UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
___________________________________________________________________________________
Form 10-K
___________________________________________________________________________________
| | | | | |
| ☑ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2024
OR | | | | | |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from_____________ to ________________
Commission file number: 001-36421
___________________________________________________________________________________
Aurinia Pharmaceuticals Inc.
(Exact name of registrant as specified in its charter)
___________________________________________________________________________________
| | | | | |
| Alberta, Canada | |
(State or other jurisdiction of
incorporation or organization) | |
#140, 14315 - 118 Avenue Edmonton, Alberta T5L 4S6 | 98-1231763 |
| (Address of principal executive offices) | (I.R.S. Employer
Identification Number) |
Registrant’s telephone number, including area code:
(250) 744-2487
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of Each Class | Symbol | Name of Each Exchange on Which Registered |
| Common shares, no par value | AUPH | The Nasdaq Global Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
___________________________________________________________________________________
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☑ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☑
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☑ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one): | | | | | | | | | | | | | | |
| Large accelerated filer | ☑ | | Accelerated filer | ☐ |
| Non-accelerated filer | ☐ | | Smaller reporting company | ☐ |
| | | Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☑
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☑
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the Registrant based on the closing price of the common shares on the Nasdaq Global Market on June 30, 2024 was $0.8 billion.
As of February 25, 2025, there were 137,339,016 of the registrant’s common shares outstanding.
DOCUMENTS INCORPORATED BY REFERENCE | | | | | | | | |
| Document Description | | 10-K Part |
| Portions of the registrant’s definitive proxy statement to be filed with the U.S. Securities and Exchange Commission pursuant to Regulation 14A within 120 days after registrant’s fiscal year end of December 31, 2024 are incorporated by reference into Part III of this Annual Report on Form 10-K. | | III |
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K for the year ended December 31, 2024 (this “Annual Report”) contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”) and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which are subject to the safe harbor provisions created by those sections, as well as “forward-looking information” as defined in applicable Canadian securities laws. Forward-looking statements can be identified by words such as “intends,” “believes,” “anticipates,” “indicates,” “plans,” “expects,” “suggests,” “may,” “should,” “potential,” “designed to,” “will” and similar expressions that predict or indicate future events and trends that do not relate to historical matters. You should not unduly rely on forward-looking statements because they involve known and unknown risks, uncertainties and other factors, some of which are beyond our control. These risks, uncertainties and other factors may cause our actual results, performance or achievements to be materially different from the anticipated future results, performance or achievements expressed or implied by the forward-looking statements.
These forward-looking statements include, but are not limited to, statements regarding:
•our ability to grow net product sales of LUPKYNIS® (voclosporin);
•our ability to maintain an effective sales and marketing organization;
•the potential market size for LUPKYNIS;
•our ability to obtain an uninterrupted supply of commercial and clinical product from our contract manufacturers;
•LUPKYNIS market exclusivity period as a result of the enforcement of regulatory exclusivity and the validity and enforceability of issued and pending patents covering LUPKYNIS;
•our ability to comply with our obligations under our collaboration and license agreement with Otsuka Pharmaceutical Co., Ltd (“Otsuka”);
•the timing and our ability to develop, obtain regulatory approvals for and commercialize AUR200;
•the rate and degree of market acceptance and clinical utility of AUR200, if approved;
•the relationship between earlier study results (preclinical and clinical) and later clinical study results;
•our ability to hire and retain key employees;
•our overall financial performance, including but not limited to, net product sales and net cash provided by or used for operating activities, including any milestone, royalty and other payments resulting from our collaboration and license agreement and commercial supply agreement with Otsuka;
•our capital requirements and our potential need for, and ability to obtain, additional financing; and
•our ability to maintain effective internal controls.
These forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results to differ materially from the anticipated future results, performance or achievements expressed or implied by any forward-looking statements, including the factors described under the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” You should evaluate all forward-looking statements made in this Annual Report, including the documents we incorporate by reference, in the context of these risks, uncertainties and other factors.
We caution you that the risks, uncertainties and other factors referred to above may not contain all of the risks, uncertainties and other factors that are important to you. In addition, we cannot assure you that we will realize the results, benefits or developments that we expect or anticipate or, even if substantially realized, that they will affect us or our business in the way expected. All forward-looking statements in this Annual Report apply only as of the date made and are expressly qualified in their entirety by the cautionary statements included in this Annual Report. We undertake no obligation to publicly update or revise any forward-looking statements to reflect subsequent events or circumstances.
PART I
In this Annual Report, references to “we,” “us,” “our,” “Aurinia” or "the Company," refer to Aurinia Pharmaceuticals Inc., an Alberta, Canada corporation, together with our wholly owned subsidiaries, Aurinia Pharma U.S., Inc., a Delaware corporation, and Aurinia Pharma Limited, a United Kingdom (“U.K.”) corporation, on a consolidated basis.
Item 1. Business
OVERVIEW
Background
Aurinia is a biopharmaceutical company focused on delivering therapies to people living with autoimmune diseases with high unmet medical needs. In January 2021, the Company introduced LUPKYNIS® (voclosporin), the first FDA-approved oral therapy for the treatment of adult patients with active lupus nephritis (“LN”). Aurinia is also developing AUR200, a dual inhibitor of B cell activating factor (“BAFF”) and a proliferation inducing ligand (“APRIL”) for the potential treatment of autoimmune diseases.
Net Product Sales
Aurinia sells LUPKYNIS to two specialty pharmacies and a specialty distributor in the U.S., and Aurinia sells LUPKYNIS inventory to its collaboration partner, Otsuka Pharmaceutical Co., Ltd. (“Otsuka”), for the European and Japanese market.
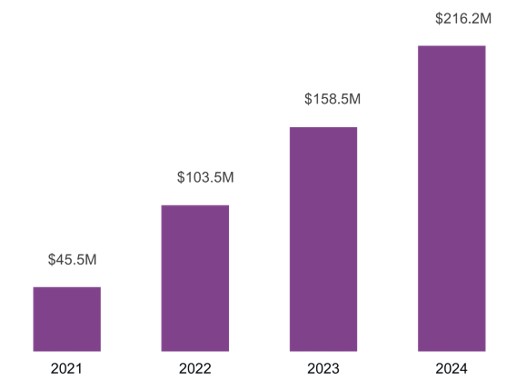
For the year ended December 31, 2024, net product sales were $216.2 million, up 36% from $158.5 million in 2023.
LUPKYNIS Net Product Sales
Cash Flow Provided by (Used in) Operating Activities
For the year ended December 31, 2024, cash flow provided by (used in) operating activities was $44.4 million, compared to $(33.5) million in 2023.
Cash Position
As of December 31, 2024, Aurinia had cash, cash equivalents, restricted cash and investments of $358.5 million, compared to $350.7 million at December 31, 2023. For the year ended December 31, 2024, the Company repurchased 6.1 million of its common shares for $41.0 million.
Otsuka Collaboration
In December 2020, Aurinia entered into a collaboration and licensing agreement with Otsuka to develop and commercialize oral voclosporin in Japan, the European Union (the “E.U.”), the U.K., Switzerland, Russia, Norway, Belarus, Iceland, Liechtenstein and Ukraine (collectively, the “Otsuka Territories”) in exchange for: (i) a $50 million upfront cash payment; (ii) regulatory and commercial milestone payments; and (iii) royalties ranging from 10% to 20% on net sales in the Otsuka Territories.
In August 2022, Aurinia entered into a commercial supply agreement with Otsuka to: (i) supply LUPKYNIS inventory to Otsuka at cost, plus a margin; and (ii) provide manufacturing and other services, including sharing the capacity of a dedicated manufacturing facility at Lonza Ltd. (“Lonza”), Aurinia’s contract manufacturing partner for voclosporin.
Otsuka has obtained regulatory approval of LUPKYNIS in Japan, the E.U., the U.K. and Switzerland.
PRODUCT PORTFOLIO
LUPKYNIS (voclosporin)
In January 2021, the Company introduced LUPKYNIS, the first FDA-approved oral therapy for the treatment of adult patients with active lupus nephritis (“LN”). The Company markets LUPKYNIS in the U.S. directly through its own commercial organization. In Japan, the European Union (the “E.U.”), the United Kingdom (the “U.K.”) and Switzerland, LUPKYNIS is marketed by Aurinia’s collaboration partner, Otsuka Pharmaceutical Co., Ltd. (“Otsuka”).
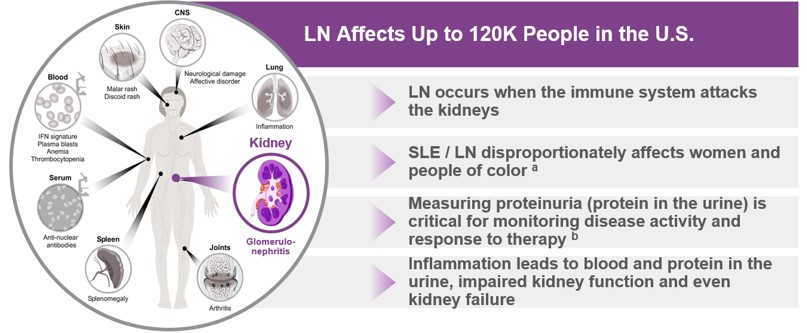
About Lupus Nephritis (LN)
LN is among the most severe and dangerous complications of systemic lupus erythematosus (“SLE”). SLE, commonly known as lupus, is a chronic autoimmune disease where the body's immune system mistakenly attacks its own healthy tissues and organs. Over 200,000 people in the United States are estimated to have SLE (U.S. Centers for Disease Control and Prevention 2024), of which 20% to 60% develop LN (KDIGO Lupus Nephritis Work Group, Kidney Int 2024;105(1S):S1-S69).
a U.S. Centers for Disease Control and Prevention 2024
b Tamirou et al., Ann Rheum Dis 2016;75:526-531
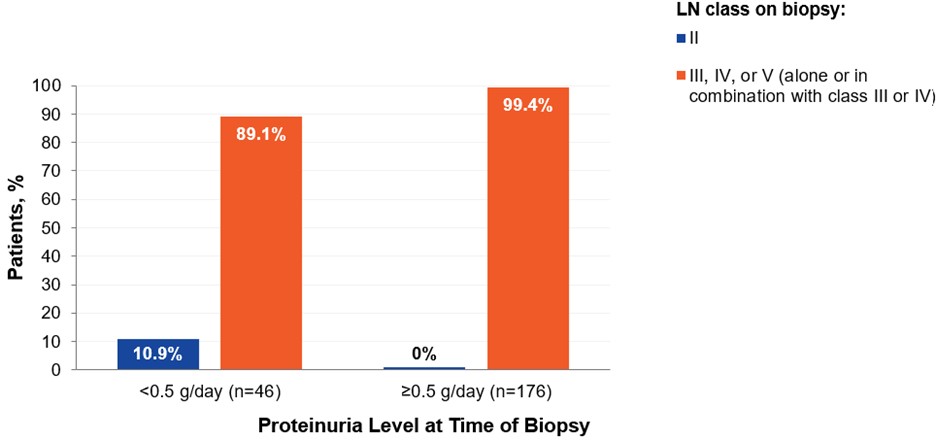
Kidney damage from LN can be progressive and is associated with long-term adverse outcomes. Proteinuria is a significant risk factor for kidney damage. Even low levels of proteinuria may be associated with significant kidney damage. Nearly 90% of SLE patients with proteinuria <0.5 g/day have been reported to have LN class III, IV, or V (alone or in combination with class III or IV) on biopsy (De Rosa et al., Kidney Int Rep., 2020;5(7):1066–1068).
LN Class by Proteinuria Level in Patients with SLE a
a De Rosa et al., Kidney Int Reports 2020;5(7):1066-1068
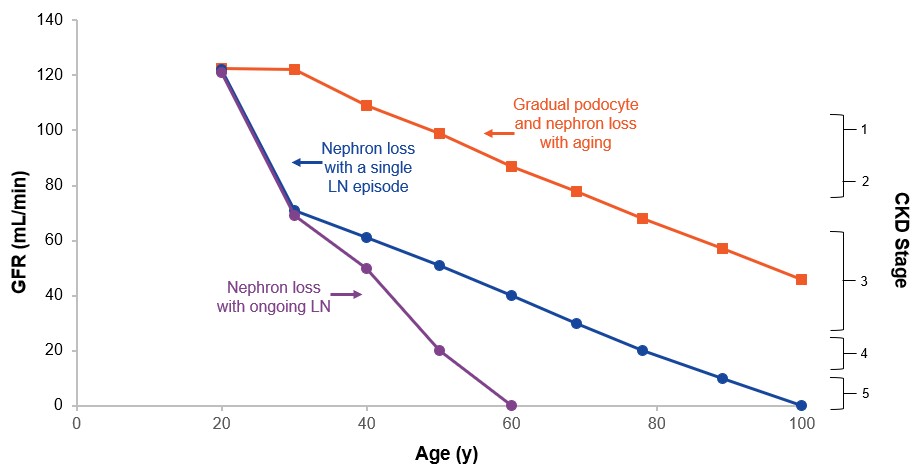
Even a single flare of LN can cause irreversible nephron loss, which can potentially shorten the lifespan of the kidneys by decades (Anders et al., Nat Rev Dis Primers 2020;6(1):7). Every subsequent flare contributes to the accrual of kidney damage, further shortening kidney lifespan and increasing the risk of adverse long-term outcomes such as end-stage kidney disease (“ESKD”) (Anders et al., Nat Rev Dis Primers 2020;6(1):7). Nephron loss and podocyte damage often lead to loss of kidney function as measured by glomerular filtration rate (“GFR”) and proteinuria (Anders et al., Nat Rev Dis Primers 2020;6(1):7 and Maria et al., Nat Rev Rheumatol 2020;16(5):255-267). Proteinuria as a marker of kidney damage routinely precedes GFR decline (Cravedi et al., Br J Clin Pharmacol 2013;76(4):516-523).
Even a Single Flare of LN Can Reduce the Lifespan of the Kidney a
a Adapted with permission from Anders et al., Nat Rev Dis Primers 2020;6(1):7; "CKD" means chronic kidney disease.
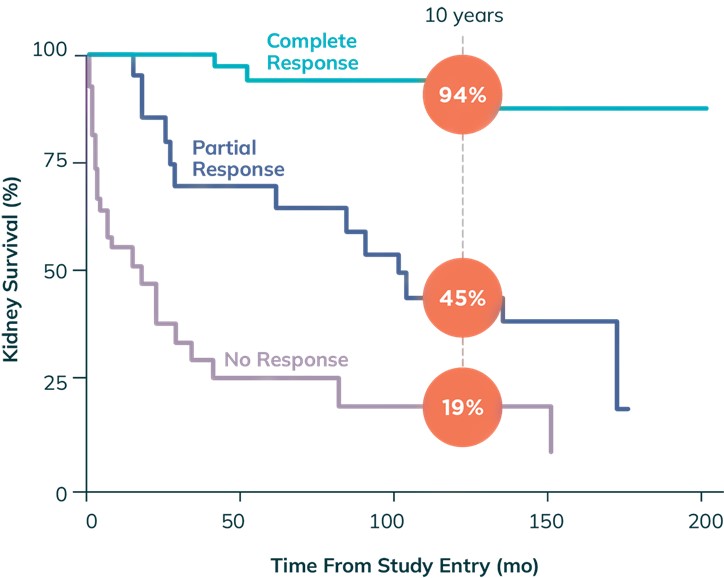
Proteinuria reduction is associated with long-term renal protection. The larger the initial reduction in proteinuria in the first several months of management, the lower the risk of ESKD (Chen et al., Clin J Am Soc Nephro 2008;3(1):46-53).
Kidney Survival Based on Proteinuria Response Status a, b
a Adapted with permission from Chen et al., Clin J Am Soc Nephro 2008;3(1):46-53
b Retrospective analysis of patients (N=86) enrolled in the prospective, controlled study of plasmapheresis in severe LN to determine long-term prognosis of achieving partial response. Complete response was defined as SCr ≤1.4 mg/dL and proteinuria ≤0.33 g/day within 5 years of study entry, and partial response was defined as ≤25% increase in baseline SCr and ≥50% reduction in baseline proteinuria to ≤1.5 g/day (but >0.33 g/day) within 5 years of entering the study. Kidney survival was determined by kidney failure (≥6 mg/dL SCr or the initiation of kidney replacement therapy).
Mycophenolate mofetil (“MMF”) and corticosteroids alone frequently fail to substantially reduce proteinuria, with only 20% to 30% of patients achieving a complete response at 1 to 2 years. Thus, the need remains for additional treatment options (Fanouriakis et al., Ann Rheum Dis 2024;83:15-29).
How LUPKYNIS Works
LUPKYNIS is a calcineurin-inhibitor immunosuppressant indicated in combination with a background immunosuppressive therapy regimen for the treatment of adult patients with active LN. LUPKYNIS targets LN with a dual mechanism of action:
1.Promotes podocyte stability, reducing proteinuria
2.Acts as an immunosuppressant through inhibition of T-cell activation and cytokine production
Clinical Study Overview of LUPKYNIS
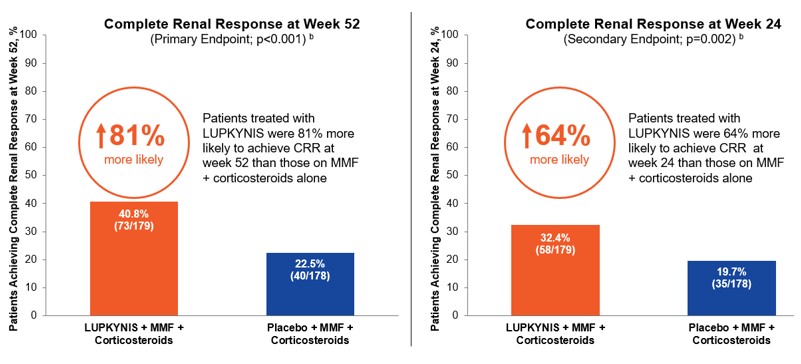
FDA approval of LUPKYNIS was based on our pivotal Phase 3 AURORA 1 study (“AURORA 1”), which demonstrated the ability of LUPKYNIS treatment to significantly improve outcomes for patients when added to the then-typical standard of care, MMF and corticosteroids. AURORA 1 was a randomized, double‑blind, placebo-controlled, Phase 3 study in 357 adults with class III, IV, or V (alone or in combination with class III or IV) LN. In this study, patients receiving LUPKYNIS with MMF plus corticosteroids compared to patients receiving MMF plus corticosteroids alone experienced a significantly higher rate of complete renal response (“CRR”) at both Week 52 (primary endpoint) and Week 24 (secondary endpoint).
Significantly More Patients on LUPKYNIS Achieved a Complete Renal Response in AURORA 1 a
a Rovin et al., Lancet 2021;397:2070-2080
b Stringent criteria of complete renal response as: Urine Protein-to-Creatinine Ratio (“UPCR”) of ≤0.5 mg/mg, maintained stable eGFR, sustained corticosteroids, and no administration of rescue medications
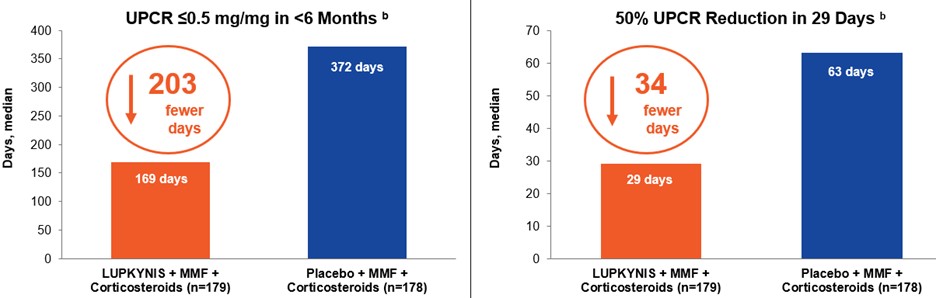
LUPKYNIS in combination with MMF and corticosteroids reduced proteinuria twice as fast as MMF and corticosteroids alone.
LUPKYNIS Rapidly Reduced Proteinuria in Fewer Days in AURORA 1 a
a Rovin et al., Lancet 2021;397:2070-2080
b Secondary endpoint
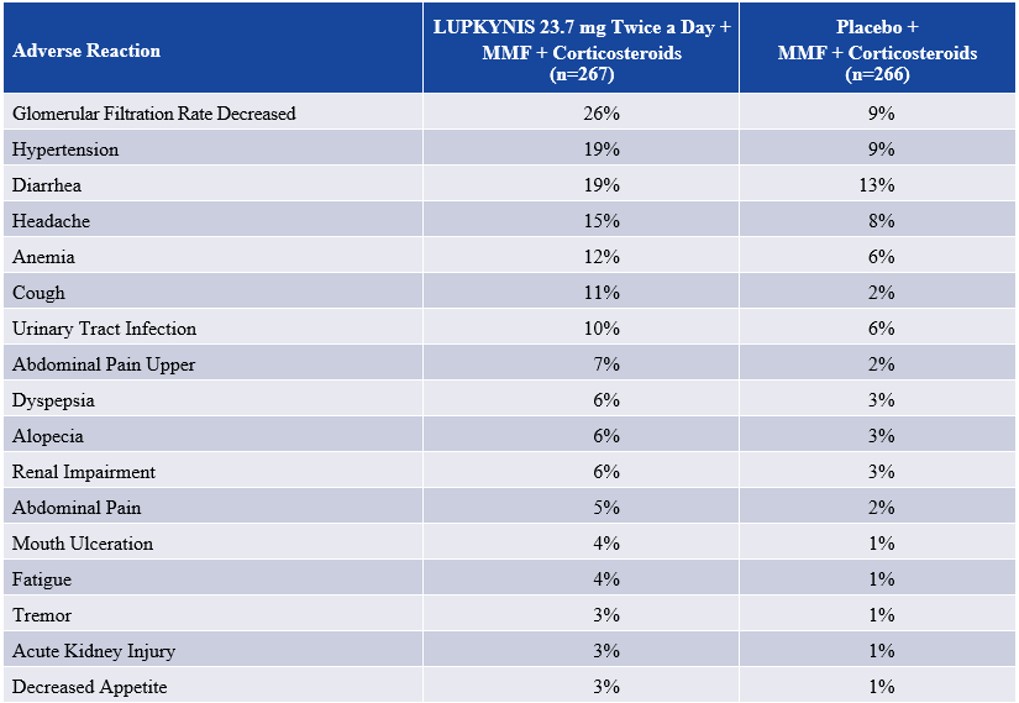
In the pivotal Phase 3 study (AURORA 1) and Phase 2 study (AURA-LV), adverse reactions occurring in ≥3% of patients treated with LUPKYNIS and ≥2% higher than placebo are shown below.
Adverse Reactions Occurring in ≥3% of Patients Treated with LUPKYNIS 23.7 mg Twice a Day and ≥2% Higher than Placebo in AURORA 1 and AURA-LV a
a LUPKYNIS Prescribing Information
In AURORA 2, a double‑blind, placebo-controlled extension study of adults with active LN who completed AURORA 1, LUPKYNIS demonstrated safety comparable to that seen in AURORA 1 with no unexpected safety signals observed through 3 years (LUPKYNIS Prescribing Information and Saxena et al., Arthritis Rheumatol 2024;76(1):59-67). The AURORA Clinical Program is the only clinical program to include 3 years of LN treatment and follow-up.
2024 American College of Rheumatology (“ACR”) Lupus Nephritis Treatment Guidelines
In part due to the clinical study results with LUPKYNIS, ACR now recommends triple immunosuppressive therapy, including treatment with a calcineurin inhibitor (the class of drugs that includes LUPKYNIS), as first-line therapy for patients with LN, with the goal of treatment being the preservation of kidney function (2024 ACR Guideline for the Screening, Treatment, and Management of Lupus Nephritis: Guideline Summary 2024). The ACR-recommended goal of therapy is to reach proteinuria ≤0.5 mg/mg by 6-12 months. LUPKYNIS’ rapid effect on reducing proteinuria facilitates achieving this treatment goal.
AUR200
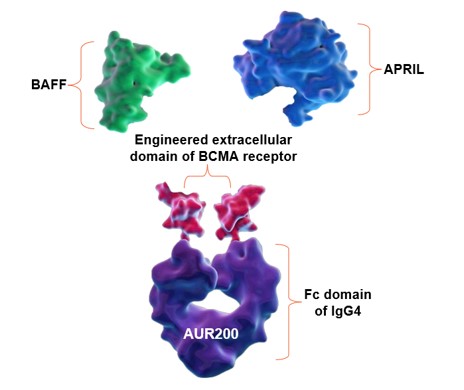
AUR200 is a dual inhibitor of B cell activating factor (“BAFF”) and a proliferation inducing ligand (“APRIL”) for the potential treatment of autoimmune diseases. AUR200 contains a B cell maturation antigen (“BCMA”)-engineered extracellular binding domain optimized for superior affinity to BAFF and APRIL (others use transmembrane activator and CAML interactor (“TACI”)-engineered extracellular binding domain). BCMA has a stronger natural affinity for APRIL than TACI (Mathur, J Clin Med 2023;12:1-18). AUR200 contains an immunoglobulin (“Ig”) G4 fragment crystallizable region (“Fc”) domain with no appreciable effector function (others use IgG1 Fc domain). IgG4 is considered the least inflammatory across the IgG subclasses, in part because it poorly activates the complement system (Oskam et al., Front Immun 2023;14:1-11).
AUR200
BAFF and APRIL are important cytokines that regulate B cell survival and differentiation (Mathur et al., J Clin Med 2023;12:1-18). BAFF and APRIL receptor targets are expressed on B cells at different stages of B cell development (Mathur et al., J Clin Med 2023;12:1-18). Targeting both BAFF and APRIL depletes a broader set of B cells than targeting a single cytokine. By inhibiting BAFF and APRIL, drugs like AUR200 may prevent the activation of autoreactive B cells and reduce their numbers and associated antibodies in the body, thereby treating autoimmune diseases.
B Cell Maturation a
a Schrezenmeier et al., J Am Soc Nephrol 2018;29:741-758
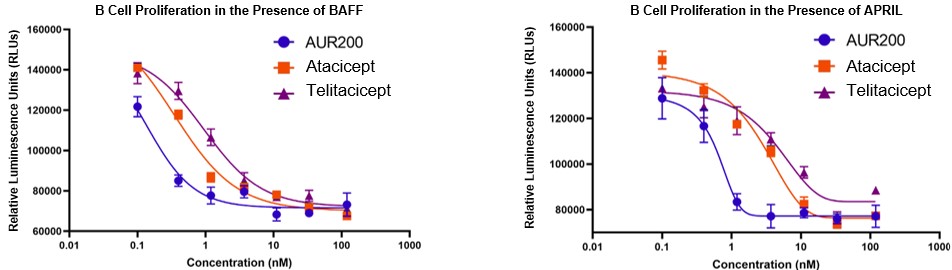
Based on preclinical in vitro testing, AUR200 has high binding affinity for both BAFF and APRIL as compared to competitor dual BAFF/APRIL inhibitors, atacicept and telitacicept.
AUR200 Is a High Affinity Dual BAFF/APRIL Inhibitor a
a Morales et al., ACR Convergence 2022
Based on preclinical in vitro testing, AUR200 potently inhibits both BAFF- and APRIL-mediated B cell proliferation as compared to competitor dual BAFF/APRIL inhibitors, atacicept and telitacicept.
AUR200 Potently Inhibits BAFF- and APRIL-Mediated B Cell Proliferation a
a Morales et al., ACR Convergence 2022
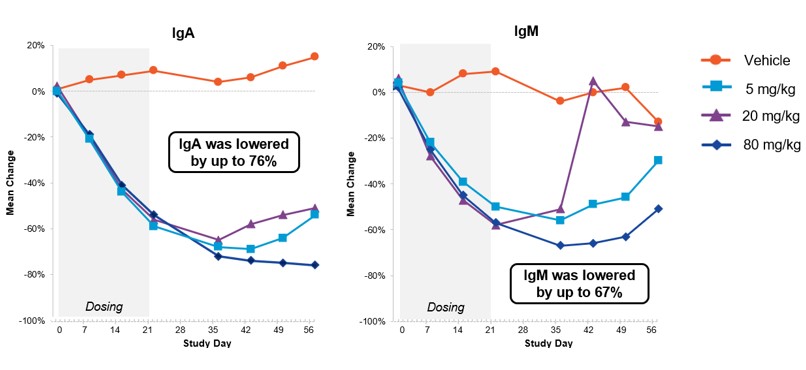
In preclinical testing in non-human primates, subcutaneous dosing of AUR200 resulted in significant and sustained reductions in the B cell antibodies immunoglobulin A (“IgA”) and immunoglobulin M (“IgM”). AUR200 was well-tolerated with no adverse findings at any of the doses tested.
AUR200 Depletes B Cell Antibodies in Non-Human Primates a
a Morales et al., ACR Convergence 2022
A single ascending dose (“SAD”) study to assess the safety, tolerability and pharmacodynamics of AUR200 in healthy volunteers was initiated in September 2024 and is ongoing. Initial results from this study are expected in the second quarter of 2025.
SALES AND MARKETING ORGANIZATION
Aurinia employs an experienced sales and marketing team dedicated to the commercialization of LUPKYNIS, supported by professionals in commercial operations, commercial supply chain, patient services and market access functions.
REGULATORY EXCLUSIVITY
We have received New Chemical Entity (“NCE”) exclusivity for LUPKYNIS in the U.S., which provides for exclusivity until January 22, 2026. In the U.S., NCEs approved by the FDA are eligible for market exclusivity under the U.S. Federal Food, Drug, and Cosmetic Act (the “FDCA”), which can prevent the approval of generic versions of the NCE for 5 to 7.5 years from the date of the initial approval of the NCE. Specifically, the FDCA provides a 5-year period of marketing exclusivity within the U.S. to the applicant that gains approval of a new drug application (“NDA”) for an NCE. A drug is an NCE if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the first 4 years of the exclusivity period, the FDA may not accept for review an Abbreviated New Drug Application (“ANDA”) or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all of the data required for approval. However, an application may be submitted 4 years after the NDA approval of the NCE if it contains a certification of patent invalidity or non-infringement. The initiation of patent litigation by the patent holder will trigger an automatic stay in the approval of any generic competition until the earlier of: (i) 30 months from the certification; or (ii) a court ruling of patent invalidity or non-infringement for the relevant patents. In the absence of a court ruling, the 30-month stay will be extended by such amount of time (if any) that is required for 7.5 years to have elapsed from the date of NDA approval of the NCE.
We also have NCE-equivalent exclusivity for voclosporin in certain European countries, which provides exclusivity for 10 years in Europe post-approval. Additionally, we have exclusivity for 8 years in Japan post-approval.
INTELLECTUAL PROPERTY
We own granted patents, including U.S. patents, covering LUPKYNIS for composition of matter and methods of use. U.S. Patent Nos. 7,332,472, 10,286,036 and 11,622,991 are listed in the U.S. FDA Orange Book.
•U.S. Patent No. 7,332,472: Patent protection for patents related to the composition of matter of voclosporin are expected to be extended in the U.S. and certain other major markets, including many European markets, until October 2027 under the Hatch-Waxman Act in the U.S., the Supplementary Protection Certificate program in the E.U. and comparable patent extension laws in other countries. In the U.S., we have applied for a patent term extension for U.S. Patent No. 7,332,472 and are awaiting confirmation from the USPTO. As the patent term extension was not granted prior to the expiry of the patent term for our composition of matter patent for voclosporin, we applied for, and have received, an interim patent term extension until October 17, 2025. If the patent term extension is not granted prior to the expiration of the interim patent term extension that was granted, we intend to file future interim patent term extensions to the extent permitted until the USPTO completes its review of the patent term extension application.
•U.S. Patent No. 10,286,036: In May 2019, we were granted U.S. Patent No. 10,286,036 with a term extending to December 2037. The patent claims are directed at the LUPKYNIS dosing protocol for LN used in our clinical trials. We have also filed for protection of this subject matter under the Patent Cooperation Treaty (“PCT”) and are applying for similar protection in certain member countries thereof. Patents issuing from this PCT application have terms extending to May 2038, and such patents have been issued in Australia, Europe, Hong Kong, Israel, Japan, Korea, Mexico, Malaysia, Russia and Singapore. Several third parties have filed oppositions against a granted European patent relating to the LUPKYNIS dosing protocol, which we are vigorously defending. We have also applied for a patent term extension for the issued Japanese counterpart patent and are awaiting confirmation from the Japan Patent Office (“JPO”).
•U.S. Patent No. 11,622,991: In April 2023, we were granted U.S. Patent No. 11,622,991 with a term extending to December 2037. Importantly, the patent claims reflect the unique and proprietary dosing regimen of LUPKYNIS that is consistent with the FDA-approved product label. This patent specifies the method of treating patients with LN by administering LUPKYNIS in combination with MMF and corticosteroids and using eGFR to pharmacodynamically dose the product. Patents claiming this subject matter have been issued in Japan and Israel, with terms extending to May 2038, and are pending in various other jurisdictions. We have also applied for a patent term extension for the issued Japanese counterpart patent and are awaiting confirmation from the JPO.
On February 25, 2025, we received a paragraph IV notice of certification (the “Notice Letter”) related to a submission of an ANDA to the FDA seeking authorization to manufacture, use or sell a generic version of LUPKYNIS in the U.S., prior to the expiry of U.S. Patent Nos. 10,286,036 and 11,622,991 (the “2037 Patents”), which are listed in the FDA's Orange Book. The Notice Letter alleges that the 2037 Patents are invalid, unenforceable and/or will not be infringed by the commercial manufacture, use or sale of the generic product described in the ANDA.
We intend to vigorously defend LUPKYNIS and our intellectual property rights protecting LUPKYNIS. In accordance with the Hatch-Waxman Act, because LUPKYNIS is an NCE, should we file a patent infringement lawsuit within 45 days of receipt of the Notice Letter, the FDA cannot approve any ANDA any earlier than 7.5 years from the approval of the LUPKYNIS NDA unless a District Court finds that all of the asserted claims of the patents-in-suit are invalid, unenforceable and/or not infringed.
COMPETITION
The pharmaceutical industry is competitive. While LUPKYNIS is the only FDA-approved oral therapy for the treatment of adult patients with active LN, BENLYSTA® (belimumab, marketed by GSK plc), an injectable treatment, is also FDA-approved for LN. Additionally, physicians continue to treat LN with an off-label combination of MMF and corticosteroids alone or in combination with first generation calcineurin inhibitors such as tacrolimus.
As a potential treatment for autoimmune disease, AUR200 is subject to competition from both FDA-approved and investigational products. Competing products include, but are not limited to, other dual BAFF/APRIL inhibitors (e.g., povetacicept, atacicept and telitacicept).
MANUFACTURING AND SUPPLY CHAIN
We rely on third-party manufacturers to supply commercial inventory for LUPKYNIS and semi-finished products and expect to continue to do so to meet our development and commercial needs. In all of our manufacturing agreements and commercial supply agreements, we require that contract manufacturers produce drug substance and drug products in accordance with cGMP and all other applicable laws and regulations. We maintain confidentiality agreements with potential and existing manufacturers to protect our proprietary rights related to LUPKYNIS. The long-term commercial success of LUPKYNIS will depend in part on the ability of our contract manufacturers to supply cGMP-compliant drug substance and drug product without interruption.
Manufacturing of Drug Substance
Voclosporin requires a specialized drug substance manufacturing process and is manufactured by Lonza, our sole supplier for drug substance. Pricing for supply is determined through supply agreements between us and Lonza and is based on the volume produced and the cost of the raw materials used in the drug substance manufacturing process. As of the date of this Annual Report, we have not experienced any difficulty in obtaining the raw materials required with respect to the manufacturing of voclosporin. We believe we have enough inventory on hand and manufacturing capacity to meet forecasted demand.
In December 2020, Aurinia entered into a manufacturing services agreement with Lonza for the construction of a dedicated manufacturing facility for voclosporin (the “Monoplant"). The construction of the Monoplant began in January 2021 and manufacturing of voclosporin began in late June 2023. The Monoplant is equipped with state-of-the-art manufacturing equipment to provide cost and production efficiency for the manufacturing of voclosporin, while expanding existing capacity and providing supply security to meet future commercial demand. Aurinia pays a quarterly fixed facility fee of 3.6 million Swiss Francs (approximately $4.0 million) for the exclusive right to use the Monoplant through March 31, 2030.
Encapsulation
Catalent Pharma Solutions (“Catalent”) is currently the sole supplier for the preparation of our voclosporin capsules. Pricing for these services is determined by a supply agreement between Catalent and us. We expect that Catalent will continue to provide contract manufacturing services with respect to encapsulating voclosporin in order to manufacture voclosporin capsules that are required for our future commercial and clinical supply needs.
Packaging
We use a sole supplier for the blistering and packaging of LUPKYNIS commercial cartons for sale in the U.S. and for the blistering of semi-finished products. Pricing for these services is determined by a supply agreement between us and our supplier. We expect no issues in obtaining contract manufacturing services with respect to the packaging of LUPKYNIS commercial cartons for the U.S. market.
GOVERNMENT REGULATION
Pharmaceutical products, including LUPKYNIS, are subject to extensive government regulation. In the U.S., the FDA regulates pharmaceutical products. FDA regulations govern the testing, research and development activities, manufacturing, quality, storage, advertising, promotion, labeling, sale and distribution of pharmaceutical products. Accordingly, there is a rigorous process for the approval of new drugs and ongoing oversight of marketed products. We may also be subject to foreign regulatory requirements governing clinical studies and drug products if products are tested or marketed abroad. The approval process outside of the U.S. varies from jurisdiction to jurisdiction and the time required may be longer or shorter than that required for FDA approval.
Regulation in the U.S.
The FDA testing and approval process requires substantial time, effort and financial resources. We cannot assure you that any of our product candidates will ever obtain approval. The FDA approval process for new drugs includes, without limitation:
•preclinical studies;
•submission in the U.S. of an investigational new drug application for clinical studies conducted in the U.S.;
•adequate and well-controlled clinical studies to establish safety and efficacy of the product;
•review and approval of an NDA in the U.S.; and
•inspection of the facilities used in the manufacturing of the drug to assess compliance with the FDA’s cGMP regulations.
Any products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including record-keeping requirements and reporting of adverse experiences with the drug. Drug manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies and are subject to periodic inspections by the FDA and certain state agencies for compliance with the FDA's current Good Manufacturing Practices (“cGMPs”), which impose certain procedural and documentation requirements on us and our third-party manufacturers. Even after regulatory approval is obtained, under certain circumstances, such as later discovery of previously unknown safety risks, the FDA can withdraw approval or subject the drug to additional restrictions.
The FDA closely regulates the marketing and promotion of drugs. Drugs may only be marketed in a manner consistent with their FDA-approved labeling. Approval may be subject to post-marketing surveillance and other record-keeping and reporting obligations. Product approvals may be withdrawn if compliance with regulatory standards is not maintained or if problems occur following initial marketing.
The failure to comply with FDA’s requirements may result in adverse publicity, warning letters, corrective advertising, restrictions on marketing or manufacturing, refusals to review pending product applications, refusals to permit the import or export of products, seizures, injunctions, and civil and criminal penalties.
Refer to the section titled “Risk Factors” in this Annual Report for a discussion of the potential impacts that compliance with government regulation may have on our business.
U.S. Health Care Fraud and Abuse Laws and Compliance Requirements
We are subject to various federal and state laws targeting fraud and abuse in the health care industry. These laws may impact, among other things, our sales and marketing efforts. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
•the federal Anti-Kickback Statute (“AKS”), which prohibits, among other things, persons from soliciting, receiving, offering or paying remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, an item or service reimbursable under a federal health care program, such as the Medicare and Medicaid programs. The term “remuneration” has been broadly interpreted to include anything of value, including for example gifts, cash payments, donations, the furnishing of supplies or equipment,
•waivers of payment, ownership interests, and providing any item, service or compensation for something other than fair market value.
•federal false claims and civil monetary penalties laws, including the federal civil False Claims Act, which prohibits anyone from, among other things, knowingly presenting, or causing to be presented, for payment to federal programs (including Medicare and Medicaid) claims for items or services that are false or fraudulent. Although we may not submit claims directly to payors, manufacturers can be held liable under these laws in a variety of ways. These include:
providing inaccurate billing or coding information to customers; improperly promoting a product’s off-label use; violating the federal Anti-Kickback Statute; or misreporting pricing information to government programs.
•provisions of the federal Health Insurance Portability and Accountability Act of 1996 (HIPAA), which created new federal criminal statutes that prohibit, among other things, knowingly and willfully executing a scheme to defraud any health care benefit program or making false statements in connection with the delivery of or payment for health care benefits, items or services.
•the federal Physician Payment Sunshine Act requirements, under the Patient Protection and Affordable Care Act (the ACA), which require manufacturers of certain drugs and biologics to track and report to U.S. Centers for Medicare & Medicaid Services (CMS) payments and other transfers of value they make to U.S. physicians and teaching hospitals as well as physician ownership and investment interests in the manufacturer.
•provisions of HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, which impose certain requirements relating to the privacy, security and transmission of individually identifiable health information.
•section 1927 of the Social Security Act, which requires that manufacturers of drugs and biological products covered by Medicaid report pricing information to CMS on a monthly and quarterly basis, including the best price available to any customer of the manufacturer, with certain exceptions for government programs, and pay prescription rebates to state Medicaid programs based on a statutory formula derived from reported pricing information.
•various state and/or foreign law equivalents of each of the above federal laws, such as the California Consumer Privacy Act, many of which differ from each other in significant ways and may not have the same effect, which complicates our compliance efforts.
Regulation in Non-U.S. Jurisdictions
In addition to regulations in the U.S., we, or our partners, may be subject to a variety of foreign regulations governing clinical studies and commercial sales and distribution of LUPKYNIS or future products. When we, or our partners, market LUPKYNIS in foreign countries, we are also subject to foreign regulatory requirements governing marketing approval for pharmaceutical products. The requirements governing the conduct of clinical studies, product approval, pricing and reimbursement vary widely from country to country. Whether or not FDA approval has been obtained, approval of a product by the regulatory authorities of foreign countries must be obtained before marketing the product in those countries. The approval process varies from country to country, and the time required for such approvals may differ substantially from that required for FDA approval. Foreign regulatory approval processes involve many of the risks associated with FDA marketing approval discussed above. There is no assurance that any FDA approval of any of our product candidates will result in similar foreign approvals or vice versa. The process for clinical studies in the European Union and other countries is similar, and studies are heavily scrutinized by the designated ethics committees and regulatory authorities. In addition, foreign regulations may include applicable post-marketing requirements, including safety surveillance, anti-fraud and abuse laws, and implementation of corporate compliance programs and reporting of payments or other transfers of value to health care professionals and entities.
In Europe, the E.U. General Data Protection Regulation (2016/679) (the “GDPR”) contains provisions specifically directed at the processing of health information. The GDPR provides for potentially significant sanctions and contains extraterritorial measures intended to bring non-E.U. companies under the regulation. In addition to the GDPR, individual countries in Europe and elsewhere in the world have enacted similar data privacy legislation. This legislation imposes increased compliance obligations and regulatory risk, including the potential for significant fines for noncompliance.
Other Laws and Regulations
We are subject to a variety of financial disclosure and securities trading regulations as a public company in the U.S., including laws relating to the oversight activities of the U.S. Securities and Exchange Commission (the “SEC”) and the regulations of the Nasdaq Global Market, on which our common shares are traded.
Coverage and Reimbursement
In the U.S. and internationally, sales of LUPKYNIS, and any other products that we market in the future, and our ability to generate revenues on such sales, are dependent, in significant part, on the availability of adequate coverage and reimbursement from third-party payors, such as state and federal governments, managed care providers and private insurance plans. These organizations routinely implement cost-cutting and reimbursement initiatives that have the ability, or potential, to impact a patient’s overall access to our product. Examples of these initiatives include, but are not limited to, establishing formularies that govern the drugs and biologics that are eligible for reimbursement and the out-of-pocket obligations of member patients for such products.
Political, economic and regulatory influences are subjecting the healthcare industry in the U.S. to fundamental changes. There have been, and we expect there will continue to be, legislative and regulatory proposals to change the healthcare system in ways that could significantly affect our future business. For example, ACA enacted in March 2010, substantially changed the way healthcare is financed by both governmental and private insurers. Among other cost containment measures, the ACA established:
•an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents;
•a Medicare Part D coverage gap discount program, in which pharmaceutical manufacturers who wish to have their drugs covered under Part D must offer discounts for eligible beneficiaries during their coverage gap period, often referred to as the donut hole; and
•a formula that increases the rebates a manufacturer must pay under the Medicaid Drug Rebate Program.
Additionally, in August 2022, the Inflation Reduction Act of 2022 (“IRA”) was passed by the U.S. Congress which, among other things, includes policies that are designed to have a direct impact on drug prices and reduce drug spending by the federal government, which took effect in 2023. This legislation contains substantial drug pricing reforms, including the establishment of a drug price negotiation program within the U.S. Department of Health and Human Services that would require manufacturers to charge a negotiated “maximum fair price” for certain selected drugs covered by Medicare or pay an excise tax for noncompliance, the establishment of rebate payment requirements on manufacturers of certain drugs payable under Medicare Parts B and D to penalize price increases that outpace inflation, and requires manufacturers to provide discounts on Part D drugs. Legislative, administrative, and private payor efforts to control drug costs span a range of proposals, including drug price negotiation, Medicare Part D redesign, drug price inflation rebates, international mechanisms, generic drug promotion and anticompetitive behavior, manufacturer reporting, and reforms that could impact therapies utilizing the accelerated approval pathway.
Individual states in the U.S. have also become increasingly active in passing legislation and implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. Recently, there has also been heightened governmental (federal and state) scrutiny over the manner in which drug manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed bills designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products.
Similar political, economic and regulatory developments are occurring in the E.U. and may affect the ability of pharmaceutical companies to profitably commercialize their products. In addition to continuing pressure on prices and cost containment measures, legislative developments at the E.U. or member state level may result in significant additional requirements or obstacles. The delivery of healthcare in the E.U., including the establishment and operation of health services and the pricing and reimbursement of medicines, is almost exclusively a matter for national, rather than E.U., law and policy. National governments and health service providers have different priorities and approaches to the delivery of health care and the pricing and reimbursement of products in that context. In general, however, the healthcare budgetary constraints in most E.U. member states have resulted in restrictions on the pricing and reimbursement of medicines by relevant health service providers. Coupled with ever-increasing E.U. and national regulatory burdens on those wishing to develop and market products, this could restrict or regulate post-approval activities and affect the ability of pharmaceutical companies to commercialize their products. In international markets, reimbursement and healthcare payment systems vary significantly by country, and many countries have instituted price ceilings on specific products and therapies.
Our ability to successfully commercialize products depends in part on the extent to which reimbursement for the costs of our products and related treatments will be available in the U.S. and worldwide from government health administration authorities, private health insurers and other organizations.
HUMAN CAPITAL
As of February 26, 2025, we had 130 employees. We also hire consultants and contract with third parties, as needed, to provide additional resources to support our business activities. None of our employees are represented by labor unions or covered by collective bargaining agreements, and we consider our relations with our employees to be good. We also hire consultants and contract with third parties, as needed, to provide additional resources to support our business activities.
Our key human capital management objectives are to identify, recruit, integrate, retain and motivate our new and existing employees. We believe that our compensation and benefit programs are appropriately designed to attract and retain qualified talent. Employees receive an annual base salary and are eligible to earn performance-based cash bonuses. To create and maintain a successful work environment, we offer a comprehensive package of additional benefits that support the physical and mental health and wellness of all of our employees and their families. Additionally, we grant equity awards in order to allow for directors, officers and employees of Aurinia to share in the performance of the Company.
CORPORATE INFORMATION
Aurinia is organized as a corporation under the Business Corporations Act (Alberta). We have one wholly owned subsidiary, Aurinia Pharma U.S., Inc., a Delaware corporation. Our principal executive office is located at #140, 14315 - 118 Avenue, Edmonton, Alberta, Canada T5L 4S6 and our phone number is +1 (250) 744-2487. Our website address is www.auriniapharma.com.
We file or furnish electronically with the SEC our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to reports, pursuant to Sections 13(a) and 15(d) of the Exchange Act. We make available on our website, free of charge, copies of these reports as soon as reasonably practicable after filing or furnishing these reports with the SEC. The SEC maintains a website at www.sec.gov that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. The information posted on, or that can be accessed through, our website is not incorporated into this Annual Report and the contents of these websites are not intended to be incorporated by reference into any report or document we file with, or furnish to, the SEC. Certain documents are also filed with securities regulators in Canada and are available under our profile at the website www.sedarplus.ca.
Item 1A. Risk Factors
An investment in our common shares involves a high degree of risk. You should carefully consider the material risks and uncertainties described below before deciding whether to purchase our common shares. Certain risks may be applicable to multiple categories but are only included once below. In assessing these risks, you should also refer to the other information contained in this Annual Report, including our audited financial statements and related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our business, financial condition, results of operations, cash flow, reputation and prospects could be materially and adversely affected by any of these risks and uncertainties, as well as other risks and uncertainties not currently known to us or that we currently do not believe to be material. In any such case, the trading price of our common shares of stock could decline, and you could lose all or part of your investment.
RISKS RELATED TO COMMERCIALIZATION
We are substantially dependent on the commercial success of LUPKYNIS.
The success of our business is substantially dependent on our ability to successfully commercialize LUPKYNIS, our sole approved product. The Company markets LUPKYNIS in the U.S. directly through its own commercial organization. The market for effective pharmaceutical sales and marketing professionals is competitive, and maintaining these capabilities is expensive and challenging. If we are unable to maintain an effective sales and marketing organization, LUPKYNIS sales could be adversely affected, and our business may suffer. LUPKYNIS’s competition as a treatment in LN patients includes BENLYSTA and physicians continuing to treat LN with an off-label combination of MMF and corticosteroids alone or in combination with first generation calcineurin inhibitors such as tacrolimus. If we are unable to further change treatment practices, further growth of LUPKYNIS net product sales will be limited, and our business may suffer. We may be subject to additional competition from future products.
In an effort to remain competitive in the marketplace, we may determine to change our pricing, dosage forms and strengths and other marketing strategies for LUPKYNIS, including altering the amount or availability of discounts or rebates. Any such changes could have short-term or long-term negative impacts on net product sales, which could cause our business and results of operations to suffer. Price increases or changes to our marketing strategies may also negatively affect our reputation and our ability to secure and maintain reimbursement coverage for LUPKYNIS, which could result in decreased demand and cause our business and results of operations to suffer. If we are unable to successfully price or market LUPKYNIS, the commercial prospects for LUPKYNIS will be limited, and our business may suffer.
Our estimates of the potential market size for LUPKYNIS are based on prescription and sales data for relevant in-market products, the results of clinical studies, medical literature and other information. If the potential market size for LUPKYNIS is smaller than we estimate, the commercial prospects for LUPKYNIS may be limited, and our business may suffer.
Product liability or other lawsuits against us could cause us to incur substantial liabilities and reduce LUPKYNIS sales.
Patients suffering from LN may become gravely ill. The most commonly reported adverse reactions in our AURORA 1 and AURORA 2 clinical studies (≥3%) were: glomerular filtration rate decreased, hypertension, diarrhea, headache, anemia, cough, urinary tract infection, abdominal pain upper, dyspepsia, alopecia, renal impairment, abdominal pain, mouth ulceration, fatigue, tremor, acute kidney injury, and decreased appetite. Some patients who are treated with LUPKYNIS may die due to their underlying illness or suffer adverse events (which may or may not be drug related).
As such, we may face product liability lawsuits. Although we carry product liability insurance, product liability lawsuits against us could cause us to incur substantial liabilities and reduce LUPKYNIS sales. Furthermore, any such lawsuits could impair our business reputation and result in the initiation of investigations by regulators.
Additionally, we may not have and may not be able to obtain insurance on acceptable terms or with adequate coverage against potential liabilities or other losses if any claim or lawsuit is brought against us, regardless of the success or failure of the claim or lawsuit. Even where claims are submitted to insurance carriers for defense and indemnity, there can be no assurance that the claims will be fully covered by insurance or that the indemnitors or insurers will remain financially viable to cover the cost of such claims. Any such claims or lawsuits could materially impact our financial condition, and our business may suffer.
The commercial success of LUPKYNIS in certain ex-U.S. territories is dependent on the fulfillment of contractual obligations under our out-license agreement and commercial supply agreement.
In December 2020, we entered into a collaboration and licensing agreement with Otsuka to develop and commercialize oral voclosporin in Japan, the E.U., the U.K., Switzerland, Russia, Norway, Belarus, Iceland, Liechtenstein and Ukraine
(collectively, the “Otsuka Territories”) in exchange for: (i) a $50 million upfront cash payment; (ii) regulatory and commercial milestone payments; and (iii) royalties ranging from 10% to 20% on net sales in the Otsuka Territories.
In August 2022, we entered into a commercial supply agreement with Otsuka to: (i) supply LUPKYNIS inventory to Otsuka at cost, plus a margin; and (ii) provide manufacturing and other services, including sharing the capacity of a dedicated manufacturing facility at Lonza, our contract manufacturing partner for voclosporin.
If we are held to not have met our commercial supply obligations, or if Otsuka is unable to successfully commercialize oral voclosporin in the Otsuka Territories, the commercial prospects for LUPKYNIS in the Otsuka Territories will be limited, and our business may suffer.
The commercial success of LUPKYNIS is dependent on pricing regulations and/or third-party coverage and reimbursement policies.
In the U.S. and markets in other countries, patients generally rely on third-party reimbursement for all or part of the costs associated with their treatment. Adequate coverage and reimbursement from governmental healthcare programs, such as Medicaid and Medicare, and commercial payors is critical to market acceptance of our products. Government authorities and other third-party payors, such as private health insurers and health maintenance organizations, decide which medication they will pay for and establish reimbursement levels.
Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular products. Increasingly, third-party payors are requiring that drug manufacturers provide them with predetermined discounts from list prices and are challenging the prices charged for products. Net prices for products may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors. Private third-party payors often rely on Medicare coverage policy and payment limitations in setting their own reimbursement policies.
If LUPKYNIS is subject to unfavorable pricing regulations and/or third-party coverage and reimbursement policies, the commercial prospects for LUPKYNIS may be limited, and our business may suffer.
If we fail to comply with our reporting and payment obligations under the Medicaid Drug Rebate Program or other governmental pricing programs in the U.S., we could be subject to additional reimbursement requirements, penalties, sanctions and fines.
We participate in the Medicaid Drug Rebate Program, administered by Centers for Medicare and Medicaid Services, and other federal and state government pricing programs in the U.S., and we may in the future participate in additional government pricing programs. These programs generally require us to pay rebates or otherwise provide discounts to government payors in connection with LUPKYNIS, which is dispensed to beneficiaries of these programs. In some cases, such as with the Medicaid Drug Rebate Program, the rebates are based on pricing and rebate calculations, which are complex.
The Office of Inspector General assesses our compliance with reporting requirements under the Medicaid Drug Rebate Program. We are liable for errors associated with our submission of pricing data and for any overcharging of government payors, which could result in a civil monetary penalty. Failure to make necessary disclosures and/or to identify overpayments could result in allegations against us under the U.S. False Claims Act (“FCA”) and other laws and regulations. Any required refunds to the U.S. government or responding to a government investigation or enforcement action would be expensive and time consuming and could have a material adverse effect on our business, results of operations and financial condition. If Centers for Medicare and Medicaid Services were to terminate our rebate agreement, no federal payments would be available under Medicaid or Medicare for LUPKYNIS, which could harm our business.
We have reported on various commercial metrics relating to LUPKYNIS, and no single metric is indicative of, or directly correlated to, our current or future financial performance.
We have reported on various commercial metrics relating to LUPKYNIS activity, including the number of prescriptions/PSFs, persistency rates, the number of patients on therapy, patient restarts and patients resulting from hospital fills. None of these metrics, in and of themselves, is indicative of current or future financial performance. Even when a patient becomes a patient on LUPKYNIS therapy, there is no guarantee that they will be a patient for which we recognize revenue, or that they will remain on therapy for any period of time. A patient on therapy who discontinues treatment generally results in zero future revenue, and discontinuations can occur at any time once a patient commences therapy.
Our net product sales are primarily the result of our net sales of LUPKYNIS to two specialty pharmacies and a specialty distributor in the U.S., and net sales of LUPKYNIS inventory to our collaboration partner, Otsuka, for the European and
Japanese market. Revenue from the two specialty pharmacies do not necessarily correlate to any of our commercial metrics. Revenue from Otsuka has no relevance to any of the above noted metrics. Our revenue could therefore fluctuate in a manner contrary to any trend of our commercial metrics.
RISKS RELATED TO PATENTS AND PROPRIETARY TECHNOLOGY
LUPKYNIS’s market exclusivity periods will depend on the validity and enforceability of issued and pending patents covering LUPKYNIS.
We depend globally on patents and other granted rights to prevent others from improperly benefiting from our commercial product, LUPKYNIS, and products or inventions that we develop or acquire. Protecting our patents and other intellectual property may require us to file infringement actions, which may be expensive and time-consuming. For material details about our intellectual property portfolio protecting LUPKYNIS, see the section titled “Intellectual Property” in Item 1.
We have and plan to file additional patent applications that, if issued, would provide further protection for LUPKYNIS. Although we believe the bases for our patents and patent applications are sound, they are untested, and there is no assurance that they will not be successfully challenged. There can be no assurance that any issued patent or any patent currently in process will protect LUPKYNIS from generic competition. If our intellectual property does not protect LUPKYNIS from generic competition, LUPKYNIS net product sales may decline, and/or we may incur additional costs for patent protection, including patent infringement litigation costs arising out of ANDA submissions by generic companies to manufacture and sell generic products or arising out of 505(b)(2) submissions, which could have a material adverse effect on our business, results of operations and financial condition, and our business may suffer.
On February 25, 2025, we received a paragraph IV notice of certification (the “Notice Letter”) related to a submission of an ANDA to the FDA seeking authorization to manufacture, use or sell a generic version of LUPKYNIS in the U.S., prior to the expiry of U.S. Patent Nos. 10,286,036 and 11,622,991 (the “2037 Patents”), which are listed in the FDA's Orange Book. The Notice Letter alleges that the 2037 Patents are invalid, unenforceable and/or will not be infringed by the commercial manufacture, use or sale of the generic product described in the ANDA. Although we intend to vigorously defend our intellectual property rights protecting LUPKYNIS, we may incur significant patent litigation costs, and, if any entity that may file an ANDA is successful in the introduction of the generic product described in its ANDA, then LUPKYNIS net product sales may decline, which could have a material adverse effect on our business, results of operations and financial condition.
If our products or our product candidates infringe the rights of others, we could be subject to expensive litigation, become liable for substantial damages, be required to obtain licenses from others or be prohibited from selling our products or product candidates altogether.
Our competitors or others may have patent rights that they choose to assert against us, licensees, suppliers, customers or potential marketing partners. Moreover, we may not know about patents or patent applications that our products or product candidates could infringe. Because patent applications do not publish for at least 18 months, if at all, and can take many years to issue, there may be currently pending applications unknown to us that may later result in issued patents that our products or product candidates could infringe. In addition, if third parties file patent applications or obtain patents claiming inventions also claimed by us in issued patents or pending applications, we may have to participate in interference proceedings in the U.S. Patent and Trademark Office (“USPTO”) to determine priority of invention. If third parties file oppositions in foreign countries, we may also have to participate in opposition proceedings in foreign tribunals to defend the patentability of claims in our foreign patent applications. If a third party claims that we infringe its proprietary rights, any of the following may occur:
•we may become involved in time-consuming and expensive litigation, even if the claim is without merit;
•we may become liable for substantial damages for past infringement if a court decides that we have infringed a patent;
•a court may prohibit us from selling or licensing our products without a license from the patent holder, which may not be available on commercially acceptable terms, if at all, or which may require us to pay substantial royalties or grant cross-licenses to our patents; or
•we may have to redesign our products or product candidates so that they do not infringe patent rights of others, which may not be possible or commercially feasible and may require new regulatory approvals.
Any of these events could have a material adverse effect on our business, results of operations and financial condition, and our business may suffer.
Patent policy and rule changes could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
Changes in either the patent laws or interpretation of the patent laws in the U.S. or other countries may diminish the value of our patents or narrow the scope of our patent protection. The laws of foreign countries may not protect our rights to the same extent as the laws of the U.S. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the U.S. and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. We therefore cannot be certain that we were the first to make the inventions claimed in our patents or pending applications, or that we were the first to file for patent protection of such inventions.
Assuming the other requirements for patentability are met, in the U.S., prior to March 15, 2013, the first to make the claimed invention is entitled to the patent, while, outside the U.S., the first to file a patent application is entitled to the patent. After March 15, 2013, under the Leahy-Smith America Invents Act (“Leahy-Smith Act”), enacted on September 16, 2011, the U.S. has moved to a first-to-file system. The Leahy-Smith Act also includes a number of significant changes that affect the way patent applications will be prosecuted and may also affect patent litigation. In general, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, results of operations and financial condition, and our business may suffer.
Among some of the other changes introduced by the Leahy-Smith Act are changes that limit where a patentee may file a patent infringement suit and provide new opportunities for third parties to challenge issued patents in the USPTO. We may be subject to the risk of third-party prior art submissions on pending applications or become a party to opposition, derivation, reexamination, inter partes review, post-grant review or interference proceedings challenging our patents for our products or product candidates. There is a lower standard of evidence necessary to invalidate a patent claim in a USPTO proceeding relative to the standard in U.S. federal courts. This could lead third parties to challenge and successfully invalidate our patents that would not otherwise be invalidated if challenged through the court system.
We may not be able to protect the confidentiality of our trade secrets.
There may be an unauthorized disclosure of confidential information under our control, such as information relating to our technology, research and development, production, marketing, and business operations and those of our collaborators, in various forms. Unauthorized disclosures of such information could subject us to complaints or lawsuits for damages, in the U.S., Canada or other jurisdictions, or could otherwise have a negative impact on our business, financial condition, results of operations, reputation and credibility, and our business may suffer.
RISKS RELATED TO FINANCIAL POSITION
Our overall financial performance may not meet our expectations.
Our overall financial performance, including but not limited to, net product sales and net cash provided by or used for operating activities, including any milestone, royalty and other payments resulting from our collaboration and license agreement and commercial supply agreement with Otsuka, is difficult to predict and may fluctuate from quarter to quarter and year to year. Historical performance may not be indicative of future performance. For example, our net product sales may be below expectations, and our costs to operate our business, including cost of product sales, research and development expenses and selling, general and administrative expenses, could exceed our estimates. If our overall performance does not meet our expectations, our business may suffer.
Our restructuring efforts and associated organizational changes may not adequately reduce our operating costs, may lead to additional workforce attrition and may cause operational disruptions.
On February 15, 2024, we announced a strategic restructuring that reduced headcount by approximately 25% and discontinued Aurinia’s AUR300 development program. On November 7, 2024, we announced another strategic restructuring that further reduced headcount by approximately 45% to sharpen the Company's focus on continued LUPKYNIS growth and the rapid development of AUR200. The restructuring efforts may not adequately reduce our operating costs and could yield unintended consequences, such as loss of institutional knowledge and expertise, employee attrition and a reduction in employee morale, as well as substantial demands on our employees, all of which may materially adversely affect our revenues, results of operations or financial condition, and our business may suffer.
Our ability to use our net operating loss carryforwards and tax credit carryforwards to offset future taxable income may be subject to certain limitations. We may also be subject to other potential tax consequences.
Under the provisions of the applicable tax legislation, our net operating loss and tax credit carryforwards are subject to review and possible adjustment by applicable tax regulatory authorities. In addition, proposed or actual changes to applicable tax legislation may significantly impact our ability to utilize our net operating losses and tax credit carryforwards to offset taxable income in the future. This could limit the amount of tax attributes that can be utilized annually to offset future taxable income or tax liabilities. The amount of the annual limitation is determined based on the value of a company immediately prior to the ownership change. Subsequent ownership changes may further affect the limitation in future years. We may not be able to use some or all of our net operating loss and tax credit carryforwards. Additionally, should an event occur that causes or is deemed to cause a change in the residency of Aurinia from Canada to the U.S., for example, we may be subject to certain tax rules that could cause a deemed disposition of our assets for tax purposes. Should that occur, we may be subject to a material amount of tax owing, without corresponding revenue from any actual disposition of our assets, which would have a material adverse effect on our business and financial condition.
RISKS RELATED TO DRUG DEVELOPMENT AND REGULATORY APPROVAL
Drug development involves a lengthy and expensive process with an uncertain outcome, and results of earlier studies may not be predictive of future study results.
Clinical testing is expensive, can take many years to complete and its outcome is inherently uncertain. Failure can occur at any time during the clinical study process. The results of nonclinical studies and early clinical studies of AUR200 may not be predictive of the results of later-stage clinical studies. Promising results shown in early-stage clinical studies may still suffer significant setbacks in subsequent clinical studies. There is a high failure rate for pharmaceutical product candidates proceeding through clinical studies, and product candidates in later stages of clinical studies may fail to show the desired safety and efficacy, despite having progressed through nonclinical studies and initial clinical studies.
A number of companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical studies due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier studies. Moreover, nonclinical and clinical data often are susceptible to varying interpretations and analyses. We do not know whether any clinical studies we may conduct will demonstrate consistent or adequate efficacy and safety sufficient to obtain regulatory approval to market AUR200.
Results from studies of AUR200 may not be sufficient to obtain regulatory approvals to market our product candidate on a timely basis, if at all.
Pharmaceutical product candidates are subject to extensive government regulations related to development, clinical studies, manufacturing and commercialization. In order to sell any product that is under development, we must first receive regulatory approval. To obtain regulatory approval, we must conduct nonclinical and clinical studies that demonstrate that AUR200 is safe and effective. The process of obtaining FDA, EC and other regulatory authority approvals is costly, time-consuming, uncertain and subject to unanticipated delays.
The FDA, EC and other regulatory authorities have substantial discretion in the approval process and may not agree that we have demonstrated that AUR200 is safe and effective. If AUR200 is not found to be safe and effective, we would be unable to obtain regulatory approval to manufacture, market and sell AUR200. We can provide no assurances that the FDA, EC or other regulatory authorities will approve AUR200 or, if approved, what the scope of the approved indication might be.
Our development of AUR200 may be delayed or halted.
Our development of AUR200 may be delayed or halted for various reasons, including:
•insufficient financial resources;
•insufficient supplies of drug product to treat the patients in the studies;
•failure of patients to enroll in the studies at the rate we expect;
•ineffectiveness of AUR200;
•patients experiencing unexpected side effects or other safety concerns being raised during treatment;
•changes in governmental regulations or administrative actions;
•failure to conduct studies in accordance with required clinical practices;
•inspection of clinical study operations or study sites by the FDA or other regulatory authorities, resulting in a clinical hold;
•political unrest effecting clinical sites;
•a shutdown of the U.S. government, including the FDA; or
•natural disasters, public health crises or other catastrophic events impacting any of our clinical sites.
If the development of AUR200 is delayed or halted, we may incur significant additional expenses, and the potential approval of AUR200 may be delayed or could be made impossible to obtain, which would have a material adverse effect on our business and financial condition, and our business may suffer.
Compliance with ongoing post-marketing obligations for LUPKYNIS may uncover new safety information that could give rise to a product recall, updated warnings or other regulatory actions.
After a regulator, such as the FDA, approves a product for marketing, the product’s sponsor must comply with post-marketing obligations. Post-marketing obligations include, but are not limited to, the reporting of adverse events to the regulator within specified timeframes, the submission of product-specific annual reports and notification when a drug product is found to have significant deviations from its approved manufacturing specifications. Such deviations may include unforeseen side effects. Our ongoing compliance with such mandatory reporting requirements could result in additional requests for information that could result in a product recall, strengthened warnings, revisions to other label information, conducting additional clinical studies or the imposition of other risk-management measures. Regulators may also require the withdrawal of the product from the market. Any of these post-marketing regulatory actions could materially affect our sales and increase our costs, and our business may suffer.
Failure to obtain regulatory approval in international jurisdictions would prevent our products, our product candidates or any other products we or our current or future out-licensees may develop from being marketed abroad.
In the event we or our current or future out-licensees (together, “Marketers”) pursue the right to market and sell our products, our product candidates or any other products we may develop (“Product Candidate”) in jurisdictions where we do not already have approval, Marketers would be required to obtain separate marketing approvals and comply with numerous and varying regulatory requirements in those jurisdictions. The approval procedures vary among jurisdictions and may involve additional testing. The time required to obtain approval may differ substantially from that required to obtain, for example, FDA or EC approval. The regulatory approval process outside the U.S. generally includes all of the risks associated with obtaining FDA approval. In addition, in many countries outside the U.S., it is required that the product be approved for reimbursement before the product can be approved for sale in that jurisdiction. In the event Marketers choose to pursue them, Marketers may not obtain approvals from regulatory authorities in such jurisdictions on a timely basis, if at all. Our existing approvals do not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the U.S. does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. If Marketers are unable in the future to obtain approval of a Product Candidate by regulatory authorities, the commercial prospects of that Product Candidate may be significantly diminished and our business may suffer.
RISKS RELATED TO OUR RELIANCE ON THIRD PARTIES
The commercial success of LUPKYNIS and the clinical success of AUR200 will depend on our ability to obtain an uninterrupted supply from our contract manufacturers.
We rely on sole-source contract manufacturers to produce LUPKYNIS and clinical drug supply and expect to continue to do so to meet our commercial and development needs. In all of our manufacturing agreements, we require that contract manufacturers produce active pharmaceutical ingredients (“APIs”) and drug products in accordance with cGMP and all other applicable laws and regulations. The long-term commercial success of LUPKYNIS and clinical success of AUR200 will depend in part on the ability of our contract manufacturers to supply cGMP-compliant API and drug product without interruption. If there is an interruption in the supply from our contract manufacturers, our business may suffer.
We rely on third parties to provide certain services relating to our commercial distribution, clinical studies and other activities. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may face delays in the studies, regulatory submissions, regulatory approval or commercialization of AUR200, or the commercialization of LUPKYNIS.
In the U.S., we rely on two specialty pharmacies to distribute LUPKYNIS to patients. If they provide us with improper information to properly estimate our inventory management, conduct themselves in a manner that violates applicable law, or cease to comply with our agreements with them, it may result in lower net product sales of LUPKYNIS, which would harm our results of operations and business.
We rely on clinical sites to comply with study protocols and regulations applicable to clinical study conduct. We and these clinical sites are required to comply with current Good Clinical Practices (“cGCP”), which are regulations and guidelines
enforced by the FDA and comparable foreign regulatory authorities for products in clinical development. Regulatory authorities enforce cGCPs through periodic inspections of study sponsors, principal investigators and clinical sites. If we, the investigators or the clinical sites fail to comply with applicable cGCPs, the clinical data generated in our clinical studies may be deemed unreliable and the regulatory authorities may require us to perform additional clinical studies before approving our marketing applications, which would delay or compromise the regulatory approval process.
We rely on clinical sites to enroll patients in our clinical studies. The rate of enrollment of patients into our clinical studies at these clinical sites is dependent on a number of factors, including the number of eligible patients and the interest level of investigators, study staff and patients in our clinical studies relative to other enrolling studies. If the clinical sites participating in our clinical studies do not enroll patients in a timely manner, we may face delays in the studies, regulatory submissions, regulatory approval or commercialization of AUR200.
We have agreements with contract research organizations and other third parties to provide services relating to our clinical programs. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards, and our reliance on third parties does not relieve us of our regulatory responsibilities. If these third parties do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the work they perform is compromised due to the failure to adhere to regulatory requirements or for other reasons, we may face delays in the studies, regulatory submissions, regulatory approval or commercialization of AUR200.
RISKS RELATED TO GOVERNMENT REGULATION
We are subject to various federal, state and foreign laws and regulations governing the health care industry that could result in substantial penalties for noncompliance.
We are subject to various federal, state and foreign laws and regulations governing the health care industry that could result in substantial penalties for noncompliance. These laws and regulations may impact our ability to operate, including our sales and marketing efforts. In addition, we may be subject to patient privacy regulation by federal, state and foreign governments that govern jurisdictions in which we conduct our business. The laws and regulations that may affect our ability to operate include:
•The AKS, which prohibits, among other things, persons from soliciting, receiving, offering or paying remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, an item or service reimbursable under a federal health care program, such as the Medicare and Medicaid programs. The term “remuneration” has been broadly interpreted to include anything of value, including for example gifts, cash payments, donations, the furnishing of supplies or equipment, waivers of payment, ownership interests, and providing any item, service or compensation for something other than fair market value.
•U.S. false claims and civil monetary penalties laws, including the FCA, which prohibits anyone from, among other things, knowingly presenting, or causing to be presented, for payment to federal programs (including Medicare and Medicaid) claims for items or services that are false or fraudulent. Although we may not submit claims directly to payors, manufacturers can be held liable under these laws in a variety of ways. These include: providing inaccurate billing or coding information to customers; improperly promoting a product’s off-label use; violating the AKS; or misreporting pricing information to government programs.
•The U.S. Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which prohibits, among other things, knowingly and willfully executing a scheme to defraud any health care benefit program or making false statements in connection with the delivery of or payment for health care benefits, items or services.
•The U.S. Physician Payment Sunshine Act requirements, under the Patient Protection and Affordable Care Act, which require manufacturers of certain drugs and biologics to track and report to U.S. Centers for Medicare & Medicaid Services payments and other transfers of value they make to U.S. physicians and teaching hospitals as well as physician ownership and investment interests in the manufacturer.
•Various federal, state and foreign data privacy and security laws and regulations. These include provisions of HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, which impose certain requirements relating to the privacy, security and transmission of individually identifiable health information in the U.S. and the General Data Protection Regulation (“GDPR”) in the European Union. We may not be directly subject to certain of these laws and regulations, such as privacy and security requirements under HIPAA; however, we may be subject to criminal penalties for knowingly, aiding and embedding these violations.
•Section 1927 of the Social Security Act, which requires that manufacturers of drugs and biological products covered by Medicaid report pricing information to CMS on a monthly and quarterly basis, including the best price available to
any customer of the manufacturer, with certain exceptions for government programs, and pay prescription rebates to state Medicaid programs based on a statutory formula derived from reported pricing information.
•Various state and/or foreign law equivalents of each of the above federal laws, such as the California Consumer Privacy Act, many of which differ from each other in significant ways and may not have the same effect, which complicates our compliance efforts.
If we are found to be in violation of any of the laws or regulations described above or any other laws or regulations that apply to us (including any changes made to such laws or regulations, or new laws or regulations implemented, by applicable government entities), we may be subject to substantial penalties, including civil and criminal penalties, damages, fines and possible exclusion from participation in Medicare, Medicaid and other federal health care programs. If we are subjected to substantial penalties, our business may suffer, and we may be forced to curtail or cease our operations.
Drugs approved by the FDA, EC and/or other regulatory agencies are subject to ongoing regulation.
Any products manufactured or distributed by us pursuant to FDA, EC and/or other regulatory agency approvals may be subject to continuing regulation by such agencies, including record-keeping requirements and reporting of adverse experiences with the drug. Drug manufacturers and their subcontractors are required to register their establishments with the FDA, EC and/or other regulatory agencies and may be subject to periodic unannounced inspections by such agencies for compliance with cGMPs, which impose certain procedural and documentation requirements on us and our third-party manufacturers. Even after regulatory approval is obtained, under certain circumstances, such as later discovery of previously unknown safety risks, the FDA, EC and/or other regulatory agencies can withdraw approval, recall the product or subject the drug to additional restrictions. In addition, governments outside of the U.S. tend to impose strict price controls, which may adversely affect our revenues or our royalty payments received from license agreements.
RISKS RELATED TO OWNERSHIP OF OUR COMMON SHARES
The volume and trading price of our common shares may fluctuate significantly, and you may lose all or part of your investment.
The volume and trading price of our common shares may fluctuate significantly, and you may lose all or part of your investment. These fluctuations could be based on various factors, including factors described elsewhere in this Annual Report and below:
•changes in analyst estimates, ratings and price targets;
•negative press reports or other negative publicity, whether or not true, about our business;
•developments concerning the pharmaceutical and biotechnology industry in general;
•market sentiment towards pharmaceutical and biotechnology stocks;
•developments concerning the overall economy; and
•market sentiment toward equity securities.
Any of these factors may result in volatile changes in the volume and trading price of our common shares. In the past, following periods of volatility in the market price of a company’s securities, shareholders have often instituted securities class action litigation against that company. If we were involved in a class action suit, it could divert the attention of management, result in negative press reports and, if adversely determined, have a material adverse effect on our results of operations and financial condition.
In addition, shareholder activists have become involved in numerous public companies. In the past two years, we have faced “withhold” campaigns against nominees for director from some of our shareholders and such actions could continue in the future. Responding to actions by shareholder activists may disrupt our business and divert the attention of management and employees, and can have an impact on the price of our common shares.
We have never paid a dividend on our common shares, and you should rely on price appreciation of our common shares for return on your investment.
We have never paid a dividend on our common shares. Even if we decide to pay dividends, the timing, amount and form of future dividends will depend on future results of operations, financial condition, contractual restrictions and other factors. You should not rely on dividend income from your investment, and should rely on price appreciation of our common shares for a return, if any, on your investment.
Our capital requirements and our potential need for, and ability to obtain, additional financing are uncertain. If we need to obtain additional financing in the future, such financing could result in dilution to your investment, adversely affect the trading price of our common shares and/or create future operating and financial restrictions.
As of December 31, 2024, we had cash, cash equivalents, restricted cash and investments of $358.5 million. LUPKYNIS is our only approved product and our only source of net product sales. Prior to the year ended December 31, 2024, we had negative operating cash flow for multiple years. The amount and timing of future funding requirements, if any, will depend on many factors, including the success of our commercialization efforts for LUPKYNIS and our ability to control expenses and our decisions on how to deploy capital. If necessary, we will raise additional capital through equity or debt financings. We can provide no assurance that additional financing will be available to us on favorable terms, or at all. If we issue additional equity securities or securities convertible into equity securities, you may suffer dilution to your investment, and such issuance may adversely affect the trading price of our common shares. Any new debt financing we enter into may involve covenants that restrict our operations, which may include limitations on borrowing and specific restrictions on the use of our assets, as well as prohibitions on our ability to create liens or pay dividends. If we need to raise additional capital and are unable to do so, we may be forced to curtail or cease our operations.
There can be no assurance that we will continue to repurchase Common Shares or that we will repurchase Common Shares at favorable prices.
Our Board has the authority to authorize share repurchase programs. In February 2024, the Board approved a share repurchase program of up to $150 million of our common shares (the “Share Repurchase Plan”). The timing and amount of repurchase transactions will be determined by management based on its evaluation of market conditions, share price, legal requirements, including applicable blackout period restrictions, and other factors. A reduction in repurchases under, or the completion of, our share repurchase programs could have a negative effect on the market price of our common shares. Additionally, the recently enacted IRA includes an excise tax on share repurchases, which will increase the cost of share repurchases. We can provide no assurance that we will repurchase common shares at favorable prices, if at all.
GENERAL RISK FACTORS
Our ability to hire and retain key employees is uncertain.
The market for effective professionals in the pharmaceutical industry is competitive and hiring and retaining these professionals is expensive and challenging. If we are unable to hire and retain key employees, we may be unable to effectively execute on our operating plan, and our business may suffer.
Our employees, consultants, contract manufacturing organizations, principal investigators, and clinical research organizations may engage in misconduct, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
We are exposed to the risk of employee, consultants, contract manufacturing organizations, principal investigators, and clinical research organizations misconduct, which could include intentional failures to comply with regulatory standards and requirements, such as FDA regulations, federal and state healthcare fraud and abuse laws and regulations, or similar laws and regulations established and enforced by comparable foreign regulatory authorities. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commissions, customer incentive programs and other business arrangements. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in protecting us from governmental actions or lawsuits. If any such actions are instituted against us, and we are not successful in defending ourselves, those actions, including the imposition of significant fines or other sanctions, could have a material adverse effect on our business and results of operations.
Business interruptions resulting from geopolitical actions, natural disasters, public health crises or other catastrophic events could have an adverse impact on our business.
Business interruptions resulting from geopolitical actions, such as war and terrorism, natural disasters, public health crises, such as a pandemic, or other catastrophic events could have an adverse impact on our business. For example, if one of these events were to adversely affect one of our contract manufacturers, our supply of LUPKYNIS could be interrupted.
You may be unable to enforce actions against us, or certain of our directors and officers under U.S. laws.
We are an Alberta, Canada corporation, and some of our directors and officers reside outside of the U.S. Because all or a substantial portion of the assets of these persons are located outside of the U.S., it may not be possible to effect service of process upon those persons. Furthermore, it may not be possible for investors to enforce judgments obtained in U.S. courts based upon the civil liability provisions U.S. laws against any of those persons. There is doubt as to the enforceability, in original actions in Canadian courts, of liabilities based upon U.S. federal securities laws and as to the enforceability in Canadian courts of judgments of U.S. courts obtained in actions based upon the civil liability provisions of U.S. laws.
Our business and operations may be materially adversely affected in the event of computer system failures or security breaches.
Despite the implementation of security measures, our internal computer systems, and those of other third parties on which we rely, are vulnerable to damage from computer viruses, unauthorized access, cyber-attacks, natural disasters, fire, terrorism, war and telecommunication and electrical failures. If such an event were to occur and interrupt our operations, it could result in a material disruption of our all or a significant part of our business. To the extent that any disruption or security breach results in a loss of or damage to our data or applications, loss of trade secrets or inappropriate disclosure of confidential or proprietary information, including protected health information or personal data of employees or former employees, access to our customer or clinical data or disruption of the manufacturing process, we could incur liability and the further development of our products or product candidates could be delayed. We may also be vulnerable to cyber-attacks or other malfeasance by hackers, employees and others. This type of breach of our cybersecurity may compromise our confidential information or our financial information and adversely affect our business or result in legal proceedings. Additionally, compliance with privacy laws, data breach notification laws, and data security requirements is rigorous, time-intensive and may increase our costs.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
We are subject to the periodic reporting requirements of the Exchange Act. Our disclosure controls and procedures are designed to reasonably assure that information required to be disclosed by us in reports we file or submit under the Exchange Act is accumulated and communicated to management and recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC.
Disclosure controls and procedures or internal controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements due to error or fraud may occur and not be detected.
Use of hazardous materials might expose us to risk in the form of damages.
Drug manufacturing processes involve the controlled use of hazardous materials. We and our third-party manufacturing contractors are subject to regulations governing the use, manufacture, storage, handling and disposal of such materials and certain waste products. Although we believe that our third-party manufacturers have the required safety procedures for handling and disposing of such materials and comply with the standards prescribed by such laws and regulations, the risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of such an accident, we could be held liable for any damages that result and such liability could exceed our resources.
Item 1B. Unresolved Staff Comments
None.
Item 1C. Cybersecurity
Risk Management and Strategy
We maintain a cybersecurity risk management program as part of the Company's overall risk management framework and related policies and processes to identify, assess and manage material risks from cybersecurity threats.
Our Information Security Policy is designed to align with certain best practices, including GDPR. This policy promotes the management and execution of our information security framework for preserving the confidentiality, integrity, availability and
privacy of our information assets, including by helping enable us to better oversee, monitor and identify certain risks related to the processing of information by authorized third-party service providers. We also have an Information Technology ("IT") Steering Committee to help ensure security and compliance across our IT services. We have in the past, and may in the future, engage third parties to assess the effectiveness of our cybersecurity prevention and response systems and processes. We implement a layered strategy for overseeing and identifying material risks from cybersecurity threats associated with our use of third party service providers, including: (i) the use of a suite of Microsoft tools (including Microsoft Defender); (ii) a cloud IT strategy that eliminates any central platform; (iii) engaging a cybersecurity firm that monitors our systems 24/7 and provides daily alerts and updates; (iv) regular cybersecurity training for all employees and contractors; and (v) policies and procedures that govern employee activities along with technical controls in place to enforce those policies and procedures.
During 2024, we refreshed our business continuity program to assess the resilience of our processes and systems against potential threats, including cyber-attacks. Our refreshed crisis management and business continuity program establishes crisis management instructions with a detailed plan for each business department outlining critical processes, internal and external dependencies and recovery strategies. In addition, routine information security training and updates are regularly rolled out to our employees, and we track certain metrics that we believe help ensure we have a strong security posture.
To date, cybersecurity threats, including those resulting from any previous cybersecurity incidents, have not materially affected our Company, including our business strategy, results of operations or financial condition. We do not believe that cybersecurity threats resulting from any previous cybersecurity incidents of which we are aware are reasonably likely to materially affect our Company. See “We rely significantly on information technology and any failure, inadequacy, or security lapse of that technology, including any cybersecurity incidents, could harm us” in the “Risk Factors” section of this Annual Report for further information.
Governance
One of the key functions of our Board is informed oversight of our risk management process. Our Board administers the risk oversight function directly through the Board, as well as through various standing committees of our Board that address risks inherent in their respective areas of oversight. The Board at least annually reviews management's annual enterprise risk assessment, business continuity process and cybersecurity posture. Our Audit Committee is responsible for overseeing the management of risks associated with our financial reporting, accounting and auditing matters, as well as business-related risks (such as leadership, continuity, cybersecurity and matters relating to our commercial activities), reviewing as required our processes around the management and monitoring of such risks, as well as conducting a risk assessment review. Our Audit Committee charter sets forth the responsibilities of the Audit Committee consistent with applicable SEC and Nasdaq rules, including reviewing our approach to risk mitigation with respect to IT and cybersecurity. An information security update is provided quarterly, or as needed, to the Audit Committee, with a detailed review provided at least annually, or as needed.
In addition, our Chief Information Officer ("CIO") is responsible for leading the assessment and management of cybersecurity risks. Our CIO, who has held this position since 2021, has over 20 years of experience in information security and holds an MBA from The George B. Delaplaine School of Business and Economics. He was previously CIO at Autolus Therapeutics from 2018 to 2021, and CIO at Sucampo Pharmaceuticals from 2015 to 2018. Prior to that, he was a Director, IT at AstraZeneca from 2008 to 2015. Our CIO regularly receives reports from our Head of Enterprise Technology along with our cybersecurity partners on cybersecurity threats and incidents, as applicable.
Item 2. Properties update
We lease 4,375 square feet of office space in Edmonton, Alberta, which serves as our principal executive office. We lease 30,531 square feet of office space in Rockville, Maryland, which serves as our commercial office. We believe these facilities are adequate to meet our current and future needs.
Item 3. Legal Proceedings
We are not currently a party to any material legal proceedings.
Item 4. Mine Safety Disclosures
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Shareholder Matters and Issuer Purchases of Equity Securities
Market Information
Our common shares are traded on The Nasdaq Global Market under the symbol “AUPH”.
Holders of Record
As of February 20, 2025, we had 112 registered holders. Certain of our common shares are held in “street” name, and, accordingly, the number of beneficial owners of such shares is not known or included in the foregoing number. The number of holders of record also does not include shareholders whose shares may be held in trust by other entities.
Dividends
We have never paid dividends on our common shares, and we do not have any plans to pay dividends. Any future determination to pay dividends will be at the discretion of our Board and will depend on then-existing conditions, including our financial condition, results of operations, projections, liquidity, contractual restrictions, legal requirements and other factors that our Board deems relevant.
Purchases of Equity Securities by the Issuer or Affiliated Purchasers
In February 2024, the Board approved a share repurchase program of up to $150 million of shares of our common shares (“Share Repurchase Plan”). Purchases under the share repurchase program commenced on February 21, 2024. For the year ended December 31, 2024, the Company repurchased 6.1 million of its common shares for $41.0 million. The timing and amount of future repurchase transactions will be determined by management based on its evaluation of market conditions, share price, legal requirements, including applicable blackout period restrictions, and other factors. Under Alberta law, the repurchased common shares are cancelled and not reissued.
The following table summarizes the common share activity of our repurchased shares under the Share Repurchase Plan.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Period | | Total number of shares purchased | | Average price paid per share in $ | | Total number of shares purchased as part of publicly announced program | | Maximum approximate dollar value of shares that may yet be purchased under program (in thousands)(1) |
| 2/21/2024-2/29/2024 | | 1,732,787 | | $5.77 | | 1,732,787 | | $140,000 |
| 3/1/2024-3/31/2024 | | 640,587 | | $4.98 | | 640,587 | | $136,809 |
| 4/1/2024-4/30/2024 | | 1,049,556 | | $4.93 | | 1,049,556 | | $131,638 |
| 5/1/2024-5/31/2024 | | 891 | | $4.99 | | 891 | | $131,633 |
| 11/1/2024-11/30/2024 | | 2,371,612 | | $8.43 | | 2,371,612 | | $111,633 |
| 12/1/2024-12/31/2024 | | 257,206 | | $8.98 | | 257,206 | | $109,325 |
| Total | | 6,052,639 | | | | 6,052,639 | | |
(1)Does not include broker commissions.
The Company has entered into a Rule 10b5-1 stock repurchase plan for the purpose of establishing a trading plan to purchase the Company’s common shares in a manner intended to satisfy the affirmative defense of Rule 10b5-1(c) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and in accordance with applicable Canadian securities laws.
Performance Graph
The following line graph compares the cumulative total shareholder return through December 31, 2024, by an investor who invested $100 on December 31, 2019 in each of: (i) our common shares; (ii) the Nasdaq Biotechnology Index; and (iii) the Nasdaq Composite Index. The historical share price performance of our common shares shown in the performance graph is not necessarily indicative of future share price performance.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Cumulative Total Return Date Ended |
| | Ticker | | December 31, 2019 | | December 31, 2020 | | December 31, 2021 | | December 31, 2022 | | December 31, 2023 | | December 31, 2024 |
| Aurinia Pharmaceuticals Inc. | | AUPH | | $ | 100.00 | | | $ | 68.26 | | | $ | 112.88 | | | $ | 21.32 | | | $ | 44.37 | | | $ | 44.32 | |
| NASDAQ Biotechnology Index | | ^NBI | | $ | 100.00 | | | $ | 125.69 | | | $ | 124.89 | | | $ | 111.27 | | | $ | 115.42 | | | $ | 113.84 | |
| NASDAQ Composite Index | | ^IXIC | | $ | 100.00 | | | $ | 143.64 | | | $ | 174.36 | | | $ | 116.65 | | | $ | 167.30 | | | $ | 215.22 | |
This performance graph shall not be deemed “soliciting material” or to be “filed” with the SEC for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities under that Section, and shall not be deemed to be incorporated by reference into any of our filings under the Securities Act, except to the extent that we specifically incorporate this information by reference therein, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
Item 6. Reserved.
Not applicable.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the notes thereto and other financial information included in this Annual Report. Some of the information contained in this discussion and analysis, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. You should review the “Risk Factors” set forth in this Annual Report for a discussion of important factors that could cause our actual results may differ materially from the results described or implied by the forward-looking statements contained in the following discussion and analysis.
The following generally discusses 2024 and 2023 items and year-to-year comparisons between 2024 and 2023. Discussion of 2022 and year-to-year comparisons between 2023 and 2022 that are not included in this discussion can be found in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on February 15, 2024.
Overview
Aurinia is a biopharmaceutical company focused on delivering therapies to people living with autoimmune diseases with high unmet medical needs. In January 2021, the Company introduced LUPKYNIS® (voclosporin), the first FDA-approved oral therapy for the treatment of adult patients with active lupus nephritis (“LN”). Aurinia is also developing AUR200, a dual inhibitor of B cell activating factor (BAFF) and a proliferation inducing ligand (APRIL) for the potential treatment of autoimmune diseases.
Results of Operations
Comparison of the Years Ended December 31, 2024 and 2023
The following table sets forth our results of operations for the years ended December 31, 2024 and 2023 (in thousands):
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | |
| 2024 | | 2023 | | Change |
| Revenue | | | | | |
| Net product sales | $ | 216,186 | | | $ | 158,533 | | | $ | 57,653 | |
| License, collaboration and royalty revenue | 18,947 | | | 16,980 | | | 1,967 | |
| Total revenue | 235,133 | | | 175,513 | | | 59,620 | |
| Operating expenses | | | | | |
| Cost of revenue | 28,248 | | | 14,148 | | | 14,100 | |
| Selling, general and administrative | 172,028 | | | 195,036 | | | (23,008) | |
| Research and development | 20,785 | | | 49,641 | | | (28,856) | |
| Restructuring | 23,106 | | | — | | | 23,106 | |
| Other (income) expense, net | (4,347) | | | 8,379 | | | (12,726) | |
| Total operating expenses | 239,820 | | | 267,204 | | | (27,384) | |
| Loss from operations | (4,687) | | | (91,691) | | | 87,004 | |
| Interest income | 16,970 | | | 16,997 | | | (27) | |
| Interest expense | (4,835) | | | (2,775) | | | (2,060) | |
| Net income (loss) before income taxes | 7,448 | | | (77,469) | | | 84,917 | |
| Income tax expense | 1,696 | | | 551 | | | 1,145 | |
| Net income (loss) | $ | 5,752 | | | $ | (78,020) | | | $ | 83,772 | |
Net Product Sales
Aurinia sells LUPKYNIS to two specialty pharmacies and a specialty distributor in the U.S., and Aurinia sells LUPKYNIS inventory to its collaboration partner, Otsuka Pharmaceutical Co., Ltd. (“Otsuka”), for the European and Japanese market. The two specialty pharmacies, specialty distributor and Otsuka are considered our customers for accounting purposes.
For the year ended December 31, 2024, net product sales were $216.2 million, up 36% from $158.5 million in 2023. The increase is primarily due to an increase in the number of LUPKYNIS cartons sold to specialty pharmacies, driven by further LN market penetration.
License, Collaboration and Royalty Revenue
License, collaboration and royalty revenue consists of revenue from a collaboration and licensing agreement with Otsuka to develop and commercialize oral voclosporin in Japan, the European Union (the “E.U.”), the United Kingdom (the “U.K.”), Switzerland, Russia, Norway, Belarus, Iceland, Liechtenstein and Ukraine (collectively, the “Otsuka Territories”) in exchange for: (i) a $50 million upfront cash payment; (ii) regulatory and commercial milestone payments; and (iii) royalties ranging from 10% to 20% on net sales in the Otsuka Territories.
License, collaboration and royalty revenue also consists of revenue from a commercial supply agreement with Otsuka to provide manufacturing and other services, including sharing the capacity of a dedicated manufacturing facility at Lonza Ltd. (“Lonza”), Aurinia’s contract manufacturing partner for voclosporin.
For the year ended December 31, 2024, license, collaboration and royalty revenue was $18.9 million, up 11% from $17.0 million in 2023. The increase is primarily due to an increase in manufacturing services provided to Otsuka for sharing the capacity of the Monoplant, which commenced in late 2023. Aurinia recognized revenue for a $10.0 million milestone in 2024 for the approval of LUPKYNIS for the treatment of LN in Japan by the Japanese Ministry of Health, Labour and Welfare and a $10.0 million milestone in 2023 for pricing and reimbursement approval in certain European jurisdictions.
Cost of Revenue
Cost of revenue consists primarily of expense associated with: (ii) amortization of the finance lease right-of-use asset recognized in connection with the Monoplant; (ii) manufacturing; and (iii) shipping, storage and distribution.
In December 2020, Aurinia entered into a manufacturing services agreement with Lonza for the construction of a dedicated manufacturing facility for voclosporin (the “Monoplant”). The construction of the Monoplant began in January 2021 and manufacturing of voclosporin began in late June 2023. The Monoplant is equipped with state-of-the-art manufacturing equipment to provide cost and production efficiency for the manufacturing of voclosporin, while expanding existing capacity and providing supply security to meet future commercial demand. Aurinia pays a quarterly fixed facility fee of 3.6 million Swiss Francs (approximately $4.0 million) for the exclusive right to use the Monoplant through March 31, 2030.
For the year ended December 31, 2024, cost of revenue was $28.2 million, compared to $14.1 million in 2023. The increase is primarily due to an increase in: (i) amortization of the finance lease right-of-use asset recognized in connection with the Monoplant, which was placed into service in late June 2023; (ii) Aurinia’s net sales of LUPKYNIS inventory to Otsuka; and (iii) Aurinia’s net sales of LUPKYNIS in the U.S.
For the year ended December 31, 2024, gross margin was 88%, compared to 92% in 2023.
Selling, General and Administrative Expense
Selling, general and administrative (“SG&A”) expense consists of personnel and non-personnel expenses to support growing sales of LUPKYNIS. Personnel-related expense includes salaries, incentive pay, benefits and share-based compensation for personnel engaged in sales, finance and administrative functions. Non-personnel-related expense includes: (i) selling, patient
services, pharmacovigilance, marketing, advertising, travel, sponsorships and trade shows; and (ii) other general and administrative costs, including consulting, legal, patent, insurance, accounting, information technology and facilities.
The following table summarizes our SG&A expense for the years ended December 31, 2024 and 2023 (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, | | |
| | 2024 | | 2023 | | Change |
| Personnel-expense: | | | | | | |
| Salaries, incentive pay and benefits | | $ | 73,231 | | | $ | 82,768 | | | $ | (9,537) | |
| Share-based compensation | | 31,641 | | | 36,511 | | | (4,870) | |
| Total personnel expense | | 104,872 | | | 119,279 | | | (14,407) | |
| Non-personnel expense: | | | | | | |
| Professional fees and services | | 33,809 | | | 32,874 | | | 935 | |
| Marketing and advertising | | 14,094 | | | 18,287 | | | (4,193) | |
| Travel, sponsorships and trade shows | | 8,605 | | | 11,281 | | | (2,676) | |
| Other | | 10,648 | | | 13,315 | | | (2,667) | |
| Total non-personnel expense | | 67,156 | | | 75,757 | | | (8,601) | |
| Total SG&A expense | | $ | 172,028 | | | $ | 195,036 | | | $ | (23,008) | |
The decrease in SG&A personnel-expense and non-personnel expense were primarily a result of lower employee-related general and administrative costs, including share-based compensation, and overhead costs resulting from our strategic restructuring efforts in 2024.
We expect our SG&A expense to decrease in 2025 as we realize the full benefits of our strategic restructuring efforts.
Research and Development Expense
Research and development (“R&D”) expense consists of personnel and non-personnel expenses. Personnel-related expense includes salaries, incentive pay, benefits and share-based compensation for personnel engaged in research and development functions. Non-personnel-related expense includes subcontractors and materials used for R&D activities, including development, clinical trials, clinical supply and distribution, and other professional services.
The following table summarizes our R&D expense for the years ended December 31, 2024 and 2023 (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, | | |
| | 2024 | | 2023 | | Change |
| Personnel expense: | | | | | | |
| Salaries, incentive pay and benefits | | $ | 6,461 | | | $ | 14,546 | | | $ | (8,085) | |
| Share-based compensation | | (1,329) | | | 7,533 | | | (8,862) | |
| Total personnel expense | | 5,132 | | | 22,079 | | | (16,947) | |
| Non-personnel expense: | | | | | | |
| Contract research organizations and developmental expenses | | 12,526 | | | 17,858 | | | (5,332) | |
| Clinical supply and distribution | | 2,530 | | | 9,104 | | | (6,574) | |
| Other | | 597 | | | 600 | | | (3) | |
| Total non-personnel expense | | 15,653 | | | 27,562 | | | (11,909) | |
| Total R&D expense | | $ | 20,785 | | | $ | 49,641 | | | $ | (28,856) | |
The decrease in R&D personnel-expense was primarily a result of a reduction of headcount from our strategic restructuring efforts in 2024, including the reversal of non-cash, share-based compensation expense related to forfeited, unvested equity awards. The decrease in R&D non-personnel expense was primarily a result of discontinuing our AUR300 development program in February 2024, and the timing of development activities for our AUR200 program.
We expect our R&D expenses to increase for the foreseeable future as we advance AUR200 through clinical development and continue to meet our post-approval obligations with the FDA related to LUPKYNIS.
Restructuring Expense
Restructuring expense consists primarily of one-time termination benefits to affected employees, including severance and health care benefits, contract terminations and other costs related to our strategic restructuring efforts in 2024. On February 15, 2024, we announced a strategic restructuring that reduced headcount by approximately 25% and discontinued Aurinia’s AUR300 development program. On November 7, 2024, we announced another strategic restructuring that further reduced headcount by approximately 45% to sharpen the Company's focus on continued LUPKYNIS growth and the rapid development of AUR200.
For the year ended December 31, 2024, restructuring expense was $23.1 million, compared to nil in 2023.
Other (Income) Expense, Net
For the year ended December 31, 2024, other (income) expense, net was $(4.3) million, compared to $8.4 million in 2023. The change is primarily due to: (i) changes in the foreign exchange remeasurement of the finance lease liability recognized in connection with the Monoplant, which commenced in late June 2023 and is denominated in Swiss Francs; (ii) changes in the fair value assumptions related to our deferred compensation liability; and (iii) a one-time expense in 2023 related to shareholder matters.
Liquidity and Capital Resources
As of December 31, 2024, Aurinia had cash, cash equivalents, restricted cash and investments of $358.5 million, compared to $350.7 million at December 31, 2023. For the year ended December 31, 2024, the Company repurchased 6.1 million of its common shares for $41.0 million. For the year ended December 31, 2024, cash flow provided by (used in) operating activities was $44.4 million, compared to $(33.5) million in 2023.
Based on our current operating plans and projections, the Company expects to fund future operations with existing cash or cash generated from operations.
The amount and timing of additional future funding needs, if any, will depend on many factors, including the success of our commercialization efforts for LUPKYNIS and our ability to control expenses. If necessary, we intend to raise additional capital through equity or debt financings. We can provide no assurance that additional financing will be available to us on favorable terms, or at all.
Refer to the Notes to Consolidated Financial Statements, including Note 5, of Item 15 of this Annual Report for Aurinia’s material cash requirements from known contractual and other obligations as of December 31, 2024.
Critical Accounting Estimates
The discussion and analysis of our financial condition and results of operations is based on our audited consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these audited consolidated financial statements requires us to make estimates, judgments and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses and related disclosure of contingent assets and liabilities. We evaluate our estimates on an ongoing basis. We base our estimates on historical experience and on other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ materially from these estimates under different assumptions or conditions.
Critical accounting estimates are those estimates made in accordance with U.S. GAAP that involve a significant level of estimation uncertainty and have had or are reasonably likely to have a material impact on our financial condition or results of operations. While our significant accounting policies are more fully described in the notes to our consolidated financial statements in Item 15 of this Annual Report, we believe that the following critical accounting policy and underlying estimates are most critical to understanding our reported financial results.
Net Product Sales
Revenue from product sales is recognized when the customer obtains control of our product, which typically occurs on delivery. Revenue from product sales is recorded at the transaction price, net of estimates for variable consideration consisting of prompt-pay discounts, customer fees, government rebates, co-payment assistance, payor rebates and administration fees for which
reserves are established. These reserves are based on estimates of the amounts earned or to be claimed on the related sales and are classified as reductions of accounts receivable (if the amount is payable to our customer) or a liability (if the amount is payable to a party other than our customer).
Variable consideration is estimated using the expected-value amount method, which is the sum of probability-weighted amounts in a range of possible consideration amounts. Significant judgment is required in estimating variable consideration. In making these estimates, we consider historical data, including patient mix and inventory sold to our customers that has not yet been dispensed. We use a data aggregator and historical claims to estimate variable consideration for inventory sold to our customers that has not yet been dispensed. Actual amounts of consideration ultimately received may differ from our estimates. If actual results vary materially from our estimates, we adjust these estimates, which will affect net product sales and earnings in the period such estimates are adjusted.
As of December 31, 2024, we did not have any material adjustments to variable consideration estimates based on actual results.
Impact of Recently Issued Accounting Pronouncements
We describe the impact of recently issued accounting pronouncements that apply to us in Note 2 of Item 15 of this Annual Report.
Item 7A. Quantitative and Qualitative Disclosures about Market Risks
Interest Rate Risk
Financial instruments that potentially expose the Company to interest rate risk consist of cash, cash equivalents, restricted cash and investments. These instruments consist of certificates of deposits, money market instruments and investments in U.S. treasury securities, U.S. government agency securities and highly rated corporate debt securities. As of December 31, 2024, these instruments had a weighted average remaining maturity of 7 months. As of December 31, 2024, a hypothetical 1% increase or decrease in interest rates would have resulted in a $2.8 million fluctuation of annual interest income in our investment portfolio. We do not believe that the results of operations or cash flows would be affected to any significant degree by a sudden change in market interest rates relative to our investment portfolio.
Foreign Currency Exchange Rate Risk
The Company’s potential exposure to foreign currency exchange rate risk consists primarily of fixed facility payments due under our manufacturing services agreement with Lonza Ltd. for the use of the Monoplant. The Monoplant agreement is denominated in Swiss Francs. As of December 31, 2024, we recognized a $72.5 million finance lease liability on our consolidated balance sheet related to the Monoplant. A hypothetical 10% increase or decrease in the Swiss Franc compared to the U.S. dollar would have a $7.5 million fluctuation in the valuation of the lease liability. As of December 31, 2024, there were no other foreign currency fluctuations that would have had a material impact on our financial condition or results of operations.
Credit Risk
Financial instruments that potentially expose the Company to credit risk consist of cash, cash equivalents, investments and accounts receivable.
The Company maintains cash balances with a limited number of highly reputable financial institutions in excess of amounts insured by the Federal Deposit Insurance Corporation and Canada Deposit Insurance Corporation. Our investment policy limits the investment of excess cash to certain types of instruments such as certificates of deposit, money market instruments, U.S. treasury securities, U.S. government agency securities and highly rated corporate debt securities, and places restrictions on maturities and concentrations by asset class and issuer. To date, the Company has not experienced any losses associated with credit risk and continues to believe that this exposure is not significant.
The Company’s major customers, which include two specialty pharmacies and our collaboration and license partner, Otsuka, accounted for the majority of our accounts receivable as of December 31, 2024. Net product sales from the two specialty pharmacies represent 88% of total revenues for the year ended December 31, 2024, compared to 91% for the same period in 2023. We monitor economic conditions and the creditworthiness of our customers. We regularly communicate with our customers regarding the status of receivable balances and have not experienced any issues with collectability. The timing between the recognition of revenue and the receipt of payment is not significant. Our standard credit terms range from 30 to 45 days. The Company has had no historical write-offs related to our customers or receivables.
ITEM 8. Financial Statements and Supplementary Data
The information required by this Item 8 is contained on pages F-1 through F-27 of this report and is incorporated herein by reference.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and principal financial officer, evaluated the effectiveness of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, as of the end of the period covered by this Annual Report on Form 10-K. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate, to allow timely decisions regarding required disclosure.
Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of December 31, 2024, our principal executive officer and principal financial officer concluded that, as of such date, our disclosure controls and procedures were effective.
Management’s Annual Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) under the Exchange Act. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with generally accepted accounting principles.
Management has assessed the effectiveness of our internal control over financial reporting based on the framework set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework (2013 framework). Based on our evaluation, management has concluded that our internal control over financial reporting was effective as of December 31, 2024.
The effectiveness of our internal control over financial reporting has been audited by PricewaterhouseCoopers LLP an independent registered public accounting firm, as stated in their attestation report herein, which appears in the "Index to Consolidated Financial Statements" in Part IV.
Inherent Limitations of Internal Controls
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risks that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Changes in Internal Control over Financial Reporting
We regularly review our system of internal control over financial reporting and make changes to our processes and systems to improve controls and increase efficiency, while ensuring that we maintain an effective internal control environment. Changes may include such activities as implementing new, more efficient systems, consolidating activities, and migrating processes.
During the quarter ended December 31, 2024, there were no changes in our internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
During the quarter ended December 31, 2024, no directors or Section 16 officers adopted or terminated any “Rule 10b5-1 trading arrangement” or any “non-Rule 10b5-1 trading arrangement,” as each term is defined in Item 408(a) of Regulation S-K.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
None.
PART III
Item 10. Directors, Executive Officers, and Corporate Governance
The information required by this Item and not set forth below will be set forth in the section headed “—Election of Directors” and “Information Regarding the Board of Directors and Corporate Governance” in our definitive Proxy Statement for our 2025 Annual Meeting of Shareholders (or amended Annual Report on Form 10-K) to be filed within 120 days of the end of our fiscal year ended December 31, 2024 (our “Future Filing”) and is incorporated in this Annual Report by reference.
We have adopted a Corporate Code of Ethics and Conduct for directors, officers (including our principal executive officer, and principal financial officer and principal accounting officer) and employees. The Corporate Code of Ethics and Conduct is available on our website at http://www.auriniapharma.com under the Corporate Governance section of our Investors page. We will promptly disclose on our website future amendment of certain provisions of the Corporate Code of Ethics and Conduct and waivers of Corporate Code of Ethics and Conduct. Shareholders may request a free copy of the Corporate Code of Ethics and Conduct from c/o Aurinia Pharmaceuticals Inc., #140, 14315 - 118 Avenue Edmonton, Alberta T5L 4S6, Attn: Corporate Secretary.
Item 11. Executive Compensation
The information required by this Item will be set forth in the section headed “Executive Compensation” in our Future Filing and is incorporated in this Annual Report by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters
The information required by this Item will be set forth in the section headed “Security Ownership of Certain Beneficial Owners and Management” in our Future Filing and is incorporated in this Annual Report by reference.
Information regarding our equity compensation plans will be set forth in the section headed “Executive Compensation” in our Future Filing and is incorporated in this Annual Report by reference.
Item 13. Certain Relationships and Related Transactions and Director Independence
The information required by this Item will be set forth in the section headed “Transactions With Related Persons” in our Future Filing and is incorporated in this Annual Report by reference.
Item 14. Principal Accountant Fees and Services
The information required by this Item will be set forth in the section headed “Appointment of Selection of Independent Registered Public Accounting Firm” in our Future Filing and is incorporated in this Annual Report by reference.
PART IV
Item 15. Exhibits and Financial Statement Schedules
a.We have filed the following documents as part of this Annual Report:
1.Consolidated Financial Statements
The following financial statements are filed as part of this report:
Our consolidated financial statements are listed under Part II, Item 8. "Index to Consolidated Financial Statements" in this Annual Report.
2.Financial Statement Schedules
All financial statement schedules have been omitted because they are not applicable, not material or the required information is shown under Part II, Item 8. “Index to Consolidated Financial Statements” in this Annual Report.
3.Exhibits
The following exhibits, as required by Item 601 of Regulation S-K, which are incorporated herein by reference, are filed or furnished with this Annual Report, in each case as indicated therein.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Incorporation by Reference |
Exhibit
Number | | Description | | Form | | SEC File No. | | Exhibit | | Filing Date |
| | | | | | | | | | | |
| 3.1 | | | | 10-K | | 001-36421 | | 3.1 | | 02/24/21 |
| | | | | | | | | | | |
| 3.2 | | | | 8-K | | 001-36421 | | 3.1 | | 09/12/2024 |
| | | | | | | | | | | |
| 4.1 | | | | 10-K | | 001-36421 | | 4.1 | | 02/24/21 |
| | | | | | | | | | | |
| 4.2 | | Reference is made to Exhibits 3.1 and 3.2 | | | | | | | | |
| | | | | | | | | | | |
| 4.3 | | | | 10-K | | 001-36421 | | 4.3 | | 02/24/21 |
| | | | | | | | | | | |
10.1+ | | | | 10-K | | 001-36421 | | 10.1 | | 02/24/21 |
| | | | | | | | | | |
10.2+ | | | | S-8 | | 333-216447 | | 99.2 | | 03/03/2017 |
| | | | | | | | | | |
10.3+ | | | | S-8 | | 333-257424 | | 10.1 | | 06/25/21 |
| | | | | | | | | | |
10.4+ | | | | S-8 | | 333-257424 | | 10.2 | | 06/25/21 |
| | | | | | | | | | |
| 10.5 | | | | 6-K | | 001-36421 | | 99.2 | | 12/30/20 |
10.6# | | | | 10-K | | 001-36421 | | 10.5 | | 02/24/21 |
| | | | | | | | | | | |
10.8# | | | | 10-K | | 001-36421 | | 10.6 | | 02/24/21 |
| | | | | | | | | | | |
10.10# | | | | 10-K | | 001-36421 | | 10.9 | | 02/24/21 |
| | | | | | | | | | |
10.12+# | | | | 10-K | | 001-36421 | | 10.11 | | 02/24/21 |
| | | | | | | | | | |
10.13+# | | | | 10-K | | 001-36421 | | 10.13 | | 02/24/21 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
10.15+# | | | | 10-K | | 001-36421 | | 10.16 | | 02/24/21 |
| | | | | | | | | | |
10.16+# | | | | 10-K | | 001-36421 | | 10.17 | | 02/24/21 |
| | | | | | | | | | |
10.17+ | | | | 10-K | | 001-36421 | | 10.20 | | 02/24/21 |
| | | | | | | | | | |
| 10.18 | | | | 10-Q | | 001-36421 | | 3.4 | | 05/02/24 |
| | | | | | | | | | |
10.20+# | | | | 10-K | | 001-36421 | | 10.20 | | 02/28/23 |
| | | | | | | | | | |
10.21+# | | | | 10-K | | 001-36421 | | 10.21 | | 02/28/23 |
| | | | | | | | | | |
| 19.1* | | | | | | | | | | |
| | | | | | | | | | |
| 21.1* | | | | | | | | | | |
| | | | | | | | | | |
| 23.1* | | | | | | | | | | |
| | | | | | | | | | |
| 24.1* | | Power of Attorney (contained in signature page of this report) | | | | | | | | |
| | | | | | | | | | |
| 31.1* | | | | | | | | | | |
| | | | | | | | | | |
| 31.2* | | | | | | | | | | |
| | | | | | | | | | |
| 32.1** | | | | | | | | | | |
| | | | | | | | | | |
| 32.2** | | | | | | | | | | |
| | | | | | | | | | |
97.1+ | | | | 10-K | | 001-36421 | | 97.1 | | 02/15/24 |
| | | | | | | | | | |
| 101.INS* | | Inline XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document | | | | | | | | |
| | | | | | | | | | |
| 101.SCH* | | Inline XBRL Taxonomy Extension Schema Document | | | | | | | | |
| | | | | | | | | | |
| 101.CAL* | | Inline XBRL Taxonomy Extension Calculation Linkbase Document | | | | | | | | |
| | | | | | | | | | |
| 101.DEF* | | Inline XBRL Taxonomy Extension Definition Linkbase Document | | | | | | | | |
| | | | | | | | | | |
| 101.LAB* | | Inline XBRL Taxonomy Extension Label Linkbase Document | | | | | | | | |
| | | | | | | | | | |
| 101.PRE* | | Inline XBRL Taxonomy Extension Presentation Linkbase Document | | | | | | | | |
| | | | | | | | | | |
| 104 | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | | | | | | | | |
| | | | | | | | | | |
| * | | Filed herewith. | | | | | | | | |
| ** | | Furnished herewith. Exhibit 32.1 and Exhibit 32.2 are being furnished and shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liability of that section, nor shall such exhibit be deemed to be incorporated by reference in any registration statement or other document filed under the Securities Act of 1933, as amended, or the Exchange Act, except as otherwise specifically stated in such filing. |
| + | | Indicates a management contract or compensatory plan. | | | | | | | | |
| # | | Certain portions of this exhibit (indicated by asterisks) have been omitted pursuant to Item 601(b)(10)(iv) of Regulation S-K because they are not material and are the type that Aurinia treats as private or confidential. |
| | | | | | | | | | |
Item 16. Form 10-K Summary
None.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | |
| | AURINIA PHARMACEUTICALS INC. |
| | |
| February 26, 2025 | By: | /s/ Peter Greenleaf |
| | | Peter Greenleaf |
| | | Chief Executive Officer |
| | (Principal Executive Officer) |
SIGNATURES AND POWER OF ATTORNEY
We, the undersigned directors and officers of Aurinia Pharmaceuticals Inc., hereby severally constitute and appoint Peter Greenleaf and Joseph Miller, and each of them singly, our true and lawful attorneys, with full power to them, and to each of them singly, to sign for us and in our names in the capacities indicated below, any and all amendments to this Annual Report on Form 10-K, and to file or cause to be filed the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as each of us might or could do in person, and hereby ratifying and confirming all that said attorneys, and each of them, or their substitute or substitutes, shall do or cause to be done by virtue of this Power of Attorney.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | | | | | | | | | | |
| Name | | Title | | Date |
| | | | |
| /s/ Peter Greenleaf | | President, Chief Executive Officer, Director | | February 26, 2025 |
| Peter Greenleaf | | (Principal Executive Officer) | | |
| | | | |
| /s/ Joseph Miller | | Chief Financial Officer | | February 26, 2025 |
| Joseph Miller | | (Principal Financial and Accounting Officer) | | |
| | | | |
| /s/ Kevin Tang | | Chair of the Board | | February 26, 2025 |
| Kevin Tang | | | | |
| | | | |
| /s/ Jeffrey A. Bailey | | Director | | February 26, 2025 |
| Jeffrey A. Bailey | | | | |
| | | | |
| /s/ David R.W. Jayne | | Director | | February 26, 2025 |
| David R.W. Jayne, M.D., FRCP, FRCPE, FMedSci | | | | |
| | | | |
| /s/ Craig Johnson | | Director | | February 26, 2025 |
| Craig Johnson | | | | |
| | | | |
| /s/ Jill Leversage | | Director | | February 26, 2025 |
| Jill Leversage | | | | |
| | | | |
| /s/ Karen Smith | | Director | | February 26, 2025 |
| Karen Smith | | | | |
/s/ PricewaterhouseCoopers LLP
Chartered Professional Accountants, Licensed Public Accountants
Toronto, Canada
February 26, 2025
We have served as the Company's auditor since at least 1997. We have not been able to determine the specific year we began serving as auditor of the Company.
We have served as the Company's auditor since at least 1997. We have not been able to determine the specific year we began serving as auditor of the Company.
| | |
AURINIA PHARMACEUTICALS INC. AND SUBSIDIARIES CONSOLIDATED BALANCE SHEETS (in thousands) |
| | | | | | | | | | | | | | |
| | As of December 31, |
| | 2024 | | 2023 |
| ASSETS | | | | |
| Current assets: | | | | |
| Cash, cash equivalents and restricted cash | | $ | 83,433 | | | $ | 48,875 | |
| Short-term investments | | 275,043 | | | 301,614 | |
| Accounts receivable, net | | 36,544 | | | 24,089 | |
| Inventory, net | | 39,228 | | | 39,705 | |
| Prepaid expenses and deposits | | 11,219 | | | 9,486 | |
| Other current assets | | 1,129 | | | 1,031 | |
| Total current assets | | 446,596 | | | 424,800 | |
| Finance right-of-use lease assets | | 92,072 | | | 108,715 | |
| Intangible assets, net | | 4,355 | | | 4,977 | |
| Operating right-of-use lease assets | | 4,068 | | | 4,498 | |
| Property and equipment, net | | 2,731 | | | 3,354 | |
| Long-term investments | | — | | | 201 | |
| Other noncurrent assets | | 823 | | | 1,517 | |
| Total assets | | $ | 550,645 | | | $ | 548,062 | |
| | | | |
| LIABILITIES AND SHAREHOLDERS' EQUITY | | | | |
| Current liabilities: | | | | |
| Accounts payable | | $ | 5,187 | | | $ | 4,327 | |
| Accrued expenses | | 64,971 | | | 50,062 | |
| Finance lease liabilities, current portion | | 14,046 | | | 14,609 | |
| Deferred revenue | | 11,002 | | | 4,813 | |
| Operating lease liabilities, current portion | | 1,026 | | | 989 | |
| Other current liabilities (of which nil and $0.8 million in 2024 and in 2023 is due to a related party, respectively) | | 1,531 | | | 2,388 | |
| Total current liabilities | | 97,763 | | | 77,188 | |
| Finance lease liabilities, less current portion | | 58,554 | | | 75,479 | |
| Deferred compensation and other noncurrent liabilities (of which nil and $7.6 million in 2024 and in 2023 is due to a related party, respectively) | | 11,107 | | | 10,911 | |
| Operating lease liabilities, less current portion | | 5,743 | | | 6,530 | |
| Total liabilities | | 173,167 | | | 170,108 | |
| Commitments and Contingencies (Note 5) | | | | |
| Shareholders' equity | | | | |
| Common shares - no par value, unlimited shares authorized, 140,883 and 143,833 shares issued and outstanding at December 31, 2024 and 2023, respectively | | 1,187,696 | | | 1,200,218 | |
| Additional paid-in capital | | 126,999 | | | 120,788 | |
| Accumulated other comprehensive loss | | (647) | | | (730) | |
| Accumulated deficit | | (936,570) | | | (942,322) | |
| Total shareholders' equity | | 377,478 | | | 377,954 | |
| Total liabilities and shareholders' equity | | $ | 550,645 | | | $ | 548,062 | |
See accompanying notes.
| | |
AURINIA PHARMACEUTICALS INC. AND SUBSIDIARIES CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME (LOSS) (in thousands, except per share data) |
| | | | | | | | | | | | | | | | | | | | |
| | Years ended December 31, |
| | 2024 | | 2023 | | 2022 |
| Revenue | | | | | | |
| Net product sales | | $ | 216,186 | | | $ | 158,533 | | | $ | 103,468 | |
| License, collaboration and royalty revenue | | 18,947 | | | 16,980 | | | 30,562 | |
| Total revenue | | 235,133 | | | 175,513 | | | 134,030 | |
| Operating expenses | | | | | | |
| Cost of revenue | | 28,248 | | | 14,148 | | | 5,664 | |
| Selling, general and administrative | | 172,028 | | | 195,036 | | | 196,371 | |
| Research and development | | 20,785 | | | 49,641 | | | 44,988 | |
| Restructuring | | 23,106 | | | — | | | — | |
| Other (income) expense, net | | (4,347) | | | 8,379 | | | (1,523) | |
| Total operating expenses | | 239,820 | | | 267,204 | | | 245,500 | |
| Loss from operations | | (4,687) | | | (91,691) | | | (111,470) | |
| Interest income | | 16,970 | | | 16,997 | | | 5,118 | |
| Interest expense | | (4,835) | | | (2,775) | | | — | |
| Net income (loss) before income taxes | | 7,448 | | | (77,469) | | | (106,352) | |
| Income tax expense | | 1,696 | | | 551 | | | 1,828 | |
| Net income (loss) | | 5,752 | | | (78,020) | | | (108,180) | |
| Other comprehensive gain (loss): | | | | | | |
| Unrealized gain (loss) on available-for-sale securities | | 83 | | | 331 | | | (209) | |
| Comprehensive income (loss) | | $ | 5,835 | | | $ | (77,689) | | | $ | (108,389) | |
| | | | | | |
| Earnings (loss) per share | | | | | | |
| Basic | | $ | 0.04 | | | $ | (0.54) | | | $ | (0.76) | |
| Diluted | | $ | 0.04 | | | $ | (0.54) | | | $ | (0.76) | |
| | | | | | |
| Shares used in computing earnings (loss) per share | | | | | | |
| Basic | | 143,057 | | 143,236 | | | 141,915 | |
| Diluted | | 146,194 | | 143,236 | | 141,915 | |
See accompanying notes.
| | |
AURINIA PHARMACEUTICALS INC. AND SUBSIDIARIES CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY (in thousands) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common Shares | | Additional Paid-In Capital | | Accumulated Other Comprehensive Loss | | Accumulated Deficit | | Total Shareholders' Equity |
| | Shares | | Amount | | | | |
| Balance at December 31, 2021 | | 141,600 | | | $ | 1,177,051 | | | $ | 59,014 | | | $ | (852) | | | $ | (756,122) | | | $ | 479,091 | |
| Issuance of common shares from exercise of stock options and vesting of restricted stock units | | 383 | | | 5,064 | | | (4,543) | | | — | | | — | | | 521 | |
| Issuance of common shares under ESPP | | 285 | | | 3,194 | | | (1,282) | | | — | | | — | | | 1,912 | |
| Share-based compensation | | — | | | — | | | 32,300 | | | — | | | — | | | 32,300 | |
| Unrealized loss on available-for-sale securities | | — | | | — | | | — | | | (209) | | | — | | | (209) | |
| Net loss | | — | | | — | | | — | | | — | | | (108,180) | | | (108,180) | |
| Balance at December 31, 2022 | | 142,268 | | | $ | 1,185,309 | | | $ | 85,489 | | | $ | (1,061) | | | $ | (864,302) | | | $ | 405,435 | |
| Issuance of common shares from exercise of stock options and vesting of restricted stock units | | 1,146 | | | 11,256 | | | (8,209) | | | — | | | — | | | 3,047 | |
| Issuance of common shares under ESPP | | 419 | | | 3,653 | | | (1,803) | | | — | | | — | | | 1,850 | |
| Share-based compensation | | — | | | — | | | 45,311 | | | — | | | — | | | 45,311 | |
| Unrealized gain on available-for-sale securities | | — | | | — | | | — | | | 331 | | | — | | | 331 | |
| Net loss | | — | | | — | | | — | | | — | | | (78,020) | | | (78,020) | |
| Balance at December 31, 2023 | | 143,833 | | | $ | 1,200,218 | | | $ | 120,788 | | | $ | (730) | | | $ | (942,322) | | | $ | 377,954 | |
| Purchases of common shares under Share Repurchase Plan | | (6,053) | | | (41,043) | | | — | | | — | | | — | | | (41,043) | |
| Issuance of common shares from exercise of stock options and vesting of restricted stock units | | 2,867 | | | 26,769 | | | (24,717) | | | — | | | — | | | 2,052 | |
| Issuance of common shares under ESPP | | 236 | | | 1,752 | | | (668) | | | — | | | — | | | 1,084 | |
| Share-based compensation | | — | | | — | | | 31,596 | | | — | | | — | | | 31,596 | |
| Unrealized gain on available-for-sale securities | | — | | | — | | | — | | | 83 | | | — | | | 83 | |
| Net income | | — | | | — | | | — | | | — | | | 5,752 | | | 5,752 | |
| Balance at December 31, 2024 | | 140,883 | | | $ | 1,187,696 | | | $ | 126,999 | | | $ | (647) | | | $ | (936,570) | | | $ | 377,478 | |
See accompanying notes.
| | |
AURINIA PHARMACEUTICALS INC. AND SUBSIDIARIES CONSOLIDATED STATEMENTS OF CASH FLOWS (in thousands) |
| | | | | | | | | | | | | | | | | | | | |
| | Years ended December 31, |
| | 2024 | | 2023 | | 2022 |
| Cash flows from operating activities: | | | | | | |
| Net income (loss) | | $ | 5,752 | | | $ | (78,020) | | | $ | (108,180) | |
| Adjustments to reconcile consolidated net income (loss) to net cash provided by (used in) operating activities: | | | | | | |
| Share-based compensation | | 31,596 | | | 45,311 | | | 32,300 | |
| Amortization and depreciation | | 19,445 | | | 11,647 | | | 2,706 | |
| Foreign exchange (gain) loss on revaluation of finance lease liability (Monoplant) | | (5,910) | | | 5,949 | | | — | |
| Net amortization of premiums and discounts on investments | | (12,731) | | | (12,141) | | | (1,572) | |
| Non-cash write-down of inventory | | — | | | 916 | | | 3,646 | |
| Other, net | | 788 | | | (1,515) | | | (1,612) | |
| Net changes in operating assets and liabilities: | | | | | | |
| Accounts receivable, net | | (12,455) | | | (10,606) | | | 1,927 | |
| Inventory, net | | 477 | | | (15,869) | | | (9,072) | |
| Prepaid expenses and other current assets | | (1,834) | | | 4,399 | | | (2,404) | |
| Other noncurrent operating assets | | 31 | | | (16) | | | (363) | |
| Accounts payable | | 860 | | | 1,240 | | | (792) | |
| Accrued expenses and other liabilities | | 13,330 | | | 12,154 | | | 1,491 | |
| Deferred revenue | | 5,789 | | | 3,763 | | | 3,048 | |
| Operating lease liabilities | | (750) | | | (673) | | | (652) | |
| Net cash provided by (used in) operating activities | | 44,388 | | | (33,461) | | | (79,529) | |
| Cash flows from investing activities: | | | | | | |
| Proceeds from the sale and maturities of investments | | 585,418 | | | 529,376 | | | 464,316 | |
| Purchases of investments | | (545,832) | | | (523,500) | | | (523,993) | |
| Upfront lease payments | | (43) | | | (11,864) | | | (663) | |
| Purchases of property, equipment and intangible assets | | (281) | | | (718) | | | (292) | |
| Net cash provided by (used in) investing activities | | 39,262 | | | (6,706) | | | (60,632) | |
| Cash flows from financing activities: | | | | | | |
| Repurchase of common shares | | (40,239) | | | — | | | — | |
| Principal portion of finance lease payments | | (11,989) | | | (10,025) | | | — | |
| Proceeds from issuance of common shares from exercise of stock options and vesting of RSUs | | 8,186 | | | 5,324 | | | 1,561 | |
| Proceeds from issuance of common shares under ESPP | | 1,084 | | | 1,850 | | | 1,912 | |
| Taxes paid related to net settlement of exercises of stock options and vesting of RSUs | | (6,134) | | | (2,279) | | | (1,040) | |
| Net cash (used in) provided by financing activities | | (49,092) | | | (5,130) | | | 2,433 | |
| Net increase (decrease) in cash, cash equivalents and restricted cash | | 34,558 | | | (45,297) | | | (137,728) | |
| Cash, cash equivalents and restricted cash, beginning of the period | | 48,875 | | | 94,172 | | | 231,900 | |
| Cash, cash equivalents and restricted cash, end of the period | | $ | 83,433 | | | $ | 48,875 | | | $ | 94,172 | |
| Supplemental cash flow information: | | | | | | |
| Finance lease liability arising from obtaining right-of-use assets | | $ | — | | | $ | 94,140 | | | $ | — | |
| Cash paid for taxes | | $ | (2,184) | | | $ | (496) | | | $ | (1,979) | |
| Reconciliation of cash, cash equivalents and restricted cash to the consolidated balance sheets | | | | | | |
| Cash and cash equivalents | | $ | 83,396 | | | $ | 48,755 | | | $ | 94,088 | |
| Restricted cash | | 37 | | | 120 | | | 84 | |
| Total cash, cash equivalents and restricted cash | | $ | 83,433 | | | $ | 48,875 | | | $ | 94,172 | |
See accompanying notes.
| | |
AURINIA PHARMACEUTICALS INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS |
1. Organization and Description of Business
Aurinia is a biopharmaceutical company focused on delivering therapies to people living with autoimmune diseases with high unmet medical needs. In January 2021, the Company introduced LUPKYNIS® (voclosporin), the first FDA-approved oral therapy for the treatment of adult patients with active lupus nephritis (“LN”). Aurinia is also developing AUR200, a dual inhibitor of B cell activating factor (BAFF) and a proliferation inducing ligand (APRIL) for the potential treatment of autoimmune diseases.
2. Basis of Presentation and Summary of Significant Accounting Policies
Basis of Presentation, Principles of Consolidation and Use of Estimates
The Company’s consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the U.S. (“U.S. GAAP”) and include the accounts of the Company and its wholly owned subsidiaries, Aurinia Pharma U.S., Inc., a Delaware corporation, and Aurinia Pharma Limited, a U.K. corporation. During 2024, Aurinia Pharma Limited was dissolved. All inter-company transactions and balances have been eliminated in consolidation. The preparation of the Company’s consolidated financial statements requires management to make estimates and assumptions that impact the reported amounts of assets, liabilities, revenue and expenses and the disclosure of contingent assets and liabilities in its consolidated financial statements and the accompanying notes. Actual results may differ materially from these estimates.
Certain prior year amounts and notes to the consolidated financial statements have been reclassified to conform with the current year presentation. Taxes paid related to the net settlement of exercises of stock options and vesting of RSUs, which was previously included in proceeds from issuance of share-based awards on the consolidated statements of cash flows, have been separately presented for the years ended December 31, 2023 and 2022. Such reclassifications did not affect net income (loss), shareholders’ equity or cash flows.
Summary of Significant Accounting Policies
Cash, Cash Equivalents and Restricted Cash
Cash and cash equivalents consist of operating accounts, money market funds and money market accounts. The Company considers all highly liquid investments with an original maturity of three months or less when purchased to be cash equivalents. Income generated from cash and cash equivalents is recorded as interest income. Cash is classified as restricted cash when certain funds are reserved for a specific purpose and are not available for immediate or general business use.
Investments
The Company invests its cash reserves in short-term, fixed rate, highly liquid financial instruments such as certificates of deposits and investments in U.S. treasury securities, U.S. government agency securities and highly rated corporate debt securities. The Company classifies its debt securities as available-for-sale in accordance with the Financial Accounting Standards Board (the “FASB”) Accounting Standards Codification (“ASC”) Topic 320, Investments—Debt Securities. Investments classified as available-for-sale investments are carried at fair value. Unrealized gain (loss) is recorded in other comprehensive loss. Realized gain (loss) is recorded in interest income. The cost of securities sold is based on the specific-identification method. Interest income is accrued when earned and the amortization of premiums and accretion of discounts to maturity arising from acquisition is included in interest income on the consolidated statements of operations and comprehensive loss.
Fair Value Measurements
The Company's financial instruments consist primarily of cash and cash equivalents, investments, accounts receivable, accounts payable and accrued liabilities. The carrying value of accounts receivable, accounts payable and accrued expenses approximate their fair values because of their short-term nature. Estimated fair values of available-for-sale debt securities are generally based on prices obtained from commercial pricing services.
FASB ASC Topic 820-10, Fair Value Measurements and Disclosures (“ASC 820-10”), defines fair value, establishes a consistent framework for measuring fair value and expands disclosure for each major asset and liability category measured at fair value on either a recurring or non-recurring basis. Fair value is defined as an exit price, representing the amount that would
be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, the accounting guidance establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
Level 1: Observable inputs such as quoted prices in active markets.
Level 2: Inputs, other than the quoted prices in active markets that are observable either directly or indirectly.
Level 3: Unobservable inputs for which there are little or no market data, which require the reporting entity to develop its own assumptions.
See Note 4 for a summary of financial assets measured at fair value on a recurring basis.
Accounts Receivable, Net
Accounts receivables are stated as amounts due, net of estimates for discounts offered in customer contracts and any expected credit losses. The Company estimates expected credit losses using the “expected loss” model, which is based on an assessment of the collectability of customer accounts, including collection history, credit quality, the age of past-due balances, current conditions, and reasonable and supportable future conditions that might impact a customer’s ability to pay. The allowance for credit losses is periodically analyzed and adjusted as needed through a charge to selling, general and administrative expense. Amounts deemed to be uncollectible are charged against the allowance for credit losses. The Company does not assess whether a contract has a significant financing component if the expectation at contract inception is such that the period between the transfer of the promised good to the customer and receipt of payment will be one year or less. As of December 31, 2024 and 2023, the Company did not record an allowance for credit losses.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash, cash equivalents, investments and accounts receivable.
The Company maintains cash balances with a limited number of highly reputable financial institutions in excess of amounts insured by the Federal Deposit Insurance Corporation and Canada Deposit Insurance Corporation. The Company's investment policy limits the investment of excess cash to certain types of financial instruments such as certificates of deposit, money market instruments, U.S. treasury securities, U.S. government agency securities and highly rated corporate debt securities, and places restrictions on maturities and concentration by type and issuer. To date, the Company has not experienced any losses associated with credit risk and continues to believe that this exposure is not significant.
The Company’s major customers, which includes two specialty pharmacies and its collaboration partner, Otsuka Pharmaceutical Co., Ltd (“Otsuka”) accounted for the majority of the Company's accounts receivable as of December 31, 2024. Net product sales from the two specialty pharmacies represent 88% of total revenues for the year ended December 31, 2024, compared to 91% for the same period in 2023. To avoid supply chain disruptions, the Company has provided a nominal additional discount to its two specialty pharmacies since March 2022. The Company monitors economic conditions and the creditworthiness of its customers. The Company regularly communicates with its customers regarding the status of receivable balances and has not experienced any issues with collectability. The timing between the recognition of revenue and the receipt of payment is not significant. The Company's standard credit terms range from 30 to 45 days. The Company has had no historical write-offs related to its customers or receivables.
Inventory, Net
Inventory is valued under a standard costing methodology on a first-in, first-out basis and is stated at the lower of cost or net realizable value. The Company determines whether to capitalize inventory costs for a product based on, among other factors, the status of regulatory approval and recoverability of costs. Capitalized costs of inventory mainly include third party manufacturing costs and allocated internal labor. Storage, transportation and insurance costs are immaterial and expensed in the period incurred. The Company assesses recoverability of inventory each reporting period and writes down inventory when inventory has become obsolete, has a cost basis in excess of its net realizable value or quantities are in excess of expected product sales.
Intangible Assets, Net
Intangible assets are amortized on a straight-line basis over the estimated useful life of the related assets. The Company evaluates the estimated remaining useful life of its intangible assets and whether events or changes in circumstances warrant a revision to the remaining period of amortization.
Acquired intellectual property or a reacquired right is initially recorded at cost. If the terms of the contract giving rise to a reacquired right are favorable relative to the terms of current market transactions for the same or similar items, the difference is recognized as a gain in the consolidated statements of operations and comprehensive loss. Acquired intellectual property and reacquired rights are amortized on a straight-line basis over periods ranging from 10 to 12 years.
External patent costs associated with preparing, filing, obtaining and protecting patents are capitalized and amortized on a straight-line basis over the shorter of the estimated useful life or the patent life, commencing in the year of patent grant. Patents do not contain the option to extend or renew. External legal costs incurred to defend a patent are capitalized when it is believed that the future economic benefit of the patent will be increased and a successful defense is probable.
Property and Equipment, Net
Property and equipment is stated at cost, less accumulated depreciation and amortization. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. Amortization of leasehold improvements is computed using the straight-line method over the shorter of the lease term or the estimated useful life of the related assets. Maintenance and repairs are charged to expense as incurred; however, maintenance and repairs that improve or extend the life of existing assets are capitalized. When assets are sold, or otherwise disposed of, the cost and related accumulated depreciation are removed from the accounts, and any gain or loss is included in other (income) expense, net in the year of sale or disposal.
Recoverability and Impairment of Long-lived Assets
ASC Topic 360 requires long-lived assets, including definite-lived intangible assets, to be evaluated for impairment at least annually or when events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. If such circumstances are determined to exist, an estimate of undiscounted future cash flows produced by the asset, including its eventual residual value, is compared to the carrying value to determine whether impairment exists. If such cash flows are not expected to be sufficient to recover the carrying amount of the assets, the assets are written down to their estimated fair values. Fair value is estimated through discounted cash flow models to project cash flows from the asset. For the years ended December 31, 2024, 2023, and 2022, the Company recognized no asset impairment charge.
Leases
The Company assesses all contracts at inception to determine whether a lease exists. The Company’s leases are classified either as operating or finance leases per ASC 842. The Company leases office space under operating leases that typically provide for the payment of minimum annual rentals and may include scheduled rent increases. The Company also entered into a manufacturing agreement that contained an embedded lease of a dedicated manufacturing facility (the “Monoplant”) that was accounted for as a finance lease when the lease commencement began (see Note 5).
Leases with an initial term of 12 months or less are not recorded on the Company's consolidated balance sheets and those lease payments are recognized on a straight-line basis over the lease term in its consolidated statements of operations and comprehensive loss. For leases other than short-term leases, at lease commencement, the Company records a lease liability based on the present value of lease payments over the expected lease term. The Company calculates the present value of lease payments using the discount rate implicit in the lease, unless that rate cannot be readily determined. In that case, the Company uses its incremental borrowing rate, which is the rate of interest that the Company would have to pay to borrow on a collateralized basis an amount equal to the lease payments over the expected lease term. The Company records a corresponding right-of-use lease asset based on the lease liability, adjusted for any lease incentives received and any initial direct costs paid to the lessor prior to the lease commencement date. The Company elected to use the practical expedient that allows lessees to treat the lease and non-lease components of leases as a single lease component.
After lease commencement, the Company measures its leases as follows: (i) the lease liability based on the present value of the remaining lease payments using the discount rate determined at lease commencement; and (ii) the right-of-use lease asset based on the remeasured lease liability, adjusted for any unamortized lease incentives received, any unamortized initial direct costs
and the cumulative difference between rent expense and amounts paid under the lease agreement. Any lease incentives received and any initial direct costs are amortized on a straight-line basis over the expected lease term. Rent expense is recorded on a straight-line basis over the expected lease term.
Deferred Compensation Arrangements
The Company records deferred compensation arrangements as liabilities for estimated future employee benefits relating to certain employee retention arrangements. Pursuant to FASB ASC Topic 710, Compensation—General, the Company recognizes future benefits provided by employee retention arrangements, as deferred compensation, which is recognized when the Company determines that it is probable to make future payments. The present value of the deferred compensation liability is measured based on an income approach using an internal risk-adjusted net present value of the future payments to be made to participants and is based on the estimated future net revenue of voclosporin. Management uses significant judgement and estimates in determining the method, assumptions and inputs that are unobservable and inherently uncertain. Significant judgements and estimates include, but are not limited to, assumptions related to future net revenues of voclosporin and the risk-adjusted discount rate. The present value of the deferred compensation liability is remeasured at each balance sheet date and adjusted for changes in estimated cash flows. The impact of the remeasurement is recorded through gain (loss) on change in present value of the deferred compensation arrangements in other (income) expense, net.
Common Shares
The Company’s shares have no par value and, therefore, all amounts related to the issuance of common shares are recorded to common shares on the balance sheet. The value of common shares includes cash amounts received or paid for such shares and the fair value of equity awards and warrants. Amounts for common shares are offset by share issuance costs for equity offerings or transaction costs associated with share repurchases.
Revenue Recognition
Pursuant to FASB ASC Topic 606, Revenue from Contracts with Customers (“ASC 606”), the Company recognizes revenue when a customer obtains control of promised goods or services. Revenue is recognized in an amount that reflects the consideration that the Company expects to receive in exchange for those goods or services. To determine revenue recognition for contracts with customers within the scope of ASC 606, the Company performs the following 5 steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) a performance obligation is satisfied.
Net Product Sales
The Company sells LUPKYNIS to two specialty pharmacies and a specialty distributor in the U.S., and the Company sells LUPKYNIS inventory to its collaboration partner, Otsuka for the European and Japanese market. The two specialty pharmacies, specialty distributor and Otsuka are considered the Company's customers for accounting purposes.
Revenue from product sales is recognized when the customer obtains control of the Company's product, which typically occurs on delivery. Revenue from product sales is recorded at the transaction price, net of estimates for variable consideration consisting of prompt-pay discounts, customer fees, government rebates, co-payment assistance and payor rebates and administration fees for which reserves are established. These reserves are based on estimates of the amounts earned or to be claimed on the related sales and are classified as reductions of accounts receivable (if the amount is payable to the customer) or a liability (if the amount is payable to a party other than the customer).
Variable consideration is estimated using the expected-value amount method, which is the sum of probability-weighted amounts in a range of possible consideration amounts. In making these estimates, the Company considers historical data, including patient mix and inventory sold to customers that has not yet been dispensed. Actual amounts of consideration ultimately received may differ from the Company’s estimates. If actual results vary materially from the Company’s estimates, the Company will adjust these estimates, which will affect net product sales and earnings in the period such estimates are adjusted. These items include:
•Prompt-Pay Discounts—The Company generally provides discounts on product sales to its customers for prompt payment. The Company estimates that its customers will earn these discounts and fees. These discounts and fees are recorded as a reduction of gross revenue and accounts receivable at the time such revenue is recognized.
•Customer Fees—The Company pays certain customer fees, such as fees for certain data that customers provide to the Company. Customer fees paid to its customers are recorded as a reduction of gross revenue and accounts receivable, unless the payment is: (i) for a distinct good or service from the customer; and (ii) the Company can reasonably estimate the fair value of the goods or services received. If both conditions are met, the Company records the consideration paid to the customer as selling, general and administrative expense.
•Government Rebates—The Company estimates government rebates, primarily Medicaid, based upon a range of possible outcomes for the estimated patient mix. Medicaid rebates relate to the Company's estimated obligations to states under reimbursement arrangements. Rebates are recorded as a reduction of gross revenue and a current liability is established and included in accrued expenses at the time such gross revenue is recognized. The amount of the rebate for each unit of product reimbursed by the state Medicaid program is established by law and is adjusted upward if the wholesale acquisition cost increases more than inflation (measured by the Consumer Price Index).The liability for Medicaid rebates consists of: (i) estimated current quarter claims; (ii) estimated prior quarter claims for which an invoice has not been received; (iii) prior quarter claims based on unpaid invoices received; and (iv) estimated claims for inventory in the distribution channel at period end.
•Co-payment Assistance—Co-payment assistance represents financial assistance to qualified patients, assisting them with prescription drug co-payments required by insurance. The program is administered by the specialty pharmacies on the Company's behalf. Co-payment assistance is recorded as a reduction of revenue and a current liability is established and included in accrued expenses at the time such revenue is recognized.
•Payor Rebates and Administration Fees—Payor rebates and administration fees represent the estimated obligations to third parties, primarily benefit managers. The payor rebates and administration fees result from formulary position, price increase limit allowances (price protection) and administration fees. The liability payor rebates and administration fees are based on the estimated payors buying patterns and the resulting applicable contractual rebate rate(s) to be earned over a contractual period. Payor rebates and administration fees are recorded as a reduction of revenue and a liability is established and included in accrued expenses at the time such revenue is recognized.
Additionally, the Company has agreements with its partners that include options related to the promise for future supply of drug substance, semi-finished goods or drug product for either clinical development or commercial supply at the licensee’s discretion. The Company assesses if these options provide a material right to the licensee and if so, they are accounted for as separate performance obligations. If the Company is entitled to additional payments when the licensee exercises these options, any additional payments are recorded as product sales when the licensee obtains control of the goods, which is typically upon delivery. Certain agreements include terms where the Company can partially bill for drug substance used before the manufacturing cycle is complete, resulting in a deferred revenue which is to be recognized once delivery occurs.
License, Collaboration and Royalty Revenue
The Company enters into out-license agreements with counterparties to develop and commercialize LUPKYNIS in certain ex-U.S. territories in exchange for: (i) upfront cash payments; (ii) regulatory and commercial milestone payments; (iii) sales-based royalties; and (iv) payments for manufacturing and other services the Company provides. Each of these payments results in license, collaboration and royalty revenue.
Licenses
If the license to the Company’s intellectual property is determined to be distinct from the other performance obligations identified in the arrangement, the Company recognizes revenues from non-refundable, upfront fees allocated to the license when the license is transferred to the licensee and the licensee is able to use and benefit from the license. For licenses that are bundled with other performance obligations, management uses judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue from non-refundable, upfront fees. The Company evaluates the measure of progress each reporting period and, if necessary, adjusts the measure of performance and related revenue recognition.
Milestones
At the inception of each arrangement that includes commercial sales milestone payments, the Company evaluates whether achieving each milestone payment is considered probable and estimates the amount to be included in the transaction price using the most likely amount method. If it is probable that a significant revenue reversal would not occur, the value of the associated milestone is included in the transaction price. The transaction price is then allocated to each performance obligation on a relative stand-alone selling price basis, for which the Company recognizes revenue as or when the performance obligations under the contract are satisfied. At the end of each subsequent reporting period, the Company re-evaluates the probability of achievement of such milestones and any related constraint, and if necessary, adjusts its estimate of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis, which would affect license, collaboration and royalty revenue in the period of adjustment. Sales-based milestone payments are recognized in the period that the milestone objectives have been achieved.
Royalties
For arrangements that include sales-based royalties, revenue is recognized when the underlying product sales have occurred. Revenue is recorded based on estimated quarterly net sales reports provided by its partner. Differences between actual results and estimated amounts are adjusted in the period in which they become known, which typically follows the quarterly period in which the estimate is made.
Manufacturing and Other Services
The Company’s agreements may include manufacturing or other services to be performed by the Company on behalf of the counterparty. If these services are determined to be distinct from the other promises or performance obligations identified in the arrangement, the Company recognizes the transaction price allocated to these services as revenue. The revenue is recognized either: (i) over time based on an appropriate measure of progress when the performance by the Company does not create an asset with an alternative use and the Company has an enforceable right to payment for the performance completed to date; (ii) or at a point in time as the related performance obligations are satisfied. Certain agreements may include terms where the Company can partially bill for manufacturing services before the serves are provided, resulting in a deferred revenue which is to be recognized once the performance obligation is satisfied.
Cost of Revenue
Cost of revenue consists primarily of the cost of inventory for LUPKYNIS and semi-finished product, which mainly includes third-party manufacturing costs and allocated internal labor. Cost of revenue also includes costs related to collaboration revenues and the amortization of the finance right-of-use asset recognized in connection with the Monoplant.
Selling, General and Administrative (“SG&A”) Expense
Selling, general and administrative (“SG&A”) expense consists of personnel and non-personnel expenses to support growing sales of LUPKYNIS. Personnel-related expense includes salaries, incentive pay, benefits and share-based compensation for personnel engaged in sales, finance and administrative functions. As the majority of the Company's contracts are short-term in nature, sales commissions are generally recorded as selling, general and administrative expense when incurred as the amortization period would have been less than one year. Non-personnel-related expense includes: (i) selling, patient services, pharmacovigilance, marketing, advertising, travel, sponsorships and trade shows; and (ii) other general and administrative costs, including consulting, legal, patent, insurance, accounting, information technology and facilities.
SG&A expenses are recognized as they are incurred. The Company uses a third-party logistics provider to perform a full order-to-cash service, which includes warehousing and shipping directly to its two specialty pharmacies and receiving orders from a specialty distributor for shipping to hospitals on their behalf. Since these costs are not integral to bringing the inventories to a salable condition, the Company elected not to treat shipping and handling costs as a fulfillment activity. Shipping and handling costs related to order fulfillment are recorded in SG&A expenses.
Research and Development (“R&D”) Expense
Research and development (“R&D”) expense consists of personnel and non-personnel expenses. Personnel-related expense includes salaries, incentive pay, benefits and share-based compensation for personnel engaged in research and development
functions. Non-personnel-related expense includes subcontractors and materials used for R&D activities, development, clinical trials, clinical supply and distribution, and other professional services.
R&D expenses are recognized as they are incurred based on actual work completed through monitoring invoices received and discussions with internal personnel and external service providers as to the progress or stage of completion of the clinical studies and the agreed-upon fee to be paid for such services. Where contingent milestone payments are due to third parties under R&D arrangements or license agreements, the milestone payment obligations are expensed when the milestone results are probable to be achieved.
Restructuring Expense
Restructuring expenses consist primarily of one-time termination benefits, including severance and healthcare benefits, contract terminations and other costs. According to ASC 420, Exit or Disposal Cost Obligations (“ASC 420”), restructuring expense is measured at fair value and recognized as a liability when incurred. One-time termination benefits are expensed on the date on which the Company notifies the affected employees of the restructuring plan, unless employees must provide future service, in which case the expense is recognized over the service period.
Shared-based Compensation Expense
The Company follows ASC Topic 718, Compensation - Stock Compensation (“ASC 718”), which requires the measurement and recognition of compensation expense, based on estimated fair values, for all share-based awards made to employees and directors. The Company records compensation expense based on the fair value on the grant date using the graded accelerated vesting method for all share-based payments related to stock options, performance awards (“PAs”), restricted stock units (“RSUs”) and purchases under the Company's 2021 Employee Stock Purchase Plan. The estimated fair value of PAs is measured on the grant date and is recognized when it is determined that it is probable that the performance condition will be achieved. The Company has elected a policy for all share-based awards to estimate forfeitures based on historical forfeiture experience at the time of grant and revise in subsequent periods if actual forfeitures differ from those estimates.
Income Taxes
The Company accounts for income taxes under the asset and liability method in accordance with ASC Topic 740, Income Taxes (“ASC 740”). Deferred tax assets and liabilities are determined based on the differences between the financial statement carrying amounts and the income tax basis of assets and liabilities. Deferred tax assets and liabilities are measured using enacted tax rates applicable to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rate is recognized in income in the period that includes the enactment date. A valuation allowance is applied against any deferred tax asset if, based on available evidence, it is “more likely than not” that some or all of the deferred tax assets will not be realized. For uncertain tax positions that meet a “more likely than not” threshold, the Company recognizes the benefit of uncertain tax positions in the consolidated financial statements. The Company’s practice is to recognize interest and penalties, if any, related to uncertain tax positions in its provision for income taxes in the consolidated statements of operations.
Foreign Currency
The functional currency and reporting currency for the Company and all of its foreign subsidiaries is determined to be the U.S. dollar. Thus, the Company does not record cumulative translations adjustment upon consolidation. All monetary assets and liabilities denominated in a foreign currency are remeasured into U.S. dollars at the exchange rate on the balance sheet date. Non-monetary assets and liabilities (along with their related expenses) are translated at the rate of exchange in effect on the date assets were acquired. Monetary income and expense items are translated at the average foreign exchange rate for the period. Foreign exchange gains and losses arising on translation or settlement of a foreign currency denominated monetary item are included in the consolidated statements of operations and comprehensive loss in other (income) expense, net.
For the years ended December 31, 2024, 2023, and 2022, the Company recognized $(5.9) million, $5.9 million and nil of foreign exchange gain (loss) related to the revaluation of its finance lease liability recognized in connection with the Monoplant, respectively (see Note 5).
Segment Information
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the Company's Chief Executive Officer, the chief operating decision-maker (“CODM”). For accounting purposes, the CODM is making decisions regarding resource allocation and assessing performance based on consolidated net income as if presented in the Company's consolidated financial statements. The Company views its operations and manages its business in one operating segment.
Recent Accounting Pronouncements
In November 2024, the FASB issued final guidance in ASU No. 2024-03, Income Statement — Reporting Comprehensive Income — Expense Disaggregation Disclosures (Subtopic 220-40): Disaggregation of Income Statement Expense which applies to all public business entities requiring additional disclosure of the nature of expenses included in the income statement in response to longstanding requests from investors for more information about an entity’s expenses. The new standard requires disclosures about specific types of expenses included in the expense captions presented on the face of the income statement as well as disclosures about selling expenses. The guidance is effective for annual reporting periods beginning after December 15, 2026 and interim reporting periods within annual reporting periods beginning after December 15, 2027. The Company is currently assessing the potential impact this ASU may have on the consolidated financial statements.
In December 2023, the FASB issued final guidance in ASU No. 2023-09, Income Taxes (ASC 740): Improvements to Income Tax Disclosures requiring entities to provide additional information in the rate reconciliation and disclosures about income taxes paid. For public business entities, the guidance is effective for annual periods beginning after December 15, 2024. The Company is currently assessing the potential impact this ASU may have on the consolidated financial statements.
In November 2023, the FASB issued ASU No. 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures which requires public entities to disclose significant segment expenses regularly provided to the CODM. Public entities with a single reporting segment have to provide all disclosures required by ASC 280, including the significant segment expense disclosures. For public business entities, the guidance is effective for annual periods beginning after December 15, 2023. The Company adopted this standard during 2024 and it did not have an impact on the consolidated financial statements.
3. Earnings (Loss) Per Share
Basic earnings (loss) per share is calculated by dividing net income (loss) by the weighted-average number of common shares outstanding without consideration of potential common shares. Diluted earnings (loss) per share is calculated by dividing net income (loss) by the weighted-average number of common shares outstanding plus potential common shares. Stock options, PAs, RSUs and ESPP are considered potential common shares and are included in the calculation of diluted earnings (loss) per share using the treasury stock method when their effect is dilutive. Potential common shares are excluded from the calculation of diluted earnings (loss) per share when their effect is anti-dilutive.
For the year ended December 31, 2024, there were 3.1 million potential dilutive common shares that were included in the calculation of diluted earnings per share, which consists of: (i) 0.3 million stock options; (ii) 0.7 million PAs; and (iii) 2.1 million RSUs. For the years ended December 31, 2024, 2023, and 2022, there were 7.3 million, 19.4 million and 15.3 million of potential common shares, respectively, that were excluded from the calculation of diluted earnings (loss) per share because their effect was anti-dilutive.
4. Balance Sheet Details
Fair Value Measurement
The following table summarizes the financial assets measured at fair value on a recurring basis (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2024 |
| | Level 1 | | Level 2 | | Level 3 | | Total |
| Cash, cash equivalents, and restricted cash | | $ | 83,433 | | | $ | — | | | $ | — | | | $ | 83,433 | |
| U.S. treasury bills | | — | | | 192,101 | | | — | | | 192,101 | |
| U.S. treasury bonds | | — | | | 81,402 | | | — | | | 81,402 | |
| Commercial paper | | — | | | 1,339 | | | — | | | 1,339 | |
| Corporate bonds | | — | | | 201 | | | — | | | 201 | |
| Total | | $ | 83,433 | | | $ | 275,043 | | | $ | — | | | $ | 358,476 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2023 |
| | Level 1 | | Level 2 | | Level 3 | | Total |
| Cash, cash equivalents and restricted cash | | $ | 48,875 | | | $ | — | | | $ | — | | | $ | 48,875 | |
| U.S. treasury bills | | — | | | 122,806 | | | — | | | 122,806 | |
| U.S. treasury bonds | | — | | | 105,924 | | | — | | | 105,924 | |
| Commercial paper | | — | | | 39,304 | | | — | | | 39,304 | |
| Corporate bonds | | — | | | 33,781 | | | — | | | 33,781 | |
| Total | | $ | 48,875 | | | $ | 301,815 | | | $ | — | | | $ | 350,690 | |
The fair value of the Company's investments classified within Level 2 is based upon observable inputs that may include benchmark yield curves, reported trades, issuer spreads, benchmark securities and reference data including market research publications.
The carrying amount and related unrealized gains (losses) by type of investment consisted of the following (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2024 |
| | Amortized Cost | | Unrealized Gains | | Unrealized Losses | | Estimated Fair Value |
| Cash, cash equivalents and restricted cash | | $ | 83,433 | | | $ | — | | | $ | — | | | $ | 83,433 | |
| U.S. treasury bills | | 192,054 | | | 47 | | | — | | | 192,101 | |
| U.S. treasury bonds | | 81,292 | | | 110 | | | — | | | 81,402 | |
| Commercial paper | | 1,339 | | | — | | | — | | | 1,339 | |
| Corporate bonds | | 200 | | | 1 | | | — | | | 201 | |
| Total cash, cash equivalents, restricted cash and short-term investments | | $ | 358,318 | | | $ | 158 | | | $ | — | | | $ | 358,476 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2023 |
| | Amortized Cost | | Unrealized Gains | | Unrealized Losses | | Estimated Fair Value |
| Cash, cash equivalents and restricted cash | | $ | 48,875 | | | $ | — | | | $ | — | | | $ | 48,875 | |
| U.S. treasury bills | | 122,757 | | | 49 | | | — | | | 122,806 | |
| U.S. treasury bonds | | 105,903 | | | 21 | | | — | | | 105,924 | |
| Commercial paper | | 39,305 | | | — | | | (1) | | | 39,304 | |
| Corporate bonds | | 33,576 | | | 4 | | | — | | | 33,580 | |
| Total cash, cash equivalents, restricted cash, and short-term investments | | 350,416 | | | 74 | | | (1) | | | 350,489 | |
| Total long-term investment corporate bond | | 200 | | | 1 | | | — | | | 201 | |
| Total cash, cash equivalents, restricted cash and investments | | $ | 350,616 | | | $ | 75 | | | $ | (1) | | | $ | 350,690 | |
As of December 31, 2024 and 2023, accrued interest receivable from the investments was $0.6 million and $0.7 million, respectively, which was included in other current assets on the consolidated balance sheets. As of December 31, 2024, short-term investments mature at various dates through September 2025. As of December 31, 2024 and 2023, no allowance for credit losses was recorded.
Inventory, Net
Inventory, net consisted of the following (in thousands):
| | | | | | | | | | | | | | |
| | December 31, 2024 | | December 31, 2023 |
| Raw materials | | $ | 1,702 | | | $ | 1,746 | |
| Work in process | | 36,623 | | | 37,376 | |
| Finished goods | | 903 | | | 583 | |
| Total inventory, net | | $ | 39,228 | | | $ | 39,705 | |
As of December 31, 2024 and 2023, total inventory is recorded net of inventory reserves of nil and $0.8 million, respectively, primarily related to potential inventory obsolescence.
Prepaid Expenses and Deposits
Prepaid expenses and deposits consisted of the following (in thousands):
| | | | | | | | | | | | | | |
| | December 31, 2024 | | December 31, 2023 |
| Prepaid manufacturing and other deposits | | $ | 5,645 | | | $ | 1,345 | |
| Prepaid insurance | | 1,186 | | | 1,249 | |
| Other prepaid expenses | | 4,388 | | | 6,892 | |
| Total prepaid expenses and deposits | | $ | 11,219 | | | $ | 9,486 | |
Intangible Assets, Net
Intangible assets, net consisted of the following (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2024 |
| | Weighted Average
Life (in years) | | Gross Carrying
Value | | Accumulated
Amortization | | Net Carrying
Amount |
| Acquired intellectual property and reacquired right | | 12 | | $ | 15,126 | | | $ | (11,535) | | | $ | 3,591 | |
| Patents | | 12 | | 2,128 | | | (1,364) | | | 764 | |
| Internal-use software implementation costs | | 3 | | 2,873 | | | (2,873) | | | — | |
| Total intangible assets, net | | | | $ | 20,127 | | | $ | (15,772) | | | $ | 4,355 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2023 |
| | Weighted Average
Life (in years) | | Gross Carrying
Value | | Accumulated
Amortization | | Net Carrying
Amount |
| Acquired intellectual property and reacquired right | | 12 | | $ | 15,126 | | | $ | (10,737) | | | $ | 4,389 | |
| Patents | | 12 | | 1,847 | | | (1,297) | | | 550 | |
| Internal-use software implementation costs | | 3 | | 2,873 | | | (2,835) | | | 38 | |
| Total intangible assets, net | | | | $ | 19,846 | | | $ | (14,869) | | | $ | 4,977 | |
For the years ended December 31, 2024, 2023 and 2022, the Company recorded amortization expense of $0.9 million, $1.7 million and $2.1 million, respectively. The estimated aggregate amortization expense for each of the next 5 succeeding years is $3.8 million.
Property and Equipment, Net
Property and equipment, net consisted of the following (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | Estimated Useful Life
(in years) | | December 31, 2024 | | December 31, 2023 |
| Leasehold improvements | | Shorter of lease or estimated useful life | | $ | 3,243 | | | $ | 3,243 | |
| Furniture | | 7 | | 1,155 | | | 1,155 | |
| Office equipment | | 5 | | 631 | | | 631 | |
| Computer equipment | | 3 | | 235 | | | 235 | |
| Total gross property and equipment | | | | 5,264 | | | 5,264 | |
| Less accumulated depreciation | | | | (2,533) | | | (1,910) | |
| Property and equipment, net | | | | $ | 2,731 | | | $ | 3,354 | |
For each of the years ended December 31, 2024, 2023 and 2022, the Company recorded depreciation expense of $0.6 million.
Accrued Expenses
Accrued expenses consisted of the following (in thousands):
| | | | | | | | | | | | | | |
| | December 31, 2024 | | December 31, 2023 |
| Accrued sales rebates and fees | | $ | 24,568 | | | $ | 14,047 | |
| Accrued payroll and related expenses | | 18,639 | | | 22,486 | |
| Accrued restructuring expense | | 10,855 | | | — | |
| Accrued R&D expenses | | 3,990 | | | 5,503 | |
| Accrued sales and marketing expenses | | 2,329 | | | 2,169 | |
| Accrued other | | 4,590 | | | 5,857 | |
| Total accrued expenses | | $ | 64,971 | | | $ | 50,062 | |
5. Commitments and Contingencies
Lease Commitments
Finance Leases
Monoplant
In December 2020, the Company entered into a manufacturing services agreement with Lonza for the construction of the Monoplant. The construction of the Monoplant began in January 2021 and manufacturing of voclosporin began in late June 2023. The Monoplant is equipped with state-of-the-art manufacturing equipment to provide cost and production efficiency for the manufacturing of voclosporin, while expanding existing capacity and providing supply security to meet future commercial demand. The Company completed a capital expenditure payment program for the Monoplant totaling $23.7 million, which included: (i) a $11.8 million payment in February 2021, which was treated as an upfront lease payment and recorded under other noncurrent assets on the consolidated balance sheets; and (ii) a $11.9 million payment when the facility fulfilled the required operational qualifications, which occurred in late June 2023. The Company has the exclusive right to use the Monoplant through March 31, 2030 by paying a quarterly fixed facility fee of 3.6 million Swiss Francs (approximately $4.0 million).
The Monoplant arrangement was determined to be an embedded lease and is accounted for as a finance lease under ASC 842. The lease term is based on the non-cancellable period for which a lessee has the right to use an underlying asset (the “Monoplant Lease”). The Company determined that the Monoplant Lease commencement occurred at the point when the FDA manufacturing validation process began, which occurred on June 26, 2023.
At lease inception, the Company recorded a finance right-of-use (“ROU”) lease asset of approximately $117.6 million and a corresponding lease liability of $94.1 million, which is the present value of the minimum lease payments beginning July 2023 and ending in 2030. The Monoplant Lease finance ROU lease asset included the present value of the minimum lease payments and the capital expenditure payment program. As of December 31, 2024, the Monoplant Lease finance ROU lease asset and corresponding lease liability balance were $91.3 million and $72.5 million, respectively.
Operating Leases
Rockville, Maryland
In March 2020, the Company entered into a lease agreement for 30,531 square feet of office space in Rockville, Maryland (the “Rockville Lease”). The Rockville Lease commenced on March 12, 2020 and expires on August 31, 2031. The Company has the option to extend the Rockville Lease for two 5-year periods at the end of the initial 11-year term and has the option to terminate after 7 years; however, such options were not recognized as part of the Company's lease liabilities and corresponding ROU lease assets. The Rockville Lease requires the Company to pay certain taxes, insurance and operating costs relating to the leased premises (“Lease Operating Costs”); however, such costs are not material to the Company’s financial position.
Edmonton, Alberta
In October 2022, the Company entered into a lease agreement for 4,375 square feet of office space in Edmonton, Alberta (the “Edmonton Lease”). The Edmonton Lease commenced on November 1, 2022 and expires on March 31, 2029. The Company has the option to renew the Edmonton Lease after 5 years; however, such options were not recognized as part of the Company's lease liabilities and corresponding ROU lease assets. The Edmonton Lease requires the Company to pay Lease Operating Costs; however, such costs are not material to the Company’s financial position.
Future minimum lease payments, excluding Lease Operating Costs, as of December 31, 2024 consisted of the following (in thousands):
| | | | | | | | | | | | | | |
| | Finance Lease Payments | | Operating Lease Payments |
| 2025 | | $ | 16,057 | | | $ | 1,139 | |
| 2026 | | 16,058 | | | 1,167 | |
| 2027 | | 16,058 | | | 1,196 | |
| 2028 | | 16,059 | | | 1,225 | |
| 2029 | | 16,059 | | | 1,223 | |
| Thereafter | | 4,063 | | | 2,079 | |
| Total lease payments | | 84,354 | | | 8,029 | |
| Less: imputed interest | | (11,754) | | | (1,260) | |
| Total | | $ | 72,600 | | | $ | 6,769 | |
For the years ended December 31, 2024, 2023, and 2022, finance lease expense related to the amortization of finance ROU lease assets was $17.4 million, $8.9 million and nil, respectively. For the years ended December 31, 2024, 2023, and 2022, finance lease expense related to the interest on finance lease liabilities was $4.8 million, $2.8 million and nil, respectively. For the years ended December 31, 2024, 2023, and 2022, cash paid for amounts included in the measurement of finance lease liabilities classified in financing cash flows was $12.0 million, $10.0 million and nil, respectively. For the years ended December 31, 2024, 2023, and 2022, cash paid for amounts included in the measurement of finance lease liabilities classified in operating cash flows was $4.6 million, $2.3 million and nil, respectively. As of December 31, 2024, 2023, and 2022, the weighted-average remaining lease term for the Company’s finance leases was 5.3 and 6.3 years, respectively. As of December 31, 2024 and 2023, the weighted-average discount rate for the Company’s finance leases was 6.19%.
For the years ended December 31, 2024, 2023, and 2022, operating lease expense was $0.8 million, $0.8 million and $1.0 million, respectively. For the years ended December 31, 2024, 2023, and 2022, cash paid for amounts included in the measurement of operating lease liabilities was $1.1 million, $1.1 million and $1.2 million, respectively. As of December 31, 2024 and 2023, the weighted-average remaining lease term for the Company’s operating leases was 6.6 and 7.6 years, respectively. As of December 31, 2024 and 2023, the weighted-average discount rate for the Company’s operating leases was 5.27% and 5.28%, respectively.
Manufacturing Commitments
In the normal course of business, the Company enters into agreements for the manufacturing and supply of commercial and clinical product. In December 2020, the Company entered into a manufacturing services agreement with Lonza to manufacture commercial and clinical product. The Company has non-cancellable purchase commitments of $11.7 million through 2025, of which $6.2 million was paid during the year ended December 31, 2024. If the Company terminates certain purchase commitments without cause, the Company is required to pay Lonza for such commercial and clinical product that is scheduled to be manufactured; however, certain amounts of such commitments are expected to be reimbursed to the Company by its collaboration partner, Otsuka.
Contingencies
From time to time, the Company may become subject to claims and litigation arising in the ordinary course of business. The Company is not a party to any material legal proceedings, nor is it aware of any material pending or threatened litigation.
6. Deferred Compensation and Other Noncurrent Liabilities
In March 2012, the Company entered into employee retention arrangements with certain executive officers, whereby the Company is required to make payments to such former officers based on net revenues of voclosporin for a certain period of time. As of December 31, 2024 and 2023, the Company recorded other deferred compensation and other noncurrent liabilities of $11.1 million and $10.9 million, respectively.
One of the former officers that entered into an employee retention arrangement was considered a related party while he served as a member of the Company's board of directors (“the Board”) from September 2023 through November 2024. As of December 31, 2024 and 2023, the Company's fair value accrual for such arrangement with a related party was nil and $8.3 million, respectively. During the years ended December 31, 2024 and 2023, the Company made deferred compensation payments to such related party of $0.6 million and $0.1 million, respectively, during the time he was considered a related party.
7. Segment Information and Geographic Data
The amounts disclosed in the consolidated financial statements represent those of the single reporting unit.
The percentage of total revenues from the Company's major customers consisted of the following:
| | | | | | | | | | | | | | | | | | | | | | | |
| | 2024 | | 2023 | | 2022 |
| Specialty pharmacy A | | 49% | | 51% | | 45% | |
| Specialty pharmacy B | | 39% | | 40% | | 35% | |
| Collaboration partnership | | 9% | | 8% | | 20% | |
Revenue by Geographic Location
Revenue by geographic location consisted of the following (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | 2024 | | 2023 | | 2022 |
| U.S. | | $ | 210,095 | | | $ | 157,958 | | | $ | 102,460 | |
| Japan | | 25,038 | | | 17,555 | | | 31,481 | |
| Other | | — | | | — | | | 89 | |
| Total revenue | | $ | 235,133 | | | $ | 175,513 | | | $ | 134,030 | |
Property, Equipment and Right-of-use Lease Assets by Geographic Location
Property, equipment and right-of-use lease assets by geographic location consisted of the following (in thousands):
| | | | | | | | | | | | | | |
| | December 31, 2024 | | December 31, 2023 |
| Switzerland | | $ | 91,290 | | | $ | 108,715 | |
| U.S. | | 6,366 | | | 7,324 | |
| France | | 782 | | | — | |
| Canada | | 433 | | | 528 | |
| Total property and equipment, net and right-of-use lease assets | | $ | 98,871 | | | $ | 116,567 | |
8. License and Collaboration Agreements
In December 2020, the Company entered into a collaboration and licensing agreement with Otsuka to develop and commercialize oral voclosporin in Japan, the E.U., the U.K., Switzerland, Russia, Norway, Belarus, Iceland, Liechtenstein and Ukraine (collectively, the “Otsuka Territories”) in exchange for: (i) a $50 million upfront cash payment; (ii) regulatory and commercial milestone payments; and (iii) royalties ranging from 10% to 20% on net sales in the Otsuka Territories.
In August 2022, the Company entered into a commercial supply agreement with Otsuka to: (i) supply LUPKYNIS inventory to Otsuka at cost plus a margin; and (ii) provide manufacturing and other services, including sharing the capacity of a dedicated manufacturing facility at Lonza, the Company's contract manufacturing partner for voclosporin.
The Company recognized: (i) a $10.0 million milestone in 2024 for the approval of LUPKYNIS for the treatment of LN in Japan by the Japanese Ministry of Health, Labour and Welfare; (ii) a $10.0 million milestone in 2023 for pricing and reimbursement approval in certain European jurisdictions; and (iii) a $30 million milestone in 2022 for the marketing authorization of LUPKYNIS by the European Commission. For the years ended December 31, 2024, 2023 and 2022, the Company recognized $8.9 million, $6.0 million and $0.5 million, respectively, of additional collaboration revenue from manufacturing and other services, which includes sharing capacity of the Monoplant.
9. Shareholders' Equity
On February 15, 2024, the Company announced that the Board approved a share repurchase program of up to $150 million of the Company’s common shares (the “Share Repurchase Plan”).
The timing and amount of future repurchase transactions will be determined by management based on its evaluation of market conditions, share price, legal requirements, including applicable blackout period restrictions, and other factors. The Company has entered into a Rule 10b5-1 stock repurchase plan for the purpose of establishing a trading plan to purchase the Company’s common shares in a manner intended to satisfy the affirmative defense of Rule 10b5-1(c)(1) under the Securities Exchange Act of 1934, as amended and in accordance with applicable Canadian laws.
For the year ended December 31, 2024, the Company repurchased 6.1 million of its common shares for $41.0 million, including commissions and excise tax. The cost of repurchased shares is reported as a reduction in common shares. Under Alberta law, the common shares were cancelled and not reissued.
10. Equity Incentive Plans
2021 Equity Incentive Plan
In June 2021, the Company adopted the Amended and Restated Equity Incentive Plan (the “2021 Equity Plan”). The 2021 Equity Plan permits the issuance of various types of share-based compensation awards, including stock options, performance awards (“PAs”) and restricted stock units (“RSUs”) that may be settled in cash and common shares. The 2021 Equity Plan allows for an issuance of up to an aggregate of 23.8 million shares.
2021 Employee Stock Purchase Plan
In June 2021, the Company adopted the Employee Stock Purchase Plan (the “2021 ESPP”). Under the 2021 ESPP, eligible employees may purchase common shares of the Company at a discounted price. During 2022, the Company amended the 2021 ESPP for current and future offerings. The amendment: (i) shortened the plan from 4 purchases over a 24-month offering period to 2 purchases over a 12-month offering period; and (ii) added a mechanism to rollover participants into a new 12-month offering period if the stock price on the purchase date is less than the offering price. The 2021 ESPP allows for the issuance of up to 2.5 million shares of which 0.2 million and 0.4 million were purchased during the years ended December 31, 2024 and 2023, respectively.
Inducement Grants
In addition to stock options, PAs and RSUs granted under the 2021 Equity Plan, the Company has granted certain stock options and RSUs as inducements to new employees entering into employment with the Company in accordance with Nasdaq Listing Rule 5635(c)(4). The inducements were granted outside of the 2021 Equity Plan during 2023 and 2024.
Stock Options
Generally, stock options have a 10-year term and vest over 3 years with one-third of the shares vesting on the 1-year anniversary and the remaining options vesting monthly thereafter.
The activity related to stock options during the year ended December 31, 2024 consisted of the following:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Number of Shares
(in thousands) | | Weighted-Average Exercise Price | | Weighted-Average Remaining Contractual Life (in years) | | Aggregate Intrinsic Value
(in thousands) |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| Outstanding at December 31, 2023 | | 11,556 | | | $ | 10.63 | | | 7.03 | | $ | 7,967 | |
| Granted | | 509 | | | $ | 6.63 | | | | | |
| Exercised/released | | (362) | | | $ | 5.50 | | | | | |
| Cancelled/forfeited | | (2,427) | | | $ | 12.64 | | | | | |
| Outstanding at December 31, 2024 | | 9,276 | | | $ | 10.08 | | | 6.18 | | $ | 8,520 | |
| Options exercisable at December 31, 2024 | | 8,346 | | | $ | 11.23 | | | | | |
| Vested and expected to vest, December 31, 2024 | | 9,187 | | | $ | 10.96 | | | | | |
During the years ended, December 31, 2024, 2023 and 2022, the intrinsic value of options exercised was $0.8 million, $2.0 million and $0.9 million, respectively. During the years ended, December 31, 2024, 2023 and 2022, the total fair value of options vested was $13.9 million, $40.9 million and $49.2 million, respectively.
For the years ended December 31, 2024, 2023 and 2022, the weighted-average grant-date fair value of stock options granted was $4.31, $5.86 and $6.52, respectively. The Company estimated the fair value of each stock option on the date of grant using the Black-Scholes option pricing model with the following assumptions:
| | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| | 2024 | | 2023 | | 2022 |
| Expected term (in years) | | 5 | | 5 | | 5 |
| Expected volatility | | 77 | % | | 71 | % | | 70 | % |
| Risk-free interest rate | | 4.09 | % | | 3.99 | % | | 2.06 | % |
| Expected dividend yield | | 0.0 | % | | 0.0 | % | | 0.0 | % |
Expected term - Expected term is based upon the contractual term, taking into account expected employee exercise and expected post-vesting employment termination behavior. The length of the expected term in 2024 is in line with historic data and what management expects in the future.
Expected volatility - The Company considers historical volatility of its common shares in estimating its future stock price volatility. The expected term of the stock option is used to determine market volatility of the underlying stock. Given the growth of the Company, the expected life used to determine previous market volatility and comparable peer group reflects an appropriate estimate of future volatility.
Risk-free interest rate - The risk-free interest rate for the expected term of the stock options was based on the yield available on government benchmark bonds with an approximate equivalent remaining term at the time of the grant.
Expected dividend yield - The Company has never paid dividends on its common shares and has no plans to pay dividends on the Company's common shares in the near future. Therefore, the Company dividend yield is nil.
Performance Awards and Restricted Stock Units
The fair value of PAs and RSUs is based on the market price of the Company's common shares on the date of the grant. The PAs and RSUs vest in 3 equal annual installments on the first, second and third anniversary of the grant date.
The PAs and RSUs activity during the year ended December 31, 2024 consisted of the following:
| | | | | | | | | | | | | | |
| | Number of Shares
(in thousands) | | Weighted-Average Grant Date Fair Value |
| Unvested balance, December 31, 2023 | | 7,807 | | | $ | 9.29 | |
| Granted | | 4,784 | | | $ | 6.92 | |
| Vested | | (2,505) | | | $ | 9.47 | |
| Forfeited | | (2,100) | | | $ | 8.42 | |
| Unvested balance, December 31, 2024 | | 7,986 | | | $ | 8.04 | |
The weighted-average grant date fair value of RSUs granted during the years December 31, 2024, 2023 and 2022 was $7.13, $9.03 and $11.16, respectively. Total intrinsic value of RSUs vested during the years December 31, 2024, 2023 and 2022, was $16.2 million, $5.4 million and $2.9 million, respectively.
Share-based Compensation Expense
The classification of share-based compensation expense consisted of the following (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| | 2024 | | 2023 | | 2022 |
| Research and development | | $ | (1,329) | | | $ | 7,533 | | | $ | 3,271 | |
| Selling, general and administrative | | 31,641 | | | 36,512 | | | 28,438 | |
| Capitalized under inventories | | 1,284 | | | 1,266 | | | 591 | |
| Share-based compensation expense | | $ | 31,596 | | | $ | 45,311 | | | $ | 32,300 | |
As of December 31, 2024, total unrecognized share-based compensation cost related to unvested stock options, PAs, RSUs and ESPP was $19.7 million, which is estimated to be recognized over a weighted-average period of 1.3 years,
11. Restructuring Expenses
On February 15, 2024, the Company announced a strategic restructuring that reduced headcount by approximately 25% and discontinued the Company's AUR300 development program (the “February Restructuring”). On November 7, 2024, the Company announced another strategic restructuring that further reduced headcount by approximately 45% to sharpen the Company's focus on continued LUPKYNIS growth and the rapid development of AUR200 (the “November Restructuring”).
For the year ended December 31, 2024, total expense for the February Restructuring was $7.8 million, which was comprised of: (i) $6.1 million for one-time termination benefits to affected employees, including severance and health care benefits; (ii) $1.1 million of contract termination costs; and (iii) $0.6 million of other restructuring costs. As of December 31, 2024, the Company had made all payments related to the February Restructuring.
For the year ended December 31, 2024, total expense for the November Restructuring was $15.3 million, which was comprised of: (i) $14.0 million for one-time termination benefits to affected employees, including severance and health care benefits; (ii) $0.7 million of contract termination costs; and (iii) $0.6 million of other restructuring costs. As of December 31, 2024, the Company paid $4.8 million related to the November Restructuring. The Company anticipates up to $19 million of total expense to be incurred related to the November Restructuring, and for restructuring activities to be substantially completed in the first half of 2025.
12. Income Taxes
Net income (loss) before income taxes consisted of the following (in thousands):
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Canada | $ | (8,416) | | | $ | (90,226) | | | $ | (112,359) | |
| Foreign | 15,864 | | | 12,757 | | | 6,007 | |
| Total net income (loss) before income taxes | $ | 7,448 | | | $ | (77,469) | | | $ | (106,352) | |
Foreign net income before income taxes is substantially all U.S. source income. For the years ended December 31, 2024, 2023 and 2022, the Company recognized income tax expense of $1.7 million, $0.6 million and $1.8 million, respectively, all of which related to foreign current income tax expense and none of which related to deferred tax expense.
Aurinia Pharmaceuticals Inc. is an Alberta, Canada corporation. The difference between the Canadian statutory and provincial rate and the effective tax rate is as follows:
| | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| | 2024 | | 2023 | | 2022 |
| Canada statutory and provincial income tax | | 24.7 | % | | 24.6 | % | | 26.7 | % |
| Effect of tax rates on foreign jurisdictions | | (7.8) | | | 0.6 | | | 0.3 | |
| Withholding taxes | | 2.0 | | | (0.2) | | | (1.1) | |
| Impact of future rates and tax rate changes | | (6.5) | | | (15.6) | | | (0.1) | |
| Foreign tax credit | | (2.0) | | | 0.2 | | | 1.1 | |
| Non-deductible share-based compensation | | 106.3 | | | (13.8) | | | (8.1) | |
| | | | | | |
| | | | | | |
| State income taxes | | 5.3 | | | (0.9) | | | (0.1) | |
| Change in valuation allowance | | (100.0) | | | 3.1 | | | (21.5) | |
| Scientific Research and Experimental Development (“SRED”) and Research Credits | | (2.1) | | | 1.3 | | | 1.1 | |
| Other | | 2.9 | | | — | | | — | |
| Effective tax rate | | 22.8 | % | | (0.7) | % | | (1.7) | % |
Deferred tax assets are as follows (in thousands):
| | | | | | | | | | | | | | |
| | December 31, 2024 | | December 31, 2023 |
| Deferred tax assets: | | | | |
| Net operating loss (“NOL”) carryforwards | | $ | 130,143 | | | $ | 137,907 | |
| Share issue costs | | 879 | | | 2,325 | |
| Lease liability | | 19,380 | | | 23,837 | |
| Intangible assets | | 1,557 | | | 1,479 | |
| Research credit carryforwards | | 8,369 | | | 8,263 | |
| | | | |
| Deferred compensation | | 2,342 | | | 2,384 | |
| Accrued expenses | | 2,458 | | | 3,646 | |
| Other | | 3,407 | | | 2,688 | |
| Gross deferred tax assets | | 168,535 | | | 182,529 | |
| Valuation allowance | | (154,432) | | | (161,898) | |
| Total deferred tax assets | | 14,103 | | | 20,631 | |
| Deferred tax liabilities: | | | | |
| Right-of-use asset | | (13,630) | | | (20,060) | |
| Property, equipment and intangible assets | | (473) | | | (571) | |
| | | | |
| Total deferred tax liabilities | | (14,103) | | | (20,631) | |
| Net deferred tax assets | | $ | — | | | $ | — | |
The Company’s valuation allowance decreased by $7.5 million in 2024 as compared to 2023 primarily due to the utilization of Canada's NOL carryforwards. As of December 31, 2024 and 2023, the Company established a full valuation allowance against its net deferred tax assets due to the uncertainty surrounding the realization of such assets.
As of December 31, 2024, the Company had $527.5 million of Canada gross NOL carryforwards and $6.6 million of Canada Investment Tax Credits and British Columbia SRED with an expiration period of 2029 through 2044. As of December 31, 2024, the Company also had $0.2 million of state NOL carryforwards. The Company’s ability to utilize the state tax attribute carryforwards to offset any taxable income or tax liability in certain taxable periods may be limited under Section 382/383 of the Internal Revenue Code.
Uncertain Income Tax Positions
The Company does not have any liabilities recorded for uncertain income tax positions. The Company is currently under examination by the Canadian Revenue Agency for the years ended December 31, 2019, 2020 and 2021. There were no updates during the year ended December 31, 2024. The Company is subject to examination in the U.S., Canada and U.K. In the U.S. and Canada, tax periods subject for examination remain open from 2020 through 2024 and 2009 through 2024, respectively, due to the tax attribute carryforwards. In the U.K., tax periods subject for examination remain open from 2022 through 2024.
13. Subsequent Events
From January 1, 2025 through February 25, 2025, the Company repurchased 3.6 million of its common shares for $29.0 million, including commissions, under its Repurchase Plan.
On February 25, 2025, we received a paragraph IV notice of certification (the “Notice Letter”) related to a submission of an ANDA to the FDA seeking authorization to manufacture, use or sell a generic version of LUPKYNIS in the U.S., prior to the expiry of U.S. Patent Nos. 10,286,036 and 11,622,991 (the “2037 Patents”), which are listed in the FDA's Orange Book. The Notice Letter alleges that the 2037 Patents are invalid, unenforceable and/or will not be infringed by the commercial manufacture, use or sale of the generic product described in the ANDA.
We intend to vigorously defend LUPKYNIS and our intellectual property rights protecting LUPKYNIS. In accordance with the Hatch-Waxman Act, because LUPKYNIS is an NCE, should we file a patent infringement lawsuit within 45 days of receipt of the Notice Letter, the FDA cannot approve any ANDA any earlier than 7.5 years from the approval of the LUPKYNIS NDA unless a District Court finds that all of the asserted claims of the patents-in-suit are invalid, unenforceable and/or not infringed.
14. Selected Quarterly Financial Information (unaudited)
Condensed quarterly financial information consisted of the following (in thousands, except per share data):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | For the three months ended, |
| | March 31, 2024 | | June 30,
2024 | | September 30, 2024 | | December 31, 2024 | | Total |
| Total revenue | | $ | 50,303 | | | $ | 57,192 | | | $ | 67,771 | | | $ | 59,867 | | | $ | 235,133 | |
| Operating expenses | | 63,556 | | | 58,705 | | | 56,023 | | | 61,536 | | | 239,820 | |
| (Loss) income from operations | | (13,253) | | | (1,513) | | | 11,748 | | | (1,669) | | | (4,687) | |
| Net (loss) income | | (10,749) | | | 722 | | | 14,350 | | | 1,429 | | | 5,752 | |
| Basic (loss) earnings per share | | $ | (0.07) | | | $ | 0.01 | | | $ | 0.10 | | | $ | 0.01 | | | $ | 0.04 | |
| Diluted (loss) earnings per share | | $ | (0.07) | | | $ | 0.01 | | | $ | 0.10 | | | $ | 0.01 | | | $ | 0.04 | |
| | | | | | | | | | |
| | For the three months ended, |
| | March 31, 2023 | | June 30,
2023 | | September 30, 2023 | | December 31, 2023 | | Total |
| Total revenue | | $ | 34,409 | | | $ | 41,494 | | | $ | 54,515 | | | $ | 45,095 | | | $ | 175,513 | |
| Operating expenses | | 63,993 | | | 57,664 | | | 70,778 | | | 74,769 | | | 267,204 | |
| Loss from operations | | (29,584) | | | (16,170) | | | (16,263) | | | (29,674) | | | (91,691) | |
| Net loss | | (26,206) | | | (11,492) | | | (13,447) | | | (26,875) | | | (78,020) | |
| Basic and diluted loss per common share | | $ | (0.18) | | | $ | (0.08) | | | $ | (0.09) | | | $ | (0.19) | | | $ | (0.54) | |
| Tables may have rounding differences | | | | | | | | | | |