Exhibit 99.2

www.matinasbiopharma.com NYSE MKT: MTNB MAT2203 - Interim Data from NIH Mucocutaneous Candidiasis Phase 2 Study

Forward Looking Statement This presentation contains "forward - looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995, including those relating to the Company’s product development, clinical and regulatory timelines, market opportunity, cash flow and other statements that are predictive in nature, that depend upon or refer to future events or conditions. All statements other than statements of historical fact are statements that could be forward - looking statements. Forward - looking statements include words such as “expects,” “anticipates,” “intends,” “plans,“ “could,” “believes,” “estimates” and similar expressions. These statements involve known and unknown risks, uncertainties and other factors which may cause actual results to be materially different from any future results expressed or implied by the forward - looking statements. Forward - looking statements are subject to a number of risks and uncertainties, including, but not limited to, our ability to obtain additional capital to meet our liquidity needs on acceptable terms, or at all, including the additional capital which will be necessary to complete the clinical trials of our product candidates; our ability to successfully complete research and further development and commercialization of our product candidates; the uncertainties inherent in clinical testing; the timing, cost and uncertainty of obtaining regulatory approvals; our ability to protect the Company's intellectual property; the loss of any executive officers or key personnel or consultants; competition; changes in the regulatory landscape or the imposition of regulations that affect the Company's products; and the other factors listed under “Risk Factors” in our filings with the SEC, including Forms 10 - K, 10 - Q and 8 - K. Investors are cautioned not to place undue reliance on such forward - looking statements, which speak only as of the date of this release. Except as may be required by law, the Company does not undertake any obligation to release publicly any revisions to such forward - looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. Matinas BioPharma’s product candidates are all in a development stage and are not available for sale or use. 2

Highlights • Interim data from first two patients in National Institutes of Health (NIH) conducted Phase 2a study of MAT2203 for the treatment of refractory mucocutaneous candidiasis infection • Two out of two patients met the primary endpoint in achieving ≥ 50% clinical response • Patients reported meaningful Quality of Life improvements • Objective evidence of effect were also observed, decreased severity of lesions, decreased quantitative fungal cultures. • No serious adverse events reported to date during the course of the study • MAT2203 was well tolerated with majority of adverse events observed to date being mild in severity and unrelated to MAT2203 • Both patients elected to enroll in the long - term extension study 3

Background • MAT2203: Novel encochleated formulation of a proven molecule, amphotericin B, a broad spectrum fungicidal agent - Novel oral delivery through nanoparticle formulation - Ability to convert an IV - only administered drug into an oral formulation - Early evidence of better tolerability with lower toxicity • Product profile fits well with difficult to evaluate and treat patient populations seen at the NIH - Patients require long term therapy due to chronic drug resistant fungal infections - MAT2203 offers convenient oral dosing of amphotericin B with the favorable safety profile needed for chronic therapy • Interim data from first two patients in NIH - conducted Phase 2a study of MAT2203 for the treatment of refractory mucocutaneous candidiasis infection • Establishes POC in nonlethal fungal infections in immunocompromised patients 4

Collaboration with NIH • Collaboration with NIH began with IND enabling studies • NIH is conducting the study • Advanced Phase 2a study with enrollment and dosing of first two patients • Study subjects chosen because of inherent impaired immuno - compensation, long term fungal infection of over 20 years and anti - fungal drug resistence except for amphotericin B • Edmund C. Tramont, MD, MACP, FIDSA - Co - Investigator of the study - National Institutes of Health, National Institute Allergy and Infectious Diseases - Associate Director for Special Projects, DCR/NIAID/NIH - Former Director, Division of AIDS, NIAID/NIH, Consultant to the Surgeon General in Infectious Diseases 5

Key Unmet Need Drivers 1. Increasing resistance to presently available antifungal therapies 2. Increasing numbers of immunocompromised hosts/patients 6
Amphotericin B Value/Strengths of Amphotericin B • Amphotericin B is the most broad spectrum fungicidal agent • Gold standard of therapy for the treatment of all fungal infections • Little to no clinical resistance has developed in over 60 years of use Limitations of Amphotericin B • IV dosing • Toxicity (renal, hypokalemia) is observed at the same dose level required for efficacy and increases with therapy over 1 - 2 weeks 7
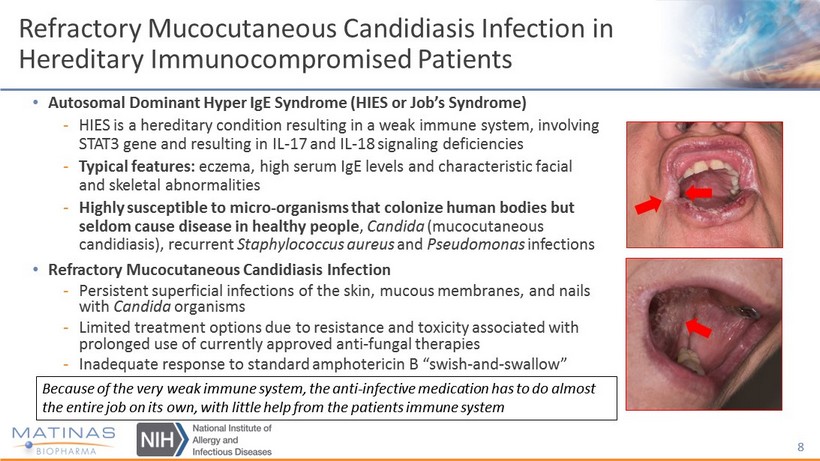
Refractory Mucocutaneous Candidiasis Infection in Hereditary Immunocompromised Patients • Autosomal Dominant Hyper IgE Syndrome (HIES or Job’s Syndrome) - HIES is a hereditary condition resulting in a weak immune system, involving STAT3 gene and resulting in IL - 17 and IL - 18 signaling deficiencies - Typical features: eczema, high serum IgE levels and characteristic facial and skeletal abnormalities - Highly susceptible to micro - organisms that colonize human bodies but seldom cause disease in healthy people , Candida (mucocutaneous candidiasis), recurrent Staphylococcus aureus and Pseudomonas infections • Refractory Mucocutaneous Candidiasis Infection - Persistent superficial infections of the skin, mucous membranes, and nails with Candida organisms - Limited treatment options due to resistance and toxicity associated with prolonged use of currently approved anti - fungal therapies - Inadequate response to standard amphotericin B “swish - and - swallow” 8 Because of the very weak immune system, the anti - infective medication has to do almost the entire job on its own, with little help from the patients immune system
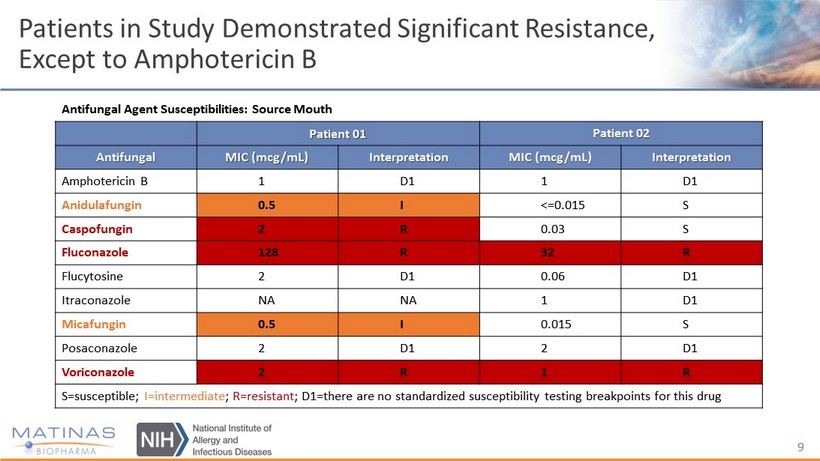
Patients in Study Demonstrated Significant Resistance, Except to Amphotericin B 9 Antifungal Agent Susceptibilities: Source Mouth Patient 01 Patient 02 Antifungal MIC (mcg/mL) Interpretation MIC (mcg/mL) Interpretation Amphotericin B 1 D1 1 D1 Anidulafungin 0.5 I <=0.015 S Caspofungin 2 R 0.03 S Fluconazole 128 R 32 R Flucytosine 2 D1 0.06 D1 Itraconazole NA NA 1 D1 Micafungin 0.5 I 0.015 S Posaconazole 2 D1 2 D1 Voriconazole 2 R 1 R S=susceptible; I=intermediate ; R=resistant ; D1=there are no standardized susceptibility testing breakpoints for this drug
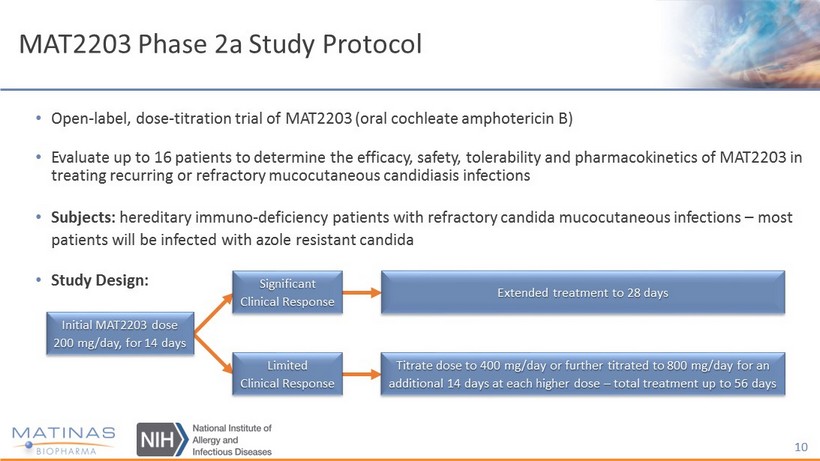
MAT2203 Phase 2a Study Protocol • Open - label, dose - titration trial of MAT2203 (oral cochleate amphotericin B) • Evaluate up to 16 patients to determine the efficacy, safety, tolerability and pharmacokinetics of MAT2203 in treating recurring or refractory mucocutaneous candidiasis infections • Subjects: hereditary immuno - deficiency patients with refractory candida mucocutaneous infections – most patients will be infected with azole resistant candida • Study Design: 10 Initial MAT2203 dose 200 mg/day, for 14 days Significant Clinical Response Limited Clinical Response Extended treatment to 28 days Titrate dose to 400 mg/day or further titrated to 800 mg/day for an additional 14 days at each higher dose – total treatment up to 56 days

Interim Data - Safety Highlights • Oral treatment with MAT2203 for up to 54 days was well tolerated • No serious adverse events reported • Reported adverse events were mostly mild in severity and unrelated to MAT2203 • No signs of nephrotoxicity, hypokalemia or hepatoxicity (measured by ALT and AST) 11
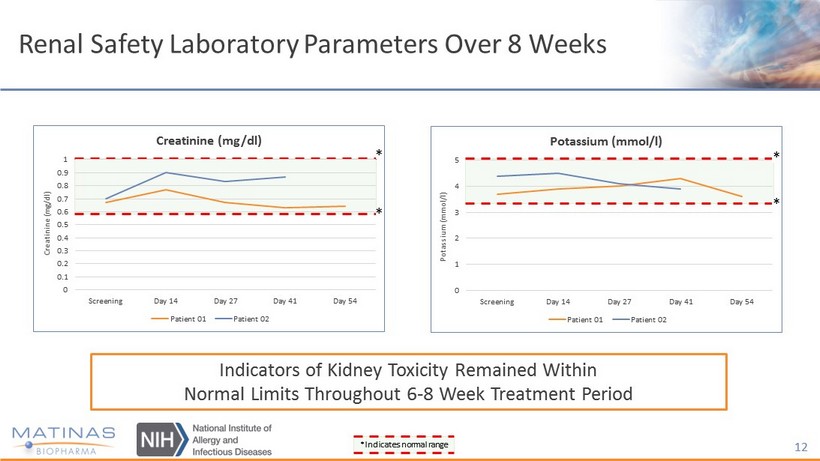
Renal Safety Laboratory Parameters Over 8 Weeks 12 Indicators of Kidney Toxicity Remained Within Normal Limits Throughout 6 - 8 Week Treatment Period 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 Screening Day 14 Day 27 Day 41 Day 54 Creatinine (mg/dl) Creatinine (mg/dl) Patient 01 Patient 02 0 1 2 3 4 5 Screening Day 14 Day 27 Day 41 Day 54 Potassium (mmol/l) Potassium ( mmol /l) Patient 01 Patient 02 *Indicates normal range * * * * * *

Hepatic Safety Laboratory Parameters Over 8 Weeks 13 *Indicates normal range 0 10 20 30 40 50 60 Screening Day 14 Day 27 Day 41 Day 54 ALT (u/l) ALT (u/l) Patient 01 Patient 02 0 5 10 15 20 25 30 35 40 Screening Day 14 Day 27 Day 41 Day 54 AST (u/l) AST (u/l) Patient 01 Patient 02 Indicators of Liver Toxicity Remained Within Normal Limits Throughout 6 - 8 Week Treatment Period * *
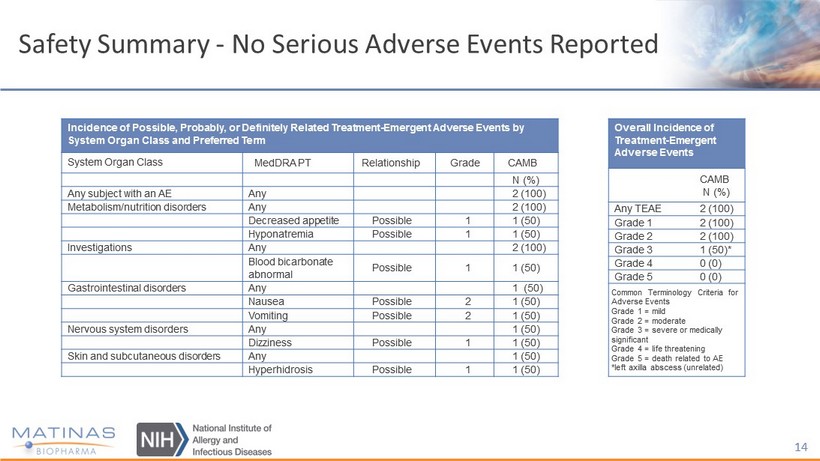
Safety Summary - No Serious Adverse Events Reported 14 Incidence of Possible, Probably, or Definitely Related Treatment - Emergent Adverse Events by System Organ Class and Preferred Term System Organ Class MedDRA PT Relationship Grade CAMB N (%) Any subject with an AE Any 2 (100) Metabolism/nutrition disorders Any 2 (100) Decreased appetite Possible 1 1 (50) Hyponatremia Possible 1 1 (50) Investigations Any 2 (100) Blood bicarbonate abnormal Possible 1 1 (50) Gastrointestinal disorders Any 1 (50) Nausea Possible 2 1 (50) Vomiting Possible 2 1 (50) Nervous system disorders Any 1 (50) Dizziness Possible 1 1 (50) Skin and subcutaneous disorders Any 1 (50) Hyperhidrosis Possible 1 1 (50) Overall Incidence of Treatment - Emergent Adverse Events CAMB N (%) Any TEAE 2 (100) Grade 1 2 (100) Grade 2 2 (100) Grade 3 1 (50)* Grade 4 0 (0) Grade 5 0 (0) Common Terminology Criteria for Adverse Events Grade 1 = mild Grade 2 = moderate Grade 3 = severe or medically significant Grade 4 = life threatening Grade 5 = death related to AE *left axilla abscess (unrelated)

Interim Data - Efficacy Highlights • Two out of two patients achieved ≥ 50% subjective and objective clinical response - Both patients reported improved quality of life, e.g. able to eat a greater variety of foods, including those that are acidic and spicy • Two out of two patients achieved objective responses, verifying the subjective responses - Both patients experienced improvements in fungal culture response - Both patients mucocutaneous lesions improved: less inflammation, less pain on scrapping of the lesions for culture 15
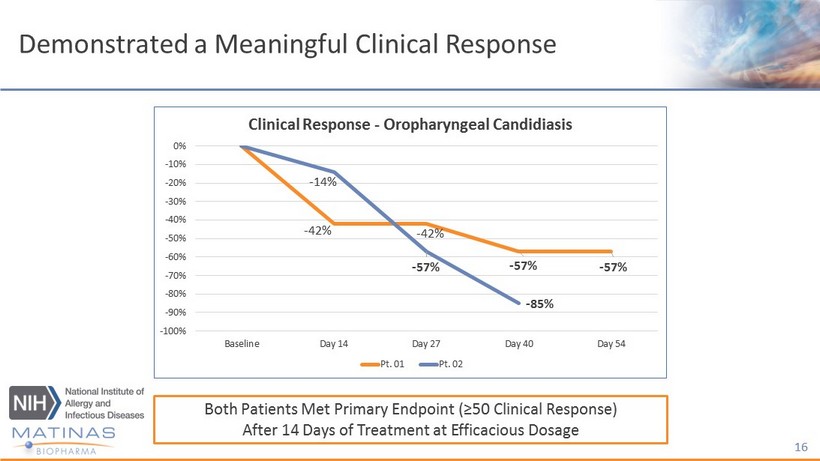
Demonstrated a Meaningful Clinical Response 16 - 42% - 42% - 57% - 57% - 14% - 57% - 85% -100% -90% -80% -70% -60% -50% -40% -30% -20% -10% 0% Baseline Day 14 Day 27 Day 40 Day 54 Clinical Response - Oropharyngeal Candidiasis Pt. 01 Pt. 02 Both Patients Met Primary Endpoint (≥50 Clinical Response) After 14 Days of Treatment at Efficacious Dosage

Patient Exam / Patient Quality of Life • Patients reported improved quality of life, e.g. as able to eat a greater variety of foods, including those that are acidic and spicy • Both patients reported less pain 17 Baseline End of Treatment Patient 02 Patient 01
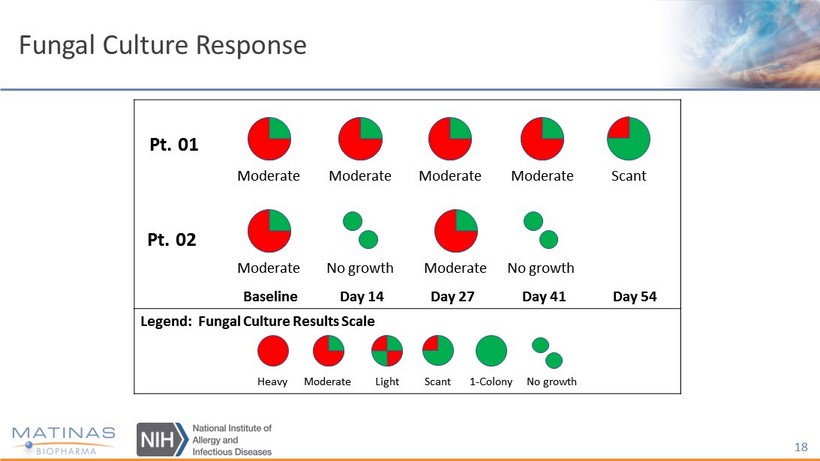
Pt. 01 Pt. 02 Baseline Day 14 Day 27 Day 41 Day 54 Legend: Fungal Culture Results Scale Moderate Moderate Moderate Moderate Scant Moderate No growth Moderate No growth Fungal Culture Response Heavy Moderate Light Scant 1 - Colony No growth 18
Interim Data Study Conclusion • Two patients with AD - HIES or Job’s Syndrome with long - standing azole resistant mucocutaneous candidiasis responded clinically to oral treatment with MAT2203 • Oral treatment with MAT2203 for up to 54 days was well tolerated - Reported adverse events were mostly mild in severity and unrelated to MAT2203 - There were no serious adverse events reported • There were no signs of nephrotoxicity, hypokalemia or hepatoxicity after oral dosing for 54 days in Patient 01 and 40 days in Patient 02 • Both patients elected to enroll in the long - term extension study 19

Clinical Unmet Needs • Increasing resistance to presently available antifungal therapies • Increasing number of patients being rendered immunocompromised by advancements in medicine - NIH is in the vanguard of studying immunocompromised patients, the number of which is expected to increase due to the growing use of immuno - compromising drugs for transplants, cancer chemotherapies, and autoimmune diseases • Since antifungal therapy usually requires prolonged administration, there is a need for an oral, safe and effective antifungal therapy, that can be dosed conveniently over time • An effective and non - toxic oral amphotericin B could address these unmet needs 20

MAT2203 Development Program and Next Steps • Company takeaways - Response results likely predictive of study outcome - Safe long - term use supports development strategy towards preventative treatment • Second Phase 2 – next data readout: vulvovaginal candidiasis (VVC) – key objectives - With oral delivery, demonstrate safety and efficacy comparable to fluconazole (gold standard for the treatment of primary/uncomplicated VVC) - Expecting data from VVC before end of June • Interim results from this study are significant steps toward: - Establishing POC for the MAT2203 product - Providing validation of technology platform - Strengthening our position to also evaluate range of fungal infections, e.g. aspergillosis, coccidiomycosis, cryptococcosis , histoplasmosis, blastomycosis , molds - Strengthening our position to impact and change treatment paradigms 21
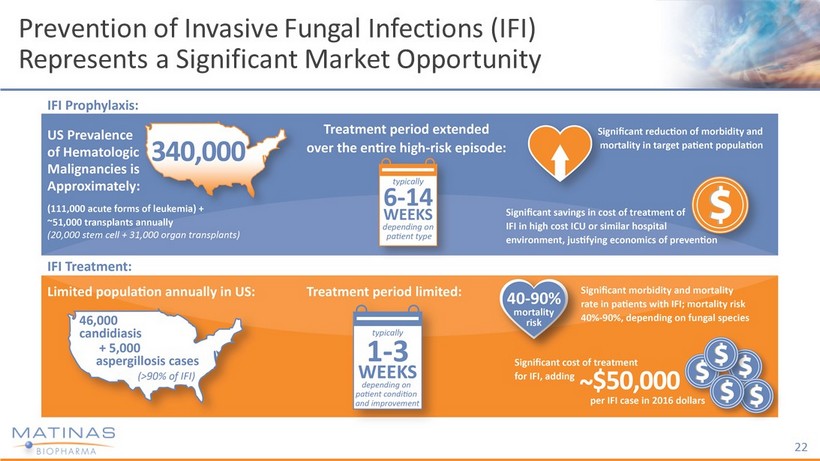
Prevention of Invasive Fungal Infections (IFI) Represents a Significant Market Opportunity 22
MAT2203: IFI Prevention is a Significant Value Driver • QIPD and Fast Track designations granted by FDA for MAT2203 - “Prevention of invasive fungal infections (IFI) due to immunosuppressive therapy” • Represents significant unmet clinical need - very few antifungals are approved for the preventative use in patients on immunosuppressive therapy • Amphotericin B (currently only available for IV infusion) is not liver - metabolized and has very few drug - drug interactions with cancer/transplant therapies • Encochleated amphotericin B (MAT2203) is designed to significantly reduce toxicities associated with the amphotericin B molecule, while making the compound absorbable in the body by convenient oral administration 23

www.matinasbiopharma.com NYSE MKT: MTNB MAT2203 - Interim Data from NIH Mucocutaneous Candidiasis Phase 2 Study