
Zynerba Pharmaceuticals Announces New ZYN002 Data from STAR 1 and STAR 2 Studies in Patients with Focal Seizures at the 2017 Annual Meeting of the American Epilepsy Society (AES)
- Clinically Meaningful Median Reduction in Seizures Compared to Baseline of 48% to 65% Achieved with Continued ZYN002 Treatment in STAR 2 Extension Study –
- Data Help Clarify Protocol Design for Planned ZYN002 Phase 2 Trial in Epilepsy -
Washington, DC., December 3, 2017 — Zynerba Pharmaceuticals, Inc. (NASDAQ:ZYNE), a clinical-stage specialty pharmaceutical company dedicated to developing and commercializing innovative pharmaceutically-produced transdermal cannabinoid treatments, today is reporting new clinical data presented at the 2017 Annual Meeting of the American Epilepsy Society (AES) in Washington, DC.
In a poster presentation entitled, “Synthetic Transdermal Cannabidiol for the Treatment of Focal Epilepsy in Adults (poster #2.428),” Terence O’Brien, MD of the Royal Melbourne Hospital at The University of Melbourne presented new data from the completed Phase 2 STAR 1 (Synthetic Transdermal Cannabidiol for the Treatment of Epilepsy) study and ongoing STAR 2 18-month open label extension study evaluating ZYN002 cannabidiol (CBD) transdermal gel in patients with focal seizures. The presentation included data through nine months of total exposure to ZYN002 (three months of treatment in STAR 1 and six months in STAR 2).
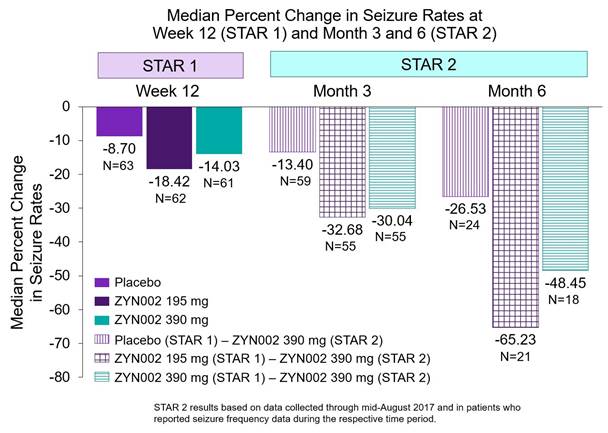
The key findings include that clinically meaningful responses to ZYN002, as measured by reductions in focal seizures from the baseline period of STAR 1, are correlated with continued treatment with ZYN002. Patients who received ZYN002 (195mg during STAR 1 for three months and 390mg for six months in STAR 2) for a total of nine months achieved a median reduction in seizures of 65%. Patients who received ZYN002 (390mg for three months in STAR 1 and six months in STAR 2) achieved a 48% median reduction in seizures from baseline. In addition, ZYN002 was shown to be very well tolerated through nine months of exposure.
“These new ZYN002 data are the first of their kind, showing that focal seizures in adults may be effectively treated by a transdermal gel delivery of pharmaceutically-produced cannabidiol,” said Terri Browning Sebree, Zynerba’s President. “In this population of patients, continued treatment with ZYN002 was shown to significantly reduce seizure rates compared to baseline. Importantly, baseline seizure frequency appears to be an important indicator of response. These are important findings that will help us finalize a new trial design. We expect to outline the design, size and timing of the trial in the first quarter of 2018, and initiate the trial later in 2018.”
“The data presented this afternoon at the American Epilepsy Society meeting are exciting as they demonstrate that ZYN002 may have an effect on focal seizures in adults suffering from refractory epilepsy,” said Terence O’Brien, MD. “The epilepsy community has been eagerly awaiting data demonstrating the potential of pharmaceutically-produced cannabidiol formulated for transdermal delivery. Evidence for the efficacy of CBD-based treatments to reduce seizures in certain epilepsy populations is emerging, but there is no previous high level clinical trial evidence for focal seizures in adults. The potential for a CBD-based treatment with an optimal tolerability profile would be significant for these patients. I look forward to participating in the next clinical trial with ZYN002, and believe that this drug holds great promise for patients suffering from refractory epilepsy.”
The data presented in the poster are as follows:
Demographics and Baseline Characteristics
· Patients randomized into STAR 1 (N=188) had a median monthly seizure frequency of 10.6 (3-335) at baseline. By group, the median monthly seizure frequency at baseline was 10.5 for the placebo group, 14.0 for the ZYN002 195 mg treatment group, and 10.14 for the ZYN002 390 mg treatment group;
· Of the 188 randomized patients, 186 were analyzed for efficacy, and 174 completed the 12-week STAR 1 study;
· 171 patients (98% of STAR 1 completers) continued into STAR 2;
· Patients were taking a wide range of antiepileptic drugs (AEDs), with a median of 3.0 AEDs; use of clobazam was excluded in both the STAR 1 and STAR 2 studies.
Efficacy
· As previously disclosed on August 7, 2017, compared with baseline, after 12 weeks of blinded treatment, the change in seizure frequency did not statistically differ between placebo and both doses of ZYN002, though there was a numerical difference favoring ZYN002;
· The lack of separation of ZYN002 from placebo in STAR 1 was likely due in part to 15 (24%) placebo-treated patients who achieved at least a 50% reduction in focal seizures; 13 of these 15 patients had a relatively low baseline seizure rate (<15 focal seizures per month);
· In STAR 1, patients with more severe epilepsy (defined as a baseline seizure frequency of ≥15 per month) taking ZYN002 had a greater percent reduction in seizures compared to patients with severe epilepsy receiving placebo;
· Continued exposure to ZYN002 in STAR 2 (all patients dosed with 390 mg/day) resulted in clinically meaningful reductions in seizures:
· Patients taking ZYN002 for six months (three months during STAR 1 and three months in STAR 2) experienced a >30% median reduction in seizures from baseline;
· Patients taking ZYN002 for nine months (three months during STAR 1 and six months in STAR 2) experienced a >65% (195 mg in STAR 1 and 390 mg in STAR 2) and >48% (390 mg in STAR 1 and STAR 2) median reduction in seizures from baseline.
· A small number of patients in STAR 2 had an increase in their background AEDs; the improvements in seizure frequency observed in STAR 2 were not due to these changes to background AEDs.
Safety
· ZYN002 was very well tolerated with an incidence of adverse events comparable to placebo and no clinically significant differences between the active treatment groups;
· The safety profile of ZYN002 was consistent with previously released data from Phase 1 and Phase 2 trials;
· There were no clinically significant changes in ECGs or laboratory results in patients receiving ZYN002.
A copy of the poster presentation is currently available on the Zynerba corporate website at http://zynerba.com/publications/
About ZYN002
Zynerba’s ZYN002 CBD gel is the first and only pharmaceutically-produced CBD formulated as a patent-protected permeation-enhanced gel and is being studied in children with Fragile X Syndrome, adult epilepsy patients with focal seizures and osteoarthritis. ZYN002 is a clear, permeation-enhanced gel that is designed to provide controlled drug delivery transdermally with once- or twice-daily dosing.
About Our Technology
Cannabinoids are a class of chemical compounds found in the Cannabis plant. The two primary cannabinoids contained in Cannabis are cannabidiol, or CBD, and ∆9-tetrahydrocannabinol, or THC. Clinical and preclinical data support the potential for CBD in treating epilepsy, arthritis and Fragile X Syndrome, and THC has positive effects on treating pain. Zynerba is developing therapeutic medicines that utilize innovative transdermal technologies that, if successful, may allow for sustained and controlled delivery of therapeutic levels of CBD and THC. Transdermal delivery of cannabinoids may have benefits over oral dosing because it allows the drug to be absorbed through the skin directly into the bloodstream. This avoids first-pass liver metabolism, potentially enabling lower dosage levels of active pharmaceutical ingredients with a higher bioavailability and improved safety profile. Transdermal delivery also avoids the gastrointestinal tract, lessening the opportunity for GI related adverse events and the potential degradation of CBD by gastric acid into THC, which may be associated with unwanted psychoactive effects.
Using an established chemical pharmaceutical process for manufacturing, Zynerba replicates the CBD and THC found in the Cannabis plant. We believe that this will allow us to meet stringent global regulatory agencies’ standards while ensuring that we can efficiently supply the amount of product required to meet the demand of the large markets that we are targeting.
About Zynerba Pharmaceuticals, Inc.
Zynerba Pharmaceuticals (NASDAQ: ZYNE) is dedicated to improving the lives of people with severe health conditions where there is a high unmet medical need by developing and commercializing pharmaceutically-produced transdermal cannabinoid medicines designed to meet the rigorous efficacy and safety standards established by global regulatory agencies. Through the discovery and development of these life-changing medicines, Zynerba seeks to improve the lives of patients battling severe, chronic health conditions including epilepsy, Fragile X syndrome, osteoarthritis, fibromyalgia and peripheral neuropathic pain. Learn more at www.zynerba.com and follow the Company on Twitter at @ZynerbaPharma.
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from the Company’s current expectations. For example, there can be no guarantee that the Company will obtain approval for ZYN002 or ZYN001 from the U.S. Food and Drug Administration (FDA) or foreign regulatory authorities; even if ZYN002 or ZYN001 are approved, the Company may not be able to obtain the label claims that it is seeking from the FDA. In addition, the Company’s cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated. Management’s expectations and, therefore, any forward-looking statements in this press release could also be affected by risks and uncertainties relating to a number of other factors, including the following: the success, cost and timing of the Company’s product development activities, studies and clinical trials; the success of competing products that are or become available; the Company’s ability to commercialize its product candidates; the size and growth potential of the markets for the Company’s product candidates, and the Company’s ability to service those markets; the Company’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators;
the rate and degree of market acceptance of the Company’s product candidates; and the Company’s expectations regarding its ability to obtain and adequately maintain sufficient intellectual property protection for its product candidates. This list is not exhaustive and these and other risks are described in the Company’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the Securities and Exchange Commission and available at www.sec.gov. Any forward-looking statements that the Company makes in this press release speak only as of the date of this press release. The Company assumes no obligation to update forward-looking statements whether as a result of new information, future events or otherwise, after the date of this press release.
Zynerba Contact
Will Roberts, VP Investor Relations and Corporate Communications
484.581.7489
robertsw@zynerba.com
Media contact
Theresa Dolge
Tonic Life Communications
Office: 215-928-2748
Theresa.Dolge@toniclc.com