| Item 7.01 | Regulation FD Disclosure. |
On July 16, 2023, the Acumen Pharmaceuticals, Inc. (“Acumen” or the “Company”) issued a press release relating to the topline results from the Phase 1 INTERCEPT-AD trial of ACU193. A copy of this press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K (this “Report”) and is incorporated by reference.
Also on July 16, 2023, the Company posted an investor presentation relating to the topline results from the Phase 1 INTERCEPT-AD trial of ACU193 to its website at https://investors.acumenpharm.com/news-events/presentations, which the Company will use in connection with an investor update call on July 17, 2023 at 8:00 AM EDT. A copy of the investor presentation is attached as Exhibit 99.2 to this Report.
The information in this Item 7.01 of this Report (including Exhibit 99.1 and Exhibit 99.2), is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that Section, nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act, except as expressly set forth by specific reference in such filing. The Company’s submission of this Report shall not be deemed an admission as to the materiality of any information required to be disclosed solely to satisfy the requirements of Regulation FD.
On July 16, 2023, the Company presented topline results from the Phase 1 INTERCEPT-AD trial of ACU193 at the Alzheimer’s Association International Conference (AAIC®) 2023, taking place in Amsterdam and online from July 16-20, 2023.
Topline results demonstrated that ACU193 met the primary objective of the Phase 1, first-in-human, randomized, double-blind, placebo-controlled study in both single and multiple doses in 60 participants with early Alzheimer’s disease (“AD”). Dose levels were 2, 10, 25 and 60 mg/kg for one to three doses administered intravenously. An analysis of change in amyloid plaque load, as measured by positron emission tomography (“PET”) SUVr, demonstrated a rapid, dose-related mean decrease at the higher dose levels studied (60 mg/kg every 4 weeks [Q4W] and 25 mg/kg every 2 weeks [Q2W]). The overall rate of amyloid related imaging abnormalities – edema (“ARIA-E”) was 10.4%, which included one case of symptomatic ARIA-E (2.1%). Pharmacokinetic (“PK”) results in serum and cerebrospinal fluid (“CSF”) demonstrated statistically significant dose proportionality and support monthly dosing of ACU193.
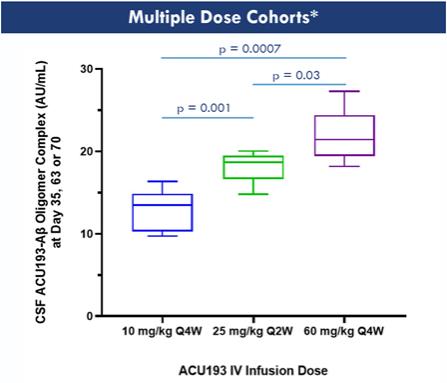
Statistically significant, dose-related central target engagement was observed as measured by ACU193-AßO complex, establishing the first target engagement assay developed that is specific to an AßO-targeting antibody. An exposure response relationship (Emax) model revealed near maximal target engagement with repeated dosing at 25 mg/kg and 60 mg/kg.
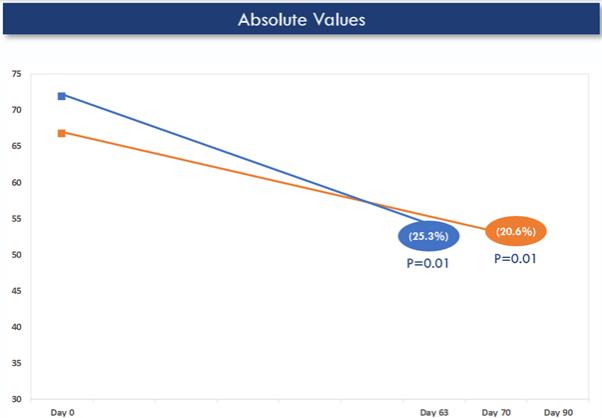
Higher doses of ACU193 (60 mg/kg Q4W and 25 mg/kg Q2W) showed a statistically significant reduction in amyloid plaque load as determined by amyloid PET after 6-12 weeks (from baseline to endpoint within cohorts (p = 0.01)). This finding provides evidence that ACU193 is active in the brain.
Mean Reduction in Amyloid Plaque (Centiloids)