
Seres Therapeutics Corporate Overview May 2023 Exhibit 99.2

Some of the statements in this presentation constitute “forward looking statements” under the Private Securities Litigation Reform Act of 1995, including, but not limited to timing of VOWST product availability; the anticipated supply and degree of market acceptance of VOWST; the potential for microbiome therapeutics to protect against infection; the timing of clinical development; our development opportunities and plans; the ultimate safety and efficacy data for our products; the sufficiency of cash to fund operations; the receipt of milestone payments and access to additional debt tranches; and other statements which are not historical fact. Such statements are subject to important factors, risks and uncertainties, such as those discussed under the caption "Risk Factors" in the Company’s Annual Report on Form 10-K filed on March 7, 2023, and its other filings with the SEC, that may cause actual results to differ materially from those expressed or implied by such forward looking statements. Any forward-looking statements included herein represent our views as of today only. We may update these statements, but we disclaim any obligation to do so. Forward Looking Statements Seres Therapeutics, Inc. © 2023
VOWSTTM is the First FDA Approved Orally Administered Microbiota-Based Therapeutic VOWSTTM is indicated to prevent the recurrence of C. difficile infection (CDI) in individuals 18 years of age or older following antibacterial treatment for recurrent CDI (rCDI). Seres is pioneering a new modality, led by VOWSTTM Seres Therapeutics, Inc. © 2023
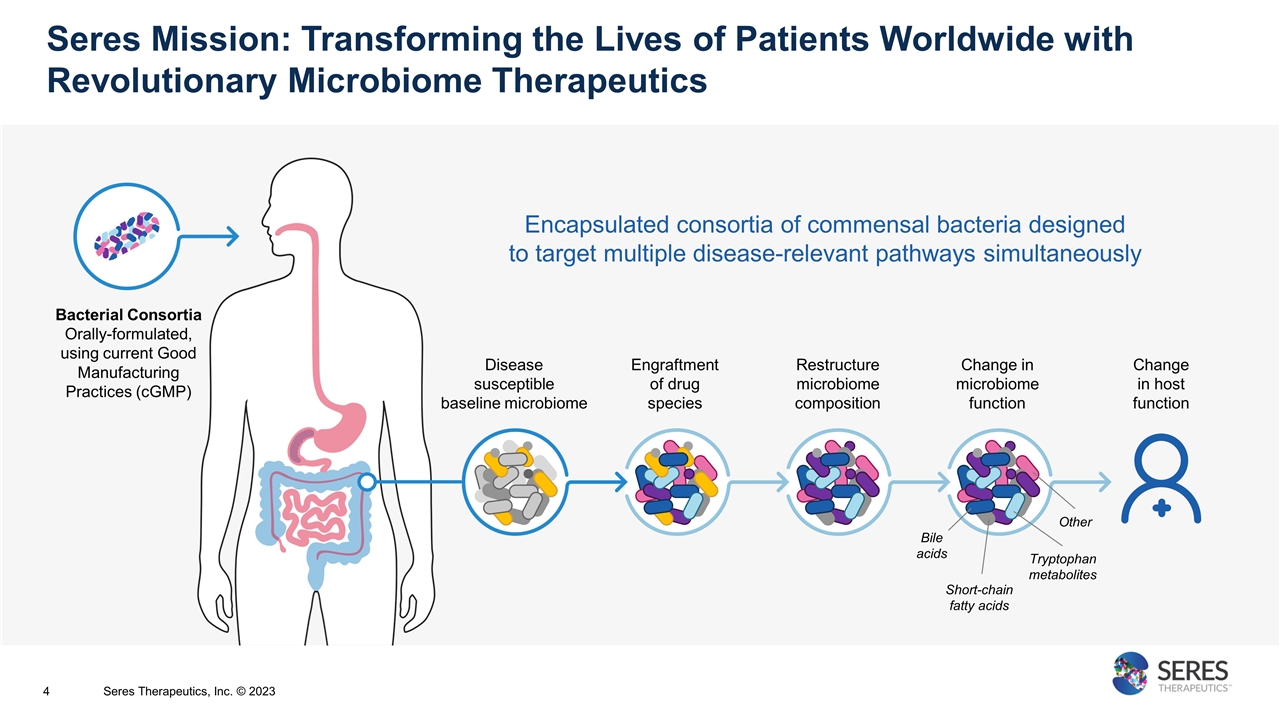
Seres Mission: Transforming the Lives of Patients Worldwide with Revolutionary Microbiome Therapeutics Encapsulated consortia of commensal bacteria designed to target multiple disease-relevant pathways simultaneously Bacterial Consortia Orally-formulated, using current Good Manufacturing Practices (cGMP) Disease susceptible baseline microbiome Engraftment of drug species Restructure microbiome composition Change in microbiome function Change in host function Bile acids Short-chain fatty acids Tryptophan metabolites Other Seres Therapeutics, Inc. © 2023
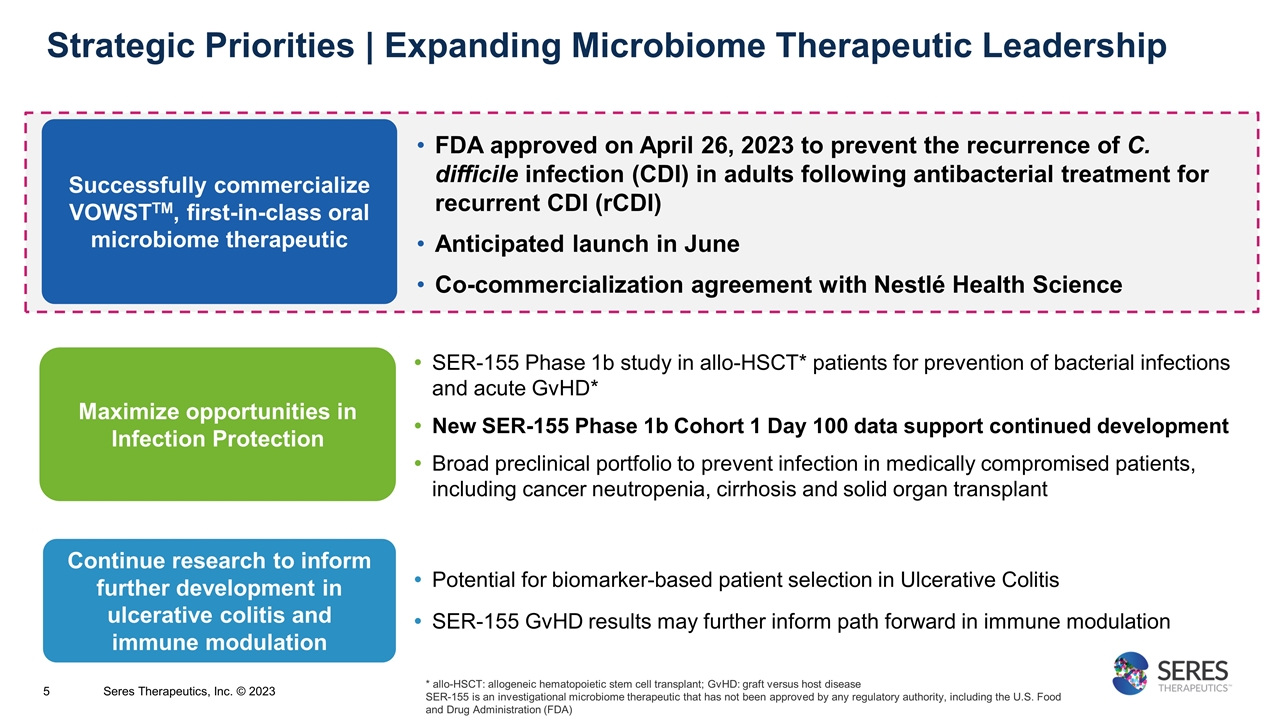
Strategic Priorities | Expanding Microbiome Therapeutic Leadership Successfully commercialize VOWSTTM, first-in-class oral microbiome therapeutic Maximize opportunities in Infection Protection Continue research to inform further development in ulcerative colitis and immune modulation FDA approved on April 26, 2023 to prevent the recurrence of C. difficile infection (CDI) in adults following antibacterial treatment for recurrent CDI (rCDI) Anticipated launch in June Co-commercialization agreement with Nestlé Health Science SER-155 Phase 1b study in allo-HSCT* patients for prevention of bacterial infections and acute GvHD* New SER-155 Phase 1b Cohort 1 Day 100 data support continued development Broad preclinical portfolio to prevent infection in medically compromised patients, including cancer neutropenia, cirrhosis and solid organ transplant Potential for biomarker-based patient selection in Ulcerative Colitis SER-155 GvHD results may further inform path forward in immune modulation * allo-HSCT: allogeneic hematopoietic stem cell transplant; GvHD: graft versus host disease SER-155 is an investigational microbiome therapeutic that has not been approved by any regulatory authority, including the U.S. Food and Drug Administration (FDA) 5 Seres Therapeutics, Inc. © 2023
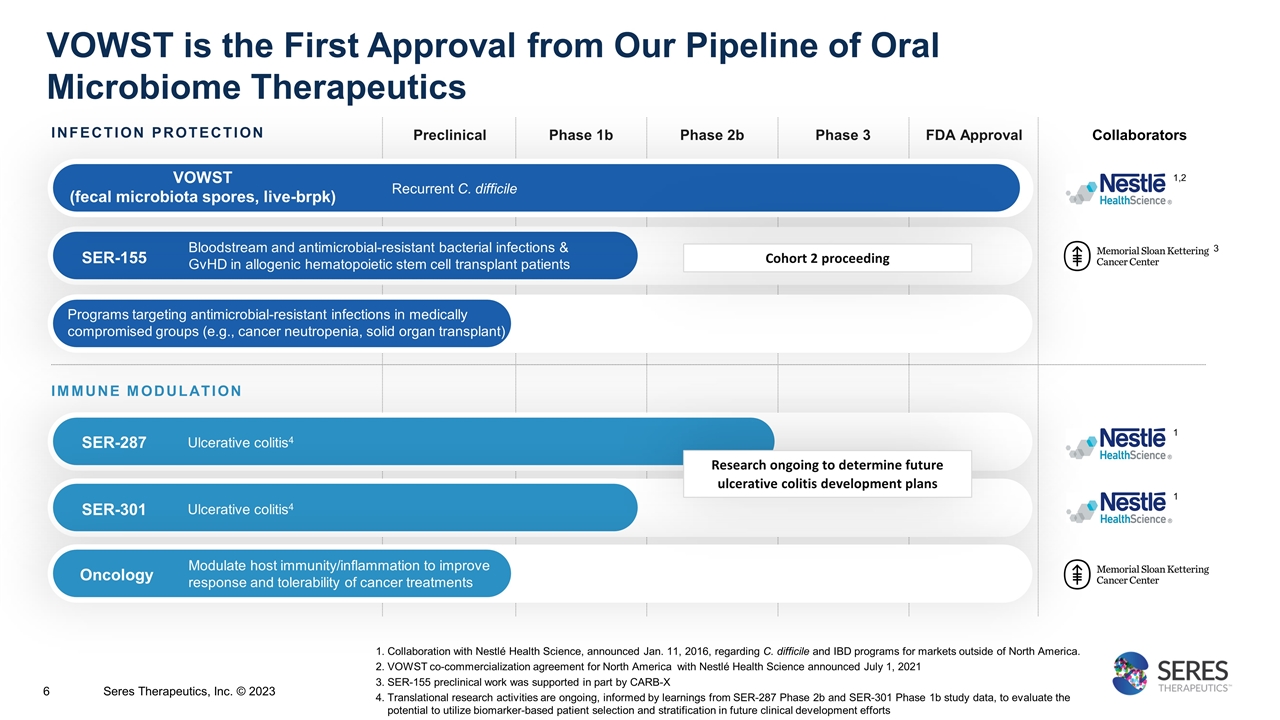
Immunotherapy Collaborators INFECTION PROTECTION Preclinical Phase 1b Phase 2b FDA Approval SER-155 Bloodstream and antimicrobial-resistant bacterial infections & GvHD in allogenic hematopoietic stem cell transplant patients IMMUNE MODULATION SER-287 Ulcerative colitis4 SER-301 Ulcerative colitis4 Modulate host immunity/inflammation to improve response and tolerability of cancer treatments Programs targeting antimicrobial-resistant infections in medically compromised groups (e.g., cancer neutropenia, solid organ transplant) VOWST (fecal microbiota spores, live-brpk) Recurrent C. difficile 1,2 3 Oncology Research ongoing to determine future ulcerative colitis development plans 1 1 Collaboration with Nestlé Health Science, announced Jan. 11, 2016, regarding C. difficile and IBD programs for markets outside of North America. VOWST co-commercialization agreement for North America with Nestlé Health Science announced July 1, 2021 SER-155 preclinical work was supported in part by CARB-X Translational research activities are ongoing, informed by learnings from SER-287 Phase 2b and SER-301 Phase 1b study data, to evaluate the potential to utilize biomarker-based patient selection and stratification in future clinical development efforts Cohort 2 proceeding VOWST is the First Approval from Our Pipeline of Oral Microbiome Therapeutics Phase 3 Seres Therapeutics, Inc. © 2023
VOWSTTM and Recurrent C. difficile Infection Seres Therapeutics, Inc. © 2023
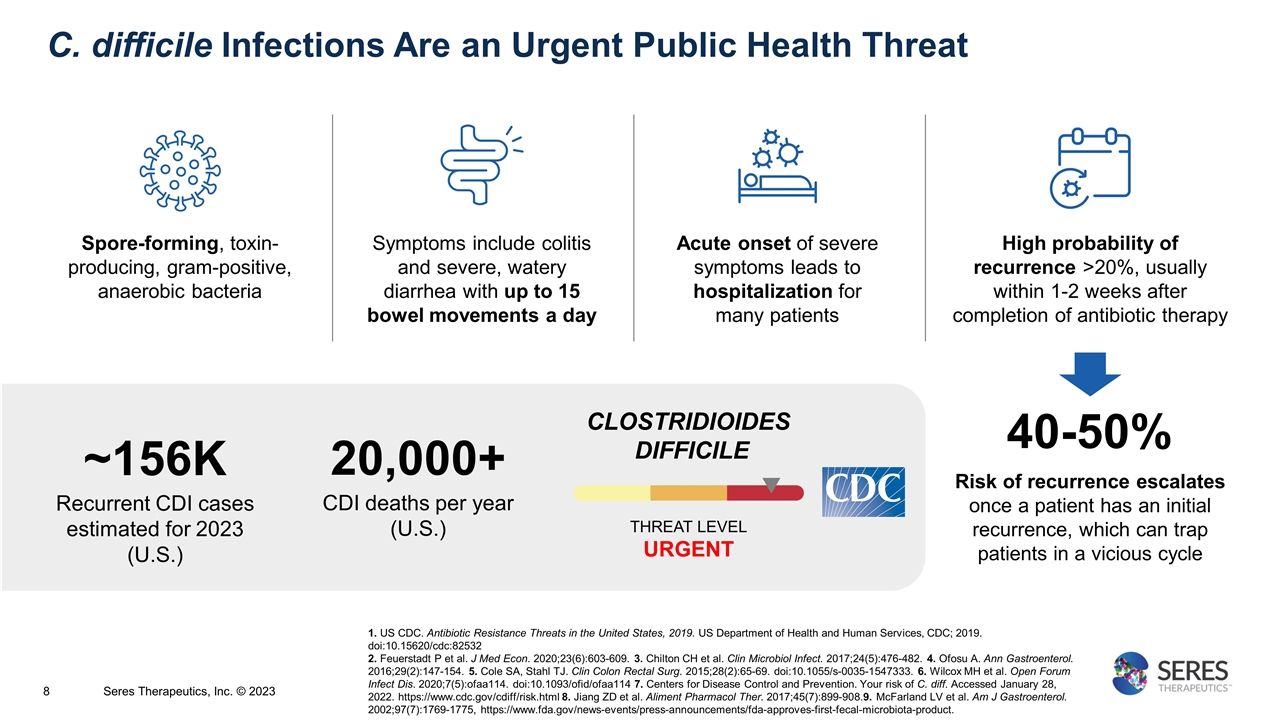
C. difficile Infections Are an Urgent Public Health Threat High probability of recurrence >20%, usually within 1-2 weeks after completion of antibiotic therapy Symptoms include colitis and severe, watery diarrhea with up to 15 bowel movements a day Spore-forming, toxin-producing, gram-positive, anaerobic bacteria Acute onset of severe symptoms leads to hospitalization for many patients Risk of recurrence escalates once a patient has an initial recurrence, which can trap patients in a vicious cycle 40-50% 20,000+ CDI deaths per year (U.S.) CLOSTRIDIOIDES DIFFICILE THREAT LEVEL URGENT ~156K Recurrent CDI cases estimated for 2023 (U.S.) 1. US CDC. Antibiotic Resistance Threats in the United States, 2019. US Department of Health and Human Services, CDC; 2019. doi:10.15620/cdc:82532 2. Feuerstadt P et al. J Med Econ. 2020;23(6):603-609. 3. Chilton CH et al. Clin Microbiol Infect. 2017;24(5):476-482. 4. Ofosu A. Ann Gastroenterol. 2016;29(2):147-154. 5. Cole SA, Stahl TJ. Clin Colon Rectal Surg. 2015;28(2):65-69. doi:10.1055/s-0035-1547333. 6. Wilcox MH et al. Open Forum Infect Dis. 2020;7(5):ofaa114. doi:10.1093/ofid/ofaa114 7. Centers for Disease Control and Prevention. Your risk of C. diff. Accessed January 28, 2022. https://www.cdc.gov/cdiff/risk.html 8. Jiang ZD et al. Aliment Pharmacol Ther. 2017;45(7):899-908.9. McFarland LV et al. Am J Gastroenterol. 2002;97(7):1769-1775, https://www.fda.gov/news-events/press-announcements/fda-approves-first-fecal-microbiota-product. Seres Therapeutics, Inc. © 2023
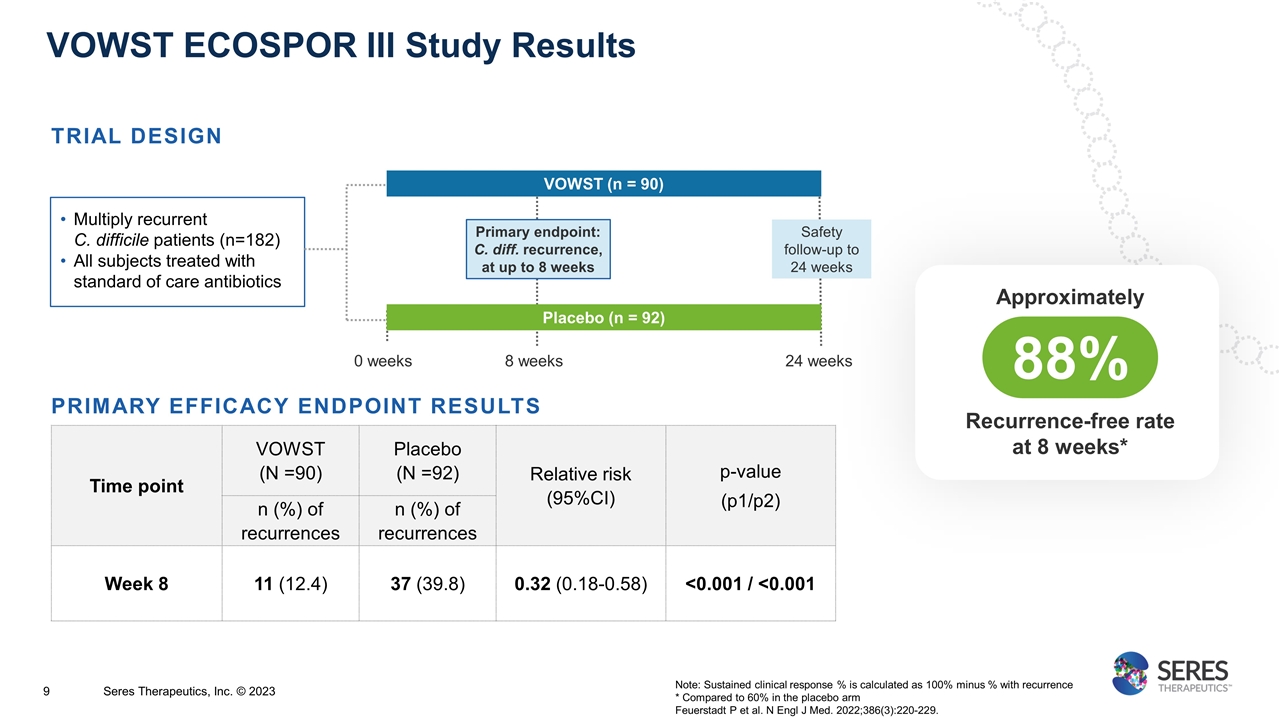
Time point VOWST (N =90) Placebo (N =92) Relative risk (95%CI) p-value (p1/p2) n (%) of recurrences n (%) of recurrences Week 8 11 (12.4) 37 (39.8) 0.32 (0.18-0.58) <0.001 / <0.001 TRIAL DESIGN VOWST ECOSPOR III Study Results Primary endpoint: C. diff. recurrence, at up to 8 weeks VOWST (n = 90) Multiply recurrent C. difficile patients (n=182) All subjects treated with standard of care antibiotics Placebo (n = 92) 0 weeks 8 weeks 24 weeks Safety follow-up to 24 weeks PRIMARY EFFICACY ENDPOINT RESULTS Note: Sustained clinical response % is calculated as 100% minus % with recurrence * Compared to 60% in the placebo arm Feuerstadt P et al. N Engl J Med. 2022;386(3):220-229. Approximately 88% Recurrence-free rate at 8 weeks* Seres Therapeutics, Inc. © 2023
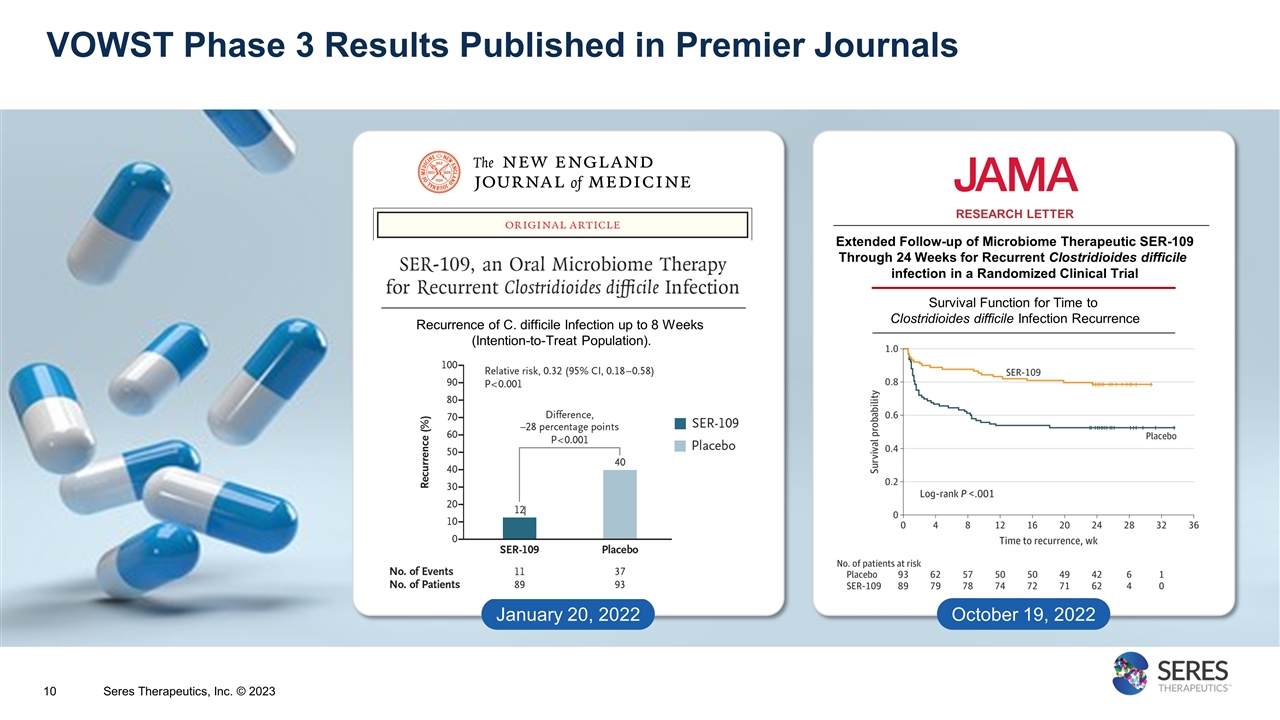
VOWST Phase 3 Results Published in Premier Journals January 20, 2022 Extended Follow-up of Microbiome Therapeutic SER-109 Through 24 Weeks for Recurrent Clostridioides difficile infection in a Randomized Clinical Trial RESEARCH LETTER Survival Function for Time to Clostridioides difficile Infection Recurrence October 19, 2022 Recurrence of C. difficile Infection up to 8 Weeks (Intention-to-Treat Population). Seres Therapeutics, Inc. © 2023
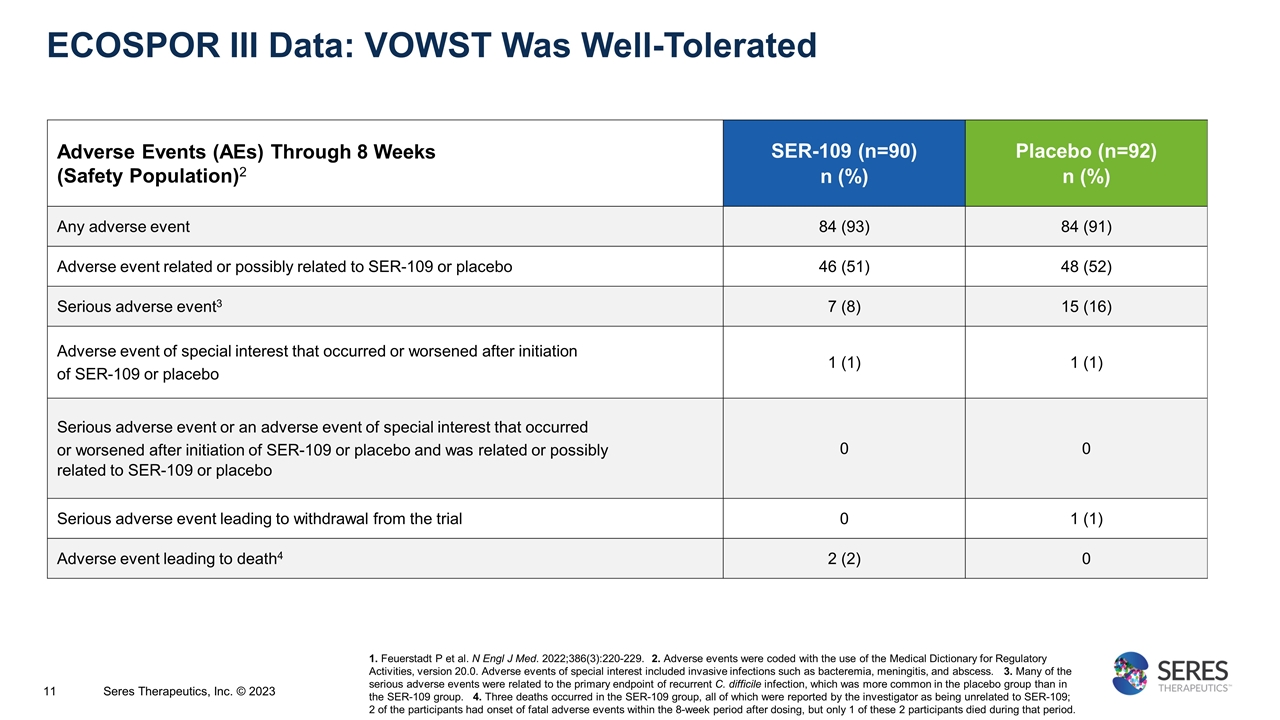
ECOSPOR III Data: VOWST Was Well-Tolerated Adverse Events (AEs) Through 8 Weeks (Safety Population)2 SER-109 (n=90) n (%) Placebo (n=92) n (%) Any adverse event 84 (93) 84 (91) Adverse event related or possibly related to SER-109 or placebo 46 (51) 48 (52) Serious adverse event3 7 (8) 15 (16) Adverse event of special interest that occurred or worsened after initiation of SER-109 or placebo 1 (1) 1 (1) Serious adverse event or an adverse event of special interest that occurred or worsened after initiation of SER-109 or placebo and was related or possibly related to SER-109 or placebo 0 0 Serious adverse event leading to withdrawal from the trial 0 1 (1) Adverse event leading to death4 2 (2) 0 1. Feuerstadt P et al. N Engl J Med. 2022;386(3):220-229. 2. Adverse events were coded with the use of the Medical Dictionary for Regulatory Activities, version 20.0. Adverse events of special interest included invasive infections such as bacteremia, meningitis, and abscess. 3. Many of the serious adverse events were related to the primary endpoint of recurrent C. difficile infection, which was more common in the placebo group than in the SER-109 group. 4. Three deaths occurred in the SER-109 group, all of which were reported by the investigator as being unrelated to SER-109; 2 of the participants had onset of fatal adverse events within the 8-week period after dosing, but only 1 of these 2 participants died during that period. Seres Therapeutics, Inc. © 2023
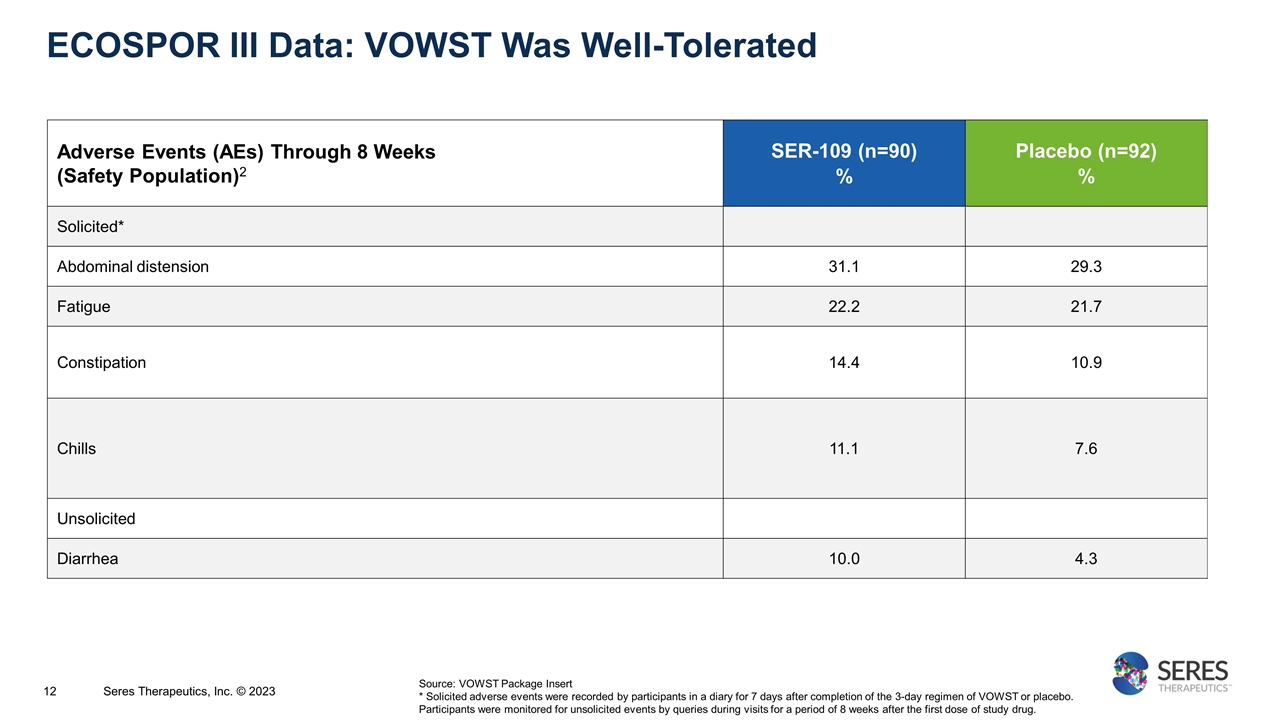
ECOSPOR III Data: VOWST Was Well-Tolerated Adverse Events (AEs) Through 8 Weeks (Safety Population)2 SER-109 (n=90) % Placebo (n=92) % Solicited* Abdominal distension 31.1 29.3 Fatigue 22.2 21.7 Constipation 14.4 10.9 Chills 11.1 7.6 Unsolicited Diarrhea 10.0 4.3 Source: VOWST Package Insert * Solicited adverse events were recorded by participants in a diary for 7 days after completion of the 3-day regimen of VOWST or placebo. Participants were monitored for unsolicited events by queries during visits for a period of 8 weeks after the first dose of study drug. Seres Therapeutics, Inc. © 2023
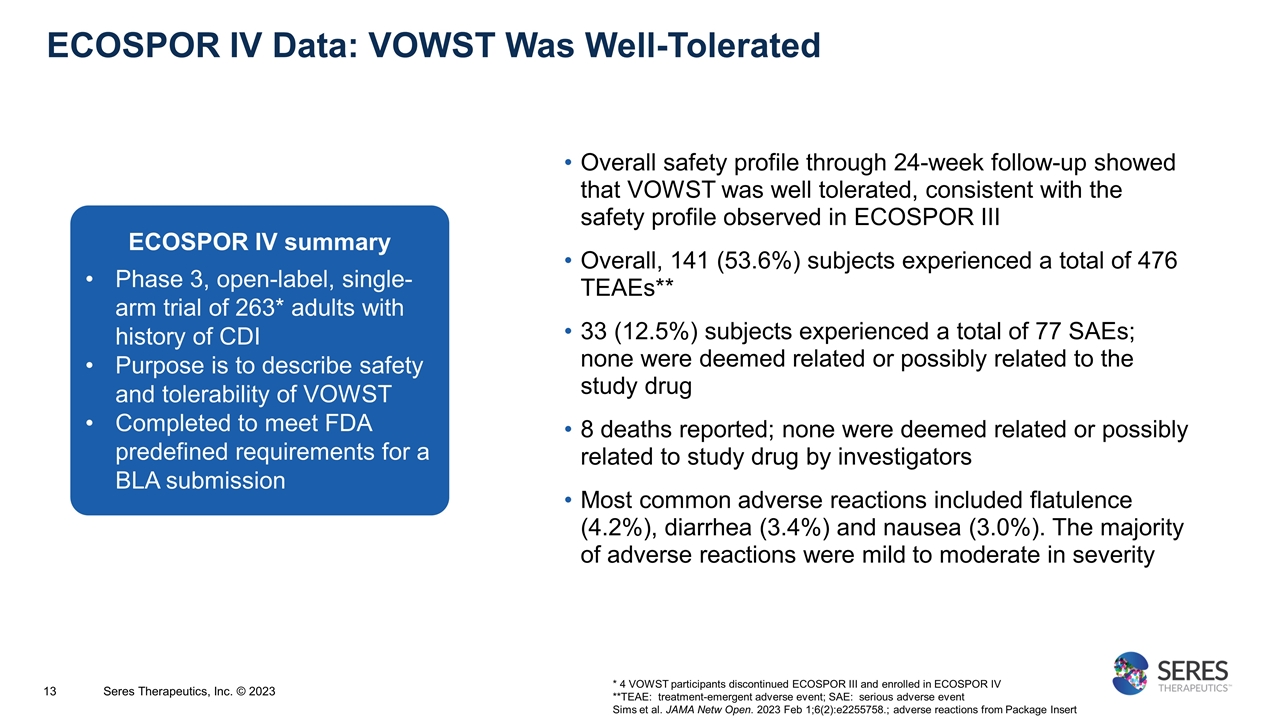
ECOSPOR IV Data: VOWST Was Well-Tolerated * 4 VOWST participants discontinued ECOSPOR III and enrolled in ECOSPOR IV **TEAE: treatment-emergent adverse event; SAE: serious adverse event Sims et al. JAMA Netw Open. 2023 Feb 1;6(2):e2255758.; adverse reactions from Package Insert Seres Therapeutics, Inc. © 2023 Overall safety profile through 24-week follow-up showed that VOWST was well tolerated, consistent with the safety profile observed in ECOSPOR III Overall, 141 (53.6%) subjects experienced a total of 476 TEAEs** 33 (12.5%) subjects experienced a total of 77 SAEs; none were deemed related or possibly related to the study drug 8 deaths reported; none were deemed related or possibly related to study drug by investigators Most common adverse reactions included flatulence (4.2%), diarrhea (3.4%) and nausea (3.0%). The majority of adverse reactions were mild to moderate in severity ECOSPOR IV summary Phase 3, open-label, single-arm trial of 263* adults with history of CDI Purpose is to describe safety and tolerability of VOWST Completed to meet FDA predefined requirements for a BLA submission
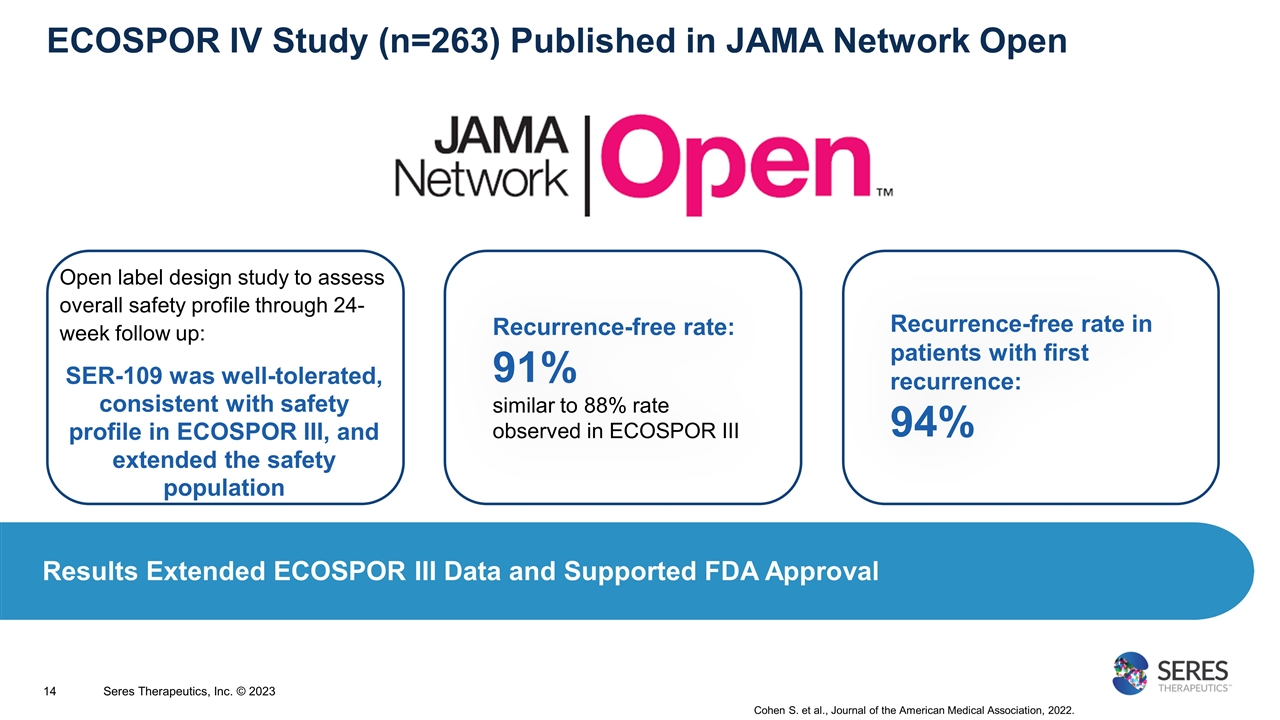
ECOSPOR IV Study (n=263) Published in JAMA Network Open Open label design study to assess overall safety profile through 24-week follow up: SER-109 was well-tolerated, consistent with safety profile in ECOSPOR III, and extended the safety population Recurrence-free rate: 91% similar to 88% rate observed in ECOSPOR III Recurrence-free rate in patients with first recurrence: 94% Cohen S. et al., Journal of the American Medical Association, 2022. Results Extended ECOSPOR III Data and Supported FDA Approval Seres Therapeutics, Inc. © 2023
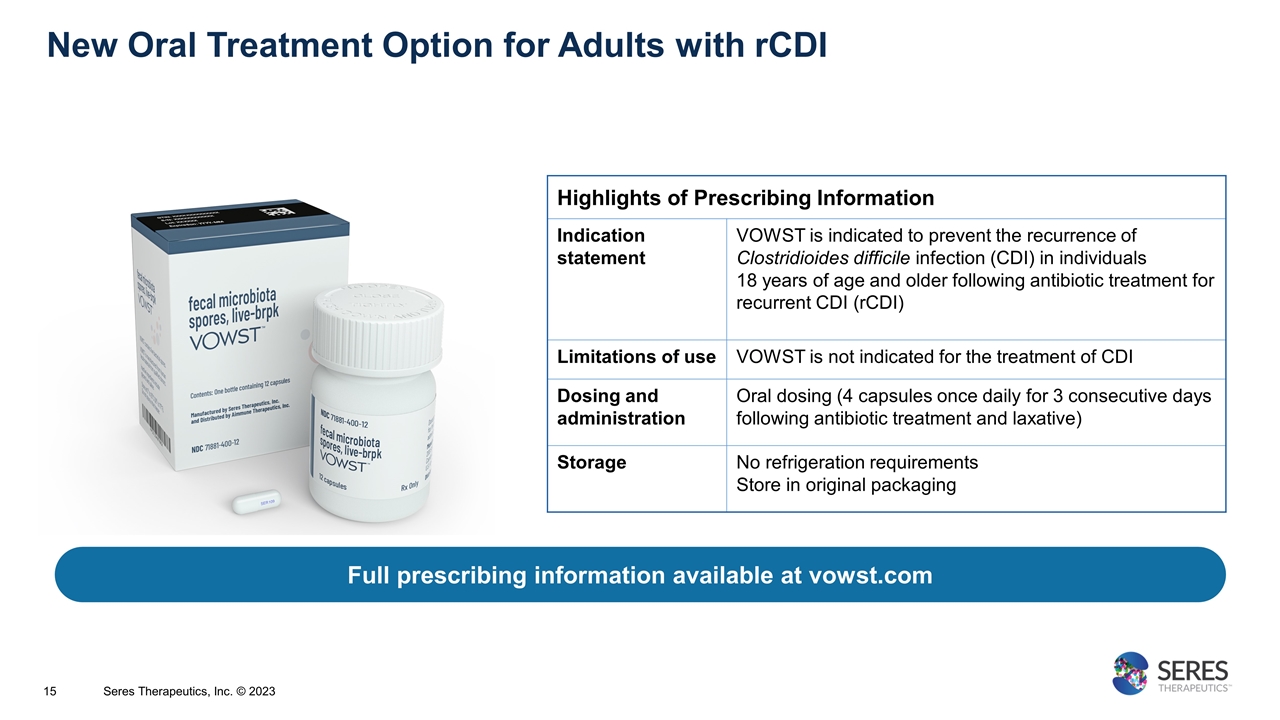
Full prescribing information available at vowst.com Highlights of Prescribing Information Indication statement VOWST is indicated to prevent the recurrence of Clostridioides difficile infection (CDI) in individuals 18 years of age and older following antibiotic treatment for recurrent CDI (rCDI) Limitations of use VOWST is not indicated for the treatment of CDI Dosing and administration Oral dosing (4 capsules once daily for 3 consecutive days following antibiotic treatment and laxative) Storage No refrigeration requirements Store in original packaging New Oral Treatment Option for Adults with rCDI Seres Therapeutics, Inc. © 2023
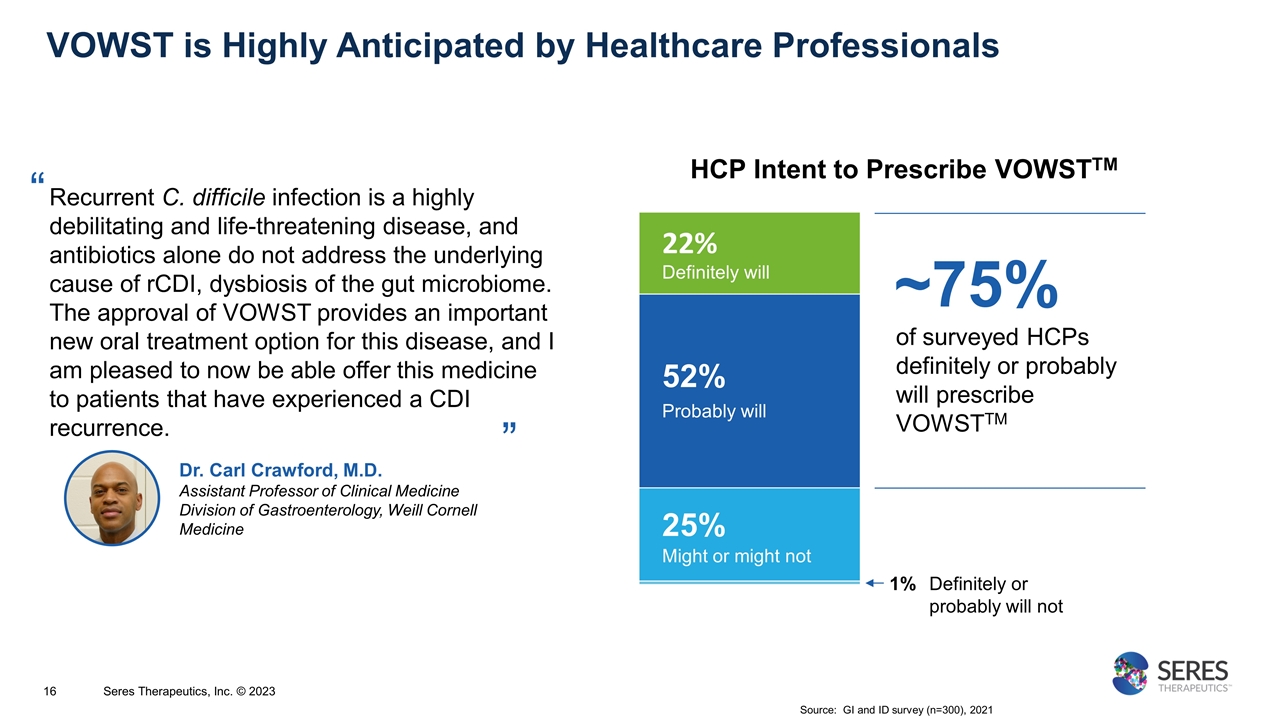
“ VOWST is Highly Anticipated by Healthcare Professionals Source: GI and ID survey (n=300), 2021 Dr. Carl Crawford, M.D. Assistant Professor of Clinical Medicine Division of Gastroenterology, Weill Cornell Medicine Recurrent C. difficile infection is a highly debilitating and life-threatening disease, and antibiotics alone do not address the underlying cause of rCDI, dysbiosis of the gut microbiome. The approval of VOWST provides an important new oral treatment option for this disease, and I am pleased to now be able offer this medicine to patients that have experienced a CDI recurrence. Definitely or probably will not Probably will Might or might not Definitely will of surveyed HCPs definitely or probably will prescribe VOWSTTM 22% 52% 25% 1% ~75% HCP Intent to Prescribe VOWSTTM ” Seres Therapeutics, Inc. © 2023
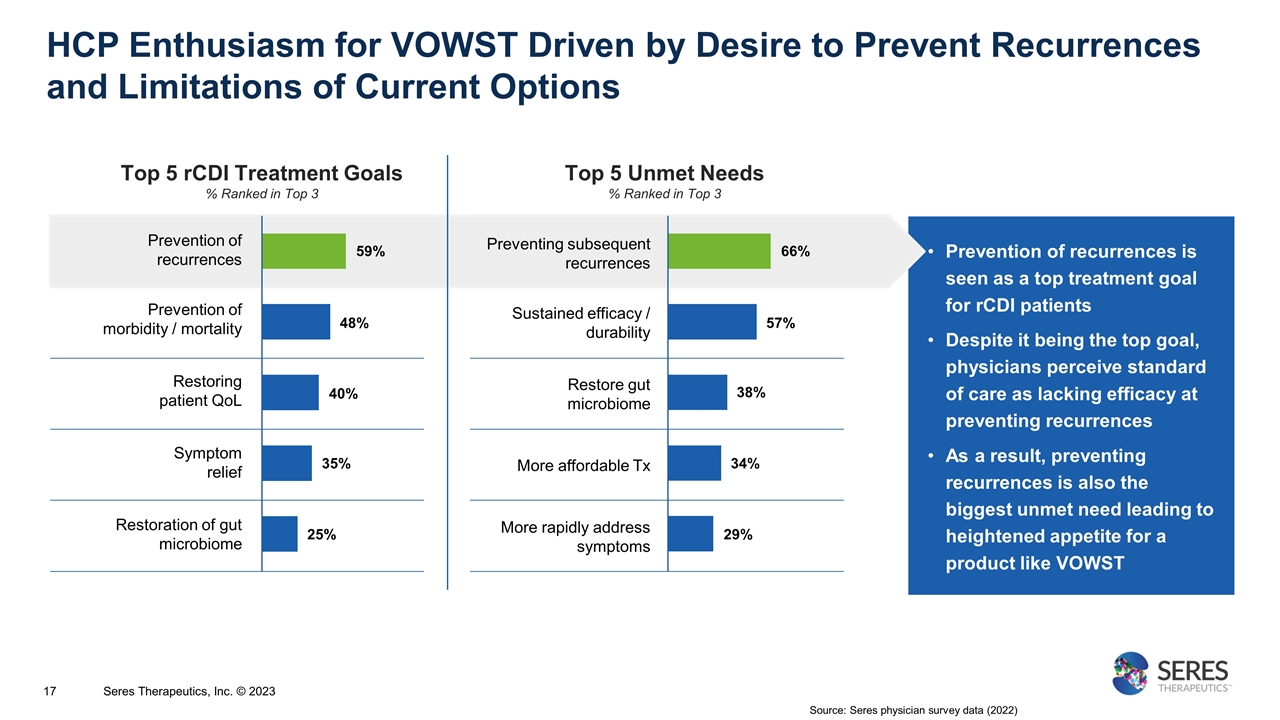
Prevention of recurrences is seen as a top treatment goal for rCDI patients Despite it being the top goal, physicians perceive standard of care as lacking efficacy at preventing recurrences As a result, preventing recurrences is also the biggest unmet need leading to heightened appetite for a product like VOWST HCP Enthusiasm for VOWST Driven by Desire to Prevent Recurrences and Limitations of Current Options 17 Top 5 rCDI Treatment Goals % Ranked in Top 3 Prevention of recurrences Prevention of morbidity / mortality Restoring patient QoL Symptom relief Restoration of gut microbiome Preventing subsequent recurrences Sustained efficacy / durability Restore gut microbiome More affordable Tx More rapidly address symptoms Top 5 Unmet Needs % Ranked in Top 3 59% 48% 40% 35% 25% 66% 57% 38% 34% 29% Source: Seres physician survey data (2022) Seres Therapeutics, Inc. © 2023
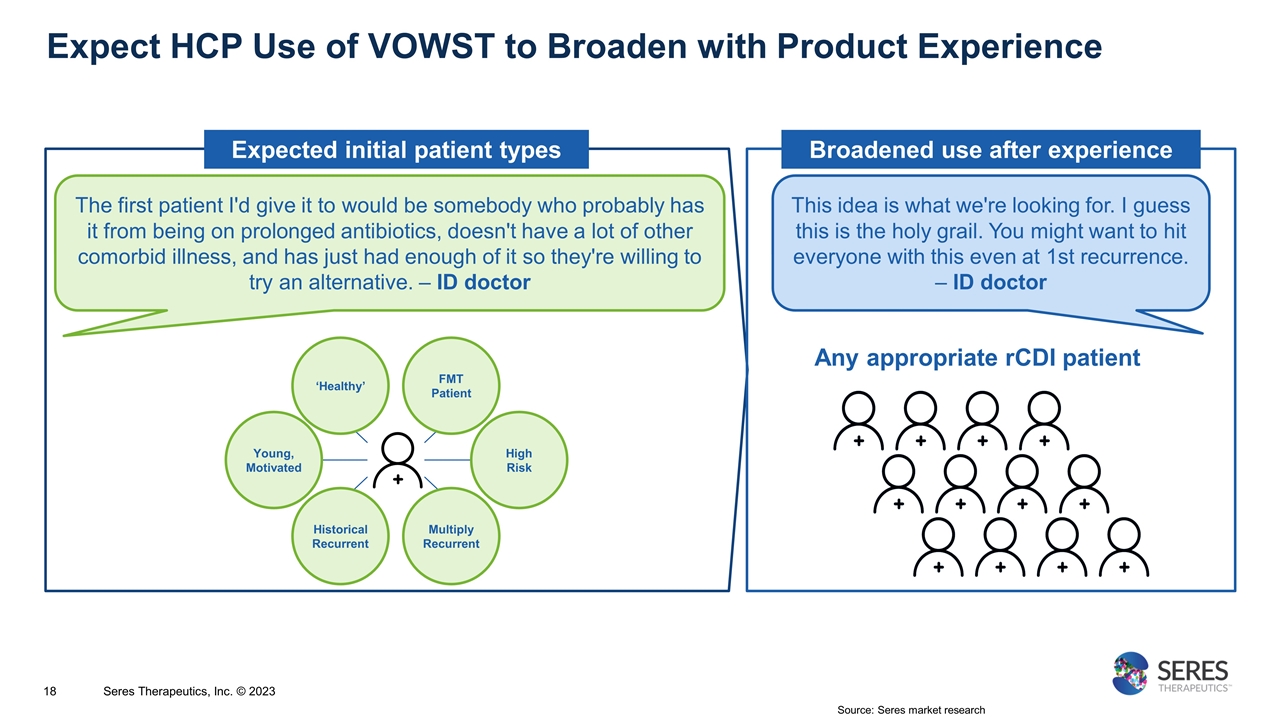
Expect HCP Use of VOWST to Broaden with Product Experience Source: Seres market research Seres Therapeutics, Inc. © 2023 Expected initial patient types The first patient I'd give it to would be somebody who probably has it from being on prolonged antibiotics, doesn't have a lot of other comorbid illness, and has just had enough of it so they're willing to try an alternative. – ID doctor This idea is what we're looking for. I guess this is the holy grail. You might want to hit everyone with this even at 1st recurrence. – ID doctor Young, Motivated High Risk Historical Recurrent ‘Healthy’ Multiply Recurrent FMT Patient Broadened use after experience Any appropriate rCDI patient
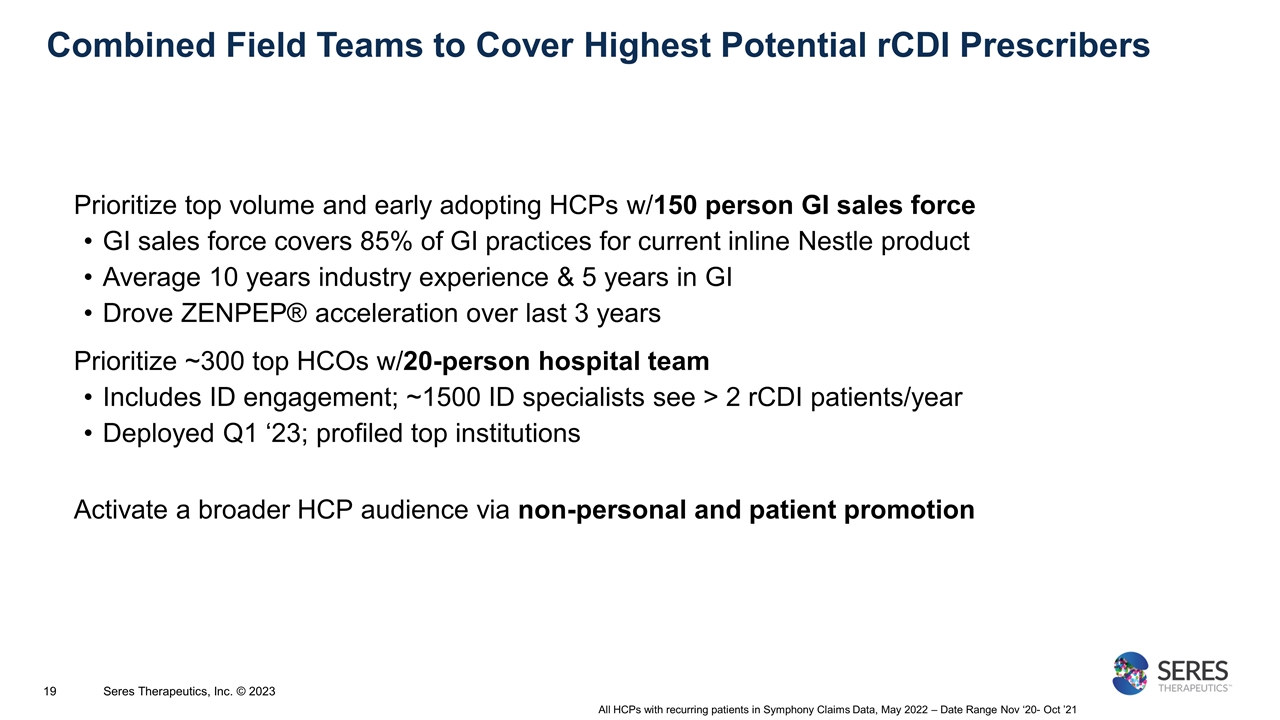
Combined Field Teams to Cover Highest Potential rCDI Prescribers Prioritize top volume and early adopting HCPs w/150 person GI sales force GI sales force covers 85% of GI practices for current inline Nestle product Average 10 years industry experience & 5 years in GI Drove ZENPEP® acceleration over last 3 years Prioritize ~300 top HCOs w/20-person hospital team Includes ID engagement; ~1500 ID specialists see > 2 rCDI patients/year Deployed Q1 ‘23; profiled top institutions Activate a broader HCP audience via non-personal and patient promotion All HCPs with recurring patients in Symphony Claims Data, May 2022 – Date Range Nov ‘20- Oct ’21 Seres Therapeutics, Inc. © 2023
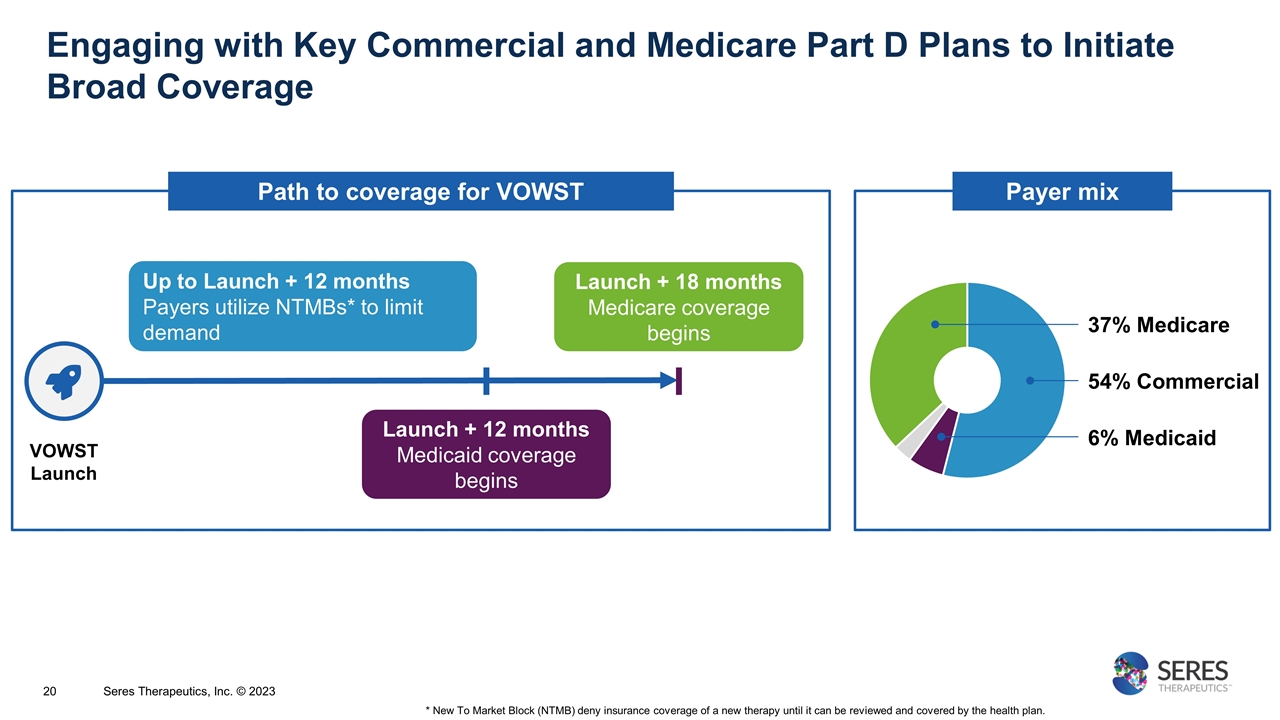
Engaging with Key Commercial and Medicare Part D Plans to Initiate Broad Coverage * New To Market Block (NTMB) deny insurance coverage of a new therapy until it can be reviewed and covered by the health plan. Seres Therapeutics, Inc. © 2023 VOWST Launch Up to Launch + 12 months Payers utilize NTMBs* to limit demand Launch + 18 months Medicare coverage begins Launch + 12 months Medicaid coverage begins 54% Commercial 37% Medicare 6% Medicaid Payer mix Path to coverage for VOWST
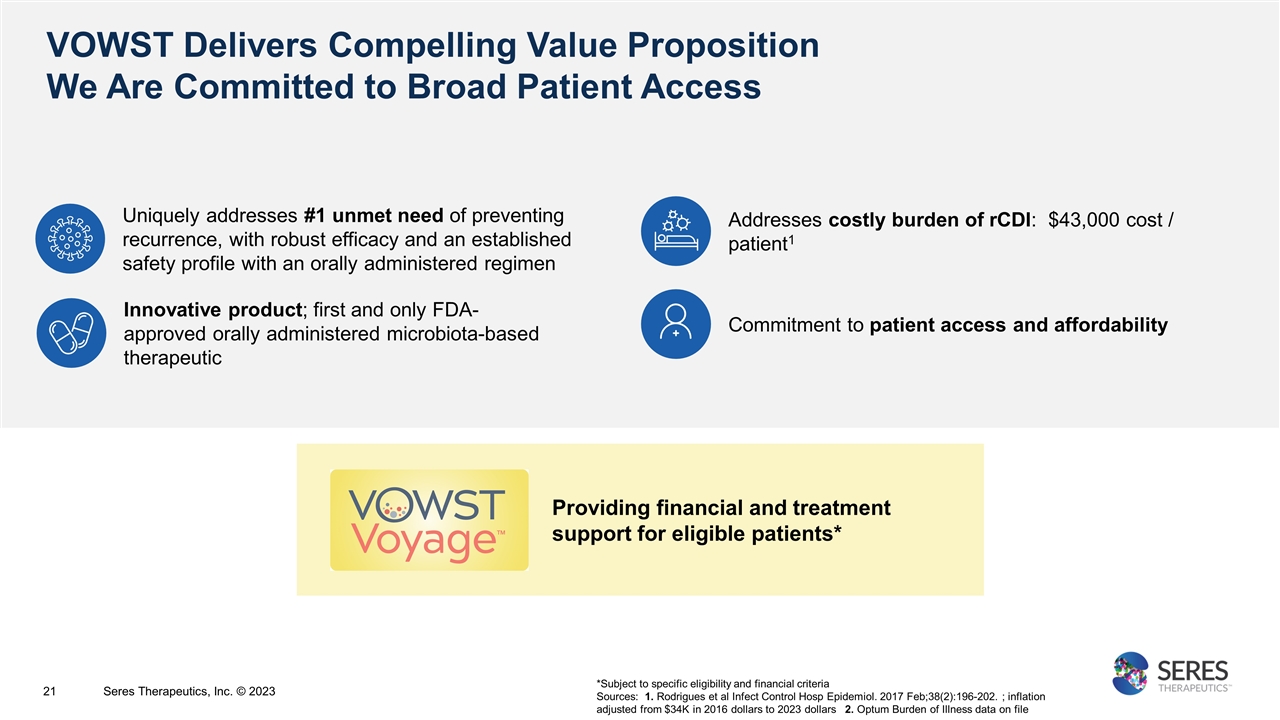
*Subject to specific eligibility and financial criteria Sources: 1. Rodrigues et al Infect Control Hosp Epidemiol. 2017 Feb;38(2):196-202. ; inflation adjusted from $34K in 2016 dollars to 2023 dollars 2. Optum Burden of Illness data on file VOWST Delivers Compelling Value Proposition We Are Committed to Broad Patient Access 21 Seres Therapeutics, Inc. © 2023 Uniquely addresses #1 unmet need of preventing recurrence, with robust efficacy and an established safety profile with an orally administered regimen Innovative product; first and only FDA-approved orally administered microbiota-based therapeutic Addresses costly burden of rCDI: $43,000 cost / patient1 Commitment to patient access and affordability Providing financial and treatment support for eligible patients*
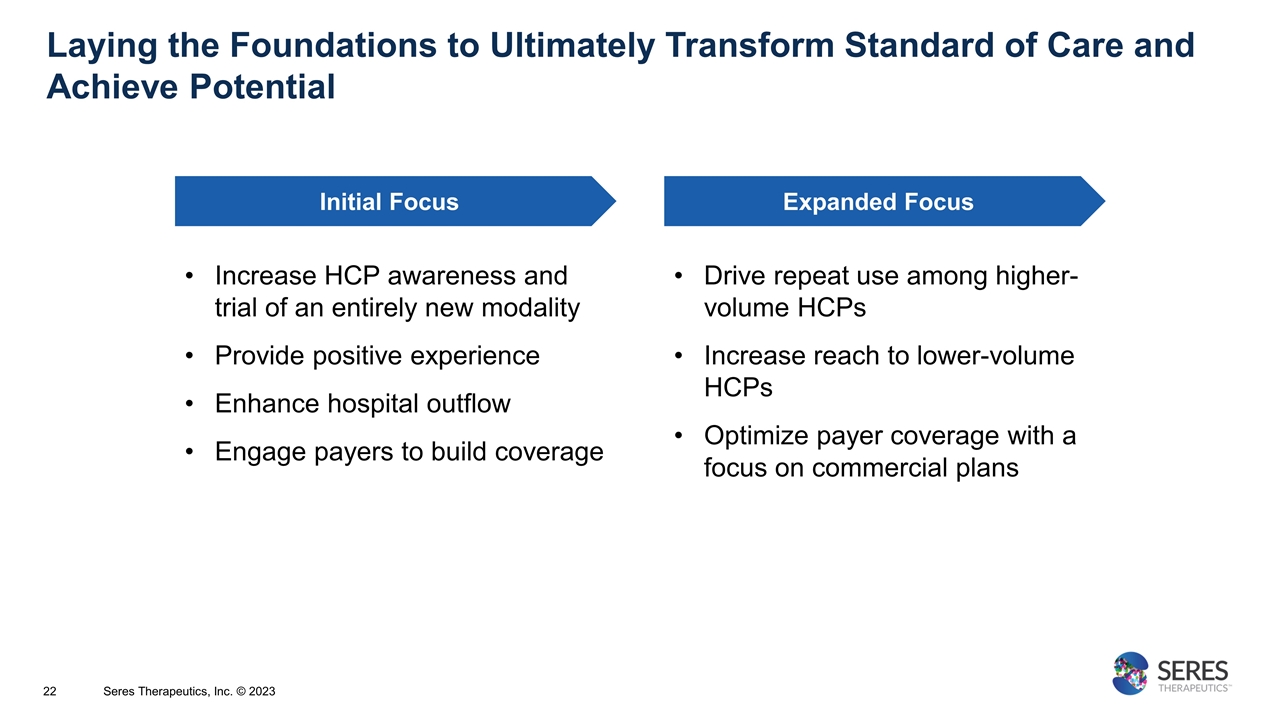
Laying the Foundations to Ultimately Transform Standard of Care and Achieve Potential Initial Focus Expanded Focus Increase HCP awareness and trial of an entirely new modality Provide positive experience Enhance hospital outflow Engage payers to build coverage Drive repeat use among higher-volume HCPs Increase reach to lower-volume HCPs Optimize payer coverage with a focus on commercial plans Seres Therapeutics, Inc. © 2023

Co-commercializing VOWST in the United States with 50/50 profit sharing per July 2021 agreement, extending our global strategic collaboration Seres Therapeutics, Inc. © 2023
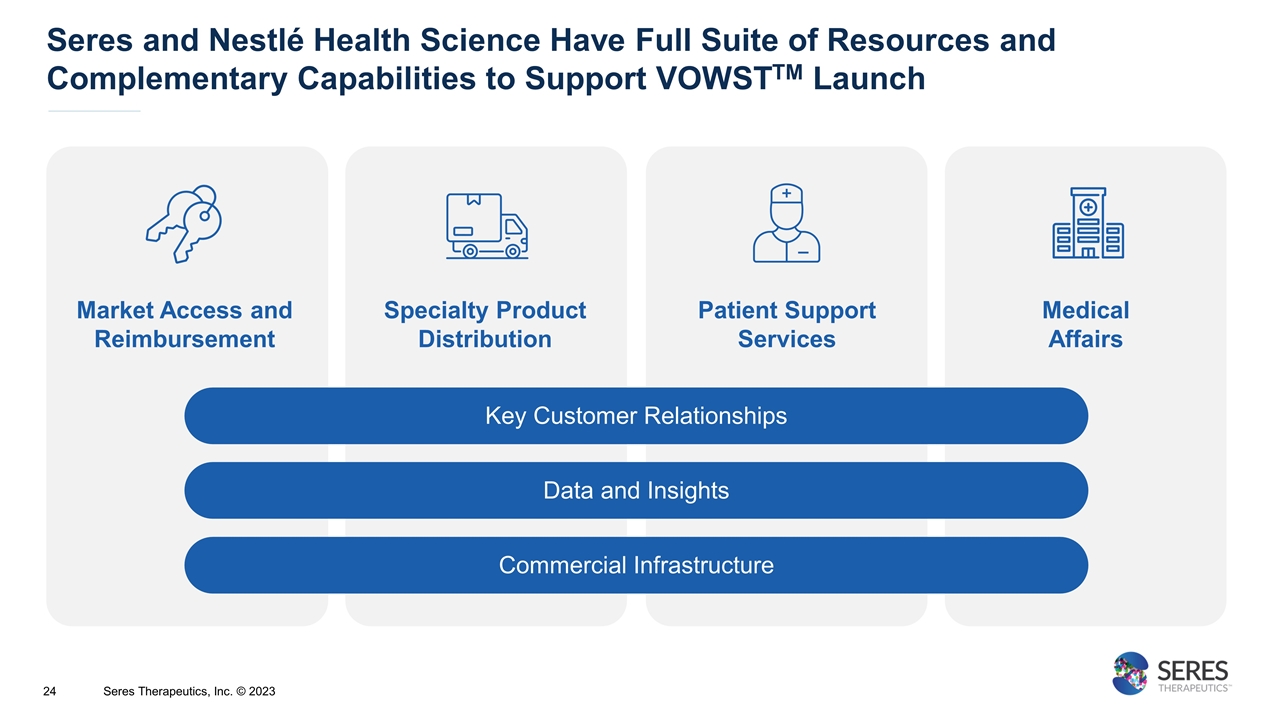
Seres and Nestlé Health Science Have Full Suite of Resources and Complementary Capabilities to Support VOWSTTM Launch Market Access and Reimbursement Specialty Product Distribution Patient Support Services Medical Affairs Data and Insights Commercial Infrastructure Key Customer Relationships Seres Therapeutics, Inc. © 2023
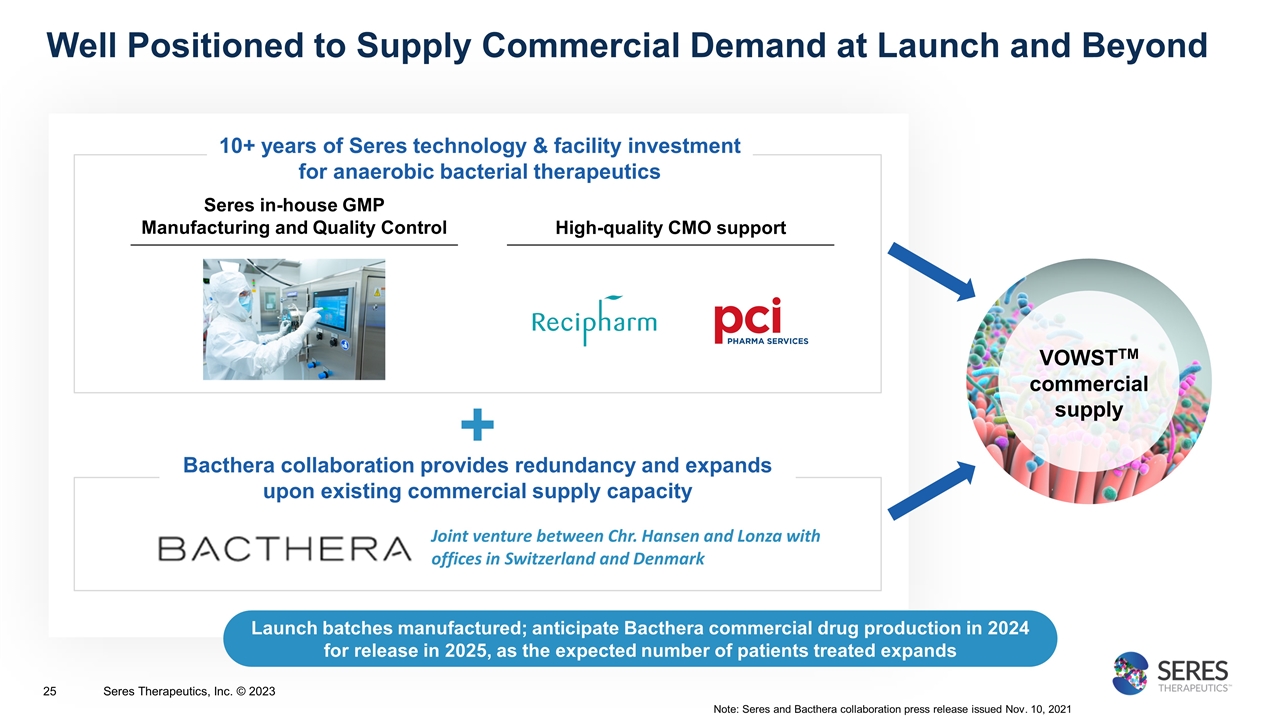
Well Positioned to Supply Commercial Demand at Launch and Beyond VOWSTTM commercial supply Note: Seres and Bacthera collaboration press release issued Nov. 10, 2021 + 10+ years of Seres technology & facility investment for anaerobic bacterial therapeutics Joint venture between Chr. Hansen and Lonza with offices in Switzerland and Denmark Bacthera collaboration provides redundancy and expands upon existing commercial supply capacity Launch batches manufactured; anticipate Bacthera commercial drug production in 2024 for release in 2025, as the expected number of patients treated expands Seres Therapeutics, Inc. © 2023 Seres in-house GMP Manufacturing and Quality Control High-quality CMO support
SER-155 and Infection Protection Franchise Seres Therapeutics, Inc. © 2023
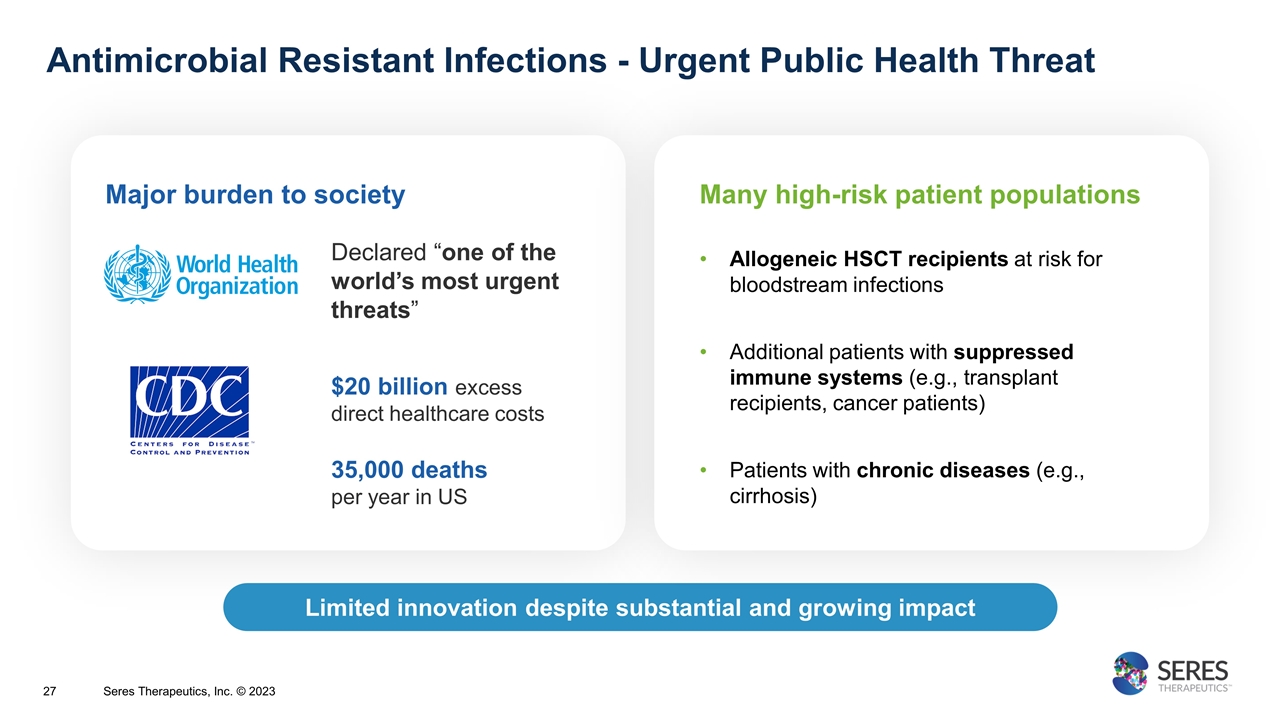
Antimicrobial Resistant Infections - Urgent Public Health Threat Limited innovation despite substantial and growing impact Declared “one of the world’s most urgent threats” $20 billion excess direct healthcare costs 35,000 deaths per year in US Major burden to society Many high-risk patient populations Allogeneic HSCT recipients at risk for bloodstream infections Additional patients with suppressed immune systems (e.g., transplant recipients, cancer patients) Patients with chronic diseases (e.g., cirrhosis) Seres Therapeutics, Inc. © 2023
SER-155 May Represent a Novel Solution to Reduce GI Pathogen Abundance and Infection & GvHD in Allogeneic HSCT Enrollment ongoing in SER-155 Phase 1b Cohort 2 a randomized, double-blind, placebo-controlled study Expect to release topline results in mid-2024 SER-155 is an oral, cultivated consortium, designed to reduce abundance of pathogens linked to infections and GvHD in allogeneic HSCT recipients* SER-155 Phase 1b study Cohort 1 SER-155 was well-tolerated through 100 Days post HSCT SER-155 bacterial strain engraftment was as expected GI pathogen domination was rare and transient in patients after SER-155 treatment compared to expected rates from prior cohort studies *Note: SER-155 is an investigational therapeutic and has not been approved by any regulatory authority, including the US Food & Drug Administration Seres Therapeutics, Inc. © 2023 Seres Therapeutics, Inc. © 2023
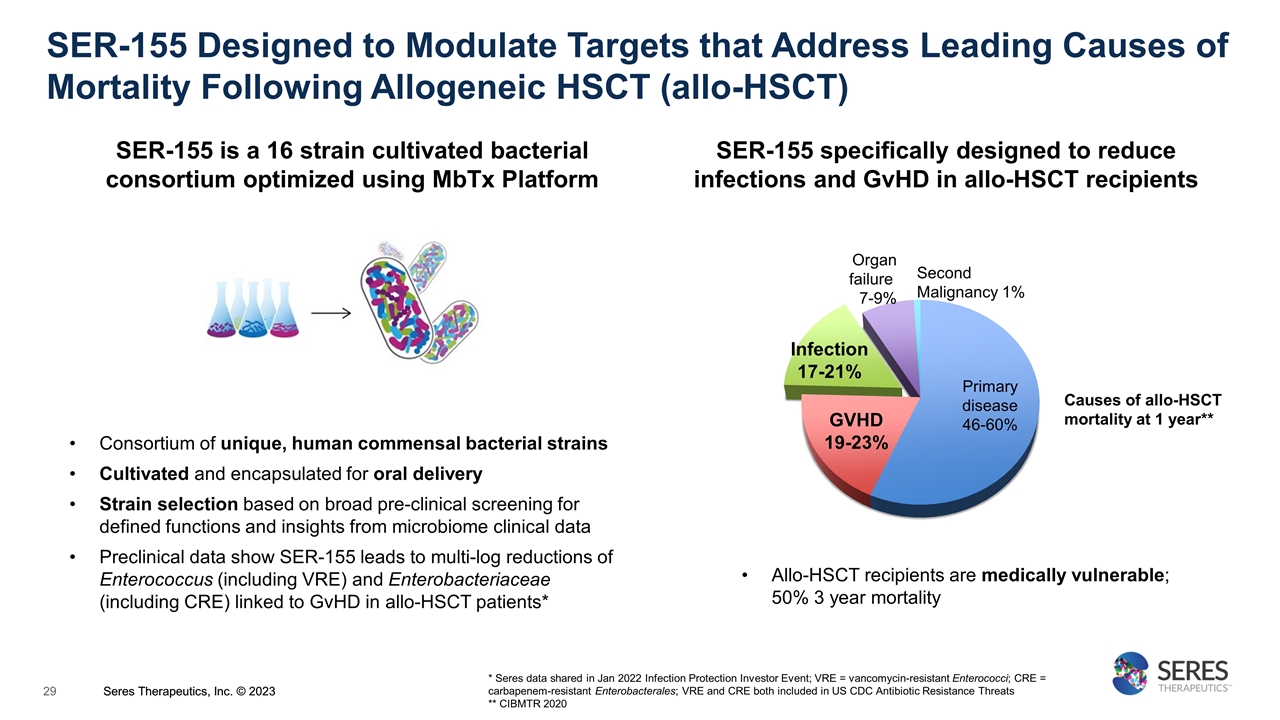
SER-155 Designed to Modulate Targets that Address Leading Causes of Mortality Following Allogeneic HSCT (allo-HSCT) Consortium of unique, human commensal bacterial strains Cultivated and encapsulated for oral delivery Strain selection based on broad pre-clinical screening for defined functions and insights from microbiome clinical data Preclinical data show SER-155 leads to multi-log reductions of Enterococcus (including VRE) and Enterobacteriaceae (including CRE) linked to GvHD in allo-HSCT patients* SER-155 is a 16 strain cultivated bacterial consortium optimized using MbTx Platform Allo-HSCT recipients are medically vulnerable; 50% 3 year mortality Organ failure 7-9% GVHD 19-23% Primary disease 46-60% Second Malignancy 1% Infection 17-21% * Seres data shared in Jan 2022 Infection Protection Investor Event; VRE = vancomycin-resistant Enterococci; CRE = carbapenem-resistant Enterobacterales; VRE and CRE both included in US CDC Antibiotic Resistance Threats ** CIBMTR 2020 SER-155 specifically designed to reduce infections and GvHD in allo-HSCT recipients Causes of allo-HSCT mortality at 1 year** Seres Therapeutics, Inc. © 2023 Seres Therapeutics, Inc. © 2023
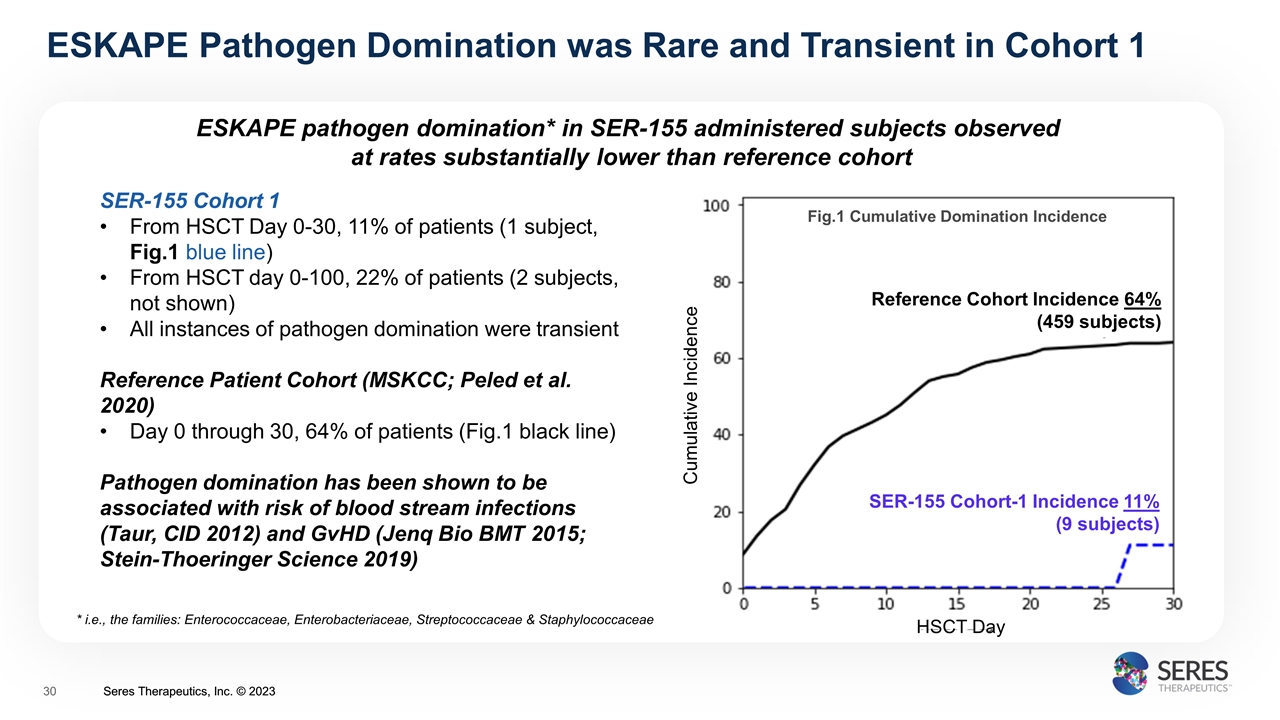
ESKAPE pathogen domination* in SER-155 administered subjects observed at rates substantially lower than reference cohort ESKAPE Pathogen Domination was Rare and Transient in Cohort 1 SER-155 Cohort 1 From HSCT Day 0-30, 11% of patients (1 subject, Fig.1 blue line) From HSCT day 0-100, 22% of patients (2 subjects, not shown) All instances of pathogen domination were transient Reference Patient Cohort (MSKCC; Peled et al. 2020) Day 0 through 30, 64% of patients (Fig.1 black line) Pathogen domination has been shown to be associated with risk of blood stream infections (Taur, CID 2012) and GvHD (Jenq Bio BMT 2015; Stein-Thoeringer Science 2019) Cumulative Incidence HSCT Day Fig.1 Cumulative Domination Incidence Reference Cohort Incidence 64% (459 subjects) SER-155 Cohort-1 Incidence 11% (9 subjects) * i.e., the families: Enterococcaceae, Enterobacteriaceae, Streptococcaceae & Staphylococcaceae Seres Therapeutics, Inc. © 2023 Seres Therapeutics, Inc. © 2023
SER-155 Was Generally Well-Tolerated in Cohort 1 (Day 100 Data) TEAE: treatment-emergent adverse event; SAE: serious adverse event; AESI: adverse event of special interest; SUSAR: suspected unexpected serious adverse reaction TEAEs observed as expected in this patient population All subjects experienced at least 1 TEAE 1 TEAE resulted in study discontinuation (unrelated to SER-155 administration) GI disorders were most common, with diarrhea being the most common AE No SAEs were considered related to SER-155 No SUSARs observed Majority of SAEs and AESIs occurred during vulnerable time for patients (from HSCT to neutrophil recovery, start of SER-155 Course 2) Data Safety Monitoring Board approved advancement to Cohort 2 Data Safety Monitoring Board met at predefined points, including at Day 100 data cut for Cohort 1, to review all safety events No deaths prior to Day 100; 3 after Day 100, none considered related to drug Seres Therapeutics, Inc. © 2023 Seres Therapeutics, Inc. © 2023
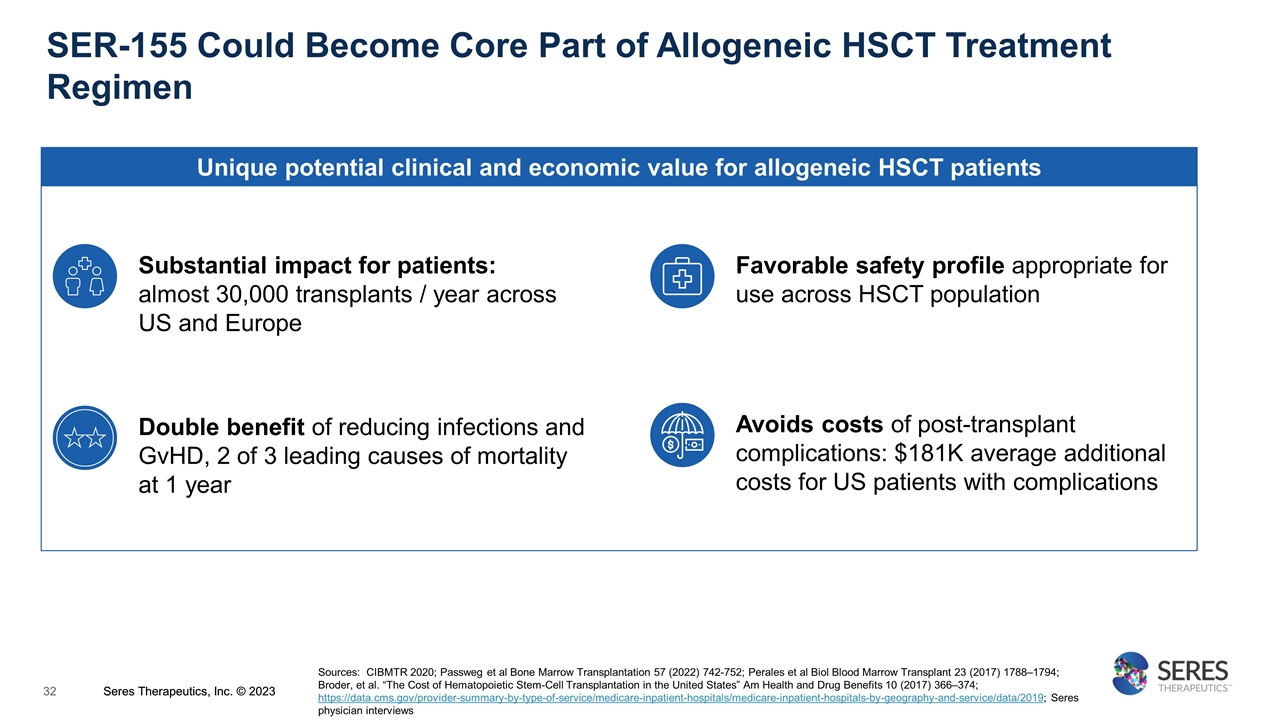
SER-155 Could Become Core Part of Allogeneic HSCT Treatment Regimen Unique potential clinical and economic value for allogeneic HSCT patients Double benefit of reducing infections and GvHD, 2 of 3 leading causes of mortality at 1 year Avoids costs of post-transplant complications: $181K average additional costs for US patients with complications Sources: CIBMTR 2020; Passweg et al Bone Marrow Transplantation 57 (2022) 742-752; Perales et al Biol Blood Marrow Transplant 23 (2017) 1788–1794; Broder, et al. “The Cost of Hematopoietic Stem-Cell Transplantation in the United States” Am Health and Drug Benefits 10 (2017) 366–374; https://data.cms.gov/provider-summary-by-type-of-service/medicare-inpatient-hospitals/medicare-inpatient-hospitals-by-geography-and-service/data/2019; Seres physician interviews Favorable safety profile appropriate for use across HSCT population Substantial impact for patients: almost 30,000 transplants / year across US and Europe Seres Therapeutics, Inc. © 2023 Seres Therapeutics, Inc. © 2023
Seres’ Path Forward Seres Therapeutics, Inc. © 2023
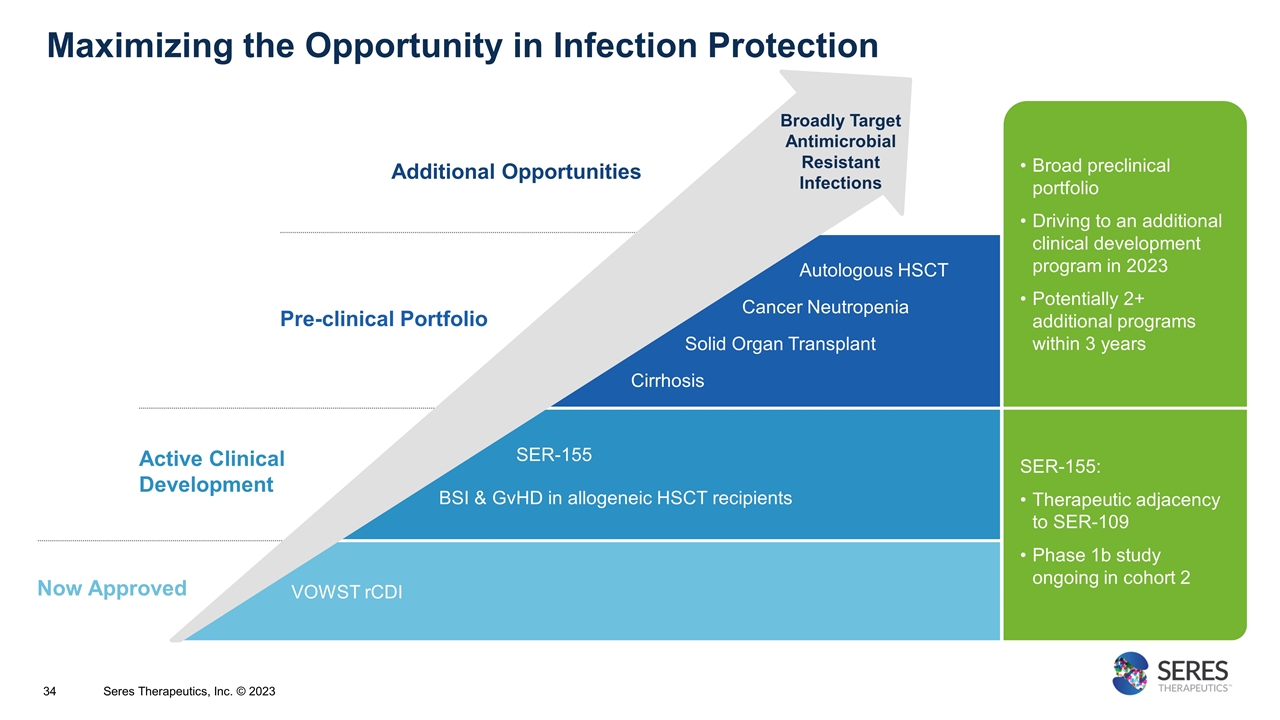
VOWST rCDI SER-155 BSI & GvHD in allogeneic HSCT recipients Broad preclinical portfolio Driving to an additional clinical development program in 2023 Potentially 2+ additional programs within 3 years SER-155: Therapeutic adjacency to SER-109 Phase 1b study ongoing in cohort 2 Additional Opportunities Now Approved Active Clinical Development Pre-clinical Portfolio Autologous HSCT Cancer Neutropenia Solid Organ Transplant Cirrhosis Maximizing the Opportunity in Infection Protection Broadly Target Antimicrobial Resistant Infections Seres Therapeutics, Inc. © 2023
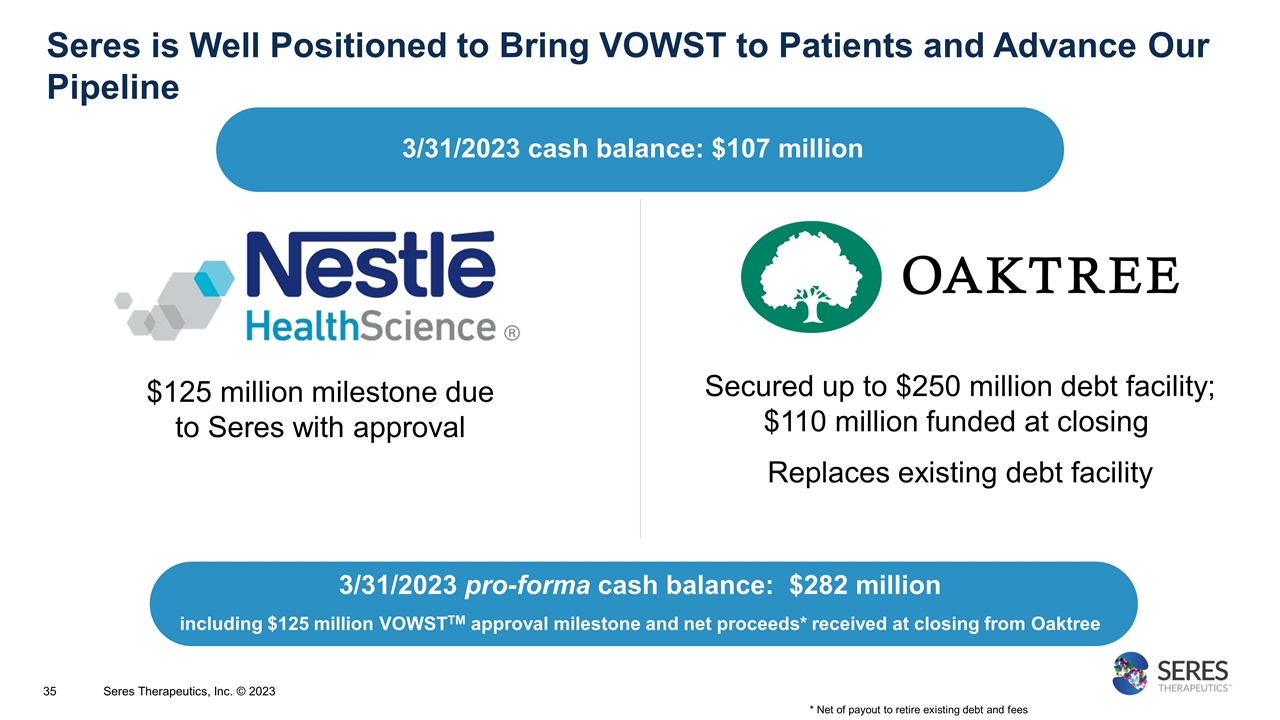
Seres is Well Positioned to Bring VOWST to Patients and Advance Our Pipeline $125 million milestone due to Seres with approval Secured up to $250 million debt facility; $110 million funded at closing Replaces existing debt facility * Net of payout to retire existing debt and fees 3/31/2023 cash balance: $107 million 3/31/2023 pro-forma cash balance: $282 million including $125 million VOWSTTM approval milestone and net proceeds* received at closing from Oaktree Seres Therapeutics, Inc. © 2023
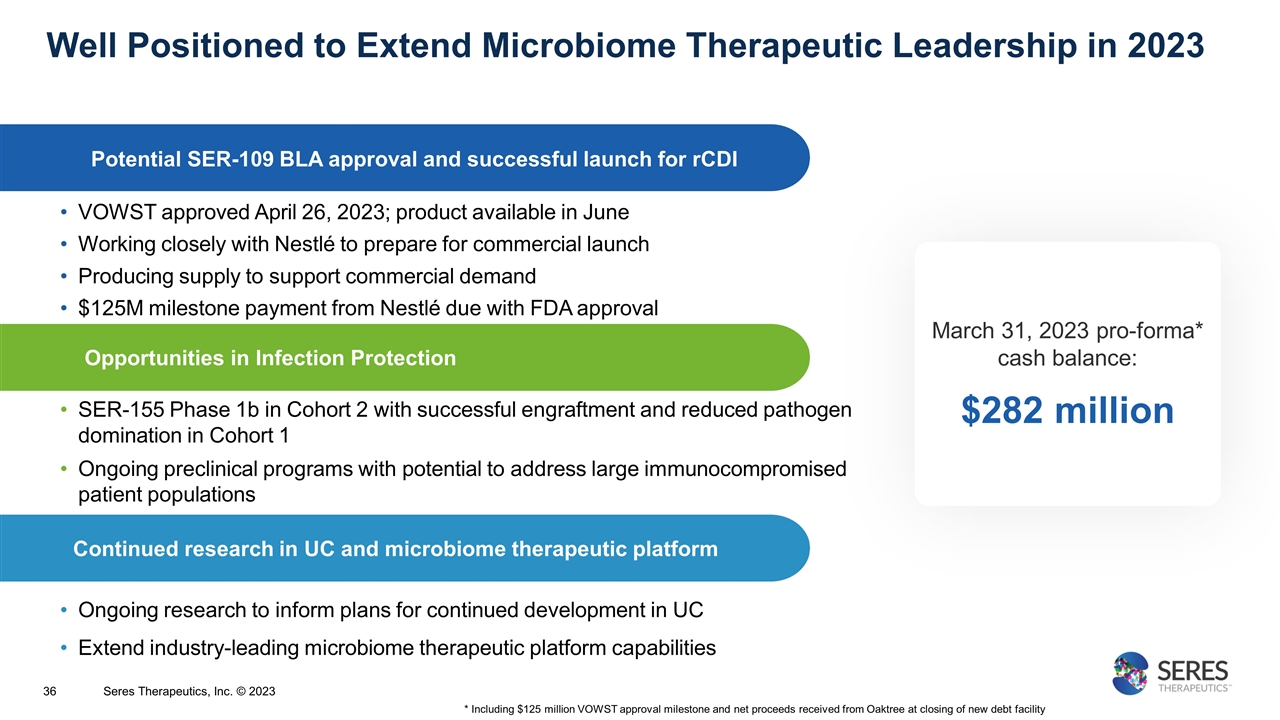
SER-155 Phase 1b in Cohort 2 with successful engraftment and reduced pathogen domination in Cohort 1 Ongoing preclinical programs with potential to address large immunocompromised patient populations Well Positioned to Extend Microbiome Therapeutic Leadership in 2023 Opportunities in Infection Protection Continued research in UC and microbiome therapeutic platform Ongoing research to inform plans for continued development in UC Extend industry-leading microbiome therapeutic platform capabilities Potential SER-109 BLA approval and successful launch for rCDI VOWST approved April 26, 2023; product available in June Working closely with Nestlé to prepare for commercial launch Producing supply to support commercial demand $125M milestone payment from Nestlé due with FDA approval March 31, 2023 pro-forma* cash balance: $282 million * Including $125 million VOWST approval milestone and net proceeds received from Oaktree at closing of new debt facility Seres Therapeutics, Inc. © 2023
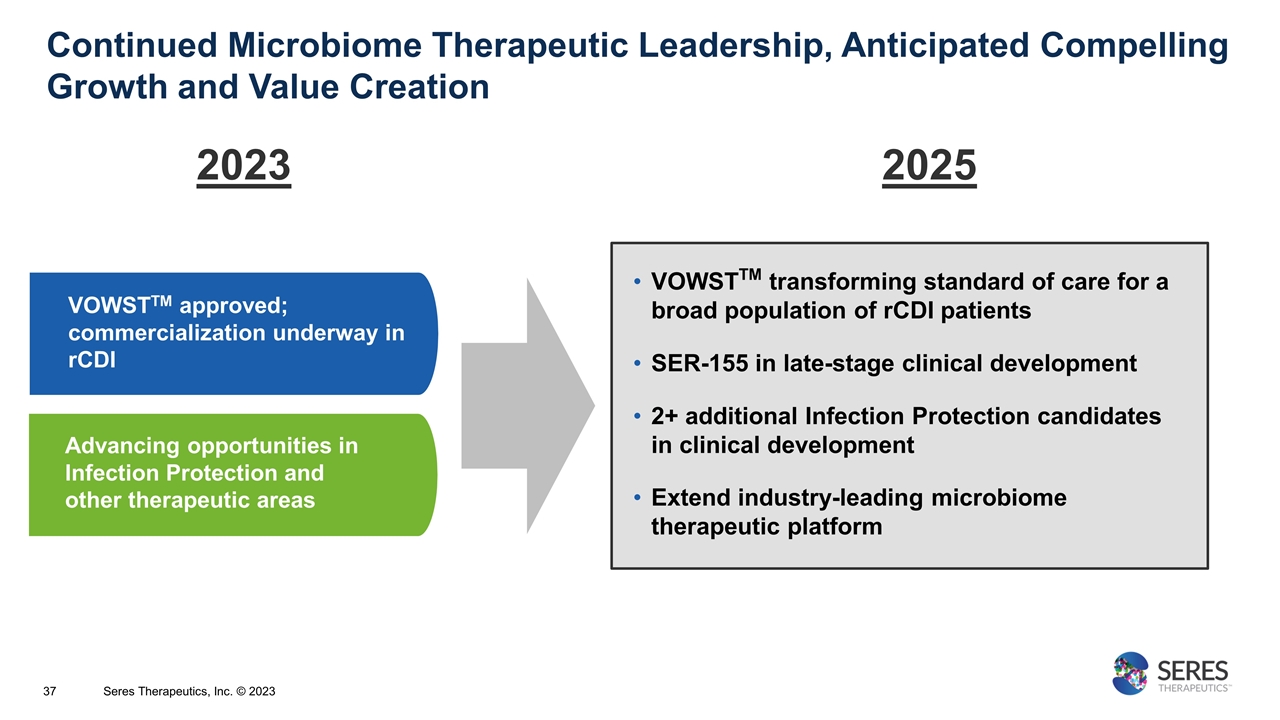
Continued Microbiome Therapeutic Leadership, Anticipated Compelling Growth and Value Creation Advancing opportunities in Infection Protection and other therapeutic areas VOWSTTM approved; commercialization underway in rCDI VOWSTTM transforming standard of care for a broad population of rCDI patients SER-155 in late-stage clinical development 2+ additional Infection Protection candidates in clinical development Extend industry-leading microbiome therapeutic platform 2023 2025 Seres Therapeutics, Inc. © 2023




































