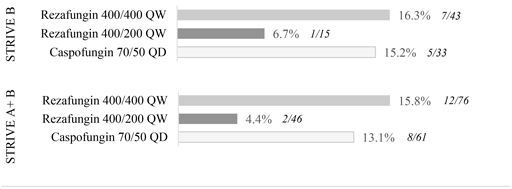
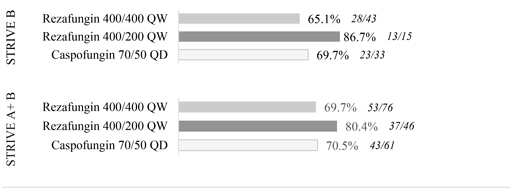
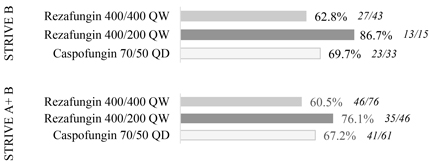
While the difference in outcomes for 400 mg/200 mg are encouraging, and the 400 mg/400 mg findings are consistent with comparator results, the size of the individual trial cohorts precludes any other conclusions pending full assessment of trial data.
There were no unanticipated or concerning adverse event trends among STRIVE B trial participants. Thetop-line results indicate that rezafungin appeared to be generally safe and well-tolerated at both dosing regimens. As expected, and observed in STRIVE A, treatment emergent adverse events (TEAEs) in the study population were observed in most patients, though study drug-related adverse events were substantially lower, with a frequency of 6.5 percent, zero percent and 14.7 percent in the 400 mg/400 mg, 400 mg/200 mg, and caspofungin groups respectively.
“There have been no new drugs approved for the treatment of seriousCandida infections or the prevention of invasive fungal infections in over a decade,” said George Thompson, M.D., associate professor of Clinical Medicine at the University of California, Davis, School of Medicine, and chair of the Mycoses Study Group Education Committee. “The positive outcomes from Part B of the STRIVE trial, which are similar to those from Part A, further substantiate the potential efficacy and safety of rezafungin in extremely ill patients affected by fungal disease. These important data also reinforce the potential of rezafungin as a practical, first-line antifungal treatment option over current standards of care, which are associated with significant limitations.”
STRIVE B Trial Design
STRIVE B was an international, multicenter, double-blind clinical trial evaluating the safety, tolerability and efficacy of once-weekly dosing of rezafungin acetate compared to once-daily dosing of caspofungin in patients with candidemia and/or invasive candidiasis. Efficacy measures in the trial included clearance ofCandida from the blood or other normally sterile sites, resolution of systemic signs attributed to theCandida infection, investigator assessment of clinical response and overall survival.
The trial enrolled 91 patients in the microbiologicalintent-to-treat, or mITT, population. Patients were randomized to receive either 400 mg of rezafungin administered intravenously once weekly for two to four weeks or daily caspofungin administered intravenously according to the approved prescribing information, with an optional step down to oral fluconazole. To align with the chosen dosing regimen in the Phase 3 program, the STRIVE B trial was amended midway to use rezafungin 400 mg for the first week followed by 200 mg once weekly for up to four weeks in total.