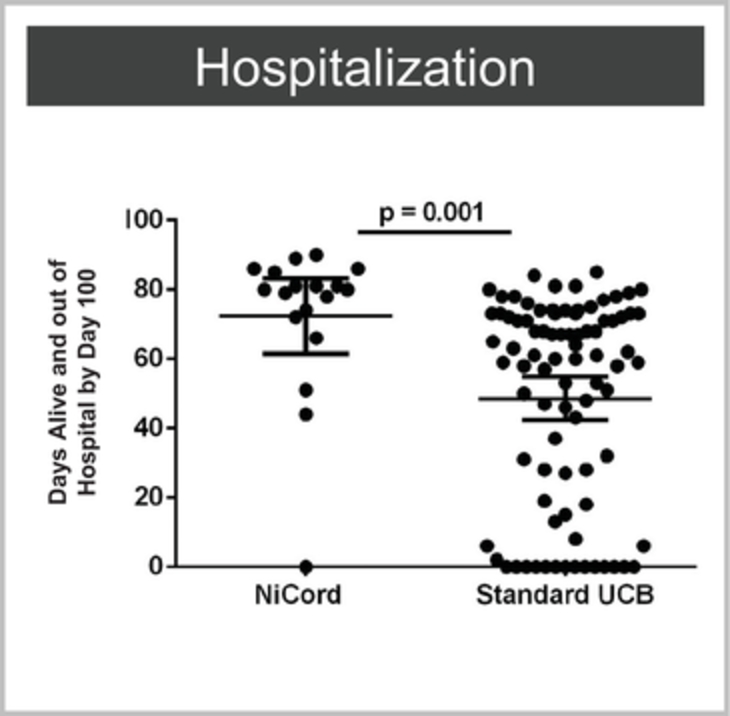
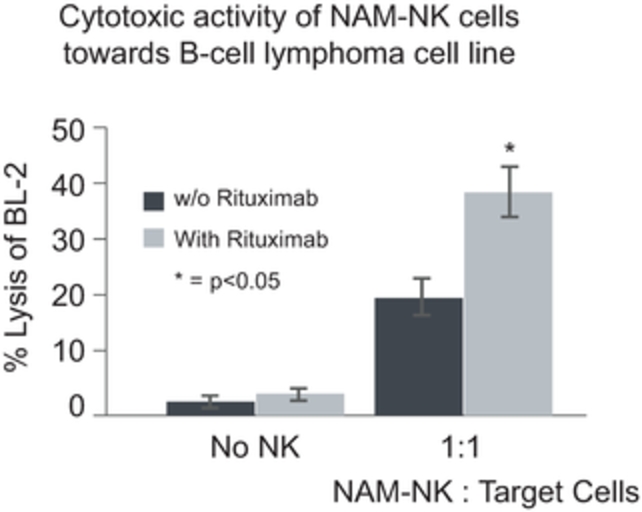
Company Limited, Miltenyi Biotec GmbH, multimmune GmbH, NantKwest, Inc., Nkarta Therapeutics, Inc., NKBio Co., Ltd., PersonGen BioTherapeutics Suzhou Co. Ltd., United Therapeutics Corporation, Y-mAbs Therapeutics, Inc., Ziopharm Oncology, Inc.
Manufacturing
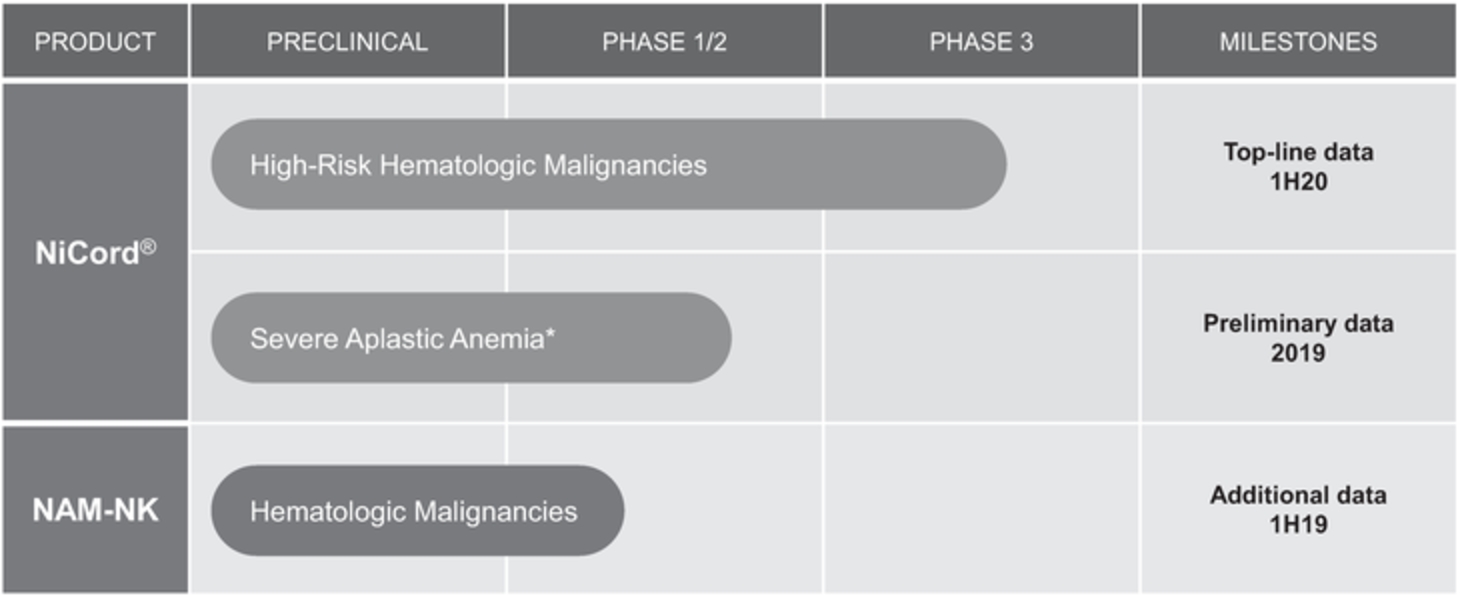
Our product candidates are currently manufactured at our Jerusalem, Israel facility using a scalable self-assembly process with well-defined unit operations. This highly specialized and precisely controlled manufacturing process enables us to manufacture product candidates reproducibly and efficiently for clinical and commercial applications.
We currently rely on a third party, Lonza Walkersville, Inc., or Lonza, to conduct a material portion of our product manufacturing for NiCord, to include CordIn, and intend to do so at Lonza or a Lonza affiliate, at least until our manufacturing facility is expected to be completed. In February 2016, and as amended, we entered into a Manufacturing Services Agreement, or the Manufacturing Agreement, with Lonza for the production of products containing human cells intended for therapeutic use in humans. Under the terms of the Manufacturing Agreement, Lonza manufactures, packages, ships, and handles quality assurance and control products, based on statements of work, which we submit with respect to each development of a process or product and as may be further be amended by change orders. Each statement of work describes the activities to be performed by the parties and is subject to the terms of the Manufacturing Agreement unless the parties have agreed otherwise.
The term of the Manufacturing Agreement is five years, unless terminated earlier pursuant to its terms. The Manufacturing Agreement may be terminated in the event of an uncured material breach by one of the parties. In addition, the Manufacturing Agreement or any statement of work thereunder may be terminated by us by providing six months prior written notice or by Lonza by providing 12 months prior written notice. In addition, the Manufacturing Agreement may be terminated if either NiCord or Cordin, which are being produced thereunder, has been or will be suspended or terminated by the FDA due to the failure of the product candidate, by providing two months prior written notice. Further, the Manufacturing Agreement may be terminated by either party upon notice in the event of dissolution, termination of existence, liquidation or business failure of the other party, the uncured appointment of a custodian or receiver to the other party or un-dismissed institution of insolvency, reorganization or bankruptcy proceedings.
As of the date of this prospectus, we have paid Lonza an aggregate of approximately $8.67 million pursuant to the Manufacturing Agreement.
Marketing, Sales and Distribution
Given our stage of development, we do not currently have any internal sales, marketing or distribution infrastructure or capabilities. We have recently formed a U.S. subsidiary, Gamida Cell Inc., to support our U.S. development and potential commercialization efforts.
In the event that we receive regulatory approvals for our products in markets outside of the United States, we intend, where appropriate, to pursue commercialization relationships, including strategic alliances and licensing, with pharmaceutical companies and other strategic partners, which are equipped to market or sell our products through their well-developed sales, marketing and distribution organizations in such countries.
Intellectual Property
We strive to protect and enhance the proprietary technologies, inventions, products and product candidates, methods of manufacture, methods of using our products and product candidates, and improvements thereof that are commercially important to our business. We protect our proprietary intellectual property by, among other things, filing patent applications in the United States and in jurisdictions outside of the United States covering our proprietary technologies, inventions, products and product candidates, methods, and improvements that are important to the development and implementation of our business.
As of April 30, 2018, we own 36 issued patents and 17 pending patent applications worldwide, including 11 U.S. issued patents, three pending U.S. non-provisional patent applications, two pending