services. Pursuant to the terms of the SAB Agreement, Dr. Buckland will receive compensation on an hourly or per diem basis, either in cash or, at Dr. Buckland’s election, in options to purchase our common stock, provided that any such compensation will not exceed $100,000 in any continuous twelve month period. The SAB Agreement has a term of two years.
In September 2013, Enumeral entered into a consulting agreement with Mr. Rothstein and his father, Norman Rothstein, to consult with Enumeral’s Chief Executive Officer and senior members of management on various financing-related matters. Each of these individuals received 500,000 shares of Enumeral common stock valued at $0.27 per share as payment for their services provided to Enumeral, with one third vesting upon the execution of the consulting agreement in September 2013, one third vesting on December 14, 2013, and one third vesting on March 10, 2014. The consulting agreement was terminated on July 30, 2014.
Director Independence
While we are currently quoted on the over-the-counter market, we have adopted the corporate governance standards of a listed company on the Nasdaq Stock Market, or Nasdaq. These standards require that a majority of the members of our board of directors be “independent,” as Nasdaq defines that term, and that our board make an affirmative determination as to the independence of each director. Consistent with these rules, our board of directors undertook its annual review of director independence in April 2016. During the review, our board considered relationships and transactions during 2015 and during the past three fiscal years between each director or any member of his or her immediate family, on the one hand, and our company and its subsidiaries and affiliates, on the other hand. The purpose of this review was to determine whether any such relationships or transactions were inconsistent with a determination that the director is independent. Based on this review, our board of directors determined that Dr. Buckland and Messrs. Easton, Sekhri, and Van Nostrand are independent under the criteria established by Nasdaq and by our board of directors.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. In accordance with SEC rules, shares of our common stock which may be acquired upon exercise of stock options or warrants which are currently exercisable or which become exercisable within 60 days of the date of the table below are deemed beneficially owned by the holders of such options and warrants and are deemed outstanding for the purpose of computing the percentage of ownership of such person, but are not treated as outstanding for the purpose of computing the percentage of ownership of any other person. Subject to community property laws, where applicable, the persons or entities named in the tables below have sole voting and investment power with respect to all shares of our common stock indicated as beneficially owned by them.
The following table sets forth information with respect to the beneficial ownership of our common stock as of July 22, 2016 (the “Determination Date”), by (i) each stockholder known by us to be the beneficial owner of more than 5% of our common stock (our only classes of voting securities), (ii) each of our directors and executive officers, and (iii) all of our directors and executive officers as a group. To the best of our knowledge, except as otherwise indicated, each of the persons named in the table has sole voting and investment power with respect to the shares of our common stock beneficially owned by such person, except to the extent such power may be shared with a spouse. To our knowledge, none of the shares listed below are held under a voting trust or similar agreement, except as noted. In addition, to our knowledge there is no arrangement, including any pledge by any person of securities of our company or any of its parents, the operation of which may at a subsequent date result in a change in control of our company.
| Title of class | | Name and address
of beneficial owner (1) | | Amount and nature
of beneficial
ownership | | | Percent of class (2) | |
| Common Stock | | Harris & Harris Group, Inc. (3)
1450 Broadway, 24th Floor
New York, NY 10018 | | | 9,801,488 | | | | 18.2 | % |
| Common Stock | | Mark Tompkins (4) | | | 1,359,219 | | | | 2.6 | % |
| Common Stock | | Allan P. Rothstein (5) | | | 2,338,136 | | | | 4.4 | % |
| Common Stock | | John J. Rydzewski (6) | | | 1,770,096 | | | | 3.4 | % |
| Common Stock | | Arthur H. Tinkelenberg, Ph.D. (7) | | | 1,819,233 | | | | 3.5 | % |
| Common Stock | | Barry Buckland, Ph.D. (8) | | | 516,526 | | | | 1.0 | % |
| Common Stock | | Robert J. Easton (9) | | | 208,985 | | | | * | |
| Common Stock | | Paul J. Sekhri (10) | | | 124,674 | | | | * | |
| Common Stock | | Robert L. Van Nostrand (11) | | | 170,129 | | | | * | |
| Common Stock | | Anhco Nguyen, Ph.D. (12) | | | 563,703 | | | | 1.1 | % |
| Common Stock | | Kevin G. Sarney (13) | | | 431,250 | | | | * | |
| Common Stock | | All of our directors and executive officers as a group (9 persons) (14) | | | 7,942,732 | | | | 14.3 | % |
| | (1) | Unless otherwise set forth above, the address for each of the persons or entities listed above is c/o Enumeral Biomedical Holdings, Inc., 200 CambridgePark Drive, Suite 2000, Cambridge, MA 02140. |
| | (2) | Applicable percentage ownership is based on 52,073,481 shares of common stock outstanding as of the Determination Date, together with securities exercisable or convertible into shares of common stock within 60 days after the Determination Date, for each shareholder. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. |
| | (3) | Consists of 7,966,368 shares of common stock owned directly by Harris & Harris Group, Inc. (“Harris”), 255,120 shares that Harris has the right to acquire upon the exercise of warrants that are exercisable within 60 days of the Determination Date, 1,500,000 shares that Harris has the right to acquire upon the exercise of PPO Warrants that are exercisable within 60 days of the Determination Date, and 80,000 shares of common stock that Daniel B. Wolfe, our former director, has the right to acquire upon the exercise of options that are exercisable within 60 days of the Determination Date (Dr. Wolfe previously assigned the economic benefit of the options to Harris). 7,966,368 shares of common stock and warrants to purchase 1,755,120 shares of common stock owned by Harris is pledged as security for a margin account. Dr. Wolfe and Douglas W. Jamison, are managing directors of Harris and as such may be deemed to have shared voting and dispositive power over the share of common stock beneficially owned by Harris. |
| | (4) | Consists of 159,219 shares of common stock owned directly by Mr. Tompkins and 1,200,000 shares that Mr. Tompkins has the right to acquire upon the exercise of warrants that are exercisable within 60 days of the Determination Date, based on information provided by Mr. Tompkins in a Schedule 13G filed with the SEC on February 16, 2016, and reflecting shares sold under the Registration Statement on Form S-1 subsequent to the date referenced in such Schedule 13G. Mr. Tompkins does not serve as an officer or director of our company. |
| | (5) | Consists of 1,824,415 shares of common stock owned directly by Mr. Rothstein, 270,427 shares that Mr. Rothstein has the right to acquire upon the exercise of warrants that are exercisable within 60 days of the Determination Date, 50,000 shares that Mr. Rothstein has the right to acquire upon the exercise of PPO warrants that are exercisable within 60 days of the Determination Date, and 193,294 shares that Mr. Rothstein has the right to acquire upon the exercise of options that are exercisable within 60 days of the Determination Date. |
| | (6) | Consists of 1,264,637 shares of common stock owned directly by Mr. Rydzewski, 188,785 shares that Mr. Rydzewski has the right to acquire upon the exercise of warrants that are exercisable within 60 days of the Determination Date, and 316,674 shares that Mr. Rydzewski has the right to acquire upon the exercise of options that are exercisable within 60 days of the Determination Date. Does not include shares that Mr. Rydzewski has the right to acquire upon exercise of options subject to performance based criteria, which criteria has not yet been achieved. |
| | (7) | Consists of 1,272,018 shares of common stock owned directly by Dr. Tinkelenberg, 172,204 shares that Dr. Tinkelenberg has the right to acquire upon the exercise of warrants that are exercisable within 60 days of the Determination Date, and 375,011 shares that Dr. Tinkelenberg has the right to acquire upon the exercise of options that are exercisable within 60 days of the Determination Date. Does not include shares that Dr. Tinkelenberg has the right to acquire upon exercise of options subject to performance based criteria, which criteria has not yet been achieved. |
| | (8) | Consists of 68,882 shares of common stock owned directly by Dr. Buckland, 175,286 shares of common stock owned by BiologicB, LLC, a company wholly-owned by Dr. Buckland, and 272,358 shares that Dr. Buckland has the right to acquire upon the exercise of options that are exercisable within 60 days of the Determination Date. |
| (9) | Consists of 208,985 shares that Mr. Easton has the right to acquire upon the exercise of options that are exercisable within 60 days of the Determination Date. |
| | |
| (10) | Consists of 124,674 shares that Mr. Sekhri has the right to acquire upon the exercise of options that are exercisable within 60 days of the Determination Date. |
| | (11) | Consists of 45,455 shares of common stock owned directly by Mr. Van Nostrand and 124,674 shares that Mr. Van Nostrand has the right to acquire upon the exercise of options that are exercisable within 60 days of the Determination Date. |
| | (12) | Consists of 563,703 shares that Dr. Nguyen has the right to acquire upon the exercise of options that are exercisable within 60 days of the Determination Date. Terms of certain options are described in such option agreements. Does not include shares that Dr. Nguyen has the right to acquire upon exercise of options subject to performance based criteria, which criteria has not yet been achieved. |
| | (13) | Consists of 431,250 shares that Mr. Sarney has the right to acquire upon the exercise of options that are exercisable within 60 days of the Determination Date. Terms of certain options are described in such option agreements. Does not include shares that Mr. Sarney has the right to acquire upon exercise of options subject to performance based criteria, which criteria has not yet been achieved. |
| | (14) | Certain shares are subject to the Voting Agreement as described above under “Directors, Executive Officers, Promoters and Control Persons - Director Relationships and Arrangements.” |
PLAN OF DISTRIBUTION
The selling stockholders may, from time to time, sell any or all of their shares of our common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. If the shares of common stock are sold through underwriters, the selling stockholders will be responsible for underwriting discounts or commissions or agent’s commissions. All selling stockholders who are broker-dealers are deemed to be underwriters. These sales may be at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale or at negotiated prices. The selling stockholders may use any one or more of the following methods when selling shares:
| · | any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale; |
| · | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| · | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| · | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| · | transactions other than on these exchanges or systems or in the over-the-counter market; |
| · | through the writing of options, whether such options are listed on an options exchange or otherwise; |
| · | an exchange distribution in accordance with the rules of the applicable exchange; |
| · | privately negotiated transactions; |
| · | broker-dealers may agree with the selling stockholders to sell a specified number of such shares at a stipulated price per share; |
| · | a combination of any such methods of sale; and |
| · | any other method permitted pursuant to applicable law. |
The selling stockholders may also sell shares under Rule 144 under the Securities Act, if available, rather than under this prospectus. Because we were at one time designated as a “shell company” under SEC regulations, pursuant to Rule 144(i), securities issued by us and registered under this Prospectus could not be sold in reliance on Rule 144 until one year after the date on which we filed current “Form 10 information” (as defined in Rule 144(i)) with the SEC reflecting that it ceased being a shell company, and provided that at the time of a proposed sale pursuant to Rule 144, the issuer has satisfied certain reporting requirements under the Exchange Act. As we filed current information satisfying the requirements of Rule 144(i) on Form 8-K on August 7, 2014, the one-year period ended on August 7, 2015.
The selling stockholders may also engage in short sales against the box, puts and calls and other transactions in our securities or derivatives of our securities and may sell or deliver shares in connection with these trades.
Broker-dealers engaged by the selling stockholders may arrange for other broker-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the selling stockholders (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated. The selling stockholders do not expect these commissions and discounts to exceed what is customary in the types of transactions involved. Any profits on the resale of shares of common stock by a broker-dealer acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act. Discounts, concessions, commissions and similar selling expenses, if any, attributable to the sale of shares will be borne by a selling stockholder. The selling stockholders may agree to indemnify any agent, dealer or broker-dealer that participates in transactions involving sales of the shares if liabilities are imposed on that person under the Securities Act.
In connection with the sale of the shares of our common stock or otherwise, the selling stockholders may enter into hedging transactions with broker-dealers, which may in turn engage in short sales of the shares of common stock in the course of hedging in positions they assume. The selling stockholders may also sell shares of common stock short and deliver shares of common stock covered by this prospectus to close out short positions and to return borrowed shares in connection with such short sales. The selling stockholders may also loan or pledge shares of common stock to broker-dealers that in turn may sell such shares.
The selling stockholders may from time to time pledge or grant a security interest in some or all of the shares of our common stock owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the shares of our common stock from time to time under this prospectus after we have filed an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending the list of selling stockholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus.
The selling stockholders also may transfer the shares of common stock in other circumstances, in which case the transferees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus and may sell the shares of common stock from time to time under this prospectus after we have filed an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending the list of selling stockholders to include the pledgees, transferees or other successors in interest as selling stockholders under this prospectus. The selling stockholders also may transfer and donate the shares of common stock in other circumstances in which case the transferees, donees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
The selling stockholders and any broker-dealers or agents that are involved in selling the shares may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions paid, or any discounts or concessions allowed to, such broker-dealers or agents and any profit realized on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. At the time a particular offering of the shares of common stock is made, a prospectus supplement, if required, will be distributed which will set forth the aggregate amount of shares of common stock being offered and the terms of the offering, including the name or names of any broker-dealers or agents, any discounts, commissions and other terms constituting compensation from the selling stockholders and any discounts, commissions or concessions allowed or re-allowed or paid to broker-dealers. Under the securities laws of some states, the shares of common stock may be sold in such states only through registered or licensed brokers or dealers. In addition, in some states the shares of common stock may not be sold unless such shares have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with. There can be no assurance that any selling stockholder will sell any or all of the shares of our common stock registered pursuant to the shelf registration statement, of which this prospectus forms a part.
Each selling stockholder has informed us that it does not have any agreement or understanding, directly or indirectly, with any person to distribute our common stock. None of the selling stockholders who are affiliates of broker-dealers, other than the initial purchasers in private transactions, purchased the shares of common stock outside of the ordinary course of business or, at the time of the purchase of the common stock, had any agreements, plans or understandings, directly or indirectly, with any person to distribute the securities.
We are required to pay all fees and expenses incident to the registration of the shares of common stock. Except as provided for indemnification of the selling stockholders, we are not obligated to pay any of the expenses of any attorney or other advisor engaged by a selling stockholder. We have agreed to indemnify the selling stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
If we are notified by any selling stockholder that any material arrangement has been entered into with a broker-dealer for the sale of shares of common stock, we will file a post-effective amendment to the registration statement. If the selling stockholders use this prospectus for any sale of the shares of our common stock, they will be subject to the prospectus delivery requirements of the Securities Act.
The anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of our common stock and activities of the selling stockholders, which may limit the timing of purchases and sales of any of the shares of common stock by the selling stockholders and any other participating person. Regulation M may also restrict the ability of any person engaged in the distribution of the shares of common stock to engage in passive market-making activities with respect to the shares of common stock. Passive market making involves transactions in which a market maker acts as both our underwriter and as a purchaser of our common stock in the secondary market. All of the foregoing may affect the marketability of the shares of common stock and the ability of any person or entity to engage in market-making activities with respect to the shares of common stock.
Once sold under the registration statement, of which this prospectus forms a part, the shares of common stock will be freely tradable in the hands of persons other than our affiliates.
Our common stock is currently quoted on the OTC Markets and trades below $5.00 per share; therefore, our common stock is considered a “penny stock” and subject to SEC rules and regulations which impose limitations upon the manner in which such shares may be publicly traded. These regulations require the delivery, prior to any transaction involving a penny stock, of a disclosure schedule explaining the penny stock market and the associated risks. Under these regulations, certain brokers who recommend such securities to persons other than established customers or certain accredited investors must make a special written suitability determination regarding such a purchaser and receive such purchaser’s written agreement to a transaction prior to sale. These regulations have the effect of limiting the trading activity of the common stock and reducing the liquidity of an investment in the common stock.
DESCRIPTION OF SECURITIES
We have authorized capital stock consisting of 300,000,000 shares of Common Stock and 10,000,000 shares of preferred stock. As of the date of this Prospectus, we had 52,073,481 shares of Common Stock issued and outstanding, and no shares of preferred stock issued and outstanding. Prior to the Merger, the Company was incorporated in Nevada, but reincorporated in Delaware in connection with the Merger.
Common Stock
The holders of outstanding shares of common stock are entitled to receive dividends out of assets or funds legally available for the payment of dividends of such times and in such amounts as Board of Directors from time to time may determine. Holders of common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders. In connection with the closing of the Merger, we implemented a staggered board consisting of three classes of directors, with Class I having an initial term expiring in 2015, Class II having an initial term expiring in 2016 and Class III having an initial term expiring in 2017. There is no cumulative voting of the election of directors then standing for election. The common stock is not entitled to pre-emptive rights and is not subject to conversion or redemption. Upon liquidation, dissolution or winding up of our company, the assets legally available for distribution to stockholders are distributable ratably among the holders of the common stock after payment of liquidation preferences, if any, on any outstanding payment of other claims of creditors.
This classification of our board of directors may prevent stockholders from changing the membership of the entire board of directors in a relatively short period of time. At least two annual meetings, instead of one, generally will be required to change the majority of directors. The classified board provisions could have the effect of prolonging the time required for a stockholder with significant voting power to gain majority representation on our board of directors. Where majority or supermajority board of directors approval is necessary for a transaction, such as in the case of an interested stockholder business combination, the inability immediately to gain majority representation on the board of directors could discourage takeovers and tender offers. See “Business - The Merger and Related Transactions—Composition of the Board; Voting Agreement.”
Preferred Stock
Shares of preferred stock may be issued from time to time in one or more series, each of which will have such distinctive designation or title as shall be determined by our Board of Directors prior to the issuance of any shares thereof. Preferred stock will have such voting powers, full or limited, or no voting powers, and such preferences and relative, participating, optional or other special rights and such qualifications, limitations or restrictions thereof, as shall be stated in such resolution or resolutions providing for the issue of such class or series of preferred stock as may be adopted from time to time by the Board of Directors prior to the issuance of any shares thereof. The number of authorized shares of preferred stock may be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of a majority of the voting power of all the then outstanding shares of our capital stock entitled to vote generally in the election of the directors, voting together as a single class, without a separate vote of the holders of the preferred stock, or any series thereof, unless a vote of any such holders is required pursuant to any preferred stock designation.
While we do not currently have any plans for the issuance of additional preferred stock, the issuance of such preferred stock could adversely affect the rights of the holders of common stock and, therefore, reduce the value of the Common Stock. It is not possible to state the actual effect of the issuance of any shares of preferred stock on the rights of holders of the Common Stock until the Board of Directors determines the specific rights of the holders of the preferred stock; however, these effects may include:
| · | Restricting dividends on the Common Stock; |
| · | Diluting the voting power of the Common Stock; |
| · | Impairing the liquidation rights of the Common Stock; or |
| · | Delaying or preventing a change in control of the Company without further action by the stockholders. |
Other than in connection with our classified board structure (as explained above) and shares of preferred stock (as explained above), which preferred stock is not currently designated nor contemplated by us, we do not believe that any provision of our charter or By-Laws would delay, defer or prevent a change in control.
Options
As of July 22, 2016, options to purchase an aggregate of 6,814,841 shares of our Common Stockhave been issued and are outstanding under the 2014 Plan. In connection with the Merger, 948,567 shares of Enumeral’s common stock issued and outstanding immediately prior to the closing of the Merger (“Pre-Merger Options”) were converted into options to purchase 1,045,419 shares of our Common Stock. These option grants retain their character as ISOs or NSOs and have a term that expires on the same date that the Pre-Merger Options would have expired. These option grants also have the same vesting schedule as the Pre-Merger Options, generally vesting over a period of four years or less with monthly vesting throughout the term. The Company has also issued options to purchase shares of our Common Stock under the 2014 Plan to employees and directors of the Company.
This summary description of the options described above is qualified in their entirety by reference to the forms of such options filed as Exhibits 10.13 and 10.16 to the Registration Statement of which this Prospectus forms a part.
Warrants
As of the date hereof:
| · | The PPO Warrants entitle their holders to purchase 21,549,510 shares of Common Stock, with a term of five years and an exercise price of $2.00 per share. |
| · | The Agent Warrants entitle their holders to purchase 2,000,000 shares of Common Stock, with a term of five years and an exercise price of $1.00 per share. |
| · | Other warrants entitle their holders to purchase 1,253,899 shares of Common Stock, with terms ranging from 7 to 10 years and a weighted average exercise price of $0.432 per share. |
The PPO Warrants, the Agent Warrants and another warrant entitling its holder to purchase 70,915 shares of Common Stock contain “weighted average” anti-dilution protection in the event that we issue Common Stock or securities convertible into or exercisable for shares of Common Stock at a price lower than the subject warrant’s exercise price, subject to certain customary exceptions, as well as customary provisions for adjustment in the event of stock splits, subdivision or combination, mergers, etc.
See “Business—The Merger and Related Transactions—Registration Rights” for a description of the registration rights granted to (among others) the holders of the PPO Warrants and the Agent Warrants, which description is incorporated herein by reference.
This summary descriptions of the warrants described above is qualified in their entirety by reference to the forms of such warrants filed as Exhibits 10.5 and 10.8 to the Registration Statement of which this Prospectus forms a part.
Convertible Notes
Prior to the Merger, Enumeral had outstanding $750,000 principal amount of 12% Convertible Promissory Notes due July 1, 2015 (the “Notes”). Upon closing of the Merger, the principal and interest on the Notes were converted into 3,230,869 shares of Company Common Stock.
Other Convertible Securities
As of the date hereof, other than the securities described above, the Company does not have any outstanding convertible securities.
Transfer Agent
The transfer agent for our Common Stock is American Stock Transfer & Trust Company, LLC. The transfer agent’s address is 6201 15th Avenue, Brooklyn, NY 11219 and its phone number is (800) 416-5585.
Restrictions on Transfer of Securities
The shares of Common Stock contained in the Units and underlying the PPO Warrants are subject to restrictions on transfer and have not been registered under the Securities Act or any state securities laws. Such securities must be held indefinitely unless:
| · | there is in effect a registration statement under the Securities Act covering the proposed disposition or transfer and such disposition or transfer is made in accordance with such registration statement; or |
| · | the shares are sold pursuant to an exemption from the registration requirements of the Securities Act and applicable state securities laws, and our counsel, or an outside counsel reasonably satisfactory to us, provides a legal opinion that such disposition is exempt from registration under the Securities Act and applicable state securities laws. |
The shares of Common Stock contained in the Units and issuable upon exercise of the PPO Warrants will bear a legend setting forth these restrictions on transfer and any legends required by state securities laws.
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
The Delaware General Corporation Law (the “DGCL”) and our Amended and Restated Certificate of Incorporation (the “Certificate”) allow us to indemnify our officers and directors from certain liabilities and our Certificate states that every director, to the fullest extent permitted by the DGCL, will not be held personally liable for a breach of fiduciary duty as a director, except for liability (i) for any breach of the director’s duty of loyalty to the Corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) under Section 174 of the DGCL or (iv) for any transaction from which the director derived any improper personal benefit. If the DGCL is amended to authorize further eliminating or limiting the personal liability of directors, then the liability of a director of the Corporation shall be eliminated or limited to the fullest extent permitted by the DGCL as so amended.
Our Certificate and our Bylaws provide that we will indemnify, to the fullest extent permitted by applicable law, any person (an “Indemnified Person”) who was or is made or is threatened to be made a party or is otherwise involved in any action, suit or proceeding, whether civil, criminal, administrative or investigative (a “Proceeding”), by reason of the fact that such person is or was a director or an officer of the Corporation or, while a director or an officer of the Corporation, is or was serving at the request of the Corporation as a director, officer, employee or agent of another corporation or of a partnership, joint venture, limited liability company, trust, enterprise or nonprofit entity, including service with respect to employee benefit plans, against all liability and loss (including liabilities arising under the Securities Act) suffered and expenses (including attorneys’ fees) reasonably incurred by such Indemnified Person in such Proceeding. Our Certificate and Bylaws also provide for the advancement of expenses (including attorney’s fees) incurred in defending any such Proceeding in advance of its final disposition.
Our Bylaws provide that if an indemnification claim is not paid in full by the Corporation within 60 days after a written claim has been received by the Corporation, except in the case of a claim for an advancement of expenses, in which case the applicable period shall be 20 days, the Indemnified Person may bring suit against the Corporation to recover the unpaid amount of the claim to the fullest extent permitted by law. If successful in whole or in part in any such suit, or in a suit brought by the Corporation to recover an advancement of expenses pursuant to the terms of an undertaking, the Indemnified Person shall be entitled to be paid also the expense of prosecuting or defending such suit.
In addition to the indemnification in our Certificate and Bylaws, we have entered into indemnification agreements with each member of our board of directors and each of our executive officers. These agreements provide for the indemnification of our directors and officers for certain expenses and liabilities (including liabilities arising under the Securities Act) incurred in connection with any action, suit, proceeding or alternative dispute resolution mechanism, or hearing, inquiry or investigation that may lead to the foregoing, to which they are a party, or are threatened to be made a party, by reason of the fact that they are or were a director, officer, employee, agent or fiduciary of our company, or any of our subsidiaries, by reason of any action or inaction by them while serving as an officer, director, agent or fiduciary, or by reason of the fact that they were serving at our request as a director, officer, employee, agent or fiduciary of another entity. In the case of an action or proceeding by or in the right of our company or any of our subsidiaries, no indemnification will be provided for any claim where a court determines that the indemnified party is prohibited from receiving indemnification. We believe that these charter and bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers.
Our Bylaws provide that we may maintain insurance, at our expense, to protect us and any director, officer, employee or agent of the Company or another corporation, partnership, joint venture, trust or other enterprise against any expense, liability or loss, whether or not we would have the power to indemnify such person against such expense, liability or loss under the DGCL. We have an insurance policy covering our directors and officers with respect to certain liabilities, including liabilities arising under the Securities Act, which might be incurred by them in such capacities and against which they cannot be indemnified by us.
The limitation of liability and indemnification provisions may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might benefit us and our stockholders. Moreover, a stockholder’s investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. There is no pending litigation or proceeding naming any of our directors or officers as to which indemnification is being sought, nor are we aware of any pending or threatened litigation that may result in claims for indemnification by any director or officer.
As discussed above, our Certificate, Bylaws, and indemnification agreement provide for indemnification of our officers or directors against liability under the Securities Act. Insofar as indemnification for liabilities arising under the Securities Act may be required or permitted to directors, officers and controlling persons of the Company pursuant to the foregoing provisions, or otherwise, the Company has been advised that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
LEGAL MATTERS
The validity of the common stock offered hereby will be passed upon for us by Duane Morris LLP, 1540 Broadway, New York, NY 10036-4086.
EXPERTS
The financial statements of Enumeral Biomedical Holdings, Inc. and its wholly owned subsidiaries, Enumeral Biomedical Corp. and Enumeral Securities Corporation, for the fiscal years ended, December 31, 2015 and 2014, appearing in the registration statement, of which this prospectus forms a part, have been audited by Friedman, LLP, an independent registered public accounting firm, as set forth in their report appearing elsewhere herein and are included in reliance on such report given on the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We file annual reports, quarterly reports, prospectus and other information with the SEC. You may read or obtain a copy of these reports at the SEC’s public reference room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may obtain information on the operation of the public reference room and their copy charges by calling the SEC at 1-800-SEC-0330. The SEC maintains a website that contains registration statements, reports, proxy information statements and other information regarding registrants that file electronically with the SEC. The address of the website is http://www.sec.gov.
We have filed with the SEC a Registration Statement on Form S-1 under the Securities Act to register the shares offered by this prospectus. The term “registration statement” means the original registration statement and any and all amendments thereto, including the schedules and exhibits to the original registration statement or any amendment. This prospectus is part of that registration statement. This prospectus does not contain all of the information set forth in the registration statement or the exhibits to the registration statement. For further information with respect to us and the shares we are offering pursuant to this prospectus, you should refer to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract, agreement or other document referred to are not necessarily complete, and you should refer to the copy of that contract or other documents filed as an exhibit to the registration statement. You may read or obtain a copy of the registration statement at the SEC’s public reference facilities and Internet site referred to above.
Enumeral Biomedical Holdings, Inc.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS FOR THE YEARS
ENDED DECEMBER 31, 2015 AND 2014
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Enumeral Biomedical Holdings, Inc.
We have audited the accompanying consolidated balance sheets of Enumeral Biomedical Holdings, Inc. as of December 31, 2015 and 2014, and the related consolidated statements of operations and comprehensive income (loss), stockholders’ equity (deficiency), and cash flows for the years ended December 31, 2015 and 2014. Enumeral Biomedical Holdings, Inc.’s management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Enumeral Biomedical Holdings, Inc. as of December 31, 2015 and 2014, and the results of its operations and its cash flows for the years ended December 31, 2015 and 2014 in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has recurring losses. These conditions raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments relating to the recoverability and classification of assets carrying amounts or the amount and classification of liabilities that might result should the Company be unable to continue as a going concern.
/s/ FRIEDMAN LLP
New York, New York
March 30, 2016
Enumeral Biomedical Holdings, Inc.
Consolidated Balance Sheets
| | | December 31, | |
| | | 2015 | | | 2014 | |
| ASSETS | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 3,596,262 | | | $ | 10,460,117 | |
| Marketable securities | | | - | | | | 3,010,119 | |
| Accounts receivable | | | 306,012 | | | | 284,401 | |
| Prepaid expenses and other current assets | | | 280,479 | | | | 202,380 | |
| Total current assets | | | 4,182,753 | | | | 13,957,017 | |
| Property and equipment, net | | | 1,511,493 | | | | 1,007,127 | |
| Other assets: | | | | | | | | |
| Restricted cash | | | 534,780 | | | | 562,410 | |
| Other assets | | | 114,572 | | | | 8,416 | |
| | | | | | | | | |
| Total assets | | $ | 6,343,598 | | | $ | 15,534,970 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIENCY) | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 343,736 | | | $ | 614,106 | |
| Accrued expenses and other current liabilities | | | 714,384 | | | | 217,141 | |
| Deferred rent | | | - | | | | 32,416 | |
| Deferred revenue | | | 130,539 | | | | 134,908 | |
| Equipment lease financing | | | 240,473 | | | | - | |
| Derivative liabilities | | | 2,138,091 | | | | 16,118,802 | |
| Total current liabilities | | | 3,567,223 | | | | 17,117,373 | |
| Deferred rent, net of current portion | | | 36,847 | | | | - | |
| Deferred revenue, net of current portion | | | - | | | | 101,180 | |
| Long-term equipment lease financing | | | 266,471 | | | | - | |
| Total liabilities | | | 3,870,541 | | | | 17,218,553 | |
| | | | | | | | | |
| Commitments and contingencies (Note 8) | | | | | | | | |
| Stockholders' equity (deficiency): | | | | | | | | |
| Preferred stock, $.001 par value; 10,000,000 shares authorized: -0- shares issued and outstanding at December 31, 2015 and 2014, respectively | | | - | | | | - | |
| Common stock, $.001 par value; 300,000,000 shares authorized: 51,932,571 and 51,588,617 shares issued and outstanding at December 31, 2015 and 2014, respectively | | | 51,933 | | | | 51,589 | |
| Additional paid-in-capital | | | 16,821,767 | | | | 15,965,252 | |
| Accumulated other comprehensive loss | | | - | | | | (9,777 | ) |
| Accumulated deficit | | | (14,400,643 | ) | | | (17,690,647 | ) |
| Total stockholders’ equity (deficiency) | | | 2,473,057 | | | | (1,683,583 | ) |
| | | | | | | | | |
| Total liabilities and stockholders’ equity (deficiency) | | $ | 6,343,598 | | | $ | 15,534,970 | |
The accompanying notes are an integral part of the consolidated financial statements
Enumeral Biomedical Holdings, Inc.
Consolidated Statements of Operations and Comprehensive Income (Loss)
| | | Years Ended
December 31, | |
| | | 2015 | | | 2014 | |
| | | | | | | |
| Revenue: | | | | | | | | |
| Collaboration and license revenues | | $ | 1,100,000 | | | $ | 115,714 | |
| Grant revenue | | | 389,385 | | | | 48,312 | |
| | | | | | | | | |
| Total revenue | | | 1,489,385 | | | | 164,026 | |
| | | | | | | | | |
| Cost of revenues and expenses: | | | | | | | | |
| Research and development | | | 6,493,859 | | | | 3,575,695 | |
| General and administrative | | | 5,695,932 | | | | 3,019,101 | |
| | | | | | | | | |
| Total cost of revenues and expenses | | | 12,189,791 | | | | 6,594,796 | |
| | | | | | | | | |
| Loss from operations | | | (10,700,406 | ) | | | (6,430,770 | ) |
| | | | | | | | | |
| Other income (expense): | | | | | | | | |
| Interest income (expense) | | | 9,699 | | | | (242,430 | ) |
| Other expense | | | - | | | | (1,690,658 | ) |
| Change in fair value of derivative liabilities | | | 13,980,711 | | | | 184,448 | |
| | | | | | | | | |
| Total other income (expense), net | | | 13,990,410 | | | | (1,748,640 | ) |
| | | | | | | | | |
| Net income (loss) before income taxes | | | 3,290,004 | | | | (8,179,410 | ) |
| Provision for income taxes | | | - | | | | - | |
| Net Income (Loss) | | $ | 3,290,004 | | | $ | (8,179,410 | ) |
| | | | | | | | | |
| Other comprehensive income (loss): | | | | | | | | |
| Reclassification for loss included in net income | | | 19,097 | | | | - | |
| Net unrealized holding losses on available-for-sale securities arising during the period | | | (9,320 | ) | | | (9,777 | ) |
| Comprehensive income (loss) | | $ | 3,299,781 | | | $ | (8,189,187 | ) |
| | | | | | | | | |
| Basic net income (loss) per share | | $ | 0.06 | | | $ | (0.26 | ) |
| | | | | | | | | |
| Diluted net income (loss) per share | | $ | 0.06 | | | $ | (0.26 | ) |
| | | | | | | | | |
| Weighted-average number of common shares attributable to common stockholders – basic | | | 51,679,230 | | | | 32,047,194 | |
| | | | | | | | | |
| Weighted-average number of common shares attributable to common stockholders – diluted | | | 52,365,494 | | | | 32,047,194 | |
The accompanying notes are an integral part of the consolidated financial statements
Enumeral Biomedical Holdings, Inc.
Consolidated Statements of Stockholders’ Equity (Deficiency)
| | | Common Stock | | | | | | Accumulated
Other | | | | | | Total
Stockholders' | |
| | | Shares | | | Amount | | | Additional Paid-In
Capital | | | Comprehensive
Loss | | | Accumulated
Deficit | | | Equity
(Deficiency) | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at December 31, 2013 | | | 16,692,093 | | | $ | 16,692 | | | $ | 9,089,324 | | | $ | - | | | $ | (9,511,237 | ) | | $ | (405,221 | ) |
| Stock-based compensation expense | | | - | | | | - | | | | 659,435 | | | | - | | | | - | | | | 659,435 | |
| Conversion of convertible promissory notes | | | 3,230,869 | | | | 3,231 | | | | 684,423 | | | | - | | | | - | | | | 687,654 | |
| Proceeds from issuance of common stock in PPO, net of issuance costs | | | 21,549,510 | | | | 21,549 | | | | 18,233,895 | | | | - | | | | - | | | | 18,255,444 | |
| Derivative liability for warrants issued to PPO and Agent | | | - | | | | - | | | | (16,261,784 | ) | | | - | | | | - | | | | (16,261,784 | ) |
| Shares issued to placement agents | | | 150,000 | | | | 150 | | | | (150 | ) | | | - | | | | - | | | | - | |
| Recapitalization for reverse merger | | | 5,497,800 | | | | 5,498 | | | | (5,498 | ) | | | - | | | | - | | | | - | |
| Exchange of Series B convertible preferred stock into common stock | | | 2,777,687 | | | | 2,778 | | | | 1,595,082 | | | | - | | | | - | | | | 1,597,860 | |
| Issuance of common stock to pre-merger shareholders | | | 1,690,658 | | | | 1,691 | | | | 1,688,967 | | | | - | | | | - | | | | 1,690,658 | |
| Beneficial conversion feature associated with convertible promissory note | | | - | | | | - | | | | 281,558 | | | | - | | | | - | | | | 281,558 | |
| Unrealized holding losses on available-for-sale securities | | | - | | | | - | | | | - | | | | (9,777 | ) | | | - | | | | (9,777 | ) |
| Net loss | | | - | | | | - | | | | - | | | | - | | | | (8,179,410 | ) | | | (8,179,410 | ) |
| Balance at December 31, 2014 | | | 51,588,617 | | | $ | 51,589 | | | $ | 15,965,252 | | | $ | (9,777 | ) | | $ | (17,690,647 | ) | | $ | (1,683,583 | ) |
| Stock-based compensation expense | | | - | | | | - | | | | 827,184 | | | | - | | | | - | | | | 827,184 | |
| Reclassification for loss included in net income | | | - | | | | - | | | | - | | | | 19,097 | | | | - | | | | 19,097 | |
| Unrealized holding losses on available-for-sale securities | | | - | | | | - | | | | - | | | | (9,320 | ) | | | - | | | | (9,320 | ) |
| Exercise of stock options | | | 124,906 | | | | 125 | | | | 29,550 | | | | - | | | | - | | | | 29,675 | |
| Issuance of restricted stock | | | 219,048 | | | | 219 | | | | (219 | ) | | | - | | | | - | | | | - | |
| Net income | | | - | | | | - | | | | - | | | | - | | | | 3,290,004 | | | | 3,290,004 | |
| Balance at December 31, 2015 | | | 51,932,571 | | | $ | 51,933 | | | $ | 16,821,767 | | | $ | - | | | $ | (14,400,643 | ) | | $ | 2,473,057 | |
The accompanying notes are an integral part of the consolidated financial statements
Enumeral Biomedical Holdings, Inc.
Consolidated Statements of Cash Flows
| | | Years Ended
December 31, | |
| | | 2015 | | | 2014 | |
| | | | | | | |
| Cash flows from operating activities: | | | | | | | | |
| Net income (loss) | | $ | 3,290,004 | | | $ | (8,179,410 | ) |
| Adjustments to reconcile net income (loss) to net cash used in operating activities: | | | | | | | | |
| Depreciation and Amortization | | | 616,523 | | | | 304,456 | |
| Exit costs associated with write-off of net carrying value of leasehold improvements | | | 22,962 | | | | - | |
| Stock-based compensation | | | 827,184 | | | | 659,435 | |
| Change in fair value of derivative liabilities | | | (13,980,711 | ) | | | (184,448 | ) |
| Non-cash costs associated with reverse merger | | | - | | | | 1,690,658 | |
| Accretion of debt discount | | | - | | | | 177,712 | |
| Realized loss on marketable securities | | | 19,097 | | | | - | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Accounts receivable | | | (21,611 | ) | | | (159,401 | ) |
| Prepaid expenses and other current assets | | | (184,255 | ) | | | (61,955 | ) |
| Accounts payable | | | (270,370 | ) | | | 271,511 | |
| Accrued expenses and other current liabilities | | | 497,243 | | | | 52,606 | |
| Deferred rent | | | 4,431 | | | | (19,952 | ) |
| Deferred revenue | | | (105,549 | ) | | | 210,374 | |
| Net cash used in operating activities | | | (9,285,052 | ) | | | (5,238,414 | ) |
| | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | |
| Purchases of property and equipment | | | (1,050,506 | ) | | | (563,695 | ) |
| Purchases of marketable securities | | | - | | | | (3,019,896 | ) |
| Proceeds from sale of marketable securities | | | 3,000,799 | | | | - | |
| Receipt of (payment of) security deposits | | | 27,630 | | | | (529,744 | ) |
| Net cash provided by (used in) investing activities | | | 1,977,923 | | | | (4,113,335 | ) |
| | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | |
| Proceeds from issuance of Series B convertible preferred stock, net of issuance costs | | | - | | | | 1,597,860 | |
| Proceeds from issuance of common stock in PPO, net of issuance costs | | | - | | | | 18,255,444 | |
| Proceeds from equipment lease financing | | | 413,599 | | | | - | |
| Proceeds from issuance of convertible promissory note | | | - | | | | 750,000 | |
| Proceeds from the exercise of stock options | | | 29,675 | | | | - | |
| Payments on long-term debt | | | - | | | | (1,055,348 | ) |
| Net cash provided by financing activities | | | 443,274 | | | | 19,547,956 | |
| | | | | | | | | |
| Net increase (decrease) in cash and cash equivalents | | | (6,863,855 | ) | | | 10,196,207 | |
| Cash and cash equivalents, beginning of period | | | 10,460,117 | | | | 263,910 | |
| Cash and cash equivalents, end of period | | $ | 3,596,262 | | | $ | 10,460,117 | |
| | | | | | | | | |
| Supplemental cash flow disclosure of investing and financing activity: | | | | | | | | |
| Cash paid for interest | | $ | 6,464 | | | $ | 73,977 | |
| Conversion of convertible note and accrued interest, net of beneficial conversion feature | | $ | - | | | $ | 687,654 | |
| Non-cash equipment financing | | $ | 93,345 | | | $ | - | |
The accompanying notes are an integral part of the consolidated financial statements
ENUMERAL BIOMEDICAL HOLDINGS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1 – NATURE OF BUSINESS
Enumeral Biomedical Corp. (“Enumeral”) was founded in 2009 in the state of Delaware as Enumeral Technologies, Inc. The name was later changed to Enumeral Biomedical Corp.
On July 31, 2014 (the “Closing Date”), Enumeral entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”) with Enumeral Biomedical Holdings, Inc., which was formerly known as Cerulean Group, Inc. (“Enumeral Biomedical” or the “Company”), and Enumeral Acquisition Corp., a wholly owned subsidiary of Enumeral Biomedical (“Acquisition Sub”), pursuant to which the Acquisition Sub merged with and into Enumeral (the “Merger”). Enumeral was the surviving corporation in the Merger and became a wholly owned subsidiary of the Company.
As a result of the Merger, all issued and outstanding common and preferred shares of Enumeral were exchanged for common shares of Enumeral Biomedical Holdings, Inc. The Merger is considered to be a recapitalization of the Company which has been retrospectively applied to these financial statements for all periods presented.
Upon the closing of the Merger and under the terms of a split-off agreement and a general release agreement (the “Split-Off Agreement”), the Company transferred all of its pre-Merger operating assets and liabilities to its wholly-owned special-purpose subsidiary, Cerulean Operating Corp. (the “Split-Off Subsidiary”). Thereafter, pursuant to the Split-Off Agreement, the Company transferred all of the outstanding shares of capital stock of Split-Off Subsidiary to the pre-Merger majority stockholder of the Company, and the former sole officer and director of the Company (the “Split-Off”), in consideration of and in exchange for (i) the surrender and cancellation of an aggregate of 23,100,000 shares of the Company’s common stock held by such stockholder (which were cancelled and will resume the status of authorized but unissued shares of the Company’s common stock) and (ii) certain representations, covenants and indemnities.
As a result of the Merger and Split-Off, the Company discontinued its pre-Merger business and acquired the business of Enumeral, and will continue the existing business operations of Enumeral as a publicly-traded company under the name Enumeral Biomedical Holdings, Inc.
Also on July 31, 2014, the Company closed a private placement offering (the “PPO”) of 21,549,510 Units (the “Units”) of its securities, at a purchase price of $1.00 per Unit, each Unit consisting of one share of the Company’s common stock and a warrant to purchase one share of the Company’s common stock at an exercise price of $2.00 per share and with a term of five years (the “PPO Warrants”). Additional information concerning the PPO and PPO Warrants is presented below in Note 10.
Also on July 31, 2014, the Company changed its fiscal year from a fiscal year ending on October 31 of each year to one ending on December 31 of each year, which is the fiscal year end of Enumeral.
Following the Merger, the Company has continued Enumeral’s business of discovering and developing novel antibody immunotherapies that help the immune system fight cancer and other diseases. The Company utilizes a proprietary platform technology that facilitates the rapid high resolution measurement of immune cell function within small tissue biopsy samples. The Company’s initial focus is on the development of a pipeline of next generation monoclonal antibody drugs targeting established and novel immune-modulatory receptors.
In its lead antibody program, the Company has characterized certain anti-PD-1 antibodies, or simply “PD-1 antibodies,” using patient biopsy samples, in an effort to identify next generation PD-1 antagonists with enhanced selectivity for the immune effector cells that carry out anti-tumor functions. The Company has identified two antagonist PD-1 antibodies that inhibit PD-1 activity in distinctly different ways. One of the antibodies blocks binding of the ligand PD-L1 to PD-1, while the other antibody does not. However, both display activity in various biological assays. In addition to its PD-1 antibody program, the Company is developing antibody drug candidates targeting TIM-3, LAG-3, OX40, TIGIT and VISTA. The Company is also pursuing several antibody programs for which it has not yet publicly disclosed the targets.
The Company’s proprietary platform technology, exclusively licensed from the Massachusetts Institute of Technology (“MIT”), is a microwell array technology that detects secreted molecules (such as antibodies and cytokines) and cell surface markers, at the level of single, live cells – and enables recovery of single, live cells of interest. The platform technology is a multipurpose tool that is valuable for activities ranging from antibody discovery to target discovery to patient stratification in clinical development. The platform yields multidimensional, functional read-outs from single live cells, such as tumor infiltrating lymphocytes, or TILs, from human tumor biopsy samples, and it enables the Company’s researchers to examine the responses of different classes of human immune cells to treatment with immune-modulators in the context of human disease, as opposed to animal models of disease.
The Company continues to be a “smaller reporting company,” as defined under the Exchange Act, following the Merger. The Company believes that as a result of the Merger, it has ceased to be a “shell company” (as such term is defined in Rule 12b-2 under the Securities and Exchange Act of 1934, as amended (the “Exchange Act”)).
2 – GOING CONCERN
The Company’s consolidated financial statements have been prepared in conformity with generally accepted accounting principles in the United States which contemplate the Company’s continuation as a going concern. As of December 31, 2015, the Company had working capital of $615,530 including $2,138,091 of derivative liabilities, and an accumulated deficit of $14,400,643. As of the date of this filing, the Company believes it only has sufficient liquidity to fund operations through June 2016. The Company is currently exploring a range of potential transactions, which may includepublic or private equity offerings, debt financings, collaborations and licensing arrangements, and/or other strategic alternatives, including a merger, sale of assets or other similar transactions. If the Company is unable to raise additional capital on terms acceptable to the Company and on a timely basis, the Company may be required to downsize or wind down its operations through liquidation, bankruptcy, or a sale of its assets. The financial statements do not include any adjustments related to the recovery and classification of asset carrying amounts or the amount and classification of liabilities that might result should the Company be unable to continue as a going concern. The Company expects to incur significant expenses and operating losses for the foreseeable future, and the Company’s net losses may fluctuate significantly from quarter to quarter and from year to year. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
The Company’sbusiness has not generated (nor does the Company anticipate that in the foreseeable future it will generate) the cash necessary to finance its operations, and the Company will require additional capital to continue its operations beyond June 2016.
The Company’s near-term capital needs depend on many factors, including:
| · | the Company’s ability to carefully manage its costs; |
| · | the amount and timing of revenue received from grants or the Company’s collaboration and license arrangements; and |
| · | the Company’s ability to raise additional capital through public or private equity offerings, debt financings, or strategic collaborations and licensing arrangements, and/or the Company’s success in promptly establishing a strategic alternative that is in its stockholders’ best interests. |
If the Company is unable to preserve or raise additional capital through one or more of the means listed above prior to the end of June 2016, the Company could face substantial liquidity problems and might be required to implement cost reduction strategies in addition to the cash conservation steps that the Company has already taken. These reductions could significantly affect the Company’s research and development activities, and could result in significant harm to the Company’s business, financial condition and results of operations. In addition, these reductions could cause the Company to further curtail its operations, or take other actions that would adversely affect the Company’s stockholders. If the Company is unable to raise additional capital on acceptable terms and on a timely basis, the Company may be required to downsize or wind down its operations through liquidation, bankruptcy, or a sale of its assets.
In addition, to the extent additional capital is raised through the sale of equity or convertible debt securities, such securities may be sold at a discount from the market price of the Company’s common stock. The issuance of these securities could also result in significant dilution to some or all of the Company’s stockholders, depending on the terms of the transaction.For example, the shares included in the Units and the warrants issued in connection with the PPO contain anti-dilution protection in the event that within certain specified time periods the Company issues common stock or securities convertible into or exercisable for shares of the Company’s common stock at a price lower than the Unit purchase price or the warrant exercise price, as applicable. The anti-dilution protection provisions apply to issuances within two years after the closing of the PPO in the case of the Units and, with respect to the warrants, prior to the warrant expiration date. In addition, the anti-dilution protection provisions are subject to exceptions for certain issuances, including but not limited to (a) shares of common stock issued in an underwritten public offering, (b) issuances of awards under the Company’s 2014 Equity Incentive Plan, and (c) other exempt issuances.
3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”). Any reference in these notes to applicable guidance is meant to refer to the authoritative United States generally accepted accounting principles as found in the Accounting Standards Codifications (“ASC”) and Accounting Standards Updates (“ASU”) of the Financial Accounting Standards Board (“FASB”).
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. On an ongoing basis, the Company’s management evaluates its estimates, which include, but are not limited to, estimates related to accruals, stock-based compensation expense, warrants to purchase securities, and reported amounts of revenues and expenses during the reported period. The Company bases its estimates on historical experience and other market-specific or other relevant assumptions that it believes to be reasonable under the circumstances. Actual results may differ from those estimates or assumptions.
Principles of Consolidation and Presentation
The consolidated financial statements include the accounts of the Company and its subsidiaries. In these consolidated financial statements, “subsidiaries” are companies that are wholly owned, the accounts of which are consolidated with those of the Company. Significant intercompany transactions and balances are eliminated in consolidation.
Segment information
Operating segments are defined as components of an enterprise about which separate discrete information is available for evaluation by the chief operating decision maker, or decision-making group, in deciding how to allocate resources and in assessing performance. The Company views its operations and manages its business in one operating segment, which is the business of discovering and developing novel antibody immunotherapies that help the immune system fight cancer and other diseases. The Company operates in only one geographic segment.
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities of 90 days or less from the purchase date to be cash equivalents. Cash and cash equivalents are held in depository and money market accounts and are reported at fair value.
Marketable Securities
Marketable securities consist of U.S. treasury securities with maturities of more than 90 days. The Company has determined the appropriate balance sheet classification of the securities as current since they are available for use in current operating activities, regardless of actual maturity dates, and are recorded on the balance sheet at fair value, with the unrealized gains and losses reported in accumulated other comprehensive income (loss), which is a separate component of stockholders’ equity. When securities are sold, the unrealized gains and losses are reclassified to net earnings.
Concentration of Credit Risk
The Company has no significant off-balance sheet concentrations of credit risk such as foreign currency exchange contracts, option contracts or other hedging arrangements. Financial instruments that subject the Company to credit risk consists primarily of cash and cash equivalents. The Company generally invests its cash in money market funds, U.S. Treasury securities and U.S. Agency securities that are subject to minimal credit and market risk. Management has established guidelines relative to credit ratings and maturities intended to safeguard principal balances and maintain liquidity. At times, the Company’s cash balances may exceed the current insured amounts under the Federal Deposit Insurance Corporation.
Fair Value of Financial Instruments
Fair values of financial instruments included in current assets and current liabilities are estimated to approximate their book values, due to the short maturity of such instruments. All debt is based on current rates at which the Company could borrow funds with similar remaining maturities and approximates fair value. The Company’s assets and liabilities that are measured at fair value on a recurring basis are measured in accordance with FASB’s Accounting Standards Codification (“ASC”) Topic 820,Fair Value Measurements and Disclosures, which establishes a three-level valuation hierarchy for measuring fair value and expands financial statement disclosures about fair value measurements. The valuation hierarchy is based upon the transparency of inputs to the valuation of an asset or liability as of the measurement date.
The valuation hierarchy is based upon the transparency of inputs to the valuation of an asset or liability as of the measurement date. The three levels are defined as follows:
| · | Level 1: Inputs to the valuation methodology are quoted prices (unadjusted) for identical assets in active markets. |
| · | Level 2: Inputs to the valuation methodology included quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument. |
| · | Level 3: Inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
The Company’s cash equivalents and marketable securities carried at fair value are primarily comprised of investments in a U.S. Treasury and federal agency backed money market funds. The valuation of the Company’s derivative liabilities is discussed below and in Note 11. The following table presents information about the Company’s financial assets and liabilities measured at a fair value on a recurring basis as of December 31, 2015 and 2014:
| | | December 31,
2015 | | | Quoted Prices in
Active Markets
(Level 1) | | | Observable
Inputs
(Level 2) | | | Unobservable
Inputs
(Level 3) | |
| Assets | | | | | | | | | | | | | | | | |
| Cash | | $ | 815,890 | | | $ | 815,890 | | | $ | - | | | $ | - | |
| Money Market funds, included in cash equivalents | | $ | 2,780,372 | | | $ | 2,780,372 | | | $ | - | | | $ | - | |
| Marketable securities – U.S. Treasuries | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| Liabilities | | | | | | | | | | | | | | | | |
| Derivative liabilities | | $ | 2,138,091 | | | $ | - | | | $ | - | | | $ | 2,138,091 | |
| | | December 31,
2014 | | | Quoted Prices in
Active Markets
(Level 1) | | | Observable
Inputs
(Level 2) | | | Unobservable
Inputs
(Level 3) | |
| Assets | | | | | | | | | | | | | | | | |
| Cash | | $ | 211,329 | | | $ | 211,329 | | | $ | - | | | $ | - | |
| Money Market funds, included in cash equivalents | | $ | 10,248,788 | | | $ | 10,248,788 | | | $ | - | | | $ | - | |
| Marketable securities – U.S. Treasuries | | $ | 3,010,119 | | | $ | 3,010,119 | | | $ | - | | | $ | - | |
| Liabilities | | | | | | | | | | | | | | | | |
| Derivative liabilities | | $ | 16,118,802 | | | $ | - | | | $ | - | | | $ | 16,118,802 | |
The following table provides a roll forward of the fair value of the Company’s warrant liabilities, using Level 3 inputs:
| Balance at December 31, 2014 | | $ | 16,118,802 | |
| Change in fair value | | | (13,980,711 | ) |
| Balance at December 31, 2015 | | $ | 2,138,091 | |
Accounts Receivable and Allowance for Doubtful Accounts
Trade receivables are recorded at the invoiced amount. The Company maintains allowances for doubtful accounts, if needed, for estimated losses resulting from the inability of customers to make required payments. This allowance is based on specific customer account reviews and historical collections experience. There was no allowance for doubtful accounts as of December 31, 2015 or December 31, 2014.
Property and Equipment
Property and equipment are recorded at cost. Expenditures for maintenance and repairs are charged to expense as incurred, whereas major betterments are capitalized as additions to property and equipment. Depreciation is recorded using the straight-line method over the estimated useful lives of the assets as follows:
| Lab equipment | | 3-5 years |
| Computer equipment and software | | 3 years |
| Furniture | | 3 years |
| Leasehold improvements | | Shorter of useful life or life of the lease |
Impairment of Long-Lived Assets
Long-lived assets are reviewed for impairment annually or whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. When such events occur, the Company compares the carrying amounts of the assets to their undiscounted expected future cash flows. If this comparison indicated that there is impairment, the amount of the impairment is calculated as the difference between the carrying value and fair value. There have been no impairments recognized during the years ended December 31, 2015 and 2014, respectively.
Revenue Recognition
Collaboration and license revenue
Non-refundable license fees are recognized as revenue when the Company has a contractual right to receive such payment, the contract price is fixed or determinable, the collection of the resulting receivable is reasonably assured and the Company has no further performance obligations under the license agreement. Multiple element arrangements, such as license and development arrangements are analyzed to determine whether the deliverables, which often include license and performance obligations such as research and steering committee services, can be separated or whether they must be accounted for as a single unit of accounting in accordance with GAAP. The Company recognizes up-front license payments as revenue upon delivery of the license only if the license has stand-alone value and the fair value of the undelivered performance obligations, typically including research and/or steering committee services, can be determined. If the fair value of the undelivered performance obligations can be determined, such obligations would then be accounted for separately as performed. If the license is considered to either (i) not have stand-alone or (ii) have stand-alone value but the fair value of any of the undelivered performance obligations cannot be determined, the arrangement would then be accounted for as a single unit of accounting and the license payments and payments for performance obligations are recognized as revenue over the estimated period of when the performance obligations are performed.
Whenever the Company determines that an arrangement should be accounted for as a single unit of accounting, it must determine the period over which the performance obligations will be performed and revenue will be recognized. Revenue will be recognized using either a relative performance or straight-line method. The Company recognizes revenue using the relative performance method provided that the Company can reasonably estimate the level of effort required to complete its performance obligations under an arrangement and such performance obligations are provided on a best-efforts basis. Direct labor hours or full-time equivalents are typically used as a measure of performance. Revenue recognized under the relative performance method would be determined by multiplying the total payments under the contract, excluding royalties and payments contingent upon achievement of substantive milestones, by the ratio of level of effort incurred to date to estimated total level of effort required to complete the Company’s performance obligations under the arrangement. Revenue is limited to the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned, as determined using the relative performance method, as of each reporting period.
If the Company cannot reasonably estimate the level of effort required to complete its performance obligations under an arrangement, the performance obligations are provided on best-efforts basis and the Company can reasonably estimate when the performance obligation ceases or the remaining obligations become inconsequential and perfunctory. At that time, the total payments under the arrangement, excluding royalties and payments contingent upon achievement of substantive milestones, would be recognized as revenue on a straight-line basis over a period the Company expects to complete its performance obligations.
Revenue is limited to the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned, as determined using the straight-line basis, as of the period ending date.
In December 2014, the Company entered into a study agreement with Merck Sharp & Dohme Corp., or Merck (the “Merck Agreement”). In February 2016, the Company and Merck subsequently amended the work plan under the Merck Agreement to also include non-small cell lung cancer tissues. Pursuant to the Merck Agreement, the Company is conducting a specified research program using its platform technology to identify functional response of single cell types in colorectal cancer and non-small cell lung cancer in the presence or absence of immunomodulatory receptor modulators identified by Merck. In this collaboration, Merck is reimbursing the Company for the cost of performing the work plan set forth in the Merck Agreement, for up to a specified number of full-time employees at a pre-determined annual rate. In addition, Merck will make certain milestone payments to the Company upon the completion of specified objectives set forth in the Merck Agreement and related work plan. In September 2015, the Company announced the achievement of the first milestone under the Merck Agreement.
In January 2016, the Company and The University of Texas M.D. Anderson Cancer Center (“MDACC”) entered into a Collaborative Research and Development Agreement (the “MDACC Agreement”). Under the MDACC Agreement, the Company and MDACC plan to collaborate on the discovery and development of novel monoclonal antibodies against selected targets in immune-oncology, utilizing the Company’s antibody discovery and immune profiling platform and MDACC’s preclinical and development expertise and infrastructure.
Pursuant to the terms of the MDACC Agreement, the Company and MDACC will share the costs of research and development activities necessary to take development candidates through successful completion of a Phase I clinical trial. The MDACC Agreement provides for a structure whereby the Company and MDACC are each granted the right to receive a percentage of the net income from product sales or any payments associated with licensing or otherwise partnering a program with a third party.
Grant Revenue
In September 2014, the Company was awarded a Phase II Small Business Innovation Research contract from the National Cancer Institute (“NCI”) for up to $999,967 over two years. Grant revenue consists of a portion of the funds received to date by the NCI. Revenue is recognized as the related research services are performed in accordance with the terms of the agreement.
Research and Development Expenses
Research and development expenditures are charged to the statement of operations as incurred. Research and development expenses are comprised of costs incurred in performing research and development activities, including salaries and benefits, facilities costs, clinical supply costs, contract services, depreciation and amortization expense and other related costs. Costs associated with acquired technology, in the form of upfront fees or milestone payments, are charged to research and development expense as incurred. Legal fees incurred in connection with patent applications, along with fees associated with the license to the Company’s core technology, are expensed as research and development expense.
Derivative Liabilities
The Company���s derivative liabilities relate to (a) warrants to purchase an aggregate of 23,549,510 shares of the Company’s common stock that were issued in connection with the July 2014 PPO (as defined below in Note 10) and (b) warrants to purchase 41,659 shares of Enumeral Series A Preferred Stock that were issued in December 2011 and June 2012 pursuant to Enumeral’s debt financing arrangement with Square 1 Bank (as further described in Note 7) that were subsequently converted into warrants to purchase 66,574 shares of the Company’s common stock in connection with the July 2014 Merger. Additional detail regarding these warrants can be found in Note 11 below.
Due to the price protection provision included in the warrant agreements, the warrants were deemed to be liabilities and, therefore, the fair value of the warrants is recorded in the current liabilities section of the balance sheet. As such, the outstanding warrants are revalued each reporting period with the resulting gains and losses recorded as the change in fair value of derivative liabilities on the consolidated statement of operations and comprehensive income (loss).
The Company used the Black-Scholes option-pricing model to estimate the fair values of the issued and outstanding warrants.
Comprehensive Income (Loss)
Other comprehensive income (loss) is comprised of unrealized holding gains and losses arising during the period on available-for-sale securities that are not other-than-temporarily impaired. The unrealized gains and losses are reported in accumulated other comprehensive income (loss), until sold or maturity, at which time they are reclassified to earnings. The Company reclassified $19,097 and $0 out of accumulated other comprehensive loss to net income during the years ended December 31, 2015 and 2014, respectively.
Stock-Based Compensation
The Company accounts for its stock-based compensation awards to employees and directors in accordance with FASB ASC Topic 718,Compensation-Stock Compensation(“ASC 718”). ASC 718 requires all stock-based payments to employees, including grants of employee stock options and restricted stock, to be recognized in the consolidated statements of operations and comprehensive income (loss) based on their grant date fair values. Compensation expense related to awards to employees is recognized on a straight-line basis based on the grant date fair value over the associated service period of the award, which is generally the vesting term. Share-based payments issued to non-employees are recorded at their fair values, and are periodically revalued as the equity instruments vest and are recognized as expense over the related service period in accordance with the provisions of ASC 718 and ASC Topic 505,Equity,and are expensed using an accelerated attribution model.
The Company estimates the fair value of its stock options using the Black-Scholes option pricing model, which requires the input of subjective assumptions, including (a) the expected stock price volatility, (b) the expected term of the award, (c) the risk-free interest rate, (d) expected dividends, and (e) the estimated fair value of its common stock on the measurement date. Due to the lack of a public market for the trading of its common stock and a lack of company specific historical and implied volatility data, the Company has based its estimate of expected volatility on the historical volatility of a group of similar companies that are publicly traded. When selecting these public companies on which it has based its expected stock price volatility, the Company selected companies with comparable characteristics to it, including enterprise value, risk profiles, position within the industry, and with historical share price information sufficient to meet the expected term of the stock-based awards. The Company computes historical volatility data using the daily closing prices for the selected companies’ shares during the equivalent period of the calculated expected term of the stock-based awards. The Company will continue to apply this process until a sufficient amount of historical information regarding the volatility of its own stock price becomes available. Due to the lack of Company specific historical option activity, the Company has estimated the expected term of its employee stock options using the “simplified” method, whereby, the expected term equals the arithmetic average of the vesting term and the original contractual term of the option. The expected term for non-employee awards is the remaining contractual term of the option. The risk-free interest rates are based on the U.S. Treasury securities with a maturity date commensurate with the expected term of the associated award. The Company has never paid dividends, and does not expect to pay dividends in the foreseeable future.
The Company is also required to estimate forfeitures at the time of grant, and revise those estimates in subsequent periods if actual forfeitures differ from its estimates. The Company uses historical data to estimate forfeitures and records stock-based compensation expense only for those awards that are expected to vest. To the extent that actual forfeitures differ from the Company’s estimates, the differences are recorded as a cumulative adjustment in the period the estimates were revised. Stock-based compensation expense recognized in the financial statements is based on awards that are ultimately expected to vest.
The Company recognizes the compensation expense of employee share-based awards on a straight-line basis over the employee’s requisite service period of each award, which is generally the vesting period. The fair value of the restricted stock awards granted to employees is based upon the fair value of the common stock on the date of grant. Expense is recognized over the vesting period.
The Company has recorded stock-based compensation expense of $827,184 and $659,435 for the years ended December 31, 2015 and 2014, respectively.
Prior to the Merger, Enumeral engaged a third party to develop an estimate of the fair value of a share of Enumeral’s common stock on a fully-diluted, minority, non-marketable basis as of December 31, 2013. Based upon unaudited historical, pro-forma and/or forecast financial and operational information, which Enumeral management represented as accurately reflecting the company’s operating results and financial position, the third party utilized both a market approach (using various financial statement metrics of similar enterprises’ equity securities to estimate the fair value of Enumeral’s equity securities) and an income approach (which bases value on expectations of future income and cash flows) in their analyses. The fair value of a single share of common stock was determined using the option pricing method, which treats common and preferred stock as call options on the aggregate enterprise value and using traditional models, including Black-Scholes or binomial models, to calculate share values.
Following the Merger, the Company’s common stock became publicly traded, and fair market value is determined based on the closing sales price of the Company’s common stock on the OTCQB.
During the year ended December 31, 2014, the Company engaged a third party to develop a binomial lattice model to estimate the fair value of options to purchase a total of 450,000 shares with vesting based on the future performance of a share of the Company’s common stock.
Earnings (Loss) Per Share
Basic earnings (loss) per common share amounts are based on weighted average number of common shares outstanding. Diluted earnings per share amounts are based on the weighted average number of common shares outstanding, plus the incremental shares that would have been outstanding upon the assumed exercise of all potentially dilutive stock options, warrants and convertible debt, subject to anti-dilution limitations. All such potentially dilutive instruments were anti-dilutive as of December 31, 2014. At December 31, 2015 and 2014, the number of shares underlying options and warrants that were anti-dilutive were approximately 26.8 million and 27.5 million, respectively.
Income Taxes
Income taxes are recorded in accordance with FASB ASC Topic 740,Income Taxes(“ACS 740”), which provides for deferred taxes using an asset and liability approach. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial reporting and the tax reporting basis of assets and liabilities and are measured using the enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. The Company provides a valuation allowance against net deferred tax assets unless, based upon the available evidence, it is more likely than not that the deferred tax assets will be realized. The Company has evaluated available evidence and concluded that the Company may not realize the benefit of its deferred tax assets; therefore a valuation allowance has been established for the full amount of the deferred tax assets. The Company accounts for uncertain tax positions in accordance with the provisions of ASC 740. When uncertain tax positions exist, the Company recognizes the tax benefit of tax positions to the extent that the benefit will more likely than not be realized. The determination as to whether the tax benefit will more likely than not be realized is based upon the technical merits of the tax position as well as consideration of the available facts and circumstances.
The Company has no uncertain tax liabilities at December 31, 2015 or December 31, 2014. The guidance requires the Company to determine whether it is more likely than not that a tax position will be sustained upon examination by the appropriate taxing authority. If a tax position meets the more likely than not recognition criteria, the guidance requires the tax position be measured at the largest amount of benefit greater than 50% likely of being realized upon ultimate settlement.
New Accounting Pronouncements
In May 2014, the FASB issued Accounting Standards Update (“ASU”) No. 2014-09,Revenue from Contracts with Customers (Topic 606). ASU No. 2014-09 provides for a single comprehensive model for use in accounting for revenue arising from contracts with customers and supersedes most current revenue recognition guidance. The accounting standard is effective for interim and annual periods beginning after December 15, 2016 with no early adoption permitted. In August 2015, the FASB issued ASU No. 2015-14,Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date,which deferred the effective date of ASU No. 2014-09 to annual periods beginning after December 15, 2017, along with an option to permit early adoption as of the original effective date. The Company is required to adopt the amendments in ASU No. 2014-09 using one of two acceptable methods. The Company’s management is currently in the process of determining which adoption method it will apply and evaluating the impact of the guidance on the Company’s consolidated financial statements.
In June 2014, the FASB issued ASU No. 2014-12,Compensation—Stock Compensation (Topic 718). ASU No 2014-12 clarifies how entities should treat performance targets that can be achieved after the requisite service period of a share-based payment award. The accounting standard is effective for interim and annual periods beginning after December 15, 2015 and is not expected to have a material impact on the Company’s consolidated financial statements.
In August 2014, the FASB issued ASU No. 2014-15,Presentation of Financial Statements—Going Concern (Subtopic 205-40). ASU No 2014-15 requires all entities to evaluate for the existence of conditions or events that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the issuance date of the financial statements. The accounting standard is effective for interim and annual periods ending after December 15, 2016, and is not expected to have a material impact on the Company’s consolidated financial statements, but may impact the Company’s footnote disclosures.
In November 2015, the FASB issued ASU No. 2015-17,Income Taxes (Topic 740). ASU No. 2015-17 requires entities to present deferred tax assets and deferred tax liabilities as noncurrent in a classified balance sheet. This guidance simplifies the current guidance in ASC Topic 740,Income Taxes, which requires entities to separately present deferred tax assets and liabilities as current and noncurrent in a classified balance sheet. This guidance is effective for fiscal years beginning after December 15, 2016, and interim periods within those annual periods, with early adoption permitted. The Company adopted the guidance early as of January 1, 2015 on a prospective basis; prior periods were not retrospectively adjusted. Due to a full valuation allowance recorded against its deferred tax assets as of December 31, 2015, the Company’s consolidated balance sheet contains no deferred tax balances.
In February 2016, the FASB issued ASU No. 2016-02,Leases (Topic 842). ASU No. 2016-02 is effective for annual periods beginning after December 15, 2018, and requires a lessee to recognize assets and liabilities for leases with a maximum possible term of more than 12 months. A lessee would recognize a liability to make lease payments (the lease liability) and a right-of-use asset representing its right to use the leased asset (the underlying asset) for the lease term. Early application is permitted. The Company is currently evaluating the impact the adoption of the accounting standard will have on its consolidated financial statements.
Other accounting standards that have been issued by the FASB or other standards-setting bodies that do not require adoption until a future date are not expected to have a material impact on the Company’s financial statements upon adoption.
4 – PROPERTY AND EQUIPMENT
Property and equipment, net consist of the following:
| | | December 31, | |
| | | 2015 | | | 2014 | |
| Laboratory equipment | | $ | 2,559,986 | | | $ | 1,611,513 | |
| Computer/office equipment and software | | | 187,337 | | | | 117,429 | |
| Furniture, fixtures and office equipment | | | 73,734 | | | | 23,526 | |
| Leasehold improvements | | | 75,262 | | | | 112,507 | |
| | | | 2,896,319 | | | | 1,864,975 | |
| Less – Accumulated depreciation and amortization | | | (1,384,826 | ) | | | (857,848 | ) |
| Total property and equipment, net | | $ | 1,511,493 | | | $ | 1,007,127 | |
The Company recognized depreciation and amortization expense of $616,523 and $304,456 for the years ended December 31, 2015 and 2014, respectively. During the year ended December 31, 2015, the Company retired leasehold improvements with a gross cost of $112,507 and expensed the remaining net carrying value of $22,962 associated with the write-down of leasehold improvements due to a relocation of the Company’s corporate office and research laboratories in March 2015 (see Note 8).
5 – RESTRICTED CASH
The Company held $534,780 and $562,410 in restricted cash as of December 31, 2015 and December 31, 2014, respectively. The balances are primarily held on deposit with a bank to collateralize standby letters of credit in the name of the Company’s facility lessors in accordance with the Company’s facility lease agreements.
6 – ACCRUED EXPENSES AND OTHER CURRENT LIABILITIES
The Company’s accrued expenses and other current liabilities consist of the following:
| | | December 31, | |
| | | 2015 | | | 2014 | |
| Wages and benefits | | $ | 447,769 | | | $ | 88,700 | |
| Accrued professional fees | | | 213,475 | | | | 125,751 | |
| Accrued other | | | 53,140 | | | | 2,690 | |
| Total accrued expenses and other current liabilities | | $ | 714,384 | | | $ | 217,141 | |
7 – DEBT
Square 1 Financing and Security Agreement
In December 2011, Enumeral entered into a $1.79 million venture debt financing with Square 1 Bank (as subsequently amended, the “Square 1 Financing”), pursuant to which Enumeral was required to comply with certain covenants on an annual basis. Enumeral was required to meet a maximum cash burn or liquidity ratio as indicated in the term loan agreement. In February 2014, Enumeral revised the terms of its Loan and Security Agreement with Square 1 Bank, whereby Enumeral agreed to complete an equity financing for gross cash proceeds of $2.0 million by March 31, 2014 and $4.0 million by June 30, 2014. Additionally, beginning on the date that Enumeral completed an equity financing for gross cash proceeds of $2.0 million, a monthly minimum unrestricted cash balance was required based upon three times the trailing three month cash burn.
In June 2014, Enumeral further revised the terms of its Loan and Security Agreement with Square 1 Bank, whereby Enumeral extended its deadline to complete an equity financing for gross cash proceeds of $4.0 million to August 1, 2014. Additionally, Enumeral amended its minimum cash requirement beginning June 26, 2014 through August 1, 2014, pursuant to which Enumeral was required to maintain a minimum of $300,000 in unrestricted cash.
In August 2014, Enumeral fully repaid this loan.
In connection with the Square 1 Financing, Enumeral issued warrants to purchase an aggregate of 41,659 shares of Enumeral Series A preferred stock that were subsequently converted into warrants to purchase 66,574 shares of the Company’s common stock in connection with the July 2014 Merger, as further described in Note 11 below.
Convertible Promissory Notes
In February 2014, Enumeral raised $750,000 by issuing convertible promissory notes at 12% interest per annum. The maturity date was July 2015. The holders of these notes had the right to convert the notes into shares of common stock at a premerger conversion price of $0.27 per share. In July 2014, the principal of $750,000 and accrued interest of $41,500, offset by $103,846 of debt discount, related to these notes were converted into 3,230,869 shares of common stock (see Note 10). If prior to maturity or conversion, Enumeral consummated a liquidation event as defined, at the election of the holder, (a) Enumeral would have been required to pay the holders an amount equal to the sum of three times the total principal amount then outstanding under these notes, plus all accrued and unpaid interest due, (b) the outstanding principal of the notes would have automatically converted into shares of Enumeral’s Series A-2 Preferred Stock at the closing of the liquidation event at the Series A-2 Original Issue Price, and (c) the outstanding principal of the notes would have automatically converted into shares of Enumeral’s common stock at the closing of the liquidation event at a price per share of $0.27.
Enumeral allocated proceeds to the convertible notes and the warrants based upon the relative fair value on the issuance date which resulted in a $140,779 debt discount on the convertible promissory notes and $140,779 allocated to the warrants, recorded in equity. The allocation of proceeds to the warrants gave rise to a beneficial conversion feature which was recorded at the intrinsic value ($140,779) calculated as the difference between the adjusted conversion price of approximately $0.22 and the estimated fair value of Enumeral’s common stock of $0.27. For the year ended December 31, 2014, the Company recorded $177,712 to interest expense as it relates to the beneficial conversion feature and debt discount associated with warrants.
Equipment Lease Financing
In December 2015, the Company and Fountain Leasing 2013 LP (“Fountain”) entered into a master lease agreement and related transaction documents, pursuant to which Fountain provided the Company with $506,944 for the purchase of research and development lab equipment (the “Fountain Lease”). Fountain’s security under the Fountain Lease is the equipment purchased and a security deposit in the amount of $101,389. The initial term of the Fountain Lease is 36 months, with payments of $21,545 per month for the first 24 months and then $1,267 for the 12 months thereafter. Pursuant to the terms of the Fountain Lease, the Company has an option at the end of the initial term to purchase the equipment for the greater of $25,347 or current fair market value, provided that such amount shall not be in excess of $152,083. In addition, the Company also has the option to extend the Fountain Lease for an additional 12 month period at a rate of $8,872 per month with the right at the end of such extension term to purchase the equipment for fair value or to return the equipment to Fountain. The Fountain Lease has a lease rate factor of 4.25% per month for the first 24 months and 0.25% for the final 12 months of the initial term.
The company has recorded current equipment lease financing of $240,473 and long-term equipment lease financing of $266,471 at December 31, 2015. The equipment has been included in property and equipment on the Company’s consolidated balance sheet.
Future principal payments on the $506,944 equipment lease financing are as follows:
| | | December 31,
2015 | |
| 2016 | | | 258,541 | |
| 2017 | | | 258,542 | |
| 2018 | | | 15,208 | |
| Total equipment lease financing payments | | | 532,291 | |
| Current equipment lease financing | | | 258,541 | |
| Less: Amount representing interest | | | (18,068 | ) |
| Current equipment lease financing, net | | | 240,473 | |
| Long-term equipment lease financing | | | 273,750 | |
| Less: Amount representing interest | | | (7,279 | ) |
| Equipment lease financing, less current portion, net | | $ | 266,471 | |
8 – COMMITMENTS
Operating Leases
In March 2015, the Company relocated its offices and research laboratories to 200 CambridgePark Drive in Cambridge, Massachusetts. The Company is leasing 16,825 square feet at this facility pursuant to Indenture of Lease (the “CambridgePark Lease”) that the Company entered into in November 2014. The term of the CambridgePark Lease is for five years, and the initial base rent is $42.50 per square foot, or approximately $715,062 on an annual basis. The base rent will increase incrementally over the term of the CambridgePark Lease, reaching approximately $804,739 on an annual basis in the fifth year of the term. In addition, the Company is obligated to pay a proportionate share of the operating expenses and applicable taxes associated with the premises, as calculated pursuant to the terms of the CambridgePark Lease. Pursuant to the terms of the CambridgePark Lease, the Company has delivered a security deposit to the landlord in the amount of $529,699, which may be reduced to $411,988 following the second anniversary of the commencement date, provided that the Company meets certain financial conditions set forth in the CambridgePark Lease. The Company has recorded deferred rent in connection with the CambridgePark Lease as of December 31, 2015 in the amount of $36,847. This amount has been recorded as a long-term liability on the Company’s consolidated balance sheet.
The Company previously occupied offices and research laboratories in approximately 4,782 square feet of space at One Kendall Square in Cambridge, Massachusetts, at an annual rent of $248,664 (the “Kendall Lease”). For the year ended December 31, 2015, the Company recorded an expense of $55,352, representing all exit costs associated with its move to new offices and research laboratories in March 2015. In June 2015, Enumeral entered into a lease termination agreement with the landlord for Enumeral’s former facility at One Kendall Square, pursuant to which the Kendall Lease was terminated as of June 17, 2015. In accordance with the terms of the lease termination agreement, Enumeral is not obligated to pay rent for the One Kendall Square facility after May 31, 2015. Enumeral had maintained a security deposit relating to the facility, recorded as restricted cash on the accompanying consolidated balance sheet. This security deposit was returned to Enumeral pursuant to the lease termination agreement.
In addition, the Company maintains a small corporate office at 1370 Broadway in New York, New York, at an annual rent of $23,100. The lease for the Company’s New York office expires on December 31, 2016.
Rent expense was $1,079,753 and $309,475 for the years ended December 31, 2015 and 2014, respectively.
Future operating lease commitments as of December 31, 2015 are as follows:
| Years Ending December 31, | | | |
| 2016 | | $ | 756,109 | |
| 2017 | | | 754,966 | |
| 2018 | | | 777,567 | |
| 2019 | | | 800,842 | |
| Thereafter | | | 134,123 | |
| | | $ | 3,223,607 | |
Employment Agreements
The Company has employment agreements with certain members of management which contain minimum annual salaries and severance benefits if their employment is terminated prior to the term of the agreements.
9 – LICENSE AGREEMENT AND RELATED-PARTY TRANSACTIONS
License Agreement
In April 2011, Enumeral licensed certain intellectual property from the Massachusetts Institute of Technology (“MIT”), then a related party (as one of Enumeral’s scientific co-founders was an employee of MIT), pursuant to an Exclusive License Agreement (the “License Agreement”), in exchange for the payment of upfront license fees and a commitment to pay annual license fees, patent costs, milestone payments, royalties on sublicense income and, upon product commercialization, royalties on the sales of products covered by the licenses or income from corporate partners, and the issuance of 66,303 shares of Enumeral common stock. This intellectual property portfolio includes patents owned by Harvard University or co-owned by MIT and The Whitehead Institute, or MIT and Massachusetts General Hospital.
All amounts paid related to the license fees have been expensed as research and development by Enumeral as incurred. The Company incurred $30,000 and $25,000 in the years ended December 31, 2015 and 2014, respectively.
In addition to potential future royalty and milestone payments that Enumeral may have to pay MIT per the terms of the License Agreement, Enumeral is obligated to pay an annual fee of $40,000 in 2016, and $50,000 every year thereafter unless the License Agreement is terminated. During the year ended December 31, 2015, the Company achieved the first milestone in the Merck Agreement and paid MIT the required percentage of the milestone payment pursuant to the terms of the License Agreement. No royalty payments have been payable as Enumeral has not commercialized any products as set forth in the License Agreement. The Company reimburses costs to MIT and Harvard University for the continued prosecution of the licensed patent estate. For the years ended December 31, 2015 and 2014, the Company reimbursed $325,945 and $505,697 to MIT and $23,637 and $173,525 to Harvard, respectively. As of December 31, 2015 and 2014, the Company had accounts payable and accrued expenses of $168,726 and $65,529, respectively, associated with the reimbursement of costs to MIT and Harvard.
The License Agreement also contained a provision whereby after the date upon which $7,500,000 of funding which was met in April of 2013, MIT and other licensing institutions set forth in the License Agreement have a right to participate in certain future equity issuances by Enumeral. In addition, pursuant to that provision Enumeral may have to issue additional shares to MIT and other licensing institutions set forth in the License Agreement if Enumeral issues common stock at a price per share that is less than the fair market value per share of the common stock issued to MIT and such licensing institutions based upon a weighted average formula set forth in the License Agreement. In March 2013, Enumeral and MIT entered into a first amendment to the License Agreement to clarify how equity issuances were to be made thereunder. In July 2014, Enumeral and MIT entered into a second amendment to the License Agreement, pursuant to which MIT’s participation rights and anti-dilution rights under the license agreement were terminated. Other than the exchange of Enumeral’s common stock for the Company’s common stock in connection with the Merger, the Company did not issue any shares of common stock to MIT and such other licensing institutions in connection with the License Agreement in 2014.
In April 2015, Enumeral and MIT entered into a third amendment to the License Agreement, which revised the timetable for Enumeral to complete certain diligence obligations relating to the initiation of clinical studies in support of obtaining regulatory approval of a Diagnostic Product (as such term is defined in the License Agreement), as well as the timetable by which Enumeral is required to make the first commercial sale of a Diagnostic Product.
Consulting Agreements
On April 19, 2011, Enumeral entered into a consulting agreement with Barry Buckland, Ph.D., a member of the board of directors. Pursuant to the original agreement, Dr. Buckland was compensated through the issuance of 159,045 shares of restricted common stock of Enumeral that vested on the following schedule: 25% on the execution of the contract, 25% on April 19, 2012 and the remaining 50% vesting in equal monthly installments through April 1, 2014. These shares were subsequently converted into 175,286 shares of the Company’s common stock in connection with the Merger. The term of the consulting agreement was three years. On February 11, 2013, the consulting agreement was amended so that Dr. Buckland would receive $75,000 per year for a period of one year. On August 14, 2013, the consulting agreement was amended so that Dr. Buckland would receive $37,500 per year for a period of one year. In September 2014, the Company and Dr. Buckland entered into a Scientific Advisory Board Agreement (the “SAB Agreement”), which supersedes the previous consulting agreement, and pursuant to which Dr. Buckland will serve as Chairman of the Company’s Scientific Advisory Board. The SAB Agreement has a term of two years. Pursuant to the terms of the SAB Agreement, Dr. Buckland will receive compensation on an hourly or per diem basis, either in cash or, at Dr. Buckland’s election, in options to purchase the Company’s common stock. The SAB Agreement limits the total amount of compensation payable to Dr. Buckland at $100,000 over any rolling 12-month period. During the years ended December 31, 2015 and 2014, the Company recognized $32,000 and $32,667 of expense, respectively, related to the consulting agreement and SAB Agreement.
On September 20, 2013, Enumeral entered into a consulting agreement with Allan Rothstein and Norman Rothstein (collectively the “Rothstein Consultants”). Pursuant to the consulting agreement, Allan Rothstein was elected as a member of the Board of Directors of Enumeral. The Rothstein Consultants were compensated through the issuance of 1,000,000 shares of restricted common stock of Enumeral, with one third vesting upon the execution of the consulting agreement, one third vesting on December 10, 2013 and one third vesting on March 10, 2014. These shares were subsequently converted into 1,102,121 shares of the Company’s common stock in connection with the Merger. The consulting agreement had a term of two years, but was terminated on July 30, 2014. During the years ended December 31, 2015 and 2014, the Company recognized $0 and $90,000 of restricted stock compensation expense, respectively, related to the shares granted in this consulting agreement.
10 – EQUITY
Common Stock
On April 8, 2014, Enumeral amended its certificate of incorporation to increase the number of authorized shares of common stock from 15,000,000 to 24,000,000.
In April 2014, Enumeral issued 948,823 shares of Series B Convertible Preferred Stock at an issue price of $2.125 per share for proceeds of $1,597,860, net of issuance costs of $418,390. The Series B Preferred Stock ranks pari passu in all respects to Enumeral’s Series A-2, Series A-1 and Series A Preferred Stock. In connection with this offering, Enumeral paid the placement agent $81,000 in cash and issued the placement agent a warrant to purchase 38,259 Series B shares exercisable at $2.125 per share for a term of five years. These costs were included in the total issuance costs. All shares and warrants were converted as part of the Merger (see Merger discussion below).
In April 2014, Enumeral issued warrants to two executive officers to purchase 105,881 shares of Convertible Preferred Series B shares in connection with Enumeral’s Series B financing. These warrants were issued in relation to these executives taking a salary reduction prior to the Series B round of financing. In connection with the Merger in July 2014, these warrants were converted into warrants to purchase 309,967 shares of the Company’s common stock (see Merger discussion below).
On July 31, 2014, Enumeral entered into the Merger Agreement, pursuant to which Enumeral became a wholly owned subsidiary of the Company. The Company’s authorized capital stock currently consists of 300,000,000 shares of common stock, par value $0.001, and 10,000,000 shares of “blank check” preferred stock, par value $0.001.
Merger
As a result of the Merger, all issued and outstanding common and preferred shares of Enumeral were exchanged for common shares of the Company as follows: (a) each share of Enumeral’s common stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.102121 shares of the Company’s common stock for a total of 4,940,744 shares post-Merger, (b) each share of Enumeral’s Series A Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.598075 shares of the Company’s common stock for a total of 4,421,744 shares post-Merger, (c) each share of Enumeral’s Series A-1 Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.790947 shares of the Company’s common stock for a total of 3,666,428 shares post-Merger, (d) each share of Enumeral’s Series A-2 Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.997594 shares of the Company’s common stock for a total of 3,663,177 shares post-Merger, (e) each share of Enumeral’s Series B Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 2.927509 shares of the Company’s common stock for a total of 2,777,687 shares post-Merger and (f) a convertible note and accrued interest was converted into 3,230,869 shares of the Company’s common stock post-Merger.
As a result of the Merger and Split-Off, the Company discontinued its pre-Merger business and acquired the business of Enumeral, and has continued the existing business operations of Enumeral as a publicly-traded company under the name Enumeral Biomedical Holdings, Inc. In accordance with “reverse merger” accounting treatment, historical financial statements for Enumeral Biomedical Holdings Inc. as of period ends, and for periods ended, prior to the Merger will be replaced with the historical financial statements of Enumeral prior to the Merger in all future filings with the SEC.
Private Placement
On July 31, 2014, the Company closed a private placement offering (the “PPO”) of 21,549,510 Units of securities, at a purchase price of $1.00 per Unit, each Unit consisting of one share of the Company’s common stock and a warrant to purchase one share of the Company’s common stock at an exercise price of $2.00 per share with a term of five years (the “PPO Warrants”). The net proceeds received from the PPO were $18,255,444. The investors in the PPO (for so long as they hold shares of the Company’s common stock) have anti-dilution protection on the shares of common stock included in the Units purchased in the PPO and not subsequently transferred or sold (other than transfers to trusts or affiliates of such investors for the purpose of estate planning) in the event that within two years after the closing of the PPO the Company issues common stock or securities convertible into or exercisable for shares of the Company’s common stock at a price lower than the Unit purchase price. The anti-dilution protection provisions are subject to exceptions for certain issuances, including but not limited to (a) shares of common stock issued in an underwritten public offering, (b) issuances of awards under the Company’s 2014 Equity Incentive Plan, and (c) other exempt issuances.
In addition, the PPO Warrants not subsequently transferred or sold (other than transfers to trusts or affiliates of such investors for the purpose of estate planning) have anti-dilution protection in the event that prior to the warrant expiration date the Company issues common stock or securities convertible into or exercisable for shares of the Company’s common stock at a price lower than the warrant exercise price, subject to the exceptions described above.
The Company agreed to pay the placement agents in the offering, registered broker-dealers, a cash commission of 10% of the gross funds raised from investors in the PPO. In addition, the placement agents received warrants exercisable for a period of five years to purchase a number of shares of the Company’s common stock equal to 10% of the number of shares of common stock with a per share exercise price of $1.00 (the “Agent Warrants”); provided, however, that the placement agents were not entitled to any warrants on the sale of Units in excess of 20,000,000. Any sub-agent of the placement agents that introduced investors to the PPO was entitled to share in the cash fees and warrants attributable to those investors as described above. The Company also reimbursed the placement agents $30,000 in the aggregate for legal expenses incurred by the placement agents’ counsels in connection with the PPO, as described in the private placement agreements. As a result of the foregoing, the placement agents were paid an aggregate cash commission of $2,154,951 and were issued Agent Warrants to purchase 2,000,000 shares of the Company’s common stock. The Agent Warrants not subsequently transferred or sold (other than transfers to trusts or affiliates of such investors for the purpose of estate planning) have anti-dilution protection until the warrant expiration date, subject to the exceptions described above for the Units. The value ascribed to the Agent Warrants are carried at fair value and reported as a derivative liability on the accompanying balance sheets.
The Company incurred approximately $500,000 of expenses in connection with the offering outside of the placement agent commissions and issued the subagent to one of the placement agents 150,000 shares of the Company’s common stock.
In addition, the Merger Agreement provided certain anti-dilution protection to the holders of the Company’s common stock immediately prior to the Merger (after giving effect to the Split-Off), in the event that the aggregate number of units sold in the PPO after the final closing thereof were to exceed 15,000,000. Accordingly, based on the final amount of gross proceeds raised in the PPO, the Company issued 1,690,658 additional shares of the Company’s common stock to holders of the Company’s common stock immediately prior to the Merger. The Company recorded $1,690,658 in other expense related to this issuance of shares at $1.00 per share.
11 – STOCK OPTIONS, RESTRICTED STOCK AND WARRANTS
Stock Options
In December 2009, Enumeral adopted the 2009 Stock Incentive Plan (the “2009 Plan”). In April 2014, Enumeral amended the 2009 Plan to increase the number of shares authorized thereunder to 3,200,437. The 2009 Plan was terminated in July 2014 in connection with the Merger.
On July 31, 2014, the Company’s Board of Directors adopted, and the Company’s stockholders approved, the 2014 Equity Incentive Plan (the “2014 Plan”), which reserves a total of 8,100,000 shares of the Company’s common stock for incentive awards. In connection with the Merger, options to purchase 948,567 shares of Enumeral common stock previously granted under the 2009 Plan were converted into options to purchase 1,045,419 shares of the Company’s common stock under the 2014 Plan.
Generally, shares that are expired, terminated, surrendered or cancelled without having been fully exercised will be available for future awards. In addition, shares of common stock that are tendered to the Company by a participant to exercise an award are added to the number of shares of common stock available for the grant of awards.
As of December 31, 2015, there are 1,399,097 shares available for issuance under the 2014 Plan to eligible employees, non-employees directors and consultants. This number is subject to adjustment in the event of a stock split, reverse stock split, stock dividend, or other change in the Company’s capitalization.
During the years ended December 31, 2015 and 2014, there were 3,444,000 and 1,922,626 stock options granted to employees, directors or consultants under the 2014 Plan, respectively, with weighted-average grant date fair values, using the Black-Scholes pricing model, of $0.31 and $0.63. The vesting of employee and director awards is generally time-based and the restrictions typically lapse 25% after 12 months and monthly thereafter for the next 36 months for employees and annually over three years for directors. In addition, certain option awards for employees provide for vesting of all or a portion of the shares underlying such option upon the achievement of certain milestones or performance-based criteria.
On March 24, 2016, the Compensation Committee of the Company’s Board of Directors granted an aggregate of 1,150,000 options to purchase shares of the Company’s common stock to employees of the Company.
The Company estimates the fair value of each stock award on the grant date using the Black-Scholes option-pricing model based on the following assumptions and the assumptions regarding the fair value of the underlying common stock on each measurement date:
| | | Years ended December 31, | |
| | | 2015 | | | 2014 | |
| | | | | | | |
| Expected Volatility | | | 104.1%-136.0 | % | | | 99.0 | % |
| Risk-free interest rate | | | 1.60%-2.22 | % | | | 1.62%-1.72 | % |
| Expected term (in years) | | | 6.00-9.59 | | | | 6.00 | |
| Expected dividend yield | | | 0 | % | | | 0 | % |
In July 2014, Enumeral modified the option grants of two former directors of Enumeral. As a result of this modification, 77,148 shares became fully vested and the Company incurred stock based compensation of $63,262 during the year ended December 31, 2014.
Stock option expense for employees was $707,325 and $339,415 for the years ended December 31, 2015 and 2014, respectively, while non-employee stock option expense was $18,474 and $25,951 for the years ended December 31, 2015 and 2014, respectively. The Company has an aggregate of $1,444,849 of unrecognized stock-based compensation expense as of December 31, 2015 to be amortized over a weighted average period of 2.73 years.
A summary stock option activity for the year ended December 31, 2015 is as follows:
| | | | | | | | | Weighted- | | | | |
| | | | | | Weighted- | | | Average | | | | |
| | | | | | Average | | | Remaining | | | Aggregate | |
| | | | | | Exercise | | | Contractual | | | Intrinsic | |
| | | Shares | | | Price | | | Term (years) | | | Value | |
| Outstanding at December 31, 2014 | | | 2,730,963 | | | $ | 0.78 | | | | 9.0 | | | $ | 763,281 | |
| Granted | | | 3,444,000 | | | $ | 0.47 | | | | | | | | | |
| Exercised | | | (124,906 | ) | | $ | 0.24 | | | | | | | | | |
| Canceled | | | (123,403 | ) | | $ | 0.44 | | | | | | | | | |
| Outstanding at December 31, 2015 | | | 5,926,654 | | | $ | 0.62 | | | | 9.0 | | | $ | 8,848 | |
| Exercisable at December 31, 2015 | | | 1,581,064 | | | $ | 0.62 | | | | 8.1 | | | $ | 8,834 | |
The aggregate intrinsic value was calculated as the difference between the exercise price of the stock options and the fair value of the underlying common stock as of the balance sheet date.
Restricted Stock
Restricted stock expense was $101,385 and $156,299 for the years ended December 31, 2015 and 2014, respectively.
A summary of restricted stock activity for the year ended December 31, 2015 is as follows:
| | | | | | Weighted- | |
| | | Number of | | | Average Grant | |
| | | Shares | | | Date Fair Value | |
| Balance of unvested restricted stock at December 31, 2014 | | | 345,699 | | | $ | 0.23 | |
| Issuance of restricted stock | | | 219,048 | | | $ | 0.30 | |
| Restrictions lapsed | | | (282,628 | ) | | $ | (0.29 | ) |
| Balance of unvested restricted stock at December 31, 2015 | | | 282,119 | | | $ | 0.24 | |
The Company has an aggregate of $51,074 of unrecognized restricted stock compensation expense as of December 31, 2015 to be amortized over a weighted average period of 0.6 years.
Warrants
As of December 31, 2015 and 2014, there were a total of 24,803,409 warrants outstanding to purchase shares of the Company’s common stock. Of these, 23,549,510 warrants were issued in connection with the PPO and 66,574 warrants were issued to Square 1 Bank and are accounted for as a derivative liability warrants. The remaining 1,187,325 warrants do not require derivative liability accounting treatment.
Derivative Liability Warrants
In connection with the PPO, the Company issued warrants to purchase an aggregate of 23,549,510 shares of the Company’s common stock. Additionally, in connection with the Square 1 Financing, Enumeral issued warrants to purchase 41,659 shares of Enumeral’s Series A preferred stock that were subsequently converted into warrants to purchase 66,574 shares of the Company’s common stock in connection with the July 2014 Merger.
PPO and Agent Warrants
In July 2014, the Company issued warrants to purchase 23,549,510 shares of the Company’s common stock in connection with the PPO, of which warrants to purchase 21,549,510 shares of the Company’s common stock had an exercise price of $2.00 per share and were issued to the investors in the PPO, and warrants to purchase 2,000,000 shares of the Company’s common stock had an exercise price of $1.00 per share and were issued to the placement agents for the PPO (or their affiliates). The estimated fair value of the warrants at the time of issuance was determined to be $16,261,784 using the Black-Scholes pricing model and the following assumptions: expected term of five years, 105.4% volatility, and a risk-free rate of 1.77%. Due to a price protection provision included in the warrant agreements, the warrants were deemed to be a liability and, therefore, the fair value of the warrants is recorded in the liability section of the balance sheet. As such, the outstanding warrants are revalued each reporting period with the resulting gains and losses recorded as the change in fair value of warrant liability on the consolidated statement of operations. The estimated fair value of the warrants at December 31, 2015 was determined to be $2,130,822 using the Black-Scholes pricing model and the following assumptions: expected remaining term of 3.58 years, 109.4% volatility, risk-free rate of 1.44%, and no expected dividends. The estimated fair value of the warrants at December 31, 2014 was determined to be $16,065,396 using the Black-Scholes pricing model and the following assumptions: expected remaining term of 4.58 years, 99.8% volatility, risk-free rate of 1.53%, and no expected dividends. All 23,549,510 warrants were outstanding as of December 31, 2015 and 2014, respectively.
Square 1 Financing
In connection with the December 2011 Square 1 Financing, Enumeral issued to Square 1 Bank warrants to purchase an aggregate of 33,944 shares of Series A convertible preferred stock at an exercise price of $1.16 per share, exercisable for seven years. In July 2014, as part of the Merger, these warrants were converted into a warrant to purchase 54,245 shares of the Company’s common stock at an exercise price of $0.73 per share. The estimated fair value of the warrants as of December 31, 2015 was determined to be $5,777 using the Black-Scholes pricing model and the following assumptions: expected term of 2.9 years, 104.6% volatility, and a risk-free rate of 1.31%. The estimated fair value of the warrants as of December 31, 2014 was determined to be $43,237 using the Black-Scholes pricing model and the following assumptions: expected term of 3.93 years, 99.8% volatility, and a risk-free rate of 1.375%. As part of a June 12, 2012 amendment to the Loan and Security Agreement between Enumeral and Square 1 Bank, Enumeral issued warrants to Square 1 Bank to purchase an aggregate of 7,715 shares of Series A convertible preferred stock at an exercise price of $1.16 per share, exercisable for seven years. In July 2014, as part of the Merger, these warrants were converted into a warrant to purchase 12,329 shares of the Company’s common stock at an exercise price of $0.73 per share. The estimated fair value of these warrants as of December 31, 2015 was determined to be $1,492 using the Black-Scholes pricing model and the following assumptions: expected term of 3.5 years, 104.6% volatility, and a risk-free rate of 1.42%. The warrants are classified as derivative liabilities in the accompanying balance sheets and measured at fair value on a recurring basis. The estimated fair value of these warrants as of December 31, 2014 was determined to be $10,169 using the Black-Scholes pricing model and the following assumptions: expected term of 4.45 years, 99.8% volatility, and a risk-free rate of 1.65%. As such, the outstanding warrants are revalued each reporting period with the resulting gains and losses recorded as the change in fair value of warrant liability on the statement of operations. As of December 31, 2015 and 2014, these warrants were outstanding and expire on December 5, 2018 and June 12, 2019.
Derivative Liability Re-Measurement
The Company used the Black-Scholes option-pricing model to estimate the fair values of the issued and outstanding warrants as of December 31, 2015 and 2014. The Company recorded income of $13,980,711 and $184,448 for the years ended December 31, 2015 and 2014, respectively, due to the change in the fair value of the warrants. Outstanding warrants are revalued each reporting period with the resulting gains and losses recorded as the change in fair value of warrant liabilities on the consolidated statements of operations.
Other Warrants
In April 2014, in connection with Enumeral’s Series B preferred stock offering, Enumeral compensated the placement agent through the issuance of a warrant for 38,259 shares of Enumeral Series B preferred stock. The estimated fair value of the warrant at the time of issuance was determined to be $49,854 using the Black-Sholes pricing model and the following assumptions: expected term of five years, exercise price of $2.125 per share, 74.6% volatility, and a risk-free rate of 1.72%. The Company recorded this value net against issuance cost in equity. In connection with the Merger in July 2014, this Series B preferred stock warrant was converted into a warrant to purchase 112,001 shares of the Company’s common stock. The exercise price of these warrants is $0.73 per share.
In April 2014, Enumeral issued warrants to two executive officers to purchase a total of 105,881 shares of Enumeral Series B preferred stock in connection with Enumeral’s Series B financing. These warrants were issued in relation to these executives taking a salary reduction prior to the Series B financing. The estimated fair value of the warrants at the time of issuance was determined to be $137,770 using the Black-Sholes pricing model and the following assumptions: expected term of five years, exercise price of $2.125 per share, 74.6% volatility, and a risk-free rate of 1.63%. These warrants vested over six months. During the year ended December 31, 2014, the Company recorded $137,770 of stock based compensation expense related to these warrants. In connection with the Merger in July 2014, these Series B warrants were converted into warrants to purchase 309,967 shares of the Company’s common stock. The exercise price of these warrants is $0.73 per share.
In April 2014, Enumeral issued warrants associated with Enumeral’s convertible promissory notes to purchase 694,443 shares of Enumeral common stock. The estimated fair value of the warrants at the time of issuance was determined to be $140,779 using the Black-Sholes pricing model and the following assumptions: expected term of ten years, exercise price of $0.27 per share, 67.6% volatility, and a risk-free rate of 2.61%. The Company recorded this value against equity. As part of the Merger, these warrants were converted into warrants to purchase 765,357 shares of the Company’s common stock. The exercise price of these warrants is $0.245 per share.
12 – INCOME TAX
There is no provision for income taxes because the Company has historically incurred operating losses and maintains a full valuation allowance against its gross deferred tax assets. The reported amount of income tax expense differs from the amount that would result from applying domestic federal statutory tax rates to pretax losses primarily because of changes in the valuation allowance.
Significant components of the Company’s net deferred tax asset at December 31, 2015 and 2014 are as follows:
| | | December 31, | |
| | | 2015 | | | 2014 | |
| Deferred tax assets: | | | | | | | | |
| Net operating losses | | $ | 4,932,778 | | | $ | 3,045,692 | |
| Accrued expenses | | | 482,130 | | | | 253,576 | |
| Stock options | | | 402,598 | | | | 251,798 | |
| Tax credit carryforwards | | | 748,255 | | | | 413,691 | |
| Depreciation and amortization | | | (23,311 | ) | | | (30,668 | ) |
| Other | | | - | | | | 135 | |
| Capitalized R&D | | | 4,070,458 | | | | 2,386,506 | |
| Total gross deferred tax assets | | | 10,612,908 | | | | 6,320,730 | |
| Valuation allowance | | | (10,612,908 | ) | | | (6,320,730 | ) |
| Total deferred tax assets | | $ | - | | | $ | - | |
The Company has evaluated the positive and negative evidence bearing upon the realizability of its deferred tax assets, which are comprised principally of net operating loss carryforwards and research and development credits. Under the applicable accounting standards, management has considered the Company’s history of losses and concluded that it is more likely than not that the Company will not recognize the benefits of federal and state deferred tax assets. Accordingly, a full valuation allowance of $10,612,908 and $6,320,730 has been established at December 31, 2015 and 2014, respectively. The valuation allowance increased $4,292,178 and $2,835,936 during the years ended December 31, 2015 and 2014, respectively.
A reconciliation of income tax expense computed at the statutory federal income tax rate to income taxes as reflected in the financial statements is as follows:
| | | December 31, | |
| | | 2015 | | | 2014 | |
| Income tax benefit computed at federal statutory tax rate | | | 34.0 | % | | | 34.0 | % |
| State taxes, net of federal benefit | | | (14.8 | )% | | | 3.8 | % |
| Non-deductible items | | | (139.2 | )% | | | (7.0 | )% |
| General business credits and other credits | | | (10.2 | )% | | | 2.1 | % |
| Change in valuation allowance | | | 130.5 | % | | | (34.5 | )% |
| Other | | | (0.3 | )% | | | 1.6 | % |
| Effective tax rate | | | 0.0 | % | | | 0.0 | % |
As of December 31, 2015, the Company had federal and state net operating loss carryforwards of $12,796,384 and $11,018,952, respectively, which begin to expire in 2030. As of December 31, 2015, the Company had federal and state research and development tax credit carryforwards of $466,599 and $358,038, respectively. These federal and state research and development tax credit carryforwards are available to reduce future income taxes payable and begin to expire in 2031 and 2026, respectively. As of December 31, 2014, the Company had federal and state net operating loss carryforwards of $7,914,557 and $6,719,602, respectively, which begin to expire in 2030. As of December 31, 2014, the company had federal and state research and development tax credit carryforwards of $259,716 and $198,897, respectively. These federal and state research and development tax credit carryforwards are available to reduce future income taxes payable and begin to expire in 2031 and 2026, respectively.
The Company adopted the authoritative guidance on accounting for and disclosure of uncertainty in tax positions on January 1, 2009, which required the Company to determine whether a tax position of the Company is more likely than not to be sustained upon examination, including resolution of any related appeals of litigation processes based on the technical merits of the position. For tax positions meeting the more likely than not threshold, the tax amount recognized in the financial statements is reduced by the largest benefit that has a greater than fifty percent likelihood of being realized upon the ultimate settlement with the relevant taxing authority. The Company determined that the adoption of this authoritative guidance did not have a material effect on the consolidated financial statements.
The Company files tax returns as prescribed by the tax laws of the jurisdictions in which it operates. In the normal course of business, the Company is subject to examination by federal and state jurisdictions, where applicable. There is currently no pending tax examination. The Company thus is still open under statute to potential examination from 2012 to the present. Earlier years may be examined to the extent that credit or loss-carry-forwards are used in it. The Company’s policy is to record interest and penalties related to income taxes as part of the tax provision.
Utilization of the net operating loss and research and development credit carryforwards may be subject to a substantial annual limitation under Section 382 of the Internal Revenue Code of 1986, as amended, due to ownership change limitations that have occurred previously or that could occur in the future. These ownership changes may limit the amount of net operating loss and research and development credit carryforwards that can be utilized annually to offset future taxable income and tax, respectively.
13 – CONCENTRATIONS
During the year ended December 31, 2015, the Company recorded revenues to two customers of $1,100,000 (74%) and $389,385 (26%) in excess of 10% of the Company’s total revenues. During the year ended December 31, 2014, the Company recorded revenues to three customers of $75,714 (46%), $48,312 (30%) and $40,000 (24%) in excess of 10% of the Company’s total revenues. At December 31, 2015, accounts receivable consisted of amounts due from two customers which represented 67% and 33% of the outstanding accounts receivable balance. At December 31, 2014, accounts receivable consisted of amounts due from one customer which represented 100% of the outstanding accounts receivable balance.
INDEX TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE MONTHS ENDED MARCH 31, 2016 AND 2015
(UNAUDITED)
CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
Enumeral Biomedical Holdings, Inc.
Condensed Consolidated Balance Sheets
(Unaudited)
| | | March 31, | | | December 31, | |
| | | 2016 | | | 2015 | |
| ASSETS | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 1,837,304 | | | $ | 3,596,262 | |
| Accounts receivable | | | 390,944 | | | | 306,012 | |
| Prepaid expenses and other current assets | | | 180,411 | | | | 280,479 | |
| Total current assets | | | 2,408,659 | | | | 4,182,753 | |
| Property and equipment, net | | | 1,325,638 | | | | 1,511,493 | |
| Other assets: | | | | | | | | |
| Restricted cash | | | 534,780 | | | | 534,780 | |
| Other assets | | | 111,556 | | | | 114,572 | |
| Total assets | | $ | 4,380,633 | | | $ | 6,343,598 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 440,113 | | | $ | 343,736 | |
| Accrued expenses | | | 648,072 | | | | 714,384 | |
| Deferred revenue | | | 87,026 | | | | 130,539 | |
| Equipment lease financing | | | 243,215 | | | | 240,473 | |
| Derivative liabilities | | | 1,459,656 | | | | 2,138,091 | |
| Total current liabilities | | | 2,878,082 | | | | 3,567,223 | |
| Deferred rent | | | 46,106 | | | | 36,847 | |
| Long-term equipment lease financing | | | 204,629 | | | | 266,471 | |
| Total liabilities | | | 3,128,817 | | | | 3,870,541 | |
| Commitments and contingencies | | | | | | | | |
| Stockholders’ equity: | | | | | | | | |
| Preferred stock, $.001 par value; 10,000,000 shares authorized: -0- shares issued and outstanding as of March 31, 2016 and December 31, 2015, respectively | | | - | | | | - | |
| Common stock, $.001 par value; 300,000,000 shares authorized: 52,073,481 and 51,932,571 shares issued and outstanding as of March 31, 2016 and December 31, 2015, respectively | | | 52,074 | | | | 51,933 | |
| Additional paid-in-capital | | | 17,163,770 | | | | 16,830,100 | |
| Accumulated deficit | | | (15,964,028 | ) | | | (14,408,976 | ) |
| Total stockholders’ equity | | | 1,251,816 | | | | 2,473,057 | |
| Total liabilities and stockholders’ equity | | $ | 4,380,633 | | | $ | 6,343,598 | |
The accompanying notes are an integral part of the condensed consolidated financial statements.
Enumeral Biomedical Holdings, Inc.
Condensed Consolidated Statements of Operations and Comprehensive Income (Loss)
(Unaudited)
| | | Three Months Ended | |
| | | March 31, | |
| | | 2016 | | | 2015 | |
| | | | | | | |
| Revenue: | | | | | | | | |
| Collaboration and license revenue | | $ | 316,018 | | | $ | 209,635 | |
| Grant revenue | | | 118,439 | | | | 65,087 | |
| Total revenue | | | 434,457 | | | | 274,722 | |
| Cost of revenue and expenses: | | | | | | | | |
| Research and development | | | 1,470,043 | | | | 1,230,504 | |
| General and administrative | | | 1,194,334 | | | | 1,424,943 | |
| Total cost of revenue and expenses | | | 2,664,377 | | | | 2,655,447 | |
| Loss from operations | | | (2,229,920 | ) | | | (2,380,725 | ) |
| Other income (expense): | | | | | | | | |
| Interest income (expense) | | | (3,567 | ) | | | 2,635 | |
| Change in fair value of derivative liabilities | | | 678,435 | | | | 4,669,884 | |
| Total other income (expense), net | | | 674,868 | | | | 4,672,519 | |
| Net income (loss) before income taxes | | | (1,555,052 | ) | | | 2,291,794 | |
| Provision for income taxes | | | - | | | | - | |
| Net income (loss) | | $ | (1,555,052 | ) | | $ | 2,291,794 | |
| Other comprehensive income (loss): | | | | | | | | |
| Reclassification for loss included in net income | | | - | | | | 6 | |
| Net unrealized holding losses on available-for-sale securities arising during the period | | | - | | | | (6,590 | ) |
| Comprehensive income (loss) | | $ | (1,555,052 | ) | | $ | 2,285,210 | |
| Basic net income (loss) per share | | $ | (0.03 | ) | | $ | 0.04 | |
| Diluted net income (loss) per share | | $ | (0.03 | ) | | $ | 0.04 | |
| Weighted-average number of common shares attributable to common stockholders - basic | | | 52,068,784 | | | | 51,607,454 | |
| Weighted-average number of common shares attributable to common stockholders - diluted | | | 52,068,784 | | | | 52,849,869 | |
The accompanying notes are an integral part of the condensed consolidated financial statements
Enumeral Biomedical Holdings, Inc.
Condensed Consolidated Statements of Cash Flows
(Unaudited)
| | | Three Months Ended | |
| | | March 31, | |
| | | 2016 | | | 2015 | |
| Cash flows from operating activities: | | | | | | | | |
| Net income (loss) | | $ | (1,555,052 | ) | | $ | 2,291,794 | |
| Adjustments to reconcile net income (loss) to net cash used in operating activities: | | | | | | | | |
| Depreciation and Amortization | | | 185,855 | | | | 136,298 | |
| Exit costs associated with write-down of net carrying value of leasehold improvements | | | - | | | | 22,962 | |
| Stock-based compensation | | | 333,811 | | | | 166,391 | |
| Change in fair value of derivative liabilities | | | (678,435 | ) | | | (4,669,884 | ) |
| Changes in operating assets and liabilities: | | | | | | | | |
| Accounts receivable | | | (84,932 | ) | | | 53,192 | |
| Prepaid expenses and other assets | | | 103,084 | | | | (44,928 | ) |
| Accounts payable | | | 96,377 | | | | (349,620 | ) |
| Accrued expenses | | | (66,312 | ) | | | 333,289 | |
| Deferred rent | | | 9,259 | | | | (28,732 | ) |
| Deferred revenue | | | (43,513 | ) | | | 24,990 | |
| Net cash used in operating activities | | | (1,699,858 | ) | | | (2,064,248 | ) |
| Cash flows from investing activities: | | | | | | | | |
| Purchases of property and equipment | | | - | | | | (391,930 | ) |
| Proceeds from sales of marketable securities | | | - | | | | 1,000,805 | |
| Net cash provided by investing activities | | | - | | | | 608,875 | |
| Cash flows from financing activities: | | | | | | | | |
| Payments on equipment lease financing | | | (59,100 | ) | | | - | |
| Net cash used in financing activities | | | (59,100 | ) | | | - | |
| Net decrease in cash and cash equivalents | | | (1,758,958 | ) | | | (1,455,373 | ) |
| Cash and cash equivalents, beginning of period | | | 3,596,262 | | | | 10,460,117 | |
| Cash and cash equivalents, end of period | | $ | 1,837,304 | | | $ | 9,004,744 | |
| | | | | | | | | |
| Supplemental cash flow disclosure of financing activities: | | | | | | | | |
| Cash paid for interest | | $ | 5,536 | | | $ | - | |
The accompanying notes are an integral part of the condensed consolidated financial statements
ENUMERAL BIOMEDICAL HOLDINGS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
1 - NATURE OF BUSINESS
Enumeral Biomedical Corp. (“Enumeral”) was founded in 2009 in the state of Delaware as Enumeral Technologies, Inc. The name was later changed to Enumeral Biomedical Corp.
On July 31, 2014 (the “Closing Date”), Enumeral entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”) with Enumeral Biomedical Holdings, Inc., which was formerly known as Cerulean Group, Inc. (“Enumeral Biomedical” or the “Company”), and Enumeral Acquisition Corp., a wholly owned subsidiary of Enumeral Biomedical (“Acquisition Sub”), pursuant to which the Acquisition Sub merged with and into Enumeral (the “Merger”). Enumeral was the surviving corporation in the Merger and became a wholly owned subsidiary of the Company.
As a result of the Merger, all issued and outstanding common and preferred shares of Enumeral were exchanged for common shares of Enumeral Biomedical Holdings, Inc. The Merger is considered to be a recapitalization of the Company which has been retrospectively applied to these unaudited condensed consolidated financial statements for all periods presented.
Upon the closing of the Merger and under the terms of a split-off agreement and a general release agreement (the “Split-Off Agreement”), the Company transferred all of its pre-Merger operating assets and liabilities to its wholly-owned special-purpose subsidiary, Cerulean Operating Corp. (the “Split-Off Subsidiary”). Thereafter, pursuant to the Split-Off Agreement, the Company transferred all of the outstanding shares of capital stock of Split-Off Subsidiary to the pre-Merger majority stockholder of the Company, and the former sole officer and director of the Company (the “Split-Off”), in consideration of and in exchange for (i) the surrender and cancellation of an aggregate of 23,100,000 shares of the Company’s common stock held by such stockholder (which were cancelled and will resume the status of authorized but unissued shares of the Company’s common stock) and (ii) certain representations, covenants and indemnities.
As a result of the Merger and Split-Off, the Company discontinued its pre-Merger business, acquired the business of Enumeral, and changed its name to Enumeral Biomedical Holdings, Inc.
Also on July 31, 2014, the Company closed a private placement offering (the “PPO”) of 21,549,510 Units (the “Units”) of its securities, at a purchase price of $1.00 per Unit, each Unit consisting of one share of the Company’s common stock and a warrant to purchase one share of the Company’s common stock at an exercise price of $2.00 per share with a term of five years (the “PPO Warrants”). Additional information concerning the PPO and PPO Warrants is presented below in Note 10.
Following the Merger, the Company has continued Enumeral’s business of discovering and developing novel antibody immunotherapies that help the immune system fight cancer and other diseases. The Company utilizes a proprietary platform technology that facilitates the rapid high resolution measurement of immune cell function within small tissue biopsy samples. The Company’s initial focus is on the development of a pipeline of next generation monoclonal antibody drugs targeting established and novel immunomodulatory receptors.
In its lead antibody program, the Company has characterized certain anti-PD-1 antibodies, or simply “PD-1 antibodies,” using patient biopsy samples, in an effort to identify next generation PD-1 antagonists with enhanced selectivity for the immune effector cells that carry out anti-tumor functions. The Company has identified two antagonist PD-1 antibodies that inhibit PD-1 activity in distinctly different ways. One of the antibodies blocks binding of the ligand PD-L1 to PD-1, while the other antibody does not. However, both display activity in various biological assays. In addition to its PD-1 antibody program, the Company is developing antibody drug candidates targeting TIM-3, LAG-3, OX40, TIGIT and VISTA. The Company is also pursuing several antibody programs for which it has not yet publicly disclosed the targets.
The Company’s proprietary platform technology, exclusively licensed from the Massachusetts Institute of Technology (“MIT”), is a microwell array technology that detects secreted molecules (such as antibodies and cytokines) and cell surface markers, at the level of single live cells and enables recovery of single live cells of interest. The platform technology is a multipurpose tool that is valuable for activities ranging from antibody discovery to target discovery and patient stratification in clinical development. The platform yields multidimensional, functional read-outs from single live cells, such as tumor infiltrating lymphocytes, or TILs, from human tumor biopsy samples, and it enables the Company’s researchers to examine the responses of different classes of human immune cells to treatment with immunomodulators in the context of human disease, as opposed to animal models of disease.
The Company continues to be a “smaller reporting company,” as defined under the Exchange Act, following the Merger. The Company believes that as a result of the Merger, it has ceased to be a “shell company” (as such term is defined in Rule 12b-2 under the Securities and Exchange Act of 1934, as amended (the “Exchange Act”)).
2 - GOING CONCERN AND LIQUIDITY
The Company’s unaudited condensed consolidated financial statements have been prepared in conformity with generally accepted accounting principles in the United States which contemplate the Company’s continuation as a going concern. As of March 31, 2016, the Company had a working capital deficit of $469,423 including $1,459,656 of derivative liabilities and an accumulated deficit of $15,964,028. As of December 31, 2015, the Company had working capital of $615,530 including $2,138,091 of derivative liabilities and an accumulated deficit of $14,408,976. As of the date of this filing, the Company believes it only has sufficient liquidity to fund operations through July 2016, which includes the receipt of the$750,000 maintenance fee from Pieris Pharmaceuticals described in Note 3 below.Even with receipt of the $750,000 maintenance fee from Pieris, the Company will still require additional capital to continue its operations through the third quarter of 2016.
The Company is currently exploring a range of potential transactions, which may include public or private equity offerings, debt financings, collaborations and licensing arrangements, and/or other strategic alternatives, including a merger, sale of assets or other similar transactions. If the Company is unable to raise additional capital on terms acceptable to the Company and on a timely basis, the Company may be required to downsize or wind down its operations through liquidation, bankruptcy, or a sale of its assets. The unaudited condensed consolidated financial statements do not include any adjustments related to the recovery and classification of asset carrying amounts or the amount and classification of liabilities that might result should the Company be unable to continue as a going concern. The Company expects to incur significant expenses and operating losses for the foreseeable future, and the Company’s net losses may fluctuate significantly from quarter to quarter and from year to year. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
The Company’s business has not generated (nor does the Company anticipate that in the foreseeable future it will generate) the cash necessary to finance its operations, and the Company will require additional capital to continue its operations beyond July 2016.
The Company’s near-term capital needs depend on many factors, including:
| · | the Company’s ability to carefully manage its costs; |
| · | the amount and timing of revenue received from grants or the Company’s collaboration and license arrangements; and |
| · | the Company’s ability to raise additional capital through public or private equity offerings, debt financings, or strategic collaborations and licensing arrangements, and/or the Company’s success in promptly establishing a strategic alternative that is in its stockholders’ best interests. |
If the Company is unable to preserve or raise additional capital through one or more of the means listed above prior to the end of July 2016, the Company could face substantial liquidity problems and might be required to implement cost reduction strategies in addition to the cash conservation steps that the Company has already taken. These reductions could significantly affect the Company’s research and development activities, and could result in significant harm to the Company’s business, financial condition and results of operations. In addition, these reductions could cause the Company to further curtail its operations, or take other actions that would adversely affect the Company’s stockholders. If the Company is unable to raise additional capital on acceptable terms and on a timely basis, the Company may be required to downsize or wind down its operations through liquidation, bankruptcy, or a sale of its assets.
In addition, to the extent additional capital is raised through the sale of equity or convertible debt securities, such securities may be sold at a discount from the market price of the Company’s common stock. The issuance of these securities could also result in significant dilution to some or all of the Company’s stockholders, depending on the terms of the transaction. For example, the shares included in the Units and the warrants issued in connection with the PPO contain anti-dilution protection in the event that within certain specified time periods the Company issues common stock or securities convertible into or exercisable for shares of the Company’s common stock at a price lower than the Unit purchase price or the warrant exercise price, as applicable. The anti-dilution protection provisions apply to issuances within two years after the closing of the PPO in the case of the Units and, with respect to the warrants, prior to the warrant expiration date. In addition, the anti-dilution protection provisions are subject to exceptions for certain issuances, including but not limited to (a) shares of common stock issued in an underwritten public offering, (b) issuances of awards under the Company’s 2014 Equity Incentive Plan, and (c) other exempt issuances.
3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying unaudited condensed consolidated financial statements were prepared using generally accepted accounting principles for interim financial information and the instructions to Form 10-Q and Regulation S-X. Accordingly, these unaudited condensed consolidated financial statements do not include all information or notes required by generally accepted accounting principles for annual financial statements and should be read in conjunction with the 2015 Financial Statements as filed on the Company's Annual Report on Form 10-K for the year ended December 31, 2015, which was filed with the SEC on March 30, 2016.
The preparation of the unaudited condensed consolidated financial statements in conformity with these accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the date of the unaudited condensed consolidated financial statements and the reported amounts of expenses during the reported period. Ultimate results could differ from the estimates of management.
In the opinion of management, the unaudited condensed consolidated financial statements included herein contain all adjustments necessary to present fairly the Company's financial position as of March 31, 2016 and the results of its operations and cash flows for the three months ended March 31, 2016 and 2015. Such adjustments are of a normal recurring nature. The results of operations for the three months ended March 31, 2016 may not be indicative of results for the full year.
Principles of Consolidation
The unaudited condensed consolidated financial statements include the accounts of the Company and its subsidiaries, Enumeral Biomedical Corp. and Enumeral Securities Corporation. In these unaudited condensed consolidated financial statements, “subsidiaries” are companies that are wholly owned, the accounts of which are consolidated with those of the Company. Intercompany transactions and balances are eliminated in consolidation.
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities of 90 days or less from the purchase date to be cash equivalents. Cash and cash equivalents are held in depository and money market accounts and are reported at fair value.
Concentration of Credit Risk
The Company has no significant off-balance sheet concentrations of credit risk such as foreign currency exchange contracts, option contracts or other hedging arrangements. Financial instruments that subject the Company to credit risk consist primarily of cash and cash equivalents. The Company generally invests its cash in money market funds, U.S. Treasury securities and U.S. Agency securities that are subject to minimal credit and market risk. Management has established guidelines relative to credit ratings and maturities intended to safeguard principal balances and maintain liquidity. At times, the Company’s cash balances may exceed the current insured amounts under the Federal Deposit Insurance Corporation.
Fair Value of Financial Instruments
Fair values of financial instruments included in current assets and current liabilities are estimated to approximate their book values, due to the short maturity of such instruments. All debt is based on current rates at which the Company could borrow funds with similar remaining maturities and approximates fair value. The Company’s assets and liabilities that are measured at fair value on a recurring basis are measured in accordance with the Financial Accounting Standards Board’s (“FASB”) Accounting Standards Codification (“ASC”) Topic 820,Fair Value Measurements and Disclosures, which establishes a three-level valuation hierarchy for measuring fair value and expands financial statement disclosures about fair value measurements.
The valuation hierarchy is based upon the transparency of inputs to the valuation of an asset or liability as of the measurement date. The three levels are defined as follows:
| | · | Level 1: Inputs to the valuation methodology are quoted prices (unadjusted) for identical assets in active markets. |
| | · | Level 2: Inputs to the valuation methodology included quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument. |
| | · | Level 3: Inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
The Company’s cash equivalents, carried at fair value, are comprised of investments in federal agency backed money market funds. The valuation of the Company’s derivative liabilities is discussed below and in Note 11. The following table presents information about the Company’s financial assets and liabilities measured at fair value on a recurring basis as of March 31, 2016 and December 31, 2015:
| | | March 31,
2016 | | | Quoted Prices in
Active Markets
(Level 1) | | | Observable
Inputs
(Level 2) | | | Unobservable
Inputs
(Level 3) | |
| Assets | | | | | | | | | | | | | | | | |
| Cash | | $ | 755,354 | | | $ | 755,354 | | | $ | - | | | $ | - | |
| Money Market funds, included in cash equivalents | | $ | 1,081,950 | | | $ | 1,081,950 | | | $ | - | | | $ | - | |
| Liabilities | | | | | | | | | | | | | | | | |
| Derivative liabilities | | $ | 1,459,656 | | | $ | - | | | $ | - | | | $ | 1,459,656 | |
| | | December 31,
2015 | | | Quoted Prices in
Active Markets
(Level 1) | | | Observable
Inputs
(Level 2) | | | Unobservable
Inputs
(Level 3) | |
| Assets | | | | | | | | | | | | | | | | |
| Cash | | $ | 815,890 | | | $ | 815,890 | | | $ | - | | | $ | - | |
| Money Market funds, included in cash equivalents | | $ | 2,780,372 | | | $ | 2,780,372 | | | $ | - | | | $ | - | |
| Liabilities | | | | | | | | | | | | | | | | |
| Derivative liabilities | | $ | 2,138,091 | | | $ | - | | | $ | - | | | $ | 2,138,091 | |
The following table provides a roll forward of the fair value of the Company’s derivative liabilities, using Level 3 inputs:
| Balance as of December 31, 2015 | | $ | 2,138,091 | |
| Change in fair value | | | (678,435 | ) |
| Balance as of March 31, 2016 | | $ | 1,459,656 | |
Accounts Receivable and Allowance for Doubtful Accounts
Trade receivables are recorded at the invoiced amount. The Company maintains allowances for doubtful accounts, if needed, for estimated losses resulting from the inability of customers to make required payments. This allowance is based on specific customer account reviews and historical collections experience. There was no allowance for doubtful accounts as of March 31, 2016 or December 31, 2015.
Property and Equipment
Property and equipment are recorded at cost. Expenditures for maintenance and repairs are charged to expense as incurred, whereas major betterments are capitalized as additions to property and equipment. Depreciation is provided using the straight-line method over the estimated useful lives of the assets as follows:
| Lab equipment | | 3-5 years |
| Computer equipment and software | | 3 years |
| Furniture | | 3 years |
| Leasehold improvements | | Shorter of useful life or life of the lease |
Impairment of Long-Lived Assets
Long-lived assets are reviewed for impairment annually or whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. When such events occur, the Company compares the carrying amounts of the assets to their undiscounted expected future cash flows. If this comparison indicated that there is impairment, the amount of the impairment is calculated as the difference between the carrying value and fair value. There have been no impairments recognized during the three months ended March 31, 2016 and 2015, respectively.
Revenue Recognition
Collaboration and license revenue
Non-refundable license fees are recognized as revenue when the Company has a contractual right to receive such payment, the contract price is fixed or determinable, the collection of the resulting receivable is reasonably assured and the Company has no further performance obligations under the license agreement. Multiple element arrangements, such as license and development arrangements are analyzed to determine whether the deliverables, which often include license and performance obligations such as research and steering committee services, can be separated or whether they must be accounted for as a single unit of accounting in accordance with GAAP.
The Company recognizes up-front license payments as revenue upon delivery of the license only if the license has stand-alone value and the fair value of the undelivered performance obligations, typically including research and/or steering committee services, can be determined. If the fair value of the undelivered performance obligations can be determined, such obligations would then be accounted for separately as performed. If the license is considered to either (i) not have stand-alone or (ii) have stand-alone value but the fair value of any of the undelivered performance obligations cannot be determined, the arrangement would then be accounted for as a single unit of accounting and the license payments and payments for performance obligations are recognized as revenue over the estimated period of when the performance obligations are performed.
Whenever the Company determines that an arrangement should be accounted for as a single unit of accounting, it must determine the period over which the performance obligations will be performed and revenue will be recognized. Revenue will be recognized using either a relative performance or straight-line method. The Company recognizes revenue using the relative performance method provided that it can reasonably estimate the level of effort required to complete its performance obligations under an arrangement and such performance obligations are provided on a best-efforts basis. Direct labor hours or full-time equivalents are typically used as a measure of performance. Revenue recognized under the relative performance method would be determined by multiplying the total payments under the contract, excluding royalties and payments contingent upon achievement of substantive milestones, by the ratio of level of effort incurred to date to estimated total level of effort required to complete the Company’s performance obligations under the arrangement. Revenue is limited to the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned, as determined using the relative performance method, as of each reporting period.
If the Company cannot reasonably estimate the level of effort required to complete its performance obligations under an arrangement, the performance obligations are provided on best-efforts basis and the Company can reasonably estimate when the performance obligation ceases or the remaining obligations become inconsequential and perfunctory. At that time, the total payments under the arrangement, excluding royalties and payments contingent upon achievement of substantive milestones, would be recognized as revenue on a straight-line basis over a period the Company expects to complete its performance obligations. Revenue is limited to the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned, as determined using the straight-line basis, as of the period ending date.
In December 2014, the Company entered into a study agreement with Merck Sharp & Dohme Corp., or Merck (the “Merck Agreement”). In February 2016, the Company and Merck subsequently amended the work plan under the Merck Agreement to also include non-small cell lung cancer tissues. Pursuant to the Merck Agreement, the Company is conducting a specified research program using its platform technology to identify functional response of single cell types in colorectal cancer and non-small cell lung cancer in the presence or absence of immunomodulatory receptor modulators identified by Merck. In this collaboration, Merck is reimbursing the Company for the cost of performing the work plan set forth in the Merck Agreement, for up to a specified number of full-time employees at a pre-determined annual rate. In addition, Merck will make certain milestone payments to the Company upon the completion of specified objectives set forth in the Merck Agreement and related work plan. In September 2015, the Company announced the achievement of the first milestone under the Merck Agreement.
In January 2016, the Company and The University of Texas M.D. Anderson Cancer Center (“MDACC”) entered into a Collaborative Research and Development Agreement (the “MDACC Agreement”). Under the MDACC Agreement, the Company and MDACC plan to collaborate on the discovery and development of novel monoclonal antibodies against selected targets in immune-oncology, utilizing the Company’s antibody discovery and immune profiling platform and MDACC’s preclinical and development expertise and infrastructure.
Pursuant to the terms of the MDACC Agreement, the Company and MDACC will share the costs of research and development activities necessary to take development candidates through successful completion of a Phase I clinical trial. The MDACC Agreement provides for a structure whereby the Company and MDACC are each granted the right to receive a percentage of the net income from product sales or any payments associated with licensing or otherwise partnering a program with a third party.
In April 2016, the Company entered into a License and Transfer Agreement (the “License Agreement”) with Pieris Pharmaceuticals, Inc. and Pieris Pharmaceuticals GmbH (collectively, “Pieris”). Pursuant to the terms and conditions of the License Agreement, Pieris is licensing from the Company specified intellectual property related to the Company’s anti-PD-1 antibody program ENUM 388D4 for the potential development and commercialization by Pieris of novel multispecific therapeutic proteins comprising fusion proteins based on Pieris’ Anticalins® class of therapeutic proteins and the Company’s antibodies in the field of oncology.
Under the License Agreement, Pieris paid the Company a $250,000 initial license fee. In June 2016, the Company entered into a Definitive License and Transfer Agreement (the “Definitive Agreement”) with Pieris, as contemplated in the License Agreement. Pieris also paid the Company a $750,000 license maintenance fee due by May 31, 2016 to continue the licensing arrangements under the License Agreement. In accordance with its terms, the Definitive Agreement supersedes the License Agreement.
Under the Definitive Agreement, the Company has granted Pieris an option until May 31, 2017 to license specified patent rights and know-how of the Company covering two additional undisclosed antibody programs on the same terms and conditions as for the Company’s 388D4 anti-PD-1 antibody (each, a “Subsequent Option”). Pieris may exercise the Subsequent Antibody Options separately and on different dates during the Option Period. Pieris will pay the Company additional license fees in the event that Pieris exercises one or both Subsequent Options.
The Company recognized $316,018 of collaboration revenue for the three month period ended March 31, 2016. The Company recognized $209,635 of collaboration revenue for the three month period ended March 31, 2015.
Grant Revenue
In September 2014, the Company was awarded a Phase II Small Business Innovation Research contract from National Cancer Institute (“NCI”), a unit of the National Institutes of Health, for up to $999,967 over two years. Grant revenue consists of a portion of the funds received to date by the NCI. Revenue is recognized as the related research services are performed in accordance with the terms of the agreement. The Company recognized $118,439 of grant revenue associated with the Phase II NCI grant for the three month period ended March 31, 2016. The Company recognized $65,087 of grant revenue associated with the Phase II NCI grant for the three month period ended March 31, 2015. The difference between the total consideration received to date and the revenue recognized is recorded as deferred revenue. Deferred revenue totaled $87,026 as of March 31, 2016 and $130,539 as of December 31, 2015.
Research and Development Expenses
Research and development expenditures are charged to the unaudited condensed consolidated statement of operations and comprehensive income (loss) as incurred. Research and development expenses are comprised of costs incurred in performing research and development activities, including salaries and benefits, facilities costs, clinical supply costs, contract services, depreciation and amortization expense and other related costs. Costs associated with acquired technology, in the form of upfront fees or milestone payments, are charged to research and development expense as incurred. Legal fees incurred in connection with patent applications, along with fees associated with the license to the Company’s core technology, are expensed as research and development expense.
Derivative Liabilities
The Company’s derivative liabilities relate to (a) warrants to purchase an aggregate of 23,549,510 shares of the Company’s common stock that were issued in connection with the PPO (as defined below in Note 10) and (b) warrants to purchase 41,659 shares of Enumeral Series A Preferred Stock that were issued in December 2011 and June 2012 pursuant to Enumeral’s debt financing arrangement with Square 1 Bank (as further described in Note 7) that were subsequently converted into warrants to purchase 66,574 shares of the Company’s common stock in connection with the July 2014 Merger. Additional detail regarding these warrants can be found in Note 11 below.
Due to the price protection provision included in the warrant agreements, the warrants were deemed to be liabilities and, therefore, the fair value of the warrants is recorded in the current liabilities section of the unaudited condensed consolidated balance sheet. As such, the outstanding warrants are revalued each reporting period with the resulting gains and losses recorded as the change in fair value of derivative liabilities on the unaudited condensed consolidated statements of operations and comprehensive income (loss).
The Company used the Black-Scholes option-pricing model to estimate the fair values of the issued and outstanding warrants. As of January 1, 2016, the Company began using a blended average of the Company’s historical volatility and the historical volatility of a group of similarly situated companies (as described in greater detail below) to calculate the expected volatility when valuing its derivative liabilities.
Comprehensive Income (Loss)
Other comprehensive income (loss) is comprised of unrealized holding gains and losses arising during the period on available-for-sale securities that are not other-than-temporarily impaired. The unrealized gains and losses are reported in accumulated other comprehensive income (loss), until sold or mature, at which time they are reclassified to earnings. The Company recognized no reclassifications out of accumulated other comprehensive loss or unrealized holding losses on available-for-sale securities for the three month period ended March 31, 2016. The Company recognized unrealized holding losses on available-for-sale securities of $6,590 and reclassified $6 out of accumulated other comprehensive loss to net income for the three months ended March 31, 2015.
Stock-Based Compensation
The Company accounts for its stock-based compensation awards to employees and directors in accordance with FASB ASC Topic 718, Compensation–Stock Compensation (“ASC 718”). ASC 718 requires all stock-based payments to employees, including grants of employee stock options and restricted stock, to be recognized in the unaudited condensed consolidated statements of operations and comprehensive income (loss) based on their grant date fair values. Compensation expense related to awards to employees is recognized on a straight-line basis based on the grant date fair value over the associated service period of the award, which is generally the vesting term. Share-based payments issued to non-employees are recorded at their fair values, and are periodically revalued as the equity instruments vest and are recognized as expense over the related service period in accordance with the provisions of ASC 718 and ASC Topic 505, Equity, and are expensed using an accelerated attribution model.
The Company estimates the fair value of its stock options using the Black-Scholes option pricing model, which requires the input of subjective assumptions, including (a) the expected stock price volatility, (b) the expected term of the award, (c) the risk-free interest rate, (d) expected dividends, and (e) the estimated fair value of its common stock on the measurement date. As of January 1, 2016, the Company began using a blended average of the Company’s historical volatility and the historical volatility of a group of similarly situated companies to calculate the expected volatility when valuing its stock options. For purposes of calculating this blended volatility, the Company selected companies with comparable characteristics to it, including enterprise value, risk profiles, position within the industry, and with historical share price information sufficient to meet the expected term of the stock-based awards. The Company computes historical volatility data using the daily closing prices for the Company’s and the selected companies’ shares during the equivalent period of the calculated expected term of the stock-based awards. Prior to January 1, 2016, due to the lack of a public market for the trading of its common stock and a lack of company specific historical and implied volatility data, the Company has based its estimate of expected volatility only on the historical volatility of a group of similarly situated companies that were publicly traded. Due to the lack of Company specific historical option activity, the Company has estimated the expected term of its employee stock options using the “simplified” method, whereby, the expected term equals the arithmetic average of the vesting term and the original contractual term of the option. The expected term for non-employee awards is the remaining contractual term of the option. The risk-free interest rates are based on the U.S. Treasury securities with a maturity date commensurate with the expected term of the associated award. The Company has never paid dividends and does not expect to pay dividends in the foreseeable future.
The fair value of the restricted stock awards granted to employees is based upon the fair value of the common stock on the date of grant. Expense is recognized over the vesting period.
The Company has recorded stock-based compensation expense of $333,811 and $166,391 for the three months ended March 31, 2016 and 2015, respectively. The Company has an aggregate of $1,359,863 of unrecognized stock-based compensation expense as of March 31, 2016 to be amortized over a weighted average period of 2.9 years.
Effective January 1, 2016, the Company has elected to account for forfeitures as they occur, as permitted by ASU 2016-09,Compensation—Stock Compensation (Topic 718), Improvements to Employee Share-Based Payment Accounting. See the Accounting Standards Adopted in the Period section below for further details.
Prior to the adoption of ASU 2016-09, the Company estimated the number of stock-based awards that were expected to vest, and only recognized compensation expense for such awards. The estimation of stock-based awards that will ultimately vest required judgment, and to the extent actual results or updated estimates differed from current estimates, such amounts were recorded as a cumulative adjustment in the period estimates were revised. The Company considered many factors when estimating expected forfeitures, including type of awards granted, employee class, and historical experience.
Earnings (Loss) Per Share
Basic earnings (loss) per common share amounts are based on the weighted average number of common shares outstanding. Diluted earnings (loss) per common share amounts are based on the weighted average number of common shares outstanding, plus the incremental shares that would have been outstanding upon the assumed exercise of all potentially dilutive stock options, warrants and convertible debt, subject to anti-dilution limitations. As of March 31, 2016 and 2015, the number of shares underlying options and warrants that were anti-dilutive were approximately 31.7 million and 25.5 million, respectively.
Income Taxes
Income taxes are recorded in accordance with FASB ASC Topic 740, Income Taxes (“ACS 740”), which provides for deferred taxes using an asset and liability approach. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial reporting and the tax reporting basis of assets and liabilities and are measured using the enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. The Company provides a valuation allowance against net deferred tax assets unless, based upon the available evidence, it is more likely than not that the deferred tax assets will be realized. The Company has evaluated available evidence and concluded that the Company may not realize the benefit of its deferred tax assets; therefore a valuation allowance has been established for the full amount of the deferred tax assets. The Company accounts for uncertain tax positions in accordance with the provisions of ASC 740. When uncertain tax positions exist, the Company recognizes the tax benefit of tax positions to the extent that the benefit will more likely than not be realized. The determination as to whether the tax benefit will more likely than not be realized is based upon the technical merits of the tax position as well as consideration of the available facts and circumstances.
The Company has no uncertain tax liabilities as of March 31, 2016 or December 31, 2015. The guidance requires the Company to determine whether it is more likely than not that a tax position will be sustained upon examination by the appropriate taxing authority. If a tax position meets the more likely than not recognition criteria, the guidance requires the tax position be measured at the largest amount of benefit greater than 50% likely of being realized upon ultimate settlement.
Recent Accounting Pronouncements
In May 2014, the FASB issued Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers (Topic 606). ASU No. 2014-09 provides for a single comprehensive model for use in accounting for revenue arising from contracts with customers and supersedes most current revenue recognition guidance. The accounting standard is effective for interim and annual periods beginning after December 15, 2016 with no early adoption permitted. In August 2015, the FASB issued ASU No. 2015-14, Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date, which deferred the effective date of ASU No. 2014-09 to annual periods beginning after December 15, 2017, along with an option to permit early adoption as of the original effective date. The Company is required to adopt the amendments in ASU No. 2014-09 using one of two acceptable methods. The Company’s management is currently in the process of determining which adoption method it will apply and evaluating the impact of the guidance on the Company’s unaudited condensed consolidated financial statements. In April 2016, the FASB issued ASU No. 2016-10,Revenue from Contracts with Customers (Topic 606): Identifying Performance Obligations and Licensing.The ASU clarifies the following two aspects of Topic 606: (a) identifying performance obligations; and (b) the licensing implementation guidance. The ASU does not change the core principle of the guidance in Topic 606. The effective date and transition requirements for the ASU are the same as the effective date and transition requirements in Topic 606. Public entities should apply the ASU for annual reporting periods beginning after December 15, 2017, including interim reporting periods therein (i.e., January 1, 2018, for a calendar year entity). Early application for public entities is permitted only as of annual reporting periods beginning after December 15, 2016, including interim reporting periods within that reporting period. The Company is currently evaluating the impact of this new standard on its unaudited condensed consolidated financial statements.
In August 2014, the FASB issued ASU No. 2014-15, Presentation of Financial Statements–Going Concern (Subtopic 205-40). ASU No 2014-15 requires all entities to evaluate for the existence of conditions or events that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the issuance date of the financial statements. The accounting standard is effective for interim and annual periods ending after December 15, 2016, and is not expected to have a material impact on the Company’s unaudited condensed consolidated financial statements, but may impact the Company’s footnote disclosures.
In February 2016, the FASB issued ASU No. 2016-02,Leases (Topic 842). ASU No. 2016-02 is effective for annual periods beginning after December 15, 2018, and requires a lessee to recognize assets and liabilities for leases with a maximum possible term of more than 12 months. A lessee would recognize a liability to make lease payments (the lease liability) and a right-of-use asset representing its right to use the leased asset (the underlying asset) for the lease term. Early application is permitted. The Company is currently evaluating the impact the adoption of the accounting standard will have on its unaudited condensed consolidated financial statements.
Accounting Standards Adopted in the Period
In March 2016 the FASB issued ASU 2016-09, which simplified several aspects of employee share-based payment accounting. In particular, the ASU permits entities to make an accounting policy election to either estimate forfeitures on share-based payment awards, as previously required, or to recognize forfeitures as they occur. Effective January 1, 2016, the Company elected to recognize forfeitures as they occur. The impact of that change in accounting policy has been recorded as an $8,333 cumulative effect adjustment to accumulated deficit, as of December 31, 2015. The Company expects that it will recognize slightly higher share-based payment expense for the remainder of 2016, relative to prior periods, as the effects of forfeitures will not be recognized until they occur, rather than being estimated at the time of grant and subsequently adjusted as and when necessary. The effects of adopting the remaining provisions in ASU 2016-09 affecting the income tax consequences of share-based payments, classification of awards as either equity or liabilities when an entity partially settles the award in cash in excess of the employer’s minimum statutory withholding requirements and classification in the statement of cash flows did not have any impact on the Company’s financial position, results of operations or cash flows.
Other accounting standards that have been issued by the FASB or other standards-setting bodies that do not require adoption until a future date are not expected to have a material impact on the Company’s unaudited condensed consolidated financial statements upon adoption.
4 - PROPERTY AND EQUIPMENT, NET
Property and equipment, net consist of the following:
| | | March 31, | | | December 31, | |
| | | 2016 | | | 2015 | |
| Laboratory equipment | | $ | 2,559,986 | | | $ | 2,559,986 | |
| Computer/office equipment and software | | | 187,337 | | | | 187,337 | |
| Furniture, fixtures and office equipment | | | 73,734 | | | | 73,734 | |
| Leasehold improvements | | | 75,262 | | | | 75,262 | |
| | | | 2,896,319 | | | | 2,896,319 | |
| Less - Accumulated depreciation and amortization | | | (1,570,681 | ) | | | (1,384,826 | ) |
| | | $ | 1,325,638 | | | $ | 1,511,493 | |
Depreciation and amortization expense for the three months ended March 31, 2016 and 2015 was $185,855 and $136,298, respectively. During the three months ended March 31, 2015, the Company expensed $22,962 associated with the write-down of leasehold improvements due to a relocation of the Company’s corporate office and research laboratories in March 2015 (see Note 8). No such write-downs occurred during the three months ended March 31, 2016.
5 – RESTRICTED CASH
The Company held $534,780 in restricted cash as of March 31, 2016 and December 31, 2015, respectively. The balances are primarily held on deposit with a bank to collateralize a standby letter of credit in the name of the Company’s facility lessor in accordance with the Company’s facility lease agreement.
6 - ACCRUED EXPENSES
The Company’s accrued expenses consist of the following:
| | | March 31, | | | December 31, | |
| | | 2016 | | | 2015 | |
| Accrued wages and benefits | | $ | 470,539 | | | $ | 447,769 | |
| Accrued professional fees | | | 117,571 | | | | 213,475 | |
| Accrued other | | | 59,962 | | | | 53,140 | |
| Total accrued expenses | | $ | 648,072 | | | $ | 714,384 | |
7 – DEBT
Equipment Lease Financing
In December 2015, the Company and Fountain Leasing 2013 LP (“Fountain”) entered into a master lease agreement and related transaction documents, pursuant to which Fountain provided the Company with $506,944 for the purchase of research and development lab equipment (the “Fountain Lease”). Fountain’s security under the Fountain Lease is the equipment purchased and a security deposit in the amount of $101,389. The initial term of the Fountain Lease is 36 months, with payments of $21,545 per month for the first 24 months and then $1,267 for the 12 months thereafter. Pursuant to the terms of the Fountain Lease, the Company has an option at the end of the initial term to purchase the equipment for the greater of $25,347 or current fair market value, provided that such amount shall not be in excess of $152,083. In addition, the Company also has the option to extend the Fountain Lease for an additional 12 month period at a rate of $8,872 per month with the right at the end of such extension term to purchase the equipment for fair value or to return the equipment to Fountain. The Fountain Lease has a lease rate factor of 4.25% per month for the first 24 months and 0.25% for the final 12 months of the initial term.
The Company has recorded current equipment lease financing of $243,215 and long-term equipment lease financing of $204,629 as of March 31, 2016. The Company has recorded current equipment lease financing of $240,473 and long-term equipment lease financing of $266,471 as of December 31, 2015. The equipment has been included in property and equipment on the Company’s unaudited condensed consolidated balance sheets.
Future principal payments on the equipment lease financing are as follows:
| For the twelve months ended March 31, | | Amount | |
| 2017 | | $ | 258,542 | |
| 2018 | | | 197,708 | |
| 2019 | | | 11,406 | |
| Total equipment lease financing payments | | | 467,656 | |
| Current equipment lease financing | | | 258,541 | |
| Less: Amount representing interest | | | (15,326 | ) |
| Current equipment lease financing, net | | | 243,215 | |
| Long-term equipment lease financing | | | 209,114 | |
| Less: Amount representing interest | | | (4,485 | ) |
| Equipment lease financing, less current portion, net | | $ | 204,629 | |
8 – COMMITMENTS
Operating Leases
In March 2015, the Company relocated its offices and research laboratories to 200 CambridgePark Drive in Cambridge, Massachusetts. The Company is leasing 16,825 square feet at this facility (the “Premises”) pursuant to Indenture of Lease (the “Lease”) that the Company entered into in November 2014. The term of the Lease is for five years, and the initial base rent is $42.50 per square foot, or approximately $715,062 on an annual basis. The base rent will increase incrementally over the term of the Lease, reaching approximately $804,739 on an annual basis in the fifth year of the term. In addition, the Company is obligated to pay a proportionate share of the operating expenses and applicable taxes associated with the premises, as calculated pursuant to the terms of the Lease. The Company is also obligated to deliver a security deposit to the landlord in the amount of $529,699, either in the form of cash or an irrevocable letter of credit, which may be reduced to $411,988 following the second anniversary of the commencement date under the Lease, provided that the Company meets certain financial conditions set forth in the Lease. The Company has recorded deferred rent in connection with the CambridgePark Lease as of March 31, 2016 and December 31, 2015 in the amount of $46,106 and $36,847, respectively. This amount has been recorded as a long-term liability on the Company’s unaudited condensed consolidated balance sheet.
The Company previously occupied offices and research laboratories in approximately 4,782 square feet of space at One Kendall Square in Cambridge, Massachusetts, at an annual rent of $248,664 (the “Kendall Lease”). For the year ended December 31, 2015, the Company recorded an expense of $55,352, representing all exit costs associated with its move to new offices and research laboratories in March 2015. For the three months ended March 31, 2015, the Company recorded an accrual of $55,352 for exit costs associated with its move to new offices and research laboratories in March 2015. The amount accrued at March 31, 2015 includes rent paid for April and May of 2015 related to the Kendall Lease. In June 2015, Enumeral entered into a lease termination agreement with the landlord for Enumeral’s former facility at One Kendall Square, pursuant to which the Kendall Lease was terminated as of June 17, 2015. In accordance with the terms of the lease termination agreement, Enumeral is not obligated to pay rent for the One Kendall Square facility after May 31, 2015. Enumeral had maintained a security deposit relating to the facility, recorded as restricted cash on the unaudited condensed consolidated balance sheet as of March 31, 2015. This security deposit was returned to Enumeral pursuant to the lease termination agreement.
In addition, the Company maintains a small corporate office at 1370 Broadway in New York, New York, at a current annual rate of $23,100. The lease for the Company’s New York office expires on December 31, 2016.
Rent expense was $332,339 and $155,264 for the three months ended March 31, 2016 and 2015, respectively.
Future operating lease commitments as of March 31, 2016 are as follows:
| For the twelve months ended March 31, | | Amount | |
| 2017 | | $ | 755,760 | |
| 2018 | | | 760,532 | |
| 2019 | | | 783,302 | |
| 2020 | | | 737,678 | |
| | | $ | 3,037,272 | |
Employment Agreements
The Company has employment agreements with members of management which contain minimum annual salaries and severance benefits if terminated prior to the term of the agreements.
9 – LICENSE AGREEMENT AND RELATED-PARTY TRANSACTIONS
License Agreement
In April 2011, Enumeral licensed certain intellectual property from the Massachusetts Institute of Technology (“MIT”), then a related party (as one of Enumeral’s scientific co-founders was an employee of MIT), pursuant to an Exclusive License Agreement (the “License Agreement”), in exchange for the payment of upfront license fees and a commitment to pay annual license fees, patent costs, milestone payments, royalties on sublicense income and, upon product commercialization, royalties on the sales of products covered by the licenses or income from corporate partners, and the issuance of 66,303 shares of Enumeral common stock. This intellectual property portfolio includes patents owned by Harvard University or co-owned by MIT and The Whitehead Institute, or MIT and Massachusetts General Hospital.
All amounts incurred related to the license fees have been expensed as research and development expenses by Enumeral as incurred. The Company incurred $10,000 and $7,500 in the three months ended March 31, 2016 and 2015, respectively.
In addition to potential future royalty and milestone payments that Enumeral may have to pay MIT per the terms of the License Agreement, Enumeral is obligated to pay an annual fee of $40,000 in 2016, and $50,000 every year thereafter unless the License Agreement is terminated. During the year ended December 31, 2015, the Company achieved the first milestone in the Merck Agreement and paid MIT the required percentage of the milestone payment pursuant to the terms of the License Agreement. No royalty payments have been payable as Enumeral has not commercialized any products as set forth in the License Agreement. Enumeral reimburses the costs to MIT and Harvard University for the continued prosecution of the licensed patent estate. For the three months ended March 31, 2016 and 2015, Enumeral paid $134,645 and $98,212 for MIT and $5,278 and $18,487 for Harvard, respectively. The Company had accounts payable and accrued expenses of $65,706 and $168,726 associated with the reimbursement of costs to MIT and Harvard as of March 31, 2016 and December 31, 2015, respectively.
The License Agreement also contained a provision whereby after the date upon which $7,500,000 of funding which was met in April of 2013, MIT and other licensing institutions set forth in the License Agreement have a right to participate in certain future equity issuances by Enumeral. In addition, pursuant to that provision Enumeral may have to issue additional shares to MIT and other licensing institutions set forth in the License Agreement if Enumeral issues common stock at a price per share that is less than the fair market value per share of the common stock issued to MIT and such licensing institutions based upon a weighted average formula set forth in the License Agreement. In March 2013, Enumeral and MIT entered into a first amendment to the License Agreement to clarify how equity issuances were to be made thereunder. In July 2014, Enumeral and MIT entered into a second amendment to the License Agreement, pursuant to which MIT’s participation rights and anti-dilution rights under the license agreement were terminated. Other than the exchange of Enumeral’s common stock for the Company’s common stock in connection with the Merger, the Company did not issue any shares of common stock to MIT and such other licensing institutions in connection with the License Agreement in 2014.
In April 2015, Enumeral and MIT entered into a third amendment to the License Agreement, which revised the timetable for Enumeral to complete certain diligence obligations relating to the initiation of clinical studies in support of obtaining regulatory approval of a Diagnostic Product (as such term is defined in the License Agreement), as well as the timetable by which Enumeral is required to make the first commercial sale of a Diagnostic Product.
In April 2016, Enumeral and MIT entered into a fourth amendment to the License Agreement, which revised the timetable for Enumeral to complete certain diligence obligations relating to the establishment of sublicenses and/or corporate partner agreements for the development of Licensed Products and/or Diagnostic Products, as well as the timetable by which an Investigational New Drug Application shall be filed on a Therapeutic Product (as such terms are defined in the Agreement).
Consulting Agreements
In September 2014, the Company and Dr. Buckland entered into a Scientific Advisory Board Agreement (the “SAB Agreement”), which replaced Dr. Buckland’s previous consulting agreement and pursuant to which Dr. Buckland serves as chairman of the Company’s Scientific Advisory Board. The SAB Agreement has a term of two years. Pursuant to the terms of the SAB Agreement, Dr. Buckland will receive compensation on an hourly or per diem basis, either in cash or, at Dr. Buckland’s election, in options to purchase the Company’s common stock. The SAB Agreement limits the total amount of compensation payable to Dr. Buckland at $100,000 over any rolling 12-month period. During the three months ended March 31, 2016 and 2015, the Company recognized $0 and $11,000 of expense related to the SAB agreement, respectively.
10 – EQUITY
Common Stock
On April 8, 2014, Enumeral amended its certificate of incorporation to increase the number of authorized shares of common stock from 15,000,000 to 24,000,000.
In April 2014, Enumeral issued 948,823 shares of Series B Convertible Preferred Stock at an issue price of $2.125 per share for proceeds of $1,597,860, net of issuance costs of $418,390. The Series B Preferred Stock ranks pari passu in all respects to Enumeral’s Series A-2, Series A-1 and Series A Preferred Stock. In connection with this offering, Enumeral paid the placement agent $81,000 in cash and issued the placement agent a warrant to purchase 38,259 Series B shares exercisable at $2.125 per share for a term of five years. These costs were included in the total issuance costs. All shares and warrants were converted as part of the Merger (see Merger discussion below).
In April 2014, Enumeral issued warrants to two executive officers to purchase 105,881 shares of Convertible Preferred Series B shares in connection with Enumeral’s Series B financing. These warrants were issued in relation to these executives taking a salary reduction prior to the Series B round of financing. In connection with the Merger in July 2014, these warrants were converted into warrants to purchase 309,967 shares of the Company’s common stock (see Merger discussion below).
On July 31, 2014, Enumeral entered into the Merger Agreement, pursuant to which Enumeral became a wholly owned subsidiary of the Company. The Company’s authorized capital stock currently consists of 300,000,000 shares of common stock, par value $0.001, and 10,000,000 shares of “blank check” preferred stock, par value $0.001.
Merger
As a result of the Merger, all issued and outstanding common and preferred shares of Enumeral were exchanged for common shares of the Company as follows: (a) each share of Enumeral’s common stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.102121 shares of the Company’s common stock for a total of 4,940,744 shares post-Merger, (b) each share of Enumeral’s Series A Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.598075 shares of the Company’s common stock for a total of 4,421,744 shares post-Merger, (c) each share of Enumeral’s Series A-1 Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.790947 shares of the Company’s common stock for a total of 3,666,428 shares post-Merger, (d) each share of Enumeral’s Series A-2 Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.997594 shares of the Company’s common stock for a total of 3,663,177 shares post-Merger, (e) each share of Enumeral’s Series B Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 2.927509 shares of the Company’s common stock for a total of 2,777,687 shares post-Merger and (f) a convertible note and accrued interest was converted into 3,230,869 shares of the Company’s common stock post-Merger.
As a result of the Merger and Split-Off, the Company discontinued its pre-Merger business and acquired the business of Enumeral, and has continued the existing business operations of Enumeral as a publicly-traded company under the name Enumeral Biomedical Holdings, Inc. In accordance with “reverse merger” accounting treatment, historical financial statements for Enumeral Biomedical Holdings Inc. as of period ends, and for periods ended, prior to the Merger will be replaced with the historical financial statements of Enumeral prior to the Merger in all future filings with the SEC.
Private Placement
On July 31, 2014, the Company closed a private placement offering (the “PPO”) of 21,549,510 Units of securities, at a purchase price of $1.00 per Unit, each Unit consisting of one share of the Company’s common stock and a warrant to purchase one share of the Company’s common stock at an exercise price of $2.00 per share with a term of five years (the “PPO Warrants”). The net proceeds received from the PPO were $18,255,444. The investors in the PPO (for so long as they hold shares of the Company’s common stock) have anti-dilution protection on the shares of common stock included in the Units purchased in the PPO and not subsequently transferred or sold (other than transfers to trusts or affiliates of such investors for the purpose of estate planning) in the event that within two years after the closing of the PPO the Company issues common stock or securities convertible into or exercisable for shares of the Company’s common stock at a price lower than the Unit purchase price. The anti-dilution protection provisions are subject to exceptions for certain issuances, including but not limited to (a) shares of common stock issued in an underwritten public offering, (b) issuances of awards under the Company’s 2014 Equity Incentive Plan, and (c) other exempt issuances.
In addition, the PPO Warrants not subsequently transferred or sold (other than transfers to trusts or affiliates of such investors for the purpose of estate planning) have anti-dilution protection in the event that prior to the warrant expiration date the Company issues common stock or securities convertible into or exercisable for shares of the Company’s common stock at a price lower than the warrant exercise price, subject to the exceptions described above.
The Company agreed to pay the placement agents in the offering, registered broker-dealers, a cash commission of 10% of the gross funds raised from investors in the PPO. In addition, the placement agents received warrants exercisable for a period of five years to purchase a number of shares of the Company’s common stock equal to 10% of the number of shares of common stock with a per share exercise price of $1.00 (the “Agent Warrants”); provided, however, that the placement agents were not entitled to any warrants on the sale of Units in excess of 20,000,000. Any sub-agent of the placement agents that introduced investors to the PPO was entitled to share in the cash fees and warrants attributable to those investors as described above. The Company also reimbursed the placement agents $30,000 in the aggregate for legal expenses incurred by the placement agents’ counsels in connection with the PPO, as described in the private placement agreements. As a result of the foregoing, the placement agents were paid an aggregate cash commission of $2,154,951 and were issued Agent Warrants to purchase 2,000,000 shares of the Company’s common stock. The Agent Warrants not subsequently transferred or sold (other than transfers to trusts or affiliates of such investors for the purpose of estate planning) have anti-dilution protection until the warrant expiration date, subject to the exceptions described above for the Units. The value ascribed to the Agent Warrants are carried at fair value and reported as a derivative liability on the accompanying unaudited condensed consolidated balance sheets.
The Company incurred approximately $500,000 of expenses in connection with the offering outside of the placement agent commissions and issued the subagent to one of the placement agents 150,000 shares of the Company’s common stock.
In addition, the Merger Agreement provided certain anti-dilution protection to the holders of the Company’s common stock immediately prior to the Merger (after giving effect to the Split-Off), in the event that the aggregate number of units sold in the PPO after the final closing thereof were to exceed 15,000,000. Accordingly, based on the final amount of gross proceeds raised in the PPO, the Company issued 1,690,658 additional shares of the Company’s common stock to holders of the Company’s common stock immediately prior to the Merger. The Company recorded $1,690,658 in other expense related to this issuance of shares at $1.00 per share.
11 - STOCK OPTIONS, RESTRICTED STOCK AND WARRANTS
Stock Options
In December 2009, Enumeral adopted the 2009 Stock Incentive Plan (the “2009 Plan”). In April 2014, Enumeral amended the 2009 Plan to increase the number of shares authorized thereunder to 3,200,437. The 2009 Plan was terminated in July 2014 in connection with the Merger.
On July 31, 2014, the Company’s Board of Directors adopted, and the Company’s stockholders approved, the 2014 Equity Incentive Plan (the “2014 Plan”), which reserves a total of 8,100,000 shares of the Company’s common stock for incentive awards. In connection with the Merger, options to purchase 948,567 shares of Enumeral common stock previously granted under the 2009 Plan were converted into options to purchase 1,045,419 shares of the Company’s common stock under the 2014 Plan.
Generally, shares that are expired, terminated, surrendered or cancelled without having been fully exercised will be available for future awards. In addition, shares of common stock that are tendered to the Company by a participant to exercise an award are added to the number of shares of common stock available for the grant of awards.
Effective January 1, 2016, the Company recognizes all share-based awards under the straight-line attribution method, assuming that all granted awards will vest. Forfeitures will be recognized in the periods when they occur. Refer to Note 3, Summary of Significant Accounting Policies, for further information. In prior periods, ASC 718 required forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The Company evaluated its forfeiture assumptions quarterly and the expected forfeiture rate was adjusted when necessary. The actual expense recognized over the vesting period is based on only those shares that vest.
In periods prior to January 1, 2016, estimates of pre-vesting option forfeitures were based on the Company’s experience. The Company used a forfeiture rate of 0 - 5% depending on when and to whom the options were granted. The Company adjusted its estimate of forfeitures over the requisite service period based on the extent to which actual forfeitures differ, or are expected to differ, from such estimates. Changes in estimated forfeitures were recognized through a cumulative adjustment in the period of change and may have impacted the amount of compensation expense to be recognized in future periods. The Company considered many factors when estimating expected forfeitures, including types of awards, employee class, and historical experience.
The Company estimates the fair value of each stock award on the grant date using the Black-Scholes option-pricing model based on the following assumptions and the assumptions regarding the fair value of the underlying common stock on each measurement date:
| | | For the three
months ended March 31, 2016 | |
| Expected Volatility | | 117.0% | |
| Risk-free interest rate | | 1.39%-1.72% | |
| Expected term (in years) | | 5.00 | |
| Expected dividend yield | | 0% | |
The Company did not grant any stock options during the three months ended March 31, 2015.
As of March 31, 2016, there were 243,268 shares available for issuance under the 2014 Plan to eligible employees, non-employee directors and consultants. This number is subject to adjustment in the event of a stock split, reverse stock split, stock dividend, or other change in the Company’s capitalization.
A summary of stock option activity for the three months ended March 31, 2016 is as follows:
| | | | | | | | | Weighted- | |
| | | | | | Weighted- | | | Average | |
| | | | | | Average | | | Remaining | |
| | | | | | Exercise | | | Contractual | |
| | | Shares | | | Price | | | Term (years) | |
| Outstanding at December 31, 2015 | | | 5,926,654 | | | $ | 0.62 | | | | 9.0 | |
| Granted | | | 1,468,182 | | | $ | 0.20 | | | | | |
| Exercised | | | - | | | $ | - | | | | | |
| Canceled | | | (453,263 | ) | | $ | 0.85 | | | | | |
| Outstanding at March 31, 2016 | | | 6,941,573 | | | $ | 0.52 | | | | 8.9 | |
| Exercisable at March 31, 2016 | | | 2,950,855 | | | $ | 0.49 | | | | 8.6 | |
Stock option compensation expense was $293,445 and $141,681 for the three months ended March 31, 2016 and 2015, respectively. The Company has an aggregate of $1,319,622 of unrecognized stock option compensation expense as of March 31, 2016 to be amortized over a weighted average period of 3.0 years.
The aggregate intrinsic value of options exercisable as of March 31, 2016 was $4,133. The aggregate intrinsic value was calculated as the difference between the exercise price of the stock options and the fair value of the underlying common stock as of the unaudited condensed consolidated balance sheet date.
��
Restricted Stock
A summary of restricted stock activity for the three months ended March 31, 2016 is as follows:
| | | | | | Weighted- | |
| | | Number of | | | Average Grant | |
| | | Shares | | | Date Fair Value | |
| Balance of unvested restricted stock at December 31, 2015 | | | 282,119 | | | $ | 0.24 | |
| Issuance of restricted stock | | | 140,910 | | | $ | 0.22 | |
| Vested | | | (213,197 | ) | | $ | 0.23 | |
| Balance of unvested restricted stock at March 31, 2016 | | | 209,832 | | | $ | 0.23 | |
Restricted stock compensation expense was $40,366 and $24,710 for the three months ended March 31, 2016 and 2015, respectively.
The Company has an aggregate of $40,241 of unrecognized restricted stock compensation expense as of March 31, 2016 to be amortized over a weighted average period of 0.5 years.
Warrants
As of March 31, 2016, there were a total of 24,803,409 warrants outstanding to purchase shares of the Company's common stock. Of these, 23,549,510 warrants were issued in connection with the PPO and 66,574 warrants were issued to Square 1 Bank (in connection with a previous financing transaction as further described below) and are accounted for as derivative liabilities. The remaining 1,187,325 warrants do not require derivative liability accounting treatment.
Derivative Liability Warrants
In connection with the PPO, the Company issued warrants to purchase an aggregate of 23,549,510 shares of the Company’s common stock. Additionally, in connection with Enumeral’s December 2011 financing transaction with Square 1 Bank, Enumeral issued warrants to purchase 41,659 shares of Enumeral’s Series A preferred stock that were subsequently converted into warrants to purchase 66,574 shares of the Company’s common stock in connection with the July 2014 Merger.
A) PPO and Agent Warrants
In July 2014, the Company issued warrants to purchase 23,549,510 shares of the Company’s common stock in connection with the PPO, of which warrants to purchase 21,549,510 shares of the Company’s common stock had an exercise price of $2.00 per share and were issued to the investors in the PPO, and warrants to purchase 2,000,000 shares of the Company’s common stock had an exercise price of $1.00 per share and were issued to the placement agents for the PPO (or their affiliates). The estimated fair value of the warrants at the time of issuance was determined to be $16,261,784 using the Black-Scholes pricing model and the following assumptions: expected term of five years, 105.4% volatility, and a risk-free rate of 1.77%. The estimated fair value of the warrants at March 31, 2016 was determined to be $1,455,450 using the Black-Scholes pricing model and the following assumptions: expected remaining term of 3.33 years, 114.6% volatility, risk-free rate of 0.93%, and no expected dividends. The estimated fair value of the warrants at December 31, 2015 was determined to be $2,130,822 using the Black-Scholes pricing model and the following assumptions: expected remaining term of 3.58 years, 109.4% volatility, risk-free rate of 1.44%, and no expected dividends. Due to a price protection provision included in the warrant agreements, the warrants were deemed to be a liability and, therefore, the fair value of the warrants is recorded in the liability section of the unaudited condensed consolidated balance sheet. As such, the outstanding warrants are revalued each reporting period with the resulting gains and losses recorded as the change in fair value of derivative liabilities on the unaudited condensed consolidated statements of operations and comprehensive income (loss). All 23,549,510 warrants were outstanding as of March 31, 2016 and December 31, 2015, respectively.
B) Square 1 Financing
In connection with the December 2011 Square 1 financing transaction, Enumeral issued to Square 1 Bank warrants to purchase an aggregate of 33,944 shares of Series A convertible preferred stock at an exercise price of $1.16 per share, exercisable for seven years. In July 2014, as part of the Merger, these warrants were converted into warrants to purchase 54,245 shares of the Company’s common stock at an exercise price of $0.73 per share. The estimated fair value of the warrants as of March 31, 2016 was determined to be $3,308 using the Black-Scholes pricing model and the following assumptions: expected term of 2.68 years, 114.6% volatility, and a risk-free rate of 0.84%. The estimated fair value of the warrants as of December 31, 2015 was determined to be $5,777 using the Black-Scholes pricing model and the following assumptions: expected term of 2.9 years, 104.6% volatility, and a risk-free rate of 1.31%. As part of a June 12, 2012 amendment to the Loan and Security Agreement between Enumeral and Square 1 Bank, Enumeral issued warrants to Square 1 Bank to purchase an aggregate of 7,715 shares of Series A convertible preferred stock at an exercise price of $1.16 per share, exercisable for seven years. In July 2014, as part of the Merger, these warrants were converted into warrants to purchase 12,329 shares of the Company’s common stock at an exercise price of $0.73 per share. The estimated fair value of these warrants as of March 31, 2016 was determined to be $898 using the Black-Scholes pricing model and the following assumptions: expected term of 3.2 years, 114.6% volatility, and a risk-free rate of 0.96%. The estimated fair value of these warrants as of December 31, 2015 was determined to be $1,492 using the Black-Scholes pricing model and the following assumptions: expected term of 3.5 years, 104.6% volatility, and a risk-free rate of 1.42%. The warrants are classified as derivative liabilities in the accompanying unaudited condensed consolidated balance sheets and measured at fair value on a recurring basis. As such, the outstanding warrants are revalued each reporting period with the resulting gains and losses recorded as the change in fair value of derivative liabilities on the unaudited condensed consolidated statements of operations and comprehensive income (loss). As of March 31, 2016 and December 31, 2015 these warrants were outstanding and expire on December 5, 2018 and June 12, 2019.
12 – CONCENTRATIONS
During the three months ended March 31, 2016, the Company recorded revenue from two entities in excess of 10% of the Company’s total revenue in the amounts of $316,018 and $118,439, which represents 73% and 27% of the Company’s total revenue for that period. During the three months ended March 31, 2015, the Company recorded revenue from two entities in excess of 10% of the Company’s total revenue in the amounts of $209,635 and $65,087, which represents 76% and 24% of the Company’s total revenue for that period.
As of March 31, 2016, accounts receivable consisted of amounts due from two entities which represented 81% and 19% of the Company’s total outstanding accounts receivable balance, respectively. As of December 31, 2015, accounts receivable consisted of amounts due from two entities which represented 67% and 33% of the Company’s total outstanding accounts receivable balance, respectively.
ENUMERAL BIOMEDICAL HOLDINGS, INC.
47,674,386 Shares of Common Stock
________________________
PROSPECTUS
________________________
July 26, 2016
















