- QURE Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
10-K/A Filing
uniQure (QURE) 10-K/A2018 FY Annual report (amended)
Filed: 29 Apr 19, 4:26pm
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington D.C. 20549
FORM 10-K/A
Amendment No. 1 to Form 10-K
(Mark One)
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2018
OR
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission file number: 001-36294
uniQure N.V.
(Exact name of Registrant as specified in its charter)
The Netherlands
(Jurisdiction of incorporation or organization)
Paasheuvelweg 25a,
1105 BP Amsterdam, The Netherlands
(Address of principal executive offices) (Zip Code)
+31-20-240-6000
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class |
| Name of Each Exchange on Which Registered |
Ordinary Shares, par value €0.05 per share |
| The NASDAQ Stock Market LLC (The NASDAQ Global Select Market) |
Securities registered under Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No o
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer x | Accelerated filer o | Non-accelerated filer o | Smaller reporting company o | Emerging growth company o |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act.) Yes o No x
The aggregate market value of the voting and non-voting ordinary shares held by non-affiliates of the registrant as of June 30, 2018 was $1,403.4 million, based on the closing price reported as of June 29, 2018 on the NASDAQ Global Select Market.
As of April 15, 2019, the registrant had 37,798,421 Ordinary Shares, par value €0.05, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None.
|
| Page |
|
|
|
| 1 | |
| 2 | |
2 | ||
7 | ||
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 36 | |
Certain Relationships and Related Transactions and Director Independence | 38 | |
40 | ||
| 41 | |
41 | ||
41 | ||
| 45 |
This Amendment No. 1 to Form 10-K (this “Amendment”) amends the Annual Report on Form 10-K for the fiscal year ended December 31, 2018 of uniQure N.V. (“uniQure” or the “Company”), as originally filed with the Securities and Exchange Commission (“SEC”) on February 28, 2019 (the “Original Form 10-K”). We are filing this Amendment to present the information required by Part III of Form 10-K that was previously omitted from the Original Form 10-K in reliance on General Instruction G.(3) to Form 10-K.
The cover page has correspondingly been amended to delete the reference in the Original Form 10-K to the incorporation by reference of the Company’s Proxy Statement for its 2019 Annual Meeting of Shareholders and to update the date as of which the number of outstanding shares of the Company’s Ordinary Shares is being provided. Part IV, Item 15(b) (Exhibits 31.3 and 31.4) have also been amended and restated in their entirety to contain the currently dated certifications from the Company’s principal executive officer and principal financial officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. The certifications of the Company’s principal executive officer and principal financial officer are attached to this Amendment No. 1 as Exhibits 31.3 and 31.4. Because no financial statements have been included in this Amendment No. 1 and this Amendment No. 1 does not contain or amend any disclosure with respect to Items 307 and 308 of Regulation S-K, paragraphs 3, 4 and 5 of the certifications have been omitted. The Exhibit Index has also been amended and restated in its entirety to include the certifications as exhibits.
Except as described above, no other changes have been made to the Original Form 10-K. This Amendment does not otherwise update information in the Original Form 10-K to reflect facts or events occurring subsequent to the filing date of the Original Form 10-K. This Amendment should be read in conjunction with the Original Form 10-K and with any of our filings made with the SEC subsequent to filing of the Original Form 10-K.
Item 10. Directors, Executive Officers and Corporate Governance
Directors and Senior Management
Set forth below are the names of our current directors and officers, their ages (as of March 31, 2019), all positions and offices that they hold with us, the period during which they have served as such, and their business experience during at least the last five years.
Name |
| Age |
| Position |
Matthew Kapusta |
| 46 |
| Chief Executive Officer, Executive Director and interim Chief Financial Officer |
Philip Astley-Sparke |
| 47 |
| Chairman, Non-Executive Director |
Madhavan Balachandran |
| 68 |
| Non-Executive Director |
Robert Gut, Ph.D. |
| 55 |
| Chief Medical Officer, Executive Director |
Jack Kaye |
| 75 |
| Non-Executive Director |
David Meek |
| 55 |
| Non-Executive Director |
David Schaffer, Ph.D. |
| 48 |
| Non-Executive Director |
Paula Soteropoulos |
| 51 |
| Non-Executive Director |
Jeremy Springhorn, Ph.D. |
| 56 |
| Non-Executive Director |
Maria Cantor |
| 51 |
| Senior Vice President, Investor Relations & Communications, |
David Cerveny |
| 52 |
| Chief Legal Officer |
Jonathan Garen |
| 53 |
| Chief Business Officer |
Sander van Deventer, M.D., Ph.D. |
| 64 |
| Chief Scientific Officer, General Manager, Amsterdam |
Christian Klemt |
| 46 |
| Chief Accounting Officer |
Alexander Kuta, Ph. D. |
| 59 |
| Senior Vice President, Regulatory Affairs |
Scott McMillan, Ph.D. |
| 60 |
| Chief Operations Officer |
MATTHEW KAPUSTA. Matthew Kapusta, age 46, joined uniQure as our Chief Financial Officer in January 2015 and was elected to our Management Board at the 2015 Annual General Meeting. In December 2016 he was appointed our Chief Executive Officer. Prior to joining uniQure, Mr. Kapusta was Senior Vice President at AngioDynamics (NASDAQ: ANGO) from 2011 to 2014, responsible for corporate development, strategic planning and national accounts. Prior to AngioDynamics, he served as Vice President, Finance and Strategic Planning and Analysis for Smith & Nephew Orthopaedics. Mr. Kapusta’s career also includes more than a decade of investment banking experience focused on emerging life sciences companies. Mr. Kapusta was Managing Director, Healthcare Investment Banking at Collins Stewart, and held various positions at Wells Fargo Securities, Robertson Stephens and PaineWebber. Mr. Kapusta holds a Master of Business Administration from New York University’s Stern School of Business, a Bachelor of Business Administration from University of Michigan’s Ross School of Business and earned his Certified Public Accountant license in 1996 while at Ernst & Young. We believe that Mr. Kapusta is qualified to serve as our CEO, Executive Director and Principal Financial Officer due to his broad expertise in the biotechnology and finance industries.
PHILIP ASTLEY-SPARKE. Philip Astley-Sparke, age 47, has served as a member of our Board since June 2015 and as chairman since 2016. He was previously president of uniQure Inc. from January 2013 until February 2015 and was responsible for building uniQure’s U.S. infrastructure. Mr. Astley-Sparke is currently Executive Chairman and co-founder of Replimune Group, Inc. (NASDAQ: REPL), a company developing second-generation oncolytic vaccines. Mr. Astley-Sparke served as Vice President and General Manager at Amgen, Inc. (NASDAQ: AMGEN), a biopharmaceutical company, until December 2011, following Amgen’s acquisition of BioVex Group, Inc., a biotechnology company, in March 2011. Mr. Astley-Sparke had been President and Chief Executive Officer of BioVex Group, which developed the first oncolytic vaccine to be approved in the western world following the approval of Imlygic in 2015. He oversaw the company’s relocation to the U.S. from the UK in 2005. Prior to BioVex, Mr. Astley-Sparke was a healthcare investment banker at Chase H&Q/Robert Fleming and qualified as a Chartered Accountant with Arthur Andersen in London. Mr. Astley-Sparke has been a Venture Partner at Forbion Capital Partners, a venture capital fund, since May 2012 and serves as Chairman of the Board of Oxyrane, a biotechnology company. We believe that Mr. Astley-Sparke is qualified to serve as a Non-Executive Director due to his expertise and experience in the biotechnology industry.
MADHAVAN BALACHANDRAN. Mr. Balachandran, age 68, has served as a member of our Board since September 2017. Mr. Balachandran has been a director of Catalent (NYSE: CTLT) since May 2017. Mr. Balachandran was Executive Vice President, Operations of Amgen Inc., a global biotechnology company, from August 2012 until July 2016 and retired as an Executive Vice President in January 2017. Mr. Balachandran joined Amgen in 1997 as Associate Director, Engineering. He became Director, Engineering in 1998, and, from 1999 to 2001, he held the position of Senior Director, Engineering and Operations Services before moving to the position of Vice President, Information Systems from 2001 to 2002. Thereafter, Mr. Balachandran was Vice President, Puerto Rico Operations from May 2002 to February 2007. From February 2007 to October 2007, Mr. Balachandran was Vice President, Site Operations, and from October 2007 to August 2012, he held the position of Senior Vice President, Manufacturing. Prior to his tenure at Amgen, Mr. Balachandran held leadership positions at Copley Pharmaceuticals, now a part of Teva Pharmaceuticals Industries Ltd., and Burroughs Welcome Company, a predecessor through mergers of GlaxoSmithKline plc. Mr. Balachandran holds a Master of Science degree in Chemical Engineering from The State University of New York at Buffalo and an MBA from East Carolina University. We believe Mr. Balachandran is qualified to serve as a Non-Executive Director due to his extensive experience in the biotechnology industry.
ROBERT GUT, M.D., PH.D. Dr. Robert Gut, age 55, joined uniQure as our Chief Medical Officer in August 2018 and was elected as an executive director to our Board at the October 2018 extraordinary general meeting. Dr. Gut was originally elected to the Board as a non-executive director in June 2018 He resigned that position in August 2018 to take the position of Chief Medical Officer because under Dutch law our non-executive directors are not able to hold executive positions with the Company. Dr. Gut has nearly 20 years of experience in the biopharmaceutical industry leading clinical development and medical affairs activities in hematology and other therapeutic areas. For the majority of his career, Dr. Gut served as Vice President, Clinical Development & Medical Affairs at Novo Nordisk Inc. (NYSE: NVO), where he headed the company’s U.S. Biopharm Medical organization with leading products in hemophilia, endocrinology and women’s health (NovoSeven®, Norditropin® and Vagifem®), totaling approximately $1.6 billion in U.S. revenue. Over his career, Dr. Gut’s contributions have helped achieve six FDA product approvals and three new product indications. Dr. Gut has supported the launch of nine new products, overseeing medical activities including medical science liaison team building and health economics and outcomes research. He has also served as a member of the Advisory Committees for Reproductive Health Drugs and Drug Safety and Risk Management for the FDA’s Center for Drug Evaluation and Research. Dr. Gut was appointed the Chief Medical Officer of Versartis, Inc. in September 2017 and received his Doctor of Medicine degree from the Medical University of Lublin, and his Doctorate degree from Lublin Institute of Medicine, Poland. He attended numerous postgraduate programs at Wharton, Stanford and Harvard Business School.
JACK KAYE. Jack Kaye, age 75, has served as a member of our Board since 2016. Mr. Kaye has also served as Chairman of the Audit Committee of Keryx Biopharmaceuticals, Inc. (NASDAQ: KERX) from 2006 to 2016 and is currently chairman of the Audit Committee and a member of the Compensation Committee of Dyadic International, Inc. (OTC: DYAI). Mr. Kaye began his career at Deloitte LLP, an international accounting, tax and consulting firm, in 1970, and was a partner in the firm from 1978 until May 2006. At Deloitte, he was responsible for servicing a diverse client base of public and private, global and domestic companies in a variety of industries. Mr. Kaye has extensive experience consulting with clients on accounting and reporting matters, private and public debt financings, SEC rules and regulations and corporate governance/Sarbanes-Oxley matters. Prior to retiring, Mr. Kaye served as Partner-in-Charge of Deloitte’s Tri-State Core Client practice, a position he held for more than 20 years. Mr. Kaye has a Bachelor of Business Administration from Baruch College and is a Certified Public Accountant. We believe that Mr. Kaye is qualified to serve as a Non-Executive Director due to his extensive accounting and financial experience.
DAVID MEEK. David Meek, age 55, has over 25 years of experience in the pharmaceutical industry where he has held various global executive positions in major pharmaceutical and biotechnology companies. Mr. Meek was appointed CEO of Ipsen in July 2016 and also serves on the Board of Directors. Prior to joining Ipsen, he was Executive Vice-President and President of the oncology division of Baxalta. Mr. Meek also spent 8 years at Novartis as a global franchise head, CEO of Novartis Canada, and region head of oncology for northern, central and Eastern Europe. He also spent 14 years at Johnson & Johnson and Janssen Pharmaceuticals, where he held a variety of senior U.S. sales and marketing positions. Mr. Meek holds a B.A. in Management from the University of Cincinnati.
DAVID SCHAFFER, PD.D. David Schaffer, age 48, has served as a member of our Board since January 2014. Dr. Schaffer is Professor of Chemical and Biomolecular Engineering, Bioengineering, and Neuroscience at University of California Berkeley, a position he has held since 2007, as well as Director of the Berkeley Stem Cell Center since 2011. Dr. Schaffer is also co-founder and the current Chief Scientific Officer of 4D Molecular Therapeutics, a company specializing proprietary technology for gene therapy products. We entered into a collaboration and license agreement with 4D Molecular Therapeutics in January 2014. Previously, Dr. Schaffer was Assistant Professor from 1999 to 2005 and Associate Professor from 2005 to 2007 at the University of California, Berkeley Department of Chemical Engineering & Helen Wills Neuroscience Institute. He has served on the boards of the American Society for Gene and Cell Therapy and the Society for Biological Engineering. He has more than 25 years of experience in chemical and molecular engineering, and stem cell and gene therapy research, has over 185 scientific publications, and serves on five journal editorial boards and five industrial scientific advisory boards. Dr. Schaffer holds a Bachelor of Science degree in Chemical Engineering from Stanford University and a Ph.D. in Chemical Engineering from the Massachusetts Institute of Technology. We believe that Dr. Schaffer is qualified to serve as a Non-Executive Director due to his experience in the biotechnology industry and his expertise in that field.
PAULA SOTEROPOULOS. Paula Soteropoulos, age 51, has served as a member of our Board since July 2013. Ms. Soteropoulos is President and Chief Executive Officer of Akcea Therapeutics (NASDAQ: AKCA), a position she has held since January 2015. From July 2013 to December 2014, she served as Senior Vice President and General Manager, Cardiometabolic Business and Strategic Alliances at Moderna Therapeutics Inc. Prior to this, Ms. Soteropoulos worked at Genzyme Corporation, a biotechnology company, from 1992 to 2013, most recently as Vice President and General Manager, Cardiovascular, Rare Diseases. Ms. Soteropoulos holds a Bachelor of Science degree in chemical engineering and a Master of Science degree in chemical and biochemical engineering, both from Tufts University, and holds an executive management certificate from the University of Virginia, Darden Graduate School of Business Administration. Ms. Soteropoulos serves on the Advisory Board for the Chemical and Biological Engineering Department of Tufts University. We believe Ms. Soteropoulos is qualified to serve as a Non-Executive Director due to her extensive experience in the biotechnology industry.
JEREMY SPRINGHORN, PH.D. Dr. Springhorn, age 56, has served as a member of our Board since September 2017. Since November 2017, Dr. Springhorn has been Chief Business Officer of Syros Pharmaceuticals (NASDAQ: SYRS), Inc. Prior to taking his position at Syros, Dr. Springhorn most recently served as Partner, Corporate Development at Flagship Pioneering from March 2015 until June 2017 where he worked with VentureLabs (in helping companies in various strategic and corporate development capacities and in creating next generation startups) and Flagship’s Corporate Limited Partners. Prior to joining Flagship, Dr. Springhorn was one of the original scientists at Alexion Pharmaceuticals, Inc. (NASDAQ: ALXN), where he played an integral role in its antibody engineering capabilities and was one of the original inventors of the drug Soliris. At Alexion Pharmaceuticals, Dr. Springhorn was Vice President of Corporate Strategy and Business Development from 2009 until March 2015 and Head of Global Business Development and Corporate Strategy from December 2006 until 2009. In 2006, Dr. Springhorn moved from research to business development, leveraging much of his drug development experience into the review of opportunities for ultra-orphan diseases. Dr. Springhorn also served as Head of Corporate Strategy as Alexion transitioned from a development firm to a global commercial stage company. Prior to 1992, Dr. Springhorn received his Ph.D. from Louisiana State University Medical Center in New Orleans and did his postdoctoral training at the Brigham and Woman’s Hospital in Boston. Dr. Springhorn currently serves on the Board of Visitors for Colby College and Board of Advisors for Mythic Therapeutics. We believe Dr. Springhorn is qualified to serve as a Non-Executive Director due to his extensive experience in the biotechnology industry.
MARIA CANTOR. Ms. Cantor, age 51, has served as our Senior Vice President, Investor Relations and Communications since June 2016. Most recently, Ms. Cantor served on the Executive Leadership Team at ARIAD Pharmaceuticals, Inc. (NASDAQ: ARIA) where she led all strategic corporate communications, first as Vice President of Corporate Communications and Investor Relations from July, 2008 to October, 2011, and later as Senior Vice President, Corporate Affairs from November, 2011 to June, 2016. She was responsible for investor relations, global communications, corporate branding and social responsibility and led the communications program for the clinical development, regulatory approval and commercial launch of the company’s first cancer therapeutic, Iclusig® (ponatinib), approved in both the U.S. and Europe. From September, 2001 to June, 2008, she served in various communications roles at Genzyme, including Senior Director, Corporate Communications, where she was involved in all aspects of corporate and product communications. Ms. Cantor also held senior communications positions within the healthcare industry including Director, Marketing and Public Relations at the St. Elizabeth Medical Center in Boston, and as Director, Marketing and Communications at Optima Healthcare Inc. in Manchester, New Hampshire. Ms. Cantor holds a Master of Science in Communications Management
from Syracuse University, S.I. Newhouse School of Public Communications in Syracuse, New York and a Bachelor of Science in Mass Communication/Broadcast Journalism from Emerson College in Boston. She is the recipient of several communications awards, including from the Associated Press of New Hampshire and the New England Society for Healthcare Communications.
DAVID CERVENY. Mr. Cerveny, aged 52, has served as our Chief Legal Officer, General Counsel and Secretary since March 2018. From 2008 until 2018, he was Chief Legal Officer, General Counsel and Secretary at ConforMIS, Inc. (NASDAQ: CFMS), a medical technology company based in Billerica, Mass. Prior to joining ConforMIS, David served as Chief Intellectual Property Counsel at Palomar Medical Technologies, Inc. Before that, David was a partner at Wilmer Cutler Pickering Hale, and Dorr LLP. Before entering law, Mr. Cerveny worked as a systems engineer developing flight control systems for McDonnell Douglas Corporation, an aerospace manufacturing corporation now part of The Boeing Company. Mr. Cerveny holds a Bachelor of Science in biomedical engineering from Marquette University and a J.D. from Boston College Law School.
JONATHAN GAREN. Mr. Garen, aged 53, has served as our Chief Business Officer since July 2016. He previously served as Chief Business Officer at Syros Pharmaceuticals (NASDAQ: SYRS) from May, 2015 to January 2016, where he was responsible for business transactions including partnering Syros’ technology platform and drug assets, and bringing in products to enhance and accelerate its pipeline. From August, 2003 to July, 2014, Mr. Garen was the Assistant Vice President of Business Development at Forest Laboratories. From July, 2014 to April 2015, he was Assistant Vice President of Business Development at Actavis, plc until following its acquisition of Forest Laboratories. From February, 2001 to June 2003, Mr. Garen was Director of Global Licensing with Pharmacia Corporation and, from September, 1999 to January 2001 was a Founder and Vice President of Technology Exchange, Inc., in New York, NY. Mr. Garen holds a Master of Environmental Science degree from Yale University and a Bachelor of Science degree in Physics from the Massachusetts Institute of Technology.
SANDER VAN DEVENTER, M.D., Ph.D. Dr. Sander van Deventer, age 64, has served as our Chief Scientific Officer and General Manager, Amsterdam since August 2017. He previously served as a member of our Board from April 2012 until September 2017 and served as member of the AMT supervisory board from April 2010 to April 2012. Dr. van Deventer was one of our co-founders. He served as our interim Chief Executive Officer from February to October 2009. He has been Professor of Translational Gastroenterology at the Leiden University Medical Center since 2008 and is a partner of Forbion Capital Partners, which he joined in 2006. He serves on the boards of enGene Inc., Argos Biotherapeutics, gICare Pharma Inc and Hookipa Biotech. He was previously a professor, head of the department of experimental medicine and chairman of the department of gastroenterology of the Academic Medical Center at the University of Amsterdam from 2002 to 2004, and subsequently professor of experimental medicine at the University of Amsterdam Medical School until 2008. Dr. van Deventer is currently a professor at Leiden University Medical Center. He has more than 15 years of experience in biotechnology product development. He is the author of more than 400 scientific articles in peer-reviewed journals, and he serves as an advisor to regulatory authorities including the EMA and FDA. Dr. van Deventer holds a degree in medicine as well as a Ph.D. from the University of Amsterdam.
CHRISTIAN KLEMT. Christian Klemt, age 46, has served as our Chief Accounting Officer since August 2017. From September 2015 until August 2017, Mr. Klemt served as our Global Controller. While serving as our Global Controller, Mr. Klemt oversaw our transition to a domestic U.S. filer and conversion to U.S. Generally Accepted Accounting Principles. Mr. Klemt joined us from CGG SA (NYSE: CGG) where he held the position of Regional Finance Director and Country Manager. Prior to this, he held various senior finance roles including Group Finance Manager at Basell Polyolefines N.V. (now LyondellBasell N.V.) (NYSE: LBI) where he led the conversion to U.S. Generally Accepted Accounting Principles following the acquisition of Lyondell and was involved in the acquisition of various petrochemical assets. Mr. Klemt holds a Master’s degree in Business Administration from the University of Muenster, Germany and qualified as a German Certified Public Accountant and Tax Advisor while employed at KPMG.
ALEXANDER KUTA, PH.D. Dr. Kuta, age 59, has served as our Senior Vice President Regulatory Affairs since January 2017. Prior to joining uniQure, he was Vice President of Research & Development Global Regulatory Affairs for EMD Serono, responsible for immune-mediated diseases, oncology and biologics regulatory CMC, from January 2016 to September 2016. He joined EMD Serono in April 2013 as Vice President, Head of US Regulatory Affairs; while at EMD Serono he served on the US Leadership Team. From April 2012 to March 2013, Dr. Kuta was Vice President of Global Regulatory Affairs and a member of the Executive Leadership Team at Lantheus Medical Imaging. His previous industry experience includes senior regulatory leadership roles at AMAG Pharmaceuticals (NASDAQ: AMAG) from August 2010 to April 2012 as well as Genzyme Corporation from August 1995 to July 2010 where he worked in the areas of rare
diseases, cell and gene therapy, therapeutic proteins and biomaterials. Prior to joining industry, he was Chief of the Cytokine and Gene Therapy Branch in the Center for Biologics at FDA from January 1993 to August 1995 and a Scientific Reviewer from January 1990 to January 1993. Dr. Kuta has served on the BIO Regulatory Affairs Leadership Committee - Cell and Gene Therapy Working Group, as reviewer for the National Gene Vector Laboratories program, on the ICH (M6) Gene Therapy Working Group and is currently on the scientific review board of the Gene Therapy Resource Program of NHLBI/NIH. Dr. Kuta holds a Bachelor of Science degree from Saint John’s University, Collegeville, MN and a Ph.D. from the Chicago Medical School at Rosalind Franklin U-Med & Science. He conducted his post-doctoral studies at the National Cancer Institute/National Institutes of Health.
SCOTT MCMILLAN, PH.D. Dr. McMillan, age 60, has served as our Chief Operating Officer since August 2017. Dr. McMillan served most recently as Senior Vice President of Quality and Technical Operations at AMAG Pharmaceuticals from February 2008 to August 2017, where he also was a member of its Executive Management Team. Before joining AMAG Pharmaceuticals, Inc. (NASDAQ: AMAG), from January 2005 to February 2008 Dr. McMillan held similar positions at AVANT Immunotherapeutics, Inc. (now Celldex Therapeutics, Inc. (NASDAQ: CLDX), and from January 2002 to January 2005 with Johnson Matthey Pharmaceutical Materials, Inc. Dr. McMillan has over 25 years of biotechnology experience in quality, process development, scale-up, technology transfer from bench to commercial scale as well as manufacturing operations. Dr. McMillan holds a Ph.D. in Chemical Engineering from Georgia Institute of Technology, a Master’s degree in Economics and Bachelor’s degree in Chemical Engineering from the University of Delaware.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our executive officers, directors and persons who beneficially own more than ten percent of our Ordinary Shares to file reports of their beneficial ownership and changes in ownership (Forms 3, 4 and 5, and any amendment thereto) with the SEC. Executive officers, directors, and greater-than-ten-percent holders are required to furnish us with copies of all Section 16(a) forms they file.
Based solely upon a review of the Forms 3, 4, and 5, as applicable, furnished to us, we believe that our executive officers, directors, and greater-than-ten-percent beneficial owners filed their beneficial ownership and change in ownership reports with the SEC in a timely manner during the 2018 calendar year.
Code of Ethics
We have adopted a code of business conduct and ethics that is applicable to all of our employees, officers, and directors, including our Chief Executive Officer and Chief Financial Officer. The code of business conduct and ethics and corporate governance guidelines and board rules are available on our website at www.uniqure.com under “Investors & Newsroom — Corporate Governance — uniQure Code of Business Conduct and Ethics.” We have also adopted corporate governance guidelines and board rules which are applicable to the company’s management.
In addition to the Listing Rules of the Nasdaq Global Select Stock Market and rules and regulations as promulgated by the SEC, as a Dutch company, our governance practices are governed by the Dutch Corporate Governance Code. The Dutch Corporate Governance Code (as amended) contains a number of principles and best practices, with an emphasis on integrity, transparency, and accountability as the primary means of achieving good governance.
There is considerable overlap between the requirements we must meet under U.S. rules and regulations and the provisions of the Dutch Corporate Governance Code. Although we apply several provisions of the Dutch Corporate Governance Code, as a “domestic” issuer, we comply with the Nasdaq corporate governance requirements.
In accordance with the Dutch Corporate Governance Code’s compliance principle of “apply-or-explain,” which permits Dutch companies to be fully compliant with the Dutch Corporate Governance Code by either applying the Dutch practices or explaining why the company has chosen to apply different practices, we disclose in our Dutch statutory annual report that accompanies our Dutch statutory annual accounts to what extent we do not apply provisions of the Dutch Corporate Governance Code, together with the reasons for those deviations. Our Dutch statutory annual report may be found on the “Investors & Newsroom — Events and Presentations” section of our website at http://www.uniqure.com/investors-newsroom/events-presentations.php.
Audit Committee
The Audit Committee is currently comprised of Jack Kaye, Philip Astley-Sparke and Jeremy Springhorn. Mr. Kaye serves as the Chair of the Audit Committee. The Audit Committee has determined that Mr. Kaye is an “audit committee financial expert” within the meaning of the SEC’s rules and regulations and has the level of financial sophistication required by Nasdaq Rule 5605(c)(2)(A). Each of Mr. Kaye, Mr. Astley-Sparke and Dr. Springhorn satisfies the director independence standards and the independence standards for members of the Audit Committee established by SEC and Nasdaq.
Item 11. Executive Compensation
COMPENSATION DISCUSSION & ANALYSIS
This Compensation Discussion and Analysis (the “CD&A”) explains our compensation philosophy, policies and decisions for 2018 for the following executives, whom we refer to in this CD&A and in the following tables as our Named Executive Officers:
Named Executive Officer |
| Title |
Matthew Kapusta |
| Chief Executive Officer and interim Chief Financial Officer |
Jonathan Garen |
| Chief Business Officer |
Scott McMillan |
| Chief Operations Officer |
Maria Cantor |
| Senior Vice President, Investor Relations & Communications |
Alexander Kuta |
| Senior Vice President, Regulatory Affairs |
Executive Summary
Our Business
We are a leader in the field of gene therapy, seeking to develop one-time administered treatments with potentially curative results for patients suffering from genetic and other devastating diseases. We are working to advance a focused pipeline of innovative gene therapies that have been developed both internally and through partnerships, such as our collaboration with Bristol Myers-Squibb focused on cardiovascular diseases. We believe our gene therapy technology platform and manufacturing capabilities provide us distinct competitive advantages, including the potential to reduce development risk, cost and time to market. We produce our adeno-associated virus based, or AAV-based, gene therapies in our own facilities with a proprietary, commercial-scale, current good manufacturing practices (“cGMP”) and compliant, manufacturing process. We believe our Lexington, Massachusetts-based facility is one of the world’s leading, most versatile, gene therapy manufacturing facilities.
2018 and Early 2019 Achievements
In 2018 and early 2019, our Named Executive Officers played critical roles in the achievement of our goal to advance and expand our pipeline of leading gene therapy product candidates. In June 2018, we announced the enrollment of the first patient in the lead-in phase of the HOPE-B pivotal study of AMT-061 in hemophilia B. This first patient was treated in January 2019 after completing the lead-in phase. In September 2018, we completed the dosing of a Phase IIb dose-confirmation study of AMT-061. In February 2019, we presented updated data from the Phase IIb dose-confirmation study, which demonstrated that all three patients experienced increasing and sustained FIX levels after a one-time administration of AMT-061.
In December 2018, we submitted to the FDA our Investigational New Drug application for AMT-130, a novel gene therapy candidate for Huntington’s disease, which was subsequently cleared in January 2019. Preparations are underway to initiate the world’s first clinical study of a one-time administered therapy for the treatment of Huntington’s disease.
In November 2018, we announced the expansion of our research pipeline to include additional novel gene therapy candidates for treating additional indications, including hemophilia A, Fabry disease and spinocerebellar ataxia Type 3 (“SCA3”).
Compensation Philosophy and Principles
We operate in a competitive, rapidly changing and heavily-regulated industry. The long-term success of our business requires us to be resourceful, adaptable, and innovative. The skills, talent, and dedication of our executive officers are critical components to our success and the future growth of the company. Therefore, our compensation program for our executive officers, including our Named Executive Officers, is designed to attract, retain, and incentivize the best possible talent.
The Compensation Committee has established core objectives for our compensation programs, which are underpinned by a focus on elements that attract and retain the talent we believe is necessary to successfully lead uniQure and our employees globally.
Pay for performance
Motivate and reward our senior management to achieve established business and individual objectives
Align interests with our shareholders
Align compensation with the value realized by our shareholders
Use “at risk” compensation to incentivize executives
Use “at risk,” or variable, compensation to align the interests with those of our shareholders over time and contribute to the achievement of both short- and long-term goals
Attract and retain talented executives
Provide compensation opportunities and policies that are competitive with similarly sized biotechnology companies
How We Determine Executive Compensation
Compensation Oversight
The Compensation Committee is composed solely of independent directors, who at the end of 2018 were Madhavan Balachandran, Jack Kaye and David Meek, with Mr. Balachandran serving as the Committee Chair. The Chair of the Board, Philip Astley-Sparke is invited to attend meetings, but is not a formal member.
Details of the Compensation Committee’s duties are fully set out in the Compensation Committee’s charter, which can be found on our website: http://uniqure.com/investors-newsroom/corporate-governance.php.
The overarching purpose of the Compensation Committee is to oversee the manner in which the Board discharges its responsibilities relating to uniQure’s compensation policies, plans and programs for uniQure’s executive officers and directors.
The Compensation Committee is wholly accountable for any changes in compensation for the Chief Executive Officer, and the Chief Executive Officer is not included in any discussions regarding changes to his own compensation. For other Named Executive Officers, recommendations are made by the Chief Executive Officer and subsequently reviewed and approved by the Compensation Committee. The practice of the Compensation Committee has been to recommend for approval by our Board the compensation of the Chief Executive Officer and other Named Executive Officers. Overall compensation for our Named Executive Officers may increase or decrease year-to-year based upon, among other things, his or her annual performance or changes in his or her responsibilities.
The Annual Committee Process
The Compensation Committee typically meets six times a year to consider the following items:
Quarter |
| Typical Meeting Topics |
Q1 |
| · Review progress compared to corporate goals for prior year; · Determine corporate goals for current year; · Determine executive compensation for current year, including base salary, and bonus for prior year, target bonus for current year, and long term equity incentives; · Determine director compensation, including cash and equity compensation; and · Determine employee equity grants; and adopt terms of annual incentive bonus plan for current year. |
|
|
|
Q2 |
| · Assess prior year activities and Compensation Committee performance; and · Plan compensation cycle through remainder of current year and into following year. |
|
|
|
Q3 |
| · Review Compensation Committee Charter; · Review with compensation consultant best practices related to disclosure and director and executive compensation; and · Engage compensation consultant for work associated with upcoming compensation cycle. |
|
|
|
Q4 |
| · Review compensation peer group; · Review information provided by compensation consultant, including comparable data related to director and executive compensation; and · Perform initial evaluations of executive compensation (including base salary, bonus for current year, target bonus for upcoming year, and long term equity incentives), director compensation (including cash and equity compensation), employee equity grants, and terms of annual incentive bonus plan for upcoming year. |
Additional meetings are scheduled on an as needed basis.
Use of an Independent Advisor
As set out in its Charter, the Compensation Committee has the authority to retain outside consultants to provide independent advice to the Committee. In 2018, the Committee retained Willis Towers Watson (“WTW”) as its independent compensation consultant. WTW reported directly to the Compensation Committee and took direction from the Chair of the Committee. Having assessed WTW’s independence pursuant to SEC rules and Nasdaq listing rules, the Compensation Committee concluded that the work of WTW did not raise any conflicts of interest.
During the year, WTW provided assistance in designing and reviewing our management and director compensation programs, including developing compensation peer groups, providing market data on all aspects of compensation, and attended Compensation Committee meetings and provided general advice.
The Compensation Committee considered the analysis and advice from WTW, as well as support and insight from management when making compensation decisions.
Managing Compensation-Related Risk
uniQure operates in a highly regulated and competitive sector, and managing risk is embedded in the manner in which the Company is run and operates. The Board has delegated to the Compensation Committee responsibility to oversee compensation-related risk.
The Compensation Committee annually evaluates whether there are potential risks arising from the Company’s compensation policies and practices as part of our annual risk assessment performed by management and reported to and discussed with the Board. The Compensation Committee has determined that uniQure’s compensation policies and practices do not encourage executives to take excessive risks given that the various elements of the policies and practices diversify the risks associated with any single element of the executives’ compensation.
Compensation Peer Group
Given the fast-paced nature of our sector, the Compensation Committee reviews the constituents of the compensation peer group on an annual basis, with the support of WTW, to ensure they remain relevant and appropriate for comparisons. The Compensation Committee, upon advice received from WTW, selected companies that comprised our 2018 peer group through a screening process that considered publicly traded biopharmaceutical companies similar to us in number of employees, market capitalization and stage of product development. The number of employees at the companies in our 2018 peer group ranged from 89 to 594 and these companies had market capitalizations that ranged from approximately $459 million to $3.16 billion. At the time the analysis was conducted in the fall of 2017, we had 217 employees and a market capitalization of approximately $1 billion.
The 2018, compensation peer group was comprised of the following 18 companies:
· Adverum Biotechnologies · Applied Genetic Technologies · Arrowhead Pharmaceuticals · Blueprint Medicines · Celldex Therapeutics · Concert Pharmaceuticals |
| · Dynavax Technologies · Epizyme · Genocea Biosciences · Invitae · NewLink Genetics · Regenxbio |
| · Revance Therapeutics · Sangamo Therapeutics · Spark Therapeutics · T2 Biosystems · Vital Therapies · XBiotech |
The Compensation Committee determined that uniQure’s size relative to the peer group was appropriate for the purpose of compensation comparisons, with our November 2017 market cap ranking at the 59th percentile, one-year Total Shareholder Return (“TSR”) ranking at the 79th percentile, revenue ranking at the 82nd percentile and headcount ranking at the 94th percentile. For roles where insufficient proxy statement data was available to inform market comparisons, the Committee additionally referenced survey data provided by WTW and Radford for similarly sized biotech and biopharma companies.
Compensation Elements
At the 2016 Annual General Meeting, uniQure shareholders approved our Remuneration Policy, which sets out the structure for the compensation granted to our senior managers, including the Chief Executive Officer and other Named Executive Officers. The full policy can be found on our website: http://uniqure.com/investors-newsroom/corporate-governance.php.
In summary, our compensation program is designed to be straightforward in nature with five core elements, the first three of which are compensation related and the last two are benefits reflecting local market practices for each Named Executive Officer.
Element |
| Purpose |
| Key Features |
Base Salary |
| Provide market-competitive fixed compensation Attract exceptional talent in the relevant market |
| · Fixed cash compensation · Reviewed annually · Value informed by market levels for executives with comparable qualifications, experience and responsibility, coupled with the nature, scope and impact of the role · Target approximately 50th percentile of market peers, considering the factors noted above |
|
|
|
|
|
Short-Term Incentive (Annual Cash Bonus) |
| Reward for achievement of pre-defined criteria in areas of strategic importance to uniQure Align compensation with Company performance |
| · Subject to the approval of the Board in its discretion · Discretionary variable cash compensation ranging from 35% to 50% of annual Base Salary for target performance in 2018 · Maximum opportunity capped at 150% of target · Objectives based solely on corporate performance for the Chief Executive Officer, and a combination of corporate (80%) and individual (20%) performance for the other Named Executive Officers · Corporate and individual targets established in the beginning of each year · Assessment against the predetermined targets informs actual cash bonus that is awarded · Target opportunity informed by levels in the market, with reference to the 50th – 75th percentile |
|
|
|
|
|
Long-Term Incentives (Equity Awards) |
| Align long-term interests with shareholders Reward sustainable value creation Encourage retention |
| · Subject to the approval of the Board in its discretion · Annual awards of variable equity-based compensation · 2018 awards were a mix of stock options, restricted stock units (RSUs) and performance stock units (PSUs) · Stock options have a ten-year term, with 25% vesting after one year and then rateably on a quarterly basis · RSUs vest rateably on an annual basis over three years · PSUs are earned based on the Company’s performance related towards corporate objectives, as determined and assessed by the Board. These awards have a pay-out range of 0% - 150% of target and vest after three years |
|
|
|
|
|
Pension and Retirement Savings Plans |
| Provide market-competitive retirement benefits |
| · Based on local market practice · U.S.-based Named Executive Officers are eligible to participate in a qualified 401(k) Plan with matching of up to 3% of base salary · Netherlands-based Named Executive Officers are eligible to participate in a defined contribution pension plan |
Other Benefits |
| Provide market competitive benefits focused on well-being |
| · An Employee Stock Purchase Plan (“ESPP”) is offered to all eligible employees, which includes eligible Named Executive Officers · ESPP allows for purchase of discounted Ordinary Shares through accumulated payroll deductions · Medical, dental and vision health care plans with premiums paid by the company for U.S.-based Named Executive Officers · Named Executive Officers participate in other programs on consistent terms with broader employee population including paid time off, company-paid life insurance and short-term and long-term disability (with employee contribution), tuition reimbursement and fitness membership reimbursement |
Target Pay Mix
A significant portion of our Named Executive Officers’ target compensation is variable and at-risk, short term incentives (“STI”) and long term incentives (“LTI”) maximizing alignment with our shareholders and long-term value creation.
In 2018, the target compensation mix for the CEO, of which 82% was at-risk, is detailed below:
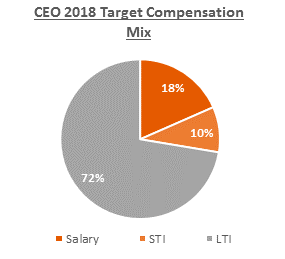
We do not specify a target mix of salary, STI and LTI compensation for our other Named Executive Officers, but we use target a range of approximately 60% - 65% for the at-risk components. The overall compensation structure is adjusted to determine an appropriate mix on a position-by-position basis based on peer group data and other comparable compensation data for each position.
2018 Compensation Decisions and Outcomes
Base Salary
As described below, our Named Executive Officers receive a base salary, the terms of which are subject to each of their individual employment agreements. The Compensation Committee annually reviews each Named Executive Officer’s base salary and may adjust such individual’s base salary after considering his or her responsibilities, performance and contributions to the Company and the Company’s overall performance. Additionally, the Compensation Committee will consider market data, with a view to ensuring base salary is set competitively, with a philosophy of targeting approximately the 50th percentile, taking into consideration the above factors. Based on that analysis and the recommendation of our Compensation Committee, the Board made adjustments from the prior year to the base salaries of our executive officers.
The 2018 base salary for our Named Executive Officers are described below:
Named Executive Officer |
| Base Salary |
| Effective Date |
| |
Matthew Kapusta |
| $ | 500,000 |
| January 2018 |
|
Jonathan Garen |
| $ | 360,500 |
| January 2018 |
|
Scott McMillan |
| $ | 364,320 |
| January 2018 |
|
Maria Cantor |
| $ | 309,000 |
| January 2018 |
|
Alexander Kuta |
| $ | 386,250 |
| January 2018 |
|
Short-term Incentive Bonus
The Company’s short-term incentives to Named Executive Officers provide an opportunity for our Named Executive Officers to earn a cash bonus, contingent on the successful achievement of goals with various program areas aligned to our strategic objectives. The award of any bonuses shall be subject to the approval of the Board in its discretion.
Any bonus for the Chief Executive Officer is based solely on the assessment of company-wide performance. For the other Named Executive Officers 80% of their opportunity is based on the same company-wide performance, with the remaining 20% based on individual performance.
Bonus opportunities for the Named Executive Officers in 2018 were as follows:
Named Executive Officer |
| Target Bonus |
| Maximum Bonus |
|
Matthew Kapusta |
| 50 | % | 75.0 | % |
Jonathan Garen |
| 35 | % | 52.5 | % |
Scott McMillan |
| 40 | % | 60.0 | % |
Maria Cantor |
| 35 | % | 52.5 | % |
Alexander Kuta |
| 35 | % | 52.5 | % |
We did not change any target bonus rates for 2018 during the year for our Named Executive Officers.
Annually, we evaluate and establish performance targets based on the corporate goals that are adopted by the Board on an annual basis. Our performance targets are generally based on the achievement of a key set of core objectives considered essential to our successful performance over a given calendar year. These core objectives are designed across the range of functions of the Company, including clinical, research and technology, regulatory, manufacturing, finance and other general and administrative functions. Our performance against targets are reviewed periodically with the Board throughout the year. At the end of the calendar year, our Board assesses the overall performance, which is then used for compensation decisions, including the payment of annual incentive bonuses.
In 2018, the Board approved the following corporate objectives:
Key Goal |
| Weighting |
| Why it Matters |
Advance our hemophilia B Program |
| 60% |
| Our AMT-061 product candidate for the treatment of hemophilia B is our lead product candidate and is currently enrolling a Phase III clinical trial. AMT-061 is the closest product to being approved for sale as a commercial product. |
|
|
|
|
|
Advance our Huntington’s Disease Program |
| 20% |
| Our AMT-130 product candidate for the treatment of Huntington’s disease is entering the clinical phase, and the associated Investigational New Drug Application recently became effective. AMT-130 also has potential to be approved for sale as a commercial product. |
|
|
|
|
|
Advance our Heart Failure Collaboration Program |
| 10% |
| During 2015, we entered into a collaboration with Bristol-Meyers Squibb Company focused primarily on cardiovascular disease. In 2018, the lead gene therapy program targeted S100A1 for congestive heart failure. |
|
|
|
|
|
Advance our Research and Technology Programs and Corporate Development |
| 10% |
| The development of enabling technologies and additional product candidates is core to our strategy. Enabling technologies include novel gene therapy components, such as AAV vectors and promoters, administration techniques and manufacturing capabilities. Our research pipeline is currently focused on liver-directed and CNS disorders, including gene therapies targeting hemophilia A, Fabry disease and spinocerebellar ataxia Type 3.
To facilitate our goals, it is also critical that we manage our corporate resources effectively, as well as develop an infrastructure and organization that anticipates emergent needs. |
We believe these four strategic areas are critical to the successful execution of our long-term strategy and the achievement of sustainable shareholder value creation. In approving the targets, each goal within a program area has an associated level of achievement and time frame. The extent to which the goal is achieved, and whether or not it is on time informs the rating assigned at year-end.
In order to achieve a threshold bonus, defined as 50% of the target bonus, the total performance must be assessed at a minimum of 50%. Amounts between threshold, target and maximum payout are interpolated to reward incremental achievement and no amounts are paid for results on a particular performance metric if actual results are below threshold. For performance assessed at below 50%, no bonus is paid, and for performance assessed at above 150%, no additional bonus is paid.
For the 2018 annual incentive bonus plan, our Board determined, based on the recommendation of the Compensation Committee, that the overall achievement of the Company relative to the target performance objectives was 140%. A summary of the performance assessment is below:
Key Goal |
| Assessment |
Advance our hemophilia B Program |
| The Board determined that there was overachievement based on:
· the successful completion of our AMT-060/AMT-061 comparability studies; · the receipt of FDA clearance to initiate our Phase 2b and phase 3 clinical studies of AMT-061; · the timely release of clinical material for the Phase 2b and Phase 3 studies; · the issuance of new patents providing broad protection of the FIX-Padua variant in gene therapy; · the initiation of patient enrollment for our Phase 3 pivotal study and achieving certain targets related to sites activated and patients enrolled at yearend; and · the completion of patient enrollment of our phase 2b study, with positive early data announced in November 2018.
The overall contribution to the final assessment was 100%. |
|
|
|
Advance our Huntington’s Disease Program |
| The Board determined there was partial achievement of our corporate goals based on:
· the successful completion of the Company’s safety and toxicology study of AMT-130; and · the submission of our Investigational New Drug Application in December 2018.
The overall contribution to the final assessment was 15%. |
|
|
|
Advance our Heart Failure Collaboration Program |
| The Board determined there was partial achievement of our corporate goals based on:
· the successful manufacture and release of AMT-126 research material; and · the timely initiation and completion of a preclinical heart function study. The results of the study demonstrated successful DNA delivery and expression of S100A1 protein, thereby validating the Company’s platform technology. However, the target did not show a material impact on heart function.
The overall contribution to the final assessment was 5%. |
|
|
|
Advance our Research and Technology Programs |
| The Board determined there was overachievement of our corporate goals based on:
· the initiation of new research programs (hemophilia A, Fabry disease, SCA3 and other indications); · the demonstration of proof of concept on two liver disease programs (hemophilia A and Fabry); · the demonstration of proof-of-concept for a next-generation, highly potent, liver-specific promoter; · the identification of an AAV vector for use in our SCA3 program; · our holding of a research and development investor day in November 2018; and · our demonstration of manufacturing production at 500 liters and with next-generation, dual-baculovirus technology.
The overall contribution to the final assessment was 13.5%. |
Advance Corporate Development Initiatives |
| The Board determined there was overachievement of our corporate goals based on:
· the completion of a follow-on public offering of $147.5 million; · the achievement of financial results within budget; · the timely completion of the organizational restructuring; and · other items associated with the internal operations of the Company.
The overall contribution to the final assessment was 6.5%. |
In respect of the individual performance component for the Named Executive Officers other than the Chief Executive Officer, the Compensation Committee noted the following achievements in approving the rating recommendation submitted by the Chief Executive Officer:
Named Executive Officer |
| Individual Goal |
| Observations |
Matthew Kapusta |
| Not applicable |
| Not applicable. |
|
|
|
|
|
Jonathan Garen |
| Exceeded goals |
| Primary achievements were leading the execution of several business opportunities, including licensing opportunities relevant to research pipeline. |
|
|
|
|
|
Scott McMillan |
| Exceeded goals |
| Primary achievements were leading the manufacture of AMT-061 and AMT-130 clinical material and successfully demonstrating production at 500-liter capacity and with a next-generation dual baculovirus process. |
|
|
|
|
|
Maria Cantor |
| Exceeded goals |
| Primary achievements were leading team the execution of a successful investor relations plan, including facilitating meaningful investor interactions, achieving new analyst coverage and coordinating a research and development investor day. |
|
|
|
|
|
Alexander Kuta |
| Exceeded goals |
| Primary achievements were leading the AMT-061 regulatory process that lead to confirmation to proceed with the Phase 2b and Phase 3 studies, the achievement of orphan drug designation in the European Union and the coordination of the AMT-130 IND submission. |
The combination of this company-wide corporate performance and individual performance resulted in the following awards in respect of 2018 performance:
Named Executive Officer |
| Actual Bonus |
| Actual Bonus |
| Actual Bonus |
| |
Matthew Kapusta |
| $ | 350,000 |
| 70 | % | 140 | % |
Jonathan Garen |
| $ | 171,598 |
| 48 | % | 136 | % |
Scott McMillan |
| $ | 198,190 |
| 54 | % | 136 | % |
Maria Cantor |
| $ | 149,247 |
| 48 | % | 138 | % |
Alexander Kuta |
| $ | 182,503 |
| 47 | % | 135 | % |
2018 Long-Term Incentive Awards
The Company’s 2014 Restated Plan provides that the Board may grant equity awards to its employees. These grants include annual and periodic equity awards linked to continued employment and, at the Board’s discretion, the achievement of certain performance targets. Such grants as they apply to our Named Executive Officers are described below. Pursuant to the 2014 Restated Plan, employees may be granted options, restricted share units (RSUs) or performance share units (PSUs). By awarding long-term incentive awards via a combination of different vehicles, the Compensation Committee can balance the objectives of driving sustainable long-term performance and shareholder value creation, encouraging retention while remaining market competitive.
The Company adopted an employee share purchase plan (the “Purchase Plan”) at the 2018 Annual General Meeting. The Purchase Plan is designed to allow eligible employees of the Company and its designated subsidiaries to purchase discounted Ordinary Shares at designated intervals through their accumulated payroll deductions. The provisions of the Purchase Plan are intended to satisfy the requirements of Section 423 of the U.S. Internal Revenue Code of 1986, as amended, with respect to U.S. participants. Favorable tax treatment is available for U.S. tax residents participating in a plan that qualifies under Section 423.
Awards are generally made annually in the first calendar quarter, taking into account impact on achieving our corporate goals, performance in the prior year and market data for the compensation peer group. The key features of each award type are as follows:
|
| · Options vest over a period of four years, with 25% of options granted becoming exercisable on the first anniversary, with the remaining options becoming exercisable pro-rata on a quarterly basis over the remaining three years. · Awards expire after ten years. · Stock options cannot be repriced, reset, or exchanged for cash if underwater without shareholder approval. |
|
|
|
|
| · RSUs vest pro-rata on an annual basis over three years. · Dividends do not accrue until shares are free from restrictions, unless expressly stated in the applicable award agreement. · Shares are issued to the participant upon vesting of the award, but may be subject to a sale of a portion of the shares to cover tax withholding requirements. |
|
| · PSUs vest after three years subject to pre-established performance conditions. · The performance conditions are determined by the Board and have historically been consistent with those established on a company-wide basis under the short-term incentive plan in the year of grant. · The payout range is 50%-150% of the target award. · Any PSUs that are earned vest on the third anniversary of grant. · Dividends do not accrue until shares are free from restrictions, unless expressly stated in the applicable award agreement. · Shares are issued to the participant upon vesting of the award but may be subject to a sale of a portion of the shares to cover tax withholding requirements. |
Target equity awards are approved each year by the Compensation Committee, based on a combination of factors including overall corporate achievement, individual performance, impact of individual on achieving the Company’s corporate goals and market competitiveness. In determining and approving award values, the Compensation Committee reviews data for our peer group and the overall total compensation of our executive officers. Once target values are approved, the Compensation Committee recommends that the Board grant the equity commesurate with the target equity award values. In light of the high overall corporate performance and individual achievement in 2017, our Compensation Committee recommended that the Board grant long-term incentive equity awards that were commensurate with the 62.5th percentile of our peer group. Accordingly, target equity awards for Named Executive Officers other than our CEO were approved at a level of approximately 150% or 175% of 2017 base salary, and target equity awards for our CEO were approved at a level of 436% of 2017 base salary. Scott McMillan’s 2018 award was pro-rated by 50% reflecting his appointment in the middle of 2017.
In establishing the mix of long-term incentives to award our Named Executive Officers, the Compensation Committee referenced market data for our peers, which found that most competitors grant awards in either stock options or a combination of stock options and restricted stock units. To further enhance the alignment of executive interests with the achievement of our corporate objectives, the Committee determined that it was appropriate for a portion of the awards to be linked to performance in the form of performance stock units, accepting that this would differentiate uniQure from typical market practice.
In 2018, equity awards had the following target mix based on fair values determined as of December 13, 2017:
|
|
Employee Share Purchase Plan
The Employee Share Purchase Plan is designed to allow eligible employees of uniQure and its designated subsidiaries to purchase discounted Ordinary Shares at designated intervals through their accumulated payroll deductions. The purpose of the Plan is to provide employees with a convenient method to invest in uniQure Ordinary Shares which will increase the equity stake of our employees and will benefit shareholders by aligning more closely the interests of our participating employees with those of our shareholders. We believe that this will help to motivate and retain highly-qualified employees.
Under the Plan, the number of Ordinary Shares initially reserved for issuance is 150,000. The purchase price of the Ordinary Shares acquired on each purchase date will be the lesser of (a) 85% of the closing price of an Ordinary Share on the first day of the offering period or (b) 85% of the closing price of an Ordinary Share on the purchase date.
CEO Pay Ratio
Under Item 402(u) of Regulation S-K adopted by the SEC pursuant to the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, we are required to disclose the ratio of the annual total compensation of our Chief Executive Officer to the annual total compensation of our median compensated employee, excluding our CEO.
Matthew Kapusta (a) |
| $ | 4,095,328 |
|
Median Employee 2018 Annual Total Compensation |
| $ | 111,598 |
|
CEO to Median Employee Pay Ratio |
| 37:1 |
| |
(a) This annual total compensation is the Total Compensation from the Summary Compensation Table.
Methodology
Our methodology for determining our CEO pay ratio relies on estimates and assumptions calculated in a manner consistent with SEC rules and guidance.
Determination of Employee Population
We determined our global employee population as of the December 31, 2018, including full-time, part-time, seasonal and temporary workers, other than our CEO. As of December 21, 2018, our total employee population, other than our CEO, consisted of 216 employees.
Calculating Median Employee Compensation
We identified the median employee based on the consistently applied compensation measure (“CACM”) of base salary obtained from our pay roll records across our global employee population. We annualized the pay of our hourly employees, adjusted the pay of employees in Europe from Euros to U.S. Dollars using the average exchange rate that we applied in 2018, and, where applicable, pro-rated any annualized base salaries of part-time employees to reflect the part-time schedule and the actual base salary being earned. Based upon the comparison using the CACM, we determined that the total compensation of our median employee was $111,598 as of December 31, 2018.
Our CEO to median employee pay ratio is 37:1.
The SEC’s rules for identifying the median compensated employee and calculating the pay ratio based on that employee’s annual total compensation allow companies to adopt a variety of methodologies, to apply certain exclusions, and to make reasonable estimates and assumptions that reflect their employee populations and compensation practices. As a result, the pay ratio reported by other companies, including our compensation peer group, may not be comparable to the pay ratio reported above, as other companies have different employee populations and compensation practices and may utilize different methodologies, exclusions, estimates and assumptions in calculating their own pay ratios.
Employment Agreements
Matthew Kapusta
Prior to becoming our Chief Executive Officer, Mr. Kapusta served as our Chief Financial Officer. On December 9, 2014, the Company entered into an employment agreement with Mr. Kapusta for the role of Chief Financial Officer (the “Kapusta CFO Agreement”). On October 19, 2017 (the “First Kapusta Amendment, March 14, 2017 (the “Second Kapusta Amendment”) and October 26, 2017 (the “Third Kapusta Amendment,” together with the First Kapusta Amendment and the Second Kapusta Amendment, the “Kapusta Agreement Amendments”), the Company entered into amendments to the Kapusta CFO Agreement in connection with his new role as Chief Executive Officer (the Kapusta CFO Agreement as amended by the Kapusta Agreement Amendments being the “Kapusta Employment Agreement”). The Kapusta Employment Agreement provides that Mr. Kapusta will earn a base salary equal to $450,000 per year, plus reimbursement of expenses incurred on the Company’s behalf. Effective January 1, 2019, Mr. Kapusta’s base salary was increased to $550,000 per year. Mr. Kapusta is also eligible for an annual performance bonus with a target for 2019 of 55% of his base salary and a grant of restricted share units as further described in the Kapusta Employment Agreement. The termination provisions of the Kapusta Employment Agreements are further discussed below. The term of the Kapusta Employment Agreement will run through December 31, 2019 or until terminated by either us or by Mr. Kapusta. A copy of the Kapusta CFO Agreement is filed as Exhibit 10.6 to the Company’s Annual Report on Form 10-K filed with the SEC on March 15, 2017. A copy of the Second Kapusta Amendment is filed as Exhibit 10.7 to the Company’s Annual Report on Form 10-K filed with the SEC on March 15, 2017. A copy of the Third Kapusta Amendment is filed as Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed with the SEC on November 1, 2017. The foregoing descriptions of the Kapusta Employment Agreement do not purport to be complete and are qualified in their entirety by reference to the full text of such agreement.
Jonathan Garen
Mr. Garen entered into an employment agreement with the Company on June 15, 2016 for the role of Chief Business Officer (the “Garen Employment Agreement”). The Garen Employment Agreement provides that Mr. Garen will receive a base salary of $340,000 per year, subject to review at the sole discretion of the Company and a discretionary bonus of up to 35% of annual base salary (with any such bonus for 2016 being pro-rated for length of service). Under the Garen Employment Agreement, Mr. Garen is also entitled to expenses and reimbursements. He was also entitled to a grant of an option to purchase 50,000 ordinary shares in the Company pursuant to the Company’s equity incentive plan and would be eligible for future grant awards. In March 2019, Mr. Garen received a letter (the “Garen 2019 Letter”), which provides that his 2019 base salary will be $378,525 and his 2018 bonus will be $171,598. The Garen 2019 Letter also provides that Mr. Garen will be entitled to participate in the 2019 equity grants. The termination provisions of the Garen Employment Agreements are further discussed below. The Garen Employment Agreement is to continue in force from year to year unless terminated in accordance with its terms.
Maria Cantor
Ms. Cantor entered into an employment agreement with the Company on May 26, 2016 for the role of Senior Vice President, Investor Relations and Communications (the “Cantor Employment Agreement”). The Cantor Employment Agreement provides that Ms. Cantor will receive a base salary of $290,000 per year, subject to review at the sole discretion of the Company and a discretionary bonus of up to 35% of annual base salary (with any such bonus for 2016 being pro-rated for length of service). Under the Cantor Employment Agreement, Ms. Cantor is also entitled to expenses and reimbursements. She was also entitled to a grant of an option to purchase 50,000 ordinary shares in the Company pursuant to the Company’s equity incentive plan and would be eligible for future grant awards. In March 2019, Ms. Cantor received a letter (the “Cantor 2019 Letter”), which provides that her 2019 base salary will be $321,360 and her 2018 bonus will be $149,247. The Cantor 2019 Letter also provides that Ms. Cantor will be entitled to participate in the 2019 equity grants. The termination provisions of the Cantor Employment Agreement are further discussed below. The Cantor Employment Agreement is to continue in force from year to year unless terminated in accordance with its terms.
Alexander E. Kuta
Mr. Kuta entered into an employment agreement with the Company on January 23, 2017 for the role of Senior Vice President, Regulatory Affairs (the “Kuta Employment Agreement”). The Kuta Employment Agreement provides that Mr. Kuta will receive a base salary of $375,000 per year, subject to review at the sole discretion of the Company and a discretionary bonus of up to 35% of annual base salary (with any such bonus for 2017 being pro-rated for length of service). Under the Kuta Employment Agreement, Mr. Kuta is also entitled to expenses and reimbursements. He was also entitled to a grant of an option to purchase 150,000 ordinary shares in the Company pursuant to the Company’s equity incentive plan and would be eligible for future grant awards. In March 2019, Mr. Kuta received a letter (the “Kuta 2019 Letter”), which provides that his 2019 base salary will be $397,838 and his 2018 bonus will be $182,503. The Kuta 2019 Letter also provides that Mr. Kuta will be entitled to participate in the 2019 equity grants. The termination provisions of the Kuta Employment Agreements are further discussed below. The Kuta Employment Agreement is to continue in force from year to year unless terminated in accordance with its terms.
Scott McMillan
Mr. McMillan entered into an employment agreement with the Company on August 7, 2017 for the role of Chief Operations Officer (the “McMillan Employment Agreement”). The McMillan Employment Agreement provides that Mr. McMillan will receive a base salary of $360,000 per year, subject to review at the sole discretion of the Company and a discretionary bonus of up to 40% of annual base salary (with any such bonus for 2017 being pro-rated for length of service). Under the McMillan Employment Agreement, Mr. McMillan is also entitled to expenses and reimbursements. He was also entitled to a grant of an option to purchase 150,000 ordinary shares in the Company pursuant to the Company’s equity incentive plan and would be eligible for future grant awards. In March 2019, Mr. McMillan received a letter (the “McMillan 2019 Letter”), which provides that his 2019 base salary will be $ 391,644 and his 2018 bonus will be $198,190. The McMillan 2019 Letter also provides that Mr. McMillan will be entitled to participate in the 2019 equity grants. The termination provisions of the McMillan Employment Agreements are further discussed below. The McMillan Employment Agreement is to continue in force from year to year unless terminated in accordance with its terms.
Other Executive Compensation Policies
Tax and Accounting Considerations
Prior to the passage of the Tax Cuts and Jobs Act of 2017, Section 162(m) of the Internal Revenue Code of 1986, as amended (the “Internal Revenue Code”), had generally disallowed a tax deduction for compensation in excess of $1.0 million paid to a company’s named executive officers, other than its chief financial officer. Historically, qualifying performance-based compensation was not subject to the deduction limitation if specified requirements were met. However, effective for taxable years beginning after December 31, 2017, the exemption for qualified performance-based compensation from the deduction limitation of Section 162(m) has been repealed, such that compensation paid to our NEOs in excess of $1 million will not be deductible unless it qualifies for the limited transition relief applicable to certain compensation arrangements in certain arrangements place as of November 2, 2017.
“Nonqualified deferred compensation” is required by Section 409A of the Internal Revenue Code to be paid under plans or arrangements that satisfy certain statutory requirements regarding timing of deferral elections, timing of payments and certain other matters. Employees and service providers who receive compensation that fails to satisfy these requirements may be subject to accelerated income tax liabilities, a 20% excise tax, penalties and interest on their compensation under such plans. The Company seeks to design and administer our compensation and benefits plans and arrangements for all of our employees and service providers, including our named executive officers, to keep them either exempt from or in compliance with the requirements of Section 409A.
Sections 280G and 4999 of the Internal Revenue Code impose certain adverse tax consequences on compensation treated as excess parachute payments. An executive is treated as having received excess parachute payments if such executive receives compensatory payments or benefits that are contingent on a change in control, and the aggregate amount of such payments and benefits equal or exceeds three times the executive’s base salary amount. The portion of the payments and benefits in excess of one times base salary amount are treated as excess parachute payments and are subject to a 20% excise tax, in addition to any applicable federal income and employment taxes.
Deferred Compensation and Retirement Plans
The Company operates a qualified 401(k) Plan for all employees at its Lexington facility in the USA. The uniQure, Inc. 401(k) Plan is an employee contribution plan only, and there are no employer contributions currently being made. The uniQure Inc. 401(k) Plan offers both a before tax and after tax (Roth) component, which are subject to IRS statutory limits for each calendar year.
The Company operates a defined contribution pension plan for all employees at its Amsterdam facility in the Netherlands, which is funded by the Company through payments to an insurance company.
Equity Incentive Plan
The 2014 Restated Plan enables the Board to among others grant options, Restricted Stock Units (RSUs) and PSUs. The purpose of the 2014 Restated Plan is to advance the interests of the Company’s shareholders by enhancing the Company’s ability to attract, retain and motivate persons who are expected to make important contributions to the group and by providing such persons with equity ownership opportunities and performance-based incentives that are intended to better align the interests of such persons with those of the Company’s shareholders.
The terms of the PSUs are further discussed above. For both RSUs and PSUs, the shares are automatically issued to the grantee upon the vesting of the award.
Under the 2014 Restated Plan, the maximum number of Ordinary Shares available is currently limited to 8,601,471. As of March 31, 2019, 3,083,113 Ordinary Shares remain available for grant under the 2014 Restated Plan.
Employee Share Purchase Plan
The ESPP is designed to allow eligible employees of the Company and its designated subsidiaries to purchase discounted Ordinary Shares at designated intervals through their accumulated payroll deductions. The purpose of the ESPP is to provide employees with a convenient method to invest in the Company’s Ordinary Shares which will increase the equity stake of the Company’s employees and will benefit shareholders by aligning more closely the interests of participating employees with those of the Company’s shareholders. The Company believes that this will help to motivate and retain highly-qualified employees.
Under the ESPP, the number of Ordinary Shares initially reserved for issuance is 150,000. The purchase price of the Ordinary Shares acquired on each purchase date will be the lesser of (a) 85% of the closing price of an Ordinary Share on the first day of the offering period or (b) 85% of the closing price of an Ordinary Share on the purchase date. As of March 31, 2019, 144,283 Ordinary Shares remain available for issuance under the ESPP.
Role of Executive Officer in Determining Executive Compensation
The Compensation Committee and Board approve all compensation decisions related to our Named Executive Offices. Such decisions by the Compensation Committee regarding compensation were made independently from our Named Executive Officers.
Stock Ownership Requirements and Hedging Policies
Currently, the Company does not have any formal stock ownership requirements or any specific hedging policies related to stock ownership.
Risk Considerations
The Compensation Committee annually evaluates whether there are potential risks arising from the Company’s compensation policies and practices. Based on such evaluation, the Compensation Committee believes that the Company’s compensation policies and practices do not encourage executives to take excessive risks because the various elements of the Company’s executive compensation policies and practices diversify the risks associated with any single element of the executive’s compensation. Instead, the elements of the Company’s executive compensation policy are, collectively, designed to achieve the Company’s annual and long-term corporate objectives and strategies.
SUMMARY COMPENSATION TABLE
The following table summarizes the annual compensation paid to our Named Executive Officers for the three fiscal years ended December 31, 2018, 2017 and 2016.
Name |
| Year |
| Salary |
| Stock |
| Option |
| Non-Equity |
| Medicare |
| All other |
| Total |
|
|
|
|
| ($) |
| ($) |
| ($) |
| ($) |
| ($) |
| ($) |
| ($) |
|
Matthew Kapusta |
| 2018 |
| 501,923 |
| 2,388,508 |
| 823,201 |
| 350,000 |
| 23,596 |
| 8,100 |
| 4,095,328 |
|
|
| 2017 |
| 468,109 |
| 2,612,217 |
| 560,496 |
| 257,624 |
| 23,745 |
| 7,343 |
| 3,929,535 |
|
|
| 2016 |
| 379,996 |
| 111,129 |
| 381,463 |
| 142,325 |
| 23.992 |
| 5,238 |
| 1,044,144 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Jonathan Garen |
| 2018 |
| 359,260 |
| 608,881 |
| 151,441 |
| 171,958 |
| 23,983 |
| 4,926 |
| 1,320,089 |
|
|
| 2017 |
| 348,500 |
| 430,027 |
| 87,469 |
| 139,659 |
| 25,227 |
| 4,816 |
| 1,035,698 |
|
|
| 2016(4) |
| 155,762 |
| 15,921 |
| 27,216 |
| 57,834 |
| 23,992 |
| 4,668 |
| 274,785 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Maria Cantor |
| 2018 |
| 310,189 |
| 584,658 |
| 147,269 |
| 149,247 |
| 23,586 |
| 8,100 |
| 1,223,049 |
|
|
| 2017 |
| 300,000 |
| 430,027 |
| 92,083 |
| 120,223 |
| 23,744 |
| 7,909 |
| 973,986 |
|
|
| 2016(5) |
| 145,139 |
| 15,921 |
| 27,216 |
| 53,444 |
| 14,237 |
| 3,393 |
| 263,949 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Alexander E. Kuta |
| 2018 |
| 387,736 |
| 166,509 |
| 183,641 |
| 182,503 |
| 16,648 |
| 8,100 |
| 945,137 |
|
|
| 2017(6) |
| 353,365 |
| — |
| 110,554 |
| 141,610 |
| 15,317 |
| 8,100 |
| 628,946 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scott McMillan |
| 2018 |
| 365,721 |
| 93,242 |
| 230,399 |
| 198,190 |
| 23,586 |
| 8,100 |
| 919,239 |
|
|
| 2017(7) |
| 145,385 |
| — |
| 53750 |
| 164,880 |
| 9,177 |
| 3,946 |
| 377,138 |
|
(1) |
| Salary is determined based on actual salary during the fiscal year 2018. |
(2) |
| The value of stock awards and stock options as reported in their respective columns is calculated using the grant date accounting fair value determined in accordance with Accounting Standards Codification 718, Compensation-Stock Compensation (“ASC 718”). Amounts reflected in the stock awards column are comprised of the accounting value of both the time-vested RSUs and PSUs granted in the years reflected. For assumptions and estimates used in determining these values, see Management’s Discussion and Analysis of Financial Condition and Results of Operations — Share-based Payments and Note 2.3.18 of the Consolidated Financial Statements in our 2018 Annual Report on Form 10-K. |
(3) |
| These amounts reflect the annual cash bonus awards granted to the Named Executive Officers pursuant to the Company’s Short-term Incentive program. |
(4) |
| Mr. Garen’s employment commenced on July 7, 2016. |
(5) |
| Mrs. Cantor’s employment commenced on June 27, 2016. |
(6) |
| Mr. Kuta’s employment commenced on January 25, 2017. |
(7) |
| Mr. McMillan’s employment commenced on August 7, 2017. |
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR END 2018
The following table contains information concerning exercisable stock options with respect to our Ordinary Shares, RSUs and PSUs granted to our named executive officers that were outstanding as of December 31, 2018.
|
|
|
| Option Awards (1) |
| Stock Awards (2) |
| ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Incentive |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Equity |
| Plan |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Incentive |
| Awards: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Plan |
| Market or |
|
|
|
|
|
|
|
|
| Equity |
|
|
|
|
| Number |
|
|
| Awards: |
| Payout |
|
|
|
|
|
|
|
|
| incentive plan |
|
|
|
|
| of |
| Market |
| Number of |
| Value of |
|
|
|
|
|
|
|
|
| awards: |
|
|
|
|
| Shares |
| Value of |
| Unearned |
| Unearned |
|
|
|
|
|
|
| Number of |
| Number of |
|
|
|
|
| or Units |
| Shares or |
| Shares, |
| Shares |
|
|
|
|
| Number of |
| securities |
| securities |
|
|
|
|
| of Stock |
| Units of |
| Units or |
| Units or |
|
|
|
|
| Securities |
| underlying |
| underlying |
|
|
|
|
| That |
| Stock |
| Rights |
| Other |
|
|
|
|
| Underlying |
| unexercised |
| unexercised |
| Option |
|
|
| Have |
| That |
| That Have |
| Rights |
|
|
|
|
| Unexercised |
| options |
| unearned |
| Exercise |
| Option |
| Not Yet |
| Have Not |
| Not Yet |
| That Have |
|
|
| Type of Equity |
| Options Exercisable |
| Unexercisable |
| options |
| Price |
| Expiration |
| Vested (3) |
| Vested |
| Vested (4) |
| Not Vested |
|
Name |
| Award |
| (#) |
| (#) |
| (#) |
| ($) |
| Date |
| (#) |
| ($) |
| (#) |
| ($) |
|
Matthew Kapusta |
| Options |
| 93,750 |
| 6,250 |
| — |
| 14.71 |
| 2025 |
| — |
| — |
| — |
| — |
|
|
| Options |
| 81,250 |
| 18,750 |
| — |
| 23.60 |
| 2025 |
| — |
| — |
| — |
| — |
|
|
| Options |
| 56,250 |
| 43,750 |
| — |
| 7.53 |
| 2026 |
| — |
| — |
| — |
| — |
|
|
| Options |
| 76,562 |
| 98,438 |
| — |
| 6.22 |
| 2027 |
| — |
| — |
| — |
| — |
|
|
| Options |
| — |
| 83,663 |
| — |
| 19.39 |
| 2028 |
| — |
| — |
| — |
| — |
|
|
| RSUs(3) |
| — |
| — |
| — |
| — |
| — |
| 87,500 |
| 2,521,750 |
| — |
| — |
|
|
| RSUs(4) |
| — |
| — |
| — |
| — |
| — |
| 31,374 |
| 904,199 |
| — |
| — |
|
|
| PSUs(6) |
| — |
| — |
| — |
| — |
| — |
| 23,064 |
| 664,704 |
| — |
| — |
|
|
| PSUs(6) |
| — |
| — |
| — |
| — |
| — |
| 209,625 |
| 6,041,393 |
| — |
| — |
|
|
| PSUs(6) |
| — |
| — |
| — |
| — |
| — |
| 43,924 |
| 1,265,890 |
| — |
| — |
|
Jonathan Garen |
| Options |
| 28,126 |
| 21,875 |
| — |
| 7.60 |
| 2026 |
| — |
| — |
| — |
| — |
|
|
| Options |
| 18,921 |
| 24,329 |
| — |
| 5.37 |
| 2027 |
| — |
| — |
| — |
| — |
|
|
| Options |
| — |
| 22,364 |
| — |
| 19.39 |
| 2028 |
| — |
| — |
| — |
| — |
|
|
| RSU’s(5) |
| — |
| — |
| — |
| — |
| — |
| 43,250 |
| 1,246,465 |
| — |
| — |
|
|
| RSU’s(4) |
| — |
| — |
| — |
| — |
| — |
| 8,387 |
| 241,713 |
| — |
| — |
|
|
| PSUs(6) |
| — |
| — |
| — |
| — |
| — |
| 56,115 |
| 1,617,234 |
| — |
| — |
|
|
| PSUs(6) |
| — |
| — |
| — |
| — |
| — |
| 11,742 |
| 338,404 |
| — |
| — |
|
Maria Cantor |
| Options |
| 31,250 |
| 18,750 |
| — |
| 8.21 |
| 2026 |
| — |
| — |
| — |
| — |
|
|
| Options |
| 18,921 |
| 24,329 |
| — |
| 5.37 |
| 2027 |
| — |
| — |
| — |
| — |
|
|
| Options |
| — |
| 19,169 |
| — |
| 19.39 |
| 2028 |
| — |
| — |
| — |
| — |
|
|
| RSU’s(5) |
| — |
| — |
| — |
| — |
| — |
| 43,250 |
| 1,246,465 |
| — |
| — |
|
|
| RSU’s(4) |
| — |
| — |
| — |
| — |
| — |
| 7,188 |
| 207,158 |
| — |
| — |
|
|
| PSUs(6) |
| — |
| — |
| — |
| — |
| — |
| 56,115 |
| 1,617,234 |
| — |
| — |
|
|
| PSUs(6) |
| — |
| — |
| — |
| — |
| — |
| 10,063 |
| 338,404 |
| — |
| — |
|
Alexander E Kuta |
| Options |
| 65,625 |
| 84,375 |
| — |
| 5.31 |
| 2027 |
| — |
| — |
| — |
| — |
|
|
| Options |
| — |
| 19.169 |
| — |
| 19.39 |
| 2028 |
| — |
| — |
| — |
| — |
|
|
| RSUs(4) |
| — |
| — |
| — |
| — |
| — |
| 8,986 |
| 258,977 |
| — |
| — |
|
|
| PSUs(6) |
| — |
| — |
| — |
| — |
| — |
| 12,580 |
| 362,556 |
| — |
| — |
|
Scott McMillan |
| Options |
| 46,875 |
| 103,125 |
| — |
| 8.49 |
| 2027 |
| — |
| — |
| — |
| — |
|
|
| Options |
| — |
| 13,419 |
| — |
| 19.39 |
| 2028 |
| — |
| — |
| — |
| — |
|
|
| RSUs(4) |
| — |
| — |
| — |
| — |
| — |
| 5,032 |
| 145,022 |
| — |
| — |
|
|
| PSUs(6) |
| — |
| — |
| — |
| — |
| — |
| 7,045 |
| 203,037 |
| — |
| — |
|
(1) The option grants typically vest over four years; 25% on the anniversary of the grant date and in equal monthly installments thereafter.
(2) RSU and PSU awards are valued based on the closing stock price of the Company on December 31, 2018 ($28.82).
(3) March 2017 RSU awards granted on March 14, 2017, vest 50% after one year, respectively two years after the grant date.
(4) 2018 RSU awards granted on January 26, 2018, vest 1/3 after each of one year, two years and three years after the grant date.
(5) 2017 PSU awards granted on January 27, 2017, were earned in December 2017 and vest three years following the date of the grant.
(6) PSU awards granted on January 26, 2018 vest three years following the date of the grant, subject to the achievement of performance metrics. The performance metrics were achieved and PSUs were earned on January 25, 2019.
GRANTS OF PLAN-BASED AWARDS FOR 2018
The following table sets forth information relating to non-equity incentives awards granted pursuant to our Short-term Incentive program and equity awards granted pursuant to our Long-term Incentive program during the year ended December 31, 2018 to each of our Named Executive Officers:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| All Other |
| All Other |
|
|
| Grant |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| stock |
| option |
|
|
| Date Fair |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Awards: |
| awards: |
|
|
| Value of |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of |
| Number of |
| Exercise or |
| Stock |
|
|
|
|
|
|
| Estimated Possible Payouts under |
| Estimated Future Payouts Under |
| shares of |
| securities |
| Base price |
| and |
| ||||||||
|
|
|
|
|
| Non-Equity Incentive Plan Awards (1) |
| Equity Incentive Plan Awards (4) |
| stock or |
| underlying |
| of Option |
| Option |
| ||||||||
|
|
|
| Grant |
| Threshold |
| Target |
| Maximum |
| Threshold |
| Target |
| Maximum |
| units |
| Option |
| Awards |
| Awards |
|
Name |
| Award |
| Dates |
| ($) |
| ($) |
| ($) |
| (#) |
| (#) |
| (#) |
| ($) |
| (#) |
| ($/sh) |
| ($) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Matthew Kapusta |
| IC (1) |
| — |
| 125,000 |
| 250,000 |
| 375,000 |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
|
|
| Option (2) |
| 01/26/18 |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
| 83,663 |
| 19.39 |
| 988,897 |
|
|
| RSU (3) |
| 01/26/18 |
| — |
| — |
| — |
| — |
| — |
| — |
| 31,374 |
| — |
| — |
| 608,342 |
|
|
| PSU (4) |
| 01/26/18 |
| — |
| — |
| — |
| 17,256 |
| 31,375 |
| 47,063 |
| 43,924 |
| — |
| — |
| 1,392,830 |
|
Jonathan Garen |
| IC (1) |
| — |
| 63,088 |
| 126,175 |
| 189,263 |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
|
|
| Option (2) |
| 01/26/18 |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
| 22,364 |
| 19.39 |
| 264,342 |
|
|
| RSU (3) |
| 01/26/18 |
| — |
| — |
| — |
| — |
| — |
| — |
| 8,387 |
| — |
| — |
| 162,624 |
|
|
| PSU (4) |
| 01/26/18 |
| — |
| — |
| — |
| 4,613 |
| 8,387 |
| 12,581 |
| 11,742 |
| — |
| — |
| 372,339 |
|
Maria Cantor |
| IC (1) |
| — |
| 63,756 |
| 127,512 |
| 191,268 |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
|
|
| Option (2) |
| 01/26/18 |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
| 19,169 |
| 19.39 |
| 226,578 |
|
|
| RSU (3) |
| 01/26/18 |
| — |
| — |
| — |
| — |
| — |
| — |
| 7,188 |
| — |
| — |
| 162,624 |
|
|
| PSU (4) |
| 01/26/18 |
| — |
| — |
| — |
| 3,953 |
| 7,188 |
| 10,782 |
| 10,063 |
| — |
| — |
| 319,098 |
|
Alexander E. Kuta |
| IC (1) |
| — |
| 54,075 |
| 108,150 |
| 162,225 |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
|
|
| Option (2) |
| 01/26/18 |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
| 23,962 |
| 19.39 |
| 283,231 |
|
|
| RSU (3) |
| 01/26/18 |
| — |
| — |
| — |
| — |
| — |
| — |
| 8,986 |
| — |
| — |
| 174,239 |
|
|
| PSU (4) |
| 01/26/18 |
| — |
| — |
| — |
| 4,942 |
| 8,986 |
| 13,479 |
| 12,580 |
| — |
| — |
| 398,912 |
|
Scott McMillan |
| IC (1) |
| — |
| 77,250 |
| 154,500 |
| 231,750 |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
|
|
| Option (2) |
| 01/26/18 |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
| 13,419 |
| 19.39 |
| 158,613 |
|
|
| RSU (3) |
| 01/26/18 |
| — |
| — |
| — |
| — |
| — |
| — |
| 5,032 |
| — |
| — |
| 97,570 |
|
|
| PSU (4) |
| 01/26/18 |
| — |
| — |
| — |
| 2,768 |
| 5,032 |
| 7,548 |
| 7,045 |
| — |
| — |
| 223,397 |
|
(1) Represents 2018 annual cash incentive awards granted under the Company’s Short-Term Incentive Plan. For additional information, please see “Compensation Discussion and Analysis—2018 Short-Term Incentive Plan”.
(2) Time-vested stock options granted under the Company’s 2014 Restated Plan. Grant date values are determined in accordance with ASC Topic 718. See “Compensation Discussion and Analysis—2018 Long-term Incentive Awards”.
(3) Time-vested RSUs granted under the Company’s 2014 Restated Plan. Grant date values are determined in accordance with ASC Topic 718. See “Compensation Discussion and Analysis—2018 Long-term Incentive Awards”.
(4) Three-year PSUs granted in 2018 under the Company’s 2014 Restated Plan for the 2018-2020 performance period were earned on January 25, 2019. Grant date values are determined in accordance with ASC Topic 718. See “Compensation Discussion and Analysis—2018 Long-term Incentive Awards”.
OPTION EXERCISES AND STOCK VESTED IN 2018
The following table discloses information for each of our Named Executive Officers regarding the exercise of stock option awards and the vesting of certain stock awards as of the end of our 2018 fiscal year.
|
| Option Awards |
| Stock Awards |
| ||||
|
| Number of |
| Value Realized on |
| Number of |
| Value Realized on |
|
|
|
|
|
|
|
|
|
|
|
Matthew Kapusta |
| — |
| — |
| 87,500 |
| 1,913,625 |
|
Jonathan Garen |
| — |
| — |
| 17,500 |
| 504,613 |
|
Maria Cantor |
| — |
| — |
| 17,500 |
| 504.613 |
|
Alexander E. Kuta |
| — |
| — |
| — |
| — |
|
Scott McMillan |
| — |
| — |
| — |
| — |
|
(1) Value realized equals number of shares vested multiplied by the closing price of our ordinary shares on the Nasdaq Global Market on the day the shares vested.
Potential Payments upon Termination or Change of Control
Pursuant to the terms of their respective employment agreements with the Company, each of our Named Executive Officers is eligible for potential payments and benefits in connection with a termination, including for Cause or for Good Reason, or in connection with a Change of Control. The following narrative and tables set forth the potential payments and value of additional benefit that each of our Named Executive Officers would receive in the scenarios contemplated. The tables below assume that employment terminated and/or the Change of Control occurred on December 31, 2018 and reflect the stock price of the Company on December 31, 2018 of $28.82. Except as otherwise provided, the following definitions apply to the potential payments upon termination.
“Accrued Benefit” means (a) payment of base salary through the termination date, (b) payment of any bonus for performance periods completed prior to the termination date, (c) any payments or benefits under the Company’s benefit plans that are vested, earned or accrued prior to the termination date (including, without limitation, earned but unused vacation); and (d) payment of unreimbursed business expenses incurred by the Named Executive Officer.
“Cause” means the good faith determination by the Company, after written notice from the Board to the Named Executive Officer that one or more of the following events has occurred and stating with reasonable specificity the actions that constitute Cause and the specific reasonable cure (related to sections (a) and (h) below): (a) the Named Executive Officer has willfully or repeatedly failed to perform his or her material duties, and such failure has not been cured after a period of thirty (30) days’ notice; (b) any reckless or grossly negligent act by the Named Executive Officer having the foreseeable effect of injuring the interest, business or reputation of the Company, or any of its parent, subsidiaries or affiliates in any material respect and which did in fact cause such material injury; (c) the Named Executive Officer’s evidenced use of any illegal drug, or illegal narcotic, or excessive amounts of alcohol (as determined by the Company in its reasonable discretion) on Company property or at a function where the Named Executive Officer is working on behalf of the Company; (d) the indictment on charges or conviction for (or the procedural equivalent or conviction for), or entering of a guilty plea or plea of no contest with respect to a felony; (e) the conviction for (or the procedural equivalent or conviction for), or entering of a guilty plea or plea of no contest with respect to a misdemeanor which, in the Board’s reasonable judgment, involves moral turpitude deceit, dishonesty or fraud, except that, in the event that the Named Executive Officer is indicted on charges for a misdemeanor set forth above, the Board may elect, in its sole discretion, to place the Named Executive Officer on administrative garden leave with continuation of full compensation and benefits under this Agreement during the pendency of the proceedings; (f) conduct by or at the direction of the Named Executive Officer constituting misappropriation or embezzlement of the property of the Company, or any of its parents or affiliates (other than the occasional, customary and de minimis use of Company property for personal purposes); (g) a breach by the Named Executive Officer of a fiduciary duty owing to the Company, including the misappropriation of (or attempted misappropriation of) a corporate opportunity or undisclosed self-dealing; (h) a material breach by the Named Executive Officer of any material provision of this Agreement, any of the Company’s written employment policies or the Named Executive Officer’s fiduciary duties to the Company, which breach, if curable, remains uncured for a period of thirty (30) days after receipt by the Named Executive Officer of written notice of such breach from the Board, which notice shall contain a reasonably specific description of such breach and the specific reasonable cure requested by the Board; and (i) any breach of their respective employment agreements.
“Change of Control” means any of the following: (a) any “person,” as such term is used in Sections 13(d) and 14(d) of the Securities Exchange Act of 1934, as amended (the “Act”) (other than the Company, any of its subsidiaries, or any trustee, fiduciary or other person or entity holding securities under any employee benefit plan or trust of the Company or any of its subsidiaries), together with all “affiliates” and “associates” (as such terms are defined in Rule 12b-2 under the Act) of such person, shall become the “beneficial owner” (as such term is defined in Rule 13d-3 under the Act), directly or indirectly, of securities of the Company representing forty (40) percent or more of the combined voting power of the Company’s then outstanding securities having the right to vote in an election of the Board (“Voting Securities”) (in such case other than as a result of an acquisition of securities directly from the Company); or (b) the date a majority of the members of the Board is replaced during any 12-month period by directors whose appointment or election is not endorsed by a majority of the members of the Board before the date of the appointment or election; or (c) the consummation of (1) any consolidation or merger of the Company where the stockholders of the Company, immediately prior to the consolidation or merger, would not, immediately after the consolidation or merger, beneficially own (as such term is defined in Rule 13d-3 under the Act), directly or indirectly, shares representing in the aggregate more than 50 percent of the voting shares of the Company issuing cash or securities in the consolidation or merger (or of its ultimate parent corporation, if any), or (2) any sale or other transfer (in one transaction or a series of transactions contemplated or arranged
by any party as a single plan) of all or substantially all of the assets of the Company.
“Change of Control Termination” means (i) any termination by the Company of the Named Executive Officer’s employment other than for Cause that occurs within 12 months after the Change of Control; or (ii) any resignation by the Named Executive Officer for Good Reason that occurs within 12 months after the Change of Control.
“Disability” means an incapacity by accident, illness or other circumstances which renders the Named Executive Officer mentally or physically incapable of performing the duties and services required of him or her on a full-time basis for a period of at least 120 days.
“Good Reason” means that the Named Executive Officer has complied with the Good Reason Process (hereinafter defined) following the occurrence of any of the following events: (a) a material diminution in the Named Executive Officer’s responsibilities, authority or duties (excluding any duties associated with any position that the Named Executive Officer may hold at the Company); (b) a diminution in the Named Executive Officer’s base salary, except for across-the-board salary reductions, based on the Company’s financial performance, similarly affecting all or substantially all other senior management employees of the Company, which reduction does not reduce the Named Executive Officer’s base salary (in the aggregate with any similar reductions during the term of employment) by more than 20% from the Named Executive Officer’s highest base salary; (c) a material change in the geographic location at which the Named Executive Officer provides services to the Company (i.e., outside a radius of fifty (50) miles from their primary business location); or (d) the material breach of their respective employment agreements by the Company (each a “Good Reason Condition”).
“Good Reason Process” means that (a) the Named Executive Officer reasonably determines in good faith that a Good Reason Condition has occurred; (b) the Named Executive Officer notifies the Board in writing of the first occurrence of the Good Reason Condition within sixty (60) days of the first occurrence of such condition; (c) the Named Executive Officer cooperates in good faith with the Company’s efforts, for a period not less than thirty (30) days following such notice (the “Cure Period”), to remedy the Good Reason Condition; (d) notwithstanding such efforts, the Good Reason Condition continues to exist; and (e) the Named Executive Officer terminates the employment within sixty (60) days after the end of the Cure Period. If the Company cures the Good Reason Condition during the Cure Period, Good Reason shall be deemed not to have occurred.
Matthew Kapusta
The following table discloses information about the benefits the Named Executive Officer would receive as of December 31, 2018, at a share price of $ 28.81 upon termination in certain circumstances, including in the event of a change in control.
|
| Termination |
| Termination in |
| Death |
| Disability(5) |
| Retirement(5) |
|
Compensation |
|
|
|
|
|
|
|
|
|
|
|
Cash severance (1) |
| 500,000 |
| 1,500,000 |
|
|
|
|
|
|
|
Pro-rata bonus (1) , (2) |
| 250,000 |
| 250,000 |
| 250,000 |
| 250,000 |
|
|
|
Long term incentive |
|
|
|
|
|
|
|
|
|
|
|
Restricted share units — unvested & accelerated |
| 3,424,760 |
| 3,424,760 |
| — |
| — |
| — |
|
Performance share units — unvested & accelerated (3) |
| 7,969,221 |
| 7,969,221 |
| 7,969,221 |
| 7,969,221 |
| — |
|
Stock options — unvested & accelerated |
| 4,128,632 |
| 4,128,632 |
| 4,128,632 |
| 4,128,632 |
| 4,128,632 |
|
Benefits and perquisites |
|
|
|
|
|
|
|
|
|
|
|
Health insurance (4) |
| 24,000 |
| 36,000 |
|
|
|
|
|
|
|
Total |
| 16,296,613 |
| 17,308,613 |
| 12,347,853 |
| 12,347,853 |
| 4,128,632 |
|
(1) |
| Cash severance and pro-rata bonus are paid as a lump sum, except in the case of base salary paid on termination without cause or for good reason, which is paid over the course of the severance period. |
(2) Pro-rata bonus amounts under the “Termination without Cause or Resignation for Good Reason” and “Death” columns are based on 2018 annual short-term incentive target.
(3) PSU amounts reflect actual earned awards for all completed tranches including the 2018 performance period.
(4) Health costs are based on individual elections and budgeted rates for 2019.
(5) The disclosure assumes the Committee did not exercise its discretion to award pro-rata short-term incentive amounts in the event of disability or retirement.
The Kapusta Employment Agreement requires us to provide compensation and/or other benefits to Mr. Kapusta during his employment and in the event of that executive’s termination of employment under certain circumstances and in the event of termination as a result of a change in control. Those arrangements are described in greater detail below. All severance payments and benefits described below (except for Accrued Benefits (defined below)) are conditioned upon the execution and delivery to the Company of a General Release of Claims.
Other than in the event of a Change of Control Termination (defined below), pursuant to the terms of the Kapusta Employment Agreement, if the Company terminates Mr. Kapusta’s employment (or fails to renew the Kapusta Employment Agreement) without Cause or if Mr. Kapusta resigns or opts not to renew the Kapusta Employment Agreement for Good Reason, then Mr. Kapusta is entitled to Accrued Benefits (defined below), twelve months of base salary, a lump sum bonus payment, accelerated vesting of options and restricted share unit awards which remain unvested as of the termination date, accelerated vesting of performance share unit awards to the extent then earned which remain unvested as of the termination date, and the continuation of certain other benefits.
If Mr. Kapusta’s employment with the Company terminates due to his death or disability, he will be entitled to Accrued Benefits and a lump sum bonus payment.
In the event of a Change of Control Termination (defined below), Mr. Kapusta will be entitled in such circumstances to a lump sum payment equal to two times Mr. Kapusta’s then-current base salary to be paid no later than sixty days after the termination date, his bonus for the year of termination pro-rated based upon Mr. Kapusta’s termination date, and a lump sum representing and additional two times Mr. Kapusta’s bonus, to be paid no later than sixty days following the termination date.
In the event that Mr. Kapusta incurs excise tax liability pursuant to section 4999 of the Internal Revenue Code, as amended, he will be entitled to certain reductions in his severance payments, which will have the result of providing him certain tax relief, all pursuant to the Kapusta Employment Agreement.
If Mr. Kapusta’s employment with the Company is terminated voluntarily without Good Reason by Mr. Kapusta, for Cause by the Company, upon a vote of the general meeting of the Company’s shareholders to dismiss him or upon a vote of the Board to recommend dismissal from his positions at the Company to the general meeting of the Company’s shareholders and /or to suspend Mr. Kapusta from his positions, then Mr. Kapusta is not entitled to any severance.
“Accrued Benefit” means (a) payment of base salary through the termination date, (b) payment of any bonus for performance periods completed prior to the termination date, (c) any payments or benefits under the Company’s benefit plans that are vested, earned or accrued prior to the termination date (including, without limitation, earned but unused vacation); (d) payment of unreimbursed business expenses incurred by Mr. Kapusta; and (e) rights to indemnification and directors’ and officers’ liability insurance coverage, under any agreement between the Company and Mr. Kapusta, and/or under any of the Company’s organizational documents.
“Change of Control Termination” means (a) any termination by the Company of Mr. Kapusta’s employment, other than for Cause, that occurs within the period that starts ninety (90) days preceding the Change of Control and ends on the one-year anniversary of the Change in Control; or (b) any resignation by Mr. Kapusta for Good Reason, that occurs within the period that starts ninety (90) days preceding the Change of Control and ends on the one-year anniversary of the Change in Control.
Jonathan Garen
The following table discloses information about the benefits the Named Executive Officer would receive as of December 31, 2018, at a share price of $ 28.81 upon termination in certain circumstances, including in the event of change in control.
|
| Termination |
| Resignation |
| Termination in |
| Death |
| Disability(1) |
| Retirement(1) |
|
Compensation |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash severance |
| 360,500 |
| 360,500 |
| 360,500 |
| — |
| — |
| — |
|
Pro-rata bonus |
| — |
| — |
| — |
| — |
| — |
| — |
|
Long term incentive |
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted share units — unvested & accelerated |
| — |
| — |
| 241,629 |
| — |
| — |
| — |
|
Performance share units — unvested & accelerated (2) |
| 1,954,960 |
| 1,954,960 |
| 1,954,960 |
| 1,954,960 |
| 1,954,960 |
| — |
|
Stock options — unvested & accelerated |
| — |
| 1,244,909 |
| 1,244,909 |
| 1,244,909 |
| 1,244,909 |
| 1,244,909 |
|
Benefits and perquisites |
|
|
|
|
|
|
|
|
|
|
|
|
|
Health insurance |
| — |
| — |
| — |
| — |
| — |
| — |
|
Total |
| 2,315,460 |
| 3,560,369 |
| 3,801,998 |
| 3,199,869 |
| 3,199,869 |
| 1,244,909 |
|
(1) The disclosure assumes the Committee did not exercise its discretion to award pro-rata short-term incentive amounts in the event of disability or retirement.
(2) PSU amounts reflect actual earned awards for all completed tranches including the 2018 performance period.
The Garen Employment Agreement requires us to provide compensation and/or other benefits to Mr. Garen during his employment and in the event of that executive’s termination of employment under certain circumstances and in the event of termination as a result of a change in control. Those arrangements are described in greater detail below. All severance payments and benefits described below (except for Accrued Benefits) are conditioned upon the execution and delivery to the Company of a General Release of Claims.
Pursuant to the terms of the Garen Employment Agreement, if Mr. Garen’s employment is terminated due to the death or Disability of Mr. Garen, then Mr. Garen is entitled to Accrued Benefits. If the Company terminates Mr. Garen’s employment without Cause or if Mr. Garen resigns for Good Reason or upon a Change of Control Termination, then Mr. Garen is entitled to Accrued Benefits and twelve months of base salary. In the event of a termination of Mr. Garen’s employment due to death or disability or if Mr. Garen resigns for Good Reason or upon a Change of Control Termination, Mr. Garen is entitled to accelerated vesting of options and earned performance share unit awards that remain unvested as of the termination date. Additionally, if Mr. Garen retires, he is entitled to accelerated vesting of options. Furthermore, in the event of a Change of Control Termination, Mr. Garen is further entitled to accelerated vesting of restricted share unit awards, and, to avoid duplication of severance payments, any amount to be paid per the above will be offset by severance amounts paid pursuant to the Company’s change of control guidelines.
Maria Cantor
The following table discloses information about the benefits the Named Executive Officer would receive as of December 31, 2018, at a share price of $ 28.81 upon termination in certain circumstances, including in the event of change in control.
|
| Termination |
| Resignation |
| Termination in |
| Death |
| Disability(1) |
| Retirement(1) |
|
Compensation |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash severance |
| 309,000 |
| 309,000 |
| 309,000 |
| — |
| — |
| — |
|
Pro-rata bonus |
| — |
| — |
| — |
| — |
| — |
| — |
|
Long term incentive |
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted share units — unvested & accelerated |
| — |
| — |
| 207,086 |
| — |
| — |
| — |
|
Performance share units — unvested & accelerated (2) |
| 1,906,588 |
| 1,906,588 |
| 1,906,588 |
| 1,906,588 |
| 1,906,588 |
| — |
|
Stock options — unvested & accelerated |
| — |
| 1,137,094 |
| 1,137,094 |
| 1,137,094 |
| 1,137,094 |
| 1,137,094 |
|
Benefits and perquisites |
|
|
|
|
|
|
|
|
|
|
|
|
|
Health insurance |
| — |
| — |
| — |
| — |
| — |
| — |
|
Total |
| 2.215.588 |
| 3,352,682 |
| 3,559,768 |
| 3,043,682 |
| 3,043,682 |
| 1,137,094 |
|
(1) The disclosure assumes the Committee did not exercise its discretion to award pro-rata short-term incentive amounts in the event of disability or retirement.
(2) PSU amounts reflect actual earned awards for all completed tranches including the 2018 performance period.
The Cantor Employment Agreement requires us to provide compensation and/or other benefits to Ms. Cantor during her employment and in the event of that executive’s termination of employment under certain circumstances and in the event of termination as a result of a change in control. Those arrangements are described in greater detail below. All severance payments and benefits described below (except for Accrued Benefits) are conditioned upon the execution and delivery to the Company of a General Release of Claims.
Pursuant to the terms of the Cantor Employment Agreement, if Ms. Cantor’s employment is terminated due to the death or Disability of Ms. Cantor, then Ms. Cantor is entitled to Accrued Benefits. If the Company terminates Ms. Cantor’s employment without Cause or if Ms. Cantor resigns for Good Reason or upon a Change of Control Termination, then Ms. Cantor is entitled to Accrued Benefits and twelve months of base salary. In the event of a termination of Ms. Cantor’s employment due to death or disability or if Ms. Cantor resigns for Good Reason or upon a Change of Control Termination, Ms. Cantor is entitled to accelerated vesting of options and earned performance share unit awards that remain unvested as of the termination date. Additionally, if Ms. Cantor retires she is entitled to accelerated vesting of options. Furthermore in the event of a Change of Control Termination, Ms. Cantor is further entitled to accelerated vesting of restricted share unit awards, and, to avoid duplication of severance payments, any amount to be paid per the above will be offset by severance amounts paid pursuant to the Company’s change of control guidelines.
Alexander E. Kuta
The following table discloses information about the benefits the Named Executive Officer would receive as of December 31, 2018, at a share price of $ 28.81 upon termination in certain circumstances, including in the event of change in control.
|
| Termination |
| Resignation |
| Termination in |
| Death |
| Disability(4) |
| Retirement(4) |
|
Compensation |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash severance |
| 386,250 |
| 386,250 |
| 386,250 |
| — |
| — |
| — |
|
Pro-rata bonus (1) |
| 135,188 |
| 135,188 |
| 135,188 |
| — |
| — |
| — |
|
Long term incentive |
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted share units — unvested & accelerated |
| — |
| — |
| 258,887 |
| — |
| — |
| — |
|
Performance share units — unvested & accelerated (2) |
| 362,430 |
| 362,430 |
| 362,430 |
| 362,430 |
| 362,430 |
| — |
|
Stock options — unvested & accelerated |
| — |
| 2,208,535 |
| 2,208,535 |
| 2,208,535 |
| 2,208,535 |
| 2,208,535 |
|
Benefits and perquisites |
|
|
|
|
|
|
|
|
|
|
|
|
|
Health insurance (3) |
| 24,000 |
| 24,000 |
| 24,000 |
| — |
| — |
| — |
|
Total |
| 907,868 |
| 3,116,403 |
| 3,375,290 |
| 2,570,965 |
| 2,570,965 |
| 2,208,535 |
|
(1) Pro-rata bonus amount under the “Termination without Cause or Resignation for Good Reason” column is based on actual 2018 annual short-term incentive pay-out.
(2) PSU amounts reflect actual earned awards for all completed tranches including the 2018 performance period.
(3) Health insurance is an estimated amount based on employee elections and paid for a period of 12 months following termination.
(4) The disclosure assumes the Committee did not exercise its discretion to award pro-rata short-term incentive amounts in the event of disability or retirement.
The Kuta Employment Agreement requires us to provide compensation and/or other benefits to Mr. Kuta during his employment and in the event of that executive’s termination of employment under certain circumstances and in the event of termination as a result of a change in control. Those arrangements are described in greater detail below. All severance payments and benefits described below (except for Accrued Benefits) are conditioned upon the execution and delivery to the Company of a General Release of Claims.
Pursuant to the terms of the Kuta Employment Agreement, if Mr. Kuta’s employment is terminated due to the death or Disability of Mr. Kuta, then Mr. Kuta is entitled to Accrued Benefits. If the Company terminates Mr. Kuta’s employment without Cause or if Mr. Kuta resigns for Good Reason or upon a Change of Control Termination, then Mr. Kuta is entitled to Accrued Benefits, twelve months of base salary, a bonus pro-rated to the date of termination and based on the target bonus amount set by the Board (currently 35%), and continued coverage through COBRA for a period of 12 months. In the event of a termination of Mr. Kuta’s employment due to death or disability or if Mr. Kuta resigns for Good Reason or upon a Change of Control Termination, Mr. Kuta is entitled to accelerated vesting of options and performance share unit awards that remain unvested as of the termination date. Additionally, if Mr. Kuta retires, he is entitled to accelerated vesting of options. Furthermore in the event of a Change of Control Termination, Mr. Kuta is further entitled to accelerated vesting of restricted share unit awards, and, to avoid duplication of severance payments, any amount to be paid per the above will be offset by severance amounts paid pursuant to the Company’s change of control guidelines.
Scott McMillan
The following table discloses information about the benefits the Named Executive Officer would as of December 31, 2018, at a share price of $ 28.81 receive upon termination in certain circumstances, including in the event of change in control.
|
| Termination |
| Resignation |
| Termination in |
| Death |
| Disability(2) |
| Retirement(2) |
|
Compensation |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash severance |
| 364,320 |
| 364,320 |
| 364,320 |
| — |
| — |
| — |
|
Pro-rata bonus |
| — |
| — |
| — |
| — |
| — |
| — |
|
Long term incentive |
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted share units — unvested & accelerated |
| — |
| — |
| 144,972 |
| — |
| — |
| — |
|
Performance share units — unvested & accelerated (1) |
| 202,966 |
| 202,966 |
| 202,966 |
| 202,966 |
| 202,966 |
| — |
|
Stock options — unvested & accelerated |
| — |
| 2,221,907 |
| 2,221,907 |
| 2,221,907 |
| 2,221,907 |
| 2,221,907 |
|
Benefits and perquisites |
|
|
|
|
|
|
|
|
|
|
|
|
|
Health insurance |
| — |
| — |
| — |
| — |
| — |
| — |
|
Total |
| 567,286 |
| 2,789,193 |
| 2,934,165 |
| 2,424,873 |
| 2,424,873 |
| 2,221,907 |
|
(1) PSU amounts reflect actual earned awards for all completed tranches including the 2018 performance period.
(2) The disclosure assumes the Committee did not exercise its discretion to award pro-rata short-term incentive amounts in the event of disability or retirement.
The McMillan Employment Agreement requires us to provide compensation and/or other benefits to Mr. McMillan during his employment and in the event of that executive’s termination of employment under certain circumstances and in the event of termination as a result of a change in control. Those arrangements are described in greater detail below. All severance payments and benefits described below (except for Accrued Benefits) are conditioned upon the execution and delivery to the Company of a General Release of Claims.
Pursuant to the terms of the McMillan Employment Agreement, if Mr. McMillan’s employment is terminated due to the death or Disability of Mr. McMillan, then Mr. McMillan is entitled to Accrued Benefits. If the Company terminates Mr. McMillan’s employment without Cause or if Mr. McMillan resigns for Good Reason or upon a Change of Control Termination, then Mr. McMillan is entitled to Accrued Benefits and twelve months of base salary. In the event of a termination of Mr. McMillan’s employment due to death or disability or if Mr. McMillan resigns for Good Reason or upon a Change of Control Termination, Mr. McMillan is entitled to accelerated vesting of options and performance share unit awards that remain unvested as of the termination date. Additionally, if Mr. McMillan retires, he is entitled to accelerated vesting of options. Furthermore in the event of a Change of Control Termination, Mr. McMillan is further entitled to accelerated vesting of restricted share unit awards, and, to avoid duplication of severance payments, any amount to be paid per the above will be offset by severance amounts paid pursuant to the Company’s change of control guidelines.
Director Compensation
Overview of Director Compensation Program
Current Director Compensation Arrangements
Our Remuneration Policy provides that our Board may determine compensation paid to non-executive directors. Other than as noted below, our Board has determined that the compensation paid to our non-executive directors will not increase in amount from that paid during the last fiscal year. Our Board-approved non-executive director compensation for their services on our Board is as follows:
· Each non-executive director received an annual retainer of $35,000 for 2018, which amount will increase to $40,000, effective as of the 2019 Annual Meeting, pro-rated for service over the course of the remainder of the year.
· The chairman of the board receives an annual retainer of $70,000, pro-rated for service over the course of the year.
· Each non-executive director who serves as member of a committee of our Board receives additional compensation as follows:
· Compensation Committee: members receive an annual retainer of $5,000; the chair receives an annual retainer of $10,000.
· Nominating and Corporate Governance Committee: members receive an annual retainer of $5,000; the chair receives an annual retainer of $10,000.
· Audit Committee: members receive an annual retainer of $7,500; the chair receives an annual retainer of $15,000.
· Each non-executive director receives an annual equity grant consisting of one-half options and one-half RSUs with a one-year vesting period for each.
The size of the annual equity grant is determined by reference to our peer group companies. In reviewing Board of Director compensation, the Compensation Committee’s independent consultant provides an analysis of cash and equity compensation practices and levels within the same compensation peer group used for the Named Executive Officers. Given the volatile nature of equity prices within our industry, for 2018 it was determined that Directors would receive a fixed value equity award. As a result, the value of the uniQure award will vary relative to our peers who predominantly use fixed share awards, which can vary dramatically in value from year-to-year.
Each annual retainer for Board and committee service is payable semi-annually.
Each member of our Board is also entitled to be reimbursed for reasonable travel and other expenses incurred in connection with attending meetings of the Board and any committee of the Board on which she or he serves.
DIRECTOR COMPENSATION TABLE
The following table summarizes the annual compensation paid to those persons who served as our non-executive directors during the fiscal year ended December 31, 2018.
Name |
| Fees Earned |
| Option Awards |
| Restricted Stock |
| Total |
|
Philip Astley-Sparke |
| 82,500 |
| 75,760 |
| 96,160 |
| 254,420 |
|
Jack Kaye |
| 57,242 |
| 75,251 |
| 90,730 |
| 223,223 |
|
David Schaffer (1) |
| — |
| 64,446 |
| 90,730 |
| 155,176 |
|
Paula Soteropoulos |
| 42,242 |
| 64,446 |
| 90,730 |
| 197,418 |
|
Madhavan Balachandran |
| 42,758 |
| 75,098 |
| 86,464 |
| 204,320 |
|
Jeremy Springhorn |
| 52,500 |
| 75,098 |
| 86,464 |
| 214,062 |
|
David Meek (3) |
| 19,305 |
| 37,694 |
| — |
| 56,999 |
|
Robert Gut (4) |
| 5,376 |
| 14,616 |
| — |
| 19,992 |
|
(1) |
| David Schaffer does not receive cash compensation by agreement due to his relationship with 4DMT. |
(2) |
| The value of stock awards and stock options as reported in their respective columns is calculated using the grant date accounting fair value determined in accordance with Accounting Standards Codification 718, Compensation-Stock Compensation (“ASC 718”). |
(3) |
| David Meek was appointed to be one of our non-executive directors at our general meeting on June 13, 2018. |
(4) |
| Robert Gut was appointed to be one of our directors at our general meeting on June 13, 2018. Mr. Gut resigned as a non-executive director August 19, 2019, in connection with his appointment as our Chief Medical Officer. Dr. Gut did not receive any additional compensation as an executive director beyond the compensation pursuant to his employment with the Company. The $37,694 relates to his share-based compensation determined in accordance with ASC 718 for the period June 13, 2018 to August 31, 2018. |
Mr. Kapusta’s compensation is disclosed above in the section titled “Management Compensation.” Dr. Gut’s compensation as a non-executive director is for the period from his election on June 13, 2018 and his resignation on August 19, 2018 (except share-based compensation, which includes the entire month of August 2018).
The following table sets forth information relating to the aggregate number of RSUs and stock options to our Ordinary Shares outstanding at December 31, 2018 for each of our non-executive directors.
Name |
| Award |
| Aggregate Number |
|
|
|
|
|
|
|
Philip Astley-Sparke |
| Option |
| 46,390 |
|
|
| RSU |
| 4,792 |
|
Jack Kaye |
| Option |
| 27,390 |
|
|
| RSU |
| 4,792 |
|
David Schaeffer |
| Option |
| 22,390 |
|
|
| RSU |
| 4,792 |
|
Paula Soteropoulos |
| Option |
| 32,390 |
|
|
| RSU |
| 4,792 |
|
Madhavan Balachandran |
| Option |
| 16,390 |
|
|
| RSU |
| 4,792 |
|
Jeremy Springhorn |
| Option |
| 16,390 |
|
|
| RSU |
| 4,792 |
|
David Meek |
| Option |
| 10,000 |
|
Robert Gut (1) |
| Option |
| 80,000 |
|
(1) Includes only awards outstanding based on service during period Dr. Gut was a non-executive director; does not include awards pertaining to period Dr. Gut was an executive director.
Compensation Committee Interlocks and Insider Participation
None of our executive officers currently serve, or have served during the last completed fiscal year, on the compensation committee or board of directors of any other entity that has one or more executive officers serving as a member of our Board or Compensation Committee.
Compensation Committee Report
The Compensation Committee Report is not “soliciting material,” is not deemed “filed” with the SEC and is not to be incorporated by reference into any filing of the Company under the Securities Act of 1933 or the Securities Exchange Act of 1934, both as amended.
We have reviewed and discussed the Compensation Discussion & Analysis contained in this Amendment with uniQure’s management, and based upon such review and discussion, we recommended to the Board that the Compensation Discussion & Analysis be included in this Amendment.
The Compensation Committee
/s/ Madhavan Balachandran |
|
Madhavan Balachandran |
|
|
|
/s/ Jack Kaye |
|
Jack Kaye |
|
|
|
/s/ David Meek |
|
David Meek |
|
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Securities Authorized for Issuance Under Equity Compensation Plans
The table below provides information about our Ordinary Shares that may be issued under our 2014 Amended and Restated Share Option Plan (the “2014 Restated Plan”), our predecessor plans and outside these plans as of April 15, 2019:
Plan Category |
| (a) Number of securities |
| (b) Weighted-average |
| (c) Number of securities |
| |
2012 Equity Incentive Plan (Equity Compensation Plan Approved by Security Holders) |
| 14,000 |
| $ | 9.15 | (2) | — |
|
2014 Restated Plan (Equity Compensation Plan Approved by Security Holders) |
| 3,618,778 |
| $ | 14.67 | (3) | 3,083,113 |
|
Equity Compensation Plans Not Approved by Security Holders (4) |
| 284,000 |
| $ | 6.99 |
| — | (5) |
Total |
| 3,916,778 |
| $ | 14.10 |
| 3,083,113 |
|
(1) |
| The exercise price for our RSU and PSU awards is $0.00 and is included in the weighted-average exercise price of outstanding options, warrants and rights. |
(2) |
| The exercise price of outstanding options is denominated in euro and translated to $at the foreign exchange rate as of April 15, 2019. |
(3) |
| These PSU Awards are measured at target for the outstanding performance-based awards. |
(4) |
| These awards include inducement grants entered into by the Company outside of the 2014 Restated Plan and the predecessor plans. |
(5) |
| At the 2018 Annual General Meeting, our Board was granted the authority to issue a maximum of 19.9% of the Company’s aggregate issued capital outside of a public offering. Ordinary Shares may be issued as part of inducement or other option grants, but are not restricted to that purpose. |
Security Ownership of Certain Beneficial Owners and Management
Based on information publicly filed and provided to us by certain holders, the following table shows the number of our Ordinary Shares beneficially owned as of April 15, 2019 by (i) each person known by us to beneficially own more than five percent of our voting securities, (ii) each Named Executive Officer, (iii) each of our directors, (iv) each of our director nominees, and (v) all of our current Named Executive Officers and directors as a group. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, Ordinary Shares that could be issued upon the exercise of outstanding equity awards and warrants held by that person that are currently exercisable or exercisable within 60 days of April 15, 2019 are considered outstanding. As of April 15, 2019, we had 37,798,421 Ordinary Shares outstanding. Unless otherwise stated in a footnote, each of the beneficial owners listed below has direct ownership of and sole voting power and investment power with respect to our Ordinary Shares.
Unless otherwise noted below, the address of each director and named executive officer is c/o uniQure N.V., Paasheuvelweg 25a, 1105 BP Amsterdam, the Netherlands.
Name and Address of |
| Ordinary Shares Beneficially Owned |
| ||
Beneficial Owner |
| Number |
| Percent |
|
5% or Greater Shareholders (“Major Shareholders”) |
|
|
|
|
|
ForUniQure B.V. (1) |
| 4,479,276 |
| 11.85 | % |
|
|
|
|
|
|
Bristol-Myers Squibb Company (2) |
| 2,388,108 |
| 6.32 | % |
|
|
|
|
|
|
FMR, LLC (3) |
| 3,659,162 |
| 9.68 | % |
|
|
|
|
|
|
Nantahala Capital Management, LLC (4) |
| 3,578,667 |
| 9.47 | % |
|
|
|
|
|
|
Consonance Capital (5) |
| 1,896,225 |
| 5.02 | % |
|
|
|
|
|
|
Directors and Named Executive Officers |
|
|
|
|
|
Matthew Kapusta |
| 504,171 |
| 1.32 | % |
|
|
|
|
|
|
Robert Gut, Ph.D. |
| 0 |
| * | % |
|
|
|
|
|
|
Philip Astley-Sparke |
| 59,932 |
| 0.16 | % |
|
|
|
|
|
|
Madhavan Balachandran |
| 13,722 |
| 0.04 | % |
|
|
|
|
|
|
Jack Kaye |
| 35,969 |
| 0.10 | % |
|
|
|
|
|
|
David Meek |
| 0 |
| * | % |
|
|
|
|
|
|
David Schaffer, Ph.D. |
| 62,481 |
| 0.17 | % |
|
|
|
|
|
|
Paula Soteropoulos |
| 49,094 |
| 0.13 | % |
|
|
|
|
|
|
Jeremy P. Springhorn, Ph.D. |
| 13,722 |
| 0.04 | % |
|
|
|
|
|
|
Maria Cantor |
| 69,555 |
| 0.18 | % |
|
|
|
|
|
|
Jonathan Garen |
| 91,355 |
| 0.24 | % |
|
|
|
|
|
|
Alexander E Kuta, Ph.D. |
| 77,806 |
| 0.21 | % |
|
|
|
|
|
|
Scott McMillan, Ph. D. |
| 61,785 |
| 0.16 | % |
|
|
|
|
|
|
Directors and Named Executive Officers Total |
| 1,039,592 |
| 2.91 | % |
|
|
|
|
|
|
Major Shareholders, Directors and Named Executive Officers Total |
| 17,041,031 |
| 45.24 | % |
* Denotes less than 0.01% beneficial ownership.
(1) |
| The registered office of Forbion 1, ForUniQure and Forbion Management is Gooimeer 2-35, 1411DC Naarden, The Netherlands. The number of shares reported is based solely on Schedules 13G/A filed by ForUniQure B.V. and Forbion I Management B.V. on February 14, 2019. Forbion’s beneficial ownership consists of (i) 4,376,883 Ordinary Shares held by ForUniQure B.V., or ForUniQure, (ii) 9,859 Ordinary Shares held by Forbion Management, and (iii) 8,789 Ordinary Shares and options to purchase 83,746 Ordinary Shares held by Dr. van Deventer, or SvD. Forbion 1 Management B.V. or Forbion 1, the director of ForUniQure and Forbion Management may be deemed to have voting and dispositive power over the Ordinary Shares held by ForUniQure and Forbion Management. Forbion 1, the director of ForUniQure, has voting and investment power over the shares held by ForUniQure, which are exercised through Forbion’s investment committee, consisting of H. A. Slootweg, M. A. van Osch, G. J. Mulder and Dr. van Deventer. None of the members of the investment committee have individual voting and investment power with respect to such shares, and the members disclaim beneficial ownership of such shares except to the extent of their proportionate pecuniary interests therein. In addition to serving on Forbion’s investment committee, Dr. van Deventer is a partner |
|
| of Forbion Capital Partners, which acts as the investment advisor to the directors of ForUniQure and Forbion 1. Dr. van Deventer disclaims beneficial ownership of such Ordinary Shares, except to the extent of his pecuniary interest therein. |
(2) |
| The registered office of Bristol-Myers Squibb Company is 345 Park Avenue, New York, NY 10154, United States. The number of shares reported is based solely on a Schedule 13G filed with the Securities and Exchange Commission by Bristol-Myers Squibb Company on August 17, 2015. |
(3) |
| The registered office of FMR, LLC is 245 Summer Street, Boston, Massachusetts 02210, United States. The number of shares reported is based solely on a Schedule 13G/A filed with the Securities and Exchange Commission by FMR, LLC on February 13, 2019. |
(4) |
| The registered office of Nantahala Capital Management, LLC is 19 Old Kings Highway S, Suite 200, Darien, CT 06820, United States. The number of shares reported is based solely on a Schedule 13G/1 filed with the Securities and Exchange Commission by Nantahala Capital Management, LLC on February 14, 2019. |
(5) |
| The registered officer of Consonance Capital Management LP (“Capital Management”), Consonance Capital Opportunity Fund Management LP (“Consonance Opportunity”), Mitchell Blutt (“Blutt”) and Consonance Capman GP LLC (“Capman”) (collectively, “Consonance Capital”) is 1370 Avenue of the Americas, Floor 33, New York, NY 10019, United States. The number of shares reported is based solely on Schedule 13G filed with the Securities and Exchange Commission by Consonance Capital on February 14, 2019. Consonance Capital’s beneficial ownership consists of (i) 1,818,531 Ordinary Shares directly held by Consonance Capital Master Account LP (“Master Account”) where Capital Management exercises voting and investment power over the Master Account Ordinary Shares, and (ii) 77,965 Ordinary Shares held directly through Consonance Opportunity. Capman is the general partner of Capital Management and Consonance Opportunity. Blutt is the Manager and Member of Capman and Chief Executive Officer of Capital Management. Capman and Blutt may be deemed to have voting and dispositive power over the Master Account Ordinary Shares and the Consonance Opportunity Ordinary Shares. Capital Management, Capman and Blutt disclaim beneficial ownership of such Ordinary Shares except to the extent of their pecuniary interest therein. |
Item 13. Certain Relationships and Related Transactions and Director Independence
Pre-Approval Policy Regarding Related Party Transactions
The Board has adopted a related party transactions policy, pursuant to which the Chief Financial Officer and the Audit Committee is charged with reviewing and approving or disapproving related party transactions. A “Related Party Transaction” under the policy means any transaction, arrangement or relationship (or any series of similar transactions, arrangements or relationships) where the amount involved or proposed to be involved exceeds $120,000 (or its equivalent in any currency), in which the Company or any of its controlled subsidiaries was, is or will be a participant (i.e., not necessarily a party) and in which any Related Party, as defined below, had, has or will have a direct or indirect material interest. The “Related Party Transactions Policy” supplements the provisions in the Company’s Code of Business Conduct and Ethics concerning potential conflict of interest situations. Pursuant to the policy, compensation of directors and senior management are reviewed and approved by the Compensation Committee.
This written policy covers transactions or series of transactions in which the Company or any subsidiary participates and a “Related Party” has or will have a direct or indirect material interest. For purposes of this policy, a “Related Party” is:
· Each director and executive officer of the Company and any person who was serving as a director and/or executive officer at any time since the beginning of the Company’s last fiscal year;
· Any nominee for election as a director of the Company;
· Any security holder who is the beneficial owner or record holder of more than 5% of any class of the Company’s voting securities;
· Any immediate family member of any of the foregoing persons. An “immediate family member” includes the spouse, parents, stepparents, children, stepchildren, siblings, mothers- and fathers-in-law, sons- and daughters-in-law, brothers- and sisters-in-law, and any person (other than a tenant or employee) sharing the household of a director, executive officer, director nominee or greater than 5% security holder of the Company; and
· Any entity that employs any person identified in the above or in which any person identified in the above directly or indirectly owns or has a material interest.
Pursuant to the Related Party Transactions Policy, each Company executive officer, director or nominee for director or any other officer or employee who intends to cause the Company to enter into a related party transaction must fully disclose to the Chief Financial Officer all material facts concerning a prospective transaction or arrangement involving the Company in which such person may have an interest. The Chief Financial Officer will review the information and make a preliminary, written conclusion as to whether the transaction is a related party transaction. If the preliminary conclusion is that the transaction would be a related party transaction, the Chief Financial Officer will present the information and his conclusion to the Audit Committee for review. If a member of the Audit Committee is involved in the transaction, that member will not participate in determining whether the related party transaction is approved or ratified by the Audit Committee. Annually, the Audit Committee will review any previously approved or ratified related party transactions that are continuing and determine based on then-existing facts and circumstances.
Before any related person transaction is approved, the following factors are to be considered:
· The Related Party’s interest in the transaction;
· The approximate value of the aggregate amount involved in the transaction;
· The approximate value of the amount of the Related Party’s interest in the transaction;
· A summary of the material terms of and facts relating to the transaction, including any documentation or proposed documentation for the transaction, and identification of the area(s) of the Company’s business directly relevant to the transaction;
· Where the transaction involves the purchase or sale of products, property or services, the availability of comparable products, property or services from or to (as applicable) unrelated third-party sources;
· Whether the transaction was undertaken in the ordinary course of business of the Company;
· An assessment of whether the transaction’s terms are comparable to terms available from or to (as applicable) unrelated third parties in an arms-length transaction;
· The purpose of, and the potential benefits to the Company of the transaction; and
· Any other information regarding the transaction or the Related Party in the context of the proposed transaction that would be material to investors in light of the circumstances of the particular transaction.
Approval of a transaction under the policy will be granted only if it is determined that, under all of the circumstances, the transaction is in, or not inconsistent with, the best interests of the Company.
Review of Related Party Transactions
Since January 1, 2018, we have engaged in the following transactions with the members of our Board, senior management, parties that held more than 5% of our Ordinary Shares during that period, and their affiliates, which we refer to as our related parties. Each of these transactions was approved in accordance with our Related Transactions Policy.
Grants of Options to Related Parties
We grant options to members of the Board and senior management. Details of options granted are included within the beneficial ownership table below.
4D Molecular Therapeutics Collaboration
In January 2014, we entered into a collaboration and license agreement with 4D Molecular Therapeutics. 4D Molecular Therapeutics is a company co-founded by Dr. David Schaffer, and he currently serves as the Chief Scientific Officer. Dr. Schaffer was appointed to our Board in January 2014 pursuant to the terms of that collaboration and license agreement. In connection with this transaction, we agreed to provide specified research and development financing, are obligated to make certain upfront, royalty and milestone payments. We continue to have rights under the collaboration and license agreement, and have been in discussions with 4DMT to potentially amend, and engage in further activity pursuant to, that agreement.
BMS
In April 2015, we and Bristol Myers Squibb (“BMS”) entered into various commercial and investment agreements providing BMS exclusive access to uniQure’s gene therapy technology platform for multiple targets in cardiovascular and other target-specific disease areas. We received $50 million in upfront payments upon effectiveness of the licensing and collaboration transaction in May 2015. An additional $15 million payment was received in July 2015 upon designation of three additional collaboration targets by BMS. In addition, pursuant to the collaboration agreements, in June 2015, BMS purchased 1,112,319 of our Ordinary Shares for aggregate consideration of $37.6 million. Immediately after the issuance, BMS owned 4.9% of our outstanding Ordinary Shares. In August 2015, we issued an additional 1,275,789 of our Ordinary Shares to BMS for aggregate consideration of $37.9 million. Immediately after the issuance, BMS owned 9.9% of our outstanding Ordinary Shares. We recognized $7.5 million in license revenue from BMS for the year ended December 31, 2018 (2017: $4.1 million).
Director Independence
Our securities are listed on the Nasdaq Global Market (“Nasdaq”) and we use the standards of “independence” prescribed by rules set forth by Nasdaq. Our Board has determined that each of Philip-Astley Sparke, Madhavan Balachandran, Jack Kaye, David Meek, Paula Soteropoulos, and Jeremy Springhorn has no relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and is independent within the meaning of the director independence standards of the Nasdaq rules and the SEC. Our Board has determined that each of Matthew Kapusta, Robert Gut and David Schaffer do not qualify as “independent” under the Nasdaq rules.
Item 14. Principal Accounting Fees and Services
Audit Fees
The following table shows the fees paid or accrued by the Company for audit and other services provided by PwC for the fiscal years ended December 31, 2018 and 2017:
|
| 2018 ($) |
| 2017 ($) |
|
|
| (in thousands) |
| ||
Audit fees |
| 1,172 |
| 595 |
|
Audit-related fees (1) |
| 122 |
| 150 |
|
Tax fees |
| — |
| — |
|
All other fees |
| — |
| — |
|
Total |
| 1,294 |
| 745 |
|
(1) |
| Audit-related fees consisted of the aggregate fees billed for assurance services rendered by PwC related to equity offering that are expensed in accordance with U.S. Generally Accepted Accounting Principles. |
Pre-Approval Policies and Procedures
The Audit Committee pre-approves all auditing services, internal control related services and permitted non-audit services (including the fees and terms thereof) to be performed, subject to the de minimis exception for non-audit services that are approved by the Audit Committee prior to the completion of an audit. The Audit Committee has adopted policies and procedures relating to the approval of all audit and non-audit services that are to be performed by the Company’s independent registered public accounting firm. This policy generally provides that the Company will not engage its independent registered public accounting firm to render audit or non-audit services unless the service is specifically approved in advance by the Audit Committee or the engagement is entered into pursuant to the pre-approval procedure described below.
From time to time, the Audit Committee may pre-approve specified types of services that are expected to be provided to the Company by its independent registered public accounting firm during the next 12 months. Any such pre-approval is detailed as to the particular service or type of services to be provided and is also generally subject to a maximum dollar amount.
The Audit Committee pre-approved all services performed since the pre-approval policy was adopted.
Item 15. Exhibits, Financial Statements Schedules.
Financial Statements and Schedules
(a) The following documents are filed as part of this report.
(1) Financial Statements.
See Index to the Consolidated Financial Statements and Supplementary Data in Item 8 of the Original Form 10-K.
All other schedules are not applicable or are omitted since either (i) the required information is not material or (ii) the information required is included in the consolidated financial statements and notes thereto in the Original Form 10-K.
(2) Exhibits.
The Exhibit Index immediately preceding the signature page of this Amendment is incorporated by reference herein.
Not applicable.
EXHIBIT INDEX
Exhibit |
| Description |
3.1 |
| |
10.1t |
| |
10.2t |
| |
10.3t |
| |
10.4t |
| |
10.5t |
| |
10.6t |
| |
10.7t |
| |
10.8t |
| |
10.10 |
| |
10.11 |
| |
10.15 |
| |
10.18 |
| |
10.19 |
|
10.20 |
| |
10.21 |
| |
10.22† |
| |
10.26 |
| |
10.27† |
| |
10.28† |
| |
10.29† |
| |
10.30† |
| |
10.31† |
| |
10.32 |
| |
10.34t |
| |
10.35t |
| |
10.36t |
| |
10.37† |
|
10.38t |
| |
10.39 |
| |
10.40 |
| |
10.41t |
| |
14.1 |
| |
21.1 |
| |
23.1 |
| |
24.1* |
| |
31.1 |
| |
31.2 |
| |
31.3* |
| Rule 13a-14(a)/15d-14(a) Certification of Chief Executive Officer. |
31.4* |
| Rule 13a-14(a)/15d-14(a) Certification of Chief Executive Officer. |
32.1 |
| |
101 |
|
† Confidential treatment requested as to certain portions, which portions have been omitted and filed separately with the Securities and Exchange Commission
* Filed herewith
t Indicates a management contract or compensatory plan or arrangement.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| UNIQURE, N.V. | |
|
| |
| By: | /s/ MATHEW KAPUSTA |
|
| Matthew Kapusta |
|
| Chief Executive Officer and interim Chief Financial Officer |
|
| (Principal Executive and Financial Officer) |
Date: April 29, 2019.