Exhibit 10.2
[***] Certain information in this document has been excluded pursuant to Regulation S-K, item 601(b)(10). Such excluded information is not material and would likely cause competitive harm to the registrant if publicly disclosed.
ADDENDUM TO THE SECOND AMENDED AND RESTATED LICENSE, PRODUCT
DEVELOPMENT AND SUPPLY UMBRELLA AGREEMENT
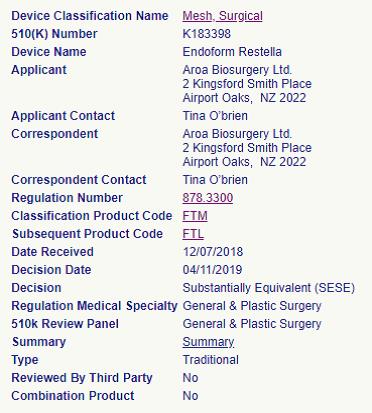
This Addendum to the Second Amended and Restated License, Product Development and Supply Umbrella Agreement (this “Addendum”) is made as of the 15th day of February, 2020, by and between TELA Bio, Inc. (“TELA Bio”), and Aroa Biosurgery Limited (“Aroa”), and sets out the terms of TELA Bio’s and Aroa’s agreements with respect to the assignment, transfer and conveyance from Aroa to TELA Bio of the FDA 510(k) clearances (K183398) for the Endoform® Restella Reconstructive Template (the “Restella Product”) as more particularly described in Exhibit B attached hereto (the “Restella Product Clearance”).
WHEREAS, TELA Bio and Aroa are parties to that certain Second Amended and Restated License, Product Development and Supply Umbrella Agreement dated as of July 16, 2015 (the “Umbrella Agreement”);
WHEREAS, TELA Bio and Aroa entered into an Addendum to the Umbrella Agreement, dated as of January 3, 2019, relating to the development of the Restella Product (“Restella Product Addendum”); and
WHEREAS, as of the date of this Addendum, the products covered by the Restella Product Clearance (each of which shall be considered a Restella Product for purposes of this Addendum) are collectively as set out in Exhibit A attached hereto;
NOW, THEREFORE, for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, and intending to be legally bound hereby, TELA Bio and Aroa agree as follows:
1. | Effective as of the date of this Addendum, Aroa hereby assigns, transfers and conveys unto TELA Bio all right, title and interest of Aroa in, to and under the Restella Product Clearance, and TELA Bio accepts such assignment, transfer and conveyance of same such that TELA Bio is and shall be, from and after the date of this Addendum, the sole and exclusive owner of the Restella Product Clearance (the “Restella Product Clearance Transfer”). |
2. | Aroa and TELA Bio will do, execute and perform all such acts, deeds, matters and things as may be reasonably required by the other to enable each of them to obtain the full benefit and advantage of the terms, intent and meaning of this Addendum, including without limitation: |
a. | Updating the establishment registration and device listing for the Restella Product, to the extent the same has not already occurred as of the date of this Addendum; |
b. | Executing an amended quality agreement (“Quality Agreement”) with regard to the Restella Product within thirty (30) days after the date of this Addendum; and |
c. | Modifying the existing labelling for the Restella Product. |
3. | All costs of the Restella Product Clearance Transfer shall be borne by TELA Bio, including the costs of modifying the existing Restella Product labelling. For the avoidance of doubt, |
neither party will charge the other for any fixed administrative or internal costs relating to the Restella Product Clearance Transfer. |
4. | TELA Bio shall be responsible and liable for all obligations relating to or in connection with the Restella Product Clearance (the “TELA Bio Restella Obligations”). TELA Bio shall indemnify and hold Aroa, its Affiliates and their respective directors, officers, employees and agents harmless from and against any and all costs, claims, liabilities or expenses (including legal costs) to the extent arising from or incidental to any breach, default or omission by TELA Bio of the TELA Bio Restella Obligations. Notwithstanding the foregoing, Aroa is and shall remain responsible and liable for all FDA obligations relating to or in connection with the Restella Product Clearance pertaining to Aroa’s roles and responsibilities with respect to its manufacture of Restella Products prior to the date of this Addendum (the “Aroa Restella Obligations”). Aroa shall indemnify and hold TELA Bio, its Affiliates and their respective directors, officers, employees and agents harmless from and against any and all costs, claims, liabilities or expenses (including legal costs) to the extent arising from or incidental to any breach, default or omission by Aroa of the Aroa Restella Obligations. |
5. | TELA Bio and Aroa agree that all documents relating to or in connection with the Restella Product Clearance (including any documents relating to or in connection with any modification or improvement of or to the Restella Product), shall be held in a shared folder accessible by both parties. All updates to such documents shall be placed in such shared folder and updated on a regular basis (quarterly or more frequently) to ensure that the documents in such shared folder are the latest versions of such documents. The shared folder shall at all times include a complete copy of the Restella Product Clearance and any additional documents that TELA Bio would be required to maintain or possess pursuant to FDA regulations. All information contained in such shared folder that is Confidential Information under the Umbrella Agreement shall be maintained as such by the parties hereto. |
6. | TELA Bio shall grant Aroa, free of charge, access to any documents, information, research or clinical trial results for the Restella Product and relating to or in connection with any other Regulatory Submissions or Regulatory Approvals for products which are modifications or improvements of or to the Restella Product, and Aroa shall be permitted to use such documents, information, research or clinical trial results solely for the purpose of: (a) developing, commercialising or seeking Regulatory Approval for any indications other than the Indications in and for the Territory; and (b) developing, commercialising or seeking Regulatory Approval for any indications (including the Indications) outside the Territory; provided, however, that any such documents, information, research or clinical trial results are being shared and/or delivered by TELA Bio to Aroa without the making or giving of any representations or warranties of any kind, express or implied, with respect thereto. All documents, information, research and clinical trial results accessed by Aroa under this Section 6 shall be Confidential Information under the Umbrella Agreement. |
7. | Capitalised terms used but not defined in this Addendum have the meanings given to those terms in the Umbrella Agreement. Except as agreed herein, the provisions of the Umbrella Agreement and any prior Addendum to the Umbrella Agreement (including without limitation, the Restella Product Addendum), are not amended and continue to be in full force and effect. This Addendum shall be governed by and construed in accordance with the laws of the State of Delaware, without regard to any conflict of law provisions. This Addendum may be executed in separate counterparts, each of which shall be an original and all of which taken together shall constitute one and the same agreement. Executed signature pages to this Addendum may be delivered by facsimile or electronic mail and any signature page so delivered shall be deemed to be an original. |
[signature page follows]
IN WITNESS WHEREOF, each of TELA Bio and Aroa has caused this Addendum to be executed by its duly authorized representative as of the date first above written.
TELA BIO, INC. |
| AROA BIOSURGERY LIMITED | ||
|
|
| ||
|
|
| ||
By: | /s/ Antony Koblish |
| By: | /s/ Brian Ward |
|
|
|
|
|
Name: | Antony Koblish |
| Name: | Brian Ward |
|
|
|
|
|
Title: | President & CEO |
| Title: | CEO |
Exhibit A
[***]
Exhibit B
Restella Product Clearance