
Zynerba Pharmaceuticals Announces Twelve Month ZYN002 Data from STAR 2 Study in Patients with
Focal Seizures at the 2018 Annual Meeting of the American Academy of Neurology (AAN)
- Continued Improvement in Seizure Control Seen in Adult Refractory Focal Seizure Patients Receiving ZYN002 through 12 Months of Open Label Exposure–
Los Angeles, CA., April 25, 2018 — Zynerba Pharmaceuticals, Inc. (NASDAQ:ZYNE), a clinical-stage specialty neuropsychiatric pharmaceutical company dedicated to developing and commercializing innovative pharmaceutically-produced transdermal cannabinoid treatments for rare and near-rare neurological and psychiatric disorders with high unmet medical needs, is reporting new longer term open label clinical data today in the Emerging Science session of the 2018 Annual Meeting of the American Academy of Neurology (AAN) in Los Angeles, CA.
In a poster presentation entitled, “Transdermal Cannabidiol (CBD) Gel for the Treatment of Focal Epilepsy in Adults” (poster P4.468), Dr. John Messenheimer presents additional data from ongoing STAR 2 (Synthetic Transdermal CAnnabidiol for the TReatment of Epilepsy) 24-month open label extension study evaluating ZYN002 cannabidiol (CBD) transdermal gel in adult patients with focal seizures. The presentation includes data through twelve months of open label exposure to ZYN002.
The key findings include that responses to ZYN002 in the STAR 2 open label extension, as measured by reductions in focal seizures from the baseline period of STAR 1, are associated with continued treatment with ZYN002. In addition, ZYN002 was shown to be well tolerated through 12 months of treatment in STAR 2.
“These data continue to suggest that focal seizures may be reduced with longer-term exposure to transdermally-delivered CBD,” said Dr. Liza Squires, Zynerba’s Chief Medical Officer. “In this population of patients, the use of ZYN002 for an additional 12 months in STAR 2 was well tolerated and appeared to result in clinically meaningful seizure reductions both across and within the originally randomized STAR 1 groups. These data continue to provide insight into the potential for ZYN002 in certain epilepsies, and we look forward to initiating a Phase 2b study in adult refractory focal seizures in the second half of 2018.”
The data presented in the poster are as follows:
· 188 patients were randomized, 186 were analyzed for efficacy, and 174 completed STAR 1;
· 171 patients (98% of STAR 1 completers) continued into STAR 2;
· As of December 12, 2017, 95 patients remained in the STAR 2 study;
· Patients were taking a wide range of antiepileptic drugs (AEDs), the most common of which are levetiracetam, carbamazepine, lamotrigine, lacosamide, and valproate.
Efficacy
· In STAR 2, longer term exposure to ZYN002 resulted in greater improvements in seizure frequency among all ZYN002-treated patients;
· Longer term exposure to ZYN002 appeared to result in clinically meaningful reductions in seizures both across and within originally randomized STAR 1 patient groups:
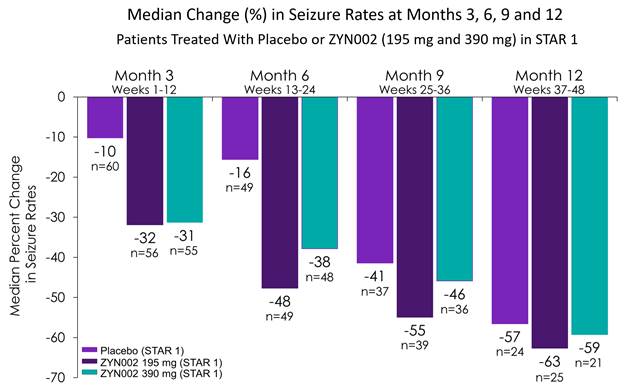
· Compared to baseline, the median changes in seizure rates at months 3, 6, 9, and 12 of STAR 2 across all ZYN002-treated patients at each time point were:
· 25% reduction at month 3 (N=171)
· 36% reduction at month 6 (N=146)
· 49% reduction at month 9 (N=112)
· 58% reduction at month 12 (N=70)

· Compared to baseline, the median changes in seizure rates at months 3, 6, 9, and 12 of STAR 2 in patients initially treated with placebo or ZYN002 in STAR 1 were:
|
| Median Change (%) in Seizure Rates in STAR 2 Study | ||||||
|
| Month 3 |
| Month 6 |
| Month 9 |
| Month 12 |
Placebo (STAR 1) |
| -10 (n=60) |
| -16 (n=49) |
| -41 (n=37) |
| -57 (n=24) |
ZYN002 195 mg (STAR 1) |
| -32 (n=56) |
| -48 (n=49) |
| -55 (n=39) |
| -63 (n=25) |
ZYN002 390 mg (STAR 1) |
| -31 (n=55) |
| -38 (n=48) |
| -46 (n=36) |
| -59 (n=21) |
Safety
· ZYN002 was well tolerated with good skin tolerability;
· In STAR 2, the most common treatment-emergent AEs (>7.5%) were upper respiratory tract infection (viral and bacterial [17%]) and headache (11%);
· One serious AE was considered possibly related to ZYN002 (increased anxiety);
· There were no clinically significant abnormal liver AEs (i.e. alanine aminotransferase/aspartate aminotransferase) >3x upper limit of normal reported for patients receiving ZYN002.
A copy of the poster presentation is currently available on the Zynerba corporate website at http://zynerba.com/publications/
About ZYN002
Zynerba’s ZYN002 CBD gel is the first and only pharmaceutically-produced CBD formulated as a patent-protected permeation-enhanced gel and is being studied in children with Fragile X Syndrome, adult epilepsy patients with focal seizures and osteoarthritis. ZYN002 is a clear, permeation-enhanced gel that is designed to provide controlled drug delivery transdermally with once- or twice-daily dosing.
About Zynerba Pharmaceuticals, Inc.
Zynerba Pharmaceuticals (NASDAQ: ZYNE) is a clinical-stage specialty pharmaceutical company dedicated to developing and commercializing innovative pharmaceutically-produced transdermal cannabinoid treatments for rare or near-rare neuropsychiatric diseases with high unmet medical needs. We are dedicated to improving the lives of people with severe health conditions by developing cannabinoid medicines designed to meet the rigorous efficacy and safety standards established by global regulatory agencies. Through the discovery and development of these potentially life-changing medicines, Zynerba seeks to improve the lives of patients battling severe, chronic health conditions including Fragile X syndrome, refractory epilepsies, Tourette Syndrome, and other neuropsychiatric disorders. Learn more at www.zynerba.com and follow the Company on Twitter at @ZynerbaPharma.
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from the Company’s current expectations. For example, there can be no guarantee that the Company will obtain approval for ZYN002 or ZYN001 from the U.S. Food and Drug Administration (FDA) or foreign regulatory authorities; even if ZYN002 or ZYN001 are approved, the Company may not be able to obtain the label claims that it is seeking from the FDA. In addition, the Company’s cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated. Management’s expectations and, therefore, any forward-looking statements in this press release could also be affected by risks and uncertainties relating to a number of other factors, including the following: the success, cost and timing of the Company’s product development activities, studies and
clinical trials; the success of competing products that are or become available; the Company’s ability to commercialize its product candidates; the size and growth potential of the markets for the Company’s product candidates, and the Company’s ability to service those markets; the Company’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators; the rate and degree of market acceptance of the Company’s product candidates; and the Company’s expectations regarding its ability to obtain and adequately maintain sufficient intellectual property protection for its product candidates. This list is not exhaustive and these and other risks are described in the Company’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the Securities and Exchange Commission and available at www.sec.gov. Any forward-looking statements that the Company makes in this press release speak only as of the date of this press release. The Company assumes no obligation to update forward-looking statements whether as a result of new information, future events or otherwise, after the date of this press release.
Zynerba Contact
Will Roberts, VP Investor Relations and Corporate Communications
484.581.7489
robertsw@zynerba.com
Media contact
Theresa Dolge
Tonic Life Communications
Office: 215-928-2748
Theresa.Dolge@toniclc.com