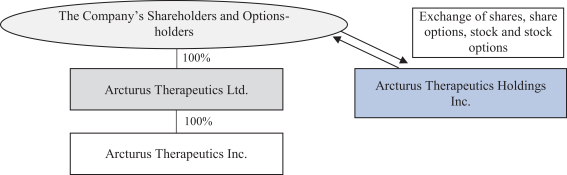
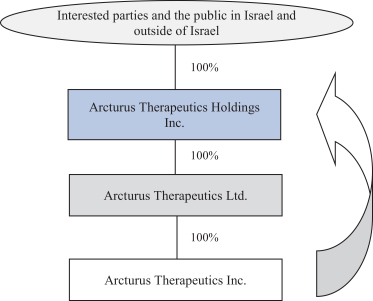
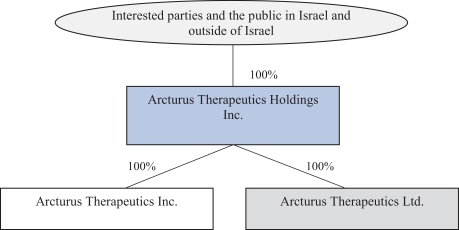
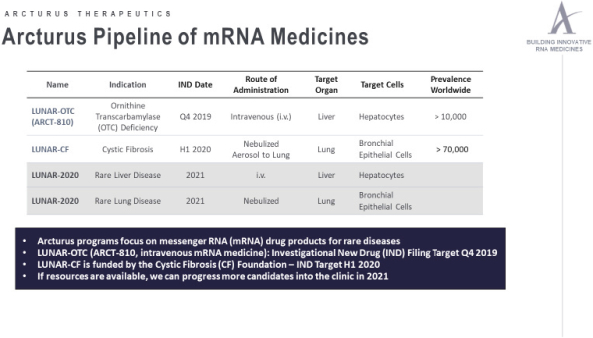
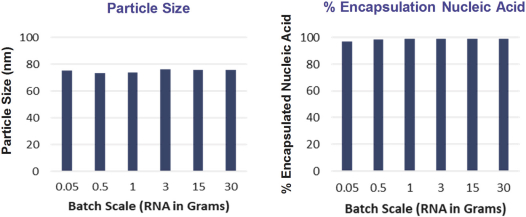
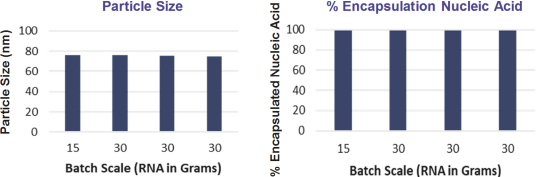
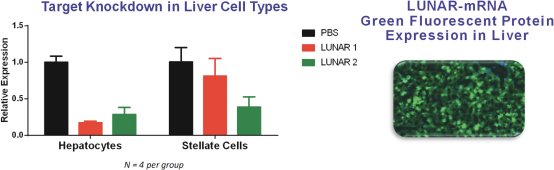
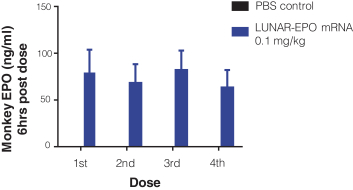
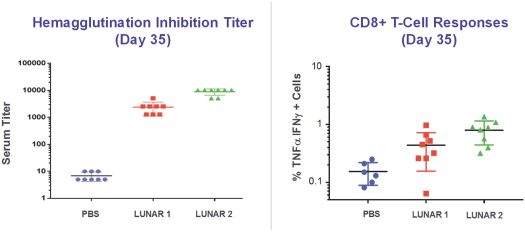
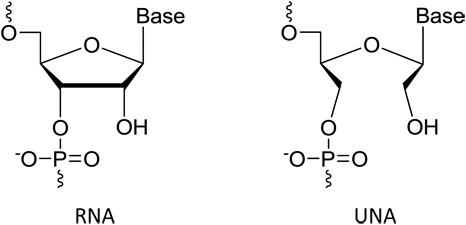
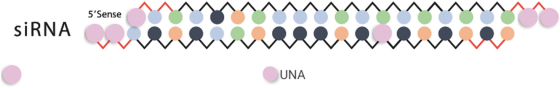
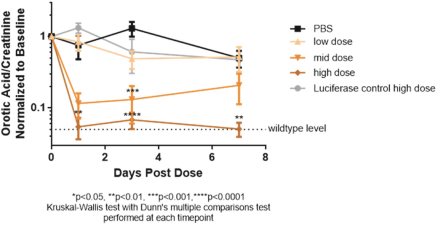
We also protect our proprietary technology and processes, in part, by confidentiality and invention assignment agreements with our employees, consultants, scientific advisors and other contractors. These agreements may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our employees, consultants, scientific advisors or other contractors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resultingknow-how and inventions.
COMPETITION
We believe that our scientific knowledge and expertise in nucleic acid-based therapies provide us with competitive advantages over the various companies and other entities that are attempting to develop similar treatments. However, we face competition at the technology platform and therapeutic indication levels from both large and small biopharmaceutical companies, academic institutions, governmental agencies and public and private research institutions. Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Our success will be based in part upon our ability to identify, develop and manage a portfolio of drugs that are safer and more effective than competing products in the treatment of our targeted patients. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, are more convenient or are less expensive than any products we may develop.
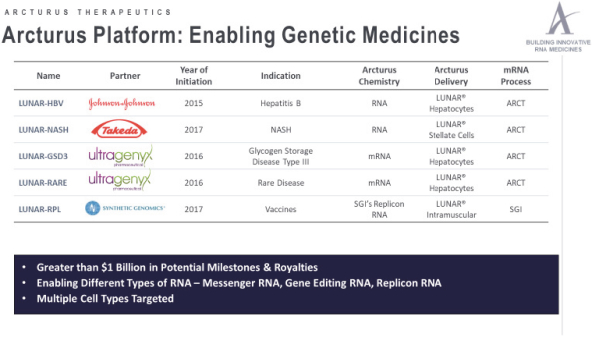
We are aware of several other companies that are working to develop nucleic acid medicines, including gene therapy, gene editing, mRNA, siRNA, and antisense therapeutics. Many of these companies, such as the newly formed Genevant, are also developing nucleic acid delivery platforms which compete with LUNAR technology.
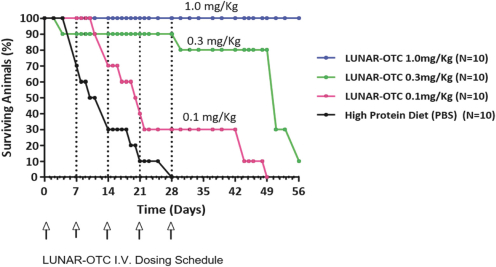
Companies currently developing mRNA therapeutics for prophylactic vaccines, cancer vaccines, or mRNA replacement therapy for rare genetic diseases include Moderna Therapeutics, Translate Bio, Ethris GmbH, CureVac GmbH, BioNTech, and eTheRNA. Translate Bio is developing mRNA replacement therapies for cystic fibrosis and OTC deficiency which are in preclinical or early clinical development, and which directly compete with ourLUNAR-OTC andLUNAR-CF programs. Ethris is in preclinical development ofETH-CFTR, a mRNA replacement therapy for cystic fibrosis. A number of companies are developing viral vector orDNA-based approaches to gene delivery for rare liver diseases, including Ultragenyx Pharmaceutical, REGENXBIO, Inc., uniQure, Vivet Therapeutics, LogicBio Therapeutics, Touchlight Genetics Ltd., Generation Bio, and Audentes Therapeutics. Ultragenyx is developing a gene therapy product for OTC deficiency which is in early clinical trials.
Companies developing siRNA therapeutics include Arbutus Biopharma, Arrowhead Pharmaceuticals, Inc, Quark Pharmaceuticals, Inc., Silence Therapeutics plc, Nitto Denko, Dicerna Pharmaceuticals, Inc., and Alnylam Pharmaceuticals, Inc. Antisense therapeutics are also in development by Ionis Pharmaceuticals, Roche Pharma, WAVE Life Sciences, Celgene Corporation, Akcea Therapeutics, Inc., Antisense Therapeutics, Ltd., ProQR, and Sarepta Therapeutics, Inc. Both Ionis Pharmaceuticals and ProQR are developing antisense therapies for cystic fibrosis which compete with ourLUNAR-CF program.
In addition, to the companies mentioned above, several companies are developingnon-nucleic acid therapies for OTC deficiency which are competitors to ourLUNAR-OTC program. For example, Synlogic’s SYNB1020 product is treating urea cycle disorders, including OTC deficiency, by introducing engineered probiotic bacteria to the gut. Promethera’s Heparesc product involves infusion of their HepaStem, liver-derived stem cells into urea
125