
SER-155 Phase 1b Cohort 1 Day 100 Data May 2023 Exhibit 99.3
SER-155 May Represent a Novel Solution to Reduce GI Pathogen Abundance and Infection & GvHD in Allogeneic HSCT Enrollment ongoing in SER-155 Phase 1b Cohort 2 a randomized, double-blind, placebo-controlled study Expect to release topline results in mid-2024 SER-155 is an oral, cultivated consortium, designed to reduce abundance of pathogens linked to infections and GvHD in allogeneic HSCT recipients* SER-155 Phase 1b study Cohort 1 SER-155 was well-tolerated through 100 Days post HSCT SER-155 bacterial strain engraftment was as expected GI pathogen domination was rare and transient in patients after SER-155 treatment compared to expected rates from prior cohort studies *Note: SER-155 is an investigational therapeutic and has not been approved by any regulatory authority, including the US Food & Drug Administration Seres Therapeutics, Inc. © 2023
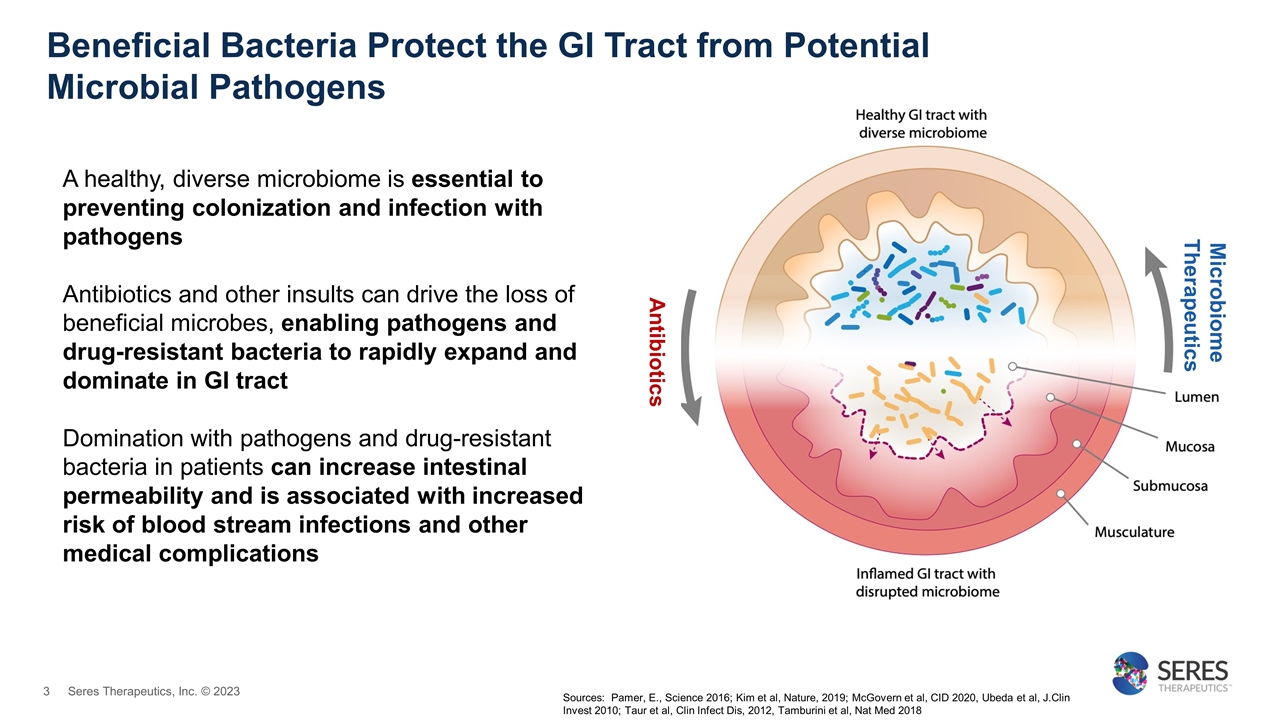
Beneficial Bacteria Protect the GI Tract from Potential Microbial Pathogens A healthy, diverse microbiome is essential to preventing colonization and infection with pathogens Antibiotics and other insults can drive the loss of beneficial microbes, enabling pathogens and drug-resistant bacteria to rapidly expand and dominate in GI tract Domination with pathogens and drug-resistant bacteria in patients can increase intestinal permeability and is associated with increased risk of blood stream infections and other medical complications Antibiotics Microbiome Therapeutics Sources: Pamer, E., Science 2016; Kim et al, Nature, 2019; McGovern et al, CID 2020, Ubeda et al, J.Clin Invest 2010; Taur et al, Clin Infect Dis, 2012, Tamburini et al, Nat Med 2018 Seres Therapeutics, Inc. © 2023
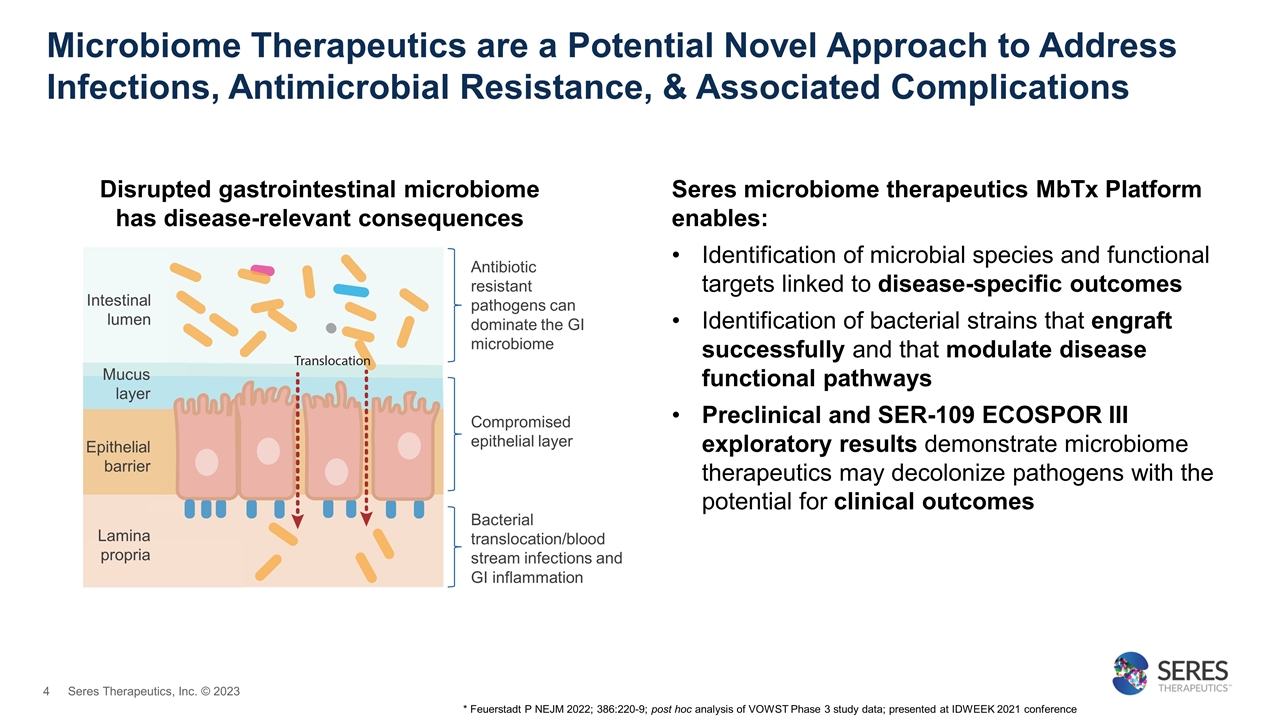
Microbiome Therapeutics are a Potential Novel Approach to Address Infections, Antimicrobial Resistance, & Associated Complications Antibiotic resistant pathogens can dominate the GI microbiome Compromised epithelial layer Bacterial translocation/blood stream infections and GI inflammation Lamina propria Intestinal lumen Epithelial barrier Mucus layer Disrupted gastrointestinal microbiome has disease-relevant consequences Seres microbiome therapeutics MbTx Platform enables: Identification of microbial species and functional targets linked to disease-specific outcomes Identification of bacterial strains that engraft successfully and that modulate disease functional pathways Preclinical and SER-109 ECOSPOR III exploratory results demonstrate microbiome therapeutics may decolonize pathogens with the potential for clinical outcomes * Feuerstadt P NEJM 2022; 386:220-9; post hoc analysis of VOWST Phase 3 study data; presented at IDWEEK 2021 conference Seres Therapeutics, Inc. © 2023
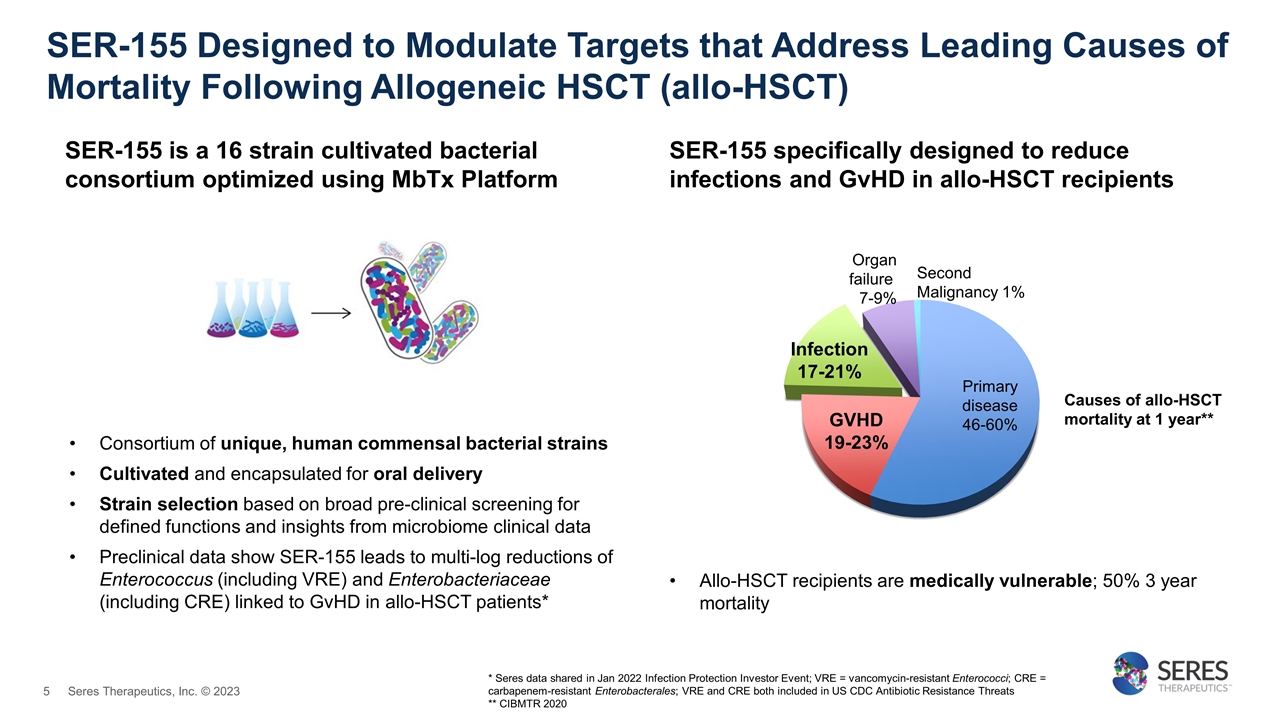
SER-155 Designed to Modulate Targets that Address Leading Causes of Mortality Following Allogeneic HSCT (allo-HSCT) Consortium of unique, human commensal bacterial strains Cultivated and encapsulated for oral delivery Strain selection based on broad pre-clinical screening for defined functions and insights from microbiome clinical data Preclinical data show SER-155 leads to multi-log reductions of Enterococcus (including VRE) and Enterobacteriaceae (including CRE) linked to GvHD in allo-HSCT patients* SER-155 is a 16 strain cultivated bacterial consortium optimized using MbTx Platform Allo-HSCT recipients are medically vulnerable; 50% 3 year mortality Organ failure 7-9% GVHD 19-23% Primary disease 46-60% Second Malignancy 1% Infection 17-21% * Seres data shared in Jan 2022 Infection Protection Investor Event; VRE = vancomycin-resistant Enterococci; CRE = carbapenem-resistant Enterobacterales; VRE and CRE both included in US CDC Antibiotic Resistance Threats ** CIBMTR 2020 SER-155 specifically designed to reduce infections and GvHD in allo-HSCT recipients Causes of allo-HSCT mortality at 1 year** Seres Therapeutics, Inc. © 2023
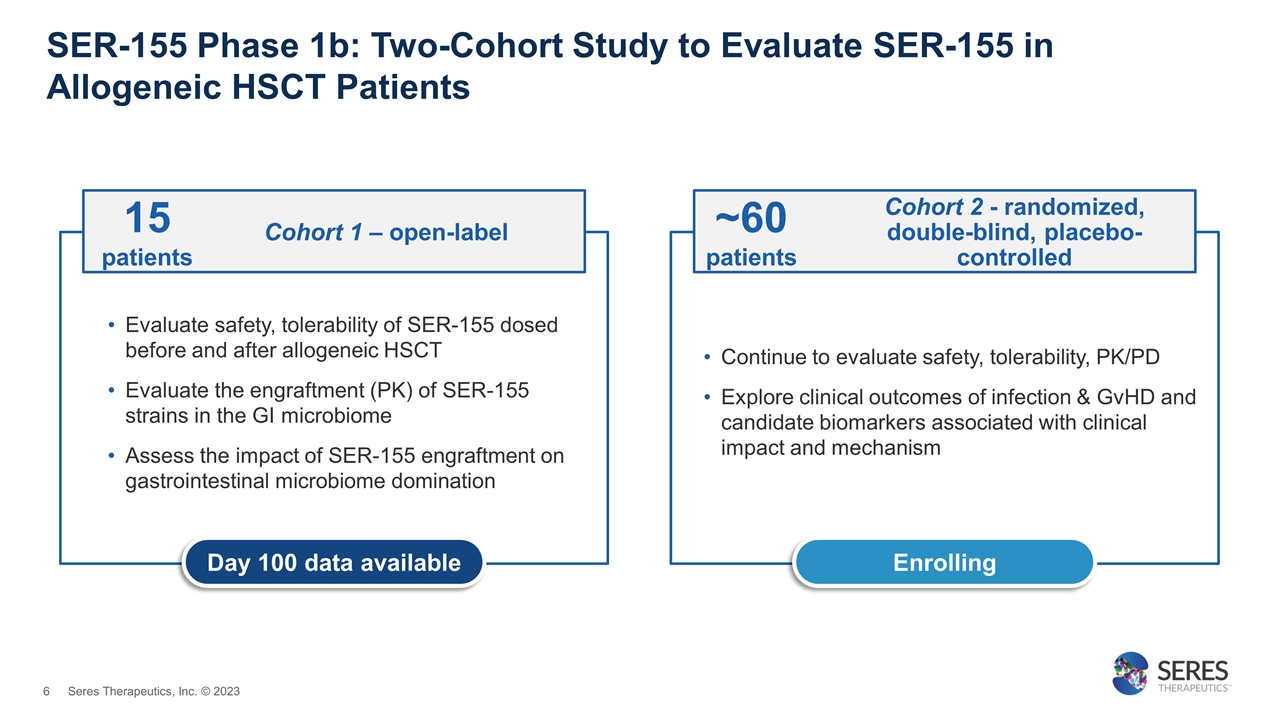
SER-155 Phase 1b: Two-Cohort Study to Evaluate SER-155 in Allogeneic HSCT Patients Evaluate safety, tolerability of SER-155 dosed before and after allogeneic HSCT Evaluate the engraftment (PK) of SER-155 strains in the GI microbiome Assess the impact of SER-155 engraftment on gastrointestinal microbiome domination Continue to evaluate safety, tolerability, PK/PD Explore clinical outcomes of infection & GvHD and candidate biomarkers associated with clinical impact and mechanism Enrolling Day 100 data available Cohort 1 – open-label 15 patients Cohort 2 - randomized, double-blind, placebo-controlled ~60 patients Seres Therapeutics, Inc. © 2023
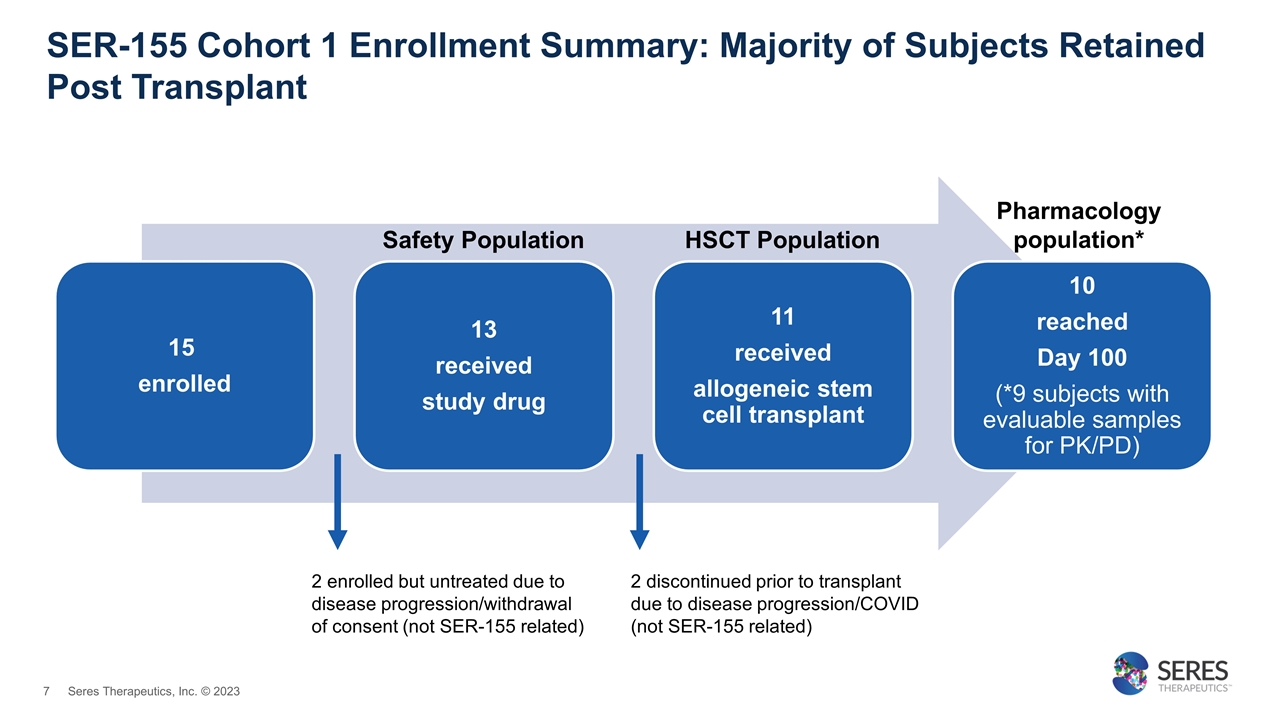
SER-155 Cohort 1 Enrollment Summary: Majority of Subjects Retained Post Transplant 15 enrolled 13 received study drug 11 received allogeneic stem cell transplant 10 reached Day 100 (*9 subjects with evaluable samples for PK/PD) 2 enrolled but untreated due to disease progression/withdrawal of consent (not SER-155 related) 2 discontinued prior to transplant due to disease progression/COVID (not SER-155 related) Pharmacology population* Safety Population HSCT Population Seres Therapeutics, Inc. © 2023
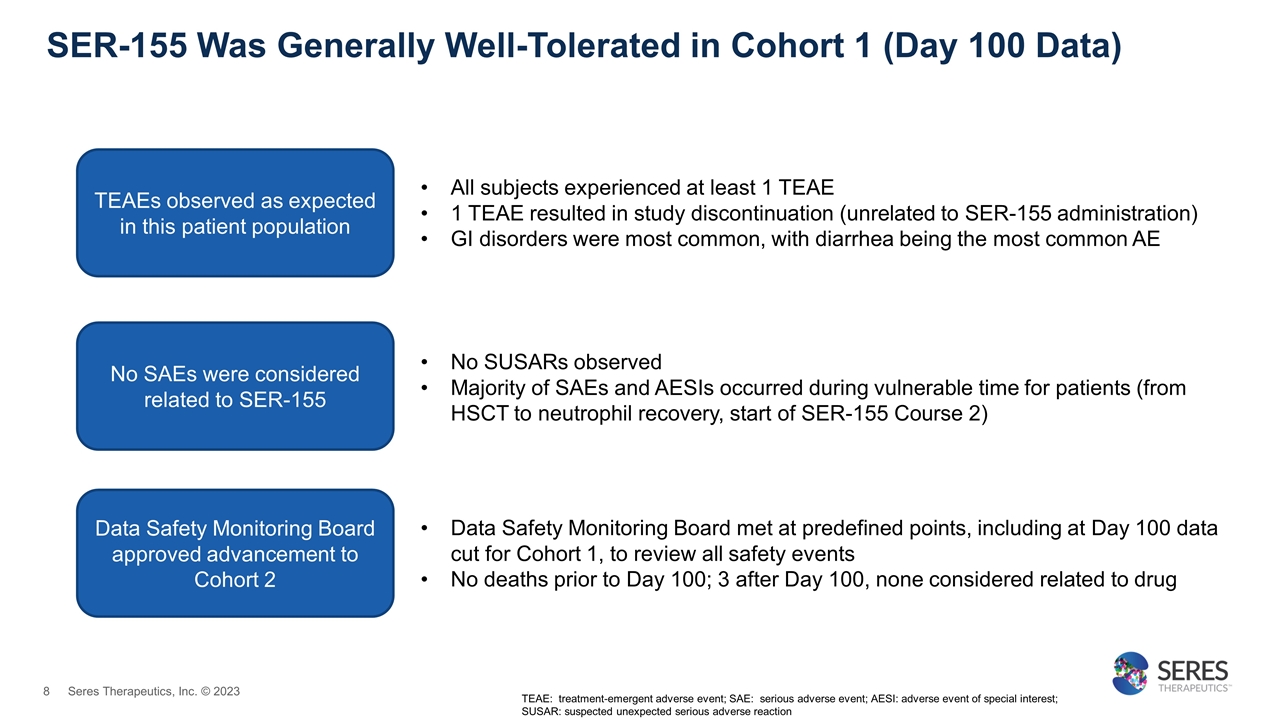
SER-155 Was Generally Well-Tolerated in Cohort 1 (Day 100 Data) TEAE: treatment-emergent adverse event; SAE: serious adverse event; AESI: adverse event of special interest; SUSAR: suspected unexpected serious adverse reaction TEAEs observed as expected in this patient population All subjects experienced at least 1 TEAE 1 TEAE resulted in study discontinuation (unrelated to SER-155 administration) GI disorders were most common, with diarrhea being the most common AE No SAEs were considered related to SER-155 No SUSARs observed Majority of SAEs and AESIs occurred during vulnerable time for patients (from HSCT to neutrophil recovery, start of SER-155 Course 2) Data Safety Monitoring Board approved advancement to Cohort 2 Data Safety Monitoring Board met at predefined points, including at Day 100 data cut for Cohort 1, to review all safety events No deaths prior to Day 100; 3 after Day 100, none considered related to drug Seres Therapeutics, Inc. © 2023
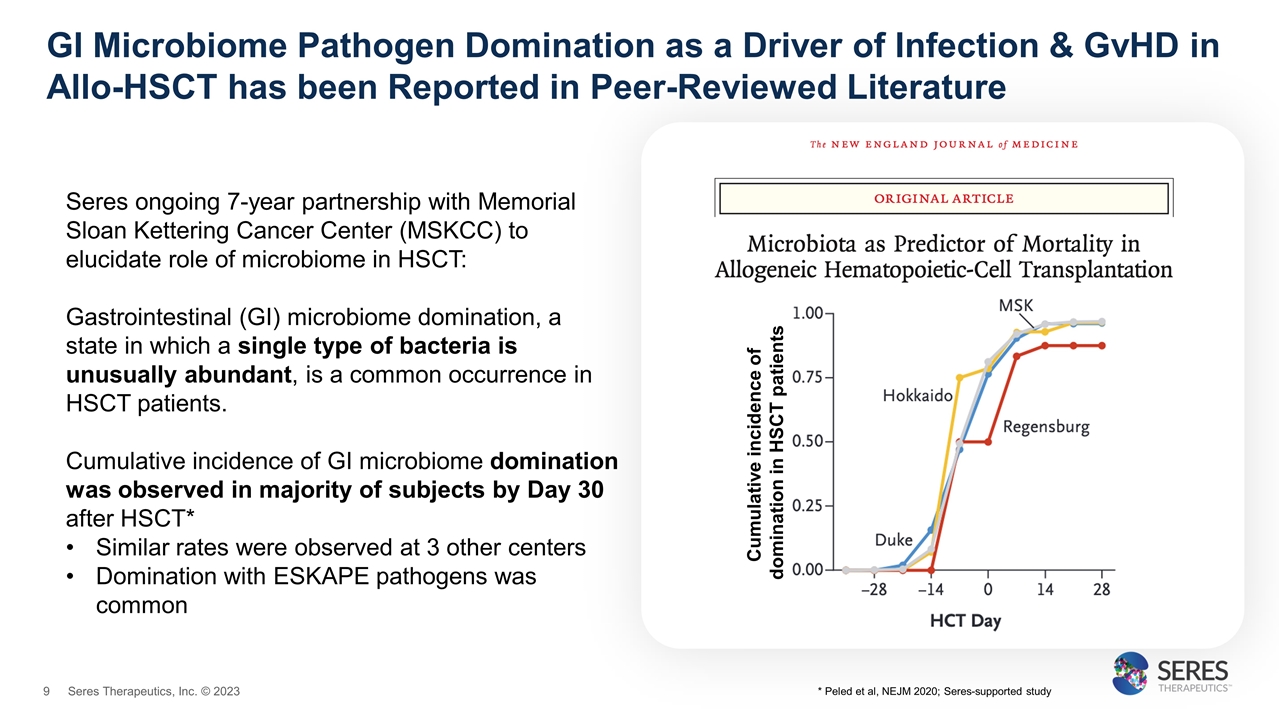
GI Microbiome Pathogen Domination as a Driver of Infection & GvHD in Allo-HSCT has been Reported in Peer-Reviewed Literature Seres ongoing 7-year partnership with Memorial Sloan Kettering Cancer Center (MSKCC) to elucidate role of microbiome in HSCT: Gastrointestinal (GI) microbiome domination, a state in which a single type of bacteria is unusually abundant, is a common occurrence in HSCT patients. Cumulative incidence of GI microbiome domination was observed in majority of subjects by Day 30 after HSCT* Similar rates were observed at 3 other centers Domination with ESKAPE pathogens was common Cumulative incidence of domination in HSCT patients * Peled et al, NEJM 2020; Seres-supported study Seres Therapeutics, Inc. © 2023
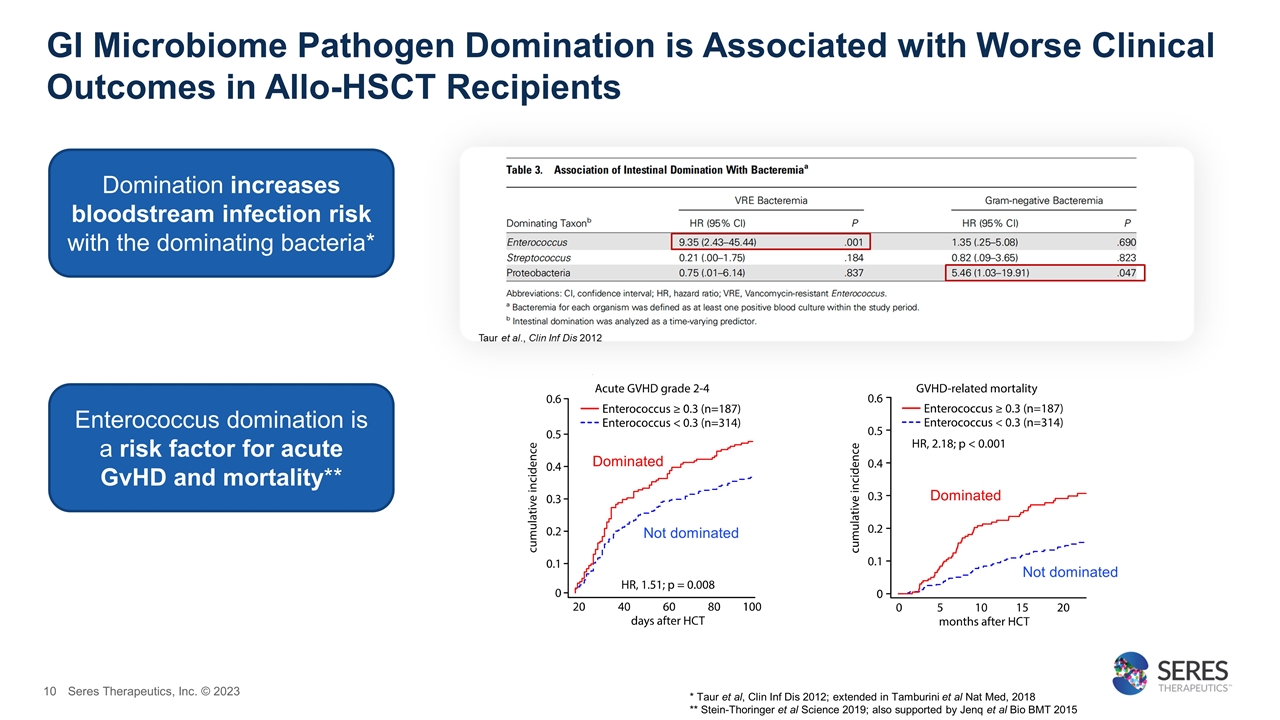
GI Microbiome Pathogen Domination is Associated with Worse Clinical Outcomes in Allo-HSCT Recipients * Taur et al, Clin Inf Dis 2012; extended in Tamburini et al Nat Med, 2018 ** Stein-Thoringer et al Science 2019; also supported by Jenq et al Bio BMT 2015 Dominated Not dominated Dominated Not dominated Domination increases bloodstream infection risk with the dominating bacteria* Enterococcus domination is a risk factor for acute GvHD and mortality** Seres Therapeutics, Inc. © 2023
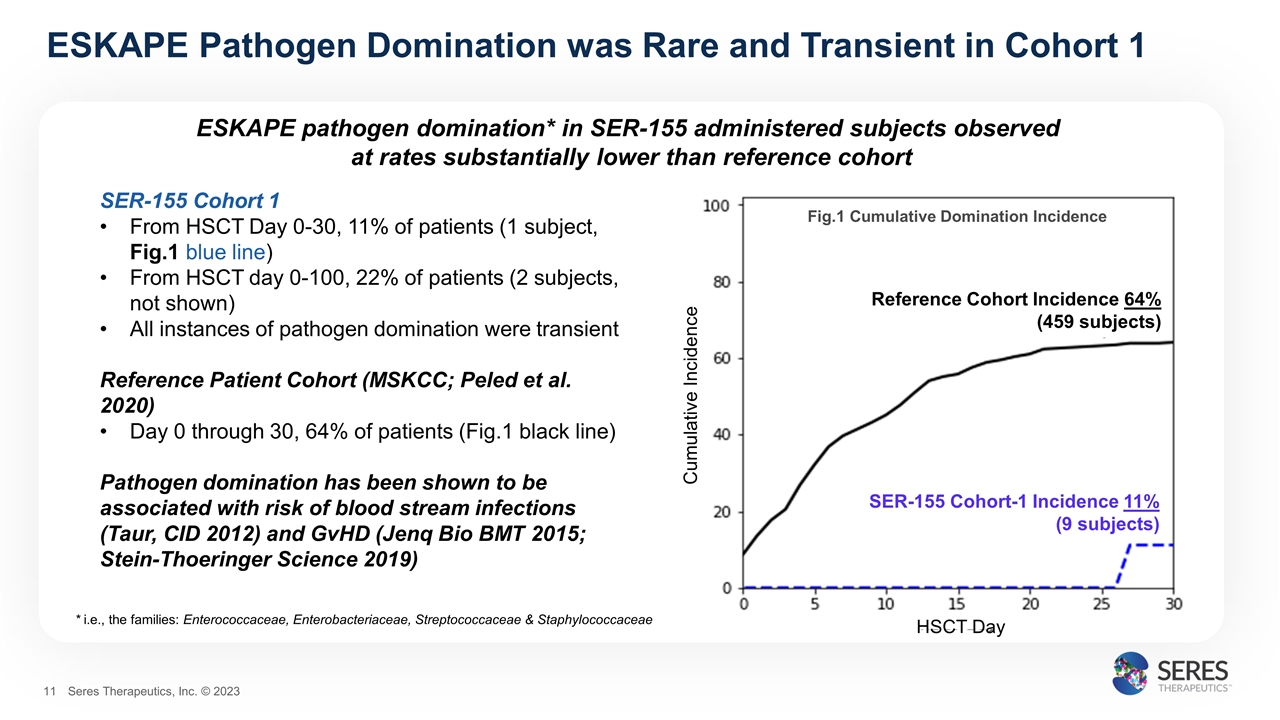
ESKAPE pathogen domination* in SER-155 administered subjects observed at rates substantially lower than reference cohort ESKAPE Pathogen Domination was Rare and Transient in Cohort 1 SER-155 Cohort 1 From HSCT Day 0-30, 11% of patients (1 subject, Fig.1 blue line) From HSCT day 0-100, 22% of patients (2 subjects, not shown) All instances of pathogen domination were transient Reference Patient Cohort (MSKCC; Peled et al. 2020) Day 0 through 30, 64% of patients (Fig.1 black line) Pathogen domination has been shown to be associated with risk of blood stream infections (Taur, CID 2012) and GvHD (Jenq Bio BMT 2015; Stein-Thoeringer Science 2019) Cumulative Incidence HSCT Day Fig.1 Cumulative Domination Incidence Reference Cohort Incidence 64% (459 subjects) SER-155 Cohort-1 Incidence 11% (9 subjects) * i.e., the families: Enterococcaceae, Enterobacteriaceae, Streptococcaceae & Staphylococcaceae Seres Therapeutics, Inc. © 2023

SER-155 Bacterial Strains Engrafted in Cohort 1 Patients Engraftment magnitude and kinetics were consistent with our expectations Engraftment is the colonization of the GI tract by metabolically active drug product strains; assessed throughout the study period via proprietary genomic technologies. Most of the strains engrafted in a majority of the individuals evaluated. These engraftment data, as well as those that will come from Cohort 2, will be used to inform Phase 2 trial design. Seres Therapeutics, Inc. © 2023

SER-155 Phase 1b Cohort 2: What We Expect to Learn SER-155 safety, strain engraftment (PK) and pathogen abundance (PD) in the context of placebo comparator arm Further elucidate mechanism of action of SER-155 Explore impact of SER-155 on clinical outcomes, including the incidence of enteric infections, BSI, BSI with enteric bacteria, and GvHD, with contemporaneous placebo rates Broaden dosing experience in allo-HSCT patients to confirm optimal dosing strategy Cohort 2 topline data expected in mid-2024 Seres Therapeutics, Inc. © 2023
SER-155 Could Become Core Part of Allogeneic HSCT Treatment Regimen Unique potential clinical and economic value for allogeneic HSCT patients Double benefit of reducing infections and GvHD, 2 of 3 leading causes of mortality at 1 year Avoids costs of post-transplant complications: $181K average additional costs for US patients with complications Sources: CIBMTR 2020; Passweg et al Bone Marrow Transplantation 57 (2022) 742-752; Perales et al Biol Blood Marrow Transplant 23 (2017) 1788–1794; Broder, et al. “The Cost of Hematopoietic Stem-Cell Transplantation in the United States” Am Health and Drug Benefits 10 (2017) 366–374; https://data.cms.gov/provider-summary-by-type-of-service/medicare-inpatient-hospitals/medicare-inpatient-hospitals-by-geography-and-service/data/2019; Seres physician interviews Favorable safety profile appropriate for use across HSCT population Substantial impact for patients: almost 30,000 transplants / year across US and Europe Seres Therapeutics, Inc. © 2023













