
Investor Conference Call to Discuss INTERCEPT-AD Results July 17, 2023 Exhibit 99.2

Forward-Looking Statements This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Any statement describing Acumen’s goals, expectations, financial or other projections, intentions or beliefs is a forward-looking statement and should be considered an at-risk statement. Words such as “believes,” “expects,” “anticipates,” “could,” “should,” “would,” “seeks,” “aims,” “plans,” “potential,” “will,” “milestone” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Forward-looking statements include statements concerning: Acumen’s business; the safety, tolerability, pharmacokinetics, target engagement and other clinical measures associated with Acumen’s product candidate, ACU193, including its performance against other antibodies; and the anticipated timeline for reporting topline data. These statements are based upon the current beliefs and expectations of Acumen management, and are subject to certain factors, risks and uncertainties, particularly those inherent in the process of discovering, developing and commercializing safe and effective human therapeutics. Such risks may be amplified by the impacts of geopolitical events and macroeconomic conditions, such as rising inflation and interest rates, supply disruptions and uncertainty of credit and financial markets. These and other risks concerning Acumen’s programs are described in additional detail in Acumen’s filings with the Securities and Exchange Commission (“SEC”), including in Acumen’s most recent Annual Report on Form 10-K, and in subsequent filings with the SEC, including Acumen’s most recent Quarterly Report on Form 10-Q. Copies of these and other documents are available from Acumen. Additional information will be made available in other filings that Acumen makes from time to time with the SEC. These forward-looking statements speak only as of the date hereof, and Acumen expressly disclaims any obligation to update or revise any forward-looking statement, except as otherwise required by law, whether, as a result of new information, future events or otherwise.

Agenda Introduction Alex Braun, Head of Investor Relations ACU193 & INTERCEPT-AD Topline Results Dan O’Connell, Chief Executive Officer Dr. Eric Siemers, Chief Medical Officer Topline Results Q&A Dr. Eric Siemers, Chief Medical Officer Dr. Steven DeKosky, Deputy Director of the McKnight Brain Institute at the University of Florida and member of Acumen’s scientific advisory board Dr. Lawrence Honig, Director of the New York State Center of Excellence for Alzheimer's Disease at Columbia University and INTERCEPT-AD trial investigator Open Q&A Dan O’Connell, Chief Executive Officer Dr. Eric Siemers, Chief Medical Officer Matt Zuga, Chief Business Officer & Chief Financial Officer Dr. Steven DeKosky Dr. Lawrence Honig

ACU193: Novel mAb Targeting Amyloid Beta Oligomers (AβOs), the Most Toxic Form of Aβ
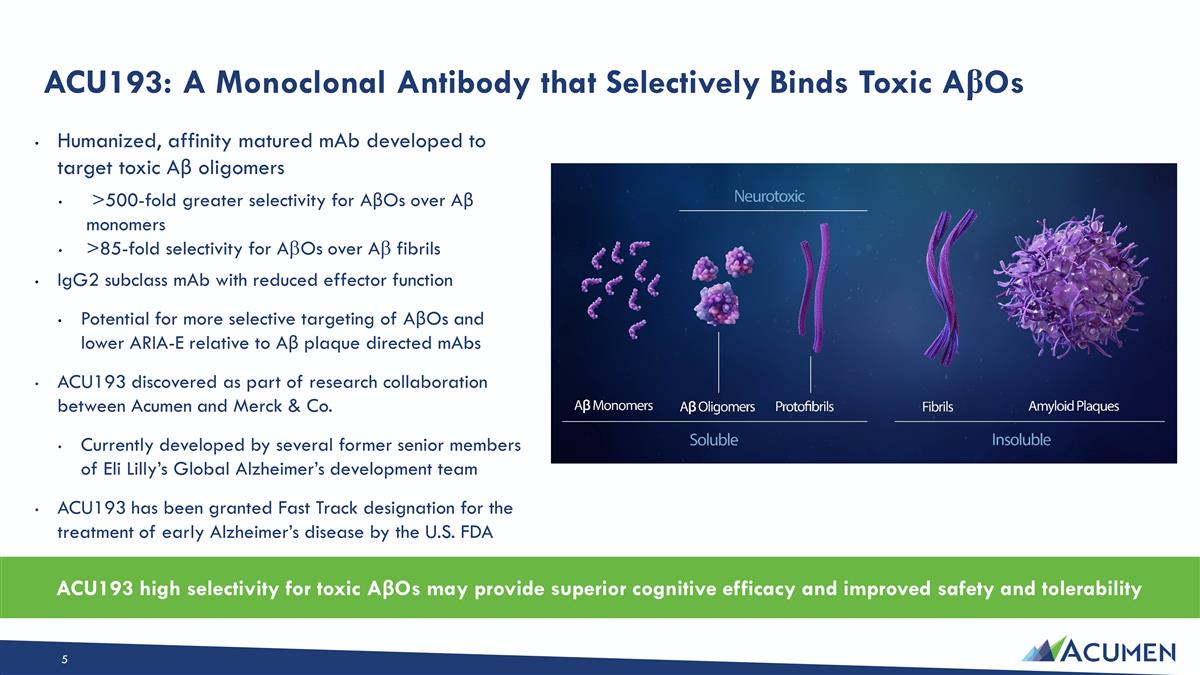
Humanized, affinity matured mAb developed to target toxic Aβ oligomers >500-fold greater selectivity for AβOs over Aβ monomers >85-fold selectivity for AbOs over Ab fibrils IgG2 subclass mAb with reduced effector function Potential for more selective targeting of AβOs and lower ARIA-E relative to Aβ plaque directed mAbs ACU193 discovered as part of research collaboration between Acumen and Merck & Co. Currently developed by several former senior members of Eli Lilly’s Global Alzheimer’s development team ACU193 has been granted Fast Track designation for the treatment of early Alzheimer’s disease by the U.S. FDA ACU193: A Monoclonal Antibody that Selectively Binds Toxic AβOs ACU193 high selectivity for toxic AβOs may provide superior cognitive efficacy and improved safety and tolerability
Rapid, dose-related, statistically significant amyloid plaque reduction observed at higher doses studied (60 mg/kg Q4W and 25 mg/kg Q2W cohorts)* at 6-12 weeks Comparable to currently approved Aβ monoclonal antibodies at similar time points in their clinical development First antibody to demonstrate target engagement of Aβ oligomers, the most toxic form of amyloid beta, in a dose-related manner Serum and CSF exposure are dose proportional; antibody concentrations significantly exceeded levels of endogenous oligomers Highest doses studied (25 mg/kg Q2W and 60 mg/kg Q4W) approached maximal target engagement (23.2 AU/mL Emax) Compelling overall safety profile, with low rate of ARIA-E No known drug-related SAEs; treatment emergent ADAs consistently low titer No cases of symptomatic ARIA-E at 10 mg/kg Q4W and 25 mg/kg Q2W doses No ARIA-E observed in ApoE4 homozygotes (n=6) Broad therapeutic index; clear path to more convenient monthly dosing regimen 1 2 3 4 *Statistically significant reduction from baseline to endpoint within cohorts (p = 0.01) INTERCEPT-AD Results Confirm Proof of Mechanism for ACU193 and Demonstrate Reduction in Amyloid Plaques at Higher Doses Studied
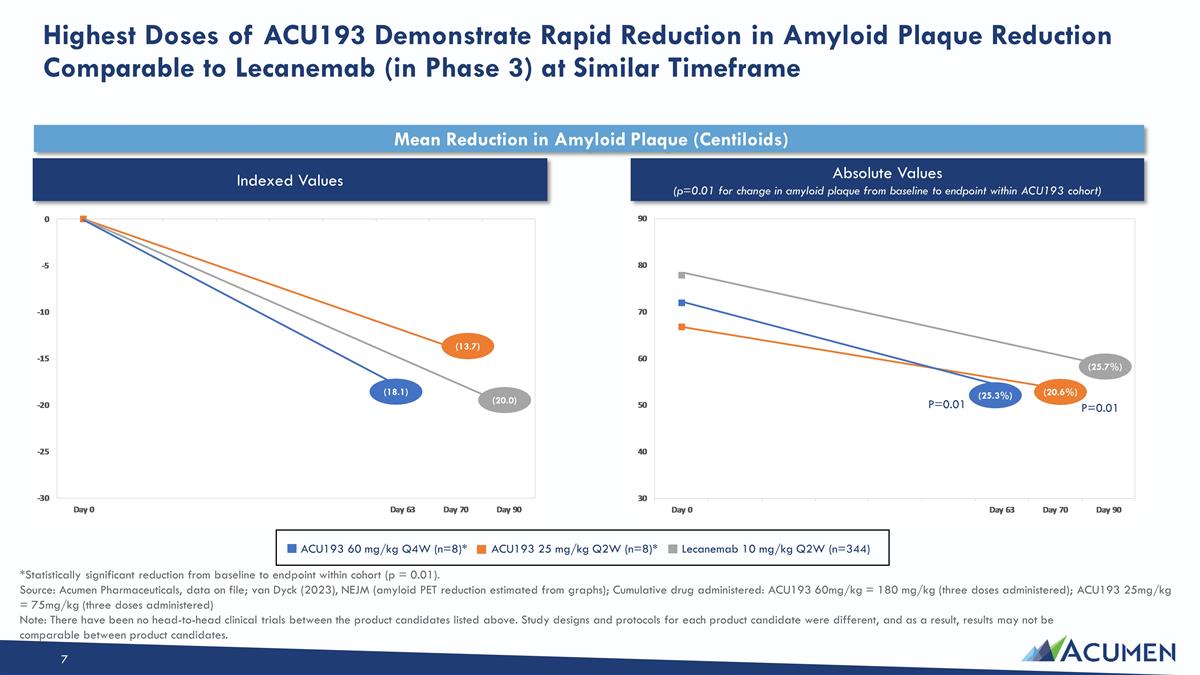
Highest Doses of ACU193 Demonstrate Rapid Reduction in Amyloid Plaque Reduction Comparable to Lecanemab (in Phase 3) at Similar Timeframe Mean Reduction in Amyloid Plaque (Centiloids) ACU193 60 mg/kg Q4W (n=8)* ACU193 25 mg/kg Q2W (n=8)* Lecanemab 10 mg/kg Q2W (n=344) Indexed Values Absolute Values (p=0.01 for change in amyloid plaque from baseline to endpoint within ACU193 cohort) (25.3%) (20.6%) (25.7%) (18.1) (13.7) (20.0) P=0.01 P=0.01 *Statistically significant reduction from baseline to endpoint within cohort (p = 0.01). Source: Acumen Pharmaceuticals, data on file; van Dyck (2023), NEJM (amyloid PET reduction estimated from graphs); Cumulative drug administered: ACU193 60mg/kg = 180 mg/kg (three doses administered); ACU193 25mg/kg = 75mg/kg (three doses administered) Note: There have been no head-to-head clinical trials between the product candidates listed above. Study designs and protocols for each product candidate were different, and as a result, results may not be comparable between product candidates.
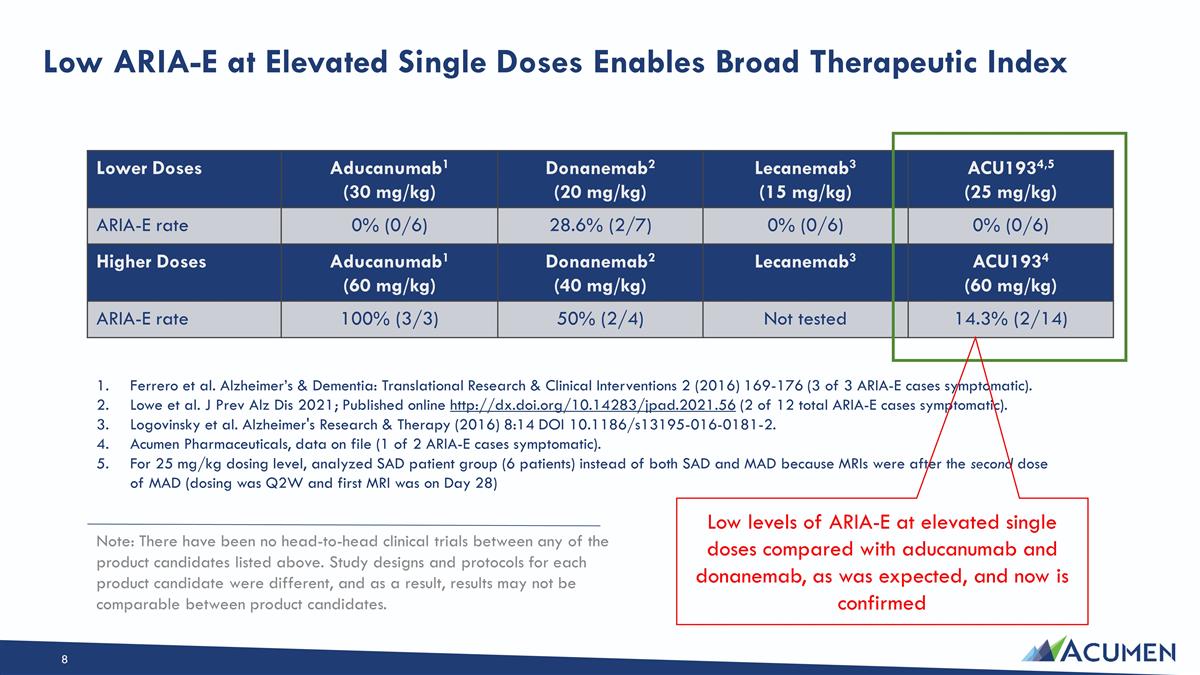
Low ARIA-E at Elevated Single Doses Enables Broad Therapeutic Index Lower Doses Aducanumab1 (30 mg/kg) Donanemab2 (20 mg/kg) Lecanemab3 (15 mg/kg) ACU1934,5 (25 mg/kg) ARIA-E rate 0% (0/6) 28.6% (2/7) 0% (0/6) 0% (0/6) Higher doses Ferrero et al. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 2 (2016) 169-176 (3 of 3 ARIA-E cases symptomatic). Lowe et al. J Prev Alz Dis 2021; Published online http://dx.doi.org/10.14283/jpad.2021.56 (2 of 12 total ARIA-E cases symptomatic). Logovinsky et al. Alzheimer's Research & Therapy (2016) 8:14 DOI 10.1186/s13195-016-0181-2. Acumen Pharmaceuticals, data on file (1 of 2 ARIA-E cases symptomatic). For 25 mg/kg dosing level, analyzed SAD patient group (6 patients) instead of both SAD and MAD because MRIs were after the second dose of MAD (dosing was Q2W and first MRI was on Day 28) Higher Doses Aducanumab1 (60 mg/kg) Donanemab2 (40 mg/kg) Lecanemab3 ACU1934 (60 mg/kg) ARIA-E rate 100% (3/3) 50% (2/4) Not tested 14.3% (2/14) Low levels of ARIA-E at elevated single doses compared with aducanumab and donanemab, as was expected, and now is confirmed Note: There have been no head-to-head clinical trials between any of the product candidates listed above. Study designs and protocols for each product candidate were different, and as a result, results may not be comparable between product candidates.
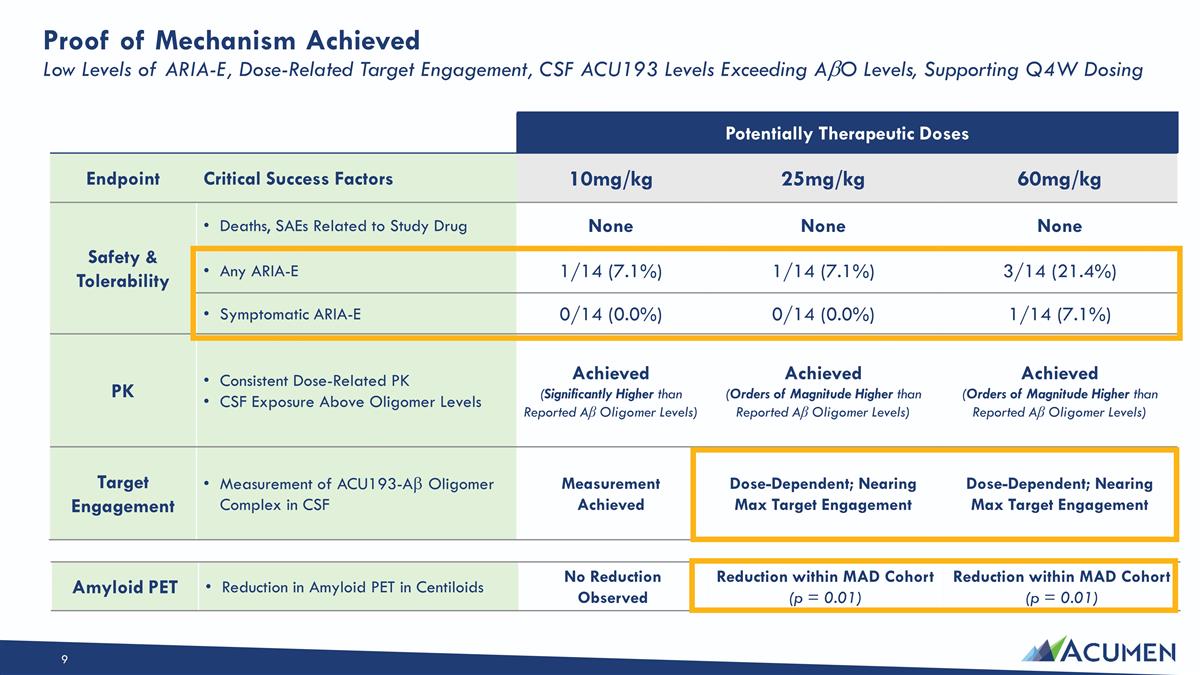
Proof of Mechanism Achieved Low Levels of ARIA-E, Dose-Related Target Engagement, CSF ACU193 Levels Exceeding AbO Levels, Supporting Q4W Dosing Potentially Therapeutic Doses Endpoint Critical Success Factors 10mg/kg 25mg/kg 60mg/kg Safety & Tolerability Deaths, SAEs Related to Study Drug None None None Any ARIA-E 1/14 (7.1%) 1/14 (7.1%) 3/14 (21.4%) Symptomatic ARIA-E 0/14 (0.0%) 0/14 (0.0%) 1/14 (7.1%) PK Consistent Dose-Related PK CSF Exposure Above Oligomer Levels Achieved (Significantly Higher than Reported Aβ Oligomer Levels) Achieved (Orders of Magnitude Higher than Reported Aβ Oligomer Levels) Achieved (Orders of Magnitude Higher than Reported Aβ Oligomer Levels) Target Engagement Measurement of ACU193-Ab Oligomer Complex in CSF Measurement Achieved Dose-Dependent; Nearing Max Target Engagement Dose-Dependent; Nearing Max Target Engagement Amyloid PET Reduction in Amyloid PET in Centiloids No Reduction Observed Reduction within MAD Cohort (p = 0.01) Reduction within MAD Cohort (p = 0.01)
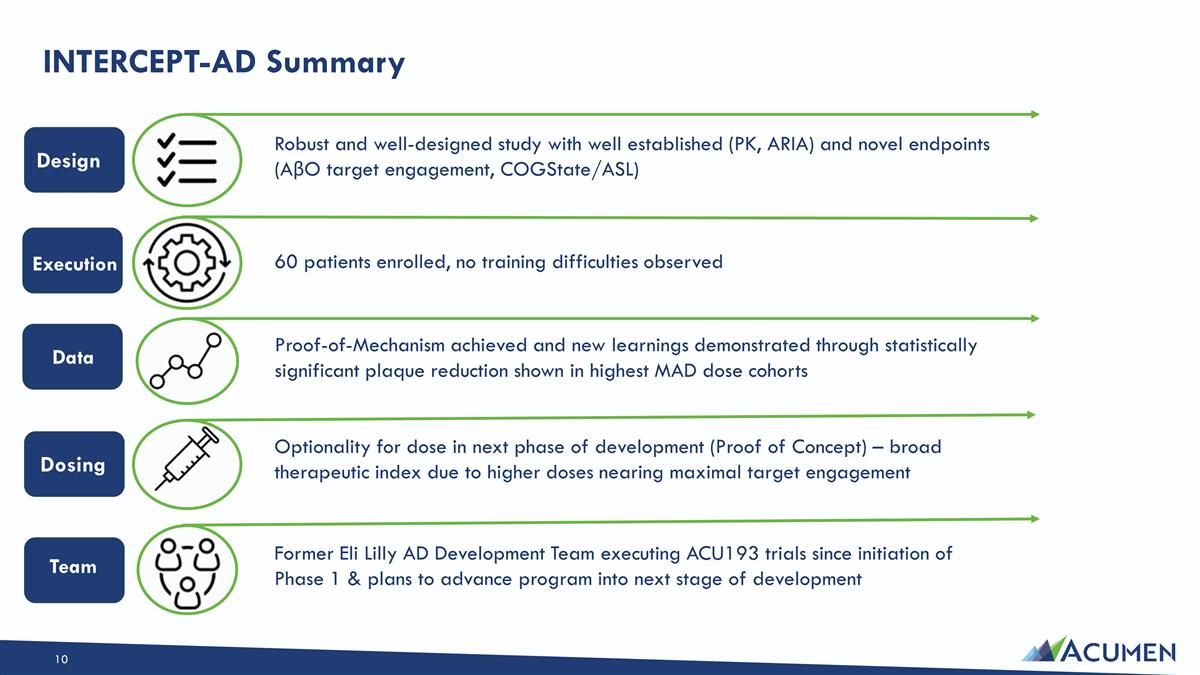
INTERCEPT-AD Summary Robust and well-designed study with well established (PK, ARIA) and novel endpoints (AβO target engagement, COGState/ASL) Execution 60 patients enrolled, no training difficulties observed Proof-of-Mechanism achieved and new learnings demonstrated through statistically significant plaque reduction shown in highest MAD dose cohorts Optionality for dose in next phase of development (Proof of Concept) – broad therapeutic index due to higher doses nearing maximal target engagement Former Eli Lilly AD Development Team executing ACU193 trials since initiation of Phase 1 & plans to advance program into next stage of development Design Dosing Data Team

INTERCEPT-AD Topline Results
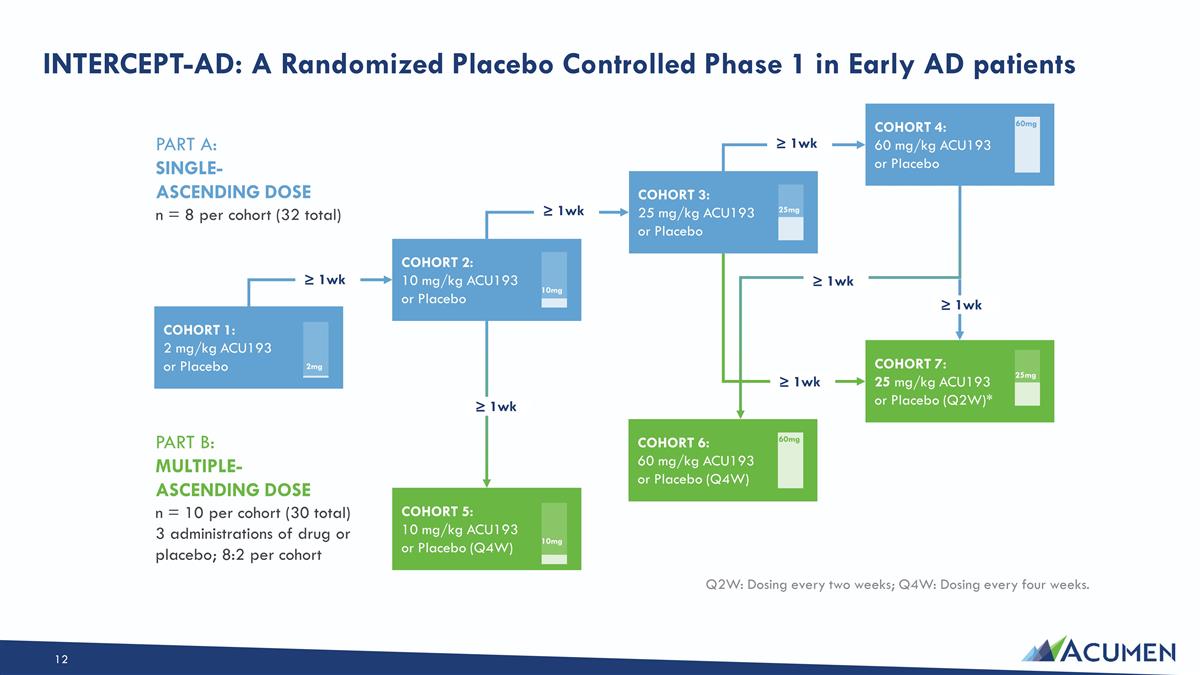
INTERCEPT-AD: A Randomized Placebo Controlled Phase 1 in Early AD patients Q2W: Dosing every two weeks; Q4W: Dosing every four weeks. PART A: SINGLE- ASCENDING DOSE n = 8 per cohort (32 total) PART B: MULTIPLE- ASCENDING DOSE n = 10 per cohort (30 total) 3 administrations of drug or placebo; 8:2 per cohort COHORT 1: 2 mg/kg ACU193 or Placebo 2mg COHORT 2: 10 mg/kg ACU193 or Placebo 10mg COHORT 3: 25 mg/kg ACU193 or Placebo 25mg COHORT 4: 60 mg/kg ACU193 or Placebo 60mg COHORT 5: 10 mg/kg ACU193 or Placebo (Q4W) 10mg COHORT 6: 60 mg/kg ACU193 or Placebo (Q4W) 60mg COHORT 7: 25 mg/kg ACU193 or Placebo (Q2W)* ≥ 1wk ≥ 1wk ≥ 1wk ≥ 1wk ≥ 1wk ≥ 1wk 25mg ≥ 1wk
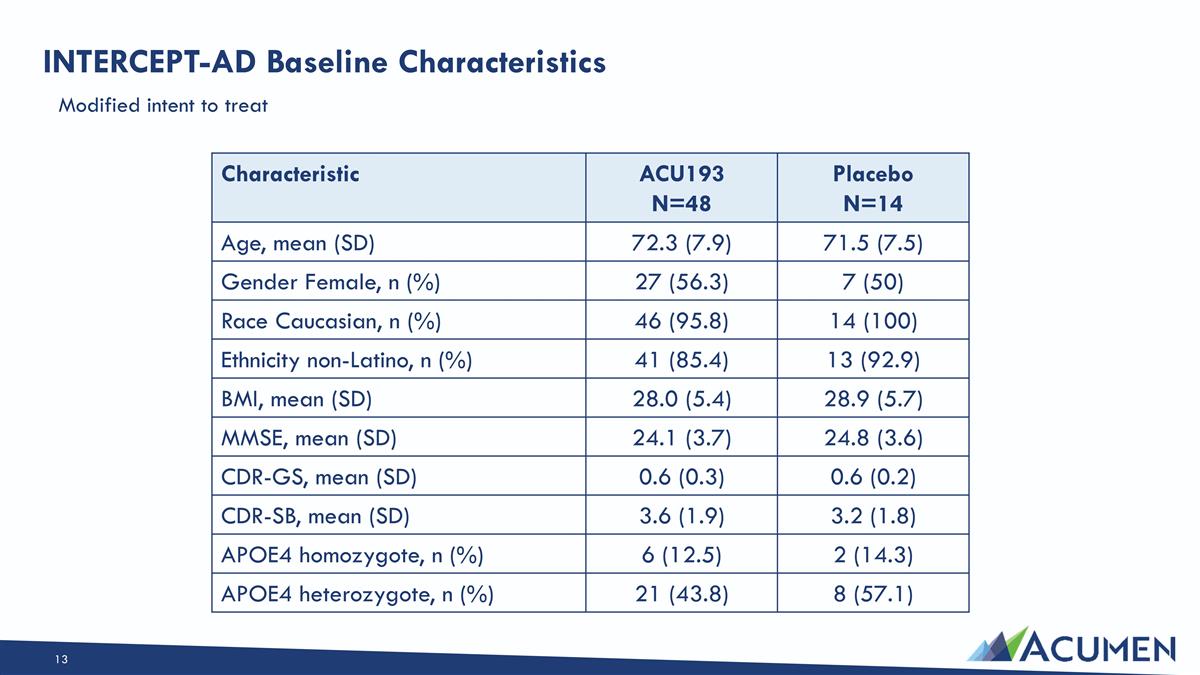
INTERCEPT-AD Baseline Characteristics Characteristic ACU193 N=48 Placebo N=14 Age, mean (SD) 72.3 (7.9) 71.5 (7.5) Gender Female, n (%) 27 (56.3) 7 (50) Race Caucasian, n (%) 46 (95.8) 14 (100) Ethnicity non-Latino, n (%) 41 (85.4) 13 (92.9) BMI, mean (SD) 28.0 (5.4) 28.9 (5.7) MMSE, mean (SD) 24.1 (3.7) 24.8 (3.6) CDR-GS, mean (SD) 0.6 (0.3) 0.6 (0.2) CDR-SB, mean (SD) 3.6 (1.9) 3.2 (1.8) APOE4 homozygote, n (%) 6 (12.5) 2 (14.3) APOE4 heterozygote, n (%) 21 (43.8) 8 (57.1) Modified intent to treat
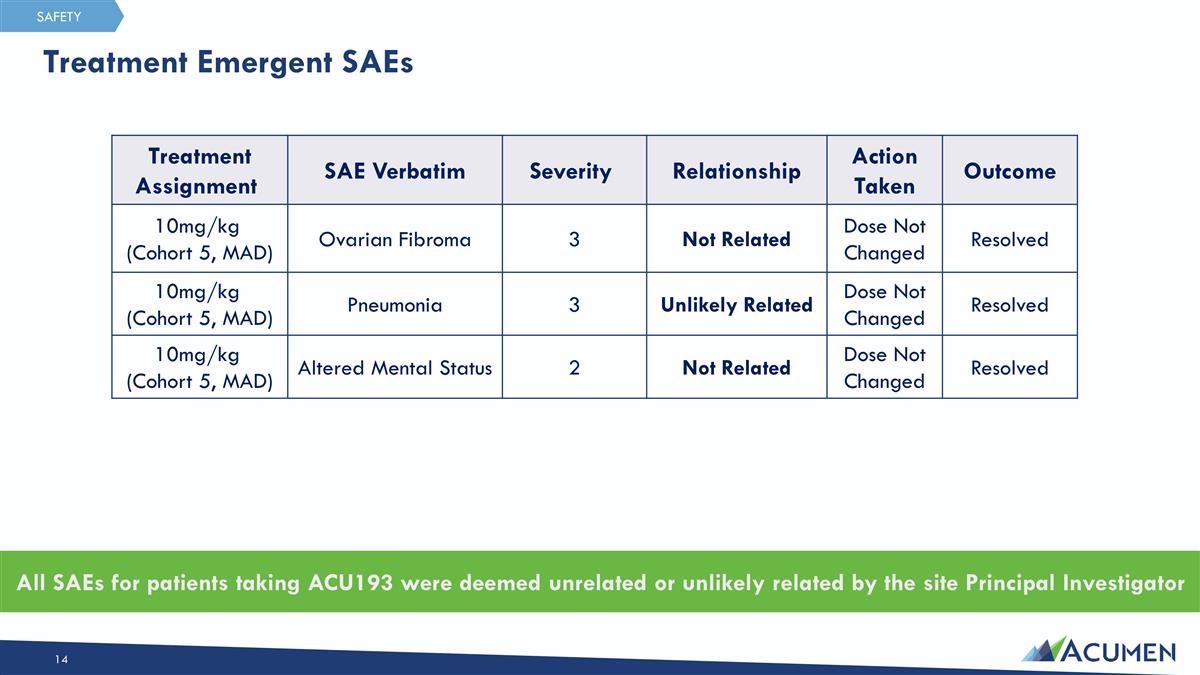
Treatment Emergent SAEs All SAEs for patients taking ACU193 were deemed unrelated or unlikely related by the site Principal Investigator Treatment Assignment SAE Verbatim Severity Relationship Action Taken Outcome 10mg/kg (Cohort 5, MAD) Ovarian Fibroma 3 Not Related Dose Not Changed Resolved 10mg/kg (Cohort 5, MAD) Pneumonia 3 Unlikely Related Dose Not Changed Resolved 10mg/kg (Cohort 5, MAD) Altered Mental Status 2 Not Related Dose Not Changed Resolved SAFETY

SAD MAD 10 mg/kg Cohorts 2, 5 25 mg/kg Cohorts 3, 7 60 mg/kg Cohorts 4, 6 NO ARIA-E Asymptomatic ARIA-E Symptomatic ARIA-E Discontinued ApoE D21 D140 3,4 PBO PBO 3,3 3,3 3,4 3,4 PBO PBO 3,4 3,4 3,4 ApoE D28 D63 D126 3,4 3,3 3,3 4,4 4,4 PBO PBO PBO 3,3 3,4 3,4 3,4 PBO PBO PBO 3,3 ApoE D28 D70 D196 2,3 3,3 3,3 4,4 3,3 PBO PBO PBO 3,4 4,4 3,4 3,3 3,4 PBO PBO PBO ApoE D21 D140 3,3 3,3 PBO PBO 4,4 3,3 2,4 3,3 PBO PBO 3,4 3,3 ApoE D28 D70 D98 3,3 3,4 3,4 3,4 3,4 3,4 PBO PBO PBO 3,3 3,4 PBO PBO PBO 4,4 4,4 ApoE D21 D140 4,4 PBO PBO 3,4 3,4 PBO PBO 3,3 3,3 3,4 2,4 3,4 2 mg/kg Cohort 1 ApoE D21 D140 3,4 3,3 PBO PBO 3,4 2,3 3,4 PBO PBO 3,3 3,3 3,3 ARIA-E Summary for INTERCEPT-AD No ε4 homozygotes developed ARIA-E despite comprising 13% in study; 4/5 ARIA-E cases are ε4 heterozygotes which comprise 47% of our study population PBO: Patient on placebo SAFETY
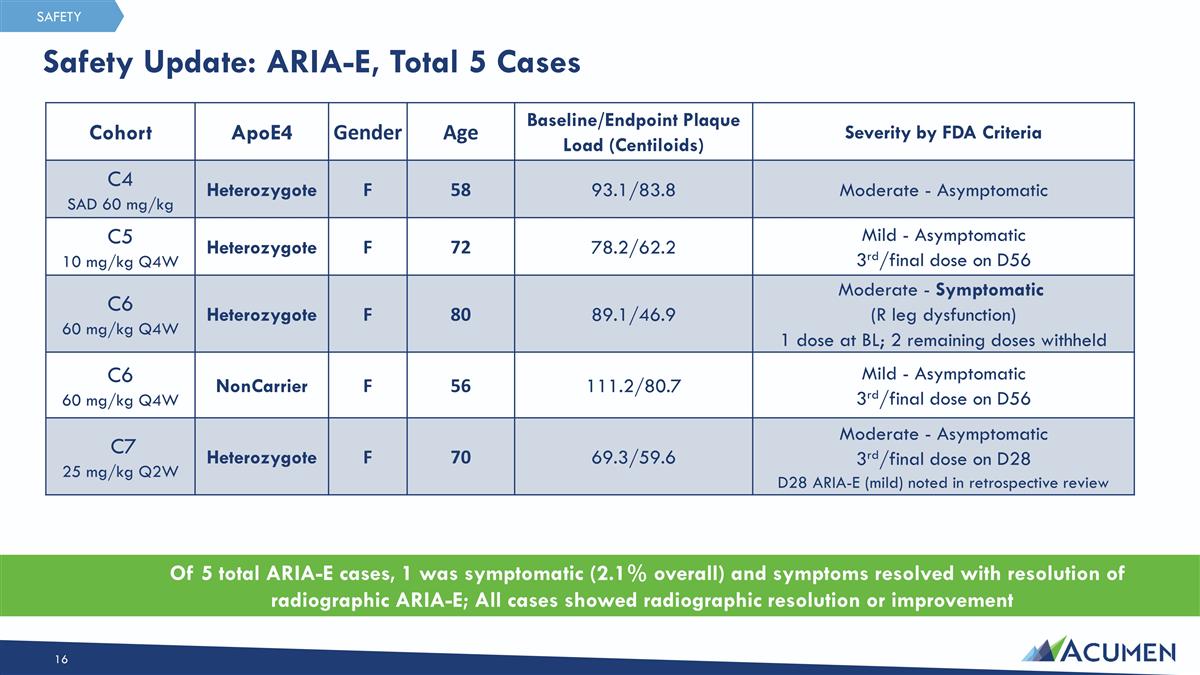
Safety Update: ARIA-E, Total 5 Cases Of 5 total ARIA-E cases, 1 was symptomatic (2.1% overall) and symptoms resolved with resolution of radiographic ARIA-E; All cases showed radiographic resolution or improvement SAFETY Cohort ApoE4 Gender Age Baseline/Endpoint Plaque Load (Centiloids) Severity by FDA Criteria C4 SAD 60 mg/kg Heterozygote F 58 93.1/83.8 Moderate - Asymptomatic C5 10 mg/kg Q4W Heterozygote F 72 78.2/62.2 Mild - Asymptomatic 3rd/final dose on D56 C6 60 mg/kg Q4W Heterozygote F 80 89.1/46.9 Moderate - Symptomatic (R leg dysfunction) 1 dose at BL; 2 remaining doses withheld C6 60 mg/kg Q4W NonCarrier F 56 111.2/80.7 Mild - Asymptomatic 3rd/final dose on D56 C7 25 mg/kg Q2W Heterozygote F 70 69.3/59.6 Moderate - Asymptomatic 3rd/final dose on D28 D28 ARIA-E (mild) noted in retrospective review
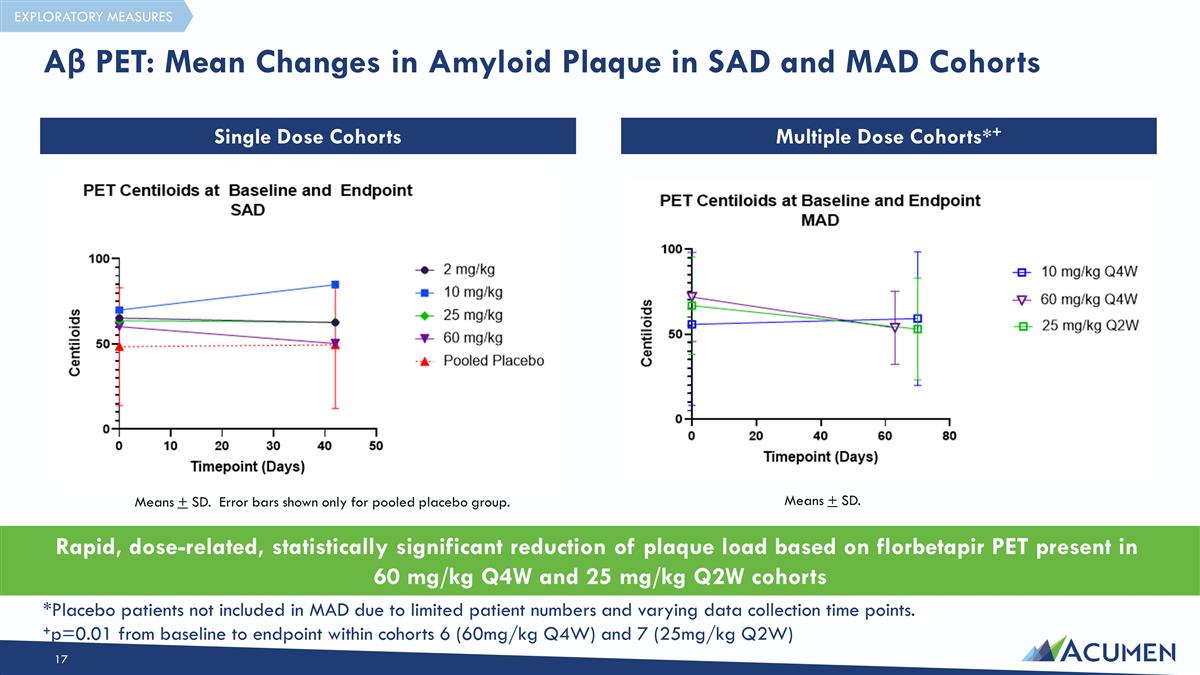
Rapid, dose-related, statistically significant reduction of plaque load based on florbetapir PET present in 60 mg/kg Q4W and 25 mg/kg Q2W cohorts Means + SD. Error bars shown only for pooled placebo group. Means + SD. *Placebo patients not included in MAD due to limited patient numbers and varying data collection time points. +p=0.01 from baseline to endpoint within cohorts 6 (60mg/kg Q4W) and 7 (25mg/kg Q2W) Aβ PET: Mean Changes in Amyloid Plaque in SAD and MAD Cohorts Single Dose Cohorts Multiple Dose Cohorts*+ EXPLORATORY MEASURES
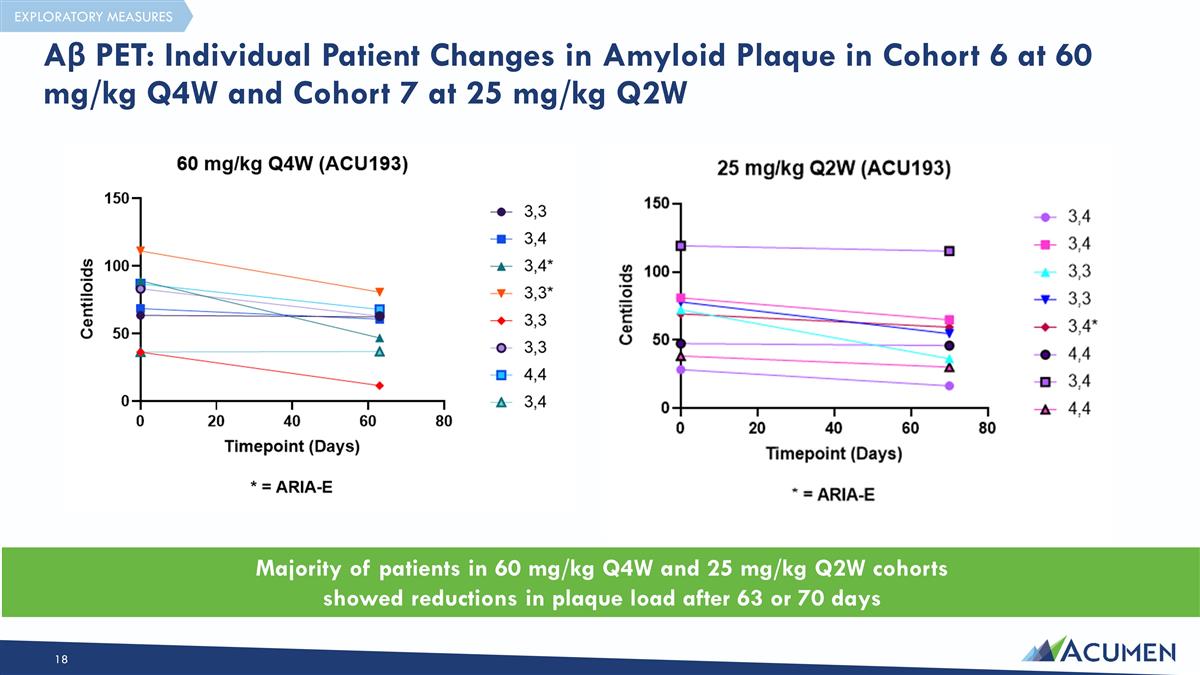
Aβ PET: Individual Patient Changes in Amyloid Plaque in Cohort 6 at 60 mg/kg Q4W and Cohort 7 at 25 mg/kg Q2W Majority of patients in 60 mg/kg Q4W and 25 mg/kg Q2W cohorts showed reductions in plaque load after 63 or 70 days EXPLORATORY MEASURES
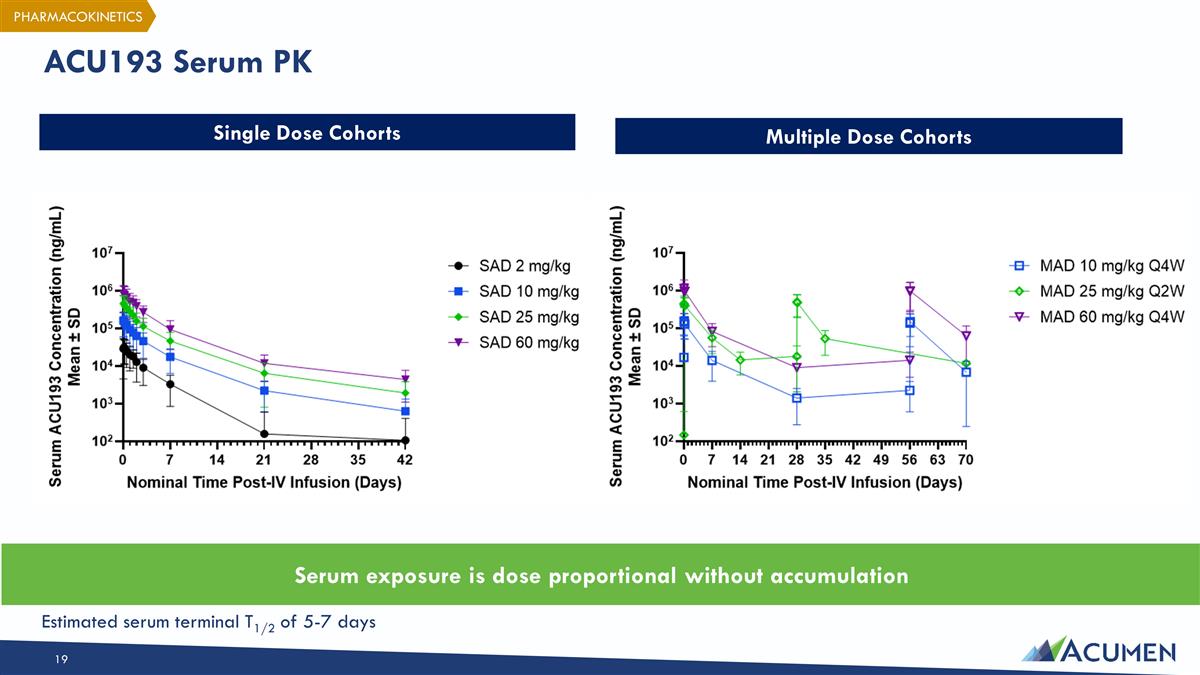
ACU193 Serum PK Single Dose Cohorts Multiple Dose Cohorts Serum exposure is dose proportional without accumulation PHARMACOKINETICS Estimated serum terminal T1/2 of 5-7 days
Immunogenicity (preliminary assessment) Evidence of low titer treatment emergent immunogenicity Some evidence of treatment emergent immunogenicity was observed Observations more common in the MAD cohorts vs. SAD cohorts Treatment emergent ADAs are consistently low titer Preliminary assessment reveals no apparent effect on serum PK PHARMACOKINETICS
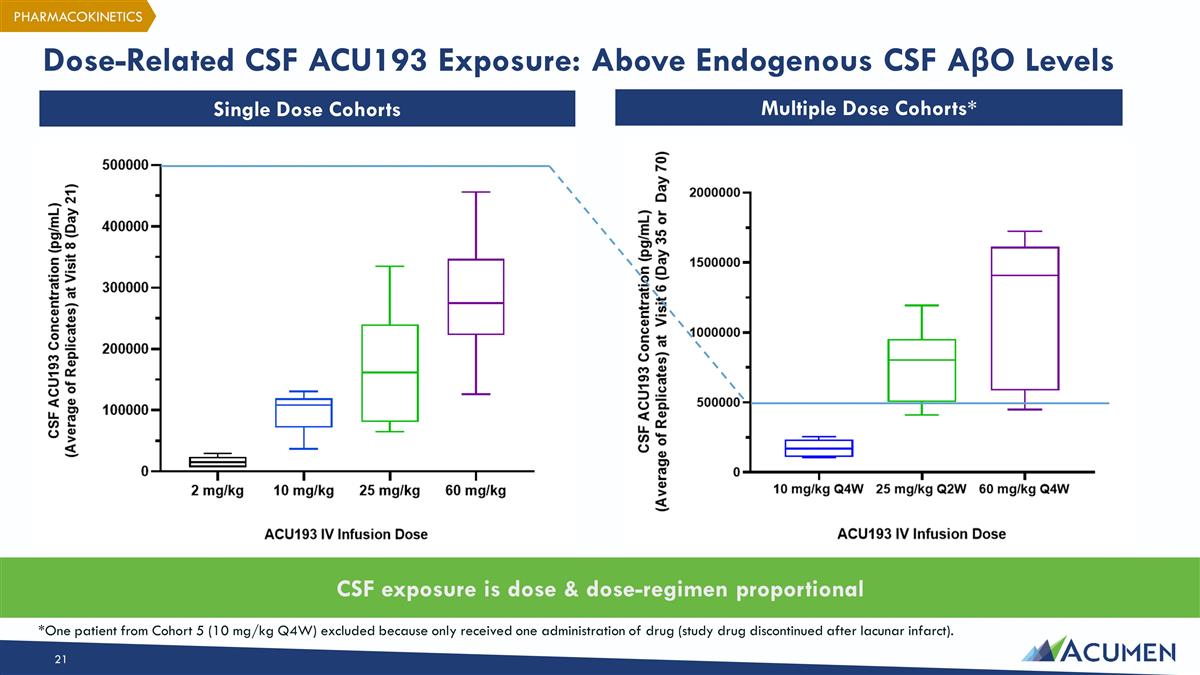
Dose-Related CSF ACU193 Exposure: Above Endogenous CSF AβO Levels Single Dose Cohorts Multiple Dose Cohorts* CSF exposure is dose & dose-regimen proportional *One patient from Cohort 5 (10 mg/kg Q4W) excluded because only received one administration of drug (study drug discontinued after lacunar infarct). PHARMACOKINETICS
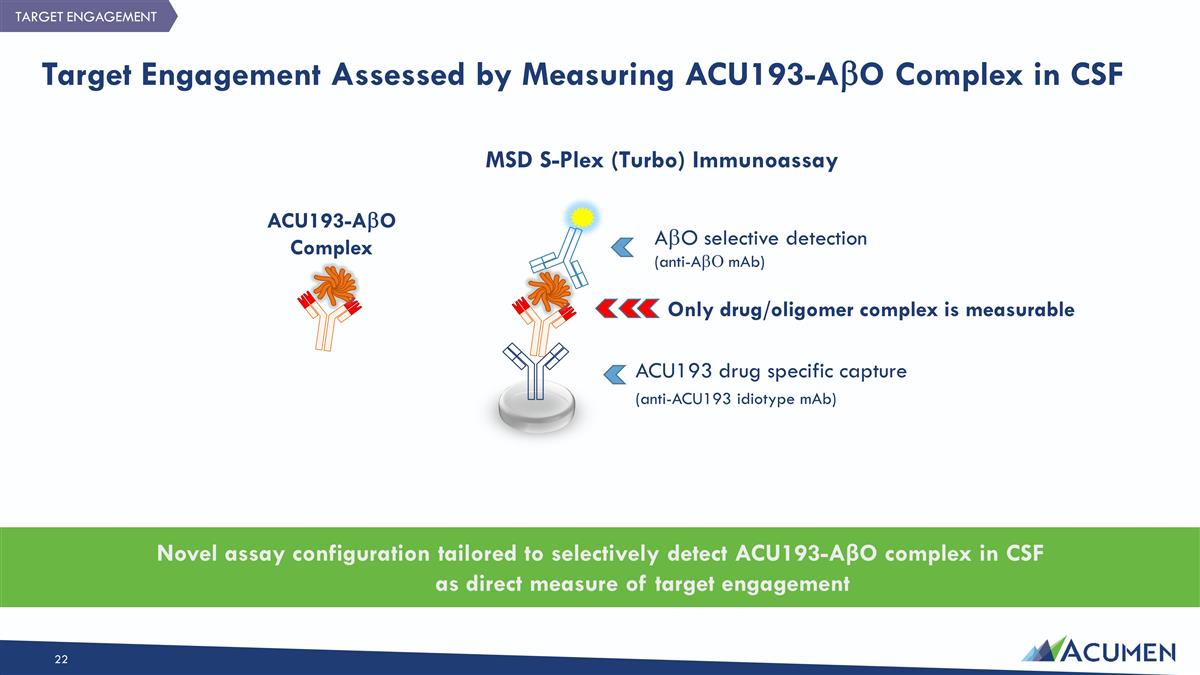
Target Engagement Assessed by Measuring ACU193-AbO Complex in CSF AbO selective detection (anti-AbO mAb) Only drug/oligomer complex is measurable ACU193 drug specific capture (anti-ACU193 idiotype mAb) Novel assay configuration tailored to selectively detect ACU193-AβO complex in CSF as direct measure of target engagement ACU193-AbO Complex MSD S-Plex (Turbo) Immunoassay TARGET ENGAGEMENT
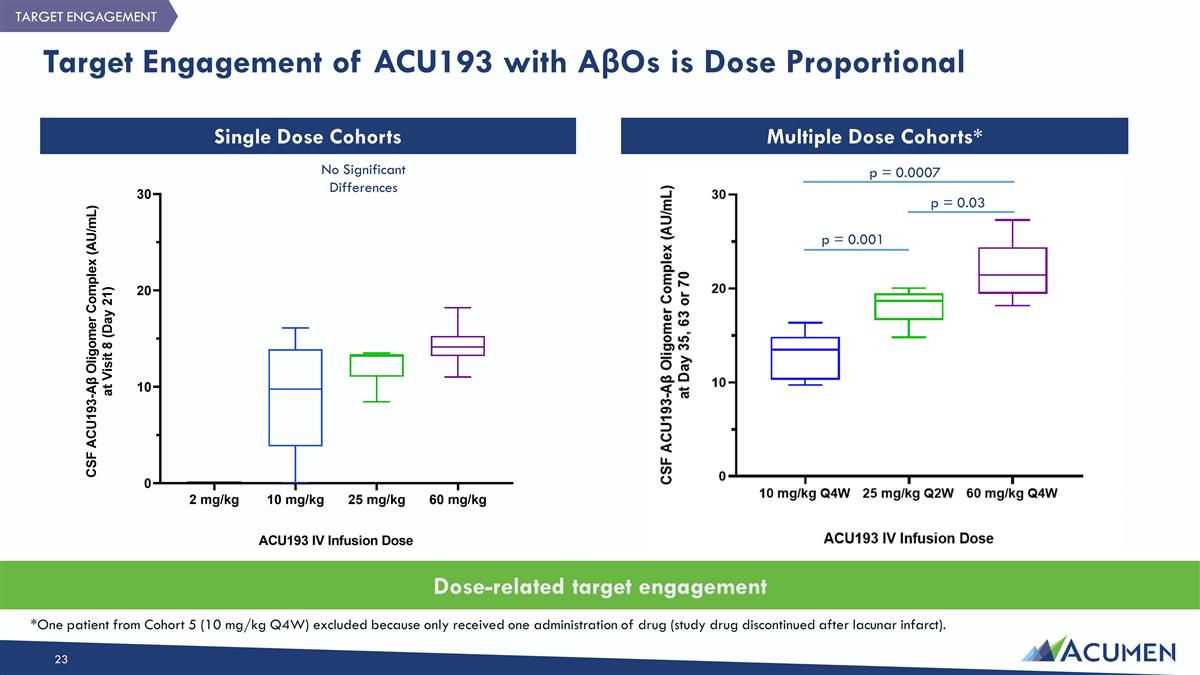
Target Engagement of ACU193 with AβOs is Dose Proportional Single Dose Cohorts Multiple Dose Cohorts* Dose-related target engagement *One patient from Cohort 5 (10 mg/kg Q4W) excluded because only received one administration of drug (study drug discontinued after lacunar infarct). TARGET ENGAGEMENT p = 0.001 p = 0.03 p = 0.0007 No Significant Differences
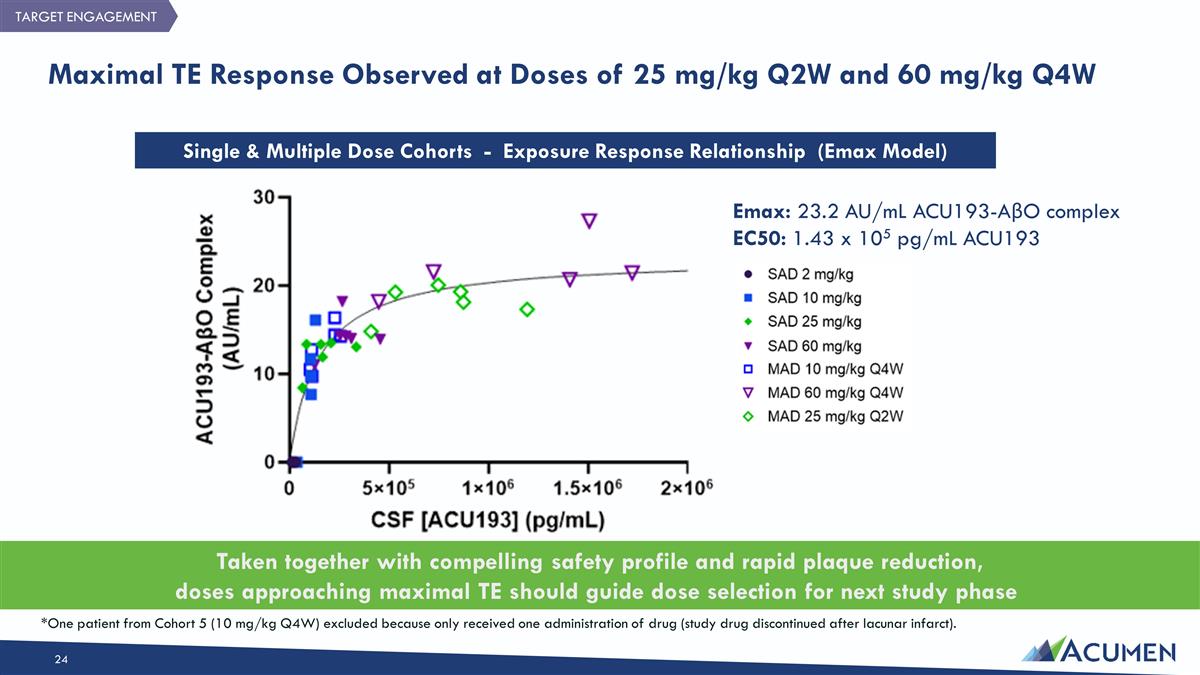
Maximal TE Response Observed at Doses of 25 mg/kg Q2W and 60 mg/kg Q4W Single & Multiple Dose Cohorts - Exposure Response Relationship (Emax Model) Emax: 23.2 AU/mL ACU193-AβO complex EC50: 1.43 x 105 pg/mL ACU193 TARGET ENGAGEMENT *One patient from Cohort 5 (10 mg/kg Q4W) excluded because only received one administration of drug (study drug discontinued after lacunar infarct). Taken together with compelling safety profile and rapid plaque reduction, doses approaching maximal TE should guide dose selection for next study phase

Phase 1 Data Supports Advancing to Phase 2/3 Rapid, dose-related, statistically significant amyloid plaque reduction observed within higher dose cohorts Topline results from INTERCEPT-AD demonstrated proof-of-mechanism for ACU193, the first clinical stage AβO-targeting antibody ACU193 well-tolerated in patients with early AD; resulted in no drug-related SAEs; low rate of ARIA-E ACU193 approached maximal central target engagement of toxic AβOs, establishing broad therapeutic index and path to convenient monthly dosing Exploratory measures: As expected, no effects observed with clinical cognitive measures As expected, no effects observed with MRI ASL pulse sequence Fluid biomarker data expected late Q3 2023 PROOF OF MECHANISM ACHIEVED RAPID, DOSE-RELATED, STATISTICALLY SIGNIFICANT AMYLOID PLAQUE REDUCTION OBSERVED AT HIGHER DOSES STUDIED Compelling Safety Profile and CNS Target Engagement; Monthly Dosing Enabled

Future Strategic Plans Anticipated interaction with the FDA in Q4 2023 to inform our proposed Phase 2/3 study Further investigate the development of a subcutaneous administration of ACU193 Evaluate potential next generation product opportunities Explore partnership opportunities that have the potential to enhance shareholder value

Q&A


























