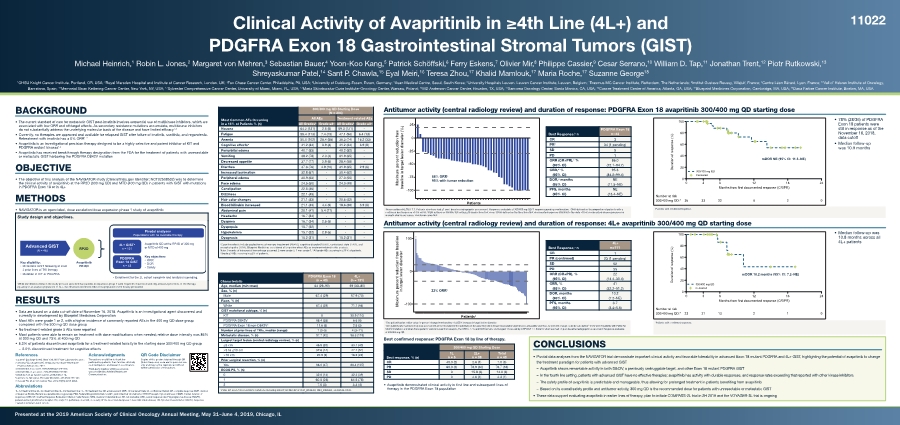
| Clinical Activity of Avapritinib in ≥4th Line (4L+) and PDGFRA Exon 18 Gastrointestinal Stromal Tumors (GIST) Michael Heinrich,1 Robin L. Jones,2 Margaret von Mehren,3 Sebastian Bauer,4 Yoon-Koo Kang,5 Patrick Schöffski,6 Ferry Eskens,7 Olivier Mir,8 Philippe Cassier,9 Cesar Serrano,10 William D. Tap,11 Jonathan Trent,12 Piotr Rutkowski,13 Shreyaskumar Patel,14 Sant P. Chawla,15 Eyal Meiri,16 Teresa Zhou,17 Khalid Mamlouk,17 Maria Roche,17 Suzanne George18 1OHSU Knight Cancer Institute, Portland, OR, USA; 2Royal Marsden Hospital and Institute of Cancer Research, London, UK; 3Fox Chase Cancer Center, Philadelphia, PA, USA; 4University of Duisburg-Essen, Essen, Germany; 5Asan Medical Centre, Seoul, South Korea; 6University Hospitals Leuven, Leuven Cancer Institute, Leuven, Belgium; 7Erasmus MC Cancer Institute, Rotterdam, The Netherlands; 8Institut Gustave Roussy, Villejuif, France; 9Centre Léon Bérard, Lyon, France; 10Vall d’ Hebron Institute of Oncology, Barcelona, Spain; 11Memorial Sloan Kettering Cancer Center, New York, NY, USA; 12Sylvester Comprehensive Cancer Center, University of Miami, Miami, FL, USA; 13Maria Sklodowska-Curie Institute–Oncology Center, Warsaw, Poland; 14MD Anderson Cancer Center, Houston, TX, USA; 15Sarcoma Oncology Center, Santa Monica, CA, USA; 16Cancer Treatment Center of America, Atlanta, GA, USA; 17Blueprint Medicines Corporation, Cambridge, MA, USA; 18Dana Farber Cancer Institute, Boston, MA, USA BACKGROUND • The current standard of care for metastatic GIST post-imatinib involves sequential use of multikinase inhibitors, which are associated with low ORR and off-target effects. As secondary resistance mutations accumulate, multikinase inhibitors do not substantially address the underlying molecular basis of the disease and have limited efficacy1-4 • Currently, no therapies are approved and available for relapsed GIST after failure of imatinib, sunitinib, and regorafenib. Retreatment with imatinib has a 0% ORR1–7 • Avapritinib is an investigational precision therapy designed to be a highly selective and potent inhibitor of KIT and PDGFRA mutant kinases1,2 • Avapritinib has received breakthrough therapy designation from the FDA for the treatment of patients with unresectable or metastatic GIST harboring the PDGFRA D842V mutation OBJECTIVE • The objective of this analysis of the NAVIGATOR study (ClinicalTrials.gov Identifier: NCT02508532) was to determine the clinical activity of avapritinib at the RP2D (300 mg QD) and MTD (400 mg QD) in patients with GIST with mutations in PDGFRA Exon 18 or in 4L+ METHODS • NAVIGATOR is an open-label, dose escalation/dose expansion phase 1 study of avapritinib Study design and objectives. 4L+ GISTa n = 121 PDGFRA Exon 18 GIST n = 43 Pivotal analyses Populations with no available therapy Avapritinib QD at the RP2D of 300 mg or MTD of 400 mg Advanced GIST (N = 46) RP2D Avapritinib PO QD Key eligibility: • Metastatic GIST following at least 2 prior lines of TKI therapy • Mutation in KIT or PDGFRA Key objectives • ORR • DOR • Safety • Enrollment for the 2L cohort complete and analysis is pending. aWhile enrollment criteria in the study protocol specified that patients in expansion group 1 were required to have received only at least 2 prior lines of TKI therapy, equating to an analysis population of 3L+, the observed enrollment reflected a population more heavily pretreated. RESULTS • Data are based on a data cut-off date of November 16, 2018. Avapritinib is an investigational agent discovered and currently in development by Blueprint Medicines Corporation • Most AEs were grade 1 or 2, with a higher incidence of commonly reported AEs in the 400 mg QD dose group compared with the 300 mg QD dose group • No treatment-related grade 5 AEs were reported • Most patients were able to remain on treatment with dose modifications when needed; relative dose intensity was 86% at 300 mg QD and 73% at 400 mg QD • 8.3% of patients discontinued avapritinib for a treatment-related toxicity in the starting dose 300/400 mg QD group – – 2.0% discontinued treatment for cognitive effects References 1. Sutent® [package insert]. New York, NY: Pfizer Laboratories; 2017. 2. Stivarga® [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2017. 3. Demetri GD, et al. Lancet. 2006;368(9544):1329-1338. 4. Demetri GD, et al. Lancet. 2013;381(9863):295-302. 5. Nishida T, et al. Gastric Cancer. 2016;19(1):3-14. 6. Serrano C, George S. Ther Adv Med Onc. 2014;6(3):115-127. 7. Cassier PA, et al. Clin Cancer Res. 2012;18(16):4458-4464. Acknowledgments The authors would like to thank the participating patients, their families, all study co-investigators, and research coordinators. Third-party medical writing assistance was provided by Ashfield Healthcare Communications. QR Code Disclaimer Copies of this poster obtained through QR (Quick Response) code are for personal use only and may not be reproduced without written permission of the authors. Abbreviations 1L, 1st treatment line; 2L, 2nd treatment line; 3L, 3rd treatment line; 4L, 4th treatment line; AE, adverse event; CBR, clinical benefit rate; CI, confidence interval; CR, complete response; DOR, duration of response; ECOG, Eastern Cooperative Oncology Group; FDA, Federal Drug Administration; GIST, gastrointestinal stromal tumor; KIT, KIT receptor tyrosine kinase; mDOR, median duration of response; mRECIST, modified Response Evaluation Criteria in Solid Tumors; MTD, maximum tolerated dose; NE, not evaluable; ORR, overall response rate; PD, progressive disease; PDGFR, platelet-derived growth factor receptor; PO, orally; PR, partial response; QD, once daily; RP2D, recommended phase 2 dose; SD, stable disease; TKI, tyrosine kinase inhibitor; RECIST, Response Evaluation Criteria in Solid Tumors. Presented at the 2019 American Society of Clinical Oncology Annual Meeting, May 31–June 4, 2019, Chicago, IL Most Common AEs Occurring in ≥ 15% of Patients % (n) 300/400 mg QD Starting Dose (N = 204) All AEs Treatment-related AEs All Gradesb Grade ≥3c All Gradesb Grade ≥3c Nausea 64.2 (131) 2.5 (5) 59.3 (121) - Fatigue 55.4 (113) 7.4 (15) 47.1 (96) 6.4 (13) Anemia 50.0 (102) 28.4 (58) 36.3 (74) 16.2 (33) Cognitive effectsa 41.2 (84) 3.9 (8) 41.2 (84) 3.9 (8) Periorbital edema 40.7 (83) - 40.2 (82) - Vomiting 38.2 (78) 2.0 (4) 31.9 (65) - Decreased appetite 37.7 (77) 2.9 (6) 28.4 (58) - Diarrhea 37.3 (76) 4.9 (10) 31.9 (65) 2.9 (6) Increased lacrimation 32.8 (67) - 30.4 (62) - Peripheral edema 30.9 (63) - 27.0 (55) - Face edema 24.5 (50) - 24.0 (49) - Constipation 22.5 (46) - - - Dizziness 22.1 (45) - - - Hair color changes 21.1 (43) - 20.6 (42) - Blood bilirubin increased 21.1 (43) 4.4 (9) 18.6 (38) 3.9 (8) Abdominal pain 20.1 (41) 5.4 (11) - - Headache 16.7 (34) - - - Dyspnea 16.7 (34) 2.5 (5) - - Dyspepsia 15.7 (32) - - - Hypokalemia 15.7 (32) 2.9 (6) - - Dysgeusia 15.2 (31) - 15.2 (31) - aCognitive effects include pooled terms of memory impairment (29.4%), cognitive disorder (10.8%), confusional state (7.4%), and encephalopathy (1.5%). Blueprint Medicines considered all cognitive effect AEs as treatment-related in this analysis. Note: 3 events of intracranial hemorrhage occurred, 2 were grade 3, 1 was grade 1. bAll grade AEs occuring in ≥15% of patients. cGrade ≥3 AEs occuring in ≥2% of patients. Characteristic PDGFRA Exon 18 (n = 43) 4L+ (n = 121) Age, median (min–max) 64 (29–90) 59 (33–80) Sex, % (n) Male 67.4 (29) 57.9 (70) Race, % (n) White 67.4 (29) 71.1 (86) GIST mutational subtype, % (n) KIT 0 90.9 (110) PDGFRA D842V 88.4 (38) 6.6 (8) PDGFRA Exon 18 non-D842Va 11.6 (5) 2.5 (3) Number of prior lines of TKIs, median (range) 1 (0–5) 4 (3–11) Metastatic disease, % (n) 97.7 (42) 98.3 (119) Largest target lesion (central radiology review), % (n) ≤5 cm 46.5 (20) 33.1 (40) >5 to ≤10 cm 32.6 (14) 47.1 (57) >10 cm 20.9 (9) 18.2 (22) Prior surgical resection, % (n) Yes 86.0 (37) 88.4 (107) ECOG PS, % (n) 0 32.6 (14) 32.2 (39) 1 60.5 (26) 64.5 (78) 2 7.0 (3) 3.3 (4) aPDGFRA Exon 18 non-D842V mutations including D842Y, DI 842-845V, I843_D846del, I843_D846del, and D842-H845. Antitumor activity (central radiology review) and duration of response: PDGFRA Exon 18 avapritinib 300/400 mg QD starting dose Antitumor activity (central radiology review) and duration of response: 4L+ avapritinib 300/400 mg QD starting dose CONCLUSIONS • Pivotal data analyses from the NAVIGATOR trial demonstrate important clinical activity and favorable tolerability in advanced Exon 18 mutant PDGFRA and 4L+ GIST, highlighting the potential of avapritinib to change the treatment paradigm for patients with advanced GIST – – Avapritinib shows remarkable activity in both D842V, a previously undruggable target, and other Exon 18 mutant PDGFRA GIST – – In the fourth line setting, patients with advanced GIST have no effective therapies; avapritinib has activity with durable responses, and response rates exceeding that reported with other kinase inhibitors – – The safety profile of avapritinib is predictable and manageable, thus allowing for prolonged treatment in patients benefiting from avapritinib – – Based on its overall safety profile and antitumor activity, 300 mg QD is the recommended dose for patients with unresectable or metastatic GIST • These data support evaluating avapritinib in earlier lines of therapy; plan to initiate COMPASS-2L trial in 2H 2019 and the VOYAGER-3L trial is ongoing Best response, % (n) 300/400 mg QD Starting Dose 1L n = 5 2L+ n = 38 Total n = 43 CR 40.0 (2) 2.6 (1) 7.0 (3) PR 60.0 (3) 78.9 (30) 76.7 (33) SD 0 15.8 (6) 14.0 (6) PD 0 2.6 (1) 2.3 (1) 11022 Best confirmed response: PDGFRA Exon 18 by line of therapy. • Avapritinib demonstrated clinical activity in first line and subsequent lines of therapy in the PDGFRA Exon 18 population Maximum percent reduction from baseline in target lesion diameter (%) -100 -75 -50 -25 0 25 Patients 86% ORRc 95% with tumor reduction Best Response,b n PDGFRA Exon 18 n=43 CR 3 PRb 34 (1 pending) SD 5 PD 1 ORR (CR+PR),c % (95% CI) 86.0 (72.1–94.7) CBR,d % (95% CI) 95.3 (84.2–99.4) DOR,e months (95% CI) NE (11.5–NE) PFS, months (95% CI) NE (13.4–NE) aAssessed by mRECIST 1.1. Patients who have had ≥1 post-baseline radiographic assessment. Response evaluable at 300/400 mg QD.b1 response pending confirmation. cORR defined as the proportion of patients with a confirmed best response of CR or PR. dCBR defined as CR/PR+SD lasting ≥16 weeks from first dose. eDOR defined as the time from first documented response (CR/PR) to the date of first documented disease progression or death due to any cause, whichever came first. Duration of response (%) Months from first documented response (CR/PR) Number at risk 300/400 mg QD:* 0 20 40 60 80 100 0 3 6 12 18 24 36 33 22 8 2 0 300/400 mg QD Censored mDOR NE (95% CI: 11.3–NE) • 78% (28/36) of PDGFRA Exon 18 patients were still in response as of the November 16, 2018, data cutoff • Median follow-up was 10.9 months *Patients with confirmed response. Best Response,c n 4L+ n=111 CR 1 PR (confirmed) 23 (1 pending) SD 52 PD 35 ORR (CR+PR), % (95% CI) 22 (14.4–30.4) CBR, % (95% CI) 41 (32.2–51.2) DOR, months (95% CI) 10.2 (7.2–NE) PFS, months (95% CI) 3.7 (3.4–5.6) Maximum percent reduction from baseline in target lesion diameter -100 0 100 Patientsa * 22% ORRb *One patient had an outlier value for percent change from baseline of >200% increase in target lesion diameter. aTwo patients who had best response assessment are not included in the waterfall plot because they did not have measurable target lesions at baseline and thus, no percent change could be calculated. bThere were 8 patients with PDGFRA D842V mutations and when these patients were removed from analysis, the ORR is 17% and DOR remains unchanged. cAssessed by mRECIST 1.1. Patients who have had ≥1 post-baseline radiographic assessment. Response evaluable at 300/400 mg QD. Duration of response (%) Months from first documented response (CR/PR) Number at risk 300/400 mg QD:* 0 20 40 60 80 100 0 3 6 12 18 24 23 21 13 2 1 0 300/400 mg QD Censored mDOR 10.2 months (95% CI: 7.2–NE) • Median follow-up was 10.8 months across all 4L+ patients *Patients with confirmed response. |
