
Summit Therapeutics Update Call from WCLC September 9, 2024

Forward-Looking Statements 2 Any statements in this presentation about the Company’s future expectations, plans and prospects, including but not limited to, statements about the clinical and preclinical development of the Company’s product candidates, entry into and actions related to the Company’s partnership with Akeso Inc., including the expected benefits of the amendment to the collaboration and license agreement, the intended use of the net proceeds from the private placement, the Company’s anticipated spending and cash runway, the therapeutic potential of the Company’s product candidates, the potential commercialization of the Company’s product candidates, the timing of initiation, completion and availability of data from clinical trials, the potential submission of applications for marketing approvals, potential acquisitions, statements about the previously disclosed At-The-Market equity offering program (“ATM Program”), the expected use of proceeds and uses thereof, and other statements containing the words "anticipate," "believe," "continue," "could," "estimate," "expect," "intend," "may," "plan," "potential," "predict," "project," "should," "target," "would," and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including the results of our evaluation of the underlying data in connection with the development and commercialization activities for ivonescimab, the outcome of discussions with regulatory authorities, including the Food and Drug Administration, the uncertainties inherent in the initiation of future clinical trials, availability and timing of data from ongoing and future clinical trials, the results of such trials, and their success, and global public health crises that may affect timing and status of our clinical trials and operations, whether preliminary results from a clinical trial will be predictive of the final results of that trial or whether results of early clinical trials or preclinical studies will be indicative of the results of later clinical trials, whether business development opportunities to expand the Company’s pipeline of drug candidates, including without limitation, through potential acquisitions of, and/or collaborations with, other entities occur, expectations for regulatory approvals, laws and regulations affecting government contracts and funding awards, availability of funding sufficient for the Company’s foreseeable and unforeseeable operating expenses and capital expenditure requirements and other factors discussed in the "Risk Factors" section of filings that the Company makes with the Securities and Exchange Commission. Any change to our ongoing trials could cause delays, affect our future expenses, and add uncertainty to our commercialization efforts, as well as to affect the likelihood of the successful completion of clinical development of ivonescimab. Accordingly, the audience should not place undue reliance on forward-looking statements or information. In addition, any forward-looking statements included in this presentation represent the Company’s views only as of the date of this presentation and should not be relied upon as representing the Company’s views as of any subsequent date. The Company specifically disclaims any obligation to update any forward-looking statements included in this presentation. Summit Confidential & Proprietary Information - Do Not Copy or Distribute Presentation Summit Update – September 2024
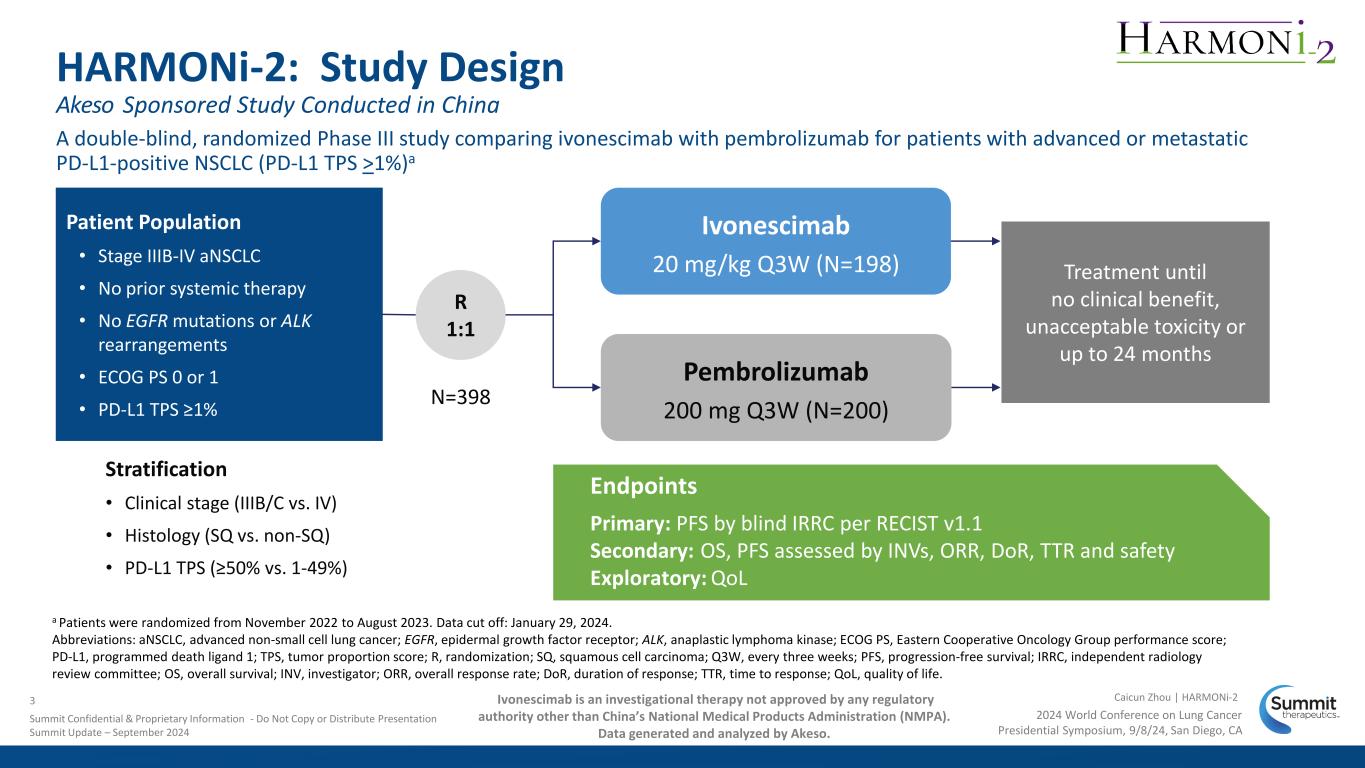
A double-blind, randomized Phase III study comparing ivonescimab with pembrolizumab for patients with advanced or metastatic PD-L1-positive NSCLC (PD-L1 TPS >1%)a Stratification • Clinical stage (IIIB/C vs. IV) • Histology (SQ vs. non-SQ) • PD-L1 TPS (≥50% vs. 1-49%) HARMONi-2: Study Design Patient Population • Stage IIIB-IV aNSCLC • No prior systemic therapy • No EGFR mutations or ALK rearrangements • ECOG PS 0 or 1 • PD-L1 TPS ≥1% R 1:1 Pembrolizumab 200 mg Q3W (N=200) Treatment until no clinical benefit, unacceptable toxicity or up to 24 months N=398 Ivonescimab 20 mg/kg Q3W (N=198) Endpoints Primary: PFS by blind IRRC per RECIST v1.1 Secondary: OS, PFS assessed by INVs, ORR, DoR, TTR and safety Exploratory: QoL a Patients were randomized from November 2022 to August 2023. Data cut off: January 29, 2024. Abbreviations: aNSCLC, advanced non-small cell lung cancer; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ECOG PS, Eastern Cooperative Oncology Group performance score; PD-L1, programmed death ligand 1; TPS, tumor proportion score; R, randomization; SQ, squamous cell carcinoma; Q3W, every three weeks; PFS, progression-free survival; IRRC, independent radiology review committee; OS, overall survival; INV, investigator; ORR, overall response rate; DoR, duration of response; TTR, time to response; QoL, quality of life. Ivonescimab is an investigational therapy not approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Data generated and analyzed by Akeso. 2024 World Conference on Lung Cancer Presidential Symposium, 9/8/24, San Diego, CA Caicun Zhou | HARMONi-2 Akeso Sponsored Study Conducted in China 3 Summit Confidential & Proprietary Information - Do Not Copy or Distribute Presentation Summit Update – September 2024
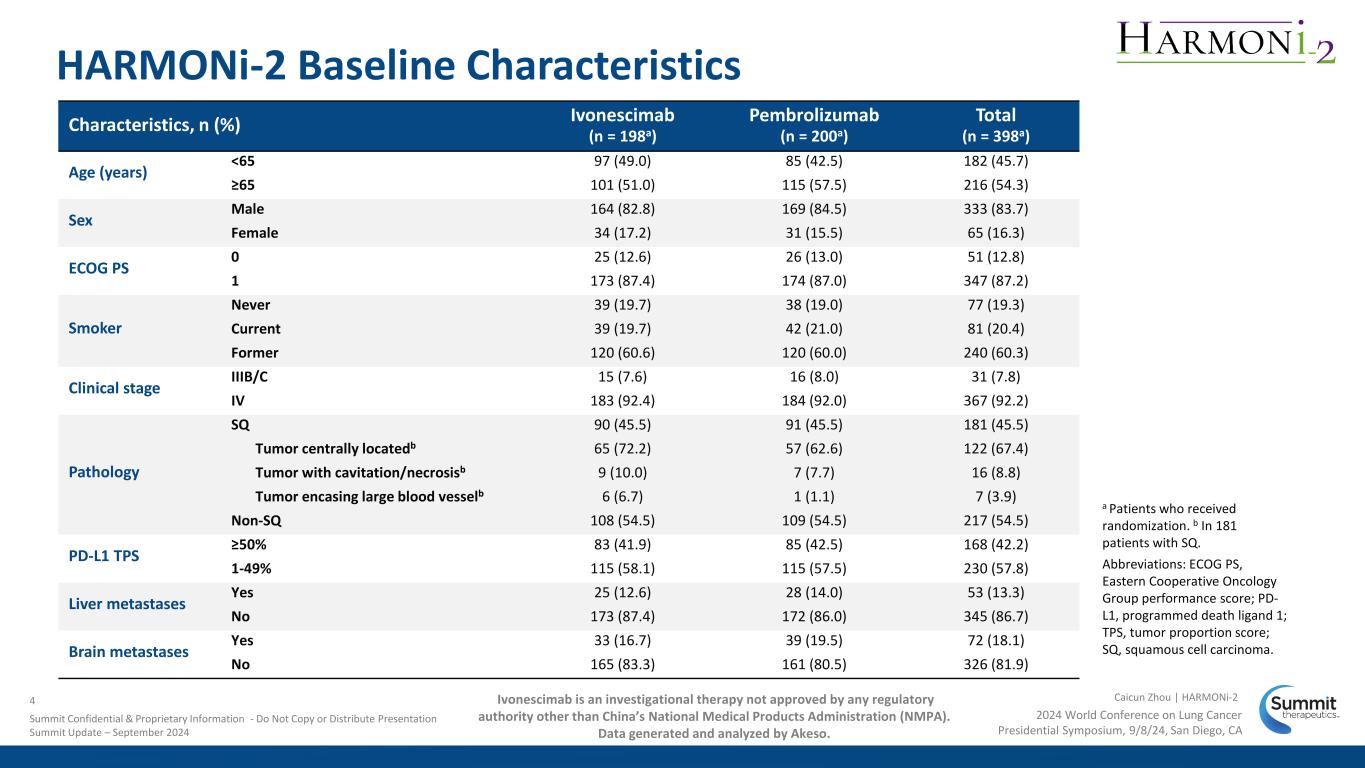
HARMONi-2 Baseline Characteristics Characteristics, n (%) Ivonescimab (n = 198a) Pembrolizumab (n = 200a) Total (n = 398a) Age (years) <65 97 (49.0) 85 (42.5) 182 (45.7) ≥65 101 (51.0) 115 (57.5) 216 (54.3) Sex Male 164 (82.8) 169 (84.5) 333 (83.7) Female 34 (17.2) 31 (15.5) 65 (16.3) ECOG PS 0 25 (12.6) 26 (13.0) 51 (12.8) 1 173 (87.4) 174 (87.0) 347 (87.2) Smoker Never 39 (19.7) 38 (19.0) 77 (19.3) Current 39 (19.7) 42 (21.0) 81 (20.4) Former 120 (60.6) 120 (60.0) 240 (60.3) Clinical stage IIIB/C 15 (7.6) 16 (8.0) 31 (7.8) IV 183 (92.4) 184 (92.0) 367 (92.2) Pathology SQ 90 (45.5) 91 (45.5) 181 (45.5) Tumor centrally locatedb 65 (72.2) 57 (62.6) 122 (67.4) Tumor with cavitation/necrosisb 9 (10.0) 7 (7.7) 16 (8.8) Tumor encasing large blood vesselb 6 (6.7) 1 (1.1) 7 (3.9) Non-SQ 108 (54.5) 109 (54.5) 217 (54.5) PD-L1 TPS ≥50% 83 (41.9) 85 (42.5) 168 (42.2) 1-49% 115 (58.1) 115 (57.5) 230 (57.8) Liver metastases Yes 25 (12.6) 28 (14.0) 53 (13.3) No 173 (87.4) 172 (86.0) 345 (86.7) Brain metastases Yes 33 (16.7) 39 (19.5) 72 (18.1) No 165 (83.3) 161 (80.5) 326 (81.9) a Patients who received randomization. b In 181 patients with SQ. Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance score; PD- L1, programmed death ligand 1; TPS, tumor proportion score; SQ, squamous cell carcinoma. 2024 World Conference on Lung Cancer Presidential Symposium, 9/8/24, San Diego, CA Caicun Zhou | HARMONi-24 Summit Confidential & Proprietary Information - Do Not Copy or Distribute Presentation Summit Update – September 2024 Ivonescimab is an investigational therapy not approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Data generated and analyzed by Akeso.
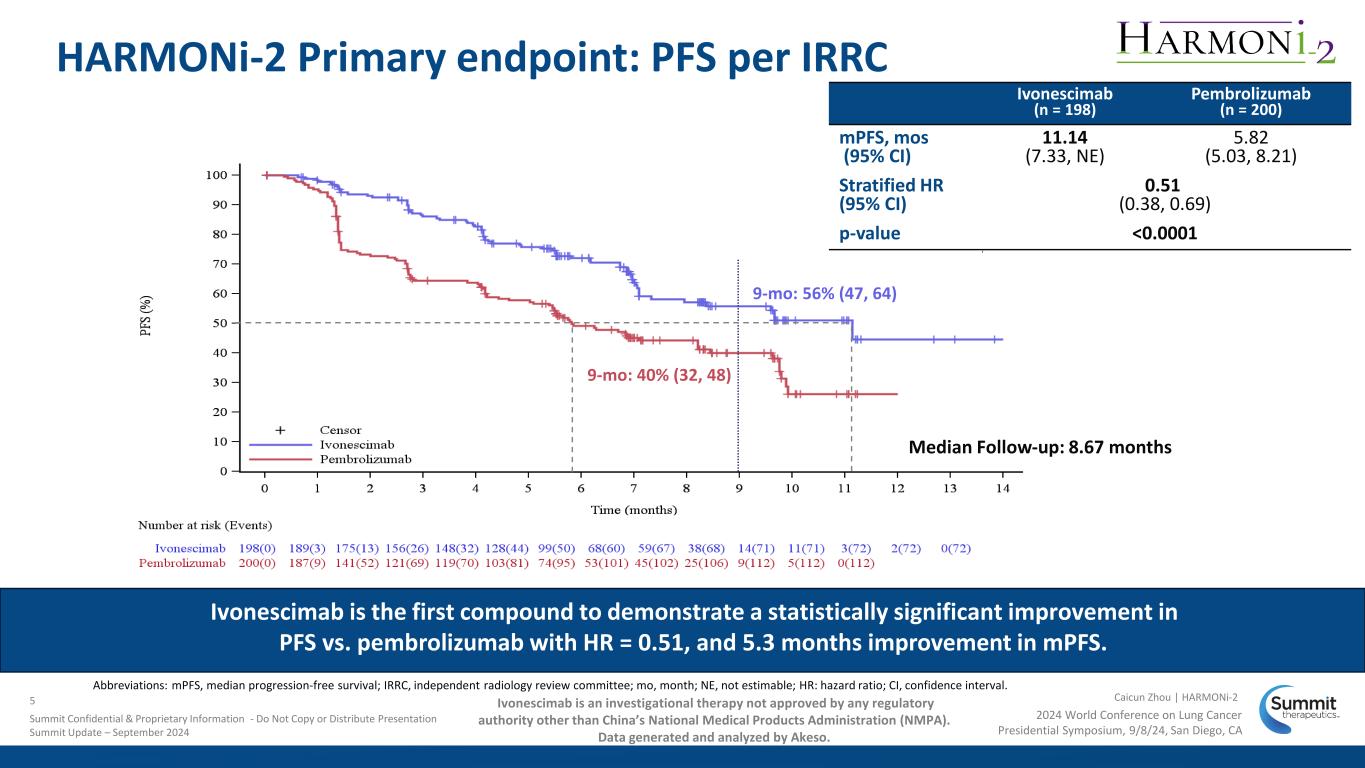
9-mo: 40% (32, 48) Median Follow-up: 8.67 months 9-mo: 56% (47, 64) HARMONi-2 Primary endpoint: PFS per IRRC Abbreviations: mPFS, median progression-free survival; IRRC, independent radiology review committee; mo, month; NE, not estimable; HR: hazard ratio; CI, confidence interval. Ivonescimab (n = 198) Pembrolizumab (n = 200) mPFS, mos (95% CI) 11.14 (7.33, NE) 5.82 (5.03, 8.21) Stratified HR (95% CI) 0.51 (0.38, 0.69) p-value <0.0001 Ivonescimab is the first compound to demonstrate a statistically significant improvement in PFS vs. pembrolizumab with HR = 0.51, and 5.3 months improvement in mPFS. 2024 World Conference on Lung Cancer Presidential Symposium, 9/8/24, San Diego, CA Caicun Zhou | HARMONi-25 Summit Confidential & Proprietary Information - Do Not Copy or Distribute Presentation Summit Update – September 2024 Ivonescimab is an investigational therapy not approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Data generated and analyzed by Akeso.
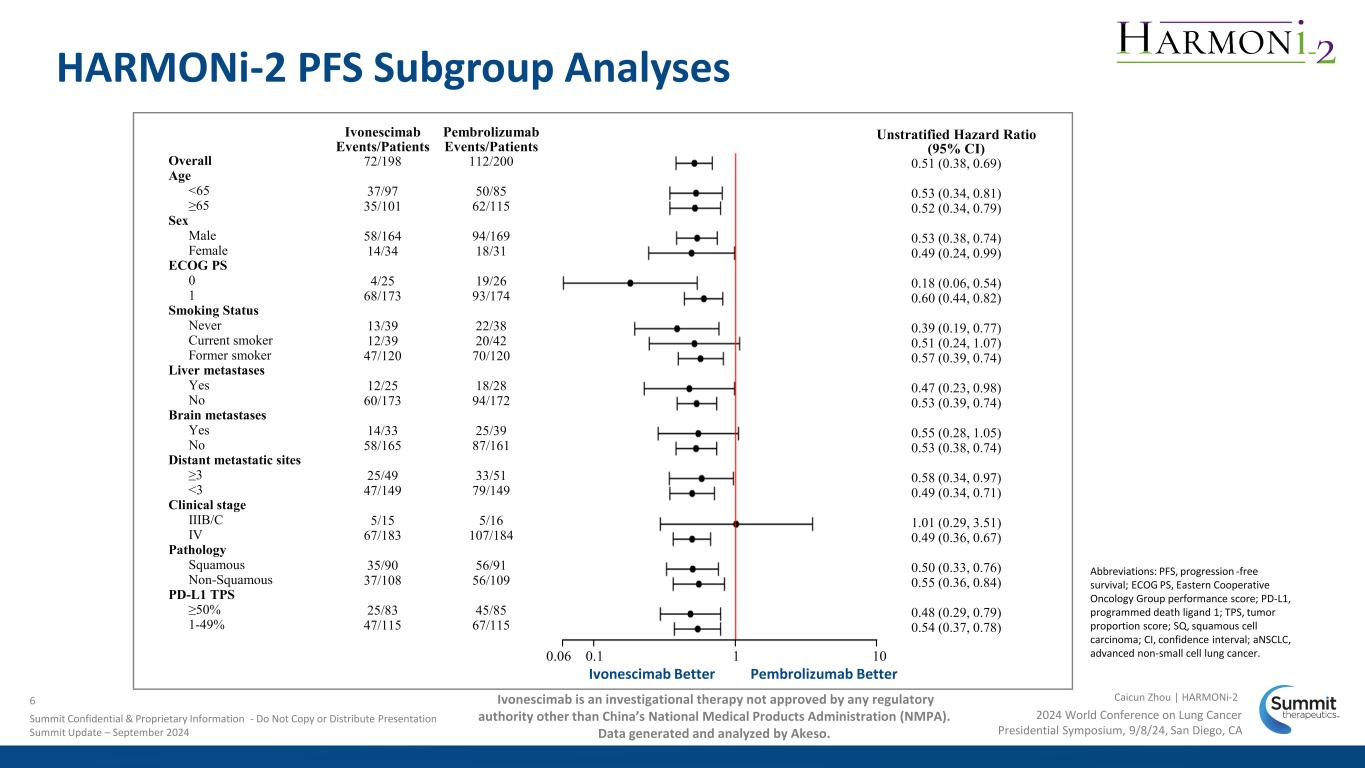
HARMONi-2 PFS Subgroup Analyses Ivonescimab Pembrolizumab Events/Patients Events/Patients Overall 72/198 112/200 Age <65 37/97 50/85 ≥65 35/101 62/115 Sex Male 58/164 94/169 Female 14/34 18/31 ECOG PS 0 4/25 19/26 1 68/173 93/174 Smoking Status Never 13/39 22/38 Current smoker 12/39 20/42 Former smoker 47/120 70/120 Liver metastases Yes 12/25 18/28 No 60/173 94/172 Brain metastases Yes 14/33 25/39 No 58/165 87/161 Distant metastatic sites ≥3 25/49 33/51 <3 47/149 79/149 Clinical stage IIIB/C 5/15 5/16 IV 67/183 107/184 Pathology Squamous 35/90 56/91 Non-Squamous 37/108 56/109 PD-L1 TPS ≥50% 25/83 45/85 1-49% 47/115 67/115 Abbreviations: PFS, progression -free survival; ECOG PS, Eastern Cooperative Oncology Group performance score; PD-L1, programmed death ligand 1; TPS, tumor proportion score; SQ, squamous cell carcinoma; CI, confidence interval; aNSCLC, advanced non-small cell lung cancer. Unstratified Hazard Ratio (95% CI) 0.51 (0.38, 0.69) 0.53 (0.34, 0.81) 0.52 (0.34, 0.79) 0.53 (0.38, 0.74) 0.49 (0.24, 0.99) 0.18 (0.06, 0.54) 0.60 (0.44, 0.82) 0.39 (0.19, 0.77) 0.51 (0.24, 1.07) 0.57 (0.39, 0.74) 0.47 (0.23, 0.98) 0.53 (0.39, 0.74) 0.55 (0.28, 1.05) 0.53 (0.38, 0.74) 0.58 (0.34, 0.97) 0.49 (0.34, 0.71) 1.01 (0.29, 3.51) 0.49 (0.36, 0.67) 0.50 (0.33, 0.76) 0.55 (0.36, 0.84) 0.48 (0.29, 0.79) 0.54 (0.37, 0.78) 0.06 0.1 1 10 Ivonescimab Better Pembrolizumab Better 2024 World Conference on Lung Cancer Presidential Symposium, 9/8/24, San Diego, CA Caicun Zhou | HARMONi-26 Summit Confidential & Proprietary Information - Do Not Copy or Distribute Presentation Summit Update – September 2024 Ivonescimab is an investigational therapy not approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Data generated and analyzed by Akeso.
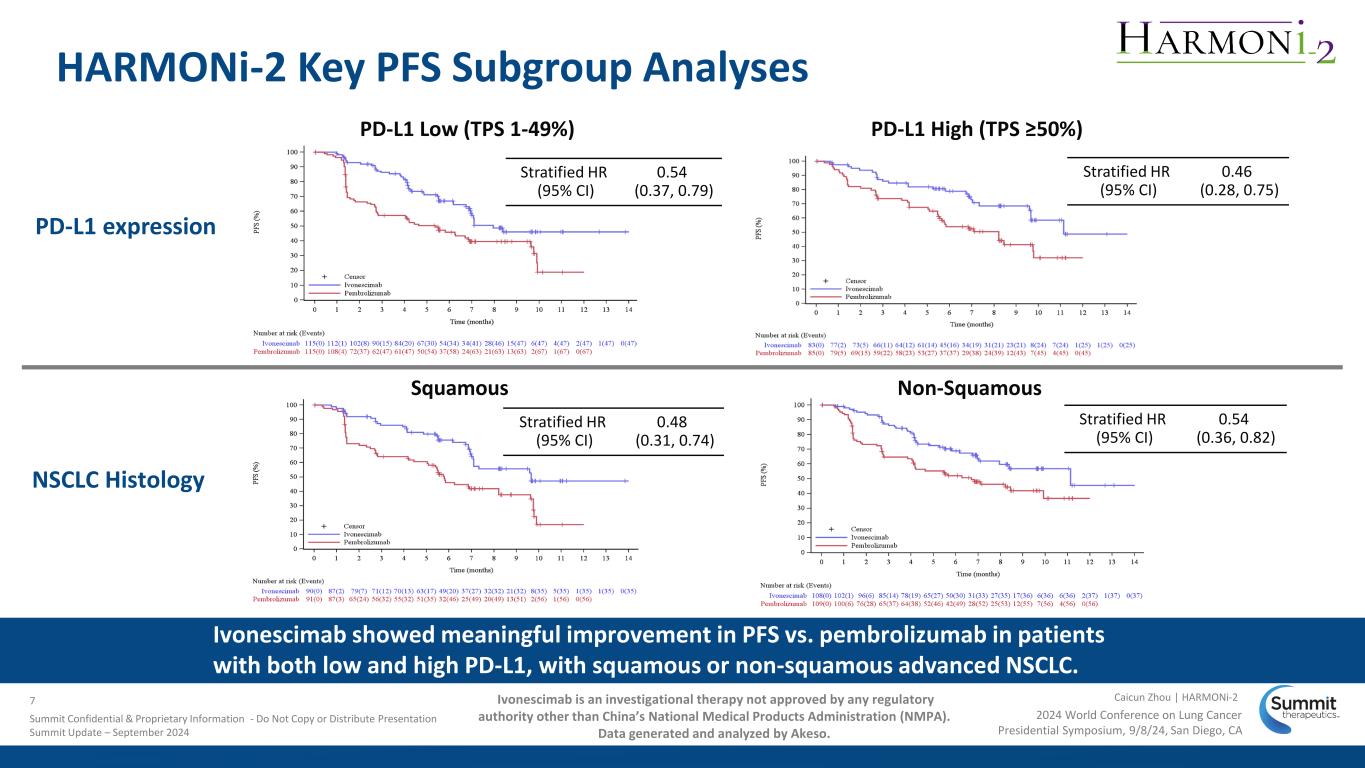
HARMONi-2 Key PFS Subgroup Analyses PD-L1 High (TPS ≥50%)PD-L1 Low (TPS 1-49%) Ivonescimab showed meaningful improvement in PFS vs. pembrolizumab in patients with both low and high PD-L1, with squamous or non-squamous advanced NSCLC. Stratified HR (95% CI) 0.54 (0.37, 0.79) Stratified HR (95% CI) 0.46 (0.28, 0.75) Stratified HR (95% CI) 0.48 (0.31, 0.74) Stratified HR (95% CI) 0.54 (0.36, 0.82) Non-SquamousSquamous NSCLC Histology PD-L1 expression 2024 World Conference on Lung Cancer Presidential Symposium, 9/8/24, San Diego, CA Caicun Zhou | HARMONi-27 Summit Confidential & Proprietary Information - Do Not Copy or Distribute Presentation Summit Update – September 2024 Ivonescimab is an investigational therapy not approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Data generated and analyzed by Akeso.
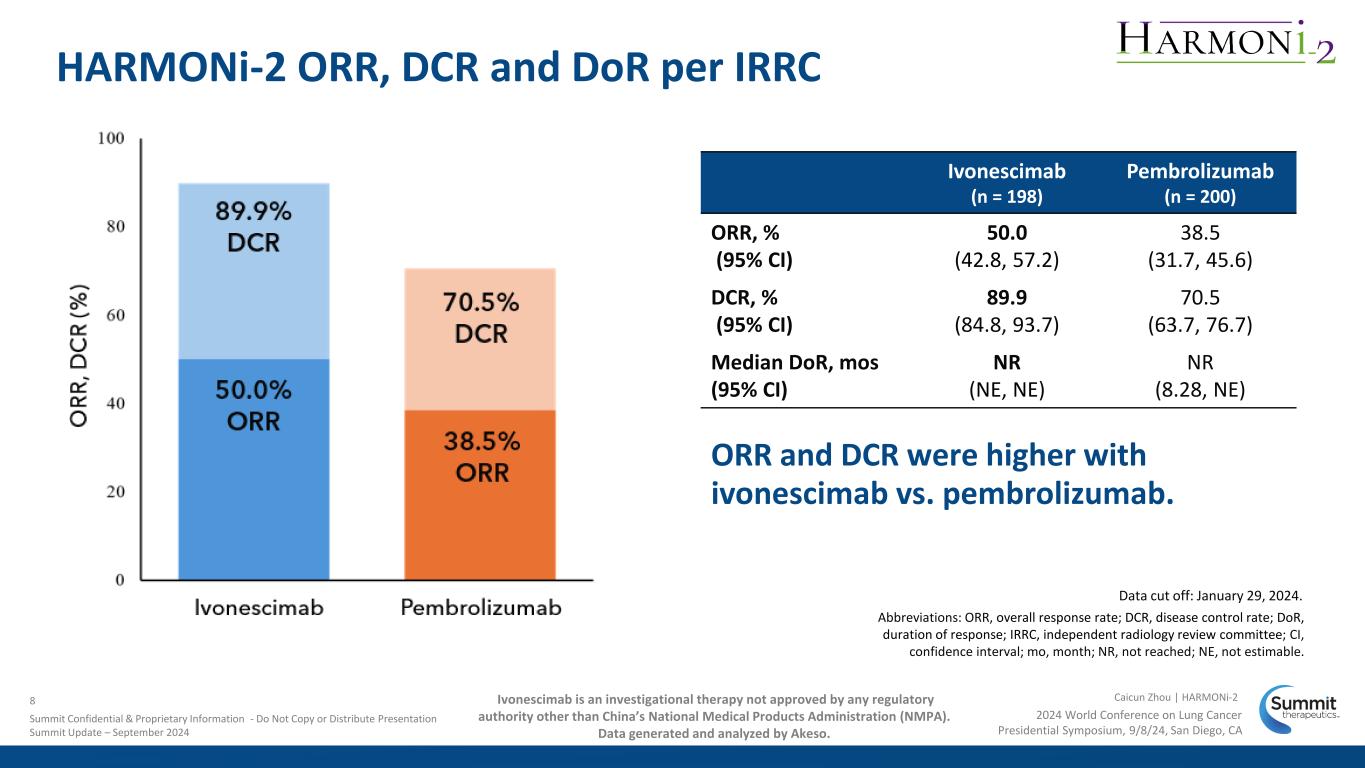
Data cut off: January 29, 2024. Abbreviations: ORR, overall response rate; DCR, disease control rate; DoR, duration of response; IRRC, independent radiology review committee; CI, confidence interval; mo, month; NR, not reached; NE, not estimable. Ivonescimab (n = 198) Pembrolizumab (n = 200) ORR, % (95% CI) 50.0 (42.8, 57.2) 38.5 (31.7, 45.6) DCR, % (95% CI) 89.9 (84.8, 93.7) 70.5 (63.7, 76.7) Median DoR, mos (95% CI) NR (NE, NE) NR (8.28, NE) ORR and DCR were higher with ivonescimab vs. pembrolizumab. HARMONi-2 ORR, DCR and DoR per IRRC 2024 World Conference on Lung Cancer Presidential Symposium, 9/8/24, San Diego, CA Caicun Zhou | HARMONi-28 Summit Confidential & Proprietary Information - Do Not Copy or Distribute Presentation Summit Update – September 2024 Ivonescimab is an investigational therapy not approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Data generated and analyzed by Akeso.
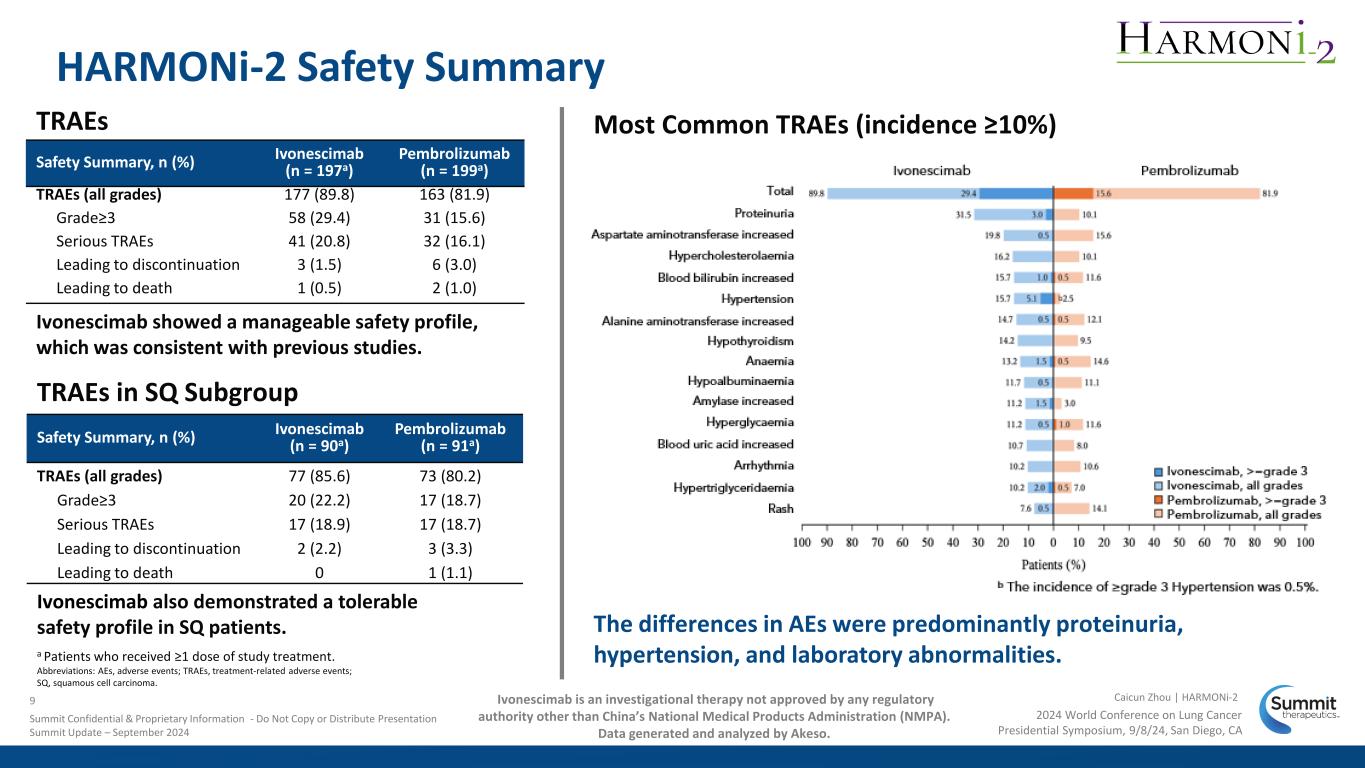
a Patients who received ≥1 dose of study treatment. Abbreviations: AEs, adverse events; TRAEs, treatment-related adverse events; SQ, squamous cell carcinoma. TRAEs HARMONi-2 Safety Summary Ivonescimab showed a manageable safety profile, which was consistent with previous studies. Safety Summary, n (%) Ivonescimab (n = 90a) Pembrolizumab (n = 91a) TRAEs (all grades) 77 (85.6) 73 (80.2) Grade≥3 20 (22.2) 17 (18.7) Serious TRAEs 17 (18.9) 17 (18.7) Leading to discontinuation 2 (2.2) 3 (3.3) Leading to death 0 1 (1.1) TRAEs in SQ Subgroup Ivonescimab also demonstrated a tolerable safety profile in SQ patients. Safety Summary, n (%) Ivonescimab (n = 197a) Pembrolizumab (n = 199a) TRAEs (all grades) 177 (89.8) 163 (81.9) Grade≥3 58 (29.4) 31 (15.6) Serious TRAEs 41 (20.8) 32 (16.1) Leading to discontinuation 3 (1.5) 6 (3.0) Leading to death 1 (0.5) 2 (1.0) The differences in AEs were predominantly proteinuria, hypertension, and laboratory abnormalities. Most Common TRAEs (incidence ≥10%) 2024 World Conference on Lung Cancer Presidential Symposium, 9/8/24, San Diego, CA Caicun Zhou | HARMONi-29 Summit Confidential & Proprietary Information - Do Not Copy or Distribute Presentation Summit Update – September 2024 Ivonescimab is an investigational therapy not approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Data generated and analyzed by Akeso.
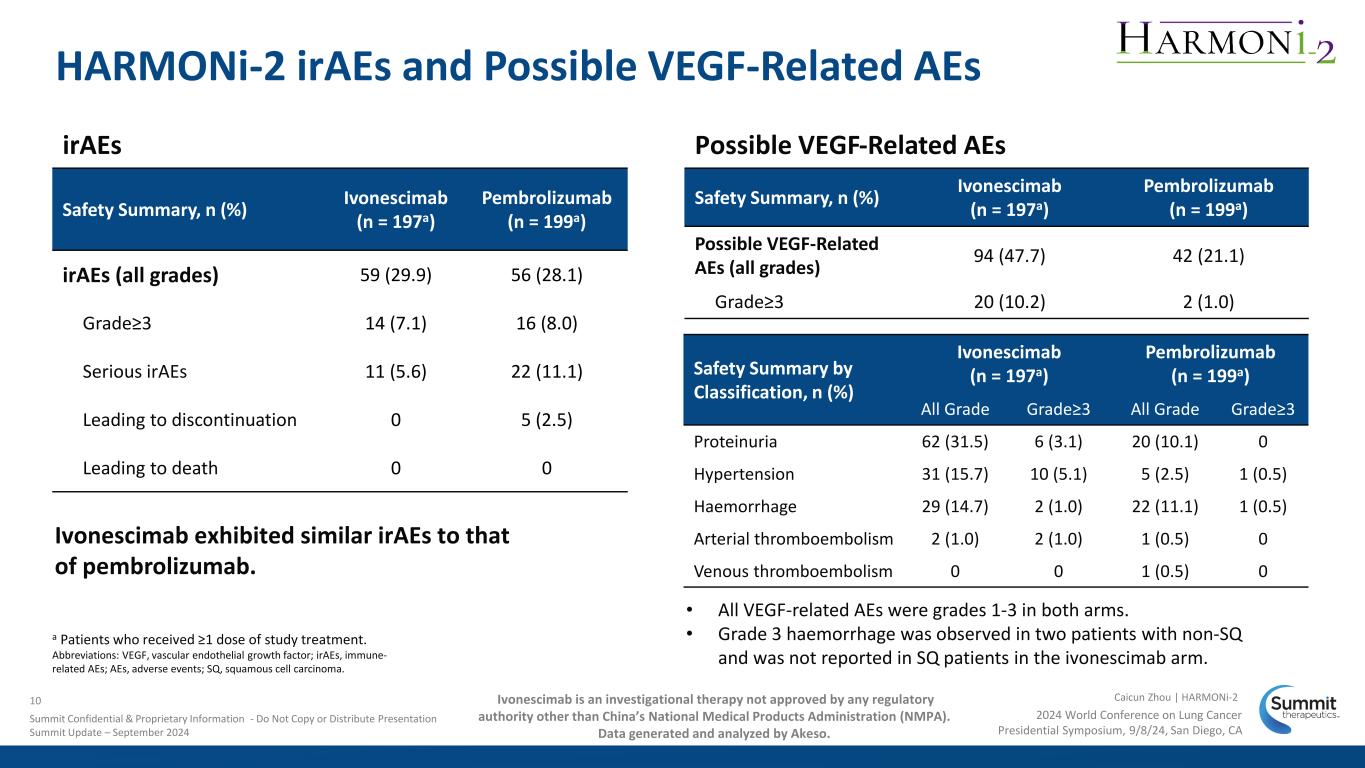
HARMONi-2 irAEs and Possible VEGF-Related AEs • All VEGF-related AEs were grades 1-3 in both arms. • Grade 3 haemorrhage was observed in two patients with non-SQ and was not reported in SQ patients in the ivonescimab arm. irAEs Safety Summary, n (%) Ivonescimab (n = 197a) Pembrolizumab (n = 199a) Possible VEGF-Related AEs (all grades) 94 (47.7) 42 (21.1) Grade≥3 20 (10.2) 2 (1.0) Possible VEGF-Related AEs Safety Summary, n (%) Ivonescimab (n = 197a) Pembrolizumab (n = 199a) irAEs (all grades) 59 (29.9) 56 (28.1) Grade≥3 14 (7.1) 16 (8.0) Serious irAEs 11 (5.6) 22 (11.1) Leading to discontinuation 0 5 (2.5) Leading to death 0 0 Safety Summary by Classification, n (%) Ivonescimab (n = 197a) Pembrolizumab (n = 199a) All Grade Grade≥3 All Grade Grade≥3 Proteinuria 62 (31.5) 6 (3.1) 20 (10.1) 0 Hypertension 31 (15.7) 10 (5.1) 5 (2.5) 1 (0.5) Haemorrhage 29 (14.7) 2 (1.0) 22 (11.1) 1 (0.5) Arterial thromboembolism 2 (1.0) 2 (1.0) 1 (0.5) 0 Venous thromboembolism 0 0 1 (0.5) 0 a Patients who received ≥1 dose of study treatment. Abbreviations: VEGF, vascular endothelial growth factor; irAEs, immune- related AEs; AEs, adverse events; SQ, squamous cell carcinoma. Ivonescimab exhibited similar irAEs to that of pembrolizumab. 2024 World Conference on Lung Cancer Presidential Symposium, 9/8/24, San Diego, CA Caicun Zhou | HARMONi-210 Summit Confidential & Proprietary Information - Do Not Copy or Distribute Presentation Summit Update – September 2024 Ivonescimab is an investigational therapy not approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Data generated and analyzed by Akeso.
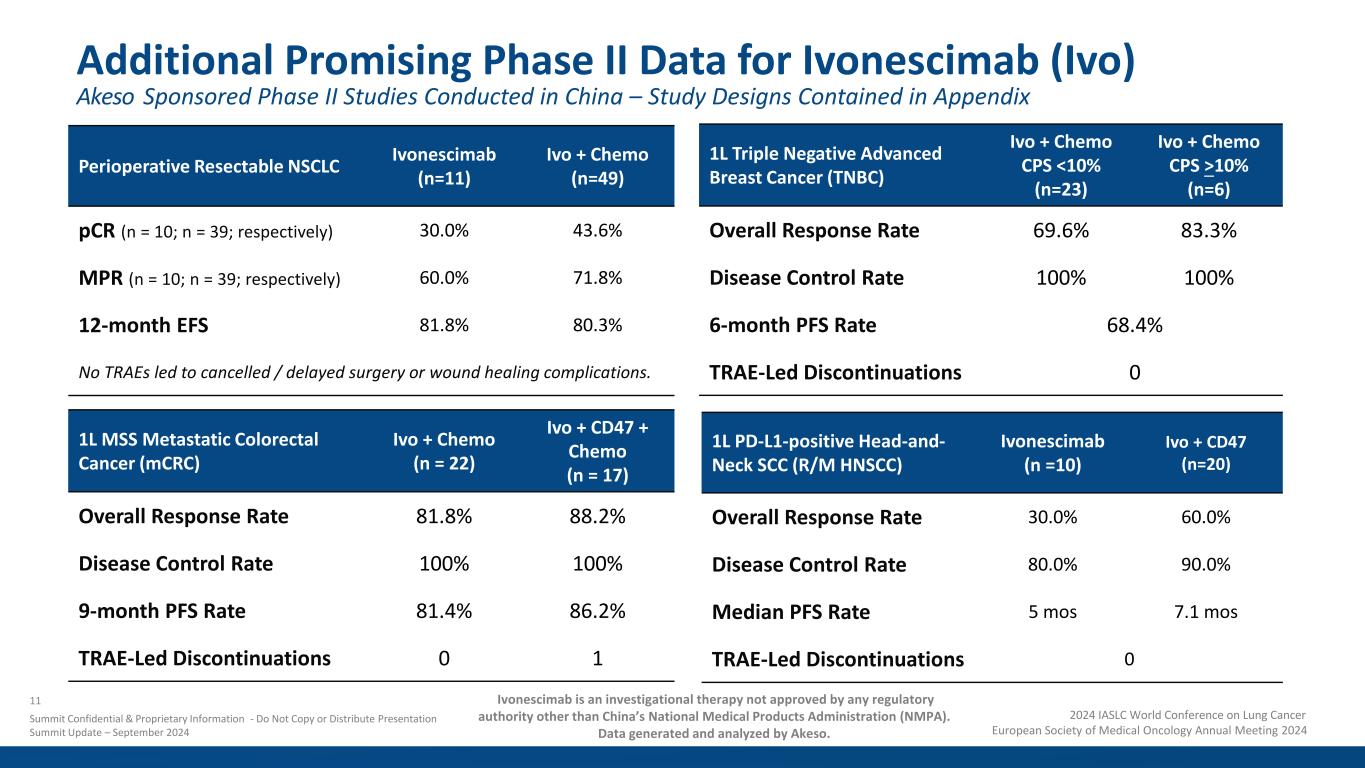
Additional Promising Phase II Data for Ivonescimab (Ivo) Perioperative Resectable NSCLC Ivonescimab (n=11) Ivo + Chemo (n=49) pCR (n = 10; n = 39; respectively) 30.0% 43.6% MPR (n = 10; n = 39; respectively) 60.0% 71.8% 12-month EFS 81.8% 80.3% No TRAEs led to cancelled / delayed surgery or wound healing complications. 1L MSS Metastatic Colorectal Cancer (mCRC) Ivo + Chemo (n = 22) Ivo + CD47 + Chemo (n = 17) Overall Response Rate 81.8% 88.2% Disease Control Rate 100% 100% 9-month PFS Rate 81.4% 86.2% TRAE-Led Discontinuations 0 1 1L PD-L1-positive Head-and- Neck SCC (R/M HNSCC) Ivonescimab (n =10) Ivo + CD47 (n=20) Overall Response Rate 30.0% 60.0% Disease Control Rate 80.0% 90.0% Median PFS Rate 5 mos 7.1 mos TRAE-Led Discontinuations 0 1L Triple Negative Advanced Breast Cancer (TNBC) Ivo + Chemo CPS <10% (n=23) Ivo + Chemo CPS >10% (n=6) Overall Response Rate 69.6% 83.3% Disease Control Rate 100% 100% 6-month PFS Rate 68.4% TRAE-Led Discontinuations 0 11 Summit Confidential & Proprietary Information - Do Not Copy or Distribute Presentation Summit Update – September 2024 2024 IASLC World Conference on Lung Cancer European Society of Medical Oncology Annual Meeting 2024 Ivonescimab is an investigational therapy not approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Data generated and analyzed by Akeso. Akeso Sponsored Phase II Studies Conducted in China – Study Designs Contained in Appendix
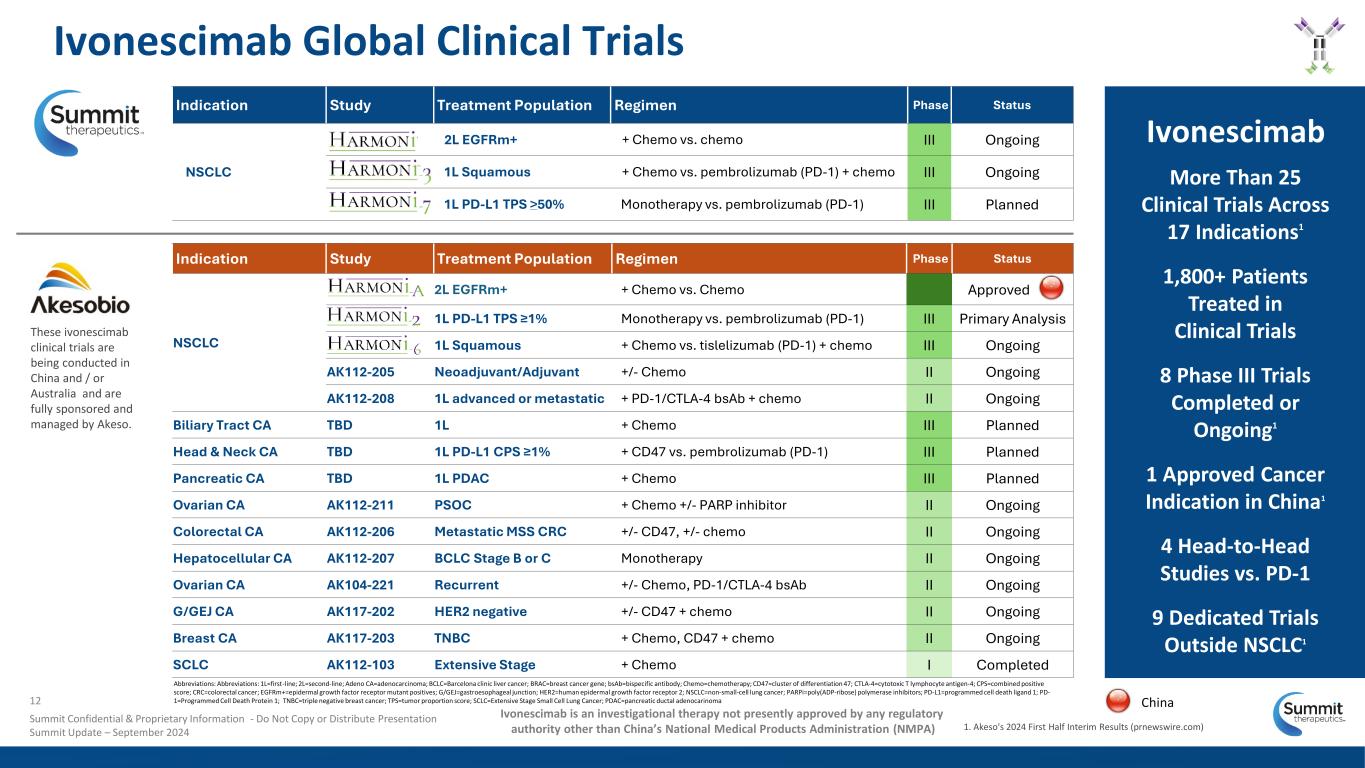
Indication Study Treatment Population Regimen Phase Status NSCLC 2L EGFRm+ + Chemo vs. Chemo Approved 1L PD-L1 TPS ≥1% Monotherapy vs. pembrolizumab (PD-1) III Primary Analysis 1L Squamous + Chemo vs. tislelizumab (PD-1) + chemo III Ongoing AK112-205 Neoadjuvant/Adjuvant +/- Chemo II Ongoing AK112-208 1L advanced or metastatic + PD-1/CTLA-4 bsAb + chemo II Ongoing Biliary Tract CA TBD 1L + Chemo III Planned Head & Neck CA TBD 1L PD-L1 CPS ≥1% + CD47 vs. pembrolizumab (PD-1) III Planned Pancreatic CA TBD 1L PDAC + Chemo III Planned Ovarian CA AK112-211 PSOC + Chemo +/- PARP inhibitor II Ongoing Colorectal CA AK112-206 Metastatic MSS CRC +/- CD47, +/- chemo II Ongoing Hepatocellular CA AK112-207 BCLC Stage B or C Monotherapy II Ongoing Ovarian CA AK104-221 Recurrent +/- Chemo, PD-1/CTLA-4 bsAb II Ongoing G/GEJ CA AK117-202 HER2 negative +/- CD47 + chemo II Ongoing Breast CA AK117-203 TNBC + Chemo, CD47 + chemo II Ongoing SCLC AK112-103 Extensive Stage + Chemo I Completed Ivonescimab Global Clinical Trials Ivonescimab More Than 25 Clinical Trials Across 17 Indications1 1,800+ Patients Treated in Clinical Trials 8 Phase III Trials Completed or Ongoing1 1 Approved Cancer Indication in China1 4 Head-to-Head Studies vs. PD-1 9 Dedicated Trials Outside NSCLC1 These ivonescimab clinical trials are being conducted in China and / or Australia and are fully sponsored and managed by Akeso. Indication Study Treatment Population Regimen Phase Status NSCLC 2L EGFRm+ + Chemo vs. chemo III Ongoing 1L Squamous + Chemo vs. pembrolizumab (PD-1) + chemo III Ongoing 1L PD-L1 TPS >50% Monotherapy vs. pembrolizumab (PD-1) III Planned 1. Akeso's 2024 First Half Interim Results (prnewswire.com) Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA) Abbreviations: Abbreviations: 1L=first-line; 2L=second-line; Adeno CA=adenocarcinoma; BCLC=Barcelona clinic liver cancer; BRAC=breast cancer gene; bsAb=bispecific antibody; Chemo=chemotherapy; CD47=cluster of differentiation 47; CTLA-4=cytotoxic T lymphocyte antigen-4; CPS=combined positive score; CRC=colorectal cancer; EGFRm+=epidermal growth factor receptor mutant positives; G/GEJ=gastroesophageal junction; HER2=human epidermal growth factor receptor 2; NSCLC=non-small-cell lung cancer; PARPi=poly(ADP-ribose) polymerase inhibitors; PD-L1=programmed cell death ligand 1; PD- 1=Programmed Cell Death Protein 1; TNBC=triple negative breast cancer; TPS=tumor proportion score; SCLC=Extensive Stage Small Cell Lung Cancer; PDAC=pancreatic ductal adenocarinoma12 Summit Confidential & Proprietary Information - Do Not Copy or Distribute Presentation Summit Update – September 2024 China

Q&A

Summit Therapeutics Update Call from WCLC September 9, 2024

Appendix
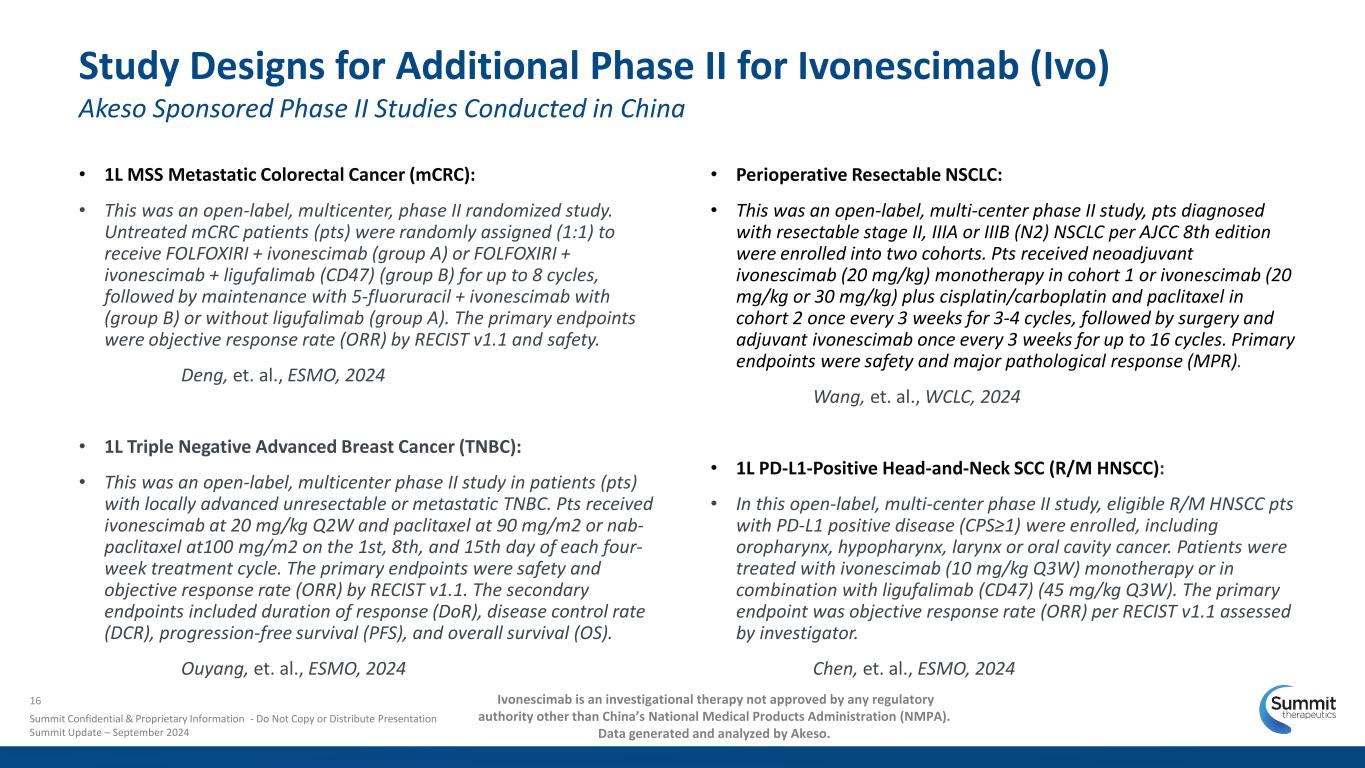
• 1L MSS Metastatic Colorectal Cancer (mCRC): • This was an open-label, multicenter, phase II randomized study. Untreated mCRC patients (pts) were randomly assigned (1:1) to receive FOLFOXIRI + ivonescimab (group A) or FOLFOXIRI + ivonescimab + ligufalimab (CD47) (group B) for up to 8 cycles, followed by maintenance with 5-fluoruracil + ivonescimab with (group B) or without ligufalimab (group A). The primary endpoints were objective response rate (ORR) by RECIST v1.1 and safety. Deng, et. al., ESMO, 2024 • 1L Triple Negative Advanced Breast Cancer (TNBC): • This was an open-label, multicenter phase II study in patients (pts) with locally advanced unresectable or metastatic TNBC. Pts received ivonescimab at 20 mg/kg Q2W and paclitaxel at 90 mg/m2 or nab- paclitaxel at100 mg/m2 on the 1st, 8th, and 15th day of each four- week treatment cycle. The primary endpoints were safety and objective response rate (ORR) by RECIST v1.1. The secondary endpoints included duration of response (DoR), disease control rate (DCR), progression-free survival (PFS), and overall survival (OS). Ouyang, et. al., ESMO, 2024 Study Designs for Additional Phase II for Ivonescimab (Ivo) Akeso Sponsored Phase II Studies Conducted in China • Perioperative Resectable NSCLC: • This was an open-label, multi-center phase II study, pts diagnosed with resectable stage II, IIIA or IIIB (N2) NSCLC per AJCC 8th edition were enrolled into two cohorts. Pts received neoadjuvant ivonescimab (20 mg/kg) monotherapy in cohort 1 or ivonescimab (20 mg/kg or 30 mg/kg) plus cisplatin/carboplatin and paclitaxel in cohort 2 once every 3 weeks for 3-4 cycles, followed by surgery and adjuvant ivonescimab once every 3 weeks for up to 16 cycles. Primary endpoints were safety and major pathological response (MPR). Wang, et. al., WCLC, 2024 • 1L PD-L1-Positive Head-and-Neck SCC (R/M HNSCC): • In this open-label, multi-center phase II study, eligible R/M HNSCC pts with PD-L1 positive disease (CPS≥1) were enrolled, including oropharynx, hypopharynx, larynx or oral cavity cancer. Patients were treated with ivonescimab (10 mg/kg Q3W) monotherapy or in combination with ligufalimab (CD47) (45 mg/kg Q3W). The primary endpoint was objective response rate (ORR) per RECIST v1.1 assessed by investigator. Chen, et. al., ESMO, 2024 16 Summit Confidential & Proprietary Information - Do Not Copy or Distribute Presentation Summit Update – September 2024 Ivonescimab is an investigational therapy not approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Data generated and analyzed by Akeso.















