
New Hope for Serious Infections Topline Results: Phase 2 STRIVE Part B Trial for Rezafungin July 29, 2019 © Cidara Therapeutics 2019 | Confidential Exhibit 99.2

Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to Cidara’s ability to develop new anti-infectives that are innovative or address unmet needs; the potential for rezafungin to successfully treat or prevent invasive fungal infections and represent an improvement over current approaches; whether the top line results of the STRIVE Part B clinical trial will be supported in the full analysis of the STRIVE Part B clinical data; whether the success of the STRIVE Part B clinical trial indicates a successful outcome in the Phase 3 ReSTORE clinical trial, including whether or not rezafungin will meet the primary endpoints in the ReSTORE trial; and whether Cidara will be able to successfully develop and commercialize rezafungin. This presentation also contains estimates and other statistical data made by independent parties and by Cidara relating to market size and growth and other data about Cidara's industryThese data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Projections, assumptions and estimates of the future performance of the markets in which Cidara operates are necessarily subject to a high degree of uncertainty and risk. Risks that contribute to the uncertain nature of the forward-looking statements include: Cidara’s abilityto obtain additional financing; the success and timing of Cidara’s preclinical studies, clinical trials and other research and development activities; receipt of necessary regulatory approvals for development and commercialization, as well as changes to applicable regulatory laws in the United States and foreign countries; changes in Cidara’s plans to develop and commercialize its product candidates; Cidara’s ability to obtain and maintain intellectual property protection for its product candidates; and the loss of key scientific or management personnel. These and other risks and uncertainties are described more fully in Cidara’s Form 10-K as most recently filed with the United States Securities and Exchange Commission (SEC), under the heading “Risk Factors.” All forward-looking statements contained in this presentation speak only as of the date on which they were made. Cidara undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.

Executive Summary STRIVE B results successfully met all efficacy and safety objectives Results corroborate those from STRIVE A Uniform improvement in all efficacy outcomes comparing the 400/200mg dose versus caspofungin comparator Well tolerated with no concerning safety signals Results warrant continuation of ongoing global ReSTORE Phase 3 trial at current 400/200mg dosing regimen
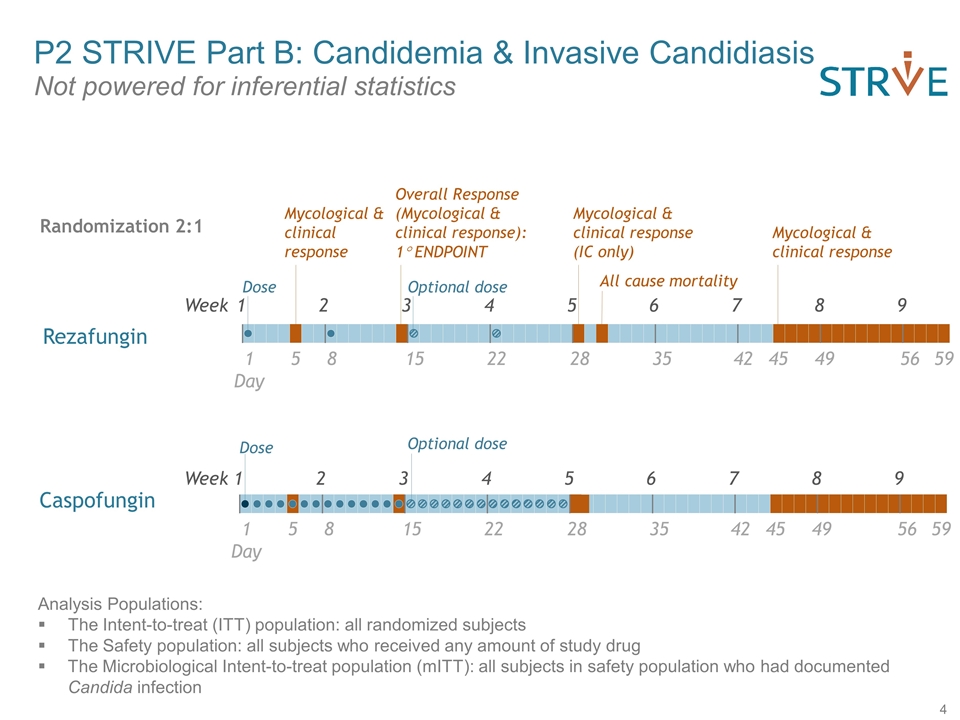
Week 1 2 3 4 5 6 7 8 9 Day 1 5 8 15 22 28 Dose Optional dose Mycological & clinical response Overall Response (Mycological & clinical response): 1° ENDPOINT Mycological & clinical response (IC only) 45 35 42 49 56 59 Mycological & clinical response Week 1 2 3 4 5 6 7 8 9 Day 1 5 8 15 22 28 45 35 42 49 56 59 Dose All cause mortality Analysis Populations: The Intent-to-treat (ITT) population: all randomized subjects The Safety population: all subjects who received any amount of study drug The Microbiological Intent-to-treat population (mITT): all subjects in safety population who had documented Candida infection Randomization 2:1 Caspofungin Rezafungin Optional dose P2 STRIVE Part B: Candidemia & Invasive Candidiasis Not powered for inferential statistics
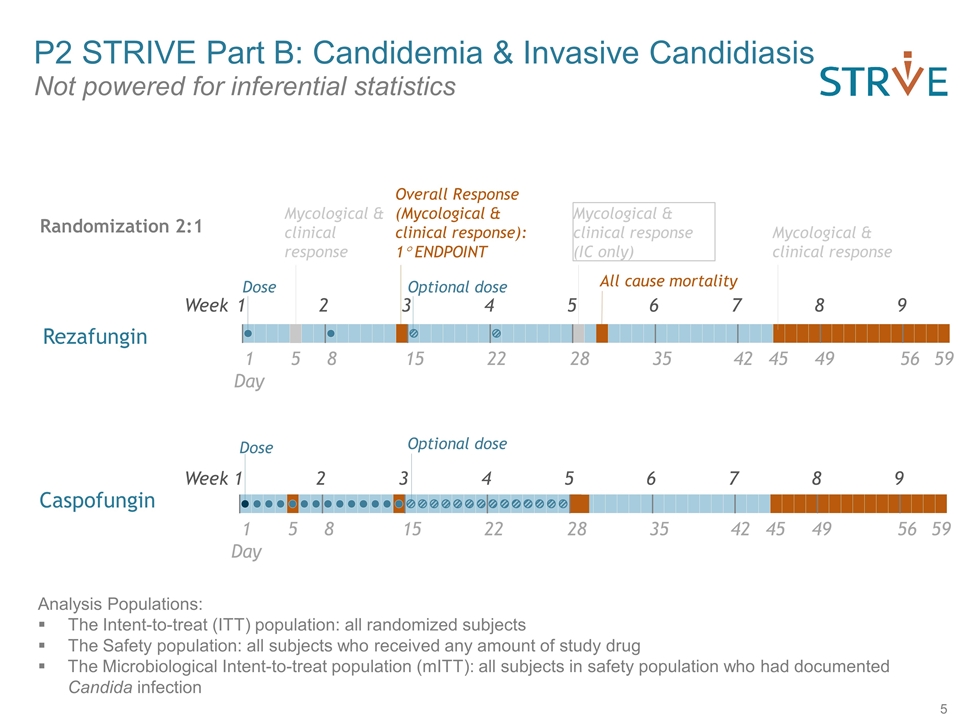
Week 1 2 3 4 5 6 7 8 9 Day 1 5 8 15 22 28 Dose Optional dose Mycological & clinical response Overall Response (Mycological & clinical response): 1° ENDPOINT Mycological & clinical response (IC only) 45 35 42 49 56 59 Mycological & clinical response Week 1 2 3 4 5 6 7 8 9 Day 1 5 8 15 22 28 45 35 42 49 56 59 Dose All cause mortality Analysis Populations: The Intent-to-treat (ITT) population: all randomized subjects The Safety population: all subjects who received any amount of study drug The Microbiological Intent-to-treat population (mITT): all subjects in safety population who had documented Candida infection P2 STRIVE Part B: Candidemia & Invasive Candidiasis Not powered for inferential statistics Caspofungin Rezafungin Optional dose Randomization 2:1
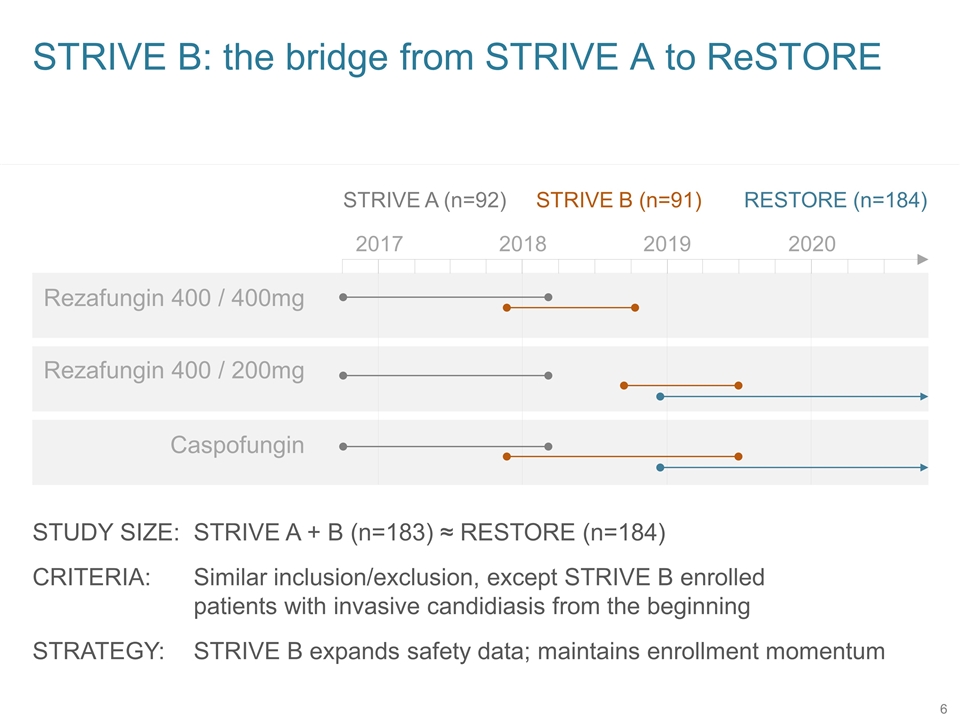
Rezafungin 400 / 200mg RESTORE (n=184) STRIVE B: the bridge from STRIVE A to ReSTORE Rezafungin 400 / 400mg Caspofungin 2017 2018 2019 2020 STRIVE B (n=91) STRIVE A (n=92) STUDY SIZE: STRIVE A + B (n=183) ≈ RESTORE (n=184) CRITERIA: Similar inclusion/exclusion, except STRIVE B enrolled patients with invasive candidiasis from the beginning STRATEGY: STRIVE B expands safety data; maintains enrollment momentum
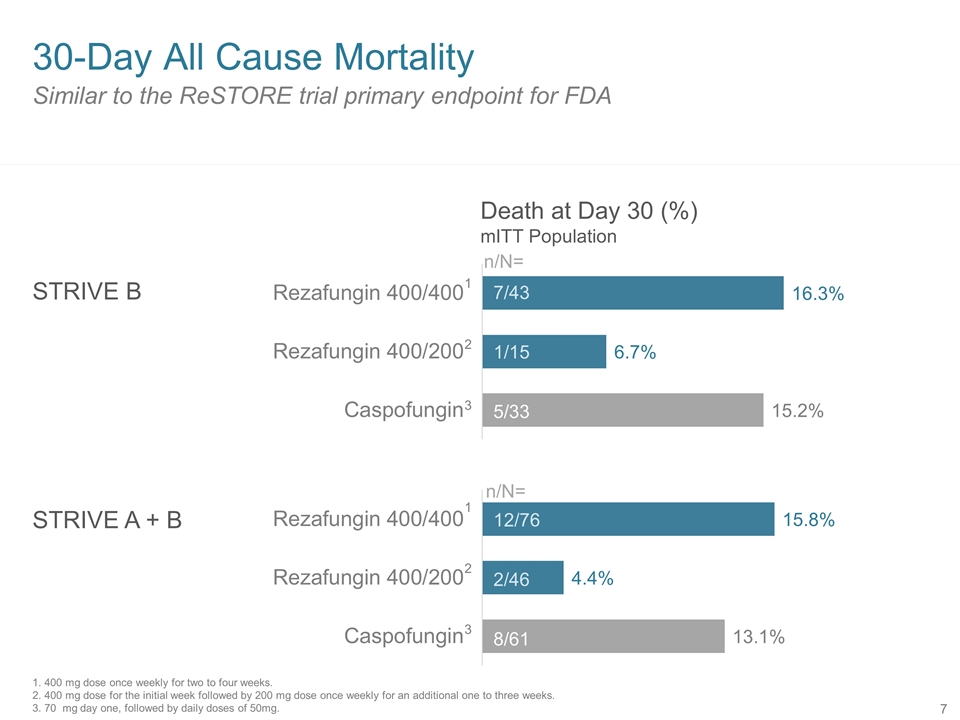
Similar to the ReSTORE trial primary endpoint for FDA 30-Day All Cause Mortality STRIVE B STRIVE A + B 7/43 1/15 5/33 12/76 2/46 8/61 n/N= n/N= Death at Day 30 (%) mITT Population 1 2 3 1 2 3 1. 400 mg dose once weekly for two to four weeks. 2. 400 mg dose for the initial week followed by 200 mg dose once weekly for an additional one to three weeks. 3. 70 mg day one, followed by daily doses of 50mg.

Similar to the ReSTORE trial primary endpoint for EMA Investigator assessment of clinical response STRIVE B STRIVE A + B 28/43 13/15 23/33 53/76 37/46 43/61 n/N= n/N= Clinical Cure (%) at Day 14 mITT Population 1. 400 mg dose once weekly for two to four weeks. 2. 400 mg dose for the initial week followed by 200 mg dose once weekly for an additional one to three weeks. 3. 70 mg day one, followed by daily doses of 50mg. 1 2 3 1 2 3
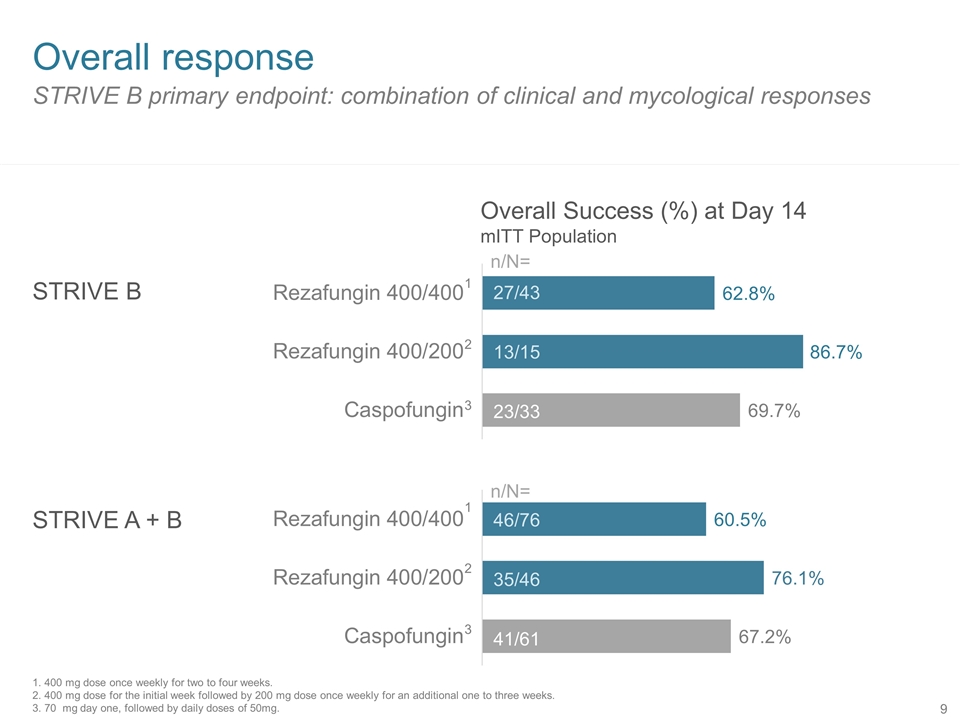
STRIVE B primary endpoint: combination of clinical and mycological responses Overall response STRIVE B STRIVE A + B 27/43 13/15 23/33 46/76 35/46 41/61 n/N= n/N= Overall Success (%) at Day 14 mITT Population 1 2 3 1 2 3 1. 400 mg dose once weekly for two to four weeks. 2. 400 mg dose for the initial week followed by 200 mg dose once weekly for an additional one to three weeks. 3. 70 mg day one, followed by daily doses of 50mg.

Topline summary of adverse events in safety population 400/400 mg (QWk) 400/200 mg (QWk) Pooled Groups N=46 N=18 N=64 n (%) All Related TEAEs 3 (6.5) 0 3 (4.7) Leading to study D/C 2 (4.3) 0 2 (3.1) Serious AE 1 (2.2) 0 1 (1.6) 70/50 mg (QD) N=34 n (%) 5 (14.7) 3 (8.8) 1 (2.9) REZAFUNGIN CASPOFUNGIN As expected and observed in STRIVE A, the majority of subjects had at least one TEAE and 40-50% had at least one Serious AE, reflecting the high morbidity of the underlying population. There were no AE trends; % of TEAEs and SAEs were approximately even across study groups. D/C=discontinuation; TEAE (treatment-emergent adverse event)=AE that occurs after first dose of study drug is administered. N=81 N=53 N=134 All Related TEAEs 7 (8.6) 6 (11.3) 13 (9.7) Leading to study D/C 3 (3.7) 0 3 (2.2) Serious AE 1 (1.2) 1 (1.9) 2 (1.5) N=68 9 (13.2) 1 (1.5) 2 (2.9) STRIVE A + B STRIVE B Study-Drug Related TEAEs
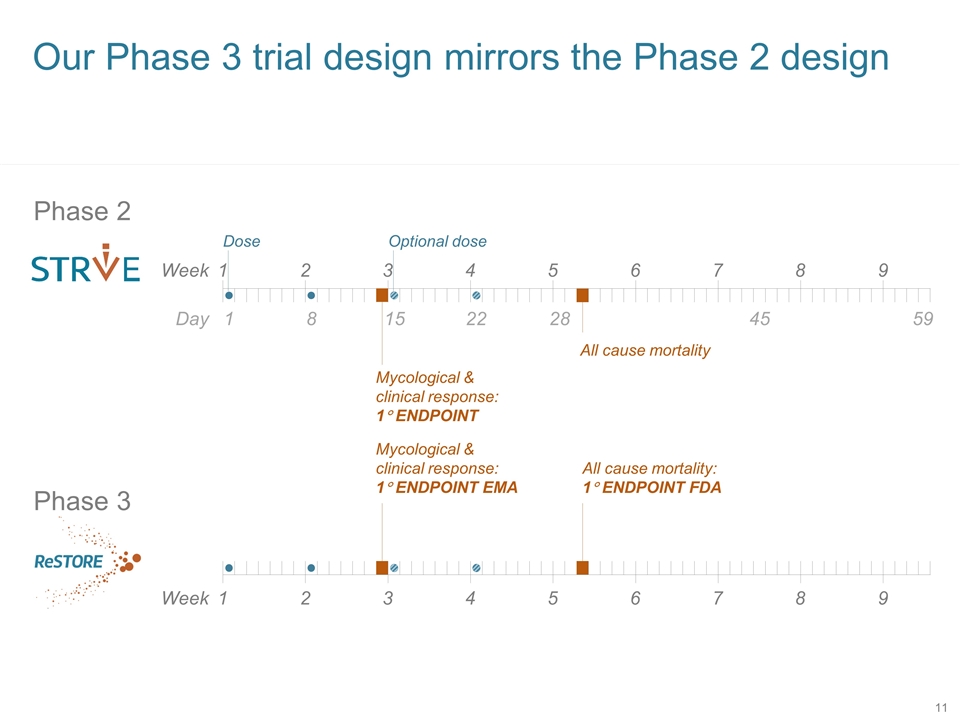
Our Phase 3 trial design mirrors the Phase 2 design Phase 2 Week 1 2 3 4 5 6 7 8 9 1 Dose Optional dose Mycological & clinical response: 1° ENDPOINT All cause mortality Day 8 15 22 28 45 59 Phase 3 Week 1 2 3 4 5 6 7 8 9 Mycological & clinical response: 1° ENDPOINT EMA All cause mortality: 1° ENDPOINT FDA
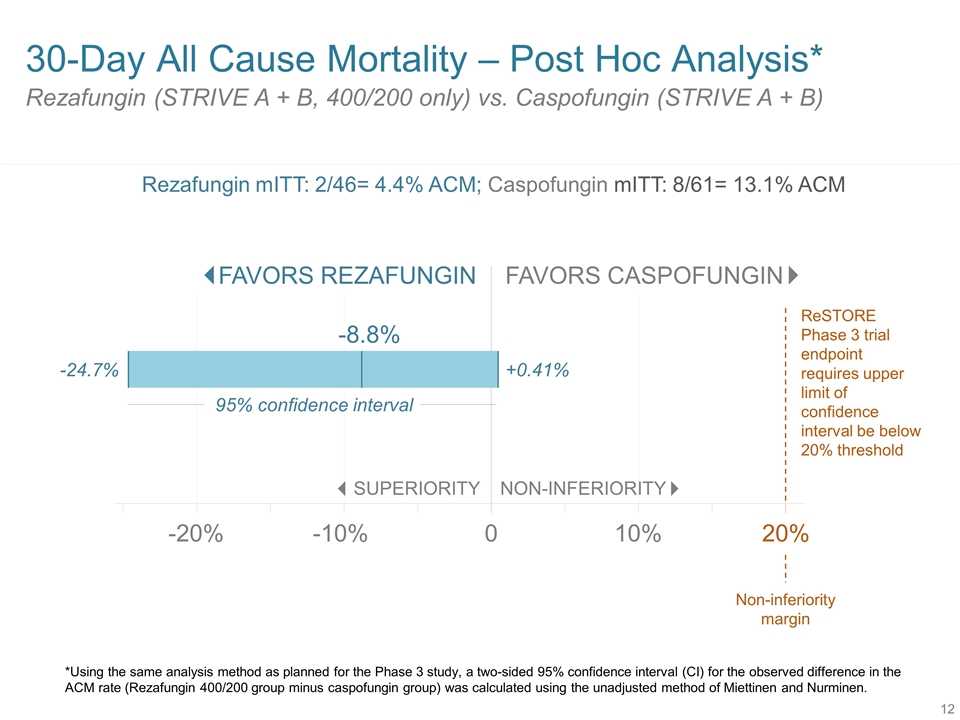
Rezafungin (STRIVE A + B, 400/200 only) vs. Caspofungin (STRIVE A + B) 30-Day All Cause Mortality – Post Hoc Analysis* SUPERIORITY NON-INFERIORITY 0 -20% 10% -10% -24.7% +0.41% FAVORS REZAFUNGIN FAVORS CASPOFUNGIN -8.8% 20% ReSTORE Phase 3 trial endpoint requires upper limit of confidence interval be below 20% threshold Non-inferiority margin 95% confidence interval *Using the same analysis method as planned for the Phase 3 study, a two-sided 95% confidence interval (CI) for the observed difference in the ACM rate (Rezafungin 400/200 group minus caspofungin group) was calculated using the unadjusted method of Miettinen and Nurminen. Rezafungin mITT: 2/46= 4.4% ACM; Caspofungin mITT: 8/61= 13.1% ACM

Conclusions Positive findings strongly support that once-weekly dosing of rezafungin is comparable to once-daily dosing of caspofungin Caspofungin cure rate at ~70% in both STRIVE A and B for Clinical Response is similar to its outcome in prior studies for candidemia/IC thereby validating the quality of the study Supports the selection of the 400/200 dosing regimen in ReSTORE Enrollment of ReSTORE Phase 3 study globally is underway

New Hope for Serious Infections Topline Results: Phase 2 STRIVE Part B Trial for Rezafungin July 29, 2019 Thank you © Cidara Therapeutics 2019 | Confidential













