
New Hope for Serious Infections Corporate Presentation August 2019 © Cidara Therapeutics 2019 | Confidential

Forward-Looking Statements Because such statements are subject to risks and and whether our Cloudbreak platform can be These slides and the uncertainties, actual results may differ materially from those expanded to identify product candidates to treat or expressed or implied by such forward-looking statements. prevent other viral diseases, such as RSV, HIV, accompanying oral Such statements include, but are not limited to, statements Dengue or Zika. This presentation also contains regarding the effectiveness, safety, and long-acting nature of estimates and other statistical data made by presentation contain rezafungin; the potential for rezafungin to treat and/or prevent independent parties and by Cidara relating to infections; whether the top line results of the STRIVE Part B market size and growth and other data about forward-looking clinical trial will be supported in the full analysis of the STRIVE Cidara's industry. These data involve a number of Part B clinical data; whether the success of the STRIVE Part assumptions and limitations, and you are cautioned statements within the B clinical trial indicates a successful outcome in the Phase 3 not to give undue weight to such estimates. ReSTORE clinical trial, including whether or not rezafungin Projections, assumptions and estimates of the meaning of the Private will meet the primary endpoints in the ReSTORE trial; and future performance of the markets in which Cidara whether Cidara will be able to successfully develop and operates are necessarily subject to a high degree Securities Litigation commercialize rezafungin; as well as the potential market size of uncertainty and risk. Risks that contribute to the for rezafungin, ability of rezafungin to capture market share uncertain nature of the forward-looking statements Reform Act of 1995. from existing therapies, and the advantages of rezafungin in include: Cidara’s ability to obtain additional other settings of care. Certain statements regarding our financing; the success and timing of Cidara’s Cloudbreak platform are also forward-looking, including preclinical studies, clinical trials and other research statements regarding whether our Cloudbreak platform can and development activities; receipt of necessary identify product candidates with intrinsic antimicrobial activity regulatory approvals for development and and immune engagement that will increase efficacy or commercialization, as well as changes to represent an improvement over existing anti-infective agents; applicable regulatory laws in the United States and whether Cloudbreak candidates, including CB-012, will foreign countries; changes in Cidara’s plans to achieve the major attributes believed to be needed in flu such develop and commercialize its product candidates; as broad spectrum, superior resistance profile, protection for Cidara’s ability to obtain and maintain intellectual high-risk populations, expanded efficacy window, long property protection for its product candidates; and duration of action and rapid onset of activity, or flexible the loss of key scientific or management personnel. administration; whether results observed with Cloudreak These and other risks and uncertainties are influenza candidates, including CB-012, in-vitro or in animal described more fully in Cidara’s Form 10-K as most studies, including, potency and broad coverage, activity recently filed with the United States Securities and against resistant strains, activity in immune compromised Exchange Commission (SEC), under the heading patients, extending the treatment window, extended half-life “Risk Factors.” All forward-looking statements and long duration of action, improved viral clearance in the contained in this presentation speak only as of the lungs, improved reduction in inflammatory cytokines, and a date on which they were made. Cidara undertakes robust safety profile or other observed attributes represent an no obligation to update such statements to reflect improvement over existing therapies or will also be observed events that occur or circumstances that exist after in human use; the date on which they were made. 2

Cidara’s pipeline addresses multiple unmet needs IND- Indication Program Discov. in vitro in vivo enable Ph 1 Ph 2 Ph 3 REZAFUNGIN Candida Intravenous Treatment Fungal Intravenous Prophylaxis CLOUDBREAK Influenza Antiviral Fc Prevention & Conjugates Treatment (AVCs) RSV, HIV, AVCs Dengue, Zika 3

Cidara addresses multiple unmet medical needs Rezafungin Cloudbreak 4
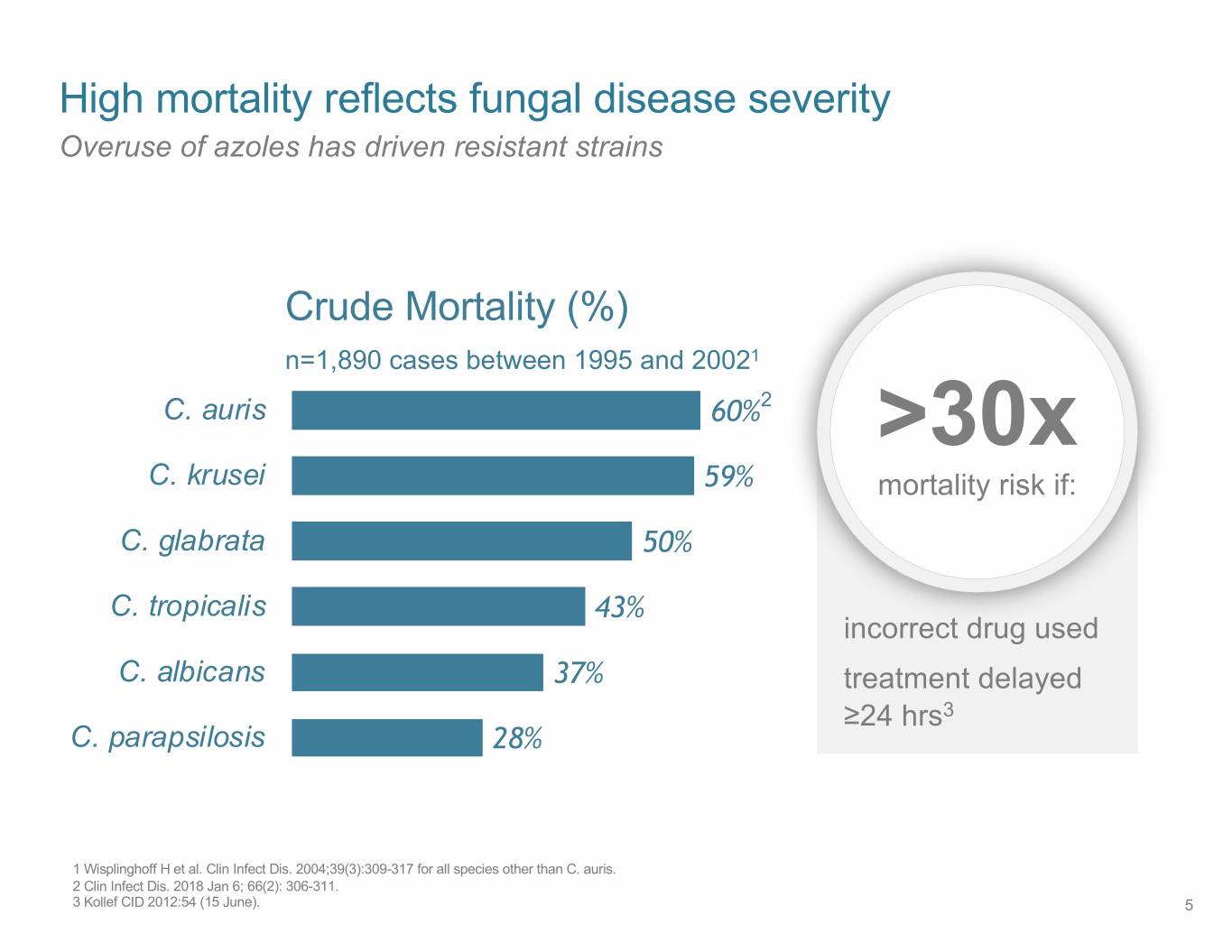
High mortality reflects fungal disease severity Overuse of azoles has driven resistant strains Crude Mortality (%) n=1,890 cases between 1995 and 20021 C. auris 60%2 >30x C. krusei 59% mortality risk if: C. glabrata 50% C. tropicalis 43% incorrect drug used C. albicans 37% treatment delayed ≥24 hrs3 C. parapsilosis 28% 1 Wisplinghoff H et al. Clin Infect Dis. 2004;39(3):309-317 for all species other than C. auris. 2 Clin Infect Dis. 2018 Jan 6; 66(2): 306-311. 3 Kollef CID 2012:54 (15 June). 5

Invasive fungal infections cause high mortality post transplant 90-day mortality % by patient category1 Bone and marrow transplantHSCT 63% Prophylaxis focus in Phase 3 trial Hematologic malignancy 52% Solid tumor 40% General medicine 38% Surgical (nontransplant) 26% HIV/AIDS 24% Solid organ transplant 23% 1 The PATH (Prospective Antifungal Therapy) Alliance registry and invasive fungal infections: update 2012 (2012). 6

Antifungal drug development has dwindled Number of New Antifungals 6 5 1 1980 1990 2000 2010 2020 amphotericin terbinafine posaconazole isavuconazole lipid forms (3) itraconazole anidulafungin fluconazole micafungin voriconazole caspofungin 7

Rezafungin for treatment in the critically ill High exposure (efficacy in critically ill) REZAFUNGIN Establish novel Once-weekly dosing (ECHINOCANDIN) drug in market (early discharge and outpatients) Under-dosed No drug-drug interactions 1ST GEN Once-daily IV dosing Established safety ECHINOCANDINS limits outpatient use High levels of resistance Oral formulation AZOLES Drug-drug interactions Hepatic toxicities Renal toxicities Spectrum POLYENES Once-daily IV dosing 8

Rezafungin for prophylaxis in hem/onc patients Host and Macro-environment • Chronic immunosuppression • Novel biologics • Epidemiology - azole resistance Antifungal therapies • Drug-drug interactions • Toxicities (bone marrow, liver, kidney) • Under-dosing 9

The Rezafungin opportunity spans ID and hematology REZAFUNGIN Cancidas Noxafil (caspofungin) (posaconazole) ~$700M ~$700M Infectious Disease Hematology Mostly Treatment Mostly Prophylaxis Source: Cancidas – IQVIA; Noxafil – 2018 Merck Annual Report 10

Rezafungin overall phase 3 development plan * Phase 3 Treatment Trial Phase 3 Prophylaxis Trial Prophylaxis against Aspergillus, Treatment of candidemia & Candida & PCP in patients Indication invasive candidiasis in patients undergoing allogeneic blood and with limited treatment options marrow transplant Phase 3 Size 184 patients ~450 patients with adaptive design Duration of 2 to 4 weeks of treatment, 90 days of prophylaxis Therapy, Day 30 all-cause mortality (US) Day 90 fungal-free survival (FFS) Endpoints, Day 14 global response (EMA) Fluconazole, posaconazole, Comparators Caspofungin Bactrim * Final trial design and timing are subject to obtaining adequate funding and the approval of regulatory authorities. 11

STRIVE B Phase 2 data in candidemia & invasive candidiasis Corroborates STRIVE A results and supports ReSTORE Phase 3 12

P2 STRIVE Part B: Candidemia & Invasive Candidiasis Not powered for inferential statistics Overall Response Mycological & (Mycological & Mycological & Randomization 2:1 clinical clinical response): clinical response Mycological & response 1° ENDPOINT (IC only) clinical response Dose Optional dose All cause mortality Week 1 2 3 4 5 6 7 8 9 Rezafungin 1 5 8 15 22 28 35 42 45 49 56 59 Day Dose Optional dose Week 1 2 3 4 5 6 7 8 9 Caspofungin 1 5 8 15 22 28 35 42 45 49 56 59 Day Analysis Populations: § The Intent-to-treat (ITT) population: all randomized subjects § The Safety population: all subjects who received any amount of study drug § The Microbiological Intent-to-treat population (mITT): all subjects in safety population who had documented Candida infection 13

STRIVE B: the bridge from STRIVE A to ReSTORE STRIVE A (n=92) STRIVE B (n=91) RESTORE (n=184) 2017 2018 2019 2020 Rezafungin 400 / 400mg Rezafungin 400 / 200mg Caspofungin STUDY SIZE: STRIVE A + B (n=183) ≈ RESTORE (n=184) CRITERIA: Similar inclusion/exclusion, except STRIVE B enrolled patients with invasive candidiasis from the beginning STRATEGY: STRIVE B expands safety data; maintains enrollment momentum 14

30-Day All Cause Mortality Similar to the ReSTORE trial primary endpoint for FDA Death at Day 30 (%) mITT Population n/N= 1 STRIVE B Rezafungin 400/400 7/43 16.3% Rezafungin 400/2002 1/15 6.7% Caspofungin3 5/33 15.2% n/N= 1 STRIVE A + B Rezafungin 400/400 12/76 15.8% 2 Rezafungin 400/200 2/46 4.4% 3 Caspofungin 8/61 13.1% 1. 400 mg dose once weekly for two to four weeks. 2. 400 mg dose for the initial week followed by 200 mg dose once weekly for an additional one to three weeks. 3. 70 mg day one, followed by daily doses of 50mg. 15

Investigator assessment of clinical response Similar to the ReSTORE trial primary endpoint for EMA Clinical Cure (%) at Day 14 mITT Population n/N= 1 STRIVE B Rezafungin 400/400 28/43 65.1% Rezafungin 400/2002 13/15 86.7% Caspofungin3 23/33 69.7% n/N= 1 STRIVE A + B Rezafungin 400/400 53/76 69.7% 2 Rezafungin 400/200 37/46 80.4% 3 Caspofungin 43/61 70.5% 1. 400 mg dose once weekly for two to four weeks. 2. 400 mg dose for the initial week followed by 200 mg dose once weekly for an additional one to three weeks. 3. 70 mg day one, followed by daily doses of 50mg. 16
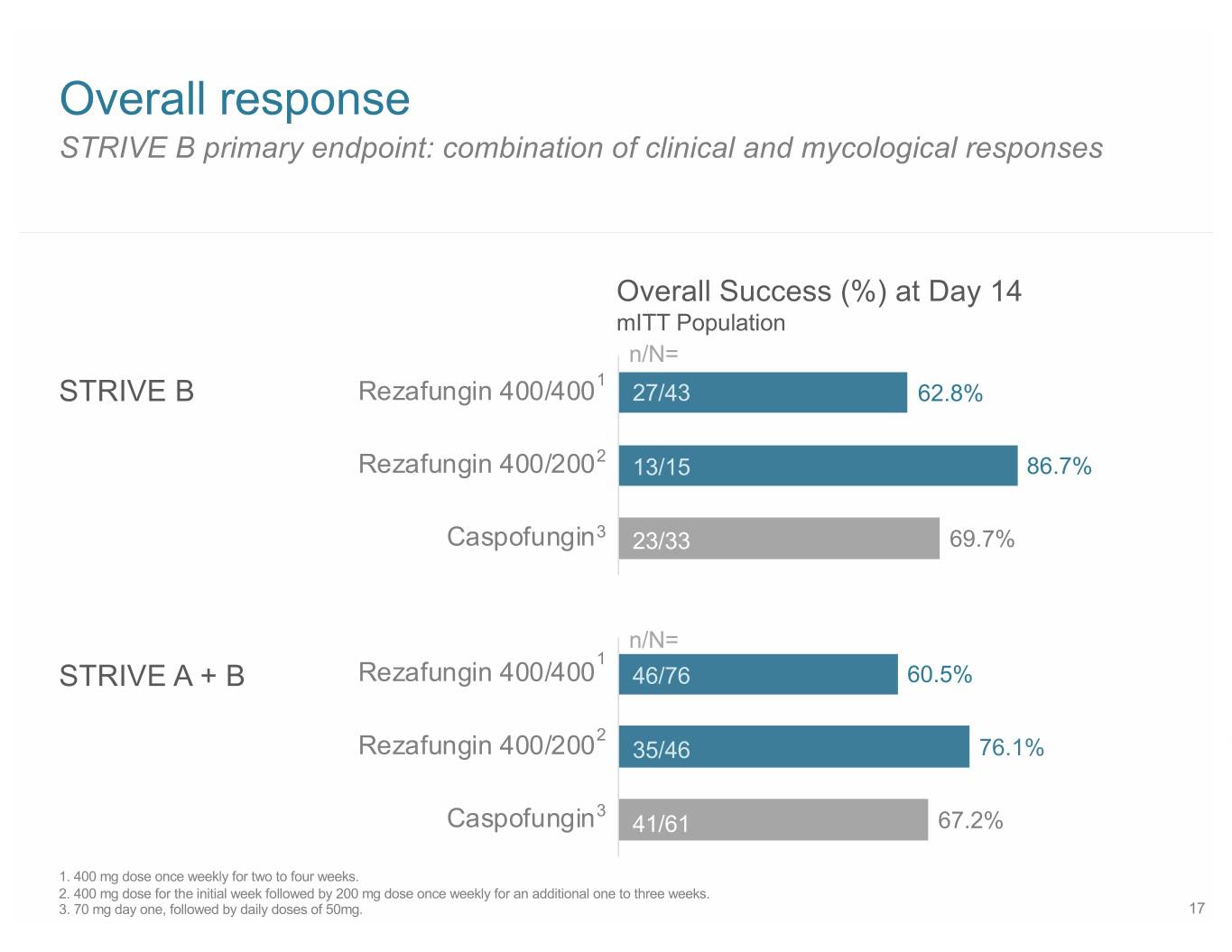
Overall response STRIVE B primary endpoint: combination of clinical and mycological responses Overall Success (%) at Day 14 mITT Population n/N= 1 STRIVE B Rezafungin 400/400 27/43 62.8% Rezafungin 400/2002 13/15 86.7% Caspofungin3 23/33 69.7% n/N= 1 STRIVE A + B Rezafungin 400/400 46/76 60.5% 2 Rezafungin 400/200 35/46 76.1% 3 Caspofungin 41/61 67.2% 1. 400 mg dose once weekly for two to four weeks. 2. 400 mg dose for the initial week followed by 200 mg dose once weekly for an additional one to three weeks. 3. 70 mg day one, followed by daily doses of 50mg. 17

Topline summary of adverse events in safety population Study-Drug Related TEAEs REZAFUNGIN CASPOFUNGIN STRIVE B As expected and 400/400 mg 400/200 mg Pooled 70/50 mg observed in STRIVE A, (QWk) (QWk) Groups (QD) thethe majoritymajority ofof subjectssubjects had at least one TEAE N=46 N=18 N=64 N=34 and 40-50% had at leastleast oneone SeriousSerious AE,AE, n (%) n (%) reflecting the high morbidity of the All Related TEAEs 3 (6.5) 0 3 (4.7) 5 (14.7) underlying population. Leading to study D/C 2 (4.3) 0 2 (3.1) 3 (8.8) There were no AE trends;trends; %% ofof TEAEsTEAEs Serious AE 1 (2.2) 0 1 (1.6) 1 (2.9) and SAEs were approximately even across study groups. STRIVE A + B N=81 N=53 N=134 N=68 D/C=discontinuation; All Related TEAEs 7 (8.6) 6 (11.3) 13 (9.7) 9 (13.2) TEAE (treatment- emergent adverse Leading to study D/C 3 (3.7) 0 3 (2.2) 1 (1.5) event)=AE that occurs after first dose of study Serious AE 1 (1.2) 1 (1.9) 2 (1.5) 2 (2.9) drug is administered. 18
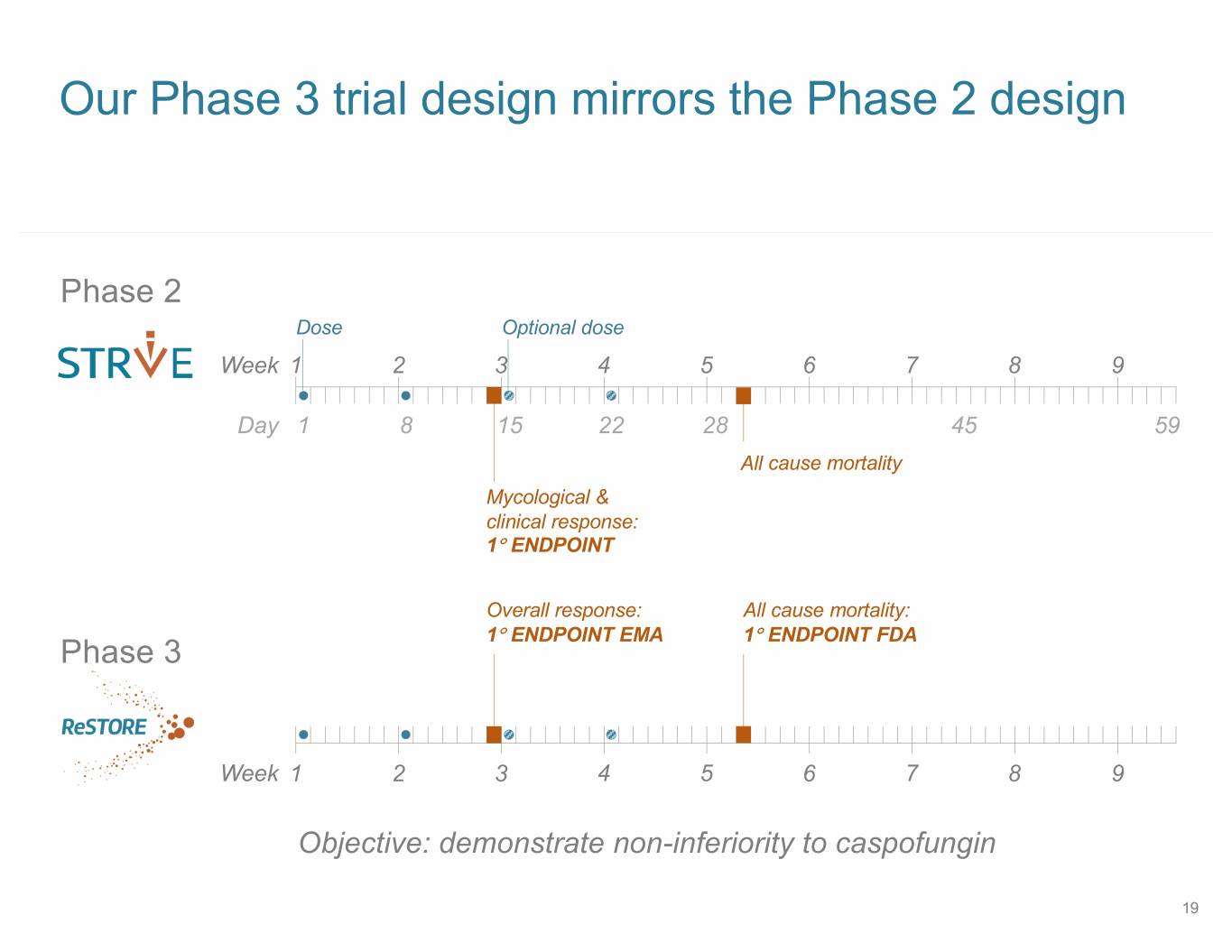
Our Phase 3 trial design mirrors the Phase 2 design Phase 2 Dose Optional dose Week 1 2 3 4 5 6 7 8 9 Day 1 8 15 22 28 45 59 All cause mortality Mycological & clinical response: 1° ENDPOINT Overall response: All cause mortality: 1° ENDPOINT EMA 1° ENDPOINT FDA Phase 3 Week 1 2 3 4 5 6 7 8 9 Objective: demonstrate non-inferiority to caspofungin 19
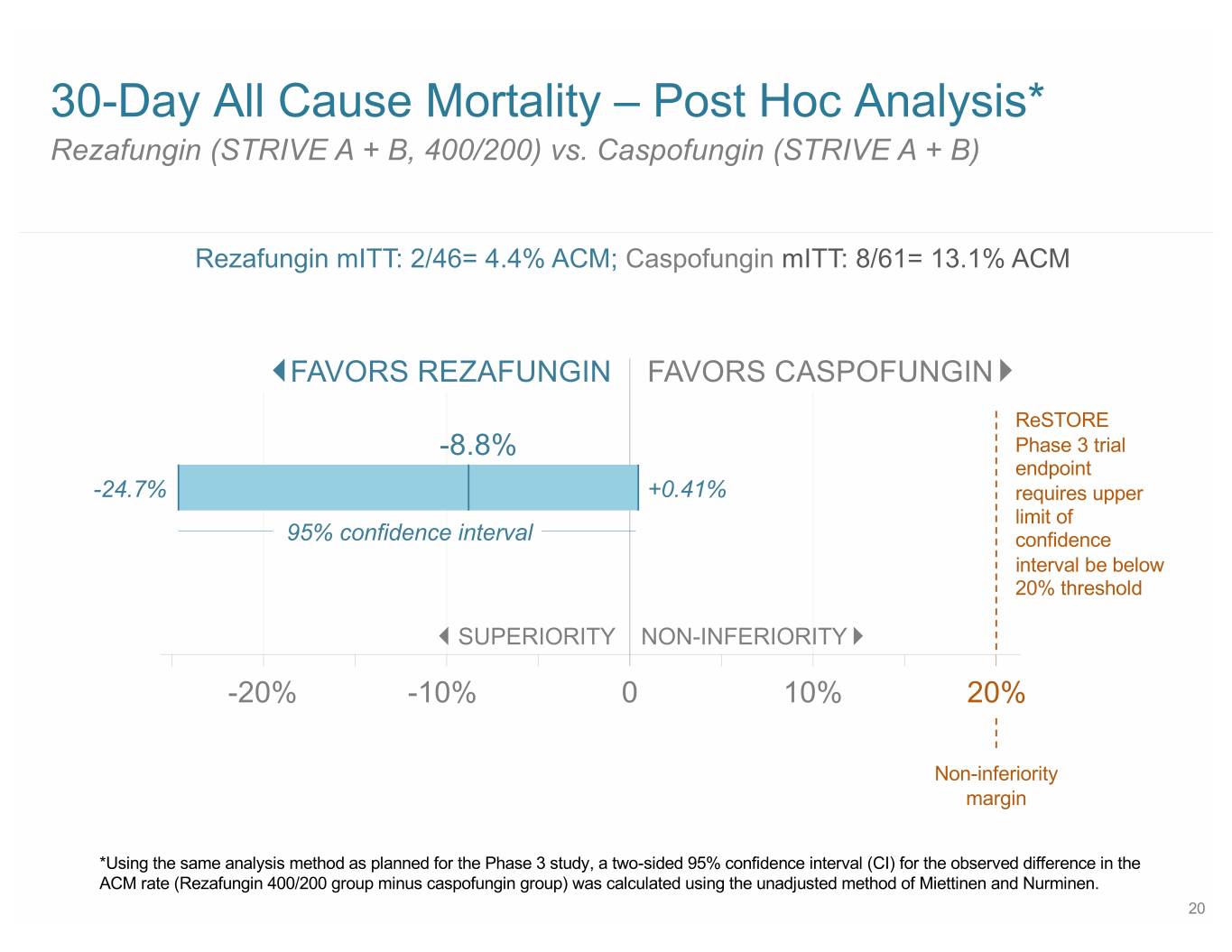
30-Day All Cause Mortality – Post Hoc Analysis* Rezafungin (STRIVE A + B, 400/200) vs. Caspofungin (STRIVE A + B) Rezafungin mITT: 2/46= 4.4% ACM; Caspofungin mITT: 8/61= 13.1% ACM FAVORS REZAFUNGIN FAVORS CASPOFUNGIN ReSTORE -8.8% Phase 3 trial endpoint -24.7% +0.41% requires upper limit of 95% confidence interval confidence interval be below 20% threshold SUPERIORITY NON-INFERIORITY -20% -10% 0 10% 20% Non-inferiority margin *Using the same analysis method as planned for the Phase 3 study, a two-sided 95% confidence interval (CI) for the observed difference in the ACM rate (Rezafungin 400/200 group minus caspofungin group) was calculated using the unadjusted method of Miettinen and Nurminen. 20
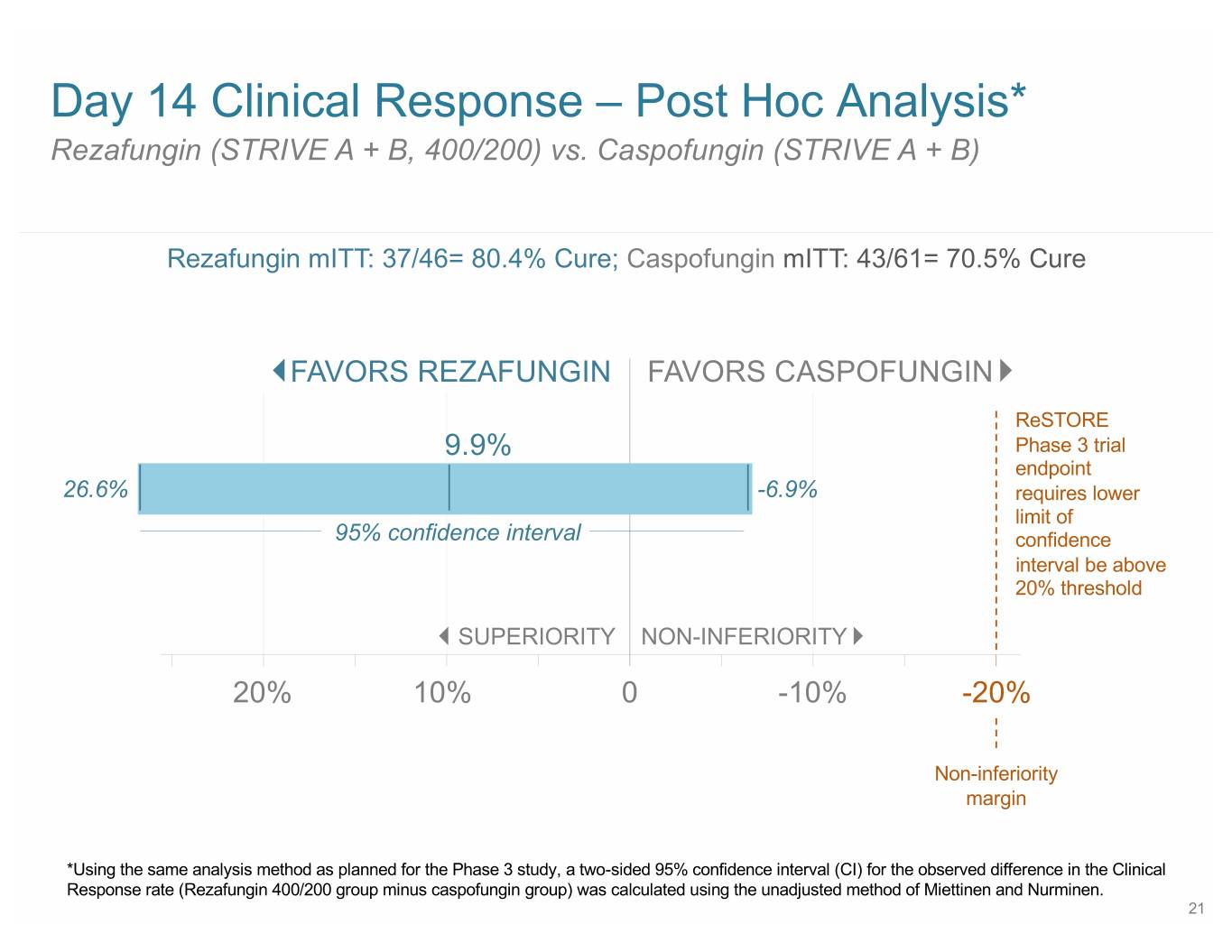
Day 14 Clinical Response – Post Hoc Analysis* Rezafungin (STRIVE A + B, 400/200) vs. Caspofungin (STRIVE A + B) Rezafungin mITT: 37/46= 80.4% Cure; Caspofungin mITT: 43/61= 70.5% Cure FAVORS REZAFUNGIN FAVORS CASPOFUNGIN ReSTORE 9.9% Phase 3 trial endpoint 26.6% -6.9% requires lower limit of 95% confidence interval confidence interval be above 20% threshold SUPERIORITY NON-INFERIORITY 20% 10% 0 -10% -20% Non-inferiority margin *Using the same analysis method as planned for the Phase 3 study, a two-sided 95% confidence interval (CI) for the observed difference in the Clinical Response rate (Rezafungin 400/200 group minus caspofungin group) was calculated using the unadjusted method of Miettinen and Nurminen. 21

Advantages of expanding outside of the in-hospital market Cidara ”Typical” Abx company Treatment: in- and outpatient Opportunity Prophylaxis: Inpatient treatment BMT/Hematology Outpatient IV pricing & Commercial Hospital inpatient DRG reimbursement (Part B) 13 years since last Candida ~15 Ph3 programs Competition or prophylaxis launch Multiple launches in 2018-19 Business ID & Hem/Onc supportive ID focused companies Development care companies 22

Antifungal (Cresemba) launch outpaces recent antibiotics U.S. Sales Post Launch: Cresemba vs. Antibiotics 30 CRESEMBA (ANTIFUNGAL) AVYCAZ Sales ($M) 20 DALVANCE ZERBAXA ANTIBIOTICS AVERAGE 10 ORBACTIV 0 1 2 3 4 5 6 7 8 9 10 11 12 13 Launch Quarter Cresemba (isavuconazole) is a triazole launched in 2015 by Astellas in the US. In 2017, Pfizer acquired rights to izavuconazole from Basilea for EU, China, 16 Asia Pac countries. Source for sales data: IMS 23
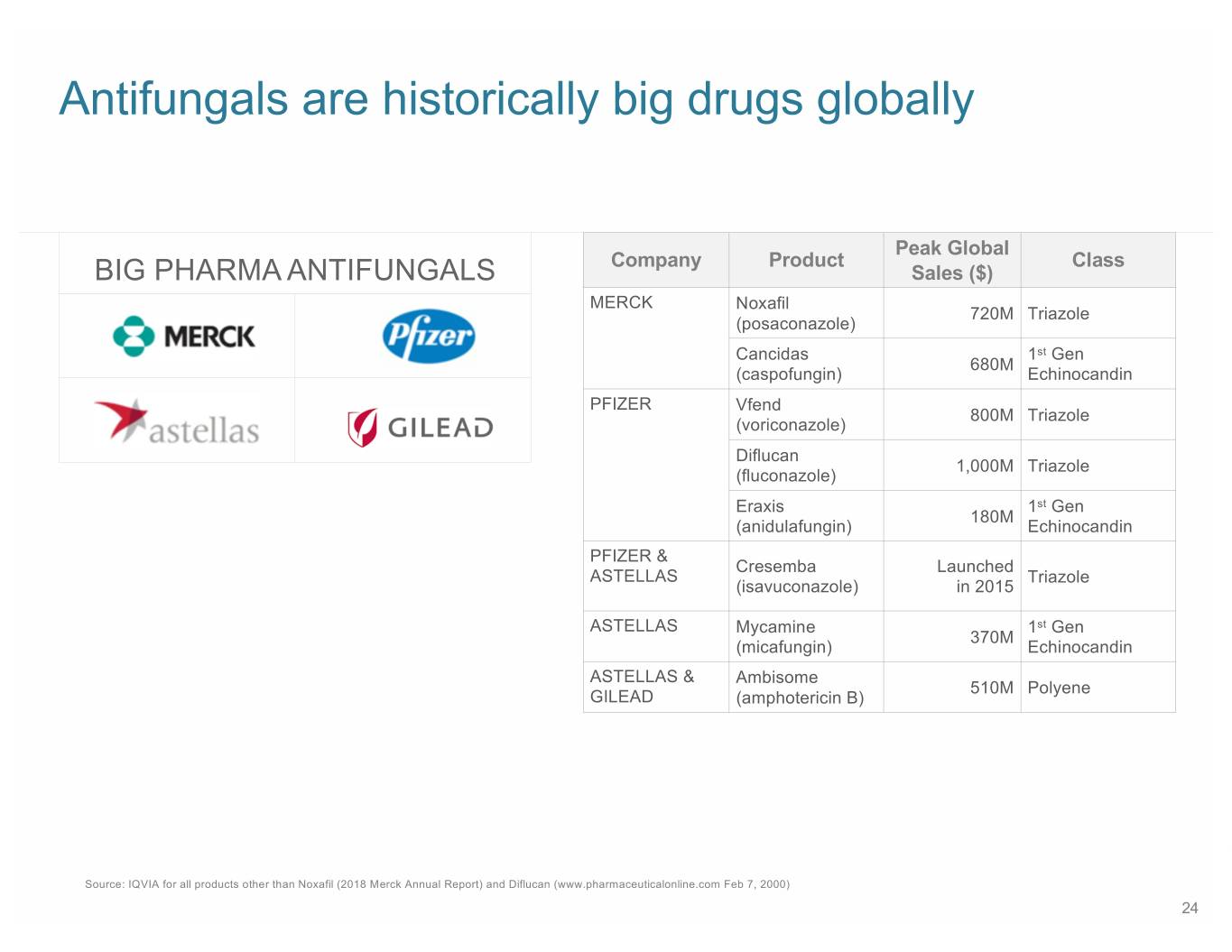
Antifungals are historically big drugs globally Peak Global Company Product Class BIG PHARMA ANTIFUNGALS Sales ($) MERCK Noxafil 720M Triazole (posaconazole) Cancidas 1st Gen 680M (caspofungin) Echinocandin PFIZER Vfend 800M Triazole (voriconazole) Diflucan 1,000M Triazole (fluconazole) Eraxis 1st Gen 180M (anidulafungin) Echinocandin PFIZER & Cresemba Launched ASTELLAS Triazole (isavuconazole) in 2015 ASTELLAS Mycamine 1st Gen 370M (micafungin) Echinocandin ASTELLAS & Ambisome 510M Polyene GILEAD (amphotericin B) Source: IQVIA for all products other than Noxafil (2018 Merck Annual Report) and Diflucan (www.pharmaceuticalonline.com Feb 7, 2000) 24

Cidara addresses multiple unmet medical needs Rezafungin Cloudbreak 25

SB 11 Vaccines for influenza have limitations Viral coverage Patient Manufacturing Strain-specific, Less effective in elderly & Challenging in a pandemic: variable coverage immune compromised long, complex production 10%-60% effective ~2-week lag time to Difficult to scale, low yields (2004-2018)1 achieve full protection2 can limit production capacity3 1. https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm 2. https://www.cdc.gov/flu/protect/keyfacts.htm 3. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm 26

SB 11 Treatments for influenza also have limitations Resistance Administration Efficacy Resistance emerges 48 hour window Limited in patients with rapidly complicated & severe disease 27

Cloudbreak antiviral conjugates (AVCs) for influenza: Potential single dose universal protection and treatment 28

SB 10 Cloudbreak platform – multimodal mechanism of action: intrinsic antimicrobial activity & immune engagement Immune Pathogen Component TARGETING MOIETY Fc MOIETY Binds conserved surface target Engages innate or Direct antimicrobial activity adaptive immune system 29

SB 10 Cloudbreak AVCs combine the power of small molecules (SMs) and monoclonal antibodies • High potency SMs • Extended half-life Intrinsic antiviral • Broad spectrum (influenza A&B) activity • Combining multiple MOAs VIRUS Conserved, essential target 30

SB 12 What would an “ideal” product look like? Broad spectrum, universal coverage Superior resistance profile Protection for High-Risk Populations Expanded efficacy window Long duration of action Rapid onset of activity Flexible administration 31
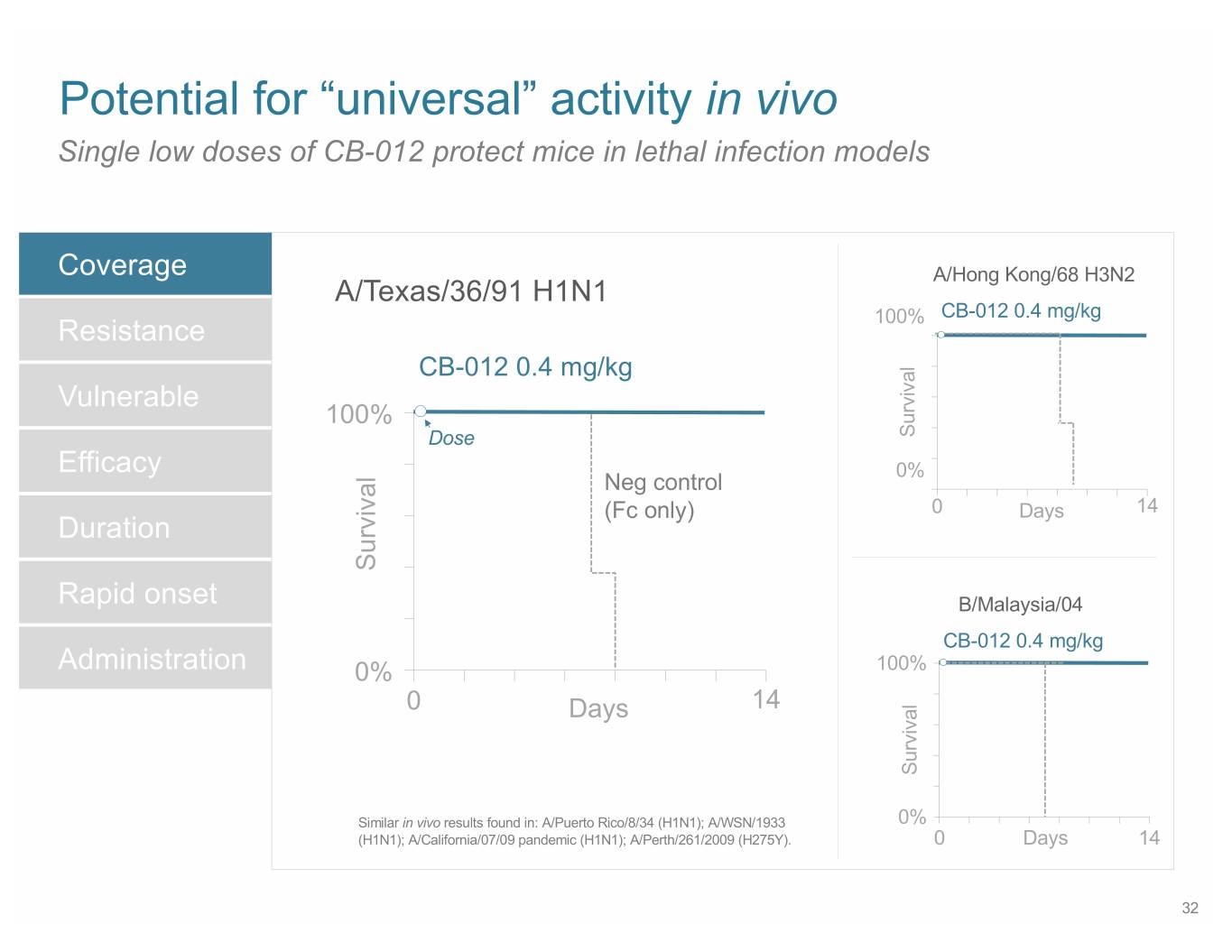
SB 14 Potential for “universal” activity in vivo Single low doses of CB-012 protect mice in lethal infection models Coverage A/Hong Kong/68 H3N2 A/Texas/36/91 H1N1 100% CB-012 0.4 mg/kg Resistance CB-012 0.4 mg/kg Vulnerable 100% Dose Survival Efficacy 0% Neg control (Fc only) 0 Days 14 Duration Survival Rapid onset B/Malaysia/04 CB-012 0.4 mg/kg Administration 0% 100% 0 Days 14 Survival Similar in vivo results found in: A/Puerto Rico/8/34 (H1N1); A/WSN/1933 0% (H1N1); A/California/07/09 pandemic (H1N1); A/Perth/261/2009 (H275Y). 0 Days 14 32
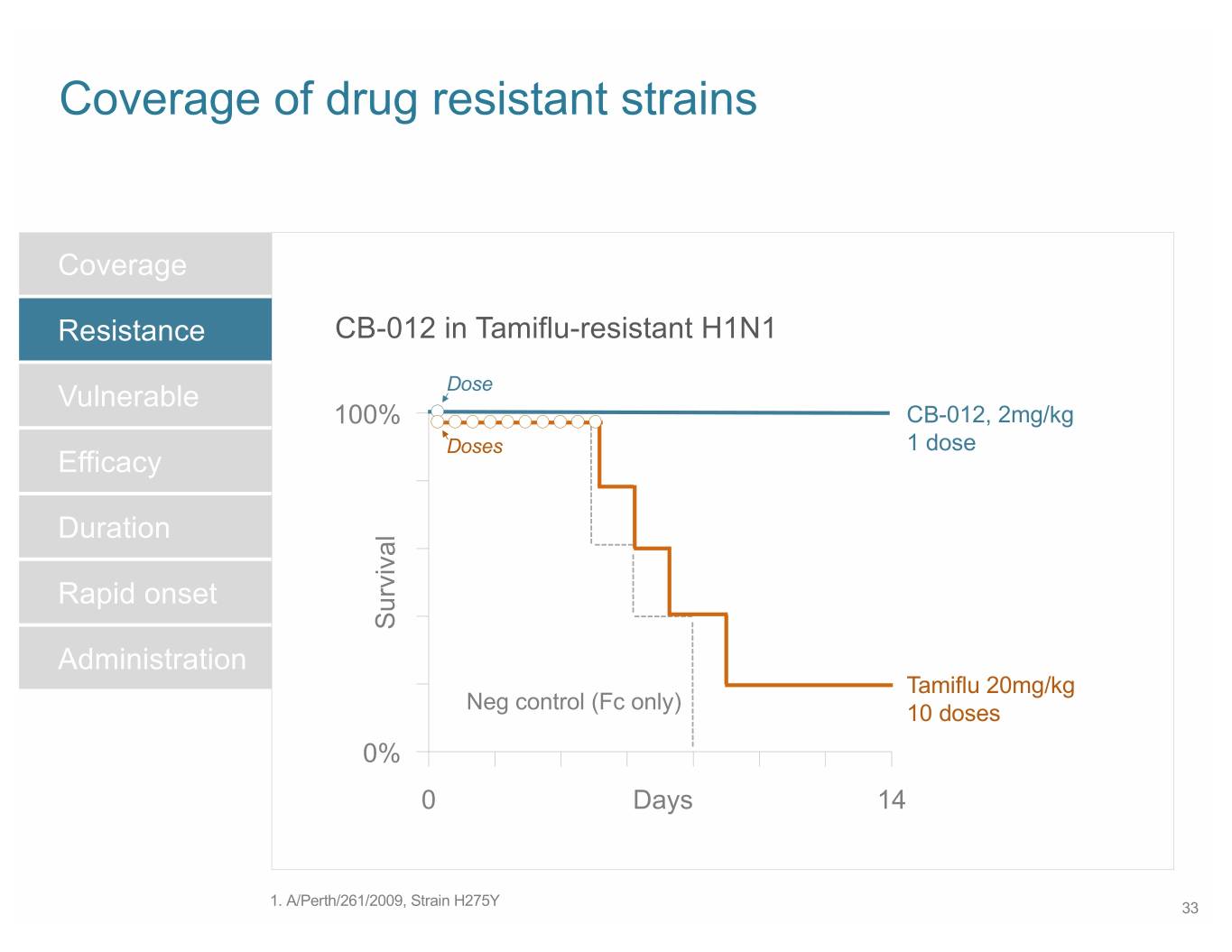
SB 14 Coverage of drug resistant strains Coverage Resistance CB-012 in Tamiflu-resistant H1N1 Vulnerable Dose 100% CB-012, 2mg/kg Doses 1 dose Efficacy Duration Rapid onset Survival Administration Tamiflu 20mg/kg Neg control (Fc only) 10 doses 0% 0 Days 14 1. A/Perth/261/2009, Strain H275Y 33

SB 14 Potential for protection in vulnerable patients Lethal influenza model (H1N1: A/Puerto Rico/8/34) Coverage BALB/c mice BALB/c SCID mice Resistance Immune competent Severe Combined ImmunoDeficiency Vulnerable Efficacy 0.3 mg/kg 0.3 mg/kg Duration 100% 100% Dose CB-012 Dose CB-012 Rapid onset Administration Survival Vehicle Survival Vehicle 0% 0% 0 Days 14 0 Days 14 34

SB 14 Expansion of the treatment window Coverage Treatment initiated 72 HOURS post-infection Resistance Dose CB-012: 10 mg/kg Vulnerable Tamiflu: 20 mg/kg Doses CB-012 Efficacy Duration Fc only Tamiflu Rapid onset Administration 0 24 48 72 96 HOURS INFECTION Mouse lethal endpoint model H1N1 35
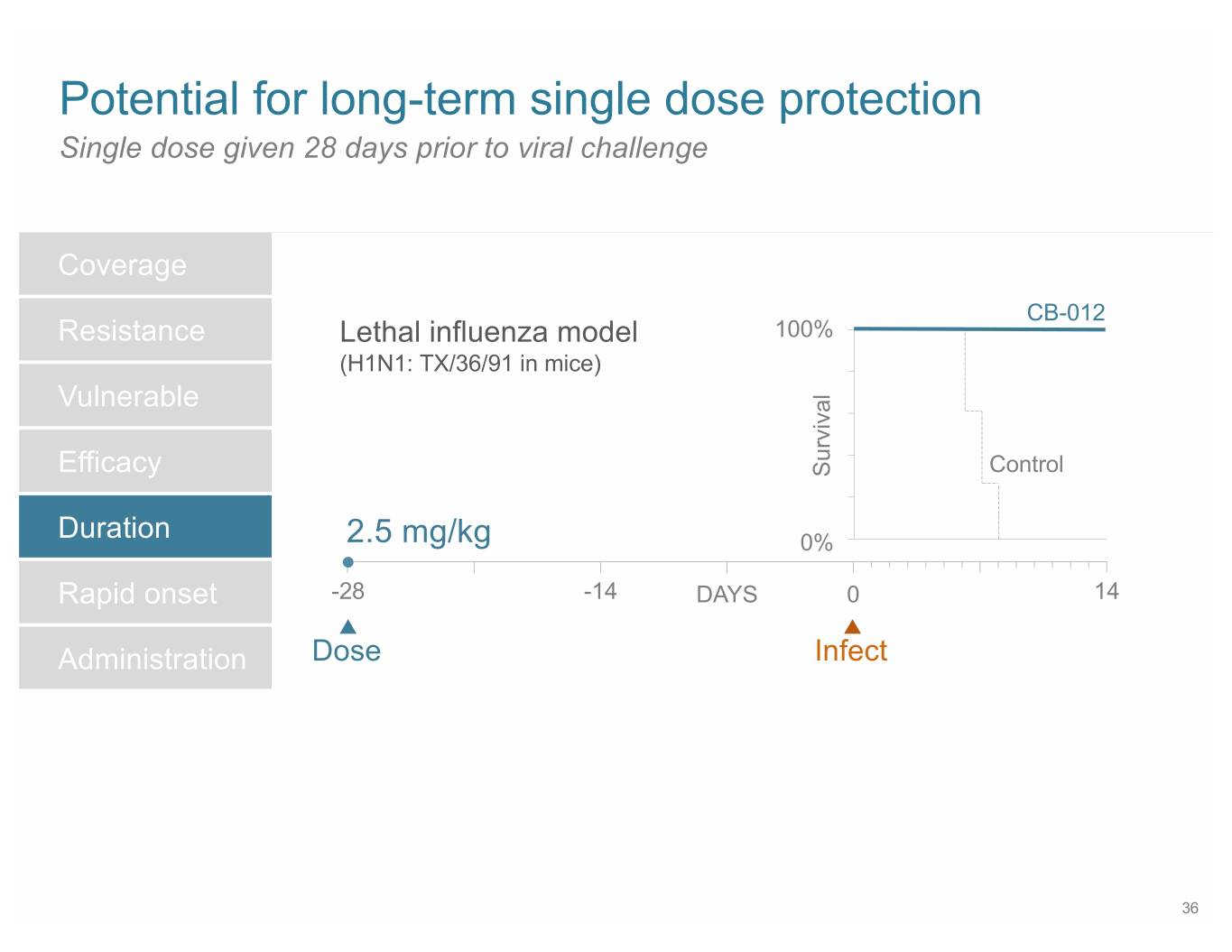
SB 14 Potential for long-term single dose protection Single dose given 28 days prior to viral challenge Coverage CB-012 Resistance Lethal influenza model 100% (H1N1: TX/36/91 in mice) Vulnerable Efficacy Survival Control Duration 2.5 mg/kg 0% Rapid onset -28 -14 DAYS 0 14 Administration Dose Infect 36

SB 14 Rapid therapeutic exposure in key tissues Coverage Resistance CB-012 lung distribution 10 mg/kg IV dose, mouse Vulnerable Efficacy Plasma Duration Concentration Cmax 1 hr CB-012: MINUTES Rapid onset Lung VACCINES: WEEKS Administration 37

SB 14 Flexible routes of administration Coverage CB-012 dosed by different routes Resistance 5 mg/kg, mouse Vulnerable Cmax 4 hr Efficacy Cmax 24 hr Duration Subq Rapid onset IM IV Administration 38

Dose-dependent viral clearance in lungs Viral burden (H1N1) in mouse lung – Day 4 post-infection PFU / g (Log10) 103 104 105 106 107 108 PBS control Fc control (3 mg/kg) Oseltamivir (5 mg/kg, BID x 4) CB-012 (0.1 mg/kg) CB-012 (0.3 mg/kg) CB-012 (1.0 mg/kg) CB-012 (3.0 mg/kg) 39

Dose-dependent reduction in inflammatory cytokines TNFa (Mouse lung) IL-6 (Mouse Lung) Concentration (pg/mL) Concentration (pg/mL) 0 400 800 0 1000 1500 PBS control Fc control (3 mg/kg) Oseltamivir (5 mg/kg, BID x 4) CB-012 (0.1 mg/kg) CB-012 (1.0 mg/kg) CB-012 (3.0 mg/kg) Uninfected 40

Broad safety margin in rats and primates Results of 14-day toxicity testing Therapeutic Margins 60000 NO FINDINGS FOR: Area under the curve (AUC) for maximum Clinical observations dose tested (hr*µg/mL) Hematology 40000 Clinical Chemistry Coagulation 15x 10x Urinalysis 20000 Histopathology AUC required for efficacy in mice (hr*µg/mL) 0 Rat Primate 41

Platform expansion beyond influenza INFLUENZA RSV HIV DENGUE ZIKA 42

Upcoming milestones 2019 2020 August Midyear Options X: new Cloudbreak data Phase 3 ReSTORE topline October IDWeek/TIMM - STRIVE A&B data 2nd half 2019 Investor Day Phase 1 subcutaneous 43

Financial overview We expect to finance our cash needs through: Summary Information ($M) June 30, 2019 • equity and debt financings, Cash $44.6 • entering into collaborations, strategic alliances and Common shares issued 26.8 licensing arrangements, • receiving government and/or Common equivalent shares issued1 32.4 charitable grants or contracts. 1 Includes 26,767,989 common shares and assumes conversion of 565,231 shares of Series X Convertible Preferred into 5,652,310 common shares at June 30, 2019. Each share of Series X Convertible Preferred is convertible into 10 shares of common. 44

SB 12 Cidara is much more than a typical ID company Strategic Focus Not a ‘typical’ Infectious Disease company Rezafungin Treatment Large market with low dev risk (STRIVE A + B) Rezafungin Prophylaxis Hem/onc supportive care, high unmet need Cloudbreak AVC Enormous influenza market potential & expansion opportunities Our Team Experienced creators of shareholder value 45

New Hope for Serious Infections Corporate Presentation August 2019 © Cidara Therapeutics 2019 | Confidential













































