
Melissa L. Johnson1; Sai-Hong Ignatius Ou2; Minal Barve3; Igor I. Rybkin4; Kyriakos P. Papadopoulos5; Ticiana A. Leal6; Karen Velastegui7; James G. Christensen7; Thian Kheoh7; Richard C. Chao7; Jared Weiss8 KRYSTAL-1: Activity and Safety of Adagrasib (MRTX849) in Patients With Colorectal Cancer (CRC) and Other Solid Tumors Harboring a KRASG12C Mutation 1Sarah Cannon Research Institute Tennessee Oncology, Nashville, Tennessee, USA. 2University of California, Irvine, Chao Family Comprehensive Cancer Center, Orange, California, USA. 3Mary Crowley Cancer Research, Dallas, Texas, USA. 4Henry Ford Cancer Institute, Detroit, Michigan, USA. 5START Center for Cancer Care, San Antonio, Texas, USA. 6University of Wisconsin Carbone Cancer Center, Madison, Wisconsin, USA. 7Mirati Therapeutics, Inc., San Diego, California, USA. 8Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, North Carolina, USA. Exhibit 99.2

Sponsored Research (Paid to Institution): AstraZeneca; Boehringer-Ingelheim; Calithera Bioeciences; EMD Serono; Roche/Genentech; GlaxoSmithKline; Gritstone Oncology; Guardant Health; Incyte; Janssen R&D; Eli Lilly and Company; Merck; Novartis; Pfizer; Sanofi-Aventis; AbbVie; Acerta Pharma; Adaptimmune; Amgen; Apexigen Array BioPharma; BeiGene; Checkpoint Therapeutics; Corvus Pharmaceuticals; CytomX; Daiichi Sankyo; Dynavax Technologies; Genmab; Genocea Biosciences; Hengrui Therapeutics; Immunocore; Jounce Therapeutics; Kadmon Pharmaceuticals; Loxo Oncology; Lycera; Mirati Therapeutics, Inc; Neovia Oncology; OncoMed Pharmaceuticals; Regeneron Pharmaceuticals; Shattuck Labs; Stem CentRx; Syndax Pharmaceuticals; Takeda Pharmaceuticals; Tarveda; University of Michigan; WindMIL Therapeutics; TCR2 Therapeutics; Arcus Biosciences; Ribon Therapeutics; Seven and Eight Biopharmaceuticals; BerGenBio; Foundation Medicine; Atreca; Rubius Therapeutics; Tmunity Therapeutics Consulting Fees (Paid to Institution): Achilles Therapeutics; AstraZeneca; Atreca, Boehringer Ingelheim; Bristol-Myers Squibb; Daiichi Sankyo; EMD Serono; G1 Therapeutics; GlaxoSmithKline; Gritstone Oncology; Incyte; Janssen; Eli Lilly and Company; Merck; Mirati Therapeutics, Inc.; Novartis; Pfizer; Ribon Therapeutics; Roche/Genentech; Sanofi-Aventis and WindMIL Therapeutics Other: Astellas Pharmaceuticals and Otsuka Pharmaceuticals (spouse) Disclosures Presented at the 32nd EORTC-NCI-AACR Symposium, October 24-25, 2020 KRYSTAL-1: Adagrasib (MRTX849) KRASG12C Inhibitor in CRC and Other Solid Tumors
KRASG12C mutations act as oncogenic drivers and occur in approximately 14% of NSCLC (adenocarcinoma), 3-4% of CRC, and 1-2% of several other cancers1-3 The KRAS protein cycles between GTP-On and GDP-Off states and has a protein resynthesis half-life of ~24 h4,5 Adagrasib is a covalent inhibitor of KRASG12C that irreversibly and selectively binds KRASG12C in its inactive, GDP-bound state6 Adagrasib was optimized for desired properties of a KRASG12C inhibitor: Potent covalent inhibitor of KRASG12C (cellular IC50: ~5 nM) High selectivity (>1000X) for the mutant KRASG12C protein vs wild-type KRAS Favorable PK properties, including oral bioavailability, long half-life (~24 h), and extensive tissue distribution Adagrasib (MRTX849) Is a Differentiated and Selective Inhibitor of KRASG12C Presented at the 32nd EORTC-NCI-AACR Symposium, October 24-25, 2020 1. Zehir A, Benayed R, Shah RH, et al. Nat Med. 2017;23(6):703–713. 2. Schirripa M, Nappo F, Cremolini C, et al. Clin Colorectal Cancer. 2020;S1533-0028(20)30067-0. 3. NIH TCGA: The Cancer Genome Atlas. 4. Bos JL, Rehmann H, Wittinghofer A. Cell. 2007;129: 865-877. 5. Shukla S, Allam US, Ahsan A, et al. Neoplasia. 2014;16(2):115-128; 6. Hallin J, Engstrom LD, Hargis L, et al. Cancer Discov. 2020;10(1): 54-71. Adagrasib Crystal Structure Hypothesis: Maintaining continuous exposure of adagrasib above a target threshold enables inhibition of KRAS-dependent signaling for the complete dose interval and maximizes depth and duration of antitumor activity KRYSTAL-1: Adagrasib (MRTX849) KRASG12C Inhibitor in CRC and Other Solid Tumors
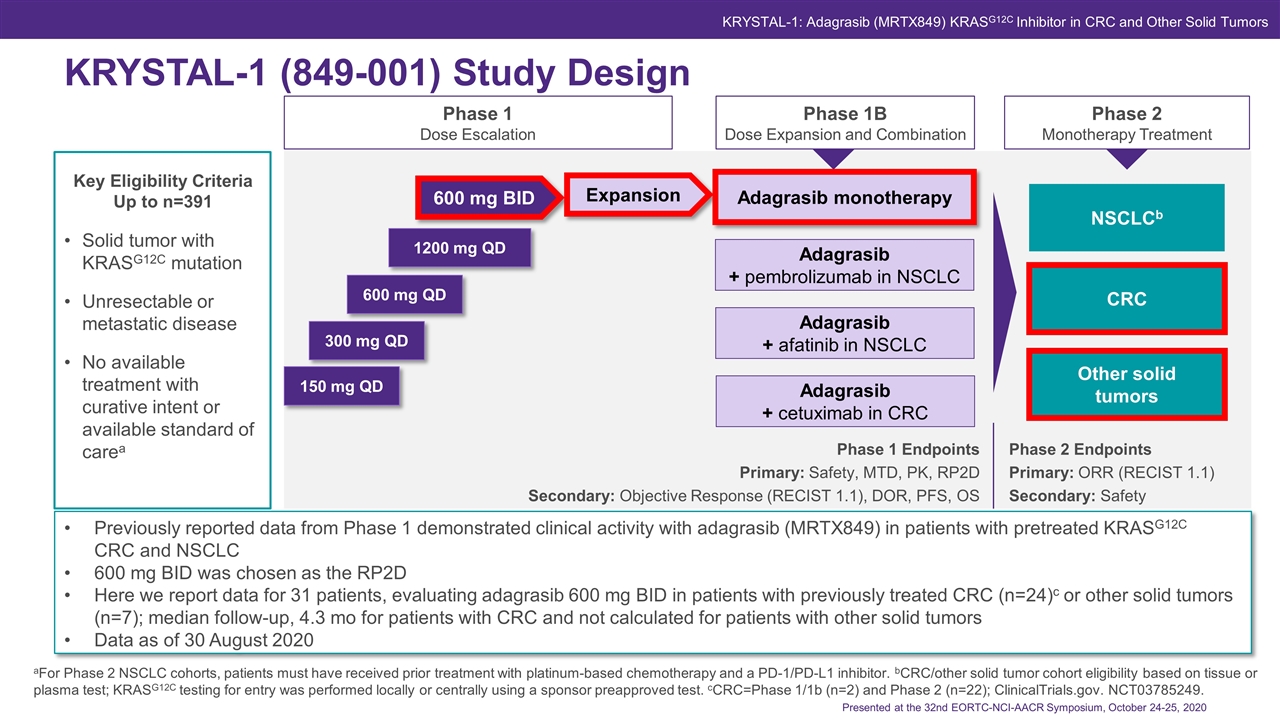
aFor Phase 2 NSCLC cohorts, patients must have received prior treatment with platinum-based chemotherapy and a PD-1/PD-L1 inhibitor. bCRC/other solid tumor cohort eligibility based on tissue or plasma test; KRASG12C testing for entry was performed locally or centrally using a sponsor preapproved test. cCRC=Phase 1/1b (n=2) and Phase 2 (n=22); ClinicalTrials.gov. NCT03785249. Phase 1 Endpoints Primary: Safety, MTD, PK, RP2D Secondary: Objective Response (RECIST 1.1), DOR, PFS, OS Phase 2 Endpoints Primary: ORR (RECIST 1.1) Secondary: Safety Solid tumor with KRASG12C mutation Unresectable or metastatic disease No available treatment with curative intent or available standard of carea Phase 1 Dose Escalation Phase 1B Dose Expansion and Combination Phase 2 Monotherapy Treatment 150 mg QD 300 mg QD 600 mg QD 1200 mg QD 600 mg BID KRYSTAL-1 (849-001) Study Design Key Eligibility Criteria Up to n=391 Expansion NSCLCb CRC* Other solid tumors*‡ Adagrasib + pembrolizumab in NSCLC Adagrasib + afatinib in NSCLC Adagrasib monotherapy Adagrasib + cetuximab in CRC CRC Other solid tumors Previously reported data from Phase 1 demonstrated clinical activity with adagrasib (MRTX849) in patients with pretreated KRASG12C CRC and NSCLC 600 mg BID was chosen as the RP2D Here we report data for 31 patients, evaluating adagrasib 600 mg BID in patients with previously treated CRC (n=24)c or other solid tumors (n=7); median follow-up, 4.3 mo for patients with CRC and not calculated for patients with other solid tumors Data as of 30 August 2020 Presented at the 32nd EORTC-NCI-AACR Symposium, October 24-25, 2020 KRYSTAL-1: Adagrasib (MRTX849) KRASG12C Inhibitor in CRC and Other Solid Tumors
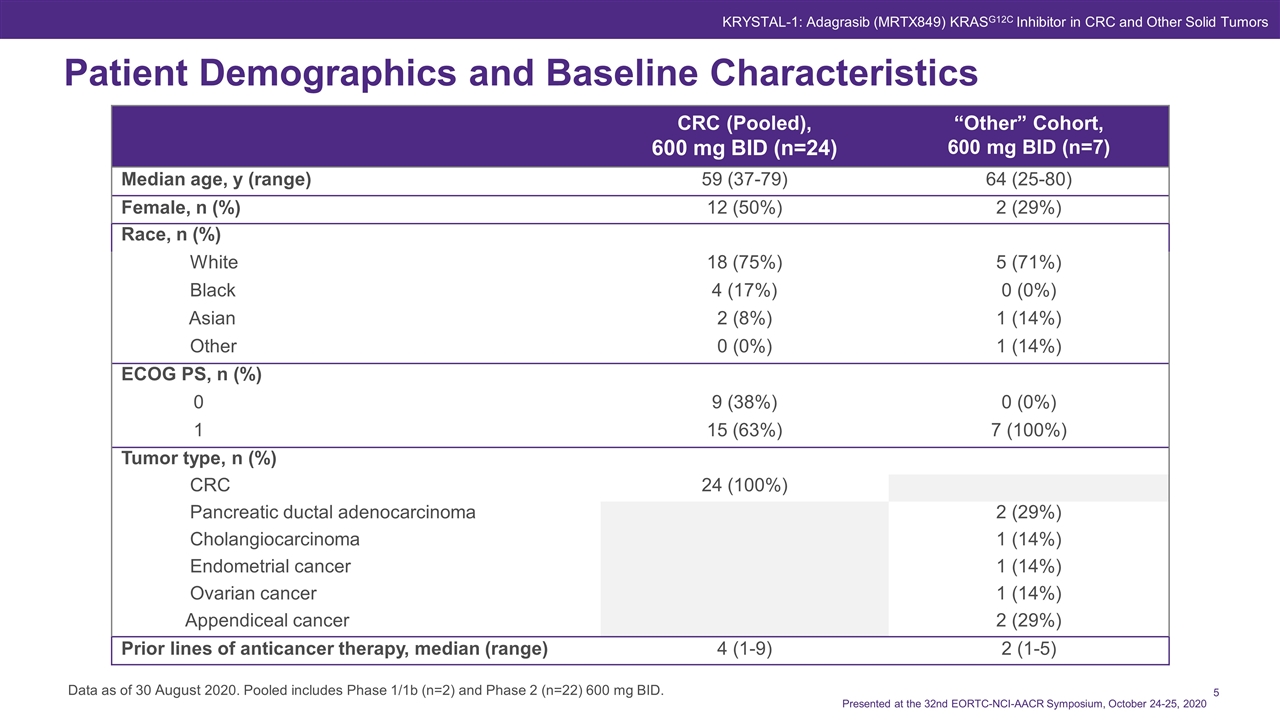
Patient Demographics and Baseline Characteristics CRC (Pooled), 600 mg BID (n=24) “Other” Cohort, 600 mg BID (n=7) Median age, y (range) 59 (37-79) 64 (25-80) Female, n (%) 12 (50%) 2 (29%) Race, n (%) White 18 (75%) 5 (71%) Black 4 (17%) 0 (0%) Asian 2 (8%) 1 (14%) Other 0 (0%) 1 (14%) ECOG PS, n (%) 0 9 (38%) 0 (0%) 1 15 (63%) 7 (100%) Tumor type, n (%) CRC 24 (100%) Pancreatic ductal adenocarcinoma 2 (29%) Cholangiocarcinoma 1 (14%) Endometrial cancer 1 (14%) Ovarian cancer 1 (14%) Appendiceal cancer 2 (29%) Prior lines of anticancer therapy, median (range) 4 (1-9) 2 (1-5) Data as of 30 August 2020. Pooled includes Phase 1/1b (n=2) and Phase 2 (n=22) 600 mg BID. Presented at the 32nd EORTC-NCI-AACR Symposium, October 24-25, 2020 KRYSTAL-1: Adagrasib (MRTX849) KRASG12C Inhibitor in CRC and Other Solid Tumors
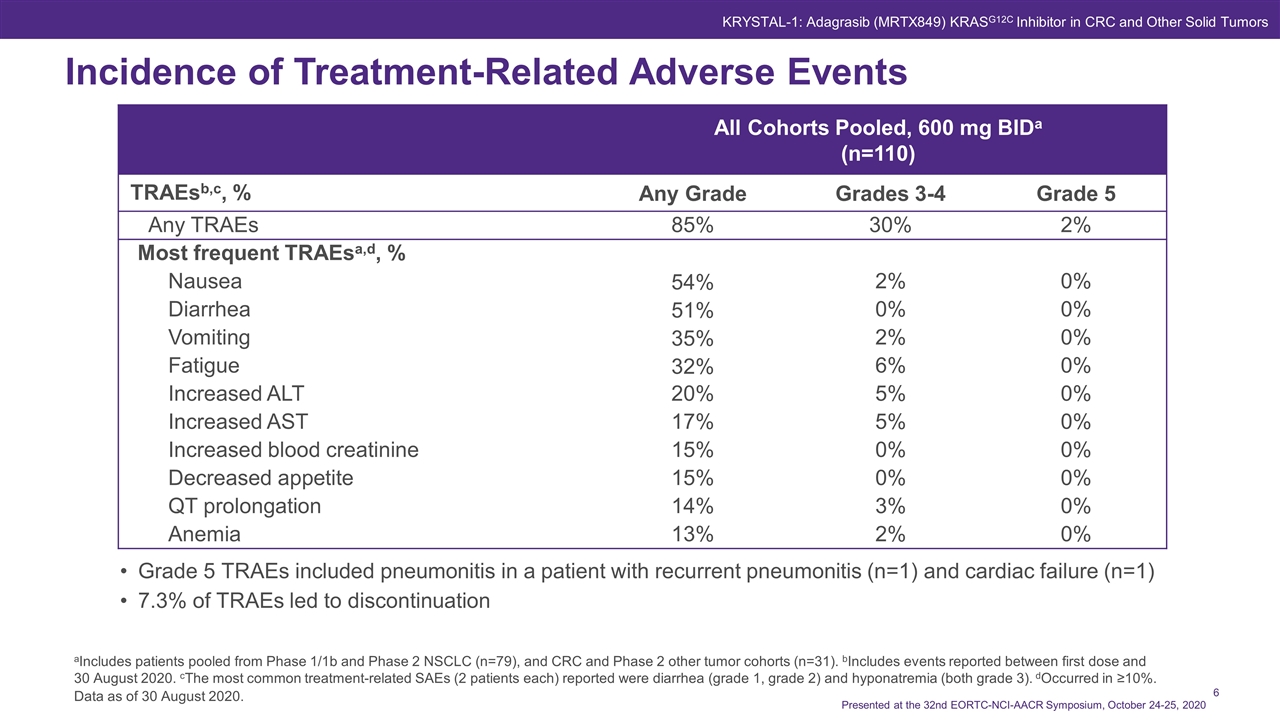
Data as of 30 August 2020. Incidence of Treatment-Related Adverse Events Presented at the 32nd EORTC-NCI-AACR Symposium, October 24-25, 2020 Grade 5 TRAEs included pneumonitis in a patient with recurrent pneumonitis (n=1) and cardiac failure (n=1) 7.3% of TRAEs led to discontinuation aIncludes patients pooled from Phase 1/1b and Phase 2 NSCLC (n=79), and CRC and Phase 2 other tumor cohorts (n=31). bIncludes events reported between first dose and 30 August 2020. cThe most common treatment-related SAEs (2 patients each) reported were diarrhea (grade 1, grade 2) and hyponatremia (both grade 3). dOccurred in ≥10%. All Cohorts Pooled, 600 mg BIDa (n=110) TRAEsb,c, % Any Grade Grades 3-4 Grade 5 Any TRAEs 85% 30% 2% Most frequent TRAEsa,d, % Nausea 54% 2% 0% Diarrhea 51% 0% 0% Vomiting 35% 2% 0% Fatigue 32% 6% 0% Increased ALT 20% 5% 0% Increased AST 17% 5% 0% Increased blood creatinine 15% 0% 0% Decreased appetite 15% 0% 0% QT prolongation 14% 3% 0% Anemia 13% 2% 0% KRYSTAL-1: Adagrasib (MRTX849) KRASG12C Inhibitor in CRC and Other Solid Tumors
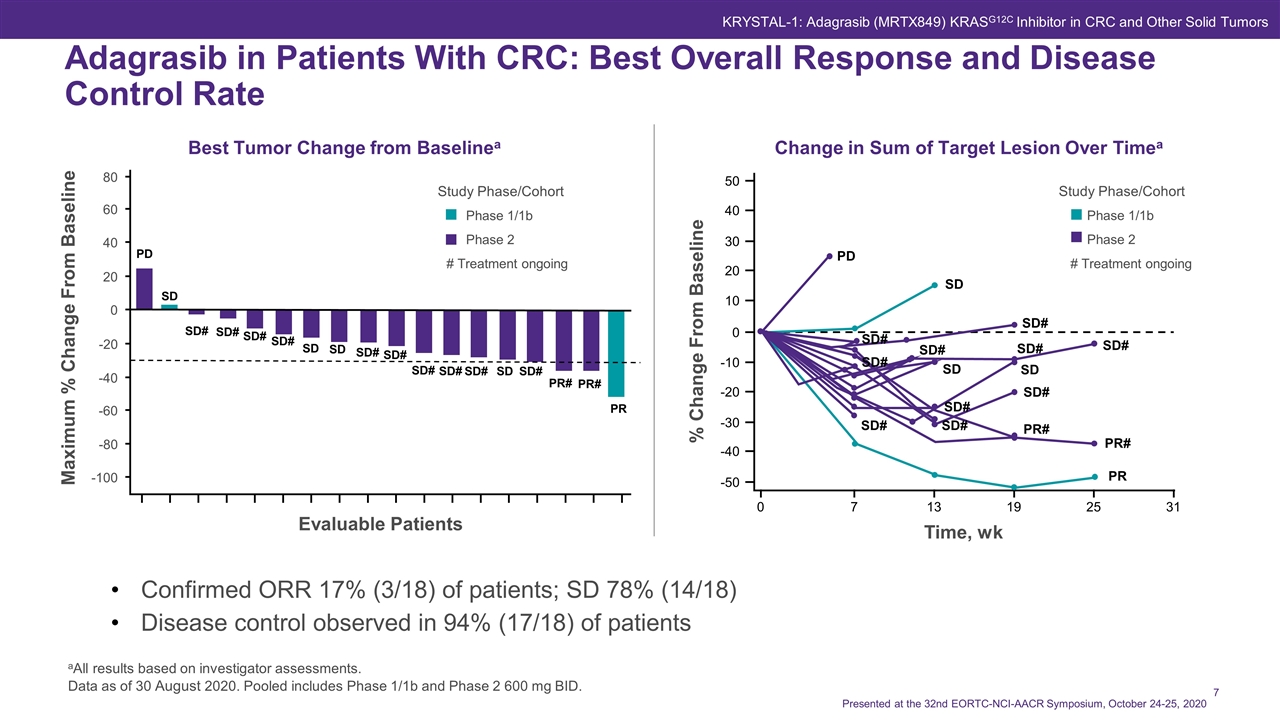
Confirmed ORR 17% (3/18) of patients; SD 78% (14/18) Disease control observed in 94% (17/18) of patients Best Tumor Change from Baselinea Adagrasib in Patients With CRC: Best Overall Response and Disease Control Rate aAll results based on investigator assessments. Data as of 30 August 2020. Pooled includes Phase 1/1b and Phase 2 600 mg BID. Change in Sum of Target Lesion Over Timea # Treatment ongoing % Change From Baseline Time, wk 50 40 30 20 0 -10 -20 -40 -50 -30 10 0 7 13 19 25 31 Study Phase/Cohort Phase 1/1b Phase 2 PD SD SD# SD# SD# SD SD# PR# PR# PR SD# SD# SD# SD# SD# SD# SD Study Phase/Cohort Phase 1/1b Phase 2 SD# SD# SD SD SD# SD# SD# SD# SD SD# PR# PR# PR PD SD Maximum % Change From Baseline Evaluable Patients 80 60 40 20 0 -20 -40 -60 -80 -100 SD# SD# # Treatment ongoing Presented at the 32nd EORTC-NCI-AACR Symposium, October 24-25, 2020 KRYSTAL-1: Adagrasib (MRTX849) KRASG12C Inhibitor in CRC and Other Solid Tumors SD#
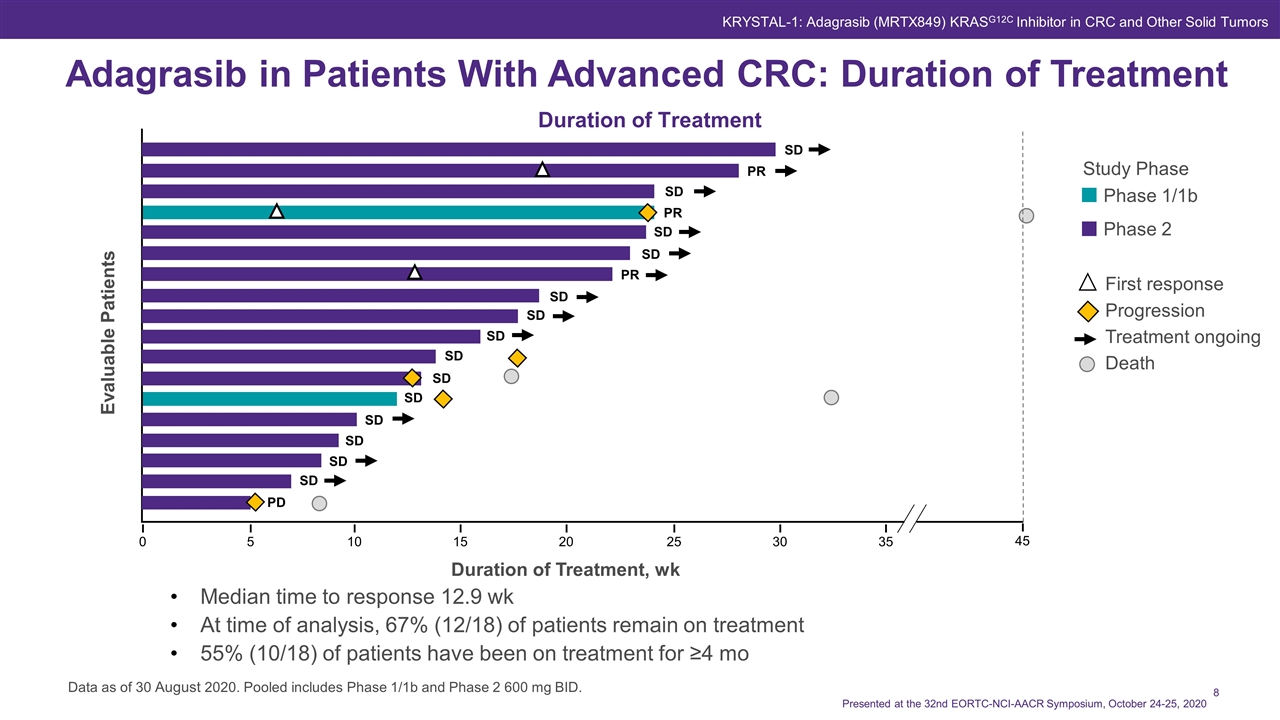
Adagrasib in Patients With Advanced CRC: Duration of Treatment Data as of 30 August 2020. Pooled includes Phase 1/1b and Phase 2 600 mg BID. Duration of Treatment Evaluable Patients 10 5 0 15 20 25 30 35 SD PR SD PR SD SD PR SD SD SD SD SD SD SD SD SD SD PD Duration of Treatment, wk Median time to response 12.9 wk At time of analysis, 67% (12/18) of patients remain on treatment 55% (10/18) of patients have been on treatment for ≥4 mo Study Phase Phase 1/1b Phase 2 First response Progression Treatment ongoing Death 45 Presented at the 32nd EORTC-NCI-AACR Symposium, October 24-25, 2020 KRYSTAL-1: Adagrasib (MRTX849) KRASG12C Inhibitor in CRC and Other Solid Tumors
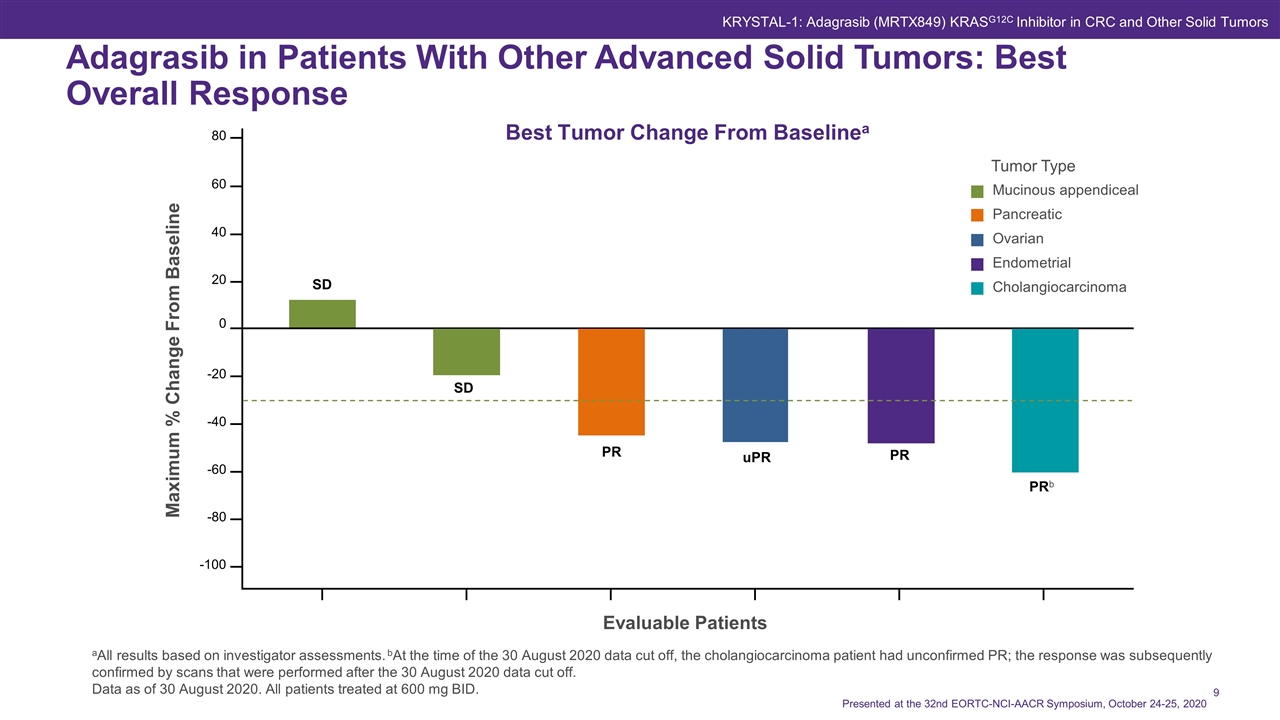
Best Tumor Change From Baselinea aAll results based on investigator assessments. bAt the time of the 30 August 2020 data cut off, the cholangiocarcinoma patient had unconfirmed PR; the response was subsequently confirmed by scans that were performed after the 30 August 2020 data cut off. Data as of 30 August 2020. All patients treated at 600 mg BID. Maximum % Change From Baseline Evaluable Patients 80 60 40 20 0 -20 -40 -60 -80 -100 SD SD PR uPR PRb PR Adagrasib in Patients With Other Advanced Solid Tumors: Best Overall Response Tumor Type Mucinous appendiceal Pancreatic Ovarian Endometrial Cholangiocarcinoma Presented at the 32nd EORTC-NCI-AACR Symposium, October 24-25, 2020 KRYSTAL-1: Adagrasib (MRTX849) KRASG12C Inhibitor in CRC and Other Solid Tumors
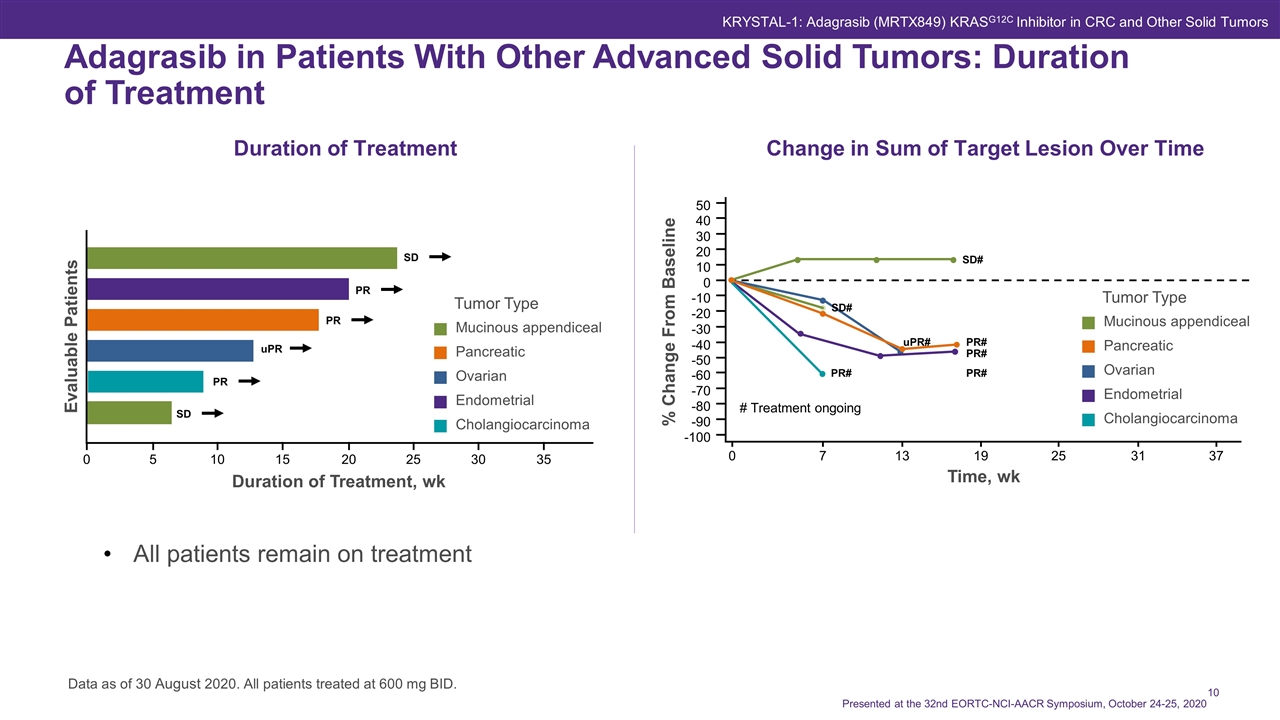
Duration of Treatment Change in Sum of Target Lesion Over Time All patients remain on treatment Data as of 30 August 2020. All patients treated at 600 mg BID. 10 5 0 15 20 25 30 35 Evaluable Patients Duration of Treatment, wk SD PR PR uPR PR SD % Change From Baseline Time, wk 40 20 50 -10 0 -50 -60 -90 -100 -80 -20 -70 -40 -30 10 30 0 7 13 19 25 31 37 # Treatment ongoing SD# SD# PR# PR# PR# PR# uPR# Adagrasib in Patients With Other Advanced Solid Tumors: Duration of Treatment Tumor Type Mucinous appendiceal Pancreatic Ovarian Endometrial Cholangiocarcinoma Tumor Type Mucinous appendiceal Pancreatic Ovarian Endometrial Cholangiocarcinoma KRYSTAL-1: Adagrasib (MRTX849) KRASG12C Inhibitor in CRC and Other Solid Tumors Presented at the 32nd EORTC-NCI-AACR Symposium, October 24-25, 2020
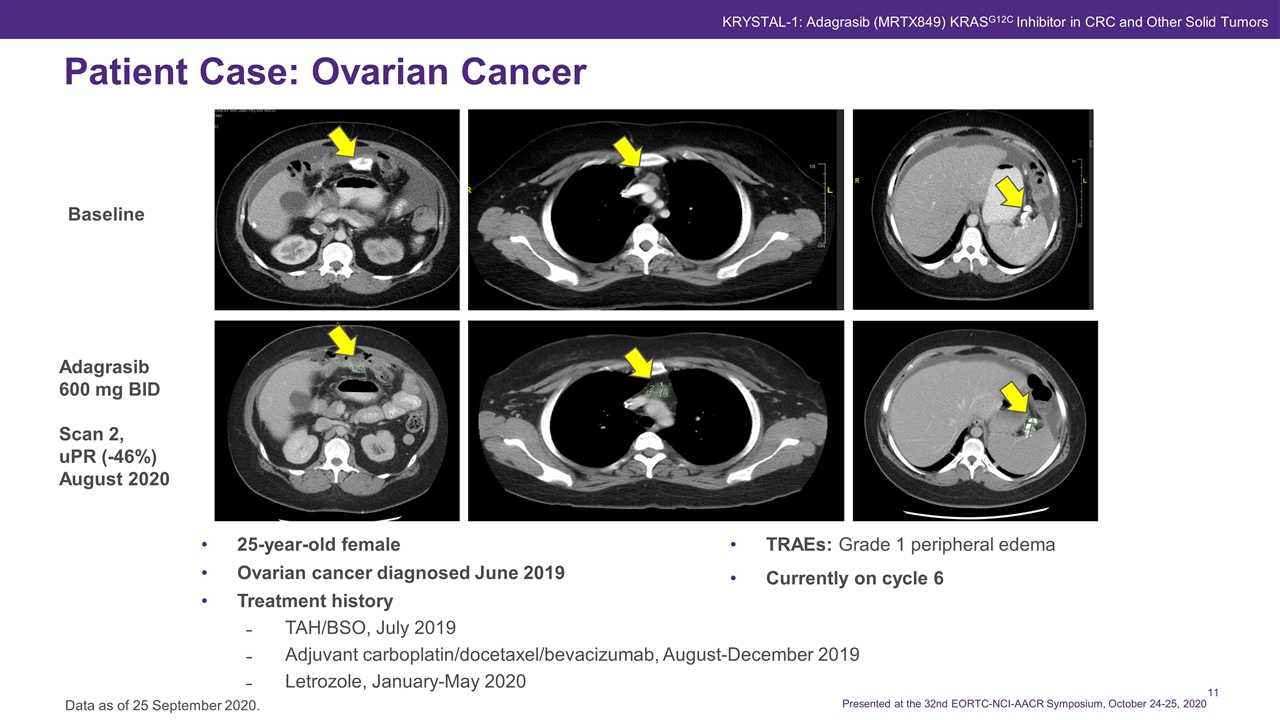
25-year-old female Ovarian cancer diagnosed June 2019 Treatment history TAH/BSO, July 2019 Adjuvant carboplatin/docetaxel/bevacizumab, August-December 2019 Letrozole, January-May 2020 Patient Case: Ovarian Cancer Data as of 25 September 2020. KRYSTAL-1: Adagrasib (MRTX849) KRASG12C Inhibitor in CRC and Other Solid Tumors Adagrasib 600 mg BID Scan 2, uPR (-46%) August 2020 Baseline TRAEs: Grade 1 peripheral edema Currently on cycle 6 Presented at the 32nd EORTC-NCI-AACR Symposium, October 24-25, 2020
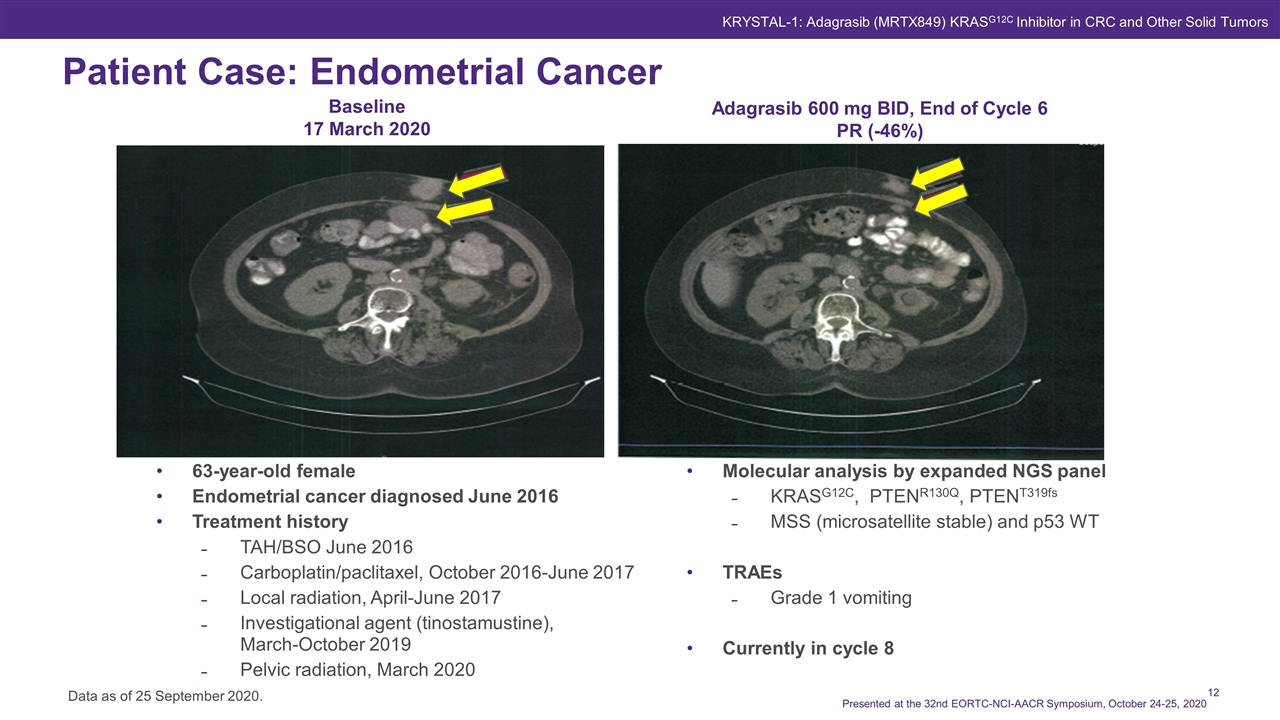
63-year-old female Endometrial cancer diagnosed June 2016 Treatment history TAH/BSO June 2016 Carboplatin/paclitaxel, October 2016-June 2017 Local radiation, April-June 2017 Investigational agent (tinostamustine), March-October 2019 Pelvic radiation, March 2020 Molecular analysis by expanded NGS panel KRASG12C, PTENR130Q, PTENT319fs MSS (microsatellite stable) and p53 WT TRAEs Grade 1 vomiting Currently in cycle 8 Patient Case: Endometrial Cancer Data as of 25 September 2020. Presented at the 32nd EORTC-NCI-AACR Symposium, October 24-25, 2020 KRYSTAL-1: Adagrasib (MRTX849) KRASG12C Inhibitor in CRC and Other Solid Tumors Baseline 17 March 2020 Adagrasib 600 mg BID, End of Cycle 6 PR (-46%)
Adagrasib is a KRASG12C-selective covalent inhibitor with a long half-life, and extensive predicted target coverage throughout the dosing interval Adagrasib is well tolerated Adagrasib provides durable benefit to patients with CRC harboring KRASG12C mutations Durable responses were observed Broad disease control rate was observed Adagrasib demonstrated clinical activity in various KRASG12C-mutated solid tumors, including pancreatic, ovarian, and endometrial cancers, and cholangiocarcinoma Enrollment in the CRC and other solid tumor monotherapy Phase 2 cohorts is ongoing Evaluation of adagrasib in combination with cetuximab (CRC) at full dose of each agent is ongoinga; a Phase 3 trial of adagrasib in combination with cetuximab is planned Conclusions Durable responses observed in NSCLC (n=23/51; 45%); See Jänne PA et al., abstract LBA-03. Presented at the 32nd EORTC-NCI-AACR Symposium, October 24-25, 2020 KRYSTAL-1: Adagrasib (MRTX849) KRASG12C Inhibitor in CRC and Other Solid Tumors aClinicalTrials.gov. NCT03785249

The patients and their families who make this trial possible The clinical study teams for their work and contributions This study is supported by Mirati Therapeutics, Inc. All authors contributed to and approved this presentation; writing and editorial assistance were provided by Rohan Gidvani of Axiom Healthcare Strategies, funded by Mirati Therapeutics, Inc. Acknowledgements Presented at the 32nd EORTC-NCI-AACR Symposium, October 24-25, 2020 KRYSTAL-1: Adagrasib (MRTX849) KRASG12C Inhibitor in CRC and Other Solid Tumors

Investigators Presented at the 32nd EORTC-NCI-AACR Symposium, October 24-25, 2020 KRYSTAL-1: Adagrasib (MRTX849) KRASG12C Inhibitor in CRC and Other Solid Tumors Harshad Amin Boca Raton Clinical Research Global USA Daniel Anderson Metro-Minnesota Community Oncology Research Consortium Minal Barve Mary Crowley Cancer Center Bruno R. Bastos Miami Cancer Institute and Baptist Health of South Florida Lyudmila Bazhenova Moores Cancer Center, University of California San Diego Tanios Bekaii-Saab Mayo Clinic David Berz Beverly Hills Cancer Center Alberto Bessudo California Cancer Associates for Research and Excellence Alejandro Calvo Kettering Cancer Center Patrick Cobb Sisters of Charity of Leavenworth Health St. Mary’s Mike Cusnir Mount Sinai Comprehensive Cancer Center Keith Eaton Seattle Cancer Care Alliance Yousuf Gaffar Maryland Oncology Hematology Navid Hafez Yale Cancer Center David Hakimian Illinois Cancer Specialists Rebecca S. Heist Massachusetts General Hospital Pasi A Jänne Dana-Farber Cancer Institute Melissa L. Johnson Sarah Cannon Research Institute Tennessee Oncology Han Koh Kaiser Permanente Scott Kruger Virginia Oncology Associates Timothy Larson Minnesota Oncology Ticiana A. Leal University of Wisconsin Carbone Cancer Center Konstantinos Leventakos Mayo Clinic Yanyan Lou Mayo Clinic Steven McCune Northwest Georgia Oncology Centers Jamal Misleh Medical Oncology Hematology Consultants Suresh Nair Lehigh Valley Physician Group Sujatha Nallapareddy Rocky Mountain Cancer Center Marcelo Negrao MD Anderson Cancer Center Gregg Newman Ridley-Tree Cancer Center Sai-Hong Ignatius Ou University of California, Irvine, Chao Family Comprehensive Cancer Center Rami Owera Woodlands Medical Specialists Jose M. Pacheco University of Colorado Anschutz Medical Campus Kyriakos P. Papadopoulos START Center for Cancer Care David Park Virginia K. Crosson Cancer Center Scott Paulson Texas Oncology, USOR Nathan Pennell Cleveland Clinic Lerner College Of Medicine Muhammad Riaz University of Cincinnati Health Barrett Cancer Center Donald Richards Texas Oncology, USOR Gregory J. Riely MSKCC, Weill Cornell Medical College Francisco Robert University of Alabama at Birmingham School of Medicine Richard Rosenberg Arizona Oncology Peter Rubin MaineHealth Cancer Care Robert Ruxer Texas Oncology Igor I. Rybkin Henry Ford Cancer Institute Joshua Sabari New York University Langone Health, New York University Perlmutter Cancer Center Alexander I. Spira Virginia Cancer Specialists, US Oncology Research Caesar Tin-U Texas Oncology Anthony Van Ho Compass Oncology Jared Weiss Lineberger Comprehensive Cancer Center, University of North Carolina John Wrangle Medical University of South Carolina Edwin Yau Roswell Park Comprehensive Cancer Center Jeffrey Yorio Texas Oncology Jun Zhang University of Kansas Medical Center
ALT = alanine aminotransferase AST = aspartate aminotransferase BID = twice daily CRC = colorectal cancer DCR = disease control rate DOR = duration of response ECOG = Eastern Cooperative Oncology Group MTD = maximum tolerated dose nM = nanomolar NSCLC = non–small-cell lung cancer ORR = objective response rate OS = overall survival PD = progressive disease Abbreviations PFS = progression-free survival PK = pharmacokinetics PR = partial response PS = performance status QD = once daily RP2D = recommended Phase 2 dose SAE = serious adverse event SD = stable disease TAH/BSO = total abdominal hysterectomy with bilateral salpingo-oophorectomy TRAE = treatment-related adverse event uPR = unconfirmed partial response KRYSTAL-1: Adagrasib (MRTX849) KRASG12C Inhibitor in CRC and Other Solid Tumors Presented at the 32nd EORTC-NCI-AACR Symposium, October 24-25, 2020















