| Item 7.01 | Regulation FD Disclosure. |
On July 29, 2019, Cidara Therapeutics, Inc. (the “Company”) issued a press release announcing the topline results from Part B of its global Phase 2 STRIVE clinical trial evaluating the Company’s lead antifungal candidate, rezafungin (“STRIVE B”). The full text of the press release is attached as Exhibit 99.1 to this Current Report on Form8-K. As indicated in the press release, on July 29, 2019 the Company will host a conference call and webcast at 8:00 a.m. Eastern time to discuss the results from STRIVE B. The slide presentation to be presented on the call is attached hereto as Exhibit 99.2.
The information contained in this Item 7.01 of this Current Reporton Form 8-K, including Exhibits 99.1 and 99.2, is being furnished pursuant to Item 7.01 and shall not be deemed “filed” for purposes of Section 18 of the Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, and it shall not be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or under the Exchange Act, whether made before or after the date hereof, except as expressly set forth by specific reference in such filing to this item of this report.
On July 29, 2019, the Company reported topline results from STRIVE B. The Company previously reported topline results from Part A of the STRIVE clinical trial (“STRIVE A”) in March 2018.
STRIVE B was an international, multicenter, double-blind clinical trial evaluating the safety, tolerability and efficacy of once-weekly dosing of rezafungin acetate compared to once-daily dosing of caspofungin in patients with candidemia and/or invasive candidiasis.
STRIVE B enrolled 91 patients in the microbiologicalintent-to-treat, or mITT, population. Patients were randomized to receive either 400 mg of rezafungin administered intravenously once weekly for two to four weeks or daily caspofungin administered intravenously according to the approved prescribing information, with an optional step down to oral fluconazole. To align with the chosen dosing regimen in the Phase 3 program, the STRIVE B trial was amended midway for Group 1 to use rezafungin 400 mg for the first week followed by 200 mg once weekly for up to four weeks in total.
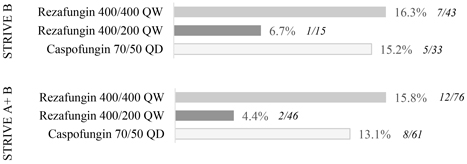
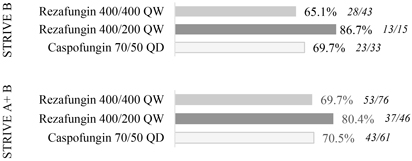
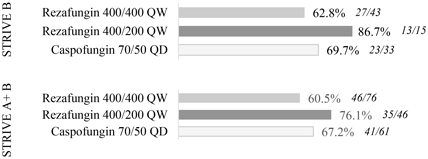
The objective of STRIVE B was to show comparability in efficacy and safety of rezafungin dosed once-weekly and caspofungin dosed once-daily. Efficacy measures in the trial included clearance ofCandida from the blood or other normally sterile sites, resolution of systemic signs attributed to theCandida infection, investigator assessment of clinical response and overall survival. The trial was not statistically powered to demonstrate superiority ornon-inferiority and therefore comparisons of efficacy are directional.
In the STRIVE B trial, rezafungin met all of its objectives for efficacy, safety and tolerability in the treatment of patients with candidemia and/or invasive candidiasis. The trial results show that patients treated with rezafungin had numerically improved outcomes as compared to caspofungin across all efficacy measures at the 400 mg/200 mg dosing regimen. In addition, an analysis combining data across STRIVE A and STRIVE B, demonstrated that rezafungin achieves meaningful improvement in outcomes compared to caspofungin on Clinical Response across all efficacy endpoints at the same 400 mg/200 mg dose.
The charts below illustrate the topline results from STRIVE B and combined results from STRIVE A and STRIVE B for critical measures of efficacy.
All-Cause Mortality – Death through Day 30a