Changli Wang | A Phase II Study of Perioperative Ivonescimab Alone or Combined with Chemotherapy in Resectable Non-Small Cell Lung Cancer 1 A Phase II Study of Perioperative Ivonescimab Alone or Combined with Chemotherapy in Resectable Non-Small Cell Lung Cancer Changli Wang1, Xiaoliang Zhao1, Lianmin Zhang1, Dongsheng Yue1, Zhenfa Zhang1, Meng Wang1, Ziqiang Tian2, Shengguang Wang1, Chong Pang1, Bin Zhang1, Qiang Zhang1, Wei Wei1, Yu Zhang1, Xiaofei Wang1, Yue Li1, Huilai Lv2, Yu Xia3, Baiyong Li3, Zhongmin Maxwell Wang3, Wenting Li3 1.Tianjin Medical University Cancer Institute & Hospital, Tianjin, P. R. China; 2.The Fourth Hospital of Hebei Medical University, Shijiazhuang, P. R. China; 3.Akeso, Inc., Zhongshan, P. R. China.

Changli Wang | A Phase II Study of Perioperative Ivonescimab Alone or Combined with Chemotherapy in Resectable Non-Small Cell Lung Cancer 2 Background • Recent phase III trials have demonstrated the efficacy of neoadjuvant PD-(L)1 inhibitors combined with chemotherapy followed by surgery and adjuvant PD-(L)1 inhibitors for resectable non-small cell lung cancer (NSCLC), including KEYNOTE 6711, CheckMate 77T2, AEGEAN3, Neotorch4, and RATIONALE 3155. • Ivonescimab is a first-in-class anti-PD-1/VEGF bispecific antibody. Ivonescimab, both as monotherapy and in combination with chemotherapy, showed promising antitumor activity and manageable safety profile in patients with advanced NSCLC6,7. • Here we report the efficacy and safety of a phase II study of perioperative ivonescimab alone or combined with chemotherapy in resectable NSCLC (NCT05247684). 1Wakelee H, et al. N Engl J Med. 2023 Aug 10;389(6):491-503. 2Cascone T, et al. N Engl J Med. 2024 May 16;390(19):1756-1769. 3Heymach JV, et al. N Engl J Med. 2023 Nov 2;389(18):1672-1684. 4Lu S, et al. JAMA. 2024 Jan 16;331(3):201-211. 5D. Yue, et al. 2023 ESMO LBA58. 6Fang W, et al. JAMA. 2024 Aug 20;332(7):561-570. 7Wang L, et al. J Thorac Oncol. 2024 Mar;19(3):465-475.
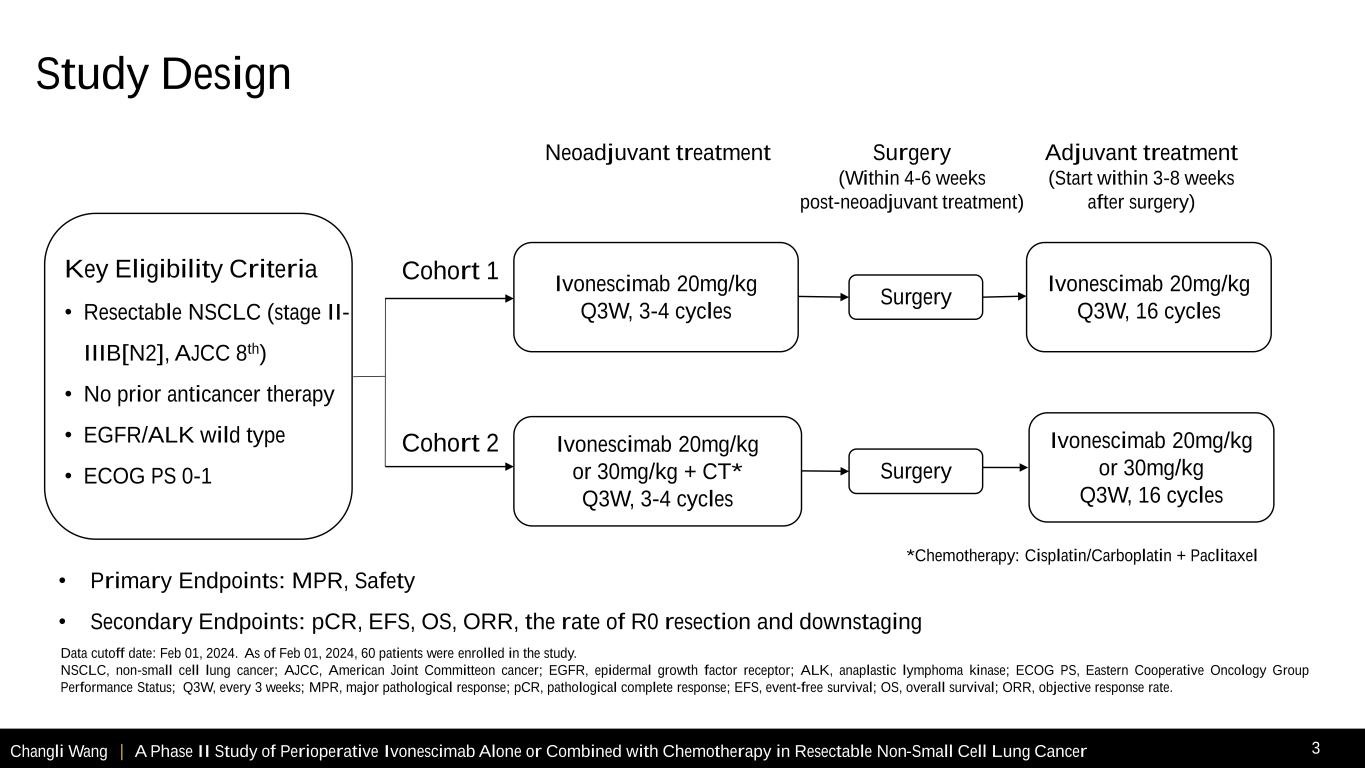
• Primary Endpoints: MPR, Safety • Secondary Endpoints: pCR, EFS, OS, ORR, the rate of R0 resection and downstaging Changli Wang | A Phase II Study of Perioperative Ivonescimab Alone or Combined with Chemotherapy in Resectable Non-Small Cell Lung Cancer 3 Key Eligibility Criteria • Resectable NSCLC (stage II- IIIB[N2], AJCC 8th) • No prior anticancer therapy • EGFR/ALK wild type • ECOG PS 0-1 *Chemotherapy: Cisplatin/Carboplatin + Paclitaxel Surgery Ivonescimab 20mg/kg or 30mg/kg + CT* Q3W, 3-4 cycles Neoadjuvant treatment Surgery (Within 4-6 weeks post-neoadjuvant treatment) Adjuvant treatment (Start within 3-8 weeks after surgery) Cohort 1 Cohort 2 Surgery Ivonescimab 20mg/kg Q3W, 3-4 cycles Ivonescimab 20mg/kg Q3W, 16 cycles Data cutoff date: Feb 01, 2024. As of Feb 01, 2024, 60 patients were enrolled in the study. NSCLC, non-small cell lung cancer; AJCC, American Joint Committeon cancer; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ECOG PS, Eastern Cooperative Oncology Group Performance Status; Q3W, every 3 weeks; MPR, major pathological response; pCR, pathological complete response; EFS, event-free survival; OS, overall survival; ORR, objective response rate. Study Design Ivonescimab 20mg/kg or 30mg/kg Q3W, 16 cycles

Changli Wang | A Phase II Study of Perioperative Ivonescimab Alone or Combined with Chemotherapy in Resectable Non-Small Cell Lung Cancer 4 60 patients were enrolled 11 were enrolled to Cohort 1 49 were enrolled to Cohort 2 1 did not complete surgery 1 Disease progression 10 did not complete surgery 6 Subject request 1 Disease progression 1 Adverse event 2 Other 1 remains on adjuvant treatment 9 end of treatment 24 remain on adjuvant treatment 12 end of treatment 39 completed R0 resection10 completed R0 resection 36 received ≥1 cycle of adjuvant treatment 3 were awaiting adjuvant treatment All received ≥1 cycle of adjuvant treatment Patient Disposition
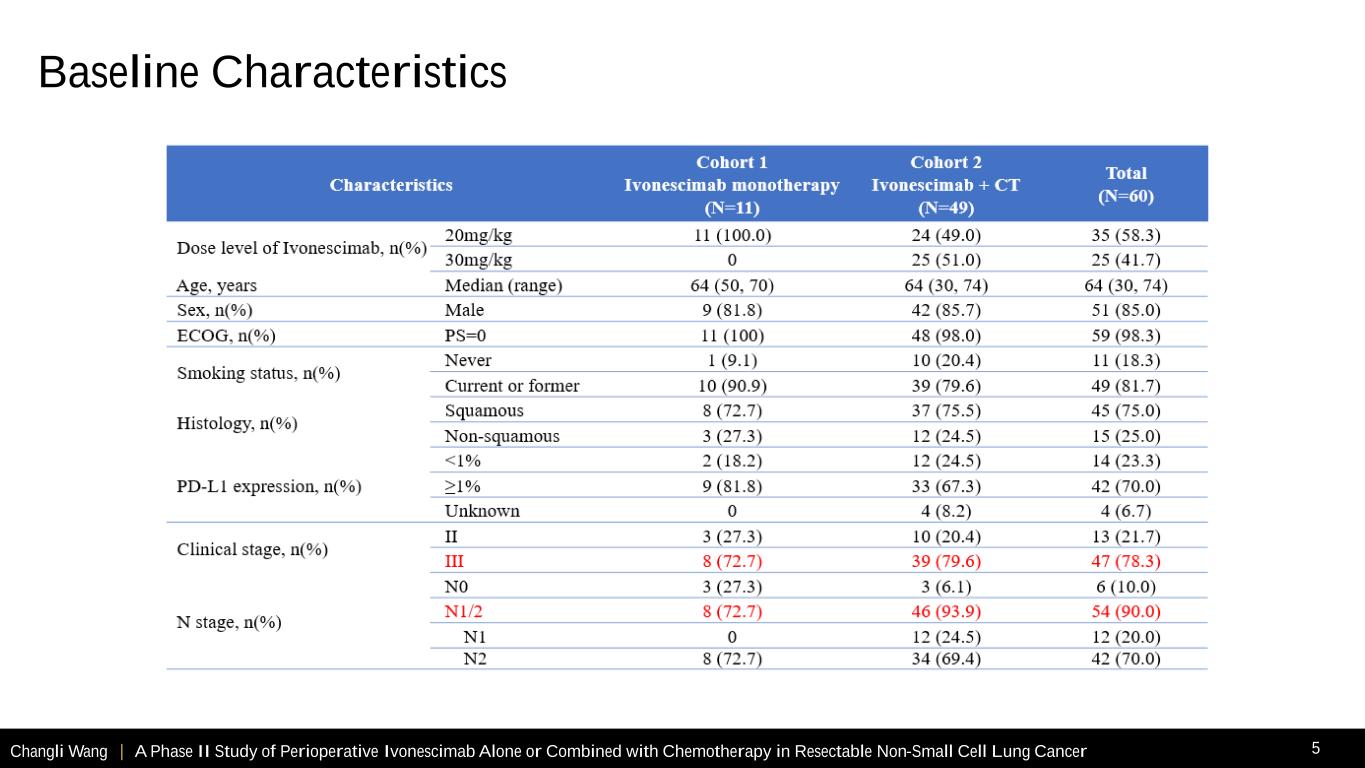
Changli Wang | A Phase II Study of Perioperative Ivonescimab Alone or Combined with Chemotherapy in Resectable Non-Small Cell Lung Cancer 5 Baseline Characteristics
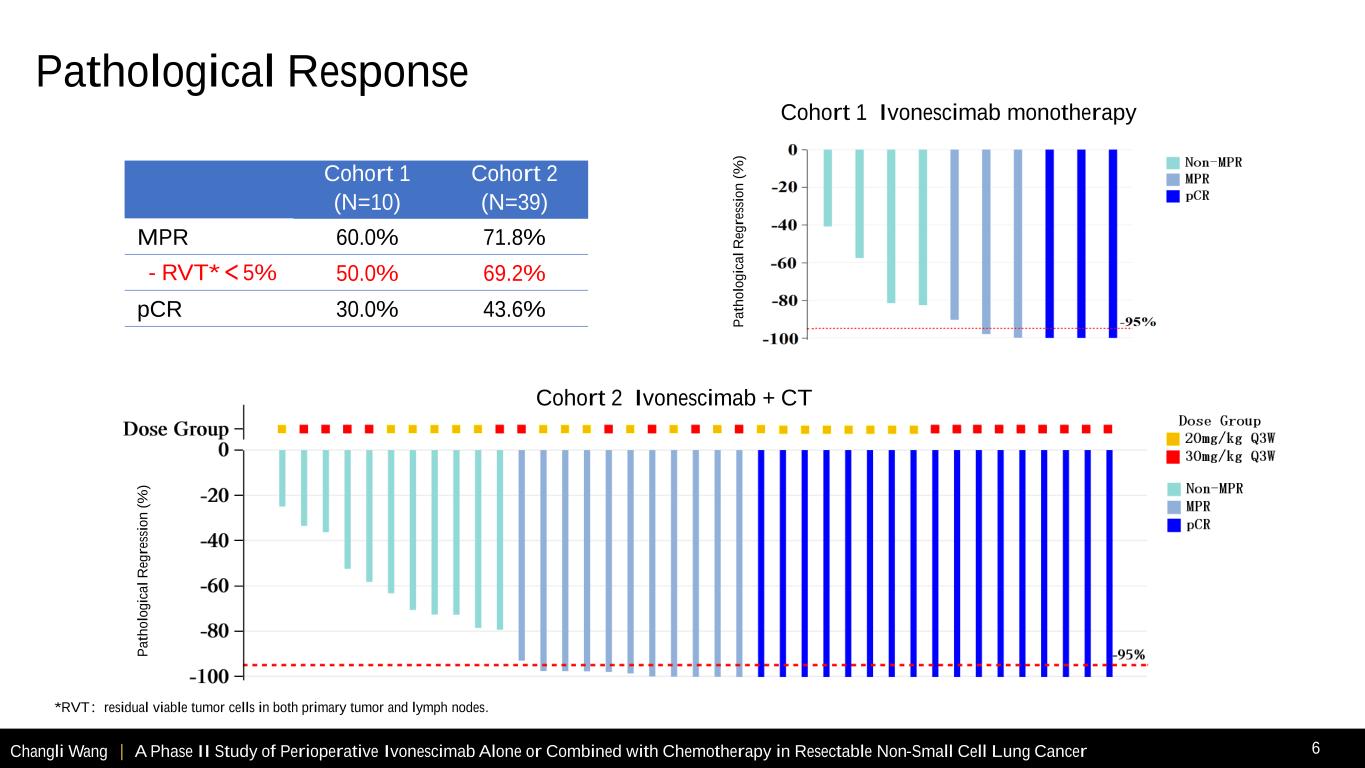
Changli Wang | A Phase II Study of Perioperative Ivonescimab Alone or Combined with Chemotherapy in Resectable Non-Small Cell Lung Cancer 6 Cohort 1 Cohort 2 (N=10) (N=39) MPR 60.0% 71.8% - RVT*<5% 50.0% 69.2% pCR 30.0% 43.6% *RVT:residual viable tumor cells in both primary tumor and lymph nodes. Cohort 1 Ivonescimab monotherapy Cohort 2 Ivonescimab + CT P a th o lo g ic a l R eg re ss io n ( % ) P a th o lo g ic a l R eg re ss io n ( % ) Pathological Response
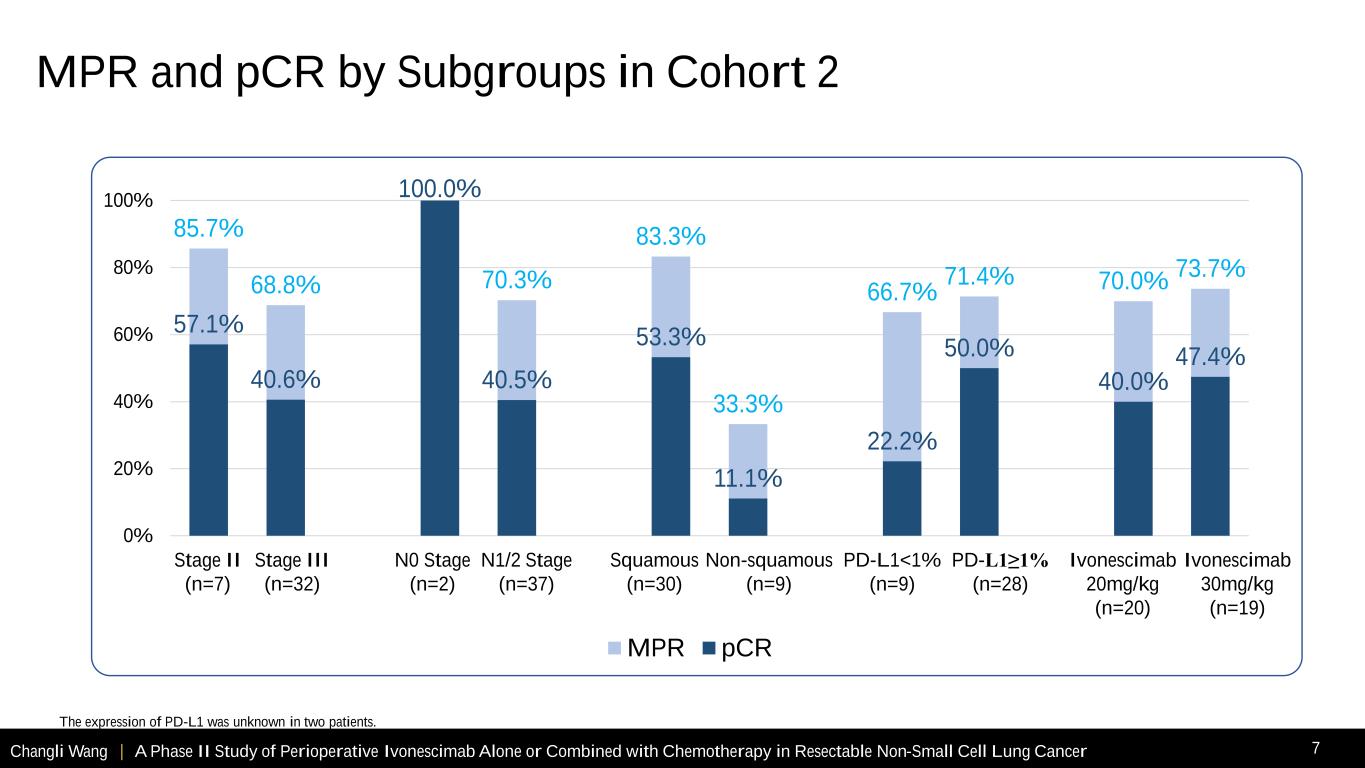
Changli Wang | A Phase II Study of Perioperative Ivonescimab Alone or Combined with Chemotherapy in Resectable Non-Small Cell Lung Cancer 7 85.7% 68.8% 100.0% 70.3% 83.3% 33.3% 66.7% 71.4% 70.0% 73.7% 57.1% 40.6% 40.5% 53.3% 11.1% 22.2% 50.0% 40.0% 47.4% 0% 20% 40% 60% 80% 100% MPR pCR Stage II (n=7) Stage III (n=32) N0 Stage (n=2) N1/2 Stage (n=37) Squamous (n=30) Non-squamous (n=9) PD-L1≥1% (n=28) PD-L1<1% (n=9) Ivonescimab 20mg/kg (n=20) Ivonescimab 30mg/kg (n=19) The expression of PD-L1 was unknown in two patients. MPR and pCR by Subgroups in Cohort 2

Changli Wang | A Phase II Study of Perioperative Ivonescimab Alone or Combined with Chemotherapy in Resectable Non-Small Cell Lung Cancer 8 E F S ( % ) Cohort 1 Ivonescimab monotherapy (n=11) Median Follow-up (95% CI) 17.64 (12.3, 21.2) No. of Events/No. of Patients (%) 4/11 (36.4%) Median EFS (95% CI) NR (6.9, NE) E F S ( % ) Cohort 2 Ivonescimab + CT (n=49) Median Follow-up (95% CI) 8.94 (6.6, 12.2) No. of Events/No. of Patients (%) 8/49 (16.3%) Median EFS (95% CI) NR (14.9, NE) EFS
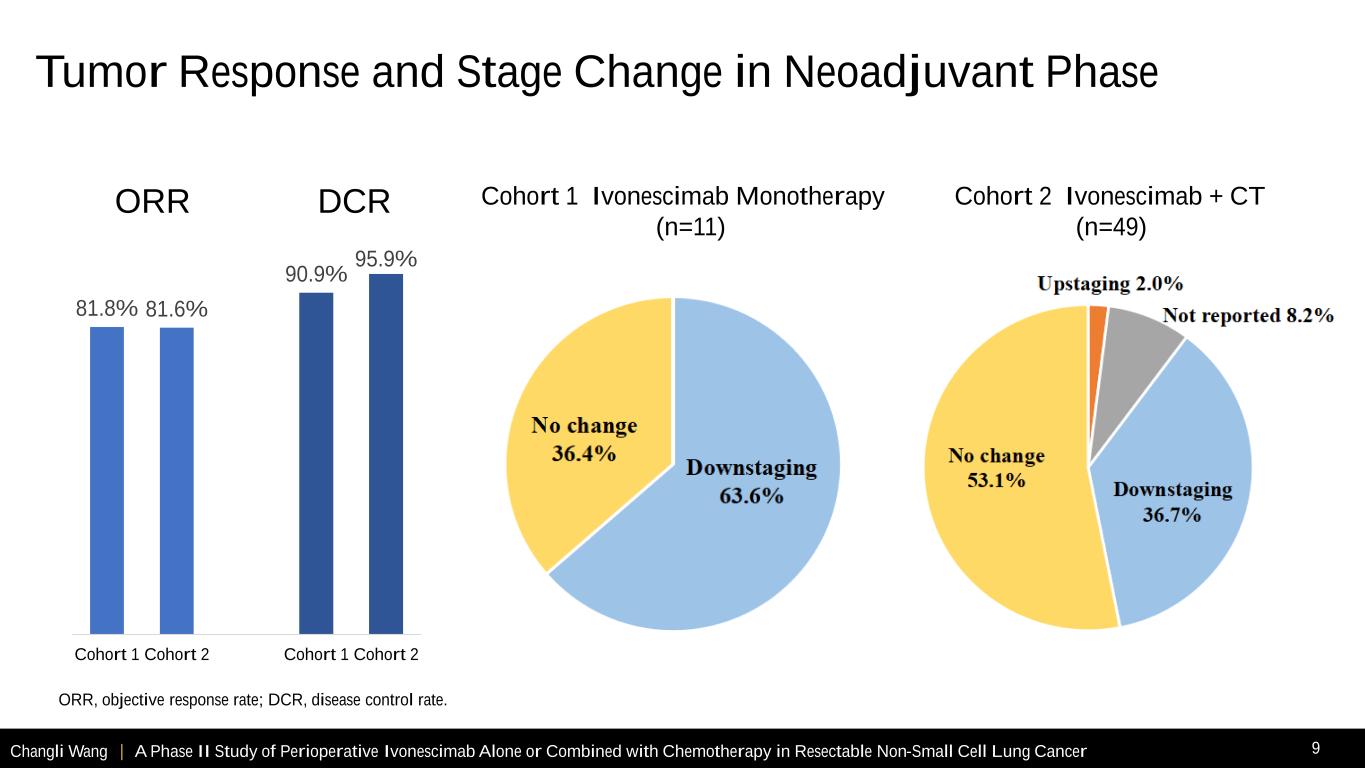
Changli Wang | A Phase II Study of Perioperative Ivonescimab Alone or Combined with Chemotherapy in Resectable Non-Small Cell Lung Cancer 9 ORR, objective response rate; DCR, disease control rate. 81.8% 81.6% 90.9% 95.9% Cohort 1 Cohort 2 Cohort 1 Cohort 2 ORR DCR Cohort 2 Ivonescimab + CT (n=49) Cohort 1 Ivonescimab Monotherapy (n=11) Tumor Response and Stage Change in Neoadjuvant Phase
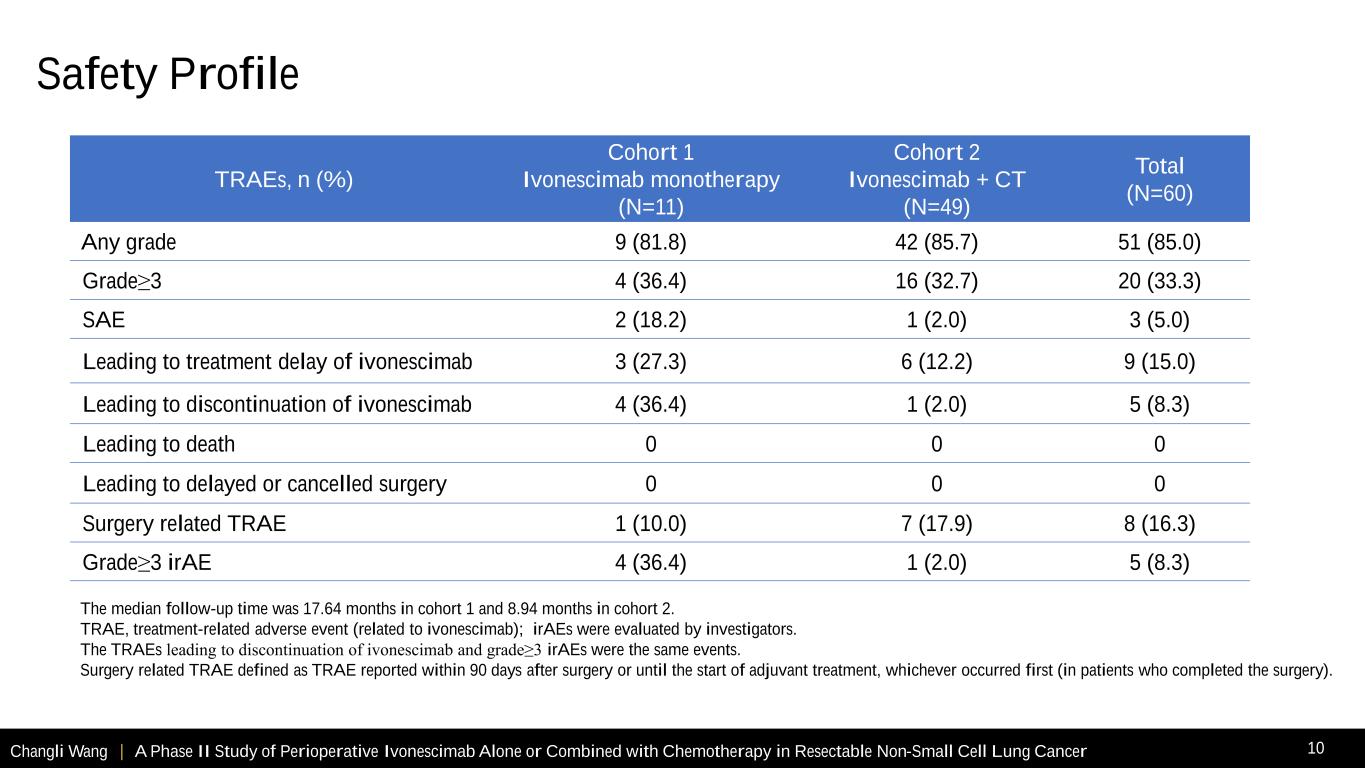
Changli Wang | A Phase II Study of Perioperative Ivonescimab Alone or Combined with Chemotherapy in Resectable Non-Small Cell Lung Cancer 10 TRAEs, n (%) Cohort 1 Ivonescimab monotherapy (N=11) Cohort 2 Ivonescimab + CT (N=49) Total (N=60) Any grade 9 (81.8) 42 (85.7) 51 (85.0) Grade≥3 4 (36.4) 16 (32.7) 20 (33.3) SAE 2 (18.2) 1 (2.0) 3 (5.0) Leading to treatment delay of ivonescimab 3 (27.3) 6 (12.2) 9 (15.0) Leading to discontinuation of ivonescimab 4 (36.4) 1 (2.0) 5 (8.3) Leading to death 0 0 0 Leading to delayed or cancelled surgery 0 0 0 Surgery related TRAE 1 (10.0) 7 (17.9) 8 (16.3) Grade≥3 irAE 4 (36.4) 1 (2.0) 5 (8.3) The median follow-up time was 17.64 months in cohort 1 and 8.94 months in cohort 2. TRAE, treatment-related adverse event (related to ivonescimab); irAEs were evaluated by investigators. The TRAEs leading to discontinuation of ivonescimab and grade≥3 irAEs were the same events. Surgery related TRAE defined as TRAE reported within 90 days after surgery or until the start of adjuvant treatment, whichever occurred first (in patients who completed the surgery). Safety Profile
Changli Wang | A Phase II Study of Perioperative Ivonescimab Alone or Combined with Chemotherapy in Resectable Non-Small Cell Lung Cancer 11 • Perioperative ivonescimab monotherapy or combined with chemotherapy for resectable NSCLC demonstrated high rates of pCR and MPR in this phase II study. • Compared with ivonescimab monotherapy, rates of MPR and pCR in ivonescimab combined with chemotherapy were numerically higher, and across tumor stage and PD-L1 expression subgroups. - Ivonescimab + chemotherapy: pCR rate was 43.6%, MPR rate was 71.8% -As of Aug 30, 2024, 55 patients in cohort 2 completed surgery, pCR and MPR rates were improved to 52.7% and 72.7%, respectively. For squamous NSCLC, pCR and MPR rates were 63.6% and 84.1%, respectively. - Ivonescimab monotherapy: pCR rate was 30.0%, MPR rate was 60.0% • Although EFS is not mature yet, higher pCR rates relative to historic studies1-3 may predict longer EFS. Additional follow-up studies are planned to confirm these results. • The safety profile was manageable. There were no TRAEs that led to cancelled or delayed surgery or wound healing complications. 1Wakelee H, et al. N Engl J Med. 2023 Aug 10;389(6):491-503. 2Cascone T, et al. N Engl J Med. 2024 May 16;390(19):1756-1769. 3Lu S, et al. JAMA. 2024 Jan 16;331(3):201-211. Conclusions

Changli Wang | A Phase II Study of Perioperative Ivonescimab Alone or Combined with Chemotherapy in Resectable Non-Small Cell Lung Cancer 12 • Thanks to all patients, their families, and their caregivers for their participation • Thanks to all investigators who supported this clinical trail • Thanks to all staff members for their contributions Acknowledgements











